User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
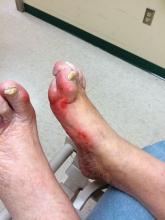
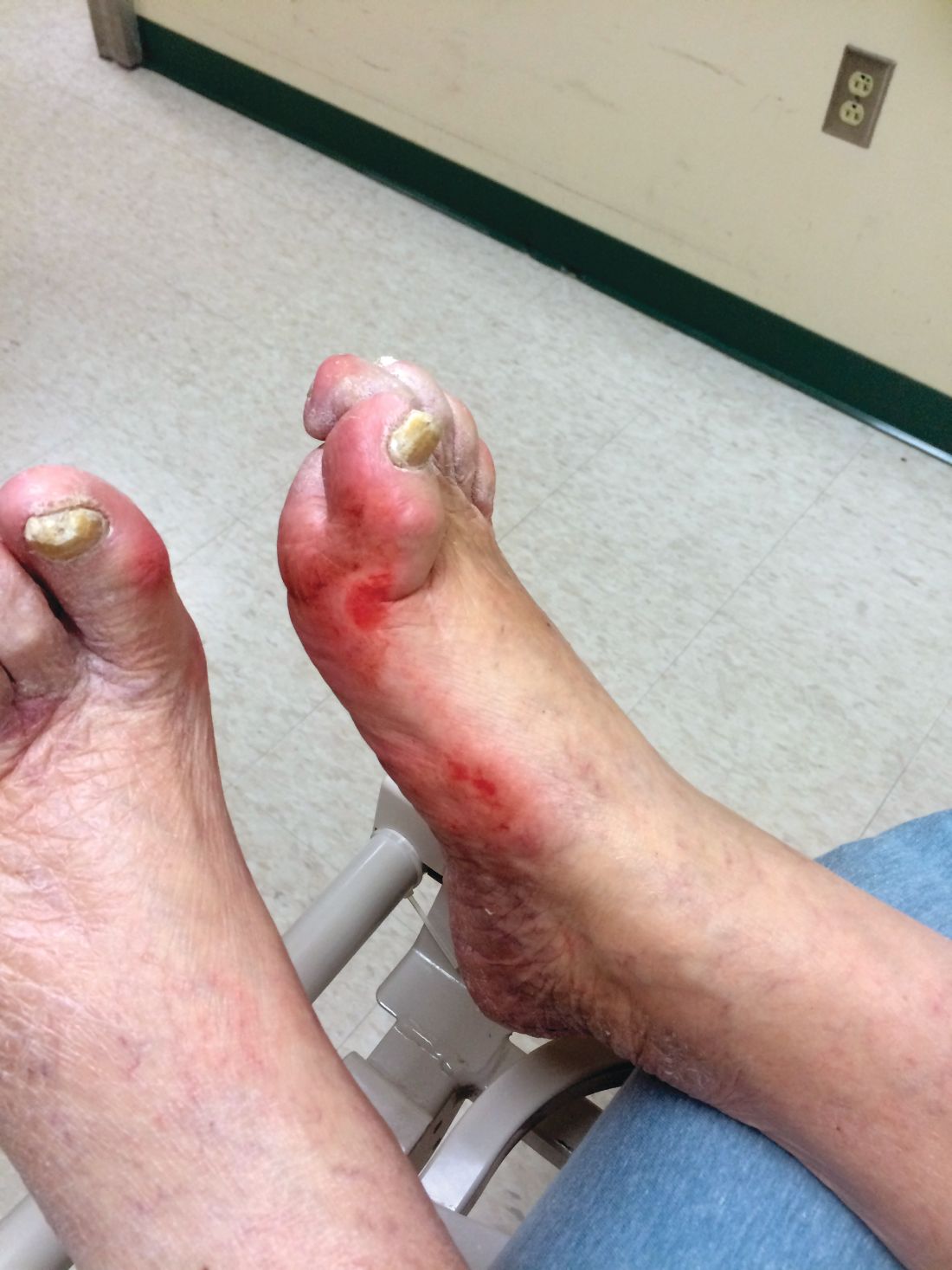
Diabetic foot ulcer healing is predictable by WIfI stage scores
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: The Wound, Ischemia, and foot Infection (WIfI) classification of diabetic foot ulcers provides a predictable primary outcome for wound healing at 1 year.
Major finding: Wound healing probability at 1 year was 94.1% for WIfI stage 1 wounds and 67.4% for stage 4 wounds.
Study details: A single-location, multidisciplinary-setting, retrospective study of 709 WIfI stage 1-4 wounds presented by 310 diabetic foot ulcer patients.
Disclosures: The authors reported no conflicts of interest.
Source: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Thyroid markers linked to risk of gestational diabetes
Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes, results of a longitudinal study suggest.
Increased levels of free triiodothyronine (fT3) and the ratio of fT3 to free thyroxine (fT4) were associated with increased risk of this common metabolic complication of pregnancy, study authors reported in the Journal of Clinical Endocrinology & Metabolism.
“To our knowledge, this is the first study to identify fT3 and the fT3:fT4 ratio measured early in pregnancy as independent risk factors of gestational diabetes,” wrote Shristi Rawal, PhD, of the National Institute of Child Health and Human Development (NICHD) , and her colleagues.
Although routine thyroid function screening during pregnancy remains controversial, Dr. Rawal and colleagues said their results support the “potential benefits” of the practice, particularly in light of other recent evidence suggesting thyroid-related adverse pregnancy outcomes.
The current case control study by Dr. Rawal and her coinvestigators included 107 women with gestational diabetes and 214 nongestational diabetes controls selected from a 12-center pregnancy cohort, which included 2,802 women aged between 18 and 40 years. The thyroid markers fT3, fT4, and thyroid-stimulating hormone (TSH) were measured at four pregnancy visits, including first trimester (weeks 10-14) and second trimester (weeks 15-26).
The fT3:fT4 ratio had the strongest association with gestational diabetes. In the second trimester measurement, women in the highest quartile had an almost 14-fold increase in risk when compared to the lowest quartile, after adjusting for potential confounders including prepregnancy body mass index and diabetes family history (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30), Dr. Rawal and her colleagues reported. The ratio of fT3:fT4 at the first trimester was also associated with increased risk (aOR, 8.63; 95% CI, 2.87-26.00).
Similarly, fT3 was positively associated with gestational diabetes at the first trimester (aOR, 4.25; 95% CI, 1.67-10.80) and second trimester (aOR, 3.89; 95% CI, 1.50-10.10), investigators reported.
By contrast, there was no association between fT4 or TSH and gestational diabetes, they found.
“These findings, in combination with previous evidence of thyroid-related adverse pregnancy outcomes, support the benefits of thyroid screening among pregnant women in early to mid pregnancy,” senior author Cuilin Zhang, MD, MPH, PhD, of the NICHD, said in a press statement.
Thyroid function abnormalities are relatively common in pregnant women and have been associated with obstetric complications such as pregnancy loss and premature delivery, investigators noted.
Previous evidence is sparse regarding a potential link between thyroid dysfunction and gestational diabetes. There are some prospective studies that show women with hypothyroidism have an increased incidence of gestational diabetes, Dr. Rawal and her colleagues wrote. Isolated hypothyroxinema, or normal TSH and low fT4, has also been linked to increased risk in some studies, but not in others, they added.
Support for the study came from NICHD and the American Recovery and Reinvestment Act research grants. The authors reported no conflicts of interest.
SOURCE: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes, results of a longitudinal study suggest.
Increased levels of free triiodothyronine (fT3) and the ratio of fT3 to free thyroxine (fT4) were associated with increased risk of this common metabolic complication of pregnancy, study authors reported in the Journal of Clinical Endocrinology & Metabolism.
“To our knowledge, this is the first study to identify fT3 and the fT3:fT4 ratio measured early in pregnancy as independent risk factors of gestational diabetes,” wrote Shristi Rawal, PhD, of the National Institute of Child Health and Human Development (NICHD) , and her colleagues.
Although routine thyroid function screening during pregnancy remains controversial, Dr. Rawal and colleagues said their results support the “potential benefits” of the practice, particularly in light of other recent evidence suggesting thyroid-related adverse pregnancy outcomes.
The current case control study by Dr. Rawal and her coinvestigators included 107 women with gestational diabetes and 214 nongestational diabetes controls selected from a 12-center pregnancy cohort, which included 2,802 women aged between 18 and 40 years. The thyroid markers fT3, fT4, and thyroid-stimulating hormone (TSH) were measured at four pregnancy visits, including first trimester (weeks 10-14) and second trimester (weeks 15-26).
The fT3:fT4 ratio had the strongest association with gestational diabetes. In the second trimester measurement, women in the highest quartile had an almost 14-fold increase in risk when compared to the lowest quartile, after adjusting for potential confounders including prepregnancy body mass index and diabetes family history (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30), Dr. Rawal and her colleagues reported. The ratio of fT3:fT4 at the first trimester was also associated with increased risk (aOR, 8.63; 95% CI, 2.87-26.00).
Similarly, fT3 was positively associated with gestational diabetes at the first trimester (aOR, 4.25; 95% CI, 1.67-10.80) and second trimester (aOR, 3.89; 95% CI, 1.50-10.10), investigators reported.
By contrast, there was no association between fT4 or TSH and gestational diabetes, they found.
“These findings, in combination with previous evidence of thyroid-related adverse pregnancy outcomes, support the benefits of thyroid screening among pregnant women in early to mid pregnancy,” senior author Cuilin Zhang, MD, MPH, PhD, of the NICHD, said in a press statement.
Thyroid function abnormalities are relatively common in pregnant women and have been associated with obstetric complications such as pregnancy loss and premature delivery, investigators noted.
Previous evidence is sparse regarding a potential link between thyroid dysfunction and gestational diabetes. There are some prospective studies that show women with hypothyroidism have an increased incidence of gestational diabetes, Dr. Rawal and her colleagues wrote. Isolated hypothyroxinema, or normal TSH and low fT4, has also been linked to increased risk in some studies, but not in others, they added.
Support for the study came from NICHD and the American Recovery and Reinvestment Act research grants. The authors reported no conflicts of interest.
SOURCE: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes, results of a longitudinal study suggest.
Increased levels of free triiodothyronine (fT3) and the ratio of fT3 to free thyroxine (fT4) were associated with increased risk of this common metabolic complication of pregnancy, study authors reported in the Journal of Clinical Endocrinology & Metabolism.
“To our knowledge, this is the first study to identify fT3 and the fT3:fT4 ratio measured early in pregnancy as independent risk factors of gestational diabetes,” wrote Shristi Rawal, PhD, of the National Institute of Child Health and Human Development (NICHD) , and her colleagues.
Although routine thyroid function screening during pregnancy remains controversial, Dr. Rawal and colleagues said their results support the “potential benefits” of the practice, particularly in light of other recent evidence suggesting thyroid-related adverse pregnancy outcomes.
The current case control study by Dr. Rawal and her coinvestigators included 107 women with gestational diabetes and 214 nongestational diabetes controls selected from a 12-center pregnancy cohort, which included 2,802 women aged between 18 and 40 years. The thyroid markers fT3, fT4, and thyroid-stimulating hormone (TSH) were measured at four pregnancy visits, including first trimester (weeks 10-14) and second trimester (weeks 15-26).
The fT3:fT4 ratio had the strongest association with gestational diabetes. In the second trimester measurement, women in the highest quartile had an almost 14-fold increase in risk when compared to the lowest quartile, after adjusting for potential confounders including prepregnancy body mass index and diabetes family history (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30), Dr. Rawal and her colleagues reported. The ratio of fT3:fT4 at the first trimester was also associated with increased risk (aOR, 8.63; 95% CI, 2.87-26.00).
Similarly, fT3 was positively associated with gestational diabetes at the first trimester (aOR, 4.25; 95% CI, 1.67-10.80) and second trimester (aOR, 3.89; 95% CI, 1.50-10.10), investigators reported.
By contrast, there was no association between fT4 or TSH and gestational diabetes, they found.
“These findings, in combination with previous evidence of thyroid-related adverse pregnancy outcomes, support the benefits of thyroid screening among pregnant women in early to mid pregnancy,” senior author Cuilin Zhang, MD, MPH, PhD, of the NICHD, said in a press statement.
Thyroid function abnormalities are relatively common in pregnant women and have been associated with obstetric complications such as pregnancy loss and premature delivery, investigators noted.
Previous evidence is sparse regarding a potential link between thyroid dysfunction and gestational diabetes. There are some prospective studies that show women with hypothyroidism have an increased incidence of gestational diabetes, Dr. Rawal and her colleagues wrote. Isolated hypothyroxinema, or normal TSH and low fT4, has also been linked to increased risk in some studies, but not in others, they added.
Support for the study came from NICHD and the American Recovery and Reinvestment Act research grants. The authors reported no conflicts of interest.
SOURCE: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM.
Key clinical point: Thyroid dysfunction early in pregnancy may increase risk of gestational diabetes.
Major finding: The triiodothyronine to thyroxine ratio in the second trimester had the strongest association with gestational diabetes (adjusted odds ratio, 13.60; 95% confidence interval, 3.97-46.30).
Study details: A case control study including 107 gestational diabetes cases and 214 nongestational diabetes controls.
Disclosures: The authors had no disclosures. Support for the study came from the National Institute of Child Health and Human Development and the American Recovery and Reinvestment Act research grants.
Source: Rawal S et al. J Clin Endocrinol Metab. 2018 Jun 7. doi: 10.1210/jc.2017-024421.
Bariatric revision mortality linked to age, comorbidities
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
REPORTING FROM DDW 2018
Key clinical point: Revisional bariatric procedures may carry a greater mortality risk than previous studies have suggested.
Major finding: The mortality rate in the sample was 2.1%.
Study details: The 2014 Nationwide Inpatient Sample database, comprising 14,280 patients who underwent revisional bariatric surgery.
Disclosures: Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
Source: Popov VB et al. DDW 2018, Abstract 324.
Headline can be up to 255 characters
- The article body can contain (4 total):
- Images
- Videos
- Poll Daddy surveys
- Hyerplinks
- And text formatting (ital, bold, underline, etc.).
- 2,500 character limit with spaces
Image
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. "Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum."
"Quote or highlighted text."
Table
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Video

Poll
[polldaddy:10018427]
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Table
| Column Header | Column Header | Column Header |
|---|---|---|
| Column Text | Column Text | Column Text |
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
- The article body can contain (4 total):
- Images
- Videos
- Poll Daddy surveys
- Hyerplinks
- And text formatting (ital, bold, underline, etc.).
- 2,500 character limit with spaces
Image
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. "Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum."
"Quote or highlighted text."
Table
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Video

Poll
[polldaddy:10018427]
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Table
| Column Header | Column Header | Column Header |
|---|---|---|
| Column Text | Column Text | Column Text |
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
- The article body can contain (4 total):
- Images
- Videos
- Poll Daddy surveys
- Hyerplinks
- And text formatting (ital, bold, underline, etc.).
- 2,500 character limit with spaces
Image
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. "Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum."
"Quote or highlighted text."
Table
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Video

Poll
[polldaddy:10018427]
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Table
| Column Header | Column Header | Column Header |
|---|---|---|
| Column Text | Column Text | Column Text |
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Beyond the sleeve and RYGB: The frontier of bariatric procedures
BOSTON – Though are the bariatric procedures most patients will receive, other surgical approaches to weight loss are occasionally performed. Knowing these various techniques and their likely efficacy and safety can help physicians who care for patients with obesity, whether a patient is considering a less common option, or whether a post-vagal blockade patient shows up on the schedule with long-term issues.
A common theme among many of these procedures is that overall numbers are low, long-term follow-up may be lacking, and research quality is variable, said Travis McKenzie, MD, speaking at a bariatric surgery-focused session of the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists.
One minimally invasive approach targets stomach functions and the appetite and satiety signaling system. In vagal blockade via an electronic implant (vBloc), an indwelling, removable device produces electronically-induced intermittent blockade of the vagal nerve.
In one randomized controlled trial, excess weight loss in patients receiving this procedure was 24%, significantly more than the 16% seen in the group that received a sham procedure (P = .002); both groups received regular follow-up and counseling, according to the study protocol. Overall, at 1 year, 52% of those in the treatment group had seen at least 20% reduction in excess weight; just 3.7% of vBloc recipients had adverse events, mostly some dyspepsia and pain at the implant site, said Dr. McKenzie, an endocrine surgeon at the Mayo Clinic, Rochester, Minn.
The vBloc procedure, said Dr. McKenzie, “demonstrated modest weight loss at 2 years, with a reasonable risk profile.”
A variation of the duodenal switch is known as single anastomosis duodeno-ileal bypass with sleeve gastrectomy, or SADI-S. This procedure both resects the greater curve of the stomach to create a gastric sleeve, and uses a single intestinal anastomosis to create a common channel of 200, 250 or 300 cm, bypassing most of the small intestine.
In this procedure, also known as the one-anastomosis duodenal switch (OADS), weight loss occurs both because of intestinal malabsorption and because of the reduced stomach volume.
Parsing safety and efficacy of this procedure isn’t easy, given the studies at hand, said Dr. McKenzie: “The data are plagued by short follow-up, low numbers, and inconsistent quality.” Of the 14 case series following 1,045 patients, none include randomized controlled data, he said.
The data that are available show total body weight loss in the 34%-39% range, with little difference between losses seen at 1 year and 2 years.
However, said Dr. McKenzie, one 100-patient case series showed that SADI-S patients averaged 2.5 bowel movements daily after the procedure, and two patients needed surgical revision because they were experiencing malnutrition. Anemia, vitamin B12 and D deficiencies, and folate deficiency are all commonly seen two years after SADI-S procedures, he said.
“The OADS procedure is very effective, although better data are needed before drawing conclusions,” said Dr. McKenzie.
A gastric bypass variation known as the “mini” bypass, or the one anastomosis gastric bypass (OAGB), is another less common bariatric technique. In this procedure, a small gastric pouch is created that forms the working stomach, which is then connected to the duodenum with bypassing of a significant portion (up to 200 cm) of the small intestine. This procedure causes both restrictive and malabsorptive weight loss, and is usually performed using minimally invasive surgery.
Four randomized controlled trials exist, said Dr. McKenzie, that compare OAGB variously to Roux-en-Y gastric bypass (RYGB) and to sleeve gastrectomy. In an 80-patient study that compared OAGB with RYGB at two years post-procedure, excess weight loss was similar, at 60% for OAGB versus 64% for RYGB ( Ann Surg. 2005;24[1]20-8). However, morbidity was less for OAGB recipients (8% vs 20%, P less than .05).
Another study looked at OAGB and sleeve gastrectomy in 60 patients, following them for 5 years. Total body weight loss was similar between groups at 20%-23%, said Dr. McKenzie (Obes Surg. 2014;24[9]1552-62).
“But what about bile reflux?” asked Dr. McKenzie. He pointed out that in OAGB, digestive juices enter the digestive path very close to the outlet of the new, surgically created stomach, affording the potential for significant reflux. Calling for further study of the frequency of bile reflux and potential long-term sequelae, he advised caution with this otherwise attractive procedure.
Those caring for bariatric patients may also see the consequences of “rogue” procedures on occasion: “Interest in metabolic surgery has led to some ‘original’ procedures, many of which are not based on firm science,” said Dr. McKenzie. An exemplar of an understudied procedure is the sleeve gastrectomy with a loop bipartition, with results that have been published in case reports, but whose longer-term outcomes are unknown.
“Caution is advised regarding operations that are devised outside of study protocols,” said Dr. McKenzie.
Dr. McKenzie reported that he had no relevant financial disclosures.
SOURCE: McKenzie, T. AACE 2018, presentation SGS4.
BOSTON – Though are the bariatric procedures most patients will receive, other surgical approaches to weight loss are occasionally performed. Knowing these various techniques and their likely efficacy and safety can help physicians who care for patients with obesity, whether a patient is considering a less common option, or whether a post-vagal blockade patient shows up on the schedule with long-term issues.
A common theme among many of these procedures is that overall numbers are low, long-term follow-up may be lacking, and research quality is variable, said Travis McKenzie, MD, speaking at a bariatric surgery-focused session of the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists.
One minimally invasive approach targets stomach functions and the appetite and satiety signaling system. In vagal blockade via an electronic implant (vBloc), an indwelling, removable device produces electronically-induced intermittent blockade of the vagal nerve.
In one randomized controlled trial, excess weight loss in patients receiving this procedure was 24%, significantly more than the 16% seen in the group that received a sham procedure (P = .002); both groups received regular follow-up and counseling, according to the study protocol. Overall, at 1 year, 52% of those in the treatment group had seen at least 20% reduction in excess weight; just 3.7% of vBloc recipients had adverse events, mostly some dyspepsia and pain at the implant site, said Dr. McKenzie, an endocrine surgeon at the Mayo Clinic, Rochester, Minn.
The vBloc procedure, said Dr. McKenzie, “demonstrated modest weight loss at 2 years, with a reasonable risk profile.”
A variation of the duodenal switch is known as single anastomosis duodeno-ileal bypass with sleeve gastrectomy, or SADI-S. This procedure both resects the greater curve of the stomach to create a gastric sleeve, and uses a single intestinal anastomosis to create a common channel of 200, 250 or 300 cm, bypassing most of the small intestine.
In this procedure, also known as the one-anastomosis duodenal switch (OADS), weight loss occurs both because of intestinal malabsorption and because of the reduced stomach volume.
Parsing safety and efficacy of this procedure isn’t easy, given the studies at hand, said Dr. McKenzie: “The data are plagued by short follow-up, low numbers, and inconsistent quality.” Of the 14 case series following 1,045 patients, none include randomized controlled data, he said.
The data that are available show total body weight loss in the 34%-39% range, with little difference between losses seen at 1 year and 2 years.
However, said Dr. McKenzie, one 100-patient case series showed that SADI-S patients averaged 2.5 bowel movements daily after the procedure, and two patients needed surgical revision because they were experiencing malnutrition. Anemia, vitamin B12 and D deficiencies, and folate deficiency are all commonly seen two years after SADI-S procedures, he said.
“The OADS procedure is very effective, although better data are needed before drawing conclusions,” said Dr. McKenzie.
A gastric bypass variation known as the “mini” bypass, or the one anastomosis gastric bypass (OAGB), is another less common bariatric technique. In this procedure, a small gastric pouch is created that forms the working stomach, which is then connected to the duodenum with bypassing of a significant portion (up to 200 cm) of the small intestine. This procedure causes both restrictive and malabsorptive weight loss, and is usually performed using minimally invasive surgery.
Four randomized controlled trials exist, said Dr. McKenzie, that compare OAGB variously to Roux-en-Y gastric bypass (RYGB) and to sleeve gastrectomy. In an 80-patient study that compared OAGB with RYGB at two years post-procedure, excess weight loss was similar, at 60% for OAGB versus 64% for RYGB ( Ann Surg. 2005;24[1]20-8). However, morbidity was less for OAGB recipients (8% vs 20%, P less than .05).
Another study looked at OAGB and sleeve gastrectomy in 60 patients, following them for 5 years. Total body weight loss was similar between groups at 20%-23%, said Dr. McKenzie (Obes Surg. 2014;24[9]1552-62).
“But what about bile reflux?” asked Dr. McKenzie. He pointed out that in OAGB, digestive juices enter the digestive path very close to the outlet of the new, surgically created stomach, affording the potential for significant reflux. Calling for further study of the frequency of bile reflux and potential long-term sequelae, he advised caution with this otherwise attractive procedure.
Those caring for bariatric patients may also see the consequences of “rogue” procedures on occasion: “Interest in metabolic surgery has led to some ‘original’ procedures, many of which are not based on firm science,” said Dr. McKenzie. An exemplar of an understudied procedure is the sleeve gastrectomy with a loop bipartition, with results that have been published in case reports, but whose longer-term outcomes are unknown.
“Caution is advised regarding operations that are devised outside of study protocols,” said Dr. McKenzie.
Dr. McKenzie reported that he had no relevant financial disclosures.
SOURCE: McKenzie, T. AACE 2018, presentation SGS4.
BOSTON – Though are the bariatric procedures most patients will receive, other surgical approaches to weight loss are occasionally performed. Knowing these various techniques and their likely efficacy and safety can help physicians who care for patients with obesity, whether a patient is considering a less common option, or whether a post-vagal blockade patient shows up on the schedule with long-term issues.
A common theme among many of these procedures is that overall numbers are low, long-term follow-up may be lacking, and research quality is variable, said Travis McKenzie, MD, speaking at a bariatric surgery-focused session of the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists.
One minimally invasive approach targets stomach functions and the appetite and satiety signaling system. In vagal blockade via an electronic implant (vBloc), an indwelling, removable device produces electronically-induced intermittent blockade of the vagal nerve.
In one randomized controlled trial, excess weight loss in patients receiving this procedure was 24%, significantly more than the 16% seen in the group that received a sham procedure (P = .002); both groups received regular follow-up and counseling, according to the study protocol. Overall, at 1 year, 52% of those in the treatment group had seen at least 20% reduction in excess weight; just 3.7% of vBloc recipients had adverse events, mostly some dyspepsia and pain at the implant site, said Dr. McKenzie, an endocrine surgeon at the Mayo Clinic, Rochester, Minn.
The vBloc procedure, said Dr. McKenzie, “demonstrated modest weight loss at 2 years, with a reasonable risk profile.”
A variation of the duodenal switch is known as single anastomosis duodeno-ileal bypass with sleeve gastrectomy, or SADI-S. This procedure both resects the greater curve of the stomach to create a gastric sleeve, and uses a single intestinal anastomosis to create a common channel of 200, 250 or 300 cm, bypassing most of the small intestine.
In this procedure, also known as the one-anastomosis duodenal switch (OADS), weight loss occurs both because of intestinal malabsorption and because of the reduced stomach volume.
Parsing safety and efficacy of this procedure isn’t easy, given the studies at hand, said Dr. McKenzie: “The data are plagued by short follow-up, low numbers, and inconsistent quality.” Of the 14 case series following 1,045 patients, none include randomized controlled data, he said.
The data that are available show total body weight loss in the 34%-39% range, with little difference between losses seen at 1 year and 2 years.
However, said Dr. McKenzie, one 100-patient case series showed that SADI-S patients averaged 2.5 bowel movements daily after the procedure, and two patients needed surgical revision because they were experiencing malnutrition. Anemia, vitamin B12 and D deficiencies, and folate deficiency are all commonly seen two years after SADI-S procedures, he said.
“The OADS procedure is very effective, although better data are needed before drawing conclusions,” said Dr. McKenzie.
A gastric bypass variation known as the “mini” bypass, or the one anastomosis gastric bypass (OAGB), is another less common bariatric technique. In this procedure, a small gastric pouch is created that forms the working stomach, which is then connected to the duodenum with bypassing of a significant portion (up to 200 cm) of the small intestine. This procedure causes both restrictive and malabsorptive weight loss, and is usually performed using minimally invasive surgery.
Four randomized controlled trials exist, said Dr. McKenzie, that compare OAGB variously to Roux-en-Y gastric bypass (RYGB) and to sleeve gastrectomy. In an 80-patient study that compared OAGB with RYGB at two years post-procedure, excess weight loss was similar, at 60% for OAGB versus 64% for RYGB ( Ann Surg. 2005;24[1]20-8). However, morbidity was less for OAGB recipients (8% vs 20%, P less than .05).
Another study looked at OAGB and sleeve gastrectomy in 60 patients, following them for 5 years. Total body weight loss was similar between groups at 20%-23%, said Dr. McKenzie (Obes Surg. 2014;24[9]1552-62).
“But what about bile reflux?” asked Dr. McKenzie. He pointed out that in OAGB, digestive juices enter the digestive path very close to the outlet of the new, surgically created stomach, affording the potential for significant reflux. Calling for further study of the frequency of bile reflux and potential long-term sequelae, he advised caution with this otherwise attractive procedure.
Those caring for bariatric patients may also see the consequences of “rogue” procedures on occasion: “Interest in metabolic surgery has led to some ‘original’ procedures, many of which are not based on firm science,” said Dr. McKenzie. An exemplar of an understudied procedure is the sleeve gastrectomy with a loop bipartition, with results that have been published in case reports, but whose longer-term outcomes are unknown.
“Caution is advised regarding operations that are devised outside of study protocols,” said Dr. McKenzie.
Dr. McKenzie reported that he had no relevant financial disclosures.
SOURCE: McKenzie, T. AACE 2018, presentation SGS4.
EXPERT ANALYSIS FROM AACE 2018
The case for bariatric surgery to manage CV risk in diabetes
BOSTON – For patients with obesity and metabolic syndrome or type 2 diabetes ( health over the lifespan.
“Behavioral changes in diet and activity may be effective over the short term, but they are often ineffective over the long term,” said Daniel L. Hurley, MD. By contrast, “Bariatric surgery is very effective long-term,” he said.
At the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists, Dr. Hurley made the case for bariatric surgery in effective and durable management of type 2 diabetes and cardiovascular risk, weighing risks and benefits for those with higher and lower levels of obesity.
Speaking during a morning session focused on bariatric surgery, Dr. Hurley, an endocrionologist at the Mayo Clinic, Rochester, Minn., noted that bariatric surgery reduces not just weight, but also visceral adiposity. This, he said, is important when thinking about type 2 diabetes (T2D), because diabetes prevalence has climbed in the United States as obesity has also increased, according to examination of data from the National Health and Nutrition Examination Survey (NHANES).
Additionally, increased abdominal adiposity is associated with increased risk for cardiovascular-related deaths, myocardial infarctions, and all-cause deaths. Some of this relationship is mediated by T2D, which itself “is a major cause of cardiovascular-related morbidity and mortality,” said Dr. Hurley.
From a population health perspective, the increased prevalence of T2D – expected to reach 10% in the United States by 2030 – will also boost cardiovascular morbidity and mortality, said Dr. Hurley. Those with T2D die 5 to 10 years earlier, and have double the risk for heart attack and stroke of their peers without diabetes. The risk of lower limb amputation can be as much as 40 times greater for an individual with T2D across the lifespan, he said.
The National Institutes of Health recognizes bariatric surgery as an appropriate weight loss therapy for individuals with a body mass index (BMI) of at least 35 kg/m2 and comorbidity. Whether bariatric surgery might be appropriate for individuals with T2D and BMIs of less than 35 kg/m2 is less settled, though at least some RCTs support the surgical approach, said Dr. Hurley.
The body of data that support long-term metabolic and cardiovascular benefits of bariatric surgery as obesity therapy is growing, said Dr. Hurley. A large prospective observational study by the American College of Surgeons’ Bariatric Surgery Center Network followed 28,616 patients, finding that Roux-en-Y gastric bypass (RYGB) was most effective in improving or resolving CVD comorbidities. At 1 year post surgery, 83% of RYGB patients saw improvement or resolution of T2D; the figure was 79% for hypertension and 66% for dyslipidemia (Ann Surg. 2011;254[3]:410-20).
Weight loss for patients receiving bariatric procedures has generally been durable: for laparoscopic RYGB patients tracked to 7 years after surgery, 75% had maintained at least a 20% weight loss (JAMA Surg. 2018;153[5]427-34).
Longer-term clinical follow-up points toward favorable metabolic and cardiovascular outcomes, said Dr. Hurley, citing data from the Swedish Obese Subjects (SOS) trial. This study followed over 4,000 patients with high BMIs (at least 34 kg/m2 for men and 38 kg/m2 for women) over 10 years. At that point, 36% of gastric bypass patients, compared with 13% of non-surgical high BMI patients, saw resolution of T2D, a significant difference. Triglyceride levels also fell significantly more for the bypass recipients. Hypertension was resolved in just 19% of patients at 10 years, a non-significant difference from the 11% of control patients. Data from the same patient set also showed a significant reduction in total cardiovascular events in the surgical versus non-surgical patients (n = 49 vs. 28, hazard ratio 0.83, log-rank P = .05). Fatal cardiovascular events were significantly lower for patients who had received bariatric surgery, with a 24% decline in mortality for bariatric surgery patients at about 11 years post surgery.
Canadian data showed even greater reductions in mortality, with an 89% decrease in mortality after RYGB, compared with non-surgical patients at the 5-year mark (Ann Surg 2004;240:416-24).
In trials that afforded a direct comparison of medical therapy and bariatric surgery obesity and diabetes, Dr. Hurley said that randomized trials generally show no change to modest change in HbA1c levels with medical management. By contrast, patients in the surgical arms showed a range of improvement ranging from a reduction of just under 1% to reductions of over 5%, with an average reduction of more than 2% across the trials.
Separating out data from the randomized controlled trials with patient BMIs averaging 35 kg/m2 or less, odds ratios still favored bariatric surgery over medication therapy for diabetes-related outcomes in this lower-BMI population, said Dr. Hurley (Diabetes Care 2016;39:924-33).
More data come from a recently reported randomized trial that assigned patients with T2D and a mean BMI of 37 kg/m2 (range, 27-43) to intensive medical therapy, or either sleeve gastrectomy (SG) or RYGB. The study, which had a 90% completion rate at the 5-year mark, found that both surgical procedures were significantly more effective at reducing HbA1c to 6% or less 12 months into the study (P less than .001).
At the 60-month mark, 45% of the RYGB and 25% of the SG patients were on no diabetes medications, while just 2% of the medical therapy arm had stopped all medications, and 40% of this group remained on insulin 5 years into the study, said Dr. Hurley (N Engl J Med. 2017;376:641-651).
“For treatment of type 2 diabetes and cardiovascular co-morbidities, long-term goals often are met following bariatric surgery versus behavior change,” said Dr. Hurley.
Dr. Hurley reported that he had no financial disclosures.
SOURCE: Hurley, D. AACE 2018, Session SGS-4.
BOSTON – For patients with obesity and metabolic syndrome or type 2 diabetes ( health over the lifespan.
“Behavioral changes in diet and activity may be effective over the short term, but they are often ineffective over the long term,” said Daniel L. Hurley, MD. By contrast, “Bariatric surgery is very effective long-term,” he said.
At the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists, Dr. Hurley made the case for bariatric surgery in effective and durable management of type 2 diabetes and cardiovascular risk, weighing risks and benefits for those with higher and lower levels of obesity.
Speaking during a morning session focused on bariatric surgery, Dr. Hurley, an endocrionologist at the Mayo Clinic, Rochester, Minn., noted that bariatric surgery reduces not just weight, but also visceral adiposity. This, he said, is important when thinking about type 2 diabetes (T2D), because diabetes prevalence has climbed in the United States as obesity has also increased, according to examination of data from the National Health and Nutrition Examination Survey (NHANES).
Additionally, increased abdominal adiposity is associated with increased risk for cardiovascular-related deaths, myocardial infarctions, and all-cause deaths. Some of this relationship is mediated by T2D, which itself “is a major cause of cardiovascular-related morbidity and mortality,” said Dr. Hurley.
From a population health perspective, the increased prevalence of T2D – expected to reach 10% in the United States by 2030 – will also boost cardiovascular morbidity and mortality, said Dr. Hurley. Those with T2D die 5 to 10 years earlier, and have double the risk for heart attack and stroke of their peers without diabetes. The risk of lower limb amputation can be as much as 40 times greater for an individual with T2D across the lifespan, he said.
The National Institutes of Health recognizes bariatric surgery as an appropriate weight loss therapy for individuals with a body mass index (BMI) of at least 35 kg/m2 and comorbidity. Whether bariatric surgery might be appropriate for individuals with T2D and BMIs of less than 35 kg/m2 is less settled, though at least some RCTs support the surgical approach, said Dr. Hurley.
The body of data that support long-term metabolic and cardiovascular benefits of bariatric surgery as obesity therapy is growing, said Dr. Hurley. A large prospective observational study by the American College of Surgeons’ Bariatric Surgery Center Network followed 28,616 patients, finding that Roux-en-Y gastric bypass (RYGB) was most effective in improving or resolving CVD comorbidities. At 1 year post surgery, 83% of RYGB patients saw improvement or resolution of T2D; the figure was 79% for hypertension and 66% for dyslipidemia (Ann Surg. 2011;254[3]:410-20).
Weight loss for patients receiving bariatric procedures has generally been durable: for laparoscopic RYGB patients tracked to 7 years after surgery, 75% had maintained at least a 20% weight loss (JAMA Surg. 2018;153[5]427-34).
Longer-term clinical follow-up points toward favorable metabolic and cardiovascular outcomes, said Dr. Hurley, citing data from the Swedish Obese Subjects (SOS) trial. This study followed over 4,000 patients with high BMIs (at least 34 kg/m2 for men and 38 kg/m2 for women) over 10 years. At that point, 36% of gastric bypass patients, compared with 13% of non-surgical high BMI patients, saw resolution of T2D, a significant difference. Triglyceride levels also fell significantly more for the bypass recipients. Hypertension was resolved in just 19% of patients at 10 years, a non-significant difference from the 11% of control patients. Data from the same patient set also showed a significant reduction in total cardiovascular events in the surgical versus non-surgical patients (n = 49 vs. 28, hazard ratio 0.83, log-rank P = .05). Fatal cardiovascular events were significantly lower for patients who had received bariatric surgery, with a 24% decline in mortality for bariatric surgery patients at about 11 years post surgery.
Canadian data showed even greater reductions in mortality, with an 89% decrease in mortality after RYGB, compared with non-surgical patients at the 5-year mark (Ann Surg 2004;240:416-24).
In trials that afforded a direct comparison of medical therapy and bariatric surgery obesity and diabetes, Dr. Hurley said that randomized trials generally show no change to modest change in HbA1c levels with medical management. By contrast, patients in the surgical arms showed a range of improvement ranging from a reduction of just under 1% to reductions of over 5%, with an average reduction of more than 2% across the trials.
Separating out data from the randomized controlled trials with patient BMIs averaging 35 kg/m2 or less, odds ratios still favored bariatric surgery over medication therapy for diabetes-related outcomes in this lower-BMI population, said Dr. Hurley (Diabetes Care 2016;39:924-33).
More data come from a recently reported randomized trial that assigned patients with T2D and a mean BMI of 37 kg/m2 (range, 27-43) to intensive medical therapy, or either sleeve gastrectomy (SG) or RYGB. The study, which had a 90% completion rate at the 5-year mark, found that both surgical procedures were significantly more effective at reducing HbA1c to 6% or less 12 months into the study (P less than .001).
At the 60-month mark, 45% of the RYGB and 25% of the SG patients were on no diabetes medications, while just 2% of the medical therapy arm had stopped all medications, and 40% of this group remained on insulin 5 years into the study, said Dr. Hurley (N Engl J Med. 2017;376:641-651).
“For treatment of type 2 diabetes and cardiovascular co-morbidities, long-term goals often are met following bariatric surgery versus behavior change,” said Dr. Hurley.
Dr. Hurley reported that he had no financial disclosures.
SOURCE: Hurley, D. AACE 2018, Session SGS-4.
BOSTON – For patients with obesity and metabolic syndrome or type 2 diabetes ( health over the lifespan.
“Behavioral changes in diet and activity may be effective over the short term, but they are often ineffective over the long term,” said Daniel L. Hurley, MD. By contrast, “Bariatric surgery is very effective long-term,” he said.
At the annual clinical and scientific meeting of the American Association of Clinical Endocrinologists, Dr. Hurley made the case for bariatric surgery in effective and durable management of type 2 diabetes and cardiovascular risk, weighing risks and benefits for those with higher and lower levels of obesity.
Speaking during a morning session focused on bariatric surgery, Dr. Hurley, an endocrionologist at the Mayo Clinic, Rochester, Minn., noted that bariatric surgery reduces not just weight, but also visceral adiposity. This, he said, is important when thinking about type 2 diabetes (T2D), because diabetes prevalence has climbed in the United States as obesity has also increased, according to examination of data from the National Health and Nutrition Examination Survey (NHANES).
Additionally, increased abdominal adiposity is associated with increased risk for cardiovascular-related deaths, myocardial infarctions, and all-cause deaths. Some of this relationship is mediated by T2D, which itself “is a major cause of cardiovascular-related morbidity and mortality,” said Dr. Hurley.
From a population health perspective, the increased prevalence of T2D – expected to reach 10% in the United States by 2030 – will also boost cardiovascular morbidity and mortality, said Dr. Hurley. Those with T2D die 5 to 10 years earlier, and have double the risk for heart attack and stroke of their peers without diabetes. The risk of lower limb amputation can be as much as 40 times greater for an individual with T2D across the lifespan, he said.
The National Institutes of Health recognizes bariatric surgery as an appropriate weight loss therapy for individuals with a body mass index (BMI) of at least 35 kg/m2 and comorbidity. Whether bariatric surgery might be appropriate for individuals with T2D and BMIs of less than 35 kg/m2 is less settled, though at least some RCTs support the surgical approach, said Dr. Hurley.
The body of data that support long-term metabolic and cardiovascular benefits of bariatric surgery as obesity therapy is growing, said Dr. Hurley. A large prospective observational study by the American College of Surgeons’ Bariatric Surgery Center Network followed 28,616 patients, finding that Roux-en-Y gastric bypass (RYGB) was most effective in improving or resolving CVD comorbidities. At 1 year post surgery, 83% of RYGB patients saw improvement or resolution of T2D; the figure was 79% for hypertension and 66% for dyslipidemia (Ann Surg. 2011;254[3]:410-20).
Weight loss for patients receiving bariatric procedures has generally been durable: for laparoscopic RYGB patients tracked to 7 years after surgery, 75% had maintained at least a 20% weight loss (JAMA Surg. 2018;153[5]427-34).
Longer-term clinical follow-up points toward favorable metabolic and cardiovascular outcomes, said Dr. Hurley, citing data from the Swedish Obese Subjects (SOS) trial. This study followed over 4,000 patients with high BMIs (at least 34 kg/m2 for men and 38 kg/m2 for women) over 10 years. At that point, 36% of gastric bypass patients, compared with 13% of non-surgical high BMI patients, saw resolution of T2D, a significant difference. Triglyceride levels also fell significantly more for the bypass recipients. Hypertension was resolved in just 19% of patients at 10 years, a non-significant difference from the 11% of control patients. Data from the same patient set also showed a significant reduction in total cardiovascular events in the surgical versus non-surgical patients (n = 49 vs. 28, hazard ratio 0.83, log-rank P = .05). Fatal cardiovascular events were significantly lower for patients who had received bariatric surgery, with a 24% decline in mortality for bariatric surgery patients at about 11 years post surgery.
Canadian data showed even greater reductions in mortality, with an 89% decrease in mortality after RYGB, compared with non-surgical patients at the 5-year mark (Ann Surg 2004;240:416-24).
In trials that afforded a direct comparison of medical therapy and bariatric surgery obesity and diabetes, Dr. Hurley said that randomized trials generally show no change to modest change in HbA1c levels with medical management. By contrast, patients in the surgical arms showed a range of improvement ranging from a reduction of just under 1% to reductions of over 5%, with an average reduction of more than 2% across the trials.
Separating out data from the randomized controlled trials with patient BMIs averaging 35 kg/m2 or less, odds ratios still favored bariatric surgery over medication therapy for diabetes-related outcomes in this lower-BMI population, said Dr. Hurley (Diabetes Care 2016;39:924-33).
More data come from a recently reported randomized trial that assigned patients with T2D and a mean BMI of 37 kg/m2 (range, 27-43) to intensive medical therapy, or either sleeve gastrectomy (SG) or RYGB. The study, which had a 90% completion rate at the 5-year mark, found that both surgical procedures were significantly more effective at reducing HbA1c to 6% or less 12 months into the study (P less than .001).
At the 60-month mark, 45% of the RYGB and 25% of the SG patients were on no diabetes medications, while just 2% of the medical therapy arm had stopped all medications, and 40% of this group remained on insulin 5 years into the study, said Dr. Hurley (N Engl J Med. 2017;376:641-651).
“For treatment of type 2 diabetes and cardiovascular co-morbidities, long-term goals often are met following bariatric surgery versus behavior change,” said Dr. Hurley.
Dr. Hurley reported that he had no financial disclosures.
SOURCE: Hurley, D. AACE 2018, Session SGS-4.
EXPERT ANALYSIS FROM AACE 2018
Diabetes places burden on patients
according to a new survey by prescription manager UpWell Health.
The time spent on activities that come with diabetes management – blood glucose monitoring, diet planning, medical appointments – can add up to several hours a week. Among the 5,255 respondents to the online survey, 18% said that such tasks took up 5-10 hours each week, 7% said it was 10-15 hours, and 9% said they spent 15 or more hours a week on diabetes-related tasks, UpWell reported.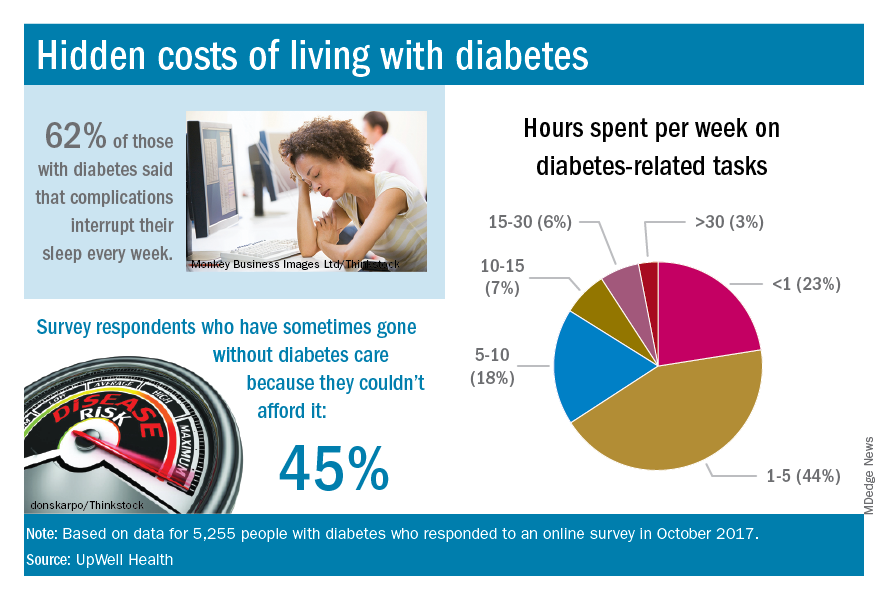
Since medical expenses aren’t always fully covered by insurance, 43% of respondents paid up to $1,000 a year out of pocket to treat diabetes complications, 16% paid $1,000 to $2,000 a year, and 4% paid more than $5,000. Five percent also paid over $5,000 a year out of pocket for diabetes care from a physician and 45% said that they had sometimes gone without diabetes care because they couldn’t afford it, the survey data showed.
“Most people with diabetes are able to manage it successfully and live active, satisfying lives. But doing so requires constant planning, vigilance, and care. They eagerly seek trustworthy resources to help them reduce the burden of living with diabetes,” UpWell said.
according to a new survey by prescription manager UpWell Health.
The time spent on activities that come with diabetes management – blood glucose monitoring, diet planning, medical appointments – can add up to several hours a week. Among the 5,255 respondents to the online survey, 18% said that such tasks took up 5-10 hours each week, 7% said it was 10-15 hours, and 9% said they spent 15 or more hours a week on diabetes-related tasks, UpWell reported.
Since medical expenses aren’t always fully covered by insurance, 43% of respondents paid up to $1,000 a year out of pocket to treat diabetes complications, 16% paid $1,000 to $2,000 a year, and 4% paid more than $5,000. Five percent also paid over $5,000 a year out of pocket for diabetes care from a physician and 45% said that they had sometimes gone without diabetes care because they couldn’t afford it, the survey data showed.
“Most people with diabetes are able to manage it successfully and live active, satisfying lives. But doing so requires constant planning, vigilance, and care. They eagerly seek trustworthy resources to help them reduce the burden of living with diabetes,” UpWell said.
according to a new survey by prescription manager UpWell Health.
The time spent on activities that come with diabetes management – blood glucose monitoring, diet planning, medical appointments – can add up to several hours a week. Among the 5,255 respondents to the online survey, 18% said that such tasks took up 5-10 hours each week, 7% said it was 10-15 hours, and 9% said they spent 15 or more hours a week on diabetes-related tasks, UpWell reported.
Since medical expenses aren’t always fully covered by insurance, 43% of respondents paid up to $1,000 a year out of pocket to treat diabetes complications, 16% paid $1,000 to $2,000 a year, and 4% paid more than $5,000. Five percent also paid over $5,000 a year out of pocket for diabetes care from a physician and 45% said that they had sometimes gone without diabetes care because they couldn’t afford it, the survey data showed.
“Most people with diabetes are able to manage it successfully and live active, satisfying lives. But doing so requires constant planning, vigilance, and care. They eagerly seek trustworthy resources to help them reduce the burden of living with diabetes,” UpWell said.
FDA alerts clinicians to gastric balloon deaths
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
according to an alert from the Food and Drug Administration issued on June 4.
Seven of these deaths occurred in patients in the United States; four involved the ORBERA Intragastric Balloon System, and three involved the ReShape Integrated Dual Balloon System.
The FDA has approved updated labeling for the ORBERA and ReShape balloon systems in the United States. The labels contain more information about possible death associated with the use of these devices in the United States. The manufacturers’ sites, Apollo Endosurgery and ReShape Lifesciences, provide more details about the new labeling.
In a letter to health care providers, the FDA advised clinicians to educate bariatric surgery patients about the symptoms of complications from balloon procedures, including not only gastric perforation but also esophageal perforation, balloon deflation, gastrointestinal obstruction, and ulceration. In addition, the FDA reminded clinicians to monitor patients during the entire course of treatment for additional complications, including acute pancreatitis and spontaneous hyperinflation.
Any adverse events involving intragastric balloon systems should be reported to the FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
Genes, not adiposity, may be driving appetite differences in obesity
BOSTON – Evidence from a twin study points to genes, rather than just adiposity, as the underlying factor in differences in appetite and satiety that have been observed in obesity.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The work adds a new dimension – and some questions – to previous research, which suggested individuals with obesity show heightened brain activation to food cues, especially calorically dense food.
“We thought it was fat mass…but when we controlled for everything that monozygotic pairs have in common, that relationship went away, implicating something that the monozygotic twins have in common, i.e., genetics,” said first author Jennifer Rosenbaum, MD, in a video interview at the annual meeting of the American Academy of Clinical Endocrinologists.
Dr. Rosenbaum, a fellow in the department of metabolism, endocrinology, and nutrition at the University of Washington, Seattle, and her collaborators made use of a statewide twin registry to conduct an extensive investigation of subjective and objective measures of appetite and satiety in the 42 twin pairs.
Twins had a mean age of 31 years; 27 of the twin pairs were monozygotic, Dr. Rosenbaum said. At least one member of each twin pair met criteria for obesity, and participants had a mean body mass index of 32.8 kg/m2.
On the study day, participants arrived in fasting state, and had a fixed-calorie breakfast equivalent to 10% of their daily caloric needs. They then underwent dual-energy x-ray absorptiometry scanning to determine adiposity, and also filled out a behavioral questionnaire.
Then, participants received the first of two functional MRI scans; during the scan, they were shown images of high calorie foods, low calorie foods, and nonfood objects, completing ratings of how appealing they found each image. After consuming another standardized meal equivalent to 20% of daily caloric needs, the fMRI scan was repeated.
Finally, participants were given access to a buffet meal and allowed to eat as much as they chose; consumption was measured. Before and after each meal and scan, and at various points during the day, the investigators also obtained blood samples and asked participants to rate their hunger on a visual analog scale.
“When compared with how much fat mass they had, there was no relationship between how hungry or full they were when they were fasting, how hungry or full they were with a snack, or when they ate the buffet. It just didn’t matter how much fat mass they had” for subjective reporting of hunger and fullness, said Dr. Rosenbaum.
However, there was a direct correlation between fat mass and amount consumed at the ad libitum buffet. Additionally, the fMRI analysis showed that “the brain activation that we would expect to go down, didn’t seem to go down as much if you had more adiposity,” she said.
As fat mass went up, areas of the brain implicated in appetite and reward showed more activity when participants were presented with the tempting images of high calorie foods, regardless of the calories consumed. These areas include the ventral and dorsal striata, the amygdala, the insula, the ventral tegmental area, and the medial orbitofrontal cortex.
Next, the researchers looked for differences within the monozygotic twin pairs, who essentially share a genome. They compared the brain activation of the twin with the higher fat mass with that of the twin with lower fat mass. Instead of seeing the same correlation between higher adiposity and greater brain activation with tempting stimuli, “Suddenly, we lost that relationship between how many calories they would eat and how their brain activated with the food,” said Dr. Rosenbaum. This is a clue, she said, that genetics, rather than simple adiposity, is driving the different responses to food cues.
The study was funded by the National Institutes of Health. Dr. Rosenbaum reported no financial disclosures.
BOSTON – Evidence from a twin study points to genes, rather than just adiposity, as the underlying factor in differences in appetite and satiety that have been observed in obesity.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The work adds a new dimension – and some questions – to previous research, which suggested individuals with obesity show heightened brain activation to food cues, especially calorically dense food.
“We thought it was fat mass…but when we controlled for everything that monozygotic pairs have in common, that relationship went away, implicating something that the monozygotic twins have in common, i.e., genetics,” said first author Jennifer Rosenbaum, MD, in a video interview at the annual meeting of the American Academy of Clinical Endocrinologists.
Dr. Rosenbaum, a fellow in the department of metabolism, endocrinology, and nutrition at the University of Washington, Seattle, and her collaborators made use of a statewide twin registry to conduct an extensive investigation of subjective and objective measures of appetite and satiety in the 42 twin pairs.
Twins had a mean age of 31 years; 27 of the twin pairs were monozygotic, Dr. Rosenbaum said. At least one member of each twin pair met criteria for obesity, and participants had a mean body mass index of 32.8 kg/m2.
On the study day, participants arrived in fasting state, and had a fixed-calorie breakfast equivalent to 10% of their daily caloric needs. They then underwent dual-energy x-ray absorptiometry scanning to determine adiposity, and also filled out a behavioral questionnaire.
Then, participants received the first of two functional MRI scans; during the scan, they were shown images of high calorie foods, low calorie foods, and nonfood objects, completing ratings of how appealing they found each image. After consuming another standardized meal equivalent to 20% of daily caloric needs, the fMRI scan was repeated.
Finally, participants were given access to a buffet meal and allowed to eat as much as they chose; consumption was measured. Before and after each meal and scan, and at various points during the day, the investigators also obtained blood samples and asked participants to rate their hunger on a visual analog scale.
“When compared with how much fat mass they had, there was no relationship between how hungry or full they were when they were fasting, how hungry or full they were with a snack, or when they ate the buffet. It just didn’t matter how much fat mass they had” for subjective reporting of hunger and fullness, said Dr. Rosenbaum.
However, there was a direct correlation between fat mass and amount consumed at the ad libitum buffet. Additionally, the fMRI analysis showed that “the brain activation that we would expect to go down, didn’t seem to go down as much if you had more adiposity,” she said.
As fat mass went up, areas of the brain implicated in appetite and reward showed more activity when participants were presented with the tempting images of high calorie foods, regardless of the calories consumed. These areas include the ventral and dorsal striata, the amygdala, the insula, the ventral tegmental area, and the medial orbitofrontal cortex.
Next, the researchers looked for differences within the monozygotic twin pairs, who essentially share a genome. They compared the brain activation of the twin with the higher fat mass with that of the twin with lower fat mass. Instead of seeing the same correlation between higher adiposity and greater brain activation with tempting stimuli, “Suddenly, we lost that relationship between how many calories they would eat and how their brain activated with the food,” said Dr. Rosenbaum. This is a clue, she said, that genetics, rather than simple adiposity, is driving the different responses to food cues.
The study was funded by the National Institutes of Health. Dr. Rosenbaum reported no financial disclosures.
BOSTON – Evidence from a twin study points to genes, rather than just adiposity, as the underlying factor in differences in appetite and satiety that have been observed in obesity.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The work adds a new dimension – and some questions – to previous research, which suggested individuals with obesity show heightened brain activation to food cues, especially calorically dense food.
“We thought it was fat mass…but when we controlled for everything that monozygotic pairs have in common, that relationship went away, implicating something that the monozygotic twins have in common, i.e., genetics,” said first author Jennifer Rosenbaum, MD, in a video interview at the annual meeting of the American Academy of Clinical Endocrinologists.
Dr. Rosenbaum, a fellow in the department of metabolism, endocrinology, and nutrition at the University of Washington, Seattle, and her collaborators made use of a statewide twin registry to conduct an extensive investigation of subjective and objective measures of appetite and satiety in the 42 twin pairs.
Twins had a mean age of 31 years; 27 of the twin pairs were monozygotic, Dr. Rosenbaum said. At least one member of each twin pair met criteria for obesity, and participants had a mean body mass index of 32.8 kg/m2.
On the study day, participants arrived in fasting state, and had a fixed-calorie breakfast equivalent to 10% of their daily caloric needs. They then underwent dual-energy x-ray absorptiometry scanning to determine adiposity, and also filled out a behavioral questionnaire.
Then, participants received the first of two functional MRI scans; during the scan, they were shown images of high calorie foods, low calorie foods, and nonfood objects, completing ratings of how appealing they found each image. After consuming another standardized meal equivalent to 20% of daily caloric needs, the fMRI scan was repeated.
Finally, participants were given access to a buffet meal and allowed to eat as much as they chose; consumption was measured. Before and after each meal and scan, and at various points during the day, the investigators also obtained blood samples and asked participants to rate their hunger on a visual analog scale.
“When compared with how much fat mass they had, there was no relationship between how hungry or full they were when they were fasting, how hungry or full they were with a snack, or when they ate the buffet. It just didn’t matter how much fat mass they had” for subjective reporting of hunger and fullness, said Dr. Rosenbaum.
However, there was a direct correlation between fat mass and amount consumed at the ad libitum buffet. Additionally, the fMRI analysis showed that “the brain activation that we would expect to go down, didn’t seem to go down as much if you had more adiposity,” she said.
As fat mass went up, areas of the brain implicated in appetite and reward showed more activity when participants were presented with the tempting images of high calorie foods, regardless of the calories consumed. These areas include the ventral and dorsal striata, the amygdala, the insula, the ventral tegmental area, and the medial orbitofrontal cortex.
Next, the researchers looked for differences within the monozygotic twin pairs, who essentially share a genome. They compared the brain activation of the twin with the higher fat mass with that of the twin with lower fat mass. Instead of seeing the same correlation between higher adiposity and greater brain activation with tempting stimuli, “Suddenly, we lost that relationship between how many calories they would eat and how their brain activated with the food,” said Dr. Rosenbaum. This is a clue, she said, that genetics, rather than simple adiposity, is driving the different responses to food cues.
The study was funded by the National Institutes of Health. Dr. Rosenbaum reported no financial disclosures.
REPORTING FROM AACE 2018
Oral diabetes drugs linked to lower levels of bone formation marker
in a Danish clinical trial.
Procollagen type 1 N-terminal peptide (P1NP) plasma concentrations were lower in patients who received either metformin or metformin plus rosiglitazone, Tore Bjerregaard Stage, PhD, a specialist in clinical pharmacology and pharmacy at the University of Southern Denmark, Odense, and his coauthors wrote in Bone.
By contrast, insulin did not appear to influence markers of bone turnover.
Improving glycemic control was associated with increased plasma concentrations of C-terminal telopeptide of collagen (CTx), a marker of bone resorption. However, this finding might reflect “normalization, rather than an abnormal increase in bone resorption,” Dr. Stage and his colleagues wrote.
These findings come from an analysis of the South Danish Diabetes Study, a 2-year, multicenter, randomized, controlled trial including 371 patients with type 2 diabetes. Patients were first randomized to receive short- or long-acting human insulin, then further randomized to metformin plus rosiglitazone, metformin plus placebo, rosiglitazone plus placebo, or two placebos.
Bone turnover markers were assessed at baseline and at 3-, 12-, and 24-month follow-ups.
Dr. Stage and his coinvestigators hoped the analysis would provide insights into how antidiabetic medication might influence bone turnover, potentially helping explain the increased risk of fracture found in patients with type 2 diabetes. “Alterations in bone metabolism due to antidiabetic medication may influence bone metabolism both directly, e.g., by insulin promoting bone formation, and indirectly by improvement of glycemic control,” they wrote.
Overall, levels of both bone turnover markers increased over time in the study. However, investigators found that concentrations of the bone formation marker P1NP were 13% lower in patients randomized to metformin alone, and 21% lower in patients randomized to metformin and rosiglitazone; no such association was found between P1NP concentrations and treatment with rosiglitazone alone. By contrast, the type of oral antidiabetic drug treatment had no effect on concentrations of CTx concentrations, the investigators said.
Type of insulin treatment received in the trial did not appear to have an impact on concentrations of either bone turnover marker, they added.
HbA1c had no influence on concentrations of P1NP; but it was inversely correlated with levels of CTx, a finding that the investigators said merits more study.
“Further clinical trials investigating the effects of improved glycemic control on bone remodeling including other biochemical markers of bone turnover are needed to confirm if lowering of glucose levels solely changes bone resorption and not formation,” Dr. Stage and his coauthors wrote.
This study was funded with grants from the Danish Council for Independent Research and the Region of Southern Denmark. Dr. Stage and coauthors reported no conflicts of interest related to the report.
SOURCE: Stage TB et al. Bone. 2018 Apr 12;112:35-41.
in a Danish clinical trial.
Procollagen type 1 N-terminal peptide (P1NP) plasma concentrations were lower in patients who received either metformin or metformin plus rosiglitazone, Tore Bjerregaard Stage, PhD, a specialist in clinical pharmacology and pharmacy at the University of Southern Denmark, Odense, and his coauthors wrote in Bone.
By contrast, insulin did not appear to influence markers of bone turnover.
Improving glycemic control was associated with increased plasma concentrations of C-terminal telopeptide of collagen (CTx), a marker of bone resorption. However, this finding might reflect “normalization, rather than an abnormal increase in bone resorption,” Dr. Stage and his colleagues wrote.
These findings come from an analysis of the South Danish Diabetes Study, a 2-year, multicenter, randomized, controlled trial including 371 patients with type 2 diabetes. Patients were first randomized to receive short- or long-acting human insulin, then further randomized to metformin plus rosiglitazone, metformin plus placebo, rosiglitazone plus placebo, or two placebos.
Bone turnover markers were assessed at baseline and at 3-, 12-, and 24-month follow-ups.
Dr. Stage and his coinvestigators hoped the analysis would provide insights into how antidiabetic medication might influence bone turnover, potentially helping explain the increased risk of fracture found in patients with type 2 diabetes. “Alterations in bone metabolism due to antidiabetic medication may influence bone metabolism both directly, e.g., by insulin promoting bone formation, and indirectly by improvement of glycemic control,” they wrote.
Overall, levels of both bone turnover markers increased over time in the study. However, investigators found that concentrations of the bone formation marker P1NP were 13% lower in patients randomized to metformin alone, and 21% lower in patients randomized to metformin and rosiglitazone; no such association was found between P1NP concentrations and treatment with rosiglitazone alone. By contrast, the type of oral antidiabetic drug treatment had no effect on concentrations of CTx concentrations, the investigators said.
Type of insulin treatment received in the trial did not appear to have an impact on concentrations of either bone turnover marker, they added.
HbA1c had no influence on concentrations of P1NP; but it was inversely correlated with levels of CTx, a finding that the investigators said merits more study.
“Further clinical trials investigating the effects of improved glycemic control on bone remodeling including other biochemical markers of bone turnover are needed to confirm if lowering of glucose levels solely changes bone resorption and not formation,” Dr. Stage and his coauthors wrote.
This study was funded with grants from the Danish Council for Independent Research and the Region of Southern Denmark. Dr. Stage and coauthors reported no conflicts of interest related to the report.
SOURCE: Stage TB et al. Bone. 2018 Apr 12;112:35-41.
in a Danish clinical trial.
Procollagen type 1 N-terminal peptide (P1NP) plasma concentrations were lower in patients who received either metformin or metformin plus rosiglitazone, Tore Bjerregaard Stage, PhD, a specialist in clinical pharmacology and pharmacy at the University of Southern Denmark, Odense, and his coauthors wrote in Bone.
By contrast, insulin did not appear to influence markers of bone turnover.
Improving glycemic control was associated with increased plasma concentrations of C-terminal telopeptide of collagen (CTx), a marker of bone resorption. However, this finding might reflect “normalization, rather than an abnormal increase in bone resorption,” Dr. Stage and his colleagues wrote.
These findings come from an analysis of the South Danish Diabetes Study, a 2-year, multicenter, randomized, controlled trial including 371 patients with type 2 diabetes. Patients were first randomized to receive short- or long-acting human insulin, then further randomized to metformin plus rosiglitazone, metformin plus placebo, rosiglitazone plus placebo, or two placebos.
Bone turnover markers were assessed at baseline and at 3-, 12-, and 24-month follow-ups.
Dr. Stage and his coinvestigators hoped the analysis would provide insights into how antidiabetic medication might influence bone turnover, potentially helping explain the increased risk of fracture found in patients with type 2 diabetes. “Alterations in bone metabolism due to antidiabetic medication may influence bone metabolism both directly, e.g., by insulin promoting bone formation, and indirectly by improvement of glycemic control,” they wrote.
Overall, levels of both bone turnover markers increased over time in the study. However, investigators found that concentrations of the bone formation marker P1NP were 13% lower in patients randomized to metformin alone, and 21% lower in patients randomized to metformin and rosiglitazone; no such association was found between P1NP concentrations and treatment with rosiglitazone alone. By contrast, the type of oral antidiabetic drug treatment had no effect on concentrations of CTx concentrations, the investigators said.
Type of insulin treatment received in the trial did not appear to have an impact on concentrations of either bone turnover marker, they added.
HbA1c had no influence on concentrations of P1NP; but it was inversely correlated with levels of CTx, a finding that the investigators said merits more study.
“Further clinical trials investigating the effects of improved glycemic control on bone remodeling including other biochemical markers of bone turnover are needed to confirm if lowering of glucose levels solely changes bone resorption and not formation,” Dr. Stage and his coauthors wrote.
This study was funded with grants from the Danish Council for Independent Research and the Region of Southern Denmark. Dr. Stage and coauthors reported no conflicts of interest related to the report.
SOURCE: Stage TB et al. Bone. 2018 Apr 12;112:35-41.
FROM BONE
Key clinical point: Treatment with oral antidiabetic drugs was associated with reductions in levels of P1NP, a marker of bone formation.
Major finding: Concentrations of P1NP were 13% lower in patients randomized to metformin and 21% lower in patients randomized to metformin and rosiglitazone.
Study details: An analysis of the South Danish Diabetes Study, a 2-year, multicenter, randomized, controlled trial of 371 patients with type 2 diabetes.
Disclosures: The authors reported no conflicts of interest related to the study.
Source: Stage TB et al. Bone. 2018 Apr 12;112:35-41.