User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Diabetes, hypertension, smoking may raise risk for late-onset epilepsy
Hypertension, diabetes, and smoking in midlife are potentially modifiable risk factors for late-onset epilepsy, according to a study that also found an increased risk with the apolipoprotein E4 (APOE4) genotype and in black individuals.
Emily L. Johnson, MD, of Johns Hopkins University, Baltimore, and her coauthors analyzed data from 10,420 participants in the Atherosclerosis Risk in Communities prospective cohort study and found 596 who developed late-onset epilepsy (LOE), defined as recurrent unprovoked seizures starting at 60 years or older.
The study, published online July 23 in JAMA Neurology, showed that individuals with hypertension at baseline had a 30% higher risk of LOE, those who smoked had a 9% higher risk, and those with diabetes had a 45% higher risk. However, the increased risk associated with smoking was evident only in women, who had a 27% higher risk.
Participants with incident stroke had a 3.38-fold higher risk of LOE, and those with dementia had a 2.56-fold higher risk, Dr. Johnson and her colleagues reported.
“The association of LOE with vascular and lifestyle risk factors persisted after participants with diagnosis of clinical stroke or dementia were included or censored, suggesting that these risk factors may contribute to LOE even in the absence of dementia or clinical stroke,” the investigators wrote.
However, higher levels of physical activity decreased the risk by 10%, and moderate alcohol consumption – defined as 1-7 standard drinks per week – was associated with a 28% reduction.
Race and geographic location both influenced the risk of LOE; black participants showed a 66% higher risk, compared with whites, and black participants in Mississippi and North Carolina had a higher risk than did North Carolina white participants. The highest risk of LOE was seen in black participants with diabetes.
“The reasons for the different incidences of LOE by race/ethnicity may be owing to differing effects of comorbidities, such as diabetes, for which we found a significantly higher effect in black individuals than in white individuals,” the authors wrote.
Individuals who had one APOE4 allele showed a 46% higher risk, and those with two alleles showed a 2.57-fold higher risk.
“The APOE4 genotype is the major genetic risk factor for Alzheimer’s disease, which is associated with epilepsy; however, no prior association between APOE4 and LOE has previously been shown,” the authors reported.
The researchers noted that their findings suggested that lifestyle modifications earlier in life could mitigate some of the risk factors associated with LOE, and could also help identify patients at higher risk for the disease.
No funding was declared. One author declared consultancy and investigator positions with private industry.
SOURCE: Johnson E et al. JAMA Neurol, 2018 July 23. doi: 10.1001/jamaneurol.2018.1935.
Hypertension, diabetes, and smoking in midlife are potentially modifiable risk factors for late-onset epilepsy, according to a study that also found an increased risk with the apolipoprotein E4 (APOE4) genotype and in black individuals.
Emily L. Johnson, MD, of Johns Hopkins University, Baltimore, and her coauthors analyzed data from 10,420 participants in the Atherosclerosis Risk in Communities prospective cohort study and found 596 who developed late-onset epilepsy (LOE), defined as recurrent unprovoked seizures starting at 60 years or older.
The study, published online July 23 in JAMA Neurology, showed that individuals with hypertension at baseline had a 30% higher risk of LOE, those who smoked had a 9% higher risk, and those with diabetes had a 45% higher risk. However, the increased risk associated with smoking was evident only in women, who had a 27% higher risk.
Participants with incident stroke had a 3.38-fold higher risk of LOE, and those with dementia had a 2.56-fold higher risk, Dr. Johnson and her colleagues reported.
“The association of LOE with vascular and lifestyle risk factors persisted after participants with diagnosis of clinical stroke or dementia were included or censored, suggesting that these risk factors may contribute to LOE even in the absence of dementia or clinical stroke,” the investigators wrote.
However, higher levels of physical activity decreased the risk by 10%, and moderate alcohol consumption – defined as 1-7 standard drinks per week – was associated with a 28% reduction.
Race and geographic location both influenced the risk of LOE; black participants showed a 66% higher risk, compared with whites, and black participants in Mississippi and North Carolina had a higher risk than did North Carolina white participants. The highest risk of LOE was seen in black participants with diabetes.
“The reasons for the different incidences of LOE by race/ethnicity may be owing to differing effects of comorbidities, such as diabetes, for which we found a significantly higher effect in black individuals than in white individuals,” the authors wrote.
Individuals who had one APOE4 allele showed a 46% higher risk, and those with two alleles showed a 2.57-fold higher risk.
“The APOE4 genotype is the major genetic risk factor for Alzheimer’s disease, which is associated with epilepsy; however, no prior association between APOE4 and LOE has previously been shown,” the authors reported.
The researchers noted that their findings suggested that lifestyle modifications earlier in life could mitigate some of the risk factors associated with LOE, and could also help identify patients at higher risk for the disease.
No funding was declared. One author declared consultancy and investigator positions with private industry.
SOURCE: Johnson E et al. JAMA Neurol, 2018 July 23. doi: 10.1001/jamaneurol.2018.1935.
Hypertension, diabetes, and smoking in midlife are potentially modifiable risk factors for late-onset epilepsy, according to a study that also found an increased risk with the apolipoprotein E4 (APOE4) genotype and in black individuals.
Emily L. Johnson, MD, of Johns Hopkins University, Baltimore, and her coauthors analyzed data from 10,420 participants in the Atherosclerosis Risk in Communities prospective cohort study and found 596 who developed late-onset epilepsy (LOE), defined as recurrent unprovoked seizures starting at 60 years or older.
The study, published online July 23 in JAMA Neurology, showed that individuals with hypertension at baseline had a 30% higher risk of LOE, those who smoked had a 9% higher risk, and those with diabetes had a 45% higher risk. However, the increased risk associated with smoking was evident only in women, who had a 27% higher risk.
Participants with incident stroke had a 3.38-fold higher risk of LOE, and those with dementia had a 2.56-fold higher risk, Dr. Johnson and her colleagues reported.
“The association of LOE with vascular and lifestyle risk factors persisted after participants with diagnosis of clinical stroke or dementia were included or censored, suggesting that these risk factors may contribute to LOE even in the absence of dementia or clinical stroke,” the investigators wrote.
However, higher levels of physical activity decreased the risk by 10%, and moderate alcohol consumption – defined as 1-7 standard drinks per week – was associated with a 28% reduction.
Race and geographic location both influenced the risk of LOE; black participants showed a 66% higher risk, compared with whites, and black participants in Mississippi and North Carolina had a higher risk than did North Carolina white participants. The highest risk of LOE was seen in black participants with diabetes.
“The reasons for the different incidences of LOE by race/ethnicity may be owing to differing effects of comorbidities, such as diabetes, for which we found a significantly higher effect in black individuals than in white individuals,” the authors wrote.
Individuals who had one APOE4 allele showed a 46% higher risk, and those with two alleles showed a 2.57-fold higher risk.
“The APOE4 genotype is the major genetic risk factor for Alzheimer’s disease, which is associated with epilepsy; however, no prior association between APOE4 and LOE has previously been shown,” the authors reported.
The researchers noted that their findings suggested that lifestyle modifications earlier in life could mitigate some of the risk factors associated with LOE, and could also help identify patients at higher risk for the disease.
No funding was declared. One author declared consultancy and investigator positions with private industry.
SOURCE: Johnson E et al. JAMA Neurol, 2018 July 23. doi: 10.1001/jamaneurol.2018.1935.
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: Midlife diabetes is associated with a 45% higher risk of late-onset epilepsy.
Study details: A prospective cohort study in 10,420 individuals in the Atherosclerosis Risk in Communities study.
Disclosures: No funding was declared. One author declared consultancy and investigator positions with private industry.
Source: Johnson E et al. JAMA Neurol. 2018 Jul 23. doi: 10.1001/jamaneurol.2018.1935.
Trial data suggest beneficial class effects of SGLT2 inhibitors, including dapagliflozin
ORLANDO – a post hoc analysis of data from the EXSCEL trial suggested.
The findings are consistent with those from published cardiovascular outcomes trials (CVOTs) of sodium-glucose transporter 2 (SGLT2) inhibitors other than dapagliflozin, real-world data, and findings from non-CVOTs of dapagliflozin, Lindsay Clegg, PhD, reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
In EXSCEL – a CVOT of once-weekly treatment with the glucagonlike peptide–1 receptor agonist exenatide added to usual care in patients with type 2 diabetes mellitus – 10% of patients took an SGLT2 inhibitor, and about half of those took dapagliflozin. For the current analysis, the effects of all SGLT2 inhibitors and dapagliflozin alone were evaluated in EXSCEL patients who received placebo.
“Just looking at that placebo data, we wanted to ask what the impact of SGLT2 inhibition was on the adjudicated cardiovascular events, as well as all-cause death and eGFR [estimated glomerular filtration rate] in this population,” Dr. Clegg, a postdoctoral fellow with the AstraZeneca Quantitative Clinical Pharmacology Group in Gaithersburg, Md., said in an interview.
In two propensity-matched cohorts, including a cohort of 709 SGLT2 inhibitor users and a cohort of 709 non-SGLT2 inhibitor users, SGLT2 inhibitors and dapagliflozin alone were found to numerically decrease the major adverse cardiac event (MACE) hazard ratio, and SGLT2 inhibitors significantly reduced all-cause mortality risk, she explained.
MACE events – a composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke – occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 3.41 vs. 4.45; adjusted HR, 0.79). Dr. Clegg noted that this hazard ratio is “very consistent with what has been seen in the CVOTs for [the SGLT2 inhibitors] empagliflozin and canagliflozin in literature.”
The corresponding figures for dapagliflozin were 11 versus 22 events (event rate per 100 patient-years, 2.69 vs. 4.54; aHR, 0.55).
“So those weren’t statistically significant, but those point estimates were very similar to literature,” she said.
All-cause mortality events occurred in 14 versus 37 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 1.61 vs. 3.34; aHR, 0.50), and in 7 versus 13 dapagliflozin patients within these groups, respectively (event rate per 100 patient-years, 1.62 vs. 2.42; aHR, 0.66).
The overall SGLT2 inhibitor all-cause mortality findings were very similar to what was seen in CVD-REAL, a real-world evidence trial which looked at cardiovascular outcomes in new users of SGLT-2 inhibitors, and the differences were statistically significant for the treatment effect.
“For dapagliflozin, the numbers were pretty similar as well. Not statistically significant, because the number of subjects was smaller, but similar,” Dr. Clegg said.
“On eGFR looking at renal function ... subjects not using an SGLT2 inhibitor had about a 1 mL/min per year decline, which is what we would expect for this population. At baseline the median eGFR was about 80, so it’s a fairly healthy population, because exenatide isn’t used in people with poor renal function,” she explained.
The effects of SGLT2 inhibitors overall, and dapagliflozin alone, were associated with the statistically significant increase in the eGFR slope over time – an outcome that the Food and Drug Administration now recognizes as a surrogate endpoint for renal outcomes, she added. “And again, that’s very consistent with what was seen for [the SGLT2 inhibitor empagliflozin] in the literature.”
Empagliflozin and canagliflozin (another SGLT2 inhibitor) have been shown to reduce MACE, all-cause mortality, and renal events in CVOTs, and real-world evidence suggests a class effect benefit, but dapagliflozin CVOT data have not yet been published.
“Overall this was a nice dataset where we had these adjudicated events to look at outcomes with SGLT2 inhibitors and with [dapagliflozin] specifically, and what we see is very encouraging and suggestive of a class effect,” she concluded, noting that findings from the ongoing phase 3 DECLARE-TIMI58 dapagliflozin CVOT should be released later this year.
Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
SOURCE: Clegg L et al. ADA 2018, Abstract 130-LB.
ORLANDO – a post hoc analysis of data from the EXSCEL trial suggested.
The findings are consistent with those from published cardiovascular outcomes trials (CVOTs) of sodium-glucose transporter 2 (SGLT2) inhibitors other than dapagliflozin, real-world data, and findings from non-CVOTs of dapagliflozin, Lindsay Clegg, PhD, reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
In EXSCEL – a CVOT of once-weekly treatment with the glucagonlike peptide–1 receptor agonist exenatide added to usual care in patients with type 2 diabetes mellitus – 10% of patients took an SGLT2 inhibitor, and about half of those took dapagliflozin. For the current analysis, the effects of all SGLT2 inhibitors and dapagliflozin alone were evaluated in EXSCEL patients who received placebo.
“Just looking at that placebo data, we wanted to ask what the impact of SGLT2 inhibition was on the adjudicated cardiovascular events, as well as all-cause death and eGFR [estimated glomerular filtration rate] in this population,” Dr. Clegg, a postdoctoral fellow with the AstraZeneca Quantitative Clinical Pharmacology Group in Gaithersburg, Md., said in an interview.
In two propensity-matched cohorts, including a cohort of 709 SGLT2 inhibitor users and a cohort of 709 non-SGLT2 inhibitor users, SGLT2 inhibitors and dapagliflozin alone were found to numerically decrease the major adverse cardiac event (MACE) hazard ratio, and SGLT2 inhibitors significantly reduced all-cause mortality risk, she explained.
MACE events – a composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke – occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 3.41 vs. 4.45; adjusted HR, 0.79). Dr. Clegg noted that this hazard ratio is “very consistent with what has been seen in the CVOTs for [the SGLT2 inhibitors] empagliflozin and canagliflozin in literature.”
The corresponding figures for dapagliflozin were 11 versus 22 events (event rate per 100 patient-years, 2.69 vs. 4.54; aHR, 0.55).
“So those weren’t statistically significant, but those point estimates were very similar to literature,” she said.
All-cause mortality events occurred in 14 versus 37 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 1.61 vs. 3.34; aHR, 0.50), and in 7 versus 13 dapagliflozin patients within these groups, respectively (event rate per 100 patient-years, 1.62 vs. 2.42; aHR, 0.66).
The overall SGLT2 inhibitor all-cause mortality findings were very similar to what was seen in CVD-REAL, a real-world evidence trial which looked at cardiovascular outcomes in new users of SGLT-2 inhibitors, and the differences were statistically significant for the treatment effect.
“For dapagliflozin, the numbers were pretty similar as well. Not statistically significant, because the number of subjects was smaller, but similar,” Dr. Clegg said.
“On eGFR looking at renal function ... subjects not using an SGLT2 inhibitor had about a 1 mL/min per year decline, which is what we would expect for this population. At baseline the median eGFR was about 80, so it’s a fairly healthy population, because exenatide isn’t used in people with poor renal function,” she explained.
The effects of SGLT2 inhibitors overall, and dapagliflozin alone, were associated with the statistically significant increase in the eGFR slope over time – an outcome that the Food and Drug Administration now recognizes as a surrogate endpoint for renal outcomes, she added. “And again, that’s very consistent with what was seen for [the SGLT2 inhibitor empagliflozin] in the literature.”
Empagliflozin and canagliflozin (another SGLT2 inhibitor) have been shown to reduce MACE, all-cause mortality, and renal events in CVOTs, and real-world evidence suggests a class effect benefit, but dapagliflozin CVOT data have not yet been published.
“Overall this was a nice dataset where we had these adjudicated events to look at outcomes with SGLT2 inhibitors and with [dapagliflozin] specifically, and what we see is very encouraging and suggestive of a class effect,” she concluded, noting that findings from the ongoing phase 3 DECLARE-TIMI58 dapagliflozin CVOT should be released later this year.
Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
SOURCE: Clegg L et al. ADA 2018, Abstract 130-LB.
ORLANDO – a post hoc analysis of data from the EXSCEL trial suggested.
The findings are consistent with those from published cardiovascular outcomes trials (CVOTs) of sodium-glucose transporter 2 (SGLT2) inhibitors other than dapagliflozin, real-world data, and findings from non-CVOTs of dapagliflozin, Lindsay Clegg, PhD, reported in a late-breaking poster at the annual scientific sessions of the American Diabetes Association.
In EXSCEL – a CVOT of once-weekly treatment with the glucagonlike peptide–1 receptor agonist exenatide added to usual care in patients with type 2 diabetes mellitus – 10% of patients took an SGLT2 inhibitor, and about half of those took dapagliflozin. For the current analysis, the effects of all SGLT2 inhibitors and dapagliflozin alone were evaluated in EXSCEL patients who received placebo.
“Just looking at that placebo data, we wanted to ask what the impact of SGLT2 inhibition was on the adjudicated cardiovascular events, as well as all-cause death and eGFR [estimated glomerular filtration rate] in this population,” Dr. Clegg, a postdoctoral fellow with the AstraZeneca Quantitative Clinical Pharmacology Group in Gaithersburg, Md., said in an interview.
In two propensity-matched cohorts, including a cohort of 709 SGLT2 inhibitor users and a cohort of 709 non-SGLT2 inhibitor users, SGLT2 inhibitors and dapagliflozin alone were found to numerically decrease the major adverse cardiac event (MACE) hazard ratio, and SGLT2 inhibitors significantly reduced all-cause mortality risk, she explained.
MACE events – a composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke – occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 3.41 vs. 4.45; adjusted HR, 0.79). Dr. Clegg noted that this hazard ratio is “very consistent with what has been seen in the CVOTs for [the SGLT2 inhibitors] empagliflozin and canagliflozin in literature.”
The corresponding figures for dapagliflozin were 11 versus 22 events (event rate per 100 patient-years, 2.69 vs. 4.54; aHR, 0.55).
“So those weren’t statistically significant, but those point estimates were very similar to literature,” she said.
All-cause mortality events occurred in 14 versus 37 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (event rate per 100 patient-years, 1.61 vs. 3.34; aHR, 0.50), and in 7 versus 13 dapagliflozin patients within these groups, respectively (event rate per 100 patient-years, 1.62 vs. 2.42; aHR, 0.66).
The overall SGLT2 inhibitor all-cause mortality findings were very similar to what was seen in CVD-REAL, a real-world evidence trial which looked at cardiovascular outcomes in new users of SGLT-2 inhibitors, and the differences were statistically significant for the treatment effect.
“For dapagliflozin, the numbers were pretty similar as well. Not statistically significant, because the number of subjects was smaller, but similar,” Dr. Clegg said.
“On eGFR looking at renal function ... subjects not using an SGLT2 inhibitor had about a 1 mL/min per year decline, which is what we would expect for this population. At baseline the median eGFR was about 80, so it’s a fairly healthy population, because exenatide isn’t used in people with poor renal function,” she explained.
The effects of SGLT2 inhibitors overall, and dapagliflozin alone, were associated with the statistically significant increase in the eGFR slope over time – an outcome that the Food and Drug Administration now recognizes as a surrogate endpoint for renal outcomes, she added. “And again, that’s very consistent with what was seen for [the SGLT2 inhibitor empagliflozin] in the literature.”
Empagliflozin and canagliflozin (another SGLT2 inhibitor) have been shown to reduce MACE, all-cause mortality, and renal events in CVOTs, and real-world evidence suggests a class effect benefit, but dapagliflozin CVOT data have not yet been published.
“Overall this was a nice dataset where we had these adjudicated events to look at outcomes with SGLT2 inhibitors and with [dapagliflozin] specifically, and what we see is very encouraging and suggestive of a class effect,” she concluded, noting that findings from the ongoing phase 3 DECLARE-TIMI58 dapagliflozin CVOT should be released later this year.
Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
SOURCE: Clegg L et al. ADA 2018, Abstract 130-LB.
REPORTING FROM ADA 2018
Key clinical point: Sodium-glucose transporter 2 inhibitors, including dapagliflozin, have beneficial class effects on major adverse cardiac events, all-cause mortality, and renal function.
Major finding: MACE occurred in 28 versus 44 patients in the SGLT2 and non-SGLT2 inhibitor groups, respectively (adjusted hazard ratio, 0.79).
Study details: A post hoc analysis of data from 1,418 EXSCEL trial subjects.
Disclosures: Dr. Clegg is employed by AstraZeneca. She reported having no other disclosures.
Source: Clegg L et al. ADA 2018, Abstract 130-LB.
Special care advised for HIV-infected patients with diabetes
ORLANDO – Research suggests that HIV-positive people who take the latest generations of AIDS medications are living almost as long as everyone else. But they still face special medical challenges, and an endocrinologist urged colleagues to adjust their approaches to diabetes in these patients.
said Todd T. Brown, MD, PhD, of Johns Hopkins Medicine, Baltimore, in a presentation at the annual scientific sessions of the American Diabetes Association.
It’s not just a matter of subbing in an alternate drug here or there. When it comes to diabetes, patients with HIV require significant adjustments to diagnosis and treatment, Dr. Brown said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In terms of diagnosis, treatment guidelines approved by the Infectious Diseases Society of America and ADA recommend that all HIV-positive patients be tested for diabetes before they begin taking antiretroviral therapy. Then, the guidelines suggest, they should be tested 4-6 weeks after initiation of therapy, and every 6-12 months going forward.
“It’s a bit of overkill to go every 6 months,” said Dr. Brown, who prefers an annual testing approach. He added that research has suggested that the 2-hour postload glucose test is more sensitive than the fasting glucose test in some HIV-positive populations. However, he believes that it’s generally fine to give a fasting glucose test before initiation of therapy – and on an annual basis afterward – rather than the more cumbersome postload test.
Still, he said, the postload test may be appropriate in a patient with impaired glucose tolerance “if you really want to make the diagnosis, and especially if you’ll change your treatment based on it.”
Ongoing treatment of HIV-positive patients also presents unique challenges, he said. For one, antiretroviral therapy seems to affect glucose metabolism and body fat, he said, and findings from a 2016 study suggest HIV-positive people who begin antiretroviral therapy face a higher risk of developing diabetes after weight gain (J Acquir Immune Defic Syndr. 2016 Oct 1;73[2]:228-36).
One option is to switch patients to integrase inhibitors, but findings from a 2017 study suggested that this may also lead to more weight gain, Dr. Brown said.
“This has been an evolving story,” he said. “The clinical consequences of this are unclear. This is a topic that’s being hotly investigated now in the HIV health world” (JAIDS. 2017 Dec 15;76[5]:527-31).
As for other diabetes management issues, Dr. Brown noted that hemoglobin A1c tests appear to underestimate glycemia in HIV-infected patients. He suggested that goal HbA1c levels should be lower in diabetic patients with HIV, especially those with CD4+ counts under 500 cells /mm3 and/or mean cell volume over 100 fL.
Research suggests that lifestyle changes seem to work well in HIV-positive patients, he said, and metformin is the ideal first-line drug treatment just as in the HIV-negative population. “It’s a good drug. We all love it,” he said. “It may improve lipohypertrophy and coronary plaque.”
He added that proteinuria and neuropathy are more common in HIV-positive patients with diabetes. He said levels of neuropathy and nephropathy could be related to AIDS drugs.
On the medication front, Dr. Brown cautioned about certain drugs in HIV-positive patients: The HIV drug dolutegravir increases metformin concentrations by about 80%, he said, and there are concerns about bone and cardiac health in HIV-positive patients who take the diabetes medications known as thiazolidinediones (glitazones).
He added that there are sparse data about the use of several types of diabetes drugs – DPP IV inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors – in HIV-positive patients.
Dr. Brown discloses consulting for Gilead Sciences, ViiV, BMS, Merck, Theratechnologies, and EMD Serono.
ORLANDO – Research suggests that HIV-positive people who take the latest generations of AIDS medications are living almost as long as everyone else. But they still face special medical challenges, and an endocrinologist urged colleagues to adjust their approaches to diabetes in these patients.
said Todd T. Brown, MD, PhD, of Johns Hopkins Medicine, Baltimore, in a presentation at the annual scientific sessions of the American Diabetes Association.
It’s not just a matter of subbing in an alternate drug here or there. When it comes to diabetes, patients with HIV require significant adjustments to diagnosis and treatment, Dr. Brown said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In terms of diagnosis, treatment guidelines approved by the Infectious Diseases Society of America and ADA recommend that all HIV-positive patients be tested for diabetes before they begin taking antiretroviral therapy. Then, the guidelines suggest, they should be tested 4-6 weeks after initiation of therapy, and every 6-12 months going forward.
“It’s a bit of overkill to go every 6 months,” said Dr. Brown, who prefers an annual testing approach. He added that research has suggested that the 2-hour postload glucose test is more sensitive than the fasting glucose test in some HIV-positive populations. However, he believes that it’s generally fine to give a fasting glucose test before initiation of therapy – and on an annual basis afterward – rather than the more cumbersome postload test.
Still, he said, the postload test may be appropriate in a patient with impaired glucose tolerance “if you really want to make the diagnosis, and especially if you’ll change your treatment based on it.”
Ongoing treatment of HIV-positive patients also presents unique challenges, he said. For one, antiretroviral therapy seems to affect glucose metabolism and body fat, he said, and findings from a 2016 study suggest HIV-positive people who begin antiretroviral therapy face a higher risk of developing diabetes after weight gain (J Acquir Immune Defic Syndr. 2016 Oct 1;73[2]:228-36).
One option is to switch patients to integrase inhibitors, but findings from a 2017 study suggested that this may also lead to more weight gain, Dr. Brown said.
“This has been an evolving story,” he said. “The clinical consequences of this are unclear. This is a topic that’s being hotly investigated now in the HIV health world” (JAIDS. 2017 Dec 15;76[5]:527-31).
As for other diabetes management issues, Dr. Brown noted that hemoglobin A1c tests appear to underestimate glycemia in HIV-infected patients. He suggested that goal HbA1c levels should be lower in diabetic patients with HIV, especially those with CD4+ counts under 500 cells /mm3 and/or mean cell volume over 100 fL.
Research suggests that lifestyle changes seem to work well in HIV-positive patients, he said, and metformin is the ideal first-line drug treatment just as in the HIV-negative population. “It’s a good drug. We all love it,” he said. “It may improve lipohypertrophy and coronary plaque.”
He added that proteinuria and neuropathy are more common in HIV-positive patients with diabetes. He said levels of neuropathy and nephropathy could be related to AIDS drugs.
On the medication front, Dr. Brown cautioned about certain drugs in HIV-positive patients: The HIV drug dolutegravir increases metformin concentrations by about 80%, he said, and there are concerns about bone and cardiac health in HIV-positive patients who take the diabetes medications known as thiazolidinediones (glitazones).
He added that there are sparse data about the use of several types of diabetes drugs – DPP IV inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors – in HIV-positive patients.
Dr. Brown discloses consulting for Gilead Sciences, ViiV, BMS, Merck, Theratechnologies, and EMD Serono.
ORLANDO – Research suggests that HIV-positive people who take the latest generations of AIDS medications are living almost as long as everyone else. But they still face special medical challenges, and an endocrinologist urged colleagues to adjust their approaches to diabetes in these patients.
said Todd T. Brown, MD, PhD, of Johns Hopkins Medicine, Baltimore, in a presentation at the annual scientific sessions of the American Diabetes Association.
It’s not just a matter of subbing in an alternate drug here or there. When it comes to diabetes, patients with HIV require significant adjustments to diagnosis and treatment, Dr. Brown said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In terms of diagnosis, treatment guidelines approved by the Infectious Diseases Society of America and ADA recommend that all HIV-positive patients be tested for diabetes before they begin taking antiretroviral therapy. Then, the guidelines suggest, they should be tested 4-6 weeks after initiation of therapy, and every 6-12 months going forward.
“It’s a bit of overkill to go every 6 months,” said Dr. Brown, who prefers an annual testing approach. He added that research has suggested that the 2-hour postload glucose test is more sensitive than the fasting glucose test in some HIV-positive populations. However, he believes that it’s generally fine to give a fasting glucose test before initiation of therapy – and on an annual basis afterward – rather than the more cumbersome postload test.
Still, he said, the postload test may be appropriate in a patient with impaired glucose tolerance “if you really want to make the diagnosis, and especially if you’ll change your treatment based on it.”
Ongoing treatment of HIV-positive patients also presents unique challenges, he said. For one, antiretroviral therapy seems to affect glucose metabolism and body fat, he said, and findings from a 2016 study suggest HIV-positive people who begin antiretroviral therapy face a higher risk of developing diabetes after weight gain (J Acquir Immune Defic Syndr. 2016 Oct 1;73[2]:228-36).
One option is to switch patients to integrase inhibitors, but findings from a 2017 study suggested that this may also lead to more weight gain, Dr. Brown said.
“This has been an evolving story,” he said. “The clinical consequences of this are unclear. This is a topic that’s being hotly investigated now in the HIV health world” (JAIDS. 2017 Dec 15;76[5]:527-31).
As for other diabetes management issues, Dr. Brown noted that hemoglobin A1c tests appear to underestimate glycemia in HIV-infected patients. He suggested that goal HbA1c levels should be lower in diabetic patients with HIV, especially those with CD4+ counts under 500 cells /mm3 and/or mean cell volume over 100 fL.
Research suggests that lifestyle changes seem to work well in HIV-positive patients, he said, and metformin is the ideal first-line drug treatment just as in the HIV-negative population. “It’s a good drug. We all love it,” he said. “It may improve lipohypertrophy and coronary plaque.”
He added that proteinuria and neuropathy are more common in HIV-positive patients with diabetes. He said levels of neuropathy and nephropathy could be related to AIDS drugs.
On the medication front, Dr. Brown cautioned about certain drugs in HIV-positive patients: The HIV drug dolutegravir increases metformin concentrations by about 80%, he said, and there are concerns about bone and cardiac health in HIV-positive patients who take the diabetes medications known as thiazolidinediones (glitazones).
He added that there are sparse data about the use of several types of diabetes drugs – DPP IV inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors – in HIV-positive patients.
Dr. Brown discloses consulting for Gilead Sciences, ViiV, BMS, Merck, Theratechnologies, and EMD Serono.
EXPERT ANALYSIS FROM ADA 2018
Closed-loop insulin control for T2DM is feasible in hospital setting
ORLANDO – (T2DM).
The findings, released at the annual scientific sessions of the American Diabetes Association and via simultaneous publication in The New England Journal of Medicine, don’t examine cost or clinical outcomes. However, “our results suggest this new technology might be another approach to manage in-patient hypoglycemia in a safe and effective way, lead author Lia Bally, MD, PhD, of the division of endocrinology, diabetes, and clinical nutrition, Bern (Switzerland ) University Hospital, said in an interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For the open-label trial, the researchers recruited 136 adults with T2DM under noncritical care at two hospitals (one in the England and the other in Switzerland). Some patients had undergone surgery, Dr. Bally said, and some others were being treated for systemic infections. Comorbidities were significantly more severe in the closed-loop group, and 43% had sepsis.
All of the subjects required subcutaneous insulin therapy.
From 2016 to 2017, patients were randomly assigned to receive normal subcutaneous insulin therapy (n = 70) or closed-loop insulin delivery (n = 66).
It took about 15 minutes to perform the procedure to implement the closed-loop insulin delivery system, Dr. Bally said. It featured a subcutaneous cannula inserted into the abdomen, a continuous glucose monitor (a device also used in the control group), and a trial insulin pump.
This was not a hybrid system, and it did not include prandial insulin boluses or input of the timing and carbohydrate content of meals. One reason behind the choice to adopt a fully automated system was to relieve the burden on both health care professionals and patients, coauthor Hood Thabit, PhD, of Wellcome Trust–MRC Institute of Metabolic Science, the Manchester Academic Health Science Center, and University of Manchester, said in an interview.
For up to 15 days or until discharge, researchers tracked how much of the time sensor glucose measurements were in a target range of 100 mg/dL to 180 mg/dL.
In the closed-loop group, glucose measurements were in the target range 66 mg/dL ± 17% of the time compared to 42 mg/dL ± 17% in the control group, a difference of 24 mg/dL ± 3% (95% confidence interval, 19-30; P less than .001).
For the closed-loop group, the average glucose level was 154 mg/dL, and it was 188 mg/dL in the control group (P less than .001).
The researchers didn’t find a statistically significant difference between the groups in duration of hypoglycemia or amount of insulin delivered.
None of the patients suffered from severe hypoglycemia or clinically significant hyperglycemia with ketonemia.
There were 18 incidents of clinically significant hyperglycemia events (capillary glucose levels of more than 360 mg/dL) in the closed-loop group, compared with 41 such events in the control group. (P = .03)
Three patients in each group had adverse trial-related device effects.
Of 62 patients in the closed-loop group who completed the trial, 87% reported being pleased by their glucose levels, and all but one reported being happy to have their levels monitored automatically. All 62 patients said they’d recommend the system to others.
Going forward, the researchers hope to launch a multicenter trial that will examine clinical outcomes such as postoperative complications, infections, mortality, and glucose control after hospital discharge, according to Dr. Bally.
The study was supported by Diabetes UK, the Swiss National Science Foundation, the European Foundation for the Study of Diabetes, the JDRF, the National Institute for Health Research Cambridge Biomedical Research Center, and a Wellcome Strategic Award. Abbott Diabetes Care supplied equipment and guidance regarding connectivity, and representatives reviewed the manuscript before submission.
Dr. Bally reported funding from the University Hospital Bern, University of Bern and the Swiss Diabetes Foundation. Dr. Thabit reported no disclosures. Other authors report no disclosures or various disclosures.
SOURCE: Bally L et al. ADA 2018 Abstract 350-OR. Published simultaneously in The New England Journal of Medicine. June 25, 2018
ORLANDO – (T2DM).
The findings, released at the annual scientific sessions of the American Diabetes Association and via simultaneous publication in The New England Journal of Medicine, don’t examine cost or clinical outcomes. However, “our results suggest this new technology might be another approach to manage in-patient hypoglycemia in a safe and effective way, lead author Lia Bally, MD, PhD, of the division of endocrinology, diabetes, and clinical nutrition, Bern (Switzerland ) University Hospital, said in an interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For the open-label trial, the researchers recruited 136 adults with T2DM under noncritical care at two hospitals (one in the England and the other in Switzerland). Some patients had undergone surgery, Dr. Bally said, and some others were being treated for systemic infections. Comorbidities were significantly more severe in the closed-loop group, and 43% had sepsis.
All of the subjects required subcutaneous insulin therapy.
From 2016 to 2017, patients were randomly assigned to receive normal subcutaneous insulin therapy (n = 70) or closed-loop insulin delivery (n = 66).
It took about 15 minutes to perform the procedure to implement the closed-loop insulin delivery system, Dr. Bally said. It featured a subcutaneous cannula inserted into the abdomen, a continuous glucose monitor (a device also used in the control group), and a trial insulin pump.
This was not a hybrid system, and it did not include prandial insulin boluses or input of the timing and carbohydrate content of meals. One reason behind the choice to adopt a fully automated system was to relieve the burden on both health care professionals and patients, coauthor Hood Thabit, PhD, of Wellcome Trust–MRC Institute of Metabolic Science, the Manchester Academic Health Science Center, and University of Manchester, said in an interview.
For up to 15 days or until discharge, researchers tracked how much of the time sensor glucose measurements were in a target range of 100 mg/dL to 180 mg/dL.
In the closed-loop group, glucose measurements were in the target range 66 mg/dL ± 17% of the time compared to 42 mg/dL ± 17% in the control group, a difference of 24 mg/dL ± 3% (95% confidence interval, 19-30; P less than .001).
For the closed-loop group, the average glucose level was 154 mg/dL, and it was 188 mg/dL in the control group (P less than .001).
The researchers didn’t find a statistically significant difference between the groups in duration of hypoglycemia or amount of insulin delivered.
None of the patients suffered from severe hypoglycemia or clinically significant hyperglycemia with ketonemia.
There were 18 incidents of clinically significant hyperglycemia events (capillary glucose levels of more than 360 mg/dL) in the closed-loop group, compared with 41 such events in the control group. (P = .03)
Three patients in each group had adverse trial-related device effects.
Of 62 patients in the closed-loop group who completed the trial, 87% reported being pleased by their glucose levels, and all but one reported being happy to have their levels monitored automatically. All 62 patients said they’d recommend the system to others.
Going forward, the researchers hope to launch a multicenter trial that will examine clinical outcomes such as postoperative complications, infections, mortality, and glucose control after hospital discharge, according to Dr. Bally.
The study was supported by Diabetes UK, the Swiss National Science Foundation, the European Foundation for the Study of Diabetes, the JDRF, the National Institute for Health Research Cambridge Biomedical Research Center, and a Wellcome Strategic Award. Abbott Diabetes Care supplied equipment and guidance regarding connectivity, and representatives reviewed the manuscript before submission.
Dr. Bally reported funding from the University Hospital Bern, University of Bern and the Swiss Diabetes Foundation. Dr. Thabit reported no disclosures. Other authors report no disclosures or various disclosures.
SOURCE: Bally L et al. ADA 2018 Abstract 350-OR. Published simultaneously in The New England Journal of Medicine. June 25, 2018
ORLANDO – (T2DM).
The findings, released at the annual scientific sessions of the American Diabetes Association and via simultaneous publication in The New England Journal of Medicine, don’t examine cost or clinical outcomes. However, “our results suggest this new technology might be another approach to manage in-patient hypoglycemia in a safe and effective way, lead author Lia Bally, MD, PhD, of the division of endocrinology, diabetes, and clinical nutrition, Bern (Switzerland ) University Hospital, said in an interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
For the open-label trial, the researchers recruited 136 adults with T2DM under noncritical care at two hospitals (one in the England and the other in Switzerland). Some patients had undergone surgery, Dr. Bally said, and some others were being treated for systemic infections. Comorbidities were significantly more severe in the closed-loop group, and 43% had sepsis.
All of the subjects required subcutaneous insulin therapy.
From 2016 to 2017, patients were randomly assigned to receive normal subcutaneous insulin therapy (n = 70) or closed-loop insulin delivery (n = 66).
It took about 15 minutes to perform the procedure to implement the closed-loop insulin delivery system, Dr. Bally said. It featured a subcutaneous cannula inserted into the abdomen, a continuous glucose monitor (a device also used in the control group), and a trial insulin pump.
This was not a hybrid system, and it did not include prandial insulin boluses or input of the timing and carbohydrate content of meals. One reason behind the choice to adopt a fully automated system was to relieve the burden on both health care professionals and patients, coauthor Hood Thabit, PhD, of Wellcome Trust–MRC Institute of Metabolic Science, the Manchester Academic Health Science Center, and University of Manchester, said in an interview.
For up to 15 days or until discharge, researchers tracked how much of the time sensor glucose measurements were in a target range of 100 mg/dL to 180 mg/dL.
In the closed-loop group, glucose measurements were in the target range 66 mg/dL ± 17% of the time compared to 42 mg/dL ± 17% in the control group, a difference of 24 mg/dL ± 3% (95% confidence interval, 19-30; P less than .001).
For the closed-loop group, the average glucose level was 154 mg/dL, and it was 188 mg/dL in the control group (P less than .001).
The researchers didn’t find a statistically significant difference between the groups in duration of hypoglycemia or amount of insulin delivered.
None of the patients suffered from severe hypoglycemia or clinically significant hyperglycemia with ketonemia.
There were 18 incidents of clinically significant hyperglycemia events (capillary glucose levels of more than 360 mg/dL) in the closed-loop group, compared with 41 such events in the control group. (P = .03)
Three patients in each group had adverse trial-related device effects.
Of 62 patients in the closed-loop group who completed the trial, 87% reported being pleased by their glucose levels, and all but one reported being happy to have their levels monitored automatically. All 62 patients said they’d recommend the system to others.
Going forward, the researchers hope to launch a multicenter trial that will examine clinical outcomes such as postoperative complications, infections, mortality, and glucose control after hospital discharge, according to Dr. Bally.
The study was supported by Diabetes UK, the Swiss National Science Foundation, the European Foundation for the Study of Diabetes, the JDRF, the National Institute for Health Research Cambridge Biomedical Research Center, and a Wellcome Strategic Award. Abbott Diabetes Care supplied equipment and guidance regarding connectivity, and representatives reviewed the manuscript before submission.
Dr. Bally reported funding from the University Hospital Bern, University of Bern and the Swiss Diabetes Foundation. Dr. Thabit reported no disclosures. Other authors report no disclosures or various disclosures.
SOURCE: Bally L et al. ADA 2018 Abstract 350-OR. Published simultaneously in The New England Journal of Medicine. June 25, 2018
REPORTING FROM ADA 2018
Key clinical point: Use of an automated closed-loop insulin delivery system may be feasible in the noncritical hospital setting.
Major finding: In the closed-loop group, glucose measurements were in the target range 66 mg/dL ± 17% of the time compared with 42 mg/dL ± 17% in the control group, a difference of 24 mg/dL ± 3% (95% CI, 19-30; P less than .001).
Study details: Randomized, open-label, two-center trial of 136 inpatients with type 2 diabetes mellitus assigned to either standard subcutaneous insulin therapy or closed-loop insulin delivery for 15 days or until discharge.
Disclosures: The study was supported by Diabetes UK, the Swiss National Science Foundation, the European Foundation for the Study of Diabetes, the JDRF, the National Institute for Health Research Cambridge Biomedical Research Center, and a Wellcome Strategic Award. Abbott Diabetes Care supplied equipment and guidance regarding connectivity, and representatives reviewed the manuscript before submission. The researchers reported no disclosures or various disclosures.
Source: Bally L et al. ADA 2018 Abstract 350-OR.
Meet the rare diabetes diagnosis that thrills patients
ORLANDO – Liana K. Billings, MD, an endocrinologist at the University of Chicago and the NorthShore University HealthSystem in Skokie, Ill., loves the thrill of letting patients know they have a rare kind of diabetes. “Once you do this once, you don’t want to stop,” she told colleagues in a presentation at the annual scientific sessions of the American Diabetes Association.
Yes, it’s true: There’s a diabetes diagnosis that spawns good feelings like delight and relief. The cause for celebration is a condition known as monogenetic diabetes, also known as maturity-onset diabetes of the young (MODY) if it develops after the neonatal period.
“The reason that getting a diagnosis of MODY can be a ‘good’ diagnosis is because the three most common forms of MODY have gene-specific treatments that typically improve patients’ glycemic control and are less onerous than the treatments patients were previously receiving when they were thought to have type 1 or type 2 diabetes,” explained Miriam S. Udler, MD, PhD, of Massachusetts General Hospital and Harvard Medical School, Boston, in an interview.
Dr. Udler and Dr. Billings spoke to colleagues about monogenetic diabetes in their presentation at the ADA meeting.
Research has suggested that 1%-4% of people with diabetes have the monogenetic form, in which the condition is caused by changes in a single gene. A 2017 British study of 1,407 patients with diabetes reported that “the minimum prevalence of monogenic diabetes is 3.6% of patients diagnosed at age 30 years or younger.”
The study, which tested a screening regimen, also turned up 17 new diagnoses of monogenetic diabetes among the 1,407 patients, doubling the total. The findings reflect an apparent fact about monogenetic diabetes: Physicians often don’t look for it, even though a diagnosis can be a godsend – especially for those who were previously diagnosed with type 1 or type 2 and placed on treatment regimens that are unnecessary at best and harmful at worst. (Diabetes Care. 2017 Aug;40[8]: 1017-25)
Patients with the MODY variant in the GCK gene, for example, “can generally stop all medications because they are not at risk for clinically significant complications of diabetes,” Dr. Udler said. “Patients with HNF1A and HNF4A variants can often be switched from insulin injections to ... a sulfonylurea, which is easier to take than insulin injections, and patients generally have better glycemic control after switching to pills.”
Unfortunately for doctors and patients, it can be complicated and costly to test for monogenetic diabetes. But screening tools are available to help physicians make choices about whether to launch testing in the first place, according to Dr. Udler and Dr. Billings.
There are two forms of monogenetic diabetes – neonatal diabetes, which is diagnosed by age 6-9 months, and MODY, which is typically diagnosed in those aged between 10 and 25 years, noted Dr. Billings.
Reasons to suspect MODY include early onset of diabetes (under 35 years), a family history of diabetes, a lack of obesity, and negative islet-cell antibodies, she said.
Obese patients may also have the condition: A 2017 American study of 488 overweight and obese children and adolescents diagnosed with type 2 diabetes found that 4.5% actually had monogenetic diabetes. (Genet Med. 2018 Jun;20[6]:583-90).
Once a physician suspects MODY, physicians may consult the University of Exeter’s risk calculator. It provides guidance about whether a test is a good idea. Dr. Billings cautioned, however, that the value of a calculator’s estimate of risk is not all-encompassing. “You should never use the calculator by itself as a reason to not pursue your intuition,” she said.
Dr. Udler noted that the University of Exeter calculator has important limitations, such as its reliance on specific genes, its lack of consideration of family history outside of parents, and its reliance on the experiences of white European patients.
As for tests, the University of Chicago and the University of Exeter both offer free genetic testing for neonatal diabetes, Dr. Billings said in her presentation.
Monogenetic diabetes tests in older children and adults are not free. However, Dr. Udler said the tests are often covered by insurance companies whether done for one or more genes.
At least one company offers a direct-to-consumer monogenetic diabetes test, according to Dr. Udler, but she recommended against it, especially in light of a curious online notice that says the test isn’t intended to be diagnostic. “I’m not sure what this would be useful for then,” she said.
For her part, Dr. Billings cautioned that test results may be inconclusive, and tests may offer different answers. She also recommended referring patients to genetic counseling.
Dr. Udler reported a board member/advisory panel relationship with Encompass Bioscience. Dr. Billings reported relationships with Novo Nordisk, Sanofi, and Dexcom.
ORLANDO – Liana K. Billings, MD, an endocrinologist at the University of Chicago and the NorthShore University HealthSystem in Skokie, Ill., loves the thrill of letting patients know they have a rare kind of diabetes. “Once you do this once, you don’t want to stop,” she told colleagues in a presentation at the annual scientific sessions of the American Diabetes Association.
Yes, it’s true: There’s a diabetes diagnosis that spawns good feelings like delight and relief. The cause for celebration is a condition known as monogenetic diabetes, also known as maturity-onset diabetes of the young (MODY) if it develops after the neonatal period.
“The reason that getting a diagnosis of MODY can be a ‘good’ diagnosis is because the three most common forms of MODY have gene-specific treatments that typically improve patients’ glycemic control and are less onerous than the treatments patients were previously receiving when they were thought to have type 1 or type 2 diabetes,” explained Miriam S. Udler, MD, PhD, of Massachusetts General Hospital and Harvard Medical School, Boston, in an interview.
Dr. Udler and Dr. Billings spoke to colleagues about monogenetic diabetes in their presentation at the ADA meeting.
Research has suggested that 1%-4% of people with diabetes have the monogenetic form, in which the condition is caused by changes in a single gene. A 2017 British study of 1,407 patients with diabetes reported that “the minimum prevalence of monogenic diabetes is 3.6% of patients diagnosed at age 30 years or younger.”
The study, which tested a screening regimen, also turned up 17 new diagnoses of monogenetic diabetes among the 1,407 patients, doubling the total. The findings reflect an apparent fact about monogenetic diabetes: Physicians often don’t look for it, even though a diagnosis can be a godsend – especially for those who were previously diagnosed with type 1 or type 2 and placed on treatment regimens that are unnecessary at best and harmful at worst. (Diabetes Care. 2017 Aug;40[8]: 1017-25)
Patients with the MODY variant in the GCK gene, for example, “can generally stop all medications because they are not at risk for clinically significant complications of diabetes,” Dr. Udler said. “Patients with HNF1A and HNF4A variants can often be switched from insulin injections to ... a sulfonylurea, which is easier to take than insulin injections, and patients generally have better glycemic control after switching to pills.”
Unfortunately for doctors and patients, it can be complicated and costly to test for monogenetic diabetes. But screening tools are available to help physicians make choices about whether to launch testing in the first place, according to Dr. Udler and Dr. Billings.
There are two forms of monogenetic diabetes – neonatal diabetes, which is diagnosed by age 6-9 months, and MODY, which is typically diagnosed in those aged between 10 and 25 years, noted Dr. Billings.
Reasons to suspect MODY include early onset of diabetes (under 35 years), a family history of diabetes, a lack of obesity, and negative islet-cell antibodies, she said.
Obese patients may also have the condition: A 2017 American study of 488 overweight and obese children and adolescents diagnosed with type 2 diabetes found that 4.5% actually had monogenetic diabetes. (Genet Med. 2018 Jun;20[6]:583-90).
Once a physician suspects MODY, physicians may consult the University of Exeter’s risk calculator. It provides guidance about whether a test is a good idea. Dr. Billings cautioned, however, that the value of a calculator’s estimate of risk is not all-encompassing. “You should never use the calculator by itself as a reason to not pursue your intuition,” she said.
Dr. Udler noted that the University of Exeter calculator has important limitations, such as its reliance on specific genes, its lack of consideration of family history outside of parents, and its reliance on the experiences of white European patients.
As for tests, the University of Chicago and the University of Exeter both offer free genetic testing for neonatal diabetes, Dr. Billings said in her presentation.
Monogenetic diabetes tests in older children and adults are not free. However, Dr. Udler said the tests are often covered by insurance companies whether done for one or more genes.
At least one company offers a direct-to-consumer monogenetic diabetes test, according to Dr. Udler, but she recommended against it, especially in light of a curious online notice that says the test isn’t intended to be diagnostic. “I’m not sure what this would be useful for then,” she said.
For her part, Dr. Billings cautioned that test results may be inconclusive, and tests may offer different answers. She also recommended referring patients to genetic counseling.
Dr. Udler reported a board member/advisory panel relationship with Encompass Bioscience. Dr. Billings reported relationships with Novo Nordisk, Sanofi, and Dexcom.
ORLANDO – Liana K. Billings, MD, an endocrinologist at the University of Chicago and the NorthShore University HealthSystem in Skokie, Ill., loves the thrill of letting patients know they have a rare kind of diabetes. “Once you do this once, you don’t want to stop,” she told colleagues in a presentation at the annual scientific sessions of the American Diabetes Association.
Yes, it’s true: There’s a diabetes diagnosis that spawns good feelings like delight and relief. The cause for celebration is a condition known as monogenetic diabetes, also known as maturity-onset diabetes of the young (MODY) if it develops after the neonatal period.
“The reason that getting a diagnosis of MODY can be a ‘good’ diagnosis is because the three most common forms of MODY have gene-specific treatments that typically improve patients’ glycemic control and are less onerous than the treatments patients were previously receiving when they were thought to have type 1 or type 2 diabetes,” explained Miriam S. Udler, MD, PhD, of Massachusetts General Hospital and Harvard Medical School, Boston, in an interview.
Dr. Udler and Dr. Billings spoke to colleagues about monogenetic diabetes in their presentation at the ADA meeting.
Research has suggested that 1%-4% of people with diabetes have the monogenetic form, in which the condition is caused by changes in a single gene. A 2017 British study of 1,407 patients with diabetes reported that “the minimum prevalence of monogenic diabetes is 3.6% of patients diagnosed at age 30 years or younger.”
The study, which tested a screening regimen, also turned up 17 new diagnoses of monogenetic diabetes among the 1,407 patients, doubling the total. The findings reflect an apparent fact about monogenetic diabetes: Physicians often don’t look for it, even though a diagnosis can be a godsend – especially for those who were previously diagnosed with type 1 or type 2 and placed on treatment regimens that are unnecessary at best and harmful at worst. (Diabetes Care. 2017 Aug;40[8]: 1017-25)
Patients with the MODY variant in the GCK gene, for example, “can generally stop all medications because they are not at risk for clinically significant complications of diabetes,” Dr. Udler said. “Patients with HNF1A and HNF4A variants can often be switched from insulin injections to ... a sulfonylurea, which is easier to take than insulin injections, and patients generally have better glycemic control after switching to pills.”
Unfortunately for doctors and patients, it can be complicated and costly to test for monogenetic diabetes. But screening tools are available to help physicians make choices about whether to launch testing in the first place, according to Dr. Udler and Dr. Billings.
There are two forms of monogenetic diabetes – neonatal diabetes, which is diagnosed by age 6-9 months, and MODY, which is typically diagnosed in those aged between 10 and 25 years, noted Dr. Billings.
Reasons to suspect MODY include early onset of diabetes (under 35 years), a family history of diabetes, a lack of obesity, and negative islet-cell antibodies, she said.
Obese patients may also have the condition: A 2017 American study of 488 overweight and obese children and adolescents diagnosed with type 2 diabetes found that 4.5% actually had monogenetic diabetes. (Genet Med. 2018 Jun;20[6]:583-90).
Once a physician suspects MODY, physicians may consult the University of Exeter’s risk calculator. It provides guidance about whether a test is a good idea. Dr. Billings cautioned, however, that the value of a calculator’s estimate of risk is not all-encompassing. “You should never use the calculator by itself as a reason to not pursue your intuition,” she said.
Dr. Udler noted that the University of Exeter calculator has important limitations, such as its reliance on specific genes, its lack of consideration of family history outside of parents, and its reliance on the experiences of white European patients.
As for tests, the University of Chicago and the University of Exeter both offer free genetic testing for neonatal diabetes, Dr. Billings said in her presentation.
Monogenetic diabetes tests in older children and adults are not free. However, Dr. Udler said the tests are often covered by insurance companies whether done for one or more genes.
At least one company offers a direct-to-consumer monogenetic diabetes test, according to Dr. Udler, but she recommended against it, especially in light of a curious online notice that says the test isn’t intended to be diagnostic. “I’m not sure what this would be useful for then,” she said.
For her part, Dr. Billings cautioned that test results may be inconclusive, and tests may offer different answers. She also recommended referring patients to genetic counseling.
Dr. Udler reported a board member/advisory panel relationship with Encompass Bioscience. Dr. Billings reported relationships with Novo Nordisk, Sanofi, and Dexcom.
EXPERT ANALYSIS FROM ADA 2018
Chronic kidney disease is 40% more common in T2DM than T1DM
ORLANDO – A new analysis of more than 1.5 million U.S. subjects with diabetes found that chronic kidney disease (CKD) is much more common in type 2 diabetes mellitus (T2DM) than in type 1 diabetes mellitus (T1DM) – 44% vs. 32%, respectively. The research also provides more evidence that albumin testing can provide crucial warning signs of future kidney trouble.
“Our data suggest – but don’t really prove – that there’s a lot more eGFR testing than there is albumin testing,” said nephrologist and study coauthor Michael Cressman, DO, of Covance, the drug development business of LabCorp, in an interview at the annual scientific sessions of the American Diabetes Association. “It is very important to measure albumin in the urine in order to identify patients who are at highest risk of progressive renal disease. There you identify people for whom you really want to maximize all the available treatments.”
According to the study, previous research has estimated that 25% of U.S. adults with diabetes have CKD (eGFR less than 60 ml/min per 1.73m2 or an albumin to creatinine ratio equal to or greater than 30 mg/g), but the difference in rates between T1DM and T2DM has been unclear.
Researchers analyzed LabCorp laboratory data on blood from for 48,036 adults with T1DM and 1,461,915 with T2DM. The analysis included ACR and CKD-EPI calculator for eGFR measurements from 2014-2017.
The researchers tracked declines in eGFR in patients who had more than three eGFR readings over at least 1 year.
Researchers found that the rate of CKD was 40% higher in patients with T2DM than it was in those with T1DM (44% vs. 32%, respectively; P less than .001), as was the prevalence of subjects considered to be at high or very high risk (18% vs. 12%, respectively; P less than .001).
These findings didn’t surprise Dr. Cressman, who said the higher ages of subjects with T2DM could explain the gap since they were more likely to have been exposed to hypertension for longer amounts of time.
Researchers also reported that the median eGFR decline (ml/min per year) was especially high in those with macroalbuminuria: –3.80 in T1DM and –3.58 in T2DM.
“Although MA [macroalbuminuria] is uncommon and most frequently observed in patients with normal or only mildly reduced eGFR, it was a potent predictor of eGFR decline in both T1DM and T2DM,” the researchers wrote.
“While it’s been known for a while that it’s bad to have albumin, this is more of a strong reinforcing piece of data,” Dr. Cressman said. “When you read about these things and it’s an epidemiological study or a clinical trial, it kind of loses its flavor. These are actual patients. A doctor could look at this data and say, ‘I ought to be checking this [albumin].’ It’s sort of an obvious rationale for what the guidelines say.”
No study funding was reported. Dr. Cressman reported employment by Covance. Other study authors variously report no disclosures or employment by Covance and its parent company LabCorp and stock/shareholding in LabCorp.
SOURCE: Cressman M et al. ADA 2018, Abstract 544-P.
ORLANDO – A new analysis of more than 1.5 million U.S. subjects with diabetes found that chronic kidney disease (CKD) is much more common in type 2 diabetes mellitus (T2DM) than in type 1 diabetes mellitus (T1DM) – 44% vs. 32%, respectively. The research also provides more evidence that albumin testing can provide crucial warning signs of future kidney trouble.
“Our data suggest – but don’t really prove – that there’s a lot more eGFR testing than there is albumin testing,” said nephrologist and study coauthor Michael Cressman, DO, of Covance, the drug development business of LabCorp, in an interview at the annual scientific sessions of the American Diabetes Association. “It is very important to measure albumin in the urine in order to identify patients who are at highest risk of progressive renal disease. There you identify people for whom you really want to maximize all the available treatments.”
According to the study, previous research has estimated that 25% of U.S. adults with diabetes have CKD (eGFR less than 60 ml/min per 1.73m2 or an albumin to creatinine ratio equal to or greater than 30 mg/g), but the difference in rates between T1DM and T2DM has been unclear.
Researchers analyzed LabCorp laboratory data on blood from for 48,036 adults with T1DM and 1,461,915 with T2DM. The analysis included ACR and CKD-EPI calculator for eGFR measurements from 2014-2017.
The researchers tracked declines in eGFR in patients who had more than three eGFR readings over at least 1 year.
Researchers found that the rate of CKD was 40% higher in patients with T2DM than it was in those with T1DM (44% vs. 32%, respectively; P less than .001), as was the prevalence of subjects considered to be at high or very high risk (18% vs. 12%, respectively; P less than .001).
These findings didn’t surprise Dr. Cressman, who said the higher ages of subjects with T2DM could explain the gap since they were more likely to have been exposed to hypertension for longer amounts of time.
Researchers also reported that the median eGFR decline (ml/min per year) was especially high in those with macroalbuminuria: –3.80 in T1DM and –3.58 in T2DM.
“Although MA [macroalbuminuria] is uncommon and most frequently observed in patients with normal or only mildly reduced eGFR, it was a potent predictor of eGFR decline in both T1DM and T2DM,” the researchers wrote.
“While it’s been known for a while that it’s bad to have albumin, this is more of a strong reinforcing piece of data,” Dr. Cressman said. “When you read about these things and it’s an epidemiological study or a clinical trial, it kind of loses its flavor. These are actual patients. A doctor could look at this data and say, ‘I ought to be checking this [albumin].’ It’s sort of an obvious rationale for what the guidelines say.”
No study funding was reported. Dr. Cressman reported employment by Covance. Other study authors variously report no disclosures or employment by Covance and its parent company LabCorp and stock/shareholding in LabCorp.
SOURCE: Cressman M et al. ADA 2018, Abstract 544-P.
ORLANDO – A new analysis of more than 1.5 million U.S. subjects with diabetes found that chronic kidney disease (CKD) is much more common in type 2 diabetes mellitus (T2DM) than in type 1 diabetes mellitus (T1DM) – 44% vs. 32%, respectively. The research also provides more evidence that albumin testing can provide crucial warning signs of future kidney trouble.
“Our data suggest – but don’t really prove – that there’s a lot more eGFR testing than there is albumin testing,” said nephrologist and study coauthor Michael Cressman, DO, of Covance, the drug development business of LabCorp, in an interview at the annual scientific sessions of the American Diabetes Association. “It is very important to measure albumin in the urine in order to identify patients who are at highest risk of progressive renal disease. There you identify people for whom you really want to maximize all the available treatments.”
According to the study, previous research has estimated that 25% of U.S. adults with diabetes have CKD (eGFR less than 60 ml/min per 1.73m2 or an albumin to creatinine ratio equal to or greater than 30 mg/g), but the difference in rates between T1DM and T2DM has been unclear.
Researchers analyzed LabCorp laboratory data on blood from for 48,036 adults with T1DM and 1,461,915 with T2DM. The analysis included ACR and CKD-EPI calculator for eGFR measurements from 2014-2017.
The researchers tracked declines in eGFR in patients who had more than three eGFR readings over at least 1 year.
Researchers found that the rate of CKD was 40% higher in patients with T2DM than it was in those with T1DM (44% vs. 32%, respectively; P less than .001), as was the prevalence of subjects considered to be at high or very high risk (18% vs. 12%, respectively; P less than .001).
These findings didn’t surprise Dr. Cressman, who said the higher ages of subjects with T2DM could explain the gap since they were more likely to have been exposed to hypertension for longer amounts of time.
Researchers also reported that the median eGFR decline (ml/min per year) was especially high in those with macroalbuminuria: –3.80 in T1DM and –3.58 in T2DM.
“Although MA [macroalbuminuria] is uncommon and most frequently observed in patients with normal or only mildly reduced eGFR, it was a potent predictor of eGFR decline in both T1DM and T2DM,” the researchers wrote.
“While it’s been known for a while that it’s bad to have albumin, this is more of a strong reinforcing piece of data,” Dr. Cressman said. “When you read about these things and it’s an epidemiological study or a clinical trial, it kind of loses its flavor. These are actual patients. A doctor could look at this data and say, ‘I ought to be checking this [albumin].’ It’s sort of an obvious rationale for what the guidelines say.”
No study funding was reported. Dr. Cressman reported employment by Covance. Other study authors variously report no disclosures or employment by Covance and its parent company LabCorp and stock/shareholding in LabCorp.
SOURCE: Cressman M et al. ADA 2018, Abstract 544-P.
REPORTING FROM ADA 2018
Key clinical point: CKD is significantly more common in patients with T2DM than those with T1DM, and albumin testing provides crucial warning signs.
Major finding: Of subjects with T2DM, 44% had signs of CKD, compared with 32% of those with T1DM.
Study details: Analysis of LabCorp blood testing of more than 1.5 million U.S. adults with diabetes from 2014-2017.
Disclosures: No study funding was reported. Authors reported various disclosures, mostly employment for Covance or its parent company, LabCorp.
Source: Cressman M et al. ADA 2018, Abstract 544-P.
Diabetes risk may rise with work hours
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.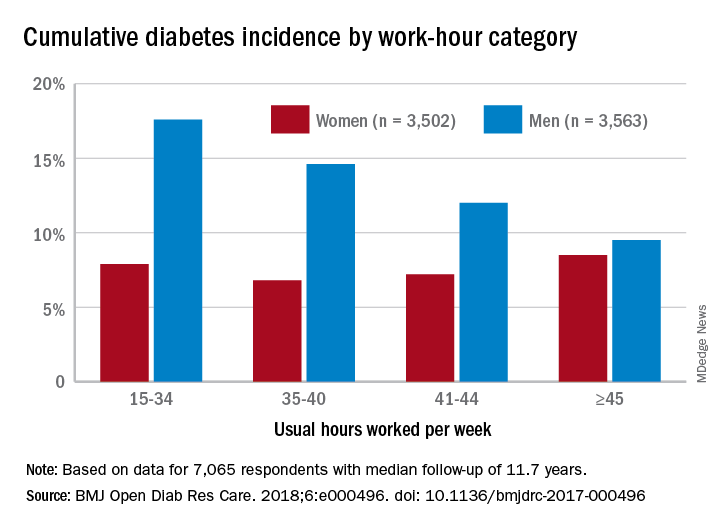
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
FROM BMJ OPEN DIABETES RESEARCH & CARE
Dietary recommendations for patients with diabetes
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2
Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes in the elderly: Matching meds to needs
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2
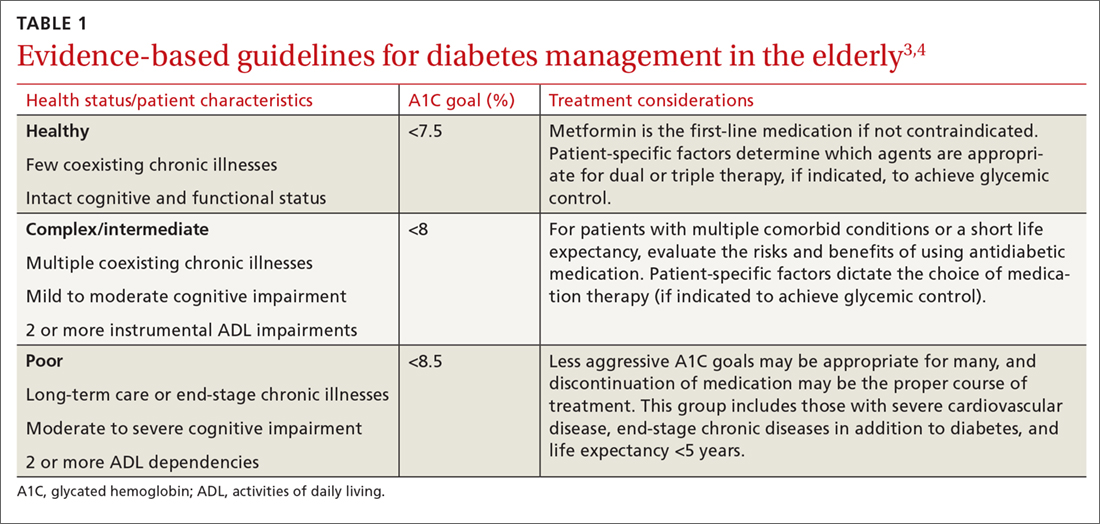
Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; [email protected].
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
From The Journal of Family Practice | 2018;67(7):408-410,412-415.
PRACTICE RECOMMENDATIONS
› Allow higher A1C goals for elderly patients who have such comorbid conditions as cognitive dysfunction, dementia, or cardiovascular or renal disease. B
› Look to metformin first in most instances if there are no contraindications. Monitor renal function frequently and vitamin B12 levels periodically. B
› Consider glucagon-like peptide-1 receptor agonists for patients who also have established cardiovascular disease, or consider starting basal insulin instead of using multiple oral agents. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Blood pressure targets: How low should you go (and for whom)?
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.
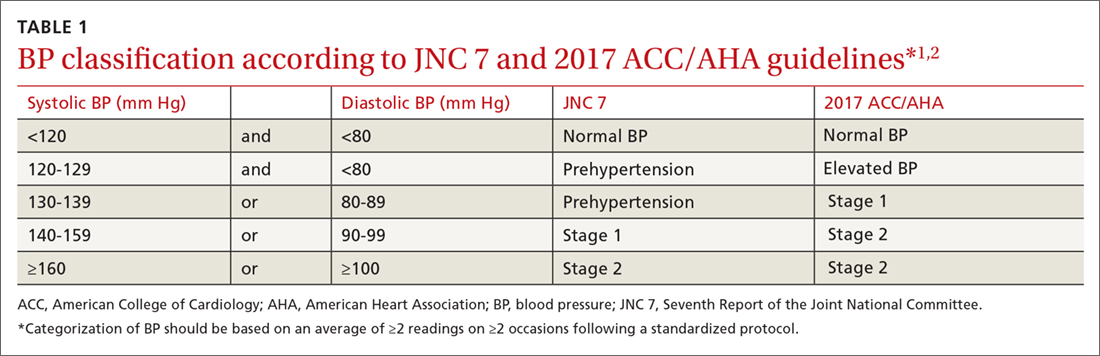
Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.
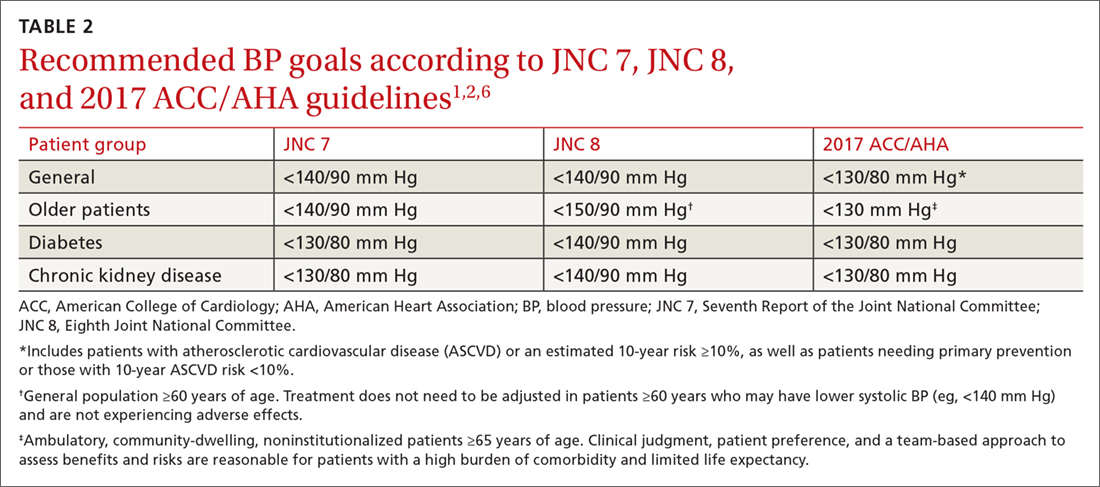
SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.
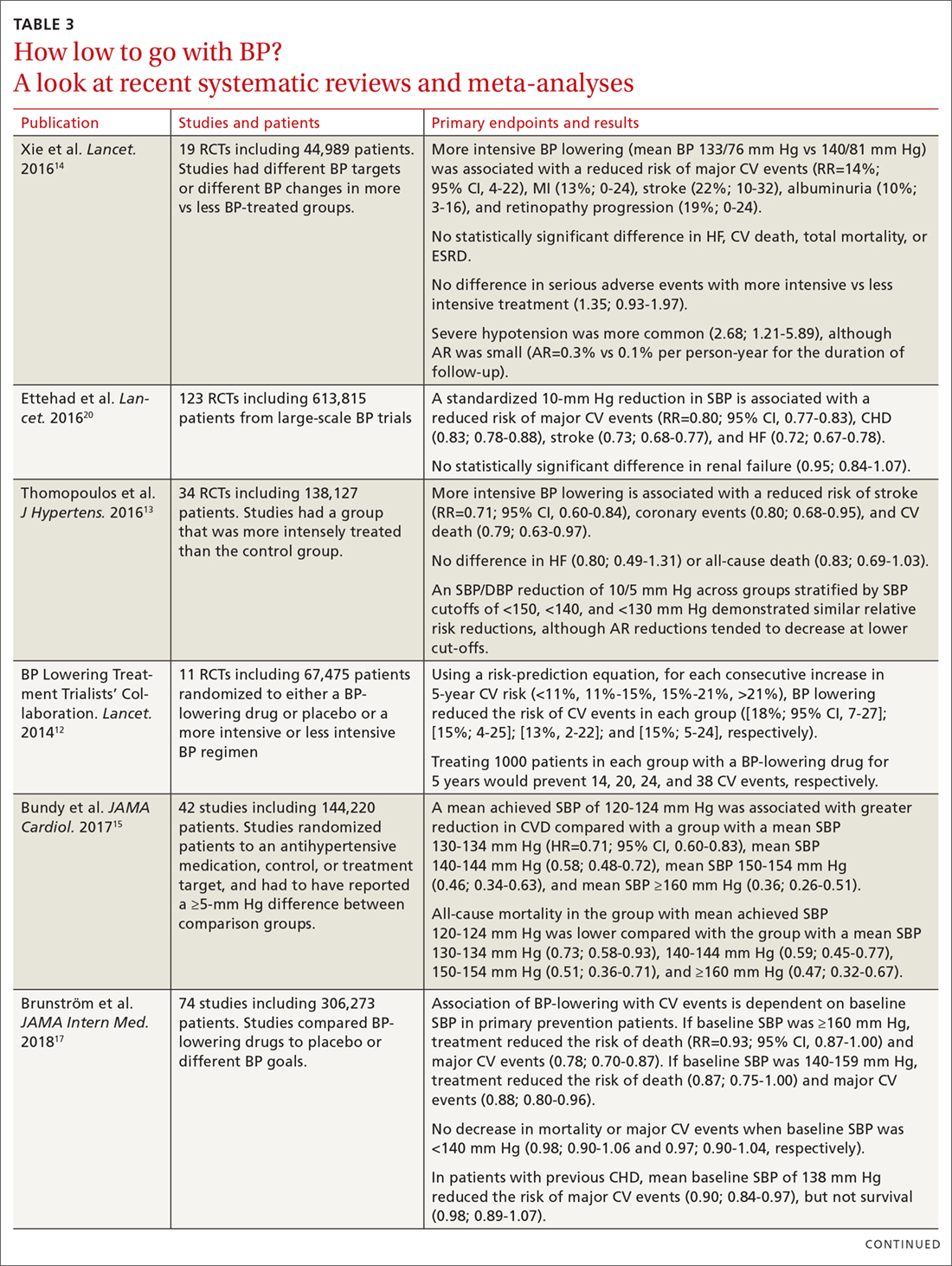
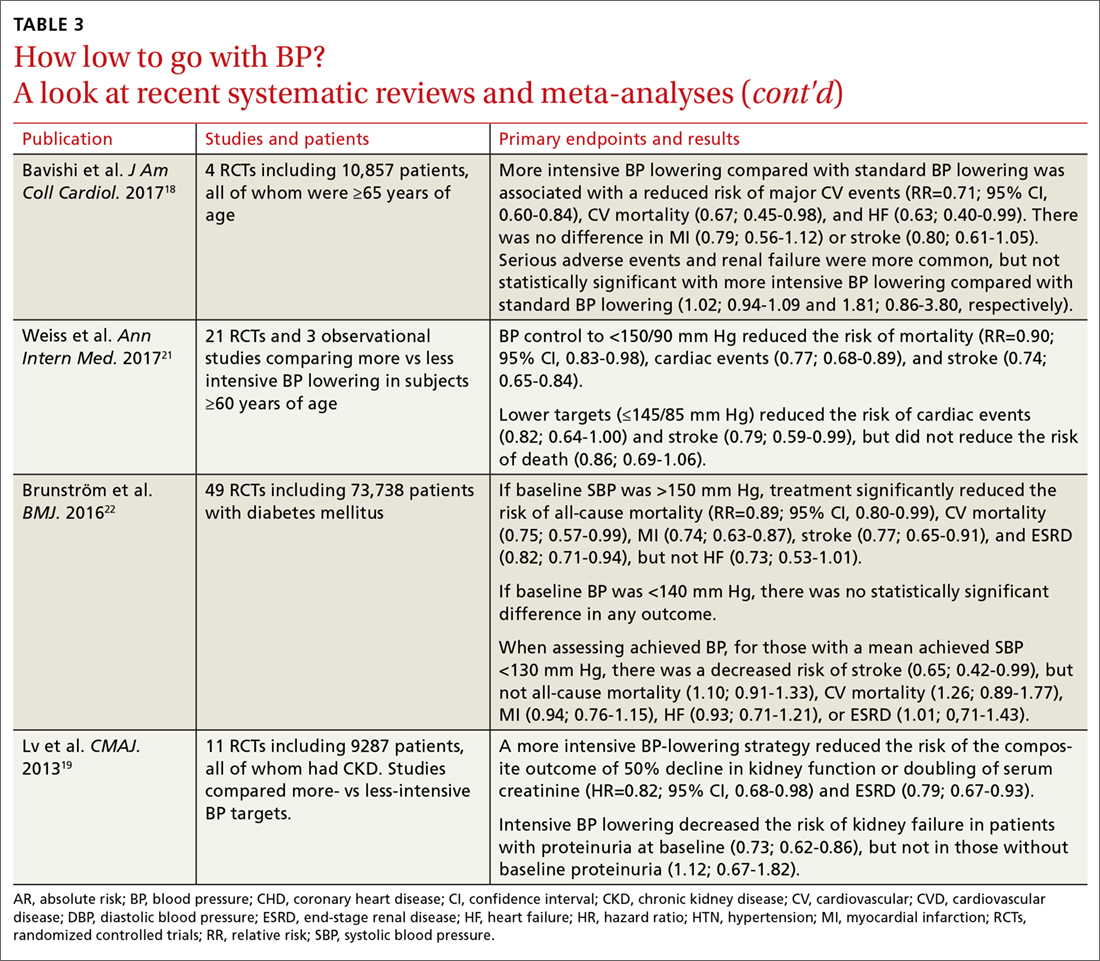
Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.
Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; [email protected].
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
PRACTICE RECOMMENDATIONS
› Treat adults with hypertension and cardiovascular disease or those at high risk (≥10%) of an atherosclerotic cardiovascular disease (ASCVD) event to a blood pressure (BP) goal <130/80 mm Hg. A for systolic BP goal; C for diastolic BP goal.
› Treat adults with hypertension and a low risk of a cardiovascular event (ie, primary prevention and ASCVD <10%) to a BP goal <130/80 mm Hg. B for systolic BP goal; C for diastolic BP goal.
› Treat ambulatory, community-dwelling, noninstitutionalized older patients to a systolic BP goal <130 mm Hg. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series