User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
VIDEO: Another worry about energy drinks in kids
VANCOUVER – At least one of the commercially available energy drinks plays havoc with blood sugar and insulin levels in teen users, according to research from the University of Calgary (Alta.).
Ten teenage boys and ten girls were randomized to one regular “5-hour energy” drink, or, as a control, the decaffeinated version of the product, and then given an oral glucose tolerance test 40 minutes later.
In the second phase, the kids switched over to the drink they didn’t get in the first go-round, and the tolerance testing was repeated. Blood testing was done at baseline and throughout the study.
Investigator Jane Shearer, Ph.D., of the university’s department of biochemistry and molecular biology, explained the results, and why she’s worried about them, in an interview at the World Diabetes Congress.
Five-hour energy is a sugar-free 2-ounce drink containing about 200 mg of caffeine in its regular-strength formulation.
The findings raise another red flag about energy drinks in kids, especially if they are prone to diabetes. Dr. Shearer also shared her thoughts on what to do to address the issue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VANCOUVER – At least one of the commercially available energy drinks plays havoc with blood sugar and insulin levels in teen users, according to research from the University of Calgary (Alta.).
Ten teenage boys and ten girls were randomized to one regular “5-hour energy” drink, or, as a control, the decaffeinated version of the product, and then given an oral glucose tolerance test 40 minutes later.
In the second phase, the kids switched over to the drink they didn’t get in the first go-round, and the tolerance testing was repeated. Blood testing was done at baseline and throughout the study.
Investigator Jane Shearer, Ph.D., of the university’s department of biochemistry and molecular biology, explained the results, and why she’s worried about them, in an interview at the World Diabetes Congress.
Five-hour energy is a sugar-free 2-ounce drink containing about 200 mg of caffeine in its regular-strength formulation.
The findings raise another red flag about energy drinks in kids, especially if they are prone to diabetes. Dr. Shearer also shared her thoughts on what to do to address the issue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VANCOUVER – At least one of the commercially available energy drinks plays havoc with blood sugar and insulin levels in teen users, according to research from the University of Calgary (Alta.).
Ten teenage boys and ten girls were randomized to one regular “5-hour energy” drink, or, as a control, the decaffeinated version of the product, and then given an oral glucose tolerance test 40 minutes later.
In the second phase, the kids switched over to the drink they didn’t get in the first go-round, and the tolerance testing was repeated. Blood testing was done at baseline and throughout the study.
Investigator Jane Shearer, Ph.D., of the university’s department of biochemistry and molecular biology, explained the results, and why she’s worried about them, in an interview at the World Diabetes Congress.
Five-hour energy is a sugar-free 2-ounce drink containing about 200 mg of caffeine in its regular-strength formulation.
The findings raise another red flag about energy drinks in kids, especially if they are prone to diabetes. Dr. Shearer also shared her thoughts on what to do to address the issue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE WORLD DIABETES CONGRESS
HbA1c strengthens diabetes predictive model more in whites than in African Americans
The addition of hemoglobin A1c to a type 2 diabetes risk prediction model greatly improved its performance among race-based populations, according to a new cohort study of white and African American populations.
However, while adding HbA1c to the existing Atherosclerosis Risk in Communities (ARIC) diabetes risk prediction model can improve its overall predictive value, the model is significantly more effective at predicting incidence of type 2 diabetes within white populations than among populations of African Americans (Diabetes Care. 2015 Dec 1. doi: 10.2337/dc15-0509).
“Results from the current analysis confirm our hypothesis that when type 2 diabetes prediction models are updated to include A1c, a reflection of current clinical practice for the diagnosis of type 2 diabetes, there exists a racial divide in model performance,” wrote Dr. Mary E. Lacy of Brown University, Providence, R.I.
The study enrolled 2,456 participants of the Coronary Artery Risk Development Study in Young Adults (CARDIA), an ongoing, multicenter, longitudinal study that began in 1985-1986 with 5,115 men and women aged 18-30 years: 2,637 African Americans and 2,478 whites. Dr. Lacy and her colleagues recruited from the year 20 group in 2005-2006. All subjects were diabetes free at baseline; the study excluded those who had prevalent diabetes or no diabetes data at year 20.
Follow-up was done after 5 years (year 25 from the start of CARDIA). The subjects had fasting and 2-hour postload glucose measured via the hexokinase ultraviolet method, as well as HbA1c, which was tested via a Tosoh G7 high-performance liquid chromatography instrument. These same measurements were taken at baseline in year 20. Model discrimination, calibration, and integrated discrimination improvement with incident diabetes were measured with these data, both before and after taking HbA1c into account, as defined by the American Diabetes Association’s 2010 guidelines.
Discrimination with the model for the overall population gave an area under the curve of 0.841, which investigators described as “good.” That number rose to 0.863 when factoring in HbA1c (P = .03). Similar increases in model discrimination were found in both white (0.875 to 0.902, P = .08) and African American (0.796 to 0.816, P = .14) populations.
“When racial subgroups in CARDIA were analyzed separately, model performance was better among whites than African Americans,” the authors concluded. “For all models that used ADA 2010 diagnostic guidelines, model discrimination was significantly higher in whites than African Americans [and] the addition of baseline A1c improved model discrimination in whites and African Americans to a similar degree.”
Integrated Discrimination Improvement analyses also showed that HbA1c substantially improved model discrimination for both the African-American and white cohorts. Furthermore, in the overall study population, “the 5-year incidence of type 2 diabetes was 3.0% (n = 74) under the ADA 2004 diagnostic guidelines versus 5.1% (n = 124) using the ADA 2010 guidelines.” African Americans were found to be slightly more at risk for developing type 2 diabetes compared with whites.
Dr. Lacy and her colleagues concluded by saying these findings highlight the need for discrimination models to use the 2010 American Diabetes Association guidelines and incorporate HbA1c as a parameter. However, further study is needed to find a predictive model as effective for African Americans and other racial minorities as the model in this trial turned out to be for whites.
The researchers did not report any conflicts of interest. The National Heart, Lung, and Blood Institute and the National Institute on Aging funded the study.
The addition of hemoglobin A1c to a type 2 diabetes risk prediction model greatly improved its performance among race-based populations, according to a new cohort study of white and African American populations.
However, while adding HbA1c to the existing Atherosclerosis Risk in Communities (ARIC) diabetes risk prediction model can improve its overall predictive value, the model is significantly more effective at predicting incidence of type 2 diabetes within white populations than among populations of African Americans (Diabetes Care. 2015 Dec 1. doi: 10.2337/dc15-0509).
“Results from the current analysis confirm our hypothesis that when type 2 diabetes prediction models are updated to include A1c, a reflection of current clinical practice for the diagnosis of type 2 diabetes, there exists a racial divide in model performance,” wrote Dr. Mary E. Lacy of Brown University, Providence, R.I.
The study enrolled 2,456 participants of the Coronary Artery Risk Development Study in Young Adults (CARDIA), an ongoing, multicenter, longitudinal study that began in 1985-1986 with 5,115 men and women aged 18-30 years: 2,637 African Americans and 2,478 whites. Dr. Lacy and her colleagues recruited from the year 20 group in 2005-2006. All subjects were diabetes free at baseline; the study excluded those who had prevalent diabetes or no diabetes data at year 20.
Follow-up was done after 5 years (year 25 from the start of CARDIA). The subjects had fasting and 2-hour postload glucose measured via the hexokinase ultraviolet method, as well as HbA1c, which was tested via a Tosoh G7 high-performance liquid chromatography instrument. These same measurements were taken at baseline in year 20. Model discrimination, calibration, and integrated discrimination improvement with incident diabetes were measured with these data, both before and after taking HbA1c into account, as defined by the American Diabetes Association’s 2010 guidelines.
Discrimination with the model for the overall population gave an area under the curve of 0.841, which investigators described as “good.” That number rose to 0.863 when factoring in HbA1c (P = .03). Similar increases in model discrimination were found in both white (0.875 to 0.902, P = .08) and African American (0.796 to 0.816, P = .14) populations.
“When racial subgroups in CARDIA were analyzed separately, model performance was better among whites than African Americans,” the authors concluded. “For all models that used ADA 2010 diagnostic guidelines, model discrimination was significantly higher in whites than African Americans [and] the addition of baseline A1c improved model discrimination in whites and African Americans to a similar degree.”
Integrated Discrimination Improvement analyses also showed that HbA1c substantially improved model discrimination for both the African-American and white cohorts. Furthermore, in the overall study population, “the 5-year incidence of type 2 diabetes was 3.0% (n = 74) under the ADA 2004 diagnostic guidelines versus 5.1% (n = 124) using the ADA 2010 guidelines.” African Americans were found to be slightly more at risk for developing type 2 diabetes compared with whites.
Dr. Lacy and her colleagues concluded by saying these findings highlight the need for discrimination models to use the 2010 American Diabetes Association guidelines and incorporate HbA1c as a parameter. However, further study is needed to find a predictive model as effective for African Americans and other racial minorities as the model in this trial turned out to be for whites.
The researchers did not report any conflicts of interest. The National Heart, Lung, and Blood Institute and the National Institute on Aging funded the study.
The addition of hemoglobin A1c to a type 2 diabetes risk prediction model greatly improved its performance among race-based populations, according to a new cohort study of white and African American populations.
However, while adding HbA1c to the existing Atherosclerosis Risk in Communities (ARIC) diabetes risk prediction model can improve its overall predictive value, the model is significantly more effective at predicting incidence of type 2 diabetes within white populations than among populations of African Americans (Diabetes Care. 2015 Dec 1. doi: 10.2337/dc15-0509).
“Results from the current analysis confirm our hypothesis that when type 2 diabetes prediction models are updated to include A1c, a reflection of current clinical practice for the diagnosis of type 2 diabetes, there exists a racial divide in model performance,” wrote Dr. Mary E. Lacy of Brown University, Providence, R.I.
The study enrolled 2,456 participants of the Coronary Artery Risk Development Study in Young Adults (CARDIA), an ongoing, multicenter, longitudinal study that began in 1985-1986 with 5,115 men and women aged 18-30 years: 2,637 African Americans and 2,478 whites. Dr. Lacy and her colleagues recruited from the year 20 group in 2005-2006. All subjects were diabetes free at baseline; the study excluded those who had prevalent diabetes or no diabetes data at year 20.
Follow-up was done after 5 years (year 25 from the start of CARDIA). The subjects had fasting and 2-hour postload glucose measured via the hexokinase ultraviolet method, as well as HbA1c, which was tested via a Tosoh G7 high-performance liquid chromatography instrument. These same measurements were taken at baseline in year 20. Model discrimination, calibration, and integrated discrimination improvement with incident diabetes were measured with these data, both before and after taking HbA1c into account, as defined by the American Diabetes Association’s 2010 guidelines.
Discrimination with the model for the overall population gave an area under the curve of 0.841, which investigators described as “good.” That number rose to 0.863 when factoring in HbA1c (P = .03). Similar increases in model discrimination were found in both white (0.875 to 0.902, P = .08) and African American (0.796 to 0.816, P = .14) populations.
“When racial subgroups in CARDIA were analyzed separately, model performance was better among whites than African Americans,” the authors concluded. “For all models that used ADA 2010 diagnostic guidelines, model discrimination was significantly higher in whites than African Americans [and] the addition of baseline A1c improved model discrimination in whites and African Americans to a similar degree.”
Integrated Discrimination Improvement analyses also showed that HbA1c substantially improved model discrimination for both the African-American and white cohorts. Furthermore, in the overall study population, “the 5-year incidence of type 2 diabetes was 3.0% (n = 74) under the ADA 2004 diagnostic guidelines versus 5.1% (n = 124) using the ADA 2010 guidelines.” African Americans were found to be slightly more at risk for developing type 2 diabetes compared with whites.
Dr. Lacy and her colleagues concluded by saying these findings highlight the need for discrimination models to use the 2010 American Diabetes Association guidelines and incorporate HbA1c as a parameter. However, further study is needed to find a predictive model as effective for African Americans and other racial minorities as the model in this trial turned out to be for whites.
The researchers did not report any conflicts of interest. The National Heart, Lung, and Blood Institute and the National Institute on Aging funded the study.
FROM DIABETES CARE
Key clinical point: Adding hemoglobin A1c to the existing Atherosclerosis Risk in Communities diabetes risk prediction model can significantly improve the accuracy of diabetes incidence prediction in white and African-American populations.
Major finding: With new parameters for prediction, model discrimination increased from 0.841 to 0.863 in the overall population, 0.796 to 0.816 among African Americans, and 0.875 to 0.902 among whites.
Data source: Cohort study of 2,456 white and African American participants in the CARDIA study in 2005-2006 who did not have diabetes.
Disclosures: The National Heart, Lung, and Blood Institute and the National Institute on Aging funded the study. The authors reported no conflicts of interest.
Lixisenatide not cardioprotective in type 2 diabetes
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding lixisenatide to usual care failed to prevent major cardiovascular events in patients with type 2 diabetes who had a recent ACS.
Major finding: Death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina occurred in 13.4% of participants receiving lixisenatide and 13.2% of those receiving placebo.
Data source: An international randomized double-blind placebo-controlled trial involving 6,068 patients followed for a median of 2 years.
Disclosures: Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
WDC: Alogliptin promotes regression of carotid atherosclerosis in diabetic patients
VANCOUVER, B.C. – Alogliptin, an oral inhibitor of dipeptidyl peptidase 4 (DPP-4) having hypoglycemic activity, promoted regression of preclinical carotid atherosclerosis in patients with type 2 diabetes, judging from the findings of the randomized Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A).
Among the 341 patients studied, all of whom were free of known cardiovascular disease at baseline, those in the alogliptin group had significant reductions in echocardiographic carotid intima-media thickness (IMT) at 2 years when compared with peers in a usual care group, according to data reported at the World Diabetes Congress.
“Our data may suggest … efficacy and benefit of alogliptin when used at an early stage of disease in preventing the progression of atherosclerosis,” commented first author Dr. Tomoya Mita, assistant professor in the department of metabolism and endocrinology at Juntendo University Graduate School of Medicine, Tokyo.
Three other trials – SAVOR TIMI 53, EXAMINE, and TECOS – have not found changes in cardiovascular outcomes with DPP-4 inhibitors among diabetic patients, he noted. Patients in those trials, however, had established cardiovascular disease or were at high risk.
“I’m not sure whether our data will translate into clinical outcome. But the magnitude of IMT reduction was identical to that of pioglitazone, [which] may reduce CV events,” Dr. Mita commented. “We are conducting a follow-up study that focuses on clinical outcome and safety, so we will find more detailed information in that study.”
“The importance of this study is really showing the limitations to cardiovascular outcome trials that have been going on,” commented Dr. Robert E. Ratner, professor of medicine at Georgetown University and senior research scientist at the MedStar Health Research Institute, both in Washington, as well as chief scientific and medical officer of the American Diabetes Association in Alexandria, Va., who was session comoderator.
Those studies have been limited by selection bias, he agreed in an interview. “Taking patients who are so far advanced in their disease really isn’t the practical solution. We need to be thinking earlier, we need to be thinking in terms of prevention.”
Patients were recruited to SPEAD-A from diabetes outpatient clinics at 11 centers in Japan. They had to be 30 years of age or older and to have diabetes inadequately controlled by diet and lifestyle measures, or agents other than DPP-4 inhibitors, but with a glycated hemoglobin level of less than 9.4%.
Trial results, reported at the meeting and simultaneously published (Diabetes Care. 2016;39:18-27. doi: 10.2337/dc15-0781), showed that, relative to peers given usual care, patients given alogliptin (U.S. brand name Nesina) had greater reductions at 2 years in levels of glycated hemoglobin (−0.3% vs. −0.1%, P = .004) without a higher incidence of hypoglycemia.
Echocardiographic data revealed differences between the alogliptin group and the usual-care group in favor of the former with respect to changes in common carotid IMT (−0.026 mm vs. +0.005 mm, P = .022), right carotid maximum IMT (−0.045 mm vs. +0.011 mm, P = .025), and left carotid maximum IMT (−0.079 mm vs. −0.015 mm, P = .013).
Body mass index increased in the alogliptin group but decreased in the usual-care group (+0.3 kg/m2 vs. –0.3 kg/m2, P = .003), Dr. Mita reported.
The groups were statistically indistinguishable with respect to insulin and fasting glucose levels, lipid profiles, blood pressure, markers of inflammation, and estimated glomerular filtration rate.
Rates of adverse events and serious adverse events were similar with alogliptin and usual care. There was no significant difference between groups in cardiovascular events, but only five patients experienced these events.
“I’m not sure how the DPP-4 inhibitor reduces the carotid intima-media thickness in this study,” Dr. Mita commented, but other research has suggested that drugs in this class decrease smooth muscle cell proliferation and inflammation and improve endothelial function.
Dr. Mita disclosed that he receives research funds from MSD and Takeda Pharma and that he receives lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Ono Pharmaceutical, and Takeda Pharmaceutical.
Financial support for the study was provided by Astellas Pharma, AstraZeneca, Bayer Holding, Daiichi Sankyo, Sumitomo Dainippon Pharma, Eli Lilly Japan, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Pfizer Japan, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, and Takeda
VANCOUVER, B.C. – Alogliptin, an oral inhibitor of dipeptidyl peptidase 4 (DPP-4) having hypoglycemic activity, promoted regression of preclinical carotid atherosclerosis in patients with type 2 diabetes, judging from the findings of the randomized Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A).
Among the 341 patients studied, all of whom were free of known cardiovascular disease at baseline, those in the alogliptin group had significant reductions in echocardiographic carotid intima-media thickness (IMT) at 2 years when compared with peers in a usual care group, according to data reported at the World Diabetes Congress.
“Our data may suggest … efficacy and benefit of alogliptin when used at an early stage of disease in preventing the progression of atherosclerosis,” commented first author Dr. Tomoya Mita, assistant professor in the department of metabolism and endocrinology at Juntendo University Graduate School of Medicine, Tokyo.
Three other trials – SAVOR TIMI 53, EXAMINE, and TECOS – have not found changes in cardiovascular outcomes with DPP-4 inhibitors among diabetic patients, he noted. Patients in those trials, however, had established cardiovascular disease or were at high risk.
“I’m not sure whether our data will translate into clinical outcome. But the magnitude of IMT reduction was identical to that of pioglitazone, [which] may reduce CV events,” Dr. Mita commented. “We are conducting a follow-up study that focuses on clinical outcome and safety, so we will find more detailed information in that study.”
“The importance of this study is really showing the limitations to cardiovascular outcome trials that have been going on,” commented Dr. Robert E. Ratner, professor of medicine at Georgetown University and senior research scientist at the MedStar Health Research Institute, both in Washington, as well as chief scientific and medical officer of the American Diabetes Association in Alexandria, Va., who was session comoderator.
Those studies have been limited by selection bias, he agreed in an interview. “Taking patients who are so far advanced in their disease really isn’t the practical solution. We need to be thinking earlier, we need to be thinking in terms of prevention.”
Patients were recruited to SPEAD-A from diabetes outpatient clinics at 11 centers in Japan. They had to be 30 years of age or older and to have diabetes inadequately controlled by diet and lifestyle measures, or agents other than DPP-4 inhibitors, but with a glycated hemoglobin level of less than 9.4%.
Trial results, reported at the meeting and simultaneously published (Diabetes Care. 2016;39:18-27. doi: 10.2337/dc15-0781), showed that, relative to peers given usual care, patients given alogliptin (U.S. brand name Nesina) had greater reductions at 2 years in levels of glycated hemoglobin (−0.3% vs. −0.1%, P = .004) without a higher incidence of hypoglycemia.
Echocardiographic data revealed differences between the alogliptin group and the usual-care group in favor of the former with respect to changes in common carotid IMT (−0.026 mm vs. +0.005 mm, P = .022), right carotid maximum IMT (−0.045 mm vs. +0.011 mm, P = .025), and left carotid maximum IMT (−0.079 mm vs. −0.015 mm, P = .013).
Body mass index increased in the alogliptin group but decreased in the usual-care group (+0.3 kg/m2 vs. –0.3 kg/m2, P = .003), Dr. Mita reported.
The groups were statistically indistinguishable with respect to insulin and fasting glucose levels, lipid profiles, blood pressure, markers of inflammation, and estimated glomerular filtration rate.
Rates of adverse events and serious adverse events were similar with alogliptin and usual care. There was no significant difference between groups in cardiovascular events, but only five patients experienced these events.
“I’m not sure how the DPP-4 inhibitor reduces the carotid intima-media thickness in this study,” Dr. Mita commented, but other research has suggested that drugs in this class decrease smooth muscle cell proliferation and inflammation and improve endothelial function.
Dr. Mita disclosed that he receives research funds from MSD and Takeda Pharma and that he receives lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Ono Pharmaceutical, and Takeda Pharmaceutical.
Financial support for the study was provided by Astellas Pharma, AstraZeneca, Bayer Holding, Daiichi Sankyo, Sumitomo Dainippon Pharma, Eli Lilly Japan, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Pfizer Japan, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, and Takeda
VANCOUVER, B.C. – Alogliptin, an oral inhibitor of dipeptidyl peptidase 4 (DPP-4) having hypoglycemic activity, promoted regression of preclinical carotid atherosclerosis in patients with type 2 diabetes, judging from the findings of the randomized Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A).
Among the 341 patients studied, all of whom were free of known cardiovascular disease at baseline, those in the alogliptin group had significant reductions in echocardiographic carotid intima-media thickness (IMT) at 2 years when compared with peers in a usual care group, according to data reported at the World Diabetes Congress.
“Our data may suggest … efficacy and benefit of alogliptin when used at an early stage of disease in preventing the progression of atherosclerosis,” commented first author Dr. Tomoya Mita, assistant professor in the department of metabolism and endocrinology at Juntendo University Graduate School of Medicine, Tokyo.
Three other trials – SAVOR TIMI 53, EXAMINE, and TECOS – have not found changes in cardiovascular outcomes with DPP-4 inhibitors among diabetic patients, he noted. Patients in those trials, however, had established cardiovascular disease or were at high risk.
“I’m not sure whether our data will translate into clinical outcome. But the magnitude of IMT reduction was identical to that of pioglitazone, [which] may reduce CV events,” Dr. Mita commented. “We are conducting a follow-up study that focuses on clinical outcome and safety, so we will find more detailed information in that study.”
“The importance of this study is really showing the limitations to cardiovascular outcome trials that have been going on,” commented Dr. Robert E. Ratner, professor of medicine at Georgetown University and senior research scientist at the MedStar Health Research Institute, both in Washington, as well as chief scientific and medical officer of the American Diabetes Association in Alexandria, Va., who was session comoderator.
Those studies have been limited by selection bias, he agreed in an interview. “Taking patients who are so far advanced in their disease really isn’t the practical solution. We need to be thinking earlier, we need to be thinking in terms of prevention.”
Patients were recruited to SPEAD-A from diabetes outpatient clinics at 11 centers in Japan. They had to be 30 years of age or older and to have diabetes inadequately controlled by diet and lifestyle measures, or agents other than DPP-4 inhibitors, but with a glycated hemoglobin level of less than 9.4%.
Trial results, reported at the meeting and simultaneously published (Diabetes Care. 2016;39:18-27. doi: 10.2337/dc15-0781), showed that, relative to peers given usual care, patients given alogliptin (U.S. brand name Nesina) had greater reductions at 2 years in levels of glycated hemoglobin (−0.3% vs. −0.1%, P = .004) without a higher incidence of hypoglycemia.
Echocardiographic data revealed differences between the alogliptin group and the usual-care group in favor of the former with respect to changes in common carotid IMT (−0.026 mm vs. +0.005 mm, P = .022), right carotid maximum IMT (−0.045 mm vs. +0.011 mm, P = .025), and left carotid maximum IMT (−0.079 mm vs. −0.015 mm, P = .013).
Body mass index increased in the alogliptin group but decreased in the usual-care group (+0.3 kg/m2 vs. –0.3 kg/m2, P = .003), Dr. Mita reported.
The groups were statistically indistinguishable with respect to insulin and fasting glucose levels, lipid profiles, blood pressure, markers of inflammation, and estimated glomerular filtration rate.
Rates of adverse events and serious adverse events were similar with alogliptin and usual care. There was no significant difference between groups in cardiovascular events, but only five patients experienced these events.
“I’m not sure how the DPP-4 inhibitor reduces the carotid intima-media thickness in this study,” Dr. Mita commented, but other research has suggested that drugs in this class decrease smooth muscle cell proliferation and inflammation and improve endothelial function.
Dr. Mita disclosed that he receives research funds from MSD and Takeda Pharma and that he receives lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Ono Pharmaceutical, and Takeda Pharmaceutical.
Financial support for the study was provided by Astellas Pharma, AstraZeneca, Bayer Holding, Daiichi Sankyo, Sumitomo Dainippon Pharma, Eli Lilly Japan, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Pfizer Japan, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, and Takeda
AT THE WORLD DIABETES CONGRESS
Key clinical point: Alogliptin holds promise for reduction of atherosclerosis in diabetic patients who do not have clinical cardiovascular disease.
Major finding: Relative to usual care, alogliptin reduced common carotid mean IMT (−0.026 mm vs. +0.005 mm), right carotid maximum IMT (−0.045 mm vs. +0.011 mm), and left carotid maximum IMT (−0.079 mm vs. −0.015 mm).
Data source: A multicenter, randomized open-label trial among 341 patients with type 2 diabetes who were free of known cardiovascular disease at baseline.
Disclosures: Dr. Mita disclosed that he receives research funds from MSD and Takeda Pharma and that he receives lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Ono Pharmaceutical, and Takeda Pharmaceutical. Financial support for the study was provided by Astellas Pharma, AstraZeneca, Bayer Holding, Daiichi Sankyo, Sumitomo Dainippon Pharma, Eli Lilly Japan, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Pfizer Japan, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, and Takeda Pharmaceutical.
Blood pressure above 140/80 worsens proteinuric diabetic kidney disease
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
SAN DIEGO – Among patients with proteinuric diabetic kidney disease, a mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes, a large national analysis showed.
“Proteinuric diabetic kidney disease frequently progresses to end-stage renal disease,” lead study author Dr. David J. Leehey said at a meeting sponsored by the American Society of Nephrology. “Control of blood pressure delays progression, but the optimal blood pressure to improve outcomes remains unclear.”
In a recent report from the Eighth Joint National Committee, panel members recommended a blood pressure of less than 140/90 mm Hg both for patients with diabetes and patients with chronic kidney disease (JAMA. 2014 Feb 5;311[5]:507-20).
“There are few data with regard to target blood pressure in proteinuric diabetic kidney disease, however, leaving physicians uncertain about how aggressively to lower blood pressure in such patients,” said Dr. Leehey, a nephrologist at the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill.
In an effort to assess the relationship between blood pressure and renal outcomes in proteinuric diabetic kidney disease, the researchers evaluated blood pressure data from all 1,448 patients who were randomized to the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone (N Engl J Med. 2013 Nov 14;369[20]:1892-1903). Randomization occurred between July 2008 and September 2012, but the trial was halted in October of 2012 due to safety reasons (acute kidney injury and hyperkalemic episodes).
For the current analysis, Dr. Leehey and his associates evaluated the associations of mean on-treatment blood pressure with the primary endpoint (defined as a decline in estimated glomerular filtration rate [eGFR], end-stage renal disease [ESRD], or death), the renal endpoint (defined as a decline in eGFR or cystatin C), the rate of eGFR decline, and mortality.
Trough sitting blood pressure measurements were obtained at every visit during the screening, titration, randomization, and treatment phases of VA NEPHRON-D. Mean blood pressure was defined as the average of all on-treatment blood pressures from the randomization visit to the last blood pressure measurement taken.
Dr. Leehey reported that there were 284 primary endpoints: 132 in the combination group and 152 in the monotherapy group. The mean blood pressure was based on a mean of 7.7 readings per participant.
Both mean systolic and mean diastolic blood pressure were strongly associated with the primary endpoint (P less than .001), univariate analysis revealed. The hazard of developing the primary endpoint increased as the mean systolic blood pressure rose from less than 120 mm Hg to greater than 150 mm Hg (P = .018), multivariate analysis showed.
A significant increase in hazard ratio was seen when mean systolic blood pressure was greater than 140 mm Hg (HR, 1.51). The researchers also observed a significant effect of mean diastolic blood pressure on the hazard of developing the primary endpoint (P = .005), with an increase in hazard ratio when mean diastolic blood pressure was greater than 80 mm Hg (HR, 1.54).
“Associations between BP and both renal endpoint and rate of eGFR decline were similar to those with the primary endpoint,” Dr. Leehey said. “There was a U-shaped relationship between rate of eGFR decline and mean diastolic blood pressure, with the lowest rate of eGFR decline seen when diastolic blood pressure was in the 70-79 mm Hg range. No effect of BP with mortality was observed, possibly because of the limited number of mortality events.”
The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: In patients with proteinuric diabetic kidney disease, a mean systolic BP greater than 140 mm Hg and a mean diastolic BP greater than 80 mm Hg were linked to worse renal outcomes.
Major finding: A mean systolic blood pressure greater than 140 mm Hg and a mean diastolic blood pressure greater than 80 mm Hg were associated with worse renal outcomes (hazard ratios of 1.51 and 1.54, respectively).
Data source: Blood pressure data from 1,448 patients who were randomized to the VA NEPHRON-D trial, which evaluated the combination of losartan with lisinopril, compared with losartan alone.
Disclosures: The study was funded by support from the Veterans Administration. Dr. Leehey reported having no financial disclosures.
AHA: Broadening evidence for CABG over PCI in diabetics
ORLANDO – New evidence further strengthens the case for coronary artery bypass grafting over percutaneous coronary intervention as the preferred revascularization strategy in diabetic patients with multivessel coronary artery disease.
An analysis of 4,819 such patients in a British Columbia province-wide registry who underwent revascularization during 2007-2014 led to the conclusion that the 30-day adjusted risk of the primary composite endpoint of death, MI, or stroke was 39% lower in those who underwent coronary artery bypass grafting (CABG), Dr. Krishnan Ramanathan reported at the American Heart Association scientific sessions.
From day 31 through 5 years of follow-up, the relative risk reduction was 36% in favor of CABG, again after adjustment for age, sex, presentation with stable ischemic heart disease or stabilized acute coronary syndrome (ACS), urgency, renal insufficiency, liver disease, peripheral arterial disease, and left ventricular ejection fraction, added Dr. Ramanathan of the University of British Columbia in Vancouver.
These results in a broad patient population in real-world clinical practice, albeit in an observational, nonrandomized setting, mirror those in the earlier randomized FREEDOM trial sponsored by the National Heart, Lung, and Blood Institute. The 5-year rate of the composite of death, MI, or stroke in FREEDOM was 18.7% in the CABG group and 26.6% with percutaneous coronary intervention (PCI). The number needed to treat with CABG instead of PCI in FREEDOM in order to avoid one case of the composite endpoint was 12.6 (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
An important difference between the British Columbia and the FREEDOM trial is that the registry contained a much higher proportion of diabetic patients with multivessel disease who presented with acute coronary syndrome. Indeed, the ratio of stabilized ACS to stable ischemic heart disease patients in the registry was 62% to 38% as compared with 36% to 64% in FREEDOM. The registry patients underwent a median of 7.8 days of stabilization from the time of initial catheterization to CABG.
The registry data really shore up the advantages of CABG over PCI in diabetics with multivessel disease and ACS, Dr. Ramanathan noted. In the registry, the 30-day composite endpoint occurred in 4.4% of CABG-treated ACS patients, compared with 8.3% with PCI. For 31 days through 5 years, the rates were 21.4% versus 34.7%. The differences favoring CABG were highly significant at both time points.
FREEDOM led to two important revisions in the 2014 guidelines on coronary revascularization, issued by the American College of Cardiology, American Heart Association, American Academy of Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiac Angiography and Interventions, and Society of Thoracic Surgeons. In a focused update issued earlier this year, a new class I recommendation was issued calling for a heart team approach to revascularization in patients with diabetes mellitus and complex multivessel coronary artery disease (CAD). And a recommendation for CABG over PCI for improving survival in diabetic patients with multivessel CAD was upgraded from class IIa to class I.
Even though FREEDOM was a major randomized trial that might be expected to be practice changing, that hasn’t happened. A report from the ACC’s National Cardiovascular Data ACTION Registry presented earlier this year at the ACC annual scientific session documented that the most common revascularization strategy for diabetic patients with multivessel CAD following a non–ST elevation ACS remains PCI, as has been the case since the registry was created. In fact, following the 2012 publication of FREEDOM, the proportion of such patients treated by PCI took a sharp uptick in 2013-2014, according to Dr. Ramanathan.
In the British Columbia registry, 64% of diabetic patients with multivessel disease and an ACS were treated by PCI, 36% by CABG.
“Overall, CABG in stabilized ACS patients is underutilized,” he said.
Discussant Dr. Marc Ruel declared, “The salient finding about the British Columbia study is not so much about the stable ischemic heart disease patients, but about the high proportion of ACS patients in the study. CABG was better than PCI for all major adverse cardiovascular events and each component of the endpoint.”
Dr. Ruel, professor and chair of cardiac surgery at the University of Ottawa Heart Institute, coauthored a meta-analysis of randomized controlled trials comparing CABG to PCI in diabetic patients. Half of the trials used later-generation drug-eluting stents. At 5 years of follow-up, the relative risk of all-cause mortality in diabetic patients allocated to CABG was 0.67 (Lancet Diabetes Endocrinol. 2013 Dec;1[4]:317-28).
“Remember that 0.67 because it’s a recurring number in our literature,” he advised.
For example, in the British Columbia study the relative risk of death, MI, or stroke from 31 days to 5 years was 0.64 in the CABG as compared with the PCI group. And in a patient-level meta-analysis of the FREEDOM, BARI-2D, and COURAGE trials presented by Dr. John Mancini earlier at the AHA scientific sessions in Orlando, CABG plus optimal medical therapy was associated with a 0.65 relative risk of death, MI, or stroke, compared with PCI plus optimal medical therapy, which in turn wasn’t significantly better than optimal medical therapy alone, Dr. Ruel noted.
The 2015 European Society of Cardiology guidelines on management of ACS state that a heart team discussion involving a cardiologist and cardiac surgeon does not need to take place for every patient presenting with multivessel disease and a non–ST elevation ACS. The British Columbia study, coupled with the other evidence, “gives new credence” to a call for revision of that recommendation in diabetic patients, he said.
The British Columbia study was supported by the British Columbia Provincial Health Services Authority. Dr. Ramanathan reported having no financial conflicts.
ORLANDO – New evidence further strengthens the case for coronary artery bypass grafting over percutaneous coronary intervention as the preferred revascularization strategy in diabetic patients with multivessel coronary artery disease.
An analysis of 4,819 such patients in a British Columbia province-wide registry who underwent revascularization during 2007-2014 led to the conclusion that the 30-day adjusted risk of the primary composite endpoint of death, MI, or stroke was 39% lower in those who underwent coronary artery bypass grafting (CABG), Dr. Krishnan Ramanathan reported at the American Heart Association scientific sessions.
From day 31 through 5 years of follow-up, the relative risk reduction was 36% in favor of CABG, again after adjustment for age, sex, presentation with stable ischemic heart disease or stabilized acute coronary syndrome (ACS), urgency, renal insufficiency, liver disease, peripheral arterial disease, and left ventricular ejection fraction, added Dr. Ramanathan of the University of British Columbia in Vancouver.
These results in a broad patient population in real-world clinical practice, albeit in an observational, nonrandomized setting, mirror those in the earlier randomized FREEDOM trial sponsored by the National Heart, Lung, and Blood Institute. The 5-year rate of the composite of death, MI, or stroke in FREEDOM was 18.7% in the CABG group and 26.6% with percutaneous coronary intervention (PCI). The number needed to treat with CABG instead of PCI in FREEDOM in order to avoid one case of the composite endpoint was 12.6 (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
An important difference between the British Columbia and the FREEDOM trial is that the registry contained a much higher proportion of diabetic patients with multivessel disease who presented with acute coronary syndrome. Indeed, the ratio of stabilized ACS to stable ischemic heart disease patients in the registry was 62% to 38% as compared with 36% to 64% in FREEDOM. The registry patients underwent a median of 7.8 days of stabilization from the time of initial catheterization to CABG.
The registry data really shore up the advantages of CABG over PCI in diabetics with multivessel disease and ACS, Dr. Ramanathan noted. In the registry, the 30-day composite endpoint occurred in 4.4% of CABG-treated ACS patients, compared with 8.3% with PCI. For 31 days through 5 years, the rates were 21.4% versus 34.7%. The differences favoring CABG were highly significant at both time points.
FREEDOM led to two important revisions in the 2014 guidelines on coronary revascularization, issued by the American College of Cardiology, American Heart Association, American Academy of Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiac Angiography and Interventions, and Society of Thoracic Surgeons. In a focused update issued earlier this year, a new class I recommendation was issued calling for a heart team approach to revascularization in patients with diabetes mellitus and complex multivessel coronary artery disease (CAD). And a recommendation for CABG over PCI for improving survival in diabetic patients with multivessel CAD was upgraded from class IIa to class I.
Even though FREEDOM was a major randomized trial that might be expected to be practice changing, that hasn’t happened. A report from the ACC’s National Cardiovascular Data ACTION Registry presented earlier this year at the ACC annual scientific session documented that the most common revascularization strategy for diabetic patients with multivessel CAD following a non–ST elevation ACS remains PCI, as has been the case since the registry was created. In fact, following the 2012 publication of FREEDOM, the proportion of such patients treated by PCI took a sharp uptick in 2013-2014, according to Dr. Ramanathan.
In the British Columbia registry, 64% of diabetic patients with multivessel disease and an ACS were treated by PCI, 36% by CABG.
“Overall, CABG in stabilized ACS patients is underutilized,” he said.
Discussant Dr. Marc Ruel declared, “The salient finding about the British Columbia study is not so much about the stable ischemic heart disease patients, but about the high proportion of ACS patients in the study. CABG was better than PCI for all major adverse cardiovascular events and each component of the endpoint.”
Dr. Ruel, professor and chair of cardiac surgery at the University of Ottawa Heart Institute, coauthored a meta-analysis of randomized controlled trials comparing CABG to PCI in diabetic patients. Half of the trials used later-generation drug-eluting stents. At 5 years of follow-up, the relative risk of all-cause mortality in diabetic patients allocated to CABG was 0.67 (Lancet Diabetes Endocrinol. 2013 Dec;1[4]:317-28).
“Remember that 0.67 because it’s a recurring number in our literature,” he advised.
For example, in the British Columbia study the relative risk of death, MI, or stroke from 31 days to 5 years was 0.64 in the CABG as compared with the PCI group. And in a patient-level meta-analysis of the FREEDOM, BARI-2D, and COURAGE trials presented by Dr. John Mancini earlier at the AHA scientific sessions in Orlando, CABG plus optimal medical therapy was associated with a 0.65 relative risk of death, MI, or stroke, compared with PCI plus optimal medical therapy, which in turn wasn’t significantly better than optimal medical therapy alone, Dr. Ruel noted.
The 2015 European Society of Cardiology guidelines on management of ACS state that a heart team discussion involving a cardiologist and cardiac surgeon does not need to take place for every patient presenting with multivessel disease and a non–ST elevation ACS. The British Columbia study, coupled with the other evidence, “gives new credence” to a call for revision of that recommendation in diabetic patients, he said.
The British Columbia study was supported by the British Columbia Provincial Health Services Authority. Dr. Ramanathan reported having no financial conflicts.
ORLANDO – New evidence further strengthens the case for coronary artery bypass grafting over percutaneous coronary intervention as the preferred revascularization strategy in diabetic patients with multivessel coronary artery disease.
An analysis of 4,819 such patients in a British Columbia province-wide registry who underwent revascularization during 2007-2014 led to the conclusion that the 30-day adjusted risk of the primary composite endpoint of death, MI, or stroke was 39% lower in those who underwent coronary artery bypass grafting (CABG), Dr. Krishnan Ramanathan reported at the American Heart Association scientific sessions.
From day 31 through 5 years of follow-up, the relative risk reduction was 36% in favor of CABG, again after adjustment for age, sex, presentation with stable ischemic heart disease or stabilized acute coronary syndrome (ACS), urgency, renal insufficiency, liver disease, peripheral arterial disease, and left ventricular ejection fraction, added Dr. Ramanathan of the University of British Columbia in Vancouver.
These results in a broad patient population in real-world clinical practice, albeit in an observational, nonrandomized setting, mirror those in the earlier randomized FREEDOM trial sponsored by the National Heart, Lung, and Blood Institute. The 5-year rate of the composite of death, MI, or stroke in FREEDOM was 18.7% in the CABG group and 26.6% with percutaneous coronary intervention (PCI). The number needed to treat with CABG instead of PCI in FREEDOM in order to avoid one case of the composite endpoint was 12.6 (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
An important difference between the British Columbia and the FREEDOM trial is that the registry contained a much higher proportion of diabetic patients with multivessel disease who presented with acute coronary syndrome. Indeed, the ratio of stabilized ACS to stable ischemic heart disease patients in the registry was 62% to 38% as compared with 36% to 64% in FREEDOM. The registry patients underwent a median of 7.8 days of stabilization from the time of initial catheterization to CABG.
The registry data really shore up the advantages of CABG over PCI in diabetics with multivessel disease and ACS, Dr. Ramanathan noted. In the registry, the 30-day composite endpoint occurred in 4.4% of CABG-treated ACS patients, compared with 8.3% with PCI. For 31 days through 5 years, the rates were 21.4% versus 34.7%. The differences favoring CABG were highly significant at both time points.
FREEDOM led to two important revisions in the 2014 guidelines on coronary revascularization, issued by the American College of Cardiology, American Heart Association, American Academy of Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiac Angiography and Interventions, and Society of Thoracic Surgeons. In a focused update issued earlier this year, a new class I recommendation was issued calling for a heart team approach to revascularization in patients with diabetes mellitus and complex multivessel coronary artery disease (CAD). And a recommendation for CABG over PCI for improving survival in diabetic patients with multivessel CAD was upgraded from class IIa to class I.
Even though FREEDOM was a major randomized trial that might be expected to be practice changing, that hasn’t happened. A report from the ACC’s National Cardiovascular Data ACTION Registry presented earlier this year at the ACC annual scientific session documented that the most common revascularization strategy for diabetic patients with multivessel CAD following a non–ST elevation ACS remains PCI, as has been the case since the registry was created. In fact, following the 2012 publication of FREEDOM, the proportion of such patients treated by PCI took a sharp uptick in 2013-2014, according to Dr. Ramanathan.
In the British Columbia registry, 64% of diabetic patients with multivessel disease and an ACS were treated by PCI, 36% by CABG.
“Overall, CABG in stabilized ACS patients is underutilized,” he said.
Discussant Dr. Marc Ruel declared, “The salient finding about the British Columbia study is not so much about the stable ischemic heart disease patients, but about the high proportion of ACS patients in the study. CABG was better than PCI for all major adverse cardiovascular events and each component of the endpoint.”
Dr. Ruel, professor and chair of cardiac surgery at the University of Ottawa Heart Institute, coauthored a meta-analysis of randomized controlled trials comparing CABG to PCI in diabetic patients. Half of the trials used later-generation drug-eluting stents. At 5 years of follow-up, the relative risk of all-cause mortality in diabetic patients allocated to CABG was 0.67 (Lancet Diabetes Endocrinol. 2013 Dec;1[4]:317-28).
“Remember that 0.67 because it’s a recurring number in our literature,” he advised.
For example, in the British Columbia study the relative risk of death, MI, or stroke from 31 days to 5 years was 0.64 in the CABG as compared with the PCI group. And in a patient-level meta-analysis of the FREEDOM, BARI-2D, and COURAGE trials presented by Dr. John Mancini earlier at the AHA scientific sessions in Orlando, CABG plus optimal medical therapy was associated with a 0.65 relative risk of death, MI, or stroke, compared with PCI plus optimal medical therapy, which in turn wasn’t significantly better than optimal medical therapy alone, Dr. Ruel noted.
The 2015 European Society of Cardiology guidelines on management of ACS state that a heart team discussion involving a cardiologist and cardiac surgeon does not need to take place for every patient presenting with multivessel disease and a non–ST elevation ACS. The British Columbia study, coupled with the other evidence, “gives new credence” to a call for revision of that recommendation in diabetic patients, he said.
The British Columbia study was supported by the British Columbia Provincial Health Services Authority. Dr. Ramanathan reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: New evidence reinforces CABG as preferred over PCI for revascularization in diabetic patients with multivessel CAD.
Major finding: Diabetic patients with multivessel CAD were 36% less likely to experience death, MI, or stroke within the next 5 years if they underwent revascularization with CABG than PCI.
Data source: This observational registry study included 4,819 diabetic patients with multivessel CAD who underwent CABG or isolated PCI for stable ischemic heart disease or stabilized acute coronary syndrome.
Disclosures: The study was supported by the British Columbia Provincial Health Services Authority. The presenter reported having no financial conflicts of interest.
Adolescents still struggling mentally years after bariatric surgery
LOS ANGELES – One in five adolescents report poor mental health 2 years after gastric bypass surgery, a Swedish study shows.
“There is an unmet need for psychiatric and psychological treatment in adolescents, and 20% is a much higher figure than you find in adults,” Kajsa Järvholm of Lund University, Sweden, said at Obesity Week 2015.
Notably, weight loss did not differ during follow-up between adolescents with poor mental health (PMH) and those with average or good mental health.
Girls were more likely than were boys to report PMH (14 vs. 2; P = .053), but no significant age difference was found.
Prior research has shown that adolescents seeking bariatric surgery have impaired mental health compared with population norms, but most adolescents experience an improvement after surgery.
A recent systematic review of psychological and social outcomes in adolescents undergoing bariatric surgery found overall quality of life improved after surgery, regardless of the surgical type, with peak improvement at months 6 to 12 (Clin Obes. 2015 Nov. 6. doi: 10.1111/cob.12119).
The current study involved 82 adolescents who were part of the larger Adolescent Morbid Obesity Surgery (AMOS) study cohort. At the time of surgery, the average age of the patients was 16.8 years and the average body mass index was 45.4 kg/m2. Two-thirds (67%) were girls.
The adolescents were assessed by self-report questionnaires at baseline, 1 year, and 2 years after laparoscopic bariatric surgery. Standardized cutoffs on two different variables, depression and obesity-related problems, were used to classify adolescents as having a PMH or average and good mental health 2 years after surgery.
Adolescents with PMH at 2 years post surgery reported significantly more symptoms of anxiety (P = .004) and depression (P = .028) already before surgery, Ms. Järvholm said.
One year after surgery, more differences were observed. Adolescents with PMH, in addition to reporting more symptoms of anxiety (P less than .0001) and depression (P = .003), also reported more anger (P = .005) and obesity-related problems (P = .006) than did adolescents with an average or good mental health.
No differences were seen at this time in self-concept or disruptive behavior, she said.
Two years after surgery, all measured aspects of mental health were worse in adolescents with PMH (all P values less than .0001), Ms. Järvholm said.
“Preoperative identification is difficult since most variables do not differ between groups, but we should take extra care with adolescents with more anxiety and more depression already before surgery,” she said at the meeting, presented by the Obesity Society and American Society for Metabolic and Bariatric Surgery.
LOS ANGELES – One in five adolescents report poor mental health 2 years after gastric bypass surgery, a Swedish study shows.
“There is an unmet need for psychiatric and psychological treatment in adolescents, and 20% is a much higher figure than you find in adults,” Kajsa Järvholm of Lund University, Sweden, said at Obesity Week 2015.
Notably, weight loss did not differ during follow-up between adolescents with poor mental health (PMH) and those with average or good mental health.
Girls were more likely than were boys to report PMH (14 vs. 2; P = .053), but no significant age difference was found.
Prior research has shown that adolescents seeking bariatric surgery have impaired mental health compared with population norms, but most adolescents experience an improvement after surgery.
A recent systematic review of psychological and social outcomes in adolescents undergoing bariatric surgery found overall quality of life improved after surgery, regardless of the surgical type, with peak improvement at months 6 to 12 (Clin Obes. 2015 Nov. 6. doi: 10.1111/cob.12119).
The current study involved 82 adolescents who were part of the larger Adolescent Morbid Obesity Surgery (AMOS) study cohort. At the time of surgery, the average age of the patients was 16.8 years and the average body mass index was 45.4 kg/m2. Two-thirds (67%) were girls.
The adolescents were assessed by self-report questionnaires at baseline, 1 year, and 2 years after laparoscopic bariatric surgery. Standardized cutoffs on two different variables, depression and obesity-related problems, were used to classify adolescents as having a PMH or average and good mental health 2 years after surgery.
Adolescents with PMH at 2 years post surgery reported significantly more symptoms of anxiety (P = .004) and depression (P = .028) already before surgery, Ms. Järvholm said.
One year after surgery, more differences were observed. Adolescents with PMH, in addition to reporting more symptoms of anxiety (P less than .0001) and depression (P = .003), also reported more anger (P = .005) and obesity-related problems (P = .006) than did adolescents with an average or good mental health.
No differences were seen at this time in self-concept or disruptive behavior, she said.
Two years after surgery, all measured aspects of mental health were worse in adolescents with PMH (all P values less than .0001), Ms. Järvholm said.
“Preoperative identification is difficult since most variables do not differ between groups, but we should take extra care with adolescents with more anxiety and more depression already before surgery,” she said at the meeting, presented by the Obesity Society and American Society for Metabolic and Bariatric Surgery.
LOS ANGELES – One in five adolescents report poor mental health 2 years after gastric bypass surgery, a Swedish study shows.
“There is an unmet need for psychiatric and psychological treatment in adolescents, and 20% is a much higher figure than you find in adults,” Kajsa Järvholm of Lund University, Sweden, said at Obesity Week 2015.
Notably, weight loss did not differ during follow-up between adolescents with poor mental health (PMH) and those with average or good mental health.
Girls were more likely than were boys to report PMH (14 vs. 2; P = .053), but no significant age difference was found.
Prior research has shown that adolescents seeking bariatric surgery have impaired mental health compared with population norms, but most adolescents experience an improvement after surgery.
A recent systematic review of psychological and social outcomes in adolescents undergoing bariatric surgery found overall quality of life improved after surgery, regardless of the surgical type, with peak improvement at months 6 to 12 (Clin Obes. 2015 Nov. 6. doi: 10.1111/cob.12119).
The current study involved 82 adolescents who were part of the larger Adolescent Morbid Obesity Surgery (AMOS) study cohort. At the time of surgery, the average age of the patients was 16.8 years and the average body mass index was 45.4 kg/m2. Two-thirds (67%) were girls.
The adolescents were assessed by self-report questionnaires at baseline, 1 year, and 2 years after laparoscopic bariatric surgery. Standardized cutoffs on two different variables, depression and obesity-related problems, were used to classify adolescents as having a PMH or average and good mental health 2 years after surgery.
Adolescents with PMH at 2 years post surgery reported significantly more symptoms of anxiety (P = .004) and depression (P = .028) already before surgery, Ms. Järvholm said.
One year after surgery, more differences were observed. Adolescents with PMH, in addition to reporting more symptoms of anxiety (P less than .0001) and depression (P = .003), also reported more anger (P = .005) and obesity-related problems (P = .006) than did adolescents with an average or good mental health.
No differences were seen at this time in self-concept or disruptive behavior, she said.
Two years after surgery, all measured aspects of mental health were worse in adolescents with PMH (all P values less than .0001), Ms. Järvholm said.
“Preoperative identification is difficult since most variables do not differ between groups, but we should take extra care with adolescents with more anxiety and more depression already before surgery,” she said at the meeting, presented by the Obesity Society and American Society for Metabolic and Bariatric Surgery.
AT OBESITY WEEK 2015
Key clinical point: A substantial number of adolescents report poor mental health 2 years after bariatric surgery.
Major finding: One in five adolescents report poor mental health 2 years after bariatric surgery.
Data source: Retrospective study of 82 adolescents.
Disclosures: The authors reported having no conflicts of interest.
VIDEO: SPRINT results rebut JNC 8’s blood pressure targets
ORLANDO – SPRINT’s hypertension-treatment results serve as a rebuttal to the systolic blood pressure target of less than 150 mm Hg for older patients set less than 2 years ago by the panel originally constituted as JNC 8, Dr. Prakash Deedwania said in an interview at the American Heart Association scientific sessions.
“We were all concerned” by the high target systolic blood pressure for patients aged 60 years or older set by the panel initially organized as JNC 8, said Dr. Deedwania, professor of medicine at the University of California, San Francisco, in Fresno. The SPRINT results, which showed incremental value for a systolic blood pressure target of less than 120 mm Hg for selected patients at risk for cardiovascular disease, will influence the new hypertension-treatment guidelines now in development by a panel formed by the American College of Cardiology and American Heart Association, he said.
While applauding SPRINT (Systolic Blood Pressure Intervention Trial) and its clear outcome, Dr. Deedwania cited several cautions to keep in mind when applying the results to practice. One of his concerns centered on driving diastolic pressure low with aggressive antihypertensive treatment, especially in elderly patients with underlying coronary artery disease who could have inadequate coronary perfusion if their diastolic pressure drops too low.
Another caution was the need to reconcile the SPRINT results with those from the ACCORD blood pressure trial results, which failed to show that a systolic blood pressure target of less than 120 mm Hg led to better outcomes than did a target of less than 140 mm Hg in patients with diabetes. Although SPRINT specifically excluded patients with diabetes, a helpful analysis that the SPRINT data should allow is assessment of outcomes in patients who were obese and had hyperlipidemia as well as hypertension, defining a subgroup of patients with metabolic syndrome that is considered a prediabetes state.
Dr. Deedwania also warned that so far researchers have not reported the SPRINT results in patients with preexisting renal dysfunction compared with patients with more normal kidney function. Patients with impaired renal function can be in jeopardy if their blood pressure gets too low, he noted.
Dr. Deedwania had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ORLANDO – SPRINT’s hypertension-treatment results serve as a rebuttal to the systolic blood pressure target of less than 150 mm Hg for older patients set less than 2 years ago by the panel originally constituted as JNC 8, Dr. Prakash Deedwania said in an interview at the American Heart Association scientific sessions.
“We were all concerned” by the high target systolic blood pressure for patients aged 60 years or older set by the panel initially organized as JNC 8, said Dr. Deedwania, professor of medicine at the University of California, San Francisco, in Fresno. The SPRINT results, which showed incremental value for a systolic blood pressure target of less than 120 mm Hg for selected patients at risk for cardiovascular disease, will influence the new hypertension-treatment guidelines now in development by a panel formed by the American College of Cardiology and American Heart Association, he said.
While applauding SPRINT (Systolic Blood Pressure Intervention Trial) and its clear outcome, Dr. Deedwania cited several cautions to keep in mind when applying the results to practice. One of his concerns centered on driving diastolic pressure low with aggressive antihypertensive treatment, especially in elderly patients with underlying coronary artery disease who could have inadequate coronary perfusion if their diastolic pressure drops too low.
Another caution was the need to reconcile the SPRINT results with those from the ACCORD blood pressure trial results, which failed to show that a systolic blood pressure target of less than 120 mm Hg led to better outcomes than did a target of less than 140 mm Hg in patients with diabetes. Although SPRINT specifically excluded patients with diabetes, a helpful analysis that the SPRINT data should allow is assessment of outcomes in patients who were obese and had hyperlipidemia as well as hypertension, defining a subgroup of patients with metabolic syndrome that is considered a prediabetes state.
Dr. Deedwania also warned that so far researchers have not reported the SPRINT results in patients with preexisting renal dysfunction compared with patients with more normal kidney function. Patients with impaired renal function can be in jeopardy if their blood pressure gets too low, he noted.
Dr. Deedwania had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ORLANDO – SPRINT’s hypertension-treatment results serve as a rebuttal to the systolic blood pressure target of less than 150 mm Hg for older patients set less than 2 years ago by the panel originally constituted as JNC 8, Dr. Prakash Deedwania said in an interview at the American Heart Association scientific sessions.
“We were all concerned” by the high target systolic blood pressure for patients aged 60 years or older set by the panel initially organized as JNC 8, said Dr. Deedwania, professor of medicine at the University of California, San Francisco, in Fresno. The SPRINT results, which showed incremental value for a systolic blood pressure target of less than 120 mm Hg for selected patients at risk for cardiovascular disease, will influence the new hypertension-treatment guidelines now in development by a panel formed by the American College of Cardiology and American Heart Association, he said.
While applauding SPRINT (Systolic Blood Pressure Intervention Trial) and its clear outcome, Dr. Deedwania cited several cautions to keep in mind when applying the results to practice. One of his concerns centered on driving diastolic pressure low with aggressive antihypertensive treatment, especially in elderly patients with underlying coronary artery disease who could have inadequate coronary perfusion if their diastolic pressure drops too low.
Another caution was the need to reconcile the SPRINT results with those from the ACCORD blood pressure trial results, which failed to show that a systolic blood pressure target of less than 120 mm Hg led to better outcomes than did a target of less than 140 mm Hg in patients with diabetes. Although SPRINT specifically excluded patients with diabetes, a helpful analysis that the SPRINT data should allow is assessment of outcomes in patients who were obese and had hyperlipidemia as well as hypertension, defining a subgroup of patients with metabolic syndrome that is considered a prediabetes state.
Dr. Deedwania also warned that so far researchers have not reported the SPRINT results in patients with preexisting renal dysfunction compared with patients with more normal kidney function. Patients with impaired renal function can be in jeopardy if their blood pressure gets too low, he noted.
Dr. Deedwania had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
U.S. hospitalization rates overestimated for some causes
Patient hospitalization rates based on discharge data that include repeat hospitalizations may overestimate disease incidence in individuals, particularly for conditions with a high proportion of repeat hospitalizations, a new study found.
Stephanie Benjamin, Ph.D., of the department of health sciences at California State University, Northridge, and her associates said that this is the first study to quantify the overestimation of hospitalization rates of individuals for common diabetes-related causes from the inclusion of repeat hospitalizations, and to determine whether this in turn affects comparisons of diabetes to nondiabetes rates. The results of the study, published online in Preventing Chronic Diseases, suggest that the use of repeat hospitalizations substantially overestimated hospitalization rates for both the diabetic and nondiabetic populations and that this overestimation varied by cause (2015 Nov 19;12:150274 ).
The research team analyzed 2011 hospitalization data for adults aged 18 years or older from the State Inpatient Databases of the Agency for Healthcare Research and Quality. The researchers examined data (a total of 10,384,306 hospital discharges) from 12 states (Arkansas, California, Florida, Hawaii, Iowa, Massachusetts, Mississippi, Nebraska, New Mexico, New York, Vermont, and Washington) whose discharge data distinguished repeat hospitalizations among individuals. They then calculated percentage increases from repeat hospitalizations in rates and compared the ratio of diabetes with nondiabetes rates while excluding and including repeat hospitalizations.
Regardless of diabetes status, hospitalization rates were considerably higher when repeat hospitalizations within a calendar year were included. The magnitude of the differences varied by condition, Dr. Benjamin and her associates found. Among adults with diabetes, rates ranged from 13.0% higher for stroke to 41.6% higher for heart failure; for adults without diabetes, these rates ranged from 9.5% higher for stroke to 25.2% higher for heart failure. Ratios of diabetes versus nondiabetes rates were similar with and without repeat hospitalizations.
While the study’s findings may not be generalizable to the national population or any one region of the country, Dr. Benjamin said, the impact of repeat hospitalizations on rates and rate ratios were consistent across all 12 states, suggesting that the relationships described “may be robust, especially because the 12 states evaluated were distributed across the country.”
Dr. Benjamin and her coauthors suggest that, when such data are used to examine trends, consideration should be given to the possible impact of any change in frequency of repeat hospitalizations. For example, successful efforts to reduce 30-day readmission rates could reduce repeat hospitalizations without reducing disease incidence rates among individuals.
Read the entire study at the PCD website.
On Twitter @richpizzi
Patient hospitalization rates based on discharge data that include repeat hospitalizations may overestimate disease incidence in individuals, particularly for conditions with a high proportion of repeat hospitalizations, a new study found.
Stephanie Benjamin, Ph.D., of the department of health sciences at California State University, Northridge, and her associates said that this is the first study to quantify the overestimation of hospitalization rates of individuals for common diabetes-related causes from the inclusion of repeat hospitalizations, and to determine whether this in turn affects comparisons of diabetes to nondiabetes rates. The results of the study, published online in Preventing Chronic Diseases, suggest that the use of repeat hospitalizations substantially overestimated hospitalization rates for both the diabetic and nondiabetic populations and that this overestimation varied by cause (2015 Nov 19;12:150274 ).
The research team analyzed 2011 hospitalization data for adults aged 18 years or older from the State Inpatient Databases of the Agency for Healthcare Research and Quality. The researchers examined data (a total of 10,384,306 hospital discharges) from 12 states (Arkansas, California, Florida, Hawaii, Iowa, Massachusetts, Mississippi, Nebraska, New Mexico, New York, Vermont, and Washington) whose discharge data distinguished repeat hospitalizations among individuals. They then calculated percentage increases from repeat hospitalizations in rates and compared the ratio of diabetes with nondiabetes rates while excluding and including repeat hospitalizations.
Regardless of diabetes status, hospitalization rates were considerably higher when repeat hospitalizations within a calendar year were included. The magnitude of the differences varied by condition, Dr. Benjamin and her associates found. Among adults with diabetes, rates ranged from 13.0% higher for stroke to 41.6% higher for heart failure; for adults without diabetes, these rates ranged from 9.5% higher for stroke to 25.2% higher for heart failure. Ratios of diabetes versus nondiabetes rates were similar with and without repeat hospitalizations.
While the study’s findings may not be generalizable to the national population or any one region of the country, Dr. Benjamin said, the impact of repeat hospitalizations on rates and rate ratios were consistent across all 12 states, suggesting that the relationships described “may be robust, especially because the 12 states evaluated were distributed across the country.”
Dr. Benjamin and her coauthors suggest that, when such data are used to examine trends, consideration should be given to the possible impact of any change in frequency of repeat hospitalizations. For example, successful efforts to reduce 30-day readmission rates could reduce repeat hospitalizations without reducing disease incidence rates among individuals.
Read the entire study at the PCD website.
On Twitter @richpizzi
Patient hospitalization rates based on discharge data that include repeat hospitalizations may overestimate disease incidence in individuals, particularly for conditions with a high proportion of repeat hospitalizations, a new study found.
Stephanie Benjamin, Ph.D., of the department of health sciences at California State University, Northridge, and her associates said that this is the first study to quantify the overestimation of hospitalization rates of individuals for common diabetes-related causes from the inclusion of repeat hospitalizations, and to determine whether this in turn affects comparisons of diabetes to nondiabetes rates. The results of the study, published online in Preventing Chronic Diseases, suggest that the use of repeat hospitalizations substantially overestimated hospitalization rates for both the diabetic and nondiabetic populations and that this overestimation varied by cause (2015 Nov 19;12:150274 ).
The research team analyzed 2011 hospitalization data for adults aged 18 years or older from the State Inpatient Databases of the Agency for Healthcare Research and Quality. The researchers examined data (a total of 10,384,306 hospital discharges) from 12 states (Arkansas, California, Florida, Hawaii, Iowa, Massachusetts, Mississippi, Nebraska, New Mexico, New York, Vermont, and Washington) whose discharge data distinguished repeat hospitalizations among individuals. They then calculated percentage increases from repeat hospitalizations in rates and compared the ratio of diabetes with nondiabetes rates while excluding and including repeat hospitalizations.
Regardless of diabetes status, hospitalization rates were considerably higher when repeat hospitalizations within a calendar year were included. The magnitude of the differences varied by condition, Dr. Benjamin and her associates found. Among adults with diabetes, rates ranged from 13.0% higher for stroke to 41.6% higher for heart failure; for adults without diabetes, these rates ranged from 9.5% higher for stroke to 25.2% higher for heart failure. Ratios of diabetes versus nondiabetes rates were similar with and without repeat hospitalizations.
While the study’s findings may not be generalizable to the national population or any one region of the country, Dr. Benjamin said, the impact of repeat hospitalizations on rates and rate ratios were consistent across all 12 states, suggesting that the relationships described “may be robust, especially because the 12 states evaluated were distributed across the country.”
Dr. Benjamin and her coauthors suggest that, when such data are used to examine trends, consideration should be given to the possible impact of any change in frequency of repeat hospitalizations. For example, successful efforts to reduce 30-day readmission rates could reduce repeat hospitalizations without reducing disease incidence rates among individuals.
Read the entire study at the PCD website.
On Twitter @richpizzi
FROM PREVENTING CHRONIC DISEASES
Reoperation risk doubled in Roux-en-Y over sleeve gastrectomy
CHICAGO – Patients undergoing Roux-en-Y gastric bypass are twice as likely to need a reoperation as those having sleeve gastrectomy, according to ACS NSQIP data.
Reoperation among Roux-en-Y patients was associated with a 10-fold increase in mortality over sleeve gastrectomy (1.2% vs. 0.1%; P less than .01) and a 3-fold increase in length of stay (6 days vs. 2 days; P less than .01), Dr. Matthew Whealon reported at the American College of Surgeons Clinical Congress
The results are consistent with prior contemporary analyses using ACS National Surgical Quality Improvement Program (NSQIP) data reporting reoperation rates of 2.5%-5.1% for Roux-en-Y gastric bypass (RYGB) and 1.6%-3% for sleeve gastrectomy. Those analyses, however, did not include the reasons for reoperation, as these data were not available until the 2012 database release, he said.
With these data now in hand, lead author Dr. Mark Hanna and his fellow investigators at the University of California, Irvine, identified 36,757 adults in the 2012-2013 database who underwent RYGB (n = 19,597) or sleeve gastrectomy (n = 17,160) for morbid obesity and performed multivariate regression analyses to identify risk factors associated with reoperation.
In all, 518 RYGB patients and 231 sleeve gastrectomy patients required an unplanned return to the operating room (2.6% vs. 1.3%), Dr. Whealon said. The mean time from the index procedure to reoperation was 7.6 days and 7.1 days, respectively.
Obstruction was the biggest driver of reoperation following RYGB, accounting for 28% of reoperations. Other causes were bleeding (14.5%), leak (13%), and other unspecified reasons (18.5%), with data missing in 12%.
Bleeding was the most common indication for reoperation after sleeve gastrectomy (25.5%), followed by other unspecified reasons(24.6%), missing data (13%), leak (12.55%), and obstruction (11.2%), he said.
In adjusted multivariate analyses, factors that significantly increased the risk for reoperation were heart failure (adjusted odds ratio, 2.3), dependent functional status (aOR, 2.1), RYGB (aOR, 1.94), chronic obstructive pulmonary disease (aOR, 1.7), open operation (aOR, 1.6), and male sex (aOR, 1.1). The P values were less than .05 for all comparisons.
Factors not significant for reoperation included body mass index, age, smoking status, bleeding disorder, steroid use, dialysis, hypertension, diabetes, preoperative sepsis, emergent admission, elective operation, and preoperative weight loss.
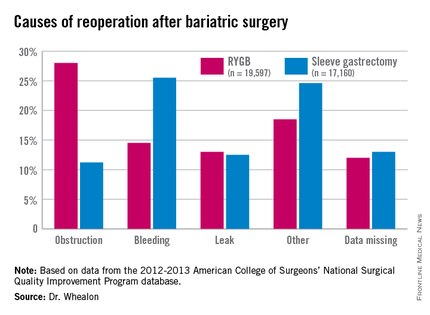
While bariatric surgery remains a safe operation with low mortality and reoperation rates, additional studies are needed, because of the increased mortality associated with reoperation, to identify ways to mitigate these complications, Dr. Whealon said.
Limitations of the study were that ICD-9 codes for postoperative hemorrhage could not differentiate between intra-abdominal and gastrointestinal bleeding, the database is subject to coding errors, and missing data may have introduced bias into the study, he noted.
Discussant Dr. Matthew Goldblatt of the Medical College of Wisconsin in Milwaukee commented that use of the ACS MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) database would have avoided some of the coding errors for reoperation.
He also questioned whether the average 7-day return to surgery interval reflects the use of endoscopy, as few surgeons would wait that long if, as the analysis suggests, a primary reason for reoperation was postoperative bleeding.
Endoscopy was included in the reoperations, Dr. Whealon said, but he could not speak to the exact percentage it comprised.
Finally, Dr. Goldblatt said, “the patients that you identified as being the highest risk for complication, as is often the case in these reviews, are really the ones most likely to gain the most from the procedure. … So how can people avoid operating on these patients when they are the ones that can get the most out of it?”
Dr. Whealon agreed that high-risk patients have the most to gain and suggested that “optimizing their comorbid conditions before operation will help reduce their risk.”
The authors reported having no conflicts of interest.
CHICAGO – Patients undergoing Roux-en-Y gastric bypass are twice as likely to need a reoperation as those having sleeve gastrectomy, according to ACS NSQIP data.
Reoperation among Roux-en-Y patients was associated with a 10-fold increase in mortality over sleeve gastrectomy (1.2% vs. 0.1%; P less than .01) and a 3-fold increase in length of stay (6 days vs. 2 days; P less than .01), Dr. Matthew Whealon reported at the American College of Surgeons Clinical Congress
The results are consistent with prior contemporary analyses using ACS National Surgical Quality Improvement Program (NSQIP) data reporting reoperation rates of 2.5%-5.1% for Roux-en-Y gastric bypass (RYGB) and 1.6%-3% for sleeve gastrectomy. Those analyses, however, did not include the reasons for reoperation, as these data were not available until the 2012 database release, he said.
With these data now in hand, lead author Dr. Mark Hanna and his fellow investigators at the University of California, Irvine, identified 36,757 adults in the 2012-2013 database who underwent RYGB (n = 19,597) or sleeve gastrectomy (n = 17,160) for morbid obesity and performed multivariate regression analyses to identify risk factors associated with reoperation.
In all, 518 RYGB patients and 231 sleeve gastrectomy patients required an unplanned return to the operating room (2.6% vs. 1.3%), Dr. Whealon said. The mean time from the index procedure to reoperation was 7.6 days and 7.1 days, respectively.
Obstruction was the biggest driver of reoperation following RYGB, accounting for 28% of reoperations. Other causes were bleeding (14.5%), leak (13%), and other unspecified reasons (18.5%), with data missing in 12%.
Bleeding was the most common indication for reoperation after sleeve gastrectomy (25.5%), followed by other unspecified reasons(24.6%), missing data (13%), leak (12.55%), and obstruction (11.2%), he said.
In adjusted multivariate analyses, factors that significantly increased the risk for reoperation were heart failure (adjusted odds ratio, 2.3), dependent functional status (aOR, 2.1), RYGB (aOR, 1.94), chronic obstructive pulmonary disease (aOR, 1.7), open operation (aOR, 1.6), and male sex (aOR, 1.1). The P values were less than .05 for all comparisons.
Factors not significant for reoperation included body mass index, age, smoking status, bleeding disorder, steroid use, dialysis, hypertension, diabetes, preoperative sepsis, emergent admission, elective operation, and preoperative weight loss.

While bariatric surgery remains a safe operation with low mortality and reoperation rates, additional studies are needed, because of the increased mortality associated with reoperation, to identify ways to mitigate these complications, Dr. Whealon said.
Limitations of the study were that ICD-9 codes for postoperative hemorrhage could not differentiate between intra-abdominal and gastrointestinal bleeding, the database is subject to coding errors, and missing data may have introduced bias into the study, he noted.
Discussant Dr. Matthew Goldblatt of the Medical College of Wisconsin in Milwaukee commented that use of the ACS MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) database would have avoided some of the coding errors for reoperation.
He also questioned whether the average 7-day return to surgery interval reflects the use of endoscopy, as few surgeons would wait that long if, as the analysis suggests, a primary reason for reoperation was postoperative bleeding.
Endoscopy was included in the reoperations, Dr. Whealon said, but he could not speak to the exact percentage it comprised.
Finally, Dr. Goldblatt said, “the patients that you identified as being the highest risk for complication, as is often the case in these reviews, are really the ones most likely to gain the most from the procedure. … So how can people avoid operating on these patients when they are the ones that can get the most out of it?”
Dr. Whealon agreed that high-risk patients have the most to gain and suggested that “optimizing their comorbid conditions before operation will help reduce their risk.”
The authors reported having no conflicts of interest.
CHICAGO – Patients undergoing Roux-en-Y gastric bypass are twice as likely to need a reoperation as those having sleeve gastrectomy, according to ACS NSQIP data.
Reoperation among Roux-en-Y patients was associated with a 10-fold increase in mortality over sleeve gastrectomy (1.2% vs. 0.1%; P less than .01) and a 3-fold increase in length of stay (6 days vs. 2 days; P less than .01), Dr. Matthew Whealon reported at the American College of Surgeons Clinical Congress
The results are consistent with prior contemporary analyses using ACS National Surgical Quality Improvement Program (NSQIP) data reporting reoperation rates of 2.5%-5.1% for Roux-en-Y gastric bypass (RYGB) and 1.6%-3% for sleeve gastrectomy. Those analyses, however, did not include the reasons for reoperation, as these data were not available until the 2012 database release, he said.
With these data now in hand, lead author Dr. Mark Hanna and his fellow investigators at the University of California, Irvine, identified 36,757 adults in the 2012-2013 database who underwent RYGB (n = 19,597) or sleeve gastrectomy (n = 17,160) for morbid obesity and performed multivariate regression analyses to identify risk factors associated with reoperation.
In all, 518 RYGB patients and 231 sleeve gastrectomy patients required an unplanned return to the operating room (2.6% vs. 1.3%), Dr. Whealon said. The mean time from the index procedure to reoperation was 7.6 days and 7.1 days, respectively.
Obstruction was the biggest driver of reoperation following RYGB, accounting for 28% of reoperations. Other causes were bleeding (14.5%), leak (13%), and other unspecified reasons (18.5%), with data missing in 12%.
Bleeding was the most common indication for reoperation after sleeve gastrectomy (25.5%), followed by other unspecified reasons(24.6%), missing data (13%), leak (12.55%), and obstruction (11.2%), he said.
In adjusted multivariate analyses, factors that significantly increased the risk for reoperation were heart failure (adjusted odds ratio, 2.3), dependent functional status (aOR, 2.1), RYGB (aOR, 1.94), chronic obstructive pulmonary disease (aOR, 1.7), open operation (aOR, 1.6), and male sex (aOR, 1.1). The P values were less than .05 for all comparisons.
Factors not significant for reoperation included body mass index, age, smoking status, bleeding disorder, steroid use, dialysis, hypertension, diabetes, preoperative sepsis, emergent admission, elective operation, and preoperative weight loss.

While bariatric surgery remains a safe operation with low mortality and reoperation rates, additional studies are needed, because of the increased mortality associated with reoperation, to identify ways to mitigate these complications, Dr. Whealon said.
Limitations of the study were that ICD-9 codes for postoperative hemorrhage could not differentiate between intra-abdominal and gastrointestinal bleeding, the database is subject to coding errors, and missing data may have introduced bias into the study, he noted.
Discussant Dr. Matthew Goldblatt of the Medical College of Wisconsin in Milwaukee commented that use of the ACS MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) database would have avoided some of the coding errors for reoperation.
He also questioned whether the average 7-day return to surgery interval reflects the use of endoscopy, as few surgeons would wait that long if, as the analysis suggests, a primary reason for reoperation was postoperative bleeding.
Endoscopy was included in the reoperations, Dr. Whealon said, but he could not speak to the exact percentage it comprised.
Finally, Dr. Goldblatt said, “the patients that you identified as being the highest risk for complication, as is often the case in these reviews, are really the ones most likely to gain the most from the procedure. … So how can people avoid operating on these patients when they are the ones that can get the most out of it?”
Dr. Whealon agreed that high-risk patients have the most to gain and suggested that “optimizing their comorbid conditions before operation will help reduce their risk.”
The authors reported having no conflicts of interest.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Patients undergoing Roux-en-Y gastric bypass were twice as likely to need a reoperation as with sleeve gastrectomy, and reoperation increased morbidity 10-fold.
Major finding: The reoperation rate for Roux-en-Y gastric bypass was 2.6% vs. 1.3% for sleeve gastrectomy.
Data source: An ACS NSQIP database analysis of 36,757 patients undergoing bariatric surgery.
Disclosures: The authors reported having no conflicts of interest.