User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Severity of obesity matters in pediatric population
Children and adolescents with more severe obesity show a higher prevalence of cardiometabolic abnormalities than those with less severe obesity, so differentiating among levels of obesity is important in the pediatric population, according to a report published online Oct. 1 in New England Journal of Medicine.
Current screening guidelines for pediatric patients use only a single category for obesity, which doesn’t take into account their varying levels of risk for obesity-related disorders. To assess the distribution of cardiometabolic risk factors in obese children and adolescents, researchers performed a cross-sectional analysis of data from the National Health and Nutrition Examination Survey for 2011-2012.
They assessed a nationally representative sample of 8,579 participants aged 3-19 years, categorizing them by age- and gender-specific body mass index percentiles: overweight (85th-94th percentile), class I obesity (95th percentile to 119% of the 95th percentile), class II obesity (120% to 139% of the 95th percentile, or BMI of 35-39, whichever was lower), or class III obesity (140% of the 95th percentile, or BMI of 40 or greater, whichever was lower).
A total of 47% of the study participants were overweight, 36% had class I obesity, 12% had class II obesity, and 5% had class III obesity. The prevalence of abnormal values for total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, systolic BP, diastolic BP, glycated hemoglobin, and fasting glucose all increased with increasing severity of obesity, at most ages and across both genders. The correlations were more pronounced in boys than in girls, said Asheley C. Skinner, Ph.D., of the department of pediatrics and the department of health policy and management, University of North Carolina at Chapel Hill, and her associates.
The findings indicate that distinguishing among at least three levels of obesity severity “provides a more fine-tuned approach to identifying patients with the greatest risk of potential complications and death,” allowing targeted interventions to those at greatest risk. This is especially important because resources are too limited to provide services for every child with obesity, the investigators said (N Engl J Med. 2015 Oct 1. doi:10.1056/NEJMoa1502821). “As older adolescents transition to young adulthood, the recognition that teens with obesity have increased cardiometabolic risk will be important,” they added.
This study did not specify a source of funding. Dr. Skinner reported having no relevant financial disclosures; one of her associates reported receiving personal fees from Nestle unrelated to this work.
Children and adolescents with more severe obesity show a higher prevalence of cardiometabolic abnormalities than those with less severe obesity, so differentiating among levels of obesity is important in the pediatric population, according to a report published online Oct. 1 in New England Journal of Medicine.
Current screening guidelines for pediatric patients use only a single category for obesity, which doesn’t take into account their varying levels of risk for obesity-related disorders. To assess the distribution of cardiometabolic risk factors in obese children and adolescents, researchers performed a cross-sectional analysis of data from the National Health and Nutrition Examination Survey for 2011-2012.
They assessed a nationally representative sample of 8,579 participants aged 3-19 years, categorizing them by age- and gender-specific body mass index percentiles: overweight (85th-94th percentile), class I obesity (95th percentile to 119% of the 95th percentile), class II obesity (120% to 139% of the 95th percentile, or BMI of 35-39, whichever was lower), or class III obesity (140% of the 95th percentile, or BMI of 40 or greater, whichever was lower).
A total of 47% of the study participants were overweight, 36% had class I obesity, 12% had class II obesity, and 5% had class III obesity. The prevalence of abnormal values for total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, systolic BP, diastolic BP, glycated hemoglobin, and fasting glucose all increased with increasing severity of obesity, at most ages and across both genders. The correlations were more pronounced in boys than in girls, said Asheley C. Skinner, Ph.D., of the department of pediatrics and the department of health policy and management, University of North Carolina at Chapel Hill, and her associates.
The findings indicate that distinguishing among at least three levels of obesity severity “provides a more fine-tuned approach to identifying patients with the greatest risk of potential complications and death,” allowing targeted interventions to those at greatest risk. This is especially important because resources are too limited to provide services for every child with obesity, the investigators said (N Engl J Med. 2015 Oct 1. doi:10.1056/NEJMoa1502821). “As older adolescents transition to young adulthood, the recognition that teens with obesity have increased cardiometabolic risk will be important,” they added.
This study did not specify a source of funding. Dr. Skinner reported having no relevant financial disclosures; one of her associates reported receiving personal fees from Nestle unrelated to this work.
Children and adolescents with more severe obesity show a higher prevalence of cardiometabolic abnormalities than those with less severe obesity, so differentiating among levels of obesity is important in the pediatric population, according to a report published online Oct. 1 in New England Journal of Medicine.
Current screening guidelines for pediatric patients use only a single category for obesity, which doesn’t take into account their varying levels of risk for obesity-related disorders. To assess the distribution of cardiometabolic risk factors in obese children and adolescents, researchers performed a cross-sectional analysis of data from the National Health and Nutrition Examination Survey for 2011-2012.
They assessed a nationally representative sample of 8,579 participants aged 3-19 years, categorizing them by age- and gender-specific body mass index percentiles: overweight (85th-94th percentile), class I obesity (95th percentile to 119% of the 95th percentile), class II obesity (120% to 139% of the 95th percentile, or BMI of 35-39, whichever was lower), or class III obesity (140% of the 95th percentile, or BMI of 40 or greater, whichever was lower).
A total of 47% of the study participants were overweight, 36% had class I obesity, 12% had class II obesity, and 5% had class III obesity. The prevalence of abnormal values for total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, systolic BP, diastolic BP, glycated hemoglobin, and fasting glucose all increased with increasing severity of obesity, at most ages and across both genders. The correlations were more pronounced in boys than in girls, said Asheley C. Skinner, Ph.D., of the department of pediatrics and the department of health policy and management, University of North Carolina at Chapel Hill, and her associates.
The findings indicate that distinguishing among at least three levels of obesity severity “provides a more fine-tuned approach to identifying patients with the greatest risk of potential complications and death,” allowing targeted interventions to those at greatest risk. This is especially important because resources are too limited to provide services for every child with obesity, the investigators said (N Engl J Med. 2015 Oct 1. doi:10.1056/NEJMoa1502821). “As older adolescents transition to young adulthood, the recognition that teens with obesity have increased cardiometabolic risk will be important,” they added.
This study did not specify a source of funding. Dr. Skinner reported having no relevant financial disclosures; one of her associates reported receiving personal fees from Nestle unrelated to this work.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Children and adolescents with more severe obesity show a higher prevalence of cardiometabolic abnormalities, so differentiating among levels of obesity is important in the pediatric population.
Major finding: Forty-seven percent of the study participants were overweight, 36% had class I obesity, 12% had class II obesity, and 5% had class III obesity.
Data source: A cross-sectional analysis of NHANES data concerning 8,579 overweight/obese participants aged 3-19 years.
Disclosures: This study did not specify a source of funding. Dr. Skinner reported having no relevant financial disclosures; one of her associates reported receiving personal fees from Nestle unrelated to this work.
EASD: Metformin-induced B12 deficiency linked to diabetic neuropathy
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
STOCKHOLM – Clinicians might want to consider more routinely monitoring levels of vitamin B12 in their diabetic patients who are using metformin, according to new data from the HOME (Hyperinsulinaemia: the Outcome of Its Metabolic Effects) randomized controlled trial, which showed that B12 depletion was linked to neurotoxicity.
The new study findings, presented at the annual meeting of the European Association for the Study of Diabetes, showed that metformin increased levels of serum methylmalonic acid (MMA), the gold standard biomarker of tissue B12 deficiency, and that this in turn was linked to neuropathy measured using a validated score.
“We earlier showed that metformin causes B12 deficiency (<150 pmol/L) with a number needed to harm of 14 after 4.3 years,” said study investigator Dr. Mattijs Out of Bethesda Diabetes Research Center in Hoogeveen, the Netherlands, referring to the original results of the trial (BMJ. 2010;340:c2181).
“Today I showed you that metformin increases MMA, dose dependently and progressively over time,” he added. “Metformin has two opposite effects on neuropathy,” he continued, “a neuroprotective effect by improving glycemic control and a neurotoxic effect by inducing B12 depletion.”
Dr. Out noted that metformin remains the cornerstone of type 2 diabetes therapy with over 100 million prescriptions per year, putting many patients at risk for B12 deficiency and its consequences.
Current guidelines from the American Diabetes Association and EASD mention B12 deficiency as a potential downside of metformin use but do not go so far as giving specific recommendations because of lack of evidence on whether screening should be routinely performed or if vitamin B12 supplements should be given.
“Consequences of vitamin B12 deficiency, such as neuropathy or mental changes, may be profound,” Dr. Out said. Changes because of B12 deficits can be difficult to diagnose because they may be ascribed to old age or diabetes itself, and they may become irreversible if unchecked, he observed. Diagnosing and managing B12 deficiency is, in theory, “easy, cheap, and effective,” he said. A new trial would be needed, however, to determine whether screening for B12 deficiency or supplementation would be the better approach.
In the current study, structural equation monitoring (SEM) was used to determine the likely effects of metformin on the Valk neuropathy score (Diabetes Med. 1992;9:716-21) directly and via effects on hemoglobin A1c and MMA. The analysis involved 390 insulin-treated patients with type 2 diabetes who were also treated with 850 mg metformin or placebo up to three times daily for 52 months. Over a 4.3-year follow-up period, patients had HbA1c and neuropathy scores measured 17 times, and MMA was measured at six visits.
Compared with placebo, metformin use was associated with a significant (P= .001) 0.039 micromol/L (95% confidence interval, 0.019-0.055) increase in MMA over the course of the study.
While there was no significant difference in neuropathy scores between the placebo- and metformin-treated groups, SEM showed that metformin had a beneficial effect on lowering the neuropathy score via lowering HbA1c and an adverse effect on the neuropathy score by increasing MMA levels. Overall, metformin was associated with a 0.25-point increase in the neuropathy score, suggesting the net effect of the oral hypoglycemic drug is a negative one as higher scores mean worse neuropathy.
After the presentation of the findings, session chair Dr. Guntram Schernthaner commented: “In my center in Vienna, we have been using metformin for 30 years, but we have never used this high dosage [850 mg]. We have suspected several times the B12 deficiency and we measured it in many cases, but we never found any cases of severe B12 deficiency.”
Dr. Schernthaner, who is professor of medicine at the University of Vienna in Austria, suggested the next step would be to perform a large trial of maybe 2,000 metformin users to see how often B12 deficiency really occurs.
Dr. Out agreed a larger trial would be the ideal and conceded that the effect size was small. However, with such a large number of patients using the drug worldwide, potentially “many patients are at risk.”
He said a direct effect of metformin on neuropathy would perhaps not be found by general screening because of its protective effect via improved glycemic control, “so you may miss B12 deficiency–induced neuropathy.” Measuring MMA, where possible, may be a solution.
AT EASD 2015
Key clinical point: Metformin has an overall detrimental effect on neuropathy mediated by its effect on MMA, a specific biomarker of B12 deficiency.
Major finding: Metformin use was associated with a significant (P = .001) 0.04 micromol/L increase in MMA over the course of the study when compared with placebo.
Data source: A prospective, randomized controlled trial of 390 insulin-treated patients with type 2 diabetes who were randomized to metformin or placebo for 4.3 years.
Disclosures: Dr. Out reported that he had no disclosures. The HOME study was supported by Takeda, Lifescan, Merck Sharpe & Dohme, and Novo Nordisk.
EASD: Liraglutide lowers HbA1c when added to insulin in longstanding type 2 diabetes
STOCKHOLM – Adding liraglutide to multiple daily insulin injections helped stabilize blood glucose in patients with type 2 diabetes, while also resulting in weight loss and reduction of daily insulin doses.
All this was accomplished without an increase in the risk of hypoglycemia, Dr. Marcus Lind said at the annual meeting of the European Association for the Study of Diabetes.
A randomized, placebo-controlled study showed that the approach was successful in patients with long-standing disease, said Dr. Lind of the University of Gothenburg, Sweden. “We used to think that incretin-based therapies were most helpful in early type 2 diabetes. This seems to confirm that they are effective during the entire disease process.”
The soon-to-be-published 24-week study examined the addition of liraglutide in 124 patients with type 2 diabetes of long duration (a mean of 17 years). They were overweight, with a mean body mass index of 33.5 (about 218 pounds). At baseline, their mean hemoglobin A1c was 9%; they were taking a mean of 105 units of insulin each day in about four injections.
The study’s primary endpoint was change in HbA1c at 24 weeks. This declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%). “This is a difference of 1.1%, which is very clinically significant,” Dr. Lind said.
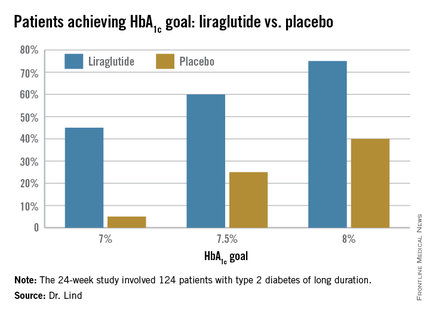
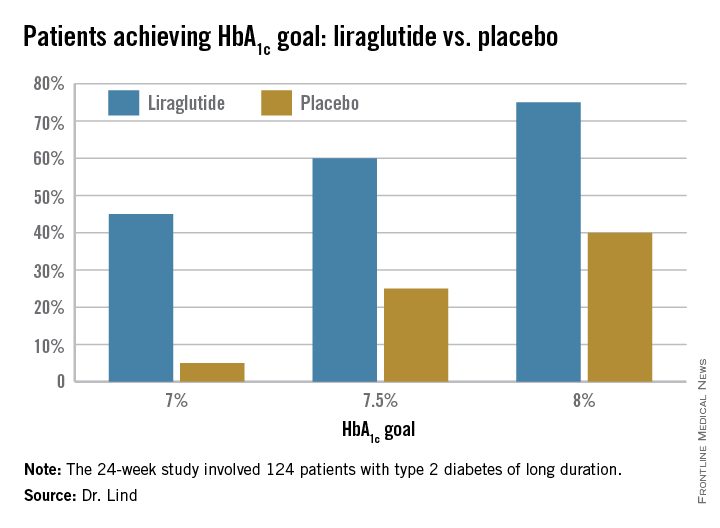
Dr. Lind assigned individualized HbA1c goals to patients, with the cutpoints of 7%, 7.5%, and 8%. Significantly more patients taking liraglutide were able to achieve those goals (see chart).
They also lost about 8 pounds more weight than did the placebo patients, and experienced a greater decrease in systolic blood pressure (–5.5 mm Hg more than placebo). There was no effect on diastolic blood pressure or lipids.
There were no severe hypoglycemic events in either group, nor any between-group difference in nonsevere events.
There were three serious adverse events among three patients taking liraglutide, and eight among four patients taking placebo; none of these were pancreatitis or pancreatic cancer.
Patients taking the study drug experienced significantly more nausea, especially at the beginning of the study, compared to the end of the study (22% vs. 5%).
The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
STOCKHOLM – Adding liraglutide to multiple daily insulin injections helped stabilize blood glucose in patients with type 2 diabetes, while also resulting in weight loss and reduction of daily insulin doses.
All this was accomplished without an increase in the risk of hypoglycemia, Dr. Marcus Lind said at the annual meeting of the European Association for the Study of Diabetes.
A randomized, placebo-controlled study showed that the approach was successful in patients with long-standing disease, said Dr. Lind of the University of Gothenburg, Sweden. “We used to think that incretin-based therapies were most helpful in early type 2 diabetes. This seems to confirm that they are effective during the entire disease process.”
The soon-to-be-published 24-week study examined the addition of liraglutide in 124 patients with type 2 diabetes of long duration (a mean of 17 years). They were overweight, with a mean body mass index of 33.5 (about 218 pounds). At baseline, their mean hemoglobin A1c was 9%; they were taking a mean of 105 units of insulin each day in about four injections.
The study’s primary endpoint was change in HbA1c at 24 weeks. This declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%). “This is a difference of 1.1%, which is very clinically significant,” Dr. Lind said.

Dr. Lind assigned individualized HbA1c goals to patients, with the cutpoints of 7%, 7.5%, and 8%. Significantly more patients taking liraglutide were able to achieve those goals (see chart).
They also lost about 8 pounds more weight than did the placebo patients, and experienced a greater decrease in systolic blood pressure (–5.5 mm Hg more than placebo). There was no effect on diastolic blood pressure or lipids.
There were no severe hypoglycemic events in either group, nor any between-group difference in nonsevere events.
There were three serious adverse events among three patients taking liraglutide, and eight among four patients taking placebo; none of these were pancreatitis or pancreatic cancer.
Patients taking the study drug experienced significantly more nausea, especially at the beginning of the study, compared to the end of the study (22% vs. 5%).
The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
STOCKHOLM – Adding liraglutide to multiple daily insulin injections helped stabilize blood glucose in patients with type 2 diabetes, while also resulting in weight loss and reduction of daily insulin doses.
All this was accomplished without an increase in the risk of hypoglycemia, Dr. Marcus Lind said at the annual meeting of the European Association for the Study of Diabetes.
A randomized, placebo-controlled study showed that the approach was successful in patients with long-standing disease, said Dr. Lind of the University of Gothenburg, Sweden. “We used to think that incretin-based therapies were most helpful in early type 2 diabetes. This seems to confirm that they are effective during the entire disease process.”
The soon-to-be-published 24-week study examined the addition of liraglutide in 124 patients with type 2 diabetes of long duration (a mean of 17 years). They were overweight, with a mean body mass index of 33.5 (about 218 pounds). At baseline, their mean hemoglobin A1c was 9%; they were taking a mean of 105 units of insulin each day in about four injections.
The study’s primary endpoint was change in HbA1c at 24 weeks. This declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%). “This is a difference of 1.1%, which is very clinically significant,” Dr. Lind said.

Dr. Lind assigned individualized HbA1c goals to patients, with the cutpoints of 7%, 7.5%, and 8%. Significantly more patients taking liraglutide were able to achieve those goals (see chart).
They also lost about 8 pounds more weight than did the placebo patients, and experienced a greater decrease in systolic blood pressure (–5.5 mm Hg more than placebo). There was no effect on diastolic blood pressure or lipids.
There were no severe hypoglycemic events in either group, nor any between-group difference in nonsevere events.
There were three serious adverse events among three patients taking liraglutide, and eight among four patients taking placebo; none of these were pancreatitis or pancreatic cancer.
Patients taking the study drug experienced significantly more nausea, especially at the beginning of the study, compared to the end of the study (22% vs. 5%).
The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
AT EASD 2015
Key clinical point: Liraglutide added to multiple daily insulin injections lowered HbA1c significantly more than did placebo in patients with type 2 diabetes.
Major finding: HbA1c at 24 weeks declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%).
Data source: A randomized, placebo-controlled study of 124 patients.
Disclosures: The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.
Evidence links common endocrine-disrupting chemicals to obesity, diabetes, reproductive disorders
Cash register receipts, tin can linings, and a range of cosmetics and other common household products increasingly are implicated in the most intractable of society’s diseases such as obesity and diabetes.
So-called endocrine-disrupting chemicals have become so common, according to the Washington-based Endocrine Society, that nearly every human alive has been exposed to at least one such chemical, probably more than once, and just as likely, over an extended period of time.
The World Health Organization reports that there are more than 800 known endocrine disrupting chemicals (EDCs) used in products globally, but only a fraction of them have been tested in humans. What effects, if any, this exposure is having on individuals is still unknown, but data linking the ability of EDCs – either singularly or in combination – to mimic, block, or otherwise interfere with the body’s natural hormone signaling has some experts sounding the alarm.
“The evidence is more definitive than ever before – EDCs disrupt hormones in a manner that harms human health,” Andrea C. Gore, Ph.D., a pharmacology professor at the University of Texas at Austin, said in a press conference. Dr. Gore chairs the Endocrine Society task force that recently released an executive summary of its second scientific statement on EDCs. The Society presented the statement, an update of one released in 2009, at this year’s International Conference on Chemicals Management annual meeting in Geneva.
Because the endocrine system’s role is to interact with the environment, it is predisposed to react to triggers increasingly found in everything from pesticides to shower curtains, which have now made their way into waterways and the food chain primarily without any studies on their impact. “Both natural hormones and EDCs have unique dose-response properties [and can] act at very-low doses,” Dr. Gore told reporters. “We’re exposed throughout our lives.”
The use of EDCs largely began post-WWII with pesticides such as DDT (dichlorodiphenyltrichloroethane), but over time came to include use as thickeners, plastic softeners, and scent in many common household items. Dr. Gore told a reporter that even though these chemicals were not intended to enter the environment at large, decades of use has made their entry into the food chain inevitable. The population health effects of this are only now becoming evident as data accumulate linking EDCs to a constellation of ill health effects.
“In humans, there are strong epidemiological associations between EDCs and chronic diseases.” Dr. Gore said.
She specifically cited obesity, diabetes, and a range of reproductive disorders, including infertility and certain hormone-related cancers. Research also shows a link between prenatal EDC exposure in animals to obesity, insulin resistance, and overabundant insulin later in life.
The Society’s meta-analysis of more than 1,300 studies published in the last 5 years also implicated EDCs in disorders of the prostate gland, the thyroid, and the neuroendocrine systems, the latter two being particularly vulnerable because of their role in hormone regulation at all stages of development.
“We’re particularly concerned about fetuses and how exposure can set the stage for later development of diseases,” Dr. Gore said, noting that human studies have shown a link between higher EDC exposures over time and cognitive deficits and other adverse neurocognitive outcomes.
Because the effect of EDC exposure differs according to the dose, length, and timing of exposure, designing studies to measure any harm has been difficult, said Dr. Gore, although in the past 5 years, there has been increased insight into the molecular mechanisms underlying EDCs.
Among the most common EDCs are bisphenol A (BPA) and phthalates, synthetic chemicals that bind to hormone receptors and depending upon the dose, either potentiate, inhibit – or both – the hormone’s effect on receptors. These EDCs frequently occur in toys, bottle nipples, rain coats, shower curtains, and in medical supplies such as blood bags, IV tubing, and catheters.
Initially registered as a pesticide, the EDC triclosan’s antimicrobial power has meant it is now used in deodorants and even in toothpaste. Triclosan has been shown to disrupt the thyroid, and to have antiestrogenic, and antiandrogenic properties. It also has been linked to asthma.
Plastic water bottles and disposable, plastic-based food packaging commonly found in microwaveable products often are high in BPAs, according to Dr. Gore, who urged all primary care physicians to counsel patients on the importance of avoiding products that contain them whenever possible, particularly in cases of pediatric obesity or diabetes, and for patients who are pregnant or in the family-planning stages. “You might not see an adverse outcome until years or decades later.”
Because many EDCs are lipophilic, our bodies often store them in our fat cells, often for years at a time.
While bisphenol A tends to exit the body quickly, we are commonly exposed to it on a daily basis, usually through a compound that leaches into our food, allowing the chemical an opportunity to exert an effect, even if the results of this effect aren’t immediately apparent. “If it’s a pregnant woman or someone planning a family, that exposure can change something,” Dr. Gore warned.
Although earlier studies had linked EDC exposure to a variety of reproductive health concerns, Dr. Gore said that since the Society’s last statement, there is much more corroborating evidence. In particular, polycystic ovarian syndrome in humans has been associated with higher body burdens of BPA and other chemicals, as have endometriosis, fibroids, and some adverse birth outcomes. Still, much of the data come from animal studies, and studies of specific links between EDCs and reproductive outcomes and cancers are inconsistent.
According to Dr. Renee Howard, a pediatric dermatologist with Sutter Health in San Francisco, who regularly gives presentations on the effects of EDCs in cosmetics and other skin care products, although much of the current research shows a link between EDCs and chronic illness, so far a causal relationship has not been established. Still, she said she routinely tells other physicians to keep an “open mind” when discussing EDCs with patients.
“We can acknowledge there is uncertainty, and we can tell patients that these chemicals are actively being studied, but that the studies are, as of now, inconclusive, and that there are still no documented adverse health effects associated with skin care products,” Dr. Howard said. Nevertheless, she said common sense dictates her to counsel patients to avoid products containing the EDC triclosan such as antimicrobials and scented products. She also advises them to eat organic produce in order to avoid pesticides.
Dr. Gore and her coauthors hope the statement will help bump EDC oversight higher up the policy chain globally.
“Exposure to endocrine disrupting chemicals during early development can have long-lasting, even permanent consequences,” Endocrine Society member Dr. Jean-Pierre Bourguignon, a professor of pediatric endocrinology at the University of Liège in Belgium, said in a statement. “The science is clear, and it’s time for policy makers to take this wealth of evidence into account as they develop legislation.”
To that end, earlier this year, twin bills are now before the House and Senate, which – if passed – will take effect in 2018, banning the sale of any personal care products containing microbeads, BPA-rich, microscopic plastic particles that have entered much of the natural water supply, threatening marine life which often mistake the tiny bits as food. Meanwhile, Minnesota has banned the use of triclosan statewide as of 2017.
Dr. Gore also said in addition to funding for this research being made a priority, it’s time to rethink who gets to be in on the science. Rather than just industrial chemists, she believes the teams should include so-called “green chemists,” basic, translational, and clinical scientists; epidemiologists, as well as public health professionals. Health care providers should be familiar with endocrine science and the latest developments in EDC research, accordingly, because, said Dr. Gore, “The [health] costs of EDCs have been estimated in the hundreds of millions of dollars. Prevention might seem expensive, but it’s far-less-expensive than all the ensuing diseases.”
Most of all, Dr. Gore and her colleagues emphasize that there will never be “absolute proof” of anything, but that taking action to stem exposure is essential.
The analysis was sponsored by the Endocrine Society. Dr. Gore is editor in chief of Endocrinology. Dr. Howard disclosed no relevant financial relationships.
On Twitter @whitneymcknight
Cash register receipts, tin can linings, and a range of cosmetics and other common household products increasingly are implicated in the most intractable of society’s diseases such as obesity and diabetes.
So-called endocrine-disrupting chemicals have become so common, according to the Washington-based Endocrine Society, that nearly every human alive has been exposed to at least one such chemical, probably more than once, and just as likely, over an extended period of time.
The World Health Organization reports that there are more than 800 known endocrine disrupting chemicals (EDCs) used in products globally, but only a fraction of them have been tested in humans. What effects, if any, this exposure is having on individuals is still unknown, but data linking the ability of EDCs – either singularly or in combination – to mimic, block, or otherwise interfere with the body’s natural hormone signaling has some experts sounding the alarm.
“The evidence is more definitive than ever before – EDCs disrupt hormones in a manner that harms human health,” Andrea C. Gore, Ph.D., a pharmacology professor at the University of Texas at Austin, said in a press conference. Dr. Gore chairs the Endocrine Society task force that recently released an executive summary of its second scientific statement on EDCs. The Society presented the statement, an update of one released in 2009, at this year’s International Conference on Chemicals Management annual meeting in Geneva.
Because the endocrine system’s role is to interact with the environment, it is predisposed to react to triggers increasingly found in everything from pesticides to shower curtains, which have now made their way into waterways and the food chain primarily without any studies on their impact. “Both natural hormones and EDCs have unique dose-response properties [and can] act at very-low doses,” Dr. Gore told reporters. “We’re exposed throughout our lives.”
The use of EDCs largely began post-WWII with pesticides such as DDT (dichlorodiphenyltrichloroethane), but over time came to include use as thickeners, plastic softeners, and scent in many common household items. Dr. Gore told a reporter that even though these chemicals were not intended to enter the environment at large, decades of use has made their entry into the food chain inevitable. The population health effects of this are only now becoming evident as data accumulate linking EDCs to a constellation of ill health effects.
“In humans, there are strong epidemiological associations between EDCs and chronic diseases.” Dr. Gore said.
She specifically cited obesity, diabetes, and a range of reproductive disorders, including infertility and certain hormone-related cancers. Research also shows a link between prenatal EDC exposure in animals to obesity, insulin resistance, and overabundant insulin later in life.
The Society’s meta-analysis of more than 1,300 studies published in the last 5 years also implicated EDCs in disorders of the prostate gland, the thyroid, and the neuroendocrine systems, the latter two being particularly vulnerable because of their role in hormone regulation at all stages of development.
“We’re particularly concerned about fetuses and how exposure can set the stage for later development of diseases,” Dr. Gore said, noting that human studies have shown a link between higher EDC exposures over time and cognitive deficits and other adverse neurocognitive outcomes.
Because the effect of EDC exposure differs according to the dose, length, and timing of exposure, designing studies to measure any harm has been difficult, said Dr. Gore, although in the past 5 years, there has been increased insight into the molecular mechanisms underlying EDCs.
Among the most common EDCs are bisphenol A (BPA) and phthalates, synthetic chemicals that bind to hormone receptors and depending upon the dose, either potentiate, inhibit – or both – the hormone’s effect on receptors. These EDCs frequently occur in toys, bottle nipples, rain coats, shower curtains, and in medical supplies such as blood bags, IV tubing, and catheters.
Initially registered as a pesticide, the EDC triclosan’s antimicrobial power has meant it is now used in deodorants and even in toothpaste. Triclosan has been shown to disrupt the thyroid, and to have antiestrogenic, and antiandrogenic properties. It also has been linked to asthma.
Plastic water bottles and disposable, plastic-based food packaging commonly found in microwaveable products often are high in BPAs, according to Dr. Gore, who urged all primary care physicians to counsel patients on the importance of avoiding products that contain them whenever possible, particularly in cases of pediatric obesity or diabetes, and for patients who are pregnant or in the family-planning stages. “You might not see an adverse outcome until years or decades later.”
Because many EDCs are lipophilic, our bodies often store them in our fat cells, often for years at a time.
While bisphenol A tends to exit the body quickly, we are commonly exposed to it on a daily basis, usually through a compound that leaches into our food, allowing the chemical an opportunity to exert an effect, even if the results of this effect aren’t immediately apparent. “If it’s a pregnant woman or someone planning a family, that exposure can change something,” Dr. Gore warned.
Although earlier studies had linked EDC exposure to a variety of reproductive health concerns, Dr. Gore said that since the Society’s last statement, there is much more corroborating evidence. In particular, polycystic ovarian syndrome in humans has been associated with higher body burdens of BPA and other chemicals, as have endometriosis, fibroids, and some adverse birth outcomes. Still, much of the data come from animal studies, and studies of specific links between EDCs and reproductive outcomes and cancers are inconsistent.
According to Dr. Renee Howard, a pediatric dermatologist with Sutter Health in San Francisco, who regularly gives presentations on the effects of EDCs in cosmetics and other skin care products, although much of the current research shows a link between EDCs and chronic illness, so far a causal relationship has not been established. Still, she said she routinely tells other physicians to keep an “open mind” when discussing EDCs with patients.
“We can acknowledge there is uncertainty, and we can tell patients that these chemicals are actively being studied, but that the studies are, as of now, inconclusive, and that there are still no documented adverse health effects associated with skin care products,” Dr. Howard said. Nevertheless, she said common sense dictates her to counsel patients to avoid products containing the EDC triclosan such as antimicrobials and scented products. She also advises them to eat organic produce in order to avoid pesticides.
Dr. Gore and her coauthors hope the statement will help bump EDC oversight higher up the policy chain globally.
“Exposure to endocrine disrupting chemicals during early development can have long-lasting, even permanent consequences,” Endocrine Society member Dr. Jean-Pierre Bourguignon, a professor of pediatric endocrinology at the University of Liège in Belgium, said in a statement. “The science is clear, and it’s time for policy makers to take this wealth of evidence into account as they develop legislation.”
To that end, earlier this year, twin bills are now before the House and Senate, which – if passed – will take effect in 2018, banning the sale of any personal care products containing microbeads, BPA-rich, microscopic plastic particles that have entered much of the natural water supply, threatening marine life which often mistake the tiny bits as food. Meanwhile, Minnesota has banned the use of triclosan statewide as of 2017.
Dr. Gore also said in addition to funding for this research being made a priority, it’s time to rethink who gets to be in on the science. Rather than just industrial chemists, she believes the teams should include so-called “green chemists,” basic, translational, and clinical scientists; epidemiologists, as well as public health professionals. Health care providers should be familiar with endocrine science and the latest developments in EDC research, accordingly, because, said Dr. Gore, “The [health] costs of EDCs have been estimated in the hundreds of millions of dollars. Prevention might seem expensive, but it’s far-less-expensive than all the ensuing diseases.”
Most of all, Dr. Gore and her colleagues emphasize that there will never be “absolute proof” of anything, but that taking action to stem exposure is essential.
The analysis was sponsored by the Endocrine Society. Dr. Gore is editor in chief of Endocrinology. Dr. Howard disclosed no relevant financial relationships.
On Twitter @whitneymcknight
Cash register receipts, tin can linings, and a range of cosmetics and other common household products increasingly are implicated in the most intractable of society’s diseases such as obesity and diabetes.
So-called endocrine-disrupting chemicals have become so common, according to the Washington-based Endocrine Society, that nearly every human alive has been exposed to at least one such chemical, probably more than once, and just as likely, over an extended period of time.
The World Health Organization reports that there are more than 800 known endocrine disrupting chemicals (EDCs) used in products globally, but only a fraction of them have been tested in humans. What effects, if any, this exposure is having on individuals is still unknown, but data linking the ability of EDCs – either singularly or in combination – to mimic, block, or otherwise interfere with the body’s natural hormone signaling has some experts sounding the alarm.
“The evidence is more definitive than ever before – EDCs disrupt hormones in a manner that harms human health,” Andrea C. Gore, Ph.D., a pharmacology professor at the University of Texas at Austin, said in a press conference. Dr. Gore chairs the Endocrine Society task force that recently released an executive summary of its second scientific statement on EDCs. The Society presented the statement, an update of one released in 2009, at this year’s International Conference on Chemicals Management annual meeting in Geneva.
Because the endocrine system’s role is to interact with the environment, it is predisposed to react to triggers increasingly found in everything from pesticides to shower curtains, which have now made their way into waterways and the food chain primarily without any studies on their impact. “Both natural hormones and EDCs have unique dose-response properties [and can] act at very-low doses,” Dr. Gore told reporters. “We’re exposed throughout our lives.”
The use of EDCs largely began post-WWII with pesticides such as DDT (dichlorodiphenyltrichloroethane), but over time came to include use as thickeners, plastic softeners, and scent in many common household items. Dr. Gore told a reporter that even though these chemicals were not intended to enter the environment at large, decades of use has made their entry into the food chain inevitable. The population health effects of this are only now becoming evident as data accumulate linking EDCs to a constellation of ill health effects.
“In humans, there are strong epidemiological associations between EDCs and chronic diseases.” Dr. Gore said.
She specifically cited obesity, diabetes, and a range of reproductive disorders, including infertility and certain hormone-related cancers. Research also shows a link between prenatal EDC exposure in animals to obesity, insulin resistance, and overabundant insulin later in life.
The Society’s meta-analysis of more than 1,300 studies published in the last 5 years also implicated EDCs in disorders of the prostate gland, the thyroid, and the neuroendocrine systems, the latter two being particularly vulnerable because of their role in hormone regulation at all stages of development.
“We’re particularly concerned about fetuses and how exposure can set the stage for later development of diseases,” Dr. Gore said, noting that human studies have shown a link between higher EDC exposures over time and cognitive deficits and other adverse neurocognitive outcomes.
Because the effect of EDC exposure differs according to the dose, length, and timing of exposure, designing studies to measure any harm has been difficult, said Dr. Gore, although in the past 5 years, there has been increased insight into the molecular mechanisms underlying EDCs.
Among the most common EDCs are bisphenol A (BPA) and phthalates, synthetic chemicals that bind to hormone receptors and depending upon the dose, either potentiate, inhibit – or both – the hormone’s effect on receptors. These EDCs frequently occur in toys, bottle nipples, rain coats, shower curtains, and in medical supplies such as blood bags, IV tubing, and catheters.
Initially registered as a pesticide, the EDC triclosan’s antimicrobial power has meant it is now used in deodorants and even in toothpaste. Triclosan has been shown to disrupt the thyroid, and to have antiestrogenic, and antiandrogenic properties. It also has been linked to asthma.
Plastic water bottles and disposable, plastic-based food packaging commonly found in microwaveable products often are high in BPAs, according to Dr. Gore, who urged all primary care physicians to counsel patients on the importance of avoiding products that contain them whenever possible, particularly in cases of pediatric obesity or diabetes, and for patients who are pregnant or in the family-planning stages. “You might not see an adverse outcome until years or decades later.”
Because many EDCs are lipophilic, our bodies often store them in our fat cells, often for years at a time.
While bisphenol A tends to exit the body quickly, we are commonly exposed to it on a daily basis, usually through a compound that leaches into our food, allowing the chemical an opportunity to exert an effect, even if the results of this effect aren’t immediately apparent. “If it’s a pregnant woman or someone planning a family, that exposure can change something,” Dr. Gore warned.
Although earlier studies had linked EDC exposure to a variety of reproductive health concerns, Dr. Gore said that since the Society’s last statement, there is much more corroborating evidence. In particular, polycystic ovarian syndrome in humans has been associated with higher body burdens of BPA and other chemicals, as have endometriosis, fibroids, and some adverse birth outcomes. Still, much of the data come from animal studies, and studies of specific links between EDCs and reproductive outcomes and cancers are inconsistent.
According to Dr. Renee Howard, a pediatric dermatologist with Sutter Health in San Francisco, who regularly gives presentations on the effects of EDCs in cosmetics and other skin care products, although much of the current research shows a link between EDCs and chronic illness, so far a causal relationship has not been established. Still, she said she routinely tells other physicians to keep an “open mind” when discussing EDCs with patients.
“We can acknowledge there is uncertainty, and we can tell patients that these chemicals are actively being studied, but that the studies are, as of now, inconclusive, and that there are still no documented adverse health effects associated with skin care products,” Dr. Howard said. Nevertheless, she said common sense dictates her to counsel patients to avoid products containing the EDC triclosan such as antimicrobials and scented products. She also advises them to eat organic produce in order to avoid pesticides.
Dr. Gore and her coauthors hope the statement will help bump EDC oversight higher up the policy chain globally.
“Exposure to endocrine disrupting chemicals during early development can have long-lasting, even permanent consequences,” Endocrine Society member Dr. Jean-Pierre Bourguignon, a professor of pediatric endocrinology at the University of Liège in Belgium, said in a statement. “The science is clear, and it’s time for policy makers to take this wealth of evidence into account as they develop legislation.”
To that end, earlier this year, twin bills are now before the House and Senate, which – if passed – will take effect in 2018, banning the sale of any personal care products containing microbeads, BPA-rich, microscopic plastic particles that have entered much of the natural water supply, threatening marine life which often mistake the tiny bits as food. Meanwhile, Minnesota has banned the use of triclosan statewide as of 2017.
Dr. Gore also said in addition to funding for this research being made a priority, it’s time to rethink who gets to be in on the science. Rather than just industrial chemists, she believes the teams should include so-called “green chemists,” basic, translational, and clinical scientists; epidemiologists, as well as public health professionals. Health care providers should be familiar with endocrine science and the latest developments in EDC research, accordingly, because, said Dr. Gore, “The [health] costs of EDCs have been estimated in the hundreds of millions of dollars. Prevention might seem expensive, but it’s far-less-expensive than all the ensuing diseases.”
Most of all, Dr. Gore and her colleagues emphasize that there will never be “absolute proof” of anything, but that taking action to stem exposure is essential.
The analysis was sponsored by the Endocrine Society. Dr. Gore is editor in chief of Endocrinology. Dr. Howard disclosed no relevant financial relationships.
On Twitter @whitneymcknight
Long-acting insulin degludec – and degludec combo drug – win FDA nod
Two new insulins have gained FDA approval.
Insulin degludec (Tresiba) and insulin degludec/insulin aspart (Ryzodeg 70/30) – both manufactured by Novo Nordisk – have been shown to improve glucose control in adults with hard-to-regulate type 1 diabetes, and in patients with advanced type 2 diabetes, according to the Food and Drug Administration statement announcing the approvals on Sept. 25.
Insulin degludec is intended to be used as add-on therapy to prandial insulin or oral antidiabetic drugs. Findings from 9 randomized studies comprising almost 4,000 patients supported the approval: three in patients with type 1 disease and six in patients with type 2 disease.
Three trials comprising 1,102 patients with type 1 diabetes examined degludec in combination with prandial insulin or oral therapy. Six trials comprising 2,702 patients with type 2 diabetes also examined the drug in combination with prandial insulin or as an add-on to oral therapy. In all of these studies, degludec was associated with reductions in HbA1c.
The combination of the long-acting insulin degludec and rapid-acting insulin aspart was evaluated in five studies – one with 362 patients with type 1 diabetes and four comprising 998 patients with type 2 disease.
In the first study, the drug was used with prandial insulin. In the second group of studies, it was administered once or twice a day as the sole treatment.
Both Tresiba and Ryzodeg have been approved in Europe, Mexico, and Japan.
The most common adverse reactions associated with both drugs in clinical trials were hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, itching, rash, edema, and weight gain, according to the FDA.
In 2013, FDA refused to approve either of these drugs, despite a positive recommendation from the review committee. The agency asked for additional cardiovascular risk data, which Novo Nordisk is now acquiring with a 7,600-patient study called DEVOTE. DEVOTE is set to be completed in 2016; it is designed to generate follow-up data for 2 additional years.
In April, the company resubmitted its new drug application based on the study’s interim results, which are not publicly available.
Two new insulins have gained FDA approval.
Insulin degludec (Tresiba) and insulin degludec/insulin aspart (Ryzodeg 70/30) – both manufactured by Novo Nordisk – have been shown to improve glucose control in adults with hard-to-regulate type 1 diabetes, and in patients with advanced type 2 diabetes, according to the Food and Drug Administration statement announcing the approvals on Sept. 25.
Insulin degludec is intended to be used as add-on therapy to prandial insulin or oral antidiabetic drugs. Findings from 9 randomized studies comprising almost 4,000 patients supported the approval: three in patients with type 1 disease and six in patients with type 2 disease.
Three trials comprising 1,102 patients with type 1 diabetes examined degludec in combination with prandial insulin or oral therapy. Six trials comprising 2,702 patients with type 2 diabetes also examined the drug in combination with prandial insulin or as an add-on to oral therapy. In all of these studies, degludec was associated with reductions in HbA1c.
The combination of the long-acting insulin degludec and rapid-acting insulin aspart was evaluated in five studies – one with 362 patients with type 1 diabetes and four comprising 998 patients with type 2 disease.
In the first study, the drug was used with prandial insulin. In the second group of studies, it was administered once or twice a day as the sole treatment.
Both Tresiba and Ryzodeg have been approved in Europe, Mexico, and Japan.
The most common adverse reactions associated with both drugs in clinical trials were hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, itching, rash, edema, and weight gain, according to the FDA.
In 2013, FDA refused to approve either of these drugs, despite a positive recommendation from the review committee. The agency asked for additional cardiovascular risk data, which Novo Nordisk is now acquiring with a 7,600-patient study called DEVOTE. DEVOTE is set to be completed in 2016; it is designed to generate follow-up data for 2 additional years.
In April, the company resubmitted its new drug application based on the study’s interim results, which are not publicly available.
Two new insulins have gained FDA approval.
Insulin degludec (Tresiba) and insulin degludec/insulin aspart (Ryzodeg 70/30) – both manufactured by Novo Nordisk – have been shown to improve glucose control in adults with hard-to-regulate type 1 diabetes, and in patients with advanced type 2 diabetes, according to the Food and Drug Administration statement announcing the approvals on Sept. 25.
Insulin degludec is intended to be used as add-on therapy to prandial insulin or oral antidiabetic drugs. Findings from 9 randomized studies comprising almost 4,000 patients supported the approval: three in patients with type 1 disease and six in patients with type 2 disease.
Three trials comprising 1,102 patients with type 1 diabetes examined degludec in combination with prandial insulin or oral therapy. Six trials comprising 2,702 patients with type 2 diabetes also examined the drug in combination with prandial insulin or as an add-on to oral therapy. In all of these studies, degludec was associated with reductions in HbA1c.
The combination of the long-acting insulin degludec and rapid-acting insulin aspart was evaluated in five studies – one with 362 patients with type 1 diabetes and four comprising 998 patients with type 2 disease.
In the first study, the drug was used with prandial insulin. In the second group of studies, it was administered once or twice a day as the sole treatment.
Both Tresiba and Ryzodeg have been approved in Europe, Mexico, and Japan.
The most common adverse reactions associated with both drugs in clinical trials were hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, itching, rash, edema, and weight gain, according to the FDA.
In 2013, FDA refused to approve either of these drugs, despite a positive recommendation from the review committee. The agency asked for additional cardiovascular risk data, which Novo Nordisk is now acquiring with a 7,600-patient study called DEVOTE. DEVOTE is set to be completed in 2016; it is designed to generate follow-up data for 2 additional years.
In April, the company resubmitted its new drug application based on the study’s interim results, which are not publicly available.
In type 2 diabetes, pump therapy costs more but saves much
STOCKHOLM – Insulin pump therapy is considerably more expensive than daily insulin injections for patients with type 2 diabetes, but the benefits it confers offset about 30% of the price difference.
A cost-modeling study showed that patients who use the pump gain almost another year of life expectancy and experience a delay in the onset of major complications, such as end-stage renal disease and amputation, Stephane Roze said at the annual meeting of the European Association for the Study of Diabetes.
Pump therapy also conferred a significantly improved quality of life over multiple daily injections, a benefit that carries its own economic value, said Mr. Roze of HEVA-HEOR, a health economics evaluation company in Lyon, France.
“Even though these patients are initiating the pump at an older age than patients with type 1 diabetes, the therapy still looks to be cost effective,” he said. “It’s a good investment for payers.”
Mr. Roze used the CORE Diabetes Model to examine the cost/benefit ratio of insulin pump therapy, compared with multiple daily injections in patients with poorly controlled type 2 diabetes who lived in the Netherlands. The CORE model comprises 14 sub-Markov models that run in parallel; it examines all of the micro- and macrovascular complications related to diabetes. Model input data were the cohort characteristics and 6-month clinical outcomes of the OpT2mise study.
The trial examined glucose variability in 331 patients with type 2 diabetes who were randomized to either pump therapy or daily insulin injections.
It is an excellent study for cost-modeling initiatives, Mr. Roze said. It’s multicenter and multinational, conducted in 36 institutions in the United States, Canada, Europe, Israel, and South Africa. The cohort is large and diverse. Importantly, all of the patients had their treatment optimized with daily insulin analogue injections before being randomized. That initial step lent credence to their baseline glucose control; despite optimization, they still had a mean hemoglobin A1cof 9%. The OpT2mise patients were a mean of 56 years old, with mean disease duration of 15 years.
At 6 months, the study showed that HbA1c had decreased significantly more in the pump group than in the injection group (1.1% vs. 0.4%). Patients using the pump also needed significantly lower daily insulin doses (97 unit/kg per day vs. 122 unit/kg per day).
These results were inputted into the CORE model, along with the costs of diabetes-related complications in the Netherlands and the costs of both pump therapy and daily insulin injections. For the first year of treatment, pump therapy more than twice as expensive as daily injections (4,407 euros vs. 1,597 euros). This difference remained when costs were extrapolated to year 2 and beyond (4,365 euros vs. 1,555 euros).
The model, however, determined that pump therapy has the potential to extend life expectancy by almost 1 year, compared with injection therapy, by reducing and/or delaying life-threatening diabetes complications. These clinical benefits translated into an additional 9.38 quality-adjusted life-years (QALY) for patients using the pump, compared with 8.95 QALY for patients using injections.
Over a lifetime of treatment (13 years), pump therapy would cost a total of 58,024 euros, compared with 20,237 euros for injection therapy – a difference of 37,787 euros. Compared with injection therapy, though, pump therapy would save almost 10,000 euros in the cost of renal complications; 625 euros in the cost of ulcer, amputation, and neuropathy complications; 152 euros in the cost of eye complications; and 410 euros in complications due to hypoglycemia. These savings reduced the total cost difference between the two to 27,051 euros, Mr. Roze said.
The benefits held over time, despite the paradox of survivorship, he said. “The longer a patient with chronic disease lives, the more opportunity they have to develop a complication.”
The incremental cost-effectiveness ratio was 62,295 euros/QALY – an amount that falls well within the Netherlands’ “willingness to pay” threshold, which is a measure of what payers or society are generally willing to accept as a good value for health care expenditures.
The newest data from OpT2mise, also released at EASD, bolster Mr. Roze’s modeling. In phase II of the study, patients who had been on injections crossed over to pump therapy. Within 6 months of crossover, these patients experienced a mean HbA1c decrease of 0.8%. By 12 months from study start, when all patients had been on pump therapy for at least 6 months, the mean HbA1chad dropped to 7.8% from baseline.
“We were reassured to see the 12-month data, which confirm the sustainability of the effect,” Mr. Roze said. “This is one of the main questions when you do health care modeling.”
Mr. Roze is a co-owner of HEVA-HEOR.
STOCKHOLM – Insulin pump therapy is considerably more expensive than daily insulin injections for patients with type 2 diabetes, but the benefits it confers offset about 30% of the price difference.
A cost-modeling study showed that patients who use the pump gain almost another year of life expectancy and experience a delay in the onset of major complications, such as end-stage renal disease and amputation, Stephane Roze said at the annual meeting of the European Association for the Study of Diabetes.
Pump therapy also conferred a significantly improved quality of life over multiple daily injections, a benefit that carries its own economic value, said Mr. Roze of HEVA-HEOR, a health economics evaluation company in Lyon, France.
“Even though these patients are initiating the pump at an older age than patients with type 1 diabetes, the therapy still looks to be cost effective,” he said. “It’s a good investment for payers.”
Mr. Roze used the CORE Diabetes Model to examine the cost/benefit ratio of insulin pump therapy, compared with multiple daily injections in patients with poorly controlled type 2 diabetes who lived in the Netherlands. The CORE model comprises 14 sub-Markov models that run in parallel; it examines all of the micro- and macrovascular complications related to diabetes. Model input data were the cohort characteristics and 6-month clinical outcomes of the OpT2mise study.
The trial examined glucose variability in 331 patients with type 2 diabetes who were randomized to either pump therapy or daily insulin injections.
It is an excellent study for cost-modeling initiatives, Mr. Roze said. It’s multicenter and multinational, conducted in 36 institutions in the United States, Canada, Europe, Israel, and South Africa. The cohort is large and diverse. Importantly, all of the patients had their treatment optimized with daily insulin analogue injections before being randomized. That initial step lent credence to their baseline glucose control; despite optimization, they still had a mean hemoglobin A1cof 9%. The OpT2mise patients were a mean of 56 years old, with mean disease duration of 15 years.
At 6 months, the study showed that HbA1c had decreased significantly more in the pump group than in the injection group (1.1% vs. 0.4%). Patients using the pump also needed significantly lower daily insulin doses (97 unit/kg per day vs. 122 unit/kg per day).
These results were inputted into the CORE model, along with the costs of diabetes-related complications in the Netherlands and the costs of both pump therapy and daily insulin injections. For the first year of treatment, pump therapy more than twice as expensive as daily injections (4,407 euros vs. 1,597 euros). This difference remained when costs were extrapolated to year 2 and beyond (4,365 euros vs. 1,555 euros).
The model, however, determined that pump therapy has the potential to extend life expectancy by almost 1 year, compared with injection therapy, by reducing and/or delaying life-threatening diabetes complications. These clinical benefits translated into an additional 9.38 quality-adjusted life-years (QALY) for patients using the pump, compared with 8.95 QALY for patients using injections.
Over a lifetime of treatment (13 years), pump therapy would cost a total of 58,024 euros, compared with 20,237 euros for injection therapy – a difference of 37,787 euros. Compared with injection therapy, though, pump therapy would save almost 10,000 euros in the cost of renal complications; 625 euros in the cost of ulcer, amputation, and neuropathy complications; 152 euros in the cost of eye complications; and 410 euros in complications due to hypoglycemia. These savings reduced the total cost difference between the two to 27,051 euros, Mr. Roze said.
The benefits held over time, despite the paradox of survivorship, he said. “The longer a patient with chronic disease lives, the more opportunity they have to develop a complication.”
The incremental cost-effectiveness ratio was 62,295 euros/QALY – an amount that falls well within the Netherlands’ “willingness to pay” threshold, which is a measure of what payers or society are generally willing to accept as a good value for health care expenditures.
The newest data from OpT2mise, also released at EASD, bolster Mr. Roze’s modeling. In phase II of the study, patients who had been on injections crossed over to pump therapy. Within 6 months of crossover, these patients experienced a mean HbA1c decrease of 0.8%. By 12 months from study start, when all patients had been on pump therapy for at least 6 months, the mean HbA1chad dropped to 7.8% from baseline.
“We were reassured to see the 12-month data, which confirm the sustainability of the effect,” Mr. Roze said. “This is one of the main questions when you do health care modeling.”
Mr. Roze is a co-owner of HEVA-HEOR.
STOCKHOLM – Insulin pump therapy is considerably more expensive than daily insulin injections for patients with type 2 diabetes, but the benefits it confers offset about 30% of the price difference.
A cost-modeling study showed that patients who use the pump gain almost another year of life expectancy and experience a delay in the onset of major complications, such as end-stage renal disease and amputation, Stephane Roze said at the annual meeting of the European Association for the Study of Diabetes.
Pump therapy also conferred a significantly improved quality of life over multiple daily injections, a benefit that carries its own economic value, said Mr. Roze of HEVA-HEOR, a health economics evaluation company in Lyon, France.
“Even though these patients are initiating the pump at an older age than patients with type 1 diabetes, the therapy still looks to be cost effective,” he said. “It’s a good investment for payers.”
Mr. Roze used the CORE Diabetes Model to examine the cost/benefit ratio of insulin pump therapy, compared with multiple daily injections in patients with poorly controlled type 2 diabetes who lived in the Netherlands. The CORE model comprises 14 sub-Markov models that run in parallel; it examines all of the micro- and macrovascular complications related to diabetes. Model input data were the cohort characteristics and 6-month clinical outcomes of the OpT2mise study.
The trial examined glucose variability in 331 patients with type 2 diabetes who were randomized to either pump therapy or daily insulin injections.
It is an excellent study for cost-modeling initiatives, Mr. Roze said. It’s multicenter and multinational, conducted in 36 institutions in the United States, Canada, Europe, Israel, and South Africa. The cohort is large and diverse. Importantly, all of the patients had their treatment optimized with daily insulin analogue injections before being randomized. That initial step lent credence to their baseline glucose control; despite optimization, they still had a mean hemoglobin A1cof 9%. The OpT2mise patients were a mean of 56 years old, with mean disease duration of 15 years.
At 6 months, the study showed that HbA1c had decreased significantly more in the pump group than in the injection group (1.1% vs. 0.4%). Patients using the pump also needed significantly lower daily insulin doses (97 unit/kg per day vs. 122 unit/kg per day).
These results were inputted into the CORE model, along with the costs of diabetes-related complications in the Netherlands and the costs of both pump therapy and daily insulin injections. For the first year of treatment, pump therapy more than twice as expensive as daily injections (4,407 euros vs. 1,597 euros). This difference remained when costs were extrapolated to year 2 and beyond (4,365 euros vs. 1,555 euros).
The model, however, determined that pump therapy has the potential to extend life expectancy by almost 1 year, compared with injection therapy, by reducing and/or delaying life-threatening diabetes complications. These clinical benefits translated into an additional 9.38 quality-adjusted life-years (QALY) for patients using the pump, compared with 8.95 QALY for patients using injections.
Over a lifetime of treatment (13 years), pump therapy would cost a total of 58,024 euros, compared with 20,237 euros for injection therapy – a difference of 37,787 euros. Compared with injection therapy, though, pump therapy would save almost 10,000 euros in the cost of renal complications; 625 euros in the cost of ulcer, amputation, and neuropathy complications; 152 euros in the cost of eye complications; and 410 euros in complications due to hypoglycemia. These savings reduced the total cost difference between the two to 27,051 euros, Mr. Roze said.
The benefits held over time, despite the paradox of survivorship, he said. “The longer a patient with chronic disease lives, the more opportunity they have to develop a complication.”
The incremental cost-effectiveness ratio was 62,295 euros/QALY – an amount that falls well within the Netherlands’ “willingness to pay” threshold, which is a measure of what payers or society are generally willing to accept as a good value for health care expenditures.
The newest data from OpT2mise, also released at EASD, bolster Mr. Roze’s modeling. In phase II of the study, patients who had been on injections crossed over to pump therapy. Within 6 months of crossover, these patients experienced a mean HbA1c decrease of 0.8%. By 12 months from study start, when all patients had been on pump therapy for at least 6 months, the mean HbA1chad dropped to 7.8% from baseline.
“We were reassured to see the 12-month data, which confirm the sustainability of the effect,” Mr. Roze said. “This is one of the main questions when you do health care modeling.”
Mr. Roze is a co-owner of HEVA-HEOR.
AT EASD 2015
Key clinical point: The benefits of insulin pump therapy for patients with type 2 diabetes make up for about 30% of the increased cost compared with insulin injections.
Major finding: Insulin pump therapy saved money by adding almost a year of life expectancy for patients with type 2 diabetes and delaying the onset of micro- and macrovascular complications.
Data source: The cost-effectiveness model was based on OpT2mise, which randomized 331 patients with type 2 diabetes to pump therapy or daily insulin injections.
Disclosures: Mr. Roze is a co-owner of HEVA-HEOR.
Diabetic foot ulcer: Early closure post debridement best
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
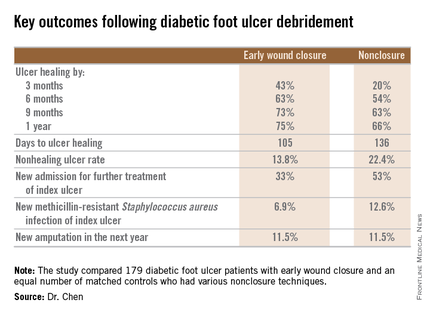
He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
AT ICAAC 2015
Key clinical point: Diabetic foot ulcers are more likely to heal with early wound closure following surgical debridement than with nonclosure techniques.
Major finding: Healing of diabetic foot ulcers after surgical debridement took an average of 105 days in patients who underwent early wound closure prior to hospital discharge, compared with 136 days in those whose wounds were managed with nonclosure techniques.
Data source: A retrospective, nonrandomized study featuring two propensity score–matched groups, with 179 patients in each, who were followed for 1 year post discharge for surgical debridement of a diabetic foot ulcer.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
EASD: SGLT2 inhibitor empagliflozin cuts CV risk in patients with type 2 diabetes
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
The EMPA-REG OUTCOME study results are spectacular in terms of the hard endpoints that were measured and the explicit reductions seen. It is exciting news because we have had quite a few negative trials with DPP4-inhibtiors where nothing much has happened and we don’t have cardiovascular outcomes. So that’s the upside, and that’s very exciting.
The downside has to be that the population studied included people who were post MI or were at higher cardiovascular risk than the general population with type 2 diabetes. So the study participants were people who are way into their diabetes pathology and are already having cardiovascular events. While it is of course good that these patients greatly benefit, one must not start to generalize and say that this shows that everyone with type 2 diabetes ought to be on an SGLT2 inhibitor.
In the future, I think one has to be very careful about the way that trials are set up and about who has gone into those trials. We still do not know very much about people who are newly onset with type 2 diabetes and if the SGLT2 inhibitors will be useful for them generally, but it is very nice to have positive outcomes in the group of patients studied.
These are big effects, which, as clinicians, we all like to see, and these are unlikely to be by chance because they are so big. The EMPA-REG OUTCOME findings clearly did not occur by chance. This is a really positive and exciting outcome for empagliflozin and it will affect the way people treat their patients. But the warning is that we must not generalize from the specifics of this trial in terms of who the patients treated were: they tended to be male, they were all people who had major events.
These remarks come from an interview with Dr. David Matthews, conducted onsite at EASD 2015 and exclusive to this news organization. Dr. Matthews is professor of diabetes and emeritus founding chairman, Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, England. He is the coinvestigator for a Janssen-sponsored canagliflozin trial and has received honoraria and support from Janssen.
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
STOCKHOLM – The combined risk for cardiovascular death, nonfatal MI, and nonfatal stroke was reduced by 14% when empagliflozin was added to standard care for type 2 diabetes in patients at high cardiovascular risk, compared with standard care alone, in the EMPA-REG OUTCOME study.
This primary composite endpoint was reached by 10.5% of the 4,687 patients treated with the antidiabetic drug versus 12.1% of the 2,333 patients given a placebo, with a hazard ratio (HR) of 0.86 and 95% confidence interval (CI) of 0.74 to 0.99 (P = .04 for superiority and P less than .001 for noninferiority).
The results of the EMPA-REG OUTCOME study, which were reported for the first time at the annual meeting of the European Association for the Study of Diabetes, were published simultaneously Sept. 17 online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1504720).
Dr. Silvio Inzucchi, professor of medicine at Yale University, New Haven, Conn., and the study investigator who presented the key outcome findings, said during the Q&A session that this was the first presentation of these data and that of course there had to be a little bit of caution before making any firm recommendations.
“It is really important that the diabetes and cardiology communities digest [these data], to try to understand them, and that we also have to be cautious here. This was a group of patients with 10 years’ duration of diabetes, all of whom had cardiovascular disease, so we can not necessarily ‘cut and paste’ these expectations to the entirety of the diabetes population.”
Putting the results into context ahead of their presentation in detail at the meeting, Dr. Naveed Sattar of the University of Glasgow, Scotland, said in an interview that there had been four other cardiovascular outcomes trials in this high-risk population of patients with type 2 diabetes and cardiovascular disease – three with DPP-4 inhibitors, namely the SAVOR-TIMI 53 trial, the EXAMINEtrial, and the recent TECOS. The fourth such trial involved a GLP-1 antagonist (ELIXA). All of these had shown modest or small additional reductions in blood glucose levels with the tested drugs versus standard of care or placebo control but had “shown unequivocally no benefit in [reducing] cardiovascular events.” Cardiovascular effects had simply been neutral or no cause for concern.
So to now have a trial with a drug that is potentially more potent at lowering blood glucose than the others and is showing a cardiovascular benefit is potentially game changing, said Dr. Sattar, professor of metabolic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow.
Evidence suggests that empagliflozin does more than just lower blood glucose, said Dr. Sattar, who was not involved in the EMPA-REG trial. “Empagliflozin lowers blood pressure, it reduces weight, so is it these added effects that have conferred this benefit or is it something else?” He added: “Most people’s perception is that it is possibly a class effect, but the data need to be seen.”
Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, singled out the EMPA-REG study as one to watch. Speaking in an interview at the annual meeting of the European Society of Cardiology in London, he said that if the cardiovascular risk reduction touted were true it would make empagliflozin “the only modern glucose-lowering drug to have shown such a capacity.”
EMPA-REG OUTCOME was a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin, given at an oral dose of 10 mg/day or 25 mg/day compared to the best usual care in patients with type 2 diabetes who were at increased cardiovascular risk due to the presence of established cardiovascular disease. The study was done at 590 sites in 42 countries across six continents and involved more than 7,000 patients observed over a median of 3.1 years.
Looking at the individual components of the primary composite endpoint, there was no significant difference in terms of the relative risk reduction for MI or stroke. However, the unexpected results were that cardiovascular death, hospitalization for heart failure, and all-cause mortality were all reduced by more than one-third, with respective relative risk reductions of 38% (HR, 0.62; 95% CI, 0.49-0.77; P less than .001), 35% (HR, 0.68; 95% CI, 0.57-0.82; P less than .001), and 32% (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Even when the results were shown separating out the two doses of empagliflozin, the HR remained highly significant for these and the primary composite endpoint.
The key secondary outcome of the trial was a composite of the primary outcome plus hospitalization for unstable angina. However, no significant difference for superiority was seen between the groups (P = .008, P less than .001 for noninferiority).
“I am here because of the importance of this,” said Dr. Andrew Boulton outgoing president of the EASD and professor of medicine at the University of Manchester, England, who introduced the EASD-sponsored symposium where the results were revealed.
In an interview, Dr. Boulton said it “looks promising.”
The results were presented by the senior authors of the study and independently commented upon afterward by the EASD-invited discussant Dr. Hertzel Gerstein of McMaster University and Hamilton (Ont.) Health Sciences. “Without question, the EMPA-REG investigators have designed a good protocol, and they have answered a very important question,” he said. “This was a well done and very important study,” he noted.
Dr. Gerstein observed that empagliflozin “clearly reduces CV death and heart failure hospitalization,” an effect that starts to appear after just 3 months. This early effect is unlikely to be down to just glucose-lowering, antihypertensive effects of the background medications used, or weight loss because of this speed of onset. It could be due to the diuretic properties of the SLGT2 inhibitor or, maybe more likely, a combination of beneficial effects. “It is probably a class effect,” he said, “but you can never be certain.”
Based on the findings, he suggested that empagliflozin “may become first-line treatment for middle aged people with type 2 diabetes at risk for CV outcomes” and that the trial had “identified a treatment that could save many lives and reduce much suffering.” The results were unexpected and will open up new avenues for research.
Dr. Bernard Zinman of Mount Sinai Hospital, Toronto, lead author for the trial, said during the session that the number of patients needed to be treated with empagliflozin for 3 years to prevent one cardiovascular death was 39. By comparison, he said, the numbers needed to treat with simvastatin or ramipril for 5 years were 30 and 59, respectively.
Good safety was observed in the trial, reported Dr. David Fitchett, fellow author. Dr. Fitchett, a cardiologist at St Michael’s Hospital and associate professor at the University of Toronto, noted that overall rates of both any adverse events and serious adverse events were similar between the groups, with the exception of genital infections that were mainly mycoses and mostly in women.
Rates of confirmed hypoglycemic events were similar, he said, and there was no increase in diabetic ketoacidosis or bone fracture, which have been the focus of recent warnings issued by the Food and Drug Administration with regard to SLGT2 inhibitors.
Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
AT EASD 2015
Key clinical point: Empagliflozin reduced cardiovascular risk in patients with type 2 diabetes who were at high cardiovascular risk, making it the first antidiabetic drug to have been shown to have such an effect.
Major finding: This primary composite endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke was reached by 10.5% (N = 4,687) of patients treated with empagliflozin versus 12.1% (N = 2,333) of those given placebo.
Data source: EMPA-REG OUTCOME: a phase III, international, multicenter, randomized, parallel group, double-blind cardiovascular safety study of empagliflozin added to usual care versus usual care alone in more than 7,000 patients with type 2 diabetes at high cardiovascular risk.
Disclosures: Boehringer Ingelheim and Eli Lilly funded the study. Dr. Sattar disclosed ties with Amgen, Sanofi, and Merck Sharp & Dohme. Dr. Sattar and Dr. Rydén were on the panel of experts invited to talk about the study in a satellite symposium sponsored by Boehringer Ingelheim and Eli Lilly. Dr. Boulton did not report having disclosures. Dr. Gerstein reported ties with Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Abbot, Bayer, Berlin Chemie, Roche, GSK, Amgen, and Kaneq Bioscience. Dr. Zinman disclosed ties with Boehringer Ingelheim. Dr. Fitchett disclosed consulting for Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Merck Sharp & Dohme. Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
When stepping up type 2 treatment, GLP-1 agonists have the edge
STOCKHOLM – Patients with poorly controlled type 2 diabetes are more likely to hit glycemic targets if their add-on therapy is a GLP-1 receptor agonist.
The GLP-1 drugs seemed slightly more effective than adding a bolus of premixed insulin; data from a recent study show that after receiving a GLP-1 receptor agonist as an add-on drug, patients achieved a mean hemoglobin A1c of 7.4% within 6 months of treatment intensification, Dr. Reimar W. Thomsen said at the annual meeting of the European Association for the Study of Diabetes. But his Danish database study wasn’t able to control for socioeconomic factors, which may influence whether patients are able to get that more expensive class of drug in Denmark.
Nevertheless, the study provides a “real-world” look at the management of patients who don’t hit therapeutic goals on basal insulin only, said Dr. Thomsen of Aarhus University, Denmark. In the cohort of 7,000 patients, fewer than half were able to reach an HbA1c of 7.5% or lower on the single treatment.
The patients were drawn from Danish national health care databases, and diagnosed with type 2 diabetes from 2000 to 2012. They were a mean of 64 years old with a mean baseline HbA1c of 9.2%. Although newly diagnosed, they had probably had the disease for a while – 26% had macrovascular complications and 47%, microvascular disease. All were started on basal insulin monotherapy. Within 6 months, the mean HbA1c had dropped to 7.6%.
However, just 45% of the group hit the treatment goal of 7.5% or lower, with 29% attaining the 7% HbA1c target.
A temporal assessment of response showed some improvements over the course of the study, Dr. Thomsen noted. In 2000-2003, about 25% hit the goal on basal insulin only; by 2010-2012, that had increased to 33%. This probably reflects an earlier diagnosis of disease, especially as the mean HbA1c at diagnosis was lower in the later years (9% vs.9.6%), he said.
In another noteworthy temporal association, choices for add-on therapy changed over the years. In the first few years, premixed insulin was the top choice, accounting for 80% of intensification prescriptions. By 2012, that had dropped to about 22%. Bolus insulin use rose from about 17% in 2000 to 35% in 2012. GLP-1 receptor agonists didn’t arrive on the scene until 2008 but gained rapid acceptance. By 2010-2012 they accounted for 30% of intensification prescriptions.
Patients whose glucose control was intensified with GLP-1 drugs (326) were the youngest (55 years) and healthiest, with only 22% having medical comorbidities. They started the add-on therapy at a mean of 27 months after diagnosis. At that time, the mean HbA1c was 8.4%; this dropped by 0.8%, landing at a mean of 7.6%.
Those who added bolus insulin (893) were a mean of 59 years old; 30% had comorbidities. They started the new treatment at a mean of 13 months after diagnosis. Their mean HbA1c at intensification was 8.2%; this dropped 0.4%, landing at 7.8%
Patients who added premixed insulin (1,798) were a mean of 64 years old; 32% had medical comorbidities. They started add-on therapy 11 months after diagnosis. Their HbA1c was a mean of 8.8% at intensification; it dropped 0.9%, landing at a mean of 7.9%.
Fifty-nine patients were intensified with dual therapy; Dr. Thomsen did not specify what combinations were employed. These patients were a mean of 61 years old; 42% of them had medical comorbidities. They had a mean HbA1c of 8.9% at the time of intensification. Add-on therapy dropped that measurement by 1.2%, landing this group at a mean HbA1c of 7.7%.
A multivariate analysis determined the likelihood of attaining the treatment target with the various add-on monotherapies; it adjusted for age, gender, diabetes complications, disease duration, medical comorbidities, and baseline HbA1c. Compared with premixed insulin, the chance of meeting goal was similar with bolus insulin (relative risk, 1.03) and higher with GLP-1 agonists (RR, 1.56 for less than 7%; RR, 1.27 for less than 7.5%).
Dr. Thomsen had no financial disclosures.
STOCKHOLM – Patients with poorly controlled type 2 diabetes are more likely to hit glycemic targets if their add-on therapy is a GLP-1 receptor agonist.
The GLP-1 drugs seemed slightly more effective than adding a bolus of premixed insulin; data from a recent study show that after receiving a GLP-1 receptor agonist as an add-on drug, patients achieved a mean hemoglobin A1c of 7.4% within 6 months of treatment intensification, Dr. Reimar W. Thomsen said at the annual meeting of the European Association for the Study of Diabetes. But his Danish database study wasn’t able to control for socioeconomic factors, which may influence whether patients are able to get that more expensive class of drug in Denmark.
Nevertheless, the study provides a “real-world” look at the management of patients who don’t hit therapeutic goals on basal insulin only, said Dr. Thomsen of Aarhus University, Denmark. In the cohort of 7,000 patients, fewer than half were able to reach an HbA1c of 7.5% or lower on the single treatment.
The patients were drawn from Danish national health care databases, and diagnosed with type 2 diabetes from 2000 to 2012. They were a mean of 64 years old with a mean baseline HbA1c of 9.2%. Although newly diagnosed, they had probably had the disease for a while – 26% had macrovascular complications and 47%, microvascular disease. All were started on basal insulin monotherapy. Within 6 months, the mean HbA1c had dropped to 7.6%.
However, just 45% of the group hit the treatment goal of 7.5% or lower, with 29% attaining the 7% HbA1c target.
A temporal assessment of response showed some improvements over the course of the study, Dr. Thomsen noted. In 2000-2003, about 25% hit the goal on basal insulin only; by 2010-2012, that had increased to 33%. This probably reflects an earlier diagnosis of disease, especially as the mean HbA1c at diagnosis was lower in the later years (9% vs.9.6%), he said.
In another noteworthy temporal association, choices for add-on therapy changed over the years. In the first few years, premixed insulin was the top choice, accounting for 80% of intensification prescriptions. By 2012, that had dropped to about 22%. Bolus insulin use rose from about 17% in 2000 to 35% in 2012. GLP-1 receptor agonists didn’t arrive on the scene until 2008 but gained rapid acceptance. By 2010-2012 they accounted for 30% of intensification prescriptions.
Patients whose glucose control was intensified with GLP-1 drugs (326) were the youngest (55 years) and healthiest, with only 22% having medical comorbidities. They started the add-on therapy at a mean of 27 months after diagnosis. At that time, the mean HbA1c was 8.4%; this dropped by 0.8%, landing at a mean of 7.6%.
Those who added bolus insulin (893) were a mean of 59 years old; 30% had comorbidities. They started the new treatment at a mean of 13 months after diagnosis. Their mean HbA1c at intensification was 8.2%; this dropped 0.4%, landing at 7.8%
Patients who added premixed insulin (1,798) were a mean of 64 years old; 32% had medical comorbidities. They started add-on therapy 11 months after diagnosis. Their HbA1c was a mean of 8.8% at intensification; it dropped 0.9%, landing at a mean of 7.9%.
Fifty-nine patients were intensified with dual therapy; Dr. Thomsen did not specify what combinations were employed. These patients were a mean of 61 years old; 42% of them had medical comorbidities. They had a mean HbA1c of 8.9% at the time of intensification. Add-on therapy dropped that measurement by 1.2%, landing this group at a mean HbA1c of 7.7%.
A multivariate analysis determined the likelihood of attaining the treatment target with the various add-on monotherapies; it adjusted for age, gender, diabetes complications, disease duration, medical comorbidities, and baseline HbA1c. Compared with premixed insulin, the chance of meeting goal was similar with bolus insulin (relative risk, 1.03) and higher with GLP-1 agonists (RR, 1.56 for less than 7%; RR, 1.27 for less than 7.5%).
Dr. Thomsen had no financial disclosures.
STOCKHOLM – Patients with poorly controlled type 2 diabetes are more likely to hit glycemic targets if their add-on therapy is a GLP-1 receptor agonist.
The GLP-1 drugs seemed slightly more effective than adding a bolus of premixed insulin; data from a recent study show that after receiving a GLP-1 receptor agonist as an add-on drug, patients achieved a mean hemoglobin A1c of 7.4% within 6 months of treatment intensification, Dr. Reimar W. Thomsen said at the annual meeting of the European Association for the Study of Diabetes. But his Danish database study wasn’t able to control for socioeconomic factors, which may influence whether patients are able to get that more expensive class of drug in Denmark.
Nevertheless, the study provides a “real-world” look at the management of patients who don’t hit therapeutic goals on basal insulin only, said Dr. Thomsen of Aarhus University, Denmark. In the cohort of 7,000 patients, fewer than half were able to reach an HbA1c of 7.5% or lower on the single treatment.
The patients were drawn from Danish national health care databases, and diagnosed with type 2 diabetes from 2000 to 2012. They were a mean of 64 years old with a mean baseline HbA1c of 9.2%. Although newly diagnosed, they had probably had the disease for a while – 26% had macrovascular complications and 47%, microvascular disease. All were started on basal insulin monotherapy. Within 6 months, the mean HbA1c had dropped to 7.6%.
However, just 45% of the group hit the treatment goal of 7.5% or lower, with 29% attaining the 7% HbA1c target.
A temporal assessment of response showed some improvements over the course of the study, Dr. Thomsen noted. In 2000-2003, about 25% hit the goal on basal insulin only; by 2010-2012, that had increased to 33%. This probably reflects an earlier diagnosis of disease, especially as the mean HbA1c at diagnosis was lower in the later years (9% vs.9.6%), he said.
In another noteworthy temporal association, choices for add-on therapy changed over the years. In the first few years, premixed insulin was the top choice, accounting for 80% of intensification prescriptions. By 2012, that had dropped to about 22%. Bolus insulin use rose from about 17% in 2000 to 35% in 2012. GLP-1 receptor agonists didn’t arrive on the scene until 2008 but gained rapid acceptance. By 2010-2012 they accounted for 30% of intensification prescriptions.
Patients whose glucose control was intensified with GLP-1 drugs (326) were the youngest (55 years) and healthiest, with only 22% having medical comorbidities. They started the add-on therapy at a mean of 27 months after diagnosis. At that time, the mean HbA1c was 8.4%; this dropped by 0.8%, landing at a mean of 7.6%.
Those who added bolus insulin (893) were a mean of 59 years old; 30% had comorbidities. They started the new treatment at a mean of 13 months after diagnosis. Their mean HbA1c at intensification was 8.2%; this dropped 0.4%, landing at 7.8%
Patients who added premixed insulin (1,798) were a mean of 64 years old; 32% had medical comorbidities. They started add-on therapy 11 months after diagnosis. Their HbA1c was a mean of 8.8% at intensification; it dropped 0.9%, landing at a mean of 7.9%.
Fifty-nine patients were intensified with dual therapy; Dr. Thomsen did not specify what combinations were employed. These patients were a mean of 61 years old; 42% of them had medical comorbidities. They had a mean HbA1c of 8.9% at the time of intensification. Add-on therapy dropped that measurement by 1.2%, landing this group at a mean HbA1c of 7.7%.
A multivariate analysis determined the likelihood of attaining the treatment target with the various add-on monotherapies; it adjusted for age, gender, diabetes complications, disease duration, medical comorbidities, and baseline HbA1c. Compared with premixed insulin, the chance of meeting goal was similar with bolus insulin (relative risk, 1.03) and higher with GLP-1 agonists (RR, 1.56 for less than 7%; RR, 1.27 for less than 7.5%).
Dr. Thomsen had no financial disclosures.
AT EASD 2015
Key clinical point: Patients with poorly controlled type 2 diabetes are more likely to hit glycemic targets if their add-on therapy is a GLP-1 receptor agonist.
Major finding: Patients with poorly controlled type 2 diabetes were 26% more likely to hit glycemic goals when their add-on therapy was a GLP-1 receptor agonist compared with another insulin.
Data source: A retrospective cohort study of 7,000 patients.
Disclosures: Dr. Thomsen had no financial disclosures.
No difference in long-term cost for diabetes treatment with bariatric surgery
There was no difference in long-term health care cost between conventional treatment and bariatric surgery in patients with diabetes, according to a study published in the Lancet online Sept. 17.
Elevated body mass index is associated with an increased risk of diabetes. However, devising effective lifestyle interventions to reduce weight in the severely obese remains a challenge. Data from the Swedish Obese Subjects (SOS) study have demonstrated prevention and remission of type 2 diabetes after bariatric surgery.
Catherine Keating, Ph.D., of the Deakin Health Economics and Baker IDI Heart and Diabetes Institute in Melbourne, and her associates sought to quantify the health care costs during a 15-year period for obese patients treated with bariatric surgery versus conventional treatment.
Data on prescription drug costs were obtained by questionnaires from participants in the SOS study and the Swedish Prescribed Drug Register. The Swedish National Patient Register was used to obtain data on the outpatient and inpatient visits. The participants were followed for up to 15 years.
The original SOS study was a prospective study that compared treatment outcomes and costs in two groups of obese adults: those who underwent bariatric surgery versus a control cohort who received conventional obesity management. The participants ranged from 37 to 60 years old with BMIs of greater than 34 kg/m2 in men and greater than 38 in women. Conventional treatment included behavior modification, lifestyle intervention, or no treatment.
The definition of diabetes was based on a self-report of taking diabetes drugs or a fasting glucose measurement.
After exclusions, 4,030 participants were included; of these, 2,836 were euglycemic, 591 had prediabetes, and 603 were diabetic, the investigators reported (Lancet 2015 Sep 16 [doi: 10.1016/
S2213-8587(15)00290-9]).
Patients in the bariatric surgery groups were on average 6.2 kg heavier (P less than .0001) and 1.5 years younger (P less than .0001) than were the controls. After the 15-year follow-up, weight loss was 16 kg greater in the diabetes subgroup, 18 kg greater in the prediabetes subgroup, and 20 kg greater in the euglycemia subgroup who had bariatric surgery versus conventional treatment (P less than .0001 for all).
After 15 years, the aggregated drug costs were not different between the conventional treatment group and the bariatric surgery group in patients with euglycemia. However, drug costs were lower in the prediabetic participants who had surgery versus conventional treatment (P = .007). Similarly, patients with diabetes who underwent bariatric surgery incurred lower drug costs after 15 years (P less than .0001).
The inpatient hospital costs after 15 years were greater in the surgery group for all glucose levels versus conventional treatment (mean, $51,225 vs. $25,313; P less than .0001).
There were no differences in outpatient costs demonstrated between the glucose subgroups. However, in the diabetes group, there were wide confidence intervals associated with outpatient costs that were thought to be secondary to end-stage renal disease visits.
Finally, the total cost of health care for the euglycemic group was higher in those who underwent surgery versus conventional treatment (P less than .0001). The prediabetic subgroup also incurred a higher total health care cost for surgery versus conventional treatment (P less than .0001). However, there were no differences in total health care cost for patients with diabetes who had surgery versus conventional treatment ($88,572 vs. $79,967, P less than .090).
The total cost was also higher in patients with diabetes for more than 1 year who had surgical intervention versus those who got conventional treatment (P less than .011). However, patients with diabetes for less than 1 year did not show differences in total cost when treated with surgery versus conventional treatments (P less than .476)
“In this study, we show that for obese patients with type 2 diabetes, the upfront costs of bariatric surgery seem to be largely offset by prevention of future health care and drug use,” the authors wrote. In addition, “long-term health care cost results support prioritization of patients with obesity and type 2 diabetes for bariatric surgery.”
This study was supported by a grant from AFA Forsakring. The authors reported multiple disclosures.
Bariatric surgery should be considered, irrespective of body mas index, in patients whose diabetes is not under control, despite the use of medications and lifestyle interventions.
Since the start of the SOS study, bariatric surgery has changed, and present day surgical costs would be lower.
Dr. Ricardo Cohen is affiliated with the Center for Obesity and Diabetes in Sao Paulo, Brazil. These comments were taken from an accompanying editorial (Lancet 2015 Sep 16 [doi: 10.1016/S2213-8587(15)00320-4]). No conflicts of interests were declared.
Bariatric surgery should be considered, irrespective of body mas index, in patients whose diabetes is not under control, despite the use of medications and lifestyle interventions.
Since the start of the SOS study, bariatric surgery has changed, and present day surgical costs would be lower.
Dr. Ricardo Cohen is affiliated with the Center for Obesity and Diabetes in Sao Paulo, Brazil. These comments were taken from an accompanying editorial (Lancet 2015 Sep 16 [doi: 10.1016/S2213-8587(15)00320-4]). No conflicts of interests were declared.
Bariatric surgery should be considered, irrespective of body mas index, in patients whose diabetes is not under control, despite the use of medications and lifestyle interventions.
Since the start of the SOS study, bariatric surgery has changed, and present day surgical costs would be lower.
Dr. Ricardo Cohen is affiliated with the Center for Obesity and Diabetes in Sao Paulo, Brazil. These comments were taken from an accompanying editorial (Lancet 2015 Sep 16 [doi: 10.1016/S2213-8587(15)00320-4]). No conflicts of interests were declared.
There was no difference in long-term health care cost between conventional treatment and bariatric surgery in patients with diabetes, according to a study published in the Lancet online Sept. 17.
Elevated body mass index is associated with an increased risk of diabetes. However, devising effective lifestyle interventions to reduce weight in the severely obese remains a challenge. Data from the Swedish Obese Subjects (SOS) study have demonstrated prevention and remission of type 2 diabetes after bariatric surgery.
Catherine Keating, Ph.D., of the Deakin Health Economics and Baker IDI Heart and Diabetes Institute in Melbourne, and her associates sought to quantify the health care costs during a 15-year period for obese patients treated with bariatric surgery versus conventional treatment.
Data on prescription drug costs were obtained by questionnaires from participants in the SOS study and the Swedish Prescribed Drug Register. The Swedish National Patient Register was used to obtain data on the outpatient and inpatient visits. The participants were followed for up to 15 years.
The original SOS study was a prospective study that compared treatment outcomes and costs in two groups of obese adults: those who underwent bariatric surgery versus a control cohort who received conventional obesity management. The participants ranged from 37 to 60 years old with BMIs of greater than 34 kg/m2 in men and greater than 38 in women. Conventional treatment included behavior modification, lifestyle intervention, or no treatment.
The definition of diabetes was based on a self-report of taking diabetes drugs or a fasting glucose measurement.
After exclusions, 4,030 participants were included; of these, 2,836 were euglycemic, 591 had prediabetes, and 603 were diabetic, the investigators reported (Lancet 2015 Sep 16 [doi: 10.1016/
S2213-8587(15)00290-9]).
Patients in the bariatric surgery groups were on average 6.2 kg heavier (P less than .0001) and 1.5 years younger (P less than .0001) than were the controls. After the 15-year follow-up, weight loss was 16 kg greater in the diabetes subgroup, 18 kg greater in the prediabetes subgroup, and 20 kg greater in the euglycemia subgroup who had bariatric surgery versus conventional treatment (P less than .0001 for all).
After 15 years, the aggregated drug costs were not different between the conventional treatment group and the bariatric surgery group in patients with euglycemia. However, drug costs were lower in the prediabetic participants who had surgery versus conventional treatment (P = .007). Similarly, patients with diabetes who underwent bariatric surgery incurred lower drug costs after 15 years (P less than .0001).
The inpatient hospital costs after 15 years were greater in the surgery group for all glucose levels versus conventional treatment (mean, $51,225 vs. $25,313; P less than .0001).
There were no differences in outpatient costs demonstrated between the glucose subgroups. However, in the diabetes group, there were wide confidence intervals associated with outpatient costs that were thought to be secondary to end-stage renal disease visits.
Finally, the total cost of health care for the euglycemic group was higher in those who underwent surgery versus conventional treatment (P less than .0001). The prediabetic subgroup also incurred a higher total health care cost for surgery versus conventional treatment (P less than .0001). However, there were no differences in total health care cost for patients with diabetes who had surgery versus conventional treatment ($88,572 vs. $79,967, P less than .090).
The total cost was also higher in patients with diabetes for more than 1 year who had surgical intervention versus those who got conventional treatment (P less than .011). However, patients with diabetes for less than 1 year did not show differences in total cost when treated with surgery versus conventional treatments (P less than .476)
“In this study, we show that for obese patients with type 2 diabetes, the upfront costs of bariatric surgery seem to be largely offset by prevention of future health care and drug use,” the authors wrote. In addition, “long-term health care cost results support prioritization of patients with obesity and type 2 diabetes for bariatric surgery.”
This study was supported by a grant from AFA Forsakring. The authors reported multiple disclosures.
There was no difference in long-term health care cost between conventional treatment and bariatric surgery in patients with diabetes, according to a study published in the Lancet online Sept. 17.
Elevated body mass index is associated with an increased risk of diabetes. However, devising effective lifestyle interventions to reduce weight in the severely obese remains a challenge. Data from the Swedish Obese Subjects (SOS) study have demonstrated prevention and remission of type 2 diabetes after bariatric surgery.
Catherine Keating, Ph.D., of the Deakin Health Economics and Baker IDI Heart and Diabetes Institute in Melbourne, and her associates sought to quantify the health care costs during a 15-year period for obese patients treated with bariatric surgery versus conventional treatment.
Data on prescription drug costs were obtained by questionnaires from participants in the SOS study and the Swedish Prescribed Drug Register. The Swedish National Patient Register was used to obtain data on the outpatient and inpatient visits. The participants were followed for up to 15 years.
The original SOS study was a prospective study that compared treatment outcomes and costs in two groups of obese adults: those who underwent bariatric surgery versus a control cohort who received conventional obesity management. The participants ranged from 37 to 60 years old with BMIs of greater than 34 kg/m2 in men and greater than 38 in women. Conventional treatment included behavior modification, lifestyle intervention, or no treatment.
The definition of diabetes was based on a self-report of taking diabetes drugs or a fasting glucose measurement.
After exclusions, 4,030 participants were included; of these, 2,836 were euglycemic, 591 had prediabetes, and 603 were diabetic, the investigators reported (Lancet 2015 Sep 16 [doi: 10.1016/
S2213-8587(15)00290-9]).
Patients in the bariatric surgery groups were on average 6.2 kg heavier (P less than .0001) and 1.5 years younger (P less than .0001) than were the controls. After the 15-year follow-up, weight loss was 16 kg greater in the diabetes subgroup, 18 kg greater in the prediabetes subgroup, and 20 kg greater in the euglycemia subgroup who had bariatric surgery versus conventional treatment (P less than .0001 for all).
After 15 years, the aggregated drug costs were not different between the conventional treatment group and the bariatric surgery group in patients with euglycemia. However, drug costs were lower in the prediabetic participants who had surgery versus conventional treatment (P = .007). Similarly, patients with diabetes who underwent bariatric surgery incurred lower drug costs after 15 years (P less than .0001).
The inpatient hospital costs after 15 years were greater in the surgery group for all glucose levels versus conventional treatment (mean, $51,225 vs. $25,313; P less than .0001).
There were no differences in outpatient costs demonstrated between the glucose subgroups. However, in the diabetes group, there were wide confidence intervals associated with outpatient costs that were thought to be secondary to end-stage renal disease visits.
Finally, the total cost of health care for the euglycemic group was higher in those who underwent surgery versus conventional treatment (P less than .0001). The prediabetic subgroup also incurred a higher total health care cost for surgery versus conventional treatment (P less than .0001). However, there were no differences in total health care cost for patients with diabetes who had surgery versus conventional treatment ($88,572 vs. $79,967, P less than .090).
The total cost was also higher in patients with diabetes for more than 1 year who had surgical intervention versus those who got conventional treatment (P less than .011). However, patients with diabetes for less than 1 year did not show differences in total cost when treated with surgery versus conventional treatments (P less than .476)
“In this study, we show that for obese patients with type 2 diabetes, the upfront costs of bariatric surgery seem to be largely offset by prevention of future health care and drug use,” the authors wrote. In addition, “long-term health care cost results support prioritization of patients with obesity and type 2 diabetes for bariatric surgery.”
This study was supported by a grant from AFA Forsakring. The authors reported multiple disclosures.
THE LANCET
Key clinical point: There was no difference in long-term health care cost between conventional treatment and bariatric surgery in patients with diabetes.
Major finding: There was no difference in total health care cost for patients with diabetes who received surgery versus conventional treatment ($88,572 vs. $79,967, P = .090).
Data source: A 15-year follow-up of Swedish Obese Subjects study participants with additional data obtained on drug costs and inpatient and outpatient visits.
Disclosures: This study was supported by a grant from AFA Forsakring. The authors reported multiple disclosures.