User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Clinical Guideline: Preventing type 2 diabetes with diet and exercise programs
Diabetes affects over 9% of Americans and is expected to double in prevalence in the next 25 years. In a country where 37% of adults are at risk for developing type 2 diabetes, programs to prevent diabetes are necessary to address the costs, morbidity, and mortality associated with this disease. Diet and exercise programs to prevent diabetes are targeted to patients with a glycosylated hemoglobin A1c level between 5.7% and 6.4% or a fasting blood glucose of 100-125 mg/dL. Many studies have shown the benefit of intensive diet and exercise programs in preventing progression to diabetes and improving weight reduction. Based on the results of a systematic review published in Annals of Internal Medicine in July 2015, the Community Preventive Services Task Force published a clinical guideline recommending the use of combined diet and exercise programs for all patients at increased risk of developing type 2 diabetes (Ann Intern Med. 2015 Jul 14. doi: 10.7326/M15-0452.).
Description of diet and exercise programs
The task force identified several critical components of successful programs: a minimum of 3 months’ duration; well-trained providers and facilitators; a mix of counseling, coaching, and extended support outside of scheduled sessions; and multiple sessions of varied content and delivery methods.
Interventions commonly featured at least one of a few other components. Most programs set a clear goal for weight loss, and many programs included goals for physical activity and diet. Many programs used a variety of different team members such as doctors, nurses, dietitians, diabetes educators, personal trainers, trained health coaches, and physiotherapists. A series of maintenance sessions after the initial intervention was noted in many of the studies. Often times, the studies modeled their intervention based upon the well-known National Diabetes Prevention Program curriculum. These materials are available free on the Centers for Disease Control and Prevention’s website. Of the 66 programs reviewed, all were live programs using some combination of individual and group sessions. Seven programs were virtual programs using a variety of modalities such as websites and apps. Two studies were conducted with groups of adolescents.
Program outcomes
The task force recommendation was based upon a systematic review of 53 studies that evaluated 66 intensive diet and exercise education programs from 1991 to 2015. The studies were conducted over 3 months to 6 years with an average length of 12 months. Only five were less than 6 months. They included randomized controlled trials, prospective nonrandomized comparison trials, and prospective single-group studies. Some were compared with usual care and some with less intensive counseling.
The systematic review supported a decrease in the risk of developing type 2 diabetes and improved hyperglycemia, blood pressure, and lipid panels. The data were not sufficient to directly comment on long-term outcomes like cardiovascular disease, diabetic compilations, or death.
It was clear that more intense programs that included more sessions, more personnel, and more individual sessions had better weight loss and diabetes prevention outcomes.
Economics
A review of 28 studies showed that these programs are cost effective. Twelve programs provided program costs, including identifying patients at risk and program costs. The average cost per participant was $653, with a range of $383-$1,160. Group-based programs were more cost effective.
Next steps
There is a large amount of evidence to support the recommendation of diet and exercise programs in the prevention of type 2 diabetes. The cost of such programs is small in comparison with the costs incurred from the long-term health effects of type 2 diabetes, but we do not yet know which components of these programs are most important. The data support that higher-intensity programs are more successful, but, at the same time, more expensive. In an era in which virtual tools are prevalent, data are currently insufficient to support their use. Identifying a structure for maintenance visits, causes of program attrition, and long-term follow-up of existing programs also may help to craft the ideal program for diabetes prevention.
The Bottom Line
Combined programs of diet and exercise promotion are very successful and cost effective in preventing patients with prediabetes from progressing to type 2 diabetes. Higher-intensity programs with more sessions, more personnel, and individual counseling are more effective but also are more expensive. All patients with an increased risk for type 2 diabetes should participate in a combined diet and exercise program.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Fidler is attending physician and assistant director in the family practice residency program at Abington Memorial Hospital.
Diabetes affects over 9% of Americans and is expected to double in prevalence in the next 25 years. In a country where 37% of adults are at risk for developing type 2 diabetes, programs to prevent diabetes are necessary to address the costs, morbidity, and mortality associated with this disease. Diet and exercise programs to prevent diabetes are targeted to patients with a glycosylated hemoglobin A1c level between 5.7% and 6.4% or a fasting blood glucose of 100-125 mg/dL. Many studies have shown the benefit of intensive diet and exercise programs in preventing progression to diabetes and improving weight reduction. Based on the results of a systematic review published in Annals of Internal Medicine in July 2015, the Community Preventive Services Task Force published a clinical guideline recommending the use of combined diet and exercise programs for all patients at increased risk of developing type 2 diabetes (Ann Intern Med. 2015 Jul 14. doi: 10.7326/M15-0452.).
Description of diet and exercise programs
The task force identified several critical components of successful programs: a minimum of 3 months’ duration; well-trained providers and facilitators; a mix of counseling, coaching, and extended support outside of scheduled sessions; and multiple sessions of varied content and delivery methods.
Interventions commonly featured at least one of a few other components. Most programs set a clear goal for weight loss, and many programs included goals for physical activity and diet. Many programs used a variety of different team members such as doctors, nurses, dietitians, diabetes educators, personal trainers, trained health coaches, and physiotherapists. A series of maintenance sessions after the initial intervention was noted in many of the studies. Often times, the studies modeled their intervention based upon the well-known National Diabetes Prevention Program curriculum. These materials are available free on the Centers for Disease Control and Prevention’s website. Of the 66 programs reviewed, all were live programs using some combination of individual and group sessions. Seven programs were virtual programs using a variety of modalities such as websites and apps. Two studies were conducted with groups of adolescents.
Program outcomes
The task force recommendation was based upon a systematic review of 53 studies that evaluated 66 intensive diet and exercise education programs from 1991 to 2015. The studies were conducted over 3 months to 6 years with an average length of 12 months. Only five were less than 6 months. They included randomized controlled trials, prospective nonrandomized comparison trials, and prospective single-group studies. Some were compared with usual care and some with less intensive counseling.
The systematic review supported a decrease in the risk of developing type 2 diabetes and improved hyperglycemia, blood pressure, and lipid panels. The data were not sufficient to directly comment on long-term outcomes like cardiovascular disease, diabetic compilations, or death.
It was clear that more intense programs that included more sessions, more personnel, and more individual sessions had better weight loss and diabetes prevention outcomes.
Economics
A review of 28 studies showed that these programs are cost effective. Twelve programs provided program costs, including identifying patients at risk and program costs. The average cost per participant was $653, with a range of $383-$1,160. Group-based programs were more cost effective.
Next steps
There is a large amount of evidence to support the recommendation of diet and exercise programs in the prevention of type 2 diabetes. The cost of such programs is small in comparison with the costs incurred from the long-term health effects of type 2 diabetes, but we do not yet know which components of these programs are most important. The data support that higher-intensity programs are more successful, but, at the same time, more expensive. In an era in which virtual tools are prevalent, data are currently insufficient to support their use. Identifying a structure for maintenance visits, causes of program attrition, and long-term follow-up of existing programs also may help to craft the ideal program for diabetes prevention.
The Bottom Line
Combined programs of diet and exercise promotion are very successful and cost effective in preventing patients with prediabetes from progressing to type 2 diabetes. Higher-intensity programs with more sessions, more personnel, and individual counseling are more effective but also are more expensive. All patients with an increased risk for type 2 diabetes should participate in a combined diet and exercise program.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Fidler is attending physician and assistant director in the family practice residency program at Abington Memorial Hospital.
Diabetes affects over 9% of Americans and is expected to double in prevalence in the next 25 years. In a country where 37% of adults are at risk for developing type 2 diabetes, programs to prevent diabetes are necessary to address the costs, morbidity, and mortality associated with this disease. Diet and exercise programs to prevent diabetes are targeted to patients with a glycosylated hemoglobin A1c level between 5.7% and 6.4% or a fasting blood glucose of 100-125 mg/dL. Many studies have shown the benefit of intensive diet and exercise programs in preventing progression to diabetes and improving weight reduction. Based on the results of a systematic review published in Annals of Internal Medicine in July 2015, the Community Preventive Services Task Force published a clinical guideline recommending the use of combined diet and exercise programs for all patients at increased risk of developing type 2 diabetes (Ann Intern Med. 2015 Jul 14. doi: 10.7326/M15-0452.).
Description of diet and exercise programs
The task force identified several critical components of successful programs: a minimum of 3 months’ duration; well-trained providers and facilitators; a mix of counseling, coaching, and extended support outside of scheduled sessions; and multiple sessions of varied content and delivery methods.
Interventions commonly featured at least one of a few other components. Most programs set a clear goal for weight loss, and many programs included goals for physical activity and diet. Many programs used a variety of different team members such as doctors, nurses, dietitians, diabetes educators, personal trainers, trained health coaches, and physiotherapists. A series of maintenance sessions after the initial intervention was noted in many of the studies. Often times, the studies modeled their intervention based upon the well-known National Diabetes Prevention Program curriculum. These materials are available free on the Centers for Disease Control and Prevention’s website. Of the 66 programs reviewed, all were live programs using some combination of individual and group sessions. Seven programs were virtual programs using a variety of modalities such as websites and apps. Two studies were conducted with groups of adolescents.
Program outcomes
The task force recommendation was based upon a systematic review of 53 studies that evaluated 66 intensive diet and exercise education programs from 1991 to 2015. The studies were conducted over 3 months to 6 years with an average length of 12 months. Only five were less than 6 months. They included randomized controlled trials, prospective nonrandomized comparison trials, and prospective single-group studies. Some were compared with usual care and some with less intensive counseling.
The systematic review supported a decrease in the risk of developing type 2 diabetes and improved hyperglycemia, blood pressure, and lipid panels. The data were not sufficient to directly comment on long-term outcomes like cardiovascular disease, diabetic compilations, or death.
It was clear that more intense programs that included more sessions, more personnel, and more individual sessions had better weight loss and diabetes prevention outcomes.
Economics
A review of 28 studies showed that these programs are cost effective. Twelve programs provided program costs, including identifying patients at risk and program costs. The average cost per participant was $653, with a range of $383-$1,160. Group-based programs were more cost effective.
Next steps
There is a large amount of evidence to support the recommendation of diet and exercise programs in the prevention of type 2 diabetes. The cost of such programs is small in comparison with the costs incurred from the long-term health effects of type 2 diabetes, but we do not yet know which components of these programs are most important. The data support that higher-intensity programs are more successful, but, at the same time, more expensive. In an era in which virtual tools are prevalent, data are currently insufficient to support their use. Identifying a structure for maintenance visits, causes of program attrition, and long-term follow-up of existing programs also may help to craft the ideal program for diabetes prevention.
The Bottom Line
Combined programs of diet and exercise promotion are very successful and cost effective in preventing patients with prediabetes from progressing to type 2 diabetes. Higher-intensity programs with more sessions, more personnel, and individual counseling are more effective but also are more expensive. All patients with an increased risk for type 2 diabetes should participate in a combined diet and exercise program.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Fidler is attending physician and assistant director in the family practice residency program at Abington Memorial Hospital.
Subclinical heart dysfunction, fatty liver linked
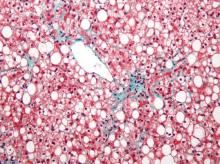
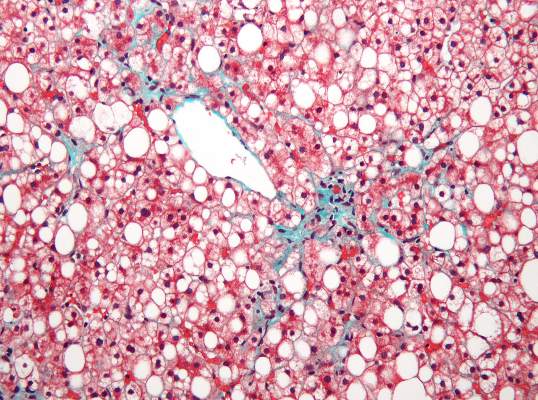
Researchers found an association between nonalcoholic fatty liver disease (NAFLD) and myocardial dysfunction and remodeling, according to a new study published in Hepatology.
“Both NAFLD and heart failure (particularly heart failure with preserved ejection fraction) are obesity-related conditions that have reached epidemic proportions. We know from epidemiologic studies that persons with NAFLD are more likely to die from cardiovascular disease than from liver-related death. This risk seems to be proportional to the amount of fat in the liver and is independent of the presence of nonalcoholic steatohepatitis (NASH). There have been numerous studies that have focused on the relationship between NAFLD and coronary artery disease, but very little work has been done to determine relationships with heart failure,” Dr. Lisa B. VanWagner of Northwestern University in Chicago noted. She continued, “There are several well-established major risk factors for the development of clinical heart failure, including coronary artery disease, diabetes, and hypertension, all of which are also closely associated with NAFLD. However, whether NAFLD is independently associated with subclinical myocardial remodeling or dysfunction that may lead to the development of clinical heart failure is unknown.”
NAFLD and heart failure are both associated with obesity. Likewise, there is evidence that NAFLD may also be related to endothelial dysfunction, coronary plaques, coronary artery calcifications, as well as being an independent risk factor for cardiovascular disease.
Dr. VanWagner and her colleagues conducted a cross-sectional study of 2,713 patients from the CARDIA (Coronary Artery Risk Development in Young Adults) study to understand any associations between NAFLD and abnormalities in left ventricular (LV) function and structure. Study participants completed CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study (Hepatology 2015;62:773-83 [doi:10.1002/hep.27869]). Participants were excluded from analysis if they had missing or incomplete imaging, pregnancy, a history of MI or heart failure, cirrhosis, hepatitis, or chronic liver disease risk factors, or if they weighed more than 450 pounds,
Of the 2,713 subjects included in analysis, 48% were black and 58.8% were female. NAFLD was detected in 10% (n = 271) of participants. Those with NAFLD were more likely to be white males with metabolic syndrome and who were obese and had higher CT-measured levels of visceral adipose tissue, and an increased waist circumference and waist-to-hip ratio. Insulin resistance markers such as elevated fasting glucose, elevated C-reactive protein, and hypertriglyceridemia were more common in the participants with NAFLD.
Study participants with NAFLD had signs of myocardial remodeling such as more left ventricular wall thickness, LV end-diastolic volume, left aortic volume index, and LV mass index. Likewise, NAFLD was associated with more circumferential strain and global longitudinal strain but no differences in ejection fraction.
Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (34.6% vs. 23.6%; P less than .0001), and elevated LV filling pressures (33.3% vs. 23.7%; P less than .001) was more common in NAFLD participants, compared with non-NAFLD subjects.
After researchers adjusted for health behaviors and demographic factors, evidence of NAFLD was associated with worse GLS (P less than .0001). Finally, NAFLD was associated with subclinical cardiac remodeling and dysfunction even after adjustment for body mass index and heart failure risk factors (P less than .01).
Dr. VanWagner summarized, “NAFLD may not necessarily be a ‘benign condition’ as previously thought. In our study, we determined liver fat by CT scan, which admittedly detects fat at a higher level (typically greater than 30%) than for example on MRI, which can detect fat as low as 5%. A fatty liver detected on CT or even on [ultrasound], which has similar sensitivity as CT for detecting liver fat should prompt evaluation for additional cardiovascular risk factors and treatment of identified abnormalities to reduce [atherosclerotic cardiovascular disease] and [heart failure] risk. Currently, our study only shows associations between liver fat and subclinical changes in the myocardium and causality cannot be determined. [On the basis] of our data, we cannot recommend screening for HF [heart failure] in this population, but future studies are needed to determine if NAFLD in fact lies in the casual pathway for the development of clinical HF.”
The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
Researchers found an association between nonalcoholic fatty liver disease (NAFLD) and myocardial dysfunction and remodeling, according to a new study published in Hepatology.
“Both NAFLD and heart failure (particularly heart failure with preserved ejection fraction) are obesity-related conditions that have reached epidemic proportions. We know from epidemiologic studies that persons with NAFLD are more likely to die from cardiovascular disease than from liver-related death. This risk seems to be proportional to the amount of fat in the liver and is independent of the presence of nonalcoholic steatohepatitis (NASH). There have been numerous studies that have focused on the relationship between NAFLD and coronary artery disease, but very little work has been done to determine relationships with heart failure,” Dr. Lisa B. VanWagner of Northwestern University in Chicago noted. She continued, “There are several well-established major risk factors for the development of clinical heart failure, including coronary artery disease, diabetes, and hypertension, all of which are also closely associated with NAFLD. However, whether NAFLD is independently associated with subclinical myocardial remodeling or dysfunction that may lead to the development of clinical heart failure is unknown.”
NAFLD and heart failure are both associated with obesity. Likewise, there is evidence that NAFLD may also be related to endothelial dysfunction, coronary plaques, coronary artery calcifications, as well as being an independent risk factor for cardiovascular disease.
Dr. VanWagner and her colleagues conducted a cross-sectional study of 2,713 patients from the CARDIA (Coronary Artery Risk Development in Young Adults) study to understand any associations between NAFLD and abnormalities in left ventricular (LV) function and structure. Study participants completed CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study (Hepatology 2015;62:773-83 [doi:10.1002/hep.27869]). Participants were excluded from analysis if they had missing or incomplete imaging, pregnancy, a history of MI or heart failure, cirrhosis, hepatitis, or chronic liver disease risk factors, or if they weighed more than 450 pounds,
Of the 2,713 subjects included in analysis, 48% were black and 58.8% were female. NAFLD was detected in 10% (n = 271) of participants. Those with NAFLD were more likely to be white males with metabolic syndrome and who were obese and had higher CT-measured levels of visceral adipose tissue, and an increased waist circumference and waist-to-hip ratio. Insulin resistance markers such as elevated fasting glucose, elevated C-reactive protein, and hypertriglyceridemia were more common in the participants with NAFLD.
Study participants with NAFLD had signs of myocardial remodeling such as more left ventricular wall thickness, LV end-diastolic volume, left aortic volume index, and LV mass index. Likewise, NAFLD was associated with more circumferential strain and global longitudinal strain but no differences in ejection fraction.
Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (34.6% vs. 23.6%; P less than .0001), and elevated LV filling pressures (33.3% vs. 23.7%; P less than .001) was more common in NAFLD participants, compared with non-NAFLD subjects.
After researchers adjusted for health behaviors and demographic factors, evidence of NAFLD was associated with worse GLS (P less than .0001). Finally, NAFLD was associated with subclinical cardiac remodeling and dysfunction even after adjustment for body mass index and heart failure risk factors (P less than .01).
Dr. VanWagner summarized, “NAFLD may not necessarily be a ‘benign condition’ as previously thought. In our study, we determined liver fat by CT scan, which admittedly detects fat at a higher level (typically greater than 30%) than for example on MRI, which can detect fat as low as 5%. A fatty liver detected on CT or even on [ultrasound], which has similar sensitivity as CT for detecting liver fat should prompt evaluation for additional cardiovascular risk factors and treatment of identified abnormalities to reduce [atherosclerotic cardiovascular disease] and [heart failure] risk. Currently, our study only shows associations between liver fat and subclinical changes in the myocardium and causality cannot be determined. [On the basis] of our data, we cannot recommend screening for HF [heart failure] in this population, but future studies are needed to determine if NAFLD in fact lies in the casual pathway for the development of clinical HF.”
The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
Researchers found an association between nonalcoholic fatty liver disease (NAFLD) and myocardial dysfunction and remodeling, according to a new study published in Hepatology.
“Both NAFLD and heart failure (particularly heart failure with preserved ejection fraction) are obesity-related conditions that have reached epidemic proportions. We know from epidemiologic studies that persons with NAFLD are more likely to die from cardiovascular disease than from liver-related death. This risk seems to be proportional to the amount of fat in the liver and is independent of the presence of nonalcoholic steatohepatitis (NASH). There have been numerous studies that have focused on the relationship between NAFLD and coronary artery disease, but very little work has been done to determine relationships with heart failure,” Dr. Lisa B. VanWagner of Northwestern University in Chicago noted. She continued, “There are several well-established major risk factors for the development of clinical heart failure, including coronary artery disease, diabetes, and hypertension, all of which are also closely associated with NAFLD. However, whether NAFLD is independently associated with subclinical myocardial remodeling or dysfunction that may lead to the development of clinical heart failure is unknown.”
NAFLD and heart failure are both associated with obesity. Likewise, there is evidence that NAFLD may also be related to endothelial dysfunction, coronary plaques, coronary artery calcifications, as well as being an independent risk factor for cardiovascular disease.
Dr. VanWagner and her colleagues conducted a cross-sectional study of 2,713 patients from the CARDIA (Coronary Artery Risk Development in Young Adults) study to understand any associations between NAFLD and abnormalities in left ventricular (LV) function and structure. Study participants completed CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study (Hepatology 2015;62:773-83 [doi:10.1002/hep.27869]). Participants were excluded from analysis if they had missing or incomplete imaging, pregnancy, a history of MI or heart failure, cirrhosis, hepatitis, or chronic liver disease risk factors, or if they weighed more than 450 pounds,
Of the 2,713 subjects included in analysis, 48% were black and 58.8% were female. NAFLD was detected in 10% (n = 271) of participants. Those with NAFLD were more likely to be white males with metabolic syndrome and who were obese and had higher CT-measured levels of visceral adipose tissue, and an increased waist circumference and waist-to-hip ratio. Insulin resistance markers such as elevated fasting glucose, elevated C-reactive protein, and hypertriglyceridemia were more common in the participants with NAFLD.
Study participants with NAFLD had signs of myocardial remodeling such as more left ventricular wall thickness, LV end-diastolic volume, left aortic volume index, and LV mass index. Likewise, NAFLD was associated with more circumferential strain and global longitudinal strain but no differences in ejection fraction.
Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (34.6% vs. 23.6%; P less than .0001), and elevated LV filling pressures (33.3% vs. 23.7%; P less than .001) was more common in NAFLD participants, compared with non-NAFLD subjects.
After researchers adjusted for health behaviors and demographic factors, evidence of NAFLD was associated with worse GLS (P less than .0001). Finally, NAFLD was associated with subclinical cardiac remodeling and dysfunction even after adjustment for body mass index and heart failure risk factors (P less than .01).
Dr. VanWagner summarized, “NAFLD may not necessarily be a ‘benign condition’ as previously thought. In our study, we determined liver fat by CT scan, which admittedly detects fat at a higher level (typically greater than 30%) than for example on MRI, which can detect fat as low as 5%. A fatty liver detected on CT or even on [ultrasound], which has similar sensitivity as CT for detecting liver fat should prompt evaluation for additional cardiovascular risk factors and treatment of identified abnormalities to reduce [atherosclerotic cardiovascular disease] and [heart failure] risk. Currently, our study only shows associations between liver fat and subclinical changes in the myocardium and causality cannot be determined. [On the basis] of our data, we cannot recommend screening for HF [heart failure] in this population, but future studies are needed to determine if NAFLD in fact lies in the casual pathway for the development of clinical HF.”
The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
FROM HEPATOLOGY
Key clinical point: Researchers found an association between nonalcoholic fatty liver disease and myocardial dysfunction and remodeling.
Major finding: Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (P less than .0001), and elevated LV filling pressures (P less than .001) was more common in participants with NAFLD than in those without.
Data source: A cross-sectional study of 2,713 patients from the CARDIA study using CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study.
Disclosures: The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
VIDEO: Newer type 2 diabetes drugs pose no significant heart failure risk
LONDON – Neither the GLP-1 receptor agonist lixisenatide nor the DPP-4 inhibitor sitagliptin significantly increased the risk of heart failure or associated hospitalizations in patients with type 2 diabetes, according to data from two large cardiovascular safety trials.
Further analysis from ELIXA (Evaluation of Lixisenatide [Lyxumia] in Acute Coronary Syndrome) showed that the hazard ratios for several secondary heart failure endpoints were 1.00 or below. Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure than in those who did not, there was there was no difference between treatment with the GLP-1 (glucagon-like peptide 1) agonist or placebo.
The findings add to those already presented in June at the annual scientific sessions of the American Diabetes Association from the 6,068-patient trial, which enrolled patients with type 2 diabetes who had experienced an acute coronary syndrome within the past 6 months.
Other reassuring data on the safety of newer diabetes medicines came from an updated analysis of TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin [Januvia]). The time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms, and there were no differences between the treatments in terms of hospitalization for heart failure or cardiovascular death or hospitalization for heart failure or all-cause death with the DPP-4 (dipeptyl peptidase 4) inhibitor. TECOS enrolled nearly 15,000 patients with type 2 diabetes and concurrent cardiovascular disease who were aged 50 years or older and the primary findings have been published (N Engl J Med. 2015 July 15;373:232-42).
These trials provide accumulating evidence that there is no heart failure risk with these particular diabetes medications, Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, commented in an interview at the annual congress of the European Society of Cardiology. Dr. Rydén added that the trial results everyone is waiting to hear about concern the cardiovascular safety of the selective sodium–glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin (Jardiance).
Advance information issued by manufacturer Boehringer-Ingelheim suggests that this drug actually may lower cardiovascular risk in patients at high risk for cardiovascular events, Dr. Rydén observed. “Now, if that is true, it is the only modern glucose-lowering drug to have shown such a capacity.” Results of the EMPA-REG OUTCOME study will be presented in September at the annual meeting of the European Association for the Study of Diabetes in Stockholm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Neither the GLP-1 receptor agonist lixisenatide nor the DPP-4 inhibitor sitagliptin significantly increased the risk of heart failure or associated hospitalizations in patients with type 2 diabetes, according to data from two large cardiovascular safety trials.
Further analysis from ELIXA (Evaluation of Lixisenatide [Lyxumia] in Acute Coronary Syndrome) showed that the hazard ratios for several secondary heart failure endpoints were 1.00 or below. Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure than in those who did not, there was there was no difference between treatment with the GLP-1 (glucagon-like peptide 1) agonist or placebo.
The findings add to those already presented in June at the annual scientific sessions of the American Diabetes Association from the 6,068-patient trial, which enrolled patients with type 2 diabetes who had experienced an acute coronary syndrome within the past 6 months.
Other reassuring data on the safety of newer diabetes medicines came from an updated analysis of TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin [Januvia]). The time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms, and there were no differences between the treatments in terms of hospitalization for heart failure or cardiovascular death or hospitalization for heart failure or all-cause death with the DPP-4 (dipeptyl peptidase 4) inhibitor. TECOS enrolled nearly 15,000 patients with type 2 diabetes and concurrent cardiovascular disease who were aged 50 years or older and the primary findings have been published (N Engl J Med. 2015 July 15;373:232-42).
These trials provide accumulating evidence that there is no heart failure risk with these particular diabetes medications, Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, commented in an interview at the annual congress of the European Society of Cardiology. Dr. Rydén added that the trial results everyone is waiting to hear about concern the cardiovascular safety of the selective sodium–glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin (Jardiance).
Advance information issued by manufacturer Boehringer-Ingelheim suggests that this drug actually may lower cardiovascular risk in patients at high risk for cardiovascular events, Dr. Rydén observed. “Now, if that is true, it is the only modern glucose-lowering drug to have shown such a capacity.” Results of the EMPA-REG OUTCOME study will be presented in September at the annual meeting of the European Association for the Study of Diabetes in Stockholm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Neither the GLP-1 receptor agonist lixisenatide nor the DPP-4 inhibitor sitagliptin significantly increased the risk of heart failure or associated hospitalizations in patients with type 2 diabetes, according to data from two large cardiovascular safety trials.
Further analysis from ELIXA (Evaluation of Lixisenatide [Lyxumia] in Acute Coronary Syndrome) showed that the hazard ratios for several secondary heart failure endpoints were 1.00 or below. Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure than in those who did not, there was there was no difference between treatment with the GLP-1 (glucagon-like peptide 1) agonist or placebo.
The findings add to those already presented in June at the annual scientific sessions of the American Diabetes Association from the 6,068-patient trial, which enrolled patients with type 2 diabetes who had experienced an acute coronary syndrome within the past 6 months.
Other reassuring data on the safety of newer diabetes medicines came from an updated analysis of TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin [Januvia]). The time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms, and there were no differences between the treatments in terms of hospitalization for heart failure or cardiovascular death or hospitalization for heart failure or all-cause death with the DPP-4 (dipeptyl peptidase 4) inhibitor. TECOS enrolled nearly 15,000 patients with type 2 diabetes and concurrent cardiovascular disease who were aged 50 years or older and the primary findings have been published (N Engl J Med. 2015 July 15;373:232-42).
These trials provide accumulating evidence that there is no heart failure risk with these particular diabetes medications, Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, commented in an interview at the annual congress of the European Society of Cardiology. Dr. Rydén added that the trial results everyone is waiting to hear about concern the cardiovascular safety of the selective sodium–glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin (Jardiance).
Advance information issued by manufacturer Boehringer-Ingelheim suggests that this drug actually may lower cardiovascular risk in patients at high risk for cardiovascular events, Dr. Rydén observed. “Now, if that is true, it is the only modern glucose-lowering drug to have shown such a capacity.” Results of the EMPA-REG OUTCOME study will be presented in September at the annual meeting of the European Association for the Study of Diabetes in Stockholm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Young hypertensives: Cut back on the caffeine
LONDON – Drinking more than three cups of coffee daily increased the risk of a cardiovascular event by 50% in a study of more than 1,000 adults aged 45 years or younger with mild, untreated hypertension.
The results of the Hypertension and Ambulatory Recording Venetia Study (HARVEST) also showed that even moderate coffee intake, defined as between one and three cups per day, could up the risk of a cardiovascular event when compared with non–coffee drinkers.
“Don’t drink a lot of coffee; reduce your intake,” Dr. Lucio Mos advised young to middle-aged hypertensive adults in a video interview at the annual congress of the European Society of Cardiology.
Heavy coffee drinking is a strong predictor of increasing blood pressure and rising blood glucose, said Dr. Mos of Hospital San Daniele del Friuli in Udine, Italy. In this study, which had a mean of 12.5 years of follow-up, there was a linear relationship between coffee intake and cardiovascular events such as heart attacks, with the risk increasing with higher coffee intake.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Drinking more than three cups of coffee daily increased the risk of a cardiovascular event by 50% in a study of more than 1,000 adults aged 45 years or younger with mild, untreated hypertension.
The results of the Hypertension and Ambulatory Recording Venetia Study (HARVEST) also showed that even moderate coffee intake, defined as between one and three cups per day, could up the risk of a cardiovascular event when compared with non–coffee drinkers.
“Don’t drink a lot of coffee; reduce your intake,” Dr. Lucio Mos advised young to middle-aged hypertensive adults in a video interview at the annual congress of the European Society of Cardiology.
Heavy coffee drinking is a strong predictor of increasing blood pressure and rising blood glucose, said Dr. Mos of Hospital San Daniele del Friuli in Udine, Italy. In this study, which had a mean of 12.5 years of follow-up, there was a linear relationship between coffee intake and cardiovascular events such as heart attacks, with the risk increasing with higher coffee intake.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Drinking more than three cups of coffee daily increased the risk of a cardiovascular event by 50% in a study of more than 1,000 adults aged 45 years or younger with mild, untreated hypertension.
The results of the Hypertension and Ambulatory Recording Venetia Study (HARVEST) also showed that even moderate coffee intake, defined as between one and three cups per day, could up the risk of a cardiovascular event when compared with non–coffee drinkers.
“Don’t drink a lot of coffee; reduce your intake,” Dr. Lucio Mos advised young to middle-aged hypertensive adults in a video interview at the annual congress of the European Society of Cardiology.
Heavy coffee drinking is a strong predictor of increasing blood pressure and rising blood glucose, said Dr. Mos of Hospital San Daniele del Friuli in Udine, Italy. In this study, which had a mean of 12.5 years of follow-up, there was a linear relationship between coffee intake and cardiovascular events such as heart attacks, with the risk increasing with higher coffee intake.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Napping – a novel nondrug antihypertensive therapy
LONDON – A regular midday nap is associated with clinically meaningful reductions in blood pressure in hypertensive patients, according to a prospective observational study presented at the annual congress of the European Society of Cardiology.
The midday nappers in the 386-patient study averaged 5 mm Hg lower daytime and 8 mm Hg lower nighttime systolic blood pressure, compared with non-nappers, Dr. Manolis Kallistratos said in a video interview.
Sleep during nap time was associated with bigger blood pressure reductions than simply resting. Sawing logs for 60 minutes or more brought the most benefits, according to Dr. Kallistratos, head of the hypertension division at Asklepieion Voula General Hospital in Athens.
Nappers also had significantly less arterial stiffness as reflected in a lower pulse wave velocity, as well as a smaller average left atrial diameter indicative of less structural heart damage, the cardiologist noted.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – A regular midday nap is associated with clinically meaningful reductions in blood pressure in hypertensive patients, according to a prospective observational study presented at the annual congress of the European Society of Cardiology.
The midday nappers in the 386-patient study averaged 5 mm Hg lower daytime and 8 mm Hg lower nighttime systolic blood pressure, compared with non-nappers, Dr. Manolis Kallistratos said in a video interview.
Sleep during nap time was associated with bigger blood pressure reductions than simply resting. Sawing logs for 60 minutes or more brought the most benefits, according to Dr. Kallistratos, head of the hypertension division at Asklepieion Voula General Hospital in Athens.
Nappers also had significantly less arterial stiffness as reflected in a lower pulse wave velocity, as well as a smaller average left atrial diameter indicative of less structural heart damage, the cardiologist noted.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – A regular midday nap is associated with clinically meaningful reductions in blood pressure in hypertensive patients, according to a prospective observational study presented at the annual congress of the European Society of Cardiology.
The midday nappers in the 386-patient study averaged 5 mm Hg lower daytime and 8 mm Hg lower nighttime systolic blood pressure, compared with non-nappers, Dr. Manolis Kallistratos said in a video interview.
Sleep during nap time was associated with bigger blood pressure reductions than simply resting. Sawing logs for 60 minutes or more brought the most benefits, according to Dr. Kallistratos, head of the hypertension division at Asklepieion Voula General Hospital in Athens.
Nappers also had significantly less arterial stiffness as reflected in a lower pulse wave velocity, as well as a smaller average left atrial diameter indicative of less structural heart damage, the cardiologist noted.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2015
FDA warns of disabling joint pain from DPP-4 inhibitors
Multiple reports of severe and disabling joint pain in some patients taking dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes have prompted the Food and Drug Administration to add a new warning and precaution for this class of drugs. Some cases were severe enough to require hospitalization, though symptoms eventually resolved after patients stopped taking the medication.
In a MedWatch Bulletin, the FDA advises that physicians should be alert for DPP-4 inhibitors as a causative factor for patients who present with severe, persistent joint pain, even for those who have been on the medication for some time.
Most patients developed symptoms within a month of beginning treatment; however, some patients had been on a DPP-4 inhibitor for as long as a year before the onset of joint pain. When the medication was stopped, arthralgia resolved within a month in all reported cases.
Of the 33 cases of severe arthralgia found in the FDA adverse events reporting database, 28 were associated with the use of sitagliptin (Januvia), with some cases also reported with saxagliptin (Onglyza), linagliptin (Tradjenta), alogliptin (Nesina), and vildagliptin (Galvus). Ten patients’ symptoms were severe enough to require hospitalization; eight experienced recurrent arthralgia when rechallenged.
A literature search conducted by FDA officials revealed seven reports of DPP-4 inhibitor–associated arthralgia, two of which also were in their reporting database.
Patients taking DPP-4 inhibitors should continue taking their medication but consult their health care providers if they experience severe, persistent joint pain, according to the FDA advisory.
On Twitter @karioakes
Multiple reports of severe and disabling joint pain in some patients taking dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes have prompted the Food and Drug Administration to add a new warning and precaution for this class of drugs. Some cases were severe enough to require hospitalization, though symptoms eventually resolved after patients stopped taking the medication.
In a MedWatch Bulletin, the FDA advises that physicians should be alert for DPP-4 inhibitors as a causative factor for patients who present with severe, persistent joint pain, even for those who have been on the medication for some time.
Most patients developed symptoms within a month of beginning treatment; however, some patients had been on a DPP-4 inhibitor for as long as a year before the onset of joint pain. When the medication was stopped, arthralgia resolved within a month in all reported cases.
Of the 33 cases of severe arthralgia found in the FDA adverse events reporting database, 28 were associated with the use of sitagliptin (Januvia), with some cases also reported with saxagliptin (Onglyza), linagliptin (Tradjenta), alogliptin (Nesina), and vildagliptin (Galvus). Ten patients’ symptoms were severe enough to require hospitalization; eight experienced recurrent arthralgia when rechallenged.
A literature search conducted by FDA officials revealed seven reports of DPP-4 inhibitor–associated arthralgia, two of which also were in their reporting database.
Patients taking DPP-4 inhibitors should continue taking their medication but consult their health care providers if they experience severe, persistent joint pain, according to the FDA advisory.
On Twitter @karioakes
Multiple reports of severe and disabling joint pain in some patients taking dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes have prompted the Food and Drug Administration to add a new warning and precaution for this class of drugs. Some cases were severe enough to require hospitalization, though symptoms eventually resolved after patients stopped taking the medication.
In a MedWatch Bulletin, the FDA advises that physicians should be alert for DPP-4 inhibitors as a causative factor for patients who present with severe, persistent joint pain, even for those who have been on the medication for some time.
Most patients developed symptoms within a month of beginning treatment; however, some patients had been on a DPP-4 inhibitor for as long as a year before the onset of joint pain. When the medication was stopped, arthralgia resolved within a month in all reported cases.
Of the 33 cases of severe arthralgia found in the FDA adverse events reporting database, 28 were associated with the use of sitagliptin (Januvia), with some cases also reported with saxagliptin (Onglyza), linagliptin (Tradjenta), alogliptin (Nesina), and vildagliptin (Galvus). Ten patients’ symptoms were severe enough to require hospitalization; eight experienced recurrent arthralgia when rechallenged.
A literature search conducted by FDA officials revealed seven reports of DPP-4 inhibitor–associated arthralgia, two of which also were in their reporting database.
Patients taking DPP-4 inhibitors should continue taking their medication but consult their health care providers if they experience severe, persistent joint pain, according to the FDA advisory.
On Twitter @karioakes
FDA approves Synjardy for type 2 diabetes
The FDA has approved Synjardy (empagliflozin/metformin hydrochloride) to help control blood glucose in adults with type 2 diabetes, the drug’s manufacturers announced Aug. 27.
Synjardy, which contains the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin, is indicated as an adjunct to diet and exercise in patients unable to achieve sufficient glycemic control on empagliflozin or metformin, or in patients already being treated with both drugs.
When combined, empagliflozin and metformin offer “complementary mechanisms of action” to help control blood glucose, according to a statement by the manufacturers, Boehringer Ingelheim Pharmaceuticals and Eli Lilly and Co.
“Empagliflozin ... removes excess glucose through the urine by blocking glucose reabsorption in the kidney,” the statement said. Metformin, frequently prescribed as a first-line treatment, lowers glucose production by the liver and its absorption in the intestine.
Synjardy contains a boxed warning for lactic acidosis and should not be used by adults with severe kidney problems or allergies to empagliflozin, metformin, or any other ingredients in the medication. It also should not be used to treat type 1 diabetes or diabetic ketoacidosis.
Common side effects from Synjardy include runny nose, sore throat, urinary tract infections, female genital infections, diarrhea, headache, nausea, and vomiting.
Visit the Boehringer Ingelheim website for full prescribing information. Adverse effects may be reported to the FDA at http://www.fda.gov/medwatch.com.
The FDA has approved Synjardy (empagliflozin/metformin hydrochloride) to help control blood glucose in adults with type 2 diabetes, the drug’s manufacturers announced Aug. 27.
Synjardy, which contains the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin, is indicated as an adjunct to diet and exercise in patients unable to achieve sufficient glycemic control on empagliflozin or metformin, or in patients already being treated with both drugs.
When combined, empagliflozin and metformin offer “complementary mechanisms of action” to help control blood glucose, according to a statement by the manufacturers, Boehringer Ingelheim Pharmaceuticals and Eli Lilly and Co.
“Empagliflozin ... removes excess glucose through the urine by blocking glucose reabsorption in the kidney,” the statement said. Metformin, frequently prescribed as a first-line treatment, lowers glucose production by the liver and its absorption in the intestine.
Synjardy contains a boxed warning for lactic acidosis and should not be used by adults with severe kidney problems or allergies to empagliflozin, metformin, or any other ingredients in the medication. It also should not be used to treat type 1 diabetes or diabetic ketoacidosis.
Common side effects from Synjardy include runny nose, sore throat, urinary tract infections, female genital infections, diarrhea, headache, nausea, and vomiting.
Visit the Boehringer Ingelheim website for full prescribing information. Adverse effects may be reported to the FDA at http://www.fda.gov/medwatch.com.
The FDA has approved Synjardy (empagliflozin/metformin hydrochloride) to help control blood glucose in adults with type 2 diabetes, the drug’s manufacturers announced Aug. 27.
Synjardy, which contains the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin, is indicated as an adjunct to diet and exercise in patients unable to achieve sufficient glycemic control on empagliflozin or metformin, or in patients already being treated with both drugs.
When combined, empagliflozin and metformin offer “complementary mechanisms of action” to help control blood glucose, according to a statement by the manufacturers, Boehringer Ingelheim Pharmaceuticals and Eli Lilly and Co.
“Empagliflozin ... removes excess glucose through the urine by blocking glucose reabsorption in the kidney,” the statement said. Metformin, frequently prescribed as a first-line treatment, lowers glucose production by the liver and its absorption in the intestine.
Synjardy contains a boxed warning for lactic acidosis and should not be used by adults with severe kidney problems or allergies to empagliflozin, metformin, or any other ingredients in the medication. It also should not be used to treat type 1 diabetes or diabetic ketoacidosis.
Common side effects from Synjardy include runny nose, sore throat, urinary tract infections, female genital infections, diarrhea, headache, nausea, and vomiting.
Visit the Boehringer Ingelheim website for full prescribing information. Adverse effects may be reported to the FDA at http://www.fda.gov/medwatch.com.
In diabetic foot ulcers, use 50% rule to gauge need for advanced therapies
VANCOUVER – When diabetic foot ulcer healing has stalled despite 4 weeks of optimal standard therapy, it’s time to seriously consider resorting to advanced therapies, according to Dr. Afsaneh Alavi of the University of Toronto.
Advanced therapy options include negative pressure wound therapy, hyperbaric oxygen, artificial skin substitutes, growth factors, collagen-based dressings, and electromagnetic therapy.
“You hear a lot about advanced therapy for diabetic foot ulcers. It’s expensive. But it can be cost-effective if used properly. It’s our duty to see who is the best person to receive this therapy,” she said at the World Congress of Dermatology.
A useful rule of thumb, the dermatologist added, is that if a healable ulcer isn’t 50% smaller at week 4 despite optimal care, it’s time to consider moving on to advanced therapies. As was shown in a well-conducted, 203-patient, randomized, prospective controlled clinical trial, a wound that hasn’t met that threshold is unlikely to be healed at 12 weeks (Diabetes Care. 2003 Jun;26(6):1879-82).
Two caveats: advanced therapy is appropriate only for healable diabetic foot ulcers, meaning those in patients with adequate peripheral circulation as defined by measurements of transcutaneous oxygen pressure, toe pressure brachial index, and Doppler studies.
“Eighty percent of diabetic patients have noncompressible arteries and therefore the ankle brachial index is not an accurate test to evaluate their circulation,” according to Dr. Avali.
The other caveat regarding advanced therapy is that appropriate candidates should have a wound edge that’s sharp, not migrating, she continued.
The 4-week cutoff for seeing substantial progress in wound healing with standard therapy before turning to advanced therapies is based in part upon an influential study of 2,517 patients with diabetic neuropathic foot ulcers who received one of three advanced biological therapies. On average, patients started advanced biological therapy within 28 days following their first visit to a specialized wound clinic. Prolonged time to initiation of advanced treatment was associated with significantly longer time to healing (Arch Dermatol. 2010 Aug;146(8):857-62).
While thinking about local wound care in a patient with a diabetic foot ulcer, Dr. Avali said it’s also important to take a step back and make sure hypertension and other conventional cardiovascular risk factors are well controlled and also to take a close look at the HbA1c. In a study of 183 patients with diabetic wounds treated at a specialized academic wound center, for every 1.0% increase in HbA1c above the mean level of 8.0%, the wound-area healing rate was reduced by 0.028 cm2 per day. The implication is that HbA1c may be a key biomarker that’s useful in predicting wound-healing rate in patients with diabetic ulcers (J. Invest Dermatol. 2011 Oct;131(10):2121-7).
She reported serving as a consultant to Acelity and Smith & Nephew.
VANCOUVER – When diabetic foot ulcer healing has stalled despite 4 weeks of optimal standard therapy, it’s time to seriously consider resorting to advanced therapies, according to Dr. Afsaneh Alavi of the University of Toronto.
Advanced therapy options include negative pressure wound therapy, hyperbaric oxygen, artificial skin substitutes, growth factors, collagen-based dressings, and electromagnetic therapy.
“You hear a lot about advanced therapy for diabetic foot ulcers. It’s expensive. But it can be cost-effective if used properly. It’s our duty to see who is the best person to receive this therapy,” she said at the World Congress of Dermatology.
A useful rule of thumb, the dermatologist added, is that if a healable ulcer isn’t 50% smaller at week 4 despite optimal care, it’s time to consider moving on to advanced therapies. As was shown in a well-conducted, 203-patient, randomized, prospective controlled clinical trial, a wound that hasn’t met that threshold is unlikely to be healed at 12 weeks (Diabetes Care. 2003 Jun;26(6):1879-82).
Two caveats: advanced therapy is appropriate only for healable diabetic foot ulcers, meaning those in patients with adequate peripheral circulation as defined by measurements of transcutaneous oxygen pressure, toe pressure brachial index, and Doppler studies.
“Eighty percent of diabetic patients have noncompressible arteries and therefore the ankle brachial index is not an accurate test to evaluate their circulation,” according to Dr. Avali.
The other caveat regarding advanced therapy is that appropriate candidates should have a wound edge that’s sharp, not migrating, she continued.
The 4-week cutoff for seeing substantial progress in wound healing with standard therapy before turning to advanced therapies is based in part upon an influential study of 2,517 patients with diabetic neuropathic foot ulcers who received one of three advanced biological therapies. On average, patients started advanced biological therapy within 28 days following their first visit to a specialized wound clinic. Prolonged time to initiation of advanced treatment was associated with significantly longer time to healing (Arch Dermatol. 2010 Aug;146(8):857-62).
While thinking about local wound care in a patient with a diabetic foot ulcer, Dr. Avali said it’s also important to take a step back and make sure hypertension and other conventional cardiovascular risk factors are well controlled and also to take a close look at the HbA1c. In a study of 183 patients with diabetic wounds treated at a specialized academic wound center, for every 1.0% increase in HbA1c above the mean level of 8.0%, the wound-area healing rate was reduced by 0.028 cm2 per day. The implication is that HbA1c may be a key biomarker that’s useful in predicting wound-healing rate in patients with diabetic ulcers (J. Invest Dermatol. 2011 Oct;131(10):2121-7).
She reported serving as a consultant to Acelity and Smith & Nephew.
VANCOUVER – When diabetic foot ulcer healing has stalled despite 4 weeks of optimal standard therapy, it’s time to seriously consider resorting to advanced therapies, according to Dr. Afsaneh Alavi of the University of Toronto.
Advanced therapy options include negative pressure wound therapy, hyperbaric oxygen, artificial skin substitutes, growth factors, collagen-based dressings, and electromagnetic therapy.
“You hear a lot about advanced therapy for diabetic foot ulcers. It’s expensive. But it can be cost-effective if used properly. It’s our duty to see who is the best person to receive this therapy,” she said at the World Congress of Dermatology.
A useful rule of thumb, the dermatologist added, is that if a healable ulcer isn’t 50% smaller at week 4 despite optimal care, it’s time to consider moving on to advanced therapies. As was shown in a well-conducted, 203-patient, randomized, prospective controlled clinical trial, a wound that hasn’t met that threshold is unlikely to be healed at 12 weeks (Diabetes Care. 2003 Jun;26(6):1879-82).
Two caveats: advanced therapy is appropriate only for healable diabetic foot ulcers, meaning those in patients with adequate peripheral circulation as defined by measurements of transcutaneous oxygen pressure, toe pressure brachial index, and Doppler studies.
“Eighty percent of diabetic patients have noncompressible arteries and therefore the ankle brachial index is not an accurate test to evaluate their circulation,” according to Dr. Avali.
The other caveat regarding advanced therapy is that appropriate candidates should have a wound edge that’s sharp, not migrating, she continued.
The 4-week cutoff for seeing substantial progress in wound healing with standard therapy before turning to advanced therapies is based in part upon an influential study of 2,517 patients with diabetic neuropathic foot ulcers who received one of three advanced biological therapies. On average, patients started advanced biological therapy within 28 days following their first visit to a specialized wound clinic. Prolonged time to initiation of advanced treatment was associated with significantly longer time to healing (Arch Dermatol. 2010 Aug;146(8):857-62).
While thinking about local wound care in a patient with a diabetic foot ulcer, Dr. Avali said it’s also important to take a step back and make sure hypertension and other conventional cardiovascular risk factors are well controlled and also to take a close look at the HbA1c. In a study of 183 patients with diabetic wounds treated at a specialized academic wound center, for every 1.0% increase in HbA1c above the mean level of 8.0%, the wound-area healing rate was reduced by 0.028 cm2 per day. The implication is that HbA1c may be a key biomarker that’s useful in predicting wound-healing rate in patients with diabetic ulcers (J. Invest Dermatol. 2011 Oct;131(10):2121-7).
She reported serving as a consultant to Acelity and Smith & Nephew.
EXPERT ANALYSIS FROM WCD 2015
Offloading is key to diabetic foot ulcer management
VANCOUVER – Offloading of plantar pressures is the key component of successful management of plantar diabetic foot ulcers, Dr. Afsaneh Alavi asserted at the World Congress of Dermatology.
“Debridement to remove the callous is the most important part of local wound care, but debridement without offloading afterwards will rebuild callous,” explained Dr. Alavi, a University of Toronto dermatologist.
Many options exist in terms of offloading devices. The total contact cast is recognized as the gold standard. It reduces pressures by 90%. Yet a national survey of more than 900 foot clinics found that less than 2% of centers utilized total contact casts for the majority of their patients with plantar diabetic foot ulcers. Only 15% of centers utilized another highly effective option, the removable cast walker, in the majority of patients (Diabetes Care. 2008 Nov; 31(11): 2118–2119). Cost is believed to be a major issue. Other options include custom orthotic inserts and forefoot and heel relief shoes.
The diabetic foot ulcer recurrence rate is extremely high: up to 83% within the first year.
“Counsel patients to use therapeutic shoes and insoles, engage in self-inspection, and get professional foot care. Shoes should be worn at all times, even in the house. Patients typically spend 30%-40% of their time at home,” Dr. Alavi continued.
The pathophysiology of diabetic foot ulcers is a vicious circle of immunopathy, neuropathy, and angiopathy. Callous formation secondary to sensory and motor neuropathy leads to subcutaneous bleeding, ulcer formation, and deeper infection, sometimes with life- or limb-threatening osteomyelitis.
“Detection of this chain of events at the stage of callous formation would make a huge difference,” according to the dermatologist. She pointed out that “Most of our health care system is focused more on management than prevention. We spend more on amputations due to diabetic foot ulcers than for prevention.”
Although many methods of callous removal are available, including autolytic therapy with hydrogels, whirlpools, and wet-to-dry dressings, Dr. Alavi said the best method is sharp surgical debridement. She highlighted a retrospective 12-week study of topical wound treatments in 310 diabetic foot ulcers and 366 chronic venous leg ulcers which found that venous leg ulcers showed a 34% greater median wound surface area reduction following office visits with surgical debridement compared with no surgical debridement. Serial surgical debridement in patients with diabetic foot ulcers was also associated with a 2.35-fold increased likelihood of wound closure (Wound Repair Regen. 2009 May-June;17(3):306-11).
She noted that it has been estimated that by 2030, at least 10% of the world’s adult population -- some 550 million people -- will have diabetes. And a diabetic individual’s lifetime risk of developing diabetic foot ulcers is up to 25%. The 5-year mortality rate following amputation related to a diabetic foot ulcer is nearly 50%, about the same as following diagnosis of colon cancer.
Dr. Alavi reported serving as a consultant to Acelity and Smith & Nephew.
VANCOUVER – Offloading of plantar pressures is the key component of successful management of plantar diabetic foot ulcers, Dr. Afsaneh Alavi asserted at the World Congress of Dermatology.
“Debridement to remove the callous is the most important part of local wound care, but debridement without offloading afterwards will rebuild callous,” explained Dr. Alavi, a University of Toronto dermatologist.
Many options exist in terms of offloading devices. The total contact cast is recognized as the gold standard. It reduces pressures by 90%. Yet a national survey of more than 900 foot clinics found that less than 2% of centers utilized total contact casts for the majority of their patients with plantar diabetic foot ulcers. Only 15% of centers utilized another highly effective option, the removable cast walker, in the majority of patients (Diabetes Care. 2008 Nov; 31(11): 2118–2119). Cost is believed to be a major issue. Other options include custom orthotic inserts and forefoot and heel relief shoes.
The diabetic foot ulcer recurrence rate is extremely high: up to 83% within the first year.
“Counsel patients to use therapeutic shoes and insoles, engage in self-inspection, and get professional foot care. Shoes should be worn at all times, even in the house. Patients typically spend 30%-40% of their time at home,” Dr. Alavi continued.
The pathophysiology of diabetic foot ulcers is a vicious circle of immunopathy, neuropathy, and angiopathy. Callous formation secondary to sensory and motor neuropathy leads to subcutaneous bleeding, ulcer formation, and deeper infection, sometimes with life- or limb-threatening osteomyelitis.
“Detection of this chain of events at the stage of callous formation would make a huge difference,” according to the dermatologist. She pointed out that “Most of our health care system is focused more on management than prevention. We spend more on amputations due to diabetic foot ulcers than for prevention.”
Although many methods of callous removal are available, including autolytic therapy with hydrogels, whirlpools, and wet-to-dry dressings, Dr. Alavi said the best method is sharp surgical debridement. She highlighted a retrospective 12-week study of topical wound treatments in 310 diabetic foot ulcers and 366 chronic venous leg ulcers which found that venous leg ulcers showed a 34% greater median wound surface area reduction following office visits with surgical debridement compared with no surgical debridement. Serial surgical debridement in patients with diabetic foot ulcers was also associated with a 2.35-fold increased likelihood of wound closure (Wound Repair Regen. 2009 May-June;17(3):306-11).
She noted that it has been estimated that by 2030, at least 10% of the world’s adult population -- some 550 million people -- will have diabetes. And a diabetic individual’s lifetime risk of developing diabetic foot ulcers is up to 25%. The 5-year mortality rate following amputation related to a diabetic foot ulcer is nearly 50%, about the same as following diagnosis of colon cancer.
Dr. Alavi reported serving as a consultant to Acelity and Smith & Nephew.
VANCOUVER – Offloading of plantar pressures is the key component of successful management of plantar diabetic foot ulcers, Dr. Afsaneh Alavi asserted at the World Congress of Dermatology.
“Debridement to remove the callous is the most important part of local wound care, but debridement without offloading afterwards will rebuild callous,” explained Dr. Alavi, a University of Toronto dermatologist.
Many options exist in terms of offloading devices. The total contact cast is recognized as the gold standard. It reduces pressures by 90%. Yet a national survey of more than 900 foot clinics found that less than 2% of centers utilized total contact casts for the majority of their patients with plantar diabetic foot ulcers. Only 15% of centers utilized another highly effective option, the removable cast walker, in the majority of patients (Diabetes Care. 2008 Nov; 31(11): 2118–2119). Cost is believed to be a major issue. Other options include custom orthotic inserts and forefoot and heel relief shoes.
The diabetic foot ulcer recurrence rate is extremely high: up to 83% within the first year.
“Counsel patients to use therapeutic shoes and insoles, engage in self-inspection, and get professional foot care. Shoes should be worn at all times, even in the house. Patients typically spend 30%-40% of their time at home,” Dr. Alavi continued.
The pathophysiology of diabetic foot ulcers is a vicious circle of immunopathy, neuropathy, and angiopathy. Callous formation secondary to sensory and motor neuropathy leads to subcutaneous bleeding, ulcer formation, and deeper infection, sometimes with life- or limb-threatening osteomyelitis.
“Detection of this chain of events at the stage of callous formation would make a huge difference,” according to the dermatologist. She pointed out that “Most of our health care system is focused more on management than prevention. We spend more on amputations due to diabetic foot ulcers than for prevention.”
Although many methods of callous removal are available, including autolytic therapy with hydrogels, whirlpools, and wet-to-dry dressings, Dr. Alavi said the best method is sharp surgical debridement. She highlighted a retrospective 12-week study of topical wound treatments in 310 diabetic foot ulcers and 366 chronic venous leg ulcers which found that venous leg ulcers showed a 34% greater median wound surface area reduction following office visits with surgical debridement compared with no surgical debridement. Serial surgical debridement in patients with diabetic foot ulcers was also associated with a 2.35-fold increased likelihood of wound closure (Wound Repair Regen. 2009 May-June;17(3):306-11).
She noted that it has been estimated that by 2030, at least 10% of the world’s adult population -- some 550 million people -- will have diabetes. And a diabetic individual’s lifetime risk of developing diabetic foot ulcers is up to 25%. The 5-year mortality rate following amputation related to a diabetic foot ulcer is nearly 50%, about the same as following diagnosis of colon cancer.
Dr. Alavi reported serving as a consultant to Acelity and Smith & Nephew.
EXPERT ANALYSIS FROM WCD 2015
Add-on liraglutide effective in obese diabetes patients
Liraglutide, used as an adjunct to a low-calorie diet and exercise, improved both weight loss and glycemic control in obese patients with type 2 diabetes, compared with placebo, according to results of a randomized trial published online Aug. in JAMA.
The SCALE Diabetes trial is the first study specifically designed to assess liraglutide’s efficacy of for weight loss in patients with type 2 diabetes, as well as the first study to evaluate the higher (3.0-mg) dose of the drug in this patient population, said Dr. Melanie J. Davies of the Diabetes Research Centre, University of Leicester (England) and her associates.
This trial, conducted at 126 sites in nine countries, involved 846 participants randomly assigned in a double-blind fashion to receive daily self-administered subcutaneous high-dose [3.0 mg] liraglutide (423 patients), low-dose [1.8 mg] liraglutide (211 patients), or placebo (212 patients) for 56 weeks, followed by a 16-week observation period to assess the effects of stopping treatment. All study participants were encouraged to walk briskly for 150 minutes or more per week and to follow a low-calorie diet containing 30% energy from fat, 20% from protein, and 50% from carbohydrates.
The mean body weight at baseline was roughly 106 kg. A total of 23% of patients taking high-dose liraglutide, 22% of those taking low-dose liraglutide, and 34% of the placebo group withdrew before completing the treatment phase of the study.
Mean weight loss was 6.0% of baseline weight (6.4 kg) for high-dose liraglutide and 4.7% (5.0 kg) for low-dose liraglutide, compared with 2.0% (2.2 kg) with placebo. Half of the patients taking high-dose liraglutide and 36% of those taking low-dose liraglutide lost 5% or more of their baseline weight, compared with 13.8% of patients taking placebo. Both active-treatment groups also showed significantly greater reductions in waist circumference, the investigators said (JAMA 2015 Aug 18 [doi:10.1001/jama.2015.9676]).
In addition, liraglutide bested placebo in glycemic control as measured by decrease in hemoglobin A1c level; in the proportion of patients achieving HbA1c targets; and in fasting plasma glucose level, fasting glucagon level, proinsulin level, and other glycemic indexes. More patients taking liraglutide than placebo were able to reduce their use of oral hypoglycemic agents. Liraglutide also improved cardiovascular measures such as systolic blood pressure, total cholesterol, triglycerides, and C-reactive protein. Furthermore, the higher dose of liraglutide significantly improved scores on two measures of health-related quality of life.
Adverse event rates were higher with liraglutide than placebo, with GI effects predominating. There were 87 hypoglycemic events per 100 patient-years of exposure to high-dose liraglutide and 95 per 100 patient-years for low-dose liraglutide, compared with 31 per 100 patient-years for placebo. Heart rate and the incidence of cardiac arrhythmias increased with use of the active drug and returned to normal after the treatment phase concluded. Similarly, serum amylase and lipase activity increased during the treatment phase and returned to baseline level afterward. The long-term clinical relevance of these effects are not yet known, and further research also is needed to assess the effects of longer-term liraglutide therapy, Dr. Davies and her associates noted.
Liraglutide 3 mg (Saxenda*, Novo Nordisk) was approved by the Food and Drug Administration in December 2014 as an adjunct to a reduced-calorie diet and increased physical activity in obese adults with a body mass index of 30 kg/m2 or greater and in overweight adults with a BMI of 27 kg/m2 or greater and at least one weight-related condition such as hypertension, type 2 diabetes, or dyslipidemia. Liraglutide up to 1.8 mg (Victoza) was approved by the FDA in 2010 as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes, and is recommended as second-line therapy.”
SCALE Diabetes was funded by Novo Nordisk. Dr. Davies and her coinvestigators reported ties to Novo Nordisk and numerous other pharmaceutical companies.
*Correction, 8/21/2015: The 3.0-mg dosage of liraglutide was incorrectly identified. The trade name is Saxenda.
Liraglutide, used as an adjunct to a low-calorie diet and exercise, improved both weight loss and glycemic control in obese patients with type 2 diabetes, compared with placebo, according to results of a randomized trial published online Aug. in JAMA.
The SCALE Diabetes trial is the first study specifically designed to assess liraglutide’s efficacy of for weight loss in patients with type 2 diabetes, as well as the first study to evaluate the higher (3.0-mg) dose of the drug in this patient population, said Dr. Melanie J. Davies of the Diabetes Research Centre, University of Leicester (England) and her associates.
This trial, conducted at 126 sites in nine countries, involved 846 participants randomly assigned in a double-blind fashion to receive daily self-administered subcutaneous high-dose [3.0 mg] liraglutide (423 patients), low-dose [1.8 mg] liraglutide (211 patients), or placebo (212 patients) for 56 weeks, followed by a 16-week observation period to assess the effects of stopping treatment. All study participants were encouraged to walk briskly for 150 minutes or more per week and to follow a low-calorie diet containing 30% energy from fat, 20% from protein, and 50% from carbohydrates.
The mean body weight at baseline was roughly 106 kg. A total of 23% of patients taking high-dose liraglutide, 22% of those taking low-dose liraglutide, and 34% of the placebo group withdrew before completing the treatment phase of the study.
Mean weight loss was 6.0% of baseline weight (6.4 kg) for high-dose liraglutide and 4.7% (5.0 kg) for low-dose liraglutide, compared with 2.0% (2.2 kg) with placebo. Half of the patients taking high-dose liraglutide and 36% of those taking low-dose liraglutide lost 5% or more of their baseline weight, compared with 13.8% of patients taking placebo. Both active-treatment groups also showed significantly greater reductions in waist circumference, the investigators said (JAMA 2015 Aug 18 [doi:10.1001/jama.2015.9676]).
In addition, liraglutide bested placebo in glycemic control as measured by decrease in hemoglobin A1c level; in the proportion of patients achieving HbA1c targets; and in fasting plasma glucose level, fasting glucagon level, proinsulin level, and other glycemic indexes. More patients taking liraglutide than placebo were able to reduce their use of oral hypoglycemic agents. Liraglutide also improved cardiovascular measures such as systolic blood pressure, total cholesterol, triglycerides, and C-reactive protein. Furthermore, the higher dose of liraglutide significantly improved scores on two measures of health-related quality of life.
Adverse event rates were higher with liraglutide than placebo, with GI effects predominating. There were 87 hypoglycemic events per 100 patient-years of exposure to high-dose liraglutide and 95 per 100 patient-years for low-dose liraglutide, compared with 31 per 100 patient-years for placebo. Heart rate and the incidence of cardiac arrhythmias increased with use of the active drug and returned to normal after the treatment phase concluded. Similarly, serum amylase and lipase activity increased during the treatment phase and returned to baseline level afterward. The long-term clinical relevance of these effects are not yet known, and further research also is needed to assess the effects of longer-term liraglutide therapy, Dr. Davies and her associates noted.
Liraglutide 3 mg (Saxenda*, Novo Nordisk) was approved by the Food and Drug Administration in December 2014 as an adjunct to a reduced-calorie diet and increased physical activity in obese adults with a body mass index of 30 kg/m2 or greater and in overweight adults with a BMI of 27 kg/m2 or greater and at least one weight-related condition such as hypertension, type 2 diabetes, or dyslipidemia. Liraglutide up to 1.8 mg (Victoza) was approved by the FDA in 2010 as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes, and is recommended as second-line therapy.”
SCALE Diabetes was funded by Novo Nordisk. Dr. Davies and her coinvestigators reported ties to Novo Nordisk and numerous other pharmaceutical companies.
*Correction, 8/21/2015: The 3.0-mg dosage of liraglutide was incorrectly identified. The trade name is Saxenda.
Liraglutide, used as an adjunct to a low-calorie diet and exercise, improved both weight loss and glycemic control in obese patients with type 2 diabetes, compared with placebo, according to results of a randomized trial published online Aug. in JAMA.
The SCALE Diabetes trial is the first study specifically designed to assess liraglutide’s efficacy of for weight loss in patients with type 2 diabetes, as well as the first study to evaluate the higher (3.0-mg) dose of the drug in this patient population, said Dr. Melanie J. Davies of the Diabetes Research Centre, University of Leicester (England) and her associates.
This trial, conducted at 126 sites in nine countries, involved 846 participants randomly assigned in a double-blind fashion to receive daily self-administered subcutaneous high-dose [3.0 mg] liraglutide (423 patients), low-dose [1.8 mg] liraglutide (211 patients), or placebo (212 patients) for 56 weeks, followed by a 16-week observation period to assess the effects of stopping treatment. All study participants were encouraged to walk briskly for 150 minutes or more per week and to follow a low-calorie diet containing 30% energy from fat, 20% from protein, and 50% from carbohydrates.
The mean body weight at baseline was roughly 106 kg. A total of 23% of patients taking high-dose liraglutide, 22% of those taking low-dose liraglutide, and 34% of the placebo group withdrew before completing the treatment phase of the study.
Mean weight loss was 6.0% of baseline weight (6.4 kg) for high-dose liraglutide and 4.7% (5.0 kg) for low-dose liraglutide, compared with 2.0% (2.2 kg) with placebo. Half of the patients taking high-dose liraglutide and 36% of those taking low-dose liraglutide lost 5% or more of their baseline weight, compared with 13.8% of patients taking placebo. Both active-treatment groups also showed significantly greater reductions in waist circumference, the investigators said (JAMA 2015 Aug 18 [doi:10.1001/jama.2015.9676]).
In addition, liraglutide bested placebo in glycemic control as measured by decrease in hemoglobin A1c level; in the proportion of patients achieving HbA1c targets; and in fasting plasma glucose level, fasting glucagon level, proinsulin level, and other glycemic indexes. More patients taking liraglutide than placebo were able to reduce their use of oral hypoglycemic agents. Liraglutide also improved cardiovascular measures such as systolic blood pressure, total cholesterol, triglycerides, and C-reactive protein. Furthermore, the higher dose of liraglutide significantly improved scores on two measures of health-related quality of life.
Adverse event rates were higher with liraglutide than placebo, with GI effects predominating. There were 87 hypoglycemic events per 100 patient-years of exposure to high-dose liraglutide and 95 per 100 patient-years for low-dose liraglutide, compared with 31 per 100 patient-years for placebo. Heart rate and the incidence of cardiac arrhythmias increased with use of the active drug and returned to normal after the treatment phase concluded. Similarly, serum amylase and lipase activity increased during the treatment phase and returned to baseline level afterward. The long-term clinical relevance of these effects are not yet known, and further research also is needed to assess the effects of longer-term liraglutide therapy, Dr. Davies and her associates noted.
Liraglutide 3 mg (Saxenda*, Novo Nordisk) was approved by the Food and Drug Administration in December 2014 as an adjunct to a reduced-calorie diet and increased physical activity in obese adults with a body mass index of 30 kg/m2 or greater and in overweight adults with a BMI of 27 kg/m2 or greater and at least one weight-related condition such as hypertension, type 2 diabetes, or dyslipidemia. Liraglutide up to 1.8 mg (Victoza) was approved by the FDA in 2010 as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes, and is recommended as second-line therapy.”
SCALE Diabetes was funded by Novo Nordisk. Dr. Davies and her coinvestigators reported ties to Novo Nordisk and numerous other pharmaceutical companies.
*Correction, 8/21/2015: The 3.0-mg dosage of liraglutide was incorrectly identified. The trade name is Saxenda.
FROM JAMA
Key clinical point: Liraglutide, along with diet and exercise, improved weight loss and glycemic control in obese patients with type 2 diabetes.
Major finding: Mean weight loss was 6.0% of baseline weight (6.4 kg) for high-dose liraglutide and 4.7% (5.0 kg) for low-dose liraglutide, compared with 2.0% (2.2 kg) with placebo.
Data source: SCALE Diabetes, an international randomized double-blind placebo-controlled clinical trial involving 846 participants treated for 56 weeks and followed for an additional 12 weeks.
Disclosures: SCALE Diabetes was funded by Novo Nordisk. Dr. Davies and her coinvestigators reported ties to Novo Nordisk and numerous other pharmaceutical companies.