User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Will ‘Dr. Disinformation’ ever face the music?
On Sept. 5, Rashid Buttar, DO, posted on Twitter that COVID-19 “was a planned operation” and shared an article alleging that most people who got the COVID vaccine would be dead by 2025.
Others include testimony in June by Sherri Jane Tenpenny, DO, before Ohio state legislators that the vaccine could cause people to become magnetized. Clips from the hearing went viral on the Internet. On April 9, 2020, Joseph Mercola, DO, posted a video titled “Could hydrogen peroxide treat coronavirus?” which was shared more than 4,600 times. In the video, Dr. Mercola said inhaling hydrogen peroxide through a nebulizer could prevent or cure COVID.
These physicians are identified as members of the “Disinformation Dozen,” a group of top superspreaders of COVID vaccine misinformation on social media, according to a 2021 report by the nonprofit Center for Countering Digital Hate. The report, based on an analysis of antivaccine content on social media platforms, found that 12 people were responsible for 65% of it. The group is composed of physicians, antivaccine activists, and people known for promoting alternative medicine.
The physician voices are of particular concern because their medical credentials lend credence to their unproven, often dangerous pronouncements. All three continue to hold medical licenses and have not faced consequences for their COVID-related statements.
But leaders of professional medical organizations increasingly are calling for that to change and urging medical oversight boards to take more aggressive action.
In July, the Federation of State Medical Boards, the national umbrella organization for the state-based boards, issued a statement making clear that doctors who generate and spread COVID misinformation could be subject to disciplinary action, including the suspension or revocation of their licenses. The American Board of Family Medicine, American Board of Internal Medicine, and American Board of Pediatrics issued a joint statement Sept. 9 in support of the state boards’ position, warning that “such unethical or unprofessional conduct may prompt their respective board to take action that could put their certification at risk.”
And the superspreaders identified by the center’s report are not alone. KHN identified 20 other doctors who have made false or misleading claims about COVID by combing through published fact checks and other news coverage.
For example, at an Indiana school board meeting in August, Dan Stock, MD, claimed the surge in covid cases this summer was due to “antibody mediated viral enhancement” from people receiving covid vaccines. PolitiFact rated his claim “Pants on Fire” false.
Stella Immanuel, MD, a member of a group America’s Frontline Doctors, which has consistently made false statements about COVID, said in a video that went viral in July 2020 that masks weren’t needed because covid could be cured by hydroxychloroquine. Dr. Immanuel’s website currently promotes a set of vitamins, as well as hydroxychloroquine and ivermectin, as COVID treatments.
Two of the doctors mentioned by name in this article responded to requests for comment. Dr. Mercola offered documents to rebut criticisms of his hydrogen peroxide COVID treatment and took issue with the center’s “Disinformation Dozen” report methodology. Dr. Buttar defended his positions, saying via email that “the science is clear and anyone who contests it, has a suspect agenda at best and/or lacks a moral compass.” He also pointed to data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Recording System, considered inconclusive by many experts.
Since the onset of the COVID pandemic, misinformation has been widespread on social media platforms. And many experts blame it for undermining efforts to curb the coronavirus’s spread. A recent poll showed that more than 50% of Americans who won’t get vaccinated cited conspiracy theories as their reasons – for example, saying the vaccines cause infertility or alter DNA.
Some physicians have gained notoriety by embracing COVID-related fringe ideas, quack treatments and falsehoods via social media, conservative talk shows, and even in person with patients. Whether promoting the use of ivermectin, an antiparasitic drug for animals, or a mix of vitamins to treat COVID, doctors’ words can be especially powerful. Public opinion polls consistently show that Americans have high trust in doctors.
“There is a sense of credibility that comes with being a doctor,” said Rachel Moran, PhD, a researcher who studies COVID misinformation at the University of Washington. “There is also a sense they have access to insider info that we don’t. This is a very confusing time, and it can seem that if anyone knows what I should be doing in this situation, it’s a doctor.”
While COVID is a novel and complicated infectious disease, physicians spreading misinformation generally have no particular expertise in infectious diseases. Scott Atlas, MD, who endorsed former President Donald Trump’s unproven statements about the course of the pandemic, is a radiation oncologist.
Traditionally, the responsibility of policing physicians has fallen to state medical boards. Beyond overseeing the licensing process, these panels investigate complaints about doctors and discipline those who engage in unethical, unprofessional or, in extreme cases, criminal activity. Any member of the public can submit a complaint about a physician.
“The boards are relatively slow and weak and it’s a long, slow process to pull somebody’s license,” said Arthur Caplan, PhD, founding head of the department of medical ethics at New York University. “In many states, they have their hands full with doctors who have committed felonies, doctors who are molesting their patients. Keeping an eye on misinformation is somewhat down on the priority list.”
To date, only two doctors have reportedly faced such sanctions. In Oregon, Steven LaTulippe, MD, had his license suspended in December 2020 for refusing to wear a face mask at his clinic and telling patients that masks were ineffective in curbing the spread of COVID, and even dangerous. Thomas Cowan, MD, a San Francisco physician who posted a YouTube video that went viral in March 2020 stating that 5G networks cause COVID, voluntarily surrendered his medical license to California’s medical board in February 2021.
Humayun Chaudhry, DO, president of the Federation of State Medical Boards, however, said it’s possible some doctors could already be the subject of inquiries and investigations, since these actions are not made public until sanctions are handed down.
KHN reached out to the medical and osteopathic boards of all 50 states and the District of Columbia to see if they had received COVID misinformation complaints. Of the 43 that responded, only a handful shared specifics.
During a 1-week period in August, Kansas’ medical board received six such complaints. In all, the state has received 35 complaints against 20 licensees about spreading covid misinformation on social media and in person. Indiana has received about 30 in the past year. South Carolina said it had about 10 since January. Rhode Island didn’t share the number of complaints but said it has taken disciplinary action against one doctor for spreading misinformation, though it hasn’t moved to suspend his license. (The disciplinary measures include a fine, a reprimand on the doctor’s record and a mandate to complete an ethics course.) Five states said they had received only a couple, and 11 states reported receiving no complaints regarding COVID misinformation.
Confidentiality laws in 13 states prevented those boards from sharing information about complaints.
Social media companies have also been slow to take action. Some doctors’ accounts – specifically those among the Disinformation Dozen – have been suspended, but others are still active and posting misinformation.
Imran Ahmed, CEO of the Center for Countering Digital Hate, said social media platforms often don’t consistently apply their rules against spreading misinformation.
“Even when it’s the same companies, Facebook will sometimes take posts down, but Instagram will not,” Mr. Ahmed said, referring to Facebook’s ownership of Instagram. “It goes to show their piecemeal, ineffective approach to enforcing their own rules.”
A Facebook spokesperson said the company has removed over 3,000 accounts, pages and groups for repeatedly violating COVID and vaccine misinformation policies since the beginning of the pandemic. Dr. Buttar’s Facebook and Instagram pages and Tenpenny’s Facebook page have been removed, while Dr. Mercola’s Facebook posts have been demoted, which means fewer people will see them. Dr. Tenpenny and Dr. Mercola still have Instagram accounts.
Part of the challenge may be that these doctors sometimes present scientific opinions that aren’t mainstream but are viewed as potentially valid by some of their colleagues.
“It can be difficult to prove that what is being said is outside the range of scientific and medical consensus,” said Dr. Caplan. “The doctors who were advising Trump – like Scott Atlas – recommended herd immunity. That was far from the consensus of epidemiologists, but you couldn’t get a board to take his license away because it was a fringe opinion.”
Even if these physicians don’t face consequences, it is likely, experts said, that the public health will.
“Medical misinformation doesn’t just result in people making bad personal and community health choices, but it also divides communities and families, leaving an emotional toll,” said Dr. Moran. “Misinformation narratives have real sticking power and impact people’s ability to make safe health choices.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On Sept. 5, Rashid Buttar, DO, posted on Twitter that COVID-19 “was a planned operation” and shared an article alleging that most people who got the COVID vaccine would be dead by 2025.
Others include testimony in June by Sherri Jane Tenpenny, DO, before Ohio state legislators that the vaccine could cause people to become magnetized. Clips from the hearing went viral on the Internet. On April 9, 2020, Joseph Mercola, DO, posted a video titled “Could hydrogen peroxide treat coronavirus?” which was shared more than 4,600 times. In the video, Dr. Mercola said inhaling hydrogen peroxide through a nebulizer could prevent or cure COVID.
These physicians are identified as members of the “Disinformation Dozen,” a group of top superspreaders of COVID vaccine misinformation on social media, according to a 2021 report by the nonprofit Center for Countering Digital Hate. The report, based on an analysis of antivaccine content on social media platforms, found that 12 people were responsible for 65% of it. The group is composed of physicians, antivaccine activists, and people known for promoting alternative medicine.
The physician voices are of particular concern because their medical credentials lend credence to their unproven, often dangerous pronouncements. All three continue to hold medical licenses and have not faced consequences for their COVID-related statements.
But leaders of professional medical organizations increasingly are calling for that to change and urging medical oversight boards to take more aggressive action.
In July, the Federation of State Medical Boards, the national umbrella organization for the state-based boards, issued a statement making clear that doctors who generate and spread COVID misinformation could be subject to disciplinary action, including the suspension or revocation of their licenses. The American Board of Family Medicine, American Board of Internal Medicine, and American Board of Pediatrics issued a joint statement Sept. 9 in support of the state boards’ position, warning that “such unethical or unprofessional conduct may prompt their respective board to take action that could put their certification at risk.”
And the superspreaders identified by the center’s report are not alone. KHN identified 20 other doctors who have made false or misleading claims about COVID by combing through published fact checks and other news coverage.
For example, at an Indiana school board meeting in August, Dan Stock, MD, claimed the surge in covid cases this summer was due to “antibody mediated viral enhancement” from people receiving covid vaccines. PolitiFact rated his claim “Pants on Fire” false.
Stella Immanuel, MD, a member of a group America’s Frontline Doctors, which has consistently made false statements about COVID, said in a video that went viral in July 2020 that masks weren’t needed because covid could be cured by hydroxychloroquine. Dr. Immanuel’s website currently promotes a set of vitamins, as well as hydroxychloroquine and ivermectin, as COVID treatments.
Two of the doctors mentioned by name in this article responded to requests for comment. Dr. Mercola offered documents to rebut criticisms of his hydrogen peroxide COVID treatment and took issue with the center’s “Disinformation Dozen” report methodology. Dr. Buttar defended his positions, saying via email that “the science is clear and anyone who contests it, has a suspect agenda at best and/or lacks a moral compass.” He also pointed to data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Recording System, considered inconclusive by many experts.
Since the onset of the COVID pandemic, misinformation has been widespread on social media platforms. And many experts blame it for undermining efforts to curb the coronavirus’s spread. A recent poll showed that more than 50% of Americans who won’t get vaccinated cited conspiracy theories as their reasons – for example, saying the vaccines cause infertility or alter DNA.
Some physicians have gained notoriety by embracing COVID-related fringe ideas, quack treatments and falsehoods via social media, conservative talk shows, and even in person with patients. Whether promoting the use of ivermectin, an antiparasitic drug for animals, or a mix of vitamins to treat COVID, doctors’ words can be especially powerful. Public opinion polls consistently show that Americans have high trust in doctors.
“There is a sense of credibility that comes with being a doctor,” said Rachel Moran, PhD, a researcher who studies COVID misinformation at the University of Washington. “There is also a sense they have access to insider info that we don’t. This is a very confusing time, and it can seem that if anyone knows what I should be doing in this situation, it’s a doctor.”
While COVID is a novel and complicated infectious disease, physicians spreading misinformation generally have no particular expertise in infectious diseases. Scott Atlas, MD, who endorsed former President Donald Trump’s unproven statements about the course of the pandemic, is a radiation oncologist.
Traditionally, the responsibility of policing physicians has fallen to state medical boards. Beyond overseeing the licensing process, these panels investigate complaints about doctors and discipline those who engage in unethical, unprofessional or, in extreme cases, criminal activity. Any member of the public can submit a complaint about a physician.
“The boards are relatively slow and weak and it’s a long, slow process to pull somebody’s license,” said Arthur Caplan, PhD, founding head of the department of medical ethics at New York University. “In many states, they have their hands full with doctors who have committed felonies, doctors who are molesting their patients. Keeping an eye on misinformation is somewhat down on the priority list.”
To date, only two doctors have reportedly faced such sanctions. In Oregon, Steven LaTulippe, MD, had his license suspended in December 2020 for refusing to wear a face mask at his clinic and telling patients that masks were ineffective in curbing the spread of COVID, and even dangerous. Thomas Cowan, MD, a San Francisco physician who posted a YouTube video that went viral in March 2020 stating that 5G networks cause COVID, voluntarily surrendered his medical license to California’s medical board in February 2021.
Humayun Chaudhry, DO, president of the Federation of State Medical Boards, however, said it’s possible some doctors could already be the subject of inquiries and investigations, since these actions are not made public until sanctions are handed down.
KHN reached out to the medical and osteopathic boards of all 50 states and the District of Columbia to see if they had received COVID misinformation complaints. Of the 43 that responded, only a handful shared specifics.
During a 1-week period in August, Kansas’ medical board received six such complaints. In all, the state has received 35 complaints against 20 licensees about spreading covid misinformation on social media and in person. Indiana has received about 30 in the past year. South Carolina said it had about 10 since January. Rhode Island didn’t share the number of complaints but said it has taken disciplinary action against one doctor for spreading misinformation, though it hasn’t moved to suspend his license. (The disciplinary measures include a fine, a reprimand on the doctor’s record and a mandate to complete an ethics course.) Five states said they had received only a couple, and 11 states reported receiving no complaints regarding COVID misinformation.
Confidentiality laws in 13 states prevented those boards from sharing information about complaints.
Social media companies have also been slow to take action. Some doctors’ accounts – specifically those among the Disinformation Dozen – have been suspended, but others are still active and posting misinformation.
Imran Ahmed, CEO of the Center for Countering Digital Hate, said social media platforms often don’t consistently apply their rules against spreading misinformation.
“Even when it’s the same companies, Facebook will sometimes take posts down, but Instagram will not,” Mr. Ahmed said, referring to Facebook’s ownership of Instagram. “It goes to show their piecemeal, ineffective approach to enforcing their own rules.”
A Facebook spokesperson said the company has removed over 3,000 accounts, pages and groups for repeatedly violating COVID and vaccine misinformation policies since the beginning of the pandemic. Dr. Buttar’s Facebook and Instagram pages and Tenpenny’s Facebook page have been removed, while Dr. Mercola’s Facebook posts have been demoted, which means fewer people will see them. Dr. Tenpenny and Dr. Mercola still have Instagram accounts.
Part of the challenge may be that these doctors sometimes present scientific opinions that aren’t mainstream but are viewed as potentially valid by some of their colleagues.
“It can be difficult to prove that what is being said is outside the range of scientific and medical consensus,” said Dr. Caplan. “The doctors who were advising Trump – like Scott Atlas – recommended herd immunity. That was far from the consensus of epidemiologists, but you couldn’t get a board to take his license away because it was a fringe opinion.”
Even if these physicians don’t face consequences, it is likely, experts said, that the public health will.
“Medical misinformation doesn’t just result in people making bad personal and community health choices, but it also divides communities and families, leaving an emotional toll,” said Dr. Moran. “Misinformation narratives have real sticking power and impact people’s ability to make safe health choices.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On Sept. 5, Rashid Buttar, DO, posted on Twitter that COVID-19 “was a planned operation” and shared an article alleging that most people who got the COVID vaccine would be dead by 2025.
Others include testimony in June by Sherri Jane Tenpenny, DO, before Ohio state legislators that the vaccine could cause people to become magnetized. Clips from the hearing went viral on the Internet. On April 9, 2020, Joseph Mercola, DO, posted a video titled “Could hydrogen peroxide treat coronavirus?” which was shared more than 4,600 times. In the video, Dr. Mercola said inhaling hydrogen peroxide through a nebulizer could prevent or cure COVID.
These physicians are identified as members of the “Disinformation Dozen,” a group of top superspreaders of COVID vaccine misinformation on social media, according to a 2021 report by the nonprofit Center for Countering Digital Hate. The report, based on an analysis of antivaccine content on social media platforms, found that 12 people were responsible for 65% of it. The group is composed of physicians, antivaccine activists, and people known for promoting alternative medicine.
The physician voices are of particular concern because their medical credentials lend credence to their unproven, often dangerous pronouncements. All three continue to hold medical licenses and have not faced consequences for their COVID-related statements.
But leaders of professional medical organizations increasingly are calling for that to change and urging medical oversight boards to take more aggressive action.
In July, the Federation of State Medical Boards, the national umbrella organization for the state-based boards, issued a statement making clear that doctors who generate and spread COVID misinformation could be subject to disciplinary action, including the suspension or revocation of their licenses. The American Board of Family Medicine, American Board of Internal Medicine, and American Board of Pediatrics issued a joint statement Sept. 9 in support of the state boards’ position, warning that “such unethical or unprofessional conduct may prompt their respective board to take action that could put their certification at risk.”
And the superspreaders identified by the center’s report are not alone. KHN identified 20 other doctors who have made false or misleading claims about COVID by combing through published fact checks and other news coverage.
For example, at an Indiana school board meeting in August, Dan Stock, MD, claimed the surge in covid cases this summer was due to “antibody mediated viral enhancement” from people receiving covid vaccines. PolitiFact rated his claim “Pants on Fire” false.
Stella Immanuel, MD, a member of a group America’s Frontline Doctors, which has consistently made false statements about COVID, said in a video that went viral in July 2020 that masks weren’t needed because covid could be cured by hydroxychloroquine. Dr. Immanuel’s website currently promotes a set of vitamins, as well as hydroxychloroquine and ivermectin, as COVID treatments.
Two of the doctors mentioned by name in this article responded to requests for comment. Dr. Mercola offered documents to rebut criticisms of his hydrogen peroxide COVID treatment and took issue with the center’s “Disinformation Dozen” report methodology. Dr. Buttar defended his positions, saying via email that “the science is clear and anyone who contests it, has a suspect agenda at best and/or lacks a moral compass.” He also pointed to data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Recording System, considered inconclusive by many experts.
Since the onset of the COVID pandemic, misinformation has been widespread on social media platforms. And many experts blame it for undermining efforts to curb the coronavirus’s spread. A recent poll showed that more than 50% of Americans who won’t get vaccinated cited conspiracy theories as their reasons – for example, saying the vaccines cause infertility or alter DNA.
Some physicians have gained notoriety by embracing COVID-related fringe ideas, quack treatments and falsehoods via social media, conservative talk shows, and even in person with patients. Whether promoting the use of ivermectin, an antiparasitic drug for animals, or a mix of vitamins to treat COVID, doctors’ words can be especially powerful. Public opinion polls consistently show that Americans have high trust in doctors.
“There is a sense of credibility that comes with being a doctor,” said Rachel Moran, PhD, a researcher who studies COVID misinformation at the University of Washington. “There is also a sense they have access to insider info that we don’t. This is a very confusing time, and it can seem that if anyone knows what I should be doing in this situation, it’s a doctor.”
While COVID is a novel and complicated infectious disease, physicians spreading misinformation generally have no particular expertise in infectious diseases. Scott Atlas, MD, who endorsed former President Donald Trump’s unproven statements about the course of the pandemic, is a radiation oncologist.
Traditionally, the responsibility of policing physicians has fallen to state medical boards. Beyond overseeing the licensing process, these panels investigate complaints about doctors and discipline those who engage in unethical, unprofessional or, in extreme cases, criminal activity. Any member of the public can submit a complaint about a physician.
“The boards are relatively slow and weak and it’s a long, slow process to pull somebody’s license,” said Arthur Caplan, PhD, founding head of the department of medical ethics at New York University. “In many states, they have their hands full with doctors who have committed felonies, doctors who are molesting their patients. Keeping an eye on misinformation is somewhat down on the priority list.”
To date, only two doctors have reportedly faced such sanctions. In Oregon, Steven LaTulippe, MD, had his license suspended in December 2020 for refusing to wear a face mask at his clinic and telling patients that masks were ineffective in curbing the spread of COVID, and even dangerous. Thomas Cowan, MD, a San Francisco physician who posted a YouTube video that went viral in March 2020 stating that 5G networks cause COVID, voluntarily surrendered his medical license to California’s medical board in February 2021.
Humayun Chaudhry, DO, president of the Federation of State Medical Boards, however, said it’s possible some doctors could already be the subject of inquiries and investigations, since these actions are not made public until sanctions are handed down.
KHN reached out to the medical and osteopathic boards of all 50 states and the District of Columbia to see if they had received COVID misinformation complaints. Of the 43 that responded, only a handful shared specifics.
During a 1-week period in August, Kansas’ medical board received six such complaints. In all, the state has received 35 complaints against 20 licensees about spreading covid misinformation on social media and in person. Indiana has received about 30 in the past year. South Carolina said it had about 10 since January. Rhode Island didn’t share the number of complaints but said it has taken disciplinary action against one doctor for spreading misinformation, though it hasn’t moved to suspend his license. (The disciplinary measures include a fine, a reprimand on the doctor’s record and a mandate to complete an ethics course.) Five states said they had received only a couple, and 11 states reported receiving no complaints regarding COVID misinformation.
Confidentiality laws in 13 states prevented those boards from sharing information about complaints.
Social media companies have also been slow to take action. Some doctors’ accounts – specifically those among the Disinformation Dozen – have been suspended, but others are still active and posting misinformation.
Imran Ahmed, CEO of the Center for Countering Digital Hate, said social media platforms often don’t consistently apply their rules against spreading misinformation.
“Even when it’s the same companies, Facebook will sometimes take posts down, but Instagram will not,” Mr. Ahmed said, referring to Facebook’s ownership of Instagram. “It goes to show their piecemeal, ineffective approach to enforcing their own rules.”
A Facebook spokesperson said the company has removed over 3,000 accounts, pages and groups for repeatedly violating COVID and vaccine misinformation policies since the beginning of the pandemic. Dr. Buttar’s Facebook and Instagram pages and Tenpenny’s Facebook page have been removed, while Dr. Mercola’s Facebook posts have been demoted, which means fewer people will see them. Dr. Tenpenny and Dr. Mercola still have Instagram accounts.
Part of the challenge may be that these doctors sometimes present scientific opinions that aren’t mainstream but are viewed as potentially valid by some of their colleagues.
“It can be difficult to prove that what is being said is outside the range of scientific and medical consensus,” said Dr. Caplan. “The doctors who were advising Trump – like Scott Atlas – recommended herd immunity. That was far from the consensus of epidemiologists, but you couldn’t get a board to take his license away because it was a fringe opinion.”
Even if these physicians don’t face consequences, it is likely, experts said, that the public health will.
“Medical misinformation doesn’t just result in people making bad personal and community health choices, but it also divides communities and families, leaving an emotional toll,” said Dr. Moran. “Misinformation narratives have real sticking power and impact people’s ability to make safe health choices.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Watchful waiting in BCC: Which patients can benefit?
Basal cell carcinomas (BCCs), the most common form of skin cancer, are generally slow-growing tumors that occur in older patients.
Given the low rates of metastasis and mortality associated with BCC, some patients do not require treatment. However, there have been no evidence-based recommendations on who may benefit from a watch-and-wait approach.
.
The investigators found that, for older people with low-grade BCCs and limited life expectancy, the risks associated with surgery – bleeding, infection, and wound dehiscence – appeared to outweigh the advantages. According to the authors, these patients “might not live long enough to benefit from treatment.”
This finding mirrors oncologists’ observations regarding low-risk prostate cancer, for which watchful waiting is now the standard of care.
“At present, however, procedure rates [for patients with BCC] increase with age, and many basal cell carcinomas are treated surgically regardless of a patient’s life expectancy,” Eleni Linos, MD, PhD, professor of dermatology at Stanford (Calif.) University, and Mary-Margaret Chren, MD, chair of dermatology at Vanderbilt University, Nashville, Tenn., write in a viewpoint article published in August in JAMA Internal Medicine.
Considering the current treatment patterns for BCC, patients would “benefit from the existence of an evidence-based standard of care that includes active surveillance,” Mackenzie Wehner, MD, assistant professor at MD Anderson Cancer Center, Houston, Tex., writes in an editorial that accompanies the article in JAMA Dermatology.
Insights from the Dutch study
The article in JAMA Dermatology presents a cohort study conducted at Radboud University Medical Center in Nijmegen, the Netherlands. The study included 89 patients who were managed with watchful waiting. The patients received no treatment for at least 3 months following their diagnoses.
The median age of the patients was 83 years. The patients had a total of 280 BCCs. The median initial diameter of the BCCs was 9.5 mm. Just over half of the patients were men, and about half of the BCCs were in the head and neck region.
The median follow-up was 9 months; the maximum follow-up was 6.5 years. Remarkably, the investigators say, more than half the tumors (53.2%) did not grow, and some even shrank. The majority of patients were asymptomatic at presentation, and fewer than 10% developed new symptoms, such as bleeding and itching, during follow-up.
Among the tumors that did grow, 70% were low-risk superficial/nodular tumors, which only increased in size by an estimated 1.06 mm over a year. Thirty percent were higher-risk micronodular/infiltrative tumors, which grew an estimated 4.46 mm over a 12-month period.
About two-thirds of patients eventually chose to have at least one of their BCCs removed after a median of about 7 months. Only three BCCs (2.8%) needed more extensive surgery – reconstructive surgery, rather than primary closure, for instance – than would have been necessary with an earlier excision.
No deaths from BCC were reported in the study.
The investigators tracked the reasons patients opted for watchful waiting. Many understood that their tumors likely would not cause problems in their remaining years. Others prioritized dealing with more pressing health or family problems. Logistics came into play for some, such as not having reliable transportation for hospital visits.
“In patients with [limited life expectancy] and asymptomatic low-risk tumors, [watchful waiting] should be discussed as a potentially appropriate approach,” the investigators, led by Marieke E. C. van Winden, MD, a dermatology resident at Radboud University, conclude.
For patients who wish to pursue a watchful waiting approach, the Dutch team recommends conducting follow-up visits every 3-6 months to see whether patients wish to continue with watchful waiting and to determine whether the risk-to-benefit ratio has shifted.
These recommendations are in line with criteria Dr. Linos and Dr. Chren propose in their viewpoint article in JAMA Internal Medicine. They characterize low-risk BCCs as asymptomatic, smaller than 1 cm in diameter, and located on the trunk or extremities in immunocompetent patients. They note that details regarding active surveillance for BCCs need to be worked out.
“Active surveillance should be studied as a management option because it is supported by the available evidence, congruent with the care of other low-risk cancers, and in accord with principles of shared decision-making,” Dr. Linos and Dr. Chren write.
No funding source was reported. Dr. Wehner, Dr. van Winden, Dr. Linos, and Dr. Chren have disclosed no relevant financial relationships. Two of Dr. van Winden’s coauthors report ties to several companies, including Sanofi Genzyme, AbbVie, Novartis, and Janssen.
A version of this article first appeared on Medscape.com.
Basal cell carcinomas (BCCs), the most common form of skin cancer, are generally slow-growing tumors that occur in older patients.
Given the low rates of metastasis and mortality associated with BCC, some patients do not require treatment. However, there have been no evidence-based recommendations on who may benefit from a watch-and-wait approach.
.
The investigators found that, for older people with low-grade BCCs and limited life expectancy, the risks associated with surgery – bleeding, infection, and wound dehiscence – appeared to outweigh the advantages. According to the authors, these patients “might not live long enough to benefit from treatment.”
This finding mirrors oncologists’ observations regarding low-risk prostate cancer, for which watchful waiting is now the standard of care.
“At present, however, procedure rates [for patients with BCC] increase with age, and many basal cell carcinomas are treated surgically regardless of a patient’s life expectancy,” Eleni Linos, MD, PhD, professor of dermatology at Stanford (Calif.) University, and Mary-Margaret Chren, MD, chair of dermatology at Vanderbilt University, Nashville, Tenn., write in a viewpoint article published in August in JAMA Internal Medicine.
Considering the current treatment patterns for BCC, patients would “benefit from the existence of an evidence-based standard of care that includes active surveillance,” Mackenzie Wehner, MD, assistant professor at MD Anderson Cancer Center, Houston, Tex., writes in an editorial that accompanies the article in JAMA Dermatology.
Insights from the Dutch study
The article in JAMA Dermatology presents a cohort study conducted at Radboud University Medical Center in Nijmegen, the Netherlands. The study included 89 patients who were managed with watchful waiting. The patients received no treatment for at least 3 months following their diagnoses.
The median age of the patients was 83 years. The patients had a total of 280 BCCs. The median initial diameter of the BCCs was 9.5 mm. Just over half of the patients were men, and about half of the BCCs were in the head and neck region.
The median follow-up was 9 months; the maximum follow-up was 6.5 years. Remarkably, the investigators say, more than half the tumors (53.2%) did not grow, and some even shrank. The majority of patients were asymptomatic at presentation, and fewer than 10% developed new symptoms, such as bleeding and itching, during follow-up.
Among the tumors that did grow, 70% were low-risk superficial/nodular tumors, which only increased in size by an estimated 1.06 mm over a year. Thirty percent were higher-risk micronodular/infiltrative tumors, which grew an estimated 4.46 mm over a 12-month period.
About two-thirds of patients eventually chose to have at least one of their BCCs removed after a median of about 7 months. Only three BCCs (2.8%) needed more extensive surgery – reconstructive surgery, rather than primary closure, for instance – than would have been necessary with an earlier excision.
No deaths from BCC were reported in the study.
The investigators tracked the reasons patients opted for watchful waiting. Many understood that their tumors likely would not cause problems in their remaining years. Others prioritized dealing with more pressing health or family problems. Logistics came into play for some, such as not having reliable transportation for hospital visits.
“In patients with [limited life expectancy] and asymptomatic low-risk tumors, [watchful waiting] should be discussed as a potentially appropriate approach,” the investigators, led by Marieke E. C. van Winden, MD, a dermatology resident at Radboud University, conclude.
For patients who wish to pursue a watchful waiting approach, the Dutch team recommends conducting follow-up visits every 3-6 months to see whether patients wish to continue with watchful waiting and to determine whether the risk-to-benefit ratio has shifted.
These recommendations are in line with criteria Dr. Linos and Dr. Chren propose in their viewpoint article in JAMA Internal Medicine. They characterize low-risk BCCs as asymptomatic, smaller than 1 cm in diameter, and located on the trunk or extremities in immunocompetent patients. They note that details regarding active surveillance for BCCs need to be worked out.
“Active surveillance should be studied as a management option because it is supported by the available evidence, congruent with the care of other low-risk cancers, and in accord with principles of shared decision-making,” Dr. Linos and Dr. Chren write.
No funding source was reported. Dr. Wehner, Dr. van Winden, Dr. Linos, and Dr. Chren have disclosed no relevant financial relationships. Two of Dr. van Winden’s coauthors report ties to several companies, including Sanofi Genzyme, AbbVie, Novartis, and Janssen.
A version of this article first appeared on Medscape.com.
Basal cell carcinomas (BCCs), the most common form of skin cancer, are generally slow-growing tumors that occur in older patients.
Given the low rates of metastasis and mortality associated with BCC, some patients do not require treatment. However, there have been no evidence-based recommendations on who may benefit from a watch-and-wait approach.
.
The investigators found that, for older people with low-grade BCCs and limited life expectancy, the risks associated with surgery – bleeding, infection, and wound dehiscence – appeared to outweigh the advantages. According to the authors, these patients “might not live long enough to benefit from treatment.”
This finding mirrors oncologists’ observations regarding low-risk prostate cancer, for which watchful waiting is now the standard of care.
“At present, however, procedure rates [for patients with BCC] increase with age, and many basal cell carcinomas are treated surgically regardless of a patient’s life expectancy,” Eleni Linos, MD, PhD, professor of dermatology at Stanford (Calif.) University, and Mary-Margaret Chren, MD, chair of dermatology at Vanderbilt University, Nashville, Tenn., write in a viewpoint article published in August in JAMA Internal Medicine.
Considering the current treatment patterns for BCC, patients would “benefit from the existence of an evidence-based standard of care that includes active surveillance,” Mackenzie Wehner, MD, assistant professor at MD Anderson Cancer Center, Houston, Tex., writes in an editorial that accompanies the article in JAMA Dermatology.
Insights from the Dutch study
The article in JAMA Dermatology presents a cohort study conducted at Radboud University Medical Center in Nijmegen, the Netherlands. The study included 89 patients who were managed with watchful waiting. The patients received no treatment for at least 3 months following their diagnoses.
The median age of the patients was 83 years. The patients had a total of 280 BCCs. The median initial diameter of the BCCs was 9.5 mm. Just over half of the patients were men, and about half of the BCCs were in the head and neck region.
The median follow-up was 9 months; the maximum follow-up was 6.5 years. Remarkably, the investigators say, more than half the tumors (53.2%) did not grow, and some even shrank. The majority of patients were asymptomatic at presentation, and fewer than 10% developed new symptoms, such as bleeding and itching, during follow-up.
Among the tumors that did grow, 70% were low-risk superficial/nodular tumors, which only increased in size by an estimated 1.06 mm over a year. Thirty percent were higher-risk micronodular/infiltrative tumors, which grew an estimated 4.46 mm over a 12-month period.
About two-thirds of patients eventually chose to have at least one of their BCCs removed after a median of about 7 months. Only three BCCs (2.8%) needed more extensive surgery – reconstructive surgery, rather than primary closure, for instance – than would have been necessary with an earlier excision.
No deaths from BCC were reported in the study.
The investigators tracked the reasons patients opted for watchful waiting. Many understood that their tumors likely would not cause problems in their remaining years. Others prioritized dealing with more pressing health or family problems. Logistics came into play for some, such as not having reliable transportation for hospital visits.
“In patients with [limited life expectancy] and asymptomatic low-risk tumors, [watchful waiting] should be discussed as a potentially appropriate approach,” the investigators, led by Marieke E. C. van Winden, MD, a dermatology resident at Radboud University, conclude.
For patients who wish to pursue a watchful waiting approach, the Dutch team recommends conducting follow-up visits every 3-6 months to see whether patients wish to continue with watchful waiting and to determine whether the risk-to-benefit ratio has shifted.
These recommendations are in line with criteria Dr. Linos and Dr. Chren propose in their viewpoint article in JAMA Internal Medicine. They characterize low-risk BCCs as asymptomatic, smaller than 1 cm in diameter, and located on the trunk or extremities in immunocompetent patients. They note that details regarding active surveillance for BCCs need to be worked out.
“Active surveillance should be studied as a management option because it is supported by the available evidence, congruent with the care of other low-risk cancers, and in accord with principles of shared decision-making,” Dr. Linos and Dr. Chren write.
No funding source was reported. Dr. Wehner, Dr. van Winden, Dr. Linos, and Dr. Chren have disclosed no relevant financial relationships. Two of Dr. van Winden’s coauthors report ties to several companies, including Sanofi Genzyme, AbbVie, Novartis, and Janssen.
A version of this article first appeared on Medscape.com.
Should I get a COVID-19 booster shot?
When I was in Florida a few weeks ago, I met a friend outside who approached me wearing an N-95 mask. He said he was wearing it because the Delta variant was running wild in Florida, and several of his younger unvaccinated employees had contracted it, and he encouraged me to get a COVID booster shot. In the late summer, although the federal government recommended booster shots for anyone 8 months after their original vaccination series, national confusion still reigns, with an Food and Drug Administration advisory panel more recently recommending against a Pfizer booster for all adults, but supporting a booster for those ages 65 and older or at a high risk for severe COVID-19.
At the end of December, I was excited when the local hospital whose staff I am on made the Moderna vaccine available. I had to wait several hours but it was worth it, and I did not care about the low-grade fever and malaise I experienced after the second dose. Astoundingly, I still have patients who have not been vaccinated, although many of them are elderly, frail, or immunocompromised. I think people who publicly argue against vaccination need to visit their local intensive care unit.
While less so than some other physicians, – and you must lean in to see anything. We take all reasonable precautions, wearing masks, wiping down exam rooms and door handles, keeping the waiting room as empty as possible, using HEPA filters, and keeping exhaust fans going in the rooms continuously. My staff have all been vaccinated (I’m lucky there).
Still, if you are seeing 30 or 40 patients a day of all age groups and working in small unventilated rooms, you could be exposed to the Delta variant. While breakthrough infections among fully vaccinated immunocompetent individuals may be rare, if you do develop a breakthrough case, even if mild or asymptomatic, CDC recommendations include quarantining for at least 10 days. Obviously, this can be disastrous to your practice as a COVID infection works through your office.
This brings us to back to booster shots. Personally, I think all health care workers should be eager to get a booster shot. I also think individuals who have wide public exposure, particularly indoors, such as teachers and retail sales workers, should be eager to get one too. Here are some of the pros, as well as some cons for boosters.
Arguments for booster shots
- Booster shots should elevate your antibody levels and make you more resistant to breakthrough infections, but this is still theoretical. Antibody levels decline over time – more rapidly in those over 56 years of age.
- Vaccine doses go to waste every month in the United States, although specific numbers are lacking.
- Vaccinated individuals almost never get hospitalized and die from COVID, presumably even fewer do so after receiving a booster.
- You could unwittingly become a vector. Many of the breakthrough infections are mild and without symptoms. If you do test positive, it could be devastating to your patients, and your medical practice.
Arguments against booster shots
- These vaccine doses should be going to other countries that have low vaccination levels where many of the nasty variants are developing.
- You may have side effects from the vaccine, though thrombosis has only been seen with the Astra-Zeneca and Johnson and Johnson vaccines. Myocarditis is usually seen in younger patients and is almost always self limited.
- Breakthrough infections are rare.
This COVID pandemic is moving and changing so fast, it is bewildering. But with a little luck, COVID could eventually become an annual nuisance like the flu, and the COVID vaccine will become an annual shot based on the newest mutations. For now, my opinion is, get your booster shot.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
When I was in Florida a few weeks ago, I met a friend outside who approached me wearing an N-95 mask. He said he was wearing it because the Delta variant was running wild in Florida, and several of his younger unvaccinated employees had contracted it, and he encouraged me to get a COVID booster shot. In the late summer, although the federal government recommended booster shots for anyone 8 months after their original vaccination series, national confusion still reigns, with an Food and Drug Administration advisory panel more recently recommending against a Pfizer booster for all adults, but supporting a booster for those ages 65 and older or at a high risk for severe COVID-19.
At the end of December, I was excited when the local hospital whose staff I am on made the Moderna vaccine available. I had to wait several hours but it was worth it, and I did not care about the low-grade fever and malaise I experienced after the second dose. Astoundingly, I still have patients who have not been vaccinated, although many of them are elderly, frail, or immunocompromised. I think people who publicly argue against vaccination need to visit their local intensive care unit.
While less so than some other physicians, – and you must lean in to see anything. We take all reasonable precautions, wearing masks, wiping down exam rooms and door handles, keeping the waiting room as empty as possible, using HEPA filters, and keeping exhaust fans going in the rooms continuously. My staff have all been vaccinated (I’m lucky there).
Still, if you are seeing 30 or 40 patients a day of all age groups and working in small unventilated rooms, you could be exposed to the Delta variant. While breakthrough infections among fully vaccinated immunocompetent individuals may be rare, if you do develop a breakthrough case, even if mild or asymptomatic, CDC recommendations include quarantining for at least 10 days. Obviously, this can be disastrous to your practice as a COVID infection works through your office.
This brings us to back to booster shots. Personally, I think all health care workers should be eager to get a booster shot. I also think individuals who have wide public exposure, particularly indoors, such as teachers and retail sales workers, should be eager to get one too. Here are some of the pros, as well as some cons for boosters.
Arguments for booster shots
- Booster shots should elevate your antibody levels and make you more resistant to breakthrough infections, but this is still theoretical. Antibody levels decline over time – more rapidly in those over 56 years of age.
- Vaccine doses go to waste every month in the United States, although specific numbers are lacking.
- Vaccinated individuals almost never get hospitalized and die from COVID, presumably even fewer do so after receiving a booster.
- You could unwittingly become a vector. Many of the breakthrough infections are mild and without symptoms. If you do test positive, it could be devastating to your patients, and your medical practice.
Arguments against booster shots
- These vaccine doses should be going to other countries that have low vaccination levels where many of the nasty variants are developing.
- You may have side effects from the vaccine, though thrombosis has only been seen with the Astra-Zeneca and Johnson and Johnson vaccines. Myocarditis is usually seen in younger patients and is almost always self limited.
- Breakthrough infections are rare.
This COVID pandemic is moving and changing so fast, it is bewildering. But with a little luck, COVID could eventually become an annual nuisance like the flu, and the COVID vaccine will become an annual shot based on the newest mutations. For now, my opinion is, get your booster shot.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
When I was in Florida a few weeks ago, I met a friend outside who approached me wearing an N-95 mask. He said he was wearing it because the Delta variant was running wild in Florida, and several of his younger unvaccinated employees had contracted it, and he encouraged me to get a COVID booster shot. In the late summer, although the federal government recommended booster shots for anyone 8 months after their original vaccination series, national confusion still reigns, with an Food and Drug Administration advisory panel more recently recommending against a Pfizer booster for all adults, but supporting a booster for those ages 65 and older or at a high risk for severe COVID-19.
At the end of December, I was excited when the local hospital whose staff I am on made the Moderna vaccine available. I had to wait several hours but it was worth it, and I did not care about the low-grade fever and malaise I experienced after the second dose. Astoundingly, I still have patients who have not been vaccinated, although many of them are elderly, frail, or immunocompromised. I think people who publicly argue against vaccination need to visit their local intensive care unit.
While less so than some other physicians, – and you must lean in to see anything. We take all reasonable precautions, wearing masks, wiping down exam rooms and door handles, keeping the waiting room as empty as possible, using HEPA filters, and keeping exhaust fans going in the rooms continuously. My staff have all been vaccinated (I’m lucky there).
Still, if you are seeing 30 or 40 patients a day of all age groups and working in small unventilated rooms, you could be exposed to the Delta variant. While breakthrough infections among fully vaccinated immunocompetent individuals may be rare, if you do develop a breakthrough case, even if mild or asymptomatic, CDC recommendations include quarantining for at least 10 days. Obviously, this can be disastrous to your practice as a COVID infection works through your office.
This brings us to back to booster shots. Personally, I think all health care workers should be eager to get a booster shot. I also think individuals who have wide public exposure, particularly indoors, such as teachers and retail sales workers, should be eager to get one too. Here are some of the pros, as well as some cons for boosters.
Arguments for booster shots
- Booster shots should elevate your antibody levels and make you more resistant to breakthrough infections, but this is still theoretical. Antibody levels decline over time – more rapidly in those over 56 years of age.
- Vaccine doses go to waste every month in the United States, although specific numbers are lacking.
- Vaccinated individuals almost never get hospitalized and die from COVID, presumably even fewer do so after receiving a booster.
- You could unwittingly become a vector. Many of the breakthrough infections are mild and without symptoms. If you do test positive, it could be devastating to your patients, and your medical practice.
Arguments against booster shots
- These vaccine doses should be going to other countries that have low vaccination levels where many of the nasty variants are developing.
- You may have side effects from the vaccine, though thrombosis has only been seen with the Astra-Zeneca and Johnson and Johnson vaccines. Myocarditis is usually seen in younger patients and is almost always self limited.
- Breakthrough infections are rare.
This COVID pandemic is moving and changing so fast, it is bewildering. But with a little luck, COVID could eventually become an annual nuisance like the flu, and the COVID vaccine will become an annual shot based on the newest mutations. For now, my opinion is, get your booster shot.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Doctor who claimed masks hurt health loses license
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Noise in medicine
A 26-year-old woman who reports a history of acyclovir-resistant herpes complains of a recurring, stinging rash around her mouth. Topical tacrolimus made it worse, she said. On exam, she has somewhat grouped pustules on her cutaneous lip. I mentioned her to colleagues, saying: “I’ve a patient with acyclovir-resistant herpes who isn’t improving on high-dose Valtrex.” They proffered a few alternative diagnoses and treatment recommendations. I tried several to no avail.
(it is after all only one condition). Nobel Prize–winning economist Daniel Kahneman, PhD, with two other authors, has written a brilliant book about this cognitive unreliability called “Noise: A Flaw in Human Judgment” (New York: Hachette Book Group, 2021).
Both bias and noise create trouble for us. Although biases get more attention, noise is both more prevalent and insidious. In a 2016 article, Dr. Kahneman and coauthors use a bathroom scale as an analogy to explain the difference. “We would say that the scale is biased if its readings are generally either too high or too low. A scale that consistently underestimates true weight by exactly 4 pounds is seriously biased but free of noise. A scale that gives two different readings when you step on it twice is noisy.” In the case presented, “measurements” by me and my colleagues were returning different “readings.” There is one true diagnosis and best treatment, yet because of noise, we waste time and resources by not getting it right the first time.
There is also evidence of bias in this case. For example, there’s probably some confirmation bias: The patient said she has a history of antiviral-resistant herpes; therefore, her rash might appear to be herpes. Also there might be salience bias: it’s easy to see how prominent pustules might be herpes simplex virus. Noise is an issue in many misdiagnoses, but trickier to see. In most instances, we don’t have the opportunity to get multiple assessments of the same case. When examined though, interrater reliability in medicine is often found to be shockingly low, an indication of how much noise there is in our clinical judgments. This leads to waste, frustration – and can even be dangerous when we’re trying to diagnose cancers such as melanoma, lung, or breast cancer.
Dr. Kahneman and colleagues have excellent recommendations on how to reduce noise, such as tips for good decision hygiene (e.g., using differential diagnoses) and using algorithms (e.g., calculating Apgar or LACE scores). I also liked their strategy of aggregating expert opinions. Fascinatingly, averaging multiple independent assessments is mathematically guaranteed to reduce noise. (God, I love economists). This is true of measurements and opinions: If you use 100 judgments for a case, you reduce noise by 90% (the noise is divided by the square root of the number of judgments averaged). So 20 colleagues’ opinions would reduce noise by almost 80%. However, those 20 opinions must be independent to avoid spurious agreement. (Again, math for the win.)
I showed photos of my patient to a few other dermatologists. They independently returned the same result: perioral dermatitis. This was the correct diagnosis and reminded me why grand rounds and tumor boards are such a great help. Multiple, independent assessments are more likely to get it right than just one opinion because we are canceling out the noise. But remember, grand rounds has to be old-school style – no looking at your coresident answers before giving yours!
Our patient cleared after restarting her topical tacrolimus and a bit of doxycycline. Credit the wisdom of the crowd. Reassuringly though, Dr. Kahneman also shows that expertise does matter in minimizing error. So that fellowship you did was still a great idea.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He reports having no conflicts of interest. Write to him at [email protected].
A 26-year-old woman who reports a history of acyclovir-resistant herpes complains of a recurring, stinging rash around her mouth. Topical tacrolimus made it worse, she said. On exam, she has somewhat grouped pustules on her cutaneous lip. I mentioned her to colleagues, saying: “I’ve a patient with acyclovir-resistant herpes who isn’t improving on high-dose Valtrex.” They proffered a few alternative diagnoses and treatment recommendations. I tried several to no avail.
(it is after all only one condition). Nobel Prize–winning economist Daniel Kahneman, PhD, with two other authors, has written a brilliant book about this cognitive unreliability called “Noise: A Flaw in Human Judgment” (New York: Hachette Book Group, 2021).
Both bias and noise create trouble for us. Although biases get more attention, noise is both more prevalent and insidious. In a 2016 article, Dr. Kahneman and coauthors use a bathroom scale as an analogy to explain the difference. “We would say that the scale is biased if its readings are generally either too high or too low. A scale that consistently underestimates true weight by exactly 4 pounds is seriously biased but free of noise. A scale that gives two different readings when you step on it twice is noisy.” In the case presented, “measurements” by me and my colleagues were returning different “readings.” There is one true diagnosis and best treatment, yet because of noise, we waste time and resources by not getting it right the first time.
There is also evidence of bias in this case. For example, there’s probably some confirmation bias: The patient said she has a history of antiviral-resistant herpes; therefore, her rash might appear to be herpes. Also there might be salience bias: it’s easy to see how prominent pustules might be herpes simplex virus. Noise is an issue in many misdiagnoses, but trickier to see. In most instances, we don’t have the opportunity to get multiple assessments of the same case. When examined though, interrater reliability in medicine is often found to be shockingly low, an indication of how much noise there is in our clinical judgments. This leads to waste, frustration – and can even be dangerous when we’re trying to diagnose cancers such as melanoma, lung, or breast cancer.
Dr. Kahneman and colleagues have excellent recommendations on how to reduce noise, such as tips for good decision hygiene (e.g., using differential diagnoses) and using algorithms (e.g., calculating Apgar or LACE scores). I also liked their strategy of aggregating expert opinions. Fascinatingly, averaging multiple independent assessments is mathematically guaranteed to reduce noise. (God, I love economists). This is true of measurements and opinions: If you use 100 judgments for a case, you reduce noise by 90% (the noise is divided by the square root of the number of judgments averaged). So 20 colleagues’ opinions would reduce noise by almost 80%. However, those 20 opinions must be independent to avoid spurious agreement. (Again, math for the win.)
I showed photos of my patient to a few other dermatologists. They independently returned the same result: perioral dermatitis. This was the correct diagnosis and reminded me why grand rounds and tumor boards are such a great help. Multiple, independent assessments are more likely to get it right than just one opinion because we are canceling out the noise. But remember, grand rounds has to be old-school style – no looking at your coresident answers before giving yours!
Our patient cleared after restarting her topical tacrolimus and a bit of doxycycline. Credit the wisdom of the crowd. Reassuringly though, Dr. Kahneman also shows that expertise does matter in minimizing error. So that fellowship you did was still a great idea.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He reports having no conflicts of interest. Write to him at [email protected].
A 26-year-old woman who reports a history of acyclovir-resistant herpes complains of a recurring, stinging rash around her mouth. Topical tacrolimus made it worse, she said. On exam, she has somewhat grouped pustules on her cutaneous lip. I mentioned her to colleagues, saying: “I’ve a patient with acyclovir-resistant herpes who isn’t improving on high-dose Valtrex.” They proffered a few alternative diagnoses and treatment recommendations. I tried several to no avail.
(it is after all only one condition). Nobel Prize–winning economist Daniel Kahneman, PhD, with two other authors, has written a brilliant book about this cognitive unreliability called “Noise: A Flaw in Human Judgment” (New York: Hachette Book Group, 2021).
Both bias and noise create trouble for us. Although biases get more attention, noise is both more prevalent and insidious. In a 2016 article, Dr. Kahneman and coauthors use a bathroom scale as an analogy to explain the difference. “We would say that the scale is biased if its readings are generally either too high or too low. A scale that consistently underestimates true weight by exactly 4 pounds is seriously biased but free of noise. A scale that gives two different readings when you step on it twice is noisy.” In the case presented, “measurements” by me and my colleagues were returning different “readings.” There is one true diagnosis and best treatment, yet because of noise, we waste time and resources by not getting it right the first time.
There is also evidence of bias in this case. For example, there’s probably some confirmation bias: The patient said she has a history of antiviral-resistant herpes; therefore, her rash might appear to be herpes. Also there might be salience bias: it’s easy to see how prominent pustules might be herpes simplex virus. Noise is an issue in many misdiagnoses, but trickier to see. In most instances, we don’t have the opportunity to get multiple assessments of the same case. When examined though, interrater reliability in medicine is often found to be shockingly low, an indication of how much noise there is in our clinical judgments. This leads to waste, frustration – and can even be dangerous when we’re trying to diagnose cancers such as melanoma, lung, or breast cancer.
Dr. Kahneman and colleagues have excellent recommendations on how to reduce noise, such as tips for good decision hygiene (e.g., using differential diagnoses) and using algorithms (e.g., calculating Apgar or LACE scores). I also liked their strategy of aggregating expert opinions. Fascinatingly, averaging multiple independent assessments is mathematically guaranteed to reduce noise. (God, I love economists). This is true of measurements and opinions: If you use 100 judgments for a case, you reduce noise by 90% (the noise is divided by the square root of the number of judgments averaged). So 20 colleagues’ opinions would reduce noise by almost 80%. However, those 20 opinions must be independent to avoid spurious agreement. (Again, math for the win.)
I showed photos of my patient to a few other dermatologists. They independently returned the same result: perioral dermatitis. This was the correct diagnosis and reminded me why grand rounds and tumor boards are such a great help. Multiple, independent assessments are more likely to get it right than just one opinion because we are canceling out the noise. But remember, grand rounds has to be old-school style – no looking at your coresident answers before giving yours!
Our patient cleared after restarting her topical tacrolimus and a bit of doxycycline. Credit the wisdom of the crowd. Reassuringly though, Dr. Kahneman also shows that expertise does matter in minimizing error. So that fellowship you did was still a great idea.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He reports having no conflicts of interest. Write to him at [email protected].
FDA approves topical ruxolitinib for atopic dermatitis, first JAK inhibitor for this indication in the U.S.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
COVID-19 claims more than 675,000 U.S. lives, surpassing the 1918 flu
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
Medicare payments for most skin procedures dropped over last 15 years
according to a new analysis.
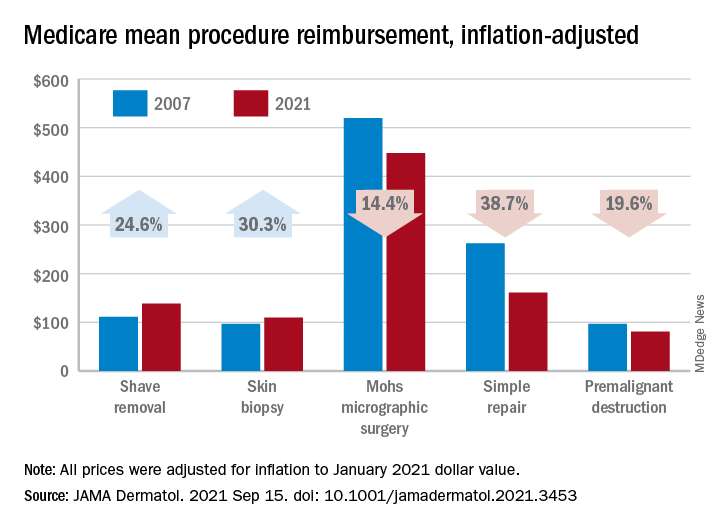
Large increases in mean reimbursement for skin biopsies (+30.3%) and shave removal (+24.6%) were not enough to offset lower rates for procedures such as simple repair (–38.7%), premalignant destruction (–19.6%), Mohs micrographic surgery (–14.4%), and flap repair (–14.1%), Rishabh S. Mazmudar, BS, of Case Western Reserve University, Cleveland, and associates said.
“Given Medicare’s contribution to a large proportion of health care expenditures, changes in Medicare reimbursement rates may result in parallel changes in private insurance reimbursement,” they wrote in JAMA Dermatology.
A recently published study showed that Medicare reimbursement for 20 dermatologic service codes had fallen by 10% between the two comparison years, 2000 and 2020. The current study expanded the number of procedures to 46 (divided into nine categories) and used historical Medicare fee schedules to examine annual trends over a 15-year period, Mr. Mazmudar and associates explained.
Other specialties have seen reimbursement fall by more than 4.8%, including emergency medicine (–21.2% from 2000 to 2020) and general surgery (–24.4% from 2000 to 2018), but “these comparisons are likely skewed by the disproportionate increase in reimbursement rates for biopsies and shave removals during this time period,” the investigators said.
If those two procedure categories were excluded from the analysis, the mean change in overall reimbursement for the remaining dermatologic procedures would be −9.6%, they noted.
Detailed payment information provided for 15 of the 46 procedures shows that only intermediate repair (+4.5%) and benign destruction (+2.3%) joined biopsies and shave excisions with increased reimbursement from 2007 to 2021. The smallest drop among the other procedures was –1.9% for malignant destruction, the research team reported.
The inflation-adjusted, year-by-year analysis showed that reimbursement for the nine procedure categories has gradually declined since peaking in 2011, with the most notable exceptions being biopsies and shave excisions. Both had been following the trend until 2013, when reimbursement for shave removals jumped by almost 30 percentage points, and 2019, when rates for biopsies soared by more than 30 percentage points, according to the investigators.
The increase for skin biopsies followed the split of the original CPT code into three categories, but “the jump in reimbursement for shave removals in 2013 requires further investigation,” Mr. Mazmudar and associates wrote.
The investigators did not disclose any conflicts of interest.
according to a new analysis.

Large increases in mean reimbursement for skin biopsies (+30.3%) and shave removal (+24.6%) were not enough to offset lower rates for procedures such as simple repair (–38.7%), premalignant destruction (–19.6%), Mohs micrographic surgery (–14.4%), and flap repair (–14.1%), Rishabh S. Mazmudar, BS, of Case Western Reserve University, Cleveland, and associates said.
“Given Medicare’s contribution to a large proportion of health care expenditures, changes in Medicare reimbursement rates may result in parallel changes in private insurance reimbursement,” they wrote in JAMA Dermatology.
A recently published study showed that Medicare reimbursement for 20 dermatologic service codes had fallen by 10% between the two comparison years, 2000 and 2020. The current study expanded the number of procedures to 46 (divided into nine categories) and used historical Medicare fee schedules to examine annual trends over a 15-year period, Mr. Mazmudar and associates explained.
Other specialties have seen reimbursement fall by more than 4.8%, including emergency medicine (–21.2% from 2000 to 2020) and general surgery (–24.4% from 2000 to 2018), but “these comparisons are likely skewed by the disproportionate increase in reimbursement rates for biopsies and shave removals during this time period,” the investigators said.
If those two procedure categories were excluded from the analysis, the mean change in overall reimbursement for the remaining dermatologic procedures would be −9.6%, they noted.
Detailed payment information provided for 15 of the 46 procedures shows that only intermediate repair (+4.5%) and benign destruction (+2.3%) joined biopsies and shave excisions with increased reimbursement from 2007 to 2021. The smallest drop among the other procedures was –1.9% for malignant destruction, the research team reported.
The inflation-adjusted, year-by-year analysis showed that reimbursement for the nine procedure categories has gradually declined since peaking in 2011, with the most notable exceptions being biopsies and shave excisions. Both had been following the trend until 2013, when reimbursement for shave removals jumped by almost 30 percentage points, and 2019, when rates for biopsies soared by more than 30 percentage points, according to the investigators.
The increase for skin biopsies followed the split of the original CPT code into three categories, but “the jump in reimbursement for shave removals in 2013 requires further investigation,” Mr. Mazmudar and associates wrote.
The investigators did not disclose any conflicts of interest.
according to a new analysis.

Large increases in mean reimbursement for skin biopsies (+30.3%) and shave removal (+24.6%) were not enough to offset lower rates for procedures such as simple repair (–38.7%), premalignant destruction (–19.6%), Mohs micrographic surgery (–14.4%), and flap repair (–14.1%), Rishabh S. Mazmudar, BS, of Case Western Reserve University, Cleveland, and associates said.
“Given Medicare’s contribution to a large proportion of health care expenditures, changes in Medicare reimbursement rates may result in parallel changes in private insurance reimbursement,” they wrote in JAMA Dermatology.
A recently published study showed that Medicare reimbursement for 20 dermatologic service codes had fallen by 10% between the two comparison years, 2000 and 2020. The current study expanded the number of procedures to 46 (divided into nine categories) and used historical Medicare fee schedules to examine annual trends over a 15-year period, Mr. Mazmudar and associates explained.
Other specialties have seen reimbursement fall by more than 4.8%, including emergency medicine (–21.2% from 2000 to 2020) and general surgery (–24.4% from 2000 to 2018), but “these comparisons are likely skewed by the disproportionate increase in reimbursement rates for biopsies and shave removals during this time period,” the investigators said.
If those two procedure categories were excluded from the analysis, the mean change in overall reimbursement for the remaining dermatologic procedures would be −9.6%, they noted.
Detailed payment information provided for 15 of the 46 procedures shows that only intermediate repair (+4.5%) and benign destruction (+2.3%) joined biopsies and shave excisions with increased reimbursement from 2007 to 2021. The smallest drop among the other procedures was –1.9% for malignant destruction, the research team reported.
The inflation-adjusted, year-by-year analysis showed that reimbursement for the nine procedure categories has gradually declined since peaking in 2011, with the most notable exceptions being biopsies and shave excisions. Both had been following the trend until 2013, when reimbursement for shave removals jumped by almost 30 percentage points, and 2019, when rates for biopsies soared by more than 30 percentage points, according to the investigators.
The increase for skin biopsies followed the split of the original CPT code into three categories, but “the jump in reimbursement for shave removals in 2013 requires further investigation,” Mr. Mazmudar and associates wrote.
The investigators did not disclose any conflicts of interest.
FROM JAMA DERMATOLOGY
Moderna vaccine more effective than Pfizer and J&J
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
Patient risk-benefit thresholds for antibiotic use in dermatologic surgery vary widely
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM DERMATOLOGIC SURGERY





