User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
ATPCI: Trimetazidine fizzles for post-PCI angina
Adding trimetazidine to optimal medical therapy does not improve outcomes following successful percutaneous coronary intervention (PCI) for stable angina or a non–ST-elevated myocardial infarction, results of the ATPCI trial show.
There was no benefit for the composite primary endpoint of cardiac death, hospitalization for cardiac events, or recurrent/persistent angina requiring an addition, switch, or increased dose of antianginal therapies, or requiring coronary angiography (hazard ratio, 0.98; 95% confidence interval, 0.88-1.09).
Further, there were no between-group differences in any of the individual components of the endpoint or any prespecified subgroups, Roberto Ferrari, MD, professor of cardiology at the University of Ferrara (Italy), reported in a Hot Line session at the digital European Society of Cardiology Congress 2020.
“I think one of the reasons why we couldn’t see any results was really due to this population was extremely well treated,” he said. “Almost all of them were receiving either a beta-blocker or calcium blocker and, on top of this, they had a successful angioplasty and that is what we should do, at least according to ESC guidelines.”
Research has shown that about 85% to 90% of patients have a change in New York Heart Association angina class within 30 days of PCI, leaving very little angina leftover to treat, observed Magnus Ohman, MD, director of the advanced coronary disease program at Duke University, Durham, N.C., who was not involved in the study.
“The fundamental question is whether this was the right study. Is this agent ineffective, or is it just that it was studied in the wrong population? That to me is really the crux of the matter,” he said in an interview.
There is potential benefit in chronic angina, which reflects the level II recommendation by the ESC, said Dr. Ohman. “Those patients typically require more therapy and, in the ideal world of treating angina, you need both physiological and metabolic agents to treat angina and trimetazidine is one metabolic agent.”
Trimetazidine is not available in the United States, but the anti-ischemic metabolic agent is recommended as second-line therapy for angina after beta-blockers and calcium-channel blockers in the 2019 ESC guidelines on chronic coronary syndrome.
Unlike other commonly used first- and second-line antianginal drugs, trimetazidine is devoid of hemodynamic effects, Dr. Ferrari said. It improves myocardial utilization by favoring glucose to fatty acids, thus allowing anaerobic adenosine triphosphate formation and preventing acidosis.
In the absence of contemporary data on the prognostic benefits of antianginal drugs in post-PCI patients, ATPCI investigators at 365 centers in 27 countries randomly assigned 6007 patients with stable angina or non–ST-segment MI after successful elective or urgent PCI to optimal medical therapy alone or with trimetazidine, 35 mg modified-release twice daily.
Patients with severe heart failure, valvular disease, arrhythmia, renal failure or acute ST-elevation MI were excluded.
Most patients (77% male) had Canadian Class Cardiovascular Society class III/IV angina (58%) and were receiving aspirin plus a P2Y12 inhibitor (97%), lipid-lowering agent (96.6%), renin-angiotensin inhibitors (82.2%), and beta-blockers (83.9%). A quarter were receiving calcium-channel blockers (27.6%). In all, 2517 patients had an urgent PCI and 3490 had an elective PCI.
After a median follow-up of 47.5 months, the composite primary endpoint occurred in 23.3% of the trimetazidine group and 23.7% of the control group, according to the study, which was published simultaneously in The Lancet.
The incidence of the individual components was similar:
- Cardiac death: 2.1% vs. 2.6% (HR, 0.81)
- Hospital admission for cardiac events: 13.4% vs. 13.4% (HR, 1.01)
- Angina leading to coronary angiography: 16.9% vs. 16.6% (HR, 1.02)
- Angina leading to increase/switch in antianginal drugs (HR, 1.01)
There was no between-group difference in the composite major secondary endpoint, which included the primary endpoint components plus ischemia leading to coronary angiography and an increase or switch in antianginal therapies. This outcome occurred in 23.5% and 24.0% of patients in the trimetazidine and control groups, respectively (HR, 0.98; 95% CI, 0.88-1.08).
Results were also similar when the primary endpoint was analyzed based on whether patients underwent elective PCI (HR, 0.94; 95% CI, 0.82-1.08) or urgent PCI (HR, 1.04; 95% CI, 0.88-1.22), Dr. Ferrari reported.
Given the lack of observed efficacy, trimetazidine has no use or place in the population studied, said Stephan Windecker, MD, the formal discussant for the study and chair of cardiology at Bern (Switzerland) University Hospital. “Notwithstanding, I think we have to recognize that the optimal medical therapy is so potent and has been well implemented in this trial that any additional medication beyond this is just unable to exploit additional benefit.”
The study was supported by Servier. Dr. Ferrari received fees, honoraria, and travel expenses from Servier. Dr. Ohman reports no relevant financial conflicts of interest. Dr. Windecker is an unpaid member of the steering/executive group for trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliancé, Medtronic, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments from any pharmaceutical or device company.
This article first appeared on Medscape.com.
Adding trimetazidine to optimal medical therapy does not improve outcomes following successful percutaneous coronary intervention (PCI) for stable angina or a non–ST-elevated myocardial infarction, results of the ATPCI trial show.
There was no benefit for the composite primary endpoint of cardiac death, hospitalization for cardiac events, or recurrent/persistent angina requiring an addition, switch, or increased dose of antianginal therapies, or requiring coronary angiography (hazard ratio, 0.98; 95% confidence interval, 0.88-1.09).
Further, there were no between-group differences in any of the individual components of the endpoint or any prespecified subgroups, Roberto Ferrari, MD, professor of cardiology at the University of Ferrara (Italy), reported in a Hot Line session at the digital European Society of Cardiology Congress 2020.
“I think one of the reasons why we couldn’t see any results was really due to this population was extremely well treated,” he said. “Almost all of them were receiving either a beta-blocker or calcium blocker and, on top of this, they had a successful angioplasty and that is what we should do, at least according to ESC guidelines.”
Research has shown that about 85% to 90% of patients have a change in New York Heart Association angina class within 30 days of PCI, leaving very little angina leftover to treat, observed Magnus Ohman, MD, director of the advanced coronary disease program at Duke University, Durham, N.C., who was not involved in the study.
“The fundamental question is whether this was the right study. Is this agent ineffective, or is it just that it was studied in the wrong population? That to me is really the crux of the matter,” he said in an interview.
There is potential benefit in chronic angina, which reflects the level II recommendation by the ESC, said Dr. Ohman. “Those patients typically require more therapy and, in the ideal world of treating angina, you need both physiological and metabolic agents to treat angina and trimetazidine is one metabolic agent.”
Trimetazidine is not available in the United States, but the anti-ischemic metabolic agent is recommended as second-line therapy for angina after beta-blockers and calcium-channel blockers in the 2019 ESC guidelines on chronic coronary syndrome.
Unlike other commonly used first- and second-line antianginal drugs, trimetazidine is devoid of hemodynamic effects, Dr. Ferrari said. It improves myocardial utilization by favoring glucose to fatty acids, thus allowing anaerobic adenosine triphosphate formation and preventing acidosis.
In the absence of contemporary data on the prognostic benefits of antianginal drugs in post-PCI patients, ATPCI investigators at 365 centers in 27 countries randomly assigned 6007 patients with stable angina or non–ST-segment MI after successful elective or urgent PCI to optimal medical therapy alone or with trimetazidine, 35 mg modified-release twice daily.
Patients with severe heart failure, valvular disease, arrhythmia, renal failure or acute ST-elevation MI were excluded.
Most patients (77% male) had Canadian Class Cardiovascular Society class III/IV angina (58%) and were receiving aspirin plus a P2Y12 inhibitor (97%), lipid-lowering agent (96.6%), renin-angiotensin inhibitors (82.2%), and beta-blockers (83.9%). A quarter were receiving calcium-channel blockers (27.6%). In all, 2517 patients had an urgent PCI and 3490 had an elective PCI.
After a median follow-up of 47.5 months, the composite primary endpoint occurred in 23.3% of the trimetazidine group and 23.7% of the control group, according to the study, which was published simultaneously in The Lancet.
The incidence of the individual components was similar:
- Cardiac death: 2.1% vs. 2.6% (HR, 0.81)
- Hospital admission for cardiac events: 13.4% vs. 13.4% (HR, 1.01)
- Angina leading to coronary angiography: 16.9% vs. 16.6% (HR, 1.02)
- Angina leading to increase/switch in antianginal drugs (HR, 1.01)
There was no between-group difference in the composite major secondary endpoint, which included the primary endpoint components plus ischemia leading to coronary angiography and an increase or switch in antianginal therapies. This outcome occurred in 23.5% and 24.0% of patients in the trimetazidine and control groups, respectively (HR, 0.98; 95% CI, 0.88-1.08).
Results were also similar when the primary endpoint was analyzed based on whether patients underwent elective PCI (HR, 0.94; 95% CI, 0.82-1.08) or urgent PCI (HR, 1.04; 95% CI, 0.88-1.22), Dr. Ferrari reported.
Given the lack of observed efficacy, trimetazidine has no use or place in the population studied, said Stephan Windecker, MD, the formal discussant for the study and chair of cardiology at Bern (Switzerland) University Hospital. “Notwithstanding, I think we have to recognize that the optimal medical therapy is so potent and has been well implemented in this trial that any additional medication beyond this is just unable to exploit additional benefit.”
The study was supported by Servier. Dr. Ferrari received fees, honoraria, and travel expenses from Servier. Dr. Ohman reports no relevant financial conflicts of interest. Dr. Windecker is an unpaid member of the steering/executive group for trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliancé, Medtronic, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments from any pharmaceutical or device company.
This article first appeared on Medscape.com.
Adding trimetazidine to optimal medical therapy does not improve outcomes following successful percutaneous coronary intervention (PCI) for stable angina or a non–ST-elevated myocardial infarction, results of the ATPCI trial show.
There was no benefit for the composite primary endpoint of cardiac death, hospitalization for cardiac events, or recurrent/persistent angina requiring an addition, switch, or increased dose of antianginal therapies, or requiring coronary angiography (hazard ratio, 0.98; 95% confidence interval, 0.88-1.09).
Further, there were no between-group differences in any of the individual components of the endpoint or any prespecified subgroups, Roberto Ferrari, MD, professor of cardiology at the University of Ferrara (Italy), reported in a Hot Line session at the digital European Society of Cardiology Congress 2020.
“I think one of the reasons why we couldn’t see any results was really due to this population was extremely well treated,” he said. “Almost all of them were receiving either a beta-blocker or calcium blocker and, on top of this, they had a successful angioplasty and that is what we should do, at least according to ESC guidelines.”
Research has shown that about 85% to 90% of patients have a change in New York Heart Association angina class within 30 days of PCI, leaving very little angina leftover to treat, observed Magnus Ohman, MD, director of the advanced coronary disease program at Duke University, Durham, N.C., who was not involved in the study.
“The fundamental question is whether this was the right study. Is this agent ineffective, or is it just that it was studied in the wrong population? That to me is really the crux of the matter,” he said in an interview.
There is potential benefit in chronic angina, which reflects the level II recommendation by the ESC, said Dr. Ohman. “Those patients typically require more therapy and, in the ideal world of treating angina, you need both physiological and metabolic agents to treat angina and trimetazidine is one metabolic agent.”
Trimetazidine is not available in the United States, but the anti-ischemic metabolic agent is recommended as second-line therapy for angina after beta-blockers and calcium-channel blockers in the 2019 ESC guidelines on chronic coronary syndrome.
Unlike other commonly used first- and second-line antianginal drugs, trimetazidine is devoid of hemodynamic effects, Dr. Ferrari said. It improves myocardial utilization by favoring glucose to fatty acids, thus allowing anaerobic adenosine triphosphate formation and preventing acidosis.
In the absence of contemporary data on the prognostic benefits of antianginal drugs in post-PCI patients, ATPCI investigators at 365 centers in 27 countries randomly assigned 6007 patients with stable angina or non–ST-segment MI after successful elective or urgent PCI to optimal medical therapy alone or with trimetazidine, 35 mg modified-release twice daily.
Patients with severe heart failure, valvular disease, arrhythmia, renal failure or acute ST-elevation MI were excluded.
Most patients (77% male) had Canadian Class Cardiovascular Society class III/IV angina (58%) and were receiving aspirin plus a P2Y12 inhibitor (97%), lipid-lowering agent (96.6%), renin-angiotensin inhibitors (82.2%), and beta-blockers (83.9%). A quarter were receiving calcium-channel blockers (27.6%). In all, 2517 patients had an urgent PCI and 3490 had an elective PCI.
After a median follow-up of 47.5 months, the composite primary endpoint occurred in 23.3% of the trimetazidine group and 23.7% of the control group, according to the study, which was published simultaneously in The Lancet.
The incidence of the individual components was similar:
- Cardiac death: 2.1% vs. 2.6% (HR, 0.81)
- Hospital admission for cardiac events: 13.4% vs. 13.4% (HR, 1.01)
- Angina leading to coronary angiography: 16.9% vs. 16.6% (HR, 1.02)
- Angina leading to increase/switch in antianginal drugs (HR, 1.01)
There was no between-group difference in the composite major secondary endpoint, which included the primary endpoint components plus ischemia leading to coronary angiography and an increase or switch in antianginal therapies. This outcome occurred in 23.5% and 24.0% of patients in the trimetazidine and control groups, respectively (HR, 0.98; 95% CI, 0.88-1.08).
Results were also similar when the primary endpoint was analyzed based on whether patients underwent elective PCI (HR, 0.94; 95% CI, 0.82-1.08) or urgent PCI (HR, 1.04; 95% CI, 0.88-1.22), Dr. Ferrari reported.
Given the lack of observed efficacy, trimetazidine has no use or place in the population studied, said Stephan Windecker, MD, the formal discussant for the study and chair of cardiology at Bern (Switzerland) University Hospital. “Notwithstanding, I think we have to recognize that the optimal medical therapy is so potent and has been well implemented in this trial that any additional medication beyond this is just unable to exploit additional benefit.”
The study was supported by Servier. Dr. Ferrari received fees, honoraria, and travel expenses from Servier. Dr. Ohman reports no relevant financial conflicts of interest. Dr. Windecker is an unpaid member of the steering/executive group for trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliancé, Medtronic, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments from any pharmaceutical or device company.
This article first appeared on Medscape.com.
Two PR employees at FDA fired after plasma therapy controversy
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
DAPA-CKD: SGLT2 inhibitor benefit extends to chronic kidney disease without diabetes
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
FROM ESC CONGRESS 2020
RATE-AF trial boosts digoxin for rate control in permanent AFib
Digoxin now deserves to be considered first-line therapy for long-term heart rate control in older patients with permanent atrial fibrillation and symptoms of heart failure, Dipak Kotecha, MBChB, PhD, MSc, declared at the virtual annual congress of the European Society of Cardiology.
He presented the 12-month results of RATE-AF (Rate Control Therapy Evaluation in Permanent Atrial Fibrillation), in which 160 seniors (mean age, 76 years) with moderate or severe symptoms caused by permanent atrial fibrillation (AFib) as well as heart failure symptoms were randomized to low-dose digoxin or the beta-blocker bisoprolol for rate control.
The open-label trial was designed to address a centuries-old unmet need: “Although digoxin has been in use since 1785, we have no longer-term clinical trials of digoxin in patients with AFib or AFib with heart failure,” noted Dr. Kotecha, professor of cardiology at the University of Birmingham (England).
Not only is digoxin greatly understudied in AFib, but permanent AFib – the most common form of the arrhythmia – has received only a tiny fraction of the research attention that’s been devoted to paroxysmal or persistent AFib, he added.
In RATE-AF, digoxin and bisoprolol proved similarly effective at reducing heart rate, from about 100 bpm at baseline to the mid-70s at 6 and 12 months. Notably, only a handful of study participants required an additional rate control drug during the 12-month study. Nor did the two drugs differ in terms of their impact on patient-reported quality of life at 6 months as reflected by their Short Form–36 physical component score, the primary study endpoint. And both drugs were well tolerated, with 96% of patients in the digoxin group still on the drug at a mean of 161 mcg/day at 6 months, and 89% still on their beta-blocker.
But that’s pretty much where the similarities in outcomes ended.
For example, at 12 months, the digoxin group scored significantly higher than the beta-blocker group on several domains of the Short Form–36 physical component score, including vitality, physical function, and global health. More than half of the digoxin group had a two-class improvement in modified European Heart Rhythm Association AFib-related symptoms at 6 months, compared with 10% of the beta-blocker group. At 12 months, nearly 70% of the digoxin group had a two-class improvement, as did 30% on bisoprolol.
Heart failure symptoms in the digoxin group improved from a mean baseline New York Heart Association class of 2.4 to 1.5 at both 6 and 12 months; the improvement was more modest in the beta-blocker group, going from NYHA 2.4 at baseline to 2.0 at both 6 and 12 months. And while N-terminal of the prohormone brain natriuretic peptide levels improved in the digoxin group from a baseline of 1,095 pg/mL to 1,058 at 6 months and 960 at 12 months, NT-proBNP actually went up in the beta-blocker group, from 1,041 to 1,209-1,250 pg/mL at 12 months.
Moreover, Dr. Kotecha continued, while RATE-AF was underpowered to assess clinical events, it’s nevertheless noteworthy that a total of 29 adverse events occurred in 12 months in the digoxin group, compared with 142 with beta-blocker therapy. There were 12 unplanned hospital admissions in the digoxin group and 28 in the beta-blocker group, and 22 primary care visits for either AFib or cardiovascular symptoms in patients on digoxin versus 64 in the beta-blocker group.
“Our results suggest a wider use of digoxin for stable patients with permanent AFib,” Dr. Kotecha concluded. However, in an interview, Jonathan Piccini, MD, had a different take on the study results.
“I don’t think this study should widely impact clinical practice in the U.S.,” according to Dr. Piccini, director of cardiac electrophysiology at Duke University, Durham, N.C.
His reservations included RATE-AF’s modest sample size as well as uncertainty as to the trial’s generalizability, given that bisoprolol isn’t much used in the United States. Also, these were elderly patients with shortness of breath, and it’s unclear how effective digoxin would be for rate control in patients with permanent AFib who are more active.
“The classic teaching is that digoxin is great for rate control at rest, but when people are active it’s not nearly as good as beta-blockers or calcium-channel blockers,” the cardiologist said.
“A beta-blocker is still going to be my first-line rate control agent. But the results of RATE-AF do open my mind that for an older sedentary patient I may very well think twice now about using digoxin, because in that situation it looks like it achieves similar goals as a beta-blocker,” Dr. Piccini added.
On the plus side for RATE-AF: “I am very pleased to see that we have a randomized controlled trial focused on rate control in permanent AFib. It also tickles me pink to see a randomized, controlled study of digoxin. And I’m really excited to see a clinical trial that focuses on quality of life. It should give some confidence to know that from a quality of life perspective clinicians can consider using either digoxin or a beta-blocker for rate control,” he said.
American College of Cardiology vice president Dipti Itchhaporia, MD, said she’d need to see a much larger randomized trial including a calcium-channel blocker as a third-rate control arm before she’d consider digoxin as first-line rate control therapy in patients with AFib with or without heart failure. Also, she has reservations about drawing definitive conclusions from an open, unblinded study in which patient-reported outcomes are the primary endpoint.
“I think these were surprising findings given what we all think about digoxin in this country. In general, digoxin fell out of favor for rate control, mainly because of observational studies showing increased mortality. So most of us choose a beta-blocker,” she observed in an interview.
But of course, a randomized trial, even a 160-patient randomized trial, constitutes a higher level of evidence.
“I don’t think I’m going to convert tomorrow and make digoxin my first-line rate control therapy without more data. But RATE-AF does makes me stop and think about using it more than I did before in some of my permanent AFib patients,” said Dr. Itchhaporia, director of disease management at Hoag Memorial Hospital in Newport Beach, Calif.
Dr. Kotecha reported having no financial conflicts regarding the study, which was funded by the U.K. National Institute for Health Research, the British Heart Foundation, and the European Union.
Digoxin now deserves to be considered first-line therapy for long-term heart rate control in older patients with permanent atrial fibrillation and symptoms of heart failure, Dipak Kotecha, MBChB, PhD, MSc, declared at the virtual annual congress of the European Society of Cardiology.
He presented the 12-month results of RATE-AF (Rate Control Therapy Evaluation in Permanent Atrial Fibrillation), in which 160 seniors (mean age, 76 years) with moderate or severe symptoms caused by permanent atrial fibrillation (AFib) as well as heart failure symptoms were randomized to low-dose digoxin or the beta-blocker bisoprolol for rate control.
The open-label trial was designed to address a centuries-old unmet need: “Although digoxin has been in use since 1785, we have no longer-term clinical trials of digoxin in patients with AFib or AFib with heart failure,” noted Dr. Kotecha, professor of cardiology at the University of Birmingham (England).
Not only is digoxin greatly understudied in AFib, but permanent AFib – the most common form of the arrhythmia – has received only a tiny fraction of the research attention that’s been devoted to paroxysmal or persistent AFib, he added.
In RATE-AF, digoxin and bisoprolol proved similarly effective at reducing heart rate, from about 100 bpm at baseline to the mid-70s at 6 and 12 months. Notably, only a handful of study participants required an additional rate control drug during the 12-month study. Nor did the two drugs differ in terms of their impact on patient-reported quality of life at 6 months as reflected by their Short Form–36 physical component score, the primary study endpoint. And both drugs were well tolerated, with 96% of patients in the digoxin group still on the drug at a mean of 161 mcg/day at 6 months, and 89% still on their beta-blocker.
But that’s pretty much where the similarities in outcomes ended.
For example, at 12 months, the digoxin group scored significantly higher than the beta-blocker group on several domains of the Short Form–36 physical component score, including vitality, physical function, and global health. More than half of the digoxin group had a two-class improvement in modified European Heart Rhythm Association AFib-related symptoms at 6 months, compared with 10% of the beta-blocker group. At 12 months, nearly 70% of the digoxin group had a two-class improvement, as did 30% on bisoprolol.
Heart failure symptoms in the digoxin group improved from a mean baseline New York Heart Association class of 2.4 to 1.5 at both 6 and 12 months; the improvement was more modest in the beta-blocker group, going from NYHA 2.4 at baseline to 2.0 at both 6 and 12 months. And while N-terminal of the prohormone brain natriuretic peptide levels improved in the digoxin group from a baseline of 1,095 pg/mL to 1,058 at 6 months and 960 at 12 months, NT-proBNP actually went up in the beta-blocker group, from 1,041 to 1,209-1,250 pg/mL at 12 months.
Moreover, Dr. Kotecha continued, while RATE-AF was underpowered to assess clinical events, it’s nevertheless noteworthy that a total of 29 adverse events occurred in 12 months in the digoxin group, compared with 142 with beta-blocker therapy. There were 12 unplanned hospital admissions in the digoxin group and 28 in the beta-blocker group, and 22 primary care visits for either AFib or cardiovascular symptoms in patients on digoxin versus 64 in the beta-blocker group.
“Our results suggest a wider use of digoxin for stable patients with permanent AFib,” Dr. Kotecha concluded. However, in an interview, Jonathan Piccini, MD, had a different take on the study results.
“I don’t think this study should widely impact clinical practice in the U.S.,” according to Dr. Piccini, director of cardiac electrophysiology at Duke University, Durham, N.C.
His reservations included RATE-AF’s modest sample size as well as uncertainty as to the trial’s generalizability, given that bisoprolol isn’t much used in the United States. Also, these were elderly patients with shortness of breath, and it’s unclear how effective digoxin would be for rate control in patients with permanent AFib who are more active.
“The classic teaching is that digoxin is great for rate control at rest, but when people are active it’s not nearly as good as beta-blockers or calcium-channel blockers,” the cardiologist said.
“A beta-blocker is still going to be my first-line rate control agent. But the results of RATE-AF do open my mind that for an older sedentary patient I may very well think twice now about using digoxin, because in that situation it looks like it achieves similar goals as a beta-blocker,” Dr. Piccini added.
On the plus side for RATE-AF: “I am very pleased to see that we have a randomized controlled trial focused on rate control in permanent AFib. It also tickles me pink to see a randomized, controlled study of digoxin. And I’m really excited to see a clinical trial that focuses on quality of life. It should give some confidence to know that from a quality of life perspective clinicians can consider using either digoxin or a beta-blocker for rate control,” he said.
American College of Cardiology vice president Dipti Itchhaporia, MD, said she’d need to see a much larger randomized trial including a calcium-channel blocker as a third-rate control arm before she’d consider digoxin as first-line rate control therapy in patients with AFib with or without heart failure. Also, she has reservations about drawing definitive conclusions from an open, unblinded study in which patient-reported outcomes are the primary endpoint.
“I think these were surprising findings given what we all think about digoxin in this country. In general, digoxin fell out of favor for rate control, mainly because of observational studies showing increased mortality. So most of us choose a beta-blocker,” she observed in an interview.
But of course, a randomized trial, even a 160-patient randomized trial, constitutes a higher level of evidence.
“I don’t think I’m going to convert tomorrow and make digoxin my first-line rate control therapy without more data. But RATE-AF does makes me stop and think about using it more than I did before in some of my permanent AFib patients,” said Dr. Itchhaporia, director of disease management at Hoag Memorial Hospital in Newport Beach, Calif.
Dr. Kotecha reported having no financial conflicts regarding the study, which was funded by the U.K. National Institute for Health Research, the British Heart Foundation, and the European Union.
Digoxin now deserves to be considered first-line therapy for long-term heart rate control in older patients with permanent atrial fibrillation and symptoms of heart failure, Dipak Kotecha, MBChB, PhD, MSc, declared at the virtual annual congress of the European Society of Cardiology.
He presented the 12-month results of RATE-AF (Rate Control Therapy Evaluation in Permanent Atrial Fibrillation), in which 160 seniors (mean age, 76 years) with moderate or severe symptoms caused by permanent atrial fibrillation (AFib) as well as heart failure symptoms were randomized to low-dose digoxin or the beta-blocker bisoprolol for rate control.
The open-label trial was designed to address a centuries-old unmet need: “Although digoxin has been in use since 1785, we have no longer-term clinical trials of digoxin in patients with AFib or AFib with heart failure,” noted Dr. Kotecha, professor of cardiology at the University of Birmingham (England).
Not only is digoxin greatly understudied in AFib, but permanent AFib – the most common form of the arrhythmia – has received only a tiny fraction of the research attention that’s been devoted to paroxysmal or persistent AFib, he added.
In RATE-AF, digoxin and bisoprolol proved similarly effective at reducing heart rate, from about 100 bpm at baseline to the mid-70s at 6 and 12 months. Notably, only a handful of study participants required an additional rate control drug during the 12-month study. Nor did the two drugs differ in terms of their impact on patient-reported quality of life at 6 months as reflected by their Short Form–36 physical component score, the primary study endpoint. And both drugs were well tolerated, with 96% of patients in the digoxin group still on the drug at a mean of 161 mcg/day at 6 months, and 89% still on their beta-blocker.
But that’s pretty much where the similarities in outcomes ended.
For example, at 12 months, the digoxin group scored significantly higher than the beta-blocker group on several domains of the Short Form–36 physical component score, including vitality, physical function, and global health. More than half of the digoxin group had a two-class improvement in modified European Heart Rhythm Association AFib-related symptoms at 6 months, compared with 10% of the beta-blocker group. At 12 months, nearly 70% of the digoxin group had a two-class improvement, as did 30% on bisoprolol.
Heart failure symptoms in the digoxin group improved from a mean baseline New York Heart Association class of 2.4 to 1.5 at both 6 and 12 months; the improvement was more modest in the beta-blocker group, going from NYHA 2.4 at baseline to 2.0 at both 6 and 12 months. And while N-terminal of the prohormone brain natriuretic peptide levels improved in the digoxin group from a baseline of 1,095 pg/mL to 1,058 at 6 months and 960 at 12 months, NT-proBNP actually went up in the beta-blocker group, from 1,041 to 1,209-1,250 pg/mL at 12 months.
Moreover, Dr. Kotecha continued, while RATE-AF was underpowered to assess clinical events, it’s nevertheless noteworthy that a total of 29 adverse events occurred in 12 months in the digoxin group, compared with 142 with beta-blocker therapy. There were 12 unplanned hospital admissions in the digoxin group and 28 in the beta-blocker group, and 22 primary care visits for either AFib or cardiovascular symptoms in patients on digoxin versus 64 in the beta-blocker group.
“Our results suggest a wider use of digoxin for stable patients with permanent AFib,” Dr. Kotecha concluded. However, in an interview, Jonathan Piccini, MD, had a different take on the study results.
“I don’t think this study should widely impact clinical practice in the U.S.,” according to Dr. Piccini, director of cardiac electrophysiology at Duke University, Durham, N.C.
His reservations included RATE-AF’s modest sample size as well as uncertainty as to the trial’s generalizability, given that bisoprolol isn’t much used in the United States. Also, these were elderly patients with shortness of breath, and it’s unclear how effective digoxin would be for rate control in patients with permanent AFib who are more active.
“The classic teaching is that digoxin is great for rate control at rest, but when people are active it’s not nearly as good as beta-blockers or calcium-channel blockers,” the cardiologist said.
“A beta-blocker is still going to be my first-line rate control agent. But the results of RATE-AF do open my mind that for an older sedentary patient I may very well think twice now about using digoxin, because in that situation it looks like it achieves similar goals as a beta-blocker,” Dr. Piccini added.
On the plus side for RATE-AF: “I am very pleased to see that we have a randomized controlled trial focused on rate control in permanent AFib. It also tickles me pink to see a randomized, controlled study of digoxin. And I’m really excited to see a clinical trial that focuses on quality of life. It should give some confidence to know that from a quality of life perspective clinicians can consider using either digoxin or a beta-blocker for rate control,” he said.
American College of Cardiology vice president Dipti Itchhaporia, MD, said she’d need to see a much larger randomized trial including a calcium-channel blocker as a third-rate control arm before she’d consider digoxin as first-line rate control therapy in patients with AFib with or without heart failure. Also, she has reservations about drawing definitive conclusions from an open, unblinded study in which patient-reported outcomes are the primary endpoint.
“I think these were surprising findings given what we all think about digoxin in this country. In general, digoxin fell out of favor for rate control, mainly because of observational studies showing increased mortality. So most of us choose a beta-blocker,” she observed in an interview.
But of course, a randomized trial, even a 160-patient randomized trial, constitutes a higher level of evidence.
“I don’t think I’m going to convert tomorrow and make digoxin my first-line rate control therapy without more data. But RATE-AF does makes me stop and think about using it more than I did before in some of my permanent AFib patients,” said Dr. Itchhaporia, director of disease management at Hoag Memorial Hospital in Newport Beach, Calif.
Dr. Kotecha reported having no financial conflicts regarding the study, which was funded by the U.K. National Institute for Health Research, the British Heart Foundation, and the European Union.
REPORTING FROM ESC CONGRESS 2020
New ESC/EACTS guideline on atrial fibrillation
New atrial fibrillation (AFib) management guidelines from the European Society of Cardiology (ESC) call for diagnostic confirmation and structured characterization of AFib and the need to streamline integrated care with the Atrial fibrillation Better Care (ABC) pathway.
“It’s as simple as CC to ABC,” quipped one task force member during the virtual unveiling of the guidelines at the ESC Congress 2020.
The guidelines were developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) and published simultaneously August 29 in the European Heart Journal.
Acknowledging the slew of novel screening tools now available and their reported sensitivity and specificity rates, the document supports opportunistic screening for AFib by pulse taking or electrocardiogram (ECG) rhythm strip in patients at least 65 years of age, with a class 1 recommendation, evidence level B.
Systematic ECG screening should also be considered to detect AFib in individuals at least 75 years of age or in those at high risk for stroke (class IIa, level B).
Other new class I screening recommendations are to inform individuals undergoing screening about the significance and treatment implications of detecting AFib and to have a structured referral platform in place for further physician-led evaluation.
A definite diagnosis of clinical AFib is established only after confirmation by a conventional 12-lead ECG or single-lead ECG strip with at least 30 seconds of AFib.
In line with ESC’s 2016 AFib guidelines, the new iteration classifies AFib as first diagnosed, paroxysmal, persistent, long-standing persistent, and permanent. But it’s also important to classify the clinical profile of AFib, task force member Giuseppe Boriani, MD, PhD, University of Modena, Italy, said in the first of five presentations.
“So the novelty of the 2020 guidelines is related to the proposal of the 4S-AF scheme for a structured characterization of atrial fibrillation that takes into account Stroke risk, severity of Symptoms, Severity of atrial fibrillation burden, and Substrate severity,” he said.
This represents a paradigm shift from a single-domain conventional classification of AFib toward a structured characterization that streamlines assessment, informs treatment decision-making, and facilitates communication among physicians of various specialties, said Tatjana Potpara, MD, PhD, guideline co-chair and head of the Department for Intensive Arrhythmia Care, Clinical Centre of Serbia, Belgrade.
“The beauty of this approach is that, at present, the assessment of the ‘S’ components are performed using available tools, but in the future, the 4S-AF has a great potential to incorporate whatever becomes available for a more precision assessment of substrate or symptoms or arrhythmia burden and so forth,” she said.
ABC pathway
The guidelines advocate the previously described ABC pathway for integrated care management, which includes ‘A’ for Anticoagulation/Avoid stroke, ‘B’ for Better symptom control, and ‘C’ for Comorbidity/Cardiovascular risk factor optimization.
The document strengthens support for formal risk score–based assessment of bleeding risk in all patients, including use of the HAS-BLED score to help address modifiable bleeding risk factors and to identify patients at high bleeding risk (HAS-BLED score ≥3) for early and more frequent follow-up.
These assessments should be done regularly, given that both stroke and bleeding risk are dynamic and change over time with aging and comorbidities, Dr. Potpara stressed. In patients with AFib initially at low risk for stroke, the next assessment should be optimally performed at 4-6 months.
The guideline also targets weight loss in patients who are obese and have AFib, particularly those being evaluated for ablation, and good blood pressure control in patients with AFib and hypertension to reduce AFib recurrences and risk for stroke and bleeding (both class I, up from IIa).
It’s particularly important that these risk factors are addressed, and that modifiable risk factors that go along with increased AFib occurrence and persistence are addressed and communicated to patients, said Gerhard Hindricks, MD, PhD, guideline cochair and medical director of the Rhythmology Department, Heart Centre Leipzig (Germany).
“I have to confess, as an interventional electrophysiologist, there has been a time where I have not appreciated these risk factors intensely enough,” he said. “But we have learned, also in the field of catheter ablation, that weight loss is an essential basis for a good procedure. If we can motivate patients to lose weight and then come to the intervention with better outcome, it’s a true benefit for the patient and addresses patient values. So I’m particularly happy we have introduced that with such intensity in the guidelines.”
Rate and rhythm control
The guidelines make no recommendation of one novel oral anticoagulant (NOAC) over another. However, in patients already receiving vitamin K antagonists with low time in the therapeutic range, they recommend switching to a different NOAC but ensuring good adherence and persistence with therapy (class I recommendation) or efforts to improve time in therapeutic range (class IIa).
Catheter ablation takes on a more prominent role for rhythm control and is now recommended after one antiarrhythmic drug therapy fails to improve symptoms of AF recurrence in patients with paroxysmal AFib, or persistent AFib with or without major risk factors for recurrence. The class I recommendation is based on results from the CAPTAF and CABANA trials, said task force member Carina Blomström-Lundqvist, MD, PhD, Uppsala University, Sweden.
Catheter ablation is also now a first-line therapy for patients with AFib who have a high likelihood of tachycardia-induced cardiomyopathy, independent of symptom status. “In this subset of patients, catheter ablation may offer a lot with respect to restoration of left ventricular function,” observed Dr. Hindricks.
Complete electrical isolation of the pulmonary veins is recommended during all AFib catheter ablation procedures (class I).
“Even as a medical conservative, I think it is totally reasonable to move to catheter ablation after a failed drug trial,” commented John Mandrola, MD, Baptist Health, Louisville, Ky., who was not a part of the guideline development.
Although the chance of a second drug working after one failure is low, he noted that operators in the United States have dofetilide, which is not used much in Europe, and sometimes works surprisingly well.
“That said, the caveat is that moving to catheter ablation after drug failure is only appropriate if we have addressed all the pertinent risk factors: sleep apnea, weight loss, lack of fitness, blood pressure control, and alcohol excess,” he said.
As for tachycardia-mediated cardiomyopathy, this too can be reasonable, Dr. Mandrola said. “I often get people ‘out of a hole’ with amiodarone plus cardioversion for a few months and then proceed to ablation.”
Notably, the 2020 iteration sharpens its recommendation that amiodarone not be used first-line for long-term rhythm control in all patients with AFib, including those with heart failure with reduced ejection fraction, given its extracardiac toxicity (class I, up from IIa).
Quality counts
In response to growing evidence that guideline-adherence is associated with significantly better outcomes in AFib, the 2020 ESC/EACTS guidelines explicitly included a recommendation on the need to measure quality of care to identify opportunities for improvement.
With this framework in mind, a task force with 23 people – including members from ESC and heart rhythm societies in the United States, Asia Pacific, and Latin America, along with patient representatives – was created to develop a list of quality indicators (QIs), ultimately settling on 17 main QIs and 17 secondary ones, said Elena Arbelo, MD, PhD, MSc, University of Barcelona.
The QIs are classified into six domains: patient assessment, anticoagulation, rate control, rhythm control, risk factor modification, and, importantly, outcome measures. A full list is accessible in a paper, simultaneously published in EP EuroPace.
Five patient-reported outcomes fall under the outcomes domain but only one – health-related quality of life – is a main quality indicator. The remaining outcomes are still important but are listed as secondary because of the lack of evidence to sustain or defend their systematic implementation, particularly evidence on how to measure them appropriately, Dr. Arbelo said.
“Hopefully, following the [class I] recommendation by the 2020 ESC guidelines to routinely collect patient-reported outcomes will allow us to collect further evidence and in the future have sufficient evidence to include these as a main outcome,” she said.
The QI work was driven in parallel with the guidelines and had a huge impact on its development, including inclusion of clear recommendations on how to measure quality, Dr. Hindricks said. “I believe that the whole issue of quality management in the treatment of patients with a focus on patient values cannot be overestimated.”
Disclosure information for all writing committee members is in the report. Dr. Mandrola is a writer and podcaster for Medscape.
A version of this article originally appeared on Medscape.com.
New atrial fibrillation (AFib) management guidelines from the European Society of Cardiology (ESC) call for diagnostic confirmation and structured characterization of AFib and the need to streamline integrated care with the Atrial fibrillation Better Care (ABC) pathway.
“It’s as simple as CC to ABC,” quipped one task force member during the virtual unveiling of the guidelines at the ESC Congress 2020.
The guidelines were developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) and published simultaneously August 29 in the European Heart Journal.
Acknowledging the slew of novel screening tools now available and their reported sensitivity and specificity rates, the document supports opportunistic screening for AFib by pulse taking or electrocardiogram (ECG) rhythm strip in patients at least 65 years of age, with a class 1 recommendation, evidence level B.
Systematic ECG screening should also be considered to detect AFib in individuals at least 75 years of age or in those at high risk for stroke (class IIa, level B).
Other new class I screening recommendations are to inform individuals undergoing screening about the significance and treatment implications of detecting AFib and to have a structured referral platform in place for further physician-led evaluation.
A definite diagnosis of clinical AFib is established only after confirmation by a conventional 12-lead ECG or single-lead ECG strip with at least 30 seconds of AFib.
In line with ESC’s 2016 AFib guidelines, the new iteration classifies AFib as first diagnosed, paroxysmal, persistent, long-standing persistent, and permanent. But it’s also important to classify the clinical profile of AFib, task force member Giuseppe Boriani, MD, PhD, University of Modena, Italy, said in the first of five presentations.
“So the novelty of the 2020 guidelines is related to the proposal of the 4S-AF scheme for a structured characterization of atrial fibrillation that takes into account Stroke risk, severity of Symptoms, Severity of atrial fibrillation burden, and Substrate severity,” he said.
This represents a paradigm shift from a single-domain conventional classification of AFib toward a structured characterization that streamlines assessment, informs treatment decision-making, and facilitates communication among physicians of various specialties, said Tatjana Potpara, MD, PhD, guideline co-chair and head of the Department for Intensive Arrhythmia Care, Clinical Centre of Serbia, Belgrade.
“The beauty of this approach is that, at present, the assessment of the ‘S’ components are performed using available tools, but in the future, the 4S-AF has a great potential to incorporate whatever becomes available for a more precision assessment of substrate or symptoms or arrhythmia burden and so forth,” she said.
ABC pathway
The guidelines advocate the previously described ABC pathway for integrated care management, which includes ‘A’ for Anticoagulation/Avoid stroke, ‘B’ for Better symptom control, and ‘C’ for Comorbidity/Cardiovascular risk factor optimization.
The document strengthens support for formal risk score–based assessment of bleeding risk in all patients, including use of the HAS-BLED score to help address modifiable bleeding risk factors and to identify patients at high bleeding risk (HAS-BLED score ≥3) for early and more frequent follow-up.
These assessments should be done regularly, given that both stroke and bleeding risk are dynamic and change over time with aging and comorbidities, Dr. Potpara stressed. In patients with AFib initially at low risk for stroke, the next assessment should be optimally performed at 4-6 months.
The guideline also targets weight loss in patients who are obese and have AFib, particularly those being evaluated for ablation, and good blood pressure control in patients with AFib and hypertension to reduce AFib recurrences and risk for stroke and bleeding (both class I, up from IIa).
It’s particularly important that these risk factors are addressed, and that modifiable risk factors that go along with increased AFib occurrence and persistence are addressed and communicated to patients, said Gerhard Hindricks, MD, PhD, guideline cochair and medical director of the Rhythmology Department, Heart Centre Leipzig (Germany).
“I have to confess, as an interventional electrophysiologist, there has been a time where I have not appreciated these risk factors intensely enough,” he said. “But we have learned, also in the field of catheter ablation, that weight loss is an essential basis for a good procedure. If we can motivate patients to lose weight and then come to the intervention with better outcome, it’s a true benefit for the patient and addresses patient values. So I’m particularly happy we have introduced that with such intensity in the guidelines.”
Rate and rhythm control
The guidelines make no recommendation of one novel oral anticoagulant (NOAC) over another. However, in patients already receiving vitamin K antagonists with low time in the therapeutic range, they recommend switching to a different NOAC but ensuring good adherence and persistence with therapy (class I recommendation) or efforts to improve time in therapeutic range (class IIa).
Catheter ablation takes on a more prominent role for rhythm control and is now recommended after one antiarrhythmic drug therapy fails to improve symptoms of AF recurrence in patients with paroxysmal AFib, or persistent AFib with or without major risk factors for recurrence. The class I recommendation is based on results from the CAPTAF and CABANA trials, said task force member Carina Blomström-Lundqvist, MD, PhD, Uppsala University, Sweden.
Catheter ablation is also now a first-line therapy for patients with AFib who have a high likelihood of tachycardia-induced cardiomyopathy, independent of symptom status. “In this subset of patients, catheter ablation may offer a lot with respect to restoration of left ventricular function,” observed Dr. Hindricks.
Complete electrical isolation of the pulmonary veins is recommended during all AFib catheter ablation procedures (class I).
“Even as a medical conservative, I think it is totally reasonable to move to catheter ablation after a failed drug trial,” commented John Mandrola, MD, Baptist Health, Louisville, Ky., who was not a part of the guideline development.
Although the chance of a second drug working after one failure is low, he noted that operators in the United States have dofetilide, which is not used much in Europe, and sometimes works surprisingly well.
“That said, the caveat is that moving to catheter ablation after drug failure is only appropriate if we have addressed all the pertinent risk factors: sleep apnea, weight loss, lack of fitness, blood pressure control, and alcohol excess,” he said.
As for tachycardia-mediated cardiomyopathy, this too can be reasonable, Dr. Mandrola said. “I often get people ‘out of a hole’ with amiodarone plus cardioversion for a few months and then proceed to ablation.”
Notably, the 2020 iteration sharpens its recommendation that amiodarone not be used first-line for long-term rhythm control in all patients with AFib, including those with heart failure with reduced ejection fraction, given its extracardiac toxicity (class I, up from IIa).
Quality counts
In response to growing evidence that guideline-adherence is associated with significantly better outcomes in AFib, the 2020 ESC/EACTS guidelines explicitly included a recommendation on the need to measure quality of care to identify opportunities for improvement.
With this framework in mind, a task force with 23 people – including members from ESC and heart rhythm societies in the United States, Asia Pacific, and Latin America, along with patient representatives – was created to develop a list of quality indicators (QIs), ultimately settling on 17 main QIs and 17 secondary ones, said Elena Arbelo, MD, PhD, MSc, University of Barcelona.
The QIs are classified into six domains: patient assessment, anticoagulation, rate control, rhythm control, risk factor modification, and, importantly, outcome measures. A full list is accessible in a paper, simultaneously published in EP EuroPace.
Five patient-reported outcomes fall under the outcomes domain but only one – health-related quality of life – is a main quality indicator. The remaining outcomes are still important but are listed as secondary because of the lack of evidence to sustain or defend their systematic implementation, particularly evidence on how to measure them appropriately, Dr. Arbelo said.
“Hopefully, following the [class I] recommendation by the 2020 ESC guidelines to routinely collect patient-reported outcomes will allow us to collect further evidence and in the future have sufficient evidence to include these as a main outcome,” she said.
The QI work was driven in parallel with the guidelines and had a huge impact on its development, including inclusion of clear recommendations on how to measure quality, Dr. Hindricks said. “I believe that the whole issue of quality management in the treatment of patients with a focus on patient values cannot be overestimated.”
Disclosure information for all writing committee members is in the report. Dr. Mandrola is a writer and podcaster for Medscape.
A version of this article originally appeared on Medscape.com.
New atrial fibrillation (AFib) management guidelines from the European Society of Cardiology (ESC) call for diagnostic confirmation and structured characterization of AFib and the need to streamline integrated care with the Atrial fibrillation Better Care (ABC) pathway.
“It’s as simple as CC to ABC,” quipped one task force member during the virtual unveiling of the guidelines at the ESC Congress 2020.
The guidelines were developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) and published simultaneously August 29 in the European Heart Journal.
Acknowledging the slew of novel screening tools now available and their reported sensitivity and specificity rates, the document supports opportunistic screening for AFib by pulse taking or electrocardiogram (ECG) rhythm strip in patients at least 65 years of age, with a class 1 recommendation, evidence level B.
Systematic ECG screening should also be considered to detect AFib in individuals at least 75 years of age or in those at high risk for stroke (class IIa, level B).
Other new class I screening recommendations are to inform individuals undergoing screening about the significance and treatment implications of detecting AFib and to have a structured referral platform in place for further physician-led evaluation.
A definite diagnosis of clinical AFib is established only after confirmation by a conventional 12-lead ECG or single-lead ECG strip with at least 30 seconds of AFib.
In line with ESC’s 2016 AFib guidelines, the new iteration classifies AFib as first diagnosed, paroxysmal, persistent, long-standing persistent, and permanent. But it’s also important to classify the clinical profile of AFib, task force member Giuseppe Boriani, MD, PhD, University of Modena, Italy, said in the first of five presentations.
“So the novelty of the 2020 guidelines is related to the proposal of the 4S-AF scheme for a structured characterization of atrial fibrillation that takes into account Stroke risk, severity of Symptoms, Severity of atrial fibrillation burden, and Substrate severity,” he said.
This represents a paradigm shift from a single-domain conventional classification of AFib toward a structured characterization that streamlines assessment, informs treatment decision-making, and facilitates communication among physicians of various specialties, said Tatjana Potpara, MD, PhD, guideline co-chair and head of the Department for Intensive Arrhythmia Care, Clinical Centre of Serbia, Belgrade.
“The beauty of this approach is that, at present, the assessment of the ‘S’ components are performed using available tools, but in the future, the 4S-AF has a great potential to incorporate whatever becomes available for a more precision assessment of substrate or symptoms or arrhythmia burden and so forth,” she said.
ABC pathway
The guidelines advocate the previously described ABC pathway for integrated care management, which includes ‘A’ for Anticoagulation/Avoid stroke, ‘B’ for Better symptom control, and ‘C’ for Comorbidity/Cardiovascular risk factor optimization.
The document strengthens support for formal risk score–based assessment of bleeding risk in all patients, including use of the HAS-BLED score to help address modifiable bleeding risk factors and to identify patients at high bleeding risk (HAS-BLED score ≥3) for early and more frequent follow-up.
These assessments should be done regularly, given that both stroke and bleeding risk are dynamic and change over time with aging and comorbidities, Dr. Potpara stressed. In patients with AFib initially at low risk for stroke, the next assessment should be optimally performed at 4-6 months.
The guideline also targets weight loss in patients who are obese and have AFib, particularly those being evaluated for ablation, and good blood pressure control in patients with AFib and hypertension to reduce AFib recurrences and risk for stroke and bleeding (both class I, up from IIa).
It’s particularly important that these risk factors are addressed, and that modifiable risk factors that go along with increased AFib occurrence and persistence are addressed and communicated to patients, said Gerhard Hindricks, MD, PhD, guideline cochair and medical director of the Rhythmology Department, Heart Centre Leipzig (Germany).
“I have to confess, as an interventional electrophysiologist, there has been a time where I have not appreciated these risk factors intensely enough,” he said. “But we have learned, also in the field of catheter ablation, that weight loss is an essential basis for a good procedure. If we can motivate patients to lose weight and then come to the intervention with better outcome, it’s a true benefit for the patient and addresses patient values. So I’m particularly happy we have introduced that with such intensity in the guidelines.”
Rate and rhythm control
The guidelines make no recommendation of one novel oral anticoagulant (NOAC) over another. However, in patients already receiving vitamin K antagonists with low time in the therapeutic range, they recommend switching to a different NOAC but ensuring good adherence and persistence with therapy (class I recommendation) or efforts to improve time in therapeutic range (class IIa).
Catheter ablation takes on a more prominent role for rhythm control and is now recommended after one antiarrhythmic drug therapy fails to improve symptoms of AF recurrence in patients with paroxysmal AFib, or persistent AFib with or without major risk factors for recurrence. The class I recommendation is based on results from the CAPTAF and CABANA trials, said task force member Carina Blomström-Lundqvist, MD, PhD, Uppsala University, Sweden.
Catheter ablation is also now a first-line therapy for patients with AFib who have a high likelihood of tachycardia-induced cardiomyopathy, independent of symptom status. “In this subset of patients, catheter ablation may offer a lot with respect to restoration of left ventricular function,” observed Dr. Hindricks.
Complete electrical isolation of the pulmonary veins is recommended during all AFib catheter ablation procedures (class I).
“Even as a medical conservative, I think it is totally reasonable to move to catheter ablation after a failed drug trial,” commented John Mandrola, MD, Baptist Health, Louisville, Ky., who was not a part of the guideline development.
Although the chance of a second drug working after one failure is low, he noted that operators in the United States have dofetilide, which is not used much in Europe, and sometimes works surprisingly well.
“That said, the caveat is that moving to catheter ablation after drug failure is only appropriate if we have addressed all the pertinent risk factors: sleep apnea, weight loss, lack of fitness, blood pressure control, and alcohol excess,” he said.
As for tachycardia-mediated cardiomyopathy, this too can be reasonable, Dr. Mandrola said. “I often get people ‘out of a hole’ with amiodarone plus cardioversion for a few months and then proceed to ablation.”
Notably, the 2020 iteration sharpens its recommendation that amiodarone not be used first-line for long-term rhythm control in all patients with AFib, including those with heart failure with reduced ejection fraction, given its extracardiac toxicity (class I, up from IIa).
Quality counts
In response to growing evidence that guideline-adherence is associated with significantly better outcomes in AFib, the 2020 ESC/EACTS guidelines explicitly included a recommendation on the need to measure quality of care to identify opportunities for improvement.
With this framework in mind, a task force with 23 people – including members from ESC and heart rhythm societies in the United States, Asia Pacific, and Latin America, along with patient representatives – was created to develop a list of quality indicators (QIs), ultimately settling on 17 main QIs and 17 secondary ones, said Elena Arbelo, MD, PhD, MSc, University of Barcelona.
The QIs are classified into six domains: patient assessment, anticoagulation, rate control, rhythm control, risk factor modification, and, importantly, outcome measures. A full list is accessible in a paper, simultaneously published in EP EuroPace.
Five patient-reported outcomes fall under the outcomes domain but only one – health-related quality of life – is a main quality indicator. The remaining outcomes are still important but are listed as secondary because of the lack of evidence to sustain or defend their systematic implementation, particularly evidence on how to measure them appropriately, Dr. Arbelo said.
“Hopefully, following the [class I] recommendation by the 2020 ESC guidelines to routinely collect patient-reported outcomes will allow us to collect further evidence and in the future have sufficient evidence to include these as a main outcome,” she said.
The QI work was driven in parallel with the guidelines and had a huge impact on its development, including inclusion of clear recommendations on how to measure quality, Dr. Hindricks said. “I believe that the whole issue of quality management in the treatment of patients with a focus on patient values cannot be overestimated.”
Disclosure information for all writing committee members is in the report. Dr. Mandrola is a writer and podcaster for Medscape.
A version of this article originally appeared on Medscape.com.
EXPLORER trial hints at potential new drug option in obstructive hypertrophic cardiomyopathy
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.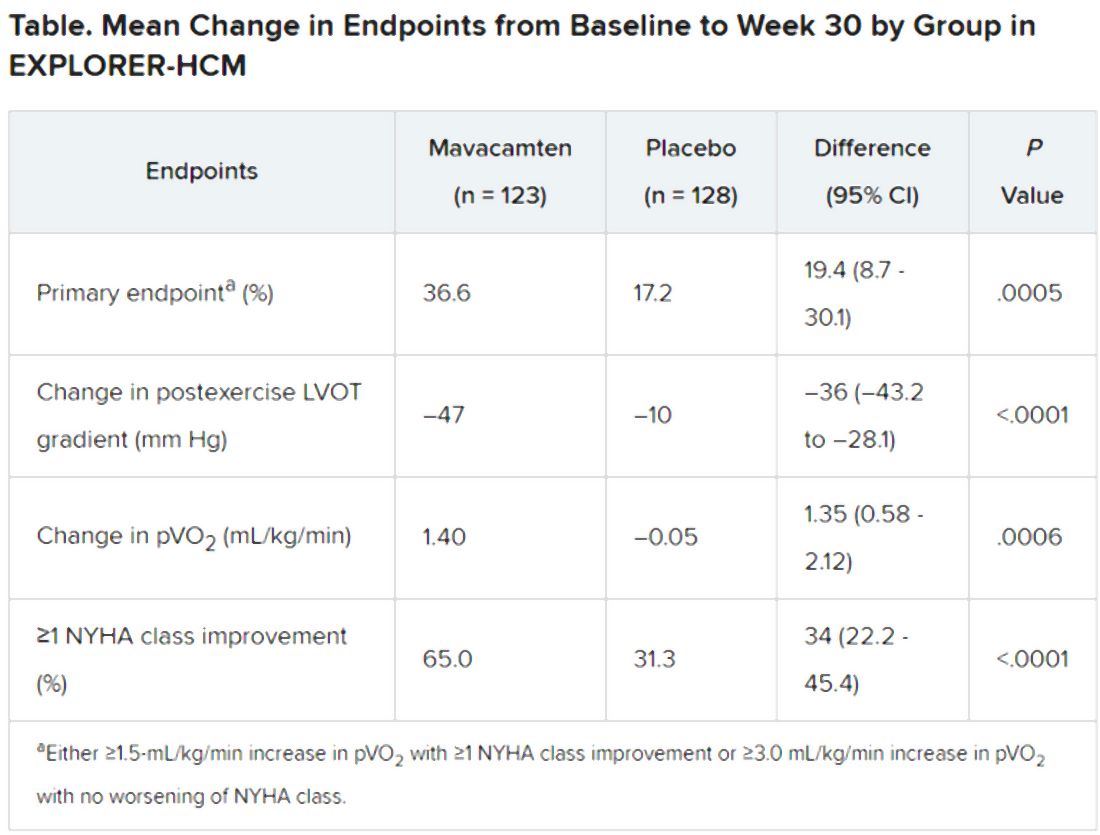
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
EMPEROR-Reduced: Empagliflozin’s HFrEF benefit solidifies class effects
The SGLT2 inhibitor drug class solidified its role as a major, new treatment for patients with heart failure with reduced ejection fraction and no diabetes, with results from a second large, controlled trial showing clear efficacy and safety in this population.
Patients with heart failure with reduced ejection fraction (HFrEF) treated with the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) had a statistically significant 25% relative cut in their incidence of cardiovascular death or first heart failure hospitalization, compared with placebo-treated controls when added on top of standard HFrEF treatment, and this benefit was consistent regardless of whether the treated patients also had type 2 diabetes, Milton Packer, MD, reported at the virtual annual congress of the European Society of Cardiology.
This 25% drop in the primary endpoint with empagliflozin treatment in the EMPEROR-Reduced trial exactly matched the cut in incidence of cardiovascular death or heart failure hospitalization produced by treatment with a another SGLT2 inhibitor, dapagliflozin (Farxiga), in the DAPA-HF trial (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
The performance of these two SGLT2 inhibitors was “incredibly consistent” across the their respective trials run in HFrEF patients with and without type 2 diabetes, and the combined evidence base of the two trials makes for “really compelling evidence” of both safety and efficacy that should prompt a change to U.S. practice, with both of these drugs forming a new cornerstone of HFrEF treatment, Dr. Packer said.
Results plant drug class firmly as HFrEF treatment
Dr. Packer stressed in his presentation that optimal treatment of patients with HFrEF now demands use of one of these two SGLT2 inhibitors, as well as sacubitril plus valsartan (Entresto), a beta-blocker, and a mineralocorticoid receptor antagonist, plus a diuretic as a fifth drug class for the many HFrEF patients who also need treatment for fluid overload. He further advocated for rapid introduction of these four cornerstone agents with proven survival benefits once a patient receives a HFrEF diagnosis, suggesting that sacubitril plus valsartan, an SGLT2 inhibitor, a beta-blocker, and a mineralocorticoid receptor antagonist could all be initiated within 6 weeks or less while acknowledging that optimal up-titration of the beta-blocker would likely take longer.
The order in which a patient starts these drugs shouldn’t matter, and there currently seems to be no evidence that clearly points toward using either dapagliflozin or empagliflozin over the other, Dr. Packer added.
In recognition of the importance of sending a message to heart failure clinicians about the newly proven efficacy of SGLT2 inhibitors in HFrEF patients, the American College of Cardiology and American Heart Association are now drafting an “expert decision pathway” to help clinicians as they enter this new prescribing space. This interim guidance should come out before the end of 2020, prior to release of fully revised HFrEF management guidelines in 2021, said Athena Poppas, MD, president of the ACC, in an interview.
“There is clearly need for education” that can help guide physicians who care for HFrEF patients on how to introduce an SGLT2 inhibitor along with the additional, lengthy list of drug classes proven to benefit these patients, noted Dr. Poppas, who is also a professor and chief of cardiology at the Brown University in Providence, R.I. Physicians may find that they need extra backup for successfully starting both sacubitril plus valsartan and an SGLT2 inhibitor in HFrEF patients because recent history has shown substantial pushback from third-party payers in reimbursing for these relatively expensive drugs, Dr. Poppas noted. She added that this is a problem that may be compounded when patients should ideally get both drug classes.
Physicians who care for heart failure patients have their own history of dragging their feet when adding new drugs to the regimens HFrEF patients receive. The angiotensin converting enzyme inhibitors and beta-blockers took about 17 years each to start reaching a majority of U.S. HFrEF patients, and sacubitril plus valsartan is now used on perhaps a quarter to a third of HFrEF patients despite receiving Food and Drug Administration approval for these patients in mid 2015, noted Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart and Vascular Institute in Fairfax, Va.
Despite dapagliflozin receiving FDA approval in May 2020 for treating HFrEF in patients without diabetes, early uptake in U.S. practice has been very slow, with findings from large U.S. patient registries suggesting that perhaps 1% of suitable HFrEF patients currently get the drug, estimated Dr. O’Connor in an interview.
Given how strong the evidence now is for benefit and safety from dapagliflozin and empagliflozin, it may take as little as 5 years to reach greater than 50% penetration of one of these drugs into U.S. HFrEF patient populations, suggested Dr. Packer, a distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
EMPEROR-Reduced outcomes
The road to routine use of these SGLT2 inhibitor drugs should be hastened by empagliflozin’s impressive performance in EMPEROR-Reduced, in which the drug scored highly significant benefits over placebo for the prespecified primary and two major secondary endpoints, one of which was a measure of preserved renal function.
The trial randomized 3,730 patients at 520 sites in 20 countries during 2017-2019 and followed them on treatment for a median of 16 months. All patients had a left ventricular ejection fraction of 40% or less, and roughly three-quarters had New York Heart Association (NYHA) class II function, nearly one-quarter had class III function, and fewer than 1% of patients fell into the class IV category.
The primary endpoint occurred in 19% of the empagliflozin-treated patients and in 25% of those who received placebo. Among the half of patients with diabetes in the trial, the relative risk reduction by empagliflozin compared with placebo was a statistically significant 28%; among those without diabetes, it was a statistically significant 22%. Concurrently with Dr. Packer’s report, the results appeared in an article posted online (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
The study also had two main prespecified secondary endpoints: the incidence of total hospitalizations for heart failure, both first and recurrent, which fell by 30% in the empagliflozin-treated patients, compared with placebo, and the rate of declining renal function during the 16 months of the study as measured by estimated glomerular filtration rate, which dropped by roughly 1 mL/min per 1.73 m2 among the empagliflozin recipients and by about 4 mL/min/ per 1.73 m2 in the placebo patients.
Treatment with empagliflozin also achieved a notable, statistically significant 50% drop in major adverse renal events, consistent with the performance of other drugs in the class.
“Renal protection is a big plus” of empagliflozin in this trial and from the other SGLT2 inhibitors in prior studies, noted Dr. O’Connor.
The EMPEROR-Reduced results also showed an important benefit for HFrEF patients from empagliflozin not previously seen as quickly with any other drug class, noted Dr. Packer. The SGLT2 inhibitor led to statistically a significant slowing in the progression of patients from NYHA class II function to class III, compared with placebo, and it also significantly promoted the recovery of patients from NYHA class III to class II, an effect that became apparent within the first month on treatment and a benefit that is a “big deal” for patients because it represents a “significant change in functional capacity.” This additional dimension of empagliflozin’s benefit “really impressed me,” Dr. Packer said.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Poppas and Dr. O’Connor had no relevant disclosures.
SOURCE: Packer M. ESC 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190.
The SGLT2 inhibitor drug class solidified its role as a major, new treatment for patients with heart failure with reduced ejection fraction and no diabetes, with results from a second large, controlled trial showing clear efficacy and safety in this population.
Patients with heart failure with reduced ejection fraction (HFrEF) treated with the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) had a statistically significant 25% relative cut in their incidence of cardiovascular death or first heart failure hospitalization, compared with placebo-treated controls when added on top of standard HFrEF treatment, and this benefit was consistent regardless of whether the treated patients also had type 2 diabetes, Milton Packer, MD, reported at the virtual annual congress of the European Society of Cardiology.
This 25% drop in the primary endpoint with empagliflozin treatment in the EMPEROR-Reduced trial exactly matched the cut in incidence of cardiovascular death or heart failure hospitalization produced by treatment with a another SGLT2 inhibitor, dapagliflozin (Farxiga), in the DAPA-HF trial (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
The performance of these two SGLT2 inhibitors was “incredibly consistent” across the their respective trials run in HFrEF patients with and without type 2 diabetes, and the combined evidence base of the two trials makes for “really compelling evidence” of both safety and efficacy that should prompt a change to U.S. practice, with both of these drugs forming a new cornerstone of HFrEF treatment, Dr. Packer said.
Results plant drug class firmly as HFrEF treatment
Dr. Packer stressed in his presentation that optimal treatment of patients with HFrEF now demands use of one of these two SGLT2 inhibitors, as well as sacubitril plus valsartan (Entresto), a beta-blocker, and a mineralocorticoid receptor antagonist, plus a diuretic as a fifth drug class for the many HFrEF patients who also need treatment for fluid overload. He further advocated for rapid introduction of these four cornerstone agents with proven survival benefits once a patient receives a HFrEF diagnosis, suggesting that sacubitril plus valsartan, an SGLT2 inhibitor, a beta-blocker, and a mineralocorticoid receptor antagonist could all be initiated within 6 weeks or less while acknowledging that optimal up-titration of the beta-blocker would likely take longer.
The order in which a patient starts these drugs shouldn’t matter, and there currently seems to be no evidence that clearly points toward using either dapagliflozin or empagliflozin over the other, Dr. Packer added.
In recognition of the importance of sending a message to heart failure clinicians about the newly proven efficacy of SGLT2 inhibitors in HFrEF patients, the American College of Cardiology and American Heart Association are now drafting an “expert decision pathway” to help clinicians as they enter this new prescribing space. This interim guidance should come out before the end of 2020, prior to release of fully revised HFrEF management guidelines in 2021, said Athena Poppas, MD, president of the ACC, in an interview.
“There is clearly need for education” that can help guide physicians who care for HFrEF patients on how to introduce an SGLT2 inhibitor along with the additional, lengthy list of drug classes proven to benefit these patients, noted Dr. Poppas, who is also a professor and chief of cardiology at the Brown University in Providence, R.I. Physicians may find that they need extra backup for successfully starting both sacubitril plus valsartan and an SGLT2 inhibitor in HFrEF patients because recent history has shown substantial pushback from third-party payers in reimbursing for these relatively expensive drugs, Dr. Poppas noted. She added that this is a problem that may be compounded when patients should ideally get both drug classes.
Physicians who care for heart failure patients have their own history of dragging their feet when adding new drugs to the regimens HFrEF patients receive. The angiotensin converting enzyme inhibitors and beta-blockers took about 17 years each to start reaching a majority of U.S. HFrEF patients, and sacubitril plus valsartan is now used on perhaps a quarter to a third of HFrEF patients despite receiving Food and Drug Administration approval for these patients in mid 2015, noted Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart and Vascular Institute in Fairfax, Va.
Despite dapagliflozin receiving FDA approval in May 2020 for treating HFrEF in patients without diabetes, early uptake in U.S. practice has been very slow, with findings from large U.S. patient registries suggesting that perhaps 1% of suitable HFrEF patients currently get the drug, estimated Dr. O’Connor in an interview.
Given how strong the evidence now is for benefit and safety from dapagliflozin and empagliflozin, it may take as little as 5 years to reach greater than 50% penetration of one of these drugs into U.S. HFrEF patient populations, suggested Dr. Packer, a distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
EMPEROR-Reduced outcomes
The road to routine use of these SGLT2 inhibitor drugs should be hastened by empagliflozin’s impressive performance in EMPEROR-Reduced, in which the drug scored highly significant benefits over placebo for the prespecified primary and two major secondary endpoints, one of which was a measure of preserved renal function.
The trial randomized 3,730 patients at 520 sites in 20 countries during 2017-2019 and followed them on treatment for a median of 16 months. All patients had a left ventricular ejection fraction of 40% or less, and roughly three-quarters had New York Heart Association (NYHA) class II function, nearly one-quarter had class III function, and fewer than 1% of patients fell into the class IV category.
The primary endpoint occurred in 19% of the empagliflozin-treated patients and in 25% of those who received placebo. Among the half of patients with diabetes in the trial, the relative risk reduction by empagliflozin compared with placebo was a statistically significant 28%; among those without diabetes, it was a statistically significant 22%. Concurrently with Dr. Packer’s report, the results appeared in an article posted online (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
The study also had two main prespecified secondary endpoints: the incidence of total hospitalizations for heart failure, both first and recurrent, which fell by 30% in the empagliflozin-treated patients, compared with placebo, and the rate of declining renal function during the 16 months of the study as measured by estimated glomerular filtration rate, which dropped by roughly 1 mL/min per 1.73 m2 among the empagliflozin recipients and by about 4 mL/min/ per 1.73 m2 in the placebo patients.
Treatment with empagliflozin also achieved a notable, statistically significant 50% drop in major adverse renal events, consistent with the performance of other drugs in the class.
“Renal protection is a big plus” of empagliflozin in this trial and from the other SGLT2 inhibitors in prior studies, noted Dr. O’Connor.
The EMPEROR-Reduced results also showed an important benefit for HFrEF patients from empagliflozin not previously seen as quickly with any other drug class, noted Dr. Packer. The SGLT2 inhibitor led to statistically a significant slowing in the progression of patients from NYHA class II function to class III, compared with placebo, and it also significantly promoted the recovery of patients from NYHA class III to class II, an effect that became apparent within the first month on treatment and a benefit that is a “big deal” for patients because it represents a “significant change in functional capacity.” This additional dimension of empagliflozin’s benefit “really impressed me,” Dr. Packer said.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Poppas and Dr. O’Connor had no relevant disclosures.
SOURCE: Packer M. ESC 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190.
The SGLT2 inhibitor drug class solidified its role as a major, new treatment for patients with heart failure with reduced ejection fraction and no diabetes, with results from a second large, controlled trial showing clear efficacy and safety in this population.
Patients with heart failure with reduced ejection fraction (HFrEF) treated with the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) had a statistically significant 25% relative cut in their incidence of cardiovascular death or first heart failure hospitalization, compared with placebo-treated controls when added on top of standard HFrEF treatment, and this benefit was consistent regardless of whether the treated patients also had type 2 diabetes, Milton Packer, MD, reported at the virtual annual congress of the European Society of Cardiology.
This 25% drop in the primary endpoint with empagliflozin treatment in the EMPEROR-Reduced trial exactly matched the cut in incidence of cardiovascular death or heart failure hospitalization produced by treatment with a another SGLT2 inhibitor, dapagliflozin (Farxiga), in the DAPA-HF trial (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
The performance of these two SGLT2 inhibitors was “incredibly consistent” across the their respective trials run in HFrEF patients with and without type 2 diabetes, and the combined evidence base of the two trials makes for “really compelling evidence” of both safety and efficacy that should prompt a change to U.S. practice, with both of these drugs forming a new cornerstone of HFrEF treatment, Dr. Packer said.
Results plant drug class firmly as HFrEF treatment
Dr. Packer stressed in his presentation that optimal treatment of patients with HFrEF now demands use of one of these two SGLT2 inhibitors, as well as sacubitril plus valsartan (Entresto), a beta-blocker, and a mineralocorticoid receptor antagonist, plus a diuretic as a fifth drug class for the many HFrEF patients who also need treatment for fluid overload. He further advocated for rapid introduction of these four cornerstone agents with proven survival benefits once a patient receives a HFrEF diagnosis, suggesting that sacubitril plus valsartan, an SGLT2 inhibitor, a beta-blocker, and a mineralocorticoid receptor antagonist could all be initiated within 6 weeks or less while acknowledging that optimal up-titration of the beta-blocker would likely take longer.
The order in which a patient starts these drugs shouldn’t matter, and there currently seems to be no evidence that clearly points toward using either dapagliflozin or empagliflozin over the other, Dr. Packer added.
In recognition of the importance of sending a message to heart failure clinicians about the newly proven efficacy of SGLT2 inhibitors in HFrEF patients, the American College of Cardiology and American Heart Association are now drafting an “expert decision pathway” to help clinicians as they enter this new prescribing space. This interim guidance should come out before the end of 2020, prior to release of fully revised HFrEF management guidelines in 2021, said Athena Poppas, MD, president of the ACC, in an interview.
“There is clearly need for education” that can help guide physicians who care for HFrEF patients on how to introduce an SGLT2 inhibitor along with the additional, lengthy list of drug classes proven to benefit these patients, noted Dr. Poppas, who is also a professor and chief of cardiology at the Brown University in Providence, R.I. Physicians may find that they need extra backup for successfully starting both sacubitril plus valsartan and an SGLT2 inhibitor in HFrEF patients because recent history has shown substantial pushback from third-party payers in reimbursing for these relatively expensive drugs, Dr. Poppas noted. She added that this is a problem that may be compounded when patients should ideally get both drug classes.
Physicians who care for heart failure patients have their own history of dragging their feet when adding new drugs to the regimens HFrEF patients receive. The angiotensin converting enzyme inhibitors and beta-blockers took about 17 years each to start reaching a majority of U.S. HFrEF patients, and sacubitril plus valsartan is now used on perhaps a quarter to a third of HFrEF patients despite receiving Food and Drug Administration approval for these patients in mid 2015, noted Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart and Vascular Institute in Fairfax, Va.
Despite dapagliflozin receiving FDA approval in May 2020 for treating HFrEF in patients without diabetes, early uptake in U.S. practice has been very slow, with findings from large U.S. patient registries suggesting that perhaps 1% of suitable HFrEF patients currently get the drug, estimated Dr. O’Connor in an interview.
Given how strong the evidence now is for benefit and safety from dapagliflozin and empagliflozin, it may take as little as 5 years to reach greater than 50% penetration of one of these drugs into U.S. HFrEF patient populations, suggested Dr. Packer, a distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
EMPEROR-Reduced outcomes
The road to routine use of these SGLT2 inhibitor drugs should be hastened by empagliflozin’s impressive performance in EMPEROR-Reduced, in which the drug scored highly significant benefits over placebo for the prespecified primary and two major secondary endpoints, one of which was a measure of preserved renal function.
The trial randomized 3,730 patients at 520 sites in 20 countries during 2017-2019 and followed them on treatment for a median of 16 months. All patients had a left ventricular ejection fraction of 40% or less, and roughly three-quarters had New York Heart Association (NYHA) class II function, nearly one-quarter had class III function, and fewer than 1% of patients fell into the class IV category.
The primary endpoint occurred in 19% of the empagliflozin-treated patients and in 25% of those who received placebo. Among the half of patients with diabetes in the trial, the relative risk reduction by empagliflozin compared with placebo was a statistically significant 28%; among those without diabetes, it was a statistically significant 22%. Concurrently with Dr. Packer’s report, the results appeared in an article posted online (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
The study also had two main prespecified secondary endpoints: the incidence of total hospitalizations for heart failure, both first and recurrent, which fell by 30% in the empagliflozin-treated patients, compared with placebo, and the rate of declining renal function during the 16 months of the study as measured by estimated glomerular filtration rate, which dropped by roughly 1 mL/min per 1.73 m2 among the empagliflozin recipients and by about 4 mL/min/ per 1.73 m2 in the placebo patients.
Treatment with empagliflozin also achieved a notable, statistically significant 50% drop in major adverse renal events, consistent with the performance of other drugs in the class.
“Renal protection is a big plus” of empagliflozin in this trial and from the other SGLT2 inhibitors in prior studies, noted Dr. O’Connor.
The EMPEROR-Reduced results also showed an important benefit for HFrEF patients from empagliflozin not previously seen as quickly with any other drug class, noted Dr. Packer. The SGLT2 inhibitor led to statistically a significant slowing in the progression of patients from NYHA class II function to class III, compared with placebo, and it also significantly promoted the recovery of patients from NYHA class III to class II, an effect that became apparent within the first month on treatment and a benefit that is a “big deal” for patients because it represents a “significant change in functional capacity.” This additional dimension of empagliflozin’s benefit “really impressed me,” Dr. Packer said.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Poppas and Dr. O’Connor had no relevant disclosures.
SOURCE: Packer M. ESC 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190.
FROM ESC CONGRESS 2020
Early rhythm control in AFib gains new life
Initiation of rhythm control with antiarrhythmic drugs and/or ablation in patients with early, recently diagnosed atrial fibrillation (AFib) led to a significantly lower risk of major adverse cardiovascular outcomes, compared with a rate-control strategy, during more than 5 years of follow-up in the large randomized EAST-AFNET 4 trial, Paulus Kirchhof, MD, said at the virtual annual congress of the European Society of Cardiology.
Previous trials of rate versus rhythm control in AFib, such as AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management), failed to show an advantage for rhythm over rate control in terms of clinical outcomes. Why was EAST-AFNET 4 different? Dr. Kirchhof offered two major reasons: The study incorporated AFib ablation as an option in the rhythm control strategy, and treatment started soon after diagnosis of the arrhythmia. Indeed, nearly 40% of patients had their first-ever AFib episode at the time of randomization, and the median time from diagnosis to randomization was just 36 days.
“Once you are in AFib for a few months, the atrium suffers severe damage, some of it irreversible, so it becomes more difficult to restore and maintain sinus rhythm when you wait longer,” explained Dr. Kirchhof, director of the department of cardiology at the University Heart and Vascular Center in Hamburg (Ger.) and professor of cardiovascular medicine at the University of Birmingham, England.
Also, epidemiologic studies show that the risk of cardiovascular complications is heightened in the first year following diagnosis of AFib. “So there’s a window of opportunity to prevent complications,” he added.
The impetus for conducting EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial ) was straightforward, according to the cardiologist: “The question of whether rhythm control is beneficial or not has been in the field for several decades. Most people, like me, always believed that maintaining sinus rhythm would help, but we didn’t have the data to show it.”
Early rhythm control shows sustained benefits
EAST-AFNET 4 was a prospective, open, blinded-outcome-assessement trial. It included 2,789 patients with early AFib and an average CHA2DS2-VASc score of 3.4 who were randomized at 135 sites in 11 European countries to early rhythm control or guideline-recommended rate control. At a median 5.1 years of follow-up, the primary outcome – a composite of cardiovascular death, stroke, acute coronary syndrome, or hospitalization for worsening heart failure – occurred at a pace of 3.9% per year in the rhythm control group and 5% per year with rate control. This translated to a statistically significant and clinically meaningful 21% relative risk reduction favoring early rhythm control.
The 28% reduction in cardiovascular death with rhythm control was statistically significant, as was the 35% reduction in stroke. However, the 19% reduction in heart failure hospitalizations and 17% decrease in hospitalizations for acute coronary syndrome were not.
The co–primary endpoint – the mean number of nights spent in the hospital per year, which served as a proxy for the cost of treatment to a health care system – didn’t differ between the two treatment arms, at roughly 5 nights per year.
The clinical benefit of early rhythm control was consistent across all 19 prespecified patient subgroups, including those who were asymptomatic and patients with or without heart failure.
Serious adverse events related to rhythm control therapy – most often drug-related bradycardia – occurred in 4.9% of patients over the course of 5.1 years, compared to a 1.4% serious event rate in patients assigned to rate control. Dr. Kirchhof called the roughly 1% per year serious event rate in the rhythm control group quite acceptable.
“To put that in perspective, the annualized rate of severe bleeds on oral anticoagulation – a very beneficial therapy used by more than 90% of participants at 2 years – is about 2%,” the cardiologist noted.
Only 8% of patients randomized to rhythm control received AFib ablation as initial therapy, consistent with current clinical practice. By 2 years, 19.4% of the rhythm control group had undergone AFib ablation. Also at that time, 15% of the rate control group was receiving rhythm control therapy to help manage AFib-related symptoms.
One of the big surprises in the study, he said, was that nearly three-quarters of patients in both groups were asymptomatic at 2 years.
“I think that shows how well we control symptoms, even without rhythm control,” he observed.
Results ‘move the field forward’
Dr. Kirchhof stressed that this was a trial of two different treatment strategies, and it’s not yet possible to single out any specific component of the rhythm control strategy as being responsible for the improved outcomes.
“I cannot tell you whether the outcome difference was due to AFib ablation or early treatment or the fact that we’re now better at using antiarrhythmic drugs than we were 20 years ago,” he said.
Asked if the EAST-AFNET 4 findings warrant more aggressive screening for AFib in order to detect and intervene early in the arrhythmia, Dr. Kirchhof replied with an unambiguous yes.
“My conclusion is that every patient with newly diagnosed AFib and a CHA2DS2-VASc score of 2 or more should not only receive anticoagulation and rate control, but should also be offered rhythm control therapy at the time of diagnosis, which also means that all of these people have to be seen by a cardiologist who has expertise in the domain of AFib management. It’s a big clinical challenge, but it leads to a 21% improvement in outcomes, and I think we have to do what’s best for our patients,” he said.
In an interview, Kalyanam Shivkumar, MD, PhD, called EAST-AFNET 4 “a very important study.”
“It moves the field forward, for sure. I think it will change clinical practice, and it should,” commented Dr. Shivkumar, who was not involved in the study.
“Now there are so many wearable technologies out there – the Apple Watch and others – which will enable rhythm abnormalities to be detected early on. This bodes well for the field,” said Dr. Shivkumar, who is editor-in-chief of JACC: Clinical Electrophysiology. He is also professor of medicine, radiology, and bioengineering at the University of California, Los Angeles, and director of the UCLA Cardiac Arrhythmia Center.
Dr. Kirchhof reported receiving research grants to conduct the EAST-AFNET 4 trial from the German Ministry of Education and Research, the German Center for Cardiovascular Research, the Atrial Fibrillation Network, the European Heart Rhythm Association, St. Jude Medical, Abbott, Sanofi, the German Heart Foundation, the European Union, the British Heart Foundation, and the Leducq Foundation.
Simultaneous with his presentation at ESC Congress 2020, the study results were published online at NEJM.org.
SOURCE: Kirchhof P. ESC Congress 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2019422.
Initiation of rhythm control with antiarrhythmic drugs and/or ablation in patients with early, recently diagnosed atrial fibrillation (AFib) led to a significantly lower risk of major adverse cardiovascular outcomes, compared with a rate-control strategy, during more than 5 years of follow-up in the large randomized EAST-AFNET 4 trial, Paulus Kirchhof, MD, said at the virtual annual congress of the European Society of Cardiology.
Previous trials of rate versus rhythm control in AFib, such as AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management), failed to show an advantage for rhythm over rate control in terms of clinical outcomes. Why was EAST-AFNET 4 different? Dr. Kirchhof offered two major reasons: The study incorporated AFib ablation as an option in the rhythm control strategy, and treatment started soon after diagnosis of the arrhythmia. Indeed, nearly 40% of patients had their first-ever AFib episode at the time of randomization, and the median time from diagnosis to randomization was just 36 days.
“Once you are in AFib for a few months, the atrium suffers severe damage, some of it irreversible, so it becomes more difficult to restore and maintain sinus rhythm when you wait longer,” explained Dr. Kirchhof, director of the department of cardiology at the University Heart and Vascular Center in Hamburg (Ger.) and professor of cardiovascular medicine at the University of Birmingham, England.
Also, epidemiologic studies show that the risk of cardiovascular complications is heightened in the first year following diagnosis of AFib. “So there’s a window of opportunity to prevent complications,” he added.
The impetus for conducting EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial ) was straightforward, according to the cardiologist: “The question of whether rhythm control is beneficial or not has been in the field for several decades. Most people, like me, always believed that maintaining sinus rhythm would help, but we didn’t have the data to show it.”
Early rhythm control shows sustained benefits
EAST-AFNET 4 was a prospective, open, blinded-outcome-assessement trial. It included 2,789 patients with early AFib and an average CHA2DS2-VASc score of 3.4 who were randomized at 135 sites in 11 European countries to early rhythm control or guideline-recommended rate control. At a median 5.1 years of follow-up, the primary outcome – a composite of cardiovascular death, stroke, acute coronary syndrome, or hospitalization for worsening heart failure – occurred at a pace of 3.9% per year in the rhythm control group and 5% per year with rate control. This translated to a statistically significant and clinically meaningful 21% relative risk reduction favoring early rhythm control.
The 28% reduction in cardiovascular death with rhythm control was statistically significant, as was the 35% reduction in stroke. However, the 19% reduction in heart failure hospitalizations and 17% decrease in hospitalizations for acute coronary syndrome were not.
The co–primary endpoint – the mean number of nights spent in the hospital per year, which served as a proxy for the cost of treatment to a health care system – didn’t differ between the two treatment arms, at roughly 5 nights per year.
The clinical benefit of early rhythm control was consistent across all 19 prespecified patient subgroups, including those who were asymptomatic and patients with or without heart failure.
Serious adverse events related to rhythm control therapy – most often drug-related bradycardia – occurred in 4.9% of patients over the course of 5.1 years, compared to a 1.4% serious event rate in patients assigned to rate control. Dr. Kirchhof called the roughly 1% per year serious event rate in the rhythm control group quite acceptable.
“To put that in perspective, the annualized rate of severe bleeds on oral anticoagulation – a very beneficial therapy used by more than 90% of participants at 2 years – is about 2%,” the cardiologist noted.
Only 8% of patients randomized to rhythm control received AFib ablation as initial therapy, consistent with current clinical practice. By 2 years, 19.4% of the rhythm control group had undergone AFib ablation. Also at that time, 15% of the rate control group was receiving rhythm control therapy to help manage AFib-related symptoms.
One of the big surprises in the study, he said, was that nearly three-quarters of patients in both groups were asymptomatic at 2 years.
“I think that shows how well we control symptoms, even without rhythm control,” he observed.
Results ‘move the field forward’
Dr. Kirchhof stressed that this was a trial of two different treatment strategies, and it’s not yet possible to single out any specific component of the rhythm control strategy as being responsible for the improved outcomes.
“I cannot tell you whether the outcome difference was due to AFib ablation or early treatment or the fact that we’re now better at using antiarrhythmic drugs than we were 20 years ago,” he said.
Asked if the EAST-AFNET 4 findings warrant more aggressive screening for AFib in order to detect and intervene early in the arrhythmia, Dr. Kirchhof replied with an unambiguous yes.
“My conclusion is that every patient with newly diagnosed AFib and a CHA2DS2-VASc score of 2 or more should not only receive anticoagulation and rate control, but should also be offered rhythm control therapy at the time of diagnosis, which also means that all of these people have to be seen by a cardiologist who has expertise in the domain of AFib management. It’s a big clinical challenge, but it leads to a 21% improvement in outcomes, and I think we have to do what’s best for our patients,” he said.
In an interview, Kalyanam Shivkumar, MD, PhD, called EAST-AFNET 4 “a very important study.”
“It moves the field forward, for sure. I think it will change clinical practice, and it should,” commented Dr. Shivkumar, who was not involved in the study.
“Now there are so many wearable technologies out there – the Apple Watch and others – which will enable rhythm abnormalities to be detected early on. This bodes well for the field,” said Dr. Shivkumar, who is editor-in-chief of JACC: Clinical Electrophysiology. He is also professor of medicine, radiology, and bioengineering at the University of California, Los Angeles, and director of the UCLA Cardiac Arrhythmia Center.
Dr. Kirchhof reported receiving research grants to conduct the EAST-AFNET 4 trial from the German Ministry of Education and Research, the German Center for Cardiovascular Research, the Atrial Fibrillation Network, the European Heart Rhythm Association, St. Jude Medical, Abbott, Sanofi, the German Heart Foundation, the European Union, the British Heart Foundation, and the Leducq Foundation.
Simultaneous with his presentation at ESC Congress 2020, the study results were published online at NEJM.org.
SOURCE: Kirchhof P. ESC Congress 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2019422.
Initiation of rhythm control with antiarrhythmic drugs and/or ablation in patients with early, recently diagnosed atrial fibrillation (AFib) led to a significantly lower risk of major adverse cardiovascular outcomes, compared with a rate-control strategy, during more than 5 years of follow-up in the large randomized EAST-AFNET 4 trial, Paulus Kirchhof, MD, said at the virtual annual congress of the European Society of Cardiology.
Previous trials of rate versus rhythm control in AFib, such as AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management), failed to show an advantage for rhythm over rate control in terms of clinical outcomes. Why was EAST-AFNET 4 different? Dr. Kirchhof offered two major reasons: The study incorporated AFib ablation as an option in the rhythm control strategy, and treatment started soon after diagnosis of the arrhythmia. Indeed, nearly 40% of patients had their first-ever AFib episode at the time of randomization, and the median time from diagnosis to randomization was just 36 days.
“Once you are in AFib for a few months, the atrium suffers severe damage, some of it irreversible, so it becomes more difficult to restore and maintain sinus rhythm when you wait longer,” explained Dr. Kirchhof, director of the department of cardiology at the University Heart and Vascular Center in Hamburg (Ger.) and professor of cardiovascular medicine at the University of Birmingham, England.
Also, epidemiologic studies show that the risk of cardiovascular complications is heightened in the first year following diagnosis of AFib. “So there’s a window of opportunity to prevent complications,” he added.
The impetus for conducting EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial ) was straightforward, according to the cardiologist: “The question of whether rhythm control is beneficial or not has been in the field for several decades. Most people, like me, always believed that maintaining sinus rhythm would help, but we didn’t have the data to show it.”
Early rhythm control shows sustained benefits
EAST-AFNET 4 was a prospective, open, blinded-outcome-assessement trial. It included 2,789 patients with early AFib and an average CHA2DS2-VASc score of 3.4 who were randomized at 135 sites in 11 European countries to early rhythm control or guideline-recommended rate control. At a median 5.1 years of follow-up, the primary outcome – a composite of cardiovascular death, stroke, acute coronary syndrome, or hospitalization for worsening heart failure – occurred at a pace of 3.9% per year in the rhythm control group and 5% per year with rate control. This translated to a statistically significant and clinically meaningful 21% relative risk reduction favoring early rhythm control.
The 28% reduction in cardiovascular death with rhythm control was statistically significant, as was the 35% reduction in stroke. However, the 19% reduction in heart failure hospitalizations and 17% decrease in hospitalizations for acute coronary syndrome were not.
The co–primary endpoint – the mean number of nights spent in the hospital per year, which served as a proxy for the cost of treatment to a health care system – didn’t differ between the two treatment arms, at roughly 5 nights per year.
The clinical benefit of early rhythm control was consistent across all 19 prespecified patient subgroups, including those who were asymptomatic and patients with or without heart failure.
Serious adverse events related to rhythm control therapy – most often drug-related bradycardia – occurred in 4.9% of patients over the course of 5.1 years, compared to a 1.4% serious event rate in patients assigned to rate control. Dr. Kirchhof called the roughly 1% per year serious event rate in the rhythm control group quite acceptable.
“To put that in perspective, the annualized rate of severe bleeds on oral anticoagulation – a very beneficial therapy used by more than 90% of participants at 2 years – is about 2%,” the cardiologist noted.
Only 8% of patients randomized to rhythm control received AFib ablation as initial therapy, consistent with current clinical practice. By 2 years, 19.4% of the rhythm control group had undergone AFib ablation. Also at that time, 15% of the rate control group was receiving rhythm control therapy to help manage AFib-related symptoms.
One of the big surprises in the study, he said, was that nearly three-quarters of patients in both groups were asymptomatic at 2 years.
“I think that shows how well we control symptoms, even without rhythm control,” he observed.
Results ‘move the field forward’
Dr. Kirchhof stressed that this was a trial of two different treatment strategies, and it’s not yet possible to single out any specific component of the rhythm control strategy as being responsible for the improved outcomes.
“I cannot tell you whether the outcome difference was due to AFib ablation or early treatment or the fact that we’re now better at using antiarrhythmic drugs than we were 20 years ago,” he said.
Asked if the EAST-AFNET 4 findings warrant more aggressive screening for AFib in order to detect and intervene early in the arrhythmia, Dr. Kirchhof replied with an unambiguous yes.
“My conclusion is that every patient with newly diagnosed AFib and a CHA2DS2-VASc score of 2 or more should not only receive anticoagulation and rate control, but should also be offered rhythm control therapy at the time of diagnosis, which also means that all of these people have to be seen by a cardiologist who has expertise in the domain of AFib management. It’s a big clinical challenge, but it leads to a 21% improvement in outcomes, and I think we have to do what’s best for our patients,” he said.
In an interview, Kalyanam Shivkumar, MD, PhD, called EAST-AFNET 4 “a very important study.”
“It moves the field forward, for sure. I think it will change clinical practice, and it should,” commented Dr. Shivkumar, who was not involved in the study.
“Now there are so many wearable technologies out there – the Apple Watch and others – which will enable rhythm abnormalities to be detected early on. This bodes well for the field,” said Dr. Shivkumar, who is editor-in-chief of JACC: Clinical Electrophysiology. He is also professor of medicine, radiology, and bioengineering at the University of California, Los Angeles, and director of the UCLA Cardiac Arrhythmia Center.
Dr. Kirchhof reported receiving research grants to conduct the EAST-AFNET 4 trial from the German Ministry of Education and Research, the German Center for Cardiovascular Research, the Atrial Fibrillation Network, the European Heart Rhythm Association, St. Jude Medical, Abbott, Sanofi, the German Heart Foundation, the European Union, the British Heart Foundation, and the Leducq Foundation.
Simultaneous with his presentation at ESC Congress 2020, the study results were published online at NEJM.org.
SOURCE: Kirchhof P. ESC Congress 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2019422.
REPORTING FROM ESC CONGRESS 2020
FDA pulls amputation boxed warning off canagliflozin label
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
ESC 2020 looks to make its mark in ‘new era’ of virtual meetings
The coronavirus may have quashed plans to socialize and stroll the canals of Amsterdam while at this year’s European Society of Cardiology (ESC) Congress, but organizers are promising a historic digital experience that will “once again, be a celebration of discovery and ground-breaking science.”
“My message — if I have to choose only one thing why ESC 2020 will be a historic event — is that the physician working at the Cleveland Clinic, who was planning to attend this year in Amsterdam, as well as the colleague in a bush hospital in Uganda, who would have never have dreamed to be part of the ESC Congress, both will have for the first time the same access at the same time to knowledge shared at the worldwide leading cardiovascular meeting,” Marco Roffi, MD, co-chair of the scientific program, told theheart.org | Medscape Cardiology.
Taking a page from the American College of Cardiology, which set the virtual bar early in the pandemic with its highly interactive ACC 2020, ESC is taking some 80 Hot Line, clinical practice guidelines, and special sessions live with question-and-answer interactions and panel discussions.
The latest COVID-19 research and four new guideline documents — including recommendations on atrial fibrillation (AF), non-ST-segment elevation acute coronary syndromes, sports cardiology and exercise in patients with cardiovascular disease, and adult congenital heart disease — will be featured at the ESC Congress 2020, scheduled for August 29 to September 1.
Presentations will be shorter and sessions more focused, but more than 500 scientific and educational sessions will be streamed in addition to more than 4000 abstracts available live or on demand as full presentations or e-posters, said Roffi, University Hospital of Geneva, Switzerland.
To pull off the virtual event, a digital studio in Amsterdam will host hundreds of key opinion leaders, and ESC employed more than 1000 satellite studios around the world to gather contributions from scientists and experts with the help of 70 behind-the-scenes experts.
Nevertheless, a “strategic decision” was made to provide free access to the event and its content for 30 days — a strategy that has attracted some 58,000 registrants thus far, up from a record 32,000 attendees at last year’s congress in Paris, Roffi said.
“Obviously, the income will not be the same as a physical congress, but we felt there was too much at stake to make a compromise,” he said. “We believe we are the leaders in cardiovascular meetings in the physical ones and we want to keep this position even in the new era. And we believe this is the beginning of a new era in whatever form will be.”
Hot Line Sessions 1-3, Saturday (14:00 CEST)
The Hot Line sessions will feature 13 clinical trials and kick off with EMPEROR-Reduced, which compared the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) added to standard care in patients with heart failure with reduced ejection fraction (HFrEF), with and without diabetes.
Eli Lilly and Boehringer Ingelheim already announced the trial met its composite primary endpoint of reducing cardiovascular (CV) death or HF hospitalization risk but the details will be important given the SGLT2 inhibitors› rapid shift beyond diabetes to HF and chronic kidney disease (CKD).
Results presented at ESC 2019 from the landmark DAPA-HF trial led to the recent new indication for dapagliflozin (Farxiga, AstraZeneca) for HFrEF in the absence of diabetes. New data will be released in a Sunday Hot Line session looking at the SGLT2 inhibitor among diabetic and nondiabetic CKD patients in DAPA-CKD, which was halted early because of overwhelming efficacy.
As the indication evolved, the SGLT2 inhibitors became truly cardiovascular disease drugs, Roffi said, “so all the cardiologists will have to become familiar with these agents.”
Hot Line 2 will look at the oral cardiac myosin inhibitor mavacamten as an alternative to surgery or percutaneous interventions to treat obstructive hypertrophic cardiomyopathy (HCM). The first-in-class investigational agent is thought to reduce the hypercontractility characteristic of HCM by inhibiting excessive actin-myosin cross-bridges, and it showed promise in the recent phase 2 dose-finding MAVERICK HCM trial.
Investigators are expected to flesh out details from the 251-patient phase 3 EXPLORER-HCM trial, which reported functional and symptomatic gains with once-daily dosing in top-line results released by developer MyoKardia.
“This is really a revolutionary way to treat — hopefully successfully — this very complex disease,” Roffi said.
Rounding out the day is the EAST-AFNET 4 trial, which has been almost 10 years in the making and examined whether early rhythm control with antiarrhythmic drugs and catheter ablation can prevent adverse outcomes in patients with AF compared with usual care alone based on the ESC 2010 AF treatment guidelines.
Hot Line Sessions 4-6, Sunday (14:00 CEST)
Hot Line 4 features the ATPCI study examining the addition of the oral antianginal agent trimetazidine to standard of care in 6007 patients with angina after recent successful percutaneous coronary intervention.
Next up is POPULAR-TAVI looking at aspirin with or without clopidogrel in patients undergoing transcatheter aortic valve implantation (TAVI).
“This is a dilemma that we have every day in the cath lab when we perform a TAVI because we have in front of us patients who, by definition, are at high bleeding risk, are old, and have comorbidities such as renal insufficiencies,” Roffi said. “I like this very much because it’s a very practical study. Whatever the response will be of this study, it will impact clinical practice.”
Hot Line 6 is devoted to the PARALLAX trial comparing sacubitril/valsartan (Entresto, Novartis) with individualized medical therapy in 2569 heart failure with preserved ejection fraction (HFpEF) patients. The primary outcomes are 12-week change in N-terminal pro-brain natriuretic peptide and 24-week change in 6-minute walk distance.
Hot Line Sessions 7-9, Monday (14:00 CEST)
The next day starts with the timely topic of inflammation with the LoDoCo2 trial, in which 5522 patients with stable coronary artery disease were randomized to low-dose colchicine 0.5 mg daily or placebo on top of optimal medical therapy. The primary composite endpoint is CV death, myocardial infarction (MI), ischemic stroke, and ischemia-driven revascularization.
Additional colchicine data also will be presented in a late-breaking science session on Saturday that includes the Australian COPS trial in acute coronary syndromes and new analyses from COLCOT, which demonstrated a 23% reduction in the risk of first ischemic CV events following an MI but no mortality benefit. The low-cost anti-inflammatory drug is also being tested in the mammoth 6000-patient phase 3 Colchicine Coronavirus SARS-CoV-2 (COLCORONA) trial, expected to be completed by the end of September.
Hot Line 8 switches gears with the open-label randomized HOME-PE trial comparing outpatient management of pulmonary embolism (PE) in 1975 select patients based on either the simplified Pulmonary Embolism Severity Index (PESI) score, featured in the most recent ESC acute PE guidelines, or the HESTIA criteria, developed in the HESTIA study. The event-driven primary end point is the composite of recurrent venous thromboembolism, major bleeding, and all-cause death at 30 days.
Last up on Monday is a new analysis on the effects of lowering blood pressure for prevention of CV events across various BP levels from the BPLTTC, which is the largest resource of patient-level randomized clinical trial data, at more than 350,000 patients.
Tuesday Hot Line Sessions 10-12 (14:00 CEST)
The final day of the Congress ends with bang, with the randomized BRACE-CORONA trial examining the effect of continuing or suspending angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in 700 patients with SARS-CoV-2 infection.
Although several cardiovascular societies including ESC recommend continuation of renin-angiotensin-aldosterone system (RAAS) antagonists in COVID-19 patients, randomized data are lacking and patients have been rattled by early observations suggesting that ACE2 upregulation from RAAS antagonists could increase the risk of developing severe COVID-19.
“BRACE-CORONA will answer the question that everybody’s been asking, whether or not you should continue ARBs and ACE inhibitors in COVID-19 patients. This is a randomized trial and we are all very excited about it,” Roffi said.
COVID-19 will also be discussed in a late-breaking science session on Sunday and in three industry Q&A sessions scattered over the 4 days.
Rounding out the last Hot Line session is IMPACT-AFib, a claims database analysis of early vs delayed educational interventions to improve oral anticoagulation use in a whopping 80,000 patients with AF, and REALITY, a much-needed cost-effectiveness analysis of liberal vs restrictive transfusion strategies in 630 patients with acute MI and anemia.
This article first appeared on Medscape.com.
The coronavirus may have quashed plans to socialize and stroll the canals of Amsterdam while at this year’s European Society of Cardiology (ESC) Congress, but organizers are promising a historic digital experience that will “once again, be a celebration of discovery and ground-breaking science.”
“My message — if I have to choose only one thing why ESC 2020 will be a historic event — is that the physician working at the Cleveland Clinic, who was planning to attend this year in Amsterdam, as well as the colleague in a bush hospital in Uganda, who would have never have dreamed to be part of the ESC Congress, both will have for the first time the same access at the same time to knowledge shared at the worldwide leading cardiovascular meeting,” Marco Roffi, MD, co-chair of the scientific program, told theheart.org | Medscape Cardiology.
Taking a page from the American College of Cardiology, which set the virtual bar early in the pandemic with its highly interactive ACC 2020, ESC is taking some 80 Hot Line, clinical practice guidelines, and special sessions live with question-and-answer interactions and panel discussions.
The latest COVID-19 research and four new guideline documents — including recommendations on atrial fibrillation (AF), non-ST-segment elevation acute coronary syndromes, sports cardiology and exercise in patients with cardiovascular disease, and adult congenital heart disease — will be featured at the ESC Congress 2020, scheduled for August 29 to September 1.
Presentations will be shorter and sessions more focused, but more than 500 scientific and educational sessions will be streamed in addition to more than 4000 abstracts available live or on demand as full presentations or e-posters, said Roffi, University Hospital of Geneva, Switzerland.
To pull off the virtual event, a digital studio in Amsterdam will host hundreds of key opinion leaders, and ESC employed more than 1000 satellite studios around the world to gather contributions from scientists and experts with the help of 70 behind-the-scenes experts.
Nevertheless, a “strategic decision” was made to provide free access to the event and its content for 30 days — a strategy that has attracted some 58,000 registrants thus far, up from a record 32,000 attendees at last year’s congress in Paris, Roffi said.
“Obviously, the income will not be the same as a physical congress, but we felt there was too much at stake to make a compromise,” he said. “We believe we are the leaders in cardiovascular meetings in the physical ones and we want to keep this position even in the new era. And we believe this is the beginning of a new era in whatever form will be.”
Hot Line Sessions 1-3, Saturday (14:00 CEST)
The Hot Line sessions will feature 13 clinical trials and kick off with EMPEROR-Reduced, which compared the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) added to standard care in patients with heart failure with reduced ejection fraction (HFrEF), with and without diabetes.
Eli Lilly and Boehringer Ingelheim already announced the trial met its composite primary endpoint of reducing cardiovascular (CV) death or HF hospitalization risk but the details will be important given the SGLT2 inhibitors› rapid shift beyond diabetes to HF and chronic kidney disease (CKD).
Results presented at ESC 2019 from the landmark DAPA-HF trial led to the recent new indication for dapagliflozin (Farxiga, AstraZeneca) for HFrEF in the absence of diabetes. New data will be released in a Sunday Hot Line session looking at the SGLT2 inhibitor among diabetic and nondiabetic CKD patients in DAPA-CKD, which was halted early because of overwhelming efficacy.
As the indication evolved, the SGLT2 inhibitors became truly cardiovascular disease drugs, Roffi said, “so all the cardiologists will have to become familiar with these agents.”
Hot Line 2 will look at the oral cardiac myosin inhibitor mavacamten as an alternative to surgery or percutaneous interventions to treat obstructive hypertrophic cardiomyopathy (HCM). The first-in-class investigational agent is thought to reduce the hypercontractility characteristic of HCM by inhibiting excessive actin-myosin cross-bridges, and it showed promise in the recent phase 2 dose-finding MAVERICK HCM trial.
Investigators are expected to flesh out details from the 251-patient phase 3 EXPLORER-HCM trial, which reported functional and symptomatic gains with once-daily dosing in top-line results released by developer MyoKardia.
“This is really a revolutionary way to treat — hopefully successfully — this very complex disease,” Roffi said.
Rounding out the day is the EAST-AFNET 4 trial, which has been almost 10 years in the making and examined whether early rhythm control with antiarrhythmic drugs and catheter ablation can prevent adverse outcomes in patients with AF compared with usual care alone based on the ESC 2010 AF treatment guidelines.
Hot Line Sessions 4-6, Sunday (14:00 CEST)
Hot Line 4 features the ATPCI study examining the addition of the oral antianginal agent trimetazidine to standard of care in 6007 patients with angina after recent successful percutaneous coronary intervention.
Next up is POPULAR-TAVI looking at aspirin with or without clopidogrel in patients undergoing transcatheter aortic valve implantation (TAVI).
“This is a dilemma that we have every day in the cath lab when we perform a TAVI because we have in front of us patients who, by definition, are at high bleeding risk, are old, and have comorbidities such as renal insufficiencies,” Roffi said. “I like this very much because it’s a very practical study. Whatever the response will be of this study, it will impact clinical practice.”
Hot Line 6 is devoted to the PARALLAX trial comparing sacubitril/valsartan (Entresto, Novartis) with individualized medical therapy in 2569 heart failure with preserved ejection fraction (HFpEF) patients. The primary outcomes are 12-week change in N-terminal pro-brain natriuretic peptide and 24-week change in 6-minute walk distance.
Hot Line Sessions 7-9, Monday (14:00 CEST)
The next day starts with the timely topic of inflammation with the LoDoCo2 trial, in which 5522 patients with stable coronary artery disease were randomized to low-dose colchicine 0.5 mg daily or placebo on top of optimal medical therapy. The primary composite endpoint is CV death, myocardial infarction (MI), ischemic stroke, and ischemia-driven revascularization.
Additional colchicine data also will be presented in a late-breaking science session on Saturday that includes the Australian COPS trial in acute coronary syndromes and new analyses from COLCOT, which demonstrated a 23% reduction in the risk of first ischemic CV events following an MI but no mortality benefit. The low-cost anti-inflammatory drug is also being tested in the mammoth 6000-patient phase 3 Colchicine Coronavirus SARS-CoV-2 (COLCORONA) trial, expected to be completed by the end of September.
Hot Line 8 switches gears with the open-label randomized HOME-PE trial comparing outpatient management of pulmonary embolism (PE) in 1975 select patients based on either the simplified Pulmonary Embolism Severity Index (PESI) score, featured in the most recent ESC acute PE guidelines, or the HESTIA criteria, developed in the HESTIA study. The event-driven primary end point is the composite of recurrent venous thromboembolism, major bleeding, and all-cause death at 30 days.
Last up on Monday is a new analysis on the effects of lowering blood pressure for prevention of CV events across various BP levels from the BPLTTC, which is the largest resource of patient-level randomized clinical trial data, at more than 350,000 patients.
Tuesday Hot Line Sessions 10-12 (14:00 CEST)
The final day of the Congress ends with bang, with the randomized BRACE-CORONA trial examining the effect of continuing or suspending angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in 700 patients with SARS-CoV-2 infection.
Although several cardiovascular societies including ESC recommend continuation of renin-angiotensin-aldosterone system (RAAS) antagonists in COVID-19 patients, randomized data are lacking and patients have been rattled by early observations suggesting that ACE2 upregulation from RAAS antagonists could increase the risk of developing severe COVID-19.
“BRACE-CORONA will answer the question that everybody’s been asking, whether or not you should continue ARBs and ACE inhibitors in COVID-19 patients. This is a randomized trial and we are all very excited about it,” Roffi said.
COVID-19 will also be discussed in a late-breaking science session on Sunday and in three industry Q&A sessions scattered over the 4 days.
Rounding out the last Hot Line session is IMPACT-AFib, a claims database analysis of early vs delayed educational interventions to improve oral anticoagulation use in a whopping 80,000 patients with AF, and REALITY, a much-needed cost-effectiveness analysis of liberal vs restrictive transfusion strategies in 630 patients with acute MI and anemia.
This article first appeared on Medscape.com.
The coronavirus may have quashed plans to socialize and stroll the canals of Amsterdam while at this year’s European Society of Cardiology (ESC) Congress, but organizers are promising a historic digital experience that will “once again, be a celebration of discovery and ground-breaking science.”
“My message — if I have to choose only one thing why ESC 2020 will be a historic event — is that the physician working at the Cleveland Clinic, who was planning to attend this year in Amsterdam, as well as the colleague in a bush hospital in Uganda, who would have never have dreamed to be part of the ESC Congress, both will have for the first time the same access at the same time to knowledge shared at the worldwide leading cardiovascular meeting,” Marco Roffi, MD, co-chair of the scientific program, told theheart.org | Medscape Cardiology.
Taking a page from the American College of Cardiology, which set the virtual bar early in the pandemic with its highly interactive ACC 2020, ESC is taking some 80 Hot Line, clinical practice guidelines, and special sessions live with question-and-answer interactions and panel discussions.
The latest COVID-19 research and four new guideline documents — including recommendations on atrial fibrillation (AF), non-ST-segment elevation acute coronary syndromes, sports cardiology and exercise in patients with cardiovascular disease, and adult congenital heart disease — will be featured at the ESC Congress 2020, scheduled for August 29 to September 1.
Presentations will be shorter and sessions more focused, but more than 500 scientific and educational sessions will be streamed in addition to more than 4000 abstracts available live or on demand as full presentations or e-posters, said Roffi, University Hospital of Geneva, Switzerland.
To pull off the virtual event, a digital studio in Amsterdam will host hundreds of key opinion leaders, and ESC employed more than 1000 satellite studios around the world to gather contributions from scientists and experts with the help of 70 behind-the-scenes experts.
Nevertheless, a “strategic decision” was made to provide free access to the event and its content for 30 days — a strategy that has attracted some 58,000 registrants thus far, up from a record 32,000 attendees at last year’s congress in Paris, Roffi said.
“Obviously, the income will not be the same as a physical congress, but we felt there was too much at stake to make a compromise,” he said. “We believe we are the leaders in cardiovascular meetings in the physical ones and we want to keep this position even in the new era. And we believe this is the beginning of a new era in whatever form will be.”
Hot Line Sessions 1-3, Saturday (14:00 CEST)
The Hot Line sessions will feature 13 clinical trials and kick off with EMPEROR-Reduced, which compared the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) added to standard care in patients with heart failure with reduced ejection fraction (HFrEF), with and without diabetes.
Eli Lilly and Boehringer Ingelheim already announced the trial met its composite primary endpoint of reducing cardiovascular (CV) death or HF hospitalization risk but the details will be important given the SGLT2 inhibitors› rapid shift beyond diabetes to HF and chronic kidney disease (CKD).
Results presented at ESC 2019 from the landmark DAPA-HF trial led to the recent new indication for dapagliflozin (Farxiga, AstraZeneca) for HFrEF in the absence of diabetes. New data will be released in a Sunday Hot Line session looking at the SGLT2 inhibitor among diabetic and nondiabetic CKD patients in DAPA-CKD, which was halted early because of overwhelming efficacy.
As the indication evolved, the SGLT2 inhibitors became truly cardiovascular disease drugs, Roffi said, “so all the cardiologists will have to become familiar with these agents.”
Hot Line 2 will look at the oral cardiac myosin inhibitor mavacamten as an alternative to surgery or percutaneous interventions to treat obstructive hypertrophic cardiomyopathy (HCM). The first-in-class investigational agent is thought to reduce the hypercontractility characteristic of HCM by inhibiting excessive actin-myosin cross-bridges, and it showed promise in the recent phase 2 dose-finding MAVERICK HCM trial.
Investigators are expected to flesh out details from the 251-patient phase 3 EXPLORER-HCM trial, which reported functional and symptomatic gains with once-daily dosing in top-line results released by developer MyoKardia.
“This is really a revolutionary way to treat — hopefully successfully — this very complex disease,” Roffi said.
Rounding out the day is the EAST-AFNET 4 trial, which has been almost 10 years in the making and examined whether early rhythm control with antiarrhythmic drugs and catheter ablation can prevent adverse outcomes in patients with AF compared with usual care alone based on the ESC 2010 AF treatment guidelines.
Hot Line Sessions 4-6, Sunday (14:00 CEST)
Hot Line 4 features the ATPCI study examining the addition of the oral antianginal agent trimetazidine to standard of care in 6007 patients with angina after recent successful percutaneous coronary intervention.
Next up is POPULAR-TAVI looking at aspirin with or without clopidogrel in patients undergoing transcatheter aortic valve implantation (TAVI).
“This is a dilemma that we have every day in the cath lab when we perform a TAVI because we have in front of us patients who, by definition, are at high bleeding risk, are old, and have comorbidities such as renal insufficiencies,” Roffi said. “I like this very much because it’s a very practical study. Whatever the response will be of this study, it will impact clinical practice.”
Hot Line 6 is devoted to the PARALLAX trial comparing sacubitril/valsartan (Entresto, Novartis) with individualized medical therapy in 2569 heart failure with preserved ejection fraction (HFpEF) patients. The primary outcomes are 12-week change in N-terminal pro-brain natriuretic peptide and 24-week change in 6-minute walk distance.
Hot Line Sessions 7-9, Monday (14:00 CEST)
The next day starts with the timely topic of inflammation with the LoDoCo2 trial, in which 5522 patients with stable coronary artery disease were randomized to low-dose colchicine 0.5 mg daily or placebo on top of optimal medical therapy. The primary composite endpoint is CV death, myocardial infarction (MI), ischemic stroke, and ischemia-driven revascularization.
Additional colchicine data also will be presented in a late-breaking science session on Saturday that includes the Australian COPS trial in acute coronary syndromes and new analyses from COLCOT, which demonstrated a 23% reduction in the risk of first ischemic CV events following an MI but no mortality benefit. The low-cost anti-inflammatory drug is also being tested in the mammoth 6000-patient phase 3 Colchicine Coronavirus SARS-CoV-2 (COLCORONA) trial, expected to be completed by the end of September.
Hot Line 8 switches gears with the open-label randomized HOME-PE trial comparing outpatient management of pulmonary embolism (PE) in 1975 select patients based on either the simplified Pulmonary Embolism Severity Index (PESI) score, featured in the most recent ESC acute PE guidelines, or the HESTIA criteria, developed in the HESTIA study. The event-driven primary end point is the composite of recurrent venous thromboembolism, major bleeding, and all-cause death at 30 days.
Last up on Monday is a new analysis on the effects of lowering blood pressure for prevention of CV events across various BP levels from the BPLTTC, which is the largest resource of patient-level randomized clinical trial data, at more than 350,000 patients.
Tuesday Hot Line Sessions 10-12 (14:00 CEST)
The final day of the Congress ends with bang, with the randomized BRACE-CORONA trial examining the effect of continuing or suspending angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in 700 patients with SARS-CoV-2 infection.
Although several cardiovascular societies including ESC recommend continuation of renin-angiotensin-aldosterone system (RAAS) antagonists in COVID-19 patients, randomized data are lacking and patients have been rattled by early observations suggesting that ACE2 upregulation from RAAS antagonists could increase the risk of developing severe COVID-19.
“BRACE-CORONA will answer the question that everybody’s been asking, whether or not you should continue ARBs and ACE inhibitors in COVID-19 patients. This is a randomized trial and we are all very excited about it,” Roffi said.
COVID-19 will also be discussed in a late-breaking science session on Sunday and in three industry Q&A sessions scattered over the 4 days.
Rounding out the last Hot Line session is IMPACT-AFib, a claims database analysis of early vs delayed educational interventions to improve oral anticoagulation use in a whopping 80,000 patients with AF, and REALITY, a much-needed cost-effectiveness analysis of liberal vs restrictive transfusion strategies in 630 patients with acute MI and anemia.
This article first appeared on Medscape.com.



















