User login
HCV Hub
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
direct-acting antivirals
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
Liver cancer risk lower after sustained response to DAAs
Individuals with hepatitis C infection who achieved a sustained virologic response (SVR) to treatment with direct-acting antivirals had a significantly lower risk of hepatocellular carcinoma (HCC), a new study suggests.
A retrospective cohort study of 22,500 U.S. veterans with hepatitis C who had been treated with direct-acting antivirals (DAAs) found those with an SVR had a 72% lower risk of HCC, compared with those who did not achieve that response (hazard ratio, 0.28; 95% confidence interval, 0.22-0.36; P less than .0001), even after adjusting for demographics as well as clinical and health utilization factors.
“These data show that successful eradication of HCV [hepatitis C virus] confers a benefit in DAA-treated patients,” wrote Fasiha Kanwal, MD, from the Michael E. DeBakey Veterans Affairs Medical Center in Houston and her coauthors. “Although a few recent studies have raised concerns that DAA might accelerate the risk of HCC in some patients early in the course of treatment, we did not find any factors that differentiated patients with HCC that developed during DAA treatment.”
The results highlighted the importance of early treatment with antivirals, beginning well before the patients showed signs of progressing to advanced fibrosis or cirrhosis, the investigators noted.
“Delaying treatment until patients progress to cirrhosis might be associated with substantial downstream costs incurred as part of lifelong HCC surveillance and/or management of HCC,” they wrote.
Sustained virologic response to DAAs also was associated with a longer time to diagnosis, and patients who didn’t achieve it showed higher rates of cancer much earlier. The most common antivirals used were sofosbuvir (75.2%; 51.1% in combination with ledipasvir), the combination of paritaprevir/ritonavir (23.3%), daclatasvir-based treatments (0.8%), and simeprevir (0.7%).
While the patients achieved SVR that showed similarly beneficial effects on HCC risk in patients with or without cirrhosis, the authors also noted that patients with cirrhosis had a nearly fivefold greater risk of developing cancer than did those without (HR, 4.73; 95% CI, 3.34-6.68). Similarly, patients with a fibrosis score (FIB-4) greater than 3.25 had a sixfold higher risk of HCC, compared with those with a value of 1.45 or lower.
Researchers commented that, at this level of risk, surveillance for HCC in these patients may be cost effective.
“Based on these data, HCC surveillance or risk modification may be needed for all patients who have progressed to cirrhosis or advanced fibrosis (as indicated by high FIB-4) at the time of SVR,” they wrote.
Alcohol use was also associated with a significantly higher annual incidence of HCC (HR, 1.56; 95% CI, 1.11-2.18).
Among the study cohort, 39% had cirrhosis, 29.7% had advanced fibrosis, and nearly one-quarter had previously been treated for hepatitis C infection. More than 40% also had diabetes, 61.4% reported alcohol use, and 54.2% had a history of drug use.
“DAAs offer a chance of cure for all patients with HCV, including patients with advanced cirrhosis, older patients, and those with alcohol use – all characteristics independently associated with risk of HCC in HCV,” the authors explained. “These data show the treated population has changed significantly in the DAA era to include many patients with other HCC risk factors; these differences likely explain why the newer cohorts of DAA-treated patients face higher absolute HCC risk than expected, based on historic data.”
The study was partly supported by the Department of Veteran Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
The availability of direct-acting antivirals (DAAs) has revolutionized treatment of hepatitis C. Sustained virologic response (SVR) can be routinely achieved in more than 95% of patients – except in those with decompensated cirrhosis – with a 12-week course of these oral drugs, which have minimal adverse effects. Thus, guidelines recommend that all patients with hepatitis C should be treated with DAAs.1 It was a shock to the medical community when the recent Cochrane review concluded there was insufficient evidence to confirm or reject an effect of DAA therapy on HCV-related morbidity or all-cause mortality.2 The authors cautioned that the lack of valid evidence for DAAs’ effectiveness and the possibility of potential harm should be considered before treating people with hepatitis C with DAAs. Their conclusion was in part based on their rejection of SVR as a valid surrogate for clinical outcome. Previous studies of interferon-based therapies showed that SVR was associated with improvement in liver histology, decreased risk of hepatocellular carcinoma (HCC), and mortality.
Treatment of hepatitis C with DAAs represents one out of a handful of cases in which we can claim that a cure for a chronic disease is possible; however, treatment must be initiated early before advanced fibrosis or cirrhosis to prevent a persistent, though greatly reduced, risk of HCC. Physicians managing patients with hepatitis C should make treatment decisions based on evidence from the entire literature – which supports claims of the DAA treatment’s benefits and refutes allegations of its harmfulness – and should not be swayed by the misguided conclusions of the Cochrane review.
References
1. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed on July 2, 2017.
2. Jakobsen J.C., Nielsen E.E., Feinberg J., et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017 Jun 6;6:CD012143.
3. Curry M.P., O’Leary J.G., Bzowej N., et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618-28.
4. Kanwal F., Kramer J., Asch S.M., et al. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology. 2017 Jun 19. pii: S0016-5085(17)35797.
Anna S. Lok, MD, AGAF, FAASLD, is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine at the University of Michigan Health System in Ann Arbor. She has received research grants from Bristol-Myers Squibb and Gilead through the University of Michigan.
The availability of direct-acting antivirals (DAAs) has revolutionized treatment of hepatitis C. Sustained virologic response (SVR) can be routinely achieved in more than 95% of patients – except in those with decompensated cirrhosis – with a 12-week course of these oral drugs, which have minimal adverse effects. Thus, guidelines recommend that all patients with hepatitis C should be treated with DAAs.1 It was a shock to the medical community when the recent Cochrane review concluded there was insufficient evidence to confirm or reject an effect of DAA therapy on HCV-related morbidity or all-cause mortality.2 The authors cautioned that the lack of valid evidence for DAAs’ effectiveness and the possibility of potential harm should be considered before treating people with hepatitis C with DAAs. Their conclusion was in part based on their rejection of SVR as a valid surrogate for clinical outcome. Previous studies of interferon-based therapies showed that SVR was associated with improvement in liver histology, decreased risk of hepatocellular carcinoma (HCC), and mortality.
Treatment of hepatitis C with DAAs represents one out of a handful of cases in which we can claim that a cure for a chronic disease is possible; however, treatment must be initiated early before advanced fibrosis or cirrhosis to prevent a persistent, though greatly reduced, risk of HCC. Physicians managing patients with hepatitis C should make treatment decisions based on evidence from the entire literature – which supports claims of the DAA treatment’s benefits and refutes allegations of its harmfulness – and should not be swayed by the misguided conclusions of the Cochrane review.
References
1. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed on July 2, 2017.
2. Jakobsen J.C., Nielsen E.E., Feinberg J., et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017 Jun 6;6:CD012143.
3. Curry M.P., O’Leary J.G., Bzowej N., et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618-28.
4. Kanwal F., Kramer J., Asch S.M., et al. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology. 2017 Jun 19. pii: S0016-5085(17)35797.
Anna S. Lok, MD, AGAF, FAASLD, is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine at the University of Michigan Health System in Ann Arbor. She has received research grants from Bristol-Myers Squibb and Gilead through the University of Michigan.
The availability of direct-acting antivirals (DAAs) has revolutionized treatment of hepatitis C. Sustained virologic response (SVR) can be routinely achieved in more than 95% of patients – except in those with decompensated cirrhosis – with a 12-week course of these oral drugs, which have minimal adverse effects. Thus, guidelines recommend that all patients with hepatitis C should be treated with DAAs.1 It was a shock to the medical community when the recent Cochrane review concluded there was insufficient evidence to confirm or reject an effect of DAA therapy on HCV-related morbidity or all-cause mortality.2 The authors cautioned that the lack of valid evidence for DAAs’ effectiveness and the possibility of potential harm should be considered before treating people with hepatitis C with DAAs. Their conclusion was in part based on their rejection of SVR as a valid surrogate for clinical outcome. Previous studies of interferon-based therapies showed that SVR was associated with improvement in liver histology, decreased risk of hepatocellular carcinoma (HCC), and mortality.
Treatment of hepatitis C with DAAs represents one out of a handful of cases in which we can claim that a cure for a chronic disease is possible; however, treatment must be initiated early before advanced fibrosis or cirrhosis to prevent a persistent, though greatly reduced, risk of HCC. Physicians managing patients with hepatitis C should make treatment decisions based on evidence from the entire literature – which supports claims of the DAA treatment’s benefits and refutes allegations of its harmfulness – and should not be swayed by the misguided conclusions of the Cochrane review.
References
1. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed on July 2, 2017.
2. Jakobsen J.C., Nielsen E.E., Feinberg J., et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017 Jun 6;6:CD012143.
3. Curry M.P., O’Leary J.G., Bzowej N., et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618-28.
4. Kanwal F., Kramer J., Asch S.M., et al. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology. 2017 Jun 19. pii: S0016-5085(17)35797.
Anna S. Lok, MD, AGAF, FAASLD, is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine at the University of Michigan Health System in Ann Arbor. She has received research grants from Bristol-Myers Squibb and Gilead through the University of Michigan.
Individuals with hepatitis C infection who achieved a sustained virologic response (SVR) to treatment with direct-acting antivirals had a significantly lower risk of hepatocellular carcinoma (HCC), a new study suggests.
A retrospective cohort study of 22,500 U.S. veterans with hepatitis C who had been treated with direct-acting antivirals (DAAs) found those with an SVR had a 72% lower risk of HCC, compared with those who did not achieve that response (hazard ratio, 0.28; 95% confidence interval, 0.22-0.36; P less than .0001), even after adjusting for demographics as well as clinical and health utilization factors.
“These data show that successful eradication of HCV [hepatitis C virus] confers a benefit in DAA-treated patients,” wrote Fasiha Kanwal, MD, from the Michael E. DeBakey Veterans Affairs Medical Center in Houston and her coauthors. “Although a few recent studies have raised concerns that DAA might accelerate the risk of HCC in some patients early in the course of treatment, we did not find any factors that differentiated patients with HCC that developed during DAA treatment.”
The results highlighted the importance of early treatment with antivirals, beginning well before the patients showed signs of progressing to advanced fibrosis or cirrhosis, the investigators noted.
“Delaying treatment until patients progress to cirrhosis might be associated with substantial downstream costs incurred as part of lifelong HCC surveillance and/or management of HCC,” they wrote.
Sustained virologic response to DAAs also was associated with a longer time to diagnosis, and patients who didn’t achieve it showed higher rates of cancer much earlier. The most common antivirals used were sofosbuvir (75.2%; 51.1% in combination with ledipasvir), the combination of paritaprevir/ritonavir (23.3%), daclatasvir-based treatments (0.8%), and simeprevir (0.7%).
While the patients achieved SVR that showed similarly beneficial effects on HCC risk in patients with or without cirrhosis, the authors also noted that patients with cirrhosis had a nearly fivefold greater risk of developing cancer than did those without (HR, 4.73; 95% CI, 3.34-6.68). Similarly, patients with a fibrosis score (FIB-4) greater than 3.25 had a sixfold higher risk of HCC, compared with those with a value of 1.45 or lower.
Researchers commented that, at this level of risk, surveillance for HCC in these patients may be cost effective.
“Based on these data, HCC surveillance or risk modification may be needed for all patients who have progressed to cirrhosis or advanced fibrosis (as indicated by high FIB-4) at the time of SVR,” they wrote.
Alcohol use was also associated with a significantly higher annual incidence of HCC (HR, 1.56; 95% CI, 1.11-2.18).
Among the study cohort, 39% had cirrhosis, 29.7% had advanced fibrosis, and nearly one-quarter had previously been treated for hepatitis C infection. More than 40% also had diabetes, 61.4% reported alcohol use, and 54.2% had a history of drug use.
“DAAs offer a chance of cure for all patients with HCV, including patients with advanced cirrhosis, older patients, and those with alcohol use – all characteristics independently associated with risk of HCC in HCV,” the authors explained. “These data show the treated population has changed significantly in the DAA era to include many patients with other HCC risk factors; these differences likely explain why the newer cohorts of DAA-treated patients face higher absolute HCC risk than expected, based on historic data.”
The study was partly supported by the Department of Veteran Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
Individuals with hepatitis C infection who achieved a sustained virologic response (SVR) to treatment with direct-acting antivirals had a significantly lower risk of hepatocellular carcinoma (HCC), a new study suggests.
A retrospective cohort study of 22,500 U.S. veterans with hepatitis C who had been treated with direct-acting antivirals (DAAs) found those with an SVR had a 72% lower risk of HCC, compared with those who did not achieve that response (hazard ratio, 0.28; 95% confidence interval, 0.22-0.36; P less than .0001), even after adjusting for demographics as well as clinical and health utilization factors.
“These data show that successful eradication of HCV [hepatitis C virus] confers a benefit in DAA-treated patients,” wrote Fasiha Kanwal, MD, from the Michael E. DeBakey Veterans Affairs Medical Center in Houston and her coauthors. “Although a few recent studies have raised concerns that DAA might accelerate the risk of HCC in some patients early in the course of treatment, we did not find any factors that differentiated patients with HCC that developed during DAA treatment.”
The results highlighted the importance of early treatment with antivirals, beginning well before the patients showed signs of progressing to advanced fibrosis or cirrhosis, the investigators noted.
“Delaying treatment until patients progress to cirrhosis might be associated with substantial downstream costs incurred as part of lifelong HCC surveillance and/or management of HCC,” they wrote.
Sustained virologic response to DAAs also was associated with a longer time to diagnosis, and patients who didn’t achieve it showed higher rates of cancer much earlier. The most common antivirals used were sofosbuvir (75.2%; 51.1% in combination with ledipasvir), the combination of paritaprevir/ritonavir (23.3%), daclatasvir-based treatments (0.8%), and simeprevir (0.7%).
While the patients achieved SVR that showed similarly beneficial effects on HCC risk in patients with or without cirrhosis, the authors also noted that patients with cirrhosis had a nearly fivefold greater risk of developing cancer than did those without (HR, 4.73; 95% CI, 3.34-6.68). Similarly, patients with a fibrosis score (FIB-4) greater than 3.25 had a sixfold higher risk of HCC, compared with those with a value of 1.45 or lower.
Researchers commented that, at this level of risk, surveillance for HCC in these patients may be cost effective.
“Based on these data, HCC surveillance or risk modification may be needed for all patients who have progressed to cirrhosis or advanced fibrosis (as indicated by high FIB-4) at the time of SVR,” they wrote.
Alcohol use was also associated with a significantly higher annual incidence of HCC (HR, 1.56; 95% CI, 1.11-2.18).
Among the study cohort, 39% had cirrhosis, 29.7% had advanced fibrosis, and nearly one-quarter had previously been treated for hepatitis C infection. More than 40% also had diabetes, 61.4% reported alcohol use, and 54.2% had a history of drug use.
“DAAs offer a chance of cure for all patients with HCV, including patients with advanced cirrhosis, older patients, and those with alcohol use – all characteristics independently associated with risk of HCC in HCV,” the authors explained. “These data show the treated population has changed significantly in the DAA era to include many patients with other HCC risk factors; these differences likely explain why the newer cohorts of DAA-treated patients face higher absolute HCC risk than expected, based on historic data.”
The study was partly supported by the Department of Veteran Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: Individuals who achieved an SVR to antiviral treatment for hepatitis C infection had a 72% lower risk of hepatocellular carcinoma than those who do not show a sustained response.
Data source: Retrospective cohort study in 22,500 U.S. veterans with hepatitis C.
Disclosures: The study was partly supported by the Department of Veterans Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
Hepatitis C is a pediatric disease now
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
HCV/HIV-coinfected teens rapidly progress to advanced liver disease
MADRID – Roughly one in four children with vertically transmitted hepatitis C virus (HCV)/HIV coinfection will progress to advanced hepatic fibrosis by age 20 years despite treatment with pegylated interferon plus ribavirin, Carolina Fernández McPhee, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This rate was nearly five times higher than in matched children with vertically acquired HCV monoinfection in a multicenter retrospective study, according to Dr. McPhee of Hospital General Universitario Gregorio Marañón in Madrid.
She presented a multicenter retrospective study of liver disease progression in 71 HCV/HIV coinfected children and 71 age- and sex-matched HCV-monoinfected children. The coinfected children are being followed in CORISPES (the Spanish Cohort of HIV-infected Children), where they receive state-of-the-art care.
All was quiet through age 9 years, with no progression to liver fibrosis in either patient group. Among patients followed to age 20 years, however, 9 (24%) of 38 HCV/HIV-coinfected patients showed progression to advanced fibrosis, compared with just 3 (6%) of 54 patients with HCV only.
Of HCV/HIV coinfected patients, 73% were infected with the hard to treat viral genotypes 1 or 4, compared with 93% of patients with HCV-only.
In the study group, 22 patients with HCV/HIV and 52 patients with HCV-only underwent treatment with pegylated interferon and ribavirin. At the time of treatment, three coinfected patients already had cirrhosis, another eight had moderate to advanced fibrosis, and half had no or mild fibrosis. In contrast, only one patient with HCV monoinfection had cirrhosis, four had moderate to advanced fibrosis, and the rest – nearly 90% of the total group – had no or mild fibrosis.
At treatment initiation, 96% of the HCV/HIV group were on antiretroviral therapy, 86% showed suppression of HIV RNA, 44% had AIDS, and 32% had a CD4 count below 500 cells/mm3.
The sustained viral response rate was similar in the two groups of patients – 41% in the HCV/HIV group and 42% in HCV-only patients – despite the fact that the HCV monoinfected patients had a higher prevalence of the tough to treat genotypes.
Roughly 12 million people worldwide are coinfected with HCV and HIV.
Dr. McPhee reported having no relevant financial disclosures.
MADRID – Roughly one in four children with vertically transmitted hepatitis C virus (HCV)/HIV coinfection will progress to advanced hepatic fibrosis by age 20 years despite treatment with pegylated interferon plus ribavirin, Carolina Fernández McPhee, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This rate was nearly five times higher than in matched children with vertically acquired HCV monoinfection in a multicenter retrospective study, according to Dr. McPhee of Hospital General Universitario Gregorio Marañón in Madrid.
She presented a multicenter retrospective study of liver disease progression in 71 HCV/HIV coinfected children and 71 age- and sex-matched HCV-monoinfected children. The coinfected children are being followed in CORISPES (the Spanish Cohort of HIV-infected Children), where they receive state-of-the-art care.
All was quiet through age 9 years, with no progression to liver fibrosis in either patient group. Among patients followed to age 20 years, however, 9 (24%) of 38 HCV/HIV-coinfected patients showed progression to advanced fibrosis, compared with just 3 (6%) of 54 patients with HCV only.
Of HCV/HIV coinfected patients, 73% were infected with the hard to treat viral genotypes 1 or 4, compared with 93% of patients with HCV-only.
In the study group, 22 patients with HCV/HIV and 52 patients with HCV-only underwent treatment with pegylated interferon and ribavirin. At the time of treatment, three coinfected patients already had cirrhosis, another eight had moderate to advanced fibrosis, and half had no or mild fibrosis. In contrast, only one patient with HCV monoinfection had cirrhosis, four had moderate to advanced fibrosis, and the rest – nearly 90% of the total group – had no or mild fibrosis.
At treatment initiation, 96% of the HCV/HIV group were on antiretroviral therapy, 86% showed suppression of HIV RNA, 44% had AIDS, and 32% had a CD4 count below 500 cells/mm3.
The sustained viral response rate was similar in the two groups of patients – 41% in the HCV/HIV group and 42% in HCV-only patients – despite the fact that the HCV monoinfected patients had a higher prevalence of the tough to treat genotypes.
Roughly 12 million people worldwide are coinfected with HCV and HIV.
Dr. McPhee reported having no relevant financial disclosures.
MADRID – Roughly one in four children with vertically transmitted hepatitis C virus (HCV)/HIV coinfection will progress to advanced hepatic fibrosis by age 20 years despite treatment with pegylated interferon plus ribavirin, Carolina Fernández McPhee, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This rate was nearly five times higher than in matched children with vertically acquired HCV monoinfection in a multicenter retrospective study, according to Dr. McPhee of Hospital General Universitario Gregorio Marañón in Madrid.
She presented a multicenter retrospective study of liver disease progression in 71 HCV/HIV coinfected children and 71 age- and sex-matched HCV-monoinfected children. The coinfected children are being followed in CORISPES (the Spanish Cohort of HIV-infected Children), where they receive state-of-the-art care.
All was quiet through age 9 years, with no progression to liver fibrosis in either patient group. Among patients followed to age 20 years, however, 9 (24%) of 38 HCV/HIV-coinfected patients showed progression to advanced fibrosis, compared with just 3 (6%) of 54 patients with HCV only.
Of HCV/HIV coinfected patients, 73% were infected with the hard to treat viral genotypes 1 or 4, compared with 93% of patients with HCV-only.
In the study group, 22 patients with HCV/HIV and 52 patients with HCV-only underwent treatment with pegylated interferon and ribavirin. At the time of treatment, three coinfected patients already had cirrhosis, another eight had moderate to advanced fibrosis, and half had no or mild fibrosis. In contrast, only one patient with HCV monoinfection had cirrhosis, four had moderate to advanced fibrosis, and the rest – nearly 90% of the total group – had no or mild fibrosis.
At treatment initiation, 96% of the HCV/HIV group were on antiretroviral therapy, 86% showed suppression of HIV RNA, 44% had AIDS, and 32% had a CD4 count below 500 cells/mm3.
The sustained viral response rate was similar in the two groups of patients – 41% in the HCV/HIV group and 42% in HCV-only patients – despite the fact that the HCV monoinfected patients had a higher prevalence of the tough to treat genotypes.
Roughly 12 million people worldwide are coinfected with HCV and HIV.
Dr. McPhee reported having no relevant financial disclosures.
AT ESPID 2017
Key clinical point:
Major finding: By age 20 years, 24% of a group of patients with vertically transmitted HCV/HIV coinfection had progressed to advanced hepatic fibrosis, compared with 6% of patients with vertically transmitted HCV monoinfection.
Data source: This retrospective, multicenter, observational Spanish study included 71 children with vertically transmitted HCV/HIV coinfection and 71 with vertically transmitted HCV monoinfection.
Disclosures: Dr. McPhee reported having no relevant financial disclosures.
Few states fully back HCV prevention, treatment
The prevalence of hepatitis C virus (HCV) varies considerably by state, and the same can be said for the state laws and policies attempting to decrease that prevalence, according to an assessment by the Centers for Disease Control and Prevention.
In 2015, incidence of acute HCV infection exceeded the national average of 0.8 per 100,000 population in 17 states, including seven with rates that at least doubled it, the report noted. New HCV infections have increased in recent years despite curative therapies “and known preventive measures to interrupt transmission.”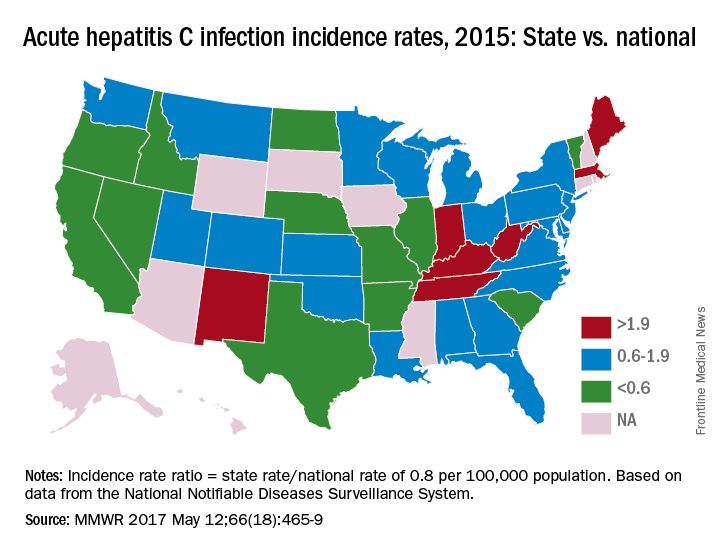
The “most comprehensive” laws on prevention through clean needle access as of 2016 were found in Maine, Nevada, and Utah, with laws in 12 other states categorized as “more comprehensive” and 18 states falling into the “least comprehensive” category. On the Medicaid side of the equation, 16 states had permissive policies that did not require sobriety or required only screening and counseling before treatment, 24 states had restrictive policies that requited sobriety, and 10 states had no policy available, the report showed (MMWR. 2017 May 12:66[18]:465-9).
Only three states – Massachusetts, New Mexico, and Washington – had a comprehensive (all three were considered “more comprehensive”) set of prevention laws and a permissive treatment policy, the investigators said, while also noting that two of the three – Massachusetts and New Mexico – were among the states with acute HCV rates that were at least twice the national average.
“Although the costs of HCV therapies have raised budgetary issues for state Medicaid programs in the past, the costs of HCV treatment have declined in recent years, increasing the cost-effectiveness of treatment, particularly among persons who inject drugs and who might serve as an ongoing source of transmission to others,” the report concluded.
The analysis examined three types of laws on access to clean needles and syringes: authorization of exchange programs, the scope of drug paraphernalia laws, and retail sale of needles and syringes. Each law was assessed for five elements, including authorization of syringe exchange statewide or in selected jurisdictions and exemption of needles or syringes from the definition of drug paraphernalia.
For the accompanying map (see “Acute hepatitis C infection incidence rates, 2015: State vs. national”), each state’s acute HCV incidence rate for 2015 was divided by the national rate to determine the incidence rate ratio, with data unavailable for 10 states.
The prevalence of hepatitis C virus (HCV) varies considerably by state, and the same can be said for the state laws and policies attempting to decrease that prevalence, according to an assessment by the Centers for Disease Control and Prevention.
In 2015, incidence of acute HCV infection exceeded the national average of 0.8 per 100,000 population in 17 states, including seven with rates that at least doubled it, the report noted. New HCV infections have increased in recent years despite curative therapies “and known preventive measures to interrupt transmission.”
The “most comprehensive” laws on prevention through clean needle access as of 2016 were found in Maine, Nevada, and Utah, with laws in 12 other states categorized as “more comprehensive” and 18 states falling into the “least comprehensive” category. On the Medicaid side of the equation, 16 states had permissive policies that did not require sobriety or required only screening and counseling before treatment, 24 states had restrictive policies that requited sobriety, and 10 states had no policy available, the report showed (MMWR. 2017 May 12:66[18]:465-9).
Only three states – Massachusetts, New Mexico, and Washington – had a comprehensive (all three were considered “more comprehensive”) set of prevention laws and a permissive treatment policy, the investigators said, while also noting that two of the three – Massachusetts and New Mexico – were among the states with acute HCV rates that were at least twice the national average.
“Although the costs of HCV therapies have raised budgetary issues for state Medicaid programs in the past, the costs of HCV treatment have declined in recent years, increasing the cost-effectiveness of treatment, particularly among persons who inject drugs and who might serve as an ongoing source of transmission to others,” the report concluded.
The analysis examined three types of laws on access to clean needles and syringes: authorization of exchange programs, the scope of drug paraphernalia laws, and retail sale of needles and syringes. Each law was assessed for five elements, including authorization of syringe exchange statewide or in selected jurisdictions and exemption of needles or syringes from the definition of drug paraphernalia.
For the accompanying map (see “Acute hepatitis C infection incidence rates, 2015: State vs. national”), each state’s acute HCV incidence rate for 2015 was divided by the national rate to determine the incidence rate ratio, with data unavailable for 10 states.
The prevalence of hepatitis C virus (HCV) varies considerably by state, and the same can be said for the state laws and policies attempting to decrease that prevalence, according to an assessment by the Centers for Disease Control and Prevention.
In 2015, incidence of acute HCV infection exceeded the national average of 0.8 per 100,000 population in 17 states, including seven with rates that at least doubled it, the report noted. New HCV infections have increased in recent years despite curative therapies “and known preventive measures to interrupt transmission.”
The “most comprehensive” laws on prevention through clean needle access as of 2016 were found in Maine, Nevada, and Utah, with laws in 12 other states categorized as “more comprehensive” and 18 states falling into the “least comprehensive” category. On the Medicaid side of the equation, 16 states had permissive policies that did not require sobriety or required only screening and counseling before treatment, 24 states had restrictive policies that requited sobriety, and 10 states had no policy available, the report showed (MMWR. 2017 May 12:66[18]:465-9).
Only three states – Massachusetts, New Mexico, and Washington – had a comprehensive (all three were considered “more comprehensive”) set of prevention laws and a permissive treatment policy, the investigators said, while also noting that two of the three – Massachusetts and New Mexico – were among the states with acute HCV rates that were at least twice the national average.
“Although the costs of HCV therapies have raised budgetary issues for state Medicaid programs in the past, the costs of HCV treatment have declined in recent years, increasing the cost-effectiveness of treatment, particularly among persons who inject drugs and who might serve as an ongoing source of transmission to others,” the report concluded.
The analysis examined three types of laws on access to clean needles and syringes: authorization of exchange programs, the scope of drug paraphernalia laws, and retail sale of needles and syringes. Each law was assessed for five elements, including authorization of syringe exchange statewide or in selected jurisdictions and exemption of needles or syringes from the definition of drug paraphernalia.
For the accompanying map (see “Acute hepatitis C infection incidence rates, 2015: State vs. national”), each state’s acute HCV incidence rate for 2015 was divided by the national rate to determine the incidence rate ratio, with data unavailable for 10 states.
FROM MMWR
HCV incidence in young women doubled from 2006 to 2014
The incidence of hepatitis C virus infection in reproductive-age women has doubled between 2006 and 2014 while the number of acute cases increased more than threefold, according to data published in the Annals of Internal Medicine.
Researchers analyzed data from the National Notifiable Diseases Surveillance System (NNDSS) from 2006 to 2014 and the Quest Diagnostics Health Trends national database from 2011 to 2014, finding 425,322 women with confirmed HCV infection, 40.4% of whom were aged 15 to 44 years.
Around half of all acute infections were in non-Hispanic white women, and of the 2,069 women with available risk information, 63% acknowledged injection drug use (Ann Intern Med. 2017 May 8. doi:10.7326/M16-2350).
The analysis also found 1,859 cases of hepatitis C infection in children aged 2-13 years. According to the Quest data, the proportion of children with current hepatitis C infection was 3.2-fold higher in children aged 2-3 years than in those aged 12-13 years.
Commenting on this age difference, Kathleen N. Ly, MPH, from the Centers for Disease Control and Prevention, and her coauthors noted that it may have been the result of decreased testing over time in children already known to have chronic hepatitis C infection, or could be caused by spontaneous remission of infection, which is more common in infants and children than in adults.
The rate of infection among pregnant women tested for hepatitis C virus between 2011 and 2014 was 0.73%, which the authors calculated would mean that overall, 29,000 women with hepatitis C virus infection gave birth during that period across the United States. Based on data from a recent systematic review and meta-analysis, which found a likely mother-to-child transmission rate of 5.8/100 live births, they estimated that 1,700 infants were born with hepatitis C infection during that period.
“In contrast, only about 200 childhood cases per year are reported to the NNDSS, which may suggest a need for wider screening for HCV in pregnant women and their infants, as is recommended for HIV and hepatitis B virus,” the authors wrote. “However, recommendations for screening in pregnant women and clearer testing guidelines for infants born to HCV-infected mothers do not exist at this time.”
The study was supported by the CDC. One author was an employee of Quest Diagnostics, but no other conflicts of interest were declared.
Recognizing hepatitis C infection in pregnant women and neonates is possible, and clinical trials of antiviral therapy may show safety and efficacy in pregnant women and in children. Rather than silence, HCV infection calls out for public health action directed at all aspects of the epidemic, including consideration of screening pregnant women. At the very least, screening of pregnant women for HCV infection risk factors, as well as risk-based testing, requires more emphasis. Another issue in need of attention is the lack of authoritative, consensus-based recommendations for the identification, testing, and case management of newborns of infected mothers.
Much work lies ahead to eradicate HCV, starting with resources for public health surveillance to monitor incidence and prevalence and to fully characterize the infection in the population. Strategies to effectively prevent or cure infection in reproductive-aged women and their sexual and needle-sharing partners are critical.
Alfred DeMaria Jr., MD, is from the Massachusetts Department of Public Health. These comments are taken from an accompanying editorial (Ann Intern Med. 2017 May 8. doi:10.7326/M17-0927). No conflicts of interest were declared.
Recognizing hepatitis C infection in pregnant women and neonates is possible, and clinical trials of antiviral therapy may show safety and efficacy in pregnant women and in children. Rather than silence, HCV infection calls out for public health action directed at all aspects of the epidemic, including consideration of screening pregnant women. At the very least, screening of pregnant women for HCV infection risk factors, as well as risk-based testing, requires more emphasis. Another issue in need of attention is the lack of authoritative, consensus-based recommendations for the identification, testing, and case management of newborns of infected mothers.
Much work lies ahead to eradicate HCV, starting with resources for public health surveillance to monitor incidence and prevalence and to fully characterize the infection in the population. Strategies to effectively prevent or cure infection in reproductive-aged women and their sexual and needle-sharing partners are critical.
Alfred DeMaria Jr., MD, is from the Massachusetts Department of Public Health. These comments are taken from an accompanying editorial (Ann Intern Med. 2017 May 8. doi:10.7326/M17-0927). No conflicts of interest were declared.
Recognizing hepatitis C infection in pregnant women and neonates is possible, and clinical trials of antiviral therapy may show safety and efficacy in pregnant women and in children. Rather than silence, HCV infection calls out for public health action directed at all aspects of the epidemic, including consideration of screening pregnant women. At the very least, screening of pregnant women for HCV infection risk factors, as well as risk-based testing, requires more emphasis. Another issue in need of attention is the lack of authoritative, consensus-based recommendations for the identification, testing, and case management of newborns of infected mothers.
Much work lies ahead to eradicate HCV, starting with resources for public health surveillance to monitor incidence and prevalence and to fully characterize the infection in the population. Strategies to effectively prevent or cure infection in reproductive-aged women and their sexual and needle-sharing partners are critical.
Alfred DeMaria Jr., MD, is from the Massachusetts Department of Public Health. These comments are taken from an accompanying editorial (Ann Intern Med. 2017 May 8. doi:10.7326/M17-0927). No conflicts of interest were declared.
The incidence of hepatitis C virus infection in reproductive-age women has doubled between 2006 and 2014 while the number of acute cases increased more than threefold, according to data published in the Annals of Internal Medicine.
Researchers analyzed data from the National Notifiable Diseases Surveillance System (NNDSS) from 2006 to 2014 and the Quest Diagnostics Health Trends national database from 2011 to 2014, finding 425,322 women with confirmed HCV infection, 40.4% of whom were aged 15 to 44 years.
Around half of all acute infections were in non-Hispanic white women, and of the 2,069 women with available risk information, 63% acknowledged injection drug use (Ann Intern Med. 2017 May 8. doi:10.7326/M16-2350).
The analysis also found 1,859 cases of hepatitis C infection in children aged 2-13 years. According to the Quest data, the proportion of children with current hepatitis C infection was 3.2-fold higher in children aged 2-3 years than in those aged 12-13 years.
Commenting on this age difference, Kathleen N. Ly, MPH, from the Centers for Disease Control and Prevention, and her coauthors noted that it may have been the result of decreased testing over time in children already known to have chronic hepatitis C infection, or could be caused by spontaneous remission of infection, which is more common in infants and children than in adults.
The rate of infection among pregnant women tested for hepatitis C virus between 2011 and 2014 was 0.73%, which the authors calculated would mean that overall, 29,000 women with hepatitis C virus infection gave birth during that period across the United States. Based on data from a recent systematic review and meta-analysis, which found a likely mother-to-child transmission rate of 5.8/100 live births, they estimated that 1,700 infants were born with hepatitis C infection during that period.
“In contrast, only about 200 childhood cases per year are reported to the NNDSS, which may suggest a need for wider screening for HCV in pregnant women and their infants, as is recommended for HIV and hepatitis B virus,” the authors wrote. “However, recommendations for screening in pregnant women and clearer testing guidelines for infants born to HCV-infected mothers do not exist at this time.”
The study was supported by the CDC. One author was an employee of Quest Diagnostics, but no other conflicts of interest were declared.
The incidence of hepatitis C virus infection in reproductive-age women has doubled between 2006 and 2014 while the number of acute cases increased more than threefold, according to data published in the Annals of Internal Medicine.
Researchers analyzed data from the National Notifiable Diseases Surveillance System (NNDSS) from 2006 to 2014 and the Quest Diagnostics Health Trends national database from 2011 to 2014, finding 425,322 women with confirmed HCV infection, 40.4% of whom were aged 15 to 44 years.
Around half of all acute infections were in non-Hispanic white women, and of the 2,069 women with available risk information, 63% acknowledged injection drug use (Ann Intern Med. 2017 May 8. doi:10.7326/M16-2350).
The analysis also found 1,859 cases of hepatitis C infection in children aged 2-13 years. According to the Quest data, the proportion of children with current hepatitis C infection was 3.2-fold higher in children aged 2-3 years than in those aged 12-13 years.
Commenting on this age difference, Kathleen N. Ly, MPH, from the Centers for Disease Control and Prevention, and her coauthors noted that it may have been the result of decreased testing over time in children already known to have chronic hepatitis C infection, or could be caused by spontaneous remission of infection, which is more common in infants and children than in adults.
The rate of infection among pregnant women tested for hepatitis C virus between 2011 and 2014 was 0.73%, which the authors calculated would mean that overall, 29,000 women with hepatitis C virus infection gave birth during that period across the United States. Based on data from a recent systematic review and meta-analysis, which found a likely mother-to-child transmission rate of 5.8/100 live births, they estimated that 1,700 infants were born with hepatitis C infection during that period.
“In contrast, only about 200 childhood cases per year are reported to the NNDSS, which may suggest a need for wider screening for HCV in pregnant women and their infants, as is recommended for HIV and hepatitis B virus,” the authors wrote. “However, recommendations for screening in pregnant women and clearer testing guidelines for infants born to HCV-infected mothers do not exist at this time.”
The study was supported by the CDC. One author was an employee of Quest Diagnostics, but no other conflicts of interest were declared.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: The incidence of hepatitis C infection increased significantly in reproductive age women during 2006-2014.
Major finding: The incidence of hepatitis C infection in reproductive-age women doubled during 2006-2014 while the number of acute cases increased more than threefold.
Data source: Analysis of data from the National Notifiable Diseases Surveillance System from 2006 to 2014 and the Quest Diagnostics Health Trends national database from 2011 to 2014.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. One author was an employee of Quest Diagnostics, but no other conflicts of interest were declared.
HCV treatment program achieves 97% SVR among homeless
A community-based primary care treatment program achieved a 97% sustained virologic response rate at 12 weeks among homeless adults with hepatitis C, according to a Research Letter to the Editor published online in JAMA Internal Medicine.
An estimated 44% of homeless adults have hepatitis C and face many barriers to effective treatment. Investigators described their experience with the Boston Health Care for the Homeless program during an 18-month period in which 64 such patients received oral direct-acting antivirals as part of integrated primary care services. Most were men, and the mean age was 55 years, said Joshua A. Barocas, MD, of the division of infectious diseases, Massachusetts General Hospital, Boston, and his associates.
Patients were selected for the therapy by clinicians – primary care physicians and nurse practitioners – based in part on their adherence to previous medical appointments. They received weekly phone calls from a care coordinator, and they were not required to maintain sobriety to remain eligible for Medicare or Medicaid coverage for the treatment.
Among the study participants, 62 of 64 (97%) achieved a sustained virologic response at 12 weeks. Only 13% reported missing more than three doses of oral antivirals. Viral testing showed genetic mutations conferring resistance to treatment in one of the two patients who did not achieve SVR, the investigators said (JAMA Intern Med. 2017 Apr 10. doi: 10.1001/jamainternmed.2017.0358).
“These findings demonstrate that with a dedicated program for treating HCV in homeless and marginally housed adults in a primary care setting, it is possible to achieve outcomes similar to those of clinical trials and other cohorts, despite significant additional barriers and competing priorities to health care faced by this population,” Dr. Barocas and his associates said.
The National Institute on Drug Abuse supported the study. Dr. Barocas and his associates reported having no relevant disclosures.
A community-based primary care treatment program achieved a 97% sustained virologic response rate at 12 weeks among homeless adults with hepatitis C, according to a Research Letter to the Editor published online in JAMA Internal Medicine.
An estimated 44% of homeless adults have hepatitis C and face many barriers to effective treatment. Investigators described their experience with the Boston Health Care for the Homeless program during an 18-month period in which 64 such patients received oral direct-acting antivirals as part of integrated primary care services. Most were men, and the mean age was 55 years, said Joshua A. Barocas, MD, of the division of infectious diseases, Massachusetts General Hospital, Boston, and his associates.
Patients were selected for the therapy by clinicians – primary care physicians and nurse practitioners – based in part on their adherence to previous medical appointments. They received weekly phone calls from a care coordinator, and they were not required to maintain sobriety to remain eligible for Medicare or Medicaid coverage for the treatment.
Among the study participants, 62 of 64 (97%) achieved a sustained virologic response at 12 weeks. Only 13% reported missing more than three doses of oral antivirals. Viral testing showed genetic mutations conferring resistance to treatment in one of the two patients who did not achieve SVR, the investigators said (JAMA Intern Med. 2017 Apr 10. doi: 10.1001/jamainternmed.2017.0358).
“These findings demonstrate that with a dedicated program for treating HCV in homeless and marginally housed adults in a primary care setting, it is possible to achieve outcomes similar to those of clinical trials and other cohorts, despite significant additional barriers and competing priorities to health care faced by this population,” Dr. Barocas and his associates said.
The National Institute on Drug Abuse supported the study. Dr. Barocas and his associates reported having no relevant disclosures.
A community-based primary care treatment program achieved a 97% sustained virologic response rate at 12 weeks among homeless adults with hepatitis C, according to a Research Letter to the Editor published online in JAMA Internal Medicine.
An estimated 44% of homeless adults have hepatitis C and face many barriers to effective treatment. Investigators described their experience with the Boston Health Care for the Homeless program during an 18-month period in which 64 such patients received oral direct-acting antivirals as part of integrated primary care services. Most were men, and the mean age was 55 years, said Joshua A. Barocas, MD, of the division of infectious diseases, Massachusetts General Hospital, Boston, and his associates.
Patients were selected for the therapy by clinicians – primary care physicians and nurse practitioners – based in part on their adherence to previous medical appointments. They received weekly phone calls from a care coordinator, and they were not required to maintain sobriety to remain eligible for Medicare or Medicaid coverage for the treatment.
Among the study participants, 62 of 64 (97%) achieved a sustained virologic response at 12 weeks. Only 13% reported missing more than three doses of oral antivirals. Viral testing showed genetic mutations conferring resistance to treatment in one of the two patients who did not achieve SVR, the investigators said (JAMA Intern Med. 2017 Apr 10. doi: 10.1001/jamainternmed.2017.0358).
“These findings demonstrate that with a dedicated program for treating HCV in homeless and marginally housed adults in a primary care setting, it is possible to achieve outcomes similar to those of clinical trials and other cohorts, despite significant additional barriers and competing priorities to health care faced by this population,” Dr. Barocas and his associates said.
The National Institute on Drug Abuse supported the study. Dr. Barocas and his associates reported having no relevant disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A community-based primary-care treatment program achieved a 97% sustained virologic response rate at 12 weeks among homeless adults with hepatitis C.
Major finding: Among the study participants, 62 of 64 (97%) achieved a sustained virologic response at 12 weeks.
Data source: A retrospective cohort study of 64 homeless adults in Boston treated during an 18-month period.
Disclosures: The National Institute on Drug Abuse supported the study. Dr. Barocas and his associates reported having no relevant disclosures.
Hepatitis B, C appear to raise Parkinson’s risk
Hepatitis B and C appear to raise the risk of later Parkinson’s disease (PD), according to a report published in Neurology.
The etiology of Parkinson’s disease is complex, and several factors, including environmental toxins and head trauma, have been proposed that may increase risk for the disorder. Two recent epidemiologic studies in Taiwan found an association between hepatitis C and Parkinson’s risk, said Julia Pakpoor, BM BCh, of the Unit of Health-Care Epidemiology, Nuffield Department of Population Health, University of Oxford (England), and her associates.
To further explore that association, the investigators performed a retrospective cohort study using data from National Health Service hospitals across England for 1999-2011. They assessed the risk for developing PD among 21,633 people with hepatitis B, 48,428 with hepatitis C, 6,225 with autoimmune hepatitis, 4,234 with chronic active hepatitis, 19,870 with HIV, and 6,132,124 control subjects with other disorders.
The risk of developing PD was elevated for only 1 or more years following hospitalization for hepatitis B (relative risk, 1.76) or hepatitis C (RR, 1.51). “These findings may be explained by a specific aspect of viral hepatitis (rather than a general hepatic inflammatory process or general use of antivirals), but whether this reflects shared disease mechanisms, shared genetic or environmental susceptibility, sequelae of viral hepatitis per se, or a consequence of treatment remains to be determined,” Dr. Pakpoor and her associates said (Neurology. 2017;88:1-4).
The reason for this association is not yet known. “Neurotropic features of hepatitis C have been described previously and include the potential for cognitive impairment, independent of hepatic encephalopathy. Further, all essential hepatitis C virus receptors have been shown to be expressed on the brain microvascular endothelium. … suggesting one mechanism by which the virus may affect the CNS,” they noted.
In addition, parkinsonism has been described as an adverse effect of treatment with interferon and ribavirin, which are commonly used in hepatitis C infection. Parkinsonism also is known to develop in association with liver cirrhosis, and cirrhosis status was not available for the members of this study cohort.
More studies are needed to confirm this association and verify that it is causal. Such research will also provide insight into pathophysiologic pathways of PD, “which may be important to understanding the development of PD more broadly,” Dr. Pakpoor and her associates said.
The English National Institute for Health Research supported the study. Dr. Pakpoor and her associates reported having no relevant financial disclosures.
The findings presented by Dr. Pakpoor and her associates justify performing deep-sequencing studies in brain tissue samples at autopsy or in cerebrospinal fluid samples from patients with PD, to detect possible links with infectious agents such as viral hepatitis.
However, for any such link to be considered conclusive, future research also must show that direct-acting antiviral therapies for chronic HCV improve PD symptoms, or epidemiology studies must demonstrate a strong association with specific hepatitis virus.
Julian Benito-Leon, MD, PhD, is in the department of neurology at Complutense University Hospital, Madrid. He reported having no relevant financial disclosures. Dr. Benito-Leon made these remarks in an editorial accompanying Dr. Pakpoor’s report (Neurology. 2017;88:1-2).
The findings presented by Dr. Pakpoor and her associates justify performing deep-sequencing studies in brain tissue samples at autopsy or in cerebrospinal fluid samples from patients with PD, to detect possible links with infectious agents such as viral hepatitis.
However, for any such link to be considered conclusive, future research also must show that direct-acting antiviral therapies for chronic HCV improve PD symptoms, or epidemiology studies must demonstrate a strong association with specific hepatitis virus.
Julian Benito-Leon, MD, PhD, is in the department of neurology at Complutense University Hospital, Madrid. He reported having no relevant financial disclosures. Dr. Benito-Leon made these remarks in an editorial accompanying Dr. Pakpoor’s report (Neurology. 2017;88:1-2).
The findings presented by Dr. Pakpoor and her associates justify performing deep-sequencing studies in brain tissue samples at autopsy or in cerebrospinal fluid samples from patients with PD, to detect possible links with infectious agents such as viral hepatitis.
However, for any such link to be considered conclusive, future research also must show that direct-acting antiviral therapies for chronic HCV improve PD symptoms, or epidemiology studies must demonstrate a strong association with specific hepatitis virus.
Julian Benito-Leon, MD, PhD, is in the department of neurology at Complutense University Hospital, Madrid. He reported having no relevant financial disclosures. Dr. Benito-Leon made these remarks in an editorial accompanying Dr. Pakpoor’s report (Neurology. 2017;88:1-2).
Hepatitis B and C appear to raise the risk of later Parkinson’s disease (PD), according to a report published in Neurology.
The etiology of Parkinson’s disease is complex, and several factors, including environmental toxins and head trauma, have been proposed that may increase risk for the disorder. Two recent epidemiologic studies in Taiwan found an association between hepatitis C and Parkinson’s risk, said Julia Pakpoor, BM BCh, of the Unit of Health-Care Epidemiology, Nuffield Department of Population Health, University of Oxford (England), and her associates.
To further explore that association, the investigators performed a retrospective cohort study using data from National Health Service hospitals across England for 1999-2011. They assessed the risk for developing PD among 21,633 people with hepatitis B, 48,428 with hepatitis C, 6,225 with autoimmune hepatitis, 4,234 with chronic active hepatitis, 19,870 with HIV, and 6,132,124 control subjects with other disorders.
The risk of developing PD was elevated for only 1 or more years following hospitalization for hepatitis B (relative risk, 1.76) or hepatitis C (RR, 1.51). “These findings may be explained by a specific aspect of viral hepatitis (rather than a general hepatic inflammatory process or general use of antivirals), but whether this reflects shared disease mechanisms, shared genetic or environmental susceptibility, sequelae of viral hepatitis per se, or a consequence of treatment remains to be determined,” Dr. Pakpoor and her associates said (Neurology. 2017;88:1-4).
The reason for this association is not yet known. “Neurotropic features of hepatitis C have been described previously and include the potential for cognitive impairment, independent of hepatic encephalopathy. Further, all essential hepatitis C virus receptors have been shown to be expressed on the brain microvascular endothelium. … suggesting one mechanism by which the virus may affect the CNS,” they noted.
In addition, parkinsonism has been described as an adverse effect of treatment with interferon and ribavirin, which are commonly used in hepatitis C infection. Parkinsonism also is known to develop in association with liver cirrhosis, and cirrhosis status was not available for the members of this study cohort.
More studies are needed to confirm this association and verify that it is causal. Such research will also provide insight into pathophysiologic pathways of PD, “which may be important to understanding the development of PD more broadly,” Dr. Pakpoor and her associates said.
The English National Institute for Health Research supported the study. Dr. Pakpoor and her associates reported having no relevant financial disclosures.
Hepatitis B and C appear to raise the risk of later Parkinson’s disease (PD), according to a report published in Neurology.
The etiology of Parkinson’s disease is complex, and several factors, including environmental toxins and head trauma, have been proposed that may increase risk for the disorder. Two recent epidemiologic studies in Taiwan found an association between hepatitis C and Parkinson’s risk, said Julia Pakpoor, BM BCh, of the Unit of Health-Care Epidemiology, Nuffield Department of Population Health, University of Oxford (England), and her associates.
To further explore that association, the investigators performed a retrospective cohort study using data from National Health Service hospitals across England for 1999-2011. They assessed the risk for developing PD among 21,633 people with hepatitis B, 48,428 with hepatitis C, 6,225 with autoimmune hepatitis, 4,234 with chronic active hepatitis, 19,870 with HIV, and 6,132,124 control subjects with other disorders.
The risk of developing PD was elevated for only 1 or more years following hospitalization for hepatitis B (relative risk, 1.76) or hepatitis C (RR, 1.51). “These findings may be explained by a specific aspect of viral hepatitis (rather than a general hepatic inflammatory process or general use of antivirals), but whether this reflects shared disease mechanisms, shared genetic or environmental susceptibility, sequelae of viral hepatitis per se, or a consequence of treatment remains to be determined,” Dr. Pakpoor and her associates said (Neurology. 2017;88:1-4).
The reason for this association is not yet known. “Neurotropic features of hepatitis C have been described previously and include the potential for cognitive impairment, independent of hepatic encephalopathy. Further, all essential hepatitis C virus receptors have been shown to be expressed on the brain microvascular endothelium. … suggesting one mechanism by which the virus may affect the CNS,” they noted.
In addition, parkinsonism has been described as an adverse effect of treatment with interferon and ribavirin, which are commonly used in hepatitis C infection. Parkinsonism also is known to develop in association with liver cirrhosis, and cirrhosis status was not available for the members of this study cohort.
More studies are needed to confirm this association and verify that it is causal. Such research will also provide insight into pathophysiologic pathways of PD, “which may be important to understanding the development of PD more broadly,” Dr. Pakpoor and her associates said.
The English National Institute for Health Research supported the study. Dr. Pakpoor and her associates reported having no relevant financial disclosures.
FROM NEUROLOGY
Key clinical point:
Major finding: The risk of developing PD was significantly elevated for only 1 or more years following hospitalization for hepatitis B (RR, 1.76) or hepatitis C (RR, 1.51).
Data source: A retrospective cohort study involving 70,061 people in the general U.K. population with hepatitis B or C, 6,225 with autoimmune hepatitis, 4,234 with chronic active hepatitis, 19,870 with HIV, and 6,132,124 control subjects hospitalized during 1999-2011.
Disclosures: The English National Institute for Health Research supported the study. Dr. Pakpoor and her associates reported having no relevant financial disclosures.
Elbasvir, grazoprevir beat HCV despite compensated cirrhosis
Twelve weeks of combination therapy with elbasvir and grazoprevir (EBR/GZR) achieved sustained virologic response in 98% of treatment-naive patients with compensated cirrhosis and chronic hepatitis C (HCV) genotype 1, 4, or 6 infections, and in 89% of treatment-experienced patients, according to a pooled analysis of six industry-sponsored trials.
Concomitant ribavirin offered “no incremental benefit” for treatment-naive patients, while 16 or 18 weeks of EBR and GZR with ribavirin achieved SVR12 in 100% of treatment-experienced patients, wrote Ira M. Jacobson, MD, of Mount Sinai Beth Israel and Icahn School of Medicine at Mount Sinai, New York, and his associates. The report was published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.01.050).
Regardless of treatment history, genotype 1a patients with resistance-associated variants (RAV) in HCV nonstructural protein 5A (NS5A) needed ribavirin to achieve sustained virologic response (SVR) rates above 90%, the researchers emphasized. “Both patients with HCV genotype 1a infection with baseline RAVs who received 16 or 18 weeks of EBR/GZR and ribavirin achieved SVR12,” the researchers noted. Elbasvir is an HCV NS5A inhibitor, and GZR is an HCV NS3/4A protease inhibitor. In 2016, the Food and Drug Administration approved them in combination (Zepatier) for chronic genotype 1 and genotype 4 HCV. Studies have confirmed the benefits of treating HCV even when patients have cirrhosis, but they can be challenging to treat, especially if they have already failed a regimen that included a direct-acting antiviral agent, the investigators noted.
To explore the efficacy of EBR/GZR in compensated, Child-Pugh A cirrhosis, they studied 402 such patients with HCV genotype 1, 4, or 6 infections whose baseline HCV RNA level exceeded 10,000 IU. Patients had participated in one of six phase II/III clinical trials and had received EBR/GZR 50 mg/100 mg once daily for 12-18 weeks, with or without ribavirin (800-1400 mg/day based on body weight). Treatment-naive patients received 12 weeks of treatment, while treatment-experienced patients received 12, 16, or 18 weeks of treatment.
Twelve weeks of ribavirin did not boost SVR for either treatment-naive (90%) or treatment-experienced patients (91%), the researchers said. However, all 49 treatment-experienced patients who received EBR/GZR plus ribavirin for 16 or 18 weeks achieved SVR12, compared to 94% of patients who received EBR/GZR without ribavirin for 16 or 18 weeks.
Virologic failure was more common with HCV genotype 1a infections than with genotype 1b or 4 infection, especially if patients previously had not responded to interferon, the researchers noted. Eight of 11 (73%) patients with HCV genotype 1a infection and baseline NS5A RAVs achieved SVR12, compared with 98% of GT1a-infected patients without RAVs at baseline. But EBR/GZR was effective in various other subgroups, including patients with less than 100,000 platelets per microL, serum albumin below 3.5 g/dL, and FibroScan scores below 25 kPa. These findings suggest that EBR/GZR remains effective in patients with advanced compensated cirrhosis, the investigators said.
Most patients tolerated therapy, but six stopped treatment because of adverse events, including one episode of severe, possibly treatment-related abdominal pain. Also, four patients had late, asymptomatic rises in alanine aminotransferase (ALT) after first normalizing on treatment, and one patient stopped treatment because of grade 4 ALT elevation with eosinophilia. “There were no decompensation events in this generally healthy cirrhotic population, and no other evidence of declining liver function while on treatment,” the researchers added.
The integrated analysis was not prespecified, nor was it powered to compare outcomes between treatment arms. Only three patients had genotype 6 infection, and all were treatment-experienced. Only 23 patients had genotype 4 infection. Also, most patients had well-compensated cirrhosis. Finally, the trials varied in terms of how they defined cirrhosis, the investigators noted.
Merck, which funded the study, makes elbasvir and grazoprevir. The investigators acknowledged medical writing and editorial assistance from ApotheCom, which Merck funded. Dr. Jacobson disclosed consulting relationships and grant funding from Merck, AbbVie, Bristol-Myers Squibb, Gilead, Intercept, Janssen, and Trek.
Twelve weeks of combination therapy with elbasvir and grazoprevir (EBR/GZR) achieved sustained virologic response in 98% of treatment-naive patients with compensated cirrhosis and chronic hepatitis C (HCV) genotype 1, 4, or 6 infections, and in 89% of treatment-experienced patients, according to a pooled analysis of six industry-sponsored trials.
Concomitant ribavirin offered “no incremental benefit” for treatment-naive patients, while 16 or 18 weeks of EBR and GZR with ribavirin achieved SVR12 in 100% of treatment-experienced patients, wrote Ira M. Jacobson, MD, of Mount Sinai Beth Israel and Icahn School of Medicine at Mount Sinai, New York, and his associates. The report was published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.01.050).
Regardless of treatment history, genotype 1a patients with resistance-associated variants (RAV) in HCV nonstructural protein 5A (NS5A) needed ribavirin to achieve sustained virologic response (SVR) rates above 90%, the researchers emphasized. “Both patients with HCV genotype 1a infection with baseline RAVs who received 16 or 18 weeks of EBR/GZR and ribavirin achieved SVR12,” the researchers noted. Elbasvir is an HCV NS5A inhibitor, and GZR is an HCV NS3/4A protease inhibitor. In 2016, the Food and Drug Administration approved them in combination (Zepatier) for chronic genotype 1 and genotype 4 HCV. Studies have confirmed the benefits of treating HCV even when patients have cirrhosis, but they can be challenging to treat, especially if they have already failed a regimen that included a direct-acting antiviral agent, the investigators noted.
To explore the efficacy of EBR/GZR in compensated, Child-Pugh A cirrhosis, they studied 402 such patients with HCV genotype 1, 4, or 6 infections whose baseline HCV RNA level exceeded 10,000 IU. Patients had participated in one of six phase II/III clinical trials and had received EBR/GZR 50 mg/100 mg once daily for 12-18 weeks, with or without ribavirin (800-1400 mg/day based on body weight). Treatment-naive patients received 12 weeks of treatment, while treatment-experienced patients received 12, 16, or 18 weeks of treatment.
Twelve weeks of ribavirin did not boost SVR for either treatment-naive (90%) or treatment-experienced patients (91%), the researchers said. However, all 49 treatment-experienced patients who received EBR/GZR plus ribavirin for 16 or 18 weeks achieved SVR12, compared to 94% of patients who received EBR/GZR without ribavirin for 16 or 18 weeks.
Virologic failure was more common with HCV genotype 1a infections than with genotype 1b or 4 infection, especially if patients previously had not responded to interferon, the researchers noted. Eight of 11 (73%) patients with HCV genotype 1a infection and baseline NS5A RAVs achieved SVR12, compared with 98% of GT1a-infected patients without RAVs at baseline. But EBR/GZR was effective in various other subgroups, including patients with less than 100,000 platelets per microL, serum albumin below 3.5 g/dL, and FibroScan scores below 25 kPa. These findings suggest that EBR/GZR remains effective in patients with advanced compensated cirrhosis, the investigators said.
Most patients tolerated therapy, but six stopped treatment because of adverse events, including one episode of severe, possibly treatment-related abdominal pain. Also, four patients had late, asymptomatic rises in alanine aminotransferase (ALT) after first normalizing on treatment, and one patient stopped treatment because of grade 4 ALT elevation with eosinophilia. “There were no decompensation events in this generally healthy cirrhotic population, and no other evidence of declining liver function while on treatment,” the researchers added.
The integrated analysis was not prespecified, nor was it powered to compare outcomes between treatment arms. Only three patients had genotype 6 infection, and all were treatment-experienced. Only 23 patients had genotype 4 infection. Also, most patients had well-compensated cirrhosis. Finally, the trials varied in terms of how they defined cirrhosis, the investigators noted.
Merck, which funded the study, makes elbasvir and grazoprevir. The investigators acknowledged medical writing and editorial assistance from ApotheCom, which Merck funded. Dr. Jacobson disclosed consulting relationships and grant funding from Merck, AbbVie, Bristol-Myers Squibb, Gilead, Intercept, Janssen, and Trek.
Twelve weeks of combination therapy with elbasvir and grazoprevir (EBR/GZR) achieved sustained virologic response in 98% of treatment-naive patients with compensated cirrhosis and chronic hepatitis C (HCV) genotype 1, 4, or 6 infections, and in 89% of treatment-experienced patients, according to a pooled analysis of six industry-sponsored trials.
Concomitant ribavirin offered “no incremental benefit” for treatment-naive patients, while 16 or 18 weeks of EBR and GZR with ribavirin achieved SVR12 in 100% of treatment-experienced patients, wrote Ira M. Jacobson, MD, of Mount Sinai Beth Israel and Icahn School of Medicine at Mount Sinai, New York, and his associates. The report was published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.01.050).
Regardless of treatment history, genotype 1a patients with resistance-associated variants (RAV) in HCV nonstructural protein 5A (NS5A) needed ribavirin to achieve sustained virologic response (SVR) rates above 90%, the researchers emphasized. “Both patients with HCV genotype 1a infection with baseline RAVs who received 16 or 18 weeks of EBR/GZR and ribavirin achieved SVR12,” the researchers noted. Elbasvir is an HCV NS5A inhibitor, and GZR is an HCV NS3/4A protease inhibitor. In 2016, the Food and Drug Administration approved them in combination (Zepatier) for chronic genotype 1 and genotype 4 HCV. Studies have confirmed the benefits of treating HCV even when patients have cirrhosis, but they can be challenging to treat, especially if they have already failed a regimen that included a direct-acting antiviral agent, the investigators noted.
To explore the efficacy of EBR/GZR in compensated, Child-Pugh A cirrhosis, they studied 402 such patients with HCV genotype 1, 4, or 6 infections whose baseline HCV RNA level exceeded 10,000 IU. Patients had participated in one of six phase II/III clinical trials and had received EBR/GZR 50 mg/100 mg once daily for 12-18 weeks, with or without ribavirin (800-1400 mg/day based on body weight). Treatment-naive patients received 12 weeks of treatment, while treatment-experienced patients received 12, 16, or 18 weeks of treatment.
Twelve weeks of ribavirin did not boost SVR for either treatment-naive (90%) or treatment-experienced patients (91%), the researchers said. However, all 49 treatment-experienced patients who received EBR/GZR plus ribavirin for 16 or 18 weeks achieved SVR12, compared to 94% of patients who received EBR/GZR without ribavirin for 16 or 18 weeks.
Virologic failure was more common with HCV genotype 1a infections than with genotype 1b or 4 infection, especially if patients previously had not responded to interferon, the researchers noted. Eight of 11 (73%) patients with HCV genotype 1a infection and baseline NS5A RAVs achieved SVR12, compared with 98% of GT1a-infected patients without RAVs at baseline. But EBR/GZR was effective in various other subgroups, including patients with less than 100,000 platelets per microL, serum albumin below 3.5 g/dL, and FibroScan scores below 25 kPa. These findings suggest that EBR/GZR remains effective in patients with advanced compensated cirrhosis, the investigators said.
Most patients tolerated therapy, but six stopped treatment because of adverse events, including one episode of severe, possibly treatment-related abdominal pain. Also, four patients had late, asymptomatic rises in alanine aminotransferase (ALT) after first normalizing on treatment, and one patient stopped treatment because of grade 4 ALT elevation with eosinophilia. “There were no decompensation events in this generally healthy cirrhotic population, and no other evidence of declining liver function while on treatment,” the researchers added.
The integrated analysis was not prespecified, nor was it powered to compare outcomes between treatment arms. Only three patients had genotype 6 infection, and all were treatment-experienced. Only 23 patients had genotype 4 infection. Also, most patients had well-compensated cirrhosis. Finally, the trials varied in terms of how they defined cirrhosis, the investigators noted.
Merck, which funded the study, makes elbasvir and grazoprevir. The investigators acknowledged medical writing and editorial assistance from ApotheCom, which Merck funded. Dr. Jacobson disclosed consulting relationships and grant funding from Merck, AbbVie, Bristol-Myers Squibb, Gilead, Intercept, Janssen, and Trek.
FROM GASTROENTEROLOGY
Key clinical point: Twelve weeks of combination therapy with elbasvir and grazoprevir was relatively well tolerated and achieved sustained viral response for most patients with chronic genotype 1, 4, or 6 hepatitis C virus infections and compensated cirrhosis.
Major finding: Rates of sustained virologic response were 98% for treatment-naive patients and 89% for treatment-experienced patients.
Data source: An integrated analysis of 402 patients with treatment-naive or treatment-experienced HCV GT1, 4, or 6 infection and compensated, Child-Pugh A cirrhosis.
Disclosures: Merck, which funded the study, makes elbasvir and grazoprevir. The investigators acknowledged medical writing and editorial assistance from ApotheCom, which Merck funded. Dr. Jacobson disclosed consulting relationships and grant funding from Merck, AbbVie, Bristol-Myers Squibb, Gilead, Intercept, Janssen, and Trek.
HCV ‘cure’ within the VA appears likely
The number of Veterans Affairs patients with hepatitis C who have achieved a sustained virologic response to antiviral therapy has escalated so rapidly and reached such a height that the disease may well be eradicated in that health care system within a few years, according to a report in Alimentary Pharmacology and Therapeutics.
The potential public health benefits are substantial, “considering that HCV infection is the most common cause of cirrhosis and liver cancer in the VA and the United States, that the benefits of SVR are long-lasting, and that HCV clearance reduces the risk of liver cancer by 76% and all-cause mortality by 50%,” said Andrew M. Moon, MD, of the division of general internal medicine, University of Washington, Seattle, and his associates.
An estimated 124,662 VA patients currently are infected, and curing them “would substantially reduce the burden of HCV within the entire country and prevent tens of thousands of deaths,” they noted.
The VA dramatically increased the number of patients who were offered treatment in recent years, because it was able to allocate nearly $700 million to offset the high costs of highly effective direct antiviral agents, which in turn made these better-tolerated drugs more widely available at clinics across the country. The VA also removed all treatment prioritization criteria, allowing all patients, not just those with severe disease, to receive highly effective direct antiviral agents. This is “in stark contrast to most health care systems, state Medicaid programs, and insurance carriers in the U.S., which still restrict access ... based on severity of liver disease,” the investigators said (Aliment Pharmacol Ther. 2017 March 8. doi: 10.1111/apt.14021).
To examine the impact of these changes, Dr. Moon and his associates performed a retrospective cohort study, analyzing the electronic medical records of all 105,369 HCV treatment regimens given to 78,947 VA patients (mean age, 56 years) during a 17-year period. They found that annual treatment rates more than doubled from 2,726 to 6,679 patients when pegylated interferon was introduced, declined for a while and then rose modestly to 4,900 patients when boceprevir and telaprevir were introduced, declined again to an all-time low of 2,609 and then rebounded to 9,180 patients when sofosbuvir and simeprevir were introduced, and finally skyrocketed to 31,028 patients when ledipasvir/sofosbuvir and paritaprevir/ritonavir/ombitasvir/dasabuvir were introduced.
Correspondingly, SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end. The improvement in SVR rates was even more pronounced among traditionally “hard to treat” cases, such as patients with concomitant cirrhosis (from 11.0% to 87.0%), decompensated cirrhosis (from 14.6% to 85.2%), highly refractory infection (from 16.4% to 89.3%), and genotype-1 infection (from 1.3% to 91.7%). “The number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015,” Dr. Moon and his associates said.
“We believe that our findings based on the VA health care system might be relevant and informative for other comprehensive health care systems,” providing proof-of-concept that similar results can be achieved if aggressive screening; affordable, tolerable treatment; and open access to all patients are implemented.
The number of Veterans Affairs patients with hepatitis C who have achieved a sustained virologic response to antiviral therapy has escalated so rapidly and reached such a height that the disease may well be eradicated in that health care system within a few years, according to a report in Alimentary Pharmacology and Therapeutics.
The potential public health benefits are substantial, “considering that HCV infection is the most common cause of cirrhosis and liver cancer in the VA and the United States, that the benefits of SVR are long-lasting, and that HCV clearance reduces the risk of liver cancer by 76% and all-cause mortality by 50%,” said Andrew M. Moon, MD, of the division of general internal medicine, University of Washington, Seattle, and his associates.
An estimated 124,662 VA patients currently are infected, and curing them “would substantially reduce the burden of HCV within the entire country and prevent tens of thousands of deaths,” they noted.
The VA dramatically increased the number of patients who were offered treatment in recent years, because it was able to allocate nearly $700 million to offset the high costs of highly effective direct antiviral agents, which in turn made these better-tolerated drugs more widely available at clinics across the country. The VA also removed all treatment prioritization criteria, allowing all patients, not just those with severe disease, to receive highly effective direct antiviral agents. This is “in stark contrast to most health care systems, state Medicaid programs, and insurance carriers in the U.S., which still restrict access ... based on severity of liver disease,” the investigators said (Aliment Pharmacol Ther. 2017 March 8. doi: 10.1111/apt.14021).
To examine the impact of these changes, Dr. Moon and his associates performed a retrospective cohort study, analyzing the electronic medical records of all 105,369 HCV treatment regimens given to 78,947 VA patients (mean age, 56 years) during a 17-year period. They found that annual treatment rates more than doubled from 2,726 to 6,679 patients when pegylated interferon was introduced, declined for a while and then rose modestly to 4,900 patients when boceprevir and telaprevir were introduced, declined again to an all-time low of 2,609 and then rebounded to 9,180 patients when sofosbuvir and simeprevir were introduced, and finally skyrocketed to 31,028 patients when ledipasvir/sofosbuvir and paritaprevir/ritonavir/ombitasvir/dasabuvir were introduced.
Correspondingly, SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end. The improvement in SVR rates was even more pronounced among traditionally “hard to treat” cases, such as patients with concomitant cirrhosis (from 11.0% to 87.0%), decompensated cirrhosis (from 14.6% to 85.2%), highly refractory infection (from 16.4% to 89.3%), and genotype-1 infection (from 1.3% to 91.7%). “The number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015,” Dr. Moon and his associates said.
“We believe that our findings based on the VA health care system might be relevant and informative for other comprehensive health care systems,” providing proof-of-concept that similar results can be achieved if aggressive screening; affordable, tolerable treatment; and open access to all patients are implemented.
The number of Veterans Affairs patients with hepatitis C who have achieved a sustained virologic response to antiviral therapy has escalated so rapidly and reached such a height that the disease may well be eradicated in that health care system within a few years, according to a report in Alimentary Pharmacology and Therapeutics.
The potential public health benefits are substantial, “considering that HCV infection is the most common cause of cirrhosis and liver cancer in the VA and the United States, that the benefits of SVR are long-lasting, and that HCV clearance reduces the risk of liver cancer by 76% and all-cause mortality by 50%,” said Andrew M. Moon, MD, of the division of general internal medicine, University of Washington, Seattle, and his associates.
An estimated 124,662 VA patients currently are infected, and curing them “would substantially reduce the burden of HCV within the entire country and prevent tens of thousands of deaths,” they noted.
The VA dramatically increased the number of patients who were offered treatment in recent years, because it was able to allocate nearly $700 million to offset the high costs of highly effective direct antiviral agents, which in turn made these better-tolerated drugs more widely available at clinics across the country. The VA also removed all treatment prioritization criteria, allowing all patients, not just those with severe disease, to receive highly effective direct antiviral agents. This is “in stark contrast to most health care systems, state Medicaid programs, and insurance carriers in the U.S., which still restrict access ... based on severity of liver disease,” the investigators said (Aliment Pharmacol Ther. 2017 March 8. doi: 10.1111/apt.14021).
To examine the impact of these changes, Dr. Moon and his associates performed a retrospective cohort study, analyzing the electronic medical records of all 105,369 HCV treatment regimens given to 78,947 VA patients (mean age, 56 years) during a 17-year period. They found that annual treatment rates more than doubled from 2,726 to 6,679 patients when pegylated interferon was introduced, declined for a while and then rose modestly to 4,900 patients when boceprevir and telaprevir were introduced, declined again to an all-time low of 2,609 and then rebounded to 9,180 patients when sofosbuvir and simeprevir were introduced, and finally skyrocketed to 31,028 patients when ledipasvir/sofosbuvir and paritaprevir/ritonavir/ombitasvir/dasabuvir were introduced.
Correspondingly, SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end. The improvement in SVR rates was even more pronounced among traditionally “hard to treat” cases, such as patients with concomitant cirrhosis (from 11.0% to 87.0%), decompensated cirrhosis (from 14.6% to 85.2%), highly refractory infection (from 16.4% to 89.3%), and genotype-1 infection (from 1.3% to 91.7%). “The number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015,” Dr. Moon and his associates said.
“We believe that our findings based on the VA health care system might be relevant and informative for other comprehensive health care systems,” providing proof-of-concept that similar results can be achieved if aggressive screening; affordable, tolerable treatment; and open access to all patients are implemented.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
Key clinical point: The number of VA patients with hepatitis C virus who have achieved a sustained virologic response has escalated so rapidly and so high that the disease may be eradicated in that health care system within a few years.
Major finding: SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end; the number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015.
Data source: A retrospective cohort study examining all 105,369 antiviral regimens administered within the VA in 1999-2016.
Disclosures: The VA Office of Research and Development funded the study. Dr. Moon and his associates reported having no relevant financial disclosures.
HCV testing stagnant among baby boomers
Despite the urging of the United States Preventive Services Task Force and other organizations in 2013, the percentage of baby boomers who underwent testing for hepatitis C (HCV) infection had barely changed 2 years later – from 12.3% in 2013 to 13.8% in 2015.
The numbers are particularly troubling because new and improved antiviral drugs offer cures that could forestall liver cancer, cirrhosis, and other potential complications, with shorter regimens and fewer side effects than older regimens.
Other reactions were more forceful. “Kind of pathetic, isn’t it?” said John D. Scott, MD, assistant director of the Hepatitis and Liver Clinic at Harborview Medical Center, and an associate professor of medicine at the University of Washington, Seattle.
The researchers analyzed 2013 and 2015 data from the National Health Interview Survey, which included records for 21,827 baby boomers with HCV testing data.
The slight increase overall of 12.3% to 13.8% was small but also statistically significant (P = .013). Some populations fared better: Compared with the privately insured, those with Medicare plus Medicaid were more likely to have been tested (prevalence ratio, 1.83; 95% confidence interval, 1.32-2.53), as were those only on Medicaid (PR, 1.35; 95% CI, 1.04-1.76), and those with military insurance (PR, 1.62; 95% CI, 1.16-2.26).
The study could be subject to recall bias, since it relied on participants’ self-reports.
The authors speculate that the higher prevalence of testing in those with military insurance may reflect efforts by the Veterans Health Administration to reduce the high prevalence of HCV-associated disease among veterans.
It’s entirely possible to increase testing rates, according to Dr. Scott, who has a grant from the Centers for Disease Control and Prevention to study ways to increase uptake. “Probably the easiest thing to do is just incorporate this information into your electronic medical record and make it part of your alerts and standard preventative practices. Try to automate a lot of this rather than remind a very busy primary care doctor of all the things they have to do,” he said.
For example, one strategy that Seattle’s King County has employed is to automatically notify the testing laboratory if an antibody test is positive. “The lab knows to keep that blood and run a second (nucleic acid) test without the patient having to come back. That has helped to get our confirmatory rates up,” said Dr. Scott.
More broadly, the importance of testing needs to be emphasized, according to Paul J. Thuluvath, MD, medical director at the Institute of Digestive Health and Liver Disease at Mercy Medical Center, Baltimore, and a professor of medicine and surgery at the University of Maryland. “We need everybody to buy into this: the primary care physicians, internists, and gynecologists. If they are not convinced of the importance of this, it’s not going to happen. And I don’t think many primary care physicians and internists are convinced yet,” he said.
Despite the urging of the United States Preventive Services Task Force and other organizations in 2013, the percentage of baby boomers who underwent testing for hepatitis C (HCV) infection had barely changed 2 years later – from 12.3% in 2013 to 13.8% in 2015.
The numbers are particularly troubling because new and improved antiviral drugs offer cures that could forestall liver cancer, cirrhosis, and other potential complications, with shorter regimens and fewer side effects than older regimens.
Other reactions were more forceful. “Kind of pathetic, isn’t it?” said John D. Scott, MD, assistant director of the Hepatitis and Liver Clinic at Harborview Medical Center, and an associate professor of medicine at the University of Washington, Seattle.
The researchers analyzed 2013 and 2015 data from the National Health Interview Survey, which included records for 21,827 baby boomers with HCV testing data.
The slight increase overall of 12.3% to 13.8% was small but also statistically significant (P = .013). Some populations fared better: Compared with the privately insured, those with Medicare plus Medicaid were more likely to have been tested (prevalence ratio, 1.83; 95% confidence interval, 1.32-2.53), as were those only on Medicaid (PR, 1.35; 95% CI, 1.04-1.76), and those with military insurance (PR, 1.62; 95% CI, 1.16-2.26).
The study could be subject to recall bias, since it relied on participants’ self-reports.
The authors speculate that the higher prevalence of testing in those with military insurance may reflect efforts by the Veterans Health Administration to reduce the high prevalence of HCV-associated disease among veterans.
It’s entirely possible to increase testing rates, according to Dr. Scott, who has a grant from the Centers for Disease Control and Prevention to study ways to increase uptake. “Probably the easiest thing to do is just incorporate this information into your electronic medical record and make it part of your alerts and standard preventative practices. Try to automate a lot of this rather than remind a very busy primary care doctor of all the things they have to do,” he said.
For example, one strategy that Seattle’s King County has employed is to automatically notify the testing laboratory if an antibody test is positive. “The lab knows to keep that blood and run a second (nucleic acid) test without the patient having to come back. That has helped to get our confirmatory rates up,” said Dr. Scott.
More broadly, the importance of testing needs to be emphasized, according to Paul J. Thuluvath, MD, medical director at the Institute of Digestive Health and Liver Disease at Mercy Medical Center, Baltimore, and a professor of medicine and surgery at the University of Maryland. “We need everybody to buy into this: the primary care physicians, internists, and gynecologists. If they are not convinced of the importance of this, it’s not going to happen. And I don’t think many primary care physicians and internists are convinced yet,” he said.
Despite the urging of the United States Preventive Services Task Force and other organizations in 2013, the percentage of baby boomers who underwent testing for hepatitis C (HCV) infection had barely changed 2 years later – from 12.3% in 2013 to 13.8% in 2015.
The numbers are particularly troubling because new and improved antiviral drugs offer cures that could forestall liver cancer, cirrhosis, and other potential complications, with shorter regimens and fewer side effects than older regimens.
Other reactions were more forceful. “Kind of pathetic, isn’t it?” said John D. Scott, MD, assistant director of the Hepatitis and Liver Clinic at Harborview Medical Center, and an associate professor of medicine at the University of Washington, Seattle.
The researchers analyzed 2013 and 2015 data from the National Health Interview Survey, which included records for 21,827 baby boomers with HCV testing data.
The slight increase overall of 12.3% to 13.8% was small but also statistically significant (P = .013). Some populations fared better: Compared with the privately insured, those with Medicare plus Medicaid were more likely to have been tested (prevalence ratio, 1.83; 95% confidence interval, 1.32-2.53), as were those only on Medicaid (PR, 1.35; 95% CI, 1.04-1.76), and those with military insurance (PR, 1.62; 95% CI, 1.16-2.26).
The study could be subject to recall bias, since it relied on participants’ self-reports.
The authors speculate that the higher prevalence of testing in those with military insurance may reflect efforts by the Veterans Health Administration to reduce the high prevalence of HCV-associated disease among veterans.
It’s entirely possible to increase testing rates, according to Dr. Scott, who has a grant from the Centers for Disease Control and Prevention to study ways to increase uptake. “Probably the easiest thing to do is just incorporate this information into your electronic medical record and make it part of your alerts and standard preventative practices. Try to automate a lot of this rather than remind a very busy primary care doctor of all the things they have to do,” he said.
For example, one strategy that Seattle’s King County has employed is to automatically notify the testing laboratory if an antibody test is positive. “The lab knows to keep that blood and run a second (nucleic acid) test without the patient having to come back. That has helped to get our confirmatory rates up,” said Dr. Scott.
More broadly, the importance of testing needs to be emphasized, according to Paul J. Thuluvath, MD, medical director at the Institute of Digestive Health and Liver Disease at Mercy Medical Center, Baltimore, and a professor of medicine and surgery at the University of Maryland. “We need everybody to buy into this: the primary care physicians, internists, and gynecologists. If they are not convinced of the importance of this, it’s not going to happen. And I don’t think many primary care physicians and internists are convinced yet,” he said.
FROM AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Primary care physicians are not yet convinced of HCV test’s value.
Major finding: Between 2013 and 2015, HCV testing rates rose from 12.3% to 13.8%.
Data source: Retrospective analysis of 21,827 baby boomers who were part of the National Health Interview Survey.
Disclosures: The study was funded by the American Cancer Society. Dr. Fedewa reported having no financial disclosures. Dr. Scott has received research funding from Merck and serves on the data safety and monitoring board for Tacere Therapeutics. Dr. Thuluvath has received funding from Gilead and has received speaking fees from Gilead and AbbVie.