User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


No sedation fails to improve mortality in mechanically ventilated patients
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
REPORTING FROM CCC49
Antibiotic resistance rises among pneumococcus strains in kids
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
FROM CLINICAL INFECTIOUS DISEASES
U.S. reports first death from COVID-19, possible outbreak at long-term care facility
The first death in the United States from the novel coronavirus (COVID-19) was a Washington state man in his 50s who had underlying health conditions, state health officials announced on Feb 29. At the same time, officials there are investigating a possible COVID-19 outbreak at a long-term care facility.
Washington state officials reported two other presumptive positive cases of COVID-19, both of whom are associated with LifeCare of Kirkland, Washington. One is a woman in her 70s who is a resident at the facility and the other is a woman in her 40s who is a health care worker at the facility.
Additionally, many residents and staff members at the facility have reported respiratory symptoms, according to Jeff Duchin, MD, health officer for public health in Seattle and King County. Among the more than 100 residents at the facility, 27 have respiratory symptoms; while among the 180 staff members, 25 have reported symptoms.
Overall, these reports bring the total number of U.S. COVID-19 cases detected by the public health system to 22, though that number is expected to climb as these investigations continue.
The general risk to the American public is still low, including residents in long-term care facilities, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during the Feb. 29 press briefing. Older people are are higher risk, however, and long-term care facilities should emphasize handwashing and the early identification of individuals with symptoms.
Dr. Duchin added that health care workers who are sick should stay home and that visitors should be screened for symptoms, the same advice offered to limit the spread of influenza at long-term care facilities.

The CDC briefing comes after President Trump held his own press conference at the White House where he identified the person who had died as being a woman in her 50s who was medically at risk.
During that press conference, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said that the current pattern of disease with COVID-19 suggests that 75%-80% of patients will have mild illness and recover, while 15%-20% will require advanced medical care.
For the most part, the more serious cases will occur in those who are elderly or have underlying medical conditions. There is “no indication” that individuals who recover from the virus are becoming re-infected, Dr. Fauci said.
The administration also announced a series of actions aimed at slowing the spread of the virus and responding to it. On March 2, President Trump will meet with leaders in the pharmaceutical industry at the White House to discuss vaccine development. The administration is also working to ensure an adequate supply of face masks. Vice President Mike Pence said there are currently more than 40 million masks available, but that the administration has received promises of 35 million more masks per month from manufacturers. Access to masks will be prioritized for high-risk health care workers, Vice President Pence said. “The average American does not need to go out and buy a mask,” he added.
Additionally, Vice President Pence announced new travel restrictions with Iran that would bar entry to the United States for any foreign national who visited Iran in the last 14 days. The federal government is also advising Americans not to travel to the regions in Italy and South Korea that have been most affected by COVID-19. The government is also working with officials in Italy and South Korea to conduct medical screening of anyone coming into the United States from those countries.
The first death in the United States from the novel coronavirus (COVID-19) was a Washington state man in his 50s who had underlying health conditions, state health officials announced on Feb 29. At the same time, officials there are investigating a possible COVID-19 outbreak at a long-term care facility.
Washington state officials reported two other presumptive positive cases of COVID-19, both of whom are associated with LifeCare of Kirkland, Washington. One is a woman in her 70s who is a resident at the facility and the other is a woman in her 40s who is a health care worker at the facility.
Additionally, many residents and staff members at the facility have reported respiratory symptoms, according to Jeff Duchin, MD, health officer for public health in Seattle and King County. Among the more than 100 residents at the facility, 27 have respiratory symptoms; while among the 180 staff members, 25 have reported symptoms.
Overall, these reports bring the total number of U.S. COVID-19 cases detected by the public health system to 22, though that number is expected to climb as these investigations continue.
The general risk to the American public is still low, including residents in long-term care facilities, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during the Feb. 29 press briefing. Older people are are higher risk, however, and long-term care facilities should emphasize handwashing and the early identification of individuals with symptoms.
Dr. Duchin added that health care workers who are sick should stay home and that visitors should be screened for symptoms, the same advice offered to limit the spread of influenza at long-term care facilities.

The CDC briefing comes after President Trump held his own press conference at the White House where he identified the person who had died as being a woman in her 50s who was medically at risk.
During that press conference, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said that the current pattern of disease with COVID-19 suggests that 75%-80% of patients will have mild illness and recover, while 15%-20% will require advanced medical care.
For the most part, the more serious cases will occur in those who are elderly or have underlying medical conditions. There is “no indication” that individuals who recover from the virus are becoming re-infected, Dr. Fauci said.
The administration also announced a series of actions aimed at slowing the spread of the virus and responding to it. On March 2, President Trump will meet with leaders in the pharmaceutical industry at the White House to discuss vaccine development. The administration is also working to ensure an adequate supply of face masks. Vice President Mike Pence said there are currently more than 40 million masks available, but that the administration has received promises of 35 million more masks per month from manufacturers. Access to masks will be prioritized for high-risk health care workers, Vice President Pence said. “The average American does not need to go out and buy a mask,” he added.
Additionally, Vice President Pence announced new travel restrictions with Iran that would bar entry to the United States for any foreign national who visited Iran in the last 14 days. The federal government is also advising Americans not to travel to the regions in Italy and South Korea that have been most affected by COVID-19. The government is also working with officials in Italy and South Korea to conduct medical screening of anyone coming into the United States from those countries.
The first death in the United States from the novel coronavirus (COVID-19) was a Washington state man in his 50s who had underlying health conditions, state health officials announced on Feb 29. At the same time, officials there are investigating a possible COVID-19 outbreak at a long-term care facility.
Washington state officials reported two other presumptive positive cases of COVID-19, both of whom are associated with LifeCare of Kirkland, Washington. One is a woman in her 70s who is a resident at the facility and the other is a woman in her 40s who is a health care worker at the facility.
Additionally, many residents and staff members at the facility have reported respiratory symptoms, according to Jeff Duchin, MD, health officer for public health in Seattle and King County. Among the more than 100 residents at the facility, 27 have respiratory symptoms; while among the 180 staff members, 25 have reported symptoms.
Overall, these reports bring the total number of U.S. COVID-19 cases detected by the public health system to 22, though that number is expected to climb as these investigations continue.
The general risk to the American public is still low, including residents in long-term care facilities, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during the Feb. 29 press briefing. Older people are are higher risk, however, and long-term care facilities should emphasize handwashing and the early identification of individuals with symptoms.
Dr. Duchin added that health care workers who are sick should stay home and that visitors should be screened for symptoms, the same advice offered to limit the spread of influenza at long-term care facilities.

The CDC briefing comes after President Trump held his own press conference at the White House where he identified the person who had died as being a woman in her 50s who was medically at risk.
During that press conference, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said that the current pattern of disease with COVID-19 suggests that 75%-80% of patients will have mild illness and recover, while 15%-20% will require advanced medical care.
For the most part, the more serious cases will occur in those who are elderly or have underlying medical conditions. There is “no indication” that individuals who recover from the virus are becoming re-infected, Dr. Fauci said.
The administration also announced a series of actions aimed at slowing the spread of the virus and responding to it. On March 2, President Trump will meet with leaders in the pharmaceutical industry at the White House to discuss vaccine development. The administration is also working to ensure an adequate supply of face masks. Vice President Mike Pence said there are currently more than 40 million masks available, but that the administration has received promises of 35 million more masks per month from manufacturers. Access to masks will be prioritized for high-risk health care workers, Vice President Pence said. “The average American does not need to go out and buy a mask,” he added.
Additionally, Vice President Pence announced new travel restrictions with Iran that would bar entry to the United States for any foreign national who visited Iran in the last 14 days. The federal government is also advising Americans not to travel to the regions in Italy and South Korea that have been most affected by COVID-19. The government is also working with officials in Italy and South Korea to conduct medical screening of anyone coming into the United States from those countries.
CDC revises COVID-19 test kits, broadens ‘person under investigation’ definition
In a telebriefing on the COVID-19 outbreak, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at .

The definition has been revised “to meet the needs of this rapidly evolving situation,” she said. The new PUI definition includes travel to more geographic areas to reflect this past week’s marked uptick in coronavirus activity in Italy and Iran. In addition to these countries and China, recent travel to Japan or South Korea also constitutes an epidemiologic risk factor which, in conjunction with clinical features, warrant an individual being classified as a PUI. These five countries each now have widespread person-to-person transmission of the virus.
Dr. Messonnier left open the possibility that the PUI definition would continue to evolve if such transmission within communities becomes more common. Asked whether the small number of U.S. cases thus might be an artifact of low test volumes, she said, “We aggressively controlled our borders to slow the spread. This was an intentional U.S. strategy. The CDC has always had the capacity to test rapidly from the time the sequence was available. ...We have been testing aggressively.”
The original PUI definition, she explained, emphasized individuals with fever, cough, or trouble breathing who had traveled recently from areas with COVID-19 activity, in particular China’s Hubei province. “We have been most focused on symptomatic people who are closely linked to, or who had, travel history, but our criteria also allow for clinical discretion,” she said. “There is no substitute for an astute clinician on the front lines of patient care.”
The first COVID-19 case from person-to-person spread was reported on Feb. 27. “At this time, we don’t know how or where this person became infected,” said Dr. Messonnier, although investigations are still underway. She responded to a question about whether the CDC delayed allowing COVID-19 testing for the patient for several days, as was reported in some media accounts. “According to CDC records, the first call we got was Feb. 23,” when public health officials in California reported a severely ill person with no travel abroad and no known contacts with individuals that would trigger suspicions for coronavirus. The CDC recommended COVID-19 testing on that day, she said.
Dr. Messonnier declined to answer questions about a whistleblower report alleging improper training and inadequate protective measures for Department of Health & Human Services workers at the quarantine center at Travis Air Force Base, Calif.
Dr. Messonnier said that the CDC has been working closely with the Food and Drug Administration to address problems with the COVID-19 test kits that were unusable because of a large number of indeterminate results. The two agencies together have determined that of the three reactions that were initially deemed necessary for a definitive COVID-19 diagnosis, just two are sufficient, so new kits that omit the problematic chemical are being manufactured and distributed.
These new kits are rapidly being made available; the goal, said Dr. Messonnier, is to have to state and local public health departments equipped with test kits by about March 7.
As local tests become available, the most updated information will be coming from state and local public health departments, she stressed, adding that the CDC would continue to update case counts on Monday, Wednesday, and Friday of each week. Procedures are being developed for the management of patients presumed to have COVID-19, where local health departments see positive tests but the mandatory CDC confirmatory test hasn’t been completed.
While new cases emerge across Europe and Asia, China’s earlier COVID-19 explosion seems to be slowing. “It’s really good news that the case counts in China are decreasing,” both for the well-being of that country’s citizens, and as a sign of the disease’s potential global effects, said Dr. Messonnier. She added that epidemiologists and mathematical modelers are parsing case fatality rates as well.
She advised health care providers and public health officials to keep abreast of changes in CDC guidance by checking frequently at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
In a telebriefing on the COVID-19 outbreak, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at .

The definition has been revised “to meet the needs of this rapidly evolving situation,” she said. The new PUI definition includes travel to more geographic areas to reflect this past week’s marked uptick in coronavirus activity in Italy and Iran. In addition to these countries and China, recent travel to Japan or South Korea also constitutes an epidemiologic risk factor which, in conjunction with clinical features, warrant an individual being classified as a PUI. These five countries each now have widespread person-to-person transmission of the virus.
Dr. Messonnier left open the possibility that the PUI definition would continue to evolve if such transmission within communities becomes more common. Asked whether the small number of U.S. cases thus might be an artifact of low test volumes, she said, “We aggressively controlled our borders to slow the spread. This was an intentional U.S. strategy. The CDC has always had the capacity to test rapidly from the time the sequence was available. ...We have been testing aggressively.”
The original PUI definition, she explained, emphasized individuals with fever, cough, or trouble breathing who had traveled recently from areas with COVID-19 activity, in particular China’s Hubei province. “We have been most focused on symptomatic people who are closely linked to, or who had, travel history, but our criteria also allow for clinical discretion,” she said. “There is no substitute for an astute clinician on the front lines of patient care.”
The first COVID-19 case from person-to-person spread was reported on Feb. 27. “At this time, we don’t know how or where this person became infected,” said Dr. Messonnier, although investigations are still underway. She responded to a question about whether the CDC delayed allowing COVID-19 testing for the patient for several days, as was reported in some media accounts. “According to CDC records, the first call we got was Feb. 23,” when public health officials in California reported a severely ill person with no travel abroad and no known contacts with individuals that would trigger suspicions for coronavirus. The CDC recommended COVID-19 testing on that day, she said.
Dr. Messonnier declined to answer questions about a whistleblower report alleging improper training and inadequate protective measures for Department of Health & Human Services workers at the quarantine center at Travis Air Force Base, Calif.
Dr. Messonnier said that the CDC has been working closely with the Food and Drug Administration to address problems with the COVID-19 test kits that were unusable because of a large number of indeterminate results. The two agencies together have determined that of the three reactions that were initially deemed necessary for a definitive COVID-19 diagnosis, just two are sufficient, so new kits that omit the problematic chemical are being manufactured and distributed.
These new kits are rapidly being made available; the goal, said Dr. Messonnier, is to have to state and local public health departments equipped with test kits by about March 7.
As local tests become available, the most updated information will be coming from state and local public health departments, she stressed, adding that the CDC would continue to update case counts on Monday, Wednesday, and Friday of each week. Procedures are being developed for the management of patients presumed to have COVID-19, where local health departments see positive tests but the mandatory CDC confirmatory test hasn’t been completed.
While new cases emerge across Europe and Asia, China’s earlier COVID-19 explosion seems to be slowing. “It’s really good news that the case counts in China are decreasing,” both for the well-being of that country’s citizens, and as a sign of the disease’s potential global effects, said Dr. Messonnier. She added that epidemiologists and mathematical modelers are parsing case fatality rates as well.
She advised health care providers and public health officials to keep abreast of changes in CDC guidance by checking frequently at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
In a telebriefing on the COVID-19 outbreak, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at .

The definition has been revised “to meet the needs of this rapidly evolving situation,” she said. The new PUI definition includes travel to more geographic areas to reflect this past week’s marked uptick in coronavirus activity in Italy and Iran. In addition to these countries and China, recent travel to Japan or South Korea also constitutes an epidemiologic risk factor which, in conjunction with clinical features, warrant an individual being classified as a PUI. These five countries each now have widespread person-to-person transmission of the virus.
Dr. Messonnier left open the possibility that the PUI definition would continue to evolve if such transmission within communities becomes more common. Asked whether the small number of U.S. cases thus might be an artifact of low test volumes, she said, “We aggressively controlled our borders to slow the spread. This was an intentional U.S. strategy. The CDC has always had the capacity to test rapidly from the time the sequence was available. ...We have been testing aggressively.”
The original PUI definition, she explained, emphasized individuals with fever, cough, or trouble breathing who had traveled recently from areas with COVID-19 activity, in particular China’s Hubei province. “We have been most focused on symptomatic people who are closely linked to, or who had, travel history, but our criteria also allow for clinical discretion,” she said. “There is no substitute for an astute clinician on the front lines of patient care.”
The first COVID-19 case from person-to-person spread was reported on Feb. 27. “At this time, we don’t know how or where this person became infected,” said Dr. Messonnier, although investigations are still underway. She responded to a question about whether the CDC delayed allowing COVID-19 testing for the patient for several days, as was reported in some media accounts. “According to CDC records, the first call we got was Feb. 23,” when public health officials in California reported a severely ill person with no travel abroad and no known contacts with individuals that would trigger suspicions for coronavirus. The CDC recommended COVID-19 testing on that day, she said.
Dr. Messonnier declined to answer questions about a whistleblower report alleging improper training and inadequate protective measures for Department of Health & Human Services workers at the quarantine center at Travis Air Force Base, Calif.
Dr. Messonnier said that the CDC has been working closely with the Food and Drug Administration to address problems with the COVID-19 test kits that were unusable because of a large number of indeterminate results. The two agencies together have determined that of the three reactions that were initially deemed necessary for a definitive COVID-19 diagnosis, just two are sufficient, so new kits that omit the problematic chemical are being manufactured and distributed.
These new kits are rapidly being made available; the goal, said Dr. Messonnier, is to have to state and local public health departments equipped with test kits by about March 7.
As local tests become available, the most updated information will be coming from state and local public health departments, she stressed, adding that the CDC would continue to update case counts on Monday, Wednesday, and Friday of each week. Procedures are being developed for the management of patients presumed to have COVID-19, where local health departments see positive tests but the mandatory CDC confirmatory test hasn’t been completed.
While new cases emerge across Europe and Asia, China’s earlier COVID-19 explosion seems to be slowing. “It’s really good news that the case counts in China are decreasing,” both for the well-being of that country’s citizens, and as a sign of the disease’s potential global effects, said Dr. Messonnier. She added that epidemiologists and mathematical modelers are parsing case fatality rates as well.
She advised health care providers and public health officials to keep abreast of changes in CDC guidance by checking frequently at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
REPORTING FROM A CDC BRIEFING
Children bearing the brunt of declining flu activity
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
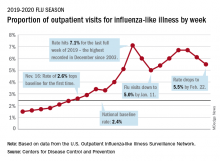
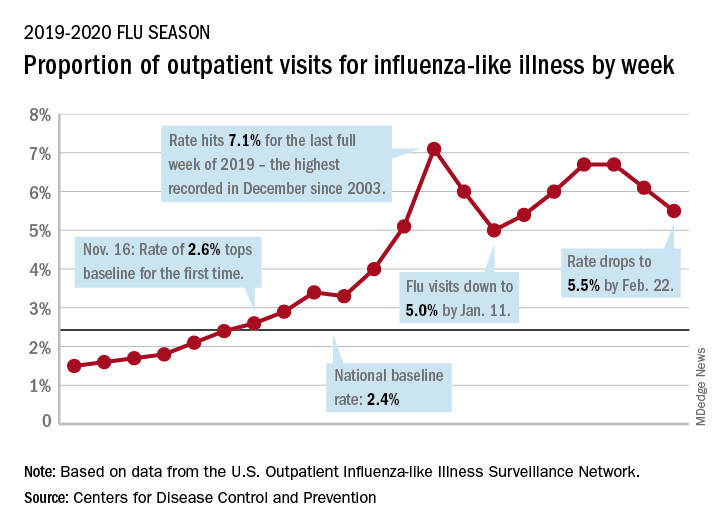
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
What hospitalists need to know about COVID-19
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
Hospitalist profile: Vineet Chopra, MD, MSc, FHM
Vineet Chopra, MD, MSc, FHM, is associate professor of medicine and chief of the Division of Hospital Medicine at Michigan Medicine and the VA Ann Arbor (Michigan) Health System. A career hospitalist, Dr. Chopra’s research is dedicated to improving the safety of hospitalized patients through prevention of hospital-acquired complications. His work focuses on identifying and preventing complications associated with central venous catheters with a particular emphasis on peripherally inserted central catheters (PICCs).
Dr. Chopra is the recipient of numerous teaching and research awards including the 2016 Kaiser Permanente Award for Clinical Teaching, the Jerome W. Conn Award for Outstanding Research in the Department of Medicine, the 2016 Society of Hospital Medicine Award for Excellence in Research, and the 2014 McDevitt Award for Research Excellence. He has published over 100 peer-reviewed articles and has served as associate editor for the American Journal of Medicine and Journal of Hospital Medicine.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
I think I knew very early – toward the middle of my intern year – that I wanted to be a hospitalist. There was much that drew me to the field. First, I loved being in the inpatient setting. The tempo of work, the unexpected nature of what may come next, and the opportunity to truly have an impact on a patients life at their time of greatest need appealed to me. I wasn’t as inclined towards the procedural fields and also loved the cognitive aspects of general medicine – doing the work up on a difficult diagnosis or medically managing a patient with acute coronary syndrome came naturally. I found myself loving the work so much so that it didn’t feel like work. And the rest was history!
What is your current role at Michigan Medicine?
I started at Michigan Medicine in 2008 as a full-time clinician taking care of patients on direct care and resident services. After 3 years of clinical work, I decided it was time to hone in on a specific skill set and went back to a research fellowship.
I become Michigan’s first fellow in hospital medicine – the guinea pig – for what would turn out to be one of the best decisions in my life. After finishing fellowship, I switched my focus from clinical work to research and rose up the ranks to receive tenure as an associate professor of medicine. After attaining tenure, I was among a handful of people in the nation who had success in both the research and the clinical arenas and leadership opportunities began to come my way.
I was fortunate to be recruited as the inaugural division chief of hospital medicine at Michigan Medicine in 2017. The Division of hospital medicine is the 13th in the department of medicine and the first one to be created in over 60 years. As division chief, I oversee all of our clinical, academic, research, and educational endeavors. Currently, we have approximately 130 hospitalists in our group and about 30 advanced practice providers (APPs) with a support and research staff of about 15 individuals. So I like to say we have a big family!
What are your favorite areas of clinical practice and/or research?
I am fortunate to have the ability to enjoy all that hospital medicine has to offer. I still appreciate the challenges that direct care brings, and I continue to do as much as I can in this area. I also enjoy working with residents and medical students at the university and at our VA site – where much of my focus is devoted to making sure all learners on the team are growing while they provide excellent patient care. To meet a new patient and work to develop a therapeutic relationship with them such that we can make positive changes in their disease trajectory remains my favorite part of clinical work.
My research work remains closely linked to my clinical interests around preventing patient harm and improving patient safety – so studying hospital-acquired infections, coming up with new ideas and strategies, and then implementing them when on clinical service represents the perfect blend of the two. My research is largely focused on intravenous devices and catheters, and I focus my work on preventing harms such as bloodstream infection, venous thrombosis, and related adverse events. I have been fortunate to receive national and international attention for my research, including adoption of my work into guidelines and changes to national policies. I am honored to serve on the most important federal advisory committee that advises the government on health care infections (the committee is called HICPAC – Healthcare Infection Control Practice Advisory Committee).
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
For me, the most challenging aspects are also the most rewarding. First and foremost, making a connection with a patient and their family to understand their concerns and define a therapeutic alliance is both challenging and rewarding. Second, ensuring that we have the ability to work efficiently and effectively to manage patient care is sometimes challenging but also the most rewarding aspect of the job. I am fortunate to work in a health system where I am surrounded by smart colleagues, important resources, advanced technology, and the support of nurses and advanced practice providers who share this zeal of patient care with me.
Finally, one the greatest challenges and rewards remains time. Our work is hard and grueling, and it is often very challenging to get things done at different times of the day. But the ability to make a diagnosis or see a patient improve makes it all worth it!
How will hospital medicine change in the next decade or two?
I predict our work will shift from a model that is reactive – taking care of patients that are sick and need hospitalization – to a proactive approach where the focus will remain on keeping people out of the hospital. This doesn’t necessarily mean that we will be out of a job – but I see the model of our work shifting to ensure that patients who are discharged remain healthy and well. This means we will need to embrace extensivist models, hospital at home care, and aspects such as bridge clinics.
I also think our work will evolve to harness some of the incredible technology that surrounds us outside health care, but has not yet permeated our work flow. To that end, aspects such as virtual consultations and patient assessments, and remote monitoring that includes biometrics, will all fall into our workflow. And of course, lets not forget about the mighty electronic medical record and how that will affect our experience and work. I see much more of our work shifting toward becoming digital experts, harnessing the power of big data and predictive analytics to provide better care for patients. These are skills that are emerging in our field, but we have not yet mastered the art of managing data.
Do you have any advice for students and residents interested in hospital medicine?
I would highly recommend taking on a rotation with a hospitalist, carrying the pager and working side-by-side with someone who truly loves what they do. Many students and residents just see the on/off nature of the work, but that is truly skin deep in terms of attraction.
The beauty of hospital medicine is that you can be everything for a patient – their doctor, their health care navigator, their friend, and their partner during their hospital stay. Find that joy – you will not regret it!
Vineet Chopra, MD, MSc, FHM, is associate professor of medicine and chief of the Division of Hospital Medicine at Michigan Medicine and the VA Ann Arbor (Michigan) Health System. A career hospitalist, Dr. Chopra’s research is dedicated to improving the safety of hospitalized patients through prevention of hospital-acquired complications. His work focuses on identifying and preventing complications associated with central venous catheters with a particular emphasis on peripherally inserted central catheters (PICCs).
Dr. Chopra is the recipient of numerous teaching and research awards including the 2016 Kaiser Permanente Award for Clinical Teaching, the Jerome W. Conn Award for Outstanding Research in the Department of Medicine, the 2016 Society of Hospital Medicine Award for Excellence in Research, and the 2014 McDevitt Award for Research Excellence. He has published over 100 peer-reviewed articles and has served as associate editor for the American Journal of Medicine and Journal of Hospital Medicine.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
I think I knew very early – toward the middle of my intern year – that I wanted to be a hospitalist. There was much that drew me to the field. First, I loved being in the inpatient setting. The tempo of work, the unexpected nature of what may come next, and the opportunity to truly have an impact on a patients life at their time of greatest need appealed to me. I wasn’t as inclined towards the procedural fields and also loved the cognitive aspects of general medicine – doing the work up on a difficult diagnosis or medically managing a patient with acute coronary syndrome came naturally. I found myself loving the work so much so that it didn’t feel like work. And the rest was history!
What is your current role at Michigan Medicine?
I started at Michigan Medicine in 2008 as a full-time clinician taking care of patients on direct care and resident services. After 3 years of clinical work, I decided it was time to hone in on a specific skill set and went back to a research fellowship.
I become Michigan’s first fellow in hospital medicine – the guinea pig – for what would turn out to be one of the best decisions in my life. After finishing fellowship, I switched my focus from clinical work to research and rose up the ranks to receive tenure as an associate professor of medicine. After attaining tenure, I was among a handful of people in the nation who had success in both the research and the clinical arenas and leadership opportunities began to come my way.
I was fortunate to be recruited as the inaugural division chief of hospital medicine at Michigan Medicine in 2017. The Division of hospital medicine is the 13th in the department of medicine and the first one to be created in over 60 years. As division chief, I oversee all of our clinical, academic, research, and educational endeavors. Currently, we have approximately 130 hospitalists in our group and about 30 advanced practice providers (APPs) with a support and research staff of about 15 individuals. So I like to say we have a big family!
What are your favorite areas of clinical practice and/or research?
I am fortunate to have the ability to enjoy all that hospital medicine has to offer. I still appreciate the challenges that direct care brings, and I continue to do as much as I can in this area. I also enjoy working with residents and medical students at the university and at our VA site – where much of my focus is devoted to making sure all learners on the team are growing while they provide excellent patient care. To meet a new patient and work to develop a therapeutic relationship with them such that we can make positive changes in their disease trajectory remains my favorite part of clinical work.
My research work remains closely linked to my clinical interests around preventing patient harm and improving patient safety – so studying hospital-acquired infections, coming up with new ideas and strategies, and then implementing them when on clinical service represents the perfect blend of the two. My research is largely focused on intravenous devices and catheters, and I focus my work on preventing harms such as bloodstream infection, venous thrombosis, and related adverse events. I have been fortunate to receive national and international attention for my research, including adoption of my work into guidelines and changes to national policies. I am honored to serve on the most important federal advisory committee that advises the government on health care infections (the committee is called HICPAC – Healthcare Infection Control Practice Advisory Committee).
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
For me, the most challenging aspects are also the most rewarding. First and foremost, making a connection with a patient and their family to understand their concerns and define a therapeutic alliance is both challenging and rewarding. Second, ensuring that we have the ability to work efficiently and effectively to manage patient care is sometimes challenging but also the most rewarding aspect of the job. I am fortunate to work in a health system where I am surrounded by smart colleagues, important resources, advanced technology, and the support of nurses and advanced practice providers who share this zeal of patient care with me.
Finally, one the greatest challenges and rewards remains time. Our work is hard and grueling, and it is often very challenging to get things done at different times of the day. But the ability to make a diagnosis or see a patient improve makes it all worth it!
How will hospital medicine change in the next decade or two?
I predict our work will shift from a model that is reactive – taking care of patients that are sick and need hospitalization – to a proactive approach where the focus will remain on keeping people out of the hospital. This doesn’t necessarily mean that we will be out of a job – but I see the model of our work shifting to ensure that patients who are discharged remain healthy and well. This means we will need to embrace extensivist models, hospital at home care, and aspects such as bridge clinics.
I also think our work will evolve to harness some of the incredible technology that surrounds us outside health care, but has not yet permeated our work flow. To that end, aspects such as virtual consultations and patient assessments, and remote monitoring that includes biometrics, will all fall into our workflow. And of course, lets not forget about the mighty electronic medical record and how that will affect our experience and work. I see much more of our work shifting toward becoming digital experts, harnessing the power of big data and predictive analytics to provide better care for patients. These are skills that are emerging in our field, but we have not yet mastered the art of managing data.
Do you have any advice for students and residents interested in hospital medicine?
I would highly recommend taking on a rotation with a hospitalist, carrying the pager and working side-by-side with someone who truly loves what they do. Many students and residents just see the on/off nature of the work, but that is truly skin deep in terms of attraction.
The beauty of hospital medicine is that you can be everything for a patient – their doctor, their health care navigator, their friend, and their partner during their hospital stay. Find that joy – you will not regret it!
Vineet Chopra, MD, MSc, FHM, is associate professor of medicine and chief of the Division of Hospital Medicine at Michigan Medicine and the VA Ann Arbor (Michigan) Health System. A career hospitalist, Dr. Chopra’s research is dedicated to improving the safety of hospitalized patients through prevention of hospital-acquired complications. His work focuses on identifying and preventing complications associated with central venous catheters with a particular emphasis on peripherally inserted central catheters (PICCs).
Dr. Chopra is the recipient of numerous teaching and research awards including the 2016 Kaiser Permanente Award for Clinical Teaching, the Jerome W. Conn Award for Outstanding Research in the Department of Medicine, the 2016 Society of Hospital Medicine Award for Excellence in Research, and the 2014 McDevitt Award for Research Excellence. He has published over 100 peer-reviewed articles and has served as associate editor for the American Journal of Medicine and Journal of Hospital Medicine.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
I think I knew very early – toward the middle of my intern year – that I wanted to be a hospitalist. There was much that drew me to the field. First, I loved being in the inpatient setting. The tempo of work, the unexpected nature of what may come next, and the opportunity to truly have an impact on a patients life at their time of greatest need appealed to me. I wasn’t as inclined towards the procedural fields and also loved the cognitive aspects of general medicine – doing the work up on a difficult diagnosis or medically managing a patient with acute coronary syndrome came naturally. I found myself loving the work so much so that it didn’t feel like work. And the rest was history!
What is your current role at Michigan Medicine?
I started at Michigan Medicine in 2008 as a full-time clinician taking care of patients on direct care and resident services. After 3 years of clinical work, I decided it was time to hone in on a specific skill set and went back to a research fellowship.
I become Michigan’s first fellow in hospital medicine – the guinea pig – for what would turn out to be one of the best decisions in my life. After finishing fellowship, I switched my focus from clinical work to research and rose up the ranks to receive tenure as an associate professor of medicine. After attaining tenure, I was among a handful of people in the nation who had success in both the research and the clinical arenas and leadership opportunities began to come my way.
I was fortunate to be recruited as the inaugural division chief of hospital medicine at Michigan Medicine in 2017. The Division of hospital medicine is the 13th in the department of medicine and the first one to be created in over 60 years. As division chief, I oversee all of our clinical, academic, research, and educational endeavors. Currently, we have approximately 130 hospitalists in our group and about 30 advanced practice providers (APPs) with a support and research staff of about 15 individuals. So I like to say we have a big family!
What are your favorite areas of clinical practice and/or research?
I am fortunate to have the ability to enjoy all that hospital medicine has to offer. I still appreciate the challenges that direct care brings, and I continue to do as much as I can in this area. I also enjoy working with residents and medical students at the university and at our VA site – where much of my focus is devoted to making sure all learners on the team are growing while they provide excellent patient care. To meet a new patient and work to develop a therapeutic relationship with them such that we can make positive changes in their disease trajectory remains my favorite part of clinical work.
My research work remains closely linked to my clinical interests around preventing patient harm and improving patient safety – so studying hospital-acquired infections, coming up with new ideas and strategies, and then implementing them when on clinical service represents the perfect blend of the two. My research is largely focused on intravenous devices and catheters, and I focus my work on preventing harms such as bloodstream infection, venous thrombosis, and related adverse events. I have been fortunate to receive national and international attention for my research, including adoption of my work into guidelines and changes to national policies. I am honored to serve on the most important federal advisory committee that advises the government on health care infections (the committee is called HICPAC – Healthcare Infection Control Practice Advisory Committee).
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
For me, the most challenging aspects are also the most rewarding. First and foremost, making a connection with a patient and their family to understand their concerns and define a therapeutic alliance is both challenging and rewarding. Second, ensuring that we have the ability to work efficiently and effectively to manage patient care is sometimes challenging but also the most rewarding aspect of the job. I am fortunate to work in a health system where I am surrounded by smart colleagues, important resources, advanced technology, and the support of nurses and advanced practice providers who share this zeal of patient care with me.
Finally, one the greatest challenges and rewards remains time. Our work is hard and grueling, and it is often very challenging to get things done at different times of the day. But the ability to make a diagnosis or see a patient improve makes it all worth it!
How will hospital medicine change in the next decade or two?
I predict our work will shift from a model that is reactive – taking care of patients that are sick and need hospitalization – to a proactive approach where the focus will remain on keeping people out of the hospital. This doesn’t necessarily mean that we will be out of a job – but I see the model of our work shifting to ensure that patients who are discharged remain healthy and well. This means we will need to embrace extensivist models, hospital at home care, and aspects such as bridge clinics.
I also think our work will evolve to harness some of the incredible technology that surrounds us outside health care, but has not yet permeated our work flow. To that end, aspects such as virtual consultations and patient assessments, and remote monitoring that includes biometrics, will all fall into our workflow. And of course, lets not forget about the mighty electronic medical record and how that will affect our experience and work. I see much more of our work shifting toward becoming digital experts, harnessing the power of big data and predictive analytics to provide better care for patients. These are skills that are emerging in our field, but we have not yet mastered the art of managing data.
Do you have any advice for students and residents interested in hospital medicine?
I would highly recommend taking on a rotation with a hospitalist, carrying the pager and working side-by-side with someone who truly loves what they do. Many students and residents just see the on/off nature of the work, but that is truly skin deep in terms of attraction.
The beauty of hospital medicine is that you can be everything for a patient – their doctor, their health care navigator, their friend, and their partner during their hospital stay. Find that joy – you will not regret it!
Labor & Delivery: An overlooked entry point for the spread of viral infection
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
OB hospitalists have a key role to play
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
Pence named COVID-19 point person as CDC reports possible community spread
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
AI algorithm finds diagnostic AFib signatures in normal ECGs
NATIONAL HARBOR, MD. – Researchers have created an artificial intelligence algorithm that can evaluate a 10-second ECG recording of a person in normal sinus rhythm and tell with a sensitivity and specificity of almost 80% whether or not that person ever had atrial fibrillation episodes some time in the past or will have a first arrhythmia episode in the near future.
Although this algorithm – derived from and then validated with a dataset of nearly 650,000 ECG recordings from more than 180,000 patients – still needs prospective validation, it offers the prospect for a potential revolution in screening for atrial fibrillation (AFib), Paul A. Friedman, MD, cautioned at the annual International AF Symposium. If initial clinical findings are confirmed, it would show that a 10-second, 12-lead ECG recording can provide the same screening scope as what otherwise takes weeks of ambulatory ECG recording with a Holter monitor or an implanted device, explained Dr. Friedman, professor of medicine and chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
This finding “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” Dr. Friedman and his associates noted in the published report of their study (Lancet. 2019 Sep 7;394[10201]:861-7). “We’re still working to define the window of ECG” recording time that provides the optimal assessment for a history of asymptomatic AFib, but the “possibilities this opens are huge,” Dr. Friedman said in his talk at the symposium. This work sprang from the premise that “subtle signatures” in a brief, apparently normal sinus rhythm ECG tracing can harbor reliable clues about AFib history or an imminent episode.
The 2019 report by Dr. Friedman and associates documented that in the validation phase of their study, the trained artificial intelligence (AI) program identified patients with a history of AFib or an impending arrhythmia event from a single, 10-second ECG that to the naked eye seemed to show normal sinus rhythm with a sensitivity of 79.0%, a specificity of 79.5%, and an accuracy of 79.4%. It also showed an area under a receiver operating characteristic curve of 0.87, meaning that screening for AFib by this method compared favorably with the area-under-the-curve (AUC) results tallied by several widely accepted screening tools, including Pap smears for cervical cancer (AUC of 0.70), mammograms for breast cancer (AUC of 0.85), and CHA2DS2-VASc scoring for estimating stroke risk in AFib patients (AUC of 0.57-0.72), Dr. Friedman said.
The researchers developed the AI algorithm with more than 450,000 10-second ECG tracings collected from roughly 126,000 patients who underwent at least one ECG recording as part of their routine care at the Mayo Clinic during 1993-2017. The goal was for the program to find and validate recurring characteristics in the ECG that consistently linked with a history of or an impending AFib episode and that did not appear in ECG recordings from people without any AFib history. The program this effort produced then underwent further adjustment with the use of more than 64,340 ECGs from an additional 18,116 patients, and then the final product underwent validation testing with a further 130,802 ECGs collected from an additional 36,280 people, the study phase that resulted in the reported sensitivity and specificity estimates.
It’s currently unclear to Dr. Friedman and associates what specific features the program uses to classify patients. It’s an important question, but if the results are reproducible and reliable, this uncertainty shouldn’t slow clinical adoption, he said in an interview.
While “this particular algorithm needs prospective vetting,” a similar algorithm developed by Dr. Friedman and the same research team that uses a 10-second ECG to identify patients with a left ventricular ejection fraction of 35% or less is further advanced in development, and a device that uses this algorithm will soon receive Food and Drug Administration review under a fast track designation that the agency approved in late 2019.
The researchers developed this algorithm for estimating left ventricular function using a strategy similar to their development of a tool for diagnosing AFib (Nat Med. 2019 Jan 7;25[1]:70-4), and results from 100 patients prospectively studied with this approach to ECG analysis and reported at the American Heart Association scientific sessions in November 2019 showed that the algorithm identified substantial left ventricular dysfunction with an AUC of 0.906 (Circulation. 2019 Nov 19;140[suppl 1]:A13447). The same team of investigators has developed an AI algorithm that can calculate a person’s physiologic age based on the ECG recording (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]: 10.1161/CIRCEP.119.007284).
The study received no commercial funding, and Dr. Friedman and coauthors had no relevant disclosures. The Mayo Clinic has licensed a related artificial intelligence algorithm to EKO, and Dr. Friedman may benefit financially from this arrangement.
NATIONAL HARBOR, MD. – Researchers have created an artificial intelligence algorithm that can evaluate a 10-second ECG recording of a person in normal sinus rhythm and tell with a sensitivity and specificity of almost 80% whether or not that person ever had atrial fibrillation episodes some time in the past or will have a first arrhythmia episode in the near future.
Although this algorithm – derived from and then validated with a dataset of nearly 650,000 ECG recordings from more than 180,000 patients – still needs prospective validation, it offers the prospect for a potential revolution in screening for atrial fibrillation (AFib), Paul A. Friedman, MD, cautioned at the annual International AF Symposium. If initial clinical findings are confirmed, it would show that a 10-second, 12-lead ECG recording can provide the same screening scope as what otherwise takes weeks of ambulatory ECG recording with a Holter monitor or an implanted device, explained Dr. Friedman, professor of medicine and chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
This finding “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” Dr. Friedman and his associates noted in the published report of their study (Lancet. 2019 Sep 7;394[10201]:861-7). “We’re still working to define the window of ECG” recording time that provides the optimal assessment for a history of asymptomatic AFib, but the “possibilities this opens are huge,” Dr. Friedman said in his talk at the symposium. This work sprang from the premise that “subtle signatures” in a brief, apparently normal sinus rhythm ECG tracing can harbor reliable clues about AFib history or an imminent episode.
The 2019 report by Dr. Friedman and associates documented that in the validation phase of their study, the trained artificial intelligence (AI) program identified patients with a history of AFib or an impending arrhythmia event from a single, 10-second ECG that to the naked eye seemed to show normal sinus rhythm with a sensitivity of 79.0%, a specificity of 79.5%, and an accuracy of 79.4%. It also showed an area under a receiver operating characteristic curve of 0.87, meaning that screening for AFib by this method compared favorably with the area-under-the-curve (AUC) results tallied by several widely accepted screening tools, including Pap smears for cervical cancer (AUC of 0.70), mammograms for breast cancer (AUC of 0.85), and CHA2DS2-VASc scoring for estimating stroke risk in AFib patients (AUC of 0.57-0.72), Dr. Friedman said.
The researchers developed the AI algorithm with more than 450,000 10-second ECG tracings collected from roughly 126,000 patients who underwent at least one ECG recording as part of their routine care at the Mayo Clinic during 1993-2017. The goal was for the program to find and validate recurring characteristics in the ECG that consistently linked with a history of or an impending AFib episode and that did not appear in ECG recordings from people without any AFib history. The program this effort produced then underwent further adjustment with the use of more than 64,340 ECGs from an additional 18,116 patients, and then the final product underwent validation testing with a further 130,802 ECGs collected from an additional 36,280 people, the study phase that resulted in the reported sensitivity and specificity estimates.
It’s currently unclear to Dr. Friedman and associates what specific features the program uses to classify patients. It’s an important question, but if the results are reproducible and reliable, this uncertainty shouldn’t slow clinical adoption, he said in an interview.
While “this particular algorithm needs prospective vetting,” a similar algorithm developed by Dr. Friedman and the same research team that uses a 10-second ECG to identify patients with a left ventricular ejection fraction of 35% or less is further advanced in development, and a device that uses this algorithm will soon receive Food and Drug Administration review under a fast track designation that the agency approved in late 2019.
The researchers developed this algorithm for estimating left ventricular function using a strategy similar to their development of a tool for diagnosing AFib (Nat Med. 2019 Jan 7;25[1]:70-4), and results from 100 patients prospectively studied with this approach to ECG analysis and reported at the American Heart Association scientific sessions in November 2019 showed that the algorithm identified substantial left ventricular dysfunction with an AUC of 0.906 (Circulation. 2019 Nov 19;140[suppl 1]:A13447). The same team of investigators has developed an AI algorithm that can calculate a person’s physiologic age based on the ECG recording (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]: 10.1161/CIRCEP.119.007284).
The study received no commercial funding, and Dr. Friedman and coauthors had no relevant disclosures. The Mayo Clinic has licensed a related artificial intelligence algorithm to EKO, and Dr. Friedman may benefit financially from this arrangement.
NATIONAL HARBOR, MD. – Researchers have created an artificial intelligence algorithm that can evaluate a 10-second ECG recording of a person in normal sinus rhythm and tell with a sensitivity and specificity of almost 80% whether or not that person ever had atrial fibrillation episodes some time in the past or will have a first arrhythmia episode in the near future.
Although this algorithm – derived from and then validated with a dataset of nearly 650,000 ECG recordings from more than 180,000 patients – still needs prospective validation, it offers the prospect for a potential revolution in screening for atrial fibrillation (AFib), Paul A. Friedman, MD, cautioned at the annual International AF Symposium. If initial clinical findings are confirmed, it would show that a 10-second, 12-lead ECG recording can provide the same screening scope as what otherwise takes weeks of ambulatory ECG recording with a Holter monitor or an implanted device, explained Dr. Friedman, professor of medicine and chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
This finding “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” Dr. Friedman and his associates noted in the published report of their study (Lancet. 2019 Sep 7;394[10201]:861-7). “We’re still working to define the window of ECG” recording time that provides the optimal assessment for a history of asymptomatic AFib, but the “possibilities this opens are huge,” Dr. Friedman said in his talk at the symposium. This work sprang from the premise that “subtle signatures” in a brief, apparently normal sinus rhythm ECG tracing can harbor reliable clues about AFib history or an imminent episode.
The 2019 report by Dr. Friedman and associates documented that in the validation phase of their study, the trained artificial intelligence (AI) program identified patients with a history of AFib or an impending arrhythmia event from a single, 10-second ECG that to the naked eye seemed to show normal sinus rhythm with a sensitivity of 79.0%, a specificity of 79.5%, and an accuracy of 79.4%. It also showed an area under a receiver operating characteristic curve of 0.87, meaning that screening for AFib by this method compared favorably with the area-under-the-curve (AUC) results tallied by several widely accepted screening tools, including Pap smears for cervical cancer (AUC of 0.70), mammograms for breast cancer (AUC of 0.85), and CHA2DS2-VASc scoring for estimating stroke risk in AFib patients (AUC of 0.57-0.72), Dr. Friedman said.
The researchers developed the AI algorithm with more than 450,000 10-second ECG tracings collected from roughly 126,000 patients who underwent at least one ECG recording as part of their routine care at the Mayo Clinic during 1993-2017. The goal was for the program to find and validate recurring characteristics in the ECG that consistently linked with a history of or an impending AFib episode and that did not appear in ECG recordings from people without any AFib history. The program this effort produced then underwent further adjustment with the use of more than 64,340 ECGs from an additional 18,116 patients, and then the final product underwent validation testing with a further 130,802 ECGs collected from an additional 36,280 people, the study phase that resulted in the reported sensitivity and specificity estimates.
It’s currently unclear to Dr. Friedman and associates what specific features the program uses to classify patients. It’s an important question, but if the results are reproducible and reliable, this uncertainty shouldn’t slow clinical adoption, he said in an interview.
While “this particular algorithm needs prospective vetting,” a similar algorithm developed by Dr. Friedman and the same research team that uses a 10-second ECG to identify patients with a left ventricular ejection fraction of 35% or less is further advanced in development, and a device that uses this algorithm will soon receive Food and Drug Administration review under a fast track designation that the agency approved in late 2019.
The researchers developed this algorithm for estimating left ventricular function using a strategy similar to their development of a tool for diagnosing AFib (Nat Med. 2019 Jan 7;25[1]:70-4), and results from 100 patients prospectively studied with this approach to ECG analysis and reported at the American Heart Association scientific sessions in November 2019 showed that the algorithm identified substantial left ventricular dysfunction with an AUC of 0.906 (Circulation. 2019 Nov 19;140[suppl 1]:A13447). The same team of investigators has developed an AI algorithm that can calculate a person’s physiologic age based on the ECG recording (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]: 10.1161/CIRCEP.119.007284).
The study received no commercial funding, and Dr. Friedman and coauthors had no relevant disclosures. The Mayo Clinic has licensed a related artificial intelligence algorithm to EKO, and Dr. Friedman may benefit financially from this arrangement.
THE AF SYMPOSIUM 2020