User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


FDA warns about fecal microbiota for transplantation
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Rapid assay distinguishes viral from bacterial infection
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
REPORTING FROM ESPID 2019
Key clinical point: A novel real-time single-gene–expression PCR test quickly distinguishes viral from bacterial infection in febrile children.
Major finding: The expression signature of the IFI44L gene rapidly distinguished bacterial from viral infection in febrile children with 91% sensitivity and 93% specificity.
Study details: This translational study included 25 febrile children with definite bacterial or viral infections and 10 healthy controls.
Disclosures: The presenter reported having no financial conflicts regarding her study, supported by institutional funding.
HM19: One chapter’s experience
The Society of Hospital Medicine is an organization vested in improving the quality of inpatient medicine by empowering its members with education and providing venues for professional development including networking, advocacy, and leadership advancement. Every year, SHM holds a national conference which is a focused meeting point for over 5,000 hospitalists.
SHM hosts more than 50 local chapters nationwide to increase networking, education, and collaboration within the hospital medicine community. The Wiregrass chapter of SHM is based in the southeast corner of Alabama, covering the counties of lower Alabama and the panhandle of Florida. This year we were recognized as a platinum status chapter, which is the highest status, based on our work and participation to improve the quality of inpatient medicine.
As part of winning the platinum ribbon, we were awarded three complimentary registration scholarships to the SHM Annual Conference in 2019. The chapter leadership met and selected three individuals who have been involved with the chapter actively but have never had an opportunity to experience SHM’s Annual Conference. We selected a first-year resident, Dr. Avani Parrekh; a hospital medicine nurse practitioner, Madison Rivenbark; and a fourth-year medical student who is about to start his internal medicine residency, William Bancroft.
After the meeting we interviewed them to better understand their experience. Below are their thoughts.
Avani Parekh, MD
First year, Internal Medicine Residency
Southeast Health Medical Center
Dothan, Ala.
I am so thankful for the opportunity that was given to me by the Wiregrass chapter by sponsoring my attendance at the 2019 SHM Annual Conference in Washington. This was my first SHM conference, and it was truly a rewarding experience.
I thoroughly enjoyed attending the lectures. They were very informative and engaging. Every presenter was so passionate and inspiring. Coming from an “all-female class” of PGY-1 at my program, I especially enjoyed the “Fe(male) in medicine” talk, as well as Quick Talks on women in medicine. The “Updates in Hospital Medicine” session on various topics such as heart failure, pneumonia, and sepsis was outstanding. I was excited to apply the knowledge I gained from this event into my patient care.
Overall, it was a well-organized and up-to-date event. I am looking forward to attending more SHM conferences in the future.
Madison Rivenbark, NP
Department of Hospital Medicine
Southeast Health Medical Center
Dothan, Ala.
I was extremely fortunate to be selected to receive a scholarship that covered the conference fee for the 2019 SHM Annual Conference. This was my first SHM conference, and it was quite the learning experience. I enjoyed each educational session that I attended. I felt like I was able to bring something home with me that I can incorporate into my practice to better care for the patients that I see each day.
As mentioned above, I learned from each session, but my personal favorite was the “Updates in Hospital Medicine” session. I was very impressed by the enthusiasm of the two speakers. The information provided was presented so that it engaged each attendee.
Not only did I learn a wealth of valuable information that will help me in my career, I gained affirmation concerning my future educational endeavors. I was inspired to pursue a higher level of learning regarding my career. I witnessed this awesome organization that is filled with encouraging and motivating people, and I realized I wanted to be more involved on a local level, and maybe one day, on a larger level. In addition, this conference inspired me to continue to be a lifetime learner and to always crave more knowledge. I am blessed to be a part of hospital medicine. I look forward to the future of this specialty.
William Bancroft, MS IV
Alabama College of Osteopathic Medicine
Dothan, Ala.
I was honored to have been chosen by the Wiregrass chapter as the medical student representative for the SHM Annual Conference. I have been serving in the local chapter during both my 3rd and 4th years in different roles, from helping as a student liaison for our medical students to executive planning coordinator for events. It was a surprise when I got asked by the chapter to be their student representative, but one that I was very excited to accept.
This was my first medical conference. I had heard about what different conferences were like from many of my attendings, so I had some expectations, but this experience was so much better. I enjoyed meeting and networking with people. I also found myself eagerly waiting to get to the next lecture because I was getting an opportunity to hear about different case studies, new research outcomes, and new standards of care.
It was a real treat to learn about all the new changes to treatment, but even more encouraging to know that most of it was just reinforcing everything my attendings have been teaching us as medical students. I enjoyed my time at the SHM Annual Conference so much that I emailed all my new coresidents and encouraged them to join the Society.
Dr. Skandhan is a hospitalist at Southeast Health Medical Center in Dothan, Ala., as well as president and founder of the Wiregrass chapter of SHM.
The Society of Hospital Medicine is an organization vested in improving the quality of inpatient medicine by empowering its members with education and providing venues for professional development including networking, advocacy, and leadership advancement. Every year, SHM holds a national conference which is a focused meeting point for over 5,000 hospitalists.
SHM hosts more than 50 local chapters nationwide to increase networking, education, and collaboration within the hospital medicine community. The Wiregrass chapter of SHM is based in the southeast corner of Alabama, covering the counties of lower Alabama and the panhandle of Florida. This year we were recognized as a platinum status chapter, which is the highest status, based on our work and participation to improve the quality of inpatient medicine.
As part of winning the platinum ribbon, we were awarded three complimentary registration scholarships to the SHM Annual Conference in 2019. The chapter leadership met and selected three individuals who have been involved with the chapter actively but have never had an opportunity to experience SHM’s Annual Conference. We selected a first-year resident, Dr. Avani Parrekh; a hospital medicine nurse practitioner, Madison Rivenbark; and a fourth-year medical student who is about to start his internal medicine residency, William Bancroft.
After the meeting we interviewed them to better understand their experience. Below are their thoughts.
Avani Parekh, MD
First year, Internal Medicine Residency
Southeast Health Medical Center
Dothan, Ala.
I am so thankful for the opportunity that was given to me by the Wiregrass chapter by sponsoring my attendance at the 2019 SHM Annual Conference in Washington. This was my first SHM conference, and it was truly a rewarding experience.
I thoroughly enjoyed attending the lectures. They were very informative and engaging. Every presenter was so passionate and inspiring. Coming from an “all-female class” of PGY-1 at my program, I especially enjoyed the “Fe(male) in medicine” talk, as well as Quick Talks on women in medicine. The “Updates in Hospital Medicine” session on various topics such as heart failure, pneumonia, and sepsis was outstanding. I was excited to apply the knowledge I gained from this event into my patient care.
Overall, it was a well-organized and up-to-date event. I am looking forward to attending more SHM conferences in the future.
Madison Rivenbark, NP
Department of Hospital Medicine
Southeast Health Medical Center
Dothan, Ala.
I was extremely fortunate to be selected to receive a scholarship that covered the conference fee for the 2019 SHM Annual Conference. This was my first SHM conference, and it was quite the learning experience. I enjoyed each educational session that I attended. I felt like I was able to bring something home with me that I can incorporate into my practice to better care for the patients that I see each day.
As mentioned above, I learned from each session, but my personal favorite was the “Updates in Hospital Medicine” session. I was very impressed by the enthusiasm of the two speakers. The information provided was presented so that it engaged each attendee.
Not only did I learn a wealth of valuable information that will help me in my career, I gained affirmation concerning my future educational endeavors. I was inspired to pursue a higher level of learning regarding my career. I witnessed this awesome organization that is filled with encouraging and motivating people, and I realized I wanted to be more involved on a local level, and maybe one day, on a larger level. In addition, this conference inspired me to continue to be a lifetime learner and to always crave more knowledge. I am blessed to be a part of hospital medicine. I look forward to the future of this specialty.
William Bancroft, MS IV
Alabama College of Osteopathic Medicine
Dothan, Ala.
I was honored to have been chosen by the Wiregrass chapter as the medical student representative for the SHM Annual Conference. I have been serving in the local chapter during both my 3rd and 4th years in different roles, from helping as a student liaison for our medical students to executive planning coordinator for events. It was a surprise when I got asked by the chapter to be their student representative, but one that I was very excited to accept.
This was my first medical conference. I had heard about what different conferences were like from many of my attendings, so I had some expectations, but this experience was so much better. I enjoyed meeting and networking with people. I also found myself eagerly waiting to get to the next lecture because I was getting an opportunity to hear about different case studies, new research outcomes, and new standards of care.
It was a real treat to learn about all the new changes to treatment, but even more encouraging to know that most of it was just reinforcing everything my attendings have been teaching us as medical students. I enjoyed my time at the SHM Annual Conference so much that I emailed all my new coresidents and encouraged them to join the Society.
Dr. Skandhan is a hospitalist at Southeast Health Medical Center in Dothan, Ala., as well as president and founder of the Wiregrass chapter of SHM.
The Society of Hospital Medicine is an organization vested in improving the quality of inpatient medicine by empowering its members with education and providing venues for professional development including networking, advocacy, and leadership advancement. Every year, SHM holds a national conference which is a focused meeting point for over 5,000 hospitalists.
SHM hosts more than 50 local chapters nationwide to increase networking, education, and collaboration within the hospital medicine community. The Wiregrass chapter of SHM is based in the southeast corner of Alabama, covering the counties of lower Alabama and the panhandle of Florida. This year we were recognized as a platinum status chapter, which is the highest status, based on our work and participation to improve the quality of inpatient medicine.
As part of winning the platinum ribbon, we were awarded three complimentary registration scholarships to the SHM Annual Conference in 2019. The chapter leadership met and selected three individuals who have been involved with the chapter actively but have never had an opportunity to experience SHM’s Annual Conference. We selected a first-year resident, Dr. Avani Parrekh; a hospital medicine nurse practitioner, Madison Rivenbark; and a fourth-year medical student who is about to start his internal medicine residency, William Bancroft.
After the meeting we interviewed them to better understand their experience. Below are their thoughts.
Avani Parekh, MD
First year, Internal Medicine Residency
Southeast Health Medical Center
Dothan, Ala.
I am so thankful for the opportunity that was given to me by the Wiregrass chapter by sponsoring my attendance at the 2019 SHM Annual Conference in Washington. This was my first SHM conference, and it was truly a rewarding experience.
I thoroughly enjoyed attending the lectures. They were very informative and engaging. Every presenter was so passionate and inspiring. Coming from an “all-female class” of PGY-1 at my program, I especially enjoyed the “Fe(male) in medicine” talk, as well as Quick Talks on women in medicine. The “Updates in Hospital Medicine” session on various topics such as heart failure, pneumonia, and sepsis was outstanding. I was excited to apply the knowledge I gained from this event into my patient care.
Overall, it was a well-organized and up-to-date event. I am looking forward to attending more SHM conferences in the future.
Madison Rivenbark, NP
Department of Hospital Medicine
Southeast Health Medical Center
Dothan, Ala.
I was extremely fortunate to be selected to receive a scholarship that covered the conference fee for the 2019 SHM Annual Conference. This was my first SHM conference, and it was quite the learning experience. I enjoyed each educational session that I attended. I felt like I was able to bring something home with me that I can incorporate into my practice to better care for the patients that I see each day.
As mentioned above, I learned from each session, but my personal favorite was the “Updates in Hospital Medicine” session. I was very impressed by the enthusiasm of the two speakers. The information provided was presented so that it engaged each attendee.
Not only did I learn a wealth of valuable information that will help me in my career, I gained affirmation concerning my future educational endeavors. I was inspired to pursue a higher level of learning regarding my career. I witnessed this awesome organization that is filled with encouraging and motivating people, and I realized I wanted to be more involved on a local level, and maybe one day, on a larger level. In addition, this conference inspired me to continue to be a lifetime learner and to always crave more knowledge. I am blessed to be a part of hospital medicine. I look forward to the future of this specialty.
William Bancroft, MS IV
Alabama College of Osteopathic Medicine
Dothan, Ala.
I was honored to have been chosen by the Wiregrass chapter as the medical student representative for the SHM Annual Conference. I have been serving in the local chapter during both my 3rd and 4th years in different roles, from helping as a student liaison for our medical students to executive planning coordinator for events. It was a surprise when I got asked by the chapter to be their student representative, but one that I was very excited to accept.
This was my first medical conference. I had heard about what different conferences were like from many of my attendings, so I had some expectations, but this experience was so much better. I enjoyed meeting and networking with people. I also found myself eagerly waiting to get to the next lecture because I was getting an opportunity to hear about different case studies, new research outcomes, and new standards of care.
It was a real treat to learn about all the new changes to treatment, but even more encouraging to know that most of it was just reinforcing everything my attendings have been teaching us as medical students. I enjoyed my time at the SHM Annual Conference so much that I emailed all my new coresidents and encouraged them to join the Society.
Dr. Skandhan is a hospitalist at Southeast Health Medical Center in Dothan, Ala., as well as president and founder of the Wiregrass chapter of SHM.
A “Ray of light”
Finding inspiration in our patients
I rush into the room at 4:30 p.m., hoping for a quick visit and maybe an early exit from the hospital; I had been asked to see Mr. Bryant in room 6765 with sigmoid volvulus.
“Hey, Dr. Hass, my brother!” he says with a huge smile. Somehow, he must have gotten a glimpse of me before I could see him. I peek over the nurse’s shoulder, and then I see that unforgettable smile with only a few teeth and big bright eyes. Immediately I recognize him and think, “How could I have forgotten his name? Ray – like a beam of light.” He certainly had not forgotten me.
“It’s been more than a year since I was last here,” he says proudly.
When we met during his last hospitalization, I was struck by a thought that implanted itself deep in my brain: This guy is the happiest person I have ever met. And after what must have been 18 hard months for him, he is still smiling – and more than that, he is radiating love.
The fact that he is the “happiest person” is made more remarkable by all the hardship he has endured. Ray was born with cerebral palsy and didn’t walk until he was 10. The continuous spasms in his muscles led to severe cervical disc disease. His worsening pain and weakness were missed by his health care providers until he had lost significant strength in his hands and legs. When he finally got an MRI and then emergency surgery, it was too late. He never regained the dexterity of his hands or the ability to walk. He can climb onto his scooter chair only with the help of a lift.
“Wow! How you been, Ray?”
He replies with a phrase that jumped back out from my memory as he was saying it: “I just wake up every day and think about what I can do to make people happy.”
The goosebumps rise on my arms; I remember feeling this same sense of awe the last time we met – a feeling of real spiritual love for this guy.
“Today I feel so much better, too. I want to thank y’all who helped my stomach go down. Man, it got so huge, I thought I might blow up.” One of the consequences of the nerve damage he sustained is a very slow gut that has led to a stretched-out colon. The other day, his big, floppy colon got twisted, and neither our gastroenterologist nor radiologist was able to untwist it. He still has a tube in his rectum to help decompress his bowel.
Ray fills me in on the details in the slightly strained and slurred speech that sometimes comes with cerebral palsy. As he relays his story, my mind goes to work trying to diagnosis this mysterious case of happiness. How can I not try to get to the origins of this wellspring of love? I can’t help but thinking: Was it Ray’s joy and his speech impediment that made him seem childlike, or was it some brain injury that blessedly knocked out his self-pity? I would be wallowing in self-pity if I were as gravely disabled as him.
After a moment’s reflection, I recall the research on the amazing stability of our happiness set point: Good things and bad only move our happiness for a while before we return to our innate level of happiness. I see I had likely fallen prey to a stereotype of the disabled as heroic for just being themselves. Ray’s happiness is largely because of his lack of self-absorption and his focus on service and love.
Finishing our conversation and leaving the room feeling enlivened, I realize that Ray‘s generous spirit is a gift.
That night, my heart aches. I think about the inadequate care that led to Ray’s profound loss of function, leading to a surge of anger toward our flawed health care system – one that routinely lets down the most vulnerable among us.
The next day, two sisters and an aunt join Ray in his room. They ask for hugs, and I happily supply them. “Ray told us about you,” says Sheila, one of his sisters.
“Well, we have been talking about him here at the hospital, because he brightens everyone’s day. He is truly amazing. Has Ray always been so full of love?” I say, hoping to get some insight into his remarkable spirit.
Tonya, his aunt, responds first. “We were raised that way – to look for the good and keep love in our hearts. But Ray has always been the best. He never, ever complains. He brings joy to so many people. You should see him every day out on his scooter. That’s how he got that big sore on his butt.”
Ray indeed had developed a pressure sore, one that was going to need some thoughtful, ongoing care.
“But I finally got the right kind of cushion, before it was real hard,” he says.
I move from hospitalist mode to primary care mode and ask about his home equipment and his dental care. But they all want to keep talking about love.
“If doctors showed more love and their human side, they could bring more healing,” his sister says.
After 20 minutes of chatting, I pause. It is my last day on service, I had run out of medical reason to stay and I have others to see. So, I reluctantly give my goodbye hugs and leave. At the door, I turn back around. “Hey, Ray, can I get a picture with you?”
“Yeah, I want one with you, too!”
So, not surprisingly, Ray never complains. Maybe his spinal cord injury wasn’t from negligent care. Maybe he was so accustomed to looking past discomfort and too busy with his ministry of love, it didn’t occur to him to seek care.
Still, such a tragedy that he lost so much of the little mobility he did have. But maybe not so bad. His injury brought him back in contact with me and our staff. He is still waking up trying to make people happy and I can see his efforts are working. “He made my day!” I hear from a nurse. There is a healthy buzz at the nurses’ station after visits to his room.
Before walking out the door, he gives me an awkward fist bump from the bed and says, “I want to thank y’all again for everything. And I want you to know I love you.”
I find myself tearing up. “I love you too, my brother. And I am the one who should be grateful, Ray.” Saying it, I feel myself playing a part in the cycle of gratitude. Even small gifts put us under an obligation to give back. With great gifts, the desire to give is inescapable.
There is only one Ray, but he has given me something to aspire toward and what feels like urgency to do it. I want to “wake up each day thinking about ways to make other people happy.”
And understanding the potency of the gift from him has alerted me to the value of looking for other gifts and other inspirations from those I care for – something those of us who tend to be in the “doing” part of the provider-patient relationship can easy miss.
I will never be the beacon of light and love that Ray is, but being compelled to be my most authentic caring self with him, I see that for years I have held back – in the name of professionalism – the positive emotions that naturally arise from the work I do. I will try to shine and try to connect with that “Ray of light” residing in all my patients. I hope, too, that the cycle of giving Ray started will continue spreading to all those I care for.
Dr. Hass is a hospitalist at Sutter Health in Oakland, Calif. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
Finding inspiration in our patients
Finding inspiration in our patients
I rush into the room at 4:30 p.m., hoping for a quick visit and maybe an early exit from the hospital; I had been asked to see Mr. Bryant in room 6765 with sigmoid volvulus.
“Hey, Dr. Hass, my brother!” he says with a huge smile. Somehow, he must have gotten a glimpse of me before I could see him. I peek over the nurse’s shoulder, and then I see that unforgettable smile with only a few teeth and big bright eyes. Immediately I recognize him and think, “How could I have forgotten his name? Ray – like a beam of light.” He certainly had not forgotten me.
“It’s been more than a year since I was last here,” he says proudly.
When we met during his last hospitalization, I was struck by a thought that implanted itself deep in my brain: This guy is the happiest person I have ever met. And after what must have been 18 hard months for him, he is still smiling – and more than that, he is radiating love.
The fact that he is the “happiest person” is made more remarkable by all the hardship he has endured. Ray was born with cerebral palsy and didn’t walk until he was 10. The continuous spasms in his muscles led to severe cervical disc disease. His worsening pain and weakness were missed by his health care providers until he had lost significant strength in his hands and legs. When he finally got an MRI and then emergency surgery, it was too late. He never regained the dexterity of his hands or the ability to walk. He can climb onto his scooter chair only with the help of a lift.
“Wow! How you been, Ray?”
He replies with a phrase that jumped back out from my memory as he was saying it: “I just wake up every day and think about what I can do to make people happy.”
The goosebumps rise on my arms; I remember feeling this same sense of awe the last time we met – a feeling of real spiritual love for this guy.
“Today I feel so much better, too. I want to thank y’all who helped my stomach go down. Man, it got so huge, I thought I might blow up.” One of the consequences of the nerve damage he sustained is a very slow gut that has led to a stretched-out colon. The other day, his big, floppy colon got twisted, and neither our gastroenterologist nor radiologist was able to untwist it. He still has a tube in his rectum to help decompress his bowel.
Ray fills me in on the details in the slightly strained and slurred speech that sometimes comes with cerebral palsy. As he relays his story, my mind goes to work trying to diagnosis this mysterious case of happiness. How can I not try to get to the origins of this wellspring of love? I can’t help but thinking: Was it Ray’s joy and his speech impediment that made him seem childlike, or was it some brain injury that blessedly knocked out his self-pity? I would be wallowing in self-pity if I were as gravely disabled as him.
After a moment’s reflection, I recall the research on the amazing stability of our happiness set point: Good things and bad only move our happiness for a while before we return to our innate level of happiness. I see I had likely fallen prey to a stereotype of the disabled as heroic for just being themselves. Ray’s happiness is largely because of his lack of self-absorption and his focus on service and love.
Finishing our conversation and leaving the room feeling enlivened, I realize that Ray‘s generous spirit is a gift.
That night, my heart aches. I think about the inadequate care that led to Ray’s profound loss of function, leading to a surge of anger toward our flawed health care system – one that routinely lets down the most vulnerable among us.
The next day, two sisters and an aunt join Ray in his room. They ask for hugs, and I happily supply them. “Ray told us about you,” says Sheila, one of his sisters.
“Well, we have been talking about him here at the hospital, because he brightens everyone’s day. He is truly amazing. Has Ray always been so full of love?” I say, hoping to get some insight into his remarkable spirit.
Tonya, his aunt, responds first. “We were raised that way – to look for the good and keep love in our hearts. But Ray has always been the best. He never, ever complains. He brings joy to so many people. You should see him every day out on his scooter. That’s how he got that big sore on his butt.”
Ray indeed had developed a pressure sore, one that was going to need some thoughtful, ongoing care.
“But I finally got the right kind of cushion, before it was real hard,” he says.
I move from hospitalist mode to primary care mode and ask about his home equipment and his dental care. But they all want to keep talking about love.
“If doctors showed more love and their human side, they could bring more healing,” his sister says.
After 20 minutes of chatting, I pause. It is my last day on service, I had run out of medical reason to stay and I have others to see. So, I reluctantly give my goodbye hugs and leave. At the door, I turn back around. “Hey, Ray, can I get a picture with you?”
“Yeah, I want one with you, too!”
So, not surprisingly, Ray never complains. Maybe his spinal cord injury wasn’t from negligent care. Maybe he was so accustomed to looking past discomfort and too busy with his ministry of love, it didn’t occur to him to seek care.
Still, such a tragedy that he lost so much of the little mobility he did have. But maybe not so bad. His injury brought him back in contact with me and our staff. He is still waking up trying to make people happy and I can see his efforts are working. “He made my day!” I hear from a nurse. There is a healthy buzz at the nurses’ station after visits to his room.
Before walking out the door, he gives me an awkward fist bump from the bed and says, “I want to thank y’all again for everything. And I want you to know I love you.”
I find myself tearing up. “I love you too, my brother. And I am the one who should be grateful, Ray.” Saying it, I feel myself playing a part in the cycle of gratitude. Even small gifts put us under an obligation to give back. With great gifts, the desire to give is inescapable.
There is only one Ray, but he has given me something to aspire toward and what feels like urgency to do it. I want to “wake up each day thinking about ways to make other people happy.”
And understanding the potency of the gift from him has alerted me to the value of looking for other gifts and other inspirations from those I care for – something those of us who tend to be in the “doing” part of the provider-patient relationship can easy miss.
I will never be the beacon of light and love that Ray is, but being compelled to be my most authentic caring self with him, I see that for years I have held back – in the name of professionalism – the positive emotions that naturally arise from the work I do. I will try to shine and try to connect with that “Ray of light” residing in all my patients. I hope, too, that the cycle of giving Ray started will continue spreading to all those I care for.
Dr. Hass is a hospitalist at Sutter Health in Oakland, Calif. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
I rush into the room at 4:30 p.m., hoping for a quick visit and maybe an early exit from the hospital; I had been asked to see Mr. Bryant in room 6765 with sigmoid volvulus.
“Hey, Dr. Hass, my brother!” he says with a huge smile. Somehow, he must have gotten a glimpse of me before I could see him. I peek over the nurse’s shoulder, and then I see that unforgettable smile with only a few teeth and big bright eyes. Immediately I recognize him and think, “How could I have forgotten his name? Ray – like a beam of light.” He certainly had not forgotten me.
“It’s been more than a year since I was last here,” he says proudly.
When we met during his last hospitalization, I was struck by a thought that implanted itself deep in my brain: This guy is the happiest person I have ever met. And after what must have been 18 hard months for him, he is still smiling – and more than that, he is radiating love.
The fact that he is the “happiest person” is made more remarkable by all the hardship he has endured. Ray was born with cerebral palsy and didn’t walk until he was 10. The continuous spasms in his muscles led to severe cervical disc disease. His worsening pain and weakness were missed by his health care providers until he had lost significant strength in his hands and legs. When he finally got an MRI and then emergency surgery, it was too late. He never regained the dexterity of his hands or the ability to walk. He can climb onto his scooter chair only with the help of a lift.
“Wow! How you been, Ray?”
He replies with a phrase that jumped back out from my memory as he was saying it: “I just wake up every day and think about what I can do to make people happy.”
The goosebumps rise on my arms; I remember feeling this same sense of awe the last time we met – a feeling of real spiritual love for this guy.
“Today I feel so much better, too. I want to thank y’all who helped my stomach go down. Man, it got so huge, I thought I might blow up.” One of the consequences of the nerve damage he sustained is a very slow gut that has led to a stretched-out colon. The other day, his big, floppy colon got twisted, and neither our gastroenterologist nor radiologist was able to untwist it. He still has a tube in his rectum to help decompress his bowel.
Ray fills me in on the details in the slightly strained and slurred speech that sometimes comes with cerebral palsy. As he relays his story, my mind goes to work trying to diagnosis this mysterious case of happiness. How can I not try to get to the origins of this wellspring of love? I can’t help but thinking: Was it Ray’s joy and his speech impediment that made him seem childlike, or was it some brain injury that blessedly knocked out his self-pity? I would be wallowing in self-pity if I were as gravely disabled as him.
After a moment’s reflection, I recall the research on the amazing stability of our happiness set point: Good things and bad only move our happiness for a while before we return to our innate level of happiness. I see I had likely fallen prey to a stereotype of the disabled as heroic for just being themselves. Ray’s happiness is largely because of his lack of self-absorption and his focus on service and love.
Finishing our conversation and leaving the room feeling enlivened, I realize that Ray‘s generous spirit is a gift.
That night, my heart aches. I think about the inadequate care that led to Ray’s profound loss of function, leading to a surge of anger toward our flawed health care system – one that routinely lets down the most vulnerable among us.
The next day, two sisters and an aunt join Ray in his room. They ask for hugs, and I happily supply them. “Ray told us about you,” says Sheila, one of his sisters.
“Well, we have been talking about him here at the hospital, because he brightens everyone’s day. He is truly amazing. Has Ray always been so full of love?” I say, hoping to get some insight into his remarkable spirit.
Tonya, his aunt, responds first. “We were raised that way – to look for the good and keep love in our hearts. But Ray has always been the best. He never, ever complains. He brings joy to so many people. You should see him every day out on his scooter. That’s how he got that big sore on his butt.”
Ray indeed had developed a pressure sore, one that was going to need some thoughtful, ongoing care.
“But I finally got the right kind of cushion, before it was real hard,” he says.
I move from hospitalist mode to primary care mode and ask about his home equipment and his dental care. But they all want to keep talking about love.
“If doctors showed more love and their human side, they could bring more healing,” his sister says.
After 20 minutes of chatting, I pause. It is my last day on service, I had run out of medical reason to stay and I have others to see. So, I reluctantly give my goodbye hugs and leave. At the door, I turn back around. “Hey, Ray, can I get a picture with you?”
“Yeah, I want one with you, too!”
So, not surprisingly, Ray never complains. Maybe his spinal cord injury wasn’t from negligent care. Maybe he was so accustomed to looking past discomfort and too busy with his ministry of love, it didn’t occur to him to seek care.
Still, such a tragedy that he lost so much of the little mobility he did have. But maybe not so bad. His injury brought him back in contact with me and our staff. He is still waking up trying to make people happy and I can see his efforts are working. “He made my day!” I hear from a nurse. There is a healthy buzz at the nurses’ station after visits to his room.
Before walking out the door, he gives me an awkward fist bump from the bed and says, “I want to thank y’all again for everything. And I want you to know I love you.”
I find myself tearing up. “I love you too, my brother. And I am the one who should be grateful, Ray.” Saying it, I feel myself playing a part in the cycle of gratitude. Even small gifts put us under an obligation to give back. With great gifts, the desire to give is inescapable.
There is only one Ray, but he has given me something to aspire toward and what feels like urgency to do it. I want to “wake up each day thinking about ways to make other people happy.”
And understanding the potency of the gift from him has alerted me to the value of looking for other gifts and other inspirations from those I care for – something those of us who tend to be in the “doing” part of the provider-patient relationship can easy miss.
I will never be the beacon of light and love that Ray is, but being compelled to be my most authentic caring self with him, I see that for years I have held back – in the name of professionalism – the positive emotions that naturally arise from the work I do. I will try to shine and try to connect with that “Ray of light” residing in all my patients. I hope, too, that the cycle of giving Ray started will continue spreading to all those I care for.
Dr. Hass is a hospitalist at Sutter Health in Oakland, Calif. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
Are hospitalists being more highly valued?
An uptrend in financial support
Since the inception of hospital medicine more than 2 decades ago, the total number of hospitalists has rapidly increased to more than 60,000. The Society of Hospital Medicine’s State of Hospital Medicine Report (SoHM), published biennially, captures new changes in our growing field and sheds light on current practice trends.
Among its findings, the 2018 SoHM Report reassuringly reveals that financial support from hospitals to hospital medicine groups (HMGs) continues to climb, even in the setting of rising health care costs and ongoing budget pressure.
The median amount of financial support per full-time equivalent (FTE) physician for HMGs serving adults was $176,658, according to the 2018 SoHM Report, which is up 12% from the 2016 median of $157,535. While there is no correlation between group sizes and the amount of financial support per FTE physician, there are significant differences across regions, with HMGs in the Midwest garnering the highest median support, at $193,121 per FTE physician.
The report also reveals big differences by employment model. For example, private multispecialty and primary care medical groups receive much less financial support ($58,396 per FTE physician) than HMGs employed by hospitals. This likely signifies that their main source of revenue is from professional service fees. Regardless of the types of employment models, past surveys have reported more than 95% of HMGs receive support from their hospitals to help cover expenses.
The median amount of financial support per FTE provider (including nurse practitioners, physician assistants, and locum tenens) was $134,300, which represents a 3.3% decrease, compared with the 2016 SoHM Report. For the first time, the 2018 SoHM also collected data on financial support per “work relative value unit” (wRVU) in addition to support per FTE physician and support per FTE provider. HMGs and their hospitals can use support per wRVU data to evaluate the support per unit of work, regardless of who (whether it is a physician, an advanced practice provider, and/or others) performed that work.
The median amount of financial support per wRVU for HMGs serving adults in 2018 was $41.92, with academic HMGs reporting a higher amount ($45.81) than nonacademic HMGs ($41.28). It will be interesting to track these numbers over time.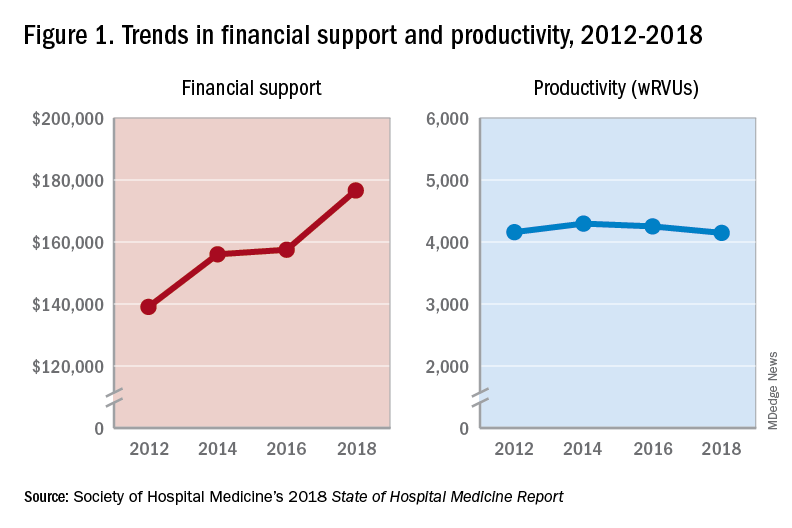
One of the most intriguing findings from the SHM’s 2018 SoHM Report is that financial support has risen despite relatively flat professional fee productivity (see Figure 1). Productivity, calculated as work relative value units (wRVUs) per physician declined slightly from 4,252 in 2016 to 4,147 in 2018.
There may be a few reasons why wRVUs per physician has remained relatively unchanged over the years. Many hospitals emphasize quality of care above provider productivity. The volume-to-value shift in theory serves as a means to reduce hospital-associated complications, length of stay, and readmission rates, thereby avoiding penalties and saving the overall costs for the hospitals in the long run.
Hospitalists involved in quality improvement projects and other essential nonclinical work perform tasks that are rarely captured in the wRVU metric. Improving patient experience, one of the Triple Aim components, necessitates extra time and effort, which also are nonbillable. In addition, increasing productivity can be challenging, a double-edged sword that may further escalate burnout and turnover rates. The static productivity may portend that it has leveled off or hit the ceiling in spite of ongoing efforts to improve efficacy.
In my view, the decision to invest in hospitalists for their contributions and dedications should not be determined based on a single metric such as wRVUs per physician. Hospitalist work on quality improvements; patient safety; efficiency, from direct bedside patient care to nonclinical efforts; teaching; research; involvements in various committees; administrative tasks; and leadership roles in improving health care systems are immeasurable. These are the reasons that most hospitals chose to adopt the hospitalist model and continue to support it. In fact, demand for hospitalists still outstrips supply, as evidenced by more than half of the hospital medicine groups with unfilled positions and an overall high turnover rate per 2018 SoHM data.
Although hospitalists are needed for the value that they provide, they should not take the status quo for granted. Instead, in return for the favorable financial support and in appreciation of being valued, hospitalists have a responsibility to prove that they are the right group chosen to do the work and help achieve their hospital’s mission and goals.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
References
Afsar N. Looking into the Future and Making History. Hospitalist. 2019;23(1):31.
Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
FitzGerald S. Not a Time for Modesty. Oct. 2009. Retrieved from https://acphospitalist.org/archives/2009/10/value.htm.
Watcher RM et al. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Eng J Med. 2016. 375(11):1009-11.
An uptrend in financial support
An uptrend in financial support
Since the inception of hospital medicine more than 2 decades ago, the total number of hospitalists has rapidly increased to more than 60,000. The Society of Hospital Medicine’s State of Hospital Medicine Report (SoHM), published biennially, captures new changes in our growing field and sheds light on current practice trends.
Among its findings, the 2018 SoHM Report reassuringly reveals that financial support from hospitals to hospital medicine groups (HMGs) continues to climb, even in the setting of rising health care costs and ongoing budget pressure.
The median amount of financial support per full-time equivalent (FTE) physician for HMGs serving adults was $176,658, according to the 2018 SoHM Report, which is up 12% from the 2016 median of $157,535. While there is no correlation between group sizes and the amount of financial support per FTE physician, there are significant differences across regions, with HMGs in the Midwest garnering the highest median support, at $193,121 per FTE physician.
The report also reveals big differences by employment model. For example, private multispecialty and primary care medical groups receive much less financial support ($58,396 per FTE physician) than HMGs employed by hospitals. This likely signifies that their main source of revenue is from professional service fees. Regardless of the types of employment models, past surveys have reported more than 95% of HMGs receive support from their hospitals to help cover expenses.
The median amount of financial support per FTE provider (including nurse practitioners, physician assistants, and locum tenens) was $134,300, which represents a 3.3% decrease, compared with the 2016 SoHM Report. For the first time, the 2018 SoHM also collected data on financial support per “work relative value unit” (wRVU) in addition to support per FTE physician and support per FTE provider. HMGs and their hospitals can use support per wRVU data to evaluate the support per unit of work, regardless of who (whether it is a physician, an advanced practice provider, and/or others) performed that work.
The median amount of financial support per wRVU for HMGs serving adults in 2018 was $41.92, with academic HMGs reporting a higher amount ($45.81) than nonacademic HMGs ($41.28). It will be interesting to track these numbers over time.
One of the most intriguing findings from the SHM’s 2018 SoHM Report is that financial support has risen despite relatively flat professional fee productivity (see Figure 1). Productivity, calculated as work relative value units (wRVUs) per physician declined slightly from 4,252 in 2016 to 4,147 in 2018.
There may be a few reasons why wRVUs per physician has remained relatively unchanged over the years. Many hospitals emphasize quality of care above provider productivity. The volume-to-value shift in theory serves as a means to reduce hospital-associated complications, length of stay, and readmission rates, thereby avoiding penalties and saving the overall costs for the hospitals in the long run.
Hospitalists involved in quality improvement projects and other essential nonclinical work perform tasks that are rarely captured in the wRVU metric. Improving patient experience, one of the Triple Aim components, necessitates extra time and effort, which also are nonbillable. In addition, increasing productivity can be challenging, a double-edged sword that may further escalate burnout and turnover rates. The static productivity may portend that it has leveled off or hit the ceiling in spite of ongoing efforts to improve efficacy.
In my view, the decision to invest in hospitalists for their contributions and dedications should not be determined based on a single metric such as wRVUs per physician. Hospitalist work on quality improvements; patient safety; efficiency, from direct bedside patient care to nonclinical efforts; teaching; research; involvements in various committees; administrative tasks; and leadership roles in improving health care systems are immeasurable. These are the reasons that most hospitals chose to adopt the hospitalist model and continue to support it. In fact, demand for hospitalists still outstrips supply, as evidenced by more than half of the hospital medicine groups with unfilled positions and an overall high turnover rate per 2018 SoHM data.
Although hospitalists are needed for the value that they provide, they should not take the status quo for granted. Instead, in return for the favorable financial support and in appreciation of being valued, hospitalists have a responsibility to prove that they are the right group chosen to do the work and help achieve their hospital’s mission and goals.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
References
Afsar N. Looking into the Future and Making History. Hospitalist. 2019;23(1):31.
Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
FitzGerald S. Not a Time for Modesty. Oct. 2009. Retrieved from https://acphospitalist.org/archives/2009/10/value.htm.
Watcher RM et al. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Eng J Med. 2016. 375(11):1009-11.
Since the inception of hospital medicine more than 2 decades ago, the total number of hospitalists has rapidly increased to more than 60,000. The Society of Hospital Medicine’s State of Hospital Medicine Report (SoHM), published biennially, captures new changes in our growing field and sheds light on current practice trends.
Among its findings, the 2018 SoHM Report reassuringly reveals that financial support from hospitals to hospital medicine groups (HMGs) continues to climb, even in the setting of rising health care costs and ongoing budget pressure.
The median amount of financial support per full-time equivalent (FTE) physician for HMGs serving adults was $176,658, according to the 2018 SoHM Report, which is up 12% from the 2016 median of $157,535. While there is no correlation between group sizes and the amount of financial support per FTE physician, there are significant differences across regions, with HMGs in the Midwest garnering the highest median support, at $193,121 per FTE physician.
The report also reveals big differences by employment model. For example, private multispecialty and primary care medical groups receive much less financial support ($58,396 per FTE physician) than HMGs employed by hospitals. This likely signifies that their main source of revenue is from professional service fees. Regardless of the types of employment models, past surveys have reported more than 95% of HMGs receive support from their hospitals to help cover expenses.
The median amount of financial support per FTE provider (including nurse practitioners, physician assistants, and locum tenens) was $134,300, which represents a 3.3% decrease, compared with the 2016 SoHM Report. For the first time, the 2018 SoHM also collected data on financial support per “work relative value unit” (wRVU) in addition to support per FTE physician and support per FTE provider. HMGs and their hospitals can use support per wRVU data to evaluate the support per unit of work, regardless of who (whether it is a physician, an advanced practice provider, and/or others) performed that work.
The median amount of financial support per wRVU for HMGs serving adults in 2018 was $41.92, with academic HMGs reporting a higher amount ($45.81) than nonacademic HMGs ($41.28). It will be interesting to track these numbers over time.
One of the most intriguing findings from the SHM’s 2018 SoHM Report is that financial support has risen despite relatively flat professional fee productivity (see Figure 1). Productivity, calculated as work relative value units (wRVUs) per physician declined slightly from 4,252 in 2016 to 4,147 in 2018.
There may be a few reasons why wRVUs per physician has remained relatively unchanged over the years. Many hospitals emphasize quality of care above provider productivity. The volume-to-value shift in theory serves as a means to reduce hospital-associated complications, length of stay, and readmission rates, thereby avoiding penalties and saving the overall costs for the hospitals in the long run.
Hospitalists involved in quality improvement projects and other essential nonclinical work perform tasks that are rarely captured in the wRVU metric. Improving patient experience, one of the Triple Aim components, necessitates extra time and effort, which also are nonbillable. In addition, increasing productivity can be challenging, a double-edged sword that may further escalate burnout and turnover rates. The static productivity may portend that it has leveled off or hit the ceiling in spite of ongoing efforts to improve efficacy.
In my view, the decision to invest in hospitalists for their contributions and dedications should not be determined based on a single metric such as wRVUs per physician. Hospitalist work on quality improvements; patient safety; efficiency, from direct bedside patient care to nonclinical efforts; teaching; research; involvements in various committees; administrative tasks; and leadership roles in improving health care systems are immeasurable. These are the reasons that most hospitals chose to adopt the hospitalist model and continue to support it. In fact, demand for hospitalists still outstrips supply, as evidenced by more than half of the hospital medicine groups with unfilled positions and an overall high turnover rate per 2018 SoHM data.
Although hospitalists are needed for the value that they provide, they should not take the status quo for granted. Instead, in return for the favorable financial support and in appreciation of being valued, hospitalists have a responsibility to prove that they are the right group chosen to do the work and help achieve their hospital’s mission and goals.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
References
Afsar N. Looking into the Future and Making History. Hospitalist. 2019;23(1):31.
Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
FitzGerald S. Not a Time for Modesty. Oct. 2009. Retrieved from https://acphospitalist.org/archives/2009/10/value.htm.
Watcher RM et al. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Eng J Med. 2016. 375(11):1009-11.
Eosinophil-guided therapy reduces corticosteroid use in COPD
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
FROM LANCET RESPIRATORY MEDICINE
FDA invites sample submission for FDA-ARGOS database
which seeks to support research and regulatory decisions regarding DNA testing for pathogens with quality-controlled and curated genomic sequence data. Such testing and devices could be used as medical countermeasures against biothreats such as Ebola and Zika.
Infectious disease next-generation sequencing could use DNA analysis to help identify pathogens – from viruses to parasites – faster and more efficiently by, in theory, accomplishing with one test what was only possible before with many, according to the FDA. In order to not only further development of such tests and devices but also aid regulatory and scientific review of them, the FDA has collaborated with the Department of Defense, the National Center for Biotechnology Information, and Institute for Genome Sciences at the University of Maryland, Baltimore, to create FDA-ARGOS.
However, the FDA and its collaborators need samples of pathogens to continue developing the database, so they’ve invited health care professionals to submit samples for that purpose. More information, including preferred organism list and submission guidelines, can be found on the FDA-ARGOS website.
which seeks to support research and regulatory decisions regarding DNA testing for pathogens with quality-controlled and curated genomic sequence data. Such testing and devices could be used as medical countermeasures against biothreats such as Ebola and Zika.
Infectious disease next-generation sequencing could use DNA analysis to help identify pathogens – from viruses to parasites – faster and more efficiently by, in theory, accomplishing with one test what was only possible before with many, according to the FDA. In order to not only further development of such tests and devices but also aid regulatory and scientific review of them, the FDA has collaborated with the Department of Defense, the National Center for Biotechnology Information, and Institute for Genome Sciences at the University of Maryland, Baltimore, to create FDA-ARGOS.
However, the FDA and its collaborators need samples of pathogens to continue developing the database, so they’ve invited health care professionals to submit samples for that purpose. More information, including preferred organism list and submission guidelines, can be found on the FDA-ARGOS website.
which seeks to support research and regulatory decisions regarding DNA testing for pathogens with quality-controlled and curated genomic sequence data. Such testing and devices could be used as medical countermeasures against biothreats such as Ebola and Zika.
Infectious disease next-generation sequencing could use DNA analysis to help identify pathogens – from viruses to parasites – faster and more efficiently by, in theory, accomplishing with one test what was only possible before with many, according to the FDA. In order to not only further development of such tests and devices but also aid regulatory and scientific review of them, the FDA has collaborated with the Department of Defense, the National Center for Biotechnology Information, and Institute for Genome Sciences at the University of Maryland, Baltimore, to create FDA-ARGOS.
However, the FDA and its collaborators need samples of pathogens to continue developing the database, so they’ve invited health care professionals to submit samples for that purpose. More information, including preferred organism list and submission guidelines, can be found on the FDA-ARGOS website.
Reducing adverse drug reactions
Easing the inpatient/outpatient transition
Adverse drug reactions are a problem hospitalists encounter often. An estimated 9% of hospital admissions in older adults are the result of adverse drug reactions, and up to one in five adults experience an adverse drug reaction during hospitalization.
“Many interventions have been tried to solve this problem, and certain of them have worked, but to date we don’t have any great solutions that meaningfully impact the rate of these events in a way that’s feasible in most health care environments, so any efforts to reduce the burden of these problems in older adults could be hugely beneficial,” said Michael Steinman, MD, author of an editorial highlighting a new approach.
His editorial in BMJ Quality & Safety cites research on the Pharm2Pharm program, implemented in six Hawaiian hospitals, in which hospital-based pharmacists identified inpatients at high risk of medication misadventures with criteria such as use of multiple medications, presence of high-risk medications such as warfarin or glucose-lowering drugs, and a history of previous acute care use resulting from medication-related problems. The hospital pharmacist would then meet with the patient to reconcile medications and facilitate a coordinated hand-off to a community pharmacist, who would meet with the patient after discharge.
In addition to a 36% reduction in the rate of medication-related hospitalizations, the intervention generated an estimated savings of $6.6 million per year in avoided hospitalizations.
There are two major takeaways, said Dr. Steinman, who is based in the division of geriatrics at the University of California, San Francisco: It’s critical to focus on transitions and coordination between inpatient and outpatient care to address medication-related problems, and pharmacists can be extremely helpful in that.
“Decisions about drug therapy in the hospital may seem reasonable in the short term but often won’t stick in the long term unless there is a coordinated care that can help ensure appropriate follow-through once patients return home,” Dr. Steinman said. “The study that the editorial references is a systems intervention that hospitalists can advocate for in their own institutions, but in the immediate day-to-day, trying to ensure solid coordination of medication management from the inpatient to outpatient setting is likely to be very helpful for their patients.”
The long-term outcomes of hospitalized patients are largely influenced by getting them set up with appropriate community resources and supports once they leave the hospital, he added, and the hospital can play a critical role in putting these pieces into place.
Reference
1. Steinman MA. Reducing hospital admissions for adverse drug events through coordinated pharmacist care: learning from Hawai’i without a field trip. BMJ Qual Saf. Epub 2018 Nov 24. doi: 10.1136/bmjqs-2018-008815. Accessed Dec. 11, 2018.
Easing the inpatient/outpatient transition
Easing the inpatient/outpatient transition
Adverse drug reactions are a problem hospitalists encounter often. An estimated 9% of hospital admissions in older adults are the result of adverse drug reactions, and up to one in five adults experience an adverse drug reaction during hospitalization.
“Many interventions have been tried to solve this problem, and certain of them have worked, but to date we don’t have any great solutions that meaningfully impact the rate of these events in a way that’s feasible in most health care environments, so any efforts to reduce the burden of these problems in older adults could be hugely beneficial,” said Michael Steinman, MD, author of an editorial highlighting a new approach.
His editorial in BMJ Quality & Safety cites research on the Pharm2Pharm program, implemented in six Hawaiian hospitals, in which hospital-based pharmacists identified inpatients at high risk of medication misadventures with criteria such as use of multiple medications, presence of high-risk medications such as warfarin or glucose-lowering drugs, and a history of previous acute care use resulting from medication-related problems. The hospital pharmacist would then meet with the patient to reconcile medications and facilitate a coordinated hand-off to a community pharmacist, who would meet with the patient after discharge.
In addition to a 36% reduction in the rate of medication-related hospitalizations, the intervention generated an estimated savings of $6.6 million per year in avoided hospitalizations.
There are two major takeaways, said Dr. Steinman, who is based in the division of geriatrics at the University of California, San Francisco: It’s critical to focus on transitions and coordination between inpatient and outpatient care to address medication-related problems, and pharmacists can be extremely helpful in that.
“Decisions about drug therapy in the hospital may seem reasonable in the short term but often won’t stick in the long term unless there is a coordinated care that can help ensure appropriate follow-through once patients return home,” Dr. Steinman said. “The study that the editorial references is a systems intervention that hospitalists can advocate for in their own institutions, but in the immediate day-to-day, trying to ensure solid coordination of medication management from the inpatient to outpatient setting is likely to be very helpful for their patients.”
The long-term outcomes of hospitalized patients are largely influenced by getting them set up with appropriate community resources and supports once they leave the hospital, he added, and the hospital can play a critical role in putting these pieces into place.
Reference
1. Steinman MA. Reducing hospital admissions for adverse drug events through coordinated pharmacist care: learning from Hawai’i without a field trip. BMJ Qual Saf. Epub 2018 Nov 24. doi: 10.1136/bmjqs-2018-008815. Accessed Dec. 11, 2018.
Adverse drug reactions are a problem hospitalists encounter often. An estimated 9% of hospital admissions in older adults are the result of adverse drug reactions, and up to one in five adults experience an adverse drug reaction during hospitalization.
“Many interventions have been tried to solve this problem, and certain of them have worked, but to date we don’t have any great solutions that meaningfully impact the rate of these events in a way that’s feasible in most health care environments, so any efforts to reduce the burden of these problems in older adults could be hugely beneficial,” said Michael Steinman, MD, author of an editorial highlighting a new approach.
His editorial in BMJ Quality & Safety cites research on the Pharm2Pharm program, implemented in six Hawaiian hospitals, in which hospital-based pharmacists identified inpatients at high risk of medication misadventures with criteria such as use of multiple medications, presence of high-risk medications such as warfarin or glucose-lowering drugs, and a history of previous acute care use resulting from medication-related problems. The hospital pharmacist would then meet with the patient to reconcile medications and facilitate a coordinated hand-off to a community pharmacist, who would meet with the patient after discharge.
In addition to a 36% reduction in the rate of medication-related hospitalizations, the intervention generated an estimated savings of $6.6 million per year in avoided hospitalizations.
There are two major takeaways, said Dr. Steinman, who is based in the division of geriatrics at the University of California, San Francisco: It’s critical to focus on transitions and coordination between inpatient and outpatient care to address medication-related problems, and pharmacists can be extremely helpful in that.
“Decisions about drug therapy in the hospital may seem reasonable in the short term but often won’t stick in the long term unless there is a coordinated care that can help ensure appropriate follow-through once patients return home,” Dr. Steinman said. “The study that the editorial references is a systems intervention that hospitalists can advocate for in their own institutions, but in the immediate day-to-day, trying to ensure solid coordination of medication management from the inpatient to outpatient setting is likely to be very helpful for their patients.”
The long-term outcomes of hospitalized patients are largely influenced by getting them set up with appropriate community resources and supports once they leave the hospital, he added, and the hospital can play a critical role in putting these pieces into place.
Reference
1. Steinman MA. Reducing hospital admissions for adverse drug events through coordinated pharmacist care: learning from Hawai’i without a field trip. BMJ Qual Saf. Epub 2018 Nov 24. doi: 10.1136/bmjqs-2018-008815. Accessed Dec. 11, 2018.
Reducing pediatric RSV burden is top priority
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.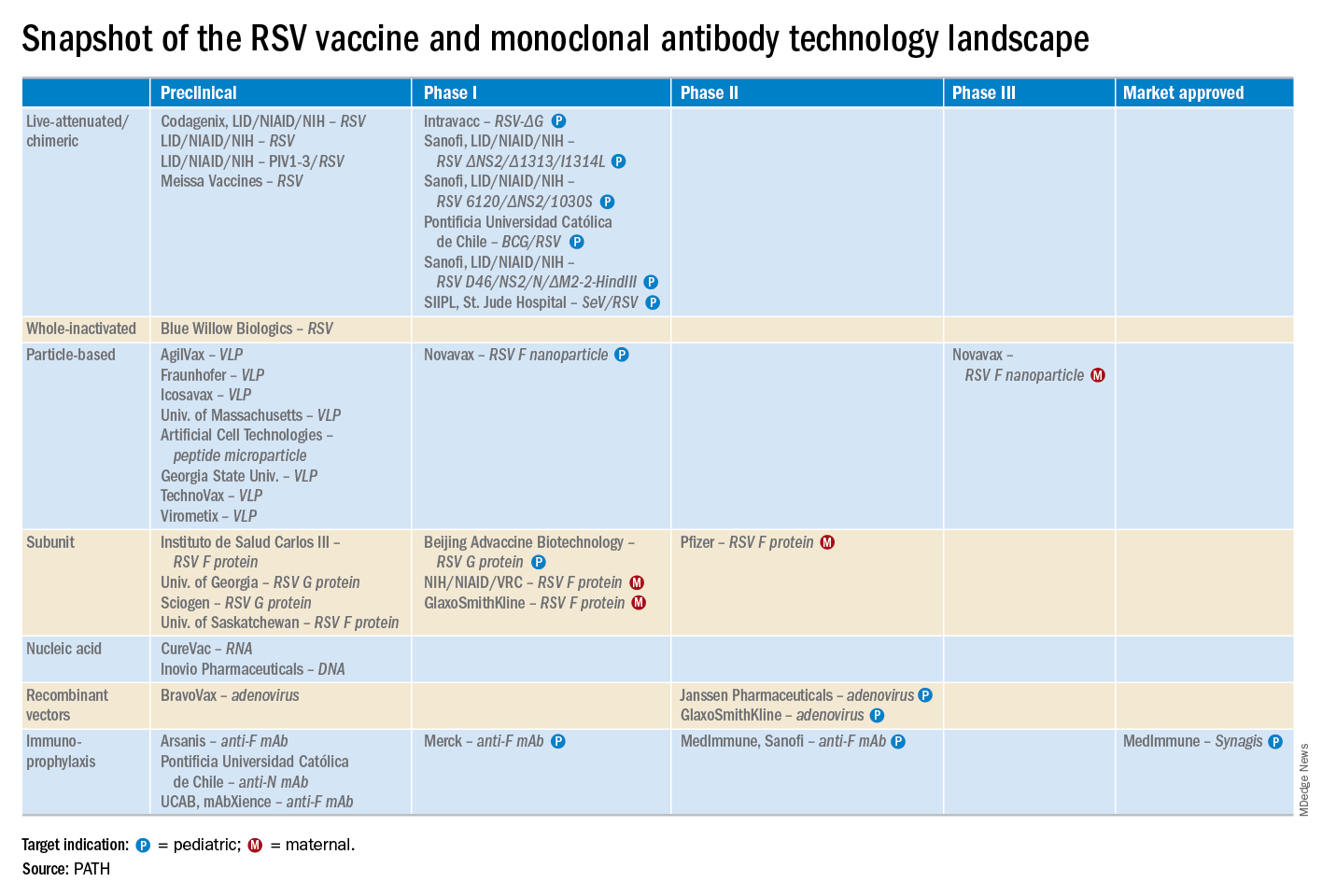
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
EXPERT ANALYSIS FROM ESPID 2019
Fewer antibiotics prescribed with PCR than conventional stool testing
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
REPORTING FROM DDW 2019