User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


No Pip/Tazo for patients with ESBL blood stream infections
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Physician burnout may be jeopardizing patient care
Clinical question: Is physician burnout associated with more patient safety issues, low professionalism, or poor patient satisfaction?
Background: Burnout is common among physicians and has a negative effect on their personal lives. It is unclear whether physician burnout is associated with poor outcomes for patients.
Study design: Meta-analysis.
Setting: Forty-seven published studies from 19 countries assessing inpatient and outpatient physicians and the relationship between physician burnout and patient care.
Synopsis: After a systematic review of the published literature, 47 studies were included to pool data from 42,473 physicians. Study subjects included residents, early-career and late-career physicians, and both hospital and outpatient physicians. All studies used validated measures of physician burnout.
Burnout was associated with a two-fold increased risk of physician-reported safety incidents (odds ratio, 1.96; 95% confidence interval, 1.59-2.40), low professionalism (OR, 2.31; 95% CI, 1.87-2.85), and likelihood of low patient-reported satisfaction (OR, 2.28; 95% CI, 1.42-3.68). There were no significant differences in these results based on country of origin of the study. Early-career physicians were more likely to have burnout associated with low professionalism than were late-career physicians.
Of the components of burnout, depersonalization was most strongly associated with these negative outcomes. Interestingly, the increased risk of patient safety incidents was associated with physician-reported, but not health care system–reported, patient safety outcomes. This raises concerns that the health care systems may not be capturing “near misses” in their metrics.
Bottom line: Physician burnout doubles the risk of being involved in a patient safety incident, low professionalism, and poor patient satisfaction.
Citation: Panagioti M et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction. JAMA Intern Med. 2018;178(10):1317-30.
Dr. Gabriel is assistant professor of medicine and director of Pre-operative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is physician burnout associated with more patient safety issues, low professionalism, or poor patient satisfaction?
Background: Burnout is common among physicians and has a negative effect on their personal lives. It is unclear whether physician burnout is associated with poor outcomes for patients.
Study design: Meta-analysis.
Setting: Forty-seven published studies from 19 countries assessing inpatient and outpatient physicians and the relationship between physician burnout and patient care.
Synopsis: After a systematic review of the published literature, 47 studies were included to pool data from 42,473 physicians. Study subjects included residents, early-career and late-career physicians, and both hospital and outpatient physicians. All studies used validated measures of physician burnout.
Burnout was associated with a two-fold increased risk of physician-reported safety incidents (odds ratio, 1.96; 95% confidence interval, 1.59-2.40), low professionalism (OR, 2.31; 95% CI, 1.87-2.85), and likelihood of low patient-reported satisfaction (OR, 2.28; 95% CI, 1.42-3.68). There were no significant differences in these results based on country of origin of the study. Early-career physicians were more likely to have burnout associated with low professionalism than were late-career physicians.
Of the components of burnout, depersonalization was most strongly associated with these negative outcomes. Interestingly, the increased risk of patient safety incidents was associated with physician-reported, but not health care system–reported, patient safety outcomes. This raises concerns that the health care systems may not be capturing “near misses” in their metrics.
Bottom line: Physician burnout doubles the risk of being involved in a patient safety incident, low professionalism, and poor patient satisfaction.
Citation: Panagioti M et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction. JAMA Intern Med. 2018;178(10):1317-30.
Dr. Gabriel is assistant professor of medicine and director of Pre-operative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is physician burnout associated with more patient safety issues, low professionalism, or poor patient satisfaction?
Background: Burnout is common among physicians and has a negative effect on their personal lives. It is unclear whether physician burnout is associated with poor outcomes for patients.
Study design: Meta-analysis.
Setting: Forty-seven published studies from 19 countries assessing inpatient and outpatient physicians and the relationship between physician burnout and patient care.
Synopsis: After a systematic review of the published literature, 47 studies were included to pool data from 42,473 physicians. Study subjects included residents, early-career and late-career physicians, and both hospital and outpatient physicians. All studies used validated measures of physician burnout.
Burnout was associated with a two-fold increased risk of physician-reported safety incidents (odds ratio, 1.96; 95% confidence interval, 1.59-2.40), low professionalism (OR, 2.31; 95% CI, 1.87-2.85), and likelihood of low patient-reported satisfaction (OR, 2.28; 95% CI, 1.42-3.68). There were no significant differences in these results based on country of origin of the study. Early-career physicians were more likely to have burnout associated with low professionalism than were late-career physicians.
Of the components of burnout, depersonalization was most strongly associated with these negative outcomes. Interestingly, the increased risk of patient safety incidents was associated with physician-reported, but not health care system–reported, patient safety outcomes. This raises concerns that the health care systems may not be capturing “near misses” in their metrics.
Bottom line: Physician burnout doubles the risk of being involved in a patient safety incident, low professionalism, and poor patient satisfaction.
Citation: Panagioti M et al. Association between physician burnout and patient safety, professionalism, and patient satisfaction. JAMA Intern Med. 2018;178(10):1317-30.
Dr. Gabriel is assistant professor of medicine and director of Pre-operative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Residents are drowning in job offers – and debt
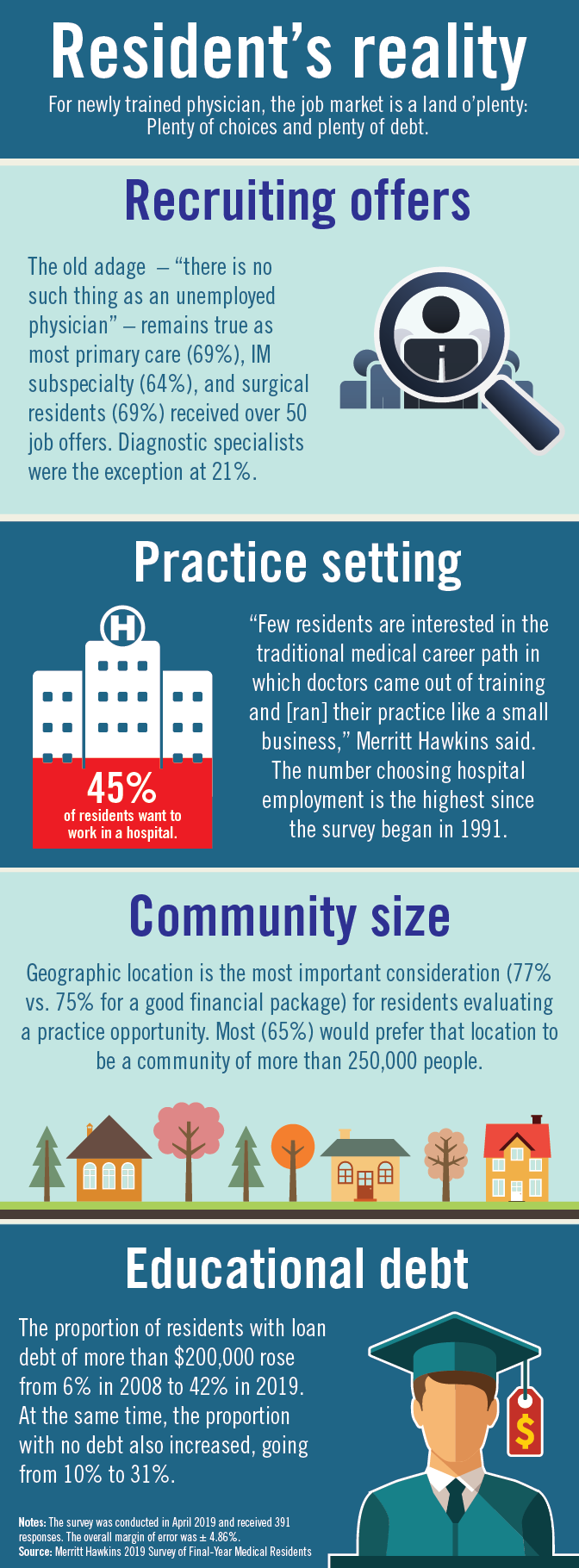
Physician search firm Merritt Hawkins did – actually, they heard from 391 residents – and 64% said that they had been contacted too many times by recruiters.
“Physicians coming out of training are being recruited like blue-chip athletes,” Travis Singleton, executive vice president of Merritt Hawkins, said in a statement. “There are simply not enough new doctors to go around.”
Merritt Hawkins asked physicians in their final year of residency about career choices, practice plans, and finances. Most said that they would prefer to be employed by a hospital or group practice, and a majority want to practice in a community with a population of 250,000 or more. More than half of the residents owed over $150,000 in student loans, but there were considerable debt differences between U.S. and international medical graduates.
The specialty distribution of respondents was 50% primary care, 30% internal medicine subspecialty/other, 15% surgical, and 5% diagnostic. About three-quarters were U.S. graduates and one-quarter of the residents were international medical graduates in this latest survey in a series that has been conducted periodically since 1991.
The survey was conducted in April 2018.
Physician search firm Merritt Hawkins did – actually, they heard from 391 residents – and 64% said that they had been contacted too many times by recruiters.
“Physicians coming out of training are being recruited like blue-chip athletes,” Travis Singleton, executive vice president of Merritt Hawkins, said in a statement. “There are simply not enough new doctors to go around.”
Merritt Hawkins asked physicians in their final year of residency about career choices, practice plans, and finances. Most said that they would prefer to be employed by a hospital or group practice, and a majority want to practice in a community with a population of 250,000 or more. More than half of the residents owed over $150,000 in student loans, but there were considerable debt differences between U.S. and international medical graduates.
The specialty distribution of respondents was 50% primary care, 30% internal medicine subspecialty/other, 15% surgical, and 5% diagnostic. About three-quarters were U.S. graduates and one-quarter of the residents were international medical graduates in this latest survey in a series that has been conducted periodically since 1991.
The survey was conducted in April 2018.

Physician search firm Merritt Hawkins did – actually, they heard from 391 residents – and 64% said that they had been contacted too many times by recruiters.
“Physicians coming out of training are being recruited like blue-chip athletes,” Travis Singleton, executive vice president of Merritt Hawkins, said in a statement. “There are simply not enough new doctors to go around.”
Merritt Hawkins asked physicians in their final year of residency about career choices, practice plans, and finances. Most said that they would prefer to be employed by a hospital or group practice, and a majority want to practice in a community with a population of 250,000 or more. More than half of the residents owed over $150,000 in student loans, but there were considerable debt differences between U.S. and international medical graduates.
The specialty distribution of respondents was 50% primary care, 30% internal medicine subspecialty/other, 15% surgical, and 5% diagnostic. About three-quarters were U.S. graduates and one-quarter of the residents were international medical graduates in this latest survey in a series that has been conducted periodically since 1991.
The survey was conducted in April 2018.

Benefits of Medicare Shared Savings Program ACOs lacking
or improving quality, according to new research.
“Our conclusion that the MSSP was not associated with improvements in spending, quality, or most measures of hospital use differ from that of previous evaluations of Medicare ACOs,” Adam Markovitz, of the University of Michigan, Ann Arbor, and colleagues wrote in a new research report published in Annals of Internal Medicine.
“Our instrumental variable model addresses selection effects not directly captured in previous evaluations,” the researchers continued.
To illustrate the point, the researchers found an association between MSSP and spending when using an adjusted longitudinal model (change, –$118; 95% confidence interval, –$151 to –$85 per beneficiary per quarter), with savings coming from reductions in inpatient services, outpatient services, and skilled nursing facility charges.
However, when employing an instrumental variable model, there was not an association with changes in total spending (change, $5; 95%CI, –$51 to $62 per beneficiary per quarter.
“The instrumental variable estimate for spending differed significantly from the adjusted estimate,” Mr. Markovitz and colleagues noted. “Estimated savings were smaller in instrumental variable models than in adjusted models across each ACO cohort.”
Similar patterns were observed in quality observations.
“The MSSP was associated with improvements in all four clinical quality indicators in the adjusted longitudinal model but not in the instrumental variable model,” the authors wrote. “The MSSP was associated with modest decreases in all-cause hospitalizations and preventable hospitalizations in the longitudinal model but not in the instrumental variable model.”
Overall, the authors noted that the results “challenge the view that MSSP ACOs have lowered spending and improved quality; they indicate that savings by MSSP ACOs may be driven by nonrandom exit of high-cost clinicians and their patient panels from this voluntary program.”
Indeed, the report states that removing “high-cost clinicians from ACO contracts could have large effects on spending estimates and may contribute to reported findings that MSSP savings grow over time.”
Primary sources of funding for the research included the Horowitz Foundation for Social Policy, Agency for Healthcare Research and Quality, and the National Institute on Aging. No relevant disclosures were made by the authors.
SOURCE: Markovitz A et al. Ann Intern Med. 2019 Jun 18. doi: 10.7326/M18-2539.
or improving quality, according to new research.
“Our conclusion that the MSSP was not associated with improvements in spending, quality, or most measures of hospital use differ from that of previous evaluations of Medicare ACOs,” Adam Markovitz, of the University of Michigan, Ann Arbor, and colleagues wrote in a new research report published in Annals of Internal Medicine.
“Our instrumental variable model addresses selection effects not directly captured in previous evaluations,” the researchers continued.
To illustrate the point, the researchers found an association between MSSP and spending when using an adjusted longitudinal model (change, –$118; 95% confidence interval, –$151 to –$85 per beneficiary per quarter), with savings coming from reductions in inpatient services, outpatient services, and skilled nursing facility charges.
However, when employing an instrumental variable model, there was not an association with changes in total spending (change, $5; 95%CI, –$51 to $62 per beneficiary per quarter.
“The instrumental variable estimate for spending differed significantly from the adjusted estimate,” Mr. Markovitz and colleagues noted. “Estimated savings were smaller in instrumental variable models than in adjusted models across each ACO cohort.”
Similar patterns were observed in quality observations.
“The MSSP was associated with improvements in all four clinical quality indicators in the adjusted longitudinal model but not in the instrumental variable model,” the authors wrote. “The MSSP was associated with modest decreases in all-cause hospitalizations and preventable hospitalizations in the longitudinal model but not in the instrumental variable model.”
Overall, the authors noted that the results “challenge the view that MSSP ACOs have lowered spending and improved quality; they indicate that savings by MSSP ACOs may be driven by nonrandom exit of high-cost clinicians and their patient panels from this voluntary program.”
Indeed, the report states that removing “high-cost clinicians from ACO contracts could have large effects on spending estimates and may contribute to reported findings that MSSP savings grow over time.”
Primary sources of funding for the research included the Horowitz Foundation for Social Policy, Agency for Healthcare Research and Quality, and the National Institute on Aging. No relevant disclosures were made by the authors.
SOURCE: Markovitz A et al. Ann Intern Med. 2019 Jun 18. doi: 10.7326/M18-2539.
or improving quality, according to new research.
“Our conclusion that the MSSP was not associated with improvements in spending, quality, or most measures of hospital use differ from that of previous evaluations of Medicare ACOs,” Adam Markovitz, of the University of Michigan, Ann Arbor, and colleagues wrote in a new research report published in Annals of Internal Medicine.
“Our instrumental variable model addresses selection effects not directly captured in previous evaluations,” the researchers continued.
To illustrate the point, the researchers found an association between MSSP and spending when using an adjusted longitudinal model (change, –$118; 95% confidence interval, –$151 to –$85 per beneficiary per quarter), with savings coming from reductions in inpatient services, outpatient services, and skilled nursing facility charges.
However, when employing an instrumental variable model, there was not an association with changes in total spending (change, $5; 95%CI, –$51 to $62 per beneficiary per quarter.
“The instrumental variable estimate for spending differed significantly from the adjusted estimate,” Mr. Markovitz and colleagues noted. “Estimated savings were smaller in instrumental variable models than in adjusted models across each ACO cohort.”
Similar patterns were observed in quality observations.
“The MSSP was associated with improvements in all four clinical quality indicators in the adjusted longitudinal model but not in the instrumental variable model,” the authors wrote. “The MSSP was associated with modest decreases in all-cause hospitalizations and preventable hospitalizations in the longitudinal model but not in the instrumental variable model.”
Overall, the authors noted that the results “challenge the view that MSSP ACOs have lowered spending and improved quality; they indicate that savings by MSSP ACOs may be driven by nonrandom exit of high-cost clinicians and their patient panels from this voluntary program.”
Indeed, the report states that removing “high-cost clinicians from ACO contracts could have large effects on spending estimates and may contribute to reported findings that MSSP savings grow over time.”
Primary sources of funding for the research included the Horowitz Foundation for Social Policy, Agency for Healthcare Research and Quality, and the National Institute on Aging. No relevant disclosures were made by the authors.
SOURCE: Markovitz A et al. Ann Intern Med. 2019 Jun 18. doi: 10.7326/M18-2539.
FROM ANNALS OF INTERNAL MEDICINE
HM19: Sepsis care update
Presenter
Patricia Kritek MD, EdM
Session Title
Sepsis update: From screening to refractory shock
Background
Each year 1.7 million adults in America develop sepsis, and 270,000 Americans die from sepsis annually. Sepsis costs U.S. health care over $27 billion dollars each year. Because of the wide range of etiologies and variation in presentation and intensity, it is a challenge to establish homogeneous evidence based guidelines.1
The definition of sepsis based on the “SIRS” criterion was developed initially in 1992, later revised as Sepsis-2 in 2001. The latest Sepsis-3 definition – “life-threatening organ dysfunction due to a dysregulated host response to infection” – was developed in 2016 by the Third International Consensus Definitions for Sepsis and Septic Shock. This newest definition has renounced the SIRS criterion and adopted the Sequential Organ Failure Assessment (SOFA) score. Treatment guidelines in sepsis were developed by the Surviving Sepsis Campaign starting with the Barcelona Declaration in 2002 and revised multiple times, with the development of 3-hour and 6-hour care bundles in 2012. The latest revision, in 2018, consolidated to a 1-hour bundle.2
Sepsis is a continuum of every severe infection, and with the combined efforts of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, evidence-based guidelines have been developed over the past 2 decades, with the latest iteration in 2018. The Centers for Medicare & Medicaid Services still uses Sepsis-2 for diagnosis, and the 3-hr/6-hr bundle compliance (2016) for expected care.3
Session summary
Dr. Kritek, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, presented to a room of over 1,000 enthusiastic hospitalists. She was able to capture everyone’s attention with a great presentation. As sepsis is one of the most common and serious conditions we encounter, most hospitalists are fairly well versed in evidence-based practices, and Dr. Kritek was able to keep us engaged, describing in detail the evolving definition, pathophysiology, and screening procedures for sepsis. She also spoke about important studies and the latest evidence that will positively impact each hospitalist’s practice in treating sepsis.
Dr. Kritek explained clearly how the Surviving Sepsis Campaign developed a vital and nontraditional guideline that “recommends health systems have a performance improvement program for sepsis including screening for high-risk patients.” In a 1-hour session, Dr. Kritek did a commendable job untangling this bewildering health care challenge, and aligned each component to explain how to best use available resources and address sepsis in individual hospitals.
She talked about the statistics and historical aspects involved in the definition of sepsis, and the Surviving Sepsis Campaign. With three good case scenarios, Dr. Kritek explained how it was difficult to accurately diagnose sepsis using the Sepsis-2/SIRS criterion, and how the SIRS criterion led to several false positives. This created a need for the new Sepsis-3 definition, which used delta SOFA score of 2 indicating “organ failure.”
Key takeaways: Screening
- Sepsis-3 with delta SOFA score of at least 2 and Quick SOFA (qSOFA) of at least 2 was best at predicting in-hospital death, ICU admission, and long ICU stay in ED.
- qSOFA was not helpful in the admitted ICU population. An increase of at least 2 points in SOFA score within 24 hours of admission to the ICU was the best predictor of in-hospital mortality and long ICU stays.
- SIRS has high sensitivity and low specificity. The Early Warning Score has accuracy similar to qSOFA.
- Understanding that there is no perfect answer regarding screening, but having a process is vital for each organization. This approach led to the Surviving Sepsis Campaign guideline: “Recommend health systems have a performance improvement program for sepsis including screening for high-risk patients.”
Key takeaways: Treatment
- Meta-analysis showed that specifically targeted, early goal–directed treatment (specifically, central venous pressure 8-12 mm Hg, central venous oxygen saturation greater than 70%, packed red blood cell inotropes used) did not show any improvement in 90-day mortality, and actually generated worse outcomes, including cirrhosis, as well as higher costs of care.
- Antibiotics: Though part of the 3-hour bundle, antibiotics are recommended to be administered within 1 hour.
- Intravenous fluids: Patients with sepsis-induced hypoperfusion need 30 mL/kg crystalloids. Normal saline and lactated ringer are preferred. Lactated ringer has the advantage over normal saline, with a reduced incidence of major adverse kidney events.
- Importance of bundle compliance: N.Y. study showed use of protocols cut mortality from 30.2% to 25.4%.
Refractory septic shock
- Adding hydrocortisone and fludrocortisone improved mortality at 28 days, helped patients get off vasopressors sooner, and ultimately resulted in less organ failure. But no difference in 90-day mortality.
- A study of vitamin C use in septic patients needs further studies to validate, as it only included 47 patients.
- Early renal replacement therapy showed no difference in mortality or length of stay.
Dr. Kritek’s presentation made a positive impact by helping to explain the reasoning behind the established and evolving best practices and guidelines for care of patients with sepsis and septic shock. Her approach will help hospitalists provide cost-effective care, by understanding which expensive interventions and practices do not make a difference in patient care.
Dr. Odeti is hospitalist medical director at Johnston Memorial Hospital in Abingdon, Va. JMH is part of Ballad Health, a health system operating 21 hospitals in northeast Tennessee and southwest Virginia.
References
1. https://www.sepsis.org/wp-content/uploads/2017/05/Sepsis-Fact-Sheet-2018.pdf.
2. http://www.survivingsepsis.org/News/Pages/SCCM-and-ACEP-Release-Joint-Statement-About-the-Surviving-Sepsis-Campaign-Hour-1-Bundle.aspx
3. Specifications Manual for National Hospital Inpatient Quality Measures Discharges 01-01-17 (1Q17) through 12-31-17 (4Q17).
Presenter
Patricia Kritek MD, EdM
Session Title
Sepsis update: From screening to refractory shock
Background
Each year 1.7 million adults in America develop sepsis, and 270,000 Americans die from sepsis annually. Sepsis costs U.S. health care over $27 billion dollars each year. Because of the wide range of etiologies and variation in presentation and intensity, it is a challenge to establish homogeneous evidence based guidelines.1
The definition of sepsis based on the “SIRS” criterion was developed initially in 1992, later revised as Sepsis-2 in 2001. The latest Sepsis-3 definition – “life-threatening organ dysfunction due to a dysregulated host response to infection” – was developed in 2016 by the Third International Consensus Definitions for Sepsis and Septic Shock. This newest definition has renounced the SIRS criterion and adopted the Sequential Organ Failure Assessment (SOFA) score. Treatment guidelines in sepsis were developed by the Surviving Sepsis Campaign starting with the Barcelona Declaration in 2002 and revised multiple times, with the development of 3-hour and 6-hour care bundles in 2012. The latest revision, in 2018, consolidated to a 1-hour bundle.2
Sepsis is a continuum of every severe infection, and with the combined efforts of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, evidence-based guidelines have been developed over the past 2 decades, with the latest iteration in 2018. The Centers for Medicare & Medicaid Services still uses Sepsis-2 for diagnosis, and the 3-hr/6-hr bundle compliance (2016) for expected care.3
Session summary
Dr. Kritek, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, presented to a room of over 1,000 enthusiastic hospitalists. She was able to capture everyone’s attention with a great presentation. As sepsis is one of the most common and serious conditions we encounter, most hospitalists are fairly well versed in evidence-based practices, and Dr. Kritek was able to keep us engaged, describing in detail the evolving definition, pathophysiology, and screening procedures for sepsis. She also spoke about important studies and the latest evidence that will positively impact each hospitalist’s practice in treating sepsis.
Dr. Kritek explained clearly how the Surviving Sepsis Campaign developed a vital and nontraditional guideline that “recommends health systems have a performance improvement program for sepsis including screening for high-risk patients.” In a 1-hour session, Dr. Kritek did a commendable job untangling this bewildering health care challenge, and aligned each component to explain how to best use available resources and address sepsis in individual hospitals.
She talked about the statistics and historical aspects involved in the definition of sepsis, and the Surviving Sepsis Campaign. With three good case scenarios, Dr. Kritek explained how it was difficult to accurately diagnose sepsis using the Sepsis-2/SIRS criterion, and how the SIRS criterion led to several false positives. This created a need for the new Sepsis-3 definition, which used delta SOFA score of 2 indicating “organ failure.”
Key takeaways: Screening
- Sepsis-3 with delta SOFA score of at least 2 and Quick SOFA (qSOFA) of at least 2 was best at predicting in-hospital death, ICU admission, and long ICU stay in ED.
- qSOFA was not helpful in the admitted ICU population. An increase of at least 2 points in SOFA score within 24 hours of admission to the ICU was the best predictor of in-hospital mortality and long ICU stays.
- SIRS has high sensitivity and low specificity. The Early Warning Score has accuracy similar to qSOFA.
- Understanding that there is no perfect answer regarding screening, but having a process is vital for each organization. This approach led to the Surviving Sepsis Campaign guideline: “Recommend health systems have a performance improvement program for sepsis including screening for high-risk patients.”
Key takeaways: Treatment
- Meta-analysis showed that specifically targeted, early goal–directed treatment (specifically, central venous pressure 8-12 mm Hg, central venous oxygen saturation greater than 70%, packed red blood cell inotropes used) did not show any improvement in 90-day mortality, and actually generated worse outcomes, including cirrhosis, as well as higher costs of care.
- Antibiotics: Though part of the 3-hour bundle, antibiotics are recommended to be administered within 1 hour.
- Intravenous fluids: Patients with sepsis-induced hypoperfusion need 30 mL/kg crystalloids. Normal saline and lactated ringer are preferred. Lactated ringer has the advantage over normal saline, with a reduced incidence of major adverse kidney events.
- Importance of bundle compliance: N.Y. study showed use of protocols cut mortality from 30.2% to 25.4%.
Refractory septic shock
- Adding hydrocortisone and fludrocortisone improved mortality at 28 days, helped patients get off vasopressors sooner, and ultimately resulted in less organ failure. But no difference in 90-day mortality.
- A study of vitamin C use in septic patients needs further studies to validate, as it only included 47 patients.
- Early renal replacement therapy showed no difference in mortality or length of stay.
Dr. Kritek’s presentation made a positive impact by helping to explain the reasoning behind the established and evolving best practices and guidelines for care of patients with sepsis and septic shock. Her approach will help hospitalists provide cost-effective care, by understanding which expensive interventions and practices do not make a difference in patient care.
Dr. Odeti is hospitalist medical director at Johnston Memorial Hospital in Abingdon, Va. JMH is part of Ballad Health, a health system operating 21 hospitals in northeast Tennessee and southwest Virginia.
References
1. https://www.sepsis.org/wp-content/uploads/2017/05/Sepsis-Fact-Sheet-2018.pdf.
2. http://www.survivingsepsis.org/News/Pages/SCCM-and-ACEP-Release-Joint-Statement-About-the-Surviving-Sepsis-Campaign-Hour-1-Bundle.aspx
3. Specifications Manual for National Hospital Inpatient Quality Measures Discharges 01-01-17 (1Q17) through 12-31-17 (4Q17).
Presenter
Patricia Kritek MD, EdM
Session Title
Sepsis update: From screening to refractory shock
Background
Each year 1.7 million adults in America develop sepsis, and 270,000 Americans die from sepsis annually. Sepsis costs U.S. health care over $27 billion dollars each year. Because of the wide range of etiologies and variation in presentation and intensity, it is a challenge to establish homogeneous evidence based guidelines.1
The definition of sepsis based on the “SIRS” criterion was developed initially in 1992, later revised as Sepsis-2 in 2001. The latest Sepsis-3 definition – “life-threatening organ dysfunction due to a dysregulated host response to infection” – was developed in 2016 by the Third International Consensus Definitions for Sepsis and Septic Shock. This newest definition has renounced the SIRS criterion and adopted the Sequential Organ Failure Assessment (SOFA) score. Treatment guidelines in sepsis were developed by the Surviving Sepsis Campaign starting with the Barcelona Declaration in 2002 and revised multiple times, with the development of 3-hour and 6-hour care bundles in 2012. The latest revision, in 2018, consolidated to a 1-hour bundle.2
Sepsis is a continuum of every severe infection, and with the combined efforts of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, evidence-based guidelines have been developed over the past 2 decades, with the latest iteration in 2018. The Centers for Medicare & Medicaid Services still uses Sepsis-2 for diagnosis, and the 3-hr/6-hr bundle compliance (2016) for expected care.3
Session summary
Dr. Kritek, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, presented to a room of over 1,000 enthusiastic hospitalists. She was able to capture everyone’s attention with a great presentation. As sepsis is one of the most common and serious conditions we encounter, most hospitalists are fairly well versed in evidence-based practices, and Dr. Kritek was able to keep us engaged, describing in detail the evolving definition, pathophysiology, and screening procedures for sepsis. She also spoke about important studies and the latest evidence that will positively impact each hospitalist’s practice in treating sepsis.
Dr. Kritek explained clearly how the Surviving Sepsis Campaign developed a vital and nontraditional guideline that “recommends health systems have a performance improvement program for sepsis including screening for high-risk patients.” In a 1-hour session, Dr. Kritek did a commendable job untangling this bewildering health care challenge, and aligned each component to explain how to best use available resources and address sepsis in individual hospitals.
She talked about the statistics and historical aspects involved in the definition of sepsis, and the Surviving Sepsis Campaign. With three good case scenarios, Dr. Kritek explained how it was difficult to accurately diagnose sepsis using the Sepsis-2/SIRS criterion, and how the SIRS criterion led to several false positives. This created a need for the new Sepsis-3 definition, which used delta SOFA score of 2 indicating “organ failure.”
Key takeaways: Screening
- Sepsis-3 with delta SOFA score of at least 2 and Quick SOFA (qSOFA) of at least 2 was best at predicting in-hospital death, ICU admission, and long ICU stay in ED.
- qSOFA was not helpful in the admitted ICU population. An increase of at least 2 points in SOFA score within 24 hours of admission to the ICU was the best predictor of in-hospital mortality and long ICU stays.
- SIRS has high sensitivity and low specificity. The Early Warning Score has accuracy similar to qSOFA.
- Understanding that there is no perfect answer regarding screening, but having a process is vital for each organization. This approach led to the Surviving Sepsis Campaign guideline: “Recommend health systems have a performance improvement program for sepsis including screening for high-risk patients.”
Key takeaways: Treatment
- Meta-analysis showed that specifically targeted, early goal–directed treatment (specifically, central venous pressure 8-12 mm Hg, central venous oxygen saturation greater than 70%, packed red blood cell inotropes used) did not show any improvement in 90-day mortality, and actually generated worse outcomes, including cirrhosis, as well as higher costs of care.
- Antibiotics: Though part of the 3-hour bundle, antibiotics are recommended to be administered within 1 hour.
- Intravenous fluids: Patients with sepsis-induced hypoperfusion need 30 mL/kg crystalloids. Normal saline and lactated ringer are preferred. Lactated ringer has the advantage over normal saline, with a reduced incidence of major adverse kidney events.
- Importance of bundle compliance: N.Y. study showed use of protocols cut mortality from 30.2% to 25.4%.
Refractory septic shock
- Adding hydrocortisone and fludrocortisone improved mortality at 28 days, helped patients get off vasopressors sooner, and ultimately resulted in less organ failure. But no difference in 90-day mortality.
- A study of vitamin C use in septic patients needs further studies to validate, as it only included 47 patients.
- Early renal replacement therapy showed no difference in mortality or length of stay.
Dr. Kritek’s presentation made a positive impact by helping to explain the reasoning behind the established and evolving best practices and guidelines for care of patients with sepsis and septic shock. Her approach will help hospitalists provide cost-effective care, by understanding which expensive interventions and practices do not make a difference in patient care.
Dr. Odeti is hospitalist medical director at Johnston Memorial Hospital in Abingdon, Va. JMH is part of Ballad Health, a health system operating 21 hospitals in northeast Tennessee and southwest Virginia.
References
1. https://www.sepsis.org/wp-content/uploads/2017/05/Sepsis-Fact-Sheet-2018.pdf.
2. http://www.survivingsepsis.org/News/Pages/SCCM-and-ACEP-Release-Joint-Statement-About-the-Surviving-Sepsis-Campaign-Hour-1-Bundle.aspx
3. Specifications Manual for National Hospital Inpatient Quality Measures Discharges 01-01-17 (1Q17) through 12-31-17 (4Q17).
Quick Byte: Conversations about cost
Patients want to talk
Seventy percent of Americans would like to have conversations about the costs of care with their health care providers, but only 28% do so, according to polling conducted for the Robert Wood Johnson Foundation (RWJF) by Avalere Health.
With those polling results in hand, 2 years ago RWJF and Avalere Health launched the Cost Conversation projects to help. Practice briefs for these kinds of conversations are now available on America’s Essential Hospitals’ website (https://essentialhospitals.org/cost-care/practice-briefs/).
Reference
1. Ganos E, Steinberg K, Seidman J, Masi D, Gomez-Rexode A, Fraser A. Talking About Costs: Innovation In Clinician-Patient Conversations. Health Affairs. Published online Nov. 27, 2018. doi: 10.1377/hblog20181126.366161. Accessed Dec. 11, 2018.
Patients want to talk
Patients want to talk
Seventy percent of Americans would like to have conversations about the costs of care with their health care providers, but only 28% do so, according to polling conducted for the Robert Wood Johnson Foundation (RWJF) by Avalere Health.
With those polling results in hand, 2 years ago RWJF and Avalere Health launched the Cost Conversation projects to help. Practice briefs for these kinds of conversations are now available on America’s Essential Hospitals’ website (https://essentialhospitals.org/cost-care/practice-briefs/).
Reference
1. Ganos E, Steinberg K, Seidman J, Masi D, Gomez-Rexode A, Fraser A. Talking About Costs: Innovation In Clinician-Patient Conversations. Health Affairs. Published online Nov. 27, 2018. doi: 10.1377/hblog20181126.366161. Accessed Dec. 11, 2018.
Seventy percent of Americans would like to have conversations about the costs of care with their health care providers, but only 28% do so, according to polling conducted for the Robert Wood Johnson Foundation (RWJF) by Avalere Health.
With those polling results in hand, 2 years ago RWJF and Avalere Health launched the Cost Conversation projects to help. Practice briefs for these kinds of conversations are now available on America’s Essential Hospitals’ website (https://essentialhospitals.org/cost-care/practice-briefs/).
Reference
1. Ganos E, Steinberg K, Seidman J, Masi D, Gomez-Rexode A, Fraser A. Talking About Costs: Innovation In Clinician-Patient Conversations. Health Affairs. Published online Nov. 27, 2018. doi: 10.1377/hblog20181126.366161. Accessed Dec. 11, 2018.
Abuse rate of gabapentin, pregabalin far below that of opioids
SAN ANTONIO – Prescription opioid abuse has continued declining since 2011, but opioids remain far more commonly abused than other prescription drugs, including gabapentin and pregabalin, new research shows.
“Both gabapentin and pregabalin are abused but at rates that are 6-56 times less frequent than for opioid analgesics,” wrote Kofi Asomaning, DSci, of Pfizer, and associates at Pfizer and Denver Health’s Rocky Mountain Poison and Drug Center.
“Gabapentin is generally more frequently abused than pregabalin,” they reported in a research poster at the annual meeting of the College on Problems of Drug Dependence.
The researchers analyzed data from the RADARS System Survey of Non-Medical Use of Prescription Drugs Program (NMURx), the RADARS System Treatment Center Programs Combined, and the American Association of Poison Control Centers National Poison Data System (NPDS).
All those use self-reported data. The first is a confidential, anonymous web-based survey used to estimate population-level prevalence, and the second surveys patients with opioid use disorder entering treatment. The NPDS tracks all cases reported to poison control centers nationally.
Analysis of the NMURx data revealed similar lifetime abuse prevalence rates for gabapentin and pregabalin at 0.4%, several magnitudes lower than the 5.3% rate identified with opioids.
Gabapentin, however, had higher rates of abuse in the past month in the Treatment Center Programs Combined. For the third to fourth quarter of 2017, 0.12 per 100,000 population reportedly abused gabapentin, compared with 0.01 per 100,000 for pregabalin. The rate for past-month abuse of opioids was 0.79 per 100,000.
A similar pattern for the same quarter emerged from the NPDS data: Rate of gabapentin abuse was 0.06 per 100,000, rate for pregabalin was 0.01 per 100,000, and rate for opioids was 0.40 per 100,000.
Both pregabalin and opioids were predominantly ingested, though a very small amount of each was inhaled and a similarly small amount of opioids was injected. Data on exposure route for gabapentin were not provided, though it was used more frequently than pregabalin.
The research was funded by Pfizer. The RADARS system is owned by Denver Health and Hospital Authority under the Colorado state government. RADARS receives some funding from pharmaceutical industry subscriptions. Dr. Asomaning and Diane L. Martire, MD, MPH, are Pfizer employees who have financial interests with Pfizer.
SAN ANTONIO – Prescription opioid abuse has continued declining since 2011, but opioids remain far more commonly abused than other prescription drugs, including gabapentin and pregabalin, new research shows.
“Both gabapentin and pregabalin are abused but at rates that are 6-56 times less frequent than for opioid analgesics,” wrote Kofi Asomaning, DSci, of Pfizer, and associates at Pfizer and Denver Health’s Rocky Mountain Poison and Drug Center.
“Gabapentin is generally more frequently abused than pregabalin,” they reported in a research poster at the annual meeting of the College on Problems of Drug Dependence.
The researchers analyzed data from the RADARS System Survey of Non-Medical Use of Prescription Drugs Program (NMURx), the RADARS System Treatment Center Programs Combined, and the American Association of Poison Control Centers National Poison Data System (NPDS).
All those use self-reported data. The first is a confidential, anonymous web-based survey used to estimate population-level prevalence, and the second surveys patients with opioid use disorder entering treatment. The NPDS tracks all cases reported to poison control centers nationally.
Analysis of the NMURx data revealed similar lifetime abuse prevalence rates for gabapentin and pregabalin at 0.4%, several magnitudes lower than the 5.3% rate identified with opioids.
Gabapentin, however, had higher rates of abuse in the past month in the Treatment Center Programs Combined. For the third to fourth quarter of 2017, 0.12 per 100,000 population reportedly abused gabapentin, compared with 0.01 per 100,000 for pregabalin. The rate for past-month abuse of opioids was 0.79 per 100,000.
A similar pattern for the same quarter emerged from the NPDS data: Rate of gabapentin abuse was 0.06 per 100,000, rate for pregabalin was 0.01 per 100,000, and rate for opioids was 0.40 per 100,000.
Both pregabalin and opioids were predominantly ingested, though a very small amount of each was inhaled and a similarly small amount of opioids was injected. Data on exposure route for gabapentin were not provided, though it was used more frequently than pregabalin.
The research was funded by Pfizer. The RADARS system is owned by Denver Health and Hospital Authority under the Colorado state government. RADARS receives some funding from pharmaceutical industry subscriptions. Dr. Asomaning and Diane L. Martire, MD, MPH, are Pfizer employees who have financial interests with Pfizer.
SAN ANTONIO – Prescription opioid abuse has continued declining since 2011, but opioids remain far more commonly abused than other prescription drugs, including gabapentin and pregabalin, new research shows.
“Both gabapentin and pregabalin are abused but at rates that are 6-56 times less frequent than for opioid analgesics,” wrote Kofi Asomaning, DSci, of Pfizer, and associates at Pfizer and Denver Health’s Rocky Mountain Poison and Drug Center.
“Gabapentin is generally more frequently abused than pregabalin,” they reported in a research poster at the annual meeting of the College on Problems of Drug Dependence.
The researchers analyzed data from the RADARS System Survey of Non-Medical Use of Prescription Drugs Program (NMURx), the RADARS System Treatment Center Programs Combined, and the American Association of Poison Control Centers National Poison Data System (NPDS).
All those use self-reported data. The first is a confidential, anonymous web-based survey used to estimate population-level prevalence, and the second surveys patients with opioid use disorder entering treatment. The NPDS tracks all cases reported to poison control centers nationally.
Analysis of the NMURx data revealed similar lifetime abuse prevalence rates for gabapentin and pregabalin at 0.4%, several magnitudes lower than the 5.3% rate identified with opioids.
Gabapentin, however, had higher rates of abuse in the past month in the Treatment Center Programs Combined. For the third to fourth quarter of 2017, 0.12 per 100,000 population reportedly abused gabapentin, compared with 0.01 per 100,000 for pregabalin. The rate for past-month abuse of opioids was 0.79 per 100,000.
A similar pattern for the same quarter emerged from the NPDS data: Rate of gabapentin abuse was 0.06 per 100,000, rate for pregabalin was 0.01 per 100,000, and rate for opioids was 0.40 per 100,000.
Both pregabalin and opioids were predominantly ingested, though a very small amount of each was inhaled and a similarly small amount of opioids was injected. Data on exposure route for gabapentin were not provided, though it was used more frequently than pregabalin.
The research was funded by Pfizer. The RADARS system is owned by Denver Health and Hospital Authority under the Colorado state government. RADARS receives some funding from pharmaceutical industry subscriptions. Dr. Asomaning and Diane L. Martire, MD, MPH, are Pfizer employees who have financial interests with Pfizer.
REPORTING FROM CPDD 2019
Adjuvant corticosteroids in hospitalized patients with CAP
When is it appropriate to treat?
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
When is it appropriate to treat?
When is it appropriate to treat?
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
Rivaroxaban tied to higher GI bleeding than other NOACs
SAN DIEGO – Patients on rivaroxaban had significantly higher rates of GI bleeding, compared with those taking apixaban or dabigatran, results from a large population-based study showed.
“This may be due to the fact that rivaroxaban is administered as a single daily dose as opposed to the other two non–vitamin K anticoagulants [NOACs], which are given twice daily,” lead study author Arnar B. Ingason said at the annual Digestive Disease Week. “This may lead to a greater variance in plasma drug concentration, making these patients more susceptible to bleeding.”
Mr. Ingason, a medical student at the University of Iceland, Reykjavik, said that although several studies have compared warfarin with NOACs, it remains unclear which NOAC has the most favorable GI profile. In an effort to improve the research in this area, he and his associates performed a nationwide, population-based study during March 2014–Jan. 2018 to compare the GI bleeding risk of patients receiving rivaroxaban to that of a combined pool of patients receiving either apixaban or dabigatran. They drew from the Icelandic Medicine Registry, which contains all outpatient drug prescriptions in the country. Next, the researchers linked the personal identification numbers of patients to the Landspitali University diagnoses registry, which includes more than 90% of all patients hospitalized for GI bleeding. They used 1:1 nearest neighbor propensity score for matching and Kaplan-Meier survival estimates and Cox regression to compare rates of GI bleeding. The study outcome of interest was any clinically relevant GI bleeding.
Mr. Ingason reported that the baseline characteristics were similar between the rivaroxaban group and the apixaban/dabigatran group. They matched for several variables, including age, sex, Charlson score, the proportion being anticoagulant naive, moderate to severe renal disease, moderate to severe liver disease, any prior bleeding, and any prior thrombotic events.
During the study period, 3,473 patients received rivaroxaban, 1,901 received apixaban, and 1,086 received dabigatran. After propensity score matching, the researchers compared 2,635 patients who received rivaroxaban with 2,365 patients who received either apixaban or dabigatran. They found that patients in the rivaroxaban group had significantly higher rates of GI bleeding, compared with in the apixaban/dabigatran group (1.2 and. 0.6 events per 100 patient-years, respectively). This yielded a hazard ratio of 2.02, “which means that patients receiving rivaroxaban are twice as likely to get GI bleeding compared to patients on apixaban or dabigatran,” Mr. Ingason said. When the researchers examined the entire unmatched cohort of patients, the rivaroxaban group also had significantly higher rates of GI bleeding, compared with the apixaban/dabigatran group (1.0 and 0.6 events per 100 patient-years; HR, 1.75).
Mr. Ingason and his colleagues observed that patients in the rivaroxaban group had higher rates of GI bleeding, compared with the apixaban/dabigatran group, during the entire follow-up period. At the end of year 4, the rivaroxaban group had a 4% cumulative event rate of GI bleeding, compared with 1.8% for the apixaban/dabigatran group, a highly significant difference at P = .0057).
When a meeting attendee asked Mr. Ingason why patients taking apixaban or dabigatran were combined into one group, he said that it was done to increase the power of their study. “Our theory was that rivaroxaban was different because it is administered as a single daily dose, while the others are given twice daily,” he said. The researchers reported having no financial disclosures.
SAN DIEGO – Patients on rivaroxaban had significantly higher rates of GI bleeding, compared with those taking apixaban or dabigatran, results from a large population-based study showed.
“This may be due to the fact that rivaroxaban is administered as a single daily dose as opposed to the other two non–vitamin K anticoagulants [NOACs], which are given twice daily,” lead study author Arnar B. Ingason said at the annual Digestive Disease Week. “This may lead to a greater variance in plasma drug concentration, making these patients more susceptible to bleeding.”
Mr. Ingason, a medical student at the University of Iceland, Reykjavik, said that although several studies have compared warfarin with NOACs, it remains unclear which NOAC has the most favorable GI profile. In an effort to improve the research in this area, he and his associates performed a nationwide, population-based study during March 2014–Jan. 2018 to compare the GI bleeding risk of patients receiving rivaroxaban to that of a combined pool of patients receiving either apixaban or dabigatran. They drew from the Icelandic Medicine Registry, which contains all outpatient drug prescriptions in the country. Next, the researchers linked the personal identification numbers of patients to the Landspitali University diagnoses registry, which includes more than 90% of all patients hospitalized for GI bleeding. They used 1:1 nearest neighbor propensity score for matching and Kaplan-Meier survival estimates and Cox regression to compare rates of GI bleeding. The study outcome of interest was any clinically relevant GI bleeding.
Mr. Ingason reported that the baseline characteristics were similar between the rivaroxaban group and the apixaban/dabigatran group. They matched for several variables, including age, sex, Charlson score, the proportion being anticoagulant naive, moderate to severe renal disease, moderate to severe liver disease, any prior bleeding, and any prior thrombotic events.
During the study period, 3,473 patients received rivaroxaban, 1,901 received apixaban, and 1,086 received dabigatran. After propensity score matching, the researchers compared 2,635 patients who received rivaroxaban with 2,365 patients who received either apixaban or dabigatran. They found that patients in the rivaroxaban group had significantly higher rates of GI bleeding, compared with in the apixaban/dabigatran group (1.2 and. 0.6 events per 100 patient-years, respectively). This yielded a hazard ratio of 2.02, “which means that patients receiving rivaroxaban are twice as likely to get GI bleeding compared to patients on apixaban or dabigatran,” Mr. Ingason said. When the researchers examined the entire unmatched cohort of patients, the rivaroxaban group also had significantly higher rates of GI bleeding, compared with the apixaban/dabigatran group (1.0 and 0.6 events per 100 patient-years; HR, 1.75).
Mr. Ingason and his colleagues observed that patients in the rivaroxaban group had higher rates of GI bleeding, compared with the apixaban/dabigatran group, during the entire follow-up period. At the end of year 4, the rivaroxaban group had a 4% cumulative event rate of GI bleeding, compared with 1.8% for the apixaban/dabigatran group, a highly significant difference at P = .0057).
When a meeting attendee asked Mr. Ingason why patients taking apixaban or dabigatran were combined into one group, he said that it was done to increase the power of their study. “Our theory was that rivaroxaban was different because it is administered as a single daily dose, while the others are given twice daily,” he said. The researchers reported having no financial disclosures.
SAN DIEGO – Patients on rivaroxaban had significantly higher rates of GI bleeding, compared with those taking apixaban or dabigatran, results from a large population-based study showed.
“This may be due to the fact that rivaroxaban is administered as a single daily dose as opposed to the other two non–vitamin K anticoagulants [NOACs], which are given twice daily,” lead study author Arnar B. Ingason said at the annual Digestive Disease Week. “This may lead to a greater variance in plasma drug concentration, making these patients more susceptible to bleeding.”
Mr. Ingason, a medical student at the University of Iceland, Reykjavik, said that although several studies have compared warfarin with NOACs, it remains unclear which NOAC has the most favorable GI profile. In an effort to improve the research in this area, he and his associates performed a nationwide, population-based study during March 2014–Jan. 2018 to compare the GI bleeding risk of patients receiving rivaroxaban to that of a combined pool of patients receiving either apixaban or dabigatran. They drew from the Icelandic Medicine Registry, which contains all outpatient drug prescriptions in the country. Next, the researchers linked the personal identification numbers of patients to the Landspitali University diagnoses registry, which includes more than 90% of all patients hospitalized for GI bleeding. They used 1:1 nearest neighbor propensity score for matching and Kaplan-Meier survival estimates and Cox regression to compare rates of GI bleeding. The study outcome of interest was any clinically relevant GI bleeding.
Mr. Ingason reported that the baseline characteristics were similar between the rivaroxaban group and the apixaban/dabigatran group. They matched for several variables, including age, sex, Charlson score, the proportion being anticoagulant naive, moderate to severe renal disease, moderate to severe liver disease, any prior bleeding, and any prior thrombotic events.
During the study period, 3,473 patients received rivaroxaban, 1,901 received apixaban, and 1,086 received dabigatran. After propensity score matching, the researchers compared 2,635 patients who received rivaroxaban with 2,365 patients who received either apixaban or dabigatran. They found that patients in the rivaroxaban group had significantly higher rates of GI bleeding, compared with in the apixaban/dabigatran group (1.2 and. 0.6 events per 100 patient-years, respectively). This yielded a hazard ratio of 2.02, “which means that patients receiving rivaroxaban are twice as likely to get GI bleeding compared to patients on apixaban or dabigatran,” Mr. Ingason said. When the researchers examined the entire unmatched cohort of patients, the rivaroxaban group also had significantly higher rates of GI bleeding, compared with the apixaban/dabigatran group (1.0 and 0.6 events per 100 patient-years; HR, 1.75).
Mr. Ingason and his colleagues observed that patients in the rivaroxaban group had higher rates of GI bleeding, compared with the apixaban/dabigatran group, during the entire follow-up period. At the end of year 4, the rivaroxaban group had a 4% cumulative event rate of GI bleeding, compared with 1.8% for the apixaban/dabigatran group, a highly significant difference at P = .0057).
When a meeting attendee asked Mr. Ingason why patients taking apixaban or dabigatran were combined into one group, he said that it was done to increase the power of their study. “Our theory was that rivaroxaban was different because it is administered as a single daily dose, while the others are given twice daily,” he said. The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
Creating better performance incentives
P4P programs suffer from several flaws
Many performance improvement programs try to create a higher value health system by incentivizing physicians and health systems to behave in particular ways. These have often been pay-for-performance programs that offer bonuses or impose penalties depending on how providers perform on various metrics.
“In theory, this makes sense,” said Dhruv Khullar, MD, MPP, lead author of a JAMA article about the future of incentives, and assistant professor at Weill Cornell Medicine in New York. “But in practice, these programs have not been successful in consistently improving quality, and sometimes they have been counterproductive. In our article, we argued that focusing too narrowly on financial rewards is not the right strategy to improve health system performance – and is sometimes at odds with the physician professionalism and what really motivates most clinicians.”
Pay-for-performance programs suffer from several fundamental flaws: they focus too narrowly on financial incentives and use centralized accountability instead of local culture, for example, Dr. Khullar said.
“A better future state would involve capitalizing on physician professionalism through nonfinancial rewards, resources for quality improvement, team-based assessments, and emphasizing continuous learning and organizational culture,” he noted. Performance programs would take a more global view of clinical care by emphasizing culture, teams, trust, and learning. Such a system would allow hospitalists and other physicians to worry less about meeting specific metrics and focus more on providing high-quality care to their patients.
“I would hope physicians, payers, and administrators would reconsider some previously held beliefs about quality improvement, especially the idea that better quality requires giving people bonus payments or imposing financial penalties,” Dr. Khullar said. “We believe the next wave of performance improvement programs should entertain other paths to better quality, which are more in line with human motivation and physician professionalism.”
Reference
1. Khullar D, Wolfson D, Casalino LP. Professionalism, Performance, and the Future of Physician Incentives. JAMA. 2018 Nov 26 (Epub ahead of print). doi: 10.1001/jama.2018.17719. Accessed Dec. 11, 2018.
P4P programs suffer from several flaws
P4P programs suffer from several flaws
Many performance improvement programs try to create a higher value health system by incentivizing physicians and health systems to behave in particular ways. These have often been pay-for-performance programs that offer bonuses or impose penalties depending on how providers perform on various metrics.
“In theory, this makes sense,” said Dhruv Khullar, MD, MPP, lead author of a JAMA article about the future of incentives, and assistant professor at Weill Cornell Medicine in New York. “But in practice, these programs have not been successful in consistently improving quality, and sometimes they have been counterproductive. In our article, we argued that focusing too narrowly on financial rewards is not the right strategy to improve health system performance – and is sometimes at odds with the physician professionalism and what really motivates most clinicians.”
Pay-for-performance programs suffer from several fundamental flaws: they focus too narrowly on financial incentives and use centralized accountability instead of local culture, for example, Dr. Khullar said.
“A better future state would involve capitalizing on physician professionalism through nonfinancial rewards, resources for quality improvement, team-based assessments, and emphasizing continuous learning and organizational culture,” he noted. Performance programs would take a more global view of clinical care by emphasizing culture, teams, trust, and learning. Such a system would allow hospitalists and other physicians to worry less about meeting specific metrics and focus more on providing high-quality care to their patients.
“I would hope physicians, payers, and administrators would reconsider some previously held beliefs about quality improvement, especially the idea that better quality requires giving people bonus payments or imposing financial penalties,” Dr. Khullar said. “We believe the next wave of performance improvement programs should entertain other paths to better quality, which are more in line with human motivation and physician professionalism.”
Reference
1. Khullar D, Wolfson D, Casalino LP. Professionalism, Performance, and the Future of Physician Incentives. JAMA. 2018 Nov 26 (Epub ahead of print). doi: 10.1001/jama.2018.17719. Accessed Dec. 11, 2018.
Many performance improvement programs try to create a higher value health system by incentivizing physicians and health systems to behave in particular ways. These have often been pay-for-performance programs that offer bonuses or impose penalties depending on how providers perform on various metrics.
“In theory, this makes sense,” said Dhruv Khullar, MD, MPP, lead author of a JAMA article about the future of incentives, and assistant professor at Weill Cornell Medicine in New York. “But in practice, these programs have not been successful in consistently improving quality, and sometimes they have been counterproductive. In our article, we argued that focusing too narrowly on financial rewards is not the right strategy to improve health system performance – and is sometimes at odds with the physician professionalism and what really motivates most clinicians.”
Pay-for-performance programs suffer from several fundamental flaws: they focus too narrowly on financial incentives and use centralized accountability instead of local culture, for example, Dr. Khullar said.
“A better future state would involve capitalizing on physician professionalism through nonfinancial rewards, resources for quality improvement, team-based assessments, and emphasizing continuous learning and organizational culture,” he noted. Performance programs would take a more global view of clinical care by emphasizing culture, teams, trust, and learning. Such a system would allow hospitalists and other physicians to worry less about meeting specific metrics and focus more on providing high-quality care to their patients.
“I would hope physicians, payers, and administrators would reconsider some previously held beliefs about quality improvement, especially the idea that better quality requires giving people bonus payments or imposing financial penalties,” Dr. Khullar said. “We believe the next wave of performance improvement programs should entertain other paths to better quality, which are more in line with human motivation and physician professionalism.”
Reference
1. Khullar D, Wolfson D, Casalino LP. Professionalism, Performance, and the Future of Physician Incentives. JAMA. 2018 Nov 26 (Epub ahead of print). doi: 10.1001/jama.2018.17719. Accessed Dec. 11, 2018.

















