User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Hospital-acquired conditions drop 8% since 2014, saving 8,000 lives and $3 billion
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
From 2014 to 2016, the rate of potentially deadly hospital-acquired conditions in the United States dropped by 8% – a change that translated into 350,000 fewer such conditions, 8,000 fewer inpatient deaths, and a national savings of almost $3 billion.
The preliminary new baseline rate for hospital-acquired conditions (HACs) is 90 per 1,000 discharges – down from 98 per 1,000 discharges at the end of 2014, according to the Agency for Healthcare Research and Quality’s new report, “AHRQ National Scorecard on Hospital-Acquired Conditions – Updated Baseline Rates and Preliminary Results 2014-2016.”
The largest improvements occurred in central line–associated bloodstream infections (down 31% from 2014), postoperative venous thromboembolism (21% decline), adverse drug events (15% decline), and pressure ulcers (10% decline). A new category, C. difficile infections, also showed a large decline over 2014 (11%).
These numbers build on earlier successes associated with a national goal set by the Centers for Medicare & Medicaid Services to reduce HACs by 20% by 2019. They should be hailed as proof that attention to prevention strategies can save lives and money, said Seema Verma, CMS administrator.
“Today’s results show that this is a tremendous accomplishment by America’s hospitals in delivering high-quality, affordable healthcare,” Ms. Verma said in a press statement. “CMS is committed to moving the healthcare system to one that improves quality and fosters innovation while reducing administrative burden and lowering costs. This work could not be accomplished without the concerted effort of our many hospital, patient, provider, private, and federal partners – all working together to ensure the best possible care by protecting patients from harm and making care safer.”
The numbers continue to go in the right direction, the report noted. Data reported in late 2016 found a 17% decline in HACs from 2010 to 2014. This equated to 2.1 million HACs, 87,000 fewer deaths, and a savings of $19.9 billion.
Much work remains to be done to achieve the stated 2019 goal, the report noted, but the rewards are great. Reaching the 20% reduction goal would secure a total decrease in the HAC rate from 98 to 78 per 1,000 discharges. This would result in 1.78 million fewer HAC in the years from 2015-2019. That decrease would ultimately save 53,000 lives and $19.1 billion over 5 years.
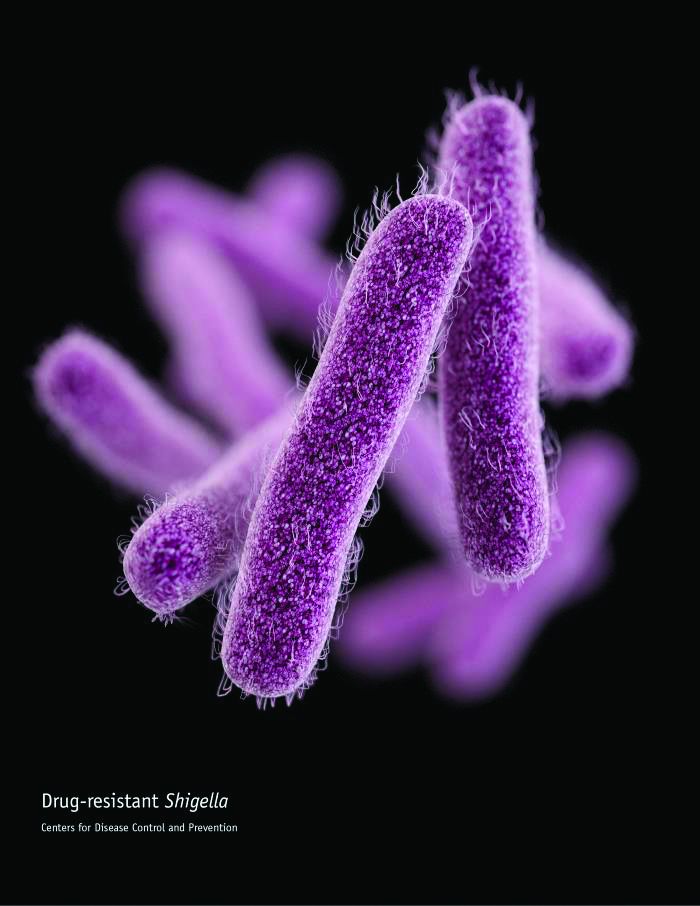
CDC concerned about multidrug-resistant Shigella
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
The Centers for Disease Control and Prevention have issued follow-up recommendations for managing and reporting Shigella infections because of concerns about increasing antibiotic resistance and the possibility of treatment failures.
Isolates with no resistance to quinolone antibiotics have ciprofloxacin minimum inhibitory concentration (MIC) values of less than 0.015 mcg/mL. However, the CDC has continued to identify isolates of Shigella that, while still within the susceptible range for the fluoroquinolone antibiotic ciprofloxacin (that is, having MIC values less than 1 mcg/mL), have MIC values for ciprofloxacin of 0.12-1.0 mcg/mL, thus appearing to harbor one or more resistance mechanisms. Furthermore, the CDC has identified an increasing number of isolates that have MIC values for azithromycin exceeding the epidemiologic cutoff value, which suggests some form of acquired resistance.
“CDC is particularly concerned about people who are at high risk for multidrug-resistant Shigella infections and are more likely to require antibiotic treatment, such as men who have sex with men, patients who are homeless, and immunocompromised patients. These patients often have more severe disease, prolonged shedding, and recurrent infections,” the recommendations stated.
More information can be found in the CDC’s Health Alert Network release.
A U.S. model for Italian hospitals?
In the United States, family physicians (general practitioners) used to manage their patients in the hospital, either as the primary care doctor or in consultation with specialists. Only since the 1990s has a new kind of physician gained widespread acceptance: the hospitalist (“specialist of inpatient care”).1
In Italy the process has not been the same. In our health care system, primary care physicians have always transferred the responsibility of hospital care to an inpatient team. Actually, our hospital-based doctors dedicate their whole working time to inpatient care, and general practitioners are not expected to go to the hospital. The patients were (and are) admitted to one ward or another according to their main clinical problem.
Little by little, a huge number of organ specialty and subspecialty wards have filled Italian hospitals. In this context, the internal medicine specialty was unable to occupy its characteristic role, so that, a few years ago, the medical community wondered if the specialty should have continued to exist.
Anyway, as a result of hyperspecialization, we have many different specialists in inpatient care who are not specialists in global inpatient care.
Nowadays, in our country we are faced with a dramatic epidemiologic change. The Italian population is aging and the majority of patients have not only one clinical problem but multiple comorbidities. When these patients reach the emergency department, it is not easy to identify the main clinical problem and assign him/her to an organ specialty unit. And when he or she eventually arrives there, a considerable number of consultants is frequently required. The vision of organ specialists is not holistic, and they are more prone to maximizing their tools than rationalizing them. So, at present, our traditional hospital model has been generating care fragmentation, overproduction of diagnoses, overprescription of drugs, and increasing costs.
It is obvious that a new model is necessary for the future, and we look with great interest at the American hospitalist model.
We need a new hospital-based clinician who has wide-ranging competencies, and is able to define priorities and appropriateness of care when a patient requires multiple specialists’ interventions; one who is autonomous in performing basic procedures and expert in perioperative medicine; prompt to communicate with primary care doctors at the time of admission and discharge; and prepared to work in managed-care organizations.
We wonder: Are Italian hospital-based internists – the only specialists in global inpatient care – suited to this role?
We think so. However, current Italian training in internal medicine is focused mainly on scientific bases of diseases, pathophysiological, and clinical aspects. Concepts such as complexity or the management of patients with comorbidities are quite difficult to teach to medical school students and therefore often neglected. As a result, internal medicine physicians require a prolonged practical training.
Inspired by the Core Competencies in Hospital Medicine published by the Society of Hospital Medicine, this year in Genoa (the birthplace of Christopher Columbus) we started a 2-year second-level University Master course, called “Hospitalist: Managing complexity in Internal Medicine inpatients” for 35 internal medicine specialists. It is the fruit of collaboration between the main association of Italian hospital-based internists (Federation of Associations of Hospital Doctors on Internal Medicine, or FADOI) and the University of Genoa’s Department of Internal Medicine, Academy of Health Management, and the Center of Simulation and Advanced Training.
In Italy, this is the first concrete initiative to train, and better define, this new type of physician expert in the management of inpatients.
According to SHM’s definition of a hospitalist, we think that the activities of this new physician should also include teaching and research related to hospital medicine. And as Dr. Steven Pantilat wrote, “patient safety, leadership, palliative care and quality improvement are the issues that pertain to all hospitalists.”2
Theoretically, the development of the hospitalist model should be easier in Italy when compared to the United States. Dr. Robert Wachter and Dr. Lee Goldman wrote in 1996 about the objections to the hospitalist model of American primary care physicians (“to preserve continuity”) and specialists (“fewer consultations, lower income”), but in Italy family doctors do not usually follow their patients in the hospital, and specialists have no incentive for in-hospital consultations.3 Moreover, patients with comorbidities, or pathologies on the border between medicine and surgery (e.g. cholecystitis, bowel obstruction, polytrauma, etc.), are already often assigned to internal medicine, and in the smallest hospitals, the internist is most of the time the only specialist doctor continually present.
Nevertheless, the Italian hospitalist model will be a challenge. We know we have to deal with organ specialists, but we strongly believe that this is the most appropriate and the most sustainable model for the future of the Italian hospitals. Our wish is not to become the “bosses” of the hospital, but to ensure global, coordinated, and respectful care to present and future patients.
Published outcomes studies demonstrate that the U.S. hospitalist model has led to consistent and pronounced cost saving with no loss in quality.4 In the United States, the hospitalist field has grown from a few hundred physicians to more than 50,000,5 making it the fastest growing physician specialty in medical history.
Why should the same not occur in Italy?
References
1. Baudendistel TE, Watcher RM. The evolution of the hospitalist movement in USA. Clin Med JRCPL. 2002;2:327-30.
2. Pantilat S. What is a Hospitalist? The Hospitalist 2006 February;2006(2).
3. Wachter RM, Goldman Lee. The emerging role of “Hospitalists” in the American Health Care System. N Engl J Med. 1996;335:514-7.
4. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Medicine. 2011;9:58:1-22. http://www.biomedcentral.com/1741-7015/9/58.
5. Wachter RM, Goldman L. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375:1009-11.
Valerio Verdiani, MD, director of internal medicine, Grosseto, Italy. Francesco Orlandini, MD, internal medicine, health administrator, ASL4 Liguria, Chiavari (GE), Italy. Micaela La Regina, MD, internal medicine, risk management and clinical governance, ASL5 Liguria, La Spezia, Italy. Giovanni Murialdo, MD, department of internal medicine and medical specialty, University of Genoa (Italy). Andrea Fontanella, MD, director of medicine department, president of the Federation of Associations of Hospital Doctors on Internal Medicine (FADOI), Naples, Italy. Mauro Silingardi, MD, director of internal medicine, director of training and refresher of FADOI, Bologna, Italy.
In the United States, family physicians (general practitioners) used to manage their patients in the hospital, either as the primary care doctor or in consultation with specialists. Only since the 1990s has a new kind of physician gained widespread acceptance: the hospitalist (“specialist of inpatient care”).1
In Italy the process has not been the same. In our health care system, primary care physicians have always transferred the responsibility of hospital care to an inpatient team. Actually, our hospital-based doctors dedicate their whole working time to inpatient care, and general practitioners are not expected to go to the hospital. The patients were (and are) admitted to one ward or another according to their main clinical problem.
Little by little, a huge number of organ specialty and subspecialty wards have filled Italian hospitals. In this context, the internal medicine specialty was unable to occupy its characteristic role, so that, a few years ago, the medical community wondered if the specialty should have continued to exist.
Anyway, as a result of hyperspecialization, we have many different specialists in inpatient care who are not specialists in global inpatient care.
Nowadays, in our country we are faced with a dramatic epidemiologic change. The Italian population is aging and the majority of patients have not only one clinical problem but multiple comorbidities. When these patients reach the emergency department, it is not easy to identify the main clinical problem and assign him/her to an organ specialty unit. And when he or she eventually arrives there, a considerable number of consultants is frequently required. The vision of organ specialists is not holistic, and they are more prone to maximizing their tools than rationalizing them. So, at present, our traditional hospital model has been generating care fragmentation, overproduction of diagnoses, overprescription of drugs, and increasing costs.
It is obvious that a new model is necessary for the future, and we look with great interest at the American hospitalist model.
We need a new hospital-based clinician who has wide-ranging competencies, and is able to define priorities and appropriateness of care when a patient requires multiple specialists’ interventions; one who is autonomous in performing basic procedures and expert in perioperative medicine; prompt to communicate with primary care doctors at the time of admission and discharge; and prepared to work in managed-care organizations.
We wonder: Are Italian hospital-based internists – the only specialists in global inpatient care – suited to this role?
We think so. However, current Italian training in internal medicine is focused mainly on scientific bases of diseases, pathophysiological, and clinical aspects. Concepts such as complexity or the management of patients with comorbidities are quite difficult to teach to medical school students and therefore often neglected. As a result, internal medicine physicians require a prolonged practical training.
Inspired by the Core Competencies in Hospital Medicine published by the Society of Hospital Medicine, this year in Genoa (the birthplace of Christopher Columbus) we started a 2-year second-level University Master course, called “Hospitalist: Managing complexity in Internal Medicine inpatients” for 35 internal medicine specialists. It is the fruit of collaboration between the main association of Italian hospital-based internists (Federation of Associations of Hospital Doctors on Internal Medicine, or FADOI) and the University of Genoa’s Department of Internal Medicine, Academy of Health Management, and the Center of Simulation and Advanced Training.
In Italy, this is the first concrete initiative to train, and better define, this new type of physician expert in the management of inpatients.
According to SHM’s definition of a hospitalist, we think that the activities of this new physician should also include teaching and research related to hospital medicine. And as Dr. Steven Pantilat wrote, “patient safety, leadership, palliative care and quality improvement are the issues that pertain to all hospitalists.”2
Theoretically, the development of the hospitalist model should be easier in Italy when compared to the United States. Dr. Robert Wachter and Dr. Lee Goldman wrote in 1996 about the objections to the hospitalist model of American primary care physicians (“to preserve continuity”) and specialists (“fewer consultations, lower income”), but in Italy family doctors do not usually follow their patients in the hospital, and specialists have no incentive for in-hospital consultations.3 Moreover, patients with comorbidities, or pathologies on the border between medicine and surgery (e.g. cholecystitis, bowel obstruction, polytrauma, etc.), are already often assigned to internal medicine, and in the smallest hospitals, the internist is most of the time the only specialist doctor continually present.
Nevertheless, the Italian hospitalist model will be a challenge. We know we have to deal with organ specialists, but we strongly believe that this is the most appropriate and the most sustainable model for the future of the Italian hospitals. Our wish is not to become the “bosses” of the hospital, but to ensure global, coordinated, and respectful care to present and future patients.
Published outcomes studies demonstrate that the U.S. hospitalist model has led to consistent and pronounced cost saving with no loss in quality.4 In the United States, the hospitalist field has grown from a few hundred physicians to more than 50,000,5 making it the fastest growing physician specialty in medical history.
Why should the same not occur in Italy?
References
1. Baudendistel TE, Watcher RM. The evolution of the hospitalist movement in USA. Clin Med JRCPL. 2002;2:327-30.
2. Pantilat S. What is a Hospitalist? The Hospitalist 2006 February;2006(2).
3. Wachter RM, Goldman Lee. The emerging role of “Hospitalists” in the American Health Care System. N Engl J Med. 1996;335:514-7.
4. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Medicine. 2011;9:58:1-22. http://www.biomedcentral.com/1741-7015/9/58.
5. Wachter RM, Goldman L. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375:1009-11.
Valerio Verdiani, MD, director of internal medicine, Grosseto, Italy. Francesco Orlandini, MD, internal medicine, health administrator, ASL4 Liguria, Chiavari (GE), Italy. Micaela La Regina, MD, internal medicine, risk management and clinical governance, ASL5 Liguria, La Spezia, Italy. Giovanni Murialdo, MD, department of internal medicine and medical specialty, University of Genoa (Italy). Andrea Fontanella, MD, director of medicine department, president of the Federation of Associations of Hospital Doctors on Internal Medicine (FADOI), Naples, Italy. Mauro Silingardi, MD, director of internal medicine, director of training and refresher of FADOI, Bologna, Italy.
In the United States, family physicians (general practitioners) used to manage their patients in the hospital, either as the primary care doctor or in consultation with specialists. Only since the 1990s has a new kind of physician gained widespread acceptance: the hospitalist (“specialist of inpatient care”).1
In Italy the process has not been the same. In our health care system, primary care physicians have always transferred the responsibility of hospital care to an inpatient team. Actually, our hospital-based doctors dedicate their whole working time to inpatient care, and general practitioners are not expected to go to the hospital. The patients were (and are) admitted to one ward or another according to their main clinical problem.
Little by little, a huge number of organ specialty and subspecialty wards have filled Italian hospitals. In this context, the internal medicine specialty was unable to occupy its characteristic role, so that, a few years ago, the medical community wondered if the specialty should have continued to exist.
Anyway, as a result of hyperspecialization, we have many different specialists in inpatient care who are not specialists in global inpatient care.
Nowadays, in our country we are faced with a dramatic epidemiologic change. The Italian population is aging and the majority of patients have not only one clinical problem but multiple comorbidities. When these patients reach the emergency department, it is not easy to identify the main clinical problem and assign him/her to an organ specialty unit. And when he or she eventually arrives there, a considerable number of consultants is frequently required. The vision of organ specialists is not holistic, and they are more prone to maximizing their tools than rationalizing them. So, at present, our traditional hospital model has been generating care fragmentation, overproduction of diagnoses, overprescription of drugs, and increasing costs.
It is obvious that a new model is necessary for the future, and we look with great interest at the American hospitalist model.
We need a new hospital-based clinician who has wide-ranging competencies, and is able to define priorities and appropriateness of care when a patient requires multiple specialists’ interventions; one who is autonomous in performing basic procedures and expert in perioperative medicine; prompt to communicate with primary care doctors at the time of admission and discharge; and prepared to work in managed-care organizations.
We wonder: Are Italian hospital-based internists – the only specialists in global inpatient care – suited to this role?
We think so. However, current Italian training in internal medicine is focused mainly on scientific bases of diseases, pathophysiological, and clinical aspects. Concepts such as complexity or the management of patients with comorbidities are quite difficult to teach to medical school students and therefore often neglected. As a result, internal medicine physicians require a prolonged practical training.
Inspired by the Core Competencies in Hospital Medicine published by the Society of Hospital Medicine, this year in Genoa (the birthplace of Christopher Columbus) we started a 2-year second-level University Master course, called “Hospitalist: Managing complexity in Internal Medicine inpatients” for 35 internal medicine specialists. It is the fruit of collaboration between the main association of Italian hospital-based internists (Federation of Associations of Hospital Doctors on Internal Medicine, or FADOI) and the University of Genoa’s Department of Internal Medicine, Academy of Health Management, and the Center of Simulation and Advanced Training.
In Italy, this is the first concrete initiative to train, and better define, this new type of physician expert in the management of inpatients.
According to SHM’s definition of a hospitalist, we think that the activities of this new physician should also include teaching and research related to hospital medicine. And as Dr. Steven Pantilat wrote, “patient safety, leadership, palliative care and quality improvement are the issues that pertain to all hospitalists.”2
Theoretically, the development of the hospitalist model should be easier in Italy when compared to the United States. Dr. Robert Wachter and Dr. Lee Goldman wrote in 1996 about the objections to the hospitalist model of American primary care physicians (“to preserve continuity”) and specialists (“fewer consultations, lower income”), but in Italy family doctors do not usually follow their patients in the hospital, and specialists have no incentive for in-hospital consultations.3 Moreover, patients with comorbidities, or pathologies on the border between medicine and surgery (e.g. cholecystitis, bowel obstruction, polytrauma, etc.), are already often assigned to internal medicine, and in the smallest hospitals, the internist is most of the time the only specialist doctor continually present.
Nevertheless, the Italian hospitalist model will be a challenge. We know we have to deal with organ specialists, but we strongly believe that this is the most appropriate and the most sustainable model for the future of the Italian hospitals. Our wish is not to become the “bosses” of the hospital, but to ensure global, coordinated, and respectful care to present and future patients.
Published outcomes studies demonstrate that the U.S. hospitalist model has led to consistent and pronounced cost saving with no loss in quality.4 In the United States, the hospitalist field has grown from a few hundred physicians to more than 50,000,5 making it the fastest growing physician specialty in medical history.
Why should the same not occur in Italy?
References
1. Baudendistel TE, Watcher RM. The evolution of the hospitalist movement in USA. Clin Med JRCPL. 2002;2:327-30.
2. Pantilat S. What is a Hospitalist? The Hospitalist 2006 February;2006(2).
3. Wachter RM, Goldman Lee. The emerging role of “Hospitalists” in the American Health Care System. N Engl J Med. 1996;335:514-7.
4. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Medicine. 2011;9:58:1-22. http://www.biomedcentral.com/1741-7015/9/58.
5. Wachter RM, Goldman L. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375:1009-11.
Valerio Verdiani, MD, director of internal medicine, Grosseto, Italy. Francesco Orlandini, MD, internal medicine, health administrator, ASL4 Liguria, Chiavari (GE), Italy. Micaela La Regina, MD, internal medicine, risk management and clinical governance, ASL5 Liguria, La Spezia, Italy. Giovanni Murialdo, MD, department of internal medicine and medical specialty, University of Genoa (Italy). Andrea Fontanella, MD, director of medicine department, president of the Federation of Associations of Hospital Doctors on Internal Medicine (FADOI), Naples, Italy. Mauro Silingardi, MD, director of internal medicine, director of training and refresher of FADOI, Bologna, Italy.
A clinical pathway to standardize use of maintenance IV fluids
Clinical question
Can an evidence-based clinical pathway improve adherence to recent recommendations to use isotonic solutions for maintenance intravenous fluids in hospitalized children?
Background
The traditional teaching regarding composition of maintenance intravenous fluids (IVF) in children has been based on the Holliday-Segar method.1 Since its publication in Pediatrics in 1957, concerns have been raised regarding the risk of iatrogenic hyponatremia caused by giving hypotonic fluids determined by this method,2 especially in patients with an elevated risk of increased antidiuretic hormone (ADH) secretion.3 Multiple recent systematic reviews and meta-analyses have confirmed that isotonic IVF reduces the risk of hyponatremia in hospitalized children.4
Study design
Interrupted time series analysis before and after pathway implementation.
Setting
370-bed tertiary care free-standing children’s hospital.
Synopsis
A multidisciplinary team was assembled, comprising physicians and nurses in hospital medicine, general pediatrics, emergency medicine, and nephrology. After a systematic review of the recent literature, a clinical algorithm and web-based training module were developed. Faculty in general pediatrics, hospital medicine, and emergency medicine were required to complete the module, while medical and surgical residents were encouraged but not required to complete the module. A maintenance IVF order set was created and embedded into all order sets previously containing IVF orders and was also available in stand-alone form.
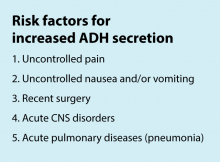
Inclusion criteria (“pathway eligible”) included being euvolemic and requiring IVF. Exclusion criteria included fluid status derangements, critical illness, severe serum sodium abnormalities (serum sodium ≥150 mEq/L or ≤130 mEq/L) use of TPN or ketogenic diet. In the order set, IVF composition was determined based on risk factors for increased ADH secretion. Inclusion of potassium in IVF was also determined by the pathway.
Over the 1-year study period, 11,602 pathway-eligible encounters in 10,287 patients were reviewed. Use of isotonic maintenance IVF increased significantly from 9.3% to 50.6%, while use of hypotonic fluids decreased from 94.2% to 56.6%. Use of potassium-containing IVF increased from 52.9% to 75.3%. Dysnatremia continued to occur due to hypotonic IVF use.
Bottom line
A combined clinical pathway and training module to standardize the composition of IVF is feasible, and results in increased use of isotonic and potassium-containing fluids.
Citation
Rooholamini S, Clifton H, Haaland W, et al. Outcomes of a clinical pathway to standardize use of maintenance intravenous fluids. Hosp Pediatr. 2017 Dec;7(12):703-9.
Dr. Chang is a pediatric hospitalist at Baystate Children’s Hospital in Springfield, Mass., and is the pediatric editor of The Hospitalist.
References
1. Holliday MA et al. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957;19:823-32.
2. Friedman JN et al. Comparison of isotonic and hypotonic intravenous maintenance fluids: a randomized clinical trial. JAMA Pediatr. 2015;169:445-51.
3. Fuchs J et al. Current Issues in Intravenous Fluid Use in Hospitalized Children. Rev Recent Clin Trials. 2017;12:284-9.
4. McNab S et al. Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. Cochrane Database. Syst Rev 2014:CD009457.
Clinical question
Can an evidence-based clinical pathway improve adherence to recent recommendations to use isotonic solutions for maintenance intravenous fluids in hospitalized children?
Background
The traditional teaching regarding composition of maintenance intravenous fluids (IVF) in children has been based on the Holliday-Segar method.1 Since its publication in Pediatrics in 1957, concerns have been raised regarding the risk of iatrogenic hyponatremia caused by giving hypotonic fluids determined by this method,2 especially in patients with an elevated risk of increased antidiuretic hormone (ADH) secretion.3 Multiple recent systematic reviews and meta-analyses have confirmed that isotonic IVF reduces the risk of hyponatremia in hospitalized children.4
Study design
Interrupted time series analysis before and after pathway implementation.
Setting
370-bed tertiary care free-standing children’s hospital.
Synopsis
A multidisciplinary team was assembled, comprising physicians and nurses in hospital medicine, general pediatrics, emergency medicine, and nephrology. After a systematic review of the recent literature, a clinical algorithm and web-based training module were developed. Faculty in general pediatrics, hospital medicine, and emergency medicine were required to complete the module, while medical and surgical residents were encouraged but not required to complete the module. A maintenance IVF order set was created and embedded into all order sets previously containing IVF orders and was also available in stand-alone form.
Inclusion criteria (“pathway eligible”) included being euvolemic and requiring IVF. Exclusion criteria included fluid status derangements, critical illness, severe serum sodium abnormalities (serum sodium ≥150 mEq/L or ≤130 mEq/L) use of TPN or ketogenic diet. In the order set, IVF composition was determined based on risk factors for increased ADH secretion. Inclusion of potassium in IVF was also determined by the pathway.
Over the 1-year study period, 11,602 pathway-eligible encounters in 10,287 patients were reviewed. Use of isotonic maintenance IVF increased significantly from 9.3% to 50.6%, while use of hypotonic fluids decreased from 94.2% to 56.6%. Use of potassium-containing IVF increased from 52.9% to 75.3%. Dysnatremia continued to occur due to hypotonic IVF use.
Bottom line
A combined clinical pathway and training module to standardize the composition of IVF is feasible, and results in increased use of isotonic and potassium-containing fluids.
Citation
Rooholamini S, Clifton H, Haaland W, et al. Outcomes of a clinical pathway to standardize use of maintenance intravenous fluids. Hosp Pediatr. 2017 Dec;7(12):703-9.
Dr. Chang is a pediatric hospitalist at Baystate Children’s Hospital in Springfield, Mass., and is the pediatric editor of The Hospitalist.
References
1. Holliday MA et al. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957;19:823-32.
2. Friedman JN et al. Comparison of isotonic and hypotonic intravenous maintenance fluids: a randomized clinical trial. JAMA Pediatr. 2015;169:445-51.
3. Fuchs J et al. Current Issues in Intravenous Fluid Use in Hospitalized Children. Rev Recent Clin Trials. 2017;12:284-9.
4. McNab S et al. Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. Cochrane Database. Syst Rev 2014:CD009457.
Clinical question
Can an evidence-based clinical pathway improve adherence to recent recommendations to use isotonic solutions for maintenance intravenous fluids in hospitalized children?
Background
The traditional teaching regarding composition of maintenance intravenous fluids (IVF) in children has been based on the Holliday-Segar method.1 Since its publication in Pediatrics in 1957, concerns have been raised regarding the risk of iatrogenic hyponatremia caused by giving hypotonic fluids determined by this method,2 especially in patients with an elevated risk of increased antidiuretic hormone (ADH) secretion.3 Multiple recent systematic reviews and meta-analyses have confirmed that isotonic IVF reduces the risk of hyponatremia in hospitalized children.4
Study design
Interrupted time series analysis before and after pathway implementation.
Setting
370-bed tertiary care free-standing children’s hospital.
Synopsis
A multidisciplinary team was assembled, comprising physicians and nurses in hospital medicine, general pediatrics, emergency medicine, and nephrology. After a systematic review of the recent literature, a clinical algorithm and web-based training module were developed. Faculty in general pediatrics, hospital medicine, and emergency medicine were required to complete the module, while medical and surgical residents were encouraged but not required to complete the module. A maintenance IVF order set was created and embedded into all order sets previously containing IVF orders and was also available in stand-alone form.
Inclusion criteria (“pathway eligible”) included being euvolemic and requiring IVF. Exclusion criteria included fluid status derangements, critical illness, severe serum sodium abnormalities (serum sodium ≥150 mEq/L or ≤130 mEq/L) use of TPN or ketogenic diet. In the order set, IVF composition was determined based on risk factors for increased ADH secretion. Inclusion of potassium in IVF was also determined by the pathway.
Over the 1-year study period, 11,602 pathway-eligible encounters in 10,287 patients were reviewed. Use of isotonic maintenance IVF increased significantly from 9.3% to 50.6%, while use of hypotonic fluids decreased from 94.2% to 56.6%. Use of potassium-containing IVF increased from 52.9% to 75.3%. Dysnatremia continued to occur due to hypotonic IVF use.
Bottom line
A combined clinical pathway and training module to standardize the composition of IVF is feasible, and results in increased use of isotonic and potassium-containing fluids.
Citation
Rooholamini S, Clifton H, Haaland W, et al. Outcomes of a clinical pathway to standardize use of maintenance intravenous fluids. Hosp Pediatr. 2017 Dec;7(12):703-9.
Dr. Chang is a pediatric hospitalist at Baystate Children’s Hospital in Springfield, Mass., and is the pediatric editor of The Hospitalist.
References
1. Holliday MA et al. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957;19:823-32.
2. Friedman JN et al. Comparison of isotonic and hypotonic intravenous maintenance fluids: a randomized clinical trial. JAMA Pediatr. 2015;169:445-51.
3. Fuchs J et al. Current Issues in Intravenous Fluid Use in Hospitalized Children. Rev Recent Clin Trials. 2017;12:284-9.
4. McNab S et al. Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. Cochrane Database. Syst Rev 2014:CD009457.
Concerns in the Management of Chronic Heart Failure - Staying on the Path to Optimal Medical Therapy
Trio of blood biomarkers elevated in children with LRTIs
TORONTO – While C-reactive protein, procalcitonin, and proadrenomedullin are associated with development of severe clinical outcomes in children with lower respiratory tract infections, proadrenomedullin is most strongly associated with disease severity, preliminary results from a prospective cohort study showed.
“Despite the fact that pneumonia guidelines call the site of care decision the most important decision in the management of pediatric pneumonia, no validated risk stratification tools exist for pediatric lower respiratory tract infections (LRTI),” lead study author Todd A. Florin, MD, said at the annual Pediatric Academic Societies meeting. “Biomarkers offer an objective means of classifying disease severity and clinical outcomes.”
PCT is a precursor of calcitonin secreted by the thyroid, lung, and intestine in response to bacterial infections. It also has been shown to be associated with adverse outcomes and mortality in adults, with results generally suggesting that it is a stronger predictor of severity than CRP. “There is limited data on the association of CRP or PCT with severe outcomes in children with LRTIs,” Dr. Florin noted. “One recent U.S. study of 532 children did demonstrate an association of elevated PCT with ICU admission, chest drainage, and hospital length of stay in children with [community-acquired pneumonia] CAP.”
ProADM, meanwhile, is a vasodilatory peptide with antimicrobial and anti-inflammatory functions synthesized during severe infections. It has a half-life of several hours and has been shown to be associated with disease severity in adults with LRTI. Recent studies have shown that it has improved prognostication over WBC, CRP, and PCT. “In two small studies of children with pneumonia, proADM levels were significantly elevated in children with complicated pneumonia, compared to those with uncomplicated pneumonia,” Dr. Florin said. “Although all three of these markers demonstrate promise in predicting severe outcomes in adults with LRTIs, very few studies have examined their association with disease severity in pediatric disease. Therefore, the aim of the current analysis was to determine the association between blood biomarkers and disease severity in children who present to the ED with lower respiratory tract infections.”
In a study known as Catalyzing Ambulatory Research in Pneumonia Etiology and Diagnostic Innovations in Emergency Medicine (CARPE DIEM), he and his associates performed a prospective cohort analysis of children with suspected CAP who were admitted to the Cincinnati Children’s Hospital ED between July 2012 and December 2017. They limited the analysis to children aged 3 months to 18 years with signs and symptoms of an LRTI, and all eligible patients were required to have a chest radiograph ordered for suspicion of CAP. They excluded children hospitalized within 14 days prior to the index ED visit, immunodeficient or immunosuppressed children, those with a history of aspiration or aspiration pneumonia, and those who weighed less than 5 kg because of blood drawing maximums. Biomarkers were measured only in children with focal findings on chest x-ray in the ED. The primary outcome was disease severity: mild (defined as discharged home), moderate (defined as hospitalized, but not severe) and severe (defined as having an ICU length of stay of greater than 48 hours, chest drainage, severe sepsis, noninvasive positive pressure ventilation, intubation, vasoactive infusions, or death). Biomarkers were obtained at the time of presentation to the ED, prior to the occurrence of clinical outcomes.
Over a period of 4.5 years, the researchers enrolled 1,142 patients. Of these, 478 had focal findings on chest x-ray and blood obtained. The median age of these 478 children was 4.4 years, 52% were male, and 82% had all three biomarkers performed. Specifically, 456 had CRP and PCT performed, while 358 had proADM performed. “Not every child had every marker performed due to challenges in obtaining sufficient blood for all three biomarkers in some children,” Dr. Florin explained.
Preliminary data that Dr. Florin presented at PAS found that the median CRP, PCT, and proADM did not differ by gender, race, ethnicity, or insurance status. “In addition, there were not significant differences in the distribution of disease severity by biomarker performed, with approximately 27% of patients being classified as mild, 66% as moderate, and 7% as severe,” he said.
The median CRP was 2.4 ng/mL in those with mild disease, 2.5 ng/mL in those with moderate disease, and 6.25 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease reaching statistical significance (P = .002). The median PCT was 0.16 ng/mL in those with mild disease, 0.26 ng/mL in those with moderate disease, and 0.49 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease reaching statistical significance (P = .047). Meanwhile, the median proADM was 0.53 ng/mL in those with mild disease, 0.59 ng/mL in those with moderate disease, and 0.81 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease also reaching statistical significance (P less than .0001).
Next, the researchers performed logistic regression of each biomarker individually and in combination. They found that and had the best ability to discriminate those developing severe vs. nonsevere disease (area under the receiving operating curve of 0.72, vs. 0.67 and 0.60, respectively). When CRP and PCT markers were combined with proADM, they were no longer associated with severe disease, while a strong association with proADM remained significant.
Dr. Florin acknowledged certain limitations of the study, including the fact that requiring collection of blood samples may have resulted in an enrollment bias toward patients receiving phlebotomy or IV line placement in the ED. “In addition, the children in the moderate-severity group are likely more heterogeneous than the other two severity groups,” he said. “Finally, given that this is a single-center study, we had a relatively small number of outcomes for some of the individual severity measures, which may have limited power and precision.”
He concluded his presentation by saying that he is “cautiously optimistic” about the study results. “As is the case in many biomarker studies, I do not anticipate that any single biomarker will be the magic bullet for predicting disease severity in pediatric CAP,” Dr. Florin said. “It will likely be a combination of clinical factors and several biomarkers that will achieve optimal prognostic ability. That said, our results suggest that similar to adult studies, proADM appears to have the strongest association with severe disease, compared with CRP and PCT. Combinations of biomarkers did not perform better than proADM alone. With the advent of rapid point-of-care diagnostics, these markers may have a role in management and site-of-care decisions for children with LRTI.”
The study received funding support from the Gerber Foundation, the National Institute of Allergy and Infectious Diseases, and Cincinnati Children’s Hospital Medical Center. Dr. Florin reported having no financial disclosures.
TORONTO – While C-reactive protein, procalcitonin, and proadrenomedullin are associated with development of severe clinical outcomes in children with lower respiratory tract infections, proadrenomedullin is most strongly associated with disease severity, preliminary results from a prospective cohort study showed.
“Despite the fact that pneumonia guidelines call the site of care decision the most important decision in the management of pediatric pneumonia, no validated risk stratification tools exist for pediatric lower respiratory tract infections (LRTI),” lead study author Todd A. Florin, MD, said at the annual Pediatric Academic Societies meeting. “Biomarkers offer an objective means of classifying disease severity and clinical outcomes.”
PCT is a precursor of calcitonin secreted by the thyroid, lung, and intestine in response to bacterial infections. It also has been shown to be associated with adverse outcomes and mortality in adults, with results generally suggesting that it is a stronger predictor of severity than CRP. “There is limited data on the association of CRP or PCT with severe outcomes in children with LRTIs,” Dr. Florin noted. “One recent U.S. study of 532 children did demonstrate an association of elevated PCT with ICU admission, chest drainage, and hospital length of stay in children with [community-acquired pneumonia] CAP.”
ProADM, meanwhile, is a vasodilatory peptide with antimicrobial and anti-inflammatory functions synthesized during severe infections. It has a half-life of several hours and has been shown to be associated with disease severity in adults with LRTI. Recent studies have shown that it has improved prognostication over WBC, CRP, and PCT. “In two small studies of children with pneumonia, proADM levels were significantly elevated in children with complicated pneumonia, compared to those with uncomplicated pneumonia,” Dr. Florin said. “Although all three of these markers demonstrate promise in predicting severe outcomes in adults with LRTIs, very few studies have examined their association with disease severity in pediatric disease. Therefore, the aim of the current analysis was to determine the association between blood biomarkers and disease severity in children who present to the ED with lower respiratory tract infections.”
In a study known as Catalyzing Ambulatory Research in Pneumonia Etiology and Diagnostic Innovations in Emergency Medicine (CARPE DIEM), he and his associates performed a prospective cohort analysis of children with suspected CAP who were admitted to the Cincinnati Children’s Hospital ED between July 2012 and December 2017. They limited the analysis to children aged 3 months to 18 years with signs and symptoms of an LRTI, and all eligible patients were required to have a chest radiograph ordered for suspicion of CAP. They excluded children hospitalized within 14 days prior to the index ED visit, immunodeficient or immunosuppressed children, those with a history of aspiration or aspiration pneumonia, and those who weighed less than 5 kg because of blood drawing maximums. Biomarkers were measured only in children with focal findings on chest x-ray in the ED. The primary outcome was disease severity: mild (defined as discharged home), moderate (defined as hospitalized, but not severe) and severe (defined as having an ICU length of stay of greater than 48 hours, chest drainage, severe sepsis, noninvasive positive pressure ventilation, intubation, vasoactive infusions, or death). Biomarkers were obtained at the time of presentation to the ED, prior to the occurrence of clinical outcomes.
Over a period of 4.5 years, the researchers enrolled 1,142 patients. Of these, 478 had focal findings on chest x-ray and blood obtained. The median age of these 478 children was 4.4 years, 52% were male, and 82% had all three biomarkers performed. Specifically, 456 had CRP and PCT performed, while 358 had proADM performed. “Not every child had every marker performed due to challenges in obtaining sufficient blood for all three biomarkers in some children,” Dr. Florin explained.
Preliminary data that Dr. Florin presented at PAS found that the median CRP, PCT, and proADM did not differ by gender, race, ethnicity, or insurance status. “In addition, there were not significant differences in the distribution of disease severity by biomarker performed, with approximately 27% of patients being classified as mild, 66% as moderate, and 7% as severe,” he said.
The median CRP was 2.4 ng/mL in those with mild disease, 2.5 ng/mL in those with moderate disease, and 6.25 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease reaching statistical significance (P = .002). The median PCT was 0.16 ng/mL in those with mild disease, 0.26 ng/mL in those with moderate disease, and 0.49 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease reaching statistical significance (P = .047). Meanwhile, the median proADM was 0.53 ng/mL in those with mild disease, 0.59 ng/mL in those with moderate disease, and 0.81 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease also reaching statistical significance (P less than .0001).
Next, the researchers performed logistic regression of each biomarker individually and in combination. They found that and had the best ability to discriminate those developing severe vs. nonsevere disease (area under the receiving operating curve of 0.72, vs. 0.67 and 0.60, respectively). When CRP and PCT markers were combined with proADM, they were no longer associated with severe disease, while a strong association with proADM remained significant.
Dr. Florin acknowledged certain limitations of the study, including the fact that requiring collection of blood samples may have resulted in an enrollment bias toward patients receiving phlebotomy or IV line placement in the ED. “In addition, the children in the moderate-severity group are likely more heterogeneous than the other two severity groups,” he said. “Finally, given that this is a single-center study, we had a relatively small number of outcomes for some of the individual severity measures, which may have limited power and precision.”
He concluded his presentation by saying that he is “cautiously optimistic” about the study results. “As is the case in many biomarker studies, I do not anticipate that any single biomarker will be the magic bullet for predicting disease severity in pediatric CAP,” Dr. Florin said. “It will likely be a combination of clinical factors and several biomarkers that will achieve optimal prognostic ability. That said, our results suggest that similar to adult studies, proADM appears to have the strongest association with severe disease, compared with CRP and PCT. Combinations of biomarkers did not perform better than proADM alone. With the advent of rapid point-of-care diagnostics, these markers may have a role in management and site-of-care decisions for children with LRTI.”
The study received funding support from the Gerber Foundation, the National Institute of Allergy and Infectious Diseases, and Cincinnati Children’s Hospital Medical Center. Dr. Florin reported having no financial disclosures.
TORONTO – While C-reactive protein, procalcitonin, and proadrenomedullin are associated with development of severe clinical outcomes in children with lower respiratory tract infections, proadrenomedullin is most strongly associated with disease severity, preliminary results from a prospective cohort study showed.
“Despite the fact that pneumonia guidelines call the site of care decision the most important decision in the management of pediatric pneumonia, no validated risk stratification tools exist for pediatric lower respiratory tract infections (LRTI),” lead study author Todd A. Florin, MD, said at the annual Pediatric Academic Societies meeting. “Biomarkers offer an objective means of classifying disease severity and clinical outcomes.”
PCT is a precursor of calcitonin secreted by the thyroid, lung, and intestine in response to bacterial infections. It also has been shown to be associated with adverse outcomes and mortality in adults, with results generally suggesting that it is a stronger predictor of severity than CRP. “There is limited data on the association of CRP or PCT with severe outcomes in children with LRTIs,” Dr. Florin noted. “One recent U.S. study of 532 children did demonstrate an association of elevated PCT with ICU admission, chest drainage, and hospital length of stay in children with [community-acquired pneumonia] CAP.”
ProADM, meanwhile, is a vasodilatory peptide with antimicrobial and anti-inflammatory functions synthesized during severe infections. It has a half-life of several hours and has been shown to be associated with disease severity in adults with LRTI. Recent studies have shown that it has improved prognostication over WBC, CRP, and PCT. “In two small studies of children with pneumonia, proADM levels were significantly elevated in children with complicated pneumonia, compared to those with uncomplicated pneumonia,” Dr. Florin said. “Although all three of these markers demonstrate promise in predicting severe outcomes in adults with LRTIs, very few studies have examined their association with disease severity in pediatric disease. Therefore, the aim of the current analysis was to determine the association between blood biomarkers and disease severity in children who present to the ED with lower respiratory tract infections.”
In a study known as Catalyzing Ambulatory Research in Pneumonia Etiology and Diagnostic Innovations in Emergency Medicine (CARPE DIEM), he and his associates performed a prospective cohort analysis of children with suspected CAP who were admitted to the Cincinnati Children’s Hospital ED between July 2012 and December 2017. They limited the analysis to children aged 3 months to 18 years with signs and symptoms of an LRTI, and all eligible patients were required to have a chest radiograph ordered for suspicion of CAP. They excluded children hospitalized within 14 days prior to the index ED visit, immunodeficient or immunosuppressed children, those with a history of aspiration or aspiration pneumonia, and those who weighed less than 5 kg because of blood drawing maximums. Biomarkers were measured only in children with focal findings on chest x-ray in the ED. The primary outcome was disease severity: mild (defined as discharged home), moderate (defined as hospitalized, but not severe) and severe (defined as having an ICU length of stay of greater than 48 hours, chest drainage, severe sepsis, noninvasive positive pressure ventilation, intubation, vasoactive infusions, or death). Biomarkers were obtained at the time of presentation to the ED, prior to the occurrence of clinical outcomes.
Over a period of 4.5 years, the researchers enrolled 1,142 patients. Of these, 478 had focal findings on chest x-ray and blood obtained. The median age of these 478 children was 4.4 years, 52% were male, and 82% had all three biomarkers performed. Specifically, 456 had CRP and PCT performed, while 358 had proADM performed. “Not every child had every marker performed due to challenges in obtaining sufficient blood for all three biomarkers in some children,” Dr. Florin explained.
Preliminary data that Dr. Florin presented at PAS found that the median CRP, PCT, and proADM did not differ by gender, race, ethnicity, or insurance status. “In addition, there were not significant differences in the distribution of disease severity by biomarker performed, with approximately 27% of patients being classified as mild, 66% as moderate, and 7% as severe,” he said.
The median CRP was 2.4 ng/mL in those with mild disease, 2.5 ng/mL in those with moderate disease, and 6.25 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease reaching statistical significance (P = .002). The median PCT was 0.16 ng/mL in those with mild disease, 0.26 ng/mL in those with moderate disease, and 0.49 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease reaching statistical significance (P = .047). Meanwhile, the median proADM was 0.53 ng/mL in those with mild disease, 0.59 ng/mL in those with moderate disease, and 0.81 ng/mL in those with severe disease, with the difference between the two subclasses of nonsevere disease and moderate disease and severe disease also reaching statistical significance (P less than .0001).
Next, the researchers performed logistic regression of each biomarker individually and in combination. They found that and had the best ability to discriminate those developing severe vs. nonsevere disease (area under the receiving operating curve of 0.72, vs. 0.67 and 0.60, respectively). When CRP and PCT markers were combined with proADM, they were no longer associated with severe disease, while a strong association with proADM remained significant.
Dr. Florin acknowledged certain limitations of the study, including the fact that requiring collection of blood samples may have resulted in an enrollment bias toward patients receiving phlebotomy or IV line placement in the ED. “In addition, the children in the moderate-severity group are likely more heterogeneous than the other two severity groups,” he said. “Finally, given that this is a single-center study, we had a relatively small number of outcomes for some of the individual severity measures, which may have limited power and precision.”
He concluded his presentation by saying that he is “cautiously optimistic” about the study results. “As is the case in many biomarker studies, I do not anticipate that any single biomarker will be the magic bullet for predicting disease severity in pediatric CAP,” Dr. Florin said. “It will likely be a combination of clinical factors and several biomarkers that will achieve optimal prognostic ability. That said, our results suggest that similar to adult studies, proADM appears to have the strongest association with severe disease, compared with CRP and PCT. Combinations of biomarkers did not perform better than proADM alone. With the advent of rapid point-of-care diagnostics, these markers may have a role in management and site-of-care decisions for children with LRTI.”
The study received funding support from the Gerber Foundation, the National Institute of Allergy and Infectious Diseases, and Cincinnati Children’s Hospital Medical Center. Dr. Florin reported having no financial disclosures.
AT PAS 18
Key clinical point: Blood biomarkers such as C-reactive protein (CRP), procalcitonin (PCT), and proadrenomedullin (proADM) may have a role in management and site-of-care decisions for children with LRTIs.
Major finding: The proADM alone was associated with the largest odds for severe disease (OR 13.1), compared with CRP alone (OR 1.6) and PCT alone (OR 1.4).
Study details: Preliminary results from prospective cohort analysis of 478 children with suspected community-acquired pneumonia who were admitted to the Cincinnati Children’s Hospital ED.
Disclosures: The study received funding support from the Gerber Foundation, the National Institute of Allergy and Infectious Diseases, and Cincinnati Children’s Hospital Medical Center. Dr. Florin reported having no financial disclosures.
Neonatal deaths lower in high-volume hospitals
AUSTIN, TEX. – A first look at the timing of neonatal deaths showed an association with weekend deliveries in one Texas county. However, birth weight and ethnicity attenuated the association, according to a recent study. Higher hospital volumes were associated with lower risk of neonatal deaths.
The retrospective, population-based cohort study, presented during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, used data from birth certificates and infant death certificates in the state of Texas. The investigators, said Elizabeth Restrepo, PhD, chose to examine data from Tarrant County, Tex., which has historically had persistently high infant mortality rates; in 2013, she said, the infant mortality rate in that county was 7.11/1,000 births – the highest in the state for that year.
The first question Dr. Restrepo and her colleagues at Texas Women’s University, Denton, wanted to answer was whether there was an association between the risk of neonatal mortality and the day of the week of the birth. For this and the study’s other research questions, she and her colleagues looked at 2012 data, matching 32,140 birth certificate records with 92 infant death certificates.
The investigators found an independent association between the risk of neonatal death and whether the birth happened on a weekday (Monday at 7:00 a.m. through Friday at 6:59 p.m.), or on a weekend (Friday at 7:00 p.m. through Monday at 6:59 a.m.). However, once birth weight and ethnicity were controlled in the statistical analysis, the association was not statistically significant despite an odds ratio of 1.44 (95% confidence interval, 0.911-2.27; P = .119).
“Births in the 12 hospitals studied appear to have been organized to take place more frequently on the working weekday rather than weekend days,” wrote Dr. Restrepo and her colleagues in the poster accompanying the presentation. Although the study wasn’t designed to answer this particular question, Dr. Restrepo said in discussion during the poster session that planned deliveries, such as inductions and cesarean deliveries, are likely to happen during the week, while the case mix is wider on weekends. Patient characteristics, as well as staffing patterns, may come into play.
The researchers also asked whether birth volume at a given institution increases the odds of neonatal death on weekends. Here, they found a significant inverse relationship between hospital birth volume and neonatal deaths (r = –0.021; P less than .001). With each additional increase of 1% in the weekday birth rate, the odds of neonatal death dropped by approximately 7.4%.
Examining the Tarrant County data further, Dr. Restrepo and her colleagues found that the hospitals with higher birth volumes had a more even distribution of births across the days of the week, with resulting lower concentrations of births during the week (r = –.394; P less than .001).
To classify infant deaths, the investigators included only ICD-10 diagnoses classified as P-codes to capture deaths occurring in the first 28 days after birth, but excluding congenital problems that are incompatible with life or that usually cause early death.
The researchers reported that they had no conflicts of interest; the study was funded by a research enhancement program award from the Texas Women’s University Office of Research and Sponsored Programs.
SOURCE: Restrepo E et al. ACOG 2018, Abstract 22R.
AUSTIN, TEX. – A first look at the timing of neonatal deaths showed an association with weekend deliveries in one Texas county. However, birth weight and ethnicity attenuated the association, according to a recent study. Higher hospital volumes were associated with lower risk of neonatal deaths.
The retrospective, population-based cohort study, presented during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, used data from birth certificates and infant death certificates in the state of Texas. The investigators, said Elizabeth Restrepo, PhD, chose to examine data from Tarrant County, Tex., which has historically had persistently high infant mortality rates; in 2013, she said, the infant mortality rate in that county was 7.11/1,000 births – the highest in the state for that year.
The first question Dr. Restrepo and her colleagues at Texas Women’s University, Denton, wanted to answer was whether there was an association between the risk of neonatal mortality and the day of the week of the birth. For this and the study’s other research questions, she and her colleagues looked at 2012 data, matching 32,140 birth certificate records with 92 infant death certificates.
The investigators found an independent association between the risk of neonatal death and whether the birth happened on a weekday (Monday at 7:00 a.m. through Friday at 6:59 p.m.), or on a weekend (Friday at 7:00 p.m. through Monday at 6:59 a.m.). However, once birth weight and ethnicity were controlled in the statistical analysis, the association was not statistically significant despite an odds ratio of 1.44 (95% confidence interval, 0.911-2.27; P = .119).
“Births in the 12 hospitals studied appear to have been organized to take place more frequently on the working weekday rather than weekend days,” wrote Dr. Restrepo and her colleagues in the poster accompanying the presentation. Although the study wasn’t designed to answer this particular question, Dr. Restrepo said in discussion during the poster session that planned deliveries, such as inductions and cesarean deliveries, are likely to happen during the week, while the case mix is wider on weekends. Patient characteristics, as well as staffing patterns, may come into play.
The researchers also asked whether birth volume at a given institution increases the odds of neonatal death on weekends. Here, they found a significant inverse relationship between hospital birth volume and neonatal deaths (r = –0.021; P less than .001). With each additional increase of 1% in the weekday birth rate, the odds of neonatal death dropped by approximately 7.4%.
Examining the Tarrant County data further, Dr. Restrepo and her colleagues found that the hospitals with higher birth volumes had a more even distribution of births across the days of the week, with resulting lower concentrations of births during the week (r = –.394; P less than .001).
To classify infant deaths, the investigators included only ICD-10 diagnoses classified as P-codes to capture deaths occurring in the first 28 days after birth, but excluding congenital problems that are incompatible with life or that usually cause early death.
The researchers reported that they had no conflicts of interest; the study was funded by a research enhancement program award from the Texas Women’s University Office of Research and Sponsored Programs.
SOURCE: Restrepo E et al. ACOG 2018, Abstract 22R.
AUSTIN, TEX. – A first look at the timing of neonatal deaths showed an association with weekend deliveries in one Texas county. However, birth weight and ethnicity attenuated the association, according to a recent study. Higher hospital volumes were associated with lower risk of neonatal deaths.
The retrospective, population-based cohort study, presented during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, used data from birth certificates and infant death certificates in the state of Texas. The investigators, said Elizabeth Restrepo, PhD, chose to examine data from Tarrant County, Tex., which has historically had persistently high infant mortality rates; in 2013, she said, the infant mortality rate in that county was 7.11/1,000 births – the highest in the state for that year.
The first question Dr. Restrepo and her colleagues at Texas Women’s University, Denton, wanted to answer was whether there was an association between the risk of neonatal mortality and the day of the week of the birth. For this and the study’s other research questions, she and her colleagues looked at 2012 data, matching 32,140 birth certificate records with 92 infant death certificates.
The investigators found an independent association between the risk of neonatal death and whether the birth happened on a weekday (Monday at 7:00 a.m. through Friday at 6:59 p.m.), or on a weekend (Friday at 7:00 p.m. through Monday at 6:59 a.m.). However, once birth weight and ethnicity were controlled in the statistical analysis, the association was not statistically significant despite an odds ratio of 1.44 (95% confidence interval, 0.911-2.27; P = .119).
“Births in the 12 hospitals studied appear to have been organized to take place more frequently on the working weekday rather than weekend days,” wrote Dr. Restrepo and her colleagues in the poster accompanying the presentation. Although the study wasn’t designed to answer this particular question, Dr. Restrepo said in discussion during the poster session that planned deliveries, such as inductions and cesarean deliveries, are likely to happen during the week, while the case mix is wider on weekends. Patient characteristics, as well as staffing patterns, may come into play.
The researchers also asked whether birth volume at a given institution increases the odds of neonatal death on weekends. Here, they found a significant inverse relationship between hospital birth volume and neonatal deaths (r = –0.021; P less than .001). With each additional increase of 1% in the weekday birth rate, the odds of neonatal death dropped by approximately 7.4%.
Examining the Tarrant County data further, Dr. Restrepo and her colleagues found that the hospitals with higher birth volumes had a more even distribution of births across the days of the week, with resulting lower concentrations of births during the week (r = –.394; P less than .001).
To classify infant deaths, the investigators included only ICD-10 diagnoses classified as P-codes to capture deaths occurring in the first 28 days after birth, but excluding congenital problems that are incompatible with life or that usually cause early death.
The researchers reported that they had no conflicts of interest; the study was funded by a research enhancement program award from the Texas Women’s University Office of Research and Sponsored Programs.
SOURCE: Restrepo E et al. ACOG 2018, Abstract 22R.
REPORTING FROM ACOG 2018
Key clinical point: Neonatal deaths were lower in hospitals with higher delivery volumes.
Major finding: Higher weekday birth volumes were associated with lower risk of neonatal death (P = .002).
Study details: Retrospective cohort study of 92 neonatal deaths in a single Texas county in 2012.
Disclosures: The study was funded by Texas Women’s University. The authors reported that they had no relevant disclosures.
Source: Restrepo E et al. ACOG 2018, Abstract 22R.
Rethinking preop testing
ORLANDO – Michael Rothberg, MD, a nocturnist who works at Presbyterian Rust Medical Center in Albuquerque, often is torn when asked to routinely perform preoperative tests, such as ECGs, on patients.
On the one hand, Dr. Rothberg knows that for many patients there is almost certainly no benefit to some of the tests. On the other hand, surgeons expect the tests to be performed – so, for the sake of collegiality, patients often have tests ordered that hospitalists suspect are unnecessary.
This was a big part of why Dr. Rothberg decided to come a day early to HM18, held in early April in Orlando, to attend the pre-course “Essentials of Perioperative Medicine and Co-Management for the Hospitalist.” He was looking for expert guidance on which patients need what tests before surgery, and also how to better determine what preoperative tests are a waste of time and money for certain patients, so that he’ll be armed with useful information when he went back to his medical center.
“I can slap something on the surgeon’s desk and say, ‘Here’s why we’re not doing it,’ ” Dr. Rothberg said.
At the HM18 pre-course, experts gave guidance on the benefits of hospitalist involvement in perioperative care and offered points to consider when assessing cardiac and pulmonary risk before surgeries. Hospitalists then broke into groups to brainstorm techniques that could improve their perioperative work.
She noted how surgical safety checklists have been shown to improve morbidity and mortality, as seen with a checklist developed by the World Health Organization and in California, where an enhanced recovery program at 20 hospitals has been successful.
“I think the reason we see changes in each of those … from pre to post when they implement, is because people start to communicate and collaborate,” she said. “I think that’s the secret sauce, and you can take that back home with you.”
Assessing risk
As for preoperative testing, history is replete with examples of tests once considered crucial but that have proven to be unimportant for many patients, including preoperative carotid endarterectomy, preop ECG, preop coronary revasularization, and preop lab work.
“I was always listening for bruits years ago,” Dr. Grant said. “I’ve sort of stopped doing that now. You’ll hear it, you won’t know what to do with it. We used to take care of those things before surgery. We now know that’s not helpful for patients without symptoms.”
Dr. Cohn’s philosophy is to not suggest a delay without firm evidence that it is necessary. “I try not to interfere with surgery unless I feel that there is significant risk,” he said.
In workshop discussions at the HM18 pre-course, hospitalists considered their contributions to preoperative care and ways they might be able to contribute more effectively. Among their ideas were better communication with anesthesiology – regarded as severely lacking by many hospitalists in the session – as well as designating smaller perioperative teams to foster knowledge and greater trust with surgeons.
Aron Mednick, MD, FHM, director of the comanagement service at Tisch Hospital, NYU Langone Medical Center, New York, said his group talked about an “identify, mitigate, propose, and resolve” method – identifying services or conditions with a high rate of preoperative problems, finding data on how to solve them, and proposing ways to get hospitalists involved in the solution.
“We noted that a lot of people experience resistance with getting hospitalists involved in care early,” he said. “So one of the ways to do this is actually to identify problems and start above the surgeon, at the CMO and COO level, and then move down through department chairs and, basically, impose our existence on the care of the patient.”
ORLANDO – Michael Rothberg, MD, a nocturnist who works at Presbyterian Rust Medical Center in Albuquerque, often is torn when asked to routinely perform preoperative tests, such as ECGs, on patients.
On the one hand, Dr. Rothberg knows that for many patients there is almost certainly no benefit to some of the tests. On the other hand, surgeons expect the tests to be performed – so, for the sake of collegiality, patients often have tests ordered that hospitalists suspect are unnecessary.
This was a big part of why Dr. Rothberg decided to come a day early to HM18, held in early April in Orlando, to attend the pre-course “Essentials of Perioperative Medicine and Co-Management for the Hospitalist.” He was looking for expert guidance on which patients need what tests before surgery, and also how to better determine what preoperative tests are a waste of time and money for certain patients, so that he’ll be armed with useful information when he went back to his medical center.
“I can slap something on the surgeon’s desk and say, ‘Here’s why we’re not doing it,’ ” Dr. Rothberg said.
At the HM18 pre-course, experts gave guidance on the benefits of hospitalist involvement in perioperative care and offered points to consider when assessing cardiac and pulmonary risk before surgeries. Hospitalists then broke into groups to brainstorm techniques that could improve their perioperative work.
She noted how surgical safety checklists have been shown to improve morbidity and mortality, as seen with a checklist developed by the World Health Organization and in California, where an enhanced recovery program at 20 hospitals has been successful.
“I think the reason we see changes in each of those … from pre to post when they implement, is because people start to communicate and collaborate,” she said. “I think that’s the secret sauce, and you can take that back home with you.”
Assessing risk
As for preoperative testing, history is replete with examples of tests once considered crucial but that have proven to be unimportant for many patients, including preoperative carotid endarterectomy, preop ECG, preop coronary revasularization, and preop lab work.
“I was always listening for bruits years ago,” Dr. Grant said. “I’ve sort of stopped doing that now. You’ll hear it, you won’t know what to do with it. We used to take care of those things before surgery. We now know that’s not helpful for patients without symptoms.”
Dr. Cohn’s philosophy is to not suggest a delay without firm evidence that it is necessary. “I try not to interfere with surgery unless I feel that there is significant risk,” he said.
In workshop discussions at the HM18 pre-course, hospitalists considered their contributions to preoperative care and ways they might be able to contribute more effectively. Among their ideas were better communication with anesthesiology – regarded as severely lacking by many hospitalists in the session – as well as designating smaller perioperative teams to foster knowledge and greater trust with surgeons.
Aron Mednick, MD, FHM, director of the comanagement service at Tisch Hospital, NYU Langone Medical Center, New York, said his group talked about an “identify, mitigate, propose, and resolve” method – identifying services or conditions with a high rate of preoperative problems, finding data on how to solve them, and proposing ways to get hospitalists involved in the solution.
“We noted that a lot of people experience resistance with getting hospitalists involved in care early,” he said. “So one of the ways to do this is actually to identify problems and start above the surgeon, at the CMO and COO level, and then move down through department chairs and, basically, impose our existence on the care of the patient.”
ORLANDO – Michael Rothberg, MD, a nocturnist who works at Presbyterian Rust Medical Center in Albuquerque, often is torn when asked to routinely perform preoperative tests, such as ECGs, on patients.
On the one hand, Dr. Rothberg knows that for many patients there is almost certainly no benefit to some of the tests. On the other hand, surgeons expect the tests to be performed – so, for the sake of collegiality, patients often have tests ordered that hospitalists suspect are unnecessary.
This was a big part of why Dr. Rothberg decided to come a day early to HM18, held in early April in Orlando, to attend the pre-course “Essentials of Perioperative Medicine and Co-Management for the Hospitalist.” He was looking for expert guidance on which patients need what tests before surgery, and also how to better determine what preoperative tests are a waste of time and money for certain patients, so that he’ll be armed with useful information when he went back to his medical center.
“I can slap something on the surgeon’s desk and say, ‘Here’s why we’re not doing it,’ ” Dr. Rothberg said.
At the HM18 pre-course, experts gave guidance on the benefits of hospitalist involvement in perioperative care and offered points to consider when assessing cardiac and pulmonary risk before surgeries. Hospitalists then broke into groups to brainstorm techniques that could improve their perioperative work.
She noted how surgical safety checklists have been shown to improve morbidity and mortality, as seen with a checklist developed by the World Health Organization and in California, where an enhanced recovery program at 20 hospitals has been successful.
“I think the reason we see changes in each of those … from pre to post when they implement, is because people start to communicate and collaborate,” she said. “I think that’s the secret sauce, and you can take that back home with you.”
Assessing risk
As for preoperative testing, history is replete with examples of tests once considered crucial but that have proven to be unimportant for many patients, including preoperative carotid endarterectomy, preop ECG, preop coronary revasularization, and preop lab work.
“I was always listening for bruits years ago,” Dr. Grant said. “I’ve sort of stopped doing that now. You’ll hear it, you won’t know what to do with it. We used to take care of those things before surgery. We now know that’s not helpful for patients without symptoms.”
Dr. Cohn’s philosophy is to not suggest a delay without firm evidence that it is necessary. “I try not to interfere with surgery unless I feel that there is significant risk,” he said.
In workshop discussions at the HM18 pre-course, hospitalists considered their contributions to preoperative care and ways they might be able to contribute more effectively. Among their ideas were better communication with anesthesiology – regarded as severely lacking by many hospitalists in the session – as well as designating smaller perioperative teams to foster knowledge and greater trust with surgeons.
Aron Mednick, MD, FHM, director of the comanagement service at Tisch Hospital, NYU Langone Medical Center, New York, said his group talked about an “identify, mitigate, propose, and resolve” method – identifying services or conditions with a high rate of preoperative problems, finding data on how to solve them, and proposing ways to get hospitalists involved in the solution.
“We noted that a lot of people experience resistance with getting hospitalists involved in care early,” he said. “So one of the ways to do this is actually to identify problems and start above the surgeon, at the CMO and COO level, and then move down through department chairs and, basically, impose our existence on the care of the patient.”
REPORTING FROM HM18
Many hospitals had no mandatory flu vaccine requirements in 2017
Many U.S. hospitals still did not have influenza vaccination requirements for health care personnel as of summer 2017, suggested the results of a national survey.
Nearly two-thirds of hospitals had mandatory influenza vaccination in place in 2017, up from just one-third in 2013, according to survey responses submitted by infection preventionists working at Veterans Affairs (VA) and non-VA hospitals.
Despite recommendations to vaccinate health care personnel against influenza, there are several challenges and barriers to implementing the practice, the authors wrote in JAMA Network Open.
“Mandating influenza vaccination remains a controversial topic, with uncertainty of the effectiveness of health care personnel influenza vaccination in reducing patient morbidity and mortality, different conclusions regarding the grading of the evidence, and numerous legal and ethical precedents to be carefully considered,” they wrote.
Their study was based on 1,062 responses to a panel survey of infection preventionists conducted every 4 years. The survey asked providers about practices used in their hospitals to prevent health care–associated infections.
Compared with 2013, when only 37.1% of non-VA hospitals had mandatory influenza vaccination requirements, the 2017 survey showed a significant increase to 61.4% (P less than .001), Dr. Greene and his colleagues wrote in their report.
By contrast, the proportion of VA hospitals with such requirements increased only slightly, from 1.3% in 2013 to just 4.1% in 2017 (P = .29), the report showed.
Penalties for not complying with the policy were not universal in hospitals with mandates, they added. Only 74% said they had such penalties, and 13% allowed health care personnel to decline influenza vaccination without a specified reason.
After the survey responses were received, the VA issued a directive stating that all health care personnel should receive annual influenza vaccination and should wear masks during influenza season, Dr. Greene noted.
That directive is in line with recommendations from the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, which have stated that all health care personnel should receive influenza vaccination each year.
In addition, the U.S. Department of Health & Human Services has set a goal of 90% of health care personnel to be vaccinated by 2020, Dr. Green and his coauthors noted.
Mandating influenza vaccination is just one proven successful strategy for increasing coverage at hospitals, according to the study authors. Other approaches include influenza education, incentives, free and easy access to vaccination, and annual campaigns directed at health care personnel, as well as written policies describing the vaccination goal.
“Regardless of whether an organization has an official mandate for vaccinations, establishing a written policy that states the organizational commitment to increasing vaccination rates is among the recommended strategies for improving vaccination coverage among health care personnel,” they wrote.
Dr. Greene and his coauthors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
SOURCE: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
This study suggests a significant increase in use of mandatory influenza vaccination policies during 2013-2017, driven mainly by increases at non–Veterans Affairs (VA) hospitals and little change at VA facilities. However, there are some caveats to the findings that should be considered, Hilary M. Babcock, MD, MPH, wrote in an editorial referencing the study.
The sample for the 2013 and 2017 surveys included different facilities and different size facilities, so direct comparisons cannot be made, according to Dr. Babcock.
Moreover, the survey questions were worded somewhat differently in the two surveys, and it does not appear that “mandate” was defined by the study authors, she said in her editorial.
The VA recently issued a directive that all health care personnel should receive influenza vaccination and wear masks during influenza season. This new directive provides an “excellent opportunity” to address knowledge gaps regarding the effects of influenza vaccination of health care personnel on patient outcomes, according to Dr. Babcock.
“While the assumption that decreasing the risk of influenza in health care personnel will result in decreased risk of influenza in patients cared for by those health care personnel is common sense, for acute care settings, it is still largely an assumption,” Dr. Babcock wrote. “Hopefully, the Veterans Health Administration will combine this initiative with thoughtful, planned, patient outcome assessments to help define the anticipated benefit of these efforts.”
Dr. Babcock is with Washington University and the BJC HealthCare Infection Prevention & Epidemiology Consortium, both in St. Louis. These comments are derived from her editorial in JAMA Network Open (2018;1[2]:e180144). Dr. Babcock reported no conflict of interest disclosures related to her editorial.
This study suggests a significant increase in use of mandatory influenza vaccination policies during 2013-2017, driven mainly by increases at non–Veterans Affairs (VA) hospitals and little change at VA facilities. However, there are some caveats to the findings that should be considered, Hilary M. Babcock, MD, MPH, wrote in an editorial referencing the study.
The sample for the 2013 and 2017 surveys included different facilities and different size facilities, so direct comparisons cannot be made, according to Dr. Babcock.
Moreover, the survey questions were worded somewhat differently in the two surveys, and it does not appear that “mandate” was defined by the study authors, she said in her editorial.
The VA recently issued a directive that all health care personnel should receive influenza vaccination and wear masks during influenza season. This new directive provides an “excellent opportunity” to address knowledge gaps regarding the effects of influenza vaccination of health care personnel on patient outcomes, according to Dr. Babcock.
“While the assumption that decreasing the risk of influenza in health care personnel will result in decreased risk of influenza in patients cared for by those health care personnel is common sense, for acute care settings, it is still largely an assumption,” Dr. Babcock wrote. “Hopefully, the Veterans Health Administration will combine this initiative with thoughtful, planned, patient outcome assessments to help define the anticipated benefit of these efforts.”
Dr. Babcock is with Washington University and the BJC HealthCare Infection Prevention & Epidemiology Consortium, both in St. Louis. These comments are derived from her editorial in JAMA Network Open (2018;1[2]:e180144). Dr. Babcock reported no conflict of interest disclosures related to her editorial.
This study suggests a significant increase in use of mandatory influenza vaccination policies during 2013-2017, driven mainly by increases at non–Veterans Affairs (VA) hospitals and little change at VA facilities. However, there are some caveats to the findings that should be considered, Hilary M. Babcock, MD, MPH, wrote in an editorial referencing the study.
The sample for the 2013 and 2017 surveys included different facilities and different size facilities, so direct comparisons cannot be made, according to Dr. Babcock.
Moreover, the survey questions were worded somewhat differently in the two surveys, and it does not appear that “mandate” was defined by the study authors, she said in her editorial.
The VA recently issued a directive that all health care personnel should receive influenza vaccination and wear masks during influenza season. This new directive provides an “excellent opportunity” to address knowledge gaps regarding the effects of influenza vaccination of health care personnel on patient outcomes, according to Dr. Babcock.
“While the assumption that decreasing the risk of influenza in health care personnel will result in decreased risk of influenza in patients cared for by those health care personnel is common sense, for acute care settings, it is still largely an assumption,” Dr. Babcock wrote. “Hopefully, the Veterans Health Administration will combine this initiative with thoughtful, planned, patient outcome assessments to help define the anticipated benefit of these efforts.”
Dr. Babcock is with Washington University and the BJC HealthCare Infection Prevention & Epidemiology Consortium, both in St. Louis. These comments are derived from her editorial in JAMA Network Open (2018;1[2]:e180144). Dr. Babcock reported no conflict of interest disclosures related to her editorial.
Many U.S. hospitals still did not have influenza vaccination requirements for health care personnel as of summer 2017, suggested the results of a national survey.
Nearly two-thirds of hospitals had mandatory influenza vaccination in place in 2017, up from just one-third in 2013, according to survey responses submitted by infection preventionists working at Veterans Affairs (VA) and non-VA hospitals.
Despite recommendations to vaccinate health care personnel against influenza, there are several challenges and barriers to implementing the practice, the authors wrote in JAMA Network Open.
“Mandating influenza vaccination remains a controversial topic, with uncertainty of the effectiveness of health care personnel influenza vaccination in reducing patient morbidity and mortality, different conclusions regarding the grading of the evidence, and numerous legal and ethical precedents to be carefully considered,” they wrote.
Their study was based on 1,062 responses to a panel survey of infection preventionists conducted every 4 years. The survey asked providers about practices used in their hospitals to prevent health care–associated infections.
Compared with 2013, when only 37.1% of non-VA hospitals had mandatory influenza vaccination requirements, the 2017 survey showed a significant increase to 61.4% (P less than .001), Dr. Greene and his colleagues wrote in their report.
By contrast, the proportion of VA hospitals with such requirements increased only slightly, from 1.3% in 2013 to just 4.1% in 2017 (P = .29), the report showed.
Penalties for not complying with the policy were not universal in hospitals with mandates, they added. Only 74% said they had such penalties, and 13% allowed health care personnel to decline influenza vaccination without a specified reason.
After the survey responses were received, the VA issued a directive stating that all health care personnel should receive annual influenza vaccination and should wear masks during influenza season, Dr. Greene noted.
That directive is in line with recommendations from the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, which have stated that all health care personnel should receive influenza vaccination each year.
In addition, the U.S. Department of Health & Human Services has set a goal of 90% of health care personnel to be vaccinated by 2020, Dr. Green and his coauthors noted.
Mandating influenza vaccination is just one proven successful strategy for increasing coverage at hospitals, according to the study authors. Other approaches include influenza education, incentives, free and easy access to vaccination, and annual campaigns directed at health care personnel, as well as written policies describing the vaccination goal.
“Regardless of whether an organization has an official mandate for vaccinations, establishing a written policy that states the organizational commitment to increasing vaccination rates is among the recommended strategies for improving vaccination coverage among health care personnel,” they wrote.
Dr. Greene and his coauthors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
SOURCE: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
Many U.S. hospitals still did not have influenza vaccination requirements for health care personnel as of summer 2017, suggested the results of a national survey.
Nearly two-thirds of hospitals had mandatory influenza vaccination in place in 2017, up from just one-third in 2013, according to survey responses submitted by infection preventionists working at Veterans Affairs (VA) and non-VA hospitals.
Despite recommendations to vaccinate health care personnel against influenza, there are several challenges and barriers to implementing the practice, the authors wrote in JAMA Network Open.
“Mandating influenza vaccination remains a controversial topic, with uncertainty of the effectiveness of health care personnel influenza vaccination in reducing patient morbidity and mortality, different conclusions regarding the grading of the evidence, and numerous legal and ethical precedents to be carefully considered,” they wrote.
Their study was based on 1,062 responses to a panel survey of infection preventionists conducted every 4 years. The survey asked providers about practices used in their hospitals to prevent health care–associated infections.
Compared with 2013, when only 37.1% of non-VA hospitals had mandatory influenza vaccination requirements, the 2017 survey showed a significant increase to 61.4% (P less than .001), Dr. Greene and his colleagues wrote in their report.
By contrast, the proportion of VA hospitals with such requirements increased only slightly, from 1.3% in 2013 to just 4.1% in 2017 (P = .29), the report showed.
Penalties for not complying with the policy were not universal in hospitals with mandates, they added. Only 74% said they had such penalties, and 13% allowed health care personnel to decline influenza vaccination without a specified reason.
After the survey responses were received, the VA issued a directive stating that all health care personnel should receive annual influenza vaccination and should wear masks during influenza season, Dr. Greene noted.
That directive is in line with recommendations from the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, which have stated that all health care personnel should receive influenza vaccination each year.
In addition, the U.S. Department of Health & Human Services has set a goal of 90% of health care personnel to be vaccinated by 2020, Dr. Green and his coauthors noted.
Mandating influenza vaccination is just one proven successful strategy for increasing coverage at hospitals, according to the study authors. Other approaches include influenza education, incentives, free and easy access to vaccination, and annual campaigns directed at health care personnel, as well as written policies describing the vaccination goal.
“Regardless of whether an organization has an official mandate for vaccinations, establishing a written policy that states the organizational commitment to increasing vaccination rates is among the recommended strategies for improving vaccination coverage among health care personnel,” they wrote.
Dr. Greene and his coauthors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
SOURCE: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
FROM JAMA Network Open
Key clinical point: Despite a significant increase in influenza vaccination at non-VA hospitals, many VA and non-VA hospitals still do not have mandatory influenza vaccination requirements for health care personnel.
Major finding: During 2013-2017, the proportion of non-VA hospitals with requirements increased from 37.1% to 61.4% (P less than .001), contrasting with a rise from 1.3% to just 4.1% at VA hospitals (P = .29).
Study details: A study of survey responses from 1,062 infection preventionists at VA and non-VA hospitals in the United States submitted between 2013 and 2017.
Disclosures: Authors reported receiving grants from the Blue Cross Blue Shield of Michigan Foundation and the U.S. Department of Veterans Affairs Patient Safety Center of Inquiry during the conduct of the study. One study coauthor reported personal fees from Jvion and from Doximity outside the submitted work.
Source: Greene MT et al. JAMA Network Open. 2018;1(2):e180143.
Severity of sepsis-associated coagulopathy predicts hospital mortality
Patients with appear to be at heightened risk of death, according to results of a large retrospective cohort study.
The risk of death in the study increased with the severity of the sepsis-associated coagulopathy, which was defined using international normalized ratio (INR) and platelet counts.
Those findings suggest that the severity of coagulation abnormalities might be used to quantify mortality risk, according to investigator Patrick G. Lyons, MD, of the division of pulmonary and critical care medicine, Washington University, St. Louis, and his coinvestigators.
“Future trials of sepsis therapies targeting the coagulation cascade should take into account the presence or absence of sepsis-associated coagulopathy, as well as the severity of sepsis-associated coagulopathy, when formulating potential trial designs,” the investigators wrote in the journal Critical Care Medicine.
Their retrospective cohort study included 6,148 consecutive patients with sepsis or septic shock hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015. Of that group, 26% had sepsis-associated coagulopathy, defined as having both an INR of 1.2 or higher and a platelet count less than 150,000/mcL. Sepsis-associated coagulopathy was classified as mild for 4%, moderate for 16%, and severe for 6% of the cohort.
Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, the research team found, increasing progressively from 27.0% for mild, 40.7% for moderate, and 56.1% for patients in the most severe category of sepsis-associated coagulopathy (P less than .001).
Hospital and ICU days also increased progressively according to the severity of coagulopathy, they reported.
Both presence and severity of sepsis-associated coagulopathy remained independently associated with hospital mortality even after adjustments were made for patient characteristics, hospitalization variables, and interactions between sepsis-associated coagulopathy and cancer, investigators said. Odds ratios ranged from 1.33 to 2.14 for presence of sepsis-associated coagulopathy, and from 1.18 to 1.51 for severity, they reported in the journal.
These data have potential implications for managing patients with sepsis, according to Dr. Lyons and coinvestigators. In particular, severity of sepsis-associated coagulopathy might be used as “another relatively simple way” to compare sepsis patient populations, similar to other markers of severity such as the Sequential Organ Failure Assessment score.
“This could have important implications for comparing the outcomes of patients with sepsis from different hospitals, especially with increasing requirements for public reporting of such data through systems such as the Severe Sepsis/Septic Shock Early Management Bundle-1 and New York State’s Rory’s Regulations,” the investigators wrote.
Reported disclosures for the study included institutional funding from Asahi Kasei Pharma America by one coauthor, and support from Barnes-Jewish Hospital Foundation by another. No other potential conflicts of interest were reported.
SOURCE: Lyons PG et al. Crit Care Med. 2018 May;46(5):736-42.
This study outlines a simplified classification scheme for coagulopathy with implications that are potentially “profound,” according to authors of an editorial accompanying the journal article.
“Despite the frequency with which hemostatic derangements occur in sepsis, there has not been a widely accepted system for stratification of coagulopathies,” said editorialists Garrett W. Britton, DO, Cody Babcock, PharmD, and Christopher J. Colombo, MD. “Of the most cited criteria, all have varying concordance, and one does not seem to have an advantage over another.”
In the present study, patients with sepsis-associated coagulopathy were stratified into mild, moderate, and severe categories based on international normalized ratio (INR) levels and platelet counts.
While the study has limitations including a sicker patient cohort and arbitrarily chosen severity thresholds, the investigators did find progressively increasing mortality rates that correlated with severity and were independent of confounding variables.
“Overall, this stratification system will prove useful in identifying target populations in future interventional studies,” the editorial authors wrote.
Since sepsis-related mortality remains high, the ultimate goal of research should be identifying varying phenotypes of the disease and targeting them with specific therapies, they added.
“Lyons et al. have aided the first steps in that process with their straightforward classification scheme for sepsis-associated coagulopathy,” they wrote. “Intelligently designed therapeutic trials ‘evaluating’ the response of these phenotypes to new (or old) pharmacotherapy should be the ultimate goal.”
Garrett W. Britton, DO, is with the department of medicine, critical care section, Walter Reed National Military Medical Center, Bethesda, Md. Cody Babcock, PharmD, and Christopher J. Colombo, MD, are with the department of medicine, critical care section, Dwight David Eisenhower Army Medical Center, Fort Gordon, Ga. These comments are derived from their editorial in Critical Care Medicine . The authors had no disclosures beyond reporting government work.
This study outlines a simplified classification scheme for coagulopathy with implications that are potentially “profound,” according to authors of an editorial accompanying the journal article.
“Despite the frequency with which hemostatic derangements occur in sepsis, there has not been a widely accepted system for stratification of coagulopathies,” said editorialists Garrett W. Britton, DO, Cody Babcock, PharmD, and Christopher J. Colombo, MD. “Of the most cited criteria, all have varying concordance, and one does not seem to have an advantage over another.”
In the present study, patients with sepsis-associated coagulopathy were stratified into mild, moderate, and severe categories based on international normalized ratio (INR) levels and platelet counts.
While the study has limitations including a sicker patient cohort and arbitrarily chosen severity thresholds, the investigators did find progressively increasing mortality rates that correlated with severity and were independent of confounding variables.
“Overall, this stratification system will prove useful in identifying target populations in future interventional studies,” the editorial authors wrote.
Since sepsis-related mortality remains high, the ultimate goal of research should be identifying varying phenotypes of the disease and targeting them with specific therapies, they added.
“Lyons et al. have aided the first steps in that process with their straightforward classification scheme for sepsis-associated coagulopathy,” they wrote. “Intelligently designed therapeutic trials ‘evaluating’ the response of these phenotypes to new (or old) pharmacotherapy should be the ultimate goal.”
Garrett W. Britton, DO, is with the department of medicine, critical care section, Walter Reed National Military Medical Center, Bethesda, Md. Cody Babcock, PharmD, and Christopher J. Colombo, MD, are with the department of medicine, critical care section, Dwight David Eisenhower Army Medical Center, Fort Gordon, Ga. These comments are derived from their editorial in Critical Care Medicine . The authors had no disclosures beyond reporting government work.
This study outlines a simplified classification scheme for coagulopathy with implications that are potentially “profound,” according to authors of an editorial accompanying the journal article.
“Despite the frequency with which hemostatic derangements occur in sepsis, there has not been a widely accepted system for stratification of coagulopathies,” said editorialists Garrett W. Britton, DO, Cody Babcock, PharmD, and Christopher J. Colombo, MD. “Of the most cited criteria, all have varying concordance, and one does not seem to have an advantage over another.”
In the present study, patients with sepsis-associated coagulopathy were stratified into mild, moderate, and severe categories based on international normalized ratio (INR) levels and platelet counts.
While the study has limitations including a sicker patient cohort and arbitrarily chosen severity thresholds, the investigators did find progressively increasing mortality rates that correlated with severity and were independent of confounding variables.
“Overall, this stratification system will prove useful in identifying target populations in future interventional studies,” the editorial authors wrote.
Since sepsis-related mortality remains high, the ultimate goal of research should be identifying varying phenotypes of the disease and targeting them with specific therapies, they added.
“Lyons et al. have aided the first steps in that process with their straightforward classification scheme for sepsis-associated coagulopathy,” they wrote. “Intelligently designed therapeutic trials ‘evaluating’ the response of these phenotypes to new (or old) pharmacotherapy should be the ultimate goal.”
Garrett W. Britton, DO, is with the department of medicine, critical care section, Walter Reed National Military Medical Center, Bethesda, Md. Cody Babcock, PharmD, and Christopher J. Colombo, MD, are with the department of medicine, critical care section, Dwight David Eisenhower Army Medical Center, Fort Gordon, Ga. These comments are derived from their editorial in Critical Care Medicine . The authors had no disclosures beyond reporting government work.
Patients with appear to be at heightened risk of death, according to results of a large retrospective cohort study.
The risk of death in the study increased with the severity of the sepsis-associated coagulopathy, which was defined using international normalized ratio (INR) and platelet counts.
Those findings suggest that the severity of coagulation abnormalities might be used to quantify mortality risk, according to investigator Patrick G. Lyons, MD, of the division of pulmonary and critical care medicine, Washington University, St. Louis, and his coinvestigators.
“Future trials of sepsis therapies targeting the coagulation cascade should take into account the presence or absence of sepsis-associated coagulopathy, as well as the severity of sepsis-associated coagulopathy, when formulating potential trial designs,” the investigators wrote in the journal Critical Care Medicine.
Their retrospective cohort study included 6,148 consecutive patients with sepsis or septic shock hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015. Of that group, 26% had sepsis-associated coagulopathy, defined as having both an INR of 1.2 or higher and a platelet count less than 150,000/mcL. Sepsis-associated coagulopathy was classified as mild for 4%, moderate for 16%, and severe for 6% of the cohort.
Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, the research team found, increasing progressively from 27.0% for mild, 40.7% for moderate, and 56.1% for patients in the most severe category of sepsis-associated coagulopathy (P less than .001).
Hospital and ICU days also increased progressively according to the severity of coagulopathy, they reported.
Both presence and severity of sepsis-associated coagulopathy remained independently associated with hospital mortality even after adjustments were made for patient characteristics, hospitalization variables, and interactions between sepsis-associated coagulopathy and cancer, investigators said. Odds ratios ranged from 1.33 to 2.14 for presence of sepsis-associated coagulopathy, and from 1.18 to 1.51 for severity, they reported in the journal.
These data have potential implications for managing patients with sepsis, according to Dr. Lyons and coinvestigators. In particular, severity of sepsis-associated coagulopathy might be used as “another relatively simple way” to compare sepsis patient populations, similar to other markers of severity such as the Sequential Organ Failure Assessment score.
“This could have important implications for comparing the outcomes of patients with sepsis from different hospitals, especially with increasing requirements for public reporting of such data through systems such as the Severe Sepsis/Septic Shock Early Management Bundle-1 and New York State’s Rory’s Regulations,” the investigators wrote.
Reported disclosures for the study included institutional funding from Asahi Kasei Pharma America by one coauthor, and support from Barnes-Jewish Hospital Foundation by another. No other potential conflicts of interest were reported.
SOURCE: Lyons PG et al. Crit Care Med. 2018 May;46(5):736-42.
Patients with appear to be at heightened risk of death, according to results of a large retrospective cohort study.
The risk of death in the study increased with the severity of the sepsis-associated coagulopathy, which was defined using international normalized ratio (INR) and platelet counts.
Those findings suggest that the severity of coagulation abnormalities might be used to quantify mortality risk, according to investigator Patrick G. Lyons, MD, of the division of pulmonary and critical care medicine, Washington University, St. Louis, and his coinvestigators.
“Future trials of sepsis therapies targeting the coagulation cascade should take into account the presence or absence of sepsis-associated coagulopathy, as well as the severity of sepsis-associated coagulopathy, when formulating potential trial designs,” the investigators wrote in the journal Critical Care Medicine.
Their retrospective cohort study included 6,148 consecutive patients with sepsis or septic shock hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015. Of that group, 26% had sepsis-associated coagulopathy, defined as having both an INR of 1.2 or higher and a platelet count less than 150,000/mcL. Sepsis-associated coagulopathy was classified as mild for 4%, moderate for 16%, and severe for 6% of the cohort.
Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, the research team found, increasing progressively from 27.0% for mild, 40.7% for moderate, and 56.1% for patients in the most severe category of sepsis-associated coagulopathy (P less than .001).
Hospital and ICU days also increased progressively according to the severity of coagulopathy, they reported.
Both presence and severity of sepsis-associated coagulopathy remained independently associated with hospital mortality even after adjustments were made for patient characteristics, hospitalization variables, and interactions between sepsis-associated coagulopathy and cancer, investigators said. Odds ratios ranged from 1.33 to 2.14 for presence of sepsis-associated coagulopathy, and from 1.18 to 1.51 for severity, they reported in the journal.
These data have potential implications for managing patients with sepsis, according to Dr. Lyons and coinvestigators. In particular, severity of sepsis-associated coagulopathy might be used as “another relatively simple way” to compare sepsis patient populations, similar to other markers of severity such as the Sequential Organ Failure Assessment score.
“This could have important implications for comparing the outcomes of patients with sepsis from different hospitals, especially with increasing requirements for public reporting of such data through systems such as the Severe Sepsis/Septic Shock Early Management Bundle-1 and New York State’s Rory’s Regulations,” the investigators wrote.
Reported disclosures for the study included institutional funding from Asahi Kasei Pharma America by one coauthor, and support from Barnes-Jewish Hospital Foundation by another. No other potential conflicts of interest were reported.
SOURCE: Lyons PG et al. Crit Care Med. 2018 May;46(5):736-42.
FROM CRITICAL CARE MEDICINE
Key clinical point: Risk of hospital mortality increased incrementally with the severity of sepsis-related coagulopathy.
Major finding: Hospital mortality was 25.4% for patients with no sepsis-associated coagulopathy, increasing progressively up to 56.1% for patients in the most severe category.
Study details: A retrospective cohort study including 6,148 consecutive patients hospitalized at a 1,300-bed urban academic medical center between 2010 and 2015.
Disclosures: One author reported institutional funding from Asahi Kasei Pharma America and another noted support from Barnes-Jewish Hospital Foundation. No other potential conflicts of interest were reported.
Source: Lyons PG et al. Crit Care Med. 2018 May;46(5):73642.