User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Study: No increased mortality with ACA-prompted readmission declines
Concerns that efforts to reduce 30-day hospital readmission rates under the Affordable Care Act’s Hospital Readmission Reduction Program might lead to unintended increases in mortality rates appear to be unfounded, according to a review of more than 6.7 million hospitalizations for heart failure, acute myocardial infarction, or pneumonia between 2008 and 2014.
In fact, reductions in 30-day readmission rates among Medicare fee-for-service beneficiaries are weakly but significantly correlated with reductions in hospital 30-day mortality rates after discharge, according to Kumar Dharmarajan, MD, of Yale New Haven (Conn.) Health, and colleagues (JAMA 2017 Jul 18;318[3]:270-8. doi: 10.1001/jama.2017.8444).
From 2008 to 2014, the RARRs declined in aggregate across hospitals (–0.053% for heart failure, –0.044% for acute MI, and –0.033% for pneumonia).
“In contrast, monthly aggregate trends across hospitals in 30-day risk-adjusted mortality rates after discharge varied by admitting condition” the investigators said.
For heart failure, acute MI, and pneumonia, there was an increase of 0.008%, a decrease of 0.003%, and an increase of 0.001%, respectively, they said. However, paired monthly trends in 30-day RARRs and 30-day RAMRs after discharge “identified concomitant reduction in readmission and mortality rates within hospitals.”
Correlation coefficients of the paired monthly trends for heart failure, acute MI, and pneumonia in 2008-2014 were 0.066, 0.067, and 0.108, respectively.
“Paired trends in hospital 30-day risk-adjusted readmission rates and both 90-day risk-adjusted mortality rates after discharge and 90-day risk-adjusted mortality rates after the admission date also identified concomitant reductions in readmission and mortality rates within hospitals,” the authors wrote.
The findings “do not support increasing postdischarge mortality related to reducing hospital readmissions,” they concluded.
The authors work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr. Dharmarajan reported serving as a consultant and scientific advisory board member for Clover Health at the time this research was performed. He is supported by grants from the National Institute on Aging and the American Federation for Aging Research, and the Yale Claude D. Pepper Older Americans Independence Center.
The findings by Dharmarajan and colleagues are “certainly good news,” Karen E. Joynt Maddox, MD, wrote in an editorial.
The study provides support for strategies that hospitals are using to reduce readmissions, and also underscores the importance of evaluating unintended consequences of policy changes such as the Affordable Care Act’s Hospital Readmissions Reduction Program (HRRP), she said (JAMA. 2017 Jul 18;318[3]:243-4).
The study did not address the possibility that attention to reducing readmissions has taken priority over reducing mortality, which could have the unintended consequence of slowing improvements in mortality, she noted, suggesting that for this and other reasons it may be “time to reexamine and reengineer the HRRP to avoid unintended consequences and to ensure that its incentives are fully aligned with the ultimate goal of improving the health outcomes of patients.
“Only with full knowledge of the advantages and disadvantages of a particular policy decision can policy makers and advocates work to craft statutes and rules that maximize benefits while minimizing harms,” she wrote.
Dr. Joynt Maddox is with Brigham and Women’s Hospital, Boston. She is supported by a grant from the National Heart, Lung, and Blood Institute.
The findings by Dharmarajan and colleagues are “certainly good news,” Karen E. Joynt Maddox, MD, wrote in an editorial.
The study provides support for strategies that hospitals are using to reduce readmissions, and also underscores the importance of evaluating unintended consequences of policy changes such as the Affordable Care Act’s Hospital Readmissions Reduction Program (HRRP), she said (JAMA. 2017 Jul 18;318[3]:243-4).
The study did not address the possibility that attention to reducing readmissions has taken priority over reducing mortality, which could have the unintended consequence of slowing improvements in mortality, she noted, suggesting that for this and other reasons it may be “time to reexamine and reengineer the HRRP to avoid unintended consequences and to ensure that its incentives are fully aligned with the ultimate goal of improving the health outcomes of patients.
“Only with full knowledge of the advantages and disadvantages of a particular policy decision can policy makers and advocates work to craft statutes and rules that maximize benefits while minimizing harms,” she wrote.
Dr. Joynt Maddox is with Brigham and Women’s Hospital, Boston. She is supported by a grant from the National Heart, Lung, and Blood Institute.
The findings by Dharmarajan and colleagues are “certainly good news,” Karen E. Joynt Maddox, MD, wrote in an editorial.
The study provides support for strategies that hospitals are using to reduce readmissions, and also underscores the importance of evaluating unintended consequences of policy changes such as the Affordable Care Act’s Hospital Readmissions Reduction Program (HRRP), she said (JAMA. 2017 Jul 18;318[3]:243-4).
The study did not address the possibility that attention to reducing readmissions has taken priority over reducing mortality, which could have the unintended consequence of slowing improvements in mortality, she noted, suggesting that for this and other reasons it may be “time to reexamine and reengineer the HRRP to avoid unintended consequences and to ensure that its incentives are fully aligned with the ultimate goal of improving the health outcomes of patients.
“Only with full knowledge of the advantages and disadvantages of a particular policy decision can policy makers and advocates work to craft statutes and rules that maximize benefits while minimizing harms,” she wrote.
Dr. Joynt Maddox is with Brigham and Women’s Hospital, Boston. She is supported by a grant from the National Heart, Lung, and Blood Institute.
Concerns that efforts to reduce 30-day hospital readmission rates under the Affordable Care Act’s Hospital Readmission Reduction Program might lead to unintended increases in mortality rates appear to be unfounded, according to a review of more than 6.7 million hospitalizations for heart failure, acute myocardial infarction, or pneumonia between 2008 and 2014.
In fact, reductions in 30-day readmission rates among Medicare fee-for-service beneficiaries are weakly but significantly correlated with reductions in hospital 30-day mortality rates after discharge, according to Kumar Dharmarajan, MD, of Yale New Haven (Conn.) Health, and colleagues (JAMA 2017 Jul 18;318[3]:270-8. doi: 10.1001/jama.2017.8444).
From 2008 to 2014, the RARRs declined in aggregate across hospitals (–0.053% for heart failure, –0.044% for acute MI, and –0.033% for pneumonia).
“In contrast, monthly aggregate trends across hospitals in 30-day risk-adjusted mortality rates after discharge varied by admitting condition” the investigators said.
For heart failure, acute MI, and pneumonia, there was an increase of 0.008%, a decrease of 0.003%, and an increase of 0.001%, respectively, they said. However, paired monthly trends in 30-day RARRs and 30-day RAMRs after discharge “identified concomitant reduction in readmission and mortality rates within hospitals.”
Correlation coefficients of the paired monthly trends for heart failure, acute MI, and pneumonia in 2008-2014 were 0.066, 0.067, and 0.108, respectively.
“Paired trends in hospital 30-day risk-adjusted readmission rates and both 90-day risk-adjusted mortality rates after discharge and 90-day risk-adjusted mortality rates after the admission date also identified concomitant reductions in readmission and mortality rates within hospitals,” the authors wrote.
The findings “do not support increasing postdischarge mortality related to reducing hospital readmissions,” they concluded.
The authors work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr. Dharmarajan reported serving as a consultant and scientific advisory board member for Clover Health at the time this research was performed. He is supported by grants from the National Institute on Aging and the American Federation for Aging Research, and the Yale Claude D. Pepper Older Americans Independence Center.
Concerns that efforts to reduce 30-day hospital readmission rates under the Affordable Care Act’s Hospital Readmission Reduction Program might lead to unintended increases in mortality rates appear to be unfounded, according to a review of more than 6.7 million hospitalizations for heart failure, acute myocardial infarction, or pneumonia between 2008 and 2014.
In fact, reductions in 30-day readmission rates among Medicare fee-for-service beneficiaries are weakly but significantly correlated with reductions in hospital 30-day mortality rates after discharge, according to Kumar Dharmarajan, MD, of Yale New Haven (Conn.) Health, and colleagues (JAMA 2017 Jul 18;318[3]:270-8. doi: 10.1001/jama.2017.8444).
From 2008 to 2014, the RARRs declined in aggregate across hospitals (–0.053% for heart failure, –0.044% for acute MI, and –0.033% for pneumonia).
“In contrast, monthly aggregate trends across hospitals in 30-day risk-adjusted mortality rates after discharge varied by admitting condition” the investigators said.
For heart failure, acute MI, and pneumonia, there was an increase of 0.008%, a decrease of 0.003%, and an increase of 0.001%, respectively, they said. However, paired monthly trends in 30-day RARRs and 30-day RAMRs after discharge “identified concomitant reduction in readmission and mortality rates within hospitals.”
Correlation coefficients of the paired monthly trends for heart failure, acute MI, and pneumonia in 2008-2014 were 0.066, 0.067, and 0.108, respectively.
“Paired trends in hospital 30-day risk-adjusted readmission rates and both 90-day risk-adjusted mortality rates after discharge and 90-day risk-adjusted mortality rates after the admission date also identified concomitant reductions in readmission and mortality rates within hospitals,” the authors wrote.
The findings “do not support increasing postdischarge mortality related to reducing hospital readmissions,” they concluded.
The authors work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr. Dharmarajan reported serving as a consultant and scientific advisory board member for Clover Health at the time this research was performed. He is supported by grants from the National Institute on Aging and the American Federation for Aging Research, and the Yale Claude D. Pepper Older Americans Independence Center.
FROM JAMA
Key clinical point:
Major finding: Correlation coefficients of the paired monthly trends for heart failure, acute myocardial infarction, and pneumonia in 2008-2014 were 0.066, 0.067, and 0.108, respectively.
Data source: A retrospective review of more than 6.7 million hospitalized Medicare fee-for-service beneficiaries.
Disclosures: The authors work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr. Dharmarajan reported serving as a consultant and scientific advisory board member for Clover Health at the time this research was performed. He is supported by grants from the National Institute on Aging and the American Federation for Aging Research, and the Yale Claude D. Pepper Older Americans Independence Center.
High Value Care curriculum reduced echocardiogram ordering
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.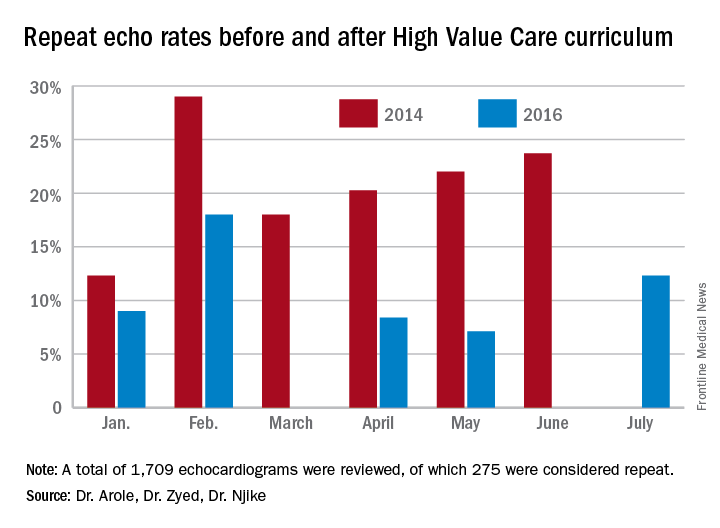
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
What is the best approach for managing CIED infections?
The case
A 72-year-old man with ischemic cardiomyopathy and left-ventricular ejection fraction of 15% had an cardioverter-defibrillator implanted five years ago for primary prevention of sudden cardiac death. He was brought to the emergency department by his daughter who noticed erythema and swelling over the generator pocket site. His temperature is 100.1° F. Vital signs are otherwise normal and stable.
What are the best initial and definitive management strategies for this patient?
When should a cardiac implanted electronic device (CIED) infection be suspected?
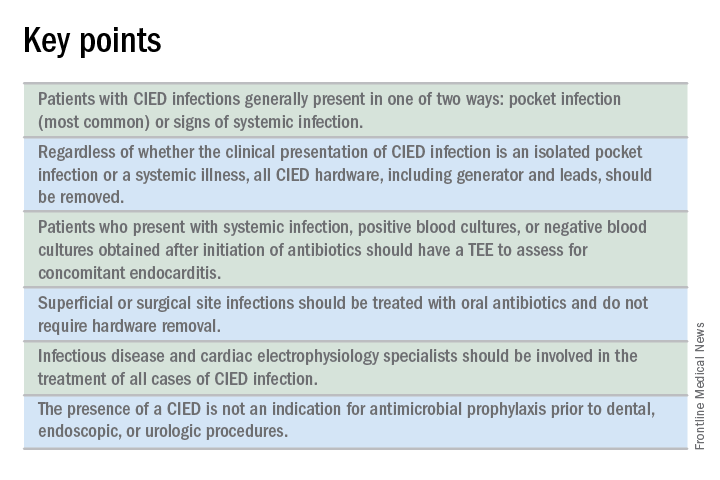
CIED infections generally present in one of two ways: as a local generator pocket infection or as a systemic illness.1 Around 70% of CIED infections present with findings related to the generator pocket site. Findings in such cases include pain, swelling, erythema, induration, and ulceration. Systemic findings range from vague constitutional symptoms to overt sepsis. It is important to note that systemic signs of infection are only present in the minority of cases. Their absence does not rule out a CIED infection.1,2 As such, hospitalists must maintain a high index of suspicion in order to avoid missing the diagnosis.
Unfortunately, it can be difficult to distinguish between a CIED infection and less severe postimplantation complications such as surgical site infections, superficial pocket site infections, and noninfected hematoma.2
What are the risk factors for CIED infections?
The risk factors for CIED infection fit into three broad categories: procedure, patient, and pathogen.
Procedural factors that increase risk of infection include lack of periprocedural antimicrobial prophylaxis, inexperienced physician operator, multiple revision procedures, hematoma formation at the pocket site, and increased amount of hardware.1
Patient factors center on medical comorbidities, including congestive heart failure, diabetes mellitus, chronic kidney disease, immunosuppression, and ongoing oral anticoagulation.1
The specific pathogen also plays a role in infection risk. In one cohort study of 1,524 patients with CIEDs, patients with Staphylococcus aureus bacteremia ended up having confirmed or suspected CIED infections in 54.6% of cases, compared with just 12.0% of patients who had bacteremia with gram-negative bacilli.3
Which bacteria cause most CIED infections?
The vast majority of CIED infections are caused by gram-positive bacteria.1,4 An Italian study of 1,204 patients with CIED infection reported that pathogens isolated from extracted leads were gram-positive bacteria in 92.5% of infections.4 Staph species are the most common pathogens. Coagulase-negative staphylococcus and Staphylococcus aureus accounted for 69% and 13.8% of all isolates, respectively. Of note, 33% of CoNS isolates and 13% of S. aureus isolates were resistant to oxacillin in that study.4
Which initial diagnostic tests should be performed?
Patients should have two sets of blood cultures drawn from two separate sites, prior to administration of antibiotics.1 Current guidelines recommend against aspiration of a suspected infected pocket site because the sensitivity for identifying the causal pathogen is low while the risk of introducing infection to the generator pocket is substantial.1 In the event of CIED removal, pocket tissue and lead tips should be sent for gram stain and culture.1
Do all patients require a transesophageal echocardiogram?
Guidelines recommend a transesophageal echocardiogram (TEE) if suspicion for CIED infection is high based on positive blood cultures, antibiotic initiation prior to culture collection, ongoing systemic illness, or other suggestive signs.1,2 Positive transthoracic echocardiogram findings (for example, a valve vegetation) do not obviate the need for TEE because of the possibility of other relevant complications (including endocarditis and perivalvular extension) for which TEE has a greater sensitivity.1
What is the approach to antimicrobial therapy?
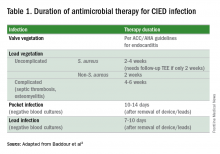
Since most infections involve staphylococcus species, initial empiric antimicrobial therapy should cover both oxacillin-sensitive and oxacillin-resistant strains. Thus, intravenous vancomycin is an appropriate initial choice.1 Culture and sensitivity results should then guide specific therapy decisions.1 Table 1 provides a summary of strategies for antimicrobial selection and duration.
Should all patients undergo complete device removal?
All patients with CIED infection require complete device removal, even if the infection is suspected to be confined to the generator pocket and blood cultures remain negative.2 Patients with superficial or surgical site infections without CIED infection do not require complete device removal. Rather, those cases can be managed with a 7- to 10-day course of oral antimicrobials.1
After device removal, what is the appropriate timing for installing a new device?
The decision to implant a replacement device is often made with input from infectious disease and cardiac electrophysiology experts. The decision must consider both infection and device-related concerns. Importantly, repeat blood cultures must be negative, and any infection in the pocket site should be controlled prior to installing a new device.2
Should all patients with a CIED receive antimicrobial prophylaxis prior to invasive dental, urologic, or endoscopic procedures?
No. Merely having a CIED is not considered an indication for antimicrobial prophylaxis.2
Back to the case
Two sets of blood cultures are drawn, and vancomycin is started as empiric therapy. The culture results show CoNS species, and antimicrobial resistance testing shows oxacillin-resistance.
TEE shows a vegetation on one of the leads but no vegetation on any of the heart valves. Cardiac electrophysiology is consulted and performs a percutaneous extraction of the entire device (generator and leads). Starting on the day of extraction, the patient undergoes two weeks of intravenous antimicrobial therapy with vancomycin. The patient, his family, and the electrophysiology team discuss the patient’s wishes, indications, and potential burdens related to device replacement.
He ultimately decides to have a replacement device installed. An infectious disease specialist is consulted to help define appropriate timing of the new device installation procedure.
Bottom line
The patient clearly had a CIED infection which required TEE, extraction of his CIED, and prolonged antimicrobial therapy.
Dr. Davisson is a primary care physician at Community Health Network in Indianapolis, Ind. Dr. Lockwood is a hospitalist at the Lexington VA Medical Center. Dr. Sweigart is a hospitalist at the University of Kentucky, Lexington, and the Lexington VA Medical Center.
References
1. Baddour LM, Epstein AE, Erickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458-77.
2. Baddour LM, Cha YM, Wilson WR. Infections of Cardiovascular Implantable Electronic Devices. N Engl J Med. 2012;367(9):842-9.
3. Uslan DZ, Sohail MR, St. Sauver JL, et al. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infection: A Population-Based Study. Arch Intern Med. 2007;167(7):669-75.
4. Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of Cardiac Implantable Electronic Device Infections. Europace. 2012;14(9):1334-9.
The case
A 72-year-old man with ischemic cardiomyopathy and left-ventricular ejection fraction of 15% had an cardioverter-defibrillator implanted five years ago for primary prevention of sudden cardiac death. He was brought to the emergency department by his daughter who noticed erythema and swelling over the generator pocket site. His temperature is 100.1° F. Vital signs are otherwise normal and stable.
What are the best initial and definitive management strategies for this patient?
When should a cardiac implanted electronic device (CIED) infection be suspected?
CIED infections generally present in one of two ways: as a local generator pocket infection or as a systemic illness.1 Around 70% of CIED infections present with findings related to the generator pocket site. Findings in such cases include pain, swelling, erythema, induration, and ulceration. Systemic findings range from vague constitutional symptoms to overt sepsis. It is important to note that systemic signs of infection are only present in the minority of cases. Their absence does not rule out a CIED infection.1,2 As such, hospitalists must maintain a high index of suspicion in order to avoid missing the diagnosis.
Unfortunately, it can be difficult to distinguish between a CIED infection and less severe postimplantation complications such as surgical site infections, superficial pocket site infections, and noninfected hematoma.2
What are the risk factors for CIED infections?
The risk factors for CIED infection fit into three broad categories: procedure, patient, and pathogen.
Procedural factors that increase risk of infection include lack of periprocedural antimicrobial prophylaxis, inexperienced physician operator, multiple revision procedures, hematoma formation at the pocket site, and increased amount of hardware.1
Patient factors center on medical comorbidities, including congestive heart failure, diabetes mellitus, chronic kidney disease, immunosuppression, and ongoing oral anticoagulation.1
The specific pathogen also plays a role in infection risk. In one cohort study of 1,524 patients with CIEDs, patients with Staphylococcus aureus bacteremia ended up having confirmed or suspected CIED infections in 54.6% of cases, compared with just 12.0% of patients who had bacteremia with gram-negative bacilli.3
Which bacteria cause most CIED infections?
The vast majority of CIED infections are caused by gram-positive bacteria.1,4 An Italian study of 1,204 patients with CIED infection reported that pathogens isolated from extracted leads were gram-positive bacteria in 92.5% of infections.4 Staph species are the most common pathogens. Coagulase-negative staphylococcus and Staphylococcus aureus accounted for 69% and 13.8% of all isolates, respectively. Of note, 33% of CoNS isolates and 13% of S. aureus isolates were resistant to oxacillin in that study.4
Which initial diagnostic tests should be performed?
Patients should have two sets of blood cultures drawn from two separate sites, prior to administration of antibiotics.1 Current guidelines recommend against aspiration of a suspected infected pocket site because the sensitivity for identifying the causal pathogen is low while the risk of introducing infection to the generator pocket is substantial.1 In the event of CIED removal, pocket tissue and lead tips should be sent for gram stain and culture.1
Do all patients require a transesophageal echocardiogram?
Guidelines recommend a transesophageal echocardiogram (TEE) if suspicion for CIED infection is high based on positive blood cultures, antibiotic initiation prior to culture collection, ongoing systemic illness, or other suggestive signs.1,2 Positive transthoracic echocardiogram findings (for example, a valve vegetation) do not obviate the need for TEE because of the possibility of other relevant complications (including endocarditis and perivalvular extension) for which TEE has a greater sensitivity.1
What is the approach to antimicrobial therapy?
Since most infections involve staphylococcus species, initial empiric antimicrobial therapy should cover both oxacillin-sensitive and oxacillin-resistant strains. Thus, intravenous vancomycin is an appropriate initial choice.1 Culture and sensitivity results should then guide specific therapy decisions.1 Table 1 provides a summary of strategies for antimicrobial selection and duration.
Should all patients undergo complete device removal?
All patients with CIED infection require complete device removal, even if the infection is suspected to be confined to the generator pocket and blood cultures remain negative.2 Patients with superficial or surgical site infections without CIED infection do not require complete device removal. Rather, those cases can be managed with a 7- to 10-day course of oral antimicrobials.1
After device removal, what is the appropriate timing for installing a new device?
The decision to implant a replacement device is often made with input from infectious disease and cardiac electrophysiology experts. The decision must consider both infection and device-related concerns. Importantly, repeat blood cultures must be negative, and any infection in the pocket site should be controlled prior to installing a new device.2
Should all patients with a CIED receive antimicrobial prophylaxis prior to invasive dental, urologic, or endoscopic procedures?
No. Merely having a CIED is not considered an indication for antimicrobial prophylaxis.2
Back to the case
Two sets of blood cultures are drawn, and vancomycin is started as empiric therapy. The culture results show CoNS species, and antimicrobial resistance testing shows oxacillin-resistance.
TEE shows a vegetation on one of the leads but no vegetation on any of the heart valves. Cardiac electrophysiology is consulted and performs a percutaneous extraction of the entire device (generator and leads). Starting on the day of extraction, the patient undergoes two weeks of intravenous antimicrobial therapy with vancomycin. The patient, his family, and the electrophysiology team discuss the patient’s wishes, indications, and potential burdens related to device replacement.
He ultimately decides to have a replacement device installed. An infectious disease specialist is consulted to help define appropriate timing of the new device installation procedure.
Bottom line
The patient clearly had a CIED infection which required TEE, extraction of his CIED, and prolonged antimicrobial therapy.
Dr. Davisson is a primary care physician at Community Health Network in Indianapolis, Ind. Dr. Lockwood is a hospitalist at the Lexington VA Medical Center. Dr. Sweigart is a hospitalist at the University of Kentucky, Lexington, and the Lexington VA Medical Center.

References
1. Baddour LM, Epstein AE, Erickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458-77.
2. Baddour LM, Cha YM, Wilson WR. Infections of Cardiovascular Implantable Electronic Devices. N Engl J Med. 2012;367(9):842-9.
3. Uslan DZ, Sohail MR, St. Sauver JL, et al. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infection: A Population-Based Study. Arch Intern Med. 2007;167(7):669-75.
4. Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of Cardiac Implantable Electronic Device Infections. Europace. 2012;14(9):1334-9.

The case
A 72-year-old man with ischemic cardiomyopathy and left-ventricular ejection fraction of 15% had an cardioverter-defibrillator implanted five years ago for primary prevention of sudden cardiac death. He was brought to the emergency department by his daughter who noticed erythema and swelling over the generator pocket site. His temperature is 100.1° F. Vital signs are otherwise normal and stable.
What are the best initial and definitive management strategies for this patient?
When should a cardiac implanted electronic device (CIED) infection be suspected?
CIED infections generally present in one of two ways: as a local generator pocket infection or as a systemic illness.1 Around 70% of CIED infections present with findings related to the generator pocket site. Findings in such cases include pain, swelling, erythema, induration, and ulceration. Systemic findings range from vague constitutional symptoms to overt sepsis. It is important to note that systemic signs of infection are only present in the minority of cases. Their absence does not rule out a CIED infection.1,2 As such, hospitalists must maintain a high index of suspicion in order to avoid missing the diagnosis.
Unfortunately, it can be difficult to distinguish between a CIED infection and less severe postimplantation complications such as surgical site infections, superficial pocket site infections, and noninfected hematoma.2
What are the risk factors for CIED infections?
The risk factors for CIED infection fit into three broad categories: procedure, patient, and pathogen.
Procedural factors that increase risk of infection include lack of periprocedural antimicrobial prophylaxis, inexperienced physician operator, multiple revision procedures, hematoma formation at the pocket site, and increased amount of hardware.1
Patient factors center on medical comorbidities, including congestive heart failure, diabetes mellitus, chronic kidney disease, immunosuppression, and ongoing oral anticoagulation.1
The specific pathogen also plays a role in infection risk. In one cohort study of 1,524 patients with CIEDs, patients with Staphylococcus aureus bacteremia ended up having confirmed or suspected CIED infections in 54.6% of cases, compared with just 12.0% of patients who had bacteremia with gram-negative bacilli.3
Which bacteria cause most CIED infections?
The vast majority of CIED infections are caused by gram-positive bacteria.1,4 An Italian study of 1,204 patients with CIED infection reported that pathogens isolated from extracted leads were gram-positive bacteria in 92.5% of infections.4 Staph species are the most common pathogens. Coagulase-negative staphylococcus and Staphylococcus aureus accounted for 69% and 13.8% of all isolates, respectively. Of note, 33% of CoNS isolates and 13% of S. aureus isolates were resistant to oxacillin in that study.4
Which initial diagnostic tests should be performed?
Patients should have two sets of blood cultures drawn from two separate sites, prior to administration of antibiotics.1 Current guidelines recommend against aspiration of a suspected infected pocket site because the sensitivity for identifying the causal pathogen is low while the risk of introducing infection to the generator pocket is substantial.1 In the event of CIED removal, pocket tissue and lead tips should be sent for gram stain and culture.1
Do all patients require a transesophageal echocardiogram?
Guidelines recommend a transesophageal echocardiogram (TEE) if suspicion for CIED infection is high based on positive blood cultures, antibiotic initiation prior to culture collection, ongoing systemic illness, or other suggestive signs.1,2 Positive transthoracic echocardiogram findings (for example, a valve vegetation) do not obviate the need for TEE because of the possibility of other relevant complications (including endocarditis and perivalvular extension) for which TEE has a greater sensitivity.1
What is the approach to antimicrobial therapy?
Since most infections involve staphylococcus species, initial empiric antimicrobial therapy should cover both oxacillin-sensitive and oxacillin-resistant strains. Thus, intravenous vancomycin is an appropriate initial choice.1 Culture and sensitivity results should then guide specific therapy decisions.1 Table 1 provides a summary of strategies for antimicrobial selection and duration.
Should all patients undergo complete device removal?
All patients with CIED infection require complete device removal, even if the infection is suspected to be confined to the generator pocket and blood cultures remain negative.2 Patients with superficial or surgical site infections without CIED infection do not require complete device removal. Rather, those cases can be managed with a 7- to 10-day course of oral antimicrobials.1
After device removal, what is the appropriate timing for installing a new device?
The decision to implant a replacement device is often made with input from infectious disease and cardiac electrophysiology experts. The decision must consider both infection and device-related concerns. Importantly, repeat blood cultures must be negative, and any infection in the pocket site should be controlled prior to installing a new device.2
Should all patients with a CIED receive antimicrobial prophylaxis prior to invasive dental, urologic, or endoscopic procedures?
No. Merely having a CIED is not considered an indication for antimicrobial prophylaxis.2
Back to the case
Two sets of blood cultures are drawn, and vancomycin is started as empiric therapy. The culture results show CoNS species, and antimicrobial resistance testing shows oxacillin-resistance.
TEE shows a vegetation on one of the leads but no vegetation on any of the heart valves. Cardiac electrophysiology is consulted and performs a percutaneous extraction of the entire device (generator and leads). Starting on the day of extraction, the patient undergoes two weeks of intravenous antimicrobial therapy with vancomycin. The patient, his family, and the electrophysiology team discuss the patient’s wishes, indications, and potential burdens related to device replacement.
He ultimately decides to have a replacement device installed. An infectious disease specialist is consulted to help define appropriate timing of the new device installation procedure.
Bottom line
The patient clearly had a CIED infection which required TEE, extraction of his CIED, and prolonged antimicrobial therapy.
Dr. Davisson is a primary care physician at Community Health Network in Indianapolis, Ind. Dr. Lockwood is a hospitalist at the Lexington VA Medical Center. Dr. Sweigart is a hospitalist at the University of Kentucky, Lexington, and the Lexington VA Medical Center.

References
1. Baddour LM, Epstein AE, Erickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458-77.
2. Baddour LM, Cha YM, Wilson WR. Infections of Cardiovascular Implantable Electronic Devices. N Engl J Med. 2012;367(9):842-9.
3. Uslan DZ, Sohail MR, St. Sauver JL, et al. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infection: A Population-Based Study. Arch Intern Med. 2007;167(7):669-75.
4. Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of Cardiac Implantable Electronic Device Infections. Europace. 2012;14(9):1334-9.

Plasma biomarker distinguishes ARDS, acute heart failure
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: An sST2 cutpoint of 271 ng/mL discriminated between ARDS and acute heart failure with 83% sensitivity and 88% specificity.
Data source: Review of 72 patients admitted for acute decompensated heart failure at one U.S. center.
Disclosures: Dr. Levy had no disclosures.
The role of NPs and PAs in hospital medicine programs
Background and growth
Hospitalist nurse practitioner (NP) and physician assistant (PA) providers have been a growing and evolving part of the inpatient medical workforce, seemingly since the inception of hospital medicine. Given the growth of these disciplines within hospital medicine, at this juncture it is helpful to look at this journey, to see what roles these providers have been serving, and to consider newer and novel trends in how NPs and PAs are being weaved into hospital medicine programs.
The drivers for growth in this provider population are not unlike those of physician hospitalists. The same milieu that provided inroads for physicians in hospital-based care have led the way for increased use of NP/PA providers. An aging physician workforce, residency work hour reforms, increasing complexity of patients and systems on the inpatient side, and the recognition that caring for inpatients is a specialty vastly different from the role of internist in primary care have all impacted the numbers of NPs and PAs in this arena.
• 2007 Today’s Hospitalist article: “Midlevels make a rocky entrance into hospital medicine”1
• 2009 ACP Hospitalist article: “When hiring midlevels, proceed with caution”2
These titles reflect the uncertainty at the time in how best to utilize NP/PA providers in hospital medicine (as well as an unfashionable vocabulary). The numbers at the time tell a similar story. In the Society of Hospital Medicine survey in 2007-2008, about 29% and 21% of hospital medicine practices utilized NPs and PAs, respectively. However, by 2014 about 50% of Veterans Affairs inpatient medical services deployed NP/PA providers, and most recent data from the Society of Hospital Medicine reveal that about 63% of groups use these advanced practice providers (APPs), with higher numbers in pediatric programs. Clearly there is evolving growth and enthusiasm for NP/PAs in hospital medicine.
Program models
Determining how best to use NP/PAs in hospital medicine programs has had a similar evolution. Reviewing past articles addressing these issues, one can see that there has been clear migration; initially NP/PAs were primarily hired to assist with late-afternoon admission surges, with about 60% of the APP workload being utilized to admit in 2007. Their role has continued to grow and change, much as hospitalist practices have; current program models consist of a few major types, with some novel models coming to the fore.
Another model is use of an NP/PA in an observation unit or with lower acuity observation patients. The majority of the management of the patients is completed and billed by the APP, with the physician available for backup. This hits the “sweet spot,” utilizing the right provider with the right skill set for the right patient. The program has to account for some reimbursement or compensation for the physician oversight time, but it is a very efficient use of APPs.
The third major deployment of APPs is with admissions. Many groups use APPs to admit into the late afternoon and evening, getting patients “tucked in,” including starting diagnostic work-ups and treatment plans. The physician hospitalist then evaluates the patient the next day and often bills for the admission. This model works in situations where the patient work-up is dependent on lab testing, imaging, or other diagnostic testing to understand and plan for the “arc” of the hospitalization; or in situations where the diagnosis is clear, but the patient needs time with treatment to determine response. The downside of this model is long-term job satisfaction for the APP (although some programs have them rotate through such a model at intervals).
Another area where APPs have made strong inroads is that of comanagement services. The NP or PA develops a long-term relationship with a surgical comanagement team, and is often highly engaged and extremely appreciated for managing chronic conditions such as hypertension and diabetes. This can be a very satisfying model for both teams. The NP/PA usually bills independently for these encounters.
APPS are also used in cross coverage and triage roles, allowing the day teams to focus on their primary patients. In a triage role, they can interface with the emergency department, providing a semi-neutral “mediator” for patient disposition.
On the more novel end of the spectrum, there is growth in more independent roles for APP hospitalists. Some groups are having success at using the paired rounding or dyad model, but having the physician see the patient every third day. This is most successful where there is strong onboarding and deep clarity for when to contact the backup physician. There are some data to support the effectiveness of this model, most recently in the Journal of Clinical Outcomes Management.3
Critical access hospitals are also having success in deploying APPs in a very independent role, staffing these hospitals at night. Smaller, rural hospitals with aging medical staff have learned to maximize the scope of practice of their APPs to remain viable and provide care for inpatients. This can be a very successful model for APPs working at the maximum scope of their practice. In addition, the use of telemedicine has been implemented to allow for remote physician backup. This may be a rapidly growing arm to hospital medicine practices in the future.
Ongoing barriers
There are many barriers to maximizing the scope of practice and efficiency of APPs in hospital medicine. They range from the “macro” to the “micro.”
On the larger stage, Medicare requires that home care orders be signed by an attending physician, which can be inefficient and difficult to accomplish. Other payers may have somewhat arcane statutes that limit billing practices, and state practice limitations vary widely. Although 22 states now allow for independent practice for NPs, other states may have a very restrictive practice environment that can impede creative care delivery models. But regardless of how liberal a practice the state allows, a hospital’s medical bylaws can still restrict the day-to-day practice of APPs. And those restrictive bylaws are emblematic of a more constant and corporeal barrier to APP practice, that of medical staff culture.
If there are physicians on the staff who fear that utilization of NP/PA providers will lead to a decay in the quality of care, or who feel threatened by the use of APPs, that can create a local stopgap to maximizing utilization of APPs. In addition, hospitalist physicians and leaders may lack knowledge or experience in APP practice. APPs take more time to successfully onboard than physicians; without clear expectations or road maps to accomplish this onboarding, leaders may feel that APP integration doesn’t work. And one bad experience can create long-term barriers for future practices.
Other barriers are the lack of standardized rigor and vigor in graduate education programs (in both educational and clinical experiences). This results in variation in the quality of NP/PA providers at graduation. Knowledge gaps may be perceived as incompetence, rather than just a lack of experience. There is a certificate for added qualification in hospital medicine for PA providers (which includes a specialty exam), and there is an acute care focus for NPs in training; however, there is no standardized licensure to ensure hospital medicine competency, creating a quagmire for hospitalist leaders who desire demonstrable competence of these providers.
Another barrier for some programs is financial; physicians may not want to give up their RVUs to an NP/PA provider. This can really inhibit a more independent role for the APP. It is important that financial incentives align with all members of the practice working at maximum scope.
Summary and future
In summary, the role of PA/NP in hospital medicine has continued to grow and evolve, to meet the needs of the industry. This includes an increase in the scope and independence of APPs, including the use of telehealth for required oversight. As a specialty, it is imperative that we continue to research APP model effectiveness, embrace innovative delivery models, and support effective onboarding and career development opportunities for our NP/PA providers.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Ms. Cardin is vice president, Advanced Practice Providers, at Sound Physicians, and is a member of SHM’s Board of Directors.
References
1. “Midlevels make a rocky entrance into hospital medicine,” by Bonnie Darves, Today’s Hospitalist, January 2007.
2. “When hiring midlevels, proceed with caution,” by Jessica Berthold, ACP Hospitalist, April 2009.
3. “A Comparison of Conventional and Expanded Physician Assistant Hospitalist Staffing Models at a Community Hospital,” J Clin Outcomes Manag. 2016 Oct 1;23[10]:455-61.
Background and growth
Hospitalist nurse practitioner (NP) and physician assistant (PA) providers have been a growing and evolving part of the inpatient medical workforce, seemingly since the inception of hospital medicine. Given the growth of these disciplines within hospital medicine, at this juncture it is helpful to look at this journey, to see what roles these providers have been serving, and to consider newer and novel trends in how NPs and PAs are being weaved into hospital medicine programs.
The drivers for growth in this provider population are not unlike those of physician hospitalists. The same milieu that provided inroads for physicians in hospital-based care have led the way for increased use of NP/PA providers. An aging physician workforce, residency work hour reforms, increasing complexity of patients and systems on the inpatient side, and the recognition that caring for inpatients is a specialty vastly different from the role of internist in primary care have all impacted the numbers of NPs and PAs in this arena.
• 2007 Today’s Hospitalist article: “Midlevels make a rocky entrance into hospital medicine”1
• 2009 ACP Hospitalist article: “When hiring midlevels, proceed with caution”2
These titles reflect the uncertainty at the time in how best to utilize NP/PA providers in hospital medicine (as well as an unfashionable vocabulary). The numbers at the time tell a similar story. In the Society of Hospital Medicine survey in 2007-2008, about 29% and 21% of hospital medicine practices utilized NPs and PAs, respectively. However, by 2014 about 50% of Veterans Affairs inpatient medical services deployed NP/PA providers, and most recent data from the Society of Hospital Medicine reveal that about 63% of groups use these advanced practice providers (APPs), with higher numbers in pediatric programs. Clearly there is evolving growth and enthusiasm for NP/PAs in hospital medicine.
Program models
Determining how best to use NP/PAs in hospital medicine programs has had a similar evolution. Reviewing past articles addressing these issues, one can see that there has been clear migration; initially NP/PAs were primarily hired to assist with late-afternoon admission surges, with about 60% of the APP workload being utilized to admit in 2007. Their role has continued to grow and change, much as hospitalist practices have; current program models consist of a few major types, with some novel models coming to the fore.
Another model is use of an NP/PA in an observation unit or with lower acuity observation patients. The majority of the management of the patients is completed and billed by the APP, with the physician available for backup. This hits the “sweet spot,” utilizing the right provider with the right skill set for the right patient. The program has to account for some reimbursement or compensation for the physician oversight time, but it is a very efficient use of APPs.
The third major deployment of APPs is with admissions. Many groups use APPs to admit into the late afternoon and evening, getting patients “tucked in,” including starting diagnostic work-ups and treatment plans. The physician hospitalist then evaluates the patient the next day and often bills for the admission. This model works in situations where the patient work-up is dependent on lab testing, imaging, or other diagnostic testing to understand and plan for the “arc” of the hospitalization; or in situations where the diagnosis is clear, but the patient needs time with treatment to determine response. The downside of this model is long-term job satisfaction for the APP (although some programs have them rotate through such a model at intervals).
Another area where APPs have made strong inroads is that of comanagement services. The NP or PA develops a long-term relationship with a surgical comanagement team, and is often highly engaged and extremely appreciated for managing chronic conditions such as hypertension and diabetes. This can be a very satisfying model for both teams. The NP/PA usually bills independently for these encounters.
APPS are also used in cross coverage and triage roles, allowing the day teams to focus on their primary patients. In a triage role, they can interface with the emergency department, providing a semi-neutral “mediator” for patient disposition.
On the more novel end of the spectrum, there is growth in more independent roles for APP hospitalists. Some groups are having success at using the paired rounding or dyad model, but having the physician see the patient every third day. This is most successful where there is strong onboarding and deep clarity for when to contact the backup physician. There are some data to support the effectiveness of this model, most recently in the Journal of Clinical Outcomes Management.3
Critical access hospitals are also having success in deploying APPs in a very independent role, staffing these hospitals at night. Smaller, rural hospitals with aging medical staff have learned to maximize the scope of practice of their APPs to remain viable and provide care for inpatients. This can be a very successful model for APPs working at the maximum scope of their practice. In addition, the use of telemedicine has been implemented to allow for remote physician backup. This may be a rapidly growing arm to hospital medicine practices in the future.
Ongoing barriers
There are many barriers to maximizing the scope of practice and efficiency of APPs in hospital medicine. They range from the “macro” to the “micro.”
On the larger stage, Medicare requires that home care orders be signed by an attending physician, which can be inefficient and difficult to accomplish. Other payers may have somewhat arcane statutes that limit billing practices, and state practice limitations vary widely. Although 22 states now allow for independent practice for NPs, other states may have a very restrictive practice environment that can impede creative care delivery models. But regardless of how liberal a practice the state allows, a hospital’s medical bylaws can still restrict the day-to-day practice of APPs. And those restrictive bylaws are emblematic of a more constant and corporeal barrier to APP practice, that of medical staff culture.
If there are physicians on the staff who fear that utilization of NP/PA providers will lead to a decay in the quality of care, or who feel threatened by the use of APPs, that can create a local stopgap to maximizing utilization of APPs. In addition, hospitalist physicians and leaders may lack knowledge or experience in APP practice. APPs take more time to successfully onboard than physicians; without clear expectations or road maps to accomplish this onboarding, leaders may feel that APP integration doesn’t work. And one bad experience can create long-term barriers for future practices.
Other barriers are the lack of standardized rigor and vigor in graduate education programs (in both educational and clinical experiences). This results in variation in the quality of NP/PA providers at graduation. Knowledge gaps may be perceived as incompetence, rather than just a lack of experience. There is a certificate for added qualification in hospital medicine for PA providers (which includes a specialty exam), and there is an acute care focus for NPs in training; however, there is no standardized licensure to ensure hospital medicine competency, creating a quagmire for hospitalist leaders who desire demonstrable competence of these providers.
Another barrier for some programs is financial; physicians may not want to give up their RVUs to an NP/PA provider. This can really inhibit a more independent role for the APP. It is important that financial incentives align with all members of the practice working at maximum scope.
Summary and future
In summary, the role of PA/NP in hospital medicine has continued to grow and evolve, to meet the needs of the industry. This includes an increase in the scope and independence of APPs, including the use of telehealth for required oversight. As a specialty, it is imperative that we continue to research APP model effectiveness, embrace innovative delivery models, and support effective onboarding and career development opportunities for our NP/PA providers.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Ms. Cardin is vice president, Advanced Practice Providers, at Sound Physicians, and is a member of SHM’s Board of Directors.
References
1. “Midlevels make a rocky entrance into hospital medicine,” by Bonnie Darves, Today’s Hospitalist, January 2007.
2. “When hiring midlevels, proceed with caution,” by Jessica Berthold, ACP Hospitalist, April 2009.
3. “A Comparison of Conventional and Expanded Physician Assistant Hospitalist Staffing Models at a Community Hospital,” J Clin Outcomes Manag. 2016 Oct 1;23[10]:455-61.
Background and growth
Hospitalist nurse practitioner (NP) and physician assistant (PA) providers have been a growing and evolving part of the inpatient medical workforce, seemingly since the inception of hospital medicine. Given the growth of these disciplines within hospital medicine, at this juncture it is helpful to look at this journey, to see what roles these providers have been serving, and to consider newer and novel trends in how NPs and PAs are being weaved into hospital medicine programs.
The drivers for growth in this provider population are not unlike those of physician hospitalists. The same milieu that provided inroads for physicians in hospital-based care have led the way for increased use of NP/PA providers. An aging physician workforce, residency work hour reforms, increasing complexity of patients and systems on the inpatient side, and the recognition that caring for inpatients is a specialty vastly different from the role of internist in primary care have all impacted the numbers of NPs and PAs in this arena.
• 2007 Today’s Hospitalist article: “Midlevels make a rocky entrance into hospital medicine”1
• 2009 ACP Hospitalist article: “When hiring midlevels, proceed with caution”2
These titles reflect the uncertainty at the time in how best to utilize NP/PA providers in hospital medicine (as well as an unfashionable vocabulary). The numbers at the time tell a similar story. In the Society of Hospital Medicine survey in 2007-2008, about 29% and 21% of hospital medicine practices utilized NPs and PAs, respectively. However, by 2014 about 50% of Veterans Affairs inpatient medical services deployed NP/PA providers, and most recent data from the Society of Hospital Medicine reveal that about 63% of groups use these advanced practice providers (APPs), with higher numbers in pediatric programs. Clearly there is evolving growth and enthusiasm for NP/PAs in hospital medicine.
Program models
Determining how best to use NP/PAs in hospital medicine programs has had a similar evolution. Reviewing past articles addressing these issues, one can see that there has been clear migration; initially NP/PAs were primarily hired to assist with late-afternoon admission surges, with about 60% of the APP workload being utilized to admit in 2007. Their role has continued to grow and change, much as hospitalist practices have; current program models consist of a few major types, with some novel models coming to the fore.
Another model is use of an NP/PA in an observation unit or with lower acuity observation patients. The majority of the management of the patients is completed and billed by the APP, with the physician available for backup. This hits the “sweet spot,” utilizing the right provider with the right skill set for the right patient. The program has to account for some reimbursement or compensation for the physician oversight time, but it is a very efficient use of APPs.
The third major deployment of APPs is with admissions. Many groups use APPs to admit into the late afternoon and evening, getting patients “tucked in,” including starting diagnostic work-ups and treatment plans. The physician hospitalist then evaluates the patient the next day and often bills for the admission. This model works in situations where the patient work-up is dependent on lab testing, imaging, or other diagnostic testing to understand and plan for the “arc” of the hospitalization; or in situations where the diagnosis is clear, but the patient needs time with treatment to determine response. The downside of this model is long-term job satisfaction for the APP (although some programs have them rotate through such a model at intervals).
Another area where APPs have made strong inroads is that of comanagement services. The NP or PA develops a long-term relationship with a surgical comanagement team, and is often highly engaged and extremely appreciated for managing chronic conditions such as hypertension and diabetes. This can be a very satisfying model for both teams. The NP/PA usually bills independently for these encounters.
APPS are also used in cross coverage and triage roles, allowing the day teams to focus on their primary patients. In a triage role, they can interface with the emergency department, providing a semi-neutral “mediator” for patient disposition.
On the more novel end of the spectrum, there is growth in more independent roles for APP hospitalists. Some groups are having success at using the paired rounding or dyad model, but having the physician see the patient every third day. This is most successful where there is strong onboarding and deep clarity for when to contact the backup physician. There are some data to support the effectiveness of this model, most recently in the Journal of Clinical Outcomes Management.3
Critical access hospitals are also having success in deploying APPs in a very independent role, staffing these hospitals at night. Smaller, rural hospitals with aging medical staff have learned to maximize the scope of practice of their APPs to remain viable and provide care for inpatients. This can be a very successful model for APPs working at the maximum scope of their practice. In addition, the use of telemedicine has been implemented to allow for remote physician backup. This may be a rapidly growing arm to hospital medicine practices in the future.
Ongoing barriers
There are many barriers to maximizing the scope of practice and efficiency of APPs in hospital medicine. They range from the “macro” to the “micro.”
On the larger stage, Medicare requires that home care orders be signed by an attending physician, which can be inefficient and difficult to accomplish. Other payers may have somewhat arcane statutes that limit billing practices, and state practice limitations vary widely. Although 22 states now allow for independent practice for NPs, other states may have a very restrictive practice environment that can impede creative care delivery models. But regardless of how liberal a practice the state allows, a hospital’s medical bylaws can still restrict the day-to-day practice of APPs. And those restrictive bylaws are emblematic of a more constant and corporeal barrier to APP practice, that of medical staff culture.
If there are physicians on the staff who fear that utilization of NP/PA providers will lead to a decay in the quality of care, or who feel threatened by the use of APPs, that can create a local stopgap to maximizing utilization of APPs. In addition, hospitalist physicians and leaders may lack knowledge or experience in APP practice. APPs take more time to successfully onboard than physicians; without clear expectations or road maps to accomplish this onboarding, leaders may feel that APP integration doesn’t work. And one bad experience can create long-term barriers for future practices.
Other barriers are the lack of standardized rigor and vigor in graduate education programs (in both educational and clinical experiences). This results in variation in the quality of NP/PA providers at graduation. Knowledge gaps may be perceived as incompetence, rather than just a lack of experience. There is a certificate for added qualification in hospital medicine for PA providers (which includes a specialty exam), and there is an acute care focus for NPs in training; however, there is no standardized licensure to ensure hospital medicine competency, creating a quagmire for hospitalist leaders who desire demonstrable competence of these providers.
Another barrier for some programs is financial; physicians may not want to give up their RVUs to an NP/PA provider. This can really inhibit a more independent role for the APP. It is important that financial incentives align with all members of the practice working at maximum scope.
Summary and future
In summary, the role of PA/NP in hospital medicine has continued to grow and evolve, to meet the needs of the industry. This includes an increase in the scope and independence of APPs, including the use of telehealth for required oversight. As a specialty, it is imperative that we continue to research APP model effectiveness, embrace innovative delivery models, and support effective onboarding and career development opportunities for our NP/PA providers.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Ms. Cardin is vice president, Advanced Practice Providers, at Sound Physicians, and is a member of SHM’s Board of Directors.
References
1. “Midlevels make a rocky entrance into hospital medicine,” by Bonnie Darves, Today’s Hospitalist, January 2007.
2. “When hiring midlevels, proceed with caution,” by Jessica Berthold, ACP Hospitalist, April 2009.
3. “A Comparison of Conventional and Expanded Physician Assistant Hospitalist Staffing Models at a Community Hospital,” J Clin Outcomes Manag. 2016 Oct 1;23[10]:455-61.
Hospitalists’ scope of services continues to evolve
Over the course of serial iterations of the State of Hospital Medicine (SOHM) Report, SHM has presented survey data that describe the evolving role hospitalists play in patient care. The 2016 SOHM Report shows the continuation of prior trends in hospital medicine groups’ (HMGs) scope of admittance and comanagement services. Some downturns are notable among previously increased specialty services.
The SOHM Report characterizes HMGs by their general scope of admitted patients – as admitters of purely traditional internal medicine or pediatrics hospitalized patients; full-range, nearly universal admitters who admit most patients within their age designation except OB and emergency surgery patients; or traditional admitters with some exceptions (for example, limited classically surgical patients).
As adult and adult-ped HMGs make up almost 97% of survey respondents, the predominance of the “some exceptions” category seems to represent a serious trend in much of Hospital Medicine practice. This could mean that HMGs have worked out more specific arrangements as to which patients they will admit or that the definitions are more in flux. It comes at a time when concerns figure prominently in national discussions over the stretching of hospitalists by their expanding scope of care and the need for ever more coordinated care between hospitalists and specialists.
Again, with these opportunities, concerns have arisen about scope-creep and its potential deleterious effects on patient care. Hospitalists have been noted to be prodded into providing critical, geriatric, and palliative care, without specialty training in these areas.1 Interestingly, however, specialty work reported by HMGs has largely shown a downturn since 2014, when most specialty services had appeared to be on the rise.
Whether this means that there is relief from scope-creep or that it is “just a blip” will remain to be seen in future data. If HMGs are able to capture the opportunity to improve outcomes through greater involvement in postacute care, this particular area may be one to watch, despite its apparent downturn since the 2014 report.
Thus, it is as imperative as ever that HMGs participate in the State of Hospital Medicine survey.
Dr. Creamer is a member of SHM’s Practice Analysis Committee. He is a hospitalist and informaticist with the MetroHealth System in Cleveland.
References
1. Wellikson, L. Hospitalists Stretched as their Responsibilities Broaden. The Hospitalist. 2016 Nov;2016(11).
Over the course of serial iterations of the State of Hospital Medicine (SOHM) Report, SHM has presented survey data that describe the evolving role hospitalists play in patient care. The 2016 SOHM Report shows the continuation of prior trends in hospital medicine groups’ (HMGs) scope of admittance and comanagement services. Some downturns are notable among previously increased specialty services.
The SOHM Report characterizes HMGs by their general scope of admitted patients – as admitters of purely traditional internal medicine or pediatrics hospitalized patients; full-range, nearly universal admitters who admit most patients within their age designation except OB and emergency surgery patients; or traditional admitters with some exceptions (for example, limited classically surgical patients).
As adult and adult-ped HMGs make up almost 97% of survey respondents, the predominance of the “some exceptions” category seems to represent a serious trend in much of Hospital Medicine practice. This could mean that HMGs have worked out more specific arrangements as to which patients they will admit or that the definitions are more in flux. It comes at a time when concerns figure prominently in national discussions over the stretching of hospitalists by their expanding scope of care and the need for ever more coordinated care between hospitalists and specialists.

Again, with these opportunities, concerns have arisen about scope-creep and its potential deleterious effects on patient care. Hospitalists have been noted to be prodded into providing critical, geriatric, and palliative care, without specialty training in these areas.1 Interestingly, however, specialty work reported by HMGs has largely shown a downturn since 2014, when most specialty services had appeared to be on the rise.

Whether this means that there is relief from scope-creep or that it is “just a blip” will remain to be seen in future data. If HMGs are able to capture the opportunity to improve outcomes through greater involvement in postacute care, this particular area may be one to watch, despite its apparent downturn since the 2014 report.
Thus, it is as imperative as ever that HMGs participate in the State of Hospital Medicine survey.
Dr. Creamer is a member of SHM’s Practice Analysis Committee. He is a hospitalist and informaticist with the MetroHealth System in Cleveland.
References
1. Wellikson, L. Hospitalists Stretched as their Responsibilities Broaden. The Hospitalist. 2016 Nov;2016(11).
Over the course of serial iterations of the State of Hospital Medicine (SOHM) Report, SHM has presented survey data that describe the evolving role hospitalists play in patient care. The 2016 SOHM Report shows the continuation of prior trends in hospital medicine groups’ (HMGs) scope of admittance and comanagement services. Some downturns are notable among previously increased specialty services.
The SOHM Report characterizes HMGs by their general scope of admitted patients – as admitters of purely traditional internal medicine or pediatrics hospitalized patients; full-range, nearly universal admitters who admit most patients within their age designation except OB and emergency surgery patients; or traditional admitters with some exceptions (for example, limited classically surgical patients).
As adult and adult-ped HMGs make up almost 97% of survey respondents, the predominance of the “some exceptions” category seems to represent a serious trend in much of Hospital Medicine practice. This could mean that HMGs have worked out more specific arrangements as to which patients they will admit or that the definitions are more in flux. It comes at a time when concerns figure prominently in national discussions over the stretching of hospitalists by their expanding scope of care and the need for ever more coordinated care between hospitalists and specialists.

Again, with these opportunities, concerns have arisen about scope-creep and its potential deleterious effects on patient care. Hospitalists have been noted to be prodded into providing critical, geriatric, and palliative care, without specialty training in these areas.1 Interestingly, however, specialty work reported by HMGs has largely shown a downturn since 2014, when most specialty services had appeared to be on the rise.

Whether this means that there is relief from scope-creep or that it is “just a blip” will remain to be seen in future data. If HMGs are able to capture the opportunity to improve outcomes through greater involvement in postacute care, this particular area may be one to watch, despite its apparent downturn since the 2014 report.
Thus, it is as imperative as ever that HMGs participate in the State of Hospital Medicine survey.
Dr. Creamer is a member of SHM’s Practice Analysis Committee. He is a hospitalist and informaticist with the MetroHealth System in Cleveland.
References
1. Wellikson, L. Hospitalists Stretched as their Responsibilities Broaden. The Hospitalist. 2016 Nov;2016(11).
HM17 session summary: Hospitalists as leaders in patient flow and hospital throughput
Presenters
Gaby Berger, MD; Aaron Hamilton, MD, FHM; Christopher Kim, MD, SFHM; Eduardo Margo, MD; Vikas Parekh, MD, FACP, SFHM; Anneliese Schleyer, MD, SFHM; Emily Wang, MD
Session Summary
This HM17 workshop brought together academic and community hospitalists to share effective strategies for improving hospital patient flow.
This was followed by a break-out session, in which hospitalists were encouraged to further explore these and other strategies for improving patient flow.
Key takeaways for HM
- Expedited discharge: Identify patients who can be safely discharged before noon. Consider creating standard work to ensure that key steps in discharge planning process, such as completion of medication reconciliation and discharge instructions and communication with patient and families and the interdisciplinary team, occur the day prior to discharge.
- Length of stay reduction strategies: Partner with utilization management to identify and develop a strategy to actively manage patients with long length of stay. Several institutions have set up committees to review such cases and address barriers, escalating requests for resources to executive leadership as needed.
- Facilitate transfers: Develop a standard process that is streamlined and patient-centered and includes established criteria for deciding whether interhospital transfers are appropriate.
- Short Stay Units: Some hospitals have had success with hospitalist-run short stay units as a strategy to decrease length of stay in observation patients. This strategy is most ideal for patients with a predictable length of stay. If you are thinking of starting an observation unit at your hospital, consider establishing criteria and protocols to expedite care.
- Hospitalist Quarterback: Given their broad perspective and clinical knowledge, hospitalists are uniquely positioned to help manage hospital, and even system-wide, capacity in real time. Some hospitals have successfully employed this strategy in some form to improve throughput. However, hospitalists need tools to help them electronically track incoming patients, integration with utilization management resources, and support from executive leadership to be successful.
Dr. Stella is a hospitalist in Denver and an editorial board member of The Hospitalist.
Presenters
Gaby Berger, MD; Aaron Hamilton, MD, FHM; Christopher Kim, MD, SFHM; Eduardo Margo, MD; Vikas Parekh, MD, FACP, SFHM; Anneliese Schleyer, MD, SFHM; Emily Wang, MD
Session Summary
This HM17 workshop brought together academic and community hospitalists to share effective strategies for improving hospital patient flow.
This was followed by a break-out session, in which hospitalists were encouraged to further explore these and other strategies for improving patient flow.
Key takeaways for HM
- Expedited discharge: Identify patients who can be safely discharged before noon. Consider creating standard work to ensure that key steps in discharge planning process, such as completion of medication reconciliation and discharge instructions and communication with patient and families and the interdisciplinary team, occur the day prior to discharge.
- Length of stay reduction strategies: Partner with utilization management to identify and develop a strategy to actively manage patients with long length of stay. Several institutions have set up committees to review such cases and address barriers, escalating requests for resources to executive leadership as needed.
- Facilitate transfers: Develop a standard process that is streamlined and patient-centered and includes established criteria for deciding whether interhospital transfers are appropriate.
- Short Stay Units: Some hospitals have had success with hospitalist-run short stay units as a strategy to decrease length of stay in observation patients. This strategy is most ideal for patients with a predictable length of stay. If you are thinking of starting an observation unit at your hospital, consider establishing criteria and protocols to expedite care.
- Hospitalist Quarterback: Given their broad perspective and clinical knowledge, hospitalists are uniquely positioned to help manage hospital, and even system-wide, capacity in real time. Some hospitals have successfully employed this strategy in some form to improve throughput. However, hospitalists need tools to help them electronically track incoming patients, integration with utilization management resources, and support from executive leadership to be successful.
Dr. Stella is a hospitalist in Denver and an editorial board member of The Hospitalist.
Presenters
Gaby Berger, MD; Aaron Hamilton, MD, FHM; Christopher Kim, MD, SFHM; Eduardo Margo, MD; Vikas Parekh, MD, FACP, SFHM; Anneliese Schleyer, MD, SFHM; Emily Wang, MD
Session Summary
This HM17 workshop brought together academic and community hospitalists to share effective strategies for improving hospital patient flow.
This was followed by a break-out session, in which hospitalists were encouraged to further explore these and other strategies for improving patient flow.
Key takeaways for HM
- Expedited discharge: Identify patients who can be safely discharged before noon. Consider creating standard work to ensure that key steps in discharge planning process, such as completion of medication reconciliation and discharge instructions and communication with patient and families and the interdisciplinary team, occur the day prior to discharge.
- Length of stay reduction strategies: Partner with utilization management to identify and develop a strategy to actively manage patients with long length of stay. Several institutions have set up committees to review such cases and address barriers, escalating requests for resources to executive leadership as needed.
- Facilitate transfers: Develop a standard process that is streamlined and patient-centered and includes established criteria for deciding whether interhospital transfers are appropriate.
- Short Stay Units: Some hospitals have had success with hospitalist-run short stay units as a strategy to decrease length of stay in observation patients. This strategy is most ideal for patients with a predictable length of stay. If you are thinking of starting an observation unit at your hospital, consider establishing criteria and protocols to expedite care.
- Hospitalist Quarterback: Given their broad perspective and clinical knowledge, hospitalists are uniquely positioned to help manage hospital, and even system-wide, capacity in real time. Some hospitals have successfully employed this strategy in some form to improve throughput. However, hospitalists need tools to help them electronically track incoming patients, integration with utilization management resources, and support from executive leadership to be successful.
Dr. Stella is a hospitalist in Denver and an editorial board member of The Hospitalist.
Hospital isolates C. difficile carriers and rates drop
NEW ORLEANS – A Montreal hospital grappling with high Clostridium difficile infections rates launched an intervention in October 2013 to screen patients at admission and detect asymptomatic carriers, and investigators found 4.8% of 7,599 people admitted through the ED over 15 months were carriers of C. difficile.
To protect Jewish General Hospital physicians, staff and other patients from potential transmission, these patients were placed in isolation. However, because they were fairly numerous – 1 in 20 admissions – and because infectious disease (ID) experts feared a substantial backlash, these patients were put in less restrictive isolation. They were permitted to share rooms as long as the dividing curtains remained drawn, for example. In addition, clinicians could skip wearing traditional isolation hats and gowns.
The ID team at the hospital considered the intervention a success. “It is estimated we prevented 64 cases over 15 months,” Dr. Longtin said during a packed session at the annual meeting of the American Society for Microbiology.
The hospital’s C. difficile rate dropped from 6.9 per 10,000 patient-days before the screening and isolation protocol to 3.0 per 10,000 during the intervention. The difference was statically significant (P less than .001).
“Compared to other hospitals in the province, we used to be in the middle of the pack [for C. difficile infection rates], and now we are the lowest,” Dr. Longtin said.
Asymptomatic carriers were detected using rectal sampling with sterile swab and polymerase chain reaction analysis. Testing was performed 7 days a week and analyzed once daily, with results generated within 24 hours and documented in the patient chart. Only patients admitted through the ED were screened, which prompted some questions from colleagues, Dr. Longtin said. However, he defends this approach because the 30% or so of patients admitted from the ED tend to spend more days on the ward. The risk of becoming colonized increases steadily with duration of hospitalization. This occurs despite isolating patients with C. difficile infection. Initial results of the study were published in JAMA Internal Medicine (2016 Jun 1;176[6]:796-804).
Risk to health care workers
C. difficile carriers are contagious, but not as much as people with C. difficile infection, Dr. Longtin said. In one study, the microorganism was present on the skin of 61% of symptomatic carriers versus 78% of those infected (Clin Infect Dis. 2007 Oct 15;45[8]:992-8). In addition, C. difficile present on patient skin can be transferred to health care worker hands, even up to 6 weeks after resolution of associated diarrhea (Infect Control Hosp Epidemiol. 2016 Apr;37[4]:475-7).
Prior to the intervention, C. difficile prevention at Jewish General involved guidelines that “have not really changed in the last 20 years,” Dr. Longtin said. Contact precautions around infected patients, hand hygiene, environmental cleaning, and antibiotic stewardship were the main strategies.
“Despite all these measures, we were not completely blocking dissemination of C difficile in our hospital,” Dr. Longtin said. He added that soap and water are better than alcohol for C. difficile, “but honestly not very good. Even the best hand hygiene technique is poorly effective to remove C. difficile. On the other hand – get it? – gloves are very effective. We felt we had to combine hand washing with gloves.”
Hand hygiene compliance increased from 37% to 50% during the intervention, and Dr. Longtin expected further improvements over time.
Risk to other patients
“Transmission of C. difficile cannot only be explained by infected patients in a hospital, so likely carriers also play a role,” Dr. Longtin said.
Another set of investigators found that hospital patients exposed to a carrier of C. difficile had nearly twice the risk of acquiring the infection (odds ratio, 1.79) (Gastroenterology. 2017 Apr;152[5]:1031-41.e2).
“For every patient with C. difficile infection, it’s estimated there are 5-7 C. difficile carriers, so they are numerous as well,” he said.
The bigger picture
During the study period, the C. difficile infection trends did not significantly change on the city level, further supporting the effectiveness of the carrier screen-and-isolate strategy.
There was slight increase in antibiotic use during the intervention period, Dr. Longtin said. “The only type of antibiotics that really decreased were vancomycin and metronidazole... which suggests in turn there were fewer cases of C. difficile infection.”
Long-term follow-up is ongoing, Dr. Longtin said. “We have more than 3 years of intervention. In the past year, our rate was 2.2 per 10,000 patient-days.”
Unanswered questions include the generalizability of the results “because we’re a very pro–infection control hospital,” he said. In addition, a formal cost-benefit analysis of this strategy would be worthwhile in the future.
Dr. Longtin is a consultant for AMG Medical and receives research support from Merck and BD Medical.
NEW ORLEANS – A Montreal hospital grappling with high Clostridium difficile infections rates launched an intervention in October 2013 to screen patients at admission and detect asymptomatic carriers, and investigators found 4.8% of 7,599 people admitted through the ED over 15 months were carriers of C. difficile.
To protect Jewish General Hospital physicians, staff and other patients from potential transmission, these patients were placed in isolation. However, because they were fairly numerous – 1 in 20 admissions – and because infectious disease (ID) experts feared a substantial backlash, these patients were put in less restrictive isolation. They were permitted to share rooms as long as the dividing curtains remained drawn, for example. In addition, clinicians could skip wearing traditional isolation hats and gowns.
The ID team at the hospital considered the intervention a success. “It is estimated we prevented 64 cases over 15 months,” Dr. Longtin said during a packed session at the annual meeting of the American Society for Microbiology.
The hospital’s C. difficile rate dropped from 6.9 per 10,000 patient-days before the screening and isolation protocol to 3.0 per 10,000 during the intervention. The difference was statically significant (P less than .001).
“Compared to other hospitals in the province, we used to be in the middle of the pack [for C. difficile infection rates], and now we are the lowest,” Dr. Longtin said.
Asymptomatic carriers were detected using rectal sampling with sterile swab and polymerase chain reaction analysis. Testing was performed 7 days a week and analyzed once daily, with results generated within 24 hours and documented in the patient chart. Only patients admitted through the ED were screened, which prompted some questions from colleagues, Dr. Longtin said. However, he defends this approach because the 30% or so of patients admitted from the ED tend to spend more days on the ward. The risk of becoming colonized increases steadily with duration of hospitalization. This occurs despite isolating patients with C. difficile infection. Initial results of the study were published in JAMA Internal Medicine (2016 Jun 1;176[6]:796-804).
Risk to health care workers
C. difficile carriers are contagious, but not as much as people with C. difficile infection, Dr. Longtin said. In one study, the microorganism was present on the skin of 61% of symptomatic carriers versus 78% of those infected (Clin Infect Dis. 2007 Oct 15;45[8]:992-8). In addition, C. difficile present on patient skin can be transferred to health care worker hands, even up to 6 weeks after resolution of associated diarrhea (Infect Control Hosp Epidemiol. 2016 Apr;37[4]:475-7).
Prior to the intervention, C. difficile prevention at Jewish General involved guidelines that “have not really changed in the last 20 years,” Dr. Longtin said. Contact precautions around infected patients, hand hygiene, environmental cleaning, and antibiotic stewardship were the main strategies.
“Despite all these measures, we were not completely blocking dissemination of C difficile in our hospital,” Dr. Longtin said. He added that soap and water are better than alcohol for C. difficile, “but honestly not very good. Even the best hand hygiene technique is poorly effective to remove C. difficile. On the other hand – get it? – gloves are very effective. We felt we had to combine hand washing with gloves.”
Hand hygiene compliance increased from 37% to 50% during the intervention, and Dr. Longtin expected further improvements over time.
Risk to other patients
“Transmission of C. difficile cannot only be explained by infected patients in a hospital, so likely carriers also play a role,” Dr. Longtin said.
Another set of investigators found that hospital patients exposed to a carrier of C. difficile had nearly twice the risk of acquiring the infection (odds ratio, 1.79) (Gastroenterology. 2017 Apr;152[5]:1031-41.e2).
“For every patient with C. difficile infection, it’s estimated there are 5-7 C. difficile carriers, so they are numerous as well,” he said.
The bigger picture
During the study period, the C. difficile infection trends did not significantly change on the city level, further supporting the effectiveness of the carrier screen-and-isolate strategy.
There was slight increase in antibiotic use during the intervention period, Dr. Longtin said. “The only type of antibiotics that really decreased were vancomycin and metronidazole... which suggests in turn there were fewer cases of C. difficile infection.”
Long-term follow-up is ongoing, Dr. Longtin said. “We have more than 3 years of intervention. In the past year, our rate was 2.2 per 10,000 patient-days.”
Unanswered questions include the generalizability of the results “because we’re a very pro–infection control hospital,” he said. In addition, a formal cost-benefit analysis of this strategy would be worthwhile in the future.
Dr. Longtin is a consultant for AMG Medical and receives research support from Merck and BD Medical.
NEW ORLEANS – A Montreal hospital grappling with high Clostridium difficile infections rates launched an intervention in October 2013 to screen patients at admission and detect asymptomatic carriers, and investigators found 4.8% of 7,599 people admitted through the ED over 15 months were carriers of C. difficile.
To protect Jewish General Hospital physicians, staff and other patients from potential transmission, these patients were placed in isolation. However, because they were fairly numerous – 1 in 20 admissions – and because infectious disease (ID) experts feared a substantial backlash, these patients were put in less restrictive isolation. They were permitted to share rooms as long as the dividing curtains remained drawn, for example. In addition, clinicians could skip wearing traditional isolation hats and gowns.
The ID team at the hospital considered the intervention a success. “It is estimated we prevented 64 cases over 15 months,” Dr. Longtin said during a packed session at the annual meeting of the American Society for Microbiology.
The hospital’s C. difficile rate dropped from 6.9 per 10,000 patient-days before the screening and isolation protocol to 3.0 per 10,000 during the intervention. The difference was statically significant (P less than .001).
“Compared to other hospitals in the province, we used to be in the middle of the pack [for C. difficile infection rates], and now we are the lowest,” Dr. Longtin said.
Asymptomatic carriers were detected using rectal sampling with sterile swab and polymerase chain reaction analysis. Testing was performed 7 days a week and analyzed once daily, with results generated within 24 hours and documented in the patient chart. Only patients admitted through the ED were screened, which prompted some questions from colleagues, Dr. Longtin said. However, he defends this approach because the 30% or so of patients admitted from the ED tend to spend more days on the ward. The risk of becoming colonized increases steadily with duration of hospitalization. This occurs despite isolating patients with C. difficile infection. Initial results of the study were published in JAMA Internal Medicine (2016 Jun 1;176[6]:796-804).
Risk to health care workers
C. difficile carriers are contagious, but not as much as people with C. difficile infection, Dr. Longtin said. In one study, the microorganism was present on the skin of 61% of symptomatic carriers versus 78% of those infected (Clin Infect Dis. 2007 Oct 15;45[8]:992-8). In addition, C. difficile present on patient skin can be transferred to health care worker hands, even up to 6 weeks after resolution of associated diarrhea (Infect Control Hosp Epidemiol. 2016 Apr;37[4]:475-7).
Prior to the intervention, C. difficile prevention at Jewish General involved guidelines that “have not really changed in the last 20 years,” Dr. Longtin said. Contact precautions around infected patients, hand hygiene, environmental cleaning, and antibiotic stewardship were the main strategies.
“Despite all these measures, we were not completely blocking dissemination of C difficile in our hospital,” Dr. Longtin said. He added that soap and water are better than alcohol for C. difficile, “but honestly not very good. Even the best hand hygiene technique is poorly effective to remove C. difficile. On the other hand – get it? – gloves are very effective. We felt we had to combine hand washing with gloves.”
Hand hygiene compliance increased from 37% to 50% during the intervention, and Dr. Longtin expected further improvements over time.
Risk to other patients
“Transmission of C. difficile cannot only be explained by infected patients in a hospital, so likely carriers also play a role,” Dr. Longtin said.
Another set of investigators found that hospital patients exposed to a carrier of C. difficile had nearly twice the risk of acquiring the infection (odds ratio, 1.79) (Gastroenterology. 2017 Apr;152[5]:1031-41.e2).
“For every patient with C. difficile infection, it’s estimated there are 5-7 C. difficile carriers, so they are numerous as well,” he said.
The bigger picture
During the study period, the C. difficile infection trends did not significantly change on the city level, further supporting the effectiveness of the carrier screen-and-isolate strategy.
There was slight increase in antibiotic use during the intervention period, Dr. Longtin said. “The only type of antibiotics that really decreased were vancomycin and metronidazole... which suggests in turn there were fewer cases of C. difficile infection.”
Long-term follow-up is ongoing, Dr. Longtin said. “We have more than 3 years of intervention. In the past year, our rate was 2.2 per 10,000 patient-days.”
Unanswered questions include the generalizability of the results “because we’re a very pro–infection control hospital,” he said. In addition, a formal cost-benefit analysis of this strategy would be worthwhile in the future.
Dr. Longtin is a consultant for AMG Medical and receives research support from Merck and BD Medical.
AT ASM MICROBE 2017
Key clinical point: Identification and isolation of asymptomatic carriers of Clostridium difficile decreased a hospital’s infection rates over time.
Major finding: (P less than .001).
Data source: A study of 7,599 people screened at admission through the ED at an acute care hospital.
Disclosures: Dr. Longtin is a consultant for AMG Medical and receives research support from Merck and BD Medical.
Idarucizumab reversed dabigatran completely and rapidly in study
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
FROM 2017 ISTH CONGRESS
Key clinical point:
Major finding: Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group, 197 patients were able to undergo urgent procedures after a median of 1.6 hours.
Data source: A multicenter, prospective, open-label study of 503 patients (RE-VERSE AD).
Disclosures: Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, BMS/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
Ceftaroline shortens duration of MRSA bacteremia
NEW ORLEANS – Ceftaroline fosamil reduced the median duration of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia by 2 days in Veterans Administration patients, a retrospective study showed.
Investigators identified 219 patients with MRSA within the Veterans Affairs (VA) medical system nationwide from 2011 to 2015. All patients received at least 48 hours of ceftaroline fosamil (Teflaro) therapy to treat MRSA bacteremia. “We know it has good activity against MRSA in vitro. We use it in bacteremia, but we don’t have a lot of clinical data to support or refute its use,” said Nicholas S. Britt, PharmD, a PGY2 infectious diseases resident at Barnes-Jewish Hospital in St. Louis.
“Ceftaroline was primarily used as second-line or salvage therapy … which is basically what we expected, based on how it’s used in clinical practice,” Dr. Britt said.
Treatment failures
A total of 88 of the 219 (40%) patients experienced treatment failure. This rate “seems kind of high, but, if you look at some of the other MRSA agents for bacteremia (vancomycin, for example), it usually has a treatment failure rate around 60%,” Dr. Britt said. “The outcomes were not as poor as I would expect with [patients] using it for second- and third-line therapy.”
Hospital-acquired infection (odds ratio, 2.11; P = .013), ICU admission (OR, 3.95; P less than .001) and infective endocarditis (OR, 4.77; P = .002) were significantly associated with treatment failure in a univariate analysis. “Admissions to the ICU and endocarditis were the big ones, factors you would associate with failure for most antibiotics,” Dr. Britt said. In a multivariate analysis, only ICU admission remained significantly associated with treatment failure (adjusted OR, 2.24; P = .028).
The investigators also looked at treatment failure with ceftaroline monotherapy, compared with its use in combination. There is in vitro data showing synergy when you add ceftaroline to daptomycin, vancomycin, or some of these other agents,” Dr. Britt said. However, he added, “We didn’t find any significant difference in outcomes when you added another agent.” Treatment failure with monotherapy was 35%, versus 46%, with combination treatment (P = .107).
“This could be because the sicker patients are the ones getting combination therapy.”
No observed differences by dosing
Dr. Britt and his colleagues also looked for any differences by dosing interval, “which hasn’t been evaluated extensively.”
The Food and Drug Administration labeled it for use every 12 hours, but treatment of MRSA bacteremia is an off-label use, Dr. Britt explained. Dosing every 8 hours instead improves the achievement of pharmacokinetic and pharmacodynamic parameters in in vitro studies. “Clinically, we’re almost always using it q8. They’re sick patients, so you don’t want to under-dose them. And ceftaroline is pretty well tolerated overall.”
“But, we didn’t really see any difference between the q8 and the q12” in terms of treatment failure. The rates were 36% and 42%, respectively, and not significantly different (P = .440). “Granted, patients who are sicker are probably going to get treated more aggressively,” Dr. Britt added.
The current research only focused on outcomes associated with ceftaroline. Going forward, Dr. Britt said, “We’re hoping to use this data to compare ceftaroline to other agents as well, probably as second-line therapy, since that’s how it’s used most often.”
Dr. Britt had no relevant financial disclosures.
NEW ORLEANS – Ceftaroline fosamil reduced the median duration of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia by 2 days in Veterans Administration patients, a retrospective study showed.
Investigators identified 219 patients with MRSA within the Veterans Affairs (VA) medical system nationwide from 2011 to 2015. All patients received at least 48 hours of ceftaroline fosamil (Teflaro) therapy to treat MRSA bacteremia. “We know it has good activity against MRSA in vitro. We use it in bacteremia, but we don’t have a lot of clinical data to support or refute its use,” said Nicholas S. Britt, PharmD, a PGY2 infectious diseases resident at Barnes-Jewish Hospital in St. Louis.
“Ceftaroline was primarily used as second-line or salvage therapy … which is basically what we expected, based on how it’s used in clinical practice,” Dr. Britt said.
Treatment failures
A total of 88 of the 219 (40%) patients experienced treatment failure. This rate “seems kind of high, but, if you look at some of the other MRSA agents for bacteremia (vancomycin, for example), it usually has a treatment failure rate around 60%,” Dr. Britt said. “The outcomes were not as poor as I would expect with [patients] using it for second- and third-line therapy.”
Hospital-acquired infection (odds ratio, 2.11; P = .013), ICU admission (OR, 3.95; P less than .001) and infective endocarditis (OR, 4.77; P = .002) were significantly associated with treatment failure in a univariate analysis. “Admissions to the ICU and endocarditis were the big ones, factors you would associate with failure for most antibiotics,” Dr. Britt said. In a multivariate analysis, only ICU admission remained significantly associated with treatment failure (adjusted OR, 2.24; P = .028).
The investigators also looked at treatment failure with ceftaroline monotherapy, compared with its use in combination. There is in vitro data showing synergy when you add ceftaroline to daptomycin, vancomycin, or some of these other agents,” Dr. Britt said. However, he added, “We didn’t find any significant difference in outcomes when you added another agent.” Treatment failure with monotherapy was 35%, versus 46%, with combination treatment (P = .107).
“This could be because the sicker patients are the ones getting combination therapy.”
No observed differences by dosing
Dr. Britt and his colleagues also looked for any differences by dosing interval, “which hasn’t been evaluated extensively.”
The Food and Drug Administration labeled it for use every 12 hours, but treatment of MRSA bacteremia is an off-label use, Dr. Britt explained. Dosing every 8 hours instead improves the achievement of pharmacokinetic and pharmacodynamic parameters in in vitro studies. “Clinically, we’re almost always using it q8. They’re sick patients, so you don’t want to under-dose them. And ceftaroline is pretty well tolerated overall.”
“But, we didn’t really see any difference between the q8 and the q12” in terms of treatment failure. The rates were 36% and 42%, respectively, and not significantly different (P = .440). “Granted, patients who are sicker are probably going to get treated more aggressively,” Dr. Britt added.
The current research only focused on outcomes associated with ceftaroline. Going forward, Dr. Britt said, “We’re hoping to use this data to compare ceftaroline to other agents as well, probably as second-line therapy, since that’s how it’s used most often.”
Dr. Britt had no relevant financial disclosures.
NEW ORLEANS – Ceftaroline fosamil reduced the median duration of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia by 2 days in Veterans Administration patients, a retrospective study showed.
Investigators identified 219 patients with MRSA within the Veterans Affairs (VA) medical system nationwide from 2011 to 2015. All patients received at least 48 hours of ceftaroline fosamil (Teflaro) therapy to treat MRSA bacteremia. “We know it has good activity against MRSA in vitro. We use it in bacteremia, but we don’t have a lot of clinical data to support or refute its use,” said Nicholas S. Britt, PharmD, a PGY2 infectious diseases resident at Barnes-Jewish Hospital in St. Louis.
“Ceftaroline was primarily used as second-line or salvage therapy … which is basically what we expected, based on how it’s used in clinical practice,” Dr. Britt said.
Treatment failures
A total of 88 of the 219 (40%) patients experienced treatment failure. This rate “seems kind of high, but, if you look at some of the other MRSA agents for bacteremia (vancomycin, for example), it usually has a treatment failure rate around 60%,” Dr. Britt said. “The outcomes were not as poor as I would expect with [patients] using it for second- and third-line therapy.”
Hospital-acquired infection (odds ratio, 2.11; P = .013), ICU admission (OR, 3.95; P less than .001) and infective endocarditis (OR, 4.77; P = .002) were significantly associated with treatment failure in a univariate analysis. “Admissions to the ICU and endocarditis were the big ones, factors you would associate with failure for most antibiotics,” Dr. Britt said. In a multivariate analysis, only ICU admission remained significantly associated with treatment failure (adjusted OR, 2.24; P = .028).
The investigators also looked at treatment failure with ceftaroline monotherapy, compared with its use in combination. There is in vitro data showing synergy when you add ceftaroline to daptomycin, vancomycin, or some of these other agents,” Dr. Britt said. However, he added, “We didn’t find any significant difference in outcomes when you added another agent.” Treatment failure with monotherapy was 35%, versus 46%, with combination treatment (P = .107).
“This could be because the sicker patients are the ones getting combination therapy.”
No observed differences by dosing
Dr. Britt and his colleagues also looked for any differences by dosing interval, “which hasn’t been evaluated extensively.”
The Food and Drug Administration labeled it for use every 12 hours, but treatment of MRSA bacteremia is an off-label use, Dr. Britt explained. Dosing every 8 hours instead improves the achievement of pharmacokinetic and pharmacodynamic parameters in in vitro studies. “Clinically, we’re almost always using it q8. They’re sick patients, so you don’t want to under-dose them. And ceftaroline is pretty well tolerated overall.”
“But, we didn’t really see any difference between the q8 and the q12” in terms of treatment failure. The rates were 36% and 42%, respectively, and not significantly different (P = .440). “Granted, patients who are sicker are probably going to get treated more aggressively,” Dr. Britt added.
The current research only focused on outcomes associated with ceftaroline. Going forward, Dr. Britt said, “We’re hoping to use this data to compare ceftaroline to other agents as well, probably as second-line therapy, since that’s how it’s used most often.”
Dr. Britt had no relevant financial disclosures.
AT ASM MICROBE 2017
Key clinical point:
Major finding: Median duration of MRSA bacteremia dropped from 2.79 days before to 1.18 days after initiation of ceftaroline (P less than .001).
Data source: A retrospective study of 219 hospitalized VA patients initiating ceftaroline for MRSA bacteremia.
Disclosures: Dr. Britt had no relevant financial disclosures.