User login
In Case You Missed It: COVID
COVID-19 in 2020: Deaths and disparities
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
FROM MMWR
Mishap ruins millions of J&J COVID vaccine doses
About 15 million doses of the Johnson & Johnson COVID-19 vaccine were ruined after workers at a manufacturing plant mixed up ingredients, The New York Times reported.
The Baltimore plant is operated by a company called Emergent BioSolutions, the Times said. The company works with both Johnson & Johnson and AstraZeneca.
The mistake has stopped shipments of the vaccine until the FDA investigates, the paper said. The mishap, however, does not affect doses of the J&J one-shot vaccine already delivered and being used.
The problem is that tens of millions of doses were supposed to come from the Baltimore plant.
The Associated Press reported that Emergent has had numerous problems with the FDA, with the agency citing the company for poorly trained employees, cracked vials and mold.
The records cover inspections at Emergent facilities, including Bayview, since 2017. Following a December 2017 inspection at an Emergent plant in Canton, Massachusetts, the FDA said the company hadn’t corrected “continued low level mold and yeast isolates” found in the facility. Nearly a year later, agency investigators questioned why Emergent had “an unwritten policy of not conducting routine compliance audits” at a separate plant in Baltimore, known as Camden, where an anthrax vaccine is filled into vials.
Meanwhile, in a statement, Johnson & Johnson said its own quality control process identified the problem in one batch of ingredients. The company said the Emergent plant in Baltimore is “not yet authorized to manufacture drug substance for our COVID-19 vaccine. This batch was never advanced to the filling and finishing stages of our manufacturing process.”
The company said it plans to still seek emergency use authorization for a different Emergent facility and will provide more experts on site at Emergent.
The Times reports that President Joe Biden’s team still believes the administration can meet its commitment to have enough vaccine doses to immunize every adult by the end of May.
Johnson & Johnson said it still plans to deliver an additional 24 million doses through April.
A version of this article first appeared on WebMD.com.
This article was updated 4/1/21.
About 15 million doses of the Johnson & Johnson COVID-19 vaccine were ruined after workers at a manufacturing plant mixed up ingredients, The New York Times reported.
The Baltimore plant is operated by a company called Emergent BioSolutions, the Times said. The company works with both Johnson & Johnson and AstraZeneca.
The mistake has stopped shipments of the vaccine until the FDA investigates, the paper said. The mishap, however, does not affect doses of the J&J one-shot vaccine already delivered and being used.
The problem is that tens of millions of doses were supposed to come from the Baltimore plant.
The Associated Press reported that Emergent has had numerous problems with the FDA, with the agency citing the company for poorly trained employees, cracked vials and mold.
The records cover inspections at Emergent facilities, including Bayview, since 2017. Following a December 2017 inspection at an Emergent plant in Canton, Massachusetts, the FDA said the company hadn’t corrected “continued low level mold and yeast isolates” found in the facility. Nearly a year later, agency investigators questioned why Emergent had “an unwritten policy of not conducting routine compliance audits” at a separate plant in Baltimore, known as Camden, where an anthrax vaccine is filled into vials.
Meanwhile, in a statement, Johnson & Johnson said its own quality control process identified the problem in one batch of ingredients. The company said the Emergent plant in Baltimore is “not yet authorized to manufacture drug substance for our COVID-19 vaccine. This batch was never advanced to the filling and finishing stages of our manufacturing process.”
The company said it plans to still seek emergency use authorization for a different Emergent facility and will provide more experts on site at Emergent.
The Times reports that President Joe Biden’s team still believes the administration can meet its commitment to have enough vaccine doses to immunize every adult by the end of May.
Johnson & Johnson said it still plans to deliver an additional 24 million doses through April.
A version of this article first appeared on WebMD.com.
This article was updated 4/1/21.
About 15 million doses of the Johnson & Johnson COVID-19 vaccine were ruined after workers at a manufacturing plant mixed up ingredients, The New York Times reported.
The Baltimore plant is operated by a company called Emergent BioSolutions, the Times said. The company works with both Johnson & Johnson and AstraZeneca.
The mistake has stopped shipments of the vaccine until the FDA investigates, the paper said. The mishap, however, does not affect doses of the J&J one-shot vaccine already delivered and being used.
The problem is that tens of millions of doses were supposed to come from the Baltimore plant.
The Associated Press reported that Emergent has had numerous problems with the FDA, with the agency citing the company for poorly trained employees, cracked vials and mold.
The records cover inspections at Emergent facilities, including Bayview, since 2017. Following a December 2017 inspection at an Emergent plant in Canton, Massachusetts, the FDA said the company hadn’t corrected “continued low level mold and yeast isolates” found in the facility. Nearly a year later, agency investigators questioned why Emergent had “an unwritten policy of not conducting routine compliance audits” at a separate plant in Baltimore, known as Camden, where an anthrax vaccine is filled into vials.
Meanwhile, in a statement, Johnson & Johnson said its own quality control process identified the problem in one batch of ingredients. The company said the Emergent plant in Baltimore is “not yet authorized to manufacture drug substance for our COVID-19 vaccine. This batch was never advanced to the filling and finishing stages of our manufacturing process.”
The company said it plans to still seek emergency use authorization for a different Emergent facility and will provide more experts on site at Emergent.
The Times reports that President Joe Biden’s team still believes the administration can meet its commitment to have enough vaccine doses to immunize every adult by the end of May.
Johnson & Johnson said it still plans to deliver an additional 24 million doses through April.
A version of this article first appeared on WebMD.com.
This article was updated 4/1/21.
Children could become eligible for a COVID-19 vaccine by fall, expert predicts
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
FROM THE SPD PRE-AAD MEETING
Pfizer: Vaccine shown 100% effective in children aged 12-15
The study enrolled 2,260 adolescents aged 12-15. No infections were reported in the group given the vaccine produced by Pfizer and its European partner, BioNTech, the release said. The placebo group reported 18 cases of COVID-19.
The vaccinated children showed a strong antibody response with no serious side effects.
Albert Bourla, PhD, chairman and CEO of Pfizer, said the company plans to seek Food and Drug Administration emergency use authorization, which could allow this age group to be vaccinated before the start of the next school year. Pfizer will also seek authorization from the European Medicines Agency.
“We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” Dr. Bourla said in the release.
The clinical trials showed a stronger response in children aged 12-15 than the 95% effectiveness reported in clinical trials in adults. The Pfizer vaccine is now authorized to be given to people aged 16 and up in the United States.
Health experts said the clinical trials – while not peer-reviewed – amounted to very good news.
“The sooner that we can get vaccines into as many people as possible, regardless of their age, the sooner we will be able to really feel like we’re ending this pandemic for good,” Angela Rasmussen, PhD, a virologist affiliated with Georgetown University in Washington, told The New York Times.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, recently said that getting children vaccinated is an important step toward achieving herd immunity.
“We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape,” he said earlier in March during a hearing of the Senate Health, Education, Labor, and Pensions Committee. “We ultimately would like to get and have to get children into that mix.”
Pfizer said it started clinical trials during the week of March 23 with children aged 5-11 and will next start trials with children aged 2-5, followed by children aged 6 months to 2 years. Vaccine makers Moderna and AstraZeneca also have started clinical trials in younger children.
A version of this article first appeared on WebMD.com.
The study enrolled 2,260 adolescents aged 12-15. No infections were reported in the group given the vaccine produced by Pfizer and its European partner, BioNTech, the release said. The placebo group reported 18 cases of COVID-19.
The vaccinated children showed a strong antibody response with no serious side effects.
Albert Bourla, PhD, chairman and CEO of Pfizer, said the company plans to seek Food and Drug Administration emergency use authorization, which could allow this age group to be vaccinated before the start of the next school year. Pfizer will also seek authorization from the European Medicines Agency.
“We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” Dr. Bourla said in the release.
The clinical trials showed a stronger response in children aged 12-15 than the 95% effectiveness reported in clinical trials in adults. The Pfizer vaccine is now authorized to be given to people aged 16 and up in the United States.
Health experts said the clinical trials – while not peer-reviewed – amounted to very good news.
“The sooner that we can get vaccines into as many people as possible, regardless of their age, the sooner we will be able to really feel like we’re ending this pandemic for good,” Angela Rasmussen, PhD, a virologist affiliated with Georgetown University in Washington, told The New York Times.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, recently said that getting children vaccinated is an important step toward achieving herd immunity.
“We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape,” he said earlier in March during a hearing of the Senate Health, Education, Labor, and Pensions Committee. “We ultimately would like to get and have to get children into that mix.”
Pfizer said it started clinical trials during the week of March 23 with children aged 5-11 and will next start trials with children aged 2-5, followed by children aged 6 months to 2 years. Vaccine makers Moderna and AstraZeneca also have started clinical trials in younger children.
A version of this article first appeared on WebMD.com.
The study enrolled 2,260 adolescents aged 12-15. No infections were reported in the group given the vaccine produced by Pfizer and its European partner, BioNTech, the release said. The placebo group reported 18 cases of COVID-19.
The vaccinated children showed a strong antibody response with no serious side effects.
Albert Bourla, PhD, chairman and CEO of Pfizer, said the company plans to seek Food and Drug Administration emergency use authorization, which could allow this age group to be vaccinated before the start of the next school year. Pfizer will also seek authorization from the European Medicines Agency.
“We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” Dr. Bourla said in the release.
The clinical trials showed a stronger response in children aged 12-15 than the 95% effectiveness reported in clinical trials in adults. The Pfizer vaccine is now authorized to be given to people aged 16 and up in the United States.
Health experts said the clinical trials – while not peer-reviewed – amounted to very good news.
“The sooner that we can get vaccines into as many people as possible, regardless of their age, the sooner we will be able to really feel like we’re ending this pandemic for good,” Angela Rasmussen, PhD, a virologist affiliated with Georgetown University in Washington, told The New York Times.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, recently said that getting children vaccinated is an important step toward achieving herd immunity.
“We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape,” he said earlier in March during a hearing of the Senate Health, Education, Labor, and Pensions Committee. “We ultimately would like to get and have to get children into that mix.”
Pfizer said it started clinical trials during the week of March 23 with children aged 5-11 and will next start trials with children aged 2-5, followed by children aged 6 months to 2 years. Vaccine makers Moderna and AstraZeneca also have started clinical trials in younger children.
A version of this article first appeared on WebMD.com.
CDC adds new medical conditions to COVID-19 high-risk list
The Centers for Disease Control and Prevention has added several new medical conditions to its list of those that predispose adults to more severe COVID-19 illness.
Conditions that had previously been categorized as “might be” placing individuals at increased risk – but now are listed as high risk – include type 1 diabetes (in addition to type 2), moderate-to-severe asthma, liver disease, dementia or other neurologic conditions, stroke/cerebrovascular disease, HIV infection, cystic fibrosis, and overweight (in addition to obesity).
Substance use disorders, which hadn’t been previously listed, are now also considered high risk.
The new list groups together certain categories, such as chronic lung diseases (chronic obstructive pulmonary disease, asthma, cystic fibrosis, etc) and heart conditions (heart failure, coronary artery disease, hypertension, etc).
Both diabetes types are now grouped under “diabetes.”
The added medical conditions were posted on the CDC website’s COVID-19 page on March 29.
Type 1 diabetes and other conditions now priority for vaccination
The CDC refers to the medical conditions list as phase 1c in regard to COVID-19 vaccine prioritization, which means that anyone with any of these conditions can now be prioritized for vaccination, following those in groups 1a (frontline essential workers and those in long-term care facilities) and 1b (people aged 65-74 years; other essential workers; and people aged 16-64 years with underlying conditions that increase the risk of serious, life-threatening complications from COVID-19).
But in many cases, multiple states have already either fully opened up vaccine eligibility to all adults or have created their own lists of underlying high-risk medical conditions, CDC spokeswoman Kristen Nordlund told this news organization.
No conditions have been removed from the list.
In January, the American Diabetes Association and 18 other organizations sent a letter to the CDC requesting that type 1 diabetes be prioritized along with type 2, based on data from studies showing people with both types to be at high risk for severe COVID-19 illness.
Now, ADA says, “this updated guidance will help to address the fact that in many states, millions of people with type 1 diabetes have not been prioritized equally, slowing their access to critical vaccines.”
While awaiting this latest CDC move, ADA had been urging state governors to prioritize type 1 and type 2 diabetes equally. As of now, 38 states and the District of Columbia had either done so or announced that they would.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has added several new medical conditions to its list of those that predispose adults to more severe COVID-19 illness.
Conditions that had previously been categorized as “might be” placing individuals at increased risk – but now are listed as high risk – include type 1 diabetes (in addition to type 2), moderate-to-severe asthma, liver disease, dementia or other neurologic conditions, stroke/cerebrovascular disease, HIV infection, cystic fibrosis, and overweight (in addition to obesity).
Substance use disorders, which hadn’t been previously listed, are now also considered high risk.
The new list groups together certain categories, such as chronic lung diseases (chronic obstructive pulmonary disease, asthma, cystic fibrosis, etc) and heart conditions (heart failure, coronary artery disease, hypertension, etc).
Both diabetes types are now grouped under “diabetes.”
The added medical conditions were posted on the CDC website’s COVID-19 page on March 29.
Type 1 diabetes and other conditions now priority for vaccination
The CDC refers to the medical conditions list as phase 1c in regard to COVID-19 vaccine prioritization, which means that anyone with any of these conditions can now be prioritized for vaccination, following those in groups 1a (frontline essential workers and those in long-term care facilities) and 1b (people aged 65-74 years; other essential workers; and people aged 16-64 years with underlying conditions that increase the risk of serious, life-threatening complications from COVID-19).
But in many cases, multiple states have already either fully opened up vaccine eligibility to all adults or have created their own lists of underlying high-risk medical conditions, CDC spokeswoman Kristen Nordlund told this news organization.
No conditions have been removed from the list.
In January, the American Diabetes Association and 18 other organizations sent a letter to the CDC requesting that type 1 diabetes be prioritized along with type 2, based on data from studies showing people with both types to be at high risk for severe COVID-19 illness.
Now, ADA says, “this updated guidance will help to address the fact that in many states, millions of people with type 1 diabetes have not been prioritized equally, slowing their access to critical vaccines.”
While awaiting this latest CDC move, ADA had been urging state governors to prioritize type 1 and type 2 diabetes equally. As of now, 38 states and the District of Columbia had either done so or announced that they would.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has added several new medical conditions to its list of those that predispose adults to more severe COVID-19 illness.
Conditions that had previously been categorized as “might be” placing individuals at increased risk – but now are listed as high risk – include type 1 diabetes (in addition to type 2), moderate-to-severe asthma, liver disease, dementia or other neurologic conditions, stroke/cerebrovascular disease, HIV infection, cystic fibrosis, and overweight (in addition to obesity).
Substance use disorders, which hadn’t been previously listed, are now also considered high risk.
The new list groups together certain categories, such as chronic lung diseases (chronic obstructive pulmonary disease, asthma, cystic fibrosis, etc) and heart conditions (heart failure, coronary artery disease, hypertension, etc).
Both diabetes types are now grouped under “diabetes.”
The added medical conditions were posted on the CDC website’s COVID-19 page on March 29.
Type 1 diabetes and other conditions now priority for vaccination
The CDC refers to the medical conditions list as phase 1c in regard to COVID-19 vaccine prioritization, which means that anyone with any of these conditions can now be prioritized for vaccination, following those in groups 1a (frontline essential workers and those in long-term care facilities) and 1b (people aged 65-74 years; other essential workers; and people aged 16-64 years with underlying conditions that increase the risk of serious, life-threatening complications from COVID-19).
But in many cases, multiple states have already either fully opened up vaccine eligibility to all adults or have created their own lists of underlying high-risk medical conditions, CDC spokeswoman Kristen Nordlund told this news organization.
No conditions have been removed from the list.
In January, the American Diabetes Association and 18 other organizations sent a letter to the CDC requesting that type 1 diabetes be prioritized along with type 2, based on data from studies showing people with both types to be at high risk for severe COVID-19 illness.
Now, ADA says, “this updated guidance will help to address the fact that in many states, millions of people with type 1 diabetes have not been prioritized equally, slowing their access to critical vaccines.”
While awaiting this latest CDC move, ADA had been urging state governors to prioritize type 1 and type 2 diabetes equally. As of now, 38 states and the District of Columbia had either done so or announced that they would.
A version of this article first appeared on Medscape.com.
Vaccine mismatch: What to do after dose 1 when plans change
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
COVID vaccines could lose their punch within a year, experts say
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
National Psoriasis Foundation recommends some stop methotrexate for 2 weeks after J&J vaccine
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
New COVID-19 cases rise again in children
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
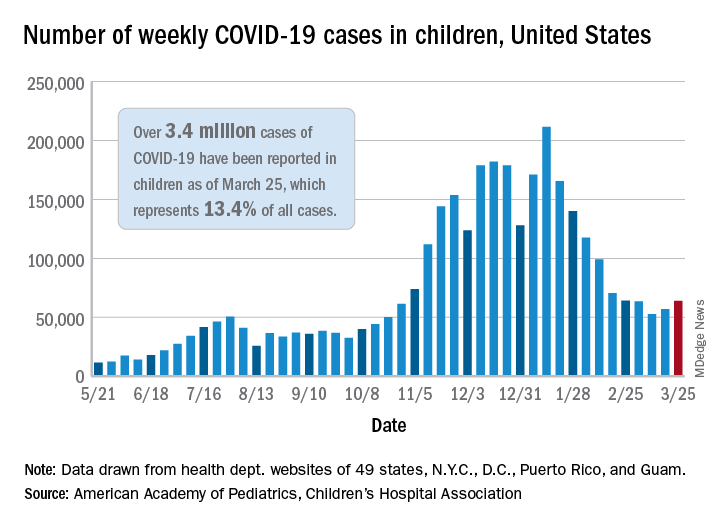
That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
Long-haul COVID-19 brings welcome attention to POTS
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.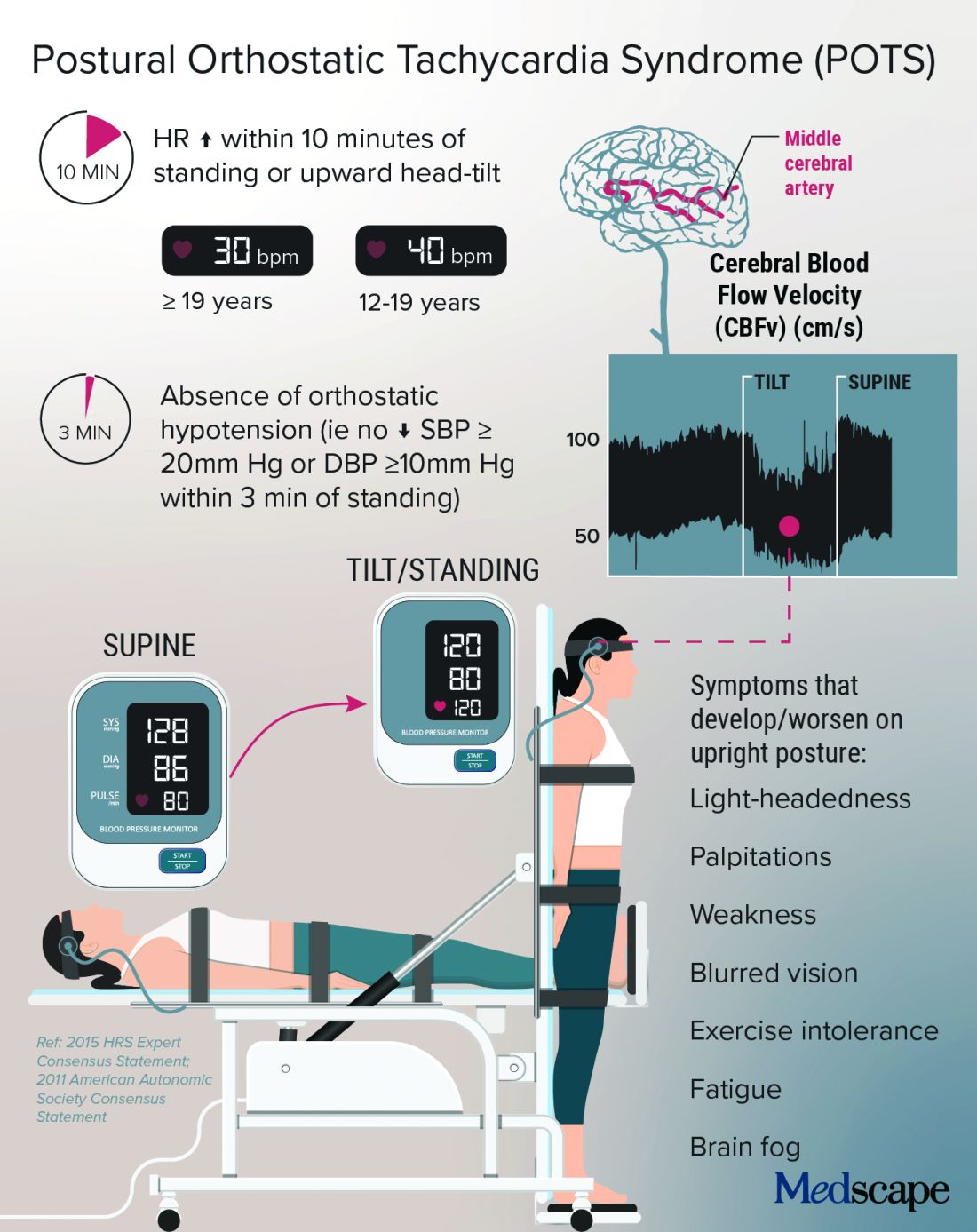
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.