User login
In Case You Missed It: COVID
COVID-19 ‘long-haul’ symptoms overlap with ME/CFS
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
People experiencing long-term symptoms following acute COVID-19 infection are increasingly meeting criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a phenomenon that highlights the need for unified research and clinical approaches, speakers said at a press briefing March 25 held by the advocacy group MEAction.
“Post-COVID lingering illness was predictable. Similar lingering fatigue syndromes have been reported in the scientific literature for nearly 100 years, following a variety of well-documented infections with viruses, bacteria, fungi, and even protozoa,” said Anthony Komaroff, MD, professor of medicine at Harvard Medical School, Boston.
Core criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for at least 6 months, postexertional malaise (PEM), or a worsening of symptoms following even minor exertion (often described as “crashes”), unrefreshing sleep, and cognitive impairment and/or orthostatic intolerance.
Patients with ME/CFS also commonly experience painful headaches, muscle or joint aches, and allergies/other sensitivities. Although many patients can trace their symptoms to an initiating infection, “the cause is often unclear because the diagnosis is often delayed for months or years after symptom onset,” said Lucinda Bateman, MD, founder of the Bateman Horne Center, Salt Lake City, who leads a clinician coalition that aims to improve ME/CFS management.
In an international survey of 3762 COVID-19 “long-haulers” published in a preprint in December of 2020, the most frequent symptoms reported at least 6 months after illness onset were fatigue in 78%, PEM in 72%, and cognitive dysfunction (“brain fog”) in 55%. At the time of the survey, 45% reported requiring reduced work schedules because of their illness, and 22% reported being unable to work at all.
Dr. Bateman said those findings align with her experience so far with 12 COVID-19 “long haulers” who self-referred to her ME/CFS and fibromyalgia specialty clinic. Nine of the 12 met criteria for postural orthostatic tachycardia syndrome (POTS) based on the 10-minute NASA Lean Test, she said, and half also met the 2016 American College of Rheumatology criteria for fibromyalgia.
“Some were severely impaired. We suspect a small fiber polyneuropathy in about half, and mast cell activation syndrome in more than half. We look forward to doing more testing,” Dr. Bateman said.
To be sure, Dr. Komaroff noted, there are some differences. “Long COVID” patients will often experience breathlessness and ongoing anosmia (loss of taste and smell), which aren’t typical of ME/CFS.
But, he said, “many of the symptoms are quite similar ... My guess is that ME/CFS is an illness with a final common pathway that can be triggered by different things,” said Dr. Komaroff, a senior physician at Brigham and Women’s Hospital in Boston, and editor-in-chief of the Harvard Health Letter.
Based on previous data about CFS suggesting a 10% rate of symptoms persisting at least a year following a variety of infectious agents and the predicted 200 million COVID-19 cases globally by the end of 2021, Dr. Komaroff estimated that about 20 million cases of “long COVID” would be expected in the next year.
‘A huge investment’
On the research side, the National Institutes of Health recently appropriated $1.15 billion dollars over the next 4 years to investigate “the heterogeneity in the recovery process after COVID and to develop treatments for those suffering from [postacute COVID-19 syndrome]” according to a Feb. 5, 2021, blog from the National Institute of Neurological Disorders and Stroke (NINDS).
That same day, another NINDS blog announced “new resources for large-scale ME/CFS research” and emphasized the tie-in with long–COVID-19 syndrome.
“That’s a huge investment. In my opinion, there will be several lingering illnesses following COVID,” Dr. Komaroff said, adding, “It’s my bet that long COVID will prove to be caused by certain kinds of abnormalities in the brain, some of the same abnormalities already identified in ME/CFS. Research will determine whether that’s right or wrong.”
In 2017, NINDS had announced a large increase in funding for ME/CFS research, including the creation of four dedicated research centers. In April 2019, NINDS held a 2-day conference highlighting that ongoing work, as reported by Medscape Medical News.
During the briefing, NINDS clinical director Avindra Nath, MD, described a comprehensive ongoing ME/CFS intramural study he’s been leading since 2016.
He’s now also overseeing two long–COVID-19 studies, one of which has a protocol similar to that of the ME/CFS study and will include individuals who are still experiencing long-term symptoms following confirmed cases of COVID-19. The aim is to screen about 1,300 patients. Several task forces are now examining all of these data together.
“Each aspect is now being analyzed … What we learn from one applies to the other,” Dr. Nath said.
Advice for clinicians
In interviews, Dr. Bateman and Dr. Nath offered clinical advice for managing patients who meet ME/CFS criteria, whether they had confirmed or suspected COVID-19, a different infection, or unknown trigger(s).
Dr. Bateman advised that clinicians assess patients for each of the symptoms individually. “Besides exercise intolerance and PEM, the most commonly missed is orthostatic intolerance. It really doesn’t matter what the cause is, it’s amenable to supportive treatment. It’s one aspect of the illness that contributes to severely impaired function. My plea to all physicians would be for sure to assess for [orthostatic intolerance], and gain an understanding about activity management and avoiding PEM symptoms.”
Dr. Nath noted that an often-challenging situation is when tests for the infectious agent and other blood work come back negative, yet the patient still reports multiple debilitating symptoms. This has been a particular issue with long COVID-19, since many patients became ill early in the pandemic before the polymerase chain reaction (PCR) tests for SARS-CoV-2 were widely available.
“The physician can only order tests that are available at their labs. I think what the physician should do is handle symptoms symptomatically but also refer patients to specialists who are taking care of these patients or to research studies,” he said.
Dr. Bateman added, “Whether they had a documented COVID infection – we just have to let go of that in 2020. Way too many people didn’t have access to a test or the timing wasn’t amenable. If people meet criteria for ME/CFS, it’s irrelevant … It’s mainly a clinical diagnosis. It’s not reliant on identifying the infectious trigger.”
Dr. Komaroff, who began caring for then-termed “chronic fatigue syndrome” patients and researching the condition more than 30 years ago, said that “every cloud has its silver lining. The increased focus on postinfectious fatigue syndrome is a silver lining in my mind around the terrible dark cloud that is the pandemic of COVID.”
Dr. Komaroff has received personal fees from Serimmune Inc., Ono Pharma, and Deallus, and grants from the NIH. Dr. Bateman is employed by the Bateman Horne Center, which receives grants from the NIH, and fees from Exagen Inc., and Teva Pharmaceutical. Dr. Nath is an NIH employee.
A version of this article first appeared on Medscape.com.
Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
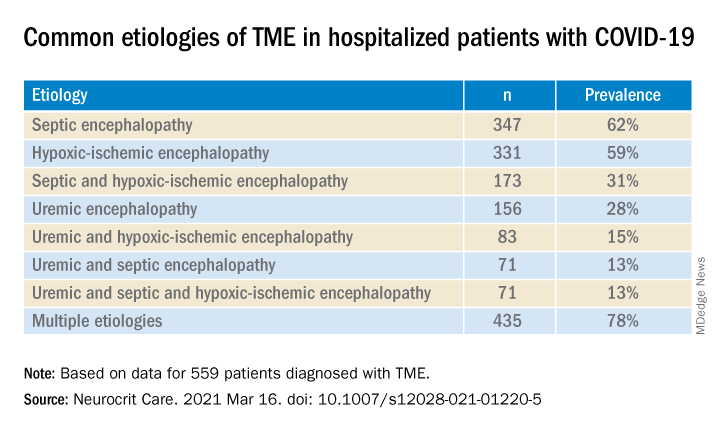
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE
COVID-19 vaccination in RMD patients: Safety data “reassuring”
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES
In U.S., lockdowns added 2 pounds per month
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Vitamin D may protect against COVID-19, especially in Black patients
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 Monoclonal Antibody Infusions: A Multidisciplinary Initiative to Operationalize EUA Novel Treatment Options
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.

Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
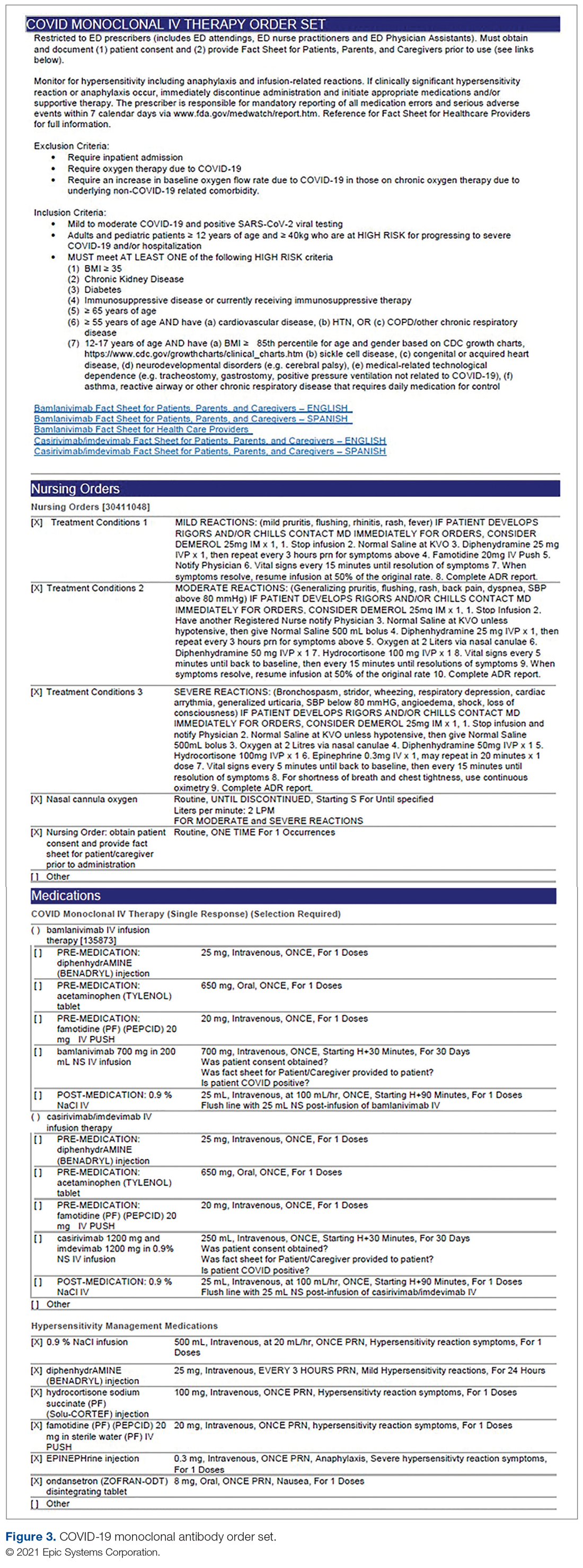
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
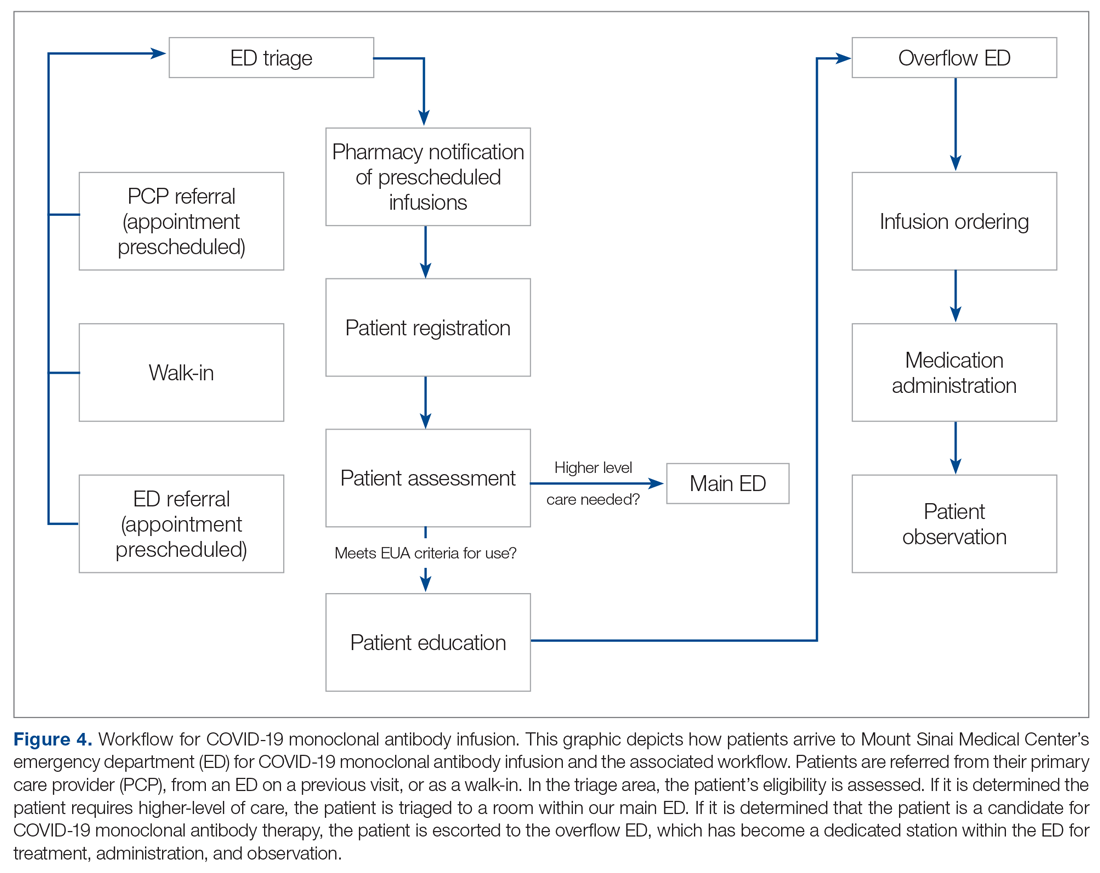
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
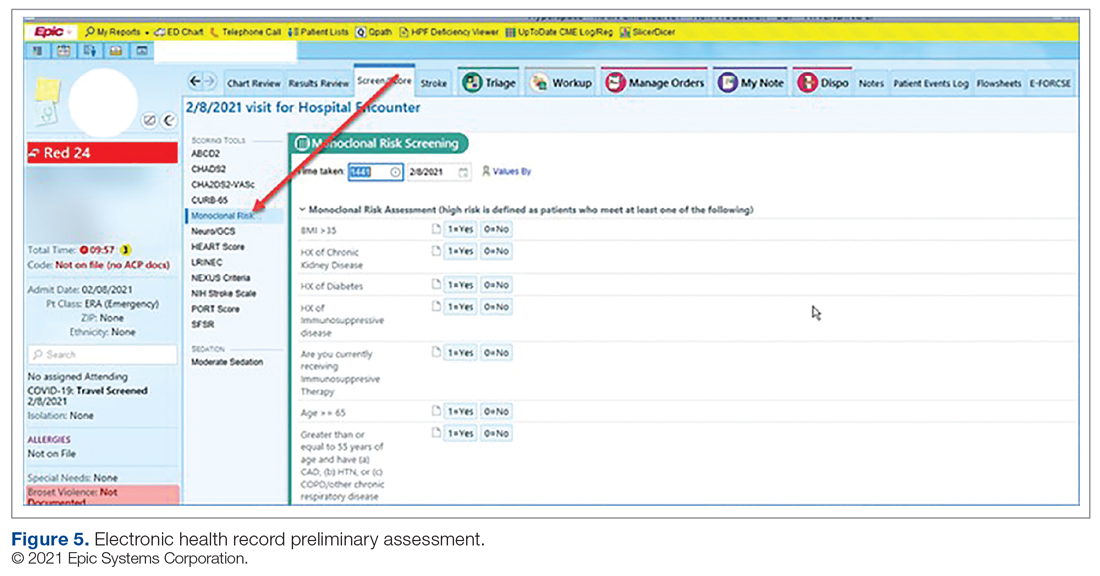
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).

The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.

On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.

Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.

The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.

Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.

Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).

The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.

On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.

Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.

The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.

Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.

Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
COVID-19 maternal antibodies transferred to fetus, newborn from pregnant and lactating vaccine recipients
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
COVID-19 variants now detected in more animals, may find hosts in mice
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
Infliximab weakens COVID-19 antibody response for IBD patients
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
Patients treated with infliximab for inflammatory bowel disease (IBD) showed significantly reduced response to COVID-19 antibodies, compared with those treated with vedolizumab, according to data from nearly 7,000 patients.
Although anti–tumor necrosis factor (anti-TNF) drugs are routinely used for patients with IBD, the impact of their immune-suppressing properties on protective immunity to COVID-19 is unknown, wrote Nicholas A. Kennedy, MD, of the University of Exeter (England) and colleagues. These drugs have been reported to impair protective immunity following vaccines for other diseases, such as those for influenza and viral hepatitis.
“By suppressing immune responses, biological and immunosuppression therapies may lead to chronic SARS-CoV-2 infection and have recently been implicated in the evolution and emergence of novel variants,” they noted, citing a study published in Cell.
In the current study, published in Gut, the researchers used data from the CLARITY IBD study to identify 6,935 patients with IBD aged 5 years and older seen at 92 hospitals in the United Kingdom between Sept. 22, 2020, and Dec. 23, 2020. Of these, 4,685 were treated with infliximab, and 2,250 received vedolizumab. The proportion of study participants with a positive anti–SARS-CoV-2 antibody test was the primary outcome, with secondary outcomes including proportion with positive antibodies following positive polymerase chain reaction test for SARS-CoV-2 and the magnitude of antibody reactivity.
Substantial seroprevalence differences seen
Overall, rates of symptomatic and proven SARS-CoV-2 infection and hospitalization were similar between infliximab-treated and vedolizumab-treated patients with IBD. However, seroprevalence was significantly lower in the infliximab group, compared with the vedolizumab group (3.4% vs. 6.0%; P < .0001). In addition, infliximab and immunomodulator use were each independently associated with lower seropositivity, compared with vedolizumab (odds ratio, 0.66 for infliximab and OR, 0.70 for immunomodulators) in a multivariate analysis.
In a sensitivity analysis, 39 of 81 infliximab-treated patients with polymerase chain reaction–confirmed COVID-19 infection seroconverted (48%), compared with 30 of 36 vedolizumab-treated patients (83%) (P < .00044). Infliximab-treated patients with confirmed infections also showed a lower magnitude of anti–SARS-CoV-2 reactivity, compared with vedolizumab-treated patients (P < .0001).
From a clinical perspective, the lower seroconversion rates and reduced levels of anti–SARS-CoV-2 antibody reactivity might increase susceptibility to recurrent COVID-19 infections in infliximab-treated IBD patients, the researchers noted. In addition, the impaired serological responses might promote chronic nasopharyngeal colonization and consequently promote the development of COVID-19 variants and drive persistent transmission, the researchers said.
The study findings were limited by several factors including lack of knowledge on the impact of attenuated immune response on infection risk, the potential for recall bias associated with patient reports, and the focus on infliximab only, the researchers pointed out. However, the key findings are likely apply to other anti-TNF monoclonal antibodies including adalimumab, certolizumab and golimumab, they suggested.
The study was strengthened by the recruitment of a large number of patients in a narrow time frame and comprehensive collection of data on patient-reported outcomes, COVID-19 testing, and serological assay results, the researchers said. Overall, the findings support the public health value of serological testing and virus surveillance to identify suboptimal vaccine response and to consider implications for practice, they added. “If attenuated serological responses following vaccination are also observed, then modified immunization strategies will need to be designed for millions of patients worldwide,” they emphasized.
Findings inform clinical practice and public health
The study is very important for many reasons, said Kim L. Isaacs, MD, PhD, AGAF, of the University of North Carolina at Chapel Hill in an interview. “It is known that there is decreased responsiveness to a number of routine vaccinations in IBD patients on immune active therapy. In terms of SARS-CoV-2, development of an immune response with infection is important in terms of severity of infection, reinfection, and possibly limiting spread of infection in this patient population,” she said. “Looking at both serum seroconversion and reactivity of immune response in patients with known SARS-CoV-2 infection will help to define clinical and public health guidance, and also may be predictive as to what might happen with SARS-CoV-2 immunization based on background biologic or immunosuppressant therapy,” she noted.
Dr. Isaacs said that she was not surprised by the study findings. “Anti-TNF, thiopurine, and methotrexate therapy are all thought to be systemically active and likely to suppress the immune response to infection and vaccination,” she said. Vedolizumab, on the other hand, is thought to be less systemically active and clinically is associated with fewer serious infections.
Data will drive patient counseling
“These results affect counseling of IBD patients on immune active therapy who have had a SARS-CoV-2 infection,” said Dr. Isaacs. “They should be made aware that infection does not indicate protection for further infection. Although the issues that are raised in this study are of concern, patients should not have clinically beneficial therapy discontinued or switched based on these results,” she said.
“Additional research is needed to determine what the seroconversion rate is with the currently available immunizations for SARS-CoV-2,” said Dr. Isaacs. More questions to address include whether there are differences in the different products available, whether immunization after SARS-CoV-2 infection improves both seroconversion and immune reactivity, and whether there is any benefit to transiently stopping dual immune active therapy during the time of immunization, she said.
Further studies can fill knowledge gaps
“There is a knowledge gap in our understanding of susceptibility to SARS-CoV-2 infections among patients with IBD who have previously been infected,” Shirley Cohen-Mekelburg, MD, MS, staff physician and research scientist in the inflammatory bowel disease program at the Veterans Affairs Ann Arbor (Mich.) Healthcare System, said in an interview. ”This is a first step in beginning to narrow this gap – to provide patients and providers with data to drive recommendations during this COVID-19 pandemic.”
She added that, while further work needs to be done, the study findings do support potential benefit for ongoing vigilance among patients receiving infliximab for IBD. “The study findings also drive us to seek answers to more questions: For example, should we consider serological testing for patients on infliximab? How does the presence or absence of anti–SARS-CoV-2 antibodies associate with susceptibility to infection for patients with infliximab?
“Further studies examining anti–SARS-CoV-2 reactivity are necessary to better understand antibody responses between patients with IBD to the general population, or between patients on immunosuppressive therapy and the general population,” she said. “Observational studies are also not designed to examine the causal relationship between infections, medications, and antibody responses. There may be some inherent differences to patients who receive infliximab as compared to vedolizumab for IBD.”
The study was supported by Biogen (Switzerland), Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, Hull University Teaching Hospital NHS Trust, and the Royal Devon and Exeter NHS Foundation Trust. The study authors disclosed financial and nonfinancial relationships with numerous companies, including AbbVie, Biogen, Celltrion Healthcare, Galapagos, F. Hoffmann-La Roche, and Immundiagnostik, as well as Janssen, who markets infliximab, and Takeda, who markets vedolizumab. Dr. Isaacs and Dr. Cohen-Mekelburg had no relevant financial conflicts to disclose.
This article was updated 3/31/21.
FROM GUT
Postintubation tracheal injury in the COVID-19 era
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY