User login
In Case You Missed It: COVID
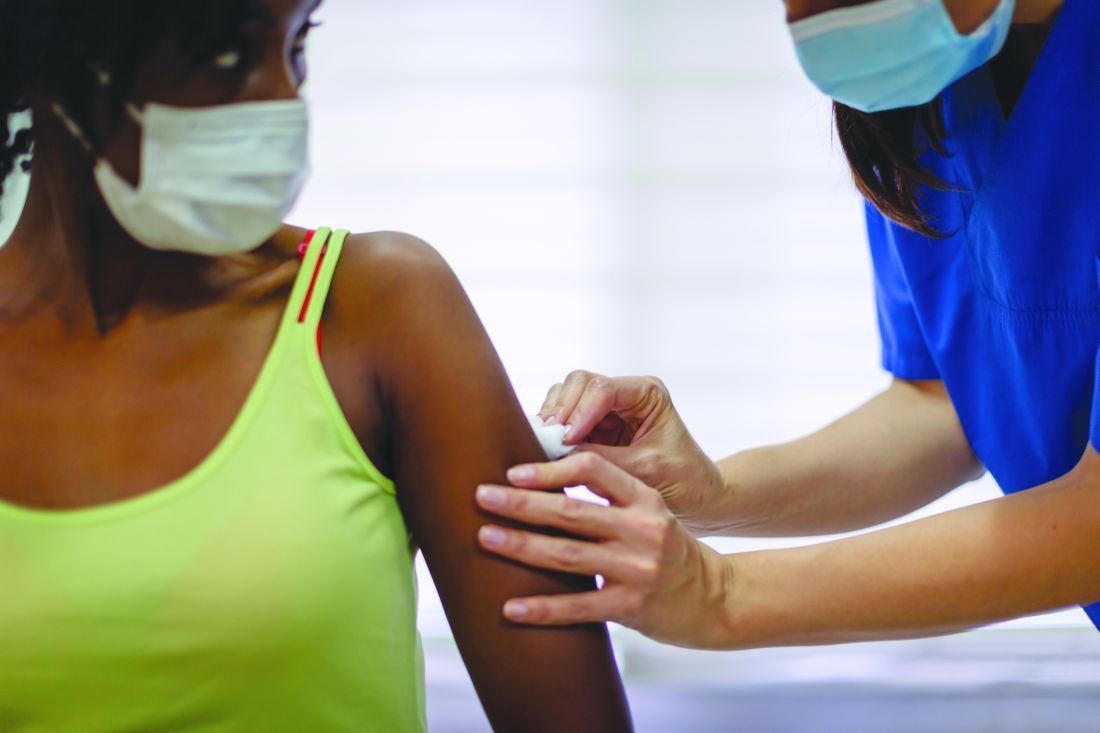
List of COVID-19 high-risk comorbidities expanded
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The COVID-19 push to evolve
Has anyone else noticed how slow it has been on your pediatric floors? Well, you are not alone.
The COVID pandemic has had a significant impact on health care volumes, with pediatric volumes decreasing across the nation. A Children’s Hospital Association CEO survey, currently unpublished, noted a 10%-20% decline in inpatient admissions and a 30%-50% decline in pediatric ED visits this past year. Even our usual respiratory surge has been disrupted. The rate of influenza tracked by the CDC is around 1%, compared with the usual seasonal flu baseline national rate of 2.6%. These COVID-related declines have occurred amidst the backdrop of already-decreasing inpatient admissions because of the great work of the pediatric hospital medicine (PHM) community in reducing unnecessary admissions and lengths of stay.
For many hospitals, several factors related to the pandemic have raised significant financial concerns. According to Becker Hospital Review, as of August 2020 over 500 hospitals had furloughed workers. While 26 of those hospitals had brought back workers by December 2020, many did not. Similar financial concerns were noted in a Kaufmann Hall report from January 2021, which showed a median drop of 55% in operating margins. The CARES Act helped reduce some of the detrimental impact on operating margins, but it did not diminish the added burden of personal protective equipment expenses, longer length of stay for COVID patients, and a reimbursement shift to more government payors and uninsured caused by pandemic-forced job losses.
COVID’s impact specific to pediatric hospital medicine has been substantial. A recent unpublished survey by the PHM Economics Research Collaborative (PERC) demonstrated how COVID has affected pediatric hospital medicine programs. Forty-five unique PHM programs from over 21 states responded, with 98% reporting a decrease in pediatric inpatient admissions as well as ED visits. About 11% reported temporary unit closures, while 51% of all programs reported staffing restrictions ranging from hiring freezes to downsizing the number of hospitalists in the group. Salaries decreased in 26% of reporting programs, and 20%-56% described reduced benefits, ranging from less CME/vacation time and stipends to retirement benefits. The three most frequent benefit losses included annual salary increases, educational stipends, and bonuses.
Community hospitals felt the palpable, financial strain of decreasing pediatric admissions well before the pandemic. Hospitals like MedStar Franklin Square Hospital in Baltimore and Harrington Hospital in Southbridge, Mass., had decided to close their pediatrics units before COVID hit. In a 2014 unpublished survey of 349 community PHM (CPHM) programs, 57% of respondents felt that finances and justification for a pediatric program were primary concerns.
Responding to financial stressors is not a novel challenge for CPHM programs. To keep these vital pediatric programs in place despite lower inpatient volumes, those of us in CPHM have learned many lessons over the years on how to adapt. Such adaptations have included diversification in procedures and multifloor coverage in the hospital. Voiding cystourethrogram catheterizations and circumcisions are now more commonly performed by CPHM providers, who may also cover multiple areas of the hospital, including the ED, NICU, and well-newborn nursery. Comanagement of subspecialty or surgical patients is yet another example of such diversification.
Furthermore, the PERC survey showed that some PHM programs temporarily covered pediatric ICUs and step-down units and began doing ED and urgent care coverage as primary providers Most programs reported no change in newborn visits while 16% reported an increase in newborn volume and 14% reported a decrease in newborn volume. My own health system was one of the groups that had an increase in newborn volume. This was caused by community pediatricians who had stopped coming in to see their own newborns. This coverage adjustment has yet to return to baseline and will likely become permanent.
There was a 11% increase from prepandemic baselines (from 9% to 20%) in programs doing telemedicine. Most respondents stated that they will continue to offer telemedicine with an additional 25% of programs considering starting. There was also a slight increase during the pandemic of coverage of mental health units (from 11% to 13%), which may have led 11% of respondents to consider the addition of this service. The survey also noted that about 28% of PHM programs performed circumcisions, frenectomies, and sedation prepandemic, and 14%-18% are considering adding these services.
Overall, the financial stressors are improving, but our need to adapt in PHM is more pressing than ever. The pandemic has given us the push for evolution and some opportunities that did not exist before. One is the use of telemedicine to expand our subspecialty support to community hospitals, as well as to children’s hospitals in areas where subspecialists are in short supply. These telemedicine consults are being reimbursed for the first time, which allows more access to these services.
With the pandemic, many hospitals are moving to single room occupancy models. Construction to add more beds is costly, and unnecessary if we can utilize community hospitals to keep appropriate patients in their home communities. The opportunity to partner with community hospital programs to provide telemedicine support should not be overlooked. This is also an opportunity for academic referral centers to have more open beds for critical care and highly specialized patients.
Another opportunity is to expand scope by changing age limits, as 18% of respondents to the PERC survey reported that they had started to care for adults since the pandemic. The Pediatric Overflow Planning Contingency Response Network (POPCoRN) has been a valuable resource for education on caring for adults, guidance on which patient populations are appropriate, and the resources needed to do this. While caring for older adults, even in their 90s, was a pandemic-related phenomenon, there is an opportunity to see if the age limit we care for should be raised to 21, or even 25, as some CPHM programs had been doing prepandemic.
Along with the expansion of age limits, there are many other areas of opportunity highlighted within the PERC survey. These include expanding coverage within pediatric ICUs, EDs, and urgent care areas, along with coverage of well newborns that were previously covered by community pediatricians. Also, the increase of mental health admissions is another area where PHM programs might expand their services.
While I hope the financial stressors improve, hope is not a plan and therefore we need to think and prepare for what the post-COVID future may look like. Some have predicted a rebound pediatric respiratory surge next year as the masks come off and children return to in-person learning and daycare. This may be true, but we would be foolish not to use lessons from the pandemic as well as the past to consider options in our toolkit to become more financially stable. POPCoRN, as well as the American Academy of Pediatrics’ listserv and subcommittees, have been a source of collaboration and shared knowledge during a time when we have needed to quickly respond to ever-changing information. These networks and information sharing should be leveraged once the dust settles for us to prepare for future challenges.
New innovations may arise as we look at how we address the growing need for mental health services and incorporate new procedures, like point of care ultrasound. As Charles Darwin said: “It is not the strongest of the species that survives nor the most intelligent that survives. It is the one that is most adaptable to change.” It is time for us to evolve.
Dr. Dias is a clinical associate professor of pediatrics at Yale University, New Haven, Conn., in the division of pediatric hospital medicine. She has practiced community pediatric hospital medicine for over 21 years in New Jersey, Pennsylvania, and Connecticut. She is the chair of the Education Working Group for the AAP’s section on hospital medicine’s subcommittee on community hospitalists as well as the cochair of the Community Hospital Operations Group of the POPCoRN network.
Has anyone else noticed how slow it has been on your pediatric floors? Well, you are not alone.
The COVID pandemic has had a significant impact on health care volumes, with pediatric volumes decreasing across the nation. A Children’s Hospital Association CEO survey, currently unpublished, noted a 10%-20% decline in inpatient admissions and a 30%-50% decline in pediatric ED visits this past year. Even our usual respiratory surge has been disrupted. The rate of influenza tracked by the CDC is around 1%, compared with the usual seasonal flu baseline national rate of 2.6%. These COVID-related declines have occurred amidst the backdrop of already-decreasing inpatient admissions because of the great work of the pediatric hospital medicine (PHM) community in reducing unnecessary admissions and lengths of stay.
For many hospitals, several factors related to the pandemic have raised significant financial concerns. According to Becker Hospital Review, as of August 2020 over 500 hospitals had furloughed workers. While 26 of those hospitals had brought back workers by December 2020, many did not. Similar financial concerns were noted in a Kaufmann Hall report from January 2021, which showed a median drop of 55% in operating margins. The CARES Act helped reduce some of the detrimental impact on operating margins, but it did not diminish the added burden of personal protective equipment expenses, longer length of stay for COVID patients, and a reimbursement shift to more government payors and uninsured caused by pandemic-forced job losses.
COVID’s impact specific to pediatric hospital medicine has been substantial. A recent unpublished survey by the PHM Economics Research Collaborative (PERC) demonstrated how COVID has affected pediatric hospital medicine programs. Forty-five unique PHM programs from over 21 states responded, with 98% reporting a decrease in pediatric inpatient admissions as well as ED visits. About 11% reported temporary unit closures, while 51% of all programs reported staffing restrictions ranging from hiring freezes to downsizing the number of hospitalists in the group. Salaries decreased in 26% of reporting programs, and 20%-56% described reduced benefits, ranging from less CME/vacation time and stipends to retirement benefits. The three most frequent benefit losses included annual salary increases, educational stipends, and bonuses.
Community hospitals felt the palpable, financial strain of decreasing pediatric admissions well before the pandemic. Hospitals like MedStar Franklin Square Hospital in Baltimore and Harrington Hospital in Southbridge, Mass., had decided to close their pediatrics units before COVID hit. In a 2014 unpublished survey of 349 community PHM (CPHM) programs, 57% of respondents felt that finances and justification for a pediatric program were primary concerns.
Responding to financial stressors is not a novel challenge for CPHM programs. To keep these vital pediatric programs in place despite lower inpatient volumes, those of us in CPHM have learned many lessons over the years on how to adapt. Such adaptations have included diversification in procedures and multifloor coverage in the hospital. Voiding cystourethrogram catheterizations and circumcisions are now more commonly performed by CPHM providers, who may also cover multiple areas of the hospital, including the ED, NICU, and well-newborn nursery. Comanagement of subspecialty or surgical patients is yet another example of such diversification.
Furthermore, the PERC survey showed that some PHM programs temporarily covered pediatric ICUs and step-down units and began doing ED and urgent care coverage as primary providers Most programs reported no change in newborn visits while 16% reported an increase in newborn volume and 14% reported a decrease in newborn volume. My own health system was one of the groups that had an increase in newborn volume. This was caused by community pediatricians who had stopped coming in to see their own newborns. This coverage adjustment has yet to return to baseline and will likely become permanent.
There was a 11% increase from prepandemic baselines (from 9% to 20%) in programs doing telemedicine. Most respondents stated that they will continue to offer telemedicine with an additional 25% of programs considering starting. There was also a slight increase during the pandemic of coverage of mental health units (from 11% to 13%), which may have led 11% of respondents to consider the addition of this service. The survey also noted that about 28% of PHM programs performed circumcisions, frenectomies, and sedation prepandemic, and 14%-18% are considering adding these services.
Overall, the financial stressors are improving, but our need to adapt in PHM is more pressing than ever. The pandemic has given us the push for evolution and some opportunities that did not exist before. One is the use of telemedicine to expand our subspecialty support to community hospitals, as well as to children’s hospitals in areas where subspecialists are in short supply. These telemedicine consults are being reimbursed for the first time, which allows more access to these services.
With the pandemic, many hospitals are moving to single room occupancy models. Construction to add more beds is costly, and unnecessary if we can utilize community hospitals to keep appropriate patients in their home communities. The opportunity to partner with community hospital programs to provide telemedicine support should not be overlooked. This is also an opportunity for academic referral centers to have more open beds for critical care and highly specialized patients.
Another opportunity is to expand scope by changing age limits, as 18% of respondents to the PERC survey reported that they had started to care for adults since the pandemic. The Pediatric Overflow Planning Contingency Response Network (POPCoRN) has been a valuable resource for education on caring for adults, guidance on which patient populations are appropriate, and the resources needed to do this. While caring for older adults, even in their 90s, was a pandemic-related phenomenon, there is an opportunity to see if the age limit we care for should be raised to 21, or even 25, as some CPHM programs had been doing prepandemic.
Along with the expansion of age limits, there are many other areas of opportunity highlighted within the PERC survey. These include expanding coverage within pediatric ICUs, EDs, and urgent care areas, along with coverage of well newborns that were previously covered by community pediatricians. Also, the increase of mental health admissions is another area where PHM programs might expand their services.
While I hope the financial stressors improve, hope is not a plan and therefore we need to think and prepare for what the post-COVID future may look like. Some have predicted a rebound pediatric respiratory surge next year as the masks come off and children return to in-person learning and daycare. This may be true, but we would be foolish not to use lessons from the pandemic as well as the past to consider options in our toolkit to become more financially stable. POPCoRN, as well as the American Academy of Pediatrics’ listserv and subcommittees, have been a source of collaboration and shared knowledge during a time when we have needed to quickly respond to ever-changing information. These networks and information sharing should be leveraged once the dust settles for us to prepare for future challenges.
New innovations may arise as we look at how we address the growing need for mental health services and incorporate new procedures, like point of care ultrasound. As Charles Darwin said: “It is not the strongest of the species that survives nor the most intelligent that survives. It is the one that is most adaptable to change.” It is time for us to evolve.
Dr. Dias is a clinical associate professor of pediatrics at Yale University, New Haven, Conn., in the division of pediatric hospital medicine. She has practiced community pediatric hospital medicine for over 21 years in New Jersey, Pennsylvania, and Connecticut. She is the chair of the Education Working Group for the AAP’s section on hospital medicine’s subcommittee on community hospitalists as well as the cochair of the Community Hospital Operations Group of the POPCoRN network.
Has anyone else noticed how slow it has been on your pediatric floors? Well, you are not alone.
The COVID pandemic has had a significant impact on health care volumes, with pediatric volumes decreasing across the nation. A Children’s Hospital Association CEO survey, currently unpublished, noted a 10%-20% decline in inpatient admissions and a 30%-50% decline in pediatric ED visits this past year. Even our usual respiratory surge has been disrupted. The rate of influenza tracked by the CDC is around 1%, compared with the usual seasonal flu baseline national rate of 2.6%. These COVID-related declines have occurred amidst the backdrop of already-decreasing inpatient admissions because of the great work of the pediatric hospital medicine (PHM) community in reducing unnecessary admissions and lengths of stay.
For many hospitals, several factors related to the pandemic have raised significant financial concerns. According to Becker Hospital Review, as of August 2020 over 500 hospitals had furloughed workers. While 26 of those hospitals had brought back workers by December 2020, many did not. Similar financial concerns were noted in a Kaufmann Hall report from January 2021, which showed a median drop of 55% in operating margins. The CARES Act helped reduce some of the detrimental impact on operating margins, but it did not diminish the added burden of personal protective equipment expenses, longer length of stay for COVID patients, and a reimbursement shift to more government payors and uninsured caused by pandemic-forced job losses.
COVID’s impact specific to pediatric hospital medicine has been substantial. A recent unpublished survey by the PHM Economics Research Collaborative (PERC) demonstrated how COVID has affected pediatric hospital medicine programs. Forty-five unique PHM programs from over 21 states responded, with 98% reporting a decrease in pediatric inpatient admissions as well as ED visits. About 11% reported temporary unit closures, while 51% of all programs reported staffing restrictions ranging from hiring freezes to downsizing the number of hospitalists in the group. Salaries decreased in 26% of reporting programs, and 20%-56% described reduced benefits, ranging from less CME/vacation time and stipends to retirement benefits. The three most frequent benefit losses included annual salary increases, educational stipends, and bonuses.
Community hospitals felt the palpable, financial strain of decreasing pediatric admissions well before the pandemic. Hospitals like MedStar Franklin Square Hospital in Baltimore and Harrington Hospital in Southbridge, Mass., had decided to close their pediatrics units before COVID hit. In a 2014 unpublished survey of 349 community PHM (CPHM) programs, 57% of respondents felt that finances and justification for a pediatric program were primary concerns.
Responding to financial stressors is not a novel challenge for CPHM programs. To keep these vital pediatric programs in place despite lower inpatient volumes, those of us in CPHM have learned many lessons over the years on how to adapt. Such adaptations have included diversification in procedures and multifloor coverage in the hospital. Voiding cystourethrogram catheterizations and circumcisions are now more commonly performed by CPHM providers, who may also cover multiple areas of the hospital, including the ED, NICU, and well-newborn nursery. Comanagement of subspecialty or surgical patients is yet another example of such diversification.
Furthermore, the PERC survey showed that some PHM programs temporarily covered pediatric ICUs and step-down units and began doing ED and urgent care coverage as primary providers Most programs reported no change in newborn visits while 16% reported an increase in newborn volume and 14% reported a decrease in newborn volume. My own health system was one of the groups that had an increase in newborn volume. This was caused by community pediatricians who had stopped coming in to see their own newborns. This coverage adjustment has yet to return to baseline and will likely become permanent.
There was a 11% increase from prepandemic baselines (from 9% to 20%) in programs doing telemedicine. Most respondents stated that they will continue to offer telemedicine with an additional 25% of programs considering starting. There was also a slight increase during the pandemic of coverage of mental health units (from 11% to 13%), which may have led 11% of respondents to consider the addition of this service. The survey also noted that about 28% of PHM programs performed circumcisions, frenectomies, and sedation prepandemic, and 14%-18% are considering adding these services.
Overall, the financial stressors are improving, but our need to adapt in PHM is more pressing than ever. The pandemic has given us the push for evolution and some opportunities that did not exist before. One is the use of telemedicine to expand our subspecialty support to community hospitals, as well as to children’s hospitals in areas where subspecialists are in short supply. These telemedicine consults are being reimbursed for the first time, which allows more access to these services.
With the pandemic, many hospitals are moving to single room occupancy models. Construction to add more beds is costly, and unnecessary if we can utilize community hospitals to keep appropriate patients in their home communities. The opportunity to partner with community hospital programs to provide telemedicine support should not be overlooked. This is also an opportunity for academic referral centers to have more open beds for critical care and highly specialized patients.
Another opportunity is to expand scope by changing age limits, as 18% of respondents to the PERC survey reported that they had started to care for adults since the pandemic. The Pediatric Overflow Planning Contingency Response Network (POPCoRN) has been a valuable resource for education on caring for adults, guidance on which patient populations are appropriate, and the resources needed to do this. While caring for older adults, even in their 90s, was a pandemic-related phenomenon, there is an opportunity to see if the age limit we care for should be raised to 21, or even 25, as some CPHM programs had been doing prepandemic.
Along with the expansion of age limits, there are many other areas of opportunity highlighted within the PERC survey. These include expanding coverage within pediatric ICUs, EDs, and urgent care areas, along with coverage of well newborns that were previously covered by community pediatricians. Also, the increase of mental health admissions is another area where PHM programs might expand their services.
While I hope the financial stressors improve, hope is not a plan and therefore we need to think and prepare for what the post-COVID future may look like. Some have predicted a rebound pediatric respiratory surge next year as the masks come off and children return to in-person learning and daycare. This may be true, but we would be foolish not to use lessons from the pandemic as well as the past to consider options in our toolkit to become more financially stable. POPCoRN, as well as the American Academy of Pediatrics’ listserv and subcommittees, have been a source of collaboration and shared knowledge during a time when we have needed to quickly respond to ever-changing information. These networks and information sharing should be leveraged once the dust settles for us to prepare for future challenges.
New innovations may arise as we look at how we address the growing need for mental health services and incorporate new procedures, like point of care ultrasound. As Charles Darwin said: “It is not the strongest of the species that survives nor the most intelligent that survives. It is the one that is most adaptable to change.” It is time for us to evolve.
Dr. Dias is a clinical associate professor of pediatrics at Yale University, New Haven, Conn., in the division of pediatric hospital medicine. She has practiced community pediatric hospital medicine for over 21 years in New Jersey, Pennsylvania, and Connecticut. She is the chair of the Education Working Group for the AAP’s section on hospital medicine’s subcommittee on community hospitalists as well as the cochair of the Community Hospital Operations Group of the POPCoRN network.
Risk for erectile dysfunction sixfold higher in men with COVID-19
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The pandemic is making periods unbearable for some women
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
COVID-19 in children: New cases back on the decline
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
Excess deaths jump 23% in U.S. in 2020, mostly because of COVID-19
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
Children likely the ‘leading edge’ in spread of COVID-19 variants
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
AstraZeneca COVID vaccine: Clotting disorder mechanism revealed?
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.