User login
In Case You Missed It: COVID
Psychiatrists’ happiness, well-being hit hard by COVID-19
Events of the past year have taken a huge toll on the happiness, wellness, and lifestyles of many, but especially those in the health care field, including psychiatrists.
The newly released Medscape Psychiatrist Lifestyle, Happiness & Burnout Report 2021 reveals how psychiatrists are coping with burnout and trying to maintain personal wellness, and how they view their workplaces and their futures amid the ongoing COVID-19 pandemic.
Before the pandemic hit in March 2020, 84% of psychiatrists who responded to the survey reported being happy outside of work, similar to the percentage (82%) of physicians overall.
But as the pandemic has worn on, feelings have shifted, and there are clear signs of strain on those in the health care field. Now, just over half (55%) of psychiatrists say they are happy outside of work, similar to the percentage (58%) of physicians overall.
Perhaps not surprising given the specific challenges of COVID-19, infectious disease physicians, pulmonologists, rheumatologists, and intensivists currently rank lowest in happiness outside of work.
Anxiety, depression, burnout
With the ongoing COVID-19 pandemic, more than three quarters (77%) of psychiatrists surveyed report experiencing some degree of anxiety about their future, the same percentage as for physicians overall.
This year, more psychiatrists reported being either burned out or burned out and depressed (41% vs. 35% last year). About two-thirds of psychiatrists said burnout has had at least a moderate impact on their lives; 5% consider the impact so severe that they are thinking of leaving medicine altogether.
The majority of burned-out psychiatrists (63%) said they felt that way even before the pandemic began; for about one-third (37%), burnout set in after the pandemic began.
in the workplace (39%) and spending too many hours at work (37%).
Psychiatrists’ top tactic to cope with burnout is talking with family or friends (53%), followed by isolating themselves from others (51%), sleeping (45%), and exercising (43%); 42% said they eat junk food to cope; 35% play music; and 25% drink alcohol.
Most psychiatrists (63%) suffering burnout and/or depression don’t plan on seeking professional help. About one-third are currently seeking help or plan to do so, the highest proportion among all specialties.
Considering their symptoms not severe enough (57%) and feeling that they could deal with the problem on their own (41%) are the top reasons for not seeking professional help; 36% said they were too busy to get help, and 17% said they didn’t want to risk disclosing a problem.
Fifteen percent of psychiatrists who are burned out, depressed, or both have contemplated suicide, and 2% have attempted suicide.
Striving for work-life balance
Work-life balance is the most pressing workplace issue for 45% of psychiatrists, and 44% would sacrifice some of their salary for better work-life balance. These figures are about the same for physicians overall.
Forty-seven percent of psychiatrists take 3-4 weeks of vacation each year; 16% take 5 or more weeks. In this there was no change from last year’s report.
About one-third (35%) of psychiatrists generally make time to focus on their own well-being, the same proportion as physicians overall.
About two-thirds (68%) of psychiatrists exercise two or more times per week. Half of psychiatrists said they are currently trying to lose weight; about one-quarter are trying to maintain their current weight.
About one-quarter (26%) of psychiatrists said they do not drink alcohol at all; 17% have five or more drinks per week.
Most psychiatrists are currently in a committed relationship, with 81% either married or living with a partner. Among psychiatrists who are married or living with a partner, 43% are with someone who also works in medicine. About 81% of psychiatrists say their marriages are very good or good. These percentages are similar to those of physicians overall (85%).
Most psychiatrists (58%) spend up to 10 hours per week online for personal reasons; 82% spend this amount of time online each week for work.
It’s likely that the amount of time spent online for work will increase, given the pandemic-fueled surge in telemedicine. Yet even when their personal and professional Internet use are combined, psychiatrists, on average, spend far less time online than the nearly 7 hours per day of the average Internet user, according to recent data.
Findings from the latest happiness, wellness, and lifestyle survey are based on 12,339 Medscape member physicians practicing in the United States who completed an online survey conducted between Aug. 30 and Nov. 5, 2020.
A version of this article first appeared on Medscape.com.
Events of the past year have taken a huge toll on the happiness, wellness, and lifestyles of many, but especially those in the health care field, including psychiatrists.
The newly released Medscape Psychiatrist Lifestyle, Happiness & Burnout Report 2021 reveals how psychiatrists are coping with burnout and trying to maintain personal wellness, and how they view their workplaces and their futures amid the ongoing COVID-19 pandemic.
Before the pandemic hit in March 2020, 84% of psychiatrists who responded to the survey reported being happy outside of work, similar to the percentage (82%) of physicians overall.
But as the pandemic has worn on, feelings have shifted, and there are clear signs of strain on those in the health care field. Now, just over half (55%) of psychiatrists say they are happy outside of work, similar to the percentage (58%) of physicians overall.
Perhaps not surprising given the specific challenges of COVID-19, infectious disease physicians, pulmonologists, rheumatologists, and intensivists currently rank lowest in happiness outside of work.
Anxiety, depression, burnout
With the ongoing COVID-19 pandemic, more than three quarters (77%) of psychiatrists surveyed report experiencing some degree of anxiety about their future, the same percentage as for physicians overall.
This year, more psychiatrists reported being either burned out or burned out and depressed (41% vs. 35% last year). About two-thirds of psychiatrists said burnout has had at least a moderate impact on their lives; 5% consider the impact so severe that they are thinking of leaving medicine altogether.
The majority of burned-out psychiatrists (63%) said they felt that way even before the pandemic began; for about one-third (37%), burnout set in after the pandemic began.
in the workplace (39%) and spending too many hours at work (37%).
Psychiatrists’ top tactic to cope with burnout is talking with family or friends (53%), followed by isolating themselves from others (51%), sleeping (45%), and exercising (43%); 42% said they eat junk food to cope; 35% play music; and 25% drink alcohol.
Most psychiatrists (63%) suffering burnout and/or depression don’t plan on seeking professional help. About one-third are currently seeking help or plan to do so, the highest proportion among all specialties.
Considering their symptoms not severe enough (57%) and feeling that they could deal with the problem on their own (41%) are the top reasons for not seeking professional help; 36% said they were too busy to get help, and 17% said they didn’t want to risk disclosing a problem.
Fifteen percent of psychiatrists who are burned out, depressed, or both have contemplated suicide, and 2% have attempted suicide.
Striving for work-life balance
Work-life balance is the most pressing workplace issue for 45% of psychiatrists, and 44% would sacrifice some of their salary for better work-life balance. These figures are about the same for physicians overall.
Forty-seven percent of psychiatrists take 3-4 weeks of vacation each year; 16% take 5 or more weeks. In this there was no change from last year’s report.
About one-third (35%) of psychiatrists generally make time to focus on their own well-being, the same proportion as physicians overall.
About two-thirds (68%) of psychiatrists exercise two or more times per week. Half of psychiatrists said they are currently trying to lose weight; about one-quarter are trying to maintain their current weight.
About one-quarter (26%) of psychiatrists said they do not drink alcohol at all; 17% have five or more drinks per week.
Most psychiatrists are currently in a committed relationship, with 81% either married or living with a partner. Among psychiatrists who are married or living with a partner, 43% are with someone who also works in medicine. About 81% of psychiatrists say their marriages are very good or good. These percentages are similar to those of physicians overall (85%).
Most psychiatrists (58%) spend up to 10 hours per week online for personal reasons; 82% spend this amount of time online each week for work.
It’s likely that the amount of time spent online for work will increase, given the pandemic-fueled surge in telemedicine. Yet even when their personal and professional Internet use are combined, psychiatrists, on average, spend far less time online than the nearly 7 hours per day of the average Internet user, according to recent data.
Findings from the latest happiness, wellness, and lifestyle survey are based on 12,339 Medscape member physicians practicing in the United States who completed an online survey conducted between Aug. 30 and Nov. 5, 2020.
A version of this article first appeared on Medscape.com.
Events of the past year have taken a huge toll on the happiness, wellness, and lifestyles of many, but especially those in the health care field, including psychiatrists.
The newly released Medscape Psychiatrist Lifestyle, Happiness & Burnout Report 2021 reveals how psychiatrists are coping with burnout and trying to maintain personal wellness, and how they view their workplaces and their futures amid the ongoing COVID-19 pandemic.
Before the pandemic hit in March 2020, 84% of psychiatrists who responded to the survey reported being happy outside of work, similar to the percentage (82%) of physicians overall.
But as the pandemic has worn on, feelings have shifted, and there are clear signs of strain on those in the health care field. Now, just over half (55%) of psychiatrists say they are happy outside of work, similar to the percentage (58%) of physicians overall.
Perhaps not surprising given the specific challenges of COVID-19, infectious disease physicians, pulmonologists, rheumatologists, and intensivists currently rank lowest in happiness outside of work.
Anxiety, depression, burnout
With the ongoing COVID-19 pandemic, more than three quarters (77%) of psychiatrists surveyed report experiencing some degree of anxiety about their future, the same percentage as for physicians overall.
This year, more psychiatrists reported being either burned out or burned out and depressed (41% vs. 35% last year). About two-thirds of psychiatrists said burnout has had at least a moderate impact on their lives; 5% consider the impact so severe that they are thinking of leaving medicine altogether.
The majority of burned-out psychiatrists (63%) said they felt that way even before the pandemic began; for about one-third (37%), burnout set in after the pandemic began.
in the workplace (39%) and spending too many hours at work (37%).
Psychiatrists’ top tactic to cope with burnout is talking with family or friends (53%), followed by isolating themselves from others (51%), sleeping (45%), and exercising (43%); 42% said they eat junk food to cope; 35% play music; and 25% drink alcohol.
Most psychiatrists (63%) suffering burnout and/or depression don’t plan on seeking professional help. About one-third are currently seeking help or plan to do so, the highest proportion among all specialties.
Considering their symptoms not severe enough (57%) and feeling that they could deal with the problem on their own (41%) are the top reasons for not seeking professional help; 36% said they were too busy to get help, and 17% said they didn’t want to risk disclosing a problem.
Fifteen percent of psychiatrists who are burned out, depressed, or both have contemplated suicide, and 2% have attempted suicide.
Striving for work-life balance
Work-life balance is the most pressing workplace issue for 45% of psychiatrists, and 44% would sacrifice some of their salary for better work-life balance. These figures are about the same for physicians overall.
Forty-seven percent of psychiatrists take 3-4 weeks of vacation each year; 16% take 5 or more weeks. In this there was no change from last year’s report.
About one-third (35%) of psychiatrists generally make time to focus on their own well-being, the same proportion as physicians overall.
About two-thirds (68%) of psychiatrists exercise two or more times per week. Half of psychiatrists said they are currently trying to lose weight; about one-quarter are trying to maintain their current weight.
About one-quarter (26%) of psychiatrists said they do not drink alcohol at all; 17% have five or more drinks per week.
Most psychiatrists are currently in a committed relationship, with 81% either married or living with a partner. Among psychiatrists who are married or living with a partner, 43% are with someone who also works in medicine. About 81% of psychiatrists say their marriages are very good or good. These percentages are similar to those of physicians overall (85%).
Most psychiatrists (58%) spend up to 10 hours per week online for personal reasons; 82% spend this amount of time online each week for work.
It’s likely that the amount of time spent online for work will increase, given the pandemic-fueled surge in telemedicine. Yet even when their personal and professional Internet use are combined, psychiatrists, on average, spend far less time online than the nearly 7 hours per day of the average Internet user, according to recent data.
Findings from the latest happiness, wellness, and lifestyle survey are based on 12,339 Medscape member physicians practicing in the United States who completed an online survey conducted between Aug. 30 and Nov. 5, 2020.
A version of this article first appeared on Medscape.com.
New cases of child COVID-19 drop for fifth straight week
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
Variants spur new FDA guidance on COVID vaccines, tests, drugs
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
Pandemic puts patients with psoriatic disease off seeking medical help
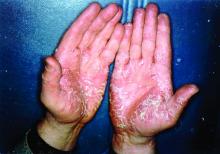
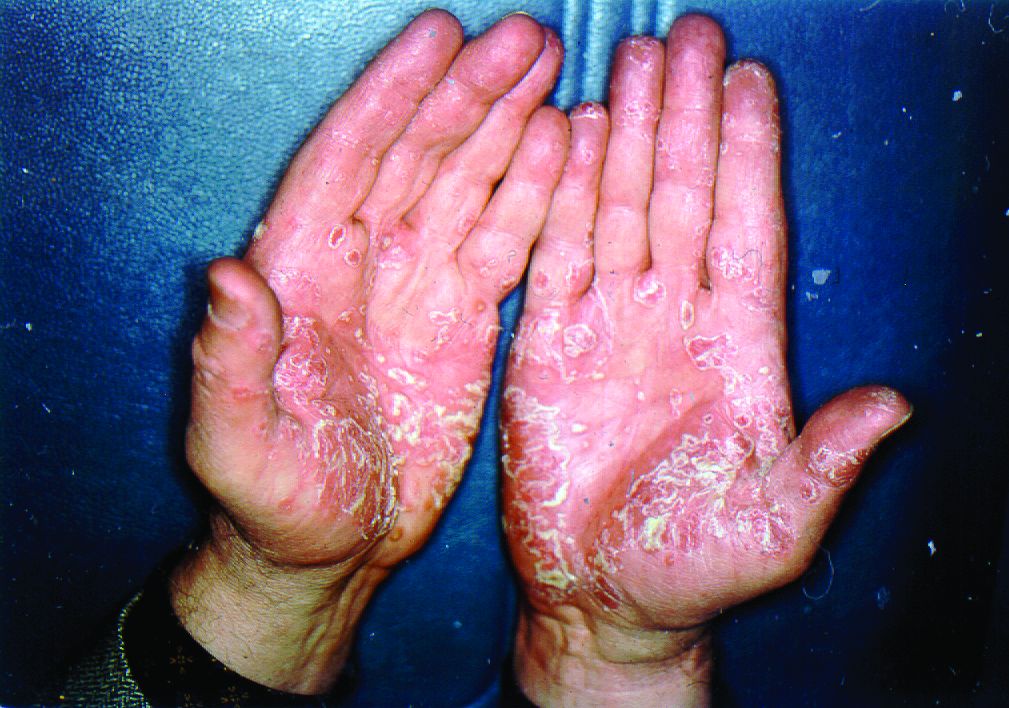
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
FROM CARC 2021
Another COVID-19 Adverse Effect: Routine Vaccinations Declined Steeply
COVID-19 upended everything last year, including routine health care such as older people receiving their pneumonia, pertussis, and shingles shots. Weekly vaccinations for Medicare beneficiaries aged > 65 years dropped in the first half of 2020 by up to 89% when compared with the first half of 2019. Researchers from the Centers for Disease Control and Prevention (CDC) studied weekly receipt of 4 vaccines: 13-valent pneumococcal conjugate vaccine, 23-valent pneumococcal polysaccharide vaccine, tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine, and recombinant zoster vaccine.
Before the national emergency was declared in March 2020, vaccination rates were consistently higher among Medicare beneficiaries than in the corresponding weeks in 2019. After the declaration, vaccination rates began dropping precipitously. In the first week alone, the rates were 25 to 62% lower than during the corresponding week in 2019.
Vaccination rates declined for all the vaccines studied, overall, and across all racial and ethnic groups. They began to recover gradually between late April and July, but were still lower in the last study week compared with 2019, except for PPSV23.
The emphasis naturally has been largely on COVID-19, but the other infections still present risks for older people. And now that states are beginning to lift restrictions, the researchers say, the likelihood of exposure to vaccine-preventable diseases is increasing. They urge health care providers to continue efforts to resolve disruptions in routine vaccinations, and to emphasize the safety of the vaccines.
Importantly, practitioners also need to explain to patients about expected reactions to some vaccines, and help them understand the potential overlap between vaccination reactions and symptoms of COVID-19.
COVID-19 upended everything last year, including routine health care such as older people receiving their pneumonia, pertussis, and shingles shots. Weekly vaccinations for Medicare beneficiaries aged > 65 years dropped in the first half of 2020 by up to 89% when compared with the first half of 2019. Researchers from the Centers for Disease Control and Prevention (CDC) studied weekly receipt of 4 vaccines: 13-valent pneumococcal conjugate vaccine, 23-valent pneumococcal polysaccharide vaccine, tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine, and recombinant zoster vaccine.
Before the national emergency was declared in March 2020, vaccination rates were consistently higher among Medicare beneficiaries than in the corresponding weeks in 2019. After the declaration, vaccination rates began dropping precipitously. In the first week alone, the rates were 25 to 62% lower than during the corresponding week in 2019.
Vaccination rates declined for all the vaccines studied, overall, and across all racial and ethnic groups. They began to recover gradually between late April and July, but were still lower in the last study week compared with 2019, except for PPSV23.
The emphasis naturally has been largely on COVID-19, but the other infections still present risks for older people. And now that states are beginning to lift restrictions, the researchers say, the likelihood of exposure to vaccine-preventable diseases is increasing. They urge health care providers to continue efforts to resolve disruptions in routine vaccinations, and to emphasize the safety of the vaccines.
Importantly, practitioners also need to explain to patients about expected reactions to some vaccines, and help them understand the potential overlap between vaccination reactions and symptoms of COVID-19.
COVID-19 upended everything last year, including routine health care such as older people receiving their pneumonia, pertussis, and shingles shots. Weekly vaccinations for Medicare beneficiaries aged > 65 years dropped in the first half of 2020 by up to 89% when compared with the first half of 2019. Researchers from the Centers for Disease Control and Prevention (CDC) studied weekly receipt of 4 vaccines: 13-valent pneumococcal conjugate vaccine, 23-valent pneumococcal polysaccharide vaccine, tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine, and recombinant zoster vaccine.
Before the national emergency was declared in March 2020, vaccination rates were consistently higher among Medicare beneficiaries than in the corresponding weeks in 2019. After the declaration, vaccination rates began dropping precipitously. In the first week alone, the rates were 25 to 62% lower than during the corresponding week in 2019.
Vaccination rates declined for all the vaccines studied, overall, and across all racial and ethnic groups. They began to recover gradually between late April and July, but were still lower in the last study week compared with 2019, except for PPSV23.
The emphasis naturally has been largely on COVID-19, but the other infections still present risks for older people. And now that states are beginning to lift restrictions, the researchers say, the likelihood of exposure to vaccine-preventable diseases is increasing. They urge health care providers to continue efforts to resolve disruptions in routine vaccinations, and to emphasize the safety of the vaccines.
Importantly, practitioners also need to explain to patients about expected reactions to some vaccines, and help them understand the potential overlap between vaccination reactions and symptoms of COVID-19.
Native Americans Embrace the COVID-19 Vaccines ‘to Protect the Community and Preserve the Culture’
A large portion of the general American public is still feeling wary of the COVID-19 vaccines. In a recent Pew Research survey, about 40% of respondents said they “would not get the vaccine” (although about half of that group allowed for flexibility and said they might when more information becomes available). In the Native American community, however, it’s a different story.
According to the Seattle-based Urban Indian Health Institute (UIHI), one of 12 Tribal Epidemiology Centers in the US, 75% of the 1,435 American Indian/Alaska Native participants in its National COVID-19 Vaccination Survey were willing to receive the vaccine. A big reason is that the emphasis in Native American communities has been on “we,” rather than “me.” Even though the respondents might feel reluctant due to “historical and current abuse from healthcare and government institutions,” the UIHI says, they ultimately felt the heavy cost of COVID-19 for them, their friends, families, and community outweighed potential risks.
Where there is hesitancy, it’s often due to concern about the exceptional speed of the clinical trials assessing the vaccines. Of those who were willing to get vaccinated, two-thirds were confident that the vaccines had been adequately tested for safety and effectiveness among Native people, in contrast to 31% of the “unwilling.” Seventy-five percent of the unwilling perceive the vaccine as dangerous to their health.
The willingness to receive a COVID-19 vaccine varied by Indian Health Services (IHS) region, with California Area having the lowest proportion (64%) and Albuquerque Area the highest (86%). The survey also asked about perceptions of COVID-19: 75% of those unwilling to get vaccinated felt they were at risk of being infected with COVID-19, compared with 85% of those willing to get a vaccine. Interestingly, though, the majority in the “unwilling” group takes the infection seriously and acknowledges the spread of COVID-19 in the state where they live.
The primary motivation for getting vaccinated, UIHI says, is a “strong sense of responsibility to protect the Native community and preserve cultural ways”—74% of all participants supported this concept. That’s a unique difference when compared with other communities of color, UIHI says. By comparison, only 36% of black communities and 53% of Latinx communities have been found to perceive vaccination as a community responsibility. The finding illustrates the importance of community in Native American culture—although that also differs within the 2 groups surveyed: Of those willing to get vaccinated, 87% believe it’s their communal responsibility, whereas 66% of the unwilling believe it’s an individual choice.
Tribal campaigns that emphasize the good individuals can do for the tribe appear to appeal. In an interview with NBC News, Abigail Echo-Hawk, director of UIHI, said the Seattle Indian Health Board, for example, went from about 7,000 calls a month about the vaccine to nearly 5,000 on 1 day.
But it isn’t just the appeal to communal feeling that spurs participation—it’s also the knowledge that protecting people protects the culture. The Cherokee Tribe, for instance, has been mobilizing to get as many people vaccinated as possible, starting with some of the “most endangered members of the tribe”: those who still speak Cherokee. “We put Cherokee-fluent speakers, most of whom are elders, at the front of the line,” Principal Chief Chuck Hoskin Jr., leader of the Cherokee Nation, told NBC News. The tribe was able to put its roughly 22,000 Cherokee speakers at the top of the list because it answers to the IHS, not the state of Oklahoma, which has people aged < 65 years in Phase 4 of its vaccine rollout.
Appealing to the reverence for Native American culture and tradition is a wise move. Not only because it protects people, but also because vaccinating elders and fluent speakers may reassure others. “When fluent speakers got the vaccine, I think that helped people’s anxiety subside,” Hoskins said. “And I think people felt sort of a renewed obligation to try and protect the culture by being vaccinated.”
Many of the survey respondents viewed getting vaccinated as an act of love, protecting others. One participant planned to get the vaccine to “protect the knowledge keepers; ensuring knowledge is passed to our future generations.”
A majority of UIHI survey respondents who were unwilling to get vaccinated indicated they would be willing at some point in the future—often at least one year from now. This, UIHI says, “may suggest with proper messaging and education on the efficacy and safety of vaccine, hesitancy can be addressed.”
That could depend on who’s delivering the message. The greatest difference between the 2 groups, the UIHI says, was that those who were willing to take vaccines trusted government organizations (ie,Centers for Disease Control and prevention, Food and Drug Administration, and National Institutes of Health) and their regular doctor. Those unwilling to get vaccinated had the highest trust in Urban Indian health clinics, their regular doctor, and Tribal clinics, respectively. The biggest divide? Almost all of the willing group “mostly” or “completely” trusts Dr. Anthony Fauci and the scientists working on the vaccines. Most of the unwilling group does not.
Factors such as convenience, cost, and advice all entered into the respondents’ decision making. But one of the UIHI’s key recommendations is to continue to draw connections between getting vaccinated and the preservation of Native traditions, cultural pride, and love and respect for family, elders, and the broader Native community. Elders, Native community leaders, and Tribal leaders were among the top ambassadors for getting the message out, the UIHI survey found.
Ultimately, each individual decides who to trust. One of the survey respondents said, “Although the US government should have and could have done so much more for all people living here, if we turn down the vaccine, we not only risk our lives and the lives of others…we undermine all the struggles our tribes have gone through to keep our people safe. Even when the US government has directly worked against our tribal checkpoints and safety efforts. To not get vaccinated, is to say the US government’s failure to protect the people is right, and our tribal efforts, wisdom, and courage is wrong.”
A large portion of the general American public is still feeling wary of the COVID-19 vaccines. In a recent Pew Research survey, about 40% of respondents said they “would not get the vaccine” (although about half of that group allowed for flexibility and said they might when more information becomes available). In the Native American community, however, it’s a different story.
According to the Seattle-based Urban Indian Health Institute (UIHI), one of 12 Tribal Epidemiology Centers in the US, 75% of the 1,435 American Indian/Alaska Native participants in its National COVID-19 Vaccination Survey were willing to receive the vaccine. A big reason is that the emphasis in Native American communities has been on “we,” rather than “me.” Even though the respondents might feel reluctant due to “historical and current abuse from healthcare and government institutions,” the UIHI says, they ultimately felt the heavy cost of COVID-19 for them, their friends, families, and community outweighed potential risks.
Where there is hesitancy, it’s often due to concern about the exceptional speed of the clinical trials assessing the vaccines. Of those who were willing to get vaccinated, two-thirds were confident that the vaccines had been adequately tested for safety and effectiveness among Native people, in contrast to 31% of the “unwilling.” Seventy-five percent of the unwilling perceive the vaccine as dangerous to their health.
The willingness to receive a COVID-19 vaccine varied by Indian Health Services (IHS) region, with California Area having the lowest proportion (64%) and Albuquerque Area the highest (86%). The survey also asked about perceptions of COVID-19: 75% of those unwilling to get vaccinated felt they were at risk of being infected with COVID-19, compared with 85% of those willing to get a vaccine. Interestingly, though, the majority in the “unwilling” group takes the infection seriously and acknowledges the spread of COVID-19 in the state where they live.
The primary motivation for getting vaccinated, UIHI says, is a “strong sense of responsibility to protect the Native community and preserve cultural ways”—74% of all participants supported this concept. That’s a unique difference when compared with other communities of color, UIHI says. By comparison, only 36% of black communities and 53% of Latinx communities have been found to perceive vaccination as a community responsibility. The finding illustrates the importance of community in Native American culture—although that also differs within the 2 groups surveyed: Of those willing to get vaccinated, 87% believe it’s their communal responsibility, whereas 66% of the unwilling believe it’s an individual choice.
Tribal campaigns that emphasize the good individuals can do for the tribe appear to appeal. In an interview with NBC News, Abigail Echo-Hawk, director of UIHI, said the Seattle Indian Health Board, for example, went from about 7,000 calls a month about the vaccine to nearly 5,000 on 1 day.
But it isn’t just the appeal to communal feeling that spurs participation—it’s also the knowledge that protecting people protects the culture. The Cherokee Tribe, for instance, has been mobilizing to get as many people vaccinated as possible, starting with some of the “most endangered members of the tribe”: those who still speak Cherokee. “We put Cherokee-fluent speakers, most of whom are elders, at the front of the line,” Principal Chief Chuck Hoskin Jr., leader of the Cherokee Nation, told NBC News. The tribe was able to put its roughly 22,000 Cherokee speakers at the top of the list because it answers to the IHS, not the state of Oklahoma, which has people aged < 65 years in Phase 4 of its vaccine rollout.
Appealing to the reverence for Native American culture and tradition is a wise move. Not only because it protects people, but also because vaccinating elders and fluent speakers may reassure others. “When fluent speakers got the vaccine, I think that helped people’s anxiety subside,” Hoskins said. “And I think people felt sort of a renewed obligation to try and protect the culture by being vaccinated.”
Many of the survey respondents viewed getting vaccinated as an act of love, protecting others. One participant planned to get the vaccine to “protect the knowledge keepers; ensuring knowledge is passed to our future generations.”
A majority of UIHI survey respondents who were unwilling to get vaccinated indicated they would be willing at some point in the future—often at least one year from now. This, UIHI says, “may suggest with proper messaging and education on the efficacy and safety of vaccine, hesitancy can be addressed.”
That could depend on who’s delivering the message. The greatest difference between the 2 groups, the UIHI says, was that those who were willing to take vaccines trusted government organizations (ie,Centers for Disease Control and prevention, Food and Drug Administration, and National Institutes of Health) and their regular doctor. Those unwilling to get vaccinated had the highest trust in Urban Indian health clinics, their regular doctor, and Tribal clinics, respectively. The biggest divide? Almost all of the willing group “mostly” or “completely” trusts Dr. Anthony Fauci and the scientists working on the vaccines. Most of the unwilling group does not.
Factors such as convenience, cost, and advice all entered into the respondents’ decision making. But one of the UIHI’s key recommendations is to continue to draw connections between getting vaccinated and the preservation of Native traditions, cultural pride, and love and respect for family, elders, and the broader Native community. Elders, Native community leaders, and Tribal leaders were among the top ambassadors for getting the message out, the UIHI survey found.
Ultimately, each individual decides who to trust. One of the survey respondents said, “Although the US government should have and could have done so much more for all people living here, if we turn down the vaccine, we not only risk our lives and the lives of others…we undermine all the struggles our tribes have gone through to keep our people safe. Even when the US government has directly worked against our tribal checkpoints and safety efforts. To not get vaccinated, is to say the US government’s failure to protect the people is right, and our tribal efforts, wisdom, and courage is wrong.”
A large portion of the general American public is still feeling wary of the COVID-19 vaccines. In a recent Pew Research survey, about 40% of respondents said they “would not get the vaccine” (although about half of that group allowed for flexibility and said they might when more information becomes available). In the Native American community, however, it’s a different story.
According to the Seattle-based Urban Indian Health Institute (UIHI), one of 12 Tribal Epidemiology Centers in the US, 75% of the 1,435 American Indian/Alaska Native participants in its National COVID-19 Vaccination Survey were willing to receive the vaccine. A big reason is that the emphasis in Native American communities has been on “we,” rather than “me.” Even though the respondents might feel reluctant due to “historical and current abuse from healthcare and government institutions,” the UIHI says, they ultimately felt the heavy cost of COVID-19 for them, their friends, families, and community outweighed potential risks.
Where there is hesitancy, it’s often due to concern about the exceptional speed of the clinical trials assessing the vaccines. Of those who were willing to get vaccinated, two-thirds were confident that the vaccines had been adequately tested for safety and effectiveness among Native people, in contrast to 31% of the “unwilling.” Seventy-five percent of the unwilling perceive the vaccine as dangerous to their health.
The willingness to receive a COVID-19 vaccine varied by Indian Health Services (IHS) region, with California Area having the lowest proportion (64%) and Albuquerque Area the highest (86%). The survey also asked about perceptions of COVID-19: 75% of those unwilling to get vaccinated felt they were at risk of being infected with COVID-19, compared with 85% of those willing to get a vaccine. Interestingly, though, the majority in the “unwilling” group takes the infection seriously and acknowledges the spread of COVID-19 in the state where they live.
The primary motivation for getting vaccinated, UIHI says, is a “strong sense of responsibility to protect the Native community and preserve cultural ways”—74% of all participants supported this concept. That’s a unique difference when compared with other communities of color, UIHI says. By comparison, only 36% of black communities and 53% of Latinx communities have been found to perceive vaccination as a community responsibility. The finding illustrates the importance of community in Native American culture—although that also differs within the 2 groups surveyed: Of those willing to get vaccinated, 87% believe it’s their communal responsibility, whereas 66% of the unwilling believe it’s an individual choice.
Tribal campaigns that emphasize the good individuals can do for the tribe appear to appeal. In an interview with NBC News, Abigail Echo-Hawk, director of UIHI, said the Seattle Indian Health Board, for example, went from about 7,000 calls a month about the vaccine to nearly 5,000 on 1 day.
But it isn’t just the appeal to communal feeling that spurs participation—it’s also the knowledge that protecting people protects the culture. The Cherokee Tribe, for instance, has been mobilizing to get as many people vaccinated as possible, starting with some of the “most endangered members of the tribe”: those who still speak Cherokee. “We put Cherokee-fluent speakers, most of whom are elders, at the front of the line,” Principal Chief Chuck Hoskin Jr., leader of the Cherokee Nation, told NBC News. The tribe was able to put its roughly 22,000 Cherokee speakers at the top of the list because it answers to the IHS, not the state of Oklahoma, which has people aged < 65 years in Phase 4 of its vaccine rollout.
Appealing to the reverence for Native American culture and tradition is a wise move. Not only because it protects people, but also because vaccinating elders and fluent speakers may reassure others. “When fluent speakers got the vaccine, I think that helped people’s anxiety subside,” Hoskins said. “And I think people felt sort of a renewed obligation to try and protect the culture by being vaccinated.”
Many of the survey respondents viewed getting vaccinated as an act of love, protecting others. One participant planned to get the vaccine to “protect the knowledge keepers; ensuring knowledge is passed to our future generations.”
A majority of UIHI survey respondents who were unwilling to get vaccinated indicated they would be willing at some point in the future—often at least one year from now. This, UIHI says, “may suggest with proper messaging and education on the efficacy and safety of vaccine, hesitancy can be addressed.”
That could depend on who’s delivering the message. The greatest difference between the 2 groups, the UIHI says, was that those who were willing to take vaccines trusted government organizations (ie,Centers for Disease Control and prevention, Food and Drug Administration, and National Institutes of Health) and their regular doctor. Those unwilling to get vaccinated had the highest trust in Urban Indian health clinics, their regular doctor, and Tribal clinics, respectively. The biggest divide? Almost all of the willing group “mostly” or “completely” trusts Dr. Anthony Fauci and the scientists working on the vaccines. Most of the unwilling group does not.
Factors such as convenience, cost, and advice all entered into the respondents’ decision making. But one of the UIHI’s key recommendations is to continue to draw connections between getting vaccinated and the preservation of Native traditions, cultural pride, and love and respect for family, elders, and the broader Native community. Elders, Native community leaders, and Tribal leaders were among the top ambassadors for getting the message out, the UIHI survey found.
Ultimately, each individual decides who to trust. One of the survey respondents said, “Although the US government should have and could have done so much more for all people living here, if we turn down the vaccine, we not only risk our lives and the lives of others…we undermine all the struggles our tribes have gone through to keep our people safe. Even when the US government has directly worked against our tribal checkpoints and safety efforts. To not get vaccinated, is to say the US government’s failure to protect the people is right, and our tribal efforts, wisdom, and courage is wrong.”
Oxford launches COVID-19 vaccine study in children
Oxford University is starting a COVID-19 vaccine study with children and young adults aged between 6 and 17 years.
At Oxford and three partner sites in London, Southampton, and Bristol, the phase 2 clinical trial will test whether kids and teens have a good immune response to the AstraZeneca vaccine. Previous trials have shown that the shot is safe in children.
“While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination,” Andrew Pollard, PhD, the chief investigator for the trial and a professor of pediatric infection and immunity at Oxford, said in a statement.
The new trial will enroll 300 volunteers, with up to 240 receiving the vaccine. The control group will receive a meningitis vaccine, which is safe in children and produces similar side effects to the COVID-19 vaccine, such as a sore arm.
COVID-19 vaccine trials have included children over age 12, so this marks the youngest group to be tested so far. Pfizer, Moderna, and Janssen have announced plans to start trials in younger children this spring, according to the Washington Post. Widespread vaccination in children likely won’t occur until 2022, the newspaper reported.
The trial launched on Feb. 12, and the first vaccinations are expected by the end of the month. Parents can visit Oxford’s COVID-19 Vaccine Trial website to sign their children up for the study.
“This study will play an important role in helping to protect children in the future,” Grace Li, a pediatric clinical research fellow for the Oxford Vaccine Group, said in the statement.
“We’ve already seen that the vaccine is safe and effective in adults, and our understanding of how children are affected by the coronavirus continues to evolve,” she said.
A version of this article first appeared on WebMD.com.
Oxford University is starting a COVID-19 vaccine study with children and young adults aged between 6 and 17 years.
At Oxford and three partner sites in London, Southampton, and Bristol, the phase 2 clinical trial will test whether kids and teens have a good immune response to the AstraZeneca vaccine. Previous trials have shown that the shot is safe in children.
“While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination,” Andrew Pollard, PhD, the chief investigator for the trial and a professor of pediatric infection and immunity at Oxford, said in a statement.
The new trial will enroll 300 volunteers, with up to 240 receiving the vaccine. The control group will receive a meningitis vaccine, which is safe in children and produces similar side effects to the COVID-19 vaccine, such as a sore arm.
COVID-19 vaccine trials have included children over age 12, so this marks the youngest group to be tested so far. Pfizer, Moderna, and Janssen have announced plans to start trials in younger children this spring, according to the Washington Post. Widespread vaccination in children likely won’t occur until 2022, the newspaper reported.
The trial launched on Feb. 12, and the first vaccinations are expected by the end of the month. Parents can visit Oxford’s COVID-19 Vaccine Trial website to sign their children up for the study.
“This study will play an important role in helping to protect children in the future,” Grace Li, a pediatric clinical research fellow for the Oxford Vaccine Group, said in the statement.
“We’ve already seen that the vaccine is safe and effective in adults, and our understanding of how children are affected by the coronavirus continues to evolve,” she said.
A version of this article first appeared on WebMD.com.
Oxford University is starting a COVID-19 vaccine study with children and young adults aged between 6 and 17 years.
At Oxford and three partner sites in London, Southampton, and Bristol, the phase 2 clinical trial will test whether kids and teens have a good immune response to the AstraZeneca vaccine. Previous trials have shown that the shot is safe in children.
“While most children are relatively unaffected by coronavirus and are unlikely to become unwell with the infection, it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination,” Andrew Pollard, PhD, the chief investigator for the trial and a professor of pediatric infection and immunity at Oxford, said in a statement.
The new trial will enroll 300 volunteers, with up to 240 receiving the vaccine. The control group will receive a meningitis vaccine, which is safe in children and produces similar side effects to the COVID-19 vaccine, such as a sore arm.
COVID-19 vaccine trials have included children over age 12, so this marks the youngest group to be tested so far. Pfizer, Moderna, and Janssen have announced plans to start trials in younger children this spring, according to the Washington Post. Widespread vaccination in children likely won’t occur until 2022, the newspaper reported.
The trial launched on Feb. 12, and the first vaccinations are expected by the end of the month. Parents can visit Oxford’s COVID-19 Vaccine Trial website to sign their children up for the study.
“This study will play an important role in helping to protect children in the future,” Grace Li, a pediatric clinical research fellow for the Oxford Vaccine Group, said in the statement.
“We’ve already seen that the vaccine is safe and effective in adults, and our understanding of how children are affected by the coronavirus continues to evolve,” she said.
A version of this article first appeared on WebMD.com.
Six-month follow-up shows continuing morbidity for COVID-19 survivors
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
FROM CCC50
COVID-19 vaccines: New candidates & answers to commonly asked questions
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
REFERENCES
- CDC. COVID-19 vaccination. Accessed February 22, 2021.
- CDC. COVID data tracker. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed February 22, 2021.
- Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed February 22, 2021.
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morbid Mortal Wkly Rep. ePub: February 19, 2021. Accessed February 22, 2021.
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. Accessed February 25, 2021.
Organ transplant patient dies after receiving COVID-19–infected lungs
Doctors say a woman in Michigan contracted COVID-19 and died last fall 2 months after receiving a tainted double-lung transplant from a donor who turned out to harbor the virus that causes the disease – despite showing no signs of illness and initially testing negative.
Officials at the University of Michigan Medical School suggested it may be the first proven case of COVID-19 in the U.S. in which the virus was transmitted via an organ transplant. A surgeon who handled the donor lungs was also infected with the virus and fell ill but later recovered.
The incident appears to be isolated – the only confirmed case among nearly 40,000 transplants in 2020. But it has led to calls for more thorough testing of lung transplant donors, with samples taken from deep within the donor lungs as well as the nose and throat, said Dr. Daniel Kaul, director of Michigan Medicine’s transplant infectious disease service.
“We would absolutely not have used the lungs if we’d had a positive COVID-19 test,” said Dr. Kaul, who coauthored a report about the case in the American Journal of Transplantation.
The virus was transmitted when lungs from a woman from the Upper Midwest, who died after suffering a severe brain injury in a car accident, were transplanted into a woman with chronic obstructive lung disease at University Hospital in Ann Arbor. The nose and throat samples routinely collected from both organ donors and recipients tested negative for SARS-CoV-2, the virus that causes covid.
“All the screening that we normally do and are able to do, we did,” Dr. Kaul said.
Three days after the operation, however, the recipient spiked a fever; her blood pressure fell and her breathing became labored. Imaging showed signs of lung infection.
As her condition worsened, the patient developed septic shock and heart function problems. Doctors decided to test for SARS-CoV-2, Dr. Kaul said. Samples from her new lungs came back positive.
Suspicious about the origin of the infection, doctors returned to samples from the transplant donor. A molecular test of a swab from the donor’s nose and throat, taken 48 hours after her lungs were procured, had been negative for SARS-Cov-2. The donor’s family told doctors she had no history of recent travel or COVID-19 symptoms and no known exposure to anyone with the disease.
But doctors had kept a sample of fluid washed from deep within the donor lungs. When they tested that fluid, it was positive for the virus. Four days after the transplant, the surgeon who handled the donor lungs and performed the surgery tested positive, too. Genetic screening revealed that the transplant recipient and the surgeon had been infected by the donor. Ten other members of the transplant team tested negative for the virus.
The transplant recipient deteriorated rapidly, developing multisystem organ failure. Doctors tried known treatments for COVID-19, including remdesivir, a newly approved drug, and convalescent blood plasma from people previously infected with the disease. Eventually, she was placed on the last-resort option of ECMO, or extracorporeal membrane oxygenation, to no avail. Life support was withdrawn, and she died 61 days after the transplant.
Dr. Kaul called the incident “a tragic case.”
While the Michigan case marks the first confirmed incident in the U.S. of transmission through a transplant, others have been suspected. A recent Centers for Disease Control and Prevention report reviewed eight possible cases of what’s known as donor-derived infection that occurred last spring, but concluded the most likely source of transmission of the COVID-19 virus in those cases was in a community or health care setting.
Before this incident, it was not clear whether the COVID-19 virus could be transmitted through solid organ transplants, though it’s well documented with other respiratory viruses. Donor transmission of H1N1 2009 pandemic influenza has been detected almost exclusively in lung transplant recipients, Dr. Kaul noted.
While it’s not surprising that SARS-CoV-2 can be transmitted through infected lungs, it remains uncertain whether other organs affected by COVID-19 – hearts, livers and kidneys, for instance – can transmit the virus, too.
“It seems for non-lung donors that it may be very difficult to transmit COVID-19, even if the donor has COVID-19,” Dr. Kaul said.
Organ donors have been tested routinely for SARS-CoV-2 during the pandemic, though it’s not required by the Organ Procurement and Transplantation Network, or OPTN, which oversees transplants in the U.S. But the Michigan case underscores the need for more extensive sampling before transplant, especially in areas with high rates of covid transmission, Dr. Kaul said.
When it comes to lungs, that means making sure to test samples from the donor’s lower respiratory tract, as well as from the nose and throat. Obtaining and testing such samples from donors can be difficult to carry out in a timely fashion. There’s also the risk of introducing infection into the donated lungs, Dr. Kaul said.
Because no organs other than lungs were used, the Michigan case doesn’t provide insight into testing protocols for other organs.
Overall, viral transmissions from organ donors to recipients remain rare, occurring in fewer than 1% of transplant recipients, research shows. The medical risks facing ailing patients who reject a donor organ are generally far higher, said Dr. David Klassen, chief medical officer with the United Network for Organ Sharing, the federal contractor that runs the OPTN.
“The risks of turning down transplants are catastrophic,” he said. “I don’t think patients should be afraid of the transplant process.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Doctors say a woman in Michigan contracted COVID-19 and died last fall 2 months after receiving a tainted double-lung transplant from a donor who turned out to harbor the virus that causes the disease – despite showing no signs of illness and initially testing negative.
Officials at the University of Michigan Medical School suggested it may be the first proven case of COVID-19 in the U.S. in which the virus was transmitted via an organ transplant. A surgeon who handled the donor lungs was also infected with the virus and fell ill but later recovered.
The incident appears to be isolated – the only confirmed case among nearly 40,000 transplants in 2020. But it has led to calls for more thorough testing of lung transplant donors, with samples taken from deep within the donor lungs as well as the nose and throat, said Dr. Daniel Kaul, director of Michigan Medicine’s transplant infectious disease service.
“We would absolutely not have used the lungs if we’d had a positive COVID-19 test,” said Dr. Kaul, who coauthored a report about the case in the American Journal of Transplantation.
The virus was transmitted when lungs from a woman from the Upper Midwest, who died after suffering a severe brain injury in a car accident, were transplanted into a woman with chronic obstructive lung disease at University Hospital in Ann Arbor. The nose and throat samples routinely collected from both organ donors and recipients tested negative for SARS-CoV-2, the virus that causes covid.
“All the screening that we normally do and are able to do, we did,” Dr. Kaul said.
Three days after the operation, however, the recipient spiked a fever; her blood pressure fell and her breathing became labored. Imaging showed signs of lung infection.
As her condition worsened, the patient developed septic shock and heart function problems. Doctors decided to test for SARS-CoV-2, Dr. Kaul said. Samples from her new lungs came back positive.
Suspicious about the origin of the infection, doctors returned to samples from the transplant donor. A molecular test of a swab from the donor’s nose and throat, taken 48 hours after her lungs were procured, had been negative for SARS-Cov-2. The donor’s family told doctors she had no history of recent travel or COVID-19 symptoms and no known exposure to anyone with the disease.
But doctors had kept a sample of fluid washed from deep within the donor lungs. When they tested that fluid, it was positive for the virus. Four days after the transplant, the surgeon who handled the donor lungs and performed the surgery tested positive, too. Genetic screening revealed that the transplant recipient and the surgeon had been infected by the donor. Ten other members of the transplant team tested negative for the virus.
The transplant recipient deteriorated rapidly, developing multisystem organ failure. Doctors tried known treatments for COVID-19, including remdesivir, a newly approved drug, and convalescent blood plasma from people previously infected with the disease. Eventually, she was placed on the last-resort option of ECMO, or extracorporeal membrane oxygenation, to no avail. Life support was withdrawn, and she died 61 days after the transplant.
Dr. Kaul called the incident “a tragic case.”
While the Michigan case marks the first confirmed incident in the U.S. of transmission through a transplant, others have been suspected. A recent Centers for Disease Control and Prevention report reviewed eight possible cases of what’s known as donor-derived infection that occurred last spring, but concluded the most likely source of transmission of the COVID-19 virus in those cases was in a community or health care setting.
Before this incident, it was not clear whether the COVID-19 virus could be transmitted through solid organ transplants, though it’s well documented with other respiratory viruses. Donor transmission of H1N1 2009 pandemic influenza has been detected almost exclusively in lung transplant recipients, Dr. Kaul noted.
While it’s not surprising that SARS-CoV-2 can be transmitted through infected lungs, it remains uncertain whether other organs affected by COVID-19 – hearts, livers and kidneys, for instance – can transmit the virus, too.
“It seems for non-lung donors that it may be very difficult to transmit COVID-19, even if the donor has COVID-19,” Dr. Kaul said.
Organ donors have been tested routinely for SARS-CoV-2 during the pandemic, though it’s not required by the Organ Procurement and Transplantation Network, or OPTN, which oversees transplants in the U.S. But the Michigan case underscores the need for more extensive sampling before transplant, especially in areas with high rates of covid transmission, Dr. Kaul said.
When it comes to lungs, that means making sure to test samples from the donor’s lower respiratory tract, as well as from the nose and throat. Obtaining and testing such samples from donors can be difficult to carry out in a timely fashion. There’s also the risk of introducing infection into the donated lungs, Dr. Kaul said.
Because no organs other than lungs were used, the Michigan case doesn’t provide insight into testing protocols for other organs.
Overall, viral transmissions from organ donors to recipients remain rare, occurring in fewer than 1% of transplant recipients, research shows. The medical risks facing ailing patients who reject a donor organ are generally far higher, said Dr. David Klassen, chief medical officer with the United Network for Organ Sharing, the federal contractor that runs the OPTN.
“The risks of turning down transplants are catastrophic,” he said. “I don’t think patients should be afraid of the transplant process.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Doctors say a woman in Michigan contracted COVID-19 and died last fall 2 months after receiving a tainted double-lung transplant from a donor who turned out to harbor the virus that causes the disease – despite showing no signs of illness and initially testing negative.
Officials at the University of Michigan Medical School suggested it may be the first proven case of COVID-19 in the U.S. in which the virus was transmitted via an organ transplant. A surgeon who handled the donor lungs was also infected with the virus and fell ill but later recovered.
The incident appears to be isolated – the only confirmed case among nearly 40,000 transplants in 2020. But it has led to calls for more thorough testing of lung transplant donors, with samples taken from deep within the donor lungs as well as the nose and throat, said Dr. Daniel Kaul, director of Michigan Medicine’s transplant infectious disease service.
“We would absolutely not have used the lungs if we’d had a positive COVID-19 test,” said Dr. Kaul, who coauthored a report about the case in the American Journal of Transplantation.
The virus was transmitted when lungs from a woman from the Upper Midwest, who died after suffering a severe brain injury in a car accident, were transplanted into a woman with chronic obstructive lung disease at University Hospital in Ann Arbor. The nose and throat samples routinely collected from both organ donors and recipients tested negative for SARS-CoV-2, the virus that causes covid.
“All the screening that we normally do and are able to do, we did,” Dr. Kaul said.
Three days after the operation, however, the recipient spiked a fever; her blood pressure fell and her breathing became labored. Imaging showed signs of lung infection.
As her condition worsened, the patient developed septic shock and heart function problems. Doctors decided to test for SARS-CoV-2, Dr. Kaul said. Samples from her new lungs came back positive.
Suspicious about the origin of the infection, doctors returned to samples from the transplant donor. A molecular test of a swab from the donor’s nose and throat, taken 48 hours after her lungs were procured, had been negative for SARS-Cov-2. The donor’s family told doctors she had no history of recent travel or COVID-19 symptoms and no known exposure to anyone with the disease.
But doctors had kept a sample of fluid washed from deep within the donor lungs. When they tested that fluid, it was positive for the virus. Four days after the transplant, the surgeon who handled the donor lungs and performed the surgery tested positive, too. Genetic screening revealed that the transplant recipient and the surgeon had been infected by the donor. Ten other members of the transplant team tested negative for the virus.
The transplant recipient deteriorated rapidly, developing multisystem organ failure. Doctors tried known treatments for COVID-19, including remdesivir, a newly approved drug, and convalescent blood plasma from people previously infected with the disease. Eventually, she was placed on the last-resort option of ECMO, or extracorporeal membrane oxygenation, to no avail. Life support was withdrawn, and she died 61 days after the transplant.
Dr. Kaul called the incident “a tragic case.”
While the Michigan case marks the first confirmed incident in the U.S. of transmission through a transplant, others have been suspected. A recent Centers for Disease Control and Prevention report reviewed eight possible cases of what’s known as donor-derived infection that occurred last spring, but concluded the most likely source of transmission of the COVID-19 virus in those cases was in a community or health care setting.
Before this incident, it was not clear whether the COVID-19 virus could be transmitted through solid organ transplants, though it’s well documented with other respiratory viruses. Donor transmission of H1N1 2009 pandemic influenza has been detected almost exclusively in lung transplant recipients, Dr. Kaul noted.
While it’s not surprising that SARS-CoV-2 can be transmitted through infected lungs, it remains uncertain whether other organs affected by COVID-19 – hearts, livers and kidneys, for instance – can transmit the virus, too.
“It seems for non-lung donors that it may be very difficult to transmit COVID-19, even if the donor has COVID-19,” Dr. Kaul said.
Organ donors have been tested routinely for SARS-CoV-2 during the pandemic, though it’s not required by the Organ Procurement and Transplantation Network, or OPTN, which oversees transplants in the U.S. But the Michigan case underscores the need for more extensive sampling before transplant, especially in areas with high rates of covid transmission, Dr. Kaul said.
When it comes to lungs, that means making sure to test samples from the donor’s lower respiratory tract, as well as from the nose and throat. Obtaining and testing such samples from donors can be difficult to carry out in a timely fashion. There’s also the risk of introducing infection into the donated lungs, Dr. Kaul said.
Because no organs other than lungs were used, the Michigan case doesn’t provide insight into testing protocols for other organs.
Overall, viral transmissions from organ donors to recipients remain rare, occurring in fewer than 1% of transplant recipients, research shows. The medical risks facing ailing patients who reject a donor organ are generally far higher, said Dr. David Klassen, chief medical officer with the United Network for Organ Sharing, the federal contractor that runs the OPTN.
“The risks of turning down transplants are catastrophic,” he said. “I don’t think patients should be afraid of the transplant process.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.