User login
In Case You Missed It: COVID
New-onset arrhythmias low in COVID-19 and flu
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Variant found in NYC, Northeast
, according to CNN.
The variant, called B.1.526, has appeared in diverse neighborhoods in New York City and is “scattered in the Northeast,” the researchers said.
“We observed a steady increase in the detection rate from late December to mid-February, with an alarming rise to 12.7% in the past two weeks,” researchers from Columbia University Medical Center wrote in a report, which was published as a preprint Feb. 25.
On Feb. 22, the team released another preprint about the B.1.1.7 and B.1.351 variants first identified in the United Kingdom and South Africa, respectively, which also mentions the B.1.526 variant in the U.S. Neither report has been peer reviewed.
Viruses mutate often, and several coronavirus variants have been identified and followed during the pandemic. Not all mutations are significant or are necessarily more contagious or dangerous. Researchers have been tracking the B.1.526 variant in the U.S. to find out if there are significant mutations that could be a cause for concern.
In the most recent preprints, the variant appears to have the same mutation found in B.1.351, called E484K, which may allow the virus to evade vaccines and the body’s natural immune response. The E484K mutation has shown up in at least 59 lines of the coronavirus, the research team said. That means the virus is evolving independently across the country and world, which could give the virus an advantage.
“A concern is that it might be beginning to overtake other strains, just like the U.K. and South African variants,” David Ho, MD, the lead study author and director of the Aaron Diamond AIDS Research Center at Columbia, told CNN.
“However, we don’t have enough data to firm up this point now,” he said.
In a separate preprint posted Feb. 23, a research team at the California Institute of Technology developed a software tool that noticed the rise of B.1.526 in the New York region. The preprint hasn’t yet been peer reviewed.
“It appears that the frequency of lineage B.1.526 has increased rapidly in New York,” they wrote.
Both teams also reported on another variant, called B.1.427/B.1.429, which appears to be increasing in California. The variant could be more contagious and cause more severe disease, they said, but the research is still in the early stages.
Researchers at the University of California, San Francisco, have tested virus samples from recent outbreaks in California and also found that the variant is becoming more common. The variant didn’t appear in samples from September but was in half of the samples by late January. It has a different pattern of mutations than other variants, and one called L452R may affect the spike protein on the virus and allow it attach to cells more easily.
“Our data shows that this is likely the key mutation that makes this variant more infectious,” Charles Chiu, MD, associate director of the clinical microbiology lab at UCSF, told CNN.
The team also noticed that patients with a B.1.427/B.1.429 infection had more severe COVID-19 cases and needed more oxygen, CNN reported. The team plans to post a preprint once public health officials in San Francisco review the report.
Right now, the CDC provides public data for three variants: B.1.1.7, B.1.351, and P.1, which was first identified in Brazil. The U.S. has reported 1,881 B.1.1.7 cases across 45 states, 46 B.1.351 cases in 14 states, and five P.1 cases in four states, according to a CDC tally as of Feb. 23.
At the moment, lab officials aren’t able to tell patients or doctors whether someone has been infected by a variant, according to Kaiser Health News. High-level labs conduct genomic sequencing on samples and aren’t able to communicate information back to individual people.
But the Association of Public Health Laboratories and public health officials in several states are pushing for federal authorization of a test that could sequence the full genome and notify doctors. The test could be available in coming weeks, the news outlet reported.
A version of this article first appeared on WebMD.com.
, according to CNN.
The variant, called B.1.526, has appeared in diverse neighborhoods in New York City and is “scattered in the Northeast,” the researchers said.
“We observed a steady increase in the detection rate from late December to mid-February, with an alarming rise to 12.7% in the past two weeks,” researchers from Columbia University Medical Center wrote in a report, which was published as a preprint Feb. 25.
On Feb. 22, the team released another preprint about the B.1.1.7 and B.1.351 variants first identified in the United Kingdom and South Africa, respectively, which also mentions the B.1.526 variant in the U.S. Neither report has been peer reviewed.
Viruses mutate often, and several coronavirus variants have been identified and followed during the pandemic. Not all mutations are significant or are necessarily more contagious or dangerous. Researchers have been tracking the B.1.526 variant in the U.S. to find out if there are significant mutations that could be a cause for concern.
In the most recent preprints, the variant appears to have the same mutation found in B.1.351, called E484K, which may allow the virus to evade vaccines and the body’s natural immune response. The E484K mutation has shown up in at least 59 lines of the coronavirus, the research team said. That means the virus is evolving independently across the country and world, which could give the virus an advantage.
“A concern is that it might be beginning to overtake other strains, just like the U.K. and South African variants,” David Ho, MD, the lead study author and director of the Aaron Diamond AIDS Research Center at Columbia, told CNN.
“However, we don’t have enough data to firm up this point now,” he said.
In a separate preprint posted Feb. 23, a research team at the California Institute of Technology developed a software tool that noticed the rise of B.1.526 in the New York region. The preprint hasn’t yet been peer reviewed.
“It appears that the frequency of lineage B.1.526 has increased rapidly in New York,” they wrote.
Both teams also reported on another variant, called B.1.427/B.1.429, which appears to be increasing in California. The variant could be more contagious and cause more severe disease, they said, but the research is still in the early stages.
Researchers at the University of California, San Francisco, have tested virus samples from recent outbreaks in California and also found that the variant is becoming more common. The variant didn’t appear in samples from September but was in half of the samples by late January. It has a different pattern of mutations than other variants, and one called L452R may affect the spike protein on the virus and allow it attach to cells more easily.
“Our data shows that this is likely the key mutation that makes this variant more infectious,” Charles Chiu, MD, associate director of the clinical microbiology lab at UCSF, told CNN.
The team also noticed that patients with a B.1.427/B.1.429 infection had more severe COVID-19 cases and needed more oxygen, CNN reported. The team plans to post a preprint once public health officials in San Francisco review the report.
Right now, the CDC provides public data for three variants: B.1.1.7, B.1.351, and P.1, which was first identified in Brazil. The U.S. has reported 1,881 B.1.1.7 cases across 45 states, 46 B.1.351 cases in 14 states, and five P.1 cases in four states, according to a CDC tally as of Feb. 23.
At the moment, lab officials aren’t able to tell patients or doctors whether someone has been infected by a variant, according to Kaiser Health News. High-level labs conduct genomic sequencing on samples and aren’t able to communicate information back to individual people.
But the Association of Public Health Laboratories and public health officials in several states are pushing for federal authorization of a test that could sequence the full genome and notify doctors. The test could be available in coming weeks, the news outlet reported.
A version of this article first appeared on WebMD.com.
, according to CNN.
The variant, called B.1.526, has appeared in diverse neighborhoods in New York City and is “scattered in the Northeast,” the researchers said.
“We observed a steady increase in the detection rate from late December to mid-February, with an alarming rise to 12.7% in the past two weeks,” researchers from Columbia University Medical Center wrote in a report, which was published as a preprint Feb. 25.
On Feb. 22, the team released another preprint about the B.1.1.7 and B.1.351 variants first identified in the United Kingdom and South Africa, respectively, which also mentions the B.1.526 variant in the U.S. Neither report has been peer reviewed.
Viruses mutate often, and several coronavirus variants have been identified and followed during the pandemic. Not all mutations are significant or are necessarily more contagious or dangerous. Researchers have been tracking the B.1.526 variant in the U.S. to find out if there are significant mutations that could be a cause for concern.
In the most recent preprints, the variant appears to have the same mutation found in B.1.351, called E484K, which may allow the virus to evade vaccines and the body’s natural immune response. The E484K mutation has shown up in at least 59 lines of the coronavirus, the research team said. That means the virus is evolving independently across the country and world, which could give the virus an advantage.
“A concern is that it might be beginning to overtake other strains, just like the U.K. and South African variants,” David Ho, MD, the lead study author and director of the Aaron Diamond AIDS Research Center at Columbia, told CNN.
“However, we don’t have enough data to firm up this point now,” he said.
In a separate preprint posted Feb. 23, a research team at the California Institute of Technology developed a software tool that noticed the rise of B.1.526 in the New York region. The preprint hasn’t yet been peer reviewed.
“It appears that the frequency of lineage B.1.526 has increased rapidly in New York,” they wrote.
Both teams also reported on another variant, called B.1.427/B.1.429, which appears to be increasing in California. The variant could be more contagious and cause more severe disease, they said, but the research is still in the early stages.
Researchers at the University of California, San Francisco, have tested virus samples from recent outbreaks in California and also found that the variant is becoming more common. The variant didn’t appear in samples from September but was in half of the samples by late January. It has a different pattern of mutations than other variants, and one called L452R may affect the spike protein on the virus and allow it attach to cells more easily.
“Our data shows that this is likely the key mutation that makes this variant more infectious,” Charles Chiu, MD, associate director of the clinical microbiology lab at UCSF, told CNN.
The team also noticed that patients with a B.1.427/B.1.429 infection had more severe COVID-19 cases and needed more oxygen, CNN reported. The team plans to post a preprint once public health officials in San Francisco review the report.
Right now, the CDC provides public data for three variants: B.1.1.7, B.1.351, and P.1, which was first identified in Brazil. The U.S. has reported 1,881 B.1.1.7 cases across 45 states, 46 B.1.351 cases in 14 states, and five P.1 cases in four states, according to a CDC tally as of Feb. 23.
At the moment, lab officials aren’t able to tell patients or doctors whether someone has been infected by a variant, according to Kaiser Health News. High-level labs conduct genomic sequencing on samples and aren’t able to communicate information back to individual people.
But the Association of Public Health Laboratories and public health officials in several states are pushing for federal authorization of a test that could sequence the full genome and notify doctors. The test could be available in coming weeks, the news outlet reported.
A version of this article first appeared on WebMD.com.
Heroes: Nurses’ sacrifice in the age of COVID-19
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
Myocardial injury seen on MRI in 54% of recovered COVID-19 patients
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
Pediatric COVID-19: Data to guide practice
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
Janssen/J&J COVID-19 vaccine cuts transmission, new data show
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ob.gyns. report high burnout prior to pandemic
Among ob.gyns. who reported burnout in the past year, 82% say they felt burned out before the advent of the coronavirus pandemic, according the Medscape Obstetrician & Gynecologist Lifestyle, Happiness, & Burnout Report.
The past year brought unusual challenges to physicians in all specialties in different ways.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” wrote Keith L. Martin and Mary Lyn Koval, both of Medscape Business of Medicine, in the introduction to the report.
Although more physicians said their burnout began prior to the pandemic, 81% of ob.gyns. reported that they were happy outside of work prior to the pandemic. However, those reporting happiness outside of work dropped to 57% after the pandemic started.
“One does not have to do a ‘deep dive’ to understand the top reasons reported for burnout,” said Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, in an interview. “Many conversations I have with colleagues are about the frustration of learning and managing electronic health records, insurance reimbursements, and a work-life balance. In addition, more physician practices are being purchased by hospitals or private-equity networks [that are] reducing and/or eliminating the autonomy of physicians.
“While all [respondents] exhibited a dramatic decline in ‘happiness’ prepandemic, compared with our current situation, ob.gyns. were no exception,” he added.
Burnout and suicidal thoughts
Overall, 26% of ob.gyn. survey respondents reported being burned out, 6% reported being depressed, and 18% reported being both burned out and depressed. Of those who reported burnout, 52% said burnout had “a strong or severe impact on my life,” while 20% reported a moderate impact and 28% reported little or no impact.
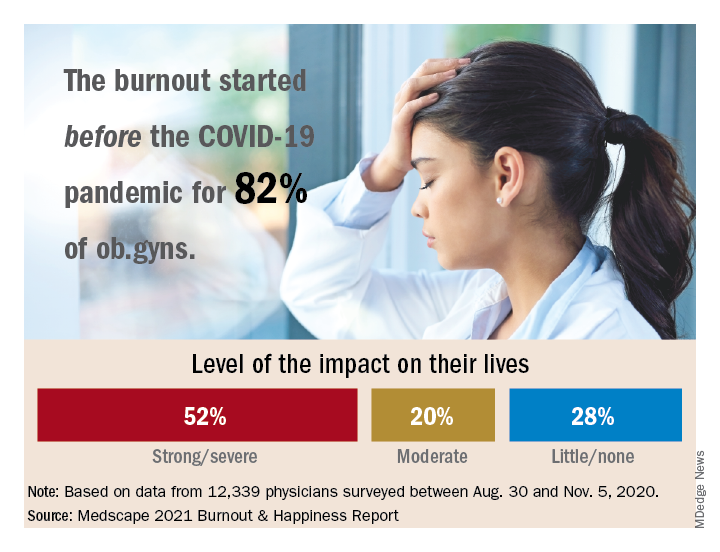
More than half (56%) of ob.gyns. who reported either depression or burnout said they had not sought professional help, although 17% reported receiving professional care in the past.
The main reason given for not seeking professional help was that burnout and depressive symptoms were not severe enough to merit it, according to 50% of respondents who reported burnout or depression but were not seeking help. In addition, 43% said they were too busy to seek help, 36% said they could deal with their symptoms without professional help, and 24% said they did not want to risk disclosure of their symptoms.
The most common cause of burnout was an overload of bureaucratic tasks, reported by 52% of respondents, followed by “lack of respect from administrators/employers, colleagues, or staff” (43%), and insufficient compensation or reimbursement (39%).
Notably, 19% of ob.gyns. reported suicidal thoughts, and 1% said they had attempted suicide.
“The most concerning statistic from this survey was in reference to suicidal ideation,” said Dr. Trolice. “Approximately one in five ob.gyns. have contemplated suicide, compared with 4.8% of adults age 18 and older in the U.S. reporting in 2019.”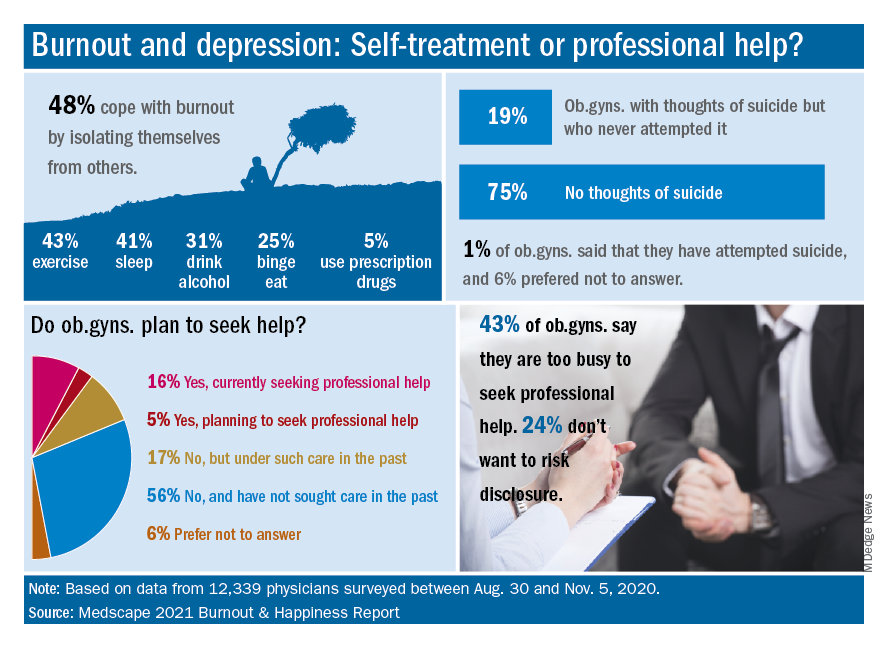
Dr. Trolice said he was not surprised that relatively few ob.gyns. sought help for mental health issues. “Physicians are very private and usually do not seek help from colleagues, presumably from hubris. While this is unfortunate, all hospitals and health care organization should implement regular assessments of physicians’ health to ensure optimal performance from a professional and personal basis.”
Balance and self-care
The top workplace concern, by a large margin, was for work-life balance, reported by 44% of respondents, followed by compensation (19%), combining work and parenting (18%), and relationships with staff and colleagues (8%).
Approximately one-third (36%) of the ob.gyn. respondents said they made time to focus on personal well-being, compared with 35% of physicians overall. Although only 13% reported exercising every day, a total of 69% exercised at least twice a week, similar to the 70% of physicians overall who reported exercising at least twice a week.
“Work-life balance is high on the list of concerns, but physicians are split 50/50 on whether they would accept a salary reduction to improve this aspect of their lives,” Dr. Trolice said.
“Social relationships are a proven value to mental health, yet nearly 50% of ob.gyns. who reported feeling burnout use isolationism as their coping skill, citing a lack of severity to require treatment,” he noted. Nevertheless, more than 80% of responders were married and described their relationship as “good or very good.”
Address burnout at individual and organizational levels
“Sadly, the findings are not surprising,” said Iris Krisha, MD, of Emory University, Atlanta, in an interview. “Burnout rates have been steadily increasing among physicians across all specialties.” Barriers to reducing burnout exist at the organizational and individual level, therefore strategies to reduce burnout should address individual and organizational solutions, Dr. Krishna emphasized. “At the organizational level, solutions may include developing manageable workloads, creating fair productivity targets, encouraging physician engagement in work structure, supporting flexible work schedules, and allowing for protected time for education and exercise. On the individual level, physicians can work to develop stress management strategies, engage in mindfulness and self-care.”
To reduce the burden of bureaucratic tasks, “health care organizations can work toward optimizing electronic medical records and hire staff to offload clerical work, and physicians can seek training in efficiency,” said Dr. Krishna. In addition, “health care organizations can reduce the stigma that may surround burnout or mental health issues, as well as promote a culture of wellness and resilience,” to help reduce and prevent burnout.
Find positivity and purpose
Improving the workplace experience so physicians feel engaged and in control as they navigate their many responsibilities may help reduce burnout, said Dr. Trolice. On the individual level, “finding your purpose to give you more meaning at work, discovering the power of hope to embrace optimism, and building friendships at work for greater engagement with others,” can help as well.
“In the face of adversity and setbacks, people in happier workplaces tend to be better at coping with and recovering from work pressure and at reconciling conflict,” Dr. Trolice emphasized. “The practice of medicine has dramatically changed for many physicians compared with the original expectations when they applied to medical school. Nevertheless, it behooves physician to adapt to 21st century medical care as they remind themselves of their purpose.”
The report included responses from 12,339 physicians across 29 specialties who completed a 10-minute online survey between Aug. 30 and Nov. 5, 2020. Participants were required to be practicing U.S. physicians.
Among ob.gyns. who reported burnout in the past year, 82% say they felt burned out before the advent of the coronavirus pandemic, according the Medscape Obstetrician & Gynecologist Lifestyle, Happiness, & Burnout Report.
The past year brought unusual challenges to physicians in all specialties in different ways.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” wrote Keith L. Martin and Mary Lyn Koval, both of Medscape Business of Medicine, in the introduction to the report.
Although more physicians said their burnout began prior to the pandemic, 81% of ob.gyns. reported that they were happy outside of work prior to the pandemic. However, those reporting happiness outside of work dropped to 57% after the pandemic started.
“One does not have to do a ‘deep dive’ to understand the top reasons reported for burnout,” said Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, in an interview. “Many conversations I have with colleagues are about the frustration of learning and managing electronic health records, insurance reimbursements, and a work-life balance. In addition, more physician practices are being purchased by hospitals or private-equity networks [that are] reducing and/or eliminating the autonomy of physicians.
“While all [respondents] exhibited a dramatic decline in ‘happiness’ prepandemic, compared with our current situation, ob.gyns. were no exception,” he added.
Burnout and suicidal thoughts
Overall, 26% of ob.gyn. survey respondents reported being burned out, 6% reported being depressed, and 18% reported being both burned out and depressed. Of those who reported burnout, 52% said burnout had “a strong or severe impact on my life,” while 20% reported a moderate impact and 28% reported little or no impact.

More than half (56%) of ob.gyns. who reported either depression or burnout said they had not sought professional help, although 17% reported receiving professional care in the past.
The main reason given for not seeking professional help was that burnout and depressive symptoms were not severe enough to merit it, according to 50% of respondents who reported burnout or depression but were not seeking help. In addition, 43% said they were too busy to seek help, 36% said they could deal with their symptoms without professional help, and 24% said they did not want to risk disclosure of their symptoms.
The most common cause of burnout was an overload of bureaucratic tasks, reported by 52% of respondents, followed by “lack of respect from administrators/employers, colleagues, or staff” (43%), and insufficient compensation or reimbursement (39%).
Notably, 19% of ob.gyns. reported suicidal thoughts, and 1% said they had attempted suicide.
“The most concerning statistic from this survey was in reference to suicidal ideation,” said Dr. Trolice. “Approximately one in five ob.gyns. have contemplated suicide, compared with 4.8% of adults age 18 and older in the U.S. reporting in 2019.”
Dr. Trolice said he was not surprised that relatively few ob.gyns. sought help for mental health issues. “Physicians are very private and usually do not seek help from colleagues, presumably from hubris. While this is unfortunate, all hospitals and health care organization should implement regular assessments of physicians’ health to ensure optimal performance from a professional and personal basis.”
Balance and self-care
The top workplace concern, by a large margin, was for work-life balance, reported by 44% of respondents, followed by compensation (19%), combining work and parenting (18%), and relationships with staff and colleagues (8%).
Approximately one-third (36%) of the ob.gyn. respondents said they made time to focus on personal well-being, compared with 35% of physicians overall. Although only 13% reported exercising every day, a total of 69% exercised at least twice a week, similar to the 70% of physicians overall who reported exercising at least twice a week.
“Work-life balance is high on the list of concerns, but physicians are split 50/50 on whether they would accept a salary reduction to improve this aspect of their lives,” Dr. Trolice said.
“Social relationships are a proven value to mental health, yet nearly 50% of ob.gyns. who reported feeling burnout use isolationism as their coping skill, citing a lack of severity to require treatment,” he noted. Nevertheless, more than 80% of responders were married and described their relationship as “good or very good.”
Address burnout at individual and organizational levels
“Sadly, the findings are not surprising,” said Iris Krisha, MD, of Emory University, Atlanta, in an interview. “Burnout rates have been steadily increasing among physicians across all specialties.” Barriers to reducing burnout exist at the organizational and individual level, therefore strategies to reduce burnout should address individual and organizational solutions, Dr. Krishna emphasized. “At the organizational level, solutions may include developing manageable workloads, creating fair productivity targets, encouraging physician engagement in work structure, supporting flexible work schedules, and allowing for protected time for education and exercise. On the individual level, physicians can work to develop stress management strategies, engage in mindfulness and self-care.”
To reduce the burden of bureaucratic tasks, “health care organizations can work toward optimizing electronic medical records and hire staff to offload clerical work, and physicians can seek training in efficiency,” said Dr. Krishna. In addition, “health care organizations can reduce the stigma that may surround burnout or mental health issues, as well as promote a culture of wellness and resilience,” to help reduce and prevent burnout.
Find positivity and purpose
Improving the workplace experience so physicians feel engaged and in control as they navigate their many responsibilities may help reduce burnout, said Dr. Trolice. On the individual level, “finding your purpose to give you more meaning at work, discovering the power of hope to embrace optimism, and building friendships at work for greater engagement with others,” can help as well.
“In the face of adversity and setbacks, people in happier workplaces tend to be better at coping with and recovering from work pressure and at reconciling conflict,” Dr. Trolice emphasized. “The practice of medicine has dramatically changed for many physicians compared with the original expectations when they applied to medical school. Nevertheless, it behooves physician to adapt to 21st century medical care as they remind themselves of their purpose.”
The report included responses from 12,339 physicians across 29 specialties who completed a 10-minute online survey between Aug. 30 and Nov. 5, 2020. Participants were required to be practicing U.S. physicians.
Among ob.gyns. who reported burnout in the past year, 82% say they felt burned out before the advent of the coronavirus pandemic, according the Medscape Obstetrician & Gynecologist Lifestyle, Happiness, & Burnout Report.
The past year brought unusual challenges to physicians in all specialties in different ways.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” wrote Keith L. Martin and Mary Lyn Koval, both of Medscape Business of Medicine, in the introduction to the report.
Although more physicians said their burnout began prior to the pandemic, 81% of ob.gyns. reported that they were happy outside of work prior to the pandemic. However, those reporting happiness outside of work dropped to 57% after the pandemic started.
“One does not have to do a ‘deep dive’ to understand the top reasons reported for burnout,” said Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, in an interview. “Many conversations I have with colleagues are about the frustration of learning and managing electronic health records, insurance reimbursements, and a work-life balance. In addition, more physician practices are being purchased by hospitals or private-equity networks [that are] reducing and/or eliminating the autonomy of physicians.
“While all [respondents] exhibited a dramatic decline in ‘happiness’ prepandemic, compared with our current situation, ob.gyns. were no exception,” he added.
Burnout and suicidal thoughts
Overall, 26% of ob.gyn. survey respondents reported being burned out, 6% reported being depressed, and 18% reported being both burned out and depressed. Of those who reported burnout, 52% said burnout had “a strong or severe impact on my life,” while 20% reported a moderate impact and 28% reported little or no impact.

More than half (56%) of ob.gyns. who reported either depression or burnout said they had not sought professional help, although 17% reported receiving professional care in the past.
The main reason given for not seeking professional help was that burnout and depressive symptoms were not severe enough to merit it, according to 50% of respondents who reported burnout or depression but were not seeking help. In addition, 43% said they were too busy to seek help, 36% said they could deal with their symptoms without professional help, and 24% said they did not want to risk disclosure of their symptoms.
The most common cause of burnout was an overload of bureaucratic tasks, reported by 52% of respondents, followed by “lack of respect from administrators/employers, colleagues, or staff” (43%), and insufficient compensation or reimbursement (39%).
Notably, 19% of ob.gyns. reported suicidal thoughts, and 1% said they had attempted suicide.
“The most concerning statistic from this survey was in reference to suicidal ideation,” said Dr. Trolice. “Approximately one in five ob.gyns. have contemplated suicide, compared with 4.8% of adults age 18 and older in the U.S. reporting in 2019.”
Dr. Trolice said he was not surprised that relatively few ob.gyns. sought help for mental health issues. “Physicians are very private and usually do not seek help from colleagues, presumably from hubris. While this is unfortunate, all hospitals and health care organization should implement regular assessments of physicians’ health to ensure optimal performance from a professional and personal basis.”
Balance and self-care
The top workplace concern, by a large margin, was for work-life balance, reported by 44% of respondents, followed by compensation (19%), combining work and parenting (18%), and relationships with staff and colleagues (8%).
Approximately one-third (36%) of the ob.gyn. respondents said they made time to focus on personal well-being, compared with 35% of physicians overall. Although only 13% reported exercising every day, a total of 69% exercised at least twice a week, similar to the 70% of physicians overall who reported exercising at least twice a week.
“Work-life balance is high on the list of concerns, but physicians are split 50/50 on whether they would accept a salary reduction to improve this aspect of their lives,” Dr. Trolice said.
“Social relationships are a proven value to mental health, yet nearly 50% of ob.gyns. who reported feeling burnout use isolationism as their coping skill, citing a lack of severity to require treatment,” he noted. Nevertheless, more than 80% of responders were married and described their relationship as “good or very good.”
Address burnout at individual and organizational levels
“Sadly, the findings are not surprising,” said Iris Krisha, MD, of Emory University, Atlanta, in an interview. “Burnout rates have been steadily increasing among physicians across all specialties.” Barriers to reducing burnout exist at the organizational and individual level, therefore strategies to reduce burnout should address individual and organizational solutions, Dr. Krishna emphasized. “At the organizational level, solutions may include developing manageable workloads, creating fair productivity targets, encouraging physician engagement in work structure, supporting flexible work schedules, and allowing for protected time for education and exercise. On the individual level, physicians can work to develop stress management strategies, engage in mindfulness and self-care.”
To reduce the burden of bureaucratic tasks, “health care organizations can work toward optimizing electronic medical records and hire staff to offload clerical work, and physicians can seek training in efficiency,” said Dr. Krishna. In addition, “health care organizations can reduce the stigma that may surround burnout or mental health issues, as well as promote a culture of wellness and resilience,” to help reduce and prevent burnout.
Find positivity and purpose
Improving the workplace experience so physicians feel engaged and in control as they navigate their many responsibilities may help reduce burnout, said Dr. Trolice. On the individual level, “finding your purpose to give you more meaning at work, discovering the power of hope to embrace optimism, and building friendships at work for greater engagement with others,” can help as well.
“In the face of adversity and setbacks, people in happier workplaces tend to be better at coping with and recovering from work pressure and at reconciling conflict,” Dr. Trolice emphasized. “The practice of medicine has dramatically changed for many physicians compared with the original expectations when they applied to medical school. Nevertheless, it behooves physician to adapt to 21st century medical care as they remind themselves of their purpose.”
The report included responses from 12,339 physicians across 29 specialties who completed a 10-minute online survey between Aug. 30 and Nov. 5, 2020. Participants were required to be practicing U.S. physicians.
COVID and schools: A pediatrician's case for a return to class
In a time when this country is struggling to find topics on which we can achieve broad consensus, the question of whether in-class learning is important stands as an outlier. Parents, teachers, students, and pediatricians all agree that having children learn in a social, face-to-face environment is critical to their education and mental health. Because school has become a de facto daycare source for many families, employers have joined in the chorus supporting a return to in-class education.
Of course, beyond that basic point of agreement the myriad of questions relating to when and how that return to the educational norm can be achieved we divide into groups with almost as many answers as there are questions. Part of the problem stems from the national leadership vacuum that fed the confusion. In this void the topic of school reopening has become politicized.
On Jan. 5, 2021, the American Academy of Pediatrics released an updated interim COVID-19 Guidance for Safe Schools at services.aap.org. It is a thorough and well thought out document that should function as a roadmap for communities and pediatricians who serve as official and unofficial advisers to their local school departments. At the very outset it reminds us that “school transmission mirrors but does not drive community transmission.”
Unfortunately, timing is everything and while the document’s salient points received some media attention it was mostly buried by the tsunami of press coverage in the wake of the storming of the Capitol the next day and the postinauguration reshuffling of the federal government. Even if it had been released on one of those seldom seen quiet news days, I fear the document’s message encouraging the return to in-class learning would have still not received the attention it deserved.
The lack of a high-visibility celebrity spokesperson and a system of short-tenure presidencies puts the AAP at a disadvantage when it comes to getting its message across to a national audience. The advocacy role filters down to those of us in our own communities who must convince school boards that not only is in-class learning critical but there are safe ways to do it.
In some communities the timing of return to in-class learning may pit pediatricians against teachers. Usually, these two groups share an enthusiastic advocacy for children. However, facing what has up to this point been a poorly defined health risk, teachers are understandably resistant to return to the classroom although they acknowledge its importance.
Armed with the AAP’s guidance document, pediatricians should encourage school boards and state and local health departments to look closely at the epidemiologic evidence and consider creative ways to prioritize teachers for what currently are limited and erratic vaccine supplies. Strategies might include offering vaccines to teachers based strictly on their age and/or health status. However, teachers and in-class education are so critical to the educational process and the national economy that an open offer to all teachers makes more sense.
While some states have already prioritized teachers for vaccines, the AAP must continue to speak loudly that in-class education is critical and urge all states to do what is necessary to make teachers feel safe to return to the classroom.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a time when this country is struggling to find topics on which we can achieve broad consensus, the question of whether in-class learning is important stands as an outlier. Parents, teachers, students, and pediatricians all agree that having children learn in a social, face-to-face environment is critical to their education and mental health. Because school has become a de facto daycare source for many families, employers have joined in the chorus supporting a return to in-class education.
Of course, beyond that basic point of agreement the myriad of questions relating to when and how that return to the educational norm can be achieved we divide into groups with almost as many answers as there are questions. Part of the problem stems from the national leadership vacuum that fed the confusion. In this void the topic of school reopening has become politicized.
On Jan. 5, 2021, the American Academy of Pediatrics released an updated interim COVID-19 Guidance for Safe Schools at services.aap.org. It is a thorough and well thought out document that should function as a roadmap for communities and pediatricians who serve as official and unofficial advisers to their local school departments. At the very outset it reminds us that “school transmission mirrors but does not drive community transmission.”
Unfortunately, timing is everything and while the document’s salient points received some media attention it was mostly buried by the tsunami of press coverage in the wake of the storming of the Capitol the next day and the postinauguration reshuffling of the federal government. Even if it had been released on one of those seldom seen quiet news days, I fear the document’s message encouraging the return to in-class learning would have still not received the attention it deserved.
The lack of a high-visibility celebrity spokesperson and a system of short-tenure presidencies puts the AAP at a disadvantage when it comes to getting its message across to a national audience. The advocacy role filters down to those of us in our own communities who must convince school boards that not only is in-class learning critical but there are safe ways to do it.
In some communities the timing of return to in-class learning may pit pediatricians against teachers. Usually, these two groups share an enthusiastic advocacy for children. However, facing what has up to this point been a poorly defined health risk, teachers are understandably resistant to return to the classroom although they acknowledge its importance.
Armed with the AAP’s guidance document, pediatricians should encourage school boards and state and local health departments to look closely at the epidemiologic evidence and consider creative ways to prioritize teachers for what currently are limited and erratic vaccine supplies. Strategies might include offering vaccines to teachers based strictly on their age and/or health status. However, teachers and in-class education are so critical to the educational process and the national economy that an open offer to all teachers makes more sense.
While some states have already prioritized teachers for vaccines, the AAP must continue to speak loudly that in-class education is critical and urge all states to do what is necessary to make teachers feel safe to return to the classroom.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a time when this country is struggling to find topics on which we can achieve broad consensus, the question of whether in-class learning is important stands as an outlier. Parents, teachers, students, and pediatricians all agree that having children learn in a social, face-to-face environment is critical to their education and mental health. Because school has become a de facto daycare source for many families, employers have joined in the chorus supporting a return to in-class education.
Of course, beyond that basic point of agreement the myriad of questions relating to when and how that return to the educational norm can be achieved we divide into groups with almost as many answers as there are questions. Part of the problem stems from the national leadership vacuum that fed the confusion. In this void the topic of school reopening has become politicized.
On Jan. 5, 2021, the American Academy of Pediatrics released an updated interim COVID-19 Guidance for Safe Schools at services.aap.org. It is a thorough and well thought out document that should function as a roadmap for communities and pediatricians who serve as official and unofficial advisers to their local school departments. At the very outset it reminds us that “school transmission mirrors but does not drive community transmission.”
Unfortunately, timing is everything and while the document’s salient points received some media attention it was mostly buried by the tsunami of press coverage in the wake of the storming of the Capitol the next day and the postinauguration reshuffling of the federal government. Even if it had been released on one of those seldom seen quiet news days, I fear the document’s message encouraging the return to in-class learning would have still not received the attention it deserved.
The lack of a high-visibility celebrity spokesperson and a system of short-tenure presidencies puts the AAP at a disadvantage when it comes to getting its message across to a national audience. The advocacy role filters down to those of us in our own communities who must convince school boards that not only is in-class learning critical but there are safe ways to do it.
In some communities the timing of return to in-class learning may pit pediatricians against teachers. Usually, these two groups share an enthusiastic advocacy for children. However, facing what has up to this point been a poorly defined health risk, teachers are understandably resistant to return to the classroom although they acknowledge its importance.
Armed with the AAP’s guidance document, pediatricians should encourage school boards and state and local health departments to look closely at the epidemiologic evidence and consider creative ways to prioritize teachers for what currently are limited and erratic vaccine supplies. Strategies might include offering vaccines to teachers based strictly on their age and/or health status. However, teachers and in-class education are so critical to the educational process and the national economy that an open offer to all teachers makes more sense.
While some states have already prioritized teachers for vaccines, the AAP must continue to speak loudly that in-class education is critical and urge all states to do what is necessary to make teachers feel safe to return to the classroom.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
COVID-19 vaccination recommended for rheumatology patients
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
Loss of smell lingers post COVID-19
The findings illustrate that olfactory problems are common not only during the acute COVID-19 phase but also “in the long run” and that these problems should be “taken into consideration” when following up these patients, study investigator Johannes Frasnelli, MD, professor, department of anatomy, Université du Québec à Trois-Rivières, said in an interview.
Loss of the sense of smell can affect quality of life because it affects eating and drinking, and may even be dangerous, said Dr. Frasnelli. “If your sense of smell is impaired, you may unknowingly eat spoiled food, or you may not smell smoke or gas in your home,” he said. In addition, Dr. Frasnelli noted that an impaired sense of smell is associated with higher rates of depression. The findings will be presented at the annual meeting of the American Academy of Neurology in April.
‘Striking’ finding
Research shows that about 60% of patients with COVID-19 lose their sense of smell to some degree during the acute phase of the disease. “But we wanted to go further and look at the longer-term effects of loss of smell and taste,” said Dr. Frasnelli.
The analysis included 813 health care workers in the province of Quebec. For all the patients, SARS-CoV-2 infection was confirmed through testing with a nasopharyngeal viral swab.
Participants completed a 64-item online questionnaire that asked about three senses: olfactory; gustatory, which includes tastes such as sweet, sour, bitter, salty, savory and umami; and trigeminal, which includes sensations such as spiciness of hot peppers and “coolness” of mint.
They were asked to rate these on a scale of 0 (no perception) to 10 (very strong perception) before the infection, during the infection, and currently. They were also asked about other symptoms, including fatigue.
Most respondents had been infected in the first wave of the virus in March and April of 2020 and responded to the questionnaire an average of 5 months later.
The vast majority of respondents (84.1%) were women, which Dr. Frasnelli said was not surprising because women predominate in the health care field.
The analysis showed that average smell ratings were 8.98 before infection, 2.85 during the acute phase, and 7.41 when respondents answered the questionnaire. The sense of taste was less affected and recovered faster than did the sense of smell. Results for taste were 9.20 before infection, 3.59 during the acute phase, and 8.05 after COVID-19.
Among 580 respondents who indicated a compromised sense of smell during the acute phase, the average smell rating when answering the questionnaire was 6.89, compared to 9.03 before the infection. More than half (51.2%) reported not regaining full olfactory function.
The fact that the sense of smell had not returned to normal for half the participants so long after being infected is “novel and quite striking,” said Dr. Frasnelli.
However, he noted, this doesn’t necessarily mean all those with a compromised sense of smell “have huge problems.” In some cases, he said, the problem “is more subtle.”
Not a CNS problem?
Respondents also completed a chemosensory dysfunction home test (CD-HT). They were asked to prepare common household food items, such as peanut butter, sugar, salt, and vinegar, in a particular way – for example, to add sugar or salt to water – and provide feedback on how they smell and taste.
For this CD-HT analysis, 18.4% of respondents reported having persistent loss of smell. This, Dr. Frasnelli said, adds to evidence from self-reported responses and suggests that in some cases, the problem is more than senses not returning to normal.
“From the questionnaires, roughly 50% said their sense of smell is still not back to normal, and when we look at the CD home test, we see that almost 20% of subjects indeed have pretty strong impairment of their sense of smell,” he said.
The results showed no sex differences, although Dr. Frasnelli noted that most of the sample were women. “It’s tricky to look at the data with regard to sex because it’s a bit skewed,” he said.
Male respondents were older than female participants, but there was no difference in impairment between age groups. Dr. Frasnelli said this was “quite interesting,” inasmuch as older people usually lose some sense of smell.
The researchers have not yet examined whether the results differ by type of health care worker.
They also have not examined in detail whether infection severity affects the risk for extended olfactory impairment. Although some research suggests that the problem with smell is more common in less severe cases, Dr. Frasnelli noted this could be because loss of smell is not a huge problem for patients battling grave health problems.
As for other symptoms, many respondents reported lingering fatigue; some reported debilitating fatigue, said Dr. Frasnelli. However, he cautioned that this is difficult to interpret, because the participants were health care workers, many of whom returned to work during the pandemic and perhaps had not fully rested.
He also noted that he and his colleagues have not “made the link” between impaired smell and the degree of fatigue.
The COVID-19 virus appears to attack supporting sustentacular cells in the olfactory epithelium, not nerve cells.
“Right now, it seems that the smell problem is not a central nervous system problem but a peripheral problem,” said Dr. Frasnelli. “But we don’t know for sure; it may be that the virus somehow gets into the brain and some symptoms are caused by the effects of the infection on the brain.”
The researchers will extend their research with another questionnaire to assess senses 10-12 months after COVID-19.
Limitations of the study include the subjective nature of the smell and taste ratings and the single time point at which data were collected.
Confirmatory findings
Commenting on the research in an interview, Thomas Hummel, MD, professor, smell and taste clinic, department of otorhinolaryngology, Technische Universität Dresden (Germany), said the new results regarding loss of smell after COVID-19 are “very congruent” with what he and his colleagues have observed.
Research shows that up to one in five of those infected with SARS-CoV-2 experience olfactory loss. “While the numbers may vary a bit from study to study or lab to lab, I think 5% to 20% of post–COVID-19 patients exhibit long-term olfactory loss,” Dr. Hummel said.
His group has observed that “many more are not back to normal,” which conforms with what Dr. Frasnelli’s study reveals, said Dr. Hummel.
Also commenting on the research, Kenneth L. Tyler, MD, professor of neurology, University of Colorado at Denver, Aurora, and a fellow of the American Academy of Neurology, said the study was relatively large and the results “interesting.”
Although it “provides more evidence there’s a subset of patients with symptoms even well past the acute phase” of COVID-19, the results are “mostly confirmatory” and include “nothing super surprising,” Dr. Tyler said in an interview.
However, the investigators did attempt to make the study “a little more quantitative” and “to confirm the self-reporting with their validated CD home test,” he said.
Dr. Tyler wondered how representative the sample was and whether the study drew more participants with impaired senses. “If I had a loss of smell or taste, maybe I would be more likely to respond to such a survey,” he said.
He also noted the difficulty of separating loss of smell from loss of taste.
“If you lose your sense of smell, things don’t taste right, so it can be confounding as to how to separate out those two,” he noted.
The study was supported by the Foundation of the Université du Québec à Trois-Rivières and the Province of Quebec. Dr. Frasnelli has received royalties from Styriabooks in Austria for a book on olfaction published in 2019 and has received honoraria for speaking engagements. Dr. Hummel and Dr. Tyler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings illustrate that olfactory problems are common not only during the acute COVID-19 phase but also “in the long run” and that these problems should be “taken into consideration” when following up these patients, study investigator Johannes Frasnelli, MD, professor, department of anatomy, Université du Québec à Trois-Rivières, said in an interview.
Loss of the sense of smell can affect quality of life because it affects eating and drinking, and may even be dangerous, said Dr. Frasnelli. “If your sense of smell is impaired, you may unknowingly eat spoiled food, or you may not smell smoke or gas in your home,” he said. In addition, Dr. Frasnelli noted that an impaired sense of smell is associated with higher rates of depression. The findings will be presented at the annual meeting of the American Academy of Neurology in April.
‘Striking’ finding
Research shows that about 60% of patients with COVID-19 lose their sense of smell to some degree during the acute phase of the disease. “But we wanted to go further and look at the longer-term effects of loss of smell and taste,” said Dr. Frasnelli.
The analysis included 813 health care workers in the province of Quebec. For all the patients, SARS-CoV-2 infection was confirmed through testing with a nasopharyngeal viral swab.
Participants completed a 64-item online questionnaire that asked about three senses: olfactory; gustatory, which includes tastes such as sweet, sour, bitter, salty, savory and umami; and trigeminal, which includes sensations such as spiciness of hot peppers and “coolness” of mint.
They were asked to rate these on a scale of 0 (no perception) to 10 (very strong perception) before the infection, during the infection, and currently. They were also asked about other symptoms, including fatigue.
Most respondents had been infected in the first wave of the virus in March and April of 2020 and responded to the questionnaire an average of 5 months later.
The vast majority of respondents (84.1%) were women, which Dr. Frasnelli said was not surprising because women predominate in the health care field.
The analysis showed that average smell ratings were 8.98 before infection, 2.85 during the acute phase, and 7.41 when respondents answered the questionnaire. The sense of taste was less affected and recovered faster than did the sense of smell. Results for taste were 9.20 before infection, 3.59 during the acute phase, and 8.05 after COVID-19.
Among 580 respondents who indicated a compromised sense of smell during the acute phase, the average smell rating when answering the questionnaire was 6.89, compared to 9.03 before the infection. More than half (51.2%) reported not regaining full olfactory function.
The fact that the sense of smell had not returned to normal for half the participants so long after being infected is “novel and quite striking,” said Dr. Frasnelli.
However, he noted, this doesn’t necessarily mean all those with a compromised sense of smell “have huge problems.” In some cases, he said, the problem “is more subtle.”
Not a CNS problem?
Respondents also completed a chemosensory dysfunction home test (CD-HT). They were asked to prepare common household food items, such as peanut butter, sugar, salt, and vinegar, in a particular way – for example, to add sugar or salt to water – and provide feedback on how they smell and taste.
For this CD-HT analysis, 18.4% of respondents reported having persistent loss of smell. This, Dr. Frasnelli said, adds to evidence from self-reported responses and suggests that in some cases, the problem is more than senses not returning to normal.
“From the questionnaires, roughly 50% said their sense of smell is still not back to normal, and when we look at the CD home test, we see that almost 20% of subjects indeed have pretty strong impairment of their sense of smell,” he said.
The results showed no sex differences, although Dr. Frasnelli noted that most of the sample were women. “It’s tricky to look at the data with regard to sex because it’s a bit skewed,” he said.
Male respondents were older than female participants, but there was no difference in impairment between age groups. Dr. Frasnelli said this was “quite interesting,” inasmuch as older people usually lose some sense of smell.
The researchers have not yet examined whether the results differ by type of health care worker.
They also have not examined in detail whether infection severity affects the risk for extended olfactory impairment. Although some research suggests that the problem with smell is more common in less severe cases, Dr. Frasnelli noted this could be because loss of smell is not a huge problem for patients battling grave health problems.
As for other symptoms, many respondents reported lingering fatigue; some reported debilitating fatigue, said Dr. Frasnelli. However, he cautioned that this is difficult to interpret, because the participants were health care workers, many of whom returned to work during the pandemic and perhaps had not fully rested.
He also noted that he and his colleagues have not “made the link” between impaired smell and the degree of fatigue.
The COVID-19 virus appears to attack supporting sustentacular cells in the olfactory epithelium, not nerve cells.
“Right now, it seems that the smell problem is not a central nervous system problem but a peripheral problem,” said Dr. Frasnelli. “But we don’t know for sure; it may be that the virus somehow gets into the brain and some symptoms are caused by the effects of the infection on the brain.”
The researchers will extend their research with another questionnaire to assess senses 10-12 months after COVID-19.
Limitations of the study include the subjective nature of the smell and taste ratings and the single time point at which data were collected.
Confirmatory findings
Commenting on the research in an interview, Thomas Hummel, MD, professor, smell and taste clinic, department of otorhinolaryngology, Technische Universität Dresden (Germany), said the new results regarding loss of smell after COVID-19 are “very congruent” with what he and his colleagues have observed.
Research shows that up to one in five of those infected with SARS-CoV-2 experience olfactory loss. “While the numbers may vary a bit from study to study or lab to lab, I think 5% to 20% of post–COVID-19 patients exhibit long-term olfactory loss,” Dr. Hummel said.
His group has observed that “many more are not back to normal,” which conforms with what Dr. Frasnelli’s study reveals, said Dr. Hummel.
Also commenting on the research, Kenneth L. Tyler, MD, professor of neurology, University of Colorado at Denver, Aurora, and a fellow of the American Academy of Neurology, said the study was relatively large and the results “interesting.”
Although it “provides more evidence there’s a subset of patients with symptoms even well past the acute phase” of COVID-19, the results are “mostly confirmatory” and include “nothing super surprising,” Dr. Tyler said in an interview.
However, the investigators did attempt to make the study “a little more quantitative” and “to confirm the self-reporting with their validated CD home test,” he said.
Dr. Tyler wondered how representative the sample was and whether the study drew more participants with impaired senses. “If I had a loss of smell or taste, maybe I would be more likely to respond to such a survey,” he said.
He also noted the difficulty of separating loss of smell from loss of taste.
“If you lose your sense of smell, things don’t taste right, so it can be confounding as to how to separate out those two,” he noted.
The study was supported by the Foundation of the Université du Québec à Trois-Rivières and the Province of Quebec. Dr. Frasnelli has received royalties from Styriabooks in Austria for a book on olfaction published in 2019 and has received honoraria for speaking engagements. Dr. Hummel and Dr. Tyler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings illustrate that olfactory problems are common not only during the acute COVID-19 phase but also “in the long run” and that these problems should be “taken into consideration” when following up these patients, study investigator Johannes Frasnelli, MD, professor, department of anatomy, Université du Québec à Trois-Rivières, said in an interview.
Loss of the sense of smell can affect quality of life because it affects eating and drinking, and may even be dangerous, said Dr. Frasnelli. “If your sense of smell is impaired, you may unknowingly eat spoiled food, or you may not smell smoke or gas in your home,” he said. In addition, Dr. Frasnelli noted that an impaired sense of smell is associated with higher rates of depression. The findings will be presented at the annual meeting of the American Academy of Neurology in April.
‘Striking’ finding
Research shows that about 60% of patients with COVID-19 lose their sense of smell to some degree during the acute phase of the disease. “But we wanted to go further and look at the longer-term effects of loss of smell and taste,” said Dr. Frasnelli.
The analysis included 813 health care workers in the province of Quebec. For all the patients, SARS-CoV-2 infection was confirmed through testing with a nasopharyngeal viral swab.
Participants completed a 64-item online questionnaire that asked about three senses: olfactory; gustatory, which includes tastes such as sweet, sour, bitter, salty, savory and umami; and trigeminal, which includes sensations such as spiciness of hot peppers and “coolness” of mint.
They were asked to rate these on a scale of 0 (no perception) to 10 (very strong perception) before the infection, during the infection, and currently. They were also asked about other symptoms, including fatigue.
Most respondents had been infected in the first wave of the virus in March and April of 2020 and responded to the questionnaire an average of 5 months later.
The vast majority of respondents (84.1%) were women, which Dr. Frasnelli said was not surprising because women predominate in the health care field.
The analysis showed that average smell ratings were 8.98 before infection, 2.85 during the acute phase, and 7.41 when respondents answered the questionnaire. The sense of taste was less affected and recovered faster than did the sense of smell. Results for taste were 9.20 before infection, 3.59 during the acute phase, and 8.05 after COVID-19.
Among 580 respondents who indicated a compromised sense of smell during the acute phase, the average smell rating when answering the questionnaire was 6.89, compared to 9.03 before the infection. More than half (51.2%) reported not regaining full olfactory function.
The fact that the sense of smell had not returned to normal for half the participants so long after being infected is “novel and quite striking,” said Dr. Frasnelli.
However, he noted, this doesn’t necessarily mean all those with a compromised sense of smell “have huge problems.” In some cases, he said, the problem “is more subtle.”
Not a CNS problem?
Respondents also completed a chemosensory dysfunction home test (CD-HT). They were asked to prepare common household food items, such as peanut butter, sugar, salt, and vinegar, in a particular way – for example, to add sugar or salt to water – and provide feedback on how they smell and taste.
For this CD-HT analysis, 18.4% of respondents reported having persistent loss of smell. This, Dr. Frasnelli said, adds to evidence from self-reported responses and suggests that in some cases, the problem is more than senses not returning to normal.
“From the questionnaires, roughly 50% said their sense of smell is still not back to normal, and when we look at the CD home test, we see that almost 20% of subjects indeed have pretty strong impairment of their sense of smell,” he said.
The results showed no sex differences, although Dr. Frasnelli noted that most of the sample were women. “It’s tricky to look at the data with regard to sex because it’s a bit skewed,” he said.
Male respondents were older than female participants, but there was no difference in impairment between age groups. Dr. Frasnelli said this was “quite interesting,” inasmuch as older people usually lose some sense of smell.
The researchers have not yet examined whether the results differ by type of health care worker.
They also have not examined in detail whether infection severity affects the risk for extended olfactory impairment. Although some research suggests that the problem with smell is more common in less severe cases, Dr. Frasnelli noted this could be because loss of smell is not a huge problem for patients battling grave health problems.
As for other symptoms, many respondents reported lingering fatigue; some reported debilitating fatigue, said Dr. Frasnelli. However, he cautioned that this is difficult to interpret, because the participants were health care workers, many of whom returned to work during the pandemic and perhaps had not fully rested.
He also noted that he and his colleagues have not “made the link” between impaired smell and the degree of fatigue.
The COVID-19 virus appears to attack supporting sustentacular cells in the olfactory epithelium, not nerve cells.
“Right now, it seems that the smell problem is not a central nervous system problem but a peripheral problem,” said Dr. Frasnelli. “But we don’t know for sure; it may be that the virus somehow gets into the brain and some symptoms are caused by the effects of the infection on the brain.”
The researchers will extend their research with another questionnaire to assess senses 10-12 months after COVID-19.
Limitations of the study include the subjective nature of the smell and taste ratings and the single time point at which data were collected.
Confirmatory findings
Commenting on the research in an interview, Thomas Hummel, MD, professor, smell and taste clinic, department of otorhinolaryngology, Technische Universität Dresden (Germany), said the new results regarding loss of smell after COVID-19 are “very congruent” with what he and his colleagues have observed.
Research shows that up to one in five of those infected with SARS-CoV-2 experience olfactory loss. “While the numbers may vary a bit from study to study or lab to lab, I think 5% to 20% of post–COVID-19 patients exhibit long-term olfactory loss,” Dr. Hummel said.
His group has observed that “many more are not back to normal,” which conforms with what Dr. Frasnelli’s study reveals, said Dr. Hummel.
Also commenting on the research, Kenneth L. Tyler, MD, professor of neurology, University of Colorado at Denver, Aurora, and a fellow of the American Academy of Neurology, said the study was relatively large and the results “interesting.”
Although it “provides more evidence there’s a subset of patients with symptoms even well past the acute phase” of COVID-19, the results are “mostly confirmatory” and include “nothing super surprising,” Dr. Tyler said in an interview.
However, the investigators did attempt to make the study “a little more quantitative” and “to confirm the self-reporting with their validated CD home test,” he said.
Dr. Tyler wondered how representative the sample was and whether the study drew more participants with impaired senses. “If I had a loss of smell or taste, maybe I would be more likely to respond to such a survey,” he said.
He also noted the difficulty of separating loss of smell from loss of taste.
“If you lose your sense of smell, things don’t taste right, so it can be confounding as to how to separate out those two,” he noted.
The study was supported by the Foundation of the Université du Québec à Trois-Rivières and the Province of Quebec. Dr. Frasnelli has received royalties from Styriabooks in Austria for a book on olfaction published in 2019 and has received honoraria for speaking engagements. Dr. Hummel and Dr. Tyler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.