User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
FDA approves point-of-care COVID-19 antigen test

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.
COVID-19 vaccine supply will be limited at first, ACIP says
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
Asymptomatic SARS-CoV-2 infections in kids tied to local rates
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Convalescent plasma actions spark trial recruitment concerns
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
Prognosis for rural hospitals worsens with pandemic
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
When viruses collide: Flu season during pandemic
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
As COVID-19 cases increase in children, deaths remain low
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
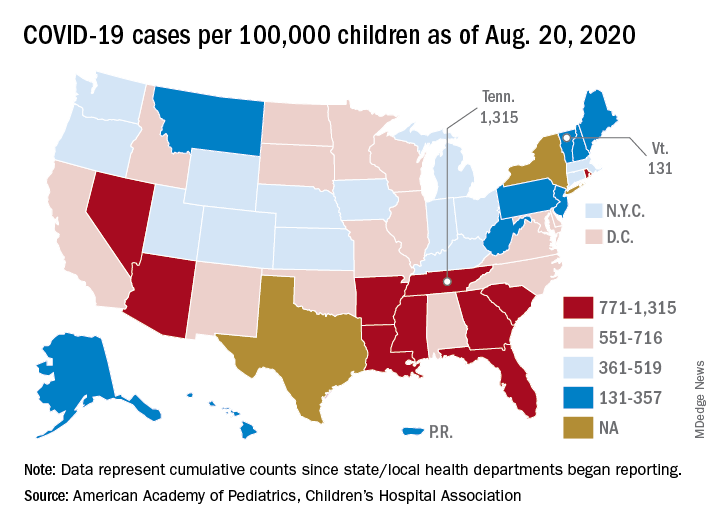
The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
First evidence of SARS-CoV-2 in heart cells
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
Famotidine associated with benefits in hospitalized COVID patients in another trial
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
It also demonstrated lower levels of serum markers for severe disease.
The findings come from an observational study of 83 hospitalized patients that was published in the American Journal of Gastroenterology.
“The mechanism of exactly how famotidine works has yet to be proven,” lead study author Jeffrey F. Mather, MS, said in an interview. “There’s thought that it works directly on the virus, and there is thought that it works through inactivating certain proteases that are required for the virus infection, but I think the most interesting [hypothesis] is by Malone et al. “They’re looking at the blocking of the histamine-2 receptor causing a decrease in the amount of histamine. It’s all speculative, but it will be interesting if that gets worked out.”
In a study that largely mimicked that of an earlier, larger published observational study on the topic (doi: 10.1053/j.gastro.2020.05.053), Mr. Mather and colleagues retrospectively evaluated 878 patients who tested positive for SARS-CoV-2 and who required admission to Hartford (Conn.) Hospital between Feb. 24, 2020, and May 14, 2020. Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug within 1 week of COVID-19 screening and/or hospital admission. Primary outcomes of interest were in-hospital death as recorded in the discharge of the patients, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes of interest were several serum markers of disease activity including white blood cell count, lymphocyte count, and eosinophil count.
Famotidine was administered orally in 83% of the patients and intravenously in the remaining 17%. Mr. Mather, director of data management in the division of research management at Hartford Hospital, and his colleagues reported that 83 of the 878 patients studied (9.5%) received famotidine. Compared with patients not treated with famotidine, those who received the drug were slightly younger (a mean of 64 vs. 68 years, respectively; P = .021); otherwise, there were no differences between the two groups in baseline demographics or in preexisting comorbidities.
The use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio, 0.37; P = .021) as well as combined death or intubation (OR, 0.47; P = .040). The outcomes were similar when the researchers performed propensity score matching to adjust for age differences between groups.
In addition, the use of famotidine was associated with lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs. 12.7 mg/dL; P =. 002), lower median procalcitonin levels (0.16 vs. 0.30 ng/mL; P = .004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs. 964 ng/mL; P = .076).
Logistic regression analysis revealed that use of famotidine was an independent predictor of both lower mortality and combined death/intubation. In addition, predictors of both adverse outcomes included older age, a body mass index of greater than 30 kg/m2, chronic kidney disease, the national early warning score, and a higher neutrophil-lymphocyte ratio.
“This is an important stepping stone, but until we have a randomized, controlled trial, we really can’t speak about causation; we can only speak about association, and that’s okay,” Brennan Spiegel, MD, MSHS, director of health services research at Cedars-Sinai, Los Angeles, who was not affiliated with the study, said in an interview. “There’s nothing wrong with association because finding associations can raise important hypotheses that can then be tested in prospective randomized trials, for example.”
In July 2020, Dr. Spiegel and his colleagues published a separate paper looking at proton pump inhibitors and the risk of COVID-19. “In that study we did look at H2 blockers, and we did find that they were slightly associated with a reduction in COVID-19,” he said. “It was a small effect, but it was a benefit. When we see consistency among studies, it’s a signal in the noise we can try and follow and see if there is something more to it.”
Mr. Mather acknowledged certain limitations of the study, including the fact that patients who did and did not receive famotidine were propensity-matched for age. “The risk factors that others have shown for adverse events are equivalent in the groups, but anytime you do a retrospective study like this there is the potential for underlying factors that may play a role in the outcomes that you’re not considering,” Mr. Mather said. “That’s why the gold standard is the randomized trial, to wash those effects out. There’s only an association here, and it supports the need for a randomized trial.”
Famotidine is currently being tested in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (NCT 04370262).
“It’s fascinating because famotidine is a safe medicine,” added Dr. Spiegel, who is also co–editor in chief of the American Journal of Gastroenterology. “There are very few side effects; it’s something we’ve been using for decades.”
Mr. Mather and his colleagues reported having no financial disclosures. Dr. Spiegel disclosed that he has served on advisory boards for Allergan, Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals.
SOURCE: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
REPORTING FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Key clinical point: Among hospitalized COVID-19 patients, famotidine use was associated with a reduction in death and either death or intubation.
Major finding: The use of famotidine was associated with a decreased risk of in-hospital mortality (OR, 0.37; P = .021), as well as the combined endpoint of death or intubation (OR, 0.47; P = .040).
Study details: A single-center observational study of 83 patients hospitalized with COVID-19.
Disclosures: The researchers reported having no financial disclosures.
Source: Mather J et al. 2020 Aug 14. Am J Gastroenterol.
FDA approves clinical trials for cannabinoid drug designed to reduce COVID-19 lung inflammation
The US Food and Drug Administration has approved phase one clinical trials for a synthetic cannabinoid drug designed to treat acute respiratory distress syndrome (ARDS), a life-threatening lung condition which may occur in severe cases of the novel coronavirus, Forbes reported.
ARDS can be triggered by over-creation of cytokines, proteins which tell the body to produce more inflammation, Forbes said.
The drug going to clinical trials, ARDS-003, would “dampen the cytokine release” and prevent development of ARDS, Tetra Bio-Pharma company CEO and chief regulatory officer Guy Chamberland, MD, said in a news release.
Consequences of ARDS include scarring of the lungs and organ injury caused by the decrease in blood to the tissue, the release said.
“The FDA repeatedly stated that they want clinical trials for COVID-19 to begin as soon as possible, as long as they meet regulatory requirements,” the news release said. “The medical community is in urgent need of drugs that can reduce the strength and duration of the severe inflammation. It is anticipated that this type of new drug would favorably impact health care and possibly reduce the negative health outcomes post infection.”
ARDS-003 works by binding to CB2 receptors, one of two main receptors in the endocannabinoid system which modulate inflammation and cytokine activity, Forbes said. CB2 receptors don’t bring on a psychoactive high.
Phase one clinical trials would begin enrolling participants in December to determine if the drug is safe, Chamberland said, according to Forbes.
If phase one is successful, phase two would test the drug on a larger group in the second quarter of 2021 to assess safety and tolerability for people who have COVID-19.
If phase two is successful, the company may seek emergency authorization through the FDA, Chamberland said. Phase three would start at the end of 2021.
Tetra Bio-Pharma says it has already contracted with Dalton Pharma Services to manufacture the active pharmaceutical ingredient (API), HU-308, and the finished drug product ARDS-003.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved phase one clinical trials for a synthetic cannabinoid drug designed to treat acute respiratory distress syndrome (ARDS), a life-threatening lung condition which may occur in severe cases of the novel coronavirus, Forbes reported.
ARDS can be triggered by over-creation of cytokines, proteins which tell the body to produce more inflammation, Forbes said.
The drug going to clinical trials, ARDS-003, would “dampen the cytokine release” and prevent development of ARDS, Tetra Bio-Pharma company CEO and chief regulatory officer Guy Chamberland, MD, said in a news release.
Consequences of ARDS include scarring of the lungs and organ injury caused by the decrease in blood to the tissue, the release said.
“The FDA repeatedly stated that they want clinical trials for COVID-19 to begin as soon as possible, as long as they meet regulatory requirements,” the news release said. “The medical community is in urgent need of drugs that can reduce the strength and duration of the severe inflammation. It is anticipated that this type of new drug would favorably impact health care and possibly reduce the negative health outcomes post infection.”
ARDS-003 works by binding to CB2 receptors, one of two main receptors in the endocannabinoid system which modulate inflammation and cytokine activity, Forbes said. CB2 receptors don’t bring on a psychoactive high.
Phase one clinical trials would begin enrolling participants in December to determine if the drug is safe, Chamberland said, according to Forbes.
If phase one is successful, phase two would test the drug on a larger group in the second quarter of 2021 to assess safety and tolerability for people who have COVID-19.
If phase two is successful, the company may seek emergency authorization through the FDA, Chamberland said. Phase three would start at the end of 2021.
Tetra Bio-Pharma says it has already contracted with Dalton Pharma Services to manufacture the active pharmaceutical ingredient (API), HU-308, and the finished drug product ARDS-003.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved phase one clinical trials for a synthetic cannabinoid drug designed to treat acute respiratory distress syndrome (ARDS), a life-threatening lung condition which may occur in severe cases of the novel coronavirus, Forbes reported.
ARDS can be triggered by over-creation of cytokines, proteins which tell the body to produce more inflammation, Forbes said.
The drug going to clinical trials, ARDS-003, would “dampen the cytokine release” and prevent development of ARDS, Tetra Bio-Pharma company CEO and chief regulatory officer Guy Chamberland, MD, said in a news release.
Consequences of ARDS include scarring of the lungs and organ injury caused by the decrease in blood to the tissue, the release said.
“The FDA repeatedly stated that they want clinical trials for COVID-19 to begin as soon as possible, as long as they meet regulatory requirements,” the news release said. “The medical community is in urgent need of drugs that can reduce the strength and duration of the severe inflammation. It is anticipated that this type of new drug would favorably impact health care and possibly reduce the negative health outcomes post infection.”
ARDS-003 works by binding to CB2 receptors, one of two main receptors in the endocannabinoid system which modulate inflammation and cytokine activity, Forbes said. CB2 receptors don’t bring on a psychoactive high.
Phase one clinical trials would begin enrolling participants in December to determine if the drug is safe, Chamberland said, according to Forbes.
If phase one is successful, phase two would test the drug on a larger group in the second quarter of 2021 to assess safety and tolerability for people who have COVID-19.
If phase two is successful, the company may seek emergency authorization through the FDA, Chamberland said. Phase three would start at the end of 2021.
Tetra Bio-Pharma says it has already contracted with Dalton Pharma Services to manufacture the active pharmaceutical ingredient (API), HU-308, and the finished drug product ARDS-003.
This article first appeared on Medscape.com.











