User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Latest report adds almost 44,000 child COVID-19 cases in 1 week
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
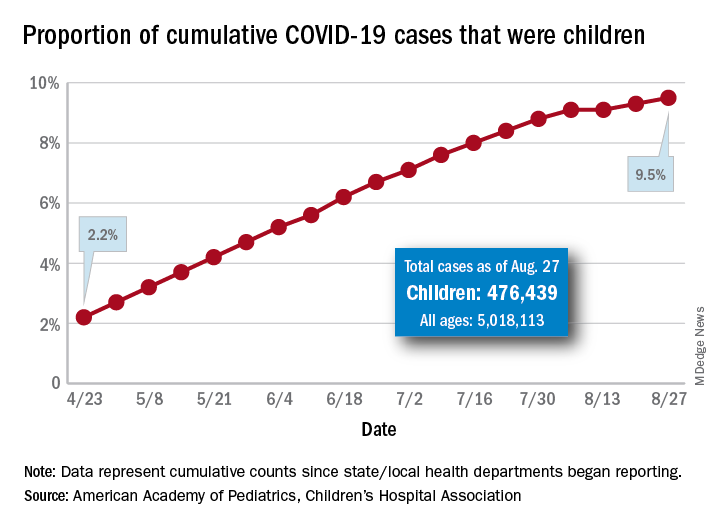
The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
First randomized trial reassures on ACEIs, ARBs in COVID-19
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Three malpractice risks of video visits
During a telemedicine visit with his physician, a 62-year-old obese patient with an ankle injury reported new swelling of his leg. Three weeks had passed since the man visited an emergency department, where he underwent surgery and had a cast applied to the wound. The physician, during the telemedicine visit, advised the patient to elevate his leg and see an orthopedist within 24 hours. A Doppler ultrasound was ordered for 12:30 p.m. that same day.
The patient never made it to the appointment. He became unresponsive and went into full arrest hours later. His death fueled a lawsuit by his family that claimed failure to diagnose and treat deep venous thrombosis. The family contended the providers involved should have referred the patient to care immediately during the video visit.
The case, which comes from the claims database of national medical liability insurer The Doctors Company, illustrates the legal risks that can stem from video visits with patients, says Richard Cahill, JD, vice president and associate general counsel for The Doctors Company.
“By evaluating the patient remotely, the physician failed to appreciate the often subtle nuances of the clinical presentation, which undoubtedly could have been more accurately assessed in the office setting, and would probably have led to more urgent evaluation and intervention, thereby likely preventing the unfortunate and otherwise avoidable result,” said Mr. Cahill.
According to a Harris poll, 42% of Americans reported using video visits during the pandemic, a trend that is likely to continue as practices reopen and virtual care becomes the norm. But as physicians conduct more video visits, so grows their risk for lawsuits associated with the technology.
“We probably will see more malpractice suits filed the more telehealth is used,” said Mei Wa Kwong, JD, executive director of the Center for Connected Health Policy. “It’s a numbers game. The more it’s used, the higher likelihood that lawsuits occur.”
Three problems in not being able to touch the patient
1. The primary challenge with video visits “is the inability to directly observe and lay hands on the patient,” says Jonathan Einbinder, MD, assistant vice president of analytics for CRICO, a medical liability insurer based in Boston.
said Dr. Einbinder, a practicing internist.
Such incomplete pictures can lead to diagnostic errors and the potential for lawsuits, as demonstrated by a recent CRICO analysis. Of 106 telemedicine-related claims from 2014 to 2018, 66% were diagnosis related, according to the analysis of claims from CRICO’s national database. Twelve percent of the telemedicine-related claims were associated with surgical treatment, 11% were related to medical treatment, and 5% were associated with medication issues. A smaller number of claims resulted from patient monitoring, ob.gyn. care, and safety and security.
Another analysis by The Doctors Company similarly determined that diagnostic errors are the most common allegation in telemedicine-related claims. In the study of 28 telemedicine-related claims from The Doctors’ database, 71% were diagnosis related, 11% were associated with mismanagement of treatment, and 7% were related to improper management of a surgical patient. Other allegations included improper performance of treatment or procedure and improper performance of surgery.
“Because a ‘typical’ exam can’t be done, there is the potential to miss things,” said David L. Feldman, MD, chief medical officer for The Doctors Company Group. “A subtlety, perhaps a lump that can’t be seen but only felt, and only by an experienced examiner, for example, may be missed.”
2. Documentation dangers also loom, said William Sullivan, DO, JD, an emergency physician and an attorney who specializes in health care. The legal risk lies in documenting a video visit in the same way the doctor would document an in-person visit, he explained.
“Investigation into a potential lawsuit begins when there is some type of bad outcome related to medical care,” Dr. Sullivan explained. “To determine whether the lawsuit has merit, patients/attorneys review the medical records to retrospectively determine the potential cause of the bad outcome. If the documentation reflects an examination that could not have been performed, a lawyer might be more likely to pursue a case, and it would be more difficult to defend the care provided.”
Dr. Sullivan provided this example: During a video visit, a patient complains of acute onset weakness. The physician documents that the patient’s heart has a “regular rate and rhythm,” and “muscle strength is equal bilaterally.” The following day, the patient’s weakness continues, and the patient goes to the emergency department where he is diagnosed with stroke. An EKG in the ED shows that the patient is in atrial fibrillation.
“The telehealth provider would have a difficult time explaining how it was determined that the patient had normal muscle strength and a normal heart rhythm over a video visit the day before,” Dr. Sullivan said. “A lawyer in a subsequent malpractice case would present the provider as careless and would argue that if the provider had only sent the patient to the emergency department after the telehealth visit instead of documenting exam findings that couldn’t have been performed, the patient could have been successfully treated for the stroke.”
3. Poorly executed informed consent can also give rise to a lawsuit. This includes informed consent regarding the use of telehealth as the accepted modality for the visit rather than traditional on-site evaluations, as well as preprocedure informed consent.
“Inadequate and/or poorly documented informed consent can result in a claim for medical battery,” Mr. Cahill said.
A medical battery allegation refers to the alleged treatment or touching of a patient’s body without that person’s consent. As the AMA Journal of Ethics explains, a patient’s consent must be given, either expressly or implicitly, before a physician may legally “interfere” with the physical body of the patient.
Ideally, the informed consent process is undertaken during a first in-person visit, before virtual visits begin, Dr. Feldman said.
“There is a lot that a patient has to understand when a visit is done virtually, which is part of the informed consent process,” Dr. Feldman said. “The pandemic has forced some physicians to do their first visit virtually, and this makes the process of informing patients more onerous. It is not a simple matter of converting an in-person office practice to a remote office practice. The work flows are different, so there are definitely legal concerns as it relates to privacy and cybersecurity to name a few.”
Waivers may be weak protection
Since the pandemic started, a number of states have enacted emergency malpractice protections to shield health professionals from lawsuits. Some protections, such as those in Massachusetts, offer immunity to health professionals who provide general care to patients during the COVID-19 emergency, in addition to treatment of COVID-19 patients. Other protections, like those in Connecticut, apply specifically to care provided in support of the state’s pandemic response.
Whether that immunity applies both to in-person visits and video visits during the pandemic is not certain, said J. Richard Moore, JD, a medical liability defense attorney based in Indianapolis. Indiana’s immunity statute for example, does not make a specific provision for telehealth, he said.
“My best prediction is that if considered by the courts, the immunity would be applied to telehealth services, so long as they are being provided ‘in response to the emergency,’ which is the scope of the immunity,” he said. “I would not consider telehealth physicians to be either more or less protected than in-person providers.”
Regulatory scrutiny for telehealth providers has also been relaxed in response to COVID-19, but experts warn not to rely on the temporary shields for ultimate protection.
In March, the U.S. Department of Health and Human Service’s Office of Civil Rights (OCR) eased enforcement actions for noncompliance with Health Insurance Portability and Accountability Act requirements in connection with the good faith provision of telehealth during the COVID-19 health crisis. Under the notice, health providers can use popular applications such as Apple FaceTime, Facebook Messenger, Zoom, or Google Hangouts, to offer telehealth care without risk that OCR will impose fines or penalties for HIPAA violations.
But once the current health care emergency is mitigated, the waivers will likely be withdrawn, and enforcement actions will probably resume, Mr. Cahill said.
“It is recommended that, to avoid potential problems going forward, practitioners use due diligence and undertake best efforts to obey existing privacy and security requirements, including the use of technology that satisfies compliance regulations, despite the waiver by OCR,” he said.
In addition, a majority of states have relaxed state-specific rules for practicing telehealth and loosened licensure requirements during the pandemic. At least 47 states have issued waivers to alter in-state licensure requirements for telemedicine in response to COVID-19, according to the Federation of State Medical Boards. Most of the waivers allow physicians licensed in other states to provide care in states where they do not hold licenses, and some enable doctors to treat patients without first having had an in-person evaluation.
But at least for now, these are temporary changes, reminds Amy Lerman, JD, a health care attorney based in Washington, who specializes in telehealth and corporate compliance. Given the current pandemic environment, a significant concern is that physicians new to the telemedicine space are reacting only to the most recent rules established in the context of the pandemic, Ms. Lerman said.
“As previously noted, the recent developments are temporary in nature – states and various federal agencies have been pretty clear in setting this temporal boundary,” she said. “It is not advisable for providers to build telepractice models around temporary sets of rules.
“Furthermore, the recent developments are not necessarily comprehensive relative to all of the state-specific and other requirements that telemedicine providers are otherwise expected to follow, so relying only on the most recent guidance may cause providers to create telepractice models that have key gaps with respect to regulatory compliance.”
How you can avoid a lawsuit
As businesses reopen and practices resume treatments, physicians should weigh the choice between in-person care and video visits very carefully, said Joseph Kvedar, MD, president of the American Telemedicine Association and a dermatology professor at Harvard Medical School, Boston.
“We have to be very thoughtful about quality in this current phase, where we are doing what I call a hybrid model,” he said. “Some services are offered by telehealth and some require patients to come into the doctor’s office. We have to be very thoughtful about what types of care we determine to be appropriate for telehealth, and that has to be based on clinical quality. And if it is, it should follow that we’ll have low incidence of liability claims.”
Data should be at the center of that conclusion, Dr. Kvedar advises.
“Think about what data is needed to make a therapeutic or diagnostic decision,” he said. “If a health care provider can gather the information needed without touching the patient, then the provider is probably on safe, solid ground making that decision via a telehealth interaction. If the patient can come into the doctor’s office, and the provider deems it necessary to see the patient in person and touch the patient in order to make that clinical decision, then the patient should come in.”
An important step to preventing liability is also having strong telehealth systems and protocols in place and the necessary support to carry them out, said Dr. Einbinder of medical liability insurer CRICO.
For example, Dr. Einbinder, who practices in a 12-doctor internal medicine group, said when he finishes a virtual visit, he enters any orders into the electronic health record. Some of the orders will result in notifications to Dr. Einbinder if they are not executed, such as a referral appointment or a procedure that was not completed.
“I also can forward my orders to a front desk pool that is responsible for making sure things get done,” he said. “And, in our hospital system, we have good case management for complex patients and population management for a variety of chronic conditions. These represent additional safety nets.”
Another liability safeguard is sending patients a “visit summary” after each virtual visit, Dr. Sullivan said. This could be in the form of an email or a text that includes a brief template including items such as diagnosis, recommendations, follow-up, and a reminder to contact the doctor or go to the emergency department if symptoms worsen or new problems develop.
“Patients tend to remember about half of what physicians tell them and half of the information patients do remember is incorrect,” he said. “Consider a few sentences in an e-mail or text message as a substitute for the after-visit instructions from an office visit to enhance patient understanding. There are several inexpensive programs/services that allow text messages to be sent from a computer using a separate dedicated phone number and pretty much every patient has a cell phone to receive the instructions.”
Dr. Sullivan suggests having a documentation template specifically for telehealth visits. He also recommends the inclusion an “informed refusal of care” in the record when necessary. Dr. Sullivan’s wife, a family physician, has encountered several patients who fear contracting COVID-19 and who have refused her recommendations for in-person visits, he said. In such cases, he said it’s a good idea to document that the patient decided to forgo the recommendations given.
“If a patient suffers a bad outcome because of a failure to seek an in-person exam, a short note in the patient’s chart would help to establish that the lack of a follow-up physical exam was the patient’s informed decision, not due to some alleged negligence of the medical provider,” he said.
Concerning informed consent, Dr. Feldman says at a minimum physicians should discuss the following with patients:
- Names and credentials of staff participating.
- The right to stop or refuse treatment by telemedicine.
- Technology that will be used.
- Privacy and security risks.
- Technology-specific risks and permission to bill.
- Alternative care in case of an emergency or technology malfunction.
- Any state-specific requirements.
“Physicians can ensure they have a strong informed consent process during video visits by taking the time to cover these points at the beginning of the first visit, and being sure the patient understands and agrees to these,” Dr. Sullivan explained. “Ideally, this conversation can be recorded for future reference if necessary or at a minimum documented in the medical record.”
Consider these extra precautions
Mr. Cahill advises that practitioners be especially mindful of their “web-side manner” and the setting in which they are communicating with virtual patients to promote confidentiality, professionalism, and uninterrupted interactions.
“Use of a headset in a quiet home office is advisable,” he said. “Physicians must also be cognizant of their physical appearance and the background behind them when the visit includes both audio and visual capability. For ‘face-to-face’ telehealth encounters, it is recommended that a white lab jacket be worn as the appropriate attire; coat and tie are unnecessary.”
Some patients may need to be reminded of the need for confidentiality during a video visit, Mr. Moore added. Physicians are typically in a position to ensure confidentiality, but some patients may not understand how to protect their privacy on their end.
“If the physician sees on the screen or hears from an audio connection that there are other people around who may be able to overhear what is communicated, the physician probably has some responsibility to remind the patient that she or he may want to go to a more private place, close the door, etc.,” he said. “While I think a claim against a physician on this basis would be pretty weak, it is still a good practice for the physician to be cognizant of those kinds of concerns even if the patient is not.”
Finally, for physicians who set up telehealth operability during the pandemic – possibly in a hurry – consider using your actual case data to take a look backward, said Ms. Lerman, the Washington-based health care attorney. Reviewing the data can help determine whether you’re in compliance with relevant state laws, she said.
“If, for example, a provider set up telehealth operations during the pandemic and can see that most of [the] patients are based in a single state, or a small group of states, it is worthwhile to take [the] time and become familiar with the telemedicine laws in those states,” she said. “If there are modifications that need to be made, it may be easier to make them incrementally before the telehealth operations grow any larger in scope.”
A version of this article originally appeared on Medscape.com.
During a telemedicine visit with his physician, a 62-year-old obese patient with an ankle injury reported new swelling of his leg. Three weeks had passed since the man visited an emergency department, where he underwent surgery and had a cast applied to the wound. The physician, during the telemedicine visit, advised the patient to elevate his leg and see an orthopedist within 24 hours. A Doppler ultrasound was ordered for 12:30 p.m. that same day.
The patient never made it to the appointment. He became unresponsive and went into full arrest hours later. His death fueled a lawsuit by his family that claimed failure to diagnose and treat deep venous thrombosis. The family contended the providers involved should have referred the patient to care immediately during the video visit.
The case, which comes from the claims database of national medical liability insurer The Doctors Company, illustrates the legal risks that can stem from video visits with patients, says Richard Cahill, JD, vice president and associate general counsel for The Doctors Company.
“By evaluating the patient remotely, the physician failed to appreciate the often subtle nuances of the clinical presentation, which undoubtedly could have been more accurately assessed in the office setting, and would probably have led to more urgent evaluation and intervention, thereby likely preventing the unfortunate and otherwise avoidable result,” said Mr. Cahill.
According to a Harris poll, 42% of Americans reported using video visits during the pandemic, a trend that is likely to continue as practices reopen and virtual care becomes the norm. But as physicians conduct more video visits, so grows their risk for lawsuits associated with the technology.
“We probably will see more malpractice suits filed the more telehealth is used,” said Mei Wa Kwong, JD, executive director of the Center for Connected Health Policy. “It’s a numbers game. The more it’s used, the higher likelihood that lawsuits occur.”
Three problems in not being able to touch the patient
1. The primary challenge with video visits “is the inability to directly observe and lay hands on the patient,” says Jonathan Einbinder, MD, assistant vice president of analytics for CRICO, a medical liability insurer based in Boston.
said Dr. Einbinder, a practicing internist.
Such incomplete pictures can lead to diagnostic errors and the potential for lawsuits, as demonstrated by a recent CRICO analysis. Of 106 telemedicine-related claims from 2014 to 2018, 66% were diagnosis related, according to the analysis of claims from CRICO’s national database. Twelve percent of the telemedicine-related claims were associated with surgical treatment, 11% were related to medical treatment, and 5% were associated with medication issues. A smaller number of claims resulted from patient monitoring, ob.gyn. care, and safety and security.
Another analysis by The Doctors Company similarly determined that diagnostic errors are the most common allegation in telemedicine-related claims. In the study of 28 telemedicine-related claims from The Doctors’ database, 71% were diagnosis related, 11% were associated with mismanagement of treatment, and 7% were related to improper management of a surgical patient. Other allegations included improper performance of treatment or procedure and improper performance of surgery.
“Because a ‘typical’ exam can’t be done, there is the potential to miss things,” said David L. Feldman, MD, chief medical officer for The Doctors Company Group. “A subtlety, perhaps a lump that can’t be seen but only felt, and only by an experienced examiner, for example, may be missed.”
2. Documentation dangers also loom, said William Sullivan, DO, JD, an emergency physician and an attorney who specializes in health care. The legal risk lies in documenting a video visit in the same way the doctor would document an in-person visit, he explained.
“Investigation into a potential lawsuit begins when there is some type of bad outcome related to medical care,” Dr. Sullivan explained. “To determine whether the lawsuit has merit, patients/attorneys review the medical records to retrospectively determine the potential cause of the bad outcome. If the documentation reflects an examination that could not have been performed, a lawyer might be more likely to pursue a case, and it would be more difficult to defend the care provided.”
Dr. Sullivan provided this example: During a video visit, a patient complains of acute onset weakness. The physician documents that the patient’s heart has a “regular rate and rhythm,” and “muscle strength is equal bilaterally.” The following day, the patient’s weakness continues, and the patient goes to the emergency department where he is diagnosed with stroke. An EKG in the ED shows that the patient is in atrial fibrillation.
“The telehealth provider would have a difficult time explaining how it was determined that the patient had normal muscle strength and a normal heart rhythm over a video visit the day before,” Dr. Sullivan said. “A lawyer in a subsequent malpractice case would present the provider as careless and would argue that if the provider had only sent the patient to the emergency department after the telehealth visit instead of documenting exam findings that couldn’t have been performed, the patient could have been successfully treated for the stroke.”
3. Poorly executed informed consent can also give rise to a lawsuit. This includes informed consent regarding the use of telehealth as the accepted modality for the visit rather than traditional on-site evaluations, as well as preprocedure informed consent.
“Inadequate and/or poorly documented informed consent can result in a claim for medical battery,” Mr. Cahill said.
A medical battery allegation refers to the alleged treatment or touching of a patient’s body without that person’s consent. As the AMA Journal of Ethics explains, a patient’s consent must be given, either expressly or implicitly, before a physician may legally “interfere” with the physical body of the patient.
Ideally, the informed consent process is undertaken during a first in-person visit, before virtual visits begin, Dr. Feldman said.
“There is a lot that a patient has to understand when a visit is done virtually, which is part of the informed consent process,” Dr. Feldman said. “The pandemic has forced some physicians to do their first visit virtually, and this makes the process of informing patients more onerous. It is not a simple matter of converting an in-person office practice to a remote office practice. The work flows are different, so there are definitely legal concerns as it relates to privacy and cybersecurity to name a few.”
Waivers may be weak protection
Since the pandemic started, a number of states have enacted emergency malpractice protections to shield health professionals from lawsuits. Some protections, such as those in Massachusetts, offer immunity to health professionals who provide general care to patients during the COVID-19 emergency, in addition to treatment of COVID-19 patients. Other protections, like those in Connecticut, apply specifically to care provided in support of the state’s pandemic response.
Whether that immunity applies both to in-person visits and video visits during the pandemic is not certain, said J. Richard Moore, JD, a medical liability defense attorney based in Indianapolis. Indiana’s immunity statute for example, does not make a specific provision for telehealth, he said.
“My best prediction is that if considered by the courts, the immunity would be applied to telehealth services, so long as they are being provided ‘in response to the emergency,’ which is the scope of the immunity,” he said. “I would not consider telehealth physicians to be either more or less protected than in-person providers.”
Regulatory scrutiny for telehealth providers has also been relaxed in response to COVID-19, but experts warn not to rely on the temporary shields for ultimate protection.
In March, the U.S. Department of Health and Human Service’s Office of Civil Rights (OCR) eased enforcement actions for noncompliance with Health Insurance Portability and Accountability Act requirements in connection with the good faith provision of telehealth during the COVID-19 health crisis. Under the notice, health providers can use popular applications such as Apple FaceTime, Facebook Messenger, Zoom, or Google Hangouts, to offer telehealth care without risk that OCR will impose fines or penalties for HIPAA violations.
But once the current health care emergency is mitigated, the waivers will likely be withdrawn, and enforcement actions will probably resume, Mr. Cahill said.
“It is recommended that, to avoid potential problems going forward, practitioners use due diligence and undertake best efforts to obey existing privacy and security requirements, including the use of technology that satisfies compliance regulations, despite the waiver by OCR,” he said.
In addition, a majority of states have relaxed state-specific rules for practicing telehealth and loosened licensure requirements during the pandemic. At least 47 states have issued waivers to alter in-state licensure requirements for telemedicine in response to COVID-19, according to the Federation of State Medical Boards. Most of the waivers allow physicians licensed in other states to provide care in states where they do not hold licenses, and some enable doctors to treat patients without first having had an in-person evaluation.
But at least for now, these are temporary changes, reminds Amy Lerman, JD, a health care attorney based in Washington, who specializes in telehealth and corporate compliance. Given the current pandemic environment, a significant concern is that physicians new to the telemedicine space are reacting only to the most recent rules established in the context of the pandemic, Ms. Lerman said.
“As previously noted, the recent developments are temporary in nature – states and various federal agencies have been pretty clear in setting this temporal boundary,” she said. “It is not advisable for providers to build telepractice models around temporary sets of rules.
“Furthermore, the recent developments are not necessarily comprehensive relative to all of the state-specific and other requirements that telemedicine providers are otherwise expected to follow, so relying only on the most recent guidance may cause providers to create telepractice models that have key gaps with respect to regulatory compliance.”
How you can avoid a lawsuit
As businesses reopen and practices resume treatments, physicians should weigh the choice between in-person care and video visits very carefully, said Joseph Kvedar, MD, president of the American Telemedicine Association and a dermatology professor at Harvard Medical School, Boston.
“We have to be very thoughtful about quality in this current phase, where we are doing what I call a hybrid model,” he said. “Some services are offered by telehealth and some require patients to come into the doctor’s office. We have to be very thoughtful about what types of care we determine to be appropriate for telehealth, and that has to be based on clinical quality. And if it is, it should follow that we’ll have low incidence of liability claims.”
Data should be at the center of that conclusion, Dr. Kvedar advises.
“Think about what data is needed to make a therapeutic or diagnostic decision,” he said. “If a health care provider can gather the information needed without touching the patient, then the provider is probably on safe, solid ground making that decision via a telehealth interaction. If the patient can come into the doctor’s office, and the provider deems it necessary to see the patient in person and touch the patient in order to make that clinical decision, then the patient should come in.”
An important step to preventing liability is also having strong telehealth systems and protocols in place and the necessary support to carry them out, said Dr. Einbinder of medical liability insurer CRICO.
For example, Dr. Einbinder, who practices in a 12-doctor internal medicine group, said when he finishes a virtual visit, he enters any orders into the electronic health record. Some of the orders will result in notifications to Dr. Einbinder if they are not executed, such as a referral appointment or a procedure that was not completed.
“I also can forward my orders to a front desk pool that is responsible for making sure things get done,” he said. “And, in our hospital system, we have good case management for complex patients and population management for a variety of chronic conditions. These represent additional safety nets.”
Another liability safeguard is sending patients a “visit summary” after each virtual visit, Dr. Sullivan said. This could be in the form of an email or a text that includes a brief template including items such as diagnosis, recommendations, follow-up, and a reminder to contact the doctor or go to the emergency department if symptoms worsen or new problems develop.
“Patients tend to remember about half of what physicians tell them and half of the information patients do remember is incorrect,” he said. “Consider a few sentences in an e-mail or text message as a substitute for the after-visit instructions from an office visit to enhance patient understanding. There are several inexpensive programs/services that allow text messages to be sent from a computer using a separate dedicated phone number and pretty much every patient has a cell phone to receive the instructions.”
Dr. Sullivan suggests having a documentation template specifically for telehealth visits. He also recommends the inclusion an “informed refusal of care” in the record when necessary. Dr. Sullivan’s wife, a family physician, has encountered several patients who fear contracting COVID-19 and who have refused her recommendations for in-person visits, he said. In such cases, he said it’s a good idea to document that the patient decided to forgo the recommendations given.
“If a patient suffers a bad outcome because of a failure to seek an in-person exam, a short note in the patient’s chart would help to establish that the lack of a follow-up physical exam was the patient’s informed decision, not due to some alleged negligence of the medical provider,” he said.
Concerning informed consent, Dr. Feldman says at a minimum physicians should discuss the following with patients:
- Names and credentials of staff participating.
- The right to stop or refuse treatment by telemedicine.
- Technology that will be used.
- Privacy and security risks.
- Technology-specific risks and permission to bill.
- Alternative care in case of an emergency or technology malfunction.
- Any state-specific requirements.
“Physicians can ensure they have a strong informed consent process during video visits by taking the time to cover these points at the beginning of the first visit, and being sure the patient understands and agrees to these,” Dr. Sullivan explained. “Ideally, this conversation can be recorded for future reference if necessary or at a minimum documented in the medical record.”
Consider these extra precautions
Mr. Cahill advises that practitioners be especially mindful of their “web-side manner” and the setting in which they are communicating with virtual patients to promote confidentiality, professionalism, and uninterrupted interactions.
“Use of a headset in a quiet home office is advisable,” he said. “Physicians must also be cognizant of their physical appearance and the background behind them when the visit includes both audio and visual capability. For ‘face-to-face’ telehealth encounters, it is recommended that a white lab jacket be worn as the appropriate attire; coat and tie are unnecessary.”
Some patients may need to be reminded of the need for confidentiality during a video visit, Mr. Moore added. Physicians are typically in a position to ensure confidentiality, but some patients may not understand how to protect their privacy on their end.
“If the physician sees on the screen or hears from an audio connection that there are other people around who may be able to overhear what is communicated, the physician probably has some responsibility to remind the patient that she or he may want to go to a more private place, close the door, etc.,” he said. “While I think a claim against a physician on this basis would be pretty weak, it is still a good practice for the physician to be cognizant of those kinds of concerns even if the patient is not.”
Finally, for physicians who set up telehealth operability during the pandemic – possibly in a hurry – consider using your actual case data to take a look backward, said Ms. Lerman, the Washington-based health care attorney. Reviewing the data can help determine whether you’re in compliance with relevant state laws, she said.
“If, for example, a provider set up telehealth operations during the pandemic and can see that most of [the] patients are based in a single state, or a small group of states, it is worthwhile to take [the] time and become familiar with the telemedicine laws in those states,” she said. “If there are modifications that need to be made, it may be easier to make them incrementally before the telehealth operations grow any larger in scope.”
A version of this article originally appeared on Medscape.com.
During a telemedicine visit with his physician, a 62-year-old obese patient with an ankle injury reported new swelling of his leg. Three weeks had passed since the man visited an emergency department, where he underwent surgery and had a cast applied to the wound. The physician, during the telemedicine visit, advised the patient to elevate his leg and see an orthopedist within 24 hours. A Doppler ultrasound was ordered for 12:30 p.m. that same day.
The patient never made it to the appointment. He became unresponsive and went into full arrest hours later. His death fueled a lawsuit by his family that claimed failure to diagnose and treat deep venous thrombosis. The family contended the providers involved should have referred the patient to care immediately during the video visit.
The case, which comes from the claims database of national medical liability insurer The Doctors Company, illustrates the legal risks that can stem from video visits with patients, says Richard Cahill, JD, vice president and associate general counsel for The Doctors Company.
“By evaluating the patient remotely, the physician failed to appreciate the often subtle nuances of the clinical presentation, which undoubtedly could have been more accurately assessed in the office setting, and would probably have led to more urgent evaluation and intervention, thereby likely preventing the unfortunate and otherwise avoidable result,” said Mr. Cahill.
According to a Harris poll, 42% of Americans reported using video visits during the pandemic, a trend that is likely to continue as practices reopen and virtual care becomes the norm. But as physicians conduct more video visits, so grows their risk for lawsuits associated with the technology.
“We probably will see more malpractice suits filed the more telehealth is used,” said Mei Wa Kwong, JD, executive director of the Center for Connected Health Policy. “It’s a numbers game. The more it’s used, the higher likelihood that lawsuits occur.”
Three problems in not being able to touch the patient
1. The primary challenge with video visits “is the inability to directly observe and lay hands on the patient,” says Jonathan Einbinder, MD, assistant vice president of analytics for CRICO, a medical liability insurer based in Boston.
said Dr. Einbinder, a practicing internist.
Such incomplete pictures can lead to diagnostic errors and the potential for lawsuits, as demonstrated by a recent CRICO analysis. Of 106 telemedicine-related claims from 2014 to 2018, 66% were diagnosis related, according to the analysis of claims from CRICO’s national database. Twelve percent of the telemedicine-related claims were associated with surgical treatment, 11% were related to medical treatment, and 5% were associated with medication issues. A smaller number of claims resulted from patient monitoring, ob.gyn. care, and safety and security.
Another analysis by The Doctors Company similarly determined that diagnostic errors are the most common allegation in telemedicine-related claims. In the study of 28 telemedicine-related claims from The Doctors’ database, 71% were diagnosis related, 11% were associated with mismanagement of treatment, and 7% were related to improper management of a surgical patient. Other allegations included improper performance of treatment or procedure and improper performance of surgery.
“Because a ‘typical’ exam can’t be done, there is the potential to miss things,” said David L. Feldman, MD, chief medical officer for The Doctors Company Group. “A subtlety, perhaps a lump that can’t be seen but only felt, and only by an experienced examiner, for example, may be missed.”
2. Documentation dangers also loom, said William Sullivan, DO, JD, an emergency physician and an attorney who specializes in health care. The legal risk lies in documenting a video visit in the same way the doctor would document an in-person visit, he explained.
“Investigation into a potential lawsuit begins when there is some type of bad outcome related to medical care,” Dr. Sullivan explained. “To determine whether the lawsuit has merit, patients/attorneys review the medical records to retrospectively determine the potential cause of the bad outcome. If the documentation reflects an examination that could not have been performed, a lawyer might be more likely to pursue a case, and it would be more difficult to defend the care provided.”
Dr. Sullivan provided this example: During a video visit, a patient complains of acute onset weakness. The physician documents that the patient’s heart has a “regular rate and rhythm,” and “muscle strength is equal bilaterally.” The following day, the patient’s weakness continues, and the patient goes to the emergency department where he is diagnosed with stroke. An EKG in the ED shows that the patient is in atrial fibrillation.
“The telehealth provider would have a difficult time explaining how it was determined that the patient had normal muscle strength and a normal heart rhythm over a video visit the day before,” Dr. Sullivan said. “A lawyer in a subsequent malpractice case would present the provider as careless and would argue that if the provider had only sent the patient to the emergency department after the telehealth visit instead of documenting exam findings that couldn’t have been performed, the patient could have been successfully treated for the stroke.”
3. Poorly executed informed consent can also give rise to a lawsuit. This includes informed consent regarding the use of telehealth as the accepted modality for the visit rather than traditional on-site evaluations, as well as preprocedure informed consent.
“Inadequate and/or poorly documented informed consent can result in a claim for medical battery,” Mr. Cahill said.
A medical battery allegation refers to the alleged treatment or touching of a patient’s body without that person’s consent. As the AMA Journal of Ethics explains, a patient’s consent must be given, either expressly or implicitly, before a physician may legally “interfere” with the physical body of the patient.
Ideally, the informed consent process is undertaken during a first in-person visit, before virtual visits begin, Dr. Feldman said.
“There is a lot that a patient has to understand when a visit is done virtually, which is part of the informed consent process,” Dr. Feldman said. “The pandemic has forced some physicians to do their first visit virtually, and this makes the process of informing patients more onerous. It is not a simple matter of converting an in-person office practice to a remote office practice. The work flows are different, so there are definitely legal concerns as it relates to privacy and cybersecurity to name a few.”
Waivers may be weak protection
Since the pandemic started, a number of states have enacted emergency malpractice protections to shield health professionals from lawsuits. Some protections, such as those in Massachusetts, offer immunity to health professionals who provide general care to patients during the COVID-19 emergency, in addition to treatment of COVID-19 patients. Other protections, like those in Connecticut, apply specifically to care provided in support of the state’s pandemic response.
Whether that immunity applies both to in-person visits and video visits during the pandemic is not certain, said J. Richard Moore, JD, a medical liability defense attorney based in Indianapolis. Indiana’s immunity statute for example, does not make a specific provision for telehealth, he said.
“My best prediction is that if considered by the courts, the immunity would be applied to telehealth services, so long as they are being provided ‘in response to the emergency,’ which is the scope of the immunity,” he said. “I would not consider telehealth physicians to be either more or less protected than in-person providers.”
Regulatory scrutiny for telehealth providers has also been relaxed in response to COVID-19, but experts warn not to rely on the temporary shields for ultimate protection.
In March, the U.S. Department of Health and Human Service’s Office of Civil Rights (OCR) eased enforcement actions for noncompliance with Health Insurance Portability and Accountability Act requirements in connection with the good faith provision of telehealth during the COVID-19 health crisis. Under the notice, health providers can use popular applications such as Apple FaceTime, Facebook Messenger, Zoom, or Google Hangouts, to offer telehealth care without risk that OCR will impose fines or penalties for HIPAA violations.
But once the current health care emergency is mitigated, the waivers will likely be withdrawn, and enforcement actions will probably resume, Mr. Cahill said.
“It is recommended that, to avoid potential problems going forward, practitioners use due diligence and undertake best efforts to obey existing privacy and security requirements, including the use of technology that satisfies compliance regulations, despite the waiver by OCR,” he said.
In addition, a majority of states have relaxed state-specific rules for practicing telehealth and loosened licensure requirements during the pandemic. At least 47 states have issued waivers to alter in-state licensure requirements for telemedicine in response to COVID-19, according to the Federation of State Medical Boards. Most of the waivers allow physicians licensed in other states to provide care in states where they do not hold licenses, and some enable doctors to treat patients without first having had an in-person evaluation.
But at least for now, these are temporary changes, reminds Amy Lerman, JD, a health care attorney based in Washington, who specializes in telehealth and corporate compliance. Given the current pandemic environment, a significant concern is that physicians new to the telemedicine space are reacting only to the most recent rules established in the context of the pandemic, Ms. Lerman said.
“As previously noted, the recent developments are temporary in nature – states and various federal agencies have been pretty clear in setting this temporal boundary,” she said. “It is not advisable for providers to build telepractice models around temporary sets of rules.
“Furthermore, the recent developments are not necessarily comprehensive relative to all of the state-specific and other requirements that telemedicine providers are otherwise expected to follow, so relying only on the most recent guidance may cause providers to create telepractice models that have key gaps with respect to regulatory compliance.”
How you can avoid a lawsuit
As businesses reopen and practices resume treatments, physicians should weigh the choice between in-person care and video visits very carefully, said Joseph Kvedar, MD, president of the American Telemedicine Association and a dermatology professor at Harvard Medical School, Boston.
“We have to be very thoughtful about quality in this current phase, where we are doing what I call a hybrid model,” he said. “Some services are offered by telehealth and some require patients to come into the doctor’s office. We have to be very thoughtful about what types of care we determine to be appropriate for telehealth, and that has to be based on clinical quality. And if it is, it should follow that we’ll have low incidence of liability claims.”
Data should be at the center of that conclusion, Dr. Kvedar advises.
“Think about what data is needed to make a therapeutic or diagnostic decision,” he said. “If a health care provider can gather the information needed without touching the patient, then the provider is probably on safe, solid ground making that decision via a telehealth interaction. If the patient can come into the doctor’s office, and the provider deems it necessary to see the patient in person and touch the patient in order to make that clinical decision, then the patient should come in.”
An important step to preventing liability is also having strong telehealth systems and protocols in place and the necessary support to carry them out, said Dr. Einbinder of medical liability insurer CRICO.
For example, Dr. Einbinder, who practices in a 12-doctor internal medicine group, said when he finishes a virtual visit, he enters any orders into the electronic health record. Some of the orders will result in notifications to Dr. Einbinder if they are not executed, such as a referral appointment or a procedure that was not completed.
“I also can forward my orders to a front desk pool that is responsible for making sure things get done,” he said. “And, in our hospital system, we have good case management for complex patients and population management for a variety of chronic conditions. These represent additional safety nets.”
Another liability safeguard is sending patients a “visit summary” after each virtual visit, Dr. Sullivan said. This could be in the form of an email or a text that includes a brief template including items such as diagnosis, recommendations, follow-up, and a reminder to contact the doctor or go to the emergency department if symptoms worsen or new problems develop.
“Patients tend to remember about half of what physicians tell them and half of the information patients do remember is incorrect,” he said. “Consider a few sentences in an e-mail or text message as a substitute for the after-visit instructions from an office visit to enhance patient understanding. There are several inexpensive programs/services that allow text messages to be sent from a computer using a separate dedicated phone number and pretty much every patient has a cell phone to receive the instructions.”
Dr. Sullivan suggests having a documentation template specifically for telehealth visits. He also recommends the inclusion an “informed refusal of care” in the record when necessary. Dr. Sullivan’s wife, a family physician, has encountered several patients who fear contracting COVID-19 and who have refused her recommendations for in-person visits, he said. In such cases, he said it’s a good idea to document that the patient decided to forgo the recommendations given.
“If a patient suffers a bad outcome because of a failure to seek an in-person exam, a short note in the patient’s chart would help to establish that the lack of a follow-up physical exam was the patient’s informed decision, not due to some alleged negligence of the medical provider,” he said.
Concerning informed consent, Dr. Feldman says at a minimum physicians should discuss the following with patients:
- Names and credentials of staff participating.
- The right to stop or refuse treatment by telemedicine.
- Technology that will be used.
- Privacy and security risks.
- Technology-specific risks and permission to bill.
- Alternative care in case of an emergency or technology malfunction.
- Any state-specific requirements.
“Physicians can ensure they have a strong informed consent process during video visits by taking the time to cover these points at the beginning of the first visit, and being sure the patient understands and agrees to these,” Dr. Sullivan explained. “Ideally, this conversation can be recorded for future reference if necessary or at a minimum documented in the medical record.”
Consider these extra precautions
Mr. Cahill advises that practitioners be especially mindful of their “web-side manner” and the setting in which they are communicating with virtual patients to promote confidentiality, professionalism, and uninterrupted interactions.
“Use of a headset in a quiet home office is advisable,” he said. “Physicians must also be cognizant of their physical appearance and the background behind them when the visit includes both audio and visual capability. For ‘face-to-face’ telehealth encounters, it is recommended that a white lab jacket be worn as the appropriate attire; coat and tie are unnecessary.”
Some patients may need to be reminded of the need for confidentiality during a video visit, Mr. Moore added. Physicians are typically in a position to ensure confidentiality, but some patients may not understand how to protect their privacy on their end.
“If the physician sees on the screen or hears from an audio connection that there are other people around who may be able to overhear what is communicated, the physician probably has some responsibility to remind the patient that she or he may want to go to a more private place, close the door, etc.,” he said. “While I think a claim against a physician on this basis would be pretty weak, it is still a good practice for the physician to be cognizant of those kinds of concerns even if the patient is not.”
Finally, for physicians who set up telehealth operability during the pandemic – possibly in a hurry – consider using your actual case data to take a look backward, said Ms. Lerman, the Washington-based health care attorney. Reviewing the data can help determine whether you’re in compliance with relevant state laws, she said.
“If, for example, a provider set up telehealth operations during the pandemic and can see that most of [the] patients are based in a single state, or a small group of states, it is worthwhile to take [the] time and become familiar with the telemedicine laws in those states,” she said. “If there are modifications that need to be made, it may be easier to make them incrementally before the telehealth operations grow any larger in scope.”
A version of this article originally appeared on Medscape.com.
FDA expands remdesivir use for all COVID-19 hospitalized patients
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
Beyond microcephaly: Zika-affected children near school age
In 2020, “the virus” has come to mean one thing: SARS-CoV-2. But just a few years ago, Zika had the world's attention, as one news report after another described children with microcephaly born to women who'd been infected while pregnant.
It can be difficult for physicians to determine whether a birth defect is the result of Zika. Most infections have few or no symptoms, and mothers may not know if they’ve been exposed. Karin Nielsen, MD, remembers one child in particular, a 9-month-old boy born with microcephaly whose parents brought the infant to her in 2018 because he had started having seizures.
The child was born in Mexico in 2017, when the Zika virus was still known to be circulating in the Americas, said Dr. Nielsen, a pediatric infectious disease specialist at the University of California, Los Angeles. Brain imaging revealed calcifications and other signs in the boy’s brain that were consistent with exposure. But his mother said she was never sick during pregnancy.
Because Zika is transmitted not just via mosquito and from mother to fetus but also sexually, Dr. Nielsen thinks the mother probably contracted an asymptomatic infection from her husband, who recalled having a rash when she was 4 months pregnant. When they participated in a research study, both parents tested positive for Zika antibodies.
“The child had the classic symptoms of congenital Zika syndrome,” Dr. Nielsen said. “He was 9 months old, he had microcephaly, and he was having mal seizures.”
Researchers have since learned that children with such classic symptoms represent only a small proportion of those affected by prenatal Zika exposure – about 3%-5%. The virus was at its height during the 2016-2016 epidemic and is not currently causing outbreaks. But as researchers have followed cohorts of children exposed to Zika in utero, they have found many subtler effects physicians will need to monitor as the children grow up.
“When we’re seeing hundreds of kids with microcephaly, we had a lot of people infected,” Dr. Nielsen said. “Microcephaly is only the tip of the iceberg.”
Early evidence
Microcephaly may be the most identifiable symptom of fetal Zika infection, but researchers tracking cohorts of exposed children have begun to build a more complete picture of what long-term effects might look like. But hundreds, if not thousands, of children have been exposed to Zika in the womb – it’s not clear how many, Dr. Nielsen said – and many show a range of effects that don’t officially qualify as congenital Zika syndrome.
Current estimates suggest about one third of exposed children have some type of neurologic or neurodevelopmental problem, even though prevalence of visible effects is much lower. Over time, the incidence of these effects has fluctuated; some developmental delays and sensory deficits began manifesting later in childhood whereas others, at least in a few children, have resolved.
“We’re just beginning to have some of the data that we need to think about the full spectrum of outcomes,” said Cindy Moore, MD, chief medical officer in the Division of Congenital and Developmental Disorders in the Centers for Disease Control and Prevention’s National Center on Birth Defects and Developmental Disabilities.
“As we’re learning more and more, we’re learning the spectrum is expanding to less severe forms,” Dr. Moore said. “We do know that with some infections, there are later onset of problems.”
Studies published in 2018 described cohorts of children whose mothers had confirmed or suspected Zika infections during pregnancy in the French Territories of America (Guadalupe, Martinique, and French Guiana) and in Salvador, Brazil. The research provided valuable early data on the incidence of microcephaly and other severe effects in newborns, but noted the need for long-term follow up.
The U.S. Zika Pregnancy and Infant Registry is one of the largest such cohorts. In August 2018, researchers made their first report on data from the registry They looked at 1450 children age 1 or older who had undergone neuroimaging or screenings (developmental, vision, hearing) or both. In 6%, at least one birth defect was linked to Zika, and 9% had at least one neurodevelopmental abnormality.
As these children age past developmental milestones, more effects will likely manifest – even in those children whose appearance and imaging presented as healthy at birth.
Longer-term follow up
Nielsen at UCLA and M. Elisabeth Lopes Moreira, MD, of the Oswaldo Cruz Foundation in Rio de Janeiro, are following a cohort of more than 100 children born in Rio de Janeiro during Brazil’s 2015-2016 epidemic to mothers with symptomatic, PCR-confirmed Zika infections during pregnancy. In December 2018, their team reported that rates of severe neurodevelopmental delay and sensory dysfunction – 14% of 131 children aged 12-18 months – were higher than those found in earlier studies.
In August 2019, the team described neurodevelopmental, vision, and hearing outcomes in 216 Zika-exposed children 2 years after birth. They used the Bayley-III Scales of Infant and Toddler Development to assess cognitive, language and motor skills in 146 of the children. Forty percent of them were below or very below average in development, more than one third (35%) had language delays, 12% percent had hearing loss, and 7% had abnormal eye anatomy, such as underdeveloped retinas.
In two of the eight children in the cohort with microcephaly, the abnormality unexpectedly resolved. Although that finding received a lot of press, Dr. Nielsen pointed out that “not all microcephalies are created equal.”
In one case, a child born small for gestational age had proportional microcephaly: the baby›s head circumference met the criteria for microcephaly, but the infant›s head was proportional to the body so, as the child grew, the apparent microcephaly disappeared.
In the other case, the child was born with craniosynostosis, in which the skull sutures fuse too early – another effect seen with prenatal Zika exposure, Dr. Nielsen said. After corrective surgery, the child’s head circumference no longer met the definition of microcephaly, but the child still had symptoms related to congenital Zika: a developmental delay and calcifications in the brain. Meanwhile, two other children in the Rio cohort developed secondary microcephaly.
In another follow-up study of children up to age 4, Dr. Nielsen and colleagues found that both clinicians and family may think that Zika-exposed infants without microcephaly are developing normally, but that may not be true. Nearly 70% of children without microcephaly had neurologic abnormalities on physical examination, and more than half had failure to thrive because of poor feeding related to neurologic abnormalities.
Initially, some children may be able to mask subtle problems. A study published in January from Sarah B. Mulkey, MD, PhD, of Children’s National Hospital in Washington, DC, and colleagues described neurodevelopmental outcomes in 70 Colombian children up to 18 months old who had been exposed to Zika in utero. The children had a normal head circumference at birth and a normal fetal MRI, but – compared with typically developing peers – their communication, social cognition, and mobility scores on standardized assessments tended to decline as they got older.
“Especially in a very young child, there’s always going to be a possibility that you can compensate for a deficit, and it appears that at least some of these children are doing so,” said William J. Muller, MD, PhD, associate professor of pediatrics at Northwestern University, Chicago. When the children are older, certain behavioral effects will become easier to assess.
“With these children now approaching school age, understanding the full spectrum of neurodevelopmental abnormalities has important public health and educational system implications,” Dr. Muller and Dr. Mulkey wrote in a commentary about one of Dr. Nielsen’s studies.
Researchers face multiple barriers to understanding the long-term effects of fetal Zika infection. Many infants known to have been exposed in utero never received the recommended early assessments and haven’t been followed long-term. Particularly in Brazil, poverty, poor access to healthcare, and overcrowding all complicate surveillance efforts, Dr. Muller said. Stigma related to children’s neurodevelopmental problems also can potentially reduce a mother’s willingness to attend all follow-ups and assessments.
Some children may have been exposed but were never recognized as such, making it difficult for researchers to track their development and assemble a complete picture of prenatal Zika infection outcomes. Asymptomatic infection occurs in about 80% of Zika infections, though it’s not clear if that number holds for infections during pregnancy as well, according to Dr. Muller and Dr. Mulkey. Because nearly all the current research involves children whose mothers had symptomatic infections, the studies’ generalizability may be limited.
Those likely asymptomatic infections are also a major reason none of the cohorts have comparison groups.
“There are literally hundreds of things that can contribute to or cause developmental problems,” said Dr. Moore of the CDC, who noted that it would be nice to have a comparison group so as to know what Zika may not be responsible for. That said, it would be difficult-to-impossible to create a control group with similar geographic and demographic characteristics as the exposed children, a group who researchers can be certain weren’t exposed.
Neurodevelopmental disabilities occur in about 15% of the general population, making it difficult to determine whether Zika causes any or all long-term, less severe developmental findings in exposed children. The difficulty only compounds with time: the older a child is when a developmental problem is recognized, the harder it is to go back and say the problem is a result of something that occurred before birth, Dr. Moore said. “It’s a challenging field to say, this is what caused that outcome.”
Exposed children need continued evaluation
Interpreting the clinical implications of available studies is also challenging. It can be difficult to distinguish between central nervous system damage and peripheral damage, leaving the true etiology of poor vision or hearing elusive. The Zika virus can attack both the optic nerve and the part of the brain that interprets what a person sees: “Are you not seeing well because that part of your brain is not developed, or is it just a problem with the eye?” Dr. Nielsen said.
When problems can’t be precisely identified, successful interventions are harder. If the cochlea is normal, for instance, but the part of the brain that interprets sound or language has deficits, a hearing aid won’t help.
The services and interventions that children need depend on their specific developmental or cognitive deficits, regardless of the cause. But if clinicians know the cause is likely Zika exposure, they also know to look for other deficits.
Children showing likely effects of congenital Zika infection should be further evaluated for other possible birth defects and referred to a developmental specialist, early intervention services, and family support services. Depending on the child, primary care providers might consider referrals to an infectious disease specialist, clinical geneticist, neurologist, or other specialists.
Even with no confirmed infection or visible signs at birth, clinicians should remain vigilant with children who had possible exposure. A recently published study of 120 children conceived during the Zika outbreak in Paraíba, Brazil, assessed as infants and then again at 2 years old, exemplifies why. Researchers identified adverse neurologic outcomes and developmental delays in several children who had no physical evidence of birth defects as newborns, but whose antibody tests showed possible infection.
“In this post-epidemic period, with decreased Zika transmission and less public awareness,” wrote Dr. Mulkey and a colleague, “follow-up of these children is now more important than ever”.
A version of this article originally appeared on Medscape.com.
In 2020, “the virus” has come to mean one thing: SARS-CoV-2. But just a few years ago, Zika had the world's attention, as one news report after another described children with microcephaly born to women who'd been infected while pregnant.
It can be difficult for physicians to determine whether a birth defect is the result of Zika. Most infections have few or no symptoms, and mothers may not know if they’ve been exposed. Karin Nielsen, MD, remembers one child in particular, a 9-month-old boy born with microcephaly whose parents brought the infant to her in 2018 because he had started having seizures.
The child was born in Mexico in 2017, when the Zika virus was still known to be circulating in the Americas, said Dr. Nielsen, a pediatric infectious disease specialist at the University of California, Los Angeles. Brain imaging revealed calcifications and other signs in the boy’s brain that were consistent with exposure. But his mother said she was never sick during pregnancy.
Because Zika is transmitted not just via mosquito and from mother to fetus but also sexually, Dr. Nielsen thinks the mother probably contracted an asymptomatic infection from her husband, who recalled having a rash when she was 4 months pregnant. When they participated in a research study, both parents tested positive for Zika antibodies.
“The child had the classic symptoms of congenital Zika syndrome,” Dr. Nielsen said. “He was 9 months old, he had microcephaly, and he was having mal seizures.”
Researchers have since learned that children with such classic symptoms represent only a small proportion of those affected by prenatal Zika exposure – about 3%-5%. The virus was at its height during the 2016-2016 epidemic and is not currently causing outbreaks. But as researchers have followed cohorts of children exposed to Zika in utero, they have found many subtler effects physicians will need to monitor as the children grow up.
“When we’re seeing hundreds of kids with microcephaly, we had a lot of people infected,” Dr. Nielsen said. “Microcephaly is only the tip of the iceberg.”
Early evidence
Microcephaly may be the most identifiable symptom of fetal Zika infection, but researchers tracking cohorts of exposed children have begun to build a more complete picture of what long-term effects might look like. But hundreds, if not thousands, of children have been exposed to Zika in the womb – it’s not clear how many, Dr. Nielsen said – and many show a range of effects that don’t officially qualify as congenital Zika syndrome.
Current estimates suggest about one third of exposed children have some type of neurologic or neurodevelopmental problem, even though prevalence of visible effects is much lower. Over time, the incidence of these effects has fluctuated; some developmental delays and sensory deficits began manifesting later in childhood whereas others, at least in a few children, have resolved.
“We’re just beginning to have some of the data that we need to think about the full spectrum of outcomes,” said Cindy Moore, MD, chief medical officer in the Division of Congenital and Developmental Disorders in the Centers for Disease Control and Prevention’s National Center on Birth Defects and Developmental Disabilities.
“As we’re learning more and more, we’re learning the spectrum is expanding to less severe forms,” Dr. Moore said. “We do know that with some infections, there are later onset of problems.”
Studies published in 2018 described cohorts of children whose mothers had confirmed or suspected Zika infections during pregnancy in the French Territories of America (Guadalupe, Martinique, and French Guiana) and in Salvador, Brazil. The research provided valuable early data on the incidence of microcephaly and other severe effects in newborns, but noted the need for long-term follow up.
The U.S. Zika Pregnancy and Infant Registry is one of the largest such cohorts. In August 2018, researchers made their first report on data from the registry They looked at 1450 children age 1 or older who had undergone neuroimaging or screenings (developmental, vision, hearing) or both. In 6%, at least one birth defect was linked to Zika, and 9% had at least one neurodevelopmental abnormality.
As these children age past developmental milestones, more effects will likely manifest – even in those children whose appearance and imaging presented as healthy at birth.
Longer-term follow up
Nielsen at UCLA and M. Elisabeth Lopes Moreira, MD, of the Oswaldo Cruz Foundation in Rio de Janeiro, are following a cohort of more than 100 children born in Rio de Janeiro during Brazil’s 2015-2016 epidemic to mothers with symptomatic, PCR-confirmed Zika infections during pregnancy. In December 2018, their team reported that rates of severe neurodevelopmental delay and sensory dysfunction – 14% of 131 children aged 12-18 months – were higher than those found in earlier studies.
In August 2019, the team described neurodevelopmental, vision, and hearing outcomes in 216 Zika-exposed children 2 years after birth. They used the Bayley-III Scales of Infant and Toddler Development to assess cognitive, language and motor skills in 146 of the children. Forty percent of them were below or very below average in development, more than one third (35%) had language delays, 12% percent had hearing loss, and 7% had abnormal eye anatomy, such as underdeveloped retinas.
In two of the eight children in the cohort with microcephaly, the abnormality unexpectedly resolved. Although that finding received a lot of press, Dr. Nielsen pointed out that “not all microcephalies are created equal.”
In one case, a child born small for gestational age had proportional microcephaly: the baby›s head circumference met the criteria for microcephaly, but the infant›s head was proportional to the body so, as the child grew, the apparent microcephaly disappeared.
In the other case, the child was born with craniosynostosis, in which the skull sutures fuse too early – another effect seen with prenatal Zika exposure, Dr. Nielsen said. After corrective surgery, the child’s head circumference no longer met the definition of microcephaly, but the child still had symptoms related to congenital Zika: a developmental delay and calcifications in the brain. Meanwhile, two other children in the Rio cohort developed secondary microcephaly.
In another follow-up study of children up to age 4, Dr. Nielsen and colleagues found that both clinicians and family may think that Zika-exposed infants without microcephaly are developing normally, but that may not be true. Nearly 70% of children without microcephaly had neurologic abnormalities on physical examination, and more than half had failure to thrive because of poor feeding related to neurologic abnormalities.
Initially, some children may be able to mask subtle problems. A study published in January from Sarah B. Mulkey, MD, PhD, of Children’s National Hospital in Washington, DC, and colleagues described neurodevelopmental outcomes in 70 Colombian children up to 18 months old who had been exposed to Zika in utero. The children had a normal head circumference at birth and a normal fetal MRI, but – compared with typically developing peers – their communication, social cognition, and mobility scores on standardized assessments tended to decline as they got older.
“Especially in a very young child, there’s always going to be a possibility that you can compensate for a deficit, and it appears that at least some of these children are doing so,” said William J. Muller, MD, PhD, associate professor of pediatrics at Northwestern University, Chicago. When the children are older, certain behavioral effects will become easier to assess.
“With these children now approaching school age, understanding the full spectrum of neurodevelopmental abnormalities has important public health and educational system implications,” Dr. Muller and Dr. Mulkey wrote in a commentary about one of Dr. Nielsen’s studies.
Researchers face multiple barriers to understanding the long-term effects of fetal Zika infection. Many infants known to have been exposed in utero never received the recommended early assessments and haven’t been followed long-term. Particularly in Brazil, poverty, poor access to healthcare, and overcrowding all complicate surveillance efforts, Dr. Muller said. Stigma related to children’s neurodevelopmental problems also can potentially reduce a mother’s willingness to attend all follow-ups and assessments.
Some children may have been exposed but were never recognized as such, making it difficult for researchers to track their development and assemble a complete picture of prenatal Zika infection outcomes. Asymptomatic infection occurs in about 80% of Zika infections, though it’s not clear if that number holds for infections during pregnancy as well, according to Dr. Muller and Dr. Mulkey. Because nearly all the current research involves children whose mothers had symptomatic infections, the studies’ generalizability may be limited.
Those likely asymptomatic infections are also a major reason none of the cohorts have comparison groups.
“There are literally hundreds of things that can contribute to or cause developmental problems,” said Dr. Moore of the CDC, who noted that it would be nice to have a comparison group so as to know what Zika may not be responsible for. That said, it would be difficult-to-impossible to create a control group with similar geographic and demographic characteristics as the exposed children, a group who researchers can be certain weren’t exposed.
Neurodevelopmental disabilities occur in about 15% of the general population, making it difficult to determine whether Zika causes any or all long-term, less severe developmental findings in exposed children. The difficulty only compounds with time: the older a child is when a developmental problem is recognized, the harder it is to go back and say the problem is a result of something that occurred before birth, Dr. Moore said. “It’s a challenging field to say, this is what caused that outcome.”
Exposed children need continued evaluation
Interpreting the clinical implications of available studies is also challenging. It can be difficult to distinguish between central nervous system damage and peripheral damage, leaving the true etiology of poor vision or hearing elusive. The Zika virus can attack both the optic nerve and the part of the brain that interprets what a person sees: “Are you not seeing well because that part of your brain is not developed, or is it just a problem with the eye?” Dr. Nielsen said.
When problems can’t be precisely identified, successful interventions are harder. If the cochlea is normal, for instance, but the part of the brain that interprets sound or language has deficits, a hearing aid won’t help.
The services and interventions that children need depend on their specific developmental or cognitive deficits, regardless of the cause. But if clinicians know the cause is likely Zika exposure, they also know to look for other deficits.
Children showing likely effects of congenital Zika infection should be further evaluated for other possible birth defects and referred to a developmental specialist, early intervention services, and family support services. Depending on the child, primary care providers might consider referrals to an infectious disease specialist, clinical geneticist, neurologist, or other specialists.
Even with no confirmed infection or visible signs at birth, clinicians should remain vigilant with children who had possible exposure. A recently published study of 120 children conceived during the Zika outbreak in Paraíba, Brazil, assessed as infants and then again at 2 years old, exemplifies why. Researchers identified adverse neurologic outcomes and developmental delays in several children who had no physical evidence of birth defects as newborns, but whose antibody tests showed possible infection.
“In this post-epidemic period, with decreased Zika transmission and less public awareness,” wrote Dr. Mulkey and a colleague, “follow-up of these children is now more important than ever”.
A version of this article originally appeared on Medscape.com.
In 2020, “the virus” has come to mean one thing: SARS-CoV-2. But just a few years ago, Zika had the world's attention, as one news report after another described children with microcephaly born to women who'd been infected while pregnant.
It can be difficult for physicians to determine whether a birth defect is the result of Zika. Most infections have few or no symptoms, and mothers may not know if they’ve been exposed. Karin Nielsen, MD, remembers one child in particular, a 9-month-old boy born with microcephaly whose parents brought the infant to her in 2018 because he had started having seizures.
The child was born in Mexico in 2017, when the Zika virus was still known to be circulating in the Americas, said Dr. Nielsen, a pediatric infectious disease specialist at the University of California, Los Angeles. Brain imaging revealed calcifications and other signs in the boy’s brain that were consistent with exposure. But his mother said she was never sick during pregnancy.
Because Zika is transmitted not just via mosquito and from mother to fetus but also sexually, Dr. Nielsen thinks the mother probably contracted an asymptomatic infection from her husband, who recalled having a rash when she was 4 months pregnant. When they participated in a research study, both parents tested positive for Zika antibodies.
“The child had the classic symptoms of congenital Zika syndrome,” Dr. Nielsen said. “He was 9 months old, he had microcephaly, and he was having mal seizures.”
Researchers have since learned that children with such classic symptoms represent only a small proportion of those affected by prenatal Zika exposure – about 3%-5%. The virus was at its height during the 2016-2016 epidemic and is not currently causing outbreaks. But as researchers have followed cohorts of children exposed to Zika in utero, they have found many subtler effects physicians will need to monitor as the children grow up.
“When we’re seeing hundreds of kids with microcephaly, we had a lot of people infected,” Dr. Nielsen said. “Microcephaly is only the tip of the iceberg.”
Early evidence
Microcephaly may be the most identifiable symptom of fetal Zika infection, but researchers tracking cohorts of exposed children have begun to build a more complete picture of what long-term effects might look like. But hundreds, if not thousands, of children have been exposed to Zika in the womb – it’s not clear how many, Dr. Nielsen said – and many show a range of effects that don’t officially qualify as congenital Zika syndrome.
Current estimates suggest about one third of exposed children have some type of neurologic or neurodevelopmental problem, even though prevalence of visible effects is much lower. Over time, the incidence of these effects has fluctuated; some developmental delays and sensory deficits began manifesting later in childhood whereas others, at least in a few children, have resolved.
“We’re just beginning to have some of the data that we need to think about the full spectrum of outcomes,” said Cindy Moore, MD, chief medical officer in the Division of Congenital and Developmental Disorders in the Centers for Disease Control and Prevention’s National Center on Birth Defects and Developmental Disabilities.
“As we’re learning more and more, we’re learning the spectrum is expanding to less severe forms,” Dr. Moore said. “We do know that with some infections, there are later onset of problems.”
Studies published in 2018 described cohorts of children whose mothers had confirmed or suspected Zika infections during pregnancy in the French Territories of America (Guadalupe, Martinique, and French Guiana) and in Salvador, Brazil. The research provided valuable early data on the incidence of microcephaly and other severe effects in newborns, but noted the need for long-term follow up.
The U.S. Zika Pregnancy and Infant Registry is one of the largest such cohorts. In August 2018, researchers made their first report on data from the registry They looked at 1450 children age 1 or older who had undergone neuroimaging or screenings (developmental, vision, hearing) or both. In 6%, at least one birth defect was linked to Zika, and 9% had at least one neurodevelopmental abnormality.
As these children age past developmental milestones, more effects will likely manifest – even in those children whose appearance and imaging presented as healthy at birth.
Longer-term follow up
Nielsen at UCLA and M. Elisabeth Lopes Moreira, MD, of the Oswaldo Cruz Foundation in Rio de Janeiro, are following a cohort of more than 100 children born in Rio de Janeiro during Brazil’s 2015-2016 epidemic to mothers with symptomatic, PCR-confirmed Zika infections during pregnancy. In December 2018, their team reported that rates of severe neurodevelopmental delay and sensory dysfunction – 14% of 131 children aged 12-18 months – were higher than those found in earlier studies.
In August 2019, the team described neurodevelopmental, vision, and hearing outcomes in 216 Zika-exposed children 2 years after birth. They used the Bayley-III Scales of Infant and Toddler Development to assess cognitive, language and motor skills in 146 of the children. Forty percent of them were below or very below average in development, more than one third (35%) had language delays, 12% percent had hearing loss, and 7% had abnormal eye anatomy, such as underdeveloped retinas.
In two of the eight children in the cohort with microcephaly, the abnormality unexpectedly resolved. Although that finding received a lot of press, Dr. Nielsen pointed out that “not all microcephalies are created equal.”
In one case, a child born small for gestational age had proportional microcephaly: the baby›s head circumference met the criteria for microcephaly, but the infant›s head was proportional to the body so, as the child grew, the apparent microcephaly disappeared.
In the other case, the child was born with craniosynostosis, in which the skull sutures fuse too early – another effect seen with prenatal Zika exposure, Dr. Nielsen said. After corrective surgery, the child’s head circumference no longer met the definition of microcephaly, but the child still had symptoms related to congenital Zika: a developmental delay and calcifications in the brain. Meanwhile, two other children in the Rio cohort developed secondary microcephaly.
In another follow-up study of children up to age 4, Dr. Nielsen and colleagues found that both clinicians and family may think that Zika-exposed infants without microcephaly are developing normally, but that may not be true. Nearly 70% of children without microcephaly had neurologic abnormalities on physical examination, and more than half had failure to thrive because of poor feeding related to neurologic abnormalities.
Initially, some children may be able to mask subtle problems. A study published in January from Sarah B. Mulkey, MD, PhD, of Children’s National Hospital in Washington, DC, and colleagues described neurodevelopmental outcomes in 70 Colombian children up to 18 months old who had been exposed to Zika in utero. The children had a normal head circumference at birth and a normal fetal MRI, but – compared with typically developing peers – their communication, social cognition, and mobility scores on standardized assessments tended to decline as they got older.
“Especially in a very young child, there’s always going to be a possibility that you can compensate for a deficit, and it appears that at least some of these children are doing so,” said William J. Muller, MD, PhD, associate professor of pediatrics at Northwestern University, Chicago. When the children are older, certain behavioral effects will become easier to assess.
“With these children now approaching school age, understanding the full spectrum of neurodevelopmental abnormalities has important public health and educational system implications,” Dr. Muller and Dr. Mulkey wrote in a commentary about one of Dr. Nielsen’s studies.
Researchers face multiple barriers to understanding the long-term effects of fetal Zika infection. Many infants known to have been exposed in utero never received the recommended early assessments and haven’t been followed long-term. Particularly in Brazil, poverty, poor access to healthcare, and overcrowding all complicate surveillance efforts, Dr. Muller said. Stigma related to children’s neurodevelopmental problems also can potentially reduce a mother’s willingness to attend all follow-ups and assessments.
Some children may have been exposed but were never recognized as such, making it difficult for researchers to track their development and assemble a complete picture of prenatal Zika infection outcomes. Asymptomatic infection occurs in about 80% of Zika infections, though it’s not clear if that number holds for infections during pregnancy as well, according to Dr. Muller and Dr. Mulkey. Because nearly all the current research involves children whose mothers had symptomatic infections, the studies’ generalizability may be limited.
Those likely asymptomatic infections are also a major reason none of the cohorts have comparison groups.
“There are literally hundreds of things that can contribute to or cause developmental problems,” said Dr. Moore of the CDC, who noted that it would be nice to have a comparison group so as to know what Zika may not be responsible for. That said, it would be difficult-to-impossible to create a control group with similar geographic and demographic characteristics as the exposed children, a group who researchers can be certain weren’t exposed.
Neurodevelopmental disabilities occur in about 15% of the general population, making it difficult to determine whether Zika causes any or all long-term, less severe developmental findings in exposed children. The difficulty only compounds with time: the older a child is when a developmental problem is recognized, the harder it is to go back and say the problem is a result of something that occurred before birth, Dr. Moore said. “It’s a challenging field to say, this is what caused that outcome.”
Exposed children need continued evaluation
Interpreting the clinical implications of available studies is also challenging. It can be difficult to distinguish between central nervous system damage and peripheral damage, leaving the true etiology of poor vision or hearing elusive. The Zika virus can attack both the optic nerve and the part of the brain that interprets what a person sees: “Are you not seeing well because that part of your brain is not developed, or is it just a problem with the eye?” Dr. Nielsen said.
When problems can’t be precisely identified, successful interventions are harder. If the cochlea is normal, for instance, but the part of the brain that interprets sound or language has deficits, a hearing aid won’t help.
The services and interventions that children need depend on their specific developmental or cognitive deficits, regardless of the cause. But if clinicians know the cause is likely Zika exposure, they also know to look for other deficits.
Children showing likely effects of congenital Zika infection should be further evaluated for other possible birth defects and referred to a developmental specialist, early intervention services, and family support services. Depending on the child, primary care providers might consider referrals to an infectious disease specialist, clinical geneticist, neurologist, or other specialists.
Even with no confirmed infection or visible signs at birth, clinicians should remain vigilant with children who had possible exposure. A recently published study of 120 children conceived during the Zika outbreak in Paraíba, Brazil, assessed as infants and then again at 2 years old, exemplifies why. Researchers identified adverse neurologic outcomes and developmental delays in several children who had no physical evidence of birth defects as newborns, but whose antibody tests showed possible infection.
“In this post-epidemic period, with decreased Zika transmission and less public awareness,” wrote Dr. Mulkey and a colleague, “follow-up of these children is now more important than ever”.
A version of this article originally appeared on Medscape.com.
Two PR employees at FDA fired after plasma therapy controversy
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
NYC public hospitals rose to the demands of the COVID-19 crisis
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
Hospitalists at the center of the storm
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
COVID-19 at home: What does optimal care look like?
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
SARS-CoV-2 appears unlikely to pass through breast milk
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
FROM JAMA
Mitigating psychiatric disorder relapse in pregnancy during pandemic
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.