User login
American Association for Geriatric Psychiatry (AAGP)
High uric acid boosts fracture risk
LAS VEGAS – A serum uric acid level of 7 mg/dL or more was independently associated with a 62% increased risk of hip fracture among older men participating in the Cardiovascular Health Study.
This finding confirms the clinical relevance of prior translational work pointing to a link between uric acid and bone health, Dr. Tapan Mehta observed at a meeting sponsored by the National Kidney Foundation.
He and his coinvestigators had suspected the existence of such a link, based upon their prior observations that uric acid inhibits expression of 1-alpha hydroxylase protein in vivo and that elevated uric acid levels are associated with higher parathyroid hormone levels in humans.
A key question now under study in clinical trials is whether lowering uric acid levels in the absence of symptomatic gout provides ancillary benefits to the kidneys.
The Cardiovascular Health Study was a National Heart, Lung, and Blood Institute–funded, population-based, observational study conducted to identify cardiovascular risk factors in men and women aged 65 years and older. In this analysis, Dr. Mehta reported on 1,963 male and 2,729 female participants with baseline serum uric acid levels available.
During a median 11 years of follow-up, 156 first-incident hip fractures occurred. The association between hyperuricemia and increased fracture risk was confined to men, 430 of whom had a baseline serum uric acid of 7 mg/dL or more.
Participants with an elevated uric acid were disproportionately black, obese, in less than good health, and had significantly higher levels of insulin, C-reactive protein, and cystatin C. In a multivariate regression analysis adjusted for demographics, body mass index, alcohol, smoking, physical activity, diabetes, C-reactive protein, hypertension, and known cardiovascular disease, men with a serum uric acid of 7 mg/dL or higher remained at 62% greater risk of hip fracture, compared with those with a uric acid below 7 mg/dL, according to Dr. Mehta of the University of Colorado, Denver.
High uric acid is known to increase the risk of gout, kidney stones, and more rapid progression of chronic kidney disease. It has also been linked to hypertension.
Possible mechanisms that might explain the link between increased serum uric acid and hip fractures include the fact that uric acid suppresses vitamin D activation. Also, uric acid inhibits expression of 1-alpha hydroxylase protein, and higher uric acid levels are associated with higher levels of parathyroid hormone. In addition, uric acid may cause inflammation in bone, which would be expected to increase fragility. But all of these thoughts on mechanism remain speculative for now, according to the nephrologist.
The absence of a relationship between hyperuricemia and hip fractures in older women in the Cardiovascular Health Study suggests other factors are more important than uric acid levels in women.
Dr. Mehta reported having no financial conflicts regarding this study.
LAS VEGAS – A serum uric acid level of 7 mg/dL or more was independently associated with a 62% increased risk of hip fracture among older men participating in the Cardiovascular Health Study.
This finding confirms the clinical relevance of prior translational work pointing to a link between uric acid and bone health, Dr. Tapan Mehta observed at a meeting sponsored by the National Kidney Foundation.
He and his coinvestigators had suspected the existence of such a link, based upon their prior observations that uric acid inhibits expression of 1-alpha hydroxylase protein in vivo and that elevated uric acid levels are associated with higher parathyroid hormone levels in humans.
A key question now under study in clinical trials is whether lowering uric acid levels in the absence of symptomatic gout provides ancillary benefits to the kidneys.
The Cardiovascular Health Study was a National Heart, Lung, and Blood Institute–funded, population-based, observational study conducted to identify cardiovascular risk factors in men and women aged 65 years and older. In this analysis, Dr. Mehta reported on 1,963 male and 2,729 female participants with baseline serum uric acid levels available.
During a median 11 years of follow-up, 156 first-incident hip fractures occurred. The association between hyperuricemia and increased fracture risk was confined to men, 430 of whom had a baseline serum uric acid of 7 mg/dL or more.
Participants with an elevated uric acid were disproportionately black, obese, in less than good health, and had significantly higher levels of insulin, C-reactive protein, and cystatin C. In a multivariate regression analysis adjusted for demographics, body mass index, alcohol, smoking, physical activity, diabetes, C-reactive protein, hypertension, and known cardiovascular disease, men with a serum uric acid of 7 mg/dL or higher remained at 62% greater risk of hip fracture, compared with those with a uric acid below 7 mg/dL, according to Dr. Mehta of the University of Colorado, Denver.
High uric acid is known to increase the risk of gout, kidney stones, and more rapid progression of chronic kidney disease. It has also been linked to hypertension.
Possible mechanisms that might explain the link between increased serum uric acid and hip fractures include the fact that uric acid suppresses vitamin D activation. Also, uric acid inhibits expression of 1-alpha hydroxylase protein, and higher uric acid levels are associated with higher levels of parathyroid hormone. In addition, uric acid may cause inflammation in bone, which would be expected to increase fragility. But all of these thoughts on mechanism remain speculative for now, according to the nephrologist.
The absence of a relationship between hyperuricemia and hip fractures in older women in the Cardiovascular Health Study suggests other factors are more important than uric acid levels in women.
Dr. Mehta reported having no financial conflicts regarding this study.
LAS VEGAS – A serum uric acid level of 7 mg/dL or more was independently associated with a 62% increased risk of hip fracture among older men participating in the Cardiovascular Health Study.
This finding confirms the clinical relevance of prior translational work pointing to a link between uric acid and bone health, Dr. Tapan Mehta observed at a meeting sponsored by the National Kidney Foundation.
He and his coinvestigators had suspected the existence of such a link, based upon their prior observations that uric acid inhibits expression of 1-alpha hydroxylase protein in vivo and that elevated uric acid levels are associated with higher parathyroid hormone levels in humans.
A key question now under study in clinical trials is whether lowering uric acid levels in the absence of symptomatic gout provides ancillary benefits to the kidneys.
The Cardiovascular Health Study was a National Heart, Lung, and Blood Institute–funded, population-based, observational study conducted to identify cardiovascular risk factors in men and women aged 65 years and older. In this analysis, Dr. Mehta reported on 1,963 male and 2,729 female participants with baseline serum uric acid levels available.
During a median 11 years of follow-up, 156 first-incident hip fractures occurred. The association between hyperuricemia and increased fracture risk was confined to men, 430 of whom had a baseline serum uric acid of 7 mg/dL or more.
Participants with an elevated uric acid were disproportionately black, obese, in less than good health, and had significantly higher levels of insulin, C-reactive protein, and cystatin C. In a multivariate regression analysis adjusted for demographics, body mass index, alcohol, smoking, physical activity, diabetes, C-reactive protein, hypertension, and known cardiovascular disease, men with a serum uric acid of 7 mg/dL or higher remained at 62% greater risk of hip fracture, compared with those with a uric acid below 7 mg/dL, according to Dr. Mehta of the University of Colorado, Denver.
High uric acid is known to increase the risk of gout, kidney stones, and more rapid progression of chronic kidney disease. It has also been linked to hypertension.
Possible mechanisms that might explain the link between increased serum uric acid and hip fractures include the fact that uric acid suppresses vitamin D activation. Also, uric acid inhibits expression of 1-alpha hydroxylase protein, and higher uric acid levels are associated with higher levels of parathyroid hormone. In addition, uric acid may cause inflammation in bone, which would be expected to increase fragility. But all of these thoughts on mechanism remain speculative for now, according to the nephrologist.
The absence of a relationship between hyperuricemia and hip fractures in older women in the Cardiovascular Health Study suggests other factors are more important than uric acid levels in women.
Dr. Mehta reported having no financial conflicts regarding this study.
AT SCM 14
Major finding: Men aged 65 years or older with a baseline serum uric acid level of 7.0 mg/dL or higher had an adjusted 62% increased risk of a first hip fracture during a median 11 years of follow-up.
Data source: This was an analysis of 1,963 men and 2,729 women, all aged 65 years or older at entry into the population-based, observational, prospective Cardiovascular Health Study.
Disclosures: The Cardiovascular Health Study was funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
Chronic kidney disease may raise cancer risk
LAS VEGAS – Patients with chronic kidney disease appear to be at increased risk for cancer, a study showed.
A retrospective analysis of data on 31,896 participants in ALLHAT (the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed that during a mean 4.9 years of in-trial follow-up, 2,529 subjects were diagnosed with various cancers.
The 5-year rate of incident cancer was 7.24 cases per 100 person-years among subjects with a baseline normal estimated glomerular filtration rate (eGFR) greater than 90 mL/min per 1.73 m2. The cancer rate rose with decreasing renal function: 8.38 cases per 100 person-years in patients with a baseline eGFR of 60-89.9, 9.18 per 100 person-years in those with an eGFR of 45-59.9, and 11.58 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, Dr. Dhruti P. Chen reported at a meeting sponsored by the National Kidney Foundation.
An additional 4 years of follow-up for mortality due to cancer was available through national databases after ALLHAT ended. During the mean total 8.9 years of follow-up, there were 2,338 cancer-related deaths. The 10-year rate of cancer mortality was 7.90 cases per 100 person-years in patients with an eGFR greater than 90; 7.71 in those with an eGFR of 60-89.9; 10.11 per 100 person-years with an eGFR of 45-59.9; and 13.19 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, added Dr. Chen of the department of medicine at Case Western Reserve University, Cleveland.
In a multivariate analysis adjusted for demographics, body mass index, diabetes, cardiovascular risk factors, blood glucose level, and the antihypertensive agent to which a patient was randomized, the association between chronic kidney disease and incident cancer was attenuated. Nonetheless, having a baseline eGFR below 45 mL/min per 1.73 m2 was independently associated with an adjusted 28% increased risk of incident cancer during 4.9 years of follow-up, compared with those with an eGFR greater than 90, as well as with a 55% increased risk of cancer mortality during 8.9 years of follow-up.
Colon cancer was the only common type of malignancy whose incidence was significantly increased in patients with chronic kidney disease, although Dr. Chen said she didn’t attach much significance to this finding, given the limited number of cancers that accrued.
In an interview, she observed that a post hoc analysis such as this can’t establish causality or the mechanisms involved. She speculated that the most likely explanation for the findings is that patients with impaired kidney function have incomplete removal of various toxins, some of which are oncogenic.
ALLHAT was funded by the National Heart, Lung, and Blood Institute. Dr. Chen reported having no financial conflicts of interest.
LAS VEGAS – Patients with chronic kidney disease appear to be at increased risk for cancer, a study showed.
A retrospective analysis of data on 31,896 participants in ALLHAT (the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed that during a mean 4.9 years of in-trial follow-up, 2,529 subjects were diagnosed with various cancers.
The 5-year rate of incident cancer was 7.24 cases per 100 person-years among subjects with a baseline normal estimated glomerular filtration rate (eGFR) greater than 90 mL/min per 1.73 m2. The cancer rate rose with decreasing renal function: 8.38 cases per 100 person-years in patients with a baseline eGFR of 60-89.9, 9.18 per 100 person-years in those with an eGFR of 45-59.9, and 11.58 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, Dr. Dhruti P. Chen reported at a meeting sponsored by the National Kidney Foundation.
An additional 4 years of follow-up for mortality due to cancer was available through national databases after ALLHAT ended. During the mean total 8.9 years of follow-up, there were 2,338 cancer-related deaths. The 10-year rate of cancer mortality was 7.90 cases per 100 person-years in patients with an eGFR greater than 90; 7.71 in those with an eGFR of 60-89.9; 10.11 per 100 person-years with an eGFR of 45-59.9; and 13.19 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, added Dr. Chen of the department of medicine at Case Western Reserve University, Cleveland.
In a multivariate analysis adjusted for demographics, body mass index, diabetes, cardiovascular risk factors, blood glucose level, and the antihypertensive agent to which a patient was randomized, the association between chronic kidney disease and incident cancer was attenuated. Nonetheless, having a baseline eGFR below 45 mL/min per 1.73 m2 was independently associated with an adjusted 28% increased risk of incident cancer during 4.9 years of follow-up, compared with those with an eGFR greater than 90, as well as with a 55% increased risk of cancer mortality during 8.9 years of follow-up.
Colon cancer was the only common type of malignancy whose incidence was significantly increased in patients with chronic kidney disease, although Dr. Chen said she didn’t attach much significance to this finding, given the limited number of cancers that accrued.
In an interview, she observed that a post hoc analysis such as this can’t establish causality or the mechanisms involved. She speculated that the most likely explanation for the findings is that patients with impaired kidney function have incomplete removal of various toxins, some of which are oncogenic.
ALLHAT was funded by the National Heart, Lung, and Blood Institute. Dr. Chen reported having no financial conflicts of interest.
LAS VEGAS – Patients with chronic kidney disease appear to be at increased risk for cancer, a study showed.
A retrospective analysis of data on 31,896 participants in ALLHAT (the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed that during a mean 4.9 years of in-trial follow-up, 2,529 subjects were diagnosed with various cancers.
The 5-year rate of incident cancer was 7.24 cases per 100 person-years among subjects with a baseline normal estimated glomerular filtration rate (eGFR) greater than 90 mL/min per 1.73 m2. The cancer rate rose with decreasing renal function: 8.38 cases per 100 person-years in patients with a baseline eGFR of 60-89.9, 9.18 per 100 person-years in those with an eGFR of 45-59.9, and 11.58 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, Dr. Dhruti P. Chen reported at a meeting sponsored by the National Kidney Foundation.
An additional 4 years of follow-up for mortality due to cancer was available through national databases after ALLHAT ended. During the mean total 8.9 years of follow-up, there were 2,338 cancer-related deaths. The 10-year rate of cancer mortality was 7.90 cases per 100 person-years in patients with an eGFR greater than 90; 7.71 in those with an eGFR of 60-89.9; 10.11 per 100 person-years with an eGFR of 45-59.9; and 13.19 per 100 person-years in patients with an eGFR below 45 mL/min per 1.73 m2, added Dr. Chen of the department of medicine at Case Western Reserve University, Cleveland.
In a multivariate analysis adjusted for demographics, body mass index, diabetes, cardiovascular risk factors, blood glucose level, and the antihypertensive agent to which a patient was randomized, the association between chronic kidney disease and incident cancer was attenuated. Nonetheless, having a baseline eGFR below 45 mL/min per 1.73 m2 was independently associated with an adjusted 28% increased risk of incident cancer during 4.9 years of follow-up, compared with those with an eGFR greater than 90, as well as with a 55% increased risk of cancer mortality during 8.9 years of follow-up.
Colon cancer was the only common type of malignancy whose incidence was significantly increased in patients with chronic kidney disease, although Dr. Chen said she didn’t attach much significance to this finding, given the limited number of cancers that accrued.
In an interview, she observed that a post hoc analysis such as this can’t establish causality or the mechanisms involved. She speculated that the most likely explanation for the findings is that patients with impaired kidney function have incomplete removal of various toxins, some of which are oncogenic.
ALLHAT was funded by the National Heart, Lung, and Blood Institute. Dr. Chen reported having no financial conflicts of interest.
AT SCM 14
Major finding: Patients with chronic kidney disease had an adjusted 55% increased risk of cancer-related mortality during a mean 8.9 years of prospective follow-up, compared with those with normal kidney function.
Data source: This was a post hoc analysis of 31,896 participants in the prospective ALLHAT study.
Disclosures: ALLHAT was funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts of interest.
Avoid these seven risk factors and slash Alzheimer’s risk
ORLANDO – Up to half of all cases of Alzheimer’s disease are attributable to seven modifiable risk factors – including depression, Dr. Kristine Yaffe asserted in her 2014 AAGP Distinguished Scientist Award Lecture.
In addition to depression, the modifiable risk factors for Alzheimer's disease backed by the best supporting evidence from observational studies are diabetes, smoking, midlife obesity, midlife hypertension, low educational attainment, and physical inactivity.
A public health campaign successful in achieving a rather modest 10% reduction across the board in these seven risk factors could prevent an estimated 184,000 cases of Alzheimer's disease in the United States alone. A more ambitious 25% reduction in the levels of these seven risk factors could prevent close to half a million cases in the United States and 3 million worldwide, she predicted at the annual meeting of the American Association for Geriatric Psychiatry.
"We’re all told by the Alzheimer’s Association and others that there’s an epidemic in dementia. And it’s true: There is an epidemic in dementia, because we’re living longer and the baby boomers are aging. We’re seeing things we didn’t see before. There wasn’t so much neurodegenerative disease when people died in their 60s and 70s. Now that we’re living into our 80s and 90s, we’re going to see a lot more neurodegenerative disease. But there are also a lot of interesting secular trends that hold promise. I think we have reason to be cautiously optimistic," declared Dr. Yaffe, professor of psychiatry, neurology, and epidemiology at the University of California, San Francisco. She is also Roy and Marie Scola Endowed Chair in Psychiatry at UCSF, and is chief of geriatric psychiatry and director of the memory disorders clinic at the San Francisco Veterans Affairs Medical Center
Dr. Yaffe is credited with having played a pivotal role in establishing many of these risk factors, including, for example, depression (Arch. Gen. Psychiatry 2012;69:493-8), limited educational attainment, or literacy (J. Gerontol. A. Biol. Sci. Med. Sci. 2013 [doi:10.1093/gerona/glt176]), and cardiorespiratory fitness as a protective factor (Neurology 2014;82:1339-46) and J. Gerontol. A. Biol. Sci. Med. Sci. 2014 [doi:10.1093/gerona/glt287]).
She has also been a leader in identifying posttraumatic stress disorder and traumatic brain injury as risk factors, although those didn’t crack her list of key risks. Her interest in developing a preventive approach targeting modifiable risk factors amenable to a public health campaign has been fueled by recognition that Alzheimer’s disease is irreversible, slow to develop, and that existing drugs are of only limited symptomatic benefit and don’t halt disease progression.
She and coinvestigator Deborah E. Barnes, Ph.D., estimated the impact of reducing levels of the risk factors both in the United States and globally by first calculating the population-attributable risk; that is, the percentage of Alzheimer’s disease cases attributable to a given risk factor. They found that, globally, lack of education and smoking are the biggest contributors to risk. In the United States, physical inactivity contributed to the biggest proportion of cases (Lancet Neurol. 2011;10:819-28).
The current favored hypothesis as to how higher educational attainment, mental stimulation, physical exercise, and other factors protect against development of Alzheimer’s disease and other forms of dementia involves what’s called "cognitive reserve." Cognitive reserve is conceptualized as a sort of buffer in the brain that protects against neuropathologic accumulation. It’s what enables many seniors to have died with the normal cognitive function despite meeting neuropathologic criteria for Alzheimer’s disease at autopsy.
Dr. Yaffe draws hope from the well-documented marked decline in deaths from heart disease and stroke in recent decades, about half of which is attributable to treatment advances and the other half to public health measures aimed at risk factor reduction.
Evidence from recent studies suggests that a parallel decline in the incidence of dementia has been underway during the past couple of decades. For example, a population-based U.S. survey found the prevalence of cognitive impairment among people aged 70 years and older fell from 12.2% in 1993 to 8.7% in 2002 (Alzheimer's Dement. 2008;4:134-44). Dutch investigators reported that in the Rotterdam Study, the incidence rate ratio of dementia fell from 6.56 cases/1,000 person-years in 1990 to 4.92 cases/1,000 person-years in 2000 (Neurology 2012;78:1456-63). In three regions of England under study, the prevalence of dementia declined from 8.3% in 1989-1994 to 6.5% in 2008-2011 (Lancet 2013;382:1405-12). And, in Stockholm, there is evidence to suggest a decreasing incidence of dementia during a recent 20-year period (Neurology 2013;80:1888-94).
The authors of these studies attributed their findings to higher educational level, a decline in stroke incidence, and adoption of healthier lifestyles.
Working against these favorable trends, at least in the United States, are the epidemics of obesity and type 2 diabetes as well as widespread sedentary behavior.
"A lot of cardiovascular risk factors are shifting earlier now, so the exposures are in the teens and 20s as opposed to the 50s and 60s. And that’s bad," Dr. Yaffe observed.
Nonetheless, she reported that she sees opportunities. Earlier this year, Dr. Yaffe together with Dr. A. David Smith, professor emeritus of pharmacology, of the University of Oxford (England) penned an appeal to the governments of the G8 countries to make prevention of dementia a major health priority. The open letter, entitled "Dementia (Including Alzheimer’s Disease) Can Be Prevented," was signed by 109 experts from 36 countries.
"There is already sufficient evidence to justify immediate action," the authors declared (J. Alzheimer's Dis. 2014;38:699-703). "Tell people that adopting a healthy lifestyle may help ward off dementia as it does for other diseases."
Dr. Yaffe reported serving as a consultant to Novartis and Pfizer. She receives grant support from the National Institute on Aging, the Alzheimer’s Association, the American Health Assistance Foundation, the California Department of Public Health, and the U.S. Department of Defense.
ORLANDO – Up to half of all cases of Alzheimer’s disease are attributable to seven modifiable risk factors – including depression, Dr. Kristine Yaffe asserted in her 2014 AAGP Distinguished Scientist Award Lecture.
In addition to depression, the modifiable risk factors for Alzheimer's disease backed by the best supporting evidence from observational studies are diabetes, smoking, midlife obesity, midlife hypertension, low educational attainment, and physical inactivity.
A public health campaign successful in achieving a rather modest 10% reduction across the board in these seven risk factors could prevent an estimated 184,000 cases of Alzheimer's disease in the United States alone. A more ambitious 25% reduction in the levels of these seven risk factors could prevent close to half a million cases in the United States and 3 million worldwide, she predicted at the annual meeting of the American Association for Geriatric Psychiatry.
"We’re all told by the Alzheimer’s Association and others that there’s an epidemic in dementia. And it’s true: There is an epidemic in dementia, because we’re living longer and the baby boomers are aging. We’re seeing things we didn’t see before. There wasn’t so much neurodegenerative disease when people died in their 60s and 70s. Now that we’re living into our 80s and 90s, we’re going to see a lot more neurodegenerative disease. But there are also a lot of interesting secular trends that hold promise. I think we have reason to be cautiously optimistic," declared Dr. Yaffe, professor of psychiatry, neurology, and epidemiology at the University of California, San Francisco. She is also Roy and Marie Scola Endowed Chair in Psychiatry at UCSF, and is chief of geriatric psychiatry and director of the memory disorders clinic at the San Francisco Veterans Affairs Medical Center
Dr. Yaffe is credited with having played a pivotal role in establishing many of these risk factors, including, for example, depression (Arch. Gen. Psychiatry 2012;69:493-8), limited educational attainment, or literacy (J. Gerontol. A. Biol. Sci. Med. Sci. 2013 [doi:10.1093/gerona/glt176]), and cardiorespiratory fitness as a protective factor (Neurology 2014;82:1339-46) and J. Gerontol. A. Biol. Sci. Med. Sci. 2014 [doi:10.1093/gerona/glt287]).
She has also been a leader in identifying posttraumatic stress disorder and traumatic brain injury as risk factors, although those didn’t crack her list of key risks. Her interest in developing a preventive approach targeting modifiable risk factors amenable to a public health campaign has been fueled by recognition that Alzheimer’s disease is irreversible, slow to develop, and that existing drugs are of only limited symptomatic benefit and don’t halt disease progression.
She and coinvestigator Deborah E. Barnes, Ph.D., estimated the impact of reducing levels of the risk factors both in the United States and globally by first calculating the population-attributable risk; that is, the percentage of Alzheimer’s disease cases attributable to a given risk factor. They found that, globally, lack of education and smoking are the biggest contributors to risk. In the United States, physical inactivity contributed to the biggest proportion of cases (Lancet Neurol. 2011;10:819-28).
The current favored hypothesis as to how higher educational attainment, mental stimulation, physical exercise, and other factors protect against development of Alzheimer’s disease and other forms of dementia involves what’s called "cognitive reserve." Cognitive reserve is conceptualized as a sort of buffer in the brain that protects against neuropathologic accumulation. It’s what enables many seniors to have died with the normal cognitive function despite meeting neuropathologic criteria for Alzheimer’s disease at autopsy.
Dr. Yaffe draws hope from the well-documented marked decline in deaths from heart disease and stroke in recent decades, about half of which is attributable to treatment advances and the other half to public health measures aimed at risk factor reduction.
Evidence from recent studies suggests that a parallel decline in the incidence of dementia has been underway during the past couple of decades. For example, a population-based U.S. survey found the prevalence of cognitive impairment among people aged 70 years and older fell from 12.2% in 1993 to 8.7% in 2002 (Alzheimer's Dement. 2008;4:134-44). Dutch investigators reported that in the Rotterdam Study, the incidence rate ratio of dementia fell from 6.56 cases/1,000 person-years in 1990 to 4.92 cases/1,000 person-years in 2000 (Neurology 2012;78:1456-63). In three regions of England under study, the prevalence of dementia declined from 8.3% in 1989-1994 to 6.5% in 2008-2011 (Lancet 2013;382:1405-12). And, in Stockholm, there is evidence to suggest a decreasing incidence of dementia during a recent 20-year period (Neurology 2013;80:1888-94).
The authors of these studies attributed their findings to higher educational level, a decline in stroke incidence, and adoption of healthier lifestyles.
Working against these favorable trends, at least in the United States, are the epidemics of obesity and type 2 diabetes as well as widespread sedentary behavior.
"A lot of cardiovascular risk factors are shifting earlier now, so the exposures are in the teens and 20s as opposed to the 50s and 60s. And that’s bad," Dr. Yaffe observed.
Nonetheless, she reported that she sees opportunities. Earlier this year, Dr. Yaffe together with Dr. A. David Smith, professor emeritus of pharmacology, of the University of Oxford (England) penned an appeal to the governments of the G8 countries to make prevention of dementia a major health priority. The open letter, entitled "Dementia (Including Alzheimer’s Disease) Can Be Prevented," was signed by 109 experts from 36 countries.
"There is already sufficient evidence to justify immediate action," the authors declared (J. Alzheimer's Dis. 2014;38:699-703). "Tell people that adopting a healthy lifestyle may help ward off dementia as it does for other diseases."
Dr. Yaffe reported serving as a consultant to Novartis and Pfizer. She receives grant support from the National Institute on Aging, the Alzheimer’s Association, the American Health Assistance Foundation, the California Department of Public Health, and the U.S. Department of Defense.
ORLANDO – Up to half of all cases of Alzheimer’s disease are attributable to seven modifiable risk factors – including depression, Dr. Kristine Yaffe asserted in her 2014 AAGP Distinguished Scientist Award Lecture.
In addition to depression, the modifiable risk factors for Alzheimer's disease backed by the best supporting evidence from observational studies are diabetes, smoking, midlife obesity, midlife hypertension, low educational attainment, and physical inactivity.
A public health campaign successful in achieving a rather modest 10% reduction across the board in these seven risk factors could prevent an estimated 184,000 cases of Alzheimer's disease in the United States alone. A more ambitious 25% reduction in the levels of these seven risk factors could prevent close to half a million cases in the United States and 3 million worldwide, she predicted at the annual meeting of the American Association for Geriatric Psychiatry.
"We’re all told by the Alzheimer’s Association and others that there’s an epidemic in dementia. And it’s true: There is an epidemic in dementia, because we’re living longer and the baby boomers are aging. We’re seeing things we didn’t see before. There wasn’t so much neurodegenerative disease when people died in their 60s and 70s. Now that we’re living into our 80s and 90s, we’re going to see a lot more neurodegenerative disease. But there are also a lot of interesting secular trends that hold promise. I think we have reason to be cautiously optimistic," declared Dr. Yaffe, professor of psychiatry, neurology, and epidemiology at the University of California, San Francisco. She is also Roy and Marie Scola Endowed Chair in Psychiatry at UCSF, and is chief of geriatric psychiatry and director of the memory disorders clinic at the San Francisco Veterans Affairs Medical Center
Dr. Yaffe is credited with having played a pivotal role in establishing many of these risk factors, including, for example, depression (Arch. Gen. Psychiatry 2012;69:493-8), limited educational attainment, or literacy (J. Gerontol. A. Biol. Sci. Med. Sci. 2013 [doi:10.1093/gerona/glt176]), and cardiorespiratory fitness as a protective factor (Neurology 2014;82:1339-46) and J. Gerontol. A. Biol. Sci. Med. Sci. 2014 [doi:10.1093/gerona/glt287]).
She has also been a leader in identifying posttraumatic stress disorder and traumatic brain injury as risk factors, although those didn’t crack her list of key risks. Her interest in developing a preventive approach targeting modifiable risk factors amenable to a public health campaign has been fueled by recognition that Alzheimer’s disease is irreversible, slow to develop, and that existing drugs are of only limited symptomatic benefit and don’t halt disease progression.
She and coinvestigator Deborah E. Barnes, Ph.D., estimated the impact of reducing levels of the risk factors both in the United States and globally by first calculating the population-attributable risk; that is, the percentage of Alzheimer’s disease cases attributable to a given risk factor. They found that, globally, lack of education and smoking are the biggest contributors to risk. In the United States, physical inactivity contributed to the biggest proportion of cases (Lancet Neurol. 2011;10:819-28).
The current favored hypothesis as to how higher educational attainment, mental stimulation, physical exercise, and other factors protect against development of Alzheimer’s disease and other forms of dementia involves what’s called "cognitive reserve." Cognitive reserve is conceptualized as a sort of buffer in the brain that protects against neuropathologic accumulation. It’s what enables many seniors to have died with the normal cognitive function despite meeting neuropathologic criteria for Alzheimer’s disease at autopsy.
Dr. Yaffe draws hope from the well-documented marked decline in deaths from heart disease and stroke in recent decades, about half of which is attributable to treatment advances and the other half to public health measures aimed at risk factor reduction.
Evidence from recent studies suggests that a parallel decline in the incidence of dementia has been underway during the past couple of decades. For example, a population-based U.S. survey found the prevalence of cognitive impairment among people aged 70 years and older fell from 12.2% in 1993 to 8.7% in 2002 (Alzheimer's Dement. 2008;4:134-44). Dutch investigators reported that in the Rotterdam Study, the incidence rate ratio of dementia fell from 6.56 cases/1,000 person-years in 1990 to 4.92 cases/1,000 person-years in 2000 (Neurology 2012;78:1456-63). In three regions of England under study, the prevalence of dementia declined from 8.3% in 1989-1994 to 6.5% in 2008-2011 (Lancet 2013;382:1405-12). And, in Stockholm, there is evidence to suggest a decreasing incidence of dementia during a recent 20-year period (Neurology 2013;80:1888-94).
The authors of these studies attributed their findings to higher educational level, a decline in stroke incidence, and adoption of healthier lifestyles.
Working against these favorable trends, at least in the United States, are the epidemics of obesity and type 2 diabetes as well as widespread sedentary behavior.
"A lot of cardiovascular risk factors are shifting earlier now, so the exposures are in the teens and 20s as opposed to the 50s and 60s. And that’s bad," Dr. Yaffe observed.
Nonetheless, she reported that she sees opportunities. Earlier this year, Dr. Yaffe together with Dr. A. David Smith, professor emeritus of pharmacology, of the University of Oxford (England) penned an appeal to the governments of the G8 countries to make prevention of dementia a major health priority. The open letter, entitled "Dementia (Including Alzheimer’s Disease) Can Be Prevented," was signed by 109 experts from 36 countries.
"There is already sufficient evidence to justify immediate action," the authors declared (J. Alzheimer's Dis. 2014;38:699-703). "Tell people that adopting a healthy lifestyle may help ward off dementia as it does for other diseases."
Dr. Yaffe reported serving as a consultant to Novartis and Pfizer. She receives grant support from the National Institute on Aging, the Alzheimer’s Association, the American Health Assistance Foundation, the California Department of Public Health, and the U.S. Department of Defense.
EXPERT ANALYSIS FROM THE AAGP ANNUAL MEETING
Geriatric depression, pain respond to various drugs
ORLANDO – Duloxetine for the treatment of late-life depression missed its primary end point in a recent large randomized trial, but it did result in consistently significant clinical improvement, compared with placebo across numerous secondary outcome measures.
The results overall provide a suggestion of antidepressant efficacy along with evidence of a meaningful reduction in pain in this elderly patient population, Dr. David C. Steffens observed at the annual meeting of the American Association for Geriatric Psychiatry.
This was a multicenter, double-blind, placebo-controlled 24-week study. It included 296 patients age 65 or older with major depressive disorder, a Montgomery-Asberg Depression Rating Scale score of 20 or above indicative of at least moderately severe depression, and a Mini-Mental Status Examination score of 20 or more. Participants were randomized 2-to-1 to duloxetine (Cymbalta) at either 60 mg/day, 120 mg/day, or placebo.
The primary end point was change in the Maier subscale of the 17-item Hamilton Depression Rating Scale (HAMD-17) at week 12, compared with placebo. The difference fell short of statistical significance at week 12, which was surprising given that the difference was significant at weeks 4, 8, 16, and 20. Moreover, significantly greater improvement in depressive symptoms as measured by the Geriatric Depression Scale was seen in the duloxetine group at weeks 2, 4, 8, 16, 20, and 24 – but once again, not at week 12, which was the point when the study allowed for dose adjustment from 60 mg/day to 120 mg/day, noted Dr. Steffens, professor and chairman of the department of psychiatry at the University of Connecticut, Farmington.
Participants had baseline mild to moderate pain as reflected in their scores on the Brief Pain Inventory and Pain Numeric Rating Scale. The duloxetine-treated group showed significant improvement in pain severity and interference, compared with controls. That’s an important finding given that close to two-thirds of patients with geriatric depression have comorbid pain that typically compounds their mood disorder.
Prior trials of duloxetine have been shorter. In this one, the remission rate, as defined by a HAMD-17 score of 7 or less among patients on duloxetine at 60 mg/day for the full 24 weeks, was 66% by week 12 and 65% at week 24. Thus, if a patient wasn’t in remission after 12 weeks at 60 mg/day, it was unlikely to happen with another 12 weeks on that regimen, and a dose increase to 120 mg/day may be warranted.
Five patients discontinued duloxetine for serious adverse events, compared with one placebo-treated control. The most prominent duloxetine-related side effects were diarrhea and dizziness. Duloxetine-treated patients showed a small increase in heart rate and a modest decrease in blood pressure, issues that need to be borne in mind in an elderly population at increased cardiovascular risk. One patient on duloxetine fell and sustained a hip fracture (Am. J. Geriatr. Psychiatry 2014;22:34-45).
Dr. Steffens, who didn’t participate in the duloxetine trial, highlighted another encouraging development in the treatment of late-life depression: a pooled subgroup analysis he and other investigators conducted using the data from three similar 14-week, prospective, randomized, double-blind trials of adjuvant aripiprazole (Abilify), compared with second-line antidepressant therapy in patients with major depressive disorder who had an incomplete response to standard antidepressant therapy. Their analysis included 679 patients under 50 years of age and 409 others aged 50-67 years. The older group was further subdivided by age: 50-55, 56-60, and 61-67 years.
"Our goal was to generate some preliminary data. Industry has been less and less interested in thinking about atypical antipsychotics in the elderly because of the black box warnings. Our reasoning was any data that we can get that might support further study in this population of older adults makes sense," the psychiatrist explained.
Older patients on aripiprazole showed significantly greater improvement on the Montgomery-Asberg Depression Rating Scale at week 14 than placebo-treated controls. Their remission rate was 33%, compared with 27% in younger aripiprazole-treated patients (Int. J. Geriatr. Psychiatry 2011;26:564-72).
These data are "as good as we have in terms of this particular research issue. We showed people are not keeling over from this therapy. We can at least say that among the few elderly patients we have here, we have preliminary data that this may be helpful and can be tolerated. There are some signals here. Subsequent studies looking at the older population are needed," according to Dr. Steffens.
In response to an audience question, Dr. Steffens said that in the 10 or 12 patients with late-life depression where he has tried using aripiprazole off-label at a starting dose of 1 or 2 mg/day he has had a fair degree of success.
Dr. Steffens reported having no financial conflicts.
ORLANDO – Duloxetine for the treatment of late-life depression missed its primary end point in a recent large randomized trial, but it did result in consistently significant clinical improvement, compared with placebo across numerous secondary outcome measures.
The results overall provide a suggestion of antidepressant efficacy along with evidence of a meaningful reduction in pain in this elderly patient population, Dr. David C. Steffens observed at the annual meeting of the American Association for Geriatric Psychiatry.
This was a multicenter, double-blind, placebo-controlled 24-week study. It included 296 patients age 65 or older with major depressive disorder, a Montgomery-Asberg Depression Rating Scale score of 20 or above indicative of at least moderately severe depression, and a Mini-Mental Status Examination score of 20 or more. Participants were randomized 2-to-1 to duloxetine (Cymbalta) at either 60 mg/day, 120 mg/day, or placebo.
The primary end point was change in the Maier subscale of the 17-item Hamilton Depression Rating Scale (HAMD-17) at week 12, compared with placebo. The difference fell short of statistical significance at week 12, which was surprising given that the difference was significant at weeks 4, 8, 16, and 20. Moreover, significantly greater improvement in depressive symptoms as measured by the Geriatric Depression Scale was seen in the duloxetine group at weeks 2, 4, 8, 16, 20, and 24 – but once again, not at week 12, which was the point when the study allowed for dose adjustment from 60 mg/day to 120 mg/day, noted Dr. Steffens, professor and chairman of the department of psychiatry at the University of Connecticut, Farmington.
Participants had baseline mild to moderate pain as reflected in their scores on the Brief Pain Inventory and Pain Numeric Rating Scale. The duloxetine-treated group showed significant improvement in pain severity and interference, compared with controls. That’s an important finding given that close to two-thirds of patients with geriatric depression have comorbid pain that typically compounds their mood disorder.
Prior trials of duloxetine have been shorter. In this one, the remission rate, as defined by a HAMD-17 score of 7 or less among patients on duloxetine at 60 mg/day for the full 24 weeks, was 66% by week 12 and 65% at week 24. Thus, if a patient wasn’t in remission after 12 weeks at 60 mg/day, it was unlikely to happen with another 12 weeks on that regimen, and a dose increase to 120 mg/day may be warranted.
Five patients discontinued duloxetine for serious adverse events, compared with one placebo-treated control. The most prominent duloxetine-related side effects were diarrhea and dizziness. Duloxetine-treated patients showed a small increase in heart rate and a modest decrease in blood pressure, issues that need to be borne in mind in an elderly population at increased cardiovascular risk. One patient on duloxetine fell and sustained a hip fracture (Am. J. Geriatr. Psychiatry 2014;22:34-45).
Dr. Steffens, who didn’t participate in the duloxetine trial, highlighted another encouraging development in the treatment of late-life depression: a pooled subgroup analysis he and other investigators conducted using the data from three similar 14-week, prospective, randomized, double-blind trials of adjuvant aripiprazole (Abilify), compared with second-line antidepressant therapy in patients with major depressive disorder who had an incomplete response to standard antidepressant therapy. Their analysis included 679 patients under 50 years of age and 409 others aged 50-67 years. The older group was further subdivided by age: 50-55, 56-60, and 61-67 years.
"Our goal was to generate some preliminary data. Industry has been less and less interested in thinking about atypical antipsychotics in the elderly because of the black box warnings. Our reasoning was any data that we can get that might support further study in this population of older adults makes sense," the psychiatrist explained.
Older patients on aripiprazole showed significantly greater improvement on the Montgomery-Asberg Depression Rating Scale at week 14 than placebo-treated controls. Their remission rate was 33%, compared with 27% in younger aripiprazole-treated patients (Int. J. Geriatr. Psychiatry 2011;26:564-72).
These data are "as good as we have in terms of this particular research issue. We showed people are not keeling over from this therapy. We can at least say that among the few elderly patients we have here, we have preliminary data that this may be helpful and can be tolerated. There are some signals here. Subsequent studies looking at the older population are needed," according to Dr. Steffens.
In response to an audience question, Dr. Steffens said that in the 10 or 12 patients with late-life depression where he has tried using aripiprazole off-label at a starting dose of 1 or 2 mg/day he has had a fair degree of success.
Dr. Steffens reported having no financial conflicts.
ORLANDO – Duloxetine for the treatment of late-life depression missed its primary end point in a recent large randomized trial, but it did result in consistently significant clinical improvement, compared with placebo across numerous secondary outcome measures.
The results overall provide a suggestion of antidepressant efficacy along with evidence of a meaningful reduction in pain in this elderly patient population, Dr. David C. Steffens observed at the annual meeting of the American Association for Geriatric Psychiatry.
This was a multicenter, double-blind, placebo-controlled 24-week study. It included 296 patients age 65 or older with major depressive disorder, a Montgomery-Asberg Depression Rating Scale score of 20 or above indicative of at least moderately severe depression, and a Mini-Mental Status Examination score of 20 or more. Participants were randomized 2-to-1 to duloxetine (Cymbalta) at either 60 mg/day, 120 mg/day, or placebo.
The primary end point was change in the Maier subscale of the 17-item Hamilton Depression Rating Scale (HAMD-17) at week 12, compared with placebo. The difference fell short of statistical significance at week 12, which was surprising given that the difference was significant at weeks 4, 8, 16, and 20. Moreover, significantly greater improvement in depressive symptoms as measured by the Geriatric Depression Scale was seen in the duloxetine group at weeks 2, 4, 8, 16, 20, and 24 – but once again, not at week 12, which was the point when the study allowed for dose adjustment from 60 mg/day to 120 mg/day, noted Dr. Steffens, professor and chairman of the department of psychiatry at the University of Connecticut, Farmington.
Participants had baseline mild to moderate pain as reflected in their scores on the Brief Pain Inventory and Pain Numeric Rating Scale. The duloxetine-treated group showed significant improvement in pain severity and interference, compared with controls. That’s an important finding given that close to two-thirds of patients with geriatric depression have comorbid pain that typically compounds their mood disorder.
Prior trials of duloxetine have been shorter. In this one, the remission rate, as defined by a HAMD-17 score of 7 or less among patients on duloxetine at 60 mg/day for the full 24 weeks, was 66% by week 12 and 65% at week 24. Thus, if a patient wasn’t in remission after 12 weeks at 60 mg/day, it was unlikely to happen with another 12 weeks on that regimen, and a dose increase to 120 mg/day may be warranted.
Five patients discontinued duloxetine for serious adverse events, compared with one placebo-treated control. The most prominent duloxetine-related side effects were diarrhea and dizziness. Duloxetine-treated patients showed a small increase in heart rate and a modest decrease in blood pressure, issues that need to be borne in mind in an elderly population at increased cardiovascular risk. One patient on duloxetine fell and sustained a hip fracture (Am. J. Geriatr. Psychiatry 2014;22:34-45).
Dr. Steffens, who didn’t participate in the duloxetine trial, highlighted another encouraging development in the treatment of late-life depression: a pooled subgroup analysis he and other investigators conducted using the data from three similar 14-week, prospective, randomized, double-blind trials of adjuvant aripiprazole (Abilify), compared with second-line antidepressant therapy in patients with major depressive disorder who had an incomplete response to standard antidepressant therapy. Their analysis included 679 patients under 50 years of age and 409 others aged 50-67 years. The older group was further subdivided by age: 50-55, 56-60, and 61-67 years.
"Our goal was to generate some preliminary data. Industry has been less and less interested in thinking about atypical antipsychotics in the elderly because of the black box warnings. Our reasoning was any data that we can get that might support further study in this population of older adults makes sense," the psychiatrist explained.
Older patients on aripiprazole showed significantly greater improvement on the Montgomery-Asberg Depression Rating Scale at week 14 than placebo-treated controls. Their remission rate was 33%, compared with 27% in younger aripiprazole-treated patients (Int. J. Geriatr. Psychiatry 2011;26:564-72).
These data are "as good as we have in terms of this particular research issue. We showed people are not keeling over from this therapy. We can at least say that among the few elderly patients we have here, we have preliminary data that this may be helpful and can be tolerated. There are some signals here. Subsequent studies looking at the older population are needed," according to Dr. Steffens.
In response to an audience question, Dr. Steffens said that in the 10 or 12 patients with late-life depression where he has tried using aripiprazole off-label at a starting dose of 1 or 2 mg/day he has had a fair degree of success.
Dr. Steffens reported having no financial conflicts.
EXPERT ANALYSIS FROM THE AAGP ANNUAL MEETING
Harness the ‘placebo’ in late-life depression
ORLANDO – The bad news about antidepressant therapy for patients whose first episode of depression occurs in late life is that the randomized trials show it’s no better than placebo. The good news is that placebo is pretty darn effective.
What are clinicians to make of these research findings?
In everyday practice, make sure to give patients with late-life depression the sort of TLC they would have received in the placebo arm of a randomized drug trial. Only don’t call it placebo therapy. First of all, it’s not ethical to give patients a placebo outside of a formal clinical trial, and secondly, what goes on in the control arm of a randomized antidepressant trial is not really a placebo anyway, Dr. J. Craig Nelson explained at the annual meeting of the American Association for Geriatric Psychiatry.
"I don’t know that it’s a mistake to give those people antidepressants. I think it is a mistake, though, not to provide the elements of clinical management that those people really seem most responsive to. So if you do use an antidepressant, don’t just prescribe it and then see that person 2 months later. You want to see the patient on a regular basis and take advantage of what’s often called a placebo response but is really a response to clinical management," said Dr. Nelson, professor of psychiatry and director of geriatric psychiatry at the University of California, San Francisco.
"Provide those elements of supportive care that are important," he urged. "It’s being human, but to be more specific it’s being human and providing attention, reassurance, support, education about depression, and monitoring of symptoms and side effects. You want to see the patient regularly and take advantage of that effect."
In his meta-analysis of 10 randomized, placebo-controlled trials of antidepressant therapy, using data on more than 2,300 outpatients aged 60 years or older with late-life major depressive disorder, he was able to identify one specific subgroup with a markedly better response to antidepressant medication than to placebo.
Among the 385 patients with at least a 10-year duration of depression along with at least moderately severe disease as defined by a baseline Hamilton Depression Rating Scale score of 21 or more, there was a 58% response rate to medication, compared with a 31% response to placebo. The number needed to treat with an antidepressant rather than placebo to achieve one additional response in this group was a very respectable four. For all others, however, the number needed to treat was a whopping 21 (Am. J. Psychiatry 2013;170:651-9).
Antidepressants are clearly ineffective in patients with depression and dementia. Dr. Nelson demonstrated this in a meta-analysis of seven randomized trials with 330 patients, in which antidepressants showed no significant benefit over placebo in terms of response or remission rates (J. Am. Geriatr. Soc. 2011;59:577-85). Moreover, a subsequent large, placebo-controlled randomized trial of sertraline or mirtazapine involving 326 patients with depression and dementia also showed no suggestion of an advantage for drug therapy over placebo (Lancet 2011;378:403-11).
Another group for whom randomized trials have shown antidepressant medications are likely to fail is patients with late-life depression plus executive dysfunction. This is a population for whom problem-solving therapy has proved to be quite effective, the psychiatrist noted.
He highlighted what he called "arguably the best controlled study of psychotherapy in older depressed adults." It included 221 subjects aged 60 years and older with executive dysfunction who were randomized to 12 weekly sessions of problem-solving therapy or supportive therapy. The two groups showed comparable reduction of depressive symptoms for the first 6 weeks, then the group assigned to problem-solving therapy pulled ahead.
At week 9 they had a response rate of 47%, compared with 29% in the supportive therapy group. By week 12 the margin had grown to 57% vs. 34%. The week 12 remission rates were 46% and 28% (Am. J. Psychiatry 2010;167:1391-8). The group that received problem-solving therapy also showed a significant advantage in terms of improvement in disability, as measured using the 12-item WHO Disability Assessment Schedule II (Arch. Gen. Psychiatry 2011;68:33-41).
Dr. Nelson reported serving as a consultant to Bristol-Myers Squibb, Eli Lilly, Lundbeck, Otsuka America, Pfizer, Shire, and Sunovion.
ORLANDO – The bad news about antidepressant therapy for patients whose first episode of depression occurs in late life is that the randomized trials show it’s no better than placebo. The good news is that placebo is pretty darn effective.
What are clinicians to make of these research findings?
In everyday practice, make sure to give patients with late-life depression the sort of TLC they would have received in the placebo arm of a randomized drug trial. Only don’t call it placebo therapy. First of all, it’s not ethical to give patients a placebo outside of a formal clinical trial, and secondly, what goes on in the control arm of a randomized antidepressant trial is not really a placebo anyway, Dr. J. Craig Nelson explained at the annual meeting of the American Association for Geriatric Psychiatry.
"I don’t know that it’s a mistake to give those people antidepressants. I think it is a mistake, though, not to provide the elements of clinical management that those people really seem most responsive to. So if you do use an antidepressant, don’t just prescribe it and then see that person 2 months later. You want to see the patient on a regular basis and take advantage of what’s often called a placebo response but is really a response to clinical management," said Dr. Nelson, professor of psychiatry and director of geriatric psychiatry at the University of California, San Francisco.
"Provide those elements of supportive care that are important," he urged. "It’s being human, but to be more specific it’s being human and providing attention, reassurance, support, education about depression, and monitoring of symptoms and side effects. You want to see the patient regularly and take advantage of that effect."
In his meta-analysis of 10 randomized, placebo-controlled trials of antidepressant therapy, using data on more than 2,300 outpatients aged 60 years or older with late-life major depressive disorder, he was able to identify one specific subgroup with a markedly better response to antidepressant medication than to placebo.
Among the 385 patients with at least a 10-year duration of depression along with at least moderately severe disease as defined by a baseline Hamilton Depression Rating Scale score of 21 or more, there was a 58% response rate to medication, compared with a 31% response to placebo. The number needed to treat with an antidepressant rather than placebo to achieve one additional response in this group was a very respectable four. For all others, however, the number needed to treat was a whopping 21 (Am. J. Psychiatry 2013;170:651-9).
Antidepressants are clearly ineffective in patients with depression and dementia. Dr. Nelson demonstrated this in a meta-analysis of seven randomized trials with 330 patients, in which antidepressants showed no significant benefit over placebo in terms of response or remission rates (J. Am. Geriatr. Soc. 2011;59:577-85). Moreover, a subsequent large, placebo-controlled randomized trial of sertraline or mirtazapine involving 326 patients with depression and dementia also showed no suggestion of an advantage for drug therapy over placebo (Lancet 2011;378:403-11).
Another group for whom randomized trials have shown antidepressant medications are likely to fail is patients with late-life depression plus executive dysfunction. This is a population for whom problem-solving therapy has proved to be quite effective, the psychiatrist noted.
He highlighted what he called "arguably the best controlled study of psychotherapy in older depressed adults." It included 221 subjects aged 60 years and older with executive dysfunction who were randomized to 12 weekly sessions of problem-solving therapy or supportive therapy. The two groups showed comparable reduction of depressive symptoms for the first 6 weeks, then the group assigned to problem-solving therapy pulled ahead.
At week 9 they had a response rate of 47%, compared with 29% in the supportive therapy group. By week 12 the margin had grown to 57% vs. 34%. The week 12 remission rates were 46% and 28% (Am. J. Psychiatry 2010;167:1391-8). The group that received problem-solving therapy also showed a significant advantage in terms of improvement in disability, as measured using the 12-item WHO Disability Assessment Schedule II (Arch. Gen. Psychiatry 2011;68:33-41).
Dr. Nelson reported serving as a consultant to Bristol-Myers Squibb, Eli Lilly, Lundbeck, Otsuka America, Pfizer, Shire, and Sunovion.
ORLANDO – The bad news about antidepressant therapy for patients whose first episode of depression occurs in late life is that the randomized trials show it’s no better than placebo. The good news is that placebo is pretty darn effective.
What are clinicians to make of these research findings?
In everyday practice, make sure to give patients with late-life depression the sort of TLC they would have received in the placebo arm of a randomized drug trial. Only don’t call it placebo therapy. First of all, it’s not ethical to give patients a placebo outside of a formal clinical trial, and secondly, what goes on in the control arm of a randomized antidepressant trial is not really a placebo anyway, Dr. J. Craig Nelson explained at the annual meeting of the American Association for Geriatric Psychiatry.
"I don’t know that it’s a mistake to give those people antidepressants. I think it is a mistake, though, not to provide the elements of clinical management that those people really seem most responsive to. So if you do use an antidepressant, don’t just prescribe it and then see that person 2 months later. You want to see the patient on a regular basis and take advantage of what’s often called a placebo response but is really a response to clinical management," said Dr. Nelson, professor of psychiatry and director of geriatric psychiatry at the University of California, San Francisco.
"Provide those elements of supportive care that are important," he urged. "It’s being human, but to be more specific it’s being human and providing attention, reassurance, support, education about depression, and monitoring of symptoms and side effects. You want to see the patient regularly and take advantage of that effect."
In his meta-analysis of 10 randomized, placebo-controlled trials of antidepressant therapy, using data on more than 2,300 outpatients aged 60 years or older with late-life major depressive disorder, he was able to identify one specific subgroup with a markedly better response to antidepressant medication than to placebo.
Among the 385 patients with at least a 10-year duration of depression along with at least moderately severe disease as defined by a baseline Hamilton Depression Rating Scale score of 21 or more, there was a 58% response rate to medication, compared with a 31% response to placebo. The number needed to treat with an antidepressant rather than placebo to achieve one additional response in this group was a very respectable four. For all others, however, the number needed to treat was a whopping 21 (Am. J. Psychiatry 2013;170:651-9).
Antidepressants are clearly ineffective in patients with depression and dementia. Dr. Nelson demonstrated this in a meta-analysis of seven randomized trials with 330 patients, in which antidepressants showed no significant benefit over placebo in terms of response or remission rates (J. Am. Geriatr. Soc. 2011;59:577-85). Moreover, a subsequent large, placebo-controlled randomized trial of sertraline or mirtazapine involving 326 patients with depression and dementia also showed no suggestion of an advantage for drug therapy over placebo (Lancet 2011;378:403-11).
Another group for whom randomized trials have shown antidepressant medications are likely to fail is patients with late-life depression plus executive dysfunction. This is a population for whom problem-solving therapy has proved to be quite effective, the psychiatrist noted.
He highlighted what he called "arguably the best controlled study of psychotherapy in older depressed adults." It included 221 subjects aged 60 years and older with executive dysfunction who were randomized to 12 weekly sessions of problem-solving therapy or supportive therapy. The two groups showed comparable reduction of depressive symptoms for the first 6 weeks, then the group assigned to problem-solving therapy pulled ahead.
At week 9 they had a response rate of 47%, compared with 29% in the supportive therapy group. By week 12 the margin had grown to 57% vs. 34%. The week 12 remission rates were 46% and 28% (Am. J. Psychiatry 2010;167:1391-8). The group that received problem-solving therapy also showed a significant advantage in terms of improvement in disability, as measured using the 12-item WHO Disability Assessment Schedule II (Arch. Gen. Psychiatry 2011;68:33-41).
Dr. Nelson reported serving as a consultant to Bristol-Myers Squibb, Eli Lilly, Lundbeck, Otsuka America, Pfizer, Shire, and Sunovion.
EXPERT OPINION FROM THE AAGP ANNUAL MEETING
Key points in managing late-life anxiety
ORLANDO – Do SSRIs actually work for late-life generalized anxiety disorder?
"I think the answer is, ‘Yes, a little.’ The benefits are limited in the randomized controlled trials and in my own clinical experience. Many people will get some benefit, but fairly few will get such a substantial benefit that that’s all they need. Maybe on the order of 40% at most," Dr. Eric J. Lenze said at the annual meeting of the American Association for Geriatric Psychiatry.
He should know. He was the principal investigator in two of the three randomized, placebo-controlled trials that have established the efficacy of selective serotonin reuptake inhibitors for later-life generalized anxiety disorder (GAD), including by far the largest study, involving escitalopram (JAMA 2009;301:295-303).
For patients who don’t achieve remission of anxiety symptoms with SSRI monotherapy, a good option – and one of proven benefit – is dual therapy along with cognitive-behavioral therapy (CBT). In a randomized, placebo-controlled clinical trial involving 73 older adults with GAD, Dr. Lenze and his coinvestigators showed that during a lead-in phase with 12 weeks of open-label escitalopram, participants showed a modest reduction in worry symptoms on the Penn State Worry Questionnaire, but if they then had CBT added, they showed a substantial further reduction in worry, compared with those who did not receive CBT. Continued escitalopram prevented relapse, but for many patients CBT allowed sustained drug-free remission (Am. J. Psychiatry 2013;170:782-9).
The concept is that starting out with an SSRI helps reduce the patient’s distress and somatization, then adding the CBT addresses the underlying pathologic worry.
"The combination seems to be effective, and it’s an attractive one. CBT has what the psychologists call ‘durable’ benefits: You get a course of CBT, it’s stopped, and those benefits are maintained," explained Dr. Lenze, professor of psychiatry at Washington University in St. Louis.
The beauty of CBT in the context of late-life anxiety is that relaxation training appears to be the single most effective component of CBT for this condition, as has been shown by investigators at the University of California, San Diego (Am. J. Geriatr. Psychiatry 2009;17:105-15).
"This is important because relaxation training – deep breathing, muscle relaxation, and pleasant imagery – is also the easiest component of CBT, which means that if I had the time in my clinic, even I could probably pull this off with patients. If you have a bevy of therapists you can refer to, your patients will get benefit from it," the psychopharmacologist continued.
Pharmacologic options beyond the three SSRIs supported by placebo-controlled, randomized trial evidence – sertraline, citalopram, and escitalopram – include the various other SSRIs. In addition, two selective norepinephrine reuptake inhibitors (SNRIs) – venlafaxine and duloxetine – are supported by retrospective analyses of earlier Food and Drug Administration approval studies that showed the drugs appeared equally efficacious in young adults and the elderly with GAD.
Also, a large multisite study of pregabalin for late-life GAD showed it was effective starting at 50 mg b.i.d. and titrating up as tolerated to 100 mg t.i.d. And a multicenter study showed quetiapine XR was effective at much lower doses than those used for schizophrenia. But neither pregabalin nor quetiapine XR approved for GAD.
The use of benzodiazepines is problematic. They induce falls and cognitive impairment at a lower dose than is effective for anxiety. And short-acting benzodiazepines are not safer than long-acting ones. Yet benzodiazepines, to Dr. Lenze’s dismay, are heavily prescribed for late-life anxiety, especially by primary care physicians.
"I sometimes do use benzos, but I would say about 10 times less than many of my colleagues. By the time a patient with anxiety gets to a psychiatrist, they’re probably already on a benzo. For most of my patients, one of the key questions in my mind is, ‘When am I going to start tapering that benzo someone else put them on?’ " according to Dr. Lenze.
That’s why "Think twice about prescribing a benzodiazepine" is on his list of eight rules for the management of anxiety.
Dr. Lenze reported having received research grants from Roche and Lundbeck.
Eight rules for managing late-life anxiety
Dr. Lenze’s work with older patients who have anxiety has led him to develop the following checklist that should help you respond to these patients with compassion:
• Include an objective measurement of severity in assessment. Patients with GAD will often come in for a follow-up visit unaware that they’re now spending much less of their day wracked by worry and that they now feel they have some ability to stop it. It helps to show them the earlier numbers.
• Think twice about prescribing a benzodiazepine.
• Provide psychoeducation about anxiety. "A lot of the bite of anxiety is defanged if you just understand what anxiety is. It’s not going to kill you. It’s a set of alarm systems in your brain that helps you survive by responding to threats at the cost of making you miserable. That’s all it is. If you can get that across to a patient and the family, that’s a crucial part of getting well and staying well," Dr. Lenze said.
Physicians often don’t have a lot of time for patient education in the office, and patients might not remember much of what was said anyway, because they were stressed out about the visit. So recommendations for good self-help books are useful, added Dr. Lenze.
• Start low and go slow in elderly patients – but go slow. "Starting low is a good exercise in graduated exposure for these patients so they feel more comfortable. But get them up to the same doses of SSRIs (selective serotonin reuptake inhibitors) and SNRIs (selective norepinephrine reuptake inhibitors) you use in younger adults as quickly as you can for their level of comfort. Ten weeks of escitalopram at 2.5 mg is not the way to treat these individuals," he continued.
• Arrange for frequent follow-up within the first month of starting therapy or a dose change in order to monitor response and encourage adherence. "This is really important," he said. "They get anxious about treatment, scared of medication, and frequently stop therapy."
• For first-line therapy, stick to what you’re used to prescribing. You’ll project more confidence when a patient calls back about side effects.
• Consider augmentation and switch strategies for inadequate responders.
• Provide maintenance therapy. "An area where many in our field make errors is in letting people drop out of long-term treatment. Antidepressants have maintenance benefits. That means they prevent relapse if you keep taking them. One place where we can all do better is in continuing to remind our patients of the benefits of maintenance treatment," Dr. Lenze emphasized.
ORLANDO – Do SSRIs actually work for late-life generalized anxiety disorder?
"I think the answer is, ‘Yes, a little.’ The benefits are limited in the randomized controlled trials and in my own clinical experience. Many people will get some benefit, but fairly few will get such a substantial benefit that that’s all they need. Maybe on the order of 40% at most," Dr. Eric J. Lenze said at the annual meeting of the American Association for Geriatric Psychiatry.
He should know. He was the principal investigator in two of the three randomized, placebo-controlled trials that have established the efficacy of selective serotonin reuptake inhibitors for later-life generalized anxiety disorder (GAD), including by far the largest study, involving escitalopram (JAMA 2009;301:295-303).
For patients who don’t achieve remission of anxiety symptoms with SSRI monotherapy, a good option – and one of proven benefit – is dual therapy along with cognitive-behavioral therapy (CBT). In a randomized, placebo-controlled clinical trial involving 73 older adults with GAD, Dr. Lenze and his coinvestigators showed that during a lead-in phase with 12 weeks of open-label escitalopram, participants showed a modest reduction in worry symptoms on the Penn State Worry Questionnaire, but if they then had CBT added, they showed a substantial further reduction in worry, compared with those who did not receive CBT. Continued escitalopram prevented relapse, but for many patients CBT allowed sustained drug-free remission (Am. J. Psychiatry 2013;170:782-9).
The concept is that starting out with an SSRI helps reduce the patient’s distress and somatization, then adding the CBT addresses the underlying pathologic worry.
"The combination seems to be effective, and it’s an attractive one. CBT has what the psychologists call ‘durable’ benefits: You get a course of CBT, it’s stopped, and those benefits are maintained," explained Dr. Lenze, professor of psychiatry at Washington University in St. Louis.
The beauty of CBT in the context of late-life anxiety is that relaxation training appears to be the single most effective component of CBT for this condition, as has been shown by investigators at the University of California, San Diego (Am. J. Geriatr. Psychiatry 2009;17:105-15).
"This is important because relaxation training – deep breathing, muscle relaxation, and pleasant imagery – is also the easiest component of CBT, which means that if I had the time in my clinic, even I could probably pull this off with patients. If you have a bevy of therapists you can refer to, your patients will get benefit from it," the psychopharmacologist continued.
Pharmacologic options beyond the three SSRIs supported by placebo-controlled, randomized trial evidence – sertraline, citalopram, and escitalopram – include the various other SSRIs. In addition, two selective norepinephrine reuptake inhibitors (SNRIs) – venlafaxine and duloxetine – are supported by retrospective analyses of earlier Food and Drug Administration approval studies that showed the drugs appeared equally efficacious in young adults and the elderly with GAD.
Also, a large multisite study of pregabalin for late-life GAD showed it was effective starting at 50 mg b.i.d. and titrating up as tolerated to 100 mg t.i.d. And a multicenter study showed quetiapine XR was effective at much lower doses than those used for schizophrenia. But neither pregabalin nor quetiapine XR approved for GAD.
The use of benzodiazepines is problematic. They induce falls and cognitive impairment at a lower dose than is effective for anxiety. And short-acting benzodiazepines are not safer than long-acting ones. Yet benzodiazepines, to Dr. Lenze’s dismay, are heavily prescribed for late-life anxiety, especially by primary care physicians.
"I sometimes do use benzos, but I would say about 10 times less than many of my colleagues. By the time a patient with anxiety gets to a psychiatrist, they’re probably already on a benzo. For most of my patients, one of the key questions in my mind is, ‘When am I going to start tapering that benzo someone else put them on?’ " according to Dr. Lenze.
That’s why "Think twice about prescribing a benzodiazepine" is on his list of eight rules for the management of anxiety.
Dr. Lenze reported having received research grants from Roche and Lundbeck.
Eight rules for managing late-life anxiety
Dr. Lenze’s work with older patients who have anxiety has led him to develop the following checklist that should help you respond to these patients with compassion:
• Include an objective measurement of severity in assessment. Patients with GAD will often come in for a follow-up visit unaware that they’re now spending much less of their day wracked by worry and that they now feel they have some ability to stop it. It helps to show them the earlier numbers.
• Think twice about prescribing a benzodiazepine.
• Provide psychoeducation about anxiety. "A lot of the bite of anxiety is defanged if you just understand what anxiety is. It’s not going to kill you. It’s a set of alarm systems in your brain that helps you survive by responding to threats at the cost of making you miserable. That’s all it is. If you can get that across to a patient and the family, that’s a crucial part of getting well and staying well," Dr. Lenze said.
Physicians often don’t have a lot of time for patient education in the office, and patients might not remember much of what was said anyway, because they were stressed out about the visit. So recommendations for good self-help books are useful, added Dr. Lenze.
• Start low and go slow in elderly patients – but go slow. "Starting low is a good exercise in graduated exposure for these patients so they feel more comfortable. But get them up to the same doses of SSRIs (selective serotonin reuptake inhibitors) and SNRIs (selective norepinephrine reuptake inhibitors) you use in younger adults as quickly as you can for their level of comfort. Ten weeks of escitalopram at 2.5 mg is not the way to treat these individuals," he continued.
• Arrange for frequent follow-up within the first month of starting therapy or a dose change in order to monitor response and encourage adherence. "This is really important," he said. "They get anxious about treatment, scared of medication, and frequently stop therapy."
• For first-line therapy, stick to what you’re used to prescribing. You’ll project more confidence when a patient calls back about side effects.
• Consider augmentation and switch strategies for inadequate responders.
• Provide maintenance therapy. "An area where many in our field make errors is in letting people drop out of long-term treatment. Antidepressants have maintenance benefits. That means they prevent relapse if you keep taking them. One place where we can all do better is in continuing to remind our patients of the benefits of maintenance treatment," Dr. Lenze emphasized.
ORLANDO – Do SSRIs actually work for late-life generalized anxiety disorder?
"I think the answer is, ‘Yes, a little.’ The benefits are limited in the randomized controlled trials and in my own clinical experience. Many people will get some benefit, but fairly few will get such a substantial benefit that that’s all they need. Maybe on the order of 40% at most," Dr. Eric J. Lenze said at the annual meeting of the American Association for Geriatric Psychiatry.
He should know. He was the principal investigator in two of the three randomized, placebo-controlled trials that have established the efficacy of selective serotonin reuptake inhibitors for later-life generalized anxiety disorder (GAD), including by far the largest study, involving escitalopram (JAMA 2009;301:295-303).
For patients who don’t achieve remission of anxiety symptoms with SSRI monotherapy, a good option – and one of proven benefit – is dual therapy along with cognitive-behavioral therapy (CBT). In a randomized, placebo-controlled clinical trial involving 73 older adults with GAD, Dr. Lenze and his coinvestigators showed that during a lead-in phase with 12 weeks of open-label escitalopram, participants showed a modest reduction in worry symptoms on the Penn State Worry Questionnaire, but if they then had CBT added, they showed a substantial further reduction in worry, compared with those who did not receive CBT. Continued escitalopram prevented relapse, but for many patients CBT allowed sustained drug-free remission (Am. J. Psychiatry 2013;170:782-9).
The concept is that starting out with an SSRI helps reduce the patient’s distress and somatization, then adding the CBT addresses the underlying pathologic worry.
"The combination seems to be effective, and it’s an attractive one. CBT has what the psychologists call ‘durable’ benefits: You get a course of CBT, it’s stopped, and those benefits are maintained," explained Dr. Lenze, professor of psychiatry at Washington University in St. Louis.
The beauty of CBT in the context of late-life anxiety is that relaxation training appears to be the single most effective component of CBT for this condition, as has been shown by investigators at the University of California, San Diego (Am. J. Geriatr. Psychiatry 2009;17:105-15).
"This is important because relaxation training – deep breathing, muscle relaxation, and pleasant imagery – is also the easiest component of CBT, which means that if I had the time in my clinic, even I could probably pull this off with patients. If you have a bevy of therapists you can refer to, your patients will get benefit from it," the psychopharmacologist continued.
Pharmacologic options beyond the three SSRIs supported by placebo-controlled, randomized trial evidence – sertraline, citalopram, and escitalopram – include the various other SSRIs. In addition, two selective norepinephrine reuptake inhibitors (SNRIs) – venlafaxine and duloxetine – are supported by retrospective analyses of earlier Food and Drug Administration approval studies that showed the drugs appeared equally efficacious in young adults and the elderly with GAD.
Also, a large multisite study of pregabalin for late-life GAD showed it was effective starting at 50 mg b.i.d. and titrating up as tolerated to 100 mg t.i.d. And a multicenter study showed quetiapine XR was effective at much lower doses than those used for schizophrenia. But neither pregabalin nor quetiapine XR approved for GAD.
The use of benzodiazepines is problematic. They induce falls and cognitive impairment at a lower dose than is effective for anxiety. And short-acting benzodiazepines are not safer than long-acting ones. Yet benzodiazepines, to Dr. Lenze’s dismay, are heavily prescribed for late-life anxiety, especially by primary care physicians.
"I sometimes do use benzos, but I would say about 10 times less than many of my colleagues. By the time a patient with anxiety gets to a psychiatrist, they’re probably already on a benzo. For most of my patients, one of the key questions in my mind is, ‘When am I going to start tapering that benzo someone else put them on?’ " according to Dr. Lenze.
That’s why "Think twice about prescribing a benzodiazepine" is on his list of eight rules for the management of anxiety.
Dr. Lenze reported having received research grants from Roche and Lundbeck.
Eight rules for managing late-life anxiety
Dr. Lenze’s work with older patients who have anxiety has led him to develop the following checklist that should help you respond to these patients with compassion:
• Include an objective measurement of severity in assessment. Patients with GAD will often come in for a follow-up visit unaware that they’re now spending much less of their day wracked by worry and that they now feel they have some ability to stop it. It helps to show them the earlier numbers.
• Think twice about prescribing a benzodiazepine.
• Provide psychoeducation about anxiety. "A lot of the bite of anxiety is defanged if you just understand what anxiety is. It’s not going to kill you. It’s a set of alarm systems in your brain that helps you survive by responding to threats at the cost of making you miserable. That’s all it is. If you can get that across to a patient and the family, that’s a crucial part of getting well and staying well," Dr. Lenze said.
Physicians often don’t have a lot of time for patient education in the office, and patients might not remember much of what was said anyway, because they were stressed out about the visit. So recommendations for good self-help books are useful, added Dr. Lenze.
• Start low and go slow in elderly patients – but go slow. "Starting low is a good exercise in graduated exposure for these patients so they feel more comfortable. But get them up to the same doses of SSRIs (selective serotonin reuptake inhibitors) and SNRIs (selective norepinephrine reuptake inhibitors) you use in younger adults as quickly as you can for their level of comfort. Ten weeks of escitalopram at 2.5 mg is not the way to treat these individuals," he continued.
• Arrange for frequent follow-up within the first month of starting therapy or a dose change in order to monitor response and encourage adherence. "This is really important," he said. "They get anxious about treatment, scared of medication, and frequently stop therapy."
• For first-line therapy, stick to what you’re used to prescribing. You’ll project more confidence when a patient calls back about side effects.
• Consider augmentation and switch strategies for inadequate responders.
• Provide maintenance therapy. "An area where many in our field make errors is in letting people drop out of long-term treatment. Antidepressants have maintenance benefits. That means they prevent relapse if you keep taking them. One place where we can all do better is in continuing to remind our patients of the benefits of maintenance treatment," Dr. Lenze emphasized.
EXPERT OPINION FROM THE AAGP ANNUAL MEETING
Elderly suicide prevention: Focus on housing transition
ORLANDO – Transition to an assisted-living facility or nursing home is a period of high risk for suicide among the elderly – as well as an opportunity for preventive intervention.
That was the key message of a study, which earned its presenter, Briana Mezuk, Ph.D., the Best Early Investigator Award for the top research study presented at the annual meeting of the American Association for Geriatric Psychiatry.
Dr. Mezuk, an epidemiologist in the department of family medicine and population health at Virginia Commonwealth University, Richmond, presented an analysis of suicide among seniors in nursing homes and assisted-living facilities in Virginia during 2003-2011. The data came from the Virginia Violent Death Reporting System, which includes detailed case narratives describing the circumstances of all violent deaths in the state.
Dr. Mezuk’s study was unusual in that it provided both quantitative data addressing the study hypothesis that nursing homes and assisted-living facilities where suicides occurred would have lower quality of care ratings than facilities without suicides – the opposite proved to be true – as well as common narrative themes gleaned from the case files that could provide clinicians with practical warning signs of impending suicidality.
"Common themes of verbalized anticipatory distress, substance abuse, caregiver burden, past suicide attempts, and history of mental illness highlight potential risk factors for suicide in later life," she reported.
Older adults have the nation’s highest suicide rate. Yet even though there are roughly 16,000 Medicare/Medicaid–certified nursing homes and 31,000 assisted-living facilities in the United States, little research has been done on the epidemiology of suicide risk in these senior residential communities, Dr. Mezuk noted.
In scrutinizing all 3,451 suicides among persons aged 50 years or older recorded in the Virginia reporting system during the 9-year study period, she was able to identify 109 suicides related to life in a senior community. Fifty-two decedents lived in an assisted-living facility or nursing home at the time of their death. Another 38 were getting ready to move into a long-term facility – and were demonstrably unhappy about it. And 19 Virginians over age 50 who committed suicide had a family member who had recently entered long-term care, most often a spouse.
Contrary to Dr. Mezuk’s expectations, nursing homes where a suicide took place had significantly better overall rating scores on the widely used
- <cf number="\"2\"">’</cf>
Center for Medicare & Medicaid Services Nursing Home Compare metrics than facilities without a suicide, by a margin of 3.92 versus 3.11 out of a possible 5. Nursing homes with a death by suicide were less likely to be for-profit and more likely to be part of a continuing care retirement community. Their average staff rating score of 3.92 out of 5 was significantly better than the 2.97 for nursing homes without a suicide.
Assisted-living facilities where a suicide occurred had an average bed size twice that of other assisted-living facilities.
The average age of individuals who committed suicide in a nursing home or assisted-living facility was 77.8 years. Of all suicides, 22% were accomplished using firearms, 20% by hanging, 17% by falls, and 15% by cutting, among other means.
Among seniors who were living in a long-term care facility at the time of suicide, common themes that emerged from the narrative reports were a history of treatment for depression, one or more prior suicide attempts while in long-term care, and complaints of chronic pain.
Recurring themes among those who committed suicide while anticipating entry into a nursing home or assisted-living facility included explicit resistance to the move, threatened self-harm to avoid the transition, a poor family support network, and despondence over the prospect of the loss of home, friends, and mobility.
Among suicides who were caregivers of a relative who had recently entered long-term care, the common themes were marked despondence over the relative’s change of living status, their own personal failing health, and a history of treatment for depression.
Dr. Mezuk’s study was funded by the Virginia Department of Health and the National Institute of Mental Health. She reported having no financial conflicts.
ORLANDO – Transition to an assisted-living facility or nursing home is a period of high risk for suicide among the elderly – as well as an opportunity for preventive intervention.
That was the key message of a study, which earned its presenter, Briana Mezuk, Ph.D., the Best Early Investigator Award for the top research study presented at the annual meeting of the American Association for Geriatric Psychiatry.
Dr. Mezuk, an epidemiologist in the department of family medicine and population health at Virginia Commonwealth University, Richmond, presented an analysis of suicide among seniors in nursing homes and assisted-living facilities in Virginia during 2003-2011. The data came from the Virginia Violent Death Reporting System, which includes detailed case narratives describing the circumstances of all violent deaths in the state.
Dr. Mezuk’s study was unusual in that it provided both quantitative data addressing the study hypothesis that nursing homes and assisted-living facilities where suicides occurred would have lower quality of care ratings than facilities without suicides – the opposite proved to be true – as well as common narrative themes gleaned from the case files that could provide clinicians with practical warning signs of impending suicidality.
"Common themes of verbalized anticipatory distress, substance abuse, caregiver burden, past suicide attempts, and history of mental illness highlight potential risk factors for suicide in later life," she reported.
Older adults have the nation’s highest suicide rate. Yet even though there are roughly 16,000 Medicare/Medicaid–certified nursing homes and 31,000 assisted-living facilities in the United States, little research has been done on the epidemiology of suicide risk in these senior residential communities, Dr. Mezuk noted.
In scrutinizing all 3,451 suicides among persons aged 50 years or older recorded in the Virginia reporting system during the 9-year study period, she was able to identify 109 suicides related to life in a senior community. Fifty-two decedents lived in an assisted-living facility or nursing home at the time of their death. Another 38 were getting ready to move into a long-term facility – and were demonstrably unhappy about it. And 19 Virginians over age 50 who committed suicide had a family member who had recently entered long-term care, most often a spouse.
Contrary to Dr. Mezuk’s expectations, nursing homes where a suicide took place had significantly better overall rating scores on the widely used
- <cf number="\"2\"">’</cf>
Center for Medicare & Medicaid Services Nursing Home Compare metrics than facilities without a suicide, by a margin of 3.92 versus 3.11 out of a possible 5. Nursing homes with a death by suicide were less likely to be for-profit and more likely to be part of a continuing care retirement community. Their average staff rating score of 3.92 out of 5 was significantly better than the 2.97 for nursing homes without a suicide.
Assisted-living facilities where a suicide occurred had an average bed size twice that of other assisted-living facilities.
The average age of individuals who committed suicide in a nursing home or assisted-living facility was 77.8 years. Of all suicides, 22% were accomplished using firearms, 20% by hanging, 17% by falls, and 15% by cutting, among other means.
Among seniors who were living in a long-term care facility at the time of suicide, common themes that emerged from the narrative reports were a history of treatment for depression, one or more prior suicide attempts while in long-term care, and complaints of chronic pain.
Recurring themes among those who committed suicide while anticipating entry into a nursing home or assisted-living facility included explicit resistance to the move, threatened self-harm to avoid the transition, a poor family support network, and despondence over the prospect of the loss of home, friends, and mobility.
Among suicides who were caregivers of a relative who had recently entered long-term care, the common themes were marked despondence over the relative’s change of living status, their own personal failing health, and a history of treatment for depression.
Dr. Mezuk’s study was funded by the Virginia Department of Health and the National Institute of Mental Health. She reported having no financial conflicts.
ORLANDO – Transition to an assisted-living facility or nursing home is a period of high risk for suicide among the elderly – as well as an opportunity for preventive intervention.
That was the key message of a study, which earned its presenter, Briana Mezuk, Ph.D., the Best Early Investigator Award for the top research study presented at the annual meeting of the American Association for Geriatric Psychiatry.
Dr. Mezuk, an epidemiologist in the department of family medicine and population health at Virginia Commonwealth University, Richmond, presented an analysis of suicide among seniors in nursing homes and assisted-living facilities in Virginia during 2003-2011. The data came from the Virginia Violent Death Reporting System, which includes detailed case narratives describing the circumstances of all violent deaths in the state.
Dr. Mezuk’s study was unusual in that it provided both quantitative data addressing the study hypothesis that nursing homes and assisted-living facilities where suicides occurred would have lower quality of care ratings than facilities without suicides – the opposite proved to be true – as well as common narrative themes gleaned from the case files that could provide clinicians with practical warning signs of impending suicidality.
"Common themes of verbalized anticipatory distress, substance abuse, caregiver burden, past suicide attempts, and history of mental illness highlight potential risk factors for suicide in later life," she reported.
Older adults have the nation’s highest suicide rate. Yet even though there are roughly 16,000 Medicare/Medicaid–certified nursing homes and 31,000 assisted-living facilities in the United States, little research has been done on the epidemiology of suicide risk in these senior residential communities, Dr. Mezuk noted.
In scrutinizing all 3,451 suicides among persons aged 50 years or older recorded in the Virginia reporting system during the 9-year study period, she was able to identify 109 suicides related to life in a senior community. Fifty-two decedents lived in an assisted-living facility or nursing home at the time of their death. Another 38 were getting ready to move into a long-term facility – and were demonstrably unhappy about it. And 19 Virginians over age 50 who committed suicide had a family member who had recently entered long-term care, most often a spouse.
Contrary to Dr. Mezuk’s expectations, nursing homes where a suicide took place had significantly better overall rating scores on the widely used
- <cf number="\"2\"">’</cf>
Center for Medicare & Medicaid Services Nursing Home Compare metrics than facilities without a suicide, by a margin of 3.92 versus 3.11 out of a possible 5. Nursing homes with a death by suicide were less likely to be for-profit and more likely to be part of a continuing care retirement community. Their average staff rating score of 3.92 out of 5 was significantly better than the 2.97 for nursing homes without a suicide.
Assisted-living facilities where a suicide occurred had an average bed size twice that of other assisted-living facilities.
The average age of individuals who committed suicide in a nursing home or assisted-living facility was 77.8 years. Of all suicides, 22% were accomplished using firearms, 20% by hanging, 17% by falls, and 15% by cutting, among other means.
Among seniors who were living in a long-term care facility at the time of suicide, common themes that emerged from the narrative reports were a history of treatment for depression, one or more prior suicide attempts while in long-term care, and complaints of chronic pain.
Recurring themes among those who committed suicide while anticipating entry into a nursing home or assisted-living facility included explicit resistance to the move, threatened self-harm to avoid the transition, a poor family support network, and despondence over the prospect of the loss of home, friends, and mobility.
Among suicides who were caregivers of a relative who had recently entered long-term care, the common themes were marked despondence over the relative’s change of living status, their own personal failing health, and a history of treatment for depression.
Dr. Mezuk’s study was funded by the Virginia Department of Health and the National Institute of Mental Health. She reported having no financial conflicts.
AT THE AAGP ANNUAL MEETING
Major finding: Nursing homes where a suicide occurred scored significantly better on measures of health quality and staff ratings than facilities without a suicide.
Data source: This retrospective study involved detailed scrutiny of 109 suicides deemed related to living in a nursing home or assisted-living facility as recorded in the Virginia Violent Death Reporting System.
Disclosures: The study was funded by the Virginia Department of Health and the National Institute of Mental Health. The presenter reported having no financial conflicts.
Antipsychotics’ risk in dementia revised upward
ORLANDO – The mortality risk associated with prescription of antipsychotics or valproic acid for behavioral disturbances in patients with dementia is considerably greater than previously estimated, according to a massive national study.
This retrospective study included 45,669 matched pairs of Veterans Affairs patients over age 65 with dementia diagnosed during 1998-2009. Thus, there were 45,669 VA patients over age 65 with dementia diagnosed during 1998-2009 who were on one of the medications under scrutiny: haloperidol, olanzapine, quetiapine, risperidone, valproic acid, or a nontricyclic antidepressant. Each of these patients was matched to an older VA patient with dementia who was not on any of those medications and had not been for at least the past 6 months.
Investigators defined the number needed to harm (NNH) as the number of dementia patients who would need to be on one of the medications for 180 days in order to result in one additional death compared with nonuser matched controls. The NNH ranged from a worst-case scenario of 15 for haloperidol to 50 for quetiapine, Dr. Donovan T. Maust reported at the annual meeting of the American Association for Geriatric Psychiatry.
The patient pairs were matched based on demographics; comorbid medical and psychiatric diagnoses, including delirium within the prior 12 months; and a history of psychiatric hospitalization, noted Dr. Maust of the section on geriatric psychiatry at the University of Michigan, Ann Arbor.
The mortality NNHs found in this large study are considerably lower – that is, more unfavorable than in the two earlier meta-analyses conducted by other investigators. In the first meta-analysis, the estimated NNH for second-generation antipsychotics versus placebo was 100 (JAMA 2005;294:1934-43). More recently, a 2011 Agency for Healthcare Research Quality comparative effectiveness review found an NNH of 87 for second-generation antipsychotics when used in treating behavioral disturbances in elderly patients with dementia. The explanation for the larger, more favorable NNHs found in these meta-analyses is that they relied mainly upon studies of 8-12 weeks’ duration and so failed to capture the additional drug-related deaths that occurred over the course of the 180-day study in VA patients, the psychiatrist said.
In addition to providing a truer picture of the mortality risks of antipsychotics and valproic acid, the other advantage of the VA study is that it delineates the mortality risks for individual medications compared with no use at all. Earlier studies lumped antipsychotics together as a class, he noted.
"You can see that the risk is basically doubled with risperidone compared to quetiapine. So clinically I would use quetiapine as my first-line agent if I had to use anything. And I’ve stopped using haloperidol altogether," Dr. Maust said in an interview.
After the Food and Drug Administration issued its 2005 black box warning about the increased mortality risk of atypical antipsychotics in elderly patients with dementia, prescription of those drugs for the treatment of agitation and other behavioral symptoms in dementia outpatients dropped off, while the use of conventional antipsychotics climbed. With the 2008 black box warning regarding conventional antipsychotics, outpatient prescriptions for those agents also tailed off, but the use of valproic acid increased. However, the NNH of 29 for valproic acid seen in the new study shows this drug is problematic, too.
"It’s a bit like Whac-a-Mole where once one drug goes off the list, then people try something else. They’re such frustrating behaviors, and it’s such a distressing situation for the patient, the family, and the providers that it makes sense that people are trying alternatives," Dr. Maust commented.
The NNH of 158 for patients on antidepressant therapy seen in this study is probably not clinically meaningful in that it approaches the background mortality risk in this elderly, sick population, he added.
While the use of antipsychotics to treat behavioral disturbances in patients with dementia may have dropped off in outpatient settings since the black box warnings, that’s not the case in the inpatient setting – at least, not in the geriatric psychiatry inpatient unit at the Mayo Clinic in Rochester, Minn., Dr. Jung In "Kristin" Lee reported in a separate presentation at the meeting.
She presented a retrospective study of all patients hospitalized in the unit with a discharge diagnosis of dementia over the course of two study years: 2002 and 2012. In 2002, well before the black box warnings, 58% of 52 dementia patients were discharged on an antipsychotic agent, with quetiapine being the most commonly prescribed. Similarly, 65% of the 43 dementia patients discharged in 2012 were on an antipsychotic, with quetiapine once again being No. 1. In both years only a single dementia patient was discharged on a conventional antipsychotic, according to Dr. Lee of the Mayo Clinic.
Dr. Maust’s study was sponsored by the National Institute of Mental Health and the University of Michigan Program for Positive Aging. He reported having no financial conflicts, as did Dr. Lee, whose study was unfunded.
ORLANDO – The mortality risk associated with prescription of antipsychotics or valproic acid for behavioral disturbances in patients with dementia is considerably greater than previously estimated, according to a massive national study.
This retrospective study included 45,669 matched pairs of Veterans Affairs patients over age 65 with dementia diagnosed during 1998-2009. Thus, there were 45,669 VA patients over age 65 with dementia diagnosed during 1998-2009 who were on one of the medications under scrutiny: haloperidol, olanzapine, quetiapine, risperidone, valproic acid, or a nontricyclic antidepressant. Each of these patients was matched to an older VA patient with dementia who was not on any of those medications and had not been for at least the past 6 months.
Investigators defined the number needed to harm (NNH) as the number of dementia patients who would need to be on one of the medications for 180 days in order to result in one additional death compared with nonuser matched controls. The NNH ranged from a worst-case scenario of 15 for haloperidol to 50 for quetiapine, Dr. Donovan T. Maust reported at the annual meeting of the American Association for Geriatric Psychiatry.
The patient pairs were matched based on demographics; comorbid medical and psychiatric diagnoses, including delirium within the prior 12 months; and a history of psychiatric hospitalization, noted Dr. Maust of the section on geriatric psychiatry at the University of Michigan, Ann Arbor.
The mortality NNHs found in this large study are considerably lower – that is, more unfavorable than in the two earlier meta-analyses conducted by other investigators. In the first meta-analysis, the estimated NNH for second-generation antipsychotics versus placebo was 100 (JAMA 2005;294:1934-43). More recently, a 2011 Agency for Healthcare Research Quality comparative effectiveness review found an NNH of 87 for second-generation antipsychotics when used in treating behavioral disturbances in elderly patients with dementia. The explanation for the larger, more favorable NNHs found in these meta-analyses is that they relied mainly upon studies of 8-12 weeks’ duration and so failed to capture the additional drug-related deaths that occurred over the course of the 180-day study in VA patients, the psychiatrist said.
In addition to providing a truer picture of the mortality risks of antipsychotics and valproic acid, the other advantage of the VA study is that it delineates the mortality risks for individual medications compared with no use at all. Earlier studies lumped antipsychotics together as a class, he noted.
"You can see that the risk is basically doubled with risperidone compared to quetiapine. So clinically I would use quetiapine as my first-line agent if I had to use anything. And I’ve stopped using haloperidol altogether," Dr. Maust said in an interview.
After the Food and Drug Administration issued its 2005 black box warning about the increased mortality risk of atypical antipsychotics in elderly patients with dementia, prescription of those drugs for the treatment of agitation and other behavioral symptoms in dementia outpatients dropped off, while the use of conventional antipsychotics climbed. With the 2008 black box warning regarding conventional antipsychotics, outpatient prescriptions for those agents also tailed off, but the use of valproic acid increased. However, the NNH of 29 for valproic acid seen in the new study shows this drug is problematic, too.
"It’s a bit like Whac-a-Mole where once one drug goes off the list, then people try something else. They’re such frustrating behaviors, and it’s such a distressing situation for the patient, the family, and the providers that it makes sense that people are trying alternatives," Dr. Maust commented.
The NNH of 158 for patients on antidepressant therapy seen in this study is probably not clinically meaningful in that it approaches the background mortality risk in this elderly, sick population, he added.
While the use of antipsychotics to treat behavioral disturbances in patients with dementia may have dropped off in outpatient settings since the black box warnings, that’s not the case in the inpatient setting – at least, not in the geriatric psychiatry inpatient unit at the Mayo Clinic in Rochester, Minn., Dr. Jung In "Kristin" Lee reported in a separate presentation at the meeting.
She presented a retrospective study of all patients hospitalized in the unit with a discharge diagnosis of dementia over the course of two study years: 2002 and 2012. In 2002, well before the black box warnings, 58% of 52 dementia patients were discharged on an antipsychotic agent, with quetiapine being the most commonly prescribed. Similarly, 65% of the 43 dementia patients discharged in 2012 were on an antipsychotic, with quetiapine once again being No. 1. In both years only a single dementia patient was discharged on a conventional antipsychotic, according to Dr. Lee of the Mayo Clinic.
Dr. Maust’s study was sponsored by the National Institute of Mental Health and the University of Michigan Program for Positive Aging. He reported having no financial conflicts, as did Dr. Lee, whose study was unfunded.
ORLANDO – The mortality risk associated with prescription of antipsychotics or valproic acid for behavioral disturbances in patients with dementia is considerably greater than previously estimated, according to a massive national study.
This retrospective study included 45,669 matched pairs of Veterans Affairs patients over age 65 with dementia diagnosed during 1998-2009. Thus, there were 45,669 VA patients over age 65 with dementia diagnosed during 1998-2009 who were on one of the medications under scrutiny: haloperidol, olanzapine, quetiapine, risperidone, valproic acid, or a nontricyclic antidepressant. Each of these patients was matched to an older VA patient with dementia who was not on any of those medications and had not been for at least the past 6 months.
Investigators defined the number needed to harm (NNH) as the number of dementia patients who would need to be on one of the medications for 180 days in order to result in one additional death compared with nonuser matched controls. The NNH ranged from a worst-case scenario of 15 for haloperidol to 50 for quetiapine, Dr. Donovan T. Maust reported at the annual meeting of the American Association for Geriatric Psychiatry.
The patient pairs were matched based on demographics; comorbid medical and psychiatric diagnoses, including delirium within the prior 12 months; and a history of psychiatric hospitalization, noted Dr. Maust of the section on geriatric psychiatry at the University of Michigan, Ann Arbor.
The mortality NNHs found in this large study are considerably lower – that is, more unfavorable than in the two earlier meta-analyses conducted by other investigators. In the first meta-analysis, the estimated NNH for second-generation antipsychotics versus placebo was 100 (JAMA 2005;294:1934-43). More recently, a 2011 Agency for Healthcare Research Quality comparative effectiveness review found an NNH of 87 for second-generation antipsychotics when used in treating behavioral disturbances in elderly patients with dementia. The explanation for the larger, more favorable NNHs found in these meta-analyses is that they relied mainly upon studies of 8-12 weeks’ duration and so failed to capture the additional drug-related deaths that occurred over the course of the 180-day study in VA patients, the psychiatrist said.
In addition to providing a truer picture of the mortality risks of antipsychotics and valproic acid, the other advantage of the VA study is that it delineates the mortality risks for individual medications compared with no use at all. Earlier studies lumped antipsychotics together as a class, he noted.
"You can see that the risk is basically doubled with risperidone compared to quetiapine. So clinically I would use quetiapine as my first-line agent if I had to use anything. And I’ve stopped using haloperidol altogether," Dr. Maust said in an interview.
After the Food and Drug Administration issued its 2005 black box warning about the increased mortality risk of atypical antipsychotics in elderly patients with dementia, prescription of those drugs for the treatment of agitation and other behavioral symptoms in dementia outpatients dropped off, while the use of conventional antipsychotics climbed. With the 2008 black box warning regarding conventional antipsychotics, outpatient prescriptions for those agents also tailed off, but the use of valproic acid increased. However, the NNH of 29 for valproic acid seen in the new study shows this drug is problematic, too.
"It’s a bit like Whac-a-Mole where once one drug goes off the list, then people try something else. They’re such frustrating behaviors, and it’s such a distressing situation for the patient, the family, and the providers that it makes sense that people are trying alternatives," Dr. Maust commented.
The NNH of 158 for patients on antidepressant therapy seen in this study is probably not clinically meaningful in that it approaches the background mortality risk in this elderly, sick population, he added.
While the use of antipsychotics to treat behavioral disturbances in patients with dementia may have dropped off in outpatient settings since the black box warnings, that’s not the case in the inpatient setting – at least, not in the geriatric psychiatry inpatient unit at the Mayo Clinic in Rochester, Minn., Dr. Jung In "Kristin" Lee reported in a separate presentation at the meeting.
She presented a retrospective study of all patients hospitalized in the unit with a discharge diagnosis of dementia over the course of two study years: 2002 and 2012. In 2002, well before the black box warnings, 58% of 52 dementia patients were discharged on an antipsychotic agent, with quetiapine being the most commonly prescribed. Similarly, 65% of the 43 dementia patients discharged in 2012 were on an antipsychotic, with quetiapine once again being No. 1. In both years only a single dementia patient was discharged on a conventional antipsychotic, according to Dr. Lee of the Mayo Clinic.
Dr. Maust’s study was sponsored by the National Institute of Mental Health and the University of Michigan Program for Positive Aging. He reported having no financial conflicts, as did Dr. Lee, whose study was unfunded.
AT THE AAGP ANNUAL MEETING
Major finding: Among elderly patients with dementia placed on an antipsychotic to treat behavioral problems, the number needed to harm – that is, the number of patients that needed to be on the medication for 180 days in order to result in one additional death compared with nontreatment – ranged from 15 with haloperidol to a best-case scenario of 50 with quetiapine.
Data source: A retrospective study involving more than 90,000 Veterans Affairs patients over age 65 with dementia: 45,669 who were placed on an antipsychotic agent, valproic acid, or an antidepressant to manage behavioral problems and an equal number of closely matched patients not on any of the medications under scrutiny.
Disclosures: The study was supported by the National Institute of Mental Health and the University of Michigan Program for Positive Aging. The presenter reported having no financial conflicts.
Asenapine often beneficial in geriatric bipolar disorder
ORLANDO – Low-dose asenapine provided global improvement in symptoms and mood in older nondemented patients with bipolar disorder with prior suboptimal response to other therapies, according to a pilot study.
This atypical antipsychotic agent, which was used in the study at a mean daily dose of just 7.4 mg, is a novel therapy worth considering in selected geriatric patients with bipolar disorder, Dr. Martha N. Sajatovic said at the annual meeting of the American Association for Geriatric Psychiatry.
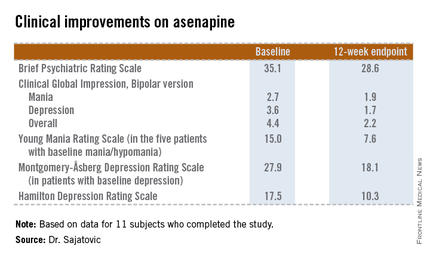
She presented a 12-week, prospective, open-label pilot study of asenapine (Saphris) in 15 nondemented patients over age 60 years with type 1 or 2 bipolar disorder who had a poor response to previous treatments. Patients started on asenapine at 5 mg/day and were titrated as indicated to a maximum of 20 mg/day, although the mean dose used was 7.4 mg/day.
The 11 subjects who completed the study showed significant improvements on the Brief Psychiatric Rating Scale, on the Clinical Global Impression, Bipolar version, and on measures of mood symptoms. No changes were found from baseline in terms of mean body mass index, abnormal movement scores, blood glucose levels, lipid levels, or functional status as measured by the Short Form–12, or on the WHO-Disability Assessment Scale II.
Of the four study dropouts, one left because of elevated liver function test results, another because of the emergence of manic symptoms while on asenapine, and two others for reasons unrelated to the study medication, according to Dr. Sajatovic, professor of psychiatry and director of the geropsychiatry program at Case Western Reserve University, Cleveland.
The most common treatment-emergent adverse event was mild, transient GI discomfort, which affected one-third of participants.
The addition of asenapine to the armamentarium for bipolar disorder in later life is a welcome development, she said. Of the 6 million American adults with bipolar disorder, roughly 1 million are over the age of 60. Traditional mood stabilizers such as lithium often have limiting side effects in the older population, and treatment failures are common, she noted.
This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.
ORLANDO – Low-dose asenapine provided global improvement in symptoms and mood in older nondemented patients with bipolar disorder with prior suboptimal response to other therapies, according to a pilot study.
This atypical antipsychotic agent, which was used in the study at a mean daily dose of just 7.4 mg, is a novel therapy worth considering in selected geriatric patients with bipolar disorder, Dr. Martha N. Sajatovic said at the annual meeting of the American Association for Geriatric Psychiatry.

She presented a 12-week, prospective, open-label pilot study of asenapine (Saphris) in 15 nondemented patients over age 60 years with type 1 or 2 bipolar disorder who had a poor response to previous treatments. Patients started on asenapine at 5 mg/day and were titrated as indicated to a maximum of 20 mg/day, although the mean dose used was 7.4 mg/day.
The 11 subjects who completed the study showed significant improvements on the Brief Psychiatric Rating Scale, on the Clinical Global Impression, Bipolar version, and on measures of mood symptoms. No changes were found from baseline in terms of mean body mass index, abnormal movement scores, blood glucose levels, lipid levels, or functional status as measured by the Short Form–12, or on the WHO-Disability Assessment Scale II.
Of the four study dropouts, one left because of elevated liver function test results, another because of the emergence of manic symptoms while on asenapine, and two others for reasons unrelated to the study medication, according to Dr. Sajatovic, professor of psychiatry and director of the geropsychiatry program at Case Western Reserve University, Cleveland.
The most common treatment-emergent adverse event was mild, transient GI discomfort, which affected one-third of participants.
The addition of asenapine to the armamentarium for bipolar disorder in later life is a welcome development, she said. Of the 6 million American adults with bipolar disorder, roughly 1 million are over the age of 60. Traditional mood stabilizers such as lithium often have limiting side effects in the older population, and treatment failures are common, she noted.
This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.
ORLANDO – Low-dose asenapine provided global improvement in symptoms and mood in older nondemented patients with bipolar disorder with prior suboptimal response to other therapies, according to a pilot study.
This atypical antipsychotic agent, which was used in the study at a mean daily dose of just 7.4 mg, is a novel therapy worth considering in selected geriatric patients with bipolar disorder, Dr. Martha N. Sajatovic said at the annual meeting of the American Association for Geriatric Psychiatry.

She presented a 12-week, prospective, open-label pilot study of asenapine (Saphris) in 15 nondemented patients over age 60 years with type 1 or 2 bipolar disorder who had a poor response to previous treatments. Patients started on asenapine at 5 mg/day and were titrated as indicated to a maximum of 20 mg/day, although the mean dose used was 7.4 mg/day.
The 11 subjects who completed the study showed significant improvements on the Brief Psychiatric Rating Scale, on the Clinical Global Impression, Bipolar version, and on measures of mood symptoms. No changes were found from baseline in terms of mean body mass index, abnormal movement scores, blood glucose levels, lipid levels, or functional status as measured by the Short Form–12, or on the WHO-Disability Assessment Scale II.
Of the four study dropouts, one left because of elevated liver function test results, another because of the emergence of manic symptoms while on asenapine, and two others for reasons unrelated to the study medication, according to Dr. Sajatovic, professor of psychiatry and director of the geropsychiatry program at Case Western Reserve University, Cleveland.
The most common treatment-emergent adverse event was mild, transient GI discomfort, which affected one-third of participants.
The addition of asenapine to the armamentarium for bipolar disorder in later life is a welcome development, she said. Of the 6 million American adults with bipolar disorder, roughly 1 million are over the age of 60. Traditional mood stabilizers such as lithium often have limiting side effects in the older population, and treatment failures are common, she noted.
This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.
AT THE AAGP ANNUAL MEETING
Major finding: The atypical antipsychotic asenapine produced significant improvement in symptoms and mood in patients with later-life bipolar disorder that previously had shown a poor response to other therapies.
Data source: A prospective, 12-week, open-label pilot study in which 15 nondemented patients over age 60 with bipolar disorder suboptimally responsive to other therapies were treated with asenapine starting at 5 mg/day.
Disclosures: This pilot study was sponsored by NV Organon/Merck. Dr. Satajovic reported receiving research grants from Merck and other companies, as well as serving as a consultant to Prophase, Otsuka, Pfizer, Amgen, and United BioSource.
Surprising new findings about late-life schizophrenia
ORLANDO – Quality of life is not static in older persons with early-onset schizophrenia, according to a prospective longitudinal study.
"What’s presented in the literature is that you reach an end stage in late-life schizophrenia, sometimes called the quiescent state, where nothing changes. Wrong! So get that out of your head. Our work and other recent studies suggest that symptoms fluctuate. Cognitive functioning fluctuates. Mood fluctuates. And quality of life and social functioning fluctuate. Our model suggests that there remain potential opportunities to impact quality of life among older adults with schizophrenia," Dr. Carl I. Cohen declared at the annual meeting of the American Association for Geriatric Psychiatry.
He presented a prospective study of 104 community-dwelling New Yorkers with schizophrenia diagnosed before age 45. They, along with 113 matched controls, were followed for a mean of 52 months starting at an average age of 61 years.
The primary outcome in this analysis was change in the Quality of Life Index (QLI), which scores four domains – health and functioning, socioeconomics, family, and psychological/spiritual life – on a 0-30 scale.
Over the course of 52 months, the mean QLI in the schizophrenia group barely budged, moving from 22.2 to 22.6. But that bland statistic hides some fascinating changes, Dr. Cohen noted. While 24% of those in the schizophrenia group experienced a significant decline in quality of life as reflected in a drop of 2.65 points or more on the QLI, which is one-half of a standard deviation, an additional 32% had a significant improvement in QLI score. Thus, nearly three-fifths of those in the schizophrenia group had more than a half standard deviation change in their QLI scores.
Moreover, 13% of the schizophrenia group had a low baseline quality of life based upon a QLI score below the mean for the control group of matched peers, but a high QLI score at follow-up. Conversely, 15% went from a high baseline QLI to a low quality of life score at follow-up, according to Dr. Cohen, professor of psychiatry and director of the division of geriatric psychiatry at SUNY (State University of New York) Downstate Medical Center, Brooklyn.
In a multivariate analysis, several predictors of a high QLI score at follow-up emerged: fewer depressive symptoms, a higher baseline QLI, and – in a novel finding – higher religiousness scores.
"This highlights the role that religious and spiritual beliefs and activities play in this population of older adults with schizophrenia," he observed.
Also, subjects who showed improvement in their QLI score over time experienced improvement in positive symptoms of schizophrenia. "Thus, enhancing quality of life has a direct clinical impact," Dr. Cohen continued.
Audience members congratulated him for shining light on an understudied population that’s often assumed to be totally burned out.
"I’ve always said the secret of schizophrenia is not so much the young people," Dr. Cohen replied, "but actually to understand the older patients who’ve gone through the illness in its different manifestations and to see how they’ve handled it. Many of them do improve in later life, and we can learn quite a bit."
The study was funded by the National Institute of General Medical Sciences. Dr. Cohen reported having no financial disclosures, although he added, "when I speak to an audience of psychiatrists, I always feel I ought to disclose something."
ORLANDO – Quality of life is not static in older persons with early-onset schizophrenia, according to a prospective longitudinal study.
"What’s presented in the literature is that you reach an end stage in late-life schizophrenia, sometimes called the quiescent state, where nothing changes. Wrong! So get that out of your head. Our work and other recent studies suggest that symptoms fluctuate. Cognitive functioning fluctuates. Mood fluctuates. And quality of life and social functioning fluctuate. Our model suggests that there remain potential opportunities to impact quality of life among older adults with schizophrenia," Dr. Carl I. Cohen declared at the annual meeting of the American Association for Geriatric Psychiatry.
He presented a prospective study of 104 community-dwelling New Yorkers with schizophrenia diagnosed before age 45. They, along with 113 matched controls, were followed for a mean of 52 months starting at an average age of 61 years.
The primary outcome in this analysis was change in the Quality of Life Index (QLI), which scores four domains – health and functioning, socioeconomics, family, and psychological/spiritual life – on a 0-30 scale.
Over the course of 52 months, the mean QLI in the schizophrenia group barely budged, moving from 22.2 to 22.6. But that bland statistic hides some fascinating changes, Dr. Cohen noted. While 24% of those in the schizophrenia group experienced a significant decline in quality of life as reflected in a drop of 2.65 points or more on the QLI, which is one-half of a standard deviation, an additional 32% had a significant improvement in QLI score. Thus, nearly three-fifths of those in the schizophrenia group had more than a half standard deviation change in their QLI scores.
Moreover, 13% of the schizophrenia group had a low baseline quality of life based upon a QLI score below the mean for the control group of matched peers, but a high QLI score at follow-up. Conversely, 15% went from a high baseline QLI to a low quality of life score at follow-up, according to Dr. Cohen, professor of psychiatry and director of the division of geriatric psychiatry at SUNY (State University of New York) Downstate Medical Center, Brooklyn.
In a multivariate analysis, several predictors of a high QLI score at follow-up emerged: fewer depressive symptoms, a higher baseline QLI, and – in a novel finding – higher religiousness scores.
"This highlights the role that religious and spiritual beliefs and activities play in this population of older adults with schizophrenia," he observed.
Also, subjects who showed improvement in their QLI score over time experienced improvement in positive symptoms of schizophrenia. "Thus, enhancing quality of life has a direct clinical impact," Dr. Cohen continued.
Audience members congratulated him for shining light on an understudied population that’s often assumed to be totally burned out.
"I’ve always said the secret of schizophrenia is not so much the young people," Dr. Cohen replied, "but actually to understand the older patients who’ve gone through the illness in its different manifestations and to see how they’ve handled it. Many of them do improve in later life, and we can learn quite a bit."
The study was funded by the National Institute of General Medical Sciences. Dr. Cohen reported having no financial disclosures, although he added, "when I speak to an audience of psychiatrists, I always feel I ought to disclose something."
ORLANDO – Quality of life is not static in older persons with early-onset schizophrenia, according to a prospective longitudinal study.
"What’s presented in the literature is that you reach an end stage in late-life schizophrenia, sometimes called the quiescent state, where nothing changes. Wrong! So get that out of your head. Our work and other recent studies suggest that symptoms fluctuate. Cognitive functioning fluctuates. Mood fluctuates. And quality of life and social functioning fluctuate. Our model suggests that there remain potential opportunities to impact quality of life among older adults with schizophrenia," Dr. Carl I. Cohen declared at the annual meeting of the American Association for Geriatric Psychiatry.
He presented a prospective study of 104 community-dwelling New Yorkers with schizophrenia diagnosed before age 45. They, along with 113 matched controls, were followed for a mean of 52 months starting at an average age of 61 years.
The primary outcome in this analysis was change in the Quality of Life Index (QLI), which scores four domains – health and functioning, socioeconomics, family, and psychological/spiritual life – on a 0-30 scale.
Over the course of 52 months, the mean QLI in the schizophrenia group barely budged, moving from 22.2 to 22.6. But that bland statistic hides some fascinating changes, Dr. Cohen noted. While 24% of those in the schizophrenia group experienced a significant decline in quality of life as reflected in a drop of 2.65 points or more on the QLI, which is one-half of a standard deviation, an additional 32% had a significant improvement in QLI score. Thus, nearly three-fifths of those in the schizophrenia group had more than a half standard deviation change in their QLI scores.
Moreover, 13% of the schizophrenia group had a low baseline quality of life based upon a QLI score below the mean for the control group of matched peers, but a high QLI score at follow-up. Conversely, 15% went from a high baseline QLI to a low quality of life score at follow-up, according to Dr. Cohen, professor of psychiatry and director of the division of geriatric psychiatry at SUNY (State University of New York) Downstate Medical Center, Brooklyn.
In a multivariate analysis, several predictors of a high QLI score at follow-up emerged: fewer depressive symptoms, a higher baseline QLI, and – in a novel finding – higher religiousness scores.
"This highlights the role that religious and spiritual beliefs and activities play in this population of older adults with schizophrenia," he observed.
Also, subjects who showed improvement in their QLI score over time experienced improvement in positive symptoms of schizophrenia. "Thus, enhancing quality of life has a direct clinical impact," Dr. Cohen continued.
Audience members congratulated him for shining light on an understudied population that’s often assumed to be totally burned out.
"I’ve always said the secret of schizophrenia is not so much the young people," Dr. Cohen replied, "but actually to understand the older patients who’ve gone through the illness in its different manifestations and to see how they’ve handled it. Many of them do improve in later life, and we can learn quite a bit."
The study was funded by the National Institute of General Medical Sciences. Dr. Cohen reported having no financial disclosures, although he added, "when I speak to an audience of psychiatrists, I always feel I ought to disclose something."
AT THE AAGP ANNUAL MEETING
Major finding: Quality of life in older persons with schizophrenia changed significantly either for better or for worse over the course of 52 months in nearly three-fifths of patients.
Data source: This was a longitudinal study of 104 community-dwelling individuals with early-onset schizophrenia who were prospectively followed for a mean of 52 months starting at an average age of 61 years.
Disclosures: The study was funded by the National Institute of General Medical Sciences. Dr. Cohen reported having no financial disclosures, although he added, "when I speak to an audience of psychiatrists, I always feel I ought to disclose something."