User login
Biomarkers predict VTE risk with menopausal oral hormone therapy
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Women in the top 25% for D-dimer level before going on menopausal hormone therapy had a 6% incidence of venous thromboembolism over 5 years.
Study details: This was a nested case-control study focused on identifying biomarkers for venous thromboembolism risk which included 1,082 participants in the Women’s Health Initiative randomized to menopausal hormone therapy or placebo.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was funded by the National Institutes of Health.
PCSK9 inhibition isn’t the answer for high Lp(a)
CHICAGO – according to the results of the ANITSCHKOW study, Erik S. Stroes, MD, PhD, reported at the American Heart Association scientific sessions.
“The reality is that for now we don’t have any drugs to significantly lower elevated Lp(a),” he said. “We can identify patients with elevated Lp(a), but we don’t have a clue how to treat them.”
Elevated Lp(a) is a highly prevalent lipid abnormality. It induces arterial wall inflammation, a known predictor of future cardiovascular events. The monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9) dramatically reduce LDL cholesterol and also reduce arterial wall inflammation. In the published studies, PCSK9 inhibitors also reduced Lp(a) by an average of 27%; however, most participants in those studies had isolated high LDL with a normal or slightly elevated Lp(a).
ANITSCHKOW was the first double-blind, randomized, placebo-controlled study to look at the effects of a PCSK9 inhibitor – in this case, evolocumab (Repatha) – in patients with severe elevations in both LDL and Lp(a). The results proved disappointing yet informative, according to Dr. Stroes, professor of internal medicine and a vascular medicine specialist at the University of Amsterdam.
The 16-week, 14-site trial included 128 Dutch, American, and Canadian patients with a mean baseline LDL of 146 mg/dL and a median Lp(a) of 202 nmol/L who were randomized to monthly subcutanous injections of evolocumab at 420 mg or placebo. All participants had evidence of significant arterial wall inflammation at baseline as measured by PET-CT. Of the subjects, 54% were on statin therapy.
Evolucumab achieved a placebo-subtracted 61% reduction in LDL to 60 mg/dL but a mere 14% reduction in Lp(a) to 188 nmol/L, still far in excess of the 50 nmol/L cutoff defining elevated Lp(a).
The primary endpoint was change in arterial wall inflammation from baseline to week 16 as measured using PET-CT. Based upon the results of other studies showing a 3.3% drop in arterial wall inflammation for every 10% reduction in LDL, Dr. Stroes and his coinvestigators expected to see a 20% decrease in arterial wall inflammation in the evolocumab group. Instead, they found a mere 8.4% reduction, which wasn’t significantly different than in placebo-treated controls. And there was no difference in arterial wall inflammation between the group on concomitant statin therapy and those who weren’t.
The implication is that the residual Lp(a) elevation despite PCSK9 inhibitor therapy might explain the discrepancy, compared with previous studies in which LDL lowering did reduce arterial wall inflammation, according to Dr. Stroes.
“Persistent arterial wall inflammation on PET-CT after evolocumab, potentially related to persistent Lp(a) elevation, implies the need for additional therapies to decrease the proinflammatory state in Lp(a) elevation,” he observed.
Lp(a) in the spotlight
An elevated Lp(a) of 50 nmol/L or more is present in 20% of the general population, according to a Danish study. More than 70% of a person’s Lp(a) level is genetically driven. And a genetically driven elevated Lp(a) has been shown to be associated with a twofold to fourfold increased risk of cardiovascular events.
Moreover, other investigators have shown that a severely elevated Lp(a) (greater than 180 nmol/L) poses a cardiovascular risk comparable with that of heterozygous familial hypercholesterolemia and is present in 1 in 100 individuals.
“We spend a lot of time on familial hypercholesterolemia, and we should. But mind you, this severe Lp(a) elevation is more frequent than heterozygous FH,” Dr. Stroes said.
Session cochair Robert H. Eckel, MD, asked the audience for a show of hands by those who regularly measure Lp(a) in their patients. Very few hands were raised.
“I measure Lp(a) frequently, and I think it’s a very important risk factor,” declared Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
The ANITSCHKOW study was sponsored by Amgen. Dr. Stroes reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
CHICAGO – according to the results of the ANITSCHKOW study, Erik S. Stroes, MD, PhD, reported at the American Heart Association scientific sessions.
“The reality is that for now we don’t have any drugs to significantly lower elevated Lp(a),” he said. “We can identify patients with elevated Lp(a), but we don’t have a clue how to treat them.”
Elevated Lp(a) is a highly prevalent lipid abnormality. It induces arterial wall inflammation, a known predictor of future cardiovascular events. The monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9) dramatically reduce LDL cholesterol and also reduce arterial wall inflammation. In the published studies, PCSK9 inhibitors also reduced Lp(a) by an average of 27%; however, most participants in those studies had isolated high LDL with a normal or slightly elevated Lp(a).
ANITSCHKOW was the first double-blind, randomized, placebo-controlled study to look at the effects of a PCSK9 inhibitor – in this case, evolocumab (Repatha) – in patients with severe elevations in both LDL and Lp(a). The results proved disappointing yet informative, according to Dr. Stroes, professor of internal medicine and a vascular medicine specialist at the University of Amsterdam.
The 16-week, 14-site trial included 128 Dutch, American, and Canadian patients with a mean baseline LDL of 146 mg/dL and a median Lp(a) of 202 nmol/L who were randomized to monthly subcutanous injections of evolocumab at 420 mg or placebo. All participants had evidence of significant arterial wall inflammation at baseline as measured by PET-CT. Of the subjects, 54% were on statin therapy.
Evolucumab achieved a placebo-subtracted 61% reduction in LDL to 60 mg/dL but a mere 14% reduction in Lp(a) to 188 nmol/L, still far in excess of the 50 nmol/L cutoff defining elevated Lp(a).
The primary endpoint was change in arterial wall inflammation from baseline to week 16 as measured using PET-CT. Based upon the results of other studies showing a 3.3% drop in arterial wall inflammation for every 10% reduction in LDL, Dr. Stroes and his coinvestigators expected to see a 20% decrease in arterial wall inflammation in the evolocumab group. Instead, they found a mere 8.4% reduction, which wasn’t significantly different than in placebo-treated controls. And there was no difference in arterial wall inflammation between the group on concomitant statin therapy and those who weren’t.
The implication is that the residual Lp(a) elevation despite PCSK9 inhibitor therapy might explain the discrepancy, compared with previous studies in which LDL lowering did reduce arterial wall inflammation, according to Dr. Stroes.
“Persistent arterial wall inflammation on PET-CT after evolocumab, potentially related to persistent Lp(a) elevation, implies the need for additional therapies to decrease the proinflammatory state in Lp(a) elevation,” he observed.
Lp(a) in the spotlight
An elevated Lp(a) of 50 nmol/L or more is present in 20% of the general population, according to a Danish study. More than 70% of a person’s Lp(a) level is genetically driven. And a genetically driven elevated Lp(a) has been shown to be associated with a twofold to fourfold increased risk of cardiovascular events.
Moreover, other investigators have shown that a severely elevated Lp(a) (greater than 180 nmol/L) poses a cardiovascular risk comparable with that of heterozygous familial hypercholesterolemia and is present in 1 in 100 individuals.
“We spend a lot of time on familial hypercholesterolemia, and we should. But mind you, this severe Lp(a) elevation is more frequent than heterozygous FH,” Dr. Stroes said.
Session cochair Robert H. Eckel, MD, asked the audience for a show of hands by those who regularly measure Lp(a) in their patients. Very few hands were raised.
“I measure Lp(a) frequently, and I think it’s a very important risk factor,” declared Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
The ANITSCHKOW study was sponsored by Amgen. Dr. Stroes reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
CHICAGO – according to the results of the ANITSCHKOW study, Erik S. Stroes, MD, PhD, reported at the American Heart Association scientific sessions.
“The reality is that for now we don’t have any drugs to significantly lower elevated Lp(a),” he said. “We can identify patients with elevated Lp(a), but we don’t have a clue how to treat them.”
Elevated Lp(a) is a highly prevalent lipid abnormality. It induces arterial wall inflammation, a known predictor of future cardiovascular events. The monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9) dramatically reduce LDL cholesterol and also reduce arterial wall inflammation. In the published studies, PCSK9 inhibitors also reduced Lp(a) by an average of 27%; however, most participants in those studies had isolated high LDL with a normal or slightly elevated Lp(a).
ANITSCHKOW was the first double-blind, randomized, placebo-controlled study to look at the effects of a PCSK9 inhibitor – in this case, evolocumab (Repatha) – in patients with severe elevations in both LDL and Lp(a). The results proved disappointing yet informative, according to Dr. Stroes, professor of internal medicine and a vascular medicine specialist at the University of Amsterdam.
The 16-week, 14-site trial included 128 Dutch, American, and Canadian patients with a mean baseline LDL of 146 mg/dL and a median Lp(a) of 202 nmol/L who were randomized to monthly subcutanous injections of evolocumab at 420 mg or placebo. All participants had evidence of significant arterial wall inflammation at baseline as measured by PET-CT. Of the subjects, 54% were on statin therapy.
Evolucumab achieved a placebo-subtracted 61% reduction in LDL to 60 mg/dL but a mere 14% reduction in Lp(a) to 188 nmol/L, still far in excess of the 50 nmol/L cutoff defining elevated Lp(a).
The primary endpoint was change in arterial wall inflammation from baseline to week 16 as measured using PET-CT. Based upon the results of other studies showing a 3.3% drop in arterial wall inflammation for every 10% reduction in LDL, Dr. Stroes and his coinvestigators expected to see a 20% decrease in arterial wall inflammation in the evolocumab group. Instead, they found a mere 8.4% reduction, which wasn’t significantly different than in placebo-treated controls. And there was no difference in arterial wall inflammation between the group on concomitant statin therapy and those who weren’t.
The implication is that the residual Lp(a) elevation despite PCSK9 inhibitor therapy might explain the discrepancy, compared with previous studies in which LDL lowering did reduce arterial wall inflammation, according to Dr. Stroes.
“Persistent arterial wall inflammation on PET-CT after evolocumab, potentially related to persistent Lp(a) elevation, implies the need for additional therapies to decrease the proinflammatory state in Lp(a) elevation,” he observed.
Lp(a) in the spotlight
An elevated Lp(a) of 50 nmol/L or more is present in 20% of the general population, according to a Danish study. More than 70% of a person’s Lp(a) level is genetically driven. And a genetically driven elevated Lp(a) has been shown to be associated with a twofold to fourfold increased risk of cardiovascular events.
Moreover, other investigators have shown that a severely elevated Lp(a) (greater than 180 nmol/L) poses a cardiovascular risk comparable with that of heterozygous familial hypercholesterolemia and is present in 1 in 100 individuals.
“We spend a lot of time on familial hypercholesterolemia, and we should. But mind you, this severe Lp(a) elevation is more frequent than heterozygous FH,” Dr. Stroes said.
Session cochair Robert H. Eckel, MD, asked the audience for a show of hands by those who regularly measure Lp(a) in their patients. Very few hands were raised.
“I measure Lp(a) frequently, and I think it’s a very important risk factor,” declared Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
The ANITSCHKOW study was sponsored by Amgen. Dr. Stroes reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Evolocumab has no effect on arterial wall inflammation in patients with severely elevated Lp(a).
Major finding: Median Lp(a) declined modestly from 202 nmol/L to 188 nmol/L in response to evolocumab.
Study details: This multicenter, 16-week, double-blind, placebo-controlled study included 128 patients with both elevated LDL and Lp(a).
Disclosures: The ANITSCHKOW study was sponsored by Amgen. The presenter reported receiving institutional research grants from and serving as a paid speaker for Amgen, Merck, Novartis, and Regeneron.
Subclinical hypothyroidism boosts immediate risk of heart failure
CHICAGO – The short-term risk of developing heart failure in patients with newly identified hypothyroidism, be it overt or subclinical, is double that of euthyroid individuals, Caroline H. Noergaard, MD, reported at the American Heart Association scientific sessions.
“This is really important clinically. The association with heart failure has previously been shown in both overt and subclinical hyperthyroidism, but it’s actually new knowledge that hypothyroidism is associated with immediate risk of heart failure. And a lot of people have subclinical hypothyroidism,” said Dr. Noergaard, a PhD student in epidemiology at Aalborg (Denmark) University.
Also at the meeting, Jeffrey L. Anderson, MD, reported that free thyroxine levels within the normal reference range were associated in graded fashion with an increased prevalence and incidence of atrial fibrillation in a large Utah study, a finding that provides independent confirmation of an earlier report by investigators from the population-based Rotterdam Study.
“These findings validate those of the Rotterdam Study in a much larger dataset and may have important clinical implications, including a redefinition of the reference range and the target-free T4 levels for thyroxine replacement therapy,” observed Dr. Anderson, professor of internal medicine at the University of Utah, Salt Lake City, and a research cardiologist at the Intermountain Medical Center Heart Institute.
Hypothyroidism and heart failure
Dr. Noergaard presented a retrospective study of over 1 million Copenhagen-area adults (mean age, 50 years) with no history of heart failure, who had their first thyroid function test. She and her coinvestigators turned to comprehensive Danish national health care registries to determine how many of these individuals were diagnosed with new-onset heart failure within 90 days after their thyroid function test.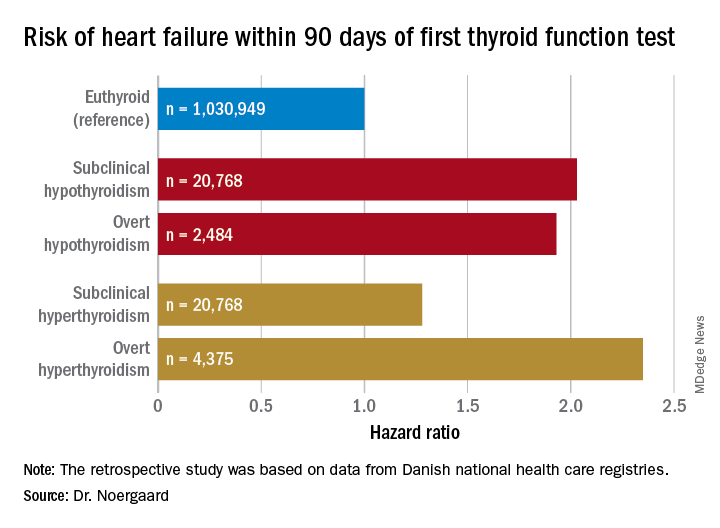
Subclinical hypothyroidism was defined by a thyroid-stimulating hormone level greater than 5 mIU/L and a free T4 of 9-22 pmol/L. Overt hypothyroidism required a TSH greater than 5 mIU/L with a free T4 less than 9 pmol/L.
Free T4 predicts atrial fibrillation risk
Dr. Anderson presented a retrospective analysis of 174,914 adult patients in the Intermountain Healthcare EMR database, none of whom were on thyroid replacement at entry. The patients, who were a mean age of 64 years and 65% women, were followed for an average of 6.3 years. Of these, 88.4% had a free T4 within the normal reference range of 0.75-1.5 ng/dL, 7.4% had a value below the cutoff for normal, and 4.2% had a free T4 above the reference range.
Upon dividing the patients within the normal range into quartiles based upon their free T4 level, he and his coinvestigators found that the baseline prevalence of atrial fibrillation was 8.7% in those in quartile 1, 9.3% in quartile 2, 10.5% in quartile 3, and 12.6% in quartile 4. In a multivariate analysis adjusted for potential confounders, the risk of prevalent atrial fibrillation was increased by 11% for patients in quartile 2, compared with those in the first quartile, by 22% in quartile 3, and by 40% in quartile 4.
The incidence of new-onset atrial fibrillation during 3 years of follow-up was 4.1% in patients in normal-range quartile 1, 4.3% in quartile 2, 4.5% in quartile 3, and 5.2% in the top normal-range quartile. The odds of developing atrial fibrillation were increased by 8% and 16% in quartiles 3 and 4, compared with quartile 1.
Serum TSH and free T3 levels showed no consistent relationship with atrial fibrillation.
The Utah findings confirm in a large U.S. population the earlier report from the Rotterdam Study (J Clin Endocrinol Metab. 2015 Oct;100(10):3718-24).
Dr. Noergaard and Dr. Anderson reported having no financial conflicts regarding their studies, which were carried out free of commercial support.
CHICAGO – The short-term risk of developing heart failure in patients with newly identified hypothyroidism, be it overt or subclinical, is double that of euthyroid individuals, Caroline H. Noergaard, MD, reported at the American Heart Association scientific sessions.
“This is really important clinically. The association with heart failure has previously been shown in both overt and subclinical hyperthyroidism, but it’s actually new knowledge that hypothyroidism is associated with immediate risk of heart failure. And a lot of people have subclinical hypothyroidism,” said Dr. Noergaard, a PhD student in epidemiology at Aalborg (Denmark) University.
Also at the meeting, Jeffrey L. Anderson, MD, reported that free thyroxine levels within the normal reference range were associated in graded fashion with an increased prevalence and incidence of atrial fibrillation in a large Utah study, a finding that provides independent confirmation of an earlier report by investigators from the population-based Rotterdam Study.
“These findings validate those of the Rotterdam Study in a much larger dataset and may have important clinical implications, including a redefinition of the reference range and the target-free T4 levels for thyroxine replacement therapy,” observed Dr. Anderson, professor of internal medicine at the University of Utah, Salt Lake City, and a research cardiologist at the Intermountain Medical Center Heart Institute.
Hypothyroidism and heart failure
Dr. Noergaard presented a retrospective study of over 1 million Copenhagen-area adults (mean age, 50 years) with no history of heart failure, who had their first thyroid function test. She and her coinvestigators turned to comprehensive Danish national health care registries to determine how many of these individuals were diagnosed with new-onset heart failure within 90 days after their thyroid function test.
Subclinical hypothyroidism was defined by a thyroid-stimulating hormone level greater than 5 mIU/L and a free T4 of 9-22 pmol/L. Overt hypothyroidism required a TSH greater than 5 mIU/L with a free T4 less than 9 pmol/L.
Free T4 predicts atrial fibrillation risk
Dr. Anderson presented a retrospective analysis of 174,914 adult patients in the Intermountain Healthcare EMR database, none of whom were on thyroid replacement at entry. The patients, who were a mean age of 64 years and 65% women, were followed for an average of 6.3 years. Of these, 88.4% had a free T4 within the normal reference range of 0.75-1.5 ng/dL, 7.4% had a value below the cutoff for normal, and 4.2% had a free T4 above the reference range.
Upon dividing the patients within the normal range into quartiles based upon their free T4 level, he and his coinvestigators found that the baseline prevalence of atrial fibrillation was 8.7% in those in quartile 1, 9.3% in quartile 2, 10.5% in quartile 3, and 12.6% in quartile 4. In a multivariate analysis adjusted for potential confounders, the risk of prevalent atrial fibrillation was increased by 11% for patients in quartile 2, compared with those in the first quartile, by 22% in quartile 3, and by 40% in quartile 4.
The incidence of new-onset atrial fibrillation during 3 years of follow-up was 4.1% in patients in normal-range quartile 1, 4.3% in quartile 2, 4.5% in quartile 3, and 5.2% in the top normal-range quartile. The odds of developing atrial fibrillation were increased by 8% and 16% in quartiles 3 and 4, compared with quartile 1.
Serum TSH and free T3 levels showed no consistent relationship with atrial fibrillation.
The Utah findings confirm in a large U.S. population the earlier report from the Rotterdam Study (J Clin Endocrinol Metab. 2015 Oct;100(10):3718-24).
Dr. Noergaard and Dr. Anderson reported having no financial conflicts regarding their studies, which were carried out free of commercial support.
CHICAGO – The short-term risk of developing heart failure in patients with newly identified hypothyroidism, be it overt or subclinical, is double that of euthyroid individuals, Caroline H. Noergaard, MD, reported at the American Heart Association scientific sessions.
“This is really important clinically. The association with heart failure has previously been shown in both overt and subclinical hyperthyroidism, but it’s actually new knowledge that hypothyroidism is associated with immediate risk of heart failure. And a lot of people have subclinical hypothyroidism,” said Dr. Noergaard, a PhD student in epidemiology at Aalborg (Denmark) University.
Also at the meeting, Jeffrey L. Anderson, MD, reported that free thyroxine levels within the normal reference range were associated in graded fashion with an increased prevalence and incidence of atrial fibrillation in a large Utah study, a finding that provides independent confirmation of an earlier report by investigators from the population-based Rotterdam Study.
“These findings validate those of the Rotterdam Study in a much larger dataset and may have important clinical implications, including a redefinition of the reference range and the target-free T4 levels for thyroxine replacement therapy,” observed Dr. Anderson, professor of internal medicine at the University of Utah, Salt Lake City, and a research cardiologist at the Intermountain Medical Center Heart Institute.
Hypothyroidism and heart failure
Dr. Noergaard presented a retrospective study of over 1 million Copenhagen-area adults (mean age, 50 years) with no history of heart failure, who had their first thyroid function test. She and her coinvestigators turned to comprehensive Danish national health care registries to determine how many of these individuals were diagnosed with new-onset heart failure within 90 days after their thyroid function test.
Subclinical hypothyroidism was defined by a thyroid-stimulating hormone level greater than 5 mIU/L and a free T4 of 9-22 pmol/L. Overt hypothyroidism required a TSH greater than 5 mIU/L with a free T4 less than 9 pmol/L.
Free T4 predicts atrial fibrillation risk
Dr. Anderson presented a retrospective analysis of 174,914 adult patients in the Intermountain Healthcare EMR database, none of whom were on thyroid replacement at entry. The patients, who were a mean age of 64 years and 65% women, were followed for an average of 6.3 years. Of these, 88.4% had a free T4 within the normal reference range of 0.75-1.5 ng/dL, 7.4% had a value below the cutoff for normal, and 4.2% had a free T4 above the reference range.
Upon dividing the patients within the normal range into quartiles based upon their free T4 level, he and his coinvestigators found that the baseline prevalence of atrial fibrillation was 8.7% in those in quartile 1, 9.3% in quartile 2, 10.5% in quartile 3, and 12.6% in quartile 4. In a multivariate analysis adjusted for potential confounders, the risk of prevalent atrial fibrillation was increased by 11% for patients in quartile 2, compared with those in the first quartile, by 22% in quartile 3, and by 40% in quartile 4.
The incidence of new-onset atrial fibrillation during 3 years of follow-up was 4.1% in patients in normal-range quartile 1, 4.3% in quartile 2, 4.5% in quartile 3, and 5.2% in the top normal-range quartile. The odds of developing atrial fibrillation were increased by 8% and 16% in quartiles 3 and 4, compared with quartile 1.
Serum TSH and free T3 levels showed no consistent relationship with atrial fibrillation.
The Utah findings confirm in a large U.S. population the earlier report from the Rotterdam Study (J Clin Endocrinol Metab. 2015 Oct;100(10):3718-24).
Dr. Noergaard and Dr. Anderson reported having no financial conflicts regarding their studies, which were carried out free of commercial support.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Both subclinical and overt hypothyroidism are associated with a 100% increased risk of being diagnosed with heart failure, compared with euthyroid individuals.
Study details: This was a retrospective study of the association between free thyroxine levels and short-term risk of developing heart failure in more than 1 million Copenhagen-area patients.
Disclosures: The presenter reported having no financial conflicts regarding the Danish study, conducted free of commercial support.
Ezetimibe found effective for primary prevention in elderly
CHICAGO – has been provided by the Japanese EWTOPIA 75 trial.

Ezetimibe (Zetia) at 10 mg/day reduced the risk of the primary endpoint, a composite of atherosclerotic cardiovascular events, by 34% compared with a dietary counseling control group over the course of 5 years of followup. Yasuyoshi Ouchi, MD, PhD, reported the findings of the 3,796-patient study at the American Heart Association scientific sessions.
There was also a 40% relative risk reduction for cardiac events in the ezetimibe group. The 22% reduction in cerebrovascular events, however, didn’t achieve statistical significance, and there was no between-group difference in all-cause mortality, said Dr. Ouchi, principal investigator in EWTOPIA 75 and professor emeritus of geriatric medicine at the University of Tokyo.
The landmark randomized clinical trials of lipid-lowering for primary cardiovascular prevention included too few elderly participants to permit assessment of its merits and possible harms in that population. This has left a major evidence gap at a time when in many parts of the world, including the United States, Europe, and Japan, the population over age 75 is growing explosively.
“Along with this population change, the number of patients age 75 and older with hypercholesterolemia has dramatically increased,” Dr. Ouchi continued.
Eligibility for EWTOPIA was restricted to patients who were at least 75 years old, had an LDL of at least 140 mg/dL, no history of CAD, and had at least one high-risk factor, such as diabetes or hypertension. Their mean age at enrollment was 80.7 years. Seventy-four percent of them were women, reflecting the significantly longer life expectancy of Japanese women compared to men.
The study design was open-label with no placebo arm. Dr. Ouchi argued that this was appropriate, given that the components of the primary composite endpoint were “entirely objective”: fatal and nonfatal MI, fatal and nonfatal stroke, sudden cardiac death, and coronary revascularization.
The mean LDL in the ezetimibe group dropped from 162 mg/dL at baseline to 120 mg/dL at 5 years, versus 131 mg/dL in the control group.
Ezetimibe was the lipid-lowering agent selected for EWTOPIA because it has an excellent safety record in older patients. There were no important differences between the two study arms in terms of adverse events, according to Dr. Ouchi.

Discussant Jennifer G. Robinson, MD, said that for a decade she has tried without success to get backing for a primary prevention statin trial in elderly U.S. patients, so congratulations to the Japanese investigators are in order.
She expressed doubts as to the generalizability of the EWTOPIA results to non-Japanese populations, however.
“Frankly, I was very surprised to see the large effect size. EWTOPIA had far more effect than we expected based on other trials of LDL-lowering agents to date,” said Dr. Robinson, professor of epidemiology and medicine and director of the Prevention Intervention Center at the University of Iowa, Iowa City.
“It’s a little better performance than we expected from that magnitude of LDL lowering, which was quite modest,” she added.
Among the possible explanations she cited for the greater magnitude of reduction in major vascular events seen in EWTOPIA as compared, for example, to the IMPROVE-IT trial, which also utilized ezetimibe, are genetic differences in the Japanese population. It’s known that the Japanese have different genetic polymorphisms of Niemann-Pick C1 Like 1 (NPC1L1), which is what ezetimibe binds to in order to inhibit small intestinal enterocyte uptake and absorption of cholesterol. Or it might just be that older adults, regardless of their ethnicity, have a more robust response to LDL lowering than the younger ones who’ve been the focus of previous trials.
“I think the LDL lowering from ezetimibe was very effective in Japanese older adults without cardiovascular disease, and I think that’s a very appropriate therapy for primary prevention moving forward in that population,” Dr. Robinson said.
As for herself, she’s awaiting confirmation in other populations. She’s particularly eager to see the outcome of the ongoing double-blind, randomized STAREE trial of atorvastatin (Lipitor) at 40 mg/day or placebo for primary prevention in 18,000 Australians age 70 and up. Results are expected in 2022.
Dr. Ouchi reported having no financial conflicts regarding the EWTOPIA study, funded by the Japanese government.
SOURCE: Ouchi Y. AHA Late Breaker 02.
CHICAGO – has been provided by the Japanese EWTOPIA 75 trial.

Ezetimibe (Zetia) at 10 mg/day reduced the risk of the primary endpoint, a composite of atherosclerotic cardiovascular events, by 34% compared with a dietary counseling control group over the course of 5 years of followup. Yasuyoshi Ouchi, MD, PhD, reported the findings of the 3,796-patient study at the American Heart Association scientific sessions.
There was also a 40% relative risk reduction for cardiac events in the ezetimibe group. The 22% reduction in cerebrovascular events, however, didn’t achieve statistical significance, and there was no between-group difference in all-cause mortality, said Dr. Ouchi, principal investigator in EWTOPIA 75 and professor emeritus of geriatric medicine at the University of Tokyo.
The landmark randomized clinical trials of lipid-lowering for primary cardiovascular prevention included too few elderly participants to permit assessment of its merits and possible harms in that population. This has left a major evidence gap at a time when in many parts of the world, including the United States, Europe, and Japan, the population over age 75 is growing explosively.
“Along with this population change, the number of patients age 75 and older with hypercholesterolemia has dramatically increased,” Dr. Ouchi continued.
Eligibility for EWTOPIA was restricted to patients who were at least 75 years old, had an LDL of at least 140 mg/dL, no history of CAD, and had at least one high-risk factor, such as diabetes or hypertension. Their mean age at enrollment was 80.7 years. Seventy-four percent of them were women, reflecting the significantly longer life expectancy of Japanese women compared to men.
The study design was open-label with no placebo arm. Dr. Ouchi argued that this was appropriate, given that the components of the primary composite endpoint were “entirely objective”: fatal and nonfatal MI, fatal and nonfatal stroke, sudden cardiac death, and coronary revascularization.
The mean LDL in the ezetimibe group dropped from 162 mg/dL at baseline to 120 mg/dL at 5 years, versus 131 mg/dL in the control group.
Ezetimibe was the lipid-lowering agent selected for EWTOPIA because it has an excellent safety record in older patients. There were no important differences between the two study arms in terms of adverse events, according to Dr. Ouchi.

Discussant Jennifer G. Robinson, MD, said that for a decade she has tried without success to get backing for a primary prevention statin trial in elderly U.S. patients, so congratulations to the Japanese investigators are in order.
She expressed doubts as to the generalizability of the EWTOPIA results to non-Japanese populations, however.
“Frankly, I was very surprised to see the large effect size. EWTOPIA had far more effect than we expected based on other trials of LDL-lowering agents to date,” said Dr. Robinson, professor of epidemiology and medicine and director of the Prevention Intervention Center at the University of Iowa, Iowa City.
“It’s a little better performance than we expected from that magnitude of LDL lowering, which was quite modest,” she added.
Among the possible explanations she cited for the greater magnitude of reduction in major vascular events seen in EWTOPIA as compared, for example, to the IMPROVE-IT trial, which also utilized ezetimibe, are genetic differences in the Japanese population. It’s known that the Japanese have different genetic polymorphisms of Niemann-Pick C1 Like 1 (NPC1L1), which is what ezetimibe binds to in order to inhibit small intestinal enterocyte uptake and absorption of cholesterol. Or it might just be that older adults, regardless of their ethnicity, have a more robust response to LDL lowering than the younger ones who’ve been the focus of previous trials.
“I think the LDL lowering from ezetimibe was very effective in Japanese older adults without cardiovascular disease, and I think that’s a very appropriate therapy for primary prevention moving forward in that population,” Dr. Robinson said.
As for herself, she’s awaiting confirmation in other populations. She’s particularly eager to see the outcome of the ongoing double-blind, randomized STAREE trial of atorvastatin (Lipitor) at 40 mg/day or placebo for primary prevention in 18,000 Australians age 70 and up. Results are expected in 2022.
Dr. Ouchi reported having no financial conflicts regarding the EWTOPIA study, funded by the Japanese government.
SOURCE: Ouchi Y. AHA Late Breaker 02.
CHICAGO – has been provided by the Japanese EWTOPIA 75 trial.

Ezetimibe (Zetia) at 10 mg/day reduced the risk of the primary endpoint, a composite of atherosclerotic cardiovascular events, by 34% compared with a dietary counseling control group over the course of 5 years of followup. Yasuyoshi Ouchi, MD, PhD, reported the findings of the 3,796-patient study at the American Heart Association scientific sessions.
There was also a 40% relative risk reduction for cardiac events in the ezetimibe group. The 22% reduction in cerebrovascular events, however, didn’t achieve statistical significance, and there was no between-group difference in all-cause mortality, said Dr. Ouchi, principal investigator in EWTOPIA 75 and professor emeritus of geriatric medicine at the University of Tokyo.
The landmark randomized clinical trials of lipid-lowering for primary cardiovascular prevention included too few elderly participants to permit assessment of its merits and possible harms in that population. This has left a major evidence gap at a time when in many parts of the world, including the United States, Europe, and Japan, the population over age 75 is growing explosively.
“Along with this population change, the number of patients age 75 and older with hypercholesterolemia has dramatically increased,” Dr. Ouchi continued.
Eligibility for EWTOPIA was restricted to patients who were at least 75 years old, had an LDL of at least 140 mg/dL, no history of CAD, and had at least one high-risk factor, such as diabetes or hypertension. Their mean age at enrollment was 80.7 years. Seventy-four percent of them were women, reflecting the significantly longer life expectancy of Japanese women compared to men.
The study design was open-label with no placebo arm. Dr. Ouchi argued that this was appropriate, given that the components of the primary composite endpoint were “entirely objective”: fatal and nonfatal MI, fatal and nonfatal stroke, sudden cardiac death, and coronary revascularization.
The mean LDL in the ezetimibe group dropped from 162 mg/dL at baseline to 120 mg/dL at 5 years, versus 131 mg/dL in the control group.
Ezetimibe was the lipid-lowering agent selected for EWTOPIA because it has an excellent safety record in older patients. There were no important differences between the two study arms in terms of adverse events, according to Dr. Ouchi.

Discussant Jennifer G. Robinson, MD, said that for a decade she has tried without success to get backing for a primary prevention statin trial in elderly U.S. patients, so congratulations to the Japanese investigators are in order.
She expressed doubts as to the generalizability of the EWTOPIA results to non-Japanese populations, however.
“Frankly, I was very surprised to see the large effect size. EWTOPIA had far more effect than we expected based on other trials of LDL-lowering agents to date,” said Dr. Robinson, professor of epidemiology and medicine and director of the Prevention Intervention Center at the University of Iowa, Iowa City.
“It’s a little better performance than we expected from that magnitude of LDL lowering, which was quite modest,” she added.
Among the possible explanations she cited for the greater magnitude of reduction in major vascular events seen in EWTOPIA as compared, for example, to the IMPROVE-IT trial, which also utilized ezetimibe, are genetic differences in the Japanese population. It’s known that the Japanese have different genetic polymorphisms of Niemann-Pick C1 Like 1 (NPC1L1), which is what ezetimibe binds to in order to inhibit small intestinal enterocyte uptake and absorption of cholesterol. Or it might just be that older adults, regardless of their ethnicity, have a more robust response to LDL lowering than the younger ones who’ve been the focus of previous trials.
“I think the LDL lowering from ezetimibe was very effective in Japanese older adults without cardiovascular disease, and I think that’s a very appropriate therapy for primary prevention moving forward in that population,” Dr. Robinson said.
As for herself, she’s awaiting confirmation in other populations. She’s particularly eager to see the outcome of the ongoing double-blind, randomized STAREE trial of atorvastatin (Lipitor) at 40 mg/day or placebo for primary prevention in 18,000 Australians age 70 and up. Results are expected in 2022.
Dr. Ouchi reported having no financial conflicts regarding the EWTOPIA study, funded by the Japanese government.
SOURCE: Ouchi Y. AHA Late Breaker 02.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: LDL-lowering for primary cardiovascular prevention in elderly patients has been shown for the first time to impart significant net benefit.
Major finding: The incidence of atherosclerotic cardiovascular events was reduced by 34% in elderly patients on ezetimibe at 10 mg/day, compared with usual care.
Study details: The 5-year prospective randomized EWTOPIA 75 trial included 3,796 Japanese patients age 75 and older with elevated LDL and no history of CAD.
Disclosures: The presenter reported having no financial conflicts regarding the study, sponsored by the Japanese government.
Source: Ouchi Y. AHA Late Breaker 02.
Obesity paradox applies to post-stroke mortality
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.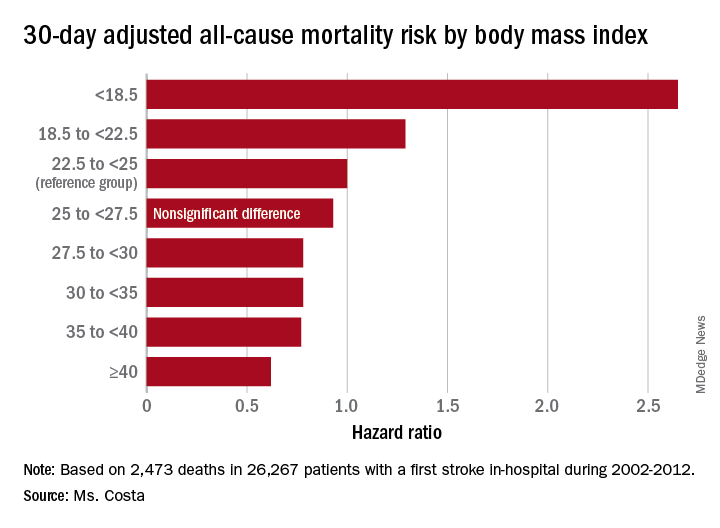
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Heavier stroke patients have lower 30-day and 1-year all-cause mortality.
Major finding: The 30-day stroke-related mortality rate after in-hospital stroke was reduced by 45% in VA patients with Class III obesity.
Study details: This retrospective study looked at the relationship between body mass index and post-stroke mortality in more than 26,000 veterans who had an inpatient stroke, with extensive adjustments made for potential confounders.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was sponsored by the Department of Veterans Affairs.
Source: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
Novel agent cut LDL in statin-intolerant patients
CHICAGO – Bempedoic acid, a novel oral lipid-lowering agent, reduced LDL cholesterol by 21% and high-sensitivity C-reactive protein by 24% with a side-effect profile similar to placebo in statin-intolerant hypercholesterolemic patients with or at high risk for atherosclerotic cardiovascular disease in the pivotal phase 3 CLEAR Serenity trial, Ulrich Laufs, MD, PhD, reported at the American Heart Association scientific sessions.
CLEAR Serenity is one of five pivotal phase 3 trials of bempedoic acid. The others evaluated bempedoic acid as add-on therapy to obtain additional lipid lowering in patients already on a maximum-dose statin, and in combination with ezetimibe (Zetia) as a single pill, also in patients on full-dose statin therapy. All of these trials met their efficacy and safety endpoints. The drug’s developer, Esperion Therapeutics, has announced plans to file for marketing approval of bempedoic acid and for the bempedoic acid/ezetimibe combo pill with the Food and Drug Administration and the European Medicines Agency in the first months of 2019.
CLEAR Serenity was a 24-week, double-blind, placebo-controlled trial conducted at 67 North American sites. The 345 statin-intolerant participants were randomized 2:1 to bempedoic acid at 180 mg once daily or placebo.
“I think the specific contribution of this study is, importantly, that myalgia and other muscle-related symptoms were not increased with bempedoic acid versus placebo in this population that’s statin intolerant, more than 90% of whom complained of statin-related muscle symptoms,” said Dr. Laufs, professor and chair of the department of cardiology at the University of Leipzig (Germany).
Rates of major adverse cardiovascular events were too low in this relatively short-term, modest-size trial to be informative, but the results of the previously presented CLEAR Harmony trial are reassuring in this regard, according to the cardiologist. CLEAR Harmony was a 52-week study that included 2,230 patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia whose LDL was inadequately controlled despite high-intensity statin therapy. They were randomized to add-on bempedoic acid or placebo. The adjudicated major adverse cardiovascular event rate was 4.6% in the bempedoic acid group and not significantly different at 5.7% in controls.
Definitive data on the effect of bempedoic acid on cardiovascular morbidity and mortality event rates will eventually be provided by an ongoing global randomized, double-blind, placebo-controlled trial expected to enroll more than 12,000 patients.
Rates of various types of adverse events were closely similar in the bempedoic acid and placebo groups in CLEAR Serenity, with a couple of intriguing exceptions, according to Dr. Laufs. For example, the rate of new-onset or worsening diabetes was 2.1% in the bempedoic acid group, compared with 4.5% in controls.
“This is consistent with results in the other studies in the overall bempedoic acid program. It will be something of great interest to follow up in the ongoing outcomes trial,” he said. “At this point I would feel comfortable in saying that there is no deterioration of glucose tolerance, unlike with statins. Whether there is an actual improvement or not needs to be characterized a little better.”
The other difference in the safety profile between the two study arms in CLEAR Serenity was a trend for higher uric acid levels and an increased risk of developing gout in the bempedoic acid group. Gout occurred in 1.7% of the bempedoic acid group and 0.9% of placebo-treated controls. A similar signal has been seen in the other pivotal trials, but a definitive answer as to gout risk must await the large ongoing outcomes trial, Dr. Laufs continued.
Bempedoic acid is a first-in-class oral inhibitor of ATP citrate lyase, an enzyme that is inactive in skeletal muscle – thus, the lack of myalgia complaints – and lies upstream of HMG-CoA reductase in cholesterol synthesis. When combined in a single pill with ezetimibe, which lowers LDL by stimulating the LDL receptor, the lipid-lowering impact is magnified over that of either drug alone. In the phase 3 program, the combination pill resulted in a further 35% reduction in LDL when added to a maximally tolerated statin and a 43% reduction in LDL when used as monotherapy.
Session cochair Robert H. Eckel, MD, commented that bempedoic acid appears to be poised to address a significant unmet need in preventive cardiology.
“We clearly need alternative therapies in patients with statin intolerance. We see a lot of these patients in referral centers. This drug looks safe and very effective at modifying LDL, about as much so as ezetimibe and maybe a little bit more,” said Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
Dr. Laufs reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
CHICAGO – Bempedoic acid, a novel oral lipid-lowering agent, reduced LDL cholesterol by 21% and high-sensitivity C-reactive protein by 24% with a side-effect profile similar to placebo in statin-intolerant hypercholesterolemic patients with or at high risk for atherosclerotic cardiovascular disease in the pivotal phase 3 CLEAR Serenity trial, Ulrich Laufs, MD, PhD, reported at the American Heart Association scientific sessions.
CLEAR Serenity is one of five pivotal phase 3 trials of bempedoic acid. The others evaluated bempedoic acid as add-on therapy to obtain additional lipid lowering in patients already on a maximum-dose statin, and in combination with ezetimibe (Zetia) as a single pill, also in patients on full-dose statin therapy. All of these trials met their efficacy and safety endpoints. The drug’s developer, Esperion Therapeutics, has announced plans to file for marketing approval of bempedoic acid and for the bempedoic acid/ezetimibe combo pill with the Food and Drug Administration and the European Medicines Agency in the first months of 2019.
CLEAR Serenity was a 24-week, double-blind, placebo-controlled trial conducted at 67 North American sites. The 345 statin-intolerant participants were randomized 2:1 to bempedoic acid at 180 mg once daily or placebo.
“I think the specific contribution of this study is, importantly, that myalgia and other muscle-related symptoms were not increased with bempedoic acid versus placebo in this population that’s statin intolerant, more than 90% of whom complained of statin-related muscle symptoms,” said Dr. Laufs, professor and chair of the department of cardiology at the University of Leipzig (Germany).
Rates of major adverse cardiovascular events were too low in this relatively short-term, modest-size trial to be informative, but the results of the previously presented CLEAR Harmony trial are reassuring in this regard, according to the cardiologist. CLEAR Harmony was a 52-week study that included 2,230 patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia whose LDL was inadequately controlled despite high-intensity statin therapy. They were randomized to add-on bempedoic acid or placebo. The adjudicated major adverse cardiovascular event rate was 4.6% in the bempedoic acid group and not significantly different at 5.7% in controls.
Definitive data on the effect of bempedoic acid on cardiovascular morbidity and mortality event rates will eventually be provided by an ongoing global randomized, double-blind, placebo-controlled trial expected to enroll more than 12,000 patients.
Rates of various types of adverse events were closely similar in the bempedoic acid and placebo groups in CLEAR Serenity, with a couple of intriguing exceptions, according to Dr. Laufs. For example, the rate of new-onset or worsening diabetes was 2.1% in the bempedoic acid group, compared with 4.5% in controls.
“This is consistent with results in the other studies in the overall bempedoic acid program. It will be something of great interest to follow up in the ongoing outcomes trial,” he said. “At this point I would feel comfortable in saying that there is no deterioration of glucose tolerance, unlike with statins. Whether there is an actual improvement or not needs to be characterized a little better.”
The other difference in the safety profile between the two study arms in CLEAR Serenity was a trend for higher uric acid levels and an increased risk of developing gout in the bempedoic acid group. Gout occurred in 1.7% of the bempedoic acid group and 0.9% of placebo-treated controls. A similar signal has been seen in the other pivotal trials, but a definitive answer as to gout risk must await the large ongoing outcomes trial, Dr. Laufs continued.
Bempedoic acid is a first-in-class oral inhibitor of ATP citrate lyase, an enzyme that is inactive in skeletal muscle – thus, the lack of myalgia complaints – and lies upstream of HMG-CoA reductase in cholesterol synthesis. When combined in a single pill with ezetimibe, which lowers LDL by stimulating the LDL receptor, the lipid-lowering impact is magnified over that of either drug alone. In the phase 3 program, the combination pill resulted in a further 35% reduction in LDL when added to a maximally tolerated statin and a 43% reduction in LDL when used as monotherapy.
Session cochair Robert H. Eckel, MD, commented that bempedoic acid appears to be poised to address a significant unmet need in preventive cardiology.
“We clearly need alternative therapies in patients with statin intolerance. We see a lot of these patients in referral centers. This drug looks safe and very effective at modifying LDL, about as much so as ezetimibe and maybe a little bit more,” said Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
Dr. Laufs reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
CHICAGO – Bempedoic acid, a novel oral lipid-lowering agent, reduced LDL cholesterol by 21% and high-sensitivity C-reactive protein by 24% with a side-effect profile similar to placebo in statin-intolerant hypercholesterolemic patients with or at high risk for atherosclerotic cardiovascular disease in the pivotal phase 3 CLEAR Serenity trial, Ulrich Laufs, MD, PhD, reported at the American Heart Association scientific sessions.
CLEAR Serenity is one of five pivotal phase 3 trials of bempedoic acid. The others evaluated bempedoic acid as add-on therapy to obtain additional lipid lowering in patients already on a maximum-dose statin, and in combination with ezetimibe (Zetia) as a single pill, also in patients on full-dose statin therapy. All of these trials met their efficacy and safety endpoints. The drug’s developer, Esperion Therapeutics, has announced plans to file for marketing approval of bempedoic acid and for the bempedoic acid/ezetimibe combo pill with the Food and Drug Administration and the European Medicines Agency in the first months of 2019.
CLEAR Serenity was a 24-week, double-blind, placebo-controlled trial conducted at 67 North American sites. The 345 statin-intolerant participants were randomized 2:1 to bempedoic acid at 180 mg once daily or placebo.
“I think the specific contribution of this study is, importantly, that myalgia and other muscle-related symptoms were not increased with bempedoic acid versus placebo in this population that’s statin intolerant, more than 90% of whom complained of statin-related muscle symptoms,” said Dr. Laufs, professor and chair of the department of cardiology at the University of Leipzig (Germany).
Rates of major adverse cardiovascular events were too low in this relatively short-term, modest-size trial to be informative, but the results of the previously presented CLEAR Harmony trial are reassuring in this regard, according to the cardiologist. CLEAR Harmony was a 52-week study that included 2,230 patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia whose LDL was inadequately controlled despite high-intensity statin therapy. They were randomized to add-on bempedoic acid or placebo. The adjudicated major adverse cardiovascular event rate was 4.6% in the bempedoic acid group and not significantly different at 5.7% in controls.
Definitive data on the effect of bempedoic acid on cardiovascular morbidity and mortality event rates will eventually be provided by an ongoing global randomized, double-blind, placebo-controlled trial expected to enroll more than 12,000 patients.
Rates of various types of adverse events were closely similar in the bempedoic acid and placebo groups in CLEAR Serenity, with a couple of intriguing exceptions, according to Dr. Laufs. For example, the rate of new-onset or worsening diabetes was 2.1% in the bempedoic acid group, compared with 4.5% in controls.
“This is consistent with results in the other studies in the overall bempedoic acid program. It will be something of great interest to follow up in the ongoing outcomes trial,” he said. “At this point I would feel comfortable in saying that there is no deterioration of glucose tolerance, unlike with statins. Whether there is an actual improvement or not needs to be characterized a little better.”
The other difference in the safety profile between the two study arms in CLEAR Serenity was a trend for higher uric acid levels and an increased risk of developing gout in the bempedoic acid group. Gout occurred in 1.7% of the bempedoic acid group and 0.9% of placebo-treated controls. A similar signal has been seen in the other pivotal trials, but a definitive answer as to gout risk must await the large ongoing outcomes trial, Dr. Laufs continued.
Bempedoic acid is a first-in-class oral inhibitor of ATP citrate lyase, an enzyme that is inactive in skeletal muscle – thus, the lack of myalgia complaints – and lies upstream of HMG-CoA reductase in cholesterol synthesis. When combined in a single pill with ezetimibe, which lowers LDL by stimulating the LDL receptor, the lipid-lowering impact is magnified over that of either drug alone. In the phase 3 program, the combination pill resulted in a further 35% reduction in LDL when added to a maximally tolerated statin and a 43% reduction in LDL when used as monotherapy.
Session cochair Robert H. Eckel, MD, commented that bempedoic acid appears to be poised to address a significant unmet need in preventive cardiology.
“We clearly need alternative therapies in patients with statin intolerance. We see a lot of these patients in referral centers. This drug looks safe and very effective at modifying LDL, about as much so as ezetimibe and maybe a little bit more,” said Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
Dr. Laufs reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Oral once-daily bempedoic acid may be an attractive option for statin-intolerant patients.
Major finding: Bempedoic acid reduced elevated LDL cholesterol by 21% in statin-intolerant patients at high cardiovascular risk.
Study details: CLEAR Serenity was a 24-week, double-blind, placebo-controlled, multicenter, pivotal phase 3 trial including 345 statin-intolerant patients at high risk for cardiovascular events.
Disclosures: The presenter reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
Biodegradable polymer shows no long-term benefit in heart stents
CHICAGO – The idea behind putting a biodegradable polymer on a drug-eluting coronary stent is that, once the antirestenosis drug elutes and the polymer that held it degrades, the bare-metal stent left behind would trigger fewer long-term episodes of in-stent thrombosis than would stents that retain their polymer coating. But 10-year follow-up from a large trial that matched two second-generation drug-eluting stents, one with a biodegradable polymer and the other with a durable polymer, showed no statistically significant difference between the two for any clinical outcome, including the incidence of in-stent thrombosis, Sebastian Kufner, MD, said at the American Heart Association scientific sessions.
The potential advantage of a biodegradable polymer “is expected to occur over time,” and hence following patients for 10 or more years should start to show a clear advantage, at least for the endpoint of stent thrombosis, but that didn’t happen. After a median follow-up of 10.6 years, the cumulative rate of definite or probable stent thrombosis was 1.8% among 1,299 patients who received a sirolimus-eluting stent with a biodegradable polymer (Yukon Choice) and 2.5% among 652 patients who received a second-generation everolimus-eluting stent with a durable polymer (Xience), a difference that was not statistically significant, reported Dr. Kufner, a cardiologist at the The German Heart Centre in Munich.
These two stents also produced comparable 10-year outcomes that showed no statistically significant differences for the outcomes of all-cause death, MI, need for target-lesion revascularization, or the combined incidence of all three of these outcomes. In contrast, both of these second-generation stents showed statistically significant improvements in the combined cardiac endpoint, as well as in all-cause death, and definite stent thrombosis compared with the 652 patients who received the first-generation sirolimus-eluting stent Cypher. Concurrently with Dr. Kufner’s report, the results appeared in an article online (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038065).
Another notable finding from the 10-year follow-up was the poor prognosis these patients faced after their interventions, including the patients who received second-generation drug-eluting stents. The 10-year rate of all cause death was 30% among patients who received Xience stents, 32% among those treated with Yukon Choice stents, and 37% among patients treated with Cypher stents.
“I’m daunted by this 10-year mortality rate even with the best current drug-eluting stents,” said Roxana Mehran, MD, professor of medicine at Icahn School of Medicine at Mount Sinai in New York and a cochair of the session. “I’m depressed about this as an interventionalist. We need to do better, although it might not just be about the revascularization.” The high mortality in these patients after 10 years may also reflect a lack of optimal medical treatment in some, she suggested.
The ISAR-TEST-4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents) trial randomized 2,603 patients during the period of September 2007–August 2008 at either of two centers in Munich. The study’s primary endpoint was the combined rate of cardiac death, MI, and target-lesion revascularization by 12 months after treatment. The 12-month results showed a similar 14% rate of this combined endpoint in the subgroup of patients treated with the biodegradable-polymer stent and in those treated with stents that used durable polymers, which proved the noninferiority of the stent with a biodegradable polymer (Eur Heart J. 2009 Oct 1;30[20]:2441-9). This initial analysis combined the patients who received Cypher and Xience stents into one comparator group: patients who received stents with durable polymers.
The new, long-term follow-up analysis included 2,153 patients (83%) followed for at least 10 years after their intervention.
ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
SOURCE: Kufner S et al. AHA scientific sessions, Abstract 18630.
The absence of incremental benefit from a stent with a biodegradable polymer compared with the second generation everolimus-eluting stent with a durable polymer in this 10-year follow-up is consistent with prior reports from medium-term follow-up. At best, drug-eluting coronary stents with a biodegradable polymer are noninferior to the current generation of those with a durable polymer. Late clinical benefit from a biodegradable polymer over a second-generation drug-eluting stent with a durable polymer remains elusive. The findings begs the public health question of whether paying a higher price for a biodegradable coronary stent with a durable-polymer is a good investment.
These 10-year results also highlight that, despite using drug-eluting stent technology that remains more or less standard of care today, the treated patients showed a staggering rate of major adverse cardiac events that exceeded 3% each year. This finding suggests an urgent need to address this residual risk through further improvements in medical therapy and stent technology.
Right now, accumulated evidence supports the notion that thinner struts are less thrombogenic than thicker struts, and that not all durable polymers are equal, with some associated with less inflammation and thrombogenicity. The next frontier for stent design seems to be ultrathin struts that are less than 70 mcm in diameter. We need to now see results from a study that compares an ultrathin-strut stent with a durable polymer against one with a biodegradable polymer.
Sripal Bangalore, MD , is an interventional cardiologist and professor of medicine at New York University. He has been a consultant or adviser to Abbott Vascular, Amgen, Biotronik, and Pfizer, and he has received research funding from Abbott Vascular. He made these comments as designated discussant for ISAR-TEST-4, and in an editorial published online concurrently with his talk (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038378 ).
The absence of incremental benefit from a stent with a biodegradable polymer compared with the second generation everolimus-eluting stent with a durable polymer in this 10-year follow-up is consistent with prior reports from medium-term follow-up. At best, drug-eluting coronary stents with a biodegradable polymer are noninferior to the current generation of those with a durable polymer. Late clinical benefit from a biodegradable polymer over a second-generation drug-eluting stent with a durable polymer remains elusive. The findings begs the public health question of whether paying a higher price for a biodegradable coronary stent with a durable-polymer is a good investment.
These 10-year results also highlight that, despite using drug-eluting stent technology that remains more or less standard of care today, the treated patients showed a staggering rate of major adverse cardiac events that exceeded 3% each year. This finding suggests an urgent need to address this residual risk through further improvements in medical therapy and stent technology.
Right now, accumulated evidence supports the notion that thinner struts are less thrombogenic than thicker struts, and that not all durable polymers are equal, with some associated with less inflammation and thrombogenicity. The next frontier for stent design seems to be ultrathin struts that are less than 70 mcm in diameter. We need to now see results from a study that compares an ultrathin-strut stent with a durable polymer against one with a biodegradable polymer.
Sripal Bangalore, MD , is an interventional cardiologist and professor of medicine at New York University. He has been a consultant or adviser to Abbott Vascular, Amgen, Biotronik, and Pfizer, and he has received research funding from Abbott Vascular. He made these comments as designated discussant for ISAR-TEST-4, and in an editorial published online concurrently with his talk (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038378 ).
The absence of incremental benefit from a stent with a biodegradable polymer compared with the second generation everolimus-eluting stent with a durable polymer in this 10-year follow-up is consistent with prior reports from medium-term follow-up. At best, drug-eluting coronary stents with a biodegradable polymer are noninferior to the current generation of those with a durable polymer. Late clinical benefit from a biodegradable polymer over a second-generation drug-eluting stent with a durable polymer remains elusive. The findings begs the public health question of whether paying a higher price for a biodegradable coronary stent with a durable-polymer is a good investment.
These 10-year results also highlight that, despite using drug-eluting stent technology that remains more or less standard of care today, the treated patients showed a staggering rate of major adverse cardiac events that exceeded 3% each year. This finding suggests an urgent need to address this residual risk through further improvements in medical therapy and stent technology.
Right now, accumulated evidence supports the notion that thinner struts are less thrombogenic than thicker struts, and that not all durable polymers are equal, with some associated with less inflammation and thrombogenicity. The next frontier for stent design seems to be ultrathin struts that are less than 70 mcm in diameter. We need to now see results from a study that compares an ultrathin-strut stent with a durable polymer against one with a biodegradable polymer.
Sripal Bangalore, MD , is an interventional cardiologist and professor of medicine at New York University. He has been a consultant or adviser to Abbott Vascular, Amgen, Biotronik, and Pfizer, and he has received research funding from Abbott Vascular. He made these comments as designated discussant for ISAR-TEST-4, and in an editorial published online concurrently with his talk (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038378 ).
CHICAGO – The idea behind putting a biodegradable polymer on a drug-eluting coronary stent is that, once the antirestenosis drug elutes and the polymer that held it degrades, the bare-metal stent left behind would trigger fewer long-term episodes of in-stent thrombosis than would stents that retain their polymer coating. But 10-year follow-up from a large trial that matched two second-generation drug-eluting stents, one with a biodegradable polymer and the other with a durable polymer, showed no statistically significant difference between the two for any clinical outcome, including the incidence of in-stent thrombosis, Sebastian Kufner, MD, said at the American Heart Association scientific sessions.
The potential advantage of a biodegradable polymer “is expected to occur over time,” and hence following patients for 10 or more years should start to show a clear advantage, at least for the endpoint of stent thrombosis, but that didn’t happen. After a median follow-up of 10.6 years, the cumulative rate of definite or probable stent thrombosis was 1.8% among 1,299 patients who received a sirolimus-eluting stent with a biodegradable polymer (Yukon Choice) and 2.5% among 652 patients who received a second-generation everolimus-eluting stent with a durable polymer (Xience), a difference that was not statistically significant, reported Dr. Kufner, a cardiologist at the The German Heart Centre in Munich.
These two stents also produced comparable 10-year outcomes that showed no statistically significant differences for the outcomes of all-cause death, MI, need for target-lesion revascularization, or the combined incidence of all three of these outcomes. In contrast, both of these second-generation stents showed statistically significant improvements in the combined cardiac endpoint, as well as in all-cause death, and definite stent thrombosis compared with the 652 patients who received the first-generation sirolimus-eluting stent Cypher. Concurrently with Dr. Kufner’s report, the results appeared in an article online (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038065).
Another notable finding from the 10-year follow-up was the poor prognosis these patients faced after their interventions, including the patients who received second-generation drug-eluting stents. The 10-year rate of all cause death was 30% among patients who received Xience stents, 32% among those treated with Yukon Choice stents, and 37% among patients treated with Cypher stents.
“I’m daunted by this 10-year mortality rate even with the best current drug-eluting stents,” said Roxana Mehran, MD, professor of medicine at Icahn School of Medicine at Mount Sinai in New York and a cochair of the session. “I’m depressed about this as an interventionalist. We need to do better, although it might not just be about the revascularization.” The high mortality in these patients after 10 years may also reflect a lack of optimal medical treatment in some, she suggested.
The ISAR-TEST-4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents) trial randomized 2,603 patients during the period of September 2007–August 2008 at either of two centers in Munich. The study’s primary endpoint was the combined rate of cardiac death, MI, and target-lesion revascularization by 12 months after treatment. The 12-month results showed a similar 14% rate of this combined endpoint in the subgroup of patients treated with the biodegradable-polymer stent and in those treated with stents that used durable polymers, which proved the noninferiority of the stent with a biodegradable polymer (Eur Heart J. 2009 Oct 1;30[20]:2441-9). This initial analysis combined the patients who received Cypher and Xience stents into one comparator group: patients who received stents with durable polymers.
The new, long-term follow-up analysis included 2,153 patients (83%) followed for at least 10 years after their intervention.
ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
SOURCE: Kufner S et al. AHA scientific sessions, Abstract 18630.
CHICAGO – The idea behind putting a biodegradable polymer on a drug-eluting coronary stent is that, once the antirestenosis drug elutes and the polymer that held it degrades, the bare-metal stent left behind would trigger fewer long-term episodes of in-stent thrombosis than would stents that retain their polymer coating. But 10-year follow-up from a large trial that matched two second-generation drug-eluting stents, one with a biodegradable polymer and the other with a durable polymer, showed no statistically significant difference between the two for any clinical outcome, including the incidence of in-stent thrombosis, Sebastian Kufner, MD, said at the American Heart Association scientific sessions.
The potential advantage of a biodegradable polymer “is expected to occur over time,” and hence following patients for 10 or more years should start to show a clear advantage, at least for the endpoint of stent thrombosis, but that didn’t happen. After a median follow-up of 10.6 years, the cumulative rate of definite or probable stent thrombosis was 1.8% among 1,299 patients who received a sirolimus-eluting stent with a biodegradable polymer (Yukon Choice) and 2.5% among 652 patients who received a second-generation everolimus-eluting stent with a durable polymer (Xience), a difference that was not statistically significant, reported Dr. Kufner, a cardiologist at the The German Heart Centre in Munich.
These two stents also produced comparable 10-year outcomes that showed no statistically significant differences for the outcomes of all-cause death, MI, need for target-lesion revascularization, or the combined incidence of all three of these outcomes. In contrast, both of these second-generation stents showed statistically significant improvements in the combined cardiac endpoint, as well as in all-cause death, and definite stent thrombosis compared with the 652 patients who received the first-generation sirolimus-eluting stent Cypher. Concurrently with Dr. Kufner’s report, the results appeared in an article online (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038065).
Another notable finding from the 10-year follow-up was the poor prognosis these patients faced after their interventions, including the patients who received second-generation drug-eluting stents. The 10-year rate of all cause death was 30% among patients who received Xience stents, 32% among those treated with Yukon Choice stents, and 37% among patients treated with Cypher stents.
“I’m daunted by this 10-year mortality rate even with the best current drug-eluting stents,” said Roxana Mehran, MD, professor of medicine at Icahn School of Medicine at Mount Sinai in New York and a cochair of the session. “I’m depressed about this as an interventionalist. We need to do better, although it might not just be about the revascularization.” The high mortality in these patients after 10 years may also reflect a lack of optimal medical treatment in some, she suggested.
The ISAR-TEST-4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents) trial randomized 2,603 patients during the period of September 2007–August 2008 at either of two centers in Munich. The study’s primary endpoint was the combined rate of cardiac death, MI, and target-lesion revascularization by 12 months after treatment. The 12-month results showed a similar 14% rate of this combined endpoint in the subgroup of patients treated with the biodegradable-polymer stent and in those treated with stents that used durable polymers, which proved the noninferiority of the stent with a biodegradable polymer (Eur Heart J. 2009 Oct 1;30[20]:2441-9). This initial analysis combined the patients who received Cypher and Xience stents into one comparator group: patients who received stents with durable polymers.
The new, long-term follow-up analysis included 2,153 patients (83%) followed for at least 10 years after their intervention.
ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
SOURCE: Kufner S et al. AHA scientific sessions, Abstract 18630.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The rate of adverse cardiac events was 48% with a biodegradable-polymer stent and 46% with a durable polymer.
Study details: Long-term follow-up of 2,153 patients in the ISAR-TEST-4 trial.
Disclosures: ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
Source: Kufner S et al. AHA 2018, Abstract 18630.
New diabetes drugs solidify their cardiovascular and renal benefits
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Ticagrelor holds no edge over aspirin in CABG patients
CHICAGO – Ticagrelor performed about as well as aspirin did as monotherapy for preventing coronary bypass graft failure during the year following surgery in a randomized, multicenter trial with almost 1,900 patients.
Ticagrelor monotherapy also produced about the same number of major bleeding events as did aspirin monotherapy, Heribert Schunkert, MD, said at the American Heart Association scientific sessions. There were two limitations of the trial: The incidence of cardiovascular disease events that served as the efficacy endpoint for the study was less than what Dr. Schunkert and his associates expected, and they enrolled about half the projected number of patients because the study lost industry support and then, a couple of years later, showed a relentlessly neutral result leading to early termination of recruitment, said Dr. Schunkert, professor of cardiology and medical director of the German Heart Center in Munich.
The TiCAB (Study Comparing Ticagrelor With Aspirin for Prevention of Vascular Events in Patients Undergoing CABG) trial randomized 1,893 patients during 2013-2017 who underwent CABG at any of 26 centers in Austria, Germany, or Switzerland. Eligible patients underwent surgery for three-vessel disease, left main disease, or had two-vessel disease plus a left ventricular ejection fraction of less than 50%. About 31% of patients had unstable angina or non-ST elevation MI, with the remaining 69% having stable angina. The study included 931 patients who received 90 mg oral ticagrelor (Brilinta) b.i.d. plus aspirin placebo, and 928 who received 100 mg aspirin once daily plus ticagrelor placebo. The study medications began prior to surgery.
The study’s primary efficacy endpoint was the combined rate of cardiovascular death, MI, stroke, or need for revascularization by 1 year after surgery. This occurred in 9.7% of the ticagrelor patients and in 8.2% of those who received aspirin, a difference that was not statistically significant. Several secondary efficacy endpoints examined also showed a neutral result. The primary safety measure was the incidence of major bleeds by the Bleeding Academic Research Consortium criteria, which occurred in 3.7% of the ticagrelor patients and 3.2% of those on aspirin, not a statistically significant difference. After the year of follow-up about 85% of patients in both treatment arms remained on their assigned regimen, Dr. Schunkert said.
TiCAB received funding from AstraZeneca, which markets ticagrelor (Brilinta). Dr. Schunkert has received honoraria and research support from, and has been a speaker on behalf of, AstraZeneca. He has also received honoraria from Amgen, Bayer Vital, Boehringer Ingelheim, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier.
SOURCE: Schunkert H et al. AHA 2018, Abstract 19561.
Because the TiCAB study was about half the size of the planned study, its power was low and yielded a result with wide confidence intervals. Despite that, I do not believe that a further, larger study is warranted. The TiCAB results are sufficient to show that monotherapy with ticagrelor is not superior to monotherapy with aspirin in patients undergoing coronary artery bypass grafting and during the year following surgery. The TiCAB results add to a larger body of evidence indicating ticagrelor’s noninferiority to and lack of superiority to aspirin as monotherapy for patients with coronary artery disease or a history of ischemic stroke or transient ischemic attack.
How can these two drugs produce similar efficacy outcomes? Aspirin is an effective antiplatelet drug, and evidence also suggests that treatment with opiates such as morphine (Circ Cardiovasc Interv. 2016 Sept;9[9]:e004229) and fentanyl (Circulation. 2018 Jan 16;137[3]:307-9) during and after surgery can interfere with the intestinal absorption of ticagrelor and other oral P2Y12 receptor antagonists, such as clopidogrel and prasugrel.
Another interesting finding in TiCAB was that aspirin and ticagrelor monotherapy produced similar rates of major bleeds. Results from prior studies had raised concerns about ticagrelor’s safety in patients undergoing coronary artery bypass surgery, but the new results show that this may be a problem when patients receive dual antiplatelet therapy but not when they receive ticagrelor monotherapy. Current evidence favors dual antiplatelet therapy to achieve a greater decrease in cardiovascular disease events, but this occurs at the expense of increased bleeding. Larger trials of dual therapy after coronary artery bypass grafting are warranted; further study of monotherapy is not.
Robert F. Storey, MD , is a professor of cardiology at the University of Sheffield (England). He has been a consultant to, and received honoraria and research support from, AstraZeneca, and he has been a consultant to Actelion, Avacta, Bayer, Bristol-Myers Squibb/Pfizer, Haemonetics, Novartis, PlaqueTec, and Thromboserin. He made these comments as designated discussant for the TiCAB report.
Because the TiCAB study was about half the size of the planned study, its power was low and yielded a result with wide confidence intervals. Despite that, I do not believe that a further, larger study is warranted. The TiCAB results are sufficient to show that monotherapy with ticagrelor is not superior to monotherapy with aspirin in patients undergoing coronary artery bypass grafting and during the year following surgery. The TiCAB results add to a larger body of evidence indicating ticagrelor’s noninferiority to and lack of superiority to aspirin as monotherapy for patients with coronary artery disease or a history of ischemic stroke or transient ischemic attack.
How can these two drugs produce similar efficacy outcomes? Aspirin is an effective antiplatelet drug, and evidence also suggests that treatment with opiates such as morphine (Circ Cardiovasc Interv. 2016 Sept;9[9]:e004229) and fentanyl (Circulation. 2018 Jan 16;137[3]:307-9) during and after surgery can interfere with the intestinal absorption of ticagrelor and other oral P2Y12 receptor antagonists, such as clopidogrel and prasugrel.
Another interesting finding in TiCAB was that aspirin and ticagrelor monotherapy produced similar rates of major bleeds. Results from prior studies had raised concerns about ticagrelor’s safety in patients undergoing coronary artery bypass surgery, but the new results show that this may be a problem when patients receive dual antiplatelet therapy but not when they receive ticagrelor monotherapy. Current evidence favors dual antiplatelet therapy to achieve a greater decrease in cardiovascular disease events, but this occurs at the expense of increased bleeding. Larger trials of dual therapy after coronary artery bypass grafting are warranted; further study of monotherapy is not.
Robert F. Storey, MD , is a professor of cardiology at the University of Sheffield (England). He has been a consultant to, and received honoraria and research support from, AstraZeneca, and he has been a consultant to Actelion, Avacta, Bayer, Bristol-Myers Squibb/Pfizer, Haemonetics, Novartis, PlaqueTec, and Thromboserin. He made these comments as designated discussant for the TiCAB report.
Because the TiCAB study was about half the size of the planned study, its power was low and yielded a result with wide confidence intervals. Despite that, I do not believe that a further, larger study is warranted. The TiCAB results are sufficient to show that monotherapy with ticagrelor is not superior to monotherapy with aspirin in patients undergoing coronary artery bypass grafting and during the year following surgery. The TiCAB results add to a larger body of evidence indicating ticagrelor’s noninferiority to and lack of superiority to aspirin as monotherapy for patients with coronary artery disease or a history of ischemic stroke or transient ischemic attack.
How can these two drugs produce similar efficacy outcomes? Aspirin is an effective antiplatelet drug, and evidence also suggests that treatment with opiates such as morphine (Circ Cardiovasc Interv. 2016 Sept;9[9]:e004229) and fentanyl (Circulation. 2018 Jan 16;137[3]:307-9) during and after surgery can interfere with the intestinal absorption of ticagrelor and other oral P2Y12 receptor antagonists, such as clopidogrel and prasugrel.
Another interesting finding in TiCAB was that aspirin and ticagrelor monotherapy produced similar rates of major bleeds. Results from prior studies had raised concerns about ticagrelor’s safety in patients undergoing coronary artery bypass surgery, but the new results show that this may be a problem when patients receive dual antiplatelet therapy but not when they receive ticagrelor monotherapy. Current evidence favors dual antiplatelet therapy to achieve a greater decrease in cardiovascular disease events, but this occurs at the expense of increased bleeding. Larger trials of dual therapy after coronary artery bypass grafting are warranted; further study of monotherapy is not.
Robert F. Storey, MD , is a professor of cardiology at the University of Sheffield (England). He has been a consultant to, and received honoraria and research support from, AstraZeneca, and he has been a consultant to Actelion, Avacta, Bayer, Bristol-Myers Squibb/Pfizer, Haemonetics, Novartis, PlaqueTec, and Thromboserin. He made these comments as designated discussant for the TiCAB report.
CHICAGO – Ticagrelor performed about as well as aspirin did as monotherapy for preventing coronary bypass graft failure during the year following surgery in a randomized, multicenter trial with almost 1,900 patients.
Ticagrelor monotherapy also produced about the same number of major bleeding events as did aspirin monotherapy, Heribert Schunkert, MD, said at the American Heart Association scientific sessions. There were two limitations of the trial: The incidence of cardiovascular disease events that served as the efficacy endpoint for the study was less than what Dr. Schunkert and his associates expected, and they enrolled about half the projected number of patients because the study lost industry support and then, a couple of years later, showed a relentlessly neutral result leading to early termination of recruitment, said Dr. Schunkert, professor of cardiology and medical director of the German Heart Center in Munich.
The TiCAB (Study Comparing Ticagrelor With Aspirin for Prevention of Vascular Events in Patients Undergoing CABG) trial randomized 1,893 patients during 2013-2017 who underwent CABG at any of 26 centers in Austria, Germany, or Switzerland. Eligible patients underwent surgery for three-vessel disease, left main disease, or had two-vessel disease plus a left ventricular ejection fraction of less than 50%. About 31% of patients had unstable angina or non-ST elevation MI, with the remaining 69% having stable angina. The study included 931 patients who received 90 mg oral ticagrelor (Brilinta) b.i.d. plus aspirin placebo, and 928 who received 100 mg aspirin once daily plus ticagrelor placebo. The study medications began prior to surgery.
The study’s primary efficacy endpoint was the combined rate of cardiovascular death, MI, stroke, or need for revascularization by 1 year after surgery. This occurred in 9.7% of the ticagrelor patients and in 8.2% of those who received aspirin, a difference that was not statistically significant. Several secondary efficacy endpoints examined also showed a neutral result. The primary safety measure was the incidence of major bleeds by the Bleeding Academic Research Consortium criteria, which occurred in 3.7% of the ticagrelor patients and 3.2% of those on aspirin, not a statistically significant difference. After the year of follow-up about 85% of patients in both treatment arms remained on their assigned regimen, Dr. Schunkert said.
TiCAB received funding from AstraZeneca, which markets ticagrelor (Brilinta). Dr. Schunkert has received honoraria and research support from, and has been a speaker on behalf of, AstraZeneca. He has also received honoraria from Amgen, Bayer Vital, Boehringer Ingelheim, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier.
SOURCE: Schunkert H et al. AHA 2018, Abstract 19561.
CHICAGO – Ticagrelor performed about as well as aspirin did as monotherapy for preventing coronary bypass graft failure during the year following surgery in a randomized, multicenter trial with almost 1,900 patients.
Ticagrelor monotherapy also produced about the same number of major bleeding events as did aspirin monotherapy, Heribert Schunkert, MD, said at the American Heart Association scientific sessions. There were two limitations of the trial: The incidence of cardiovascular disease events that served as the efficacy endpoint for the study was less than what Dr. Schunkert and his associates expected, and they enrolled about half the projected number of patients because the study lost industry support and then, a couple of years later, showed a relentlessly neutral result leading to early termination of recruitment, said Dr. Schunkert, professor of cardiology and medical director of the German Heart Center in Munich.
The TiCAB (Study Comparing Ticagrelor With Aspirin for Prevention of Vascular Events in Patients Undergoing CABG) trial randomized 1,893 patients during 2013-2017 who underwent CABG at any of 26 centers in Austria, Germany, or Switzerland. Eligible patients underwent surgery for three-vessel disease, left main disease, or had two-vessel disease plus a left ventricular ejection fraction of less than 50%. About 31% of patients had unstable angina or non-ST elevation MI, with the remaining 69% having stable angina. The study included 931 patients who received 90 mg oral ticagrelor (Brilinta) b.i.d. plus aspirin placebo, and 928 who received 100 mg aspirin once daily plus ticagrelor placebo. The study medications began prior to surgery.
The study’s primary efficacy endpoint was the combined rate of cardiovascular death, MI, stroke, or need for revascularization by 1 year after surgery. This occurred in 9.7% of the ticagrelor patients and in 8.2% of those who received aspirin, a difference that was not statistically significant. Several secondary efficacy endpoints examined also showed a neutral result. The primary safety measure was the incidence of major bleeds by the Bleeding Academic Research Consortium criteria, which occurred in 3.7% of the ticagrelor patients and 3.2% of those on aspirin, not a statistically significant difference. After the year of follow-up about 85% of patients in both treatment arms remained on their assigned regimen, Dr. Schunkert said.
TiCAB received funding from AstraZeneca, which markets ticagrelor (Brilinta). Dr. Schunkert has received honoraria and research support from, and has been a speaker on behalf of, AstraZeneca. He has also received honoraria from Amgen, Bayer Vital, Boehringer Ingelheim, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier.
SOURCE: Schunkert H et al. AHA 2018, Abstract 19561.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Ticagrelor was similar to aspirin for preventing graft failure in CABG patients.
Major finding: After 1 year, the combined cardiovascular disease endpoint occurred in 9.7% of ticagrelor patients and in 8.2% on aspirin.
Study details: TiCAB, a multicenter, randomized trial with 1,893 patients.
Disclosures: TiCAB received funding from AstraZeneca, which markets ticagrelor (Brilinta). Dr. Schunkert has received honoraria and research support from, and has been a speaker on behalf of, AstraZeneca. He has received honoraria from Amgen, Bayer Vital, Boehringer Ingelheim, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier.
Source: Schunkert H et al. AHA 2018, Abstract 19561.
Firibastat looking good for difficult-to-treat hypertension
CHICAGO – Firibastat, a first-in-class oral antihypertensive drug, proved safe, effective, and well-tolerated in NEW-HOPE, a phase 2b clinical trial focused on an understudied and underserved patient population composed largely of overweight or obese, high-risk, hypertensive racial minorities,
Firibastat is the first brain aminopeptidase A inhibitor. It selectively and specifically inhibits conversion of angiotensin II to angiotensin III, which exerts tonic stimulation over blood pressure. Decreased angiotensin III means less release of vasopressin, reduced sympathetic nervous system activity, and increased baroreflex activity, all of which add up to lower blood pressure, Keith C. Ferdinand, MD, explained at the American Heart Association scientific sessions.
NEW-HOPE (Novel Evaluation With QGC001 in Hypertensive Overweight Patients of Multiple Ethnic origins) was an open-label multicenter study including 254 middle-aged or older hypertensive patients. Two-thirds were obese, the rest overweight. Participants had a mean body mass index of 33 kg/m2 and a baseline automated office blood pressure (AOBP) of 153.9/91.5 mm Hg. A total of 46% were women, and 38% were black. Indeed, blacks and other minorities made up 54% of the NEW-HOPE population.
“Minority populations in many of the clinical trials in hypertension have been underrepresented,” observed Dr. Ferdinand, professor of medicine at Tulane University in New Orleans.
After a 2-week washout period, all patients were placed on firibastat at 250 mg b.i.d. for 2 weeks. If at that point their AOBP was more than 140/90 mm Hg they were bumped up to 500 mg b.i.d. Hydrochlorothiazide at 25 mg/day could be added 1 month into the trial if a patient’s systolic blood pressure was 160 mm Hg or more or their diastolic blood pressure was at least 100 mm Hg. Of note, 85% of patients were able to remain on firibastat monotherapy throughout the study.
The primary endpoint was change from baseline in systolic AOBP at 8 weeks. By week 8, the mean systolic blood pressure (SBP) had fallen by 9.7 mm Hg from a baseline of 153.9, which in hypertension circles is deemed a clinically meaningful improvement. Mean diastolic AOBP fell from 91.5 to 87.2 mm Hg, for a 4.3–mm Hg reduction.
“The diastolic number was smaller, but remember, this was a middle-aged and older population, and SBP is the most important endpoint in patients of that age, not just in clinical trials but in terms of its effects on morbidity and mortality,” Dr. Ferdinand said.
A word about the rigorous AOBP protocol used in NEW-HOPE: It was similar to that used in the landmark SPRINT trial. Patients were required to rest seated with back support in a quiet room with no talking for 5 minutes. Then six measurements were taken at 1-minute intervals, with patients’ legs uncrossed and feet on the floor the whole time. The first measurement was discarded, and the next five were averaged.
“This correlates very well with daytime ambulatory blood pressure, which is the gold standard,” the cardiologist noted.
In a prespecified subgroup analysis, systolic AOBP was reduced significantly more in obese than in overweight patients. Black patients averaged a 10.5–mm Hg decrease, nonblacks a 4.1–mm Hg reduction. But black patients also averaged a 2.0 kg/m2 higher body mass index.
“ACE inhibitors and angiotensin receptor blockers have been shown to be less efficacious in black patients. Firibastat, however, had similar effects in both black and nonblack patients. And this is probably one of the main take-away points of the study: This is a drug based on the phenotype of obesity and increased blood pressure, and the drug had efficacy regardless of self-identified race,” the cardiologist continued.
The most common treatment-emergent adverse events were headache in 4% of patients and skin reactions in 3%. There were no cases of angioedema. The only serious adverse event was one case of erythema multiforme. There were no clinically meaningful changes in any laboratory parameters.
Based upon these encouraging results, a pivotal phase 3 clinical trial is being planned in patients with difficult-to-treat or resistant hypertension. Dr. Ferdinand sees firibastat as being particularly useful as part of a two-drug treatment strategy, probably with the addition of a calcium channel blocker or potent diuretic, since “two drugs is the way to go in resistant hypertension.”
Audience members called the study “very hopeful,” but wondered about the absence of a placebo control arm. Reservations were also voiced about the need for twice-daily dosing of firibastat because it’s well established that adherence drops off when an antihypertensive agent has to be taken more than once daily.
Dr. Ferdinand said the lack of a placebo arm in NEW-HOPE was endorsed by the Food and Drug Administration because of ethical concerns surrounding use of a placebo in a high-risk population such as this. Obesity is known to increase the risk of resistant hypertension fivefold. Obesity is more common in blacks and Hispanics, with a prevalence of 47%, than in whites, where the prevalence is 38%.
As for the b.i.d. dosing, Quantum Genomics, the company that sponsored NEW-HOPE, is developing a sustained-release, once-daily formulation of firibastat that’s better suited for clinical practice, he added.
NEW-HOPE was carried out in collaboration with the Association of Black Cardiologists. Dr. Ferdinand reported serving as a consultant to Quantum Genomics and a handful of other pharmaceutical companies.
CHICAGO – Firibastat, a first-in-class oral antihypertensive drug, proved safe, effective, and well-tolerated in NEW-HOPE, a phase 2b clinical trial focused on an understudied and underserved patient population composed largely of overweight or obese, high-risk, hypertensive racial minorities,
Firibastat is the first brain aminopeptidase A inhibitor. It selectively and specifically inhibits conversion of angiotensin II to angiotensin III, which exerts tonic stimulation over blood pressure. Decreased angiotensin III means less release of vasopressin, reduced sympathetic nervous system activity, and increased baroreflex activity, all of which add up to lower blood pressure, Keith C. Ferdinand, MD, explained at the American Heart Association scientific sessions.
NEW-HOPE (Novel Evaluation With QGC001 in Hypertensive Overweight Patients of Multiple Ethnic origins) was an open-label multicenter study including 254 middle-aged or older hypertensive patients. Two-thirds were obese, the rest overweight. Participants had a mean body mass index of 33 kg/m2 and a baseline automated office blood pressure (AOBP) of 153.9/91.5 mm Hg. A total of 46% were women, and 38% were black. Indeed, blacks and other minorities made up 54% of the NEW-HOPE population.
“Minority populations in many of the clinical trials in hypertension have been underrepresented,” observed Dr. Ferdinand, professor of medicine at Tulane University in New Orleans.
After a 2-week washout period, all patients were placed on firibastat at 250 mg b.i.d. for 2 weeks. If at that point their AOBP was more than 140/90 mm Hg they were bumped up to 500 mg b.i.d. Hydrochlorothiazide at 25 mg/day could be added 1 month into the trial if a patient’s systolic blood pressure was 160 mm Hg or more or their diastolic blood pressure was at least 100 mm Hg. Of note, 85% of patients were able to remain on firibastat monotherapy throughout the study.
The primary endpoint was change from baseline in systolic AOBP at 8 weeks. By week 8, the mean systolic blood pressure (SBP) had fallen by 9.7 mm Hg from a baseline of 153.9, which in hypertension circles is deemed a clinically meaningful improvement. Mean diastolic AOBP fell from 91.5 to 87.2 mm Hg, for a 4.3–mm Hg reduction.
“The diastolic number was smaller, but remember, this was a middle-aged and older population, and SBP is the most important endpoint in patients of that age, not just in clinical trials but in terms of its effects on morbidity and mortality,” Dr. Ferdinand said.
A word about the rigorous AOBP protocol used in NEW-HOPE: It was similar to that used in the landmark SPRINT trial. Patients were required to rest seated with back support in a quiet room with no talking for 5 minutes. Then six measurements were taken at 1-minute intervals, with patients’ legs uncrossed and feet on the floor the whole time. The first measurement was discarded, and the next five were averaged.
“This correlates very well with daytime ambulatory blood pressure, which is the gold standard,” the cardiologist noted.
In a prespecified subgroup analysis, systolic AOBP was reduced significantly more in obese than in overweight patients. Black patients averaged a 10.5–mm Hg decrease, nonblacks a 4.1–mm Hg reduction. But black patients also averaged a 2.0 kg/m2 higher body mass index.
“ACE inhibitors and angiotensin receptor blockers have been shown to be less efficacious in black patients. Firibastat, however, had similar effects in both black and nonblack patients. And this is probably one of the main take-away points of the study: This is a drug based on the phenotype of obesity and increased blood pressure, and the drug had efficacy regardless of self-identified race,” the cardiologist continued.
The most common treatment-emergent adverse events were headache in 4% of patients and skin reactions in 3%. There were no cases of angioedema. The only serious adverse event was one case of erythema multiforme. There were no clinically meaningful changes in any laboratory parameters.
Based upon these encouraging results, a pivotal phase 3 clinical trial is being planned in patients with difficult-to-treat or resistant hypertension. Dr. Ferdinand sees firibastat as being particularly useful as part of a two-drug treatment strategy, probably with the addition of a calcium channel blocker or potent diuretic, since “two drugs is the way to go in resistant hypertension.”
Audience members called the study “very hopeful,” but wondered about the absence of a placebo control arm. Reservations were also voiced about the need for twice-daily dosing of firibastat because it’s well established that adherence drops off when an antihypertensive agent has to be taken more than once daily.
Dr. Ferdinand said the lack of a placebo arm in NEW-HOPE was endorsed by the Food and Drug Administration because of ethical concerns surrounding use of a placebo in a high-risk population such as this. Obesity is known to increase the risk of resistant hypertension fivefold. Obesity is more common in blacks and Hispanics, with a prevalence of 47%, than in whites, where the prevalence is 38%.
As for the b.i.d. dosing, Quantum Genomics, the company that sponsored NEW-HOPE, is developing a sustained-release, once-daily formulation of firibastat that’s better suited for clinical practice, he added.
NEW-HOPE was carried out in collaboration with the Association of Black Cardiologists. Dr. Ferdinand reported serving as a consultant to Quantum Genomics and a handful of other pharmaceutical companies.
CHICAGO – Firibastat, a first-in-class oral antihypertensive drug, proved safe, effective, and well-tolerated in NEW-HOPE, a phase 2b clinical trial focused on an understudied and underserved patient population composed largely of overweight or obese, high-risk, hypertensive racial minorities,
Firibastat is the first brain aminopeptidase A inhibitor. It selectively and specifically inhibits conversion of angiotensin II to angiotensin III, which exerts tonic stimulation over blood pressure. Decreased angiotensin III means less release of vasopressin, reduced sympathetic nervous system activity, and increased baroreflex activity, all of which add up to lower blood pressure, Keith C. Ferdinand, MD, explained at the American Heart Association scientific sessions.
NEW-HOPE (Novel Evaluation With QGC001 in Hypertensive Overweight Patients of Multiple Ethnic origins) was an open-label multicenter study including 254 middle-aged or older hypertensive patients. Two-thirds were obese, the rest overweight. Participants had a mean body mass index of 33 kg/m2 and a baseline automated office blood pressure (AOBP) of 153.9/91.5 mm Hg. A total of 46% were women, and 38% were black. Indeed, blacks and other minorities made up 54% of the NEW-HOPE population.
“Minority populations in many of the clinical trials in hypertension have been underrepresented,” observed Dr. Ferdinand, professor of medicine at Tulane University in New Orleans.
After a 2-week washout period, all patients were placed on firibastat at 250 mg b.i.d. for 2 weeks. If at that point their AOBP was more than 140/90 mm Hg they were bumped up to 500 mg b.i.d. Hydrochlorothiazide at 25 mg/day could be added 1 month into the trial if a patient’s systolic blood pressure was 160 mm Hg or more or their diastolic blood pressure was at least 100 mm Hg. Of note, 85% of patients were able to remain on firibastat monotherapy throughout the study.
The primary endpoint was change from baseline in systolic AOBP at 8 weeks. By week 8, the mean systolic blood pressure (SBP) had fallen by 9.7 mm Hg from a baseline of 153.9, which in hypertension circles is deemed a clinically meaningful improvement. Mean diastolic AOBP fell from 91.5 to 87.2 mm Hg, for a 4.3–mm Hg reduction.
“The diastolic number was smaller, but remember, this was a middle-aged and older population, and SBP is the most important endpoint in patients of that age, not just in clinical trials but in terms of its effects on morbidity and mortality,” Dr. Ferdinand said.
A word about the rigorous AOBP protocol used in NEW-HOPE: It was similar to that used in the landmark SPRINT trial. Patients were required to rest seated with back support in a quiet room with no talking for 5 minutes. Then six measurements were taken at 1-minute intervals, with patients’ legs uncrossed and feet on the floor the whole time. The first measurement was discarded, and the next five were averaged.
“This correlates very well with daytime ambulatory blood pressure, which is the gold standard,” the cardiologist noted.
In a prespecified subgroup analysis, systolic AOBP was reduced significantly more in obese than in overweight patients. Black patients averaged a 10.5–mm Hg decrease, nonblacks a 4.1–mm Hg reduction. But black patients also averaged a 2.0 kg/m2 higher body mass index.
“ACE inhibitors and angiotensin receptor blockers have been shown to be less efficacious in black patients. Firibastat, however, had similar effects in both black and nonblack patients. And this is probably one of the main take-away points of the study: This is a drug based on the phenotype of obesity and increased blood pressure, and the drug had efficacy regardless of self-identified race,” the cardiologist continued.
The most common treatment-emergent adverse events were headache in 4% of patients and skin reactions in 3%. There were no cases of angioedema. The only serious adverse event was one case of erythema multiforme. There were no clinically meaningful changes in any laboratory parameters.
Based upon these encouraging results, a pivotal phase 3 clinical trial is being planned in patients with difficult-to-treat or resistant hypertension. Dr. Ferdinand sees firibastat as being particularly useful as part of a two-drug treatment strategy, probably with the addition of a calcium channel blocker or potent diuretic, since “two drugs is the way to go in resistant hypertension.”
Audience members called the study “very hopeful,” but wondered about the absence of a placebo control arm. Reservations were also voiced about the need for twice-daily dosing of firibastat because it’s well established that adherence drops off when an antihypertensive agent has to be taken more than once daily.
Dr. Ferdinand said the lack of a placebo arm in NEW-HOPE was endorsed by the Food and Drug Administration because of ethical concerns surrounding use of a placebo in a high-risk population such as this. Obesity is known to increase the risk of resistant hypertension fivefold. Obesity is more common in blacks and Hispanics, with a prevalence of 47%, than in whites, where the prevalence is 38%.
As for the b.i.d. dosing, Quantum Genomics, the company that sponsored NEW-HOPE, is developing a sustained-release, once-daily formulation of firibastat that’s better suited for clinical practice, he added.
NEW-HOPE was carried out in collaboration with the Association of Black Cardiologists. Dr. Ferdinand reported serving as a consultant to Quantum Genomics and a handful of other pharmaceutical companies.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: A first-in-class oral brain angiotensin III inhibitor shows considerable promise in difficult-to-treat hypertension.
Major finding: Mean systolic blood pressure fell by 9.7 mm Hg at 8 weeks from a baseline of 153.9 mm Hg in response to firibastat.
Study details: This 8-week, open-label, multicenter trial included 254 obese or overweight patients with difficult-to-treat hypertension.
Disclosures: The presenter reported serving as a consultant to Quantum Genomics, the study sponsor.






















