User login
Bariatric Surgery Good Deal for Diabetes, but …
NEW ORLEANS – If the yardstick for measuring the cost-effectiveness of an operation or a medical treatment is that it costs less than $50,000 for each quality-adjusted life-year gained, then weight-loss surgery as a treatment for type 2 diabetes is cost-effective.
However, more long-term follow-up is needed to determine the true value of metabolic or bariatric surgery such as gastric bypass, compared with medical treatment for type 2 diabetes. Studies of bariatric surgery in the nondiabetic population found it was most cost-effective in the following scenarios: in women; in the morbidly obese vs. the moderately obese; in patients with obesity-related comorbidities including diabetes; when the procedures were performed laparoscopically; and when the studies themselves received industry support.
In people with diabetes, the results were similar. “Diabetes metabolic surgery is more cost-effective early in the course of type 2 diabetes compared to later in the course, when performed laparoscopically, and again when the study received support from industry,” reported Dr. William H. Herman, professor of epidemiology and internal medicine at the University of Michigan School of Public Health in Ann Arbor and director of the Michigan Center for Diabetes Translational Research.
He reviewed 11 economic analyses of bariatric surgery and concluded that all exceeded the benchmark for cost-effectiveness based on the cost per quality-adjusted life-year (QALY) gained. Six studies evaluated the general population of obese people and found that the cost-effectiveness ratios ranged from $1,600 to $44,000 per QALY gained. The remaining five studies involved obese patients with type 2 diabetes, two of which reported cost-effectiveness ratios of $2,000 to $23,000 per QALY gained; and the remaining three studies actually reporting a cost-savings. “In other words, the money spent on these interventions was more than recouped in the savings resulting from reduced downstream medical costs,” Dr. Herman reported at the American Diabetes Association scientific sessions.
The studies that found gastric bypass cost-saving in diabetes are noteworthy, Dr. Herman said. “If an intervention is more effective and less costly than a comparator intervention, then it is cost-saving, and that really is an unusual finding in health or medicine; perhaps 10% or 15% of interventions turn out to be cost-saving,” he said. “These are interventions that we want to adopt and put into practice pretty much without question.”
By the same measure, if an intervention is more costly and less effective, it’s easy to dismiss “out of hand,” Dr. Herman said. However, interpreting some of the studies he evaluated was more nuanced. “The problem occurs when a new treatment is both more effective but more costly, which was the case with two of the five analyses of metabolic surgery, and all of the analysis of bariatric surgery in the nondiabetic population,” he said
While gastric bypass surgery for type 2 diabetes is a good value, Dr. Herman added a few caveats. “When one looks at other interventions in similar categories, metformin for diabetes prevention has recently been shown to be cost-saving,” he said. He also said surgery is more cost-effective than marginally cost-effective interventions like intensive glycemic management for people with newly diagnosed type 2 diabetes or retinal screening every year vs. every 2 years.
One key issue with the existing evidence on cost-effectiveness of metabolic surgery for type 2 diabetes that Dr. Herman elucidated is how the studies accounted for participants lost to follow-up. “We know that a patient lost to follow-up may have a less favorable outcome than one who returns for follow-up,” he said. There are two ways studies can account for lost patients: the available-case analysis, which assumes that the patients lost to follow-up have the same rates of remission; and the attrition-adjusted available case follow-up, which uses a worst-case imputation. “I would argue that to account for attrition bias, remission rates calculated using the cases available for follow-up should be adjusted using worst-case imputation,” Dr. Herman said.
He pointed out another limitation when calculating the value of gastric bypass surgery for type 2 diabetes: “There are no randomized clinical trials of metabolic surgery that describe its long-term impact on diabetes treatments, complications, comorbidities, and survival. And it really is going be very important to get these data to confirm the cost-effectiveness of metabolic surgery.”
Among the shortcomings of the existing literature he noted are the assumptions that treatment-related adverse events are self-limited, that body mass index (BMI) achieved up to 5 years after surgery will remain stable, and that diabetes will not relapse. “The data are pretty good now on reversal, remission, hernia repair, and those sorts of things, but we need to look at longer downstream costs associated with surgery, including the need for cholecystectomy, joint replacements, and nutritional deficiencies that may occur and do clearly have financial implications,” he said.
At the same time, the analyses on gastric bypass surgery for type 2 diabetes could be more favorable if they account for improvements in health-related quality-of-life and rely less on cross-sectional data. Dr. Herman said, “I would argue that using cross-sectional data to estimate changes in health-related quality of life as a function of BMI underestimates the improvements on health-related quality-of-life associated with weight loss and will in fact underestimate the cost utility of interventions for obesity treatment,” he said.
Dr. Herman added, “Clearly the evidence to date suggests that metabolic surgery is cost-effective, but I’ll be more assured when I see longer-term follow-up.”
Dr. Herman has no financial relationships to disclose.
NEW ORLEANS – If the yardstick for measuring the cost-effectiveness of an operation or a medical treatment is that it costs less than $50,000 for each quality-adjusted life-year gained, then weight-loss surgery as a treatment for type 2 diabetes is cost-effective.
However, more long-term follow-up is needed to determine the true value of metabolic or bariatric surgery such as gastric bypass, compared with medical treatment for type 2 diabetes. Studies of bariatric surgery in the nondiabetic population found it was most cost-effective in the following scenarios: in women; in the morbidly obese vs. the moderately obese; in patients with obesity-related comorbidities including diabetes; when the procedures were performed laparoscopically; and when the studies themselves received industry support.
In people with diabetes, the results were similar. “Diabetes metabolic surgery is more cost-effective early in the course of type 2 diabetes compared to later in the course, when performed laparoscopically, and again when the study received support from industry,” reported Dr. William H. Herman, professor of epidemiology and internal medicine at the University of Michigan School of Public Health in Ann Arbor and director of the Michigan Center for Diabetes Translational Research.
He reviewed 11 economic analyses of bariatric surgery and concluded that all exceeded the benchmark for cost-effectiveness based on the cost per quality-adjusted life-year (QALY) gained. Six studies evaluated the general population of obese people and found that the cost-effectiveness ratios ranged from $1,600 to $44,000 per QALY gained. The remaining five studies involved obese patients with type 2 diabetes, two of which reported cost-effectiveness ratios of $2,000 to $23,000 per QALY gained; and the remaining three studies actually reporting a cost-savings. “In other words, the money spent on these interventions was more than recouped in the savings resulting from reduced downstream medical costs,” Dr. Herman reported at the American Diabetes Association scientific sessions.
The studies that found gastric bypass cost-saving in diabetes are noteworthy, Dr. Herman said. “If an intervention is more effective and less costly than a comparator intervention, then it is cost-saving, and that really is an unusual finding in health or medicine; perhaps 10% or 15% of interventions turn out to be cost-saving,” he said. “These are interventions that we want to adopt and put into practice pretty much without question.”
By the same measure, if an intervention is more costly and less effective, it’s easy to dismiss “out of hand,” Dr. Herman said. However, interpreting some of the studies he evaluated was more nuanced. “The problem occurs when a new treatment is both more effective but more costly, which was the case with two of the five analyses of metabolic surgery, and all of the analysis of bariatric surgery in the nondiabetic population,” he said
While gastric bypass surgery for type 2 diabetes is a good value, Dr. Herman added a few caveats. “When one looks at other interventions in similar categories, metformin for diabetes prevention has recently been shown to be cost-saving,” he said. He also said surgery is more cost-effective than marginally cost-effective interventions like intensive glycemic management for people with newly diagnosed type 2 diabetes or retinal screening every year vs. every 2 years.
One key issue with the existing evidence on cost-effectiveness of metabolic surgery for type 2 diabetes that Dr. Herman elucidated is how the studies accounted for participants lost to follow-up. “We know that a patient lost to follow-up may have a less favorable outcome than one who returns for follow-up,” he said. There are two ways studies can account for lost patients: the available-case analysis, which assumes that the patients lost to follow-up have the same rates of remission; and the attrition-adjusted available case follow-up, which uses a worst-case imputation. “I would argue that to account for attrition bias, remission rates calculated using the cases available for follow-up should be adjusted using worst-case imputation,” Dr. Herman said.
He pointed out another limitation when calculating the value of gastric bypass surgery for type 2 diabetes: “There are no randomized clinical trials of metabolic surgery that describe its long-term impact on diabetes treatments, complications, comorbidities, and survival. And it really is going be very important to get these data to confirm the cost-effectiveness of metabolic surgery.”
Among the shortcomings of the existing literature he noted are the assumptions that treatment-related adverse events are self-limited, that body mass index (BMI) achieved up to 5 years after surgery will remain stable, and that diabetes will not relapse. “The data are pretty good now on reversal, remission, hernia repair, and those sorts of things, but we need to look at longer downstream costs associated with surgery, including the need for cholecystectomy, joint replacements, and nutritional deficiencies that may occur and do clearly have financial implications,” he said.
At the same time, the analyses on gastric bypass surgery for type 2 diabetes could be more favorable if they account for improvements in health-related quality-of-life and rely less on cross-sectional data. Dr. Herman said, “I would argue that using cross-sectional data to estimate changes in health-related quality of life as a function of BMI underestimates the improvements on health-related quality-of-life associated with weight loss and will in fact underestimate the cost utility of interventions for obesity treatment,” he said.
Dr. Herman added, “Clearly the evidence to date suggests that metabolic surgery is cost-effective, but I’ll be more assured when I see longer-term follow-up.”
Dr. Herman has no financial relationships to disclose.
NEW ORLEANS – If the yardstick for measuring the cost-effectiveness of an operation or a medical treatment is that it costs less than $50,000 for each quality-adjusted life-year gained, then weight-loss surgery as a treatment for type 2 diabetes is cost-effective.
However, more long-term follow-up is needed to determine the true value of metabolic or bariatric surgery such as gastric bypass, compared with medical treatment for type 2 diabetes. Studies of bariatric surgery in the nondiabetic population found it was most cost-effective in the following scenarios: in women; in the morbidly obese vs. the moderately obese; in patients with obesity-related comorbidities including diabetes; when the procedures were performed laparoscopically; and when the studies themselves received industry support.
In people with diabetes, the results were similar. “Diabetes metabolic surgery is more cost-effective early in the course of type 2 diabetes compared to later in the course, when performed laparoscopically, and again when the study received support from industry,” reported Dr. William H. Herman, professor of epidemiology and internal medicine at the University of Michigan School of Public Health in Ann Arbor and director of the Michigan Center for Diabetes Translational Research.
He reviewed 11 economic analyses of bariatric surgery and concluded that all exceeded the benchmark for cost-effectiveness based on the cost per quality-adjusted life-year (QALY) gained. Six studies evaluated the general population of obese people and found that the cost-effectiveness ratios ranged from $1,600 to $44,000 per QALY gained. The remaining five studies involved obese patients with type 2 diabetes, two of which reported cost-effectiveness ratios of $2,000 to $23,000 per QALY gained; and the remaining three studies actually reporting a cost-savings. “In other words, the money spent on these interventions was more than recouped in the savings resulting from reduced downstream medical costs,” Dr. Herman reported at the American Diabetes Association scientific sessions.
The studies that found gastric bypass cost-saving in diabetes are noteworthy, Dr. Herman said. “If an intervention is more effective and less costly than a comparator intervention, then it is cost-saving, and that really is an unusual finding in health or medicine; perhaps 10% or 15% of interventions turn out to be cost-saving,” he said. “These are interventions that we want to adopt and put into practice pretty much without question.”
By the same measure, if an intervention is more costly and less effective, it’s easy to dismiss “out of hand,” Dr. Herman said. However, interpreting some of the studies he evaluated was more nuanced. “The problem occurs when a new treatment is both more effective but more costly, which was the case with two of the five analyses of metabolic surgery, and all of the analysis of bariatric surgery in the nondiabetic population,” he said
While gastric bypass surgery for type 2 diabetes is a good value, Dr. Herman added a few caveats. “When one looks at other interventions in similar categories, metformin for diabetes prevention has recently been shown to be cost-saving,” he said. He also said surgery is more cost-effective than marginally cost-effective interventions like intensive glycemic management for people with newly diagnosed type 2 diabetes or retinal screening every year vs. every 2 years.
One key issue with the existing evidence on cost-effectiveness of metabolic surgery for type 2 diabetes that Dr. Herman elucidated is how the studies accounted for participants lost to follow-up. “We know that a patient lost to follow-up may have a less favorable outcome than one who returns for follow-up,” he said. There are two ways studies can account for lost patients: the available-case analysis, which assumes that the patients lost to follow-up have the same rates of remission; and the attrition-adjusted available case follow-up, which uses a worst-case imputation. “I would argue that to account for attrition bias, remission rates calculated using the cases available for follow-up should be adjusted using worst-case imputation,” Dr. Herman said.
He pointed out another limitation when calculating the value of gastric bypass surgery for type 2 diabetes: “There are no randomized clinical trials of metabolic surgery that describe its long-term impact on diabetes treatments, complications, comorbidities, and survival. And it really is going be very important to get these data to confirm the cost-effectiveness of metabolic surgery.”
Among the shortcomings of the existing literature he noted are the assumptions that treatment-related adverse events are self-limited, that body mass index (BMI) achieved up to 5 years after surgery will remain stable, and that diabetes will not relapse. “The data are pretty good now on reversal, remission, hernia repair, and those sorts of things, but we need to look at longer downstream costs associated with surgery, including the need for cholecystectomy, joint replacements, and nutritional deficiencies that may occur and do clearly have financial implications,” he said.
At the same time, the analyses on gastric bypass surgery for type 2 diabetes could be more favorable if they account for improvements in health-related quality-of-life and rely less on cross-sectional data. Dr. Herman said, “I would argue that using cross-sectional data to estimate changes in health-related quality of life as a function of BMI underestimates the improvements on health-related quality-of-life associated with weight loss and will in fact underestimate the cost utility of interventions for obesity treatment,” he said.
Dr. Herman added, “Clearly the evidence to date suggests that metabolic surgery is cost-effective, but I’ll be more assured when I see longer-term follow-up.”
Dr. Herman has no financial relationships to disclose.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Bariatric surgery good deal for diabetes, but…
NEW ORLEANS – If the yardstick for measuring the cost-effectiveness of an operation or a medical treatment is that it costs less than $50,000 for each quality-adjusted life-year gained, then weight-loss surgery as a treatment for type 2 diabetes is cost-effective.
However, more long-term follow-up is needed to determine the true value of metabolic or bariatric surgery such as gastric bypass, compared with medical treatment for type 2 diabetes. Studies of bariatric surgery in the nondiabetic population found it was most cost-effective in the following scenarios: in women; in the morbidly obese vs. the moderately obese; in patients with obesity-related comorbidities including diabetes; when the procedures were performed laparoscopically; and when the studies themselves received industry support.
In people with diabetes, the results were similar. “Diabetes metabolic surgery is more cost-effective early in the course of type 2 diabetes compared to later in the course, when performed laparoscopically, and again when the study received support from industry,” reported Dr. William H. Herman, professor of epidemiology and internal medicine at the University of Michigan School of Public Health in Ann Arbor and director of the Michigan Center for Diabetes Translational Research.
He reviewed 11 economic analyses of bariatric surgery and concluded that all exceeded the benchmark for cost-effectiveness based on the cost per quality-adjusted life-year (QALY) gained. Six studies evaluated the general population of obese people and found that the cost-effectiveness ratios ranged from $1,600 to $44,000 per QALY gained. The remaining five studies involved obese patients with type 2 diabetes, two of which reported cost-effectiveness ratios of $2,000 to $23,000 per QALY gained; and the remaining three studies actually reporting a cost-savings. “In other words, the money spent on these interventions was more than recouped in the savings resulting from reduced downstream medical costs,” Dr. Herman reported at the American Diabetes Association scientific sessions.
The studies that found gastric bypass cost-saving in diabetes are noteworthy, Dr. Herman said. “If an intervention is more effective and less costly than a comparator intervention, then it is cost-saving, and that really is an unusual finding in health or medicine; perhaps 10% or 15% of interventions turn out to be cost-saving,” he said. “These are interventions that we want to adopt and put into practice pretty much without question.”
By the same measure, if an intervention is more costly and less effective, it’s easy to dismiss “out of hand,” Dr. Herman said. However, interpreting some of the studies he evaluated was more nuanced. “The problem occurs when a new treatment is both more effective but more costly, which was the case with two of the five analyses of metabolic surgery, and all of the analysis of bariatric surgery in the nondiabetic population,” he said
While gastric bypass surgery for type 2 diabetes is a good value, Dr. Herman added a few caveats. “When one looks at other interventions in similar categories, metformin for diabetes prevention has recently been shown to be cost-saving,” he said. He also said surgery is more cost-effective than marginally cost-effective interventions like intensive glycemic management for people with newly diagnosed type 2 diabetes or retinal screening every year vs. every 2 years.
One key issue with the existing evidence on cost-effectiveness of metabolic surgery for type 2 diabetes that Dr. Herman elucidated is how the studies accounted for participants lost to follow-up. “We know that a patient lost to follow-up may have a less favorable outcome than one who returns for follow-up,” he said. There are two ways studies can account for lost patients: the available-case analysis, which assumes that the patients lost to follow-up have the same rates of remission; and the attrition-adjusted available case follow-up, which uses a worst-case imputation. “I would argue that to account for attrition bias, remission rates calculated using the cases available for follow-up should be adjusted using worst-case imputation,” Dr. Herman said.
He pointed out another limitation when calculating the value of gastric bypass surgery for type 2 diabetes: “There are no randomized clinical trials of metabolic surgery that describe its long-term impact on diabetes treatments, complications, comorbidities, and survival. And it really is going be very important to get these data to confirm the cost-effectiveness of metabolic surgery.”
Among the shortcomings of the existing literature he noted are the assumptions that treatment-related adverse events are self-limited, that body mass index (BMI) achieved up to 5 years after surgery will remain stable, and that diabetes will not relapse. “The data are pretty good now on reversal, remission, hernia repair, and those sorts of things, but we need to look at longer downstream costs associated with surgery, including the need for cholecystectomy, joint replacements, and nutritional deficiencies that may occur and do clearly have financial implications,” he said.
At the same time, the analyses on gastric bypass surgery for type 2 diabetes could be more favorable if they account for improvements in health-related quality-of-life and rely less on cross-sectional data. Dr. Herman said, “I would argue that using cross-sectional data to estimate changes in health-related quality of life as a function of BMI underestimates the improvements on health-related quality-of-life associated with weight loss and will in fact underestimate the cost utility of interventions for obesity treatment,” he said.
Dr. Herman added, “Clearly the evidence to date suggests that metabolic surgery is cost-effective, but I’ll be more assured when I see longer-term follow-up.”
Dr. Herman has no financial relationships to disclose.
NEW ORLEANS – If the yardstick for measuring the cost-effectiveness of an operation or a medical treatment is that it costs less than $50,000 for each quality-adjusted life-year gained, then weight-loss surgery as a treatment for type 2 diabetes is cost-effective.
However, more long-term follow-up is needed to determine the true value of metabolic or bariatric surgery such as gastric bypass, compared with medical treatment for type 2 diabetes. Studies of bariatric surgery in the nondiabetic population found it was most cost-effective in the following scenarios: in women; in the morbidly obese vs. the moderately obese; in patients with obesity-related comorbidities including diabetes; when the procedures were performed laparoscopically; and when the studies themselves received industry support.
In people with diabetes, the results were similar. “Diabetes metabolic surgery is more cost-effective early in the course of type 2 diabetes compared to later in the course, when performed laparoscopically, and again when the study received support from industry,” reported Dr. William H. Herman, professor of epidemiology and internal medicine at the University of Michigan School of Public Health in Ann Arbor and director of the Michigan Center for Diabetes Translational Research.
He reviewed 11 economic analyses of bariatric surgery and concluded that all exceeded the benchmark for cost-effectiveness based on the cost per quality-adjusted life-year (QALY) gained. Six studies evaluated the general population of obese people and found that the cost-effectiveness ratios ranged from $1,600 to $44,000 per QALY gained. The remaining five studies involved obese patients with type 2 diabetes, two of which reported cost-effectiveness ratios of $2,000 to $23,000 per QALY gained; and the remaining three studies actually reporting a cost-savings. “In other words, the money spent on these interventions was more than recouped in the savings resulting from reduced downstream medical costs,” Dr. Herman reported at the American Diabetes Association scientific sessions.
The studies that found gastric bypass cost-saving in diabetes are noteworthy, Dr. Herman said. “If an intervention is more effective and less costly than a comparator intervention, then it is cost-saving, and that really is an unusual finding in health or medicine; perhaps 10% or 15% of interventions turn out to be cost-saving,” he said. “These are interventions that we want to adopt and put into practice pretty much without question.”
By the same measure, if an intervention is more costly and less effective, it’s easy to dismiss “out of hand,” Dr. Herman said. However, interpreting some of the studies he evaluated was more nuanced. “The problem occurs when a new treatment is both more effective but more costly, which was the case with two of the five analyses of metabolic surgery, and all of the analysis of bariatric surgery in the nondiabetic population,” he said
While gastric bypass surgery for type 2 diabetes is a good value, Dr. Herman added a few caveats. “When one looks at other interventions in similar categories, metformin for diabetes prevention has recently been shown to be cost-saving,” he said. He also said surgery is more cost-effective than marginally cost-effective interventions like intensive glycemic management for people with newly diagnosed type 2 diabetes or retinal screening every year vs. every 2 years.
One key issue with the existing evidence on cost-effectiveness of metabolic surgery for type 2 diabetes that Dr. Herman elucidated is how the studies accounted for participants lost to follow-up. “We know that a patient lost to follow-up may have a less favorable outcome than one who returns for follow-up,” he said. There are two ways studies can account for lost patients: the available-case analysis, which assumes that the patients lost to follow-up have the same rates of remission; and the attrition-adjusted available case follow-up, which uses a worst-case imputation. “I would argue that to account for attrition bias, remission rates calculated using the cases available for follow-up should be adjusted using worst-case imputation,” Dr. Herman said.
He pointed out another limitation when calculating the value of gastric bypass surgery for type 2 diabetes: “There are no randomized clinical trials of metabolic surgery that describe its long-term impact on diabetes treatments, complications, comorbidities, and survival. And it really is going be very important to get these data to confirm the cost-effectiveness of metabolic surgery.”
Among the shortcomings of the existing literature he noted are the assumptions that treatment-related adverse events are self-limited, that body mass index (BMI) achieved up to 5 years after surgery will remain stable, and that diabetes will not relapse. “The data are pretty good now on reversal, remission, hernia repair, and those sorts of things, but we need to look at longer downstream costs associated with surgery, including the need for cholecystectomy, joint replacements, and nutritional deficiencies that may occur and do clearly have financial implications,” he said.
At the same time, the analyses on gastric bypass surgery for type 2 diabetes could be more favorable if they account for improvements in health-related quality-of-life and rely less on cross-sectional data. Dr. Herman said, “I would argue that using cross-sectional data to estimate changes in health-related quality of life as a function of BMI underestimates the improvements on health-related quality-of-life associated with weight loss and will in fact underestimate the cost utility of interventions for obesity treatment,” he said.
Dr. Herman added, “Clearly the evidence to date suggests that metabolic surgery is cost-effective, but I’ll be more assured when I see longer-term follow-up.”
Dr. Herman has no financial relationships to disclose.
NEW ORLEANS – If the yardstick for measuring the cost-effectiveness of an operation or a medical treatment is that it costs less than $50,000 for each quality-adjusted life-year gained, then weight-loss surgery as a treatment for type 2 diabetes is cost-effective.
However, more long-term follow-up is needed to determine the true value of metabolic or bariatric surgery such as gastric bypass, compared with medical treatment for type 2 diabetes. Studies of bariatric surgery in the nondiabetic population found it was most cost-effective in the following scenarios: in women; in the morbidly obese vs. the moderately obese; in patients with obesity-related comorbidities including diabetes; when the procedures were performed laparoscopically; and when the studies themselves received industry support.
In people with diabetes, the results were similar. “Diabetes metabolic surgery is more cost-effective early in the course of type 2 diabetes compared to later in the course, when performed laparoscopically, and again when the study received support from industry,” reported Dr. William H. Herman, professor of epidemiology and internal medicine at the University of Michigan School of Public Health in Ann Arbor and director of the Michigan Center for Diabetes Translational Research.
He reviewed 11 economic analyses of bariatric surgery and concluded that all exceeded the benchmark for cost-effectiveness based on the cost per quality-adjusted life-year (QALY) gained. Six studies evaluated the general population of obese people and found that the cost-effectiveness ratios ranged from $1,600 to $44,000 per QALY gained. The remaining five studies involved obese patients with type 2 diabetes, two of which reported cost-effectiveness ratios of $2,000 to $23,000 per QALY gained; and the remaining three studies actually reporting a cost-savings. “In other words, the money spent on these interventions was more than recouped in the savings resulting from reduced downstream medical costs,” Dr. Herman reported at the American Diabetes Association scientific sessions.
The studies that found gastric bypass cost-saving in diabetes are noteworthy, Dr. Herman said. “If an intervention is more effective and less costly than a comparator intervention, then it is cost-saving, and that really is an unusual finding in health or medicine; perhaps 10% or 15% of interventions turn out to be cost-saving,” he said. “These are interventions that we want to adopt and put into practice pretty much without question.”
By the same measure, if an intervention is more costly and less effective, it’s easy to dismiss “out of hand,” Dr. Herman said. However, interpreting some of the studies he evaluated was more nuanced. “The problem occurs when a new treatment is both more effective but more costly, which was the case with two of the five analyses of metabolic surgery, and all of the analysis of bariatric surgery in the nondiabetic population,” he said
While gastric bypass surgery for type 2 diabetes is a good value, Dr. Herman added a few caveats. “When one looks at other interventions in similar categories, metformin for diabetes prevention has recently been shown to be cost-saving,” he said. He also said surgery is more cost-effective than marginally cost-effective interventions like intensive glycemic management for people with newly diagnosed type 2 diabetes or retinal screening every year vs. every 2 years.
One key issue with the existing evidence on cost-effectiveness of metabolic surgery for type 2 diabetes that Dr. Herman elucidated is how the studies accounted for participants lost to follow-up. “We know that a patient lost to follow-up may have a less favorable outcome than one who returns for follow-up,” he said. There are two ways studies can account for lost patients: the available-case analysis, which assumes that the patients lost to follow-up have the same rates of remission; and the attrition-adjusted available case follow-up, which uses a worst-case imputation. “I would argue that to account for attrition bias, remission rates calculated using the cases available for follow-up should be adjusted using worst-case imputation,” Dr. Herman said.
He pointed out another limitation when calculating the value of gastric bypass surgery for type 2 diabetes: “There are no randomized clinical trials of metabolic surgery that describe its long-term impact on diabetes treatments, complications, comorbidities, and survival. And it really is going be very important to get these data to confirm the cost-effectiveness of metabolic surgery.”
Among the shortcomings of the existing literature he noted are the assumptions that treatment-related adverse events are self-limited, that body mass index (BMI) achieved up to 5 years after surgery will remain stable, and that diabetes will not relapse. “The data are pretty good now on reversal, remission, hernia repair, and those sorts of things, but we need to look at longer downstream costs associated with surgery, including the need for cholecystectomy, joint replacements, and nutritional deficiencies that may occur and do clearly have financial implications,” he said.
At the same time, the analyses on gastric bypass surgery for type 2 diabetes could be more favorable if they account for improvements in health-related quality-of-life and rely less on cross-sectional data. Dr. Herman said, “I would argue that using cross-sectional data to estimate changes in health-related quality of life as a function of BMI underestimates the improvements on health-related quality-of-life associated with weight loss and will in fact underestimate the cost utility of interventions for obesity treatment,” he said.
Dr. Herman added, “Clearly the evidence to date suggests that metabolic surgery is cost-effective, but I’ll be more assured when I see longer-term follow-up.”
Dr. Herman has no financial relationships to disclose.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Bariatric or metabolic surgery is a cost-effective treatment for type 2 diabetes.
Major finding: Cost-effectiveness ratios of $2,000-$23,000 for bariatric surgery in people with type 2 diabetes fall below the cost-effectiveness threshold.
Data source: Review of 11 economic analyses of bariatric surgery, including six studies of bariatric surgery in people with type 2 diabetes.
Disclosures: Dr. Herman reported having no financial disclosures.
Benefits of Lifestyle Intervention Only Brief in Some T2DM Patients
NEW ORLEANS – Underserved African Americans with type 2 diabetes mellitus who participated in a year-long intensive self-management program did not experience sustained serum glucose control, compared with a control group receiving only two diabetes education classes.
“Relative to non-Hispanic whites, African Americans with type 2 diabetes experience more diabetes-related complications and higher rates of diabetes hospitalization,” lead study author Elizabeth B. Lynch, Ph.D., said at the annual scientific sessions of the American Diabetes Association. “These disparities are even greater for underserved disadvantaged African American populations.”
Dr. Lynch, a psychologist who directs the section of community health in the department of preventive medicine at Rush University Medical Center, Chicago, noted that several self-management interventions for diabetes have demonstrated efficacy at improving glucose control at 6 months. “However, there have not been any diabetes self-management interventions specifically targeting African Americans that have achieved sustained blood glucose control,” she said.
In a trial known as Lifestyle Intervention Through Food and Exercise (LIFE), the researchers examined the effect of a group-based intervention on glucose control at 12 months in a population of low-income African Americans. The intervention components consisted of cognitively tailored nutrition education taught by a registered dietitian, behavioral modification, social support, and peer support. “This education curriculum was based on a series of studies that were done using cognitive anthropological methods with low-income African Americans looking at beliefs and knowledge about the relationship between food and health,” Dr. Lynch said. “We used those studies to design an intervention with the aim of reducing cognitive load among participants when they’re learning new information about nutrition, so essentially making the information easier for people to understand.” Behavioral modification techniques included goal setting, self-monitoring, and problem solving. “We also had social support, and there was a peer supporter who was an individual from the community with type 2 diabetes who was assigned to each of the participants and called them on a regular basis to check in with them on their goals and encourage them,” she said.
The LIFE program consisted of 20 group sessions in the first 6 months and 8 sessions in the second 6 months, while a control group received 2 group-based education classes in the first 6 months only. The researchers conducted assessments at baseline, 6 months, and 12 months.
Individuals were eligible for the trial if they were African American, were a patient of a community clinic affiliated with Cook County Healthcare System, had a clinical diagnosis of type 2 diabetes, and had a hemoglobin A1c level of 7% or greater. Of 1,403 initially screened for the trial, 603 were found to be eligible. Of these, 211 were randomized and enrolled: 106 to the treatment group and 105 to the control group. There was 94% follow-up at 6 and 12 months.
At baseline, the mean age of study participants was 55 years, 70% were female, 46% had a high school education or less, 60% had an annual income of less than $24,000, 65% were uninsured, and 39% had limited health literacy. Baseline food intake as reported by two 24-hour food recalls consisted of a diet high in saturated fat and low in fiber, with a moderate intake of carbohydrates and underconsumption of fruits, vegetables, and dairy products. The baseline level of daily physical activity as measured by accelerometry revealed sedentary activity that exceeded 7 hours per day, 3,614 steps per day, and only 14 minutes per day of moderate-level activity. Study enrollees had a baseline HbA1c level of 9% and a diabetes duration of 11 years; 45% used insulin, and 48% had poor medication adherence. Their mean body mass index was 35.6 kg/m2, and 91% had hypertension.
More than half of individuals in the intervention group attended each of the 20 group sessions, and 90% attended at least 1. At the same time, 68% of individuals in the control group attended both educational sessions. Dr. Lynch reported that compared with the control group, the intervention group had a significantly greater reduction in HbA1c at 6 months (–0.76 vs. –0.21, respectively; P = .026) but not at 12 months (–0.63 vs. –0.45; P = .47). In addition, a higher percentage of individuals in the treatment group had a 0.5% or more decline in HbA1c level at 6 months (63% vs. 42%, P = .005) but not at 12 months (53% vs. 51%, P = .89). The fact that the control group also had a reduction in HbA1c presented a conundrum for the researchers. “One possible explanation for the decrease in A1c in the control group is that medication adherence increased in this group, relative to the intervention group,” Dr. Lynch explained in a press release. “Additional research is needed to identify the most effective strategies to achieve sustained A1c control in African Americans with type 2 diabetes.”
No changes were observed in blood pressure, weight, or physical activity over the course of 12 months in either group.
Although LIFE lacked a third study arm that received usual care, one possible implication of the current findings “may be that diabetes education of any type may be helpful in improving glycemic control, especially in a population that does not normally receive any education,” she said. “Medication adherence may be an easier and more effective strategy to improve glycemic control in this population.”
LIFE was supported by grants from the National Institutes of Health. Dr. Lynch reported having no relevant financial disclosures.
NEW ORLEANS – Underserved African Americans with type 2 diabetes mellitus who participated in a year-long intensive self-management program did not experience sustained serum glucose control, compared with a control group receiving only two diabetes education classes.
“Relative to non-Hispanic whites, African Americans with type 2 diabetes experience more diabetes-related complications and higher rates of diabetes hospitalization,” lead study author Elizabeth B. Lynch, Ph.D., said at the annual scientific sessions of the American Diabetes Association. “These disparities are even greater for underserved disadvantaged African American populations.”
Dr. Lynch, a psychologist who directs the section of community health in the department of preventive medicine at Rush University Medical Center, Chicago, noted that several self-management interventions for diabetes have demonstrated efficacy at improving glucose control at 6 months. “However, there have not been any diabetes self-management interventions specifically targeting African Americans that have achieved sustained blood glucose control,” she said.
In a trial known as Lifestyle Intervention Through Food and Exercise (LIFE), the researchers examined the effect of a group-based intervention on glucose control at 12 months in a population of low-income African Americans. The intervention components consisted of cognitively tailored nutrition education taught by a registered dietitian, behavioral modification, social support, and peer support. “This education curriculum was based on a series of studies that were done using cognitive anthropological methods with low-income African Americans looking at beliefs and knowledge about the relationship between food and health,” Dr. Lynch said. “We used those studies to design an intervention with the aim of reducing cognitive load among participants when they’re learning new information about nutrition, so essentially making the information easier for people to understand.” Behavioral modification techniques included goal setting, self-monitoring, and problem solving. “We also had social support, and there was a peer supporter who was an individual from the community with type 2 diabetes who was assigned to each of the participants and called them on a regular basis to check in with them on their goals and encourage them,” she said.
The LIFE program consisted of 20 group sessions in the first 6 months and 8 sessions in the second 6 months, while a control group received 2 group-based education classes in the first 6 months only. The researchers conducted assessments at baseline, 6 months, and 12 months.
Individuals were eligible for the trial if they were African American, were a patient of a community clinic affiliated with Cook County Healthcare System, had a clinical diagnosis of type 2 diabetes, and had a hemoglobin A1c level of 7% or greater. Of 1,403 initially screened for the trial, 603 were found to be eligible. Of these, 211 were randomized and enrolled: 106 to the treatment group and 105 to the control group. There was 94% follow-up at 6 and 12 months.
At baseline, the mean age of study participants was 55 years, 70% were female, 46% had a high school education or less, 60% had an annual income of less than $24,000, 65% were uninsured, and 39% had limited health literacy. Baseline food intake as reported by two 24-hour food recalls consisted of a diet high in saturated fat and low in fiber, with a moderate intake of carbohydrates and underconsumption of fruits, vegetables, and dairy products. The baseline level of daily physical activity as measured by accelerometry revealed sedentary activity that exceeded 7 hours per day, 3,614 steps per day, and only 14 minutes per day of moderate-level activity. Study enrollees had a baseline HbA1c level of 9% and a diabetes duration of 11 years; 45% used insulin, and 48% had poor medication adherence. Their mean body mass index was 35.6 kg/m2, and 91% had hypertension.
More than half of individuals in the intervention group attended each of the 20 group sessions, and 90% attended at least 1. At the same time, 68% of individuals in the control group attended both educational sessions. Dr. Lynch reported that compared with the control group, the intervention group had a significantly greater reduction in HbA1c at 6 months (–0.76 vs. –0.21, respectively; P = .026) but not at 12 months (–0.63 vs. –0.45; P = .47). In addition, a higher percentage of individuals in the treatment group had a 0.5% or more decline in HbA1c level at 6 months (63% vs. 42%, P = .005) but not at 12 months (53% vs. 51%, P = .89). The fact that the control group also had a reduction in HbA1c presented a conundrum for the researchers. “One possible explanation for the decrease in A1c in the control group is that medication adherence increased in this group, relative to the intervention group,” Dr. Lynch explained in a press release. “Additional research is needed to identify the most effective strategies to achieve sustained A1c control in African Americans with type 2 diabetes.”
No changes were observed in blood pressure, weight, or physical activity over the course of 12 months in either group.
Although LIFE lacked a third study arm that received usual care, one possible implication of the current findings “may be that diabetes education of any type may be helpful in improving glycemic control, especially in a population that does not normally receive any education,” she said. “Medication adherence may be an easier and more effective strategy to improve glycemic control in this population.”
LIFE was supported by grants from the National Institutes of Health. Dr. Lynch reported having no relevant financial disclosures.
NEW ORLEANS – Underserved African Americans with type 2 diabetes mellitus who participated in a year-long intensive self-management program did not experience sustained serum glucose control, compared with a control group receiving only two diabetes education classes.
“Relative to non-Hispanic whites, African Americans with type 2 diabetes experience more diabetes-related complications and higher rates of diabetes hospitalization,” lead study author Elizabeth B. Lynch, Ph.D., said at the annual scientific sessions of the American Diabetes Association. “These disparities are even greater for underserved disadvantaged African American populations.”
Dr. Lynch, a psychologist who directs the section of community health in the department of preventive medicine at Rush University Medical Center, Chicago, noted that several self-management interventions for diabetes have demonstrated efficacy at improving glucose control at 6 months. “However, there have not been any diabetes self-management interventions specifically targeting African Americans that have achieved sustained blood glucose control,” she said.
In a trial known as Lifestyle Intervention Through Food and Exercise (LIFE), the researchers examined the effect of a group-based intervention on glucose control at 12 months in a population of low-income African Americans. The intervention components consisted of cognitively tailored nutrition education taught by a registered dietitian, behavioral modification, social support, and peer support. “This education curriculum was based on a series of studies that were done using cognitive anthropological methods with low-income African Americans looking at beliefs and knowledge about the relationship between food and health,” Dr. Lynch said. “We used those studies to design an intervention with the aim of reducing cognitive load among participants when they’re learning new information about nutrition, so essentially making the information easier for people to understand.” Behavioral modification techniques included goal setting, self-monitoring, and problem solving. “We also had social support, and there was a peer supporter who was an individual from the community with type 2 diabetes who was assigned to each of the participants and called them on a regular basis to check in with them on their goals and encourage them,” she said.
The LIFE program consisted of 20 group sessions in the first 6 months and 8 sessions in the second 6 months, while a control group received 2 group-based education classes in the first 6 months only. The researchers conducted assessments at baseline, 6 months, and 12 months.
Individuals were eligible for the trial if they were African American, were a patient of a community clinic affiliated with Cook County Healthcare System, had a clinical diagnosis of type 2 diabetes, and had a hemoglobin A1c level of 7% or greater. Of 1,403 initially screened for the trial, 603 were found to be eligible. Of these, 211 were randomized and enrolled: 106 to the treatment group and 105 to the control group. There was 94% follow-up at 6 and 12 months.
At baseline, the mean age of study participants was 55 years, 70% were female, 46% had a high school education or less, 60% had an annual income of less than $24,000, 65% were uninsured, and 39% had limited health literacy. Baseline food intake as reported by two 24-hour food recalls consisted of a diet high in saturated fat and low in fiber, with a moderate intake of carbohydrates and underconsumption of fruits, vegetables, and dairy products. The baseline level of daily physical activity as measured by accelerometry revealed sedentary activity that exceeded 7 hours per day, 3,614 steps per day, and only 14 minutes per day of moderate-level activity. Study enrollees had a baseline HbA1c level of 9% and a diabetes duration of 11 years; 45% used insulin, and 48% had poor medication adherence. Their mean body mass index was 35.6 kg/m2, and 91% had hypertension.
More than half of individuals in the intervention group attended each of the 20 group sessions, and 90% attended at least 1. At the same time, 68% of individuals in the control group attended both educational sessions. Dr. Lynch reported that compared with the control group, the intervention group had a significantly greater reduction in HbA1c at 6 months (–0.76 vs. –0.21, respectively; P = .026) but not at 12 months (–0.63 vs. –0.45; P = .47). In addition, a higher percentage of individuals in the treatment group had a 0.5% or more decline in HbA1c level at 6 months (63% vs. 42%, P = .005) but not at 12 months (53% vs. 51%, P = .89). The fact that the control group also had a reduction in HbA1c presented a conundrum for the researchers. “One possible explanation for the decrease in A1c in the control group is that medication adherence increased in this group, relative to the intervention group,” Dr. Lynch explained in a press release. “Additional research is needed to identify the most effective strategies to achieve sustained A1c control in African Americans with type 2 diabetes.”
No changes were observed in blood pressure, weight, or physical activity over the course of 12 months in either group.
Although LIFE lacked a third study arm that received usual care, one possible implication of the current findings “may be that diabetes education of any type may be helpful in improving glycemic control, especially in a population that does not normally receive any education,” she said. “Medication adherence may be an easier and more effective strategy to improve glycemic control in this population.”
LIFE was supported by grants from the National Institutes of Health. Dr. Lynch reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Benefits of lifestyle intervention only brief in some patients with type 2 diabetes
NEW ORLEANS – Underserved African Americans with type 2 diabetes mellitus who participated in a year-long intensive self-management program did not experience sustained serum glucose control, compared with a control group receiving only two diabetes education classes.
“Relative to non-Hispanic whites, African Americans with type 2 diabetes experience more diabetes-related complications and higher rates of diabetes hospitalization,” lead study author Elizabeth B. Lynch, Ph.D., said at the annual scientific sessions of the American Diabetes Association. “These disparities are even greater for underserved disadvantaged African American populations.”
Dr. Lynch, a psychologist who directs the section of community health in the department of preventive medicine at Rush University Medical Center, Chicago, noted that several self-management interventions for diabetes have demonstrated efficacy at improving glucose control at 6 months. “However, there have not been any diabetes self-management interventions specifically targeting African Americans that have achieved sustained blood glucose control,” she said.
In a trial known as Lifestyle Intervention Through Food and Exercise (LIFE), the researchers examined the effect of a group-based intervention on glucose control at 12 months in a population of low-income African Americans. The intervention components consisted of cognitively tailored nutrition education taught by a registered dietitian, behavioral modification, social support, and peer support. “This education curriculum was based on a series of studies that were done using cognitive anthropological methods with low-income African Americans looking at beliefs and knowledge about the relationship between food and health,” Dr. Lynch said. “We used those studies to design an intervention with the aim of reducing cognitive load among participants when they’re learning new information about nutrition, so essentially making the information easier for people to understand.” Behavioral modification techniques included goal setting, self-monitoring, and problem solving. “We also had social support, and there was a peer supporter who was an individual from the community with type 2 diabetes who was assigned to each of the participants and called them on a regular basis to check in with them on their goals and encourage them,” she said.
The LIFE program consisted of 20 group sessions in the first 6 months and 8 sessions in the second 6 months, while a control group received 2 group-based education classes in the first 6 months only. The researchers conducted assessments at baseline, 6 months, and 12 months.
Individuals were eligible for the trial if they were African American, were a patient of a community clinic affiliated with Cook County Healthcare System, had a clinical diagnosis of type 2 diabetes, and had a hemoglobin A1c level of 7% or greater. Of 1,403 initially screened for the trial, 603 were found to be eligible. Of these, 211 were randomized and enrolled: 106 to the treatment group and 105 to the control group. There was 94% follow-up at 6 and 12 months.
At baseline, the mean age of study participants was 55 years, 70% were female, 46% had a high school education or less, 60% had an annual income of less than $24,000, 65% were uninsured, and 39% had limited health literacy. Baseline food intake as reported by two 24-hour food recalls consisted of a diet high in saturated fat and low in fiber, with a moderate intake of carbohydrates and underconsumption of fruits, vegetables, and dairy products. The baseline level of daily physical activity as measured by accelerometry revealed sedentary activity that exceeded 7 hours per day, 3,614 steps per day, and only 14 minutes per day of moderate-level activity. Study enrollees had a baseline HbA1c level of 9% and a diabetes duration of 11 years; 45% used insulin, and 48% had poor medication adherence. Their mean body mass index was 35.6 kg/m2, and 91% had hypertension.
More than half of individuals in the intervention group attended each of the 20 group sessions, and 90% attended at least 1. At the same time, 68% of individuals in the control group attended both educational sessions. Dr. Lynch reported that compared with the control group, the intervention group had a significantly greater reduction in HbA1c at 6 months (–0.76 vs. –0.21, respectively; P = .026) but not at 12 months (–0.63 vs. –0.45; P = .47). In addition, a higher percentage of individuals in the treatment group had a 0.5% or more decline in HbA1c level at 6 months (63% vs. 42%, P = .005) but not at 12 months (53% vs. 51%, P = .89). The fact that the control group also had a reduction in HbA1c presented a conundrum for the researchers. “One possible explanation for the decrease in A1c in the control group is that medication adherence increased in this group, relative to the intervention group,” Dr. Lynch explained in a press release. “Additional research is needed to identify the most effective strategies to achieve sustained A1c control in African Americans with type 2 diabetes.”
No changes were observed in blood pressure, weight, or physical activity over the course of 12 months in either group.
Although LIFE lacked a third study arm that received usual care, one possible implication of the current findings “may be that diabetes education of any type may be helpful in improving glycemic control, especially in a population that does not normally receive any education,” she said. “Medication adherence may be an easier and more effective strategy to improve glycemic control in this population.”
LIFE was supported by grants from the National Institutes of Health. Dr. Lynch reported having no relevant financial disclosures.
NEW ORLEANS – Underserved African Americans with type 2 diabetes mellitus who participated in a year-long intensive self-management program did not experience sustained serum glucose control, compared with a control group receiving only two diabetes education classes.
“Relative to non-Hispanic whites, African Americans with type 2 diabetes experience more diabetes-related complications and higher rates of diabetes hospitalization,” lead study author Elizabeth B. Lynch, Ph.D., said at the annual scientific sessions of the American Diabetes Association. “These disparities are even greater for underserved disadvantaged African American populations.”
Dr. Lynch, a psychologist who directs the section of community health in the department of preventive medicine at Rush University Medical Center, Chicago, noted that several self-management interventions for diabetes have demonstrated efficacy at improving glucose control at 6 months. “However, there have not been any diabetes self-management interventions specifically targeting African Americans that have achieved sustained blood glucose control,” she said.
In a trial known as Lifestyle Intervention Through Food and Exercise (LIFE), the researchers examined the effect of a group-based intervention on glucose control at 12 months in a population of low-income African Americans. The intervention components consisted of cognitively tailored nutrition education taught by a registered dietitian, behavioral modification, social support, and peer support. “This education curriculum was based on a series of studies that were done using cognitive anthropological methods with low-income African Americans looking at beliefs and knowledge about the relationship between food and health,” Dr. Lynch said. “We used those studies to design an intervention with the aim of reducing cognitive load among participants when they’re learning new information about nutrition, so essentially making the information easier for people to understand.” Behavioral modification techniques included goal setting, self-monitoring, and problem solving. “We also had social support, and there was a peer supporter who was an individual from the community with type 2 diabetes who was assigned to each of the participants and called them on a regular basis to check in with them on their goals and encourage them,” she said.
The LIFE program consisted of 20 group sessions in the first 6 months and 8 sessions in the second 6 months, while a control group received 2 group-based education classes in the first 6 months only. The researchers conducted assessments at baseline, 6 months, and 12 months.
Individuals were eligible for the trial if they were African American, were a patient of a community clinic affiliated with Cook County Healthcare System, had a clinical diagnosis of type 2 diabetes, and had a hemoglobin A1c level of 7% or greater. Of 1,403 initially screened for the trial, 603 were found to be eligible. Of these, 211 were randomized and enrolled: 106 to the treatment group and 105 to the control group. There was 94% follow-up at 6 and 12 months.
At baseline, the mean age of study participants was 55 years, 70% were female, 46% had a high school education or less, 60% had an annual income of less than $24,000, 65% were uninsured, and 39% had limited health literacy. Baseline food intake as reported by two 24-hour food recalls consisted of a diet high in saturated fat and low in fiber, with a moderate intake of carbohydrates and underconsumption of fruits, vegetables, and dairy products. The baseline level of daily physical activity as measured by accelerometry revealed sedentary activity that exceeded 7 hours per day, 3,614 steps per day, and only 14 minutes per day of moderate-level activity. Study enrollees had a baseline HbA1c level of 9% and a diabetes duration of 11 years; 45% used insulin, and 48% had poor medication adherence. Their mean body mass index was 35.6 kg/m2, and 91% had hypertension.
More than half of individuals in the intervention group attended each of the 20 group sessions, and 90% attended at least 1. At the same time, 68% of individuals in the control group attended both educational sessions. Dr. Lynch reported that compared with the control group, the intervention group had a significantly greater reduction in HbA1c at 6 months (–0.76 vs. –0.21, respectively; P = .026) but not at 12 months (–0.63 vs. –0.45; P = .47). In addition, a higher percentage of individuals in the treatment group had a 0.5% or more decline in HbA1c level at 6 months (63% vs. 42%, P = .005) but not at 12 months (53% vs. 51%, P = .89). The fact that the control group also had a reduction in HbA1c presented a conundrum for the researchers. “One possible explanation for the decrease in A1c in the control group is that medication adherence increased in this group, relative to the intervention group,” Dr. Lynch explained in a press release. “Additional research is needed to identify the most effective strategies to achieve sustained A1c control in African Americans with type 2 diabetes.”
No changes were observed in blood pressure, weight, or physical activity over the course of 12 months in either group.
Although LIFE lacked a third study arm that received usual care, one possible implication of the current findings “may be that diabetes education of any type may be helpful in improving glycemic control, especially in a population that does not normally receive any education,” she said. “Medication adherence may be an easier and more effective strategy to improve glycemic control in this population.”
LIFE was supported by grants from the National Institutes of Health. Dr. Lynch reported having no relevant financial disclosures.
NEW ORLEANS – Underserved African Americans with type 2 diabetes mellitus who participated in a year-long intensive self-management program did not experience sustained serum glucose control, compared with a control group receiving only two diabetes education classes.
“Relative to non-Hispanic whites, African Americans with type 2 diabetes experience more diabetes-related complications and higher rates of diabetes hospitalization,” lead study author Elizabeth B. Lynch, Ph.D., said at the annual scientific sessions of the American Diabetes Association. “These disparities are even greater for underserved disadvantaged African American populations.”
Dr. Lynch, a psychologist who directs the section of community health in the department of preventive medicine at Rush University Medical Center, Chicago, noted that several self-management interventions for diabetes have demonstrated efficacy at improving glucose control at 6 months. “However, there have not been any diabetes self-management interventions specifically targeting African Americans that have achieved sustained blood glucose control,” she said.
In a trial known as Lifestyle Intervention Through Food and Exercise (LIFE), the researchers examined the effect of a group-based intervention on glucose control at 12 months in a population of low-income African Americans. The intervention components consisted of cognitively tailored nutrition education taught by a registered dietitian, behavioral modification, social support, and peer support. “This education curriculum was based on a series of studies that were done using cognitive anthropological methods with low-income African Americans looking at beliefs and knowledge about the relationship between food and health,” Dr. Lynch said. “We used those studies to design an intervention with the aim of reducing cognitive load among participants when they’re learning new information about nutrition, so essentially making the information easier for people to understand.” Behavioral modification techniques included goal setting, self-monitoring, and problem solving. “We also had social support, and there was a peer supporter who was an individual from the community with type 2 diabetes who was assigned to each of the participants and called them on a regular basis to check in with them on their goals and encourage them,” she said.
The LIFE program consisted of 20 group sessions in the first 6 months and 8 sessions in the second 6 months, while a control group received 2 group-based education classes in the first 6 months only. The researchers conducted assessments at baseline, 6 months, and 12 months.
Individuals were eligible for the trial if they were African American, were a patient of a community clinic affiliated with Cook County Healthcare System, had a clinical diagnosis of type 2 diabetes, and had a hemoglobin A1c level of 7% or greater. Of 1,403 initially screened for the trial, 603 were found to be eligible. Of these, 211 were randomized and enrolled: 106 to the treatment group and 105 to the control group. There was 94% follow-up at 6 and 12 months.
At baseline, the mean age of study participants was 55 years, 70% were female, 46% had a high school education or less, 60% had an annual income of less than $24,000, 65% were uninsured, and 39% had limited health literacy. Baseline food intake as reported by two 24-hour food recalls consisted of a diet high in saturated fat and low in fiber, with a moderate intake of carbohydrates and underconsumption of fruits, vegetables, and dairy products. The baseline level of daily physical activity as measured by accelerometry revealed sedentary activity that exceeded 7 hours per day, 3,614 steps per day, and only 14 minutes per day of moderate-level activity. Study enrollees had a baseline HbA1c level of 9% and a diabetes duration of 11 years; 45% used insulin, and 48% had poor medication adherence. Their mean body mass index was 35.6 kg/m2, and 91% had hypertension.
More than half of individuals in the intervention group attended each of the 20 group sessions, and 90% attended at least 1. At the same time, 68% of individuals in the control group attended both educational sessions. Dr. Lynch reported that compared with the control group, the intervention group had a significantly greater reduction in HbA1c at 6 months (–0.76 vs. –0.21, respectively; P = .026) but not at 12 months (–0.63 vs. –0.45; P = .47). In addition, a higher percentage of individuals in the treatment group had a 0.5% or more decline in HbA1c level at 6 months (63% vs. 42%, P = .005) but not at 12 months (53% vs. 51%, P = .89). The fact that the control group also had a reduction in HbA1c presented a conundrum for the researchers. “One possible explanation for the decrease in A1c in the control group is that medication adherence increased in this group, relative to the intervention group,” Dr. Lynch explained in a press release. “Additional research is needed to identify the most effective strategies to achieve sustained A1c control in African Americans with type 2 diabetes.”
No changes were observed in blood pressure, weight, or physical activity over the course of 12 months in either group.
Although LIFE lacked a third study arm that received usual care, one possible implication of the current findings “may be that diabetes education of any type may be helpful in improving glycemic control, especially in a population that does not normally receive any education,” she said. “Medication adherence may be an easier and more effective strategy to improve glycemic control in this population.”
LIFE was supported by grants from the National Institutes of Health. Dr. Lynch reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: A lifestyle intervention led to a pronounced reduction in hemoglobin A1c level after 6 months but not after 1 year for African Americans with type 2 diabetes.
Major finding: Compared with patients in the control group, those in the intensive intervention group had a significantly more sizable reduction in HbA1c level at 6 months (P = .026) but not at 12 months (P = .47).
Data source: A trial of 211 patients with type 2 diabetes who were randomized to either a year-long diabetes self-management training program or to two diabetes education classes.
Disclosures: LIFE was supported by grants from the National Institutes of Health. Dr. Lynch reported having no relevant financial disclosures.
Long-term Metformin Use Protective Against Neurodegenerative Disease
NEW ORLEANS – The use of metformin for at least 2 years had a protective effect on the incidence of neurodegenerative disease among elderly veterans, according to results from a large analysis of Veterans Affairs data.
At the annual scientific sessions of the American Diabetes Association, lead study author Qian Shi said that according to the current medical literature, diabetes increases one’s risk of Alzheimer’s disease (by 1.46- to 1.56-fold), all types of dementia (by 1.51- to 1.73-fold), vascular dementia (by 2.27- to 2.48-fold), and mild cognitive impairment (by 1.21-fold). “Metformin can cross the blood-brain barrier having specific effects on the central nervous system. But the exact mechanism and sites of its action remain unknown, and there are conflicting results,” said Ms. Shi, a PhD candidate in the department of global health policy and management at Tulane University School of Public Health and Tropical Medicine, New Orleans.
In an effort to examine the impact of receiving metformin treatment on the incidence of neurodegenerative disease and the association between length of metformin exposure and the risk of neurodegenerative diseases, the researchers used the Veterans Affairs database from 2004 to 2010 to study 6,046 patients who were at least 50 years of age with type 2 diabetes mellitus and were receiving long-term insulin treatment.
The length of metformin exposure was categorized by exposure years over the study period from baseline to the time of the first diagnosis of neurodegenerative disease, which included Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, dementia, and cognitive impairment. The five categories of metformin exposure time were no metformin treatment, less than 1 year, 1-2 years, 2-4 years, and 4 years or more. The mean age of patients was 63 years, 98% were male, and they were followed for a median of 5.3 years.
Of the 6,046 patients, 433 developed neurodegenerative disease during the study period, primarily dementia (334 cases). Other diagnoses included Parkinson’s disease (100 cases), Alzheimer’s disease (71 cases), and cognitive impairment (19 cases).
Ms. Shi reported that the adjusted incidence rates of neurodegenerative disease by cohort were 2.08 cases per 100 person-years for those who received no metformin treatment, 2.47 per 100 person-years for those treated with metformin for less than 1 year, 1.61 per 100 person-years for those treated 1-2 years, 1.30 per 100 person-years for those treated 2-4 years, and 0.49 person-years for those treated 4 years or more. The longer patients took metformin, the less likely they were to develop neurodegenerative disease, she said.
When comparing patients who received metformin treatment with those who did not on Cox regression analysis, the hazard ratio was 0.686 for neurodegenerative disease, 0.644 for dementia, and 0.611 for Parkinson’s disease. The risk reduction was not as robust for those with Alzheimer’s disease and cognitive impairment, most likely because of the limited number of cases, Ms. Shi said. Renal disease had no significant association with the risk of neurodegenerative disease, and it was balanced across metformin exposure groups.
She acknowledged certain limitations of the study, including its retrospective design, the high proportion of males, and the fact that data on diabetes duration and serum vitamin B level were not available.
The researchers reported having no relevant financial disclosures.
NEW ORLEANS – The use of metformin for at least 2 years had a protective effect on the incidence of neurodegenerative disease among elderly veterans, according to results from a large analysis of Veterans Affairs data.
At the annual scientific sessions of the American Diabetes Association, lead study author Qian Shi said that according to the current medical literature, diabetes increases one’s risk of Alzheimer’s disease (by 1.46- to 1.56-fold), all types of dementia (by 1.51- to 1.73-fold), vascular dementia (by 2.27- to 2.48-fold), and mild cognitive impairment (by 1.21-fold). “Metformin can cross the blood-brain barrier having specific effects on the central nervous system. But the exact mechanism and sites of its action remain unknown, and there are conflicting results,” said Ms. Shi, a PhD candidate in the department of global health policy and management at Tulane University School of Public Health and Tropical Medicine, New Orleans.
In an effort to examine the impact of receiving metformin treatment on the incidence of neurodegenerative disease and the association between length of metformin exposure and the risk of neurodegenerative diseases, the researchers used the Veterans Affairs database from 2004 to 2010 to study 6,046 patients who were at least 50 years of age with type 2 diabetes mellitus and were receiving long-term insulin treatment.
The length of metformin exposure was categorized by exposure years over the study period from baseline to the time of the first diagnosis of neurodegenerative disease, which included Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, dementia, and cognitive impairment. The five categories of metformin exposure time were no metformin treatment, less than 1 year, 1-2 years, 2-4 years, and 4 years or more. The mean age of patients was 63 years, 98% were male, and they were followed for a median of 5.3 years.
Of the 6,046 patients, 433 developed neurodegenerative disease during the study period, primarily dementia (334 cases). Other diagnoses included Parkinson’s disease (100 cases), Alzheimer’s disease (71 cases), and cognitive impairment (19 cases).
Ms. Shi reported that the adjusted incidence rates of neurodegenerative disease by cohort were 2.08 cases per 100 person-years for those who received no metformin treatment, 2.47 per 100 person-years for those treated with metformin for less than 1 year, 1.61 per 100 person-years for those treated 1-2 years, 1.30 per 100 person-years for those treated 2-4 years, and 0.49 person-years for those treated 4 years or more. The longer patients took metformin, the less likely they were to develop neurodegenerative disease, she said.
When comparing patients who received metformin treatment with those who did not on Cox regression analysis, the hazard ratio was 0.686 for neurodegenerative disease, 0.644 for dementia, and 0.611 for Parkinson’s disease. The risk reduction was not as robust for those with Alzheimer’s disease and cognitive impairment, most likely because of the limited number of cases, Ms. Shi said. Renal disease had no significant association with the risk of neurodegenerative disease, and it was balanced across metformin exposure groups.
She acknowledged certain limitations of the study, including its retrospective design, the high proportion of males, and the fact that data on diabetes duration and serum vitamin B level were not available.
The researchers reported having no relevant financial disclosures.
NEW ORLEANS – The use of metformin for at least 2 years had a protective effect on the incidence of neurodegenerative disease among elderly veterans, according to results from a large analysis of Veterans Affairs data.
At the annual scientific sessions of the American Diabetes Association, lead study author Qian Shi said that according to the current medical literature, diabetes increases one’s risk of Alzheimer’s disease (by 1.46- to 1.56-fold), all types of dementia (by 1.51- to 1.73-fold), vascular dementia (by 2.27- to 2.48-fold), and mild cognitive impairment (by 1.21-fold). “Metformin can cross the blood-brain barrier having specific effects on the central nervous system. But the exact mechanism and sites of its action remain unknown, and there are conflicting results,” said Ms. Shi, a PhD candidate in the department of global health policy and management at Tulane University School of Public Health and Tropical Medicine, New Orleans.
In an effort to examine the impact of receiving metformin treatment on the incidence of neurodegenerative disease and the association between length of metformin exposure and the risk of neurodegenerative diseases, the researchers used the Veterans Affairs database from 2004 to 2010 to study 6,046 patients who were at least 50 years of age with type 2 diabetes mellitus and were receiving long-term insulin treatment.
The length of metformin exposure was categorized by exposure years over the study period from baseline to the time of the first diagnosis of neurodegenerative disease, which included Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, dementia, and cognitive impairment. The five categories of metformin exposure time were no metformin treatment, less than 1 year, 1-2 years, 2-4 years, and 4 years or more. The mean age of patients was 63 years, 98% were male, and they were followed for a median of 5.3 years.
Of the 6,046 patients, 433 developed neurodegenerative disease during the study period, primarily dementia (334 cases). Other diagnoses included Parkinson’s disease (100 cases), Alzheimer’s disease (71 cases), and cognitive impairment (19 cases).
Ms. Shi reported that the adjusted incidence rates of neurodegenerative disease by cohort were 2.08 cases per 100 person-years for those who received no metformin treatment, 2.47 per 100 person-years for those treated with metformin for less than 1 year, 1.61 per 100 person-years for those treated 1-2 years, 1.30 per 100 person-years for those treated 2-4 years, and 0.49 person-years for those treated 4 years or more. The longer patients took metformin, the less likely they were to develop neurodegenerative disease, she said.
When comparing patients who received metformin treatment with those who did not on Cox regression analysis, the hazard ratio was 0.686 for neurodegenerative disease, 0.644 for dementia, and 0.611 for Parkinson’s disease. The risk reduction was not as robust for those with Alzheimer’s disease and cognitive impairment, most likely because of the limited number of cases, Ms. Shi said. Renal disease had no significant association with the risk of neurodegenerative disease, and it was balanced across metformin exposure groups.
She acknowledged certain limitations of the study, including its retrospective design, the high proportion of males, and the fact that data on diabetes duration and serum vitamin B level were not available.
The researchers reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Long-term metformin use protective against neurodegenerative disease
NEW ORLEANS – The use of metformin for at least 2 years had a protective effect on the incidence of neurodegenerative disease among elderly veterans, according to results from a large analysis of Veterans Affairs data.
At the annual scientific sessions of the American Diabetes Association, lead study author Qian Shi said that according to the current medical literature, diabetes increases one’s risk of Alzheimer’s disease (by 1.46- to 1.56-fold), all types of dementia (by 1.51- to 1.73-fold), vascular dementia (by 2.27- to 2.48-fold), and mild cognitive impairment (by 1.21-fold). “Metformin can cross the blood-brain barrier having specific effects on the central nervous system. But the exact mechanism and sites of its action remain unknown, and there are conflicting results,” said Ms. Shi, a PhD candidate in the department of global health policy and management at Tulane University School of Public Health and Tropical Medicine, New Orleans.
In an effort to examine the impact of receiving metformin treatment on the incidence of neurodegenerative disease and the association between length of metformin exposure and the risk of neurodegenerative diseases, the researchers used the Veterans Affairs database from 2004 to 2010 to study 6,046 patients who were at least 50 years of age with type 2 diabetes mellitus and were receiving long-term insulin treatment.
The length of metformin exposure was categorized by exposure years over the study period from baseline to the time of the first diagnosis of neurodegenerative disease, which included Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, dementia, and cognitive impairment. The five categories of metformin exposure time were no metformin treatment, less than 1 year, 1-2 years, 2-4 years, and 4 years or more. The mean age of patients was 63 years, 98% were male, and they were followed for a median of 5.3 years.
Of the 6,046 patients, 433 developed neurodegenerative disease during the study period, primarily dementia (334 cases). Other diagnoses included Parkinson’s disease (100 cases), Alzheimer’s disease (71 cases), and cognitive impairment (19 cases).
Ms. Shi reported that the adjusted incidence rates of neurodegenerative disease by cohort were 2.08 cases per 100 person-years for those who received no metformin treatment, 2.47 per 100 person-years for those treated with metformin for less than 1 year, 1.61 per 100 person-years for those treated 1-2 years, 1.30 per 100 person-years for those treated 2-4 years, and 0.49 person-years for those treated 4 years or more. The longer patients took metformin, the less likely they were to develop neurodegenerative disease, she said.
When comparing patients who received metformin treatment with those who did not on Cox regression analysis, the hazard ratio was 0.686 for neurodegenerative disease, 0.644 for dementia, and 0.611 for Parkinson’s disease. The risk reduction was not as robust for those with Alzheimer’s disease and cognitive impairment, most likely because of the limited number of cases, Ms. Shi said. Renal disease had no significant association with the risk of neurodegenerative disease, and it was balanced across metformin exposure groups.
She acknowledged certain limitations of the study, including its retrospective design, the high proportion of males, and the fact that data on diabetes duration and serum vitamin B level were not available.
The researchers reported having no relevant financial disclosures.
NEW ORLEANS – The use of metformin for at least 2 years had a protective effect on the incidence of neurodegenerative disease among elderly veterans, according to results from a large analysis of Veterans Affairs data.
At the annual scientific sessions of the American Diabetes Association, lead study author Qian Shi said that according to the current medical literature, diabetes increases one’s risk of Alzheimer’s disease (by 1.46- to 1.56-fold), all types of dementia (by 1.51- to 1.73-fold), vascular dementia (by 2.27- to 2.48-fold), and mild cognitive impairment (by 1.21-fold). “Metformin can cross the blood-brain barrier having specific effects on the central nervous system. But the exact mechanism and sites of its action remain unknown, and there are conflicting results,” said Ms. Shi, a PhD candidate in the department of global health policy and management at Tulane University School of Public Health and Tropical Medicine, New Orleans.
In an effort to examine the impact of receiving metformin treatment on the incidence of neurodegenerative disease and the association between length of metformin exposure and the risk of neurodegenerative diseases, the researchers used the Veterans Affairs database from 2004 to 2010 to study 6,046 patients who were at least 50 years of age with type 2 diabetes mellitus and were receiving long-term insulin treatment.
The length of metformin exposure was categorized by exposure years over the study period from baseline to the time of the first diagnosis of neurodegenerative disease, which included Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, dementia, and cognitive impairment. The five categories of metformin exposure time were no metformin treatment, less than 1 year, 1-2 years, 2-4 years, and 4 years or more. The mean age of patients was 63 years, 98% were male, and they were followed for a median of 5.3 years.
Of the 6,046 patients, 433 developed neurodegenerative disease during the study period, primarily dementia (334 cases). Other diagnoses included Parkinson’s disease (100 cases), Alzheimer’s disease (71 cases), and cognitive impairment (19 cases).
Ms. Shi reported that the adjusted incidence rates of neurodegenerative disease by cohort were 2.08 cases per 100 person-years for those who received no metformin treatment, 2.47 per 100 person-years for those treated with metformin for less than 1 year, 1.61 per 100 person-years for those treated 1-2 years, 1.30 per 100 person-years for those treated 2-4 years, and 0.49 person-years for those treated 4 years or more. The longer patients took metformin, the less likely they were to develop neurodegenerative disease, she said.
When comparing patients who received metformin treatment with those who did not on Cox regression analysis, the hazard ratio was 0.686 for neurodegenerative disease, 0.644 for dementia, and 0.611 for Parkinson’s disease. The risk reduction was not as robust for those with Alzheimer’s disease and cognitive impairment, most likely because of the limited number of cases, Ms. Shi said. Renal disease had no significant association with the risk of neurodegenerative disease, and it was balanced across metformin exposure groups.
She acknowledged certain limitations of the study, including its retrospective design, the high proportion of males, and the fact that data on diabetes duration and serum vitamin B level were not available.
The researchers reported having no relevant financial disclosures.
NEW ORLEANS – The use of metformin for at least 2 years had a protective effect on the incidence of neurodegenerative disease among elderly veterans, according to results from a large analysis of Veterans Affairs data.
At the annual scientific sessions of the American Diabetes Association, lead study author Qian Shi said that according to the current medical literature, diabetes increases one’s risk of Alzheimer’s disease (by 1.46- to 1.56-fold), all types of dementia (by 1.51- to 1.73-fold), vascular dementia (by 2.27- to 2.48-fold), and mild cognitive impairment (by 1.21-fold). “Metformin can cross the blood-brain barrier having specific effects on the central nervous system. But the exact mechanism and sites of its action remain unknown, and there are conflicting results,” said Ms. Shi, a PhD candidate in the department of global health policy and management at Tulane University School of Public Health and Tropical Medicine, New Orleans.
In an effort to examine the impact of receiving metformin treatment on the incidence of neurodegenerative disease and the association between length of metformin exposure and the risk of neurodegenerative diseases, the researchers used the Veterans Affairs database from 2004 to 2010 to study 6,046 patients who were at least 50 years of age with type 2 diabetes mellitus and were receiving long-term insulin treatment.
The length of metformin exposure was categorized by exposure years over the study period from baseline to the time of the first diagnosis of neurodegenerative disease, which included Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, dementia, and cognitive impairment. The five categories of metformin exposure time were no metformin treatment, less than 1 year, 1-2 years, 2-4 years, and 4 years or more. The mean age of patients was 63 years, 98% were male, and they were followed for a median of 5.3 years.
Of the 6,046 patients, 433 developed neurodegenerative disease during the study period, primarily dementia (334 cases). Other diagnoses included Parkinson’s disease (100 cases), Alzheimer’s disease (71 cases), and cognitive impairment (19 cases).
Ms. Shi reported that the adjusted incidence rates of neurodegenerative disease by cohort were 2.08 cases per 100 person-years for those who received no metformin treatment, 2.47 per 100 person-years for those treated with metformin for less than 1 year, 1.61 per 100 person-years for those treated 1-2 years, 1.30 per 100 person-years for those treated 2-4 years, and 0.49 person-years for those treated 4 years or more. The longer patients took metformin, the less likely they were to develop neurodegenerative disease, she said.
When comparing patients who received metformin treatment with those who did not on Cox regression analysis, the hazard ratio was 0.686 for neurodegenerative disease, 0.644 for dementia, and 0.611 for Parkinson’s disease. The risk reduction was not as robust for those with Alzheimer’s disease and cognitive impairment, most likely because of the limited number of cases, Ms. Shi said. Renal disease had no significant association with the risk of neurodegenerative disease, and it was balanced across metformin exposure groups.
She acknowledged certain limitations of the study, including its retrospective design, the high proportion of males, and the fact that data on diabetes duration and serum vitamin B level were not available.
The researchers reported having no relevant financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Use of metformin for at least 2 years was protective against the onset of neurodegenerative disease.
Major finding: The adjusted incidence rates of neurodegenerative disease ranged from 2.47 cases per 100 person-years for those treated with metformin for less than 1 year to 0.49 cases per 100 person-years for those treated for 4 years or longer.
Data source: A longitudinal study of 6,046 patients at least 50 years of age with type 2 diabetes mellitus who were receiving long-term insulin treatment.
Disclosures: The researchers reported having no relevant financial disclosures.
Artificial pancreas can improve inpatient glycemic control in type 2 diabetes
NEW ORLEANS – Having people with type 2 diabetes mellitus use an artificial pancreas during hospitalization has the potential to improve control of their glycemia when compared with conventional insulin therapy, based on the results of a small study of inpatients in the United Kingdom.
“This is the first study to show that automated subcutaneous closed-loop insulin delivery without meal-time insulin is feasible and safe in patients with insulin-treated type 2 diabetes in the general wards,” Dr. Hood Thabit of the University of Cambridge (England) reported at the ADA annual scientific sessions. “Closed-loop [delivery] increased time in target, with reduced glucose variability and reduced time spent hyperglycemic without actually increasing time spent hypoglycemic,” he said.
The study involved 40 general ward inpatients evenly assigned to the closed-loop system and conventional insulin therapy for 72 hours.
Dr. Thabit said hyperglycemia in hospital patients is a common problem that’s poorly managed. “There’s an unmet need for an effective and safe glucose control, specifically in the underserved and understudied population of type 2 diabetes in the general wards,” he said. The use of the closed-loop system in inpatients with type 2 diabetes “remains untested until now,” Dr. Thabit added.
The 20 patients randomized to the closed-loop system spent an average of 61% of the whole study period within the sensor glucose target vs. 38% of those on conventional insulin therapy. The closed-loop patients also used comparable insulin daily on average: 62.6 U (±36.3 U) vs. 66.0 U (±39.6 U), Dr. Thabit said.
He noted that those on the closed-loop system did not have to announce meals to the control algorithm, or give any meal-time insulin – “we didn’t want to trouble our nurses with this, due to the increasing workload that health care professionals in the hospital currently face,” he said – and showed “significantly improved” nighttime control of glucose while “simultaneously reducing the risk of nocturnal hypoglycemia”. “The closed loop may potentially be an effective and safe tool to manage hospital inpatient hyperglycemia in this particularly underserved population of patients whilst easing the burden of health care professionals in hospital,” he said.
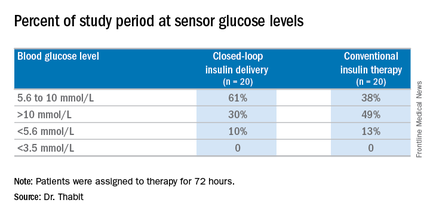
The cost is not insignificant. The pump and sensor devices together with related consumables can cost up to £6,000 (about $8,600), but he did note the artificial pancreas device itself is reusable. The cost of the automated closed-loop glucose control system can also potentially be offset by the reduced time of health care professionals spent managing inpatient hyperglycemia safely. The study investigators are in the process of planning a larger trial, Dr. Thabit said.
Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
NEW ORLEANS – Having people with type 2 diabetes mellitus use an artificial pancreas during hospitalization has the potential to improve control of their glycemia when compared with conventional insulin therapy, based on the results of a small study of inpatients in the United Kingdom.
“This is the first study to show that automated subcutaneous closed-loop insulin delivery without meal-time insulin is feasible and safe in patients with insulin-treated type 2 diabetes in the general wards,” Dr. Hood Thabit of the University of Cambridge (England) reported at the ADA annual scientific sessions. “Closed-loop [delivery] increased time in target, with reduced glucose variability and reduced time spent hyperglycemic without actually increasing time spent hypoglycemic,” he said.
The study involved 40 general ward inpatients evenly assigned to the closed-loop system and conventional insulin therapy for 72 hours.
Dr. Thabit said hyperglycemia in hospital patients is a common problem that’s poorly managed. “There’s an unmet need for an effective and safe glucose control, specifically in the underserved and understudied population of type 2 diabetes in the general wards,” he said. The use of the closed-loop system in inpatients with type 2 diabetes “remains untested until now,” Dr. Thabit added.
The 20 patients randomized to the closed-loop system spent an average of 61% of the whole study period within the sensor glucose target vs. 38% of those on conventional insulin therapy. The closed-loop patients also used comparable insulin daily on average: 62.6 U (±36.3 U) vs. 66.0 U (±39.6 U), Dr. Thabit said.
He noted that those on the closed-loop system did not have to announce meals to the control algorithm, or give any meal-time insulin – “we didn’t want to trouble our nurses with this, due to the increasing workload that health care professionals in the hospital currently face,” he said – and showed “significantly improved” nighttime control of glucose while “simultaneously reducing the risk of nocturnal hypoglycemia”. “The closed loop may potentially be an effective and safe tool to manage hospital inpatient hyperglycemia in this particularly underserved population of patients whilst easing the burden of health care professionals in hospital,” he said.

The cost is not insignificant. The pump and sensor devices together with related consumables can cost up to £6,000 (about $8,600), but he did note the artificial pancreas device itself is reusable. The cost of the automated closed-loop glucose control system can also potentially be offset by the reduced time of health care professionals spent managing inpatient hyperglycemia safely. The study investigators are in the process of planning a larger trial, Dr. Thabit said.
Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
NEW ORLEANS – Having people with type 2 diabetes mellitus use an artificial pancreas during hospitalization has the potential to improve control of their glycemia when compared with conventional insulin therapy, based on the results of a small study of inpatients in the United Kingdom.
“This is the first study to show that automated subcutaneous closed-loop insulin delivery without meal-time insulin is feasible and safe in patients with insulin-treated type 2 diabetes in the general wards,” Dr. Hood Thabit of the University of Cambridge (England) reported at the ADA annual scientific sessions. “Closed-loop [delivery] increased time in target, with reduced glucose variability and reduced time spent hyperglycemic without actually increasing time spent hypoglycemic,” he said.
The study involved 40 general ward inpatients evenly assigned to the closed-loop system and conventional insulin therapy for 72 hours.
Dr. Thabit said hyperglycemia in hospital patients is a common problem that’s poorly managed. “There’s an unmet need for an effective and safe glucose control, specifically in the underserved and understudied population of type 2 diabetes in the general wards,” he said. The use of the closed-loop system in inpatients with type 2 diabetes “remains untested until now,” Dr. Thabit added.
The 20 patients randomized to the closed-loop system spent an average of 61% of the whole study period within the sensor glucose target vs. 38% of those on conventional insulin therapy. The closed-loop patients also used comparable insulin daily on average: 62.6 U (±36.3 U) vs. 66.0 U (±39.6 U), Dr. Thabit said.
He noted that those on the closed-loop system did not have to announce meals to the control algorithm, or give any meal-time insulin – “we didn’t want to trouble our nurses with this, due to the increasing workload that health care professionals in the hospital currently face,” he said – and showed “significantly improved” nighttime control of glucose while “simultaneously reducing the risk of nocturnal hypoglycemia”. “The closed loop may potentially be an effective and safe tool to manage hospital inpatient hyperglycemia in this particularly underserved population of patients whilst easing the burden of health care professionals in hospital,” he said.

The cost is not insignificant. The pump and sensor devices together with related consumables can cost up to £6,000 (about $8,600), but he did note the artificial pancreas device itself is reusable. The cost of the automated closed-loop glucose control system can also potentially be offset by the reduced time of health care professionals spent managing inpatient hyperglycemia safely. The study investigators are in the process of planning a larger trial, Dr. Thabit said.
Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point:Hospitalized patients in the general ward with type 2 diabetes mellitus can maintain better glycemic control by using an artificial pancreas than by taking conventional therapy.
Major finding: Patients on the closed-loop system spent an average of 61% of the study period within the sensor glucose target vs. 38% for those on conventional insulin therapy.
Data source: A single-center study of 40 patients randomized to wear the artificial pancreas or take conventional therapy.
Disclosures: Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
Study Plots Long-term Financial Impact of Diabetes
NEW ORLEANs – Between 2001 and 2013, a cohort of persons with newly diagnosed diabetes spent $3,489 more on average on medical costs in the first year after their diagnosis than they had in the year preceding it. Comparied with their diabetes-free counterparts, patients spent $6,424 more on average in the first year following diagnosis, results from a large data analysis found. In addition, during the period of 9 years before and 9 years after the diagnosis of diabetes, per capita total medical expenditures for the diabetes cohort increased annually by $382, compared with an increase of $177 for the cohort who did not have the condition.
“We know that people with diagnosed diabetes spend more on medical care than those without diagnosed diabetes because of the additional costs associated with managing diabetes and diabetes complications,” lead study author Sundar S. Shrestha, Ph.D., said in an interview in advance of the annual scientific sessions of the American Diabetes Association. “However, little information is available on how much more those with diagnosed diabetes spend after diagnosis than before diagnosis. Also, little information is available on how much more those with diagnosed diabetes spend on medical care, compared with those without diagnosed diabetes.”
Dr. Shrestha, a health economist at the Centers for Disease Control and Prevention, and his associates, analyzed the MarketScan Commercial Claims and Encounters database for the period 2001-2013 to compare the trajectory of medical expenditures (in 2013 U.S. dollars) among a diabetes cohort 9 years before and 9 years after diagnosis with a matched cohort of individuals without diabetes for U.S. adults aged 25-64 years. They defined diabetes incidence as having two or more outpatient claims 30 days apart or at least one inpatient claim with diabetes codes during the case identification period that spanned up to 2 calendar years after the first diabetes claim with at least 2 previous years without any diabetes claim.
Dr. Shrestha reported on 415,728 patient-years of data. The diabetes cohort spent an additional $51,000 on average during the 9 years before and after diagnosis, compared with their counterparts who had no diabetes diagnosis. Overall medical expenditures after diagnosis were also 2.3 times higher than before diagnosis.
“Although the additional expenditure after diagnosis was much higher for people with diagnosed diabetes, after the first year of diagnosis, it did not increase with the duration of diabetes during the study period,” Dr. Shrestha said. “However, the composition of expenditures differed, increasing for prescription drugs and decreasing for inpatient care.” He noted that the estimated excess medical expenditures described in the study indicate that “identifying those at high risk of diabetes, delaying/preventing development of diabetes through a lifestyle change program or other intervention, and managing diabetes effectively could reduce health care costs substantially.”
He acknowledged certain limitations of the study, including the fact that the data were drawn from a privately insured adult population and therefore may not be generalizable to the entire population of the United States. “Additionally, the data do not allow us to distinguish between type 1 and type 2 diabetes,” Dr. Shrestha said. He reported having no financial disclosures.
NEW ORLEANs – Between 2001 and 2013, a cohort of persons with newly diagnosed diabetes spent $3,489 more on average on medical costs in the first year after their diagnosis than they had in the year preceding it. Comparied with their diabetes-free counterparts, patients spent $6,424 more on average in the first year following diagnosis, results from a large data analysis found. In addition, during the period of 9 years before and 9 years after the diagnosis of diabetes, per capita total medical expenditures for the diabetes cohort increased annually by $382, compared with an increase of $177 for the cohort who did not have the condition.
“We know that people with diagnosed diabetes spend more on medical care than those without diagnosed diabetes because of the additional costs associated with managing diabetes and diabetes complications,” lead study author Sundar S. Shrestha, Ph.D., said in an interview in advance of the annual scientific sessions of the American Diabetes Association. “However, little information is available on how much more those with diagnosed diabetes spend after diagnosis than before diagnosis. Also, little information is available on how much more those with diagnosed diabetes spend on medical care, compared with those without diagnosed diabetes.”
Dr. Shrestha, a health economist at the Centers for Disease Control and Prevention, and his associates, analyzed the MarketScan Commercial Claims and Encounters database for the period 2001-2013 to compare the trajectory of medical expenditures (in 2013 U.S. dollars) among a diabetes cohort 9 years before and 9 years after diagnosis with a matched cohort of individuals without diabetes for U.S. adults aged 25-64 years. They defined diabetes incidence as having two or more outpatient claims 30 days apart or at least one inpatient claim with diabetes codes during the case identification period that spanned up to 2 calendar years after the first diabetes claim with at least 2 previous years without any diabetes claim.
Dr. Shrestha reported on 415,728 patient-years of data. The diabetes cohort spent an additional $51,000 on average during the 9 years before and after diagnosis, compared with their counterparts who had no diabetes diagnosis. Overall medical expenditures after diagnosis were also 2.3 times higher than before diagnosis.
“Although the additional expenditure after diagnosis was much higher for people with diagnosed diabetes, after the first year of diagnosis, it did not increase with the duration of diabetes during the study period,” Dr. Shrestha said. “However, the composition of expenditures differed, increasing for prescription drugs and decreasing for inpatient care.” He noted that the estimated excess medical expenditures described in the study indicate that “identifying those at high risk of diabetes, delaying/preventing development of diabetes through a lifestyle change program or other intervention, and managing diabetes effectively could reduce health care costs substantially.”
He acknowledged certain limitations of the study, including the fact that the data were drawn from a privately insured adult population and therefore may not be generalizable to the entire population of the United States. “Additionally, the data do not allow us to distinguish between type 1 and type 2 diabetes,” Dr. Shrestha said. He reported having no financial disclosures.
NEW ORLEANs – Between 2001 and 2013, a cohort of persons with newly diagnosed diabetes spent $3,489 more on average on medical costs in the first year after their diagnosis than they had in the year preceding it. Comparied with their diabetes-free counterparts, patients spent $6,424 more on average in the first year following diagnosis, results from a large data analysis found. In addition, during the period of 9 years before and 9 years after the diagnosis of diabetes, per capita total medical expenditures for the diabetes cohort increased annually by $382, compared with an increase of $177 for the cohort who did not have the condition.
“We know that people with diagnosed diabetes spend more on medical care than those without diagnosed diabetes because of the additional costs associated with managing diabetes and diabetes complications,” lead study author Sundar S. Shrestha, Ph.D., said in an interview in advance of the annual scientific sessions of the American Diabetes Association. “However, little information is available on how much more those with diagnosed diabetes spend after diagnosis than before diagnosis. Also, little information is available on how much more those with diagnosed diabetes spend on medical care, compared with those without diagnosed diabetes.”
Dr. Shrestha, a health economist at the Centers for Disease Control and Prevention, and his associates, analyzed the MarketScan Commercial Claims and Encounters database for the period 2001-2013 to compare the trajectory of medical expenditures (in 2013 U.S. dollars) among a diabetes cohort 9 years before and 9 years after diagnosis with a matched cohort of individuals without diabetes for U.S. adults aged 25-64 years. They defined diabetes incidence as having two or more outpatient claims 30 days apart or at least one inpatient claim with diabetes codes during the case identification period that spanned up to 2 calendar years after the first diabetes claim with at least 2 previous years without any diabetes claim.
Dr. Shrestha reported on 415,728 patient-years of data. The diabetes cohort spent an additional $51,000 on average during the 9 years before and after diagnosis, compared with their counterparts who had no diabetes diagnosis. Overall medical expenditures after diagnosis were also 2.3 times higher than before diagnosis.
“Although the additional expenditure after diagnosis was much higher for people with diagnosed diabetes, after the first year of diagnosis, it did not increase with the duration of diabetes during the study period,” Dr. Shrestha said. “However, the composition of expenditures differed, increasing for prescription drugs and decreasing for inpatient care.” He noted that the estimated excess medical expenditures described in the study indicate that “identifying those at high risk of diabetes, delaying/preventing development of diabetes through a lifestyle change program or other intervention, and managing diabetes effectively could reduce health care costs substantially.”
He acknowledged certain limitations of the study, including the fact that the data were drawn from a privately insured adult population and therefore may not be generalizable to the entire population of the United States. “Additionally, the data do not allow us to distinguish between type 1 and type 2 diabetes,” Dr. Shrestha said. He reported having no financial disclosures.
AT THE ADA SCIENTIFIC SESSIONS
Study plots long-term financial impact of diabetes
NEW ORLEANs – Between 2001 and 2013, a cohort of persons with newly diagnosed diabetes spent $3,489 more on average on medical costs in the first year after their diagnosis than they had in the year preceding it. Comparied with their diabetes-free counterparts, patients spent $6,424 more on average in the first year following diagnosis, results from a large data analysis found. In addition, during the period of 9 years before and 9 years after the diagnosis of diabetes, per capita total medical expenditures for the diabetes cohort increased annually by $382, compared with an increase of $177 for the cohort who did not have the condition.
“We know that people with diagnosed diabetes spend more on medical care than those without diagnosed diabetes because of the additional costs associated with managing diabetes and diabetes complications,” lead study author Sundar S. Shrestha, Ph.D., said in an interview in advance of the annual scientific sessions of the American Diabetes Association. “However, little information is available on how much more those with diagnosed diabetes spend after diagnosis than before diagnosis. Also, little information is available on how much more those with diagnosed diabetes spend on medical care, compared with those without diagnosed diabetes.”
Dr. Shrestha, a health economist at the Centers for Disease Control and Prevention, and his associates, analyzed the MarketScan Commercial Claims and Encounters database for the period 2001-2013 to compare the trajectory of medical expenditures (in 2013 U.S. dollars) among a diabetes cohort 9 years before and 9 years after diagnosis with a matched cohort of individuals without diabetes for U.S. adults aged 25-64 years. They defined diabetes incidence as having two or more outpatient claims 30 days apart or at least one inpatient claim with diabetes codes during the case identification period that spanned up to 2 calendar years after the first diabetes claim with at least 2 previous years without any diabetes claim.
Dr. Shrestha reported on 415,728 patient-years of data. The diabetes cohort spent an additional $51,000 on average during the 9 years before and after diagnosis, compared with their counterparts who had no diabetes diagnosis. Overall medical expenditures after diagnosis were also 2.3 times higher than before diagnosis.
“Although the additional expenditure after diagnosis was much higher for people with diagnosed diabetes, after the first year of diagnosis, it did not increase with the duration of diabetes during the study period,” Dr. Shrestha said. “However, the composition of expenditures differed, increasing for prescription drugs and decreasing for inpatient care.” He noted that the estimated excess medical expenditures described in the study indicate that “identifying those at high risk of diabetes, delaying/preventing development of diabetes through a lifestyle change program or other intervention, and managing diabetes effectively could reduce health care costs substantially.”
He acknowledged certain limitations of the study, including the fact that the data were drawn from a privately insured adult population and therefore may not be generalizable to the entire population of the United States. “Additionally, the data do not allow us to distinguish between type 1 and type 2 diabetes,” Dr. Shrestha said. He reported having no financial disclosures.
NEW ORLEANs – Between 2001 and 2013, a cohort of persons with newly diagnosed diabetes spent $3,489 more on average on medical costs in the first year after their diagnosis than they had in the year preceding it. Comparied with their diabetes-free counterparts, patients spent $6,424 more on average in the first year following diagnosis, results from a large data analysis found. In addition, during the period of 9 years before and 9 years after the diagnosis of diabetes, per capita total medical expenditures for the diabetes cohort increased annually by $382, compared with an increase of $177 for the cohort who did not have the condition.
“We know that people with diagnosed diabetes spend more on medical care than those without diagnosed diabetes because of the additional costs associated with managing diabetes and diabetes complications,” lead study author Sundar S. Shrestha, Ph.D., said in an interview in advance of the annual scientific sessions of the American Diabetes Association. “However, little information is available on how much more those with diagnosed diabetes spend after diagnosis than before diagnosis. Also, little information is available on how much more those with diagnosed diabetes spend on medical care, compared with those without diagnosed diabetes.”
Dr. Shrestha, a health economist at the Centers for Disease Control and Prevention, and his associates, analyzed the MarketScan Commercial Claims and Encounters database for the period 2001-2013 to compare the trajectory of medical expenditures (in 2013 U.S. dollars) among a diabetes cohort 9 years before and 9 years after diagnosis with a matched cohort of individuals without diabetes for U.S. adults aged 25-64 years. They defined diabetes incidence as having two or more outpatient claims 30 days apart or at least one inpatient claim with diabetes codes during the case identification period that spanned up to 2 calendar years after the first diabetes claim with at least 2 previous years without any diabetes claim.
Dr. Shrestha reported on 415,728 patient-years of data. The diabetes cohort spent an additional $51,000 on average during the 9 years before and after diagnosis, compared with their counterparts who had no diabetes diagnosis. Overall medical expenditures after diagnosis were also 2.3 times higher than before diagnosis.
“Although the additional expenditure after diagnosis was much higher for people with diagnosed diabetes, after the first year of diagnosis, it did not increase with the duration of diabetes during the study period,” Dr. Shrestha said. “However, the composition of expenditures differed, increasing for prescription drugs and decreasing for inpatient care.” He noted that the estimated excess medical expenditures described in the study indicate that “identifying those at high risk of diabetes, delaying/preventing development of diabetes through a lifestyle change program or other intervention, and managing diabetes effectively could reduce health care costs substantially.”
He acknowledged certain limitations of the study, including the fact that the data were drawn from a privately insured adult population and therefore may not be generalizable to the entire population of the United States. “Additionally, the data do not allow us to distinguish between type 1 and type 2 diabetes,” Dr. Shrestha said. He reported having no financial disclosures.
NEW ORLEANs – Between 2001 and 2013, a cohort of persons with newly diagnosed diabetes spent $3,489 more on average on medical costs in the first year after their diagnosis than they had in the year preceding it. Comparied with their diabetes-free counterparts, patients spent $6,424 more on average in the first year following diagnosis, results from a large data analysis found. In addition, during the period of 9 years before and 9 years after the diagnosis of diabetes, per capita total medical expenditures for the diabetes cohort increased annually by $382, compared with an increase of $177 for the cohort who did not have the condition.
“We know that people with diagnosed diabetes spend more on medical care than those without diagnosed diabetes because of the additional costs associated with managing diabetes and diabetes complications,” lead study author Sundar S. Shrestha, Ph.D., said in an interview in advance of the annual scientific sessions of the American Diabetes Association. “However, little information is available on how much more those with diagnosed diabetes spend after diagnosis than before diagnosis. Also, little information is available on how much more those with diagnosed diabetes spend on medical care, compared with those without diagnosed diabetes.”
Dr. Shrestha, a health economist at the Centers for Disease Control and Prevention, and his associates, analyzed the MarketScan Commercial Claims and Encounters database for the period 2001-2013 to compare the trajectory of medical expenditures (in 2013 U.S. dollars) among a diabetes cohort 9 years before and 9 years after diagnosis with a matched cohort of individuals without diabetes for U.S. adults aged 25-64 years. They defined diabetes incidence as having two or more outpatient claims 30 days apart or at least one inpatient claim with diabetes codes during the case identification period that spanned up to 2 calendar years after the first diabetes claim with at least 2 previous years without any diabetes claim.
Dr. Shrestha reported on 415,728 patient-years of data. The diabetes cohort spent an additional $51,000 on average during the 9 years before and after diagnosis, compared with their counterparts who had no diabetes diagnosis. Overall medical expenditures after diagnosis were also 2.3 times higher than before diagnosis.
“Although the additional expenditure after diagnosis was much higher for people with diagnosed diabetes, after the first year of diagnosis, it did not increase with the duration of diabetes during the study period,” Dr. Shrestha said. “However, the composition of expenditures differed, increasing for prescription drugs and decreasing for inpatient care.” He noted that the estimated excess medical expenditures described in the study indicate that “identifying those at high risk of diabetes, delaying/preventing development of diabetes through a lifestyle change program or other intervention, and managing diabetes effectively could reduce health care costs substantially.”
He acknowledged certain limitations of the study, including the fact that the data were drawn from a privately insured adult population and therefore may not be generalizable to the entire population of the United States. “Additionally, the data do not allow us to distinguish between type 1 and type 2 diabetes,” Dr. Shrestha said. He reported having no financial disclosures.
AT THE ADA SCIENTIFIC SESSIONS
Key clinical point: Between 2001 and 2013, medical expenditures were significantly greater for patients with diabetes than for those who did not have the condition.
Major finding: The diabetes cohort spent an additional $51,000 on average during the 9 years before and after diagnosis, compared with their diabetes-free counterparts.
Data source: An analysis of 415,728 patient-years of data from the MarketScan Commercial Claims and Encounters database for the period 2001-2013.
Disclosures: Dr. Shrestha reported having no financial disclosures.
Comprehensive Diabetic Retinopathy Screening Challenging
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS



















