User login
Mini X-Ray Device Treats Nonmelanoma Skin Cancers
MIAMI BEACH – An x-ray tube about the size of a sewing needle can safely deliver radiation to nonmelanoma skin tumors on the nose, scalp, or other highly visible areas of skin with good efficacy and good to excellent cosmetic results, an investigator reported at the annual meeting of the American Society for Radiation Oncology.
About 93% of patients treated with an electronic brachytherapy system from Xoft had cosmetic results judged to meet Radiation Therapy Oncology Group (RTOG) criteria for "excellent" at 1 year, said Dr. Ajay Bhatnagar of Cancer Treatment Services Arizona, Casa Grande.
"To date, the treatment of nonmelanoma skin cancer with electronic brachytherapy is comparable to traditional brachytherapy, with excellent cosmesis, acceptable acute toxicities, and no recurrences to date. Brachytherapy is an excellent modality for elderly patients with nonmelanoma skin cancers in cosmetically sensitive locations," he said.
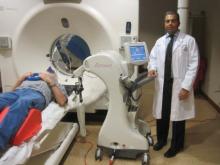
The electronic brachytherapy system delivers a radiation dose to tumors equivalent to that of an iridium-192, high dose rate surface (Leipzig) applicator, but without the need for a radioisotope. The electronic device is a miniature, high dose rate, low-energy x-ray tube that produces x-rays with a maximum of 50 keV of energy.
Dr. Bhatnagar looked at data on 102 patients treated with the device for 120 nonmelanoma skin cancer lesions. All patients received 5 Gy in eight dose fractions for a total of 40 Gy delivered in two fractions per week for 4 weeks. The prescription depth, based on CT simulation, varied from 3 to 7 mm. The treatment goal was to achieve a minimum 5-mm treatment margin wherever possible.
The device was placed with the patient immobilized and wearing a flex shield that prevents excessive radiation exposure to the clinician. Skin care included application of petrolatum ointment during treatment and aloe vera gel for 1 month afterward.
Of the 120 lesions treated, 59% had basal cell histology, 36% were squamous cell, 2% were Merkel cell carcinomas, 3% were cutaneous T-cell lymphoma lesions, and 1% had a mixed basal-squamous histology.
About one-third of the lesions were on the nose, another third on the face, with the remainder on the ears, extremities, scalp, and torso.
Nearly all patients (91%) experienced an acute rash with treatment, and 25% had itching of the treatment site. The most frequent late adverse effect was hypopigmentation of the treated skin, which occurred in 11% of lesions from 3 to 6 months.
At 1 month post treatment, cosmesis was rated as excellent (no change to slight atrophy or pigment change; slight hair loss; or no changes to slight induration or loss of subcutaneous fat) in about 77% of 81 patients evaluated; the remainder had "good" cosmesis (patch atrophy, moderate telangiectasia, total hair loss; moderate fibrosis but asymptomatic, slight field contracture with less than 10% linear reduction).
A physician who was not involved in the study commented that radiation therapy offers certain advantages over surgery when tumors are located in prominent locations, such as the face.
"We’ve been treating these lesions for close to 100 years now, and radiation does very well," said Dr. Keith A. Cengel, of the University of Pennsylvania School of Medicine in Philadelphia. "The one drawback – and I tell this to my patients when I treat with radiation – is that [subsequent] surgery can be more difficult and have a higher complication rate if you have a recurrence."
Mohs surgery, the most common form of treatment for basal or squamous cell skin cancers, may itself leave a comparatively deep and wide wound that may not heal as smoothly as a radiation-treated lesion, he added.
Dr. Cengel comoderated the session in which the electronic brachytherapy data were presented.
The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
MIAMI BEACH – An x-ray tube about the size of a sewing needle can safely deliver radiation to nonmelanoma skin tumors on the nose, scalp, or other highly visible areas of skin with good efficacy and good to excellent cosmetic results, an investigator reported at the annual meeting of the American Society for Radiation Oncology.
About 93% of patients treated with an electronic brachytherapy system from Xoft had cosmetic results judged to meet Radiation Therapy Oncology Group (RTOG) criteria for "excellent" at 1 year, said Dr. Ajay Bhatnagar of Cancer Treatment Services Arizona, Casa Grande.
"To date, the treatment of nonmelanoma skin cancer with electronic brachytherapy is comparable to traditional brachytherapy, with excellent cosmesis, acceptable acute toxicities, and no recurrences to date. Brachytherapy is an excellent modality for elderly patients with nonmelanoma skin cancers in cosmetically sensitive locations," he said.
The electronic brachytherapy system delivers a radiation dose to tumors equivalent to that of an iridium-192, high dose rate surface (Leipzig) applicator, but without the need for a radioisotope. The electronic device is a miniature, high dose rate, low-energy x-ray tube that produces x-rays with a maximum of 50 keV of energy.
Dr. Bhatnagar looked at data on 102 patients treated with the device for 120 nonmelanoma skin cancer lesions. All patients received 5 Gy in eight dose fractions for a total of 40 Gy delivered in two fractions per week for 4 weeks. The prescription depth, based on CT simulation, varied from 3 to 7 mm. The treatment goal was to achieve a minimum 5-mm treatment margin wherever possible.
The device was placed with the patient immobilized and wearing a flex shield that prevents excessive radiation exposure to the clinician. Skin care included application of petrolatum ointment during treatment and aloe vera gel for 1 month afterward.
Of the 120 lesions treated, 59% had basal cell histology, 36% were squamous cell, 2% were Merkel cell carcinomas, 3% were cutaneous T-cell lymphoma lesions, and 1% had a mixed basal-squamous histology.
About one-third of the lesions were on the nose, another third on the face, with the remainder on the ears, extremities, scalp, and torso.
Nearly all patients (91%) experienced an acute rash with treatment, and 25% had itching of the treatment site. The most frequent late adverse effect was hypopigmentation of the treated skin, which occurred in 11% of lesions from 3 to 6 months.
At 1 month post treatment, cosmesis was rated as excellent (no change to slight atrophy or pigment change; slight hair loss; or no changes to slight induration or loss of subcutaneous fat) in about 77% of 81 patients evaluated; the remainder had "good" cosmesis (patch atrophy, moderate telangiectasia, total hair loss; moderate fibrosis but asymptomatic, slight field contracture with less than 10% linear reduction).
A physician who was not involved in the study commented that radiation therapy offers certain advantages over surgery when tumors are located in prominent locations, such as the face.
"We’ve been treating these lesions for close to 100 years now, and radiation does very well," said Dr. Keith A. Cengel, of the University of Pennsylvania School of Medicine in Philadelphia. "The one drawback – and I tell this to my patients when I treat with radiation – is that [subsequent] surgery can be more difficult and have a higher complication rate if you have a recurrence."
Mohs surgery, the most common form of treatment for basal or squamous cell skin cancers, may itself leave a comparatively deep and wide wound that may not heal as smoothly as a radiation-treated lesion, he added.
Dr. Cengel comoderated the session in which the electronic brachytherapy data were presented.
The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
MIAMI BEACH – An x-ray tube about the size of a sewing needle can safely deliver radiation to nonmelanoma skin tumors on the nose, scalp, or other highly visible areas of skin with good efficacy and good to excellent cosmetic results, an investigator reported at the annual meeting of the American Society for Radiation Oncology.
About 93% of patients treated with an electronic brachytherapy system from Xoft had cosmetic results judged to meet Radiation Therapy Oncology Group (RTOG) criteria for "excellent" at 1 year, said Dr. Ajay Bhatnagar of Cancer Treatment Services Arizona, Casa Grande.
"To date, the treatment of nonmelanoma skin cancer with electronic brachytherapy is comparable to traditional brachytherapy, with excellent cosmesis, acceptable acute toxicities, and no recurrences to date. Brachytherapy is an excellent modality for elderly patients with nonmelanoma skin cancers in cosmetically sensitive locations," he said.
The electronic brachytherapy system delivers a radiation dose to tumors equivalent to that of an iridium-192, high dose rate surface (Leipzig) applicator, but without the need for a radioisotope. The electronic device is a miniature, high dose rate, low-energy x-ray tube that produces x-rays with a maximum of 50 keV of energy.
Dr. Bhatnagar looked at data on 102 patients treated with the device for 120 nonmelanoma skin cancer lesions. All patients received 5 Gy in eight dose fractions for a total of 40 Gy delivered in two fractions per week for 4 weeks. The prescription depth, based on CT simulation, varied from 3 to 7 mm. The treatment goal was to achieve a minimum 5-mm treatment margin wherever possible.
The device was placed with the patient immobilized and wearing a flex shield that prevents excessive radiation exposure to the clinician. Skin care included application of petrolatum ointment during treatment and aloe vera gel for 1 month afterward.
Of the 120 lesions treated, 59% had basal cell histology, 36% were squamous cell, 2% were Merkel cell carcinomas, 3% were cutaneous T-cell lymphoma lesions, and 1% had a mixed basal-squamous histology.
About one-third of the lesions were on the nose, another third on the face, with the remainder on the ears, extremities, scalp, and torso.
Nearly all patients (91%) experienced an acute rash with treatment, and 25% had itching of the treatment site. The most frequent late adverse effect was hypopigmentation of the treated skin, which occurred in 11% of lesions from 3 to 6 months.
At 1 month post treatment, cosmesis was rated as excellent (no change to slight atrophy or pigment change; slight hair loss; or no changes to slight induration or loss of subcutaneous fat) in about 77% of 81 patients evaluated; the remainder had "good" cosmesis (patch atrophy, moderate telangiectasia, total hair loss; moderate fibrosis but asymptomatic, slight field contracture with less than 10% linear reduction).
A physician who was not involved in the study commented that radiation therapy offers certain advantages over surgery when tumors are located in prominent locations, such as the face.
"We’ve been treating these lesions for close to 100 years now, and radiation does very well," said Dr. Keith A. Cengel, of the University of Pennsylvania School of Medicine in Philadelphia. "The one drawback – and I tell this to my patients when I treat with radiation – is that [subsequent] surgery can be more difficult and have a higher complication rate if you have a recurrence."
Mohs surgery, the most common form of treatment for basal or squamous cell skin cancers, may itself leave a comparatively deep and wide wound that may not heal as smoothly as a radiation-treated lesion, he added.
Dr. Cengel comoderated the session in which the electronic brachytherapy data were presented.
The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Approximately 93% of patients treated for nonmelanoma skin cancers with an electronic brachytherapy device had cosmetic results rated as excellent at 1 year, according to Radiation Therapy Oncology Group criteria.
Data Source: Retrospective case study of 102 patients with 120 basal cell, squamous cell, or other nonmelanoma cancers
Disclosures: The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
Mini X-Ray Device Treats Nonmelanoma Skin Cancers
MIAMI BEACH – An x-ray tube about the size of a sewing needle can safely deliver radiation to nonmelanoma skin tumors on the nose, scalp, or other highly visible areas of skin with good efficacy and good to excellent cosmetic results, an investigator reported at the annual meeting of the American Society for Radiation Oncology.
About 93% of patients treated with an electronic brachytherapy system from Xoft had cosmetic results judged to meet Radiation Therapy Oncology Group (RTOG) criteria for "excellent" at 1 year, said Dr. Ajay Bhatnagar of Cancer Treatment Services Arizona, Casa Grande.
"To date, the treatment of nonmelanoma skin cancer with electronic brachytherapy is comparable to traditional brachytherapy, with excellent cosmesis, acceptable acute toxicities, and no recurrences to date. Brachytherapy is an excellent modality for elderly patients with nonmelanoma skin cancers in cosmetically sensitive locations," he said.
The electronic brachytherapy system delivers a radiation dose to tumors equivalent to that of an iridium-192, high dose rate surface (Leipzig) applicator, but without the need for a radioisotope. The electronic device is a miniature, high dose rate, low-energy x-ray tube that produces x-rays with a maximum of 50 keV of energy.
Dr. Bhatnagar looked at data on 102 patients treated with the device for 120 nonmelanoma skin cancer lesions. All patients received 5 Gy in eight dose fractions for a total of 40 Gy delivered in two fractions per week for 4 weeks. The prescription depth, based on CT simulation, varied from 3 to 7 mm. The treatment goal was to achieve a minimum 5-mm treatment margin wherever possible.
The device was placed with the patient immobilized and wearing a flex shield that prevents excessive radiation exposure to the clinician. Skin care included application of petrolatum ointment during treatment and aloe vera gel for 1 month afterward.
Of the 120 lesions treated, 59% had basal cell histology, 36% were squamous cell, 2% were Merkel cell carcinomas, 3% were cutaneous T-cell lymphoma lesions, and 1% had a mixed basal-squamous histology.
About one-third of the lesions were on the nose, another third on the face, with the remainder on the ears, extremities, scalp, and torso.
Nearly all patients (91%) experienced an acute rash with treatment, and 25% had itching of the treatment site. The most frequent late adverse effect was hypopigmentation of the treated skin, which occurred in 11% of lesions from 3 to 6 months.
At 1 month post treatment, cosmesis was rated as excellent (no change to slight atrophy or pigment change; slight hair loss; or no changes to slight induration or loss of subcutaneous fat) in about 77% of 81 patients evaluated; the remainder had "good" cosmesis (patch atrophy, moderate telangiectasia, total hair loss; moderate fibrosis but asymptomatic, slight field contracture with less than 10% linear reduction).
Dr. Bhatnagar also launched a salvo in the turf battle between radiation oncologists and surgeons by urging his colleagues to take on more skin cancer cases. "Despite skin cancer being the most common malignancy in the world, it’s under-represented in our radiation oncology clinics. As radiation oncologists, we need to play a more prominent role in the treatment of nonmelanoma skin cancers," he said in his presentation.
A radiation oncologist who was not involved in the study commented that radiation therapy offers certain advantages over surgery when tumors are located in prominent locations, such as the face.
"We’ve been treating these lesions for close to 100 years now, and radiation does very well," said Dr. Keith A. Cengel, of the University of Pennsylvania School of Medicine in Philadelphia. "The one drawback – and I tell this to my patients when I treat with radiation – is that [subsequent] surgery can be more difficult and have a higher complication rate if you have a recurrence."
Mohs surgery, the most common form of treatment for basal or squamous cell skin cancers, may itself leave a comparatively deep and wide wound that may not heal as smoothly as a radiation-treated lesion, he added.
Dr. Cengel comoderated the session in which the electronic brachytherapy data were presented.
The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
MIAMI BEACH – An x-ray tube about the size of a sewing needle can safely deliver radiation to nonmelanoma skin tumors on the nose, scalp, or other highly visible areas of skin with good efficacy and good to excellent cosmetic results, an investigator reported at the annual meeting of the American Society for Radiation Oncology.
About 93% of patients treated with an electronic brachytherapy system from Xoft had cosmetic results judged to meet Radiation Therapy Oncology Group (RTOG) criteria for "excellent" at 1 year, said Dr. Ajay Bhatnagar of Cancer Treatment Services Arizona, Casa Grande.
"To date, the treatment of nonmelanoma skin cancer with electronic brachytherapy is comparable to traditional brachytherapy, with excellent cosmesis, acceptable acute toxicities, and no recurrences to date. Brachytherapy is an excellent modality for elderly patients with nonmelanoma skin cancers in cosmetically sensitive locations," he said.
The electronic brachytherapy system delivers a radiation dose to tumors equivalent to that of an iridium-192, high dose rate surface (Leipzig) applicator, but without the need for a radioisotope. The electronic device is a miniature, high dose rate, low-energy x-ray tube that produces x-rays with a maximum of 50 keV of energy.
Dr. Bhatnagar looked at data on 102 patients treated with the device for 120 nonmelanoma skin cancer lesions. All patients received 5 Gy in eight dose fractions for a total of 40 Gy delivered in two fractions per week for 4 weeks. The prescription depth, based on CT simulation, varied from 3 to 7 mm. The treatment goal was to achieve a minimum 5-mm treatment margin wherever possible.
The device was placed with the patient immobilized and wearing a flex shield that prevents excessive radiation exposure to the clinician. Skin care included application of petrolatum ointment during treatment and aloe vera gel for 1 month afterward.
Of the 120 lesions treated, 59% had basal cell histology, 36% were squamous cell, 2% were Merkel cell carcinomas, 3% were cutaneous T-cell lymphoma lesions, and 1% had a mixed basal-squamous histology.
About one-third of the lesions were on the nose, another third on the face, with the remainder on the ears, extremities, scalp, and torso.
Nearly all patients (91%) experienced an acute rash with treatment, and 25% had itching of the treatment site. The most frequent late adverse effect was hypopigmentation of the treated skin, which occurred in 11% of lesions from 3 to 6 months.
At 1 month post treatment, cosmesis was rated as excellent (no change to slight atrophy or pigment change; slight hair loss; or no changes to slight induration or loss of subcutaneous fat) in about 77% of 81 patients evaluated; the remainder had "good" cosmesis (patch atrophy, moderate telangiectasia, total hair loss; moderate fibrosis but asymptomatic, slight field contracture with less than 10% linear reduction).
Dr. Bhatnagar also launched a salvo in the turf battle between radiation oncologists and surgeons by urging his colleagues to take on more skin cancer cases. "Despite skin cancer being the most common malignancy in the world, it’s under-represented in our radiation oncology clinics. As radiation oncologists, we need to play a more prominent role in the treatment of nonmelanoma skin cancers," he said in his presentation.
A radiation oncologist who was not involved in the study commented that radiation therapy offers certain advantages over surgery when tumors are located in prominent locations, such as the face.
"We’ve been treating these lesions for close to 100 years now, and radiation does very well," said Dr. Keith A. Cengel, of the University of Pennsylvania School of Medicine in Philadelphia. "The one drawback – and I tell this to my patients when I treat with radiation – is that [subsequent] surgery can be more difficult and have a higher complication rate if you have a recurrence."
Mohs surgery, the most common form of treatment for basal or squamous cell skin cancers, may itself leave a comparatively deep and wide wound that may not heal as smoothly as a radiation-treated lesion, he added.
Dr. Cengel comoderated the session in which the electronic brachytherapy data were presented.
The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
MIAMI BEACH – An x-ray tube about the size of a sewing needle can safely deliver radiation to nonmelanoma skin tumors on the nose, scalp, or other highly visible areas of skin with good efficacy and good to excellent cosmetic results, an investigator reported at the annual meeting of the American Society for Radiation Oncology.
About 93% of patients treated with an electronic brachytherapy system from Xoft had cosmetic results judged to meet Radiation Therapy Oncology Group (RTOG) criteria for "excellent" at 1 year, said Dr. Ajay Bhatnagar of Cancer Treatment Services Arizona, Casa Grande.
"To date, the treatment of nonmelanoma skin cancer with electronic brachytherapy is comparable to traditional brachytherapy, with excellent cosmesis, acceptable acute toxicities, and no recurrences to date. Brachytherapy is an excellent modality for elderly patients with nonmelanoma skin cancers in cosmetically sensitive locations," he said.
The electronic brachytherapy system delivers a radiation dose to tumors equivalent to that of an iridium-192, high dose rate surface (Leipzig) applicator, but without the need for a radioisotope. The electronic device is a miniature, high dose rate, low-energy x-ray tube that produces x-rays with a maximum of 50 keV of energy.
Dr. Bhatnagar looked at data on 102 patients treated with the device for 120 nonmelanoma skin cancer lesions. All patients received 5 Gy in eight dose fractions for a total of 40 Gy delivered in two fractions per week for 4 weeks. The prescription depth, based on CT simulation, varied from 3 to 7 mm. The treatment goal was to achieve a minimum 5-mm treatment margin wherever possible.
The device was placed with the patient immobilized and wearing a flex shield that prevents excessive radiation exposure to the clinician. Skin care included application of petrolatum ointment during treatment and aloe vera gel for 1 month afterward.
Of the 120 lesions treated, 59% had basal cell histology, 36% were squamous cell, 2% were Merkel cell carcinomas, 3% were cutaneous T-cell lymphoma lesions, and 1% had a mixed basal-squamous histology.
About one-third of the lesions were on the nose, another third on the face, with the remainder on the ears, extremities, scalp, and torso.
Nearly all patients (91%) experienced an acute rash with treatment, and 25% had itching of the treatment site. The most frequent late adverse effect was hypopigmentation of the treated skin, which occurred in 11% of lesions from 3 to 6 months.
At 1 month post treatment, cosmesis was rated as excellent (no change to slight atrophy or pigment change; slight hair loss; or no changes to slight induration or loss of subcutaneous fat) in about 77% of 81 patients evaluated; the remainder had "good" cosmesis (patch atrophy, moderate telangiectasia, total hair loss; moderate fibrosis but asymptomatic, slight field contracture with less than 10% linear reduction).
Dr. Bhatnagar also launched a salvo in the turf battle between radiation oncologists and surgeons by urging his colleagues to take on more skin cancer cases. "Despite skin cancer being the most common malignancy in the world, it’s under-represented in our radiation oncology clinics. As radiation oncologists, we need to play a more prominent role in the treatment of nonmelanoma skin cancers," he said in his presentation.
A radiation oncologist who was not involved in the study commented that radiation therapy offers certain advantages over surgery when tumors are located in prominent locations, such as the face.
"We’ve been treating these lesions for close to 100 years now, and radiation does very well," said Dr. Keith A. Cengel, of the University of Pennsylvania School of Medicine in Philadelphia. "The one drawback – and I tell this to my patients when I treat with radiation – is that [subsequent] surgery can be more difficult and have a higher complication rate if you have a recurrence."
Mohs surgery, the most common form of treatment for basal or squamous cell skin cancers, may itself leave a comparatively deep and wide wound that may not heal as smoothly as a radiation-treated lesion, he added.
Dr. Cengel comoderated the session in which the electronic brachytherapy data were presented.
The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Approximately 93% of patients treated for nonmelanoma skin cancers with an electronic brachytherapy device had cosmetic results rated as excellent at 1 year, according to Radiation Therapy Oncology Group criteria.
Data Source: Retrospective case study of 102 patients with 120 basal cell, squamous cell, or other nonmelanoma cancers
Disclosures: The study was sponsored by Xoft, manufacturer of the brachytherapy device. Dr. Bhatnagar receives funding for serving as principal investigator of a prospective multicenter study of the device.
Protons Added to Photon Radiation Improve Chordoma Control
MIAMI BEACH – Where conventional radiation and surgery often fail, high-dose proton–based radiation therapy can succeed at providing local control of spinal chordomas, according to investigators at the annual meeting of the American Society for Radiation Oncology.
Among 126 patients with 127 localized chordomas, those who received a combination of pre- and postoperative radiation with protons added to photon radiation had a 5-year local control rate of 85%, reported Dr. Ronny L. Rotondo of the Stephen Harris Chordoma Center at Massachusetts General Hospital in Boston and his colleagues.
"Local control of spinal chordomas remains quite poor with surgery and photon-radiation therapy at conventional doses less than 60 Gy, with a number of series reporting local failure rates as high as 75%-100%. More recently, a number of centers have reported encouraging results with particle therapy, including protons and carbon ions," Dr. Rotondo said.
Chordomas are rare cancers that arise from the remnants of the fetal notochord, a structure that normally exists only during embryonic development. Although they do not tend to metastasize, chordomas incapacitate patients with locally aggressive growth.
Surgical resection is the mainstay of treatment, but local recurrences are common, and salvage therapy after local failure is often unsuccessful, hence the need for adjuvant radiation therapy, Dr. Rotondo said.
The unique physical properties of proton energy deposition in tissue makes proton therapy well suited for treatment of chordomas; most of the dose is deposited in the target tissue with little or no exit dose, allowing for higher doses and better local control than is possible with conventional photon therapy, he said.
He and his colleagues looked at clinical outcomes and clinical and pathologic prognostic factors in patients who underwent high-dose proton–based therapy with or without surgery for primary or recurrent chordomas of the thoracic, lumbar, or sacrococcygeal spine.
The patients were treated from 1982 through 2011 at either the Harvard Cyclotron Laboratory in Cambridge, Mass., or more recently at Massachusetts General Hospital’s proton therapy center in Boston. Their mean age at diagnosis was 53 years (range, 5-88 years), and the mean maximum size of lesions was 7 cm (range, 1.6 cm-21.7 cm).
In all, 45% of patients had surgery followed by radiation; 48% had preoperative radiation and surgery, followed by postoperative radiation; and 7% had radiation for recurrent tumors. About half of all of the surgeries (49%) were performed with en bloc resection, and 49% were intralesional (in the remaining 2% the surgery type was unknown).
Clear surgical margins (R0) were achieved in 27% of patients, with 45% having R1 resections, 24% having R2 margins, and 4% having unknown margin status.
Although some received protons* exclusively, most patients received a combination of photons and protons, with the protons comprising about 45% of the energy delivered in both pre- and postoperative treatment. The mean total dose delivered was 72.4 Gy.
Among patients still alive, median follow-up was 47 months with a median of 5.7 months since the last follow-up.
For the entire cohort, overall survival was 81% at 5 years and 53% at 10 years. The local control rate was 62% at 5 years and 49% at 10 years. Distant control was 77% at 5 years and 63% at 10 years.
Comparing patients by presentation, the investigators found that the 5-year local rate was 68% for patients with primary tumors, compared with 49% for those with recurrent tumors (P = .058). Respective 5-year event-free survival rates by presentation were 51% and 34% (P = .035).
Local control rates were also significantly better among patients who had undergone en bloc resection, at 72% at 5 years, compared with 55% for those who underwent an intralesional procedure (P = .016).
There were no significant differences in local control by surgical margin size, although there was a trend favoring R0, compared with R1 or R2, resections.
Preoperative radiotherapy also offered a significant benefit in both local and locoregional control of primary tumors. Local control at 5 years was 85% for those who received preoperative radiation in addition to postoperative, compared with 56% for those who did not (P = .019). Respective locoregional control rates were 79% and 56% at 5 years (P = .034).
The review was internally funded. Dr. Rotondo reported having no relevant conflicts of interest.
*Correction, 10/25/2011: An earlier version of this story incorrectly stated that some patients received photons exclusively.
MIAMI BEACH – Where conventional radiation and surgery often fail, high-dose proton–based radiation therapy can succeed at providing local control of spinal chordomas, according to investigators at the annual meeting of the American Society for Radiation Oncology.
Among 126 patients with 127 localized chordomas, those who received a combination of pre- and postoperative radiation with protons added to photon radiation had a 5-year local control rate of 85%, reported Dr. Ronny L. Rotondo of the Stephen Harris Chordoma Center at Massachusetts General Hospital in Boston and his colleagues.
"Local control of spinal chordomas remains quite poor with surgery and photon-radiation therapy at conventional doses less than 60 Gy, with a number of series reporting local failure rates as high as 75%-100%. More recently, a number of centers have reported encouraging results with particle therapy, including protons and carbon ions," Dr. Rotondo said.
Chordomas are rare cancers that arise from the remnants of the fetal notochord, a structure that normally exists only during embryonic development. Although they do not tend to metastasize, chordomas incapacitate patients with locally aggressive growth.
Surgical resection is the mainstay of treatment, but local recurrences are common, and salvage therapy after local failure is often unsuccessful, hence the need for adjuvant radiation therapy, Dr. Rotondo said.
The unique physical properties of proton energy deposition in tissue makes proton therapy well suited for treatment of chordomas; most of the dose is deposited in the target tissue with little or no exit dose, allowing for higher doses and better local control than is possible with conventional photon therapy, he said.
He and his colleagues looked at clinical outcomes and clinical and pathologic prognostic factors in patients who underwent high-dose proton–based therapy with or without surgery for primary or recurrent chordomas of the thoracic, lumbar, or sacrococcygeal spine.
The patients were treated from 1982 through 2011 at either the Harvard Cyclotron Laboratory in Cambridge, Mass., or more recently at Massachusetts General Hospital’s proton therapy center in Boston. Their mean age at diagnosis was 53 years (range, 5-88 years), and the mean maximum size of lesions was 7 cm (range, 1.6 cm-21.7 cm).
In all, 45% of patients had surgery followed by radiation; 48% had preoperative radiation and surgery, followed by postoperative radiation; and 7% had radiation for recurrent tumors. About half of all of the surgeries (49%) were performed with en bloc resection, and 49% were intralesional (in the remaining 2% the surgery type was unknown).
Clear surgical margins (R0) were achieved in 27% of patients, with 45% having R1 resections, 24% having R2 margins, and 4% having unknown margin status.
Although some received protons* exclusively, most patients received a combination of photons and protons, with the protons comprising about 45% of the energy delivered in both pre- and postoperative treatment. The mean total dose delivered was 72.4 Gy.
Among patients still alive, median follow-up was 47 months with a median of 5.7 months since the last follow-up.
For the entire cohort, overall survival was 81% at 5 years and 53% at 10 years. The local control rate was 62% at 5 years and 49% at 10 years. Distant control was 77% at 5 years and 63% at 10 years.
Comparing patients by presentation, the investigators found that the 5-year local rate was 68% for patients with primary tumors, compared with 49% for those with recurrent tumors (P = .058). Respective 5-year event-free survival rates by presentation were 51% and 34% (P = .035).
Local control rates were also significantly better among patients who had undergone en bloc resection, at 72% at 5 years, compared with 55% for those who underwent an intralesional procedure (P = .016).
There were no significant differences in local control by surgical margin size, although there was a trend favoring R0, compared with R1 or R2, resections.
Preoperative radiotherapy also offered a significant benefit in both local and locoregional control of primary tumors. Local control at 5 years was 85% for those who received preoperative radiation in addition to postoperative, compared with 56% for those who did not (P = .019). Respective locoregional control rates were 79% and 56% at 5 years (P = .034).
The review was internally funded. Dr. Rotondo reported having no relevant conflicts of interest.
*Correction, 10/25/2011: An earlier version of this story incorrectly stated that some patients received photons exclusively.
MIAMI BEACH – Where conventional radiation and surgery often fail, high-dose proton–based radiation therapy can succeed at providing local control of spinal chordomas, according to investigators at the annual meeting of the American Society for Radiation Oncology.
Among 126 patients with 127 localized chordomas, those who received a combination of pre- and postoperative radiation with protons added to photon radiation had a 5-year local control rate of 85%, reported Dr. Ronny L. Rotondo of the Stephen Harris Chordoma Center at Massachusetts General Hospital in Boston and his colleagues.
"Local control of spinal chordomas remains quite poor with surgery and photon-radiation therapy at conventional doses less than 60 Gy, with a number of series reporting local failure rates as high as 75%-100%. More recently, a number of centers have reported encouraging results with particle therapy, including protons and carbon ions," Dr. Rotondo said.
Chordomas are rare cancers that arise from the remnants of the fetal notochord, a structure that normally exists only during embryonic development. Although they do not tend to metastasize, chordomas incapacitate patients with locally aggressive growth.
Surgical resection is the mainstay of treatment, but local recurrences are common, and salvage therapy after local failure is often unsuccessful, hence the need for adjuvant radiation therapy, Dr. Rotondo said.
The unique physical properties of proton energy deposition in tissue makes proton therapy well suited for treatment of chordomas; most of the dose is deposited in the target tissue with little or no exit dose, allowing for higher doses and better local control than is possible with conventional photon therapy, he said.
He and his colleagues looked at clinical outcomes and clinical and pathologic prognostic factors in patients who underwent high-dose proton–based therapy with or without surgery for primary or recurrent chordomas of the thoracic, lumbar, or sacrococcygeal spine.
The patients were treated from 1982 through 2011 at either the Harvard Cyclotron Laboratory in Cambridge, Mass., or more recently at Massachusetts General Hospital’s proton therapy center in Boston. Their mean age at diagnosis was 53 years (range, 5-88 years), and the mean maximum size of lesions was 7 cm (range, 1.6 cm-21.7 cm).
In all, 45% of patients had surgery followed by radiation; 48% had preoperative radiation and surgery, followed by postoperative radiation; and 7% had radiation for recurrent tumors. About half of all of the surgeries (49%) were performed with en bloc resection, and 49% were intralesional (in the remaining 2% the surgery type was unknown).
Clear surgical margins (R0) were achieved in 27% of patients, with 45% having R1 resections, 24% having R2 margins, and 4% having unknown margin status.
Although some received protons* exclusively, most patients received a combination of photons and protons, with the protons comprising about 45% of the energy delivered in both pre- and postoperative treatment. The mean total dose delivered was 72.4 Gy.
Among patients still alive, median follow-up was 47 months with a median of 5.7 months since the last follow-up.
For the entire cohort, overall survival was 81% at 5 years and 53% at 10 years. The local control rate was 62% at 5 years and 49% at 10 years. Distant control was 77% at 5 years and 63% at 10 years.
Comparing patients by presentation, the investigators found that the 5-year local rate was 68% for patients with primary tumors, compared with 49% for those with recurrent tumors (P = .058). Respective 5-year event-free survival rates by presentation were 51% and 34% (P = .035).
Local control rates were also significantly better among patients who had undergone en bloc resection, at 72% at 5 years, compared with 55% for those who underwent an intralesional procedure (P = .016).
There were no significant differences in local control by surgical margin size, although there was a trend favoring R0, compared with R1 or R2, resections.
Preoperative radiotherapy also offered a significant benefit in both local and locoregional control of primary tumors. Local control at 5 years was 85% for those who received preoperative radiation in addition to postoperative, compared with 56% for those who did not (P = .019). Respective locoregional control rates were 79% and 56% at 5 years (P = .034).
The review was internally funded. Dr. Rotondo reported having no relevant conflicts of interest.
*Correction, 10/25/2011: An earlier version of this story incorrectly stated that some patients received photons exclusively.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Local control of spinal chordomas at 5 years was 85% for patients who received preoperative and postoperative radiation with protons and photons, compared with 56% for those who received only postoperative radiation (P = .019).
Data Source: Retrospective review of a case series of 126 patients with 127 chordomas.
Disclosures: The review was internally funded. Dr. Rotondo reported having no relevant conflicts of interest.
Early Trial Supports Bevacizumab in Head and Neck Cancer
MIAMI BEACH – Bevacizumab added to docetaxel and definitive radiotherapy was an effective and safe non–platinum-based regimen for locoregionally advanced squamous cell carcinoma of the head and neck in a small phase II trial that was presented at the annual meeting of the American Society for Radiation Oncology.
The disease-free survival rate at 2 years (the primary end point) was 75% among 28 patients who had completed the full course of chemoradiation in the phase II study, said Dr. Nicholas Galanopoulos of University Hospitals Seidman Cancer Center in Cleveland. In this as-treated population, 85% of patients were alive at 2 years, locoregional control was achieved in 85% of patients, and 81.5% were free of distant metastases at 2 years.
The numbers were slightly lower when two additional patients who dropped out of the study (one for unspecified reasons and one who developed aspiration pneumonia and was hospitalized) were included in an intention-to-treat analysis. Among all 30 patients, the disease-free survival rate at 2 years was 63% and overall survival was 72%, although locoregional control and distant-metastasis-free rates remained unchanged at 85% and 81.5%, respectively.
"Future trials of concurrent chemoradiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer," Dr. Galanopoulos said.
The rationale for trying bevacizumab (Avastin) in chemoradiation regimens for head and neck tumors is based on its demonstrated ability to inhibit endothelial cell proliferation and blood vessel formation, and to increase radiosensitivity of tumors when administered concurrently with radiation, he noted.
In all, 30 treatment-naive patients (26 men, 4 women) with locally advanced stage III (6 patients) or IV (24 patients) squamous cell carcinoma of the head and neck were enrolled. The patients had good performance status and a life expectancy greater than 12 weeks.
They received 70.2 Gy radiation in 1.8-Gy fractions, docetaxel (Taxotere) 20 mg/m2 per week, and bevacizumab 5 mg/kg every 2 weeks, with bevacizumab continued for up to 1 year following chemoradiation (median, 7 months).
"Future trials of concurrent chemo-radiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer."
Results were reported as of a median follow-up of 24.4 months.
Adjuvant bevacizumab was stopped 4 weeks before surgery in those patients who required neck dissection, and the drug was discontinued in three patients (one for radiation necrosis of the larynx requiring laryngectomy, one for severe pharyngoesophageal stenosis, and one for hemorrhagic cholecystits requiring cholecystectomy).
Major toxicities associated with the regimen included grade 4 bleeding in two patients (the episode of hemorrhagic cholecystitis already noted), and one oropharyngeal hemorrhage in field during chemoradiation.
Late grade 3 or 4 dysphagia occurred in 7 of 19 patients who had been treated with 3-D conformal radiation therapy, but in none of 11 patients who received intensity-modulated radiation (P less than .05). Patients with laryngeal primary tumors were more likely to develop serious late dysphagia, compared with patients with oropharyngeal primaries (58%% vs. 15%; P less than .05).
The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
MIAMI BEACH – Bevacizumab added to docetaxel and definitive radiotherapy was an effective and safe non–platinum-based regimen for locoregionally advanced squamous cell carcinoma of the head and neck in a small phase II trial that was presented at the annual meeting of the American Society for Radiation Oncology.
The disease-free survival rate at 2 years (the primary end point) was 75% among 28 patients who had completed the full course of chemoradiation in the phase II study, said Dr. Nicholas Galanopoulos of University Hospitals Seidman Cancer Center in Cleveland. In this as-treated population, 85% of patients were alive at 2 years, locoregional control was achieved in 85% of patients, and 81.5% were free of distant metastases at 2 years.
The numbers were slightly lower when two additional patients who dropped out of the study (one for unspecified reasons and one who developed aspiration pneumonia and was hospitalized) were included in an intention-to-treat analysis. Among all 30 patients, the disease-free survival rate at 2 years was 63% and overall survival was 72%, although locoregional control and distant-metastasis-free rates remained unchanged at 85% and 81.5%, respectively.
"Future trials of concurrent chemoradiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer," Dr. Galanopoulos said.
The rationale for trying bevacizumab (Avastin) in chemoradiation regimens for head and neck tumors is based on its demonstrated ability to inhibit endothelial cell proliferation and blood vessel formation, and to increase radiosensitivity of tumors when administered concurrently with radiation, he noted.
In all, 30 treatment-naive patients (26 men, 4 women) with locally advanced stage III (6 patients) or IV (24 patients) squamous cell carcinoma of the head and neck were enrolled. The patients had good performance status and a life expectancy greater than 12 weeks.
They received 70.2 Gy radiation in 1.8-Gy fractions, docetaxel (Taxotere) 20 mg/m2 per week, and bevacizumab 5 mg/kg every 2 weeks, with bevacizumab continued for up to 1 year following chemoradiation (median, 7 months).
"Future trials of concurrent chemo-radiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer."
Results were reported as of a median follow-up of 24.4 months.
Adjuvant bevacizumab was stopped 4 weeks before surgery in those patients who required neck dissection, and the drug was discontinued in three patients (one for radiation necrosis of the larynx requiring laryngectomy, one for severe pharyngoesophageal stenosis, and one for hemorrhagic cholecystits requiring cholecystectomy).
Major toxicities associated with the regimen included grade 4 bleeding in two patients (the episode of hemorrhagic cholecystitis already noted), and one oropharyngeal hemorrhage in field during chemoradiation.
Late grade 3 or 4 dysphagia occurred in 7 of 19 patients who had been treated with 3-D conformal radiation therapy, but in none of 11 patients who received intensity-modulated radiation (P less than .05). Patients with laryngeal primary tumors were more likely to develop serious late dysphagia, compared with patients with oropharyngeal primaries (58%% vs. 15%; P less than .05).
The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
MIAMI BEACH – Bevacizumab added to docetaxel and definitive radiotherapy was an effective and safe non–platinum-based regimen for locoregionally advanced squamous cell carcinoma of the head and neck in a small phase II trial that was presented at the annual meeting of the American Society for Radiation Oncology.
The disease-free survival rate at 2 years (the primary end point) was 75% among 28 patients who had completed the full course of chemoradiation in the phase II study, said Dr. Nicholas Galanopoulos of University Hospitals Seidman Cancer Center in Cleveland. In this as-treated population, 85% of patients were alive at 2 years, locoregional control was achieved in 85% of patients, and 81.5% were free of distant metastases at 2 years.
The numbers were slightly lower when two additional patients who dropped out of the study (one for unspecified reasons and one who developed aspiration pneumonia and was hospitalized) were included in an intention-to-treat analysis. Among all 30 patients, the disease-free survival rate at 2 years was 63% and overall survival was 72%, although locoregional control and distant-metastasis-free rates remained unchanged at 85% and 81.5%, respectively.
"Future trials of concurrent chemoradiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer," Dr. Galanopoulos said.
The rationale for trying bevacizumab (Avastin) in chemoradiation regimens for head and neck tumors is based on its demonstrated ability to inhibit endothelial cell proliferation and blood vessel formation, and to increase radiosensitivity of tumors when administered concurrently with radiation, he noted.
In all, 30 treatment-naive patients (26 men, 4 women) with locally advanced stage III (6 patients) or IV (24 patients) squamous cell carcinoma of the head and neck were enrolled. The patients had good performance status and a life expectancy greater than 12 weeks.
They received 70.2 Gy radiation in 1.8-Gy fractions, docetaxel (Taxotere) 20 mg/m2 per week, and bevacizumab 5 mg/kg every 2 weeks, with bevacizumab continued for up to 1 year following chemoradiation (median, 7 months).
"Future trials of concurrent chemo-radiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer."
Results were reported as of a median follow-up of 24.4 months.
Adjuvant bevacizumab was stopped 4 weeks before surgery in those patients who required neck dissection, and the drug was discontinued in three patients (one for radiation necrosis of the larynx requiring laryngectomy, one for severe pharyngoesophageal stenosis, and one for hemorrhagic cholecystits requiring cholecystectomy).
Major toxicities associated with the regimen included grade 4 bleeding in two patients (the episode of hemorrhagic cholecystitis already noted), and one oropharyngeal hemorrhage in field during chemoradiation.
Late grade 3 or 4 dysphagia occurred in 7 of 19 patients who had been treated with 3-D conformal radiation therapy, but in none of 11 patients who received intensity-modulated radiation (P less than .05). Patients with laryngeal primary tumors were more likely to develop serious late dysphagia, compared with patients with oropharyngeal primaries (58%% vs. 15%; P less than .05).
The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: The 2-year disease-free survival rate was 75% in an as-treated population with locoregionally advanced squamous cell carcinoma of the head and neck, and 63% in an intention-to-treat analysis.
Data Source: A phase II clinical trial evaluating the addition of bevacizumab to chemoradiation with docetaxel.
Disclosures: The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
Neoadjuvant Therapy Improves Survival of Large Sarcomas in Extremities
MIAMI BEACH – Preoperative chemotherapy or radiation – take your pick or both – improves overall survival of patients with large, soft-tissue sarcomas of the extremities, retrospective studies from two cancer centers suggest.
Looking at a cohort of 112 patients, investigators at the Mayo Clinic in Scottsdale, Ariz., saw no significant differences by treatment type in local control rates or overall survival.
But among patients with tumors measuring longer than 5 cm, the 3-year overall survival rate was 63% for those who had neoadjuvant radiation and surgery and 70% for those who had chemoradiotherapy and surgery, versus 40% with surgery alone (P = .03), radiation oncologist Jonathan B. Ashman and colleagues reported at the annual meeting of the American Society for Radiation Oncology.
"We found that patients who are treated with either neoadjuvant strategy – either radiation or chemotherapy/radiation – have bigger tumors, so they’re more aggressive tumors, but the local control is the same, so we think that the neoadjuvant therapy is helping more than we would expect from surgery alone," Dr. Ashman said in an interview.
In a separate study, Johns Hopkins Hospital investigators reported excellent local control and good overall survival rates for patients with large sarcomas of the extremities that were treated with neoadjuvant chemoradiotherapy, surgery, and adjuvant chemotherapy.
The estimated 3-year overall survival rate was 66%, the estimated disease-free survival rate 58%, and estimated the local control rate 100% among 16 patients treated with interdigitated neoadjuvant chemotherapy and radiation using the MAID protocol (mesna, doxorubicin, ifosfamide and dacarbazine), reported radiation oncologist Dr. Raju Raval and colleagues from Johns Hopkins Hospital in Baltimore.
The Mayo Experience
The Mayo investigators retrospectively looked at whether adding chemotherapy to external beam radiation therapy and limb-sparing surgery added benefit for patients with stage II or III soft-tissue sarcomas of the extremities. The center’s sarcoma team recommends either neoadjuvant radiation or chemoradiotherapy for patients who have high-grade tumors and are likely to have narrow resection margins based on preoperative MRI imaging showing large tumor size or unfavorable location of the tumor relative to bone or to neurovascular structures.
"We found that patients who are treated with either neoadjuvant strategy – either radiation or chemotherapy/ radiation – have bigger tumors."
The investigators reviewed the charts of 91 patients treated for primary disease, and 21 treated for tumor recurrence from 1998 through 2009. Median follow-up was 22.1 months. Overall, 39 of the patients received neoadjuvant chemoradiotherapy, 37 neoadjuvant radiation, and 36 surgery alone. The majority of tumors in each treatment group were pathologic grade 3 or 4, and most were 5 cm or greater in their longest dimension.
Of the 39 patients who received some form of chemotherapy, 20 had concurrent weekly cisplatin 20-40 mg/m2, with the remainder receiving other protocols, including MAID and MAP (methotrexate, doxorubicin, and cisplatin).
Dr. Ashman said that although there is no clear evidence to support the use of weekly cisplatin in sarcoma, "our practice has been over the last 5-8 years to have patients who have large and high-risk tumors but otherwise have good performance status, with good renal function and no other contraindications to receive weekly cisplatin, because it’s such a well-tolerated regimen. The rationale is that there hasn’t been really good data to show a benefit from the more toxic protocols."
Cisplatin is known to sensitize tumor cells to radiation, he noted. "When you’re looking at just radiosensitization, and you’re looking at just local outcomes and not trying to affect the distant metastasis rate, then let’s try to use a therapy that’s less toxic, so that patients don’t have to break therapy."
In all, 92 patients had surgery with limb-preservation attempt, and the majority (88%) had disease-free surgical margins (R0 resections); rates of R0 resections did not differ among the treatment groups.
The overall 3-year local control rate was 87%, distant metastasis-free survival was 69%, and overall survival was 68%. Patients who received a neoadjuvant therapy had higher rates of wound complications, which occurred in 11% of surgery-only patients, compared with 50% of patients who received chemoradiotherapy (P = .003) and 42% of those who received radiation (P = .02). There was no significant difference in wound complications between the two neoadjuvant therapy types, however, and no factors predicted these complications.
The Johns Hopkins Experience
Dr. Raval and colleagues from Johns Hopkins reported on 16 patients with high-grade soft-tissue sarcomas treated with interdigitated neoadjuvant chemotherapy and radiation, surgery, and adjuvant chemotherapy.
The therapy consists of three cycles of chemotherapy, with 44 Gy radiation divided into 22 fractions, 11 of which are delivered following the first chemotherapy cycle, and 11 following the second cycle. Surgery is performed about 80 days after the start of therapy, and an additional 11 Gy or radiation is given if an R0 resection was not achieved. If the patient can tolerate it, three additional adjuvant chemotherapy cycles are given.
Estimated 3-year overall survival was 66%, disease-free survival was 58%, and the local control rate was 100%. No local recurrences had been seen at the longest follow-up of 160 months.
The Hopkins investigators compared their results with those from a pilot study using the same protocol at Massachusetts General Hospital in Boston. The MGH investigators had an 87% 5-year overall survival rate, 70% disease-free survival rate, and 92% local-control rate. Among MGH historical controls treated with surgery only, the respective rates were 58%, 42%, and 86%.
"This combined-modality approach to high grade soft-tissue sarcoma continues to play a role in the treatment of patients with this rare soft tissue neoplasm," the investigators concluded.
Both studies were internally funded. Dr. Ashman and Dr. Raval reported that they had no relevant financial disclosures.
MIAMI BEACH – Preoperative chemotherapy or radiation – take your pick or both – improves overall survival of patients with large, soft-tissue sarcomas of the extremities, retrospective studies from two cancer centers suggest.
Looking at a cohort of 112 patients, investigators at the Mayo Clinic in Scottsdale, Ariz., saw no significant differences by treatment type in local control rates or overall survival.
But among patients with tumors measuring longer than 5 cm, the 3-year overall survival rate was 63% for those who had neoadjuvant radiation and surgery and 70% for those who had chemoradiotherapy and surgery, versus 40% with surgery alone (P = .03), radiation oncologist Jonathan B. Ashman and colleagues reported at the annual meeting of the American Society for Radiation Oncology.
"We found that patients who are treated with either neoadjuvant strategy – either radiation or chemotherapy/radiation – have bigger tumors, so they’re more aggressive tumors, but the local control is the same, so we think that the neoadjuvant therapy is helping more than we would expect from surgery alone," Dr. Ashman said in an interview.
In a separate study, Johns Hopkins Hospital investigators reported excellent local control and good overall survival rates for patients with large sarcomas of the extremities that were treated with neoadjuvant chemoradiotherapy, surgery, and adjuvant chemotherapy.
The estimated 3-year overall survival rate was 66%, the estimated disease-free survival rate 58%, and estimated the local control rate 100% among 16 patients treated with interdigitated neoadjuvant chemotherapy and radiation using the MAID protocol (mesna, doxorubicin, ifosfamide and dacarbazine), reported radiation oncologist Dr. Raju Raval and colleagues from Johns Hopkins Hospital in Baltimore.
The Mayo Experience
The Mayo investigators retrospectively looked at whether adding chemotherapy to external beam radiation therapy and limb-sparing surgery added benefit for patients with stage II or III soft-tissue sarcomas of the extremities. The center’s sarcoma team recommends either neoadjuvant radiation or chemoradiotherapy for patients who have high-grade tumors and are likely to have narrow resection margins based on preoperative MRI imaging showing large tumor size or unfavorable location of the tumor relative to bone or to neurovascular structures.
"We found that patients who are treated with either neoadjuvant strategy – either radiation or chemotherapy/ radiation – have bigger tumors."
The investigators reviewed the charts of 91 patients treated for primary disease, and 21 treated for tumor recurrence from 1998 through 2009. Median follow-up was 22.1 months. Overall, 39 of the patients received neoadjuvant chemoradiotherapy, 37 neoadjuvant radiation, and 36 surgery alone. The majority of tumors in each treatment group were pathologic grade 3 or 4, and most were 5 cm or greater in their longest dimension.
Of the 39 patients who received some form of chemotherapy, 20 had concurrent weekly cisplatin 20-40 mg/m2, with the remainder receiving other protocols, including MAID and MAP (methotrexate, doxorubicin, and cisplatin).
Dr. Ashman said that although there is no clear evidence to support the use of weekly cisplatin in sarcoma, "our practice has been over the last 5-8 years to have patients who have large and high-risk tumors but otherwise have good performance status, with good renal function and no other contraindications to receive weekly cisplatin, because it’s such a well-tolerated regimen. The rationale is that there hasn’t been really good data to show a benefit from the more toxic protocols."
Cisplatin is known to sensitize tumor cells to radiation, he noted. "When you’re looking at just radiosensitization, and you’re looking at just local outcomes and not trying to affect the distant metastasis rate, then let’s try to use a therapy that’s less toxic, so that patients don’t have to break therapy."
In all, 92 patients had surgery with limb-preservation attempt, and the majority (88%) had disease-free surgical margins (R0 resections); rates of R0 resections did not differ among the treatment groups.
The overall 3-year local control rate was 87%, distant metastasis-free survival was 69%, and overall survival was 68%. Patients who received a neoadjuvant therapy had higher rates of wound complications, which occurred in 11% of surgery-only patients, compared with 50% of patients who received chemoradiotherapy (P = .003) and 42% of those who received radiation (P = .02). There was no significant difference in wound complications between the two neoadjuvant therapy types, however, and no factors predicted these complications.
The Johns Hopkins Experience
Dr. Raval and colleagues from Johns Hopkins reported on 16 patients with high-grade soft-tissue sarcomas treated with interdigitated neoadjuvant chemotherapy and radiation, surgery, and adjuvant chemotherapy.
The therapy consists of three cycles of chemotherapy, with 44 Gy radiation divided into 22 fractions, 11 of which are delivered following the first chemotherapy cycle, and 11 following the second cycle. Surgery is performed about 80 days after the start of therapy, and an additional 11 Gy or radiation is given if an R0 resection was not achieved. If the patient can tolerate it, three additional adjuvant chemotherapy cycles are given.
Estimated 3-year overall survival was 66%, disease-free survival was 58%, and the local control rate was 100%. No local recurrences had been seen at the longest follow-up of 160 months.
The Hopkins investigators compared their results with those from a pilot study using the same protocol at Massachusetts General Hospital in Boston. The MGH investigators had an 87% 5-year overall survival rate, 70% disease-free survival rate, and 92% local-control rate. Among MGH historical controls treated with surgery only, the respective rates were 58%, 42%, and 86%.
"This combined-modality approach to high grade soft-tissue sarcoma continues to play a role in the treatment of patients with this rare soft tissue neoplasm," the investigators concluded.
Both studies were internally funded. Dr. Ashman and Dr. Raval reported that they had no relevant financial disclosures.
MIAMI BEACH – Preoperative chemotherapy or radiation – take your pick or both – improves overall survival of patients with large, soft-tissue sarcomas of the extremities, retrospective studies from two cancer centers suggest.
Looking at a cohort of 112 patients, investigators at the Mayo Clinic in Scottsdale, Ariz., saw no significant differences by treatment type in local control rates or overall survival.
But among patients with tumors measuring longer than 5 cm, the 3-year overall survival rate was 63% for those who had neoadjuvant radiation and surgery and 70% for those who had chemoradiotherapy and surgery, versus 40% with surgery alone (P = .03), radiation oncologist Jonathan B. Ashman and colleagues reported at the annual meeting of the American Society for Radiation Oncology.
"We found that patients who are treated with either neoadjuvant strategy – either radiation or chemotherapy/radiation – have bigger tumors, so they’re more aggressive tumors, but the local control is the same, so we think that the neoadjuvant therapy is helping more than we would expect from surgery alone," Dr. Ashman said in an interview.
In a separate study, Johns Hopkins Hospital investigators reported excellent local control and good overall survival rates for patients with large sarcomas of the extremities that were treated with neoadjuvant chemoradiotherapy, surgery, and adjuvant chemotherapy.
The estimated 3-year overall survival rate was 66%, the estimated disease-free survival rate 58%, and estimated the local control rate 100% among 16 patients treated with interdigitated neoadjuvant chemotherapy and radiation using the MAID protocol (mesna, doxorubicin, ifosfamide and dacarbazine), reported radiation oncologist Dr. Raju Raval and colleagues from Johns Hopkins Hospital in Baltimore.
The Mayo Experience
The Mayo investigators retrospectively looked at whether adding chemotherapy to external beam radiation therapy and limb-sparing surgery added benefit for patients with stage II or III soft-tissue sarcomas of the extremities. The center’s sarcoma team recommends either neoadjuvant radiation or chemoradiotherapy for patients who have high-grade tumors and are likely to have narrow resection margins based on preoperative MRI imaging showing large tumor size or unfavorable location of the tumor relative to bone or to neurovascular structures.
"We found that patients who are treated with either neoadjuvant strategy – either radiation or chemotherapy/ radiation – have bigger tumors."
The investigators reviewed the charts of 91 patients treated for primary disease, and 21 treated for tumor recurrence from 1998 through 2009. Median follow-up was 22.1 months. Overall, 39 of the patients received neoadjuvant chemoradiotherapy, 37 neoadjuvant radiation, and 36 surgery alone. The majority of tumors in each treatment group were pathologic grade 3 or 4, and most were 5 cm or greater in their longest dimension.
Of the 39 patients who received some form of chemotherapy, 20 had concurrent weekly cisplatin 20-40 mg/m2, with the remainder receiving other protocols, including MAID and MAP (methotrexate, doxorubicin, and cisplatin).
Dr. Ashman said that although there is no clear evidence to support the use of weekly cisplatin in sarcoma, "our practice has been over the last 5-8 years to have patients who have large and high-risk tumors but otherwise have good performance status, with good renal function and no other contraindications to receive weekly cisplatin, because it’s such a well-tolerated regimen. The rationale is that there hasn’t been really good data to show a benefit from the more toxic protocols."
Cisplatin is known to sensitize tumor cells to radiation, he noted. "When you’re looking at just radiosensitization, and you’re looking at just local outcomes and not trying to affect the distant metastasis rate, then let’s try to use a therapy that’s less toxic, so that patients don’t have to break therapy."
In all, 92 patients had surgery with limb-preservation attempt, and the majority (88%) had disease-free surgical margins (R0 resections); rates of R0 resections did not differ among the treatment groups.
The overall 3-year local control rate was 87%, distant metastasis-free survival was 69%, and overall survival was 68%. Patients who received a neoadjuvant therapy had higher rates of wound complications, which occurred in 11% of surgery-only patients, compared with 50% of patients who received chemoradiotherapy (P = .003) and 42% of those who received radiation (P = .02). There was no significant difference in wound complications between the two neoadjuvant therapy types, however, and no factors predicted these complications.
The Johns Hopkins Experience
Dr. Raval and colleagues from Johns Hopkins reported on 16 patients with high-grade soft-tissue sarcomas treated with interdigitated neoadjuvant chemotherapy and radiation, surgery, and adjuvant chemotherapy.
The therapy consists of three cycles of chemotherapy, with 44 Gy radiation divided into 22 fractions, 11 of which are delivered following the first chemotherapy cycle, and 11 following the second cycle. Surgery is performed about 80 days after the start of therapy, and an additional 11 Gy or radiation is given if an R0 resection was not achieved. If the patient can tolerate it, three additional adjuvant chemotherapy cycles are given.
Estimated 3-year overall survival was 66%, disease-free survival was 58%, and the local control rate was 100%. No local recurrences had been seen at the longest follow-up of 160 months.
The Hopkins investigators compared their results with those from a pilot study using the same protocol at Massachusetts General Hospital in Boston. The MGH investigators had an 87% 5-year overall survival rate, 70% disease-free survival rate, and 92% local-control rate. Among MGH historical controls treated with surgery only, the respective rates were 58%, 42%, and 86%.
"This combined-modality approach to high grade soft-tissue sarcoma continues to play a role in the treatment of patients with this rare soft tissue neoplasm," the investigators concluded.
Both studies were internally funded. Dr. Ashman and Dr. Raval reported that they had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Among patients with soft-tissue sarcomas of the extremities measuring longer than 5 cm, 3-year overall survival was 63% for those who had neoadjuvant radiation and surgery and 70% for those who had chemoradiotherapy and surgery, vs. 40% for surgery alone (P = .03).
Data Source: Retrospective cases reviews from the Mayo Clinic Scottsdale, Ariz., and Johns Hopkins Hospital in Baltimore.
Disclosures: Both studies were internally funded. Dr. Ashman and Dr. Raval reported that they had no relevant financial disclosures.
Screen Carotids After Head and Neck Radiation
MIAMI BEACH – Head and neck cancer patients treated with radiation should be screened routinely for carotid artery stenosis, investigators recommended at the annual meeting of the American Society for Radiation Oncology.
Among 225 patients who had received radiation and were screened, an estimated 18% had significant asymptomatic stenosis (50% or greater narrowing) of one or both carotid arteries 3 years after treatment said Dr. Jennifer Dorth, a resident in radiation oncology at Duke University Medical Center in Durham, N.C.
"We recommend screening for head and neck cancer patients given that there are high rates of stenosis as well as high rates of progression of stenosis," she said.
Factors significantly associated with risk for stenosis included Framingham risk factors (smoking history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular/peripheral vascular disease, and atrial fibrillation) and radiation dose.
The investigators retrospectively reviewed outcomes of asymptomatic, disease-free head and neck cancer patients who had received radiation with curative intent to the neck. The patients were screened with carotid Doppler ultrasound at or after the 1-year follow-up visit, and this was repeated every 2-3 years. Patients with ultrasound evidence of 50% or greater stenosis were referred to vascular surgery.
The study identified 225 patients, 139 of whom had received intensity-modulated radiation therapy (IMRT), with the dose calculated separately for each side of the neck. Because of the separate treatment planning, the investigators analyzed the data by creating two separate models: one looking at all patients, and the other looking at 278 treatments in the 139 patients with IMRT.
In each model, about 85% of patients had stage III or IV disease and about 58% had cancer in the oropharynx, followed in order of frequency by the larynx, oral cavity, nasopharynx, or other sites.
A total of 33 patients had stenosis in 51 arteries. The median time between completion of radiation therapy and the last follow-up screening was 2 years. The median time to stenosis was 3 years.
Actuarial estimates of carotid artery stenosis were 2% at 1 year, 6% at 2 years, and 18% at 3 years.
In univariate analysis, factors associated with stenosis included male gender (P = .02), hypertension (P = .003), vascular disease (P less than .001), and Framingham score (P less than .001).
In the multivariate model looking at all patients, each Framingham risk factor was associated with a near doubling of stenosis risk (hazard ratio 1.8, P = .0003). In the model focusing on the IMRT population (adjusted for Framingham score), only radiation dose was significantly associated with stenosis (HR 1.07/Gy, P = .02).
Of the 33 patients with stenosis, 8 had no further follow-up imaging, 8 had stable stenosis, and 17 had progressive stenosis, 2 of whom had a cerebrovascular event. Eight patients with progressive stenosis received medical management only, and nine went on to surgery (three endarterectomies and six stent placements).
"Of the nine patients who underwent surgical management, there was a high rate of restenosis in 30% of patients at a year median follow-up, and this is consistent, unfortunately, with other series looking at rates of restenosis," Dr. Dorth said.
The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.
MIAMI BEACH – Head and neck cancer patients treated with radiation should be screened routinely for carotid artery stenosis, investigators recommended at the annual meeting of the American Society for Radiation Oncology.
Among 225 patients who had received radiation and were screened, an estimated 18% had significant asymptomatic stenosis (50% or greater narrowing) of one or both carotid arteries 3 years after treatment said Dr. Jennifer Dorth, a resident in radiation oncology at Duke University Medical Center in Durham, N.C.
"We recommend screening for head and neck cancer patients given that there are high rates of stenosis as well as high rates of progression of stenosis," she said.
Factors significantly associated with risk for stenosis included Framingham risk factors (smoking history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular/peripheral vascular disease, and atrial fibrillation) and radiation dose.
The investigators retrospectively reviewed outcomes of asymptomatic, disease-free head and neck cancer patients who had received radiation with curative intent to the neck. The patients were screened with carotid Doppler ultrasound at or after the 1-year follow-up visit, and this was repeated every 2-3 years. Patients with ultrasound evidence of 50% or greater stenosis were referred to vascular surgery.
The study identified 225 patients, 139 of whom had received intensity-modulated radiation therapy (IMRT), with the dose calculated separately for each side of the neck. Because of the separate treatment planning, the investigators analyzed the data by creating two separate models: one looking at all patients, and the other looking at 278 treatments in the 139 patients with IMRT.
In each model, about 85% of patients had stage III or IV disease and about 58% had cancer in the oropharynx, followed in order of frequency by the larynx, oral cavity, nasopharynx, or other sites.
A total of 33 patients had stenosis in 51 arteries. The median time between completion of radiation therapy and the last follow-up screening was 2 years. The median time to stenosis was 3 years.
Actuarial estimates of carotid artery stenosis were 2% at 1 year, 6% at 2 years, and 18% at 3 years.
In univariate analysis, factors associated with stenosis included male gender (P = .02), hypertension (P = .003), vascular disease (P less than .001), and Framingham score (P less than .001).
In the multivariate model looking at all patients, each Framingham risk factor was associated with a near doubling of stenosis risk (hazard ratio 1.8, P = .0003). In the model focusing on the IMRT population (adjusted for Framingham score), only radiation dose was significantly associated with stenosis (HR 1.07/Gy, P = .02).
Of the 33 patients with stenosis, 8 had no further follow-up imaging, 8 had stable stenosis, and 17 had progressive stenosis, 2 of whom had a cerebrovascular event. Eight patients with progressive stenosis received medical management only, and nine went on to surgery (three endarterectomies and six stent placements).
"Of the nine patients who underwent surgical management, there was a high rate of restenosis in 30% of patients at a year median follow-up, and this is consistent, unfortunately, with other series looking at rates of restenosis," Dr. Dorth said.
The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.
MIAMI BEACH – Head and neck cancer patients treated with radiation should be screened routinely for carotid artery stenosis, investigators recommended at the annual meeting of the American Society for Radiation Oncology.
Among 225 patients who had received radiation and were screened, an estimated 18% had significant asymptomatic stenosis (50% or greater narrowing) of one or both carotid arteries 3 years after treatment said Dr. Jennifer Dorth, a resident in radiation oncology at Duke University Medical Center in Durham, N.C.
"We recommend screening for head and neck cancer patients given that there are high rates of stenosis as well as high rates of progression of stenosis," she said.
Factors significantly associated with risk for stenosis included Framingham risk factors (smoking history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular/peripheral vascular disease, and atrial fibrillation) and radiation dose.
The investigators retrospectively reviewed outcomes of asymptomatic, disease-free head and neck cancer patients who had received radiation with curative intent to the neck. The patients were screened with carotid Doppler ultrasound at or after the 1-year follow-up visit, and this was repeated every 2-3 years. Patients with ultrasound evidence of 50% or greater stenosis were referred to vascular surgery.
The study identified 225 patients, 139 of whom had received intensity-modulated radiation therapy (IMRT), with the dose calculated separately for each side of the neck. Because of the separate treatment planning, the investigators analyzed the data by creating two separate models: one looking at all patients, and the other looking at 278 treatments in the 139 patients with IMRT.
In each model, about 85% of patients had stage III or IV disease and about 58% had cancer in the oropharynx, followed in order of frequency by the larynx, oral cavity, nasopharynx, or other sites.
A total of 33 patients had stenosis in 51 arteries. The median time between completion of radiation therapy and the last follow-up screening was 2 years. The median time to stenosis was 3 years.
Actuarial estimates of carotid artery stenosis were 2% at 1 year, 6% at 2 years, and 18% at 3 years.
In univariate analysis, factors associated with stenosis included male gender (P = .02), hypertension (P = .003), vascular disease (P less than .001), and Framingham score (P less than .001).
In the multivariate model looking at all patients, each Framingham risk factor was associated with a near doubling of stenosis risk (hazard ratio 1.8, P = .0003). In the model focusing on the IMRT population (adjusted for Framingham score), only radiation dose was significantly associated with stenosis (HR 1.07/Gy, P = .02).
Of the 33 patients with stenosis, 8 had no further follow-up imaging, 8 had stable stenosis, and 17 had progressive stenosis, 2 of whom had a cerebrovascular event. Eight patients with progressive stenosis received medical management only, and nine went on to surgery (three endarterectomies and six stent placements).
"Of the nine patients who underwent surgical management, there was a high rate of restenosis in 30% of patients at a year median follow-up, and this is consistent, unfortunately, with other series looking at rates of restenosis," Dr. Dorth said.
The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: The estimated risk for stenosis of the carotid arteries 3 years after irradiation for head and neck cancer was 18%.
Data Source: Retrospective review of data on 225 patients who received irradiation for head and neck cancer.
Disclosures: The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.
Nix Oxaliplatin, Up Radiation in Locally Advanced Rectal Cancer
MIAMI BEACH – For patients with locally advanced rectal cancer, hold the oxaliplatin, but up the preoperative radiation dose from 45 to 50 Gy, French investigators advised oncologists at a meeting here.
Chemoradiation with a less-toxic capecitabine (Xeloda)–containing regimen and 50 Gy radiation should become the standard of therapy for preoperative chemoradiation in patients with locally advanced rectal cancer, according to Dr. Jean-Pierre Gerard and his colleagues.
The assertion is based on evidence from a large phase III trial presented by Dr. Gerard and from earlier American and Italian studies suggesting better local control with higher radiation doses, but added toxicity from oxaliplatin (Eloxatin) without additional therapeutic benefit.
The evidence also suggests that capecitabine, which is given orally, can replace intravenous 5-fluourouracil (5-FU) infusions in this clinical setting, said Dr. Gerard, a radiation oncologist at the Centre Antoine-Lacassagne in Nice, France.
"When we take all the trials together, we propose the regimen we call ‘CAP50,’ where we give 5 weeks of treatment with 50 Gy, not 45 Gy. You sterilize more tumor, have only 4% local recurrence, replace 5-FU infusion with capecitabine by mouth – it’s very easy – and you delete oxaliplatin," Dr. Gerard said in a briefing at annual meeting of the American Society for Radiation Oncology, where he gave 3-year results from the ACCORD 12 trial.
Not everyone was convinced. Dr. Karyn A. Goodman, the invited discussant of Dr. Gerard’s featured talk in the meeting’s plenary session, said it’s difficult to tease out this conclusion from the data presented because the ACCORD 12 investigators, in an attempt to conduct a "pragmatic trial," moved the goalposts by changing the treatment parameters three times.
"The investigators took several leaps in the design of the study which make interpretation of their results more difficult," said Dr. Goodman from Memorial Sloan-Kettering Cancer Center in New York.
"First, they simultaneously increased the radiation dose to 50 Gy in 2-Gy fractions and added oxaliplatin in the experimental arm," she explained.
"Second, they changed the baseline chemotherapy from 5-FU to capecitabine, which is at a slightly lower dose than has been given in other preoperative chemoradiotherapy trials.
"Third, the primary end point was pathologic complete response, a marker for response that has not been validated as a surrogate for overall survival, and has even been shown ... to have not yet fulfilled the criteria to be a surrogate for overall survival or local control."
In addition, the study was powered to detect a large difference in pathological complete response rate, thus allowing for a smaller sample than would ordinarily be required to evaluate the effect of adjuvant therapy on colorectal cancer, Dr. Goodman added.
The rationale for ACCORD 12 came from large studies showing that even with total mesorectal excision, preoperative radiotherapy decreased local recurrence rates and that chemoradiotherapy – particularly preoperatively – was better than radiotherapy alone.
The trial designers emulated the design of the Italian STAR 01 trial (which was ongoing at the time the ACCORD 12 was started) by including oxaliplatin and with 50 Gy radiation prior to surgery in 747 patients. That trial, results of which were reported at the 2009 ASCO annual meeting, showed that oxaliplatin added nothing but toxicity to the therapeutic regimen
Dr. Gerard presented 3-year results from the study on 598 patients in an intention-to-treat population. The patients were randomized to receive either preoperative capecitabine 1600 mg/m2 for 5 days plus 45 Gy radiation in 1.8-Gy fractions or capecitabine at the same dose plus oxaliplatin 50 mg/m2 weekly plus 50 Gy divided into 2-Gy fractions. All patients then underwent total mesorectal excision, with adjuvant therapy at the treating center\'s discretion.
Early results, published in 2010 (J. Clin. Oncol. 2010;28:1638-44) showed no significant differences between capecitabine and 45 Gy (Cap45) and capecitabine plus oxaliplatin and 50 Gy (Capox50) for the primary end point of pathological complete response rates according to the Dworak criteria: 13.9% for 287 patients treated with Cap45 vs. 19.2% for 287 patients treated with Capox50 (P = .09). Grade 3 or 4 toxicities, however, were twice as high in the Capox50 patients, at 25%, compared with 11% of patients treated with Cap45 (P less than .001). This analysis was for the as-treated population.
At 3 years (intention-to-treat population, 299 patients in each arm), Dr. Gerard reported no significant differences between Cap45 and Capox50 in local recurrence (6.1% vs. 4.4%), distant metastases (25% vs. 21%), disease-free survival (71% vs. 73%), overall survival (85% vs. 88%), or grade 3 or great toxicities (2.7%, 4 patients, vs. 1.3%, 2 patients).
In exploratory analyses, significant predictors for 3-year disease-free survival were pathological stage (T0-1 vs. T2 or T3; P less than .00001), nodal status (N0 vs. N1-2; P less than .0001), and close margins (1 mm or less vs. greater than 1 mm; P less than .0001).
The investigations concluded that oxaliplatin increases toxicity (primarily diarrhea) without affecting pathological response, that 50 Gy over 5 weeks is compatible with surgery and may help to sterilize the surgical sample, and that capecitabine has activity equivalent to that of 5-FU, without the need for intravenous infusion.
The ACCORD 12 trial was supported in part by Roche and Sanofi-Aventis together with a grant of the French National Program of Research Programmes Hospitaliers de Recherche Clinique. It was conducted under the auspices of Institut National du Cancer. Dr. Gerard and Dr. Goodman reported that they had no relevant financial disclosures.
MIAMI BEACH – For patients with locally advanced rectal cancer, hold the oxaliplatin, but up the preoperative radiation dose from 45 to 50 Gy, French investigators advised oncologists at a meeting here.
Chemoradiation with a less-toxic capecitabine (Xeloda)–containing regimen and 50 Gy radiation should become the standard of therapy for preoperative chemoradiation in patients with locally advanced rectal cancer, according to Dr. Jean-Pierre Gerard and his colleagues.
The assertion is based on evidence from a large phase III trial presented by Dr. Gerard and from earlier American and Italian studies suggesting better local control with higher radiation doses, but added toxicity from oxaliplatin (Eloxatin) without additional therapeutic benefit.
The evidence also suggests that capecitabine, which is given orally, can replace intravenous 5-fluourouracil (5-FU) infusions in this clinical setting, said Dr. Gerard, a radiation oncologist at the Centre Antoine-Lacassagne in Nice, France.
"When we take all the trials together, we propose the regimen we call ‘CAP50,’ where we give 5 weeks of treatment with 50 Gy, not 45 Gy. You sterilize more tumor, have only 4% local recurrence, replace 5-FU infusion with capecitabine by mouth – it’s very easy – and you delete oxaliplatin," Dr. Gerard said in a briefing at annual meeting of the American Society for Radiation Oncology, where he gave 3-year results from the ACCORD 12 trial.
Not everyone was convinced. Dr. Karyn A. Goodman, the invited discussant of Dr. Gerard’s featured talk in the meeting’s plenary session, said it’s difficult to tease out this conclusion from the data presented because the ACCORD 12 investigators, in an attempt to conduct a "pragmatic trial," moved the goalposts by changing the treatment parameters three times.
"The investigators took several leaps in the design of the study which make interpretation of their results more difficult," said Dr. Goodman from Memorial Sloan-Kettering Cancer Center in New York.
"First, they simultaneously increased the radiation dose to 50 Gy in 2-Gy fractions and added oxaliplatin in the experimental arm," she explained.
"Second, they changed the baseline chemotherapy from 5-FU to capecitabine, which is at a slightly lower dose than has been given in other preoperative chemoradiotherapy trials.
"Third, the primary end point was pathologic complete response, a marker for response that has not been validated as a surrogate for overall survival, and has even been shown ... to have not yet fulfilled the criteria to be a surrogate for overall survival or local control."
In addition, the study was powered to detect a large difference in pathological complete response rate, thus allowing for a smaller sample than would ordinarily be required to evaluate the effect of adjuvant therapy on colorectal cancer, Dr. Goodman added.
The rationale for ACCORD 12 came from large studies showing that even with total mesorectal excision, preoperative radiotherapy decreased local recurrence rates and that chemoradiotherapy – particularly preoperatively – was better than radiotherapy alone.
The trial designers emulated the design of the Italian STAR 01 trial (which was ongoing at the time the ACCORD 12 was started) by including oxaliplatin and with 50 Gy radiation prior to surgery in 747 patients. That trial, results of which were reported at the 2009 ASCO annual meeting, showed that oxaliplatin added nothing but toxicity to the therapeutic regimen
Dr. Gerard presented 3-year results from the study on 598 patients in an intention-to-treat population. The patients were randomized to receive either preoperative capecitabine 1600 mg/m2 for 5 days plus 45 Gy radiation in 1.8-Gy fractions or capecitabine at the same dose plus oxaliplatin 50 mg/m2 weekly plus 50 Gy divided into 2-Gy fractions. All patients then underwent total mesorectal excision, with adjuvant therapy at the treating center\'s discretion.
Early results, published in 2010 (J. Clin. Oncol. 2010;28:1638-44) showed no significant differences between capecitabine and 45 Gy (Cap45) and capecitabine plus oxaliplatin and 50 Gy (Capox50) for the primary end point of pathological complete response rates according to the Dworak criteria: 13.9% for 287 patients treated with Cap45 vs. 19.2% for 287 patients treated with Capox50 (P = .09). Grade 3 or 4 toxicities, however, were twice as high in the Capox50 patients, at 25%, compared with 11% of patients treated with Cap45 (P less than .001). This analysis was for the as-treated population.
At 3 years (intention-to-treat population, 299 patients in each arm), Dr. Gerard reported no significant differences between Cap45 and Capox50 in local recurrence (6.1% vs. 4.4%), distant metastases (25% vs. 21%), disease-free survival (71% vs. 73%), overall survival (85% vs. 88%), or grade 3 or great toxicities (2.7%, 4 patients, vs. 1.3%, 2 patients).
In exploratory analyses, significant predictors for 3-year disease-free survival were pathological stage (T0-1 vs. T2 or T3; P less than .00001), nodal status (N0 vs. N1-2; P less than .0001), and close margins (1 mm or less vs. greater than 1 mm; P less than .0001).
The investigations concluded that oxaliplatin increases toxicity (primarily diarrhea) without affecting pathological response, that 50 Gy over 5 weeks is compatible with surgery and may help to sterilize the surgical sample, and that capecitabine has activity equivalent to that of 5-FU, without the need for intravenous infusion.
The ACCORD 12 trial was supported in part by Roche and Sanofi-Aventis together with a grant of the French National Program of Research Programmes Hospitaliers de Recherche Clinique. It was conducted under the auspices of Institut National du Cancer. Dr. Gerard and Dr. Goodman reported that they had no relevant financial disclosures.
MIAMI BEACH – For patients with locally advanced rectal cancer, hold the oxaliplatin, but up the preoperative radiation dose from 45 to 50 Gy, French investigators advised oncologists at a meeting here.
Chemoradiation with a less-toxic capecitabine (Xeloda)–containing regimen and 50 Gy radiation should become the standard of therapy for preoperative chemoradiation in patients with locally advanced rectal cancer, according to Dr. Jean-Pierre Gerard and his colleagues.
The assertion is based on evidence from a large phase III trial presented by Dr. Gerard and from earlier American and Italian studies suggesting better local control with higher radiation doses, but added toxicity from oxaliplatin (Eloxatin) without additional therapeutic benefit.
The evidence also suggests that capecitabine, which is given orally, can replace intravenous 5-fluourouracil (5-FU) infusions in this clinical setting, said Dr. Gerard, a radiation oncologist at the Centre Antoine-Lacassagne in Nice, France.
"When we take all the trials together, we propose the regimen we call ‘CAP50,’ where we give 5 weeks of treatment with 50 Gy, not 45 Gy. You sterilize more tumor, have only 4% local recurrence, replace 5-FU infusion with capecitabine by mouth – it’s very easy – and you delete oxaliplatin," Dr. Gerard said in a briefing at annual meeting of the American Society for Radiation Oncology, where he gave 3-year results from the ACCORD 12 trial.
Not everyone was convinced. Dr. Karyn A. Goodman, the invited discussant of Dr. Gerard’s featured talk in the meeting’s plenary session, said it’s difficult to tease out this conclusion from the data presented because the ACCORD 12 investigators, in an attempt to conduct a "pragmatic trial," moved the goalposts by changing the treatment parameters three times.
"The investigators took several leaps in the design of the study which make interpretation of their results more difficult," said Dr. Goodman from Memorial Sloan-Kettering Cancer Center in New York.
"First, they simultaneously increased the radiation dose to 50 Gy in 2-Gy fractions and added oxaliplatin in the experimental arm," she explained.
"Second, they changed the baseline chemotherapy from 5-FU to capecitabine, which is at a slightly lower dose than has been given in other preoperative chemoradiotherapy trials.
"Third, the primary end point was pathologic complete response, a marker for response that has not been validated as a surrogate for overall survival, and has even been shown ... to have not yet fulfilled the criteria to be a surrogate for overall survival or local control."
In addition, the study was powered to detect a large difference in pathological complete response rate, thus allowing for a smaller sample than would ordinarily be required to evaluate the effect of adjuvant therapy on colorectal cancer, Dr. Goodman added.
The rationale for ACCORD 12 came from large studies showing that even with total mesorectal excision, preoperative radiotherapy decreased local recurrence rates and that chemoradiotherapy – particularly preoperatively – was better than radiotherapy alone.
The trial designers emulated the design of the Italian STAR 01 trial (which was ongoing at the time the ACCORD 12 was started) by including oxaliplatin and with 50 Gy radiation prior to surgery in 747 patients. That trial, results of which were reported at the 2009 ASCO annual meeting, showed that oxaliplatin added nothing but toxicity to the therapeutic regimen
Dr. Gerard presented 3-year results from the study on 598 patients in an intention-to-treat population. The patients were randomized to receive either preoperative capecitabine 1600 mg/m2 for 5 days plus 45 Gy radiation in 1.8-Gy fractions or capecitabine at the same dose plus oxaliplatin 50 mg/m2 weekly plus 50 Gy divided into 2-Gy fractions. All patients then underwent total mesorectal excision, with adjuvant therapy at the treating center\'s discretion.
Early results, published in 2010 (J. Clin. Oncol. 2010;28:1638-44) showed no significant differences between capecitabine and 45 Gy (Cap45) and capecitabine plus oxaliplatin and 50 Gy (Capox50) for the primary end point of pathological complete response rates according to the Dworak criteria: 13.9% for 287 patients treated with Cap45 vs. 19.2% for 287 patients treated with Capox50 (P = .09). Grade 3 or 4 toxicities, however, were twice as high in the Capox50 patients, at 25%, compared with 11% of patients treated with Cap45 (P less than .001). This analysis was for the as-treated population.
At 3 years (intention-to-treat population, 299 patients in each arm), Dr. Gerard reported no significant differences between Cap45 and Capox50 in local recurrence (6.1% vs. 4.4%), distant metastases (25% vs. 21%), disease-free survival (71% vs. 73%), overall survival (85% vs. 88%), or grade 3 or great toxicities (2.7%, 4 patients, vs. 1.3%, 2 patients).
In exploratory analyses, significant predictors for 3-year disease-free survival were pathological stage (T0-1 vs. T2 or T3; P less than .00001), nodal status (N0 vs. N1-2; P less than .0001), and close margins (1 mm or less vs. greater than 1 mm; P less than .0001).
The investigations concluded that oxaliplatin increases toxicity (primarily diarrhea) without affecting pathological response, that 50 Gy over 5 weeks is compatible with surgery and may help to sterilize the surgical sample, and that capecitabine has activity equivalent to that of 5-FU, without the need for intravenous infusion.
The ACCORD 12 trial was supported in part by Roche and Sanofi-Aventis together with a grant of the French National Program of Research Programmes Hospitaliers de Recherche Clinique. It was conducted under the auspices of Institut National du Cancer. Dr. Gerard and Dr. Goodman reported that they had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: At 3 years, there were no significant differences between Cap45 and Capox50 in local recurrence (6.1% vs. 4.4%), distant metastases (25% vs. 21%), disease-free survival (71% vs. 73%), overall survival (85% vs. 88%), or grade 3 or great toxicities (2.7% vs. 1.3%).
Data Source: 598 patients with advanced rectal cancer in the phase III, randomized ACCORD 12 trial.
Disclosures: The trial was supported in part by Roche and Sanofi- Aventis together with a grant of the French National Program of Research Programmes. It was conducted under the auspices of Institut National du Cancer. Dr. Gerard and Dr. Goodman reported that they had no relevant financial disclosures.
Less Toxic IMRT Controls Cervical Cancer After Hysterectomy
MIAMI BEACH – For women with cervical cancer, intensity-modulated radiation therapy to the pelvis after surgery provides disease control similar to that seen with standard external beam radiation therapy, but with lower acute bowel toxicity, investigators reported here.
Two-year results from the phase II Radiation Therapy Oncology Group (RTOG) 0418 trial showed that the combination of the newer radiation technique and weekly cisplatin chemotherapy was associated with an estimated disease-free survival rate of 86.9%. This compares with historic data, Dr. Lorraine Portelance told attendees at the annual meeting of the American Society for Radiation Oncology (ASTRO).
The RTOG investigators had previously reported that pelvic intensity-modulated radiation therapy (IMRT) to 50.4 Gy delivered over 28 fractions plus weekly cisplatin 40 mg/m2 with or without vaginal brachytherapy was associated with a significant reduction in grade 2 or greater short-term bowel toxicity compared with historical controls.
"One very important question remained, though: were we able to achieve decreased toxicity and good chemotherapy compliance at a cost of disease control loss? We have now reached the point where we could analyze the secondary end point of disease control, which is of prime importance to determine if this disease approach is viable," said Dr. Portelance from the University of Miami’s Sylvester Comprehensive Cancer Center.
A total of 40 patients from 25 institutions were eligible for analysis of the secondary end point. All had hysterectomies (3 total abdominal, 3 vaginal, 28 radical, and 6 laparoscopic assisted).
Two patients (5%) had International Federation of Gynecologists and Obstetricians (FIGO) stage IA disease; 31 (77.5%) had stage IB; 4 (10%) had stage IIA; and 3 (7.5) had stage IIB disease. In 25 patients there was no nodal involvement; 15 had 1 involved node.
At a median 2.68 years’ follow-up, there were 5 local-regional relapses (estimated 2-year rate, 10.6%); 3 para-aortic nodes involved (5.3%); and 4 distant metastases (excluding the para-aortic nodes; 10.3%).
Dr. Portelance noted that this translated into an estimated 2-year disease-free survival rate of 86.9%, which is comparable to that shown in an intergroup trial, published in 2000, that compared concurrent chemotherapy and pelvic radiation therapy with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cervical cancer (J. Clin. Oncol. 2000;18:1606-13).
In the RTOG 0418 trial, the estimated 2-year overall survival rate is 94.6%, again comparable with previously published data, Dr. Portelance said.
"This ROTG 0418 study provides level II evidence that, when done under clear guidelines, postoperative pelvic intensity-modulated radiation could lead to decreased toxicity and excellent chemotherapy without any cost in disease control," she concluded.
The study was supported by grants from the National Cancer Institute. Dr. Portelance said she had no conflicts of interest.
MIAMI BEACH – For women with cervical cancer, intensity-modulated radiation therapy to the pelvis after surgery provides disease control similar to that seen with standard external beam radiation therapy, but with lower acute bowel toxicity, investigators reported here.
Two-year results from the phase II Radiation Therapy Oncology Group (RTOG) 0418 trial showed that the combination of the newer radiation technique and weekly cisplatin chemotherapy was associated with an estimated disease-free survival rate of 86.9%. This compares with historic data, Dr. Lorraine Portelance told attendees at the annual meeting of the American Society for Radiation Oncology (ASTRO).
The RTOG investigators had previously reported that pelvic intensity-modulated radiation therapy (IMRT) to 50.4 Gy delivered over 28 fractions plus weekly cisplatin 40 mg/m2 with or without vaginal brachytherapy was associated with a significant reduction in grade 2 or greater short-term bowel toxicity compared with historical controls.
"One very important question remained, though: were we able to achieve decreased toxicity and good chemotherapy compliance at a cost of disease control loss? We have now reached the point where we could analyze the secondary end point of disease control, which is of prime importance to determine if this disease approach is viable," said Dr. Portelance from the University of Miami’s Sylvester Comprehensive Cancer Center.
A total of 40 patients from 25 institutions were eligible for analysis of the secondary end point. All had hysterectomies (3 total abdominal, 3 vaginal, 28 radical, and 6 laparoscopic assisted).
Two patients (5%) had International Federation of Gynecologists and Obstetricians (FIGO) stage IA disease; 31 (77.5%) had stage IB; 4 (10%) had stage IIA; and 3 (7.5) had stage IIB disease. In 25 patients there was no nodal involvement; 15 had 1 involved node.
At a median 2.68 years’ follow-up, there were 5 local-regional relapses (estimated 2-year rate, 10.6%); 3 para-aortic nodes involved (5.3%); and 4 distant metastases (excluding the para-aortic nodes; 10.3%).
Dr. Portelance noted that this translated into an estimated 2-year disease-free survival rate of 86.9%, which is comparable to that shown in an intergroup trial, published in 2000, that compared concurrent chemotherapy and pelvic radiation therapy with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cervical cancer (J. Clin. Oncol. 2000;18:1606-13).
In the RTOG 0418 trial, the estimated 2-year overall survival rate is 94.6%, again comparable with previously published data, Dr. Portelance said.
"This ROTG 0418 study provides level II evidence that, when done under clear guidelines, postoperative pelvic intensity-modulated radiation could lead to decreased toxicity and excellent chemotherapy without any cost in disease control," she concluded.
The study was supported by grants from the National Cancer Institute. Dr. Portelance said she had no conflicts of interest.
MIAMI BEACH – For women with cervical cancer, intensity-modulated radiation therapy to the pelvis after surgery provides disease control similar to that seen with standard external beam radiation therapy, but with lower acute bowel toxicity, investigators reported here.
Two-year results from the phase II Radiation Therapy Oncology Group (RTOG) 0418 trial showed that the combination of the newer radiation technique and weekly cisplatin chemotherapy was associated with an estimated disease-free survival rate of 86.9%. This compares with historic data, Dr. Lorraine Portelance told attendees at the annual meeting of the American Society for Radiation Oncology (ASTRO).
The RTOG investigators had previously reported that pelvic intensity-modulated radiation therapy (IMRT) to 50.4 Gy delivered over 28 fractions plus weekly cisplatin 40 mg/m2 with or without vaginal brachytherapy was associated with a significant reduction in grade 2 or greater short-term bowel toxicity compared with historical controls.
"One very important question remained, though: were we able to achieve decreased toxicity and good chemotherapy compliance at a cost of disease control loss? We have now reached the point where we could analyze the secondary end point of disease control, which is of prime importance to determine if this disease approach is viable," said Dr. Portelance from the University of Miami’s Sylvester Comprehensive Cancer Center.
A total of 40 patients from 25 institutions were eligible for analysis of the secondary end point. All had hysterectomies (3 total abdominal, 3 vaginal, 28 radical, and 6 laparoscopic assisted).
Two patients (5%) had International Federation of Gynecologists and Obstetricians (FIGO) stage IA disease; 31 (77.5%) had stage IB; 4 (10%) had stage IIA; and 3 (7.5) had stage IIB disease. In 25 patients there was no nodal involvement; 15 had 1 involved node.
At a median 2.68 years’ follow-up, there were 5 local-regional relapses (estimated 2-year rate, 10.6%); 3 para-aortic nodes involved (5.3%); and 4 distant metastases (excluding the para-aortic nodes; 10.3%).
Dr. Portelance noted that this translated into an estimated 2-year disease-free survival rate of 86.9%, which is comparable to that shown in an intergroup trial, published in 2000, that compared concurrent chemotherapy and pelvic radiation therapy with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cervical cancer (J. Clin. Oncol. 2000;18:1606-13).
In the RTOG 0418 trial, the estimated 2-year overall survival rate is 94.6%, again comparable with previously published data, Dr. Portelance said.
"This ROTG 0418 study provides level II evidence that, when done under clear guidelines, postoperative pelvic intensity-modulated radiation could lead to decreased toxicity and excellent chemotherapy without any cost in disease control," she concluded.
The study was supported by grants from the National Cancer Institute. Dr. Portelance said she had no conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Intensity-modulated radiation therapy following surgery was associated with an estimated 2-year disease-free survival rate of 86.9%, and overall survival rate of 94.6%, in patients with cervical cancer.
Data Source: Follow-up of the prospective multi-institutional RTOG 0418 trial.
Disclosures: The study was supported by grants from the National Cancer Institute. Dr. Portelance had no conflict of interest disclosures.
Multimodal DCIS Therapy, Tamoxifen Cuts Breast Cancer Deaths
MIAMI BEACH – Adding radiotherapy and tamoxifen to breast-conserving surgery significantly reduces the local recurrence rate and the breast cancer–specific death rate in women with ductal carcinoma in situ, according to a systematic review and meta-analysis.
The review of 22 studies with a minimum of 10-year follow-up data showed that surgery plus radiation therapy nearly halved the rate of ipsilateral local recurrence from 23.5% with surgery alone to 13.5%, and the addition of tamoxifen cut the rate even further, to 9.2%.
The addition of tamoxifen to surgery and radiation also reduced breast cancer death rates from 3.1% without the drug to 1.5% with it, reported Dr. Kirsty Stuart of the Westmead Breast Cancer Institute at Westmead Hospital in Sydney, Australia.
"DCIS [ductal carcinoma in situ] treatment, however, will ultimately depend on the individual patient, their general condition, their tumor, and their fears," she told attendees at the annual meeting of the American Society for Radiation Oncology.
Dr. Stuart and colleagues conducted a meta-analysis of published randomized or nonrandomized trials of long-term outcomes in DCIS to determine the benefits of adjuvant radiotherapy and tamoxifen, a selective estrogen receptor modulator. The subjects all had pure DCIS with a minimum of 10-years’ follow-up, with data on treatment type and local recurrence. All studies were peer reviewed.
The investigators defined local recurrence as subsequent ipsilateral breast or chest wall disease (DCIS or invasive), and calculated the breast cancer death rate as the number of deaths from breast cancer divided by the total number of DCIS cases.
They identified a total of 22 qualifying studies dating from 1974 through 2011 with 6,167 patients. In all, 4.9% of patients had mastectomies, 51.8% had conservative surgery plus radiation, 41.2% had conservative surgery alone, and 2.1% had biopsy alone.
Among all cases, ipsilateral local recurrence was seen in 3.3% of mastectomy patients, 13.5% of patients who had surgery and radiation, 23.5% of surgery only patients, and 35.1% of biopsy only patients. Between-treatment comparisons showed that mastectomy was significantly better than each of the forms of therapy, both at preventing all cases of ipsilateral local recurrences and all cases of invasive local recurrence.
Looking at the addition of tamoxifen to surgery with or without radiation, the authors found that the drug significantly reduced the rate of local recurrence, from 24.1% with surgery alone to 19.8% with surgery and tamoxifen, and from 14.9% for the surgery/radiation combination to 9.2% for the two modalities plus tamoxifen.
Between-treatment comparisons showed that adding tamoxifen to radiation and surgery significantly improved recurrence rates over surgery plus radiation (P = .037), surgery plus tamoxifen (P = .0086), or surgery alone (P less than .000001). Compared with surgery only, the relative risk for invasive local recurrence was 0.71 for surgery plus tamoxifen, 0.63 for surgery plus radiotherapy, and 0.35 for all three treatments.
Invasive breast cancer death rates were also significantly lower when tamoxifen was added to surgery and radiation, decreasing from 8.4% without the drug to 4.3% with it.
"From the pooled data, conservative surgery alone for DCIS has a high recurrence rate that is partly reduced with tamoxifen," Dr. Stuart said.
"DCIS treatment will ultimately depend on the individual patient, their general condition, their tumor, and their fears."
"Conservative surgery plus radiation therapy almost halves the ipsilateral recurrence rate, and has a breast cancer death rate that is equivalent to that of the mastectomy population.
"Conservative surgery and radiation therapy plus tamoxifen halves the invasive local recurrence rate, from 8% to 4%, and halves the breast cancer death rate, from 3% to 1.5%."
In a separate study Dr. Julia Wong of the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, both in Boston, presented 8-year follow-up data on wide-area excision alone in 132 patients treated for DCIS. The investigators found that 19 patients had a local recurrence. The cumulative 8-year local recurrence rate was 14.4%. A total of 13 of the recurrences were DCIS, and 6 were invasive disease. All but one of the recurrences was detectable by mammogram, and one was palpable.
A total of 14 of the recurrences were in the same quadrant as the original tumor, and 5 were elsewhere in the same breast. Of the six patients with invasive disease, none had axillary involvement, and no patients developed distant metastases.
Other events seen in the study included 13 contralateral breast cancers (4 DCIS, 9 invasive), 1 other cancer, and 3 deaths from other causes.
"Even in this highly selected group of patients with small grade 1 or 2 DCIS treated with wide excision alone and margins 1 cm or greater, there is a substantial local recurrence rate, especially in the same quadrant," Dr. Wong said.
The meta-analysis was internally funded. Dr. Stuart reported having no relevant financial disclosures. Dr. Wong’s study was funded by participating institutions. Dr. Wong reported no other relevant financial disclosures.
MIAMI BEACH – Adding radiotherapy and tamoxifen to breast-conserving surgery significantly reduces the local recurrence rate and the breast cancer–specific death rate in women with ductal carcinoma in situ, according to a systematic review and meta-analysis.
The review of 22 studies with a minimum of 10-year follow-up data showed that surgery plus radiation therapy nearly halved the rate of ipsilateral local recurrence from 23.5% with surgery alone to 13.5%, and the addition of tamoxifen cut the rate even further, to 9.2%.
The addition of tamoxifen to surgery and radiation also reduced breast cancer death rates from 3.1% without the drug to 1.5% with it, reported Dr. Kirsty Stuart of the Westmead Breast Cancer Institute at Westmead Hospital in Sydney, Australia.
"DCIS [ductal carcinoma in situ] treatment, however, will ultimately depend on the individual patient, their general condition, their tumor, and their fears," she told attendees at the annual meeting of the American Society for Radiation Oncology.
Dr. Stuart and colleagues conducted a meta-analysis of published randomized or nonrandomized trials of long-term outcomes in DCIS to determine the benefits of adjuvant radiotherapy and tamoxifen, a selective estrogen receptor modulator. The subjects all had pure DCIS with a minimum of 10-years’ follow-up, with data on treatment type and local recurrence. All studies were peer reviewed.
The investigators defined local recurrence as subsequent ipsilateral breast or chest wall disease (DCIS or invasive), and calculated the breast cancer death rate as the number of deaths from breast cancer divided by the total number of DCIS cases.
They identified a total of 22 qualifying studies dating from 1974 through 2011 with 6,167 patients. In all, 4.9% of patients had mastectomies, 51.8% had conservative surgery plus radiation, 41.2% had conservative surgery alone, and 2.1% had biopsy alone.
Among all cases, ipsilateral local recurrence was seen in 3.3% of mastectomy patients, 13.5% of patients who had surgery and radiation, 23.5% of surgery only patients, and 35.1% of biopsy only patients. Between-treatment comparisons showed that mastectomy was significantly better than each of the forms of therapy, both at preventing all cases of ipsilateral local recurrences and all cases of invasive local recurrence.
Looking at the addition of tamoxifen to surgery with or without radiation, the authors found that the drug significantly reduced the rate of local recurrence, from 24.1% with surgery alone to 19.8% with surgery and tamoxifen, and from 14.9% for the surgery/radiation combination to 9.2% for the two modalities plus tamoxifen.
Between-treatment comparisons showed that adding tamoxifen to radiation and surgery significantly improved recurrence rates over surgery plus radiation (P = .037), surgery plus tamoxifen (P = .0086), or surgery alone (P less than .000001). Compared with surgery only, the relative risk for invasive local recurrence was 0.71 for surgery plus tamoxifen, 0.63 for surgery plus radiotherapy, and 0.35 for all three treatments.
Invasive breast cancer death rates were also significantly lower when tamoxifen was added to surgery and radiation, decreasing from 8.4% without the drug to 4.3% with it.
"From the pooled data, conservative surgery alone for DCIS has a high recurrence rate that is partly reduced with tamoxifen," Dr. Stuart said.
"DCIS treatment will ultimately depend on the individual patient, their general condition, their tumor, and their fears."
"Conservative surgery plus radiation therapy almost halves the ipsilateral recurrence rate, and has a breast cancer death rate that is equivalent to that of the mastectomy population.
"Conservative surgery and radiation therapy plus tamoxifen halves the invasive local recurrence rate, from 8% to 4%, and halves the breast cancer death rate, from 3% to 1.5%."
In a separate study Dr. Julia Wong of the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, both in Boston, presented 8-year follow-up data on wide-area excision alone in 132 patients treated for DCIS. The investigators found that 19 patients had a local recurrence. The cumulative 8-year local recurrence rate was 14.4%. A total of 13 of the recurrences were DCIS, and 6 were invasive disease. All but one of the recurrences was detectable by mammogram, and one was palpable.
A total of 14 of the recurrences were in the same quadrant as the original tumor, and 5 were elsewhere in the same breast. Of the six patients with invasive disease, none had axillary involvement, and no patients developed distant metastases.
Other events seen in the study included 13 contralateral breast cancers (4 DCIS, 9 invasive), 1 other cancer, and 3 deaths from other causes.
"Even in this highly selected group of patients with small grade 1 or 2 DCIS treated with wide excision alone and margins 1 cm or greater, there is a substantial local recurrence rate, especially in the same quadrant," Dr. Wong said.
The meta-analysis was internally funded. Dr. Stuart reported having no relevant financial disclosures. Dr. Wong’s study was funded by participating institutions. Dr. Wong reported no other relevant financial disclosures.
MIAMI BEACH – Adding radiotherapy and tamoxifen to breast-conserving surgery significantly reduces the local recurrence rate and the breast cancer–specific death rate in women with ductal carcinoma in situ, according to a systematic review and meta-analysis.
The review of 22 studies with a minimum of 10-year follow-up data showed that surgery plus radiation therapy nearly halved the rate of ipsilateral local recurrence from 23.5% with surgery alone to 13.5%, and the addition of tamoxifen cut the rate even further, to 9.2%.
The addition of tamoxifen to surgery and radiation also reduced breast cancer death rates from 3.1% without the drug to 1.5% with it, reported Dr. Kirsty Stuart of the Westmead Breast Cancer Institute at Westmead Hospital in Sydney, Australia.
"DCIS [ductal carcinoma in situ] treatment, however, will ultimately depend on the individual patient, their general condition, their tumor, and their fears," she told attendees at the annual meeting of the American Society for Radiation Oncology.
Dr. Stuart and colleagues conducted a meta-analysis of published randomized or nonrandomized trials of long-term outcomes in DCIS to determine the benefits of adjuvant radiotherapy and tamoxifen, a selective estrogen receptor modulator. The subjects all had pure DCIS with a minimum of 10-years’ follow-up, with data on treatment type and local recurrence. All studies were peer reviewed.
The investigators defined local recurrence as subsequent ipsilateral breast or chest wall disease (DCIS or invasive), and calculated the breast cancer death rate as the number of deaths from breast cancer divided by the total number of DCIS cases.
They identified a total of 22 qualifying studies dating from 1974 through 2011 with 6,167 patients. In all, 4.9% of patients had mastectomies, 51.8% had conservative surgery plus radiation, 41.2% had conservative surgery alone, and 2.1% had biopsy alone.
Among all cases, ipsilateral local recurrence was seen in 3.3% of mastectomy patients, 13.5% of patients who had surgery and radiation, 23.5% of surgery only patients, and 35.1% of biopsy only patients. Between-treatment comparisons showed that mastectomy was significantly better than each of the forms of therapy, both at preventing all cases of ipsilateral local recurrences and all cases of invasive local recurrence.
Looking at the addition of tamoxifen to surgery with or without radiation, the authors found that the drug significantly reduced the rate of local recurrence, from 24.1% with surgery alone to 19.8% with surgery and tamoxifen, and from 14.9% for the surgery/radiation combination to 9.2% for the two modalities plus tamoxifen.
Between-treatment comparisons showed that adding tamoxifen to radiation and surgery significantly improved recurrence rates over surgery plus radiation (P = .037), surgery plus tamoxifen (P = .0086), or surgery alone (P less than .000001). Compared with surgery only, the relative risk for invasive local recurrence was 0.71 for surgery plus tamoxifen, 0.63 for surgery plus radiotherapy, and 0.35 for all three treatments.
Invasive breast cancer death rates were also significantly lower when tamoxifen was added to surgery and radiation, decreasing from 8.4% without the drug to 4.3% with it.
"From the pooled data, conservative surgery alone for DCIS has a high recurrence rate that is partly reduced with tamoxifen," Dr. Stuart said.
"DCIS treatment will ultimately depend on the individual patient, their general condition, their tumor, and their fears."
"Conservative surgery plus radiation therapy almost halves the ipsilateral recurrence rate, and has a breast cancer death rate that is equivalent to that of the mastectomy population.
"Conservative surgery and radiation therapy plus tamoxifen halves the invasive local recurrence rate, from 8% to 4%, and halves the breast cancer death rate, from 3% to 1.5%."
In a separate study Dr. Julia Wong of the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, both in Boston, presented 8-year follow-up data on wide-area excision alone in 132 patients treated for DCIS. The investigators found that 19 patients had a local recurrence. The cumulative 8-year local recurrence rate was 14.4%. A total of 13 of the recurrences were DCIS, and 6 were invasive disease. All but one of the recurrences was detectable by mammogram, and one was palpable.
A total of 14 of the recurrences were in the same quadrant as the original tumor, and 5 were elsewhere in the same breast. Of the six patients with invasive disease, none had axillary involvement, and no patients developed distant metastases.
Other events seen in the study included 13 contralateral breast cancers (4 DCIS, 9 invasive), 1 other cancer, and 3 deaths from other causes.
"Even in this highly selected group of patients with small grade 1 or 2 DCIS treated with wide excision alone and margins 1 cm or greater, there is a substantial local recurrence rate, especially in the same quadrant," Dr. Wong said.
The meta-analysis was internally funded. Dr. Stuart reported having no relevant financial disclosures. Dr. Wong’s study was funded by participating institutions. Dr. Wong reported no other relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: The addition of tamoxifen to surgery and radiation reduced breast cancer death rates from 3.1% without the drug to 1.5% with it.
Data Source: Systematic review and meta-analysis of 22 studies with long-term follow-up of women treated for ductal carcinoma in situ.
Disclosures: The meta-analysis was internally funded. Dr. Stuart reported having no relevant financial disclosures. Dr. Wong's study was funded by the participating institutions. Dr. Wong reported having no other relevant financial disclosures.
Lower Radiation Dose Improves Lung Cancer Survival
MIAMI BEACH – Less turned out to be better in a large clinical trial comparing radiation doses in patients treated with radiation and chemotherapy for stage III non–small cell lung cancer, investigators reported here.
The median overall survival rate at 1 year was 81% for patients treated with standard-dose (60 Gy) radiation, compared with 70.4% for those who received the high dose (74 Gy), according to preliminary findings from the radiation-dose arm of the ongoing phase III Radiation Therapy Oncology Group (RTOG) 0617 trial. The respective median survival rates were 21.7 months and 20.7 months (P = .02).
A planned interim analysis from the trial showed that the radiation comparison had crossed the prespecified boundary for futility, and the high-dose arm was stopped in June 2011, reported Dr. Jeffrey Bradley from Washington University in St. Louis.
"I think this changes practice: If [cancer centers] weren’t using 60 Gray before, perhaps they should go back to using 60 Gray, because it does not appear that a higher dose is better," Dr. Bradley commented at the annual meeting of the American Society of Radiation Oncology (ASTRO).
Dr. Tim R. Williams, from the Lynn Cancer Institute at Boca Raton (Fla.) Regional Hospital, the immediate-past chairman of ASTRO, noted that his center has used high-dose radiation in stage III non–small cell lung cancer (NSCLC) patients for about 5 years. Although practice patterns vary, it’s likely that many treatment centers currently use the higher dose, he said.
In RTOG 0617, a total of 500 patients with stage IIIA/IIIB NSCLC were scheduled for randomization to one of four arms in a 2 x 2 factorial design with patients assigned to receive either 74 Gy or 60 Gy radiation with or without cetuximab (Erbitux), on a background chemotherapy regimen of weekly paclitaxel (45 mg/m2) and carboplatin (titrated to an area-under-the-curve of 2).
The radiation was delivered in 2 Gy fractions over 30 to 37 fractions.
The analysis was performed on 426 patients who had been enrolled in the study before June 17, 2011.
Seeking to understand why the higher radiation dose was not better – the investigators had originally hypothesized that 74 Gy would result in a 7-month improvement in overall survival vs. 64 Gy.– they performed univariate analyses, and found that significant predictors for better outcomes included continuous therapy, nonsquamous histology, and, female gender. In multivariate analysis, radiation dose (60 Gy vs. 74 Gy) was associated with a hazard ratio for overall survival of 1.48 (P = .038),
nonsquamous histology versus squamous was associated with an HR of 1.52 (P = .025), and gross or internal tumor volume had a small but significant HR of 1.002 (P = .011).
Dr. Benjamin Movsas, chair of radiation oncology at the Henry Ford Health System in Detroit, the invited discussant, said that "as of 2011, level I evidence demonstrates no role for dose escalation in stage III non–small cell lung cancer."
He noted that although there were small differences between the radiation dose groups in terms of tumor histology, gross tumor volume, and other factors, they were not large enough to explain the differences in outcomes.
Citing the advice of his late father, also a physician, Dr. Movsas reminded the audience that "More is not always better."
The trial is continuing with patients assigned to 60 Gy radiation only, with the goal of evaluating the secondary study end point of overall survival of patients with or without cetuximab added to concurrent chemoradiotherapy.
The RTOG 0617 trial is supported by grants from the U.S. National Cancer Institute, with additional support from Bristol-Myers Squibb and ImClone.
Dr. Bradley and Dr. Williams had no disclosures. Dr. Movsas disclosed departmental research support from Varian and Philips. He also has served as a chair of an RTOG committee, but was not involved in the 0617 study.
MIAMI BEACH – Less turned out to be better in a large clinical trial comparing radiation doses in patients treated with radiation and chemotherapy for stage III non–small cell lung cancer, investigators reported here.
The median overall survival rate at 1 year was 81% for patients treated with standard-dose (60 Gy) radiation, compared with 70.4% for those who received the high dose (74 Gy), according to preliminary findings from the radiation-dose arm of the ongoing phase III Radiation Therapy Oncology Group (RTOG) 0617 trial. The respective median survival rates were 21.7 months and 20.7 months (P = .02).
A planned interim analysis from the trial showed that the radiation comparison had crossed the prespecified boundary for futility, and the high-dose arm was stopped in June 2011, reported Dr. Jeffrey Bradley from Washington University in St. Louis.
"I think this changes practice: If [cancer centers] weren’t using 60 Gray before, perhaps they should go back to using 60 Gray, because it does not appear that a higher dose is better," Dr. Bradley commented at the annual meeting of the American Society of Radiation Oncology (ASTRO).
Dr. Tim R. Williams, from the Lynn Cancer Institute at Boca Raton (Fla.) Regional Hospital, the immediate-past chairman of ASTRO, noted that his center has used high-dose radiation in stage III non–small cell lung cancer (NSCLC) patients for about 5 years. Although practice patterns vary, it’s likely that many treatment centers currently use the higher dose, he said.
In RTOG 0617, a total of 500 patients with stage IIIA/IIIB NSCLC were scheduled for randomization to one of four arms in a 2 x 2 factorial design with patients assigned to receive either 74 Gy or 60 Gy radiation with or without cetuximab (Erbitux), on a background chemotherapy regimen of weekly paclitaxel (45 mg/m2) and carboplatin (titrated to an area-under-the-curve of 2).
The radiation was delivered in 2 Gy fractions over 30 to 37 fractions.
The analysis was performed on 426 patients who had been enrolled in the study before June 17, 2011.
Seeking to understand why the higher radiation dose was not better – the investigators had originally hypothesized that 74 Gy would result in a 7-month improvement in overall survival vs. 64 Gy.– they performed univariate analyses, and found that significant predictors for better outcomes included continuous therapy, nonsquamous histology, and, female gender. In multivariate analysis, radiation dose (60 Gy vs. 74 Gy) was associated with a hazard ratio for overall survival of 1.48 (P = .038),
nonsquamous histology versus squamous was associated with an HR of 1.52 (P = .025), and gross or internal tumor volume had a small but significant HR of 1.002 (P = .011).
Dr. Benjamin Movsas, chair of radiation oncology at the Henry Ford Health System in Detroit, the invited discussant, said that "as of 2011, level I evidence demonstrates no role for dose escalation in stage III non–small cell lung cancer."
He noted that although there were small differences between the radiation dose groups in terms of tumor histology, gross tumor volume, and other factors, they were not large enough to explain the differences in outcomes.
Citing the advice of his late father, also a physician, Dr. Movsas reminded the audience that "More is not always better."
The trial is continuing with patients assigned to 60 Gy radiation only, with the goal of evaluating the secondary study end point of overall survival of patients with or without cetuximab added to concurrent chemoradiotherapy.
The RTOG 0617 trial is supported by grants from the U.S. National Cancer Institute, with additional support from Bristol-Myers Squibb and ImClone.
Dr. Bradley and Dr. Williams had no disclosures. Dr. Movsas disclosed departmental research support from Varian and Philips. He also has served as a chair of an RTOG committee, but was not involved in the 0617 study.
MIAMI BEACH – Less turned out to be better in a large clinical trial comparing radiation doses in patients treated with radiation and chemotherapy for stage III non–small cell lung cancer, investigators reported here.
The median overall survival rate at 1 year was 81% for patients treated with standard-dose (60 Gy) radiation, compared with 70.4% for those who received the high dose (74 Gy), according to preliminary findings from the radiation-dose arm of the ongoing phase III Radiation Therapy Oncology Group (RTOG) 0617 trial. The respective median survival rates were 21.7 months and 20.7 months (P = .02).
A planned interim analysis from the trial showed that the radiation comparison had crossed the prespecified boundary for futility, and the high-dose arm was stopped in June 2011, reported Dr. Jeffrey Bradley from Washington University in St. Louis.
"I think this changes practice: If [cancer centers] weren’t using 60 Gray before, perhaps they should go back to using 60 Gray, because it does not appear that a higher dose is better," Dr. Bradley commented at the annual meeting of the American Society of Radiation Oncology (ASTRO).
Dr. Tim R. Williams, from the Lynn Cancer Institute at Boca Raton (Fla.) Regional Hospital, the immediate-past chairman of ASTRO, noted that his center has used high-dose radiation in stage III non–small cell lung cancer (NSCLC) patients for about 5 years. Although practice patterns vary, it’s likely that many treatment centers currently use the higher dose, he said.
In RTOG 0617, a total of 500 patients with stage IIIA/IIIB NSCLC were scheduled for randomization to one of four arms in a 2 x 2 factorial design with patients assigned to receive either 74 Gy or 60 Gy radiation with or without cetuximab (Erbitux), on a background chemotherapy regimen of weekly paclitaxel (45 mg/m2) and carboplatin (titrated to an area-under-the-curve of 2).
The radiation was delivered in 2 Gy fractions over 30 to 37 fractions.
The analysis was performed on 426 patients who had been enrolled in the study before June 17, 2011.
Seeking to understand why the higher radiation dose was not better – the investigators had originally hypothesized that 74 Gy would result in a 7-month improvement in overall survival vs. 64 Gy.– they performed univariate analyses, and found that significant predictors for better outcomes included continuous therapy, nonsquamous histology, and, female gender. In multivariate analysis, radiation dose (60 Gy vs. 74 Gy) was associated with a hazard ratio for overall survival of 1.48 (P = .038),
nonsquamous histology versus squamous was associated with an HR of 1.52 (P = .025), and gross or internal tumor volume had a small but significant HR of 1.002 (P = .011).
Dr. Benjamin Movsas, chair of radiation oncology at the Henry Ford Health System in Detroit, the invited discussant, said that "as of 2011, level I evidence demonstrates no role for dose escalation in stage III non–small cell lung cancer."
He noted that although there were small differences between the radiation dose groups in terms of tumor histology, gross tumor volume, and other factors, they were not large enough to explain the differences in outcomes.
Citing the advice of his late father, also a physician, Dr. Movsas reminded the audience that "More is not always better."
The trial is continuing with patients assigned to 60 Gy radiation only, with the goal of evaluating the secondary study end point of overall survival of patients with or without cetuximab added to concurrent chemoradiotherapy.
The RTOG 0617 trial is supported by grants from the U.S. National Cancer Institute, with additional support from Bristol-Myers Squibb and ImClone.
Dr. Bradley and Dr. Williams had no disclosures. Dr. Movsas disclosed departmental research support from Varian and Philips. He also has served as a chair of an RTOG committee, but was not involved in the 0617 study.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Median overall survival among patients with stage III NSCLC treated with chemotherapy was 20.7 months with high-dose radiation (74 Gy), vs. 21.7 months with standard-dose radiation (60 Gy; P =.02).
Data Source: 426 patients enrolled in the randomized controlled RTOG 0617 trial.
Disclosures: Dr. Bradley and Dr. Williams had no disclosures. Dr. Movsas disclosed departmental research support from Varian and Philips. He also has served as a chair of an RTOG committee, but was not involved in the 0617 study.