User login
Investigational JAK inhibitor reduced PASI scores
PRAGUE – The major role that the Janus kinases play in the pathogenesis of psoriasis is confirmed, based on the results of a phase II study of the investigational oral agent ASP015K, according to Dr. Bernhardt Zeiher.
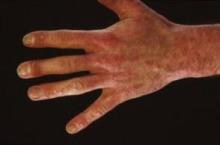
ASP015K inhibits Janus kinase (JAK) 1 and 3, with relative sparing of JAK 2. In a 6-week dose-ranging study of 124 patients with moderate to severe psoriasis, all tested doses – 10, 25, 60, and 100 mg twice daily as well as 50 mg once daily – significantly outperformed placebo in terms of improvement in Psoriasis Area and Severity Index (PASI) scores.
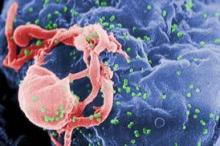
Moreover, immunohistochemistry of skin biopsies gathered at baseline and at days 14 and 42 after initiation of ASP015K showed the drug reduced epidermal thickness, epidermal proliferation as measured by Ki67, and infiltration of CD3 T cells and CD11c dendritic cells. The greatest changes at the cellular level were seen at the highest dose, 100 mg twice daily.
"Although treatment was only for 6 weeks, the defining pathology of psoriasis was reversed in 62% of patients in the 100-mg b.i.d. cohort. In these patients, epidermal thickness was normalized and was comparable with nonlesional skin after treatment, parakeratosis was eliminated, and a granular layer was restored. Keratinocyte proliferation, judged by Ki67 staining, was reversed to levels of nonlesional skin. Major reductions in the number of T cells and inflammatory CD11c-positive dendritic cells were also measured in day 42 biopsies, along with elimination of organized lymphoid structures," according to Dr. Zeiher of Astellas Pharma, Deerfield, Ill., which is developing the JAK 1/3 inhibitor.
Improvements in cellular findings and PASI scores were largely dose-dependent.
Mean PASI scores fell by 12 points over a 6-week period in patients assigned to ASP015K at 100 mg b.i.d.; a 4-point drop in mean PASI scores was seen in placebo-treated controls.
Yet histologic improvements associated with the 50-mg once-daily dose were second in magnitude only to those seen with the 100-mg b.i.d. dose.
"When you look at the kinetics of the drug, you have more JAK inhibition holiday with once-a-day dosing," Dr. Zeiher explained. The notion that a daily JAK inhibition holiday provides added therapeutic benefit is both interesting and readily testable. A planned phase IIb study will evaluate once-daily oral dosing of ASP015K at a range of doses, with 150 mg as the maximum.
The JAK 1/3 inhibitor was well tolerated overall. Three subjects dropped out of the study because of drug-related side effects: two with decreased neutrophil counts but not frank neutropenia on doses of 60 and 100 mg b.i.d., and one as a result of GI upset while on 100 mg b.i.d.
skin biopsies, epidermal thickness, epidermal proliferation, Ki67, CD3 T cells, CD11c dendritic cells,
PRAGUE – The major role that the Janus kinases play in the pathogenesis of psoriasis is confirmed, based on the results of a phase II study of the investigational oral agent ASP015K, according to Dr. Bernhardt Zeiher.
ASP015K inhibits Janus kinase (JAK) 1 and 3, with relative sparing of JAK 2. In a 6-week dose-ranging study of 124 patients with moderate to severe psoriasis, all tested doses – 10, 25, 60, and 100 mg twice daily as well as 50 mg once daily – significantly outperformed placebo in terms of improvement in Psoriasis Area and Severity Index (PASI) scores.
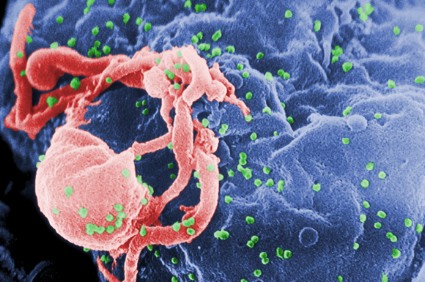
Moreover, immunohistochemistry of skin biopsies gathered at baseline and at days 14 and 42 after initiation of ASP015K showed the drug reduced epidermal thickness, epidermal proliferation as measured by Ki67, and infiltration of CD3 T cells and CD11c dendritic cells. The greatest changes at the cellular level were seen at the highest dose, 100 mg twice daily.
"Although treatment was only for 6 weeks, the defining pathology of psoriasis was reversed in 62% of patients in the 100-mg b.i.d. cohort. In these patients, epidermal thickness was normalized and was comparable with nonlesional skin after treatment, parakeratosis was eliminated, and a granular layer was restored. Keratinocyte proliferation, judged by Ki67 staining, was reversed to levels of nonlesional skin. Major reductions in the number of T cells and inflammatory CD11c-positive dendritic cells were also measured in day 42 biopsies, along with elimination of organized lymphoid structures," according to Dr. Zeiher of Astellas Pharma, Deerfield, Ill., which is developing the JAK 1/3 inhibitor.
Improvements in cellular findings and PASI scores were largely dose-dependent.
Mean PASI scores fell by 12 points over a 6-week period in patients assigned to ASP015K at 100 mg b.i.d.; a 4-point drop in mean PASI scores was seen in placebo-treated controls.
Yet histologic improvements associated with the 50-mg once-daily dose were second in magnitude only to those seen with the 100-mg b.i.d. dose.
"When you look at the kinetics of the drug, you have more JAK inhibition holiday with once-a-day dosing," Dr. Zeiher explained. The notion that a daily JAK inhibition holiday provides added therapeutic benefit is both interesting and readily testable. A planned phase IIb study will evaluate once-daily oral dosing of ASP015K at a range of doses, with 150 mg as the maximum.
The JAK 1/3 inhibitor was well tolerated overall. Three subjects dropped out of the study because of drug-related side effects: two with decreased neutrophil counts but not frank neutropenia on doses of 60 and 100 mg b.i.d., and one as a result of GI upset while on 100 mg b.i.d.
PRAGUE – The major role that the Janus kinases play in the pathogenesis of psoriasis is confirmed, based on the results of a phase II study of the investigational oral agent ASP015K, according to Dr. Bernhardt Zeiher.
ASP015K inhibits Janus kinase (JAK) 1 and 3, with relative sparing of JAK 2. In a 6-week dose-ranging study of 124 patients with moderate to severe psoriasis, all tested doses – 10, 25, 60, and 100 mg twice daily as well as 50 mg once daily – significantly outperformed placebo in terms of improvement in Psoriasis Area and Severity Index (PASI) scores.
Moreover, immunohistochemistry of skin biopsies gathered at baseline and at days 14 and 42 after initiation of ASP015K showed the drug reduced epidermal thickness, epidermal proliferation as measured by Ki67, and infiltration of CD3 T cells and CD11c dendritic cells. The greatest changes at the cellular level were seen at the highest dose, 100 mg twice daily.
"Although treatment was only for 6 weeks, the defining pathology of psoriasis was reversed in 62% of patients in the 100-mg b.i.d. cohort. In these patients, epidermal thickness was normalized and was comparable with nonlesional skin after treatment, parakeratosis was eliminated, and a granular layer was restored. Keratinocyte proliferation, judged by Ki67 staining, was reversed to levels of nonlesional skin. Major reductions in the number of T cells and inflammatory CD11c-positive dendritic cells were also measured in day 42 biopsies, along with elimination of organized lymphoid structures," according to Dr. Zeiher of Astellas Pharma, Deerfield, Ill., which is developing the JAK 1/3 inhibitor.
Improvements in cellular findings and PASI scores were largely dose-dependent.
Mean PASI scores fell by 12 points over a 6-week period in patients assigned to ASP015K at 100 mg b.i.d.; a 4-point drop in mean PASI scores was seen in placebo-treated controls.
Yet histologic improvements associated with the 50-mg once-daily dose were second in magnitude only to those seen with the 100-mg b.i.d. dose.
"When you look at the kinetics of the drug, you have more JAK inhibition holiday with once-a-day dosing," Dr. Zeiher explained. The notion that a daily JAK inhibition holiday provides added therapeutic benefit is both interesting and readily testable. A planned phase IIb study will evaluate once-daily oral dosing of ASP015K at a range of doses, with 150 mg as the maximum.
The JAK 1/3 inhibitor was well tolerated overall. Three subjects dropped out of the study because of drug-related side effects: two with decreased neutrophil counts but not frank neutropenia on doses of 60 and 100 mg b.i.d., and one as a result of GI upset while on 100 mg b.i.d.
skin biopsies, epidermal thickness, epidermal proliferation, Ki67, CD3 T cells, CD11c dendritic cells,
skin biopsies, epidermal thickness, epidermal proliferation, Ki67, CD3 T cells, CD11c dendritic cells,
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Mean PASI scores fell by 12 points over a 6-week period in patients assigned to ASP015K at 100 mg b.i.d.; a 4-point drop in mean PASI scores was seen in placebo-treated controls.
Data Source: A phase II dose-ranging study involving 124 patients with moderate to severe psoriasis.
Disclosures: The study was sponsored by Astellas Pharma, and the results were presented by a company employee.
Risk of anal cancer is high in HIV-infected people
Curbing the high rates of anal cancer in HIV-infected individuals won’t be accomplished through targeted efforts focused on HIV-positive men who have sex with men, according to Dr. Michel Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections – Europe.
Anal cancer rates among HIV-infected persons are indeed highest among men who have sex with men (MSM), but anal cancer rates also are quite high among other HIV-infected men and women, based on results of a large study.
The findings take on added significance given the availability of the HPV vaccine, although the efficacy of the vaccine in HIV-positive individuals remains to be seen. In addition, pilot projects testing the benefits of periodic anal histology screening in HIV-infected individuals are underway, Dr. Janier observed at the annual congress of the European Academy of Dermatology and Venereology.
The study by Dr. Michael J. Silverberg of Kaiser Permanente, Oakland, Calif., and the North American AIDS Cohort Collaboration on Research and Design (NA–ACCORD) of IeDEA included 36,189 HIV-infected North Americans and 114,260 uninfected controls followed during 1996-2007. The HIV-positive group comprised 55% MSM, 19% heterosexual men, and 26% women.
The unadjusted anal cancer incidence rates per 100,000 person-years were 131 for HIV-positive MSM, 46 for HIV-infected heterosexual men, and 2 for uninfected men. This translated to a demographically adjusted rate ratio of 80.3 for MSM compared with uninfected men, and to a 26.7-fold increased risk of anal cancer in HIV-infected straight men compared with uninfected heterosexual men.
HIV-infected women had an anal cancer incidence rate of 30 cases per 100,000 person-years. A rate ratio could not be determined because no cases occurred in uninfected women (Clin. Infect. Dis. 2012;54:1026-34).
Among HIV-infected individuals, the adjusted relative risk of anal cancer doubled from 1996-1999 to 2000-2003, then leveled off. This is in line with the findings of the large French Hospital Database Study, which also observed a jump in anal cancer rates among HIV-infected individuals in the late 1990s followed by more recent stabilization in the era of widely available antiretroviral therapy (AIDS 2008;22:1203-11).
The National Institutes of Health was the chief sponsor of the study. Dr. Janier reported having no financial conflicts.
Curbing the high rates of anal cancer in HIV-infected individuals won’t be accomplished through targeted efforts focused on HIV-positive men who have sex with men, according to Dr. Michel Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections – Europe.
Anal cancer rates among HIV-infected persons are indeed highest among men who have sex with men (MSM), but anal cancer rates also are quite high among other HIV-infected men and women, based on results of a large study.
The findings take on added significance given the availability of the HPV vaccine, although the efficacy of the vaccine in HIV-positive individuals remains to be seen. In addition, pilot projects testing the benefits of periodic anal histology screening in HIV-infected individuals are underway, Dr. Janier observed at the annual congress of the European Academy of Dermatology and Venereology.
The study by Dr. Michael J. Silverberg of Kaiser Permanente, Oakland, Calif., and the North American AIDS Cohort Collaboration on Research and Design (NA–ACCORD) of IeDEA included 36,189 HIV-infected North Americans and 114,260 uninfected controls followed during 1996-2007. The HIV-positive group comprised 55% MSM, 19% heterosexual men, and 26% women.
The unadjusted anal cancer incidence rates per 100,000 person-years were 131 for HIV-positive MSM, 46 for HIV-infected heterosexual men, and 2 for uninfected men. This translated to a demographically adjusted rate ratio of 80.3 for MSM compared with uninfected men, and to a 26.7-fold increased risk of anal cancer in HIV-infected straight men compared with uninfected heterosexual men.
HIV-infected women had an anal cancer incidence rate of 30 cases per 100,000 person-years. A rate ratio could not be determined because no cases occurred in uninfected women (Clin. Infect. Dis. 2012;54:1026-34).
Among HIV-infected individuals, the adjusted relative risk of anal cancer doubled from 1996-1999 to 2000-2003, then leveled off. This is in line with the findings of the large French Hospital Database Study, which also observed a jump in anal cancer rates among HIV-infected individuals in the late 1990s followed by more recent stabilization in the era of widely available antiretroviral therapy (AIDS 2008;22:1203-11).
The National Institutes of Health was the chief sponsor of the study. Dr. Janier reported having no financial conflicts.
Curbing the high rates of anal cancer in HIV-infected individuals won’t be accomplished through targeted efforts focused on HIV-positive men who have sex with men, according to Dr. Michel Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections – Europe.
Anal cancer rates among HIV-infected persons are indeed highest among men who have sex with men (MSM), but anal cancer rates also are quite high among other HIV-infected men and women, based on results of a large study.
The findings take on added significance given the availability of the HPV vaccine, although the efficacy of the vaccine in HIV-positive individuals remains to be seen. In addition, pilot projects testing the benefits of periodic anal histology screening in HIV-infected individuals are underway, Dr. Janier observed at the annual congress of the European Academy of Dermatology and Venereology.
The study by Dr. Michael J. Silverberg of Kaiser Permanente, Oakland, Calif., and the North American AIDS Cohort Collaboration on Research and Design (NA–ACCORD) of IeDEA included 36,189 HIV-infected North Americans and 114,260 uninfected controls followed during 1996-2007. The HIV-positive group comprised 55% MSM, 19% heterosexual men, and 26% women.
The unadjusted anal cancer incidence rates per 100,000 person-years were 131 for HIV-positive MSM, 46 for HIV-infected heterosexual men, and 2 for uninfected men. This translated to a demographically adjusted rate ratio of 80.3 for MSM compared with uninfected men, and to a 26.7-fold increased risk of anal cancer in HIV-infected straight men compared with uninfected heterosexual men.
HIV-infected women had an anal cancer incidence rate of 30 cases per 100,000 person-years. A rate ratio could not be determined because no cases occurred in uninfected women (Clin. Infect. Dis. 2012;54:1026-34).
Among HIV-infected individuals, the adjusted relative risk of anal cancer doubled from 1996-1999 to 2000-2003, then leveled off. This is in line with the findings of the large French Hospital Database Study, which also observed a jump in anal cancer rates among HIV-infected individuals in the late 1990s followed by more recent stabilization in the era of widely available antiretroviral therapy (AIDS 2008;22:1203-11).
The National Institutes of Health was the chief sponsor of the study. Dr. Janier reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Psoriasis drug pipeline extrudes progress
PRAGUE – Two biologic agents addressing novel therapeutic targets in psoriasis continue to advance in the developmental pipeline, as highlighted in studies presented at the annual congress of the European Academy of Dermatology and Venereology.
Merck’s MK-3222 is a monoclonal antibody directed against the interleukin-23 p19 subunit. MK-3222 specifically binds and neutralizes IL23, and thereby inhibits IL23-dependent Th17 cells, which mediate inflammatory injury in psoriasis.
Dr. Tamara Kopp of the Medical University of Vienna presented a proof-of-concept study which suggested that targeting IL23p19 via MK-3222 could be an important new form of therapy for moderate to severe psoriasis. But there was a potential red flag: Eighteen percent of patients treated with just three doses of the antibody over the course of up to 12 weeks developed antidrug antibodies. The clinical significance of these antibodies will require additional study.
Separately, Dr. Richard Langley presented a secondary analysis of data from a phase II, 125-patient, double-blind, placebo-controlled, dose-ranging study of secukinumab, a fully human monoclonal antibody directed against the proinflammatory cytokine IL17A (Br. J. Dermatol. 2012 Oct. 27 [doi: 10.1111/bjd.12110]). The objective was to determine how long it takes for psoriasis symptoms to return after 8 weeks of therapy.
The answer is that relapse takes a very long time.
At the most effective dose tested in the trial, which was 150 mg of secukinumab given subcutaneously at weeks 0, 4, and 8, a Psoriasis Area and Severity Index (PASI) 75 response was seen at week 12 in 82% of patients assigned to the monoclonal antibody, compared with 9% of placebo-treated controls. A PASI 90 response – that is, at least a 90% reduction in the PASI score compared with baseline – was documented at week 12 in 52% of patients on secukinumab and 5% of controls.
At week 32, fully 24 weeks since the final 150-mg dose, only half of PASI 75 responders to secukinumab had relapsed as defined by loss of at least 50% of the maximum PASI improvement seen at week 12. Median time to relapse was 174 days, reported Dr. Langley, director of research in the division of clinical dermatology and cutaneous science at Dalhousie University in Halifax, N.S.
Secukinumab was quite well tolerated. Indeed, the overall adverse events and serious adverse events were generally similar in nature to placebo, but less frequent.
Based upon the phase II time-to-relapse analysis, it’s likely that two maintenance therapy schedules will be tested in larger studies: one involving retreatment at 4-week intervals, and another in which retreatment is held in abeyance until relapse commences, according to Dr. Langley.
Dr. Kopp reported that the MK-3222 study involved 77 enrollees, 66 of whom completed the 16-week dose-ranging study. Only 2 of the 11 dropouts did so because of adverse events.
All 11 patients who received intravenous MK-3222 at either 3 or 10 mg/kg on days 1, 56, and 84 had a PASI 75 response at week 16. So did 10 of 15 who received 3 mg/kg and 13 of 14 who received 10 mg/kg on days 1, 28, and 56.
The anti-IL23p19 antibody was generally well tolerated, with no dose-related increase in adverse events and no ECG or laboratory abnormalities.
Of the nine patients who developed antidrug antibodies during the study, five had serum drug concentrations that were significantly lower than those of subjects without antidrug antibodies; two of these five patients – those with the most pronounced reduction in drug concentrations – also had neutralizing antidrug antibodies to MK-3222. However, patients with antidrug antibodies did not differ from the others in terms of their clinical response to treatment as reflected in PASI improvement, and their adverse event profile was not outstanding.
Dr. Kopp serves as a consultant to Merck, which is developing MK-3222. The secukinumab study was funded by Novartis. Dr. Langley is a consultant to the company.
PRAGUE – Two biologic agents addressing novel therapeutic targets in psoriasis continue to advance in the developmental pipeline, as highlighted in studies presented at the annual congress of the European Academy of Dermatology and Venereology.
Merck’s MK-3222 is a monoclonal antibody directed against the interleukin-23 p19 subunit. MK-3222 specifically binds and neutralizes IL23, and thereby inhibits IL23-dependent Th17 cells, which mediate inflammatory injury in psoriasis.
Dr. Tamara Kopp of the Medical University of Vienna presented a proof-of-concept study which suggested that targeting IL23p19 via MK-3222 could be an important new form of therapy for moderate to severe psoriasis. But there was a potential red flag: Eighteen percent of patients treated with just three doses of the antibody over the course of up to 12 weeks developed antidrug antibodies. The clinical significance of these antibodies will require additional study.
Separately, Dr. Richard Langley presented a secondary analysis of data from a phase II, 125-patient, double-blind, placebo-controlled, dose-ranging study of secukinumab, a fully human monoclonal antibody directed against the proinflammatory cytokine IL17A (Br. J. Dermatol. 2012 Oct. 27 [doi: 10.1111/bjd.12110]). The objective was to determine how long it takes for psoriasis symptoms to return after 8 weeks of therapy.
The answer is that relapse takes a very long time.
At the most effective dose tested in the trial, which was 150 mg of secukinumab given subcutaneously at weeks 0, 4, and 8, a Psoriasis Area and Severity Index (PASI) 75 response was seen at week 12 in 82% of patients assigned to the monoclonal antibody, compared with 9% of placebo-treated controls. A PASI 90 response – that is, at least a 90% reduction in the PASI score compared with baseline – was documented at week 12 in 52% of patients on secukinumab and 5% of controls.
At week 32, fully 24 weeks since the final 150-mg dose, only half of PASI 75 responders to secukinumab had relapsed as defined by loss of at least 50% of the maximum PASI improvement seen at week 12. Median time to relapse was 174 days, reported Dr. Langley, director of research in the division of clinical dermatology and cutaneous science at Dalhousie University in Halifax, N.S.
Secukinumab was quite well tolerated. Indeed, the overall adverse events and serious adverse events were generally similar in nature to placebo, but less frequent.
Based upon the phase II time-to-relapse analysis, it’s likely that two maintenance therapy schedules will be tested in larger studies: one involving retreatment at 4-week intervals, and another in which retreatment is held in abeyance until relapse commences, according to Dr. Langley.
Dr. Kopp reported that the MK-3222 study involved 77 enrollees, 66 of whom completed the 16-week dose-ranging study. Only 2 of the 11 dropouts did so because of adverse events.
All 11 patients who received intravenous MK-3222 at either 3 or 10 mg/kg on days 1, 56, and 84 had a PASI 75 response at week 16. So did 10 of 15 who received 3 mg/kg and 13 of 14 who received 10 mg/kg on days 1, 28, and 56.
The anti-IL23p19 antibody was generally well tolerated, with no dose-related increase in adverse events and no ECG or laboratory abnormalities.
Of the nine patients who developed antidrug antibodies during the study, five had serum drug concentrations that were significantly lower than those of subjects without antidrug antibodies; two of these five patients – those with the most pronounced reduction in drug concentrations – also had neutralizing antidrug antibodies to MK-3222. However, patients with antidrug antibodies did not differ from the others in terms of their clinical response to treatment as reflected in PASI improvement, and their adverse event profile was not outstanding.
Dr. Kopp serves as a consultant to Merck, which is developing MK-3222. The secukinumab study was funded by Novartis. Dr. Langley is a consultant to the company.
PRAGUE – Two biologic agents addressing novel therapeutic targets in psoriasis continue to advance in the developmental pipeline, as highlighted in studies presented at the annual congress of the European Academy of Dermatology and Venereology.
Merck’s MK-3222 is a monoclonal antibody directed against the interleukin-23 p19 subunit. MK-3222 specifically binds and neutralizes IL23, and thereby inhibits IL23-dependent Th17 cells, which mediate inflammatory injury in psoriasis.
Dr. Tamara Kopp of the Medical University of Vienna presented a proof-of-concept study which suggested that targeting IL23p19 via MK-3222 could be an important new form of therapy for moderate to severe psoriasis. But there was a potential red flag: Eighteen percent of patients treated with just three doses of the antibody over the course of up to 12 weeks developed antidrug antibodies. The clinical significance of these antibodies will require additional study.
Separately, Dr. Richard Langley presented a secondary analysis of data from a phase II, 125-patient, double-blind, placebo-controlled, dose-ranging study of secukinumab, a fully human monoclonal antibody directed against the proinflammatory cytokine IL17A (Br. J. Dermatol. 2012 Oct. 27 [doi: 10.1111/bjd.12110]). The objective was to determine how long it takes for psoriasis symptoms to return after 8 weeks of therapy.
The answer is that relapse takes a very long time.
At the most effective dose tested in the trial, which was 150 mg of secukinumab given subcutaneously at weeks 0, 4, and 8, a Psoriasis Area and Severity Index (PASI) 75 response was seen at week 12 in 82% of patients assigned to the monoclonal antibody, compared with 9% of placebo-treated controls. A PASI 90 response – that is, at least a 90% reduction in the PASI score compared with baseline – was documented at week 12 in 52% of patients on secukinumab and 5% of controls.
At week 32, fully 24 weeks since the final 150-mg dose, only half of PASI 75 responders to secukinumab had relapsed as defined by loss of at least 50% of the maximum PASI improvement seen at week 12. Median time to relapse was 174 days, reported Dr. Langley, director of research in the division of clinical dermatology and cutaneous science at Dalhousie University in Halifax, N.S.
Secukinumab was quite well tolerated. Indeed, the overall adverse events and serious adverse events were generally similar in nature to placebo, but less frequent.
Based upon the phase II time-to-relapse analysis, it’s likely that two maintenance therapy schedules will be tested in larger studies: one involving retreatment at 4-week intervals, and another in which retreatment is held in abeyance until relapse commences, according to Dr. Langley.
Dr. Kopp reported that the MK-3222 study involved 77 enrollees, 66 of whom completed the 16-week dose-ranging study. Only 2 of the 11 dropouts did so because of adverse events.
All 11 patients who received intravenous MK-3222 at either 3 or 10 mg/kg on days 1, 56, and 84 had a PASI 75 response at week 16. So did 10 of 15 who received 3 mg/kg and 13 of 14 who received 10 mg/kg on days 1, 28, and 56.
The anti-IL23p19 antibody was generally well tolerated, with no dose-related increase in adverse events and no ECG or laboratory abnormalities.
Of the nine patients who developed antidrug antibodies during the study, five had serum drug concentrations that were significantly lower than those of subjects without antidrug antibodies; two of these five patients – those with the most pronounced reduction in drug concentrations – also had neutralizing antidrug antibodies to MK-3222. However, patients with antidrug antibodies did not differ from the others in terms of their clinical response to treatment as reflected in PASI improvement, and their adverse event profile was not outstanding.
Dr. Kopp serves as a consultant to Merck, which is developing MK-3222. The secukinumab study was funded by Novartis. Dr. Langley is a consultant to the company.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Contact dermatitis due to fragrances has morphed
PRAGUE – Reliance upon the time-honored tools for diagnosis of contact allergy due to fragrances will cause physicians to miss many cases, Dr. David E. Cohen said at the annual congress of the European Academy of Dermatology and Venereology.
"The old fragrance mix – fragrance mix I – and the use of balsam of Peru as fragrance screening tools really won’t capture contact dermatitis involving allergens to more modern fragrances," cautioned Dr. Cohen, professor and vice chair of the department of dermatology at New York University.
"I remember my grandmother always smelling like rosewater and naphthalene – like mothballs and rosewater. But we don’t smell like that anymore. Now we tend to use more botanical extracts. We see tea tree oil and jasmine appearing in our personal care products, and if we don’t test for those more contemporary fragrances, we will miss a very important source of contact dermatitis," he continued.
That’s why fragrance mix II (FM II) was developed. FM II has been incorporated into the North American Contact Dermatitis Group’s standard screening tray. It is not, however, part of the widely used T.R.U.E. (Thin-Layer Rapid Use Epicutaneous) test, nor is it included in the European standard screening series.
Multiple studies have documented that a substantial proportion of patients with allergic contact dermatitis will react only to the newer fragrances, and not to FM I or balsam of Peru. For example, a multicenter Hungarian study published earlier this year found that of 565 patients patch tested because of skin symptoms provoked by scented products, 17% exhibited contact hypersensitivity to one or more components of FM II. Moreover, 48% of FM II–positive patients reacted only to FM II, not FM I, balsam of Peru, or other test materials (Dermatitis 2012;23:71-4).
Similarly, a Mayo Clinic study of 945 patients patch tested using both the standard screening tray with FM II and the T.R.U.E. test found that 49% of patients reacted to one or more preservatives and 31% reacted to at least one fragrance or botanical additive. However, the T.R.U.E. test didn’t capture 23% of patients with a preservative allergy and 11% of those with a fragrance or botanical allergy (J. Am. Acad. Dermatol. 2010;63:789-98).
Fragrances pose one of the greatest challenges in the field of contact dermatitis. That’s because there are roughly 3,000 fragrance chemicals utilized in personal care products and cosmetics, and at least 100 of them have been described as causing contact dermatitis in patients. It can be difficult to assess the clinical relevance of positive patch test reactions because physicians don’t know what’s contained in North American fragrances. The fragrance industry is lucrative, secretive, and self-regulated.
Fragrance allergy is on the rise in children and adolescents, probably because they are being exposed to an onslaught of fragrance chemicals at a younger and younger age.
"When I grew up there was a bar of soap in the shower and, if we were lucky, shampoo; and if there was no shampoo we used the bar of soap. I have 19- and 17-year-old daughters, and if you go into their bathroom there are four different shampoos and three conditioners. There are things with glitter in there, and moisturizers and cosmetics, most of which I don’t even know what they’re for. All of them are fragrances. Folks are starting their exposure when they’re 7 and 8 years old. That was never the case before," said Dr. Cohen, who is also director of occupational, environmental, and allergic dermatology at the university.
The commercially available six-ingredient FM II panel is a 14% concentration composed of citronellol 0.5%, hydroxyisohexyl 3-cyclohexenecarboxyaldehyde (Lyral) 2.5%, hexyl cinnamal 5.0%, citral 1.0%, coumarin 2.5%, and farnesol 2.5%.
A long-standing point of contention has been the question of whether patients with atopic disease are more likely than nonatopic individuals to experience contact sensitivity, or less. The latest evidence suggests patients with asthma or severe atopic dermatitis have an overall lower prevalence of contact sensitization compared with nonatopic controls, with one striking exception.
In this very large study conducted by investigators at the Danish National Allergy Research Center, Copenhagen, an inverse association was found between atopic disease and contact allergy to metals and all groups of chemicals ... except fragrances. The prevalence of contact sensitization to fragrances was significantly higher in patients with atopic dermatitis than in controls. The investigators recommended that patients with atopic dermatitis be instructed to avoid scented moisturizers (Allergy 2012;67:1157-64).
The Danes found that patients with severe atopic dermatitis were 30% less likely than controls to be patch test–positive for contact sensitivity overall, whereas mild to moderate atopic dermatitis did not suppress contact sensitization. Again, fragrances constituted the exception: Not only did patients with severe atopic dermatitis have an increased prevalence of contact sensitization to fragrance chemicals, those with mild or moderate atopic dermatitis did, too, Dr. Cohen noted.
He reported having no financial conflicts.
PRAGUE – Reliance upon the time-honored tools for diagnosis of contact allergy due to fragrances will cause physicians to miss many cases, Dr. David E. Cohen said at the annual congress of the European Academy of Dermatology and Venereology.
"The old fragrance mix – fragrance mix I – and the use of balsam of Peru as fragrance screening tools really won’t capture contact dermatitis involving allergens to more modern fragrances," cautioned Dr. Cohen, professor and vice chair of the department of dermatology at New York University.
"I remember my grandmother always smelling like rosewater and naphthalene – like mothballs and rosewater. But we don’t smell like that anymore. Now we tend to use more botanical extracts. We see tea tree oil and jasmine appearing in our personal care products, and if we don’t test for those more contemporary fragrances, we will miss a very important source of contact dermatitis," he continued.
That’s why fragrance mix II (FM II) was developed. FM II has been incorporated into the North American Contact Dermatitis Group’s standard screening tray. It is not, however, part of the widely used T.R.U.E. (Thin-Layer Rapid Use Epicutaneous) test, nor is it included in the European standard screening series.
Multiple studies have documented that a substantial proportion of patients with allergic contact dermatitis will react only to the newer fragrances, and not to FM I or balsam of Peru. For example, a multicenter Hungarian study published earlier this year found that of 565 patients patch tested because of skin symptoms provoked by scented products, 17% exhibited contact hypersensitivity to one or more components of FM II. Moreover, 48% of FM II–positive patients reacted only to FM II, not FM I, balsam of Peru, or other test materials (Dermatitis 2012;23:71-4).
Similarly, a Mayo Clinic study of 945 patients patch tested using both the standard screening tray with FM II and the T.R.U.E. test found that 49% of patients reacted to one or more preservatives and 31% reacted to at least one fragrance or botanical additive. However, the T.R.U.E. test didn’t capture 23% of patients with a preservative allergy and 11% of those with a fragrance or botanical allergy (J. Am. Acad. Dermatol. 2010;63:789-98).
Fragrances pose one of the greatest challenges in the field of contact dermatitis. That’s because there are roughly 3,000 fragrance chemicals utilized in personal care products and cosmetics, and at least 100 of them have been described as causing contact dermatitis in patients. It can be difficult to assess the clinical relevance of positive patch test reactions because physicians don’t know what’s contained in North American fragrances. The fragrance industry is lucrative, secretive, and self-regulated.
Fragrance allergy is on the rise in children and adolescents, probably because they are being exposed to an onslaught of fragrance chemicals at a younger and younger age.
"When I grew up there was a bar of soap in the shower and, if we were lucky, shampoo; and if there was no shampoo we used the bar of soap. I have 19- and 17-year-old daughters, and if you go into their bathroom there are four different shampoos and three conditioners. There are things with glitter in there, and moisturizers and cosmetics, most of which I don’t even know what they’re for. All of them are fragrances. Folks are starting their exposure when they’re 7 and 8 years old. That was never the case before," said Dr. Cohen, who is also director of occupational, environmental, and allergic dermatology at the university.
The commercially available six-ingredient FM II panel is a 14% concentration composed of citronellol 0.5%, hydroxyisohexyl 3-cyclohexenecarboxyaldehyde (Lyral) 2.5%, hexyl cinnamal 5.0%, citral 1.0%, coumarin 2.5%, and farnesol 2.5%.
A long-standing point of contention has been the question of whether patients with atopic disease are more likely than nonatopic individuals to experience contact sensitivity, or less. The latest evidence suggests patients with asthma or severe atopic dermatitis have an overall lower prevalence of contact sensitization compared with nonatopic controls, with one striking exception.
In this very large study conducted by investigators at the Danish National Allergy Research Center, Copenhagen, an inverse association was found between atopic disease and contact allergy to metals and all groups of chemicals ... except fragrances. The prevalence of contact sensitization to fragrances was significantly higher in patients with atopic dermatitis than in controls. The investigators recommended that patients with atopic dermatitis be instructed to avoid scented moisturizers (Allergy 2012;67:1157-64).
The Danes found that patients with severe atopic dermatitis were 30% less likely than controls to be patch test–positive for contact sensitivity overall, whereas mild to moderate atopic dermatitis did not suppress contact sensitization. Again, fragrances constituted the exception: Not only did patients with severe atopic dermatitis have an increased prevalence of contact sensitization to fragrance chemicals, those with mild or moderate atopic dermatitis did, too, Dr. Cohen noted.
He reported having no financial conflicts.
PRAGUE – Reliance upon the time-honored tools for diagnosis of contact allergy due to fragrances will cause physicians to miss many cases, Dr. David E. Cohen said at the annual congress of the European Academy of Dermatology and Venereology.
"The old fragrance mix – fragrance mix I – and the use of balsam of Peru as fragrance screening tools really won’t capture contact dermatitis involving allergens to more modern fragrances," cautioned Dr. Cohen, professor and vice chair of the department of dermatology at New York University.
"I remember my grandmother always smelling like rosewater and naphthalene – like mothballs and rosewater. But we don’t smell like that anymore. Now we tend to use more botanical extracts. We see tea tree oil and jasmine appearing in our personal care products, and if we don’t test for those more contemporary fragrances, we will miss a very important source of contact dermatitis," he continued.
That’s why fragrance mix II (FM II) was developed. FM II has been incorporated into the North American Contact Dermatitis Group’s standard screening tray. It is not, however, part of the widely used T.R.U.E. (Thin-Layer Rapid Use Epicutaneous) test, nor is it included in the European standard screening series.
Multiple studies have documented that a substantial proportion of patients with allergic contact dermatitis will react only to the newer fragrances, and not to FM I or balsam of Peru. For example, a multicenter Hungarian study published earlier this year found that of 565 patients patch tested because of skin symptoms provoked by scented products, 17% exhibited contact hypersensitivity to one or more components of FM II. Moreover, 48% of FM II–positive patients reacted only to FM II, not FM I, balsam of Peru, or other test materials (Dermatitis 2012;23:71-4).
Similarly, a Mayo Clinic study of 945 patients patch tested using both the standard screening tray with FM II and the T.R.U.E. test found that 49% of patients reacted to one or more preservatives and 31% reacted to at least one fragrance or botanical additive. However, the T.R.U.E. test didn’t capture 23% of patients with a preservative allergy and 11% of those with a fragrance or botanical allergy (J. Am. Acad. Dermatol. 2010;63:789-98).
Fragrances pose one of the greatest challenges in the field of contact dermatitis. That’s because there are roughly 3,000 fragrance chemicals utilized in personal care products and cosmetics, and at least 100 of them have been described as causing contact dermatitis in patients. It can be difficult to assess the clinical relevance of positive patch test reactions because physicians don’t know what’s contained in North American fragrances. The fragrance industry is lucrative, secretive, and self-regulated.
Fragrance allergy is on the rise in children and adolescents, probably because they are being exposed to an onslaught of fragrance chemicals at a younger and younger age.
"When I grew up there was a bar of soap in the shower and, if we were lucky, shampoo; and if there was no shampoo we used the bar of soap. I have 19- and 17-year-old daughters, and if you go into their bathroom there are four different shampoos and three conditioners. There are things with glitter in there, and moisturizers and cosmetics, most of which I don’t even know what they’re for. All of them are fragrances. Folks are starting their exposure when they’re 7 and 8 years old. That was never the case before," said Dr. Cohen, who is also director of occupational, environmental, and allergic dermatology at the university.
The commercially available six-ingredient FM II panel is a 14% concentration composed of citronellol 0.5%, hydroxyisohexyl 3-cyclohexenecarboxyaldehyde (Lyral) 2.5%, hexyl cinnamal 5.0%, citral 1.0%, coumarin 2.5%, and farnesol 2.5%.
A long-standing point of contention has been the question of whether patients with atopic disease are more likely than nonatopic individuals to experience contact sensitivity, or less. The latest evidence suggests patients with asthma or severe atopic dermatitis have an overall lower prevalence of contact sensitization compared with nonatopic controls, with one striking exception.
In this very large study conducted by investigators at the Danish National Allergy Research Center, Copenhagen, an inverse association was found between atopic disease and contact allergy to metals and all groups of chemicals ... except fragrances. The prevalence of contact sensitization to fragrances was significantly higher in patients with atopic dermatitis than in controls. The investigators recommended that patients with atopic dermatitis be instructed to avoid scented moisturizers (Allergy 2012;67:1157-64).
The Danes found that patients with severe atopic dermatitis were 30% less likely than controls to be patch test–positive for contact sensitivity overall, whereas mild to moderate atopic dermatitis did not suppress contact sensitization. Again, fragrances constituted the exception: Not only did patients with severe atopic dermatitis have an increased prevalence of contact sensitization to fragrance chemicals, those with mild or moderate atopic dermatitis did, too, Dr. Cohen noted.
He reported having no financial conflicts.
EXPERT OPINION FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Stroke caution on thalidomide for cutaneous LE
PRAGUE – Low-dose thalidomide for refractory cutaneous lupus erythematosus is best used together with hydroxychloroquine or another antimalarial agent rather than as monotherapy, Dr. Victoria P. Werth asserted at the annual congress of the European Academy of Dermatology and Venereology.
When prescribing thalidomide for a patient with refractory cutaneous lupus erythematosus (CLE), many physicians discontinue antimalarial therapy, reasoning that since the patient wasn’t responsive to monotherapy, there’s no point in continued exposure to the potential risks. But that’s probably a mistake. Combination therapy acting through different mechanisms may boost the likelihood of a good response; plus, the antiplatelet action of hydroxychloroquine or another antimalarial agent will help counteract thalidomide’s prothrombotic effects, said Dr. Werth, professor of dermatology at the University of Pennsylvania, Philadelphia.
Thalidomide is unquestionably an effective therapy in patients with refractory CLE. But it’s also a drug with big problems, including perhaps an increased stroke risk, as highlighted in a recent Spanish study, she noted.
The Spanish study included 60 consecutive patients with refractory CLE who were treated with thalidomide at 100 mg/day and followed for up to 8 years. One dropped out due to side effects. Fifty-eight of the remaining 59 experienced significant clinical improvement, including 49 (85%) with a complete response as defined by a CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index) activity score of 0.
Relapse occurred in most patients, usually about 5 months after thalidomide dose reduction or withdrawal. Patients with subacute CLE were 30-fold more likely to remain in remission after drug discontinuation; those with discoid LE were at increased risk for relapse (Br. J. Dermatol. 2012;166:616-23).
Of particular concern to Dr. Werth was the finding that two patients had a stroke while on the drug. Neither had antiphospholipid antibodies, and one was quite young to have had a stroke, although both were heavy smokers.
Prescribing a drug such as thalidomide that promotes a hypercoagulable state to patients with refractory CLE is problematic because they often already have multiple risk factors for thrombosis. For one thing, treatment-refractory CLE patients tend to be smokers. Many of them are women on oral contraceptives. And there is an increased prevalence of antiphospholipid antibodies in patients with CLE, according to Dr. Werth.
Neuropathy is another major issue with thalidomide. In the Spanish study, 11 of 60 patients (18%) developed paresthesias; nerve conduction studies confirmed sensory polyneuropathy in 5 of the 11. Fortunately, the neurologic symptoms resolved in an average of 12 months after drug withdrawal.
Of course, thalidomide is a notorious teratogen. It can also cause premature ovarian failure, although this is usually reversible upon drug discontinuation.
"Obviously we need better therapies than thalidomide," Dr. Werth concluded.
Toward that end, interest is growing in thalidomide analogues as a novel potential therapy for refractory CLE. These analogues are up to 50,000 times more active than thalidomide, and are potentially less neurotoxic. One of them, lenalidomide (Revlimid), is marketed as a treatment for multiple myeloma and myelodysplastic syndrome. Others are in the development pipeline.
Several small observational studies have recently reported favorable results with lenalidomide in patients with refractory CLE. For example, investigators at Vall d’Hebron University Hospital in Barcelona reported on 15 patients treated open label with lenalidomide at 5-10 mg/day, with a follow-up of 15 months. One patient dropped out early due to side effects, but the other 14 saw clinical improvement within the first 2 weeks. Twelve patients, or 86%, achieved a CLASI score of 0. However, 9 of 12 complete responders experienced clinical relapse, usually 2-8 weeks after the drug was tapered and discontinued. Side effects were mild and infrequent, with no thrombosis or polyneuropathy (Arthritis Res. Ther. 2012;14:R265).
In another series, 4 of 5 lenalidomide-treated patients showed significant skin improvement, although one eventually developed symptoms of SLE (J. Am. Acad. Dermatol. 2012;66:571-82).
Based upon these and other promising reports, Celgene, which markets lenalidomide, recently launched the first-ever phase II study of a thalidomide analogue for the treatment of CLE.
Dr. Werth reported having no financial conflicts.
PRAGUE – Low-dose thalidomide for refractory cutaneous lupus erythematosus is best used together with hydroxychloroquine or another antimalarial agent rather than as monotherapy, Dr. Victoria P. Werth asserted at the annual congress of the European Academy of Dermatology and Venereology.
When prescribing thalidomide for a patient with refractory cutaneous lupus erythematosus (CLE), many physicians discontinue antimalarial therapy, reasoning that since the patient wasn’t responsive to monotherapy, there’s no point in continued exposure to the potential risks. But that’s probably a mistake. Combination therapy acting through different mechanisms may boost the likelihood of a good response; plus, the antiplatelet action of hydroxychloroquine or another antimalarial agent will help counteract thalidomide’s prothrombotic effects, said Dr. Werth, professor of dermatology at the University of Pennsylvania, Philadelphia.
Thalidomide is unquestionably an effective therapy in patients with refractory CLE. But it’s also a drug with big problems, including perhaps an increased stroke risk, as highlighted in a recent Spanish study, she noted.
The Spanish study included 60 consecutive patients with refractory CLE who were treated with thalidomide at 100 mg/day and followed for up to 8 years. One dropped out due to side effects. Fifty-eight of the remaining 59 experienced significant clinical improvement, including 49 (85%) with a complete response as defined by a CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index) activity score of 0.
Relapse occurred in most patients, usually about 5 months after thalidomide dose reduction or withdrawal. Patients with subacute CLE were 30-fold more likely to remain in remission after drug discontinuation; those with discoid LE were at increased risk for relapse (Br. J. Dermatol. 2012;166:616-23).
Of particular concern to Dr. Werth was the finding that two patients had a stroke while on the drug. Neither had antiphospholipid antibodies, and one was quite young to have had a stroke, although both were heavy smokers.
Prescribing a drug such as thalidomide that promotes a hypercoagulable state to patients with refractory CLE is problematic because they often already have multiple risk factors for thrombosis. For one thing, treatment-refractory CLE patients tend to be smokers. Many of them are women on oral contraceptives. And there is an increased prevalence of antiphospholipid antibodies in patients with CLE, according to Dr. Werth.
Neuropathy is another major issue with thalidomide. In the Spanish study, 11 of 60 patients (18%) developed paresthesias; nerve conduction studies confirmed sensory polyneuropathy in 5 of the 11. Fortunately, the neurologic symptoms resolved in an average of 12 months after drug withdrawal.
Of course, thalidomide is a notorious teratogen. It can also cause premature ovarian failure, although this is usually reversible upon drug discontinuation.
"Obviously we need better therapies than thalidomide," Dr. Werth concluded.
Toward that end, interest is growing in thalidomide analogues as a novel potential therapy for refractory CLE. These analogues are up to 50,000 times more active than thalidomide, and are potentially less neurotoxic. One of them, lenalidomide (Revlimid), is marketed as a treatment for multiple myeloma and myelodysplastic syndrome. Others are in the development pipeline.
Several small observational studies have recently reported favorable results with lenalidomide in patients with refractory CLE. For example, investigators at Vall d’Hebron University Hospital in Barcelona reported on 15 patients treated open label with lenalidomide at 5-10 mg/day, with a follow-up of 15 months. One patient dropped out early due to side effects, but the other 14 saw clinical improvement within the first 2 weeks. Twelve patients, or 86%, achieved a CLASI score of 0. However, 9 of 12 complete responders experienced clinical relapse, usually 2-8 weeks after the drug was tapered and discontinued. Side effects were mild and infrequent, with no thrombosis or polyneuropathy (Arthritis Res. Ther. 2012;14:R265).
In another series, 4 of 5 lenalidomide-treated patients showed significant skin improvement, although one eventually developed symptoms of SLE (J. Am. Acad. Dermatol. 2012;66:571-82).
Based upon these and other promising reports, Celgene, which markets lenalidomide, recently launched the first-ever phase II study of a thalidomide analogue for the treatment of CLE.
Dr. Werth reported having no financial conflicts.
PRAGUE – Low-dose thalidomide for refractory cutaneous lupus erythematosus is best used together with hydroxychloroquine or another antimalarial agent rather than as monotherapy, Dr. Victoria P. Werth asserted at the annual congress of the European Academy of Dermatology and Venereology.
When prescribing thalidomide for a patient with refractory cutaneous lupus erythematosus (CLE), many physicians discontinue antimalarial therapy, reasoning that since the patient wasn’t responsive to monotherapy, there’s no point in continued exposure to the potential risks. But that’s probably a mistake. Combination therapy acting through different mechanisms may boost the likelihood of a good response; plus, the antiplatelet action of hydroxychloroquine or another antimalarial agent will help counteract thalidomide’s prothrombotic effects, said Dr. Werth, professor of dermatology at the University of Pennsylvania, Philadelphia.
Thalidomide is unquestionably an effective therapy in patients with refractory CLE. But it’s also a drug with big problems, including perhaps an increased stroke risk, as highlighted in a recent Spanish study, she noted.
The Spanish study included 60 consecutive patients with refractory CLE who were treated with thalidomide at 100 mg/day and followed for up to 8 years. One dropped out due to side effects. Fifty-eight of the remaining 59 experienced significant clinical improvement, including 49 (85%) with a complete response as defined by a CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index) activity score of 0.
Relapse occurred in most patients, usually about 5 months after thalidomide dose reduction or withdrawal. Patients with subacute CLE were 30-fold more likely to remain in remission after drug discontinuation; those with discoid LE were at increased risk for relapse (Br. J. Dermatol. 2012;166:616-23).
Of particular concern to Dr. Werth was the finding that two patients had a stroke while on the drug. Neither had antiphospholipid antibodies, and one was quite young to have had a stroke, although both were heavy smokers.
Prescribing a drug such as thalidomide that promotes a hypercoagulable state to patients with refractory CLE is problematic because they often already have multiple risk factors for thrombosis. For one thing, treatment-refractory CLE patients tend to be smokers. Many of them are women on oral contraceptives. And there is an increased prevalence of antiphospholipid antibodies in patients with CLE, according to Dr. Werth.
Neuropathy is another major issue with thalidomide. In the Spanish study, 11 of 60 patients (18%) developed paresthesias; nerve conduction studies confirmed sensory polyneuropathy in 5 of the 11. Fortunately, the neurologic symptoms resolved in an average of 12 months after drug withdrawal.
Of course, thalidomide is a notorious teratogen. It can also cause premature ovarian failure, although this is usually reversible upon drug discontinuation.
"Obviously we need better therapies than thalidomide," Dr. Werth concluded.
Toward that end, interest is growing in thalidomide analogues as a novel potential therapy for refractory CLE. These analogues are up to 50,000 times more active than thalidomide, and are potentially less neurotoxic. One of them, lenalidomide (Revlimid), is marketed as a treatment for multiple myeloma and myelodysplastic syndrome. Others are in the development pipeline.
Several small observational studies have recently reported favorable results with lenalidomide in patients with refractory CLE. For example, investigators at Vall d’Hebron University Hospital in Barcelona reported on 15 patients treated open label with lenalidomide at 5-10 mg/day, with a follow-up of 15 months. One patient dropped out early due to side effects, but the other 14 saw clinical improvement within the first 2 weeks. Twelve patients, or 86%, achieved a CLASI score of 0. However, 9 of 12 complete responders experienced clinical relapse, usually 2-8 weeks after the drug was tapered and discontinued. Side effects were mild and infrequent, with no thrombosis or polyneuropathy (Arthritis Res. Ther. 2012;14:R265).
In another series, 4 of 5 lenalidomide-treated patients showed significant skin improvement, although one eventually developed symptoms of SLE (J. Am. Acad. Dermatol. 2012;66:571-82).
Based upon these and other promising reports, Celgene, which markets lenalidomide, recently launched the first-ever phase II study of a thalidomide analogue for the treatment of CLE.
Dr. Werth reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Psoriasis Patients Have Low Rates of Common Cancers
PRAGUE – Rates of three common malignancies – breast cancer, prostate cancer, and colorectal cancer – appear to be lower in psoriasis patients than in the general population of the Canadian maritime provinces of Newfoundland and Labrador, a study has shown.
In addition, only a single case of lymphoma occurred in 3,289 psoriasis patients followed for an average of 10 years, Dr. Wayne Gulliver said in presenting preliminary results of the ongoing study at the annual congress of the European Academy of Dermatology and Venereology.
The issue of a possible elevated risk of lymphoma in psoriasis patients has been controversial. Findings have been inconsistent. Some studies have reported a significantly increased risk. Among these, notably, was a population-based cohort study of more than 153,000 psoriasis patients and close to 800,000 controls in the U.K. General Practice Research Database. Investigators found an age- and sex-adjusted 35% increased risk of lymphoma in the group with psoriasis (J. Invest. Dermatol. 2006;126:2194-201).
However, lymphoma is an uncommon disease and a 35% increased risk is, from an epidemiologic perspective, only modest. Some studies have not found a significant association between psoriasis and lymphoma. The Canadian maritime study can be added to their ranks, according to Dr. Gulliver, professor of medicine and chairman of the division of dermatology at Memorial University of Newfoundland, St. John’s.
He matched records from a comprehensive research database of psoriasis patients in Newfoundland and Labrador for 1989-2010 with Canadian national and provincial cancer statistics. The purpose of the study was straightforward: "Our patients want to know if their psoriasis affects their risk of cancer, especially with the treatments we’re offering them these days," Dr. Gulliver said.
There were 232 cancer cases in 3,289 psoriasis patients, for a cumulative incidence rate of 588/100,000 population with psoriasis. Although the psoriasis population was divided nearly equally between men and women, 145 men developed cancer compared with 87 women.
Cancer rates were similar regardless of whether patients had mild psoriasis – meaning they received no systemic therapies – or moderate to severe psoriasis.
The cumulative incidence rate of breast cancer among psoriasis patients was 52/100,000 patients with mild psoriasis and 64/100,000 with moderate to severe disease compared with 96/100,000 in the general Canadian population and 84/100,000 among residents of Newfoundland and Labrador. The prostate cancer rate was 103/100,000 patients with mild psoriasis and 75/100,000 with moderate to severe psoriasis as opposed to 121/100,000 nationwide and 129/100,000 men in the general population of the two maritime provinces. The cumulative incidence rate for colorectal cancer followed the same pattern: lower in the psoriasis patients than in the general population.
Nonmelanoma skin cancer rates were relatively high: 142/100,000 patients with mild psoriasis and 122/100,000 with moderate to severe disease. That’s consistent with findings from other studies. It comes as no surprise given the common use of UV therapy and sunlight exposure in psoriasis patients.
Dr. Gulliver noted the relatively small number of cancers to date is a significant study limitation. But that number will grow as the psoriasis patients age; plus, more psoriasis patients are being added to the provincial database. He added that the database provides fertile ground for planned future studies. He said he plans soon to compare cancer rates in psoriasis patients on immunosuppressive therapy with biologic agents or cyclosporine to rates in patients on other agents.
One audience member observed that it seems more than coincidental that increased rates of breast, prostate, and colorectal cancers in the general population have previously been linked to low vitamin D levels. Perhaps the same factor that drives the increase in nonmelanoma skin cancer in psoriasis patients – that is, UV exposure – is responsible for their lower risks of those three common internal malignancies through a mechanism involving enhanced serum vitamin D levels, he speculated.
Dr. Gulliver called that a fascinating hypothesis and one he is now eager to investigate, since he has access to blood samples for the study population.
The study was free of commercial involvement, and the presenter reported having no relevant financial conflicts.
PRAGUE – Rates of three common malignancies – breast cancer, prostate cancer, and colorectal cancer – appear to be lower in psoriasis patients than in the general population of the Canadian maritime provinces of Newfoundland and Labrador, a study has shown.
In addition, only a single case of lymphoma occurred in 3,289 psoriasis patients followed for an average of 10 years, Dr. Wayne Gulliver said in presenting preliminary results of the ongoing study at the annual congress of the European Academy of Dermatology and Venereology.
The issue of a possible elevated risk of lymphoma in psoriasis patients has been controversial. Findings have been inconsistent. Some studies have reported a significantly increased risk. Among these, notably, was a population-based cohort study of more than 153,000 psoriasis patients and close to 800,000 controls in the U.K. General Practice Research Database. Investigators found an age- and sex-adjusted 35% increased risk of lymphoma in the group with psoriasis (J. Invest. Dermatol. 2006;126:2194-201).
However, lymphoma is an uncommon disease and a 35% increased risk is, from an epidemiologic perspective, only modest. Some studies have not found a significant association between psoriasis and lymphoma. The Canadian maritime study can be added to their ranks, according to Dr. Gulliver, professor of medicine and chairman of the division of dermatology at Memorial University of Newfoundland, St. John’s.
He matched records from a comprehensive research database of psoriasis patients in Newfoundland and Labrador for 1989-2010 with Canadian national and provincial cancer statistics. The purpose of the study was straightforward: "Our patients want to know if their psoriasis affects their risk of cancer, especially with the treatments we’re offering them these days," Dr. Gulliver said.
There were 232 cancer cases in 3,289 psoriasis patients, for a cumulative incidence rate of 588/100,000 population with psoriasis. Although the psoriasis population was divided nearly equally between men and women, 145 men developed cancer compared with 87 women.
Cancer rates were similar regardless of whether patients had mild psoriasis – meaning they received no systemic therapies – or moderate to severe psoriasis.
The cumulative incidence rate of breast cancer among psoriasis patients was 52/100,000 patients with mild psoriasis and 64/100,000 with moderate to severe disease compared with 96/100,000 in the general Canadian population and 84/100,000 among residents of Newfoundland and Labrador. The prostate cancer rate was 103/100,000 patients with mild psoriasis and 75/100,000 with moderate to severe psoriasis as opposed to 121/100,000 nationwide and 129/100,000 men in the general population of the two maritime provinces. The cumulative incidence rate for colorectal cancer followed the same pattern: lower in the psoriasis patients than in the general population.
Nonmelanoma skin cancer rates were relatively high: 142/100,000 patients with mild psoriasis and 122/100,000 with moderate to severe disease. That’s consistent with findings from other studies. It comes as no surprise given the common use of UV therapy and sunlight exposure in psoriasis patients.
Dr. Gulliver noted the relatively small number of cancers to date is a significant study limitation. But that number will grow as the psoriasis patients age; plus, more psoriasis patients are being added to the provincial database. He added that the database provides fertile ground for planned future studies. He said he plans soon to compare cancer rates in psoriasis patients on immunosuppressive therapy with biologic agents or cyclosporine to rates in patients on other agents.
One audience member observed that it seems more than coincidental that increased rates of breast, prostate, and colorectal cancers in the general population have previously been linked to low vitamin D levels. Perhaps the same factor that drives the increase in nonmelanoma skin cancer in psoriasis patients – that is, UV exposure – is responsible for their lower risks of those three common internal malignancies through a mechanism involving enhanced serum vitamin D levels, he speculated.
Dr. Gulliver called that a fascinating hypothesis and one he is now eager to investigate, since he has access to blood samples for the study population.
The study was free of commercial involvement, and the presenter reported having no relevant financial conflicts.
PRAGUE – Rates of three common malignancies – breast cancer, prostate cancer, and colorectal cancer – appear to be lower in psoriasis patients than in the general population of the Canadian maritime provinces of Newfoundland and Labrador, a study has shown.
In addition, only a single case of lymphoma occurred in 3,289 psoriasis patients followed for an average of 10 years, Dr. Wayne Gulliver said in presenting preliminary results of the ongoing study at the annual congress of the European Academy of Dermatology and Venereology.
The issue of a possible elevated risk of lymphoma in psoriasis patients has been controversial. Findings have been inconsistent. Some studies have reported a significantly increased risk. Among these, notably, was a population-based cohort study of more than 153,000 psoriasis patients and close to 800,000 controls in the U.K. General Practice Research Database. Investigators found an age- and sex-adjusted 35% increased risk of lymphoma in the group with psoriasis (J. Invest. Dermatol. 2006;126:2194-201).
However, lymphoma is an uncommon disease and a 35% increased risk is, from an epidemiologic perspective, only modest. Some studies have not found a significant association between psoriasis and lymphoma. The Canadian maritime study can be added to their ranks, according to Dr. Gulliver, professor of medicine and chairman of the division of dermatology at Memorial University of Newfoundland, St. John’s.
He matched records from a comprehensive research database of psoriasis patients in Newfoundland and Labrador for 1989-2010 with Canadian national and provincial cancer statistics. The purpose of the study was straightforward: "Our patients want to know if their psoriasis affects their risk of cancer, especially with the treatments we’re offering them these days," Dr. Gulliver said.
There were 232 cancer cases in 3,289 psoriasis patients, for a cumulative incidence rate of 588/100,000 population with psoriasis. Although the psoriasis population was divided nearly equally between men and women, 145 men developed cancer compared with 87 women.
Cancer rates were similar regardless of whether patients had mild psoriasis – meaning they received no systemic therapies – or moderate to severe psoriasis.
The cumulative incidence rate of breast cancer among psoriasis patients was 52/100,000 patients with mild psoriasis and 64/100,000 with moderate to severe disease compared with 96/100,000 in the general Canadian population and 84/100,000 among residents of Newfoundland and Labrador. The prostate cancer rate was 103/100,000 patients with mild psoriasis and 75/100,000 with moderate to severe psoriasis as opposed to 121/100,000 nationwide and 129/100,000 men in the general population of the two maritime provinces. The cumulative incidence rate for colorectal cancer followed the same pattern: lower in the psoriasis patients than in the general population.
Nonmelanoma skin cancer rates were relatively high: 142/100,000 patients with mild psoriasis and 122/100,000 with moderate to severe disease. That’s consistent with findings from other studies. It comes as no surprise given the common use of UV therapy and sunlight exposure in psoriasis patients.
Dr. Gulliver noted the relatively small number of cancers to date is a significant study limitation. But that number will grow as the psoriasis patients age; plus, more psoriasis patients are being added to the provincial database. He added that the database provides fertile ground for planned future studies. He said he plans soon to compare cancer rates in psoriasis patients on immunosuppressive therapy with biologic agents or cyclosporine to rates in patients on other agents.
One audience member observed that it seems more than coincidental that increased rates of breast, prostate, and colorectal cancers in the general population have previously been linked to low vitamin D levels. Perhaps the same factor that drives the increase in nonmelanoma skin cancer in psoriasis patients – that is, UV exposure – is responsible for their lower risks of those three common internal malignancies through a mechanism involving enhanced serum vitamin D levels, he speculated.
Dr. Gulliver called that a fascinating hypothesis and one he is now eager to investigate, since he has access to blood samples for the study population.
The study was free of commercial involvement, and the presenter reported having no relevant financial conflicts.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: There were 232 cancer cases in 3,289 psoriasis patients, for a cumulative incidence rate of 588/100,000 population with psoriasis, a finding that suggests these patients have lower-than-average risks of prostate, breast, and colorectal cancers.
Data Source: This was a population-based cohort study involving 3,289 psoriasis patients in Newfoundland and Labrador. Their risk of various cancers during roughly 32,000 person-years of follow-up was determined by analysis of the comprehensive provincial and national cancer registries.
Disclosures: The study was free of commercial involvement, and the presenter reported having no relevant financial conflicts.
Nongonococcal urethritis: Time to ditch azithromycin?
PRAGUE – Recent evidence dictates the need to reassess the roles of doxycycline and azithromycin in treating Chlamydia trachomatis nongonococcal urethritis, according to Dr. Michel Janier.
Current first-line therapy for nongonococcal urethritis (NGU), as recommended by the Centers for Disease Control and Prevention and other major groups, features a choice: a single 1-g dose of azithromycin, or doxycycline at 100 mg b.i.d. for 7 days. Single-dose azithromycin is a popular option, given its convenience and likely better adherence.
But a couple of important head-to-head comparative trials published recently are making waves in the infectious disease world. Doxycycline proved significantly more effective than azithromycin in clearing chlamydial NGU in one study and had a markedly lower C. trachomatis persistence rate 45 days post treatment in the other, said Dr. Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections–Europe.
One study was a phase IIb randomized, double-blind trial involving 305 men with NGU at sexually transmitted disease clinics in four U.S. cities. They were assigned to guideline-recommended treatment with either azithromycin or doxycycline alone or with a single 2-g dose of tinidazole, an antitrichomonal agent, in order to test the hypothesis that adding the second agent would boost cure rates. As it turned out, it did not.
Among the 43% of men with C. trachomatis NGU, the chlamydial clearance rate was 94.8% in the doxycycline arm compared with 77.4% with azithromycin.
While doxycycline outperformed azithromycin in men with C. trachomatis NGU, the converse was true among the 31% of participants with Mycoplasma genitalium NGU. The clearance rate was 30.8% in the doxycycline arm compared with 66.7% in the azithromycin arm (Clin. Infect. Dis. 2011;52:163-70).
The same group of investigators recently analyzed data on post-treatment persistence of NGU in a study involving 293 heterosexual men treated for NGU at STD clinics. Among the 129 men with C. trachomatis NGU, persistent C. trachomatis infection was detected via nucleic acid amplification testing 4 weeks post treatment in 23% of those who received azithromycin compared with just 5% treated with doxycycline (J. Infect. Dis. 2012;206:357-65).
The explanation for the observed higher failure rate with azithromycin compared with doxycycline in treating chlamydial NGU is probably twofold: homotypic resistance of the organism to a single dose of a bacteriostatic antibiotic, coupled with heterotopic resistance stemming from a persistent subpopulation of cells having a reduced growth rate within the larger pathogen load, Dr. Janier said.
He added that while it’s important not to overreact to a couple of studies carried out by a single group, there is intense interest on the part of many infectious disease experts in taking a closer look at the possibility that azithromycin may need to be dethroned as first-line therapy.
The dermatologist also highlighted another recent study that bears on the treatment of uncomplicated C. trachomatis NGU. The double-blind, randomized, double-dummy multicenter trial included 323 men and nonpregnant women with urogenital chlamydia. They were randomized to 7 days of once-daily delayed-release doxycycline (Doryx) at 200 mg or to 100 mg b.i.d. of standard-release doxycycline (Vibramycin).
The primary outcome, microbial cure by nucleic acid amplification testing on day 28, occurred in 95.5% of the delayed-release doxycycline group and a virtually identical 95.2% on Vibramycin. However, the delayed-release formulation was significantly better tolerated, with a 13% nausea rate compared with 21% in the Vibramycin group. Vomiting occurred in 8% of patients on delayed-release doxycycline, a significantly lower rate than the 12% with Vibramycin.
The investigators concluded that delayed-release doxycycline, with its once-daily dosing and better tolerability, could improve treatment adherence (Clin. Infect. Dis. 2012; 55: 82-8).
Dr. Janier reported having no relevant financial disclosures.
PRAGUE – Recent evidence dictates the need to reassess the roles of doxycycline and azithromycin in treating Chlamydia trachomatis nongonococcal urethritis, according to Dr. Michel Janier.
Current first-line therapy for nongonococcal urethritis (NGU), as recommended by the Centers for Disease Control and Prevention and other major groups, features a choice: a single 1-g dose of azithromycin, or doxycycline at 100 mg b.i.d. for 7 days. Single-dose azithromycin is a popular option, given its convenience and likely better adherence.
But a couple of important head-to-head comparative trials published recently are making waves in the infectious disease world. Doxycycline proved significantly more effective than azithromycin in clearing chlamydial NGU in one study and had a markedly lower C. trachomatis persistence rate 45 days post treatment in the other, said Dr. Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections–Europe.
One study was a phase IIb randomized, double-blind trial involving 305 men with NGU at sexually transmitted disease clinics in four U.S. cities. They were assigned to guideline-recommended treatment with either azithromycin or doxycycline alone or with a single 2-g dose of tinidazole, an antitrichomonal agent, in order to test the hypothesis that adding the second agent would boost cure rates. As it turned out, it did not.
Among the 43% of men with C. trachomatis NGU, the chlamydial clearance rate was 94.8% in the doxycycline arm compared with 77.4% with azithromycin.
While doxycycline outperformed azithromycin in men with C. trachomatis NGU, the converse was true among the 31% of participants with Mycoplasma genitalium NGU. The clearance rate was 30.8% in the doxycycline arm compared with 66.7% in the azithromycin arm (Clin. Infect. Dis. 2011;52:163-70).
The same group of investigators recently analyzed data on post-treatment persistence of NGU in a study involving 293 heterosexual men treated for NGU at STD clinics. Among the 129 men with C. trachomatis NGU, persistent C. trachomatis infection was detected via nucleic acid amplification testing 4 weeks post treatment in 23% of those who received azithromycin compared with just 5% treated with doxycycline (J. Infect. Dis. 2012;206:357-65).
The explanation for the observed higher failure rate with azithromycin compared with doxycycline in treating chlamydial NGU is probably twofold: homotypic resistance of the organism to a single dose of a bacteriostatic antibiotic, coupled with heterotopic resistance stemming from a persistent subpopulation of cells having a reduced growth rate within the larger pathogen load, Dr. Janier said.
He added that while it’s important not to overreact to a couple of studies carried out by a single group, there is intense interest on the part of many infectious disease experts in taking a closer look at the possibility that azithromycin may need to be dethroned as first-line therapy.
The dermatologist also highlighted another recent study that bears on the treatment of uncomplicated C. trachomatis NGU. The double-blind, randomized, double-dummy multicenter trial included 323 men and nonpregnant women with urogenital chlamydia. They were randomized to 7 days of once-daily delayed-release doxycycline (Doryx) at 200 mg or to 100 mg b.i.d. of standard-release doxycycline (Vibramycin).
The primary outcome, microbial cure by nucleic acid amplification testing on day 28, occurred in 95.5% of the delayed-release doxycycline group and a virtually identical 95.2% on Vibramycin. However, the delayed-release formulation was significantly better tolerated, with a 13% nausea rate compared with 21% in the Vibramycin group. Vomiting occurred in 8% of patients on delayed-release doxycycline, a significantly lower rate than the 12% with Vibramycin.
The investigators concluded that delayed-release doxycycline, with its once-daily dosing and better tolerability, could improve treatment adherence (Clin. Infect. Dis. 2012; 55: 82-8).
Dr. Janier reported having no relevant financial disclosures.
PRAGUE – Recent evidence dictates the need to reassess the roles of doxycycline and azithromycin in treating Chlamydia trachomatis nongonococcal urethritis, according to Dr. Michel Janier.
Current first-line therapy for nongonococcal urethritis (NGU), as recommended by the Centers for Disease Control and Prevention and other major groups, features a choice: a single 1-g dose of azithromycin, or doxycycline at 100 mg b.i.d. for 7 days. Single-dose azithromycin is a popular option, given its convenience and likely better adherence.
But a couple of important head-to-head comparative trials published recently are making waves in the infectious disease world. Doxycycline proved significantly more effective than azithromycin in clearing chlamydial NGU in one study and had a markedly lower C. trachomatis persistence rate 45 days post treatment in the other, said Dr. Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections–Europe.
One study was a phase IIb randomized, double-blind trial involving 305 men with NGU at sexually transmitted disease clinics in four U.S. cities. They were assigned to guideline-recommended treatment with either azithromycin or doxycycline alone or with a single 2-g dose of tinidazole, an antitrichomonal agent, in order to test the hypothesis that adding the second agent would boost cure rates. As it turned out, it did not.
Among the 43% of men with C. trachomatis NGU, the chlamydial clearance rate was 94.8% in the doxycycline arm compared with 77.4% with azithromycin.
While doxycycline outperformed azithromycin in men with C. trachomatis NGU, the converse was true among the 31% of participants with Mycoplasma genitalium NGU. The clearance rate was 30.8% in the doxycycline arm compared with 66.7% in the azithromycin arm (Clin. Infect. Dis. 2011;52:163-70).
The same group of investigators recently analyzed data on post-treatment persistence of NGU in a study involving 293 heterosexual men treated for NGU at STD clinics. Among the 129 men with C. trachomatis NGU, persistent C. trachomatis infection was detected via nucleic acid amplification testing 4 weeks post treatment in 23% of those who received azithromycin compared with just 5% treated with doxycycline (J. Infect. Dis. 2012;206:357-65).
The explanation for the observed higher failure rate with azithromycin compared with doxycycline in treating chlamydial NGU is probably twofold: homotypic resistance of the organism to a single dose of a bacteriostatic antibiotic, coupled with heterotopic resistance stemming from a persistent subpopulation of cells having a reduced growth rate within the larger pathogen load, Dr. Janier said.
He added that while it’s important not to overreact to a couple of studies carried out by a single group, there is intense interest on the part of many infectious disease experts in taking a closer look at the possibility that azithromycin may need to be dethroned as first-line therapy.
The dermatologist also highlighted another recent study that bears on the treatment of uncomplicated C. trachomatis NGU. The double-blind, randomized, double-dummy multicenter trial included 323 men and nonpregnant women with urogenital chlamydia. They were randomized to 7 days of once-daily delayed-release doxycycline (Doryx) at 200 mg or to 100 mg b.i.d. of standard-release doxycycline (Vibramycin).
The primary outcome, microbial cure by nucleic acid amplification testing on day 28, occurred in 95.5% of the delayed-release doxycycline group and a virtually identical 95.2% on Vibramycin. However, the delayed-release formulation was significantly better tolerated, with a 13% nausea rate compared with 21% in the Vibramycin group. Vomiting occurred in 8% of patients on delayed-release doxycycline, a significantly lower rate than the 12% with Vibramycin.
The investigators concluded that delayed-release doxycycline, with its once-daily dosing and better tolerability, could improve treatment adherence (Clin. Infect. Dis. 2012; 55: 82-8).
Dr. Janier reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Optimized Pulsed Light Clears Vascular Lesions
PRAGUE – Optimized pulsed light therapy using Palomar Medical Technologies’ proprietary MaxG handpiece proved safe and efficient for the treatment of port wine stains and capillary malformations in an open study.
The MaxG is next-generation intense pulsed light therapy. The advance lies in a handpiece optimized so as to use a dual-band spectrum with two peaks: one at 500-670 nm and another at 870-1,200 nm. The peak in the visible light range targets small, superficial vessels, while the one in the near infrared addresses deeper vessels. Thus, optimized pulsed light (OPL) is able to achieve uniform heating and pulse width matched to a target vessel’s depth and size, Dr. Maurice A. Adatto explained at the annual congress of the European Academy of Dermatology and Venereology.
For years, pulsed dye laser therapy has been considered the gold standard in the treatment of vascular lesions. But recent published data demonstrate that while the laser can coagulate vessels quite nicely at the surface, the coagulation isn’t nearly as good for deeper vessels. In animal models, the OPL achieves greater increases in temperature compared with the pulsed dye laser at the purpuric threshold fluence in deeper and larger capillaries, said Dr. Adatto, who is medical director of the SkinPulse Dermatology & Laser Center in Geneva.
Dr. Adatto presented a two-center series of 16 OPL-treated adults and adolescents with vascular lesions on the face, neck, trunk, and lower limbs. The procedures were performed by him at the center in Geneva, and by his coinvestigator Dr. David Friedman in Jerusalem. The OPL handpiece was attached to Palomar’s Icon intense pulsed light device.
Outcomes were objectively assessed using the Antera 3D camera, made by Miravex, which provides high-definition clinical photographs along with quantitative measurement of hemoglobin and melanin clearance in treated areas. Assessments were done at 2-4 days and 1-2 months post-treatment.
The efficacy was impressive, and the side effects were far milder and more transitory than can occur with pulsed dye laser therapy, according to the dermatologist.
The majority of patients – 10 of 16 – achieved 50% or greater improvement in one to four OPL sessions. After the first treatment, four patients had roughly a 20% improvement, five were 25%-49% better, three showed 50%-74% clearance, and two patients showed 80% and 100% clearance.
Side effects consisted of 3-5 days of purpura and 1-3 days of local edema. To date, OPL for vascular lesions hasn’t resulted in any scars or in hypo- or hyperpigmentation.
As for the technical details, Dr. Adatto utilized one pass with 50 J/cm2 at 10 ms, while Dr. Friedman used a two-pass technique: the first at 34-36 J/cm2 at 10 ms, followed by a second at 22-28 J/cm2 at 5 ms. Treatment sessions were carried out at 4- to 6-week intervals.
In response to audience questions, Dr. Adatto said that his anecdotal experience has been that once a patient achieves greater than about 50% improvement, be it with a single OPL treatment session or after three, the therapeutic gain of additional sessions is smaller than with the initial ones.
"You can gain another 10% or so with another session for someone who has 70% improvement, but that’s it. You will reach a plateau," he said.
Dr. Adatto is now planning a similar study using OPL to treat vascular lesions in a pediatric population.
The study was funded by Palomar Medical Technologies. Dr. Adatto has received research funds from and is an adviser to Palomar and numerous other laser and intense pulsed light device manufacturers.
PRAGUE – Optimized pulsed light therapy using Palomar Medical Technologies’ proprietary MaxG handpiece proved safe and efficient for the treatment of port wine stains and capillary malformations in an open study.
The MaxG is next-generation intense pulsed light therapy. The advance lies in a handpiece optimized so as to use a dual-band spectrum with two peaks: one at 500-670 nm and another at 870-1,200 nm. The peak in the visible light range targets small, superficial vessels, while the one in the near infrared addresses deeper vessels. Thus, optimized pulsed light (OPL) is able to achieve uniform heating and pulse width matched to a target vessel’s depth and size, Dr. Maurice A. Adatto explained at the annual congress of the European Academy of Dermatology and Venereology.
For years, pulsed dye laser therapy has been considered the gold standard in the treatment of vascular lesions. But recent published data demonstrate that while the laser can coagulate vessels quite nicely at the surface, the coagulation isn’t nearly as good for deeper vessels. In animal models, the OPL achieves greater increases in temperature compared with the pulsed dye laser at the purpuric threshold fluence in deeper and larger capillaries, said Dr. Adatto, who is medical director of the SkinPulse Dermatology & Laser Center in Geneva.
Dr. Adatto presented a two-center series of 16 OPL-treated adults and adolescents with vascular lesions on the face, neck, trunk, and lower limbs. The procedures were performed by him at the center in Geneva, and by his coinvestigator Dr. David Friedman in Jerusalem. The OPL handpiece was attached to Palomar’s Icon intense pulsed light device.
Outcomes were objectively assessed using the Antera 3D camera, made by Miravex, which provides high-definition clinical photographs along with quantitative measurement of hemoglobin and melanin clearance in treated areas. Assessments were done at 2-4 days and 1-2 months post-treatment.
The efficacy was impressive, and the side effects were far milder and more transitory than can occur with pulsed dye laser therapy, according to the dermatologist.
The majority of patients – 10 of 16 – achieved 50% or greater improvement in one to four OPL sessions. After the first treatment, four patients had roughly a 20% improvement, five were 25%-49% better, three showed 50%-74% clearance, and two patients showed 80% and 100% clearance.
Side effects consisted of 3-5 days of purpura and 1-3 days of local edema. To date, OPL for vascular lesions hasn’t resulted in any scars or in hypo- or hyperpigmentation.
As for the technical details, Dr. Adatto utilized one pass with 50 J/cm2 at 10 ms, while Dr. Friedman used a two-pass technique: the first at 34-36 J/cm2 at 10 ms, followed by a second at 22-28 J/cm2 at 5 ms. Treatment sessions were carried out at 4- to 6-week intervals.
In response to audience questions, Dr. Adatto said that his anecdotal experience has been that once a patient achieves greater than about 50% improvement, be it with a single OPL treatment session or after three, the therapeutic gain of additional sessions is smaller than with the initial ones.
"You can gain another 10% or so with another session for someone who has 70% improvement, but that’s it. You will reach a plateau," he said.
Dr. Adatto is now planning a similar study using OPL to treat vascular lesions in a pediatric population.
The study was funded by Palomar Medical Technologies. Dr. Adatto has received research funds from and is an adviser to Palomar and numerous other laser and intense pulsed light device manufacturers.
PRAGUE – Optimized pulsed light therapy using Palomar Medical Technologies’ proprietary MaxG handpiece proved safe and efficient for the treatment of port wine stains and capillary malformations in an open study.
The MaxG is next-generation intense pulsed light therapy. The advance lies in a handpiece optimized so as to use a dual-band spectrum with two peaks: one at 500-670 nm and another at 870-1,200 nm. The peak in the visible light range targets small, superficial vessels, while the one in the near infrared addresses deeper vessels. Thus, optimized pulsed light (OPL) is able to achieve uniform heating and pulse width matched to a target vessel’s depth and size, Dr. Maurice A. Adatto explained at the annual congress of the European Academy of Dermatology and Venereology.
For years, pulsed dye laser therapy has been considered the gold standard in the treatment of vascular lesions. But recent published data demonstrate that while the laser can coagulate vessels quite nicely at the surface, the coagulation isn’t nearly as good for deeper vessels. In animal models, the OPL achieves greater increases in temperature compared with the pulsed dye laser at the purpuric threshold fluence in deeper and larger capillaries, said Dr. Adatto, who is medical director of the SkinPulse Dermatology & Laser Center in Geneva.
Dr. Adatto presented a two-center series of 16 OPL-treated adults and adolescents with vascular lesions on the face, neck, trunk, and lower limbs. The procedures were performed by him at the center in Geneva, and by his coinvestigator Dr. David Friedman in Jerusalem. The OPL handpiece was attached to Palomar’s Icon intense pulsed light device.
Outcomes were objectively assessed using the Antera 3D camera, made by Miravex, which provides high-definition clinical photographs along with quantitative measurement of hemoglobin and melanin clearance in treated areas. Assessments were done at 2-4 days and 1-2 months post-treatment.
The efficacy was impressive, and the side effects were far milder and more transitory than can occur with pulsed dye laser therapy, according to the dermatologist.
The majority of patients – 10 of 16 – achieved 50% or greater improvement in one to four OPL sessions. After the first treatment, four patients had roughly a 20% improvement, five were 25%-49% better, three showed 50%-74% clearance, and two patients showed 80% and 100% clearance.
Side effects consisted of 3-5 days of purpura and 1-3 days of local edema. To date, OPL for vascular lesions hasn’t resulted in any scars or in hypo- or hyperpigmentation.
As for the technical details, Dr. Adatto utilized one pass with 50 J/cm2 at 10 ms, while Dr. Friedman used a two-pass technique: the first at 34-36 J/cm2 at 10 ms, followed by a second at 22-28 J/cm2 at 5 ms. Treatment sessions were carried out at 4- to 6-week intervals.
In response to audience questions, Dr. Adatto said that his anecdotal experience has been that once a patient achieves greater than about 50% improvement, be it with a single OPL treatment session or after three, the therapeutic gain of additional sessions is smaller than with the initial ones.
"You can gain another 10% or so with another session for someone who has 70% improvement, but that’s it. You will reach a plateau," he said.
Dr. Adatto is now planning a similar study using OPL to treat vascular lesions in a pediatric population.
The study was funded by Palomar Medical Technologies. Dr. Adatto has received research funds from and is an adviser to Palomar and numerous other laser and intense pulsed light device manufacturers.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Ten of 16 adults and adolescents who underwent dual-spectrum optimized pulsed light therapy for a variety of vascular lesions demonstrated 50% or greater improvement after one to four treatment sessions. Side effects were considerably milder than typically seen following pulsed dye laser therapy.
Data Source: This was an open study conducted at dermatology centers in Geneva and Jerusalem.
Disclosures: The study was funded by Palomar Medical Technologies, which markets the MaxG optimized pulsed light handpiece. The presenter is an advisor to Palomar and numerous other laser and intense pulsed light device manufacturers.
Secukinumab Tames Hand, Foot Psoriasis
PRAGUE – The investigational anti-interleukin-17A biologic agent secukinumab holds particular promise for the treatment of psoriasis in two of its most challenging, tough-to-tame manifestations: hand and foot psoriasis and nail involvement, a study has shown.
A post hoc analysis of a previously reported phase II, double-blind, placebo-controlled, 12-week dose-finding study in 404 patients with moderate to severe plaque psoriasis focused in part on the 131 subjects with hand and/or foot involvement. The same regimen that achieved the best outcomes in the main study – 150 mg of secukinumab given subcutaneously at weeks 0, 1, 2, and 4 – showed the greatest beneficial effect for hand and foot psoriasis, Dr. Carle Paul reported at the annual congress of the European Academy of Dermatology and Venereology.
Fifty-four percent of patients randomized to this optimal secukinumab dosing regimen achieved a positive investigator global response of their moderate to severe hand and foot psoriasis at week 12 compared with 19% assigned to placebo. An investigator global response required at least a 2-point improvement on a 5-point scale along with a score of 0 or 1, meaning clear or only minimal disease, explained Dr. Paul, a dermatologist at the University of Toulouse (France).
The PASI 75 and PASI 90 rates in patients with hand and foot psoriasis – that is, the percentage of patients with at least a 75% or 90% improvement over baseline scores on the Psoriasis Area and Severity Index – were similar to the 55% and 32% rates observed in the overall study population, he added.
The phase II study also included 304 patients with nail psoriasis. At week 12, those patients treated with secukinumab at weeks 0, 1, 2, and 4 showed a mean 19% improvement on a composite fingernail involvement score, compared with a 14% worsening with placebo.
These findings are especially encouraging because hand, foot, and nail psoriasis affect an estimated 10%-55% of all psoriasis patients. Involvement at these sites causes considerable functional disability, including difficulty in walking and using the hands. In addition, hand, foot, and nail psoriasis are notoriously difficult to treat.
Secukinumab’s side effect pattern in the large phase II study was similar to that for placebo. Definitive safety and efficacy results will come from ongoing pivotal phase III clinical trials totaling more than 3,000 psoriasis patients worldwide. The results are anticipated next year.
In addition to being used for psoriasis, secukinumab is being developed as a novel treatment for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ongoing phase III studies are expected to report results in 2014. Also, phase II studies of secukinumab for multiple sclerosis are underway.
Secukinumab is a fully human anti-interleukin-17A monoclonal antibody being developed by Novartis.
The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.
PRAGUE – The investigational anti-interleukin-17A biologic agent secukinumab holds particular promise for the treatment of psoriasis in two of its most challenging, tough-to-tame manifestations: hand and foot psoriasis and nail involvement, a study has shown.
A post hoc analysis of a previously reported phase II, double-blind, placebo-controlled, 12-week dose-finding study in 404 patients with moderate to severe plaque psoriasis focused in part on the 131 subjects with hand and/or foot involvement. The same regimen that achieved the best outcomes in the main study – 150 mg of secukinumab given subcutaneously at weeks 0, 1, 2, and 4 – showed the greatest beneficial effect for hand and foot psoriasis, Dr. Carle Paul reported at the annual congress of the European Academy of Dermatology and Venereology.
Fifty-four percent of patients randomized to this optimal secukinumab dosing regimen achieved a positive investigator global response of their moderate to severe hand and foot psoriasis at week 12 compared with 19% assigned to placebo. An investigator global response required at least a 2-point improvement on a 5-point scale along with a score of 0 or 1, meaning clear or only minimal disease, explained Dr. Paul, a dermatologist at the University of Toulouse (France).
The PASI 75 and PASI 90 rates in patients with hand and foot psoriasis – that is, the percentage of patients with at least a 75% or 90% improvement over baseline scores on the Psoriasis Area and Severity Index – were similar to the 55% and 32% rates observed in the overall study population, he added.
The phase II study also included 304 patients with nail psoriasis. At week 12, those patients treated with secukinumab at weeks 0, 1, 2, and 4 showed a mean 19% improvement on a composite fingernail involvement score, compared with a 14% worsening with placebo.
These findings are especially encouraging because hand, foot, and nail psoriasis affect an estimated 10%-55% of all psoriasis patients. Involvement at these sites causes considerable functional disability, including difficulty in walking and using the hands. In addition, hand, foot, and nail psoriasis are notoriously difficult to treat.
Secukinumab’s side effect pattern in the large phase II study was similar to that for placebo. Definitive safety and efficacy results will come from ongoing pivotal phase III clinical trials totaling more than 3,000 psoriasis patients worldwide. The results are anticipated next year.
In addition to being used for psoriasis, secukinumab is being developed as a novel treatment for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ongoing phase III studies are expected to report results in 2014. Also, phase II studies of secukinumab for multiple sclerosis are underway.
Secukinumab is a fully human anti-interleukin-17A monoclonal antibody being developed by Novartis.
The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.
PRAGUE – The investigational anti-interleukin-17A biologic agent secukinumab holds particular promise for the treatment of psoriasis in two of its most challenging, tough-to-tame manifestations: hand and foot psoriasis and nail involvement, a study has shown.
A post hoc analysis of a previously reported phase II, double-blind, placebo-controlled, 12-week dose-finding study in 404 patients with moderate to severe plaque psoriasis focused in part on the 131 subjects with hand and/or foot involvement. The same regimen that achieved the best outcomes in the main study – 150 mg of secukinumab given subcutaneously at weeks 0, 1, 2, and 4 – showed the greatest beneficial effect for hand and foot psoriasis, Dr. Carle Paul reported at the annual congress of the European Academy of Dermatology and Venereology.
Fifty-four percent of patients randomized to this optimal secukinumab dosing regimen achieved a positive investigator global response of their moderate to severe hand and foot psoriasis at week 12 compared with 19% assigned to placebo. An investigator global response required at least a 2-point improvement on a 5-point scale along with a score of 0 or 1, meaning clear or only minimal disease, explained Dr. Paul, a dermatologist at the University of Toulouse (France).
The PASI 75 and PASI 90 rates in patients with hand and foot psoriasis – that is, the percentage of patients with at least a 75% or 90% improvement over baseline scores on the Psoriasis Area and Severity Index – were similar to the 55% and 32% rates observed in the overall study population, he added.
The phase II study also included 304 patients with nail psoriasis. At week 12, those patients treated with secukinumab at weeks 0, 1, 2, and 4 showed a mean 19% improvement on a composite fingernail involvement score, compared with a 14% worsening with placebo.
These findings are especially encouraging because hand, foot, and nail psoriasis affect an estimated 10%-55% of all psoriasis patients. Involvement at these sites causes considerable functional disability, including difficulty in walking and using the hands. In addition, hand, foot, and nail psoriasis are notoriously difficult to treat.
Secukinumab’s side effect pattern in the large phase II study was similar to that for placebo. Definitive safety and efficacy results will come from ongoing pivotal phase III clinical trials totaling more than 3,000 psoriasis patients worldwide. The results are anticipated next year.
In addition to being used for psoriasis, secukinumab is being developed as a novel treatment for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ongoing phase III studies are expected to report results in 2014. Also, phase II studies of secukinumab for multiple sclerosis are underway.
Secukinumab is a fully human anti-interleukin-17A monoclonal antibody being developed by Novartis.
The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Fifty-four percent of patients with moderate to severe hand and foot psoriasis were rated clear or had only minimal disease at those sites at week 12 after being treated with secukinumab on weeks 0, 1, 2, and 4. Only 19% of placebo-treated subjects met that standard.
Data Source: This was a post hoc secondary analysis of 131 psoriasis patients with hand and foot involvement. They were part of a larger 404-patient, double-blind, randomized phase II study.
Disclosures: The phase II study and post hoc analysis were sponsored by Novartis. Dr. Paul is a recipient of research grants from and is a consultant to the company.
Evidence Backs Some Drugs for Hidradenitis Suppurativa
PRAGUE – A handful of drugs are backed by solid-quality evidence for the treatment of hidradenitis suppurativa, although to date no form of therapy is approved for this indication.
Topping the list are the tumor necrosis factor (TNF)-alpha inhibitors. They have a plausible mechanism of benefit in hidradenitis suppurativa (HS). They’ve also demonstrated effectiveness for this common inflammatory skin disease in randomized, placebo-controlled clinical trials, Dr. Gregor B. Jemec said at the annual congress of the European Academy of Dermatology and Venereology.
In addition, one older randomized trial suggests that topical clindamycin is effective, mainly in milder cases. The most promising antibiotic regimen, consisting of oral clindamycin at 300 mg twice daily plus rifampicin at 600 mg daily, has not been studied in a randomized trial. But several large, rigorous case series have demonstrated "dramatic improvement" in response to the combination, according to Dr. Jemec of the University of Copenhagen.
TNF Inhibitors
The discovery that anti-TNF therapy is effective for HS was a serendipitous finding that occurred when physicians noted marked improvement in the skin disease in a patient taking infliximab for Crohn’s disease. The biologic plausibility of the observed benefit is underscored by the fact that TNF levels are actually more elevated in patients with HS than in those with psoriasis, Dr. Jemec said.
Infliximab missed its primary efficacy end point of clear or almost clear in a 38-patient randomized trial, but it did hit several clinically important secondary end points, including pain reduction and improved quality of life (J. Am. Acad. Dermatol. 2010;62:205-17).
On the other hand, etanercept showed no benefit in a 20-patient pilot randomized trial (Arch. Dermatol. 2010;146:501-4).
By far the best studied TNF inhibitor in HS is adalimumab (Humira). It is the subject of two ongoing, pivotal phase III randomized, double-blind, placebo-controlled, multinational clinical trials aimed at winning adalimumab regulatory approval as the first-ever drug indicated for the treatment of HS.
Last year, Dr. Alexa B. Kimball of Harvard Medical School, Boston, presented the primary end point of a 16-week, randomized, double-blind phase II clinical trial involving 154 patients with HS who were placed on adalimumab 40 mg weekly, adalimumab 40 mg every other week, or placebo. At week 16, a Physician Global Assessment of clear, minimal, or mild disease with at least a 2-grade improvement over baseline was documented in 23.5% of patients on placebo, 21.2% of those on alternate-week adalimumab, and 49% of those on adalimumab 40 mg every week.
With a therapy that achieves clear or close to it in only half of treated patients, other end points take on added importance. At this year’s EADV (European Academy of Dermatology and Venereology) congress, Dr. Kimball and others presented several key secondary outcomes from the phase II study. One involved pain, a particularly prominent feature in HS. From a mean baseline pain score of 52 on a 0-100 visual analog scale, the weekly adalimumab group’s score dropped by a placebo-subtracted 15.8 points by week 2, 18.4 points at week 4, 11.7 points by week 8, 20.7 points at week 12, and by 11 points at week 20.
The minimum clinically important difference, predefined as at least a 15.4-point improvement in self-rated pain scores, occurred in 33% of the weekly adalimumab group, compared with 15% of patients on placebo at week 2. By week 12 this end point had been achieved in 63% of the weekly adalimumab group and 26% of the placebo group. Adalimumab every other week didn’t consistently improve pain relative to placebo, she reported.
Dr. Ulrich Mrowietz reported separately that the impaired work productivity that often affects patients with HS was significantly lessened by treatment with adalimumab 40 mg weekly. Total work productivity impairment as measured at week 16 by the Work Productivity and Activity Impairment Questionnaire improved by a mean of 15 points in the weekly adalimumab group while worsening by 2.7 points with placebo; the net 17.7-unit difference exceeded the minimum clinically important difference threshold of 16.2 points.
Patients assigned to weekly adalimumab also experienced significantly improved scores on the Dermatology Life Quality Index beginning on week 2 and lasting throughout the 16-week study. At week 16, for example, they enjoyed a mean 5.5-point improvement relative to baseline, compared with a 1.6-point improvement with placebo, according to Dr. Mrowietz of University Medical Center in Kiel, Germany.
Oral Antibiotics
Dr. Jemec highlighted as particularly impressive a retrospective French case series of 116 patients with severe HS treated using the combination of clindamycin 300 mg twice daily and rifampicin 600 mg daily. Severity scores decreased as a result by 50%. Seven percent of patients dropped the therapy due to diarrhea (Dermatology 2009;219:148-54).
Two smaller published case series have boosted to 164 the total number of HS patients treated with similarly "dramatic" results, noted Dr. Jemec. He was an investigator in one of these studies, in which 10 weeks on the antibiotic combination resulted in at least partial improvement in 28 of 34 patients and total remission in 16 (47%). Eight of the 16 relapsed after a mean of 5 months (Dermatology 2009;219:143-7).
Another approach has involved an intensive oral regimen of broad-spectrum antibiotics consisting of rifampin at 10 mg/kg once daily, moxifloxacin at 400 mg/day, and metronidazole at 500 mg three times daily. In a series of 28 consecutive patients with long-standing refractory HS, French investigators prescribed this regimen for 6-12 weeks before switching to consolidation therapy with rifampin and moxifloxacin for 6 more weeks. Complete remission was achieved in 16 of the 28 patients (Dermatology 2011;222:49-58).
"This therapy has yielded remarkably good results in terms of clearance and maintenance of clearance," commented Dr. Jemec.
The main side effects were diarrhea, a complaint in nearly two-thirds of patients, and vaginal candidiasis, which occurred in one-third of treated women.
Topical Clindamycin
Dr. Jemec was a coinvestigator in an early double-blind, 46-patient study that established topical clindamycin as being as effective as oral tetracycline in patients with mild disease (J. Am. Acad. Dermatol. 1998;39:971-4).
Beyond this point, the strength of evidence for proposed HS therapies tails off considerably. But Dr. Jemec deemed a couple of agents worthy of mention on the basis of intriguing yet less than persuasive supporting data:
• Acitretin. In a case series of 12 HS patients treated for 9-12 months at the relatively high dose of 0.6 mg/kg per day of acitretin, with stringent 4-year follow-up, all patients went into remission. In eight patients, the remission lasted for at least 1 year, including a single patient who has been in remission for 4 years and counting (Br. J. Dermatol. 2011;164:170-5). "Remarkable," Dr. Jemec commented.
• Dapsone. This drug is frequently reported anecdotally as being useful in HS, typically with an n-of-1 experience. Dr. Jemec and his coworkers recently published a case series of 24 treated patients, 9 of whom (38%) showed improvement (Dermatology 2011;222:342-6). None of the four severely affected patients improved, however. It was Dr. Jemec’s impression that the degree of improvement was less than he’s seen with combined oral clindamycin and rifampicin. He’d like to see a head-to-head comparison before reaching a final judgment as to a possible role for dapsone.
Dr. Jemec has received research grants from and serves as a consultant to Abbott Laboratories, which is developing adalimumab as a treatment for HS, as well as a dozen other pharmaceutical companies. Dr. Mrowietz is a consultant to Abbott, which funded his study, and to other dermatologic pharmaceutical companies.
PRAGUE – A handful of drugs are backed by solid-quality evidence for the treatment of hidradenitis suppurativa, although to date no form of therapy is approved for this indication.
Topping the list are the tumor necrosis factor (TNF)-alpha inhibitors. They have a plausible mechanism of benefit in hidradenitis suppurativa (HS). They’ve also demonstrated effectiveness for this common inflammatory skin disease in randomized, placebo-controlled clinical trials, Dr. Gregor B. Jemec said at the annual congress of the European Academy of Dermatology and Venereology.
In addition, one older randomized trial suggests that topical clindamycin is effective, mainly in milder cases. The most promising antibiotic regimen, consisting of oral clindamycin at 300 mg twice daily plus rifampicin at 600 mg daily, has not been studied in a randomized trial. But several large, rigorous case series have demonstrated "dramatic improvement" in response to the combination, according to Dr. Jemec of the University of Copenhagen.
TNF Inhibitors
The discovery that anti-TNF therapy is effective for HS was a serendipitous finding that occurred when physicians noted marked improvement in the skin disease in a patient taking infliximab for Crohn’s disease. The biologic plausibility of the observed benefit is underscored by the fact that TNF levels are actually more elevated in patients with HS than in those with psoriasis, Dr. Jemec said.
Infliximab missed its primary efficacy end point of clear or almost clear in a 38-patient randomized trial, but it did hit several clinically important secondary end points, including pain reduction and improved quality of life (J. Am. Acad. Dermatol. 2010;62:205-17).
On the other hand, etanercept showed no benefit in a 20-patient pilot randomized trial (Arch. Dermatol. 2010;146:501-4).
By far the best studied TNF inhibitor in HS is adalimumab (Humira). It is the subject of two ongoing, pivotal phase III randomized, double-blind, placebo-controlled, multinational clinical trials aimed at winning adalimumab regulatory approval as the first-ever drug indicated for the treatment of HS.
Last year, Dr. Alexa B. Kimball of Harvard Medical School, Boston, presented the primary end point of a 16-week, randomized, double-blind phase II clinical trial involving 154 patients with HS who were placed on adalimumab 40 mg weekly, adalimumab 40 mg every other week, or placebo. At week 16, a Physician Global Assessment of clear, minimal, or mild disease with at least a 2-grade improvement over baseline was documented in 23.5% of patients on placebo, 21.2% of those on alternate-week adalimumab, and 49% of those on adalimumab 40 mg every week.
With a therapy that achieves clear or close to it in only half of treated patients, other end points take on added importance. At this year’s EADV (European Academy of Dermatology and Venereology) congress, Dr. Kimball and others presented several key secondary outcomes from the phase II study. One involved pain, a particularly prominent feature in HS. From a mean baseline pain score of 52 on a 0-100 visual analog scale, the weekly adalimumab group’s score dropped by a placebo-subtracted 15.8 points by week 2, 18.4 points at week 4, 11.7 points by week 8, 20.7 points at week 12, and by 11 points at week 20.
The minimum clinically important difference, predefined as at least a 15.4-point improvement in self-rated pain scores, occurred in 33% of the weekly adalimumab group, compared with 15% of patients on placebo at week 2. By week 12 this end point had been achieved in 63% of the weekly adalimumab group and 26% of the placebo group. Adalimumab every other week didn’t consistently improve pain relative to placebo, she reported.
Dr. Ulrich Mrowietz reported separately that the impaired work productivity that often affects patients with HS was significantly lessened by treatment with adalimumab 40 mg weekly. Total work productivity impairment as measured at week 16 by the Work Productivity and Activity Impairment Questionnaire improved by a mean of 15 points in the weekly adalimumab group while worsening by 2.7 points with placebo; the net 17.7-unit difference exceeded the minimum clinically important difference threshold of 16.2 points.
Patients assigned to weekly adalimumab also experienced significantly improved scores on the Dermatology Life Quality Index beginning on week 2 and lasting throughout the 16-week study. At week 16, for example, they enjoyed a mean 5.5-point improvement relative to baseline, compared with a 1.6-point improvement with placebo, according to Dr. Mrowietz of University Medical Center in Kiel, Germany.
Oral Antibiotics
Dr. Jemec highlighted as particularly impressive a retrospective French case series of 116 patients with severe HS treated using the combination of clindamycin 300 mg twice daily and rifampicin 600 mg daily. Severity scores decreased as a result by 50%. Seven percent of patients dropped the therapy due to diarrhea (Dermatology 2009;219:148-54).
Two smaller published case series have boosted to 164 the total number of HS patients treated with similarly "dramatic" results, noted Dr. Jemec. He was an investigator in one of these studies, in which 10 weeks on the antibiotic combination resulted in at least partial improvement in 28 of 34 patients and total remission in 16 (47%). Eight of the 16 relapsed after a mean of 5 months (Dermatology 2009;219:143-7).
Another approach has involved an intensive oral regimen of broad-spectrum antibiotics consisting of rifampin at 10 mg/kg once daily, moxifloxacin at 400 mg/day, and metronidazole at 500 mg three times daily. In a series of 28 consecutive patients with long-standing refractory HS, French investigators prescribed this regimen for 6-12 weeks before switching to consolidation therapy with rifampin and moxifloxacin for 6 more weeks. Complete remission was achieved in 16 of the 28 patients (Dermatology 2011;222:49-58).
"This therapy has yielded remarkably good results in terms of clearance and maintenance of clearance," commented Dr. Jemec.
The main side effects were diarrhea, a complaint in nearly two-thirds of patients, and vaginal candidiasis, which occurred in one-third of treated women.
Topical Clindamycin
Dr. Jemec was a coinvestigator in an early double-blind, 46-patient study that established topical clindamycin as being as effective as oral tetracycline in patients with mild disease (J. Am. Acad. Dermatol. 1998;39:971-4).
Beyond this point, the strength of evidence for proposed HS therapies tails off considerably. But Dr. Jemec deemed a couple of agents worthy of mention on the basis of intriguing yet less than persuasive supporting data:
• Acitretin. In a case series of 12 HS patients treated for 9-12 months at the relatively high dose of 0.6 mg/kg per day of acitretin, with stringent 4-year follow-up, all patients went into remission. In eight patients, the remission lasted for at least 1 year, including a single patient who has been in remission for 4 years and counting (Br. J. Dermatol. 2011;164:170-5). "Remarkable," Dr. Jemec commented.
• Dapsone. This drug is frequently reported anecdotally as being useful in HS, typically with an n-of-1 experience. Dr. Jemec and his coworkers recently published a case series of 24 treated patients, 9 of whom (38%) showed improvement (Dermatology 2011;222:342-6). None of the four severely affected patients improved, however. It was Dr. Jemec’s impression that the degree of improvement was less than he’s seen with combined oral clindamycin and rifampicin. He’d like to see a head-to-head comparison before reaching a final judgment as to a possible role for dapsone.
Dr. Jemec has received research grants from and serves as a consultant to Abbott Laboratories, which is developing adalimumab as a treatment for HS, as well as a dozen other pharmaceutical companies. Dr. Mrowietz is a consultant to Abbott, which funded his study, and to other dermatologic pharmaceutical companies.
PRAGUE – A handful of drugs are backed by solid-quality evidence for the treatment of hidradenitis suppurativa, although to date no form of therapy is approved for this indication.
Topping the list are the tumor necrosis factor (TNF)-alpha inhibitors. They have a plausible mechanism of benefit in hidradenitis suppurativa (HS). They’ve also demonstrated effectiveness for this common inflammatory skin disease in randomized, placebo-controlled clinical trials, Dr. Gregor B. Jemec said at the annual congress of the European Academy of Dermatology and Venereology.
In addition, one older randomized trial suggests that topical clindamycin is effective, mainly in milder cases. The most promising antibiotic regimen, consisting of oral clindamycin at 300 mg twice daily plus rifampicin at 600 mg daily, has not been studied in a randomized trial. But several large, rigorous case series have demonstrated "dramatic improvement" in response to the combination, according to Dr. Jemec of the University of Copenhagen.
TNF Inhibitors
The discovery that anti-TNF therapy is effective for HS was a serendipitous finding that occurred when physicians noted marked improvement in the skin disease in a patient taking infliximab for Crohn’s disease. The biologic plausibility of the observed benefit is underscored by the fact that TNF levels are actually more elevated in patients with HS than in those with psoriasis, Dr. Jemec said.
Infliximab missed its primary efficacy end point of clear or almost clear in a 38-patient randomized trial, but it did hit several clinically important secondary end points, including pain reduction and improved quality of life (J. Am. Acad. Dermatol. 2010;62:205-17).
On the other hand, etanercept showed no benefit in a 20-patient pilot randomized trial (Arch. Dermatol. 2010;146:501-4).
By far the best studied TNF inhibitor in HS is adalimumab (Humira). It is the subject of two ongoing, pivotal phase III randomized, double-blind, placebo-controlled, multinational clinical trials aimed at winning adalimumab regulatory approval as the first-ever drug indicated for the treatment of HS.
Last year, Dr. Alexa B. Kimball of Harvard Medical School, Boston, presented the primary end point of a 16-week, randomized, double-blind phase II clinical trial involving 154 patients with HS who were placed on adalimumab 40 mg weekly, adalimumab 40 mg every other week, or placebo. At week 16, a Physician Global Assessment of clear, minimal, or mild disease with at least a 2-grade improvement over baseline was documented in 23.5% of patients on placebo, 21.2% of those on alternate-week adalimumab, and 49% of those on adalimumab 40 mg every week.
With a therapy that achieves clear or close to it in only half of treated patients, other end points take on added importance. At this year’s EADV (European Academy of Dermatology and Venereology) congress, Dr. Kimball and others presented several key secondary outcomes from the phase II study. One involved pain, a particularly prominent feature in HS. From a mean baseline pain score of 52 on a 0-100 visual analog scale, the weekly adalimumab group’s score dropped by a placebo-subtracted 15.8 points by week 2, 18.4 points at week 4, 11.7 points by week 8, 20.7 points at week 12, and by 11 points at week 20.
The minimum clinically important difference, predefined as at least a 15.4-point improvement in self-rated pain scores, occurred in 33% of the weekly adalimumab group, compared with 15% of patients on placebo at week 2. By week 12 this end point had been achieved in 63% of the weekly adalimumab group and 26% of the placebo group. Adalimumab every other week didn’t consistently improve pain relative to placebo, she reported.
Dr. Ulrich Mrowietz reported separately that the impaired work productivity that often affects patients with HS was significantly lessened by treatment with adalimumab 40 mg weekly. Total work productivity impairment as measured at week 16 by the Work Productivity and Activity Impairment Questionnaire improved by a mean of 15 points in the weekly adalimumab group while worsening by 2.7 points with placebo; the net 17.7-unit difference exceeded the minimum clinically important difference threshold of 16.2 points.
Patients assigned to weekly adalimumab also experienced significantly improved scores on the Dermatology Life Quality Index beginning on week 2 and lasting throughout the 16-week study. At week 16, for example, they enjoyed a mean 5.5-point improvement relative to baseline, compared with a 1.6-point improvement with placebo, according to Dr. Mrowietz of University Medical Center in Kiel, Germany.
Oral Antibiotics
Dr. Jemec highlighted as particularly impressive a retrospective French case series of 116 patients with severe HS treated using the combination of clindamycin 300 mg twice daily and rifampicin 600 mg daily. Severity scores decreased as a result by 50%. Seven percent of patients dropped the therapy due to diarrhea (Dermatology 2009;219:148-54).
Two smaller published case series have boosted to 164 the total number of HS patients treated with similarly "dramatic" results, noted Dr. Jemec. He was an investigator in one of these studies, in which 10 weeks on the antibiotic combination resulted in at least partial improvement in 28 of 34 patients and total remission in 16 (47%). Eight of the 16 relapsed after a mean of 5 months (Dermatology 2009;219:143-7).
Another approach has involved an intensive oral regimen of broad-spectrum antibiotics consisting of rifampin at 10 mg/kg once daily, moxifloxacin at 400 mg/day, and metronidazole at 500 mg three times daily. In a series of 28 consecutive patients with long-standing refractory HS, French investigators prescribed this regimen for 6-12 weeks before switching to consolidation therapy with rifampin and moxifloxacin for 6 more weeks. Complete remission was achieved in 16 of the 28 patients (Dermatology 2011;222:49-58).
"This therapy has yielded remarkably good results in terms of clearance and maintenance of clearance," commented Dr. Jemec.
The main side effects were diarrhea, a complaint in nearly two-thirds of patients, and vaginal candidiasis, which occurred in one-third of treated women.
Topical Clindamycin
Dr. Jemec was a coinvestigator in an early double-blind, 46-patient study that established topical clindamycin as being as effective as oral tetracycline in patients with mild disease (J. Am. Acad. Dermatol. 1998;39:971-4).
Beyond this point, the strength of evidence for proposed HS therapies tails off considerably. But Dr. Jemec deemed a couple of agents worthy of mention on the basis of intriguing yet less than persuasive supporting data:
• Acitretin. In a case series of 12 HS patients treated for 9-12 months at the relatively high dose of 0.6 mg/kg per day of acitretin, with stringent 4-year follow-up, all patients went into remission. In eight patients, the remission lasted for at least 1 year, including a single patient who has been in remission for 4 years and counting (Br. J. Dermatol. 2011;164:170-5). "Remarkable," Dr. Jemec commented.
• Dapsone. This drug is frequently reported anecdotally as being useful in HS, typically with an n-of-1 experience. Dr. Jemec and his coworkers recently published a case series of 24 treated patients, 9 of whom (38%) showed improvement (Dermatology 2011;222:342-6). None of the four severely affected patients improved, however. It was Dr. Jemec’s impression that the degree of improvement was less than he’s seen with combined oral clindamycin and rifampicin. He’d like to see a head-to-head comparison before reaching a final judgment as to a possible role for dapsone.
Dr. Jemec has received research grants from and serves as a consultant to Abbott Laboratories, which is developing adalimumab as a treatment for HS, as well as a dozen other pharmaceutical companies. Dr. Mrowietz is a consultant to Abbott, which funded his study, and to other dermatologic pharmaceutical companies.
EXPERT OPINION FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY