User login
Guselkumab achieves highest-ever response rates in psoriasis
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
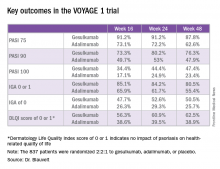
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
Key clinical point:
Major finding: The PASI 90 response rate at 24 weeks was 80% in psoriasis patients on guselkumab compared with 53% in those on adalimumab.
Data source: A randomized, multinational, 48-week, pivotal phase III clinical trial involving 837 psoriasis patients assigned to guselkumab, adalimumab, or placebo.
Disclosures: The VOYAGE 1 trial was funded by Janssen, which is developing guselkumab. The study presenter reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
Birth outcomes unaffected by paternal immunosuppressive therapy
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Adjusted rates of congenital anomalies, preterm birth, and low birthweight are not increased in children with paternal use of azathioprine, methotrexate, or cyclosporine prior to conception.
Data source: This retrospective study utilized linked Danish national registries to compare rates of low birthweight, congenital anomalies, and preterm birth in all Danish children born in 2004-2010 depending upon whether or not the father had been on methotrexate, azathioprine, or cyclosporine prior to the pregnancy.
Disclosures: The study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from, and serving as a consultant to, Pfizer and Eli Lilly.
Atopic dermatitis: Pivotal dupilumab results create sensation
VIENNA – The marquee event at this year’s annual congress of the European Academy of Dermatology and Venereology – the one everyone was eagerly awaiting – was the first presentation of two large, international, pivotal phase III randomized trials of dupilumab for treatment of inadequately controlled moderate to severe atopic dermatitis in adults.
Attendees at EADV 2016 understood that, if positive, these studies, known as SOLO 1 and SOLO 2, would be transformative. They would herald a new era of highly effective targeted biologic therapy for this common and often debilitating chronic relapsing skin disease, akin to what occurred in psoriasis therapy well over a decade ago.
The results did not disappoint.
“Dual targeting of interleukin-4 and -13 represents a therapeutic option for patients with moderate to severe atopic dermatitis,” added Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland.
These results have implications extending beyond atopic dermatitis. Asthma, chronic sinusitis with nasal polyposis, and eosinophilic esophagitis are other conditions where the type 2 inflammatory cytokines IL-4 and -13 are believed to be important drivers of disease activity. Clinical trials of dupilumab in those diseases are underway.
Dupilumab, a fully human monoclonal antibody that binds specifically to the shared alpha chain subunit of the IL-4 and -13 receptors, hit all of its primary and secondary outcome measures in SOLO 1 and SOLO 2. Moreover, some of these “secondary” endpoints are consistently reported in patient surveys to be among what they consider to be the most troublesome aspects of atopic dermatitis, including intense itching, disrupted sleep, clinically significant anxiety and/or depression, and generally diminished quality of life.
SOLO 1 and SOLO 2 were identically designed, independent, randomized, double-blind, placebo-controlled clinical trials of 16 weeks’ duration. Conducted in North America, Europe, and Asia, they included a total of 1,379 patients, split roughly 50/50 between those with moderate or severe atopic dermatitis. Their average disease duration was 26 years. Participants were randomized to subcutaneous injection of dupilumab at 300 mg once weekly or every 2 weeks or to matching placebo.
The primary endpoint was a score of clear or almost clear – 0 or 1 – on the Investigator’s Global Assessment (IGA) at week 16 accompanied by a reduction of at least 2 points from baseline. A key secondary endpoint was at least a 75% improvement in the Eczema Area and Severity Index (EASI-75), considered a coprimary endpoint by regulators in Japan and the European Union.
The use of topical agents for atopic dermatitis was not permitted except as rescue therapy for uncontrolled symptoms. An IGA of 0 or 1 with at least a 2-point drop from baseline was a high bar to reach, given that a median of 50% of participants’ body surface area was affected. But in SOLO 1, that target was achieved in 37.9% of subjects on dupilumab every other week, 37.2% with weekly therapy, and just 10.3% of placebo-treated controls. Similarly, in SOLO 2, the rates were 36.1%, 36.4%, and 8.5%, respectively.
Of note, there were essentially no differences in outcomes across the board with weekly versus biweekly dosing of dupilumab.
From a median baseline EASI score of 30, an EASI-75 was achieved at 16 weeks in 51.3% of patients on dupilumab every other week, 52.5% on weekly injections, and 14.7% of controls in SOLO 1. In SOLO 2, the corresponding EASI-75 rates were 44.2%, 48.1%, and 11.9%, respectively.
Itch is described by most patients with moderate to severe atopic dermatitis as their No. 1 issue. From a baseline median peak score of 7.7 on a 0-10 numerical rating scale for pruritus, week 16 scores dropped by a median of 51% in patients on dupilumab every 2 weeks, 48.9% with weekly therapy, and 26.1% with placebo in SOLO 1. Results in SOLO 2 mirrored those in SOLO 1.
Particularly noteworthy was the finding that a significant reduction in itch severity was documented by week 2 in both dupilumab treatment arms, Dr. Simpson observed.
Just under half of study participants had a baseline score of 8 or more on the Hospital Anxiety and Depression Scale Anxiety subscale or HADS Depression subscale, considered the cutoff for a clinically significant mood disorder. Among affected patients, a score of less than 8 was achieved at 16 weeks without the use of psychotropic medications in 12.4% of SOLO 1 participants on placebo, 41% on biweekly dupilumab, and 36.3% with weekly dupilumab. In SOLO 2, the rates were 6.1% with placebo, 39.5% with biweekly dupilumab, and 41.2% with once-weekly dupilumab.
The median baseline Dermatology Life Quality Index score was 15 across the two parallel trials. The collective proportion of patients who experienced at least a 4-point improvement, which is considered a clinically meaningful response, was 29.1% in controls, compared with 68.6% in patients dupilumab every other week and 60.2% with weekly dupilumab.
On the Patient-Oriented Eczema Measure, a composite yardstick that emphasizes sleep symptoms, the median baseline score was 22 out of a possible 28. An improvement of 4 points or more, defined as a minimal clinically important difference, was achieved in a collective 25.6% of controls, 69.6% of patients on biweekly dupilumab, and 63.6% on weekly dupilumab.
Regarding safety, no increase in infections was seen with dupilumab. In fact, only two adverse events were more frequent than with placebo. One was injection-site reactions, which were two- to threefold more common than in controls, and all of which were mild to moderate. The other safety issue was conjunctivitis, which occurred in three patients in the control arms of SOLO 1 and 2, compared with 36 in the dupilumab arms.
Asked about the mechanism of this conjunctivitis, Dr. Simpson said it remains unknown. There was no signal of an issue in the phase II studies.
“Ongoing studies are attempting to further characterize the affected patients. I would say the comforting thing is that most cases have been mild to moderate and have responded to topical steroids or topical cyclosporine. Only one patient had to discontinue dupilumab,” according to the dermatologist.
In any event, 16 weeks of treatment is not sufficient to determine the safety of long-term therapy. Long-term extension studies of SOLO 1 and 2 are well underway, as are earlier stage clinical trials in pediatric patients with moderate to severe atopic dermatitis.
In response to another audience question, Dr. Simpson said he and his coinvestigators plan to drill down into the data to see if patients with severe atopic dermatitis obtained significantly more benefits from weekly as compared with biweekly therapy, or if treatment every 2 weeks was as good as weekly therapy across the board. It’s an important question, but the study finished so recently that the investigators haven’t yet had time to conduct the analysis.
The pivotal phase III dupilumab findings met with an enthusiastic reception.
“Biologic therapy for atopic dermatitis is the light at the end of the tunnel,” declared session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
“Seminal work,” commented David M. Pariser, MD, professor of dermatology at Eastern Virginia Medical School in Norfolk.
Dr. Simpson’s presentation of the pivotal dupilumab studies was but one of the highlights of a horn-of-plenty late-breaking clinical trials session held on the final full day of EADV 2016. As attendees mingled in the hall afterward, a palpable sense of pride in their profession was evident. It was borne of the knowledge that their field not only includes basic and translational scientists capable of unraveling the inflammatory pathways involved in a challenging disease like atopic dermatitis, where there is a long-standing unmet need for new therapies, but also that their specialty includes experienced clinical trialists who can put those novel targeted therapies to the test.
There was also a sense of satisfaction that, although dermatology is a small specialty, these accomplishments are drawing favorable attention throughout the broader medical community. Pivotal trials of novel treatments for important dermatologic diseases are regularly getting published in prominent nondermatology journals. For instance, simultaneous with Dr. Simpson’s presentation in Vienna at EADV 2016, the SOLO 1 and 2 results were published online in the New England Journal of Medicine (doi. 10.1056/NEJMoa1610020).
“The online publication occurred a few minutes ago, at the start of my presentation. I didn’t say anything then because I didn’t want everybody looking at their cell phones,” he quipped.
The Food and Drug Administration has granted dupilumab a breakthrough therapy designation; a decision on the application for approval is expected by March 29, 2017.
The phase III dupilumab trials were funded by Sanofi and Regeneron Pharmaceuticals. Dr. Simpson reported having received research grants from and serving as a consultant to Regeneron and more than a dozen other pharmaceutical companies.
VIENNA – The marquee event at this year’s annual congress of the European Academy of Dermatology and Venereology – the one everyone was eagerly awaiting – was the first presentation of two large, international, pivotal phase III randomized trials of dupilumab for treatment of inadequately controlled moderate to severe atopic dermatitis in adults.
Attendees at EADV 2016 understood that, if positive, these studies, known as SOLO 1 and SOLO 2, would be transformative. They would herald a new era of highly effective targeted biologic therapy for this common and often debilitating chronic relapsing skin disease, akin to what occurred in psoriasis therapy well over a decade ago.
The results did not disappoint.
“Dual targeting of interleukin-4 and -13 represents a therapeutic option for patients with moderate to severe atopic dermatitis,” added Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland.
These results have implications extending beyond atopic dermatitis. Asthma, chronic sinusitis with nasal polyposis, and eosinophilic esophagitis are other conditions where the type 2 inflammatory cytokines IL-4 and -13 are believed to be important drivers of disease activity. Clinical trials of dupilumab in those diseases are underway.
Dupilumab, a fully human monoclonal antibody that binds specifically to the shared alpha chain subunit of the IL-4 and -13 receptors, hit all of its primary and secondary outcome measures in SOLO 1 and SOLO 2. Moreover, some of these “secondary” endpoints are consistently reported in patient surveys to be among what they consider to be the most troublesome aspects of atopic dermatitis, including intense itching, disrupted sleep, clinically significant anxiety and/or depression, and generally diminished quality of life.
SOLO 1 and SOLO 2 were identically designed, independent, randomized, double-blind, placebo-controlled clinical trials of 16 weeks’ duration. Conducted in North America, Europe, and Asia, they included a total of 1,379 patients, split roughly 50/50 between those with moderate or severe atopic dermatitis. Their average disease duration was 26 years. Participants were randomized to subcutaneous injection of dupilumab at 300 mg once weekly or every 2 weeks or to matching placebo.
The primary endpoint was a score of clear or almost clear – 0 or 1 – on the Investigator’s Global Assessment (IGA) at week 16 accompanied by a reduction of at least 2 points from baseline. A key secondary endpoint was at least a 75% improvement in the Eczema Area and Severity Index (EASI-75), considered a coprimary endpoint by regulators in Japan and the European Union.
The use of topical agents for atopic dermatitis was not permitted except as rescue therapy for uncontrolled symptoms. An IGA of 0 or 1 with at least a 2-point drop from baseline was a high bar to reach, given that a median of 50% of participants’ body surface area was affected. But in SOLO 1, that target was achieved in 37.9% of subjects on dupilumab every other week, 37.2% with weekly therapy, and just 10.3% of placebo-treated controls. Similarly, in SOLO 2, the rates were 36.1%, 36.4%, and 8.5%, respectively.
Of note, there were essentially no differences in outcomes across the board with weekly versus biweekly dosing of dupilumab.
From a median baseline EASI score of 30, an EASI-75 was achieved at 16 weeks in 51.3% of patients on dupilumab every other week, 52.5% on weekly injections, and 14.7% of controls in SOLO 1. In SOLO 2, the corresponding EASI-75 rates were 44.2%, 48.1%, and 11.9%, respectively.
Itch is described by most patients with moderate to severe atopic dermatitis as their No. 1 issue. From a baseline median peak score of 7.7 on a 0-10 numerical rating scale for pruritus, week 16 scores dropped by a median of 51% in patients on dupilumab every 2 weeks, 48.9% with weekly therapy, and 26.1% with placebo in SOLO 1. Results in SOLO 2 mirrored those in SOLO 1.
Particularly noteworthy was the finding that a significant reduction in itch severity was documented by week 2 in both dupilumab treatment arms, Dr. Simpson observed.
Just under half of study participants had a baseline score of 8 or more on the Hospital Anxiety and Depression Scale Anxiety subscale or HADS Depression subscale, considered the cutoff for a clinically significant mood disorder. Among affected patients, a score of less than 8 was achieved at 16 weeks without the use of psychotropic medications in 12.4% of SOLO 1 participants on placebo, 41% on biweekly dupilumab, and 36.3% with weekly dupilumab. In SOLO 2, the rates were 6.1% with placebo, 39.5% with biweekly dupilumab, and 41.2% with once-weekly dupilumab.
The median baseline Dermatology Life Quality Index score was 15 across the two parallel trials. The collective proportion of patients who experienced at least a 4-point improvement, which is considered a clinically meaningful response, was 29.1% in controls, compared with 68.6% in patients dupilumab every other week and 60.2% with weekly dupilumab.
On the Patient-Oriented Eczema Measure, a composite yardstick that emphasizes sleep symptoms, the median baseline score was 22 out of a possible 28. An improvement of 4 points or more, defined as a minimal clinically important difference, was achieved in a collective 25.6% of controls, 69.6% of patients on biweekly dupilumab, and 63.6% on weekly dupilumab.
Regarding safety, no increase in infections was seen with dupilumab. In fact, only two adverse events were more frequent than with placebo. One was injection-site reactions, which were two- to threefold more common than in controls, and all of which were mild to moderate. The other safety issue was conjunctivitis, which occurred in three patients in the control arms of SOLO 1 and 2, compared with 36 in the dupilumab arms.
Asked about the mechanism of this conjunctivitis, Dr. Simpson said it remains unknown. There was no signal of an issue in the phase II studies.
“Ongoing studies are attempting to further characterize the affected patients. I would say the comforting thing is that most cases have been mild to moderate and have responded to topical steroids or topical cyclosporine. Only one patient had to discontinue dupilumab,” according to the dermatologist.
In any event, 16 weeks of treatment is not sufficient to determine the safety of long-term therapy. Long-term extension studies of SOLO 1 and 2 are well underway, as are earlier stage clinical trials in pediatric patients with moderate to severe atopic dermatitis.
In response to another audience question, Dr. Simpson said he and his coinvestigators plan to drill down into the data to see if patients with severe atopic dermatitis obtained significantly more benefits from weekly as compared with biweekly therapy, or if treatment every 2 weeks was as good as weekly therapy across the board. It’s an important question, but the study finished so recently that the investigators haven’t yet had time to conduct the analysis.
The pivotal phase III dupilumab findings met with an enthusiastic reception.
“Biologic therapy for atopic dermatitis is the light at the end of the tunnel,” declared session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
“Seminal work,” commented David M. Pariser, MD, professor of dermatology at Eastern Virginia Medical School in Norfolk.
Dr. Simpson’s presentation of the pivotal dupilumab studies was but one of the highlights of a horn-of-plenty late-breaking clinical trials session held on the final full day of EADV 2016. As attendees mingled in the hall afterward, a palpable sense of pride in their profession was evident. It was borne of the knowledge that their field not only includes basic and translational scientists capable of unraveling the inflammatory pathways involved in a challenging disease like atopic dermatitis, where there is a long-standing unmet need for new therapies, but also that their specialty includes experienced clinical trialists who can put those novel targeted therapies to the test.
There was also a sense of satisfaction that, although dermatology is a small specialty, these accomplishments are drawing favorable attention throughout the broader medical community. Pivotal trials of novel treatments for important dermatologic diseases are regularly getting published in prominent nondermatology journals. For instance, simultaneous with Dr. Simpson’s presentation in Vienna at EADV 2016, the SOLO 1 and 2 results were published online in the New England Journal of Medicine (doi. 10.1056/NEJMoa1610020).
“The online publication occurred a few minutes ago, at the start of my presentation. I didn’t say anything then because I didn’t want everybody looking at their cell phones,” he quipped.
The Food and Drug Administration has granted dupilumab a breakthrough therapy designation; a decision on the application for approval is expected by March 29, 2017.
The phase III dupilumab trials were funded by Sanofi and Regeneron Pharmaceuticals. Dr. Simpson reported having received research grants from and serving as a consultant to Regeneron and more than a dozen other pharmaceutical companies.
VIENNA – The marquee event at this year’s annual congress of the European Academy of Dermatology and Venereology – the one everyone was eagerly awaiting – was the first presentation of two large, international, pivotal phase III randomized trials of dupilumab for treatment of inadequately controlled moderate to severe atopic dermatitis in adults.
Attendees at EADV 2016 understood that, if positive, these studies, known as SOLO 1 and SOLO 2, would be transformative. They would herald a new era of highly effective targeted biologic therapy for this common and often debilitating chronic relapsing skin disease, akin to what occurred in psoriasis therapy well over a decade ago.
The results did not disappoint.
“Dual targeting of interleukin-4 and -13 represents a therapeutic option for patients with moderate to severe atopic dermatitis,” added Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland.
These results have implications extending beyond atopic dermatitis. Asthma, chronic sinusitis with nasal polyposis, and eosinophilic esophagitis are other conditions where the type 2 inflammatory cytokines IL-4 and -13 are believed to be important drivers of disease activity. Clinical trials of dupilumab in those diseases are underway.
Dupilumab, a fully human monoclonal antibody that binds specifically to the shared alpha chain subunit of the IL-4 and -13 receptors, hit all of its primary and secondary outcome measures in SOLO 1 and SOLO 2. Moreover, some of these “secondary” endpoints are consistently reported in patient surveys to be among what they consider to be the most troublesome aspects of atopic dermatitis, including intense itching, disrupted sleep, clinically significant anxiety and/or depression, and generally diminished quality of life.
SOLO 1 and SOLO 2 were identically designed, independent, randomized, double-blind, placebo-controlled clinical trials of 16 weeks’ duration. Conducted in North America, Europe, and Asia, they included a total of 1,379 patients, split roughly 50/50 between those with moderate or severe atopic dermatitis. Their average disease duration was 26 years. Participants were randomized to subcutaneous injection of dupilumab at 300 mg once weekly or every 2 weeks or to matching placebo.
The primary endpoint was a score of clear or almost clear – 0 or 1 – on the Investigator’s Global Assessment (IGA) at week 16 accompanied by a reduction of at least 2 points from baseline. A key secondary endpoint was at least a 75% improvement in the Eczema Area and Severity Index (EASI-75), considered a coprimary endpoint by regulators in Japan and the European Union.
The use of topical agents for atopic dermatitis was not permitted except as rescue therapy for uncontrolled symptoms. An IGA of 0 or 1 with at least a 2-point drop from baseline was a high bar to reach, given that a median of 50% of participants’ body surface area was affected. But in SOLO 1, that target was achieved in 37.9% of subjects on dupilumab every other week, 37.2% with weekly therapy, and just 10.3% of placebo-treated controls. Similarly, in SOLO 2, the rates were 36.1%, 36.4%, and 8.5%, respectively.
Of note, there were essentially no differences in outcomes across the board with weekly versus biweekly dosing of dupilumab.
From a median baseline EASI score of 30, an EASI-75 was achieved at 16 weeks in 51.3% of patients on dupilumab every other week, 52.5% on weekly injections, and 14.7% of controls in SOLO 1. In SOLO 2, the corresponding EASI-75 rates were 44.2%, 48.1%, and 11.9%, respectively.
Itch is described by most patients with moderate to severe atopic dermatitis as their No. 1 issue. From a baseline median peak score of 7.7 on a 0-10 numerical rating scale for pruritus, week 16 scores dropped by a median of 51% in patients on dupilumab every 2 weeks, 48.9% with weekly therapy, and 26.1% with placebo in SOLO 1. Results in SOLO 2 mirrored those in SOLO 1.
Particularly noteworthy was the finding that a significant reduction in itch severity was documented by week 2 in both dupilumab treatment arms, Dr. Simpson observed.
Just under half of study participants had a baseline score of 8 or more on the Hospital Anxiety and Depression Scale Anxiety subscale or HADS Depression subscale, considered the cutoff for a clinically significant mood disorder. Among affected patients, a score of less than 8 was achieved at 16 weeks without the use of psychotropic medications in 12.4% of SOLO 1 participants on placebo, 41% on biweekly dupilumab, and 36.3% with weekly dupilumab. In SOLO 2, the rates were 6.1% with placebo, 39.5% with biweekly dupilumab, and 41.2% with once-weekly dupilumab.
The median baseline Dermatology Life Quality Index score was 15 across the two parallel trials. The collective proportion of patients who experienced at least a 4-point improvement, which is considered a clinically meaningful response, was 29.1% in controls, compared with 68.6% in patients dupilumab every other week and 60.2% with weekly dupilumab.
On the Patient-Oriented Eczema Measure, a composite yardstick that emphasizes sleep symptoms, the median baseline score was 22 out of a possible 28. An improvement of 4 points or more, defined as a minimal clinically important difference, was achieved in a collective 25.6% of controls, 69.6% of patients on biweekly dupilumab, and 63.6% on weekly dupilumab.
Regarding safety, no increase in infections was seen with dupilumab. In fact, only two adverse events were more frequent than with placebo. One was injection-site reactions, which were two- to threefold more common than in controls, and all of which were mild to moderate. The other safety issue was conjunctivitis, which occurred in three patients in the control arms of SOLO 1 and 2, compared with 36 in the dupilumab arms.
Asked about the mechanism of this conjunctivitis, Dr. Simpson said it remains unknown. There was no signal of an issue in the phase II studies.
“Ongoing studies are attempting to further characterize the affected patients. I would say the comforting thing is that most cases have been mild to moderate and have responded to topical steroids or topical cyclosporine. Only one patient had to discontinue dupilumab,” according to the dermatologist.
In any event, 16 weeks of treatment is not sufficient to determine the safety of long-term therapy. Long-term extension studies of SOLO 1 and 2 are well underway, as are earlier stage clinical trials in pediatric patients with moderate to severe atopic dermatitis.
In response to another audience question, Dr. Simpson said he and his coinvestigators plan to drill down into the data to see if patients with severe atopic dermatitis obtained significantly more benefits from weekly as compared with biweekly therapy, or if treatment every 2 weeks was as good as weekly therapy across the board. It’s an important question, but the study finished so recently that the investigators haven’t yet had time to conduct the analysis.
The pivotal phase III dupilumab findings met with an enthusiastic reception.
“Biologic therapy for atopic dermatitis is the light at the end of the tunnel,” declared session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
“Seminal work,” commented David M. Pariser, MD, professor of dermatology at Eastern Virginia Medical School in Norfolk.
Dr. Simpson’s presentation of the pivotal dupilumab studies was but one of the highlights of a horn-of-plenty late-breaking clinical trials session held on the final full day of EADV 2016. As attendees mingled in the hall afterward, a palpable sense of pride in their profession was evident. It was borne of the knowledge that their field not only includes basic and translational scientists capable of unraveling the inflammatory pathways involved in a challenging disease like atopic dermatitis, where there is a long-standing unmet need for new therapies, but also that their specialty includes experienced clinical trialists who can put those novel targeted therapies to the test.
There was also a sense of satisfaction that, although dermatology is a small specialty, these accomplishments are drawing favorable attention throughout the broader medical community. Pivotal trials of novel treatments for important dermatologic diseases are regularly getting published in prominent nondermatology journals. For instance, simultaneous with Dr. Simpson’s presentation in Vienna at EADV 2016, the SOLO 1 and 2 results were published online in the New England Journal of Medicine (doi. 10.1056/NEJMoa1610020).
“The online publication occurred a few minutes ago, at the start of my presentation. I didn’t say anything then because I didn’t want everybody looking at their cell phones,” he quipped.
The Food and Drug Administration has granted dupilumab a breakthrough therapy designation; a decision on the application for approval is expected by March 29, 2017.
The phase III dupilumab trials were funded by Sanofi and Regeneron Pharmaceuticals. Dr. Simpson reported having received research grants from and serving as a consultant to Regeneron and more than a dozen other pharmaceutical companies.
Key clinical point:
Major finding: After 16 weeks of weekly or biweekly subcutaneous injections of dupilumab, 36%-38% of patients with baseline moderate or severe atopic dermatitis were clear or almost clear, compared with 8%-10% of placebo-treated controls.
Data source: The SOLO 1 and SOLO 2 pivotal phase III randomized, double-blind, placebo-controlled clinical trials included a total of 1,379 adults with inadequately controlled moderate or severe atopic dermatitis on three continents.
Disclosures: The trials were funded by Sanofi and Regeneron Pharmaceuticals. The presenter reported having received research grants from and serving as a consultant to Regeneron and more than a dozen other pharmaceutical companies.