User login
Why STEMI patients benefit from PCI of nonculprit lesions
PHILADELPHIA – Nearly half of patients with ST-elevation MI and multivessel coronary artery disease in the landmark COMPLETE trial had an obstructive coronary lesion with vulnerable plaque morphology in a segment far from the culprit lesion, Natalia Pinilla-Echeverri, MD, reported at the American Heart Association scientific sessions.
This novel finding from an optical coherence tomography (OCT) substudy of COMPLETE provides a likely mechanistic explanation for the major clinical benefits documented in the full COMPLETE trial, noted Dr. Pinilla-Echeverri, a cardiologist at the Population Health Research Institute at McMaster University, Hamilton, Ont.
COMPLETE was a multinational trial which randomized 4,041 ST-elevation MI (STEMI) patients with multivessel disease to culprit lesion–only percutaneous coronary intervention (PCI) or additional routine angiography–guided staged PCI of nonculprit obstructive lesions with at least 70% stenosis. As previously reported, the risk of the coprimary composite endpoint comprising cardiovascular death, new MI, or ischemia-driven revascularization was reduced by 49% over 3 years of follow-up in the group with staged PCI of nonculprit lesions, with an impressive number needed to treat of just 13 (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
Dr. Pinella-Echeverri reported on the 93 patients who participated in the OCT substudy, the purpose of which was to determine the prevalence of high-risk, vulnerable plaque in obstructive and nonobstructive nonculprit lesions. For this purpose, vulnerable plaque was defined as thin-cap fibroatheroma (TCFA), a coronary lesion known to pose high risk of worsening stenosis, plaque rupture, and cardiovascular events.
Of note, these 93 patients had a total of 425 diseased segments: 150 obstructive and 275 nonobstructive.
“This is reassuring that the concept of acute coronary syndrome implies a diffuse pathophysiology of affecting not only the culprit segment but the coronary vasculature as a whole,” Dr. Pinella-Echeverri observed.
The main study finding, however, was that TCFA was significantly more prevalent in obstructive, compared with nonobstructive, nonculprit lesions by a margin of 35% to 23%. The obstructive and nonobstructive TCFA lesions had a similar lipid-rich composition; however, the obstructive ones were significantly longer and had a smaller mean lumen area.
When breaking down the prevalence of TCFA per patient, 47% of patients had a nonculprit obstructive lesion with vulnerable plaque morphology. Another 20% had nonobstructive TCFA lesions. And only 32% of the STEMI patients had no TCFA in their obstructive or nonobstructive segments.
Discussant Frans Van de Werf, MD, PhD, commented: “This [OCT substudy result] immediately explains the clinical benefit observed with preventive PCI in STEMI patients with obstructive multivessel disease.”
The finding that 20% of the STEMI patients had nonobstructive lesions with vulnerable plaque morphology by OCT provides powerful support for the current guideline-recommended strategy of immediately starting STEMI patients on intensive lipid-lowering therapy, added Dr. Van de Werf, professor of medicine at the Catholic University of Leuven (Belgium).
He argued that the decision to revascularize nonculprit lesions by means of PCI versus the more complete revascularization achieved via coronary artery bypass graft surgery shouldn’t be made during the initial primary PCI, citing evidence that when the decision gets made at that time, coronary artery bypass grafting (CABG) is less likely to be chosen.
“I believe that OCT and [fractional flow reserve] should not be performed during the index primary PCI, not only for the comfort of the patient, but also for the better selection of complete revascularization. Interventional cardiologists should not forget that CABG might be a better revascularization treatment in some cases, such as left main disease and diabetes mellitus,” the cardiologist cautioned.
The COMPLETE OCT Substudy was supported by Abbott Vascular, the Population Health Research Institute, Hamilton Health Sciences, and the Canadian Institutes of Health Research.
PHILADELPHIA – Nearly half of patients with ST-elevation MI and multivessel coronary artery disease in the landmark COMPLETE trial had an obstructive coronary lesion with vulnerable plaque morphology in a segment far from the culprit lesion, Natalia Pinilla-Echeverri, MD, reported at the American Heart Association scientific sessions.
This novel finding from an optical coherence tomography (OCT) substudy of COMPLETE provides a likely mechanistic explanation for the major clinical benefits documented in the full COMPLETE trial, noted Dr. Pinilla-Echeverri, a cardiologist at the Population Health Research Institute at McMaster University, Hamilton, Ont.
COMPLETE was a multinational trial which randomized 4,041 ST-elevation MI (STEMI) patients with multivessel disease to culprit lesion–only percutaneous coronary intervention (PCI) or additional routine angiography–guided staged PCI of nonculprit obstructive lesions with at least 70% stenosis. As previously reported, the risk of the coprimary composite endpoint comprising cardiovascular death, new MI, or ischemia-driven revascularization was reduced by 49% over 3 years of follow-up in the group with staged PCI of nonculprit lesions, with an impressive number needed to treat of just 13 (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
Dr. Pinella-Echeverri reported on the 93 patients who participated in the OCT substudy, the purpose of which was to determine the prevalence of high-risk, vulnerable plaque in obstructive and nonobstructive nonculprit lesions. For this purpose, vulnerable plaque was defined as thin-cap fibroatheroma (TCFA), a coronary lesion known to pose high risk of worsening stenosis, plaque rupture, and cardiovascular events.
Of note, these 93 patients had a total of 425 diseased segments: 150 obstructive and 275 nonobstructive.
“This is reassuring that the concept of acute coronary syndrome implies a diffuse pathophysiology of affecting not only the culprit segment but the coronary vasculature as a whole,” Dr. Pinella-Echeverri observed.
The main study finding, however, was that TCFA was significantly more prevalent in obstructive, compared with nonobstructive, nonculprit lesions by a margin of 35% to 23%. The obstructive and nonobstructive TCFA lesions had a similar lipid-rich composition; however, the obstructive ones were significantly longer and had a smaller mean lumen area.
When breaking down the prevalence of TCFA per patient, 47% of patients had a nonculprit obstructive lesion with vulnerable plaque morphology. Another 20% had nonobstructive TCFA lesions. And only 32% of the STEMI patients had no TCFA in their obstructive or nonobstructive segments.
Discussant Frans Van de Werf, MD, PhD, commented: “This [OCT substudy result] immediately explains the clinical benefit observed with preventive PCI in STEMI patients with obstructive multivessel disease.”
The finding that 20% of the STEMI patients had nonobstructive lesions with vulnerable plaque morphology by OCT provides powerful support for the current guideline-recommended strategy of immediately starting STEMI patients on intensive lipid-lowering therapy, added Dr. Van de Werf, professor of medicine at the Catholic University of Leuven (Belgium).
He argued that the decision to revascularize nonculprit lesions by means of PCI versus the more complete revascularization achieved via coronary artery bypass graft surgery shouldn’t be made during the initial primary PCI, citing evidence that when the decision gets made at that time, coronary artery bypass grafting (CABG) is less likely to be chosen.
“I believe that OCT and [fractional flow reserve] should not be performed during the index primary PCI, not only for the comfort of the patient, but also for the better selection of complete revascularization. Interventional cardiologists should not forget that CABG might be a better revascularization treatment in some cases, such as left main disease and diabetes mellitus,” the cardiologist cautioned.
The COMPLETE OCT Substudy was supported by Abbott Vascular, the Population Health Research Institute, Hamilton Health Sciences, and the Canadian Institutes of Health Research.
PHILADELPHIA – Nearly half of patients with ST-elevation MI and multivessel coronary artery disease in the landmark COMPLETE trial had an obstructive coronary lesion with vulnerable plaque morphology in a segment far from the culprit lesion, Natalia Pinilla-Echeverri, MD, reported at the American Heart Association scientific sessions.
This novel finding from an optical coherence tomography (OCT) substudy of COMPLETE provides a likely mechanistic explanation for the major clinical benefits documented in the full COMPLETE trial, noted Dr. Pinilla-Echeverri, a cardiologist at the Population Health Research Institute at McMaster University, Hamilton, Ont.
COMPLETE was a multinational trial which randomized 4,041 ST-elevation MI (STEMI) patients with multivessel disease to culprit lesion–only percutaneous coronary intervention (PCI) or additional routine angiography–guided staged PCI of nonculprit obstructive lesions with at least 70% stenosis. As previously reported, the risk of the coprimary composite endpoint comprising cardiovascular death, new MI, or ischemia-driven revascularization was reduced by 49% over 3 years of follow-up in the group with staged PCI of nonculprit lesions, with an impressive number needed to treat of just 13 (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
Dr. Pinella-Echeverri reported on the 93 patients who participated in the OCT substudy, the purpose of which was to determine the prevalence of high-risk, vulnerable plaque in obstructive and nonobstructive nonculprit lesions. For this purpose, vulnerable plaque was defined as thin-cap fibroatheroma (TCFA), a coronary lesion known to pose high risk of worsening stenosis, plaque rupture, and cardiovascular events.
Of note, these 93 patients had a total of 425 diseased segments: 150 obstructive and 275 nonobstructive.
“This is reassuring that the concept of acute coronary syndrome implies a diffuse pathophysiology of affecting not only the culprit segment but the coronary vasculature as a whole,” Dr. Pinella-Echeverri observed.
The main study finding, however, was that TCFA was significantly more prevalent in obstructive, compared with nonobstructive, nonculprit lesions by a margin of 35% to 23%. The obstructive and nonobstructive TCFA lesions had a similar lipid-rich composition; however, the obstructive ones were significantly longer and had a smaller mean lumen area.
When breaking down the prevalence of TCFA per patient, 47% of patients had a nonculprit obstructive lesion with vulnerable plaque morphology. Another 20% had nonobstructive TCFA lesions. And only 32% of the STEMI patients had no TCFA in their obstructive or nonobstructive segments.
Discussant Frans Van de Werf, MD, PhD, commented: “This [OCT substudy result] immediately explains the clinical benefit observed with preventive PCI in STEMI patients with obstructive multivessel disease.”
The finding that 20% of the STEMI patients had nonobstructive lesions with vulnerable plaque morphology by OCT provides powerful support for the current guideline-recommended strategy of immediately starting STEMI patients on intensive lipid-lowering therapy, added Dr. Van de Werf, professor of medicine at the Catholic University of Leuven (Belgium).
He argued that the decision to revascularize nonculprit lesions by means of PCI versus the more complete revascularization achieved via coronary artery bypass graft surgery shouldn’t be made during the initial primary PCI, citing evidence that when the decision gets made at that time, coronary artery bypass grafting (CABG) is less likely to be chosen.
“I believe that OCT and [fractional flow reserve] should not be performed during the index primary PCI, not only for the comfort of the patient, but also for the better selection of complete revascularization. Interventional cardiologists should not forget that CABG might be a better revascularization treatment in some cases, such as left main disease and diabetes mellitus,” the cardiologist cautioned.
The COMPLETE OCT Substudy was supported by Abbott Vascular, the Population Health Research Institute, Hamilton Health Sciences, and the Canadian Institutes of Health Research.
REPORTING FROM AHA 2019
Cardiac biomarkers refine antihypertensive drug initiation decisions
PHILADELPHIA – Incorporation of cardiac biomarkers into current guideline-based decision-making regarding initiation of antihypertensive medication in patients with previously untreated mild or moderate high blood pressure leads to more appropriate and selective matching of intensive blood pressure control with true patient risk, Ambarish Pandey, MD, reported at the American Heart Association scientific sessions.
That’s because the 2017 American College of Cardiology/AHA blood pressure guidelines recommend incorporating the ACC/AHA 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Calculator into decision making as to whether to start antihypertensive drug therapy in patients with stage 1 hypertension (130-139/80-89 mm Hg), but the risk calculator doesn’t account for the risk of heart failure.
Yet by far the greatest benefit of intensive BP lowering is in reducing the risk of developing heart failure, as demonstrated in the landmark SPRINT trial, which showed that intensive BP lowering achieved much greater risk reduction in new-onset heart failure than in atherosclerotic cardiovascular events.
Thus, there’s a need for better strategies to guide antihypertensive therapy. And therein lies the rationale for incorporating into the risk assessment an individual’s values for N-terminal pro–brain natriuretic peptide (NT-proBNP), which reflects chronic myocardial stress, and high-sensitivity cardiac troponin T (hs-cTnT), which when elevated signals myocardial injury.
“Cardiac biomarkers are intermediate phenotypes from hypertension to future cardiovascular events. They can identify individuals at increased risk for atherosclerotic events, and at even higher risk for heart failure events,” explained Dr. Pandey, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
He presented a study of 12,987 participants in three major U.S. cohort studies: the Atherosclerosis Risk In Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Dallas Heart Study. At baseline, none of the participants were on antihypertensive therapy or had known cardiovascular disease. During 10 years of prospective follow-up, 825 of them experienced a first cardiovascular disease event: 251 developed heart failure and 574 had an MI, stroke, or cardiovascular death. Dr. Pandey and his coworkers calculated the cardiovascular event incidence rate and number-needed-to-treat with intensive antihypertensive drug therapy to prevent a first cardiovascular disease event on the basis of whether patients in the various BP categories were positive or negative for one or more biomarkers.
The results
Fifty-four percent of subjects had normal BP, defined in the guidelines as less than 120/80 mm Hg. Another 3% had BP in excess of 160/100 mm Hg. No controversy exists regarding pharmacotherapy in either of these groups: It’s not warranted in the former, essential in the latter.
Another 3,000 individuals had what the ACC/AHA guidelines define as elevated BP, meaning 120-129/<80 mm Hg, or low-risk stage 1 hypertension of 130-139/80-89 mm Hg and a 10-year ASCVD risk score of less than 10%. Initiation of antihypertensive medication in these groups is not recommended in the guidelines. Yet 36% of these individuals had at least one positive cardiac biomarker. And here’s the eye-opening finding: Notably, the 10-year cardiovascular event incidence rate in this biomarker group not currently recommended for antihypertensive pharmacotherapy was 11%, more than double the 4.6% rate in the biomarker-negative group, which in turn was comparable to the 3.8% in the normal BP participants.
Antihypertensive therapy was recommended according to the guidelines in 20% of the total study population, comprising patients with stage 1 hypertension who had an ASCVD risk score of 10% or more as well as those with stage 2 hypertension, defined as BP greater than 140/90 mm Hg but less than 160/100 mm Hg. Forty-eight percent of these subjects were positive for at least one biomarker. Their cardiovascular incidence rate was 15.1%, compared to the 7.9% rate in biomarker-negative individuals.
The estimated number-needed-to-treat (NNT) with intensive blood pressure–lowering therapy to a target systolic BP of less than 120 mm Hg, as in SPRINT, to prevent one cardiovascular event in individuals not currently guideline-recommended for antihypertensive medications was 86 in those who were biomarker-negative. The NNT dropped to 36 in the biomarker-positive subgroup, a far more attractive figure that suggests a reasonable likelihood of benefit from intensive blood pressure control, in Dr. Pandey’s view.
Similarly, among individuals currently recommended for pharmacotherapy initiation, the NNTs were 49 if biomarker-negative, improving to 26 in those positive for one or both biomarkers, which was comparable to the NNT of 22 in the group with blood pressures greater than 160/100 mm Hg. The NNT of 49 in the biomarker-negative subgroup is in a borderline gray zone warranting individualized shared decision-making regarding pharmacotherapy, Dr. Pandey said.
In this study, an elevated hs-cTnT was defined as 6 ng/L or more, while an elevated NT-proBNP was considered to be at least 100 pg/mL.
“It’s noteworthy that the degree of elevation in hs-cTnT and NT-proBNP which were observed in our study were pretty subtle and much below the threshold used for diagnosis of ischemic events or heart failure. Thus, these elevations were largely representative of subtle chronic injury and not acute events,” according to the cardiologist.
One audience member asked if the elevated biomarkers could simply be a surrogate for longer duration of exposure of the heart to high BP. Sure, Dr. Pandey replied, pointing to the 6-year greater average age of the biomarker-positive participants.
“It is likely that biomarker-positive status is capturing the culmination of longstanding exposure. But the thing about hypertension is there are no symptoms that can signal to the patient or the doctor that they have this disease, so testing for the biomarkers can actually capture the high-risk group that may have had hypertension for a long duration but now needs to be treated in order to prevent the advance of downstream adverse events,” he said.
Dr. Pandey reported having no financial conflicts of interest regarding his study, conducted free of commercial support.
SOURCE: Pandey A. AHA 2019 Abstract EP.AOS.521.141
PHILADELPHIA – Incorporation of cardiac biomarkers into current guideline-based decision-making regarding initiation of antihypertensive medication in patients with previously untreated mild or moderate high blood pressure leads to more appropriate and selective matching of intensive blood pressure control with true patient risk, Ambarish Pandey, MD, reported at the American Heart Association scientific sessions.
That’s because the 2017 American College of Cardiology/AHA blood pressure guidelines recommend incorporating the ACC/AHA 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Calculator into decision making as to whether to start antihypertensive drug therapy in patients with stage 1 hypertension (130-139/80-89 mm Hg), but the risk calculator doesn’t account for the risk of heart failure.
Yet by far the greatest benefit of intensive BP lowering is in reducing the risk of developing heart failure, as demonstrated in the landmark SPRINT trial, which showed that intensive BP lowering achieved much greater risk reduction in new-onset heart failure than in atherosclerotic cardiovascular events.
Thus, there’s a need for better strategies to guide antihypertensive therapy. And therein lies the rationale for incorporating into the risk assessment an individual’s values for N-terminal pro–brain natriuretic peptide (NT-proBNP), which reflects chronic myocardial stress, and high-sensitivity cardiac troponin T (hs-cTnT), which when elevated signals myocardial injury.
“Cardiac biomarkers are intermediate phenotypes from hypertension to future cardiovascular events. They can identify individuals at increased risk for atherosclerotic events, and at even higher risk for heart failure events,” explained Dr. Pandey, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
He presented a study of 12,987 participants in three major U.S. cohort studies: the Atherosclerosis Risk In Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Dallas Heart Study. At baseline, none of the participants were on antihypertensive therapy or had known cardiovascular disease. During 10 years of prospective follow-up, 825 of them experienced a first cardiovascular disease event: 251 developed heart failure and 574 had an MI, stroke, or cardiovascular death. Dr. Pandey and his coworkers calculated the cardiovascular event incidence rate and number-needed-to-treat with intensive antihypertensive drug therapy to prevent a first cardiovascular disease event on the basis of whether patients in the various BP categories were positive or negative for one or more biomarkers.
The results
Fifty-four percent of subjects had normal BP, defined in the guidelines as less than 120/80 mm Hg. Another 3% had BP in excess of 160/100 mm Hg. No controversy exists regarding pharmacotherapy in either of these groups: It’s not warranted in the former, essential in the latter.
Another 3,000 individuals had what the ACC/AHA guidelines define as elevated BP, meaning 120-129/<80 mm Hg, or low-risk stage 1 hypertension of 130-139/80-89 mm Hg and a 10-year ASCVD risk score of less than 10%. Initiation of antihypertensive medication in these groups is not recommended in the guidelines. Yet 36% of these individuals had at least one positive cardiac biomarker. And here’s the eye-opening finding: Notably, the 10-year cardiovascular event incidence rate in this biomarker group not currently recommended for antihypertensive pharmacotherapy was 11%, more than double the 4.6% rate in the biomarker-negative group, which in turn was comparable to the 3.8% in the normal BP participants.
Antihypertensive therapy was recommended according to the guidelines in 20% of the total study population, comprising patients with stage 1 hypertension who had an ASCVD risk score of 10% or more as well as those with stage 2 hypertension, defined as BP greater than 140/90 mm Hg but less than 160/100 mm Hg. Forty-eight percent of these subjects were positive for at least one biomarker. Their cardiovascular incidence rate was 15.1%, compared to the 7.9% rate in biomarker-negative individuals.
The estimated number-needed-to-treat (NNT) with intensive blood pressure–lowering therapy to a target systolic BP of less than 120 mm Hg, as in SPRINT, to prevent one cardiovascular event in individuals not currently guideline-recommended for antihypertensive medications was 86 in those who were biomarker-negative. The NNT dropped to 36 in the biomarker-positive subgroup, a far more attractive figure that suggests a reasonable likelihood of benefit from intensive blood pressure control, in Dr. Pandey’s view.
Similarly, among individuals currently recommended for pharmacotherapy initiation, the NNTs were 49 if biomarker-negative, improving to 26 in those positive for one or both biomarkers, which was comparable to the NNT of 22 in the group with blood pressures greater than 160/100 mm Hg. The NNT of 49 in the biomarker-negative subgroup is in a borderline gray zone warranting individualized shared decision-making regarding pharmacotherapy, Dr. Pandey said.
In this study, an elevated hs-cTnT was defined as 6 ng/L or more, while an elevated NT-proBNP was considered to be at least 100 pg/mL.
“It’s noteworthy that the degree of elevation in hs-cTnT and NT-proBNP which were observed in our study were pretty subtle and much below the threshold used for diagnosis of ischemic events or heart failure. Thus, these elevations were largely representative of subtle chronic injury and not acute events,” according to the cardiologist.
One audience member asked if the elevated biomarkers could simply be a surrogate for longer duration of exposure of the heart to high BP. Sure, Dr. Pandey replied, pointing to the 6-year greater average age of the biomarker-positive participants.
“It is likely that biomarker-positive status is capturing the culmination of longstanding exposure. But the thing about hypertension is there are no symptoms that can signal to the patient or the doctor that they have this disease, so testing for the biomarkers can actually capture the high-risk group that may have had hypertension for a long duration but now needs to be treated in order to prevent the advance of downstream adverse events,” he said.
Dr. Pandey reported having no financial conflicts of interest regarding his study, conducted free of commercial support.
SOURCE: Pandey A. AHA 2019 Abstract EP.AOS.521.141
PHILADELPHIA – Incorporation of cardiac biomarkers into current guideline-based decision-making regarding initiation of antihypertensive medication in patients with previously untreated mild or moderate high blood pressure leads to more appropriate and selective matching of intensive blood pressure control with true patient risk, Ambarish Pandey, MD, reported at the American Heart Association scientific sessions.
That’s because the 2017 American College of Cardiology/AHA blood pressure guidelines recommend incorporating the ACC/AHA 10-Year Atherosclerotic Cardiovascular Disease (ASCVD) Risk Calculator into decision making as to whether to start antihypertensive drug therapy in patients with stage 1 hypertension (130-139/80-89 mm Hg), but the risk calculator doesn’t account for the risk of heart failure.
Yet by far the greatest benefit of intensive BP lowering is in reducing the risk of developing heart failure, as demonstrated in the landmark SPRINT trial, which showed that intensive BP lowering achieved much greater risk reduction in new-onset heart failure than in atherosclerotic cardiovascular events.
Thus, there’s a need for better strategies to guide antihypertensive therapy. And therein lies the rationale for incorporating into the risk assessment an individual’s values for N-terminal pro–brain natriuretic peptide (NT-proBNP), which reflects chronic myocardial stress, and high-sensitivity cardiac troponin T (hs-cTnT), which when elevated signals myocardial injury.
“Cardiac biomarkers are intermediate phenotypes from hypertension to future cardiovascular events. They can identify individuals at increased risk for atherosclerotic events, and at even higher risk for heart failure events,” explained Dr. Pandey, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
He presented a study of 12,987 participants in three major U.S. cohort studies: the Atherosclerosis Risk In Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Dallas Heart Study. At baseline, none of the participants were on antihypertensive therapy or had known cardiovascular disease. During 10 years of prospective follow-up, 825 of them experienced a first cardiovascular disease event: 251 developed heart failure and 574 had an MI, stroke, or cardiovascular death. Dr. Pandey and his coworkers calculated the cardiovascular event incidence rate and number-needed-to-treat with intensive antihypertensive drug therapy to prevent a first cardiovascular disease event on the basis of whether patients in the various BP categories were positive or negative for one or more biomarkers.
The results
Fifty-four percent of subjects had normal BP, defined in the guidelines as less than 120/80 mm Hg. Another 3% had BP in excess of 160/100 mm Hg. No controversy exists regarding pharmacotherapy in either of these groups: It’s not warranted in the former, essential in the latter.
Another 3,000 individuals had what the ACC/AHA guidelines define as elevated BP, meaning 120-129/<80 mm Hg, or low-risk stage 1 hypertension of 130-139/80-89 mm Hg and a 10-year ASCVD risk score of less than 10%. Initiation of antihypertensive medication in these groups is not recommended in the guidelines. Yet 36% of these individuals had at least one positive cardiac biomarker. And here’s the eye-opening finding: Notably, the 10-year cardiovascular event incidence rate in this biomarker group not currently recommended for antihypertensive pharmacotherapy was 11%, more than double the 4.6% rate in the biomarker-negative group, which in turn was comparable to the 3.8% in the normal BP participants.
Antihypertensive therapy was recommended according to the guidelines in 20% of the total study population, comprising patients with stage 1 hypertension who had an ASCVD risk score of 10% or more as well as those with stage 2 hypertension, defined as BP greater than 140/90 mm Hg but less than 160/100 mm Hg. Forty-eight percent of these subjects were positive for at least one biomarker. Their cardiovascular incidence rate was 15.1%, compared to the 7.9% rate in biomarker-negative individuals.
The estimated number-needed-to-treat (NNT) with intensive blood pressure–lowering therapy to a target systolic BP of less than 120 mm Hg, as in SPRINT, to prevent one cardiovascular event in individuals not currently guideline-recommended for antihypertensive medications was 86 in those who were biomarker-negative. The NNT dropped to 36 in the biomarker-positive subgroup, a far more attractive figure that suggests a reasonable likelihood of benefit from intensive blood pressure control, in Dr. Pandey’s view.
Similarly, among individuals currently recommended for pharmacotherapy initiation, the NNTs were 49 if biomarker-negative, improving to 26 in those positive for one or both biomarkers, which was comparable to the NNT of 22 in the group with blood pressures greater than 160/100 mm Hg. The NNT of 49 in the biomarker-negative subgroup is in a borderline gray zone warranting individualized shared decision-making regarding pharmacotherapy, Dr. Pandey said.
In this study, an elevated hs-cTnT was defined as 6 ng/L or more, while an elevated NT-proBNP was considered to be at least 100 pg/mL.
“It’s noteworthy that the degree of elevation in hs-cTnT and NT-proBNP which were observed in our study were pretty subtle and much below the threshold used for diagnosis of ischemic events or heart failure. Thus, these elevations were largely representative of subtle chronic injury and not acute events,” according to the cardiologist.
One audience member asked if the elevated biomarkers could simply be a surrogate for longer duration of exposure of the heart to high BP. Sure, Dr. Pandey replied, pointing to the 6-year greater average age of the biomarker-positive participants.
“It is likely that biomarker-positive status is capturing the culmination of longstanding exposure. But the thing about hypertension is there are no symptoms that can signal to the patient or the doctor that they have this disease, so testing for the biomarkers can actually capture the high-risk group that may have had hypertension for a long duration but now needs to be treated in order to prevent the advance of downstream adverse events,” he said.
Dr. Pandey reported having no financial conflicts of interest regarding his study, conducted free of commercial support.
SOURCE: Pandey A. AHA 2019 Abstract EP.AOS.521.141
REPORTING FROM AHA 2019
Renal denervation rebounds
PHILADELPHIA – Enthusiasm for catheter-based renal denervation as a potential nondrug treatment for hypertension is once again on the rise, Michael Bohm, MD, observed at the American Heart Association scientific sessions.
The field experienced “a big depression” in 2014 with the publication of the unexpectedly negative results of the Symplicity HTN-3 trial (N Engl J Med. 2014;370:1393-401), he said. But post hoc analysis of the trial revealed significant shortcomings in design and execution.
“All of the flaws of this trial have been eliminated and now there is a very tightly controlled program to show whether renal denervation will work or not,” according to Dr. Bohm, director of the department of internal medicine and professor of cardiology at Saarland University in Homburg, Germany.
Indeed, three randomized, double-blind, sham-controlled, proof-of-concept clinical trials – all with strongly positive results – were published in Lancet in 2017 and 2018: SPYRAL HTN-OFF (2017 Nov 11;390:2160-70), RADIANCE SOLO (2018 Jun 9;391:2335-45), and SPYRAL HTN-ON (2018 May 23;391:2346-55). Based on the encouraging findings, four large pivotal trials of renal denervation (RDN) for hypertension are ongoing: RADIANCE HTN, REQUIRE, RADIANCE II, and SPYRAL HTN-ON MED. In addition, the SPYRAL HTN-OFF MED pivotal trial has been completed and will be presented soon, Dr. Bohm said.
Defining who’s most likely to benefit
Treatment response has been quite variable within the various RDN trials. A reliable predictor of response would be an important advance because it would enable physicians to select the best candidates for treatment while sparing others from an invasive procedure – albeit a relatively safe one – that they may not benefit from. On this front, Dr. Bohm and colleagues have recently reported that a baseline 24-hour heart rate above the median value of 73.5 bpm in the SPYRAL HTN-OFF MED trial – a marker for sympathetic overdrive – was associated with a 10.7/7.5 mm Hg greater reduction in average ambulatory blood pressures post-RDN than with a sham procedure. In contrast, blood pressure changes in RDN recipients with a below-median baseline 24-hour heart rate weren’t significant (Eur Heart J. 2019 Mar 1;40:743-51).
“Although this is a little bit rough, there is no other really true and reliable marker,” the cardiologist observed.
A pressing need exists for a reliable intraprocedural indicator of success. Dr. Bohm noted that Australian investigators are pursuing a promising approach in animal studies: intraprocedural transvascular high-frequency pacing of the aorticorenal ganglia. Abolition of the pacing-induced increase in blood pressure may be an indicator of complete RDN (JACC Cardiovasc Interv. 2019 Jun 24;12:1109-20).
Applications other than hypertension
Renal denervation is under early-stage investigation for a range of other cardiovascular diseases in which sympathetic overdrive figures prominently.
“The truly interesting things in renal denervation are what happens beyond hypertension. There are a lot of potential applications,” according to Dr. Bohm.
For example, when RDN was performed alongside pulmonary vein isolation for treatment of paroxysmal atrial fibrillation in hypertensive patients, the arrhythmia recurrence rate was significantly reduced during 1 year of follow-up, compared with AF ablation alone, in the randomized, multicenter, 302-patient ERADICATE-AF trial, presented at the most recent meeting of the Heart Rhythm Society.
Also, a small, uncontrolled registry study of RDN in patients with cardiomyopathy and electrical storm suggests the procedure may have an immediate anti–ventricular arrhythmia effect.
Meanwhile, Dr. Bohm is pressing the German government to sponsor an independent randomized controlled trial of RDN for heart failure. He and others have shown in small pilot studies a promising signal that the treatment may improve myocardial function and the signs and symptoms of heart failure in both patients with reduced and preserved left ventricular ejection fraction – and without reducing their blood pressure, which is often already low.
Dr. Bohm and others have also been exploring the impact of RDN in patients with metabolic syndrome. The treatment has a sound pathophysiologic rationale because insulin resistance is dependent upon sympathetic nervous system activation. Preliminary reports show improved insulin sensitivity in response to RDN. Patients also report better quality of life, presumably because of the reduction in sympathetic overactivity.
A couple of small Chinese studies suggest denervating the pulmonary vein in patients with pulmonary hypertension leads to a salutary reduction in pulmonary blood pressures.
“We haven’t done that yet. There is no properly designed catheter. They’ve used a Spyra unipolar catheter. It could work, but it hasn’t been rigorously investigated,” the cardiologist said.
Dr. Bohm reported serving as a scientific adviser to Abbott, AstraZeneca, BMS, Boehringer Ingelheim, and Servier.
PHILADELPHIA – Enthusiasm for catheter-based renal denervation as a potential nondrug treatment for hypertension is once again on the rise, Michael Bohm, MD, observed at the American Heart Association scientific sessions.
The field experienced “a big depression” in 2014 with the publication of the unexpectedly negative results of the Symplicity HTN-3 trial (N Engl J Med. 2014;370:1393-401), he said. But post hoc analysis of the trial revealed significant shortcomings in design and execution.
“All of the flaws of this trial have been eliminated and now there is a very tightly controlled program to show whether renal denervation will work or not,” according to Dr. Bohm, director of the department of internal medicine and professor of cardiology at Saarland University in Homburg, Germany.
Indeed, three randomized, double-blind, sham-controlled, proof-of-concept clinical trials – all with strongly positive results – were published in Lancet in 2017 and 2018: SPYRAL HTN-OFF (2017 Nov 11;390:2160-70), RADIANCE SOLO (2018 Jun 9;391:2335-45), and SPYRAL HTN-ON (2018 May 23;391:2346-55). Based on the encouraging findings, four large pivotal trials of renal denervation (RDN) for hypertension are ongoing: RADIANCE HTN, REQUIRE, RADIANCE II, and SPYRAL HTN-ON MED. In addition, the SPYRAL HTN-OFF MED pivotal trial has been completed and will be presented soon, Dr. Bohm said.
Defining who’s most likely to benefit
Treatment response has been quite variable within the various RDN trials. A reliable predictor of response would be an important advance because it would enable physicians to select the best candidates for treatment while sparing others from an invasive procedure – albeit a relatively safe one – that they may not benefit from. On this front, Dr. Bohm and colleagues have recently reported that a baseline 24-hour heart rate above the median value of 73.5 bpm in the SPYRAL HTN-OFF MED trial – a marker for sympathetic overdrive – was associated with a 10.7/7.5 mm Hg greater reduction in average ambulatory blood pressures post-RDN than with a sham procedure. In contrast, blood pressure changes in RDN recipients with a below-median baseline 24-hour heart rate weren’t significant (Eur Heart J. 2019 Mar 1;40:743-51).
“Although this is a little bit rough, there is no other really true and reliable marker,” the cardiologist observed.
A pressing need exists for a reliable intraprocedural indicator of success. Dr. Bohm noted that Australian investigators are pursuing a promising approach in animal studies: intraprocedural transvascular high-frequency pacing of the aorticorenal ganglia. Abolition of the pacing-induced increase in blood pressure may be an indicator of complete RDN (JACC Cardiovasc Interv. 2019 Jun 24;12:1109-20).
Applications other than hypertension
Renal denervation is under early-stage investigation for a range of other cardiovascular diseases in which sympathetic overdrive figures prominently.
“The truly interesting things in renal denervation are what happens beyond hypertension. There are a lot of potential applications,” according to Dr. Bohm.
For example, when RDN was performed alongside pulmonary vein isolation for treatment of paroxysmal atrial fibrillation in hypertensive patients, the arrhythmia recurrence rate was significantly reduced during 1 year of follow-up, compared with AF ablation alone, in the randomized, multicenter, 302-patient ERADICATE-AF trial, presented at the most recent meeting of the Heart Rhythm Society.
Also, a small, uncontrolled registry study of RDN in patients with cardiomyopathy and electrical storm suggests the procedure may have an immediate anti–ventricular arrhythmia effect.
Meanwhile, Dr. Bohm is pressing the German government to sponsor an independent randomized controlled trial of RDN for heart failure. He and others have shown in small pilot studies a promising signal that the treatment may improve myocardial function and the signs and symptoms of heart failure in both patients with reduced and preserved left ventricular ejection fraction – and without reducing their blood pressure, which is often already low.
Dr. Bohm and others have also been exploring the impact of RDN in patients with metabolic syndrome. The treatment has a sound pathophysiologic rationale because insulin resistance is dependent upon sympathetic nervous system activation. Preliminary reports show improved insulin sensitivity in response to RDN. Patients also report better quality of life, presumably because of the reduction in sympathetic overactivity.
A couple of small Chinese studies suggest denervating the pulmonary vein in patients with pulmonary hypertension leads to a salutary reduction in pulmonary blood pressures.
“We haven’t done that yet. There is no properly designed catheter. They’ve used a Spyra unipolar catheter. It could work, but it hasn’t been rigorously investigated,” the cardiologist said.
Dr. Bohm reported serving as a scientific adviser to Abbott, AstraZeneca, BMS, Boehringer Ingelheim, and Servier.
PHILADELPHIA – Enthusiasm for catheter-based renal denervation as a potential nondrug treatment for hypertension is once again on the rise, Michael Bohm, MD, observed at the American Heart Association scientific sessions.
The field experienced “a big depression” in 2014 with the publication of the unexpectedly negative results of the Symplicity HTN-3 trial (N Engl J Med. 2014;370:1393-401), he said. But post hoc analysis of the trial revealed significant shortcomings in design and execution.
“All of the flaws of this trial have been eliminated and now there is a very tightly controlled program to show whether renal denervation will work or not,” according to Dr. Bohm, director of the department of internal medicine and professor of cardiology at Saarland University in Homburg, Germany.
Indeed, three randomized, double-blind, sham-controlled, proof-of-concept clinical trials – all with strongly positive results – were published in Lancet in 2017 and 2018: SPYRAL HTN-OFF (2017 Nov 11;390:2160-70), RADIANCE SOLO (2018 Jun 9;391:2335-45), and SPYRAL HTN-ON (2018 May 23;391:2346-55). Based on the encouraging findings, four large pivotal trials of renal denervation (RDN) for hypertension are ongoing: RADIANCE HTN, REQUIRE, RADIANCE II, and SPYRAL HTN-ON MED. In addition, the SPYRAL HTN-OFF MED pivotal trial has been completed and will be presented soon, Dr. Bohm said.
Defining who’s most likely to benefit
Treatment response has been quite variable within the various RDN trials. A reliable predictor of response would be an important advance because it would enable physicians to select the best candidates for treatment while sparing others from an invasive procedure – albeit a relatively safe one – that they may not benefit from. On this front, Dr. Bohm and colleagues have recently reported that a baseline 24-hour heart rate above the median value of 73.5 bpm in the SPYRAL HTN-OFF MED trial – a marker for sympathetic overdrive – was associated with a 10.7/7.5 mm Hg greater reduction in average ambulatory blood pressures post-RDN than with a sham procedure. In contrast, blood pressure changes in RDN recipients with a below-median baseline 24-hour heart rate weren’t significant (Eur Heart J. 2019 Mar 1;40:743-51).
“Although this is a little bit rough, there is no other really true and reliable marker,” the cardiologist observed.
A pressing need exists for a reliable intraprocedural indicator of success. Dr. Bohm noted that Australian investigators are pursuing a promising approach in animal studies: intraprocedural transvascular high-frequency pacing of the aorticorenal ganglia. Abolition of the pacing-induced increase in blood pressure may be an indicator of complete RDN (JACC Cardiovasc Interv. 2019 Jun 24;12:1109-20).
Applications other than hypertension
Renal denervation is under early-stage investigation for a range of other cardiovascular diseases in which sympathetic overdrive figures prominently.
“The truly interesting things in renal denervation are what happens beyond hypertension. There are a lot of potential applications,” according to Dr. Bohm.
For example, when RDN was performed alongside pulmonary vein isolation for treatment of paroxysmal atrial fibrillation in hypertensive patients, the arrhythmia recurrence rate was significantly reduced during 1 year of follow-up, compared with AF ablation alone, in the randomized, multicenter, 302-patient ERADICATE-AF trial, presented at the most recent meeting of the Heart Rhythm Society.
Also, a small, uncontrolled registry study of RDN in patients with cardiomyopathy and electrical storm suggests the procedure may have an immediate anti–ventricular arrhythmia effect.
Meanwhile, Dr. Bohm is pressing the German government to sponsor an independent randomized controlled trial of RDN for heart failure. He and others have shown in small pilot studies a promising signal that the treatment may improve myocardial function and the signs and symptoms of heart failure in both patients with reduced and preserved left ventricular ejection fraction – and without reducing their blood pressure, which is often already low.
Dr. Bohm and others have also been exploring the impact of RDN in patients with metabolic syndrome. The treatment has a sound pathophysiologic rationale because insulin resistance is dependent upon sympathetic nervous system activation. Preliminary reports show improved insulin sensitivity in response to RDN. Patients also report better quality of life, presumably because of the reduction in sympathetic overactivity.
A couple of small Chinese studies suggest denervating the pulmonary vein in patients with pulmonary hypertension leads to a salutary reduction in pulmonary blood pressures.
“We haven’t done that yet. There is no properly designed catheter. They’ve used a Spyra unipolar catheter. It could work, but it hasn’t been rigorously investigated,” the cardiologist said.
Dr. Bohm reported serving as a scientific adviser to Abbott, AstraZeneca, BMS, Boehringer Ingelheim, and Servier.
EXPERT ANALYSIS FROM AHA 2019
Framingham: Exercise lessens cardiometabolic risk of poor diet
PHILADELPHIA – Engaging in at least 150 minutes of moderate to vigorous physical activity per week as recommended in national guidelines appears to mitigate the cardiometabolic risks associated with poor diet quality in middle-aged and older adults, according to an analysis of Framingham Heart Study data.
“Our findings suggest adherence to physical activity guidelines may have a protective effect on cardiometabolic health regardless of diet quality,” Joowon Lee, PhD, declared at the American Heart Association scientific sessions.
He presented separate cross-sectional analyses of the risks of metabolic syndrome in 2,379 middle-aged participants in the National Heart, Lung, and Blood Institute–sponsored Framingham (Mass.) Third Generation Study and 1,180 older participants in the Framingham Offspring Study.
The two analyses showed the same thing across a broad age spectrum: The highest prevalence of metabolic syndrome as defined by Adult Treatment Panel III criteria was present among those individuals who got less than 150 minutes of physical activity per week and were also in the lowest tertile in terms of diet quality, while the lowest prevalence of metabolic syndrome occurred in participants in the top tertile for diet quality who engaged in at least 150 minutes per week of moderate to vigorous physical activity in accord with the Department of Health & Human Services 2018 Physical Activity Guidelines for Americans.
In both the middle-aged and older populations, optimal physical activity – that is, at least 150 minutes per week – appeared to override the adverse impact of suboptimal diet quality. Physically active individuals with moderate or even poor diet quality had a significantly lower prevalence of metabolic syndrome than did the reference group constituted by participants with poor diet quality who didn’t exercise for 150 minutes per week.
But the converse didn’t hold true: Individuals with optimal diet quality who didn’t reach the physical activity threshold had no reduction in metabolic syndrome, compared with the reference group, according to Dr. Lee of Boston University.
For example, among the Framingham Offspring Study participants, whose mean age was 69 years at the time of their ninth formal examination in 2014, the prevalence of metabolic syndrome was 59% in those who got less than 150 minutes of moderate to vigorous physical activity weekly as assessed by accelerometer and who were also in the lowest tertile for diet quality as self-reported on the DGAI-2010 (Dietary Guidelines Adherence Index) semiquantitative food frequency questionnaire. The relative risk of metabolic syndrome was reduced by 61% in participants with both optimal physical activity and diet quality, by 49% in those with at least 150 minutes of physical activity but only moderate diet quality, and by 39% in those with optimal exercise and poor diet quality. In contrast, individuals in the top or middle tertiles for diet quality who didn’t meet the physical activity standard had a metabolic syndrome rate that wasn’t significantly lower than the reference group.
Dr. Lee observed that his analyses are best viewed as hypothesis generating. Their cross-sectional format precludes firm conclusions as to causality.
His findings prompted session comoderator Satyam Sarma, MD, of the University of Texas, Dallas, to make one of the most memorable comments heard at AHA 2019: that the Framingham findings suggest it may be possible to outrun a bad diet.
Dr. Lee reported having no financial conflicts regarding his study, supported by Boston University.
SOURCE: Lee J. AHA 2019, Abstract RF244.
PHILADELPHIA – Engaging in at least 150 minutes of moderate to vigorous physical activity per week as recommended in national guidelines appears to mitigate the cardiometabolic risks associated with poor diet quality in middle-aged and older adults, according to an analysis of Framingham Heart Study data.
“Our findings suggest adherence to physical activity guidelines may have a protective effect on cardiometabolic health regardless of diet quality,” Joowon Lee, PhD, declared at the American Heart Association scientific sessions.
He presented separate cross-sectional analyses of the risks of metabolic syndrome in 2,379 middle-aged participants in the National Heart, Lung, and Blood Institute–sponsored Framingham (Mass.) Third Generation Study and 1,180 older participants in the Framingham Offspring Study.
The two analyses showed the same thing across a broad age spectrum: The highest prevalence of metabolic syndrome as defined by Adult Treatment Panel III criteria was present among those individuals who got less than 150 minutes of physical activity per week and were also in the lowest tertile in terms of diet quality, while the lowest prevalence of metabolic syndrome occurred in participants in the top tertile for diet quality who engaged in at least 150 minutes per week of moderate to vigorous physical activity in accord with the Department of Health & Human Services 2018 Physical Activity Guidelines for Americans.
In both the middle-aged and older populations, optimal physical activity – that is, at least 150 minutes per week – appeared to override the adverse impact of suboptimal diet quality. Physically active individuals with moderate or even poor diet quality had a significantly lower prevalence of metabolic syndrome than did the reference group constituted by participants with poor diet quality who didn’t exercise for 150 minutes per week.
But the converse didn’t hold true: Individuals with optimal diet quality who didn’t reach the physical activity threshold had no reduction in metabolic syndrome, compared with the reference group, according to Dr. Lee of Boston University.
For example, among the Framingham Offspring Study participants, whose mean age was 69 years at the time of their ninth formal examination in 2014, the prevalence of metabolic syndrome was 59% in those who got less than 150 minutes of moderate to vigorous physical activity weekly as assessed by accelerometer and who were also in the lowest tertile for diet quality as self-reported on the DGAI-2010 (Dietary Guidelines Adherence Index) semiquantitative food frequency questionnaire. The relative risk of metabolic syndrome was reduced by 61% in participants with both optimal physical activity and diet quality, by 49% in those with at least 150 minutes of physical activity but only moderate diet quality, and by 39% in those with optimal exercise and poor diet quality. In contrast, individuals in the top or middle tertiles for diet quality who didn’t meet the physical activity standard had a metabolic syndrome rate that wasn’t significantly lower than the reference group.
Dr. Lee observed that his analyses are best viewed as hypothesis generating. Their cross-sectional format precludes firm conclusions as to causality.
His findings prompted session comoderator Satyam Sarma, MD, of the University of Texas, Dallas, to make one of the most memorable comments heard at AHA 2019: that the Framingham findings suggest it may be possible to outrun a bad diet.
Dr. Lee reported having no financial conflicts regarding his study, supported by Boston University.
SOURCE: Lee J. AHA 2019, Abstract RF244.
PHILADELPHIA – Engaging in at least 150 minutes of moderate to vigorous physical activity per week as recommended in national guidelines appears to mitigate the cardiometabolic risks associated with poor diet quality in middle-aged and older adults, according to an analysis of Framingham Heart Study data.
“Our findings suggest adherence to physical activity guidelines may have a protective effect on cardiometabolic health regardless of diet quality,” Joowon Lee, PhD, declared at the American Heart Association scientific sessions.
He presented separate cross-sectional analyses of the risks of metabolic syndrome in 2,379 middle-aged participants in the National Heart, Lung, and Blood Institute–sponsored Framingham (Mass.) Third Generation Study and 1,180 older participants in the Framingham Offspring Study.
The two analyses showed the same thing across a broad age spectrum: The highest prevalence of metabolic syndrome as defined by Adult Treatment Panel III criteria was present among those individuals who got less than 150 minutes of physical activity per week and were also in the lowest tertile in terms of diet quality, while the lowest prevalence of metabolic syndrome occurred in participants in the top tertile for diet quality who engaged in at least 150 minutes per week of moderate to vigorous physical activity in accord with the Department of Health & Human Services 2018 Physical Activity Guidelines for Americans.
In both the middle-aged and older populations, optimal physical activity – that is, at least 150 minutes per week – appeared to override the adverse impact of suboptimal diet quality. Physically active individuals with moderate or even poor diet quality had a significantly lower prevalence of metabolic syndrome than did the reference group constituted by participants with poor diet quality who didn’t exercise for 150 minutes per week.
But the converse didn’t hold true: Individuals with optimal diet quality who didn’t reach the physical activity threshold had no reduction in metabolic syndrome, compared with the reference group, according to Dr. Lee of Boston University.
For example, among the Framingham Offspring Study participants, whose mean age was 69 years at the time of their ninth formal examination in 2014, the prevalence of metabolic syndrome was 59% in those who got less than 150 minutes of moderate to vigorous physical activity weekly as assessed by accelerometer and who were also in the lowest tertile for diet quality as self-reported on the DGAI-2010 (Dietary Guidelines Adherence Index) semiquantitative food frequency questionnaire. The relative risk of metabolic syndrome was reduced by 61% in participants with both optimal physical activity and diet quality, by 49% in those with at least 150 minutes of physical activity but only moderate diet quality, and by 39% in those with optimal exercise and poor diet quality. In contrast, individuals in the top or middle tertiles for diet quality who didn’t meet the physical activity standard had a metabolic syndrome rate that wasn’t significantly lower than the reference group.
Dr. Lee observed that his analyses are best viewed as hypothesis generating. Their cross-sectional format precludes firm conclusions as to causality.
His findings prompted session comoderator Satyam Sarma, MD, of the University of Texas, Dallas, to make one of the most memorable comments heard at AHA 2019: that the Framingham findings suggest it may be possible to outrun a bad diet.
Dr. Lee reported having no financial conflicts regarding his study, supported by Boston University.
SOURCE: Lee J. AHA 2019, Abstract RF244.
REPORTING FROM AHA 2019
RECOVERY: Early SAVR benefits asymptomatic severe AS patients
PHILADELPHIA – Early aortic valve replacement surgery for patients with asymptomatic, severe aortic stenosis has been a controversial strategy, but the first randomized trial comparing early surgery with conservative management found that early-surgery patients had a 17-fold improved survival at 8 years, according to trial results reported at the American Heart Association scientific sessions.
Albeit small – 145 patients randomized to early surgery or conservative therapy – the RECOVERY trial results provide important evidence to support early preemptive aortic valve replacement for patients with asymptomatic but severe aortic stenosis, said Duk-Hyun Kang, MD, of the division of cardiology, Asan Medical Center in Seoul, South Korea.
The trial randomized 73 patients to early surgical aortic valve replacement (SAVR) and 72 to conventional treatment. Baseline characteristics were similar between the two groups, with a mean EuroSCORE II of 0.9, peak aortic-valve jet velocity (Vmax) of 5.1 m/sec, and mean aortic-valve area of 0.63 cm2. Fifty-three conventional treatment patients went on to have SAVR when symptoms developed during follow-up. There were no operation-related deaths in either group, although one early surgery patient had a stroke and one conventional treatment patients had an MI during the operative period.
At a median follow-up of 6.2 years, 1 early-surgery patient (1.4%) and 11 conventional-therapy patients (15.3%) died from operative or cardiovascular death, Dr. Kang said (P = 0.003). “The number needed to treat to prevent one cardiovascular death within 4 years was 20 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidences of operative CV or death were 1.4% for both in the early-surgery group and 5.7% and 25.5% in the conventional-treatment group, Dr. Kang said (P = 0.003).
Rates of all-cause mortality were 6.8% and 20.8% (P = 0.030) in the respective groups. “The number needed to treat to save one life in 4 years was 16 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidence of any-cause death was 4.1% and 10.2% in the early-surgery patients and 9.7% and 31.8% in the conventional group (P = 0.018).
Among the trial limitations Dr. Kang acknowledged were that the population had severe AS with aortic velocity of 4.5 m/sec or greater. “The benefit of early surgery may be relatively smaller in asymptomatic patients with less severe aortic stenosis,” he said.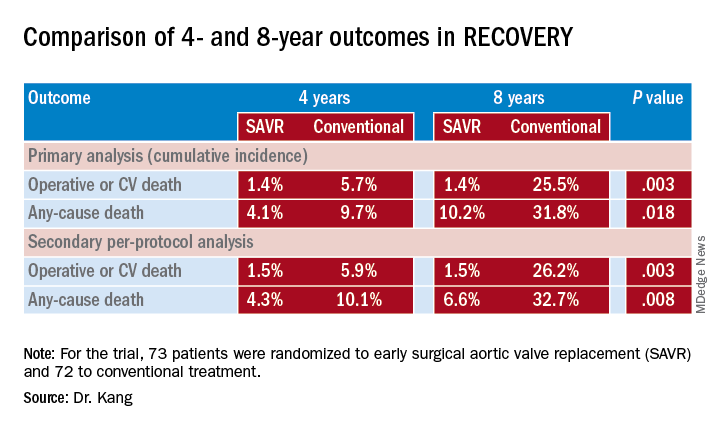
Not all patients had an exercise test to confirm their asymptomatic status, but as discussant Robert Bonow, MD, of Northwestern University in Chicago pointed out, “this is what we’re dealing with clinically.” Also the mean age of the patient population – 64.9 years – is relatively young, and they had a high incidence of bicuspid aortic valve, few comorbidities, and low operative risk, Dr. Kang said. “Thus, our study population is quite different from the populations enrolled in the TAVR [transcatheter AVR] trials, and the results of our study cannot be directly applied to early TAVR for asymptomatic severe aortic stenosis,” he said.
The mean age in both the PARTNER 3 (N Engl J Med. 2019;380:1695-705) and EVOLUT (N Engl J Med. 2019;380:1706-15) trials comparing TAVR and SAVR in low-risk patients was 73.6 years, and they had higher rates of comorbidities than did the RECOVERY patients.
Dr. Bonow said the RECOVERY findings add to the PARTNER 3 and EVOLUT findings and raise the question about rethinking guideline indications for AVR in asymptomatic severe AS patients. “Should we be talking about moving our Vmax threshold down to 4.5 m/sec, or maybe increasing the Class IIa indications to Class I?” he said. “I think we need to wait to see more data to support these excellent results.”
However, most of these asymptomatic patients do eventually have surgery “in a very short period of time,” Dr. Bonow said. “So from a clinical management point of view, I think we already have the data suggesting that we could move the ball forward, and now we have these excellent outcome data from Korea as well.”
Results were published simultaneously in the New England Journal of Medicine (2019 Nov 16. doi: 10.1056/NEJMoa1912846).
Dr. Kang and Dr. Bonow had no financial relationships to disclose.
PHILADELPHIA – Early aortic valve replacement surgery for patients with asymptomatic, severe aortic stenosis has been a controversial strategy, but the first randomized trial comparing early surgery with conservative management found that early-surgery patients had a 17-fold improved survival at 8 years, according to trial results reported at the American Heart Association scientific sessions.
Albeit small – 145 patients randomized to early surgery or conservative therapy – the RECOVERY trial results provide important evidence to support early preemptive aortic valve replacement for patients with asymptomatic but severe aortic stenosis, said Duk-Hyun Kang, MD, of the division of cardiology, Asan Medical Center in Seoul, South Korea.
The trial randomized 73 patients to early surgical aortic valve replacement (SAVR) and 72 to conventional treatment. Baseline characteristics were similar between the two groups, with a mean EuroSCORE II of 0.9, peak aortic-valve jet velocity (Vmax) of 5.1 m/sec, and mean aortic-valve area of 0.63 cm2. Fifty-three conventional treatment patients went on to have SAVR when symptoms developed during follow-up. There were no operation-related deaths in either group, although one early surgery patient had a stroke and one conventional treatment patients had an MI during the operative period.
At a median follow-up of 6.2 years, 1 early-surgery patient (1.4%) and 11 conventional-therapy patients (15.3%) died from operative or cardiovascular death, Dr. Kang said (P = 0.003). “The number needed to treat to prevent one cardiovascular death within 4 years was 20 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidences of operative CV or death were 1.4% for both in the early-surgery group and 5.7% and 25.5% in the conventional-treatment group, Dr. Kang said (P = 0.003).
Rates of all-cause mortality were 6.8% and 20.8% (P = 0.030) in the respective groups. “The number needed to treat to save one life in 4 years was 16 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidence of any-cause death was 4.1% and 10.2% in the early-surgery patients and 9.7% and 31.8% in the conventional group (P = 0.018).
Among the trial limitations Dr. Kang acknowledged were that the population had severe AS with aortic velocity of 4.5 m/sec or greater. “The benefit of early surgery may be relatively smaller in asymptomatic patients with less severe aortic stenosis,” he said.
Not all patients had an exercise test to confirm their asymptomatic status, but as discussant Robert Bonow, MD, of Northwestern University in Chicago pointed out, “this is what we’re dealing with clinically.” Also the mean age of the patient population – 64.9 years – is relatively young, and they had a high incidence of bicuspid aortic valve, few comorbidities, and low operative risk, Dr. Kang said. “Thus, our study population is quite different from the populations enrolled in the TAVR [transcatheter AVR] trials, and the results of our study cannot be directly applied to early TAVR for asymptomatic severe aortic stenosis,” he said.
The mean age in both the PARTNER 3 (N Engl J Med. 2019;380:1695-705) and EVOLUT (N Engl J Med. 2019;380:1706-15) trials comparing TAVR and SAVR in low-risk patients was 73.6 years, and they had higher rates of comorbidities than did the RECOVERY patients.
Dr. Bonow said the RECOVERY findings add to the PARTNER 3 and EVOLUT findings and raise the question about rethinking guideline indications for AVR in asymptomatic severe AS patients. “Should we be talking about moving our Vmax threshold down to 4.5 m/sec, or maybe increasing the Class IIa indications to Class I?” he said. “I think we need to wait to see more data to support these excellent results.”
However, most of these asymptomatic patients do eventually have surgery “in a very short period of time,” Dr. Bonow said. “So from a clinical management point of view, I think we already have the data suggesting that we could move the ball forward, and now we have these excellent outcome data from Korea as well.”
Results were published simultaneously in the New England Journal of Medicine (2019 Nov 16. doi: 10.1056/NEJMoa1912846).
Dr. Kang and Dr. Bonow had no financial relationships to disclose.
PHILADELPHIA – Early aortic valve replacement surgery for patients with asymptomatic, severe aortic stenosis has been a controversial strategy, but the first randomized trial comparing early surgery with conservative management found that early-surgery patients had a 17-fold improved survival at 8 years, according to trial results reported at the American Heart Association scientific sessions.
Albeit small – 145 patients randomized to early surgery or conservative therapy – the RECOVERY trial results provide important evidence to support early preemptive aortic valve replacement for patients with asymptomatic but severe aortic stenosis, said Duk-Hyun Kang, MD, of the division of cardiology, Asan Medical Center in Seoul, South Korea.
The trial randomized 73 patients to early surgical aortic valve replacement (SAVR) and 72 to conventional treatment. Baseline characteristics were similar between the two groups, with a mean EuroSCORE II of 0.9, peak aortic-valve jet velocity (Vmax) of 5.1 m/sec, and mean aortic-valve area of 0.63 cm2. Fifty-three conventional treatment patients went on to have SAVR when symptoms developed during follow-up. There were no operation-related deaths in either group, although one early surgery patient had a stroke and one conventional treatment patients had an MI during the operative period.
At a median follow-up of 6.2 years, 1 early-surgery patient (1.4%) and 11 conventional-therapy patients (15.3%) died from operative or cardiovascular death, Dr. Kang said (P = 0.003). “The number needed to treat to prevent one cardiovascular death within 4 years was 20 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidences of operative CV or death were 1.4% for both in the early-surgery group and 5.7% and 25.5% in the conventional-treatment group, Dr. Kang said (P = 0.003).
Rates of all-cause mortality were 6.8% and 20.8% (P = 0.030) in the respective groups. “The number needed to treat to save one life in 4 years was 16 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidence of any-cause death was 4.1% and 10.2% in the early-surgery patients and 9.7% and 31.8% in the conventional group (P = 0.018).
Among the trial limitations Dr. Kang acknowledged were that the population had severe AS with aortic velocity of 4.5 m/sec or greater. “The benefit of early surgery may be relatively smaller in asymptomatic patients with less severe aortic stenosis,” he said.
Not all patients had an exercise test to confirm their asymptomatic status, but as discussant Robert Bonow, MD, of Northwestern University in Chicago pointed out, “this is what we’re dealing with clinically.” Also the mean age of the patient population – 64.9 years – is relatively young, and they had a high incidence of bicuspid aortic valve, few comorbidities, and low operative risk, Dr. Kang said. “Thus, our study population is quite different from the populations enrolled in the TAVR [transcatheter AVR] trials, and the results of our study cannot be directly applied to early TAVR for asymptomatic severe aortic stenosis,” he said.
The mean age in both the PARTNER 3 (N Engl J Med. 2019;380:1695-705) and EVOLUT (N Engl J Med. 2019;380:1706-15) trials comparing TAVR and SAVR in low-risk patients was 73.6 years, and they had higher rates of comorbidities than did the RECOVERY patients.
Dr. Bonow said the RECOVERY findings add to the PARTNER 3 and EVOLUT findings and raise the question about rethinking guideline indications for AVR in asymptomatic severe AS patients. “Should we be talking about moving our Vmax threshold down to 4.5 m/sec, or maybe increasing the Class IIa indications to Class I?” he said. “I think we need to wait to see more data to support these excellent results.”
However, most of these asymptomatic patients do eventually have surgery “in a very short period of time,” Dr. Bonow said. “So from a clinical management point of view, I think we already have the data suggesting that we could move the ball forward, and now we have these excellent outcome data from Korea as well.”
Results were published simultaneously in the New England Journal of Medicine (2019 Nov 16. doi: 10.1056/NEJMoa1912846).
Dr. Kang and Dr. Bonow had no financial relationships to disclose.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Testosterone gel increases LV mass in older men
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
REPORTING FROM AHA 2019
Coronary function testing during angiography boosts 1-year angina outcomes
PHILADELPHIA – Longer and more extensive follow-up of the positive CorMicA trial confirmed that clinicians who receive added information on the presence or absence of microvascular coronary disease or coronary vasospasm in patients with chronic, stable angina but without detectable coronary obstruction adjust patient treatment in ways that produce better outcomes.
For example, expanded 1-year follow-up of the 151-patient, multicenter, CorMicA randomized study showed that stable angina patients without coronary obstruction treated by clinicians aware of microvascular or vasospastic coronary disease had blood pressures that averaged about 12/5 mm Hg lower than those of patients in the control arm, in which this information was kept blinded.
Clinicians in the study who received information about diagnoses of microvascular angina, vasospastic angina, both, or neither, generally better tailored the treatments they gave these patients, were more likely to prescribe cardiac rehabilitation to these patients, and successfully capped increases in blood pressure that occurred in the control patients, Colin Berry, MD, said at the American Heart Association scientific sessions. The investigators made the diagnoses of microvascular and vasospastic angina using an “invasive diagnostic procedure” that involved placing a wire with pressure and temperature sensors in a selected coronary artery and measuring flow patterns that resulted from a systemic infusion of adenosine and from incremental, intracoronary infusions of acetylcholine.
The CorMicA results “tell us that, if we use existing drugs and nonpharmacologic treatments, we can improve patients’ quality of life,” said Dr. Berry, co-lead investigator of the study and professor of cardiology and imaging at the University of Glasgow. “There were probably two drivers of change in treatment” between the intervention arm where clinicians learned whether their patients had microvascular angina or vasospasm, and the control arm where this information was kept blinded. Some patients reclassified as having microvascular disease or vasospasm were restarted on standard agents for treatment of coronary artery disease such as statins and ACE inhibitors that had previously been stopped, noted Dr. Berry. The identification of a specific type of vascular disorder also helped guide treatment. For example, patients identified as having vasospasm were taken off of beta-blockers, which are contraindicated in these patients, and instead began treatment with a calcium channel antagonist, he said.
“CorMicA was a landmark trial. The interventional cardiologists at my center believe that the testing [for microvascular and vasospastic angina] should be used routinely, based on the reported data,” said Harmony R. Reynolds, MD, a cardiologist at NYU Langone Health in New York.
The CorMicA (Coronary Microvascular Angina) trial enrolled 391 patients at either of two hospitals in Scotland during November 2016–November 2017. All patients were scheduled for clinically indicated, elective diagnostic angiography for suspected stable angina. Clinicians identified a coronary obstruction in 206 patients that excluded them from the main CorMicA analysis. They focused on the 181 without coronary obstruction visible by angiography, and specifically the 151 of these patients who underwent the invasive diagnostic procedure, with 76 randomized to have their diagnostic-procedure results relayed to their treating physicians and 75 patients whose diagnostic results remained blinded. The study’s primary endpoint was the mean difference in angina severity at 6 months after treatment assessed by the Seattle Angina Questionnaire (SAQ) summary score. After 6 months, the between-group difference in average SAQ summary scores was 11.7 units, a statistically significant as well as clinically meaningful benefit that linked with knowledge of the coronary function of patients based on invasive testing (J Am Coll Cardiol. 2018 Dec 11;72[23 Part A]:2841-55).
The prespecified 1-year follow-up with another SAQ took place for 142 of the 151 randomized patients, and showed ongoing separation of SAQ scores between the intervention and control patients, reaching a statistically significant mean difference of 13.6 units, Dr. Berry reported. Other benefits that linked with dissemination of results from the invasive diagnostic procedure included maintenance of the quality-of-life benefit seen after 6 months, an incremental further reduction in illness perception from 6 to 12 months, and a substantial further improvement in global treatment satisfaction, which grew from a 30% higher level in the intervention group compared with controls at 6 months to a 44% between-group difference after 12 months. Concurrently with Dr. Berry’s report, the results appeared online (JACC: Cardiovasc Interv. 2020 Jan 13;13[1] 33-45).
Additional findings for various clinical measures that were not part of the 6-month findings provided further insight into the impact that clinician knowledge about microvascular disease or vasospasm had on patients. These metrics suggested that the benefits in the intervention patients may have largely resulted from their avoiding elevations in risk markers that occurred among controls who received routine care.
For example, after 12 months, average 12/5 mm Hg differences in systolic and diastolic blood pressures between the two arms was nearly all because of pressure increases from baseline in the control patients with only very small drops in pressure from baseline among the intervention patients. Weight measurements after 1 year showed that the control patients were on average 1.3 kg heavier than the intervention patients, again mostly because of weight increases among the controls. Enrollment in a cardiac rehabilitation program at 12 months had occurred for 40% of the intervention patients and 16% of the controls, a 73% relative increase in the intervention arm, Dr. Berry reported.
The invasive, coronary, physiological assessments used in CorMicA led to stratified medical therapy and created an opportunity for better long-term angina treatment in patients without obstructive coronary disease, Dr. Berry concluded. He stressed the need to further test this hypothesis in larger, prospective studies, and to assess its cost effectiveness. But the proof-of-concept demonstrated by the CorMicA results indicate a role for more thorough investigation of coronary dysfunction when obstructive disease is absent, with the opportunity to use this information to better tailor treatment. The findings also highlight the potential for new drug development that can address nonobstructive microvascular or vasospastic coronary disease, he said.
Dr. Berry has been a speaker on behalf of Abbott Vascular, and he has received research funding from AstraZeneca, GlaxoSmithKline, HeartFlow, Novartis, and Siemens Healthcare. Dr. Reynolds had no disclosures.
SOURCE: Ford TJ. AHA 2019, session FS.AOS.03 abstract.
PHILADELPHIA – Longer and more extensive follow-up of the positive CorMicA trial confirmed that clinicians who receive added information on the presence or absence of microvascular coronary disease or coronary vasospasm in patients with chronic, stable angina but without detectable coronary obstruction adjust patient treatment in ways that produce better outcomes.
For example, expanded 1-year follow-up of the 151-patient, multicenter, CorMicA randomized study showed that stable angina patients without coronary obstruction treated by clinicians aware of microvascular or vasospastic coronary disease had blood pressures that averaged about 12/5 mm Hg lower than those of patients in the control arm, in which this information was kept blinded.
Clinicians in the study who received information about diagnoses of microvascular angina, vasospastic angina, both, or neither, generally better tailored the treatments they gave these patients, were more likely to prescribe cardiac rehabilitation to these patients, and successfully capped increases in blood pressure that occurred in the control patients, Colin Berry, MD, said at the American Heart Association scientific sessions. The investigators made the diagnoses of microvascular and vasospastic angina using an “invasive diagnostic procedure” that involved placing a wire with pressure and temperature sensors in a selected coronary artery and measuring flow patterns that resulted from a systemic infusion of adenosine and from incremental, intracoronary infusions of acetylcholine.
The CorMicA results “tell us that, if we use existing drugs and nonpharmacologic treatments, we can improve patients’ quality of life,” said Dr. Berry, co-lead investigator of the study and professor of cardiology and imaging at the University of Glasgow. “There were probably two drivers of change in treatment” between the intervention arm where clinicians learned whether their patients had microvascular angina or vasospasm, and the control arm where this information was kept blinded. Some patients reclassified as having microvascular disease or vasospasm were restarted on standard agents for treatment of coronary artery disease such as statins and ACE inhibitors that had previously been stopped, noted Dr. Berry. The identification of a specific type of vascular disorder also helped guide treatment. For example, patients identified as having vasospasm were taken off of beta-blockers, which are contraindicated in these patients, and instead began treatment with a calcium channel antagonist, he said.
“CorMicA was a landmark trial. The interventional cardiologists at my center believe that the testing [for microvascular and vasospastic angina] should be used routinely, based on the reported data,” said Harmony R. Reynolds, MD, a cardiologist at NYU Langone Health in New York.
The CorMicA (Coronary Microvascular Angina) trial enrolled 391 patients at either of two hospitals in Scotland during November 2016–November 2017. All patients were scheduled for clinically indicated, elective diagnostic angiography for suspected stable angina. Clinicians identified a coronary obstruction in 206 patients that excluded them from the main CorMicA analysis. They focused on the 181 without coronary obstruction visible by angiography, and specifically the 151 of these patients who underwent the invasive diagnostic procedure, with 76 randomized to have their diagnostic-procedure results relayed to their treating physicians and 75 patients whose diagnostic results remained blinded. The study’s primary endpoint was the mean difference in angina severity at 6 months after treatment assessed by the Seattle Angina Questionnaire (SAQ) summary score. After 6 months, the between-group difference in average SAQ summary scores was 11.7 units, a statistically significant as well as clinically meaningful benefit that linked with knowledge of the coronary function of patients based on invasive testing (J Am Coll Cardiol. 2018 Dec 11;72[23 Part A]:2841-55).
The prespecified 1-year follow-up with another SAQ took place for 142 of the 151 randomized patients, and showed ongoing separation of SAQ scores between the intervention and control patients, reaching a statistically significant mean difference of 13.6 units, Dr. Berry reported. Other benefits that linked with dissemination of results from the invasive diagnostic procedure included maintenance of the quality-of-life benefit seen after 6 months, an incremental further reduction in illness perception from 6 to 12 months, and a substantial further improvement in global treatment satisfaction, which grew from a 30% higher level in the intervention group compared with controls at 6 months to a 44% between-group difference after 12 months. Concurrently with Dr. Berry’s report, the results appeared online (JACC: Cardiovasc Interv. 2020 Jan 13;13[1] 33-45).
Additional findings for various clinical measures that were not part of the 6-month findings provided further insight into the impact that clinician knowledge about microvascular disease or vasospasm had on patients. These metrics suggested that the benefits in the intervention patients may have largely resulted from their avoiding elevations in risk markers that occurred among controls who received routine care.
For example, after 12 months, average 12/5 mm Hg differences in systolic and diastolic blood pressures between the two arms was nearly all because of pressure increases from baseline in the control patients with only very small drops in pressure from baseline among the intervention patients. Weight measurements after 1 year showed that the control patients were on average 1.3 kg heavier than the intervention patients, again mostly because of weight increases among the controls. Enrollment in a cardiac rehabilitation program at 12 months had occurred for 40% of the intervention patients and 16% of the controls, a 73% relative increase in the intervention arm, Dr. Berry reported.
The invasive, coronary, physiological assessments used in CorMicA led to stratified medical therapy and created an opportunity for better long-term angina treatment in patients without obstructive coronary disease, Dr. Berry concluded. He stressed the need to further test this hypothesis in larger, prospective studies, and to assess its cost effectiveness. But the proof-of-concept demonstrated by the CorMicA results indicate a role for more thorough investigation of coronary dysfunction when obstructive disease is absent, with the opportunity to use this information to better tailor treatment. The findings also highlight the potential for new drug development that can address nonobstructive microvascular or vasospastic coronary disease, he said.
Dr. Berry has been a speaker on behalf of Abbott Vascular, and he has received research funding from AstraZeneca, GlaxoSmithKline, HeartFlow, Novartis, and Siemens Healthcare. Dr. Reynolds had no disclosures.
SOURCE: Ford TJ. AHA 2019, session FS.AOS.03 abstract.
PHILADELPHIA – Longer and more extensive follow-up of the positive CorMicA trial confirmed that clinicians who receive added information on the presence or absence of microvascular coronary disease or coronary vasospasm in patients with chronic, stable angina but without detectable coronary obstruction adjust patient treatment in ways that produce better outcomes.
For example, expanded 1-year follow-up of the 151-patient, multicenter, CorMicA randomized study showed that stable angina patients without coronary obstruction treated by clinicians aware of microvascular or vasospastic coronary disease had blood pressures that averaged about 12/5 mm Hg lower than those of patients in the control arm, in which this information was kept blinded.
Clinicians in the study who received information about diagnoses of microvascular angina, vasospastic angina, both, or neither, generally better tailored the treatments they gave these patients, were more likely to prescribe cardiac rehabilitation to these patients, and successfully capped increases in blood pressure that occurred in the control patients, Colin Berry, MD, said at the American Heart Association scientific sessions. The investigators made the diagnoses of microvascular and vasospastic angina using an “invasive diagnostic procedure” that involved placing a wire with pressure and temperature sensors in a selected coronary artery and measuring flow patterns that resulted from a systemic infusion of adenosine and from incremental, intracoronary infusions of acetylcholine.
The CorMicA results “tell us that, if we use existing drugs and nonpharmacologic treatments, we can improve patients’ quality of life,” said Dr. Berry, co-lead investigator of the study and professor of cardiology and imaging at the University of Glasgow. “There were probably two drivers of change in treatment” between the intervention arm where clinicians learned whether their patients had microvascular angina or vasospasm, and the control arm where this information was kept blinded. Some patients reclassified as having microvascular disease or vasospasm were restarted on standard agents for treatment of coronary artery disease such as statins and ACE inhibitors that had previously been stopped, noted Dr. Berry. The identification of a specific type of vascular disorder also helped guide treatment. For example, patients identified as having vasospasm were taken off of beta-blockers, which are contraindicated in these patients, and instead began treatment with a calcium channel antagonist, he said.
“CorMicA was a landmark trial. The interventional cardiologists at my center believe that the testing [for microvascular and vasospastic angina] should be used routinely, based on the reported data,” said Harmony R. Reynolds, MD, a cardiologist at NYU Langone Health in New York.
The CorMicA (Coronary Microvascular Angina) trial enrolled 391 patients at either of two hospitals in Scotland during November 2016–November 2017. All patients were scheduled for clinically indicated, elective diagnostic angiography for suspected stable angina. Clinicians identified a coronary obstruction in 206 patients that excluded them from the main CorMicA analysis. They focused on the 181 without coronary obstruction visible by angiography, and specifically the 151 of these patients who underwent the invasive diagnostic procedure, with 76 randomized to have their diagnostic-procedure results relayed to their treating physicians and 75 patients whose diagnostic results remained blinded. The study’s primary endpoint was the mean difference in angina severity at 6 months after treatment assessed by the Seattle Angina Questionnaire (SAQ) summary score. After 6 months, the between-group difference in average SAQ summary scores was 11.7 units, a statistically significant as well as clinically meaningful benefit that linked with knowledge of the coronary function of patients based on invasive testing (J Am Coll Cardiol. 2018 Dec 11;72[23 Part A]:2841-55).
The prespecified 1-year follow-up with another SAQ took place for 142 of the 151 randomized patients, and showed ongoing separation of SAQ scores between the intervention and control patients, reaching a statistically significant mean difference of 13.6 units, Dr. Berry reported. Other benefits that linked with dissemination of results from the invasive diagnostic procedure included maintenance of the quality-of-life benefit seen after 6 months, an incremental further reduction in illness perception from 6 to 12 months, and a substantial further improvement in global treatment satisfaction, which grew from a 30% higher level in the intervention group compared with controls at 6 months to a 44% between-group difference after 12 months. Concurrently with Dr. Berry’s report, the results appeared online (JACC: Cardiovasc Interv. 2020 Jan 13;13[1] 33-45).
Additional findings for various clinical measures that were not part of the 6-month findings provided further insight into the impact that clinician knowledge about microvascular disease or vasospasm had on patients. These metrics suggested that the benefits in the intervention patients may have largely resulted from their avoiding elevations in risk markers that occurred among controls who received routine care.
For example, after 12 months, average 12/5 mm Hg differences in systolic and diastolic blood pressures between the two arms was nearly all because of pressure increases from baseline in the control patients with only very small drops in pressure from baseline among the intervention patients. Weight measurements after 1 year showed that the control patients were on average 1.3 kg heavier than the intervention patients, again mostly because of weight increases among the controls. Enrollment in a cardiac rehabilitation program at 12 months had occurred for 40% of the intervention patients and 16% of the controls, a 73% relative increase in the intervention arm, Dr. Berry reported.
The invasive, coronary, physiological assessments used in CorMicA led to stratified medical therapy and created an opportunity for better long-term angina treatment in patients without obstructive coronary disease, Dr. Berry concluded. He stressed the need to further test this hypothesis in larger, prospective studies, and to assess its cost effectiveness. But the proof-of-concept demonstrated by the CorMicA results indicate a role for more thorough investigation of coronary dysfunction when obstructive disease is absent, with the opportunity to use this information to better tailor treatment. The findings also highlight the potential for new drug development that can address nonobstructive microvascular or vasospastic coronary disease, he said.
Dr. Berry has been a speaker on behalf of Abbott Vascular, and he has received research funding from AstraZeneca, GlaxoSmithKline, HeartFlow, Novartis, and Siemens Healthcare. Dr. Reynolds had no disclosures.
SOURCE: Ford TJ. AHA 2019, session FS.AOS.03 abstract.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
SPRINT-type BP control provides up to 3 years of additional life
PHILADELPHIA – in age-dependent fashion, compared with the older target standard BP, according to a novel analysis of data from the landmark SPRINT trial.
SPRINT randomized 9,361 hypertensive patients aged 50 years or older with at least one additional cardiovascular risk factor to intensive control with a target systolic BP of less than 120 mm Hg or to the then-standard target of less than 140 mm Hg. The trial was stopped early for ethical reasons when an interim analysis showed intensive control was associated with a 27% reduction in mortality. But that 27% reduction in mortality risk is a tough concept for many patients to interpret in practical terms, Muthiah Vaduganathan, MD, observed at the American Heart Association scientific sessions.
So he and his coinvestigators sliced and diced the mountainous SPRINT data in a novel way, using an actuarial statistical analysis.
“These actuarial data from SPRINT support the survival benefits of intensive blood pressure control, especially when initiated in middle-aged, high-risk adults. Our analysis really reaffirms the original SPRINT trial results [N Engl J Med. 2015 Nov 26;373(22):2103-16] and helps present them in an alternative format that can potentially be more easily communicated to clinicians, patients, and the public at large,” explained Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
The impact of intensive BP control on residual survival was magnified in patients who were younger, since they intrinsically have a longer expected survival and will apply the antihypertensive regimen over a longer period. For example, the actuarial analysis concluded that the mean survival benefit of starting intensive BP lowering, rather than settling for a target systolic BP of less than 140 mm Hg starting at age 50 years, was 3 additional years of life, as compared with 1.1 additional years in 65-year-olds and 0.5 years in patients aged 85 years or older. The same approach can be applied to patients at any individual age from 50 to 95 years at the time of enrollment.
“This is very helpful in conveying messages to individual patients. Often if you tell a patient: ‘Your risk is going to go down by 27%,’ it’s tough for them to recognize what the baseline is and if that actually applied to them. So this may personalize that decision-making conversation,” according to the cardiologist.
One audience member commented that this SPRINT analysis might actually underestimate the true survival advantage of intensive BP lowering. He noted that SPRINT, which was halted after an average of 3.3 years, didn’t show a significant benefit for intensive BP lowering in terms of stroke reduction, whereas the ACCORD trial did, but that benefit didn’t occur until after 3 years into the study (Hypertension. 2018 Aug;72[2]:323-30).
Dr. Vaduganathan conceded that’s a limitation of his analysis.
“The assumption we’ve used is that long-term cardiovascular benefits are going to be as seen in the trial, but since SPRINT was stopped early, some benefits may be exaggerated and some may not have been observed yet,” he agreed.
Another audience member observed, “I think a lot of patients will think: ‘Okay, you’re tacking on a year at the end, when I’m going to be 89 and demented.’ The National Institute on Aging is focusing a lot more now on nondisabled life expectancy or healthy life expectancy.’”
Dr. Vaduganathan offered a degree of reassurance on this score. Because of time limitations, he said, he only presented the life expectancy results. But he and his coworkers have performed the same actuarial analysis of the SPRINT data for other endpoints related to freedom from various forms of disease or disability and found a consistent effect: Intensive BP control was associated with a longer time to onset of morbidity.
SPRINT was sponsored primarily by the National Heart, Lung, and Blood Institute. Dr. Vaduganathan reported that he receives research support from/and or serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer, Boehringer Ingelheim, and Relypsa.
SOURCE: Vaduganathan M et al. AHA 2019, Abstract MDP233.
PHILADELPHIA – in age-dependent fashion, compared with the older target standard BP, according to a novel analysis of data from the landmark SPRINT trial.
SPRINT randomized 9,361 hypertensive patients aged 50 years or older with at least one additional cardiovascular risk factor to intensive control with a target systolic BP of less than 120 mm Hg or to the then-standard target of less than 140 mm Hg. The trial was stopped early for ethical reasons when an interim analysis showed intensive control was associated with a 27% reduction in mortality. But that 27% reduction in mortality risk is a tough concept for many patients to interpret in practical terms, Muthiah Vaduganathan, MD, observed at the American Heart Association scientific sessions.
So he and his coinvestigators sliced and diced the mountainous SPRINT data in a novel way, using an actuarial statistical analysis.
“These actuarial data from SPRINT support the survival benefits of intensive blood pressure control, especially when initiated in middle-aged, high-risk adults. Our analysis really reaffirms the original SPRINT trial results [N Engl J Med. 2015 Nov 26;373(22):2103-16] and helps present them in an alternative format that can potentially be more easily communicated to clinicians, patients, and the public at large,” explained Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
The impact of intensive BP control on residual survival was magnified in patients who were younger, since they intrinsically have a longer expected survival and will apply the antihypertensive regimen over a longer period. For example, the actuarial analysis concluded that the mean survival benefit of starting intensive BP lowering, rather than settling for a target systolic BP of less than 140 mm Hg starting at age 50 years, was 3 additional years of life, as compared with 1.1 additional years in 65-year-olds and 0.5 years in patients aged 85 years or older. The same approach can be applied to patients at any individual age from 50 to 95 years at the time of enrollment.
“This is very helpful in conveying messages to individual patients. Often if you tell a patient: ‘Your risk is going to go down by 27%,’ it’s tough for them to recognize what the baseline is and if that actually applied to them. So this may personalize that decision-making conversation,” according to the cardiologist.
One audience member commented that this SPRINT analysis might actually underestimate the true survival advantage of intensive BP lowering. He noted that SPRINT, which was halted after an average of 3.3 years, didn’t show a significant benefit for intensive BP lowering in terms of stroke reduction, whereas the ACCORD trial did, but that benefit didn’t occur until after 3 years into the study (Hypertension. 2018 Aug;72[2]:323-30).
Dr. Vaduganathan conceded that’s a limitation of his analysis.
“The assumption we’ve used is that long-term cardiovascular benefits are going to be as seen in the trial, but since SPRINT was stopped early, some benefits may be exaggerated and some may not have been observed yet,” he agreed.
Another audience member observed, “I think a lot of patients will think: ‘Okay, you’re tacking on a year at the end, when I’m going to be 89 and demented.’ The National Institute on Aging is focusing a lot more now on nondisabled life expectancy or healthy life expectancy.’”
Dr. Vaduganathan offered a degree of reassurance on this score. Because of time limitations, he said, he only presented the life expectancy results. But he and his coworkers have performed the same actuarial analysis of the SPRINT data for other endpoints related to freedom from various forms of disease or disability and found a consistent effect: Intensive BP control was associated with a longer time to onset of morbidity.
SPRINT was sponsored primarily by the National Heart, Lung, and Blood Institute. Dr. Vaduganathan reported that he receives research support from/and or serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer, Boehringer Ingelheim, and Relypsa.
SOURCE: Vaduganathan M et al. AHA 2019, Abstract MDP233.
PHILADELPHIA – in age-dependent fashion, compared with the older target standard BP, according to a novel analysis of data from the landmark SPRINT trial.
SPRINT randomized 9,361 hypertensive patients aged 50 years or older with at least one additional cardiovascular risk factor to intensive control with a target systolic BP of less than 120 mm Hg or to the then-standard target of less than 140 mm Hg. The trial was stopped early for ethical reasons when an interim analysis showed intensive control was associated with a 27% reduction in mortality. But that 27% reduction in mortality risk is a tough concept for many patients to interpret in practical terms, Muthiah Vaduganathan, MD, observed at the American Heart Association scientific sessions.
So he and his coinvestigators sliced and diced the mountainous SPRINT data in a novel way, using an actuarial statistical analysis.
“These actuarial data from SPRINT support the survival benefits of intensive blood pressure control, especially when initiated in middle-aged, high-risk adults. Our analysis really reaffirms the original SPRINT trial results [N Engl J Med. 2015 Nov 26;373(22):2103-16] and helps present them in an alternative format that can potentially be more easily communicated to clinicians, patients, and the public at large,” explained Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
The impact of intensive BP control on residual survival was magnified in patients who were younger, since they intrinsically have a longer expected survival and will apply the antihypertensive regimen over a longer period. For example, the actuarial analysis concluded that the mean survival benefit of starting intensive BP lowering, rather than settling for a target systolic BP of less than 140 mm Hg starting at age 50 years, was 3 additional years of life, as compared with 1.1 additional years in 65-year-olds and 0.5 years in patients aged 85 years or older. The same approach can be applied to patients at any individual age from 50 to 95 years at the time of enrollment.
“This is very helpful in conveying messages to individual patients. Often if you tell a patient: ‘Your risk is going to go down by 27%,’ it’s tough for them to recognize what the baseline is and if that actually applied to them. So this may personalize that decision-making conversation,” according to the cardiologist.
One audience member commented that this SPRINT analysis might actually underestimate the true survival advantage of intensive BP lowering. He noted that SPRINT, which was halted after an average of 3.3 years, didn’t show a significant benefit for intensive BP lowering in terms of stroke reduction, whereas the ACCORD trial did, but that benefit didn’t occur until after 3 years into the study (Hypertension. 2018 Aug;72[2]:323-30).
Dr. Vaduganathan conceded that’s a limitation of his analysis.
“The assumption we’ve used is that long-term cardiovascular benefits are going to be as seen in the trial, but since SPRINT was stopped early, some benefits may be exaggerated and some may not have been observed yet,” he agreed.
Another audience member observed, “I think a lot of patients will think: ‘Okay, you’re tacking on a year at the end, when I’m going to be 89 and demented.’ The National Institute on Aging is focusing a lot more now on nondisabled life expectancy or healthy life expectancy.’”
Dr. Vaduganathan offered a degree of reassurance on this score. Because of time limitations, he said, he only presented the life expectancy results. But he and his coworkers have performed the same actuarial analysis of the SPRINT data for other endpoints related to freedom from various forms of disease or disability and found a consistent effect: Intensive BP control was associated with a longer time to onset of morbidity.
SPRINT was sponsored primarily by the National Heart, Lung, and Blood Institute. Dr. Vaduganathan reported that he receives research support from/and or serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer, Boehringer Ingelheim, and Relypsa.
SOURCE: Vaduganathan M et al. AHA 2019, Abstract MDP233.
REPORTING FROM AHA 2019
COACT: No benefit of immediate PCI for non–ST-elevation cardiac arrest at 1 year
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
REPORTING FROM AHA 2019
Pharmacist BP telemonitoring cut cardiovascular events, turned profit
PHILADELPHIA – A home blood pressure telemonitoring program featuring pharmacist management of patients with uncontrolled hypertension reduced cardiovascular events by half and was cost saving over the course of 5 years, even though the intervention ended after year 1, Karen L. Margolis, MD, reported at the American Heart Association scientific sessions.
“The return on investment was 126%. That means that for every dollar spent on the intervention, that dollar was recouped by $1.00 plus another $1.26,” explained Dr. Margolis, a general internist who serves as executive director for research at the HealthPartners Institute in Bloomington, Minn., and professor of medicine at the University of Minnesota, Minneapolis.
She presented 5-year follow-up data from the Hyperlink (Home Blood Pressure Telemonitoring and Case Management to Control Hypertension) study, a cluster randomized controlled trial involving 16 primary care clinics. Half of the clinics were randomized to the intervention, which entailed home blood pressure telemonitoring and pharmacist-led case management in collaboration with the primary care team. The other eight clinics provided usual care. The intervention portion of the trial, which lasted for 12 months, included 450 adults with uncontrolled hypertension as defined by repeated on-treatment blood pressure readings of 140/90 mm Hg or more. Participants’ baseline mean blood pressure was 148/85 mm Hg while on an average of one and a half antihypertensive drug classes. On average, pharmacists ended up adding one additional drug from a different antihypertensive drug class to achieve improved blood pressure control.
The details of the intervention and the short-term blood pressure results have previously been reported (JAMA. 2013 Jul 3;310[1]:46-56). Briefly, 6 months into the study, patients in the intervention arm averaged 11/6 mm Hg lower blood pressure than did the usual care controls. At 12 months – when the intervention ended – the between-group difference was similar at 10/5 mm Hg. At 18 months, the difference, while attenuated, remained significant at 7/3 mm Hg in favor of the intervention group. However, at 54 months, the intervention group’s advantage – a 3–mm Hg lower SBP and a 1–mm Hg lower DBP than in controls – was no longer significant.
The exciting new findings Dr. Margolis presented at the AHA scientific sessions focused on 5-year outcomes. Since HealthPartners is an integrated health care system, follow-up was essentially complete.
“None of the other telemetry studies I’m aware of have published anything on cardiovascular events. And we were somewhat surprised when we looked at our data to see fairly substantial differences in our primary outcome,” she noted.
That outcome was a composite of MI, stroke, heart failure, or cardiovascular death occurring over 5 years. The rate was 4.4% in the intervention group and nearly double at 8.6% in controls. That translated to a 51% relative risk reduction. The biggest difference was in stroke: 4 cases in the intervention arm, 12 in usual care controls.
The 5-year coronary revascularization rate was 5.3% in the intervention arm and 10.4% in controls, for a 52% relative risk reduction.
A major caveat regarding the Hyperlink trial was that, even at 450 patients and 5 years of follow-up, the study was underpowered to show significant differences in event rates, with P =.09 for the primary endpoint.
That being said, the financial results were striking. The intervention cost $1,511 per patient in 2017 U.S. dollars. The cost of treatment for major adverse cardiovascular events totaled $758,000 in the intervention group and $1,538,000 in usual care controls. That works out to $3,420 less per patient in the intervention arm. Offset by the cost of the intervention, that spells a net savings of $1,908 per patient achieved by implementing the year-long intervention. It’s a rare instance in health care of an intervention that actually makes money.
These results were unusual enough that Dr. Margolis and her coinvestigators decided to feed their wealth of SBP readings into a microsimulation model, which they ran 1,000 times. The model predicted – in light of the fact that patients in the intervention group were on average 2 years older than the controls were – that the expected reduction in the primary endpoint was 12% rather than the observed 51% relative risk reduction.
How to explain the discrepancy? The Hyperlink results could have been due to chance. Or it could be, Dr. Margolis surmised, that the pharmacists helped accomplish improvements in other cardiovascular risk factors, such as hyperlipidemia, smoking, or sedentary behavior. That’s unknown, since the investigators focused on changes in blood pressure only. Future studies of home telemonitoring and pharmacist case management of uncontrolled hypertension should be powered to detect significant differences in cardiovascular events and should track additional risk factors, she concluded.
She reported having no financial conflicts regarding the study.
SOURCE: Margolis KL. AHA 2019. Abstract MDP232.
PHILADELPHIA – A home blood pressure telemonitoring program featuring pharmacist management of patients with uncontrolled hypertension reduced cardiovascular events by half and was cost saving over the course of 5 years, even though the intervention ended after year 1, Karen L. Margolis, MD, reported at the American Heart Association scientific sessions.
“The return on investment was 126%. That means that for every dollar spent on the intervention, that dollar was recouped by $1.00 plus another $1.26,” explained Dr. Margolis, a general internist who serves as executive director for research at the HealthPartners Institute in Bloomington, Minn., and professor of medicine at the University of Minnesota, Minneapolis.
She presented 5-year follow-up data from the Hyperlink (Home Blood Pressure Telemonitoring and Case Management to Control Hypertension) study, a cluster randomized controlled trial involving 16 primary care clinics. Half of the clinics were randomized to the intervention, which entailed home blood pressure telemonitoring and pharmacist-led case management in collaboration with the primary care team. The other eight clinics provided usual care. The intervention portion of the trial, which lasted for 12 months, included 450 adults with uncontrolled hypertension as defined by repeated on-treatment blood pressure readings of 140/90 mm Hg or more. Participants’ baseline mean blood pressure was 148/85 mm Hg while on an average of one and a half antihypertensive drug classes. On average, pharmacists ended up adding one additional drug from a different antihypertensive drug class to achieve improved blood pressure control.
The details of the intervention and the short-term blood pressure results have previously been reported (JAMA. 2013 Jul 3;310[1]:46-56). Briefly, 6 months into the study, patients in the intervention arm averaged 11/6 mm Hg lower blood pressure than did the usual care controls. At 12 months – when the intervention ended – the between-group difference was similar at 10/5 mm Hg. At 18 months, the difference, while attenuated, remained significant at 7/3 mm Hg in favor of the intervention group. However, at 54 months, the intervention group’s advantage – a 3–mm Hg lower SBP and a 1–mm Hg lower DBP than in controls – was no longer significant.
The exciting new findings Dr. Margolis presented at the AHA scientific sessions focused on 5-year outcomes. Since HealthPartners is an integrated health care system, follow-up was essentially complete.
“None of the other telemetry studies I’m aware of have published anything on cardiovascular events. And we were somewhat surprised when we looked at our data to see fairly substantial differences in our primary outcome,” she noted.
That outcome was a composite of MI, stroke, heart failure, or cardiovascular death occurring over 5 years. The rate was 4.4% in the intervention group and nearly double at 8.6% in controls. That translated to a 51% relative risk reduction. The biggest difference was in stroke: 4 cases in the intervention arm, 12 in usual care controls.
The 5-year coronary revascularization rate was 5.3% in the intervention arm and 10.4% in controls, for a 52% relative risk reduction.
A major caveat regarding the Hyperlink trial was that, even at 450 patients and 5 years of follow-up, the study was underpowered to show significant differences in event rates, with P =.09 for the primary endpoint.
That being said, the financial results were striking. The intervention cost $1,511 per patient in 2017 U.S. dollars. The cost of treatment for major adverse cardiovascular events totaled $758,000 in the intervention group and $1,538,000 in usual care controls. That works out to $3,420 less per patient in the intervention arm. Offset by the cost of the intervention, that spells a net savings of $1,908 per patient achieved by implementing the year-long intervention. It’s a rare instance in health care of an intervention that actually makes money.
These results were unusual enough that Dr. Margolis and her coinvestigators decided to feed their wealth of SBP readings into a microsimulation model, which they ran 1,000 times. The model predicted – in light of the fact that patients in the intervention group were on average 2 years older than the controls were – that the expected reduction in the primary endpoint was 12% rather than the observed 51% relative risk reduction.
How to explain the discrepancy? The Hyperlink results could have been due to chance. Or it could be, Dr. Margolis surmised, that the pharmacists helped accomplish improvements in other cardiovascular risk factors, such as hyperlipidemia, smoking, or sedentary behavior. That’s unknown, since the investigators focused on changes in blood pressure only. Future studies of home telemonitoring and pharmacist case management of uncontrolled hypertension should be powered to detect significant differences in cardiovascular events and should track additional risk factors, she concluded.
She reported having no financial conflicts regarding the study.
SOURCE: Margolis KL. AHA 2019. Abstract MDP232.
PHILADELPHIA – A home blood pressure telemonitoring program featuring pharmacist management of patients with uncontrolled hypertension reduced cardiovascular events by half and was cost saving over the course of 5 years, even though the intervention ended after year 1, Karen L. Margolis, MD, reported at the American Heart Association scientific sessions.
“The return on investment was 126%. That means that for every dollar spent on the intervention, that dollar was recouped by $1.00 plus another $1.26,” explained Dr. Margolis, a general internist who serves as executive director for research at the HealthPartners Institute in Bloomington, Minn., and professor of medicine at the University of Minnesota, Minneapolis.
She presented 5-year follow-up data from the Hyperlink (Home Blood Pressure Telemonitoring and Case Management to Control Hypertension) study, a cluster randomized controlled trial involving 16 primary care clinics. Half of the clinics were randomized to the intervention, which entailed home blood pressure telemonitoring and pharmacist-led case management in collaboration with the primary care team. The other eight clinics provided usual care. The intervention portion of the trial, which lasted for 12 months, included 450 adults with uncontrolled hypertension as defined by repeated on-treatment blood pressure readings of 140/90 mm Hg or more. Participants’ baseline mean blood pressure was 148/85 mm Hg while on an average of one and a half antihypertensive drug classes. On average, pharmacists ended up adding one additional drug from a different antihypertensive drug class to achieve improved blood pressure control.
The details of the intervention and the short-term blood pressure results have previously been reported (JAMA. 2013 Jul 3;310[1]:46-56). Briefly, 6 months into the study, patients in the intervention arm averaged 11/6 mm Hg lower blood pressure than did the usual care controls. At 12 months – when the intervention ended – the between-group difference was similar at 10/5 mm Hg. At 18 months, the difference, while attenuated, remained significant at 7/3 mm Hg in favor of the intervention group. However, at 54 months, the intervention group’s advantage – a 3–mm Hg lower SBP and a 1–mm Hg lower DBP than in controls – was no longer significant.
The exciting new findings Dr. Margolis presented at the AHA scientific sessions focused on 5-year outcomes. Since HealthPartners is an integrated health care system, follow-up was essentially complete.
“None of the other telemetry studies I’m aware of have published anything on cardiovascular events. And we were somewhat surprised when we looked at our data to see fairly substantial differences in our primary outcome,” she noted.
That outcome was a composite of MI, stroke, heart failure, or cardiovascular death occurring over 5 years. The rate was 4.4% in the intervention group and nearly double at 8.6% in controls. That translated to a 51% relative risk reduction. The biggest difference was in stroke: 4 cases in the intervention arm, 12 in usual care controls.
The 5-year coronary revascularization rate was 5.3% in the intervention arm and 10.4% in controls, for a 52% relative risk reduction.
A major caveat regarding the Hyperlink trial was that, even at 450 patients and 5 years of follow-up, the study was underpowered to show significant differences in event rates, with P =.09 for the primary endpoint.
That being said, the financial results were striking. The intervention cost $1,511 per patient in 2017 U.S. dollars. The cost of treatment for major adverse cardiovascular events totaled $758,000 in the intervention group and $1,538,000 in usual care controls. That works out to $3,420 less per patient in the intervention arm. Offset by the cost of the intervention, that spells a net savings of $1,908 per patient achieved by implementing the year-long intervention. It’s a rare instance in health care of an intervention that actually makes money.
These results were unusual enough that Dr. Margolis and her coinvestigators decided to feed their wealth of SBP readings into a microsimulation model, which they ran 1,000 times. The model predicted – in light of the fact that patients in the intervention group were on average 2 years older than the controls were – that the expected reduction in the primary endpoint was 12% rather than the observed 51% relative risk reduction.
How to explain the discrepancy? The Hyperlink results could have been due to chance. Or it could be, Dr. Margolis surmised, that the pharmacists helped accomplish improvements in other cardiovascular risk factors, such as hyperlipidemia, smoking, or sedentary behavior. That’s unknown, since the investigators focused on changes in blood pressure only. Future studies of home telemonitoring and pharmacist case management of uncontrolled hypertension should be powered to detect significant differences in cardiovascular events and should track additional risk factors, she concluded.
She reported having no financial conflicts regarding the study.
SOURCE: Margolis KL. AHA 2019. Abstract MDP232.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS





















