User login
FUEL trial: Post-Fontan udenafil shows mixed results
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
REPORTING FROM AHA 2019
PACT-HF: Transitional care derives no overall benefit
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
Women respond more to intervention
Women respond more to intervention
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
PHILADELPHIA – A clinical trial of a program that transitions heart failure patients after they’re discharged from the hospital didn’t result in any appreciable improvement in all-cause death, readmissions or emergency department visits after 6 months overall, but it did show that women responded more favorably than men.
Harriette G.C. Van Spall, MD, MPH, reported 6-month results of the Patient-Centered Transitional Care Services in Heart Failure (PACT-HF) trial of 2,494 HF patients at 10 hospitals in Ontario during February 2015 to March 2016. They were randomized to the care-transition program or usual care. The findings, she said at the American Heart Association scientific sessions, “highlight the gap between efficacy that’s often demonstrated in mechanistic clinical trials and effectiveness when we aim to implement these results in real-world settings.” Three-month PACT-HF results were reported previously (JAMA. 2019 Feb 26;321:753-61).
The transitional-care model consisted of a comprehensive needs assessment by a nurse who also provided self-care education, a patient-centered discharge summary, and follow-up with a family physician within 7 days of discharge, which Dr. Van Spall noted “is not current practice in our health care system.”
Patients deemed high risk for readmission or death also received nurse home visits and scheduled visits to a multidisciplinary heart function clinic within 2-4 weeks of discharge and continuing as long as clinically suitable, said Dr. Van Spall, a principal investigator at the Population Health Research Institute, Hamilton, Ont., and assistant professor in cardiology at McMaster University in Hamilton.
The trial found no difference between the intervention and usual-care groups in the two composite endpoints at 6 months, Dr. Van Spall said: all-cause death, readmissions, or ED visits (63.1% and 64.5%, respectively; P = .50); or all-cause readmissions or ED visits (60.8% and 62.4%; P = .36).
“Despite the mutual overall clinical outcomes, we noted specific differences in response to treatment,” she said. With regard to the composite endpoint that included all-cause death, “Men had an attenuated response to the treatment with a hazard ratio of 1.05 (95% confidence interval, 0.87-1.26), whereas women had a hazard ratio of 0.85 (95% CI, 0.71-1.03), demonstrating that women have more of a treatment response to this health care service,” she said.
In men, rates for the first primary composite outcome were 66.3% and 64.1% in the intervention and usual-care groups, whereas in women those rates were 59.9% and 64.8% (P = .04 for sex interaction).
In the second composite endpoint, all-cause readmission or ED visit, “again, men had an attenuated response” with a HR of 1.03, whereas women had a HR of 0.83. Results were similar to those for the first primary composite outcome: 63.4% and 61.7% for intervention and usual care in men and 57.7% and 63% in women (P = .03 for sex interaction).
In putting the findings into context, Dr. Van Spall said tailoring services to risk in HF patients may be fraught with pitfalls. “We delivered intensive services to those patients at high risk of readmission or death, but it is quite possible they are the least likely to derive benefit by virtue of their advanced heart failure,” she said. “It may be that more benefit would have been derived had we chosen low- or moderate-risk patients to receive the intervention.”
She also said the sex-specific outcomes must be interpreted with caution. “But they do give us pause to consider that services could be titrated more effectively if delivered to patients who are more likely to derive benefit,” Dr. Van Spall said. The finding that women derived more of a benefit is in line with other prospective and observational studies that have found that women have a higher sense of self-care, self-efficacy, and confidence in managing their own health care needs than men.
Dr. Van Spall has no financial relationships to disclose.
REPORTING FROM AHA 2019
Analyses clarify who benefits from ARNI-ARB combination
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – Two clinical trials of the combination therapy of the neprilysin inhibitor sacubitril and the angiotensin II receptor blocker valsartan in patients with heart failure and reduced ejection fraction found that it lowered rates of all-cause death, compared to a renin-angiotensin-system inhibitor alone.
Furthermore, the treatment produced a more beneficial effect in women, who are more prone to heart failure and preserved ejection fraction (HFpEF), lead investigators reported at the American Heart Association scientific sessions.
A prespecified subgroup analysis of 4,796 patients in the PARAGON-HF trial found that the sacubitril/valsartan, or sac/val, combination had a significantly more beneficial risk reduction of first and recurrent hospitalizations for heart failure, as well as cardiovascular death, in women than men. A prespecified pooled analysis of 13,195 patients in the PARAGON-HF and the PARADIGM-HF trials also found women derived a greater benefit from the combination therapy than men, but also concluded that patients with heart failure and even mildly reduced ejection fraction had better outcomes. The results of both studies were published simultaneously with the presentations on Nov. 17 in Circulation (doi: 10.1161/circulationaha.119.044491; doi: 10.1161/circulationaha.119.044586).
The findings underscore the effectiveness of sac/val combination in patients with HF and EF in the lower ranges, defined as 40% or less, commented discussant Lynne Warner Stevenson, MD, of Vanderbilt Heart and Vascular Institute in Nashville, Tenn. “We all agree now that the use of sacubitril/valsartan is very appropriate to improve outcomes in those patients, even if they’ve never been hospitalized,” she said in an interview.
PARAGON-HF subanalysis
John J.V. McMurray, MD, of the University of Glasgow presented the PARAGON-HF subgroup analysis. He said it initially focused on 12 subgroups, but that only two baseline variables showed a modified effect of sac/val: sex and left-ventricle ejection fraction (LVEF). The findings, he said, “stood up in a very robust, multivariable analysis.”
The women in the subgroup analysis were older, had higher baseline New York Heart Association class status, and worse quality of life as measured by Kansas City Cardiomyopathy Questionnaire clinical summary score. At baseline, women also had higher average LVEF (59% vs. 56%), lower N-terminal prohormone brain natriuretic peptide levels, and higher rates of renal dysfunction and chronic kidney disease, but lower incidence of a previous MI and coronary artery disease. Prestudy treatments were similar between the sexes.
In terms of the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – “there was an apparent 27% relative risk reduction in women and no overall effect in men,” Dr. McMurray said of the treatment group. “The difference was driven completely by hospitalizations.” Rates of CV death were similar between the valsartan-only and sac/val groups in both men and women, he said.
In the analysis of LVEF, women in the treatment group seemed to cross over to a heightened risk of hospitalization and CV death at an LVEF in the 60%-65% range, Dr. McMurray said, whereas men made that cross over in the 50%-55% range. “It looks as though women might be getting more benefit from this treatment up to a higher EF than in men,” he said.
However, the differences between men and women did not hold up in the analysis of secondary outcomes. At 8 months, women in the sac/val group had a 0.6-point greater decline than did the valsartan-only patients in KCCQ-CSS score, whereas men on sac/val had a 2.8-point lesser decline than did those on valsartan only. Similar differences were seen between the treatment and valsartan-only groups within the sexes, with women showing a noticeable improvement surpassing the men.
Posttreatment hypotension rates in both sexes were higher in the sac/val groups, and the risk of renal dysfunction was a bit less in both treatment groups. Women in the treatment group had significantly higher rates of angioedema than did the valsartan-only group and men in either group.
“Compared to valsartan, it’s important to say that sacubitril/valsartan seemed to reduce the risk of heart failure and hospitalization more in women than men, but we didn’t find a similar differential for other endpoints,” Dr. McMurray said. “Therefore, we’re not sure this is a real effect or a chance finding. It’s very statistically robust, but it could still be a chance finding.”
A possible explanation could be than men may not be responding to sac/val, or that valsartan alone may be more effective in men than women, he said. “This possible effect modification of sac/val vs. valsartan by sex deserves further investigation,” he said.
PARAGON-HF and PARADIGM-HF pooled analysis
Likewise, the prespecified pooled analysis of the PARADIGM-HF and PARAGON-HF trials found a greater benefit of sac/val in women, according to results presented by Scott D. Solomon, MD, of Brigham and Women’s Hospital in Boston. Where PARAGON-HF compared combination therapy with valsartan 160 mg twice daily alone, PARADIGM-HF used enalapril 10 mg twice daily alone as the comparator renin-angiotensin-system (RAS) inhibitor.
“These data suggest that the therapeutic effect of sacubitril/valsartan vs. RAS inhibition alone appear to extend to patients with heart failure and mildly reduced EF, with therapeutic benefits that extend to a higher left-ventricle EF range in women compared to men,” Dr. Solomon said.
The pooled analysis divided patients into six different EF groups: up to 22.5%, then in 10-point increments from 22.5% to 62.5%, and 62.5% or greater. PARADIGM-HF enrolled patients age 18 years and older, whereas PARAGON-HF involved those aged 50 years and older.
The analysis showed that, as LVEF rates increased across the EF groups, the rates of the primary composite outcome – HF hospitalizations, CV death, and all-cause mortality – decreased, but the decline was greatest for CV death and less so for HF hospitalization. And while rates of all-cause mortality decreased as EF increased, rates of non-CV death increased substantially with increasing LVEF.
“For each of these endpoints, there are significant benefits to sacubitril/valsartan in the pooled analysis, and this includes HF hospitalization, CV death, either total or first events, and all-cause mortality, which was reduced overall by 12% in the combination group,” Dr. Solomon said. That benefit was seen in the first five categories of EF, but all but disappeared in the highest category (at least 62.5%), he said.
At the lower end of the EF spectrum, the effect of sac/val is more pronounced and similar for men and women, Dr. Solomon said. “But as EF goes up, we see an attenuation of that effect in both men and women, but it occurs at a different point,” he said. “Women seem to derive a benefit to a higher ejection fraction than men.” As in Dr. McMurray’s research, the benefit seems to extend to LVEF of 55%-60% in men and 65%-70% in women.
“These findings were driven by an observed benefit in patients with chronic heart failure and LVEF below the normal range,” he said. “The benefit in the EF range above the ranking ‘reduced’ but below normal was driven primarily by reduction in HF hospitalization.”
Dr. Stevenson said that these findings indicate that a previous hospitalization for HF with preserved EF may be a telling marker for the effectiveness of sac/val. “As opposed to the patient who has exertional dyspnea but has never decompensated to the level needing hospitalization, if they have pEF, our current analyses would suggest sac/val may not offer them much benefit,” she said.
In real-world practice, cost would be an issue, Dr. Stevenson said. “This drug is very expensive; the majority of patients pay more than $100 a month in out-of-pocket costs, and we have to recognize this is not a therapy that everyone can afford,” she said in an interview. “In many areas, and particularly in the disadvantaged populations, this is not going to be a therapy that we’re going to be able to offer everyone, and that gives me great concern as we move toward trying to treat the whole disease that we’re developing therapies that will be limited by finance rather than by physiology. That’s a major call to action for all of us.”
Novartis sponsored the studies. Dr. McMurray has no disclosures. Dr. Solomon disclosed financial relationships with trial sponsor Novartis along with numerous pharmaceutical companies and the National Heart, Lung, and Blood Institute.
SOURCE: McMurray JJ and Solomon SD. AHA 2019, Late Breaking Science Session 5.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
ODYSSEY Outcomes: Alirocumab cut stroke, PAD, VTE
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
REPORTING FROM THE AHA 2019
New hypertension performance measures boost 130/80 mm Hg target
PHILADELPHIA – The American Heart Association and American College of Cardiology took a big step toward facilitating widespread U.S. application of the hypertension management guideline that the societies issued in 2017 by releasing a set of performance and quality measures for adults with high blood pressure based on the 2017 guideline.
This guideline notably set a treatment target for patients diagnosed with hypertension of less than 130/80 mg/dL, and also lowered the threshold for diagnosing stage 1 hypertension to a blood pressure at or above 130/80 mm Hg, adding in a stroke about 31 million adults with hypertension to the U.S. total.
Having performance and quality measures based on the guideline is “critical, because how else would you know whether you’re having an effect on accurately diagnosing and properly controlling hypertension?” said Donald E. Casey Jr., MD, chair of the performance measures writing committee. The next step is field testing of the measures “to show they are reliable and effective,” as well as other steps to encourage widespread U.S. uptake of the performance and quality measures and the specifics of the 2017 guideline, Dr. Casey said during a presentation of the revised measures at the American Heart Association scientific sessions.
He especially highlighted the important role of Target: BP, an education, recognition, and quality improvement program run by the AHA and American Medical Association, as a tool that medical practices, health systems, and even payers and employers can use to begin to apply the new performance and quality measures (J Am Coll Cardiol. 2019 Nov 26;74[21]:2661-706) and better align with the recommendations of the 2017 high blood pressure guideline (J Am Coll Cardiol. 2018 May;71[19]:e127-248).
“We’re trying now to promote Target: BP; it’s something you can take off the shelf and get going if it’s embedded in a real-life delivery model. I think Target: BP is the secret sauce. It will be the way we’ll convince people to adopt this,” said Dr. Casey, principal and founder of IPO 4 Health, a Chicago-based health care consulting firm.
He also advised practices and health systems not to feel compelled to introduce all of the specific performance and quality measures at once. “We don’t believe everyone has the resources to do all of it at once; the point is to move toward this system of care. We understand that people don’t have the resources to get it all done” immediately, Dr. Casey said in an interview.
A report during another session at the meeting documented the potential impact that Target: BP can have on blood pressure control within a health system. The Trinity Health of New England medical group based in Springfield, Mass., a system with about 140,000 patients – including 20,000 adults diagnosed with hypertension – and served by 230 health care providers in 13 offices in western Massachusetts, began using Target: BP’s MAP improvement program in its practices in November 2018. (MAP stands for measure accurately, act rapidly, and partner with patients.) Just before the MAP program began, 72% of patients diagnosed with hypertension in the medical group were at their goal blood pressure. Less than a year later, in September 2019, the hypertension control rate had jumped to 84%, a 12 percentage point improvement in control in practices that already had been doing a relatively good job, said Daniel W. Weiswasser, MD, director of quality and clinical informatics at Trinity Health of New England. Based on this success, Trinity Health plans to next involve the remaining regions of Trinity Health of New England in Target: BP, followed by the other regions of Trinity’s national organization, which operates in 21 states with nearly 4,000 staff physicians and about half a million patients diagnosed with hypertension, Dr. Weiswasser said.
“If clinicians do the three steps of the MAP then we will see substantial drops in blood pressures. It will occur,” declared Brent M. Egan, MD, vice president for cardiovascular disease prevention of the AMA in Greenville, S.C.
The new report includes six performance measures based on the strongest guideline recommendations and designed to document adherence levels for the purposes of public reporting and pay-for-performance programs. It also includes 16 quality measures designed for local quality review purposes, with 6 process quality measures and 10 structural quality measures. The report spells out that the authors designed the performance measures for use by major national organizations such as the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance (NCQA), while the quality measures are designed to support quality improvement efforts in any care-delivery setting.
The authors said that the writing committee is sensitive to the fact that the 2019 performance measures for controlling high blood pressure developed by the NCQA for the Healthcare Effectiveness Data and Information Set and currently in use in 2019 by CMS also does not incorporate the 2017 Hypertension Clinical Practice Guidelines classification scheme. “It is well understood that these measures are already in widespread use, especially for quality-related payment programs promulgated by CMS, such as the Medicare Advantage ‘Stars’ ratings, the Medicare Shared Savings Program, and the Physician Quality Payment Program, as well as many other programs promoted by commercial health insurers. In particular, the widespread use of the 2017 Hypertension Clinical Practice Guidelines classification scheme will also help to guide decision making about when to prescribe antihypertensive medications in accordance with its current recommendations for the ACC/AHA “stages” of stage 1 and stage 2 hypertension and elevated blood pressure,” they added.
The report also says that “the writing committee was sensitive to the fact that there is currently not complete consensus among other guidelines from the American College of Physicians and the American Academy of Family Physicians, and also the European Society of Cardiology and the European Society of Hypertension. Nonetheless, despite this ongoing debate, the writing committee felt strongly that it is now time to move the U.S. health care system ahead to reflect these differing points of view and expects that widespread use of this new measure set will help to achieve this goal.” The new report revises hypertension performance measures developed by the ACC and AHA in 2011 (J Am Coll Cardiol. 2011 Jul 12;58[3]: 316-36).
In short, the performance and quality measures give all the diverse components of the U.S. health care delivery system a road map for implementing the 2017 High Blood Pressure Guideline in a format that depends on those components electing to adopt and adhere to the 2017 guideline. (Although one of the new performance measures, 1a, harmonizes with an existing and widely applied performance measure.)
“Who is the audience for this, and how will they respond? These performance measures need to be appropriated” by health systems and by performance-assessment groups. “I hope the NCQA will adopt it,” said William C. Cushman, MD, professor of preventive medicine at the University of Tennessee Health Science Center in Memphis, and chief of preventive medicine at the Memphis Veterans Affairs Medical Center. “There are some negatives to performance measures, but on balance they have done good things and led to better care.” Dr. Cushman also approved of several specific performance and quality measures included in the report. “Most of what they emphasized is good,” particularly the importance of accurate pressure measurement, he said in an interview.
“Process drives outcomes” in hypertension management, and the new performance and quality measures “have some very good process metrics,” commented Dr. Egan. “I’d encourage health systems to select two or three measures that are key to what they do and make sense in their setting rather than try to implement it all at once,” he advised, echoing what Dr. Casey had suggested. “It’s ideal to do everything, but we know that if you give physicians a long list of performance measures they just get overwhelmed. The nice thing about hypertension is that we know that process drives outcomes. In the past, we’ve had some process metrics that did not drive outcomes. Getting these processes implemented will lead to better patient outcomes and save a ton of money.”
“We have introduced the 2017 guideline recommendations throughout Target: BP, but like any quality improvement program there is a question of how does it spread,” said Gregory Wozniak, PhD, director of outcomes analytics for the AMA in Chicago. “Our goal for Target: BP is to be impacting 20 million patients by 2021.”
Dr. Casey, Dr. Weiswasser, and Dr. Wozniak had no disclosures. Dr. Cushman has received honoraria as a speaker from Arbor and Sanofi-Aventis, and travel and research support from Eli Lilly. Dr. Egan has been a consultant to and speaker on behalf of Merck and a speaker for Emcure.
SOURCE: Casey DE et al. J Am Coll Cardiol. 2019 Nov 26;74[21]: 2661-706.
PHILADELPHIA – The American Heart Association and American College of Cardiology took a big step toward facilitating widespread U.S. application of the hypertension management guideline that the societies issued in 2017 by releasing a set of performance and quality measures for adults with high blood pressure based on the 2017 guideline.
This guideline notably set a treatment target for patients diagnosed with hypertension of less than 130/80 mg/dL, and also lowered the threshold for diagnosing stage 1 hypertension to a blood pressure at or above 130/80 mm Hg, adding in a stroke about 31 million adults with hypertension to the U.S. total.
Having performance and quality measures based on the guideline is “critical, because how else would you know whether you’re having an effect on accurately diagnosing and properly controlling hypertension?” said Donald E. Casey Jr., MD, chair of the performance measures writing committee. The next step is field testing of the measures “to show they are reliable and effective,” as well as other steps to encourage widespread U.S. uptake of the performance and quality measures and the specifics of the 2017 guideline, Dr. Casey said during a presentation of the revised measures at the American Heart Association scientific sessions.
He especially highlighted the important role of Target: BP, an education, recognition, and quality improvement program run by the AHA and American Medical Association, as a tool that medical practices, health systems, and even payers and employers can use to begin to apply the new performance and quality measures (J Am Coll Cardiol. 2019 Nov 26;74[21]:2661-706) and better align with the recommendations of the 2017 high blood pressure guideline (J Am Coll Cardiol. 2018 May;71[19]:e127-248).
“We’re trying now to promote Target: BP; it’s something you can take off the shelf and get going if it’s embedded in a real-life delivery model. I think Target: BP is the secret sauce. It will be the way we’ll convince people to adopt this,” said Dr. Casey, principal and founder of IPO 4 Health, a Chicago-based health care consulting firm.
He also advised practices and health systems not to feel compelled to introduce all of the specific performance and quality measures at once. “We don’t believe everyone has the resources to do all of it at once; the point is to move toward this system of care. We understand that people don’t have the resources to get it all done” immediately, Dr. Casey said in an interview.
A report during another session at the meeting documented the potential impact that Target: BP can have on blood pressure control within a health system. The Trinity Health of New England medical group based in Springfield, Mass., a system with about 140,000 patients – including 20,000 adults diagnosed with hypertension – and served by 230 health care providers in 13 offices in western Massachusetts, began using Target: BP’s MAP improvement program in its practices in November 2018. (MAP stands for measure accurately, act rapidly, and partner with patients.) Just before the MAP program began, 72% of patients diagnosed with hypertension in the medical group were at their goal blood pressure. Less than a year later, in September 2019, the hypertension control rate had jumped to 84%, a 12 percentage point improvement in control in practices that already had been doing a relatively good job, said Daniel W. Weiswasser, MD, director of quality and clinical informatics at Trinity Health of New England. Based on this success, Trinity Health plans to next involve the remaining regions of Trinity Health of New England in Target: BP, followed by the other regions of Trinity’s national organization, which operates in 21 states with nearly 4,000 staff physicians and about half a million patients diagnosed with hypertension, Dr. Weiswasser said.
“If clinicians do the three steps of the MAP then we will see substantial drops in blood pressures. It will occur,” declared Brent M. Egan, MD, vice president for cardiovascular disease prevention of the AMA in Greenville, S.C.
The new report includes six performance measures based on the strongest guideline recommendations and designed to document adherence levels for the purposes of public reporting and pay-for-performance programs. It also includes 16 quality measures designed for local quality review purposes, with 6 process quality measures and 10 structural quality measures. The report spells out that the authors designed the performance measures for use by major national organizations such as the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance (NCQA), while the quality measures are designed to support quality improvement efforts in any care-delivery setting.
The authors said that the writing committee is sensitive to the fact that the 2019 performance measures for controlling high blood pressure developed by the NCQA for the Healthcare Effectiveness Data and Information Set and currently in use in 2019 by CMS also does not incorporate the 2017 Hypertension Clinical Practice Guidelines classification scheme. “It is well understood that these measures are already in widespread use, especially for quality-related payment programs promulgated by CMS, such as the Medicare Advantage ‘Stars’ ratings, the Medicare Shared Savings Program, and the Physician Quality Payment Program, as well as many other programs promoted by commercial health insurers. In particular, the widespread use of the 2017 Hypertension Clinical Practice Guidelines classification scheme will also help to guide decision making about when to prescribe antihypertensive medications in accordance with its current recommendations for the ACC/AHA “stages” of stage 1 and stage 2 hypertension and elevated blood pressure,” they added.
The report also says that “the writing committee was sensitive to the fact that there is currently not complete consensus among other guidelines from the American College of Physicians and the American Academy of Family Physicians, and also the European Society of Cardiology and the European Society of Hypertension. Nonetheless, despite this ongoing debate, the writing committee felt strongly that it is now time to move the U.S. health care system ahead to reflect these differing points of view and expects that widespread use of this new measure set will help to achieve this goal.” The new report revises hypertension performance measures developed by the ACC and AHA in 2011 (J Am Coll Cardiol. 2011 Jul 12;58[3]: 316-36).
In short, the performance and quality measures give all the diverse components of the U.S. health care delivery system a road map for implementing the 2017 High Blood Pressure Guideline in a format that depends on those components electing to adopt and adhere to the 2017 guideline. (Although one of the new performance measures, 1a, harmonizes with an existing and widely applied performance measure.)
“Who is the audience for this, and how will they respond? These performance measures need to be appropriated” by health systems and by performance-assessment groups. “I hope the NCQA will adopt it,” said William C. Cushman, MD, professor of preventive medicine at the University of Tennessee Health Science Center in Memphis, and chief of preventive medicine at the Memphis Veterans Affairs Medical Center. “There are some negatives to performance measures, but on balance they have done good things and led to better care.” Dr. Cushman also approved of several specific performance and quality measures included in the report. “Most of what they emphasized is good,” particularly the importance of accurate pressure measurement, he said in an interview.
“Process drives outcomes” in hypertension management, and the new performance and quality measures “have some very good process metrics,” commented Dr. Egan. “I’d encourage health systems to select two or three measures that are key to what they do and make sense in their setting rather than try to implement it all at once,” he advised, echoing what Dr. Casey had suggested. “It’s ideal to do everything, but we know that if you give physicians a long list of performance measures they just get overwhelmed. The nice thing about hypertension is that we know that process drives outcomes. In the past, we’ve had some process metrics that did not drive outcomes. Getting these processes implemented will lead to better patient outcomes and save a ton of money.”
“We have introduced the 2017 guideline recommendations throughout Target: BP, but like any quality improvement program there is a question of how does it spread,” said Gregory Wozniak, PhD, director of outcomes analytics for the AMA in Chicago. “Our goal for Target: BP is to be impacting 20 million patients by 2021.”
Dr. Casey, Dr. Weiswasser, and Dr. Wozniak had no disclosures. Dr. Cushman has received honoraria as a speaker from Arbor and Sanofi-Aventis, and travel and research support from Eli Lilly. Dr. Egan has been a consultant to and speaker on behalf of Merck and a speaker for Emcure.
SOURCE: Casey DE et al. J Am Coll Cardiol. 2019 Nov 26;74[21]: 2661-706.
PHILADELPHIA – The American Heart Association and American College of Cardiology took a big step toward facilitating widespread U.S. application of the hypertension management guideline that the societies issued in 2017 by releasing a set of performance and quality measures for adults with high blood pressure based on the 2017 guideline.
This guideline notably set a treatment target for patients diagnosed with hypertension of less than 130/80 mg/dL, and also lowered the threshold for diagnosing stage 1 hypertension to a blood pressure at or above 130/80 mm Hg, adding in a stroke about 31 million adults with hypertension to the U.S. total.
Having performance and quality measures based on the guideline is “critical, because how else would you know whether you’re having an effect on accurately diagnosing and properly controlling hypertension?” said Donald E. Casey Jr., MD, chair of the performance measures writing committee. The next step is field testing of the measures “to show they are reliable and effective,” as well as other steps to encourage widespread U.S. uptake of the performance and quality measures and the specifics of the 2017 guideline, Dr. Casey said during a presentation of the revised measures at the American Heart Association scientific sessions.
He especially highlighted the important role of Target: BP, an education, recognition, and quality improvement program run by the AHA and American Medical Association, as a tool that medical practices, health systems, and even payers and employers can use to begin to apply the new performance and quality measures (J Am Coll Cardiol. 2019 Nov 26;74[21]:2661-706) and better align with the recommendations of the 2017 high blood pressure guideline (J Am Coll Cardiol. 2018 May;71[19]:e127-248).
“We’re trying now to promote Target: BP; it’s something you can take off the shelf and get going if it’s embedded in a real-life delivery model. I think Target: BP is the secret sauce. It will be the way we’ll convince people to adopt this,” said Dr. Casey, principal and founder of IPO 4 Health, a Chicago-based health care consulting firm.
He also advised practices and health systems not to feel compelled to introduce all of the specific performance and quality measures at once. “We don’t believe everyone has the resources to do all of it at once; the point is to move toward this system of care. We understand that people don’t have the resources to get it all done” immediately, Dr. Casey said in an interview.
A report during another session at the meeting documented the potential impact that Target: BP can have on blood pressure control within a health system. The Trinity Health of New England medical group based in Springfield, Mass., a system with about 140,000 patients – including 20,000 adults diagnosed with hypertension – and served by 230 health care providers in 13 offices in western Massachusetts, began using Target: BP’s MAP improvement program in its practices in November 2018. (MAP stands for measure accurately, act rapidly, and partner with patients.) Just before the MAP program began, 72% of patients diagnosed with hypertension in the medical group were at their goal blood pressure. Less than a year later, in September 2019, the hypertension control rate had jumped to 84%, a 12 percentage point improvement in control in practices that already had been doing a relatively good job, said Daniel W. Weiswasser, MD, director of quality and clinical informatics at Trinity Health of New England. Based on this success, Trinity Health plans to next involve the remaining regions of Trinity Health of New England in Target: BP, followed by the other regions of Trinity’s national organization, which operates in 21 states with nearly 4,000 staff physicians and about half a million patients diagnosed with hypertension, Dr. Weiswasser said.
“If clinicians do the three steps of the MAP then we will see substantial drops in blood pressures. It will occur,” declared Brent M. Egan, MD, vice president for cardiovascular disease prevention of the AMA in Greenville, S.C.
The new report includes six performance measures based on the strongest guideline recommendations and designed to document adherence levels for the purposes of public reporting and pay-for-performance programs. It also includes 16 quality measures designed for local quality review purposes, with 6 process quality measures and 10 structural quality measures. The report spells out that the authors designed the performance measures for use by major national organizations such as the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance (NCQA), while the quality measures are designed to support quality improvement efforts in any care-delivery setting.
The authors said that the writing committee is sensitive to the fact that the 2019 performance measures for controlling high blood pressure developed by the NCQA for the Healthcare Effectiveness Data and Information Set and currently in use in 2019 by CMS also does not incorporate the 2017 Hypertension Clinical Practice Guidelines classification scheme. “It is well understood that these measures are already in widespread use, especially for quality-related payment programs promulgated by CMS, such as the Medicare Advantage ‘Stars’ ratings, the Medicare Shared Savings Program, and the Physician Quality Payment Program, as well as many other programs promoted by commercial health insurers. In particular, the widespread use of the 2017 Hypertension Clinical Practice Guidelines classification scheme will also help to guide decision making about when to prescribe antihypertensive medications in accordance with its current recommendations for the ACC/AHA “stages” of stage 1 and stage 2 hypertension and elevated blood pressure,” they added.
The report also says that “the writing committee was sensitive to the fact that there is currently not complete consensus among other guidelines from the American College of Physicians and the American Academy of Family Physicians, and also the European Society of Cardiology and the European Society of Hypertension. Nonetheless, despite this ongoing debate, the writing committee felt strongly that it is now time to move the U.S. health care system ahead to reflect these differing points of view and expects that widespread use of this new measure set will help to achieve this goal.” The new report revises hypertension performance measures developed by the ACC and AHA in 2011 (J Am Coll Cardiol. 2011 Jul 12;58[3]: 316-36).
In short, the performance and quality measures give all the diverse components of the U.S. health care delivery system a road map for implementing the 2017 High Blood Pressure Guideline in a format that depends on those components electing to adopt and adhere to the 2017 guideline. (Although one of the new performance measures, 1a, harmonizes with an existing and widely applied performance measure.)
“Who is the audience for this, and how will they respond? These performance measures need to be appropriated” by health systems and by performance-assessment groups. “I hope the NCQA will adopt it,” said William C. Cushman, MD, professor of preventive medicine at the University of Tennessee Health Science Center in Memphis, and chief of preventive medicine at the Memphis Veterans Affairs Medical Center. “There are some negatives to performance measures, but on balance they have done good things and led to better care.” Dr. Cushman also approved of several specific performance and quality measures included in the report. “Most of what they emphasized is good,” particularly the importance of accurate pressure measurement, he said in an interview.
“Process drives outcomes” in hypertension management, and the new performance and quality measures “have some very good process metrics,” commented Dr. Egan. “I’d encourage health systems to select two or three measures that are key to what they do and make sense in their setting rather than try to implement it all at once,” he advised, echoing what Dr. Casey had suggested. “It’s ideal to do everything, but we know that if you give physicians a long list of performance measures they just get overwhelmed. The nice thing about hypertension is that we know that process drives outcomes. In the past, we’ve had some process metrics that did not drive outcomes. Getting these processes implemented will lead to better patient outcomes and save a ton of money.”
“We have introduced the 2017 guideline recommendations throughout Target: BP, but like any quality improvement program there is a question of how does it spread,” said Gregory Wozniak, PhD, director of outcomes analytics for the AMA in Chicago. “Our goal for Target: BP is to be impacting 20 million patients by 2021.”
Dr. Casey, Dr. Weiswasser, and Dr. Wozniak had no disclosures. Dr. Cushman has received honoraria as a speaker from Arbor and Sanofi-Aventis, and travel and research support from Eli Lilly. Dr. Egan has been a consultant to and speaker on behalf of Merck and a speaker for Emcure.
SOURCE: Casey DE et al. J Am Coll Cardiol. 2019 Nov 26;74[21]: 2661-706.
REPORTING FROM AHA 2019
Navigators improve medication adherence in HFrEF
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.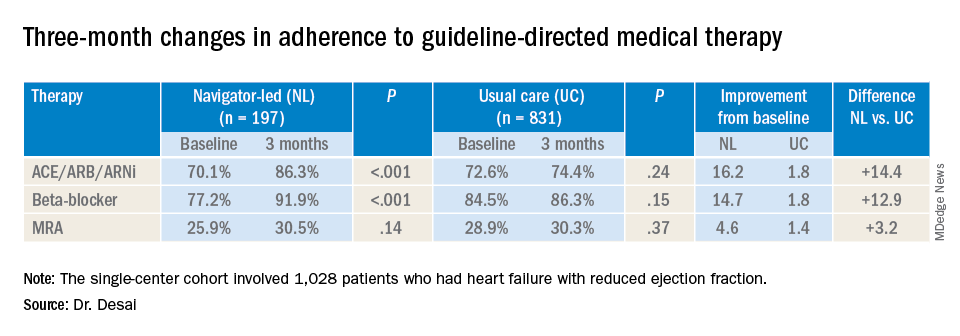
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
PHILADELPHIA – Treatment guidelines are clear about optimal treatment of heart failure in patients with reduced ejection fraction (HFrEF), but adherence breakdowns often occur.
So, Brigham and Women’s Hospital in Boston implemented a navigator-administered patient outreach program that led to improved medication adherence over usual care, according to study results reported at the American Heart Association scientific sessions.
Although the study was done at a major academic center, the findings have implications for community practitioners, lead study author Akshay S. Desai, MD, MPH, said in an interview. “The impact of the intervention is clearly greater in those practitioners who manage heart failure and have the least support around them,” he said.
“Our sense is that the kind of population where this intervention would have the greater impact would be a community-dwelling heart failure population managed by community cardiologists, where the infrastructure to provide longitudinal heart failure care is less robust than may be in an academic center,” Dr. Desai said.
The study evaluated adherence in guideline-directed medical therapy (GDMT) at 3 months. “The navigator-led remote medication optimization strategy improved utilization and dosing of all categories of GDMP and was associated with a lower rate of adverse events,” Dr. Desai said. “The impact was more pronounced in patients followed by general practitioners than by a HF specialist.” In the outreach, health navigators contacted patients by phone and managed medications based on remote surveillance of labs, blood pressure, and symptoms under supervision of a pharmacist, nurse practitioner, and heart failure specialist.
The study included 1,028 patients with chronic HFrEF who’d visited a cardiologist at Brigham and Women’s in the year prior to the study: 197 patients and their providers consented to participate in the program with the remainder serving as the reference usual-care group. Most HF specialists at Brigham and Women’s declined to participate in the navigator-led program, Dr. Desai said.
Treating providers were approached for consent to adjust medical therapy according to a sequential, stepped titration algorithm modeled on the current American College of Cardiology/American Heart Association HF Guidelines. The study population did not include patients with end-stage HF, those with a severe noncardiac illness with a life expectancy of less than a year, and patients with a pattern of nonadherence. Baseline characteristics of the two groups were well balanced, Dr. Desai said.
At baseline, 74% (759) participants were treated with ACE inhibitors/angiotensin receptor blockers/angiotensin-receptor neprilysin inhibitors (ACE/ARB/ARNi), 73% (746) with guideline-directed beta-blockers, and 29% (303) with mineralocorticoid receptor antagonists (MRAs), with 10% (107) and 11% (117) treated with target doses of ACE/ARB/ARNi and beta-blockers, respectively.
In the navigator-led group, beta-blocker adherence improved from 77.2% at baseline to 91.9% at 3 months (P less than 0.001) compared with an increase from 84.5% to 86.3% in the usual-care patients (P = 0.15), Dr. Desai said. ACE/ARB/ARNi adherence increased 16.2 percentage points to 86.3% (P less than 0.001) in the navigator-group versus 1.8 percentage points to 74.4% (P = 0.24) for usual care. In the MRA subgroup, 3-month adherence to GDMT was almost identical: 30.5% (P = 0.14) and 30.3% (P = 0.37) for the two treatment groups, respectively, although the navigator-led patients averaged a larger increase of 4.6 versus 1.4 percentage points from baseline.
Adverse event rates were similar in both groups, although the navigator group had “slightly higher rates” of hypotension and hyperkalemia but no serious events, Dr. Desai said. This group also had similarly higher rates of worsening renal function, but most were asymptomatic change in creatinine that was addressed with medication changes, he said. There were no hospitalizations for adverse events.
He said the navigator-led optimization has potential in a community setting because the referral nature of Brigham and Women’s HF population “reflects potentially a worst-case scenario for such a program.” The greatest impact was seen in patients managed by general cardiologists, he said. “If we were to move this forward, which we hope to do with scale, the impact might be greater in a community population where there are fewer specialists and less severe illnesses present.”
This study represents a proof of concept, Dr. Desai said in an interview. “What we would like to do is demonstrate that this can be done on a larger scale,” he said. “That might involve partnership with a payer or health care system to see if we can replicate these findings across a broader range of providers.”
Dr. Desai disclosed financial relationships with Novartis, AstraZeneca, Abbott, Boehringer-Ingelheim, Coston Scientific, Biofourmis, DalCor, Relypsa, Regeneron, and Alnylam. Novartis provided an unrestricted grant for the investigator-initiated trial.
SOURCE: Desai AS. AHA 2019 Featured Science session AOS.07.
REPORTING FROM AHA 2019
Mechanical circulatory support in PCI needs clearer guidance
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
REPORTING FROM AHA 2019
Bariatric surgery tied to fewer cerebrovascular events
PHILADELPHIA – Obese people living in the United Kingdom who underwent bariatric surgery had a two-thirds lower rate of major cerebrovascular events than that of a matched group of obese residents who did not undergo bariatric surgery, in a retrospective study of 8,424 people followed for a mean of just over 11 years.
Although the cut in cerebrovascular events that linked with bariatric surgery shown by the analysis was mostly driven by a reduced rate of transient ischemic attacks, a potentially unreliable diagnosis, the results showed consistent reductions in the rates of acute ischemic strokes as well as in acute, nontraumatic intracranial hemorrhages, two other components of the combined primary endpoint, Maddalena Ardissino, MBBS, said at the American Heart Association scientific sessions.
This finding of an apparent benefit from bariatric surgery in obese patients in a large U.K. database confirms other findings from a “fast-growing” evidence base showing benefits from bariatric surgery for reducing other types of cardiovascular disease events, said Dr. Ardissino, a researcher at Imperial College, London. However, the impact of bariatric surgery specifically on cerebrovascular events had not received much attention in published studies, she noted.
Her study used data collected by the Clinical Practice Research Datalink, which has primary and secondary care health records for about 42 million U.K. residents. The researchers focused on more than 251,000 obese U.K. adults (body mass index of 30 kg/m2 or greater) without a history of a cerebrovascular event who had at least 1 year of follow-up, a data file that included 4,212 adults who had undergone bariatric surgery. Their analysis matched these surgical patients with an equal number of obese adults who did not have surgery, pairing the cases and controls based on age, sex, and BMI. The resulting matched cohorts each averaged 50 years old, with a mean BMI of 40.5 kg/m2.
During just over 11 years of average follow-up, the incidence of acute ischemic stroke, acute intracranial hemorrhage, subarachnoid hemorrhage, or transient ischemic attack was about 1.3% in those without bariatric surgery and about 0.4% in those who had surgery, an absolute risk reduction of 0.9 linked with surgery and a relative risk reduction of 65% that was statistically significant, Dr. Ardissino reported. All-cause mortality was about 70% lower in the group that underwent bariatric surgery compared with those who did not have surgery, a finding that confirmed prior reports. She cautioned that the analysis was limited by a relatively low number of total events, and by the small number of criteria used for cohort matching that might have left unadjusted certain potential confounders such as the level of engagement people had with their medical care.
SOURCE: Ardissino M. AHA 2019, Abstract 335.
PHILADELPHIA – Obese people living in the United Kingdom who underwent bariatric surgery had a two-thirds lower rate of major cerebrovascular events than that of a matched group of obese residents who did not undergo bariatric surgery, in a retrospective study of 8,424 people followed for a mean of just over 11 years.
Although the cut in cerebrovascular events that linked with bariatric surgery shown by the analysis was mostly driven by a reduced rate of transient ischemic attacks, a potentially unreliable diagnosis, the results showed consistent reductions in the rates of acute ischemic strokes as well as in acute, nontraumatic intracranial hemorrhages, two other components of the combined primary endpoint, Maddalena Ardissino, MBBS, said at the American Heart Association scientific sessions.
This finding of an apparent benefit from bariatric surgery in obese patients in a large U.K. database confirms other findings from a “fast-growing” evidence base showing benefits from bariatric surgery for reducing other types of cardiovascular disease events, said Dr. Ardissino, a researcher at Imperial College, London. However, the impact of bariatric surgery specifically on cerebrovascular events had not received much attention in published studies, she noted.
Her study used data collected by the Clinical Practice Research Datalink, which has primary and secondary care health records for about 42 million U.K. residents. The researchers focused on more than 251,000 obese U.K. adults (body mass index of 30 kg/m2 or greater) without a history of a cerebrovascular event who had at least 1 year of follow-up, a data file that included 4,212 adults who had undergone bariatric surgery. Their analysis matched these surgical patients with an equal number of obese adults who did not have surgery, pairing the cases and controls based on age, sex, and BMI. The resulting matched cohorts each averaged 50 years old, with a mean BMI of 40.5 kg/m2.
During just over 11 years of average follow-up, the incidence of acute ischemic stroke, acute intracranial hemorrhage, subarachnoid hemorrhage, or transient ischemic attack was about 1.3% in those without bariatric surgery and about 0.4% in those who had surgery, an absolute risk reduction of 0.9 linked with surgery and a relative risk reduction of 65% that was statistically significant, Dr. Ardissino reported. All-cause mortality was about 70% lower in the group that underwent bariatric surgery compared with those who did not have surgery, a finding that confirmed prior reports. She cautioned that the analysis was limited by a relatively low number of total events, and by the small number of criteria used for cohort matching that might have left unadjusted certain potential confounders such as the level of engagement people had with their medical care.
SOURCE: Ardissino M. AHA 2019, Abstract 335.
PHILADELPHIA – Obese people living in the United Kingdom who underwent bariatric surgery had a two-thirds lower rate of major cerebrovascular events than that of a matched group of obese residents who did not undergo bariatric surgery, in a retrospective study of 8,424 people followed for a mean of just over 11 years.
Although the cut in cerebrovascular events that linked with bariatric surgery shown by the analysis was mostly driven by a reduced rate of transient ischemic attacks, a potentially unreliable diagnosis, the results showed consistent reductions in the rates of acute ischemic strokes as well as in acute, nontraumatic intracranial hemorrhages, two other components of the combined primary endpoint, Maddalena Ardissino, MBBS, said at the American Heart Association scientific sessions.
This finding of an apparent benefit from bariatric surgery in obese patients in a large U.K. database confirms other findings from a “fast-growing” evidence base showing benefits from bariatric surgery for reducing other types of cardiovascular disease events, said Dr. Ardissino, a researcher at Imperial College, London. However, the impact of bariatric surgery specifically on cerebrovascular events had not received much attention in published studies, she noted.
Her study used data collected by the Clinical Practice Research Datalink, which has primary and secondary care health records for about 42 million U.K. residents. The researchers focused on more than 251,000 obese U.K. adults (body mass index of 30 kg/m2 or greater) without a history of a cerebrovascular event who had at least 1 year of follow-up, a data file that included 4,212 adults who had undergone bariatric surgery. Their analysis matched these surgical patients with an equal number of obese adults who did not have surgery, pairing the cases and controls based on age, sex, and BMI. The resulting matched cohorts each averaged 50 years old, with a mean BMI of 40.5 kg/m2.
During just over 11 years of average follow-up, the incidence of acute ischemic stroke, acute intracranial hemorrhage, subarachnoid hemorrhage, or transient ischemic attack was about 1.3% in those without bariatric surgery and about 0.4% in those who had surgery, an absolute risk reduction of 0.9 linked with surgery and a relative risk reduction of 65% that was statistically significant, Dr. Ardissino reported. All-cause mortality was about 70% lower in the group that underwent bariatric surgery compared with those who did not have surgery, a finding that confirmed prior reports. She cautioned that the analysis was limited by a relatively low number of total events, and by the small number of criteria used for cohort matching that might have left unadjusted certain potential confounders such as the level of engagement people had with their medical care.
SOURCE: Ardissino M. AHA 2019, Abstract 335.
REPORTING FROM AHA 2019
Genetic test stratified AFib patients with low CHA2DS2-VASc scores
PHILADELPHIA – A 32-gene screening test for stroke risk identified a subgroup of atrial fibrillation (AFib) patients with an elevated rate of ischemic strokes despite having a low stroke risk by conventional criteria by their CHA2DS2-VASc score in a post-hoc analysis of more than 11,000 patients enrolled in a recent drug trial.
Overall, AFib patients in the highest tertile for genetic risk based on a 32 gene-loci test had a 31% increase rate of ischemic stroke during a median 2.8 years of follow-up compared with patients in the tertile with the lowest risk based on the 32-loci screen, Nicholas A. Marston, MD, said at the American Heart Association scientific sessions. This suggested that the genetic test had roughly the same association with an increased stroke risk as several components of the CHA2DS2-VASc score, such as female sex, an age of 65-74 years old, or having heart failure as a comorbidity, each of which links with an increased risk for ischemic stroke of about 31%-38%, Dr. Marston noted.
The genetic test produced even sharper discrimination among the patients with the lowest stroke risk as measured by their CHA2DS2-VASc score (Circulation. 2012 Aug 14;126[7]: 860-5). Among the slightly more than 3,000 patients in the study with a CHA2DS2-VASc score of three or less, those in the subgroup with the highest risk by the 32-loci screen had a stroke rate during follow-up that was 76% higher than those in the low or intermediate tertile for their genetic score. Among the 796 patients with a CHA2DS2-VASc score of just 1 or 2, those who also fell into the highest level of risk on the 32-loci screen had a stroke rate 3.5-fold higher than those with a similar CHA2DS2-VASc score but in the low and intermediate tertiles by the 32-loci screen.
The additional risk prediction provided by the 32-loci test was statistically significant in the analysis of the 3,071 patients with a CHA2DS2-VASc score of 3 or less after adjustment for age, sex, ancestry, and the individual components of the CHA2DS2-VASc score, which includes factors such as hypertension, diabetes, and heart failure, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. The 3.5-fold elevation among patients with a high genetic-risk score in the cohort of 796 patients with a CHA2DS2-VASc score of 1 or 2 just missed statistical significance (P = .06), possibly because the number of patients in the analysis was relatively low. Future research should explore the predictive value of the genetic risk score in patients with a CHA2DS2-VASc score of 0 or 1, the “group where therapeutic decisions could be altered” depending on the genetic risk score, he explained.
Dr. Marston and his associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which was designed to assess the safety and efficacy of the direct-acting oral anticoagulant edoxaban in patients with AFib (New Engl J Med. 2013 Nov 28; 369[21]: 2091-2104). The 32-loci panel to measure a person’s genetic risk for stroke came from a 2018 report by a multinational team of researchers (Nature Genetics. 2018 Apr;50[4]: 524-37). The new analysis applied this 32-loci genetic test panel to 11.164 unrelated AFib patients with European ancestry from the ENGAGE AF-TIMIT 48 database. They divided this cohort into tertiles based on having a low, intermediate, or high stroke risk as assessed by the 32-loci genetic test. The analysis focused on patients enrolled in the trial who had European ancestry because the 32-loci screening test relied predominantly on data collected from people with this genetic background, Dr. Marston said.
SOURCE: Marston NA et al. AHA 2019, Abstract 336.
PHILADELPHIA – A 32-gene screening test for stroke risk identified a subgroup of atrial fibrillation (AFib) patients with an elevated rate of ischemic strokes despite having a low stroke risk by conventional criteria by their CHA2DS2-VASc score in a post-hoc analysis of more than 11,000 patients enrolled in a recent drug trial.
Overall, AFib patients in the highest tertile for genetic risk based on a 32 gene-loci test had a 31% increase rate of ischemic stroke during a median 2.8 years of follow-up compared with patients in the tertile with the lowest risk based on the 32-loci screen, Nicholas A. Marston, MD, said at the American Heart Association scientific sessions. This suggested that the genetic test had roughly the same association with an increased stroke risk as several components of the CHA2DS2-VASc score, such as female sex, an age of 65-74 years old, or having heart failure as a comorbidity, each of which links with an increased risk for ischemic stroke of about 31%-38%, Dr. Marston noted.
The genetic test produced even sharper discrimination among the patients with the lowest stroke risk as measured by their CHA2DS2-VASc score (Circulation. 2012 Aug 14;126[7]: 860-5). Among the slightly more than 3,000 patients in the study with a CHA2DS2-VASc score of three or less, those in the subgroup with the highest risk by the 32-loci screen had a stroke rate during follow-up that was 76% higher than those in the low or intermediate tertile for their genetic score. Among the 796 patients with a CHA2DS2-VASc score of just 1 or 2, those who also fell into the highest level of risk on the 32-loci screen had a stroke rate 3.5-fold higher than those with a similar CHA2DS2-VASc score but in the low and intermediate tertiles by the 32-loci screen.
The additional risk prediction provided by the 32-loci test was statistically significant in the analysis of the 3,071 patients with a CHA2DS2-VASc score of 3 or less after adjustment for age, sex, ancestry, and the individual components of the CHA2DS2-VASc score, which includes factors such as hypertension, diabetes, and heart failure, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. The 3.5-fold elevation among patients with a high genetic-risk score in the cohort of 796 patients with a CHA2DS2-VASc score of 1 or 2 just missed statistical significance (P = .06), possibly because the number of patients in the analysis was relatively low. Future research should explore the predictive value of the genetic risk score in patients with a CHA2DS2-VASc score of 0 or 1, the “group where therapeutic decisions could be altered” depending on the genetic risk score, he explained.
Dr. Marston and his associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which was designed to assess the safety and efficacy of the direct-acting oral anticoagulant edoxaban in patients with AFib (New Engl J Med. 2013 Nov 28; 369[21]: 2091-2104). The 32-loci panel to measure a person’s genetic risk for stroke came from a 2018 report by a multinational team of researchers (Nature Genetics. 2018 Apr;50[4]: 524-37). The new analysis applied this 32-loci genetic test panel to 11.164 unrelated AFib patients with European ancestry from the ENGAGE AF-TIMIT 48 database. They divided this cohort into tertiles based on having a low, intermediate, or high stroke risk as assessed by the 32-loci genetic test. The analysis focused on patients enrolled in the trial who had European ancestry because the 32-loci screening test relied predominantly on data collected from people with this genetic background, Dr. Marston said.
SOURCE: Marston NA et al. AHA 2019, Abstract 336.
PHILADELPHIA – A 32-gene screening test for stroke risk identified a subgroup of atrial fibrillation (AFib) patients with an elevated rate of ischemic strokes despite having a low stroke risk by conventional criteria by their CHA2DS2-VASc score in a post-hoc analysis of more than 11,000 patients enrolled in a recent drug trial.
Overall, AFib patients in the highest tertile for genetic risk based on a 32 gene-loci test had a 31% increase rate of ischemic stroke during a median 2.8 years of follow-up compared with patients in the tertile with the lowest risk based on the 32-loci screen, Nicholas A. Marston, MD, said at the American Heart Association scientific sessions. This suggested that the genetic test had roughly the same association with an increased stroke risk as several components of the CHA2DS2-VASc score, such as female sex, an age of 65-74 years old, or having heart failure as a comorbidity, each of which links with an increased risk for ischemic stroke of about 31%-38%, Dr. Marston noted.
The genetic test produced even sharper discrimination among the patients with the lowest stroke risk as measured by their CHA2DS2-VASc score (Circulation. 2012 Aug 14;126[7]: 860-5). Among the slightly more than 3,000 patients in the study with a CHA2DS2-VASc score of three or less, those in the subgroup with the highest risk by the 32-loci screen had a stroke rate during follow-up that was 76% higher than those in the low or intermediate tertile for their genetic score. Among the 796 patients with a CHA2DS2-VASc score of just 1 or 2, those who also fell into the highest level of risk on the 32-loci screen had a stroke rate 3.5-fold higher than those with a similar CHA2DS2-VASc score but in the low and intermediate tertiles by the 32-loci screen.
The additional risk prediction provided by the 32-loci test was statistically significant in the analysis of the 3,071 patients with a CHA2DS2-VASc score of 3 or less after adjustment for age, sex, ancestry, and the individual components of the CHA2DS2-VASc score, which includes factors such as hypertension, diabetes, and heart failure, said Dr. Marston, a cardiologist at Brigham and Women’s Hospital in Boston. The 3.5-fold elevation among patients with a high genetic-risk score in the cohort of 796 patients with a CHA2DS2-VASc score of 1 or 2 just missed statistical significance (P = .06), possibly because the number of patients in the analysis was relatively low. Future research should explore the predictive value of the genetic risk score in patients with a CHA2DS2-VASc score of 0 or 1, the “group where therapeutic decisions could be altered” depending on the genetic risk score, he explained.
Dr. Marston and his associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which was designed to assess the safety and efficacy of the direct-acting oral anticoagulant edoxaban in patients with AFib (New Engl J Med. 2013 Nov 28; 369[21]: 2091-2104). The 32-loci panel to measure a person’s genetic risk for stroke came from a 2018 report by a multinational team of researchers (Nature Genetics. 2018 Apr;50[4]: 524-37). The new analysis applied this 32-loci genetic test panel to 11.164 unrelated AFib patients with European ancestry from the ENGAGE AF-TIMIT 48 database. They divided this cohort into tertiles based on having a low, intermediate, or high stroke risk as assessed by the 32-loci genetic test. The analysis focused on patients enrolled in the trial who had European ancestry because the 32-loci screening test relied predominantly on data collected from people with this genetic background, Dr. Marston said.
SOURCE: Marston NA et al. AHA 2019, Abstract 336.
REPORTING FROM AHA 2019
New heart failure trial data presage guideline revisions
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
EXPERT ANALYSIS FROM AHA 2019




















