User login
23rd World Congress of Dermatology
Expert touts ivermectin 1% cream as treatment of choice for rosacea
VANCOUVER, B.C. – A persuasive case can be made for ivermectin 1% cream as the new treatment of choice for papulopustular rosacea, Dr. Leon Kircik asserted at the World Congress of Dermatology.
He cited the results of a large recent head-to-head randomized trial in which ivermectin 1% cream (Soolantra), approved last December by the Food and Drug Administration, proved superior to metronidazole 0.75% cream (Metrocream), which is the market leader and until now the topical agent most physicians have considered their first-line treatment for papulopustular rosacea.
The study, known as ATTRACT (Assessment of a Topical Treatment in Rosacea: Activity, Compliance, Tolerability), was a 962-patient, phase III, single-blind, European randomized trial. Ivermectin demonstrated faster onset of action, a greater clinical success rate, delayed time to relapse following treatment discontinuation, a lower relapse rate, and better tolerability (Br. J. Dermatol. 2015;172:1103-10).
Dr. Kircik, a Louisville dermatologist in private practice, and a clinical trialist, was not involved in the ATTRACT study but was impressed that it was undertaken by Galderma, which markets both drugs.
“We don’t get many head-to-head studies in dermatology. Those are typically studies no one wants to sponsor. And most of what few head-to-head studies are done are designed as noninferiority studies. In this case, ATTRACT was designed as a superiority study. That’s a higher bar to reach,” noted Dr. Kircik, a consultant to, and member of the speakers bureau for, Galderma and other pharmaceutical companies.
ATTRACT was a two-part study. In part A, 962 patients with moderate to severe rosacea were randomized to ivermectin 1% cream once daily or metronidazole 0.75% cream b.i.d. for 16 weeks. As early as the first assessment at 3 weeks, a significant difference in favor of ivermectin was evident in terms of reduction in inflammatory lesion count. At week 16, the ivermectin group showed an 83% reduction from baseline in inflammatory lesions, significantly better than the 73.7% reduction with metronidazole. Moreover, 84.9% of the ivermectin group was rated clear or almost clear by Investigator’s Global Assessment (IGA) compared with 75.4% of the metronidazole group.
In addition, 34.9% of ivermectin-treated patients achieved an IGA of 0 by 16 weeks, meaning they were totally lesion free, compared with 21.7% of the metronidazole group. That “completely clear” status is really important psychosocially to a significant proportion of patients, said Dr. Kircik.
“How many times have you had a patient come in asking for an intralesional steroid injection for their single remaining lesion after months of topical treatment?” he asked rhetorically.
Ivermectin was better tolerated than was metronidazole. The rate of treatment-related adverse events leading to discontinuation – typically for skin irritation, redness, or itching – was 0.6% in the ivermectin group compared with 2.1% with metronidazole.
Part B of the ATTRACT study was an unusually designed 36-week extension study.
“In my mind, part B is much more important and much more relevant because rosacea is a chronic disease. It doesn’t last for just 16 weeks. Part B asks what happens after we stop therapy. This is the rare clinical trial that actually mimics real life,” Dr. Kircik said.
Despite physicians’ standard advice to continue with maintenance therapy after clearing, the reality is that most patients stop treatment once they clear, figuring they’ll resume if they relapse, he asserted. Part B of ATTRACT reflected that approach. Seven hundred fifty-seven patients who were clear/almost clear as defined by an IGA of 0/1 at the end of 16 weeks enrolled in the 36-week extension study. They surrendered their medication and returned to their physician monthly. If they had relapsed during that month, meaning they showed up with an IGA of 2 or more, they got their medication back; if they were still IGA 0/1, they did not.
The median time to relapse off treatment in the ivermectin group was 115 days, a full month later than the 85 days for the metronidazole group. Also, the relapse rate in the ivermectin group was significantly lower: 62.7% compared with 68.4% in the metronidazole group.
“The impact on both clinical practice and pharmacoeconomic practice is huge here. It makes sense to switch from metronidazole to ivermectin as first-line therapy because you know that your patients will do better and will relapse later and less,” Dr. Kircik declared.
He was a coinvestigator in the twin pivotal phase III randomized Galderma-funded trials that led to FDA approval of ivermectin for papulopustular rosacea. Although those studies have been published (J. Drugs Dermatol. 2014;13:1380-6), Dr. Kircik highlighted a couple of findings he said haven’t drawn the attention they deserve. Both came from the long-term 40-week extension that followed the initial 12-week, double-blind stage.
One of these underappreciated findings concerned safety. The rate of treatment-related dermatologic adverse events during the double-blind first 3 months of the study was 2.5% in the ivermectin group compared with 6.3% in vehicle-treated controls.
“That’s pretty impressive. That the active treatment arm of the study had less treatment-related dermatologic adverse events than placebo has never been seen before in any other topical study. It tells you something: The assumption here is that the potent inherent anti-inflammatory activity of ivermectin is overwhelming,” the dermatologist said.
The other particularly noteworthy finding came in the long-term, 40-week extension study that followed the initial 12-week, double-blind stage. What was impressive here was the way the proportion of patients who were IGA clear/almost clear rose steadily throughout, he noted. At the end of the initial 3-month phase of the two studies, 38%-40% of ivermectin-treated patients were clear/almost clear. At 12 months, nearly 70% were.
“In most studies, efficacy sort of plateaus at some point. Here it keeps going up through 12 months. So if somebody comes to your office after 3 months using ivermectin and says, ‘Eh, I’m okay, but I’m not clear or almost clear,’ there’s no reason to switch to another medication, because if they continue you know there’s a high chance they will become clear/almost clear,” Dr. Kircik said.
On the other hand, study participants who were still IGA ‘severe’ after 3 months remained severe after 12 months on the drug. So the message here is if a patient still has severe rosacea after 3 months on ivermectin it’s time to change drugs, he added.
To say that ivermectin should be the treatment of choice because it outperformed metronidazole cream .75% seems a bit overzealous. The reality is, and I believe most of my colleagues agree, metronidazole .75% cream is not very effective. We give it because it is what insurances force us to give, or we gave it because we had nothing else to give. Fortunately this has changed over the years with the advent of azelaic gel and now ivermectin 1% cream.
I do agree, however, that it is rare to see a company structure a superiority head-to-head study, so I will give credit where it is due. However, my guess here is that it was anticipated that ivermectin would at the very least prove noninferior, if not superior, given the poor success rate of this long-standing workhorse. This should not distract from the fact that a) the studies were thorough and well structured and b) held for a good time frame. The data are certainly compelling, so I don’t want that to be overshadowed by the heavy focus on comparing to metronidazole twice a day. To me, that’s a red herring; had they only compared to placebo, we wouldn’t be having this discussion.
The data herein presented are more than supportive of its addition to our limited armamentarium, but to say first line is premature at this early stage. The once-daily dosing and limited adverse events are supportive features as patient compliance is always an issue. Probably more important, and only time will tell, is will insurance companies cover it? Or, will they reject our prescriptions and continue the current trend of recommending medications that bear no similarity to mechanism of action or efficacy. I am suddenly reminded of the all too frequent notice sent, stating that I should give an acne patient benzoyl peroxide, instead of the retinoid I initially selected.
Kudos to Galderma for keeping innovation alive and bringing a new topical drug forward – curious to see if I can actually prescribe it.
Dr. Adam Friedman is associate professor of dermatology and director of translational research in the department of dermatology at George Washington University, Washington, D.C.
To say that ivermectin should be the treatment of choice because it outperformed metronidazole cream .75% seems a bit overzealous. The reality is, and I believe most of my colleagues agree, metronidazole .75% cream is not very effective. We give it because it is what insurances force us to give, or we gave it because we had nothing else to give. Fortunately this has changed over the years with the advent of azelaic gel and now ivermectin 1% cream.
I do agree, however, that it is rare to see a company structure a superiority head-to-head study, so I will give credit where it is due. However, my guess here is that it was anticipated that ivermectin would at the very least prove noninferior, if not superior, given the poor success rate of this long-standing workhorse. This should not distract from the fact that a) the studies were thorough and well structured and b) held for a good time frame. The data are certainly compelling, so I don’t want that to be overshadowed by the heavy focus on comparing to metronidazole twice a day. To me, that’s a red herring; had they only compared to placebo, we wouldn’t be having this discussion.
The data herein presented are more than supportive of its addition to our limited armamentarium, but to say first line is premature at this early stage. The once-daily dosing and limited adverse events are supportive features as patient compliance is always an issue. Probably more important, and only time will tell, is will insurance companies cover it? Or, will they reject our prescriptions and continue the current trend of recommending medications that bear no similarity to mechanism of action or efficacy. I am suddenly reminded of the all too frequent notice sent, stating that I should give an acne patient benzoyl peroxide, instead of the retinoid I initially selected.
Kudos to Galderma for keeping innovation alive and bringing a new topical drug forward – curious to see if I can actually prescribe it.
Dr. Adam Friedman is associate professor of dermatology and director of translational research in the department of dermatology at George Washington University, Washington, D.C.
To say that ivermectin should be the treatment of choice because it outperformed metronidazole cream .75% seems a bit overzealous. The reality is, and I believe most of my colleagues agree, metronidazole .75% cream is not very effective. We give it because it is what insurances force us to give, or we gave it because we had nothing else to give. Fortunately this has changed over the years with the advent of azelaic gel and now ivermectin 1% cream.
I do agree, however, that it is rare to see a company structure a superiority head-to-head study, so I will give credit where it is due. However, my guess here is that it was anticipated that ivermectin would at the very least prove noninferior, if not superior, given the poor success rate of this long-standing workhorse. This should not distract from the fact that a) the studies were thorough and well structured and b) held for a good time frame. The data are certainly compelling, so I don’t want that to be overshadowed by the heavy focus on comparing to metronidazole twice a day. To me, that’s a red herring; had they only compared to placebo, we wouldn’t be having this discussion.
The data herein presented are more than supportive of its addition to our limited armamentarium, but to say first line is premature at this early stage. The once-daily dosing and limited adverse events are supportive features as patient compliance is always an issue. Probably more important, and only time will tell, is will insurance companies cover it? Or, will they reject our prescriptions and continue the current trend of recommending medications that bear no similarity to mechanism of action or efficacy. I am suddenly reminded of the all too frequent notice sent, stating that I should give an acne patient benzoyl peroxide, instead of the retinoid I initially selected.
Kudos to Galderma for keeping innovation alive and bringing a new topical drug forward – curious to see if I can actually prescribe it.
Dr. Adam Friedman is associate professor of dermatology and director of translational research in the department of dermatology at George Washington University, Washington, D.C.
VANCOUVER, B.C. – A persuasive case can be made for ivermectin 1% cream as the new treatment of choice for papulopustular rosacea, Dr. Leon Kircik asserted at the World Congress of Dermatology.
He cited the results of a large recent head-to-head randomized trial in which ivermectin 1% cream (Soolantra), approved last December by the Food and Drug Administration, proved superior to metronidazole 0.75% cream (Metrocream), which is the market leader and until now the topical agent most physicians have considered their first-line treatment for papulopustular rosacea.
The study, known as ATTRACT (Assessment of a Topical Treatment in Rosacea: Activity, Compliance, Tolerability), was a 962-patient, phase III, single-blind, European randomized trial. Ivermectin demonstrated faster onset of action, a greater clinical success rate, delayed time to relapse following treatment discontinuation, a lower relapse rate, and better tolerability (Br. J. Dermatol. 2015;172:1103-10).
Dr. Kircik, a Louisville dermatologist in private practice, and a clinical trialist, was not involved in the ATTRACT study but was impressed that it was undertaken by Galderma, which markets both drugs.
“We don’t get many head-to-head studies in dermatology. Those are typically studies no one wants to sponsor. And most of what few head-to-head studies are done are designed as noninferiority studies. In this case, ATTRACT was designed as a superiority study. That’s a higher bar to reach,” noted Dr. Kircik, a consultant to, and member of the speakers bureau for, Galderma and other pharmaceutical companies.
ATTRACT was a two-part study. In part A, 962 patients with moderate to severe rosacea were randomized to ivermectin 1% cream once daily or metronidazole 0.75% cream b.i.d. for 16 weeks. As early as the first assessment at 3 weeks, a significant difference in favor of ivermectin was evident in terms of reduction in inflammatory lesion count. At week 16, the ivermectin group showed an 83% reduction from baseline in inflammatory lesions, significantly better than the 73.7% reduction with metronidazole. Moreover, 84.9% of the ivermectin group was rated clear or almost clear by Investigator’s Global Assessment (IGA) compared with 75.4% of the metronidazole group.
In addition, 34.9% of ivermectin-treated patients achieved an IGA of 0 by 16 weeks, meaning they were totally lesion free, compared with 21.7% of the metronidazole group. That “completely clear” status is really important psychosocially to a significant proportion of patients, said Dr. Kircik.
“How many times have you had a patient come in asking for an intralesional steroid injection for their single remaining lesion after months of topical treatment?” he asked rhetorically.
Ivermectin was better tolerated than was metronidazole. The rate of treatment-related adverse events leading to discontinuation – typically for skin irritation, redness, or itching – was 0.6% in the ivermectin group compared with 2.1% with metronidazole.
Part B of the ATTRACT study was an unusually designed 36-week extension study.
“In my mind, part B is much more important and much more relevant because rosacea is a chronic disease. It doesn’t last for just 16 weeks. Part B asks what happens after we stop therapy. This is the rare clinical trial that actually mimics real life,” Dr. Kircik said.
Despite physicians’ standard advice to continue with maintenance therapy after clearing, the reality is that most patients stop treatment once they clear, figuring they’ll resume if they relapse, he asserted. Part B of ATTRACT reflected that approach. Seven hundred fifty-seven patients who were clear/almost clear as defined by an IGA of 0/1 at the end of 16 weeks enrolled in the 36-week extension study. They surrendered their medication and returned to their physician monthly. If they had relapsed during that month, meaning they showed up with an IGA of 2 or more, they got their medication back; if they were still IGA 0/1, they did not.
The median time to relapse off treatment in the ivermectin group was 115 days, a full month later than the 85 days for the metronidazole group. Also, the relapse rate in the ivermectin group was significantly lower: 62.7% compared with 68.4% in the metronidazole group.
“The impact on both clinical practice and pharmacoeconomic practice is huge here. It makes sense to switch from metronidazole to ivermectin as first-line therapy because you know that your patients will do better and will relapse later and less,” Dr. Kircik declared.
He was a coinvestigator in the twin pivotal phase III randomized Galderma-funded trials that led to FDA approval of ivermectin for papulopustular rosacea. Although those studies have been published (J. Drugs Dermatol. 2014;13:1380-6), Dr. Kircik highlighted a couple of findings he said haven’t drawn the attention they deserve. Both came from the long-term 40-week extension that followed the initial 12-week, double-blind stage.
One of these underappreciated findings concerned safety. The rate of treatment-related dermatologic adverse events during the double-blind first 3 months of the study was 2.5% in the ivermectin group compared with 6.3% in vehicle-treated controls.
“That’s pretty impressive. That the active treatment arm of the study had less treatment-related dermatologic adverse events than placebo has never been seen before in any other topical study. It tells you something: The assumption here is that the potent inherent anti-inflammatory activity of ivermectin is overwhelming,” the dermatologist said.
The other particularly noteworthy finding came in the long-term, 40-week extension study that followed the initial 12-week, double-blind stage. What was impressive here was the way the proportion of patients who were IGA clear/almost clear rose steadily throughout, he noted. At the end of the initial 3-month phase of the two studies, 38%-40% of ivermectin-treated patients were clear/almost clear. At 12 months, nearly 70% were.
“In most studies, efficacy sort of plateaus at some point. Here it keeps going up through 12 months. So if somebody comes to your office after 3 months using ivermectin and says, ‘Eh, I’m okay, but I’m not clear or almost clear,’ there’s no reason to switch to another medication, because if they continue you know there’s a high chance they will become clear/almost clear,” Dr. Kircik said.
On the other hand, study participants who were still IGA ‘severe’ after 3 months remained severe after 12 months on the drug. So the message here is if a patient still has severe rosacea after 3 months on ivermectin it’s time to change drugs, he added.
VANCOUVER, B.C. – A persuasive case can be made for ivermectin 1% cream as the new treatment of choice for papulopustular rosacea, Dr. Leon Kircik asserted at the World Congress of Dermatology.
He cited the results of a large recent head-to-head randomized trial in which ivermectin 1% cream (Soolantra), approved last December by the Food and Drug Administration, proved superior to metronidazole 0.75% cream (Metrocream), which is the market leader and until now the topical agent most physicians have considered their first-line treatment for papulopustular rosacea.
The study, known as ATTRACT (Assessment of a Topical Treatment in Rosacea: Activity, Compliance, Tolerability), was a 962-patient, phase III, single-blind, European randomized trial. Ivermectin demonstrated faster onset of action, a greater clinical success rate, delayed time to relapse following treatment discontinuation, a lower relapse rate, and better tolerability (Br. J. Dermatol. 2015;172:1103-10).
Dr. Kircik, a Louisville dermatologist in private practice, and a clinical trialist, was not involved in the ATTRACT study but was impressed that it was undertaken by Galderma, which markets both drugs.
“We don’t get many head-to-head studies in dermatology. Those are typically studies no one wants to sponsor. And most of what few head-to-head studies are done are designed as noninferiority studies. In this case, ATTRACT was designed as a superiority study. That’s a higher bar to reach,” noted Dr. Kircik, a consultant to, and member of the speakers bureau for, Galderma and other pharmaceutical companies.
ATTRACT was a two-part study. In part A, 962 patients with moderate to severe rosacea were randomized to ivermectin 1% cream once daily or metronidazole 0.75% cream b.i.d. for 16 weeks. As early as the first assessment at 3 weeks, a significant difference in favor of ivermectin was evident in terms of reduction in inflammatory lesion count. At week 16, the ivermectin group showed an 83% reduction from baseline in inflammatory lesions, significantly better than the 73.7% reduction with metronidazole. Moreover, 84.9% of the ivermectin group was rated clear or almost clear by Investigator’s Global Assessment (IGA) compared with 75.4% of the metronidazole group.
In addition, 34.9% of ivermectin-treated patients achieved an IGA of 0 by 16 weeks, meaning they were totally lesion free, compared with 21.7% of the metronidazole group. That “completely clear” status is really important psychosocially to a significant proportion of patients, said Dr. Kircik.
“How many times have you had a patient come in asking for an intralesional steroid injection for their single remaining lesion after months of topical treatment?” he asked rhetorically.
Ivermectin was better tolerated than was metronidazole. The rate of treatment-related adverse events leading to discontinuation – typically for skin irritation, redness, or itching – was 0.6% in the ivermectin group compared with 2.1% with metronidazole.
Part B of the ATTRACT study was an unusually designed 36-week extension study.
“In my mind, part B is much more important and much more relevant because rosacea is a chronic disease. It doesn’t last for just 16 weeks. Part B asks what happens after we stop therapy. This is the rare clinical trial that actually mimics real life,” Dr. Kircik said.
Despite physicians’ standard advice to continue with maintenance therapy after clearing, the reality is that most patients stop treatment once they clear, figuring they’ll resume if they relapse, he asserted. Part B of ATTRACT reflected that approach. Seven hundred fifty-seven patients who were clear/almost clear as defined by an IGA of 0/1 at the end of 16 weeks enrolled in the 36-week extension study. They surrendered their medication and returned to their physician monthly. If they had relapsed during that month, meaning they showed up with an IGA of 2 or more, they got their medication back; if they were still IGA 0/1, they did not.
The median time to relapse off treatment in the ivermectin group was 115 days, a full month later than the 85 days for the metronidazole group. Also, the relapse rate in the ivermectin group was significantly lower: 62.7% compared with 68.4% in the metronidazole group.
“The impact on both clinical practice and pharmacoeconomic practice is huge here. It makes sense to switch from metronidazole to ivermectin as first-line therapy because you know that your patients will do better and will relapse later and less,” Dr. Kircik declared.
He was a coinvestigator in the twin pivotal phase III randomized Galderma-funded trials that led to FDA approval of ivermectin for papulopustular rosacea. Although those studies have been published (J. Drugs Dermatol. 2014;13:1380-6), Dr. Kircik highlighted a couple of findings he said haven’t drawn the attention they deserve. Both came from the long-term 40-week extension that followed the initial 12-week, double-blind stage.
One of these underappreciated findings concerned safety. The rate of treatment-related dermatologic adverse events during the double-blind first 3 months of the study was 2.5% in the ivermectin group compared with 6.3% in vehicle-treated controls.
“That’s pretty impressive. That the active treatment arm of the study had less treatment-related dermatologic adverse events than placebo has never been seen before in any other topical study. It tells you something: The assumption here is that the potent inherent anti-inflammatory activity of ivermectin is overwhelming,” the dermatologist said.
The other particularly noteworthy finding came in the long-term, 40-week extension study that followed the initial 12-week, double-blind stage. What was impressive here was the way the proportion of patients who were IGA clear/almost clear rose steadily throughout, he noted. At the end of the initial 3-month phase of the two studies, 38%-40% of ivermectin-treated patients were clear/almost clear. At 12 months, nearly 70% were.
“In most studies, efficacy sort of plateaus at some point. Here it keeps going up through 12 months. So if somebody comes to your office after 3 months using ivermectin and says, ‘Eh, I’m okay, but I’m not clear or almost clear,’ there’s no reason to switch to another medication, because if they continue you know there’s a high chance they will become clear/almost clear,” Dr. Kircik said.
On the other hand, study participants who were still IGA ‘severe’ after 3 months remained severe after 12 months on the drug. So the message here is if a patient still has severe rosacea after 3 months on ivermectin it’s time to change drugs, he added.
EXPERT ANALYSIS FROM WCD 2015
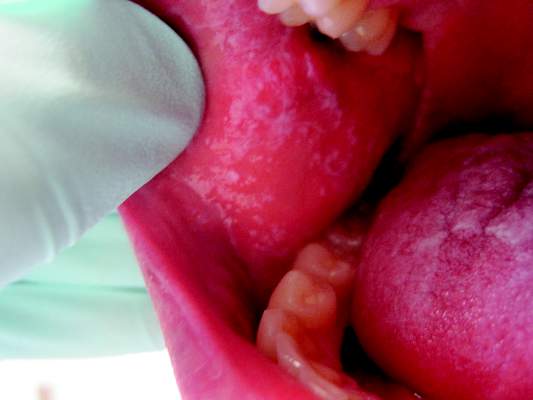
WCD: Red gums raise red flag in oral lichen planus
VANCOUVER – Gum involvement in oral lichen planus raises the risk of multisite or systemic disease, according to Dr. Roy Rogers, professor of dermatology at the Mayo Clinic in Rochester, Minn.
“When you see gingival involvement, this should stimulate you to look very carefully” for disease elsewhere, he said at the World Congress of Dermatology.
About 5% of oral lichen planus patients have involvement in three or more sites, and about 16% have cutaneous lesions, according to Dr. Rogers. Skin, eyes, ears, and nails are all potential targets, as is the esophagus. In fact, a complete oral exam may reveal erosive esophagitis to be an extension of oral lichen planus, which in some patients is painless and overlooked, he said.
Genital lesions are one of the most frequent extraoral manifestations of disease, and are easy to misdiagnose, especially in women. Dr. Rogers recalled a case of a woman who had several vulvovaginal surgeries before her lesions were recognized as lichen planus, and surgical trauma probably made her lesions worse. “A surgical approach is inappropriate until the disease is brought under control,” he said.
Lichen planus and its cutaneous manifestations are associated with hepatitis C, especially the Middle East, Southeast Asia, and the Mediterranean. Screening patients from those areas – as well as those with other risk factors – is appropriate.
Potentially 1% of lesions turn cancerous. Although the risk is low, “this requires regular follow-up either by you, or the patient’s dentist, or both” to monitor lesions, Dr. Rogers emphasized. “I encourage patients to see their dentist twice yearly and me at least yearly, if not more frequently, to renew their prescriptions and make sure everything is going well and not evolving into something else. If something’s not healing, a biopsy is indicated,” he said.
Good dental hygiene and care is a must for patients with oral lichen planus, because any inflammation in the mouth can exacerbate the condition. “Gingivitis, and certainly periodontal disease, should be controlled,” he added.
Fluorinated topical corticosteroids remain the standard of care, applied several times a day to lesions in the mouth or elsewhere to induce a remission, and then less often to maintain remission. Once the condition is under control, some patients need topical steroids only a few times a week.
When used in the mouth, topical corticosteroids can trigger secondary oral candidiasis, which patients might mistake for a flare. If their disease is under control, it almost always turns out to be thrush, said Dr. Rogers.
As an alternative to topical steroids, “I am an enthusiastic user of topical calcineurin inhibitors in oral lichen planus,” such as tacrolimus ointment, he said.
When an audience member asked about the risk of cancer from topical calcineurin inhibitors, Dr. Rogers responded, “it’s very, very low” because little is absorbed systemically. “It’s much more important to control the disease than allow it to continue” because the disease itself can lead to cancer, and treatment reduces the risk, he said.
Calcineurin inhibitors may, however, bump a precancerous lesion into a cancerous one, which is another reason for regular follow-up visits, and systemic treatment is called for in cases extensive disease, he added.
Dr. Rogers had no relevant disclosures.
VANCOUVER – Gum involvement in oral lichen planus raises the risk of multisite or systemic disease, according to Dr. Roy Rogers, professor of dermatology at the Mayo Clinic in Rochester, Minn.
“When you see gingival involvement, this should stimulate you to look very carefully” for disease elsewhere, he said at the World Congress of Dermatology.
About 5% of oral lichen planus patients have involvement in three or more sites, and about 16% have cutaneous lesions, according to Dr. Rogers. Skin, eyes, ears, and nails are all potential targets, as is the esophagus. In fact, a complete oral exam may reveal erosive esophagitis to be an extension of oral lichen planus, which in some patients is painless and overlooked, he said.
Genital lesions are one of the most frequent extraoral manifestations of disease, and are easy to misdiagnose, especially in women. Dr. Rogers recalled a case of a woman who had several vulvovaginal surgeries before her lesions were recognized as lichen planus, and surgical trauma probably made her lesions worse. “A surgical approach is inappropriate until the disease is brought under control,” he said.
Lichen planus and its cutaneous manifestations are associated with hepatitis C, especially the Middle East, Southeast Asia, and the Mediterranean. Screening patients from those areas – as well as those with other risk factors – is appropriate.
Potentially 1% of lesions turn cancerous. Although the risk is low, “this requires regular follow-up either by you, or the patient’s dentist, or both” to monitor lesions, Dr. Rogers emphasized. “I encourage patients to see their dentist twice yearly and me at least yearly, if not more frequently, to renew their prescriptions and make sure everything is going well and not evolving into something else. If something’s not healing, a biopsy is indicated,” he said.
Good dental hygiene and care is a must for patients with oral lichen planus, because any inflammation in the mouth can exacerbate the condition. “Gingivitis, and certainly periodontal disease, should be controlled,” he added.
Fluorinated topical corticosteroids remain the standard of care, applied several times a day to lesions in the mouth or elsewhere to induce a remission, and then less often to maintain remission. Once the condition is under control, some patients need topical steroids only a few times a week.
When used in the mouth, topical corticosteroids can trigger secondary oral candidiasis, which patients might mistake for a flare. If their disease is under control, it almost always turns out to be thrush, said Dr. Rogers.
As an alternative to topical steroids, “I am an enthusiastic user of topical calcineurin inhibitors in oral lichen planus,” such as tacrolimus ointment, he said.
When an audience member asked about the risk of cancer from topical calcineurin inhibitors, Dr. Rogers responded, “it’s very, very low” because little is absorbed systemically. “It’s much more important to control the disease than allow it to continue” because the disease itself can lead to cancer, and treatment reduces the risk, he said.
Calcineurin inhibitors may, however, bump a precancerous lesion into a cancerous one, which is another reason for regular follow-up visits, and systemic treatment is called for in cases extensive disease, he added.
Dr. Rogers had no relevant disclosures.
VANCOUVER – Gum involvement in oral lichen planus raises the risk of multisite or systemic disease, according to Dr. Roy Rogers, professor of dermatology at the Mayo Clinic in Rochester, Minn.
“When you see gingival involvement, this should stimulate you to look very carefully” for disease elsewhere, he said at the World Congress of Dermatology.
About 5% of oral lichen planus patients have involvement in three or more sites, and about 16% have cutaneous lesions, according to Dr. Rogers. Skin, eyes, ears, and nails are all potential targets, as is the esophagus. In fact, a complete oral exam may reveal erosive esophagitis to be an extension of oral lichen planus, which in some patients is painless and overlooked, he said.
Genital lesions are one of the most frequent extraoral manifestations of disease, and are easy to misdiagnose, especially in women. Dr. Rogers recalled a case of a woman who had several vulvovaginal surgeries before her lesions were recognized as lichen planus, and surgical trauma probably made her lesions worse. “A surgical approach is inappropriate until the disease is brought under control,” he said.
Lichen planus and its cutaneous manifestations are associated with hepatitis C, especially the Middle East, Southeast Asia, and the Mediterranean. Screening patients from those areas – as well as those with other risk factors – is appropriate.
Potentially 1% of lesions turn cancerous. Although the risk is low, “this requires regular follow-up either by you, or the patient’s dentist, or both” to monitor lesions, Dr. Rogers emphasized. “I encourage patients to see their dentist twice yearly and me at least yearly, if not more frequently, to renew their prescriptions and make sure everything is going well and not evolving into something else. If something’s not healing, a biopsy is indicated,” he said.
Good dental hygiene and care is a must for patients with oral lichen planus, because any inflammation in the mouth can exacerbate the condition. “Gingivitis, and certainly periodontal disease, should be controlled,” he added.
Fluorinated topical corticosteroids remain the standard of care, applied several times a day to lesions in the mouth or elsewhere to induce a remission, and then less often to maintain remission. Once the condition is under control, some patients need topical steroids only a few times a week.
When used in the mouth, topical corticosteroids can trigger secondary oral candidiasis, which patients might mistake for a flare. If their disease is under control, it almost always turns out to be thrush, said Dr. Rogers.
As an alternative to topical steroids, “I am an enthusiastic user of topical calcineurin inhibitors in oral lichen planus,” such as tacrolimus ointment, he said.
When an audience member asked about the risk of cancer from topical calcineurin inhibitors, Dr. Rogers responded, “it’s very, very low” because little is absorbed systemically. “It’s much more important to control the disease than allow it to continue” because the disease itself can lead to cancer, and treatment reduces the risk, he said.
Calcineurin inhibitors may, however, bump a precancerous lesion into a cancerous one, which is another reason for regular follow-up visits, and systemic treatment is called for in cases extensive disease, he added.
Dr. Rogers had no relevant disclosures.
AT WCD 2015
WCD: Restoring microbiome might ease atopic dermatitis
VANCOUVER, B.C. – Manipulating the skin microbiome might one day help control atopic dermatitis, Dr. Thomas Bieber said at the World Congress of Dermatology.
Scientists used to focus mainly on pathogens when considering how skin microbes affect atopic dermatitis (AD), said Dr. Bieber, professor of dermatology at the University of Bonn (Germany). But while Staphylococcus aureus is “the constant companion” of patients with AD, data from recent studies have shown that commensal microorganisms found on and within healthy skin undergo a “dialogue” with epithelial cells to help regulate antimicrobial peptides, which in turn inhibit pathogen growth, he said.
Flares in atopic dermatitis are marked by dramatic rises in S. aureus and corresponding decreases in commensal microbes, he noted. Whether an AD flare causes this microbial shift or results from it remains unclear, “but based on this knowledge, we are thinking about strategies to modulate and restore the microbiome. We have discovered a new kind of link to how the microbiome might affect atopic dermatitis,” Dr. Bieber explained.
In the future, such strategies might include formulating emollients to rebalance microbial diversity, he said, pointing to the landmark trial of fecal transplantation in patients with recurrent Clostridium difficile infections (N. Engl. J. Med. 2013;368:407-15). “That study was very impressive – the protocol was stopped due to the overwhelming benefits,” he said. “In terms of skin, could we imagine something similar?”
Restoring skin microbial diversity also might help prevent flares and worsening of AD, said Dr. Bieber. “We can control atopic dermatitis by controlling inflammation, dryness, improving the microbiota with appropriate emollients, and trying to hit early and efficiently,” he said. “We should not only think in terms of treatment, but in terms of prevention. Proactive management of atopic dermatitis does not restore or improve the diversity of the microbiome. It is probably not sufficient, and it’s just one part of the approach.”
Other future treatments for AD might target the dendritic cells that normally “bridge” the innate and adaptive immune systems, or the toll-like receptors of innate immune cells, which help regulate production of antimicrobial peptides by commensal microorganisms, Dr. Bieber said.
Recent research has shown that Th2 T cells inhibit antimicrobial peptide production in patients with atopic dermatitis, and that their dendritic cells have a “defective sensing mechanism toward staphylococcal-derived products,” he noted. “They are kind of neutralized or paralyzed, are not able to induce th17 immune response, and are not able to turn on antimicrobial production in the skin.” Restoring this sensing mechanism might one day help reestablish the normal, healthy diversity of the epidermal microbiome and prevent or alleviate AD, he said.
Dr. Bieber reported serving as a consultant, advisory board member, or speaker bureau member for L’Oreal, Galderma, Sanofi, Regeneron, Pfizer, and Novartis.
VANCOUVER, B.C. – Manipulating the skin microbiome might one day help control atopic dermatitis, Dr. Thomas Bieber said at the World Congress of Dermatology.
Scientists used to focus mainly on pathogens when considering how skin microbes affect atopic dermatitis (AD), said Dr. Bieber, professor of dermatology at the University of Bonn (Germany). But while Staphylococcus aureus is “the constant companion” of patients with AD, data from recent studies have shown that commensal microorganisms found on and within healthy skin undergo a “dialogue” with epithelial cells to help regulate antimicrobial peptides, which in turn inhibit pathogen growth, he said.
Flares in atopic dermatitis are marked by dramatic rises in S. aureus and corresponding decreases in commensal microbes, he noted. Whether an AD flare causes this microbial shift or results from it remains unclear, “but based on this knowledge, we are thinking about strategies to modulate and restore the microbiome. We have discovered a new kind of link to how the microbiome might affect atopic dermatitis,” Dr. Bieber explained.
In the future, such strategies might include formulating emollients to rebalance microbial diversity, he said, pointing to the landmark trial of fecal transplantation in patients with recurrent Clostridium difficile infections (N. Engl. J. Med. 2013;368:407-15). “That study was very impressive – the protocol was stopped due to the overwhelming benefits,” he said. “In terms of skin, could we imagine something similar?”
Restoring skin microbial diversity also might help prevent flares and worsening of AD, said Dr. Bieber. “We can control atopic dermatitis by controlling inflammation, dryness, improving the microbiota with appropriate emollients, and trying to hit early and efficiently,” he said. “We should not only think in terms of treatment, but in terms of prevention. Proactive management of atopic dermatitis does not restore or improve the diversity of the microbiome. It is probably not sufficient, and it’s just one part of the approach.”
Other future treatments for AD might target the dendritic cells that normally “bridge” the innate and adaptive immune systems, or the toll-like receptors of innate immune cells, which help regulate production of antimicrobial peptides by commensal microorganisms, Dr. Bieber said.
Recent research has shown that Th2 T cells inhibit antimicrobial peptide production in patients with atopic dermatitis, and that their dendritic cells have a “defective sensing mechanism toward staphylococcal-derived products,” he noted. “They are kind of neutralized or paralyzed, are not able to induce th17 immune response, and are not able to turn on antimicrobial production in the skin.” Restoring this sensing mechanism might one day help reestablish the normal, healthy diversity of the epidermal microbiome and prevent or alleviate AD, he said.
Dr. Bieber reported serving as a consultant, advisory board member, or speaker bureau member for L’Oreal, Galderma, Sanofi, Regeneron, Pfizer, and Novartis.
VANCOUVER, B.C. – Manipulating the skin microbiome might one day help control atopic dermatitis, Dr. Thomas Bieber said at the World Congress of Dermatology.
Scientists used to focus mainly on pathogens when considering how skin microbes affect atopic dermatitis (AD), said Dr. Bieber, professor of dermatology at the University of Bonn (Germany). But while Staphylococcus aureus is “the constant companion” of patients with AD, data from recent studies have shown that commensal microorganisms found on and within healthy skin undergo a “dialogue” with epithelial cells to help regulate antimicrobial peptides, which in turn inhibit pathogen growth, he said.
Flares in atopic dermatitis are marked by dramatic rises in S. aureus and corresponding decreases in commensal microbes, he noted. Whether an AD flare causes this microbial shift or results from it remains unclear, “but based on this knowledge, we are thinking about strategies to modulate and restore the microbiome. We have discovered a new kind of link to how the microbiome might affect atopic dermatitis,” Dr. Bieber explained.
In the future, such strategies might include formulating emollients to rebalance microbial diversity, he said, pointing to the landmark trial of fecal transplantation in patients with recurrent Clostridium difficile infections (N. Engl. J. Med. 2013;368:407-15). “That study was very impressive – the protocol was stopped due to the overwhelming benefits,” he said. “In terms of skin, could we imagine something similar?”
Restoring skin microbial diversity also might help prevent flares and worsening of AD, said Dr. Bieber. “We can control atopic dermatitis by controlling inflammation, dryness, improving the microbiota with appropriate emollients, and trying to hit early and efficiently,” he said. “We should not only think in terms of treatment, but in terms of prevention. Proactive management of atopic dermatitis does not restore or improve the diversity of the microbiome. It is probably not sufficient, and it’s just one part of the approach.”
Other future treatments for AD might target the dendritic cells that normally “bridge” the innate and adaptive immune systems, or the toll-like receptors of innate immune cells, which help regulate production of antimicrobial peptides by commensal microorganisms, Dr. Bieber said.
Recent research has shown that Th2 T cells inhibit antimicrobial peptide production in patients with atopic dermatitis, and that their dendritic cells have a “defective sensing mechanism toward staphylococcal-derived products,” he noted. “They are kind of neutralized or paralyzed, are not able to induce th17 immune response, and are not able to turn on antimicrobial production in the skin.” Restoring this sensing mechanism might one day help reestablish the normal, healthy diversity of the epidermal microbiome and prevent or alleviate AD, he said.
Dr. Bieber reported serving as a consultant, advisory board member, or speaker bureau member for L’Oreal, Galderma, Sanofi, Regeneron, Pfizer, and Novartis.
EXPERT ANALYSIS FROM WCD 2015
WCD: Dermatologists Underappreciate Psoriatic Itch, Patients Say
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
AT WCD 2015
WCD: Dermatologists underappreciate psoriatic itch, patients say
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
AT WCD 2015
Key clinical point: Dermatologists and psoriasis patients don’t see eye to eye when it comes to the importance of itch as the defining symptom of disease severity.
Major finding: Thirty-eight percent of psoriasis patients consider itch to be the most troublesome aspect of their disease and the most important determinant of disease severity, as do 7.4% of dermatologists.
Data source: A random survey of 391 North American and European dermatologists, 390 rheumatologists, and more than 3,000 psoriasis or psoriatic arthritis patients.
Disclosures: The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof is a consultant to and recipient of research grants from Celgene and numerous other pharmaceutical companies.
WCD: Topical squaric Acid May Help Alopecia Areata in Kids
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
AT WCD 2015
WCD: Topical squaric acid may help alopecia areata in kids
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
AT WCD 2015
Key clinical point: Squaric acid may work when other treatments fail in kids with alopecia areata.
Major finding: Fifty percent of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome with squaric acid.
Data source: A study of 10 children with alopecia aged 3-12 years.
Disclosures: There was no outside funding for the work, and Dr. Vatanchi reported having no relevant financial disclosures.
WCD: Psoriasis plus depression magnifies MI risk
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.
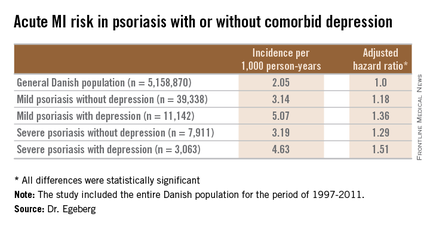
He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.

He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.

He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
AT WCD 2015
Key clinical point: In psoriasis patients, comorbid depression is associated with an increased risk of a first acute MI.
Major finding: The incidence rate of acute MI was 2.05 cases per 1,000 person-years in the general Danish population, climbing to 3.14/1,000 in those with mild psoriasis without diagnosed depression, and 5.07/1,000 for mild psoriasis with depression.
Data source: A retrospective, population-based, cohort study of the entire Danish adult population – more than 5.1 million individuals – during 1997-2011.
Disclosures: The presenter is an employee of Pfizer, which supported the study.
Regular, outcomes-based treatment effective in vulvar lichen sclerosus
VANCOUVER, B.C. – Women with vulvar lichen sclerosus (VLS) who consistently used topical corticosteroids that had been titrated to normalize skin color and texture had significantly better outcomes than patients who sometimes skipped treatment, a prospective single-center cohort study found.
Most notably, none of the treatment-compliant patients developed vulvar carcinoma during an average of almost 5 years of follow-up, compared with 4.7% of the partially compliant group (P < .001), reported Dr. Jason Lee, a dermatologist with the University of Sydney, Australia.
“We are proposing a paradigm shift to change the way we manage this condition,” Dr. Lee said at the World Congress of Dermatology. “Clinicians should aim for individual regular treatment of patients with vulvar lichen sclerosus. The treatment is safe, and the course of the disease can be altered by treatment.”
The report appeared simultaneously in JAMA Dermatology (2015 June 12 [doi:10.1001/jamadermatol.2015.0643]).
Vulvar lichen sclerosus is an uncommon disease that causes vulvar itching, pain, and sexual dysfunction, and can significantly alter the vulvar architecture. Before clinicians began treating VLS with superpotent topical corticosteroids, about 5% of cases were thought to progress to vulvar intraepithelial neoplasia or invasive squamous cell carcinoma.
Furthermore, even fairly mild cases can become cancerous, Dr. Lee noted. “Until now, it was thought that cancer was inevitable in some women with VLS,” he added. “Short-term control is easy, but long-term management lacks data, and research has not addressed whether treatment can prevent scarring or cancer.”
To assess the impact of individualized topical corticosteroid therapy with regular follow-up, Dr. Lee and his associates studied 507 women with biopsy-proven VLS during 2008-2014. Patients averaged 55 years of age at presentation and had an average symptom history of 5 years.
The investigators graded cases as mild, moderate, severe, or very severe depending on degree of hyperkeratosis, and titrated treatment with the goal of normalizing skin color and texture. They classified patients as treatment-compliant if they said they followed treatment directions most or all of the time, and as partially compliant if they said they followed directions some, little, or none of the time. They saw patients at least once a year for follow-up.
Most patients initially needed superpotent or ultrapotent topical corticosteroids to control their disease and then switched to moderate or mild strength formulations to maintain results, Dr. Lee and his associates reported.
About 30% of patients were only partially compliant with treatment. These patients were significantly less likely to achieve symptom resolution (58% vs. 93%) and resolution of dyspareunia (66% vs. 94%), and were more likely to develop adhesions and scars during follow-up (40% vs. 3%), compared with fully compliant patients (P < .001 for all), the researchers said. Rates of steroid dermatitis and reversible steroid-induced cutaneous atrophy were similar between the fully and partially compliant groups (2.2% vs. 4%, and 1.1% vs. 2%, respectively).
Based on the findings, clinicians would need to treat 21 cases of VLS to prevent 1 case of vulvar cancer and 2.7 cases to prevent scarring, said Dr. Lee.
“This is the first study adequately powered to demonstrate that cancer and scarring can be prevented by regular treatment according to clinical outcome, not symptoms,” he added.
But Dr. Lee acknowledged that selection bias could have affected the results. “We did find that patients who were not complying with treatment were more likely to have severe disease at the start of the study,” he said. “They probably were at increased risk of cancer to start with.”
The dermatology department of Royal North Shore Hospital partially funded the work. The investigators reported having no relevant conflicts of interest.
We congratulate the authors on an article that has profound implications for patients with vulvar lichen sclerosus and their physicians worldwide. Until now, suppression of the disease has concerned physicians and has often been inadequate. The reduction in cancer among patients with VLS who were treated with [topical corticosteroids] is no longer speculative. This focus of concern can effectively communicate the message that long-term use of a TCS likely prevents the progression of VLS and the development of squamous cell carcinoma.
In the study, cohort treatment was tailored to the severity of disease, using the presence or absence and degree of hyperkeratosis as the principal marker of severity. The most severe cases with extensive hyperkeratosis were treated twice daily with a superpotent TCS and less severe cases with a reduced-potency TCS for the initial treatment period.
This use is in contrast to the recommendations of guidelines. Starting with a superpotent corticosteroid has been advocated for all patients either as an initial 3-month once-daily regimen or a tapered regimen for 3 months. Signs at presentation vary considerably, with some women displaying severe widespread genital pallor, hyperkeratosis, fissures, and erosions, whereas others show subtle pallor; there are yet others who are asymptomatic. Hyperkeratosis alone may not be a sufficient marker of active disease, because ecchymoses and erosions may also be important. Tailoring treatment to the severity of the disease makes sense, because in other inflammatory diseases, such as atopic eczema, dermatologists routinely tailor the potency of the corticosteroid used to the severity of the disease.
This article is an important contribution to our knowledge, providing the first evidence in women of the association between malignant neoplasms and poor compliance with treatment. This information will allow us to better inform our patients and encourage those who find it difficult to comply for various reasons.
Dr. Susan M. Cooper is with the department of dermatology, Oxford University Hospitals Trust, Oxford, England. Dr. Nina Madnani is with the department of dermatology, P.D. Hinduja National Hospital and Medical Research Center, Mumbai. Dr. Lynnette Margesson is with the Geisel School of Medicine, Dartmouth College, Hanover, N.H. They disclosed no conflicts of interest. These comments were excerpted from their accompanying editorial (JAMA Dermatol. 2015 June 12 [doi:10.1001/jamadermatol.2015.0644]).
We congratulate the authors on an article that has profound implications for patients with vulvar lichen sclerosus and their physicians worldwide. Until now, suppression of the disease has concerned physicians and has often been inadequate. The reduction in cancer among patients with VLS who were treated with [topical corticosteroids] is no longer speculative. This focus of concern can effectively communicate the message that long-term use of a TCS likely prevents the progression of VLS and the development of squamous cell carcinoma.
In the study, cohort treatment was tailored to the severity of disease, using the presence or absence and degree of hyperkeratosis as the principal marker of severity. The most severe cases with extensive hyperkeratosis were treated twice daily with a superpotent TCS and less severe cases with a reduced-potency TCS for the initial treatment period.
This use is in contrast to the recommendations of guidelines. Starting with a superpotent corticosteroid has been advocated for all patients either as an initial 3-month once-daily regimen or a tapered regimen for 3 months. Signs at presentation vary considerably, with some women displaying severe widespread genital pallor, hyperkeratosis, fissures, and erosions, whereas others show subtle pallor; there are yet others who are asymptomatic. Hyperkeratosis alone may not be a sufficient marker of active disease, because ecchymoses and erosions may also be important. Tailoring treatment to the severity of the disease makes sense, because in other inflammatory diseases, such as atopic eczema, dermatologists routinely tailor the potency of the corticosteroid used to the severity of the disease.
This article is an important contribution to our knowledge, providing the first evidence in women of the association between malignant neoplasms and poor compliance with treatment. This information will allow us to better inform our patients and encourage those who find it difficult to comply for various reasons.
Dr. Susan M. Cooper is with the department of dermatology, Oxford University Hospitals Trust, Oxford, England. Dr. Nina Madnani is with the department of dermatology, P.D. Hinduja National Hospital and Medical Research Center, Mumbai. Dr. Lynnette Margesson is with the Geisel School of Medicine, Dartmouth College, Hanover, N.H. They disclosed no conflicts of interest. These comments were excerpted from their accompanying editorial (JAMA Dermatol. 2015 June 12 [doi:10.1001/jamadermatol.2015.0644]).
We congratulate the authors on an article that has profound implications for patients with vulvar lichen sclerosus and their physicians worldwide. Until now, suppression of the disease has concerned physicians and has often been inadequate. The reduction in cancer among patients with VLS who were treated with [topical corticosteroids] is no longer speculative. This focus of concern can effectively communicate the message that long-term use of a TCS likely prevents the progression of VLS and the development of squamous cell carcinoma.
In the study, cohort treatment was tailored to the severity of disease, using the presence or absence and degree of hyperkeratosis as the principal marker of severity. The most severe cases with extensive hyperkeratosis were treated twice daily with a superpotent TCS and less severe cases with a reduced-potency TCS for the initial treatment period.
This use is in contrast to the recommendations of guidelines. Starting with a superpotent corticosteroid has been advocated for all patients either as an initial 3-month once-daily regimen or a tapered regimen for 3 months. Signs at presentation vary considerably, with some women displaying severe widespread genital pallor, hyperkeratosis, fissures, and erosions, whereas others show subtle pallor; there are yet others who are asymptomatic. Hyperkeratosis alone may not be a sufficient marker of active disease, because ecchymoses and erosions may also be important. Tailoring treatment to the severity of the disease makes sense, because in other inflammatory diseases, such as atopic eczema, dermatologists routinely tailor the potency of the corticosteroid used to the severity of the disease.
This article is an important contribution to our knowledge, providing the first evidence in women of the association between malignant neoplasms and poor compliance with treatment. This information will allow us to better inform our patients and encourage those who find it difficult to comply for various reasons.
Dr. Susan M. Cooper is with the department of dermatology, Oxford University Hospitals Trust, Oxford, England. Dr. Nina Madnani is with the department of dermatology, P.D. Hinduja National Hospital and Medical Research Center, Mumbai. Dr. Lynnette Margesson is with the Geisel School of Medicine, Dartmouth College, Hanover, N.H. They disclosed no conflicts of interest. These comments were excerpted from their accompanying editorial (JAMA Dermatol. 2015 June 12 [doi:10.1001/jamadermatol.2015.0644]).
VANCOUVER, B.C. – Women with vulvar lichen sclerosus (VLS) who consistently used topical corticosteroids that had been titrated to normalize skin color and texture had significantly better outcomes than patients who sometimes skipped treatment, a prospective single-center cohort study found.
Most notably, none of the treatment-compliant patients developed vulvar carcinoma during an average of almost 5 years of follow-up, compared with 4.7% of the partially compliant group (P < .001), reported Dr. Jason Lee, a dermatologist with the University of Sydney, Australia.
“We are proposing a paradigm shift to change the way we manage this condition,” Dr. Lee said at the World Congress of Dermatology. “Clinicians should aim for individual regular treatment of patients with vulvar lichen sclerosus. The treatment is safe, and the course of the disease can be altered by treatment.”
The report appeared simultaneously in JAMA Dermatology (2015 June 12 [doi:10.1001/jamadermatol.2015.0643]).
Vulvar lichen sclerosus is an uncommon disease that causes vulvar itching, pain, and sexual dysfunction, and can significantly alter the vulvar architecture. Before clinicians began treating VLS with superpotent topical corticosteroids, about 5% of cases were thought to progress to vulvar intraepithelial neoplasia or invasive squamous cell carcinoma.
Furthermore, even fairly mild cases can become cancerous, Dr. Lee noted. “Until now, it was thought that cancer was inevitable in some women with VLS,” he added. “Short-term control is easy, but long-term management lacks data, and research has not addressed whether treatment can prevent scarring or cancer.”
To assess the impact of individualized topical corticosteroid therapy with regular follow-up, Dr. Lee and his associates studied 507 women with biopsy-proven VLS during 2008-2014. Patients averaged 55 years of age at presentation and had an average symptom history of 5 years.
The investigators graded cases as mild, moderate, severe, or very severe depending on degree of hyperkeratosis, and titrated treatment with the goal of normalizing skin color and texture. They classified patients as treatment-compliant if they said they followed treatment directions most or all of the time, and as partially compliant if they said they followed directions some, little, or none of the time. They saw patients at least once a year for follow-up.
Most patients initially needed superpotent or ultrapotent topical corticosteroids to control their disease and then switched to moderate or mild strength formulations to maintain results, Dr. Lee and his associates reported.
About 30% of patients were only partially compliant with treatment. These patients were significantly less likely to achieve symptom resolution (58% vs. 93%) and resolution of dyspareunia (66% vs. 94%), and were more likely to develop adhesions and scars during follow-up (40% vs. 3%), compared with fully compliant patients (P < .001 for all), the researchers said. Rates of steroid dermatitis and reversible steroid-induced cutaneous atrophy were similar between the fully and partially compliant groups (2.2% vs. 4%, and 1.1% vs. 2%, respectively).
Based on the findings, clinicians would need to treat 21 cases of VLS to prevent 1 case of vulvar cancer and 2.7 cases to prevent scarring, said Dr. Lee.
“This is the first study adequately powered to demonstrate that cancer and scarring can be prevented by regular treatment according to clinical outcome, not symptoms,” he added.
But Dr. Lee acknowledged that selection bias could have affected the results. “We did find that patients who were not complying with treatment were more likely to have severe disease at the start of the study,” he said. “They probably were at increased risk of cancer to start with.”
The dermatology department of Royal North Shore Hospital partially funded the work. The investigators reported having no relevant conflicts of interest.
VANCOUVER, B.C. – Women with vulvar lichen sclerosus (VLS) who consistently used topical corticosteroids that had been titrated to normalize skin color and texture had significantly better outcomes than patients who sometimes skipped treatment, a prospective single-center cohort study found.
Most notably, none of the treatment-compliant patients developed vulvar carcinoma during an average of almost 5 years of follow-up, compared with 4.7% of the partially compliant group (P < .001), reported Dr. Jason Lee, a dermatologist with the University of Sydney, Australia.
“We are proposing a paradigm shift to change the way we manage this condition,” Dr. Lee said at the World Congress of Dermatology. “Clinicians should aim for individual regular treatment of patients with vulvar lichen sclerosus. The treatment is safe, and the course of the disease can be altered by treatment.”
The report appeared simultaneously in JAMA Dermatology (2015 June 12 [doi:10.1001/jamadermatol.2015.0643]).
Vulvar lichen sclerosus is an uncommon disease that causes vulvar itching, pain, and sexual dysfunction, and can significantly alter the vulvar architecture. Before clinicians began treating VLS with superpotent topical corticosteroids, about 5% of cases were thought to progress to vulvar intraepithelial neoplasia or invasive squamous cell carcinoma.
Furthermore, even fairly mild cases can become cancerous, Dr. Lee noted. “Until now, it was thought that cancer was inevitable in some women with VLS,” he added. “Short-term control is easy, but long-term management lacks data, and research has not addressed whether treatment can prevent scarring or cancer.”
To assess the impact of individualized topical corticosteroid therapy with regular follow-up, Dr. Lee and his associates studied 507 women with biopsy-proven VLS during 2008-2014. Patients averaged 55 years of age at presentation and had an average symptom history of 5 years.
The investigators graded cases as mild, moderate, severe, or very severe depending on degree of hyperkeratosis, and titrated treatment with the goal of normalizing skin color and texture. They classified patients as treatment-compliant if they said they followed treatment directions most or all of the time, and as partially compliant if they said they followed directions some, little, or none of the time. They saw patients at least once a year for follow-up.
Most patients initially needed superpotent or ultrapotent topical corticosteroids to control their disease and then switched to moderate or mild strength formulations to maintain results, Dr. Lee and his associates reported.
About 30% of patients were only partially compliant with treatment. These patients were significantly less likely to achieve symptom resolution (58% vs. 93%) and resolution of dyspareunia (66% vs. 94%), and were more likely to develop adhesions and scars during follow-up (40% vs. 3%), compared with fully compliant patients (P < .001 for all), the researchers said. Rates of steroid dermatitis and reversible steroid-induced cutaneous atrophy were similar between the fully and partially compliant groups (2.2% vs. 4%, and 1.1% vs. 2%, respectively).
Based on the findings, clinicians would need to treat 21 cases of VLS to prevent 1 case of vulvar cancer and 2.7 cases to prevent scarring, said Dr. Lee.
“This is the first study adequately powered to demonstrate that cancer and scarring can be prevented by regular treatment according to clinical outcome, not symptoms,” he added.
But Dr. Lee acknowledged that selection bias could have affected the results. “We did find that patients who were not complying with treatment were more likely to have severe disease at the start of the study,” he said. “They probably were at increased risk of cancer to start with.”
The dermatology department of Royal North Shore Hospital partially funded the work. The investigators reported having no relevant conflicts of interest.
AT WCD 2015
Key clinical point: Women with vulvar lichen sclerosus who consistently used topical corticosteroids titrated to normalize skin color and texture had significantly better symptoms, scarring, and rates of vulvar cancer compared with partially compliant patients.
Major finding: None of the women who consistently followed their individualized treatment plan developed vulvar carcinoma, compared with 4.7% of partially compliant patients (P < .001).
Data source: Single-center prospective, observational cohort study of 507 women with biopsy-proven vulvar lichen sclerosus.
Disclosures: The dermatology department of Royal North Shore Hospital partially funded the work. Dr. Lee reported having no relevant conflicts of interest.
Expert critiques isotretinoin controversies
VANCOUVER – Controversies surrounding isotretinoin are mostly unfounded, Dr. Neil Shear said at the World Congress of Dermatology.
“Isotretinoin is the most effective treatment for all grades of acne vulgaris,” added Dr. Shear, professor and chief of dermatology at the University of Toronto Medical School. “Isotretinoin causes fetal malformations, and most preventative strategies around pregnancy have not been completely successful. Isotretinoin does not cause inflammatory disease, nor irritable bowel, but it can, rarely, cause depression,” he said. “If you agree with all those things, I don’t find it very controversial.”
In fact, there exists “a very good argument” for using isotretinoin as a first-line therapy for some patients with acne, said Dr. Shear. “I would be for it,” he said. “It has its issues, and they do have to be managed, but we know the issues behind systemic antibiotics might be larger than the issues behind isotretinoin.”
Safety concerns about isotretinoin have spurred dozens of lawsuits in the United States, many of which allege that the medication caused inflammatory bowel disease (IBD). But despite split verdicts and millions of dollars awarded to some plaintiffs, the best available evidence does not support a causal association between isotretinoin and IBD, Dr. Shear asserted. He pointed to a large population-based cohort study that found no association between isotretinoin and IBD in its primary analysis, although prespecified secondary analyses linked IBD with both isotretinoin and topical acne medications. “The conclusion could only be that if there is increased IBD, it is associated with acne, not with the therapy,” Dr. Shear said. “The arrow has been pointing us in this direction for over a decade now, and I am hoping there will be bigger studies to show the blame is with acne, not isotretinoin.”
Isotretinoin has been associated with clinical depression, which is sometimes preceded by onset of new headache, Dr. Shear said. He has seen some patients become depressed on the medication, he added. “But untreated acne is associated with depression and even suicidal ideation sometimes. I think it’s quite manageable, personally,” he said. “It’s all part of the risk management of using a medication like isotretinoin. Patients who are depressed with acne, even when they have dry lips and other side effects, are so much happier with isotretinoin.”
In fact, Dr. Shear would not rule out isotretinoin for acne patients who have a history of clinical depression, although he would first consider other options such as decreasing dietary glycemic load or retrying “failed” treatments to ensure that patients gave them an adequate trial, he said.
Because of its high risk of severe fetal malformations, dermatologists who prescribe isotretinoin need to ensure that female patients have a specific plan for using effective birth control, Dr. Shear emphasized. But the risk of birth defects does not negate the need for the drug, he said. “Companies have tried hard to make a similar retinoid that does not affect the fetus and cause birth defects and have not been successful.”
The hypothesis that isotretinoin adversely affects pediatric bone growth has been debunked, Dr. Shear said, in response to a question from the audience, citing a study published in the journal Osteoporosis International.
Dr. Shear reported having no relevant conflicts of interest except having worked with Roche on rituximab. Roche formerly manufactured and marketed isotretinoin (Accutane) in the United States, but no longer does so.
VANCOUVER – Controversies surrounding isotretinoin are mostly unfounded, Dr. Neil Shear said at the World Congress of Dermatology.
“Isotretinoin is the most effective treatment for all grades of acne vulgaris,” added Dr. Shear, professor and chief of dermatology at the University of Toronto Medical School. “Isotretinoin causes fetal malformations, and most preventative strategies around pregnancy have not been completely successful. Isotretinoin does not cause inflammatory disease, nor irritable bowel, but it can, rarely, cause depression,” he said. “If you agree with all those things, I don’t find it very controversial.”
In fact, there exists “a very good argument” for using isotretinoin as a first-line therapy for some patients with acne, said Dr. Shear. “I would be for it,” he said. “It has its issues, and they do have to be managed, but we know the issues behind systemic antibiotics might be larger than the issues behind isotretinoin.”
Safety concerns about isotretinoin have spurred dozens of lawsuits in the United States, many of which allege that the medication caused inflammatory bowel disease (IBD). But despite split verdicts and millions of dollars awarded to some plaintiffs, the best available evidence does not support a causal association between isotretinoin and IBD, Dr. Shear asserted. He pointed to a large population-based cohort study that found no association between isotretinoin and IBD in its primary analysis, although prespecified secondary analyses linked IBD with both isotretinoin and topical acne medications. “The conclusion could only be that if there is increased IBD, it is associated with acne, not with the therapy,” Dr. Shear said. “The arrow has been pointing us in this direction for over a decade now, and I am hoping there will be bigger studies to show the blame is with acne, not isotretinoin.”
Isotretinoin has been associated with clinical depression, which is sometimes preceded by onset of new headache, Dr. Shear said. He has seen some patients become depressed on the medication, he added. “But untreated acne is associated with depression and even suicidal ideation sometimes. I think it’s quite manageable, personally,” he said. “It’s all part of the risk management of using a medication like isotretinoin. Patients who are depressed with acne, even when they have dry lips and other side effects, are so much happier with isotretinoin.”
In fact, Dr. Shear would not rule out isotretinoin for acne patients who have a history of clinical depression, although he would first consider other options such as decreasing dietary glycemic load or retrying “failed” treatments to ensure that patients gave them an adequate trial, he said.
Because of its high risk of severe fetal malformations, dermatologists who prescribe isotretinoin need to ensure that female patients have a specific plan for using effective birth control, Dr. Shear emphasized. But the risk of birth defects does not negate the need for the drug, he said. “Companies have tried hard to make a similar retinoid that does not affect the fetus and cause birth defects and have not been successful.”
The hypothesis that isotretinoin adversely affects pediatric bone growth has been debunked, Dr. Shear said, in response to a question from the audience, citing a study published in the journal Osteoporosis International.
Dr. Shear reported having no relevant conflicts of interest except having worked with Roche on rituximab. Roche formerly manufactured and marketed isotretinoin (Accutane) in the United States, but no longer does so.
VANCOUVER – Controversies surrounding isotretinoin are mostly unfounded, Dr. Neil Shear said at the World Congress of Dermatology.
“Isotretinoin is the most effective treatment for all grades of acne vulgaris,” added Dr. Shear, professor and chief of dermatology at the University of Toronto Medical School. “Isotretinoin causes fetal malformations, and most preventative strategies around pregnancy have not been completely successful. Isotretinoin does not cause inflammatory disease, nor irritable bowel, but it can, rarely, cause depression,” he said. “If you agree with all those things, I don’t find it very controversial.”
In fact, there exists “a very good argument” for using isotretinoin as a first-line therapy for some patients with acne, said Dr. Shear. “I would be for it,” he said. “It has its issues, and they do have to be managed, but we know the issues behind systemic antibiotics might be larger than the issues behind isotretinoin.”
Safety concerns about isotretinoin have spurred dozens of lawsuits in the United States, many of which allege that the medication caused inflammatory bowel disease (IBD). But despite split verdicts and millions of dollars awarded to some plaintiffs, the best available evidence does not support a causal association between isotretinoin and IBD, Dr. Shear asserted. He pointed to a large population-based cohort study that found no association between isotretinoin and IBD in its primary analysis, although prespecified secondary analyses linked IBD with both isotretinoin and topical acne medications. “The conclusion could only be that if there is increased IBD, it is associated with acne, not with the therapy,” Dr. Shear said. “The arrow has been pointing us in this direction for over a decade now, and I am hoping there will be bigger studies to show the blame is with acne, not isotretinoin.”
Isotretinoin has been associated with clinical depression, which is sometimes preceded by onset of new headache, Dr. Shear said. He has seen some patients become depressed on the medication, he added. “But untreated acne is associated with depression and even suicidal ideation sometimes. I think it’s quite manageable, personally,” he said. “It’s all part of the risk management of using a medication like isotretinoin. Patients who are depressed with acne, even when they have dry lips and other side effects, are so much happier with isotretinoin.”
In fact, Dr. Shear would not rule out isotretinoin for acne patients who have a history of clinical depression, although he would first consider other options such as decreasing dietary glycemic load or retrying “failed” treatments to ensure that patients gave them an adequate trial, he said.
Because of its high risk of severe fetal malformations, dermatologists who prescribe isotretinoin need to ensure that female patients have a specific plan for using effective birth control, Dr. Shear emphasized. But the risk of birth defects does not negate the need for the drug, he said. “Companies have tried hard to make a similar retinoid that does not affect the fetus and cause birth defects and have not been successful.”
The hypothesis that isotretinoin adversely affects pediatric bone growth has been debunked, Dr. Shear said, in response to a question from the audience, citing a study published in the journal Osteoporosis International.
Dr. Shear reported having no relevant conflicts of interest except having worked with Roche on rituximab. Roche formerly manufactured and marketed isotretinoin (Accutane) in the United States, but no longer does so.
AT WCD 2015