User login
The Official Newspaper of the American Association for Thoracic Surgery
Apply for the Cardiothoracic Ethics Forum Scholarship
The Cardiothoracic Ethics Forum Scholarship, supported by the AATS and STS, provides opportunities for intellectual development and preparation for leadership roles in CT ethics.
The scholarships of up to $10,000 give interested CT surgeons the opportunity to obtain formal education and training in biomedical ethics through any of the several programs offered by leading ethics centers in North America.
To be eligible to apply, applicants must be a member of either the AATS or STS. The application deadline is July 1.
For more information and to apply, please visit:
www.ctsnet.org/cardiothoracic-ethics-forum-scholarship
The Cardiothoracic Ethics Forum Scholarship, supported by the AATS and STS, provides opportunities for intellectual development and preparation for leadership roles in CT ethics.
The scholarships of up to $10,000 give interested CT surgeons the opportunity to obtain formal education and training in biomedical ethics through any of the several programs offered by leading ethics centers in North America.
To be eligible to apply, applicants must be a member of either the AATS or STS. The application deadline is July 1.
For more information and to apply, please visit:
www.ctsnet.org/cardiothoracic-ethics-forum-scholarship
The Cardiothoracic Ethics Forum Scholarship, supported by the AATS and STS, provides opportunities for intellectual development and preparation for leadership roles in CT ethics.
The scholarships of up to $10,000 give interested CT surgeons the opportunity to obtain formal education and training in biomedical ethics through any of the several programs offered by leading ethics centers in North America.
To be eligible to apply, applicants must be a member of either the AATS or STS. The application deadline is July 1.
For more information and to apply, please visit:
www.ctsnet.org/cardiothoracic-ethics-forum-scholarship
Save the Date: 2017 AATS Focus on Thoracic Surgery: Mastering Surgical Innovation
Mark your calendar for the 2017 AATS Focus on Thoracic Surgery meeting taking place in a new location this year - Las Vegas.
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
For more informaton, go to:
aats.org/focus
Mark your calendar for the 2017 AATS Focus on Thoracic Surgery meeting taking place in a new location this year - Las Vegas.
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
For more informaton, go to:
aats.org/focus
Mark your calendar for the 2017 AATS Focus on Thoracic Surgery meeting taking place in a new location this year - Las Vegas.
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
For more informaton, go to:
aats.org/focus
Register for the 2017 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer
You can now register for the 2017 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting.
Lung cancer and esophageal cancer remain a global concern for patients and thoracic surgeons as two of the deadliest malignancies worldwide. The 2017 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting will concentrate on the complex issues involved with treating both lung and esophageal malignancies, while also discussing innovative approaches to improve quality and outcomes.
The two-day program features a mixture of lectures, relevant case studies, interactive panel discussions and videos. The faculty includes Chinese and internationally recognized experts in lung and esophageal disease ensuring attendees will receive insights from a broad spectrum of leaders in thoracic surgery.
June 30 - July 1, 2017
Beijing, National Conference Center
Beijing, China
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Ke-Neng Chen
Jie He
For more information, visit: aats.org/focuschina
You can now register for the 2017 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting.
Lung cancer and esophageal cancer remain a global concern for patients and thoracic surgeons as two of the deadliest malignancies worldwide. The 2017 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting will concentrate on the complex issues involved with treating both lung and esophageal malignancies, while also discussing innovative approaches to improve quality and outcomes.
The two-day program features a mixture of lectures, relevant case studies, interactive panel discussions and videos. The faculty includes Chinese and internationally recognized experts in lung and esophageal disease ensuring attendees will receive insights from a broad spectrum of leaders in thoracic surgery.
June 30 - July 1, 2017
Beijing, National Conference Center
Beijing, China
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Ke-Neng Chen
Jie He
For more information, visit: aats.org/focuschina
You can now register for the 2017 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting.
Lung cancer and esophageal cancer remain a global concern for patients and thoracic surgeons as two of the deadliest malignancies worldwide. The 2017 AATS Focus on Thoracic Surgery: Lung and Esophageal Cancer meeting will concentrate on the complex issues involved with treating both lung and esophageal malignancies, while also discussing innovative approaches to improve quality and outcomes.
The two-day program features a mixture of lectures, relevant case studies, interactive panel discussions and videos. The faculty includes Chinese and internationally recognized experts in lung and esophageal disease ensuring attendees will receive insights from a broad spectrum of leaders in thoracic surgery.
June 30 - July 1, 2017
Beijing, National Conference Center
Beijing, China
Program Directors
G. Alexander Patterson
David J. Sugarbaker
Ke-Neng Chen
Jie He
For more information, visit: aats.org/focuschina
Supreme Court: Faith-based hospitals are exempt from federal pension requirements
The U.S. Supreme Court has ruled that faith-based hospitals are exempt from federal pension requirements, holding that employees of religious-affiliated entities are not protected under the Employee Retirement Income Security Act (ERISA). The unanimous decision overturns rulings in the three circuit courts of appeals.
The opinion preserves the status quo, so it should not have an immediate effect on the affected employers and employees, said Ronald J. Mann, a law professor at Columbia University in New York who has written about one case, Advocate Health Care Network v. Stapleton, for Scotusblog.com.
The high court’s opinion centers on logic and the plain language of the ERISA statute, which is sensible, said Paul Secunda, a law professor and director of the labor and employment law program at Marquette University in Milwaukee. However, the decision does not address the larger question that matters to physicians and hospitals, he said.
“Some of these church-affiliated hospitals look an awful lot like their secular competitors,” he said. “They have thousands of employees. They make billions of dollars in revenue.”
Associate Justice Sonia Sotomayor raised similar concerns in a concurring opinion to the decision. While she agreed with her fellow justices on the ruling, she said the case outcome was still troubling.
“Despite their relationship to churches, organizations such as petitioners operate for-profit subsidiaries ... and compete in the secular market with companies that must bear the cost of complying with ERISA,” Justice Sotomayor wrote.
The Supreme Court ruling comes after current and former employees of three hospital chains – Dignity Health, Advocate Health Care, and Saint Peter’s Healthcare System – sued their employers in an effort to make them comply with ERISA. The employees argued that pension plans established by the large health care providers should not fall under ERISA’s “church plan” exception because the plans were not established by churches. ERISA requires that all private employers offering pension plans adhere to federal requirements designed to ensure plan solvency and protect plan participants.
The hospitals argued that pension plans do not have to be established by a church for the ERISA exemption to apply because the plans are maintained by qualifying church-affiliated organizations. The defendants based their position on a 1980 ERISA amendment that included the “maintained” exception. Since the amendment, the federal agencies charged with interpreting ERISA – the IRS, the Department of Labor, and the Pension Benefit Guaranty Corporation – have issued opinion after opinion reaffirming that view, the defendants noted.
Courts of appeals for the Third, Seventh, and Ninth circuits agreed with the employees, concluding that ERISA’s “plain text” requires that a pension plan be established by a church to qualify for the church-plan exemption.
The Supreme Court disagreed, however, ruling that a defined-benefit pension plan maintained by a principal-purpose organization – one controlled by or associate
However, the hospital sector has changed dramatically since the 1980 ERISA amendment, Mr. Secunda pointed out. “If you think back to when this reform was passed ... almost 40 years ago, we have a whole different animal [today] with regard to these large church-oriented hospital organizations.”
Justice Sotomayor raised similar concerns. “These organizations thus bear little resemblance to those Congress considered when enacting the 1980 amendment to the church plan definition. This current reality might prompt Congress to take a different path,” she wrote.
The legal battle over whether faith-based hospitals should comply with ERISA is not likely over, according to Mr. Secunda.
“This is not the end of the story,” he said. “This is just the beginning, and now the next big issue to be decided will be, okay, principal purpose organizations come under the church plan exemption, but do these large billion-dollar hospitals qualify? Are they principal purpose organizations? I think that’s a very open question.”
[email protected]
On Twitter @legal_med
The U.S. Supreme Court has ruled that faith-based hospitals are exempt from federal pension requirements, holding that employees of religious-affiliated entities are not protected under the Employee Retirement Income Security Act (ERISA). The unanimous decision overturns rulings in the three circuit courts of appeals.
The opinion preserves the status quo, so it should not have an immediate effect on the affected employers and employees, said Ronald J. Mann, a law professor at Columbia University in New York who has written about one case, Advocate Health Care Network v. Stapleton, for Scotusblog.com.
The high court’s opinion centers on logic and the plain language of the ERISA statute, which is sensible, said Paul Secunda, a law professor and director of the labor and employment law program at Marquette University in Milwaukee. However, the decision does not address the larger question that matters to physicians and hospitals, he said.
“Some of these church-affiliated hospitals look an awful lot like their secular competitors,” he said. “They have thousands of employees. They make billions of dollars in revenue.”
Associate Justice Sonia Sotomayor raised similar concerns in a concurring opinion to the decision. While she agreed with her fellow justices on the ruling, she said the case outcome was still troubling.
“Despite their relationship to churches, organizations such as petitioners operate for-profit subsidiaries ... and compete in the secular market with companies that must bear the cost of complying with ERISA,” Justice Sotomayor wrote.
The Supreme Court ruling comes after current and former employees of three hospital chains – Dignity Health, Advocate Health Care, and Saint Peter’s Healthcare System – sued their employers in an effort to make them comply with ERISA. The employees argued that pension plans established by the large health care providers should not fall under ERISA’s “church plan” exception because the plans were not established by churches. ERISA requires that all private employers offering pension plans adhere to federal requirements designed to ensure plan solvency and protect plan participants.
The hospitals argued that pension plans do not have to be established by a church for the ERISA exemption to apply because the plans are maintained by qualifying church-affiliated organizations. The defendants based their position on a 1980 ERISA amendment that included the “maintained” exception. Since the amendment, the federal agencies charged with interpreting ERISA – the IRS, the Department of Labor, and the Pension Benefit Guaranty Corporation – have issued opinion after opinion reaffirming that view, the defendants noted.
Courts of appeals for the Third, Seventh, and Ninth circuits agreed with the employees, concluding that ERISA’s “plain text” requires that a pension plan be established by a church to qualify for the church-plan exemption.
The Supreme Court disagreed, however, ruling that a defined-benefit pension plan maintained by a principal-purpose organization – one controlled by or associate
However, the hospital sector has changed dramatically since the 1980 ERISA amendment, Mr. Secunda pointed out. “If you think back to when this reform was passed ... almost 40 years ago, we have a whole different animal [today] with regard to these large church-oriented hospital organizations.”
Justice Sotomayor raised similar concerns. “These organizations thus bear little resemblance to those Congress considered when enacting the 1980 amendment to the church plan definition. This current reality might prompt Congress to take a different path,” she wrote.
The legal battle over whether faith-based hospitals should comply with ERISA is not likely over, according to Mr. Secunda.
“This is not the end of the story,” he said. “This is just the beginning, and now the next big issue to be decided will be, okay, principal purpose organizations come under the church plan exemption, but do these large billion-dollar hospitals qualify? Are they principal purpose organizations? I think that’s a very open question.”
[email protected]
On Twitter @legal_med
The U.S. Supreme Court has ruled that faith-based hospitals are exempt from federal pension requirements, holding that employees of religious-affiliated entities are not protected under the Employee Retirement Income Security Act (ERISA). The unanimous decision overturns rulings in the three circuit courts of appeals.
The opinion preserves the status quo, so it should not have an immediate effect on the affected employers and employees, said Ronald J. Mann, a law professor at Columbia University in New York who has written about one case, Advocate Health Care Network v. Stapleton, for Scotusblog.com.
The high court’s opinion centers on logic and the plain language of the ERISA statute, which is sensible, said Paul Secunda, a law professor and director of the labor and employment law program at Marquette University in Milwaukee. However, the decision does not address the larger question that matters to physicians and hospitals, he said.
“Some of these church-affiliated hospitals look an awful lot like their secular competitors,” he said. “They have thousands of employees. They make billions of dollars in revenue.”
Associate Justice Sonia Sotomayor raised similar concerns in a concurring opinion to the decision. While she agreed with her fellow justices on the ruling, she said the case outcome was still troubling.
“Despite their relationship to churches, organizations such as petitioners operate for-profit subsidiaries ... and compete in the secular market with companies that must bear the cost of complying with ERISA,” Justice Sotomayor wrote.
The Supreme Court ruling comes after current and former employees of three hospital chains – Dignity Health, Advocate Health Care, and Saint Peter’s Healthcare System – sued their employers in an effort to make them comply with ERISA. The employees argued that pension plans established by the large health care providers should not fall under ERISA’s “church plan” exception because the plans were not established by churches. ERISA requires that all private employers offering pension plans adhere to federal requirements designed to ensure plan solvency and protect plan participants.
The hospitals argued that pension plans do not have to be established by a church for the ERISA exemption to apply because the plans are maintained by qualifying church-affiliated organizations. The defendants based their position on a 1980 ERISA amendment that included the “maintained” exception. Since the amendment, the federal agencies charged with interpreting ERISA – the IRS, the Department of Labor, and the Pension Benefit Guaranty Corporation – have issued opinion after opinion reaffirming that view, the defendants noted.
Courts of appeals for the Third, Seventh, and Ninth circuits agreed with the employees, concluding that ERISA’s “plain text” requires that a pension plan be established by a church to qualify for the church-plan exemption.
The Supreme Court disagreed, however, ruling that a defined-benefit pension plan maintained by a principal-purpose organization – one controlled by or associate
However, the hospital sector has changed dramatically since the 1980 ERISA amendment, Mr. Secunda pointed out. “If you think back to when this reform was passed ... almost 40 years ago, we have a whole different animal [today] with regard to these large church-oriented hospital organizations.”
Justice Sotomayor raised similar concerns. “These organizations thus bear little resemblance to those Congress considered when enacting the 1980 amendment to the church plan definition. This current reality might prompt Congress to take a different path,” she wrote.
The legal battle over whether faith-based hospitals should comply with ERISA is not likely over, according to Mr. Secunda.
“This is not the end of the story,” he said. “This is just the beginning, and now the next big issue to be decided will be, okay, principal purpose organizations come under the church plan exemption, but do these large billion-dollar hospitals qualify? Are they principal purpose organizations? I think that’s a very open question.”
[email protected]
On Twitter @legal_med
Low-dose aspirin bests dual-antiplatelet therapy in TAVR
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.

All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.

All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.

All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
AT EUROPCR
Key clinical point:
Major finding: The 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack occurred in 15.3% of TAVR patients randomized to DAPT with low-dose aspirin plus clopidogrel, compared with 7.2% on aspirin only.
Data source: A randomized, multicenter, international, prospective open-label trial in 222 TAVR patients.
Disclosures: The presenter reported receiving research grants from Edwards Lifesciences and the Quebec Heart and Lung Institute, which supported the ARTE trial.
FDA approves Sapien 3 transcatheter valve for bioprosthetic valve failure
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
Medicare Advantage enrollment up again in 2017
Enrollment in Medicare Advantage plans increased for the 13th consecutive year in 2017 and now represents one-third of all Medicare beneficiaries, according to the Kaiser Family Foundation.
Currently, 19 million beneficiaries are enrolled in Medicare Advantage, more than triple the number who were enrolled when the program hit its low point of 5.3 million (13% of all beneficiaries) in 2003 and 2004 and 71% higher since the Affordable Care Act was passed in 2010, Kaiser said in a recent report.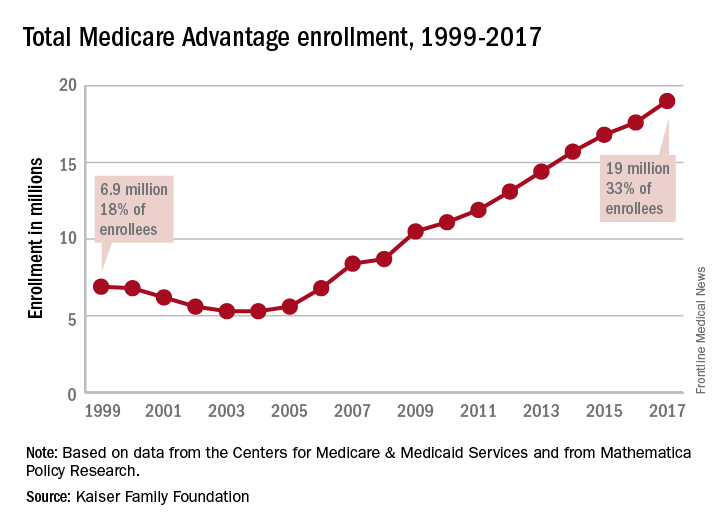
As growth continues, however, so does concentration among the private firms administering the plans. In 2017, UnitedHealthcare and Humana together account for 41% of enrollment in Medicare Advantage, “and, in 17 states, one company has more than half of all Medicare Advantage enrollment – an indicator that these markets may not be very competitive,” they noted.
Enrollment in Medicare Advantage plans increased for the 13th consecutive year in 2017 and now represents one-third of all Medicare beneficiaries, according to the Kaiser Family Foundation.
Currently, 19 million beneficiaries are enrolled in Medicare Advantage, more than triple the number who were enrolled when the program hit its low point of 5.3 million (13% of all beneficiaries) in 2003 and 2004 and 71% higher since the Affordable Care Act was passed in 2010, Kaiser said in a recent report.
As growth continues, however, so does concentration among the private firms administering the plans. In 2017, UnitedHealthcare and Humana together account for 41% of enrollment in Medicare Advantage, “and, in 17 states, one company has more than half of all Medicare Advantage enrollment – an indicator that these markets may not be very competitive,” they noted.
Enrollment in Medicare Advantage plans increased for the 13th consecutive year in 2017 and now represents one-third of all Medicare beneficiaries, according to the Kaiser Family Foundation.
Currently, 19 million beneficiaries are enrolled in Medicare Advantage, more than triple the number who were enrolled when the program hit its low point of 5.3 million (13% of all beneficiaries) in 2003 and 2004 and 71% higher since the Affordable Care Act was passed in 2010, Kaiser said in a recent report.
As growth continues, however, so does concentration among the private firms administering the plans. In 2017, UnitedHealthcare and Humana together account for 41% of enrollment in Medicare Advantage, “and, in 17 states, one company has more than half of all Medicare Advantage enrollment – an indicator that these markets may not be very competitive,” they noted.
Lung cancer linked to suicide
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: During 1973-2013, suicide among U.S. lung cancer patients was four times higher than the general adult U.S. population.
Data source: Statistics on more than 3.6 million U.S. cancer patients in the SEER Program.
Disclosures: Dr. Rahouma had no disclosures.
Alectinib in ALK+ NSCLC is a watershed moment
CHICAGO – In what’s being hailed as practice-changing findings, the anaplastic lymphoma kinase inhibitor alectinib (Alecensa) was associated with more than doubled progression-free survival (PFS), compared with crizotinib (Xalkori), the current standard of care, in patients with treatment-naive non–small cell lung cancer (NSCLC) positive for ALK.
Additionally, in the global, phase III trial, alectinib was associated with a significantly lower risk of progression to CNS metastases, a common complication of advanced ALK+ NSCLC, reported Alice T. Shaw, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, on behalf of investigators in the ALEX trial.
“I view this as a watershed moment for the treatment of ALK mutant–positive lung cancer,” commented ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
Unlike other head-to-head studies of similar drugs that frequently show only incremental benefit, the ALEX results showed a dramatic difference in outcomes for patients treated with alectinib, he said.
By comparison, the median PFS difference between chemotherapy and crizotinib in the PROFILE 1014 in patients with ALK-positive NSCLC trial was 10.9 vs. 7.0 months, Dr. Heymach pointed out.
The ALEX investigators enrolled 303 patients with untreated ALK-positive NSCLC confirmed by a central immunohistochemistry lab and randomly assigned them to treatment with either oral alectinib 600 mg twice daily or crizotinib 250 mg b.i.d.
At the primary data cutoff in February 2017, median PFS, the primary endpoint, was 11.1 months for patients treated with crizotinib, versus not reached for those treated with alectinib, translating into a hazard ratio for alectinib of 0.47 (P less than .0001).
Based on an independent review, the median PFS was determined to be 10.4 months for crizotinib, vs. 25.7 months with alectinib (HR, 0.50; P not shown).
The cumulative incidence of CNS progression, a secondary endpoint, was 41.4% in the crizotinib arm, vs. 9.41% in the alectinib arm (cause-specific HR, 0.16; P not shown).
In each arm, 97% of patients had any adverse event, and the incidence of serious adverse events was similar between the arms, at 29% for crizotinib and 28% for alectinib.
Adverse events leading to treatment discontinuation, dose reduction, or dose interruption were more frequent with crizotinib.
In the question and answer portion of the briefing, Dr. Shaw was asked whether crizotinib still had a role in this population.
“Going forward, I think that it’s pretty clear, if you have a newly diagnosed patient with metastatic ALK-positive lung cancer, that likely alectinib would be the preferred first choice,” she said.
The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships.
CHICAGO – In what’s being hailed as practice-changing findings, the anaplastic lymphoma kinase inhibitor alectinib (Alecensa) was associated with more than doubled progression-free survival (PFS), compared with crizotinib (Xalkori), the current standard of care, in patients with treatment-naive non–small cell lung cancer (NSCLC) positive for ALK.
Additionally, in the global, phase III trial, alectinib was associated with a significantly lower risk of progression to CNS metastases, a common complication of advanced ALK+ NSCLC, reported Alice T. Shaw, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, on behalf of investigators in the ALEX trial.
“I view this as a watershed moment for the treatment of ALK mutant–positive lung cancer,” commented ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
Unlike other head-to-head studies of similar drugs that frequently show only incremental benefit, the ALEX results showed a dramatic difference in outcomes for patients treated with alectinib, he said.
By comparison, the median PFS difference between chemotherapy and crizotinib in the PROFILE 1014 in patients with ALK-positive NSCLC trial was 10.9 vs. 7.0 months, Dr. Heymach pointed out.
The ALEX investigators enrolled 303 patients with untreated ALK-positive NSCLC confirmed by a central immunohistochemistry lab and randomly assigned them to treatment with either oral alectinib 600 mg twice daily or crizotinib 250 mg b.i.d.
At the primary data cutoff in February 2017, median PFS, the primary endpoint, was 11.1 months for patients treated with crizotinib, versus not reached for those treated with alectinib, translating into a hazard ratio for alectinib of 0.47 (P less than .0001).
Based on an independent review, the median PFS was determined to be 10.4 months for crizotinib, vs. 25.7 months with alectinib (HR, 0.50; P not shown).
The cumulative incidence of CNS progression, a secondary endpoint, was 41.4% in the crizotinib arm, vs. 9.41% in the alectinib arm (cause-specific HR, 0.16; P not shown).
In each arm, 97% of patients had any adverse event, and the incidence of serious adverse events was similar between the arms, at 29% for crizotinib and 28% for alectinib.
Adverse events leading to treatment discontinuation, dose reduction, or dose interruption were more frequent with crizotinib.
In the question and answer portion of the briefing, Dr. Shaw was asked whether crizotinib still had a role in this population.
“Going forward, I think that it’s pretty clear, if you have a newly diagnosed patient with metastatic ALK-positive lung cancer, that likely alectinib would be the preferred first choice,” she said.
The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships.
CHICAGO – In what’s being hailed as practice-changing findings, the anaplastic lymphoma kinase inhibitor alectinib (Alecensa) was associated with more than doubled progression-free survival (PFS), compared with crizotinib (Xalkori), the current standard of care, in patients with treatment-naive non–small cell lung cancer (NSCLC) positive for ALK.
Additionally, in the global, phase III trial, alectinib was associated with a significantly lower risk of progression to CNS metastases, a common complication of advanced ALK+ NSCLC, reported Alice T. Shaw, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, on behalf of investigators in the ALEX trial.
“I view this as a watershed moment for the treatment of ALK mutant–positive lung cancer,” commented ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
Unlike other head-to-head studies of similar drugs that frequently show only incremental benefit, the ALEX results showed a dramatic difference in outcomes for patients treated with alectinib, he said.
By comparison, the median PFS difference between chemotherapy and crizotinib in the PROFILE 1014 in patients with ALK-positive NSCLC trial was 10.9 vs. 7.0 months, Dr. Heymach pointed out.
The ALEX investigators enrolled 303 patients with untreated ALK-positive NSCLC confirmed by a central immunohistochemistry lab and randomly assigned them to treatment with either oral alectinib 600 mg twice daily or crizotinib 250 mg b.i.d.
At the primary data cutoff in February 2017, median PFS, the primary endpoint, was 11.1 months for patients treated with crizotinib, versus not reached for those treated with alectinib, translating into a hazard ratio for alectinib of 0.47 (P less than .0001).
Based on an independent review, the median PFS was determined to be 10.4 months for crizotinib, vs. 25.7 months with alectinib (HR, 0.50; P not shown).
The cumulative incidence of CNS progression, a secondary endpoint, was 41.4% in the crizotinib arm, vs. 9.41% in the alectinib arm (cause-specific HR, 0.16; P not shown).
In each arm, 97% of patients had any adverse event, and the incidence of serious adverse events was similar between the arms, at 29% for crizotinib and 28% for alectinib.
Adverse events leading to treatment discontinuation, dose reduction, or dose interruption were more frequent with crizotinib.
In the question and answer portion of the briefing, Dr. Shaw was asked whether crizotinib still had a role in this population.
“Going forward, I think that it’s pretty clear, if you have a newly diagnosed patient with metastatic ALK-positive lung cancer, that likely alectinib would be the preferred first choice,” she said.
The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships.
AT ASCO 2017
Key clinical point: Alectinib was associated with more than double the progression-free survival of standard of care crizotinib in patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase.
Major finding: Median PFS by independent review was 10.4 months with crizotinib vs. 25.7 months with alectinib.
Data source: The ALEX trial, a phase III trial of 303 patients with ALK-positive NSCLC.
Disclosures: The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships
BIO-RESORT: A mandate to prescreen PCI patients for silent diabetes
PARIS – Undetected diabetes and prediabetes are pervasive in patients undergoing percutaneous coronary intervention, and they’re associated with a sharply increased risk of major adverse cardiovascular events, according to the results of the potentially practice-changing BIO-RESORT Silent Diabetes Study, Clemens von Birgelen, MD, PhD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Our data support screening PCI all-comers for silent diabetes, which may help identify patients with an increased event risk and improve their therapy,” said Dr. von Birgelen, professor of cardiology at the Thoraxcentrum of Twente, a high-volume center for cardiac interventions in Enschede, the Netherlands.
A substantial one-third of subjects turned out to have abnormal glucose tolerance according to World Health Organization criteria and an International Expert Committee Report (Diabetes Care. 2009 Jul;32[7]:1327-34). In a multivariate analysis, their 1-year rate of the primary study endpoint – target vessel failure, a composite of cardiac death, target vessel-related MI, or target vessel revascularization – was an adjusted 2.2 times greater than in the 788 normoglycemic patients.
Moreover, among the 7% of study participants who met diagnostic criteria for silent diabetes, the risk of target vessel failure was more than 4.4 times greater than in the normoglycemic group.
“To a very great extent, periprocedural MI is the driving force behind this difference that we saw. From a biological point of view, I think that the vulnerability of the vessel in the diabetic or prediabetic patient features more brittle plaque with a higher risk of cholesterol embolization, and with more plaque mass that can be pushed to the side so that side branch vessels can become occluded, leading to periprocedural MI,” he observed.
Glucose metabolism was assessed in all participants by two methods using the conventional cutoffs: a 2-hour oral glucose tolerance test (OGTT), and the combination of fasting plasma glucose and hemoglobin A1c. By OGTT, 7% of patients had silent, previously unrecognized diabetes and another 13% had prediabetes. Using the combination of fasting plasma glucose and HbA1c, a total of 25% of subjects had silent diabetes or prediabetes. Fully 33% of participants had abnormal glucose metabolism by one yardstick or the other.
“What we have seen is there is a group of patients that are missed with either. With the OGTT you don’t see all the diabetics, and with HbA1c and fasting blood glucose you also miss some patients,” said Dr. von Birgelen.
The 1-year cumulative incidence of target vessel failure was 13.2% in patients with silent diabetes as identified by the OGTT and 12.1% in those detected by the alternative method, compared with rates of 2.8% and 3.1%, respectively, in normoglycemic PCI patients. The event rate was 6.1% in patients with prediabetes by OGTT and similar at 5.5% in those found to be prediabetic based on fasting blood glucose and HbA1c, versus rates of 2.8% and 3.1%, respectively, in normoglycemic patients.
“The findings of this study suggest that post-PCI event risk associated with hyperglycemia is a continuum without a clear threshold effect, extending well beyond the threshold that currently defines diabetes,” Dr. von Birgelen said.
Once again, it’s worth emphasizing that the elevated target vessel failure rates seen in patients with abnormal glucose metabolism were due mostly to increased rates of acute MI within the first 24 hours after PCI. The target vessel–related MI rate was 10.3% in patients with silent diabetes, compared with just 1.8% in normoglycemic controls.
Asked what the take-home message for clinicians is from this study, he noted that the Netherlands has a relatively low prevalence of diabetes, and a highly developed primary care medicine system.
“We have a very good one-to-one relationship between the patient and the GP. So if we find 7% silent diabetes and up to one-third of patients with undetected abnormal glucose tolerance in a country with a relatively low prevalence of diabetes, you may expect that in other countries with a higher prevalence and perhaps a less developed primary care system the rate may be much, much higher,” Dr. von Birgelen cautioned.
The implications for the daily clinical practice of interventional cardiology are clear, he continued: “We’ve seen in several trials that the new stents are doing a fantastic job. So if we want to further improve the outcomes in our patients we have to do something else. We should look for subgroups of our PCI patients who have a particularly high risk. And we all realize that diabetics are such a problem, but I think we have shown that the prediabetic patients are also important. So we should identify and pretreat these patients, perhaps with aggressive lipid-lowering therapy during the weeks before a scheduled elective PCI.”
“There are data showing that with aggressive lipid-lowering you might reduce the risk of periprocedural MI,” the cardiologist noted.
As a practical matter, screening via fasting blood glucose and HbA1c is probably the way to go in clinical practice, according to Dr. von Birgelen.
“In this study, we performed the OGTT because it is still considered by many the gold standard. But there is increasing evidence favoring HbA1c data and fasting blood glucose,” he said.
Other possible pre-PCI interventions worthy of consideration in patients found to have previously unsuspected abnormal glucose tolerance might include medical therapy aimed at normalizing glucose metabolism, as well as perhaps resorting to the most potent forms of dual-antiplatelet therapy in patients with stable angina who have impaired glucose tolerance. However, these are possibilities that should be tested in randomized controlled trials before widespread adoption, he added.
The BIO-RESORT Silent Diabetes Study, which will continue for 5 years of post-PCI follow-up, is a prespecified substudy of the previously reported BIO-RESORT trial, which addressed another issue entirely. It was a three-arm, patient-blinded clinical trial comparing 1-year safety and efficacy outcomes in nearly 3,500 PCI patients randomized to PCI with very thin strut biodegradable polymer everolimus- or sirolimus-eluting stents or a durable polymer zotarolimus-eluting stent. Outcomes proved noninferior across the three treatment groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
Dr. von Birgelen observed that the silent diabetes study broke new ground. Prior studies of PCI outcomes in patients with unrecognized diabetes were limited to recipients of plain old balloon angioplasty, bare metal, or first-generation drug-eluting stents. And studies of PCI in patients with unrecognized prediabetes are virtually nonexistent.
As the principal investigator for both the parent BIO-RESORT trial and the silent diabetes substudy, Dr. von Birgelen received research grants from Biotronik, Boston Scientific, and Medtronic, the cosponsors. He applauded the three companies for funding the silent diabetes substudy in the interest of science even though it had no commercial relevance to their stent businesses.
PARIS – Undetected diabetes and prediabetes are pervasive in patients undergoing percutaneous coronary intervention, and they’re associated with a sharply increased risk of major adverse cardiovascular events, according to the results of the potentially practice-changing BIO-RESORT Silent Diabetes Study, Clemens von Birgelen, MD, PhD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Our data support screening PCI all-comers for silent diabetes, which may help identify patients with an increased event risk and improve their therapy,” said Dr. von Birgelen, professor of cardiology at the Thoraxcentrum of Twente, a high-volume center for cardiac interventions in Enschede, the Netherlands.
A substantial one-third of subjects turned out to have abnormal glucose tolerance according to World Health Organization criteria and an International Expert Committee Report (Diabetes Care. 2009 Jul;32[7]:1327-34). In a multivariate analysis, their 1-year rate of the primary study endpoint – target vessel failure, a composite of cardiac death, target vessel-related MI, or target vessel revascularization – was an adjusted 2.2 times greater than in the 788 normoglycemic patients.
Moreover, among the 7% of study participants who met diagnostic criteria for silent diabetes, the risk of target vessel failure was more than 4.4 times greater than in the normoglycemic group.
“To a very great extent, periprocedural MI is the driving force behind this difference that we saw. From a biological point of view, I think that the vulnerability of the vessel in the diabetic or prediabetic patient features more brittle plaque with a higher risk of cholesterol embolization, and with more plaque mass that can be pushed to the side so that side branch vessels can become occluded, leading to periprocedural MI,” he observed.
Glucose metabolism was assessed in all participants by two methods using the conventional cutoffs: a 2-hour oral glucose tolerance test (OGTT), and the combination of fasting plasma glucose and hemoglobin A1c. By OGTT, 7% of patients had silent, previously unrecognized diabetes and another 13% had prediabetes. Using the combination of fasting plasma glucose and HbA1c, a total of 25% of subjects had silent diabetes or prediabetes. Fully 33% of participants had abnormal glucose metabolism by one yardstick or the other.
“What we have seen is there is a group of patients that are missed with either. With the OGTT you don’t see all the diabetics, and with HbA1c and fasting blood glucose you also miss some patients,” said Dr. von Birgelen.
The 1-year cumulative incidence of target vessel failure was 13.2% in patients with silent diabetes as identified by the OGTT and 12.1% in those detected by the alternative method, compared with rates of 2.8% and 3.1%, respectively, in normoglycemic PCI patients. The event rate was 6.1% in patients with prediabetes by OGTT and similar at 5.5% in those found to be prediabetic based on fasting blood glucose and HbA1c, versus rates of 2.8% and 3.1%, respectively, in normoglycemic patients.
“The findings of this study suggest that post-PCI event risk associated with hyperglycemia is a continuum without a clear threshold effect, extending well beyond the threshold that currently defines diabetes,” Dr. von Birgelen said.
Once again, it’s worth emphasizing that the elevated target vessel failure rates seen in patients with abnormal glucose metabolism were due mostly to increased rates of acute MI within the first 24 hours after PCI. The target vessel–related MI rate was 10.3% in patients with silent diabetes, compared with just 1.8% in normoglycemic controls.
Asked what the take-home message for clinicians is from this study, he noted that the Netherlands has a relatively low prevalence of diabetes, and a highly developed primary care medicine system.
“We have a very good one-to-one relationship between the patient and the GP. So if we find 7% silent diabetes and up to one-third of patients with undetected abnormal glucose tolerance in a country with a relatively low prevalence of diabetes, you may expect that in other countries with a higher prevalence and perhaps a less developed primary care system the rate may be much, much higher,” Dr. von Birgelen cautioned.
The implications for the daily clinical practice of interventional cardiology are clear, he continued: “We’ve seen in several trials that the new stents are doing a fantastic job. So if we want to further improve the outcomes in our patients we have to do something else. We should look for subgroups of our PCI patients who have a particularly high risk. And we all realize that diabetics are such a problem, but I think we have shown that the prediabetic patients are also important. So we should identify and pretreat these patients, perhaps with aggressive lipid-lowering therapy during the weeks before a scheduled elective PCI.”
“There are data showing that with aggressive lipid-lowering you might reduce the risk of periprocedural MI,” the cardiologist noted.
As a practical matter, screening via fasting blood glucose and HbA1c is probably the way to go in clinical practice, according to Dr. von Birgelen.
“In this study, we performed the OGTT because it is still considered by many the gold standard. But there is increasing evidence favoring HbA1c data and fasting blood glucose,” he said.
Other possible pre-PCI interventions worthy of consideration in patients found to have previously unsuspected abnormal glucose tolerance might include medical therapy aimed at normalizing glucose metabolism, as well as perhaps resorting to the most potent forms of dual-antiplatelet therapy in patients with stable angina who have impaired glucose tolerance. However, these are possibilities that should be tested in randomized controlled trials before widespread adoption, he added.
The BIO-RESORT Silent Diabetes Study, which will continue for 5 years of post-PCI follow-up, is a prespecified substudy of the previously reported BIO-RESORT trial, which addressed another issue entirely. It was a three-arm, patient-blinded clinical trial comparing 1-year safety and efficacy outcomes in nearly 3,500 PCI patients randomized to PCI with very thin strut biodegradable polymer everolimus- or sirolimus-eluting stents or a durable polymer zotarolimus-eluting stent. Outcomes proved noninferior across the three treatment groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
Dr. von Birgelen observed that the silent diabetes study broke new ground. Prior studies of PCI outcomes in patients with unrecognized diabetes were limited to recipients of plain old balloon angioplasty, bare metal, or first-generation drug-eluting stents. And studies of PCI in patients with unrecognized prediabetes are virtually nonexistent.
As the principal investigator for both the parent BIO-RESORT trial and the silent diabetes substudy, Dr. von Birgelen received research grants from Biotronik, Boston Scientific, and Medtronic, the cosponsors. He applauded the three companies for funding the silent diabetes substudy in the interest of science even though it had no commercial relevance to their stent businesses.
PARIS – Undetected diabetes and prediabetes are pervasive in patients undergoing percutaneous coronary intervention, and they’re associated with a sharply increased risk of major adverse cardiovascular events, according to the results of the potentially practice-changing BIO-RESORT Silent Diabetes Study, Clemens von Birgelen, MD, PhD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Our data support screening PCI all-comers for silent diabetes, which may help identify patients with an increased event risk and improve their therapy,” said Dr. von Birgelen, professor of cardiology at the Thoraxcentrum of Twente, a high-volume center for cardiac interventions in Enschede, the Netherlands.
A substantial one-third of subjects turned out to have abnormal glucose tolerance according to World Health Organization criteria and an International Expert Committee Report (Diabetes Care. 2009 Jul;32[7]:1327-34). In a multivariate analysis, their 1-year rate of the primary study endpoint – target vessel failure, a composite of cardiac death, target vessel-related MI, or target vessel revascularization – was an adjusted 2.2 times greater than in the 788 normoglycemic patients.
Moreover, among the 7% of study participants who met diagnostic criteria for silent diabetes, the risk of target vessel failure was more than 4.4 times greater than in the normoglycemic group.
“To a very great extent, periprocedural MI is the driving force behind this difference that we saw. From a biological point of view, I think that the vulnerability of the vessel in the diabetic or prediabetic patient features more brittle plaque with a higher risk of cholesterol embolization, and with more plaque mass that can be pushed to the side so that side branch vessels can become occluded, leading to periprocedural MI,” he observed.
Glucose metabolism was assessed in all participants by two methods using the conventional cutoffs: a 2-hour oral glucose tolerance test (OGTT), and the combination of fasting plasma glucose and hemoglobin A1c. By OGTT, 7% of patients had silent, previously unrecognized diabetes and another 13% had prediabetes. Using the combination of fasting plasma glucose and HbA1c, a total of 25% of subjects had silent diabetes or prediabetes. Fully 33% of participants had abnormal glucose metabolism by one yardstick or the other.
“What we have seen is there is a group of patients that are missed with either. With the OGTT you don’t see all the diabetics, and with HbA1c and fasting blood glucose you also miss some patients,” said Dr. von Birgelen.
The 1-year cumulative incidence of target vessel failure was 13.2% in patients with silent diabetes as identified by the OGTT and 12.1% in those detected by the alternative method, compared with rates of 2.8% and 3.1%, respectively, in normoglycemic PCI patients. The event rate was 6.1% in patients with prediabetes by OGTT and similar at 5.5% in those found to be prediabetic based on fasting blood glucose and HbA1c, versus rates of 2.8% and 3.1%, respectively, in normoglycemic patients.
“The findings of this study suggest that post-PCI event risk associated with hyperglycemia is a continuum without a clear threshold effect, extending well beyond the threshold that currently defines diabetes,” Dr. von Birgelen said.
Once again, it’s worth emphasizing that the elevated target vessel failure rates seen in patients with abnormal glucose metabolism were due mostly to increased rates of acute MI within the first 24 hours after PCI. The target vessel–related MI rate was 10.3% in patients with silent diabetes, compared with just 1.8% in normoglycemic controls.
Asked what the take-home message for clinicians is from this study, he noted that the Netherlands has a relatively low prevalence of diabetes, and a highly developed primary care medicine system.
“We have a very good one-to-one relationship between the patient and the GP. So if we find 7% silent diabetes and up to one-third of patients with undetected abnormal glucose tolerance in a country with a relatively low prevalence of diabetes, you may expect that in other countries with a higher prevalence and perhaps a less developed primary care system the rate may be much, much higher,” Dr. von Birgelen cautioned.
The implications for the daily clinical practice of interventional cardiology are clear, he continued: “We’ve seen in several trials that the new stents are doing a fantastic job. So if we want to further improve the outcomes in our patients we have to do something else. We should look for subgroups of our PCI patients who have a particularly high risk. And we all realize that diabetics are such a problem, but I think we have shown that the prediabetic patients are also important. So we should identify and pretreat these patients, perhaps with aggressive lipid-lowering therapy during the weeks before a scheduled elective PCI.”
“There are data showing that with aggressive lipid-lowering you might reduce the risk of periprocedural MI,” the cardiologist noted.
As a practical matter, screening via fasting blood glucose and HbA1c is probably the way to go in clinical practice, according to Dr. von Birgelen.
“In this study, we performed the OGTT because it is still considered by many the gold standard. But there is increasing evidence favoring HbA1c data and fasting blood glucose,” he said.
Other possible pre-PCI interventions worthy of consideration in patients found to have previously unsuspected abnormal glucose tolerance might include medical therapy aimed at normalizing glucose metabolism, as well as perhaps resorting to the most potent forms of dual-antiplatelet therapy in patients with stable angina who have impaired glucose tolerance. However, these are possibilities that should be tested in randomized controlled trials before widespread adoption, he added.
The BIO-RESORT Silent Diabetes Study, which will continue for 5 years of post-PCI follow-up, is a prespecified substudy of the previously reported BIO-RESORT trial, which addressed another issue entirely. It was a three-arm, patient-blinded clinical trial comparing 1-year safety and efficacy outcomes in nearly 3,500 PCI patients randomized to PCI with very thin strut biodegradable polymer everolimus- or sirolimus-eluting stents or a durable polymer zotarolimus-eluting stent. Outcomes proved noninferior across the three treatment groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
Dr. von Birgelen observed that the silent diabetes study broke new ground. Prior studies of PCI outcomes in patients with unrecognized diabetes were limited to recipients of plain old balloon angioplasty, bare metal, or first-generation drug-eluting stents. And studies of PCI in patients with unrecognized prediabetes are virtually nonexistent.
As the principal investigator for both the parent BIO-RESORT trial and the silent diabetes substudy, Dr. von Birgelen received research grants from Biotronik, Boston Scientific, and Medtronic, the cosponsors. He applauded the three companies for funding the silent diabetes substudy in the interest of science even though it had no commercial relevance to their stent businesses.
AT EUROPCR
Key clinical point:
Major finding: One-third of patients undergoing PCI have unsuspected silent diabetes or prediabetes, placing them at increased risk for major adverse cardiac events.
Data source: This prospective observational study included 988 patients not known to have diabetes who underwent screening for abnormal glucose tolerance 6 weeks after PCI with stenting.
Disclosures: The study was cosponsored by Biotronik, Boston Scientific, and Medtronic.












