User login
The Official Newspaper of the American Association for Thoracic Surgery
Urgent surgery said to deserve separate classification
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: Urgent surgery deserves a separate classification from elective and emergency surgeries for assessments of healthcare quality and performance.
Major finding: Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality, approximately double the morbidity rate and 6 times the mortality rate of elective surgeries (6.7% and 0.4%, respectively).
Data source: A retrospective analysis of outcomes for 173,643 general surgeries performed during a 1-year period at 435 hospitals across U.S.
Disclosures: This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Fewer early neurologic complications with TAVR than SAVR
PARIS – Patients with severe aortic stenosis at intermediate operative risk have a significantly lower 30-day risk of stroke and other neurologic complications with transcatheter aortic valve replacement than with surgical replacement, according to new results from the landmark SURTAVI trial.
“This is the first time the stroke rate has been shown to be lower with TAVR than with surgery,” A. Pieter Kappetein, MD, noted in presenting the results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
It’s a finding that adds to the momentum for studying TAVR in low-surgical-risk patients, he added.
“As we move toward lower-risk patients it will become even more important to see whether there is a difference in stroke. Suppose the stroke rate in SURTAVI had been a little higher with TAVR than SAVR? It would really make us more cautious about moving toward lower-risk patients. Now that we see that in intermediate-risk patients the stroke rate is actually a little bit lower than with surgery, I think we’ll feel more comfortable moving toward lower-risk patients,” according to Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University Medical Center in Rotterdam.
SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) involved randomization of 1,660 patients with severe symptomatic aortic stenosis to TAVR or SAVR. All participants were deemed to be at intermediate operative risk based upon a predicted surgical mortality of 3%-15%. The primary outcome -- a composite of all-cause mortality and disabling stroke at 2 years -- was presented at the 2017 meeting of the American College of Cardiology and simultaneously published (N Engl J Med. 2017 Apr 6;376(14):1321-1331). The rate was 12.6% with TAVR using the self-expanding CoreValve or Evolut R bioprosthesis and noninferior at 14% with SAVR.
Dr. Kappetein presented a prespecified secondary analysis of the 30-day rate of all neurologic complications, including nondisabling strokes and encephalopathy. He and the other SURTAVI organizers felt this was an important outcome because these early neurologic events have a major impact upon quality of life, including whether a patient will be discharged home or to a rehabilitation clinic or skilled nursing facility following aortic valve replacement.
The 30-day incidence of all stroke was 3.3% in the TAVR patients, significantly lower than the 5.4% rate in the SAVR group. By the 2-year mark, however, the difference was no longer statistically significant, with a rate of 6.3% in the TAVR group compared with 8.0% with SAVR.
Ninety-five percent of the early strokes were ischemic.
The 30-day incidence of disabling stroke was 1.2% with TAVR and 2.4% with SAVR, a difference that was not significant (P=0.057). The 2-year rate was 2.4% in the TAVR arm and 4.5% with SAVR, again not significantly different.
Half of the strokes in the TAVR group had a modified Rankin score of 0-1 at 30 days, meaning no or only minimal signs of stroke. In contrast, most of the strokes in the SAVR group were disabling, with a modified Rankin score of 2-6.
Only 36% of patients who had an early stroke were discharged home, compared with 87% of patients without a stroke. Not surprisingly, quality of life as assessed using the SF-36 physical summary was significantly worse in the early-stroke group. However, with or without stroke, TAVR patients recovered quality of life faster than SAVR patients.
He noted that the timing of the early strokes differed between the two groups. The great majority of both disabling and nondisabling strokes in the TAVR patients were periprocedural, occurring on the day of TAVR or the next day. Strokes in the SAVR group occurred then as well, but also on days 2-6.
One reason why SURTAVI is the first study to show a lower stroke risk with TAVR is that it was the first TAVR-versus-SAVR study to feature comprehensive neurologic testing pre- and post-procedure, along with evaluation of all suspected events by a neurologist or stroke specialist, according to Dr. Kappetein.
“As surgeons we all have said the stroke rate after SAVR is 1%-1.5%, but only when the patient wasn’t waving to us the next morning would we say, ‘Oh, that patient may have a stroke.’ Then we would call a neurologist. So there were many more subtle strokes that we never actually detected. If you do a proper examination of the patient before and after the procedure you’ll find many more strokes,” he said.
He and his coinvestigators systematically searched in vain for predictors of increased stroke risk among the TAVR and SAVR patients.
“Actually, the stroke risk is present for every patient we treat with TAVR or SAVR,” the surgeon continued.
However, discussant Adnan Kastrati, MD, chief physician at the German Heart Center in Munich, thought he spied in the SURTAVI data a potential opportunity to reduce early strokes in SAVR patients. He noted that new-onset atrial fibrillation is consistently more common in SAVR than TAVR patients, and that many of the strokes in the SAVR group occurred on days 2-6. When do heart surgeons typically start oral anticoagulation in their patients with postsurgical atrial fibrillation? he asked.
Not until after 48 hours, Dr. Kappetein replied.
“Those strokes on days 4, 5, and 6 might have to do with atrial fibrillation, and we may need to be more aggressive as surgeons in anticoagulating patients with atrial fibrillation after surgery,” he said.
Dr. Kappetein reported receiving research grant support from Medtronic, sponsor of SURTAVI.
This article was updated July 28, 2107.
PARIS – Patients with severe aortic stenosis at intermediate operative risk have a significantly lower 30-day risk of stroke and other neurologic complications with transcatheter aortic valve replacement than with surgical replacement, according to new results from the landmark SURTAVI trial.
“This is the first time the stroke rate has been shown to be lower with TAVR than with surgery,” A. Pieter Kappetein, MD, noted in presenting the results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
It’s a finding that adds to the momentum for studying TAVR in low-surgical-risk patients, he added.
“As we move toward lower-risk patients it will become even more important to see whether there is a difference in stroke. Suppose the stroke rate in SURTAVI had been a little higher with TAVR than SAVR? It would really make us more cautious about moving toward lower-risk patients. Now that we see that in intermediate-risk patients the stroke rate is actually a little bit lower than with surgery, I think we’ll feel more comfortable moving toward lower-risk patients,” according to Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University Medical Center in Rotterdam.
SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) involved randomization of 1,660 patients with severe symptomatic aortic stenosis to TAVR or SAVR. All participants were deemed to be at intermediate operative risk based upon a predicted surgical mortality of 3%-15%. The primary outcome -- a composite of all-cause mortality and disabling stroke at 2 years -- was presented at the 2017 meeting of the American College of Cardiology and simultaneously published (N Engl J Med. 2017 Apr 6;376(14):1321-1331). The rate was 12.6% with TAVR using the self-expanding CoreValve or Evolut R bioprosthesis and noninferior at 14% with SAVR.
Dr. Kappetein presented a prespecified secondary analysis of the 30-day rate of all neurologic complications, including nondisabling strokes and encephalopathy. He and the other SURTAVI organizers felt this was an important outcome because these early neurologic events have a major impact upon quality of life, including whether a patient will be discharged home or to a rehabilitation clinic or skilled nursing facility following aortic valve replacement.
The 30-day incidence of all stroke was 3.3% in the TAVR patients, significantly lower than the 5.4% rate in the SAVR group. By the 2-year mark, however, the difference was no longer statistically significant, with a rate of 6.3% in the TAVR group compared with 8.0% with SAVR.
Ninety-five percent of the early strokes were ischemic.
The 30-day incidence of disabling stroke was 1.2% with TAVR and 2.4% with SAVR, a difference that was not significant (P=0.057). The 2-year rate was 2.4% in the TAVR arm and 4.5% with SAVR, again not significantly different.
Half of the strokes in the TAVR group had a modified Rankin score of 0-1 at 30 days, meaning no or only minimal signs of stroke. In contrast, most of the strokes in the SAVR group were disabling, with a modified Rankin score of 2-6.
Only 36% of patients who had an early stroke were discharged home, compared with 87% of patients without a stroke. Not surprisingly, quality of life as assessed using the SF-36 physical summary was significantly worse in the early-stroke group. However, with or without stroke, TAVR patients recovered quality of life faster than SAVR patients.
He noted that the timing of the early strokes differed between the two groups. The great majority of both disabling and nondisabling strokes in the TAVR patients were periprocedural, occurring on the day of TAVR or the next day. Strokes in the SAVR group occurred then as well, but also on days 2-6.
One reason why SURTAVI is the first study to show a lower stroke risk with TAVR is that it was the first TAVR-versus-SAVR study to feature comprehensive neurologic testing pre- and post-procedure, along with evaluation of all suspected events by a neurologist or stroke specialist, according to Dr. Kappetein.
“As surgeons we all have said the stroke rate after SAVR is 1%-1.5%, but only when the patient wasn’t waving to us the next morning would we say, ‘Oh, that patient may have a stroke.’ Then we would call a neurologist. So there were many more subtle strokes that we never actually detected. If you do a proper examination of the patient before and after the procedure you’ll find many more strokes,” he said.
He and his coinvestigators systematically searched in vain for predictors of increased stroke risk among the TAVR and SAVR patients.
“Actually, the stroke risk is present for every patient we treat with TAVR or SAVR,” the surgeon continued.
However, discussant Adnan Kastrati, MD, chief physician at the German Heart Center in Munich, thought he spied in the SURTAVI data a potential opportunity to reduce early strokes in SAVR patients. He noted that new-onset atrial fibrillation is consistently more common in SAVR than TAVR patients, and that many of the strokes in the SAVR group occurred on days 2-6. When do heart surgeons typically start oral anticoagulation in their patients with postsurgical atrial fibrillation? he asked.
Not until after 48 hours, Dr. Kappetein replied.
“Those strokes on days 4, 5, and 6 might have to do with atrial fibrillation, and we may need to be more aggressive as surgeons in anticoagulating patients with atrial fibrillation after surgery,” he said.
Dr. Kappetein reported receiving research grant support from Medtronic, sponsor of SURTAVI.
This article was updated July 28, 2107.
PARIS – Patients with severe aortic stenosis at intermediate operative risk have a significantly lower 30-day risk of stroke and other neurologic complications with transcatheter aortic valve replacement than with surgical replacement, according to new results from the landmark SURTAVI trial.
“This is the first time the stroke rate has been shown to be lower with TAVR than with surgery,” A. Pieter Kappetein, MD, noted in presenting the results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
It’s a finding that adds to the momentum for studying TAVR in low-surgical-risk patients, he added.
“As we move toward lower-risk patients it will become even more important to see whether there is a difference in stroke. Suppose the stroke rate in SURTAVI had been a little higher with TAVR than SAVR? It would really make us more cautious about moving toward lower-risk patients. Now that we see that in intermediate-risk patients the stroke rate is actually a little bit lower than with surgery, I think we’ll feel more comfortable moving toward lower-risk patients,” according to Dr. Kappetein, professor of cardiothoracic surgery at Erasmus University Medical Center in Rotterdam.
SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) involved randomization of 1,660 patients with severe symptomatic aortic stenosis to TAVR or SAVR. All participants were deemed to be at intermediate operative risk based upon a predicted surgical mortality of 3%-15%. The primary outcome -- a composite of all-cause mortality and disabling stroke at 2 years -- was presented at the 2017 meeting of the American College of Cardiology and simultaneously published (N Engl J Med. 2017 Apr 6;376(14):1321-1331). The rate was 12.6% with TAVR using the self-expanding CoreValve or Evolut R bioprosthesis and noninferior at 14% with SAVR.
Dr. Kappetein presented a prespecified secondary analysis of the 30-day rate of all neurologic complications, including nondisabling strokes and encephalopathy. He and the other SURTAVI organizers felt this was an important outcome because these early neurologic events have a major impact upon quality of life, including whether a patient will be discharged home or to a rehabilitation clinic or skilled nursing facility following aortic valve replacement.
The 30-day incidence of all stroke was 3.3% in the TAVR patients, significantly lower than the 5.4% rate in the SAVR group. By the 2-year mark, however, the difference was no longer statistically significant, with a rate of 6.3% in the TAVR group compared with 8.0% with SAVR.
Ninety-five percent of the early strokes were ischemic.
The 30-day incidence of disabling stroke was 1.2% with TAVR and 2.4% with SAVR, a difference that was not significant (P=0.057). The 2-year rate was 2.4% in the TAVR arm and 4.5% with SAVR, again not significantly different.
Half of the strokes in the TAVR group had a modified Rankin score of 0-1 at 30 days, meaning no or only minimal signs of stroke. In contrast, most of the strokes in the SAVR group were disabling, with a modified Rankin score of 2-6.
Only 36% of patients who had an early stroke were discharged home, compared with 87% of patients without a stroke. Not surprisingly, quality of life as assessed using the SF-36 physical summary was significantly worse in the early-stroke group. However, with or without stroke, TAVR patients recovered quality of life faster than SAVR patients.
He noted that the timing of the early strokes differed between the two groups. The great majority of both disabling and nondisabling strokes in the TAVR patients were periprocedural, occurring on the day of TAVR or the next day. Strokes in the SAVR group occurred then as well, but also on days 2-6.
One reason why SURTAVI is the first study to show a lower stroke risk with TAVR is that it was the first TAVR-versus-SAVR study to feature comprehensive neurologic testing pre- and post-procedure, along with evaluation of all suspected events by a neurologist or stroke specialist, according to Dr. Kappetein.
“As surgeons we all have said the stroke rate after SAVR is 1%-1.5%, but only when the patient wasn’t waving to us the next morning would we say, ‘Oh, that patient may have a stroke.’ Then we would call a neurologist. So there were many more subtle strokes that we never actually detected. If you do a proper examination of the patient before and after the procedure you’ll find many more strokes,” he said.
He and his coinvestigators systematically searched in vain for predictors of increased stroke risk among the TAVR and SAVR patients.
“Actually, the stroke risk is present for every patient we treat with TAVR or SAVR,” the surgeon continued.
However, discussant Adnan Kastrati, MD, chief physician at the German Heart Center in Munich, thought he spied in the SURTAVI data a potential opportunity to reduce early strokes in SAVR patients. He noted that new-onset atrial fibrillation is consistently more common in SAVR than TAVR patients, and that many of the strokes in the SAVR group occurred on days 2-6. When do heart surgeons typically start oral anticoagulation in their patients with postsurgical atrial fibrillation? he asked.
Not until after 48 hours, Dr. Kappetein replied.
“Those strokes on days 4, 5, and 6 might have to do with atrial fibrillation, and we may need to be more aggressive as surgeons in anticoagulating patients with atrial fibrillation after surgery,” he said.
Dr. Kappetein reported receiving research grant support from Medtronic, sponsor of SURTAVI.
This article was updated July 28, 2107.
AT EUROPCR
Key clinical point:
Major finding: The combined incidence of disabling and nondisabling stroke within 30 days of TAVR was 3.3%, significantly better than the 5.4% rate in patients who underwent SAVR.
Data source: SURTAVI was a multicenter trial which included 1,660 patients with severe aortic stenosis who were at intermediate operative risk and were randomized to TAVR or SAVR.
Disclosures: The study presenter reported receiving research grant support from Medtronic, sponsor of the SURTAVI trial.
In 8 years, TMVR means more procedures
NEW YORK – Since the commercialization of transcatheter mitral valve repair, the share of these procedures among all mitral operations has grown exponentially and has also contributed to an increase in the number of overall mitral procedures, including surgical repair, according to an analysis of a national German database reported at the American Association for Thoracic Surgery Mitral Conclave here.
Transcatheter mitral valve repair (TMVR) using the MitraClip device (Abbott Vascular) was first commercialized in Germany in 2008, and in the years since the share of TMVR procedures among all mitral operations increased from 0.3% to 18.1% in 2015 throughout Germany, said Lenard Conradi, MD, surgical director of minimally-invasive and transcatheter heart valve procedures at the University Heart Center Hamburg.
The goal of the study was to gain insights into how cardiothoracic surgeons in Germany and at Dr. Conradi’s center in particular were approaching TMVR and what types of patients were having the procedure, Dr. Conradi said.
“While the EuroSCORE I of patients we operated on didn’t change at all between these two time frames, there were subtle changes in the patients that we operated on,” he said. “Before the commercialization of TMVR, we tended to have a ratio of organic vs. functional MR [mitral regurgitation] of 50-50; after commercialization of TMVR, many of the functional MR patients were allocated to TMVR.”
Dr. Conradi noted the profile of patients who had mitral valve repair also changed once the transcatheter approach became available. “Ischemic disease with coronary artery disease, previous infarction, or previous cardiac surgery – mostly coronary artery bypass grafting – were much less prevalent in this surgery population after TMVR became available,” he said.
The study also found that 30-day mortality declined from 7% to 4% during the study period. “That was probably due to more adequate patient selection because we had a more appropriate treatment that we could offer these high-risk patients as an alternative to high-risk surgical approaches,” Dr. Conradi said.
The analysis stratified procedure volumes and growth by four age groups: younger than 65 years; 65-74; 75-84; and greater than ore equal to 85. Older patients were more likely to have TMVR. Surgical procedure volumes increased most in the less than 65 group and least in the greater than or equal to 85 group.
In Germany, transcatheter mitral valve procedures are reimbursed at a higher rate than surgical procedures, but that doesn’t fully explain the uptake in TMVR, Dr. Conradi said. “The patients receiving the transcatheter approach vs. surgery still differ fundamentally, but the addition of an interventional program decreases the surgical patient’s mean risk profile and thus optimizes surgical results,” he said. “I think this can only happen if surgeons are closely involved. The indications will broaden for these therapies. There’s no doubt about that.”
Dr. Conradi disclosed receiving travel support and lecture fees from Abbott Vascular.
NEW YORK – Since the commercialization of transcatheter mitral valve repair, the share of these procedures among all mitral operations has grown exponentially and has also contributed to an increase in the number of overall mitral procedures, including surgical repair, according to an analysis of a national German database reported at the American Association for Thoracic Surgery Mitral Conclave here.
Transcatheter mitral valve repair (TMVR) using the MitraClip device (Abbott Vascular) was first commercialized in Germany in 2008, and in the years since the share of TMVR procedures among all mitral operations increased from 0.3% to 18.1% in 2015 throughout Germany, said Lenard Conradi, MD, surgical director of minimally-invasive and transcatheter heart valve procedures at the University Heart Center Hamburg.
The goal of the study was to gain insights into how cardiothoracic surgeons in Germany and at Dr. Conradi’s center in particular were approaching TMVR and what types of patients were having the procedure, Dr. Conradi said.
“While the EuroSCORE I of patients we operated on didn’t change at all between these two time frames, there were subtle changes in the patients that we operated on,” he said. “Before the commercialization of TMVR, we tended to have a ratio of organic vs. functional MR [mitral regurgitation] of 50-50; after commercialization of TMVR, many of the functional MR patients were allocated to TMVR.”
Dr. Conradi noted the profile of patients who had mitral valve repair also changed once the transcatheter approach became available. “Ischemic disease with coronary artery disease, previous infarction, or previous cardiac surgery – mostly coronary artery bypass grafting – were much less prevalent in this surgery population after TMVR became available,” he said.
The study also found that 30-day mortality declined from 7% to 4% during the study period. “That was probably due to more adequate patient selection because we had a more appropriate treatment that we could offer these high-risk patients as an alternative to high-risk surgical approaches,” Dr. Conradi said.
The analysis stratified procedure volumes and growth by four age groups: younger than 65 years; 65-74; 75-84; and greater than ore equal to 85. Older patients were more likely to have TMVR. Surgical procedure volumes increased most in the less than 65 group and least in the greater than or equal to 85 group.
In Germany, transcatheter mitral valve procedures are reimbursed at a higher rate than surgical procedures, but that doesn’t fully explain the uptake in TMVR, Dr. Conradi said. “The patients receiving the transcatheter approach vs. surgery still differ fundamentally, but the addition of an interventional program decreases the surgical patient’s mean risk profile and thus optimizes surgical results,” he said. “I think this can only happen if surgeons are closely involved. The indications will broaden for these therapies. There’s no doubt about that.”
Dr. Conradi disclosed receiving travel support and lecture fees from Abbott Vascular.
NEW YORK – Since the commercialization of transcatheter mitral valve repair, the share of these procedures among all mitral operations has grown exponentially and has also contributed to an increase in the number of overall mitral procedures, including surgical repair, according to an analysis of a national German database reported at the American Association for Thoracic Surgery Mitral Conclave here.
Transcatheter mitral valve repair (TMVR) using the MitraClip device (Abbott Vascular) was first commercialized in Germany in 2008, and in the years since the share of TMVR procedures among all mitral operations increased from 0.3% to 18.1% in 2015 throughout Germany, said Lenard Conradi, MD, surgical director of minimally-invasive and transcatheter heart valve procedures at the University Heart Center Hamburg.
The goal of the study was to gain insights into how cardiothoracic surgeons in Germany and at Dr. Conradi’s center in particular were approaching TMVR and what types of patients were having the procedure, Dr. Conradi said.
“While the EuroSCORE I of patients we operated on didn’t change at all between these two time frames, there were subtle changes in the patients that we operated on,” he said. “Before the commercialization of TMVR, we tended to have a ratio of organic vs. functional MR [mitral regurgitation] of 50-50; after commercialization of TMVR, many of the functional MR patients were allocated to TMVR.”
Dr. Conradi noted the profile of patients who had mitral valve repair also changed once the transcatheter approach became available. “Ischemic disease with coronary artery disease, previous infarction, or previous cardiac surgery – mostly coronary artery bypass grafting – were much less prevalent in this surgery population after TMVR became available,” he said.
The study also found that 30-day mortality declined from 7% to 4% during the study period. “That was probably due to more adequate patient selection because we had a more appropriate treatment that we could offer these high-risk patients as an alternative to high-risk surgical approaches,” Dr. Conradi said.
The analysis stratified procedure volumes and growth by four age groups: younger than 65 years; 65-74; 75-84; and greater than ore equal to 85. Older patients were more likely to have TMVR. Surgical procedure volumes increased most in the less than 65 group and least in the greater than or equal to 85 group.
In Germany, transcatheter mitral valve procedures are reimbursed at a higher rate than surgical procedures, but that doesn’t fully explain the uptake in TMVR, Dr. Conradi said. “The patients receiving the transcatheter approach vs. surgery still differ fundamentally, but the addition of an interventional program decreases the surgical patient’s mean risk profile and thus optimizes surgical results,” he said. “I think this can only happen if surgeons are closely involved. The indications will broaden for these therapies. There’s no doubt about that.”
Dr. Conradi disclosed receiving travel support and lecture fees from Abbott Vascular.
AT THE AATS MITRAL CONCLAVE 2017
Key clinical point: Since its commercialization, transcatheter mitral valve repair (TMVR) has accounted for an increasing share of all mitral surgeries in Germany and driven greater mitral surgery volume overall.
Major finding: Volume of surgical mitral valve procedures increased 70% overall and TMVR procedures 57% in Germany since 2008.
Data source: Analysis of data from German Federal statistics office.
Disclosures: Dr. Conradi reported receiving travel support and lecture fees from Abbott Vascular.
What’s driving the ‘failure’ of the ACA marketplaces?
Two reports from the Centers for Medicare & Medicaid Services show declines in the number of consumers who are activating the health insurance purchased via an Affordable Care Act exchange, a development Trump administration officials say underlines the failure of the ACA.
As of March 15, 10.3 million people paid premiums to activate coverage they purchased on the exchanges out of 12.2 million who enrolled in plans as of the Jan. 31 deadline for 2017, according to a June 12 report from CMS.
Further, some consumers who began paying premiums stopped.
Two key factors are behind the drop off, according to a second CMS report.
According to a voluntary online survey of consumers who left the exchanges between August 2016 and April 2017, 46% who canceled coverage prior to paying premiums cited cost as the main reason, including 20% who cited increased premiums over the previous year and 17% who claimed ineligibility for financial assistance.
Of those who activated their coverage by paying premiums, half (49%) said they stopped because they became eligible and received coverage elsewhere, mostly through an employer or Medicare. About a quarter (27%) cited affordability as the reason why they dropped coverage.
According to the Commonwealth Fund, CMS officials missed a few big reasons why people did not consistently pay for their coverage: Many were reacting to uncertainty around the Trump administration’s ACA repeal and replace efforts. Others were missed because of policy changes undertaken by the administration.
“One of the things that we know affected enrollment this year was the pullback of outreach efforts in the last weekend of the open enrollment period by the Trump administration,” Sara Collins, PhD, vice president of health care access and coverage at the Commonwealth Fund, said in an interview. “It did have an affect on enrollment and might be part of the reason why we are seeing – compared to last year – a lower effectuate enrollment.”
Other administration actions are taking a toll, Dr. Collins said.
There is a “general uncertainty that the administration is generating with respect to the enforcement of the individual mandate and also the uncertainty that is affecting insurers’ commitment to the marketplaces for 2018 [cost sharing reduction payments],” she said, noting both insurers and consumers are affected by this uncertainty.
While acknowledging that the main reason consumers dropped exchange policies was gaining coverage from source, Dr. Collins noted that “we are in such a strange environment, where it’s not clear what Congress is going to do or what the administration is going to do. It probably has confused the public somewhat about what their options are and maybe a concern even if they stay in a plan whether or not they will continue to get their tax credits. There has just been a general sense of uncertainty that has been created by the Trump administration and Congress in terms of the future of marketplace plans.”
Two reports from the Centers for Medicare & Medicaid Services show declines in the number of consumers who are activating the health insurance purchased via an Affordable Care Act exchange, a development Trump administration officials say underlines the failure of the ACA.
As of March 15, 10.3 million people paid premiums to activate coverage they purchased on the exchanges out of 12.2 million who enrolled in plans as of the Jan. 31 deadline for 2017, according to a June 12 report from CMS.
Further, some consumers who began paying premiums stopped.
Two key factors are behind the drop off, according to a second CMS report.
According to a voluntary online survey of consumers who left the exchanges between August 2016 and April 2017, 46% who canceled coverage prior to paying premiums cited cost as the main reason, including 20% who cited increased premiums over the previous year and 17% who claimed ineligibility for financial assistance.
Of those who activated their coverage by paying premiums, half (49%) said they stopped because they became eligible and received coverage elsewhere, mostly through an employer or Medicare. About a quarter (27%) cited affordability as the reason why they dropped coverage.
According to the Commonwealth Fund, CMS officials missed a few big reasons why people did not consistently pay for their coverage: Many were reacting to uncertainty around the Trump administration’s ACA repeal and replace efforts. Others were missed because of policy changes undertaken by the administration.
“One of the things that we know affected enrollment this year was the pullback of outreach efforts in the last weekend of the open enrollment period by the Trump administration,” Sara Collins, PhD, vice president of health care access and coverage at the Commonwealth Fund, said in an interview. “It did have an affect on enrollment and might be part of the reason why we are seeing – compared to last year – a lower effectuate enrollment.”
Other administration actions are taking a toll, Dr. Collins said.
There is a “general uncertainty that the administration is generating with respect to the enforcement of the individual mandate and also the uncertainty that is affecting insurers’ commitment to the marketplaces for 2018 [cost sharing reduction payments],” she said, noting both insurers and consumers are affected by this uncertainty.
While acknowledging that the main reason consumers dropped exchange policies was gaining coverage from source, Dr. Collins noted that “we are in such a strange environment, where it’s not clear what Congress is going to do or what the administration is going to do. It probably has confused the public somewhat about what their options are and maybe a concern even if they stay in a plan whether or not they will continue to get their tax credits. There has just been a general sense of uncertainty that has been created by the Trump administration and Congress in terms of the future of marketplace plans.”
Two reports from the Centers for Medicare & Medicaid Services show declines in the number of consumers who are activating the health insurance purchased via an Affordable Care Act exchange, a development Trump administration officials say underlines the failure of the ACA.
As of March 15, 10.3 million people paid premiums to activate coverage they purchased on the exchanges out of 12.2 million who enrolled in plans as of the Jan. 31 deadline for 2017, according to a June 12 report from CMS.
Further, some consumers who began paying premiums stopped.
Two key factors are behind the drop off, according to a second CMS report.
According to a voluntary online survey of consumers who left the exchanges between August 2016 and April 2017, 46% who canceled coverage prior to paying premiums cited cost as the main reason, including 20% who cited increased premiums over the previous year and 17% who claimed ineligibility for financial assistance.
Of those who activated their coverage by paying premiums, half (49%) said they stopped because they became eligible and received coverage elsewhere, mostly through an employer or Medicare. About a quarter (27%) cited affordability as the reason why they dropped coverage.
According to the Commonwealth Fund, CMS officials missed a few big reasons why people did not consistently pay for their coverage: Many were reacting to uncertainty around the Trump administration’s ACA repeal and replace efforts. Others were missed because of policy changes undertaken by the administration.
“One of the things that we know affected enrollment this year was the pullback of outreach efforts in the last weekend of the open enrollment period by the Trump administration,” Sara Collins, PhD, vice president of health care access and coverage at the Commonwealth Fund, said in an interview. “It did have an affect on enrollment and might be part of the reason why we are seeing – compared to last year – a lower effectuate enrollment.”
Other administration actions are taking a toll, Dr. Collins said.
There is a “general uncertainty that the administration is generating with respect to the enforcement of the individual mandate and also the uncertainty that is affecting insurers’ commitment to the marketplaces for 2018 [cost sharing reduction payments],” she said, noting both insurers and consumers are affected by this uncertainty.
While acknowledging that the main reason consumers dropped exchange policies was gaining coverage from source, Dr. Collins noted that “we are in such a strange environment, where it’s not clear what Congress is going to do or what the administration is going to do. It probably has confused the public somewhat about what their options are and maybe a concern even if they stay in a plan whether or not they will continue to get their tax credits. There has just been a general sense of uncertainty that has been created by the Trump administration and Congress in terms of the future of marketplace plans.”
HFrEF mortality halved when treatment matches guidelines
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.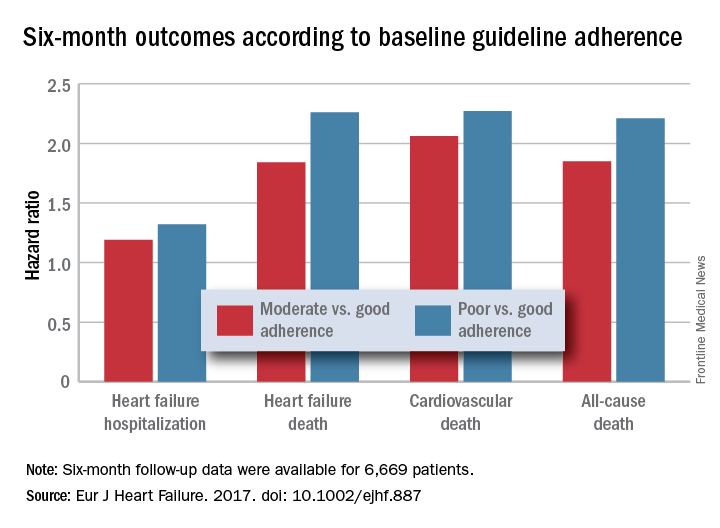
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: Six-month all-cause, cardiovascular, and heart failure mortalities were doubled in patients not on guideline-adherent therapy and dosages.
Data source: QUANTIFY, an international registry with 6,669 HFrEF patients followed for 6 months.
Disclosures: QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
Maryland passes generic drug anti–price gouging law
Maryland has become the first state to prohibit unreasonable price increases on essential off-patent or generic drugs.
The anti–price gouging law authorizes Maryland’s Attorney General to take legal action against manufacturers or distributors that raise drug prices in noncompetitive drug markets if the firms can’t prove the legitimacy of their price increases. Companies could face a civil penalty of up to $10,000 for each violation, according to the law, which goes into effect in October.
Stephen Rockower, MD, president of MedChi, the Maryland State Medical Society, said the medical society has long supported the bill and hailed its passage as beneficial for doctors and their patients.
“It’s been a long time coming,” Dr. Rockower said in an interview. “There have been lots of problems with the huge rising costs of drugs. Manufacturers and pharmacy benefit managers are completely uncontrolled in terms of their pricing. [With this law], we’ll be able to take care of our patients better. It’s hard to prescribe things if it’s going to cost patients $700 a day [for] a drug that used to cost $5 a day. Patients have to be able to afford their medicine, or they don’t take it.”
Chester “Chip” Davis, Jr. president and CEO of the Association for Accessible Medicines, a trade association for manufacturers and distributors of generic drugs, criticized the law.
“By giving the Attorney General unbounded and unprecedented authority to control pricing in a competitive free market, generic companies will be exposed to a level of risk in Maryland that will require them to evaluate whether they want to continue to market affordable medicines within the state,” Mr. Davis said in a statement. “One day, in the not too distant future, [Maryland lawmakers] should be prepared to defend why Maryland was the first state to lead the nation in creating less market-based competition, higher overall prescription drug costs, while also simultaneously increasing the risk of future drug shortages for Maryland’s patients.”
The law has two provisions. It prevents a generic drug manufacturer or wholesale distributor from engaging in price gouging in the sale of an essential off-patent or generic drug, defined as a prescription drug that no longer has market exclusivity and has been designated as essential for treating a life-threatening or chronic health condition by the Maryland Secretary of Health and Mental Hygiene, is actively marketed in the United States by three or fewer manufacturers, and is available for sale in Maryland.
The law also authorizes the Maryland Medical Assistance Program to notify the Attorney General of a price increase when the wholesale acquisition cost of a prescription drug increases by at least 50% in a given year for medications that cost more than $80 per 30-day course.
This first-of-its-kind law is a way to deter manufacturers from exploiting noncompetitive drug markets for short-term profit through unconscionable behavior that “imperils public health and individual welfare,” according to Jeremy A. Greene, MD, PhD, and William V. Padula, PhD.
“Perhaps, [the law will] help reestablish public trust in U.S. policy’s balancing of innovation and access, by reaffirming that older drugs of proven value should be accessible and subject to competition so that they are priced as the commodities they’ve become,” the authors wrote in a perspective published in the New England Journal of Medicine (doi: 10.1056/NEJMp1704907).
Maryland’s law is part of a growing movement among states to address unreasonable pricing spikes in prescription drugs, noted Dr. Greene and Dr. Padula, both of Johns Hopkins University in Baltimore. In April, Louisiana health officials requested feedback about the possibility of invoking an obscure U.S. patent and copyright law to ensure better affordability of hepatitis drugs. In May, the Nevada Senate passed a bill that would force drug makers to publish the list prices they set and the profits they make on insulin, as well as the amount of insulin discounts they give third parties. Additional pharmaceutical price-transparency laws have been proposed in 16 states and Puerto Rico.
“My first concern relates to the provisions of the legislation which directly regulate interstate commerce and pricing by prohibiting and penalizing manufacturer pricing which may occur outside of Maryland,” Gov. Hogan wrote. “These provisions would likely violate the dormant commerce clause of the Constitution. I am also concerned that the definition of ‘unconscionable increase’ and ‘excessive’ are vague, and would likely not stand a ‘vagueness challenge’ under the procedural due process concepts of the 14th Amendment.”
Gov. Hogan added that he is not convinced that the legislation is a solution to Marylanders having better access to medications and that the law may even have the unintended consequences of harming patients by restricting drug access.
[email protected]
On Twitter @legal_med
Maryland has become the first state to prohibit unreasonable price increases on essential off-patent or generic drugs.
The anti–price gouging law authorizes Maryland’s Attorney General to take legal action against manufacturers or distributors that raise drug prices in noncompetitive drug markets if the firms can’t prove the legitimacy of their price increases. Companies could face a civil penalty of up to $10,000 for each violation, according to the law, which goes into effect in October.
Stephen Rockower, MD, president of MedChi, the Maryland State Medical Society, said the medical society has long supported the bill and hailed its passage as beneficial for doctors and their patients.
“It’s been a long time coming,” Dr. Rockower said in an interview. “There have been lots of problems with the huge rising costs of drugs. Manufacturers and pharmacy benefit managers are completely uncontrolled in terms of their pricing. [With this law], we’ll be able to take care of our patients better. It’s hard to prescribe things if it’s going to cost patients $700 a day [for] a drug that used to cost $5 a day. Patients have to be able to afford their medicine, or they don’t take it.”
Chester “Chip” Davis, Jr. president and CEO of the Association for Accessible Medicines, a trade association for manufacturers and distributors of generic drugs, criticized the law.
“By giving the Attorney General unbounded and unprecedented authority to control pricing in a competitive free market, generic companies will be exposed to a level of risk in Maryland that will require them to evaluate whether they want to continue to market affordable medicines within the state,” Mr. Davis said in a statement. “One day, in the not too distant future, [Maryland lawmakers] should be prepared to defend why Maryland was the first state to lead the nation in creating less market-based competition, higher overall prescription drug costs, while also simultaneously increasing the risk of future drug shortages for Maryland’s patients.”
The law has two provisions. It prevents a generic drug manufacturer or wholesale distributor from engaging in price gouging in the sale of an essential off-patent or generic drug, defined as a prescription drug that no longer has market exclusivity and has been designated as essential for treating a life-threatening or chronic health condition by the Maryland Secretary of Health and Mental Hygiene, is actively marketed in the United States by three or fewer manufacturers, and is available for sale in Maryland.
The law also authorizes the Maryland Medical Assistance Program to notify the Attorney General of a price increase when the wholesale acquisition cost of a prescription drug increases by at least 50% in a given year for medications that cost more than $80 per 30-day course.
This first-of-its-kind law is a way to deter manufacturers from exploiting noncompetitive drug markets for short-term profit through unconscionable behavior that “imperils public health and individual welfare,” according to Jeremy A. Greene, MD, PhD, and William V. Padula, PhD.
“Perhaps, [the law will] help reestablish public trust in U.S. policy’s balancing of innovation and access, by reaffirming that older drugs of proven value should be accessible and subject to competition so that they are priced as the commodities they’ve become,” the authors wrote in a perspective published in the New England Journal of Medicine (doi: 10.1056/NEJMp1704907).
Maryland’s law is part of a growing movement among states to address unreasonable pricing spikes in prescription drugs, noted Dr. Greene and Dr. Padula, both of Johns Hopkins University in Baltimore. In April, Louisiana health officials requested feedback about the possibility of invoking an obscure U.S. patent and copyright law to ensure better affordability of hepatitis drugs. In May, the Nevada Senate passed a bill that would force drug makers to publish the list prices they set and the profits they make on insulin, as well as the amount of insulin discounts they give third parties. Additional pharmaceutical price-transparency laws have been proposed in 16 states and Puerto Rico.
“My first concern relates to the provisions of the legislation which directly regulate interstate commerce and pricing by prohibiting and penalizing manufacturer pricing which may occur outside of Maryland,” Gov. Hogan wrote. “These provisions would likely violate the dormant commerce clause of the Constitution. I am also concerned that the definition of ‘unconscionable increase’ and ‘excessive’ are vague, and would likely not stand a ‘vagueness challenge’ under the procedural due process concepts of the 14th Amendment.”
Gov. Hogan added that he is not convinced that the legislation is a solution to Marylanders having better access to medications and that the law may even have the unintended consequences of harming patients by restricting drug access.
[email protected]
On Twitter @legal_med
Maryland has become the first state to prohibit unreasonable price increases on essential off-patent or generic drugs.
The anti–price gouging law authorizes Maryland’s Attorney General to take legal action against manufacturers or distributors that raise drug prices in noncompetitive drug markets if the firms can’t prove the legitimacy of their price increases. Companies could face a civil penalty of up to $10,000 for each violation, according to the law, which goes into effect in October.
Stephen Rockower, MD, president of MedChi, the Maryland State Medical Society, said the medical society has long supported the bill and hailed its passage as beneficial for doctors and their patients.
“It’s been a long time coming,” Dr. Rockower said in an interview. “There have been lots of problems with the huge rising costs of drugs. Manufacturers and pharmacy benefit managers are completely uncontrolled in terms of their pricing. [With this law], we’ll be able to take care of our patients better. It’s hard to prescribe things if it’s going to cost patients $700 a day [for] a drug that used to cost $5 a day. Patients have to be able to afford their medicine, or they don’t take it.”
Chester “Chip” Davis, Jr. president and CEO of the Association for Accessible Medicines, a trade association for manufacturers and distributors of generic drugs, criticized the law.
“By giving the Attorney General unbounded and unprecedented authority to control pricing in a competitive free market, generic companies will be exposed to a level of risk in Maryland that will require them to evaluate whether they want to continue to market affordable medicines within the state,” Mr. Davis said in a statement. “One day, in the not too distant future, [Maryland lawmakers] should be prepared to defend why Maryland was the first state to lead the nation in creating less market-based competition, higher overall prescription drug costs, while also simultaneously increasing the risk of future drug shortages for Maryland’s patients.”
The law has two provisions. It prevents a generic drug manufacturer or wholesale distributor from engaging in price gouging in the sale of an essential off-patent or generic drug, defined as a prescription drug that no longer has market exclusivity and has been designated as essential for treating a life-threatening or chronic health condition by the Maryland Secretary of Health and Mental Hygiene, is actively marketed in the United States by three or fewer manufacturers, and is available for sale in Maryland.
The law also authorizes the Maryland Medical Assistance Program to notify the Attorney General of a price increase when the wholesale acquisition cost of a prescription drug increases by at least 50% in a given year for medications that cost more than $80 per 30-day course.
This first-of-its-kind law is a way to deter manufacturers from exploiting noncompetitive drug markets for short-term profit through unconscionable behavior that “imperils public health and individual welfare,” according to Jeremy A. Greene, MD, PhD, and William V. Padula, PhD.
“Perhaps, [the law will] help reestablish public trust in U.S. policy’s balancing of innovation and access, by reaffirming that older drugs of proven value should be accessible and subject to competition so that they are priced as the commodities they’ve become,” the authors wrote in a perspective published in the New England Journal of Medicine (doi: 10.1056/NEJMp1704907).
Maryland’s law is part of a growing movement among states to address unreasonable pricing spikes in prescription drugs, noted Dr. Greene and Dr. Padula, both of Johns Hopkins University in Baltimore. In April, Louisiana health officials requested feedback about the possibility of invoking an obscure U.S. patent and copyright law to ensure better affordability of hepatitis drugs. In May, the Nevada Senate passed a bill that would force drug makers to publish the list prices they set and the profits they make on insulin, as well as the amount of insulin discounts they give third parties. Additional pharmaceutical price-transparency laws have been proposed in 16 states and Puerto Rico.
“My first concern relates to the provisions of the legislation which directly regulate interstate commerce and pricing by prohibiting and penalizing manufacturer pricing which may occur outside of Maryland,” Gov. Hogan wrote. “These provisions would likely violate the dormant commerce clause of the Constitution. I am also concerned that the definition of ‘unconscionable increase’ and ‘excessive’ are vague, and would likely not stand a ‘vagueness challenge’ under the procedural due process concepts of the 14th Amendment.”
Gov. Hogan added that he is not convinced that the legislation is a solution to Marylanders having better access to medications and that the law may even have the unintended consequences of harming patients by restricting drug access.
[email protected]
On Twitter @legal_med
Personalized snoring video boosts CPAP adherence
BOSTON – Showing patients videos of themselves having apneic episodes may convince them to use continuous positive airway pressure (CPAP), suggests the first results of an ongoing randomized clinical trial.
The investigators based their research project design on a previous pilot study that showed improved adherence to CPAP in patients who were shown videos of themselves sleeping while participating in a sleep study, Mark S. Aloia, PhD, said in a presentation at the annual meeting of the Associated Professional Sleep Societies.
In the new study, patients who had been recently diagnosed with sleep apnea were randomly assigned to participate in one of the three treatment groups. All three groups received sleep apnea and CPAP education prior to the use of CPAP. One group also watched videos of themselves sleeping, snoring, and gasping for air, and another group watched videos of a stranger sleeping and having apneic events.
After adjustment for age, educational level, and baseline sleep apnea severity, those who watched videos of themselves still used their CPAP devices more than 2 hours per night longer than did patients in each of the groups receiving the other two interventions (P = .02).
Both video interventions involved watching 30 minutes of sleep footage shown to each patient once before starting CPAP therapy. CPAP adherence was measured by downloaded data from PAP devices over the first 90 days of use.
The average age of the patients was 50 years, and they had moderate or severe sleep apnea, with mean apnea hypopnea indices ranging from 26.5 to 33.3 in the three study arms. The majority of patients had body mass indexes over 30.
Adherence to CPAP treatment is often poor, with many patients failing to use the device for even 4 hours per night, said Dr. Aloia, a psychologist at National Jewish Health in Denver. Many patients prescribed CPAP for OSA will undergo an educational component that may include watching a video of someone with OSA sleeping and having apneic events, he added. They often have “dramatic responses” to these videos, but then fail to positively change their own behavior.
“Many times we think that if our patient just knew what we know, he or she would use CPAP more, but there is evidence that doctors don’t take their medications any more than patients do, so it is not just a matter of education, it is a little bit deeper than that and it has to be personalized,” he said.
“The use of a personalized video is promising … we hope to present more data next year,” said Dr. Aloia, who has board certification in behavioral sleep medicine,
He noted that the video technique used may be jeopardized as more and more patients partake in home-based rather than lab-based sleep studies. That said, he also reported that the research team had to exclude several patients from the study because they had already viewed videos of themselves sleeping and snoring that had been recorded by their partners.
If the intervention proves effective, Dr. Aloia said he thinks it can be modified for use in home testing.
The study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
BOSTON – Showing patients videos of themselves having apneic episodes may convince them to use continuous positive airway pressure (CPAP), suggests the first results of an ongoing randomized clinical trial.
The investigators based their research project design on a previous pilot study that showed improved adherence to CPAP in patients who were shown videos of themselves sleeping while participating in a sleep study, Mark S. Aloia, PhD, said in a presentation at the annual meeting of the Associated Professional Sleep Societies.
In the new study, patients who had been recently diagnosed with sleep apnea were randomly assigned to participate in one of the three treatment groups. All three groups received sleep apnea and CPAP education prior to the use of CPAP. One group also watched videos of themselves sleeping, snoring, and gasping for air, and another group watched videos of a stranger sleeping and having apneic events.
After adjustment for age, educational level, and baseline sleep apnea severity, those who watched videos of themselves still used their CPAP devices more than 2 hours per night longer than did patients in each of the groups receiving the other two interventions (P = .02).
Both video interventions involved watching 30 minutes of sleep footage shown to each patient once before starting CPAP therapy. CPAP adherence was measured by downloaded data from PAP devices over the first 90 days of use.
The average age of the patients was 50 years, and they had moderate or severe sleep apnea, with mean apnea hypopnea indices ranging from 26.5 to 33.3 in the three study arms. The majority of patients had body mass indexes over 30.
Adherence to CPAP treatment is often poor, with many patients failing to use the device for even 4 hours per night, said Dr. Aloia, a psychologist at National Jewish Health in Denver. Many patients prescribed CPAP for OSA will undergo an educational component that may include watching a video of someone with OSA sleeping and having apneic events, he added. They often have “dramatic responses” to these videos, but then fail to positively change their own behavior.
“Many times we think that if our patient just knew what we know, he or she would use CPAP more, but there is evidence that doctors don’t take their medications any more than patients do, so it is not just a matter of education, it is a little bit deeper than that and it has to be personalized,” he said.
“The use of a personalized video is promising … we hope to present more data next year,” said Dr. Aloia, who has board certification in behavioral sleep medicine,
He noted that the video technique used may be jeopardized as more and more patients partake in home-based rather than lab-based sleep studies. That said, he also reported that the research team had to exclude several patients from the study because they had already viewed videos of themselves sleeping and snoring that had been recorded by their partners.
If the intervention proves effective, Dr. Aloia said he thinks it can be modified for use in home testing.
The study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
BOSTON – Showing patients videos of themselves having apneic episodes may convince them to use continuous positive airway pressure (CPAP), suggests the first results of an ongoing randomized clinical trial.
The investigators based their research project design on a previous pilot study that showed improved adherence to CPAP in patients who were shown videos of themselves sleeping while participating in a sleep study, Mark S. Aloia, PhD, said in a presentation at the annual meeting of the Associated Professional Sleep Societies.
In the new study, patients who had been recently diagnosed with sleep apnea were randomly assigned to participate in one of the three treatment groups. All three groups received sleep apnea and CPAP education prior to the use of CPAP. One group also watched videos of themselves sleeping, snoring, and gasping for air, and another group watched videos of a stranger sleeping and having apneic events.
After adjustment for age, educational level, and baseline sleep apnea severity, those who watched videos of themselves still used their CPAP devices more than 2 hours per night longer than did patients in each of the groups receiving the other two interventions (P = .02).
Both video interventions involved watching 30 minutes of sleep footage shown to each patient once before starting CPAP therapy. CPAP adherence was measured by downloaded data from PAP devices over the first 90 days of use.
The average age of the patients was 50 years, and they had moderate or severe sleep apnea, with mean apnea hypopnea indices ranging from 26.5 to 33.3 in the three study arms. The majority of patients had body mass indexes over 30.
Adherence to CPAP treatment is often poor, with many patients failing to use the device for even 4 hours per night, said Dr. Aloia, a psychologist at National Jewish Health in Denver. Many patients prescribed CPAP for OSA will undergo an educational component that may include watching a video of someone with OSA sleeping and having apneic events, he added. They often have “dramatic responses” to these videos, but then fail to positively change their own behavior.
“Many times we think that if our patient just knew what we know, he or she would use CPAP more, but there is evidence that doctors don’t take their medications any more than patients do, so it is not just a matter of education, it is a little bit deeper than that and it has to be personalized,” he said.
“The use of a personalized video is promising … we hope to present more data next year,” said Dr. Aloia, who has board certification in behavioral sleep medicine,
He noted that the video technique used may be jeopardized as more and more patients partake in home-based rather than lab-based sleep studies. That said, he also reported that the research team had to exclude several patients from the study because they had already viewed videos of themselves sleeping and snoring that had been recorded by their partners.
If the intervention proves effective, Dr. Aloia said he thinks it can be modified for use in home testing.
The study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
AT SLEEP 2017
Key clinical point: Showing patients videos of themselves having an apneic event during CPAP initiation resulted in greater treatment adherence over 3 months, compared to standard ways of initiating CPAP therapy.
Major finding: Patients who watched personalized sleep videos used their CPAP devices more than 2 hours per night longer, compared with those who were not shown a personalized video.
Data source: Randomized controlled trial with 3-month data on 24 individuals out of a planned enrollment of 300 patients.
Disclosures: This ongoing study is supported by a grant from the National Heart, Lung, and Blood Institute. Dr. Aloia disclosed that he is a paid employee of Phillips, but that the study used both Phillips and ResMed CPAP devices.
T-DMI doesn’t wow in HER2-overexpressing NSCLC
CHICAGO –Beyond its role in advanced breast cancer, the antibody-drug conjugate ado-trastuzumab-emtansine (T-DM1; Kadcyla) showed modest activity in some patients with metastatic non–small cell lung cancer (mNSCLC) overexpressing the human epidermal growth factor-2 (HER2) receptor, investigators reported.
In an ongoing phase II study, 4 of 20 patients with mNSCLC whose tumors expressed the highest levels of HER2 had partial responses. In contrast, none of the 20 patients with tumors overexpressing HER2 at lower levels had responses, reported Thomas E. Stinchcombe, MD, of Duke University, Durham, N.C.
Previous studies have shown that HER2 overexpression by immunohistochemistry (IHC) is associated with poor prognosis in patients with NSCLC, but in contrast to breast and gastric cancers, HER2 overexpression in NSCLC is not always accompanied by HER2 amplifications.
“HER2 amplifications and HER2 mutations are generally mutually exclusive in NSCLC. Given the known mechanism of action of T-DM1, HER2 overexpression was chosen as an inclusion criterion for this study,” Dr. Stinchcombe said.
They enrolled patients with HER2-overexpressing mNSCLC who had disease progression following platinum-based chemotherapy. The patients were assigned to one of two 20-patient cohorts based on IHC2+ (10% or more of cells stained with 2+ intensity), or IHC3+ (10% or more of cells stained with 3+intensity).
For the primary endpoint of treatment response none of the patients in the IHC2+ cohort had objective responses by Response Evaluation Criteria in Solid Tumors (RECIST), although eight patients in this cohort had stable disease, including one patient who remained in stable disease status on treatment out to 21 months at last follow-up.
In the IHC3+ cohort, four patients had partial responses, for an objective response rate of 20%. The median duration of response was 7.3 months. Two patients in this cohort had stable disease.
Median PFS was similar between the cohorts, at 2.6 months for IHC2+ and 2.7 months for IHC3+. The respective median OS durations were 12.2 and 12.1 months.
The safety profile of T-DM1 in this population was similar to that seen in breast cancer. There were two grade 3 serious adverse events: one infusion-related hypersensitivity reaction, and one case of thrombocytopenia. There were 10 grade 3 events of any kind, 1 grade 4 event, and 1 treatment withdrawal due to a grade 2 infusion reaction.
“I think it’s honestly a little hard to tell from this study, which is very small, whether there is really a role of [HER2] overexpression in actually driving oncogenesis and being a target for T-DM1,” she said.
Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
CHICAGO –Beyond its role in advanced breast cancer, the antibody-drug conjugate ado-trastuzumab-emtansine (T-DM1; Kadcyla) showed modest activity in some patients with metastatic non–small cell lung cancer (mNSCLC) overexpressing the human epidermal growth factor-2 (HER2) receptor, investigators reported.
In an ongoing phase II study, 4 of 20 patients with mNSCLC whose tumors expressed the highest levels of HER2 had partial responses. In contrast, none of the 20 patients with tumors overexpressing HER2 at lower levels had responses, reported Thomas E. Stinchcombe, MD, of Duke University, Durham, N.C.
Previous studies have shown that HER2 overexpression by immunohistochemistry (IHC) is associated with poor prognosis in patients with NSCLC, but in contrast to breast and gastric cancers, HER2 overexpression in NSCLC is not always accompanied by HER2 amplifications.
“HER2 amplifications and HER2 mutations are generally mutually exclusive in NSCLC. Given the known mechanism of action of T-DM1, HER2 overexpression was chosen as an inclusion criterion for this study,” Dr. Stinchcombe said.
They enrolled patients with HER2-overexpressing mNSCLC who had disease progression following platinum-based chemotherapy. The patients were assigned to one of two 20-patient cohorts based on IHC2+ (10% or more of cells stained with 2+ intensity), or IHC3+ (10% or more of cells stained with 3+intensity).
For the primary endpoint of treatment response none of the patients in the IHC2+ cohort had objective responses by Response Evaluation Criteria in Solid Tumors (RECIST), although eight patients in this cohort had stable disease, including one patient who remained in stable disease status on treatment out to 21 months at last follow-up.
In the IHC3+ cohort, four patients had partial responses, for an objective response rate of 20%. The median duration of response was 7.3 months. Two patients in this cohort had stable disease.
Median PFS was similar between the cohorts, at 2.6 months for IHC2+ and 2.7 months for IHC3+. The respective median OS durations were 12.2 and 12.1 months.
The safety profile of T-DM1 in this population was similar to that seen in breast cancer. There were two grade 3 serious adverse events: one infusion-related hypersensitivity reaction, and one case of thrombocytopenia. There were 10 grade 3 events of any kind, 1 grade 4 event, and 1 treatment withdrawal due to a grade 2 infusion reaction.
“I think it’s honestly a little hard to tell from this study, which is very small, whether there is really a role of [HER2] overexpression in actually driving oncogenesis and being a target for T-DM1,” she said.
Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
CHICAGO –Beyond its role in advanced breast cancer, the antibody-drug conjugate ado-trastuzumab-emtansine (T-DM1; Kadcyla) showed modest activity in some patients with metastatic non–small cell lung cancer (mNSCLC) overexpressing the human epidermal growth factor-2 (HER2) receptor, investigators reported.
In an ongoing phase II study, 4 of 20 patients with mNSCLC whose tumors expressed the highest levels of HER2 had partial responses. In contrast, none of the 20 patients with tumors overexpressing HER2 at lower levels had responses, reported Thomas E. Stinchcombe, MD, of Duke University, Durham, N.C.
Previous studies have shown that HER2 overexpression by immunohistochemistry (IHC) is associated with poor prognosis in patients with NSCLC, but in contrast to breast and gastric cancers, HER2 overexpression in NSCLC is not always accompanied by HER2 amplifications.
“HER2 amplifications and HER2 mutations are generally mutually exclusive in NSCLC. Given the known mechanism of action of T-DM1, HER2 overexpression was chosen as an inclusion criterion for this study,” Dr. Stinchcombe said.
They enrolled patients with HER2-overexpressing mNSCLC who had disease progression following platinum-based chemotherapy. The patients were assigned to one of two 20-patient cohorts based on IHC2+ (10% or more of cells stained with 2+ intensity), or IHC3+ (10% or more of cells stained with 3+intensity).
For the primary endpoint of treatment response none of the patients in the IHC2+ cohort had objective responses by Response Evaluation Criteria in Solid Tumors (RECIST), although eight patients in this cohort had stable disease, including one patient who remained in stable disease status on treatment out to 21 months at last follow-up.
In the IHC3+ cohort, four patients had partial responses, for an objective response rate of 20%. The median duration of response was 7.3 months. Two patients in this cohort had stable disease.
Median PFS was similar between the cohorts, at 2.6 months for IHC2+ and 2.7 months for IHC3+. The respective median OS durations were 12.2 and 12.1 months.
The safety profile of T-DM1 in this population was similar to that seen in breast cancer. There were two grade 3 serious adverse events: one infusion-related hypersensitivity reaction, and one case of thrombocytopenia. There were 10 grade 3 events of any kind, 1 grade 4 event, and 1 treatment withdrawal due to a grade 2 infusion reaction.
“I think it’s honestly a little hard to tell from this study, which is very small, whether there is really a role of [HER2] overexpression in actually driving oncogenesis and being a target for T-DM1,” she said.
Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
AT ASCO 2017
Key clinical point: Some non–small cell lung cancer (NSCLC) tumors overexpress HER2, suggesting the use of T-DM1, an anti-HER2 antibody drug conjugate.
Major finding: Four of 20 patients with HER2-overexpressing NSCLC has partial responses to T-DM1 therapy.
Data source: Open-label prospective trial of 40 patients with NSCLC positive for HER2.
Disclosures: Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
Mild OSA linked to hypertension
BOSTON – Sleep apnea doesn’t have to be severe or even symptomatic to increase the risk of hypertension and diabetes, according to a pair of new studies.
“We found that even mild sleep apnea was strongly associated with increased risk of developing hypertension by four times, compared to individuals without sleep apnea,” said principal investigator and top sleep researcher Alexandros N. Vgontzas, MD, of Pennsylvania State University College of Medicine in a SLEEP press release. “Similarly, moderate sleep apnea was associated with increased risk of developing diabetes by almost three times, compared to individuals without sleep apnea.”
Dr. Vgontzas presented his team’s results on the link between mild to moderate OSA and hypertension at the annual meeting of the American Academy of Sleep Medicine. In a separate session, his colleague at Penn State, Yun Li, MD, presented the diabetes-related findings of the same study.
After multivariate adjustment, including controlling for change in body mass index over time, both mild and moderate OSA were significantly associated with increased odds for developing hypertension, compared with controls without OSA (odds ratios, 4.36 and 3.46, respectively.).
The researchers found their test for an age interaction was also significant, indicating that younger adults with nonsevere OSA were at increased risk of hypertension, while those over 60 years of age were not.
[polldaddy:9792720]
“In young and middle-aged adults, our findings suggest that early detection and treatment of mild to moderate sleep apnea is warranted in order to prevent future cardiometabolic disease,” said Dr. Li in a press release. “Given the stronger association of sleep apnea with metabolic abnormalities in this age group, emphasis should be placed on yearly monitoring of indices of metabolic symptoms and lifestyle interventions, such as weight control, healthy diet, regular exercise, and stress management.”
For diabetes, moderate OSA was significantly associated with an almost threefold increased odds for developing diabetes after adjusting for a range of baseline and follow-up variables (OR, 2.78), but mild OSA was not associated with incident diabetes (OR, 0.47).
Both studies utilized data from the Penn State Adult Cohort, a random general population sample of 1,741 adults who underwent an overnight polysomnography sleep study and had a detailed medical history interview at baseline. Mild and moderate OSA were defined as an apnea hypopnea index from 5 to 14.9 and from 15 to 29.9, respectively. The presence of hypertension or diabetes at baseline and follow-up was defined by a self-report of receiving treatment for or having a physician diagnosis of either condition.
The age range of the studied population was wide (20-84 years), with a mean age of about 47 years. The incidence of diabetes was 10.2% at follow-up, while hypertension was found in 34.2% of patients. Dr. Vgontzas said the percentage of patients with hypertension was roughly what he had expected for this population.
“Our conclusion is that, the younger a person is, the stronger is the need for detection and treatment of sleep apnea,” said Dr. Vgontzas, though he acknowledged that putting these millions of people on continuous positive airway pressure therapy is not an easy proposition.
The study was supported by National Institutes of Health grants. Dr. Vgontzas reported no conflicts of interest.
BOSTON – Sleep apnea doesn’t have to be severe or even symptomatic to increase the risk of hypertension and diabetes, according to a pair of new studies.
“We found that even mild sleep apnea was strongly associated with increased risk of developing hypertension by four times, compared to individuals without sleep apnea,” said principal investigator and top sleep researcher Alexandros N. Vgontzas, MD, of Pennsylvania State University College of Medicine in a SLEEP press release. “Similarly, moderate sleep apnea was associated with increased risk of developing diabetes by almost three times, compared to individuals without sleep apnea.”
Dr. Vgontzas presented his team’s results on the link between mild to moderate OSA and hypertension at the annual meeting of the American Academy of Sleep Medicine. In a separate session, his colleague at Penn State, Yun Li, MD, presented the diabetes-related findings of the same study.
After multivariate adjustment, including controlling for change in body mass index over time, both mild and moderate OSA were significantly associated with increased odds for developing hypertension, compared with controls without OSA (odds ratios, 4.36 and 3.46, respectively.).
The researchers found their test for an age interaction was also significant, indicating that younger adults with nonsevere OSA were at increased risk of hypertension, while those over 60 years of age were not.
[polldaddy:9792720]
“In young and middle-aged adults, our findings suggest that early detection and treatment of mild to moderate sleep apnea is warranted in order to prevent future cardiometabolic disease,” said Dr. Li in a press release. “Given the stronger association of sleep apnea with metabolic abnormalities in this age group, emphasis should be placed on yearly monitoring of indices of metabolic symptoms and lifestyle interventions, such as weight control, healthy diet, regular exercise, and stress management.”
For diabetes, moderate OSA was significantly associated with an almost threefold increased odds for developing diabetes after adjusting for a range of baseline and follow-up variables (OR, 2.78), but mild OSA was not associated with incident diabetes (OR, 0.47).
Both studies utilized data from the Penn State Adult Cohort, a random general population sample of 1,741 adults who underwent an overnight polysomnography sleep study and had a detailed medical history interview at baseline. Mild and moderate OSA were defined as an apnea hypopnea index from 5 to 14.9 and from 15 to 29.9, respectively. The presence of hypertension or diabetes at baseline and follow-up was defined by a self-report of receiving treatment for or having a physician diagnosis of either condition.
The age range of the studied population was wide (20-84 years), with a mean age of about 47 years. The incidence of diabetes was 10.2% at follow-up, while hypertension was found in 34.2% of patients. Dr. Vgontzas said the percentage of patients with hypertension was roughly what he had expected for this population.
“Our conclusion is that, the younger a person is, the stronger is the need for detection and treatment of sleep apnea,” said Dr. Vgontzas, though he acknowledged that putting these millions of people on continuous positive airway pressure therapy is not an easy proposition.
The study was supported by National Institutes of Health grants. Dr. Vgontzas reported no conflicts of interest.
BOSTON – Sleep apnea doesn’t have to be severe or even symptomatic to increase the risk of hypertension and diabetes, according to a pair of new studies.
“We found that even mild sleep apnea was strongly associated with increased risk of developing hypertension by four times, compared to individuals without sleep apnea,” said principal investigator and top sleep researcher Alexandros N. Vgontzas, MD, of Pennsylvania State University College of Medicine in a SLEEP press release. “Similarly, moderate sleep apnea was associated with increased risk of developing diabetes by almost three times, compared to individuals without sleep apnea.”
Dr. Vgontzas presented his team’s results on the link between mild to moderate OSA and hypertension at the annual meeting of the American Academy of Sleep Medicine. In a separate session, his colleague at Penn State, Yun Li, MD, presented the diabetes-related findings of the same study.
After multivariate adjustment, including controlling for change in body mass index over time, both mild and moderate OSA were significantly associated with increased odds for developing hypertension, compared with controls without OSA (odds ratios, 4.36 and 3.46, respectively.).
The researchers found their test for an age interaction was also significant, indicating that younger adults with nonsevere OSA were at increased risk of hypertension, while those over 60 years of age were not.
[polldaddy:9792720]
“In young and middle-aged adults, our findings suggest that early detection and treatment of mild to moderate sleep apnea is warranted in order to prevent future cardiometabolic disease,” said Dr. Li in a press release. “Given the stronger association of sleep apnea with metabolic abnormalities in this age group, emphasis should be placed on yearly monitoring of indices of metabolic symptoms and lifestyle interventions, such as weight control, healthy diet, regular exercise, and stress management.”
For diabetes, moderate OSA was significantly associated with an almost threefold increased odds for developing diabetes after adjusting for a range of baseline and follow-up variables (OR, 2.78), but mild OSA was not associated with incident diabetes (OR, 0.47).
Both studies utilized data from the Penn State Adult Cohort, a random general population sample of 1,741 adults who underwent an overnight polysomnography sleep study and had a detailed medical history interview at baseline. Mild and moderate OSA were defined as an apnea hypopnea index from 5 to 14.9 and from 15 to 29.9, respectively. The presence of hypertension or diabetes at baseline and follow-up was defined by a self-report of receiving treatment for or having a physician diagnosis of either condition.
The age range of the studied population was wide (20-84 years), with a mean age of about 47 years. The incidence of diabetes was 10.2% at follow-up, while hypertension was found in 34.2% of patients. Dr. Vgontzas said the percentage of patients with hypertension was roughly what he had expected for this population.
“Our conclusion is that, the younger a person is, the stronger is the need for detection and treatment of sleep apnea,” said Dr. Vgontzas, though he acknowledged that putting these millions of people on continuous positive airway pressure therapy is not an easy proposition.
The study was supported by National Institutes of Health grants. Dr. Vgontzas reported no conflicts of interest.
AT SLEEP 2017
Key clinical point: In a random sample of adults, the presence of mild to moderate OSA was associated with a significantly higher risk of hypertension and diabetes.
Major finding: Individuals with mild to moderate OSA had more than a fourfold increased risk of hypertension over 10 years of follow-up (OR, 4.36). Moderate OSA was significantly associated with incident diabetes risk (OR, 2.78), but mild OSA was not.
Data source: An observational study including a random sample of 1,741 adults between the ages of 20 and 84 years.
Disclosures: The study was supported by National Institutes of Health grants. Dr. Vgontzas reported no conflicts of interest.
Save the Date: 2017 AATS Focus on Thoracic Surgery: Mastering Surgical Innovation
Mark your calendar for the 2017 AATS Focus on Thoracic Surgery meeting taking place in a new location this year - Las Vegas.
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
For more informaton, go to:
aats.org/focus
Mark your calendar for the 2017 AATS Focus on Thoracic Surgery meeting taking place in a new location this year - Las Vegas.
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
For more informaton, go to:
aats.org/focus
Mark your calendar for the 2017 AATS Focus on Thoracic Surgery meeting taking place in a new location this year - Las Vegas.
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
For more informaton, go to:
aats.org/focus