User login
PTSD Rates Soar Among College Students
TOPLINE:
Posttraumatic stress disorder (PTSD) rates among college students more than doubled between 2017 and 2022, new data showed. Rates of acute stress disorder (ASD) also increased during that time.
METHODOLOGY:
- Researchers conducted five waves of cross-sectional study from 2017 to 2022, involving 392,377 participants across 332 colleges and universities.
- The study utilized the Healthy Minds Study data, ensuring representativeness by applying sample weights based on institutional demographics.
- Outcome variables were diagnoses of PTSD and ASD, confirmed by healthcare practitioners, with statistical analysis assessing change in odds of estimated prevalence during 2017-2022.
TAKEAWAY:
- The prevalence of PTSD among US college students increased from 3.4% in 2017-2018 to 7.5% in 2021-2022.
- ASD diagnoses also rose from 0.2% in 2017-2018 to 0.7% in 2021-2022, with both increases remaining statistically significant after adjusting for demographic differences.
- Investigators noted that these findings underscore the need for targeted, trauma-informed intervention strategies in college settings.
IN PRACTICE:
“These trends highlight the escalating mental health challenges among college students, which is consistent with recent research reporting a surge in psychiatric diagnoses,” the authors wrote. “Factors contributing to this rise may include pandemic-related stressors (eg, loss of loved ones) and the effect of traumatic events (eg, campus shootings and racial trauma),” they added.
SOURCE:
The study was led by Yusen Zhai, PhD, University of Alabama at Birmingham. It was published online on May 30, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on self-reported data and single questions for diagnosed PTSD and ASD may have limited the accuracy of the findings. The retrospective design and the absence of longitudinal follow-up may have restricted the ability to infer causality from the observed trends.
DISCLOSURES:
No disclosures were reported. No funding information was available.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Posttraumatic stress disorder (PTSD) rates among college students more than doubled between 2017 and 2022, new data showed. Rates of acute stress disorder (ASD) also increased during that time.
METHODOLOGY:
- Researchers conducted five waves of cross-sectional study from 2017 to 2022, involving 392,377 participants across 332 colleges and universities.
- The study utilized the Healthy Minds Study data, ensuring representativeness by applying sample weights based on institutional demographics.
- Outcome variables were diagnoses of PTSD and ASD, confirmed by healthcare practitioners, with statistical analysis assessing change in odds of estimated prevalence during 2017-2022.
TAKEAWAY:
- The prevalence of PTSD among US college students increased from 3.4% in 2017-2018 to 7.5% in 2021-2022.
- ASD diagnoses also rose from 0.2% in 2017-2018 to 0.7% in 2021-2022, with both increases remaining statistically significant after adjusting for demographic differences.
- Investigators noted that these findings underscore the need for targeted, trauma-informed intervention strategies in college settings.
IN PRACTICE:
“These trends highlight the escalating mental health challenges among college students, which is consistent with recent research reporting a surge in psychiatric diagnoses,” the authors wrote. “Factors contributing to this rise may include pandemic-related stressors (eg, loss of loved ones) and the effect of traumatic events (eg, campus shootings and racial trauma),” they added.
SOURCE:
The study was led by Yusen Zhai, PhD, University of Alabama at Birmingham. It was published online on May 30, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on self-reported data and single questions for diagnosed PTSD and ASD may have limited the accuracy of the findings. The retrospective design and the absence of longitudinal follow-up may have restricted the ability to infer causality from the observed trends.
DISCLOSURES:
No disclosures were reported. No funding information was available.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Posttraumatic stress disorder (PTSD) rates among college students more than doubled between 2017 and 2022, new data showed. Rates of acute stress disorder (ASD) also increased during that time.
METHODOLOGY:
- Researchers conducted five waves of cross-sectional study from 2017 to 2022, involving 392,377 participants across 332 colleges and universities.
- The study utilized the Healthy Minds Study data, ensuring representativeness by applying sample weights based on institutional demographics.
- Outcome variables were diagnoses of PTSD and ASD, confirmed by healthcare practitioners, with statistical analysis assessing change in odds of estimated prevalence during 2017-2022.
TAKEAWAY:
- The prevalence of PTSD among US college students increased from 3.4% in 2017-2018 to 7.5% in 2021-2022.
- ASD diagnoses also rose from 0.2% in 2017-2018 to 0.7% in 2021-2022, with both increases remaining statistically significant after adjusting for demographic differences.
- Investigators noted that these findings underscore the need for targeted, trauma-informed intervention strategies in college settings.
IN PRACTICE:
“These trends highlight the escalating mental health challenges among college students, which is consistent with recent research reporting a surge in psychiatric diagnoses,” the authors wrote. “Factors contributing to this rise may include pandemic-related stressors (eg, loss of loved ones) and the effect of traumatic events (eg, campus shootings and racial trauma),” they added.
SOURCE:
The study was led by Yusen Zhai, PhD, University of Alabama at Birmingham. It was published online on May 30, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on self-reported data and single questions for diagnosed PTSD and ASD may have limited the accuracy of the findings. The retrospective design and the absence of longitudinal follow-up may have restricted the ability to infer causality from the observed trends.
DISCLOSURES:
No disclosures were reported. No funding information was available.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Early Memory Problems Linked to Increased Tau
Reports from older adults and their partners of early memory issues are associated with higher levels of tau neurofibrillary tangles in the brain, new research suggests.
The findings show that in addition to beta-amyloid, tau is implicated in cognitive decline even in the absence of overt clinical symptoms.
“Understanding the earliest signs of Alzheimer’s disease is even more important now that new disease-modifying drugs are becoming available,” study author
Rebecca E. Amariglio, PhD, clinical neuropsychologist at Brigham and Women’s Hospital and the Massachusetts General Hospital and assistant professor in neurology at Harvard Medical School, Boston, said in a news release. “Our study found early suspicions of memory problems by both participants and the people who knew them well were linked to higher levels of tau tangles in the brain.”
The study was published online in Neurology.
Subjective Cognitive Decline
Beta-amyloid plaque accumulations and tau neurofibrillary tangles both underlie the clinical continuum of Alzheimer’s disease (AD). Previous studies have investigated beta-amyloid burden and self- and partner-reported cognitive decline, but fewer have examined regional tau.
Subjective cognitive decline may be an early sign of AD, but self-awareness declines as individuals become increasingly symptomatic. So, a report from a partner about the participant’s level of cognitive functioning is often required in studies of mild cognitive impairment and dementia. The relevance of this model during the preclinical stage is less clear.
For the multicohort, cross-sectional study, investigators studied 675 cognitively unimpaired older adults (mean age, 72 years; 59% female), including persons with nonelevated beta-amyloid levels and those with elevated beta-amyloid levels, as determined by PET.
Participants brought a spouse, adult child, or other study partner with them to answer questions about the participant’s cognitive abilities and their ability to complete daily tasks. About 65% of participants lived with their partners and both completed the Cognitive Function Index (CFI) to assess cognitive decline, with higher scores indicating greater cognitive decline.
Covariates included age, sex, education, and cohort as well as objective cognitive performance.
The Value of Partner Reporting
Investigators found that higher tau levels were associated with greater self- and partner-reported cognitive decline (P < .001 for both).
Significant associations between self- and partner-reported CFI measures were driven by elevated beta-amyloid levels, with continuous beta-amyloid levels showing an independent effect on CFI in addition to tau.
“Our findings suggest that asking older people who have elevated Alzheimer’s disease biomarkers about subjective cognitive decline may be valuable for early detection,” Dr. Amariglio said.
Limitations include the fact that most participants were White and highly educated. Future studies should include participants from more diverse racial and ethnic groups and people with diverse levels of education, researchers noted.
“Although this study was cross-sectional, findings suggest that among older CU individuals who at risk for AD dementia, capturing self-report and study partner report of cognitive function may be valuable for understanding the relationship between early pathophysiologic progression and the emergence of functional impairment,” the authors concluded.
The study was funded in part by the National Institute on Aging, Eli Lily, and the Alzheimer’s Association, among others. Dr. Amariglio receives research funding from the National Institute on Aging. Complete study funding and other authors’ disclosures are listed in the original paper.
A version of this article first appeared on Medscape.com.
Reports from older adults and their partners of early memory issues are associated with higher levels of tau neurofibrillary tangles in the brain, new research suggests.
The findings show that in addition to beta-amyloid, tau is implicated in cognitive decline even in the absence of overt clinical symptoms.
“Understanding the earliest signs of Alzheimer’s disease is even more important now that new disease-modifying drugs are becoming available,” study author
Rebecca E. Amariglio, PhD, clinical neuropsychologist at Brigham and Women’s Hospital and the Massachusetts General Hospital and assistant professor in neurology at Harvard Medical School, Boston, said in a news release. “Our study found early suspicions of memory problems by both participants and the people who knew them well were linked to higher levels of tau tangles in the brain.”
The study was published online in Neurology.
Subjective Cognitive Decline
Beta-amyloid plaque accumulations and tau neurofibrillary tangles both underlie the clinical continuum of Alzheimer’s disease (AD). Previous studies have investigated beta-amyloid burden and self- and partner-reported cognitive decline, but fewer have examined regional tau.
Subjective cognitive decline may be an early sign of AD, but self-awareness declines as individuals become increasingly symptomatic. So, a report from a partner about the participant’s level of cognitive functioning is often required in studies of mild cognitive impairment and dementia. The relevance of this model during the preclinical stage is less clear.
For the multicohort, cross-sectional study, investigators studied 675 cognitively unimpaired older adults (mean age, 72 years; 59% female), including persons with nonelevated beta-amyloid levels and those with elevated beta-amyloid levels, as determined by PET.
Participants brought a spouse, adult child, or other study partner with them to answer questions about the participant’s cognitive abilities and their ability to complete daily tasks. About 65% of participants lived with their partners and both completed the Cognitive Function Index (CFI) to assess cognitive decline, with higher scores indicating greater cognitive decline.
Covariates included age, sex, education, and cohort as well as objective cognitive performance.
The Value of Partner Reporting
Investigators found that higher tau levels were associated with greater self- and partner-reported cognitive decline (P < .001 for both).
Significant associations between self- and partner-reported CFI measures were driven by elevated beta-amyloid levels, with continuous beta-amyloid levels showing an independent effect on CFI in addition to tau.
“Our findings suggest that asking older people who have elevated Alzheimer’s disease biomarkers about subjective cognitive decline may be valuable for early detection,” Dr. Amariglio said.
Limitations include the fact that most participants were White and highly educated. Future studies should include participants from more diverse racial and ethnic groups and people with diverse levels of education, researchers noted.
“Although this study was cross-sectional, findings suggest that among older CU individuals who at risk for AD dementia, capturing self-report and study partner report of cognitive function may be valuable for understanding the relationship between early pathophysiologic progression and the emergence of functional impairment,” the authors concluded.
The study was funded in part by the National Institute on Aging, Eli Lily, and the Alzheimer’s Association, among others. Dr. Amariglio receives research funding from the National Institute on Aging. Complete study funding and other authors’ disclosures are listed in the original paper.
A version of this article first appeared on Medscape.com.
Reports from older adults and their partners of early memory issues are associated with higher levels of tau neurofibrillary tangles in the brain, new research suggests.
The findings show that in addition to beta-amyloid, tau is implicated in cognitive decline even in the absence of overt clinical symptoms.
“Understanding the earliest signs of Alzheimer’s disease is even more important now that new disease-modifying drugs are becoming available,” study author
Rebecca E. Amariglio, PhD, clinical neuropsychologist at Brigham and Women’s Hospital and the Massachusetts General Hospital and assistant professor in neurology at Harvard Medical School, Boston, said in a news release. “Our study found early suspicions of memory problems by both participants and the people who knew them well were linked to higher levels of tau tangles in the brain.”
The study was published online in Neurology.
Subjective Cognitive Decline
Beta-amyloid plaque accumulations and tau neurofibrillary tangles both underlie the clinical continuum of Alzheimer’s disease (AD). Previous studies have investigated beta-amyloid burden and self- and partner-reported cognitive decline, but fewer have examined regional tau.
Subjective cognitive decline may be an early sign of AD, but self-awareness declines as individuals become increasingly symptomatic. So, a report from a partner about the participant’s level of cognitive functioning is often required in studies of mild cognitive impairment and dementia. The relevance of this model during the preclinical stage is less clear.
For the multicohort, cross-sectional study, investigators studied 675 cognitively unimpaired older adults (mean age, 72 years; 59% female), including persons with nonelevated beta-amyloid levels and those with elevated beta-amyloid levels, as determined by PET.
Participants brought a spouse, adult child, or other study partner with them to answer questions about the participant’s cognitive abilities and their ability to complete daily tasks. About 65% of participants lived with their partners and both completed the Cognitive Function Index (CFI) to assess cognitive decline, with higher scores indicating greater cognitive decline.
Covariates included age, sex, education, and cohort as well as objective cognitive performance.
The Value of Partner Reporting
Investigators found that higher tau levels were associated with greater self- and partner-reported cognitive decline (P < .001 for both).
Significant associations between self- and partner-reported CFI measures were driven by elevated beta-amyloid levels, with continuous beta-amyloid levels showing an independent effect on CFI in addition to tau.
“Our findings suggest that asking older people who have elevated Alzheimer’s disease biomarkers about subjective cognitive decline may be valuable for early detection,” Dr. Amariglio said.
Limitations include the fact that most participants were White and highly educated. Future studies should include participants from more diverse racial and ethnic groups and people with diverse levels of education, researchers noted.
“Although this study was cross-sectional, findings suggest that among older CU individuals who at risk for AD dementia, capturing self-report and study partner report of cognitive function may be valuable for understanding the relationship between early pathophysiologic progression and the emergence of functional impairment,” the authors concluded.
The study was funded in part by the National Institute on Aging, Eli Lily, and the Alzheimer’s Association, among others. Dr. Amariglio receives research funding from the National Institute on Aging. Complete study funding and other authors’ disclosures are listed in the original paper.
A version of this article first appeared on Medscape.com.
Knowing My Limits
The records came in by fax. A patient who’d recently moved here and needed to connect with a local neurologist.
When I had time, I flipped through the records. He needed ongoing treatment for a rare neurological disease that I’d heard of, but wasn’t otherwise familiar with. It didn’t even exist in the textbooks or conferences when I was in residency. I’d never seen a case of it, just read about it here and there in journals.
I looked it up, reviewed current treatment options, monitoring, and other knowledge about it, then stared at the notes for a minute. Finally, after thinking it over, I attached a sticky note for my secretary that, if the person called, to redirect them to one of the local subspecialty neurology centers.
I have nothing against this patient, but realistically he would be better served seeing someone with time to keep up on advancements in esoteric disorders, not a general neurologist like myself.
Isn’t that why we have subspecialty centers?
Some of it is also me. There was a time in my career when keeping up on newly discovered disorders and their treatments was, well, cool. But after 25 years in practice, that changes.
It’s important to be at least somewhat aware of new developments (such as in this case) as you may encounter them, and need to know when it’s something you can handle and when to send it elsewhere.
Driving home that afternoon I thought, “I’m an old dog. I don’t want to learn new tricks.” Maybe that’s all it is. There are other neurologists my age and older who thrive on the challenge of learning about and treating new and rare disorders that were unknown when they started out. There’s nothing wrong with that.
But I’ve never pretended to be an academic or sub-sub-specialist. My patients depend on me to stay up to date on the large number of commonly seen neurological disorders, and I do my best to do that.
It ain’t easy being an old dog.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
The records came in by fax. A patient who’d recently moved here and needed to connect with a local neurologist.
When I had time, I flipped through the records. He needed ongoing treatment for a rare neurological disease that I’d heard of, but wasn’t otherwise familiar with. It didn’t even exist in the textbooks or conferences when I was in residency. I’d never seen a case of it, just read about it here and there in journals.
I looked it up, reviewed current treatment options, monitoring, and other knowledge about it, then stared at the notes for a minute. Finally, after thinking it over, I attached a sticky note for my secretary that, if the person called, to redirect them to one of the local subspecialty neurology centers.
I have nothing against this patient, but realistically he would be better served seeing someone with time to keep up on advancements in esoteric disorders, not a general neurologist like myself.
Isn’t that why we have subspecialty centers?
Some of it is also me. There was a time in my career when keeping up on newly discovered disorders and their treatments was, well, cool. But after 25 years in practice, that changes.
It’s important to be at least somewhat aware of new developments (such as in this case) as you may encounter them, and need to know when it’s something you can handle and when to send it elsewhere.
Driving home that afternoon I thought, “I’m an old dog. I don’t want to learn new tricks.” Maybe that’s all it is. There are other neurologists my age and older who thrive on the challenge of learning about and treating new and rare disorders that were unknown when they started out. There’s nothing wrong with that.
But I’ve never pretended to be an academic or sub-sub-specialist. My patients depend on me to stay up to date on the large number of commonly seen neurological disorders, and I do my best to do that.
It ain’t easy being an old dog.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
The records came in by fax. A patient who’d recently moved here and needed to connect with a local neurologist.
When I had time, I flipped through the records. He needed ongoing treatment for a rare neurological disease that I’d heard of, but wasn’t otherwise familiar with. It didn’t even exist in the textbooks or conferences when I was in residency. I’d never seen a case of it, just read about it here and there in journals.
I looked it up, reviewed current treatment options, monitoring, and other knowledge about it, then stared at the notes for a minute. Finally, after thinking it over, I attached a sticky note for my secretary that, if the person called, to redirect them to one of the local subspecialty neurology centers.
I have nothing against this patient, but realistically he would be better served seeing someone with time to keep up on advancements in esoteric disorders, not a general neurologist like myself.
Isn’t that why we have subspecialty centers?
Some of it is also me. There was a time in my career when keeping up on newly discovered disorders and their treatments was, well, cool. But after 25 years in practice, that changes.
It’s important to be at least somewhat aware of new developments (such as in this case) as you may encounter them, and need to know when it’s something you can handle and when to send it elsewhere.
Driving home that afternoon I thought, “I’m an old dog. I don’t want to learn new tricks.” Maybe that’s all it is. There are other neurologists my age and older who thrive on the challenge of learning about and treating new and rare disorders that were unknown when they started out. There’s nothing wrong with that.
But I’ve never pretended to be an academic or sub-sub-specialist. My patients depend on me to stay up to date on the large number of commonly seen neurological disorders, and I do my best to do that.
It ain’t easy being an old dog.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
New Era? ‘Double Selective’ Antibiotic Spares the Microbiome
A new antibiotic uses a never-before-seen mechanism to deliver a direct hit on tough-to-treat infections while leaving beneficial microbes alone. The strategy could lead to a new class of antibiotics that attack dangerous bacteria in a powerful new way, overcoming current drug resistance while sparing the gut microbiome.
“The biggest takeaway is the double-selective component,” said co-lead author Kristen A. Muñoz, PhD, who performed the research as a doctoral student at University of Illinois at Urbana-Champaign (UIUC). “We were able to develop a drug that not only targets problematic pathogens, but because it is selective for these pathogens only, we can spare the good bacteria and preserve the integrity of the microbiome.”
The drug goes after Gram-negative bacteria — pathogens responsible for debilitating and even fatal infections like gastroenteritis, urinary tract infections, pneumonia, sepsis, and cholera. The arsenal of antibiotics against them is old, with no new classes specifically targeting these bacteria coming on the market since 1968.
Many of these bugs have become resistant to one or more antibiotics, with deadly consequences. And antibiotics against them can also wipe out beneficial gut bacteria, allowing serious secondary infections to flare up.
In a study published in Nature, the drug lolamicin knocked out or reduced 130 strains of antibiotic-resistant Gram-negative bacteria in cell cultures. It also successfully treated drug-resistant bloodstream infections and pneumonia in mice while sparing their gut microbiome.
With their microbiomes intact, the mice then fought off secondary infection with Clostridioides difficile (a leading cause of opportunistic and sometimes fatal infections in US health care facilities), while mice treated with other compounds that damaged their microbiome succumbed.
How It Works
Like a well-built medieval castle, Gram-negative bacteria are encased in two protective walls, or membranes. Dr. Muñoz and her team at UIUC set out to breach this defense by finding compounds that hinder the “Lol system,” which ferries lipoproteins between them.
From one compound they constructed lolamicin, which can stop Gram-negative pathogens — with little effect on Gram-negative beneficial bacteria and no effect on Gram-positive bacteria.
“Gram-positive bacteria do not have an outer membrane, so they do not possess the Lol system,” Dr. Muñoz said. “When we compared the sequences of the Lol system in certain Gram-negative pathogens to Gram-negative commensal [beneficial] gut bacteria, we saw that the Lol systems were pretty different.”
Tossing a monkey wrench into the Lol system may be the study’s biggest contribution to future antibiotic development, said Kim Lewis, PhD, professor of Biology and director of Antimicrobial Discovery Center at Northeastern University, Boston, who has discovered several antibiotics now in preclinical research. One, darobactin, targets Gram-negative bugs without affecting the gut microbiome. Another, teixobactin, takes down Gram-positive bacteria without causing drug resistance.
“Lolamicin hits a novel target. I would say that’s the most significant study finding,” said Dr. Lewis, who was not involved in the study. “That is rare. If you look at antibiotics introduced since 1968, they have been modifications of existing antibiotics or, rarely, new chemically but hitting the same proven targets. This one hits something properly new, and [that’s] what I found perhaps the most original and interesting.”
Kirk E. Hevener, PharmD, PhD, associate professor of Pharmaceutical Sciences at the University of Tennessee Health Science Center, Memphis, Tennessee, agreed. (Dr. Hevener also was not involved in the study.) “Lolamicin works by targeting a unique Gram-negative transport system. No currently approved antibacterials work in this way, meaning it potentially represents the first of a new class of antibacterials with narrow-spectrum Gram-negative activity and low gastrointestinal disturbance,” said Dr. Hevener, whose research looks at new antimicrobial drug targets.
The UIUC researchers noted that lolamicin has one drawback: Bacteria frequently developed resistance to it. But in future work, it could be tweaked, combined with other antibiotics, or used as a template for finding other Lol system attackers, they said.
“There is still a good amount of work cut out for us in terms of assessing the clinical translatability of lolamicin, but we are hopeful for the future of this drug,” Dr. Muñoz said.
Addressing a Dire Need
Bringing such a drug to market — from discovery to Food and Drug Administration approval — could take more than a decade, said Dr. Hevener. And new agents, especially for Gram-negative bugs, are sorely needed.
Not only do these bacteria shield themselves with a double membrane but they also “have more complex resistance mechanisms including special pumps that can remove antibacterial drugs from the cell before they can be effective,” Dr. Hevener said.
As a result, drug-resistant Gram-negative bacteria are making treatment of severe infections such as sepsis and pneumonia in health care settings difficult.
Bloodstream infections with drug-resistant Klebsiella pneumoniae have a 40% mortality rate, Dr. Lewis said. And microbiome damage caused by antibiotics is also widespread and deadly, wiping out communities of helpful, protective gut bacteria. That contributes to over half of the C. difficile infections that affect 500,000 people and kill 30,000 a year in the United States.
“Our arsenal of antibacterials that can be used to treat Gram-negative infections is dangerously low,” Dr. Hevener said. “Research will always be needed to develop new antibacterials with novel mechanisms of activity that can bypass bacterial resistance mechanisms.”
A version of this article appeared on Medscape.com.
A new antibiotic uses a never-before-seen mechanism to deliver a direct hit on tough-to-treat infections while leaving beneficial microbes alone. The strategy could lead to a new class of antibiotics that attack dangerous bacteria in a powerful new way, overcoming current drug resistance while sparing the gut microbiome.
“The biggest takeaway is the double-selective component,” said co-lead author Kristen A. Muñoz, PhD, who performed the research as a doctoral student at University of Illinois at Urbana-Champaign (UIUC). “We were able to develop a drug that not only targets problematic pathogens, but because it is selective for these pathogens only, we can spare the good bacteria and preserve the integrity of the microbiome.”
The drug goes after Gram-negative bacteria — pathogens responsible for debilitating and even fatal infections like gastroenteritis, urinary tract infections, pneumonia, sepsis, and cholera. The arsenal of antibiotics against them is old, with no new classes specifically targeting these bacteria coming on the market since 1968.
Many of these bugs have become resistant to one or more antibiotics, with deadly consequences. And antibiotics against them can also wipe out beneficial gut bacteria, allowing serious secondary infections to flare up.
In a study published in Nature, the drug lolamicin knocked out or reduced 130 strains of antibiotic-resistant Gram-negative bacteria in cell cultures. It also successfully treated drug-resistant bloodstream infections and pneumonia in mice while sparing their gut microbiome.
With their microbiomes intact, the mice then fought off secondary infection with Clostridioides difficile (a leading cause of opportunistic and sometimes fatal infections in US health care facilities), while mice treated with other compounds that damaged their microbiome succumbed.
How It Works
Like a well-built medieval castle, Gram-negative bacteria are encased in two protective walls, or membranes. Dr. Muñoz and her team at UIUC set out to breach this defense by finding compounds that hinder the “Lol system,” which ferries lipoproteins between them.
From one compound they constructed lolamicin, which can stop Gram-negative pathogens — with little effect on Gram-negative beneficial bacteria and no effect on Gram-positive bacteria.
“Gram-positive bacteria do not have an outer membrane, so they do not possess the Lol system,” Dr. Muñoz said. “When we compared the sequences of the Lol system in certain Gram-negative pathogens to Gram-negative commensal [beneficial] gut bacteria, we saw that the Lol systems were pretty different.”
Tossing a monkey wrench into the Lol system may be the study’s biggest contribution to future antibiotic development, said Kim Lewis, PhD, professor of Biology and director of Antimicrobial Discovery Center at Northeastern University, Boston, who has discovered several antibiotics now in preclinical research. One, darobactin, targets Gram-negative bugs without affecting the gut microbiome. Another, teixobactin, takes down Gram-positive bacteria without causing drug resistance.
“Lolamicin hits a novel target. I would say that’s the most significant study finding,” said Dr. Lewis, who was not involved in the study. “That is rare. If you look at antibiotics introduced since 1968, they have been modifications of existing antibiotics or, rarely, new chemically but hitting the same proven targets. This one hits something properly new, and [that’s] what I found perhaps the most original and interesting.”
Kirk E. Hevener, PharmD, PhD, associate professor of Pharmaceutical Sciences at the University of Tennessee Health Science Center, Memphis, Tennessee, agreed. (Dr. Hevener also was not involved in the study.) “Lolamicin works by targeting a unique Gram-negative transport system. No currently approved antibacterials work in this way, meaning it potentially represents the first of a new class of antibacterials with narrow-spectrum Gram-negative activity and low gastrointestinal disturbance,” said Dr. Hevener, whose research looks at new antimicrobial drug targets.
The UIUC researchers noted that lolamicin has one drawback: Bacteria frequently developed resistance to it. But in future work, it could be tweaked, combined with other antibiotics, or used as a template for finding other Lol system attackers, they said.
“There is still a good amount of work cut out for us in terms of assessing the clinical translatability of lolamicin, but we are hopeful for the future of this drug,” Dr. Muñoz said.
Addressing a Dire Need
Bringing such a drug to market — from discovery to Food and Drug Administration approval — could take more than a decade, said Dr. Hevener. And new agents, especially for Gram-negative bugs, are sorely needed.
Not only do these bacteria shield themselves with a double membrane but they also “have more complex resistance mechanisms including special pumps that can remove antibacterial drugs from the cell before they can be effective,” Dr. Hevener said.
As a result, drug-resistant Gram-negative bacteria are making treatment of severe infections such as sepsis and pneumonia in health care settings difficult.
Bloodstream infections with drug-resistant Klebsiella pneumoniae have a 40% mortality rate, Dr. Lewis said. And microbiome damage caused by antibiotics is also widespread and deadly, wiping out communities of helpful, protective gut bacteria. That contributes to over half of the C. difficile infections that affect 500,000 people and kill 30,000 a year in the United States.
“Our arsenal of antibacterials that can be used to treat Gram-negative infections is dangerously low,” Dr. Hevener said. “Research will always be needed to develop new antibacterials with novel mechanisms of activity that can bypass bacterial resistance mechanisms.”
A version of this article appeared on Medscape.com.
A new antibiotic uses a never-before-seen mechanism to deliver a direct hit on tough-to-treat infections while leaving beneficial microbes alone. The strategy could lead to a new class of antibiotics that attack dangerous bacteria in a powerful new way, overcoming current drug resistance while sparing the gut microbiome.
“The biggest takeaway is the double-selective component,” said co-lead author Kristen A. Muñoz, PhD, who performed the research as a doctoral student at University of Illinois at Urbana-Champaign (UIUC). “We were able to develop a drug that not only targets problematic pathogens, but because it is selective for these pathogens only, we can spare the good bacteria and preserve the integrity of the microbiome.”
The drug goes after Gram-negative bacteria — pathogens responsible for debilitating and even fatal infections like gastroenteritis, urinary tract infections, pneumonia, sepsis, and cholera. The arsenal of antibiotics against them is old, with no new classes specifically targeting these bacteria coming on the market since 1968.
Many of these bugs have become resistant to one or more antibiotics, with deadly consequences. And antibiotics against them can also wipe out beneficial gut bacteria, allowing serious secondary infections to flare up.
In a study published in Nature, the drug lolamicin knocked out or reduced 130 strains of antibiotic-resistant Gram-negative bacteria in cell cultures. It also successfully treated drug-resistant bloodstream infections and pneumonia in mice while sparing their gut microbiome.
With their microbiomes intact, the mice then fought off secondary infection with Clostridioides difficile (a leading cause of opportunistic and sometimes fatal infections in US health care facilities), while mice treated with other compounds that damaged their microbiome succumbed.
How It Works
Like a well-built medieval castle, Gram-negative bacteria are encased in two protective walls, or membranes. Dr. Muñoz and her team at UIUC set out to breach this defense by finding compounds that hinder the “Lol system,” which ferries lipoproteins between them.
From one compound they constructed lolamicin, which can stop Gram-negative pathogens — with little effect on Gram-negative beneficial bacteria and no effect on Gram-positive bacteria.
“Gram-positive bacteria do not have an outer membrane, so they do not possess the Lol system,” Dr. Muñoz said. “When we compared the sequences of the Lol system in certain Gram-negative pathogens to Gram-negative commensal [beneficial] gut bacteria, we saw that the Lol systems were pretty different.”
Tossing a monkey wrench into the Lol system may be the study’s biggest contribution to future antibiotic development, said Kim Lewis, PhD, professor of Biology and director of Antimicrobial Discovery Center at Northeastern University, Boston, who has discovered several antibiotics now in preclinical research. One, darobactin, targets Gram-negative bugs without affecting the gut microbiome. Another, teixobactin, takes down Gram-positive bacteria without causing drug resistance.
“Lolamicin hits a novel target. I would say that’s the most significant study finding,” said Dr. Lewis, who was not involved in the study. “That is rare. If you look at antibiotics introduced since 1968, they have been modifications of existing antibiotics or, rarely, new chemically but hitting the same proven targets. This one hits something properly new, and [that’s] what I found perhaps the most original and interesting.”
Kirk E. Hevener, PharmD, PhD, associate professor of Pharmaceutical Sciences at the University of Tennessee Health Science Center, Memphis, Tennessee, agreed. (Dr. Hevener also was not involved in the study.) “Lolamicin works by targeting a unique Gram-negative transport system. No currently approved antibacterials work in this way, meaning it potentially represents the first of a new class of antibacterials with narrow-spectrum Gram-negative activity and low gastrointestinal disturbance,” said Dr. Hevener, whose research looks at new antimicrobial drug targets.
The UIUC researchers noted that lolamicin has one drawback: Bacteria frequently developed resistance to it. But in future work, it could be tweaked, combined with other antibiotics, or used as a template for finding other Lol system attackers, they said.
“There is still a good amount of work cut out for us in terms of assessing the clinical translatability of lolamicin, but we are hopeful for the future of this drug,” Dr. Muñoz said.
Addressing a Dire Need
Bringing such a drug to market — from discovery to Food and Drug Administration approval — could take more than a decade, said Dr. Hevener. And new agents, especially for Gram-negative bugs, are sorely needed.
Not only do these bacteria shield themselves with a double membrane but they also “have more complex resistance mechanisms including special pumps that can remove antibacterial drugs from the cell before they can be effective,” Dr. Hevener said.
As a result, drug-resistant Gram-negative bacteria are making treatment of severe infections such as sepsis and pneumonia in health care settings difficult.
Bloodstream infections with drug-resistant Klebsiella pneumoniae have a 40% mortality rate, Dr. Lewis said. And microbiome damage caused by antibiotics is also widespread and deadly, wiping out communities of helpful, protective gut bacteria. That contributes to over half of the C. difficile infections that affect 500,000 people and kill 30,000 a year in the United States.
“Our arsenal of antibacterials that can be used to treat Gram-negative infections is dangerously low,” Dr. Hevener said. “Research will always be needed to develop new antibacterials with novel mechanisms of activity that can bypass bacterial resistance mechanisms.”
A version of this article appeared on Medscape.com.
Vitamin D Test Inaccuracies Persist Despite Gains in Field: CDC
Some vitamin D tests may give misleading results despite progress made in recent years to improve the quality of these assays, according to the US Centers for Disease Control and Prevention (CDC).
Otoe Sugahara manager of the CDC Vitamin D Standardization-Certification Program (VDSCP), presented an update of her group’s work at ENDO 2024, the Endocrine Society’s annual meeting in Boston.
“Though most vitamin D tests in our program have improved, there still remain some sample-specific inaccuracies. The CDC is working with program participants to address these situations,” Ms. Sugahara said in a statement released by the Endocrine Society.
For example, some assays measure other compounds besides 25-hydroxyvitamin D, which can falsely elevate results of some blood samples, Ms. Sugahara reported. Thus, some tests may be misclassified, with results seen as sufficient from samples that should have indicated a vitamin D deficiency.
“While most vitamin D tests are effective, it is important for healthcare providers to be aware of the potential inconsistencies associated with vitamin D tests to avoid misclassification of the patients,” Ms. Sugahara and coauthors said in an abstract provided by the Endocrine Society.
Ms. Sugahara’s report provided a snapshot of the state of longstanding efforts to improve the quality of a widely performed service in US healthcare: testing vitamin D levels.
These include an international collaboration that gave rise in 2010 to a vitamin D standardization program, from which the CDC’s VDSCP certification emerged. Among the leaders of these efforts was Christopher Sempos, PhD, then with the Office of Dietary Supplements at the National Institutes of Health.
Many clinicians may not be aware of the concerns about the accuracy of vitamin D tests that led to the drive for standardization, Dr. Sempos, now retired, said in an interview. And, in his view, it’s something that busy practitioners should not have to consider.
“They have literally thousands of diseases they have to be able to recognize and diagnose,” Dr. Sempos said. “They should be able to count on the laboratory system to give them accurate and precise data.”
‘Nudging’ Toward Better Results
The CDC’s certification program gives labs and companies detailed information about the analytical accuracy and precision of their vitamin D tests.
This feedback has paid off with improved results, Andy Hoofnagle, MD, PhD, professor of laboratory medicine and pathology at the University of Washington in Seattle, told this news organization. It helps by “nudging manufacturers in the right direction,” he said.
“Some manufacturers reformulated, others recalibrated, which is a lot of effort on their part, so that when the patient get a number, it actually means the right thing,” said Dr. Hoofnagle, who is also chair of the Accuracy-Based Programs Committee of the College of American Pathologists.
“There are still many immunoassays on the market that aren’t giving the correct results, unfortunately, but the standardization certification program has really pushed the field in the right direction,” he said.
US scientists use two main types of technologies to measure vitamin D in the blood, Ms. Sugahara said. One is mass spectrometry, which separately measures 25-hydroxyvitamin D2 and D3 and sums the values. The other type, immunoassay, measures both compounds at the same time and reports one result for total 25-hydroxyvitamin D.
At the ENDO 2024 meeting, Ms. Sugahara reported generally positive trends seen in the VDSCP. For example, the program looks at specific tests’ bias, or the deviation of test results from the true value, as determined with the CDC’s reference method for vitamin D.
Average calibration bias was less than 1% for all assays in the VDSCP in 2022, Ms. Sugahara said. The average calibration bias for immunoassays was 0.86%, and for assays using mass spectrometry, it was 0.55%, Ms. Sugahara reported.
These are improved results compared with 2019 data, in which mass spectrometry–based assays had a mean bias of 1.9% and immunoassays had a mean bias of 2.4%, the CDC told this news organization in an email exchange.
The CDC said the VDSCP supports laboratories and researchers from around the world, including ones based in the US, China, Australia, Japan, and Korea.
Call for Research
Vitamin D tests are widely administered despite questions about their benefit for people who do not appear likely to be deficient of it.
The Endocrine Society’s newly released practice guideline recommends against routine testing of blood vitamin D levels in the general population.
Laboratory testing has increased over the years owing to studies reporting associations between blood vitamin D [25(OH)D] levels and a variety of common disorders, including musculoskeletal, metabolic, cardiovascular, malignant, autoimmune, and infectious diseases, wrote Marie B. Demay, MD, of Harvard Medical School in Boston, and coauthors in the new guideline. It was published on June 3 in The Journal of Clinical Endocrinology & Metabolism.
‘”Although a causal link between serum 25(OH)D concentrations and many disorders has not been clearly established, these associations have led to widespread supplementation with vitamin D and increased laboratory testing for 25(OH)D in the general population,” they wrote.
It’s uncertain that “any putative benefits of screening would outweigh the increased burden and cost, and whether implementation of universal 25(OH)D screening would be feasible from a societal perspective,” Dr. Demay and coauthors added.
They noted that the influential US Preventive Services Task Force also has raised doubts about widespread use of vitamin D tests.
The USPSTF has a somewhat different take from the Endocrine Society. The task force in 2021 reiterated its view that there is not enough evidence to recommend for or against widespread vitamin D testing for adults. The task force gave this test an I grade, meaning there is insufficient evidence to weigh the risks and benefits. That’s the same grade the task force gave it in 2014.
The USPSTF uses a grade of D to recommend against use of a test or service.
In an interview with this news organization, John Wong, MD, vice chair of the USPSTF, reiterated his group’s call for more research into the potential benefits and harms of vitamin D screening.
One of the challenges in addressing this issue, Dr. Wong noted, has been the variability of test results. Therefore, efforts such as the CDC’s VDSCP in improving test quality may help in eventually building up the kind of evidence base needed for the task force to offer a more definitive judgment on the tests, he said.
Wong acknowledged it must be frustrating for clinicians and patients to hear that experts don’t have the evidence needed to make a broad call about whether routine vitamin D tests are beneficial.
“We really would like to have that evidence because we recognize that it’s an important health question to help everybody in this nation stay healthy and live longer,” Dr. Wong said.
A version of this article appeared on Medscape.com.
Some vitamin D tests may give misleading results despite progress made in recent years to improve the quality of these assays, according to the US Centers for Disease Control and Prevention (CDC).
Otoe Sugahara manager of the CDC Vitamin D Standardization-Certification Program (VDSCP), presented an update of her group’s work at ENDO 2024, the Endocrine Society’s annual meeting in Boston.
“Though most vitamin D tests in our program have improved, there still remain some sample-specific inaccuracies. The CDC is working with program participants to address these situations,” Ms. Sugahara said in a statement released by the Endocrine Society.
For example, some assays measure other compounds besides 25-hydroxyvitamin D, which can falsely elevate results of some blood samples, Ms. Sugahara reported. Thus, some tests may be misclassified, with results seen as sufficient from samples that should have indicated a vitamin D deficiency.
“While most vitamin D tests are effective, it is important for healthcare providers to be aware of the potential inconsistencies associated with vitamin D tests to avoid misclassification of the patients,” Ms. Sugahara and coauthors said in an abstract provided by the Endocrine Society.
Ms. Sugahara’s report provided a snapshot of the state of longstanding efforts to improve the quality of a widely performed service in US healthcare: testing vitamin D levels.
These include an international collaboration that gave rise in 2010 to a vitamin D standardization program, from which the CDC’s VDSCP certification emerged. Among the leaders of these efforts was Christopher Sempos, PhD, then with the Office of Dietary Supplements at the National Institutes of Health.
Many clinicians may not be aware of the concerns about the accuracy of vitamin D tests that led to the drive for standardization, Dr. Sempos, now retired, said in an interview. And, in his view, it’s something that busy practitioners should not have to consider.
“They have literally thousands of diseases they have to be able to recognize and diagnose,” Dr. Sempos said. “They should be able to count on the laboratory system to give them accurate and precise data.”
‘Nudging’ Toward Better Results
The CDC’s certification program gives labs and companies detailed information about the analytical accuracy and precision of their vitamin D tests.
This feedback has paid off with improved results, Andy Hoofnagle, MD, PhD, professor of laboratory medicine and pathology at the University of Washington in Seattle, told this news organization. It helps by “nudging manufacturers in the right direction,” he said.
“Some manufacturers reformulated, others recalibrated, which is a lot of effort on their part, so that when the patient get a number, it actually means the right thing,” said Dr. Hoofnagle, who is also chair of the Accuracy-Based Programs Committee of the College of American Pathologists.
“There are still many immunoassays on the market that aren’t giving the correct results, unfortunately, but the standardization certification program has really pushed the field in the right direction,” he said.
US scientists use two main types of technologies to measure vitamin D in the blood, Ms. Sugahara said. One is mass spectrometry, which separately measures 25-hydroxyvitamin D2 and D3 and sums the values. The other type, immunoassay, measures both compounds at the same time and reports one result for total 25-hydroxyvitamin D.
At the ENDO 2024 meeting, Ms. Sugahara reported generally positive trends seen in the VDSCP. For example, the program looks at specific tests’ bias, or the deviation of test results from the true value, as determined with the CDC’s reference method for vitamin D.
Average calibration bias was less than 1% for all assays in the VDSCP in 2022, Ms. Sugahara said. The average calibration bias for immunoassays was 0.86%, and for assays using mass spectrometry, it was 0.55%, Ms. Sugahara reported.
These are improved results compared with 2019 data, in which mass spectrometry–based assays had a mean bias of 1.9% and immunoassays had a mean bias of 2.4%, the CDC told this news organization in an email exchange.
The CDC said the VDSCP supports laboratories and researchers from around the world, including ones based in the US, China, Australia, Japan, and Korea.
Call for Research
Vitamin D tests are widely administered despite questions about their benefit for people who do not appear likely to be deficient of it.
The Endocrine Society’s newly released practice guideline recommends against routine testing of blood vitamin D levels in the general population.
Laboratory testing has increased over the years owing to studies reporting associations between blood vitamin D [25(OH)D] levels and a variety of common disorders, including musculoskeletal, metabolic, cardiovascular, malignant, autoimmune, and infectious diseases, wrote Marie B. Demay, MD, of Harvard Medical School in Boston, and coauthors in the new guideline. It was published on June 3 in The Journal of Clinical Endocrinology & Metabolism.
‘”Although a causal link between serum 25(OH)D concentrations and many disorders has not been clearly established, these associations have led to widespread supplementation with vitamin D and increased laboratory testing for 25(OH)D in the general population,” they wrote.
It’s uncertain that “any putative benefits of screening would outweigh the increased burden and cost, and whether implementation of universal 25(OH)D screening would be feasible from a societal perspective,” Dr. Demay and coauthors added.
They noted that the influential US Preventive Services Task Force also has raised doubts about widespread use of vitamin D tests.
The USPSTF has a somewhat different take from the Endocrine Society. The task force in 2021 reiterated its view that there is not enough evidence to recommend for or against widespread vitamin D testing for adults. The task force gave this test an I grade, meaning there is insufficient evidence to weigh the risks and benefits. That’s the same grade the task force gave it in 2014.
The USPSTF uses a grade of D to recommend against use of a test or service.
In an interview with this news organization, John Wong, MD, vice chair of the USPSTF, reiterated his group’s call for more research into the potential benefits and harms of vitamin D screening.
One of the challenges in addressing this issue, Dr. Wong noted, has been the variability of test results. Therefore, efforts such as the CDC’s VDSCP in improving test quality may help in eventually building up the kind of evidence base needed for the task force to offer a more definitive judgment on the tests, he said.
Wong acknowledged it must be frustrating for clinicians and patients to hear that experts don’t have the evidence needed to make a broad call about whether routine vitamin D tests are beneficial.
“We really would like to have that evidence because we recognize that it’s an important health question to help everybody in this nation stay healthy and live longer,” Dr. Wong said.
A version of this article appeared on Medscape.com.
Some vitamin D tests may give misleading results despite progress made in recent years to improve the quality of these assays, according to the US Centers for Disease Control and Prevention (CDC).
Otoe Sugahara manager of the CDC Vitamin D Standardization-Certification Program (VDSCP), presented an update of her group’s work at ENDO 2024, the Endocrine Society’s annual meeting in Boston.
“Though most vitamin D tests in our program have improved, there still remain some sample-specific inaccuracies. The CDC is working with program participants to address these situations,” Ms. Sugahara said in a statement released by the Endocrine Society.
For example, some assays measure other compounds besides 25-hydroxyvitamin D, which can falsely elevate results of some blood samples, Ms. Sugahara reported. Thus, some tests may be misclassified, with results seen as sufficient from samples that should have indicated a vitamin D deficiency.
“While most vitamin D tests are effective, it is important for healthcare providers to be aware of the potential inconsistencies associated with vitamin D tests to avoid misclassification of the patients,” Ms. Sugahara and coauthors said in an abstract provided by the Endocrine Society.
Ms. Sugahara’s report provided a snapshot of the state of longstanding efforts to improve the quality of a widely performed service in US healthcare: testing vitamin D levels.
These include an international collaboration that gave rise in 2010 to a vitamin D standardization program, from which the CDC’s VDSCP certification emerged. Among the leaders of these efforts was Christopher Sempos, PhD, then with the Office of Dietary Supplements at the National Institutes of Health.
Many clinicians may not be aware of the concerns about the accuracy of vitamin D tests that led to the drive for standardization, Dr. Sempos, now retired, said in an interview. And, in his view, it’s something that busy practitioners should not have to consider.
“They have literally thousands of diseases they have to be able to recognize and diagnose,” Dr. Sempos said. “They should be able to count on the laboratory system to give them accurate and precise data.”
‘Nudging’ Toward Better Results
The CDC’s certification program gives labs and companies detailed information about the analytical accuracy and precision of their vitamin D tests.
This feedback has paid off with improved results, Andy Hoofnagle, MD, PhD, professor of laboratory medicine and pathology at the University of Washington in Seattle, told this news organization. It helps by “nudging manufacturers in the right direction,” he said.
“Some manufacturers reformulated, others recalibrated, which is a lot of effort on their part, so that when the patient get a number, it actually means the right thing,” said Dr. Hoofnagle, who is also chair of the Accuracy-Based Programs Committee of the College of American Pathologists.
“There are still many immunoassays on the market that aren’t giving the correct results, unfortunately, but the standardization certification program has really pushed the field in the right direction,” he said.
US scientists use two main types of technologies to measure vitamin D in the blood, Ms. Sugahara said. One is mass spectrometry, which separately measures 25-hydroxyvitamin D2 and D3 and sums the values. The other type, immunoassay, measures both compounds at the same time and reports one result for total 25-hydroxyvitamin D.
At the ENDO 2024 meeting, Ms. Sugahara reported generally positive trends seen in the VDSCP. For example, the program looks at specific tests’ bias, or the deviation of test results from the true value, as determined with the CDC’s reference method for vitamin D.
Average calibration bias was less than 1% for all assays in the VDSCP in 2022, Ms. Sugahara said. The average calibration bias for immunoassays was 0.86%, and for assays using mass spectrometry, it was 0.55%, Ms. Sugahara reported.
These are improved results compared with 2019 data, in which mass spectrometry–based assays had a mean bias of 1.9% and immunoassays had a mean bias of 2.4%, the CDC told this news organization in an email exchange.
The CDC said the VDSCP supports laboratories and researchers from around the world, including ones based in the US, China, Australia, Japan, and Korea.
Call for Research
Vitamin D tests are widely administered despite questions about their benefit for people who do not appear likely to be deficient of it.
The Endocrine Society’s newly released practice guideline recommends against routine testing of blood vitamin D levels in the general population.
Laboratory testing has increased over the years owing to studies reporting associations between blood vitamin D [25(OH)D] levels and a variety of common disorders, including musculoskeletal, metabolic, cardiovascular, malignant, autoimmune, and infectious diseases, wrote Marie B. Demay, MD, of Harvard Medical School in Boston, and coauthors in the new guideline. It was published on June 3 in The Journal of Clinical Endocrinology & Metabolism.
‘”Although a causal link between serum 25(OH)D concentrations and many disorders has not been clearly established, these associations have led to widespread supplementation with vitamin D and increased laboratory testing for 25(OH)D in the general population,” they wrote.
It’s uncertain that “any putative benefits of screening would outweigh the increased burden and cost, and whether implementation of universal 25(OH)D screening would be feasible from a societal perspective,” Dr. Demay and coauthors added.
They noted that the influential US Preventive Services Task Force also has raised doubts about widespread use of vitamin D tests.
The USPSTF has a somewhat different take from the Endocrine Society. The task force in 2021 reiterated its view that there is not enough evidence to recommend for or against widespread vitamin D testing for adults. The task force gave this test an I grade, meaning there is insufficient evidence to weigh the risks and benefits. That’s the same grade the task force gave it in 2014.
The USPSTF uses a grade of D to recommend against use of a test or service.
In an interview with this news organization, John Wong, MD, vice chair of the USPSTF, reiterated his group’s call for more research into the potential benefits and harms of vitamin D screening.
One of the challenges in addressing this issue, Dr. Wong noted, has been the variability of test results. Therefore, efforts such as the CDC’s VDSCP in improving test quality may help in eventually building up the kind of evidence base needed for the task force to offer a more definitive judgment on the tests, he said.
Wong acknowledged it must be frustrating for clinicians and patients to hear that experts don’t have the evidence needed to make a broad call about whether routine vitamin D tests are beneficial.
“We really would like to have that evidence because we recognize that it’s an important health question to help everybody in this nation stay healthy and live longer,” Dr. Wong said.
A version of this article appeared on Medscape.com.
Early-Life Exposure to Pollution Linked to Psychosis, Anxiety, Depression
Early-life exposure to air and noise pollution is associated with a higher risk for psychosis, depression, and anxiety in adolescence and early adulthood, results from a longitudinal birth cohort study showed.
While air pollution was associated primarily with psychotic experiences and depression, noise pollution was more likely to be associated with anxiety in adolescence and early adulthood.
“Early-life exposure could be detrimental to mental health given the extensive brain development and epigenetic processes that occur in utero and during infancy,” the researchers, led by Joanne Newbury, PhD, of Bristol Medical School, University of Bristol, England, wrote, adding that “the results of this cohort study provide novel evidence that early-life exposure to particulate matter is prospectively associated with the development of psychotic experiences and depression in youth.”
The findings were published online on May 28 in JAMA Network Open.
Large, Longitudinal Study
To learn more about how air and noise pollution may affect the brain from an early age, the investigators used data from the Avon Longitudinal Study of Parents and Children, an ongoing longitudinal birth cohort capturing data on new births in Southwest England from 1991 to 1992.
Investigators captured levels of air pollutants, which included nitrogen dioxide and fine particulate matter with a diameter smaller than 2.5 µm (PM2.5), in the areas where expectant mothers lived and where their children lived until age 12.
They also collected decibel levels of noise pollution in neighborhoods where expectant mothers and their children lived.
Participants were assessed for psychotic experiences, depression, and anxiety when they were 13, 18, and 24 years old.
Among the 9065 participants who had mental health data, 20% reported psychotic experiences, 11% reported depression, and 10% reported anxiety. About 60% of the participants had a family history of mental illness.
When they were age 13, 13.6% of participants reported psychotic experiences; 9.2% reported them at age 18, and 12.6% at age 24.
A lower number of participants reported feeling depressed and anxious at 13 years (5.6% for depression and 3.6% for anxiety) and 18 years (7.9% for depression and 5.7% for anxiety).
After adjusting for individual and family-level variables, including family psychiatric history, maternal social class, and neighborhood deprivation, elevated PM2.5 levels during pregnancy (P = .002) and childhood (P = .04) were associated with a significantly increased risk for psychotic experiences later in life. Pregnancy PM2.5 exposure was also associated with depression (P = .01).
Participants exposed to higher noise pollution in childhood and adolescence had an increased risk for anxiety (P = .03) as teenagers.
Vulnerability of the Developing Brain
The investigators noted that more information is needed to understand the underlying mechanisms behind these associations but noted that early-life exposure could be detrimental to mental health given “extensive brain development and epigenetic processes that occur in utero.”
They also noted that air pollution could lead to restricted fetal growth and premature birth, both of which are risk factors for psychopathology.
Martin Clift, PhD, of Swansea University in Swansea, Wales, who was not involved in the study, said that the paper highlights the need for more consideration of health consequences related to these exposures.
“As noted by the authors, this is an area that has received a lot of recent attention, yet there remains a large void of knowledge,” Dr. Clift said in a UK Science Media Centre release. “It highlights that some of the most dominant air pollutants can impact different mental health diagnoses, but that time-of-life is particularly important as to how each individual air pollutant may impact this diagnosis.”
Study limitations included limitations to generalizability of the data — the families in the study were more affluent and less diverse than the UK population overall.
The study was funded by the UK Medical Research Council, Wellcome Trust, and University of Bristol. Disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
Early-life exposure to air and noise pollution is associated with a higher risk for psychosis, depression, and anxiety in adolescence and early adulthood, results from a longitudinal birth cohort study showed.
While air pollution was associated primarily with psychotic experiences and depression, noise pollution was more likely to be associated with anxiety in adolescence and early adulthood.
“Early-life exposure could be detrimental to mental health given the extensive brain development and epigenetic processes that occur in utero and during infancy,” the researchers, led by Joanne Newbury, PhD, of Bristol Medical School, University of Bristol, England, wrote, adding that “the results of this cohort study provide novel evidence that early-life exposure to particulate matter is prospectively associated with the development of psychotic experiences and depression in youth.”
The findings were published online on May 28 in JAMA Network Open.
Large, Longitudinal Study
To learn more about how air and noise pollution may affect the brain from an early age, the investigators used data from the Avon Longitudinal Study of Parents and Children, an ongoing longitudinal birth cohort capturing data on new births in Southwest England from 1991 to 1992.
Investigators captured levels of air pollutants, which included nitrogen dioxide and fine particulate matter with a diameter smaller than 2.5 µm (PM2.5), in the areas where expectant mothers lived and where their children lived until age 12.
They also collected decibel levels of noise pollution in neighborhoods where expectant mothers and their children lived.
Participants were assessed for psychotic experiences, depression, and anxiety when they were 13, 18, and 24 years old.
Among the 9065 participants who had mental health data, 20% reported psychotic experiences, 11% reported depression, and 10% reported anxiety. About 60% of the participants had a family history of mental illness.
When they were age 13, 13.6% of participants reported psychotic experiences; 9.2% reported them at age 18, and 12.6% at age 24.
A lower number of participants reported feeling depressed and anxious at 13 years (5.6% for depression and 3.6% for anxiety) and 18 years (7.9% for depression and 5.7% for anxiety).
After adjusting for individual and family-level variables, including family psychiatric history, maternal social class, and neighborhood deprivation, elevated PM2.5 levels during pregnancy (P = .002) and childhood (P = .04) were associated with a significantly increased risk for psychotic experiences later in life. Pregnancy PM2.5 exposure was also associated with depression (P = .01).
Participants exposed to higher noise pollution in childhood and adolescence had an increased risk for anxiety (P = .03) as teenagers.
Vulnerability of the Developing Brain
The investigators noted that more information is needed to understand the underlying mechanisms behind these associations but noted that early-life exposure could be detrimental to mental health given “extensive brain development and epigenetic processes that occur in utero.”
They also noted that air pollution could lead to restricted fetal growth and premature birth, both of which are risk factors for psychopathology.
Martin Clift, PhD, of Swansea University in Swansea, Wales, who was not involved in the study, said that the paper highlights the need for more consideration of health consequences related to these exposures.
“As noted by the authors, this is an area that has received a lot of recent attention, yet there remains a large void of knowledge,” Dr. Clift said in a UK Science Media Centre release. “It highlights that some of the most dominant air pollutants can impact different mental health diagnoses, but that time-of-life is particularly important as to how each individual air pollutant may impact this diagnosis.”
Study limitations included limitations to generalizability of the data — the families in the study were more affluent and less diverse than the UK population overall.
The study was funded by the UK Medical Research Council, Wellcome Trust, and University of Bristol. Disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
Early-life exposure to air and noise pollution is associated with a higher risk for psychosis, depression, and anxiety in adolescence and early adulthood, results from a longitudinal birth cohort study showed.
While air pollution was associated primarily with psychotic experiences and depression, noise pollution was more likely to be associated with anxiety in adolescence and early adulthood.
“Early-life exposure could be detrimental to mental health given the extensive brain development and epigenetic processes that occur in utero and during infancy,” the researchers, led by Joanne Newbury, PhD, of Bristol Medical School, University of Bristol, England, wrote, adding that “the results of this cohort study provide novel evidence that early-life exposure to particulate matter is prospectively associated with the development of psychotic experiences and depression in youth.”
The findings were published online on May 28 in JAMA Network Open.
Large, Longitudinal Study
To learn more about how air and noise pollution may affect the brain from an early age, the investigators used data from the Avon Longitudinal Study of Parents and Children, an ongoing longitudinal birth cohort capturing data on new births in Southwest England from 1991 to 1992.
Investigators captured levels of air pollutants, which included nitrogen dioxide and fine particulate matter with a diameter smaller than 2.5 µm (PM2.5), in the areas where expectant mothers lived and where their children lived until age 12.
They also collected decibel levels of noise pollution in neighborhoods where expectant mothers and their children lived.
Participants were assessed for psychotic experiences, depression, and anxiety when they were 13, 18, and 24 years old.
Among the 9065 participants who had mental health data, 20% reported psychotic experiences, 11% reported depression, and 10% reported anxiety. About 60% of the participants had a family history of mental illness.
When they were age 13, 13.6% of participants reported psychotic experiences; 9.2% reported them at age 18, and 12.6% at age 24.
A lower number of participants reported feeling depressed and anxious at 13 years (5.6% for depression and 3.6% for anxiety) and 18 years (7.9% for depression and 5.7% for anxiety).
After adjusting for individual and family-level variables, including family psychiatric history, maternal social class, and neighborhood deprivation, elevated PM2.5 levels during pregnancy (P = .002) and childhood (P = .04) were associated with a significantly increased risk for psychotic experiences later in life. Pregnancy PM2.5 exposure was also associated with depression (P = .01).
Participants exposed to higher noise pollution in childhood and adolescence had an increased risk for anxiety (P = .03) as teenagers.
Vulnerability of the Developing Brain
The investigators noted that more information is needed to understand the underlying mechanisms behind these associations but noted that early-life exposure could be detrimental to mental health given “extensive brain development and epigenetic processes that occur in utero.”
They also noted that air pollution could lead to restricted fetal growth and premature birth, both of which are risk factors for psychopathology.
Martin Clift, PhD, of Swansea University in Swansea, Wales, who was not involved in the study, said that the paper highlights the need for more consideration of health consequences related to these exposures.
“As noted by the authors, this is an area that has received a lot of recent attention, yet there remains a large void of knowledge,” Dr. Clift said in a UK Science Media Centre release. “It highlights that some of the most dominant air pollutants can impact different mental health diagnoses, but that time-of-life is particularly important as to how each individual air pollutant may impact this diagnosis.”
Study limitations included limitations to generalizability of the data — the families in the study were more affluent and less diverse than the UK population overall.
The study was funded by the UK Medical Research Council, Wellcome Trust, and University of Bristol. Disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
Oxidative Stress in Patients With Melasma: An Evaluation of the Correlation of the Thiol/Disulfide Homeostasis Parameters and Modified MASI Score
Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4
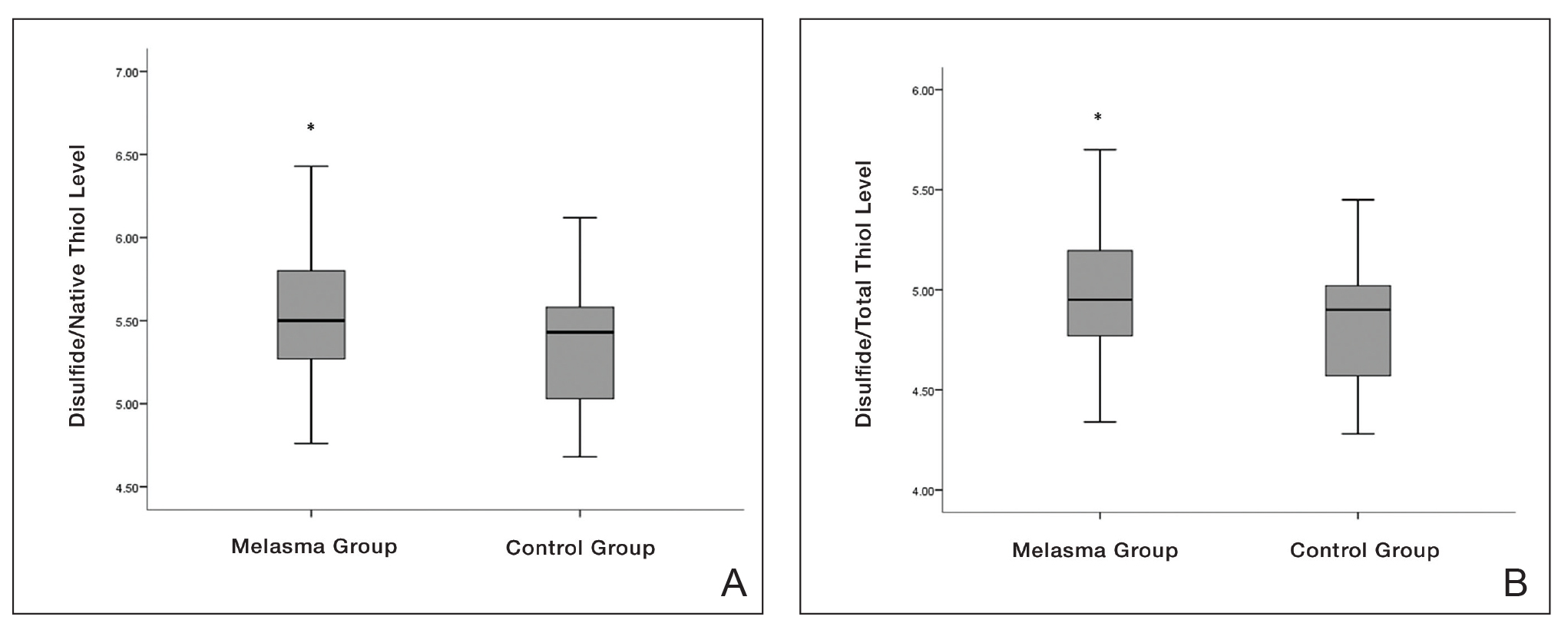

Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reports in the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.

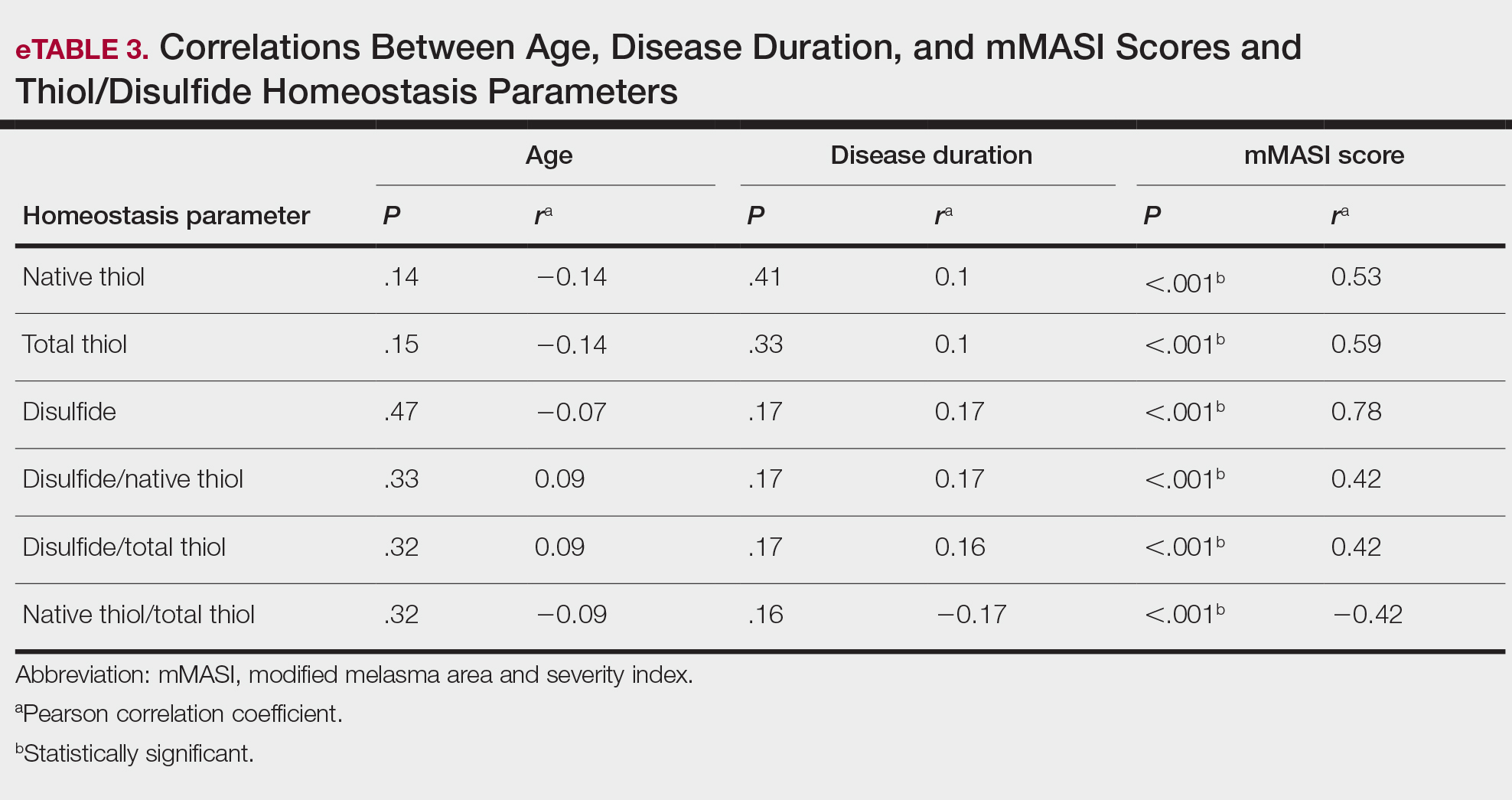
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4


Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reports in the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.



Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4


Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reports in the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.



- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
Practice Points
- Melasma is a common pigmentation disorder that causes brown or grayish patches on the skin.
- Disulfide/native thiol and disulfide/total thiol ratios were higher in patients with melasma compared with controls, which indicated the presence of oxidative stress in melasma.
- The evaluation of modified melasma area and severity index score with disulfide/native thiol and disulfide/total thiol values suggests that oxidative stress is correlated with melasma disease severity.
Need a Wood Lamp Alternative? Grab Your Smartphone
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).
Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
FDA Approves First-in-Class Drug for Lower-Risk Myelodysplastic Syndromes
The US Food and Drug Administration (FDA) has approved imetelstat (Rytelo, Geron Corporation) for certain patients with relapsed or refractory low- to intermediate-risk myelodysplastic syndromes (MDS).
Specifically, the first-in-class oligonucleotide telomerase inhibitor, which received orphan drug designation, is indicated for adults with MDS who have transfusion-dependent anemia requiring four or more red blood cell units over 8 weeks and who have not responded to erythropoiesis-stimulating agents or who have lost response to or are not eligible for erythropoiesis-stimulating agents, according to an FDA press release.
“For patients with lower-risk MDS and anemia who are transfusion dependent, we have very few options today and often cycle through available therapies, making the approval of RYTELO potentially practice changing for us,” co-investigator Rami Komrokji, MD, of Moffitt Cancer Center, Tampa, Florida, said in the Geron Corporation’s announcement of the approval.
Approval was based on efficacy and safety findings from the randomized, placebo-controlled, phase 3 IMerge trial, which found significantly improved red blood cell transfusion independence with treatment vs with placebo.
Overall, 178 patients were randomly assigned to the imetelstat arm (n = 118) and the placebo arm (n = 60). The median follow-up was 19.5 months in the treatment arm and 17.5 months in the placebo arm.
Patients received infusions of either 7.1 mg/kg of imetelstat or placebo in 28-day cycles until disease progression or unacceptable toxicity. All patients received supportive care, including red blood cell transfusions.
The rate of 8-week-or-greater red blood cell transfusion independence was 39.8% in the imetelstat vs 15% placebo arm. The rate of 24-week-or-greater red blood cell transfusion independence was 28% in the treatment arm vs 3.3% in the placebo arm.
An exploratory analysis among patients who achieved at least 8 weeks of red blood cell transfusion independence revealed that median increases in hemoglobin were 3.6 g/dL in the treatment group vs 0.8 g/dL in the placebo group.
Adverse reactions, occurring in at least 10% of patients and in at least 5% more patients in the treatment arm than in the placebo arm, included decreased platelets, white blood cells, and neutrophils; increased aspartate aminotransferase, alkaline phosphatase, and alanine aminotransferase; and fatigue, prolonged partial thromboplastin time, arthralgia/myalgia, COVID-19, and headache.
The recommended imetelstat dose is 7.1 mg/kg administered as an intravenous infusion over 2 hours every 28 days, according to the full prescribing information.
“What is exciting about RYTELO is the totality of the clinical benefit across [lower risk] MDS patients irrespective of ring sideroblast status or high transfusion burden, including sustained and durable transfusion independence and increases in hemoglobin levels, all within a well-characterized safety profile of generally manageable cytopenias,” Dr. Komrokji stated. The treatment goal for patients with this condition “is transfusion-independence and before today, this wasn’t possible for many patients.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved imetelstat (Rytelo, Geron Corporation) for certain patients with relapsed or refractory low- to intermediate-risk myelodysplastic syndromes (MDS).
Specifically, the first-in-class oligonucleotide telomerase inhibitor, which received orphan drug designation, is indicated for adults with MDS who have transfusion-dependent anemia requiring four or more red blood cell units over 8 weeks and who have not responded to erythropoiesis-stimulating agents or who have lost response to or are not eligible for erythropoiesis-stimulating agents, according to an FDA press release.
“For patients with lower-risk MDS and anemia who are transfusion dependent, we have very few options today and often cycle through available therapies, making the approval of RYTELO potentially practice changing for us,” co-investigator Rami Komrokji, MD, of Moffitt Cancer Center, Tampa, Florida, said in the Geron Corporation’s announcement of the approval.
Approval was based on efficacy and safety findings from the randomized, placebo-controlled, phase 3 IMerge trial, which found significantly improved red blood cell transfusion independence with treatment vs with placebo.
Overall, 178 patients were randomly assigned to the imetelstat arm (n = 118) and the placebo arm (n = 60). The median follow-up was 19.5 months in the treatment arm and 17.5 months in the placebo arm.
Patients received infusions of either 7.1 mg/kg of imetelstat or placebo in 28-day cycles until disease progression or unacceptable toxicity. All patients received supportive care, including red blood cell transfusions.
The rate of 8-week-or-greater red blood cell transfusion independence was 39.8% in the imetelstat vs 15% placebo arm. The rate of 24-week-or-greater red blood cell transfusion independence was 28% in the treatment arm vs 3.3% in the placebo arm.
An exploratory analysis among patients who achieved at least 8 weeks of red blood cell transfusion independence revealed that median increases in hemoglobin were 3.6 g/dL in the treatment group vs 0.8 g/dL in the placebo group.
Adverse reactions, occurring in at least 10% of patients and in at least 5% more patients in the treatment arm than in the placebo arm, included decreased platelets, white blood cells, and neutrophils; increased aspartate aminotransferase, alkaline phosphatase, and alanine aminotransferase; and fatigue, prolonged partial thromboplastin time, arthralgia/myalgia, COVID-19, and headache.
The recommended imetelstat dose is 7.1 mg/kg administered as an intravenous infusion over 2 hours every 28 days, according to the full prescribing information.
“What is exciting about RYTELO is the totality of the clinical benefit across [lower risk] MDS patients irrespective of ring sideroblast status or high transfusion burden, including sustained and durable transfusion independence and increases in hemoglobin levels, all within a well-characterized safety profile of generally manageable cytopenias,” Dr. Komrokji stated. The treatment goal for patients with this condition “is transfusion-independence and before today, this wasn’t possible for many patients.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved imetelstat (Rytelo, Geron Corporation) for certain patients with relapsed or refractory low- to intermediate-risk myelodysplastic syndromes (MDS).
Specifically, the first-in-class oligonucleotide telomerase inhibitor, which received orphan drug designation, is indicated for adults with MDS who have transfusion-dependent anemia requiring four or more red blood cell units over 8 weeks and who have not responded to erythropoiesis-stimulating agents or who have lost response to or are not eligible for erythropoiesis-stimulating agents, according to an FDA press release.
“For patients with lower-risk MDS and anemia who are transfusion dependent, we have very few options today and often cycle through available therapies, making the approval of RYTELO potentially practice changing for us,” co-investigator Rami Komrokji, MD, of Moffitt Cancer Center, Tampa, Florida, said in the Geron Corporation’s announcement of the approval.
Approval was based on efficacy and safety findings from the randomized, placebo-controlled, phase 3 IMerge trial, which found significantly improved red blood cell transfusion independence with treatment vs with placebo.
Overall, 178 patients were randomly assigned to the imetelstat arm (n = 118) and the placebo arm (n = 60). The median follow-up was 19.5 months in the treatment arm and 17.5 months in the placebo arm.
Patients received infusions of either 7.1 mg/kg of imetelstat or placebo in 28-day cycles until disease progression or unacceptable toxicity. All patients received supportive care, including red blood cell transfusions.
The rate of 8-week-or-greater red blood cell transfusion independence was 39.8% in the imetelstat vs 15% placebo arm. The rate of 24-week-or-greater red blood cell transfusion independence was 28% in the treatment arm vs 3.3% in the placebo arm.
An exploratory analysis among patients who achieved at least 8 weeks of red blood cell transfusion independence revealed that median increases in hemoglobin were 3.6 g/dL in the treatment group vs 0.8 g/dL in the placebo group.
Adverse reactions, occurring in at least 10% of patients and in at least 5% more patients in the treatment arm than in the placebo arm, included decreased platelets, white blood cells, and neutrophils; increased aspartate aminotransferase, alkaline phosphatase, and alanine aminotransferase; and fatigue, prolonged partial thromboplastin time, arthralgia/myalgia, COVID-19, and headache.
The recommended imetelstat dose is 7.1 mg/kg administered as an intravenous infusion over 2 hours every 28 days, according to the full prescribing information.
“What is exciting about RYTELO is the totality of the clinical benefit across [lower risk] MDS patients irrespective of ring sideroblast status or high transfusion burden, including sustained and durable transfusion independence and increases in hemoglobin levels, all within a well-characterized safety profile of generally manageable cytopenias,” Dr. Komrokji stated. The treatment goal for patients with this condition “is transfusion-independence and before today, this wasn’t possible for many patients.”
A version of this article appeared on Medscape.com.
Fine Particulate Matter Raises Type 2 Diabetes Risk in Women
TOPLINE:
Long-term exposure to fine particulate matter is associated with higher fasting blood glucose (FBG) levels and an increased type 2 diabetes risk, significantly contributing to the diabetes-related health burden among women of reproductive age.
METHODOLOGY:
- Exposure to fine particulate matter < 2.5 µm (PM2.5) is a known risk factor for type 2 diabetes, but its effect on women of reproductive age, who undergo hormonal fluctuations during reproductive events, is not well studied.
- Researchers evaluated the association of long-term exposure to PM2.5 with FBG levels and diabetes risk in 20,076,032 eligible women of reproductive age (average age, 27.04 years) across 350 cities in China between 2010 and 2015.
- They assessed PM2.5 exposure at the participants’ residential addresses and calculated average long-term exposure at 1 (lag 1 year), 2 (lag 2 years), and 3 years (lag 3 years) before the survey date, as defined by the World Health Organization (WHO).
- The primary outcomes were FBG levels and diabetes prevalence (FBG, ≥ 7 mmol/L, classified as diabetes; FBG, 6.1-7 mmol/L, classified as prediabetes).
- The study also evaluated the diabetes burden attributed to long-term PM2.5 exposure as per the Chinese National Ambient Air Quality Standards (annual mean PM2.5 exposure limit, > 35 µg/m3) and the WHO air quality guideline (annual mean PM2.5 exposure limit, > 5 µg/m3).
TAKEAWAY:
- The median PM2.5 exposure levels over lag periods of 1, 2, and 3 years were 67, 67, and 66 µg/m3, respectively, exceeding the WHO limit by more than 13-fold.
- Each interquartile range increase in the 3-year average PM2.5 exposure by 27 μg/m3 raised FBG levels by 0.078 mmol/L (P < .05), risk for diabetes by 18% (odds ratio [OR], 1.18; 95% CI, 1.16-1.19), and risk for prediabetes by 5% (OR, 1.05; 95% CI, 1.04-1.05).
- Long-term exposure to PM2.5 > 5 µg/m3 and 35 µg/m3 in the previous 3 years corresponded to an additional 41.7 (95% CI, 39.3-44.0) and 78.6 (95% CI, 74.5-82.6) thousand cases of diabetes nationwide, respectively.
- A higher PM2.5 exposure increased FBG levels and risk for diabetes in women with overweight or obesity vs those without and in those aged ≥ 35 years vs < 35 years (P < .001).
IN PRACTICE:
“These findings carry significant public health implications for formulating effective intervention strategies and environmental policies to better protect women’s health, particularly in countries with relatively high levels of air pollution and a large population with diabetes, such as China,” the authors wrote.
SOURCE:
The study, led by Yang Shen, Key Laboratory of Public Health Safety of the Ministry of Education and National Health Commission Key Laboratory of Health Technology Assessment, School of Public Health, Fudan University, Shanghai, China, was published online in Diabetes Care.
LIMITATIONS:
An error in the measurement of particulate matter exposure may have been possible as residential address estimates were used as a proxy for actual personal exposure. Questionnaires were used to retrospectively collect information on parameters such as smoking and alcohol consumption, which may have introduced recall bias. Data on potential confounders, such as diet and physical activity, were not included. Distinction between type 1 and type 2 diabetes was not reported owing to data collection–related limitations.
DISCLOSURES:
The study was supported by the National Key Research and Development Program of China, Henan Key Research and Development Program, State Key Laboratory of Resources and Environmental Information System, and Three-Year Public Health Action Plan of Shanghai. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term exposure to fine particulate matter is associated with higher fasting blood glucose (FBG) levels and an increased type 2 diabetes risk, significantly contributing to the diabetes-related health burden among women of reproductive age.
METHODOLOGY:
- Exposure to fine particulate matter < 2.5 µm (PM2.5) is a known risk factor for type 2 diabetes, but its effect on women of reproductive age, who undergo hormonal fluctuations during reproductive events, is not well studied.
- Researchers evaluated the association of long-term exposure to PM2.5 with FBG levels and diabetes risk in 20,076,032 eligible women of reproductive age (average age, 27.04 years) across 350 cities in China between 2010 and 2015.
- They assessed PM2.5 exposure at the participants’ residential addresses and calculated average long-term exposure at 1 (lag 1 year), 2 (lag 2 years), and 3 years (lag 3 years) before the survey date, as defined by the World Health Organization (WHO).
- The primary outcomes were FBG levels and diabetes prevalence (FBG, ≥ 7 mmol/L, classified as diabetes; FBG, 6.1-7 mmol/L, classified as prediabetes).
- The study also evaluated the diabetes burden attributed to long-term PM2.5 exposure as per the Chinese National Ambient Air Quality Standards (annual mean PM2.5 exposure limit, > 35 µg/m3) and the WHO air quality guideline (annual mean PM2.5 exposure limit, > 5 µg/m3).
TAKEAWAY:
- The median PM2.5 exposure levels over lag periods of 1, 2, and 3 years were 67, 67, and 66 µg/m3, respectively, exceeding the WHO limit by more than 13-fold.
- Each interquartile range increase in the 3-year average PM2.5 exposure by 27 μg/m3 raised FBG levels by 0.078 mmol/L (P < .05), risk for diabetes by 18% (odds ratio [OR], 1.18; 95% CI, 1.16-1.19), and risk for prediabetes by 5% (OR, 1.05; 95% CI, 1.04-1.05).
- Long-term exposure to PM2.5 > 5 µg/m3 and 35 µg/m3 in the previous 3 years corresponded to an additional 41.7 (95% CI, 39.3-44.0) and 78.6 (95% CI, 74.5-82.6) thousand cases of diabetes nationwide, respectively.
- A higher PM2.5 exposure increased FBG levels and risk for diabetes in women with overweight or obesity vs those without and in those aged ≥ 35 years vs < 35 years (P < .001).
IN PRACTICE:
“These findings carry significant public health implications for formulating effective intervention strategies and environmental policies to better protect women’s health, particularly in countries with relatively high levels of air pollution and a large population with diabetes, such as China,” the authors wrote.
SOURCE:
The study, led by Yang Shen, Key Laboratory of Public Health Safety of the Ministry of Education and National Health Commission Key Laboratory of Health Technology Assessment, School of Public Health, Fudan University, Shanghai, China, was published online in Diabetes Care.
LIMITATIONS:
An error in the measurement of particulate matter exposure may have been possible as residential address estimates were used as a proxy for actual personal exposure. Questionnaires were used to retrospectively collect information on parameters such as smoking and alcohol consumption, which may have introduced recall bias. Data on potential confounders, such as diet and physical activity, were not included. Distinction between type 1 and type 2 diabetes was not reported owing to data collection–related limitations.
DISCLOSURES:
The study was supported by the National Key Research and Development Program of China, Henan Key Research and Development Program, State Key Laboratory of Resources and Environmental Information System, and Three-Year Public Health Action Plan of Shanghai. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term exposure to fine particulate matter is associated with higher fasting blood glucose (FBG) levels and an increased type 2 diabetes risk, significantly contributing to the diabetes-related health burden among women of reproductive age.
METHODOLOGY:
- Exposure to fine particulate matter < 2.5 µm (PM2.5) is a known risk factor for type 2 diabetes, but its effect on women of reproductive age, who undergo hormonal fluctuations during reproductive events, is not well studied.
- Researchers evaluated the association of long-term exposure to PM2.5 with FBG levels and diabetes risk in 20,076,032 eligible women of reproductive age (average age, 27.04 years) across 350 cities in China between 2010 and 2015.
- They assessed PM2.5 exposure at the participants’ residential addresses and calculated average long-term exposure at 1 (lag 1 year), 2 (lag 2 years), and 3 years (lag 3 years) before the survey date, as defined by the World Health Organization (WHO).
- The primary outcomes were FBG levels and diabetes prevalence (FBG, ≥ 7 mmol/L, classified as diabetes; FBG, 6.1-7 mmol/L, classified as prediabetes).
- The study also evaluated the diabetes burden attributed to long-term PM2.5 exposure as per the Chinese National Ambient Air Quality Standards (annual mean PM2.5 exposure limit, > 35 µg/m3) and the WHO air quality guideline (annual mean PM2.5 exposure limit, > 5 µg/m3).
TAKEAWAY:
- The median PM2.5 exposure levels over lag periods of 1, 2, and 3 years were 67, 67, and 66 µg/m3, respectively, exceeding the WHO limit by more than 13-fold.
- Each interquartile range increase in the 3-year average PM2.5 exposure by 27 μg/m3 raised FBG levels by 0.078 mmol/L (P < .05), risk for diabetes by 18% (odds ratio [OR], 1.18; 95% CI, 1.16-1.19), and risk for prediabetes by 5% (OR, 1.05; 95% CI, 1.04-1.05).
- Long-term exposure to PM2.5 > 5 µg/m3 and 35 µg/m3 in the previous 3 years corresponded to an additional 41.7 (95% CI, 39.3-44.0) and 78.6 (95% CI, 74.5-82.6) thousand cases of diabetes nationwide, respectively.
- A higher PM2.5 exposure increased FBG levels and risk for diabetes in women with overweight or obesity vs those without and in those aged ≥ 35 years vs < 35 years (P < .001).
IN PRACTICE:
“These findings carry significant public health implications for formulating effective intervention strategies and environmental policies to better protect women’s health, particularly in countries with relatively high levels of air pollution and a large population with diabetes, such as China,” the authors wrote.
SOURCE:
The study, led by Yang Shen, Key Laboratory of Public Health Safety of the Ministry of Education and National Health Commission Key Laboratory of Health Technology Assessment, School of Public Health, Fudan University, Shanghai, China, was published online in Diabetes Care.
LIMITATIONS:
An error in the measurement of particulate matter exposure may have been possible as residential address estimates were used as a proxy for actual personal exposure. Questionnaires were used to retrospectively collect information on parameters such as smoking and alcohol consumption, which may have introduced recall bias. Data on potential confounders, such as diet and physical activity, were not included. Distinction between type 1 and type 2 diabetes was not reported owing to data collection–related limitations.
DISCLOSURES:
The study was supported by the National Key Research and Development Program of China, Henan Key Research and Development Program, State Key Laboratory of Resources and Environmental Information System, and Three-Year Public Health Action Plan of Shanghai. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.