User login
CDC: Vaccinated? You don’t need a mask indoors
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
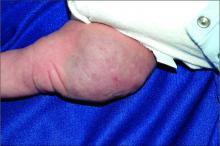
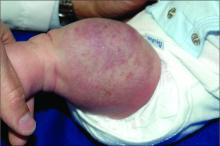
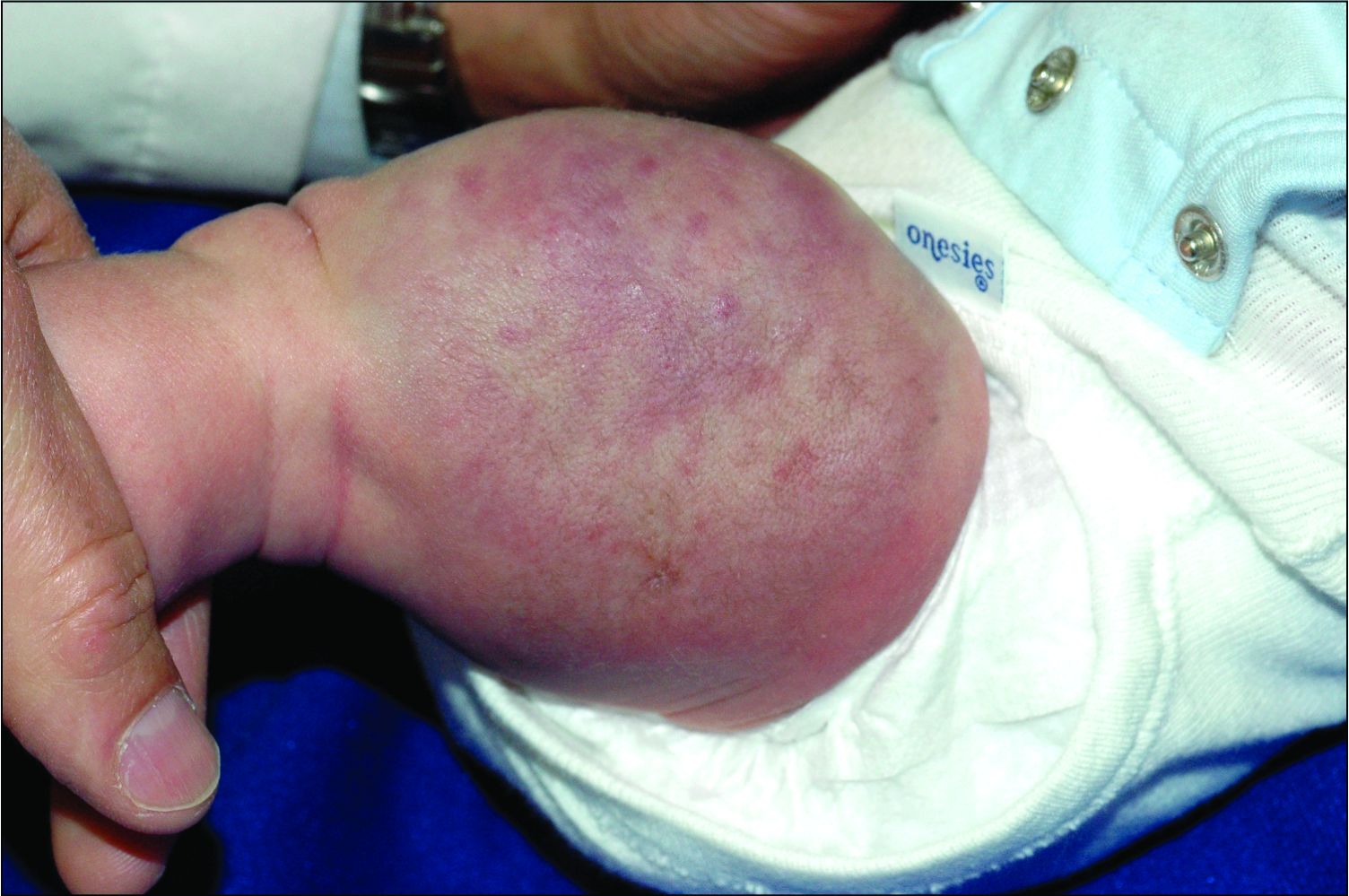
An infant girl presents with a growing pink-red leg nodule
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
On physical exam, you see an infant with a mass of the left lower extremity. Close examination reveals an approximately 7 cm x 8 cm poorly defined mass with overlying central erythematous to violaceous color of the left anterior upper leg with a lumpy texture. The lesion is moderately firm and mildly tender on palpation.
New guideline provides recommendations on reconstruction after skin cancer resection
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Severe Asthma – Treatment and Management
Vegetarians have better cholesterol levels, and more, than meat eaters
Vegetarians have more favorable levels of a number of biomarkers including cardiovascular-linked ones – total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein A and B – than meat eaters, according to results of the largest study of its kind to date.
Results of the cross-sectional, observational study of 178,000 participants were presented as an electronic poster at this year’s online European Congress on Obesity by Jirapitcha Boonpor of the Institute of Cardiovascular & Medical Sciences, University of Glasgow (Scotland).
“We found that the health benefits of becoming a vegetarian were independent of adiposity and other sociodemographic and lifestyle-related confounding factors,” senior author Carlos Celis-Morales, PhD, also from the University of Glasgow, said in an interview.
Total cholesterol and LDL cholesterol concentrations for vegetarians were 21% and 16.4% lower than in meat eaters. But some biomarkers considered beneficial – including vitamin D concentrations – were lower in vegetarians, while some considered unhealthy – including triglycerides and cystatin-C levels – were higher.
Vegetarian diets have recently become much more popular, but there is insufficient information about the health benefits. Prior reports of associations between biomarkers and a vegetarian diet were unclear, including evidence of any metabolic benefits, noted Dr. Celis-Morales.
Importantly, participants in the study had followed a vegetarian or meat-eater diet for at least 5 years before their biomarkers in blood and urine were assessed.
“If you modify your diet, then, 2 weeks later, you can see changes in some metabolic markers, but changes in markers of cardiovascular disease will take 5-10 years,” he explained.
No single biomarker can assess health
Asked to comment on the findings, John C. Mathers, PhD, noted that they clearly confirm the importance of not reading any biomarker result in isolation.
Health is complex and individual markers tell you just part of the story,” said Dr. Mathers of the Human Nutrition Research Centre, Newcastle (England) University.
He says a vegetarian diet can be nourishing but cautioned that “just because someone excludes meat from their diet does not mean necessarily that they will be eating a healthy diet.”
“Some of the biomarker differences seen in this work – such as the lower concentrations of total cholesterol and LDL cholesterol, GGT [gamma-glutamyl transferase], and ALT [alanine transaminase] – are indicators that the vegetarians were healthier than the meat eaters. However, other differences were less encouraging, including the lower concentrations of vitamin D and higher concentrations of triglycerides and cystatin-C.”
Also reflecting on the results, Jose Lara Gallegos, PhD, senior lecturer in human nutrition at Northumbria University, Newcastle upon Tyne, England, said they support previous evidence from large studies such as the European Prospective Investigation into Cancer and Nutrition (EPIC), which showed that a vegetarian diet is associated with a lower risk of heart disease.
“A vegetarian diet might also be associated with lower risk for liver diseases such as nonalcoholic fatty liver disease,” Dr. Gallegos said, but added that some levels of biomarkers considered to be “healthy” were lower in the vegetarians, and it is important to remember that strictly restricted diets might be associated with potential risks of nutritional inadequacies.
“Other, less restrictive dietary patterns, such as a Mediterranean diet, are also associated with ... health benefits,” he observed.
Large data sample from the UK Biobank study
“Specifically, we wanted to know if vegetarians were healthier because they are generally leaner and lead healthier lives, or whether their diet specifically was responsible for their improved metabolic and cardiovascular health,” Dr. Celis-Morales explained.
Data were included from 177,723 healthy participants from the UK Biobank study who were aged 37-73 years and had reported no major dietary changes over the last 5 years. In total, 4,111 participants were self-reported vegetarians who followed a diet without red meat, poultry, or fish, and 166,516 participants were meat eaters.
Nineteen biomarkers related to diabetes, hypertension, cardiovascular diseases, cancer, and liver and renal function were included, and the associations between vegetarian diet and biomarkers, compared with meat eaters, were examined.
To minimize confounding, the findings were adjusted for age, sex, deprivation, education, ethnicity, smoking, total sedentary time, type of physical activity, alcohol intake, body mass index, and waist circumference.
Compared with meat eaters, vegetarians had significantly lower concentrations of 14 biomarkers, including total cholesterol (21% lower); LDL (16% lower); lipoprotein A (1% lower), lipoprotein B (4% lower), and liver function markers (GGT: 354% lower, and ALT: 153% lower), IGF-1 (134% lower), urate (122% lower), total protein (29% lower), creatinine (607% lower), and C-reactive protein (10% lower).
However, the researchers found that, compared with meat eaters, vegetarians had significantly higher concentrations of some unhealthy biomarkers, including triglycerides (15% higher) and cystatin-C (4% higher), and lower levels of some beneficial biomarkers including high-density lipoprotein (HDL) cholesterol (5% lower), vitamin D (635% lower), and calcium (0.7% lower).
No associations were found for hemoglobin A1c, systolic blood pressure, and aminotransferase.
“Some biomarkers, for example urate, were very low in vegetarians, and this served to verify our results because we expected meat eaters to have higher levels of urate,” remarked Dr. Celis-Morales.
Diet commitment and cardiovascular outcomes
Many people, whether vegetarians or meat-eaters, follow short-term diets, for example, the Atkins or the 5:2 diet, and often lack continuity switching from one diet to the next, or back to regular eating.
“They are healthy, but they do not commit for long enough to make a difference to metabolic markers or potentially long-term health. In contrast, vegetarians are usually fully committed but the reasons behind this commitment might be a concern for the environment or animal welfare, for example,” Dr. Celis-Morales pointed out.
However, he added that many vegetarians replace the meat in their diet with unhealthy alternatives. “They often eat too much pasta or potatoes, or other high-energy food with low nutritional value.”
Having identified metabolic markers specific to long-term vegetarian diets, Dr. Celis-Morales wanted to know what happens to vegetarians’ long-term cardiovascular health. He analyzed and published these outcomes in a separate study published in December 2020.
“Over 9 years of follow-up, we have found that vegetarians have a lower risk in terms of myocardial infarction in the long-term, as well as other cardiovascular disease,” he reported.
Asked whether there was an optimum age or time in life to become a vegetarian to improve health, Dr. Celis-Morales explained that the healthier you are, the less likely you will reap the health benefits of dietary changes – for example to being a vegetarian.
“It is more likely that those people who have unhealthy lifestyle risk factors, such as smoking, and high consumption of high-energy foods or processed meat are more likely to see positive health effects,” he said.
Lifestyle changes to improve cardiovascular outcomes are usually more likely to be required at 40 or 50 years old than at younger ages. He also noted that metabolic markers tend to show clear improvement at around 3 months after adopting a particular diet but improvements in disease outcomes take a lot longer to become evident.
Dr. Celis-Morales and his team are currently conducting a further analysis to understand if the vegetarian diet is also associated with a lower risk of cancer, depression, and dementia, compared with meat-eaters.
Dr. Celis-Morales, Dr. Mathers, and Dr. Gallegos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vegetarians have more favorable levels of a number of biomarkers including cardiovascular-linked ones – total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein A and B – than meat eaters, according to results of the largest study of its kind to date.
Results of the cross-sectional, observational study of 178,000 participants were presented as an electronic poster at this year’s online European Congress on Obesity by Jirapitcha Boonpor of the Institute of Cardiovascular & Medical Sciences, University of Glasgow (Scotland).
“We found that the health benefits of becoming a vegetarian were independent of adiposity and other sociodemographic and lifestyle-related confounding factors,” senior author Carlos Celis-Morales, PhD, also from the University of Glasgow, said in an interview.
Total cholesterol and LDL cholesterol concentrations for vegetarians were 21% and 16.4% lower than in meat eaters. But some biomarkers considered beneficial – including vitamin D concentrations – were lower in vegetarians, while some considered unhealthy – including triglycerides and cystatin-C levels – were higher.
Vegetarian diets have recently become much more popular, but there is insufficient information about the health benefits. Prior reports of associations between biomarkers and a vegetarian diet were unclear, including evidence of any metabolic benefits, noted Dr. Celis-Morales.
Importantly, participants in the study had followed a vegetarian or meat-eater diet for at least 5 years before their biomarkers in blood and urine were assessed.
“If you modify your diet, then, 2 weeks later, you can see changes in some metabolic markers, but changes in markers of cardiovascular disease will take 5-10 years,” he explained.
No single biomarker can assess health
Asked to comment on the findings, John C. Mathers, PhD, noted that they clearly confirm the importance of not reading any biomarker result in isolation.
Health is complex and individual markers tell you just part of the story,” said Dr. Mathers of the Human Nutrition Research Centre, Newcastle (England) University.
He says a vegetarian diet can be nourishing but cautioned that “just because someone excludes meat from their diet does not mean necessarily that they will be eating a healthy diet.”
“Some of the biomarker differences seen in this work – such as the lower concentrations of total cholesterol and LDL cholesterol, GGT [gamma-glutamyl transferase], and ALT [alanine transaminase] – are indicators that the vegetarians were healthier than the meat eaters. However, other differences were less encouraging, including the lower concentrations of vitamin D and higher concentrations of triglycerides and cystatin-C.”
Also reflecting on the results, Jose Lara Gallegos, PhD, senior lecturer in human nutrition at Northumbria University, Newcastle upon Tyne, England, said they support previous evidence from large studies such as the European Prospective Investigation into Cancer and Nutrition (EPIC), which showed that a vegetarian diet is associated with a lower risk of heart disease.
“A vegetarian diet might also be associated with lower risk for liver diseases such as nonalcoholic fatty liver disease,” Dr. Gallegos said, but added that some levels of biomarkers considered to be “healthy” were lower in the vegetarians, and it is important to remember that strictly restricted diets might be associated with potential risks of nutritional inadequacies.
“Other, less restrictive dietary patterns, such as a Mediterranean diet, are also associated with ... health benefits,” he observed.
Large data sample from the UK Biobank study
“Specifically, we wanted to know if vegetarians were healthier because they are generally leaner and lead healthier lives, or whether their diet specifically was responsible for their improved metabolic and cardiovascular health,” Dr. Celis-Morales explained.
Data were included from 177,723 healthy participants from the UK Biobank study who were aged 37-73 years and had reported no major dietary changes over the last 5 years. In total, 4,111 participants were self-reported vegetarians who followed a diet without red meat, poultry, or fish, and 166,516 participants were meat eaters.
Nineteen biomarkers related to diabetes, hypertension, cardiovascular diseases, cancer, and liver and renal function were included, and the associations between vegetarian diet and biomarkers, compared with meat eaters, were examined.
To minimize confounding, the findings were adjusted for age, sex, deprivation, education, ethnicity, smoking, total sedentary time, type of physical activity, alcohol intake, body mass index, and waist circumference.
Compared with meat eaters, vegetarians had significantly lower concentrations of 14 biomarkers, including total cholesterol (21% lower); LDL (16% lower); lipoprotein A (1% lower), lipoprotein B (4% lower), and liver function markers (GGT: 354% lower, and ALT: 153% lower), IGF-1 (134% lower), urate (122% lower), total protein (29% lower), creatinine (607% lower), and C-reactive protein (10% lower).
However, the researchers found that, compared with meat eaters, vegetarians had significantly higher concentrations of some unhealthy biomarkers, including triglycerides (15% higher) and cystatin-C (4% higher), and lower levels of some beneficial biomarkers including high-density lipoprotein (HDL) cholesterol (5% lower), vitamin D (635% lower), and calcium (0.7% lower).
No associations were found for hemoglobin A1c, systolic blood pressure, and aminotransferase.
“Some biomarkers, for example urate, were very low in vegetarians, and this served to verify our results because we expected meat eaters to have higher levels of urate,” remarked Dr. Celis-Morales.
Diet commitment and cardiovascular outcomes
Many people, whether vegetarians or meat-eaters, follow short-term diets, for example, the Atkins or the 5:2 diet, and often lack continuity switching from one diet to the next, or back to regular eating.
“They are healthy, but they do not commit for long enough to make a difference to metabolic markers or potentially long-term health. In contrast, vegetarians are usually fully committed but the reasons behind this commitment might be a concern for the environment or animal welfare, for example,” Dr. Celis-Morales pointed out.
However, he added that many vegetarians replace the meat in their diet with unhealthy alternatives. “They often eat too much pasta or potatoes, or other high-energy food with low nutritional value.”
Having identified metabolic markers specific to long-term vegetarian diets, Dr. Celis-Morales wanted to know what happens to vegetarians’ long-term cardiovascular health. He analyzed and published these outcomes in a separate study published in December 2020.
“Over 9 years of follow-up, we have found that vegetarians have a lower risk in terms of myocardial infarction in the long-term, as well as other cardiovascular disease,” he reported.
Asked whether there was an optimum age or time in life to become a vegetarian to improve health, Dr. Celis-Morales explained that the healthier you are, the less likely you will reap the health benefits of dietary changes – for example to being a vegetarian.
“It is more likely that those people who have unhealthy lifestyle risk factors, such as smoking, and high consumption of high-energy foods or processed meat are more likely to see positive health effects,” he said.
Lifestyle changes to improve cardiovascular outcomes are usually more likely to be required at 40 or 50 years old than at younger ages. He also noted that metabolic markers tend to show clear improvement at around 3 months after adopting a particular diet but improvements in disease outcomes take a lot longer to become evident.
Dr. Celis-Morales and his team are currently conducting a further analysis to understand if the vegetarian diet is also associated with a lower risk of cancer, depression, and dementia, compared with meat-eaters.
Dr. Celis-Morales, Dr. Mathers, and Dr. Gallegos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vegetarians have more favorable levels of a number of biomarkers including cardiovascular-linked ones – total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein A and B – than meat eaters, according to results of the largest study of its kind to date.
Results of the cross-sectional, observational study of 178,000 participants were presented as an electronic poster at this year’s online European Congress on Obesity by Jirapitcha Boonpor of the Institute of Cardiovascular & Medical Sciences, University of Glasgow (Scotland).
“We found that the health benefits of becoming a vegetarian were independent of adiposity and other sociodemographic and lifestyle-related confounding factors,” senior author Carlos Celis-Morales, PhD, also from the University of Glasgow, said in an interview.
Total cholesterol and LDL cholesterol concentrations for vegetarians were 21% and 16.4% lower than in meat eaters. But some biomarkers considered beneficial – including vitamin D concentrations – were lower in vegetarians, while some considered unhealthy – including triglycerides and cystatin-C levels – were higher.
Vegetarian diets have recently become much more popular, but there is insufficient information about the health benefits. Prior reports of associations between biomarkers and a vegetarian diet were unclear, including evidence of any metabolic benefits, noted Dr. Celis-Morales.
Importantly, participants in the study had followed a vegetarian or meat-eater diet for at least 5 years before their biomarkers in blood and urine were assessed.
“If you modify your diet, then, 2 weeks later, you can see changes in some metabolic markers, but changes in markers of cardiovascular disease will take 5-10 years,” he explained.
No single biomarker can assess health
Asked to comment on the findings, John C. Mathers, PhD, noted that they clearly confirm the importance of not reading any biomarker result in isolation.
Health is complex and individual markers tell you just part of the story,” said Dr. Mathers of the Human Nutrition Research Centre, Newcastle (England) University.
He says a vegetarian diet can be nourishing but cautioned that “just because someone excludes meat from their diet does not mean necessarily that they will be eating a healthy diet.”
“Some of the biomarker differences seen in this work – such as the lower concentrations of total cholesterol and LDL cholesterol, GGT [gamma-glutamyl transferase], and ALT [alanine transaminase] – are indicators that the vegetarians were healthier than the meat eaters. However, other differences were less encouraging, including the lower concentrations of vitamin D and higher concentrations of triglycerides and cystatin-C.”
Also reflecting on the results, Jose Lara Gallegos, PhD, senior lecturer in human nutrition at Northumbria University, Newcastle upon Tyne, England, said they support previous evidence from large studies such as the European Prospective Investigation into Cancer and Nutrition (EPIC), which showed that a vegetarian diet is associated with a lower risk of heart disease.
“A vegetarian diet might also be associated with lower risk for liver diseases such as nonalcoholic fatty liver disease,” Dr. Gallegos said, but added that some levels of biomarkers considered to be “healthy” were lower in the vegetarians, and it is important to remember that strictly restricted diets might be associated with potential risks of nutritional inadequacies.
“Other, less restrictive dietary patterns, such as a Mediterranean diet, are also associated with ... health benefits,” he observed.
Large data sample from the UK Biobank study
“Specifically, we wanted to know if vegetarians were healthier because they are generally leaner and lead healthier lives, or whether their diet specifically was responsible for their improved metabolic and cardiovascular health,” Dr. Celis-Morales explained.
Data were included from 177,723 healthy participants from the UK Biobank study who were aged 37-73 years and had reported no major dietary changes over the last 5 years. In total, 4,111 participants were self-reported vegetarians who followed a diet without red meat, poultry, or fish, and 166,516 participants were meat eaters.
Nineteen biomarkers related to diabetes, hypertension, cardiovascular diseases, cancer, and liver and renal function were included, and the associations between vegetarian diet and biomarkers, compared with meat eaters, were examined.
To minimize confounding, the findings were adjusted for age, sex, deprivation, education, ethnicity, smoking, total sedentary time, type of physical activity, alcohol intake, body mass index, and waist circumference.
Compared with meat eaters, vegetarians had significantly lower concentrations of 14 biomarkers, including total cholesterol (21% lower); LDL (16% lower); lipoprotein A (1% lower), lipoprotein B (4% lower), and liver function markers (GGT: 354% lower, and ALT: 153% lower), IGF-1 (134% lower), urate (122% lower), total protein (29% lower), creatinine (607% lower), and C-reactive protein (10% lower).
However, the researchers found that, compared with meat eaters, vegetarians had significantly higher concentrations of some unhealthy biomarkers, including triglycerides (15% higher) and cystatin-C (4% higher), and lower levels of some beneficial biomarkers including high-density lipoprotein (HDL) cholesterol (5% lower), vitamin D (635% lower), and calcium (0.7% lower).
No associations were found for hemoglobin A1c, systolic blood pressure, and aminotransferase.
“Some biomarkers, for example urate, were very low in vegetarians, and this served to verify our results because we expected meat eaters to have higher levels of urate,” remarked Dr. Celis-Morales.
Diet commitment and cardiovascular outcomes
Many people, whether vegetarians or meat-eaters, follow short-term diets, for example, the Atkins or the 5:2 diet, and often lack continuity switching from one diet to the next, or back to regular eating.
“They are healthy, but they do not commit for long enough to make a difference to metabolic markers or potentially long-term health. In contrast, vegetarians are usually fully committed but the reasons behind this commitment might be a concern for the environment or animal welfare, for example,” Dr. Celis-Morales pointed out.
However, he added that many vegetarians replace the meat in their diet with unhealthy alternatives. “They often eat too much pasta or potatoes, or other high-energy food with low nutritional value.”
Having identified metabolic markers specific to long-term vegetarian diets, Dr. Celis-Morales wanted to know what happens to vegetarians’ long-term cardiovascular health. He analyzed and published these outcomes in a separate study published in December 2020.
“Over 9 years of follow-up, we have found that vegetarians have a lower risk in terms of myocardial infarction in the long-term, as well as other cardiovascular disease,” he reported.
Asked whether there was an optimum age or time in life to become a vegetarian to improve health, Dr. Celis-Morales explained that the healthier you are, the less likely you will reap the health benefits of dietary changes – for example to being a vegetarian.
“It is more likely that those people who have unhealthy lifestyle risk factors, such as smoking, and high consumption of high-energy foods or processed meat are more likely to see positive health effects,” he said.
Lifestyle changes to improve cardiovascular outcomes are usually more likely to be required at 40 or 50 years old than at younger ages. He also noted that metabolic markers tend to show clear improvement at around 3 months after adopting a particular diet but improvements in disease outcomes take a lot longer to become evident.
Dr. Celis-Morales and his team are currently conducting a further analysis to understand if the vegetarian diet is also associated with a lower risk of cancer, depression, and dementia, compared with meat-eaters.
Dr. Celis-Morales, Dr. Mathers, and Dr. Gallegos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
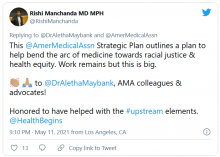
AMA announces major commitment to health equity
The 82-page report, which was created by the association’s Center for Health Equity, argues for both internal changes at the AMA and changes in how the association addresses race-based inequities in general.
The report was released just 2 months after this news organization reported that a podcast hosted by AMA’s top journal was lambasted as racist and out of touch. In the podcast – entitled “Stuctural Racism for Doctors – What Is It?” – one JAMA editor argued that structural racism doesn’t exist. He eventually resigned and the journal’s top editor was placed on administration leave.
The new AMA report’s strategic framework “is driven by the immense need for equity-centered solutions to confront harms produced by systemic racism and other forms of oppression for Black, Latinx, Indigenous, Asian, and other people of color, as well as people who identify as LGBTQ+ and people with disabilities,” the AMA said in a news release. “Its urgency is underscored by ongoing circumstances including inequities exacerbated by the COVID-19 pandemic, ongoing police brutality, and hate crimes targeting Asian, Black, and Brown communities.”
The plan includes five main approaches to addressing inequities in health care and the AMA:
- Implement antiracist equity strategies through AMA practices, programming, policies, and culture.
- Build alliances with marginalized doctors and other stakeholders to elevate the experiences and ideas of historically marginalized and minority health care leaders.
- Strengthen, empower, and equip doctors with the knowledge and tools to dismantle structural and social health inequities.
- Ensure equitable opportunities in innovation.
- Foster truth, racial healing, reconciliation, and transformation for AMA’s past by accounting for how policies and processes excluded, discriminated, and harmed communities.
As the report acknowledges, the AMA has a long history of exclusion of and discrimination against Black physicians, for which the association publicly apologized in 2008. Within the past year, the AMA has reaffirmed its commitment to addressing this legacy and to be proactive on health equity.
Among other things, the association has described racism as a public health crisis, stated that race has nothing to do with biology, said police brutality is a product of structural racism, and called on the federal government to collect and release COVID-19 race/ethnicity data. It also removed the name of AMA founder Nathan Davis, MD, from an annual award and display because of his contribution to explicit racist practices.
Equity-centered solutions
The AMA launched its Center for Health Equity in 2019 with a mandate “to embed health equity across the organization.” Aletha Maybank, MD, was named the AMA’s chief health equity officer to lead the center.
In the report that Dr. Maybank helped write, the AMA discusses the consequences of individual and systemic injustice toward minorities. Among these consequences, the report said, is “segregated and inequitable health care systems.”
The “equity-centered solutions” listed in the report include:
- End segregated health care.
- Establish national health care equity and racial justice standards.
- End the use of race-based clinical decision models.
- Eliminate all forms of discrimination, exclusion and oppression in medical and physician education, training, hiring, and promotion.
- Prevent exclusion of and ensure equal representation of Black, Indigenous and Latinx people in medical school admissions as well as medical school and hospital leadership ranks.
- Ensure equity in innovation, including design, development, implementation along with support for equitable innovation opportunities and entrepreneurship.
- Solidify connections and coordination between health care and public health.
- Acknowledge and repair past harms committed by institutions.
Changing medical education
In an exclusive interview, Gerald E. Harmon, MD, president-elect of the AMA, singled out medical education as an area that is ripe for change. “One of the most threatened phenotypes on the planet is the Black male physician,” he said. “Their numbers among medical school applicants continue to drop. We have increasing numbers of women in medical schools – over 50% of trainees are women – and more Black women are entering medical school, but Black men in medical school are an endangered species.
“We’re trying to get the physician workforce to look like the patient workforce.”
Dr. Harmon cited the “pipeline program” at the Morehouse School of Medicine in Atlanta and the AMA’s “doctors back to school” program as examples of efforts to attract minority high school students to health care careers. Much more needs to be done, he added. “We have to put equity and representation into our medical workforce so we can provide better high quality, more reliable care for underrepresented patients.”
Putting the AMA’s house in order
In its report, the AMA also makes recommendations about how it can improve equity within its own organization. Over the next 3 years, among other things, the association plans to improve the diversity of leadership at the AMA and its journal, JAMA; train all staff on equity requirements; and develop a plan to recruit more racial and ethnic minorities, LGBTQ+ people, and disabled people.
Dr. Maybank, the AMA’s chief health equity officer, said in an interview that she wouldn’t describe these efforts as affirmative action. “This is beyond affirmative action. It’s about intentional activity and action to ensure equity and justice within the AMA.”
The AMA has to thoroughly examine its own processes and determine “how inequity shows up on a day-to-day basis,” she said. “Whether it’s through hiring, innovation, publishing or communications, everybody needs to know how inequity shows up and how their own mental models can exacerbate inequities. People need tools to challenge themselves and ask themselves critical questions about racism in their processes and what they can do to mitigate those.”
A version of this article first appeared on WebMD.com.
The 82-page report, which was created by the association’s Center for Health Equity, argues for both internal changes at the AMA and changes in how the association addresses race-based inequities in general.
The report was released just 2 months after this news organization reported that a podcast hosted by AMA’s top journal was lambasted as racist and out of touch. In the podcast – entitled “Stuctural Racism for Doctors – What Is It?” – one JAMA editor argued that structural racism doesn’t exist. He eventually resigned and the journal’s top editor was placed on administration leave.
The new AMA report’s strategic framework “is driven by the immense need for equity-centered solutions to confront harms produced by systemic racism and other forms of oppression for Black, Latinx, Indigenous, Asian, and other people of color, as well as people who identify as LGBTQ+ and people with disabilities,” the AMA said in a news release. “Its urgency is underscored by ongoing circumstances including inequities exacerbated by the COVID-19 pandemic, ongoing police brutality, and hate crimes targeting Asian, Black, and Brown communities.”
The plan includes five main approaches to addressing inequities in health care and the AMA:
- Implement antiracist equity strategies through AMA practices, programming, policies, and culture.
- Build alliances with marginalized doctors and other stakeholders to elevate the experiences and ideas of historically marginalized and minority health care leaders.
- Strengthen, empower, and equip doctors with the knowledge and tools to dismantle structural and social health inequities.
- Ensure equitable opportunities in innovation.
- Foster truth, racial healing, reconciliation, and transformation for AMA’s past by accounting for how policies and processes excluded, discriminated, and harmed communities.
As the report acknowledges, the AMA has a long history of exclusion of and discrimination against Black physicians, for which the association publicly apologized in 2008. Within the past year, the AMA has reaffirmed its commitment to addressing this legacy and to be proactive on health equity.
Among other things, the association has described racism as a public health crisis, stated that race has nothing to do with biology, said police brutality is a product of structural racism, and called on the federal government to collect and release COVID-19 race/ethnicity data. It also removed the name of AMA founder Nathan Davis, MD, from an annual award and display because of his contribution to explicit racist practices.
Equity-centered solutions
The AMA launched its Center for Health Equity in 2019 with a mandate “to embed health equity across the organization.” Aletha Maybank, MD, was named the AMA’s chief health equity officer to lead the center.
In the report that Dr. Maybank helped write, the AMA discusses the consequences of individual and systemic injustice toward minorities. Among these consequences, the report said, is “segregated and inequitable health care systems.”
The “equity-centered solutions” listed in the report include:
- End segregated health care.
- Establish national health care equity and racial justice standards.
- End the use of race-based clinical decision models.
- Eliminate all forms of discrimination, exclusion and oppression in medical and physician education, training, hiring, and promotion.
- Prevent exclusion of and ensure equal representation of Black, Indigenous and Latinx people in medical school admissions as well as medical school and hospital leadership ranks.
- Ensure equity in innovation, including design, development, implementation along with support for equitable innovation opportunities and entrepreneurship.
- Solidify connections and coordination between health care and public health.
- Acknowledge and repair past harms committed by institutions.
Changing medical education
In an exclusive interview, Gerald E. Harmon, MD, president-elect of the AMA, singled out medical education as an area that is ripe for change. “One of the most threatened phenotypes on the planet is the Black male physician,” he said. “Their numbers among medical school applicants continue to drop. We have increasing numbers of women in medical schools – over 50% of trainees are women – and more Black women are entering medical school, but Black men in medical school are an endangered species.
“We’re trying to get the physician workforce to look like the patient workforce.”
Dr. Harmon cited the “pipeline program” at the Morehouse School of Medicine in Atlanta and the AMA’s “doctors back to school” program as examples of efforts to attract minority high school students to health care careers. Much more needs to be done, he added. “We have to put equity and representation into our medical workforce so we can provide better high quality, more reliable care for underrepresented patients.”
Putting the AMA’s house in order
In its report, the AMA also makes recommendations about how it can improve equity within its own organization. Over the next 3 years, among other things, the association plans to improve the diversity of leadership at the AMA and its journal, JAMA; train all staff on equity requirements; and develop a plan to recruit more racial and ethnic minorities, LGBTQ+ people, and disabled people.
Dr. Maybank, the AMA’s chief health equity officer, said in an interview that she wouldn’t describe these efforts as affirmative action. “This is beyond affirmative action. It’s about intentional activity and action to ensure equity and justice within the AMA.”
The AMA has to thoroughly examine its own processes and determine “how inequity shows up on a day-to-day basis,” she said. “Whether it’s through hiring, innovation, publishing or communications, everybody needs to know how inequity shows up and how their own mental models can exacerbate inequities. People need tools to challenge themselves and ask themselves critical questions about racism in their processes and what they can do to mitigate those.”
A version of this article first appeared on WebMD.com.
The 82-page report, which was created by the association’s Center for Health Equity, argues for both internal changes at the AMA and changes in how the association addresses race-based inequities in general.
The report was released just 2 months after this news organization reported that a podcast hosted by AMA’s top journal was lambasted as racist and out of touch. In the podcast – entitled “Stuctural Racism for Doctors – What Is It?” – one JAMA editor argued that structural racism doesn’t exist. He eventually resigned and the journal’s top editor was placed on administration leave.
The new AMA report’s strategic framework “is driven by the immense need for equity-centered solutions to confront harms produced by systemic racism and other forms of oppression for Black, Latinx, Indigenous, Asian, and other people of color, as well as people who identify as LGBTQ+ and people with disabilities,” the AMA said in a news release. “Its urgency is underscored by ongoing circumstances including inequities exacerbated by the COVID-19 pandemic, ongoing police brutality, and hate crimes targeting Asian, Black, and Brown communities.”
The plan includes five main approaches to addressing inequities in health care and the AMA:
- Implement antiracist equity strategies through AMA practices, programming, policies, and culture.
- Build alliances with marginalized doctors and other stakeholders to elevate the experiences and ideas of historically marginalized and minority health care leaders.
- Strengthen, empower, and equip doctors with the knowledge and tools to dismantle structural and social health inequities.
- Ensure equitable opportunities in innovation.
- Foster truth, racial healing, reconciliation, and transformation for AMA’s past by accounting for how policies and processes excluded, discriminated, and harmed communities.
As the report acknowledges, the AMA has a long history of exclusion of and discrimination against Black physicians, for which the association publicly apologized in 2008. Within the past year, the AMA has reaffirmed its commitment to addressing this legacy and to be proactive on health equity.
Among other things, the association has described racism as a public health crisis, stated that race has nothing to do with biology, said police brutality is a product of structural racism, and called on the federal government to collect and release COVID-19 race/ethnicity data. It also removed the name of AMA founder Nathan Davis, MD, from an annual award and display because of his contribution to explicit racist practices.
Equity-centered solutions
The AMA launched its Center for Health Equity in 2019 with a mandate “to embed health equity across the organization.” Aletha Maybank, MD, was named the AMA’s chief health equity officer to lead the center.
In the report that Dr. Maybank helped write, the AMA discusses the consequences of individual and systemic injustice toward minorities. Among these consequences, the report said, is “segregated and inequitable health care systems.”
The “equity-centered solutions” listed in the report include:
- End segregated health care.
- Establish national health care equity and racial justice standards.
- End the use of race-based clinical decision models.
- Eliminate all forms of discrimination, exclusion and oppression in medical and physician education, training, hiring, and promotion.
- Prevent exclusion of and ensure equal representation of Black, Indigenous and Latinx people in medical school admissions as well as medical school and hospital leadership ranks.
- Ensure equity in innovation, including design, development, implementation along with support for equitable innovation opportunities and entrepreneurship.
- Solidify connections and coordination between health care and public health.
- Acknowledge and repair past harms committed by institutions.
Changing medical education
In an exclusive interview, Gerald E. Harmon, MD, president-elect of the AMA, singled out medical education as an area that is ripe for change. “One of the most threatened phenotypes on the planet is the Black male physician,” he said. “Their numbers among medical school applicants continue to drop. We have increasing numbers of women in medical schools – over 50% of trainees are women – and more Black women are entering medical school, but Black men in medical school are an endangered species.
“We’re trying to get the physician workforce to look like the patient workforce.”
Dr. Harmon cited the “pipeline program” at the Morehouse School of Medicine in Atlanta and the AMA’s “doctors back to school” program as examples of efforts to attract minority high school students to health care careers. Much more needs to be done, he added. “We have to put equity and representation into our medical workforce so we can provide better high quality, more reliable care for underrepresented patients.”
Putting the AMA’s house in order
In its report, the AMA also makes recommendations about how it can improve equity within its own organization. Over the next 3 years, among other things, the association plans to improve the diversity of leadership at the AMA and its journal, JAMA; train all staff on equity requirements; and develop a plan to recruit more racial and ethnic minorities, LGBTQ+ people, and disabled people.
Dr. Maybank, the AMA’s chief health equity officer, said in an interview that she wouldn’t describe these efforts as affirmative action. “This is beyond affirmative action. It’s about intentional activity and action to ensure equity and justice within the AMA.”
The AMA has to thoroughly examine its own processes and determine “how inequity shows up on a day-to-day basis,” she said. “Whether it’s through hiring, innovation, publishing or communications, everybody needs to know how inequity shows up and how their own mental models can exacerbate inequities. People need tools to challenge themselves and ask themselves critical questions about racism in their processes and what they can do to mitigate those.”
A version of this article first appeared on WebMD.com.
BERENICE: Further evidence of heart safety of dual HER2 blockade
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
FROM ESMO BREAST CANCER 2021
Photoprotection recommended for people of color
and applying a tinted sunscreen with an SPF of 30 or greater to exposed areas, according to Henry W. Lim, MD.
In addition, “with rigorous photoprotection, vitamin D supplementation should be advised to patients,” Dr. Lim, a former chair of the department of dermatology at Henry Ford Health System, Detroit, said during the Society for Pediatric Dermatology pre-AAD meeting. “One multivitamin a day should be sufficient for most patients. This is especially relevant because we do know that skin of color patients tend to have lower vitamin D levels to start with.”
Photoprotection for people of color helps minimize the development of photodermatoses, postinflammatory hyperpigmentation, polymorphous light eruption, and chronic actinic dermatitis, he said. In a retrospective chart review of 1,080 people conducted at four academic medical centers in the United States, Dr. Lim and colleagues found a higher proportion of polymorphous light eruption and chronic actinic dermatitis in Black individuals, and a higher proportion of photoallergic contact dermatitis, phototoxic drug eruptions, phytophotodermatitis, porphyria, and solar urticaria in White individuals.
“Another pediatric photodermatosis, actinic prurigo, tends to occur most often in Mestizo individuals, patients of American Indian heritage,” he added. “This is a significant issue, especially in Latin America.”
In a systematic review of 20 studies in the medical literature, researchers assessed the quality of life and psychological impact of photodermatoses in affected patients. Studies included in the review drew from 2,487 adults and 119 children. Among adults, the self-administered Dermatology Life Quality Index (DLQI) revealed that photodermatoses adversely affected employment, education, and leisure activities in adults. Among children, the condition adversely affected outdoor activities and exacerbated symptoms in those with erythropoietic protoporphyria (EPP).
As for skin cancer risk, the association between UV light exposure and the development of melanoma is not as strong in people with skin of color, compared with light-skinned individuals. In a recent systematic review of 13 studies on the topic, 11 showed no association, one showed a small positive relationship in Black males and 1 showed a weak association in Hispanic males.
“The conclusion from this review is that UV protection for melanoma prevention in people of color is not supported by most studies,” said Dr. Lim, who was not affiliated with the review. “The authors also noted, however, that the evidence is of moderate to low quality. Larger studies should be done.”
The association between UV exposure and the development to squamous cell cancer in skin of color is also not strong. “However, we do know that sun exposure is associated with the development of basal cell carcinoma in this population,” he said.
Sunscreen ingredient studies
Dr. Lim also highlighted findings from two studies related to the effect of sunscreen application on plasma concentration of sunscreen active ingredients, both in adults. In the most recent analysis, scientists at the Food and Drug Administration and colleagues conducted a randomized clinical trial in 48 individuals with skin types II-IV.
Participants applied sunscreen at 2 mg/cm2 to 75% of body surface area at 0 hours on day 1 and 4 times on day 2 through day 4 at 2-hour intervals. Over the course of 21 days, the researchers collected 34 blood samples from each participant, and evaluated six active ingredients in four sunscreen products: avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate.
For all active ingredients, levels of greater than 0.5 ng/mL were detected after a single application on day 1. Levels of greater than 0.5 ng/mL were detected up to day 7, and up to day 21 for oxybenzone. All were detected in skin on days 7 and 14 via tape stripping. The authors called for further studies to determine the clinical significance of these findings and emphasized that the results “do not indicate that individuals should refrain from the use of sunscreen.”
The FDA is asking for additional studies on the safety of these 12 filters, noted Dr. Lim, who is a past president of the American Academy of Dermatology. On Feb. 26, 2019, the FDA issued a proposed rule regarding sunscreen drug products for over-the-counter human use. It proposes that the 16 UV filters be classified into one of 3 categories. Category I would include zinc oxide and titanium dioxide, which are generally recognized as safe and effective (GRASE). Category II would include PABA and trolamine salicylate, which are not used in the United States and are not GRASE. Category III would include 12 filters that lack insufficient safety data to make a determination regarding GRASE.
The final FDA rule was scheduled to be released in September of 2020, but a result of the Coronavirus Aid, Relief, and Economic Security (CARES) Act, the FDA “will be moving from a laborious rulemaking process to an administrative order process, which means it should not take as long to implement a monograph,” Dr. Lim said. “The FDA has decided that there will not be a final rule regarding sunscreen drug products,” but is required to issue a proposed administrative order by Sept. 27, 2021, he said.
When the final administrative order has been issued, manufacturers would have at least 1 year to comply with sunscreen products offered in the United States. “The approximate timeline is probably going to be 2023,” he said.
Dr. Lim disclosed that he is an investigator for Incyte, L’Oreal, Pfizer, and the Patient-centered Outcomes Research Institute, and a consultant for Pierre Fabre, ISDIN, Ferndale, La Roche–Posay, and Beiersdorf. He has been a speaker at general educational sessions sponsored by La Roche–Posay and Cantabria Labs.
and applying a tinted sunscreen with an SPF of 30 or greater to exposed areas, according to Henry W. Lim, MD.
In addition, “with rigorous photoprotection, vitamin D supplementation should be advised to patients,” Dr. Lim, a former chair of the department of dermatology at Henry Ford Health System, Detroit, said during the Society for Pediatric Dermatology pre-AAD meeting. “One multivitamin a day should be sufficient for most patients. This is especially relevant because we do know that skin of color patients tend to have lower vitamin D levels to start with.”
Photoprotection for people of color helps minimize the development of photodermatoses, postinflammatory hyperpigmentation, polymorphous light eruption, and chronic actinic dermatitis, he said. In a retrospective chart review of 1,080 people conducted at four academic medical centers in the United States, Dr. Lim and colleagues found a higher proportion of polymorphous light eruption and chronic actinic dermatitis in Black individuals, and a higher proportion of photoallergic contact dermatitis, phototoxic drug eruptions, phytophotodermatitis, porphyria, and solar urticaria in White individuals.
“Another pediatric photodermatosis, actinic prurigo, tends to occur most often in Mestizo individuals, patients of American Indian heritage,” he added. “This is a significant issue, especially in Latin America.”
In a systematic review of 20 studies in the medical literature, researchers assessed the quality of life and psychological impact of photodermatoses in affected patients. Studies included in the review drew from 2,487 adults and 119 children. Among adults, the self-administered Dermatology Life Quality Index (DLQI) revealed that photodermatoses adversely affected employment, education, and leisure activities in adults. Among children, the condition adversely affected outdoor activities and exacerbated symptoms in those with erythropoietic protoporphyria (EPP).
As for skin cancer risk, the association between UV light exposure and the development of melanoma is not as strong in people with skin of color, compared with light-skinned individuals. In a recent systematic review of 13 studies on the topic, 11 showed no association, one showed a small positive relationship in Black males and 1 showed a weak association in Hispanic males.
“The conclusion from this review is that UV protection for melanoma prevention in people of color is not supported by most studies,” said Dr. Lim, who was not affiliated with the review. “The authors also noted, however, that the evidence is of moderate to low quality. Larger studies should be done.”
The association between UV exposure and the development to squamous cell cancer in skin of color is also not strong. “However, we do know that sun exposure is associated with the development of basal cell carcinoma in this population,” he said.
Sunscreen ingredient studies
Dr. Lim also highlighted findings from two studies related to the effect of sunscreen application on plasma concentration of sunscreen active ingredients, both in adults. In the most recent analysis, scientists at the Food and Drug Administration and colleagues conducted a randomized clinical trial in 48 individuals with skin types II-IV.
Participants applied sunscreen at 2 mg/cm2 to 75% of body surface area at 0 hours on day 1 and 4 times on day 2 through day 4 at 2-hour intervals. Over the course of 21 days, the researchers collected 34 blood samples from each participant, and evaluated six active ingredients in four sunscreen products: avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate.
For all active ingredients, levels of greater than 0.5 ng/mL were detected after a single application on day 1. Levels of greater than 0.5 ng/mL were detected up to day 7, and up to day 21 for oxybenzone. All were detected in skin on days 7 and 14 via tape stripping. The authors called for further studies to determine the clinical significance of these findings and emphasized that the results “do not indicate that individuals should refrain from the use of sunscreen.”
The FDA is asking for additional studies on the safety of these 12 filters, noted Dr. Lim, who is a past president of the American Academy of Dermatology. On Feb. 26, 2019, the FDA issued a proposed rule regarding sunscreen drug products for over-the-counter human use. It proposes that the 16 UV filters be classified into one of 3 categories. Category I would include zinc oxide and titanium dioxide, which are generally recognized as safe and effective (GRASE). Category II would include PABA and trolamine salicylate, which are not used in the United States and are not GRASE. Category III would include 12 filters that lack insufficient safety data to make a determination regarding GRASE.
The final FDA rule was scheduled to be released in September of 2020, but a result of the Coronavirus Aid, Relief, and Economic Security (CARES) Act, the FDA “will be moving from a laborious rulemaking process to an administrative order process, which means it should not take as long to implement a monograph,” Dr. Lim said. “The FDA has decided that there will not be a final rule regarding sunscreen drug products,” but is required to issue a proposed administrative order by Sept. 27, 2021, he said.
When the final administrative order has been issued, manufacturers would have at least 1 year to comply with sunscreen products offered in the United States. “The approximate timeline is probably going to be 2023,” he said.
Dr. Lim disclosed that he is an investigator for Incyte, L’Oreal, Pfizer, and the Patient-centered Outcomes Research Institute, and a consultant for Pierre Fabre, ISDIN, Ferndale, La Roche–Posay, and Beiersdorf. He has been a speaker at general educational sessions sponsored by La Roche–Posay and Cantabria Labs.
and applying a tinted sunscreen with an SPF of 30 or greater to exposed areas, according to Henry W. Lim, MD.
In addition, “with rigorous photoprotection, vitamin D supplementation should be advised to patients,” Dr. Lim, a former chair of the department of dermatology at Henry Ford Health System, Detroit, said during the Society for Pediatric Dermatology pre-AAD meeting. “One multivitamin a day should be sufficient for most patients. This is especially relevant because we do know that skin of color patients tend to have lower vitamin D levels to start with.”
Photoprotection for people of color helps minimize the development of photodermatoses, postinflammatory hyperpigmentation, polymorphous light eruption, and chronic actinic dermatitis, he said. In a retrospective chart review of 1,080 people conducted at four academic medical centers in the United States, Dr. Lim and colleagues found a higher proportion of polymorphous light eruption and chronic actinic dermatitis in Black individuals, and a higher proportion of photoallergic contact dermatitis, phototoxic drug eruptions, phytophotodermatitis, porphyria, and solar urticaria in White individuals.
“Another pediatric photodermatosis, actinic prurigo, tends to occur most often in Mestizo individuals, patients of American Indian heritage,” he added. “This is a significant issue, especially in Latin America.”
In a systematic review of 20 studies in the medical literature, researchers assessed the quality of life and psychological impact of photodermatoses in affected patients. Studies included in the review drew from 2,487 adults and 119 children. Among adults, the self-administered Dermatology Life Quality Index (DLQI) revealed that photodermatoses adversely affected employment, education, and leisure activities in adults. Among children, the condition adversely affected outdoor activities and exacerbated symptoms in those with erythropoietic protoporphyria (EPP).
As for skin cancer risk, the association between UV light exposure and the development of melanoma is not as strong in people with skin of color, compared with light-skinned individuals. In a recent systematic review of 13 studies on the topic, 11 showed no association, one showed a small positive relationship in Black males and 1 showed a weak association in Hispanic males.
“The conclusion from this review is that UV protection for melanoma prevention in people of color is not supported by most studies,” said Dr. Lim, who was not affiliated with the review. “The authors also noted, however, that the evidence is of moderate to low quality. Larger studies should be done.”
The association between UV exposure and the development to squamous cell cancer in skin of color is also not strong. “However, we do know that sun exposure is associated with the development of basal cell carcinoma in this population,” he said.
Sunscreen ingredient studies
Dr. Lim also highlighted findings from two studies related to the effect of sunscreen application on plasma concentration of sunscreen active ingredients, both in adults. In the most recent analysis, scientists at the Food and Drug Administration and colleagues conducted a randomized clinical trial in 48 individuals with skin types II-IV.
Participants applied sunscreen at 2 mg/cm2 to 75% of body surface area at 0 hours on day 1 and 4 times on day 2 through day 4 at 2-hour intervals. Over the course of 21 days, the researchers collected 34 blood samples from each participant, and evaluated six active ingredients in four sunscreen products: avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate.
For all active ingredients, levels of greater than 0.5 ng/mL were detected after a single application on day 1. Levels of greater than 0.5 ng/mL were detected up to day 7, and up to day 21 for oxybenzone. All were detected in skin on days 7 and 14 via tape stripping. The authors called for further studies to determine the clinical significance of these findings and emphasized that the results “do not indicate that individuals should refrain from the use of sunscreen.”
The FDA is asking for additional studies on the safety of these 12 filters, noted Dr. Lim, who is a past president of the American Academy of Dermatology. On Feb. 26, 2019, the FDA issued a proposed rule regarding sunscreen drug products for over-the-counter human use. It proposes that the 16 UV filters be classified into one of 3 categories. Category I would include zinc oxide and titanium dioxide, which are generally recognized as safe and effective (GRASE). Category II would include PABA and trolamine salicylate, which are not used in the United States and are not GRASE. Category III would include 12 filters that lack insufficient safety data to make a determination regarding GRASE.
The final FDA rule was scheduled to be released in September of 2020, but a result of the Coronavirus Aid, Relief, and Economic Security (CARES) Act, the FDA “will be moving from a laborious rulemaking process to an administrative order process, which means it should not take as long to implement a monograph,” Dr. Lim said. “The FDA has decided that there will not be a final rule regarding sunscreen drug products,” but is required to issue a proposed administrative order by Sept. 27, 2021, he said.
When the final administrative order has been issued, manufacturers would have at least 1 year to comply with sunscreen products offered in the United States. “The approximate timeline is probably going to be 2023,” he said.
Dr. Lim disclosed that he is an investigator for Incyte, L’Oreal, Pfizer, and the Patient-centered Outcomes Research Institute, and a consultant for Pierre Fabre, ISDIN, Ferndale, La Roche–Posay, and Beiersdorf. He has been a speaker at general educational sessions sponsored by La Roche–Posay and Cantabria Labs.
FROM THE SPD PRE-AAD MEETING
Which comes first in osteoarthritis: The damage or the pain?
Is innervation of cartilage the driving force behind development of osteoarthritis and subsequent pain, or is the degeneration of joints in osteoarthritis affecting nerves and creating pain?
This was the question underpinning a fascinating debate at the OARSI 2021 World Congress, featuring two giants of the OA research community: Anne-Marie Malfait, MD, PhD, professor of medicine in the division of rheumatology at Rush Medical College, Chicago, and Stefan Lohmander, MD, PhD, professor emeritus of orthopedics at Lund (Sweden) University in Sweden.
At stake in the discussion is a greater understanding of the physiological processes that underpin both the development of OA in joints and the experience of pain in patients with OA.
Dr. Lohmander started by pointing out that, while pain is the primary symptoms of OA, it does not always overlap with the physiological processes of the disease, as measured by techniques such as MRI, x-ray, biomarkers, and gait analysis.
“This lack of complete overlap is often a problem when doing our clinical trials,” Dr. Lohmander told the conference, sponsored by Osteoarthritis Research Society International. “When talking about osteoarthritis, we also need to remind ourselves every so often that we are speaking of either the symptoms or the disease and maybe not always the both of them.”
While a healthy joint has pain receptors everywhere but the cartilage, studies have found that the osteoarthritic joint brings blood vessels, sensory nerves, and cells expressing nerve growth factor from the subchondral bone into even noncalcified articular cartilage, he said.
These nociceptor neurons are mechanosensitive, so mechanical injury to the joint triggers inflammation, and the inflammatory proteins themselves act on the nociceptors to generate pain signals in the brain, “so clearly, it is the joint that signals the brain,” Dr. Lohmander said.
However, Dr. Malfait pointed out that there is a body of evidence from animal studies showing that the absence of sensory nerves in joints – either from disease or removal – is associated with the onset or worsening of OA.
“Healthy nerves are really important to ensure healthy joints,” Dr. Malfait said. She said age-related loss of sensory nerves always preceded age-related OA, and was also associated with age-related loss of proprioception and vibratory perception.
Interestingly, animal studies suggest that removing intra-articular nociceptors can actually have a protective effect on the osteoarthritic joint, Dr. Malfait said. Studies in humans who have experienced neurologic lesions also suggests improvement in conditions such as rheumatoid arthritis.
She raised the idea of neurogenic inflammation: that peripheral neurons are releasing vasoactive mediators that contribute to inflammation in tissues. “These nerves and nerve products are talking to all the different cells in the joints,” she said.
Defending his argument that joint pathology is the cause of pain, not the pain causing the joint pathology, Dr. Lohmander gave the example of studies that looked at radiographic abnormalities between two knees of the same patient who also had discordant pain measures for each knee. This research “showed strong association between radiographic osteoarthritis and knee pain, supporting the argument that structural abnormalities cause knee pain,” he said.
Martin van der Esch, PhD, of the Amsterdam University of Applied Sciences, said the debate was one of the highlights of the conference because it addressed such an important and longstanding question in OA.
“Is osteoarthritis leading to a generalized pain, so involvement of the nervous system, but the source – the causality – is in the joint?” he said in an interview. “Or is it the other way around, so that means is there first a problem inside the nervous system – including also the vascular system – and which is presented in the joint?”
It is more than an academic discussion because the conclusions of that could mean different treatment approaches are needed for different groups of patients, and raises the different ways of thinking about OA, he said.
None of the sources for this story declared having any relevant conflicts of interest.
Is innervation of cartilage the driving force behind development of osteoarthritis and subsequent pain, or is the degeneration of joints in osteoarthritis affecting nerves and creating pain?
This was the question underpinning a fascinating debate at the OARSI 2021 World Congress, featuring two giants of the OA research community: Anne-Marie Malfait, MD, PhD, professor of medicine in the division of rheumatology at Rush Medical College, Chicago, and Stefan Lohmander, MD, PhD, professor emeritus of orthopedics at Lund (Sweden) University in Sweden.
At stake in the discussion is a greater understanding of the physiological processes that underpin both the development of OA in joints and the experience of pain in patients with OA.
Dr. Lohmander started by pointing out that, while pain is the primary symptoms of OA, it does not always overlap with the physiological processes of the disease, as measured by techniques such as MRI, x-ray, biomarkers, and gait analysis.
“This lack of complete overlap is often a problem when doing our clinical trials,” Dr. Lohmander told the conference, sponsored by Osteoarthritis Research Society International. “When talking about osteoarthritis, we also need to remind ourselves every so often that we are speaking of either the symptoms or the disease and maybe not always the both of them.”
While a healthy joint has pain receptors everywhere but the cartilage, studies have found that the osteoarthritic joint brings blood vessels, sensory nerves, and cells expressing nerve growth factor from the subchondral bone into even noncalcified articular cartilage, he said.
These nociceptor neurons are mechanosensitive, so mechanical injury to the joint triggers inflammation, and the inflammatory proteins themselves act on the nociceptors to generate pain signals in the brain, “so clearly, it is the joint that signals the brain,” Dr. Lohmander said.
However, Dr. Malfait pointed out that there is a body of evidence from animal studies showing that the absence of sensory nerves in joints – either from disease or removal – is associated with the onset or worsening of OA.
“Healthy nerves are really important to ensure healthy joints,” Dr. Malfait said. She said age-related loss of sensory nerves always preceded age-related OA, and was also associated with age-related loss of proprioception and vibratory perception.
Interestingly, animal studies suggest that removing intra-articular nociceptors can actually have a protective effect on the osteoarthritic joint, Dr. Malfait said. Studies in humans who have experienced neurologic lesions also suggests improvement in conditions such as rheumatoid arthritis.
She raised the idea of neurogenic inflammation: that peripheral neurons are releasing vasoactive mediators that contribute to inflammation in tissues. “These nerves and nerve products are talking to all the different cells in the joints,” she said.
Defending his argument that joint pathology is the cause of pain, not the pain causing the joint pathology, Dr. Lohmander gave the example of studies that looked at radiographic abnormalities between two knees of the same patient who also had discordant pain measures for each knee. This research “showed strong association between radiographic osteoarthritis and knee pain, supporting the argument that structural abnormalities cause knee pain,” he said.
Martin van der Esch, PhD, of the Amsterdam University of Applied Sciences, said the debate was one of the highlights of the conference because it addressed such an important and longstanding question in OA.
“Is osteoarthritis leading to a generalized pain, so involvement of the nervous system, but the source – the causality – is in the joint?” he said in an interview. “Or is it the other way around, so that means is there first a problem inside the nervous system – including also the vascular system – and which is presented in the joint?”
It is more than an academic discussion because the conclusions of that could mean different treatment approaches are needed for different groups of patients, and raises the different ways of thinking about OA, he said.
None of the sources for this story declared having any relevant conflicts of interest.
Is innervation of cartilage the driving force behind development of osteoarthritis and subsequent pain, or is the degeneration of joints in osteoarthritis affecting nerves and creating pain?
This was the question underpinning a fascinating debate at the OARSI 2021 World Congress, featuring two giants of the OA research community: Anne-Marie Malfait, MD, PhD, professor of medicine in the division of rheumatology at Rush Medical College, Chicago, and Stefan Lohmander, MD, PhD, professor emeritus of orthopedics at Lund (Sweden) University in Sweden.
At stake in the discussion is a greater understanding of the physiological processes that underpin both the development of OA in joints and the experience of pain in patients with OA.
Dr. Lohmander started by pointing out that, while pain is the primary symptoms of OA, it does not always overlap with the physiological processes of the disease, as measured by techniques such as MRI, x-ray, biomarkers, and gait analysis.
“This lack of complete overlap is often a problem when doing our clinical trials,” Dr. Lohmander told the conference, sponsored by Osteoarthritis Research Society International. “When talking about osteoarthritis, we also need to remind ourselves every so often that we are speaking of either the symptoms or the disease and maybe not always the both of them.”
While a healthy joint has pain receptors everywhere but the cartilage, studies have found that the osteoarthritic joint brings blood vessels, sensory nerves, and cells expressing nerve growth factor from the subchondral bone into even noncalcified articular cartilage, he said.
These nociceptor neurons are mechanosensitive, so mechanical injury to the joint triggers inflammation, and the inflammatory proteins themselves act on the nociceptors to generate pain signals in the brain, “so clearly, it is the joint that signals the brain,” Dr. Lohmander said.
However, Dr. Malfait pointed out that there is a body of evidence from animal studies showing that the absence of sensory nerves in joints – either from disease or removal – is associated with the onset or worsening of OA.
“Healthy nerves are really important to ensure healthy joints,” Dr. Malfait said. She said age-related loss of sensory nerves always preceded age-related OA, and was also associated with age-related loss of proprioception and vibratory perception.
Interestingly, animal studies suggest that removing intra-articular nociceptors can actually have a protective effect on the osteoarthritic joint, Dr. Malfait said. Studies in humans who have experienced neurologic lesions also suggests improvement in conditions such as rheumatoid arthritis.
She raised the idea of neurogenic inflammation: that peripheral neurons are releasing vasoactive mediators that contribute to inflammation in tissues. “These nerves and nerve products are talking to all the different cells in the joints,” she said.
Defending his argument that joint pathology is the cause of pain, not the pain causing the joint pathology, Dr. Lohmander gave the example of studies that looked at radiographic abnormalities between two knees of the same patient who also had discordant pain measures for each knee. This research “showed strong association between radiographic osteoarthritis and knee pain, supporting the argument that structural abnormalities cause knee pain,” he said.
Martin van der Esch, PhD, of the Amsterdam University of Applied Sciences, said the debate was one of the highlights of the conference because it addressed such an important and longstanding question in OA.
“Is osteoarthritis leading to a generalized pain, so involvement of the nervous system, but the source – the causality – is in the joint?” he said in an interview. “Or is it the other way around, so that means is there first a problem inside the nervous system – including also the vascular system – and which is presented in the joint?”
It is more than an academic discussion because the conclusions of that could mean different treatment approaches are needed for different groups of patients, and raises the different ways of thinking about OA, he said.
None of the sources for this story declared having any relevant conflicts of interest.
FROM OARSI 2021
Trends in hospital medicine program operations during COVID-19
Staffing was a challenge for most groups
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
Staffing was a challenge for most groups
Staffing was a challenge for most groups
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.