User login
CHEST Foundation reimagines events during the pandemic
Feeling lonely is one of the biggest challenges that we are faced with during this pandemic. It doesn’t matter who you are – a patient, a caregiver, or a physician – it affects us all.
Social distancing practices make it almost impossible to host in-person gatherings, which is hard on everyone, but as a philanthropic organization that focuses on community events, it’s down-right devastating. Not only does the Foundation look to events to help form a sense of camaraderie among our donors, we rely on them to help fund our projects.
That’s why we had to get creative last year and quickly reimagine our events in a totally new space ... cyberspace to be exact.
New takes on old favorites
We’re proud to say that we hosted seven online events in 2020, including Irv Feldman’s Poker Tournament, one of our most popular fundraisers. “We wanted to continue our traditions but knew we had to do it in a different format. We learned to pivot quickly and get everything online, but we then had to cross our fingers that our donors would get onboard,” said Angela Perillo, Director, Development & Foundation Operations. To the Foundation’s delight, the events not only piqued people’s interest, they brought in more than $150,000!
The impact of your ticket purchase
The Foundation has a new motto in 2021: “When you attend an event, you tend to our mission.” In other words, every event we host raises funds for our initiatives. “We want our donors to know that while they’re having a great time, they’re also doing their part in helping the Foundation enable more people to get access to the resources they need. A ticket sale today might help a patient get better care tomorrow, “ said Perillo.
Now’s the time to attend
Several events have been planned for this spring and summer. We hope you’ll join us by registering at chestfoundation.org and following #CHESTFoundation25 on social media:
- Irv’s Spring Splash Poker Tournament: Thursday, May 20 at 7 pm CT
- Belmont Stakes Reception & Auction: June 5 at 5 pm CT
- Irv’s Spring Splash Poker Tournament: June 18 at 7 pm CT
- Wine Tasting: June 24 at 7 pm CT
- Trivia Night: July 21 at 7 pm CT
Feeling lonely is one of the biggest challenges that we are faced with during this pandemic. It doesn’t matter who you are – a patient, a caregiver, or a physician – it affects us all.
Social distancing practices make it almost impossible to host in-person gatherings, which is hard on everyone, but as a philanthropic organization that focuses on community events, it’s down-right devastating. Not only does the Foundation look to events to help form a sense of camaraderie among our donors, we rely on them to help fund our projects.
That’s why we had to get creative last year and quickly reimagine our events in a totally new space ... cyberspace to be exact.
New takes on old favorites
We’re proud to say that we hosted seven online events in 2020, including Irv Feldman’s Poker Tournament, one of our most popular fundraisers. “We wanted to continue our traditions but knew we had to do it in a different format. We learned to pivot quickly and get everything online, but we then had to cross our fingers that our donors would get onboard,” said Angela Perillo, Director, Development & Foundation Operations. To the Foundation’s delight, the events not only piqued people’s interest, they brought in more than $150,000!
The impact of your ticket purchase
The Foundation has a new motto in 2021: “When you attend an event, you tend to our mission.” In other words, every event we host raises funds for our initiatives. “We want our donors to know that while they’re having a great time, they’re also doing their part in helping the Foundation enable more people to get access to the resources they need. A ticket sale today might help a patient get better care tomorrow, “ said Perillo.
Now’s the time to attend
Several events have been planned for this spring and summer. We hope you’ll join us by registering at chestfoundation.org and following #CHESTFoundation25 on social media:
- Irv’s Spring Splash Poker Tournament: Thursday, May 20 at 7 pm CT
- Belmont Stakes Reception & Auction: June 5 at 5 pm CT
- Irv’s Spring Splash Poker Tournament: June 18 at 7 pm CT
- Wine Tasting: June 24 at 7 pm CT
- Trivia Night: July 21 at 7 pm CT
Feeling lonely is one of the biggest challenges that we are faced with during this pandemic. It doesn’t matter who you are – a patient, a caregiver, or a physician – it affects us all.
Social distancing practices make it almost impossible to host in-person gatherings, which is hard on everyone, but as a philanthropic organization that focuses on community events, it’s down-right devastating. Not only does the Foundation look to events to help form a sense of camaraderie among our donors, we rely on them to help fund our projects.
That’s why we had to get creative last year and quickly reimagine our events in a totally new space ... cyberspace to be exact.
New takes on old favorites
We’re proud to say that we hosted seven online events in 2020, including Irv Feldman’s Poker Tournament, one of our most popular fundraisers. “We wanted to continue our traditions but knew we had to do it in a different format. We learned to pivot quickly and get everything online, but we then had to cross our fingers that our donors would get onboard,” said Angela Perillo, Director, Development & Foundation Operations. To the Foundation’s delight, the events not only piqued people’s interest, they brought in more than $150,000!
The impact of your ticket purchase
The Foundation has a new motto in 2021: “When you attend an event, you tend to our mission.” In other words, every event we host raises funds for our initiatives. “We want our donors to know that while they’re having a great time, they’re also doing their part in helping the Foundation enable more people to get access to the resources they need. A ticket sale today might help a patient get better care tomorrow, “ said Perillo.
Now’s the time to attend
Several events have been planned for this spring and summer. We hope you’ll join us by registering at chestfoundation.org and following #CHESTFoundation25 on social media:
- Irv’s Spring Splash Poker Tournament: Thursday, May 20 at 7 pm CT
- Belmont Stakes Reception & Auction: June 5 at 5 pm CT
- Irv’s Spring Splash Poker Tournament: June 18 at 7 pm CT
- Wine Tasting: June 24 at 7 pm CT
- Trivia Night: July 21 at 7 pm CT
CPT® and COVID-19 vaccination
COVID-19 vaccination efforts were initially restricted to health department control, and physician practices were not often included as vaccination sites. However, as vaccine availability improves ,physician offices will become a place where vaccines can be delivered conveniently and efficiently. It is important to understand the current and future coding and billing requirements for COVID-19 vaccination so that one’s practice may be appropriately reimbursed.
The provision of COVID-19 vaccination in an office setting is not as simple as influenza or pneumonia vaccination. One can find useful information about all vaccines and specifically about COVID-19 vaccines at https://www.cdc.gov/vaccines/ed/index.html. This site includes video training modules and downloadable resources for clinical use, as well as patient education. This information is important as providing vaccinations may require a change in infrastructure, equipment, and clinical flow. It may not be financially advantageous for one’s practice to provide COVID-19 vaccination.
If the decision is made to provide COVID-19 vaccinations, there are specific CPT codes for each vaccine and its administration (Table 1). These codes are valid for the vaccines with emergency use authorization (Pfizer, Moderna, Janssen) but not yet for as yet unauthorized vaccines (AstraZeneca). Should additional vaccines be authorized, it is expected that new CPT codes will be added.
When a patient is vaccinated, only the administration code is used at this time. The CPT codes for the vaccine (91300-3) should not be used because the cost of the vaccine is currently born by the federal government. When the vaccines are available for purchase by a practice, it will then be appropriate to use the vaccine CPT code. If an evaluation and management (E/M) service is performed, the appropriate E/M service code should be reported in addition to the vaccine administration code.
For payment of the vaccine administration by Medicare, either a single claim or roster claim can be submitted. When five or more patients are vaccinated using the same vaccine on the same day, one may submit a roster claim. Instructions on how to appropriately bill the various Medicare plans can be found at https://tinyurl.com/hfya8888. Guidelines for payment by private insurers should also be reviewed as well, as they will have their own requirements. If a vaccine is given to an individual who does not have any insurance coverage, reimbursement may be available through the Provider Relief Fund. These funds were made available by legislation, including the CARES act and information about claim submittal for the uninsured can be found at https://www.hrsa.gov/CovidUninsuredClaim.
COVID-19 vaccination efforts were initially restricted to health department control, and physician practices were not often included as vaccination sites. However, as vaccine availability improves ,physician offices will become a place where vaccines can be delivered conveniently and efficiently. It is important to understand the current and future coding and billing requirements for COVID-19 vaccination so that one’s practice may be appropriately reimbursed.
The provision of COVID-19 vaccination in an office setting is not as simple as influenza or pneumonia vaccination. One can find useful information about all vaccines and specifically about COVID-19 vaccines at https://www.cdc.gov/vaccines/ed/index.html. This site includes video training modules and downloadable resources for clinical use, as well as patient education. This information is important as providing vaccinations may require a change in infrastructure, equipment, and clinical flow. It may not be financially advantageous for one’s practice to provide COVID-19 vaccination.
If the decision is made to provide COVID-19 vaccinations, there are specific CPT codes for each vaccine and its administration (Table 1). These codes are valid for the vaccines with emergency use authorization (Pfizer, Moderna, Janssen) but not yet for as yet unauthorized vaccines (AstraZeneca). Should additional vaccines be authorized, it is expected that new CPT codes will be added.
When a patient is vaccinated, only the administration code is used at this time. The CPT codes for the vaccine (91300-3) should not be used because the cost of the vaccine is currently born by the federal government. When the vaccines are available for purchase by a practice, it will then be appropriate to use the vaccine CPT code. If an evaluation and management (E/M) service is performed, the appropriate E/M service code should be reported in addition to the vaccine administration code.
For payment of the vaccine administration by Medicare, either a single claim or roster claim can be submitted. When five or more patients are vaccinated using the same vaccine on the same day, one may submit a roster claim. Instructions on how to appropriately bill the various Medicare plans can be found at https://tinyurl.com/hfya8888. Guidelines for payment by private insurers should also be reviewed as well, as they will have their own requirements. If a vaccine is given to an individual who does not have any insurance coverage, reimbursement may be available through the Provider Relief Fund. These funds were made available by legislation, including the CARES act and information about claim submittal for the uninsured can be found at https://www.hrsa.gov/CovidUninsuredClaim.
COVID-19 vaccination efforts were initially restricted to health department control, and physician practices were not often included as vaccination sites. However, as vaccine availability improves ,physician offices will become a place where vaccines can be delivered conveniently and efficiently. It is important to understand the current and future coding and billing requirements for COVID-19 vaccination so that one’s practice may be appropriately reimbursed.
The provision of COVID-19 vaccination in an office setting is not as simple as influenza or pneumonia vaccination. One can find useful information about all vaccines and specifically about COVID-19 vaccines at https://www.cdc.gov/vaccines/ed/index.html. This site includes video training modules and downloadable resources for clinical use, as well as patient education. This information is important as providing vaccinations may require a change in infrastructure, equipment, and clinical flow. It may not be financially advantageous for one’s practice to provide COVID-19 vaccination.
If the decision is made to provide COVID-19 vaccinations, there are specific CPT codes for each vaccine and its administration (Table 1). These codes are valid for the vaccines with emergency use authorization (Pfizer, Moderna, Janssen) but not yet for as yet unauthorized vaccines (AstraZeneca). Should additional vaccines be authorized, it is expected that new CPT codes will be added.
When a patient is vaccinated, only the administration code is used at this time. The CPT codes for the vaccine (91300-3) should not be used because the cost of the vaccine is currently born by the federal government. When the vaccines are available for purchase by a practice, it will then be appropriate to use the vaccine CPT code. If an evaluation and management (E/M) service is performed, the appropriate E/M service code should be reported in addition to the vaccine administration code.
For payment of the vaccine administration by Medicare, either a single claim or roster claim can be submitted. When five or more patients are vaccinated using the same vaccine on the same day, one may submit a roster claim. Instructions on how to appropriately bill the various Medicare plans can be found at https://tinyurl.com/hfya8888. Guidelines for payment by private insurers should also be reviewed as well, as they will have their own requirements. If a vaccine is given to an individual who does not have any insurance coverage, reimbursement may be available through the Provider Relief Fund. These funds were made available by legislation, including the CARES act and information about claim submittal for the uninsured can be found at https://www.hrsa.gov/CovidUninsuredClaim.
ABIM Extends MOC Requirement Deadlines: Prepares to Launch the Longitudinal Knowledge Assessment
Recognizing that caring for patients with COVID continues to be the focus of many physicians, in March, the American Board of Internal Medicine (ABIM) announced that it extended all MOC requirement deadlines until 12/31/22. For those ABIM Board Certified in Critical Care Medicine, Hospital Medicine, Infectious Disease, or Pulmonary Disease, MOC requirements have been extended until the end of 2023.
In a letter to the internal medicine community, Richard J. Baron, MD, MACP, ABIM President and CEO; and Marianne M. Green, MD, Chair of the ABIM Board of Directors, said, “We know internists and internal medicine subspecialists have been on the front lines meeting the country’s needs, many experiencing the tragedy of COVID in deeply personal ways…We also recognize the high levels of stress you may have faced over the last 12 months, and that it will likely be some time until it subsides…We hope this gives you one less thing to worry about.”
The decision means that nobody will lose ABIM certification if they are unable to complete MOC requirements this year. Recognizing every physician’s situation is different, all ABIM MOC exams will be administered as scheduled in 2021 for those who wish to take one.
In January 2022, ABIM will launch a new Longitudinal Knowledge Assessment (LKATM) (www.abim.org/lka/), a more flexible and convenient way to maintain certification. Physicians who decide to delay their 2021 assessment will be able to enroll in the LKA when it rolls out (pending availability), or can choose to take the traditional, 10-year MOC exam if they prefer.
The LKA for Critical Care, Hospital Medicine, Infectious Disease, and Pulmonary Disease will launch in January 2023. As these were among the disciplines most impacted by COVID, additional time is needed to create the requisite content for a high-quality assessment and is why MOC requirement deadlines for these specialties is extended an additional year to provide a transition pathway to the LKA.
Through the LKA, questions can be answered on almost any internet-connected device at any time, and physicians can access all the resources used in practice (except another person). ABIM will release 30 questions each quarter that can be answered a few at a time, or all at once. Immediate feedback with rationale and reference will be provided. As long as at least 500 of the 600 questions are answered over the 5-year cycle, the LKA Participation Requirement will be met.
ABIM is in the process of updating the Physician Portal in light of the MOC requirements deadline extension. If you have any questions about your requirements, call 1-800-441-ABIM or email [email protected]. For further information about the LKA, visit abim.org/lka/.
Recognizing that caring for patients with COVID continues to be the focus of many physicians, in March, the American Board of Internal Medicine (ABIM) announced that it extended all MOC requirement deadlines until 12/31/22. For those ABIM Board Certified in Critical Care Medicine, Hospital Medicine, Infectious Disease, or Pulmonary Disease, MOC requirements have been extended until the end of 2023.
In a letter to the internal medicine community, Richard J. Baron, MD, MACP, ABIM President and CEO; and Marianne M. Green, MD, Chair of the ABIM Board of Directors, said, “We know internists and internal medicine subspecialists have been on the front lines meeting the country’s needs, many experiencing the tragedy of COVID in deeply personal ways…We also recognize the high levels of stress you may have faced over the last 12 months, and that it will likely be some time until it subsides…We hope this gives you one less thing to worry about.”
The decision means that nobody will lose ABIM certification if they are unable to complete MOC requirements this year. Recognizing every physician’s situation is different, all ABIM MOC exams will be administered as scheduled in 2021 for those who wish to take one.
In January 2022, ABIM will launch a new Longitudinal Knowledge Assessment (LKATM) (www.abim.org/lka/), a more flexible and convenient way to maintain certification. Physicians who decide to delay their 2021 assessment will be able to enroll in the LKA when it rolls out (pending availability), or can choose to take the traditional, 10-year MOC exam if they prefer.
The LKA for Critical Care, Hospital Medicine, Infectious Disease, and Pulmonary Disease will launch in January 2023. As these were among the disciplines most impacted by COVID, additional time is needed to create the requisite content for a high-quality assessment and is why MOC requirement deadlines for these specialties is extended an additional year to provide a transition pathway to the LKA.
Through the LKA, questions can be answered on almost any internet-connected device at any time, and physicians can access all the resources used in practice (except another person). ABIM will release 30 questions each quarter that can be answered a few at a time, or all at once. Immediate feedback with rationale and reference will be provided. As long as at least 500 of the 600 questions are answered over the 5-year cycle, the LKA Participation Requirement will be met.
ABIM is in the process of updating the Physician Portal in light of the MOC requirements deadline extension. If you have any questions about your requirements, call 1-800-441-ABIM or email [email protected]. For further information about the LKA, visit abim.org/lka/.
Recognizing that caring for patients with COVID continues to be the focus of many physicians, in March, the American Board of Internal Medicine (ABIM) announced that it extended all MOC requirement deadlines until 12/31/22. For those ABIM Board Certified in Critical Care Medicine, Hospital Medicine, Infectious Disease, or Pulmonary Disease, MOC requirements have been extended until the end of 2023.
In a letter to the internal medicine community, Richard J. Baron, MD, MACP, ABIM President and CEO; and Marianne M. Green, MD, Chair of the ABIM Board of Directors, said, “We know internists and internal medicine subspecialists have been on the front lines meeting the country’s needs, many experiencing the tragedy of COVID in deeply personal ways…We also recognize the high levels of stress you may have faced over the last 12 months, and that it will likely be some time until it subsides…We hope this gives you one less thing to worry about.”
The decision means that nobody will lose ABIM certification if they are unable to complete MOC requirements this year. Recognizing every physician’s situation is different, all ABIM MOC exams will be administered as scheduled in 2021 for those who wish to take one.
In January 2022, ABIM will launch a new Longitudinal Knowledge Assessment (LKATM) (www.abim.org/lka/), a more flexible and convenient way to maintain certification. Physicians who decide to delay their 2021 assessment will be able to enroll in the LKA when it rolls out (pending availability), or can choose to take the traditional, 10-year MOC exam if they prefer.
The LKA for Critical Care, Hospital Medicine, Infectious Disease, and Pulmonary Disease will launch in January 2023. As these were among the disciplines most impacted by COVID, additional time is needed to create the requisite content for a high-quality assessment and is why MOC requirement deadlines for these specialties is extended an additional year to provide a transition pathway to the LKA.
Through the LKA, questions can be answered on almost any internet-connected device at any time, and physicians can access all the resources used in practice (except another person). ABIM will release 30 questions each quarter that can be answered a few at a time, or all at once. Immediate feedback with rationale and reference will be provided. As long as at least 500 of the 600 questions are answered over the 5-year cycle, the LKA Participation Requirement will be met.
ABIM is in the process of updating the Physician Portal in light of the MOC requirements deadline extension. If you have any questions about your requirements, call 1-800-441-ABIM or email [email protected]. For further information about the LKA, visit abim.org/lka/.
Message from CHEST 2021 Co-Chair, Chris Carroll, MD, FCCP
A little over a year ago, none of us imagined we’d be where we are right now. The pandemic has deeply affected us all, and there have been so many losses, both professional and personal. I’m proud of how our CHEST community responded to the pandemic. The incredibly rapid pace of knowledge acquisition and the speed at which we disseminated that knowledge took a lot of combined effort, but that’s nothing new to our CHEST community.
Throughout the pandemic, CHEST pushed digital education with an array of webinars, podcasts, bite-sized educational modules, and infographics. We held a highly successful, well-received CHEST 2020 online conference with just a few months of planning. I’m so excited to take what we learned about offering high-quality, digital education and turn that into a hybrid meeting for CHEST 2021 that meets the educational needs of every participant!
At CHEST 2021, you will be presented with the latest in pulmonary, critical care, and sleep medicine for clinicians at all levels. Whether you are a trainee or an experienced clinician, there is something to learn at CHEST 2021. We are packing the agenda with experiences from live learning and simulation to high-quality education sessions and smaller problem-based learning classes.
On top of this, you have an amazing opportunity to network and reconnect with colleagues you haven’t seen in months! Whether at Experience CHEST, in the gaming area, the Trainee and Transition Lounge, and more, CHEST 2021, as always, is the best at providing top-tier education, team-based learning, and community connections.
This will be the first hybrid meeting put on by CHEST. We came to the decision knowing that while some people are hungry to get back to having an in-person experience, others found that an online conference better fits their needs. I strongly encourage you to join us October 17-20 in Orlando, Florida, to experience the networking and growth opportunities that come from attending in person. We are following strict protocols, as recommended by the CDC, and will be requiring all attendees to attest to being vaccinated. However, if travel isn’t possible, join us for livestreamed, immersive digital learning from wherever you are in the world. Regardless of your choice, both options will allow you to engage in fun experiences, learn, and connect.
As Co-Chair of CHEST 2021, I’d like to personally invite you to participate, whether this is your first time or you’ve lost count how many times you’ve attended our annual meeting. The community at CHEST is what makes the CHEST conference special, and we are proud to be able to keep you all connected despite geographic restrictions.
Looking forward to seeing you there and connecting on Twitter at #CHEST2021.
Chris Carroll, MD
Co-Chair, CHEST 2021
A little over a year ago, none of us imagined we’d be where we are right now. The pandemic has deeply affected us all, and there have been so many losses, both professional and personal. I’m proud of how our CHEST community responded to the pandemic. The incredibly rapid pace of knowledge acquisition and the speed at which we disseminated that knowledge took a lot of combined effort, but that’s nothing new to our CHEST community.
Throughout the pandemic, CHEST pushed digital education with an array of webinars, podcasts, bite-sized educational modules, and infographics. We held a highly successful, well-received CHEST 2020 online conference with just a few months of planning. I’m so excited to take what we learned about offering high-quality, digital education and turn that into a hybrid meeting for CHEST 2021 that meets the educational needs of every participant!
At CHEST 2021, you will be presented with the latest in pulmonary, critical care, and sleep medicine for clinicians at all levels. Whether you are a trainee or an experienced clinician, there is something to learn at CHEST 2021. We are packing the agenda with experiences from live learning and simulation to high-quality education sessions and smaller problem-based learning classes.
On top of this, you have an amazing opportunity to network and reconnect with colleagues you haven’t seen in months! Whether at Experience CHEST, in the gaming area, the Trainee and Transition Lounge, and more, CHEST 2021, as always, is the best at providing top-tier education, team-based learning, and community connections.
This will be the first hybrid meeting put on by CHEST. We came to the decision knowing that while some people are hungry to get back to having an in-person experience, others found that an online conference better fits their needs. I strongly encourage you to join us October 17-20 in Orlando, Florida, to experience the networking and growth opportunities that come from attending in person. We are following strict protocols, as recommended by the CDC, and will be requiring all attendees to attest to being vaccinated. However, if travel isn’t possible, join us for livestreamed, immersive digital learning from wherever you are in the world. Regardless of your choice, both options will allow you to engage in fun experiences, learn, and connect.
As Co-Chair of CHEST 2021, I’d like to personally invite you to participate, whether this is your first time or you’ve lost count how many times you’ve attended our annual meeting. The community at CHEST is what makes the CHEST conference special, and we are proud to be able to keep you all connected despite geographic restrictions.
Looking forward to seeing you there and connecting on Twitter at #CHEST2021.
Chris Carroll, MD
Co-Chair, CHEST 2021
A little over a year ago, none of us imagined we’d be where we are right now. The pandemic has deeply affected us all, and there have been so many losses, both professional and personal. I’m proud of how our CHEST community responded to the pandemic. The incredibly rapid pace of knowledge acquisition and the speed at which we disseminated that knowledge took a lot of combined effort, but that’s nothing new to our CHEST community.
Throughout the pandemic, CHEST pushed digital education with an array of webinars, podcasts, bite-sized educational modules, and infographics. We held a highly successful, well-received CHEST 2020 online conference with just a few months of planning. I’m so excited to take what we learned about offering high-quality, digital education and turn that into a hybrid meeting for CHEST 2021 that meets the educational needs of every participant!
At CHEST 2021, you will be presented with the latest in pulmonary, critical care, and sleep medicine for clinicians at all levels. Whether you are a trainee or an experienced clinician, there is something to learn at CHEST 2021. We are packing the agenda with experiences from live learning and simulation to high-quality education sessions and smaller problem-based learning classes.
On top of this, you have an amazing opportunity to network and reconnect with colleagues you haven’t seen in months! Whether at Experience CHEST, in the gaming area, the Trainee and Transition Lounge, and more, CHEST 2021, as always, is the best at providing top-tier education, team-based learning, and community connections.
This will be the first hybrid meeting put on by CHEST. We came to the decision knowing that while some people are hungry to get back to having an in-person experience, others found that an online conference better fits their needs. I strongly encourage you to join us October 17-20 in Orlando, Florida, to experience the networking and growth opportunities that come from attending in person. We are following strict protocols, as recommended by the CDC, and will be requiring all attendees to attest to being vaccinated. However, if travel isn’t possible, join us for livestreamed, immersive digital learning from wherever you are in the world. Regardless of your choice, both options will allow you to engage in fun experiences, learn, and connect.
As Co-Chair of CHEST 2021, I’d like to personally invite you to participate, whether this is your first time or you’ve lost count how many times you’ve attended our annual meeting. The community at CHEST is what makes the CHEST conference special, and we are proud to be able to keep you all connected despite geographic restrictions.
Looking forward to seeing you there and connecting on Twitter at #CHEST2021.
Chris Carroll, MD
Co-Chair, CHEST 2021
Multiple sclerosis updates from AAN 2021
Mitzi Joi Williams, MD, Medical Director and CEO of Joi Life Wellness Group in Atlanta, GA, discusses treatment data and other timely updates in multiple sclerosis (MS) coming out of the American Academy of Neurology (AAN) 2021 Annual Meeting.
Dr. Williams introduces the new National African Americans with Multiple Sclerosis Registry, an important initiative aimed at expanding evidence-based knowledge of MS in African American patients, increasing clinical trial participation, and engaging in research that will benefit this community.
In a review of a long-term analysis of evobrutinib, Dr. Williams shares the positive efficacy and safety outcomes that were observed and maintained in patients receiving evobrutinib 75 mg twice daily throughout the duration of the 48-week double-blind, randomized, phase 2 trial and the 60-week open-label extension portions of the study.
Next, Dr. Williams highlights a study of patients taking disease-modifying therapies (DMTs) at the NYU MS Care Center. The analysis found factors such as public insurance status, younger age, and Hispanic ethnicity to be larger predictors of COVID-19 infection in this patient population than any of the DMTs that were studied.
Lastly, Dr. Williams discusses an analysis of racial and ethnic differences in patients receiving ofatumumab in the ASCLEPIOS I/II and APOLITOS trials. The study revealed no clinically relevant differences in annualized relapse rate, pharmacokinetics, B-cell depletion, or safety profile between the study populations.
--
Mitzi Joi Williams, MD, Medical Director, CEO; Department of Neurology, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen Idec; EMD Serono; Genentech; Novartis; Bristol-Myers Squibb; AbbVie; Alexion; Novartis.
Serve(d) as a speaker or a member of a speakers bureau for: Biogen Idec; EMD Serono; Genentech Roche; Novartis; Bristol-Myers Squibb.
Mitzi Joi Williams, MD, Medical Director and CEO of Joi Life Wellness Group in Atlanta, GA, discusses treatment data and other timely updates in multiple sclerosis (MS) coming out of the American Academy of Neurology (AAN) 2021 Annual Meeting.
Dr. Williams introduces the new National African Americans with Multiple Sclerosis Registry, an important initiative aimed at expanding evidence-based knowledge of MS in African American patients, increasing clinical trial participation, and engaging in research that will benefit this community.
In a review of a long-term analysis of evobrutinib, Dr. Williams shares the positive efficacy and safety outcomes that were observed and maintained in patients receiving evobrutinib 75 mg twice daily throughout the duration of the 48-week double-blind, randomized, phase 2 trial and the 60-week open-label extension portions of the study.
Next, Dr. Williams highlights a study of patients taking disease-modifying therapies (DMTs) at the NYU MS Care Center. The analysis found factors such as public insurance status, younger age, and Hispanic ethnicity to be larger predictors of COVID-19 infection in this patient population than any of the DMTs that were studied.
Lastly, Dr. Williams discusses an analysis of racial and ethnic differences in patients receiving ofatumumab in the ASCLEPIOS I/II and APOLITOS trials. The study revealed no clinically relevant differences in annualized relapse rate, pharmacokinetics, B-cell depletion, or safety profile between the study populations.
--
Mitzi Joi Williams, MD, Medical Director, CEO; Department of Neurology, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen Idec; EMD Serono; Genentech; Novartis; Bristol-Myers Squibb; AbbVie; Alexion; Novartis.
Serve(d) as a speaker or a member of a speakers bureau for: Biogen Idec; EMD Serono; Genentech Roche; Novartis; Bristol-Myers Squibb.
Mitzi Joi Williams, MD, Medical Director and CEO of Joi Life Wellness Group in Atlanta, GA, discusses treatment data and other timely updates in multiple sclerosis (MS) coming out of the American Academy of Neurology (AAN) 2021 Annual Meeting.
Dr. Williams introduces the new National African Americans with Multiple Sclerosis Registry, an important initiative aimed at expanding evidence-based knowledge of MS in African American patients, increasing clinical trial participation, and engaging in research that will benefit this community.
In a review of a long-term analysis of evobrutinib, Dr. Williams shares the positive efficacy and safety outcomes that were observed and maintained in patients receiving evobrutinib 75 mg twice daily throughout the duration of the 48-week double-blind, randomized, phase 2 trial and the 60-week open-label extension portions of the study.
Next, Dr. Williams highlights a study of patients taking disease-modifying therapies (DMTs) at the NYU MS Care Center. The analysis found factors such as public insurance status, younger age, and Hispanic ethnicity to be larger predictors of COVID-19 infection in this patient population than any of the DMTs that were studied.
Lastly, Dr. Williams discusses an analysis of racial and ethnic differences in patients receiving ofatumumab in the ASCLEPIOS I/II and APOLITOS trials. The study revealed no clinically relevant differences in annualized relapse rate, pharmacokinetics, B-cell depletion, or safety profile between the study populations.
--
Mitzi Joi Williams, MD, Medical Director, CEO; Department of Neurology, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen Idec; EMD Serono; Genentech; Novartis; Bristol-Myers Squibb; AbbVie; Alexion; Novartis.
Serve(d) as a speaker or a member of a speakers bureau for: Biogen Idec; EMD Serono; Genentech Roche; Novartis; Bristol-Myers Squibb.

Bridging the Gap: Multidisciplinary Management of NSCLC
Numerous advances in diagnosis, staging, and treatment have evolved over the past several years for non-small cell lung cancer (NSCLC) patients. Because of the new developments, input from a team of specialists is required to treat each patient. Many hospitals have implemented a multidisciplinary team approach to treat these complex cases.
In this ReCAP, Dr Nicole Tanner, a pulmonary critical care specialist from the Medical University of South Carolina, hosts thoracic oncologist Dr Carrie Lee and thoracic surgeon Dr Jason Long, colleagues from the UNC Lineberger Comprehensive Cancer Center, in a discussion of the advantages of multidisciplinary teams in the treatment of NSCLC patients.
Their discussion ranges from identifying key multidisciplinary team members, individual practitioner responsibilities, benefits of multidisciplinary teams to patients and clinicians alike, and the advantages of incorporating telehealth visits into standard practice after the pandemic.
--
Jason M. Long, MD, MPH, FCCP, Associate Professor, Department of Surgery, University of North Carolina School of Medicine; Staff Physician, Department of Surgery, UNC Lineberger Comprehensive Cancer Center, Chapel Hill, North Carolina
Jason M. Long, MD, MPH, FCCP, has disclosed no relevant financial relationships.
Carrie B. Lee, MD, MPH, Associate Professor, Department of Medicine, University of North Carolina, Chapel Hill; Medical Director, Clinical Protocol Office, UNC Lineberger Comprehensive Cancer Center, North Carolina Cancer Hospital, Chapel Hill, North Carolina
Carrie B. Lee, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as the chair of the Data and Safety Monitoring Board for: Delcath, Inc.
Nichole T. Tanner, MD, MSCR, FCCP, Associate Professor, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina
Nichole T. Tanner, MD, MSCR, FCCP, has disclosed no relevant financial relationships.
Numerous advances in diagnosis, staging, and treatment have evolved over the past several years for non-small cell lung cancer (NSCLC) patients. Because of the new developments, input from a team of specialists is required to treat each patient. Many hospitals have implemented a multidisciplinary team approach to treat these complex cases.
In this ReCAP, Dr Nicole Tanner, a pulmonary critical care specialist from the Medical University of South Carolina, hosts thoracic oncologist Dr Carrie Lee and thoracic surgeon Dr Jason Long, colleagues from the UNC Lineberger Comprehensive Cancer Center, in a discussion of the advantages of multidisciplinary teams in the treatment of NSCLC patients.
Their discussion ranges from identifying key multidisciplinary team members, individual practitioner responsibilities, benefits of multidisciplinary teams to patients and clinicians alike, and the advantages of incorporating telehealth visits into standard practice after the pandemic.
--
Jason M. Long, MD, MPH, FCCP, Associate Professor, Department of Surgery, University of North Carolina School of Medicine; Staff Physician, Department of Surgery, UNC Lineberger Comprehensive Cancer Center, Chapel Hill, North Carolina
Jason M. Long, MD, MPH, FCCP, has disclosed no relevant financial relationships.
Carrie B. Lee, MD, MPH, Associate Professor, Department of Medicine, University of North Carolina, Chapel Hill; Medical Director, Clinical Protocol Office, UNC Lineberger Comprehensive Cancer Center, North Carolina Cancer Hospital, Chapel Hill, North Carolina
Carrie B. Lee, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as the chair of the Data and Safety Monitoring Board for: Delcath, Inc.
Nichole T. Tanner, MD, MSCR, FCCP, Associate Professor, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina
Nichole T. Tanner, MD, MSCR, FCCP, has disclosed no relevant financial relationships.
Numerous advances in diagnosis, staging, and treatment have evolved over the past several years for non-small cell lung cancer (NSCLC) patients. Because of the new developments, input from a team of specialists is required to treat each patient. Many hospitals have implemented a multidisciplinary team approach to treat these complex cases.
In this ReCAP, Dr Nicole Tanner, a pulmonary critical care specialist from the Medical University of South Carolina, hosts thoracic oncologist Dr Carrie Lee and thoracic surgeon Dr Jason Long, colleagues from the UNC Lineberger Comprehensive Cancer Center, in a discussion of the advantages of multidisciplinary teams in the treatment of NSCLC patients.
Their discussion ranges from identifying key multidisciplinary team members, individual practitioner responsibilities, benefits of multidisciplinary teams to patients and clinicians alike, and the advantages of incorporating telehealth visits into standard practice after the pandemic.
--
Jason M. Long, MD, MPH, FCCP, Associate Professor, Department of Surgery, University of North Carolina School of Medicine; Staff Physician, Department of Surgery, UNC Lineberger Comprehensive Cancer Center, Chapel Hill, North Carolina
Jason M. Long, MD, MPH, FCCP, has disclosed no relevant financial relationships.
Carrie B. Lee, MD, MPH, Associate Professor, Department of Medicine, University of North Carolina, Chapel Hill; Medical Director, Clinical Protocol Office, UNC Lineberger Comprehensive Cancer Center, North Carolina Cancer Hospital, Chapel Hill, North Carolina
Carrie B. Lee, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as the chair of the Data and Safety Monitoring Board for: Delcath, Inc.
Nichole T. Tanner, MD, MSCR, FCCP, Associate Professor, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina
Nichole T. Tanner, MD, MSCR, FCCP, has disclosed no relevant financial relationships.

DEMO: Dyspnea and intercostal retractions
The patient is probably having a delayed allergenic response to an indoor mold allergen. Early reactions can occur immediately following allergen inhalation but can also manifest 4-10 hours after exposure to wet and damp areas, such as bathrooms and basements.
Allergic asthma represents the most common asthma phenotype. The average age of onset is younger than that of nonallergic asthma, with allergens triggering exacerbations in 60%-90% of children compared with 50% of adults. Triggers can include mold spores, seasonal pollen, dust mites, and animal allergens. There is also an increased incidence of co-occurring allergic rhinoconjunctivitis and atopic dermatitis in patients with allergic asthma.
During an acute asthma exacerbation, lung examination findings may include wheezing, rhonchi, hyperinflation, or a prolonged expiratory phase. In children, supraclavicular and intercostal retractions and nasal flaring, as well as abdominal breathing, are particularly telling. Total serum immunoglobulin E levels are usually higher in allergic vs nonallergic asthma (> 100 IU), although this marker is not specific to asthma.
The allergic asthma phenotype usually responds to inhaled corticosteroid therapy. Although evidence suggests that allergic vs nonallergic asthma is less severe in many cases, compelling data point to a causal relationship between mold allergy and asthma severity in a subgroup of younger asthma patients; specifically, mold sensitization may be associated with asthma attacks requiring hospital admission in this population.
Although spirometry assessments should be obtained as the primary test to establish the asthma diagnosis, to test for allergic sensitivity to specific allergens in the environment, allergy skin tests and blood radioallergosorbent tests may be used. Up to 85% of patients with asthma demonstrate positive skin test results, and in children, this sensitization is reflective of disease activity. Such testing for allergen-specific immunoglobulin E is critical in prescribing allergen avoidance techniques and allergen immunotherapy regimens, although the method shows a relatively high false positive rate and should not be performed during an exacerbation.
The patient is probably having a delayed allergenic response to an indoor mold allergen. Early reactions can occur immediately following allergen inhalation but can also manifest 4-10 hours after exposure to wet and damp areas, such as bathrooms and basements.
Allergic asthma represents the most common asthma phenotype. The average age of onset is younger than that of nonallergic asthma, with allergens triggering exacerbations in 60%-90% of children compared with 50% of adults. Triggers can include mold spores, seasonal pollen, dust mites, and animal allergens. There is also an increased incidence of co-occurring allergic rhinoconjunctivitis and atopic dermatitis in patients with allergic asthma.
During an acute asthma exacerbation, lung examination findings may include wheezing, rhonchi, hyperinflation, or a prolonged expiratory phase. In children, supraclavicular and intercostal retractions and nasal flaring, as well as abdominal breathing, are particularly telling. Total serum immunoglobulin E levels are usually higher in allergic vs nonallergic asthma (> 100 IU), although this marker is not specific to asthma.
The allergic asthma phenotype usually responds to inhaled corticosteroid therapy. Although evidence suggests that allergic vs nonallergic asthma is less severe in many cases, compelling data point to a causal relationship between mold allergy and asthma severity in a subgroup of younger asthma patients; specifically, mold sensitization may be associated with asthma attacks requiring hospital admission in this population.
Although spirometry assessments should be obtained as the primary test to establish the asthma diagnosis, to test for allergic sensitivity to specific allergens in the environment, allergy skin tests and blood radioallergosorbent tests may be used. Up to 85% of patients with asthma demonstrate positive skin test results, and in children, this sensitization is reflective of disease activity. Such testing for allergen-specific immunoglobulin E is critical in prescribing allergen avoidance techniques and allergen immunotherapy regimens, although the method shows a relatively high false positive rate and should not be performed during an exacerbation.
The patient is probably having a delayed allergenic response to an indoor mold allergen. Early reactions can occur immediately following allergen inhalation but can also manifest 4-10 hours after exposure to wet and damp areas, such as bathrooms and basements.
Allergic asthma represents the most common asthma phenotype. The average age of onset is younger than that of nonallergic asthma, with allergens triggering exacerbations in 60%-90% of children compared with 50% of adults. Triggers can include mold spores, seasonal pollen, dust mites, and animal allergens. There is also an increased incidence of co-occurring allergic rhinoconjunctivitis and atopic dermatitis in patients with allergic asthma.
During an acute asthma exacerbation, lung examination findings may include wheezing, rhonchi, hyperinflation, or a prolonged expiratory phase. In children, supraclavicular and intercostal retractions and nasal flaring, as well as abdominal breathing, are particularly telling. Total serum immunoglobulin E levels are usually higher in allergic vs nonallergic asthma (> 100 IU), although this marker is not specific to asthma.
The allergic asthma phenotype usually responds to inhaled corticosteroid therapy. Although evidence suggests that allergic vs nonallergic asthma is less severe in many cases, compelling data point to a causal relationship between mold allergy and asthma severity in a subgroup of younger asthma patients; specifically, mold sensitization may be associated with asthma attacks requiring hospital admission in this population.
Although spirometry assessments should be obtained as the primary test to establish the asthma diagnosis, to test for allergic sensitivity to specific allergens in the environment, allergy skin tests and blood radioallergosorbent tests may be used. Up to 85% of patients with asthma demonstrate positive skin test results, and in children, this sensitization is reflective of disease activity. Such testing for allergen-specific immunoglobulin E is critical in prescribing allergen avoidance techniques and allergen immunotherapy regimens, although the method shows a relatively high false positive rate and should not be performed during an exacerbation.
A 17-year-old boy came to the hospital with shortness of breath and loud wheezing. His mother explained that earlier that day, he visited a friend’s house where they played video games together in the basement. When he returned home, he noticed that he was becoming breathless while talking. His chest radiograph findings were normal, but intercostal retractions were observed. His heart rate is 120 beats/minute.
DEMO: Persistent cough and dyspnea
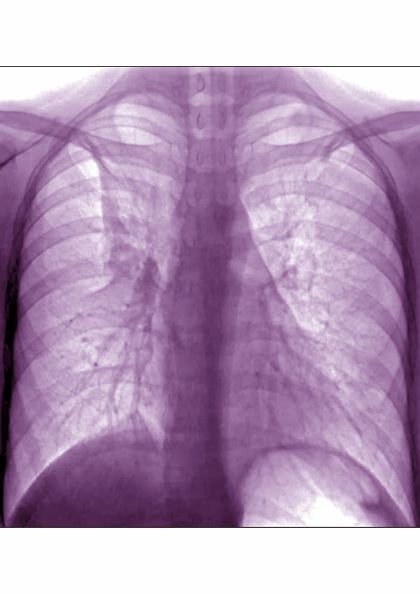
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
Based on the patient’s history of asthma, physical examination, and radiograph findings, a diagnosis of right middle lung collapse was determined. Right middle lobe syndrome (RMLS) refers to chronic or recurrent atelectasis in the right middle lobe and can stem from numerous etiologies. It is characterized by wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. Atelectasis typically occurs in the right middle lobe, but the lingula may be involved as well.
Asthma may predispose patients to atelectasis, resulting from bronchial inflammation that produces cellular debris, mucus plugs, and edema. Children are also more prone to developing atelectasis than adults because of smaller and more collapsible airways, among other features.
Lateral chest radiography is the most effective imaging technique in patients presenting with RMLS, revealing the condition's hallmark findings: a loss of volume in the right middle lobe and a blurred right heart border.
For appropriate treatment, a precise diagnosis of the pathology underlying the RMLS must be determined. This case represents a complication of uncontrolled asthma. The cornerstone of therapy is chest physical therapy and postural drainage, and the addition of mucolytics and dornase alfa may further clear airways. Children with asthma should be treated with aggressive anti-inflammatory therapy such as inhaled steroids, and the clinician may consider the addition of systemic steroids.
Cases of RMLS involving neoplastic origin or bronchiectasis should be given special consideration. Diagnosis can be confirmed with high-resolution chest CT scanning, which is safer for younger patients or those with asthma than traditional bronchography.
If a patient’s symptoms are not responsive to therapy or if the patient has predisposition to airway colonization, the clinician should perform a bronchoalveolar lavage culture to determine an appropriate antibiotic. In children with asthma, there is an association between right middle lobe collapse and infection.
Long-term follow-up has demonstrated that with treatment, RMLS typically resolves within 90 days. Soyer and colleagues also determined that baseline treatment of asthma with anti-inflammatory medications can accelerate the resolution of atelectasis. However, recurrent infections may cause parenchymal damage and bronchiectasis.
A 6-year-old girl came to the hospital with a persistent cough, episodes of dyspnea, and a slight fever. Her mother reported that she had been diagnosed with asthma when she was 4 years old. Despite strict adherence to anti-inflammatory medication, she experiences frequent symptoms, especially at night, and she had been hospitalized for an asthma exacerbation 8 months ago. Posterior-anterior chest radiograph revealed blurring of the right border of the heart.
Late-breaking news on trajectory of ADHD remission headlines world conference
Most patients will not make a full recovery from attention-deficit/hyperactivity disorder in adulthood. This late-breaking finding headlined the World Congress on ADHD – Virtual Event. Held under the specter of SARS-CoV-2, the virtual program delved into the latest research on ADHD pathophysiology, imaging, genetics, and issues on medical and psychiatric comorbidities.
However, one of the conference’s highlights was a piece of unpublished work on remission patterns by Margaret Sibley, PhD, associate professor of psychiatry and behavioral sciences at the University of Washington, Seattle.
Anywhere from 65% to 67% of young adults have desistant ADHD – meaning that they no longer meet criteria. Only up to 23% experience full remission, said Dr. Sibley during a special late-breaking session. All research on remission and most on persistence consider just one endpoint – nothing is known about longitudinal fluctuations in remission status over time.
Her research sought to answer a key question: Do people fully recover from ADHD?
Using data from the Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) Study, Dr. Sibley prospectively followed over 550 children aged 7-9.9 years with DSM-IV combined-type ADHD over 14 years, until 16 years after baseline, using interviews, questionnaires, and rating scales to track symptoms, impairment, and treatment history.
The researchers also came up with a “winning” definition for full remission, which included three or fewer symptoms of inattention and hyperactive impulsivity from all available reporters, negligible ADHD-related impairment based on preestablished impairment rating thresholds, and discontinuation of medication and behavioral treatments for at least a month prior to assessment.
In the longitudinal results, Dr. Sibley and colleagues reported that the majority (63.8%) demonstrated fluctuations between full or partial remission and ADHD recurrence. Only 9.1% sustained full remission over the course of the study. From these findings, ADHD appears to be a fluctuating disorder. While it continues into adulthood for most people, there may also be periods of remission or “good functioning.”
Most desistance from ADHD represents partial, not full remission, said Dr. Sibley. The results also show that recovery by young adulthood is very rare – most patients with remitted ADHD have recurrences.
These are important findings, said Luis Augusto Rohde, MD, PhD, who co-organized the congress’ scientific program committee with Manfred Gerlach, PhD. It shows that a patient’s ADHD may sometimes be more definitive and at other times, no clear phenotype expression emerges.
COVID’s influence
COVID-19 greatly influenced this year’s program’s agenda, said Dr. Rohde. “There’s a lot of evidence that ADHD patients are at greater risk for COVID-19, which is not a surprise,” said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry in Porto Alegre, Brazil.
ADHD is a combination of genetic liability and the demands of the environment. “In times like we are living in right now, if you have increasing demands and stress from the environment, you trigger symptoms in those even with lower genetic liability,” he said. ADHD’s pathophysiology involves attention and executive deficit disorder, which means these patients may not follow strategies to avoid infection.
This shows why COVID was so important to the discussion of program topics, he said.
Two experts addressed this subject head on in a point-counterpoint debate, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?” James Swanson, PhD, professor of pediatrics at the University of California, Irvine, projected that biological coeffects of COVID-19 will lead to ADHD symptoms, generating potentially 5 million new ADHD cases.
David Coghill, MBChB, MD, a professor of child adolescent mental health at the University of Melbourne, countered that not enough data are available yet to back this hypothesis. “Researchers are asking this question, but clinically we don’t know enough.”
While the COVID virus might not directly lead to more cases of ADHD, this could potentially happen indirectly through environmental agents of the pandemic, offered Dr. Rohde. “We’ve clearly seen in our appointments with families and children that they can’t face the amount of schooling and working from home,” he said.
Novel treatments
The conference also addressed new treatments and nonpharmacologic interventions in the pipeline for ADHD. “We had a chance to discuss the possibilities about new medications that address the problems in the current market and to show the potential usefulness of nonpharma interventions such as neuromodulations in ADHD,” said Dr. Rohde. Speakers discussed strategies ranging from family-based mindfulness interventions to oligoantigenic diets in children with ADHD.
Other researchers are looking at novel digital tools to help patients manage and treat ADHD. Adherence is a major problem in chronic disorders like hypertension, diabetes, epilepsy, and ADHD, said Dr. Rohde. “Due to ADHD symptomatology including inattention, novelty-seeking, executive deficits, and difficulties in persistence, it is an even bigger problem in this disorder.”
Speakers at the “ADHD in the digital age – From pitfalls to challenges” session discussed video game strategies to reduce ADHD impairment, and a texting app to improve adherence. Dr. Rohde talked about the FOCUS app, which fosters collaboration between patients, families, and caregivers to efficiently track ADHD symptoms and help customize treatments.
Studies suggest these tools can significantly improve adherence. They’re also well accepted by patients, said Dr. Rohde. While the expectations are high, digital interventions are not a substitute for medication. “More data is needed to include them as part of the clinical interventions for ADHD.”
Dr. Sibley received book royalties from Guilford Press. Dr. Rohde has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Bial, Medice, Novartis/Sandoz, Pfizer, and Shire/Takeda in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr. Rohde have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Novartis/Sandoz and Shire/Takeda. Dr. Rohde has received authorship royalties from Oxford Press and ArtMed and travel grants from Shire to take part in the 2018 APA annual meeting. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth of infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). He has received travel support from Medice and has done legal review for NLS. Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to the session debate on the pandemic.
Most patients will not make a full recovery from attention-deficit/hyperactivity disorder in adulthood. This late-breaking finding headlined the World Congress on ADHD – Virtual Event. Held under the specter of SARS-CoV-2, the virtual program delved into the latest research on ADHD pathophysiology, imaging, genetics, and issues on medical and psychiatric comorbidities.
However, one of the conference’s highlights was a piece of unpublished work on remission patterns by Margaret Sibley, PhD, associate professor of psychiatry and behavioral sciences at the University of Washington, Seattle.
Anywhere from 65% to 67% of young adults have desistant ADHD – meaning that they no longer meet criteria. Only up to 23% experience full remission, said Dr. Sibley during a special late-breaking session. All research on remission and most on persistence consider just one endpoint – nothing is known about longitudinal fluctuations in remission status over time.
Her research sought to answer a key question: Do people fully recover from ADHD?
Using data from the Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) Study, Dr. Sibley prospectively followed over 550 children aged 7-9.9 years with DSM-IV combined-type ADHD over 14 years, until 16 years after baseline, using interviews, questionnaires, and rating scales to track symptoms, impairment, and treatment history.
The researchers also came up with a “winning” definition for full remission, which included three or fewer symptoms of inattention and hyperactive impulsivity from all available reporters, negligible ADHD-related impairment based on preestablished impairment rating thresholds, and discontinuation of medication and behavioral treatments for at least a month prior to assessment.
In the longitudinal results, Dr. Sibley and colleagues reported that the majority (63.8%) demonstrated fluctuations between full or partial remission and ADHD recurrence. Only 9.1% sustained full remission over the course of the study. From these findings, ADHD appears to be a fluctuating disorder. While it continues into adulthood for most people, there may also be periods of remission or “good functioning.”
Most desistance from ADHD represents partial, not full remission, said Dr. Sibley. The results also show that recovery by young adulthood is very rare – most patients with remitted ADHD have recurrences.
These are important findings, said Luis Augusto Rohde, MD, PhD, who co-organized the congress’ scientific program committee with Manfred Gerlach, PhD. It shows that a patient’s ADHD may sometimes be more definitive and at other times, no clear phenotype expression emerges.
COVID’s influence
COVID-19 greatly influenced this year’s program’s agenda, said Dr. Rohde. “There’s a lot of evidence that ADHD patients are at greater risk for COVID-19, which is not a surprise,” said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry in Porto Alegre, Brazil.
ADHD is a combination of genetic liability and the demands of the environment. “In times like we are living in right now, if you have increasing demands and stress from the environment, you trigger symptoms in those even with lower genetic liability,” he said. ADHD’s pathophysiology involves attention and executive deficit disorder, which means these patients may not follow strategies to avoid infection.
This shows why COVID was so important to the discussion of program topics, he said.
Two experts addressed this subject head on in a point-counterpoint debate, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?” James Swanson, PhD, professor of pediatrics at the University of California, Irvine, projected that biological coeffects of COVID-19 will lead to ADHD symptoms, generating potentially 5 million new ADHD cases.
David Coghill, MBChB, MD, a professor of child adolescent mental health at the University of Melbourne, countered that not enough data are available yet to back this hypothesis. “Researchers are asking this question, but clinically we don’t know enough.”
While the COVID virus might not directly lead to more cases of ADHD, this could potentially happen indirectly through environmental agents of the pandemic, offered Dr. Rohde. “We’ve clearly seen in our appointments with families and children that they can’t face the amount of schooling and working from home,” he said.
Novel treatments
The conference also addressed new treatments and nonpharmacologic interventions in the pipeline for ADHD. “We had a chance to discuss the possibilities about new medications that address the problems in the current market and to show the potential usefulness of nonpharma interventions such as neuromodulations in ADHD,” said Dr. Rohde. Speakers discussed strategies ranging from family-based mindfulness interventions to oligoantigenic diets in children with ADHD.
Other researchers are looking at novel digital tools to help patients manage and treat ADHD. Adherence is a major problem in chronic disorders like hypertension, diabetes, epilepsy, and ADHD, said Dr. Rohde. “Due to ADHD symptomatology including inattention, novelty-seeking, executive deficits, and difficulties in persistence, it is an even bigger problem in this disorder.”
Speakers at the “ADHD in the digital age – From pitfalls to challenges” session discussed video game strategies to reduce ADHD impairment, and a texting app to improve adherence. Dr. Rohde talked about the FOCUS app, which fosters collaboration between patients, families, and caregivers to efficiently track ADHD symptoms and help customize treatments.
Studies suggest these tools can significantly improve adherence. They’re also well accepted by patients, said Dr. Rohde. While the expectations are high, digital interventions are not a substitute for medication. “More data is needed to include them as part of the clinical interventions for ADHD.”
Dr. Sibley received book royalties from Guilford Press. Dr. Rohde has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Bial, Medice, Novartis/Sandoz, Pfizer, and Shire/Takeda in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr. Rohde have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Novartis/Sandoz and Shire/Takeda. Dr. Rohde has received authorship royalties from Oxford Press and ArtMed and travel grants from Shire to take part in the 2018 APA annual meeting. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth of infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). He has received travel support from Medice and has done legal review for NLS. Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to the session debate on the pandemic.
Most patients will not make a full recovery from attention-deficit/hyperactivity disorder in adulthood. This late-breaking finding headlined the World Congress on ADHD – Virtual Event. Held under the specter of SARS-CoV-2, the virtual program delved into the latest research on ADHD pathophysiology, imaging, genetics, and issues on medical and psychiatric comorbidities.
However, one of the conference’s highlights was a piece of unpublished work on remission patterns by Margaret Sibley, PhD, associate professor of psychiatry and behavioral sciences at the University of Washington, Seattle.
Anywhere from 65% to 67% of young adults have desistant ADHD – meaning that they no longer meet criteria. Only up to 23% experience full remission, said Dr. Sibley during a special late-breaking session. All research on remission and most on persistence consider just one endpoint – nothing is known about longitudinal fluctuations in remission status over time.
Her research sought to answer a key question: Do people fully recover from ADHD?
Using data from the Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) Study, Dr. Sibley prospectively followed over 550 children aged 7-9.9 years with DSM-IV combined-type ADHD over 14 years, until 16 years after baseline, using interviews, questionnaires, and rating scales to track symptoms, impairment, and treatment history.
The researchers also came up with a “winning” definition for full remission, which included three or fewer symptoms of inattention and hyperactive impulsivity from all available reporters, negligible ADHD-related impairment based on preestablished impairment rating thresholds, and discontinuation of medication and behavioral treatments for at least a month prior to assessment.
In the longitudinal results, Dr. Sibley and colleagues reported that the majority (63.8%) demonstrated fluctuations between full or partial remission and ADHD recurrence. Only 9.1% sustained full remission over the course of the study. From these findings, ADHD appears to be a fluctuating disorder. While it continues into adulthood for most people, there may also be periods of remission or “good functioning.”
Most desistance from ADHD represents partial, not full remission, said Dr. Sibley. The results also show that recovery by young adulthood is very rare – most patients with remitted ADHD have recurrences.
These are important findings, said Luis Augusto Rohde, MD, PhD, who co-organized the congress’ scientific program committee with Manfred Gerlach, PhD. It shows that a patient’s ADHD may sometimes be more definitive and at other times, no clear phenotype expression emerges.
COVID’s influence
COVID-19 greatly influenced this year’s program’s agenda, said Dr. Rohde. “There’s a lot of evidence that ADHD patients are at greater risk for COVID-19, which is not a surprise,” said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry in Porto Alegre, Brazil.
ADHD is a combination of genetic liability and the demands of the environment. “In times like we are living in right now, if you have increasing demands and stress from the environment, you trigger symptoms in those even with lower genetic liability,” he said. ADHD’s pathophysiology involves attention and executive deficit disorder, which means these patients may not follow strategies to avoid infection.
This shows why COVID was so important to the discussion of program topics, he said.
Two experts addressed this subject head on in a point-counterpoint debate, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?” James Swanson, PhD, professor of pediatrics at the University of California, Irvine, projected that biological coeffects of COVID-19 will lead to ADHD symptoms, generating potentially 5 million new ADHD cases.
David Coghill, MBChB, MD, a professor of child adolescent mental health at the University of Melbourne, countered that not enough data are available yet to back this hypothesis. “Researchers are asking this question, but clinically we don’t know enough.”
While the COVID virus might not directly lead to more cases of ADHD, this could potentially happen indirectly through environmental agents of the pandemic, offered Dr. Rohde. “We’ve clearly seen in our appointments with families and children that they can’t face the amount of schooling and working from home,” he said.
Novel treatments
The conference also addressed new treatments and nonpharmacologic interventions in the pipeline for ADHD. “We had a chance to discuss the possibilities about new medications that address the problems in the current market and to show the potential usefulness of nonpharma interventions such as neuromodulations in ADHD,” said Dr. Rohde. Speakers discussed strategies ranging from family-based mindfulness interventions to oligoantigenic diets in children with ADHD.
Other researchers are looking at novel digital tools to help patients manage and treat ADHD. Adherence is a major problem in chronic disorders like hypertension, diabetes, epilepsy, and ADHD, said Dr. Rohde. “Due to ADHD symptomatology including inattention, novelty-seeking, executive deficits, and difficulties in persistence, it is an even bigger problem in this disorder.”
Speakers at the “ADHD in the digital age – From pitfalls to challenges” session discussed video game strategies to reduce ADHD impairment, and a texting app to improve adherence. Dr. Rohde talked about the FOCUS app, which fosters collaboration between patients, families, and caregivers to efficiently track ADHD symptoms and help customize treatments.
Studies suggest these tools can significantly improve adherence. They’re also well accepted by patients, said Dr. Rohde. While the expectations are high, digital interventions are not a substitute for medication. “More data is needed to include them as part of the clinical interventions for ADHD.”
Dr. Sibley received book royalties from Guilford Press. Dr. Rohde has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Bial, Medice, Novartis/Sandoz, Pfizer, and Shire/Takeda in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr. Rohde have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Novartis/Sandoz and Shire/Takeda. Dr. Rohde has received authorship royalties from Oxford Press and ArtMed and travel grants from Shire to take part in the 2018 APA annual meeting. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth of infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). He has received travel support from Medice and has done legal review for NLS. Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to the session debate on the pandemic.
FROM ADHD 2021
HHS prohibits discrimination against LGBTQ patients: Action reverses Trump-era policy
The Biden administration is reversing a Trump-era policy that allowed health care providers to bar services to lesbian, gay, bisexual, transgender, or queer (LGBTQ) patients.
The U.S. Department of Health and Human Services gave notice on Monday that it would interpret the Affordable Care Act’s Section 1557 – which bars discrimination on the basis of sex – to include discrimination on the basis of sexual orientation or gender identity. The department said its position is consistent with a June 2020 U.S. Supreme Court ruling in Bostock v. Clayton County, GA. The ruling determined that the Civil Rights Act’s prohibition of employment discrimination on the basis of sex includes sexual orientation and gender identity.
“The mission of our Department is to enhance the health and well-being of all Americans, no matter their gender identity or sexual orientation,” said HHS Assistant Secretary for Health Rachel Levine, MD, in a statement released Monday.
“All people need access to health care services to fix a broken bone, protect their heart health, and screen for cancer risk,” she said. “No one should be discriminated against when seeking medical services because of who they are.”
Many physician organizations applauded the decision.
“The Biden administration did the right thing by terminating a short-lived effort to allow discrimination based on gender or sexual orientation when seeking health care,” said Susan R. Bailey, MD, president of the American Medical Association, in a statement.
When, in 2019, the Trump administration proposed to allow providers to deny care to LGBTQ people, the AMA said in a letter to the HHS that its interpretation “was contrary to the intent and the plain language of the law.”
Now, said Bailey, the AMA welcomes the Biden administration’s interpretation. It “is a victory for health equity and ends a dismal chapter in which a federal agency sought to remove civil rights protections,” she said.
An alliance of patient groups – including the American Cancer Society, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association, the Epilepsy Foundation, the National Multiple Sclerosis Society, and the National Organization for Rare Disorders – also applauded the new policy. “This community already faces significant health disparities,” the groups noted in a statement. People with chronic illness such as HIV and cancer “need to be able to access care quickly and without fear of discrimination,” they said.
The groups had filed a friend of the court brief in a case against the Trump administration rule.
“We welcome this positive step to ensure access is preserved without hindrance, as intended by the health care law,” they said.
Twenty-two states and Washington, D.C. – led by former California Attorney General Xavier Becerra, who is now HHS secretary – sued the Trump administration in July 2020, aiming to overturn the rule.
Chase Strangio, deputy director for Trans Justice with the American Civil Liberties Union LGBTQ & HIV Project, noted that the HHS announcement was crucial in the face of efforts in multiple states to bar health care for transgender youth. “The Biden administration has affirmed what courts have said for decades: Discrimination against LGBTQ people is against the law. It also affirms what transgender people have long said: Gender-affirming care is life-saving care,” he said in a statement.
Lambda Legal, which led another lawsuit against the Trump administration rule, said it welcomed the HHS action but noted in a statement by the organization’s senior attorney, Omar Gonzalez-Pagan, that it “does not address significant aspects of the Trump-era rule that we and others have challenged in court.”
The Trump rule also “limited the remedies available to people who face health disparities, limited access to health care for people with Limited English Proficiency, unlawfully incorporated religious exemptions, and dramatically reduced the number of health care entities and insurance subject to the rule, all of which today’s action does not address,” said Gonzalez-Pagan.
“We encourage Secretary Xavier Becerra and the Biden administration to take additional steps to ensure that all LGBTQ people are completely covered wherever and whenever they may encounter discrimination during some of the most delicate and precarious moments of their lives: When seeking health care,” he said.
A version of this article first appeared on Medscape.com.
The Biden administration is reversing a Trump-era policy that allowed health care providers to bar services to lesbian, gay, bisexual, transgender, or queer (LGBTQ) patients.
The U.S. Department of Health and Human Services gave notice on Monday that it would interpret the Affordable Care Act’s Section 1557 – which bars discrimination on the basis of sex – to include discrimination on the basis of sexual orientation or gender identity. The department said its position is consistent with a June 2020 U.S. Supreme Court ruling in Bostock v. Clayton County, GA. The ruling determined that the Civil Rights Act’s prohibition of employment discrimination on the basis of sex includes sexual orientation and gender identity.
“The mission of our Department is to enhance the health and well-being of all Americans, no matter their gender identity or sexual orientation,” said HHS Assistant Secretary for Health Rachel Levine, MD, in a statement released Monday.
“All people need access to health care services to fix a broken bone, protect their heart health, and screen for cancer risk,” she said. “No one should be discriminated against when seeking medical services because of who they are.”
Many physician organizations applauded the decision.
“The Biden administration did the right thing by terminating a short-lived effort to allow discrimination based on gender or sexual orientation when seeking health care,” said Susan R. Bailey, MD, president of the American Medical Association, in a statement.
When, in 2019, the Trump administration proposed to allow providers to deny care to LGBTQ people, the AMA said in a letter to the HHS that its interpretation “was contrary to the intent and the plain language of the law.”
Now, said Bailey, the AMA welcomes the Biden administration’s interpretation. It “is a victory for health equity and ends a dismal chapter in which a federal agency sought to remove civil rights protections,” she said.
An alliance of patient groups – including the American Cancer Society, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association, the Epilepsy Foundation, the National Multiple Sclerosis Society, and the National Organization for Rare Disorders – also applauded the new policy. “This community already faces significant health disparities,” the groups noted in a statement. People with chronic illness such as HIV and cancer “need to be able to access care quickly and without fear of discrimination,” they said.
The groups had filed a friend of the court brief in a case against the Trump administration rule.
“We welcome this positive step to ensure access is preserved without hindrance, as intended by the health care law,” they said.
Twenty-two states and Washington, D.C. – led by former California Attorney General Xavier Becerra, who is now HHS secretary – sued the Trump administration in July 2020, aiming to overturn the rule.
Chase Strangio, deputy director for Trans Justice with the American Civil Liberties Union LGBTQ & HIV Project, noted that the HHS announcement was crucial in the face of efforts in multiple states to bar health care for transgender youth. “The Biden administration has affirmed what courts have said for decades: Discrimination against LGBTQ people is against the law. It also affirms what transgender people have long said: Gender-affirming care is life-saving care,” he said in a statement.
Lambda Legal, which led another lawsuit against the Trump administration rule, said it welcomed the HHS action but noted in a statement by the organization’s senior attorney, Omar Gonzalez-Pagan, that it “does not address significant aspects of the Trump-era rule that we and others have challenged in court.”
The Trump rule also “limited the remedies available to people who face health disparities, limited access to health care for people with Limited English Proficiency, unlawfully incorporated religious exemptions, and dramatically reduced the number of health care entities and insurance subject to the rule, all of which today’s action does not address,” said Gonzalez-Pagan.
“We encourage Secretary Xavier Becerra and the Biden administration to take additional steps to ensure that all LGBTQ people are completely covered wherever and whenever they may encounter discrimination during some of the most delicate and precarious moments of their lives: When seeking health care,” he said.
A version of this article first appeared on Medscape.com.
The Biden administration is reversing a Trump-era policy that allowed health care providers to bar services to lesbian, gay, bisexual, transgender, or queer (LGBTQ) patients.
The U.S. Department of Health and Human Services gave notice on Monday that it would interpret the Affordable Care Act’s Section 1557 – which bars discrimination on the basis of sex – to include discrimination on the basis of sexual orientation or gender identity. The department said its position is consistent with a June 2020 U.S. Supreme Court ruling in Bostock v. Clayton County, GA. The ruling determined that the Civil Rights Act’s prohibition of employment discrimination on the basis of sex includes sexual orientation and gender identity.
“The mission of our Department is to enhance the health and well-being of all Americans, no matter their gender identity or sexual orientation,” said HHS Assistant Secretary for Health Rachel Levine, MD, in a statement released Monday.
“All people need access to health care services to fix a broken bone, protect their heart health, and screen for cancer risk,” she said. “No one should be discriminated against when seeking medical services because of who they are.”
Many physician organizations applauded the decision.
“The Biden administration did the right thing by terminating a short-lived effort to allow discrimination based on gender or sexual orientation when seeking health care,” said Susan R. Bailey, MD, president of the American Medical Association, in a statement.
When, in 2019, the Trump administration proposed to allow providers to deny care to LGBTQ people, the AMA said in a letter to the HHS that its interpretation “was contrary to the intent and the plain language of the law.”
Now, said Bailey, the AMA welcomes the Biden administration’s interpretation. It “is a victory for health equity and ends a dismal chapter in which a federal agency sought to remove civil rights protections,” she said.
An alliance of patient groups – including the American Cancer Society, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association, the Epilepsy Foundation, the National Multiple Sclerosis Society, and the National Organization for Rare Disorders – also applauded the new policy. “This community already faces significant health disparities,” the groups noted in a statement. People with chronic illness such as HIV and cancer “need to be able to access care quickly and without fear of discrimination,” they said.
The groups had filed a friend of the court brief in a case against the Trump administration rule.
“We welcome this positive step to ensure access is preserved without hindrance, as intended by the health care law,” they said.
Twenty-two states and Washington, D.C. – led by former California Attorney General Xavier Becerra, who is now HHS secretary – sued the Trump administration in July 2020, aiming to overturn the rule.
Chase Strangio, deputy director for Trans Justice with the American Civil Liberties Union LGBTQ & HIV Project, noted that the HHS announcement was crucial in the face of efforts in multiple states to bar health care for transgender youth. “The Biden administration has affirmed what courts have said for decades: Discrimination against LGBTQ people is against the law. It also affirms what transgender people have long said: Gender-affirming care is life-saving care,” he said in a statement.
Lambda Legal, which led another lawsuit against the Trump administration rule, said it welcomed the HHS action but noted in a statement by the organization’s senior attorney, Omar Gonzalez-Pagan, that it “does not address significant aspects of the Trump-era rule that we and others have challenged in court.”
The Trump rule also “limited the remedies available to people who face health disparities, limited access to health care for people with Limited English Proficiency, unlawfully incorporated religious exemptions, and dramatically reduced the number of health care entities and insurance subject to the rule, all of which today’s action does not address,” said Gonzalez-Pagan.
“We encourage Secretary Xavier Becerra and the Biden administration to take additional steps to ensure that all LGBTQ people are completely covered wherever and whenever they may encounter discrimination during some of the most delicate and precarious moments of their lives: When seeking health care,” he said.
A version of this article first appeared on Medscape.com.