User login
Exercise and diet intervention fail to improve fatigue in breast cancer patients on chemotherapy
Key clinical point: An exercise and diet intervention had no significant impact on fatigue in women with breast cancer who were undergoing chemotherapy or radiotherapy.
Major finding: Based on the general cancer-related fatigue score using the MFI-20 questionnaire, general fatigue levels were not significantly different between groups of breast cancer patients randomized to an Adapted Physical Activity Diet (APAD) program and controls (P = 0.274).
Study details: The data come from a randomized, controlled trial of 360 adult women with early breast cancer designed to evaluate the impact of an exercise and nutrition intervention on fatigue during 6 months of chemotherapy and radiotherapy.
Disclosures: The study was supported by the INCa-DGOS and by a Montpellier Cancer SIRIC grant. The researchers had no financial conflicts to disclose.
Citation: Jacot W et al. Nutrients. 2020 Oct 9. doi: 10.3390/nu12103081.
Key clinical point: An exercise and diet intervention had no significant impact on fatigue in women with breast cancer who were undergoing chemotherapy or radiotherapy.
Major finding: Based on the general cancer-related fatigue score using the MFI-20 questionnaire, general fatigue levels were not significantly different between groups of breast cancer patients randomized to an Adapted Physical Activity Diet (APAD) program and controls (P = 0.274).
Study details: The data come from a randomized, controlled trial of 360 adult women with early breast cancer designed to evaluate the impact of an exercise and nutrition intervention on fatigue during 6 months of chemotherapy and radiotherapy.
Disclosures: The study was supported by the INCa-DGOS and by a Montpellier Cancer SIRIC grant. The researchers had no financial conflicts to disclose.
Citation: Jacot W et al. Nutrients. 2020 Oct 9. doi: 10.3390/nu12103081.
Key clinical point: An exercise and diet intervention had no significant impact on fatigue in women with breast cancer who were undergoing chemotherapy or radiotherapy.
Major finding: Based on the general cancer-related fatigue score using the MFI-20 questionnaire, general fatigue levels were not significantly different between groups of breast cancer patients randomized to an Adapted Physical Activity Diet (APAD) program and controls (P = 0.274).
Study details: The data come from a randomized, controlled trial of 360 adult women with early breast cancer designed to evaluate the impact of an exercise and nutrition intervention on fatigue during 6 months of chemotherapy and radiotherapy.
Disclosures: The study was supported by the INCa-DGOS and by a Montpellier Cancer SIRIC grant. The researchers had no financial conflicts to disclose.
Citation: Jacot W et al. Nutrients. 2020 Oct 9. doi: 10.3390/nu12103081.
DNA-based model predicts overall survival in breast cancer
Key clinical point: A prognostic signature including 8 DNA repair-related genes (MDC1, RPA3, MED17, DDB2, SFPQ, XRCC4, CYP19A1, and PARP3) predicted overall survival in breast cancer patients
Major finding: The areas under the curve were for 3-year survival and 5-year survival were 0.717 and 0.772, respectively, in the GSE9893 data set, and 0.691 and 0.718, respectively, in the GSE42568 data set.
Study details: The data come from 1,096 women with breast cancer; gene expression profiles and clinical data were collected from a Chinese health database between October 9, 2019, and February 3, 2020.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Zhang D et al. JAMA Netw Open. 2020 Oct 5. doi:10.1001/jamanetworkopen.2020.14622.
Key clinical point: A prognostic signature including 8 DNA repair-related genes (MDC1, RPA3, MED17, DDB2, SFPQ, XRCC4, CYP19A1, and PARP3) predicted overall survival in breast cancer patients
Major finding: The areas under the curve were for 3-year survival and 5-year survival were 0.717 and 0.772, respectively, in the GSE9893 data set, and 0.691 and 0.718, respectively, in the GSE42568 data set.
Study details: The data come from 1,096 women with breast cancer; gene expression profiles and clinical data were collected from a Chinese health database between October 9, 2019, and February 3, 2020.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Zhang D et al. JAMA Netw Open. 2020 Oct 5. doi:10.1001/jamanetworkopen.2020.14622.
Key clinical point: A prognostic signature including 8 DNA repair-related genes (MDC1, RPA3, MED17, DDB2, SFPQ, XRCC4, CYP19A1, and PARP3) predicted overall survival in breast cancer patients
Major finding: The areas under the curve were for 3-year survival and 5-year survival were 0.717 and 0.772, respectively, in the GSE9893 data set, and 0.691 and 0.718, respectively, in the GSE42568 data set.
Study details: The data come from 1,096 women with breast cancer; gene expression profiles and clinical data were collected from a Chinese health database between October 9, 2019, and February 3, 2020.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Zhang D et al. JAMA Netw Open. 2020 Oct 5. doi:10.1001/jamanetworkopen.2020.14622.
Textured breast implants may raise risk of cancer relapse
Key clinical point: The use of textured implants in reconstructive surgery after breast cancer mastectomy was significantly associated with lower rates of disease-free survival compared to smooth implants, but no difference was noted in local and regional recurrence-free survival based on implant texture.
Major finding: Rates of disease-free survival were significantly lower among breast cancer patients who received textured breast implants compared to those who received smooth implants (hazard ratio 3.054); the association was even stronger among patients with stage II or III tumors (HR 8.874).
Study details: The data come from a cohort study of 650 women representing 687 cases of breast cancer who were treated at a single center in South Korea between January 1, 2011, and December 31, 2016.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Lee K-T et al. JAMA Surg. 2020 Oct 7. doi: 10.1001/jamasurg.2020.4124.
Key clinical point: The use of textured implants in reconstructive surgery after breast cancer mastectomy was significantly associated with lower rates of disease-free survival compared to smooth implants, but no difference was noted in local and regional recurrence-free survival based on implant texture.
Major finding: Rates of disease-free survival were significantly lower among breast cancer patients who received textured breast implants compared to those who received smooth implants (hazard ratio 3.054); the association was even stronger among patients with stage II or III tumors (HR 8.874).
Study details: The data come from a cohort study of 650 women representing 687 cases of breast cancer who were treated at a single center in South Korea between January 1, 2011, and December 31, 2016.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Lee K-T et al. JAMA Surg. 2020 Oct 7. doi: 10.1001/jamasurg.2020.4124.
Key clinical point: The use of textured implants in reconstructive surgery after breast cancer mastectomy was significantly associated with lower rates of disease-free survival compared to smooth implants, but no difference was noted in local and regional recurrence-free survival based on implant texture.
Major finding: Rates of disease-free survival were significantly lower among breast cancer patients who received textured breast implants compared to those who received smooth implants (hazard ratio 3.054); the association was even stronger among patients with stage II or III tumors (HR 8.874).
Study details: The data come from a cohort study of 650 women representing 687 cases of breast cancer who were treated at a single center in South Korea between January 1, 2011, and December 31, 2016.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Lee K-T et al. JAMA Surg. 2020 Oct 7. doi: 10.1001/jamasurg.2020.4124.
Vacuum-assisted biopsy predicts post-treatment residual disease in breast cancer
Key clinical point: Vacuum-assisted biopsy accurately predicted residual disease in breast cancer patients after neoadjuvant chemotherapy.
Major finding: The overall false negative rate using vacuum-assisted biopsy (VAB) was 18.7%; in a subgroup of patients with a complete/partial response and imaging abnormalities of 2 cm or smaller, the false negative rate was 3.2% and the negative predictive value was 97.4% for an overall accuracy of 89.5% with VAB.
Study details: The data come from a diagnostic study of 166 women with breast cancer who received neoadjuvant chemotherapy followed by image-guided biopsy.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Tasoulis MK et al. JAMA Surg. 2020 Oct 7. doi: 10.1001/jamasurg.2020.4103.
Key clinical point: Vacuum-assisted biopsy accurately predicted residual disease in breast cancer patients after neoadjuvant chemotherapy.
Major finding: The overall false negative rate using vacuum-assisted biopsy (VAB) was 18.7%; in a subgroup of patients with a complete/partial response and imaging abnormalities of 2 cm or smaller, the false negative rate was 3.2% and the negative predictive value was 97.4% for an overall accuracy of 89.5% with VAB.
Study details: The data come from a diagnostic study of 166 women with breast cancer who received neoadjuvant chemotherapy followed by image-guided biopsy.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Tasoulis MK et al. JAMA Surg. 2020 Oct 7. doi: 10.1001/jamasurg.2020.4103.
Key clinical point: Vacuum-assisted biopsy accurately predicted residual disease in breast cancer patients after neoadjuvant chemotherapy.
Major finding: The overall false negative rate using vacuum-assisted biopsy (VAB) was 18.7%; in a subgroup of patients with a complete/partial response and imaging abnormalities of 2 cm or smaller, the false negative rate was 3.2% and the negative predictive value was 97.4% for an overall accuracy of 89.5% with VAB.
Study details: The data come from a diagnostic study of 166 women with breast cancer who received neoadjuvant chemotherapy followed by image-guided biopsy.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Tasoulis MK et al. JAMA Surg. 2020 Oct 7. doi: 10.1001/jamasurg.2020.4103.
Immediate breast reconstruction after chemotherapy doesn’t hurt survival
Key clinical point: Outcomes including recurrence, disease-free survival, and overall survival were similar for breast cancer patients who underwent immediate breast reconstruction (IBR) with nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) following neoadjuvant chemotherapy.
Major finding: Breast cancer patients who underwent IBR with NSM/SSM as surgical treatment showed similar rates of overall survival at 5 years compared to those who underwent conventional mastectomy (92.0% vs. 89.3%).
Study details: The data come from a retrospective, case-control study of 1,266 breast cancer patients who underwent neoadjuvant chemotherapy followed by immediate breast reconstruction or conventional mastectomy at a single center between January 1, 2010, and November 30, 2016.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Wu Z-Y et al. JAMA Surg. 2020 Oct 14. doi: 10.1001/jamasurg.2020.4132.
Key clinical point: Outcomes including recurrence, disease-free survival, and overall survival were similar for breast cancer patients who underwent immediate breast reconstruction (IBR) with nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) following neoadjuvant chemotherapy.
Major finding: Breast cancer patients who underwent IBR with NSM/SSM as surgical treatment showed similar rates of overall survival at 5 years compared to those who underwent conventional mastectomy (92.0% vs. 89.3%).
Study details: The data come from a retrospective, case-control study of 1,266 breast cancer patients who underwent neoadjuvant chemotherapy followed by immediate breast reconstruction or conventional mastectomy at a single center between January 1, 2010, and November 30, 2016.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Wu Z-Y et al. JAMA Surg. 2020 Oct 14. doi: 10.1001/jamasurg.2020.4132.
Key clinical point: Outcomes including recurrence, disease-free survival, and overall survival were similar for breast cancer patients who underwent immediate breast reconstruction (IBR) with nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) following neoadjuvant chemotherapy.
Major finding: Breast cancer patients who underwent IBR with NSM/SSM as surgical treatment showed similar rates of overall survival at 5 years compared to those who underwent conventional mastectomy (92.0% vs. 89.3%).
Study details: The data come from a retrospective, case-control study of 1,266 breast cancer patients who underwent neoadjuvant chemotherapy followed by immediate breast reconstruction or conventional mastectomy at a single center between January 1, 2010, and November 30, 2016.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Citation: Wu Z-Y et al. JAMA Surg. 2020 Oct 14. doi: 10.1001/jamasurg.2020.4132.
Black women show higher rates of three breast cancer subtypes
Key clinical point: Breast cancer subtypes may be associated with race and ethnicity; certain subtypes were significantly more common in Black women and infiltrating duct carcinoma compared to White women, but lobular carcinoma, and tubular adenocarcinoma were less common in Hispanic women compared to nonHispanic White women.
Major finding: The incidence of HR-negative and ERBB2-positive; HR-positive and ERBB2-positive; and triple negative breast cancer was significantly higher among Black women compared to nonHispanic White women, but the incidence of the HR-positive and ERBB2-negative subtype in Black women was lower (incidence rate ratios of 1.12, 1.46, 2.07, and 0.86, respectively).
Study details: The data come from a population-based cohort study of 239,211 women with breast cancer diagnosed between January 1, 2010, and December 31, 2015.
Disclosures: The study was supported by grants to the researchers from several organizations including the Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Special Research Fund for Central Universities, Peking Union Medical College, the Beijing Hope Run Special Fund of Cancer Foundation of China, and the PhD Innovation Fund of Cancer Hospital, Chinese Academy of Medical Sciences. The researchers had no financial conflicts to disclose.
Citation: Kong X et al. JAMA Netw Open. 2020 Oct 19. doi:10.1001/jamanetworkopen.2020.20303.
Key clinical point: Breast cancer subtypes may be associated with race and ethnicity; certain subtypes were significantly more common in Black women and infiltrating duct carcinoma compared to White women, but lobular carcinoma, and tubular adenocarcinoma were less common in Hispanic women compared to nonHispanic White women.
Major finding: The incidence of HR-negative and ERBB2-positive; HR-positive and ERBB2-positive; and triple negative breast cancer was significantly higher among Black women compared to nonHispanic White women, but the incidence of the HR-positive and ERBB2-negative subtype in Black women was lower (incidence rate ratios of 1.12, 1.46, 2.07, and 0.86, respectively).
Study details: The data come from a population-based cohort study of 239,211 women with breast cancer diagnosed between January 1, 2010, and December 31, 2015.
Disclosures: The study was supported by grants to the researchers from several organizations including the Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Special Research Fund for Central Universities, Peking Union Medical College, the Beijing Hope Run Special Fund of Cancer Foundation of China, and the PhD Innovation Fund of Cancer Hospital, Chinese Academy of Medical Sciences. The researchers had no financial conflicts to disclose.
Citation: Kong X et al. JAMA Netw Open. 2020 Oct 19. doi:10.1001/jamanetworkopen.2020.20303.
Key clinical point: Breast cancer subtypes may be associated with race and ethnicity; certain subtypes were significantly more common in Black women and infiltrating duct carcinoma compared to White women, but lobular carcinoma, and tubular adenocarcinoma were less common in Hispanic women compared to nonHispanic White women.
Major finding: The incidence of HR-negative and ERBB2-positive; HR-positive and ERBB2-positive; and triple negative breast cancer was significantly higher among Black women compared to nonHispanic White women, but the incidence of the HR-positive and ERBB2-negative subtype in Black women was lower (incidence rate ratios of 1.12, 1.46, 2.07, and 0.86, respectively).
Study details: The data come from a population-based cohort study of 239,211 women with breast cancer diagnosed between January 1, 2010, and December 31, 2015.
Disclosures: The study was supported by grants to the researchers from several organizations including the Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Special Research Fund for Central Universities, Peking Union Medical College, the Beijing Hope Run Special Fund of Cancer Foundation of China, and the PhD Innovation Fund of Cancer Hospital, Chinese Academy of Medical Sciences. The researchers had no financial conflicts to disclose.
Citation: Kong X et al. JAMA Netw Open. 2020 Oct 19. doi:10.1001/jamanetworkopen.2020.20303.
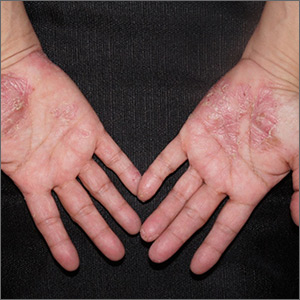
Painful pustules on hands
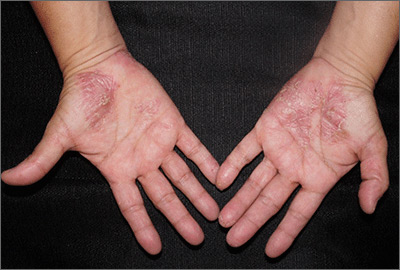
Close inspection of the plaques revealed multiple small pustules and mahogany colored macules on a broad, well-demarcated scaly red plaque. This pattern was consistent with palmoplantar pustular psoriasis.
Palmoplantar psoriasis is a chronic disease that may manifest at any age and in both sexes. The diagnosis may be clinical, as it was in this case. Bacterial culture of a pustule may demonstrate staphylococcus superinfection—a problem more likely to occur in smokers. More widespread disease, spiking fevers, or tachycardia could indicate a need for hospitalization for further work-up and management.
With disease localized to this patient’s hands and feet, the physician considered dyshidrotic eczema as part of the differential diagnosis. However dyshidrotic eczema, which presents with clear tapioca-like vesicles, is profoundly itchy; pustular psoriasis is often painful. Other differences: The vesicles in dyshidrotic eczema do not uniformly form pustules nor do they form brown macules upon healing, as occurs in palmoplantar pustular psoriasis.
Treatment for very limited disease includes topical ultrapotent steroids, topical retinoids, or phototherapy—either alone or in combination. However, more extensive disease, even if limited to hands and feet, merits systemic therapy with acitretin, methotrexate, or biologic agents. (Acitretin and methotrexate are reliable teratogens and should not be used in women who are, or may become, pregnant.) Individuals with known superinfection often benefit from antibiotic therapy, as well.
In this case, the patient had already undergone a tubal ligation for contraception. Therefore, she was started on acitretin 25 mg PO daily. Patients on systemic retinoids undergo laboratory surveillance for transaminitis and hypertriglyceridemia regularly. Adverse effects are generally well tolerated, but include photosensitivity and dry skin, lips, and eyes. The patient improved significantly on 25 mg/d and the dosage was reduced to 10 mg/d after 3 months. She currently remains clear on this regimen.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21:355-370.

Close inspection of the plaques revealed multiple small pustules and mahogany colored macules on a broad, well-demarcated scaly red plaque. This pattern was consistent with palmoplantar pustular psoriasis.
Palmoplantar psoriasis is a chronic disease that may manifest at any age and in both sexes. The diagnosis may be clinical, as it was in this case. Bacterial culture of a pustule may demonstrate staphylococcus superinfection—a problem more likely to occur in smokers. More widespread disease, spiking fevers, or tachycardia could indicate a need for hospitalization for further work-up and management.
With disease localized to this patient’s hands and feet, the physician considered dyshidrotic eczema as part of the differential diagnosis. However dyshidrotic eczema, which presents with clear tapioca-like vesicles, is profoundly itchy; pustular psoriasis is often painful. Other differences: The vesicles in dyshidrotic eczema do not uniformly form pustules nor do they form brown macules upon healing, as occurs in palmoplantar pustular psoriasis.
Treatment for very limited disease includes topical ultrapotent steroids, topical retinoids, or phototherapy—either alone or in combination. However, more extensive disease, even if limited to hands and feet, merits systemic therapy with acitretin, methotrexate, or biologic agents. (Acitretin and methotrexate are reliable teratogens and should not be used in women who are, or may become, pregnant.) Individuals with known superinfection often benefit from antibiotic therapy, as well.
In this case, the patient had already undergone a tubal ligation for contraception. Therefore, she was started on acitretin 25 mg PO daily. Patients on systemic retinoids undergo laboratory surveillance for transaminitis and hypertriglyceridemia regularly. Adverse effects are generally well tolerated, but include photosensitivity and dry skin, lips, and eyes. The patient improved significantly on 25 mg/d and the dosage was reduced to 10 mg/d after 3 months. She currently remains clear on this regimen.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

Close inspection of the plaques revealed multiple small pustules and mahogany colored macules on a broad, well-demarcated scaly red plaque. This pattern was consistent with palmoplantar pustular psoriasis.
Palmoplantar psoriasis is a chronic disease that may manifest at any age and in both sexes. The diagnosis may be clinical, as it was in this case. Bacterial culture of a pustule may demonstrate staphylococcus superinfection—a problem more likely to occur in smokers. More widespread disease, spiking fevers, or tachycardia could indicate a need for hospitalization for further work-up and management.
With disease localized to this patient’s hands and feet, the physician considered dyshidrotic eczema as part of the differential diagnosis. However dyshidrotic eczema, which presents with clear tapioca-like vesicles, is profoundly itchy; pustular psoriasis is often painful. Other differences: The vesicles in dyshidrotic eczema do not uniformly form pustules nor do they form brown macules upon healing, as occurs in palmoplantar pustular psoriasis.
Treatment for very limited disease includes topical ultrapotent steroids, topical retinoids, or phototherapy—either alone or in combination. However, more extensive disease, even if limited to hands and feet, merits systemic therapy with acitretin, methotrexate, or biologic agents. (Acitretin and methotrexate are reliable teratogens and should not be used in women who are, or may become, pregnant.) Individuals with known superinfection often benefit from antibiotic therapy, as well.
In this case, the patient had already undergone a tubal ligation for contraception. Therefore, she was started on acitretin 25 mg PO daily. Patients on systemic retinoids undergo laboratory surveillance for transaminitis and hypertriglyceridemia regularly. Adverse effects are generally well tolerated, but include photosensitivity and dry skin, lips, and eyes. The patient improved significantly on 25 mg/d and the dosage was reduced to 10 mg/d after 3 months. She currently remains clear on this regimen.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21:355-370.
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21:355-370.
Mixed outcomes in tenofovir trial for chronic hepatitis B
About one-third of patients with chronic hepatitis B maintained a profile consistent with inactive disease 1 year after withdrawal from treatment in the randomized HBRN trial, which compared tenofovir with and without pegylated interferon (PEG-IFN). The two treatment groups, however, had similarly low rates of hepatitis B surface antigen (HBsAg) loss, the trial’s primary end point.
The successful withdrawals could inform discussions with patients who are “very motivated to have a finite treatment course,” said investigator Norah Terrault, MD, from the University of Southern California, Los Angeles. The results might “help patients in talking about expectations,” she said, because “there’s a one in three chance they won’t go back on treatment” if they meet specific metrics.
In HBRN, the metrics for withdrawal from treatment after 192 weeks included low levels of viral DNA (<1,000 IU/mL) for at least 24 weeks, no cirrhosis, negative week 144 test results for the hepatitis B envelope antigen (HBeAg), and week 180 conversion to anti-HBe positivity.
Of 102 patients who received tenofovir monotherapy for 192 weeks and who completed the trial, 51 met these criteria. After withdrawal from treatment, 30% still had DNA levels below 1,000 IU/mL and normal ALT at week 240, which is consistent with inactive chronic hepatitis B.
Of the 99 participants in the combination group – who received PEG-IFN for the first 24 of 192 weeks in addition to tenofovir – 60 met the withdrawal criteria at 192 weeks. At week 240, 39% of this withdrawal group still had DNA and ALT values consistent with inactive disease.
Rates of HBsAg loss, which signals functional cure, were low in the two groups, however. At week 240, fewer patients in the tenofovir monotherapy group tested negative for HBsAg than in the tenofovir plus PEG-IFN combination group, but the difference was not significant (4.5% vs. 5.7%).
The timing of HBsAg loss differed between the groups. In the combination group, the loss largely occurred before treatment withdrawal, likely because of the antiviral effects of interferon, Dr. Terrault said in an interview. In the monotherapy group, the loss occurred after 192 weeks, possibly reflecting the immunologic consequences of treatment withdrawal.
The timing of ALT flares also differed between groups. In the combination group, 58% of flares occurred during the 24-week PEG-IFN period. In the monotherapy group, 70% of flares occurred after tenofovir was stopped at 192 weeks.
The flare picture is a tricky one, said Dr. Terrault. The episodes might be a positive factor in HBsAg loss, but severe flares carry a risk for decompensation. Good predictors of the severity of flares are lacking, and “that is the hurdle” to finding a balance with these trade-offs.
‘Partially a failure and partially a success’
The findings are “partially a failure and partially a success,” said Robert Gish, MD, from Loma Linda (Calif.) University of Health, who was not involved in the study.
The low rates of HBsAg loss and the similarity between the two treatment groups represent the failure, he explained. The success is for the patients who were HBeAg-positive when the study began because they had high HBeAg loss rates in both the monotherapy and combination groups (41% vs. 61%; P = .06).
Loss of HBeAg was numerically higher in the combination group because of the interferon effect. That could be viewed as a “subjective benefit” of PEG-IFN, even though the difference wasn’t statistically significant, said Dr. Gish.
The low rates of HBsAg loss could relate to two features of the patient profile, he explained. At study entry, the participants had moderately high levels of quantitative HBsAg and were predominately of Asian ancestry, which are predisposing factors for limited HBsAg loss.
Previous studies have suggested that peak HBsAg loss could take 2-3 years to develop after treatment withdrawal in a trial population. In the HBRN trial, rates almost 1 year after withdrawal are similar to 1-year rates from other studies, Dr. Terrault said. How these results for HBsAg loss in the two treatment groups will look at the 3-year mark is not known.
The trial design standardized withdrawal protocol and the length of time patients were on treatment before withdrawal was attempted, which are strengths of this study, said Dr. Terrault. And “a triumph of this study is execution of a standard for nucleic acid treatment in a protocolized way, followed by withdrawal. That is something we are happy about.”
Dr. Terrault reported receiving institutional grant support from Roche/Genentech and Gilead Sciences. Dr. Gish reported receiving research support from Gilead Sciences and serving as a consultant and on advisory boards for several pharmaceutical companies.
This article first appeared on Medscape.com.
About one-third of patients with chronic hepatitis B maintained a profile consistent with inactive disease 1 year after withdrawal from treatment in the randomized HBRN trial, which compared tenofovir with and without pegylated interferon (PEG-IFN). The two treatment groups, however, had similarly low rates of hepatitis B surface antigen (HBsAg) loss, the trial’s primary end point.
The successful withdrawals could inform discussions with patients who are “very motivated to have a finite treatment course,” said investigator Norah Terrault, MD, from the University of Southern California, Los Angeles. The results might “help patients in talking about expectations,” she said, because “there’s a one in three chance they won’t go back on treatment” if they meet specific metrics.
In HBRN, the metrics for withdrawal from treatment after 192 weeks included low levels of viral DNA (<1,000 IU/mL) for at least 24 weeks, no cirrhosis, negative week 144 test results for the hepatitis B envelope antigen (HBeAg), and week 180 conversion to anti-HBe positivity.
Of 102 patients who received tenofovir monotherapy for 192 weeks and who completed the trial, 51 met these criteria. After withdrawal from treatment, 30% still had DNA levels below 1,000 IU/mL and normal ALT at week 240, which is consistent with inactive chronic hepatitis B.
Of the 99 participants in the combination group – who received PEG-IFN for the first 24 of 192 weeks in addition to tenofovir – 60 met the withdrawal criteria at 192 weeks. At week 240, 39% of this withdrawal group still had DNA and ALT values consistent with inactive disease.
Rates of HBsAg loss, which signals functional cure, were low in the two groups, however. At week 240, fewer patients in the tenofovir monotherapy group tested negative for HBsAg than in the tenofovir plus PEG-IFN combination group, but the difference was not significant (4.5% vs. 5.7%).
The timing of HBsAg loss differed between the groups. In the combination group, the loss largely occurred before treatment withdrawal, likely because of the antiviral effects of interferon, Dr. Terrault said in an interview. In the monotherapy group, the loss occurred after 192 weeks, possibly reflecting the immunologic consequences of treatment withdrawal.
The timing of ALT flares also differed between groups. In the combination group, 58% of flares occurred during the 24-week PEG-IFN period. In the monotherapy group, 70% of flares occurred after tenofovir was stopped at 192 weeks.
The flare picture is a tricky one, said Dr. Terrault. The episodes might be a positive factor in HBsAg loss, but severe flares carry a risk for decompensation. Good predictors of the severity of flares are lacking, and “that is the hurdle” to finding a balance with these trade-offs.
‘Partially a failure and partially a success’
The findings are “partially a failure and partially a success,” said Robert Gish, MD, from Loma Linda (Calif.) University of Health, who was not involved in the study.
The low rates of HBsAg loss and the similarity between the two treatment groups represent the failure, he explained. The success is for the patients who were HBeAg-positive when the study began because they had high HBeAg loss rates in both the monotherapy and combination groups (41% vs. 61%; P = .06).
Loss of HBeAg was numerically higher in the combination group because of the interferon effect. That could be viewed as a “subjective benefit” of PEG-IFN, even though the difference wasn’t statistically significant, said Dr. Gish.
The low rates of HBsAg loss could relate to two features of the patient profile, he explained. At study entry, the participants had moderately high levels of quantitative HBsAg and were predominately of Asian ancestry, which are predisposing factors for limited HBsAg loss.
Previous studies have suggested that peak HBsAg loss could take 2-3 years to develop after treatment withdrawal in a trial population. In the HBRN trial, rates almost 1 year after withdrawal are similar to 1-year rates from other studies, Dr. Terrault said. How these results for HBsAg loss in the two treatment groups will look at the 3-year mark is not known.
The trial design standardized withdrawal protocol and the length of time patients were on treatment before withdrawal was attempted, which are strengths of this study, said Dr. Terrault. And “a triumph of this study is execution of a standard for nucleic acid treatment in a protocolized way, followed by withdrawal. That is something we are happy about.”
Dr. Terrault reported receiving institutional grant support from Roche/Genentech and Gilead Sciences. Dr. Gish reported receiving research support from Gilead Sciences and serving as a consultant and on advisory boards for several pharmaceutical companies.
This article first appeared on Medscape.com.
About one-third of patients with chronic hepatitis B maintained a profile consistent with inactive disease 1 year after withdrawal from treatment in the randomized HBRN trial, which compared tenofovir with and without pegylated interferon (PEG-IFN). The two treatment groups, however, had similarly low rates of hepatitis B surface antigen (HBsAg) loss, the trial’s primary end point.
The successful withdrawals could inform discussions with patients who are “very motivated to have a finite treatment course,” said investigator Norah Terrault, MD, from the University of Southern California, Los Angeles. The results might “help patients in talking about expectations,” she said, because “there’s a one in three chance they won’t go back on treatment” if they meet specific metrics.
In HBRN, the metrics for withdrawal from treatment after 192 weeks included low levels of viral DNA (<1,000 IU/mL) for at least 24 weeks, no cirrhosis, negative week 144 test results for the hepatitis B envelope antigen (HBeAg), and week 180 conversion to anti-HBe positivity.
Of 102 patients who received tenofovir monotherapy for 192 weeks and who completed the trial, 51 met these criteria. After withdrawal from treatment, 30% still had DNA levels below 1,000 IU/mL and normal ALT at week 240, which is consistent with inactive chronic hepatitis B.
Of the 99 participants in the combination group – who received PEG-IFN for the first 24 of 192 weeks in addition to tenofovir – 60 met the withdrawal criteria at 192 weeks. At week 240, 39% of this withdrawal group still had DNA and ALT values consistent with inactive disease.
Rates of HBsAg loss, which signals functional cure, were low in the two groups, however. At week 240, fewer patients in the tenofovir monotherapy group tested negative for HBsAg than in the tenofovir plus PEG-IFN combination group, but the difference was not significant (4.5% vs. 5.7%).
The timing of HBsAg loss differed between the groups. In the combination group, the loss largely occurred before treatment withdrawal, likely because of the antiviral effects of interferon, Dr. Terrault said in an interview. In the monotherapy group, the loss occurred after 192 weeks, possibly reflecting the immunologic consequences of treatment withdrawal.
The timing of ALT flares also differed between groups. In the combination group, 58% of flares occurred during the 24-week PEG-IFN period. In the monotherapy group, 70% of flares occurred after tenofovir was stopped at 192 weeks.
The flare picture is a tricky one, said Dr. Terrault. The episodes might be a positive factor in HBsAg loss, but severe flares carry a risk for decompensation. Good predictors of the severity of flares are lacking, and “that is the hurdle” to finding a balance with these trade-offs.
‘Partially a failure and partially a success’
The findings are “partially a failure and partially a success,” said Robert Gish, MD, from Loma Linda (Calif.) University of Health, who was not involved in the study.
The low rates of HBsAg loss and the similarity between the two treatment groups represent the failure, he explained. The success is for the patients who were HBeAg-positive when the study began because they had high HBeAg loss rates in both the monotherapy and combination groups (41% vs. 61%; P = .06).
Loss of HBeAg was numerically higher in the combination group because of the interferon effect. That could be viewed as a “subjective benefit” of PEG-IFN, even though the difference wasn’t statistically significant, said Dr. Gish.
The low rates of HBsAg loss could relate to two features of the patient profile, he explained. At study entry, the participants had moderately high levels of quantitative HBsAg and were predominately of Asian ancestry, which are predisposing factors for limited HBsAg loss.
Previous studies have suggested that peak HBsAg loss could take 2-3 years to develop after treatment withdrawal in a trial population. In the HBRN trial, rates almost 1 year after withdrawal are similar to 1-year rates from other studies, Dr. Terrault said. How these results for HBsAg loss in the two treatment groups will look at the 3-year mark is not known.
The trial design standardized withdrawal protocol and the length of time patients were on treatment before withdrawal was attempted, which are strengths of this study, said Dr. Terrault. And “a triumph of this study is execution of a standard for nucleic acid treatment in a protocolized way, followed by withdrawal. That is something we are happy about.”
Dr. Terrault reported receiving institutional grant support from Roche/Genentech and Gilead Sciences. Dr. Gish reported receiving research support from Gilead Sciences and serving as a consultant and on advisory boards for several pharmaceutical companies.
This article first appeared on Medscape.com.
‘Hospital at home’ increases COVID capacity in large study
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
Predicting patient risk of medication-related harm
A new tool is the first of its kind
“An increasing number of older adults are using multiple medicines, and it is important that the benefits are outweighing the risks,” said Nikesh Parekh, MBBS, MPH, lead author of a recent study of a new predictive tool. The study was done in the context of the World Health Organization campaign to halve the incidence of medication-related harm (MRH) by 2022 – reducing MRH following hospital discharge was identified as a priority area.
This works allows clinicians to calculate the risk of a patient suffering MRH post-discharge requiring health care, said Dr. Parekh, a research fellow at Brighton and Sussex Medical School in Great Britain. “This enables practitioners and policy makers to target interventions to reduce MRH at those with highest risk. This should support the delivery of cost-effective care. The knowledge of individual risk can also prompt clinicians to reconsider any high-risk medicines that they intend on prescribing at discharge.”
This is the first prediction tool to calculate individual patient risk of serious MRH post-discharge, he added.The high readmission rate for older adults is often an avoidable pressure for hospitalists, particularly where MRH is the underlying cause. “The prediction tool has the potential to significantly reduce this burden for hospitalists/patients by identifying those individuals at high risk upon discharge and ensuring that monitoring and additional support is provided to them in the community with their medications,” Dr. Parekh said.
This electronic tool could be integrated into the electronic discharge summaries so that the information can be shared with primary care clinicians in a straightforward way. “The risk score should be calculated automatically by a self-population of the tool’s fields from information that exists on the patient within the electronic discharge system.”The tool now needs to be externally validated through testing in new settings to assess its validity and reliability in new populations. “If the tool is found to be usable by hospitalists and demonstrates reasonable predictive accuracy, then it should be implemented widely to reduce the incidence of MRH,” Dr. Parekh said.
Reference
1. Parekh N, et al. Medication-related harm in older adults following hospital discharge: development and validation of a prediction tool. BMJ Qual Saf. Published Online First 2019 Sept 16. doi: 10.1136/bmjqs-2019-009587.
A new tool is the first of its kind
A new tool is the first of its kind
“An increasing number of older adults are using multiple medicines, and it is important that the benefits are outweighing the risks,” said Nikesh Parekh, MBBS, MPH, lead author of a recent study of a new predictive tool. The study was done in the context of the World Health Organization campaign to halve the incidence of medication-related harm (MRH) by 2022 – reducing MRH following hospital discharge was identified as a priority area.
This works allows clinicians to calculate the risk of a patient suffering MRH post-discharge requiring health care, said Dr. Parekh, a research fellow at Brighton and Sussex Medical School in Great Britain. “This enables practitioners and policy makers to target interventions to reduce MRH at those with highest risk. This should support the delivery of cost-effective care. The knowledge of individual risk can also prompt clinicians to reconsider any high-risk medicines that they intend on prescribing at discharge.”
This is the first prediction tool to calculate individual patient risk of serious MRH post-discharge, he added.The high readmission rate for older adults is often an avoidable pressure for hospitalists, particularly where MRH is the underlying cause. “The prediction tool has the potential to significantly reduce this burden for hospitalists/patients by identifying those individuals at high risk upon discharge and ensuring that monitoring and additional support is provided to them in the community with their medications,” Dr. Parekh said.
This electronic tool could be integrated into the electronic discharge summaries so that the information can be shared with primary care clinicians in a straightforward way. “The risk score should be calculated automatically by a self-population of the tool’s fields from information that exists on the patient within the electronic discharge system.”The tool now needs to be externally validated through testing in new settings to assess its validity and reliability in new populations. “If the tool is found to be usable by hospitalists and demonstrates reasonable predictive accuracy, then it should be implemented widely to reduce the incidence of MRH,” Dr. Parekh said.
Reference
1. Parekh N, et al. Medication-related harm in older adults following hospital discharge: development and validation of a prediction tool. BMJ Qual Saf. Published Online First 2019 Sept 16. doi: 10.1136/bmjqs-2019-009587.
“An increasing number of older adults are using multiple medicines, and it is important that the benefits are outweighing the risks,” said Nikesh Parekh, MBBS, MPH, lead author of a recent study of a new predictive tool. The study was done in the context of the World Health Organization campaign to halve the incidence of medication-related harm (MRH) by 2022 – reducing MRH following hospital discharge was identified as a priority area.
This works allows clinicians to calculate the risk of a patient suffering MRH post-discharge requiring health care, said Dr. Parekh, a research fellow at Brighton and Sussex Medical School in Great Britain. “This enables practitioners and policy makers to target interventions to reduce MRH at those with highest risk. This should support the delivery of cost-effective care. The knowledge of individual risk can also prompt clinicians to reconsider any high-risk medicines that they intend on prescribing at discharge.”
This is the first prediction tool to calculate individual patient risk of serious MRH post-discharge, he added.The high readmission rate for older adults is often an avoidable pressure for hospitalists, particularly where MRH is the underlying cause. “The prediction tool has the potential to significantly reduce this burden for hospitalists/patients by identifying those individuals at high risk upon discharge and ensuring that monitoring and additional support is provided to them in the community with their medications,” Dr. Parekh said.
This electronic tool could be integrated into the electronic discharge summaries so that the information can be shared with primary care clinicians in a straightforward way. “The risk score should be calculated automatically by a self-population of the tool’s fields from information that exists on the patient within the electronic discharge system.”The tool now needs to be externally validated through testing in new settings to assess its validity and reliability in new populations. “If the tool is found to be usable by hospitalists and demonstrates reasonable predictive accuracy, then it should be implemented widely to reduce the incidence of MRH,” Dr. Parekh said.
Reference
1. Parekh N, et al. Medication-related harm in older adults following hospital discharge: development and validation of a prediction tool. BMJ Qual Saf. Published Online First 2019 Sept 16. doi: 10.1136/bmjqs-2019-009587.