User login
Can receiving HSCT care at home reduce the risk of GVHD and COVID-19?
Researchers are conducting phase 2 trials to find out.
Anthony D. Sung, MD, of Duke University, Durham, N.C., described this research to David H. Henry, MD, of Penn Medicine in Philadelphia, host of the Blood & Cancer podcast.
On the Nov. 12 episode of Blood & Cancer, Dr. Sung outlined the process of receiving post-HSCT care at home and discussed Duke’s clinical trials assessing the impact of home care on costs, quality of life, the microbiome, graft-versus-host disease (GVHD), and other outcomes. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Welcome to this podcast. We’re delighted to have you listening today because we’re going to be speaking with Dr. Anthony Sung from Duke University, where he is assistant professor of medicine in the division of hematologic malignancies and cellular therapies.
So let’s get right into it. I’m a generalist at Pennsylvania Hospital in Philadelphia, where we do auto [autologous] transplants at the main university hospital, autos and allos [allogeneic], and these patients are in [hospital] anywhere from a little bit to a long time. And I’ve often thought to try and do some of this as outpatient. But I think you have a project, which I’m going to ask you to describe, where you try and do most [treatment] outpatient. So tell me what this project is all about, and we’ll skip through how it works.
Anthony Sung, MD: Absolutely. So this is focused on both autologous as well as allogeneic stem cell transplant patients at Duke and a few other centers around the country. Duke University has actually had a long history of an outpatient transplant program. This program is based in a day hospital, which is basically like a high-functioning clinic that’s open 7 days a week. Patients can come into the hospital and receive blood transfusions, IV infusions, and any other therapies that they would need as part of their stem cell transplant treatment in the outpatient setting, returning to their home or to a furnished apartment, temporary lodging, while they’re receiving their care.
What we have done, however, is to take this a step further and deliver care within the patient’s own home. In a sense, we’re returning to an older form of medicine where doctors would make house calls. Within our home-transplant program, instead of the patients having to be in the hospital or instead of having to come back and forth to the outpatient hospital every day, which places additional stresses and strains upon them, our providers will make house calls to the patient’s homes, will draw their labs right there, do a history and physical exam, assess and attend to any of the needs that they have.
Then in the afternoon, the providers will return, have the labs run in the hospital, as they would normally do, a CBC, CMP [comprehensive metabolic panel], and so forth. And then a nurse would return to the patient’s home if needed to deliver any interventions, such as blood transfusions, intravenous fluids, or electrolytes, right there in the comfort of the patient’s own home.
Dr. Henry: So let’s then take it through what happens. Say I am a patient with myeloma. I’ve had various therapies, and it’s time for me to get an autotransplant, let’s say. And so I need to do a couple of things. I need to get my stem cells collected. I need to then get my high-dose [conditioning] therapy, and then follows the stem cell therapy reinfusion. So can you take me through each step? Where is that done?
Dr. Sung: Absolutely. So the collection will occur in the outpatient setting, typically after mobilization with G-CSF [granulocyte colony–stimulating factor] and/or plerixafor. That will occur in our outpatient clinic with one of our leukapheresis machines. And the patient will then return to that same outpatient clinic, which is the same building, the same facility as the hospital, to receive melphalan conditioning. And then, following conditioning, about 24 hours after, day 0, that’s the day of their stem cell transplant infusion, which we do in the hospital setting just because of the potential for reactions associated with that.
But everything after that, from day 1 onwards, we try to keep them at home. And as I said, they will stay in their home. One of our nurse practitioners or physician assistants will visit them in the morning, do the assessment and draw the labs. And nurses will return in the afternoon to deliver any supportive care that they need.
Dr. Henry: So let’s define “home.” So I’m a Philadelphia resident and I say to you, Dr. Sung, I want to go home. You say, well, Philadelphia is too far. What is close enough and not too far, when you say home?
Dr. Sung: Absolutely. So when we originally conceived the program, we focused on patients who lived within an hour of our transplant center. And in part, that was because, as you know, unfortunately, things can sometimes go wrong during transplant. One of the most concerning ones is infections. And if a patient were to develop a neutropenic fever, we would want them to be seen as urgently as possible within an hour. And that’s where our limitation comes from.
So for our patients who live more than an hour away, those are the ones that we will have relocate to temporary lodging near our transplant center. And we’ve worked with several facilities in the area that have clean, furnished units that are available for rent. Many insurances also include lodging benefits for patients during stem cell transplant, recognizing this need. And historically, those [patients] were not considered part of our transplant patient cohorts.
I have not mentioned, but we initially did this in a phase 1 study, and we’re now studying it in a series of randomized, phase 2 studies that I can go into detail later on. And because they were not necessarily in their home, but a temporary lodging environment, those patients who relocated to Durham were not eligible for a home transplant study.
However, in the setting of the COVID-19 pandemic, we’ve actually pivoted our program in many ways. Specifically, if you think about a patient who’s coming into contact with the medical system, they come to the hospital, they meet someone at the door who is screening them for COVID-19. They see someone who checks them in at the front desk. A medical assistant takes them in the back. Someone calls their labs and phlebotomy. They may encounter other patients and environmental services, other individuals in the setting. You’re talking about dozens of different encounters. Who knows how many surfaces that potentially someone with COVID-19 has coughed on or contaminated?
And in contrast, you have house calls, which even if they are located in the temporary lodging, that’s just one or two individuals going into their living environment. They’re not encountering any different surfaces. And so, in the setting of COVID-19, we felt that this platform had the potential to help protect all our transplant patients who are among the most vulnerable patients, the most immunocompromised patients, and so we expanded our program to include those individuals as well.
Dr. Henry: So ... what are the actual outcomes of your patients in terms of how they’re doing, engrafting, and getting cured of their malignancy?
Dr. Sung: So as I mentioned, we first did this in a phase 1 safety and feasibility pilot study of both autologous and allo-transplant patients. This was presented at the annual meeting of the American Society of Hematology [Blood. 2017;130:745]. And we’re actually about ready to submit our manuscript on this.
And we found no difference in outcomes between patients who received care in the home transplant setting versus those who received conventional care either in the day hospital or hospital environment. The process appeared safe. Patients did just as well, if not better. Certainly, anecdotally, patients would talk about feeling so much more comfortable and happier being cared for in that home environment.
And we are now in the process of formally studying these outcomes in two NIH [National Institutes of Health]-funded clinical trials, one focused on allogeneic transplant patients [NCT02218151] and the other focused on autologous transplant patients [NCT01725022].
Dr. Henry: So of course, I’m waiting for this next question, which is cost. The services are the same, but you have people traveling, people who are highly skilled caregivers. Have you looked at cost differences from hospital versus home?
Dr. Sung: Absolutely. So you do have increased upfront costs because you have travel time for advanced practice providers and nurses. Not only that, but when a nurse is helping to give a patient a blood transfusion in the home environment, they’re 1:1 with that patient as opposed to the day hospital where a nurse could help with transfusions simultaneously for multiple patients. At the same time, by keeping patients out of the hospital, you have drastic, significant cost savings in that way.
In addition, I should mention, part of why we’re conducting these randomized, phase 2 clinical trials is we believe home care actually has the potential to decrease complications. One area of my research is on the impact of the microbiome, the bacteria in the gut, on transplant outcomes. And we’ve done a number of studies, many in collaboration with Memorial Sloan Kettering, showing that disruption of the microbiota, the bacteria in the gut, is associated with increased infections, graft-versus-host disease, and treatment-related mortality if we’re able to keep patients in their home setting.
However, I actually should go back a step. It’s well known that, if you take an individual from their home setting and put them in a foreign environment such as the hospital, that new environment, that new diet, hospital food as opposed to home food, and so forth, can dramatically shift the microbiome. Our hypothesis is that, by keeping patients in the home environment, their familiar environment will be able to help preserve their microbiome, thus decreasing infections, graft-versus-host disease, and other complications. That’s actually the goal of our studies: to see if we can preserve the microbiome and decrease complications.
Dr. Henry: So how will you evaluate that? Are you doing fecal studies, patient culture studies? How are you testing that?
Dr. Sung: So we have a very broad biobank program where we collect stool on our transplantations, pretransplant, day 0, weekly for the first month. And then, in the case of our allogeneic transplant patients, day 60, 90, 180, and 365.
And we do that both in our home transplant patients as well as their matched controls on the phase 2 studies. And we also collect it on a lot of our other transplant patients as part of our biobanking programs and our observational studies to try to understand what’s going on during transplant and how to help improve transplant outcomes.
Dr. Henry: Do you have any results of that? You’re probably showing a difference.
Dr. Sung: We think so, on some preliminary results, but those were based on small numbers of patients. And we’re really hoping that these randomized clinical trials with the larger numbers of patients enrolled will help show that difference.
But getting back to your earlier question about cost, a case of graft-versus-host disease, grade 2 or higher, can add about $100,000 to the cost of care. So if you prevent one case of bad gut or liver graft-versus-host disease, those are your cost savings right there.
The randomized, phase 2 trial for allogeneic transplant patients, the primary endpoint is graft-versus-host disease. So we’re looking at the microbiome and those associations and the prevention of GVHD. For the randomized clinical trial in autologous transplant patients – with autologous stem cells, you’re not going to get GVHD – but we do hope to improve quality of life and long-term outcomes in those patients as well.
Dr. Henry: Wonderful. Well, Tony, I really want to thank you so much for talking with us today.
Dr. Sung: Thank you very much for this opportunity. And again, I also want to just thank everyone who’s been involved in these studies, the advanced practice providers and nurses who are caring for our patients at home, the study staff who have been involved. Particularly, I’d like to highlight the role of both Nelson Chao, who’s our division chief and my mentor who piloted and first developed home transplant, and Kristin Nichols, our research nurse who has really led the drive forward.
Dr. Sung and Dr. Henry have no relevant disclosures. The trials are funded by grants from the National Institutes of Health.
Researchers are conducting phase 2 trials to find out.
Anthony D. Sung, MD, of Duke University, Durham, N.C., described this research to David H. Henry, MD, of Penn Medicine in Philadelphia, host of the Blood & Cancer podcast.
On the Nov. 12 episode of Blood & Cancer, Dr. Sung outlined the process of receiving post-HSCT care at home and discussed Duke’s clinical trials assessing the impact of home care on costs, quality of life, the microbiome, graft-versus-host disease (GVHD), and other outcomes. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Welcome to this podcast. We’re delighted to have you listening today because we’re going to be speaking with Dr. Anthony Sung from Duke University, where he is assistant professor of medicine in the division of hematologic malignancies and cellular therapies.
So let’s get right into it. I’m a generalist at Pennsylvania Hospital in Philadelphia, where we do auto [autologous] transplants at the main university hospital, autos and allos [allogeneic], and these patients are in [hospital] anywhere from a little bit to a long time. And I’ve often thought to try and do some of this as outpatient. But I think you have a project, which I’m going to ask you to describe, where you try and do most [treatment] outpatient. So tell me what this project is all about, and we’ll skip through how it works.
Anthony Sung, MD: Absolutely. So this is focused on both autologous as well as allogeneic stem cell transplant patients at Duke and a few other centers around the country. Duke University has actually had a long history of an outpatient transplant program. This program is based in a day hospital, which is basically like a high-functioning clinic that’s open 7 days a week. Patients can come into the hospital and receive blood transfusions, IV infusions, and any other therapies that they would need as part of their stem cell transplant treatment in the outpatient setting, returning to their home or to a furnished apartment, temporary lodging, while they’re receiving their care.
What we have done, however, is to take this a step further and deliver care within the patient’s own home. In a sense, we’re returning to an older form of medicine where doctors would make house calls. Within our home-transplant program, instead of the patients having to be in the hospital or instead of having to come back and forth to the outpatient hospital every day, which places additional stresses and strains upon them, our providers will make house calls to the patient’s homes, will draw their labs right there, do a history and physical exam, assess and attend to any of the needs that they have.
Then in the afternoon, the providers will return, have the labs run in the hospital, as they would normally do, a CBC, CMP [comprehensive metabolic panel], and so forth. And then a nurse would return to the patient’s home if needed to deliver any interventions, such as blood transfusions, intravenous fluids, or electrolytes, right there in the comfort of the patient’s own home.
Dr. Henry: So let’s then take it through what happens. Say I am a patient with myeloma. I’ve had various therapies, and it’s time for me to get an autotransplant, let’s say. And so I need to do a couple of things. I need to get my stem cells collected. I need to then get my high-dose [conditioning] therapy, and then follows the stem cell therapy reinfusion. So can you take me through each step? Where is that done?
Dr. Sung: Absolutely. So the collection will occur in the outpatient setting, typically after mobilization with G-CSF [granulocyte colony–stimulating factor] and/or plerixafor. That will occur in our outpatient clinic with one of our leukapheresis machines. And the patient will then return to that same outpatient clinic, which is the same building, the same facility as the hospital, to receive melphalan conditioning. And then, following conditioning, about 24 hours after, day 0, that’s the day of their stem cell transplant infusion, which we do in the hospital setting just because of the potential for reactions associated with that.
But everything after that, from day 1 onwards, we try to keep them at home. And as I said, they will stay in their home. One of our nurse practitioners or physician assistants will visit them in the morning, do the assessment and draw the labs. And nurses will return in the afternoon to deliver any supportive care that they need.
Dr. Henry: So let’s define “home.” So I’m a Philadelphia resident and I say to you, Dr. Sung, I want to go home. You say, well, Philadelphia is too far. What is close enough and not too far, when you say home?
Dr. Sung: Absolutely. So when we originally conceived the program, we focused on patients who lived within an hour of our transplant center. And in part, that was because, as you know, unfortunately, things can sometimes go wrong during transplant. One of the most concerning ones is infections. And if a patient were to develop a neutropenic fever, we would want them to be seen as urgently as possible within an hour. And that’s where our limitation comes from.
So for our patients who live more than an hour away, those are the ones that we will have relocate to temporary lodging near our transplant center. And we’ve worked with several facilities in the area that have clean, furnished units that are available for rent. Many insurances also include lodging benefits for patients during stem cell transplant, recognizing this need. And historically, those [patients] were not considered part of our transplant patient cohorts.
I have not mentioned, but we initially did this in a phase 1 study, and we’re now studying it in a series of randomized, phase 2 studies that I can go into detail later on. And because they were not necessarily in their home, but a temporary lodging environment, those patients who relocated to Durham were not eligible for a home transplant study.
However, in the setting of the COVID-19 pandemic, we’ve actually pivoted our program in many ways. Specifically, if you think about a patient who’s coming into contact with the medical system, they come to the hospital, they meet someone at the door who is screening them for COVID-19. They see someone who checks them in at the front desk. A medical assistant takes them in the back. Someone calls their labs and phlebotomy. They may encounter other patients and environmental services, other individuals in the setting. You’re talking about dozens of different encounters. Who knows how many surfaces that potentially someone with COVID-19 has coughed on or contaminated?
And in contrast, you have house calls, which even if they are located in the temporary lodging, that’s just one or two individuals going into their living environment. They’re not encountering any different surfaces. And so, in the setting of COVID-19, we felt that this platform had the potential to help protect all our transplant patients who are among the most vulnerable patients, the most immunocompromised patients, and so we expanded our program to include those individuals as well.
Dr. Henry: So ... what are the actual outcomes of your patients in terms of how they’re doing, engrafting, and getting cured of their malignancy?
Dr. Sung: So as I mentioned, we first did this in a phase 1 safety and feasibility pilot study of both autologous and allo-transplant patients. This was presented at the annual meeting of the American Society of Hematology [Blood. 2017;130:745]. And we’re actually about ready to submit our manuscript on this.
And we found no difference in outcomes between patients who received care in the home transplant setting versus those who received conventional care either in the day hospital or hospital environment. The process appeared safe. Patients did just as well, if not better. Certainly, anecdotally, patients would talk about feeling so much more comfortable and happier being cared for in that home environment.
And we are now in the process of formally studying these outcomes in two NIH [National Institutes of Health]-funded clinical trials, one focused on allogeneic transplant patients [NCT02218151] and the other focused on autologous transplant patients [NCT01725022].
Dr. Henry: So of course, I’m waiting for this next question, which is cost. The services are the same, but you have people traveling, people who are highly skilled caregivers. Have you looked at cost differences from hospital versus home?
Dr. Sung: Absolutely. So you do have increased upfront costs because you have travel time for advanced practice providers and nurses. Not only that, but when a nurse is helping to give a patient a blood transfusion in the home environment, they’re 1:1 with that patient as opposed to the day hospital where a nurse could help with transfusions simultaneously for multiple patients. At the same time, by keeping patients out of the hospital, you have drastic, significant cost savings in that way.
In addition, I should mention, part of why we’re conducting these randomized, phase 2 clinical trials is we believe home care actually has the potential to decrease complications. One area of my research is on the impact of the microbiome, the bacteria in the gut, on transplant outcomes. And we’ve done a number of studies, many in collaboration with Memorial Sloan Kettering, showing that disruption of the microbiota, the bacteria in the gut, is associated with increased infections, graft-versus-host disease, and treatment-related mortality if we’re able to keep patients in their home setting.
However, I actually should go back a step. It’s well known that, if you take an individual from their home setting and put them in a foreign environment such as the hospital, that new environment, that new diet, hospital food as opposed to home food, and so forth, can dramatically shift the microbiome. Our hypothesis is that, by keeping patients in the home environment, their familiar environment will be able to help preserve their microbiome, thus decreasing infections, graft-versus-host disease, and other complications. That’s actually the goal of our studies: to see if we can preserve the microbiome and decrease complications.
Dr. Henry: So how will you evaluate that? Are you doing fecal studies, patient culture studies? How are you testing that?
Dr. Sung: So we have a very broad biobank program where we collect stool on our transplantations, pretransplant, day 0, weekly for the first month. And then, in the case of our allogeneic transplant patients, day 60, 90, 180, and 365.
And we do that both in our home transplant patients as well as their matched controls on the phase 2 studies. And we also collect it on a lot of our other transplant patients as part of our biobanking programs and our observational studies to try to understand what’s going on during transplant and how to help improve transplant outcomes.
Dr. Henry: Do you have any results of that? You’re probably showing a difference.
Dr. Sung: We think so, on some preliminary results, but those were based on small numbers of patients. And we’re really hoping that these randomized clinical trials with the larger numbers of patients enrolled will help show that difference.
But getting back to your earlier question about cost, a case of graft-versus-host disease, grade 2 or higher, can add about $100,000 to the cost of care. So if you prevent one case of bad gut or liver graft-versus-host disease, those are your cost savings right there.
The randomized, phase 2 trial for allogeneic transplant patients, the primary endpoint is graft-versus-host disease. So we’re looking at the microbiome and those associations and the prevention of GVHD. For the randomized clinical trial in autologous transplant patients – with autologous stem cells, you’re not going to get GVHD – but we do hope to improve quality of life and long-term outcomes in those patients as well.
Dr. Henry: Wonderful. Well, Tony, I really want to thank you so much for talking with us today.
Dr. Sung: Thank you very much for this opportunity. And again, I also want to just thank everyone who’s been involved in these studies, the advanced practice providers and nurses who are caring for our patients at home, the study staff who have been involved. Particularly, I’d like to highlight the role of both Nelson Chao, who’s our division chief and my mentor who piloted and first developed home transplant, and Kristin Nichols, our research nurse who has really led the drive forward.
Dr. Sung and Dr. Henry have no relevant disclosures. The trials are funded by grants from the National Institutes of Health.
Researchers are conducting phase 2 trials to find out.
Anthony D. Sung, MD, of Duke University, Durham, N.C., described this research to David H. Henry, MD, of Penn Medicine in Philadelphia, host of the Blood & Cancer podcast.
On the Nov. 12 episode of Blood & Cancer, Dr. Sung outlined the process of receiving post-HSCT care at home and discussed Duke’s clinical trials assessing the impact of home care on costs, quality of life, the microbiome, graft-versus-host disease (GVHD), and other outcomes. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Welcome to this podcast. We’re delighted to have you listening today because we’re going to be speaking with Dr. Anthony Sung from Duke University, where he is assistant professor of medicine in the division of hematologic malignancies and cellular therapies.
So let’s get right into it. I’m a generalist at Pennsylvania Hospital in Philadelphia, where we do auto [autologous] transplants at the main university hospital, autos and allos [allogeneic], and these patients are in [hospital] anywhere from a little bit to a long time. And I’ve often thought to try and do some of this as outpatient. But I think you have a project, which I’m going to ask you to describe, where you try and do most [treatment] outpatient. So tell me what this project is all about, and we’ll skip through how it works.
Anthony Sung, MD: Absolutely. So this is focused on both autologous as well as allogeneic stem cell transplant patients at Duke and a few other centers around the country. Duke University has actually had a long history of an outpatient transplant program. This program is based in a day hospital, which is basically like a high-functioning clinic that’s open 7 days a week. Patients can come into the hospital and receive blood transfusions, IV infusions, and any other therapies that they would need as part of their stem cell transplant treatment in the outpatient setting, returning to their home or to a furnished apartment, temporary lodging, while they’re receiving their care.
What we have done, however, is to take this a step further and deliver care within the patient’s own home. In a sense, we’re returning to an older form of medicine where doctors would make house calls. Within our home-transplant program, instead of the patients having to be in the hospital or instead of having to come back and forth to the outpatient hospital every day, which places additional stresses and strains upon them, our providers will make house calls to the patient’s homes, will draw their labs right there, do a history and physical exam, assess and attend to any of the needs that they have.
Then in the afternoon, the providers will return, have the labs run in the hospital, as they would normally do, a CBC, CMP [comprehensive metabolic panel], and so forth. And then a nurse would return to the patient’s home if needed to deliver any interventions, such as blood transfusions, intravenous fluids, or electrolytes, right there in the comfort of the patient’s own home.
Dr. Henry: So let’s then take it through what happens. Say I am a patient with myeloma. I’ve had various therapies, and it’s time for me to get an autotransplant, let’s say. And so I need to do a couple of things. I need to get my stem cells collected. I need to then get my high-dose [conditioning] therapy, and then follows the stem cell therapy reinfusion. So can you take me through each step? Where is that done?
Dr. Sung: Absolutely. So the collection will occur in the outpatient setting, typically after mobilization with G-CSF [granulocyte colony–stimulating factor] and/or plerixafor. That will occur in our outpatient clinic with one of our leukapheresis machines. And the patient will then return to that same outpatient clinic, which is the same building, the same facility as the hospital, to receive melphalan conditioning. And then, following conditioning, about 24 hours after, day 0, that’s the day of their stem cell transplant infusion, which we do in the hospital setting just because of the potential for reactions associated with that.
But everything after that, from day 1 onwards, we try to keep them at home. And as I said, they will stay in their home. One of our nurse practitioners or physician assistants will visit them in the morning, do the assessment and draw the labs. And nurses will return in the afternoon to deliver any supportive care that they need.
Dr. Henry: So let’s define “home.” So I’m a Philadelphia resident and I say to you, Dr. Sung, I want to go home. You say, well, Philadelphia is too far. What is close enough and not too far, when you say home?
Dr. Sung: Absolutely. So when we originally conceived the program, we focused on patients who lived within an hour of our transplant center. And in part, that was because, as you know, unfortunately, things can sometimes go wrong during transplant. One of the most concerning ones is infections. And if a patient were to develop a neutropenic fever, we would want them to be seen as urgently as possible within an hour. And that’s where our limitation comes from.
So for our patients who live more than an hour away, those are the ones that we will have relocate to temporary lodging near our transplant center. And we’ve worked with several facilities in the area that have clean, furnished units that are available for rent. Many insurances also include lodging benefits for patients during stem cell transplant, recognizing this need. And historically, those [patients] were not considered part of our transplant patient cohorts.
I have not mentioned, but we initially did this in a phase 1 study, and we’re now studying it in a series of randomized, phase 2 studies that I can go into detail later on. And because they were not necessarily in their home, but a temporary lodging environment, those patients who relocated to Durham were not eligible for a home transplant study.
However, in the setting of the COVID-19 pandemic, we’ve actually pivoted our program in many ways. Specifically, if you think about a patient who’s coming into contact with the medical system, they come to the hospital, they meet someone at the door who is screening them for COVID-19. They see someone who checks them in at the front desk. A medical assistant takes them in the back. Someone calls their labs and phlebotomy. They may encounter other patients and environmental services, other individuals in the setting. You’re talking about dozens of different encounters. Who knows how many surfaces that potentially someone with COVID-19 has coughed on or contaminated?
And in contrast, you have house calls, which even if they are located in the temporary lodging, that’s just one or two individuals going into their living environment. They’re not encountering any different surfaces. And so, in the setting of COVID-19, we felt that this platform had the potential to help protect all our transplant patients who are among the most vulnerable patients, the most immunocompromised patients, and so we expanded our program to include those individuals as well.
Dr. Henry: So ... what are the actual outcomes of your patients in terms of how they’re doing, engrafting, and getting cured of their malignancy?
Dr. Sung: So as I mentioned, we first did this in a phase 1 safety and feasibility pilot study of both autologous and allo-transplant patients. This was presented at the annual meeting of the American Society of Hematology [Blood. 2017;130:745]. And we’re actually about ready to submit our manuscript on this.
And we found no difference in outcomes between patients who received care in the home transplant setting versus those who received conventional care either in the day hospital or hospital environment. The process appeared safe. Patients did just as well, if not better. Certainly, anecdotally, patients would talk about feeling so much more comfortable and happier being cared for in that home environment.
And we are now in the process of formally studying these outcomes in two NIH [National Institutes of Health]-funded clinical trials, one focused on allogeneic transplant patients [NCT02218151] and the other focused on autologous transplant patients [NCT01725022].
Dr. Henry: So of course, I’m waiting for this next question, which is cost. The services are the same, but you have people traveling, people who are highly skilled caregivers. Have you looked at cost differences from hospital versus home?
Dr. Sung: Absolutely. So you do have increased upfront costs because you have travel time for advanced practice providers and nurses. Not only that, but when a nurse is helping to give a patient a blood transfusion in the home environment, they’re 1:1 with that patient as opposed to the day hospital where a nurse could help with transfusions simultaneously for multiple patients. At the same time, by keeping patients out of the hospital, you have drastic, significant cost savings in that way.
In addition, I should mention, part of why we’re conducting these randomized, phase 2 clinical trials is we believe home care actually has the potential to decrease complications. One area of my research is on the impact of the microbiome, the bacteria in the gut, on transplant outcomes. And we’ve done a number of studies, many in collaboration with Memorial Sloan Kettering, showing that disruption of the microbiota, the bacteria in the gut, is associated with increased infections, graft-versus-host disease, and treatment-related mortality if we’re able to keep patients in their home setting.
However, I actually should go back a step. It’s well known that, if you take an individual from their home setting and put them in a foreign environment such as the hospital, that new environment, that new diet, hospital food as opposed to home food, and so forth, can dramatically shift the microbiome. Our hypothesis is that, by keeping patients in the home environment, their familiar environment will be able to help preserve their microbiome, thus decreasing infections, graft-versus-host disease, and other complications. That’s actually the goal of our studies: to see if we can preserve the microbiome and decrease complications.
Dr. Henry: So how will you evaluate that? Are you doing fecal studies, patient culture studies? How are you testing that?
Dr. Sung: So we have a very broad biobank program where we collect stool on our transplantations, pretransplant, day 0, weekly for the first month. And then, in the case of our allogeneic transplant patients, day 60, 90, 180, and 365.
And we do that both in our home transplant patients as well as their matched controls on the phase 2 studies. And we also collect it on a lot of our other transplant patients as part of our biobanking programs and our observational studies to try to understand what’s going on during transplant and how to help improve transplant outcomes.
Dr. Henry: Do you have any results of that? You’re probably showing a difference.
Dr. Sung: We think so, on some preliminary results, but those were based on small numbers of patients. And we’re really hoping that these randomized clinical trials with the larger numbers of patients enrolled will help show that difference.
But getting back to your earlier question about cost, a case of graft-versus-host disease, grade 2 or higher, can add about $100,000 to the cost of care. So if you prevent one case of bad gut or liver graft-versus-host disease, those are your cost savings right there.
The randomized, phase 2 trial for allogeneic transplant patients, the primary endpoint is graft-versus-host disease. So we’re looking at the microbiome and those associations and the prevention of GVHD. For the randomized clinical trial in autologous transplant patients – with autologous stem cells, you’re not going to get GVHD – but we do hope to improve quality of life and long-term outcomes in those patients as well.
Dr. Henry: Wonderful. Well, Tony, I really want to thank you so much for talking with us today.
Dr. Sung: Thank you very much for this opportunity. And again, I also want to just thank everyone who’s been involved in these studies, the advanced practice providers and nurses who are caring for our patients at home, the study staff who have been involved. Particularly, I’d like to highlight the role of both Nelson Chao, who’s our division chief and my mentor who piloted and first developed home transplant, and Kristin Nichols, our research nurse who has really led the drive forward.
Dr. Sung and Dr. Henry have no relevant disclosures. The trials are funded by grants from the National Institutes of Health.
For SCC, legs are a high-risk anatomic site in women
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Keeping the Differential at Hand
ANSWER
The correct answer is granuloma annulare (GA; choice “a”).
DISCUSSION
One of the most difficult concepts to grasp in dermatologic diagnosis is that almost all lesions and conditions—even the most common—have a broad range of morphologic presentations. These will often differ from the textbook photos. In this digital age, a simple Internet search will provide many results showing a diverse morphologic spectrum for many diseases, conditions, and lesions.
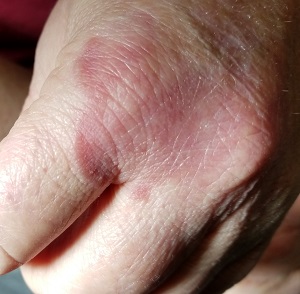
GA is a good example of how the presentation can vary. It has raised rolled margins and delled (gently concave) centers. The brownish red color is typical, but this patient showed a deeper red than most cases of GA. Occasionally, the red color is even deeper, with large patches of darkened skin and no palpable component.
Unfortunately, the misdiagnosis of fungal infection in this patient is typical. But dermatophytosis (otherwise known as “ringworm”)—the most common fungal skin infection—involves the epidermis. This means the patient would have scaly skin, probably with a well-defined margin—factors missing in this case. In addition, this patient reported no contact with sources of infection: animals, children, or immunosuppressive agents.
Ultimately, the most basic information that this patient's past providers neglected was a differential diagnosis. By establishing a differential, there was a clear decision to biopsy, which not only revealed the correct diagnosis but effectively ruled out the other options.
In defense of the patient’s other providers, I must admit that as a young primary care provider, I made this same mistake for exactly the same reasons.
TREATMENT
Most cases of GA are mild and self-limited, requiring no treatment. This is fortunate because no effective treatment exists. Topical steroids and cryotherapy will lighten the lesion, but GA nearly always resolves on its own.
ANSWER
The correct answer is granuloma annulare (GA; choice “a”).
DISCUSSION
One of the most difficult concepts to grasp in dermatologic diagnosis is that almost all lesions and conditions—even the most common—have a broad range of morphologic presentations. These will often differ from the textbook photos. In this digital age, a simple Internet search will provide many results showing a diverse morphologic spectrum for many diseases, conditions, and lesions.
GA is a good example of how the presentation can vary. It has raised rolled margins and delled (gently concave) centers. The brownish red color is typical, but this patient showed a deeper red than most cases of GA. Occasionally, the red color is even deeper, with large patches of darkened skin and no palpable component.
Unfortunately, the misdiagnosis of fungal infection in this patient is typical. But dermatophytosis (otherwise known as “ringworm”)—the most common fungal skin infection—involves the epidermis. This means the patient would have scaly skin, probably with a well-defined margin—factors missing in this case. In addition, this patient reported no contact with sources of infection: animals, children, or immunosuppressive agents.
Ultimately, the most basic information that this patient's past providers neglected was a differential diagnosis. By establishing a differential, there was a clear decision to biopsy, which not only revealed the correct diagnosis but effectively ruled out the other options.
In defense of the patient’s other providers, I must admit that as a young primary care provider, I made this same mistake for exactly the same reasons.
TREATMENT
Most cases of GA are mild and self-limited, requiring no treatment. This is fortunate because no effective treatment exists. Topical steroids and cryotherapy will lighten the lesion, but GA nearly always resolves on its own.
ANSWER
The correct answer is granuloma annulare (GA; choice “a”).
DISCUSSION
One of the most difficult concepts to grasp in dermatologic diagnosis is that almost all lesions and conditions—even the most common—have a broad range of morphologic presentations. These will often differ from the textbook photos. In this digital age, a simple Internet search will provide many results showing a diverse morphologic spectrum for many diseases, conditions, and lesions.
GA is a good example of how the presentation can vary. It has raised rolled margins and delled (gently concave) centers. The brownish red color is typical, but this patient showed a deeper red than most cases of GA. Occasionally, the red color is even deeper, with large patches of darkened skin and no palpable component.
Unfortunately, the misdiagnosis of fungal infection in this patient is typical. But dermatophytosis (otherwise known as “ringworm”)—the most common fungal skin infection—involves the epidermis. This means the patient would have scaly skin, probably with a well-defined margin—factors missing in this case. In addition, this patient reported no contact with sources of infection: animals, children, or immunosuppressive agents.
Ultimately, the most basic information that this patient's past providers neglected was a differential diagnosis. By establishing a differential, there was a clear decision to biopsy, which not only revealed the correct diagnosis but effectively ruled out the other options.
In defense of the patient’s other providers, I must admit that as a young primary care provider, I made this same mistake for exactly the same reasons.
TREATMENT
Most cases of GA are mild and self-limited, requiring no treatment. This is fortunate because no effective treatment exists. Topical steroids and cryotherapy will lighten the lesion, but GA nearly always resolves on its own.
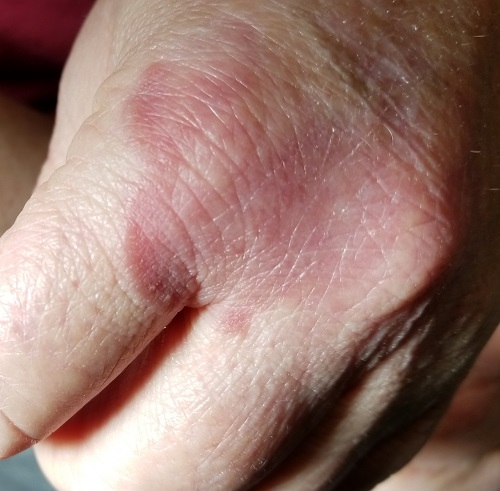
Several months ago, an asymptomatic rash slowly manifested on a 60-year-old woman’s hand. The rash—diagnosed previously as a fungal infection—continues to grow despite application of multiple antifungal creams, including tolnaftate, clotrimazole, and terbinafine. In addition, she was treated with a 1-month course of oral terbinafine 250 mg/d. Unfortunately, no treatment has provided her relief.
The patient is in otherwise good health. She denies any injury to the area. She also reports no exposure to children or animals.
The rash is a reddish brown, annular patch of skin that covers most of the dorsum of her left hand. The borders are slightly raised and thickened. It is nontender and readily blanchable. It is intradermal, with no surface disturbance such as scaling.
Punch biopsy shows palisaded granulomatous features, with no epidermal changes. Stains for fungi and bacteria fail to demonstrate any organisms.
Pfizer’s COVID-19 vaccine 95% effective in final phase 3 results
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
GLIMMER of hope for itch in primary biliary cholangitis
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Studies gauge toll of pausing fertility treatment during pandemic
More than 60% of patients at a center for reproductive medicine in Utah who had fertility treatments canceled because of the COVID-19 pandemic opted to resume treatment once the suspension was lifted about 7 weeks later.
At another fertility center in New York, a survey found that 96% of respondents who had a cycle canceled because of the pandemic found it upsetting, and 22% found it extremely upsetting, with extremely upsetting defined as equivalent to the loss of a child.
The indefinite time frame for resuming treatment when the New York survey was conducted may have been a major source of distress for patients, one of the researchers said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“They don’t know when they might have that chance again,” said Jenna M. Turocy, MD, of Columbia University Fertility Center, New York.
COVID-19 guidelines published by ASRM on March 17 recommended the suspension of new treatment cycles, including ovulation induction, intrauterine inseminations, and in vitro fertilization (IVF).
An ASRM COVID-19 task force has since supported “the measured resumption of fertility care following the easing of restrictions,” said Paul C. Lin, MD, president of the Society for Assisted Reproductive Technology and a member of the task force.
“Over the past several months, significant knowledge has been gained regarding the COVID-19 virus and its impact on patients and the medical system,” he said in a news release about the two studies that assessed the pandemic’s effects.
Certain precautions remain. “It has become clear that we will need to be practicing COVID-19 protocols at least until an effective and safe vaccine or broadly effective treatment becomes widely available,” Dr. Lin said.
Desire to proceed during a pandemic
The center continued to offer IVF cycles for oncofertility patients on an urgent basis.
In early May, patients whose cycles had been suspended had the option to receive treatment.
“Upon reopening, every patient received standardized counseling from their primary IVF physician,” Lauren Verrilli, MD, a reproductive endocrinology and infertility fellow at the University of Utah, Salt Lake City, said at the virtual meeting.
Doctors explained that much remained unknown about COVID-19 in pregnancy, and that it was unclear whether the clinic would need to shut down again. In addition, patients had to undergo COVID-19 testing.
To identify factors associated with proceeding with treatment after the suspension, the researchers compared patients who resumed treatment with patients who did not.
Their analysis included 278 patients who had planned an IVF cycle or frozen embryo transfer (FET) prior to the shutdown. The researchers examined factors such as age, parity, anti-Müllerian hormone, antral follicle count, history of prior IVF cycles or FET, number of frozen blastocysts, gamete source, and use of a gestational carrier.
In all, 62% of patients opted to receive treatment once restrictions were lifted, including 69 of the 133 (52%) patients with planned fresh cycles and 104 of the 145 (72%) patients with planned FET cycles.
Among those with planned fresh cycles, those who opted to resume treatment tended to be older than those who did not resume treatment, with a median age of 37 years versus 35 years, but the difference was not statistically significant.
Among patients with planned FET cycles, those who did not resume treatment were more likely to have a gestational carrier, compared with those who resumed treatment (7% vs. 1%). In some cases, gestational carriers lived in another state and the pandemic complicated travel arrangements, which contributed to delays, Dr. Verrilli said.
The analysis did not include information about income or socioeconomic status, which may play a role in patients’ decisions, Dr. Verrilli said.
Emotional impact of indefinite delay
Fertility treatment is often time sensitive, particularly for patients with advanced reproductive age or diminished ovarian reserve, and indefinite postponement of fertility treatment potentially could lead some patients to lose the ability to conceive with their own gametes, Dr. Turocy said.
In early April, Dr. Turocy and colleagues surveyed patients at their academic fertility center in New York City to assess patients’ reactions to the ASRM recommendations. They decided to conduct the study after they realized that treatment cancellations were having a significant emotional effect.
Investigators emailed an 18-item survey to more than 3,000 patients.
In all, 518 patients completed the survey, a response rate of 17%. Patients had an average age of 37 years (range, 23-52 years), and 92% were female. About 24% had children, and 66% had received at least one fertility treatment.
Half had a cycle canceled because of the COVID-19 pandemic, including timed intercourse cycles (5%), intrauterine insemination cycles (23%), IVF cycles with a planned fresh embryo transfer (10%), IVF with all frozen embryos (27%), egg freeze cycles (3%), and FET cycles (30%).
In response to survey questions about whether they agreed with ASRM recommendations, “the reactions were mixed,” Dr. Turocy said.
About 36% of patients agreed all fertility cycles should be canceled, 22% were unsure, and 43% disagreed with the recommendation. Patients who had a cycle canceled were slightly more likely to agree with the cancellations (40% agreed) than those who did not have a cycle canceled (30% agreed), Dr. Turocy said.
Most respondents would have preferred an option to start a treatment cycle in consultation with their doctor. Half “would have chosen to start a new cycle during the height of the pandemic in New York City,” Dr. Turocy said.
Patient opinions may vary by region and depend on the severity of COVID-19 outbreaks there, and they also might change over time, Dr. Turocy suggested. In addition, the opinions and characteristics of patients who responded to the anonymous survey may differ from those of patients who did not respond.
Dr. Verrilli, Dr. Turocy, and Dr. Lin had no relevant financial disclosures.
More than 60% of patients at a center for reproductive medicine in Utah who had fertility treatments canceled because of the COVID-19 pandemic opted to resume treatment once the suspension was lifted about 7 weeks later.
At another fertility center in New York, a survey found that 96% of respondents who had a cycle canceled because of the pandemic found it upsetting, and 22% found it extremely upsetting, with extremely upsetting defined as equivalent to the loss of a child.
The indefinite time frame for resuming treatment when the New York survey was conducted may have been a major source of distress for patients, one of the researchers said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“They don’t know when they might have that chance again,” said Jenna M. Turocy, MD, of Columbia University Fertility Center, New York.
COVID-19 guidelines published by ASRM on March 17 recommended the suspension of new treatment cycles, including ovulation induction, intrauterine inseminations, and in vitro fertilization (IVF).
An ASRM COVID-19 task force has since supported “the measured resumption of fertility care following the easing of restrictions,” said Paul C. Lin, MD, president of the Society for Assisted Reproductive Technology and a member of the task force.
“Over the past several months, significant knowledge has been gained regarding the COVID-19 virus and its impact on patients and the medical system,” he said in a news release about the two studies that assessed the pandemic’s effects.
Certain precautions remain. “It has become clear that we will need to be practicing COVID-19 protocols at least until an effective and safe vaccine or broadly effective treatment becomes widely available,” Dr. Lin said.
Desire to proceed during a pandemic
The center continued to offer IVF cycles for oncofertility patients on an urgent basis.
In early May, patients whose cycles had been suspended had the option to receive treatment.
“Upon reopening, every patient received standardized counseling from their primary IVF physician,” Lauren Verrilli, MD, a reproductive endocrinology and infertility fellow at the University of Utah, Salt Lake City, said at the virtual meeting.
Doctors explained that much remained unknown about COVID-19 in pregnancy, and that it was unclear whether the clinic would need to shut down again. In addition, patients had to undergo COVID-19 testing.
To identify factors associated with proceeding with treatment after the suspension, the researchers compared patients who resumed treatment with patients who did not.
Their analysis included 278 patients who had planned an IVF cycle or frozen embryo transfer (FET) prior to the shutdown. The researchers examined factors such as age, parity, anti-Müllerian hormone, antral follicle count, history of prior IVF cycles or FET, number of frozen blastocysts, gamete source, and use of a gestational carrier.
In all, 62% of patients opted to receive treatment once restrictions were lifted, including 69 of the 133 (52%) patients with planned fresh cycles and 104 of the 145 (72%) patients with planned FET cycles.
Among those with planned fresh cycles, those who opted to resume treatment tended to be older than those who did not resume treatment, with a median age of 37 years versus 35 years, but the difference was not statistically significant.
Among patients with planned FET cycles, those who did not resume treatment were more likely to have a gestational carrier, compared with those who resumed treatment (7% vs. 1%). In some cases, gestational carriers lived in another state and the pandemic complicated travel arrangements, which contributed to delays, Dr. Verrilli said.
The analysis did not include information about income or socioeconomic status, which may play a role in patients’ decisions, Dr. Verrilli said.
Emotional impact of indefinite delay
Fertility treatment is often time sensitive, particularly for patients with advanced reproductive age or diminished ovarian reserve, and indefinite postponement of fertility treatment potentially could lead some patients to lose the ability to conceive with their own gametes, Dr. Turocy said.
In early April, Dr. Turocy and colleagues surveyed patients at their academic fertility center in New York City to assess patients’ reactions to the ASRM recommendations. They decided to conduct the study after they realized that treatment cancellations were having a significant emotional effect.
Investigators emailed an 18-item survey to more than 3,000 patients.
In all, 518 patients completed the survey, a response rate of 17%. Patients had an average age of 37 years (range, 23-52 years), and 92% were female. About 24% had children, and 66% had received at least one fertility treatment.
Half had a cycle canceled because of the COVID-19 pandemic, including timed intercourse cycles (5%), intrauterine insemination cycles (23%), IVF cycles with a planned fresh embryo transfer (10%), IVF with all frozen embryos (27%), egg freeze cycles (3%), and FET cycles (30%).
In response to survey questions about whether they agreed with ASRM recommendations, “the reactions were mixed,” Dr. Turocy said.
About 36% of patients agreed all fertility cycles should be canceled, 22% were unsure, and 43% disagreed with the recommendation. Patients who had a cycle canceled were slightly more likely to agree with the cancellations (40% agreed) than those who did not have a cycle canceled (30% agreed), Dr. Turocy said.
Most respondents would have preferred an option to start a treatment cycle in consultation with their doctor. Half “would have chosen to start a new cycle during the height of the pandemic in New York City,” Dr. Turocy said.
Patient opinions may vary by region and depend on the severity of COVID-19 outbreaks there, and they also might change over time, Dr. Turocy suggested. In addition, the opinions and characteristics of patients who responded to the anonymous survey may differ from those of patients who did not respond.
Dr. Verrilli, Dr. Turocy, and Dr. Lin had no relevant financial disclosures.
More than 60% of patients at a center for reproductive medicine in Utah who had fertility treatments canceled because of the COVID-19 pandemic opted to resume treatment once the suspension was lifted about 7 weeks later.
At another fertility center in New York, a survey found that 96% of respondents who had a cycle canceled because of the pandemic found it upsetting, and 22% found it extremely upsetting, with extremely upsetting defined as equivalent to the loss of a child.
The indefinite time frame for resuming treatment when the New York survey was conducted may have been a major source of distress for patients, one of the researchers said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“They don’t know when they might have that chance again,” said Jenna M. Turocy, MD, of Columbia University Fertility Center, New York.
COVID-19 guidelines published by ASRM on March 17 recommended the suspension of new treatment cycles, including ovulation induction, intrauterine inseminations, and in vitro fertilization (IVF).
An ASRM COVID-19 task force has since supported “the measured resumption of fertility care following the easing of restrictions,” said Paul C. Lin, MD, president of the Society for Assisted Reproductive Technology and a member of the task force.
“Over the past several months, significant knowledge has been gained regarding the COVID-19 virus and its impact on patients and the medical system,” he said in a news release about the two studies that assessed the pandemic’s effects.
Certain precautions remain. “It has become clear that we will need to be practicing COVID-19 protocols at least until an effective and safe vaccine or broadly effective treatment becomes widely available,” Dr. Lin said.
Desire to proceed during a pandemic
The center continued to offer IVF cycles for oncofertility patients on an urgent basis.
In early May, patients whose cycles had been suspended had the option to receive treatment.
“Upon reopening, every patient received standardized counseling from their primary IVF physician,” Lauren Verrilli, MD, a reproductive endocrinology and infertility fellow at the University of Utah, Salt Lake City, said at the virtual meeting.
Doctors explained that much remained unknown about COVID-19 in pregnancy, and that it was unclear whether the clinic would need to shut down again. In addition, patients had to undergo COVID-19 testing.
To identify factors associated with proceeding with treatment after the suspension, the researchers compared patients who resumed treatment with patients who did not.
Their analysis included 278 patients who had planned an IVF cycle or frozen embryo transfer (FET) prior to the shutdown. The researchers examined factors such as age, parity, anti-Müllerian hormone, antral follicle count, history of prior IVF cycles or FET, number of frozen blastocysts, gamete source, and use of a gestational carrier.
In all, 62% of patients opted to receive treatment once restrictions were lifted, including 69 of the 133 (52%) patients with planned fresh cycles and 104 of the 145 (72%) patients with planned FET cycles.
Among those with planned fresh cycles, those who opted to resume treatment tended to be older than those who did not resume treatment, with a median age of 37 years versus 35 years, but the difference was not statistically significant.
Among patients with planned FET cycles, those who did not resume treatment were more likely to have a gestational carrier, compared with those who resumed treatment (7% vs. 1%). In some cases, gestational carriers lived in another state and the pandemic complicated travel arrangements, which contributed to delays, Dr. Verrilli said.
The analysis did not include information about income or socioeconomic status, which may play a role in patients’ decisions, Dr. Verrilli said.
Emotional impact of indefinite delay
Fertility treatment is often time sensitive, particularly for patients with advanced reproductive age or diminished ovarian reserve, and indefinite postponement of fertility treatment potentially could lead some patients to lose the ability to conceive with their own gametes, Dr. Turocy said.
In early April, Dr. Turocy and colleagues surveyed patients at their academic fertility center in New York City to assess patients’ reactions to the ASRM recommendations. They decided to conduct the study after they realized that treatment cancellations were having a significant emotional effect.
Investigators emailed an 18-item survey to more than 3,000 patients.
In all, 518 patients completed the survey, a response rate of 17%. Patients had an average age of 37 years (range, 23-52 years), and 92% were female. About 24% had children, and 66% had received at least one fertility treatment.
Half had a cycle canceled because of the COVID-19 pandemic, including timed intercourse cycles (5%), intrauterine insemination cycles (23%), IVF cycles with a planned fresh embryo transfer (10%), IVF with all frozen embryos (27%), egg freeze cycles (3%), and FET cycles (30%).
In response to survey questions about whether they agreed with ASRM recommendations, “the reactions were mixed,” Dr. Turocy said.
About 36% of patients agreed all fertility cycles should be canceled, 22% were unsure, and 43% disagreed with the recommendation. Patients who had a cycle canceled were slightly more likely to agree with the cancellations (40% agreed) than those who did not have a cycle canceled (30% agreed), Dr. Turocy said.
Most respondents would have preferred an option to start a treatment cycle in consultation with their doctor. Half “would have chosen to start a new cycle during the height of the pandemic in New York City,” Dr. Turocy said.
Patient opinions may vary by region and depend on the severity of COVID-19 outbreaks there, and they also might change over time, Dr. Turocy suggested. In addition, the opinions and characteristics of patients who responded to the anonymous survey may differ from those of patients who did not respond.
Dr. Verrilli, Dr. Turocy, and Dr. Lin had no relevant financial disclosures.
FROM ASRM 2020
Dermatologists and the history of skin care and beauty devices: Part 4
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
Breast Cancer Journal Scans: November 2020
The utilization of neoadjuvant chemotherapy is increasing, and is considered standard of care for the majority of patients with HER2-positive and triple-negative breast cancer subtypes. A significant benefit of neoadjuvant systemic therapy is assessment of the tumor biology via chemotherapy response, which has prognostic implications and additionally can help tailor therapy in the adjuvant setting. In the era of precision medicine, de-escalation strategies are considered to provide patients with efficacious treatment and spare toxicity. Tasoulis et al assessed the accuracy of image-guided biopsy after neoadjuvant therapy to predict residual disease, and found a false negative rate (FNR) of 3.2% and negative predictive value (NPV) of 97.4% when the residual imaging abnormality was 2cm or smaller with a minimum of 6 vacuum-assisted biopsies performed. They also demonstrated a FNR of 4.2% and NPV of 97.2% in the subgroup of patients with ERBB2-positive and triple-negative subtypes, those most likely to achieve a pathologic complete response. These findings suggest that local therapy de-escalation may be a relevant strategy for those patients who have excellent responses to neoadjuvant chemotherapy.
Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) have historically been, and still are, considered important prognostic and predictive factors. Beyond these, the role of genomic assays in breast cancer is evolving, and the 21-gene recurrence score is a valuable prognostic tool to assess chemotherapy benefit in women with early-stage ER-positive, HER2-negative breast cancer. Precision medicine in oncology strives to identify and apply an individualized approach to cancer care based on strong scientific data. Zhang and colleagues demonstrated the prognostic ability of an 8 DNA repair-related gene signature that was constructed from gene expression profiles from over 1,000 women diagnosed with breast cancer. These genes have roles in ER-mediated transactivation, DNA damage repair and cell adhesion processes. Additionally, Kudela et al showed that microRNAs (mRNAs), which are small RNAs that regulate gene expression, are able to classify intrinsic breast cancer subtype, play a role in endocrine resistance, and may also enhance the function of predictive models such as the 21-gene recurrence score (Kudela). Ongoing research in the field of tumor genomics will help advance the field of personalized treatment for breast cancer.
Erin Roesch, MD
The Cleveland Clinic
References:
Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, Chagpar AB, Pusztai L, Lannin DR. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol. 2015;33:4267-76.
Heil J, Schaefgen B, Sinn P, Harcos A, Gomez C, Stieber A, Hennings A, Schuetz F, Sohn C, Schneeweiss A, Golatta M. Diagnosis of pathological complete response to neoadjuvant chemotherapy in breast cancer by minimal invasive biopsy techniques. Br J Cancer. 2015;113:1565-70.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111-21.
Emmadi R, Canestrari E, Arbieva ZH, Mu W, Dai Y, Frasor J, Wiley E. Correlative analysis of miRNA expression and Oncotype Dx Recurrence Score in estrogen receptor positive breast carcinomas. PLoS One. 2015;10:e0145346.
The utilization of neoadjuvant chemotherapy is increasing, and is considered standard of care for the majority of patients with HER2-positive and triple-negative breast cancer subtypes. A significant benefit of neoadjuvant systemic therapy is assessment of the tumor biology via chemotherapy response, which has prognostic implications and additionally can help tailor therapy in the adjuvant setting. In the era of precision medicine, de-escalation strategies are considered to provide patients with efficacious treatment and spare toxicity. Tasoulis et al assessed the accuracy of image-guided biopsy after neoadjuvant therapy to predict residual disease, and found a false negative rate (FNR) of 3.2% and negative predictive value (NPV) of 97.4% when the residual imaging abnormality was 2cm or smaller with a minimum of 6 vacuum-assisted biopsies performed. They also demonstrated a FNR of 4.2% and NPV of 97.2% in the subgroup of patients with ERBB2-positive and triple-negative subtypes, those most likely to achieve a pathologic complete response. These findings suggest that local therapy de-escalation may be a relevant strategy for those patients who have excellent responses to neoadjuvant chemotherapy.
Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) have historically been, and still are, considered important prognostic and predictive factors. Beyond these, the role of genomic assays in breast cancer is evolving, and the 21-gene recurrence score is a valuable prognostic tool to assess chemotherapy benefit in women with early-stage ER-positive, HER2-negative breast cancer. Precision medicine in oncology strives to identify and apply an individualized approach to cancer care based on strong scientific data. Zhang and colleagues demonstrated the prognostic ability of an 8 DNA repair-related gene signature that was constructed from gene expression profiles from over 1,000 women diagnosed with breast cancer. These genes have roles in ER-mediated transactivation, DNA damage repair and cell adhesion processes. Additionally, Kudela et al showed that microRNAs (mRNAs), which are small RNAs that regulate gene expression, are able to classify intrinsic breast cancer subtype, play a role in endocrine resistance, and may also enhance the function of predictive models such as the 21-gene recurrence score (Kudela). Ongoing research in the field of tumor genomics will help advance the field of personalized treatment for breast cancer.
Erin Roesch, MD
The Cleveland Clinic
References:
Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, Chagpar AB, Pusztai L, Lannin DR. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol. 2015;33:4267-76.
Heil J, Schaefgen B, Sinn P, Harcos A, Gomez C, Stieber A, Hennings A, Schuetz F, Sohn C, Schneeweiss A, Golatta M. Diagnosis of pathological complete response to neoadjuvant chemotherapy in breast cancer by minimal invasive biopsy techniques. Br J Cancer. 2015;113:1565-70.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111-21.
Emmadi R, Canestrari E, Arbieva ZH, Mu W, Dai Y, Frasor J, Wiley E. Correlative analysis of miRNA expression and Oncotype Dx Recurrence Score in estrogen receptor positive breast carcinomas. PLoS One. 2015;10:e0145346.
The utilization of neoadjuvant chemotherapy is increasing, and is considered standard of care for the majority of patients with HER2-positive and triple-negative breast cancer subtypes. A significant benefit of neoadjuvant systemic therapy is assessment of the tumor biology via chemotherapy response, which has prognostic implications and additionally can help tailor therapy in the adjuvant setting. In the era of precision medicine, de-escalation strategies are considered to provide patients with efficacious treatment and spare toxicity. Tasoulis et al assessed the accuracy of image-guided biopsy after neoadjuvant therapy to predict residual disease, and found a false negative rate (FNR) of 3.2% and negative predictive value (NPV) of 97.4% when the residual imaging abnormality was 2cm or smaller with a minimum of 6 vacuum-assisted biopsies performed. They also demonstrated a FNR of 4.2% and NPV of 97.2% in the subgroup of patients with ERBB2-positive and triple-negative subtypes, those most likely to achieve a pathologic complete response. These findings suggest that local therapy de-escalation may be a relevant strategy for those patients who have excellent responses to neoadjuvant chemotherapy.
Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) have historically been, and still are, considered important prognostic and predictive factors. Beyond these, the role of genomic assays in breast cancer is evolving, and the 21-gene recurrence score is a valuable prognostic tool to assess chemotherapy benefit in women with early-stage ER-positive, HER2-negative breast cancer. Precision medicine in oncology strives to identify and apply an individualized approach to cancer care based on strong scientific data. Zhang and colleagues demonstrated the prognostic ability of an 8 DNA repair-related gene signature that was constructed from gene expression profiles from over 1,000 women diagnosed with breast cancer. These genes have roles in ER-mediated transactivation, DNA damage repair and cell adhesion processes. Additionally, Kudela et al showed that microRNAs (mRNAs), which are small RNAs that regulate gene expression, are able to classify intrinsic breast cancer subtype, play a role in endocrine resistance, and may also enhance the function of predictive models such as the 21-gene recurrence score (Kudela). Ongoing research in the field of tumor genomics will help advance the field of personalized treatment for breast cancer.
Erin Roesch, MD
The Cleveland Clinic
References:
Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, Chagpar AB, Pusztai L, Lannin DR. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol. 2015;33:4267-76.
Heil J, Schaefgen B, Sinn P, Harcos A, Gomez C, Stieber A, Hennings A, Schuetz F, Sohn C, Schneeweiss A, Golatta M. Diagnosis of pathological complete response to neoadjuvant chemotherapy in breast cancer by minimal invasive biopsy techniques. Br J Cancer. 2015;113:1565-70.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111-21.
Emmadi R, Canestrari E, Arbieva ZH, Mu W, Dai Y, Frasor J, Wiley E. Correlative analysis of miRNA expression and Oncotype Dx Recurrence Score in estrogen receptor positive breast carcinomas. PLoS One. 2015;10:e0145346.
MicroRNAs show promising predictive value for early breast cancer
Key clinical point: Differences in microRNAs (mRNAs), a group of small RNAs that regulate gene expression, can be used to distinguish among breast cancer subtypes.
Major finding: Altered expression of microRNAs distinguished between cancer and healthy samples, and also identified breast cancer subtypes including HER2, Luminal A, Luminal B, and triple negative breast cancer, according to a retrospective study of 740 breast cancer cases included in the review.
Study details: The data come from a review of the latest research on the prognostic and predictive value of microRNAs in patients with luminal A breast cancer.
Disclosures: The study was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic, the Slovak Research and Development Agency, and the Operational Programme Research and Innovation funded by the ERDF.
Citation: Kudela E et al. Int J Mol Sci. 2020 Oct 17. doi: 10.3390/ijms21207691.
Key clinical point: Differences in microRNAs (mRNAs), a group of small RNAs that regulate gene expression, can be used to distinguish among breast cancer subtypes.
Major finding: Altered expression of microRNAs distinguished between cancer and healthy samples, and also identified breast cancer subtypes including HER2, Luminal A, Luminal B, and triple negative breast cancer, according to a retrospective study of 740 breast cancer cases included in the review.
Study details: The data come from a review of the latest research on the prognostic and predictive value of microRNAs in patients with luminal A breast cancer.
Disclosures: The study was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic, the Slovak Research and Development Agency, and the Operational Programme Research and Innovation funded by the ERDF.
Citation: Kudela E et al. Int J Mol Sci. 2020 Oct 17. doi: 10.3390/ijms21207691.
Key clinical point: Differences in microRNAs (mRNAs), a group of small RNAs that regulate gene expression, can be used to distinguish among breast cancer subtypes.
Major finding: Altered expression of microRNAs distinguished between cancer and healthy samples, and also identified breast cancer subtypes including HER2, Luminal A, Luminal B, and triple negative breast cancer, according to a retrospective study of 740 breast cancer cases included in the review.
Study details: The data come from a review of the latest research on the prognostic and predictive value of microRNAs in patients with luminal A breast cancer.
Disclosures: The study was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic, the Slovak Research and Development Agency, and the Operational Programme Research and Innovation funded by the ERDF.
Citation: Kudela E et al. Int J Mol Sci. 2020 Oct 17. doi: 10.3390/ijms21207691.
Income loss shows no link to stress levels in young women with breast cancer
Key clinical point: Over a 12-month period, 15.4% of women with early breast cancer reported losing income. Although stress, anxiety, and depression were not association with household income changes (risk ratios 2.42, 1.12, and 1.41, respectively), the proportion of women reporting high stress was greatest among those who lost income (13.2%, compared to 3.1% among women maintaining an income of $100,000 or higher).
Major finding: Women with a household income below $50,000 had a higher risk of losing household income compared to those with incomes of $50,000 or higher, suggesting that lower income women may be more vulnerable to income loss after diagnosis with breast cancer.
Study details: The data come from a prospective, longitudinal cohort study including 467 women with early breast cancer enrolled in the Young and Strong cohort trial from 2012 to 2013.
Disclosures: The study was supported by an ASCO Improving Cancer Care grant, the National Institutes of Health, an NIH training grants. The researchers had no financial conflicts to disclose.
Citation: Cook EE et al. BMC Public Health. 2020 Oct 6. doi: 10.1186/s12889-020-09562-z.
Key clinical point: Over a 12-month period, 15.4% of women with early breast cancer reported losing income. Although stress, anxiety, and depression were not association with household income changes (risk ratios 2.42, 1.12, and 1.41, respectively), the proportion of women reporting high stress was greatest among those who lost income (13.2%, compared to 3.1% among women maintaining an income of $100,000 or higher).
Major finding: Women with a household income below $50,000 had a higher risk of losing household income compared to those with incomes of $50,000 or higher, suggesting that lower income women may be more vulnerable to income loss after diagnosis with breast cancer.
Study details: The data come from a prospective, longitudinal cohort study including 467 women with early breast cancer enrolled in the Young and Strong cohort trial from 2012 to 2013.
Disclosures: The study was supported by an ASCO Improving Cancer Care grant, the National Institutes of Health, an NIH training grants. The researchers had no financial conflicts to disclose.
Citation: Cook EE et al. BMC Public Health. 2020 Oct 6. doi: 10.1186/s12889-020-09562-z.
Key clinical point: Over a 12-month period, 15.4% of women with early breast cancer reported losing income. Although stress, anxiety, and depression were not association with household income changes (risk ratios 2.42, 1.12, and 1.41, respectively), the proportion of women reporting high stress was greatest among those who lost income (13.2%, compared to 3.1% among women maintaining an income of $100,000 or higher).
Major finding: Women with a household income below $50,000 had a higher risk of losing household income compared to those with incomes of $50,000 or higher, suggesting that lower income women may be more vulnerable to income loss after diagnosis with breast cancer.
Study details: The data come from a prospective, longitudinal cohort study including 467 women with early breast cancer enrolled in the Young and Strong cohort trial from 2012 to 2013.
Disclosures: The study was supported by an ASCO Improving Cancer Care grant, the National Institutes of Health, an NIH training grants. The researchers had no financial conflicts to disclose.
Citation: Cook EE et al. BMC Public Health. 2020 Oct 6. doi: 10.1186/s12889-020-09562-z.