User login
FDA approves first at-home COVID-19 test kit
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
Tildrakizumab for psoriasis shows durable efficacy over 5 years
The full during more than 5,400 patient-years of prospective follow-up, Diamont Thaçi, MD, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
For example, 89% of patients who had a PASI-75 response on the 100-mg dose of tildrakizumab (Ilumya) – the dose approved in the United States – at week 28 in the parent reSURFACE 1 and reSURFACE 2 trials maintained their PASI-75 response throughout the next 4½ years in the long-term extension study, as did 93% of those with a week 28 PASI-75 response on 200 mg, a dose approved elsewhere, said Dr. Thaçi, professor of dermatology and director of the Comprehensive Center for Inflammation Medicine at Lübeck (Germany) University.
The same held true for PASI-90, a response achieved by 71% of participants on 100 mg of tildrakizumab at week 28 and 66% at week 244, and by 73% of those on the 200-mg dose at week 28 and 70% at 5 years. A PASI-100 response was documented at week 28 in 29% of patients on the lower dose and 37% of those on 200 mg, with week 244 PASI-100 rates of 33% and 41%, respectively.
The long-term extension study enrolled 622 patients with moderate to severe chronic plaque psoriasis with at least a PASI-75 response to 100 mg or 200 mg of the humanized monoclonal antibody interleukin-23p19 inhibitor at week 28 in reSURFACE 1 or 2, or who were partial or nonresponders to etanercept in reSURFACE 2 and were then switched to tildrakizumab at 200 mg. Five hundred and forty-five of the 622 patients (88%) completed the full 5 years of the extension study.
Very few patients left the study because of loss of efficacy or adverse events. Indeed, the exposure-adjusted rate of drug-related serious adverse events was 0.8 cases per 100 patient-years at tildrakizumab 100 mg and 0.5 per 100 patient-years at 200 mg. Moreover, the rates of drug-related serious adverse events leading to treatment continuation were 0.3 and 0.2 per 100 patient-years at the 100-mg and 200-mg doses. Rates of treatment-emergent severe infection were 1.2 and 1.3 per 100 patient-years on the lower and higher doses. Major adverse cardiovascular events occurred at rates of 0.5 and 0.7 cases per 100 patient-years.
“I think the adverse events are generally similar to what has been seen with other biologics, but slightly less with tildrakizumab. Registries will provide a clearer picture. What’s interesting is that even if you double the dosage you don’t see an increase in side effects,” Dr. Thaçi said.
Asked what happens when a tildrakizumab responder stops taking the monoclonal antibody, he replied, “This is something very interesting we see with the IL-23 inhibitors: The disease comes back very slowly. It takes months, and sometimes years, for the patient to lose the PASI-75 or even the PASI-90 response. But we still consider that continuous treatment is probably the better way to go because we cannot be sure who will lose or regain response. At the moment we don’t have a biomarker to tell us what we should do in our daily practice.”
Dr. Thaçi reported serving as an adviser to and paid investigator for Almirall, the study sponsor, and approximately 20 other pharmaceutical companies.
The full during more than 5,400 patient-years of prospective follow-up, Diamont Thaçi, MD, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
For example, 89% of patients who had a PASI-75 response on the 100-mg dose of tildrakizumab (Ilumya) – the dose approved in the United States – at week 28 in the parent reSURFACE 1 and reSURFACE 2 trials maintained their PASI-75 response throughout the next 4½ years in the long-term extension study, as did 93% of those with a week 28 PASI-75 response on 200 mg, a dose approved elsewhere, said Dr. Thaçi, professor of dermatology and director of the Comprehensive Center for Inflammation Medicine at Lübeck (Germany) University.
The same held true for PASI-90, a response achieved by 71% of participants on 100 mg of tildrakizumab at week 28 and 66% at week 244, and by 73% of those on the 200-mg dose at week 28 and 70% at 5 years. A PASI-100 response was documented at week 28 in 29% of patients on the lower dose and 37% of those on 200 mg, with week 244 PASI-100 rates of 33% and 41%, respectively.
The long-term extension study enrolled 622 patients with moderate to severe chronic plaque psoriasis with at least a PASI-75 response to 100 mg or 200 mg of the humanized monoclonal antibody interleukin-23p19 inhibitor at week 28 in reSURFACE 1 or 2, or who were partial or nonresponders to etanercept in reSURFACE 2 and were then switched to tildrakizumab at 200 mg. Five hundred and forty-five of the 622 patients (88%) completed the full 5 years of the extension study.
Very few patients left the study because of loss of efficacy or adverse events. Indeed, the exposure-adjusted rate of drug-related serious adverse events was 0.8 cases per 100 patient-years at tildrakizumab 100 mg and 0.5 per 100 patient-years at 200 mg. Moreover, the rates of drug-related serious adverse events leading to treatment continuation were 0.3 and 0.2 per 100 patient-years at the 100-mg and 200-mg doses. Rates of treatment-emergent severe infection were 1.2 and 1.3 per 100 patient-years on the lower and higher doses. Major adverse cardiovascular events occurred at rates of 0.5 and 0.7 cases per 100 patient-years.
“I think the adverse events are generally similar to what has been seen with other biologics, but slightly less with tildrakizumab. Registries will provide a clearer picture. What’s interesting is that even if you double the dosage you don’t see an increase in side effects,” Dr. Thaçi said.
Asked what happens when a tildrakizumab responder stops taking the monoclonal antibody, he replied, “This is something very interesting we see with the IL-23 inhibitors: The disease comes back very slowly. It takes months, and sometimes years, for the patient to lose the PASI-75 or even the PASI-90 response. But we still consider that continuous treatment is probably the better way to go because we cannot be sure who will lose or regain response. At the moment we don’t have a biomarker to tell us what we should do in our daily practice.”
Dr. Thaçi reported serving as an adviser to and paid investigator for Almirall, the study sponsor, and approximately 20 other pharmaceutical companies.
The full during more than 5,400 patient-years of prospective follow-up, Diamont Thaçi, MD, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
For example, 89% of patients who had a PASI-75 response on the 100-mg dose of tildrakizumab (Ilumya) – the dose approved in the United States – at week 28 in the parent reSURFACE 1 and reSURFACE 2 trials maintained their PASI-75 response throughout the next 4½ years in the long-term extension study, as did 93% of those with a week 28 PASI-75 response on 200 mg, a dose approved elsewhere, said Dr. Thaçi, professor of dermatology and director of the Comprehensive Center for Inflammation Medicine at Lübeck (Germany) University.
The same held true for PASI-90, a response achieved by 71% of participants on 100 mg of tildrakizumab at week 28 and 66% at week 244, and by 73% of those on the 200-mg dose at week 28 and 70% at 5 years. A PASI-100 response was documented at week 28 in 29% of patients on the lower dose and 37% of those on 200 mg, with week 244 PASI-100 rates of 33% and 41%, respectively.
The long-term extension study enrolled 622 patients with moderate to severe chronic plaque psoriasis with at least a PASI-75 response to 100 mg or 200 mg of the humanized monoclonal antibody interleukin-23p19 inhibitor at week 28 in reSURFACE 1 or 2, or who were partial or nonresponders to etanercept in reSURFACE 2 and were then switched to tildrakizumab at 200 mg. Five hundred and forty-five of the 622 patients (88%) completed the full 5 years of the extension study.
Very few patients left the study because of loss of efficacy or adverse events. Indeed, the exposure-adjusted rate of drug-related serious adverse events was 0.8 cases per 100 patient-years at tildrakizumab 100 mg and 0.5 per 100 patient-years at 200 mg. Moreover, the rates of drug-related serious adverse events leading to treatment continuation were 0.3 and 0.2 per 100 patient-years at the 100-mg and 200-mg doses. Rates of treatment-emergent severe infection were 1.2 and 1.3 per 100 patient-years on the lower and higher doses. Major adverse cardiovascular events occurred at rates of 0.5 and 0.7 cases per 100 patient-years.
“I think the adverse events are generally similar to what has been seen with other biologics, but slightly less with tildrakizumab. Registries will provide a clearer picture. What’s interesting is that even if you double the dosage you don’t see an increase in side effects,” Dr. Thaçi said.
Asked what happens when a tildrakizumab responder stops taking the monoclonal antibody, he replied, “This is something very interesting we see with the IL-23 inhibitors: The disease comes back very slowly. It takes months, and sometimes years, for the patient to lose the PASI-75 or even the PASI-90 response. But we still consider that continuous treatment is probably the better way to go because we cannot be sure who will lose or regain response. At the moment we don’t have a biomarker to tell us what we should do in our daily practice.”
Dr. Thaçi reported serving as an adviser to and paid investigator for Almirall, the study sponsor, and approximately 20 other pharmaceutical companies.
FROM THE EADV CONGRESS
Risk factors for severe immune-related AEs identified
The first nationwide study of severe immune-related adverse events among cancer patients treated with immune checkpoint inhibitors helps identify those at elevated risk. The findings were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Immune-related adverse events are a very serious side effect of immune checkpoint inhibitor therapy, and as this therapy has become more common for treating advanced cancers, the incidence of immune-related adverse events has increased as well,” said presenting author William Murphy, a dual MD and MBA student at Harvard Medical School and Harvard Business School, both in Boston.
“However, because there is no ICD code for immune-related adverse events, it’s very difficult to study them at a population level. Most of the current literature around the incidence of immune-related adverse events and factors that are predictive of incidence are based on clinical trials and small studies,” Mr. Murphy noted.
He and his colleagues analyzed claims data from a U.S. nationwide health insurance plan for 14,378 patients who had a primary cancer and received at least one administration of an immune checkpoint inhibitor – an inhibitor of PD-1, PD-L1, or CTLA4 – during 2011-2019.
Over 19,117 patient-years of follow-up, 504 patients (3.5%) developed a severe immune-related adverse event (irAE), defined as one occurring within 2 years of their treatment and requiring inpatient hospitalization and new immunosuppression.
The incidence of severe irAEs per patient treatment year was 2.6% overall, rising from 0% in 2011 to 3.7% in 2016.
In multivariate analysis, patients had an elevated risk of severe irAEs if they received combination immunotherapy as compared with monotherapy (odds ratio, 2.44; P < .001).
On the other hand, risk fell with advancing age (OR, 0.98 per additional year; P < .001). And risk was lower for patients with melanoma (OR, 0.70; P = .01), renal cell carcinoma (OR, 0.71; P = .03), and other cancers (OR, 0.50; P < .001), compared with lung cancer.
Sex, geographic region, income, employment status, and comorbidity were not significantly associated with the risk of severe irAEs.
“We hope that patients and providers can use this evidence from a nationwide study of severe irAEs to guide treatment and management decisions,” Mr. Murphy concluded.
Real-world evidence
“As the use of immune checkpoint inhibitors increases for patients with a variety of different tumor types, there is increasing need for population-level evidence for patients treated outside of clinical trials,” said Allison Betof Warner, MD, PhD, an assistant attending physician with the melanoma service at Memorial Sloan Kettering Cancer Center in New York.
“This is a well-conducted study with an innovative approach to using real-world evidence to examine immune-related adverse events,” she added.
To her knowledge, it is the first study to look at multiple cancers for which immunotherapy is approved, Dr. Betof Warner said. This approach resulted in a large patient sample, giving power to detect differences between groups.
“The authors’ finding that combination immunotherapy is associated with more severe irAEs is in line with our clinical experience and other data sets, and the data regarding increased odds of severe irAEs in younger patients and those with lung cancer raise interesting biological questions about the etiology of irAEs,” Dr. Betof Warner noted.
However, certain factors complicate interpretation of the study’s findings, she cautioned. One such factor is requiring hospitalization to define an irAE.
“Practice patterns regarding hospitalization vary quite widely from center to center. For example, in some centers, all patients with immune-mediated colitis are hospitalized, whereas in others, these patients are managed predominantly in the outpatient setting, even in cases of high-grade toxicity,” she explained. “Practice patterns have also changed drastically over time as oncologists have grown more comfortable managing immune-related adverse events.”
Another factor is potential confounding. For example, patients with melanoma are more likely to receive combination immunotherapy given its longstanding approval for this cancer, whereas it is comparatively new for other cancers. Also, age may differ across cancers.
“The data the authors have provided are a great starting point, but I think further analysis is needed before these observations can be validated and integrated into practice,” Dr. Betof Warner concluded.
This study did not receive any specific funding. Mr. Murphy and Dr. Betof Warner disclosed no relevant conflicts of interest.
SOURCE: Murphy W et al. SITC 2020, Abstract 854.
The first nationwide study of severe immune-related adverse events among cancer patients treated with immune checkpoint inhibitors helps identify those at elevated risk. The findings were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Immune-related adverse events are a very serious side effect of immune checkpoint inhibitor therapy, and as this therapy has become more common for treating advanced cancers, the incidence of immune-related adverse events has increased as well,” said presenting author William Murphy, a dual MD and MBA student at Harvard Medical School and Harvard Business School, both in Boston.
“However, because there is no ICD code for immune-related adverse events, it’s very difficult to study them at a population level. Most of the current literature around the incidence of immune-related adverse events and factors that are predictive of incidence are based on clinical trials and small studies,” Mr. Murphy noted.
He and his colleagues analyzed claims data from a U.S. nationwide health insurance plan for 14,378 patients who had a primary cancer and received at least one administration of an immune checkpoint inhibitor – an inhibitor of PD-1, PD-L1, or CTLA4 – during 2011-2019.
Over 19,117 patient-years of follow-up, 504 patients (3.5%) developed a severe immune-related adverse event (irAE), defined as one occurring within 2 years of their treatment and requiring inpatient hospitalization and new immunosuppression.
The incidence of severe irAEs per patient treatment year was 2.6% overall, rising from 0% in 2011 to 3.7% in 2016.
In multivariate analysis, patients had an elevated risk of severe irAEs if they received combination immunotherapy as compared with monotherapy (odds ratio, 2.44; P < .001).
On the other hand, risk fell with advancing age (OR, 0.98 per additional year; P < .001). And risk was lower for patients with melanoma (OR, 0.70; P = .01), renal cell carcinoma (OR, 0.71; P = .03), and other cancers (OR, 0.50; P < .001), compared with lung cancer.
Sex, geographic region, income, employment status, and comorbidity were not significantly associated with the risk of severe irAEs.
“We hope that patients and providers can use this evidence from a nationwide study of severe irAEs to guide treatment and management decisions,” Mr. Murphy concluded.
Real-world evidence
“As the use of immune checkpoint inhibitors increases for patients with a variety of different tumor types, there is increasing need for population-level evidence for patients treated outside of clinical trials,” said Allison Betof Warner, MD, PhD, an assistant attending physician with the melanoma service at Memorial Sloan Kettering Cancer Center in New York.
“This is a well-conducted study with an innovative approach to using real-world evidence to examine immune-related adverse events,” she added.
To her knowledge, it is the first study to look at multiple cancers for which immunotherapy is approved, Dr. Betof Warner said. This approach resulted in a large patient sample, giving power to detect differences between groups.
“The authors’ finding that combination immunotherapy is associated with more severe irAEs is in line with our clinical experience and other data sets, and the data regarding increased odds of severe irAEs in younger patients and those with lung cancer raise interesting biological questions about the etiology of irAEs,” Dr. Betof Warner noted.
However, certain factors complicate interpretation of the study’s findings, she cautioned. One such factor is requiring hospitalization to define an irAE.
“Practice patterns regarding hospitalization vary quite widely from center to center. For example, in some centers, all patients with immune-mediated colitis are hospitalized, whereas in others, these patients are managed predominantly in the outpatient setting, even in cases of high-grade toxicity,” she explained. “Practice patterns have also changed drastically over time as oncologists have grown more comfortable managing immune-related adverse events.”
Another factor is potential confounding. For example, patients with melanoma are more likely to receive combination immunotherapy given its longstanding approval for this cancer, whereas it is comparatively new for other cancers. Also, age may differ across cancers.
“The data the authors have provided are a great starting point, but I think further analysis is needed before these observations can be validated and integrated into practice,” Dr. Betof Warner concluded.
This study did not receive any specific funding. Mr. Murphy and Dr. Betof Warner disclosed no relevant conflicts of interest.
SOURCE: Murphy W et al. SITC 2020, Abstract 854.
The first nationwide study of severe immune-related adverse events among cancer patients treated with immune checkpoint inhibitors helps identify those at elevated risk. The findings were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Immune-related adverse events are a very serious side effect of immune checkpoint inhibitor therapy, and as this therapy has become more common for treating advanced cancers, the incidence of immune-related adverse events has increased as well,” said presenting author William Murphy, a dual MD and MBA student at Harvard Medical School and Harvard Business School, both in Boston.
“However, because there is no ICD code for immune-related adverse events, it’s very difficult to study them at a population level. Most of the current literature around the incidence of immune-related adverse events and factors that are predictive of incidence are based on clinical trials and small studies,” Mr. Murphy noted.
He and his colleagues analyzed claims data from a U.S. nationwide health insurance plan for 14,378 patients who had a primary cancer and received at least one administration of an immune checkpoint inhibitor – an inhibitor of PD-1, PD-L1, or CTLA4 – during 2011-2019.
Over 19,117 patient-years of follow-up, 504 patients (3.5%) developed a severe immune-related adverse event (irAE), defined as one occurring within 2 years of their treatment and requiring inpatient hospitalization and new immunosuppression.
The incidence of severe irAEs per patient treatment year was 2.6% overall, rising from 0% in 2011 to 3.7% in 2016.
In multivariate analysis, patients had an elevated risk of severe irAEs if they received combination immunotherapy as compared with monotherapy (odds ratio, 2.44; P < .001).
On the other hand, risk fell with advancing age (OR, 0.98 per additional year; P < .001). And risk was lower for patients with melanoma (OR, 0.70; P = .01), renal cell carcinoma (OR, 0.71; P = .03), and other cancers (OR, 0.50; P < .001), compared with lung cancer.
Sex, geographic region, income, employment status, and comorbidity were not significantly associated with the risk of severe irAEs.
“We hope that patients and providers can use this evidence from a nationwide study of severe irAEs to guide treatment and management decisions,” Mr. Murphy concluded.
Real-world evidence
“As the use of immune checkpoint inhibitors increases for patients with a variety of different tumor types, there is increasing need for population-level evidence for patients treated outside of clinical trials,” said Allison Betof Warner, MD, PhD, an assistant attending physician with the melanoma service at Memorial Sloan Kettering Cancer Center in New York.
“This is a well-conducted study with an innovative approach to using real-world evidence to examine immune-related adverse events,” she added.
To her knowledge, it is the first study to look at multiple cancers for which immunotherapy is approved, Dr. Betof Warner said. This approach resulted in a large patient sample, giving power to detect differences between groups.
“The authors’ finding that combination immunotherapy is associated with more severe irAEs is in line with our clinical experience and other data sets, and the data regarding increased odds of severe irAEs in younger patients and those with lung cancer raise interesting biological questions about the etiology of irAEs,” Dr. Betof Warner noted.
However, certain factors complicate interpretation of the study’s findings, she cautioned. One such factor is requiring hospitalization to define an irAE.
“Practice patterns regarding hospitalization vary quite widely from center to center. For example, in some centers, all patients with immune-mediated colitis are hospitalized, whereas in others, these patients are managed predominantly in the outpatient setting, even in cases of high-grade toxicity,” she explained. “Practice patterns have also changed drastically over time as oncologists have grown more comfortable managing immune-related adverse events.”
Another factor is potential confounding. For example, patients with melanoma are more likely to receive combination immunotherapy given its longstanding approval for this cancer, whereas it is comparatively new for other cancers. Also, age may differ across cancers.
“The data the authors have provided are a great starting point, but I think further analysis is needed before these observations can be validated and integrated into practice,” Dr. Betof Warner concluded.
This study did not receive any specific funding. Mr. Murphy and Dr. Betof Warner disclosed no relevant conflicts of interest.
SOURCE: Murphy W et al. SITC 2020, Abstract 854.
FROM SITC 2020
Inattention to heightened CV risk common theme in clozapine deaths teaser
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.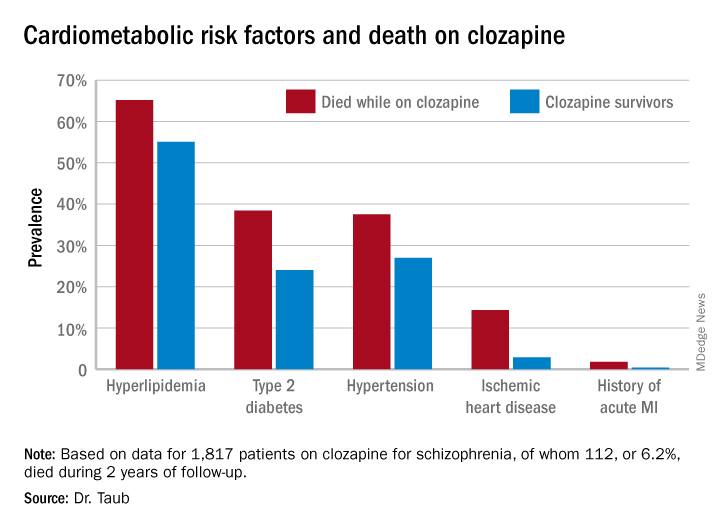
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
Death while on clozapine for schizophrenia is often associated with substandard treatment of cardiometabolic risk factors, Sharon Taub, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Inadequate treatment for metabolic syndrome was found to be a mortality predictor while on clozapine therapy. Patients who died were less likely to receive appropriate treatment for hyperlipidemia and type 2 diabetes, despite having been diagnosed with those conditions,” she said in presenting the results of her retrospective cohort study.
“Better preventive care, with special attention to those conditions, might prevent morbidity and improve life expectancy in this population,” concluded Dr. Taub of Geha Mental Health Center in Petah Tikva, Israel, and Tel Aviv University.
She reported on all 1,817 patients on clozapine for schizophrenia included in a large Israeli health care electronic medical records database, of whom 112, or 6.2%, died during 2 years of follow-up. Mortality while on the atypical antipsychotic was associated with a higher prevalence of hyperlipidemia, type 2 diabetes, hypertension, known ischemic heart disease, and a history of acute MI, compared with survivors.
Similarly, only 16.3% of those known to have type 2 diabetes who died while on clozapine were on hypoglycemic agents, compared with 67.1% of diabetic survivors. The between-group difference in the use of antihypertensive drug therapy for patients diagnosed with hypertension – 28.6% in nonsurvivors on clozapine, 40.1% in survivors – did not achieve statistical significance.
In a multivariate analysis adjusted for age, sex, and socioeconomic status, schizophrenia patients with type 2 diabetes who weren’t on hypoglycemic medication were at 695% increased risk of mortality, compared with those who were. Similarly, hyperlipidemic patients on clozapine who weren’t on a statin had a 579% increase in mortality risk.
Patients who died while on clozapine had no increased risk of use of medical services while living in the community.
This evidence of a pattern of inadequate care with regard to management of cardiometabolic risk factors in patients on clozapine is disturbing for several reasons. For one, clozapine is known to be associated with increased risk of serious side effects, including development or worsening of metabolic syndrome. Also, clozapine is an important drug in psychiatric practice: “Clozapine is the only antipsychotic indicated for refractory schizophrenia. It is highly effective in treatment-resistant disease, present in 25%-30% of individuals with schizophrenia,” Dr. Taub noted. “Clozapine is underused, mostly because of severe side effects. Its administration is often postponed.”
She reported having no financial conflicts regarding her study, which was voted by conference attendees one of the top presentations at ECNP 2020.
FROM ECNP 2020
Telehealth finds acceptance among patients with CF, clinicians
(CF) and the physicians who treat them, according to three new studies. The surveys examined attitudes during the COVID-19 pandemic, which complicates interpretation of the survey, but the results nevertheless bode well for telehealth’s future in the management of CF.
“Patients could be responding positively just because they could have a visit during the pandemic,” said Andrew NeSmith, during a presentation of a survey of adults with CF at the virtual North American Cystic Fibrosis Conference. Mr. NeSmith is the clinical data coordinator at the University of Alabama at Birmingham Cystic Fibrosis Center.
Other posters at the conference examined attitudes among pediatric populations and treating physicians, with generally positive results, which has generated optimism that telehealth could become an important element of care after the pandemic fades. “This data suggests that telehealth could be integrated into routine follow-up care in the CF chronic care model,” said Mr. NeSmith.
His team collected responses from 119 individuals at the University of Alabama at Birmingham; Boston Children’s Hospital; Brigham and Women’s Hospital, Boston; Virginia Commonwealth University, Richmond; and West Virginia University, Morgantown. A total of 28% had conducted a prior telehealth visit before the study; 92% of visits were conducted with a medical doctor. Only 13% reported experiencing difficulties with their first telehealth visit. Eighty-five percent rated convenience, and 77% rated their satisfaction with telehealth as “high.” Most (92%) said they were able to see their desired disciplines, 95% felt all of their issues had been addressed, and 83% strongly agreed that telehealth visits were of adequate length.
Not everything was rosy. A total of 48% of participants expressed at least moderate concern over a lack of pulmonary function test or throat/sputum culture. There were much fewer concerns over missing vital signs or weight measurements.
The overall results weren’t surprising to Robert Giusti, MD, clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center, New York, who was not involved in the study. “I was expecting that patients were going to like it. It makes their life easier,” he said in an interview.
A survey of families of pediatric individuals with CF at seven centers found similar levels of satisfaction. A total of 23% had used telehealth previously; 96% rated convenience, and 93% rated satisfaction as “high.” Almost all (99%) felt that all concerns were met, 98% said that sessions were adequately long, and 87% had no trouble connecting to the visit.
Some participants in this survey had concerns about what might be missing with a televisit. Half (52%) had at least a moderate concern over lack of pulmonary function tests, 45% over lack of vital signs, 29% about lack of weight measurements, and 64% about the need for throat/sputum culture. Despite those issues, 69% preferred that “some” and 22% preferred that “most” future visits be conducted by telehealth.
A survey of physicians who used telehealth with CF patients also found broad support. They reported some challenges, with 70% saying they experienced technical difficulty, and 77% saying it “took time” to resolve a visit with only 18% reporting that visits were “quickly resolved.” Most (86%) said they were satisfied with telehealth for care delivery, and 78% said it was appropriate for most patients. Most said telehealth improved the patient-physician relationship, and they believed visits were more efficient when conducted via telehealth than in person. A majority (81%) endorsed using telehealth for some visits, and 12% for most visits.
A key question will be how telehealth affects patient outcomes, according to Ryan Perkins, MD, who was a coauthor of the survey of physicians. “If they’re not doing as well from an outcomes perspective, that would be a huge limitation to our patients,” said Dr. Perkins, who is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital.
Although the study examined only models of care that were entirely virtual, Dr. Perkins noted that hybrid in-person/virtual care models are also possible. “Do we have better outcomes doing it that way? Is there higher patient satisfaction? I’m sure that will be a hot topic moving forward.”
Dr. Perkins noted that patients expressed concern about not being able to get sputum cultures and spirometry recordings during telehealth sessions. “That’s not really surprising to me, but I think it raises the question as we’re imagining care models for the future – how can we implement those components into future care delivery?”
Another hurdle will be insurance coverage. “My fear is that insurance companies are going to cut down the amount of reimbursement for telehealth visits in the future and just going to make it more complicated,” said Dr. Giusti. “Certainly, though, I think telehealth is an important outreach that we’d like to continue with our patients.”
Mr. NeSmith, Dr. Giusti, and Dr. Perkins reported no relevant financial disclosures.
SOURCE: NeSmith A et al. NACFC 2020, Abstracts 797, 799, 810.
(CF) and the physicians who treat them, according to three new studies. The surveys examined attitudes during the COVID-19 pandemic, which complicates interpretation of the survey, but the results nevertheless bode well for telehealth’s future in the management of CF.
“Patients could be responding positively just because they could have a visit during the pandemic,” said Andrew NeSmith, during a presentation of a survey of adults with CF at the virtual North American Cystic Fibrosis Conference. Mr. NeSmith is the clinical data coordinator at the University of Alabama at Birmingham Cystic Fibrosis Center.
Other posters at the conference examined attitudes among pediatric populations and treating physicians, with generally positive results, which has generated optimism that telehealth could become an important element of care after the pandemic fades. “This data suggests that telehealth could be integrated into routine follow-up care in the CF chronic care model,” said Mr. NeSmith.
His team collected responses from 119 individuals at the University of Alabama at Birmingham; Boston Children’s Hospital; Brigham and Women’s Hospital, Boston; Virginia Commonwealth University, Richmond; and West Virginia University, Morgantown. A total of 28% had conducted a prior telehealth visit before the study; 92% of visits were conducted with a medical doctor. Only 13% reported experiencing difficulties with their first telehealth visit. Eighty-five percent rated convenience, and 77% rated their satisfaction with telehealth as “high.” Most (92%) said they were able to see their desired disciplines, 95% felt all of their issues had been addressed, and 83% strongly agreed that telehealth visits were of adequate length.
Not everything was rosy. A total of 48% of participants expressed at least moderate concern over a lack of pulmonary function test or throat/sputum culture. There were much fewer concerns over missing vital signs or weight measurements.
The overall results weren’t surprising to Robert Giusti, MD, clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center, New York, who was not involved in the study. “I was expecting that patients were going to like it. It makes their life easier,” he said in an interview.
A survey of families of pediatric individuals with CF at seven centers found similar levels of satisfaction. A total of 23% had used telehealth previously; 96% rated convenience, and 93% rated satisfaction as “high.” Almost all (99%) felt that all concerns were met, 98% said that sessions were adequately long, and 87% had no trouble connecting to the visit.
Some participants in this survey had concerns about what might be missing with a televisit. Half (52%) had at least a moderate concern over lack of pulmonary function tests, 45% over lack of vital signs, 29% about lack of weight measurements, and 64% about the need for throat/sputum culture. Despite those issues, 69% preferred that “some” and 22% preferred that “most” future visits be conducted by telehealth.
A survey of physicians who used telehealth with CF patients also found broad support. They reported some challenges, with 70% saying they experienced technical difficulty, and 77% saying it “took time” to resolve a visit with only 18% reporting that visits were “quickly resolved.” Most (86%) said they were satisfied with telehealth for care delivery, and 78% said it was appropriate for most patients. Most said telehealth improved the patient-physician relationship, and they believed visits were more efficient when conducted via telehealth than in person. A majority (81%) endorsed using telehealth for some visits, and 12% for most visits.
A key question will be how telehealth affects patient outcomes, according to Ryan Perkins, MD, who was a coauthor of the survey of physicians. “If they’re not doing as well from an outcomes perspective, that would be a huge limitation to our patients,” said Dr. Perkins, who is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital.
Although the study examined only models of care that were entirely virtual, Dr. Perkins noted that hybrid in-person/virtual care models are also possible. “Do we have better outcomes doing it that way? Is there higher patient satisfaction? I’m sure that will be a hot topic moving forward.”
Dr. Perkins noted that patients expressed concern about not being able to get sputum cultures and spirometry recordings during telehealth sessions. “That’s not really surprising to me, but I think it raises the question as we’re imagining care models for the future – how can we implement those components into future care delivery?”
Another hurdle will be insurance coverage. “My fear is that insurance companies are going to cut down the amount of reimbursement for telehealth visits in the future and just going to make it more complicated,” said Dr. Giusti. “Certainly, though, I think telehealth is an important outreach that we’d like to continue with our patients.”
Mr. NeSmith, Dr. Giusti, and Dr. Perkins reported no relevant financial disclosures.
SOURCE: NeSmith A et al. NACFC 2020, Abstracts 797, 799, 810.
(CF) and the physicians who treat them, according to three new studies. The surveys examined attitudes during the COVID-19 pandemic, which complicates interpretation of the survey, but the results nevertheless bode well for telehealth’s future in the management of CF.
“Patients could be responding positively just because they could have a visit during the pandemic,” said Andrew NeSmith, during a presentation of a survey of adults with CF at the virtual North American Cystic Fibrosis Conference. Mr. NeSmith is the clinical data coordinator at the University of Alabama at Birmingham Cystic Fibrosis Center.
Other posters at the conference examined attitudes among pediatric populations and treating physicians, with generally positive results, which has generated optimism that telehealth could become an important element of care after the pandemic fades. “This data suggests that telehealth could be integrated into routine follow-up care in the CF chronic care model,” said Mr. NeSmith.
His team collected responses from 119 individuals at the University of Alabama at Birmingham; Boston Children’s Hospital; Brigham and Women’s Hospital, Boston; Virginia Commonwealth University, Richmond; and West Virginia University, Morgantown. A total of 28% had conducted a prior telehealth visit before the study; 92% of visits were conducted with a medical doctor. Only 13% reported experiencing difficulties with their first telehealth visit. Eighty-five percent rated convenience, and 77% rated their satisfaction with telehealth as “high.” Most (92%) said they were able to see their desired disciplines, 95% felt all of their issues had been addressed, and 83% strongly agreed that telehealth visits were of adequate length.
Not everything was rosy. A total of 48% of participants expressed at least moderate concern over a lack of pulmonary function test or throat/sputum culture. There were much fewer concerns over missing vital signs or weight measurements.
The overall results weren’t surprising to Robert Giusti, MD, clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center, New York, who was not involved in the study. “I was expecting that patients were going to like it. It makes their life easier,” he said in an interview.
A survey of families of pediatric individuals with CF at seven centers found similar levels of satisfaction. A total of 23% had used telehealth previously; 96% rated convenience, and 93% rated satisfaction as “high.” Almost all (99%) felt that all concerns were met, 98% said that sessions were adequately long, and 87% had no trouble connecting to the visit.
Some participants in this survey had concerns about what might be missing with a televisit. Half (52%) had at least a moderate concern over lack of pulmonary function tests, 45% over lack of vital signs, 29% about lack of weight measurements, and 64% about the need for throat/sputum culture. Despite those issues, 69% preferred that “some” and 22% preferred that “most” future visits be conducted by telehealth.
A survey of physicians who used telehealth with CF patients also found broad support. They reported some challenges, with 70% saying they experienced technical difficulty, and 77% saying it “took time” to resolve a visit with only 18% reporting that visits were “quickly resolved.” Most (86%) said they were satisfied with telehealth for care delivery, and 78% said it was appropriate for most patients. Most said telehealth improved the patient-physician relationship, and they believed visits were more efficient when conducted via telehealth than in person. A majority (81%) endorsed using telehealth for some visits, and 12% for most visits.
A key question will be how telehealth affects patient outcomes, according to Ryan Perkins, MD, who was a coauthor of the survey of physicians. “If they’re not doing as well from an outcomes perspective, that would be a huge limitation to our patients,” said Dr. Perkins, who is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital.
Although the study examined only models of care that were entirely virtual, Dr. Perkins noted that hybrid in-person/virtual care models are also possible. “Do we have better outcomes doing it that way? Is there higher patient satisfaction? I’m sure that will be a hot topic moving forward.”
Dr. Perkins noted that patients expressed concern about not being able to get sputum cultures and spirometry recordings during telehealth sessions. “That’s not really surprising to me, but I think it raises the question as we’re imagining care models for the future – how can we implement those components into future care delivery?”
Another hurdle will be insurance coverage. “My fear is that insurance companies are going to cut down the amount of reimbursement for telehealth visits in the future and just going to make it more complicated,” said Dr. Giusti. “Certainly, though, I think telehealth is an important outreach that we’d like to continue with our patients.”
Mr. NeSmith, Dr. Giusti, and Dr. Perkins reported no relevant financial disclosures.
SOURCE: NeSmith A et al. NACFC 2020, Abstracts 797, 799, 810.
FROM NACFC 2020
Half of women treated for gynecologic cancers miss or skip doses of oral drugs
Oral agents are taking on an ever-greater role in the management of gynecologic cancers. However, women being treated for these cancers have less than ideal adherence to such medications, a new study has found – with just over half reporting taking them exactly as prescribed.
The findings reported in Obstetrics & Gynecology are consistent with reports from other populations taking oral anticancer agents, in which adherence is generally lower than with intravenous therapies.
For their research, Catherine Watson, MD, and colleagues at Duke University, Durham, N.C., recruited 100 women at their institution taking a variety of oral anticancer agents for uterine or ovarian cancer for 30 days or more (median time, 6 months). The women answered a questionnaire that measured adherence as well as health literacy, quality of life, and distress. The researchers also collected information on the subjects’ race, age, insurance type, medication burden, and medication costs.
Fourteen of the women in the study additionally underwent qualitative interviews about their experiences with oral anticancer drugs. The researchers also queried physicians and nurse practitioners about their thoughts on adherence.
Dr. Watson and colleagues reported that 54% of women self-reported perfect adherence to their medication in the previous week, while 21% had missed or skipped one dose, and 25% reported skipping or missing more than one dose.
The researchers saw no significant differences between the adherent and nonadherent groups corresponding with race, age, or other demographic or clinical characteristics, but they noted that their study was not powered to detect such associations. The small sample size and self-reported data were among this study’s limitations, Dr. Watson and colleagues acknowledged.
Interviews with patients revealed some surprising reasons for the less-than-optimal adherence, with 43% of women reporting feeling anxiety about the burden of administering medication at home. While patients acknowledged the convenience of oral regimens, some also expressed a wish for more physician contact and support. Some women who were nonadherent said they perceived the efficacy of oral agents to be less than intravenous therapies.
Physicians and nurse practitioners interviewed by the researchers “tended to assume that their patients were adherent to oral anticancer therapy because of the therapy’s importance, and many did not routinely ask their patients about adherence,” Dr. Watson and colleagues wrote.
Emma Rossi, MD, a gynecologic oncologist at the University of North Carolina at Chapel Hill, commented in an interview that the study highlighted “the importance of not generalizing our perception of patients. As providers we have to spend the time asking patients where they’re at and how they’re thinking about taking an oral medication, with special attention to their fears, their expectations, and their concerns. We should be touching base with them not just before starting drugs, but during the course of treatment.”
Dr. Rossi stressed that compliance in real-life settings is likely to be different from that seen in clinical trials of oral anticancer drugs. “It’s important to recognize that real-world efficacy of treatments may be different from trial efficacy. We see these differences a lot in medicine where studies show a large magnitude of effect that doesn’t play out in real life practice – because of factors like this.”
Some 57% of the women in the study were taking their medications as maintenance, while the rest were taking the medications as active treatment. This too might have an effect on adherence, she said, with the active-treatment group potentially more motivated to maintain perfect adherence.
“You see in the interviews that doctors assume patients would be compliant because of the seriousness of the disease,” Dr. Rossi said. “But some patients said they perceived oral drugs as less strong or effective. If a patient is cancer free on a scan and doesn’t have measurable disease, prescribing an oral medication may be sending the subliminal message that it’s not as important.”
Physicians “may need to take the extra steps to individualize our counseling of patients – especially with therapies that they’re responsible for administering,” Dr. Rossi continued. “As this study shows, every patient sees treatment through her own individual lens. We really need to meet them where they’re at to make them comfortable with their treatment and optimize compliance.”
Dr. Watson and colleagues’ study was supported by their institution. One coauthor reported financial ties with drug manufacturers in the form of grant and clinical trial support and honoraria. Another coauthor is the member of various boards and steering committees, which are uncompensated. Dr. Rossi reported no financial conflicts of interest.
SOURCE: Watson C et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004170.
Oral agents are taking on an ever-greater role in the management of gynecologic cancers. However, women being treated for these cancers have less than ideal adherence to such medications, a new study has found – with just over half reporting taking them exactly as prescribed.
The findings reported in Obstetrics & Gynecology are consistent with reports from other populations taking oral anticancer agents, in which adherence is generally lower than with intravenous therapies.
For their research, Catherine Watson, MD, and colleagues at Duke University, Durham, N.C., recruited 100 women at their institution taking a variety of oral anticancer agents for uterine or ovarian cancer for 30 days or more (median time, 6 months). The women answered a questionnaire that measured adherence as well as health literacy, quality of life, and distress. The researchers also collected information on the subjects’ race, age, insurance type, medication burden, and medication costs.
Fourteen of the women in the study additionally underwent qualitative interviews about their experiences with oral anticancer drugs. The researchers also queried physicians and nurse practitioners about their thoughts on adherence.
Dr. Watson and colleagues reported that 54% of women self-reported perfect adherence to their medication in the previous week, while 21% had missed or skipped one dose, and 25% reported skipping or missing more than one dose.
The researchers saw no significant differences between the adherent and nonadherent groups corresponding with race, age, or other demographic or clinical characteristics, but they noted that their study was not powered to detect such associations. The small sample size and self-reported data were among this study’s limitations, Dr. Watson and colleagues acknowledged.
Interviews with patients revealed some surprising reasons for the less-than-optimal adherence, with 43% of women reporting feeling anxiety about the burden of administering medication at home. While patients acknowledged the convenience of oral regimens, some also expressed a wish for more physician contact and support. Some women who were nonadherent said they perceived the efficacy of oral agents to be less than intravenous therapies.
Physicians and nurse practitioners interviewed by the researchers “tended to assume that their patients were adherent to oral anticancer therapy because of the therapy’s importance, and many did not routinely ask their patients about adherence,” Dr. Watson and colleagues wrote.
Emma Rossi, MD, a gynecologic oncologist at the University of North Carolina at Chapel Hill, commented in an interview that the study highlighted “the importance of not generalizing our perception of patients. As providers we have to spend the time asking patients where they’re at and how they’re thinking about taking an oral medication, with special attention to their fears, their expectations, and their concerns. We should be touching base with them not just before starting drugs, but during the course of treatment.”
Dr. Rossi stressed that compliance in real-life settings is likely to be different from that seen in clinical trials of oral anticancer drugs. “It’s important to recognize that real-world efficacy of treatments may be different from trial efficacy. We see these differences a lot in medicine where studies show a large magnitude of effect that doesn’t play out in real life practice – because of factors like this.”
Some 57% of the women in the study were taking their medications as maintenance, while the rest were taking the medications as active treatment. This too might have an effect on adherence, she said, with the active-treatment group potentially more motivated to maintain perfect adherence.
“You see in the interviews that doctors assume patients would be compliant because of the seriousness of the disease,” Dr. Rossi said. “But some patients said they perceived oral drugs as less strong or effective. If a patient is cancer free on a scan and doesn’t have measurable disease, prescribing an oral medication may be sending the subliminal message that it’s not as important.”
Physicians “may need to take the extra steps to individualize our counseling of patients – especially with therapies that they’re responsible for administering,” Dr. Rossi continued. “As this study shows, every patient sees treatment through her own individual lens. We really need to meet them where they’re at to make them comfortable with their treatment and optimize compliance.”
Dr. Watson and colleagues’ study was supported by their institution. One coauthor reported financial ties with drug manufacturers in the form of grant and clinical trial support and honoraria. Another coauthor is the member of various boards and steering committees, which are uncompensated. Dr. Rossi reported no financial conflicts of interest.
SOURCE: Watson C et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004170.
Oral agents are taking on an ever-greater role in the management of gynecologic cancers. However, women being treated for these cancers have less than ideal adherence to such medications, a new study has found – with just over half reporting taking them exactly as prescribed.
The findings reported in Obstetrics & Gynecology are consistent with reports from other populations taking oral anticancer agents, in which adherence is generally lower than with intravenous therapies.
For their research, Catherine Watson, MD, and colleagues at Duke University, Durham, N.C., recruited 100 women at their institution taking a variety of oral anticancer agents for uterine or ovarian cancer for 30 days or more (median time, 6 months). The women answered a questionnaire that measured adherence as well as health literacy, quality of life, and distress. The researchers also collected information on the subjects’ race, age, insurance type, medication burden, and medication costs.
Fourteen of the women in the study additionally underwent qualitative interviews about their experiences with oral anticancer drugs. The researchers also queried physicians and nurse practitioners about their thoughts on adherence.
Dr. Watson and colleagues reported that 54% of women self-reported perfect adherence to their medication in the previous week, while 21% had missed or skipped one dose, and 25% reported skipping or missing more than one dose.
The researchers saw no significant differences between the adherent and nonadherent groups corresponding with race, age, or other demographic or clinical characteristics, but they noted that their study was not powered to detect such associations. The small sample size and self-reported data were among this study’s limitations, Dr. Watson and colleagues acknowledged.
Interviews with patients revealed some surprising reasons for the less-than-optimal adherence, with 43% of women reporting feeling anxiety about the burden of administering medication at home. While patients acknowledged the convenience of oral regimens, some also expressed a wish for more physician contact and support. Some women who were nonadherent said they perceived the efficacy of oral agents to be less than intravenous therapies.
Physicians and nurse practitioners interviewed by the researchers “tended to assume that their patients were adherent to oral anticancer therapy because of the therapy’s importance, and many did not routinely ask their patients about adherence,” Dr. Watson and colleagues wrote.
Emma Rossi, MD, a gynecologic oncologist at the University of North Carolina at Chapel Hill, commented in an interview that the study highlighted “the importance of not generalizing our perception of patients. As providers we have to spend the time asking patients where they’re at and how they’re thinking about taking an oral medication, with special attention to their fears, their expectations, and their concerns. We should be touching base with them not just before starting drugs, but during the course of treatment.”
Dr. Rossi stressed that compliance in real-life settings is likely to be different from that seen in clinical trials of oral anticancer drugs. “It’s important to recognize that real-world efficacy of treatments may be different from trial efficacy. We see these differences a lot in medicine where studies show a large magnitude of effect that doesn’t play out in real life practice – because of factors like this.”
Some 57% of the women in the study were taking their medications as maintenance, while the rest were taking the medications as active treatment. This too might have an effect on adherence, she said, with the active-treatment group potentially more motivated to maintain perfect adherence.
“You see in the interviews that doctors assume patients would be compliant because of the seriousness of the disease,” Dr. Rossi said. “But some patients said they perceived oral drugs as less strong or effective. If a patient is cancer free on a scan and doesn’t have measurable disease, prescribing an oral medication may be sending the subliminal message that it’s not as important.”
Physicians “may need to take the extra steps to individualize our counseling of patients – especially with therapies that they’re responsible for administering,” Dr. Rossi continued. “As this study shows, every patient sees treatment through her own individual lens. We really need to meet them where they’re at to make them comfortable with their treatment and optimize compliance.”
Dr. Watson and colleagues’ study was supported by their institution. One coauthor reported financial ties with drug manufacturers in the form of grant and clinical trial support and honoraria. Another coauthor is the member of various boards and steering committees, which are uncompensated. Dr. Rossi reported no financial conflicts of interest.
SOURCE: Watson C et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004170.
FROM OBSTETRICS & GYNECOLOGY
Sleep apnea may correlate with anxiety, depression in patients with PCOS
a study suggests.
This finding could have implications for screening and treatment, Diana Xiaojie Zhou, MD, said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“Routine OSA screening in women with PCOS should be considered in the setting of existing depression and anxiety,” said Dr. Zhou, a reproductive endocrinology and infertility fellow at the University of California, San Francisco. “Referral for OSA diagnosis and treatment in those who screen positive may have added psychological benefits in this population, as has been seen in the general population.”
Patients with PCOS experience a range of comorbidities, including higher rates of psychological disorders and OSA, she said.
OSA has been associated with depression and anxiety in the general population, and research indicates that treatment, such as with continuous positive airway pressure (CPAP), may have psychological benefits, such as reduced depression symptoms.
PCOS guidelines recommend screening for OSA to identify and alleviate symptoms such as fatigue that may to contribute to mood disorders. “However, there is a lack of studies assessing the relationship between OSA and depression and anxiety specifically in women with PCOS,” Dr. Zhou said.
A cross-sectional study
To evaluate whether OSA is associated with depression and anxiety in women with PCOS, Dr. Zhou and colleagues conducted a cross-sectional study of all women seen at a multidisciplinary PCOS clinic at university between June 2017 and June 2020.
Participants had a diagnosis of PCOS clinically confirmed by the Rotterdam criteria. Researchers determined OSA risk using the Berlin questionnaire, which is divided into three domains. A positive score in two or more domains indicates a high risk of OSA.
The investigators used the Patient Health Questionnaire-9 (PHQ-9) to assess depression symptoms, and they used the Generalized Anxiety Disorder-7 (GAD-7) to assess anxiety symptoms.
Researchers used two-sided t-test, chi-square test, and Fisher’s exact test to evaluate for differences in patient characteristics. They performed multivariate logistic regression analyses to determine the odds of moderate to severe symptoms of depression (that is, a PHQ-9 score of 10 or greater) and anxiety (a GAD-7 score of 10 or greater) among patients with a high risk of OSA, compared with patients with a low risk of OSA. They adjusted for age, body mass index, free testosterone level, and insulin resistance using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
The researchers examined data from 201 patients: 125 with a low risk of OSA and 76 with a high risk of OSA. The average age of the patients was 28 years.
On average, patients in the high-risk OSA group had a greater body mass index (37.9 vs. 26.5), a higher level of free testosterone (6.5 ng/dL vs. 4.5 ng/dL), and a higher HOMA-IR score (7 vs. 3.1), relative to those with a low risk of OSA. In addition, a greater percentage of patients with a high risk of OSA experienced oligomenorrhea (84.9% vs. 70.5%).
The average PHQ-9 score was significantly higher in the high-risk OSA group (12 vs. 8.3), as was the average GAD-7 score (8.9 vs. 6.1).
In univariate analyses, having a high risk of OSA increased the likelihood of moderate or severe depression or anxiety approximately threefold.
In multivariate analyses, a high risk of OSA remained significantly associated with moderate or severe depression or anxiety, with an odds ratio of about 2.5. “Of note, BMI was a statistically significant predictor in the univariate analyses, but not so in the multivariate analyses,” Dr. Zhou said.
Although the investigators assessed OSA, depression, and anxiety using validated questionnaires, a study with clinically confirmed diagnoses of those conditions would strengthen these findings, she said.
Various possible links
Investigators have proposed various links between PCOS, OSA, and depression and anxiety, Dr. Zhou noted. Features of PCOS such as insulin resistance, obesity, and hyperandrogenemia increase the risk of OSA. “The sleep loss and fragmentation and hypoxia that define OSA then serve to increase sympathetic tone and oxidative stress, which then potentially can lead to an increase in depression and anxiety,” Dr. Zhou said.
The results suggests that treating OSA “may have added psychological benefits for women with PCOS and highlights the broad health implications of this condition,” Marla Lujan, PhD, chair of the ASRM’s androgen excess special interest group, said in a society news release.
“The cause of PCOS is still not well understood, but we do know that 1 in 10 women in their childbearing years suffer from PCOS,” said Dr. Lujan, of Cornell University, Ithaca, N.Y. “In addition to infertility, PCOS is also associated with type 2 diabetes and cardiovascular complications such as hypertension and abnormal blood lipids.”
In a discussion following Dr. Zhou’s presentation, Alice D. Domar, PhD, said the study was eye opening.
Dr. Domar, director of integrative care at Boston IVF and associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, said that she does not typically discuss sleep apnea with patients. “For those of us who routinely work with PCOS patients, we are always looking for more information.”
Although PCOS guidelines mention screening for OSA, Dr. Zhou expects that few generalists who see PCOS patients or even subspecialists actually do.
Nevertheless, the potential for intervention is fascinating, she said. And if treating OSA also reduced a patient’s need for psychiatric medications, there could be added benefit in PCOS due to the metabolic side effects that accompany some of the drugs.
Dr. Zhou and Dr. Lujan had no relevant disclosures. Dr. Domar is a co-owner of FertiCalm, FertiStrong, and Aliz Health Apps, and a speaker for Ferring, EMD Serono, Merck, and Abbott.
SOURCE: Zhou DX et al. ASRM 2020. Abstract O-146.
a study suggests.
This finding could have implications for screening and treatment, Diana Xiaojie Zhou, MD, said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“Routine OSA screening in women with PCOS should be considered in the setting of existing depression and anxiety,” said Dr. Zhou, a reproductive endocrinology and infertility fellow at the University of California, San Francisco. “Referral for OSA diagnosis and treatment in those who screen positive may have added psychological benefits in this population, as has been seen in the general population.”
Patients with PCOS experience a range of comorbidities, including higher rates of psychological disorders and OSA, she said.
OSA has been associated with depression and anxiety in the general population, and research indicates that treatment, such as with continuous positive airway pressure (CPAP), may have psychological benefits, such as reduced depression symptoms.
PCOS guidelines recommend screening for OSA to identify and alleviate symptoms such as fatigue that may to contribute to mood disorders. “However, there is a lack of studies assessing the relationship between OSA and depression and anxiety specifically in women with PCOS,” Dr. Zhou said.
A cross-sectional study
To evaluate whether OSA is associated with depression and anxiety in women with PCOS, Dr. Zhou and colleagues conducted a cross-sectional study of all women seen at a multidisciplinary PCOS clinic at university between June 2017 and June 2020.
Participants had a diagnosis of PCOS clinically confirmed by the Rotterdam criteria. Researchers determined OSA risk using the Berlin questionnaire, which is divided into three domains. A positive score in two or more domains indicates a high risk of OSA.
The investigators used the Patient Health Questionnaire-9 (PHQ-9) to assess depression symptoms, and they used the Generalized Anxiety Disorder-7 (GAD-7) to assess anxiety symptoms.
Researchers used two-sided t-test, chi-square test, and Fisher’s exact test to evaluate for differences in patient characteristics. They performed multivariate logistic regression analyses to determine the odds of moderate to severe symptoms of depression (that is, a PHQ-9 score of 10 or greater) and anxiety (a GAD-7 score of 10 or greater) among patients with a high risk of OSA, compared with patients with a low risk of OSA. They adjusted for age, body mass index, free testosterone level, and insulin resistance using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
The researchers examined data from 201 patients: 125 with a low risk of OSA and 76 with a high risk of OSA. The average age of the patients was 28 years.
On average, patients in the high-risk OSA group had a greater body mass index (37.9 vs. 26.5), a higher level of free testosterone (6.5 ng/dL vs. 4.5 ng/dL), and a higher HOMA-IR score (7 vs. 3.1), relative to those with a low risk of OSA. In addition, a greater percentage of patients with a high risk of OSA experienced oligomenorrhea (84.9% vs. 70.5%).
The average PHQ-9 score was significantly higher in the high-risk OSA group (12 vs. 8.3), as was the average GAD-7 score (8.9 vs. 6.1).
In univariate analyses, having a high risk of OSA increased the likelihood of moderate or severe depression or anxiety approximately threefold.
In multivariate analyses, a high risk of OSA remained significantly associated with moderate or severe depression or anxiety, with an odds ratio of about 2.5. “Of note, BMI was a statistically significant predictor in the univariate analyses, but not so in the multivariate analyses,” Dr. Zhou said.
Although the investigators assessed OSA, depression, and anxiety using validated questionnaires, a study with clinically confirmed diagnoses of those conditions would strengthen these findings, she said.
Various possible links
Investigators have proposed various links between PCOS, OSA, and depression and anxiety, Dr. Zhou noted. Features of PCOS such as insulin resistance, obesity, and hyperandrogenemia increase the risk of OSA. “The sleep loss and fragmentation and hypoxia that define OSA then serve to increase sympathetic tone and oxidative stress, which then potentially can lead to an increase in depression and anxiety,” Dr. Zhou said.
The results suggests that treating OSA “may have added psychological benefits for women with PCOS and highlights the broad health implications of this condition,” Marla Lujan, PhD, chair of the ASRM’s androgen excess special interest group, said in a society news release.
“The cause of PCOS is still not well understood, but we do know that 1 in 10 women in their childbearing years suffer from PCOS,” said Dr. Lujan, of Cornell University, Ithaca, N.Y. “In addition to infertility, PCOS is also associated with type 2 diabetes and cardiovascular complications such as hypertension and abnormal blood lipids.”
In a discussion following Dr. Zhou’s presentation, Alice D. Domar, PhD, said the study was eye opening.
Dr. Domar, director of integrative care at Boston IVF and associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, said that she does not typically discuss sleep apnea with patients. “For those of us who routinely work with PCOS patients, we are always looking for more information.”
Although PCOS guidelines mention screening for OSA, Dr. Zhou expects that few generalists who see PCOS patients or even subspecialists actually do.
Nevertheless, the potential for intervention is fascinating, she said. And if treating OSA also reduced a patient’s need for psychiatric medications, there could be added benefit in PCOS due to the metabolic side effects that accompany some of the drugs.
Dr. Zhou and Dr. Lujan had no relevant disclosures. Dr. Domar is a co-owner of FertiCalm, FertiStrong, and Aliz Health Apps, and a speaker for Ferring, EMD Serono, Merck, and Abbott.
SOURCE: Zhou DX et al. ASRM 2020. Abstract O-146.
a study suggests.
This finding could have implications for screening and treatment, Diana Xiaojie Zhou, MD, said at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
“Routine OSA screening in women with PCOS should be considered in the setting of existing depression and anxiety,” said Dr. Zhou, a reproductive endocrinology and infertility fellow at the University of California, San Francisco. “Referral for OSA diagnosis and treatment in those who screen positive may have added psychological benefits in this population, as has been seen in the general population.”
Patients with PCOS experience a range of comorbidities, including higher rates of psychological disorders and OSA, she said.
OSA has been associated with depression and anxiety in the general population, and research indicates that treatment, such as with continuous positive airway pressure (CPAP), may have psychological benefits, such as reduced depression symptoms.
PCOS guidelines recommend screening for OSA to identify and alleviate symptoms such as fatigue that may to contribute to mood disorders. “However, there is a lack of studies assessing the relationship between OSA and depression and anxiety specifically in women with PCOS,” Dr. Zhou said.
A cross-sectional study
To evaluate whether OSA is associated with depression and anxiety in women with PCOS, Dr. Zhou and colleagues conducted a cross-sectional study of all women seen at a multidisciplinary PCOS clinic at university between June 2017 and June 2020.
Participants had a diagnosis of PCOS clinically confirmed by the Rotterdam criteria. Researchers determined OSA risk using the Berlin questionnaire, which is divided into three domains. A positive score in two or more domains indicates a high risk of OSA.
The investigators used the Patient Health Questionnaire-9 (PHQ-9) to assess depression symptoms, and they used the Generalized Anxiety Disorder-7 (GAD-7) to assess anxiety symptoms.
Researchers used two-sided t-test, chi-square test, and Fisher’s exact test to evaluate for differences in patient characteristics. They performed multivariate logistic regression analyses to determine the odds of moderate to severe symptoms of depression (that is, a PHQ-9 score of 10 or greater) and anxiety (a GAD-7 score of 10 or greater) among patients with a high risk of OSA, compared with patients with a low risk of OSA. They adjusted for age, body mass index, free testosterone level, and insulin resistance using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
The researchers examined data from 201 patients: 125 with a low risk of OSA and 76 with a high risk of OSA. The average age of the patients was 28 years.
On average, patients in the high-risk OSA group had a greater body mass index (37.9 vs. 26.5), a higher level of free testosterone (6.5 ng/dL vs. 4.5 ng/dL), and a higher HOMA-IR score (7 vs. 3.1), relative to those with a low risk of OSA. In addition, a greater percentage of patients with a high risk of OSA experienced oligomenorrhea (84.9% vs. 70.5%).
The average PHQ-9 score was significantly higher in the high-risk OSA group (12 vs. 8.3), as was the average GAD-7 score (8.9 vs. 6.1).
In univariate analyses, having a high risk of OSA increased the likelihood of moderate or severe depression or anxiety approximately threefold.
In multivariate analyses, a high risk of OSA remained significantly associated with moderate or severe depression or anxiety, with an odds ratio of about 2.5. “Of note, BMI was a statistically significant predictor in the univariate analyses, but not so in the multivariate analyses,” Dr. Zhou said.
Although the investigators assessed OSA, depression, and anxiety using validated questionnaires, a study with clinically confirmed diagnoses of those conditions would strengthen these findings, she said.
Various possible links
Investigators have proposed various links between PCOS, OSA, and depression and anxiety, Dr. Zhou noted. Features of PCOS such as insulin resistance, obesity, and hyperandrogenemia increase the risk of OSA. “The sleep loss and fragmentation and hypoxia that define OSA then serve to increase sympathetic tone and oxidative stress, which then potentially can lead to an increase in depression and anxiety,” Dr. Zhou said.
The results suggests that treating OSA “may have added psychological benefits for women with PCOS and highlights the broad health implications of this condition,” Marla Lujan, PhD, chair of the ASRM’s androgen excess special interest group, said in a society news release.
“The cause of PCOS is still not well understood, but we do know that 1 in 10 women in their childbearing years suffer from PCOS,” said Dr. Lujan, of Cornell University, Ithaca, N.Y. “In addition to infertility, PCOS is also associated with type 2 diabetes and cardiovascular complications such as hypertension and abnormal blood lipids.”
In a discussion following Dr. Zhou’s presentation, Alice D. Domar, PhD, said the study was eye opening.
Dr. Domar, director of integrative care at Boston IVF and associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston, said that she does not typically discuss sleep apnea with patients. “For those of us who routinely work with PCOS patients, we are always looking for more information.”
Although PCOS guidelines mention screening for OSA, Dr. Zhou expects that few generalists who see PCOS patients or even subspecialists actually do.
Nevertheless, the potential for intervention is fascinating, she said. And if treating OSA also reduced a patient’s need for psychiatric medications, there could be added benefit in PCOS due to the metabolic side effects that accompany some of the drugs.
Dr. Zhou and Dr. Lujan had no relevant disclosures. Dr. Domar is a co-owner of FertiCalm, FertiStrong, and Aliz Health Apps, and a speaker for Ferring, EMD Serono, Merck, and Abbott.
SOURCE: Zhou DX et al. ASRM 2020. Abstract O-146.
FROM ASRM 2020
Debate: After methotrexate failure, is JAK inhibitor or biologic next?
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.
Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.

Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.

Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
FROM ACR 2020
Stenotic lesion outcomes better if fractional flow reserve guides PCI
Restricting percutaneous interventions (PCI) to only those stenotic lesions that are ischemic by fractional flow reserve (FFR) thresholds is associated with better 5-year outcomes whether or not PCI is deployed, according to a cohort study presented at the American Heart Association scientific sessions.
For those that met the FFR threshold for ischemia, defined as up to 0.80, PCI reduced the risk of a major adverse cardiac event (MACE) at 5 years by 23% (hazard ratio, 0.77) relative to no PCI. Conversely, those not indicated for PCI because of a higher FFR had a 37% higher risk of MACE (HR, 1.37) at 5 years if treated with PCI relative to those who were not.
“The story of overuse of PCI is important,” reported the senior author Dennis Ko, MD, a scientist affiliated with the Schulich Heart Research Program, Sunnybrook Research Institute, University of Toronto, Canada. “We as interventionalists often think that putting in a stent is not harmful, and that turned out not to be the case.”
The FFR threshold for intervening with PCI is evidence based. Several trials, including one published in 2014, have associated PCI with better outcomes relative to medical therapy when FFR is 0.80 or lower. Other evidence suggests no advantage and possible harm for PCI performed if FFR is higher. Multiple guidelines, including those from the AHA, recommend against PCI if FFR is more than 0.80.
“As FRR is gaining in popularity, we were interested in whether physicians follow the thresholds in routine clinical practice and what happens to patient outcomes [if they are or are not followed],” Dr. Ko explained.
In this retrospective study by Dr. Ko’s trainee, Maneesh Sud, MD, and simultaneously published in JAMA, the answer was that there is deviation, and deviation leads to bad outcomes.
The 9,106 coronary artery disease patients included in the study underwent single-vessel FFR assessment within a 5-year period in Canada. The two cohorts evaluated were those with a lesional FFR of 0.80 or less, defined as ischemic, and those with a lesion with higher FFR, defined as nonischemic. The primary MACE outcome comprised death, myocardial infarction, unstable angina, or urgent coronary revascularization.
Of the 2,693 patients who met the FFR threshold of ischemia, 75.3% received PCI, and 24.7% were treated with medical therapy only. Of the 6,413 patients with nonischemic FFR, 87.4% were treated with medical therapy and 12.6% received PCI.
In those with ischemic FFR, event curves for MACE separated rapidly. At 30 days, the risk of MACE was 53% lower (HR, 0.47) in those receiving PCI. By 1 year, the advantage was less (HR, 0.76), but it was steady thereafter and remained about the same at 5 years (HR, 0.77; 95% confidence interval, 0.63-0.94). Relative advantages for each component of MACE went in the same direction. At 5 years, PCI exerted its greatest numerical advantage for the outcome or urgent coronary revascularization (HR, 0.71) and its least numerical advantage for MI (HR, 0.92), but none of these differences reached statistical significance.
In those with nonischemic coronary lesions on FFR, PCI was associated with more than twice the risk for MACE at 30 days (HR, 2.11), but the increase in risk relative to medical management fell at 1 year (HR 1.67) and 5 years (HR, 1.37). All of the individual components of MACE were numerically increased at all time points except for death, which was numerically lower at 30 days (HR, 0.41) and 5 years (HR, 0.94), even though these differences were not significant.
It could not be ascertained from these data why PCI was not performed when there was an indication or why it was performed when there was not. The investigators speculated that some clinicians may decide against PCI for ischemic lesions in the absence of symptoms or when concerned about comorbidities. They might offer PCI in nonischemic lesions because of symptoms, positive tests other than FFR, or FFR values near the threshold.
“I think the main message of our paper is that adherence of the FFR threshold as established by clinical trials is important,” Dr. Ko said in an interview. This not only means performing PCI when it is indicated, but refraining from PCI when it is not.
Basically, this study confirms that the guideline thresholds are valid, according to Jared M. O’Leary, MD, who is experienced with FFR and is Medical Director for Quality at the Vanderbilt Heart and Vascular Institute, Nashville, Tenn.
“It confirms the utility of FFR in the real world,” he said, adding that the results are “totally consistent with our practice.” He called FFR “an important tool in the cardiac cath lab” not only for determining when revascularization will benefit the patient but the opposite.
“The flip side is also true: Stenting should be avoided if a negative FFR is obtained,” he said, calling this technique “particularly useful for lesions that appear borderline by visual estimation alone.”
SOURCE: Sud M et al. AHA 2020. JAMA. 2020 Nov 13. doi: 10.1001/jama.2020.22708.
Restricting percutaneous interventions (PCI) to only those stenotic lesions that are ischemic by fractional flow reserve (FFR) thresholds is associated with better 5-year outcomes whether or not PCI is deployed, according to a cohort study presented at the American Heart Association scientific sessions.
For those that met the FFR threshold for ischemia, defined as up to 0.80, PCI reduced the risk of a major adverse cardiac event (MACE) at 5 years by 23% (hazard ratio, 0.77) relative to no PCI. Conversely, those not indicated for PCI because of a higher FFR had a 37% higher risk of MACE (HR, 1.37) at 5 years if treated with PCI relative to those who were not.
“The story of overuse of PCI is important,” reported the senior author Dennis Ko, MD, a scientist affiliated with the Schulich Heart Research Program, Sunnybrook Research Institute, University of Toronto, Canada. “We as interventionalists often think that putting in a stent is not harmful, and that turned out not to be the case.”
The FFR threshold for intervening with PCI is evidence based. Several trials, including one published in 2014, have associated PCI with better outcomes relative to medical therapy when FFR is 0.80 or lower. Other evidence suggests no advantage and possible harm for PCI performed if FFR is higher. Multiple guidelines, including those from the AHA, recommend against PCI if FFR is more than 0.80.
“As FRR is gaining in popularity, we were interested in whether physicians follow the thresholds in routine clinical practice and what happens to patient outcomes [if they are or are not followed],” Dr. Ko explained.
In this retrospective study by Dr. Ko’s trainee, Maneesh Sud, MD, and simultaneously published in JAMA, the answer was that there is deviation, and deviation leads to bad outcomes.
The 9,106 coronary artery disease patients included in the study underwent single-vessel FFR assessment within a 5-year period in Canada. The two cohorts evaluated were those with a lesional FFR of 0.80 or less, defined as ischemic, and those with a lesion with higher FFR, defined as nonischemic. The primary MACE outcome comprised death, myocardial infarction, unstable angina, or urgent coronary revascularization.
Of the 2,693 patients who met the FFR threshold of ischemia, 75.3% received PCI, and 24.7% were treated with medical therapy only. Of the 6,413 patients with nonischemic FFR, 87.4% were treated with medical therapy and 12.6% received PCI.
In those with ischemic FFR, event curves for MACE separated rapidly. At 30 days, the risk of MACE was 53% lower (HR, 0.47) in those receiving PCI. By 1 year, the advantage was less (HR, 0.76), but it was steady thereafter and remained about the same at 5 years (HR, 0.77; 95% confidence interval, 0.63-0.94). Relative advantages for each component of MACE went in the same direction. At 5 years, PCI exerted its greatest numerical advantage for the outcome or urgent coronary revascularization (HR, 0.71) and its least numerical advantage for MI (HR, 0.92), but none of these differences reached statistical significance.
In those with nonischemic coronary lesions on FFR, PCI was associated with more than twice the risk for MACE at 30 days (HR, 2.11), but the increase in risk relative to medical management fell at 1 year (HR 1.67) and 5 years (HR, 1.37). All of the individual components of MACE were numerically increased at all time points except for death, which was numerically lower at 30 days (HR, 0.41) and 5 years (HR, 0.94), even though these differences were not significant.
It could not be ascertained from these data why PCI was not performed when there was an indication or why it was performed when there was not. The investigators speculated that some clinicians may decide against PCI for ischemic lesions in the absence of symptoms or when concerned about comorbidities. They might offer PCI in nonischemic lesions because of symptoms, positive tests other than FFR, or FFR values near the threshold.
“I think the main message of our paper is that adherence of the FFR threshold as established by clinical trials is important,” Dr. Ko said in an interview. This not only means performing PCI when it is indicated, but refraining from PCI when it is not.
Basically, this study confirms that the guideline thresholds are valid, according to Jared M. O’Leary, MD, who is experienced with FFR and is Medical Director for Quality at the Vanderbilt Heart and Vascular Institute, Nashville, Tenn.
“It confirms the utility of FFR in the real world,” he said, adding that the results are “totally consistent with our practice.” He called FFR “an important tool in the cardiac cath lab” not only for determining when revascularization will benefit the patient but the opposite.
“The flip side is also true: Stenting should be avoided if a negative FFR is obtained,” he said, calling this technique “particularly useful for lesions that appear borderline by visual estimation alone.”
SOURCE: Sud M et al. AHA 2020. JAMA. 2020 Nov 13. doi: 10.1001/jama.2020.22708.
Restricting percutaneous interventions (PCI) to only those stenotic lesions that are ischemic by fractional flow reserve (FFR) thresholds is associated with better 5-year outcomes whether or not PCI is deployed, according to a cohort study presented at the American Heart Association scientific sessions.
For those that met the FFR threshold for ischemia, defined as up to 0.80, PCI reduced the risk of a major adverse cardiac event (MACE) at 5 years by 23% (hazard ratio, 0.77) relative to no PCI. Conversely, those not indicated for PCI because of a higher FFR had a 37% higher risk of MACE (HR, 1.37) at 5 years if treated with PCI relative to those who were not.
“The story of overuse of PCI is important,” reported the senior author Dennis Ko, MD, a scientist affiliated with the Schulich Heart Research Program, Sunnybrook Research Institute, University of Toronto, Canada. “We as interventionalists often think that putting in a stent is not harmful, and that turned out not to be the case.”
The FFR threshold for intervening with PCI is evidence based. Several trials, including one published in 2014, have associated PCI with better outcomes relative to medical therapy when FFR is 0.80 or lower. Other evidence suggests no advantage and possible harm for PCI performed if FFR is higher. Multiple guidelines, including those from the AHA, recommend against PCI if FFR is more than 0.80.
“As FRR is gaining in popularity, we were interested in whether physicians follow the thresholds in routine clinical practice and what happens to patient outcomes [if they are or are not followed],” Dr. Ko explained.
In this retrospective study by Dr. Ko’s trainee, Maneesh Sud, MD, and simultaneously published in JAMA, the answer was that there is deviation, and deviation leads to bad outcomes.
The 9,106 coronary artery disease patients included in the study underwent single-vessel FFR assessment within a 5-year period in Canada. The two cohorts evaluated were those with a lesional FFR of 0.80 or less, defined as ischemic, and those with a lesion with higher FFR, defined as nonischemic. The primary MACE outcome comprised death, myocardial infarction, unstable angina, or urgent coronary revascularization.
Of the 2,693 patients who met the FFR threshold of ischemia, 75.3% received PCI, and 24.7% were treated with medical therapy only. Of the 6,413 patients with nonischemic FFR, 87.4% were treated with medical therapy and 12.6% received PCI.
In those with ischemic FFR, event curves for MACE separated rapidly. At 30 days, the risk of MACE was 53% lower (HR, 0.47) in those receiving PCI. By 1 year, the advantage was less (HR, 0.76), but it was steady thereafter and remained about the same at 5 years (HR, 0.77; 95% confidence interval, 0.63-0.94). Relative advantages for each component of MACE went in the same direction. At 5 years, PCI exerted its greatest numerical advantage for the outcome or urgent coronary revascularization (HR, 0.71) and its least numerical advantage for MI (HR, 0.92), but none of these differences reached statistical significance.
In those with nonischemic coronary lesions on FFR, PCI was associated with more than twice the risk for MACE at 30 days (HR, 2.11), but the increase in risk relative to medical management fell at 1 year (HR 1.67) and 5 years (HR, 1.37). All of the individual components of MACE were numerically increased at all time points except for death, which was numerically lower at 30 days (HR, 0.41) and 5 years (HR, 0.94), even though these differences were not significant.
It could not be ascertained from these data why PCI was not performed when there was an indication or why it was performed when there was not. The investigators speculated that some clinicians may decide against PCI for ischemic lesions in the absence of symptoms or when concerned about comorbidities. They might offer PCI in nonischemic lesions because of symptoms, positive tests other than FFR, or FFR values near the threshold.
“I think the main message of our paper is that adherence of the FFR threshold as established by clinical trials is important,” Dr. Ko said in an interview. This not only means performing PCI when it is indicated, but refraining from PCI when it is not.
Basically, this study confirms that the guideline thresholds are valid, according to Jared M. O’Leary, MD, who is experienced with FFR and is Medical Director for Quality at the Vanderbilt Heart and Vascular Institute, Nashville, Tenn.
“It confirms the utility of FFR in the real world,” he said, adding that the results are “totally consistent with our practice.” He called FFR “an important tool in the cardiac cath lab” not only for determining when revascularization will benefit the patient but the opposite.
“The flip side is also true: Stenting should be avoided if a negative FFR is obtained,” he said, calling this technique “particularly useful for lesions that appear borderline by visual estimation alone.”
SOURCE: Sud M et al. AHA 2020. JAMA. 2020 Nov 13. doi: 10.1001/jama.2020.22708.
FROM AHA 2020
Can a probiotic prevent COVID-19?
On the Nov. 12 episode of the Blood & Cancer podcast, Anthony D. Sung, MD, of Duke University, Durham, N.C., joined host David H. Henry, MD, of Penn Medicine in Philadelphia, to discuss the trial of LGG as well as other research. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Here we are in COVID. We’re recording this the first week in November. Sadly, cases are spiking in the country. And I understand you’ve got some information that you might share about how manipulating ... the microbiome that we all exist with inside our gut might somehow play into doing better or worse with COVID.
Anthony Sung, MD: Absolutely. So, as associate director of the Duke Microbiome Center, I was approached by one of my colleagues, Paul Wischmeyer, who is a professor of anesthesiology and critical care medicine at Duke. Paul had previously done some very nice murine studies with the probiotic Lactobacillus rhamnosus GG, or LGG.
He showed, in a murine model of pseudomonas pneumonia, that giving LGG to mice would help modulate their microbiome and, in turn, their immune system, leading to decreased inflammation, decreased TNF-alpha, IL [interleukin]-2, and IL-6, [and] increased Treg cells [Clin Nutr. 2017;36[6]:1549-57]. This also helped prevent lung injury, and it actually significantly improved survival in mice receiving LGG [Shock. 2013;40[6]:496-503].
In addition, there has been a randomized clinical trial of LGG showing that its administration would help prevent ventilator-associated pneumonia, or VAP [Am J Respir Crit Care Med. 2010 Oct 15;182[8]:1058-64].
And a few years ago, there was another RCT [randomized, controlled trial], published in Nature, showing that another Lactobacillus product significantly decreased the combined endpoint of sepsis and mortality, primarily by reducing lower respiratory tract infection [Nature. 2017 Aug 24;548[7668]:407-12].
Dr. Henry: And how is that working? What is the bacillus doing to help us?
Dr. Sung: We think it’s through modulating the immune system. As mentioned in Paul’s studies, we saw significantly decreased amounts of TNF-alpha, IL-2, and IL-6, which are the same cytokines that have been implicated in COVID-19 and associated with increased lung injury in patients during this pandemic.
And we believe that by giving individuals this probiotic, LGG, we may help modulate the immune system, decrease lung injury and symptoms, and maybe even prevent COVID-19.
So with support from the Duke Microbiome Center, as well as private donations and philanthropy, we are conducting a randomized clinical trial of LGG to prevent COVID-19 in household contacts who’ve been exposed to the disease. In other words, if someone in the house gets COVID-19, we want to try to prophylax everybody else living in that house and prevent them from coming down with the same infection.
Dr. Henry: And this is an oral administration?
Dr. Sung: Correct. This is an oral pill, two pills once a day.
Dr. Henry: And it’s an ongoing study, of course, in COVID right now?
Dr. Sung: Correct. So we have an IND [investigational new drug application] from the FDA [Food and Drug Administration], and we are actively recruiting subjects both at Duke University, but also due to the unique study design, we can enroll patients anywhere across the country. Because of the importance of social distancing, everything is done remotely.
So a household contact can hear about us, either through your podcast or one of our Facebook ads or through other media. They can reach out to our study website, which is https://sites.duke.edu/protectehc, or reach out to us at our study email, [email protected].
And we can go ahead and screen them for eligibility in our trial. And if they are eligible and they consent to participate, we will mail them a package basically overnight, FedEx, containing either LGG or placebo, as well as kits so that they can self-collect their stool and nasal swabs so we can test it for SARS-CoV-2 by PCR [polymerase chain reaction] and look at the microbiome.
Dr. Sung and Dr. Henry have no relevant disclosures. Funding for the trial is provided by the Duke Microbiome Center and philanthropic giving. The LGG and placebo used in the trial are provided by DSM.
On the Nov. 12 episode of the Blood & Cancer podcast, Anthony D. Sung, MD, of Duke University, Durham, N.C., joined host David H. Henry, MD, of Penn Medicine in Philadelphia, to discuss the trial of LGG as well as other research. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Here we are in COVID. We’re recording this the first week in November. Sadly, cases are spiking in the country. And I understand you’ve got some information that you might share about how manipulating ... the microbiome that we all exist with inside our gut might somehow play into doing better or worse with COVID.
Anthony Sung, MD: Absolutely. So, as associate director of the Duke Microbiome Center, I was approached by one of my colleagues, Paul Wischmeyer, who is a professor of anesthesiology and critical care medicine at Duke. Paul had previously done some very nice murine studies with the probiotic Lactobacillus rhamnosus GG, or LGG.
He showed, in a murine model of pseudomonas pneumonia, that giving LGG to mice would help modulate their microbiome and, in turn, their immune system, leading to decreased inflammation, decreased TNF-alpha, IL [interleukin]-2, and IL-6, [and] increased Treg cells [Clin Nutr. 2017;36[6]:1549-57]. This also helped prevent lung injury, and it actually significantly improved survival in mice receiving LGG [Shock. 2013;40[6]:496-503].
In addition, there has been a randomized clinical trial of LGG showing that its administration would help prevent ventilator-associated pneumonia, or VAP [Am J Respir Crit Care Med. 2010 Oct 15;182[8]:1058-64].
And a few years ago, there was another RCT [randomized, controlled trial], published in Nature, showing that another Lactobacillus product significantly decreased the combined endpoint of sepsis and mortality, primarily by reducing lower respiratory tract infection [Nature. 2017 Aug 24;548[7668]:407-12].
Dr. Henry: And how is that working? What is the bacillus doing to help us?
Dr. Sung: We think it’s through modulating the immune system. As mentioned in Paul’s studies, we saw significantly decreased amounts of TNF-alpha, IL-2, and IL-6, which are the same cytokines that have been implicated in COVID-19 and associated with increased lung injury in patients during this pandemic.
And we believe that by giving individuals this probiotic, LGG, we may help modulate the immune system, decrease lung injury and symptoms, and maybe even prevent COVID-19.
So with support from the Duke Microbiome Center, as well as private donations and philanthropy, we are conducting a randomized clinical trial of LGG to prevent COVID-19 in household contacts who’ve been exposed to the disease. In other words, if someone in the house gets COVID-19, we want to try to prophylax everybody else living in that house and prevent them from coming down with the same infection.
Dr. Henry: And this is an oral administration?
Dr. Sung: Correct. This is an oral pill, two pills once a day.
Dr. Henry: And it’s an ongoing study, of course, in COVID right now?
Dr. Sung: Correct. So we have an IND [investigational new drug application] from the FDA [Food and Drug Administration], and we are actively recruiting subjects both at Duke University, but also due to the unique study design, we can enroll patients anywhere across the country. Because of the importance of social distancing, everything is done remotely.
So a household contact can hear about us, either through your podcast or one of our Facebook ads or through other media. They can reach out to our study website, which is https://sites.duke.edu/protectehc, or reach out to us at our study email, [email protected].
And we can go ahead and screen them for eligibility in our trial. And if they are eligible and they consent to participate, we will mail them a package basically overnight, FedEx, containing either LGG or placebo, as well as kits so that they can self-collect their stool and nasal swabs so we can test it for SARS-CoV-2 by PCR [polymerase chain reaction] and look at the microbiome.
Dr. Sung and Dr. Henry have no relevant disclosures. Funding for the trial is provided by the Duke Microbiome Center and philanthropic giving. The LGG and placebo used in the trial are provided by DSM.
On the Nov. 12 episode of the Blood & Cancer podcast, Anthony D. Sung, MD, of Duke University, Durham, N.C., joined host David H. Henry, MD, of Penn Medicine in Philadelphia, to discuss the trial of LGG as well as other research. The following transcript of that discussion has been edited for length and clarity.
David Henry, MD: Here we are in COVID. We’re recording this the first week in November. Sadly, cases are spiking in the country. And I understand you’ve got some information that you might share about how manipulating ... the microbiome that we all exist with inside our gut might somehow play into doing better or worse with COVID.
Anthony Sung, MD: Absolutely. So, as associate director of the Duke Microbiome Center, I was approached by one of my colleagues, Paul Wischmeyer, who is a professor of anesthesiology and critical care medicine at Duke. Paul had previously done some very nice murine studies with the probiotic Lactobacillus rhamnosus GG, or LGG.
He showed, in a murine model of pseudomonas pneumonia, that giving LGG to mice would help modulate their microbiome and, in turn, their immune system, leading to decreased inflammation, decreased TNF-alpha, IL [interleukin]-2, and IL-6, [and] increased Treg cells [Clin Nutr. 2017;36[6]:1549-57]. This also helped prevent lung injury, and it actually significantly improved survival in mice receiving LGG [Shock. 2013;40[6]:496-503].
In addition, there has been a randomized clinical trial of LGG showing that its administration would help prevent ventilator-associated pneumonia, or VAP [Am J Respir Crit Care Med. 2010 Oct 15;182[8]:1058-64].
And a few years ago, there was another RCT [randomized, controlled trial], published in Nature, showing that another Lactobacillus product significantly decreased the combined endpoint of sepsis and mortality, primarily by reducing lower respiratory tract infection [Nature. 2017 Aug 24;548[7668]:407-12].
Dr. Henry: And how is that working? What is the bacillus doing to help us?
Dr. Sung: We think it’s through modulating the immune system. As mentioned in Paul’s studies, we saw significantly decreased amounts of TNF-alpha, IL-2, and IL-6, which are the same cytokines that have been implicated in COVID-19 and associated with increased lung injury in patients during this pandemic.
And we believe that by giving individuals this probiotic, LGG, we may help modulate the immune system, decrease lung injury and symptoms, and maybe even prevent COVID-19.
So with support from the Duke Microbiome Center, as well as private donations and philanthropy, we are conducting a randomized clinical trial of LGG to prevent COVID-19 in household contacts who’ve been exposed to the disease. In other words, if someone in the house gets COVID-19, we want to try to prophylax everybody else living in that house and prevent them from coming down with the same infection.
Dr. Henry: And this is an oral administration?
Dr. Sung: Correct. This is an oral pill, two pills once a day.
Dr. Henry: And it’s an ongoing study, of course, in COVID right now?
Dr. Sung: Correct. So we have an IND [investigational new drug application] from the FDA [Food and Drug Administration], and we are actively recruiting subjects both at Duke University, but also due to the unique study design, we can enroll patients anywhere across the country. Because of the importance of social distancing, everything is done remotely.
So a household contact can hear about us, either through your podcast or one of our Facebook ads or through other media. They can reach out to our study website, which is https://sites.duke.edu/protectehc, or reach out to us at our study email, [email protected].
And we can go ahead and screen them for eligibility in our trial. And if they are eligible and they consent to participate, we will mail them a package basically overnight, FedEx, containing either LGG or placebo, as well as kits so that they can self-collect their stool and nasal swabs so we can test it for SARS-CoV-2 by PCR [polymerase chain reaction] and look at the microbiome.
Dr. Sung and Dr. Henry have no relevant disclosures. Funding for the trial is provided by the Duke Microbiome Center and philanthropic giving. The LGG and placebo used in the trial are provided by DSM.








