User login
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Measles
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
© 2020 Society of Hospital Medicine
A Plea to Reconsider the Diagnosis
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
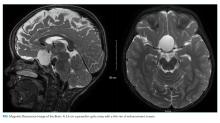
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
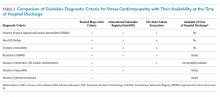
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
© 2019 Society of Hospital Medicine
Utility of ICD Codes for Stress Cardiomyopathy in Hospital Administrative Databases: What Do They Signify?
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
© 2020 Society of Hospital Medicine
Prediction of Disposition Within 48 Hours of Hospital Admission Using Patient Mobility Scores
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
© 2019 Society of Hospital Medicine
Low RAAS inhibitor dosing linked to MACE risk
Suboptimal dosing of renin-angiotensin-aldosterone system (RAAS) inhibitors to reduce the risk of hyperkalemia could increase the risk of major adverse cardiac events (MACE) and all-cause mortality in patients with chronic kidney disease (CKD) or heart failure.
Researchers reported the outcomes of an observational study that explored the real-world associations between RAAS inhibitor dose, hyperkalemia, and clinical outcomes.
RAAS inhibitors – such as ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists – are known to reduce potassium excretion and therefore increase the risk of high potassium levels.
Dr. Cecilia Linde, from the Karolinska University Hospital and Karolinska Institutet in Stockholm, and coauthors wrote that management of serum potassium levels often requires reducing the dosage of RAAS inhibitors or stopping them altogether. However, this is also associated with risks in patients with heart failure or CKD.
In this study, researchers looked at data from 100,572 people with nondialysis CKD and 13,113 with new-onset heart failure who were prescribed RAAS inhibitors during 2006-2015.
Overall, 58% of patients with CKD and 63% of patients with heart failure spent the majority of follow-up on prescribed optimal doses of RAAS inhibitors – defined as at least 50% of the guidelines-recommended dose.
Patients with hyperkalemia were more likely to have down-titrations or discontinue their RAAS inhibitors, and this increased with increasing hyperkalemia severity.
The study found consistently lower mortality rates among patients who spent most of their follow-up time on at least 50% of the guideline-recommended dose of RAAS inhibitors.
In patients with CKD, mortality rates were 7.2 deaths per 1,000 patient-years in those taking at least 50% of the recommended dose, compared with 57.7 deaths per 1,000 patient-years for those on suboptimal doses. The rates of MACE were 73 and 130 per 1,000 patient-years, respectively.
The differences were even more pronounced in patients with heart failure. Those taking at least 50% of the recommended dose had mortality rates of 12.5 per 1000 patient-years, compared with 141.7 among those on suboptimal doses. The rates of MACE were 148.5 and 290.4, respectively.
“The results highlight the potential negative impact of suboptimal RAASi dosing, indicate the generalizability of [European Society of Cardiology–recommended] RAASi doses in HF to CKD patients, and emphasize the need for strategies that allow patients to be maintained on appropriate therapy, avoiding RAASi dose modification or discontinuation,” the authors wrote.
The study was funded by AstraZeneca. One author was an employee and stockholder of AstraZeneca, and five authors declared funding and support from the pharmaceutical sector, including AstraZeneca.
SOURCE: Linde C et al. J Am Heart Assoc. 2019 Nov 12. doi: 10.1161/JAHA.119.012655.
Suboptimal dosing of renin-angiotensin-aldosterone system (RAAS) inhibitors to reduce the risk of hyperkalemia could increase the risk of major adverse cardiac events (MACE) and all-cause mortality in patients with chronic kidney disease (CKD) or heart failure.
Researchers reported the outcomes of an observational study that explored the real-world associations between RAAS inhibitor dose, hyperkalemia, and clinical outcomes.
RAAS inhibitors – such as ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists – are known to reduce potassium excretion and therefore increase the risk of high potassium levels.
Dr. Cecilia Linde, from the Karolinska University Hospital and Karolinska Institutet in Stockholm, and coauthors wrote that management of serum potassium levels often requires reducing the dosage of RAAS inhibitors or stopping them altogether. However, this is also associated with risks in patients with heart failure or CKD.
In this study, researchers looked at data from 100,572 people with nondialysis CKD and 13,113 with new-onset heart failure who were prescribed RAAS inhibitors during 2006-2015.
Overall, 58% of patients with CKD and 63% of patients with heart failure spent the majority of follow-up on prescribed optimal doses of RAAS inhibitors – defined as at least 50% of the guidelines-recommended dose.
Patients with hyperkalemia were more likely to have down-titrations or discontinue their RAAS inhibitors, and this increased with increasing hyperkalemia severity.
The study found consistently lower mortality rates among patients who spent most of their follow-up time on at least 50% of the guideline-recommended dose of RAAS inhibitors.
In patients with CKD, mortality rates were 7.2 deaths per 1,000 patient-years in those taking at least 50% of the recommended dose, compared with 57.7 deaths per 1,000 patient-years for those on suboptimal doses. The rates of MACE were 73 and 130 per 1,000 patient-years, respectively.
The differences were even more pronounced in patients with heart failure. Those taking at least 50% of the recommended dose had mortality rates of 12.5 per 1000 patient-years, compared with 141.7 among those on suboptimal doses. The rates of MACE were 148.5 and 290.4, respectively.
“The results highlight the potential negative impact of suboptimal RAASi dosing, indicate the generalizability of [European Society of Cardiology–recommended] RAASi doses in HF to CKD patients, and emphasize the need for strategies that allow patients to be maintained on appropriate therapy, avoiding RAASi dose modification or discontinuation,” the authors wrote.
The study was funded by AstraZeneca. One author was an employee and stockholder of AstraZeneca, and five authors declared funding and support from the pharmaceutical sector, including AstraZeneca.
SOURCE: Linde C et al. J Am Heart Assoc. 2019 Nov 12. doi: 10.1161/JAHA.119.012655.
Suboptimal dosing of renin-angiotensin-aldosterone system (RAAS) inhibitors to reduce the risk of hyperkalemia could increase the risk of major adverse cardiac events (MACE) and all-cause mortality in patients with chronic kidney disease (CKD) or heart failure.
Researchers reported the outcomes of an observational study that explored the real-world associations between RAAS inhibitor dose, hyperkalemia, and clinical outcomes.
RAAS inhibitors – such as ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists – are known to reduce potassium excretion and therefore increase the risk of high potassium levels.
Dr. Cecilia Linde, from the Karolinska University Hospital and Karolinska Institutet in Stockholm, and coauthors wrote that management of serum potassium levels often requires reducing the dosage of RAAS inhibitors or stopping them altogether. However, this is also associated with risks in patients with heart failure or CKD.
In this study, researchers looked at data from 100,572 people with nondialysis CKD and 13,113 with new-onset heart failure who were prescribed RAAS inhibitors during 2006-2015.
Overall, 58% of patients with CKD and 63% of patients with heart failure spent the majority of follow-up on prescribed optimal doses of RAAS inhibitors – defined as at least 50% of the guidelines-recommended dose.
Patients with hyperkalemia were more likely to have down-titrations or discontinue their RAAS inhibitors, and this increased with increasing hyperkalemia severity.
The study found consistently lower mortality rates among patients who spent most of their follow-up time on at least 50% of the guideline-recommended dose of RAAS inhibitors.
In patients with CKD, mortality rates were 7.2 deaths per 1,000 patient-years in those taking at least 50% of the recommended dose, compared with 57.7 deaths per 1,000 patient-years for those on suboptimal doses. The rates of MACE were 73 and 130 per 1,000 patient-years, respectively.
The differences were even more pronounced in patients with heart failure. Those taking at least 50% of the recommended dose had mortality rates of 12.5 per 1000 patient-years, compared with 141.7 among those on suboptimal doses. The rates of MACE were 148.5 and 290.4, respectively.
“The results highlight the potential negative impact of suboptimal RAASi dosing, indicate the generalizability of [European Society of Cardiology–recommended] RAASi doses in HF to CKD patients, and emphasize the need for strategies that allow patients to be maintained on appropriate therapy, avoiding RAASi dose modification or discontinuation,” the authors wrote.
The study was funded by AstraZeneca. One author was an employee and stockholder of AstraZeneca, and five authors declared funding and support from the pharmaceutical sector, including AstraZeneca.
SOURCE: Linde C et al. J Am Heart Assoc. 2019 Nov 12. doi: 10.1161/JAHA.119.012655.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Age, sex, and other factors linked to risk of intracranial hemorrhage in ITP
ORLANDO – A large, retrospective study suggests several factors are associated with an increased risk of intracranial hemorrhage in patients with immune thrombocytopenia.
Data on more than 300,000 immune thrombocytopenia (ITP) hospitalizations indicated that older age, male sex, not having private insurance, having a gastrointestinal or “other” bleed, and receiving treatment at a hospital in the western United States, a medium- or large-sized hospital, or an urban teaching hospital were all associated with an increased risk of intracranial hemorrhage (ICH).
Mayank Sharma, of the University of Miami, detailed these findings at the annual meeting of the American Society of Hematology.
Mr. Sharma and colleagues analyzed data from the National Inpatient Sample database from 2007 to 2016. Of the 348,906 ITP hospitalizations included, there were 3,408 (0.98%) cases of ICH.
The overall incidence of ICH was low and remained stable over time, “which is reassuring,” Mr. Sharma said. However, the mortality rate was higher among patients with ICH than among those without it – 26.7% and 3.2%, respectively.
A multivariate analysis showed that female patients had a decreased likelihood of ICH, with an odds ratio of 0.81 (95% confidence interval, 0.68-0.97). Patients with private insurance had a decreased likelihood of ICH as well, with an OR of 0.81 (95% CI, 0.61-1.08).
Conversely, older patients had an increased likelihood of ICH. The OR was 2.23 (95% CI, 1.51-3.31) for patients aged 25-64 years, and the OR was 3.69 (95% CI, 2.34-5.84) for patients aged 65 years and older.
Patients with a gastrointestinal bleed or an other bleed (not including hematuria or epistaxis) had an increased likelihood of ICH. The ORs were 1.60 (95% CI, 1.18-2.16) and 1.69 (95% CI, 1.19-2.42), respectively.
Patients hospitalized in the western United States (OR, 1.62; 95% CI, 1.26-2.08), at a medium-sized hospital (OR, 1.64; 95% CI, 1.08-2.47), at a large hospital (OR, 2.42; 95% CI, 1.65-3.55), or at an urban teaching hospital (OR, 2.73; 95% CI, 1.80-4.13) all had an increased likelihood of ICH.
“Our second objective was to study the factors associated with mortality in ITP patients with ICH,” Mr. Sharma said. “We found female gender and Medicaid, private, or self-pay as primary payers to be associated with a lower mortality in ITP with ICH.
“[A]ge of 25-64 and 65 years and above, coexistence of a GI bleed or other bleed, and admission to a large or urban teaching hospital were associated with a higher mortality,” he added.
Mr. Sharma said the study’s strengths are that it is the most recent study on trends in ITP/ICH hospitalizations, and that it’s a longitudinal assessment of data from a nationally representative database.
The study’s limitations include its retrospective nature and the use of ICD codes, which could lead to inaccuracies. Data on prior therapies and long-term outcomes were not available, and the researchers were unable to differentiate between acute and chronic ITP.
Mr. Sharma said he had no relevant conflicts of interest.
SOURCE: Sharma M et al. ASH 2019, Abstract 55.
ORLANDO – A large, retrospective study suggests several factors are associated with an increased risk of intracranial hemorrhage in patients with immune thrombocytopenia.
Data on more than 300,000 immune thrombocytopenia (ITP) hospitalizations indicated that older age, male sex, not having private insurance, having a gastrointestinal or “other” bleed, and receiving treatment at a hospital in the western United States, a medium- or large-sized hospital, or an urban teaching hospital were all associated with an increased risk of intracranial hemorrhage (ICH).
Mayank Sharma, of the University of Miami, detailed these findings at the annual meeting of the American Society of Hematology.
Mr. Sharma and colleagues analyzed data from the National Inpatient Sample database from 2007 to 2016. Of the 348,906 ITP hospitalizations included, there were 3,408 (0.98%) cases of ICH.
The overall incidence of ICH was low and remained stable over time, “which is reassuring,” Mr. Sharma said. However, the mortality rate was higher among patients with ICH than among those without it – 26.7% and 3.2%, respectively.
A multivariate analysis showed that female patients had a decreased likelihood of ICH, with an odds ratio of 0.81 (95% confidence interval, 0.68-0.97). Patients with private insurance had a decreased likelihood of ICH as well, with an OR of 0.81 (95% CI, 0.61-1.08).
Conversely, older patients had an increased likelihood of ICH. The OR was 2.23 (95% CI, 1.51-3.31) for patients aged 25-64 years, and the OR was 3.69 (95% CI, 2.34-5.84) for patients aged 65 years and older.
Patients with a gastrointestinal bleed or an other bleed (not including hematuria or epistaxis) had an increased likelihood of ICH. The ORs were 1.60 (95% CI, 1.18-2.16) and 1.69 (95% CI, 1.19-2.42), respectively.
Patients hospitalized in the western United States (OR, 1.62; 95% CI, 1.26-2.08), at a medium-sized hospital (OR, 1.64; 95% CI, 1.08-2.47), at a large hospital (OR, 2.42; 95% CI, 1.65-3.55), or at an urban teaching hospital (OR, 2.73; 95% CI, 1.80-4.13) all had an increased likelihood of ICH.
“Our second objective was to study the factors associated with mortality in ITP patients with ICH,” Mr. Sharma said. “We found female gender and Medicaid, private, or self-pay as primary payers to be associated with a lower mortality in ITP with ICH.
“[A]ge of 25-64 and 65 years and above, coexistence of a GI bleed or other bleed, and admission to a large or urban teaching hospital were associated with a higher mortality,” he added.
Mr. Sharma said the study’s strengths are that it is the most recent study on trends in ITP/ICH hospitalizations, and that it’s a longitudinal assessment of data from a nationally representative database.
The study’s limitations include its retrospective nature and the use of ICD codes, which could lead to inaccuracies. Data on prior therapies and long-term outcomes were not available, and the researchers were unable to differentiate between acute and chronic ITP.
Mr. Sharma said he had no relevant conflicts of interest.
SOURCE: Sharma M et al. ASH 2019, Abstract 55.
ORLANDO – A large, retrospective study suggests several factors are associated with an increased risk of intracranial hemorrhage in patients with immune thrombocytopenia.
Data on more than 300,000 immune thrombocytopenia (ITP) hospitalizations indicated that older age, male sex, not having private insurance, having a gastrointestinal or “other” bleed, and receiving treatment at a hospital in the western United States, a medium- or large-sized hospital, or an urban teaching hospital were all associated with an increased risk of intracranial hemorrhage (ICH).
Mayank Sharma, of the University of Miami, detailed these findings at the annual meeting of the American Society of Hematology.
Mr. Sharma and colleagues analyzed data from the National Inpatient Sample database from 2007 to 2016. Of the 348,906 ITP hospitalizations included, there were 3,408 (0.98%) cases of ICH.
The overall incidence of ICH was low and remained stable over time, “which is reassuring,” Mr. Sharma said. However, the mortality rate was higher among patients with ICH than among those without it – 26.7% and 3.2%, respectively.
A multivariate analysis showed that female patients had a decreased likelihood of ICH, with an odds ratio of 0.81 (95% confidence interval, 0.68-0.97). Patients with private insurance had a decreased likelihood of ICH as well, with an OR of 0.81 (95% CI, 0.61-1.08).
Conversely, older patients had an increased likelihood of ICH. The OR was 2.23 (95% CI, 1.51-3.31) for patients aged 25-64 years, and the OR was 3.69 (95% CI, 2.34-5.84) for patients aged 65 years and older.
Patients with a gastrointestinal bleed or an other bleed (not including hematuria or epistaxis) had an increased likelihood of ICH. The ORs were 1.60 (95% CI, 1.18-2.16) and 1.69 (95% CI, 1.19-2.42), respectively.
Patients hospitalized in the western United States (OR, 1.62; 95% CI, 1.26-2.08), at a medium-sized hospital (OR, 1.64; 95% CI, 1.08-2.47), at a large hospital (OR, 2.42; 95% CI, 1.65-3.55), or at an urban teaching hospital (OR, 2.73; 95% CI, 1.80-4.13) all had an increased likelihood of ICH.
“Our second objective was to study the factors associated with mortality in ITP patients with ICH,” Mr. Sharma said. “We found female gender and Medicaid, private, or self-pay as primary payers to be associated with a lower mortality in ITP with ICH.
“[A]ge of 25-64 and 65 years and above, coexistence of a GI bleed or other bleed, and admission to a large or urban teaching hospital were associated with a higher mortality,” he added.
Mr. Sharma said the study’s strengths are that it is the most recent study on trends in ITP/ICH hospitalizations, and that it’s a longitudinal assessment of data from a nationally representative database.
The study’s limitations include its retrospective nature and the use of ICD codes, which could lead to inaccuracies. Data on prior therapies and long-term outcomes were not available, and the researchers were unable to differentiate between acute and chronic ITP.
Mr. Sharma said he had no relevant conflicts of interest.
SOURCE: Sharma M et al. ASH 2019, Abstract 55.
REPORTING FROM ASH 2019
Iscalimab normalizes thyroid hormone levels in some patients with Graves disease
CHICAGO – The investigational monoclonal antibody iscalimab reduced levels of thyroid hormone and thyroid-stimulating hormone–receptor antibodies (TSHR-Ab) in some patients with Graves disease in a small study.
Of 15 patients with Graves disease, 7 patients, or 47%, saw their thyroid hormone levels normalize, and levels of TSHR-Ab normalized in 4 patients, or 27% of the cohort. In addition, mean levels of a chemokine associated with Graves disease activity dropped.
“These results suggest that iscalimab may be an effective and attractive immunomodulation strategy for Graves disease,” said George Kahaly, MD, PhD, in his presentation of the phase 2 results at the annual meeting of the American Thyroid Association (J Clin Endocrinol Metab. 2019 Sep 12. doi: 10.1210/clinem/dgz013).
Overall, patients who responded had lower levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSHR-Ab and lower thyroid volume at baseline.
Iscalimab is a fully human monoclonal antibody that is active against the costimulatory protein CD40 that is present on the surface of antigen-presenting cells. Dr. Kahaly, professor of endocrinology at Johannes Gutenberg University Medical Center, Mainz, Germany, explained that in primate studies, iscalimab inhibits the T cell–dependent antibody response to an antigen, without depletion of B cells. However, iscalimab would be expected to block B-cell activation and differentiation, “leading to reduced de novo TSHR antibody production,” said Dr. Kahaly. Inhibition of T cell–dependent antibody response was seen when iscalimab was given at a dose of 3 mg/kg in healthy human study participants.
The study results presented by Dr. Kahaly were drawn from a single-arm, proof-of-concept study that enrolled 15 patients with Graves disease to 12 weeks of treatment with iscalimab. The participants were followed for an additional 24 weeks after receiving intravenous iscalimab at 10 mg/kg on study days 1, 15, 29, 57, and 85.
All participants were receiving beta blockers at enrollment; four patients had new-onset Graves disease, and the rest were experiencing a treatment relapse.
The participants were a median 49 years old, and all but two were female. One patient was Asian, and the remainder were white. They were mostly normal weight, with a mean body mass index of about 23 kg/m2.
A group of seven patients who were clear responders to iscalimab saw normalization of FT4 levels; of the eight patients considered to be nonresponders, six required rescue medication with antithyroid drugs.
For responders, the initial mean FT4 level was 33.5 pmol/L, whereas for nonresponders, it was 51.3 pmol/L (P less than .05). Similarly, mean FT3 levels were 13.6 pmol/L in responders, compared with 22 pmol/L in nonresponders (P less than .05).
Mean thyroid volume was 14.5 ml in responders, compared with 26 ml in nonresponders (P less than .005).
A subgroup of four patients within the responder group became TSHR-Ab negative, with sustained low antibody levels seen during the follow-up period. All but one of the eight nonresponders had initial TSHR-Ab levels of more than 20 U/L, whereas the seven responders began with TSHR-Ab levels of about 10 U/L or less. Mean TSHR-Ab levels at baseline were 5.6 IU/L for responders, compared with 27.3 IU/L for nonresponders (P less than .001).
Most responders also had lower initial levels of antithyroid peroxidase IgG antibodies, compared with the nonresponder group.
Levels of chemokine (motif C-X-C) ligand 13 (CXCL13) fell throughout the study period. Higher CXCL13 levels are associated with lymphocytic infiltrates seen in autoimmune thyroiditis.
Occupancy of CD40 was initially measured at week 4 of the study and it remained high until week 16, when free CD40 receptors rose rapidly for several participants in both the responder and nonresponder groups. “The iscalimab intervention resulted in complete CD40 engagement for up to 20 weeks,” wrote Dr. Kahaly and colleagues in the abstract accompanying the presentation.
In assessing CD40 target engagement, the investigators found that total soluble CD40 levels climbed during the treatment period, reaching peaks as high as 400-500 ng/mL, and then plummeted back to zero by study’s end for all participants.
A pharmacokinetic analysis revealed expected peaks of serum iscalimab after treatments, with levels dropping sharply at the end of the study period and falling to levels approaching zero by week 24 for most participants.
In terms of safety, 12 patients experienced at least one adverse event, with 3 participants reporting an episode of cystitis during the study. Fatigue, headache, insomnia, nausea, and viral upper respiratory infection were each reported by 2 patients. No injection site reactions were seen. All adverse events were mild or moderate, did not result in study withdrawal, and resolved by the end of the study period, Dr. Kahaly noted.
“These encouraging results suggest that iscalimab should be tested further to understand better its potential therapeutic benefit,” the investigators wrote.
The study was funded by Novartis, which is developing iscalimab for Graves disease, other autoimmune disorders, and as an antirejection drug for patients with kidney transplants.
CHICAGO – The investigational monoclonal antibody iscalimab reduced levels of thyroid hormone and thyroid-stimulating hormone–receptor antibodies (TSHR-Ab) in some patients with Graves disease in a small study.
Of 15 patients with Graves disease, 7 patients, or 47%, saw their thyroid hormone levels normalize, and levels of TSHR-Ab normalized in 4 patients, or 27% of the cohort. In addition, mean levels of a chemokine associated with Graves disease activity dropped.
“These results suggest that iscalimab may be an effective and attractive immunomodulation strategy for Graves disease,” said George Kahaly, MD, PhD, in his presentation of the phase 2 results at the annual meeting of the American Thyroid Association (J Clin Endocrinol Metab. 2019 Sep 12. doi: 10.1210/clinem/dgz013).
Overall, patients who responded had lower levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSHR-Ab and lower thyroid volume at baseline.
Iscalimab is a fully human monoclonal antibody that is active against the costimulatory protein CD40 that is present on the surface of antigen-presenting cells. Dr. Kahaly, professor of endocrinology at Johannes Gutenberg University Medical Center, Mainz, Germany, explained that in primate studies, iscalimab inhibits the T cell–dependent antibody response to an antigen, without depletion of B cells. However, iscalimab would be expected to block B-cell activation and differentiation, “leading to reduced de novo TSHR antibody production,” said Dr. Kahaly. Inhibition of T cell–dependent antibody response was seen when iscalimab was given at a dose of 3 mg/kg in healthy human study participants.
The study results presented by Dr. Kahaly were drawn from a single-arm, proof-of-concept study that enrolled 15 patients with Graves disease to 12 weeks of treatment with iscalimab. The participants were followed for an additional 24 weeks after receiving intravenous iscalimab at 10 mg/kg on study days 1, 15, 29, 57, and 85.
All participants were receiving beta blockers at enrollment; four patients had new-onset Graves disease, and the rest were experiencing a treatment relapse.
The participants were a median 49 years old, and all but two were female. One patient was Asian, and the remainder were white. They were mostly normal weight, with a mean body mass index of about 23 kg/m2.
A group of seven patients who were clear responders to iscalimab saw normalization of FT4 levels; of the eight patients considered to be nonresponders, six required rescue medication with antithyroid drugs.
For responders, the initial mean FT4 level was 33.5 pmol/L, whereas for nonresponders, it was 51.3 pmol/L (P less than .05). Similarly, mean FT3 levels were 13.6 pmol/L in responders, compared with 22 pmol/L in nonresponders (P less than .05).
Mean thyroid volume was 14.5 ml in responders, compared with 26 ml in nonresponders (P less than .005).
A subgroup of four patients within the responder group became TSHR-Ab negative, with sustained low antibody levels seen during the follow-up period. All but one of the eight nonresponders had initial TSHR-Ab levels of more than 20 U/L, whereas the seven responders began with TSHR-Ab levels of about 10 U/L or less. Mean TSHR-Ab levels at baseline were 5.6 IU/L for responders, compared with 27.3 IU/L for nonresponders (P less than .001).
Most responders also had lower initial levels of antithyroid peroxidase IgG antibodies, compared with the nonresponder group.
Levels of chemokine (motif C-X-C) ligand 13 (CXCL13) fell throughout the study period. Higher CXCL13 levels are associated with lymphocytic infiltrates seen in autoimmune thyroiditis.
Occupancy of CD40 was initially measured at week 4 of the study and it remained high until week 16, when free CD40 receptors rose rapidly for several participants in both the responder and nonresponder groups. “The iscalimab intervention resulted in complete CD40 engagement for up to 20 weeks,” wrote Dr. Kahaly and colleagues in the abstract accompanying the presentation.
In assessing CD40 target engagement, the investigators found that total soluble CD40 levels climbed during the treatment period, reaching peaks as high as 400-500 ng/mL, and then plummeted back to zero by study’s end for all participants.
A pharmacokinetic analysis revealed expected peaks of serum iscalimab after treatments, with levels dropping sharply at the end of the study period and falling to levels approaching zero by week 24 for most participants.
In terms of safety, 12 patients experienced at least one adverse event, with 3 participants reporting an episode of cystitis during the study. Fatigue, headache, insomnia, nausea, and viral upper respiratory infection were each reported by 2 patients. No injection site reactions were seen. All adverse events were mild or moderate, did not result in study withdrawal, and resolved by the end of the study period, Dr. Kahaly noted.
“These encouraging results suggest that iscalimab should be tested further to understand better its potential therapeutic benefit,” the investigators wrote.
The study was funded by Novartis, which is developing iscalimab for Graves disease, other autoimmune disorders, and as an antirejection drug for patients with kidney transplants.
CHICAGO – The investigational monoclonal antibody iscalimab reduced levels of thyroid hormone and thyroid-stimulating hormone–receptor antibodies (TSHR-Ab) in some patients with Graves disease in a small study.
Of 15 patients with Graves disease, 7 patients, or 47%, saw their thyroid hormone levels normalize, and levels of TSHR-Ab normalized in 4 patients, or 27% of the cohort. In addition, mean levels of a chemokine associated with Graves disease activity dropped.
“These results suggest that iscalimab may be an effective and attractive immunomodulation strategy for Graves disease,” said George Kahaly, MD, PhD, in his presentation of the phase 2 results at the annual meeting of the American Thyroid Association (J Clin Endocrinol Metab. 2019 Sep 12. doi: 10.1210/clinem/dgz013).
Overall, patients who responded had lower levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSHR-Ab and lower thyroid volume at baseline.
Iscalimab is a fully human monoclonal antibody that is active against the costimulatory protein CD40 that is present on the surface of antigen-presenting cells. Dr. Kahaly, professor of endocrinology at Johannes Gutenberg University Medical Center, Mainz, Germany, explained that in primate studies, iscalimab inhibits the T cell–dependent antibody response to an antigen, without depletion of B cells. However, iscalimab would be expected to block B-cell activation and differentiation, “leading to reduced de novo TSHR antibody production,” said Dr. Kahaly. Inhibition of T cell–dependent antibody response was seen when iscalimab was given at a dose of 3 mg/kg in healthy human study participants.
The study results presented by Dr. Kahaly were drawn from a single-arm, proof-of-concept study that enrolled 15 patients with Graves disease to 12 weeks of treatment with iscalimab. The participants were followed for an additional 24 weeks after receiving intravenous iscalimab at 10 mg/kg on study days 1, 15, 29, 57, and 85.
All participants were receiving beta blockers at enrollment; four patients had new-onset Graves disease, and the rest were experiencing a treatment relapse.
The participants were a median 49 years old, and all but two were female. One patient was Asian, and the remainder were white. They were mostly normal weight, with a mean body mass index of about 23 kg/m2.
A group of seven patients who were clear responders to iscalimab saw normalization of FT4 levels; of the eight patients considered to be nonresponders, six required rescue medication with antithyroid drugs.
For responders, the initial mean FT4 level was 33.5 pmol/L, whereas for nonresponders, it was 51.3 pmol/L (P less than .05). Similarly, mean FT3 levels were 13.6 pmol/L in responders, compared with 22 pmol/L in nonresponders (P less than .05).
Mean thyroid volume was 14.5 ml in responders, compared with 26 ml in nonresponders (P less than .005).
A subgroup of four patients within the responder group became TSHR-Ab negative, with sustained low antibody levels seen during the follow-up period. All but one of the eight nonresponders had initial TSHR-Ab levels of more than 20 U/L, whereas the seven responders began with TSHR-Ab levels of about 10 U/L or less. Mean TSHR-Ab levels at baseline were 5.6 IU/L for responders, compared with 27.3 IU/L for nonresponders (P less than .001).
Most responders also had lower initial levels of antithyroid peroxidase IgG antibodies, compared with the nonresponder group.
Levels of chemokine (motif C-X-C) ligand 13 (CXCL13) fell throughout the study period. Higher CXCL13 levels are associated with lymphocytic infiltrates seen in autoimmune thyroiditis.
Occupancy of CD40 was initially measured at week 4 of the study and it remained high until week 16, when free CD40 receptors rose rapidly for several participants in both the responder and nonresponder groups. “The iscalimab intervention resulted in complete CD40 engagement for up to 20 weeks,” wrote Dr. Kahaly and colleagues in the abstract accompanying the presentation.
In assessing CD40 target engagement, the investigators found that total soluble CD40 levels climbed during the treatment period, reaching peaks as high as 400-500 ng/mL, and then plummeted back to zero by study’s end for all participants.
A pharmacokinetic analysis revealed expected peaks of serum iscalimab after treatments, with levels dropping sharply at the end of the study period and falling to levels approaching zero by week 24 for most participants.
In terms of safety, 12 patients experienced at least one adverse event, with 3 participants reporting an episode of cystitis during the study. Fatigue, headache, insomnia, nausea, and viral upper respiratory infection were each reported by 2 patients. No injection site reactions were seen. All adverse events were mild or moderate, did not result in study withdrawal, and resolved by the end of the study period, Dr. Kahaly noted.
“These encouraging results suggest that iscalimab should be tested further to understand better its potential therapeutic benefit,” the investigators wrote.
The study was funded by Novartis, which is developing iscalimab for Graves disease, other autoimmune disorders, and as an antirejection drug for patients with kidney transplants.
REPORTING FROM ATA 2019
Hydroxychloroquine prevents congenital heart block recurrence in anti-Ro pregnancies
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
REPORTING FROM ACR 2019
Quick Byte: Act locally
To solve our most pressing national health issues, we must start locally, according to a Health Affairs blog post.
“For example, in [Mecklenburg County] North Carolina, African Americans face rates of cardiovascular disease 22% higher than their white counterparts do. To fight this, an organization called Village HeartBEAT joined forces with more than 60 faith-based groups to reach more than 20,000 people – connecting them with health resources to reduce their cardiovascular risk. As a direct result, rates of smoking decreased from 17.4% to 13.9%, and obesity rates fell from 70% to 64.7%.”
Mecklenburg County is a winner of the Healthiest Cities & Counties Challenge, a collaboration between the Aetna Foundation, the American Public Health Association, and the National Association of Counties, which has awarded more than $1.5 million in grants and prizes over the last 2 years.
Reference
1. Graham G, Benjamin G. “Winning Local Solutions to Our Most Pressing Public Health Needs.” Health Affairs. https://www.healthaffairs.org/do/10.1377/hblog20190423.202497/full/. Published April 25, 2019.
To solve our most pressing national health issues, we must start locally, according to a Health Affairs blog post.
“For example, in [Mecklenburg County] North Carolina, African Americans face rates of cardiovascular disease 22% higher than their white counterparts do. To fight this, an organization called Village HeartBEAT joined forces with more than 60 faith-based groups to reach more than 20,000 people – connecting them with health resources to reduce their cardiovascular risk. As a direct result, rates of smoking decreased from 17.4% to 13.9%, and obesity rates fell from 70% to 64.7%.”
Mecklenburg County is a winner of the Healthiest Cities & Counties Challenge, a collaboration between the Aetna Foundation, the American Public Health Association, and the National Association of Counties, which has awarded more than $1.5 million in grants and prizes over the last 2 years.
Reference
1. Graham G, Benjamin G. “Winning Local Solutions to Our Most Pressing Public Health Needs.” Health Affairs. https://www.healthaffairs.org/do/10.1377/hblog20190423.202497/full/. Published April 25, 2019.
To solve our most pressing national health issues, we must start locally, according to a Health Affairs blog post.
“For example, in [Mecklenburg County] North Carolina, African Americans face rates of cardiovascular disease 22% higher than their white counterparts do. To fight this, an organization called Village HeartBEAT joined forces with more than 60 faith-based groups to reach more than 20,000 people – connecting them with health resources to reduce their cardiovascular risk. As a direct result, rates of smoking decreased from 17.4% to 13.9%, and obesity rates fell from 70% to 64.7%.”
Mecklenburg County is a winner of the Healthiest Cities & Counties Challenge, a collaboration between the Aetna Foundation, the American Public Health Association, and the National Association of Counties, which has awarded more than $1.5 million in grants and prizes over the last 2 years.
Reference
1. Graham G, Benjamin G. “Winning Local Solutions to Our Most Pressing Public Health Needs.” Health Affairs. https://www.healthaffairs.org/do/10.1377/hblog20190423.202497/full/. Published April 25, 2019.
ACR and EULAR release first classification criteria for IgG4-related disease
The American College of Rheumatology and the European League Against Rheumatism have released the first classification criteria for IgG4-related disease.
Although it was first recognized as a distinct disease in 2003, investigators have since learned that IgG4-related disease (IgG4-RD) is not particularly rare. Specialists across many different fields of medicine now treat IgG4-RD, which affects multiple organ systems, and the pancreas, kidneys, and orbits are most commonly affected by severe disease.
“IgG4-RD has proven to be a remarkable window into human immunology, and the insights investigators have made from studying this disease have already led to important discoveries in other rheumatic diseases, such as scleroderma,” John H. Stone, MD, professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital, both in Boston, said in an interview.
To develop the classification criteria, 86 experts from five continents across various subspecialties including rheumatology, internal medicine, ophthalmology, pathology, gastroenterology, allergology, pulmonology, radiology, neurology, nephrology, and others met as a working group in 2016, achieving consensus on 79 criteria. They then narrowed down the number of items to 8 domains and 29 items within a set of inclusion and exclusion criteria for the draft classification criteria. For the final classification criteria, the working group applied weighting to each inclusion criteria item within a domain on a Likert scale (–5 to 5 range), removing items that were not significantly attributable to IgG4-RD classification (those with –2 to 2 scores).
The final IgG4-RD criteria are divided into three classification steps: entry criteria, exclusion criteria, and inclusion criteria. Patients who meet the entry criteria should have clinical or radiologic involvement of one or more organs consistent with IgG4-RD, such as the pancreas, salivary glands, bile ducts, orbits, kidney, lung, aorta, retroperitoneum, pachymeninges, or thyroid gland. Patients could alternatively meet the entry criteria by having “pathologic evidence of an inflammatory process accompanied by a lymphoplasmacytic infiltrate of uncertain etiology in one of these same organs,” the authors wrote.
If a patient meets the entry criteria, their case is examined against 32 clinical, serologic, radiologic, and pathologic items and specific disease inclusions. Any exclusion criteria present in a case means the patient does not meet the criteria for IgG4-RD classification.
The third step is to evaluate whether a patient meets inclusion criteria consisting of clinical findings, serologic results, radiology assessments, and pathology interpretations across eight domains: immunostaining, head and neck gland involvement, chest, pancreas and biliary tree, kidney, and the retroperitoneum. Each criterion has a weight, and if a patient has a score of 20 or higher, they meet the classification criteria for IgG4-RD.
“The final criteria set is easy to use and lends itself well to adaptation in an electronic format, which we have already instituted at my hospital,” said Dr. Stone, who is also director of the international panel of experts who created the criteria.
Two cohorts were used to validate the IgG4-RD classification criteria. In the first cohort, investigators used 771 patients (85% of the total cohort) in whom they were “confident” or “very confident” of a diagnosis of IgG4-RD or a mimicker to assess the test performance with a classification threshold of 20 points. The researchers found the criteria had a specificity of 99.2% (95% confidence interval, 97.2%-99.8%) and a sensitivity of 85.5% (95% CI, 81.9%-88.5%). The experts used a second validation cohort of 402 additional patients (83% of the total cohort) with suspected IgG4-RD or a mimicker using the same confident and very confident metric. The panel assembled this cohort because of minor definition changes in inclusion and exclusion criteria that had been made after the derivation set of patients had been collected, but the definitions of inclusion and exclusion criteria used in the two validation cohorts were exactly the same. Overall, the specificity of the criteria was 97.8% (95% CI, 93.7%-99.2%) and the sensitivity was 82.0% (95% CI, 77.0%-86.1%) for IgG4-RD classification in this second group.
Dr. Stone said that more investigations, including multicenter clinical trials, are being organized for patients with IgG4-RD, and these classification criteria will help to identify which patients to include in these studies.
“These rigorous ACR/EULAR classification criteria will help guide us through some of the most important challenges of studying this disease well,” Dr. Stone said. “I’m anticipating major advances in this field in the years to come, triggered in part by the strength of having sound classification criteria.”
The authors reported no relevant conflicts of interest.
SOURCE: Wallace ZS et al. Arthritis Rheumatol. 2019 Dec 2. doi: 10.1002/art.41120.
The American College of Rheumatology and the European League Against Rheumatism have released the first classification criteria for IgG4-related disease.
Although it was first recognized as a distinct disease in 2003, investigators have since learned that IgG4-related disease (IgG4-RD) is not particularly rare. Specialists across many different fields of medicine now treat IgG4-RD, which affects multiple organ systems, and the pancreas, kidneys, and orbits are most commonly affected by severe disease.
“IgG4-RD has proven to be a remarkable window into human immunology, and the insights investigators have made from studying this disease have already led to important discoveries in other rheumatic diseases, such as scleroderma,” John H. Stone, MD, professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital, both in Boston, said in an interview.
To develop the classification criteria, 86 experts from five continents across various subspecialties including rheumatology, internal medicine, ophthalmology, pathology, gastroenterology, allergology, pulmonology, radiology, neurology, nephrology, and others met as a working group in 2016, achieving consensus on 79 criteria. They then narrowed down the number of items to 8 domains and 29 items within a set of inclusion and exclusion criteria for the draft classification criteria. For the final classification criteria, the working group applied weighting to each inclusion criteria item within a domain on a Likert scale (–5 to 5 range), removing items that were not significantly attributable to IgG4-RD classification (those with –2 to 2 scores).
The final IgG4-RD criteria are divided into three classification steps: entry criteria, exclusion criteria, and inclusion criteria. Patients who meet the entry criteria should have clinical or radiologic involvement of one or more organs consistent with IgG4-RD, such as the pancreas, salivary glands, bile ducts, orbits, kidney, lung, aorta, retroperitoneum, pachymeninges, or thyroid gland. Patients could alternatively meet the entry criteria by having “pathologic evidence of an inflammatory process accompanied by a lymphoplasmacytic infiltrate of uncertain etiology in one of these same organs,” the authors wrote.
If a patient meets the entry criteria, their case is examined against 32 clinical, serologic, radiologic, and pathologic items and specific disease inclusions. Any exclusion criteria present in a case means the patient does not meet the criteria for IgG4-RD classification.
The third step is to evaluate whether a patient meets inclusion criteria consisting of clinical findings, serologic results, radiology assessments, and pathology interpretations across eight domains: immunostaining, head and neck gland involvement, chest, pancreas and biliary tree, kidney, and the retroperitoneum. Each criterion has a weight, and if a patient has a score of 20 or higher, they meet the classification criteria for IgG4-RD.
“The final criteria set is easy to use and lends itself well to adaptation in an electronic format, which we have already instituted at my hospital,” said Dr. Stone, who is also director of the international panel of experts who created the criteria.
Two cohorts were used to validate the IgG4-RD classification criteria. In the first cohort, investigators used 771 patients (85% of the total cohort) in whom they were “confident” or “very confident” of a diagnosis of IgG4-RD or a mimicker to assess the test performance with a classification threshold of 20 points. The researchers found the criteria had a specificity of 99.2% (95% confidence interval, 97.2%-99.8%) and a sensitivity of 85.5% (95% CI, 81.9%-88.5%). The experts used a second validation cohort of 402 additional patients (83% of the total cohort) with suspected IgG4-RD or a mimicker using the same confident and very confident metric. The panel assembled this cohort because of minor definition changes in inclusion and exclusion criteria that had been made after the derivation set of patients had been collected, but the definitions of inclusion and exclusion criteria used in the two validation cohorts were exactly the same. Overall, the specificity of the criteria was 97.8% (95% CI, 93.7%-99.2%) and the sensitivity was 82.0% (95% CI, 77.0%-86.1%) for IgG4-RD classification in this second group.
Dr. Stone said that more investigations, including multicenter clinical trials, are being organized for patients with IgG4-RD, and these classification criteria will help to identify which patients to include in these studies.
“These rigorous ACR/EULAR classification criteria will help guide us through some of the most important challenges of studying this disease well,” Dr. Stone said. “I’m anticipating major advances in this field in the years to come, triggered in part by the strength of having sound classification criteria.”
The authors reported no relevant conflicts of interest.
SOURCE: Wallace ZS et al. Arthritis Rheumatol. 2019 Dec 2. doi: 10.1002/art.41120.
The American College of Rheumatology and the European League Against Rheumatism have released the first classification criteria for IgG4-related disease.
Although it was first recognized as a distinct disease in 2003, investigators have since learned that IgG4-related disease (IgG4-RD) is not particularly rare. Specialists across many different fields of medicine now treat IgG4-RD, which affects multiple organ systems, and the pancreas, kidneys, and orbits are most commonly affected by severe disease.
“IgG4-RD has proven to be a remarkable window into human immunology, and the insights investigators have made from studying this disease have already led to important discoveries in other rheumatic diseases, such as scleroderma,” John H. Stone, MD, professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital, both in Boston, said in an interview.
To develop the classification criteria, 86 experts from five continents across various subspecialties including rheumatology, internal medicine, ophthalmology, pathology, gastroenterology, allergology, pulmonology, radiology, neurology, nephrology, and others met as a working group in 2016, achieving consensus on 79 criteria. They then narrowed down the number of items to 8 domains and 29 items within a set of inclusion and exclusion criteria for the draft classification criteria. For the final classification criteria, the working group applied weighting to each inclusion criteria item within a domain on a Likert scale (–5 to 5 range), removing items that were not significantly attributable to IgG4-RD classification (those with –2 to 2 scores).
The final IgG4-RD criteria are divided into three classification steps: entry criteria, exclusion criteria, and inclusion criteria. Patients who meet the entry criteria should have clinical or radiologic involvement of one or more organs consistent with IgG4-RD, such as the pancreas, salivary glands, bile ducts, orbits, kidney, lung, aorta, retroperitoneum, pachymeninges, or thyroid gland. Patients could alternatively meet the entry criteria by having “pathologic evidence of an inflammatory process accompanied by a lymphoplasmacytic infiltrate of uncertain etiology in one of these same organs,” the authors wrote.
If a patient meets the entry criteria, their case is examined against 32 clinical, serologic, radiologic, and pathologic items and specific disease inclusions. Any exclusion criteria present in a case means the patient does not meet the criteria for IgG4-RD classification.
The third step is to evaluate whether a patient meets inclusion criteria consisting of clinical findings, serologic results, radiology assessments, and pathology interpretations across eight domains: immunostaining, head and neck gland involvement, chest, pancreas and biliary tree, kidney, and the retroperitoneum. Each criterion has a weight, and if a patient has a score of 20 or higher, they meet the classification criteria for IgG4-RD.
“The final criteria set is easy to use and lends itself well to adaptation in an electronic format, which we have already instituted at my hospital,” said Dr. Stone, who is also director of the international panel of experts who created the criteria.
Two cohorts were used to validate the IgG4-RD classification criteria. In the first cohort, investigators used 771 patients (85% of the total cohort) in whom they were “confident” or “very confident” of a diagnosis of IgG4-RD or a mimicker to assess the test performance with a classification threshold of 20 points. The researchers found the criteria had a specificity of 99.2% (95% confidence interval, 97.2%-99.8%) and a sensitivity of 85.5% (95% CI, 81.9%-88.5%). The experts used a second validation cohort of 402 additional patients (83% of the total cohort) with suspected IgG4-RD or a mimicker using the same confident and very confident metric. The panel assembled this cohort because of minor definition changes in inclusion and exclusion criteria that had been made after the derivation set of patients had been collected, but the definitions of inclusion and exclusion criteria used in the two validation cohorts were exactly the same. Overall, the specificity of the criteria was 97.8% (95% CI, 93.7%-99.2%) and the sensitivity was 82.0% (95% CI, 77.0%-86.1%) for IgG4-RD classification in this second group.
Dr. Stone said that more investigations, including multicenter clinical trials, are being organized for patients with IgG4-RD, and these classification criteria will help to identify which patients to include in these studies.
“These rigorous ACR/EULAR classification criteria will help guide us through some of the most important challenges of studying this disease well,” Dr. Stone said. “I’m anticipating major advances in this field in the years to come, triggered in part by the strength of having sound classification criteria.”
The authors reported no relevant conflicts of interest.
SOURCE: Wallace ZS et al. Arthritis Rheumatol. 2019 Dec 2. doi: 10.1002/art.41120.
FROM ARTHRITIS & RHEUMATOLOGY