User login
Cancer deaths cost over $94 billion in lost earnings in 2015
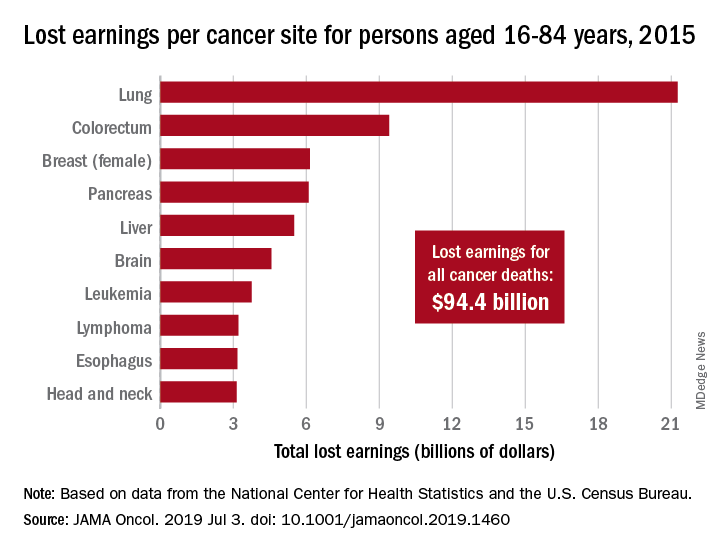
Cancer led to 492,000 deaths for Americans aged 16-84 years in 2015, and those deaths cost $94.4 billion in lost earnings that year, according to a study published in JAMA Oncology.
Cancer also took more than 8.7 million years of life from those individuals, with lung cancer being the most costly in terms of both lost earnings and years of life lost, said Farhad Islami, MD, PhD, and associates at the American Cancer Society, Atlanta.
“Person-years of life lost and lost earnings were high for many cancers associated with modifiable risk factors and effective screening and treatment, suggesting that a substantial proportion of the mortality burden is potentially avoidable,” they wrote, adding that “implementation of comprehensive cancer prevention interventions and equitable access to high-quality care across all states could reduce the burden of cancer and associated geographic and other differences in the country.”
In 2015, lung cancer took more than 2.2 million years of life and $21.3 billion in earnings from Americans aged 16-84 years. Colorectal cancer was next with 766,000 years of life lost and $9.4 billion in lost earnings, followed by female breast cancer with losses of 746,000 years of life and 6.2 billion in earnings, Dr. Islami and associated reported.
For all cancers, the cost in lost earnings per death was almost $192,000, with the highest costs coming from cancers of the brain and nervous system ($315,000) and cervix ($311,000). On that basis, lung cancer cost was lower than average at $159,000 per death, they noted.
At the state level, lost-earnings rates were lowest in Utah ($19.6 million per 100,000 persons) and highest in Kentucky ($35.3 million per 100,000). “If all states had Utah’s lost earnings rate in 2015, lost earnings in the U.S. would have been reduced by 29.3%, or $27.7 billion, and life years lost nationwide in 2015 would be reduced by 2.4 million,” Dr. Islami and his associates said in a written statement.
Data for the study were obtained from the National Center for Health Statistics (mortality) and the U.S. Census Bureau (earnings). The study was supported by the Intramural Research Department of the American Cancer Society, and all of the investigators are employees of the society.
SOURCE: Islami F et al. JAMA Oncol. 2019 Jul 3. doi: 10.1001/jamaoncol.2019.1460.
Cancer led to 492,000 deaths for Americans aged 16-84 years in 2015, and those deaths cost $94.4 billion in lost earnings that year, according to a study published in JAMA Oncology.

Cancer also took more than 8.7 million years of life from those individuals, with lung cancer being the most costly in terms of both lost earnings and years of life lost, said Farhad Islami, MD, PhD, and associates at the American Cancer Society, Atlanta.
“Person-years of life lost and lost earnings were high for many cancers associated with modifiable risk factors and effective screening and treatment, suggesting that a substantial proportion of the mortality burden is potentially avoidable,” they wrote, adding that “implementation of comprehensive cancer prevention interventions and equitable access to high-quality care across all states could reduce the burden of cancer and associated geographic and other differences in the country.”
In 2015, lung cancer took more than 2.2 million years of life and $21.3 billion in earnings from Americans aged 16-84 years. Colorectal cancer was next with 766,000 years of life lost and $9.4 billion in lost earnings, followed by female breast cancer with losses of 746,000 years of life and 6.2 billion in earnings, Dr. Islami and associated reported.
For all cancers, the cost in lost earnings per death was almost $192,000, with the highest costs coming from cancers of the brain and nervous system ($315,000) and cervix ($311,000). On that basis, lung cancer cost was lower than average at $159,000 per death, they noted.
At the state level, lost-earnings rates were lowest in Utah ($19.6 million per 100,000 persons) and highest in Kentucky ($35.3 million per 100,000). “If all states had Utah’s lost earnings rate in 2015, lost earnings in the U.S. would have been reduced by 29.3%, or $27.7 billion, and life years lost nationwide in 2015 would be reduced by 2.4 million,” Dr. Islami and his associates said in a written statement.
Data for the study were obtained from the National Center for Health Statistics (mortality) and the U.S. Census Bureau (earnings). The study was supported by the Intramural Research Department of the American Cancer Society, and all of the investigators are employees of the society.
SOURCE: Islami F et al. JAMA Oncol. 2019 Jul 3. doi: 10.1001/jamaoncol.2019.1460.
Cancer led to 492,000 deaths for Americans aged 16-84 years in 2015, and those deaths cost $94.4 billion in lost earnings that year, according to a study published in JAMA Oncology.

Cancer also took more than 8.7 million years of life from those individuals, with lung cancer being the most costly in terms of both lost earnings and years of life lost, said Farhad Islami, MD, PhD, and associates at the American Cancer Society, Atlanta.
“Person-years of life lost and lost earnings were high for many cancers associated with modifiable risk factors and effective screening and treatment, suggesting that a substantial proportion of the mortality burden is potentially avoidable,” they wrote, adding that “implementation of comprehensive cancer prevention interventions and equitable access to high-quality care across all states could reduce the burden of cancer and associated geographic and other differences in the country.”
In 2015, lung cancer took more than 2.2 million years of life and $21.3 billion in earnings from Americans aged 16-84 years. Colorectal cancer was next with 766,000 years of life lost and $9.4 billion in lost earnings, followed by female breast cancer with losses of 746,000 years of life and 6.2 billion in earnings, Dr. Islami and associated reported.
For all cancers, the cost in lost earnings per death was almost $192,000, with the highest costs coming from cancers of the brain and nervous system ($315,000) and cervix ($311,000). On that basis, lung cancer cost was lower than average at $159,000 per death, they noted.
At the state level, lost-earnings rates were lowest in Utah ($19.6 million per 100,000 persons) and highest in Kentucky ($35.3 million per 100,000). “If all states had Utah’s lost earnings rate in 2015, lost earnings in the U.S. would have been reduced by 29.3%, or $27.7 billion, and life years lost nationwide in 2015 would be reduced by 2.4 million,” Dr. Islami and his associates said in a written statement.
Data for the study were obtained from the National Center for Health Statistics (mortality) and the U.S. Census Bureau (earnings). The study was supported by the Intramural Research Department of the American Cancer Society, and all of the investigators are employees of the society.
SOURCE: Islami F et al. JAMA Oncol. 2019 Jul 3. doi: 10.1001/jamaoncol.2019.1460.
FROM JAMA ONCOLOGY
Is the vaginal or buccal route more effective when administering prostaglandins for cervical ripening at term?
Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
EXPERT COMMENTARY
Cervical ripening is routine practice in women undergoing induction of labor who have an unfavorable cervical examination.1 This is because generating contractions against a long thick cervix is more likely to lead to failed induction and cesarean delivery. Cervical ripening can be achieved using mechanical or pharmacologic methods.
Misoprostol (a prostaglandin E1 [PGE1] analog) is approved by the US Food and Drug Administration for the treatment of peptic ulcer disease, but it also is widely used off-label for cervical ripening, partly due to its low cost. Misoprostol’s optimal dosing regimen and route of administration are not known. The IMPROVE trial was designed to address this knowledge gap, specifically to compare the efficacy and safety of VM versus BM in women undergoing labor induction at term.
Details of the study
The IMPROVE trial was a prospective, randomized, noninferiority, triple-masked, placebo-controlled trial of 300 women with a singleton vertex fetus requiring cervical ripening for induction of labor at term.2 Enrolled women were randomly assigned to VM or BM (same dosing regimen) and to a matching placebo administered via the opposite route.
Primary outcomes included time-to-vaginal-delivery from first dose, which was reduced in VM vs BM (20.1 vs 28.1 hours; P = .006), and urgent cesarean delivery for nonreassuring fetal testing, which was similarly reduced in VM (3.3% vs 9.5%; P = .33). These differences persisted after controlling for covariates. There was also a greater difference seen in multiparous versus nulliparous women.
Secondary outcomes also favored VM over BM, including more vaginal deliverieswithin 24 hours, fewer doses to achieve active labor, and a lower maximum dose of oxytocin.
Overall cesarean delivery rates were similar in the 2 groups (VM, 15.8%; BM, 22.3%; P = .15). There were no significant differences in other delivery characteristics or in maternal or fetal adverse events.
While a number of studies have evaluated the risk of cesarean delivery (CD) with the use of cervical ripening agents by different routes of administration, Handal-Orefice and colleagues studied this outcome specifically in a predominantly overweight population at a tertiary care center.1
The retrospective study included 276 women, of whom 91% had a body mass index (BMI) of 25 kg/m2 or more and 61% had a BMI of 30 kg/m2 or more at the time of delivery.
For cervical ripening, 138 women received vaginal misoprostol (25 µg) and 138 received oral misoprostol (50 µg). The frequency of CD (the primary study outcome) was significantly higher with oral compared with vaginal misoprostol use (32% vs 21%; P = .04). When the analysis was adjusted for age, BMI, parity, indication for induction, and Foley catheter use, the risk of CD remained significantly higher for the oral misoprostol group (adjusted odds ratio [aOR], 2.01; 95% confidence interval [CI], 1.07-3.76).
Other key findings:
- frequency of CD among nulliparous women: 41% in the oral misoprostol group, 28% in the vaginal misoprostol group (aOR, 2.79; 95% CI, 1.26-6.19)
- time to vaginal delivery: 41 hours for the oral misoprostol group, 31 hours for the vaginal misoprostol group (P = .01)
- uterine tachysystole: 11% in the oral misoprostol group, 20% in the vaginal misoprostol group (P = .04).
The authors noted that the strengths of the study, including the racial and ethnic diversity of the population (72% of women were of either black or Hispanic race or ethnicity), the commonly used doses of misoprostol, and the performance of inductions outside a research protocol, add to the generalizability of the results.
Reference
1. Handal-Orefice R, Friedman AM, Chouinard SM, et al. Oral or vaginal misoprostol for labor induction and cesarean delivery risk. Obstet Gynecol. 2019. doi:10.1097/AOG.0000000000003274.
Continue to: Study strengths and limitations...
Study strengths and limitations
The IMPROVE trial had a triple-blinded study design with an intention-to-treat paradigm and good follow-up. There was also standardization of PGE1 administration criteria, which was consistent with the American College of Obstetricians and Gynecologists standards of care. Results were similar to those of prior studies regarding rates of tachysystole, urgent cesarean delivery, and vaginal delivery.
The study has good generalizability as it included both elective and medically indicated inductions; however, patients with ruptured membranes were excluded. Although there was no difference in the overall cesarean delivery rates, the study was underpowered to look at this outcome. The authors included a patient satisfaction survey, but this is hard to interpret since study participants all received tablets orally and vaginally. The study did not address efficacy of VM versus BM administration at different doses or time intervals.
Labor induction has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.3 This number is likely to increase given recent data suggesting that routine induction at 39 weeks may significantly decrease cesarean delivery rates.4 It is critical, therefore, that we identify the optimal technique for cervical ripening, including the ideal dosing regimen and route of administration. Results of the IMPROVE trial suggest that vaginal administration of misoprostol (25 μg initial dose, 50 μg subsequent doses) may be superior to the buccal route, with more rapid vaginal delivery, more vaginal deliveries within 24 hours, and fewer urgent cesareans for nonreassuring fetal testing (although the overall cesarean delivery rate was not significantly different).
ERROL R. NORWITZ, MD, PHD, MBA; JULIE M. STONE, MD
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 107. Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
- Martin JA, Hamilton BE, Osterman M, et al. Births: final data for 2016. Nat Vital Stat Rep. 2018;67:1-55.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
EXPERT COMMENTARY
Cervical ripening is routine practice in women undergoing induction of labor who have an unfavorable cervical examination.1 This is because generating contractions against a long thick cervix is more likely to lead to failed induction and cesarean delivery. Cervical ripening can be achieved using mechanical or pharmacologic methods.
Misoprostol (a prostaglandin E1 [PGE1] analog) is approved by the US Food and Drug Administration for the treatment of peptic ulcer disease, but it also is widely used off-label for cervical ripening, partly due to its low cost. Misoprostol’s optimal dosing regimen and route of administration are not known. The IMPROVE trial was designed to address this knowledge gap, specifically to compare the efficacy and safety of VM versus BM in women undergoing labor induction at term.
Details of the study
The IMPROVE trial was a prospective, randomized, noninferiority, triple-masked, placebo-controlled trial of 300 women with a singleton vertex fetus requiring cervical ripening for induction of labor at term.2 Enrolled women were randomly assigned to VM or BM (same dosing regimen) and to a matching placebo administered via the opposite route.
Primary outcomes included time-to-vaginal-delivery from first dose, which was reduced in VM vs BM (20.1 vs 28.1 hours; P = .006), and urgent cesarean delivery for nonreassuring fetal testing, which was similarly reduced in VM (3.3% vs 9.5%; P = .33). These differences persisted after controlling for covariates. There was also a greater difference seen in multiparous versus nulliparous women.
Secondary outcomes also favored VM over BM, including more vaginal deliverieswithin 24 hours, fewer doses to achieve active labor, and a lower maximum dose of oxytocin.
Overall cesarean delivery rates were similar in the 2 groups (VM, 15.8%; BM, 22.3%; P = .15). There were no significant differences in other delivery characteristics or in maternal or fetal adverse events.
While a number of studies have evaluated the risk of cesarean delivery (CD) with the use of cervical ripening agents by different routes of administration, Handal-Orefice and colleagues studied this outcome specifically in a predominantly overweight population at a tertiary care center.1
The retrospective study included 276 women, of whom 91% had a body mass index (BMI) of 25 kg/m2 or more and 61% had a BMI of 30 kg/m2 or more at the time of delivery.
For cervical ripening, 138 women received vaginal misoprostol (25 µg) and 138 received oral misoprostol (50 µg). The frequency of CD (the primary study outcome) was significantly higher with oral compared with vaginal misoprostol use (32% vs 21%; P = .04). When the analysis was adjusted for age, BMI, parity, indication for induction, and Foley catheter use, the risk of CD remained significantly higher for the oral misoprostol group (adjusted odds ratio [aOR], 2.01; 95% confidence interval [CI], 1.07-3.76).
Other key findings:
- frequency of CD among nulliparous women: 41% in the oral misoprostol group, 28% in the vaginal misoprostol group (aOR, 2.79; 95% CI, 1.26-6.19)
- time to vaginal delivery: 41 hours for the oral misoprostol group, 31 hours for the vaginal misoprostol group (P = .01)
- uterine tachysystole: 11% in the oral misoprostol group, 20% in the vaginal misoprostol group (P = .04).
The authors noted that the strengths of the study, including the racial and ethnic diversity of the population (72% of women were of either black or Hispanic race or ethnicity), the commonly used doses of misoprostol, and the performance of inductions outside a research protocol, add to the generalizability of the results.
Reference
1. Handal-Orefice R, Friedman AM, Chouinard SM, et al. Oral or vaginal misoprostol for labor induction and cesarean delivery risk. Obstet Gynecol. 2019. doi:10.1097/AOG.0000000000003274.
Continue to: Study strengths and limitations...
Study strengths and limitations
The IMPROVE trial had a triple-blinded study design with an intention-to-treat paradigm and good follow-up. There was also standardization of PGE1 administration criteria, which was consistent with the American College of Obstetricians and Gynecologists standards of care. Results were similar to those of prior studies regarding rates of tachysystole, urgent cesarean delivery, and vaginal delivery.
The study has good generalizability as it included both elective and medically indicated inductions; however, patients with ruptured membranes were excluded. Although there was no difference in the overall cesarean delivery rates, the study was underpowered to look at this outcome. The authors included a patient satisfaction survey, but this is hard to interpret since study participants all received tablets orally and vaginally. The study did not address efficacy of VM versus BM administration at different doses or time intervals.
Labor induction has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.3 This number is likely to increase given recent data suggesting that routine induction at 39 weeks may significantly decrease cesarean delivery rates.4 It is critical, therefore, that we identify the optimal technique for cervical ripening, including the ideal dosing regimen and route of administration. Results of the IMPROVE trial suggest that vaginal administration of misoprostol (25 μg initial dose, 50 μg subsequent doses) may be superior to the buccal route, with more rapid vaginal delivery, more vaginal deliveries within 24 hours, and fewer urgent cesareans for nonreassuring fetal testing (although the overall cesarean delivery rate was not significantly different).
ERROL R. NORWITZ, MD, PHD, MBA; JULIE M. STONE, MD
Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
EXPERT COMMENTARY
Cervical ripening is routine practice in women undergoing induction of labor who have an unfavorable cervical examination.1 This is because generating contractions against a long thick cervix is more likely to lead to failed induction and cesarean delivery. Cervical ripening can be achieved using mechanical or pharmacologic methods.
Misoprostol (a prostaglandin E1 [PGE1] analog) is approved by the US Food and Drug Administration for the treatment of peptic ulcer disease, but it also is widely used off-label for cervical ripening, partly due to its low cost. Misoprostol’s optimal dosing regimen and route of administration are not known. The IMPROVE trial was designed to address this knowledge gap, specifically to compare the efficacy and safety of VM versus BM in women undergoing labor induction at term.
Details of the study
The IMPROVE trial was a prospective, randomized, noninferiority, triple-masked, placebo-controlled trial of 300 women with a singleton vertex fetus requiring cervical ripening for induction of labor at term.2 Enrolled women were randomly assigned to VM or BM (same dosing regimen) and to a matching placebo administered via the opposite route.
Primary outcomes included time-to-vaginal-delivery from first dose, which was reduced in VM vs BM (20.1 vs 28.1 hours; P = .006), and urgent cesarean delivery for nonreassuring fetal testing, which was similarly reduced in VM (3.3% vs 9.5%; P = .33). These differences persisted after controlling for covariates. There was also a greater difference seen in multiparous versus nulliparous women.
Secondary outcomes also favored VM over BM, including more vaginal deliverieswithin 24 hours, fewer doses to achieve active labor, and a lower maximum dose of oxytocin.
Overall cesarean delivery rates were similar in the 2 groups (VM, 15.8%; BM, 22.3%; P = .15). There were no significant differences in other delivery characteristics or in maternal or fetal adverse events.
While a number of studies have evaluated the risk of cesarean delivery (CD) with the use of cervical ripening agents by different routes of administration, Handal-Orefice and colleagues studied this outcome specifically in a predominantly overweight population at a tertiary care center.1
The retrospective study included 276 women, of whom 91% had a body mass index (BMI) of 25 kg/m2 or more and 61% had a BMI of 30 kg/m2 or more at the time of delivery.
For cervical ripening, 138 women received vaginal misoprostol (25 µg) and 138 received oral misoprostol (50 µg). The frequency of CD (the primary study outcome) was significantly higher with oral compared with vaginal misoprostol use (32% vs 21%; P = .04). When the analysis was adjusted for age, BMI, parity, indication for induction, and Foley catheter use, the risk of CD remained significantly higher for the oral misoprostol group (adjusted odds ratio [aOR], 2.01; 95% confidence interval [CI], 1.07-3.76).
Other key findings:
- frequency of CD among nulliparous women: 41% in the oral misoprostol group, 28% in the vaginal misoprostol group (aOR, 2.79; 95% CI, 1.26-6.19)
- time to vaginal delivery: 41 hours for the oral misoprostol group, 31 hours for the vaginal misoprostol group (P = .01)
- uterine tachysystole: 11% in the oral misoprostol group, 20% in the vaginal misoprostol group (P = .04).
The authors noted that the strengths of the study, including the racial and ethnic diversity of the population (72% of women were of either black or Hispanic race or ethnicity), the commonly used doses of misoprostol, and the performance of inductions outside a research protocol, add to the generalizability of the results.
Reference
1. Handal-Orefice R, Friedman AM, Chouinard SM, et al. Oral or vaginal misoprostol for labor induction and cesarean delivery risk. Obstet Gynecol. 2019. doi:10.1097/AOG.0000000000003274.
Continue to: Study strengths and limitations...
Study strengths and limitations
The IMPROVE trial had a triple-blinded study design with an intention-to-treat paradigm and good follow-up. There was also standardization of PGE1 administration criteria, which was consistent with the American College of Obstetricians and Gynecologists standards of care. Results were similar to those of prior studies regarding rates of tachysystole, urgent cesarean delivery, and vaginal delivery.
The study has good generalizability as it included both elective and medically indicated inductions; however, patients with ruptured membranes were excluded. Although there was no difference in the overall cesarean delivery rates, the study was underpowered to look at this outcome. The authors included a patient satisfaction survey, but this is hard to interpret since study participants all received tablets orally and vaginally. The study did not address efficacy of VM versus BM administration at different doses or time intervals.
Labor induction has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.3 This number is likely to increase given recent data suggesting that routine induction at 39 weeks may significantly decrease cesarean delivery rates.4 It is critical, therefore, that we identify the optimal technique for cervical ripening, including the ideal dosing regimen and route of administration. Results of the IMPROVE trial suggest that vaginal administration of misoprostol (25 μg initial dose, 50 μg subsequent doses) may be superior to the buccal route, with more rapid vaginal delivery, more vaginal deliveries within 24 hours, and fewer urgent cesareans for nonreassuring fetal testing (although the overall cesarean delivery rate was not significantly different).
ERROL R. NORWITZ, MD, PHD, MBA; JULIE M. STONE, MD
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 107. Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
- Martin JA, Hamilton BE, Osterman M, et al. Births: final data for 2016. Nat Vital Stat Rep. 2018;67:1-55.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 107. Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple masked randomized controlled trial. Am J Obstet Gynecol. 2019. doi:10.1016/j.ajog.2019.04.037.
- Martin JA, Hamilton BE, Osterman M, et al. Births: final data for 2016. Nat Vital Stat Rep. 2018;67:1-55.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
Adjustment for characteristics not used by Medicare reduces hospital variations in readmission rates
Clinical question: Can differences in hospital readmission rates be explained by patient characteristics not accounted for by Medicare?
Background: In its Pay for Performance program, Medicare ties payments to readmission rates but adjusts these rates only for limited patient characteristics. Hospitals serving higher-risk patients have received greater penalties. These programs may have the unintended consequence of penalizing hospitals that provide care to higher-risk patients.
Study design: Observational study.
Setting: Medicare admissions claims from 2013 through 2014 in 2,215 hospitals.
Synopsis: Using Medicare claims for admission and linked U.S. census data, the study assessed several clinical and social characteristics not currently used for risk adjustment. A sample of 1,169,014 index admissions among 1,003,664 unique beneficiaries was analyzed. The study compared rates with and without these additional adjustments.
Additional adjustments reduced overall variation in hospital readmission by 9.6%, changed rates upward or downward by 0.4%-0.7% for the 10% of hospitals most affected by the readjustments, and they would be expected to reduce penalties by 52%, 46%, and 41% for hospitals with the largest 1%, 5%, and 10% of penalty reductions, respectively.
Bottom line: Hospitals serving higher-risk patients may be penalized because of the patients they serve rather that the quality of care they provide.
Citation: Roberts ET et al. Assessment of the effect of adjustment for patient characteristics on hospital readmission rates: Implications for Pay for Performance. JAMA Intern Med. 2018;178(11)1498-1507.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Can differences in hospital readmission rates be explained by patient characteristics not accounted for by Medicare?
Background: In its Pay for Performance program, Medicare ties payments to readmission rates but adjusts these rates only for limited patient characteristics. Hospitals serving higher-risk patients have received greater penalties. These programs may have the unintended consequence of penalizing hospitals that provide care to higher-risk patients.
Study design: Observational study.
Setting: Medicare admissions claims from 2013 through 2014 in 2,215 hospitals.
Synopsis: Using Medicare claims for admission and linked U.S. census data, the study assessed several clinical and social characteristics not currently used for risk adjustment. A sample of 1,169,014 index admissions among 1,003,664 unique beneficiaries was analyzed. The study compared rates with and without these additional adjustments.
Additional adjustments reduced overall variation in hospital readmission by 9.6%, changed rates upward or downward by 0.4%-0.7% for the 10% of hospitals most affected by the readjustments, and they would be expected to reduce penalties by 52%, 46%, and 41% for hospitals with the largest 1%, 5%, and 10% of penalty reductions, respectively.
Bottom line: Hospitals serving higher-risk patients may be penalized because of the patients they serve rather that the quality of care they provide.
Citation: Roberts ET et al. Assessment of the effect of adjustment for patient characteristics on hospital readmission rates: Implications for Pay for Performance. JAMA Intern Med. 2018;178(11)1498-1507.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Can differences in hospital readmission rates be explained by patient characteristics not accounted for by Medicare?
Background: In its Pay for Performance program, Medicare ties payments to readmission rates but adjusts these rates only for limited patient characteristics. Hospitals serving higher-risk patients have received greater penalties. These programs may have the unintended consequence of penalizing hospitals that provide care to higher-risk patients.
Study design: Observational study.
Setting: Medicare admissions claims from 2013 through 2014 in 2,215 hospitals.
Synopsis: Using Medicare claims for admission and linked U.S. census data, the study assessed several clinical and social characteristics not currently used for risk adjustment. A sample of 1,169,014 index admissions among 1,003,664 unique beneficiaries was analyzed. The study compared rates with and without these additional adjustments.
Additional adjustments reduced overall variation in hospital readmission by 9.6%, changed rates upward or downward by 0.4%-0.7% for the 10% of hospitals most affected by the readjustments, and they would be expected to reduce penalties by 52%, 46%, and 41% for hospitals with the largest 1%, 5%, and 10% of penalty reductions, respectively.
Bottom line: Hospitals serving higher-risk patients may be penalized because of the patients they serve rather that the quality of care they provide.
Citation: Roberts ET et al. Assessment of the effect of adjustment for patient characteristics on hospital readmission rates: Implications for Pay for Performance. JAMA Intern Med. 2018;178(11)1498-1507.
Dr. Asuen is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Uterus-sparing interventions to treat postpartum hemorrhage during cesarean delivery surgery
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Genomic study reveals five subtypes of colorectal cancer
Colorectal cancer can be divided into five DNA methylation subtypes that predict molecular and clinical behavior and may offer future therapeutic targets, according to investigators.
In 216 unselected colorectal cancers, five subtypes of the CpG island methylator phenotype (CIMP) showed “striking” associations with sex, age, and tumor location, reported lead author Lochlan Fennell, MD, of the QIMR Berghofer Medical Research Institute in Queensland, Australia, and colleagues. CIMP level increased with age in a stepwise fashion, they noted.
Further associations with CIMP subtype and BRAF mutation status support the investigators’ recent report that sessile serrated adenomas are rare in young patients and pose little risk of malignancy. With additional research, these findings could “inform the development of patient-centric surveillance for young and older patients who present with sessile serrated adenomas,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology.
“CIMP can be detected using a standardized marker panel to stratify tumors as CIMP-high, CIMP-low, or CIMP-negative.” In the present study, the investigators expanded these three existing subtypes into five subtypes, allowing for better prediction of clinical and molecular characteristics associated with disease progression.
Initial genomic testing showed that 13.4% of cases carried a BRAF V600E mutation, 34.7% were mutated at KRAS codon 12 or 13, and almost half of the patients (42.2%) had a TP53 mutation. Sorted into the three previously described subtypes, CIMP negative was most common (68.5%), followed by CIMP low (20.4%), and CIMP high (11.1%). About two-thirds (66%) of BRAF mutant cancers were CIMP high, compared with just 3% of BRAF wild-type cases (P less than .0001). KRAS mutated cases were more often CIMP-low than KRAS wild-type cancers (34.6% vs. 12.8%; P less than .001).
With use of Illumina HumanMethylation450 Bead Chip arrays and recursively partitioned mixed model clustering, five methylation clusters were identified; specifically, these were CIMP-H1 and CIMP-H2 (high methylation levels), CIMP-L1 and CIMP-L2 (intermediate methylation levels), and CIMP-negative (low methylation level). As described above, methylation level demonstrated a direct relationship with age, ranging from CIMP-negative (61.9 years) to CIMP-H1 (75.2 years). The investigators also reported unique characteristics of each new subtype. For instance, the CIMP-H1 cluster had many features in common with cases of serrated neoplasia, such as BRAF mutation positivity (73.9%; P less than .0001).
“BRAF mutations are a hallmark of the serrated neoplasia pathway, and indicate that these cancers probably arose in serrated precursor lesions,” the investigators wrote. “We previously showed that the colonoscopic incidence of sessile serrated adenomas does not differ between patients aged in their 30s and patients who are much older, whereas BRAF mutant cancers were restricted to older individuals, suggesting these BRAF mutant polyps may have limited malignant potential in young patients.”
In contrast with the CIMP-H1 cases, CIMP-H2 cancers were more often KRAS mutant (54.5% vs. 17.4%). Other findings revealed associations with subtype and location; for example, CIMP-L1 cases were located equally in the distal and proximal colon, whereas CIMP-L2 cases more often localized to the distal colon and rectum. Of note for CIMP-negative cancers, most (62.3%) occurred in the distal colon, and none had a BRAF mutation.
The five methylation subtypes also showed associations with consensus molecular subtypes (CMS) to varying degrees. The two strongest correlations were found in CIMP-H1 cancers and CIMP-H2 cancers, which were most frequently classified as CMS1 (69.6%) and CMS3 (54.5%), respectively.
Using CIBERSORT, the investigators detected a variety of associations between the five subtypes and stromal immune cell composition. For example, CIMP-H1 cases were enriched for macrophages, compared with the other subtypes, except CIMP-L2. Mast cells showed a stepwise relationship with subtype; they contributed the most to the immune microenvironment of CIMP-negative cancers and the least to cases classified as CIMP-H1. A converse trend was found with natural killer cells.
Of note, in CIMP-H1 and CIMP-H2 cancers, oncogenes were significantly more likely than tumor-suppressor genes to undergo gene body methylation, which is positively correlated with gene expression, and oncogenes in these subtypes had significantly greater gene body methylation than normal colonic mucosa.
“The five subtypes identified in this study are highly correlated with key clinical and molecular features, including patient age, tumor location, microsatellite instability, and oncogenic mitogen-activated protein kinase mutations,” they wrote. “We show that cancers with high DNA methylation show an increased preponderance for mutating genes involved in epigenetic regulation, and namely those that are implicated in the chromatin remodeling process.”
Concluding, the investigators explained the role of their research in future therapy development. “Our analyses have identified potentially druggable vulnerabilities in cancers of different methylation subtypes,” they wrote. “Inhibitors targeting synthetic lethalities, such as SWI/SNF component inhibitors for those with ARID mutations, should be evaluated because these agents may be clinically beneficial to certain patient subsets.”
The study was funded by the National Health and Medical Research Council, the US National Institutes of Health, Pathology Queensland, and others. The investigators disclosed no conflicts of interest.
SOURCE: Fennell L et al. CMGH. 2019 Apr 4. doi: 10.1016/j.jcmgh.2019.04.002.
Genomic, epigenomic, and transcriptomic information has revealed molecular subclasses of CRC, which has refined our understanding of the molecular and cellular biology of CRC and improved our treatment of patients with CRC. Several reliable and clinically useful molecular subtypes of colorectal cancer have been identified, including microsatellite unstable (MSI), chromosomal unstable (CIN), CpG island methylator phenotype (CIMP), and CMS 1-4 subtypes. Despite these substantial advances, it is also clear that we still only partially grasp the molecular and cellular biology driving CRC.
The studies by Fennell et al. provide new insights into the CIMP subtype of CRC that address this knowledge gap. Using a large CRC cohort and more detailed molecular information than available in prior studies, they have identified previously unrecognized CRC CIMP subtypes that have unique methylomes and mutation patterns. These 5 CIMP subclasses vary with regard to location in the colon, frequency of mutations in KRAS, BRAF, and MSI, as well as alterations in epigenetic regulatory genes. The observations related to differences in frequencies of MSI, and mutations in KRAS and BRAF help demystify the heterogeneity in clinical and cellular behavior that has been seen in the broader class of CIMP cancers. Perhaps most importantly, their studies identify plausible driver molecular alterations unique to the CIMP subclasses, such as subclass specific mutations in epigenetic regulatory genes and activated oncogenes. These are promising novel targets for chemoprevention strategies and therapies. Fennell and colleagues have now set the stage for functional studies of these molecular alterations to determine their true role in the cellular and clinical behavior of CRC.
William M. Grady, MD, is the Rodger C. Haggitt Professor of Medicine, department of medicine, division of gastroenterology, University of Washington School of Medicine, and clinical research division, Fred Hutchinson Cancer Research Center, Seattle. He is an advisory board member for Freenome and SEngine; has consulted for DiaCarta, Boehringer Ingelheim, and Guardant Health; and has conducted industry-sponsored research for Jannsenn and Cambridge Epigenetic.
Genomic, epigenomic, and transcriptomic information has revealed molecular subclasses of CRC, which has refined our understanding of the molecular and cellular biology of CRC and improved our treatment of patients with CRC. Several reliable and clinically useful molecular subtypes of colorectal cancer have been identified, including microsatellite unstable (MSI), chromosomal unstable (CIN), CpG island methylator phenotype (CIMP), and CMS 1-4 subtypes. Despite these substantial advances, it is also clear that we still only partially grasp the molecular and cellular biology driving CRC.
The studies by Fennell et al. provide new insights into the CIMP subtype of CRC that address this knowledge gap. Using a large CRC cohort and more detailed molecular information than available in prior studies, they have identified previously unrecognized CRC CIMP subtypes that have unique methylomes and mutation patterns. These 5 CIMP subclasses vary with regard to location in the colon, frequency of mutations in KRAS, BRAF, and MSI, as well as alterations in epigenetic regulatory genes. The observations related to differences in frequencies of MSI, and mutations in KRAS and BRAF help demystify the heterogeneity in clinical and cellular behavior that has been seen in the broader class of CIMP cancers. Perhaps most importantly, their studies identify plausible driver molecular alterations unique to the CIMP subclasses, such as subclass specific mutations in epigenetic regulatory genes and activated oncogenes. These are promising novel targets for chemoprevention strategies and therapies. Fennell and colleagues have now set the stage for functional studies of these molecular alterations to determine their true role in the cellular and clinical behavior of CRC.
William M. Grady, MD, is the Rodger C. Haggitt Professor of Medicine, department of medicine, division of gastroenterology, University of Washington School of Medicine, and clinical research division, Fred Hutchinson Cancer Research Center, Seattle. He is an advisory board member for Freenome and SEngine; has consulted for DiaCarta, Boehringer Ingelheim, and Guardant Health; and has conducted industry-sponsored research for Jannsenn and Cambridge Epigenetic.
Genomic, epigenomic, and transcriptomic information has revealed molecular subclasses of CRC, which has refined our understanding of the molecular and cellular biology of CRC and improved our treatment of patients with CRC. Several reliable and clinically useful molecular subtypes of colorectal cancer have been identified, including microsatellite unstable (MSI), chromosomal unstable (CIN), CpG island methylator phenotype (CIMP), and CMS 1-4 subtypes. Despite these substantial advances, it is also clear that we still only partially grasp the molecular and cellular biology driving CRC.
The studies by Fennell et al. provide new insights into the CIMP subtype of CRC that address this knowledge gap. Using a large CRC cohort and more detailed molecular information than available in prior studies, they have identified previously unrecognized CRC CIMP subtypes that have unique methylomes and mutation patterns. These 5 CIMP subclasses vary with regard to location in the colon, frequency of mutations in KRAS, BRAF, and MSI, as well as alterations in epigenetic regulatory genes. The observations related to differences in frequencies of MSI, and mutations in KRAS and BRAF help demystify the heterogeneity in clinical and cellular behavior that has been seen in the broader class of CIMP cancers. Perhaps most importantly, their studies identify plausible driver molecular alterations unique to the CIMP subclasses, such as subclass specific mutations in epigenetic regulatory genes and activated oncogenes. These are promising novel targets for chemoprevention strategies and therapies. Fennell and colleagues have now set the stage for functional studies of these molecular alterations to determine their true role in the cellular and clinical behavior of CRC.
William M. Grady, MD, is the Rodger C. Haggitt Professor of Medicine, department of medicine, division of gastroenterology, University of Washington School of Medicine, and clinical research division, Fred Hutchinson Cancer Research Center, Seattle. He is an advisory board member for Freenome and SEngine; has consulted for DiaCarta, Boehringer Ingelheim, and Guardant Health; and has conducted industry-sponsored research for Jannsenn and Cambridge Epigenetic.
Colorectal cancer can be divided into five DNA methylation subtypes that predict molecular and clinical behavior and may offer future therapeutic targets, according to investigators.
In 216 unselected colorectal cancers, five subtypes of the CpG island methylator phenotype (CIMP) showed “striking” associations with sex, age, and tumor location, reported lead author Lochlan Fennell, MD, of the QIMR Berghofer Medical Research Institute in Queensland, Australia, and colleagues. CIMP level increased with age in a stepwise fashion, they noted.
Further associations with CIMP subtype and BRAF mutation status support the investigators’ recent report that sessile serrated adenomas are rare in young patients and pose little risk of malignancy. With additional research, these findings could “inform the development of patient-centric surveillance for young and older patients who present with sessile serrated adenomas,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology.
“CIMP can be detected using a standardized marker panel to stratify tumors as CIMP-high, CIMP-low, or CIMP-negative.” In the present study, the investigators expanded these three existing subtypes into five subtypes, allowing for better prediction of clinical and molecular characteristics associated with disease progression.
Initial genomic testing showed that 13.4% of cases carried a BRAF V600E mutation, 34.7% were mutated at KRAS codon 12 or 13, and almost half of the patients (42.2%) had a TP53 mutation. Sorted into the three previously described subtypes, CIMP negative was most common (68.5%), followed by CIMP low (20.4%), and CIMP high (11.1%). About two-thirds (66%) of BRAF mutant cancers were CIMP high, compared with just 3% of BRAF wild-type cases (P less than .0001). KRAS mutated cases were more often CIMP-low than KRAS wild-type cancers (34.6% vs. 12.8%; P less than .001).
With use of Illumina HumanMethylation450 Bead Chip arrays and recursively partitioned mixed model clustering, five methylation clusters were identified; specifically, these were CIMP-H1 and CIMP-H2 (high methylation levels), CIMP-L1 and CIMP-L2 (intermediate methylation levels), and CIMP-negative (low methylation level). As described above, methylation level demonstrated a direct relationship with age, ranging from CIMP-negative (61.9 years) to CIMP-H1 (75.2 years). The investigators also reported unique characteristics of each new subtype. For instance, the CIMP-H1 cluster had many features in common with cases of serrated neoplasia, such as BRAF mutation positivity (73.9%; P less than .0001).
“BRAF mutations are a hallmark of the serrated neoplasia pathway, and indicate that these cancers probably arose in serrated precursor lesions,” the investigators wrote. “We previously showed that the colonoscopic incidence of sessile serrated adenomas does not differ between patients aged in their 30s and patients who are much older, whereas BRAF mutant cancers were restricted to older individuals, suggesting these BRAF mutant polyps may have limited malignant potential in young patients.”
In contrast with the CIMP-H1 cases, CIMP-H2 cancers were more often KRAS mutant (54.5% vs. 17.4%). Other findings revealed associations with subtype and location; for example, CIMP-L1 cases were located equally in the distal and proximal colon, whereas CIMP-L2 cases more often localized to the distal colon and rectum. Of note for CIMP-negative cancers, most (62.3%) occurred in the distal colon, and none had a BRAF mutation.
The five methylation subtypes also showed associations with consensus molecular subtypes (CMS) to varying degrees. The two strongest correlations were found in CIMP-H1 cancers and CIMP-H2 cancers, which were most frequently classified as CMS1 (69.6%) and CMS3 (54.5%), respectively.
Using CIBERSORT, the investigators detected a variety of associations between the five subtypes and stromal immune cell composition. For example, CIMP-H1 cases were enriched for macrophages, compared with the other subtypes, except CIMP-L2. Mast cells showed a stepwise relationship with subtype; they contributed the most to the immune microenvironment of CIMP-negative cancers and the least to cases classified as CIMP-H1. A converse trend was found with natural killer cells.
Of note, in CIMP-H1 and CIMP-H2 cancers, oncogenes were significantly more likely than tumor-suppressor genes to undergo gene body methylation, which is positively correlated with gene expression, and oncogenes in these subtypes had significantly greater gene body methylation than normal colonic mucosa.
“The five subtypes identified in this study are highly correlated with key clinical and molecular features, including patient age, tumor location, microsatellite instability, and oncogenic mitogen-activated protein kinase mutations,” they wrote. “We show that cancers with high DNA methylation show an increased preponderance for mutating genes involved in epigenetic regulation, and namely those that are implicated in the chromatin remodeling process.”
Concluding, the investigators explained the role of their research in future therapy development. “Our analyses have identified potentially druggable vulnerabilities in cancers of different methylation subtypes,” they wrote. “Inhibitors targeting synthetic lethalities, such as SWI/SNF component inhibitors for those with ARID mutations, should be evaluated because these agents may be clinically beneficial to certain patient subsets.”
The study was funded by the National Health and Medical Research Council, the US National Institutes of Health, Pathology Queensland, and others. The investigators disclosed no conflicts of interest.
SOURCE: Fennell L et al. CMGH. 2019 Apr 4. doi: 10.1016/j.jcmgh.2019.04.002.
Colorectal cancer can be divided into five DNA methylation subtypes that predict molecular and clinical behavior and may offer future therapeutic targets, according to investigators.
In 216 unselected colorectal cancers, five subtypes of the CpG island methylator phenotype (CIMP) showed “striking” associations with sex, age, and tumor location, reported lead author Lochlan Fennell, MD, of the QIMR Berghofer Medical Research Institute in Queensland, Australia, and colleagues. CIMP level increased with age in a stepwise fashion, they noted.
Further associations with CIMP subtype and BRAF mutation status support the investigators’ recent report that sessile serrated adenomas are rare in young patients and pose little risk of malignancy. With additional research, these findings could “inform the development of patient-centric surveillance for young and older patients who present with sessile serrated adenomas,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology.
“CIMP can be detected using a standardized marker panel to stratify tumors as CIMP-high, CIMP-low, or CIMP-negative.” In the present study, the investigators expanded these three existing subtypes into five subtypes, allowing for better prediction of clinical and molecular characteristics associated with disease progression.
Initial genomic testing showed that 13.4% of cases carried a BRAF V600E mutation, 34.7% were mutated at KRAS codon 12 or 13, and almost half of the patients (42.2%) had a TP53 mutation. Sorted into the three previously described subtypes, CIMP negative was most common (68.5%), followed by CIMP low (20.4%), and CIMP high (11.1%). About two-thirds (66%) of BRAF mutant cancers were CIMP high, compared with just 3% of BRAF wild-type cases (P less than .0001). KRAS mutated cases were more often CIMP-low than KRAS wild-type cancers (34.6% vs. 12.8%; P less than .001).
With use of Illumina HumanMethylation450 Bead Chip arrays and recursively partitioned mixed model clustering, five methylation clusters were identified; specifically, these were CIMP-H1 and CIMP-H2 (high methylation levels), CIMP-L1 and CIMP-L2 (intermediate methylation levels), and CIMP-negative (low methylation level). As described above, methylation level demonstrated a direct relationship with age, ranging from CIMP-negative (61.9 years) to CIMP-H1 (75.2 years). The investigators also reported unique characteristics of each new subtype. For instance, the CIMP-H1 cluster had many features in common with cases of serrated neoplasia, such as BRAF mutation positivity (73.9%; P less than .0001).
“BRAF mutations are a hallmark of the serrated neoplasia pathway, and indicate that these cancers probably arose in serrated precursor lesions,” the investigators wrote. “We previously showed that the colonoscopic incidence of sessile serrated adenomas does not differ between patients aged in their 30s and patients who are much older, whereas BRAF mutant cancers were restricted to older individuals, suggesting these BRAF mutant polyps may have limited malignant potential in young patients.”
In contrast with the CIMP-H1 cases, CIMP-H2 cancers were more often KRAS mutant (54.5% vs. 17.4%). Other findings revealed associations with subtype and location; for example, CIMP-L1 cases were located equally in the distal and proximal colon, whereas CIMP-L2 cases more often localized to the distal colon and rectum. Of note for CIMP-negative cancers, most (62.3%) occurred in the distal colon, and none had a BRAF mutation.
The five methylation subtypes also showed associations with consensus molecular subtypes (CMS) to varying degrees. The two strongest correlations were found in CIMP-H1 cancers and CIMP-H2 cancers, which were most frequently classified as CMS1 (69.6%) and CMS3 (54.5%), respectively.
Using CIBERSORT, the investigators detected a variety of associations between the five subtypes and stromal immune cell composition. For example, CIMP-H1 cases were enriched for macrophages, compared with the other subtypes, except CIMP-L2. Mast cells showed a stepwise relationship with subtype; they contributed the most to the immune microenvironment of CIMP-negative cancers and the least to cases classified as CIMP-H1. A converse trend was found with natural killer cells.
Of note, in CIMP-H1 and CIMP-H2 cancers, oncogenes were significantly more likely than tumor-suppressor genes to undergo gene body methylation, which is positively correlated with gene expression, and oncogenes in these subtypes had significantly greater gene body methylation than normal colonic mucosa.
“The five subtypes identified in this study are highly correlated with key clinical and molecular features, including patient age, tumor location, microsatellite instability, and oncogenic mitogen-activated protein kinase mutations,” they wrote. “We show that cancers with high DNA methylation show an increased preponderance for mutating genes involved in epigenetic regulation, and namely those that are implicated in the chromatin remodeling process.”
Concluding, the investigators explained the role of their research in future therapy development. “Our analyses have identified potentially druggable vulnerabilities in cancers of different methylation subtypes,” they wrote. “Inhibitors targeting synthetic lethalities, such as SWI/SNF component inhibitors for those with ARID mutations, should be evaluated because these agents may be clinically beneficial to certain patient subsets.”
The study was funded by the National Health and Medical Research Council, the US National Institutes of Health, Pathology Queensland, and others. The investigators disclosed no conflicts of interest.
SOURCE: Fennell L et al. CMGH. 2019 Apr 4. doi: 10.1016/j.jcmgh.2019.04.002.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
The mesh mess, enmeshed in controversy
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
SGLT2 inhibitors for type 1 diabetes: Doctors debate the merits
SAN FRANCISCO – At first, the diabetes professionals in the audience at the annual scientific sessions of the American Diabetes Association overwhelmingly raised their hands to say they would support using SGLT2 inhibitors as adjunctive therapy in patients with type 1 diabetes. Then two physicians debated whether the drugs were too risky – predictably, one said yes, the other said no. In the end, most of the audience was unconvinced by one of the doctors. Which one? Well, we’ll get to that.
First, let’s look at the issue that divided the two physicians: Should the sodium-glucose cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – now commonly used to treat patients with type 2 diabetes – also be prescribed for patients with type 1 diabetes?
The drugs are not cleared in the United States for use in patients with type 1 diabetes, although drug makers are seeking approval. Earlier in 2019, the Food and Drug Administration turned down a request for the approval of sotagliflozin (Zynquista), a dual SGLT1 and SGLT2 inhibitor, for adults with type 1 diabetes. However, the drug has been approved in the European Union for certain overweight patients with type 1 diabetes.
In addition, the drugs are very costly, compared with some of the other diabetes medications, and physicians say that puts them out of reach for some patients.
The case for ...
In arguing that SGLT2 inhibitors would be appropriate as a therapy for patients with type 1 diabetes, Bruce A. Perkins, MD, MPH, professor and clinician-scientist at Leadership Sinai Center for Diabetes at the University of Toronto, emphasized the need for new treatments in type 1 diabetes.
“Even today, people with type 1 tell us they feel isolated, they fear hypoglycemia, they fear complications. And they have this undue burden of self-management,” he said. “We can do much better. Insulin therapy still needs us desperately needing more.”
Dr. Perkins highlighted the drugs’ widely lauded effects on cardiac and renal health and noted that a 2019 meta-analysis of 10 trials found that, compared with placebo, the drugs were associated with mean reductions in hemoglobin A1c (–0.39%; 95% confidence interval, –0.43 to –0.36) and body weight (–3.47%; 95% CI, –3.78 to –3.16).
That analysis also showed a higher risk of genital infection (3.57; 95% CI, 2.97-4.29) and diabetic ketoacidosis (DKA; 3.11; 95% CI, 2.11-4.58) with SGLT inhibitors, but the authors concluded that, despite the adverse events, the available data suggested that adding the inhibitors to basal insulin could be beneficial in patients with type 1 diabetes (Diabetes Metab Res Rev. 2019 Apr 11. doi: 10.1002/dmrr.3169).
In reference to the findings on DKA, Dr. Perkins said recent research has suggested that the DKA risk could be lowered by decreasing the dose of the SGLT2 inhibitors. “[DKA] is a problem, there’s no question, but there’s a background population risk. Whether we introduce an SGLT2 or not, we have to deal with this issue. We can deal with and overcome the excess DKA risk.”
In the big picture, he said, “it would be a crime not to make this treatment available to some patients. Meaningful benefits far outweigh the risks.”
The case against ...
On the other side of the debate was David M. Nathan, MD, of Harvard Medical School and the Clinical Research Center and Diabetes Center at Massachusetts General Hospital, Boston, who acknowledged the benefits of the SGLT2 inhibitors in type 2 diabetes.
However, he pointed to findings from a 2015 trial of canagliflozin as an add-on in type 1 diabetes (Diabetes Care. 2015;38[12]:2258-65). In that 18-week, randomized phase 2 trial, the investigators found that patients who took the drug had significantly higher rates of serious adverse events (7.7% or 6.8%, depending on dose, vs. 0% for placebo), urinary tract infections (4.3% and 5.1% vs. 1.7%), and DKA (4.3% and 6.0% vs. 0%).
“It would have cost $400 a month for the ‘pleasure’ of those side effects,” Dr. Nathan said.
He also noted a 2015 report on a 29-day, randomized, placebo-controlled study of sotagliflozin, the dual SGLT1 and SGLT2 inhibitor drug, as an add-on treatment for type 1 diabetes, in which investigators reported two episodes of DKA (13%) in the SGLT2 group, compared with none in placebo (Diabetes Care. 2015;38[7]:1181-8).
Dr. Nathan also pointed to a recent FDA warning about cases of Fournier gangrene, a rare type of serious genital infection, in patients taking SGLT2 inhibitors.
“To me, the risk [of using SGLT2 inhibitors in type 1 diabetes] outweighs the benefit by a lot,” he said, echoing comments he made in an editorial he wrote in 2017, that “any added benefits of adjunctive therapies for type 1 diabetes must be carefully balanced against their added risk and cost. Physicians and patients should beware” (N Engl J Med. 2017; 377:2390-1).
The outcome...
The audience was not sufficiently convinced by Dr. Nathan to swing the final vote fully in his favor, but he did manage to dent the initial support for using SGLT2 inhibitors in patients with type 1 disease. Before the debate, the show of hands suggested that roughly 80% of the audience thought SGLT2 inhibitors would be an appropriate therapy option for patients with type 1 diabetes. When the moderator asked the same question again after the arguments had been presented, that initial support had been eroded to about 70%. Dr. Nathan had clearly raised some doubts among the attendees, but Dr. Perkins’ perspective prevailed.
Dr. Perkins reported speaker fees from Medtronic, Abbott, Sanofi and Lilly; advisory panel service for Abbott, Boehringer Ingelheim, and Insulet; and research support to his institution from Boehringer Ingelheim and Bank of Montreal. Dr. Nathan reports no disclosures.
SAN FRANCISCO – At first, the diabetes professionals in the audience at the annual scientific sessions of the American Diabetes Association overwhelmingly raised their hands to say they would support using SGLT2 inhibitors as adjunctive therapy in patients with type 1 diabetes. Then two physicians debated whether the drugs were too risky – predictably, one said yes, the other said no. In the end, most of the audience was unconvinced by one of the doctors. Which one? Well, we’ll get to that.
First, let’s look at the issue that divided the two physicians: Should the sodium-glucose cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – now commonly used to treat patients with type 2 diabetes – also be prescribed for patients with type 1 diabetes?
The drugs are not cleared in the United States for use in patients with type 1 diabetes, although drug makers are seeking approval. Earlier in 2019, the Food and Drug Administration turned down a request for the approval of sotagliflozin (Zynquista), a dual SGLT1 and SGLT2 inhibitor, for adults with type 1 diabetes. However, the drug has been approved in the European Union for certain overweight patients with type 1 diabetes.
In addition, the drugs are very costly, compared with some of the other diabetes medications, and physicians say that puts them out of reach for some patients.
The case for ...
In arguing that SGLT2 inhibitors would be appropriate as a therapy for patients with type 1 diabetes, Bruce A. Perkins, MD, MPH, professor and clinician-scientist at Leadership Sinai Center for Diabetes at the University of Toronto, emphasized the need for new treatments in type 1 diabetes.
“Even today, people with type 1 tell us they feel isolated, they fear hypoglycemia, they fear complications. And they have this undue burden of self-management,” he said. “We can do much better. Insulin therapy still needs us desperately needing more.”
Dr. Perkins highlighted the drugs’ widely lauded effects on cardiac and renal health and noted that a 2019 meta-analysis of 10 trials found that, compared with placebo, the drugs were associated with mean reductions in hemoglobin A1c (–0.39%; 95% confidence interval, –0.43 to –0.36) and body weight (–3.47%; 95% CI, –3.78 to –3.16).
That analysis also showed a higher risk of genital infection (3.57; 95% CI, 2.97-4.29) and diabetic ketoacidosis (DKA; 3.11; 95% CI, 2.11-4.58) with SGLT inhibitors, but the authors concluded that, despite the adverse events, the available data suggested that adding the inhibitors to basal insulin could be beneficial in patients with type 1 diabetes (Diabetes Metab Res Rev. 2019 Apr 11. doi: 10.1002/dmrr.3169).
In reference to the findings on DKA, Dr. Perkins said recent research has suggested that the DKA risk could be lowered by decreasing the dose of the SGLT2 inhibitors. “[DKA] is a problem, there’s no question, but there’s a background population risk. Whether we introduce an SGLT2 or not, we have to deal with this issue. We can deal with and overcome the excess DKA risk.”
In the big picture, he said, “it would be a crime not to make this treatment available to some patients. Meaningful benefits far outweigh the risks.”
The case against ...
On the other side of the debate was David M. Nathan, MD, of Harvard Medical School and the Clinical Research Center and Diabetes Center at Massachusetts General Hospital, Boston, who acknowledged the benefits of the SGLT2 inhibitors in type 2 diabetes.
However, he pointed to findings from a 2015 trial of canagliflozin as an add-on in type 1 diabetes (Diabetes Care. 2015;38[12]:2258-65). In that 18-week, randomized phase 2 trial, the investigators found that patients who took the drug had significantly higher rates of serious adverse events (7.7% or 6.8%, depending on dose, vs. 0% for placebo), urinary tract infections (4.3% and 5.1% vs. 1.7%), and DKA (4.3% and 6.0% vs. 0%).
“It would have cost $400 a month for the ‘pleasure’ of those side effects,” Dr. Nathan said.
He also noted a 2015 report on a 29-day, randomized, placebo-controlled study of sotagliflozin, the dual SGLT1 and SGLT2 inhibitor drug, as an add-on treatment for type 1 diabetes, in which investigators reported two episodes of DKA (13%) in the SGLT2 group, compared with none in placebo (Diabetes Care. 2015;38[7]:1181-8).
Dr. Nathan also pointed to a recent FDA warning about cases of Fournier gangrene, a rare type of serious genital infection, in patients taking SGLT2 inhibitors.
“To me, the risk [of using SGLT2 inhibitors in type 1 diabetes] outweighs the benefit by a lot,” he said, echoing comments he made in an editorial he wrote in 2017, that “any added benefits of adjunctive therapies for type 1 diabetes must be carefully balanced against their added risk and cost. Physicians and patients should beware” (N Engl J Med. 2017; 377:2390-1).
The outcome...
The audience was not sufficiently convinced by Dr. Nathan to swing the final vote fully in his favor, but he did manage to dent the initial support for using SGLT2 inhibitors in patients with type 1 disease. Before the debate, the show of hands suggested that roughly 80% of the audience thought SGLT2 inhibitors would be an appropriate therapy option for patients with type 1 diabetes. When the moderator asked the same question again after the arguments had been presented, that initial support had been eroded to about 70%. Dr. Nathan had clearly raised some doubts among the attendees, but Dr. Perkins’ perspective prevailed.
Dr. Perkins reported speaker fees from Medtronic, Abbott, Sanofi and Lilly; advisory panel service for Abbott, Boehringer Ingelheim, and Insulet; and research support to his institution from Boehringer Ingelheim and Bank of Montreal. Dr. Nathan reports no disclosures.
SAN FRANCISCO – At first, the diabetes professionals in the audience at the annual scientific sessions of the American Diabetes Association overwhelmingly raised their hands to say they would support using SGLT2 inhibitors as adjunctive therapy in patients with type 1 diabetes. Then two physicians debated whether the drugs were too risky – predictably, one said yes, the other said no. In the end, most of the audience was unconvinced by one of the doctors. Which one? Well, we’ll get to that.
First, let’s look at the issue that divided the two physicians: Should the sodium-glucose cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – now commonly used to treat patients with type 2 diabetes – also be prescribed for patients with type 1 diabetes?
The drugs are not cleared in the United States for use in patients with type 1 diabetes, although drug makers are seeking approval. Earlier in 2019, the Food and Drug Administration turned down a request for the approval of sotagliflozin (Zynquista), a dual SGLT1 and SGLT2 inhibitor, for adults with type 1 diabetes. However, the drug has been approved in the European Union for certain overweight patients with type 1 diabetes.
In addition, the drugs are very costly, compared with some of the other diabetes medications, and physicians say that puts them out of reach for some patients.
The case for ...
In arguing that SGLT2 inhibitors would be appropriate as a therapy for patients with type 1 diabetes, Bruce A. Perkins, MD, MPH, professor and clinician-scientist at Leadership Sinai Center for Diabetes at the University of Toronto, emphasized the need for new treatments in type 1 diabetes.
“Even today, people with type 1 tell us they feel isolated, they fear hypoglycemia, they fear complications. And they have this undue burden of self-management,” he said. “We can do much better. Insulin therapy still needs us desperately needing more.”
Dr. Perkins highlighted the drugs’ widely lauded effects on cardiac and renal health and noted that a 2019 meta-analysis of 10 trials found that, compared with placebo, the drugs were associated with mean reductions in hemoglobin A1c (–0.39%; 95% confidence interval, –0.43 to –0.36) and body weight (–3.47%; 95% CI, –3.78 to –3.16).
That analysis also showed a higher risk of genital infection (3.57; 95% CI, 2.97-4.29) and diabetic ketoacidosis (DKA; 3.11; 95% CI, 2.11-4.58) with SGLT inhibitors, but the authors concluded that, despite the adverse events, the available data suggested that adding the inhibitors to basal insulin could be beneficial in patients with type 1 diabetes (Diabetes Metab Res Rev. 2019 Apr 11. doi: 10.1002/dmrr.3169).
In reference to the findings on DKA, Dr. Perkins said recent research has suggested that the DKA risk could be lowered by decreasing the dose of the SGLT2 inhibitors. “[DKA] is a problem, there’s no question, but there’s a background population risk. Whether we introduce an SGLT2 or not, we have to deal with this issue. We can deal with and overcome the excess DKA risk.”
In the big picture, he said, “it would be a crime not to make this treatment available to some patients. Meaningful benefits far outweigh the risks.”
The case against ...
On the other side of the debate was David M. Nathan, MD, of Harvard Medical School and the Clinical Research Center and Diabetes Center at Massachusetts General Hospital, Boston, who acknowledged the benefits of the SGLT2 inhibitors in type 2 diabetes.
However, he pointed to findings from a 2015 trial of canagliflozin as an add-on in type 1 diabetes (Diabetes Care. 2015;38[12]:2258-65). In that 18-week, randomized phase 2 trial, the investigators found that patients who took the drug had significantly higher rates of serious adverse events (7.7% or 6.8%, depending on dose, vs. 0% for placebo), urinary tract infections (4.3% and 5.1% vs. 1.7%), and DKA (4.3% and 6.0% vs. 0%).
“It would have cost $400 a month for the ‘pleasure’ of those side effects,” Dr. Nathan said.
He also noted a 2015 report on a 29-day, randomized, placebo-controlled study of sotagliflozin, the dual SGLT1 and SGLT2 inhibitor drug, as an add-on treatment for type 1 diabetes, in which investigators reported two episodes of DKA (13%) in the SGLT2 group, compared with none in placebo (Diabetes Care. 2015;38[7]:1181-8).
Dr. Nathan also pointed to a recent FDA warning about cases of Fournier gangrene, a rare type of serious genital infection, in patients taking SGLT2 inhibitors.
“To me, the risk [of using SGLT2 inhibitors in type 1 diabetes] outweighs the benefit by a lot,” he said, echoing comments he made in an editorial he wrote in 2017, that “any added benefits of adjunctive therapies for type 1 diabetes must be carefully balanced against their added risk and cost. Physicians and patients should beware” (N Engl J Med. 2017; 377:2390-1).
The outcome...
The audience was not sufficiently convinced by Dr. Nathan to swing the final vote fully in his favor, but he did manage to dent the initial support for using SGLT2 inhibitors in patients with type 1 disease. Before the debate, the show of hands suggested that roughly 80% of the audience thought SGLT2 inhibitors would be an appropriate therapy option for patients with type 1 diabetes. When the moderator asked the same question again after the arguments had been presented, that initial support had been eroded to about 70%. Dr. Nathan had clearly raised some doubts among the attendees, but Dr. Perkins’ perspective prevailed.
Dr. Perkins reported speaker fees from Medtronic, Abbott, Sanofi and Lilly; advisory panel service for Abbott, Boehringer Ingelheim, and Insulet; and research support to his institution from Boehringer Ingelheim and Bank of Montreal. Dr. Nathan reports no disclosures.
EXPERT ANALYSIS FROM ADA 2019
Some burnout factors are within a physician’s control
SAN DIEGO – Eat a healthy lunch. Get more sleep. Move your body. How many times in the course of a week do you give patients gentle reminders to practice these most basic steps of self-care? And how many times in the course of a week do you allow these basics to go by the wayside for yourself?
Self-care is one of the elements that can defend against physician burnout, Carol Burke, MD, said at a session on physician burnout held during the annual Digestive Disease Week®. Personal self-care can make a real difference, and shouldn’t be ignored as the profession works to reel back some of the institutional changes that challenge physicians today.
In the workplace, unhealthy stress levels can contribute to burnout, disruptive behavior, decreased productivity, and disengagement. Burnout – a response to chronic stress characterized by a diminished sense of personal accomplishment and emotional exhaustion – can result in cynicism, a lack of compassion, and feelings of depersonalization, said Dr. Burke.
Contributors to physician stress have been well documented, said Dr. Burke, a professor of gastroenterology at the Cleveland Clinic. These range from personal debt and the struggle for work-life balance to an increased focus on metrics and documentation at the expense of authentic patient engagement. All of these factors are measurable by means of the validated Maslach Burnout Inventory, said Dr. Burke. A recent survey that used this measure indicated that nearly half of physician respondents report experiencing burnout.
In 2017, Dr. Burke led a survey of American College of Gastroenterology members that showed 49.3% of respondents reported feeling emotional exhaustion and/or depersonalization. Some key themes emerged from the survey, she said. Women and younger physicians were more likely to experience burnout. Having children in the middle years (11-15 years old) and spending more time on domestic duties and child care increased the risk of burnout.
And doing patient-related work at home or having a spouse or partner bring work home also upped burnout risk. Skipping breakfast and lunch during the workweek was another risk factor, which highlights the importance of basic self-care as armor against the administrative onslaught, said Dr. Burke.
Measured by volume alone, physician work can be overwhelming: 45% of physicians in the United States work more than 60 hours weekly, compared with fewer than 10% of the general workforce, said Dr. Burke.
What factors within the control of an individual practitioner can reduce the risk of debilitating burnout and improve quality of life? Physicians who do report a high quality of life, said Dr. Burke, are more likely to have a positive outlook. They also practice basic self-care like taking vacations, exercising regularly, and engaging in hobbies outside of work.
For exhausted, overworked clinicians, getting a good night’s sleep is a critical form of self-care. But erratic schedules, stress, and family demands can all sabotage plans for better sleep hygiene. Still, attending to sleep is important, said Dr. Burke. Individuals with disturbed sleep are less mindful and have less self-compassion. Sleep disturbance is also strongly correlated with perceived stress.
She also reported that the odds ratio for burnout was 14.7 for physicians who reported insomnia when compared with those without sleep disturbance, and it was 9.9 for those who reported nonrestorative sleep.
Physical activity can help sleep and also help other markers of burnout. Dr. Burke pointed to a recent study of 4,402 medical students. Participants were able to reduce burnout risk when they met the Centers for Disease Control and Prevention recommendations of achieving at least 150 minutes/week of moderate exercise or 75 minutes/week of vigorous exercise, plus at least 2 days/week of strength training (P less than .001; Acad Med. 2017;92:1006).
These physician-targeted programs can work, she said: “Faciliated interventions improve well-being, attitudes associated with patient-centered care, meaning and engagement in work, and reduce burnout.”
Practice-focused interventions to reclaim a semblance of control over one’s time are varied, and some are easier to implement than others. Virtual visits and group visits are surprisingly well received by patients, and each can be huge time-savers for physicians, said Dr. Burke. There are billing and workflow pitfalls to avoid, but group visits, in particular, can be practice changing for those who have heavy backlogs and see many patients with the same condition.
Medical scribes can improve productivity and reduce physician time spent on documentation. Also, said Dr. Burke, visits can appropriately be billed at a higher level of complexity when contemporaneous documentation is thorough. Clinicians overall feel that they can engage more fully with patients, and also feel more effective, when well-trained scribes are integrated into a practice, she said.
Female physicians have repeatedly been shown to have patient panels that are more demanding, and male and female patients alike expect more empathy and social support from their physicians, said Dr. Burke. When psychosocial complexities are interwoven with patient care, as they are more frequently for female providers, a 15-minute visit can easily run twice that – or more. Dr. Burke is among the physicians advocating for recognition of this invisible burden on female clinicians, either through adaptive scheduling or differential productivity expectations. This approach is not without controversy, she acknowledged; still, practices should acknowledge that clinic flow can be very different for male and female gastroenterologists, she said.
Dr. Burke reported no relevant conflicts of interest.
Read tips for how to balance family and personal lives with a professional career in order to avoid burnout at https://www.ddwnews.org/news/aga-symposium-provides-practical-tips-to-avoid-physician-burnout/.
SAN DIEGO – Eat a healthy lunch. Get more sleep. Move your body. How many times in the course of a week do you give patients gentle reminders to practice these most basic steps of self-care? And how many times in the course of a week do you allow these basics to go by the wayside for yourself?
Self-care is one of the elements that can defend against physician burnout, Carol Burke, MD, said at a session on physician burnout held during the annual Digestive Disease Week®. Personal self-care can make a real difference, and shouldn’t be ignored as the profession works to reel back some of the institutional changes that challenge physicians today.
In the workplace, unhealthy stress levels can contribute to burnout, disruptive behavior, decreased productivity, and disengagement. Burnout – a response to chronic stress characterized by a diminished sense of personal accomplishment and emotional exhaustion – can result in cynicism, a lack of compassion, and feelings of depersonalization, said Dr. Burke.
Contributors to physician stress have been well documented, said Dr. Burke, a professor of gastroenterology at the Cleveland Clinic. These range from personal debt and the struggle for work-life balance to an increased focus on metrics and documentation at the expense of authentic patient engagement. All of these factors are measurable by means of the validated Maslach Burnout Inventory, said Dr. Burke. A recent survey that used this measure indicated that nearly half of physician respondents report experiencing burnout.
In 2017, Dr. Burke led a survey of American College of Gastroenterology members that showed 49.3% of respondents reported feeling emotional exhaustion and/or depersonalization. Some key themes emerged from the survey, she said. Women and younger physicians were more likely to experience burnout. Having children in the middle years (11-15 years old) and spending more time on domestic duties and child care increased the risk of burnout.
And doing patient-related work at home or having a spouse or partner bring work home also upped burnout risk. Skipping breakfast and lunch during the workweek was another risk factor, which highlights the importance of basic self-care as armor against the administrative onslaught, said Dr. Burke.
Measured by volume alone, physician work can be overwhelming: 45% of physicians in the United States work more than 60 hours weekly, compared with fewer than 10% of the general workforce, said Dr. Burke.
What factors within the control of an individual practitioner can reduce the risk of debilitating burnout and improve quality of life? Physicians who do report a high quality of life, said Dr. Burke, are more likely to have a positive outlook. They also practice basic self-care like taking vacations, exercising regularly, and engaging in hobbies outside of work.
For exhausted, overworked clinicians, getting a good night’s sleep is a critical form of self-care. But erratic schedules, stress, and family demands can all sabotage plans for better sleep hygiene. Still, attending to sleep is important, said Dr. Burke. Individuals with disturbed sleep are less mindful and have less self-compassion. Sleep disturbance is also strongly correlated with perceived stress.
She also reported that the odds ratio for burnout was 14.7 for physicians who reported insomnia when compared with those without sleep disturbance, and it was 9.9 for those who reported nonrestorative sleep.
Physical activity can help sleep and also help other markers of burnout. Dr. Burke pointed to a recent study of 4,402 medical students. Participants were able to reduce burnout risk when they met the Centers for Disease Control and Prevention recommendations of achieving at least 150 minutes/week of moderate exercise or 75 minutes/week of vigorous exercise, plus at least 2 days/week of strength training (P less than .001; Acad Med. 2017;92:1006).
These physician-targeted programs can work, she said: “Faciliated interventions improve well-being, attitudes associated with patient-centered care, meaning and engagement in work, and reduce burnout.”
Practice-focused interventions to reclaim a semblance of control over one’s time are varied, and some are easier to implement than others. Virtual visits and group visits are surprisingly well received by patients, and each can be huge time-savers for physicians, said Dr. Burke. There are billing and workflow pitfalls to avoid, but group visits, in particular, can be practice changing for those who have heavy backlogs and see many patients with the same condition.
Medical scribes can improve productivity and reduce physician time spent on documentation. Also, said Dr. Burke, visits can appropriately be billed at a higher level of complexity when contemporaneous documentation is thorough. Clinicians overall feel that they can engage more fully with patients, and also feel more effective, when well-trained scribes are integrated into a practice, she said.
Female physicians have repeatedly been shown to have patient panels that are more demanding, and male and female patients alike expect more empathy and social support from their physicians, said Dr. Burke. When psychosocial complexities are interwoven with patient care, as they are more frequently for female providers, a 15-minute visit can easily run twice that – or more. Dr. Burke is among the physicians advocating for recognition of this invisible burden on female clinicians, either through adaptive scheduling or differential productivity expectations. This approach is not without controversy, she acknowledged; still, practices should acknowledge that clinic flow can be very different for male and female gastroenterologists, she said.
Dr. Burke reported no relevant conflicts of interest.
Read tips for how to balance family and personal lives with a professional career in order to avoid burnout at https://www.ddwnews.org/news/aga-symposium-provides-practical-tips-to-avoid-physician-burnout/.
SAN DIEGO – Eat a healthy lunch. Get more sleep. Move your body. How many times in the course of a week do you give patients gentle reminders to practice these most basic steps of self-care? And how many times in the course of a week do you allow these basics to go by the wayside for yourself?
Self-care is one of the elements that can defend against physician burnout, Carol Burke, MD, said at a session on physician burnout held during the annual Digestive Disease Week®. Personal self-care can make a real difference, and shouldn’t be ignored as the profession works to reel back some of the institutional changes that challenge physicians today.
In the workplace, unhealthy stress levels can contribute to burnout, disruptive behavior, decreased productivity, and disengagement. Burnout – a response to chronic stress characterized by a diminished sense of personal accomplishment and emotional exhaustion – can result in cynicism, a lack of compassion, and feelings of depersonalization, said Dr. Burke.
Contributors to physician stress have been well documented, said Dr. Burke, a professor of gastroenterology at the Cleveland Clinic. These range from personal debt and the struggle for work-life balance to an increased focus on metrics and documentation at the expense of authentic patient engagement. All of these factors are measurable by means of the validated Maslach Burnout Inventory, said Dr. Burke. A recent survey that used this measure indicated that nearly half of physician respondents report experiencing burnout.
In 2017, Dr. Burke led a survey of American College of Gastroenterology members that showed 49.3% of respondents reported feeling emotional exhaustion and/or depersonalization. Some key themes emerged from the survey, she said. Women and younger physicians were more likely to experience burnout. Having children in the middle years (11-15 years old) and spending more time on domestic duties and child care increased the risk of burnout.
And doing patient-related work at home or having a spouse or partner bring work home also upped burnout risk. Skipping breakfast and lunch during the workweek was another risk factor, which highlights the importance of basic self-care as armor against the administrative onslaught, said Dr. Burke.
Measured by volume alone, physician work can be overwhelming: 45% of physicians in the United States work more than 60 hours weekly, compared with fewer than 10% of the general workforce, said Dr. Burke.
What factors within the control of an individual practitioner can reduce the risk of debilitating burnout and improve quality of life? Physicians who do report a high quality of life, said Dr. Burke, are more likely to have a positive outlook. They also practice basic self-care like taking vacations, exercising regularly, and engaging in hobbies outside of work.
For exhausted, overworked clinicians, getting a good night’s sleep is a critical form of self-care. But erratic schedules, stress, and family demands can all sabotage plans for better sleep hygiene. Still, attending to sleep is important, said Dr. Burke. Individuals with disturbed sleep are less mindful and have less self-compassion. Sleep disturbance is also strongly correlated with perceived stress.
She also reported that the odds ratio for burnout was 14.7 for physicians who reported insomnia when compared with those without sleep disturbance, and it was 9.9 for those who reported nonrestorative sleep.
Physical activity can help sleep and also help other markers of burnout. Dr. Burke pointed to a recent study of 4,402 medical students. Participants were able to reduce burnout risk when they met the Centers for Disease Control and Prevention recommendations of achieving at least 150 minutes/week of moderate exercise or 75 minutes/week of vigorous exercise, plus at least 2 days/week of strength training (P less than .001; Acad Med. 2017;92:1006).
These physician-targeted programs can work, she said: “Faciliated interventions improve well-being, attitudes associated with patient-centered care, meaning and engagement in work, and reduce burnout.”
Practice-focused interventions to reclaim a semblance of control over one’s time are varied, and some are easier to implement than others. Virtual visits and group visits are surprisingly well received by patients, and each can be huge time-savers for physicians, said Dr. Burke. There are billing and workflow pitfalls to avoid, but group visits, in particular, can be practice changing for those who have heavy backlogs and see many patients with the same condition.
Medical scribes can improve productivity and reduce physician time spent on documentation. Also, said Dr. Burke, visits can appropriately be billed at a higher level of complexity when contemporaneous documentation is thorough. Clinicians overall feel that they can engage more fully with patients, and also feel more effective, when well-trained scribes are integrated into a practice, she said.
Female physicians have repeatedly been shown to have patient panels that are more demanding, and male and female patients alike expect more empathy and social support from their physicians, said Dr. Burke. When psychosocial complexities are interwoven with patient care, as they are more frequently for female providers, a 15-minute visit can easily run twice that – or more. Dr. Burke is among the physicians advocating for recognition of this invisible burden on female clinicians, either through adaptive scheduling or differential productivity expectations. This approach is not without controversy, she acknowledged; still, practices should acknowledge that clinic flow can be very different for male and female gastroenterologists, she said.
Dr. Burke reported no relevant conflicts of interest.
Read tips for how to balance family and personal lives with a professional career in order to avoid burnout at https://www.ddwnews.org/news/aga-symposium-provides-practical-tips-to-avoid-physician-burnout/.
EXPERT ANALYSIS FROM DDW 2019
Improved Patient Outcomes and Reduced Wait Times: Transforming a VA Outpatient Substance Use Disorder Program
Substance use disorders (SUDs) are an increasing public health concern in the US. The 2015 National Survey on Drug Use and Health indicated that 27 million people (8% of the US population) reported current use of recreational drugs or misuse of alcohol or prescription medications.1 The 2013 National Survey on Drug Use and Health indicated that 1.5 million veterans (roughly 6.6%) met the criteria for a SUD.2 More than 50% of patients awaiting entry into a SUD treatment program will never achieve admission due, in part, to long wait times.3-5
National attention has been focused on increasing veteran access to quality treatment based on evidence-based practices (EBPs). Several national legislative measures and treatment protocols have been implemented: the Uniform Mental Health Services in US Department of Veterans Affairs (VA) medical centers and clinics; Veterans Access, Choice, and Accountability Act (2014); Cognitive Behavioral Therapy for Substance Use Disorders (CBT-SUD) Training Program; and the Psychotropic Drug Safety Initiative (PDSI).6-8 Consistent with these directives and in line with American Society of Addiction Medicine (ASAM) and Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines for medication-assisted therapies (MAT),the James A. Haley Veterans’ Hospital (JAHVH) Mental Health and Behavioral Sciences Service (MH&BSS) Substance Use Disorders Service (SUDS) in Tampa, Florida, implemented an evidence-based, treatment-on-demand model of care.9-11
Meeting SUD Treatment Needs
What does the new supervisor of a clinical program do when a 24-employee outpatient VA Alcohol and Drug Addiction Treatment Program (ADATP) has an average 33-day wait time for treatment with 54% of patients lost to care between initial evaluation and admission?12 Patients lacked consistent access to SUD pharmacotherapy. The national VA clinical performance indicators were substandard and there are no additional resources available to apply to the program.
At JAHVH the program supervisor enlisted hospital leadership to support program redesign. The redesign sought to improve operational efficiency and eliminate patient wait time; adopt national standards for assessment and treatment developed by ASAM; implement strictly evidence-based psychotherapeutic treatments; educate program psychiatrists about evidence-based psychopharmacologic treatments and hold them accountable for patient adherence; streamline documentation templates; free clinical providers from nonclinical tasks; create an inpatient addiction consult team to diagnose and treat chronic hospitalized patients with SUDs; ensure continuity of care; and, standardize consistent, objective measures of patient response to treatment to track the program’s effectiveness.
In this article, the authors provide an explanation of the clinical, theoretical foundation and the practical steps taken to design and implement this transformation. They then describe the lessons learned, hoping that their process will serve as a model for those in similar situations.
Program Redesign
July 1, 2015, a new program supervisor was hired and began a 2-month evaluation and analysis of the program with input from leadership, staff, and hospital/community stakeholders. September 1, the monthlong process of developing the redesign began. On September 30 the plan was presented to, and approved by, MH&BSS leadership. October was spent preparing for change with an implementation date of November 2 selected. On November 2, 2015, the complete redesign was implemented.
Needs Assessment
A needs assessment yielded improvement opportunities in program structure (levels of care); clinical content; staff and resource allocation, including clinical workflow and management systems. Staff identified philosophical and practical variance in the program, often leading to confusion for patients and clinicians and potentially resulting in disparate quality care and patient outcomes. Recommendations for addressing these needs included incorporating ASAM guidelines for assignment to clinically appropriate levels of care, implementation of consistent EBPs for SUD and comorbid conditions,9 and emphasis on staff training and development to champion evidence-based program philosophy and service delivery.
The assessment determined that the average waitlist time was 33 days, and patients were required to abstain from substance or alcohol use prior to admission to the Intensive Outpatient Program. If a waitlisted patient relapsed, she or he was removed from the waitlist and denied admission. A study conducted at JAHVH reported that 54% of waitlisted patients in this clinic (prior to November 2, 2015) never were admitted to the program.12 Access to care was considered a significant issue.
Program Implementation
September was spent developing a comprehensive redesign of the SUD clinic. The vision included incorporating all ASAM levels of care; creating an evidence-based, treatment-on-demand model of care; and, securing the support of MH&BSS leadership team, staff, and patients for the redesign. The supervisory clinician interviewed staff both individually and as a group. Clinicians were provided extensive training on EBP for SUDs, including psychotherapies, psychosocial treatments, and psychopharmacologic interventions. A journal club was started with staff-generated topics that offered articles sharing current research, EBPs, and psychotherapeutic techniques, continuing education on substances, and management of coexisting diagnoses. Clinicians increased the frequency of SUD in-service trainings. Psychiatrists provided several Grand Rounds to the MH&BSS service. All counselors were assigned to 1 of the program’s 3 clinical psychologists for individual weekly clinical supervision.
By providing all staff with current, evidence-based, clinically relevant treatment information and emphasizing its relationship to successful patient outcomes, program leadership energized staff support. Staff were encouraged to perform at the top of their scope of practice and engage in training and consultation. Each staff member was delegated a role in the process to inspire buy-in.
Preparation for the Shift
October was spent preparing for a seamless, one-day implementation of proposed changes, including implementation of updated clinical policies, procedures, and document templates (rewritten to include only clinically appropriate information required by VA policy or the Joint Commission); streamlined staff schedules; and utilization of staff-developed and research/policy-driven EBP handbook. Finally, the Brief Addiction Monitor (BAM) was selected as objective criteria to consistently assess patient progress in treatment, and staff were instructed to use this measure at regular intervals and for all levels of care.
Emphasis was placed on ongoing fortification of staff and patient support for the reorganization. For example, the Addiction Severity Index, though not required by policy, was historically used, adding 90 minutes to the evaluation and admission session. Staff agreed to remove this measure to improve clinician availability. Staff were also empowered to rename the redesigned program, and chose Substance Use Disorders Service (SUDS).
Process Changes
To achieve same-day access to clinical care, program leadership created a daily morning orientation group. Patients are scheduled or may attend as a walk-in. The orientation’s purpose is to explain what services are available and to offer each patient an opportunity for immediate evaluation and treatment. Staff schedules were modified to provide patient evaluation appointment slots immediately following orientation. The number of immediate evaluation slots was initially assessed by analyzing the demand for treatment over the previous 6 months, determining the daily mean, and setting the number of slots to accommodate 3 standard deviations above the daily mean. If a patient in a daily orientation group expresses a willingness to engage in treatment, he or she is immediately evaluated by a counselor during a 90-minute session and seen by a psychiatrist to determine whether pharmacologic treatment would be appropriate. If needed, the medication is prescribed that day. The primary purpose of the patient’s initial clinical evaluation is to determine the most appropriate level of care based on ASAM criteria. Also available were 90-minute afternoon evaluation appointments with psychiatrists for patients who walk into the clinic after the morning orientation group had ended.
Prior to the redesign, clinic psychiatrists were minimally prescribing evidence-based pharmacotherapy for sobriety support. At the time of redesign, only 8% of patients diagnosed with opioid use disorders (OUDs) were prescribed buprenorphine/naloxone or naltrexone. Just 1.9% of patients diagnosed with alcohol use disorder (AUD) were prescribed naltrexone or acamprosate. With the redesign, access to these medications has significantly expanded.
All templates were redesigned to ensure consistent documentation. This change decreased the overall provider task burden, and explicitly supported the use of ASAM multidimensional criteria and the Brief Addiction Monitor (BAM) to identify a pretreatment baseline score and track each patient’s clinical progress.13 Evidence-based written curricula were standardized for individual and group psychotherapies to reduce provider and programmatic variation.
The redesign creates distinct levels of care based on ASAM criteria, including harm reduction, ambulatory detoxification, outpatient group and individual psychotherapy, an evidence-based Intensive Outpatient Program (IOP), and aftercare. Application of the ASAM standards has allowed clinicians to make accurate placement decisions that best meet individual patient needs and to serve as effective stewards even with limited treatment and financial resources. Although JAHVH does not have a residential SUD program, procedures were developed to refer veterans to community-based residential treatment programs when appropriate.
Group Therapies
With the redesign, SUDS was no longer exclusively a 12-step program; however, it still supported and recognized the value of this approach for some patients. A psychologist periodically audits group sessions to prevent drift from that group’s curriculum. Counselors are assigned to weekly hour-long clinical supervision sessions with a psychologist to review patient care and reinforce the application of evidence-based individualized treatment.
After reviewing empirical literature and VA directives, CBT-SUD was adopted. It encompasses individual and group interventions, such as motivational interviewing (MI), contingency management (CM), and medication-assisted therapies as primary therapeutic treatment modalities, all of which have demonstrated efficacy as measured by length of sobriety postintervention.9,14,15
Clinical Staff Improvements
Staff were reorganized into 3 interdisciplinary treatment teams. A weekly team meeting is scheduled to coordinate care and discuss the treatment of complex patients. Clinical staff focus has shifted from case-management to diagnosis and treatment; now patients are referred to their primary care team’s social worker for case management services. Allowing clinical staff to focus solely on the diagnosis and clinical treatment of SUDs has significantly enhanced productivity and morale.
Staff receive training in the newly adopted interventions during brief monthly refresher courses provided by inhouse psychologists. Additional training includes participation in local and national SUD teleconferences and onsite meetings with experts in harm reduction and motivational interventions. During the transition, clinicians were encouraged to attend staff resiliency training. Continuing education was available to the SUDS psychiatrists and all inpatient and outpatient psychiatrists at JAHVH. Recently, this educational initiative was expanded to include all primary care and inpatient internal medicine physicians.
Implementation
On November 2, 2015, all planned programmatic changes were simultaneously implemented. On that day, clinician and patient schedules changed, the new EBP curriculum was administered, the use of streamlined documentation procedures began, and daily orientation groups followed by same-day evaluations were initiated.
The pretreatment sobriety requirement was eliminated as a barrier to care, and the program began to use a harm-reduction treatment track as recommended by ASAM guidelines. Patients with urgent or emergent medical or psychiatric problems were immediately assessed by SUDS health care providers and treated in the clinic or transported to the emergency department. Previously unavailable, patient access to ambulatory detoxification was initiated. The prescription of buprenorphine/naloxone for the treatment of OUD treatments increased from 1 prescriber to all 3.
Three months after program reorganization, the leadership reviewed overall workflow, conducted patient satisfaction surveys, and evaluated facility use and productivity. To address patient needs and facilitate optimal use of space, the number of same-day evaluation slots was reduced while the number of individual therapy slots was increased.
Staff meet in workgroups to discuss EBPs and further refine content with feedback from the supervisory clinician and team psychologists who routinely audit group therapy sessions. Staff report ongoing benefit from weekly supervision with a clinical psychologist. An inpatient addiction consultation team that uses existing manpower and resources has been developed.
Program Goals and Outcomes
The SUDS program serves more patients at multiple levels of standardized care with 2 fewer full-time positions. One counselor and one advanced practice registered nurse were reallocated to different programs within the JAHVH VA mental health clinic. Following a review of all program clinic profiles in the VA’s Computerized Patient Record System (CPRS) for utilization, accuracy, and necessity, and allowing for accurate program data capture, the transition resulted in a reduction of distinct clinics from 114 to 67 (-58.7%). In fiscal year 2018, review of CPRS yielded 19,786 total visits (3,645 unique visits).
Eliminate Patient Wait Tme
Patient wait time, as measured in CPRS from date of initial evaluation to date of treatment was reduced from an average of 33 days to 0 within 2 weeks of program implementation. A review of CPRS data also indicated that preadmission attrition dropped from 54% to < 1%; all patients desiring treatment are assigned a counselor and treatment is initiated the same day.
Adopt ASAM Criteria
After the redesign, patients have received more appropriate care based on individualized treatment plans. Due to the implementation of a fluid and supportive model, patients can move through levels of care as clinical need dictates rather than failing treatment and having to reengage. Staff receive ongoing education on the use of ASAM. Evaluation and treatment plan templates now reflect assignment to level of care rationale using ASAM guidelines.
Use of Evidence-Based Psychotherapeutic Treatments
More consistent, coordinated, and effective psychotherapies have improved patient care. The program’s previous issues with patients receiving conflicting treatment guidance from different providers has been resolved. Duplicate and ineffective treatments, including multiple readmissions to the IOP level of care, overemphasis of abstinence-based modalities for patients in active use, and referrals to inpatient SUD care under the assumption that “higher level of care is better” have ceased through staff education, leadership support, and appropriate staffing and communication. Review of patient advocate complaints tracked by and resolved by the service demonstrated an 80% decrease in patient advocate complaints regarding SUD clinic services.
Implement Evidence-Based Psychopharmacologic Treatments
The pharmacotherapy education initiative yielded tangible benefits and is likely a significant contributor to the program’s improved clinical outcomes. Prescription of pharmacotherapy for patients with OUD has risen from 8% to 25.1% in eligible patients. Appropriate medication prescription for the treatment of AUD has risen from 1.9% to 9.8% in eligible patients. These data are reflected in the VA Pharmaceutical Drug Safety Initiative (PDSI) dashboard.
Streamline Documentation
Significantly reducing the charting burden was likely a significant contributor to increased provider productivity and improved patient outcomes. Regular meetings between SUDS leadership and clinical informatics ensure that standardized note templates meet hospital policy and gather all necessary accreditation information.
Improve Employee Morale
Increased staff morale is indicated by a noticeable reduction in employee sick days; a decrease of > 20% (over the same time period the previous year), per the VA electronic timekeeping system, during the first 6 months following the November 2 program implementation.
SUDS Inpatient Addiction Consult Team
In January of 2017, SUDS began an inpatient medicine consultation service to offer evaluation, pharmacotherapy, and supportive counseling to patients diagnosed with SUDs who had been admitted to inpatient medical and surgical services. This team includes existing SUDS staff members reallocated to the inpatient service, is led by a SUDS psychiatrist, and includes 3 multidisciplinary clinicians with extensive training in assessment, diagnosis, and treatment planning of SUDs and comorbid conditions. Prior to implementation, the SUDS inpatient addiction consult team met with hospital leadership and attending physicians for inpatient medicine and psychiatry physicians.
To access the SUDS inpatient addiction consult team, physicians request a consult. Patients are offered an evaluation and are assigned to a level of care with orders for outpatient appointments with a counselor and psychiatrist within 7 days of hospital discharge. Medication-assisted treatment for chronic SUDs is implemented while patients remain admitted to the inpatient medical service. In fiscal year 2018, the SUDS inpatient addiction consult team performed 1,428 inpatient evaluations.
Consistent Treatment Outcome Measures
The BAM is a clinical tool designed to measure patient outcomes in substance use disorders.13 Its 17-item scale measures substance use risk factors that may lead to relapse, and protective factors that are recovery-oriented behaviors that help prevent relapse. It demonstrates sensitivity to change and has excellent test-retest reliability. The BAM has been in use in the addictions treatment program since 2011 but was previously administered only after admission to the IOP and again after a 30- to 90-day follow-up period. Since the program redesign, all SUDS patients are administered the BAM at their initial evaluation and at each individual appointment thereafter. The initial BAM assessment encompasses the previous 30 days; this 30-day version is also used for monthly follow-ups. For BAM assessments that occur within 30 days from the time of the last evaluation, a 7-day version is used. Prior to the redesign, about 24% of patients received a follow-up 30-day BAM assessment.12 Per CPRS review of veterans participating in continued treatment, the rate rose to 100% 3 months after the redesign.
When program staff compared preredesign and postredesign BAM data, they detected significant clinical differences. Data demonstrate a 22.2% improvement in protective factors, including patient confidence in their ability to remain abstinent; engaging in self-help activities, such as attending Alcoholics Anonymous meetings; engaging in organized spiritual activities; going to school, working, or volunteering; securing a regular income; and time spent with friends or family who are supportive of recovery.
The data also show a marked reduction in substance use at follow-up points in treatment and a corresponding decrease in risk factors. One item of the BAM assesses patient level of satisfaction with their treatment. Since the redesign, patients report that they are “considerably” satisfied with their SUD treatment.
Currently, program staff are conducting a review of BAM scores by level of care to further parse the impact of various treatments and best target patient need using measurement-based care and EBP, such as contingency management, which provides small monetary incentives when patients maintain clean urine drug screens.16 In addition, the program plans to achieve more uniformity in BAM assessment intervals at all levels of care, and possibly also integrate BAM data into SUD group therapies. Correlation of the BAM scores to other metrics, such as readmission to inpatient medicine, relapse, urine drug screen, or critical laboratory values, will provide additional insight into impact of programmatic changes.
Discussion
Feedback from other clinics and services within the hospital has been very positive. Some providers have reported that they appreciate the ease and availability of access to SUDS. Additionally, patients engaged in treatment prior to the redesign have been contacted for an updated evaluation and assignment to a counselor and appropriate level of care. From the staff’s perspective, the shift to immediate access to care has allowed a more streamlined process with fewer hurdles for patient admission. Staff report that they now feel empowered to meet the needs of veterans in a comprehensive, same-day fashion.
The success of our redesign was contingent on internal and external sta
The successful implementation of these changes has revealed several important elements regarding patient care. The first lesson was that improving access and integrating best practices is possible without additional resources, outside monies, or disruption to patient services. With the support of MH&BSS leadership, the program streamlined existing processes and used both staff and clinic resources more efficiently.
The second lesson involved the importance of continually reviewing and revising standard operating procedures to match the needs of the current patient population. Policies and procedures that once were viewed as potential barriers to change have been replaced with a more flexible approach and willingness to evolve.
As a result, far fewer patients have been lost to treatment. The time and resources that staff historically dedicated to nonclinical patient care are now redirected to immediate service provision. This increase in operational efficiency and treatment efficacy has resulted in a boost to staff morale, even during a time of immense change and increased productivity. Program staff are now able to personally witness the significant changes in their patients’ lives and feel a sense of pride at being a member of a hard-working team that provides the highest quality of substance use treatment. This is critical to job satisfaction and meets the VA mission to provide timely, effective, and evidence-based treatments to patients.
Conclusion
JAHVH strives to continue to provide the highest quality of SUD treatment available. Future directions aim to streamline clinic operations by constantly monitoring and reviewing workloads, while also considering patient feedback. A continuous review of EBP is part of our clinic’s culture. Program leadership endeavors to promote an open environment where providers can share their triumphs and frustrations and foster a team approach to problem solving. Further plans include expanding the range of treatment levels offered by developing a residential SUD treatment facility.
1. Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health: Summary of the Effects of the 2015 NSDUH Questionnaire Redesign: Implications for Data Users. https://www.samhsa.gov/data/sites/default/files/NSDUH-TrendBreak-2015.pdf. Published June 2016. Accessed June 12, 2019.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan PDSKL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96(8):1149-1160.
4. Hser Y, Maglione M, Polinsky ML, Anglin MD. Predicting treatment entry among treatment-seeking drug abusers. J Subst Abuse Treatment. 1997;15(3):213-220.
5. Stark MJ, Campbell BK, Brinkerhoff CV. “Hello, may we help you?” A study of attrition prevention at the time of the first phone contact with substance-abusing clients. Am J Drug Alcohol Abuse. 1990;16:67-76.
6. US Department of Veterans Affairs. Uniform Mental Health Services in VA Medical Centers and Clinics. VHA Handbook 1160.01. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762. Updated November 2015. Accessed December 12, 2017.
7. Veterans Access, Choice and Accountability Act of 2014, 2 USC § 933.
8. DeMarce JM, Gnys M, Raffa SD, Karlin, BE. Cognitive Behavioral Therapy for Substance Use Disorders Among Veterans: Therapist Manual. Washington, DC: US Department of Veterans Affairs; 2014.
9. Mee-Lee D, Shulman GD, Fishman MJ, Gastfriend DR, Miller MM, eds. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions. 3rd ed. Carson City, NV: The Change Companies; 2013.
10. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
11. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for Opioid Use Disorder – Full Document. HHS Publication No. (SMA) 18-5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
12. Winn JL, Shealy SE, Kropp GJ, Felkins-Dohm D, Gonzales-Nolas C, Francis E. Housing assistance and case management: Improving access to substance use disorder treatment for homeless veterans. Psychological Serv. 2013;10(2):233-240.
13. Cacciola JS, Alterman AI, DePhilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treatment. 2013;44(3):256-263.
14. McHugh RK, Hearon BA, Otto MW. Cognitive-behavioral therapy for substance use disorders, Psychiatr Clinics North Am. 2010;33:511–525.
15. Karlin, BE, Cross, G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs health care system. Am Psychol. 2014;69:19-33.
16. DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes, Drug Alcohol Dependence. 2018;185:367-373.
Substance use disorders (SUDs) are an increasing public health concern in the US. The 2015 National Survey on Drug Use and Health indicated that 27 million people (8% of the US population) reported current use of recreational drugs or misuse of alcohol or prescription medications.1 The 2013 National Survey on Drug Use and Health indicated that 1.5 million veterans (roughly 6.6%) met the criteria for a SUD.2 More than 50% of patients awaiting entry into a SUD treatment program will never achieve admission due, in part, to long wait times.3-5
National attention has been focused on increasing veteran access to quality treatment based on evidence-based practices (EBPs). Several national legislative measures and treatment protocols have been implemented: the Uniform Mental Health Services in US Department of Veterans Affairs (VA) medical centers and clinics; Veterans Access, Choice, and Accountability Act (2014); Cognitive Behavioral Therapy for Substance Use Disorders (CBT-SUD) Training Program; and the Psychotropic Drug Safety Initiative (PDSI).6-8 Consistent with these directives and in line with American Society of Addiction Medicine (ASAM) and Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines for medication-assisted therapies (MAT),the James A. Haley Veterans’ Hospital (JAHVH) Mental Health and Behavioral Sciences Service (MH&BSS) Substance Use Disorders Service (SUDS) in Tampa, Florida, implemented an evidence-based, treatment-on-demand model of care.9-11
Meeting SUD Treatment Needs
What does the new supervisor of a clinical program do when a 24-employee outpatient VA Alcohol and Drug Addiction Treatment Program (ADATP) has an average 33-day wait time for treatment with 54% of patients lost to care between initial evaluation and admission?12 Patients lacked consistent access to SUD pharmacotherapy. The national VA clinical performance indicators were substandard and there are no additional resources available to apply to the program.
At JAHVH the program supervisor enlisted hospital leadership to support program redesign. The redesign sought to improve operational efficiency and eliminate patient wait time; adopt national standards for assessment and treatment developed by ASAM; implement strictly evidence-based psychotherapeutic treatments; educate program psychiatrists about evidence-based psychopharmacologic treatments and hold them accountable for patient adherence; streamline documentation templates; free clinical providers from nonclinical tasks; create an inpatient addiction consult team to diagnose and treat chronic hospitalized patients with SUDs; ensure continuity of care; and, standardize consistent, objective measures of patient response to treatment to track the program’s effectiveness.
In this article, the authors provide an explanation of the clinical, theoretical foundation and the practical steps taken to design and implement this transformation. They then describe the lessons learned, hoping that their process will serve as a model for those in similar situations.
Program Redesign
July 1, 2015, a new program supervisor was hired and began a 2-month evaluation and analysis of the program with input from leadership, staff, and hospital/community stakeholders. September 1, the monthlong process of developing the redesign began. On September 30 the plan was presented to, and approved by, MH&BSS leadership. October was spent preparing for change with an implementation date of November 2 selected. On November 2, 2015, the complete redesign was implemented.
Needs Assessment
A needs assessment yielded improvement opportunities in program structure (levels of care); clinical content; staff and resource allocation, including clinical workflow and management systems. Staff identified philosophical and practical variance in the program, often leading to confusion for patients and clinicians and potentially resulting in disparate quality care and patient outcomes. Recommendations for addressing these needs included incorporating ASAM guidelines for assignment to clinically appropriate levels of care, implementation of consistent EBPs for SUD and comorbid conditions,9 and emphasis on staff training and development to champion evidence-based program philosophy and service delivery.
The assessment determined that the average waitlist time was 33 days, and patients were required to abstain from substance or alcohol use prior to admission to the Intensive Outpatient Program. If a waitlisted patient relapsed, she or he was removed from the waitlist and denied admission. A study conducted at JAHVH reported that 54% of waitlisted patients in this clinic (prior to November 2, 2015) never were admitted to the program.12 Access to care was considered a significant issue.
Program Implementation
September was spent developing a comprehensive redesign of the SUD clinic. The vision included incorporating all ASAM levels of care; creating an evidence-based, treatment-on-demand model of care; and, securing the support of MH&BSS leadership team, staff, and patients for the redesign. The supervisory clinician interviewed staff both individually and as a group. Clinicians were provided extensive training on EBP for SUDs, including psychotherapies, psychosocial treatments, and psychopharmacologic interventions. A journal club was started with staff-generated topics that offered articles sharing current research, EBPs, and psychotherapeutic techniques, continuing education on substances, and management of coexisting diagnoses. Clinicians increased the frequency of SUD in-service trainings. Psychiatrists provided several Grand Rounds to the MH&BSS service. All counselors were assigned to 1 of the program’s 3 clinical psychologists for individual weekly clinical supervision.
By providing all staff with current, evidence-based, clinically relevant treatment information and emphasizing its relationship to successful patient outcomes, program leadership energized staff support. Staff were encouraged to perform at the top of their scope of practice and engage in training and consultation. Each staff member was delegated a role in the process to inspire buy-in.
Preparation for the Shift
October was spent preparing for a seamless, one-day implementation of proposed changes, including implementation of updated clinical policies, procedures, and document templates (rewritten to include only clinically appropriate information required by VA policy or the Joint Commission); streamlined staff schedules; and utilization of staff-developed and research/policy-driven EBP handbook. Finally, the Brief Addiction Monitor (BAM) was selected as objective criteria to consistently assess patient progress in treatment, and staff were instructed to use this measure at regular intervals and for all levels of care.
Emphasis was placed on ongoing fortification of staff and patient support for the reorganization. For example, the Addiction Severity Index, though not required by policy, was historically used, adding 90 minutes to the evaluation and admission session. Staff agreed to remove this measure to improve clinician availability. Staff were also empowered to rename the redesigned program, and chose Substance Use Disorders Service (SUDS).
Process Changes
To achieve same-day access to clinical care, program leadership created a daily morning orientation group. Patients are scheduled or may attend as a walk-in. The orientation’s purpose is to explain what services are available and to offer each patient an opportunity for immediate evaluation and treatment. Staff schedules were modified to provide patient evaluation appointment slots immediately following orientation. The number of immediate evaluation slots was initially assessed by analyzing the demand for treatment over the previous 6 months, determining the daily mean, and setting the number of slots to accommodate 3 standard deviations above the daily mean. If a patient in a daily orientation group expresses a willingness to engage in treatment, he or she is immediately evaluated by a counselor during a 90-minute session and seen by a psychiatrist to determine whether pharmacologic treatment would be appropriate. If needed, the medication is prescribed that day. The primary purpose of the patient’s initial clinical evaluation is to determine the most appropriate level of care based on ASAM criteria. Also available were 90-minute afternoon evaluation appointments with psychiatrists for patients who walk into the clinic after the morning orientation group had ended.
Prior to the redesign, clinic psychiatrists were minimally prescribing evidence-based pharmacotherapy for sobriety support. At the time of redesign, only 8% of patients diagnosed with opioid use disorders (OUDs) were prescribed buprenorphine/naloxone or naltrexone. Just 1.9% of patients diagnosed with alcohol use disorder (AUD) were prescribed naltrexone or acamprosate. With the redesign, access to these medications has significantly expanded.
All templates were redesigned to ensure consistent documentation. This change decreased the overall provider task burden, and explicitly supported the use of ASAM multidimensional criteria and the Brief Addiction Monitor (BAM) to identify a pretreatment baseline score and track each patient’s clinical progress.13 Evidence-based written curricula were standardized for individual and group psychotherapies to reduce provider and programmatic variation.
The redesign creates distinct levels of care based on ASAM criteria, including harm reduction, ambulatory detoxification, outpatient group and individual psychotherapy, an evidence-based Intensive Outpatient Program (IOP), and aftercare. Application of the ASAM standards has allowed clinicians to make accurate placement decisions that best meet individual patient needs and to serve as effective stewards even with limited treatment and financial resources. Although JAHVH does not have a residential SUD program, procedures were developed to refer veterans to community-based residential treatment programs when appropriate.
Group Therapies
With the redesign, SUDS was no longer exclusively a 12-step program; however, it still supported and recognized the value of this approach for some patients. A psychologist periodically audits group sessions to prevent drift from that group’s curriculum. Counselors are assigned to weekly hour-long clinical supervision sessions with a psychologist to review patient care and reinforce the application of evidence-based individualized treatment.
After reviewing empirical literature and VA directives, CBT-SUD was adopted. It encompasses individual and group interventions, such as motivational interviewing (MI), contingency management (CM), and medication-assisted therapies as primary therapeutic treatment modalities, all of which have demonstrated efficacy as measured by length of sobriety postintervention.9,14,15
Clinical Staff Improvements
Staff were reorganized into 3 interdisciplinary treatment teams. A weekly team meeting is scheduled to coordinate care and discuss the treatment of complex patients. Clinical staff focus has shifted from case-management to diagnosis and treatment; now patients are referred to their primary care team’s social worker for case management services. Allowing clinical staff to focus solely on the diagnosis and clinical treatment of SUDs has significantly enhanced productivity and morale.
Staff receive training in the newly adopted interventions during brief monthly refresher courses provided by inhouse psychologists. Additional training includes participation in local and national SUD teleconferences and onsite meetings with experts in harm reduction and motivational interventions. During the transition, clinicians were encouraged to attend staff resiliency training. Continuing education was available to the SUDS psychiatrists and all inpatient and outpatient psychiatrists at JAHVH. Recently, this educational initiative was expanded to include all primary care and inpatient internal medicine physicians.
Implementation
On November 2, 2015, all planned programmatic changes were simultaneously implemented. On that day, clinician and patient schedules changed, the new EBP curriculum was administered, the use of streamlined documentation procedures began, and daily orientation groups followed by same-day evaluations were initiated.
The pretreatment sobriety requirement was eliminated as a barrier to care, and the program began to use a harm-reduction treatment track as recommended by ASAM guidelines. Patients with urgent or emergent medical or psychiatric problems were immediately assessed by SUDS health care providers and treated in the clinic or transported to the emergency department. Previously unavailable, patient access to ambulatory detoxification was initiated. The prescription of buprenorphine/naloxone for the treatment of OUD treatments increased from 1 prescriber to all 3.
Three months after program reorganization, the leadership reviewed overall workflow, conducted patient satisfaction surveys, and evaluated facility use and productivity. To address patient needs and facilitate optimal use of space, the number of same-day evaluation slots was reduced while the number of individual therapy slots was increased.
Staff meet in workgroups to discuss EBPs and further refine content with feedback from the supervisory clinician and team psychologists who routinely audit group therapy sessions. Staff report ongoing benefit from weekly supervision with a clinical psychologist. An inpatient addiction consultation team that uses existing manpower and resources has been developed.
Program Goals and Outcomes
The SUDS program serves more patients at multiple levels of standardized care with 2 fewer full-time positions. One counselor and one advanced practice registered nurse were reallocated to different programs within the JAHVH VA mental health clinic. Following a review of all program clinic profiles in the VA’s Computerized Patient Record System (CPRS) for utilization, accuracy, and necessity, and allowing for accurate program data capture, the transition resulted in a reduction of distinct clinics from 114 to 67 (-58.7%). In fiscal year 2018, review of CPRS yielded 19,786 total visits (3,645 unique visits).
Eliminate Patient Wait Tme
Patient wait time, as measured in CPRS from date of initial evaluation to date of treatment was reduced from an average of 33 days to 0 within 2 weeks of program implementation. A review of CPRS data also indicated that preadmission attrition dropped from 54% to < 1%; all patients desiring treatment are assigned a counselor and treatment is initiated the same day.
Adopt ASAM Criteria
After the redesign, patients have received more appropriate care based on individualized treatment plans. Due to the implementation of a fluid and supportive model, patients can move through levels of care as clinical need dictates rather than failing treatment and having to reengage. Staff receive ongoing education on the use of ASAM. Evaluation and treatment plan templates now reflect assignment to level of care rationale using ASAM guidelines.
Use of Evidence-Based Psychotherapeutic Treatments
More consistent, coordinated, and effective psychotherapies have improved patient care. The program’s previous issues with patients receiving conflicting treatment guidance from different providers has been resolved. Duplicate and ineffective treatments, including multiple readmissions to the IOP level of care, overemphasis of abstinence-based modalities for patients in active use, and referrals to inpatient SUD care under the assumption that “higher level of care is better” have ceased through staff education, leadership support, and appropriate staffing and communication. Review of patient advocate complaints tracked by and resolved by the service demonstrated an 80% decrease in patient advocate complaints regarding SUD clinic services.
Implement Evidence-Based Psychopharmacologic Treatments
The pharmacotherapy education initiative yielded tangible benefits and is likely a significant contributor to the program’s improved clinical outcomes. Prescription of pharmacotherapy for patients with OUD has risen from 8% to 25.1% in eligible patients. Appropriate medication prescription for the treatment of AUD has risen from 1.9% to 9.8% in eligible patients. These data are reflected in the VA Pharmaceutical Drug Safety Initiative (PDSI) dashboard.
Streamline Documentation
Significantly reducing the charting burden was likely a significant contributor to increased provider productivity and improved patient outcomes. Regular meetings between SUDS leadership and clinical informatics ensure that standardized note templates meet hospital policy and gather all necessary accreditation information.
Improve Employee Morale
Increased staff morale is indicated by a noticeable reduction in employee sick days; a decrease of > 20% (over the same time period the previous year), per the VA electronic timekeeping system, during the first 6 months following the November 2 program implementation.
SUDS Inpatient Addiction Consult Team
In January of 2017, SUDS began an inpatient medicine consultation service to offer evaluation, pharmacotherapy, and supportive counseling to patients diagnosed with SUDs who had been admitted to inpatient medical and surgical services. This team includes existing SUDS staff members reallocated to the inpatient service, is led by a SUDS psychiatrist, and includes 3 multidisciplinary clinicians with extensive training in assessment, diagnosis, and treatment planning of SUDs and comorbid conditions. Prior to implementation, the SUDS inpatient addiction consult team met with hospital leadership and attending physicians for inpatient medicine and psychiatry physicians.
To access the SUDS inpatient addiction consult team, physicians request a consult. Patients are offered an evaluation and are assigned to a level of care with orders for outpatient appointments with a counselor and psychiatrist within 7 days of hospital discharge. Medication-assisted treatment for chronic SUDs is implemented while patients remain admitted to the inpatient medical service. In fiscal year 2018, the SUDS inpatient addiction consult team performed 1,428 inpatient evaluations.
Consistent Treatment Outcome Measures
The BAM is a clinical tool designed to measure patient outcomes in substance use disorders.13 Its 17-item scale measures substance use risk factors that may lead to relapse, and protective factors that are recovery-oriented behaviors that help prevent relapse. It demonstrates sensitivity to change and has excellent test-retest reliability. The BAM has been in use in the addictions treatment program since 2011 but was previously administered only after admission to the IOP and again after a 30- to 90-day follow-up period. Since the program redesign, all SUDS patients are administered the BAM at their initial evaluation and at each individual appointment thereafter. The initial BAM assessment encompasses the previous 30 days; this 30-day version is also used for monthly follow-ups. For BAM assessments that occur within 30 days from the time of the last evaluation, a 7-day version is used. Prior to the redesign, about 24% of patients received a follow-up 30-day BAM assessment.12 Per CPRS review of veterans participating in continued treatment, the rate rose to 100% 3 months after the redesign.
When program staff compared preredesign and postredesign BAM data, they detected significant clinical differences. Data demonstrate a 22.2% improvement in protective factors, including patient confidence in their ability to remain abstinent; engaging in self-help activities, such as attending Alcoholics Anonymous meetings; engaging in organized spiritual activities; going to school, working, or volunteering; securing a regular income; and time spent with friends or family who are supportive of recovery.
The data also show a marked reduction in substance use at follow-up points in treatment and a corresponding decrease in risk factors. One item of the BAM assesses patient level of satisfaction with their treatment. Since the redesign, patients report that they are “considerably” satisfied with their SUD treatment.
Currently, program staff are conducting a review of BAM scores by level of care to further parse the impact of various treatments and best target patient need using measurement-based care and EBP, such as contingency management, which provides small monetary incentives when patients maintain clean urine drug screens.16 In addition, the program plans to achieve more uniformity in BAM assessment intervals at all levels of care, and possibly also integrate BAM data into SUD group therapies. Correlation of the BAM scores to other metrics, such as readmission to inpatient medicine, relapse, urine drug screen, or critical laboratory values, will provide additional insight into impact of programmatic changes.
Discussion
Feedback from other clinics and services within the hospital has been very positive. Some providers have reported that they appreciate the ease and availability of access to SUDS. Additionally, patients engaged in treatment prior to the redesign have been contacted for an updated evaluation and assignment to a counselor and appropriate level of care. From the staff’s perspective, the shift to immediate access to care has allowed a more streamlined process with fewer hurdles for patient admission. Staff report that they now feel empowered to meet the needs of veterans in a comprehensive, same-day fashion.
The success of our redesign was contingent on internal and external sta
The successful implementation of these changes has revealed several important elements regarding patient care. The first lesson was that improving access and integrating best practices is possible without additional resources, outside monies, or disruption to patient services. With the support of MH&BSS leadership, the program streamlined existing processes and used both staff and clinic resources more efficiently.
The second lesson involved the importance of continually reviewing and revising standard operating procedures to match the needs of the current patient population. Policies and procedures that once were viewed as potential barriers to change have been replaced with a more flexible approach and willingness to evolve.
As a result, far fewer patients have been lost to treatment. The time and resources that staff historically dedicated to nonclinical patient care are now redirected to immediate service provision. This increase in operational efficiency and treatment efficacy has resulted in a boost to staff morale, even during a time of immense change and increased productivity. Program staff are now able to personally witness the significant changes in their patients’ lives and feel a sense of pride at being a member of a hard-working team that provides the highest quality of substance use treatment. This is critical to job satisfaction and meets the VA mission to provide timely, effective, and evidence-based treatments to patients.
Conclusion
JAHVH strives to continue to provide the highest quality of SUD treatment available. Future directions aim to streamline clinic operations by constantly monitoring and reviewing workloads, while also considering patient feedback. A continuous review of EBP is part of our clinic’s culture. Program leadership endeavors to promote an open environment where providers can share their triumphs and frustrations and foster a team approach to problem solving. Further plans include expanding the range of treatment levels offered by developing a residential SUD treatment facility.
Substance use disorders (SUDs) are an increasing public health concern in the US. The 2015 National Survey on Drug Use and Health indicated that 27 million people (8% of the US population) reported current use of recreational drugs or misuse of alcohol or prescription medications.1 The 2013 National Survey on Drug Use and Health indicated that 1.5 million veterans (roughly 6.6%) met the criteria for a SUD.2 More than 50% of patients awaiting entry into a SUD treatment program will never achieve admission due, in part, to long wait times.3-5
National attention has been focused on increasing veteran access to quality treatment based on evidence-based practices (EBPs). Several national legislative measures and treatment protocols have been implemented: the Uniform Mental Health Services in US Department of Veterans Affairs (VA) medical centers and clinics; Veterans Access, Choice, and Accountability Act (2014); Cognitive Behavioral Therapy for Substance Use Disorders (CBT-SUD) Training Program; and the Psychotropic Drug Safety Initiative (PDSI).6-8 Consistent with these directives and in line with American Society of Addiction Medicine (ASAM) and Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines for medication-assisted therapies (MAT),the James A. Haley Veterans’ Hospital (JAHVH) Mental Health and Behavioral Sciences Service (MH&BSS) Substance Use Disorders Service (SUDS) in Tampa, Florida, implemented an evidence-based, treatment-on-demand model of care.9-11
Meeting SUD Treatment Needs
What does the new supervisor of a clinical program do when a 24-employee outpatient VA Alcohol and Drug Addiction Treatment Program (ADATP) has an average 33-day wait time for treatment with 54% of patients lost to care between initial evaluation and admission?12 Patients lacked consistent access to SUD pharmacotherapy. The national VA clinical performance indicators were substandard and there are no additional resources available to apply to the program.
At JAHVH the program supervisor enlisted hospital leadership to support program redesign. The redesign sought to improve operational efficiency and eliminate patient wait time; adopt national standards for assessment and treatment developed by ASAM; implement strictly evidence-based psychotherapeutic treatments; educate program psychiatrists about evidence-based psychopharmacologic treatments and hold them accountable for patient adherence; streamline documentation templates; free clinical providers from nonclinical tasks; create an inpatient addiction consult team to diagnose and treat chronic hospitalized patients with SUDs; ensure continuity of care; and, standardize consistent, objective measures of patient response to treatment to track the program’s effectiveness.
In this article, the authors provide an explanation of the clinical, theoretical foundation and the practical steps taken to design and implement this transformation. They then describe the lessons learned, hoping that their process will serve as a model for those in similar situations.
Program Redesign
July 1, 2015, a new program supervisor was hired and began a 2-month evaluation and analysis of the program with input from leadership, staff, and hospital/community stakeholders. September 1, the monthlong process of developing the redesign began. On September 30 the plan was presented to, and approved by, MH&BSS leadership. October was spent preparing for change with an implementation date of November 2 selected. On November 2, 2015, the complete redesign was implemented.
Needs Assessment
A needs assessment yielded improvement opportunities in program structure (levels of care); clinical content; staff and resource allocation, including clinical workflow and management systems. Staff identified philosophical and practical variance in the program, often leading to confusion for patients and clinicians and potentially resulting in disparate quality care and patient outcomes. Recommendations for addressing these needs included incorporating ASAM guidelines for assignment to clinically appropriate levels of care, implementation of consistent EBPs for SUD and comorbid conditions,9 and emphasis on staff training and development to champion evidence-based program philosophy and service delivery.
The assessment determined that the average waitlist time was 33 days, and patients were required to abstain from substance or alcohol use prior to admission to the Intensive Outpatient Program. If a waitlisted patient relapsed, she or he was removed from the waitlist and denied admission. A study conducted at JAHVH reported that 54% of waitlisted patients in this clinic (prior to November 2, 2015) never were admitted to the program.12 Access to care was considered a significant issue.
Program Implementation
September was spent developing a comprehensive redesign of the SUD clinic. The vision included incorporating all ASAM levels of care; creating an evidence-based, treatment-on-demand model of care; and, securing the support of MH&BSS leadership team, staff, and patients for the redesign. The supervisory clinician interviewed staff both individually and as a group. Clinicians were provided extensive training on EBP for SUDs, including psychotherapies, psychosocial treatments, and psychopharmacologic interventions. A journal club was started with staff-generated topics that offered articles sharing current research, EBPs, and psychotherapeutic techniques, continuing education on substances, and management of coexisting diagnoses. Clinicians increased the frequency of SUD in-service trainings. Psychiatrists provided several Grand Rounds to the MH&BSS service. All counselors were assigned to 1 of the program’s 3 clinical psychologists for individual weekly clinical supervision.
By providing all staff with current, evidence-based, clinically relevant treatment information and emphasizing its relationship to successful patient outcomes, program leadership energized staff support. Staff were encouraged to perform at the top of their scope of practice and engage in training and consultation. Each staff member was delegated a role in the process to inspire buy-in.
Preparation for the Shift
October was spent preparing for a seamless, one-day implementation of proposed changes, including implementation of updated clinical policies, procedures, and document templates (rewritten to include only clinically appropriate information required by VA policy or the Joint Commission); streamlined staff schedules; and utilization of staff-developed and research/policy-driven EBP handbook. Finally, the Brief Addiction Monitor (BAM) was selected as objective criteria to consistently assess patient progress in treatment, and staff were instructed to use this measure at regular intervals and for all levels of care.
Emphasis was placed on ongoing fortification of staff and patient support for the reorganization. For example, the Addiction Severity Index, though not required by policy, was historically used, adding 90 minutes to the evaluation and admission session. Staff agreed to remove this measure to improve clinician availability. Staff were also empowered to rename the redesigned program, and chose Substance Use Disorders Service (SUDS).
Process Changes
To achieve same-day access to clinical care, program leadership created a daily morning orientation group. Patients are scheduled or may attend as a walk-in. The orientation’s purpose is to explain what services are available and to offer each patient an opportunity for immediate evaluation and treatment. Staff schedules were modified to provide patient evaluation appointment slots immediately following orientation. The number of immediate evaluation slots was initially assessed by analyzing the demand for treatment over the previous 6 months, determining the daily mean, and setting the number of slots to accommodate 3 standard deviations above the daily mean. If a patient in a daily orientation group expresses a willingness to engage in treatment, he or she is immediately evaluated by a counselor during a 90-minute session and seen by a psychiatrist to determine whether pharmacologic treatment would be appropriate. If needed, the medication is prescribed that day. The primary purpose of the patient’s initial clinical evaluation is to determine the most appropriate level of care based on ASAM criteria. Also available were 90-minute afternoon evaluation appointments with psychiatrists for patients who walk into the clinic after the morning orientation group had ended.
Prior to the redesign, clinic psychiatrists were minimally prescribing evidence-based pharmacotherapy for sobriety support. At the time of redesign, only 8% of patients diagnosed with opioid use disorders (OUDs) were prescribed buprenorphine/naloxone or naltrexone. Just 1.9% of patients diagnosed with alcohol use disorder (AUD) were prescribed naltrexone or acamprosate. With the redesign, access to these medications has significantly expanded.
All templates were redesigned to ensure consistent documentation. This change decreased the overall provider task burden, and explicitly supported the use of ASAM multidimensional criteria and the Brief Addiction Monitor (BAM) to identify a pretreatment baseline score and track each patient’s clinical progress.13 Evidence-based written curricula were standardized for individual and group psychotherapies to reduce provider and programmatic variation.
The redesign creates distinct levels of care based on ASAM criteria, including harm reduction, ambulatory detoxification, outpatient group and individual psychotherapy, an evidence-based Intensive Outpatient Program (IOP), and aftercare. Application of the ASAM standards has allowed clinicians to make accurate placement decisions that best meet individual patient needs and to serve as effective stewards even with limited treatment and financial resources. Although JAHVH does not have a residential SUD program, procedures were developed to refer veterans to community-based residential treatment programs when appropriate.
Group Therapies
With the redesign, SUDS was no longer exclusively a 12-step program; however, it still supported and recognized the value of this approach for some patients. A psychologist periodically audits group sessions to prevent drift from that group’s curriculum. Counselors are assigned to weekly hour-long clinical supervision sessions with a psychologist to review patient care and reinforce the application of evidence-based individualized treatment.
After reviewing empirical literature and VA directives, CBT-SUD was adopted. It encompasses individual and group interventions, such as motivational interviewing (MI), contingency management (CM), and medication-assisted therapies as primary therapeutic treatment modalities, all of which have demonstrated efficacy as measured by length of sobriety postintervention.9,14,15
Clinical Staff Improvements
Staff were reorganized into 3 interdisciplinary treatment teams. A weekly team meeting is scheduled to coordinate care and discuss the treatment of complex patients. Clinical staff focus has shifted from case-management to diagnosis and treatment; now patients are referred to their primary care team’s social worker for case management services. Allowing clinical staff to focus solely on the diagnosis and clinical treatment of SUDs has significantly enhanced productivity and morale.
Staff receive training in the newly adopted interventions during brief monthly refresher courses provided by inhouse psychologists. Additional training includes participation in local and national SUD teleconferences and onsite meetings with experts in harm reduction and motivational interventions. During the transition, clinicians were encouraged to attend staff resiliency training. Continuing education was available to the SUDS psychiatrists and all inpatient and outpatient psychiatrists at JAHVH. Recently, this educational initiative was expanded to include all primary care and inpatient internal medicine physicians.
Implementation
On November 2, 2015, all planned programmatic changes were simultaneously implemented. On that day, clinician and patient schedules changed, the new EBP curriculum was administered, the use of streamlined documentation procedures began, and daily orientation groups followed by same-day evaluations were initiated.
The pretreatment sobriety requirement was eliminated as a barrier to care, and the program began to use a harm-reduction treatment track as recommended by ASAM guidelines. Patients with urgent or emergent medical or psychiatric problems were immediately assessed by SUDS health care providers and treated in the clinic or transported to the emergency department. Previously unavailable, patient access to ambulatory detoxification was initiated. The prescription of buprenorphine/naloxone for the treatment of OUD treatments increased from 1 prescriber to all 3.
Three months after program reorganization, the leadership reviewed overall workflow, conducted patient satisfaction surveys, and evaluated facility use and productivity. To address patient needs and facilitate optimal use of space, the number of same-day evaluation slots was reduced while the number of individual therapy slots was increased.
Staff meet in workgroups to discuss EBPs and further refine content with feedback from the supervisory clinician and team psychologists who routinely audit group therapy sessions. Staff report ongoing benefit from weekly supervision with a clinical psychologist. An inpatient addiction consultation team that uses existing manpower and resources has been developed.
Program Goals and Outcomes
The SUDS program serves more patients at multiple levels of standardized care with 2 fewer full-time positions. One counselor and one advanced practice registered nurse were reallocated to different programs within the JAHVH VA mental health clinic. Following a review of all program clinic profiles in the VA’s Computerized Patient Record System (CPRS) for utilization, accuracy, and necessity, and allowing for accurate program data capture, the transition resulted in a reduction of distinct clinics from 114 to 67 (-58.7%). In fiscal year 2018, review of CPRS yielded 19,786 total visits (3,645 unique visits).
Eliminate Patient Wait Tme
Patient wait time, as measured in CPRS from date of initial evaluation to date of treatment was reduced from an average of 33 days to 0 within 2 weeks of program implementation. A review of CPRS data also indicated that preadmission attrition dropped from 54% to < 1%; all patients desiring treatment are assigned a counselor and treatment is initiated the same day.
Adopt ASAM Criteria
After the redesign, patients have received more appropriate care based on individualized treatment plans. Due to the implementation of a fluid and supportive model, patients can move through levels of care as clinical need dictates rather than failing treatment and having to reengage. Staff receive ongoing education on the use of ASAM. Evaluation and treatment plan templates now reflect assignment to level of care rationale using ASAM guidelines.
Use of Evidence-Based Psychotherapeutic Treatments
More consistent, coordinated, and effective psychotherapies have improved patient care. The program’s previous issues with patients receiving conflicting treatment guidance from different providers has been resolved. Duplicate and ineffective treatments, including multiple readmissions to the IOP level of care, overemphasis of abstinence-based modalities for patients in active use, and referrals to inpatient SUD care under the assumption that “higher level of care is better” have ceased through staff education, leadership support, and appropriate staffing and communication. Review of patient advocate complaints tracked by and resolved by the service demonstrated an 80% decrease in patient advocate complaints regarding SUD clinic services.
Implement Evidence-Based Psychopharmacologic Treatments
The pharmacotherapy education initiative yielded tangible benefits and is likely a significant contributor to the program’s improved clinical outcomes. Prescription of pharmacotherapy for patients with OUD has risen from 8% to 25.1% in eligible patients. Appropriate medication prescription for the treatment of AUD has risen from 1.9% to 9.8% in eligible patients. These data are reflected in the VA Pharmaceutical Drug Safety Initiative (PDSI) dashboard.
Streamline Documentation
Significantly reducing the charting burden was likely a significant contributor to increased provider productivity and improved patient outcomes. Regular meetings between SUDS leadership and clinical informatics ensure that standardized note templates meet hospital policy and gather all necessary accreditation information.
Improve Employee Morale
Increased staff morale is indicated by a noticeable reduction in employee sick days; a decrease of > 20% (over the same time period the previous year), per the VA electronic timekeeping system, during the first 6 months following the November 2 program implementation.
SUDS Inpatient Addiction Consult Team
In January of 2017, SUDS began an inpatient medicine consultation service to offer evaluation, pharmacotherapy, and supportive counseling to patients diagnosed with SUDs who had been admitted to inpatient medical and surgical services. This team includes existing SUDS staff members reallocated to the inpatient service, is led by a SUDS psychiatrist, and includes 3 multidisciplinary clinicians with extensive training in assessment, diagnosis, and treatment planning of SUDs and comorbid conditions. Prior to implementation, the SUDS inpatient addiction consult team met with hospital leadership and attending physicians for inpatient medicine and psychiatry physicians.
To access the SUDS inpatient addiction consult team, physicians request a consult. Patients are offered an evaluation and are assigned to a level of care with orders for outpatient appointments with a counselor and psychiatrist within 7 days of hospital discharge. Medication-assisted treatment for chronic SUDs is implemented while patients remain admitted to the inpatient medical service. In fiscal year 2018, the SUDS inpatient addiction consult team performed 1,428 inpatient evaluations.
Consistent Treatment Outcome Measures
The BAM is a clinical tool designed to measure patient outcomes in substance use disorders.13 Its 17-item scale measures substance use risk factors that may lead to relapse, and protective factors that are recovery-oriented behaviors that help prevent relapse. It demonstrates sensitivity to change and has excellent test-retest reliability. The BAM has been in use in the addictions treatment program since 2011 but was previously administered only after admission to the IOP and again after a 30- to 90-day follow-up period. Since the program redesign, all SUDS patients are administered the BAM at their initial evaluation and at each individual appointment thereafter. The initial BAM assessment encompasses the previous 30 days; this 30-day version is also used for monthly follow-ups. For BAM assessments that occur within 30 days from the time of the last evaluation, a 7-day version is used. Prior to the redesign, about 24% of patients received a follow-up 30-day BAM assessment.12 Per CPRS review of veterans participating in continued treatment, the rate rose to 100% 3 months after the redesign.
When program staff compared preredesign and postredesign BAM data, they detected significant clinical differences. Data demonstrate a 22.2% improvement in protective factors, including patient confidence in their ability to remain abstinent; engaging in self-help activities, such as attending Alcoholics Anonymous meetings; engaging in organized spiritual activities; going to school, working, or volunteering; securing a regular income; and time spent with friends or family who are supportive of recovery.
The data also show a marked reduction in substance use at follow-up points in treatment and a corresponding decrease in risk factors. One item of the BAM assesses patient level of satisfaction with their treatment. Since the redesign, patients report that they are “considerably” satisfied with their SUD treatment.
Currently, program staff are conducting a review of BAM scores by level of care to further parse the impact of various treatments and best target patient need using measurement-based care and EBP, such as contingency management, which provides small monetary incentives when patients maintain clean urine drug screens.16 In addition, the program plans to achieve more uniformity in BAM assessment intervals at all levels of care, and possibly also integrate BAM data into SUD group therapies. Correlation of the BAM scores to other metrics, such as readmission to inpatient medicine, relapse, urine drug screen, or critical laboratory values, will provide additional insight into impact of programmatic changes.
Discussion
Feedback from other clinics and services within the hospital has been very positive. Some providers have reported that they appreciate the ease and availability of access to SUDS. Additionally, patients engaged in treatment prior to the redesign have been contacted for an updated evaluation and assignment to a counselor and appropriate level of care. From the staff’s perspective, the shift to immediate access to care has allowed a more streamlined process with fewer hurdles for patient admission. Staff report that they now feel empowered to meet the needs of veterans in a comprehensive, same-day fashion.
The success of our redesign was contingent on internal and external sta
The successful implementation of these changes has revealed several important elements regarding patient care. The first lesson was that improving access and integrating best practices is possible without additional resources, outside monies, or disruption to patient services. With the support of MH&BSS leadership, the program streamlined existing processes and used both staff and clinic resources more efficiently.
The second lesson involved the importance of continually reviewing and revising standard operating procedures to match the needs of the current patient population. Policies and procedures that once were viewed as potential barriers to change have been replaced with a more flexible approach and willingness to evolve.
As a result, far fewer patients have been lost to treatment. The time and resources that staff historically dedicated to nonclinical patient care are now redirected to immediate service provision. This increase in operational efficiency and treatment efficacy has resulted in a boost to staff morale, even during a time of immense change and increased productivity. Program staff are now able to personally witness the significant changes in their patients’ lives and feel a sense of pride at being a member of a hard-working team that provides the highest quality of substance use treatment. This is critical to job satisfaction and meets the VA mission to provide timely, effective, and evidence-based treatments to patients.
Conclusion
JAHVH strives to continue to provide the highest quality of SUD treatment available. Future directions aim to streamline clinic operations by constantly monitoring and reviewing workloads, while also considering patient feedback. A continuous review of EBP is part of our clinic’s culture. Program leadership endeavors to promote an open environment where providers can share their triumphs and frustrations and foster a team approach to problem solving. Further plans include expanding the range of treatment levels offered by developing a residential SUD treatment facility.
1. Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health: Summary of the Effects of the 2015 NSDUH Questionnaire Redesign: Implications for Data Users. https://www.samhsa.gov/data/sites/default/files/NSDUH-TrendBreak-2015.pdf. Published June 2016. Accessed June 12, 2019.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan PDSKL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96(8):1149-1160.
4. Hser Y, Maglione M, Polinsky ML, Anglin MD. Predicting treatment entry among treatment-seeking drug abusers. J Subst Abuse Treatment. 1997;15(3):213-220.
5. Stark MJ, Campbell BK, Brinkerhoff CV. “Hello, may we help you?” A study of attrition prevention at the time of the first phone contact with substance-abusing clients. Am J Drug Alcohol Abuse. 1990;16:67-76.
6. US Department of Veterans Affairs. Uniform Mental Health Services in VA Medical Centers and Clinics. VHA Handbook 1160.01. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762. Updated November 2015. Accessed December 12, 2017.
7. Veterans Access, Choice and Accountability Act of 2014, 2 USC § 933.
8. DeMarce JM, Gnys M, Raffa SD, Karlin, BE. Cognitive Behavioral Therapy for Substance Use Disorders Among Veterans: Therapist Manual. Washington, DC: US Department of Veterans Affairs; 2014.
9. Mee-Lee D, Shulman GD, Fishman MJ, Gastfriend DR, Miller MM, eds. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions. 3rd ed. Carson City, NV: The Change Companies; 2013.
10. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
11. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for Opioid Use Disorder – Full Document. HHS Publication No. (SMA) 18-5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
12. Winn JL, Shealy SE, Kropp GJ, Felkins-Dohm D, Gonzales-Nolas C, Francis E. Housing assistance and case management: Improving access to substance use disorder treatment for homeless veterans. Psychological Serv. 2013;10(2):233-240.
13. Cacciola JS, Alterman AI, DePhilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treatment. 2013;44(3):256-263.
14. McHugh RK, Hearon BA, Otto MW. Cognitive-behavioral therapy for substance use disorders, Psychiatr Clinics North Am. 2010;33:511–525.
15. Karlin, BE, Cross, G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs health care system. Am Psychol. 2014;69:19-33.
16. DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes, Drug Alcohol Dependence. 2018;185:367-373.
1. Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health: Summary of the Effects of the 2015 NSDUH Questionnaire Redesign: Implications for Data Users. https://www.samhsa.gov/data/sites/default/files/NSDUH-TrendBreak-2015.pdf. Published June 2016. Accessed June 12, 2019.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan PDSKL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96(8):1149-1160.
4. Hser Y, Maglione M, Polinsky ML, Anglin MD. Predicting treatment entry among treatment-seeking drug abusers. J Subst Abuse Treatment. 1997;15(3):213-220.
5. Stark MJ, Campbell BK, Brinkerhoff CV. “Hello, may we help you?” A study of attrition prevention at the time of the first phone contact with substance-abusing clients. Am J Drug Alcohol Abuse. 1990;16:67-76.
6. US Department of Veterans Affairs. Uniform Mental Health Services in VA Medical Centers and Clinics. VHA Handbook 1160.01. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762. Updated November 2015. Accessed December 12, 2017.
7. Veterans Access, Choice and Accountability Act of 2014, 2 USC § 933.
8. DeMarce JM, Gnys M, Raffa SD, Karlin, BE. Cognitive Behavioral Therapy for Substance Use Disorders Among Veterans: Therapist Manual. Washington, DC: US Department of Veterans Affairs; 2014.
9. Mee-Lee D, Shulman GD, Fishman MJ, Gastfriend DR, Miller MM, eds. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions. 3rd ed. Carson City, NV: The Change Companies; 2013.
10. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
11. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for Opioid Use Disorder – Full Document. HHS Publication No. (SMA) 18-5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
12. Winn JL, Shealy SE, Kropp GJ, Felkins-Dohm D, Gonzales-Nolas C, Francis E. Housing assistance and case management: Improving access to substance use disorder treatment for homeless veterans. Psychological Serv. 2013;10(2):233-240.
13. Cacciola JS, Alterman AI, DePhilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treatment. 2013;44(3):256-263.
14. McHugh RK, Hearon BA, Otto MW. Cognitive-behavioral therapy for substance use disorders, Psychiatr Clinics North Am. 2010;33:511–525.
15. Karlin, BE, Cross, G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs health care system. Am Psychol. 2014;69:19-33.
16. DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes, Drug Alcohol Dependence. 2018;185:367-373.
Clinically Impressive Tophaceous Gout With Significant Bony Destruction
Gout is an in inflammatory condition that is generally characterized by red, hot, swollen, and painful joints. The disease is often associated with increased serum uric acid levels; which are considered elevated when they are > 6 mg/dL in women and > 7 mg/dL in men. When gout affects joints, the subchondral bone may be involved, leading to destructive, painful changes. This article presents the case of a patient diagnosed with tophaceous gout of the left second toe with bony erosive changes and calcified nodules noted on magnetic resonance images (MRI).
Case Presentation
A 70-year-old white male presented to the podiatry clinic for a left second-toe mass that was diagnosed as tophaceous gout after being seen by his primary care physician. The patient reported that the mass had slowly grown over the past 10 years. At presentation, he had a 0.2-cm ulcer on the dorsal aspect of the left second-toe mass. The patient stated that the ulcer had recently appeared with some exudate; however, there was no active drainage of material. The patient had a 20-year history of gout that was untreated with dietary modifications or medication. The patient also stated that although the left second-toe mass did not cause any pain on rest, it did cause pain with shoe gear and during ambulation. A community-based podiatrist had recommended amputation of the second toe and as a result the patient was seeking a second opinion at the US Department of Veterans Affairs (VA) Lebanon VA Medical Center (VAMC) in Pennsylvania. The patient had not had acute gouty attacks during the past 10 years.
The patient’s medical history was significant for uncontrolled gout, hyperlipidemia, coronary artery disease with a 4-vessel coronary artery bypass grafting, impaired fasting glucose, prostate cancer that was in remission, alcohol misuse (currently limited to ≤ 2 drinks per night), and 30-year history of cigarette smoking (quit 2 months prior to visit).1,2
At his first visit to the clinic, an examination revealed distinct evidence of bulging of the soft tissues of the second toe of the left foot with a dry sinus tract that was not malodorous (Figure 1). The left second toe was erythematous and edematous. A local increase in skin temperature was present on the second toe of the left foot compared with that of the contralateral foot and other toes. The dorsalis pedis and tibialis posterior pulses were easily palpated, and the capillary return was within normal limits. Palpation of the left second-toe plantar elicited mild tenderness. Crepitation was not present at the left second metatarsophalangeal joint (MPJ) nor at the interphalangeal joint. There was restricted range of motion at the left second MPJ compared with that of the right foot and no motion at the proximal interphalangeal joint. The movement at the left second metatarsophalangeal elicited tenderness. The mass on the left second toe was firm, nonpulsatile, oval-shaped, with a white pigmented consistency that measured 2 cm x 2.5 cm.
There were no deficits present on the neurologic examination, which was noncontributory. There also was no gross evidence of motor weakness. His initial temporal temperature was 98.2° F. The initial laboratory findings were uric acid, 9.5 mg/dL; fasting glucose, 117 g/dL; estimated glomerular filtration rate, 55 mL/min/1.73 m2; erythrocyte sedimentation rate, 6.5 mm/h; and white blood count, 6.6 K/uL.3,4-6
Diagnostic imaging included X-rays of the patient’s feet and a MRI of the left foot. The X-rays showed diffusely osteopenic bones with severe soft tissue swelling surrounding the second proximal interphalangeal joint. Also present was moderate soft tissue swelling at the level of the first metatarsophalangeal joint accompanied by extensive erosions at both of these joints, most pronounced at the second proximal interphalangeal joint. Also, there was narrowing at the first MPJ and the first interphalangeal joint. Erosive changes at the tarsometatarsal articulations and small lucencies within the navicular/midfoot joint were suggestive of additional gouty erosions. A small-to-moderate posterior calcaneal enthesophyte was present as well as a tiny calcaneal enthesophyte (Figure 2).
A MRI showed a destructive soft tissue mass, resulting in overhanging edges, with foci of calcifications centered about the proximal interphalangeal joint of the second toe, which is consistent with a calcified tophaceous gout nodule. The widest dimension of the mass measured 3.2 cm. There also was a less prominent calcified tophaceous gout nodule at the first MPJ. There were additional small punched-out lesions involving the bases of the first through fourth metatarsi and at the distal aspect of the first cuneiform in keeping with gouty arthropathy (Figure 3).4,7-10
The initial treatment plan presented to the patient was to amputate the left second toe. But the patient decided against amputation. Treatment guidelines for allopurinol are to titrate in 100-mg increments every 2 weeks until the serum uric acid levels are consistently < 6, tophi resolve, and the patient should be free of gout attacks.11 We initiated uric acid-lowering therapy with allopurinol at 50 mg/d for 7 days, increasing to 100 mg/d for 7 days, then to 200 mg/d for 10 days. The patient’s serum uric acid level was checked at 200 mg/d. Our patient could not tolerate the allopurinol and decided to discontinue treatment. After 1 year he started having severe pain and returned to have the toe amputated. The patient healed uneventfully.
Discussion
Tophaceous gout is characterized by collections of solid urate accompanied by chronic inflammatory and often destructive changes in the surrounding tissue brought on by periods of increased uric acid levels. Due to the patient’s 20-year history of untreated tophaceous gout, we saw the extent of bony and soft tissue destruction that this pathology created. This patient’s uric acid laboratory value of 9.5 mg/dL was well above the normal reference values of 2.6 to 7.2 mg/dL. The X-rays performed suggested that there was not only bony destruction, but also deformity.
The destruction to the surrounding soft tissues noted as advanced nonhealing wounds formed to the area of the tophi. The size of the second digit also was impressive, causing displacement of the other digits. As stated in the literature, tophaceous gout is usually painless as was the case in our patient. It is the combination of the relatively painless nature of this pathology accompanied by no treatment over many years that led to the patient’s level of deformity and tissue destruction.
Conclusion
We describe a common presentation of bone involvement secondary to significant tophaceous gout in the absence osteomyelitis. The goal of treatment was to maintain a functional foot free of major deformity, pain, or associated risk factors that could lead to a more significant surgical procedure, such as a proximal amputation.11 Given the destructive nature of this pathology, it is important to educate the patient, perform regular examinations, and start medications early to control uric acid levels. These measures will improve the patient’s prognosis and avoid severe sequelae.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155-175.
3. Choi H. Epidemiology of crystal arthropathy. Rheum Dis Clin North Am. 2006;32(2):255-273.
4. Nakayama DA, Barthelemy C, Carrera G, Lightfoot RW Jr, Wortmann RL. Tophaceous gout: a clinical and radiographic assessment. Arthritis Rheum. 1984;27(4):468-471.
5. Dalbeth N, Haskard DO. Pathophysiology of crystal-induced arthritis. In: Wortmann RL, Schumacher HR Jr, Becker MA, Ryan LM, eds. Crystal-induced Arthropathies. New York: Taylor & Francis; 2006.
6. Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum. 2010;62(5):1549-1556.
7. Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW. Tophaceous gout of the spine: MR imaging features. Clin Radiol. 2002;57(10):919-925.
8. Schumacher HR Jr, Becker MA, Edwards NL, et al. Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract. 2006;60(4):408-414.
9. McQueen FM, Doyle A, Dalbeth N. Imaging in the crystal arthropathies. Rheum Dis Clin North Am. 2014;40(2):231-249.
10. Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68(10):1609-1612.
11. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
Gout is an in inflammatory condition that is generally characterized by red, hot, swollen, and painful joints. The disease is often associated with increased serum uric acid levels; which are considered elevated when they are > 6 mg/dL in women and > 7 mg/dL in men. When gout affects joints, the subchondral bone may be involved, leading to destructive, painful changes. This article presents the case of a patient diagnosed with tophaceous gout of the left second toe with bony erosive changes and calcified nodules noted on magnetic resonance images (MRI).
Case Presentation
A 70-year-old white male presented to the podiatry clinic for a left second-toe mass that was diagnosed as tophaceous gout after being seen by his primary care physician. The patient reported that the mass had slowly grown over the past 10 years. At presentation, he had a 0.2-cm ulcer on the dorsal aspect of the left second-toe mass. The patient stated that the ulcer had recently appeared with some exudate; however, there was no active drainage of material. The patient had a 20-year history of gout that was untreated with dietary modifications or medication. The patient also stated that although the left second-toe mass did not cause any pain on rest, it did cause pain with shoe gear and during ambulation. A community-based podiatrist had recommended amputation of the second toe and as a result the patient was seeking a second opinion at the US Department of Veterans Affairs (VA) Lebanon VA Medical Center (VAMC) in Pennsylvania. The patient had not had acute gouty attacks during the past 10 years.
The patient’s medical history was significant for uncontrolled gout, hyperlipidemia, coronary artery disease with a 4-vessel coronary artery bypass grafting, impaired fasting glucose, prostate cancer that was in remission, alcohol misuse (currently limited to ≤ 2 drinks per night), and 30-year history of cigarette smoking (quit 2 months prior to visit).1,2
At his first visit to the clinic, an examination revealed distinct evidence of bulging of the soft tissues of the second toe of the left foot with a dry sinus tract that was not malodorous (Figure 1). The left second toe was erythematous and edematous. A local increase in skin temperature was present on the second toe of the left foot compared with that of the contralateral foot and other toes. The dorsalis pedis and tibialis posterior pulses were easily palpated, and the capillary return was within normal limits. Palpation of the left second-toe plantar elicited mild tenderness. Crepitation was not present at the left second metatarsophalangeal joint (MPJ) nor at the interphalangeal joint. There was restricted range of motion at the left second MPJ compared with that of the right foot and no motion at the proximal interphalangeal joint. The movement at the left second metatarsophalangeal elicited tenderness. The mass on the left second toe was firm, nonpulsatile, oval-shaped, with a white pigmented consistency that measured 2 cm x 2.5 cm.
There were no deficits present on the neurologic examination, which was noncontributory. There also was no gross evidence of motor weakness. His initial temporal temperature was 98.2° F. The initial laboratory findings were uric acid, 9.5 mg/dL; fasting glucose, 117 g/dL; estimated glomerular filtration rate, 55 mL/min/1.73 m2; erythrocyte sedimentation rate, 6.5 mm/h; and white blood count, 6.6 K/uL.3,4-6
Diagnostic imaging included X-rays of the patient’s feet and a MRI of the left foot. The X-rays showed diffusely osteopenic bones with severe soft tissue swelling surrounding the second proximal interphalangeal joint. Also present was moderate soft tissue swelling at the level of the first metatarsophalangeal joint accompanied by extensive erosions at both of these joints, most pronounced at the second proximal interphalangeal joint. Also, there was narrowing at the first MPJ and the first interphalangeal joint. Erosive changes at the tarsometatarsal articulations and small lucencies within the navicular/midfoot joint were suggestive of additional gouty erosions. A small-to-moderate posterior calcaneal enthesophyte was present as well as a tiny calcaneal enthesophyte (Figure 2).
A MRI showed a destructive soft tissue mass, resulting in overhanging edges, with foci of calcifications centered about the proximal interphalangeal joint of the second toe, which is consistent with a calcified tophaceous gout nodule. The widest dimension of the mass measured 3.2 cm. There also was a less prominent calcified tophaceous gout nodule at the first MPJ. There were additional small punched-out lesions involving the bases of the first through fourth metatarsi and at the distal aspect of the first cuneiform in keeping with gouty arthropathy (Figure 3).4,7-10
The initial treatment plan presented to the patient was to amputate the left second toe. But the patient decided against amputation. Treatment guidelines for allopurinol are to titrate in 100-mg increments every 2 weeks until the serum uric acid levels are consistently < 6, tophi resolve, and the patient should be free of gout attacks.11 We initiated uric acid-lowering therapy with allopurinol at 50 mg/d for 7 days, increasing to 100 mg/d for 7 days, then to 200 mg/d for 10 days. The patient’s serum uric acid level was checked at 200 mg/d. Our patient could not tolerate the allopurinol and decided to discontinue treatment. After 1 year he started having severe pain and returned to have the toe amputated. The patient healed uneventfully.
Discussion
Tophaceous gout is characterized by collections of solid urate accompanied by chronic inflammatory and often destructive changes in the surrounding tissue brought on by periods of increased uric acid levels. Due to the patient’s 20-year history of untreated tophaceous gout, we saw the extent of bony and soft tissue destruction that this pathology created. This patient’s uric acid laboratory value of 9.5 mg/dL was well above the normal reference values of 2.6 to 7.2 mg/dL. The X-rays performed suggested that there was not only bony destruction, but also deformity.
The destruction to the surrounding soft tissues noted as advanced nonhealing wounds formed to the area of the tophi. The size of the second digit also was impressive, causing displacement of the other digits. As stated in the literature, tophaceous gout is usually painless as was the case in our patient. It is the combination of the relatively painless nature of this pathology accompanied by no treatment over many years that led to the patient’s level of deformity and tissue destruction.
Conclusion
We describe a common presentation of bone involvement secondary to significant tophaceous gout in the absence osteomyelitis. The goal of treatment was to maintain a functional foot free of major deformity, pain, or associated risk factors that could lead to a more significant surgical procedure, such as a proximal amputation.11 Given the destructive nature of this pathology, it is important to educate the patient, perform regular examinations, and start medications early to control uric acid levels. These measures will improve the patient’s prognosis and avoid severe sequelae.
Gout is an in inflammatory condition that is generally characterized by red, hot, swollen, and painful joints. The disease is often associated with increased serum uric acid levels; which are considered elevated when they are > 6 mg/dL in women and > 7 mg/dL in men. When gout affects joints, the subchondral bone may be involved, leading to destructive, painful changes. This article presents the case of a patient diagnosed with tophaceous gout of the left second toe with bony erosive changes and calcified nodules noted on magnetic resonance images (MRI).
Case Presentation
A 70-year-old white male presented to the podiatry clinic for a left second-toe mass that was diagnosed as tophaceous gout after being seen by his primary care physician. The patient reported that the mass had slowly grown over the past 10 years. At presentation, he had a 0.2-cm ulcer on the dorsal aspect of the left second-toe mass. The patient stated that the ulcer had recently appeared with some exudate; however, there was no active drainage of material. The patient had a 20-year history of gout that was untreated with dietary modifications or medication. The patient also stated that although the left second-toe mass did not cause any pain on rest, it did cause pain with shoe gear and during ambulation. A community-based podiatrist had recommended amputation of the second toe and as a result the patient was seeking a second opinion at the US Department of Veterans Affairs (VA) Lebanon VA Medical Center (VAMC) in Pennsylvania. The patient had not had acute gouty attacks during the past 10 years.
The patient’s medical history was significant for uncontrolled gout, hyperlipidemia, coronary artery disease with a 4-vessel coronary artery bypass grafting, impaired fasting glucose, prostate cancer that was in remission, alcohol misuse (currently limited to ≤ 2 drinks per night), and 30-year history of cigarette smoking (quit 2 months prior to visit).1,2
At his first visit to the clinic, an examination revealed distinct evidence of bulging of the soft tissues of the second toe of the left foot with a dry sinus tract that was not malodorous (Figure 1). The left second toe was erythematous and edematous. A local increase in skin temperature was present on the second toe of the left foot compared with that of the contralateral foot and other toes. The dorsalis pedis and tibialis posterior pulses were easily palpated, and the capillary return was within normal limits. Palpation of the left second-toe plantar elicited mild tenderness. Crepitation was not present at the left second metatarsophalangeal joint (MPJ) nor at the interphalangeal joint. There was restricted range of motion at the left second MPJ compared with that of the right foot and no motion at the proximal interphalangeal joint. The movement at the left second metatarsophalangeal elicited tenderness. The mass on the left second toe was firm, nonpulsatile, oval-shaped, with a white pigmented consistency that measured 2 cm x 2.5 cm.
There were no deficits present on the neurologic examination, which was noncontributory. There also was no gross evidence of motor weakness. His initial temporal temperature was 98.2° F. The initial laboratory findings were uric acid, 9.5 mg/dL; fasting glucose, 117 g/dL; estimated glomerular filtration rate, 55 mL/min/1.73 m2; erythrocyte sedimentation rate, 6.5 mm/h; and white blood count, 6.6 K/uL.3,4-6
Diagnostic imaging included X-rays of the patient’s feet and a MRI of the left foot. The X-rays showed diffusely osteopenic bones with severe soft tissue swelling surrounding the second proximal interphalangeal joint. Also present was moderate soft tissue swelling at the level of the first metatarsophalangeal joint accompanied by extensive erosions at both of these joints, most pronounced at the second proximal interphalangeal joint. Also, there was narrowing at the first MPJ and the first interphalangeal joint. Erosive changes at the tarsometatarsal articulations and small lucencies within the navicular/midfoot joint were suggestive of additional gouty erosions. A small-to-moderate posterior calcaneal enthesophyte was present as well as a tiny calcaneal enthesophyte (Figure 2).
A MRI showed a destructive soft tissue mass, resulting in overhanging edges, with foci of calcifications centered about the proximal interphalangeal joint of the second toe, which is consistent with a calcified tophaceous gout nodule. The widest dimension of the mass measured 3.2 cm. There also was a less prominent calcified tophaceous gout nodule at the first MPJ. There were additional small punched-out lesions involving the bases of the first through fourth metatarsi and at the distal aspect of the first cuneiform in keeping with gouty arthropathy (Figure 3).4,7-10
The initial treatment plan presented to the patient was to amputate the left second toe. But the patient decided against amputation. Treatment guidelines for allopurinol are to titrate in 100-mg increments every 2 weeks until the serum uric acid levels are consistently < 6, tophi resolve, and the patient should be free of gout attacks.11 We initiated uric acid-lowering therapy with allopurinol at 50 mg/d for 7 days, increasing to 100 mg/d for 7 days, then to 200 mg/d for 10 days. The patient’s serum uric acid level was checked at 200 mg/d. Our patient could not tolerate the allopurinol and decided to discontinue treatment. After 1 year he started having severe pain and returned to have the toe amputated. The patient healed uneventfully.
Discussion
Tophaceous gout is characterized by collections of solid urate accompanied by chronic inflammatory and often destructive changes in the surrounding tissue brought on by periods of increased uric acid levels. Due to the patient’s 20-year history of untreated tophaceous gout, we saw the extent of bony and soft tissue destruction that this pathology created. This patient’s uric acid laboratory value of 9.5 mg/dL was well above the normal reference values of 2.6 to 7.2 mg/dL. The X-rays performed suggested that there was not only bony destruction, but also deformity.
The destruction to the surrounding soft tissues noted as advanced nonhealing wounds formed to the area of the tophi. The size of the second digit also was impressive, causing displacement of the other digits. As stated in the literature, tophaceous gout is usually painless as was the case in our patient. It is the combination of the relatively painless nature of this pathology accompanied by no treatment over many years that led to the patient’s level of deformity and tissue destruction.
Conclusion
We describe a common presentation of bone involvement secondary to significant tophaceous gout in the absence osteomyelitis. The goal of treatment was to maintain a functional foot free of major deformity, pain, or associated risk factors that could lead to a more significant surgical procedure, such as a proximal amputation.11 Given the destructive nature of this pathology, it is important to educate the patient, perform regular examinations, and start medications early to control uric acid levels. These measures will improve the patient’s prognosis and avoid severe sequelae.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155-175.
3. Choi H. Epidemiology of crystal arthropathy. Rheum Dis Clin North Am. 2006;32(2):255-273.
4. Nakayama DA, Barthelemy C, Carrera G, Lightfoot RW Jr, Wortmann RL. Tophaceous gout: a clinical and radiographic assessment. Arthritis Rheum. 1984;27(4):468-471.
5. Dalbeth N, Haskard DO. Pathophysiology of crystal-induced arthritis. In: Wortmann RL, Schumacher HR Jr, Becker MA, Ryan LM, eds. Crystal-induced Arthropathies. New York: Taylor & Francis; 2006.
6. Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum. 2010;62(5):1549-1556.
7. Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW. Tophaceous gout of the spine: MR imaging features. Clin Radiol. 2002;57(10):919-925.
8. Schumacher HR Jr, Becker MA, Edwards NL, et al. Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract. 2006;60(4):408-414.
9. McQueen FM, Doyle A, Dalbeth N. Imaging in the crystal arthropathies. Rheum Dis Clin North Am. 2014;40(2):231-249.
10. Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68(10):1609-1612.
11. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155-175.
3. Choi H. Epidemiology of crystal arthropathy. Rheum Dis Clin North Am. 2006;32(2):255-273.
4. Nakayama DA, Barthelemy C, Carrera G, Lightfoot RW Jr, Wortmann RL. Tophaceous gout: a clinical and radiographic assessment. Arthritis Rheum. 1984;27(4):468-471.
5. Dalbeth N, Haskard DO. Pathophysiology of crystal-induced arthritis. In: Wortmann RL, Schumacher HR Jr, Becker MA, Ryan LM, eds. Crystal-induced Arthropathies. New York: Taylor & Francis; 2006.
6. Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum. 2010;62(5):1549-1556.
7. Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW. Tophaceous gout of the spine: MR imaging features. Clin Radiol. 2002;57(10):919-925.
8. Schumacher HR Jr, Becker MA, Edwards NL, et al. Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract. 2006;60(4):408-414.
9. McQueen FM, Doyle A, Dalbeth N. Imaging in the crystal arthropathies. Rheum Dis Clin North Am. 2014;40(2):231-249.
10. Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68(10):1609-1612.
11. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431-1446.