User login
Unacceptable pain despite inflammation control commonly occurs in PsA patients
MADRID – A considerable number of patients with psoriatic arthritis starting their first biologic treatment report unacceptable pain throughout the first year of treatment, even when their inflammation is controlled, according to Swedish researchers.
“Despite this often efficient therapy, 40% of patients still had unacceptable pain after 1 year, and pain with features indicative of a noninflammatory mechanism accounted for more than 60% of this pain load,” senior study author Tor Olofsson, MD, a rheumatologist and doctoral student at Lund (Sweden) University, said in an interview in advance of his presentation at the European Congress of Rheumatology.
“Within rheumatology, today we are generally very good at treating inflammation in many of the arthritides, but we have a lot of patients with persistent pain despite being well treated for their inflammation,” Dr. Olofsson said. “In psoriatic arthritis patients, this remaining pain seems to be even more frequent than in rheumatoid arthritis with the capturing instruments we use here.”
Dr. Olofsson and his colleagues studied prospectively collected records from 352 psoriatic arthritis patients (48% women) participating in the South Swedish Arthritis Group register who started a first anti–tumor necrosis factor (anti-TNF) therapy during 2004-2010. Participants had a mean age of 47 years and a mean disease duration of 10 years. At the start of anti-TNF therapy, 63% of patients were taking methotrexate, and 68% were taking any conventional disease-modifying antirheumatic drug (DMARD).
Based on the Patient Acceptable Symptom State, unacceptable pain was defined as greater than 40 mm on a 0-100 mm Visual Analog Scale (VAS). Inflammation control was captured through C-reactive protein level less than 10 mg/L in combination with one or no swollen joints. Assessments were performed at baseline, 1.5, 3, 6, and 12 months after the start of the first anti-TNF agent. Analyses were also conducted in relation to European League Against Rheumatism (EULAR)–defined treatment response after 3 months (good, moderate, or no response).
At the start of anti-TNF therapy, 85% of patients reported unacceptable pain, which declined to 43% after 3 months and then remained stable, reaching 39% at 12 months. The fraction of patients who had unacceptable pain despite inflammation control was largely unchanged over the study period (24% at treatment start, 27% at 3 months, and 26% at 12 months). Unacceptable pain at 3 months was strongly related to EULAR 3-month response (24% of good responders vs. 79% of nonresponders; P less than .001). This relationship was less pronounced among patients with unacceptable pain despite inflammation control (19% of good responders vs. 37% of nonresponders; P = .016). Among EULAR good responders, unacceptable pain despite inflammation control constituted 81% of all unacceptable pain at 3 months.
Dr. Olofsson said he was surprised by the high levels of pain despite inflammation control reported by these patients. A similar study he and others conducted in rheumatoid arthritis patients a year ago, soon to be published, found that only 12% had unacceptable pain despite inflammation control 1 year after start of a first anti-TNF agent, “so captured by the same instruments, it looks like this problem might be even bigger among patients with psoriatic arthritis.”
There is a possibility that psoriatic arthritis patients may have ongoing pain from low-grade inflammation, he said, but another hypothesis is that many psoriatic arthritis patients develop a more generalized pain condition in line with fibromyalgia. It could be that, if inflammation isn’t treated quickly enough in the beginning of the disease, it could sensitize the central pain system, he said, and it may not be reversible after it has developed.
Alternative treatment strategies are often needed in affected patients, Dr. Olofsson added. This could include regular painkillers or medicines used for more generalized, noninflammatory pain states, such as amitriptyline or duloxetine, as well as nonpharmacologic treatment options.
“The bottom line here is that, if patients are treated aggressively early enough, we might be able to prevent development of this sensitization process,” Dr. Olofsson said. “If we can also do predictive studies to describe which patients have a higher risk of developing this, then maybe we can be even more focused in the initial management before they become centrally sensitized.”
Dr. Olofsson had no financial conflicts to disclose. Two of his coauthors reported relationships with AbbVie, Eli Lilly, Celgene, Novartis, UCB, and Sandoz.
Mitchel L. Zoler contributed to this report.
SOURCE: Roseman C et al. Ann Rheum Dis. 2019 Jun;78(Suppl 2):129-30. Abstract OP0112, doi: 10.1136/annrheumdis-2019-eular.1839.
MADRID – A considerable number of patients with psoriatic arthritis starting their first biologic treatment report unacceptable pain throughout the first year of treatment, even when their inflammation is controlled, according to Swedish researchers.
“Despite this often efficient therapy, 40% of patients still had unacceptable pain after 1 year, and pain with features indicative of a noninflammatory mechanism accounted for more than 60% of this pain load,” senior study author Tor Olofsson, MD, a rheumatologist and doctoral student at Lund (Sweden) University, said in an interview in advance of his presentation at the European Congress of Rheumatology.
“Within rheumatology, today we are generally very good at treating inflammation in many of the arthritides, but we have a lot of patients with persistent pain despite being well treated for their inflammation,” Dr. Olofsson said. “In psoriatic arthritis patients, this remaining pain seems to be even more frequent than in rheumatoid arthritis with the capturing instruments we use here.”
Dr. Olofsson and his colleagues studied prospectively collected records from 352 psoriatic arthritis patients (48% women) participating in the South Swedish Arthritis Group register who started a first anti–tumor necrosis factor (anti-TNF) therapy during 2004-2010. Participants had a mean age of 47 years and a mean disease duration of 10 years. At the start of anti-TNF therapy, 63% of patients were taking methotrexate, and 68% were taking any conventional disease-modifying antirheumatic drug (DMARD).
Based on the Patient Acceptable Symptom State, unacceptable pain was defined as greater than 40 mm on a 0-100 mm Visual Analog Scale (VAS). Inflammation control was captured through C-reactive protein level less than 10 mg/L in combination with one or no swollen joints. Assessments were performed at baseline, 1.5, 3, 6, and 12 months after the start of the first anti-TNF agent. Analyses were also conducted in relation to European League Against Rheumatism (EULAR)–defined treatment response after 3 months (good, moderate, or no response).
At the start of anti-TNF therapy, 85% of patients reported unacceptable pain, which declined to 43% after 3 months and then remained stable, reaching 39% at 12 months. The fraction of patients who had unacceptable pain despite inflammation control was largely unchanged over the study period (24% at treatment start, 27% at 3 months, and 26% at 12 months). Unacceptable pain at 3 months was strongly related to EULAR 3-month response (24% of good responders vs. 79% of nonresponders; P less than .001). This relationship was less pronounced among patients with unacceptable pain despite inflammation control (19% of good responders vs. 37% of nonresponders; P = .016). Among EULAR good responders, unacceptable pain despite inflammation control constituted 81% of all unacceptable pain at 3 months.
Dr. Olofsson said he was surprised by the high levels of pain despite inflammation control reported by these patients. A similar study he and others conducted in rheumatoid arthritis patients a year ago, soon to be published, found that only 12% had unacceptable pain despite inflammation control 1 year after start of a first anti-TNF agent, “so captured by the same instruments, it looks like this problem might be even bigger among patients with psoriatic arthritis.”
There is a possibility that psoriatic arthritis patients may have ongoing pain from low-grade inflammation, he said, but another hypothesis is that many psoriatic arthritis patients develop a more generalized pain condition in line with fibromyalgia. It could be that, if inflammation isn’t treated quickly enough in the beginning of the disease, it could sensitize the central pain system, he said, and it may not be reversible after it has developed.
Alternative treatment strategies are often needed in affected patients, Dr. Olofsson added. This could include regular painkillers or medicines used for more generalized, noninflammatory pain states, such as amitriptyline or duloxetine, as well as nonpharmacologic treatment options.
“The bottom line here is that, if patients are treated aggressively early enough, we might be able to prevent development of this sensitization process,” Dr. Olofsson said. “If we can also do predictive studies to describe which patients have a higher risk of developing this, then maybe we can be even more focused in the initial management before they become centrally sensitized.”
Dr. Olofsson had no financial conflicts to disclose. Two of his coauthors reported relationships with AbbVie, Eli Lilly, Celgene, Novartis, UCB, and Sandoz.
Mitchel L. Zoler contributed to this report.
SOURCE: Roseman C et al. Ann Rheum Dis. 2019 Jun;78(Suppl 2):129-30. Abstract OP0112, doi: 10.1136/annrheumdis-2019-eular.1839.
MADRID – A considerable number of patients with psoriatic arthritis starting their first biologic treatment report unacceptable pain throughout the first year of treatment, even when their inflammation is controlled, according to Swedish researchers.
“Despite this often efficient therapy, 40% of patients still had unacceptable pain after 1 year, and pain with features indicative of a noninflammatory mechanism accounted for more than 60% of this pain load,” senior study author Tor Olofsson, MD, a rheumatologist and doctoral student at Lund (Sweden) University, said in an interview in advance of his presentation at the European Congress of Rheumatology.
“Within rheumatology, today we are generally very good at treating inflammation in many of the arthritides, but we have a lot of patients with persistent pain despite being well treated for their inflammation,” Dr. Olofsson said. “In psoriatic arthritis patients, this remaining pain seems to be even more frequent than in rheumatoid arthritis with the capturing instruments we use here.”
Dr. Olofsson and his colleagues studied prospectively collected records from 352 psoriatic arthritis patients (48% women) participating in the South Swedish Arthritis Group register who started a first anti–tumor necrosis factor (anti-TNF) therapy during 2004-2010. Participants had a mean age of 47 years and a mean disease duration of 10 years. At the start of anti-TNF therapy, 63% of patients were taking methotrexate, and 68% were taking any conventional disease-modifying antirheumatic drug (DMARD).
Based on the Patient Acceptable Symptom State, unacceptable pain was defined as greater than 40 mm on a 0-100 mm Visual Analog Scale (VAS). Inflammation control was captured through C-reactive protein level less than 10 mg/L in combination with one or no swollen joints. Assessments were performed at baseline, 1.5, 3, 6, and 12 months after the start of the first anti-TNF agent. Analyses were also conducted in relation to European League Against Rheumatism (EULAR)–defined treatment response after 3 months (good, moderate, or no response).
At the start of anti-TNF therapy, 85% of patients reported unacceptable pain, which declined to 43% after 3 months and then remained stable, reaching 39% at 12 months. The fraction of patients who had unacceptable pain despite inflammation control was largely unchanged over the study period (24% at treatment start, 27% at 3 months, and 26% at 12 months). Unacceptable pain at 3 months was strongly related to EULAR 3-month response (24% of good responders vs. 79% of nonresponders; P less than .001). This relationship was less pronounced among patients with unacceptable pain despite inflammation control (19% of good responders vs. 37% of nonresponders; P = .016). Among EULAR good responders, unacceptable pain despite inflammation control constituted 81% of all unacceptable pain at 3 months.
Dr. Olofsson said he was surprised by the high levels of pain despite inflammation control reported by these patients. A similar study he and others conducted in rheumatoid arthritis patients a year ago, soon to be published, found that only 12% had unacceptable pain despite inflammation control 1 year after start of a first anti-TNF agent, “so captured by the same instruments, it looks like this problem might be even bigger among patients with psoriatic arthritis.”
There is a possibility that psoriatic arthritis patients may have ongoing pain from low-grade inflammation, he said, but another hypothesis is that many psoriatic arthritis patients develop a more generalized pain condition in line with fibromyalgia. It could be that, if inflammation isn’t treated quickly enough in the beginning of the disease, it could sensitize the central pain system, he said, and it may not be reversible after it has developed.
Alternative treatment strategies are often needed in affected patients, Dr. Olofsson added. This could include regular painkillers or medicines used for more generalized, noninflammatory pain states, such as amitriptyline or duloxetine, as well as nonpharmacologic treatment options.
“The bottom line here is that, if patients are treated aggressively early enough, we might be able to prevent development of this sensitization process,” Dr. Olofsson said. “If we can also do predictive studies to describe which patients have a higher risk of developing this, then maybe we can be even more focused in the initial management before they become centrally sensitized.”
Dr. Olofsson had no financial conflicts to disclose. Two of his coauthors reported relationships with AbbVie, Eli Lilly, Celgene, Novartis, UCB, and Sandoz.
Mitchel L. Zoler contributed to this report.
SOURCE: Roseman C et al. Ann Rheum Dis. 2019 Jun;78(Suppl 2):129-30. Abstract OP0112, doi: 10.1136/annrheumdis-2019-eular.1839.
REPORTING FROM EULAR 2019 CONGRESS
Legislative, educational interventions influenced vaccine status of California kindergartners
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
After California lawmakers implemented policies to limit and eventually eliminate nonmedical exemptions for childhood vaccinations, the proportion of kindergartners who were not up to date for recommended vaccinations fell from 10% in 2013 to 5% in 2017.
At the same time, the
The findings come from an observational study that used cross-sectional school-entry data from 2000 to 2017 to calculate the rates of kindergartners attending California schools who were not up to date on required vaccinations.
“Large-scale vaccination programs that included school-entry mandates have been essential to maintaining high levels of immunization coverage and low rates of vaccine-preventable diseases,” researchers led by S. Cassandra Pingali, MPH, MS, wrote in JAMA. “However, an increasing number of parents are not vaccinating their children over concerns about potential adverse effects. These parental actions threaten the herd immunity established by decades of high vaccine uptake and increase the potential for disease outbreaks.”
Ms. Pingali, of the department of epidemiology at Emory University, Atlanta, and colleagues conducted an observational analysis of California kindergartners who were not up to date on one or more of the required vaccinations during the course of three interventions implemented in the state. The first was Assembly Bill 2109 (AB 2109), which was passed in 2014. It required parents to show proof they had discussed the risks of not vaccinating their children with a health care practitioner before they obtained a personal belief exemption. The second intervention was a campaign carried out in 2015 by the California Department of Public Health and local health departments, designed to educate school staff on the proper application of the conditional admission criteria, which allowed students additional time to catch up on vaccination. The third intervention was the implementation of Senate bill 277 (SB 277), which banned all personal belief exemptions.
Between 2000 and 2017, the researchers reported that the yearly mean kindergarten enrollment in California was 517,962 and the mean number of schools was 7,278. Over this time, the yearly rate of students without up-to-date vaccination status rose from 8% during 2000 to 10% during 2013, before decreasing to 5% during 2017. Ms. Pingali and associates also found that average percentage chance of any within-school contact for a student without up-to-date vaccination status with another student with the same status was 19% during 2000, and increased steadily to 26% during 2014, the first year of AB 2109. The values decreased to 3% (the first year of SB 277), before increasing slightly to 5% during 2017.
“Across the interventions, the percentage of kindergartners attending schools with an up-to-date vaccination status percentage that was greater than the herd immunity threshold also increased for various vaccine-preventable diseases,” the researchers wrote. “Overall, the results suggest that the risk of disease outbreak via potential contact among susceptible children decreased over the course of the interventions.”
The way Matthew M. Davis, MD and Seema K. Shah, JD, see it, the current outbreak of measles in the United States is rooted in the failure of parents to vaccinate their children against the disease based on their beliefs rather than medical contraindications.
“The public health implications of such decisions are amplified because parents who share belief systems about childhood vaccinations tend to congregate socially and residentially, thereby forming clusters of unvaccinated children who are at elevated health risks when exposed to vaccine-preventable diseases,” the authors wrote in an accompanying editorial.
While the study reported by Pingali et al. did not measure actual outbreaks of disease, “reductions in children’s risk of contracting measles are a promising outcome in California resulting from policy changes,” wrote Dr. Davis and Ms. Shah, both of Northwestern University, Chicago (JAMA. 2019;322[1]:33-4). “Yet, because of the ease of domestic and international travel, the mobile nature of young families, and the inability of all states to implement this approach, changes made in each state for nonmedical exemptions may not ensure sufficiently high protection against measles for children across all jurisdictions in the United States. Although states have historically made their own decisions about vaccination exemptions linked to day care or school entry because states exercise primary authority over educational matters, childhood vaccination is a national matter in many respects.”
The best way to remedy the current system failure regarding measles vaccination, they continued, may be to adopt a unified national approach to prohibit nonmedical exemptions. They pointed to the fact that the United States previously achieved virtual eradication of measles as recently as 2000. “Following that achievement, state-level policy changes relaxed immunization requirements and set the stage for progressively larger outbreaks in the United States in recent years. Such system failures result when the products, processes, and people (including the public) that comprise systems do not function or behave in ways that protect health optimally.”
The study was supported by a grant from the National Institutes of Health. One coauthor reported having received consulting fees from Merck and grants from Pfizer and Walgreens. Another reported receiving grants from Pfizer, Merck, GlaxoSmithKline, Sanofi Pasteur, Protein Science, Dynavax, and MedImmune. The remaining coauthors reported no relevant financial disclosures.
The editorialists reported having no financial disclosures.
SOURCE: Pingali SC et al. JAMA. 2019 Jul 2. doi: 10.1001/jama.2019.7924.
FROM JAMA
Claims data suggest endometriosis ups risk of chronic opioid use
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
REPORTING FROM ACOG 2019
Static and Dynamic Functional Connectivity in Migraine
Resting-state functional imaging revealed static functional connectivity and dynamic functional connectivity differences between migraine and persistent post-traumatic headache for regions involved in pain processing, a new study found. The case-control study integrated the static functional connectivity and dynamic functional connectivity patterns of 59 a priori selected regions of interest involved in pain processing. Pairwise connectivity differences between migraine (n=33) and persistent post-traumatic headache (n=44) were determined and compared to healthy controls (n=36) with ANOVA and subsequent t-tests. Researchers found:
- Significant differences in static functional connectivity between migraine and persistent post-traumatic headache were found for 17 region pairs.
- Significant differences in dynamic functional connectivity between migraine and persistent post-traumatic headache were found for 10 region pairs.
- These differences in functional connectivity may be indicative of pathophysiology associated with migraine vs persistent post-traumatic headache.
Dumkrieger G, et al. Static and dynamic functional connectivity differences between migraine and persistent post-traumatic headache: A resting-state magnetic resonance imaging study. [Published online ahead of print May 1, 2019]. Cephalalgia. doi: 10.1177/0333102419847728.
Resting-state functional imaging revealed static functional connectivity and dynamic functional connectivity differences between migraine and persistent post-traumatic headache for regions involved in pain processing, a new study found. The case-control study integrated the static functional connectivity and dynamic functional connectivity patterns of 59 a priori selected regions of interest involved in pain processing. Pairwise connectivity differences between migraine (n=33) and persistent post-traumatic headache (n=44) were determined and compared to healthy controls (n=36) with ANOVA and subsequent t-tests. Researchers found:
- Significant differences in static functional connectivity between migraine and persistent post-traumatic headache were found for 17 region pairs.
- Significant differences in dynamic functional connectivity between migraine and persistent post-traumatic headache were found for 10 region pairs.
- These differences in functional connectivity may be indicative of pathophysiology associated with migraine vs persistent post-traumatic headache.
Dumkrieger G, et al. Static and dynamic functional connectivity differences between migraine and persistent post-traumatic headache: A resting-state magnetic resonance imaging study. [Published online ahead of print May 1, 2019]. Cephalalgia. doi: 10.1177/0333102419847728.
Resting-state functional imaging revealed static functional connectivity and dynamic functional connectivity differences between migraine and persistent post-traumatic headache for regions involved in pain processing, a new study found. The case-control study integrated the static functional connectivity and dynamic functional connectivity patterns of 59 a priori selected regions of interest involved in pain processing. Pairwise connectivity differences between migraine (n=33) and persistent post-traumatic headache (n=44) were determined and compared to healthy controls (n=36) with ANOVA and subsequent t-tests. Researchers found:
- Significant differences in static functional connectivity between migraine and persistent post-traumatic headache were found for 17 region pairs.
- Significant differences in dynamic functional connectivity between migraine and persistent post-traumatic headache were found for 10 region pairs.
- These differences in functional connectivity may be indicative of pathophysiology associated with migraine vs persistent post-traumatic headache.
Dumkrieger G, et al. Static and dynamic functional connectivity differences between migraine and persistent post-traumatic headache: A resting-state magnetic resonance imaging study. [Published online ahead of print May 1, 2019]. Cephalalgia. doi: 10.1177/0333102419847728.
Characteristics of Adolescents with Prolonged Headache
High levels of disability and low quality of life (QOL) were reported among children and adolescents with migraine utilizing infusion treatment, a new study found. Patients aged 6-19 years treated in an outpatient headache infusion center were included. A subset of these patients completed a behavioral health evaluation (treatment group) and were compared with a control group of similar age and gender to patients not seeking infusion treatment. Among the findings:
- 284 patients were included in the study (n=227 treatment group; n=57 controls).
- There was a promising difference in the Pediatric Pain Coping Inventory (PPCI) Distraction subscale, with a mean rank score of 61.90 for the treatment group vs 50.21 for the control group.
- There was a statistically significant difference on the Social Support subscale, with a mean rank score of 65.92 for the treatment group vs 46.26 for the control group.
- Patient-reported data also revealed a significantly higher level of disability among those seeking infusion treatment compared to the non-infusion group.
Woods K, et al. Psychosocial and demographic characteristics of children and adolescents with headache presenting for treatment in a headache infusion center. Headache. 2019;59(6):858-868. doi: 10.1111/head.13537.
High levels of disability and low quality of life (QOL) were reported among children and adolescents with migraine utilizing infusion treatment, a new study found. Patients aged 6-19 years treated in an outpatient headache infusion center were included. A subset of these patients completed a behavioral health evaluation (treatment group) and were compared with a control group of similar age and gender to patients not seeking infusion treatment. Among the findings:
- 284 patients were included in the study (n=227 treatment group; n=57 controls).
- There was a promising difference in the Pediatric Pain Coping Inventory (PPCI) Distraction subscale, with a mean rank score of 61.90 for the treatment group vs 50.21 for the control group.
- There was a statistically significant difference on the Social Support subscale, with a mean rank score of 65.92 for the treatment group vs 46.26 for the control group.
- Patient-reported data also revealed a significantly higher level of disability among those seeking infusion treatment compared to the non-infusion group.
Woods K, et al. Psychosocial and demographic characteristics of children and adolescents with headache presenting for treatment in a headache infusion center. Headache. 2019;59(6):858-868. doi: 10.1111/head.13537.
High levels of disability and low quality of life (QOL) were reported among children and adolescents with migraine utilizing infusion treatment, a new study found. Patients aged 6-19 years treated in an outpatient headache infusion center were included. A subset of these patients completed a behavioral health evaluation (treatment group) and were compared with a control group of similar age and gender to patients not seeking infusion treatment. Among the findings:
- 284 patients were included in the study (n=227 treatment group; n=57 controls).
- There was a promising difference in the Pediatric Pain Coping Inventory (PPCI) Distraction subscale, with a mean rank score of 61.90 for the treatment group vs 50.21 for the control group.
- There was a statistically significant difference on the Social Support subscale, with a mean rank score of 65.92 for the treatment group vs 46.26 for the control group.
- Patient-reported data also revealed a significantly higher level of disability among those seeking infusion treatment compared to the non-infusion group.
Woods K, et al. Psychosocial and demographic characteristics of children and adolescents with headache presenting for treatment in a headache infusion center. Headache. 2019;59(6):858-868. doi: 10.1111/head.13537.
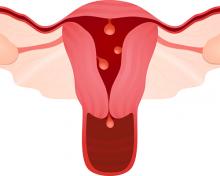
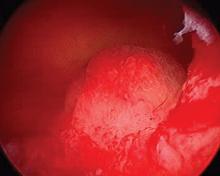
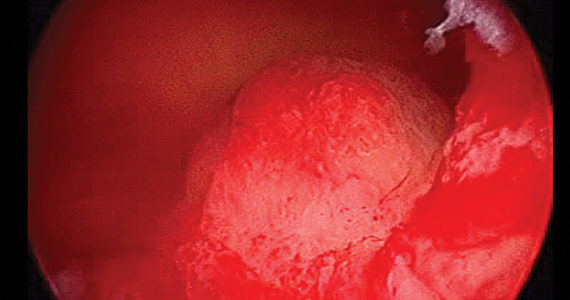
2019 Update on abnormal uterine bleeding
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
VOC-sniffing necklace may support early detection of hypoglycemia
SAN FRANCISCO – according to investigators from Indiana University-Purdue University, Indianapolis.
The device would detect changes in the volatile organic compounds (VOCs) that patients exhale as their plasma glucose level drops. In an insulin clamp study in 11 people with type 1 diabetes, the Indianapolis team found a marked shift in VOCs at a plasma glucose level of 90 mg/dL that persisted all the way down to a level of 50 mg/dL.
The team is now working on a sensor to detect that shift and alert patients. It’s the same trick that diabetes alert dogs do – minus the pup.
The device would be worn like a necklace, and “sense the air around your breath every 15 minutes or so,” said lead investigator Amanda P. Siegel, PhD, an analytical chemist and assistant research professor at the university.
Some continuous glucose monitors already warn of impending hypoglycemia, but the interstitial glucose levels on which they rely lag behind plasma glucose level by about 15 minutes or so. A VOC sniffer offers the hope of a real-time warning, Dr. Siegel said at the annual scientific sessions of the American Diabetes Association.
The team used gas chromatography–mass spectrometry to analyze 94 breath samples from the 11 participants, starting at a median fasting plasma glucose level of 150 mg/dL all the way down to 50 mg/dL, and back up to recovery. Samples collected at the 90-mg/dL and 80-mg/dL levels demonstrated VOC concentrations very similar to those at and below the hypoglycemia threshold of 70 mg/dL.
Even at 90 mg/dL, the VOC profile “looked like patients were already low. These volatile compounds change early” and stay on the breath as plasma glucose drops. “They are different from normal levels for the entire time, and separate out nicely,” Dr. Siegel said.
The team is not saying which volatile compounds are involved while the sensor is under development. They are looking for funding, and if all goes well, they hope to submit a device application to the Food and Drug Administration in 2020.
Other teams are also looking to VOCs to replace poor Fido, but he can alert to hyperglycemia and other problems as well, so his job is safe for now.
The work has been supported by the National Science Foundation. Dr. Siegel did not have any disclosures.
SOURCE: Siegel AP et al. ADA 2019, Abstract 968-P.
SAN FRANCISCO – according to investigators from Indiana University-Purdue University, Indianapolis.
The device would detect changes in the volatile organic compounds (VOCs) that patients exhale as their plasma glucose level drops. In an insulin clamp study in 11 people with type 1 diabetes, the Indianapolis team found a marked shift in VOCs at a plasma glucose level of 90 mg/dL that persisted all the way down to a level of 50 mg/dL.
The team is now working on a sensor to detect that shift and alert patients. It’s the same trick that diabetes alert dogs do – minus the pup.
The device would be worn like a necklace, and “sense the air around your breath every 15 minutes or so,” said lead investigator Amanda P. Siegel, PhD, an analytical chemist and assistant research professor at the university.
Some continuous glucose monitors already warn of impending hypoglycemia, but the interstitial glucose levels on which they rely lag behind plasma glucose level by about 15 minutes or so. A VOC sniffer offers the hope of a real-time warning, Dr. Siegel said at the annual scientific sessions of the American Diabetes Association.
The team used gas chromatography–mass spectrometry to analyze 94 breath samples from the 11 participants, starting at a median fasting plasma glucose level of 150 mg/dL all the way down to 50 mg/dL, and back up to recovery. Samples collected at the 90-mg/dL and 80-mg/dL levels demonstrated VOC concentrations very similar to those at and below the hypoglycemia threshold of 70 mg/dL.
Even at 90 mg/dL, the VOC profile “looked like patients were already low. These volatile compounds change early” and stay on the breath as plasma glucose drops. “They are different from normal levels for the entire time, and separate out nicely,” Dr. Siegel said.
The team is not saying which volatile compounds are involved while the sensor is under development. They are looking for funding, and if all goes well, they hope to submit a device application to the Food and Drug Administration in 2020.
Other teams are also looking to VOCs to replace poor Fido, but he can alert to hyperglycemia and other problems as well, so his job is safe for now.
The work has been supported by the National Science Foundation. Dr. Siegel did not have any disclosures.
SOURCE: Siegel AP et al. ADA 2019, Abstract 968-P.
SAN FRANCISCO – according to investigators from Indiana University-Purdue University, Indianapolis.
The device would detect changes in the volatile organic compounds (VOCs) that patients exhale as their plasma glucose level drops. In an insulin clamp study in 11 people with type 1 diabetes, the Indianapolis team found a marked shift in VOCs at a plasma glucose level of 90 mg/dL that persisted all the way down to a level of 50 mg/dL.
The team is now working on a sensor to detect that shift and alert patients. It’s the same trick that diabetes alert dogs do – minus the pup.
The device would be worn like a necklace, and “sense the air around your breath every 15 minutes or so,” said lead investigator Amanda P. Siegel, PhD, an analytical chemist and assistant research professor at the university.
Some continuous glucose monitors already warn of impending hypoglycemia, but the interstitial glucose levels on which they rely lag behind plasma glucose level by about 15 minutes or so. A VOC sniffer offers the hope of a real-time warning, Dr. Siegel said at the annual scientific sessions of the American Diabetes Association.
The team used gas chromatography–mass spectrometry to analyze 94 breath samples from the 11 participants, starting at a median fasting plasma glucose level of 150 mg/dL all the way down to 50 mg/dL, and back up to recovery. Samples collected at the 90-mg/dL and 80-mg/dL levels demonstrated VOC concentrations very similar to those at and below the hypoglycemia threshold of 70 mg/dL.
Even at 90 mg/dL, the VOC profile “looked like patients were already low. These volatile compounds change early” and stay on the breath as plasma glucose drops. “They are different from normal levels for the entire time, and separate out nicely,” Dr. Siegel said.
The team is not saying which volatile compounds are involved while the sensor is under development. They are looking for funding, and if all goes well, they hope to submit a device application to the Food and Drug Administration in 2020.
Other teams are also looking to VOCs to replace poor Fido, but he can alert to hyperglycemia and other problems as well, so his job is safe for now.
The work has been supported by the National Science Foundation. Dr. Siegel did not have any disclosures.
SOURCE: Siegel AP et al. ADA 2019, Abstract 968-P.
REPORTING FROM ADA 2019
The hospitalist role in treating opioid use disorder
Screen patients at the time of admission
Let’s begin with a brief case. A 25-year-old patient with a history of injection heroin use is in your care. He is admitted for treatment of endocarditis and will remain in the hospital for intravenous antibiotics for several weeks. Over the first few days of hospitalization, he frequently asks for pain medicine, stating that he is in severe pain, withdrawal, and having opioid cravings. On day 3, he leaves the hospital against medical advice. After 2 weeks, he presents to the ED in septic shock and spends several weeks in the ICU. Or, alternatively, he is found down in the community and pronounced dead from a heroin overdose.
These cases occur all too often, and hospitalists across the nation are actively building knowledge and programs to improve care for patients with opioid use disorder (OUD). It is evident that opioid misuse is the public health crisis of our time. In 2017, over 70,000 patients died from an overdose, and over 2 million patients in the United States have a diagnosis of OUD.1,2 Many of these patients interact with the hospital at some point during the course of their illness for management of overdose, withdrawal, and other complications of OUD, including endocarditis, osteomyelitis, and skin and soft tissue infections. Moreover, just 20% of the 580,000 patients hospitalized with OUD in 2015 presented as a direct sequelae of the disease.3 Patients with OUD are often admitted for unrelated reasons, but their addiction goes unaddressed.
Opioid use disorder, like many of the other conditions we see, is a chronic relapsing remitting medical disease and a risk factor for premature mortality. When a patient with diabetes is admitted with cellulitis, we might check an A1C, provide diabetic counseling, and offer evidence-based diabetes treatment, including medications like insulin. We rarely build similar systems of care within the walls of our hospitals to treat OUD like we do for diabetes or other commonly encountered diseases like heart failure and chronic obstructive pulmonary disease.
We should be intentional about separating prevention from treatment. Significant work has gone into reducing the availability of prescription opioids and increasing utilization of prescription drug monitoring programs. As a result, the average morphine milligram equivalent per opioid prescription has decreased since 2010.4 An unintended consequence of restricting legal opioids is potentially pushing patients with opioid addiction towards heroin and fentanyl. Limiting opioid prescriptions alone will only decrease opioid overdose mortality by 5% through 2025.5 Thus, treatment of OUD is critical and something that hospitalists should be trained and engaged in.
Food and Drug Administration–approved OUD treatment includes buprenorphine, methadone, and extended-release naltrexone. Buprenorphine is a partial opioid agonist that treats withdrawal and cravings. Buprenorphine started in the hospital reduces mortality, increases time spent in outpatient treatment after discharge, and reduces opioid-related 30-day readmissions by over 50%.6-8 The number needed to treat with buprenorphine to prevent return to illicit opioid use is two.9 While physicians require an 8-hour “x-waiver” training (physician assistants and nurse practitioners require a 24-hour training) to prescribe buprenorphine for the outpatient treatment of OUD, such certification is not required to order the medication as part of an acute hospitalization.
Hospitalization represents a reachable moment and unique opportunity to start treatment for OUD. Patients are away from triggering environments and surrounded by supportive staff. Unfortunately, up to 30% of these patients leave the hospital against medical advice because of inadequately treated withdrawal, unaddressed cravings, and fear of mistreatment.10 Buprenorphine therapy may help tackle the physiological piece of hospital-based treatment, but we also must work on shifting the culture of our institutions. Importantly, OUD is a medical diagnosis. These patients must receive the same dignity, autonomy, and meaningful care afforded to patients with other medical diagnoses. Patients with OUD are not “addicts,” “abusers,” or “frequent fliers.”
Hospitalists have a clear and compelling role in treating OUD. The National Academy of Medicine recently held a workshop where they compared similarities of the HIV crisis with today’s opioid epidemic. The Academy advocated for the development of hospital-based protocols that empower physicians, physician assistants, and nurse practitioners to integrate the treatment of OUD into their practice.11 Some in our field may feel that treating underlying addiction is a role for behavioral health practitioners. This is akin to having said that HIV specialists should be the only providers to treat patients with HIV during its peak. There are simply not enough psychiatrists or addiction medicine specialists to treat all of the patients who need us during this time of national urgency.
There are several examples of institutions that are laying the groundwork for this important work. The University of California, San Francisco; Oregon Health and Science University, Portland; the University of Colorado at Denver, Aurora; Rush Medical College, Boston; Boston Medical Center; the Icahn School of Medicine at Mount Sinai, New York; and the University of Texas at Austin – to name a few. Offering OUD treatment in the hospital setting must be our new and only acceptable standard of care.
What is next? We can start by screening patients for OUD at the time of admission. This can be accomplished by asking two questions: Does the patient misuse prescription or nonprescription opioids? And if so, does the patient become sick if they abruptly stop? If the patient says yes to both, steps should be taken to provide direct and purposeful care related to OUD. Hospitalists should become familiar with buprenorphine therapy and work to reduce stigma by using people-first language with patients, staff, and in medical documentation.
As a society, we should balance our past focus on optimizing opioid prescribing with current efforts to bolster treatment. To that end, a group of SHM members applied to establish a Substance Use Disorder Special Interest Group, which was recently approved by the SHM board of directors. Details on its rollout will be announced shortly. The intention is that this group will serve as a resource to SHM membership and leadership
As practitioners of hospital medicine, we may not have anticipated playing a direct role in treating patients’ underlying addiction. By empowering hospitalists and wisely using medical hospitalization as a time to treat OUD, we can all have an incredible impact on our patients. Let’s get to work.
Mr. Bottner is a hospitalist at Dell Seton Medical Center, Austin, Texas, and clinical assistant professor at the University of Texas at Austin.
References
1. Katz J. You draw it: Just how bad is the drug overdose epidemic? New York Times. https://www.nytimes.com/interactive/2017/04/14/upshot/drug-overdose-epidemic-you-draw-it.html. Published Oct 26, 2017.
2. National Institute on Drug Abuse. Ohio – Opioid summaries by state. 2018. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/ohio_2018.pdf.
3. Peterson C et al. U.S. hospital discharges documenting patient opioid use disorder without opioid overdose or treatment services, 2011-2015. J Subst Abuse Treat. 2018;92:35-39. doi: 10.1016/j.jsat.2018.06.008.
4. Guy GP. Vital Signs: Changes in opioid prescribing in the United States, 2006-2015. Morb Mortal Wkly Rep. 2017;66. doi: 10.15585/mmwr.mm6626a4.
5. Chen Q et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. doi: 10.1001/jamanetworkopen.2018.7621.
6. Liebschutz J et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-76.
7. Moreno JL et al. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019. doi: 10.1097/ADM.0000000000000499.
8. Larochelle MR et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Ann Intern Med. June 2018. doi: 10.7326/M17-3107.
9. Raleigh MF. Buprenorphine maintenance vs. placebo for opioid dependence. Am Fam Physician. 2017;95(5). https://www.aafp.org/afp/2017/0301/od1.html. Accessed May 12, 2019.
10. Ti L et al. Leaving the hospital against medical advice among people who use illicit drugs: A systematic review. Am J Public Health. 2015;105(12):2587. doi: 10.2105/AJPH.2015.302885a.
11. Springer SAM et al. Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: A call for action after a National Academies of Sciences, Engineering, and Medicine workshop. Ann Intern Med. 2018;169(5):335-6. doi: 10.7326/M18-1203.
Screen patients at the time of admission
Screen patients at the time of admission
Let’s begin with a brief case. A 25-year-old patient with a history of injection heroin use is in your care. He is admitted for treatment of endocarditis and will remain in the hospital for intravenous antibiotics for several weeks. Over the first few days of hospitalization, he frequently asks for pain medicine, stating that he is in severe pain, withdrawal, and having opioid cravings. On day 3, he leaves the hospital against medical advice. After 2 weeks, he presents to the ED in septic shock and spends several weeks in the ICU. Or, alternatively, he is found down in the community and pronounced dead from a heroin overdose.
These cases occur all too often, and hospitalists across the nation are actively building knowledge and programs to improve care for patients with opioid use disorder (OUD). It is evident that opioid misuse is the public health crisis of our time. In 2017, over 70,000 patients died from an overdose, and over 2 million patients in the United States have a diagnosis of OUD.1,2 Many of these patients interact with the hospital at some point during the course of their illness for management of overdose, withdrawal, and other complications of OUD, including endocarditis, osteomyelitis, and skin and soft tissue infections. Moreover, just 20% of the 580,000 patients hospitalized with OUD in 2015 presented as a direct sequelae of the disease.3 Patients with OUD are often admitted for unrelated reasons, but their addiction goes unaddressed.
Opioid use disorder, like many of the other conditions we see, is a chronic relapsing remitting medical disease and a risk factor for premature mortality. When a patient with diabetes is admitted with cellulitis, we might check an A1C, provide diabetic counseling, and offer evidence-based diabetes treatment, including medications like insulin. We rarely build similar systems of care within the walls of our hospitals to treat OUD like we do for diabetes or other commonly encountered diseases like heart failure and chronic obstructive pulmonary disease.
We should be intentional about separating prevention from treatment. Significant work has gone into reducing the availability of prescription opioids and increasing utilization of prescription drug monitoring programs. As a result, the average morphine milligram equivalent per opioid prescription has decreased since 2010.4 An unintended consequence of restricting legal opioids is potentially pushing patients with opioid addiction towards heroin and fentanyl. Limiting opioid prescriptions alone will only decrease opioid overdose mortality by 5% through 2025.5 Thus, treatment of OUD is critical and something that hospitalists should be trained and engaged in.
Food and Drug Administration–approved OUD treatment includes buprenorphine, methadone, and extended-release naltrexone. Buprenorphine is a partial opioid agonist that treats withdrawal and cravings. Buprenorphine started in the hospital reduces mortality, increases time spent in outpatient treatment after discharge, and reduces opioid-related 30-day readmissions by over 50%.6-8 The number needed to treat with buprenorphine to prevent return to illicit opioid use is two.9 While physicians require an 8-hour “x-waiver” training (physician assistants and nurse practitioners require a 24-hour training) to prescribe buprenorphine for the outpatient treatment of OUD, such certification is not required to order the medication as part of an acute hospitalization.
Hospitalization represents a reachable moment and unique opportunity to start treatment for OUD. Patients are away from triggering environments and surrounded by supportive staff. Unfortunately, up to 30% of these patients leave the hospital against medical advice because of inadequately treated withdrawal, unaddressed cravings, and fear of mistreatment.10 Buprenorphine therapy may help tackle the physiological piece of hospital-based treatment, but we also must work on shifting the culture of our institutions. Importantly, OUD is a medical diagnosis. These patients must receive the same dignity, autonomy, and meaningful care afforded to patients with other medical diagnoses. Patients with OUD are not “addicts,” “abusers,” or “frequent fliers.”
Hospitalists have a clear and compelling role in treating OUD. The National Academy of Medicine recently held a workshop where they compared similarities of the HIV crisis with today’s opioid epidemic. The Academy advocated for the development of hospital-based protocols that empower physicians, physician assistants, and nurse practitioners to integrate the treatment of OUD into their practice.11 Some in our field may feel that treating underlying addiction is a role for behavioral health practitioners. This is akin to having said that HIV specialists should be the only providers to treat patients with HIV during its peak. There are simply not enough psychiatrists or addiction medicine specialists to treat all of the patients who need us during this time of national urgency.
There are several examples of institutions that are laying the groundwork for this important work. The University of California, San Francisco; Oregon Health and Science University, Portland; the University of Colorado at Denver, Aurora; Rush Medical College, Boston; Boston Medical Center; the Icahn School of Medicine at Mount Sinai, New York; and the University of Texas at Austin – to name a few. Offering OUD treatment in the hospital setting must be our new and only acceptable standard of care.
What is next? We can start by screening patients for OUD at the time of admission. This can be accomplished by asking two questions: Does the patient misuse prescription or nonprescription opioids? And if so, does the patient become sick if they abruptly stop? If the patient says yes to both, steps should be taken to provide direct and purposeful care related to OUD. Hospitalists should become familiar with buprenorphine therapy and work to reduce stigma by using people-first language with patients, staff, and in medical documentation.
As a society, we should balance our past focus on optimizing opioid prescribing with current efforts to bolster treatment. To that end, a group of SHM members applied to establish a Substance Use Disorder Special Interest Group, which was recently approved by the SHM board of directors. Details on its rollout will be announced shortly. The intention is that this group will serve as a resource to SHM membership and leadership
As practitioners of hospital medicine, we may not have anticipated playing a direct role in treating patients’ underlying addiction. By empowering hospitalists and wisely using medical hospitalization as a time to treat OUD, we can all have an incredible impact on our patients. Let’s get to work.
Mr. Bottner is a hospitalist at Dell Seton Medical Center, Austin, Texas, and clinical assistant professor at the University of Texas at Austin.
References
1. Katz J. You draw it: Just how bad is the drug overdose epidemic? New York Times. https://www.nytimes.com/interactive/2017/04/14/upshot/drug-overdose-epidemic-you-draw-it.html. Published Oct 26, 2017.
2. National Institute on Drug Abuse. Ohio – Opioid summaries by state. 2018. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/ohio_2018.pdf.
3. Peterson C et al. U.S. hospital discharges documenting patient opioid use disorder without opioid overdose or treatment services, 2011-2015. J Subst Abuse Treat. 2018;92:35-39. doi: 10.1016/j.jsat.2018.06.008.
4. Guy GP. Vital Signs: Changes in opioid prescribing in the United States, 2006-2015. Morb Mortal Wkly Rep. 2017;66. doi: 10.15585/mmwr.mm6626a4.
5. Chen Q et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. doi: 10.1001/jamanetworkopen.2018.7621.
6. Liebschutz J et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-76.
7. Moreno JL et al. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019. doi: 10.1097/ADM.0000000000000499.
8. Larochelle MR et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Ann Intern Med. June 2018. doi: 10.7326/M17-3107.
9. Raleigh MF. Buprenorphine maintenance vs. placebo for opioid dependence. Am Fam Physician. 2017;95(5). https://www.aafp.org/afp/2017/0301/od1.html. Accessed May 12, 2019.
10. Ti L et al. Leaving the hospital against medical advice among people who use illicit drugs: A systematic review. Am J Public Health. 2015;105(12):2587. doi: 10.2105/AJPH.2015.302885a.
11. Springer SAM et al. Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: A call for action after a National Academies of Sciences, Engineering, and Medicine workshop. Ann Intern Med. 2018;169(5):335-6. doi: 10.7326/M18-1203.
Let’s begin with a brief case. A 25-year-old patient with a history of injection heroin use is in your care. He is admitted for treatment of endocarditis and will remain in the hospital for intravenous antibiotics for several weeks. Over the first few days of hospitalization, he frequently asks for pain medicine, stating that he is in severe pain, withdrawal, and having opioid cravings. On day 3, he leaves the hospital against medical advice. After 2 weeks, he presents to the ED in septic shock and spends several weeks in the ICU. Or, alternatively, he is found down in the community and pronounced dead from a heroin overdose.
These cases occur all too often, and hospitalists across the nation are actively building knowledge and programs to improve care for patients with opioid use disorder (OUD). It is evident that opioid misuse is the public health crisis of our time. In 2017, over 70,000 patients died from an overdose, and over 2 million patients in the United States have a diagnosis of OUD.1,2 Many of these patients interact with the hospital at some point during the course of their illness for management of overdose, withdrawal, and other complications of OUD, including endocarditis, osteomyelitis, and skin and soft tissue infections. Moreover, just 20% of the 580,000 patients hospitalized with OUD in 2015 presented as a direct sequelae of the disease.3 Patients with OUD are often admitted for unrelated reasons, but their addiction goes unaddressed.
Opioid use disorder, like many of the other conditions we see, is a chronic relapsing remitting medical disease and a risk factor for premature mortality. When a patient with diabetes is admitted with cellulitis, we might check an A1C, provide diabetic counseling, and offer evidence-based diabetes treatment, including medications like insulin. We rarely build similar systems of care within the walls of our hospitals to treat OUD like we do for diabetes or other commonly encountered diseases like heart failure and chronic obstructive pulmonary disease.
We should be intentional about separating prevention from treatment. Significant work has gone into reducing the availability of prescription opioids and increasing utilization of prescription drug monitoring programs. As a result, the average morphine milligram equivalent per opioid prescription has decreased since 2010.4 An unintended consequence of restricting legal opioids is potentially pushing patients with opioid addiction towards heroin and fentanyl. Limiting opioid prescriptions alone will only decrease opioid overdose mortality by 5% through 2025.5 Thus, treatment of OUD is critical and something that hospitalists should be trained and engaged in.
Food and Drug Administration–approved OUD treatment includes buprenorphine, methadone, and extended-release naltrexone. Buprenorphine is a partial opioid agonist that treats withdrawal and cravings. Buprenorphine started in the hospital reduces mortality, increases time spent in outpatient treatment after discharge, and reduces opioid-related 30-day readmissions by over 50%.6-8 The number needed to treat with buprenorphine to prevent return to illicit opioid use is two.9 While physicians require an 8-hour “x-waiver” training (physician assistants and nurse practitioners require a 24-hour training) to prescribe buprenorphine for the outpatient treatment of OUD, such certification is not required to order the medication as part of an acute hospitalization.
Hospitalization represents a reachable moment and unique opportunity to start treatment for OUD. Patients are away from triggering environments and surrounded by supportive staff. Unfortunately, up to 30% of these patients leave the hospital against medical advice because of inadequately treated withdrawal, unaddressed cravings, and fear of mistreatment.10 Buprenorphine therapy may help tackle the physiological piece of hospital-based treatment, but we also must work on shifting the culture of our institutions. Importantly, OUD is a medical diagnosis. These patients must receive the same dignity, autonomy, and meaningful care afforded to patients with other medical diagnoses. Patients with OUD are not “addicts,” “abusers,” or “frequent fliers.”
Hospitalists have a clear and compelling role in treating OUD. The National Academy of Medicine recently held a workshop where they compared similarities of the HIV crisis with today’s opioid epidemic. The Academy advocated for the development of hospital-based protocols that empower physicians, physician assistants, and nurse practitioners to integrate the treatment of OUD into their practice.11 Some in our field may feel that treating underlying addiction is a role for behavioral health practitioners. This is akin to having said that HIV specialists should be the only providers to treat patients with HIV during its peak. There are simply not enough psychiatrists or addiction medicine specialists to treat all of the patients who need us during this time of national urgency.
There are several examples of institutions that are laying the groundwork for this important work. The University of California, San Francisco; Oregon Health and Science University, Portland; the University of Colorado at Denver, Aurora; Rush Medical College, Boston; Boston Medical Center; the Icahn School of Medicine at Mount Sinai, New York; and the University of Texas at Austin – to name a few. Offering OUD treatment in the hospital setting must be our new and only acceptable standard of care.
What is next? We can start by screening patients for OUD at the time of admission. This can be accomplished by asking two questions: Does the patient misuse prescription or nonprescription opioids? And if so, does the patient become sick if they abruptly stop? If the patient says yes to both, steps should be taken to provide direct and purposeful care related to OUD. Hospitalists should become familiar with buprenorphine therapy and work to reduce stigma by using people-first language with patients, staff, and in medical documentation.
As a society, we should balance our past focus on optimizing opioid prescribing with current efforts to bolster treatment. To that end, a group of SHM members applied to establish a Substance Use Disorder Special Interest Group, which was recently approved by the SHM board of directors. Details on its rollout will be announced shortly. The intention is that this group will serve as a resource to SHM membership and leadership
As practitioners of hospital medicine, we may not have anticipated playing a direct role in treating patients’ underlying addiction. By empowering hospitalists and wisely using medical hospitalization as a time to treat OUD, we can all have an incredible impact on our patients. Let’s get to work.
Mr. Bottner is a hospitalist at Dell Seton Medical Center, Austin, Texas, and clinical assistant professor at the University of Texas at Austin.
References
1. Katz J. You draw it: Just how bad is the drug overdose epidemic? New York Times. https://www.nytimes.com/interactive/2017/04/14/upshot/drug-overdose-epidemic-you-draw-it.html. Published Oct 26, 2017.
2. National Institute on Drug Abuse. Ohio – Opioid summaries by state. 2018. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/ohio_2018.pdf.
3. Peterson C et al. U.S. hospital discharges documenting patient opioid use disorder without opioid overdose or treatment services, 2011-2015. J Subst Abuse Treat. 2018;92:35-39. doi: 10.1016/j.jsat.2018.06.008.
4. Guy GP. Vital Signs: Changes in opioid prescribing in the United States, 2006-2015. Morb Mortal Wkly Rep. 2017;66. doi: 10.15585/mmwr.mm6626a4.
5. Chen Q et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. doi: 10.1001/jamanetworkopen.2018.7621.
6. Liebschutz J et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-76.
7. Moreno JL et al. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019. doi: 10.1097/ADM.0000000000000499.
8. Larochelle MR et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Ann Intern Med. June 2018. doi: 10.7326/M17-3107.
9. Raleigh MF. Buprenorphine maintenance vs. placebo for opioid dependence. Am Fam Physician. 2017;95(5). https://www.aafp.org/afp/2017/0301/od1.html. Accessed May 12, 2019.
10. Ti L et al. Leaving the hospital against medical advice among people who use illicit drugs: A systematic review. Am J Public Health. 2015;105(12):2587. doi: 10.2105/AJPH.2015.302885a.
11. Springer SAM et al. Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: A call for action after a National Academies of Sciences, Engineering, and Medicine workshop. Ann Intern Med. 2018;169(5):335-6. doi: 10.7326/M18-1203.
Same-day discharge after elective PCI has increased value and patient satisfaction
Background: SDDs are as safe as non-SDDs (NSDDs) in patients after elective PCI, yet there has been only a modest increase in SDD.
Study design: Observational cross-sectional cohort study.
Setting: 493 hospitals in the United States.
Synopsis: With use of the national Premier Healthcare Database, 672,470 elective PCIs from January 2006 to December 2015 with 1-year follow-up showed a wide variation in SDD from 0% to 83% among hospitals with the overall corrected rate of 3.5%. Low-volume PCI hospitals did not increase the rate. Additionally, the cost of SDD patients was $5,128 less than NSDD patients. There was cost saving even with higher-risk transfemoral approaches and patients needing periprocedural hemodynamic or ventilatory support. Complications (death, bleeding, acute kidney injury, or acute MI at 30, 90, and 365 days) were not higher for SDD than for NSDD patients.
Limitations include that 2015 data may not reflect current practices. ICD 9 codes used for obtaining complications data can be misclassified. Cost savings are variable. Patients with periprocedural complications were not candidates for SDD but were included in the data. The study does not account for variation in technique, PCI characteristics, or SDD criteria of hospitals.
Bottom line: Prevalence of SDDs for elective PCI patients varies by institution and is an underutilized opportunity to significantly reduce hospital costs and increase patient satisfaction while maintaining the safety of patients.
Citation: Amin AP et al. Association of same-day discharge after elective percutaneous coronary intervention in the United States with costs and outcomes. JAMA Cardiol. Published online 2018 Sep 26. doi: 10.1001/jamacardio.2018.3029.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: SDDs are as safe as non-SDDs (NSDDs) in patients after elective PCI, yet there has been only a modest increase in SDD.
Study design: Observational cross-sectional cohort study.
Setting: 493 hospitals in the United States.
Synopsis: With use of the national Premier Healthcare Database, 672,470 elective PCIs from January 2006 to December 2015 with 1-year follow-up showed a wide variation in SDD from 0% to 83% among hospitals with the overall corrected rate of 3.5%. Low-volume PCI hospitals did not increase the rate. Additionally, the cost of SDD patients was $5,128 less than NSDD patients. There was cost saving even with higher-risk transfemoral approaches and patients needing periprocedural hemodynamic or ventilatory support. Complications (death, bleeding, acute kidney injury, or acute MI at 30, 90, and 365 days) were not higher for SDD than for NSDD patients.
Limitations include that 2015 data may not reflect current practices. ICD 9 codes used for obtaining complications data can be misclassified. Cost savings are variable. Patients with periprocedural complications were not candidates for SDD but were included in the data. The study does not account for variation in technique, PCI characteristics, or SDD criteria of hospitals.
Bottom line: Prevalence of SDDs for elective PCI patients varies by institution and is an underutilized opportunity to significantly reduce hospital costs and increase patient satisfaction while maintaining the safety of patients.
Citation: Amin AP et al. Association of same-day discharge after elective percutaneous coronary intervention in the United States with costs and outcomes. JAMA Cardiol. Published online 2018 Sep 26. doi: 10.1001/jamacardio.2018.3029.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: SDDs are as safe as non-SDDs (NSDDs) in patients after elective PCI, yet there has been only a modest increase in SDD.
Study design: Observational cross-sectional cohort study.
Setting: 493 hospitals in the United States.
Synopsis: With use of the national Premier Healthcare Database, 672,470 elective PCIs from January 2006 to December 2015 with 1-year follow-up showed a wide variation in SDD from 0% to 83% among hospitals with the overall corrected rate of 3.5%. Low-volume PCI hospitals did not increase the rate. Additionally, the cost of SDD patients was $5,128 less than NSDD patients. There was cost saving even with higher-risk transfemoral approaches and patients needing periprocedural hemodynamic or ventilatory support. Complications (death, bleeding, acute kidney injury, or acute MI at 30, 90, and 365 days) were not higher for SDD than for NSDD patients.
Limitations include that 2015 data may not reflect current practices. ICD 9 codes used for obtaining complications data can be misclassified. Cost savings are variable. Patients with periprocedural complications were not candidates for SDD but were included in the data. The study does not account for variation in technique, PCI characteristics, or SDD criteria of hospitals.
Bottom line: Prevalence of SDDs for elective PCI patients varies by institution and is an underutilized opportunity to significantly reduce hospital costs and increase patient satisfaction while maintaining the safety of patients.
Citation: Amin AP et al. Association of same-day discharge after elective percutaneous coronary intervention in the United States with costs and outcomes. JAMA Cardiol. Published online 2018 Sep 26. doi: 10.1001/jamacardio.2018.3029.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
‘Tis the season … for fireworks injuries
according to the Consumer Product Safety Commission.
Of the estimated 9,100 fireworks-related injuries treated in emergency departments last year, 5,600 (62%) occurred between June 22 and July 22, 2018. That works out to a rate of 1.7 ED-treated injuries per 100,000 people for that 1 month and a rate of 2.8 per 100,000 for the entire year, the CPSC said in its 2018 Fireworks Annual Report.
Children had higher injury rates than adults in the Fourth of July window, and those aged 10-14 years had the highest rate of all, 5.2 injuries per 100,000 population. They were followed by teens aged 15-19 years (3.1 per 100,000) and children aged 5-9 (2.7 per 100,000), the CPSC investigators said based on data from the National Electronic Injury Surveillance System.
A deeper dive into the data pool shows that firecrackers caused more injuries – 19% of the total for the month – than any other type of firework device (reloadable shells were second at 12%). Burns were the most common type of injury, making up 44% of the total, and hands and fingers were the body parts most often injured (28% of the total), they reported.
There were five fireworks-related deaths last year – below the average of 7.6 per year since 2003 – but the total for 2018 may go up because reporting for the year is not yet complete. In one of the 2018 cases, an 18-year-old taped a tube to a football helmet and tried to launch a mortar shell while wearing the helmet. The first one worked, but the second shell got stuck and exploded in the tube, the CPSC said.
“CPSC works year-round to help prevent deaths and injuries from fireworks, by verifying fireworks meet safety regulations in our ports, marketplace, and on the road,” acting CPSC Chairman Ann Marie Buerkle said in a written statement. “Beyond CPSC’s efforts, we want to make sure everyone takes simple safety steps to celebrate safely with their family and friends.”
according to the Consumer Product Safety Commission.

Of the estimated 9,100 fireworks-related injuries treated in emergency departments last year, 5,600 (62%) occurred between June 22 and July 22, 2018. That works out to a rate of 1.7 ED-treated injuries per 100,000 people for that 1 month and a rate of 2.8 per 100,000 for the entire year, the CPSC said in its 2018 Fireworks Annual Report.
Children had higher injury rates than adults in the Fourth of July window, and those aged 10-14 years had the highest rate of all, 5.2 injuries per 100,000 population. They were followed by teens aged 15-19 years (3.1 per 100,000) and children aged 5-9 (2.7 per 100,000), the CPSC investigators said based on data from the National Electronic Injury Surveillance System.
A deeper dive into the data pool shows that firecrackers caused more injuries – 19% of the total for the month – than any other type of firework device (reloadable shells were second at 12%). Burns were the most common type of injury, making up 44% of the total, and hands and fingers were the body parts most often injured (28% of the total), they reported.
There were five fireworks-related deaths last year – below the average of 7.6 per year since 2003 – but the total for 2018 may go up because reporting for the year is not yet complete. In one of the 2018 cases, an 18-year-old taped a tube to a football helmet and tried to launch a mortar shell while wearing the helmet. The first one worked, but the second shell got stuck and exploded in the tube, the CPSC said.
“CPSC works year-round to help prevent deaths and injuries from fireworks, by verifying fireworks meet safety regulations in our ports, marketplace, and on the road,” acting CPSC Chairman Ann Marie Buerkle said in a written statement. “Beyond CPSC’s efforts, we want to make sure everyone takes simple safety steps to celebrate safely with their family and friends.”
according to the Consumer Product Safety Commission.

Of the estimated 9,100 fireworks-related injuries treated in emergency departments last year, 5,600 (62%) occurred between June 22 and July 22, 2018. That works out to a rate of 1.7 ED-treated injuries per 100,000 people for that 1 month and a rate of 2.8 per 100,000 for the entire year, the CPSC said in its 2018 Fireworks Annual Report.
Children had higher injury rates than adults in the Fourth of July window, and those aged 10-14 years had the highest rate of all, 5.2 injuries per 100,000 population. They were followed by teens aged 15-19 years (3.1 per 100,000) and children aged 5-9 (2.7 per 100,000), the CPSC investigators said based on data from the National Electronic Injury Surveillance System.
A deeper dive into the data pool shows that firecrackers caused more injuries – 19% of the total for the month – than any other type of firework device (reloadable shells were second at 12%). Burns were the most common type of injury, making up 44% of the total, and hands and fingers were the body parts most often injured (28% of the total), they reported.
There were five fireworks-related deaths last year – below the average of 7.6 per year since 2003 – but the total for 2018 may go up because reporting for the year is not yet complete. In one of the 2018 cases, an 18-year-old taped a tube to a football helmet and tried to launch a mortar shell while wearing the helmet. The first one worked, but the second shell got stuck and exploded in the tube, the CPSC said.
“CPSC works year-round to help prevent deaths and injuries from fireworks, by verifying fireworks meet safety regulations in our ports, marketplace, and on the road,” acting CPSC Chairman Ann Marie Buerkle said in a written statement. “Beyond CPSC’s efforts, we want to make sure everyone takes simple safety steps to celebrate safely with their family and friends.”