User login
Population Management of Nonalcoholic Fatty Liver Disease
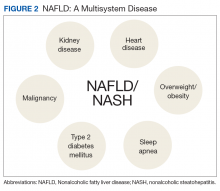
Nonalcoholic fatty liver disease (NAFLD) is an umbrella term that covers a spectrum of phenotypes ranging from nonalcoholic fatty liver or simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) defined by histologic findings of steatosis, lobular inflammation, cytologic ballooning, and some degree of fibrosis.1 While frequently observed in patients with at least 1 risk factor (eg, obesity, diabetes mellitus [DM], dyslipidemia, hypertension), NAFLD also is an independent risk factor for type 2 DM (T2DM), chronic kidney disease, and cardiovascular disease.2 At early disease stages with absence of liver fibrosis, mortality is linked to cardiovascular and not liver disease. However, in the presence of NASH, fibrosis progression to liver cirrhosis, or hepatocellular carcinoma (HCC) represent the most important liver-related outcomes that determine morbidity and mortality.3 Mirroring the obesity and T2DM epidemics, the health care burden is projected to dramatically rise.
In the following article, we will discuss how the Veterans Health Administration (VHA) is well positioned to implement an organizational strategy of comprehensive care for veterans with NAFLD. This comprehensive care strategy should include the development of a NAFLD clinic offering care for comorbid conditions frequently present in these patients, point-of-care testing, access to clinical trials, and outcomes monitoring as a key performance target for providers and the respective facility.
NAFLD disease burden
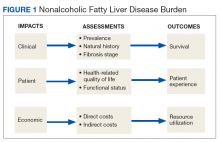
To fully appreciate the burden of a chronic disease like NAFLD, it is important to assess its long- and short-term consequences in a comprehensive manner with regard to its clinical impact, impact on the patient, and economic impact (Figure 1).
Clinical Impact
Clinical impact is assessed based on the prevalence and natural history of NAFLD and the liver fibrosis stage and determines patient survival. Coinciding with the epidemic of obesity and T2DM, the prevalence of NAFLD in the general population in North America is 24% and even higher with older age and higher body mass index (BMI).4,5 The prevalence for NAFLD is particularly high in patients with T2DM (47%). Of patients with T2DM and NAFLD, 65% have biopsy-proven NASH of which 15% have bridging fibrosis or liver cirrhosis.6
NAFLD is the fastest growing cause of cirrhosis in the US with a forecasted NAFLD population of 101 million by 2030.7 At the same time, the number of patients with NASH will rise to 27 million of which > 7 million will have bridging fibrosis or liver cirrhosis; hepatic decompensation events are estimated to occur in 105,430 patients with liver cirrhosis, posing a major public health threat related to organ availability for liver transplantation.8 Since 2013, NAFLD has been the second leading cause for liver transplantation and the top reason for transplantation in patients aged < 50 years.9,10 As many patients with NAFLD are diagnosed with HCC at stages where liver transplantation is not an option, mortality from HCC in NAFLD patients is higher than with other etiologies as treatment options are restricted.11,12
Compared with that of the general population, veterans seeking care are older and sicker with 43% of veterans taking > 5 prescribed medications.13 Of those receiving VHA care, 6.6 million veterans are either overweight or obese; 165,000 are morbidly obese with a BMI > 40.14 In addition, veterans are 2.5 times more likely to have T2DM compared with that of nonveterans. Because T2DM and obesity are the most common risk factors for NAFLD, it is not surprising that NAFLD prevalence among veterans rose 3-fold from 2003 to 2011.15 It is now estimated that 540,000 veterans will progress to NASH and 108,000 will develop bridging fibrosis or liver cirrhosis by 2030.8 Similar to that of the general population, liver cirrhosis is attributed to NAFLD in 15% of veterans.15,16 NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 years and 70 years, respectively.16,17 Shockingly, 20% of HCCs were not linked to liver cirrhosis and escaped recommended HCC screening for patients with cirrhosis.18,19
Patient Impact
Assessment of disease burden should not be restricted to clinical outcomes as patients can experience a range of symptoms that may have significant impact on their health-related quality of life (QOL) and functional status.20 Using general but not disease-specific instruments, NAFLD patients reported outcomes score low regarding fatigue, activity, and emotions.21 More disease-specific questionnaires may provide better and disease-specific insights as how NASH impacts patients’ QOL.22-24
Economic Impact
There is mounting evidence that the clinical implications of NAFLD directly influence the economic burden of NAFLD.25 The annual burden associated with all incident and prevalent NAFLD cases in the US has been estimated at $103 billion, and projections suggest that the expected 10-year burden of NAFLD may increase to $1.005 trillion.26 It is anticipated that increased NAFLD costs will affect the VHA with billions of dollars in annual expenditures in addition to the $1.5 billion already spent annually for T2DM care (4% of the VA pharmacy budget is spent on T2DM treatment).27-29
Current Patient Care
Obesity, DM, and dyslipidemia are common conditions managed by primary care providers (PCPs). Given the close association of these conditions with NAFLD, the PCP is often the first point of medical contact for patients with or at risk for NAFLD.30 For that reason, PCP awareness of NAFLD is critical for effective management of these patients. PCPs should be actively involved in the management of patients with NAFLD with pathways in place for identifying patients at high risk of liver disease for timely referral to a specialist and adequate education on the follow-up and treatment of low-risk patients. Instead, diagnosis of NAFLD is primarily triggered by either abnormal aminotransferases or detection of steatosis on imaging performed for other indications.
Barriers to optimal management of NAFLD by PCPs have been identified and occur at different levels of patient care. In the absence of clinical practice guidelines by the American Association of Family Practice covering NAFLD and a substantial latency period without signs of symptoms, NAFLD may not be perceived as a potentially serious condition by PCPs and their patients; interestingly this holds true even for some medical specialties.31-39 More than half of PCPs do not test their patients at highest risk for NAFLD (eg, patients with obesity or T2DM) and may be unaware of practice guidelines.40-42
Guidelines from Europe and the US are not completely in accordance. The US guidelines are vague regarding screening and are supported by only 1 medical society, due to the lack of NASH-specific drug therapies. The European guidelines are built on the support of 3 different stakeholders covering liver diseases, obesity, and DM and the experience using noninvasive liver fibrosis assessments for patients with NAFLD. To overcome this apparent conflict, a more practical and risk-stratified approach is warranted.41,42
Making the diagnosis can be challenging in cases with competing etiologies, such as T2DM and alcohol misuse. There also is an overreliance on aminotransferase levels to diagnose NAFLD. Significant liver disease can exist in the presence of normal aminotransferases, and this may be attributed to either spontaneous aminotransferase fluctuations or upper limits of normal that have been chosen too high.43-47 Often additional workup by PCPs depends on the magnitude of aminotransferase abnormalities.
Even if NAFLD has been diagnosed by PCPs, identifying those with NASH is hindered by the absence of an accurate noninvasive diagnostic method and the need to perform a liver biopsy. Liver biopsy is often not considered or delayed to monitor patients with serial aminotransferases, regardless of the patient’s metabolic comorbidity profile or baseline aminotransferases.32 As a result, referral to a specialist often depends on the magnitude of the aminotransferase abnormality,30,48 and often occurs when advanced liver disease is already present.49 Finally, providers may not be aware of beneficial effects of lifestyle interventions and certain medications, including statins on NASH and liver fibrosis.50-53 As NAFLD is associated with excess cardiovascular- and cancer-related morbidity and mortality, it is possible that regression of NAFLD may improve associated risk for these outcomes as well.
Framework for Comprehensive NAFLD Care
Chronic liver diseases and associated comorbidities have long been addressed by PCPs and specialty providers working in isolation and within the narrow focus of each discipline. Contrary to working in silos of the past, a coordinated management strategy with other disciplines that cover these comorbidities needs to be established, or alternatively the PCP must be aware of the management of comorbidities to execute them independently. Integration of hepatology-driven NAFLD care with other specialties involves communication, collaboration, and sharing of resources and expertise that will address patient care needs. Obviously, this cannot be undertaken in a single outpatient visit and requires vertical and longitudinal follow-up over time. One important aspect of comprehensive NAFLD care is the targeting of a particular patient population rather than being seen as a panacea for all; cost-utility analysis is hampered by uncertainties around accuracy of noninvasive biomarkers reflecting liver injury and a lack of effectiveness data for treatment. However, it seems reasonable to screen patients at high risk for NASH and adverse clinical outcomes. Such a risk stratification approach should be cost-effective.
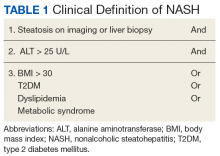
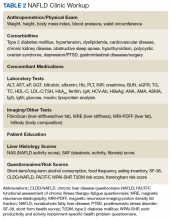
A first key step by the PCP is to identify whether a patient is at risk, especially patients with NASH. The majority of patients at risk are already seen by PCPs. While there is no consensus on ideal screening for NAFLD by PCPs, the use of ultrasound in the at-risk population is recommended in Europe.42 Although NASH remains a histopathologic diagnosis, a reasonable approach is to define NASH based on clinical criteria as done similarly in a real-world observational NAFLD cohort study.54 In the absence of chronic alcohol consumption and viral hepatitis and in a real-world scenario, NASH can be defined as steatosis shown on liver imaging or biopsy and alanine aminotransferase (ALT) levels of > 25 U/L. In addition, ≥ 1 of the following criteria must be met: BMI > 30, T2DM, dyslipidemia, or metabolic syndrome (Table 1).
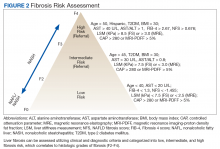
In the absence of easy-to-use validated tests, all patients with NAFLD need to be assessed with simple, noninvasive scores for the presence of clinically relevant liver fibrosis (F2-portal fibrosis with septa; F3-bridging fibrosis; F4-liver cirrhosis); those that meet the fibrosis criteria should receive further assessment usually only offered in a comprehensive NAFLD clinic.1 PCPs should focus on addressing 2 aspects related to NAFLD: (1) Does my patient have NASH based on clinical criteria; and (2) Is my patient at risk for clinically relevant liver fibrosis? PCPs are integral in optimal management of comorbidities and metabolic syndrome abnormalities with lifestyle and exercise interventions.
The care needs of a typical patient with NAFLD can be classified into 3 categories: liver disease (NAFLD) management, addressing NAFLD associated comorbidities, and attending to the personal care needs of the patient. With considerable interactions between these categories, interventions done within the framework of 1 category can influence the needs pertaining to another, requiring closer monitoring of the patient and potentially modifying care. For example, initiating a low carbohydrate diet in a patient with DM and NAFLD who is on antidiabetic medication may require adjusting the medication; disease progression or failure to achieve treatment goals may affect the emotional state of the patient, which can affect adherence.
Referrals to a comprehensive NAFLD clinic need to be standardized. Clearly, the referral process depends in part on local resources, comprehensiveness of available services, and patient characteristics, among others. Most often, PCPs refer patients with suspected diagnosis of NAFLD, with or without abnormal aminotransferases, to a hepatologist to confirm the diagnosis and for disease staging and liver disease management. This may have the advantage of greatest extent of access and should limit the number of patients with advanced liver fibrosis who otherwise may have been missed. On the other hand, different thresholds of PCPs for referrals may delay the patient’s access to comprehensive NAFLD care. Of those referred by primary care, the hepatologist identifies patients with NAFLD who benefit most from a comprehensive care approach. This automated referral process without predefined criteria remains more a vision than reality as it would require an infrastructure and resources that no health care system can provide currently.
The alternative approach of automatic referral may use predefined criteria related to patients’ diagnoses and prognoses (Figure 2).
Patient-Centered Care
At present the narrow focus of VHA specialty outpatient clinics associated with time constraints of providers and gaps in NAFLD awareness clearly does not address the complex metabolic needs of veterans with NAFLD. This is in striking contrast to the comprehensive care offered to patients with cancer. To overcome these limitations, new care delivery models need to be explored. At first it seems attractive to embed NAFLD patient care geographically into a hepatology clinic with the potential advantages of improving volume and timeliness of referral and reinforcing communication among specialty providers while maximizing convenience for patients. However, this is resource intensive not only concerning clinic space, but also in terms of staffing clinics with specialty providers.
Patient-centered care for veterans with NAFLD seems to be best organized around a comprehensive NAFLD clinic with access to specialized diagnostics and knowledge in day-to-day NAFLD management. This evolving care concept has been developed already for patients with liver cirrhosis and inflammatory bowel disease and considers NAFLD a chronic disease that cannot be addressed sufficiently by providing episodic care.55,56 The development of comprehensive NAFLD care can build on the great success of the Hepatitis Innovation Team Collaborative that employed lean management strategies with local and regional teams to facilitate efforts to make chronic hepatitis C virus a rare disease in the VHA.57
NAFLD Care Team
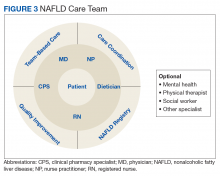
Given the central role of the liver and gastrointestinal tract in the field of nutrition, knowledge of the pathophysiology of the liver and digestive tract as well as emerging therapeutic options offered via metabolic endoscopy uniquely positions the hepatologist/gastroenterologist to take the lead in managing NAFLD. Treating NAFLD is best accomplished when the specialist partners with other health care providers who have expertise in the nutritional, behavioral, and physical activity aspects of treatment. The composition of the NAFLD care team and the roles that different providers fulfill can vary depending on the clinical setting; however, the hepatologist/gastroenterologist is best suited to lead the team, or alternatively, this role can be fulfilled by a provider with liver disease expertise.
Based on experiences from the United Kingdom, the minimum staffing of a NAFLD clinic should include a physician and nurse practitioner who has expertise in managing patients with chronic liver disease, a registered nurse, a dietitian, and a clinical pharmacy specialist (CPS).58 With coexistent diseases common and many veterans who have > 5 prescribed medications, risk of polypharmacy and adverse drug reactions are a concern, particularly since adherence in patients with chronic diseases has been reported to be as low as 43%.59-61 Risk of medication errors and serious adverse effects are magnified by difficulties with patient adherence, medication interactions, and potential need for frequent dose adjustments, particularly when on a weight-loss diet.
Without doubt, comprehensive medication management, offered by a highly trained CPS with independent prescriptive authority occurring while the veteran is in the NAFLD clinic, is highly desirable. Establishing a functional statement and care coordination agreement could describe the role of the CPS as a member of the NAFLD provider team.
Patient Evaluation
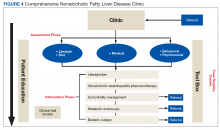
After being referred to the NAFLD clinic, the veteran should have a thorough assessment, including medical, nutritional, physical activity, exercise, and psychosocial evaluations (Figure 4).
The assessment also should include patient education to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals. Educating on NAFLD is critical as most patients with NAFLD do not think of themselves as sick and have limited readiness for lifestyle changes.63,64 A better understanding of NAFLD combined with a higher self-efficacy seems to be positively linked to better nutritional habits.65
An online patient-reported outcomes measurement information system for a patient with NAFLD (eg, assessmentcenter.net) may be beneficial and can be applied within a routine NAFLD clinic visit because of its multidimensionality and compatibility with other chronic diseases.66-68 Other tools to assess health-related QOL include questionnaires, such as the functional assessment of chronic illness therapy-fatigue, work productivity and activity impairment questionnaire: specific health problem, Short Form-36, and chronic liver disease questionnaire-NAFLD.23,69
The medical evaluation includes assessment of secondary causes of NAFLD and identification of NAFLD-related comorbidities. Weight, height, blood pressure, waist circumference, and BMI should be recorded. The physical exam should focus on signs of chronic liver disease and include inspection for acanthosis nigricans, hirsutism, and large neck circumference, which are associated with insulin resistance, polycystic ovarian syndrome, and obstructive sleep apnea, respectively. NAFLD-associated comorbidities may contribute to frailty or physical limitations that affect treatment with diet and exercise and need to be assessed. A thorough medication reconciliation will reveal whether the patient is prescribed obesogenic medications and whether comorbidities (eg, DM and dyslipidemia) are being treated optimally and according to current society guidelines.
Making the diagnosis of NAFLD requires excluding other (concomitant) chronic liver diseases. While often this is done indirectly using order sets with a panoply of available serologic tests without accounting for risks for rare causes of liver injury, a more focused and cost-effective approach is warranted. As most patients will already have had imaging studies that show fatty liver, assessment of liver fibrosis is an important step for risk stratification. Noninvasive scores (eg, FIB-4) can be used by the PCP to identify high-risk patients requiring further workup and referral.1,70 More sophisticated tools, including transient elastography and/or magnetic resonance elastography are applied for more sophisticated risk stratification and liver disease management (Table 2).71
A nutritional evaluation includes information about eating behavior and food choices, body composition analysis, and an assessment of short- and long-term alcohol consumption. Presence of bilateral muscle wasting, subcutaneous fat loss, and signs of micronutrient deficiencies also should be explored. The lifestyle evaluation should include the patient’s typical physical activity and exercise as well as limiting factors.
Finally, and equally important, the patient’s psychosocial situation should be assessed, as motivation and accountability are key to success and may require behavioral modification. Assessing readiness is done best with motivational interviewing, the 5As counseling framework (Ask, Advise, Assess, Assist, Arrange) or using open-ended questions, affirmation, reflections, and summaries.72,73 Even if not personally delivering behavioral treatment, such an approach also can help move patients toward addressing important health-related behaviors.
Personalized Interventions
If available, patients should be offered participation in NAFLD clinical trials. A personalized treatment plan should be developed for each patient with input from all NAFLD care team members. The patient and providers should work together to make important decisions about the treatment plan and goals of care. Making the patient an active participant in their treatment rather than the passive recipient will lead to improvement in adherence and outcomes. Patients will engage when they are comfortable speaking with providers and are sufficiently educated about their disease.
Personalized interventions may be built by combining different strategies, such as lifestyle and dietary interventions, NASH-specific pharmacotherapy, comorbidity management, metabolic endoscopy, and bariatric surgery. Although NASH-specific medications are not currently available, approved medications, including pioglitazone or liraglutide, can be considered for therapy.74,75 Ideally, the NAFLD team CPS would manage comorbidities, such as T2DM and dyslipidemia, but this also can be done by a hepatologist or other specialist. Metabolic endoscopy (eg, intragastric balloons) or bariatric surgery would be done by referral.
Resource-Limited Settings
Although the VHA offers care at > 150 medical centers and > 1,000 outpatient clinics, specialty care such as hepatology and sophisticated and novel testing modalities are not available at many facilities. In 2011 VHA launched the Specialty Care Access Network Extension for Community Healthcare Outcomes to bring hepatitis C therapy and liver transplantation evaluations to rural areas without specialists.76-78 It is logical to explore how telehealth can be used for NAFLD care that requires complex management using new treatments and has a high societal impact, particularly when left untreated.
Telehealth must be easy to use and integrated into everyday routines to be useful for NAFLD management by addressing different aspects of promoting self-management, optimizing therapy, and care coordination. Participation in a structured face-to-face or group-based lifestyle program is often jeopardized by time and job constraints but can be successfully overcome using online approaches.79 The Internet-based VA Video Connect videoconferencing, which incorporates cell phone, laptop, or tablet use could help expand lifestyle interventions to a much larger community of patients with NAFLD and overcome local resource constraints. Finally, e-consultation also can be used in circumstances where synchronous communication with specialists may not be necessary.
Patient Monitoring and Quality Metrics
Monitoring of the patient after initiation of an intervention is variable but occurs more frequently at the beginning. For high-intensity dietary interventions, weekly monitoring for the first several weeks can ensure ongoing motivation, and accountability may increase the patient’s confidence and provide encouragement for further weight loss. It also is an opportunity to reestablish goals with patients with declining motivation. Long-term monitoring of patients may occur in 6- to 12-month intervals to document patient-reported outcomes, liver-related mortality, cardiovascular events, malignancies, and disease progression or regression.
While quality indicators have been proposed for cirrhosis care, such indicators have yet to be defined for NALD care.80 Such quality indicators assessed with validated questionnaires should include knowledge about NAFLD, satisfaction with care, perception of quality of care, and patient-reported outcomes. Other indicators may include use of therapies to treat dyslipidemia and T2DM. Last and likely the most important indicator of improved liver health in NAFLD will be either histologic improvement of NASH or improvement of the fibrosis risk category.
Outlook
With the enormous burden of NAFLD on the rise for many more years to come, quality care delivered to patients with NAFLD warrants resource-adaptive population health management strategies. With a limited number of providers specialized in liver disease, provider education assisted by clinical guidelines and decision support tools, development of referral and access to care mechanisms through integrated care, remote monitoring strategies as well as development of patient self-management and community resources will become more important. We have outlined essential components of an effective population health management strategy for NAFLD and actionable items for the VHA to consider when implementing these strategies. This is the time for the VHA to invest in efforts for NAFLD population care. Clearly, consideration must be given to local needs and resources and integration of technology platforms. Addressing NAFLD at a population level will provide yet another opportunity to demonstrate that VHA performs better on quality when compared with care systems in the private sector.81
1. Hunt CM, Turner MJ, Gifford EJ, Britt RB, Su GL. Identifying and treating nonalcoholic fatty liver disease. Fed Pract. 2019;36(1):20-29.
2. Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and non-alcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36(2):64-71.
3. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443-457.e17.
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
5. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901-910.
6. Golabi P, Shahab O, Stepanova M, Sayiner M, Clement SC, Younossi ZM. Long-term outcomes of diabetic patients with non-alcoholic fatty liver disease (NAFLD) [abstract]. Hepatology. 2017;66(suppl 1):1142A-1143A.
7. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-2195.
8. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
9. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
10. Banini B, Mota M, Behnke M, Sharma A, Sanyal AJ. Nonalcoholic steatohepatitis (NASH) has surpassed hepatitis C as the leading cause for listing for liver transplant: implications for NASH in children and young adults. Presented at the American College of Gastroenterology Annual Scientific Meeting, Las Vegas, NV, October 18, 2016. Abstract 46. https://www.eventscribe.com/2016/ACG/QRcode.asp?Pres=199366. Accessed January 15, 2019.
11. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
12. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004-2009. Hepatology. 2015;62(6):1723-1730.
13. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
14. Gunnar W. Bariatric surgery provided by the Veterans Health Administration: current state and a look to the future. J Gen Intern Med. 2017;32(suppl 1):4-5.
15. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Seraq HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.e1-2.
16. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the wait list for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.e1.
17. Beste L, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
18. Mittal S, El-Seraq HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
19. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;55(6):1828-1837.e2.
20. David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB; NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904-1912.
21. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19(5):544-551.
22. Younossi ZM, Henry L. Economic and quality-of-life implications of nonalcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245-1253.
23. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209-1218.
24. Chawla KS, Talwalkar JA, Keach JC, Malinchoc M, Lindor KD, Jorgensen R. Reliability and validity of the chronic liver disease questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol. 2016;3(1):e000069.
25. Shetty A, Syn WK. Health, and economic burden of nonalcoholic fatty liver disease in the United States and its impact on Veterans. Fed Pract. 2019;36(1):14-19.
26. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
27. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with non-alcoholic steatohepatitis (NASH) in the United States. Hepatology. 2018. [Epub ahead of print.]
28. Allen AM, van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. claims database. Hepatology. 2018;68(6):2230-2238.
29. Diabetes mellitus. http://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20. Published July 2017. Accessed January 15, 2019.
30. Grattagliano I, D’Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P; “Steatostop Project” Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17(4):389-394.
31. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157.
32. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med. 2018;48(2):144-151.
33. Standing HC, Jarvis H, Orr J, et al. GPs’ experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68(676):e743-e749.
34. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(10):2809-2816.
35. Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
36. Ratziu V, Cadranel JF, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57(2):376-383.
37. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Seraq H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10-14.
38. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern Med J. 2013;43(3):247-253.
39. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758-765.
40. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
41. NICE National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. https://www.nice.org.uk/guidance/ng49. Published July 2016. Accessed January 15, 2019.
42. European Association for the Study of the Liver (EASL), European Association for the Study of diabetes (EASD), European Association for the study of obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
43. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
44. Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138-147.
45. Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359-1368.
46. Harman DJ, Ryder SD, James MW, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther. 2018;47(4):504-515.
47. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1-10.
48. Rinella ME, Lominadze Z, Loomba R, et al. Practice pattern in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4-12.
49. El-Atem NA, Wojcik K, Horsfall L, et al. Patterns of service utilization within Australian hepatology clinics: high prevalence of advanced liver disease. Intern Med. 2016;46(4):420-426.
50. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705-712.
51. Nascimbeni F, Aron-Wisnewsky J, Pais R, et al; LIDO Study Group. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075.
52. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829-846.
53. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
54. Barritt AS 4th, Gitlin N, Klein S, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemp Clin Trials. 2017;61:33-38.
55. Meier SK, Shah ND, Talwalkar JA. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(4):492-496.
56. Dulai PS, Singh S, Ohno-Machado L, Sandborn WJ. Population health management for inflammatory bowel disease. Gastroenterology. 2018;154(1):37-45.
57. Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis C in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35(7):24-29.
58. Cobbold JFL, Raveendran S, Peake CM, Anstee QM, Yee MS, Thursz MR. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol. 2013;4(4):263-269.
59. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487-497.
60. Harrison SA. NASH, from diagnosis to treatment: where do we stand? Hepatology. 2015;62(6):1652-1655.
61. Patel PJ, Hayward KL, Rudra R, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: implications for disease severity and management. Medicine (Baltimore). 2017;96(26):e6761.
62. Kanwal F, Mapashki S, Smith D, et al. Implementation of a population-based cirrhosis identification and management system. Clin Gastroenterol Hepatol. 2018;16(8):1182-1186.e2.
63. Mlynarski L, Schlesinger D, Lotan R, et al. Non-alcoholic fatty liver disease is not associated with a lower health perception. World J Gastroenterol. 2016;22(17):4362-4372.
64. Centis E, Moscatiello S, Bugianesi E, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58(4):771-777.
65. Zelber-Sagi S, Bord S, Dror-Lavi G, et al. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol. 2017;23(10):1881-1890.
66. Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerized adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34(9):1123-1132.
67. Verma M, Stites S, Navarro V. Bringing assessment of patient-reported outcomes to hepatology practice. Clin Gastroenterol Hepatol. 2018;16(3):447-448.
68. Ahmed S, Ware P, Gardner W, et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 8: patient-reported outcomes in electronic health records can inform clinical and policy decisions. J Clin Epidemiol. 2017;89:160-167.
69. Younossi ZM, Stepanova M, Lawitz E, et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38(10):1849-1859.
70. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259-2266.
71. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2018;pii:S1542-3565(18)30613-X. [Epub ahead of print.]
72. Searight R. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79(4):277-284.
73. Vallis M, Piccinini-Vallis H, Sharma AM, Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam Physician. 2013:59(1):27-31.
74. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
75. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
76. Salgia RJ, Mullan PB, McCurdy H, Sales A, Moseley RH, Su GL. The educational impact of the specialty care access network-extension of community healthcare outcomes program. Telemed J E Health. 2014;20(11):1004-1008.
77. Konjeti VR, Heuman D, Bajaj J, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol. 2019;17(1):207-209.e1
78. Su GL, Glass L, Tapper EB, Van T, Waljee AK, Sales AE. Virtual consultations through the Veterans Administration SCAN-ECHO project improves survival for veterans with liver disease. Hepatology. 2018;68(6):2317-2324.
79. Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155-1163.
80. Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010,8(8):709-717.
81. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs Non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885.
Nonalcoholic fatty liver disease (NAFLD) is an umbrella term that covers a spectrum of phenotypes ranging from nonalcoholic fatty liver or simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) defined by histologic findings of steatosis, lobular inflammation, cytologic ballooning, and some degree of fibrosis.1 While frequently observed in patients with at least 1 risk factor (eg, obesity, diabetes mellitus [DM], dyslipidemia, hypertension), NAFLD also is an independent risk factor for type 2 DM (T2DM), chronic kidney disease, and cardiovascular disease.2 At early disease stages with absence of liver fibrosis, mortality is linked to cardiovascular and not liver disease. However, in the presence of NASH, fibrosis progression to liver cirrhosis, or hepatocellular carcinoma (HCC) represent the most important liver-related outcomes that determine morbidity and mortality.3 Mirroring the obesity and T2DM epidemics, the health care burden is projected to dramatically rise.
In the following article, we will discuss how the Veterans Health Administration (VHA) is well positioned to implement an organizational strategy of comprehensive care for veterans with NAFLD. This comprehensive care strategy should include the development of a NAFLD clinic offering care for comorbid conditions frequently present in these patients, point-of-care testing, access to clinical trials, and outcomes monitoring as a key performance target for providers and the respective facility.
NAFLD disease burden
To fully appreciate the burden of a chronic disease like NAFLD, it is important to assess its long- and short-term consequences in a comprehensive manner with regard to its clinical impact, impact on the patient, and economic impact (Figure 1).
Clinical Impact
Clinical impact is assessed based on the prevalence and natural history of NAFLD and the liver fibrosis stage and determines patient survival. Coinciding with the epidemic of obesity and T2DM, the prevalence of NAFLD in the general population in North America is 24% and even higher with older age and higher body mass index (BMI).4,5 The prevalence for NAFLD is particularly high in patients with T2DM (47%). Of patients with T2DM and NAFLD, 65% have biopsy-proven NASH of which 15% have bridging fibrosis or liver cirrhosis.6
NAFLD is the fastest growing cause of cirrhosis in the US with a forecasted NAFLD population of 101 million by 2030.7 At the same time, the number of patients with NASH will rise to 27 million of which > 7 million will have bridging fibrosis or liver cirrhosis; hepatic decompensation events are estimated to occur in 105,430 patients with liver cirrhosis, posing a major public health threat related to organ availability for liver transplantation.8 Since 2013, NAFLD has been the second leading cause for liver transplantation and the top reason for transplantation in patients aged < 50 years.9,10 As many patients with NAFLD are diagnosed with HCC at stages where liver transplantation is not an option, mortality from HCC in NAFLD patients is higher than with other etiologies as treatment options are restricted.11,12
Compared with that of the general population, veterans seeking care are older and sicker with 43% of veterans taking > 5 prescribed medications.13 Of those receiving VHA care, 6.6 million veterans are either overweight or obese; 165,000 are morbidly obese with a BMI > 40.14 In addition, veterans are 2.5 times more likely to have T2DM compared with that of nonveterans. Because T2DM and obesity are the most common risk factors for NAFLD, it is not surprising that NAFLD prevalence among veterans rose 3-fold from 2003 to 2011.15 It is now estimated that 540,000 veterans will progress to NASH and 108,000 will develop bridging fibrosis or liver cirrhosis by 2030.8 Similar to that of the general population, liver cirrhosis is attributed to NAFLD in 15% of veterans.15,16 NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 years and 70 years, respectively.16,17 Shockingly, 20% of HCCs were not linked to liver cirrhosis and escaped recommended HCC screening for patients with cirrhosis.18,19
Patient Impact
Assessment of disease burden should not be restricted to clinical outcomes as patients can experience a range of symptoms that may have significant impact on their health-related quality of life (QOL) and functional status.20 Using general but not disease-specific instruments, NAFLD patients reported outcomes score low regarding fatigue, activity, and emotions.21 More disease-specific questionnaires may provide better and disease-specific insights as how NASH impacts patients’ QOL.22-24
Economic Impact
There is mounting evidence that the clinical implications of NAFLD directly influence the economic burden of NAFLD.25 The annual burden associated with all incident and prevalent NAFLD cases in the US has been estimated at $103 billion, and projections suggest that the expected 10-year burden of NAFLD may increase to $1.005 trillion.26 It is anticipated that increased NAFLD costs will affect the VHA with billions of dollars in annual expenditures in addition to the $1.5 billion already spent annually for T2DM care (4% of the VA pharmacy budget is spent on T2DM treatment).27-29
Current Patient Care
Obesity, DM, and dyslipidemia are common conditions managed by primary care providers (PCPs). Given the close association of these conditions with NAFLD, the PCP is often the first point of medical contact for patients with or at risk for NAFLD.30 For that reason, PCP awareness of NAFLD is critical for effective management of these patients. PCPs should be actively involved in the management of patients with NAFLD with pathways in place for identifying patients at high risk of liver disease for timely referral to a specialist and adequate education on the follow-up and treatment of low-risk patients. Instead, diagnosis of NAFLD is primarily triggered by either abnormal aminotransferases or detection of steatosis on imaging performed for other indications.
Barriers to optimal management of NAFLD by PCPs have been identified and occur at different levels of patient care. In the absence of clinical practice guidelines by the American Association of Family Practice covering NAFLD and a substantial latency period without signs of symptoms, NAFLD may not be perceived as a potentially serious condition by PCPs and their patients; interestingly this holds true even for some medical specialties.31-39 More than half of PCPs do not test their patients at highest risk for NAFLD (eg, patients with obesity or T2DM) and may be unaware of practice guidelines.40-42
Guidelines from Europe and the US are not completely in accordance. The US guidelines are vague regarding screening and are supported by only 1 medical society, due to the lack of NASH-specific drug therapies. The European guidelines are built on the support of 3 different stakeholders covering liver diseases, obesity, and DM and the experience using noninvasive liver fibrosis assessments for patients with NAFLD. To overcome this apparent conflict, a more practical and risk-stratified approach is warranted.41,42
Making the diagnosis can be challenging in cases with competing etiologies, such as T2DM and alcohol misuse. There also is an overreliance on aminotransferase levels to diagnose NAFLD. Significant liver disease can exist in the presence of normal aminotransferases, and this may be attributed to either spontaneous aminotransferase fluctuations or upper limits of normal that have been chosen too high.43-47 Often additional workup by PCPs depends on the magnitude of aminotransferase abnormalities.
Even if NAFLD has been diagnosed by PCPs, identifying those with NASH is hindered by the absence of an accurate noninvasive diagnostic method and the need to perform a liver biopsy. Liver biopsy is often not considered or delayed to monitor patients with serial aminotransferases, regardless of the patient’s metabolic comorbidity profile or baseline aminotransferases.32 As a result, referral to a specialist often depends on the magnitude of the aminotransferase abnormality,30,48 and often occurs when advanced liver disease is already present.49 Finally, providers may not be aware of beneficial effects of lifestyle interventions and certain medications, including statins on NASH and liver fibrosis.50-53 As NAFLD is associated with excess cardiovascular- and cancer-related morbidity and mortality, it is possible that regression of NAFLD may improve associated risk for these outcomes as well.
Framework for Comprehensive NAFLD Care
Chronic liver diseases and associated comorbidities have long been addressed by PCPs and specialty providers working in isolation and within the narrow focus of each discipline. Contrary to working in silos of the past, a coordinated management strategy with other disciplines that cover these comorbidities needs to be established, or alternatively the PCP must be aware of the management of comorbidities to execute them independently. Integration of hepatology-driven NAFLD care with other specialties involves communication, collaboration, and sharing of resources and expertise that will address patient care needs. Obviously, this cannot be undertaken in a single outpatient visit and requires vertical and longitudinal follow-up over time. One important aspect of comprehensive NAFLD care is the targeting of a particular patient population rather than being seen as a panacea for all; cost-utility analysis is hampered by uncertainties around accuracy of noninvasive biomarkers reflecting liver injury and a lack of effectiveness data for treatment. However, it seems reasonable to screen patients at high risk for NASH and adverse clinical outcomes. Such a risk stratification approach should be cost-effective.
A first key step by the PCP is to identify whether a patient is at risk, especially patients with NASH. The majority of patients at risk are already seen by PCPs. While there is no consensus on ideal screening for NAFLD by PCPs, the use of ultrasound in the at-risk population is recommended in Europe.42 Although NASH remains a histopathologic diagnosis, a reasonable approach is to define NASH based on clinical criteria as done similarly in a real-world observational NAFLD cohort study.54 In the absence of chronic alcohol consumption and viral hepatitis and in a real-world scenario, NASH can be defined as steatosis shown on liver imaging or biopsy and alanine aminotransferase (ALT) levels of > 25 U/L. In addition, ≥ 1 of the following criteria must be met: BMI > 30, T2DM, dyslipidemia, or metabolic syndrome (Table 1).
In the absence of easy-to-use validated tests, all patients with NAFLD need to be assessed with simple, noninvasive scores for the presence of clinically relevant liver fibrosis (F2-portal fibrosis with septa; F3-bridging fibrosis; F4-liver cirrhosis); those that meet the fibrosis criteria should receive further assessment usually only offered in a comprehensive NAFLD clinic.1 PCPs should focus on addressing 2 aspects related to NAFLD: (1) Does my patient have NASH based on clinical criteria; and (2) Is my patient at risk for clinically relevant liver fibrosis? PCPs are integral in optimal management of comorbidities and metabolic syndrome abnormalities with lifestyle and exercise interventions.
The care needs of a typical patient with NAFLD can be classified into 3 categories: liver disease (NAFLD) management, addressing NAFLD associated comorbidities, and attending to the personal care needs of the patient. With considerable interactions between these categories, interventions done within the framework of 1 category can influence the needs pertaining to another, requiring closer monitoring of the patient and potentially modifying care. For example, initiating a low carbohydrate diet in a patient with DM and NAFLD who is on antidiabetic medication may require adjusting the medication; disease progression or failure to achieve treatment goals may affect the emotional state of the patient, which can affect adherence.
Referrals to a comprehensive NAFLD clinic need to be standardized. Clearly, the referral process depends in part on local resources, comprehensiveness of available services, and patient characteristics, among others. Most often, PCPs refer patients with suspected diagnosis of NAFLD, with or without abnormal aminotransferases, to a hepatologist to confirm the diagnosis and for disease staging and liver disease management. This may have the advantage of greatest extent of access and should limit the number of patients with advanced liver fibrosis who otherwise may have been missed. On the other hand, different thresholds of PCPs for referrals may delay the patient’s access to comprehensive NAFLD care. Of those referred by primary care, the hepatologist identifies patients with NAFLD who benefit most from a comprehensive care approach. This automated referral process without predefined criteria remains more a vision than reality as it would require an infrastructure and resources that no health care system can provide currently.
The alternative approach of automatic referral may use predefined criteria related to patients’ diagnoses and prognoses (Figure 2).
Patient-Centered Care
At present the narrow focus of VHA specialty outpatient clinics associated with time constraints of providers and gaps in NAFLD awareness clearly does not address the complex metabolic needs of veterans with NAFLD. This is in striking contrast to the comprehensive care offered to patients with cancer. To overcome these limitations, new care delivery models need to be explored. At first it seems attractive to embed NAFLD patient care geographically into a hepatology clinic with the potential advantages of improving volume and timeliness of referral and reinforcing communication among specialty providers while maximizing convenience for patients. However, this is resource intensive not only concerning clinic space, but also in terms of staffing clinics with specialty providers.
Patient-centered care for veterans with NAFLD seems to be best organized around a comprehensive NAFLD clinic with access to specialized diagnostics and knowledge in day-to-day NAFLD management. This evolving care concept has been developed already for patients with liver cirrhosis and inflammatory bowel disease and considers NAFLD a chronic disease that cannot be addressed sufficiently by providing episodic care.55,56 The development of comprehensive NAFLD care can build on the great success of the Hepatitis Innovation Team Collaborative that employed lean management strategies with local and regional teams to facilitate efforts to make chronic hepatitis C virus a rare disease in the VHA.57
NAFLD Care Team
Given the central role of the liver and gastrointestinal tract in the field of nutrition, knowledge of the pathophysiology of the liver and digestive tract as well as emerging therapeutic options offered via metabolic endoscopy uniquely positions the hepatologist/gastroenterologist to take the lead in managing NAFLD. Treating NAFLD is best accomplished when the specialist partners with other health care providers who have expertise in the nutritional, behavioral, and physical activity aspects of treatment. The composition of the NAFLD care team and the roles that different providers fulfill can vary depending on the clinical setting; however, the hepatologist/gastroenterologist is best suited to lead the team, or alternatively, this role can be fulfilled by a provider with liver disease expertise.
Based on experiences from the United Kingdom, the minimum staffing of a NAFLD clinic should include a physician and nurse practitioner who has expertise in managing patients with chronic liver disease, a registered nurse, a dietitian, and a clinical pharmacy specialist (CPS).58 With coexistent diseases common and many veterans who have > 5 prescribed medications, risk of polypharmacy and adverse drug reactions are a concern, particularly since adherence in patients with chronic diseases has been reported to be as low as 43%.59-61 Risk of medication errors and serious adverse effects are magnified by difficulties with patient adherence, medication interactions, and potential need for frequent dose adjustments, particularly when on a weight-loss diet.
Without doubt, comprehensive medication management, offered by a highly trained CPS with independent prescriptive authority occurring while the veteran is in the NAFLD clinic, is highly desirable. Establishing a functional statement and care coordination agreement could describe the role of the CPS as a member of the NAFLD provider team.
Patient Evaluation
After being referred to the NAFLD clinic, the veteran should have a thorough assessment, including medical, nutritional, physical activity, exercise, and psychosocial evaluations (Figure 4).
The assessment also should include patient education to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals. Educating on NAFLD is critical as most patients with NAFLD do not think of themselves as sick and have limited readiness for lifestyle changes.63,64 A better understanding of NAFLD combined with a higher self-efficacy seems to be positively linked to better nutritional habits.65
An online patient-reported outcomes measurement information system for a patient with NAFLD (eg, assessmentcenter.net) may be beneficial and can be applied within a routine NAFLD clinic visit because of its multidimensionality and compatibility with other chronic diseases.66-68 Other tools to assess health-related QOL include questionnaires, such as the functional assessment of chronic illness therapy-fatigue, work productivity and activity impairment questionnaire: specific health problem, Short Form-36, and chronic liver disease questionnaire-NAFLD.23,69
The medical evaluation includes assessment of secondary causes of NAFLD and identification of NAFLD-related comorbidities. Weight, height, blood pressure, waist circumference, and BMI should be recorded. The physical exam should focus on signs of chronic liver disease and include inspection for acanthosis nigricans, hirsutism, and large neck circumference, which are associated with insulin resistance, polycystic ovarian syndrome, and obstructive sleep apnea, respectively. NAFLD-associated comorbidities may contribute to frailty or physical limitations that affect treatment with diet and exercise and need to be assessed. A thorough medication reconciliation will reveal whether the patient is prescribed obesogenic medications and whether comorbidities (eg, DM and dyslipidemia) are being treated optimally and according to current society guidelines.
Making the diagnosis of NAFLD requires excluding other (concomitant) chronic liver diseases. While often this is done indirectly using order sets with a panoply of available serologic tests without accounting for risks for rare causes of liver injury, a more focused and cost-effective approach is warranted. As most patients will already have had imaging studies that show fatty liver, assessment of liver fibrosis is an important step for risk stratification. Noninvasive scores (eg, FIB-4) can be used by the PCP to identify high-risk patients requiring further workup and referral.1,70 More sophisticated tools, including transient elastography and/or magnetic resonance elastography are applied for more sophisticated risk stratification and liver disease management (Table 2).71
A nutritional evaluation includes information about eating behavior and food choices, body composition analysis, and an assessment of short- and long-term alcohol consumption. Presence of bilateral muscle wasting, subcutaneous fat loss, and signs of micronutrient deficiencies also should be explored. The lifestyle evaluation should include the patient’s typical physical activity and exercise as well as limiting factors.
Finally, and equally important, the patient’s psychosocial situation should be assessed, as motivation and accountability are key to success and may require behavioral modification. Assessing readiness is done best with motivational interviewing, the 5As counseling framework (Ask, Advise, Assess, Assist, Arrange) or using open-ended questions, affirmation, reflections, and summaries.72,73 Even if not personally delivering behavioral treatment, such an approach also can help move patients toward addressing important health-related behaviors.
Personalized Interventions
If available, patients should be offered participation in NAFLD clinical trials. A personalized treatment plan should be developed for each patient with input from all NAFLD care team members. The patient and providers should work together to make important decisions about the treatment plan and goals of care. Making the patient an active participant in their treatment rather than the passive recipient will lead to improvement in adherence and outcomes. Patients will engage when they are comfortable speaking with providers and are sufficiently educated about their disease.
Personalized interventions may be built by combining different strategies, such as lifestyle and dietary interventions, NASH-specific pharmacotherapy, comorbidity management, metabolic endoscopy, and bariatric surgery. Although NASH-specific medications are not currently available, approved medications, including pioglitazone or liraglutide, can be considered for therapy.74,75 Ideally, the NAFLD team CPS would manage comorbidities, such as T2DM and dyslipidemia, but this also can be done by a hepatologist or other specialist. Metabolic endoscopy (eg, intragastric balloons) or bariatric surgery would be done by referral.
Resource-Limited Settings
Although the VHA offers care at > 150 medical centers and > 1,000 outpatient clinics, specialty care such as hepatology and sophisticated and novel testing modalities are not available at many facilities. In 2011 VHA launched the Specialty Care Access Network Extension for Community Healthcare Outcomes to bring hepatitis C therapy and liver transplantation evaluations to rural areas without specialists.76-78 It is logical to explore how telehealth can be used for NAFLD care that requires complex management using new treatments and has a high societal impact, particularly when left untreated.
Telehealth must be easy to use and integrated into everyday routines to be useful for NAFLD management by addressing different aspects of promoting self-management, optimizing therapy, and care coordination. Participation in a structured face-to-face or group-based lifestyle program is often jeopardized by time and job constraints but can be successfully overcome using online approaches.79 The Internet-based VA Video Connect videoconferencing, which incorporates cell phone, laptop, or tablet use could help expand lifestyle interventions to a much larger community of patients with NAFLD and overcome local resource constraints. Finally, e-consultation also can be used in circumstances where synchronous communication with specialists may not be necessary.
Patient Monitoring and Quality Metrics
Monitoring of the patient after initiation of an intervention is variable but occurs more frequently at the beginning. For high-intensity dietary interventions, weekly monitoring for the first several weeks can ensure ongoing motivation, and accountability may increase the patient’s confidence and provide encouragement for further weight loss. It also is an opportunity to reestablish goals with patients with declining motivation. Long-term monitoring of patients may occur in 6- to 12-month intervals to document patient-reported outcomes, liver-related mortality, cardiovascular events, malignancies, and disease progression or regression.
While quality indicators have been proposed for cirrhosis care, such indicators have yet to be defined for NALD care.80 Such quality indicators assessed with validated questionnaires should include knowledge about NAFLD, satisfaction with care, perception of quality of care, and patient-reported outcomes. Other indicators may include use of therapies to treat dyslipidemia and T2DM. Last and likely the most important indicator of improved liver health in NAFLD will be either histologic improvement of NASH or improvement of the fibrosis risk category.
Outlook
With the enormous burden of NAFLD on the rise for many more years to come, quality care delivered to patients with NAFLD warrants resource-adaptive population health management strategies. With a limited number of providers specialized in liver disease, provider education assisted by clinical guidelines and decision support tools, development of referral and access to care mechanisms through integrated care, remote monitoring strategies as well as development of patient self-management and community resources will become more important. We have outlined essential components of an effective population health management strategy for NAFLD and actionable items for the VHA to consider when implementing these strategies. This is the time for the VHA to invest in efforts for NAFLD population care. Clearly, consideration must be given to local needs and resources and integration of technology platforms. Addressing NAFLD at a population level will provide yet another opportunity to demonstrate that VHA performs better on quality when compared with care systems in the private sector.81
Nonalcoholic fatty liver disease (NAFLD) is an umbrella term that covers a spectrum of phenotypes ranging from nonalcoholic fatty liver or simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) defined by histologic findings of steatosis, lobular inflammation, cytologic ballooning, and some degree of fibrosis.1 While frequently observed in patients with at least 1 risk factor (eg, obesity, diabetes mellitus [DM], dyslipidemia, hypertension), NAFLD also is an independent risk factor for type 2 DM (T2DM), chronic kidney disease, and cardiovascular disease.2 At early disease stages with absence of liver fibrosis, mortality is linked to cardiovascular and not liver disease. However, in the presence of NASH, fibrosis progression to liver cirrhosis, or hepatocellular carcinoma (HCC) represent the most important liver-related outcomes that determine morbidity and mortality.3 Mirroring the obesity and T2DM epidemics, the health care burden is projected to dramatically rise.
In the following article, we will discuss how the Veterans Health Administration (VHA) is well positioned to implement an organizational strategy of comprehensive care for veterans with NAFLD. This comprehensive care strategy should include the development of a NAFLD clinic offering care for comorbid conditions frequently present in these patients, point-of-care testing, access to clinical trials, and outcomes monitoring as a key performance target for providers and the respective facility.
NAFLD disease burden
To fully appreciate the burden of a chronic disease like NAFLD, it is important to assess its long- and short-term consequences in a comprehensive manner with regard to its clinical impact, impact on the patient, and economic impact (Figure 1).
Clinical Impact
Clinical impact is assessed based on the prevalence and natural history of NAFLD and the liver fibrosis stage and determines patient survival. Coinciding with the epidemic of obesity and T2DM, the prevalence of NAFLD in the general population in North America is 24% and even higher with older age and higher body mass index (BMI).4,5 The prevalence for NAFLD is particularly high in patients with T2DM (47%). Of patients with T2DM and NAFLD, 65% have biopsy-proven NASH of which 15% have bridging fibrosis or liver cirrhosis.6
NAFLD is the fastest growing cause of cirrhosis in the US with a forecasted NAFLD population of 101 million by 2030.7 At the same time, the number of patients with NASH will rise to 27 million of which > 7 million will have bridging fibrosis or liver cirrhosis; hepatic decompensation events are estimated to occur in 105,430 patients with liver cirrhosis, posing a major public health threat related to organ availability for liver transplantation.8 Since 2013, NAFLD has been the second leading cause for liver transplantation and the top reason for transplantation in patients aged < 50 years.9,10 As many patients with NAFLD are diagnosed with HCC at stages where liver transplantation is not an option, mortality from HCC in NAFLD patients is higher than with other etiologies as treatment options are restricted.11,12
Compared with that of the general population, veterans seeking care are older and sicker with 43% of veterans taking > 5 prescribed medications.13 Of those receiving VHA care, 6.6 million veterans are either overweight or obese; 165,000 are morbidly obese with a BMI > 40.14 In addition, veterans are 2.5 times more likely to have T2DM compared with that of nonveterans. Because T2DM and obesity are the most common risk factors for NAFLD, it is not surprising that NAFLD prevalence among veterans rose 3-fold from 2003 to 2011.15 It is now estimated that 540,000 veterans will progress to NASH and 108,000 will develop bridging fibrosis or liver cirrhosis by 2030.8 Similar to that of the general population, liver cirrhosis is attributed to NAFLD in 15% of veterans.15,16 NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 years and 70 years, respectively.16,17 Shockingly, 20% of HCCs were not linked to liver cirrhosis and escaped recommended HCC screening for patients with cirrhosis.18,19
Patient Impact
Assessment of disease burden should not be restricted to clinical outcomes as patients can experience a range of symptoms that may have significant impact on their health-related quality of life (QOL) and functional status.20 Using general but not disease-specific instruments, NAFLD patients reported outcomes score low regarding fatigue, activity, and emotions.21 More disease-specific questionnaires may provide better and disease-specific insights as how NASH impacts patients’ QOL.22-24
Economic Impact
There is mounting evidence that the clinical implications of NAFLD directly influence the economic burden of NAFLD.25 The annual burden associated with all incident and prevalent NAFLD cases in the US has been estimated at $103 billion, and projections suggest that the expected 10-year burden of NAFLD may increase to $1.005 trillion.26 It is anticipated that increased NAFLD costs will affect the VHA with billions of dollars in annual expenditures in addition to the $1.5 billion already spent annually for T2DM care (4% of the VA pharmacy budget is spent on T2DM treatment).27-29
Current Patient Care
Obesity, DM, and dyslipidemia are common conditions managed by primary care providers (PCPs). Given the close association of these conditions with NAFLD, the PCP is often the first point of medical contact for patients with or at risk for NAFLD.30 For that reason, PCP awareness of NAFLD is critical for effective management of these patients. PCPs should be actively involved in the management of patients with NAFLD with pathways in place for identifying patients at high risk of liver disease for timely referral to a specialist and adequate education on the follow-up and treatment of low-risk patients. Instead, diagnosis of NAFLD is primarily triggered by either abnormal aminotransferases or detection of steatosis on imaging performed for other indications.
Barriers to optimal management of NAFLD by PCPs have been identified and occur at different levels of patient care. In the absence of clinical practice guidelines by the American Association of Family Practice covering NAFLD and a substantial latency period without signs of symptoms, NAFLD may not be perceived as a potentially serious condition by PCPs and their patients; interestingly this holds true even for some medical specialties.31-39 More than half of PCPs do not test their patients at highest risk for NAFLD (eg, patients with obesity or T2DM) and may be unaware of practice guidelines.40-42
Guidelines from Europe and the US are not completely in accordance. The US guidelines are vague regarding screening and are supported by only 1 medical society, due to the lack of NASH-specific drug therapies. The European guidelines are built on the support of 3 different stakeholders covering liver diseases, obesity, and DM and the experience using noninvasive liver fibrosis assessments for patients with NAFLD. To overcome this apparent conflict, a more practical and risk-stratified approach is warranted.41,42
Making the diagnosis can be challenging in cases with competing etiologies, such as T2DM and alcohol misuse. There also is an overreliance on aminotransferase levels to diagnose NAFLD. Significant liver disease can exist in the presence of normal aminotransferases, and this may be attributed to either spontaneous aminotransferase fluctuations or upper limits of normal that have been chosen too high.43-47 Often additional workup by PCPs depends on the magnitude of aminotransferase abnormalities.
Even if NAFLD has been diagnosed by PCPs, identifying those with NASH is hindered by the absence of an accurate noninvasive diagnostic method and the need to perform a liver biopsy. Liver biopsy is often not considered or delayed to monitor patients with serial aminotransferases, regardless of the patient’s metabolic comorbidity profile or baseline aminotransferases.32 As a result, referral to a specialist often depends on the magnitude of the aminotransferase abnormality,30,48 and often occurs when advanced liver disease is already present.49 Finally, providers may not be aware of beneficial effects of lifestyle interventions and certain medications, including statins on NASH and liver fibrosis.50-53 As NAFLD is associated with excess cardiovascular- and cancer-related morbidity and mortality, it is possible that regression of NAFLD may improve associated risk for these outcomes as well.
Framework for Comprehensive NAFLD Care
Chronic liver diseases and associated comorbidities have long been addressed by PCPs and specialty providers working in isolation and within the narrow focus of each discipline. Contrary to working in silos of the past, a coordinated management strategy with other disciplines that cover these comorbidities needs to be established, or alternatively the PCP must be aware of the management of comorbidities to execute them independently. Integration of hepatology-driven NAFLD care with other specialties involves communication, collaboration, and sharing of resources and expertise that will address patient care needs. Obviously, this cannot be undertaken in a single outpatient visit and requires vertical and longitudinal follow-up over time. One important aspect of comprehensive NAFLD care is the targeting of a particular patient population rather than being seen as a panacea for all; cost-utility analysis is hampered by uncertainties around accuracy of noninvasive biomarkers reflecting liver injury and a lack of effectiveness data for treatment. However, it seems reasonable to screen patients at high risk for NASH and adverse clinical outcomes. Such a risk stratification approach should be cost-effective.
A first key step by the PCP is to identify whether a patient is at risk, especially patients with NASH. The majority of patients at risk are already seen by PCPs. While there is no consensus on ideal screening for NAFLD by PCPs, the use of ultrasound in the at-risk population is recommended in Europe.42 Although NASH remains a histopathologic diagnosis, a reasonable approach is to define NASH based on clinical criteria as done similarly in a real-world observational NAFLD cohort study.54 In the absence of chronic alcohol consumption and viral hepatitis and in a real-world scenario, NASH can be defined as steatosis shown on liver imaging or biopsy and alanine aminotransferase (ALT) levels of > 25 U/L. In addition, ≥ 1 of the following criteria must be met: BMI > 30, T2DM, dyslipidemia, or metabolic syndrome (Table 1).
In the absence of easy-to-use validated tests, all patients with NAFLD need to be assessed with simple, noninvasive scores for the presence of clinically relevant liver fibrosis (F2-portal fibrosis with septa; F3-bridging fibrosis; F4-liver cirrhosis); those that meet the fibrosis criteria should receive further assessment usually only offered in a comprehensive NAFLD clinic.1 PCPs should focus on addressing 2 aspects related to NAFLD: (1) Does my patient have NASH based on clinical criteria; and (2) Is my patient at risk for clinically relevant liver fibrosis? PCPs are integral in optimal management of comorbidities and metabolic syndrome abnormalities with lifestyle and exercise interventions.
The care needs of a typical patient with NAFLD can be classified into 3 categories: liver disease (NAFLD) management, addressing NAFLD associated comorbidities, and attending to the personal care needs of the patient. With considerable interactions between these categories, interventions done within the framework of 1 category can influence the needs pertaining to another, requiring closer monitoring of the patient and potentially modifying care. For example, initiating a low carbohydrate diet in a patient with DM and NAFLD who is on antidiabetic medication may require adjusting the medication; disease progression or failure to achieve treatment goals may affect the emotional state of the patient, which can affect adherence.
Referrals to a comprehensive NAFLD clinic need to be standardized. Clearly, the referral process depends in part on local resources, comprehensiveness of available services, and patient characteristics, among others. Most often, PCPs refer patients with suspected diagnosis of NAFLD, with or without abnormal aminotransferases, to a hepatologist to confirm the diagnosis and for disease staging and liver disease management. This may have the advantage of greatest extent of access and should limit the number of patients with advanced liver fibrosis who otherwise may have been missed. On the other hand, different thresholds of PCPs for referrals may delay the patient’s access to comprehensive NAFLD care. Of those referred by primary care, the hepatologist identifies patients with NAFLD who benefit most from a comprehensive care approach. This automated referral process without predefined criteria remains more a vision than reality as it would require an infrastructure and resources that no health care system can provide currently.
The alternative approach of automatic referral may use predefined criteria related to patients’ diagnoses and prognoses (Figure 2).
Patient-Centered Care
At present the narrow focus of VHA specialty outpatient clinics associated with time constraints of providers and gaps in NAFLD awareness clearly does not address the complex metabolic needs of veterans with NAFLD. This is in striking contrast to the comprehensive care offered to patients with cancer. To overcome these limitations, new care delivery models need to be explored. At first it seems attractive to embed NAFLD patient care geographically into a hepatology clinic with the potential advantages of improving volume and timeliness of referral and reinforcing communication among specialty providers while maximizing convenience for patients. However, this is resource intensive not only concerning clinic space, but also in terms of staffing clinics with specialty providers.
Patient-centered care for veterans with NAFLD seems to be best organized around a comprehensive NAFLD clinic with access to specialized diagnostics and knowledge in day-to-day NAFLD management. This evolving care concept has been developed already for patients with liver cirrhosis and inflammatory bowel disease and considers NAFLD a chronic disease that cannot be addressed sufficiently by providing episodic care.55,56 The development of comprehensive NAFLD care can build on the great success of the Hepatitis Innovation Team Collaborative that employed lean management strategies with local and regional teams to facilitate efforts to make chronic hepatitis C virus a rare disease in the VHA.57
NAFLD Care Team
Given the central role of the liver and gastrointestinal tract in the field of nutrition, knowledge of the pathophysiology of the liver and digestive tract as well as emerging therapeutic options offered via metabolic endoscopy uniquely positions the hepatologist/gastroenterologist to take the lead in managing NAFLD. Treating NAFLD is best accomplished when the specialist partners with other health care providers who have expertise in the nutritional, behavioral, and physical activity aspects of treatment. The composition of the NAFLD care team and the roles that different providers fulfill can vary depending on the clinical setting; however, the hepatologist/gastroenterologist is best suited to lead the team, or alternatively, this role can be fulfilled by a provider with liver disease expertise.
Based on experiences from the United Kingdom, the minimum staffing of a NAFLD clinic should include a physician and nurse practitioner who has expertise in managing patients with chronic liver disease, a registered nurse, a dietitian, and a clinical pharmacy specialist (CPS).58 With coexistent diseases common and many veterans who have > 5 prescribed medications, risk of polypharmacy and adverse drug reactions are a concern, particularly since adherence in patients with chronic diseases has been reported to be as low as 43%.59-61 Risk of medication errors and serious adverse effects are magnified by difficulties with patient adherence, medication interactions, and potential need for frequent dose adjustments, particularly when on a weight-loss diet.
Without doubt, comprehensive medication management, offered by a highly trained CPS with independent prescriptive authority occurring while the veteran is in the NAFLD clinic, is highly desirable. Establishing a functional statement and care coordination agreement could describe the role of the CPS as a member of the NAFLD provider team.
Patient Evaluation
After being referred to the NAFLD clinic, the veteran should have a thorough assessment, including medical, nutritional, physical activity, exercise, and psychosocial evaluations (Figure 4).
The assessment also should include patient education to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals. Educating on NAFLD is critical as most patients with NAFLD do not think of themselves as sick and have limited readiness for lifestyle changes.63,64 A better understanding of NAFLD combined with a higher self-efficacy seems to be positively linked to better nutritional habits.65
An online patient-reported outcomes measurement information system for a patient with NAFLD (eg, assessmentcenter.net) may be beneficial and can be applied within a routine NAFLD clinic visit because of its multidimensionality and compatibility with other chronic diseases.66-68 Other tools to assess health-related QOL include questionnaires, such as the functional assessment of chronic illness therapy-fatigue, work productivity and activity impairment questionnaire: specific health problem, Short Form-36, and chronic liver disease questionnaire-NAFLD.23,69
The medical evaluation includes assessment of secondary causes of NAFLD and identification of NAFLD-related comorbidities. Weight, height, blood pressure, waist circumference, and BMI should be recorded. The physical exam should focus on signs of chronic liver disease and include inspection for acanthosis nigricans, hirsutism, and large neck circumference, which are associated with insulin resistance, polycystic ovarian syndrome, and obstructive sleep apnea, respectively. NAFLD-associated comorbidities may contribute to frailty or physical limitations that affect treatment with diet and exercise and need to be assessed. A thorough medication reconciliation will reveal whether the patient is prescribed obesogenic medications and whether comorbidities (eg, DM and dyslipidemia) are being treated optimally and according to current society guidelines.
Making the diagnosis of NAFLD requires excluding other (concomitant) chronic liver diseases. While often this is done indirectly using order sets with a panoply of available serologic tests without accounting for risks for rare causes of liver injury, a more focused and cost-effective approach is warranted. As most patients will already have had imaging studies that show fatty liver, assessment of liver fibrosis is an important step for risk stratification. Noninvasive scores (eg, FIB-4) can be used by the PCP to identify high-risk patients requiring further workup and referral.1,70 More sophisticated tools, including transient elastography and/or magnetic resonance elastography are applied for more sophisticated risk stratification and liver disease management (Table 2).71
A nutritional evaluation includes information about eating behavior and food choices, body composition analysis, and an assessment of short- and long-term alcohol consumption. Presence of bilateral muscle wasting, subcutaneous fat loss, and signs of micronutrient deficiencies also should be explored. The lifestyle evaluation should include the patient’s typical physical activity and exercise as well as limiting factors.
Finally, and equally important, the patient’s psychosocial situation should be assessed, as motivation and accountability are key to success and may require behavioral modification. Assessing readiness is done best with motivational interviewing, the 5As counseling framework (Ask, Advise, Assess, Assist, Arrange) or using open-ended questions, affirmation, reflections, and summaries.72,73 Even if not personally delivering behavioral treatment, such an approach also can help move patients toward addressing important health-related behaviors.
Personalized Interventions
If available, patients should be offered participation in NAFLD clinical trials. A personalized treatment plan should be developed for each patient with input from all NAFLD care team members. The patient and providers should work together to make important decisions about the treatment plan and goals of care. Making the patient an active participant in their treatment rather than the passive recipient will lead to improvement in adherence and outcomes. Patients will engage when they are comfortable speaking with providers and are sufficiently educated about their disease.
Personalized interventions may be built by combining different strategies, such as lifestyle and dietary interventions, NASH-specific pharmacotherapy, comorbidity management, metabolic endoscopy, and bariatric surgery. Although NASH-specific medications are not currently available, approved medications, including pioglitazone or liraglutide, can be considered for therapy.74,75 Ideally, the NAFLD team CPS would manage comorbidities, such as T2DM and dyslipidemia, but this also can be done by a hepatologist or other specialist. Metabolic endoscopy (eg, intragastric balloons) or bariatric surgery would be done by referral.
Resource-Limited Settings
Although the VHA offers care at > 150 medical centers and > 1,000 outpatient clinics, specialty care such as hepatology and sophisticated and novel testing modalities are not available at many facilities. In 2011 VHA launched the Specialty Care Access Network Extension for Community Healthcare Outcomes to bring hepatitis C therapy and liver transplantation evaluations to rural areas without specialists.76-78 It is logical to explore how telehealth can be used for NAFLD care that requires complex management using new treatments and has a high societal impact, particularly when left untreated.
Telehealth must be easy to use and integrated into everyday routines to be useful for NAFLD management by addressing different aspects of promoting self-management, optimizing therapy, and care coordination. Participation in a structured face-to-face or group-based lifestyle program is often jeopardized by time and job constraints but can be successfully overcome using online approaches.79 The Internet-based VA Video Connect videoconferencing, which incorporates cell phone, laptop, or tablet use could help expand lifestyle interventions to a much larger community of patients with NAFLD and overcome local resource constraints. Finally, e-consultation also can be used in circumstances where synchronous communication with specialists may not be necessary.
Patient Monitoring and Quality Metrics
Monitoring of the patient after initiation of an intervention is variable but occurs more frequently at the beginning. For high-intensity dietary interventions, weekly monitoring for the first several weeks can ensure ongoing motivation, and accountability may increase the patient’s confidence and provide encouragement for further weight loss. It also is an opportunity to reestablish goals with patients with declining motivation. Long-term monitoring of patients may occur in 6- to 12-month intervals to document patient-reported outcomes, liver-related mortality, cardiovascular events, malignancies, and disease progression or regression.
While quality indicators have been proposed for cirrhosis care, such indicators have yet to be defined for NALD care.80 Such quality indicators assessed with validated questionnaires should include knowledge about NAFLD, satisfaction with care, perception of quality of care, and patient-reported outcomes. Other indicators may include use of therapies to treat dyslipidemia and T2DM. Last and likely the most important indicator of improved liver health in NAFLD will be either histologic improvement of NASH or improvement of the fibrosis risk category.
Outlook
With the enormous burden of NAFLD on the rise for many more years to come, quality care delivered to patients with NAFLD warrants resource-adaptive population health management strategies. With a limited number of providers specialized in liver disease, provider education assisted by clinical guidelines and decision support tools, development of referral and access to care mechanisms through integrated care, remote monitoring strategies as well as development of patient self-management and community resources will become more important. We have outlined essential components of an effective population health management strategy for NAFLD and actionable items for the VHA to consider when implementing these strategies. This is the time for the VHA to invest in efforts for NAFLD population care. Clearly, consideration must be given to local needs and resources and integration of technology platforms. Addressing NAFLD at a population level will provide yet another opportunity to demonstrate that VHA performs better on quality when compared with care systems in the private sector.81
1. Hunt CM, Turner MJ, Gifford EJ, Britt RB, Su GL. Identifying and treating nonalcoholic fatty liver disease. Fed Pract. 2019;36(1):20-29.
2. Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and non-alcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36(2):64-71.
3. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443-457.e17.
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
5. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901-910.
6. Golabi P, Shahab O, Stepanova M, Sayiner M, Clement SC, Younossi ZM. Long-term outcomes of diabetic patients with non-alcoholic fatty liver disease (NAFLD) [abstract]. Hepatology. 2017;66(suppl 1):1142A-1143A.
7. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-2195.
8. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
9. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
10. Banini B, Mota M, Behnke M, Sharma A, Sanyal AJ. Nonalcoholic steatohepatitis (NASH) has surpassed hepatitis C as the leading cause for listing for liver transplant: implications for NASH in children and young adults. Presented at the American College of Gastroenterology Annual Scientific Meeting, Las Vegas, NV, October 18, 2016. Abstract 46. https://www.eventscribe.com/2016/ACG/QRcode.asp?Pres=199366. Accessed January 15, 2019.
11. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
12. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004-2009. Hepatology. 2015;62(6):1723-1730.
13. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
14. Gunnar W. Bariatric surgery provided by the Veterans Health Administration: current state and a look to the future. J Gen Intern Med. 2017;32(suppl 1):4-5.
15. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Seraq HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.e1-2.
16. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the wait list for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.e1.
17. Beste L, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
18. Mittal S, El-Seraq HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
19. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;55(6):1828-1837.e2.
20. David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB; NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904-1912.
21. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19(5):544-551.
22. Younossi ZM, Henry L. Economic and quality-of-life implications of nonalcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245-1253.
23. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209-1218.
24. Chawla KS, Talwalkar JA, Keach JC, Malinchoc M, Lindor KD, Jorgensen R. Reliability and validity of the chronic liver disease questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol. 2016;3(1):e000069.
25. Shetty A, Syn WK. Health, and economic burden of nonalcoholic fatty liver disease in the United States and its impact on Veterans. Fed Pract. 2019;36(1):14-19.
26. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
27. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with non-alcoholic steatohepatitis (NASH) in the United States. Hepatology. 2018. [Epub ahead of print.]
28. Allen AM, van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. claims database. Hepatology. 2018;68(6):2230-2238.
29. Diabetes mellitus. http://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20. Published July 2017. Accessed January 15, 2019.
30. Grattagliano I, D’Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P; “Steatostop Project” Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17(4):389-394.
31. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157.
32. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med. 2018;48(2):144-151.
33. Standing HC, Jarvis H, Orr J, et al. GPs’ experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68(676):e743-e749.
34. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(10):2809-2816.
35. Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
36. Ratziu V, Cadranel JF, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57(2):376-383.
37. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Seraq H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10-14.
38. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern Med J. 2013;43(3):247-253.
39. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758-765.
40. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
41. NICE National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. https://www.nice.org.uk/guidance/ng49. Published July 2016. Accessed January 15, 2019.
42. European Association for the Study of the Liver (EASL), European Association for the Study of diabetes (EASD), European Association for the study of obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
43. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
44. Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138-147.
45. Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359-1368.
46. Harman DJ, Ryder SD, James MW, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther. 2018;47(4):504-515.
47. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1-10.
48. Rinella ME, Lominadze Z, Loomba R, et al. Practice pattern in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4-12.
49. El-Atem NA, Wojcik K, Horsfall L, et al. Patterns of service utilization within Australian hepatology clinics: high prevalence of advanced liver disease. Intern Med. 2016;46(4):420-426.
50. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705-712.
51. Nascimbeni F, Aron-Wisnewsky J, Pais R, et al; LIDO Study Group. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075.
52. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829-846.
53. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
54. Barritt AS 4th, Gitlin N, Klein S, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemp Clin Trials. 2017;61:33-38.
55. Meier SK, Shah ND, Talwalkar JA. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(4):492-496.
56. Dulai PS, Singh S, Ohno-Machado L, Sandborn WJ. Population health management for inflammatory bowel disease. Gastroenterology. 2018;154(1):37-45.
57. Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis C in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35(7):24-29.
58. Cobbold JFL, Raveendran S, Peake CM, Anstee QM, Yee MS, Thursz MR. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol. 2013;4(4):263-269.
59. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487-497.
60. Harrison SA. NASH, from diagnosis to treatment: where do we stand? Hepatology. 2015;62(6):1652-1655.
61. Patel PJ, Hayward KL, Rudra R, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: implications for disease severity and management. Medicine (Baltimore). 2017;96(26):e6761.
62. Kanwal F, Mapashki S, Smith D, et al. Implementation of a population-based cirrhosis identification and management system. Clin Gastroenterol Hepatol. 2018;16(8):1182-1186.e2.
63. Mlynarski L, Schlesinger D, Lotan R, et al. Non-alcoholic fatty liver disease is not associated with a lower health perception. World J Gastroenterol. 2016;22(17):4362-4372.
64. Centis E, Moscatiello S, Bugianesi E, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58(4):771-777.
65. Zelber-Sagi S, Bord S, Dror-Lavi G, et al. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol. 2017;23(10):1881-1890.
66. Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerized adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34(9):1123-1132.
67. Verma M, Stites S, Navarro V. Bringing assessment of patient-reported outcomes to hepatology practice. Clin Gastroenterol Hepatol. 2018;16(3):447-448.
68. Ahmed S, Ware P, Gardner W, et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 8: patient-reported outcomes in electronic health records can inform clinical and policy decisions. J Clin Epidemiol. 2017;89:160-167.
69. Younossi ZM, Stepanova M, Lawitz E, et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38(10):1849-1859.
70. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259-2266.
71. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2018;pii:S1542-3565(18)30613-X. [Epub ahead of print.]
72. Searight R. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79(4):277-284.
73. Vallis M, Piccinini-Vallis H, Sharma AM, Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam Physician. 2013:59(1):27-31.
74. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
75. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
76. Salgia RJ, Mullan PB, McCurdy H, Sales A, Moseley RH, Su GL. The educational impact of the specialty care access network-extension of community healthcare outcomes program. Telemed J E Health. 2014;20(11):1004-1008.
77. Konjeti VR, Heuman D, Bajaj J, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol. 2019;17(1):207-209.e1
78. Su GL, Glass L, Tapper EB, Van T, Waljee AK, Sales AE. Virtual consultations through the Veterans Administration SCAN-ECHO project improves survival for veterans with liver disease. Hepatology. 2018;68(6):2317-2324.
79. Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155-1163.
80. Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010,8(8):709-717.
81. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs Non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885.
1. Hunt CM, Turner MJ, Gifford EJ, Britt RB, Su GL. Identifying and treating nonalcoholic fatty liver disease. Fed Pract. 2019;36(1):20-29.
2. Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and non-alcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract. 2019;36(2):64-71.
3. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443-457.e17.
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
5. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901-910.
6. Golabi P, Shahab O, Stepanova M, Sayiner M, Clement SC, Younossi ZM. Long-term outcomes of diabetic patients with non-alcoholic fatty liver disease (NAFLD) [abstract]. Hepatology. 2017;66(suppl 1):1142A-1143A.
7. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-2195.
8. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
9. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
10. Banini B, Mota M, Behnke M, Sharma A, Sanyal AJ. Nonalcoholic steatohepatitis (NASH) has surpassed hepatitis C as the leading cause for listing for liver transplant: implications for NASH in children and young adults. Presented at the American College of Gastroenterology Annual Scientific Meeting, Las Vegas, NV, October 18, 2016. Abstract 46. https://www.eventscribe.com/2016/ACG/QRcode.asp?Pres=199366. Accessed January 15, 2019.
11. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
12. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004-2009. Hepatology. 2015;62(6):1723-1730.
13. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
14. Gunnar W. Bariatric surgery provided by the Veterans Health Administration: current state and a look to the future. J Gen Intern Med. 2017;32(suppl 1):4-5.
15. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Seraq HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.e1-2.
16. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the wait list for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.e1.
17. Beste L, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
18. Mittal S, El-Seraq HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
19. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;55(6):1828-1837.e2.
20. David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB; NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904-1912.
21. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19(5):544-551.
22. Younossi ZM, Henry L. Economic and quality-of-life implications of nonalcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245-1253.
23. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209-1218.
24. Chawla KS, Talwalkar JA, Keach JC, Malinchoc M, Lindor KD, Jorgensen R. Reliability and validity of the chronic liver disease questionnaire (CLDQ) in adults with non-alcoholic steatohepatitis (NASH). BMJ Open Gastroenterol. 2016;3(1):e000069.
25. Shetty A, Syn WK. Health, and economic burden of nonalcoholic fatty liver disease in the United States and its impact on Veterans. Fed Pract. 2019;36(1):14-19.
26. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
27. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with non-alcoholic steatohepatitis (NASH) in the United States. Hepatology. 2018. [Epub ahead of print.]
28. Allen AM, van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large U.S. claims database. Hepatology. 2018;68(6):2230-2238.
29. Diabetes mellitus. http://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20. Published July 2017. Accessed January 15, 2019.
30. Grattagliano I, D’Ambrosio G, Palmieri VO, Moschetta A, Palasciano G, Portincasa P; “Steatostop Project” Group. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17(4):389-394.
31. Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157.
32. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med. 2018;48(2):144-151.
33. Standing HC, Jarvis H, Orr J, et al. GPs’ experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68(676):e743-e749.
34. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(10):2809-2816.
35. Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
36. Ratziu V, Cadranel JF, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57(2):376-383.
37. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Seraq H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10-14.
38. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern Med J. 2013;43(3):247-253.
39. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758-765.
40. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
41. NICE National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. https://www.nice.org.uk/guidance/ng49. Published July 2016. Accessed January 15, 2019.
42. European Association for the Study of the Liver (EASL), European Association for the Study of diabetes (EASD), European Association for the study of obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
43. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
44. Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63(1):138-147.
45. Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359-1368.
46. Harman DJ, Ryder SD, James MW, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther. 2018;47(4):504-515.
47. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1-10.
48. Rinella ME, Lominadze Z, Loomba R, et al. Practice pattern in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9(1):4-12.
49. El-Atem NA, Wojcik K, Horsfall L, et al. Patterns of service utilization within Australian hepatology clinics: high prevalence of advanced liver disease. Intern Med. 2016;46(4):420-426.
50. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and nonalcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705-712.
51. Nascimbeni F, Aron-Wisnewsky J, Pais R, et al; LIDO Study Group. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075.
52. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829-846.
53. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
54. Barritt AS 4th, Gitlin N, Klein S, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemp Clin Trials. 2017;61:33-38.
55. Meier SK, Shah ND, Talwalkar JA. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(4):492-496.
56. Dulai PS, Singh S, Ohno-Machado L, Sandborn WJ. Population health management for inflammatory bowel disease. Gastroenterology. 2018;154(1):37-45.
57. Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis C in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35(7):24-29.
58. Cobbold JFL, Raveendran S, Peake CM, Anstee QM, Yee MS, Thursz MR. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol. 2013;4(4):263-269.
59. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487-497.
60. Harrison SA. NASH, from diagnosis to treatment: where do we stand? Hepatology. 2015;62(6):1652-1655.
61. Patel PJ, Hayward KL, Rudra R, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: implications for disease severity and management. Medicine (Baltimore). 2017;96(26):e6761.
62. Kanwal F, Mapashki S, Smith D, et al. Implementation of a population-based cirrhosis identification and management system. Clin Gastroenterol Hepatol. 2018;16(8):1182-1186.e2.
63. Mlynarski L, Schlesinger D, Lotan R, et al. Non-alcoholic fatty liver disease is not associated with a lower health perception. World J Gastroenterol. 2016;22(17):4362-4372.
64. Centis E, Moscatiello S, Bugianesi E, et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J Hepatol. 2013;58(4):771-777.
65. Zelber-Sagi S, Bord S, Dror-Lavi G, et al. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol. 2017;23(10):1881-1890.
66. Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerized adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34(9):1123-1132.
67. Verma M, Stites S, Navarro V. Bringing assessment of patient-reported outcomes to hepatology practice. Clin Gastroenterol Hepatol. 2018;16(3):447-448.
68. Ahmed S, Ware P, Gardner W, et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 8: patient-reported outcomes in electronic health records can inform clinical and policy decisions. J Clin Epidemiol. 2017;89:160-167.
69. Younossi ZM, Stepanova M, Lawitz E, et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38(10):1849-1859.
70. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259-2266.
71. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2018;pii:S1542-3565(18)30613-X. [Epub ahead of print.]
72. Searight R. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79(4):277-284.
73. Vallis M, Piccinini-Vallis H, Sharma AM, Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam Physician. 2013:59(1):27-31.
74. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
75. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
76. Salgia RJ, Mullan PB, McCurdy H, Sales A, Moseley RH, Su GL. The educational impact of the specialty care access network-extension of community healthcare outcomes program. Telemed J E Health. 2014;20(11):1004-1008.
77. Konjeti VR, Heuman D, Bajaj J, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol. 2019;17(1):207-209.e1
78. Su GL, Glass L, Tapper EB, Van T, Waljee AK, Sales AE. Virtual consultations through the Veterans Administration SCAN-ECHO project improves survival for veterans with liver disease. Hepatology. 2018;68(6):2317-2324.
79. Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155-1163.
80. Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010,8(8):709-717.
81. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs Non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885.
International survey probes oxygen’s efficacy for cluster headache
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
FROM HEADACHE
Key clinical point: Oxygen is a highly effective treatment for cluster headache with few complications.
Major finding: More than half of respondents (54%) reported that triptans and oxygen were completely or very effective.
Study details: Analysis of data from 1,604 people with cluster headache who completed the online Cluster Headache Questionnaire.
Disclosures: The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
Source: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
Comorbidities and Nonalcoholic Fatty Liver Disease: The Chicken, the Egg, or Both?
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
Physiological versus pathological cardiac remodeling in athletes
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Raising More Than Moods: Escitalopram-Associated Priapism
Selective serotonin reuptake inhibitors are a common first choice medication for anxiety and depression treatment, but health care providers should be aware of priapism as a possible adverse effect.
Case Presentation
An 80-year-old white male was evaluated in a primary care clinic following a recent hospitalization for a suicide attempt. His past medical history included type 2 diabetes mellitus, chronic atrial fibrillation, essential hypertension and hyperlipidemia, and no prior psychiatric illness. Six weeks after his wife died of cancer, the patient attempted suicide by slitting his wrists, which resulted in significant blood loss and tendon damage.
After medical stabilization he was treated at an inpatient psychiatric facility for 10 days. There was no evidence of impaired memory nor psychosis during his hospitalization. He was prescribed doxazosin 1 mg twice daily and finasteride 5 mg daily for obstructive urinary symptoms, along with escitalopram 5 mg daily for depression and continuation of prior medications, including glipizide 10 mg twice daily, simvastatin 20 mg daily, metformin 500 mg twice daily, and lisinopril 20 mg daily. The patient’s estimated glomerular filtration rate was 85 at the time of these events.
He was evaluated by the mental health staff at the time of his primary care outpatient visit and noted to have a Patient Health Questionnaire (PHQ-9) score of 5 (mild depression symptoms) and a Generalized Anxiety Disorder 7 Item Scale (GAD-7) score of 1 (minimum anxiety symptoms). Eleven days later during his counseling appointment, he mentioned to staff that he had experienced a painful erection the day before, which lasted 4 hours. The primary care pharmacist was consulted for review of potential medication triggers. It was noted that there was a low frequency of priapism with both doxazosin and escitalopram, a selective serotonin reuptake inhibitor (SSRI). The provider team felt that the α blocker (doxazosin) was more likely than was the SSRI to cause the reported priapism event. Doxazosin was discontinued, and escitalopram 5 mg daily was maintained. His mood remained stable with no further suicidal ideation.
Eighteen days after discontinuation of doxazosin, the patient experienced a second priapism episode. He reported 2 days later that he experienced a prolonged, painful erection that lasted 4 hours and resolved without intervention. The patient’s mood continued without further suicidal thoughts, his appetite was normal, he had good social support and played cards with friends regularly. At that time, the decision was made to discontinue the escitalopram. The SSRI was felt to be a possible cause of priapism due to the length of time off doxazosin in relation to the second event.
The patient continued to do well 15 months after discontinuation of these medications. Unfortunately, he did not seek medical care during either episode of priapism, but he was felt to be reliable in his report based on a normal mental status exam. He does not have any of the other known risk factors for priapism, suggesting a possible association with his α blocker and SSRI.
Discussion
Priapism is a prolonged, painful erection lasting more than 4 hours and is considered a urologic emergency. It is divided into ischemic and nonischemic types. Ischemic priapism occurs with blood dyscrasias, such as sickle cell disease, thalassemia, leukemia, neurologic conditions affecting the spinal cord, and malignancies of bladder/prostate. The lifetime probability of priapism in patients affected by sickle cell disease is estimated at 29% to 42%.1 Medications associated with priapism include cocaine, ondansetron, antipsychotics, excessive use of erectile dysfunction drugs, and increasingly, antidepressants.2-8
Nonischemic priapism is usually associated with pelvic trauma. Cavernous blood gas obtained at the time of the event can help distinguish between the 2 types. The color of the aspirated blood sample is black in patients with ischemic priapism. Corporal blood gas analysis shows hypoxemia and acidemia. The color of blood is red in patients with nonischemic priapism and shows normal oxygen and pH. Priapism is a urologic emergency requiring aspiration of blood from the cavernous sinus to prevent ischemic tissue damage. At times surgical decompression may be required if aspiration is not successful.
Adrenergic α-blocking agents were developed for treatment of hypertension. They have become popular for management of lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Doxazosin, prazosin, and terazosin are nonuroselective and have a higher risk of cardiac adverse effects (AEs), including dizziness and orthostatic hypotension. Lexicomp lists < 1% incidence of priapism associated with doxazosin.9 The drug is metabolized by CYP3A4 with secondary pathways, including CYP2D6 and 2C9 with a drug half-life of 22 hours. Newer agents (eg, tamsulosin, alfuzosin) are considered more uroselective, targeting the α-1b receptors. The older agents have more effect on the α-1a receptors, which are also present at higher level in the cardiovascular system.10 By blocking sympathetic stimuli responsible for penile detumescence, the nonselective α blockers have a higher propensity to cause priapism. There seems to be a direct correlation between higher doses and increased risk of priapism.11 Our patient was at a relatively low dose (1 mg twice daily) of the nonselective agent doxazosin for treatment of his LUTS.
Primary care providers and psychiatrists treating depression are familiar with common sexual AEs of the SSRI class of medications. Decreased sexual desire and delayed orgasm and ejaculation are all issues that lead patients to discontinue treatment. Although SSRIs are considered first-line treatment for depression, reports indicate that up to 60% of patients with prior normal sexual function started on paroxetine may experience sexual AEs.12 The exact frequency is difficult to estimate due to underreporting of these issues by patients.
A review of the literature for cases of priapism associated with SSRIs shows that often there is more than 1 possible drug trigger, and medications used in combination may be a risk factor. It is hypothesized that SSRI action on 5-HT3 receptors may be responsible for priapism occurring in patients treated with SSRIs.13 One study cites a case of priapism in a veteran being treated with escitalopram, prazosin, and trazodone for posttraumatic stress disorder.14 Trazodone inhibits the neuronal uptake of serotonin and is used to treat depression in addition to off-label use in treatment of insomnia. Trazodone is implicated in cases of priapism via its α-blocking properties. In the aforementioned case, trazodone was initially thought to be the causative agent and was discontinued. The patient had recurrent symptoms at which time his prazosin was discontinued, and he had no further events.
Another case cites citalopram-induced priapism that occurred with an accidental overdose of citalopram 80 mg, when a patient confused his antidepressant with 81-mg aspirin tablets.15 He also had a prior history of priapism while taking trazodone. We found only 1 case listing escitalopram as the probable causative agent of priapism.16 Similar to our patient, that case had no risk factors prior to escitalopram administration. Lexicomp notes < 1% incidence of priapism reported in postmarketing studies.
Our patient had been off doxazosin for 18 days when his second event of priapism occurred. It is less likely given the half-life of doxazosin (t ½ = 22 hours) that the α blocker was the causative agent, though a combination of the 2 agents cannot be excluded as a significant factor. The Naranjo Score is an algorithm for determining the likelihood of whether an adverse drug reaction is due to the drug or other factors.17 Scoring ranks the event as probable, possible, or doubtful. This case scored +3 (+2 = appeared after suspected drug given, +1 = improved when drug discontinued), indicating possible association of escitalopram and priapism.
Conclusion
In view of the frequent use of SSRIs in treatment of depression, it may be prudent to advise patients of this uncommon but serious medication AE. Recent use of α blockers may be a risk factor in combination with SSRI therapy. Patients should be counseled to seek emergency care in the event of prolonged erection when discussing potential AEs of SSRI therapy.
1. Manjunath AS, Hofer MD. Urologic emergencies. Med Clin North Am. 2018;102(2):373-385.
2. Altman AL, Seftel AD, Brown SL, et al. Cocaine associated priapism. J Urol. 1999;161(6):1817-1818.
3. Pivot D, Javot L, Swiegot D, et al. Two Cases of recurrent priapism during antineoplastic chemotherapy: think about ondansetron. Therapie. 2013;68(6):409-410.
4. Fu E, Kovach JG, Dubin WR. Priapism associate with antipsychotic medication use: case report. J Clin Pshychopharmacol. 2017;37(4):477-478.
5. Saghafi O, Kao A, Druck J. Recurrent priapism from therapeutic quetiapine. West J Emerg Med, 2014;15(1):114-116.
6. King SH, Hallock M, Strote J, et al. Tadalafil-associated priapism. Urology. 2005;66(2):432.
7. Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64(2):240-255.
8. Bhat IA, Shannon KD, Ara A, et al. Ninety-six hours ordeal of priapism induced by paroxetine: a case report and literature review. Int J Psychiatry Med. 2015;50(3):326-334.
9. Cardura [package insert]. New York, NY: Pfizer; 2009.
10. Spagnul SJ, Cabral PH, Verndl DO, Glina S. Adrenergic α-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23(3):95-98.
11. Avisrro MU, Fernandez IA, Sánchez AS, García-Pando AC, Arias LM, del Pozo JG. Doxazosin and priapism. J Urol. 2000;163(1): 238.
12. Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatments. Drug Healthc Patient Saf. 2010;2:141-150.
13. Bonnot O, Warot D, Cohen D. Priapism associated with sertraline. J Am Acad Child Adolesc Psychiatry. 2007;46(7):790-791.
14. Mann RA, George AK. Recurrent priapism in a military veteran receiving treatment for PTSD. Mil Med. 2017;182(11):e2014-e2017.
15. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
16. Tulachan P, Chapagain M, Ojha SP, Dhungana S. Escitalopram induced priapism. J Inst Med. 2014;36(1):118-120.
17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
Selective serotonin reuptake inhibitors are a common first choice medication for anxiety and depression treatment, but health care providers should be aware of priapism as a possible adverse effect.
Selective serotonin reuptake inhibitors are a common first choice medication for anxiety and depression treatment, but health care providers should be aware of priapism as a possible adverse effect.
Case Presentation
An 80-year-old white male was evaluated in a primary care clinic following a recent hospitalization for a suicide attempt. His past medical history included type 2 diabetes mellitus, chronic atrial fibrillation, essential hypertension and hyperlipidemia, and no prior psychiatric illness. Six weeks after his wife died of cancer, the patient attempted suicide by slitting his wrists, which resulted in significant blood loss and tendon damage.
After medical stabilization he was treated at an inpatient psychiatric facility for 10 days. There was no evidence of impaired memory nor psychosis during his hospitalization. He was prescribed doxazosin 1 mg twice daily and finasteride 5 mg daily for obstructive urinary symptoms, along with escitalopram 5 mg daily for depression and continuation of prior medications, including glipizide 10 mg twice daily, simvastatin 20 mg daily, metformin 500 mg twice daily, and lisinopril 20 mg daily. The patient’s estimated glomerular filtration rate was 85 at the time of these events.
He was evaluated by the mental health staff at the time of his primary care outpatient visit and noted to have a Patient Health Questionnaire (PHQ-9) score of 5 (mild depression symptoms) and a Generalized Anxiety Disorder 7 Item Scale (GAD-7) score of 1 (minimum anxiety symptoms). Eleven days later during his counseling appointment, he mentioned to staff that he had experienced a painful erection the day before, which lasted 4 hours. The primary care pharmacist was consulted for review of potential medication triggers. It was noted that there was a low frequency of priapism with both doxazosin and escitalopram, a selective serotonin reuptake inhibitor (SSRI). The provider team felt that the α blocker (doxazosin) was more likely than was the SSRI to cause the reported priapism event. Doxazosin was discontinued, and escitalopram 5 mg daily was maintained. His mood remained stable with no further suicidal ideation.
Eighteen days after discontinuation of doxazosin, the patient experienced a second priapism episode. He reported 2 days later that he experienced a prolonged, painful erection that lasted 4 hours and resolved without intervention. The patient’s mood continued without further suicidal thoughts, his appetite was normal, he had good social support and played cards with friends regularly. At that time, the decision was made to discontinue the escitalopram. The SSRI was felt to be a possible cause of priapism due to the length of time off doxazosin in relation to the second event.
The patient continued to do well 15 months after discontinuation of these medications. Unfortunately, he did not seek medical care during either episode of priapism, but he was felt to be reliable in his report based on a normal mental status exam. He does not have any of the other known risk factors for priapism, suggesting a possible association with his α blocker and SSRI.
Discussion
Priapism is a prolonged, painful erection lasting more than 4 hours and is considered a urologic emergency. It is divided into ischemic and nonischemic types. Ischemic priapism occurs with blood dyscrasias, such as sickle cell disease, thalassemia, leukemia, neurologic conditions affecting the spinal cord, and malignancies of bladder/prostate. The lifetime probability of priapism in patients affected by sickle cell disease is estimated at 29% to 42%.1 Medications associated with priapism include cocaine, ondansetron, antipsychotics, excessive use of erectile dysfunction drugs, and increasingly, antidepressants.2-8
Nonischemic priapism is usually associated with pelvic trauma. Cavernous blood gas obtained at the time of the event can help distinguish between the 2 types. The color of the aspirated blood sample is black in patients with ischemic priapism. Corporal blood gas analysis shows hypoxemia and acidemia. The color of blood is red in patients with nonischemic priapism and shows normal oxygen and pH. Priapism is a urologic emergency requiring aspiration of blood from the cavernous sinus to prevent ischemic tissue damage. At times surgical decompression may be required if aspiration is not successful.
Adrenergic α-blocking agents were developed for treatment of hypertension. They have become popular for management of lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Doxazosin, prazosin, and terazosin are nonuroselective and have a higher risk of cardiac adverse effects (AEs), including dizziness and orthostatic hypotension. Lexicomp lists < 1% incidence of priapism associated with doxazosin.9 The drug is metabolized by CYP3A4 with secondary pathways, including CYP2D6 and 2C9 with a drug half-life of 22 hours. Newer agents (eg, tamsulosin, alfuzosin) are considered more uroselective, targeting the α-1b receptors. The older agents have more effect on the α-1a receptors, which are also present at higher level in the cardiovascular system.10 By blocking sympathetic stimuli responsible for penile detumescence, the nonselective α blockers have a higher propensity to cause priapism. There seems to be a direct correlation between higher doses and increased risk of priapism.11 Our patient was at a relatively low dose (1 mg twice daily) of the nonselective agent doxazosin for treatment of his LUTS.
Primary care providers and psychiatrists treating depression are familiar with common sexual AEs of the SSRI class of medications. Decreased sexual desire and delayed orgasm and ejaculation are all issues that lead patients to discontinue treatment. Although SSRIs are considered first-line treatment for depression, reports indicate that up to 60% of patients with prior normal sexual function started on paroxetine may experience sexual AEs.12 The exact frequency is difficult to estimate due to underreporting of these issues by patients.
A review of the literature for cases of priapism associated with SSRIs shows that often there is more than 1 possible drug trigger, and medications used in combination may be a risk factor. It is hypothesized that SSRI action on 5-HT3 receptors may be responsible for priapism occurring in patients treated with SSRIs.13 One study cites a case of priapism in a veteran being treated with escitalopram, prazosin, and trazodone for posttraumatic stress disorder.14 Trazodone inhibits the neuronal uptake of serotonin and is used to treat depression in addition to off-label use in treatment of insomnia. Trazodone is implicated in cases of priapism via its α-blocking properties. In the aforementioned case, trazodone was initially thought to be the causative agent and was discontinued. The patient had recurrent symptoms at which time his prazosin was discontinued, and he had no further events.
Another case cites citalopram-induced priapism that occurred with an accidental overdose of citalopram 80 mg, when a patient confused his antidepressant with 81-mg aspirin tablets.15 He also had a prior history of priapism while taking trazodone. We found only 1 case listing escitalopram as the probable causative agent of priapism.16 Similar to our patient, that case had no risk factors prior to escitalopram administration. Lexicomp notes < 1% incidence of priapism reported in postmarketing studies.
Our patient had been off doxazosin for 18 days when his second event of priapism occurred. It is less likely given the half-life of doxazosin (t ½ = 22 hours) that the α blocker was the causative agent, though a combination of the 2 agents cannot be excluded as a significant factor. The Naranjo Score is an algorithm for determining the likelihood of whether an adverse drug reaction is due to the drug or other factors.17 Scoring ranks the event as probable, possible, or doubtful. This case scored +3 (+2 = appeared after suspected drug given, +1 = improved when drug discontinued), indicating possible association of escitalopram and priapism.
Conclusion
In view of the frequent use of SSRIs in treatment of depression, it may be prudent to advise patients of this uncommon but serious medication AE. Recent use of α blockers may be a risk factor in combination with SSRI therapy. Patients should be counseled to seek emergency care in the event of prolonged erection when discussing potential AEs of SSRI therapy.
Case Presentation
An 80-year-old white male was evaluated in a primary care clinic following a recent hospitalization for a suicide attempt. His past medical history included type 2 diabetes mellitus, chronic atrial fibrillation, essential hypertension and hyperlipidemia, and no prior psychiatric illness. Six weeks after his wife died of cancer, the patient attempted suicide by slitting his wrists, which resulted in significant blood loss and tendon damage.
After medical stabilization he was treated at an inpatient psychiatric facility for 10 days. There was no evidence of impaired memory nor psychosis during his hospitalization. He was prescribed doxazosin 1 mg twice daily and finasteride 5 mg daily for obstructive urinary symptoms, along with escitalopram 5 mg daily for depression and continuation of prior medications, including glipizide 10 mg twice daily, simvastatin 20 mg daily, metformin 500 mg twice daily, and lisinopril 20 mg daily. The patient’s estimated glomerular filtration rate was 85 at the time of these events.
He was evaluated by the mental health staff at the time of his primary care outpatient visit and noted to have a Patient Health Questionnaire (PHQ-9) score of 5 (mild depression symptoms) and a Generalized Anxiety Disorder 7 Item Scale (GAD-7) score of 1 (minimum anxiety symptoms). Eleven days later during his counseling appointment, he mentioned to staff that he had experienced a painful erection the day before, which lasted 4 hours. The primary care pharmacist was consulted for review of potential medication triggers. It was noted that there was a low frequency of priapism with both doxazosin and escitalopram, a selective serotonin reuptake inhibitor (SSRI). The provider team felt that the α blocker (doxazosin) was more likely than was the SSRI to cause the reported priapism event. Doxazosin was discontinued, and escitalopram 5 mg daily was maintained. His mood remained stable with no further suicidal ideation.
Eighteen days after discontinuation of doxazosin, the patient experienced a second priapism episode. He reported 2 days later that he experienced a prolonged, painful erection that lasted 4 hours and resolved without intervention. The patient’s mood continued without further suicidal thoughts, his appetite was normal, he had good social support and played cards with friends regularly. At that time, the decision was made to discontinue the escitalopram. The SSRI was felt to be a possible cause of priapism due to the length of time off doxazosin in relation to the second event.
The patient continued to do well 15 months after discontinuation of these medications. Unfortunately, he did not seek medical care during either episode of priapism, but he was felt to be reliable in his report based on a normal mental status exam. He does not have any of the other known risk factors for priapism, suggesting a possible association with his α blocker and SSRI.
Discussion
Priapism is a prolonged, painful erection lasting more than 4 hours and is considered a urologic emergency. It is divided into ischemic and nonischemic types. Ischemic priapism occurs with blood dyscrasias, such as sickle cell disease, thalassemia, leukemia, neurologic conditions affecting the spinal cord, and malignancies of bladder/prostate. The lifetime probability of priapism in patients affected by sickle cell disease is estimated at 29% to 42%.1 Medications associated with priapism include cocaine, ondansetron, antipsychotics, excessive use of erectile dysfunction drugs, and increasingly, antidepressants.2-8
Nonischemic priapism is usually associated with pelvic trauma. Cavernous blood gas obtained at the time of the event can help distinguish between the 2 types. The color of the aspirated blood sample is black in patients with ischemic priapism. Corporal blood gas analysis shows hypoxemia and acidemia. The color of blood is red in patients with nonischemic priapism and shows normal oxygen and pH. Priapism is a urologic emergency requiring aspiration of blood from the cavernous sinus to prevent ischemic tissue damage. At times surgical decompression may be required if aspiration is not successful.
Adrenergic α-blocking agents were developed for treatment of hypertension. They have become popular for management of lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Doxazosin, prazosin, and terazosin are nonuroselective and have a higher risk of cardiac adverse effects (AEs), including dizziness and orthostatic hypotension. Lexicomp lists < 1% incidence of priapism associated with doxazosin.9 The drug is metabolized by CYP3A4 with secondary pathways, including CYP2D6 and 2C9 with a drug half-life of 22 hours. Newer agents (eg, tamsulosin, alfuzosin) are considered more uroselective, targeting the α-1b receptors. The older agents have more effect on the α-1a receptors, which are also present at higher level in the cardiovascular system.10 By blocking sympathetic stimuli responsible for penile detumescence, the nonselective α blockers have a higher propensity to cause priapism. There seems to be a direct correlation between higher doses and increased risk of priapism.11 Our patient was at a relatively low dose (1 mg twice daily) of the nonselective agent doxazosin for treatment of his LUTS.
Primary care providers and psychiatrists treating depression are familiar with common sexual AEs of the SSRI class of medications. Decreased sexual desire and delayed orgasm and ejaculation are all issues that lead patients to discontinue treatment. Although SSRIs are considered first-line treatment for depression, reports indicate that up to 60% of patients with prior normal sexual function started on paroxetine may experience sexual AEs.12 The exact frequency is difficult to estimate due to underreporting of these issues by patients.
A review of the literature for cases of priapism associated with SSRIs shows that often there is more than 1 possible drug trigger, and medications used in combination may be a risk factor. It is hypothesized that SSRI action on 5-HT3 receptors may be responsible for priapism occurring in patients treated with SSRIs.13 One study cites a case of priapism in a veteran being treated with escitalopram, prazosin, and trazodone for posttraumatic stress disorder.14 Trazodone inhibits the neuronal uptake of serotonin and is used to treat depression in addition to off-label use in treatment of insomnia. Trazodone is implicated in cases of priapism via its α-blocking properties. In the aforementioned case, trazodone was initially thought to be the causative agent and was discontinued. The patient had recurrent symptoms at which time his prazosin was discontinued, and he had no further events.
Another case cites citalopram-induced priapism that occurred with an accidental overdose of citalopram 80 mg, when a patient confused his antidepressant with 81-mg aspirin tablets.15 He also had a prior history of priapism while taking trazodone. We found only 1 case listing escitalopram as the probable causative agent of priapism.16 Similar to our patient, that case had no risk factors prior to escitalopram administration. Lexicomp notes < 1% incidence of priapism reported in postmarketing studies.
Our patient had been off doxazosin for 18 days when his second event of priapism occurred. It is less likely given the half-life of doxazosin (t ½ = 22 hours) that the α blocker was the causative agent, though a combination of the 2 agents cannot be excluded as a significant factor. The Naranjo Score is an algorithm for determining the likelihood of whether an adverse drug reaction is due to the drug or other factors.17 Scoring ranks the event as probable, possible, or doubtful. This case scored +3 (+2 = appeared after suspected drug given, +1 = improved when drug discontinued), indicating possible association of escitalopram and priapism.
Conclusion
In view of the frequent use of SSRIs in treatment of depression, it may be prudent to advise patients of this uncommon but serious medication AE. Recent use of α blockers may be a risk factor in combination with SSRI therapy. Patients should be counseled to seek emergency care in the event of prolonged erection when discussing potential AEs of SSRI therapy.
1. Manjunath AS, Hofer MD. Urologic emergencies. Med Clin North Am. 2018;102(2):373-385.
2. Altman AL, Seftel AD, Brown SL, et al. Cocaine associated priapism. J Urol. 1999;161(6):1817-1818.
3. Pivot D, Javot L, Swiegot D, et al. Two Cases of recurrent priapism during antineoplastic chemotherapy: think about ondansetron. Therapie. 2013;68(6):409-410.
4. Fu E, Kovach JG, Dubin WR. Priapism associate with antipsychotic medication use: case report. J Clin Pshychopharmacol. 2017;37(4):477-478.
5. Saghafi O, Kao A, Druck J. Recurrent priapism from therapeutic quetiapine. West J Emerg Med, 2014;15(1):114-116.
6. King SH, Hallock M, Strote J, et al. Tadalafil-associated priapism. Urology. 2005;66(2):432.
7. Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64(2):240-255.
8. Bhat IA, Shannon KD, Ara A, et al. Ninety-six hours ordeal of priapism induced by paroxetine: a case report and literature review. Int J Psychiatry Med. 2015;50(3):326-334.
9. Cardura [package insert]. New York, NY: Pfizer; 2009.
10. Spagnul SJ, Cabral PH, Verndl DO, Glina S. Adrenergic α-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23(3):95-98.
11. Avisrro MU, Fernandez IA, Sánchez AS, García-Pando AC, Arias LM, del Pozo JG. Doxazosin and priapism. J Urol. 2000;163(1): 238.
12. Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatments. Drug Healthc Patient Saf. 2010;2:141-150.
13. Bonnot O, Warot D, Cohen D. Priapism associated with sertraline. J Am Acad Child Adolesc Psychiatry. 2007;46(7):790-791.
14. Mann RA, George AK. Recurrent priapism in a military veteran receiving treatment for PTSD. Mil Med. 2017;182(11):e2014-e2017.
15. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
16. Tulachan P, Chapagain M, Ojha SP, Dhungana S. Escitalopram induced priapism. J Inst Med. 2014;36(1):118-120.
17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
1. Manjunath AS, Hofer MD. Urologic emergencies. Med Clin North Am. 2018;102(2):373-385.
2. Altman AL, Seftel AD, Brown SL, et al. Cocaine associated priapism. J Urol. 1999;161(6):1817-1818.
3. Pivot D, Javot L, Swiegot D, et al. Two Cases of recurrent priapism during antineoplastic chemotherapy: think about ondansetron. Therapie. 2013;68(6):409-410.
4. Fu E, Kovach JG, Dubin WR. Priapism associate with antipsychotic medication use: case report. J Clin Pshychopharmacol. 2017;37(4):477-478.
5. Saghafi O, Kao A, Druck J. Recurrent priapism from therapeutic quetiapine. West J Emerg Med, 2014;15(1):114-116.
6. King SH, Hallock M, Strote J, et al. Tadalafil-associated priapism. Urology. 2005;66(2):432.
7. Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64(2):240-255.
8. Bhat IA, Shannon KD, Ara A, et al. Ninety-six hours ordeal of priapism induced by paroxetine: a case report and literature review. Int J Psychiatry Med. 2015;50(3):326-334.
9. Cardura [package insert]. New York, NY: Pfizer; 2009.
10. Spagnul SJ, Cabral PH, Verndl DO, Glina S. Adrenergic α-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23(3):95-98.
11. Avisrro MU, Fernandez IA, Sánchez AS, García-Pando AC, Arias LM, del Pozo JG. Doxazosin and priapism. J Urol. 2000;163(1): 238.
12. Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatments. Drug Healthc Patient Saf. 2010;2:141-150.
13. Bonnot O, Warot D, Cohen D. Priapism associated with sertraline. J Am Acad Child Adolesc Psychiatry. 2007;46(7):790-791.
14. Mann RA, George AK. Recurrent priapism in a military veteran receiving treatment for PTSD. Mil Med. 2017;182(11):e2014-e2017.
15. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
16. Tulachan P, Chapagain M, Ojha SP, Dhungana S. Escitalopram induced priapism. J Inst Med. 2014;36(1):118-120.
17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
Adult HIV patients should receive standard vaccinations, with caveats
Patients infected with HIV have an increased risk of mortality and morbidity from diseases that are preventable with vaccines. Undervaccination of these patients poses a major concern, according to a literature review of the vaccine response in the adult patient with HIV published in The American Journal of Medicine.
Despite the fact that data are limited, patients infected with HIV are advised to receive their age-specific and risk group−based vaccines, according to Firas El Chaer, MD, of the University of Maryland, Baltimore, and his colleague.
HIV patients are of particular concern regarding vaccination, because, despite the use of retroviral therapy, CD4+ T-lymphocytes in individuals infected with HIV remain lower than in those without HIV. In addition, HIV causes an inappropriate response to B-cell stimulation, which results in suboptimal primary and secondary response to vaccination, according to Dr. El Chaer and his colleague. Despite this and initial concerns about vaccine safety in this population, it is now recommended that adult patients infected with HIV receive their age-specific and risk group−based vaccines, they stated.
Inactivated or subunit vaccines
Haemophilus influenzae type b vaccine is not recommended under current guidelines for individuals older than age 18 with HIV infection, unless they have a clinical indication.
Vaccination against hepatitis A virus is recommended for HIV-infected patients who are hepatitis A virus seronegative and have chronic liver disease, men who have sex with men, intravenous drug users, and travelers to endemic regions. However, research has shown that the immunogenicity of the vaccine is lower in patients with HIV than in uninfected individuals. It was found that the CD4 count at the time of vaccination, not the CD4 low point, was the major predictor of the immune response.
Patients coinfected with HIV and hepatitis B virus have an 8-fold and 19-fold increase in mortality, respectively, compared with either virus monoinfection. Although vaccination is recommended, the optimal hepatitis B virus vaccination schedule in patients with HIV remains controversial, according to the authors. They indicated that new strategies to improve hepatitis B virus vaccine immunogenicity for those infected with HIV are needed.
Individuals infected with HIV have been found to have a higher risk of human papillomavirus (HPV) infection. The safety and immunogenicity results and prospect of benefits has led to a consensus on the benefit of vaccinating HIV-infected patients who meet the HPV vaccine age criteria, the authors indicated.
With regard to standard flu vaccinations: “An annual inactivated influenza vaccine is recommended during the influenza season for all adult individuals with HIV; however, a live attenuated influenza vaccine is contraindicated in this population,” according to the review.
Patients with HIV have a more than 10-fold increased risk of invasive meningococcal disease, compared with the general population, with the risk being particularly higher in those individuals with CD4 counts less than 200 cells/mm3 and in men who have sex with men in cities with meningococcal outbreaks. For these reasons, the “quadrivalent meningococcal vaccine is recommended for all patients with HIV regardless of their CD4 count, with 2-dose primary series at least 2 months apart and with a booster every 5 years.”
Pneumonia is known to be especially dangerous in the HIV-infected population. With regard to pneumonia vaccination, the 13-valent pneumococcal conjugate vaccine is recommended for all patients with HIV, regardless of their CD4 cell counts. According to Dr. El Chaer and his colleague, it should be followed by the 23-valent pneumococcal polysaccharide vaccine at least 8 weeks later as a prime-boost regimen, preferably when CD4 counts are greater than 200 cells/mm3 and in patients receiving ART.
“Tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccines are recommended once for all individuals infected with HIV, regardless of the CD4 count, with a tetanus toxoid and diphtheria toxoid booster every 10 years,” according to the review.
Live vaccines
Live vaccines are a concerning issue for HIV-infected adults and recommendations for use are generally tied to the CD4 T-cell count. The measles, mumps, and rubella vaccine seems to be safe in patients infected with HIV with a CD4 count greater than 200 cells/mm3, according to Dr. El Chaer and his colleague. Similarly, patients with HIV with CD4 counts greater than 200 cells/mm3 and no evidence of documented immunity to varicella should receive the varicella vaccine.
In contrast, the live, attenuated varicella zoster virus vaccine is not recommended for patients infected with HIV, and it is contraindicated if CD4 count is less than 200 cells/mm3. Recently, a herpes zoster subunit vaccine (HZ/su) was tested in a phase 1/2a randomized, placebo-controlled study and was found to be safe and immunogenic regardless of CD4 count, although it has not yet been given a specific recommendation for immunocompromised patients.
“With the widespread use of ART resulting in better HIV control, ,” the authors concluded.
The study was not sponsored. Dr. El Chaer and his colleague reported that they had no conflicts.
SOURCE: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
Patients infected with HIV have an increased risk of mortality and morbidity from diseases that are preventable with vaccines. Undervaccination of these patients poses a major concern, according to a literature review of the vaccine response in the adult patient with HIV published in The American Journal of Medicine.
Despite the fact that data are limited, patients infected with HIV are advised to receive their age-specific and risk group−based vaccines, according to Firas El Chaer, MD, of the University of Maryland, Baltimore, and his colleague.
HIV patients are of particular concern regarding vaccination, because, despite the use of retroviral therapy, CD4+ T-lymphocytes in individuals infected with HIV remain lower than in those without HIV. In addition, HIV causes an inappropriate response to B-cell stimulation, which results in suboptimal primary and secondary response to vaccination, according to Dr. El Chaer and his colleague. Despite this and initial concerns about vaccine safety in this population, it is now recommended that adult patients infected with HIV receive their age-specific and risk group−based vaccines, they stated.
Inactivated or subunit vaccines
Haemophilus influenzae type b vaccine is not recommended under current guidelines for individuals older than age 18 with HIV infection, unless they have a clinical indication.
Vaccination against hepatitis A virus is recommended for HIV-infected patients who are hepatitis A virus seronegative and have chronic liver disease, men who have sex with men, intravenous drug users, and travelers to endemic regions. However, research has shown that the immunogenicity of the vaccine is lower in patients with HIV than in uninfected individuals. It was found that the CD4 count at the time of vaccination, not the CD4 low point, was the major predictor of the immune response.
Patients coinfected with HIV and hepatitis B virus have an 8-fold and 19-fold increase in mortality, respectively, compared with either virus monoinfection. Although vaccination is recommended, the optimal hepatitis B virus vaccination schedule in patients with HIV remains controversial, according to the authors. They indicated that new strategies to improve hepatitis B virus vaccine immunogenicity for those infected with HIV are needed.
Individuals infected with HIV have been found to have a higher risk of human papillomavirus (HPV) infection. The safety and immunogenicity results and prospect of benefits has led to a consensus on the benefit of vaccinating HIV-infected patients who meet the HPV vaccine age criteria, the authors indicated.
With regard to standard flu vaccinations: “An annual inactivated influenza vaccine is recommended during the influenza season for all adult individuals with HIV; however, a live attenuated influenza vaccine is contraindicated in this population,” according to the review.
Patients with HIV have a more than 10-fold increased risk of invasive meningococcal disease, compared with the general population, with the risk being particularly higher in those individuals with CD4 counts less than 200 cells/mm3 and in men who have sex with men in cities with meningococcal outbreaks. For these reasons, the “quadrivalent meningococcal vaccine is recommended for all patients with HIV regardless of their CD4 count, with 2-dose primary series at least 2 months apart and with a booster every 5 years.”
Pneumonia is known to be especially dangerous in the HIV-infected population. With regard to pneumonia vaccination, the 13-valent pneumococcal conjugate vaccine is recommended for all patients with HIV, regardless of their CD4 cell counts. According to Dr. El Chaer and his colleague, it should be followed by the 23-valent pneumococcal polysaccharide vaccine at least 8 weeks later as a prime-boost regimen, preferably when CD4 counts are greater than 200 cells/mm3 and in patients receiving ART.
“Tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccines are recommended once for all individuals infected with HIV, regardless of the CD4 count, with a tetanus toxoid and diphtheria toxoid booster every 10 years,” according to the review.
Live vaccines
Live vaccines are a concerning issue for HIV-infected adults and recommendations for use are generally tied to the CD4 T-cell count. The measles, mumps, and rubella vaccine seems to be safe in patients infected with HIV with a CD4 count greater than 200 cells/mm3, according to Dr. El Chaer and his colleague. Similarly, patients with HIV with CD4 counts greater than 200 cells/mm3 and no evidence of documented immunity to varicella should receive the varicella vaccine.
In contrast, the live, attenuated varicella zoster virus vaccine is not recommended for patients infected with HIV, and it is contraindicated if CD4 count is less than 200 cells/mm3. Recently, a herpes zoster subunit vaccine (HZ/su) was tested in a phase 1/2a randomized, placebo-controlled study and was found to be safe and immunogenic regardless of CD4 count, although it has not yet been given a specific recommendation for immunocompromised patients.
“With the widespread use of ART resulting in better HIV control, ,” the authors concluded.
The study was not sponsored. Dr. El Chaer and his colleague reported that they had no conflicts.
SOURCE: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
Patients infected with HIV have an increased risk of mortality and morbidity from diseases that are preventable with vaccines. Undervaccination of these patients poses a major concern, according to a literature review of the vaccine response in the adult patient with HIV published in The American Journal of Medicine.
Despite the fact that data are limited, patients infected with HIV are advised to receive their age-specific and risk group−based vaccines, according to Firas El Chaer, MD, of the University of Maryland, Baltimore, and his colleague.
HIV patients are of particular concern regarding vaccination, because, despite the use of retroviral therapy, CD4+ T-lymphocytes in individuals infected with HIV remain lower than in those without HIV. In addition, HIV causes an inappropriate response to B-cell stimulation, which results in suboptimal primary and secondary response to vaccination, according to Dr. El Chaer and his colleague. Despite this and initial concerns about vaccine safety in this population, it is now recommended that adult patients infected with HIV receive their age-specific and risk group−based vaccines, they stated.
Inactivated or subunit vaccines
Haemophilus influenzae type b vaccine is not recommended under current guidelines for individuals older than age 18 with HIV infection, unless they have a clinical indication.
Vaccination against hepatitis A virus is recommended for HIV-infected patients who are hepatitis A virus seronegative and have chronic liver disease, men who have sex with men, intravenous drug users, and travelers to endemic regions. However, research has shown that the immunogenicity of the vaccine is lower in patients with HIV than in uninfected individuals. It was found that the CD4 count at the time of vaccination, not the CD4 low point, was the major predictor of the immune response.
Patients coinfected with HIV and hepatitis B virus have an 8-fold and 19-fold increase in mortality, respectively, compared with either virus monoinfection. Although vaccination is recommended, the optimal hepatitis B virus vaccination schedule in patients with HIV remains controversial, according to the authors. They indicated that new strategies to improve hepatitis B virus vaccine immunogenicity for those infected with HIV are needed.
Individuals infected with HIV have been found to have a higher risk of human papillomavirus (HPV) infection. The safety and immunogenicity results and prospect of benefits has led to a consensus on the benefit of vaccinating HIV-infected patients who meet the HPV vaccine age criteria, the authors indicated.
With regard to standard flu vaccinations: “An annual inactivated influenza vaccine is recommended during the influenza season for all adult individuals with HIV; however, a live attenuated influenza vaccine is contraindicated in this population,” according to the review.
Patients with HIV have a more than 10-fold increased risk of invasive meningococcal disease, compared with the general population, with the risk being particularly higher in those individuals with CD4 counts less than 200 cells/mm3 and in men who have sex with men in cities with meningococcal outbreaks. For these reasons, the “quadrivalent meningococcal vaccine is recommended for all patients with HIV regardless of their CD4 count, with 2-dose primary series at least 2 months apart and with a booster every 5 years.”
Pneumonia is known to be especially dangerous in the HIV-infected population. With regard to pneumonia vaccination, the 13-valent pneumococcal conjugate vaccine is recommended for all patients with HIV, regardless of their CD4 cell counts. According to Dr. El Chaer and his colleague, it should be followed by the 23-valent pneumococcal polysaccharide vaccine at least 8 weeks later as a prime-boost regimen, preferably when CD4 counts are greater than 200 cells/mm3 and in patients receiving ART.
“Tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccines are recommended once for all individuals infected with HIV, regardless of the CD4 count, with a tetanus toxoid and diphtheria toxoid booster every 10 years,” according to the review.
Live vaccines
Live vaccines are a concerning issue for HIV-infected adults and recommendations for use are generally tied to the CD4 T-cell count. The measles, mumps, and rubella vaccine seems to be safe in patients infected with HIV with a CD4 count greater than 200 cells/mm3, according to Dr. El Chaer and his colleague. Similarly, patients with HIV with CD4 counts greater than 200 cells/mm3 and no evidence of documented immunity to varicella should receive the varicella vaccine.
In contrast, the live, attenuated varicella zoster virus vaccine is not recommended for patients infected with HIV, and it is contraindicated if CD4 count is less than 200 cells/mm3. Recently, a herpes zoster subunit vaccine (HZ/su) was tested in a phase 1/2a randomized, placebo-controlled study and was found to be safe and immunogenic regardless of CD4 count, although it has not yet been given a specific recommendation for immunocompromised patients.
“With the widespread use of ART resulting in better HIV control, ,” the authors concluded.
The study was not sponsored. Dr. El Chaer and his colleague reported that they had no conflicts.
SOURCE: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
FROM THE AMERICAN JOURNAL OF MEDICINE
Key clinical point: Undervaccination is too common among HIV-infected patients.
Major finding: Data on vaccine effectiveness in HIV patients are limited, but do not contraindicate the need for vaccination.
Study details: Literature review of immunogenicity and vaccine efficacy in HIV-infected adults.
Disclosures: The study was unsponsored and the authors reported they had no conflicts.
Source: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
Failure to launch can happen to college students
March often is the time of year when college freshmen truly begin to feel comfortable in their new settings. Many students report feeling excited to get back to campus after the long winter break, and once into their second semester, they feel more comfortable with the independence from family and high school supports. It also is a time for some college freshmen to return home after failing to manage this major transition.

Of the latter group, many will have had difficult months of depression, anxiety, or substance use, and most will be suffering from a deep sense of shame after failing to navigate this long-anticipated transition. Asking detailed questions about their academic challenges, social lives, self-care, and sleep while they were on campus will help you make thoughtful recommendations to your patients and their parents about how they might best get back on track.
Some students will report a great social experience, but academic struggles. They will report some normal ups and downs emotionally, but most of their distress will have been focused on their academic performance. Many 18-year-olds have not had to organize their time and effort around homework without the attention and support of parents and teachers. College often has much bigger classes, with less personal attention. There is a lot of assigned reading, but no regular incremental homework, only a major midterm and final exam, or a substantial paper. For a student who gets anxious about performance, or one with organizational challenges, this can lead to procrastination and poor performance.
Find out details about how they did academically. Did they fail one class or many classes? Did they receive some incompletes in their first semester and then struggle to catch up with them while keeping up with their second semester work? Did they have tutoring or support? Were they unrealistic about their course load? Or did they have their first serious relationship and not spend enough time on homework? Did they spend too much time partying with their new friends and not enough time sleeping and getting their homework done?
It is important to dig deeper if patients report regular or binge drug and alcohol use that interfered with their academic performance, as they may need more substantial substance use disorder treatment. Most students, though, will not have a substance use disorder. Instead, their academic failure could represent something as simple as the need for more academic support and time management support. Many schools have such programs to help students learn how to better manage their time and effort as they take fuller responsibility than they had for it in high school.
For other students, you will learn that their emotional distress preceded their academic troubles. The stress of the transition to college may be enough to trigger an episode of depression or to exacerbate a mood or anxiety disorder that was subclinical or in remission before school started. These students usually will report that sadness, intense anxiety, or loss of interest came early in their semester; perhaps they were even doing well academically when these problems started.
Ask about how their sleep was. Often they had difficulty falling asleep or woke up often at night, unlike most college students, whose sleep is compromised because they stay up late with new friends or because they are hard at work, but could easily sleep at any time.
Find out about their eating habits. Did they lose their appetite? Lose weight? Did they become preoccupied with weight or body image issues and begin restricting their intake? Eating disorders can begin in college when vulnerable students are stressed and have more control over their diet. While weight gain is more common in freshman year, it often is connected to poor stress management skills, and is more often a marker of a student who was struggling academically and then managing stress by overeating.
In the case where the distress came first, it is critical that your patients have a thorough psychiatric evaluation and treatment. It may be possible for them to return to school quickly, but it is most important that they are engaged in effective treatment and in at last partial remission before adding to their stress by attempting to return to school. Often, ambitious students and their parents need to hear this message very clearly from a pediatrician. A rushed return to school may be a set-up for a more protracted and difficult course of illness. For these students, it may be better to have a fresh start in a new semester. Help them (and their parents) to understand that they should use their time off to focus on treatment and good self-care so they might benefit from the many opportunities of college.
For a small minority of college students who do not succeed at college, their social withdrawal, academic deterioration, anxiety, and loss of interest in previous passions may occur alongside more serious psychiatric symptoms such as auditory hallucinations, paranoia, or grandiosity. Any time there is a suggestion of psychotic symptoms in a previously healthy person in the late teens or early 20s, a prompt comprehensive psychiatric evaluation is critical. These years are when most chronic psychotic disorders, such as schizophrenia, are likely to emerge. These patients require a thorough evaluation to distinguish these disorders from other illnesses, especially when they occur with substance use. And these patients require specialized care.
If your patient appears to have any psychotic symptoms, it is critical that you help the family find an excellent psychiatrist, or even a clinic that specializes in thought disorders so that he or she may get the best possible care early.
There is another class of students who withdraw from college who will need more comprehensive remediation, but not connected to any psychiatric diagnosis. Some young people may not be developmentally ready for college. These are your patients who often were excellent performers in high school, perhaps academically and athletically, but whose performance was more connected to pleasing important adults than to genuine motivating passions or sense of purpose. These young adults may have been drawn into the intense, results-oriented forces that are powerful in many of our high schools. If they did not have enough time or space to explore a host of interests, and to then manage the routine failures, setbacks, and disappointments that are essential to healthy adolescent development, they are going to run out of fuel in college. Such students often are quite dependent on their parents, and struggle with the independence college offers.
If your patients report that they could not muster the same intense work ethic they previously had, without any evidence of a psychiatric illness interfering with motivation, they may need time to finish the developmental work of cultivating a deep and rich sense of their own identity. Some students can do this at college, provided they, their parents and their school offer them adequate time before they have to declare a major. Other students will need to get a job and explore interests with a few courses at a community college, cultivating independence while learning about their own strengths and weaknesses and their genuine interests. This way, when they return to school, they will be motivated by a genuine sense of purpose and self-knowledge.
“Failure to launch” is a critical symptom at a key transitional moment. Pediatric providers can be essential to their patients and families by clarifying the nature of the difficulty and coordinating a reasonable plan to get these young adults back on track to healthy adulthood.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
March often is the time of year when college freshmen truly begin to feel comfortable in their new settings. Many students report feeling excited to get back to campus after the long winter break, and once into their second semester, they feel more comfortable with the independence from family and high school supports. It also is a time for some college freshmen to return home after failing to manage this major transition.

Of the latter group, many will have had difficult months of depression, anxiety, or substance use, and most will be suffering from a deep sense of shame after failing to navigate this long-anticipated transition. Asking detailed questions about their academic challenges, social lives, self-care, and sleep while they were on campus will help you make thoughtful recommendations to your patients and their parents about how they might best get back on track.
Some students will report a great social experience, but academic struggles. They will report some normal ups and downs emotionally, but most of their distress will have been focused on their academic performance. Many 18-year-olds have not had to organize their time and effort around homework without the attention and support of parents and teachers. College often has much bigger classes, with less personal attention. There is a lot of assigned reading, but no regular incremental homework, only a major midterm and final exam, or a substantial paper. For a student who gets anxious about performance, or one with organizational challenges, this can lead to procrastination and poor performance.
Find out details about how they did academically. Did they fail one class or many classes? Did they receive some incompletes in their first semester and then struggle to catch up with them while keeping up with their second semester work? Did they have tutoring or support? Were they unrealistic about their course load? Or did they have their first serious relationship and not spend enough time on homework? Did they spend too much time partying with their new friends and not enough time sleeping and getting their homework done?
It is important to dig deeper if patients report regular or binge drug and alcohol use that interfered with their academic performance, as they may need more substantial substance use disorder treatment. Most students, though, will not have a substance use disorder. Instead, their academic failure could represent something as simple as the need for more academic support and time management support. Many schools have such programs to help students learn how to better manage their time and effort as they take fuller responsibility than they had for it in high school.
For other students, you will learn that their emotional distress preceded their academic troubles. The stress of the transition to college may be enough to trigger an episode of depression or to exacerbate a mood or anxiety disorder that was subclinical or in remission before school started. These students usually will report that sadness, intense anxiety, or loss of interest came early in their semester; perhaps they were even doing well academically when these problems started.
Ask about how their sleep was. Often they had difficulty falling asleep or woke up often at night, unlike most college students, whose sleep is compromised because they stay up late with new friends or because they are hard at work, but could easily sleep at any time.
Find out about their eating habits. Did they lose their appetite? Lose weight? Did they become preoccupied with weight or body image issues and begin restricting their intake? Eating disorders can begin in college when vulnerable students are stressed and have more control over their diet. While weight gain is more common in freshman year, it often is connected to poor stress management skills, and is more often a marker of a student who was struggling academically and then managing stress by overeating.
In the case where the distress came first, it is critical that your patients have a thorough psychiatric evaluation and treatment. It may be possible for them to return to school quickly, but it is most important that they are engaged in effective treatment and in at last partial remission before adding to their stress by attempting to return to school. Often, ambitious students and their parents need to hear this message very clearly from a pediatrician. A rushed return to school may be a set-up for a more protracted and difficult course of illness. For these students, it may be better to have a fresh start in a new semester. Help them (and their parents) to understand that they should use their time off to focus on treatment and good self-care so they might benefit from the many opportunities of college.
For a small minority of college students who do not succeed at college, their social withdrawal, academic deterioration, anxiety, and loss of interest in previous passions may occur alongside more serious psychiatric symptoms such as auditory hallucinations, paranoia, or grandiosity. Any time there is a suggestion of psychotic symptoms in a previously healthy person in the late teens or early 20s, a prompt comprehensive psychiatric evaluation is critical. These years are when most chronic psychotic disorders, such as schizophrenia, are likely to emerge. These patients require a thorough evaluation to distinguish these disorders from other illnesses, especially when they occur with substance use. And these patients require specialized care.
If your patient appears to have any psychotic symptoms, it is critical that you help the family find an excellent psychiatrist, or even a clinic that specializes in thought disorders so that he or she may get the best possible care early.
There is another class of students who withdraw from college who will need more comprehensive remediation, but not connected to any psychiatric diagnosis. Some young people may not be developmentally ready for college. These are your patients who often were excellent performers in high school, perhaps academically and athletically, but whose performance was more connected to pleasing important adults than to genuine motivating passions or sense of purpose. These young adults may have been drawn into the intense, results-oriented forces that are powerful in many of our high schools. If they did not have enough time or space to explore a host of interests, and to then manage the routine failures, setbacks, and disappointments that are essential to healthy adolescent development, they are going to run out of fuel in college. Such students often are quite dependent on their parents, and struggle with the independence college offers.
If your patients report that they could not muster the same intense work ethic they previously had, without any evidence of a psychiatric illness interfering with motivation, they may need time to finish the developmental work of cultivating a deep and rich sense of their own identity. Some students can do this at college, provided they, their parents and their school offer them adequate time before they have to declare a major. Other students will need to get a job and explore interests with a few courses at a community college, cultivating independence while learning about their own strengths and weaknesses and their genuine interests. This way, when they return to school, they will be motivated by a genuine sense of purpose and self-knowledge.
“Failure to launch” is a critical symptom at a key transitional moment. Pediatric providers can be essential to their patients and families by clarifying the nature of the difficulty and coordinating a reasonable plan to get these young adults back on track to healthy adulthood.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
March often is the time of year when college freshmen truly begin to feel comfortable in their new settings. Many students report feeling excited to get back to campus after the long winter break, and once into their second semester, they feel more comfortable with the independence from family and high school supports. It also is a time for some college freshmen to return home after failing to manage this major transition.

Of the latter group, many will have had difficult months of depression, anxiety, or substance use, and most will be suffering from a deep sense of shame after failing to navigate this long-anticipated transition. Asking detailed questions about their academic challenges, social lives, self-care, and sleep while they were on campus will help you make thoughtful recommendations to your patients and their parents about how they might best get back on track.
Some students will report a great social experience, but academic struggles. They will report some normal ups and downs emotionally, but most of their distress will have been focused on their academic performance. Many 18-year-olds have not had to organize their time and effort around homework without the attention and support of parents and teachers. College often has much bigger classes, with less personal attention. There is a lot of assigned reading, but no regular incremental homework, only a major midterm and final exam, or a substantial paper. For a student who gets anxious about performance, or one with organizational challenges, this can lead to procrastination and poor performance.
Find out details about how they did academically. Did they fail one class or many classes? Did they receive some incompletes in their first semester and then struggle to catch up with them while keeping up with their second semester work? Did they have tutoring or support? Were they unrealistic about their course load? Or did they have their first serious relationship and not spend enough time on homework? Did they spend too much time partying with their new friends and not enough time sleeping and getting their homework done?
It is important to dig deeper if patients report regular or binge drug and alcohol use that interfered with their academic performance, as they may need more substantial substance use disorder treatment. Most students, though, will not have a substance use disorder. Instead, their academic failure could represent something as simple as the need for more academic support and time management support. Many schools have such programs to help students learn how to better manage their time and effort as they take fuller responsibility than they had for it in high school.
For other students, you will learn that their emotional distress preceded their academic troubles. The stress of the transition to college may be enough to trigger an episode of depression or to exacerbate a mood or anxiety disorder that was subclinical or in remission before school started. These students usually will report that sadness, intense anxiety, or loss of interest came early in their semester; perhaps they were even doing well academically when these problems started.
Ask about how their sleep was. Often they had difficulty falling asleep or woke up often at night, unlike most college students, whose sleep is compromised because they stay up late with new friends or because they are hard at work, but could easily sleep at any time.
Find out about their eating habits. Did they lose their appetite? Lose weight? Did they become preoccupied with weight or body image issues and begin restricting their intake? Eating disorders can begin in college when vulnerable students are stressed and have more control over their diet. While weight gain is more common in freshman year, it often is connected to poor stress management skills, and is more often a marker of a student who was struggling academically and then managing stress by overeating.
In the case where the distress came first, it is critical that your patients have a thorough psychiatric evaluation and treatment. It may be possible for them to return to school quickly, but it is most important that they are engaged in effective treatment and in at last partial remission before adding to their stress by attempting to return to school. Often, ambitious students and their parents need to hear this message very clearly from a pediatrician. A rushed return to school may be a set-up for a more protracted and difficult course of illness. For these students, it may be better to have a fresh start in a new semester. Help them (and their parents) to understand that they should use their time off to focus on treatment and good self-care so they might benefit from the many opportunities of college.
For a small minority of college students who do not succeed at college, their social withdrawal, academic deterioration, anxiety, and loss of interest in previous passions may occur alongside more serious psychiatric symptoms such as auditory hallucinations, paranoia, or grandiosity. Any time there is a suggestion of psychotic symptoms in a previously healthy person in the late teens or early 20s, a prompt comprehensive psychiatric evaluation is critical. These years are when most chronic psychotic disorders, such as schizophrenia, are likely to emerge. These patients require a thorough evaluation to distinguish these disorders from other illnesses, especially when they occur with substance use. And these patients require specialized care.
If your patient appears to have any psychotic symptoms, it is critical that you help the family find an excellent psychiatrist, or even a clinic that specializes in thought disorders so that he or she may get the best possible care early.
There is another class of students who withdraw from college who will need more comprehensive remediation, but not connected to any psychiatric diagnosis. Some young people may not be developmentally ready for college. These are your patients who often were excellent performers in high school, perhaps academically and athletically, but whose performance was more connected to pleasing important adults than to genuine motivating passions or sense of purpose. These young adults may have been drawn into the intense, results-oriented forces that are powerful in many of our high schools. If they did not have enough time or space to explore a host of interests, and to then manage the routine failures, setbacks, and disappointments that are essential to healthy adolescent development, they are going to run out of fuel in college. Such students often are quite dependent on their parents, and struggle with the independence college offers.
If your patients report that they could not muster the same intense work ethic they previously had, without any evidence of a psychiatric illness interfering with motivation, they may need time to finish the developmental work of cultivating a deep and rich sense of their own identity. Some students can do this at college, provided they, their parents and their school offer them adequate time before they have to declare a major. Other students will need to get a job and explore interests with a few courses at a community college, cultivating independence while learning about their own strengths and weaknesses and their genuine interests. This way, when they return to school, they will be motivated by a genuine sense of purpose and self-knowledge.
“Failure to launch” is a critical symptom at a key transitional moment. Pediatric providers can be essential to their patients and families by clarifying the nature of the difficulty and coordinating a reasonable plan to get these young adults back on track to healthy adulthood.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
In California, opioids most often prescribed in low-income, mostly white areas
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The most common users of opioids according to prescription drug records are residents of mostly low-income, white neighborhoods.
Major finding: Compared with 23.6% of all Californians, 44.2% of individuals in zip codes containing mostly low-income, white residents had at least one opioid prescription each year, compared with 16.1% of individuals in high-income zip codes with the lowest population of white residents.
Study details: An analysis of 29.7 million opioid prescription drug records by race and income in California during 2011-2015.
Disclosures: Dr. Schriger reported support from the Korein Foundation for his time working on the study by Friedman et al. The other authors from Friedman et al. reported no conflicts of interest.
Pembrolizumab-axitinib nearly halves risk of death in RCC
SAN FRANCISCO – When used as first-line therapy for renal cell carcinoma (RCC), the combination of pembrolizumab and axitinib has similar safety and better efficacy than single-agent sunitinib, the current standard of care, according to findings of the KEYNOTE-426 trial that will be reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
“Axitinib is usually licensed and usually used in sunitinib-refractory disease. However, there is data for both pembrolizumab and axitinib in the frontline setting,” said lead author Thomas Powles, MBBS, MRCP, MD, of Barts Health and the Royal Free NHS Trusts, Barts Cancer Institute, and Queen Mary University of London. A phase 1b trial testing the combination showed an impressive 73% objective response rate and acceptable toxicity (Lancet Oncol. 2018;19:405-15), prompting further investigation.
The 861 patients in KEYNOTE-426, a phase 3, randomized, controlled trial, were evenly assigned to combination therapy with the immune checkpoint inhibitor pembrolizumab (Keytruda), which targets programmed death–1, plus the tyrosine kinase inhibitor axitinib (Inlyta), which targets vascular endothelial growth factor and platelet-derived growth factor, or to monotherapy with the tyrosine kinase inhibitor sunitinib (Sutent), which also targets those growth factors.
Main results reported in a presscast held before the symposium showed that, with a median follow-up of 12.8 months, pembrolizumab-axitinib reduced the risk of progression-free survival events by a relative 31% and the risk of death by a relative 47%, compared with sunitinib. The combination had a rate of grade 3-5 treatment-related adverse events similar to the rate with sunitinib alone.
“The benefit of pembrolizumab plus axitinib was seen irrespective of IMDC [International Metastatic Renal Cell Carcinoma Database Consortium] risk group or PD-L1 [programmed death–ligand 1] status,” Dr. Powles noted. “Pembrolizumab and axitinib should be a standard of care in this setting, in my opinion.”
“This is a very significant trial, and it’s going to impact on patient management going forward, as it works through the regulatory process,” commented ASCO Expert and presscast moderator Robert Dreicer, MD, MS, MACP, FASCO, who is also deputy director and associate director of clinical research at the University of Virginia Cancer Center and a professor of medicine and urology at the University of Virginia, Charlottesville.
Study details
Patients in KEYNOTE-426 had newly diagnosed or recurrent stage IV clear cell RCC and had not received any previous systemic treatment for their advanced disease. They were randomized to pembrolizumab (200 mg intravenously every 3 weeks up to 35 cycles) plus axitinib (5 mg orally twice daily), or to sunitinib (50 mg orally once daily for first 4 weeks of each 6-week cycle).
Median overall survival was not reached in either group, but the 12-month rate was 89.9% with pembrolizumab-axitinib versus 78.3% with sunitinib, Dr. Powles reported in the presscast. The difference corresponded to a near halving of the risk of death with the combination (hazard ratio, 0.53; P less than .0001).
Median progression-free survival was 15.1 months with pembrolizumab-axitinib and 11.1 months with sunitinib. The difference corresponded to a nearly one-third reduction in the risk of events with the combination (HR, 0.69; P = .0001). “The 11.1 months is quite long for a control arm, so there’s nothing from these data to suggest that sunitinib underperformed in this trial,” he noted.
Pembrolizumab-axitinib was also associated with a higher objective response rate (59.3% vs. 35.7%; P less than .0001). The median duration of response was not reached with the former, compared with 15.2 months with the latter.
“Pembrolizumab and axitinib had a manageable safety profile,” Dr. Powles said. The rate of grade 3-5 treatment-related adverse events was 62.9% with the combination and 58.1% with sunitinib monotherapy.
The rate of events leading to death was similar at 0.9% and 1.6%, respectively. The rate of events leading to discontinuation of any treatment was 25.9% for pembrolizumab-axitinib and 10.1% for sunitinib, and the rate of events leading to discontinuation of both drugs in the combination was 8.2%.
Dr. Powles reported that he has a consulting or advisory role with Genentech/Roche, Bristol-Myers Squibb, Merck, Novartis, and AstraZeneca; has a nonspecified relationship with Ipsen and Bristol-Myers Squibb; receives honoraria from Roche/Genentech, Bristol-Myers Squibb, and Merck; and receives research funding from Astra-Zeneca/MedImmune and Roche/Genentech. The study was funded by Merck.
SOURCE: Powles T et al. GUCS 2019, Abstract 543.
SAN FRANCISCO – When used as first-line therapy for renal cell carcinoma (RCC), the combination of pembrolizumab and axitinib has similar safety and better efficacy than single-agent sunitinib, the current standard of care, according to findings of the KEYNOTE-426 trial that will be reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
“Axitinib is usually licensed and usually used in sunitinib-refractory disease. However, there is data for both pembrolizumab and axitinib in the frontline setting,” said lead author Thomas Powles, MBBS, MRCP, MD, of Barts Health and the Royal Free NHS Trusts, Barts Cancer Institute, and Queen Mary University of London. A phase 1b trial testing the combination showed an impressive 73% objective response rate and acceptable toxicity (Lancet Oncol. 2018;19:405-15), prompting further investigation.
The 861 patients in KEYNOTE-426, a phase 3, randomized, controlled trial, were evenly assigned to combination therapy with the immune checkpoint inhibitor pembrolizumab (Keytruda), which targets programmed death–1, plus the tyrosine kinase inhibitor axitinib (Inlyta), which targets vascular endothelial growth factor and platelet-derived growth factor, or to monotherapy with the tyrosine kinase inhibitor sunitinib (Sutent), which also targets those growth factors.
Main results reported in a presscast held before the symposium showed that, with a median follow-up of 12.8 months, pembrolizumab-axitinib reduced the risk of progression-free survival events by a relative 31% and the risk of death by a relative 47%, compared with sunitinib. The combination had a rate of grade 3-5 treatment-related adverse events similar to the rate with sunitinib alone.
“The benefit of pembrolizumab plus axitinib was seen irrespective of IMDC [International Metastatic Renal Cell Carcinoma Database Consortium] risk group or PD-L1 [programmed death–ligand 1] status,” Dr. Powles noted. “Pembrolizumab and axitinib should be a standard of care in this setting, in my opinion.”
“This is a very significant trial, and it’s going to impact on patient management going forward, as it works through the regulatory process,” commented ASCO Expert and presscast moderator Robert Dreicer, MD, MS, MACP, FASCO, who is also deputy director and associate director of clinical research at the University of Virginia Cancer Center and a professor of medicine and urology at the University of Virginia, Charlottesville.
Study details
Patients in KEYNOTE-426 had newly diagnosed or recurrent stage IV clear cell RCC and had not received any previous systemic treatment for their advanced disease. They were randomized to pembrolizumab (200 mg intravenously every 3 weeks up to 35 cycles) plus axitinib (5 mg orally twice daily), or to sunitinib (50 mg orally once daily for first 4 weeks of each 6-week cycle).
Median overall survival was not reached in either group, but the 12-month rate was 89.9% with pembrolizumab-axitinib versus 78.3% with sunitinib, Dr. Powles reported in the presscast. The difference corresponded to a near halving of the risk of death with the combination (hazard ratio, 0.53; P less than .0001).
Median progression-free survival was 15.1 months with pembrolizumab-axitinib and 11.1 months with sunitinib. The difference corresponded to a nearly one-third reduction in the risk of events with the combination (HR, 0.69; P = .0001). “The 11.1 months is quite long for a control arm, so there’s nothing from these data to suggest that sunitinib underperformed in this trial,” he noted.
Pembrolizumab-axitinib was also associated with a higher objective response rate (59.3% vs. 35.7%; P less than .0001). The median duration of response was not reached with the former, compared with 15.2 months with the latter.
“Pembrolizumab and axitinib had a manageable safety profile,” Dr. Powles said. The rate of grade 3-5 treatment-related adverse events was 62.9% with the combination and 58.1% with sunitinib monotherapy.
The rate of events leading to death was similar at 0.9% and 1.6%, respectively. The rate of events leading to discontinuation of any treatment was 25.9% for pembrolizumab-axitinib and 10.1% for sunitinib, and the rate of events leading to discontinuation of both drugs in the combination was 8.2%.
Dr. Powles reported that he has a consulting or advisory role with Genentech/Roche, Bristol-Myers Squibb, Merck, Novartis, and AstraZeneca; has a nonspecified relationship with Ipsen and Bristol-Myers Squibb; receives honoraria from Roche/Genentech, Bristol-Myers Squibb, and Merck; and receives research funding from Astra-Zeneca/MedImmune and Roche/Genentech. The study was funded by Merck.
SOURCE: Powles T et al. GUCS 2019, Abstract 543.
SAN FRANCISCO – When used as first-line therapy for renal cell carcinoma (RCC), the combination of pembrolizumab and axitinib has similar safety and better efficacy than single-agent sunitinib, the current standard of care, according to findings of the KEYNOTE-426 trial that will be reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
“Axitinib is usually licensed and usually used in sunitinib-refractory disease. However, there is data for both pembrolizumab and axitinib in the frontline setting,” said lead author Thomas Powles, MBBS, MRCP, MD, of Barts Health and the Royal Free NHS Trusts, Barts Cancer Institute, and Queen Mary University of London. A phase 1b trial testing the combination showed an impressive 73% objective response rate and acceptable toxicity (Lancet Oncol. 2018;19:405-15), prompting further investigation.
The 861 patients in KEYNOTE-426, a phase 3, randomized, controlled trial, were evenly assigned to combination therapy with the immune checkpoint inhibitor pembrolizumab (Keytruda), which targets programmed death–1, plus the tyrosine kinase inhibitor axitinib (Inlyta), which targets vascular endothelial growth factor and platelet-derived growth factor, or to monotherapy with the tyrosine kinase inhibitor sunitinib (Sutent), which also targets those growth factors.
Main results reported in a presscast held before the symposium showed that, with a median follow-up of 12.8 months, pembrolizumab-axitinib reduced the risk of progression-free survival events by a relative 31% and the risk of death by a relative 47%, compared with sunitinib. The combination had a rate of grade 3-5 treatment-related adverse events similar to the rate with sunitinib alone.
“The benefit of pembrolizumab plus axitinib was seen irrespective of IMDC [International Metastatic Renal Cell Carcinoma Database Consortium] risk group or PD-L1 [programmed death–ligand 1] status,” Dr. Powles noted. “Pembrolizumab and axitinib should be a standard of care in this setting, in my opinion.”
“This is a very significant trial, and it’s going to impact on patient management going forward, as it works through the regulatory process,” commented ASCO Expert and presscast moderator Robert Dreicer, MD, MS, MACP, FASCO, who is also deputy director and associate director of clinical research at the University of Virginia Cancer Center and a professor of medicine and urology at the University of Virginia, Charlottesville.
Study details
Patients in KEYNOTE-426 had newly diagnosed or recurrent stage IV clear cell RCC and had not received any previous systemic treatment for their advanced disease. They were randomized to pembrolizumab (200 mg intravenously every 3 weeks up to 35 cycles) plus axitinib (5 mg orally twice daily), or to sunitinib (50 mg orally once daily for first 4 weeks of each 6-week cycle).
Median overall survival was not reached in either group, but the 12-month rate was 89.9% with pembrolizumab-axitinib versus 78.3% with sunitinib, Dr. Powles reported in the presscast. The difference corresponded to a near halving of the risk of death with the combination (hazard ratio, 0.53; P less than .0001).
Median progression-free survival was 15.1 months with pembrolizumab-axitinib and 11.1 months with sunitinib. The difference corresponded to a nearly one-third reduction in the risk of events with the combination (HR, 0.69; P = .0001). “The 11.1 months is quite long for a control arm, so there’s nothing from these data to suggest that sunitinib underperformed in this trial,” he noted.
Pembrolizumab-axitinib was also associated with a higher objective response rate (59.3% vs. 35.7%; P less than .0001). The median duration of response was not reached with the former, compared with 15.2 months with the latter.
“Pembrolizumab and axitinib had a manageable safety profile,” Dr. Powles said. The rate of grade 3-5 treatment-related adverse events was 62.9% with the combination and 58.1% with sunitinib monotherapy.
The rate of events leading to death was similar at 0.9% and 1.6%, respectively. The rate of events leading to discontinuation of any treatment was 25.9% for pembrolizumab-axitinib and 10.1% for sunitinib, and the rate of events leading to discontinuation of both drugs in the combination was 8.2%.
Dr. Powles reported that he has a consulting or advisory role with Genentech/Roche, Bristol-Myers Squibb, Merck, Novartis, and AstraZeneca; has a nonspecified relationship with Ipsen and Bristol-Myers Squibb; receives honoraria from Roche/Genentech, Bristol-Myers Squibb, and Merck; and receives research funding from Astra-Zeneca/MedImmune and Roche/Genentech. The study was funded by Merck.
SOURCE: Powles T et al. GUCS 2019, Abstract 543.
REPORTING FROM GUCS 2019
Key clinical point: The combination of pembrolizumab and axitinib may become a new first-line standard of care in advanced renal cell carcinoma.
Major finding: Compared with sunitinib monotherapy, pembrolizumab and axitinib combination therapy prolonged progression-free survival (hazard ratio, 0.69; P = .0001) and overall survival (HR, 0.53; P less than .0001).
Study details: A phase 3, randomized, controlled trial among 861 patients with untreated locally advanced or metastatic renal cell carcinoma (KEYNOTE-426).
Disclosures: Dr. Powles reported that he has a consulting or advisory role with Genentech/Roche, Bristol-Myers Squibb, Merck, Novartis, and AstraZeneca; has a nonspecified relationship with Ipsen and Bristol-Myers Squibb; receives honoraria from Roche/Genentech, Bristol-Myers Squibb, and Merck; and receives research funding from AstraZeneca/MedImmune and Roche/Genentech. The study was funded by Merck.
Source: Powles T et al. GUCS 2019, Abstract 543.
Survey: Reproductive counseling is often MIA in IBD
LAS VEGAS – Inflammatory bowel disease (IBD) can disrupt both fertility and pregnancy, especially if it’s not fully controlled, and there’s a risk that the condition can be passed onto an unborn child. Still a new study suggests many patients with IBD don’t receive appropriate reproductive counseling.
Nearly two-thirds of 100 patients surveyed at a single center reported that no physician had talked to them about reproductive topics, and some said they’d considered not having children because of the condition. “Really fundamental subjects have not made their way into the interactions between patients and their care teams,” coauthor and gastroenterologist Sarah Streett, MD, AGAF, of Stanford (Calif.) University, said in an interview before the study was presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
IBD can lower fertility in both sexes and boost complications in pregnancy. “The good news is that almost all the medications used for IBD appear safe,” Dr. Streett said. “In fact, the safety risks for the baby and the pregnancy revolve around not having IBD under good control.”
Unfortunately, she said, misinformation is common. “Patients who become pregnant or are trying to become pregnant, and are worried about potential harm to the baby, will stop the medications due to incorrect information. Or they’ll be told by their health care team to stop their medications.”
Dr. Streett and study lead author Aarti Rao, MD, a gastroenterology fellow at Stanford, launched their study of IBD clinic patients to gain more understanding about patient knowledge. “We know from research already published that those with IBD have a lot of concerns about starting families and don’t have a lot of information to base their decision making on,” Dr. Streett said. “We wanted to evaluate that in our population and see how much people knew and what the need was.”
In 2018 and 2019, Dr. Streett and Dr. Rao gave an anonymous, validated 17-question survey to patients aged 18-45 with IBD. One hundred patients responded (median age = 30, 54% female, 59% white, 66% with incomes over $100,000, 52% with ulcerative colitis, 21% with prior IBD surgery, 71% with prior IBD hospitalization).
Just over a third – 35% – of the patients said they’d been counseled about reproductive health by a physician. This finding reflects findings in previous research, said Dr. Rao, who spoke in an interview.
Just 15% of those who’d undergone IBD surgery reported getting guidance about the effects of surgery on fertility.
More than a third (35%) of women and 15% of men said they’d considered not having children because of their IBD. In fact, “most potential dads and moms have the chance to do very well,” Dr. Streett said.
Without reproductive counseling, she added, parents won’t know about the risks of passing on IBD. According to Dr. Rao, there’s an estimated less than 5% chance that IBD will be passed on to children if one parent has the condition; the risk is much higher if both parents have it.
Going forward, “there’s a really urgent need for proactive counseling on the part of gastroenterologists and health care teams to give people of childbearing age the right information so they can be informed to make the best decisions,” Dr. Streett said.
The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
SOURCE: Rao A et al. Crohn’s & Colitis Congress, Abstract P009.
LAS VEGAS – Inflammatory bowel disease (IBD) can disrupt both fertility and pregnancy, especially if it’s not fully controlled, and there’s a risk that the condition can be passed onto an unborn child. Still a new study suggests many patients with IBD don’t receive appropriate reproductive counseling.
Nearly two-thirds of 100 patients surveyed at a single center reported that no physician had talked to them about reproductive topics, and some said they’d considered not having children because of the condition. “Really fundamental subjects have not made their way into the interactions between patients and their care teams,” coauthor and gastroenterologist Sarah Streett, MD, AGAF, of Stanford (Calif.) University, said in an interview before the study was presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
IBD can lower fertility in both sexes and boost complications in pregnancy. “The good news is that almost all the medications used for IBD appear safe,” Dr. Streett said. “In fact, the safety risks for the baby and the pregnancy revolve around not having IBD under good control.”
Unfortunately, she said, misinformation is common. “Patients who become pregnant or are trying to become pregnant, and are worried about potential harm to the baby, will stop the medications due to incorrect information. Or they’ll be told by their health care team to stop their medications.”
Dr. Streett and study lead author Aarti Rao, MD, a gastroenterology fellow at Stanford, launched their study of IBD clinic patients to gain more understanding about patient knowledge. “We know from research already published that those with IBD have a lot of concerns about starting families and don’t have a lot of information to base their decision making on,” Dr. Streett said. “We wanted to evaluate that in our population and see how much people knew and what the need was.”
In 2018 and 2019, Dr. Streett and Dr. Rao gave an anonymous, validated 17-question survey to patients aged 18-45 with IBD. One hundred patients responded (median age = 30, 54% female, 59% white, 66% with incomes over $100,000, 52% with ulcerative colitis, 21% with prior IBD surgery, 71% with prior IBD hospitalization).
Just over a third – 35% – of the patients said they’d been counseled about reproductive health by a physician. This finding reflects findings in previous research, said Dr. Rao, who spoke in an interview.
Just 15% of those who’d undergone IBD surgery reported getting guidance about the effects of surgery on fertility.
More than a third (35%) of women and 15% of men said they’d considered not having children because of their IBD. In fact, “most potential dads and moms have the chance to do very well,” Dr. Streett said.
Without reproductive counseling, she added, parents won’t know about the risks of passing on IBD. According to Dr. Rao, there’s an estimated less than 5% chance that IBD will be passed on to children if one parent has the condition; the risk is much higher if both parents have it.
Going forward, “there’s a really urgent need for proactive counseling on the part of gastroenterologists and health care teams to give people of childbearing age the right information so they can be informed to make the best decisions,” Dr. Streett said.
The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
SOURCE: Rao A et al. Crohn’s & Colitis Congress, Abstract P009.
LAS VEGAS – Inflammatory bowel disease (IBD) can disrupt both fertility and pregnancy, especially if it’s not fully controlled, and there’s a risk that the condition can be passed onto an unborn child. Still a new study suggests many patients with IBD don’t receive appropriate reproductive counseling.
Nearly two-thirds of 100 patients surveyed at a single center reported that no physician had talked to them about reproductive topics, and some said they’d considered not having children because of the condition. “Really fundamental subjects have not made their way into the interactions between patients and their care teams,” coauthor and gastroenterologist Sarah Streett, MD, AGAF, of Stanford (Calif.) University, said in an interview before the study was presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
IBD can lower fertility in both sexes and boost complications in pregnancy. “The good news is that almost all the medications used for IBD appear safe,” Dr. Streett said. “In fact, the safety risks for the baby and the pregnancy revolve around not having IBD under good control.”
Unfortunately, she said, misinformation is common. “Patients who become pregnant or are trying to become pregnant, and are worried about potential harm to the baby, will stop the medications due to incorrect information. Or they’ll be told by their health care team to stop their medications.”
Dr. Streett and study lead author Aarti Rao, MD, a gastroenterology fellow at Stanford, launched their study of IBD clinic patients to gain more understanding about patient knowledge. “We know from research already published that those with IBD have a lot of concerns about starting families and don’t have a lot of information to base their decision making on,” Dr. Streett said. “We wanted to evaluate that in our population and see how much people knew and what the need was.”
In 2018 and 2019, Dr. Streett and Dr. Rao gave an anonymous, validated 17-question survey to patients aged 18-45 with IBD. One hundred patients responded (median age = 30, 54% female, 59% white, 66% with incomes over $100,000, 52% with ulcerative colitis, 21% with prior IBD surgery, 71% with prior IBD hospitalization).
Just over a third – 35% – of the patients said they’d been counseled about reproductive health by a physician. This finding reflects findings in previous research, said Dr. Rao, who spoke in an interview.
Just 15% of those who’d undergone IBD surgery reported getting guidance about the effects of surgery on fertility.
More than a third (35%) of women and 15% of men said they’d considered not having children because of their IBD. In fact, “most potential dads and moms have the chance to do very well,” Dr. Streett said.
Without reproductive counseling, she added, parents won’t know about the risks of passing on IBD. According to Dr. Rao, there’s an estimated less than 5% chance that IBD will be passed on to children if one parent has the condition; the risk is much higher if both parents have it.
Going forward, “there’s a really urgent need for proactive counseling on the part of gastroenterologists and health care teams to give people of childbearing age the right information so they can be informed to make the best decisions,” Dr. Streett said.
The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
SOURCE: Rao A et al. Crohn’s & Colitis Congress, Abstract P009.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Patients with inflammatory bowel disease aren’t getting proper guidance regarding fertility, pregnancy, and genetic risks.
Major finding: Among surveyed patients, 65% said they’d never received reproductive counseling from a physician.
Study details: Single-center survey of 100 patients (median age = 30, 54% female).
Disclosures: The study was funded by a philanthropic grant. The study authors report no relevant disclosures.
Source: Rao A et al. Crohn’s & Colitis Congress 2019, Abstract P009.