User login
New myeloma drugs improve response and extend survival
DR HENRY I thought we might discuss some cases of patients with myeloma, starting with a relatively simple case and ending with one that is a little more complicated. For the first case, we have a 56-year-old healthy man with IgG kappa myeloma whose work-up shows he has multiple lytic bone lesions. He has normal renal function, normal calcium, and he’s transplant-eligible by other health issues. I’ll leave the cytogenetics up to you if that changes your approach. How would you develop or pose some options for this man’s treatment to begin with?
DR ANDERSON It’s important to start out by saying that we, in myeloma, have many new classes of drugs and many new opportunities to choose from to treat this patient.1 As you know, we have proteasome inhibitors, the first-generation bortezomib, then carfilzomib and ixazomib. We have the immunomodulatory drugs (IMiDs), thalidomide, and now lenalidomide and pomalidomide. We have a histone deacetylase (HDAC) inhibitor approved called panobinostat, and we have 2 monoclonal antibodies approved, elotuzumab and daratumumab. These classes of medicine have made it possible for 20 different Food and Drug Administration (FDA) approvals in the last 10-15 years. These agents, having been tested in advanced myeloma, have moved toward initial management.
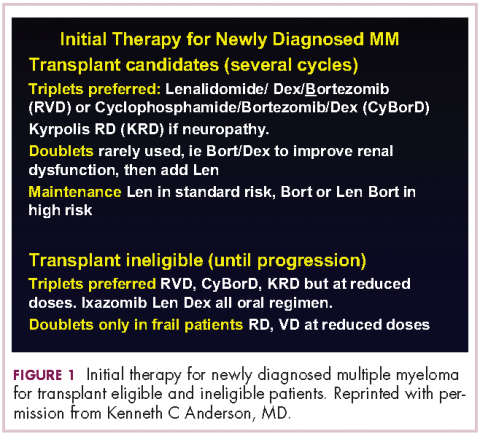
This person is 50 years old. He has adequate liver, heart, lung, and kidney function, so he would be eligible for high-dose therapy and stem-cell transplantation. In terms of initial management, there are many options (Figure 1). We strongly recommend that triplet therapy be used initially. The most common triplets would be lenalidomide, bortezomib, and dexamethasone (RVD)2,3 or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).4 If this man had neuropathy, perhaps carfilzomib, the second-generation proteasome inhibitor, with lenalidomide and dexamethasone could have been used. Why do we use these? The extent and frequency of response with these triplets is nearly universal overall response rate, with three-quarters very good partial and half-complete responses, including minimal residual disease negative responses. In this patient, we would therefore recommend treatment with either RVD or CyBorD for several cycles to maximal response.
He would then have autologous stem cells collected, and it is still the standard of care to proceed to high-dose melphalan and a single high-dose therapy and stem-cell transplantation. The cytogenetics are important: if this patient has standard-risk multiple myeloma, then lenalidomide maintenance would be given after transplant. It is now FDA-approved for this purpose because it can prolong both progression-free and – most importantly – overall survival.5 Standard-risk cytogenetics might, for example, include hyperdiploidy or translocation 11;14. On the other hand, if his myeloma were high-risk and characterized, for example, by 17p deletion, we would carry out the same induction and transplantation, but we would alter the maintenance to incorporate a proteasome inhibitor. Lenalidomide and bortezomib, for example, could be combined. Early data show that using combined maintenance therapy with lenalidomide and bortezomib, can overcome the early relapses that are characteristic of high-risk disease.6
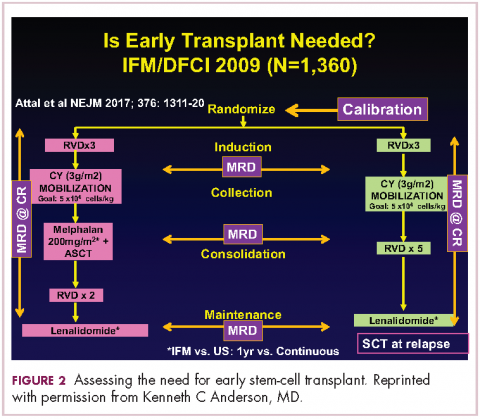
Because of the extent and frequency of response to combination novel therapies, we have undertaken with our French colleagues a clinical trial of RVD in newly diagnosed patients – such as this patient – followed by stem-cell collection in all patients (Figure 2). Then there is a randomization to either early high-dose therapy, melphalan, and autologous stem-cell transplantation, followed by lenalidomide maintenance; or in the other cohort, harvesting of stem cells, additional RVD, and then maintenance with lenalidomide, saving the stem-cell transplant for later.
The French portion of this trial was reported in the New England Journal of Medicine earlier in 2017.7 It showed that patients who received RVD, high-dose melphalan, stem-cell transplant, and had 1 year of lenalidomide maintenance, had a progression-free survival advantage of about 1 year, without an overall survival advantage; compared with those patients who received RVD and lenalidomide maintenance, saving the transplant for later. I would hasten to add that lenalidomide maintenance was given for only 1 year in this trial, and patients in the RVD-only or RVD-and-transplant arms of this trial relapsed after the lenalidomide maintenance was discontinued.
The American portion of this trial is identical. That is, RVD induction is being given and all patients have a stem-cell collection. Half of the patients then go to high-dose melphalan and stem-cell transplant early, and half of them have the transplant only later at the time of relapse. A major difference, however, is that in both the RVD-only and RVD-and-transplant cohorts, patients receive lenalidomide maintenance until progression. This trial has been ongoing since 2009 and is still ongoing, which tells us that patients in both arms – the RVD-only as well as the RVD-and-transplant arms – are doing well.
In the recent STAMINA trial, all patients underwent a single high-dose therapy and transplant. Then there was a randomization to lenalidomide maintenance only in 1 cohort; a randomization to consolidation with RVD posttransplant followed by lenalidomide maintenance in the second cohort; or a randomization to a second high-dose melphalan and stem-cell transplant followed by lenalidomide maintenance in the third cohort.8 I mention this because the outcomes in all 3 cohorts was similar.
I believe this tells us strongly that high-dose therapy and stem-cell transplantation twice – so-called tandem transplant – is no longer a major option in multiple myeloma. For now, however, in this patient, the standard of care would be to undergo induction therapy with triplet, novel combination treatment. Then, stem cells would be collected and high-dose therapy stem-cell transplant would be done, followed either by lenalidomide maintenance for standard disease or lenalidomide and bortezomib maintenance for high-risk disease. We won’t really know if we can delay transplant until the trials I’ve mentioned totally read out. In my clinical practice, if patients have had a major response to their induction therapy and have stem cells harvested, we can then offer them the opportunity to use maintenance therapy and save the transplant as a potential option for later, when myeloma relapses.
DR HENRY In summary then, this would be, in 2017, off-protocol while the data is pending: it’s reasonable to get a deep induction response, collect stem cells, have a discussion with the patient, and then consider high-dose therapy or not.
DR ANDERSON Yes. I think it’s reasonable to discuss it. We need to be open and honest with patients that the standard of care remains transplant, that you incorporate novel treatments before the transplant and novel treatments as maintenance after the transplant. The happy news is that the outcome, especially for patients who have standard-risk myeloma, is at least a decade or longer progression-free survival. It’s an optimistic picture. The data in terms of transplant being needed or not, will come within the next several years.
For now, it is a standard of care to use 1 high-dose melphalan and stem-cell transplant in this setting. I will add into our discussion with patients – besides the opportunity to harvest stem cells and think about whether one needs to do a transplant early on or not – is the issue of toxicity. High-dose melphalan by itself has a small but real secondary incidence of cancer, myelodysplasia, or leukemia. If one uses lenalidomide maintenance after melphalan transplantation treatment, that risk of secondary cancer is slightly increased.
In my experience, if patients have achieved a complete response with induction therapy only, it’s not unreasonable to offer early transplant and be clear that’s the standard of care. The alternative is maintenance with lenalidomide, knowing once the stem cells have been harvested, that transplantation can be an option to treat relapsed myeloma. We have many other options available as well. Time will tell in terms of the ongoing randomized trials as to whether transplant remains central to our treatment paradigm.
DR HENRY This leads us to our second patient. Here we have an older man of 74 years. He’s a professional piano player, so we want to try to avoid peripheral neuropathy in him. He has some mild renal insufficiency and some coronary artery disease, so he’s deemed transplant-ineligible. He has IgG kappa myeloma, and he’s brand new. What would you consider to be options for him for treatment?
DR ANDERSON This brings up the issue of a transplant-ineligible patient. He has significant comorbidity that would make transplantation an increased risk. What we would recommend in such a patient is still triplet induction therapy incorporating novel agents (Figure 1). Lenalidomide, the immunomodulatory drug, can safely be given in the context of neuropathy because it does not cause significant neuropathy. It would need to be dose modified, depending on the degree of renal insufficiency. We would recommend also including proteasome inhibitors. Bortezomib, the first-generation proteasome inhibitor, would be contraindicated because it does have a small but real attendant neuropathy. If, however, it is given weekly and subcutaneously, the risk of attendant neuropathy is quite low. In this patient, therefore, one could start with lenalidomide and bortezomib weekly and subcutaneously,1,2 with a very early and vigilant follow-up for the earliest signs of neuropathy, so as not to allow it to develop and compromise his career.
Alternatively, one could use a proteasome inhibitor that does not have attendant neuropathy. Carfilzomib, the second-generation proteasome inhibitor, does not have neuropathy.9 But we would need to have caution here, because this patient has a history of coronary artery disease, and carfilzomib has a very small, but real, incidence of cardiac toxicity so would need to be used judiciously in this setting. The third proteasome inhibitor, ixazomib, is the next-generation bortezomib-class proteasome inhibitor, and it’s oral.10 It has less neuropathy than does bortezomib, so in my view is a very realistic option for him together with lenalidomide. It does have a small incidence of neuropathy, so close monitoring for neuropathy would be indicated. We could use lenalidomide–dexamethasone as a doublet and avoid neuropathy,11 but usually doublets are reserved only for frail patients.
My recommendation, therefore, would be RVD with the bortezomib weekly or subcutaneously, or alternatively, lenalidomide, ixazomib, dexamethasone as an all-oral regimen as induction therapy. In my view, this 74-year-old patient with comorbidity is not a transplant candidate. However, one can be very optimistic with this patient. The likelihood that he could have myeloma as a chronic illness and die from something else is quite high. Initial induction triplet therapy would achieve a very high response extent and frequency. The durability would be long, especially with lenalidomide maintenance if it’s standard-risk myeloma or lenalidomide and a proteasome inhibitor, probably ixazomib in this setting, if he were to have high-risk myeloma.
If myeloma relapses, then there are many options that could be used in this patient and achieve years of progression-free and overall survival. Indeed, he is 74 years old and will respond very well to induction triplet therapy, with many years’ duration of response due to continuous lenalidomide or lenalidomide and proteasome inhibitor maintenance. Then there are many effective options to treat relapsed therapy using triplet novel agents. Therefore, his lifespan is unlikely to be shortened by multiple myeloma.
DR HENRY It’s so incredible compared with what it was when I trained. The next patient, a 45-year-old woman with IgG lambda myeloma, has had RVD induction and responded. She had lenalidomide maintenance, but then she progressed, and she got her stem-cell transplant, and she’s progressing after that. I guess we’re looking here to fold in some of the newer agents. How you would you do that in this patient?DR ANDERSON Yes. I think one of the most remarkable and exciting developments with myeloma is the rapid approval of the novel classes of agents that I mentioned earlier – the proteasome inhibitors, the immunomodulatory drugs, the HDAC inhibitor, and the monoclonal antibodies.1 They’re particularly relevant in a patient such as this one, whose myeloma has relapsed after what would be considered standard therapy for a young person with standard-risk myeloma. This patient had RVD and maintenance therapy, and then progressed. The transplant was given for relapsed myeloma. The opportunity to use stem-cell transplant in patients when myeloma becomes active after maintenance should not be forgotten as it can be very effective. In all the trials done to date in which early versus late transplant are compared, there have been similar outcomes. Therefore, if the transplant isn’t done early, don’t forget that it’s an option at the time the myeloma progresses. I do want to mention, that there are lots of options for relapsed myeloma (Figures 3 and 4). I mentioned RVD or CyBorD as initial triplet therapies.2-4 In North America, those are the 2 most common regimens. If myeloma then relapses and is resistant to RVD or to CyBorD, then we need to identify alternatives.
We also need to think about the comorbidities in the patient – issues such as age, neuropathy, presence of renal dysfunction, and other clinical factors. And we need to think about what treatment they’ve had in the past. This patient has had RVD, maintenance with lenalidomide, and a stem-cell transplant. We can offer patients a variety of therapies, but in the context of resistance to the first-generation proteasome inhibitor bortezomib and the first-generation immunomodulatory drug lenalidomide, we would strongly recommend the second-generation immunomodulatory drug pomalidomide12 together with a second-generation proteasome inhibitor, be that carfilzomib13 or ixazomib.14 When one uses the second-generation IMiDs and proteasome inhibitors together, there’s a very high frequency of response in the order of 70%-80%, which lasts years.
Besides carfilzomib and ixazomib proteasome inhibitors, we also have elotuzumab and daratumumab, the monoclonal antibodies.15-17 These agents have been FDA approved to treat patients such as this one who has had 1-3 previous therapies for their myeloma. All of them have been approved in randomized phase 3 trials compared with lenalidomide-dexamethasone in the control arm.13-15,17 They’ve all been found to be superior. Although lenalidomide-dexamethasone combined with daratumumab, ixazomib, elotuzumab, or carfilzomib is superior to lenalidomide in relapsed myeloma, the situation in North America, as in this patient, is usually that patients have had lenalidomide-dexamethasone as part of their initial treatment and their myeloma is refractory to lenalidomide.
Hence, we recommend, that we go to the second-generation pomalidomide and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Having said that, the treatment paradigm is evolving. For example, the monoclonal antibody daratumumab was initially approved by the FDA in multiply relapsed disease as a single agent because it achieves a 30% response rate.16 It now has been moved earlier into the first relapse of multiple myeloma, where it achieves much higher response rates when combined with lenalidomide–dexamethasone or combined with bortezomib–dexamethasone.17,18 Response rates of 70%-80% can be achieved, including minimal residual disease negative complete responses.
Today, in a patient who has had RVD transplant and myeloma has returned, we would recommend second-generation IMiDs, pomalidomide, and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Data for daratumumab combined with lenalidomide-dexamethasone or with bortezomib-dexamethasone, look very promising. We need, however, to see more experience of daratumumab together with lenalidomide-dexamethasone or daratumumab together with bortezomib-dexamethasone in patients whose myeloma is refractory to RVD, that is, patients whose myeloma has returned after RVD induction treatment. Of note, pomalidomide, dexamethasone, and daratumumab have just been approved by the FDA and may also be active even in myeloma recurring after RVD treatment.19
Daratumumab in combination will be moving earlier and earlier and may be appropriate to treat the first relapse. I do want to stress, however, that at present I save daratumumab for the second or greater relapse. Daratumumab is active even when relapse occurs after treatment with second-generation IMiDs and proteasome inhibitors.
DR HENRY Before we close, I have a couple practical questions with these antibodies. Daratumumab has the track record of first-treatment severe reactions and long infusion times. How long are you anticipating the first daratumumab treatment takes? There has been some talk that maybe splitting it in half and going over 2 days is easier on the patient and the infusion center. Have you done that?
DR ANDERSON Yes, I think that’s a very important point. We need to be thinking – first and foremost – about efficacy of our therapy. Equally important, however, are the safety profile and the user-friendliness for the patient. Daratumumab infusions are quite long – on the order of 8 hours or longer on day 1 of infusion. And to date, all the clinical trials have used daratumumab infusions weekly for 8 treatments, followed by 8 treatments given every 2 weeks. Then monthly daratumumab is given as a maintenance therapy. Thus, there is a requirement for multiple outpatient clinic visits that can be prolonged.
One of the opportunities that’s being tested is to give daratumumab subcutaneously. While this is being evaluated in protocols now, the results that have been reported at our national meetings look to be quite promising in terms of efficacy, similar to results with the intravenous administration. Obviously, this would allow for a much more convenient clinic visit and shorter time for the patients being treated.
I should mention that the other antibody, elotuzumab, has been approved in combination with lenalidomide and dexamethasone.15 The infusions with lenalidomide, dexamethasone, and the antibody elotuzumab are much shorter, on the order of 2- or 3-hour visits. The place for elotuzumab in the management of relapsed myeloma is yet to be totally defined. We tend to use it now in the setting of more indolent relapses, where patients might have a slowly rising monoclonal protein. Elotuzumab-lenalidomide-dexamethasone has maintained an overall survival advantage at 4 years compared with lenalidomide-dexamethasone when used in relapsed myeloma.
We are quite excited about both antibodies. Daratumumab tends to get most of the activity, as it achieves responses as a single agent,16 and the depth of the responses are markedly increased when it’s combined with lenalidomide-dexamethasone or bortezomib-dexamethasone.17,18 However, one shouldn’t forget elotuzumab15 based on its tolerability and the survival advantage I mentioned at 4 years.
The final point is that we think about myeloma genetically at the time of diagnosis and relapse in terms of standard or high-risk disease. One of the hallmarks of high-risk disease has been 17P deletion or P53 dysfunction. One of the most exciting outcomes of the development of monoclonal antibodies has been the responses observed even in the context of P53 deletion. Clearly, antibody-mediated cellular cytotoxicity, complement-mediated cytotoxicity, and other mechanisms of action of these antibodies do not require normal P53 function. The important point, therefore, is that what has previously been thought of as high-risk disease can nowadays be effectively treated with these new immune treatments, correlating with the marked improvement in survival and overall outcome.
DR HENRY We have outlined 3 kinds of myeloma patients we see, and especially interesting is the last patient, who has relapsed and then progressed, and in whom newer drugs have a role. Thank you for such a complete and thorough discussion, Dr Anderson.
1. Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:230-269.
2. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly-diagnosed multiple myeloma. Blood. 2010;116:679-686.
3. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777: a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
4. Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569-2574.
5. McCarthy PL, Owzar K, Hofmeister CC, et al. A phase III study of lenalidomide after transplant for multiple myeloma. N Engl J Med. 2012;366:1770-1781.
6. Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib, and dexamethasone (RVD) in high risk myeloma patients. Leukemia. 2014;28:690-693.
7. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib and dexamethasone with transplantation in myeloma. N Engl J Med. 2017;376:1311-1320.
8. Stadtmauer EA, Pasquini MC, Blackwell B, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Abstract LBA-1. Presented at the 2016 ASH Annual Meeting, December 6, 2016; San Diego, CA.
9. Dytfeld D, Jasielec J, Griffith KA, et al: Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162-164.
10. Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503-1512
11. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. for the FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917.
12. Richardson P, Siegel D, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832.
13. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. for the ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142-152
14. Moreau P, Masszi T, Grzasko N, et al. for the TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;28;374:1621-1634.
15. Lonial S, Dimopoulos M, Palumbo A, et al. for the ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621-631.
16. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD 38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207-1219.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
18. Palumbo A, Chanan-Khan A, Weisel K, et al. for the CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
19. Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981.
DR HENRY I thought we might discuss some cases of patients with myeloma, starting with a relatively simple case and ending with one that is a little more complicated. For the first case, we have a 56-year-old healthy man with IgG kappa myeloma whose work-up shows he has multiple lytic bone lesions. He has normal renal function, normal calcium, and he’s transplant-eligible by other health issues. I’ll leave the cytogenetics up to you if that changes your approach. How would you develop or pose some options for this man’s treatment to begin with?
DR ANDERSON It’s important to start out by saying that we, in myeloma, have many new classes of drugs and many new opportunities to choose from to treat this patient.1 As you know, we have proteasome inhibitors, the first-generation bortezomib, then carfilzomib and ixazomib. We have the immunomodulatory drugs (IMiDs), thalidomide, and now lenalidomide and pomalidomide. We have a histone deacetylase (HDAC) inhibitor approved called panobinostat, and we have 2 monoclonal antibodies approved, elotuzumab and daratumumab. These classes of medicine have made it possible for 20 different Food and Drug Administration (FDA) approvals in the last 10-15 years. These agents, having been tested in advanced myeloma, have moved toward initial management.
This person is 50 years old. He has adequate liver, heart, lung, and kidney function, so he would be eligible for high-dose therapy and stem-cell transplantation. In terms of initial management, there are many options (Figure 1). We strongly recommend that triplet therapy be used initially. The most common triplets would be lenalidomide, bortezomib, and dexamethasone (RVD)2,3 or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).4 If this man had neuropathy, perhaps carfilzomib, the second-generation proteasome inhibitor, with lenalidomide and dexamethasone could have been used. Why do we use these? The extent and frequency of response with these triplets is nearly universal overall response rate, with three-quarters very good partial and half-complete responses, including minimal residual disease negative responses. In this patient, we would therefore recommend treatment with either RVD or CyBorD for several cycles to maximal response.
He would then have autologous stem cells collected, and it is still the standard of care to proceed to high-dose melphalan and a single high-dose therapy and stem-cell transplantation. The cytogenetics are important: if this patient has standard-risk multiple myeloma, then lenalidomide maintenance would be given after transplant. It is now FDA-approved for this purpose because it can prolong both progression-free and – most importantly – overall survival.5 Standard-risk cytogenetics might, for example, include hyperdiploidy or translocation 11;14. On the other hand, if his myeloma were high-risk and characterized, for example, by 17p deletion, we would carry out the same induction and transplantation, but we would alter the maintenance to incorporate a proteasome inhibitor. Lenalidomide and bortezomib, for example, could be combined. Early data show that using combined maintenance therapy with lenalidomide and bortezomib, can overcome the early relapses that are characteristic of high-risk disease.6
Because of the extent and frequency of response to combination novel therapies, we have undertaken with our French colleagues a clinical trial of RVD in newly diagnosed patients – such as this patient – followed by stem-cell collection in all patients (Figure 2). Then there is a randomization to either early high-dose therapy, melphalan, and autologous stem-cell transplantation, followed by lenalidomide maintenance; or in the other cohort, harvesting of stem cells, additional RVD, and then maintenance with lenalidomide, saving the stem-cell transplant for later.
The French portion of this trial was reported in the New England Journal of Medicine earlier in 2017.7 It showed that patients who received RVD, high-dose melphalan, stem-cell transplant, and had 1 year of lenalidomide maintenance, had a progression-free survival advantage of about 1 year, without an overall survival advantage; compared with those patients who received RVD and lenalidomide maintenance, saving the transplant for later. I would hasten to add that lenalidomide maintenance was given for only 1 year in this trial, and patients in the RVD-only or RVD-and-transplant arms of this trial relapsed after the lenalidomide maintenance was discontinued.
The American portion of this trial is identical. That is, RVD induction is being given and all patients have a stem-cell collection. Half of the patients then go to high-dose melphalan and stem-cell transplant early, and half of them have the transplant only later at the time of relapse. A major difference, however, is that in both the RVD-only and RVD-and-transplant cohorts, patients receive lenalidomide maintenance until progression. This trial has been ongoing since 2009 and is still ongoing, which tells us that patients in both arms – the RVD-only as well as the RVD-and-transplant arms – are doing well.
In the recent STAMINA trial, all patients underwent a single high-dose therapy and transplant. Then there was a randomization to lenalidomide maintenance only in 1 cohort; a randomization to consolidation with RVD posttransplant followed by lenalidomide maintenance in the second cohort; or a randomization to a second high-dose melphalan and stem-cell transplant followed by lenalidomide maintenance in the third cohort.8 I mention this because the outcomes in all 3 cohorts was similar.
I believe this tells us strongly that high-dose therapy and stem-cell transplantation twice – so-called tandem transplant – is no longer a major option in multiple myeloma. For now, however, in this patient, the standard of care would be to undergo induction therapy with triplet, novel combination treatment. Then, stem cells would be collected and high-dose therapy stem-cell transplant would be done, followed either by lenalidomide maintenance for standard disease or lenalidomide and bortezomib maintenance for high-risk disease. We won’t really know if we can delay transplant until the trials I’ve mentioned totally read out. In my clinical practice, if patients have had a major response to their induction therapy and have stem cells harvested, we can then offer them the opportunity to use maintenance therapy and save the transplant as a potential option for later, when myeloma relapses.
DR HENRY In summary then, this would be, in 2017, off-protocol while the data is pending: it’s reasonable to get a deep induction response, collect stem cells, have a discussion with the patient, and then consider high-dose therapy or not.
DR ANDERSON Yes. I think it’s reasonable to discuss it. We need to be open and honest with patients that the standard of care remains transplant, that you incorporate novel treatments before the transplant and novel treatments as maintenance after the transplant. The happy news is that the outcome, especially for patients who have standard-risk myeloma, is at least a decade or longer progression-free survival. It’s an optimistic picture. The data in terms of transplant being needed or not, will come within the next several years.
For now, it is a standard of care to use 1 high-dose melphalan and stem-cell transplant in this setting. I will add into our discussion with patients – besides the opportunity to harvest stem cells and think about whether one needs to do a transplant early on or not – is the issue of toxicity. High-dose melphalan by itself has a small but real secondary incidence of cancer, myelodysplasia, or leukemia. If one uses lenalidomide maintenance after melphalan transplantation treatment, that risk of secondary cancer is slightly increased.
In my experience, if patients have achieved a complete response with induction therapy only, it’s not unreasonable to offer early transplant and be clear that’s the standard of care. The alternative is maintenance with lenalidomide, knowing once the stem cells have been harvested, that transplantation can be an option to treat relapsed myeloma. We have many other options available as well. Time will tell in terms of the ongoing randomized trials as to whether transplant remains central to our treatment paradigm.
DR HENRY This leads us to our second patient. Here we have an older man of 74 years. He’s a professional piano player, so we want to try to avoid peripheral neuropathy in him. He has some mild renal insufficiency and some coronary artery disease, so he’s deemed transplant-ineligible. He has IgG kappa myeloma, and he’s brand new. What would you consider to be options for him for treatment?
DR ANDERSON This brings up the issue of a transplant-ineligible patient. He has significant comorbidity that would make transplantation an increased risk. What we would recommend in such a patient is still triplet induction therapy incorporating novel agents (Figure 1). Lenalidomide, the immunomodulatory drug, can safely be given in the context of neuropathy because it does not cause significant neuropathy. It would need to be dose modified, depending on the degree of renal insufficiency. We would recommend also including proteasome inhibitors. Bortezomib, the first-generation proteasome inhibitor, would be contraindicated because it does have a small but real attendant neuropathy. If, however, it is given weekly and subcutaneously, the risk of attendant neuropathy is quite low. In this patient, therefore, one could start with lenalidomide and bortezomib weekly and subcutaneously,1,2 with a very early and vigilant follow-up for the earliest signs of neuropathy, so as not to allow it to develop and compromise his career.
Alternatively, one could use a proteasome inhibitor that does not have attendant neuropathy. Carfilzomib, the second-generation proteasome inhibitor, does not have neuropathy.9 But we would need to have caution here, because this patient has a history of coronary artery disease, and carfilzomib has a very small, but real, incidence of cardiac toxicity so would need to be used judiciously in this setting. The third proteasome inhibitor, ixazomib, is the next-generation bortezomib-class proteasome inhibitor, and it’s oral.10 It has less neuropathy than does bortezomib, so in my view is a very realistic option for him together with lenalidomide. It does have a small incidence of neuropathy, so close monitoring for neuropathy would be indicated. We could use lenalidomide–dexamethasone as a doublet and avoid neuropathy,11 but usually doublets are reserved only for frail patients.
My recommendation, therefore, would be RVD with the bortezomib weekly or subcutaneously, or alternatively, lenalidomide, ixazomib, dexamethasone as an all-oral regimen as induction therapy. In my view, this 74-year-old patient with comorbidity is not a transplant candidate. However, one can be very optimistic with this patient. The likelihood that he could have myeloma as a chronic illness and die from something else is quite high. Initial induction triplet therapy would achieve a very high response extent and frequency. The durability would be long, especially with lenalidomide maintenance if it’s standard-risk myeloma or lenalidomide and a proteasome inhibitor, probably ixazomib in this setting, if he were to have high-risk myeloma.
If myeloma relapses, then there are many options that could be used in this patient and achieve years of progression-free and overall survival. Indeed, he is 74 years old and will respond very well to induction triplet therapy, with many years’ duration of response due to continuous lenalidomide or lenalidomide and proteasome inhibitor maintenance. Then there are many effective options to treat relapsed therapy using triplet novel agents. Therefore, his lifespan is unlikely to be shortened by multiple myeloma.
DR HENRY It’s so incredible compared with what it was when I trained. The next patient, a 45-year-old woman with IgG lambda myeloma, has had RVD induction and responded. She had lenalidomide maintenance, but then she progressed, and she got her stem-cell transplant, and she’s progressing after that. I guess we’re looking here to fold in some of the newer agents. How you would you do that in this patient?DR ANDERSON Yes. I think one of the most remarkable and exciting developments with myeloma is the rapid approval of the novel classes of agents that I mentioned earlier – the proteasome inhibitors, the immunomodulatory drugs, the HDAC inhibitor, and the monoclonal antibodies.1 They’re particularly relevant in a patient such as this one, whose myeloma has relapsed after what would be considered standard therapy for a young person with standard-risk myeloma. This patient had RVD and maintenance therapy, and then progressed. The transplant was given for relapsed myeloma. The opportunity to use stem-cell transplant in patients when myeloma becomes active after maintenance should not be forgotten as it can be very effective. In all the trials done to date in which early versus late transplant are compared, there have been similar outcomes. Therefore, if the transplant isn’t done early, don’t forget that it’s an option at the time the myeloma progresses. I do want to mention, that there are lots of options for relapsed myeloma (Figures 3 and 4). I mentioned RVD or CyBorD as initial triplet therapies.2-4 In North America, those are the 2 most common regimens. If myeloma then relapses and is resistant to RVD or to CyBorD, then we need to identify alternatives.
We also need to think about the comorbidities in the patient – issues such as age, neuropathy, presence of renal dysfunction, and other clinical factors. And we need to think about what treatment they’ve had in the past. This patient has had RVD, maintenance with lenalidomide, and a stem-cell transplant. We can offer patients a variety of therapies, but in the context of resistance to the first-generation proteasome inhibitor bortezomib and the first-generation immunomodulatory drug lenalidomide, we would strongly recommend the second-generation immunomodulatory drug pomalidomide12 together with a second-generation proteasome inhibitor, be that carfilzomib13 or ixazomib.14 When one uses the second-generation IMiDs and proteasome inhibitors together, there’s a very high frequency of response in the order of 70%-80%, which lasts years.
Besides carfilzomib and ixazomib proteasome inhibitors, we also have elotuzumab and daratumumab, the monoclonal antibodies.15-17 These agents have been FDA approved to treat patients such as this one who has had 1-3 previous therapies for their myeloma. All of them have been approved in randomized phase 3 trials compared with lenalidomide-dexamethasone in the control arm.13-15,17 They’ve all been found to be superior. Although lenalidomide-dexamethasone combined with daratumumab, ixazomib, elotuzumab, or carfilzomib is superior to lenalidomide in relapsed myeloma, the situation in North America, as in this patient, is usually that patients have had lenalidomide-dexamethasone as part of their initial treatment and their myeloma is refractory to lenalidomide.
Hence, we recommend, that we go to the second-generation pomalidomide and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Having said that, the treatment paradigm is evolving. For example, the monoclonal antibody daratumumab was initially approved by the FDA in multiply relapsed disease as a single agent because it achieves a 30% response rate.16 It now has been moved earlier into the first relapse of multiple myeloma, where it achieves much higher response rates when combined with lenalidomide–dexamethasone or combined with bortezomib–dexamethasone.17,18 Response rates of 70%-80% can be achieved, including minimal residual disease negative complete responses.
Today, in a patient who has had RVD transplant and myeloma has returned, we would recommend second-generation IMiDs, pomalidomide, and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Data for daratumumab combined with lenalidomide-dexamethasone or with bortezomib-dexamethasone, look very promising. We need, however, to see more experience of daratumumab together with lenalidomide-dexamethasone or daratumumab together with bortezomib-dexamethasone in patients whose myeloma is refractory to RVD, that is, patients whose myeloma has returned after RVD induction treatment. Of note, pomalidomide, dexamethasone, and daratumumab have just been approved by the FDA and may also be active even in myeloma recurring after RVD treatment.19
Daratumumab in combination will be moving earlier and earlier and may be appropriate to treat the first relapse. I do want to stress, however, that at present I save daratumumab for the second or greater relapse. Daratumumab is active even when relapse occurs after treatment with second-generation IMiDs and proteasome inhibitors.
DR HENRY Before we close, I have a couple practical questions with these antibodies. Daratumumab has the track record of first-treatment severe reactions and long infusion times. How long are you anticipating the first daratumumab treatment takes? There has been some talk that maybe splitting it in half and going over 2 days is easier on the patient and the infusion center. Have you done that?
DR ANDERSON Yes, I think that’s a very important point. We need to be thinking – first and foremost – about efficacy of our therapy. Equally important, however, are the safety profile and the user-friendliness for the patient. Daratumumab infusions are quite long – on the order of 8 hours or longer on day 1 of infusion. And to date, all the clinical trials have used daratumumab infusions weekly for 8 treatments, followed by 8 treatments given every 2 weeks. Then monthly daratumumab is given as a maintenance therapy. Thus, there is a requirement for multiple outpatient clinic visits that can be prolonged.
One of the opportunities that’s being tested is to give daratumumab subcutaneously. While this is being evaluated in protocols now, the results that have been reported at our national meetings look to be quite promising in terms of efficacy, similar to results with the intravenous administration. Obviously, this would allow for a much more convenient clinic visit and shorter time for the patients being treated.
I should mention that the other antibody, elotuzumab, has been approved in combination with lenalidomide and dexamethasone.15 The infusions with lenalidomide, dexamethasone, and the antibody elotuzumab are much shorter, on the order of 2- or 3-hour visits. The place for elotuzumab in the management of relapsed myeloma is yet to be totally defined. We tend to use it now in the setting of more indolent relapses, where patients might have a slowly rising monoclonal protein. Elotuzumab-lenalidomide-dexamethasone has maintained an overall survival advantage at 4 years compared with lenalidomide-dexamethasone when used in relapsed myeloma.
We are quite excited about both antibodies. Daratumumab tends to get most of the activity, as it achieves responses as a single agent,16 and the depth of the responses are markedly increased when it’s combined with lenalidomide-dexamethasone or bortezomib-dexamethasone.17,18 However, one shouldn’t forget elotuzumab15 based on its tolerability and the survival advantage I mentioned at 4 years.
The final point is that we think about myeloma genetically at the time of diagnosis and relapse in terms of standard or high-risk disease. One of the hallmarks of high-risk disease has been 17P deletion or P53 dysfunction. One of the most exciting outcomes of the development of monoclonal antibodies has been the responses observed even in the context of P53 deletion. Clearly, antibody-mediated cellular cytotoxicity, complement-mediated cytotoxicity, and other mechanisms of action of these antibodies do not require normal P53 function. The important point, therefore, is that what has previously been thought of as high-risk disease can nowadays be effectively treated with these new immune treatments, correlating with the marked improvement in survival and overall outcome.
DR HENRY We have outlined 3 kinds of myeloma patients we see, and especially interesting is the last patient, who has relapsed and then progressed, and in whom newer drugs have a role. Thank you for such a complete and thorough discussion, Dr Anderson.
DR HENRY I thought we might discuss some cases of patients with myeloma, starting with a relatively simple case and ending with one that is a little more complicated. For the first case, we have a 56-year-old healthy man with IgG kappa myeloma whose work-up shows he has multiple lytic bone lesions. He has normal renal function, normal calcium, and he’s transplant-eligible by other health issues. I’ll leave the cytogenetics up to you if that changes your approach. How would you develop or pose some options for this man’s treatment to begin with?
DR ANDERSON It’s important to start out by saying that we, in myeloma, have many new classes of drugs and many new opportunities to choose from to treat this patient.1 As you know, we have proteasome inhibitors, the first-generation bortezomib, then carfilzomib and ixazomib. We have the immunomodulatory drugs (IMiDs), thalidomide, and now lenalidomide and pomalidomide. We have a histone deacetylase (HDAC) inhibitor approved called panobinostat, and we have 2 monoclonal antibodies approved, elotuzumab and daratumumab. These classes of medicine have made it possible for 20 different Food and Drug Administration (FDA) approvals in the last 10-15 years. These agents, having been tested in advanced myeloma, have moved toward initial management.
This person is 50 years old. He has adequate liver, heart, lung, and kidney function, so he would be eligible for high-dose therapy and stem-cell transplantation. In terms of initial management, there are many options (Figure 1). We strongly recommend that triplet therapy be used initially. The most common triplets would be lenalidomide, bortezomib, and dexamethasone (RVD)2,3 or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).4 If this man had neuropathy, perhaps carfilzomib, the second-generation proteasome inhibitor, with lenalidomide and dexamethasone could have been used. Why do we use these? The extent and frequency of response with these triplets is nearly universal overall response rate, with three-quarters very good partial and half-complete responses, including minimal residual disease negative responses. In this patient, we would therefore recommend treatment with either RVD or CyBorD for several cycles to maximal response.
He would then have autologous stem cells collected, and it is still the standard of care to proceed to high-dose melphalan and a single high-dose therapy and stem-cell transplantation. The cytogenetics are important: if this patient has standard-risk multiple myeloma, then lenalidomide maintenance would be given after transplant. It is now FDA-approved for this purpose because it can prolong both progression-free and – most importantly – overall survival.5 Standard-risk cytogenetics might, for example, include hyperdiploidy or translocation 11;14. On the other hand, if his myeloma were high-risk and characterized, for example, by 17p deletion, we would carry out the same induction and transplantation, but we would alter the maintenance to incorporate a proteasome inhibitor. Lenalidomide and bortezomib, for example, could be combined. Early data show that using combined maintenance therapy with lenalidomide and bortezomib, can overcome the early relapses that are characteristic of high-risk disease.6
Because of the extent and frequency of response to combination novel therapies, we have undertaken with our French colleagues a clinical trial of RVD in newly diagnosed patients – such as this patient – followed by stem-cell collection in all patients (Figure 2). Then there is a randomization to either early high-dose therapy, melphalan, and autologous stem-cell transplantation, followed by lenalidomide maintenance; or in the other cohort, harvesting of stem cells, additional RVD, and then maintenance with lenalidomide, saving the stem-cell transplant for later.
The French portion of this trial was reported in the New England Journal of Medicine earlier in 2017.7 It showed that patients who received RVD, high-dose melphalan, stem-cell transplant, and had 1 year of lenalidomide maintenance, had a progression-free survival advantage of about 1 year, without an overall survival advantage; compared with those patients who received RVD and lenalidomide maintenance, saving the transplant for later. I would hasten to add that lenalidomide maintenance was given for only 1 year in this trial, and patients in the RVD-only or RVD-and-transplant arms of this trial relapsed after the lenalidomide maintenance was discontinued.
The American portion of this trial is identical. That is, RVD induction is being given and all patients have a stem-cell collection. Half of the patients then go to high-dose melphalan and stem-cell transplant early, and half of them have the transplant only later at the time of relapse. A major difference, however, is that in both the RVD-only and RVD-and-transplant cohorts, patients receive lenalidomide maintenance until progression. This trial has been ongoing since 2009 and is still ongoing, which tells us that patients in both arms – the RVD-only as well as the RVD-and-transplant arms – are doing well.
In the recent STAMINA trial, all patients underwent a single high-dose therapy and transplant. Then there was a randomization to lenalidomide maintenance only in 1 cohort; a randomization to consolidation with RVD posttransplant followed by lenalidomide maintenance in the second cohort; or a randomization to a second high-dose melphalan and stem-cell transplant followed by lenalidomide maintenance in the third cohort.8 I mention this because the outcomes in all 3 cohorts was similar.
I believe this tells us strongly that high-dose therapy and stem-cell transplantation twice – so-called tandem transplant – is no longer a major option in multiple myeloma. For now, however, in this patient, the standard of care would be to undergo induction therapy with triplet, novel combination treatment. Then, stem cells would be collected and high-dose therapy stem-cell transplant would be done, followed either by lenalidomide maintenance for standard disease or lenalidomide and bortezomib maintenance for high-risk disease. We won’t really know if we can delay transplant until the trials I’ve mentioned totally read out. In my clinical practice, if patients have had a major response to their induction therapy and have stem cells harvested, we can then offer them the opportunity to use maintenance therapy and save the transplant as a potential option for later, when myeloma relapses.
DR HENRY In summary then, this would be, in 2017, off-protocol while the data is pending: it’s reasonable to get a deep induction response, collect stem cells, have a discussion with the patient, and then consider high-dose therapy or not.
DR ANDERSON Yes. I think it’s reasonable to discuss it. We need to be open and honest with patients that the standard of care remains transplant, that you incorporate novel treatments before the transplant and novel treatments as maintenance after the transplant. The happy news is that the outcome, especially for patients who have standard-risk myeloma, is at least a decade or longer progression-free survival. It’s an optimistic picture. The data in terms of transplant being needed or not, will come within the next several years.
For now, it is a standard of care to use 1 high-dose melphalan and stem-cell transplant in this setting. I will add into our discussion with patients – besides the opportunity to harvest stem cells and think about whether one needs to do a transplant early on or not – is the issue of toxicity. High-dose melphalan by itself has a small but real secondary incidence of cancer, myelodysplasia, or leukemia. If one uses lenalidomide maintenance after melphalan transplantation treatment, that risk of secondary cancer is slightly increased.
In my experience, if patients have achieved a complete response with induction therapy only, it’s not unreasonable to offer early transplant and be clear that’s the standard of care. The alternative is maintenance with lenalidomide, knowing once the stem cells have been harvested, that transplantation can be an option to treat relapsed myeloma. We have many other options available as well. Time will tell in terms of the ongoing randomized trials as to whether transplant remains central to our treatment paradigm.
DR HENRY This leads us to our second patient. Here we have an older man of 74 years. He’s a professional piano player, so we want to try to avoid peripheral neuropathy in him. He has some mild renal insufficiency and some coronary artery disease, so he’s deemed transplant-ineligible. He has IgG kappa myeloma, and he’s brand new. What would you consider to be options for him for treatment?
DR ANDERSON This brings up the issue of a transplant-ineligible patient. He has significant comorbidity that would make transplantation an increased risk. What we would recommend in such a patient is still triplet induction therapy incorporating novel agents (Figure 1). Lenalidomide, the immunomodulatory drug, can safely be given in the context of neuropathy because it does not cause significant neuropathy. It would need to be dose modified, depending on the degree of renal insufficiency. We would recommend also including proteasome inhibitors. Bortezomib, the first-generation proteasome inhibitor, would be contraindicated because it does have a small but real attendant neuropathy. If, however, it is given weekly and subcutaneously, the risk of attendant neuropathy is quite low. In this patient, therefore, one could start with lenalidomide and bortezomib weekly and subcutaneously,1,2 with a very early and vigilant follow-up for the earliest signs of neuropathy, so as not to allow it to develop and compromise his career.
Alternatively, one could use a proteasome inhibitor that does not have attendant neuropathy. Carfilzomib, the second-generation proteasome inhibitor, does not have neuropathy.9 But we would need to have caution here, because this patient has a history of coronary artery disease, and carfilzomib has a very small, but real, incidence of cardiac toxicity so would need to be used judiciously in this setting. The third proteasome inhibitor, ixazomib, is the next-generation bortezomib-class proteasome inhibitor, and it’s oral.10 It has less neuropathy than does bortezomib, so in my view is a very realistic option for him together with lenalidomide. It does have a small incidence of neuropathy, so close monitoring for neuropathy would be indicated. We could use lenalidomide–dexamethasone as a doublet and avoid neuropathy,11 but usually doublets are reserved only for frail patients.
My recommendation, therefore, would be RVD with the bortezomib weekly or subcutaneously, or alternatively, lenalidomide, ixazomib, dexamethasone as an all-oral regimen as induction therapy. In my view, this 74-year-old patient with comorbidity is not a transplant candidate. However, one can be very optimistic with this patient. The likelihood that he could have myeloma as a chronic illness and die from something else is quite high. Initial induction triplet therapy would achieve a very high response extent and frequency. The durability would be long, especially with lenalidomide maintenance if it’s standard-risk myeloma or lenalidomide and a proteasome inhibitor, probably ixazomib in this setting, if he were to have high-risk myeloma.
If myeloma relapses, then there are many options that could be used in this patient and achieve years of progression-free and overall survival. Indeed, he is 74 years old and will respond very well to induction triplet therapy, with many years’ duration of response due to continuous lenalidomide or lenalidomide and proteasome inhibitor maintenance. Then there are many effective options to treat relapsed therapy using triplet novel agents. Therefore, his lifespan is unlikely to be shortened by multiple myeloma.
DR HENRY It’s so incredible compared with what it was when I trained. The next patient, a 45-year-old woman with IgG lambda myeloma, has had RVD induction and responded. She had lenalidomide maintenance, but then she progressed, and she got her stem-cell transplant, and she’s progressing after that. I guess we’re looking here to fold in some of the newer agents. How you would you do that in this patient?DR ANDERSON Yes. I think one of the most remarkable and exciting developments with myeloma is the rapid approval of the novel classes of agents that I mentioned earlier – the proteasome inhibitors, the immunomodulatory drugs, the HDAC inhibitor, and the monoclonal antibodies.1 They’re particularly relevant in a patient such as this one, whose myeloma has relapsed after what would be considered standard therapy for a young person with standard-risk myeloma. This patient had RVD and maintenance therapy, and then progressed. The transplant was given for relapsed myeloma. The opportunity to use stem-cell transplant in patients when myeloma becomes active after maintenance should not be forgotten as it can be very effective. In all the trials done to date in which early versus late transplant are compared, there have been similar outcomes. Therefore, if the transplant isn’t done early, don’t forget that it’s an option at the time the myeloma progresses. I do want to mention, that there are lots of options for relapsed myeloma (Figures 3 and 4). I mentioned RVD or CyBorD as initial triplet therapies.2-4 In North America, those are the 2 most common regimens. If myeloma then relapses and is resistant to RVD or to CyBorD, then we need to identify alternatives.
We also need to think about the comorbidities in the patient – issues such as age, neuropathy, presence of renal dysfunction, and other clinical factors. And we need to think about what treatment they’ve had in the past. This patient has had RVD, maintenance with lenalidomide, and a stem-cell transplant. We can offer patients a variety of therapies, but in the context of resistance to the first-generation proteasome inhibitor bortezomib and the first-generation immunomodulatory drug lenalidomide, we would strongly recommend the second-generation immunomodulatory drug pomalidomide12 together with a second-generation proteasome inhibitor, be that carfilzomib13 or ixazomib.14 When one uses the second-generation IMiDs and proteasome inhibitors together, there’s a very high frequency of response in the order of 70%-80%, which lasts years.
Besides carfilzomib and ixazomib proteasome inhibitors, we also have elotuzumab and daratumumab, the monoclonal antibodies.15-17 These agents have been FDA approved to treat patients such as this one who has had 1-3 previous therapies for their myeloma. All of them have been approved in randomized phase 3 trials compared with lenalidomide-dexamethasone in the control arm.13-15,17 They’ve all been found to be superior. Although lenalidomide-dexamethasone combined with daratumumab, ixazomib, elotuzumab, or carfilzomib is superior to lenalidomide in relapsed myeloma, the situation in North America, as in this patient, is usually that patients have had lenalidomide-dexamethasone as part of their initial treatment and their myeloma is refractory to lenalidomide.
Hence, we recommend, that we go to the second-generation pomalidomide and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Having said that, the treatment paradigm is evolving. For example, the monoclonal antibody daratumumab was initially approved by the FDA in multiply relapsed disease as a single agent because it achieves a 30% response rate.16 It now has been moved earlier into the first relapse of multiple myeloma, where it achieves much higher response rates when combined with lenalidomide–dexamethasone or combined with bortezomib–dexamethasone.17,18 Response rates of 70%-80% can be achieved, including minimal residual disease negative complete responses.
Today, in a patient who has had RVD transplant and myeloma has returned, we would recommend second-generation IMiDs, pomalidomide, and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Data for daratumumab combined with lenalidomide-dexamethasone or with bortezomib-dexamethasone, look very promising. We need, however, to see more experience of daratumumab together with lenalidomide-dexamethasone or daratumumab together with bortezomib-dexamethasone in patients whose myeloma is refractory to RVD, that is, patients whose myeloma has returned after RVD induction treatment. Of note, pomalidomide, dexamethasone, and daratumumab have just been approved by the FDA and may also be active even in myeloma recurring after RVD treatment.19
Daratumumab in combination will be moving earlier and earlier and may be appropriate to treat the first relapse. I do want to stress, however, that at present I save daratumumab for the second or greater relapse. Daratumumab is active even when relapse occurs after treatment with second-generation IMiDs and proteasome inhibitors.
DR HENRY Before we close, I have a couple practical questions with these antibodies. Daratumumab has the track record of first-treatment severe reactions and long infusion times. How long are you anticipating the first daratumumab treatment takes? There has been some talk that maybe splitting it in half and going over 2 days is easier on the patient and the infusion center. Have you done that?
DR ANDERSON Yes, I think that’s a very important point. We need to be thinking – first and foremost – about efficacy of our therapy. Equally important, however, are the safety profile and the user-friendliness for the patient. Daratumumab infusions are quite long – on the order of 8 hours or longer on day 1 of infusion. And to date, all the clinical trials have used daratumumab infusions weekly for 8 treatments, followed by 8 treatments given every 2 weeks. Then monthly daratumumab is given as a maintenance therapy. Thus, there is a requirement for multiple outpatient clinic visits that can be prolonged.
One of the opportunities that’s being tested is to give daratumumab subcutaneously. While this is being evaluated in protocols now, the results that have been reported at our national meetings look to be quite promising in terms of efficacy, similar to results with the intravenous administration. Obviously, this would allow for a much more convenient clinic visit and shorter time for the patients being treated.
I should mention that the other antibody, elotuzumab, has been approved in combination with lenalidomide and dexamethasone.15 The infusions with lenalidomide, dexamethasone, and the antibody elotuzumab are much shorter, on the order of 2- or 3-hour visits. The place for elotuzumab in the management of relapsed myeloma is yet to be totally defined. We tend to use it now in the setting of more indolent relapses, where patients might have a slowly rising monoclonal protein. Elotuzumab-lenalidomide-dexamethasone has maintained an overall survival advantage at 4 years compared with lenalidomide-dexamethasone when used in relapsed myeloma.
We are quite excited about both antibodies. Daratumumab tends to get most of the activity, as it achieves responses as a single agent,16 and the depth of the responses are markedly increased when it’s combined with lenalidomide-dexamethasone or bortezomib-dexamethasone.17,18 However, one shouldn’t forget elotuzumab15 based on its tolerability and the survival advantage I mentioned at 4 years.
The final point is that we think about myeloma genetically at the time of diagnosis and relapse in terms of standard or high-risk disease. One of the hallmarks of high-risk disease has been 17P deletion or P53 dysfunction. One of the most exciting outcomes of the development of monoclonal antibodies has been the responses observed even in the context of P53 deletion. Clearly, antibody-mediated cellular cytotoxicity, complement-mediated cytotoxicity, and other mechanisms of action of these antibodies do not require normal P53 function. The important point, therefore, is that what has previously been thought of as high-risk disease can nowadays be effectively treated with these new immune treatments, correlating with the marked improvement in survival and overall outcome.
DR HENRY We have outlined 3 kinds of myeloma patients we see, and especially interesting is the last patient, who has relapsed and then progressed, and in whom newer drugs have a role. Thank you for such a complete and thorough discussion, Dr Anderson.
1. Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:230-269.
2. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly-diagnosed multiple myeloma. Blood. 2010;116:679-686.
3. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777: a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
4. Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569-2574.
5. McCarthy PL, Owzar K, Hofmeister CC, et al. A phase III study of lenalidomide after transplant for multiple myeloma. N Engl J Med. 2012;366:1770-1781.
6. Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib, and dexamethasone (RVD) in high risk myeloma patients. Leukemia. 2014;28:690-693.
7. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib and dexamethasone with transplantation in myeloma. N Engl J Med. 2017;376:1311-1320.
8. Stadtmauer EA, Pasquini MC, Blackwell B, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Abstract LBA-1. Presented at the 2016 ASH Annual Meeting, December 6, 2016; San Diego, CA.
9. Dytfeld D, Jasielec J, Griffith KA, et al: Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162-164.
10. Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503-1512
11. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. for the FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917.
12. Richardson P, Siegel D, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832.
13. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. for the ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142-152
14. Moreau P, Masszi T, Grzasko N, et al. for the TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;28;374:1621-1634.
15. Lonial S, Dimopoulos M, Palumbo A, et al. for the ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621-631.
16. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD 38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207-1219.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
18. Palumbo A, Chanan-Khan A, Weisel K, et al. for the CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
19. Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981.
1. Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:230-269.
2. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly-diagnosed multiple myeloma. Blood. 2010;116:679-686.
3. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777: a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
4. Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569-2574.
5. McCarthy PL, Owzar K, Hofmeister CC, et al. A phase III study of lenalidomide after transplant for multiple myeloma. N Engl J Med. 2012;366:1770-1781.
6. Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib, and dexamethasone (RVD) in high risk myeloma patients. Leukemia. 2014;28:690-693.
7. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib and dexamethasone with transplantation in myeloma. N Engl J Med. 2017;376:1311-1320.
8. Stadtmauer EA, Pasquini MC, Blackwell B, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Abstract LBA-1. Presented at the 2016 ASH Annual Meeting, December 6, 2016; San Diego, CA.
9. Dytfeld D, Jasielec J, Griffith KA, et al: Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162-164.
10. Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503-1512
11. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. for the FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917.
12. Richardson P, Siegel D, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832.
13. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. for the ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142-152
14. Moreau P, Masszi T, Grzasko N, et al. for the TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;28;374:1621-1634.
15. Lonial S, Dimopoulos M, Palumbo A, et al. for the ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621-631.
16. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD 38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207-1219.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
18. Palumbo A, Chanan-Khan A, Weisel K, et al. for the CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
19. Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981.
Immunotherapies shape the treatment landscape for hematologic malignancies
The treatment landscape for hematologic malignancies is evolving faster than ever before, with a range of available therapeutic options that is now almost as diverse as this group of tumors. Immunotherapy in particular is front and center in the battle to control these diseases. Here, we describe the latest promising developments.
Exploiting T cells
The treatment landscape for hematologic malignancies is diverse, but one particular type of therapy has led the charge in improving patient outcomes. Several features of hematologic malignancies may make them particularly amenable to immunotherapy, including the fact that they are derived from corrupt immune cells and come into constant contact with other immune cells within the hematopoietic environment in which they reside. One of the oldest forms of immunotherapy, hematopoietic stem-cell transplantation (HSCT), remains the only curative option for many patients with hematologic malignancies.1,2
Given the central role of T lymphocytes in antitumor immunity, research efforts have focused on harnessing their activity for cancer treatment. One example of this is adoptive cellular therapy (ACT), in which T cells are collected from a patient, grown outside the body to increase their number and then reinfused back to the patient. Allogeneic HSCT, in which the stem cells are collected from a matching donor and transplanted into the patient, is a crude example of ACT. The graft-versus-tumor effect is driven by donor cells present in the transplant, but is limited by the development of graft-versus-host disease (GvHD), whereby the donor T cells attack healthy host tissue.
Other types of ACT have been developed in an effort to capitalize on the anti-tumor effects of the patients own T cells and thus avoid the potentially fatal complication of GvHD. Tumor-infiltrating lymphocyte (TIL) therapy was developed to exploit the presence of tumor-specific T cells in the tumor microenvironment. To date, the efficacy of TIL therapy has been predominantly limited to melanoma.1,3,4
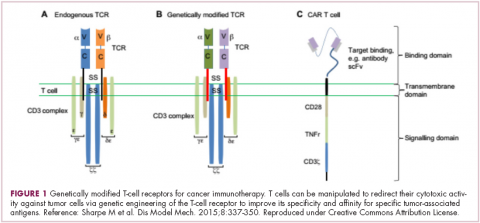
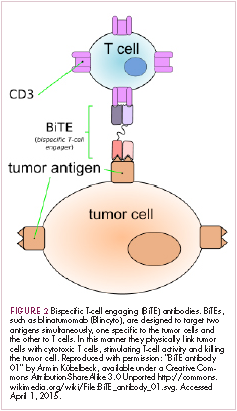
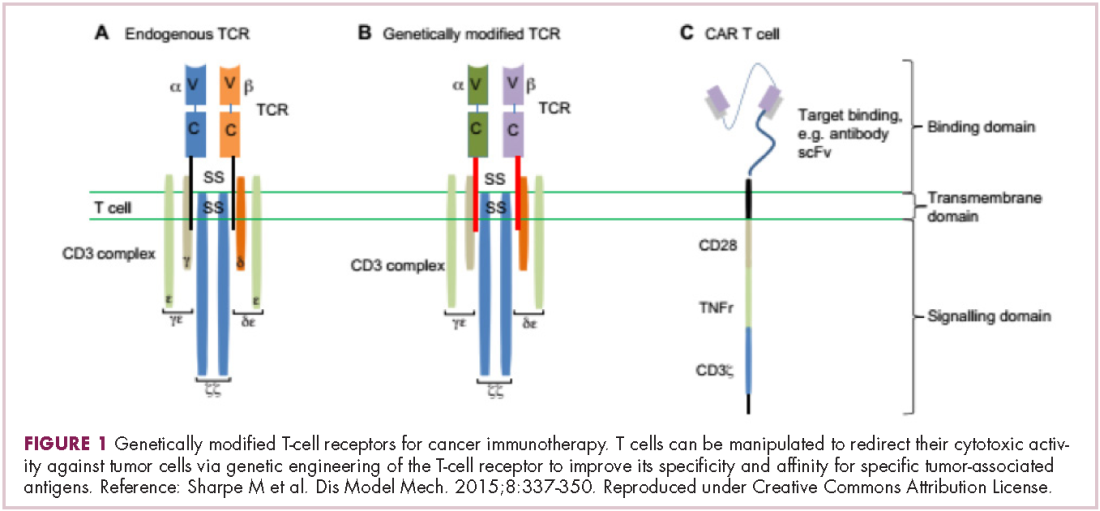
Most recently, there has been a substantial buzz around the idea of genetically engineering T cells before they are reintroduced into the patient, to increase their anti-tumor efficacy and minimize damage to healthy tissue. This is achieved either by manipulating the antigen binding portion of the T-cell receptor to alter its specificity (TCR T cells) or by generating artificial fusion receptors known as chimeric antigen receptors (CAR T cells; Figure 1). The former is limited by the need for the TCR to be genetically matched to the patient’s immune type, whereas the latter is more flexible in this regard and has proved most successful.
CARs are formed by fusing part of the single-chain variable fragment of a monoclonal antibody to part of the TCR and one or more costimulatory molecules. In this way, the T cell is guided to the tumor through antibody recognition of a particular tumor-associated antigen, whereupon its effector functions are activated by engagement of the TCR and costimulatory signal.5
Headlining advancements with CAR T cells
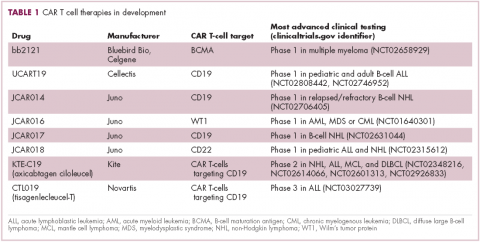
CAR T cells directed against the CD19 antigen, found on the surface of many hematologic malignancies, are the most clinically advanced in this rapidly evolving field (Table 1). Durable remissions have been demonstrated in patients with relapsed and refractory hematologic malignancies, including non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), and acute lymphoblastic lymphoma (ALL), with efficacy in both the pre- and posttransplant setting and in patients with chemotherapy-refractory disease.4,5
CTL019, a CD19-targeted CAR-T cell therapy, also known as tisagenlecleucel-T, has received breakthrough therapy designation from the US Food and Drug Administration (FDA) for the treatment of pediatric and adult patients with relapsed/refractory B-cell ALL and, more recently, for the treatment of adult patients with relapsed/refractory diffuse large B cell lymphoma.6
It is edging closer to FDA approval for the ALL indication, having been granted priority review in March on the basis of the phase 2 ELIANA trial, in which 50 patients received a single infusion of CTL019. Data presented at the American Society of Hematology annual meeting in December 2016 showed that 82% of patients achieved either complete remission (CR) or CR with incomplete blood count recovery (CRi) 3 months after treatment.7
Meanwhile, Kite Pharma has a rolling submission with the FDA for KTE-C19 (axicabtagene ciloleucel) for the treatment of patients with relapsed/refractory B-cell NHL who are ineligible for HSCT. In the ZUMA-1 trial, this therapy demonstrated an overall response rate (ORR) of 71%.8 Juno Therapeutics is developing several CAR T-cell therapies, including JCAR017, which elicited CR in 60% of patients with relapsed/refractory NHL.9
Target antigens other than CD19 are being explored, but these are mostly in the early stages of clinical development. While the focus has predominantly been on the treatment of lymphoma and leukemia, a presentation at the American Society for Clinical Oncology annual meeting in June reported the efficacy of a CAR-T cell therapy targeting the B-cell maturation antigen in patients with multiple myeloma. Results from 19 patients enrolled in an ongoing phase 1 trial in China showed that 14 had achieved stringent CR, 1 partial remission (PR) and 4 very good partial remission (VGPR).10
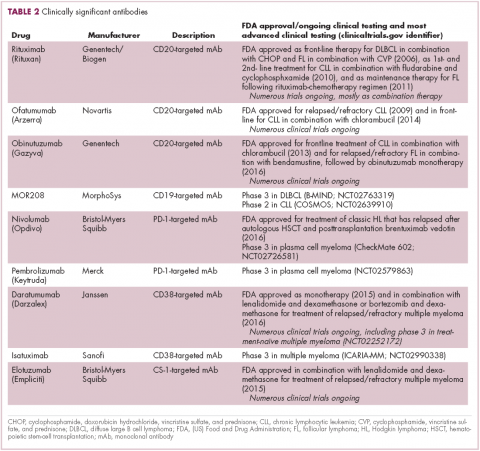
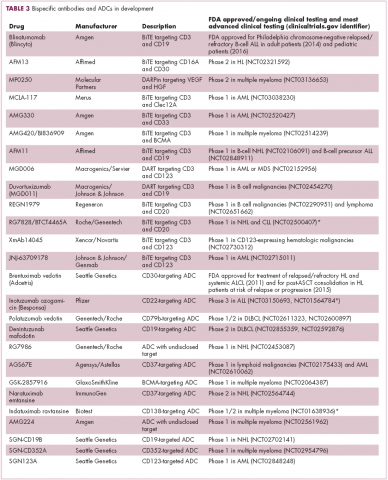
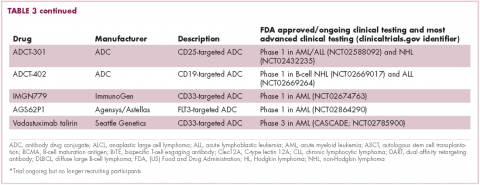
Antibodies evolve
Another type of immunotherapy that has revolutionized the treatment of hematologic malignancies is monoclonal antibodies (mAbs), targeting antigens on the surface of malignant B and T cells, in particular CD20. The approval of CD20-targeting mAb rituximab in 1997 was the first coup for the development of immunotherapy for the treatment of hematologic malignancies. It has become part of the standard treatment regimen for B-cell malignancies, including NHL and CLL, in combination with various types of chemotherapy.
Several other CD20-targeting antibodies have been developed (Table 2), some of which work in the same way as rituximab (eg, ofatumumab) and some that have a slightly different mechanism of action (eg, obinutuzumab).11 Both types of antibody have proved highly effective; ofatumumab is FDA approved for the treatment of advanced CLL and is being evaluated in phase 3 trials in other hematologic malignancies, while obinutuzumab has received regulatory approval for the first-line treatment of CLL, replacing the standard rituximab-containing regimen.12
The use of ofatumumab as maintenance therapy is supported by the results of the phase 3 PROLONG study in which 474 patients were randomly assigned to ofatumumab maintenance for 2 years or observation. Over a median follow-up of close to 20 months, ofatumumab-treated patients experienced improved progression-free survival (PFS; median PFS: 29.4 months vs 15.2 months; hazard ratio [HR], 0.50; P < .0001).13 Obinutuzumab’s new indication is based on data from the phase 3 GADOLIN trial, in which the obinutuzumab arm showed improved 3-year PFS compared with rituximab.14Until recently, multiple myeloma had proven relatively resistant to mAb therapy, but two new drug targets have dramatically altered the treatment landscape for this type of hematologic malignancy. CD2 subset 1 (CS1), also known as signaling lymphocytic activation molecule 7 (SLAMF7), and CD38 are glycoproteins expressed highly and nearly uniformly on the surface of multiple myeloma cells and only at low levels on other lymphoid and myeloid cells.15
Several antibodies directed at these targets are in clinical development, but daratumumab and elotuzumab, targeting CD38 and CS1, respectively, are both newly approved by the FDA for relapsed/refractory disease, daratumumab as monotherapy and elotuzumab in combination with lenalidomide and dexamethasone.
The indication for daratumumab was subsequently expanded to include its use in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone. Support for this new indication came from 2 pivotal phase 3 trials. In the CASTOR trial, the combination of daratumumab with bortezomib–dexamethasone reduced the risk of disease progression or death by 61%, compared with bortezomib–dexamethasone alone, whereas daratumumab with lenalidomide–dexamethasone reduced the risk of disease progression or death by 63% in the POLLUX trial.16,17
Numerous clinical trials for both drugs are ongoing, including in the front-line setting in multiple myeloma, as well as trials in other types of B-cell malignancy, and several other CD38-targeting mAbs are also in development, including isatuximab, which has reached the phase 3 stage (NCT02990338).
Innovative design
Newer drug designs, which have sought to take mAb therapy to the next level, have also shown significant efficacy in hematologic malignancies. Antibody-drug conjugates (ADCs) combine the cytotoxic efficacy of chemotherapeutic agents with the specificity of a mAb targeting a tumor-specific antigen. This essentially creates a targeted payload that improves upon the efficacy of mAb monotherapy but mitigates some of the side effects of chemotherapy related to their indiscriminate killing of both cancerous and healthy cells.
The development of ADCs has been somewhat of a rollercoaster ride, with the approval and subsequent withdrawal of the first-in-class drug gemtuzumab ozogamicin in 2010, but the field was reinvigorated with the successful development of brentuximab vedotin, which targets the CD30 antigen and is approved for the treatment of multiple different hematologic malignancies, including, most recently, for posttransplant consolidation therapy in patients with Hodgkin lymphoma at high risk of relapse or progression.18
Brentuximab vedotin may soon be joined by another FDA-approved ADC, this one targeting CD22. Inotuzumab ozogamicin was recently granted priority review for the treatment of relapsed/refractory ALL. The FDA is reviewing data from the phase 3 INO-VATE study in which inotuzumab ozogamicin reduced the risk of disease progression or death by 55% compared with standard therapy, and a decision is expected by August.19 Other ADC targets being investigated in clinical trials include CD138, CD19, and CD33 (Table 3). Meanwhile, a meta-analysis of randomized trials suggested that the withdrawal of gemtuzumab ozogamicin may have been premature, indicating that it does improve long-term overall survival (OS) and reduces the risk of relapse.20
Bispecific antibodies that link natural killer (NK) cells to tumor cells, by targeting the NK-cell receptor CD16, known as BiKEs, are also in development in an attempt to harness the power of the innate immune response.
B-cell signaling a ripe target
Beyond immunotherapy, molecularly targeted drugs directed against key drivers of hematologic malignancies are also showing great promise. In particular, the B-cell receptor (BCR) signaling pathway, a central regulator of B-cell function, and its constituent kinases that are frequently dysregulated in B cell malignancies, has emerged as an exciting therapeutic avenue.
A variety of small molecule inhibitors targeting different nodes of the BCR pathway have been developed (Table 4), but the greatest success to date has been achieved with drugs targeting Bruton’s tyrosine kinase (BTK). Their clinical development culminated in the approval of ibrutinib for the treatment of patients with mantle cell lymphoma in 2013 and subsequently for patients with CLL, Waldenström macroglobulinemia, and most recently for patients with marginal zone lymphoma.
More than 100 clinical trials of ibrutinib are ongoing in an effort to further clarify its role in a variety of different disease settings. Furthermore, in an effort to address some of the toxicity concerns with ibrutinib, more specific BTK inhibitors are also being developed.
Other kinases that orchestrate the BCR pathway, including phosphatidylinositol-3-kinase (PI3K) and SYK, are also being targeted. The delta isoform of PI3K is expressed exclusively in hematopoietic cells and a number of PI3K delta inhibitors have been developed. Idelalisib received regulatory approval for the treatment of patients with CLL in combination with rituximab, and for patients with follicular lymphoma and small lymphocytic leukemia.
As with ibrutinib, a plethora of clinical trials are ongoing, however a major setback was suffered in the frontline setting when Gilead Sciences halted 6 clinical trials due to reports of increased rates of adverse events, including deaths.26 Meanwhile, SYK inhibitors have lagged behind somewhat in their development, but one such offering, entospletinib, is showing promise in patients with AML.27
Finally, there has been some success in targeting one of the downstream targets of the BCR signaling pathway, the Bcl2 protein that is involved in the regulation of apoptosis. Venetoclax was approved last year for the treatment of patients with relapsed/refractory CLL in patients who have a chromosome 17p deletion, based on the demonstration of impressive, durable responses.28
1. Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haemato- logical malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15(4):201-215.
2. Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94.
3. Gill S. Planes, trains, and automobiles: perspectives on CAR T cells and other cellular therapies for hematologic malignancies. Curr Hematol Malig Rep. 2016;11(4):318-325.
4. Ye B, Stary CM, Gao Q, et al. Genetically modified T-cell-based adoptive immunotherapy in hematological malignancies. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5237740/. Published January 2, 2017. Accessed July 22, 2017.
5. Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
6. Novartis. Novartis personalized cell therapy CTL019 receives FDA breakthrough therapy designation. https://www.novartis.com/news/media-releases/novartis-personalized-cell-therapy-ctl019-receivesfda-breakthrough-therapy. Published July 7, 2014. Accessed June 19,
2017.
7. Novartis. Novartis presents results from first global registration trial of CTL019 in pediatric and young adult patients with r/r B-ALL. https://www.novartis.com/news/media-releases/novartis-presentsresults-first-global-registration-trial-ctl019-pediatric-and. Published December 4, 2016. Accessed June 19, 2017.
8. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 Results of ZUMA1: a multicenter study of KTE-C19 Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295.
9. Abramson JS, Palomba L, Gordon L. Transcend NHL 001: immunotherapy with the CD19-Directd CAR T-cell product JCAR017 results in high complete response rates in relapsed or refractory B-cell non-Hodgkin lymphoma. Paper presented at 58th American Society of Hematology Annual Meeting; December 3-6, 2016; San Diego, CA.
10. Fan F, Zhao W, Liu J, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol. 2017;35(suppl;):Abstr LBA3001.
11. Okroj M, Osterborg A, Blom AM. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat Rev. 2013;39(6):632-639.
12. Safdari Y, Ahmadzadeh V, Farajnia S. CD20-targeting in B-cell malignancies: novel prospects for antibodies and combination therapies. Invest New Drugs. 2016;34(4):497-512.
13. van Oers MH, Kuliczkowski K, Smolej L, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2015;16(13):1370-1379.
14. Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081-1093.
15. Touzeau C, Moreau P, Dumontet C. Monoclonal antibody therapy in multiple myeloma. Leukemia. 2017;31(5):1039-1047.
16. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331.
18. Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337.
19. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753.
20. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
21. Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93(3):290-296.
22. Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847.
23. Koehrer S, Burger JA. B-cell receptor signaling in chronic lymphocytic leukemia and other B-cell malignancies. Clin Adv Hematol Oncol. 2016;14(1):55-65.
24. Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94(3):193-205.
25. Bojarczuk K, Bobrowicz M, Dwojak M, et al. B-cell receptor signaling in the pathogenesis of lymphoid malignancies. Blood Cells Mol Dis. 2015;55(3):255-265.
26. Medscape Medical News. Gilead stops six trials adding idelalisib to other drugs. http://www.medscape.com/viewarticle/860372. Published March 14, 2016. Accessed June 19, 2017.
27. Sharman J, Di Paolo J. Targeting B-cell receptor signaling kinases in chronic lymphocytic leukemia: the promise of entospletinib. Ther Adv Hematol. 2016;7(3):157-170.
28. Food and Drug Administration. FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm495253.htm. Released April 11, 2016. Accessed June 19, 2017.
The treatment landscape for hematologic malignancies is evolving faster than ever before, with a range of available therapeutic options that is now almost as diverse as this group of tumors. Immunotherapy in particular is front and center in the battle to control these diseases. Here, we describe the latest promising developments.
Exploiting T cells
The treatment landscape for hematologic malignancies is diverse, but one particular type of therapy has led the charge in improving patient outcomes. Several features of hematologic malignancies may make them particularly amenable to immunotherapy, including the fact that they are derived from corrupt immune cells and come into constant contact with other immune cells within the hematopoietic environment in which they reside. One of the oldest forms of immunotherapy, hematopoietic stem-cell transplantation (HSCT), remains the only curative option for many patients with hematologic malignancies.1,2
Given the central role of T lymphocytes in antitumor immunity, research efforts have focused on harnessing their activity for cancer treatment. One example of this is adoptive cellular therapy (ACT), in which T cells are collected from a patient, grown outside the body to increase their number and then reinfused back to the patient. Allogeneic HSCT, in which the stem cells are collected from a matching donor and transplanted into the patient, is a crude example of ACT. The graft-versus-tumor effect is driven by donor cells present in the transplant, but is limited by the development of graft-versus-host disease (GvHD), whereby the donor T cells attack healthy host tissue.
Other types of ACT have been developed in an effort to capitalize on the anti-tumor effects of the patients own T cells and thus avoid the potentially fatal complication of GvHD. Tumor-infiltrating lymphocyte (TIL) therapy was developed to exploit the presence of tumor-specific T cells in the tumor microenvironment. To date, the efficacy of TIL therapy has been predominantly limited to melanoma.1,3,4
Most recently, there has been a substantial buzz around the idea of genetically engineering T cells before they are reintroduced into the patient, to increase their anti-tumor efficacy and minimize damage to healthy tissue. This is achieved either by manipulating the antigen binding portion of the T-cell receptor to alter its specificity (TCR T cells) or by generating artificial fusion receptors known as chimeric antigen receptors (CAR T cells; Figure 1). The former is limited by the need for the TCR to be genetically matched to the patient’s immune type, whereas the latter is more flexible in this regard and has proved most successful.
CARs are formed by fusing part of the single-chain variable fragment of a monoclonal antibody to part of the TCR and one or more costimulatory molecules. In this way, the T cell is guided to the tumor through antibody recognition of a particular tumor-associated antigen, whereupon its effector functions are activated by engagement of the TCR and costimulatory signal.5
Headlining advancements with CAR T cells
CAR T cells directed against the CD19 antigen, found on the surface of many hematologic malignancies, are the most clinically advanced in this rapidly evolving field (Table 1). Durable remissions have been demonstrated in patients with relapsed and refractory hematologic malignancies, including non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), and acute lymphoblastic lymphoma (ALL), with efficacy in both the pre- and posttransplant setting and in patients with chemotherapy-refractory disease.4,5
CTL019, a CD19-targeted CAR-T cell therapy, also known as tisagenlecleucel-T, has received breakthrough therapy designation from the US Food and Drug Administration (FDA) for the treatment of pediatric and adult patients with relapsed/refractory B-cell ALL and, more recently, for the treatment of adult patients with relapsed/refractory diffuse large B cell lymphoma.6
It is edging closer to FDA approval for the ALL indication, having been granted priority review in March on the basis of the phase 2 ELIANA trial, in which 50 patients received a single infusion of CTL019. Data presented at the American Society of Hematology annual meeting in December 2016 showed that 82% of patients achieved either complete remission (CR) or CR with incomplete blood count recovery (CRi) 3 months after treatment.7
Meanwhile, Kite Pharma has a rolling submission with the FDA for KTE-C19 (axicabtagene ciloleucel) for the treatment of patients with relapsed/refractory B-cell NHL who are ineligible for HSCT. In the ZUMA-1 trial, this therapy demonstrated an overall response rate (ORR) of 71%.8 Juno Therapeutics is developing several CAR T-cell therapies, including JCAR017, which elicited CR in 60% of patients with relapsed/refractory NHL.9
Target antigens other than CD19 are being explored, but these are mostly in the early stages of clinical development. While the focus has predominantly been on the treatment of lymphoma and leukemia, a presentation at the American Society for Clinical Oncology annual meeting in June reported the efficacy of a CAR-T cell therapy targeting the B-cell maturation antigen in patients with multiple myeloma. Results from 19 patients enrolled in an ongoing phase 1 trial in China showed that 14 had achieved stringent CR, 1 partial remission (PR) and 4 very good partial remission (VGPR).10
Antibodies evolve
Another type of immunotherapy that has revolutionized the treatment of hematologic malignancies is monoclonal antibodies (mAbs), targeting antigens on the surface of malignant B and T cells, in particular CD20. The approval of CD20-targeting mAb rituximab in 1997 was the first coup for the development of immunotherapy for the treatment of hematologic malignancies. It has become part of the standard treatment regimen for B-cell malignancies, including NHL and CLL, in combination with various types of chemotherapy.
Several other CD20-targeting antibodies have been developed (Table 2), some of which work in the same way as rituximab (eg, ofatumumab) and some that have a slightly different mechanism of action (eg, obinutuzumab).11 Both types of antibody have proved highly effective; ofatumumab is FDA approved for the treatment of advanced CLL and is being evaluated in phase 3 trials in other hematologic malignancies, while obinutuzumab has received regulatory approval for the first-line treatment of CLL, replacing the standard rituximab-containing regimen.12
The use of ofatumumab as maintenance therapy is supported by the results of the phase 3 PROLONG study in which 474 patients were randomly assigned to ofatumumab maintenance for 2 years or observation. Over a median follow-up of close to 20 months, ofatumumab-treated patients experienced improved progression-free survival (PFS; median PFS: 29.4 months vs 15.2 months; hazard ratio [HR], 0.50; P < .0001).13 Obinutuzumab’s new indication is based on data from the phase 3 GADOLIN trial, in which the obinutuzumab arm showed improved 3-year PFS compared with rituximab.14Until recently, multiple myeloma had proven relatively resistant to mAb therapy, but two new drug targets have dramatically altered the treatment landscape for this type of hematologic malignancy. CD2 subset 1 (CS1), also known as signaling lymphocytic activation molecule 7 (SLAMF7), and CD38 are glycoproteins expressed highly and nearly uniformly on the surface of multiple myeloma cells and only at low levels on other lymphoid and myeloid cells.15
Several antibodies directed at these targets are in clinical development, but daratumumab and elotuzumab, targeting CD38 and CS1, respectively, are both newly approved by the FDA for relapsed/refractory disease, daratumumab as monotherapy and elotuzumab in combination with lenalidomide and dexamethasone.
The indication for daratumumab was subsequently expanded to include its use in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone. Support for this new indication came from 2 pivotal phase 3 trials. In the CASTOR trial, the combination of daratumumab with bortezomib–dexamethasone reduced the risk of disease progression or death by 61%, compared with bortezomib–dexamethasone alone, whereas daratumumab with lenalidomide–dexamethasone reduced the risk of disease progression or death by 63% in the POLLUX trial.16,17
Numerous clinical trials for both drugs are ongoing, including in the front-line setting in multiple myeloma, as well as trials in other types of B-cell malignancy, and several other CD38-targeting mAbs are also in development, including isatuximab, which has reached the phase 3 stage (NCT02990338).
Innovative design
Newer drug designs, which have sought to take mAb therapy to the next level, have also shown significant efficacy in hematologic malignancies. Antibody-drug conjugates (ADCs) combine the cytotoxic efficacy of chemotherapeutic agents with the specificity of a mAb targeting a tumor-specific antigen. This essentially creates a targeted payload that improves upon the efficacy of mAb monotherapy but mitigates some of the side effects of chemotherapy related to their indiscriminate killing of both cancerous and healthy cells.
The development of ADCs has been somewhat of a rollercoaster ride, with the approval and subsequent withdrawal of the first-in-class drug gemtuzumab ozogamicin in 2010, but the field was reinvigorated with the successful development of brentuximab vedotin, which targets the CD30 antigen and is approved for the treatment of multiple different hematologic malignancies, including, most recently, for posttransplant consolidation therapy in patients with Hodgkin lymphoma at high risk of relapse or progression.18
Brentuximab vedotin may soon be joined by another FDA-approved ADC, this one targeting CD22. Inotuzumab ozogamicin was recently granted priority review for the treatment of relapsed/refractory ALL. The FDA is reviewing data from the phase 3 INO-VATE study in which inotuzumab ozogamicin reduced the risk of disease progression or death by 55% compared with standard therapy, and a decision is expected by August.19 Other ADC targets being investigated in clinical trials include CD138, CD19, and CD33 (Table 3). Meanwhile, a meta-analysis of randomized trials suggested that the withdrawal of gemtuzumab ozogamicin may have been premature, indicating that it does improve long-term overall survival (OS) and reduces the risk of relapse.20
Bispecific antibodies that link natural killer (NK) cells to tumor cells, by targeting the NK-cell receptor CD16, known as BiKEs, are also in development in an attempt to harness the power of the innate immune response.
B-cell signaling a ripe target
Beyond immunotherapy, molecularly targeted drugs directed against key drivers of hematologic malignancies are also showing great promise. In particular, the B-cell receptor (BCR) signaling pathway, a central regulator of B-cell function, and its constituent kinases that are frequently dysregulated in B cell malignancies, has emerged as an exciting therapeutic avenue.
A variety of small molecule inhibitors targeting different nodes of the BCR pathway have been developed (Table 4), but the greatest success to date has been achieved with drugs targeting Bruton’s tyrosine kinase (BTK). Their clinical development culminated in the approval of ibrutinib for the treatment of patients with mantle cell lymphoma in 2013 and subsequently for patients with CLL, Waldenström macroglobulinemia, and most recently for patients with marginal zone lymphoma.
More than 100 clinical trials of ibrutinib are ongoing in an effort to further clarify its role in a variety of different disease settings. Furthermore, in an effort to address some of the toxicity concerns with ibrutinib, more specific BTK inhibitors are also being developed.
Other kinases that orchestrate the BCR pathway, including phosphatidylinositol-3-kinase (PI3K) and SYK, are also being targeted. The delta isoform of PI3K is expressed exclusively in hematopoietic cells and a number of PI3K delta inhibitors have been developed. Idelalisib received regulatory approval for the treatment of patients with CLL in combination with rituximab, and for patients with follicular lymphoma and small lymphocytic leukemia.
As with ibrutinib, a plethora of clinical trials are ongoing, however a major setback was suffered in the frontline setting when Gilead Sciences halted 6 clinical trials due to reports of increased rates of adverse events, including deaths.26 Meanwhile, SYK inhibitors have lagged behind somewhat in their development, but one such offering, entospletinib, is showing promise in patients with AML.27
Finally, there has been some success in targeting one of the downstream targets of the BCR signaling pathway, the Bcl2 protein that is involved in the regulation of apoptosis. Venetoclax was approved last year for the treatment of patients with relapsed/refractory CLL in patients who have a chromosome 17p deletion, based on the demonstration of impressive, durable responses.28
The treatment landscape for hematologic malignancies is evolving faster than ever before, with a range of available therapeutic options that is now almost as diverse as this group of tumors. Immunotherapy in particular is front and center in the battle to control these diseases. Here, we describe the latest promising developments.
Exploiting T cells
The treatment landscape for hematologic malignancies is diverse, but one particular type of therapy has led the charge in improving patient outcomes. Several features of hematologic malignancies may make them particularly amenable to immunotherapy, including the fact that they are derived from corrupt immune cells and come into constant contact with other immune cells within the hematopoietic environment in which they reside. One of the oldest forms of immunotherapy, hematopoietic stem-cell transplantation (HSCT), remains the only curative option for many patients with hematologic malignancies.1,2
Given the central role of T lymphocytes in antitumor immunity, research efforts have focused on harnessing their activity for cancer treatment. One example of this is adoptive cellular therapy (ACT), in which T cells are collected from a patient, grown outside the body to increase their number and then reinfused back to the patient. Allogeneic HSCT, in which the stem cells are collected from a matching donor and transplanted into the patient, is a crude example of ACT. The graft-versus-tumor effect is driven by donor cells present in the transplant, but is limited by the development of graft-versus-host disease (GvHD), whereby the donor T cells attack healthy host tissue.
Other types of ACT have been developed in an effort to capitalize on the anti-tumor effects of the patients own T cells and thus avoid the potentially fatal complication of GvHD. Tumor-infiltrating lymphocyte (TIL) therapy was developed to exploit the presence of tumor-specific T cells in the tumor microenvironment. To date, the efficacy of TIL therapy has been predominantly limited to melanoma.1,3,4
Most recently, there has been a substantial buzz around the idea of genetically engineering T cells before they are reintroduced into the patient, to increase their anti-tumor efficacy and minimize damage to healthy tissue. This is achieved either by manipulating the antigen binding portion of the T-cell receptor to alter its specificity (TCR T cells) or by generating artificial fusion receptors known as chimeric antigen receptors (CAR T cells; Figure 1). The former is limited by the need for the TCR to be genetically matched to the patient’s immune type, whereas the latter is more flexible in this regard and has proved most successful.
CARs are formed by fusing part of the single-chain variable fragment of a monoclonal antibody to part of the TCR and one or more costimulatory molecules. In this way, the T cell is guided to the tumor through antibody recognition of a particular tumor-associated antigen, whereupon its effector functions are activated by engagement of the TCR and costimulatory signal.5
Headlining advancements with CAR T cells
CAR T cells directed against the CD19 antigen, found on the surface of many hematologic malignancies, are the most clinically advanced in this rapidly evolving field (Table 1). Durable remissions have been demonstrated in patients with relapsed and refractory hematologic malignancies, including non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), and acute lymphoblastic lymphoma (ALL), with efficacy in both the pre- and posttransplant setting and in patients with chemotherapy-refractory disease.4,5
CTL019, a CD19-targeted CAR-T cell therapy, also known as tisagenlecleucel-T, has received breakthrough therapy designation from the US Food and Drug Administration (FDA) for the treatment of pediatric and adult patients with relapsed/refractory B-cell ALL and, more recently, for the treatment of adult patients with relapsed/refractory diffuse large B cell lymphoma.6
It is edging closer to FDA approval for the ALL indication, having been granted priority review in March on the basis of the phase 2 ELIANA trial, in which 50 patients received a single infusion of CTL019. Data presented at the American Society of Hematology annual meeting in December 2016 showed that 82% of patients achieved either complete remission (CR) or CR with incomplete blood count recovery (CRi) 3 months after treatment.7
Meanwhile, Kite Pharma has a rolling submission with the FDA for KTE-C19 (axicabtagene ciloleucel) for the treatment of patients with relapsed/refractory B-cell NHL who are ineligible for HSCT. In the ZUMA-1 trial, this therapy demonstrated an overall response rate (ORR) of 71%.8 Juno Therapeutics is developing several CAR T-cell therapies, including JCAR017, which elicited CR in 60% of patients with relapsed/refractory NHL.9
Target antigens other than CD19 are being explored, but these are mostly in the early stages of clinical development. While the focus has predominantly been on the treatment of lymphoma and leukemia, a presentation at the American Society for Clinical Oncology annual meeting in June reported the efficacy of a CAR-T cell therapy targeting the B-cell maturation antigen in patients with multiple myeloma. Results from 19 patients enrolled in an ongoing phase 1 trial in China showed that 14 had achieved stringent CR, 1 partial remission (PR) and 4 very good partial remission (VGPR).10
Antibodies evolve
Another type of immunotherapy that has revolutionized the treatment of hematologic malignancies is monoclonal antibodies (mAbs), targeting antigens on the surface of malignant B and T cells, in particular CD20. The approval of CD20-targeting mAb rituximab in 1997 was the first coup for the development of immunotherapy for the treatment of hematologic malignancies. It has become part of the standard treatment regimen for B-cell malignancies, including NHL and CLL, in combination with various types of chemotherapy.
Several other CD20-targeting antibodies have been developed (Table 2), some of which work in the same way as rituximab (eg, ofatumumab) and some that have a slightly different mechanism of action (eg, obinutuzumab).11 Both types of antibody have proved highly effective; ofatumumab is FDA approved for the treatment of advanced CLL and is being evaluated in phase 3 trials in other hematologic malignancies, while obinutuzumab has received regulatory approval for the first-line treatment of CLL, replacing the standard rituximab-containing regimen.12
The use of ofatumumab as maintenance therapy is supported by the results of the phase 3 PROLONG study in which 474 patients were randomly assigned to ofatumumab maintenance for 2 years or observation. Over a median follow-up of close to 20 months, ofatumumab-treated patients experienced improved progression-free survival (PFS; median PFS: 29.4 months vs 15.2 months; hazard ratio [HR], 0.50; P < .0001).13 Obinutuzumab’s new indication is based on data from the phase 3 GADOLIN trial, in which the obinutuzumab arm showed improved 3-year PFS compared with rituximab.14Until recently, multiple myeloma had proven relatively resistant to mAb therapy, but two new drug targets have dramatically altered the treatment landscape for this type of hematologic malignancy. CD2 subset 1 (CS1), also known as signaling lymphocytic activation molecule 7 (SLAMF7), and CD38 are glycoproteins expressed highly and nearly uniformly on the surface of multiple myeloma cells and only at low levels on other lymphoid and myeloid cells.15
Several antibodies directed at these targets are in clinical development, but daratumumab and elotuzumab, targeting CD38 and CS1, respectively, are both newly approved by the FDA for relapsed/refractory disease, daratumumab as monotherapy and elotuzumab in combination with lenalidomide and dexamethasone.
The indication for daratumumab was subsequently expanded to include its use in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone. Support for this new indication came from 2 pivotal phase 3 trials. In the CASTOR trial, the combination of daratumumab with bortezomib–dexamethasone reduced the risk of disease progression or death by 61%, compared with bortezomib–dexamethasone alone, whereas daratumumab with lenalidomide–dexamethasone reduced the risk of disease progression or death by 63% in the POLLUX trial.16,17
Numerous clinical trials for both drugs are ongoing, including in the front-line setting in multiple myeloma, as well as trials in other types of B-cell malignancy, and several other CD38-targeting mAbs are also in development, including isatuximab, which has reached the phase 3 stage (NCT02990338).
Innovative design
Newer drug designs, which have sought to take mAb therapy to the next level, have also shown significant efficacy in hematologic malignancies. Antibody-drug conjugates (ADCs) combine the cytotoxic efficacy of chemotherapeutic agents with the specificity of a mAb targeting a tumor-specific antigen. This essentially creates a targeted payload that improves upon the efficacy of mAb monotherapy but mitigates some of the side effects of chemotherapy related to their indiscriminate killing of both cancerous and healthy cells.
The development of ADCs has been somewhat of a rollercoaster ride, with the approval and subsequent withdrawal of the first-in-class drug gemtuzumab ozogamicin in 2010, but the field was reinvigorated with the successful development of brentuximab vedotin, which targets the CD30 antigen and is approved for the treatment of multiple different hematologic malignancies, including, most recently, for posttransplant consolidation therapy in patients with Hodgkin lymphoma at high risk of relapse or progression.18
Brentuximab vedotin may soon be joined by another FDA-approved ADC, this one targeting CD22. Inotuzumab ozogamicin was recently granted priority review for the treatment of relapsed/refractory ALL. The FDA is reviewing data from the phase 3 INO-VATE study in which inotuzumab ozogamicin reduced the risk of disease progression or death by 55% compared with standard therapy, and a decision is expected by August.19 Other ADC targets being investigated in clinical trials include CD138, CD19, and CD33 (Table 3). Meanwhile, a meta-analysis of randomized trials suggested that the withdrawal of gemtuzumab ozogamicin may have been premature, indicating that it does improve long-term overall survival (OS) and reduces the risk of relapse.20
Bispecific antibodies that link natural killer (NK) cells to tumor cells, by targeting the NK-cell receptor CD16, known as BiKEs, are also in development in an attempt to harness the power of the innate immune response.
B-cell signaling a ripe target
Beyond immunotherapy, molecularly targeted drugs directed against key drivers of hematologic malignancies are also showing great promise. In particular, the B-cell receptor (BCR) signaling pathway, a central regulator of B-cell function, and its constituent kinases that are frequently dysregulated in B cell malignancies, has emerged as an exciting therapeutic avenue.
A variety of small molecule inhibitors targeting different nodes of the BCR pathway have been developed (Table 4), but the greatest success to date has been achieved with drugs targeting Bruton’s tyrosine kinase (BTK). Their clinical development culminated in the approval of ibrutinib for the treatment of patients with mantle cell lymphoma in 2013 and subsequently for patients with CLL, Waldenström macroglobulinemia, and most recently for patients with marginal zone lymphoma.
More than 100 clinical trials of ibrutinib are ongoing in an effort to further clarify its role in a variety of different disease settings. Furthermore, in an effort to address some of the toxicity concerns with ibrutinib, more specific BTK inhibitors are also being developed.
Other kinases that orchestrate the BCR pathway, including phosphatidylinositol-3-kinase (PI3K) and SYK, are also being targeted. The delta isoform of PI3K is expressed exclusively in hematopoietic cells and a number of PI3K delta inhibitors have been developed. Idelalisib received regulatory approval for the treatment of patients with CLL in combination with rituximab, and for patients with follicular lymphoma and small lymphocytic leukemia.
As with ibrutinib, a plethora of clinical trials are ongoing, however a major setback was suffered in the frontline setting when Gilead Sciences halted 6 clinical trials due to reports of increased rates of adverse events, including deaths.26 Meanwhile, SYK inhibitors have lagged behind somewhat in their development, but one such offering, entospletinib, is showing promise in patients with AML.27
Finally, there has been some success in targeting one of the downstream targets of the BCR signaling pathway, the Bcl2 protein that is involved in the regulation of apoptosis. Venetoclax was approved last year for the treatment of patients with relapsed/refractory CLL in patients who have a chromosome 17p deletion, based on the demonstration of impressive, durable responses.28
1. Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haemato- logical malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15(4):201-215.
2. Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94.
3. Gill S. Planes, trains, and automobiles: perspectives on CAR T cells and other cellular therapies for hematologic malignancies. Curr Hematol Malig Rep. 2016;11(4):318-325.
4. Ye B, Stary CM, Gao Q, et al. Genetically modified T-cell-based adoptive immunotherapy in hematological malignancies. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5237740/. Published January 2, 2017. Accessed July 22, 2017.
5. Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
6. Novartis. Novartis personalized cell therapy CTL019 receives FDA breakthrough therapy designation. https://www.novartis.com/news/media-releases/novartis-personalized-cell-therapy-ctl019-receivesfda-breakthrough-therapy. Published July 7, 2014. Accessed June 19,
2017.
7. Novartis. Novartis presents results from first global registration trial of CTL019 in pediatric and young adult patients with r/r B-ALL. https://www.novartis.com/news/media-releases/novartis-presentsresults-first-global-registration-trial-ctl019-pediatric-and. Published December 4, 2016. Accessed June 19, 2017.
8. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 Results of ZUMA1: a multicenter study of KTE-C19 Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295.
9. Abramson JS, Palomba L, Gordon L. Transcend NHL 001: immunotherapy with the CD19-Directd CAR T-cell product JCAR017 results in high complete response rates in relapsed or refractory B-cell non-Hodgkin lymphoma. Paper presented at 58th American Society of Hematology Annual Meeting; December 3-6, 2016; San Diego, CA.
10. Fan F, Zhao W, Liu J, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol. 2017;35(suppl;):Abstr LBA3001.
11. Okroj M, Osterborg A, Blom AM. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat Rev. 2013;39(6):632-639.
12. Safdari Y, Ahmadzadeh V, Farajnia S. CD20-targeting in B-cell malignancies: novel prospects for antibodies and combination therapies. Invest New Drugs. 2016;34(4):497-512.
13. van Oers MH, Kuliczkowski K, Smolej L, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2015;16(13):1370-1379.
14. Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081-1093.
15. Touzeau C, Moreau P, Dumontet C. Monoclonal antibody therapy in multiple myeloma. Leukemia. 2017;31(5):1039-1047.
16. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331.
18. Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337.
19. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753.
20. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
21. Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93(3):290-296.
22. Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847.
23. Koehrer S, Burger JA. B-cell receptor signaling in chronic lymphocytic leukemia and other B-cell malignancies. Clin Adv Hematol Oncol. 2016;14(1):55-65.
24. Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94(3):193-205.
25. Bojarczuk K, Bobrowicz M, Dwojak M, et al. B-cell receptor signaling in the pathogenesis of lymphoid malignancies. Blood Cells Mol Dis. 2015;55(3):255-265.
26. Medscape Medical News. Gilead stops six trials adding idelalisib to other drugs. http://www.medscape.com/viewarticle/860372. Published March 14, 2016. Accessed June 19, 2017.
27. Sharman J, Di Paolo J. Targeting B-cell receptor signaling kinases in chronic lymphocytic leukemia: the promise of entospletinib. Ther Adv Hematol. 2016;7(3):157-170.
28. Food and Drug Administration. FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm495253.htm. Released April 11, 2016. Accessed June 19, 2017.
1. Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haemato- logical malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15(4):201-215.
2. Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94.
3. Gill S. Planes, trains, and automobiles: perspectives on CAR T cells and other cellular therapies for hematologic malignancies. Curr Hematol Malig Rep. 2016;11(4):318-325.
4. Ye B, Stary CM, Gao Q, et al. Genetically modified T-cell-based adoptive immunotherapy in hematological malignancies. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5237740/. Published January 2, 2017. Accessed July 22, 2017.
5. Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
6. Novartis. Novartis personalized cell therapy CTL019 receives FDA breakthrough therapy designation. https://www.novartis.com/news/media-releases/novartis-personalized-cell-therapy-ctl019-receivesfda-breakthrough-therapy. Published July 7, 2014. Accessed June 19,
2017.
7. Novartis. Novartis presents results from first global registration trial of CTL019 in pediatric and young adult patients with r/r B-ALL. https://www.novartis.com/news/media-releases/novartis-presentsresults-first-global-registration-trial-ctl019-pediatric-and. Published December 4, 2016. Accessed June 19, 2017.
8. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 Results of ZUMA1: a multicenter study of KTE-C19 Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295.
9. Abramson JS, Palomba L, Gordon L. Transcend NHL 001: immunotherapy with the CD19-Directd CAR T-cell product JCAR017 results in high complete response rates in relapsed or refractory B-cell non-Hodgkin lymphoma. Paper presented at 58th American Society of Hematology Annual Meeting; December 3-6, 2016; San Diego, CA.
10. Fan F, Zhao W, Liu J, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol. 2017;35(suppl;):Abstr LBA3001.
11. Okroj M, Osterborg A, Blom AM. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat Rev. 2013;39(6):632-639.
12. Safdari Y, Ahmadzadeh V, Farajnia S. CD20-targeting in B-cell malignancies: novel prospects for antibodies and combination therapies. Invest New Drugs. 2016;34(4):497-512.
13. van Oers MH, Kuliczkowski K, Smolej L, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2015;16(13):1370-1379.
14. Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081-1093.
15. Touzeau C, Moreau P, Dumontet C. Monoclonal antibody therapy in multiple myeloma. Leukemia. 2017;31(5):1039-1047.
16. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331.
18. Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337.
19. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753.
20. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
21. Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93(3):290-296.
22. Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847.
23. Koehrer S, Burger JA. B-cell receptor signaling in chronic lymphocytic leukemia and other B-cell malignancies. Clin Adv Hematol Oncol. 2016;14(1):55-65.
24. Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94(3):193-205.
25. Bojarczuk K, Bobrowicz M, Dwojak M, et al. B-cell receptor signaling in the pathogenesis of lymphoid malignancies. Blood Cells Mol Dis. 2015;55(3):255-265.
26. Medscape Medical News. Gilead stops six trials adding idelalisib to other drugs. http://www.medscape.com/viewarticle/860372. Published March 14, 2016. Accessed June 19, 2017.
27. Sharman J, Di Paolo J. Targeting B-cell receptor signaling kinases in chronic lymphocytic leukemia: the promise of entospletinib. Ther Adv Hematol. 2016;7(3):157-170.
28. Food and Drug Administration. FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm495253.htm. Released April 11, 2016. Accessed June 19, 2017.
Team reports ‘simple’ way for GPs to rule out MM
Researchers say they have determined which blood tests can help general practitioners (GPs) rule out a diagnosis of multiple myeloma (MM).
The team discovered that plasma viscosity (PV) and erythrocyte sedimentation rate (ESR) were more helpful in ruling out MM than a C-reactive protein (CRP) test.
Furthermore, the possibility of MM “is extremely low” in patients with normal hemoglobin (Hb) and normal PV or ESR.
“Ordinarily, a GP will see a patient with myeloma every 5 years, and early diagnosis matters,” said study author William Hamilton, MD, of the University of Exeter Medical School in the UK.
“We report a simple way a GP can check patients presenting symptoms such as back, rib, and chest pain or recurrent chest infections and determine whether they have myeloma or not.”
Dr Hamilton and his colleagues reported their findings in the British Journal of General Practice.
The researchers analyzed blood tests performed on 2703 MM patients up to 5 years prior to diagnosis. The team then compared results in the MM cases to blood test results in 12,157 patients without MM, matched for age and other relevant parameters.
The researchers used likelihood ratios (LRs) to classify tests as useful for ruling in or ruling out MM.
The team explained that positive likelihood (LR+) tests indicate how many times more likely a positive test occurs in individuals with MM than in those without the disease. Negative likelihood (LR–) tests indicate how many times less likely a negative result will occur in individuals with MM than in those without MM.
A test was defined as useful for ruling in MM if the LR+ was ≥ 5 and useful for ruling out MM if the LR– was ≤ 0.2.
Results
None of the inflammatory markers analyzed proved useful (LR+ ≥ 5) for ruling in MM.
The LR+ was:
- 2.0 for raised PV
- 1.9 for raised ESR
- 1.2 for raised CRP.
Similarly, none of the tests alone was useful (LR– ≤ 0.2) for ruling out MM.
The LR– was:
- 0.42 for normal Hb
- 0.81 for normal calcium
- 0.80 for normal creatinine
- 0.28 for normal ESR
- 0.32 for normal PV
- 0.87 for normal CRP.
However, several combinations of tests were useful for ruling out MM.
| Tests | LR– (95% CI) |
| Hb and PV | 0.12 (0.07 to 0.23) |
| Hb and ESR | 0.16 (0.13 to 0.21 |
| Hb, creatinine, and PV | 0.09 (0.04 to 0.20) |
| Hb, calcium, and PV | 0.06 (0.02 to 0.18 |
| Hb, creatinine, and ESR | 0.16 (0.12 to 0.21) |
| Hb, calcium, and ESR | 0.16 (0.12 to 0.22) |
| Hb, calcium, creatinine, and PV | 0.06 (0.02 to 0.19) |
| Hb, calcium, creatinine, and ESR | 0.17 (0.12 to 0.24) |
| Hb and CRP* | 0.39 (0.32 to 0.47)* |
| Hb, creatinine, and CRP* | 0.33 (0.26 to 0.42)* |
| Hb, calcium, and CRP* | 0.33 (0.25 to 0.42)* |
| Hb, calcium, creatinine, and CRP* | 0.31 (0.22 to 0.42)* |
| *Not useful (LR– ≤0.2) | |
Conclusions/implications
The researchers concluded that, with normal Hb and normal PV or ESR, the possibility of MM is very low, and assessing CRP or creatinine as well increases the sensitivity of testing only slightly.
“The combination of levels of hemoglobin . . . and 1 of 2 inflammatory markers [ESR or PV] are a sufficient test rule out myeloma,” said study author Constantinos Koshiaris, of the University of Oxford in the UK.
“If abnormalities are detected in this test, it should lead to urgent urine protein tests, which can help speed up diagnosis.”
The researchers also recommend adding calcium tests if patients have certain symptoms, such as back pain, rib pain, joint pain, and fracture.
Researchers say they have determined which blood tests can help general practitioners (GPs) rule out a diagnosis of multiple myeloma (MM).
The team discovered that plasma viscosity (PV) and erythrocyte sedimentation rate (ESR) were more helpful in ruling out MM than a C-reactive protein (CRP) test.
Furthermore, the possibility of MM “is extremely low” in patients with normal hemoglobin (Hb) and normal PV or ESR.
“Ordinarily, a GP will see a patient with myeloma every 5 years, and early diagnosis matters,” said study author William Hamilton, MD, of the University of Exeter Medical School in the UK.
“We report a simple way a GP can check patients presenting symptoms such as back, rib, and chest pain or recurrent chest infections and determine whether they have myeloma or not.”
Dr Hamilton and his colleagues reported their findings in the British Journal of General Practice.
The researchers analyzed blood tests performed on 2703 MM patients up to 5 years prior to diagnosis. The team then compared results in the MM cases to blood test results in 12,157 patients without MM, matched for age and other relevant parameters.
The researchers used likelihood ratios (LRs) to classify tests as useful for ruling in or ruling out MM.
The team explained that positive likelihood (LR+) tests indicate how many times more likely a positive test occurs in individuals with MM than in those without the disease. Negative likelihood (LR–) tests indicate how many times less likely a negative result will occur in individuals with MM than in those without MM.
A test was defined as useful for ruling in MM if the LR+ was ≥ 5 and useful for ruling out MM if the LR– was ≤ 0.2.
Results
None of the inflammatory markers analyzed proved useful (LR+ ≥ 5) for ruling in MM.
The LR+ was:
- 2.0 for raised PV
- 1.9 for raised ESR
- 1.2 for raised CRP.
Similarly, none of the tests alone was useful (LR– ≤ 0.2) for ruling out MM.
The LR– was:
- 0.42 for normal Hb
- 0.81 for normal calcium
- 0.80 for normal creatinine
- 0.28 for normal ESR
- 0.32 for normal PV
- 0.87 for normal CRP.
However, several combinations of tests were useful for ruling out MM.
| Tests | LR– (95% CI) |
| Hb and PV | 0.12 (0.07 to 0.23) |
| Hb and ESR | 0.16 (0.13 to 0.21 |
| Hb, creatinine, and PV | 0.09 (0.04 to 0.20) |
| Hb, calcium, and PV | 0.06 (0.02 to 0.18 |
| Hb, creatinine, and ESR | 0.16 (0.12 to 0.21) |
| Hb, calcium, and ESR | 0.16 (0.12 to 0.22) |
| Hb, calcium, creatinine, and PV | 0.06 (0.02 to 0.19) |
| Hb, calcium, creatinine, and ESR | 0.17 (0.12 to 0.24) |
| Hb and CRP* | 0.39 (0.32 to 0.47)* |
| Hb, creatinine, and CRP* | 0.33 (0.26 to 0.42)* |
| Hb, calcium, and CRP* | 0.33 (0.25 to 0.42)* |
| Hb, calcium, creatinine, and CRP* | 0.31 (0.22 to 0.42)* |
| *Not useful (LR– ≤0.2) | |
Conclusions/implications
The researchers concluded that, with normal Hb and normal PV or ESR, the possibility of MM is very low, and assessing CRP or creatinine as well increases the sensitivity of testing only slightly.
“The combination of levels of hemoglobin . . . and 1 of 2 inflammatory markers [ESR or PV] are a sufficient test rule out myeloma,” said study author Constantinos Koshiaris, of the University of Oxford in the UK.
“If abnormalities are detected in this test, it should lead to urgent urine protein tests, which can help speed up diagnosis.”
The researchers also recommend adding calcium tests if patients have certain symptoms, such as back pain, rib pain, joint pain, and fracture.
Researchers say they have determined which blood tests can help general practitioners (GPs) rule out a diagnosis of multiple myeloma (MM).
The team discovered that plasma viscosity (PV) and erythrocyte sedimentation rate (ESR) were more helpful in ruling out MM than a C-reactive protein (CRP) test.
Furthermore, the possibility of MM “is extremely low” in patients with normal hemoglobin (Hb) and normal PV or ESR.
“Ordinarily, a GP will see a patient with myeloma every 5 years, and early diagnosis matters,” said study author William Hamilton, MD, of the University of Exeter Medical School in the UK.
“We report a simple way a GP can check patients presenting symptoms such as back, rib, and chest pain or recurrent chest infections and determine whether they have myeloma or not.”
Dr Hamilton and his colleagues reported their findings in the British Journal of General Practice.
The researchers analyzed blood tests performed on 2703 MM patients up to 5 years prior to diagnosis. The team then compared results in the MM cases to blood test results in 12,157 patients without MM, matched for age and other relevant parameters.
The researchers used likelihood ratios (LRs) to classify tests as useful for ruling in or ruling out MM.
The team explained that positive likelihood (LR+) tests indicate how many times more likely a positive test occurs in individuals with MM than in those without the disease. Negative likelihood (LR–) tests indicate how many times less likely a negative result will occur in individuals with MM than in those without MM.
A test was defined as useful for ruling in MM if the LR+ was ≥ 5 and useful for ruling out MM if the LR– was ≤ 0.2.
Results
None of the inflammatory markers analyzed proved useful (LR+ ≥ 5) for ruling in MM.
The LR+ was:
- 2.0 for raised PV
- 1.9 for raised ESR
- 1.2 for raised CRP.
Similarly, none of the tests alone was useful (LR– ≤ 0.2) for ruling out MM.
The LR– was:
- 0.42 for normal Hb
- 0.81 for normal calcium
- 0.80 for normal creatinine
- 0.28 for normal ESR
- 0.32 for normal PV
- 0.87 for normal CRP.
However, several combinations of tests were useful for ruling out MM.
| Tests | LR– (95% CI) |
| Hb and PV | 0.12 (0.07 to 0.23) |
| Hb and ESR | 0.16 (0.13 to 0.21 |
| Hb, creatinine, and PV | 0.09 (0.04 to 0.20) |
| Hb, calcium, and PV | 0.06 (0.02 to 0.18 |
| Hb, creatinine, and ESR | 0.16 (0.12 to 0.21) |
| Hb, calcium, and ESR | 0.16 (0.12 to 0.22) |
| Hb, calcium, creatinine, and PV | 0.06 (0.02 to 0.19) |
| Hb, calcium, creatinine, and ESR | 0.17 (0.12 to 0.24) |
| Hb and CRP* | 0.39 (0.32 to 0.47)* |
| Hb, creatinine, and CRP* | 0.33 (0.26 to 0.42)* |
| Hb, calcium, and CRP* | 0.33 (0.25 to 0.42)* |
| Hb, calcium, creatinine, and CRP* | 0.31 (0.22 to 0.42)* |
| *Not useful (LR– ≤0.2) | |
Conclusions/implications
The researchers concluded that, with normal Hb and normal PV or ESR, the possibility of MM is very low, and assessing CRP or creatinine as well increases the sensitivity of testing only slightly.
“The combination of levels of hemoglobin . . . and 1 of 2 inflammatory markers [ESR or PV] are a sufficient test rule out myeloma,” said study author Constantinos Koshiaris, of the University of Oxford in the UK.
“If abnormalities are detected in this test, it should lead to urgent urine protein tests, which can help speed up diagnosis.”
The researchers also recommend adding calcium tests if patients have certain symptoms, such as back pain, rib pain, joint pain, and fracture.
Study links communication, outcomes in cancer
Better communication between cancer patients and healthcare providers may provide tangible benefits, according to research published in JNCCN.
Cancer survivors who reported greater satisfaction in communicating with healthcare providers had better general health and mental health, fewer doctor visits, and reduced healthcare spending, when compared to patients who were less satisfied with communication.
“Our study suggests that when cancer care providers are more effective communicators, their patients are more likely to follow medical advice and medication protocols,” said study author Ashish Rai, PhD, of the American Cancer Society in Framingham, Massachusetts.
For this study, Dr Rai and his colleagues analyzed data from the Medical Expenditure Panel Survey (MEPS) from 2008 through 2014.
The researchers evaluated 4588 cancer survivors, dividing them into non-elderly and elderly groups. The non-elderly patients (n=2257) had a median age of 54 (range, 18-64), and the elderly patients (n=2331) had a median age of 75.
Communication satisfaction was measured by the Consumer Assessment of Healthcare Providers and Systems (CAHPS), in conjunction with the MEPS data.
Patients used a 4-point scale ranging from “never” to “always” to track whether their providers did the following:
- Listened carefully
- Explained things in a way that was easy to understand
- Showed respect for what the respondent had to say
- Spent enough time with the respondent.
A global satisfaction rating scale (0 to 10) was factored into a composite score and tracked across 12 months.
The researchers then assessed various patient outcomes.
Satisfaction and outcomes
Overall, patients who were the most satisfied with communication had the best outcomes with regard to general, physical, and mental health; fewer emergency department, hospital, and office visits; and reduced drug, out-of-pocket, and total healthcare expenditures.
However, the associations between communication satisfaction and outcomes were not always significant.
In an adjusted analysis, the elderly patients who were more satisfied with communication in year 1 had significantly better outcomes in year 2 for general health, mental health, and total healthcare expenditures.
| Elderly patients 65+ | |||
| Least satisfied
(tertile 1) |
Moderately satisfied (tertile 2) | Most satisfied
(tertile 3) |
|
| Excellent/very good general health | |||
| Unadjusted proportion | 23.6% | 31.8% | 45.8% |
| Predictive margin | 30.3
(95% CI 26.0–34.6) |
32.2
(95% CI 28.9–35.5) |
38.9
(95% CI 35.1–42.7) |
| P value | Reference | 0.466 | 0.007 |
| Highest quartile of mental health | |||
| Unadjusted proportion | 22.9% | 34.8% | 41.7% |
| Predictive margin | 27.1
(95% CI 22.1–32.1) |
35.5
(95% CI 31.5–39.5) |
37.0
(95% CI 32.7–41.4) |
| P value | Reference | 0.013 | 0.005 |
| Total healthcare expenditure | |||
| Unadjusted mean | $33,558 | $27,341 | $29,591 |
| Predictive margin | $34,071 ($29,011–$39,131) | $28,230 ($22,907–$33,553) | $26,995 ($22,568–$31,422) |
| P value | Reference | 0.301 | 0.049 |
For the non-elderly patients, those who were more satisfied with communication in year 1 had significantly better outcomes in year 2 for physician office visits and mental health.
| Non-elderly patients (18-64) | |||
| Least satisfied (tertile 1) | Moderately satisfied (tertile 2) | Most satisfied (tertile 3) | |
| Total physician office visits | |||
| Unadjusted mean | 7.96 | 6.96 | 5.85 |
| Predictive margin | 7.42
(95% CI 6.78–8.06) |
6.60
(95% CI 5.98–7.22) |
6.26
(95% CI 5.47–7.05) |
| P value | Reference | 0.211 | 0.038 |
| Highest quartile of mental health | |||
| Unadjusted proportion | 23.5% | 35.5% | 41.1% |
| Predictive margin | 29.7
(95% CI 25.3–34.1) |
36.0
(95% CI 31.3–40.7) |
34.0
(95% CI 29.5–38.4) |
| P value | Reference | 0.036 | 0.187 |
Baseline health and satisfaction
In both age groups, patients with better baseline health reported higher satisfaction with communication. Conversely, the more comorbidities patients had, the lower their satisfaction rating.
The researchers said this suggests that more complex circumstances negatively impacted patients’ perception of their communication, and the finding highlights the importance of coordinating care across a team of providers.
“The results of this study present an interesting challenge: those survivors most in need of good communication about complex medical issues may not be receiving the information they seek in a manner that they find helpful. That, in turn, results in higher healthcare utilization and expenditure,” said Crystal Denlinger, MD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania, who was not involved in this study.
“This could be due to many factors, including time constraints, competing priorities, and increasingly complex cancer therapies. This study highlights the need for additional research into how to tailor the healthcare experience both during and after cancer treatment in order to communicate more effectively.”
Conclusions
“Communication needs vary from patient to patient,” Dr Rai noted. “While time constraints do pose a challenge, the amount of time spent is only one of the attributes of effective communication. By tailoring their communication strategy to a patient’s specific needs, providers may be able to communicate more effectively in the same amount of time.”
Dr Rai also pointed out the importance of delegating both clinical and communication duties as needed. Dr Rai and his colleagues also cited earlier research demonstrating better outcomes for patients who had the option of communicating with their provider electronically.1,2
Ultimately, the researchers concluded that effective provider communication can improve outcomes by streamlining care, alleviating anxiety, boosting mutual trust, and increasing treatment adherence.
1. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198.
2. Smith AB, Basch E. Role of patient-reported outcomes in postsurgical monitoring in oncology. J Oncol Pract 2017;13:535–538.
Better communication between cancer patients and healthcare providers may provide tangible benefits, according to research published in JNCCN.
Cancer survivors who reported greater satisfaction in communicating with healthcare providers had better general health and mental health, fewer doctor visits, and reduced healthcare spending, when compared to patients who were less satisfied with communication.
“Our study suggests that when cancer care providers are more effective communicators, their patients are more likely to follow medical advice and medication protocols,” said study author Ashish Rai, PhD, of the American Cancer Society in Framingham, Massachusetts.
For this study, Dr Rai and his colleagues analyzed data from the Medical Expenditure Panel Survey (MEPS) from 2008 through 2014.
The researchers evaluated 4588 cancer survivors, dividing them into non-elderly and elderly groups. The non-elderly patients (n=2257) had a median age of 54 (range, 18-64), and the elderly patients (n=2331) had a median age of 75.
Communication satisfaction was measured by the Consumer Assessment of Healthcare Providers and Systems (CAHPS), in conjunction with the MEPS data.
Patients used a 4-point scale ranging from “never” to “always” to track whether their providers did the following:
- Listened carefully
- Explained things in a way that was easy to understand
- Showed respect for what the respondent had to say
- Spent enough time with the respondent.
A global satisfaction rating scale (0 to 10) was factored into a composite score and tracked across 12 months.
The researchers then assessed various patient outcomes.
Satisfaction and outcomes
Overall, patients who were the most satisfied with communication had the best outcomes with regard to general, physical, and mental health; fewer emergency department, hospital, and office visits; and reduced drug, out-of-pocket, and total healthcare expenditures.
However, the associations between communication satisfaction and outcomes were not always significant.
In an adjusted analysis, the elderly patients who were more satisfied with communication in year 1 had significantly better outcomes in year 2 for general health, mental health, and total healthcare expenditures.
| Elderly patients 65+ | |||
| Least satisfied
(tertile 1) |
Moderately satisfied (tertile 2) | Most satisfied
(tertile 3) |
|
| Excellent/very good general health | |||
| Unadjusted proportion | 23.6% | 31.8% | 45.8% |
| Predictive margin | 30.3
(95% CI 26.0–34.6) |
32.2
(95% CI 28.9–35.5) |
38.9
(95% CI 35.1–42.7) |
| P value | Reference | 0.466 | 0.007 |
| Highest quartile of mental health | |||
| Unadjusted proportion | 22.9% | 34.8% | 41.7% |
| Predictive margin | 27.1
(95% CI 22.1–32.1) |
35.5
(95% CI 31.5–39.5) |
37.0
(95% CI 32.7–41.4) |
| P value | Reference | 0.013 | 0.005 |
| Total healthcare expenditure | |||
| Unadjusted mean | $33,558 | $27,341 | $29,591 |
| Predictive margin | $34,071 ($29,011–$39,131) | $28,230 ($22,907–$33,553) | $26,995 ($22,568–$31,422) |
| P value | Reference | 0.301 | 0.049 |
For the non-elderly patients, those who were more satisfied with communication in year 1 had significantly better outcomes in year 2 for physician office visits and mental health.
| Non-elderly patients (18-64) | |||
| Least satisfied (tertile 1) | Moderately satisfied (tertile 2) | Most satisfied (tertile 3) | |
| Total physician office visits | |||
| Unadjusted mean | 7.96 | 6.96 | 5.85 |
| Predictive margin | 7.42
(95% CI 6.78–8.06) |
6.60
(95% CI 5.98–7.22) |
6.26
(95% CI 5.47–7.05) |
| P value | Reference | 0.211 | 0.038 |
| Highest quartile of mental health | |||
| Unadjusted proportion | 23.5% | 35.5% | 41.1% |
| Predictive margin | 29.7
(95% CI 25.3–34.1) |
36.0
(95% CI 31.3–40.7) |
34.0
(95% CI 29.5–38.4) |
| P value | Reference | 0.036 | 0.187 |
Baseline health and satisfaction
In both age groups, patients with better baseline health reported higher satisfaction with communication. Conversely, the more comorbidities patients had, the lower their satisfaction rating.
The researchers said this suggests that more complex circumstances negatively impacted patients’ perception of their communication, and the finding highlights the importance of coordinating care across a team of providers.
“The results of this study present an interesting challenge: those survivors most in need of good communication about complex medical issues may not be receiving the information they seek in a manner that they find helpful. That, in turn, results in higher healthcare utilization and expenditure,” said Crystal Denlinger, MD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania, who was not involved in this study.
“This could be due to many factors, including time constraints, competing priorities, and increasingly complex cancer therapies. This study highlights the need for additional research into how to tailor the healthcare experience both during and after cancer treatment in order to communicate more effectively.”
Conclusions
“Communication needs vary from patient to patient,” Dr Rai noted. “While time constraints do pose a challenge, the amount of time spent is only one of the attributes of effective communication. By tailoring their communication strategy to a patient’s specific needs, providers may be able to communicate more effectively in the same amount of time.”
Dr Rai also pointed out the importance of delegating both clinical and communication duties as needed. Dr Rai and his colleagues also cited earlier research demonstrating better outcomes for patients who had the option of communicating with their provider electronically.1,2
Ultimately, the researchers concluded that effective provider communication can improve outcomes by streamlining care, alleviating anxiety, boosting mutual trust, and increasing treatment adherence.
1. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198.
2. Smith AB, Basch E. Role of patient-reported outcomes in postsurgical monitoring in oncology. J Oncol Pract 2017;13:535–538.
Better communication between cancer patients and healthcare providers may provide tangible benefits, according to research published in JNCCN.
Cancer survivors who reported greater satisfaction in communicating with healthcare providers had better general health and mental health, fewer doctor visits, and reduced healthcare spending, when compared to patients who were less satisfied with communication.
“Our study suggests that when cancer care providers are more effective communicators, their patients are more likely to follow medical advice and medication protocols,” said study author Ashish Rai, PhD, of the American Cancer Society in Framingham, Massachusetts.
For this study, Dr Rai and his colleagues analyzed data from the Medical Expenditure Panel Survey (MEPS) from 2008 through 2014.
The researchers evaluated 4588 cancer survivors, dividing them into non-elderly and elderly groups. The non-elderly patients (n=2257) had a median age of 54 (range, 18-64), and the elderly patients (n=2331) had a median age of 75.
Communication satisfaction was measured by the Consumer Assessment of Healthcare Providers and Systems (CAHPS), in conjunction with the MEPS data.
Patients used a 4-point scale ranging from “never” to “always” to track whether their providers did the following:
- Listened carefully
- Explained things in a way that was easy to understand
- Showed respect for what the respondent had to say
- Spent enough time with the respondent.
A global satisfaction rating scale (0 to 10) was factored into a composite score and tracked across 12 months.
The researchers then assessed various patient outcomes.
Satisfaction and outcomes
Overall, patients who were the most satisfied with communication had the best outcomes with regard to general, physical, and mental health; fewer emergency department, hospital, and office visits; and reduced drug, out-of-pocket, and total healthcare expenditures.
However, the associations between communication satisfaction and outcomes were not always significant.
In an adjusted analysis, the elderly patients who were more satisfied with communication in year 1 had significantly better outcomes in year 2 for general health, mental health, and total healthcare expenditures.
| Elderly patients 65+ | |||
| Least satisfied
(tertile 1) |
Moderately satisfied (tertile 2) | Most satisfied
(tertile 3) |
|
| Excellent/very good general health | |||
| Unadjusted proportion | 23.6% | 31.8% | 45.8% |
| Predictive margin | 30.3
(95% CI 26.0–34.6) |
32.2
(95% CI 28.9–35.5) |
38.9
(95% CI 35.1–42.7) |
| P value | Reference | 0.466 | 0.007 |
| Highest quartile of mental health | |||
| Unadjusted proportion | 22.9% | 34.8% | 41.7% |
| Predictive margin | 27.1
(95% CI 22.1–32.1) |
35.5
(95% CI 31.5–39.5) |
37.0
(95% CI 32.7–41.4) |
| P value | Reference | 0.013 | 0.005 |
| Total healthcare expenditure | |||
| Unadjusted mean | $33,558 | $27,341 | $29,591 |
| Predictive margin | $34,071 ($29,011–$39,131) | $28,230 ($22,907–$33,553) | $26,995 ($22,568–$31,422) |
| P value | Reference | 0.301 | 0.049 |
For the non-elderly patients, those who were more satisfied with communication in year 1 had significantly better outcomes in year 2 for physician office visits and mental health.
| Non-elderly patients (18-64) | |||
| Least satisfied (tertile 1) | Moderately satisfied (tertile 2) | Most satisfied (tertile 3) | |
| Total physician office visits | |||
| Unadjusted mean | 7.96 | 6.96 | 5.85 |
| Predictive margin | 7.42
(95% CI 6.78–8.06) |
6.60
(95% CI 5.98–7.22) |
6.26
(95% CI 5.47–7.05) |
| P value | Reference | 0.211 | 0.038 |
| Highest quartile of mental health | |||
| Unadjusted proportion | 23.5% | 35.5% | 41.1% |
| Predictive margin | 29.7
(95% CI 25.3–34.1) |
36.0
(95% CI 31.3–40.7) |
34.0
(95% CI 29.5–38.4) |
| P value | Reference | 0.036 | 0.187 |
Baseline health and satisfaction
In both age groups, patients with better baseline health reported higher satisfaction with communication. Conversely, the more comorbidities patients had, the lower their satisfaction rating.
The researchers said this suggests that more complex circumstances negatively impacted patients’ perception of their communication, and the finding highlights the importance of coordinating care across a team of providers.
“The results of this study present an interesting challenge: those survivors most in need of good communication about complex medical issues may not be receiving the information they seek in a manner that they find helpful. That, in turn, results in higher healthcare utilization and expenditure,” said Crystal Denlinger, MD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania, who was not involved in this study.
“This could be due to many factors, including time constraints, competing priorities, and increasingly complex cancer therapies. This study highlights the need for additional research into how to tailor the healthcare experience both during and after cancer treatment in order to communicate more effectively.”
Conclusions
“Communication needs vary from patient to patient,” Dr Rai noted. “While time constraints do pose a challenge, the amount of time spent is only one of the attributes of effective communication. By tailoring their communication strategy to a patient’s specific needs, providers may be able to communicate more effectively in the same amount of time.”
Dr Rai also pointed out the importance of delegating both clinical and communication duties as needed. Dr Rai and his colleagues also cited earlier research demonstrating better outcomes for patients who had the option of communicating with their provider electronically.1,2
Ultimately, the researchers concluded that effective provider communication can improve outcomes by streamlining care, alleviating anxiety, boosting mutual trust, and increasing treatment adherence.
1. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198.
2. Smith AB, Basch E. Role of patient-reported outcomes in postsurgical monitoring in oncology. J Oncol Pract 2017;13:535–538.
Drug under priority review for BPDCN
The US Food and Drug Administration(FDA) has accepted for priority review the biologics license application seeking approval for tagraxofusp (Elzonris, SL-401) to treat blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The FDA expects to make a decision on this application by February 21, 2019.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
About tagraxofusp
Tagraxofusp is a targeted therapy directed to CD123, a cell surface receptor expressed on a range of malignancies. The drug is being developed by Stemline Therapeutics, Inc.
In addition to priority review, tagraxofusp has breakthrough therapy designation and orphan drug designation from the FDA.
Tagraxofusp has produced favorable early results in a phase 2 trial of patients with BPDCN. Results from this trial were presented at the 23rd Congress of the European Hematology Association (EHA) in June.
Results were presented for 45 patients—32 with previously untreated BPDCN and 13 with relapsed/refractory BPDCN.
Three patients received tagraxofusp at 7 μg/kg/day on days 1 to 5 of a 21-day cycle, and the rest received the drug at 12 μg/kg on days 1 to 5 of a 21-day cycle.
Among patients who received the 12 μg/kg/day dose, the overall response rate was 83% (35/42). The overall response rate was 90% (26/29) in the previously untreated patients and 69% (9/13) in relapsed/refractory patients.
The composite complete response rate was 62% (n=26) overall, 72% (n=21) in previously untreated patients, and 38% (n=5) in relapsed/refractory patients.
Fourteen patients went on to stem cell transplant, 1 of whom had relapsed/refractory disease at baseline.
Overall survival results were only available for the 29 previously untreated patients who received tagraxofusp at 12 μg/kg/day. In this group, the median overall survival had not been reached at a median follow-up of 13.8 months (range, 0.2 to 37.4 months).
Safety results were presented for 114 patients who have received tagraxofusp at 12 μg/kg/day on all trials of the drug. These data include patients with diseases other than BPDCN, although adverse events (AEs) were similar regardless of disease.
Common treatment-related AEs (of any grade, occurring in at least 15% of patients) included hypoalbuminemia (49%), ALT increase (48%), AST increase (48%), thrombocytopenia (29%), nausea (27%), pyrexia (25%), chills (23%), fatigue (23%), weight increase (19%), hypotension (18%), peripheral edema (17%), and vomiting (15%).
Another common AE was capillary leak syndrome (CLS), which occurred in 20% of patients (n=23). Most cases of CLS were grade 1 or 2, but there were grade 3 (n=5) and 4 (n=2) cases, as well as a single case of grade 5 CLS that occurred in a BPDCN patient.
Researchers found they could manage the CLS with monitoring and protocol adjustments.
The US Food and Drug Administration(FDA) has accepted for priority review the biologics license application seeking approval for tagraxofusp (Elzonris, SL-401) to treat blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The FDA expects to make a decision on this application by February 21, 2019.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
About tagraxofusp
Tagraxofusp is a targeted therapy directed to CD123, a cell surface receptor expressed on a range of malignancies. The drug is being developed by Stemline Therapeutics, Inc.
In addition to priority review, tagraxofusp has breakthrough therapy designation and orphan drug designation from the FDA.
Tagraxofusp has produced favorable early results in a phase 2 trial of patients with BPDCN. Results from this trial were presented at the 23rd Congress of the European Hematology Association (EHA) in June.
Results were presented for 45 patients—32 with previously untreated BPDCN and 13 with relapsed/refractory BPDCN.
Three patients received tagraxofusp at 7 μg/kg/day on days 1 to 5 of a 21-day cycle, and the rest received the drug at 12 μg/kg on days 1 to 5 of a 21-day cycle.
Among patients who received the 12 μg/kg/day dose, the overall response rate was 83% (35/42). The overall response rate was 90% (26/29) in the previously untreated patients and 69% (9/13) in relapsed/refractory patients.
The composite complete response rate was 62% (n=26) overall, 72% (n=21) in previously untreated patients, and 38% (n=5) in relapsed/refractory patients.
Fourteen patients went on to stem cell transplant, 1 of whom had relapsed/refractory disease at baseline.
Overall survival results were only available for the 29 previously untreated patients who received tagraxofusp at 12 μg/kg/day. In this group, the median overall survival had not been reached at a median follow-up of 13.8 months (range, 0.2 to 37.4 months).
Safety results were presented for 114 patients who have received tagraxofusp at 12 μg/kg/day on all trials of the drug. These data include patients with diseases other than BPDCN, although adverse events (AEs) were similar regardless of disease.
Common treatment-related AEs (of any grade, occurring in at least 15% of patients) included hypoalbuminemia (49%), ALT increase (48%), AST increase (48%), thrombocytopenia (29%), nausea (27%), pyrexia (25%), chills (23%), fatigue (23%), weight increase (19%), hypotension (18%), peripheral edema (17%), and vomiting (15%).
Another common AE was capillary leak syndrome (CLS), which occurred in 20% of patients (n=23). Most cases of CLS were grade 1 or 2, but there were grade 3 (n=5) and 4 (n=2) cases, as well as a single case of grade 5 CLS that occurred in a BPDCN patient.
Researchers found they could manage the CLS with monitoring and protocol adjustments.
The US Food and Drug Administration(FDA) has accepted for priority review the biologics license application seeking approval for tagraxofusp (Elzonris, SL-401) to treat blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The FDA expects to make a decision on this application by February 21, 2019.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
About tagraxofusp
Tagraxofusp is a targeted therapy directed to CD123, a cell surface receptor expressed on a range of malignancies. The drug is being developed by Stemline Therapeutics, Inc.
In addition to priority review, tagraxofusp has breakthrough therapy designation and orphan drug designation from the FDA.
Tagraxofusp has produced favorable early results in a phase 2 trial of patients with BPDCN. Results from this trial were presented at the 23rd Congress of the European Hematology Association (EHA) in June.
Results were presented for 45 patients—32 with previously untreated BPDCN and 13 with relapsed/refractory BPDCN.
Three patients received tagraxofusp at 7 μg/kg/day on days 1 to 5 of a 21-day cycle, and the rest received the drug at 12 μg/kg on days 1 to 5 of a 21-day cycle.
Among patients who received the 12 μg/kg/day dose, the overall response rate was 83% (35/42). The overall response rate was 90% (26/29) in the previously untreated patients and 69% (9/13) in relapsed/refractory patients.
The composite complete response rate was 62% (n=26) overall, 72% (n=21) in previously untreated patients, and 38% (n=5) in relapsed/refractory patients.
Fourteen patients went on to stem cell transplant, 1 of whom had relapsed/refractory disease at baseline.
Overall survival results were only available for the 29 previously untreated patients who received tagraxofusp at 12 μg/kg/day. In this group, the median overall survival had not been reached at a median follow-up of 13.8 months (range, 0.2 to 37.4 months).
Safety results were presented for 114 patients who have received tagraxofusp at 12 μg/kg/day on all trials of the drug. These data include patients with diseases other than BPDCN, although adverse events (AEs) were similar regardless of disease.
Common treatment-related AEs (of any grade, occurring in at least 15% of patients) included hypoalbuminemia (49%), ALT increase (48%), AST increase (48%), thrombocytopenia (29%), nausea (27%), pyrexia (25%), chills (23%), fatigue (23%), weight increase (19%), hypotension (18%), peripheral edema (17%), and vomiting (15%).
Another common AE was capillary leak syndrome (CLS), which occurred in 20% of patients (n=23). Most cases of CLS were grade 1 or 2, but there were grade 3 (n=5) and 4 (n=2) cases, as well as a single case of grade 5 CLS that occurred in a BPDCN patient.
Researchers found they could manage the CLS with monitoring and protocol adjustments.
Helping survivors in the aftermath of suicide loss
The loss of a loved one to suicide is often experienced as “devastating.”1 While survivors of suicide loss may be able to move through the grief process without clinical support,2 the traumatic and stigmatizing nature of suicide is likely to make its aftermath more challenging to navigate than other types of loss. Sanford et al3 found that more than two-thirds of suicide loss survivors sought therapy after their loss. Further, when individuals facing these challenges present for treatment, clinicians often face challenges of their own.
Very few clinicians are trained in general grief processes,4 and even those specifically trained in grief and loss have been shown to “miss” several of the common clinical features that are unique to suicide loss.3 In my professional experience, the intensity and duration of suicide grief are often greater than they are for other losses, and many survivors of suicide loss have reported that others, including clinicians, have “pathologized” this, rather than having understood it as normative under the circumstances.
Although there is extensive literature on the aftermath of suicide for surviving loved ones, very few controlled studies have assessed interventions specifically for this population. Yet the U.S. Guidelines for Suicide Postvention5 explicitly call for improved training for those who work with suicide loss survivors, as well as research on these interventions. Jordan and McGann6 noted, “Without a full knowledge of suicide and its aftermath, it is very possible to make clinical errors which can hamper treatment.” This article summarizes what is currently known about the general experience of suicide bereavement and optimal interventions in treatment.
What makes suicide loss unique?
Suicide bereavement is distinct from other types of loss in 3 significant ways7:
- the thematic content of the grief
- the social processes surrounding the survivor
- the impact that suicide has on family systems.
Additionally, the perceived intentionality and preventability of a suicide death, as well as its stigmatized and traumatic nature, differentiate it from other types of traumatic loss.7 These elements are all likely to affect the nature, intensity, and duration of the grief.
Stigma and suicide. Stigma associated with suicide is well documented.8 Former U.S. Surgeon General David Satcher9 specifically described stigma toward suicide as one of the biggest barriers to prevention. In addition, researchers have found that the stigma associated with suicide “spills over” to the bereaved family members. Doka10,11 refers to “disenfranchised grief,” in which bereaved individuals receive the message that their grief is not legitimate, and as a result, they are likely to internalize this view. Studies have shown that individuals bereaved by suicide are also stigmatized, and are believed to be more psychologically disturbed, less likable, more blameworthy, more ashamed, and more in need of professional help than other bereaved individuals.8,12-20
These judgments often mirror suicide loss survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Thus, it is not uncommon for survivors of suicide loss to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. To the extent that they are actively grieving, survivors of suicide loss often feel that they must do so in isolation. Thus, the perception of stigma, whether external or internalized, can have a profound effect on decisions about disclosure, requesting support, and ultimately on one’s ability to integrate the loss. Indeed, Feigelman et al21 found that stigmatization after suicide was specifically associated with ongoing grief difficulties, depression, and suicidal ideation.
Continue to: Traumatic nature of suicide
Traumatic nature of suicide. Suicide loss is also quite traumatic, and posttraumatic stress disorder (PTSD) symptoms such as shock, horror, disbelief, and intrusive/perseverative thoughts and questions, particularly in the earlier stages of grief, are common. Sanford et al3 found that the higher the level of “perceived closeness” to the deceased, the more likely that survivors of suicide loss would experience PTSD symptoms. In addition, the dramatic loss of social support following a suicide loss may itself be traumatic, which can serve to compound these difficulties. Notably, Sanford et al3 found that even for those survivors of suicide loss in treatment who endorsed PTSD symptoms, many of their treating clinicians did not assess or diagnose this disorder, thus missing an important component for treatment.
Increased risk for suicidality. Studies have shown that individuals who have lost a loved one to suicide are themselves at heightened risk for suicidal ideation and behaviors.22-27 Therefore, an assessment for suicide risk is always advisable. However, it is important to note that suicidal ideation is not uncommon and can serve different functions for survivors of suicide loss without necessarily progressing to a plan for acting on such ideations. Survivors of suicide loss may wish to “join” their loved one; to understand or identify with the mental state of the deceased; to punish themselves for failing to prevent the suicide; or to end their own pain through death. Therefore, it is crucial to assess the nature and function of expressed ideation (in addition to the presence or absence of plans) before assigning the level of risk.
Elements of suicide grief
After the loss of a loved one to suicide, the path to healing is often complex, with survivors of suicide loss enduring the following challenges:
Existential assumptions are shattered. Several authors28-30 have found that suicide loss is also likely to shatter survivors’ existential assumptions regarding their worldviews, roles, and identities, as well as religious and spiritual beliefs. As one survivor of suicide loss in my practice noted, “The world is gone, nothing is predictable anymore, and it’s no longer safe to assume anything.” Others have described feeling “fragmented” in ways they had never before experienced, and many have reported difficulties in “trusting” their own judgment, the stability of the world, and relationships. “Why?” becomes an emergent and insistent question in the survivor’s efforts to understand the suicide and (ideally) reassemble a coherent narrative around the loss.
Increased duration and intensity of grief. The duration of the grief process is likely to be affected by the traumatic nature of suicide loss, the differential social support accorded to its survivors, and the difficulty in finding systems that can validate and normalize the unique elements in suicide bereavement. The stigmatized reactions of others, particularly when internalized, can present barriers to the processing of grief. In addition, the intensity of the trauma and existential impact, as well as the perseverative nature of several of the unique themes (Box 1), can also prolong the processing and increase the intensity of suicide grief. Clinicians would do well to recognize the relatively “normative” nature of the increased duration and intensity, rather than seeing it as immediately indicative of a DSM diagnosis of complicated/prolonged grief disorder.
Box 1
Common themes in the suicide grief process
Several common themes are likely to emerge during the suicide grief process. Guilt and a sense of failure—around what one did and did not do—can be pervasive and persistent, and are often present even when not objectively warranted.
Anger and blame directed towards the deceased, other family members, and clinicians who had been treating the deceased may also be present, and may be used in efforts to deflect guilt. Any of these themes may be enlisted to create a deceptively simple narrative for understanding the reasons for the suicide.
Shame is often present, and certainly exacerbated by both external and internalized stigma. Feelings of rejection, betrayal, and abandonment by the deceased are also common, as well as fear/hypervigilance regarding the possibility of losing others to suicide. Given the intensity of suicide grief, it has been my observation that there may also be fear in relation to one's own mental status, as many otherwise healthy survivors of suicide loss have described feeling like they're "going crazy." Finally, there may also be relief, particularly if the deceased had been suffering from chronic psychiatric distress or had been cruel or abusive.
Continue to: Family disruption
Family disruption. It is not uncommon for a suicide loss to result in family disruption.6,31-32 This may manifest in the blaming of family members for “sins of omission or commission,”6 conflicts around the disclosure of the suicide both within and outside of the family, discordant grieving styles, and difficulties in understanding and attending to the needs of one’s children while grieving oneself.
Despite the common elements often seen in suicide grief, it is crucial to recognize that this process is not “one size fits all.” Not only are there individual variants, but Grad et al33 found gender-based differences in grieving styles, and cultural issues such as the “meanings” assigned to suicide, and culturally sanctioned grief rituals and behaviors that are also likely to affect how grief is experienced and expressed. In addition, personal variants such as closeness/conflicts with the deceased, histories of previous trauma or loss, pre-existing psychiatric disorders, and attachment orientation34 are likely to impact the grief process.
Losing close friends and colleagues may be similarly traumatic, but these survivors of suicide loss often receive even less social support than those who have kinship losses. Finally, when a suicide loss occurs in a professional capacity (such as the loss of a patient), this is likely to have many additional implications for one’s professional functions and identity.35
Interventions to help survivors
Several goals and “tasks” are involved in the suicide bereavement process (Box 21,6,30,36-40). These can be achieved through the following interventions: Support groups. Many survivors find that support groups that focus on suicide loss are extremely helpful, and research has supported this.1,4,41-44 Interactions with other suicide loss survivors provide hope, connection, and an “antidote” to stigma and shame. Optimally, group facilitators provide education, validation and normalization of the grief trajectory, and facilitate the sharing of both loss experiences and current functioning between group members. As a result, group participants often report renewed connections, increased efficacy in giving and accepting support, and decreased distress (including reductions in PTSD and depressive symptoms). The American Association of Suicidology (www.suicidology.org) and American Foundation of Suicide Prevention (www.afsp.org) provide contact information for suicide loss survivor groups (by geographical area) as well as information about online support groups.
Box 2
Goals and 'tasks' in suicide bereavement
The following goals and "tasks" should be part of the process of suicide bereavement:
- Reduce symptoms of posttraumatic stress disorder and other psychiatric disorders. Given the traumatic nature of the loss, an important goal is to understand and reduce posttraumatic stress disorder and other psychiatric symptoms, and incrementally improving functionality in relation to these.
- Integrate the loss. Recent authors36-38 have highlighted the need for survivors of suicide loss to "bear" and integrate the loss, as opposed to the concept of "getting over it." In these paradigms, the loss becomes an important part of one's identity, and eventually ceases to define it. Optimally, the "whole person" is remembered, not just the suicide. Part of this involves a reinvestment in life, with the acceptance of a "new normal" that takes the loss into account. It is not unusual for survivors of suicide loss to report some guilt in "moving on" and/or experiencing pleasure; often this is felt as a "betrayal" of the deceased.
- Create meaning from the loss. A major goal for those who have lost a loved one to suicide is the ability to find and/or create meaning from the loss. This would include the creation of a loss narrative39 that incorporates both ambiguity and complexity, as well as a regained/renewed sense of purpose in ongoing life.
- Develop ambiguity tolerance. A related "task" in suicide grief is the development of ambiguity tolerance, which generally includes an understanding of the complexity underlying suicide, the ability to offer oneself a "fair trial"30 in relation to one's realistic degree of responsibility, and the acceptance that many questions may remain unanswerable. In addition, in concert with the current understanding of "continuing bonds,"40 survivors should attempt to attend to the ongoing relationship with the deceased, including any "unfinished business."6
- Develop skills to manage stigmatized social responses and/or changes in family and social relationships.
- Memorialize and honor the deceased. Healing for survivors is facilitated by memorializations, which both validate the mourning process itself while also paying tribute to the richness of the deceased person's life.
- Post-traumatic growth. The relatively new understanding of "post-traumatic" growth is certainly applicable to the "unexpected gifts" many survivors of suicide loss report after they have moved through suicide grief. This includes greater understanding toward oneself, other survivors of suicide loss, and suicidal individuals; gratitude toward those who have provided support; and a desire to "use" their newfound understanding of suicide and suicide grief in ways to honor the deceased and benefit others. Feigelman et al1 found that many longer-term survivors of suicide loss engaged in both direct service and social activism around suicide pre- and postvention.
Individual treatment. The limited research on individual treatment for suicide loss survivors suggests that while most participants find it generally helpful, a significant number of others report that their therapists lack knowledge of suicide grief and endorse stigmatizing attitudes toward suicide and suicide loss survivors.45-46 In addition, Sanford et al3 found that survivors of suicide loss who endorsed PTSD symptoms were not assessed, diagnosed, or treated for these symptoms.
Continue to: This speaks to the importance of understanding what is...
This speaks to the importance of understanding what is “normative” for survivors of suicide loss. In general, “normalization” and psychoeducation about the suicide grief trajectory can play an important role in work with survivors of suicide loss, even in the presence of diagnosable disorders. While PTSD, depressive symptoms, and suicidal ideation are not uncommon in suicide loss survivors, and certainly may warrant clinical assessment and treatment, it can be helpful (and less stigmatizing) for your patients to know that these diagnoses are relatively common and understandable in the context of this devastating experience. For instance, survivors of suicide loss often report feeling relieved when clinicians explain the connections between traumatic loss and PTSD and/or depressive symptoms, and this can also help to relieve secondary anxiety about “going crazy.” Many survivors of suicide loss also describe feeling as though they are functioning on “autopilot” in the earlier stages of grief; it can help them understand the “function” of compartmentalization as potentially adaptive in the short run.
Suicide loss survivors may also find it helpful to learn about suicidal states of mind and their relationships to any types of mental illness their loved ones had struggled with.47
Your role: Help survivors integrate the loss
Before beginning treatment with an individual who has lost a loved one to suicide, clinicians should thoroughly explore their own understanding of and experience with suicide, including assumptions around causation, internalized stigma about suicidal individuals and survivors of suicide loss, their own history of suicide loss or suicidality, cultural/religious attitudes, and anxiety/defenses associated with the topic of suicide. These factors, particularly when unexamined, are likely to impact the treatment relationship and one’s clinical efficacy.
In concert with the existing literature, consider the potential goals and tasks involved in the integration of the individual’s suicide loss, along with any individual factors/variants that may impact the grief trajectory. Kosminsky and Jordon34 described the role of the clinician in this situation as a “transitional attachment figure” who facilitates the management and integration of the loss into the creation of what survivors of suicide loss have termed a “new normal.”
Because suicide loss is often associated with PTSD and other psychiatric illnesses (eg, depression, suicidality, substance abuse), it is essential to balance the assessment and treatment of these issues with attention to grief issues as needed. Again, to the extent that such issues have arisen primarily in the wake of the suicide loss, it can be helpful for patients to understand their connection to the context of the loss.
Continue to: Ideally, the clinician should...
Ideally, the clinician should be “present” with the patient’s pain, normative guilt, and rumination, without attempting to quickly eliminate or “fix” it or provide premature reassurance that the survivor of suicide loss “did nothing wrong.” Rather, as Jordan6 suggests, the clinician should act to promote a “fair trial” with respect to the patient’s guilt and blame, with an understanding of the “tyranny of hindsight.” The promotion of ambiguity tolerance should also play a role in coming to terms with many questions that may remain unanswered.
Optimally, clinicians should encourage patients to attend to their ongoing relationship with the deceased, particularly in the service of resolving “unfinished business,” ultimately integrating the loss into memories of the whole person. In line with this, survivors of suicide loss may be encouraged to create a narrative of the loss that incorporates both complexity and ambiguity. In the service of supporting the suicide loss survivor’s reinvestment in life, it is often helpful to facilitate their ability to anticipate and cope with triggers, such as anniversaries, birthdays, or holidays, as well as to develop and use skills for managing difficult or stigmatizing social or cultural reactions.
Any disruptions in family functioning should also be addressed. Psychoeducation about discordant grieving styles (particularly around gender) and the support of children’s grief may be helpful, and referrals to family or couples therapists should be considered as needed. Finally, the facilitation of suicide loss survivors’ creation of memorializations or rituals can help promote healing and make their loss meaningful.
Bottom Line
Losing a loved one to suicide is often a devastating and traumatic experience, but with optimal support, most survivors are ultimately able to integrate the loss and grow as a result. Understanding the suicide grief trajectory, as well as general guidelines for treatment, will facilitate healing and growth in the aftermath of suicide loss.
Related Resources
- Jordan JR, McIntosh JL. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011.
- American Association of Suicidology. http://www.suicidology.org/.
- American Foundation for Suicide Prevention. https://afsp.org/.
1. Feigelman W, Jordan JR, McIntosh JL, et al. Devastating losses: how parents cope with the death of a child to suicide or drugs. New York, NY: Springer; 2012.
2. McIntosh JL. Research on survivors of suicide. In: Stimming MT, Stimming M, eds. Before their time: adult children’s experiences of parental suicide. Philadelphia, PA: Temple University Press; 1999:157-180.
3. Sanford RL, Cerel J, McGann VL, et al. Suicide loss survivors’ experiences with therapy: Implications for clinical practice. Community Ment Health J. 2016;5(2):551-558.
4. Jordan JR, McMenamy J. Interventions for suicide survivors: a review of the literature. Suicide Life Threat Behav. 2004;34(4):337-349.
5. Survivors of Suicide Loss Task Force. Responding to grief, trauma, & distress after a suicide: U.S. national guidelines. Washington, DC: National Action Alliance for Suicide Prevention; 2015.
6. Jordan JR, McGann V. Clinical work with suicide loss survivors: implications of the U.S. postvention guidelines. Death Stud. 2017;41(10):659-672.
7. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
8. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
9. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
10. Doka KJ. Disenfranchised grief: recognizing hidden sorrow. Lexington, MA: Lexington; 1989.
11. Doka KJ. Disenfranchised grief: new directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
12. McIntosh JL. Suicide survivors: the aftermath of suicide and suicidal behavior. In: Bryant CD, ed. Handbook of death & dying. Vol. 1. Thousand Oaks, CA: SAGE Publications; 2003:339-350.
13. Jordan, JR, McIntosh, JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:19-42.
14. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton & Co.; 1987.
15. Harwood D, Hawton K, Hope T, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
16. Armour, M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
17. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
18. Calhoun LG, Allen BG. Social reactions to the survivor of a suicide in the family: A review of the literature. Omega – Journal of Death and Dying. 1991;23(2):95-107.
19. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
20. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
21. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
22. Shneidman ES. Foreword. In: Cain AC, ed. Survivors of suicide. Springfield, IL: Charles C. Thomas; 1972:ix-xi.
23. Jordan JR, McIntosh, JL. Suicide bereavement: Why study survivors of suicide loss? In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:3-18.
24. Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Community Health. 2005;59(5):407-412.
25. Hedström P, Liu KY, Nordvik MK. Interaction domains and suicide: a population-based panel study of suicides in Stockholm, 1991-1999. Soc Forces. 2008;87(2):713-740.
26. Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360(9340):1126-1130.
27. Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797-802.
28. Neimeyer RA, Sands D. Suicide loss and the quest for meaning. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:71-84.
29. Sands DC, Jordan JR, Neimeyer RA. The meanings of suicide: A narrative approach to healing. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:249-282.
30. Jordan JR. Principles of grief counseling with adult survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:179-224.
31. Cerel J, Jordan JR, Duberstein PR. The impact of suicide on the family. Crisis. 2008;29:38-44.
32. Kaslow NJ, Samples TC, Rhodes M, et al. A family-oriented and culturally sensitive postvention approach with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:301-323.
33. Grad OT, Treven M, Krysinska K. Suicide bereavement and gender. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:39-49.
34. Kosminsky PS, Jordan JR. Attachment-informed grief therapy: the clinician’s guide to foundations and applications. New York, NY: Routledge; 2016.
35. Gutin N, McGann VL, Jordan JR. The impact of suicide on professional caregivers. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:93-111.
36. Jordan JR. Bereavement after suicide. Psychiatr Ann. 2008;38(10):679-685.
37. Jordan JR. After suicide: clinical work with survivors. Grief Matters. 2009;12(1):4-9.
38. Neimeyer, RA. Traumatic loss and the reconstruction of meaning. J Palliat Med. 2002;5(6):935-942; discussion 942-943.
39. Neimeyer R, ed. Meaning reconstruction & the experience of loss. Washington, DC: American Psychological Association; 2001.
40. Klass, D. Sorrow and solace: Neglected areas in bereavement research. Death Stud. 2013;37(7):597-616.
41. Farberow NL. The Los Angeles Survivors-After-Suicide program: an evaluation. Crisis. 1992;13(1):23-34.
42. McDaid C, Trowman R, Golder S, et al. Interventions for people bereaved through suicide: systematic review. Br J Psychiatry. 2008;193(6):438-443.
43. Groos AD, Shakespeare-Finch J. Positive experiences for participants in suicide bereavement groups: a grounded theory model. Death Stud. 2013;37(1):1-24.
44. Jordan JR. Group work with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:283-300.
45. Wilson A, Marshall A. The support needs and experiences of suicidally bereaved family and friends. Death Stud. 2010;34(7):625-640.
46. McKinnon JM, Chonody J. Exploring the formal supports used by people bereaved through suicide: a qualitative study. Soc Work Ment Health. 2014;12(3):231-248.
47. Myers MF, Fine C. Touched by suicide: hope and healing after loss. New York, NY: Gotham Books; 2006.
The loss of a loved one to suicide is often experienced as “devastating.”1 While survivors of suicide loss may be able to move through the grief process without clinical support,2 the traumatic and stigmatizing nature of suicide is likely to make its aftermath more challenging to navigate than other types of loss. Sanford et al3 found that more than two-thirds of suicide loss survivors sought therapy after their loss. Further, when individuals facing these challenges present for treatment, clinicians often face challenges of their own.
Very few clinicians are trained in general grief processes,4 and even those specifically trained in grief and loss have been shown to “miss” several of the common clinical features that are unique to suicide loss.3 In my professional experience, the intensity and duration of suicide grief are often greater than they are for other losses, and many survivors of suicide loss have reported that others, including clinicians, have “pathologized” this, rather than having understood it as normative under the circumstances.
Although there is extensive literature on the aftermath of suicide for surviving loved ones, very few controlled studies have assessed interventions specifically for this population. Yet the U.S. Guidelines for Suicide Postvention5 explicitly call for improved training for those who work with suicide loss survivors, as well as research on these interventions. Jordan and McGann6 noted, “Without a full knowledge of suicide and its aftermath, it is very possible to make clinical errors which can hamper treatment.” This article summarizes what is currently known about the general experience of suicide bereavement and optimal interventions in treatment.
What makes suicide loss unique?
Suicide bereavement is distinct from other types of loss in 3 significant ways7:
- the thematic content of the grief
- the social processes surrounding the survivor
- the impact that suicide has on family systems.
Additionally, the perceived intentionality and preventability of a suicide death, as well as its stigmatized and traumatic nature, differentiate it from other types of traumatic loss.7 These elements are all likely to affect the nature, intensity, and duration of the grief.
Stigma and suicide. Stigma associated with suicide is well documented.8 Former U.S. Surgeon General David Satcher9 specifically described stigma toward suicide as one of the biggest barriers to prevention. In addition, researchers have found that the stigma associated with suicide “spills over” to the bereaved family members. Doka10,11 refers to “disenfranchised grief,” in which bereaved individuals receive the message that their grief is not legitimate, and as a result, they are likely to internalize this view. Studies have shown that individuals bereaved by suicide are also stigmatized, and are believed to be more psychologically disturbed, less likable, more blameworthy, more ashamed, and more in need of professional help than other bereaved individuals.8,12-20
These judgments often mirror suicide loss survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Thus, it is not uncommon for survivors of suicide loss to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. To the extent that they are actively grieving, survivors of suicide loss often feel that they must do so in isolation. Thus, the perception of stigma, whether external or internalized, can have a profound effect on decisions about disclosure, requesting support, and ultimately on one’s ability to integrate the loss. Indeed, Feigelman et al21 found that stigmatization after suicide was specifically associated with ongoing grief difficulties, depression, and suicidal ideation.
Continue to: Traumatic nature of suicide
Traumatic nature of suicide. Suicide loss is also quite traumatic, and posttraumatic stress disorder (PTSD) symptoms such as shock, horror, disbelief, and intrusive/perseverative thoughts and questions, particularly in the earlier stages of grief, are common. Sanford et al3 found that the higher the level of “perceived closeness” to the deceased, the more likely that survivors of suicide loss would experience PTSD symptoms. In addition, the dramatic loss of social support following a suicide loss may itself be traumatic, which can serve to compound these difficulties. Notably, Sanford et al3 found that even for those survivors of suicide loss in treatment who endorsed PTSD symptoms, many of their treating clinicians did not assess or diagnose this disorder, thus missing an important component for treatment.
Increased risk for suicidality. Studies have shown that individuals who have lost a loved one to suicide are themselves at heightened risk for suicidal ideation and behaviors.22-27 Therefore, an assessment for suicide risk is always advisable. However, it is important to note that suicidal ideation is not uncommon and can serve different functions for survivors of suicide loss without necessarily progressing to a plan for acting on such ideations. Survivors of suicide loss may wish to “join” their loved one; to understand or identify with the mental state of the deceased; to punish themselves for failing to prevent the suicide; or to end their own pain through death. Therefore, it is crucial to assess the nature and function of expressed ideation (in addition to the presence or absence of plans) before assigning the level of risk.
Elements of suicide grief
After the loss of a loved one to suicide, the path to healing is often complex, with survivors of suicide loss enduring the following challenges:
Existential assumptions are shattered. Several authors28-30 have found that suicide loss is also likely to shatter survivors’ existential assumptions regarding their worldviews, roles, and identities, as well as religious and spiritual beliefs. As one survivor of suicide loss in my practice noted, “The world is gone, nothing is predictable anymore, and it’s no longer safe to assume anything.” Others have described feeling “fragmented” in ways they had never before experienced, and many have reported difficulties in “trusting” their own judgment, the stability of the world, and relationships. “Why?” becomes an emergent and insistent question in the survivor’s efforts to understand the suicide and (ideally) reassemble a coherent narrative around the loss.
Increased duration and intensity of grief. The duration of the grief process is likely to be affected by the traumatic nature of suicide loss, the differential social support accorded to its survivors, and the difficulty in finding systems that can validate and normalize the unique elements in suicide bereavement. The stigmatized reactions of others, particularly when internalized, can present barriers to the processing of grief. In addition, the intensity of the trauma and existential impact, as well as the perseverative nature of several of the unique themes (Box 1), can also prolong the processing and increase the intensity of suicide grief. Clinicians would do well to recognize the relatively “normative” nature of the increased duration and intensity, rather than seeing it as immediately indicative of a DSM diagnosis of complicated/prolonged grief disorder.
Box 1
Common themes in the suicide grief process
Several common themes are likely to emerge during the suicide grief process. Guilt and a sense of failure—around what one did and did not do—can be pervasive and persistent, and are often present even when not objectively warranted.
Anger and blame directed towards the deceased, other family members, and clinicians who had been treating the deceased may also be present, and may be used in efforts to deflect guilt. Any of these themes may be enlisted to create a deceptively simple narrative for understanding the reasons for the suicide.
Shame is often present, and certainly exacerbated by both external and internalized stigma. Feelings of rejection, betrayal, and abandonment by the deceased are also common, as well as fear/hypervigilance regarding the possibility of losing others to suicide. Given the intensity of suicide grief, it has been my observation that there may also be fear in relation to one's own mental status, as many otherwise healthy survivors of suicide loss have described feeling like they're "going crazy." Finally, there may also be relief, particularly if the deceased had been suffering from chronic psychiatric distress or had been cruel or abusive.
Continue to: Family disruption
Family disruption. It is not uncommon for a suicide loss to result in family disruption.6,31-32 This may manifest in the blaming of family members for “sins of omission or commission,”6 conflicts around the disclosure of the suicide both within and outside of the family, discordant grieving styles, and difficulties in understanding and attending to the needs of one’s children while grieving oneself.
Despite the common elements often seen in suicide grief, it is crucial to recognize that this process is not “one size fits all.” Not only are there individual variants, but Grad et al33 found gender-based differences in grieving styles, and cultural issues such as the “meanings” assigned to suicide, and culturally sanctioned grief rituals and behaviors that are also likely to affect how grief is experienced and expressed. In addition, personal variants such as closeness/conflicts with the deceased, histories of previous trauma or loss, pre-existing psychiatric disorders, and attachment orientation34 are likely to impact the grief process.
Losing close friends and colleagues may be similarly traumatic, but these survivors of suicide loss often receive even less social support than those who have kinship losses. Finally, when a suicide loss occurs in a professional capacity (such as the loss of a patient), this is likely to have many additional implications for one’s professional functions and identity.35
Interventions to help survivors
Several goals and “tasks” are involved in the suicide bereavement process (Box 21,6,30,36-40). These can be achieved through the following interventions: Support groups. Many survivors find that support groups that focus on suicide loss are extremely helpful, and research has supported this.1,4,41-44 Interactions with other suicide loss survivors provide hope, connection, and an “antidote” to stigma and shame. Optimally, group facilitators provide education, validation and normalization of the grief trajectory, and facilitate the sharing of both loss experiences and current functioning between group members. As a result, group participants often report renewed connections, increased efficacy in giving and accepting support, and decreased distress (including reductions in PTSD and depressive symptoms). The American Association of Suicidology (www.suicidology.org) and American Foundation of Suicide Prevention (www.afsp.org) provide contact information for suicide loss survivor groups (by geographical area) as well as information about online support groups.
Box 2
Goals and 'tasks' in suicide bereavement
The following goals and "tasks" should be part of the process of suicide bereavement:
- Reduce symptoms of posttraumatic stress disorder and other psychiatric disorders. Given the traumatic nature of the loss, an important goal is to understand and reduce posttraumatic stress disorder and other psychiatric symptoms, and incrementally improving functionality in relation to these.
- Integrate the loss. Recent authors36-38 have highlighted the need for survivors of suicide loss to "bear" and integrate the loss, as opposed to the concept of "getting over it." In these paradigms, the loss becomes an important part of one's identity, and eventually ceases to define it. Optimally, the "whole person" is remembered, not just the suicide. Part of this involves a reinvestment in life, with the acceptance of a "new normal" that takes the loss into account. It is not unusual for survivors of suicide loss to report some guilt in "moving on" and/or experiencing pleasure; often this is felt as a "betrayal" of the deceased.
- Create meaning from the loss. A major goal for those who have lost a loved one to suicide is the ability to find and/or create meaning from the loss. This would include the creation of a loss narrative39 that incorporates both ambiguity and complexity, as well as a regained/renewed sense of purpose in ongoing life.
- Develop ambiguity tolerance. A related "task" in suicide grief is the development of ambiguity tolerance, which generally includes an understanding of the complexity underlying suicide, the ability to offer oneself a "fair trial"30 in relation to one's realistic degree of responsibility, and the acceptance that many questions may remain unanswerable. In addition, in concert with the current understanding of "continuing bonds,"40 survivors should attempt to attend to the ongoing relationship with the deceased, including any "unfinished business."6
- Develop skills to manage stigmatized social responses and/or changes in family and social relationships.
- Memorialize and honor the deceased. Healing for survivors is facilitated by memorializations, which both validate the mourning process itself while also paying tribute to the richness of the deceased person's life.
- Post-traumatic growth. The relatively new understanding of "post-traumatic" growth is certainly applicable to the "unexpected gifts" many survivors of suicide loss report after they have moved through suicide grief. This includes greater understanding toward oneself, other survivors of suicide loss, and suicidal individuals; gratitude toward those who have provided support; and a desire to "use" their newfound understanding of suicide and suicide grief in ways to honor the deceased and benefit others. Feigelman et al1 found that many longer-term survivors of suicide loss engaged in both direct service and social activism around suicide pre- and postvention.
Individual treatment. The limited research on individual treatment for suicide loss survivors suggests that while most participants find it generally helpful, a significant number of others report that their therapists lack knowledge of suicide grief and endorse stigmatizing attitudes toward suicide and suicide loss survivors.45-46 In addition, Sanford et al3 found that survivors of suicide loss who endorsed PTSD symptoms were not assessed, diagnosed, or treated for these symptoms.
Continue to: This speaks to the importance of understanding what is...
This speaks to the importance of understanding what is “normative” for survivors of suicide loss. In general, “normalization” and psychoeducation about the suicide grief trajectory can play an important role in work with survivors of suicide loss, even in the presence of diagnosable disorders. While PTSD, depressive symptoms, and suicidal ideation are not uncommon in suicide loss survivors, and certainly may warrant clinical assessment and treatment, it can be helpful (and less stigmatizing) for your patients to know that these diagnoses are relatively common and understandable in the context of this devastating experience. For instance, survivors of suicide loss often report feeling relieved when clinicians explain the connections between traumatic loss and PTSD and/or depressive symptoms, and this can also help to relieve secondary anxiety about “going crazy.” Many survivors of suicide loss also describe feeling as though they are functioning on “autopilot” in the earlier stages of grief; it can help them understand the “function” of compartmentalization as potentially adaptive in the short run.
Suicide loss survivors may also find it helpful to learn about suicidal states of mind and their relationships to any types of mental illness their loved ones had struggled with.47
Your role: Help survivors integrate the loss
Before beginning treatment with an individual who has lost a loved one to suicide, clinicians should thoroughly explore their own understanding of and experience with suicide, including assumptions around causation, internalized stigma about suicidal individuals and survivors of suicide loss, their own history of suicide loss or suicidality, cultural/religious attitudes, and anxiety/defenses associated with the topic of suicide. These factors, particularly when unexamined, are likely to impact the treatment relationship and one’s clinical efficacy.
In concert with the existing literature, consider the potential goals and tasks involved in the integration of the individual’s suicide loss, along with any individual factors/variants that may impact the grief trajectory. Kosminsky and Jordon34 described the role of the clinician in this situation as a “transitional attachment figure” who facilitates the management and integration of the loss into the creation of what survivors of suicide loss have termed a “new normal.”
Because suicide loss is often associated with PTSD and other psychiatric illnesses (eg, depression, suicidality, substance abuse), it is essential to balance the assessment and treatment of these issues with attention to grief issues as needed. Again, to the extent that such issues have arisen primarily in the wake of the suicide loss, it can be helpful for patients to understand their connection to the context of the loss.
Continue to: Ideally, the clinician should...
Ideally, the clinician should be “present” with the patient’s pain, normative guilt, and rumination, without attempting to quickly eliminate or “fix” it or provide premature reassurance that the survivor of suicide loss “did nothing wrong.” Rather, as Jordan6 suggests, the clinician should act to promote a “fair trial” with respect to the patient’s guilt and blame, with an understanding of the “tyranny of hindsight.” The promotion of ambiguity tolerance should also play a role in coming to terms with many questions that may remain unanswered.
Optimally, clinicians should encourage patients to attend to their ongoing relationship with the deceased, particularly in the service of resolving “unfinished business,” ultimately integrating the loss into memories of the whole person. In line with this, survivors of suicide loss may be encouraged to create a narrative of the loss that incorporates both complexity and ambiguity. In the service of supporting the suicide loss survivor’s reinvestment in life, it is often helpful to facilitate their ability to anticipate and cope with triggers, such as anniversaries, birthdays, or holidays, as well as to develop and use skills for managing difficult or stigmatizing social or cultural reactions.
Any disruptions in family functioning should also be addressed. Psychoeducation about discordant grieving styles (particularly around gender) and the support of children’s grief may be helpful, and referrals to family or couples therapists should be considered as needed. Finally, the facilitation of suicide loss survivors’ creation of memorializations or rituals can help promote healing and make their loss meaningful.
Bottom Line
Losing a loved one to suicide is often a devastating and traumatic experience, but with optimal support, most survivors are ultimately able to integrate the loss and grow as a result. Understanding the suicide grief trajectory, as well as general guidelines for treatment, will facilitate healing and growth in the aftermath of suicide loss.
Related Resources
- Jordan JR, McIntosh JL. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011.
- American Association of Suicidology. http://www.suicidology.org/.
- American Foundation for Suicide Prevention. https://afsp.org/.
The loss of a loved one to suicide is often experienced as “devastating.”1 While survivors of suicide loss may be able to move through the grief process without clinical support,2 the traumatic and stigmatizing nature of suicide is likely to make its aftermath more challenging to navigate than other types of loss. Sanford et al3 found that more than two-thirds of suicide loss survivors sought therapy after their loss. Further, when individuals facing these challenges present for treatment, clinicians often face challenges of their own.
Very few clinicians are trained in general grief processes,4 and even those specifically trained in grief and loss have been shown to “miss” several of the common clinical features that are unique to suicide loss.3 In my professional experience, the intensity and duration of suicide grief are often greater than they are for other losses, and many survivors of suicide loss have reported that others, including clinicians, have “pathologized” this, rather than having understood it as normative under the circumstances.
Although there is extensive literature on the aftermath of suicide for surviving loved ones, very few controlled studies have assessed interventions specifically for this population. Yet the U.S. Guidelines for Suicide Postvention5 explicitly call for improved training for those who work with suicide loss survivors, as well as research on these interventions. Jordan and McGann6 noted, “Without a full knowledge of suicide and its aftermath, it is very possible to make clinical errors which can hamper treatment.” This article summarizes what is currently known about the general experience of suicide bereavement and optimal interventions in treatment.
What makes suicide loss unique?
Suicide bereavement is distinct from other types of loss in 3 significant ways7:
- the thematic content of the grief
- the social processes surrounding the survivor
- the impact that suicide has on family systems.
Additionally, the perceived intentionality and preventability of a suicide death, as well as its stigmatized and traumatic nature, differentiate it from other types of traumatic loss.7 These elements are all likely to affect the nature, intensity, and duration of the grief.
Stigma and suicide. Stigma associated with suicide is well documented.8 Former U.S. Surgeon General David Satcher9 specifically described stigma toward suicide as one of the biggest barriers to prevention. In addition, researchers have found that the stigma associated with suicide “spills over” to the bereaved family members. Doka10,11 refers to “disenfranchised grief,” in which bereaved individuals receive the message that their grief is not legitimate, and as a result, they are likely to internalize this view. Studies have shown that individuals bereaved by suicide are also stigmatized, and are believed to be more psychologically disturbed, less likable, more blameworthy, more ashamed, and more in need of professional help than other bereaved individuals.8,12-20
These judgments often mirror suicide loss survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Thus, it is not uncommon for survivors of suicide loss to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. To the extent that they are actively grieving, survivors of suicide loss often feel that they must do so in isolation. Thus, the perception of stigma, whether external or internalized, can have a profound effect on decisions about disclosure, requesting support, and ultimately on one’s ability to integrate the loss. Indeed, Feigelman et al21 found that stigmatization after suicide was specifically associated with ongoing grief difficulties, depression, and suicidal ideation.
Continue to: Traumatic nature of suicide
Traumatic nature of suicide. Suicide loss is also quite traumatic, and posttraumatic stress disorder (PTSD) symptoms such as shock, horror, disbelief, and intrusive/perseverative thoughts and questions, particularly in the earlier stages of grief, are common. Sanford et al3 found that the higher the level of “perceived closeness” to the deceased, the more likely that survivors of suicide loss would experience PTSD symptoms. In addition, the dramatic loss of social support following a suicide loss may itself be traumatic, which can serve to compound these difficulties. Notably, Sanford et al3 found that even for those survivors of suicide loss in treatment who endorsed PTSD symptoms, many of their treating clinicians did not assess or diagnose this disorder, thus missing an important component for treatment.
Increased risk for suicidality. Studies have shown that individuals who have lost a loved one to suicide are themselves at heightened risk for suicidal ideation and behaviors.22-27 Therefore, an assessment for suicide risk is always advisable. However, it is important to note that suicidal ideation is not uncommon and can serve different functions for survivors of suicide loss without necessarily progressing to a plan for acting on such ideations. Survivors of suicide loss may wish to “join” their loved one; to understand or identify with the mental state of the deceased; to punish themselves for failing to prevent the suicide; or to end their own pain through death. Therefore, it is crucial to assess the nature and function of expressed ideation (in addition to the presence or absence of plans) before assigning the level of risk.
Elements of suicide grief
After the loss of a loved one to suicide, the path to healing is often complex, with survivors of suicide loss enduring the following challenges:
Existential assumptions are shattered. Several authors28-30 have found that suicide loss is also likely to shatter survivors’ existential assumptions regarding their worldviews, roles, and identities, as well as religious and spiritual beliefs. As one survivor of suicide loss in my practice noted, “The world is gone, nothing is predictable anymore, and it’s no longer safe to assume anything.” Others have described feeling “fragmented” in ways they had never before experienced, and many have reported difficulties in “trusting” their own judgment, the stability of the world, and relationships. “Why?” becomes an emergent and insistent question in the survivor’s efforts to understand the suicide and (ideally) reassemble a coherent narrative around the loss.
Increased duration and intensity of grief. The duration of the grief process is likely to be affected by the traumatic nature of suicide loss, the differential social support accorded to its survivors, and the difficulty in finding systems that can validate and normalize the unique elements in suicide bereavement. The stigmatized reactions of others, particularly when internalized, can present barriers to the processing of grief. In addition, the intensity of the trauma and existential impact, as well as the perseverative nature of several of the unique themes (Box 1), can also prolong the processing and increase the intensity of suicide grief. Clinicians would do well to recognize the relatively “normative” nature of the increased duration and intensity, rather than seeing it as immediately indicative of a DSM diagnosis of complicated/prolonged grief disorder.
Box 1
Common themes in the suicide grief process
Several common themes are likely to emerge during the suicide grief process. Guilt and a sense of failure—around what one did and did not do—can be pervasive and persistent, and are often present even when not objectively warranted.
Anger and blame directed towards the deceased, other family members, and clinicians who had been treating the deceased may also be present, and may be used in efforts to deflect guilt. Any of these themes may be enlisted to create a deceptively simple narrative for understanding the reasons for the suicide.
Shame is often present, and certainly exacerbated by both external and internalized stigma. Feelings of rejection, betrayal, and abandonment by the deceased are also common, as well as fear/hypervigilance regarding the possibility of losing others to suicide. Given the intensity of suicide grief, it has been my observation that there may also be fear in relation to one's own mental status, as many otherwise healthy survivors of suicide loss have described feeling like they're "going crazy." Finally, there may also be relief, particularly if the deceased had been suffering from chronic psychiatric distress or had been cruel or abusive.
Continue to: Family disruption
Family disruption. It is not uncommon for a suicide loss to result in family disruption.6,31-32 This may manifest in the blaming of family members for “sins of omission or commission,”6 conflicts around the disclosure of the suicide both within and outside of the family, discordant grieving styles, and difficulties in understanding and attending to the needs of one’s children while grieving oneself.
Despite the common elements often seen in suicide grief, it is crucial to recognize that this process is not “one size fits all.” Not only are there individual variants, but Grad et al33 found gender-based differences in grieving styles, and cultural issues such as the “meanings” assigned to suicide, and culturally sanctioned grief rituals and behaviors that are also likely to affect how grief is experienced and expressed. In addition, personal variants such as closeness/conflicts with the deceased, histories of previous trauma or loss, pre-existing psychiatric disorders, and attachment orientation34 are likely to impact the grief process.
Losing close friends and colleagues may be similarly traumatic, but these survivors of suicide loss often receive even less social support than those who have kinship losses. Finally, when a suicide loss occurs in a professional capacity (such as the loss of a patient), this is likely to have many additional implications for one’s professional functions and identity.35
Interventions to help survivors
Several goals and “tasks” are involved in the suicide bereavement process (Box 21,6,30,36-40). These can be achieved through the following interventions: Support groups. Many survivors find that support groups that focus on suicide loss are extremely helpful, and research has supported this.1,4,41-44 Interactions with other suicide loss survivors provide hope, connection, and an “antidote” to stigma and shame. Optimally, group facilitators provide education, validation and normalization of the grief trajectory, and facilitate the sharing of both loss experiences and current functioning between group members. As a result, group participants often report renewed connections, increased efficacy in giving and accepting support, and decreased distress (including reductions in PTSD and depressive symptoms). The American Association of Suicidology (www.suicidology.org) and American Foundation of Suicide Prevention (www.afsp.org) provide contact information for suicide loss survivor groups (by geographical area) as well as information about online support groups.
Box 2
Goals and 'tasks' in suicide bereavement
The following goals and "tasks" should be part of the process of suicide bereavement:
- Reduce symptoms of posttraumatic stress disorder and other psychiatric disorders. Given the traumatic nature of the loss, an important goal is to understand and reduce posttraumatic stress disorder and other psychiatric symptoms, and incrementally improving functionality in relation to these.
- Integrate the loss. Recent authors36-38 have highlighted the need for survivors of suicide loss to "bear" and integrate the loss, as opposed to the concept of "getting over it." In these paradigms, the loss becomes an important part of one's identity, and eventually ceases to define it. Optimally, the "whole person" is remembered, not just the suicide. Part of this involves a reinvestment in life, with the acceptance of a "new normal" that takes the loss into account. It is not unusual for survivors of suicide loss to report some guilt in "moving on" and/or experiencing pleasure; often this is felt as a "betrayal" of the deceased.
- Create meaning from the loss. A major goal for those who have lost a loved one to suicide is the ability to find and/or create meaning from the loss. This would include the creation of a loss narrative39 that incorporates both ambiguity and complexity, as well as a regained/renewed sense of purpose in ongoing life.
- Develop ambiguity tolerance. A related "task" in suicide grief is the development of ambiguity tolerance, which generally includes an understanding of the complexity underlying suicide, the ability to offer oneself a "fair trial"30 in relation to one's realistic degree of responsibility, and the acceptance that many questions may remain unanswerable. In addition, in concert with the current understanding of "continuing bonds,"40 survivors should attempt to attend to the ongoing relationship with the deceased, including any "unfinished business."6
- Develop skills to manage stigmatized social responses and/or changes in family and social relationships.
- Memorialize and honor the deceased. Healing for survivors is facilitated by memorializations, which both validate the mourning process itself while also paying tribute to the richness of the deceased person's life.
- Post-traumatic growth. The relatively new understanding of "post-traumatic" growth is certainly applicable to the "unexpected gifts" many survivors of suicide loss report after they have moved through suicide grief. This includes greater understanding toward oneself, other survivors of suicide loss, and suicidal individuals; gratitude toward those who have provided support; and a desire to "use" their newfound understanding of suicide and suicide grief in ways to honor the deceased and benefit others. Feigelman et al1 found that many longer-term survivors of suicide loss engaged in both direct service and social activism around suicide pre- and postvention.
Individual treatment. The limited research on individual treatment for suicide loss survivors suggests that while most participants find it generally helpful, a significant number of others report that their therapists lack knowledge of suicide grief and endorse stigmatizing attitudes toward suicide and suicide loss survivors.45-46 In addition, Sanford et al3 found that survivors of suicide loss who endorsed PTSD symptoms were not assessed, diagnosed, or treated for these symptoms.
Continue to: This speaks to the importance of understanding what is...
This speaks to the importance of understanding what is “normative” for survivors of suicide loss. In general, “normalization” and psychoeducation about the suicide grief trajectory can play an important role in work with survivors of suicide loss, even in the presence of diagnosable disorders. While PTSD, depressive symptoms, and suicidal ideation are not uncommon in suicide loss survivors, and certainly may warrant clinical assessment and treatment, it can be helpful (and less stigmatizing) for your patients to know that these diagnoses are relatively common and understandable in the context of this devastating experience. For instance, survivors of suicide loss often report feeling relieved when clinicians explain the connections between traumatic loss and PTSD and/or depressive symptoms, and this can also help to relieve secondary anxiety about “going crazy.” Many survivors of suicide loss also describe feeling as though they are functioning on “autopilot” in the earlier stages of grief; it can help them understand the “function” of compartmentalization as potentially adaptive in the short run.
Suicide loss survivors may also find it helpful to learn about suicidal states of mind and their relationships to any types of mental illness their loved ones had struggled with.47
Your role: Help survivors integrate the loss
Before beginning treatment with an individual who has lost a loved one to suicide, clinicians should thoroughly explore their own understanding of and experience with suicide, including assumptions around causation, internalized stigma about suicidal individuals and survivors of suicide loss, their own history of suicide loss or suicidality, cultural/religious attitudes, and anxiety/defenses associated with the topic of suicide. These factors, particularly when unexamined, are likely to impact the treatment relationship and one’s clinical efficacy.
In concert with the existing literature, consider the potential goals and tasks involved in the integration of the individual’s suicide loss, along with any individual factors/variants that may impact the grief trajectory. Kosminsky and Jordon34 described the role of the clinician in this situation as a “transitional attachment figure” who facilitates the management and integration of the loss into the creation of what survivors of suicide loss have termed a “new normal.”
Because suicide loss is often associated with PTSD and other psychiatric illnesses (eg, depression, suicidality, substance abuse), it is essential to balance the assessment and treatment of these issues with attention to grief issues as needed. Again, to the extent that such issues have arisen primarily in the wake of the suicide loss, it can be helpful for patients to understand their connection to the context of the loss.
Continue to: Ideally, the clinician should...
Ideally, the clinician should be “present” with the patient’s pain, normative guilt, and rumination, without attempting to quickly eliminate or “fix” it or provide premature reassurance that the survivor of suicide loss “did nothing wrong.” Rather, as Jordan6 suggests, the clinician should act to promote a “fair trial” with respect to the patient’s guilt and blame, with an understanding of the “tyranny of hindsight.” The promotion of ambiguity tolerance should also play a role in coming to terms with many questions that may remain unanswered.
Optimally, clinicians should encourage patients to attend to their ongoing relationship with the deceased, particularly in the service of resolving “unfinished business,” ultimately integrating the loss into memories of the whole person. In line with this, survivors of suicide loss may be encouraged to create a narrative of the loss that incorporates both complexity and ambiguity. In the service of supporting the suicide loss survivor’s reinvestment in life, it is often helpful to facilitate their ability to anticipate and cope with triggers, such as anniversaries, birthdays, or holidays, as well as to develop and use skills for managing difficult or stigmatizing social or cultural reactions.
Any disruptions in family functioning should also be addressed. Psychoeducation about discordant grieving styles (particularly around gender) and the support of children’s grief may be helpful, and referrals to family or couples therapists should be considered as needed. Finally, the facilitation of suicide loss survivors’ creation of memorializations or rituals can help promote healing and make their loss meaningful.
Bottom Line
Losing a loved one to suicide is often a devastating and traumatic experience, but with optimal support, most survivors are ultimately able to integrate the loss and grow as a result. Understanding the suicide grief trajectory, as well as general guidelines for treatment, will facilitate healing and growth in the aftermath of suicide loss.
Related Resources
- Jordan JR, McIntosh JL. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011.
- American Association of Suicidology. http://www.suicidology.org/.
- American Foundation for Suicide Prevention. https://afsp.org/.
1. Feigelman W, Jordan JR, McIntosh JL, et al. Devastating losses: how parents cope with the death of a child to suicide or drugs. New York, NY: Springer; 2012.
2. McIntosh JL. Research on survivors of suicide. In: Stimming MT, Stimming M, eds. Before their time: adult children’s experiences of parental suicide. Philadelphia, PA: Temple University Press; 1999:157-180.
3. Sanford RL, Cerel J, McGann VL, et al. Suicide loss survivors’ experiences with therapy: Implications for clinical practice. Community Ment Health J. 2016;5(2):551-558.
4. Jordan JR, McMenamy J. Interventions for suicide survivors: a review of the literature. Suicide Life Threat Behav. 2004;34(4):337-349.
5. Survivors of Suicide Loss Task Force. Responding to grief, trauma, & distress after a suicide: U.S. national guidelines. Washington, DC: National Action Alliance for Suicide Prevention; 2015.
6. Jordan JR, McGann V. Clinical work with suicide loss survivors: implications of the U.S. postvention guidelines. Death Stud. 2017;41(10):659-672.
7. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
8. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
9. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
10. Doka KJ. Disenfranchised grief: recognizing hidden sorrow. Lexington, MA: Lexington; 1989.
11. Doka KJ. Disenfranchised grief: new directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
12. McIntosh JL. Suicide survivors: the aftermath of suicide and suicidal behavior. In: Bryant CD, ed. Handbook of death & dying. Vol. 1. Thousand Oaks, CA: SAGE Publications; 2003:339-350.
13. Jordan, JR, McIntosh, JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:19-42.
14. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton & Co.; 1987.
15. Harwood D, Hawton K, Hope T, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
16. Armour, M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
17. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
18. Calhoun LG, Allen BG. Social reactions to the survivor of a suicide in the family: A review of the literature. Omega – Journal of Death and Dying. 1991;23(2):95-107.
19. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
20. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
21. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
22. Shneidman ES. Foreword. In: Cain AC, ed. Survivors of suicide. Springfield, IL: Charles C. Thomas; 1972:ix-xi.
23. Jordan JR, McIntosh, JL. Suicide bereavement: Why study survivors of suicide loss? In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:3-18.
24. Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Community Health. 2005;59(5):407-412.
25. Hedström P, Liu KY, Nordvik MK. Interaction domains and suicide: a population-based panel study of suicides in Stockholm, 1991-1999. Soc Forces. 2008;87(2):713-740.
26. Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360(9340):1126-1130.
27. Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797-802.
28. Neimeyer RA, Sands D. Suicide loss and the quest for meaning. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:71-84.
29. Sands DC, Jordan JR, Neimeyer RA. The meanings of suicide: A narrative approach to healing. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:249-282.
30. Jordan JR. Principles of grief counseling with adult survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:179-224.
31. Cerel J, Jordan JR, Duberstein PR. The impact of suicide on the family. Crisis. 2008;29:38-44.
32. Kaslow NJ, Samples TC, Rhodes M, et al. A family-oriented and culturally sensitive postvention approach with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:301-323.
33. Grad OT, Treven M, Krysinska K. Suicide bereavement and gender. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:39-49.
34. Kosminsky PS, Jordan JR. Attachment-informed grief therapy: the clinician’s guide to foundations and applications. New York, NY: Routledge; 2016.
35. Gutin N, McGann VL, Jordan JR. The impact of suicide on professional caregivers. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:93-111.
36. Jordan JR. Bereavement after suicide. Psychiatr Ann. 2008;38(10):679-685.
37. Jordan JR. After suicide: clinical work with survivors. Grief Matters. 2009;12(1):4-9.
38. Neimeyer, RA. Traumatic loss and the reconstruction of meaning. J Palliat Med. 2002;5(6):935-942; discussion 942-943.
39. Neimeyer R, ed. Meaning reconstruction & the experience of loss. Washington, DC: American Psychological Association; 2001.
40. Klass, D. Sorrow and solace: Neglected areas in bereavement research. Death Stud. 2013;37(7):597-616.
41. Farberow NL. The Los Angeles Survivors-After-Suicide program: an evaluation. Crisis. 1992;13(1):23-34.
42. McDaid C, Trowman R, Golder S, et al. Interventions for people bereaved through suicide: systematic review. Br J Psychiatry. 2008;193(6):438-443.
43. Groos AD, Shakespeare-Finch J. Positive experiences for participants in suicide bereavement groups: a grounded theory model. Death Stud. 2013;37(1):1-24.
44. Jordan JR. Group work with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:283-300.
45. Wilson A, Marshall A. The support needs and experiences of suicidally bereaved family and friends. Death Stud. 2010;34(7):625-640.
46. McKinnon JM, Chonody J. Exploring the formal supports used by people bereaved through suicide: a qualitative study. Soc Work Ment Health. 2014;12(3):231-248.
47. Myers MF, Fine C. Touched by suicide: hope and healing after loss. New York, NY: Gotham Books; 2006.
1. Feigelman W, Jordan JR, McIntosh JL, et al. Devastating losses: how parents cope with the death of a child to suicide or drugs. New York, NY: Springer; 2012.
2. McIntosh JL. Research on survivors of suicide. In: Stimming MT, Stimming M, eds. Before their time: adult children’s experiences of parental suicide. Philadelphia, PA: Temple University Press; 1999:157-180.
3. Sanford RL, Cerel J, McGann VL, et al. Suicide loss survivors’ experiences with therapy: Implications for clinical practice. Community Ment Health J. 2016;5(2):551-558.
4. Jordan JR, McMenamy J. Interventions for suicide survivors: a review of the literature. Suicide Life Threat Behav. 2004;34(4):337-349.
5. Survivors of Suicide Loss Task Force. Responding to grief, trauma, & distress after a suicide: U.S. national guidelines. Washington, DC: National Action Alliance for Suicide Prevention; 2015.
6. Jordan JR, McGann V. Clinical work with suicide loss survivors: implications of the U.S. postvention guidelines. Death Stud. 2017;41(10):659-672.
7. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
8. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
9. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
10. Doka KJ. Disenfranchised grief: recognizing hidden sorrow. Lexington, MA: Lexington; 1989.
11. Doka KJ. Disenfranchised grief: new directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
12. McIntosh JL. Suicide survivors: the aftermath of suicide and suicidal behavior. In: Bryant CD, ed. Handbook of death & dying. Vol. 1. Thousand Oaks, CA: SAGE Publications; 2003:339-350.
13. Jordan, JR, McIntosh, JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:19-42.
14. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton & Co.; 1987.
15. Harwood D, Hawton K, Hope T, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
16. Armour, M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
17. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
18. Calhoun LG, Allen BG. Social reactions to the survivor of a suicide in the family: A review of the literature. Omega – Journal of Death and Dying. 1991;23(2):95-107.
19. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
20. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
21. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
22. Shneidman ES. Foreword. In: Cain AC, ed. Survivors of suicide. Springfield, IL: Charles C. Thomas; 1972:ix-xi.
23. Jordan JR, McIntosh, JL. Suicide bereavement: Why study survivors of suicide loss? In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:3-18.
24. Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Community Health. 2005;59(5):407-412.
25. Hedström P, Liu KY, Nordvik MK. Interaction domains and suicide: a population-based panel study of suicides in Stockholm, 1991-1999. Soc Forces. 2008;87(2):713-740.
26. Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360(9340):1126-1130.
27. Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797-802.
28. Neimeyer RA, Sands D. Suicide loss and the quest for meaning. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:71-84.
29. Sands DC, Jordan JR, Neimeyer RA. The meanings of suicide: A narrative approach to healing. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:249-282.
30. Jordan JR. Principles of grief counseling with adult survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:179-224.
31. Cerel J, Jordan JR, Duberstein PR. The impact of suicide on the family. Crisis. 2008;29:38-44.
32. Kaslow NJ, Samples TC, Rhodes M, et al. A family-oriented and culturally sensitive postvention approach with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:301-323.
33. Grad OT, Treven M, Krysinska K. Suicide bereavement and gender. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:39-49.
34. Kosminsky PS, Jordan JR. Attachment-informed grief therapy: the clinician’s guide to foundations and applications. New York, NY: Routledge; 2016.
35. Gutin N, McGann VL, Jordan JR. The impact of suicide on professional caregivers. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:93-111.
36. Jordan JR. Bereavement after suicide. Psychiatr Ann. 2008;38(10):679-685.
37. Jordan JR. After suicide: clinical work with survivors. Grief Matters. 2009;12(1):4-9.
38. Neimeyer, RA. Traumatic loss and the reconstruction of meaning. J Palliat Med. 2002;5(6):935-942; discussion 942-943.
39. Neimeyer R, ed. Meaning reconstruction & the experience of loss. Washington, DC: American Psychological Association; 2001.
40. Klass, D. Sorrow and solace: Neglected areas in bereavement research. Death Stud. 2013;37(7):597-616.
41. Farberow NL. The Los Angeles Survivors-After-Suicide program: an evaluation. Crisis. 1992;13(1):23-34.
42. McDaid C, Trowman R, Golder S, et al. Interventions for people bereaved through suicide: systematic review. Br J Psychiatry. 2008;193(6):438-443.
43. Groos AD, Shakespeare-Finch J. Positive experiences for participants in suicide bereavement groups: a grounded theory model. Death Stud. 2013;37(1):1-24.
44. Jordan JR. Group work with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:283-300.
45. Wilson A, Marshall A. The support needs and experiences of suicidally bereaved family and friends. Death Stud. 2010;34(7):625-640.
46. McKinnon JM, Chonody J. Exploring the formal supports used by people bereaved through suicide: a qualitative study. Soc Work Ment Health. 2014;12(3):231-248.
47. Myers MF, Fine C. Touched by suicide: hope and healing after loss. New York, NY: Gotham Books; 2006.
U.S. HIV clinical care fails the hardest hit
AMSTERDAM – The U.S. HIV epidemic during this decade has shown an overall slow but steady decline, with national incidence rates dropping by 5% during 2011-2016. But preventing new HIV infections is clearly falling short for a few, key demographic slices that make up the vast majority of the roughly 40,000 new U.S. HIV cases annually in recent years: black/African American men, Hispanic/Latino men, men who have sex with men (MSM), and young adults aged 25-34 years.
What’s especially glaring in data from the Centers for Disease Control and Prevention are the increasing incidence rates when these groups overlap, compared with falling rates of new HIV infections for most everyone else. The CDC’s numbers for 2010-2015 show statistically significant increases in HIV incidence among MSM aged 25-34 years who are black/African American or Hispanic/Latino. Black/African American MSM accounted for 26% of the total 39,782 U.S. HIV diagnoses in 2016, and Hispanic/Latino MSM accounted for 19%, according to CDC statistics, while all blacks/African Americans accounted for 44% of 2016’s new HIV infections and all Hispanic/Latinos had 25%.
Why are these relatively small demographic subgroups bucking the overall national trend toward fewer infections? Why are their incidence rates so stubbornly high?
A major reason is that the U.S. medical community that delivers HIV prevention, diagnosis, and treatment has fallen short in reaching out to, engaging, and facilitating HIV services to these hardest-hit groups, David Malebranche, MD, said in a plenary talk at the 22nd International AIDS Conference. He cited several factors that contribute to the HIV high-risk populations from getting the care they need.
First, there is HIV stigma, which can be especially acute when accompanied by stigmatization that’s also based on race, ethnicity, and sexual orientation.
“HIV stigma causes difficulties in navigating the HIV treatment cascade. HIV stigma can adversely affect testing behavior, disclosure to sex partners and loved ones, and medical care access,” said Dr. Malebranche, an internal medicine physician and HIV specialist at Morehouse School of Medicine in Atlanta who described himself during his talk as a “ black, same-gender-loving man.” People diagnosed with HIV “will consciously choose to live in a trancelike denial of HIV,” he noted. He also cited criminalization of HIV status as another driver of stigmatization, with 34 states having HIV-specific criminal laws, sentence enhancements, or both.
Beyond HIV status, other stigmas permeate clinics based on race, sex, sexual orientation, immigrant status, and gender identity. A need exists to “study what patients experience in clinics, to identify the barriers to patient engagement and antiretroviral therapy,” but Dr. Malebranche suggested that the HIV medical community is “afraid to evaluate and assess their systems because they’re scared of what they’ll see.” U.S. HIV care has been too focused on scientific advances while “losing sight of the larger social picture,” he maintained.
“The power differential between medical systems and vulnerable communities may be partly responsible for why viral suppressions rates are so low.”
Dr. Malebranche placed much of the blame on the HIV clinical community for failing to engage patients in the most affected demographic groups.
“If a health system or clinic has problems with patient adherence, it needs to self-examine the service it provides. Most research into black MSM and the HIV treatment cascade has looked at what’s wrong in the community first and has only looked at the medical community much later, if at all,” Dr. Malebranche said. “Medical systems say things like ‘they are hard to reach,’ and ‘they don’t trust doctors,’ and ‘they are uneducated.’ Medical systems do this to avoid holding themselves accountable.”
But the people who run these systems often don’t question whether the paperwork is too onerous, certification to receive care is too cumbersome, the location too stigmatizing, and whether the staff is trained in cultural humility. “Blaming the victim doesn’t get us far in public health,” he said. The people who run and staff U.S. HIV programs “should consider the power dynamics created by some of their spaces, employees, and protocols that make them complicit in perpetuating vulnerability, and this may be why patients fall out of care. Don’t call black and same-gender-loving people ‘hard to reach’ just because you don’t know how to reach them,” he said.
In addition to calling for better appreciation of the target populations and better accommodation of their needs, Dr. Malebranche suggested greater use of peer navigators, case managers, social workers, rapid test and treat programs, and attention to overall patient health and not just their HIV status. Fundamentally, he said, the HIV medical community needs to go beyond thinking in terms of just HIV suppression and “practice the art of medicine to keep patients engaged.”
SOURCE: Malebranche D, AIDS 2018, Abstract THPL0107.
AMSTERDAM – The U.S. HIV epidemic during this decade has shown an overall slow but steady decline, with national incidence rates dropping by 5% during 2011-2016. But preventing new HIV infections is clearly falling short for a few, key demographic slices that make up the vast majority of the roughly 40,000 new U.S. HIV cases annually in recent years: black/African American men, Hispanic/Latino men, men who have sex with men (MSM), and young adults aged 25-34 years.
What’s especially glaring in data from the Centers for Disease Control and Prevention are the increasing incidence rates when these groups overlap, compared with falling rates of new HIV infections for most everyone else. The CDC’s numbers for 2010-2015 show statistically significant increases in HIV incidence among MSM aged 25-34 years who are black/African American or Hispanic/Latino. Black/African American MSM accounted for 26% of the total 39,782 U.S. HIV diagnoses in 2016, and Hispanic/Latino MSM accounted for 19%, according to CDC statistics, while all blacks/African Americans accounted for 44% of 2016’s new HIV infections and all Hispanic/Latinos had 25%.
Why are these relatively small demographic subgroups bucking the overall national trend toward fewer infections? Why are their incidence rates so stubbornly high?
A major reason is that the U.S. medical community that delivers HIV prevention, diagnosis, and treatment has fallen short in reaching out to, engaging, and facilitating HIV services to these hardest-hit groups, David Malebranche, MD, said in a plenary talk at the 22nd International AIDS Conference. He cited several factors that contribute to the HIV high-risk populations from getting the care they need.
First, there is HIV stigma, which can be especially acute when accompanied by stigmatization that’s also based on race, ethnicity, and sexual orientation.
“HIV stigma causes difficulties in navigating the HIV treatment cascade. HIV stigma can adversely affect testing behavior, disclosure to sex partners and loved ones, and medical care access,” said Dr. Malebranche, an internal medicine physician and HIV specialist at Morehouse School of Medicine in Atlanta who described himself during his talk as a “ black, same-gender-loving man.” People diagnosed with HIV “will consciously choose to live in a trancelike denial of HIV,” he noted. He also cited criminalization of HIV status as another driver of stigmatization, with 34 states having HIV-specific criminal laws, sentence enhancements, or both.
Beyond HIV status, other stigmas permeate clinics based on race, sex, sexual orientation, immigrant status, and gender identity. A need exists to “study what patients experience in clinics, to identify the barriers to patient engagement and antiretroviral therapy,” but Dr. Malebranche suggested that the HIV medical community is “afraid to evaluate and assess their systems because they’re scared of what they’ll see.” U.S. HIV care has been too focused on scientific advances while “losing sight of the larger social picture,” he maintained.
“The power differential between medical systems and vulnerable communities may be partly responsible for why viral suppressions rates are so low.”
Dr. Malebranche placed much of the blame on the HIV clinical community for failing to engage patients in the most affected demographic groups.
“If a health system or clinic has problems with patient adherence, it needs to self-examine the service it provides. Most research into black MSM and the HIV treatment cascade has looked at what’s wrong in the community first and has only looked at the medical community much later, if at all,” Dr. Malebranche said. “Medical systems say things like ‘they are hard to reach,’ and ‘they don’t trust doctors,’ and ‘they are uneducated.’ Medical systems do this to avoid holding themselves accountable.”
But the people who run these systems often don’t question whether the paperwork is too onerous, certification to receive care is too cumbersome, the location too stigmatizing, and whether the staff is trained in cultural humility. “Blaming the victim doesn’t get us far in public health,” he said. The people who run and staff U.S. HIV programs “should consider the power dynamics created by some of their spaces, employees, and protocols that make them complicit in perpetuating vulnerability, and this may be why patients fall out of care. Don’t call black and same-gender-loving people ‘hard to reach’ just because you don’t know how to reach them,” he said.
In addition to calling for better appreciation of the target populations and better accommodation of their needs, Dr. Malebranche suggested greater use of peer navigators, case managers, social workers, rapid test and treat programs, and attention to overall patient health and not just their HIV status. Fundamentally, he said, the HIV medical community needs to go beyond thinking in terms of just HIV suppression and “practice the art of medicine to keep patients engaged.”
SOURCE: Malebranche D, AIDS 2018, Abstract THPL0107.
AMSTERDAM – The U.S. HIV epidemic during this decade has shown an overall slow but steady decline, with national incidence rates dropping by 5% during 2011-2016. But preventing new HIV infections is clearly falling short for a few, key demographic slices that make up the vast majority of the roughly 40,000 new U.S. HIV cases annually in recent years: black/African American men, Hispanic/Latino men, men who have sex with men (MSM), and young adults aged 25-34 years.
What’s especially glaring in data from the Centers for Disease Control and Prevention are the increasing incidence rates when these groups overlap, compared with falling rates of new HIV infections for most everyone else. The CDC’s numbers for 2010-2015 show statistically significant increases in HIV incidence among MSM aged 25-34 years who are black/African American or Hispanic/Latino. Black/African American MSM accounted for 26% of the total 39,782 U.S. HIV diagnoses in 2016, and Hispanic/Latino MSM accounted for 19%, according to CDC statistics, while all blacks/African Americans accounted for 44% of 2016’s new HIV infections and all Hispanic/Latinos had 25%.
Why are these relatively small demographic subgroups bucking the overall national trend toward fewer infections? Why are their incidence rates so stubbornly high?
A major reason is that the U.S. medical community that delivers HIV prevention, diagnosis, and treatment has fallen short in reaching out to, engaging, and facilitating HIV services to these hardest-hit groups, David Malebranche, MD, said in a plenary talk at the 22nd International AIDS Conference. He cited several factors that contribute to the HIV high-risk populations from getting the care they need.
First, there is HIV stigma, which can be especially acute when accompanied by stigmatization that’s also based on race, ethnicity, and sexual orientation.
“HIV stigma causes difficulties in navigating the HIV treatment cascade. HIV stigma can adversely affect testing behavior, disclosure to sex partners and loved ones, and medical care access,” said Dr. Malebranche, an internal medicine physician and HIV specialist at Morehouse School of Medicine in Atlanta who described himself during his talk as a “ black, same-gender-loving man.” People diagnosed with HIV “will consciously choose to live in a trancelike denial of HIV,” he noted. He also cited criminalization of HIV status as another driver of stigmatization, with 34 states having HIV-specific criminal laws, sentence enhancements, or both.
Beyond HIV status, other stigmas permeate clinics based on race, sex, sexual orientation, immigrant status, and gender identity. A need exists to “study what patients experience in clinics, to identify the barriers to patient engagement and antiretroviral therapy,” but Dr. Malebranche suggested that the HIV medical community is “afraid to evaluate and assess their systems because they’re scared of what they’ll see.” U.S. HIV care has been too focused on scientific advances while “losing sight of the larger social picture,” he maintained.
“The power differential between medical systems and vulnerable communities may be partly responsible for why viral suppressions rates are so low.”
Dr. Malebranche placed much of the blame on the HIV clinical community for failing to engage patients in the most affected demographic groups.
“If a health system or clinic has problems with patient adherence, it needs to self-examine the service it provides. Most research into black MSM and the HIV treatment cascade has looked at what’s wrong in the community first and has only looked at the medical community much later, if at all,” Dr. Malebranche said. “Medical systems say things like ‘they are hard to reach,’ and ‘they don’t trust doctors,’ and ‘they are uneducated.’ Medical systems do this to avoid holding themselves accountable.”
But the people who run these systems often don’t question whether the paperwork is too onerous, certification to receive care is too cumbersome, the location too stigmatizing, and whether the staff is trained in cultural humility. “Blaming the victim doesn’t get us far in public health,” he said. The people who run and staff U.S. HIV programs “should consider the power dynamics created by some of their spaces, employees, and protocols that make them complicit in perpetuating vulnerability, and this may be why patients fall out of care. Don’t call black and same-gender-loving people ‘hard to reach’ just because you don’t know how to reach them,” he said.
In addition to calling for better appreciation of the target populations and better accommodation of their needs, Dr. Malebranche suggested greater use of peer navigators, case managers, social workers, rapid test and treat programs, and attention to overall patient health and not just their HIV status. Fundamentally, he said, the HIV medical community needs to go beyond thinking in terms of just HIV suppression and “practice the art of medicine to keep patients engaged.”
SOURCE: Malebranche D, AIDS 2018, Abstract THPL0107.
REPORTING FROM AIDS 2018
For people with SMI, disclosure still challenging
ROCKVILLE, MD. – People working in the mental health field are more likely to disclose their past or present treatment for psychosis than are professionals in other fields, a researcher said at a National Institute of Mental Health conference on mental health services research.
The researcher, Nev Jones, PhD, presented the findings of a small survey she conducted in 2014 and 2015 of adults with current or past experiences of psychotic disorder who described themselves as having had or having a successful career. The research was not conducted for publication purposes but as part of an effort to develop tools for students with psychosis as they continued in higher education, said Dr. Jones, of the department of mental health law and policy at the University of South Florida, Tampa.
One of those tools was a closed program that was akin to Facebook; people with early psychosis could use this to “look at successful adults across a wide range of careers and how they had navigated accommodations, disclosure, education as well as vocational choice,” she said.
Dr. Jones did not ask participants about their gender and race, but she did query them on highest degree earned. The poll was disseminated with the assistance of the National Alliance on Mental Illness and that of Stanford (Calif.) University, where Dr. Jones was a postdoctoral fellow. Of the sample presented, 33% had a masters degree (MSW, MBA), and 15% had a doctoral level degree (JD, MD, PhD).
People who worked in fields outside of mental health care were far less likely to have revealed their conditions to colleagues or employers, with 14 of 67 participants having made no disclosure. Of 14 who had made no disclosure, 12 were in fields such as banking, economics, secondary education, nursing, pediatrics, and computer programming.
Dr. Jones said she received several calls from students and staff at Stanford who were unwilling to fill out the survey.
“They were very concerned about the risks of inadvertent disclosure, even though it was anonymous, because they had unique, potentially identifiable career paths that they could not lay out in their responses without the fear that that would disclose [identify] them,” Dr. Jones said.
An additional 17 of the 67 participants made what Dr. Jones termed “selective disclosures,” such as telling a coworker who was considered a friend or a supportive boss. The majority of the respondents to Jones’s survey – 36 of the 67 participants – were open about their conditions. All but one of the respondents in this broad-disclosure group worked in mental health fields.
Dr. Jones described the broad-disclosure designation as “meaning that there is nobody in their life who doesn’t know.”
“They’re out professionally. They’ve published a book. They speak,” Dr. Jones said. “If you Google them on the Internet, you would quickly learn that they had a psychiatric disability or psychosis.”
Dr. Jones herself falls into that camp. She’s told media outlets, including the online newspaper MinnPost, about her own experience being diagnosed with schizophrenia while a PhD student. The online magazine Pacific Standard ran a full-length feature about her return to academia.
About 100,000 adolescents and young adults in the United States experience first-episode psychosis each year, and the peak onset hits between 15 and 25 years of age, according to the NIMH. About a decade ago, the NIMH launched its Recovery After an Initial Schizophrenia Episode (RAISE) initiative to examine use of coordinated specialty care treatments for people who were experiencing a first episode of psychosis. Congress in 2014 moved to provide a stream of federal funding for those kinds of efforts.
“We’re going to be soon starting to discharge, on an annual basis, potentially tens of thousands of young people from these specialized early intervention programs,” Dr. Jones said. “So it becomes really pressing to understand what’s happening to them in the context of reintegration.”
Another presenter at the panel, Marjorie L. Baldwin, PhD, of Arizona State University, Tempe, is an economist who has published a book based, in part, on her son’s struggles, “Beyond Schizophrenia: Living and Working With a Serious Mental Illness” (Lanham, Md.: Rowman & Littlefield Publishers, 2016).
She presented findings from a pilot study for a larger project looking at the issue of disclosures of serious mental illness in the workplace. She and her colleague in this work, Steven C. Marcus, PhD, of the University of Pennsylvania, Philadelphia, separately spoke about the difficulties in securing funding for the project, including five failed R01 grant applications.
“The 6th time was the charm with NIH,” Dr. Baldwin said.
An initial hurdle was finding a cost-effective way to identify workers with serious mental illness who hold or have held what she termed “competitive jobs,” which Dr. Baldwin described as those that paid at least minimum wage and are not subsidized for people with disabilities.
“You cannot do this kind of a study with random dialing because it would be way too expensive,” she said. “Schizophrenia and serious mental illnesses are not rare, but they are fairly uncommon.”
Several years ago, though, she learned of a long-running health survey into which she could “piggyback” questions on mental health status. She presented results of a pilot study with about 230 people with serious mental illness who had held or had competitive jobs. Of this group, 52% had left their most recent job for reasons other than mental illness, while those conditions had caused an additional 21% to leave. But Dr. Baldwin and her colleagues found 27% still working.
Like Dr. Jones, Dr. Baldwin said some of those workers were in professional fields, such as accounting, law, education; others worked in the service and construction industries.
“Contrary to the stereotypes, people with serious mental illness whose symptoms are reasonably well controlled can work, and many are capable of supporting themselves in mainstream competitive jobs,” Dr. Baldwin said.
Dr. Jones had no disclosures tied to her survey. Dr. Baldwin and Dr. Marcus had no disclosures other than the NIH R01 grant.
ROCKVILLE, MD. – People working in the mental health field are more likely to disclose their past or present treatment for psychosis than are professionals in other fields, a researcher said at a National Institute of Mental Health conference on mental health services research.
The researcher, Nev Jones, PhD, presented the findings of a small survey she conducted in 2014 and 2015 of adults with current or past experiences of psychotic disorder who described themselves as having had or having a successful career. The research was not conducted for publication purposes but as part of an effort to develop tools for students with psychosis as they continued in higher education, said Dr. Jones, of the department of mental health law and policy at the University of South Florida, Tampa.
One of those tools was a closed program that was akin to Facebook; people with early psychosis could use this to “look at successful adults across a wide range of careers and how they had navigated accommodations, disclosure, education as well as vocational choice,” she said.
Dr. Jones did not ask participants about their gender and race, but she did query them on highest degree earned. The poll was disseminated with the assistance of the National Alliance on Mental Illness and that of Stanford (Calif.) University, where Dr. Jones was a postdoctoral fellow. Of the sample presented, 33% had a masters degree (MSW, MBA), and 15% had a doctoral level degree (JD, MD, PhD).
People who worked in fields outside of mental health care were far less likely to have revealed their conditions to colleagues or employers, with 14 of 67 participants having made no disclosure. Of 14 who had made no disclosure, 12 were in fields such as banking, economics, secondary education, nursing, pediatrics, and computer programming.
Dr. Jones said she received several calls from students and staff at Stanford who were unwilling to fill out the survey.
“They were very concerned about the risks of inadvertent disclosure, even though it was anonymous, because they had unique, potentially identifiable career paths that they could not lay out in their responses without the fear that that would disclose [identify] them,” Dr. Jones said.
An additional 17 of the 67 participants made what Dr. Jones termed “selective disclosures,” such as telling a coworker who was considered a friend or a supportive boss. The majority of the respondents to Jones’s survey – 36 of the 67 participants – were open about their conditions. All but one of the respondents in this broad-disclosure group worked in mental health fields.
Dr. Jones described the broad-disclosure designation as “meaning that there is nobody in their life who doesn’t know.”
“They’re out professionally. They’ve published a book. They speak,” Dr. Jones said. “If you Google them on the Internet, you would quickly learn that they had a psychiatric disability or psychosis.”
Dr. Jones herself falls into that camp. She’s told media outlets, including the online newspaper MinnPost, about her own experience being diagnosed with schizophrenia while a PhD student. The online magazine Pacific Standard ran a full-length feature about her return to academia.
About 100,000 adolescents and young adults in the United States experience first-episode psychosis each year, and the peak onset hits between 15 and 25 years of age, according to the NIMH. About a decade ago, the NIMH launched its Recovery After an Initial Schizophrenia Episode (RAISE) initiative to examine use of coordinated specialty care treatments for people who were experiencing a first episode of psychosis. Congress in 2014 moved to provide a stream of federal funding for those kinds of efforts.
“We’re going to be soon starting to discharge, on an annual basis, potentially tens of thousands of young people from these specialized early intervention programs,” Dr. Jones said. “So it becomes really pressing to understand what’s happening to them in the context of reintegration.”
Another presenter at the panel, Marjorie L. Baldwin, PhD, of Arizona State University, Tempe, is an economist who has published a book based, in part, on her son’s struggles, “Beyond Schizophrenia: Living and Working With a Serious Mental Illness” (Lanham, Md.: Rowman & Littlefield Publishers, 2016).
She presented findings from a pilot study for a larger project looking at the issue of disclosures of serious mental illness in the workplace. She and her colleague in this work, Steven C. Marcus, PhD, of the University of Pennsylvania, Philadelphia, separately spoke about the difficulties in securing funding for the project, including five failed R01 grant applications.
“The 6th time was the charm with NIH,” Dr. Baldwin said.
An initial hurdle was finding a cost-effective way to identify workers with serious mental illness who hold or have held what she termed “competitive jobs,” which Dr. Baldwin described as those that paid at least minimum wage and are not subsidized for people with disabilities.
“You cannot do this kind of a study with random dialing because it would be way too expensive,” she said. “Schizophrenia and serious mental illnesses are not rare, but they are fairly uncommon.”
Several years ago, though, she learned of a long-running health survey into which she could “piggyback” questions on mental health status. She presented results of a pilot study with about 230 people with serious mental illness who had held or had competitive jobs. Of this group, 52% had left their most recent job for reasons other than mental illness, while those conditions had caused an additional 21% to leave. But Dr. Baldwin and her colleagues found 27% still working.
Like Dr. Jones, Dr. Baldwin said some of those workers were in professional fields, such as accounting, law, education; others worked in the service and construction industries.
“Contrary to the stereotypes, people with serious mental illness whose symptoms are reasonably well controlled can work, and many are capable of supporting themselves in mainstream competitive jobs,” Dr. Baldwin said.
Dr. Jones had no disclosures tied to her survey. Dr. Baldwin and Dr. Marcus had no disclosures other than the NIH R01 grant.
ROCKVILLE, MD. – People working in the mental health field are more likely to disclose their past or present treatment for psychosis than are professionals in other fields, a researcher said at a National Institute of Mental Health conference on mental health services research.
The researcher, Nev Jones, PhD, presented the findings of a small survey she conducted in 2014 and 2015 of adults with current or past experiences of psychotic disorder who described themselves as having had or having a successful career. The research was not conducted for publication purposes but as part of an effort to develop tools for students with psychosis as they continued in higher education, said Dr. Jones, of the department of mental health law and policy at the University of South Florida, Tampa.
One of those tools was a closed program that was akin to Facebook; people with early psychosis could use this to “look at successful adults across a wide range of careers and how they had navigated accommodations, disclosure, education as well as vocational choice,” she said.
Dr. Jones did not ask participants about their gender and race, but she did query them on highest degree earned. The poll was disseminated with the assistance of the National Alliance on Mental Illness and that of Stanford (Calif.) University, where Dr. Jones was a postdoctoral fellow. Of the sample presented, 33% had a masters degree (MSW, MBA), and 15% had a doctoral level degree (JD, MD, PhD).
People who worked in fields outside of mental health care were far less likely to have revealed their conditions to colleagues or employers, with 14 of 67 participants having made no disclosure. Of 14 who had made no disclosure, 12 were in fields such as banking, economics, secondary education, nursing, pediatrics, and computer programming.
Dr. Jones said she received several calls from students and staff at Stanford who were unwilling to fill out the survey.
“They were very concerned about the risks of inadvertent disclosure, even though it was anonymous, because they had unique, potentially identifiable career paths that they could not lay out in their responses without the fear that that would disclose [identify] them,” Dr. Jones said.
An additional 17 of the 67 participants made what Dr. Jones termed “selective disclosures,” such as telling a coworker who was considered a friend or a supportive boss. The majority of the respondents to Jones’s survey – 36 of the 67 participants – were open about their conditions. All but one of the respondents in this broad-disclosure group worked in mental health fields.
Dr. Jones described the broad-disclosure designation as “meaning that there is nobody in their life who doesn’t know.”
“They’re out professionally. They’ve published a book. They speak,” Dr. Jones said. “If you Google them on the Internet, you would quickly learn that they had a psychiatric disability or psychosis.”
Dr. Jones herself falls into that camp. She’s told media outlets, including the online newspaper MinnPost, about her own experience being diagnosed with schizophrenia while a PhD student. The online magazine Pacific Standard ran a full-length feature about her return to academia.
About 100,000 adolescents and young adults in the United States experience first-episode psychosis each year, and the peak onset hits between 15 and 25 years of age, according to the NIMH. About a decade ago, the NIMH launched its Recovery After an Initial Schizophrenia Episode (RAISE) initiative to examine use of coordinated specialty care treatments for people who were experiencing a first episode of psychosis. Congress in 2014 moved to provide a stream of federal funding for those kinds of efforts.
“We’re going to be soon starting to discharge, on an annual basis, potentially tens of thousands of young people from these specialized early intervention programs,” Dr. Jones said. “So it becomes really pressing to understand what’s happening to them in the context of reintegration.”
Another presenter at the panel, Marjorie L. Baldwin, PhD, of Arizona State University, Tempe, is an economist who has published a book based, in part, on her son’s struggles, “Beyond Schizophrenia: Living and Working With a Serious Mental Illness” (Lanham, Md.: Rowman & Littlefield Publishers, 2016).
She presented findings from a pilot study for a larger project looking at the issue of disclosures of serious mental illness in the workplace. She and her colleague in this work, Steven C. Marcus, PhD, of the University of Pennsylvania, Philadelphia, separately spoke about the difficulties in securing funding for the project, including five failed R01 grant applications.
“The 6th time was the charm with NIH,” Dr. Baldwin said.
An initial hurdle was finding a cost-effective way to identify workers with serious mental illness who hold or have held what she termed “competitive jobs,” which Dr. Baldwin described as those that paid at least minimum wage and are not subsidized for people with disabilities.
“You cannot do this kind of a study with random dialing because it would be way too expensive,” she said. “Schizophrenia and serious mental illnesses are not rare, but they are fairly uncommon.”
Several years ago, though, she learned of a long-running health survey into which she could “piggyback” questions on mental health status. She presented results of a pilot study with about 230 people with serious mental illness who had held or had competitive jobs. Of this group, 52% had left their most recent job for reasons other than mental illness, while those conditions had caused an additional 21% to leave. But Dr. Baldwin and her colleagues found 27% still working.
Like Dr. Jones, Dr. Baldwin said some of those workers were in professional fields, such as accounting, law, education; others worked in the service and construction industries.
“Contrary to the stereotypes, people with serious mental illness whose symptoms are reasonably well controlled can work, and many are capable of supporting themselves in mainstream competitive jobs,” Dr. Baldwin said.
Dr. Jones had no disclosures tied to her survey. Dr. Baldwin and Dr. Marcus had no disclosures other than the NIH R01 grant.
EXPERT ANALYSIS FROM AN NIMH CONFERENCE
Low-dose CT fails to improve small cell lung cancer survival
Early had no significant impact on patient survival, based on data from a randomized trial.
Although early detection of small-cell lung cancer might improve outcomes for patients given the metastatic nature of the disease, previous studies on the value and impact of low-dose CT have been divergent, wrote Anish Thomas, MD, of the National Cancer Institute and his colleagues.
In a study published in Chest, the researchers reviewed characteristics of low-dose CT–detected small cell lung cancer (SCLC) in the randomized National Lung Screening Trial (NLST). The trial compared low-dose CT (LDCT) with chest radiography to assess patients at high risk for developing lung cancer. The study population included adults aged 55-74 years with a history of smoking of at least 30 pack-years, and former smokers who had quit within the past 15 years.
A total of 26,722 participants were randomized to the LDCT treatment arm with a median follow-up of 6.5 years; 143 SCLC cases and 926 non–small cell lung cancer (NSCLC) cases were identified in this group.
Of the 143 SCLC cases, 49 (34.2%) were screen detected, 15 (10.5%) were interval detected and 79 (55.2%) were not screened or were identified post screening.
A cancer was defined as screen detected if it was diagnosed within 1 year of a positive screening or diagnosed after a longer period but with no time gap between diagnostic procedures of more than 1 year; an interval cancer was diagnosed within a year of a negative screen, and non-screened cancers were those found in patients who received no NLST screening or other post screening.
Significantly more of the SCLC cases were at stage III or IV than the NSCLC cases (86% vs. 36%). Unfavorable stage III or IV lung cancers were identified in 80% of screen detected, 86% of interval detected, and 90% of nonscreened or postscreened cases.
In addition, 31 (63%) of the 49 screen-detected SCLC cases had at least one noncalcified nodule in the cancer lobe and 24 (49%) had a single NCN in the cancer lobe.
The 3-year cancer-specific survival rates were not significantly different for screen-detected, interval-detected, and nonscreened or postscreened cases (15.3%, 20.0%, and 13.8%, respectively).
“As expected, the majority of SCLC were late-stage cancers, but surprisingly the unfavorable stage distribution was present regardless of whether the cancer was screen-, interval- or post-screen detected,” the researchers noted.
The findings differed from the results of previous smaller studies showing more favorable stage distribution for SCLC cases detected by LDCT, but this difference may be accounted for by various factors including study populations that were smaller, younger, and lacking well-defined risk factors, the researchers said.
“Our findings underscore and provide further granularity to the premise that SCLC is a very aggressive neoplasm, and contrary to smaller prior studies, indicate that LDCT is an ineffective tool to screen for SCLC,” they said. “If early detection of SCLC were to be realistic, it would need to be detected before [lesions] are visible on LDCT,” they emphasized.
The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
SOURCE: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
Early had no significant impact on patient survival, based on data from a randomized trial.
Although early detection of small-cell lung cancer might improve outcomes for patients given the metastatic nature of the disease, previous studies on the value and impact of low-dose CT have been divergent, wrote Anish Thomas, MD, of the National Cancer Institute and his colleagues.
In a study published in Chest, the researchers reviewed characteristics of low-dose CT–detected small cell lung cancer (SCLC) in the randomized National Lung Screening Trial (NLST). The trial compared low-dose CT (LDCT) with chest radiography to assess patients at high risk for developing lung cancer. The study population included adults aged 55-74 years with a history of smoking of at least 30 pack-years, and former smokers who had quit within the past 15 years.
A total of 26,722 participants were randomized to the LDCT treatment arm with a median follow-up of 6.5 years; 143 SCLC cases and 926 non–small cell lung cancer (NSCLC) cases were identified in this group.
Of the 143 SCLC cases, 49 (34.2%) were screen detected, 15 (10.5%) were interval detected and 79 (55.2%) were not screened or were identified post screening.
A cancer was defined as screen detected if it was diagnosed within 1 year of a positive screening or diagnosed after a longer period but with no time gap between diagnostic procedures of more than 1 year; an interval cancer was diagnosed within a year of a negative screen, and non-screened cancers were those found in patients who received no NLST screening or other post screening.
Significantly more of the SCLC cases were at stage III or IV than the NSCLC cases (86% vs. 36%). Unfavorable stage III or IV lung cancers were identified in 80% of screen detected, 86% of interval detected, and 90% of nonscreened or postscreened cases.
In addition, 31 (63%) of the 49 screen-detected SCLC cases had at least one noncalcified nodule in the cancer lobe and 24 (49%) had a single NCN in the cancer lobe.
The 3-year cancer-specific survival rates were not significantly different for screen-detected, interval-detected, and nonscreened or postscreened cases (15.3%, 20.0%, and 13.8%, respectively).
“As expected, the majority of SCLC were late-stage cancers, but surprisingly the unfavorable stage distribution was present regardless of whether the cancer was screen-, interval- or post-screen detected,” the researchers noted.
The findings differed from the results of previous smaller studies showing more favorable stage distribution for SCLC cases detected by LDCT, but this difference may be accounted for by various factors including study populations that were smaller, younger, and lacking well-defined risk factors, the researchers said.
“Our findings underscore and provide further granularity to the premise that SCLC is a very aggressive neoplasm, and contrary to smaller prior studies, indicate that LDCT is an ineffective tool to screen for SCLC,” they said. “If early detection of SCLC were to be realistic, it would need to be detected before [lesions] are visible on LDCT,” they emphasized.
The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
SOURCE: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
Early had no significant impact on patient survival, based on data from a randomized trial.
Although early detection of small-cell lung cancer might improve outcomes for patients given the metastatic nature of the disease, previous studies on the value and impact of low-dose CT have been divergent, wrote Anish Thomas, MD, of the National Cancer Institute and his colleagues.
In a study published in Chest, the researchers reviewed characteristics of low-dose CT–detected small cell lung cancer (SCLC) in the randomized National Lung Screening Trial (NLST). The trial compared low-dose CT (LDCT) with chest radiography to assess patients at high risk for developing lung cancer. The study population included adults aged 55-74 years with a history of smoking of at least 30 pack-years, and former smokers who had quit within the past 15 years.
A total of 26,722 participants were randomized to the LDCT treatment arm with a median follow-up of 6.5 years; 143 SCLC cases and 926 non–small cell lung cancer (NSCLC) cases were identified in this group.
Of the 143 SCLC cases, 49 (34.2%) were screen detected, 15 (10.5%) were interval detected and 79 (55.2%) were not screened or were identified post screening.
A cancer was defined as screen detected if it was diagnosed within 1 year of a positive screening or diagnosed after a longer period but with no time gap between diagnostic procedures of more than 1 year; an interval cancer was diagnosed within a year of a negative screen, and non-screened cancers were those found in patients who received no NLST screening or other post screening.
Significantly more of the SCLC cases were at stage III or IV than the NSCLC cases (86% vs. 36%). Unfavorable stage III or IV lung cancers were identified in 80% of screen detected, 86% of interval detected, and 90% of nonscreened or postscreened cases.
In addition, 31 (63%) of the 49 screen-detected SCLC cases had at least one noncalcified nodule in the cancer lobe and 24 (49%) had a single NCN in the cancer lobe.
The 3-year cancer-specific survival rates were not significantly different for screen-detected, interval-detected, and nonscreened or postscreened cases (15.3%, 20.0%, and 13.8%, respectively).
“As expected, the majority of SCLC were late-stage cancers, but surprisingly the unfavorable stage distribution was present regardless of whether the cancer was screen-, interval- or post-screen detected,” the researchers noted.
The findings differed from the results of previous smaller studies showing more favorable stage distribution for SCLC cases detected by LDCT, but this difference may be accounted for by various factors including study populations that were smaller, younger, and lacking well-defined risk factors, the researchers said.
“Our findings underscore and provide further granularity to the premise that SCLC is a very aggressive neoplasm, and contrary to smaller prior studies, indicate that LDCT is an ineffective tool to screen for SCLC,” they said. “If early detection of SCLC were to be realistic, it would need to be detected before [lesions] are visible on LDCT,” they emphasized.
The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
SOURCE: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
FROM CHEST
Key clinical point: No significant difference in survival occurred in SCLC cancer patients identified early by low-dose CT vs. those identified at interval or post screening.
Major finding: Unfavorable stage III or IV lung cancers were identified in 80% of screen-detected, 86% of interval-detected, and 90% of nonscreened or postscreened cases.
Study details: The data come from 143 small cell lung cancer cases identified in the randomized National Lung Screening Trial.
Disclosures: The study was supported in part by the National Cancer Institute. The researchers had no financial conflicts to disclose.
Source: Thomas A et al. Chest. 2018 Aug 3. doi: 10.1016/j.chest.2018.07.029.
Pediatricians doing good globally
Do the images of Syrian refugee children kindle a desire to leave the privileged suburban-dwelling patients in your practice for just a few weeks and donate your clinical skills to help the victims of political conflict? Or, are you looking for a way to give your post-retirement life some meaning by volunteering on a typhoon-ravaged island in Southeast Asia?
Before you ask your partners for a 1-month leave of absence or try to convince your spouse that camping out in a refugee camp would be a better way to spend this year’s travel budget, I urge you to read Pediatricians and Global Health: Opportunities and Considerations for Meaningful Engagement, published in Pediatrics. This lengthy and thorough report by the American Board of Pediatrics Global Health Task Force will give you pause and should serve as an important reality check before you run off to buy the plane tickets for your personal mission of mercy.
Gitanjli Arora, MD, of the Children’s Hospital of Los Angeles, and her coauthors point out that, just because there appears to be a need for people with clinical expertise does not mean that your little black bag of skills honed in the privileged bubble of a first-world country is going to be of much help. This question is one I have pondered on several occasions when I have been offered what might be called “medical voluntourism” opportunities.
If I have distinguished myself as a clinician, that skill has been based on my ability to listen and communicate. Dropped into a community where I have little or no language facility and cultural awareness, I would need to rely on my observational skills. While I pride myself on my ability to make a visual diagnosis, the truth is that my diagnostic successes based solely on observation are almost exclusively dermatologic ones. And, let me add, my familiarity with tropical skin rashes is severely limited. I’m sure I could learn but not in a stay as short as a month.
Taking a history and communicating a treatment plan would force me to rely on the skills of a translator. In settings dominated by upheaval, many of the presenting complaints are going to be the result of, or at least heavily colored by, the chaos induced by anxiety. The ability to sort out where the mind and body connect is difficult enough in a community in which I have spent 40 years and speak the language. Regardless of how much sympathy I feel for the victims of tragic events, my clinical skills would have little value in the short term. Even worse, I would probably just be in the way. As the Task Force points out, my presence could also be squandering local resources as on-site providers worked to get me up to speed.
The authors also explore the numerous other complicating factors that must be considered by those of us who feel the pull to help the victims of global strife and natural disaster. While the authors discourage physicians like you and me from “dropping in” to help, they offer an abundance of suggestions on how we can act globally and stay locally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Do the images of Syrian refugee children kindle a desire to leave the privileged suburban-dwelling patients in your practice for just a few weeks and donate your clinical skills to help the victims of political conflict? Or, are you looking for a way to give your post-retirement life some meaning by volunteering on a typhoon-ravaged island in Southeast Asia?
Before you ask your partners for a 1-month leave of absence or try to convince your spouse that camping out in a refugee camp would be a better way to spend this year’s travel budget, I urge you to read Pediatricians and Global Health: Opportunities and Considerations for Meaningful Engagement, published in Pediatrics. This lengthy and thorough report by the American Board of Pediatrics Global Health Task Force will give you pause and should serve as an important reality check before you run off to buy the plane tickets for your personal mission of mercy.
Gitanjli Arora, MD, of the Children’s Hospital of Los Angeles, and her coauthors point out that, just because there appears to be a need for people with clinical expertise does not mean that your little black bag of skills honed in the privileged bubble of a first-world country is going to be of much help. This question is one I have pondered on several occasions when I have been offered what might be called “medical voluntourism” opportunities.
If I have distinguished myself as a clinician, that skill has been based on my ability to listen and communicate. Dropped into a community where I have little or no language facility and cultural awareness, I would need to rely on my observational skills. While I pride myself on my ability to make a visual diagnosis, the truth is that my diagnostic successes based solely on observation are almost exclusively dermatologic ones. And, let me add, my familiarity with tropical skin rashes is severely limited. I’m sure I could learn but not in a stay as short as a month.
Taking a history and communicating a treatment plan would force me to rely on the skills of a translator. In settings dominated by upheaval, many of the presenting complaints are going to be the result of, or at least heavily colored by, the chaos induced by anxiety. The ability to sort out where the mind and body connect is difficult enough in a community in which I have spent 40 years and speak the language. Regardless of how much sympathy I feel for the victims of tragic events, my clinical skills would have little value in the short term. Even worse, I would probably just be in the way. As the Task Force points out, my presence could also be squandering local resources as on-site providers worked to get me up to speed.
The authors also explore the numerous other complicating factors that must be considered by those of us who feel the pull to help the victims of global strife and natural disaster. While the authors discourage physicians like you and me from “dropping in” to help, they offer an abundance of suggestions on how we can act globally and stay locally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Do the images of Syrian refugee children kindle a desire to leave the privileged suburban-dwelling patients in your practice for just a few weeks and donate your clinical skills to help the victims of political conflict? Or, are you looking for a way to give your post-retirement life some meaning by volunteering on a typhoon-ravaged island in Southeast Asia?
Before you ask your partners for a 1-month leave of absence or try to convince your spouse that camping out in a refugee camp would be a better way to spend this year’s travel budget, I urge you to read Pediatricians and Global Health: Opportunities and Considerations for Meaningful Engagement, published in Pediatrics. This lengthy and thorough report by the American Board of Pediatrics Global Health Task Force will give you pause and should serve as an important reality check before you run off to buy the plane tickets for your personal mission of mercy.
Gitanjli Arora, MD, of the Children’s Hospital of Los Angeles, and her coauthors point out that, just because there appears to be a need for people with clinical expertise does not mean that your little black bag of skills honed in the privileged bubble of a first-world country is going to be of much help. This question is one I have pondered on several occasions when I have been offered what might be called “medical voluntourism” opportunities.
If I have distinguished myself as a clinician, that skill has been based on my ability to listen and communicate. Dropped into a community where I have little or no language facility and cultural awareness, I would need to rely on my observational skills. While I pride myself on my ability to make a visual diagnosis, the truth is that my diagnostic successes based solely on observation are almost exclusively dermatologic ones. And, let me add, my familiarity with tropical skin rashes is severely limited. I’m sure I could learn but not in a stay as short as a month.
Taking a history and communicating a treatment plan would force me to rely on the skills of a translator. In settings dominated by upheaval, many of the presenting complaints are going to be the result of, or at least heavily colored by, the chaos induced by anxiety. The ability to sort out where the mind and body connect is difficult enough in a community in which I have spent 40 years and speak the language. Regardless of how much sympathy I feel for the victims of tragic events, my clinical skills would have little value in the short term. Even worse, I would probably just be in the way. As the Task Force points out, my presence could also be squandering local resources as on-site providers worked to get me up to speed.
The authors also explore the numerous other complicating factors that must be considered by those of us who feel the pull to help the victims of global strife and natural disaster. While the authors discourage physicians like you and me from “dropping in” to help, they offer an abundance of suggestions on how we can act globally and stay locally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].