User login
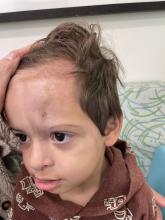
An 18-month-old male presents with a red mark on the forehead and nose
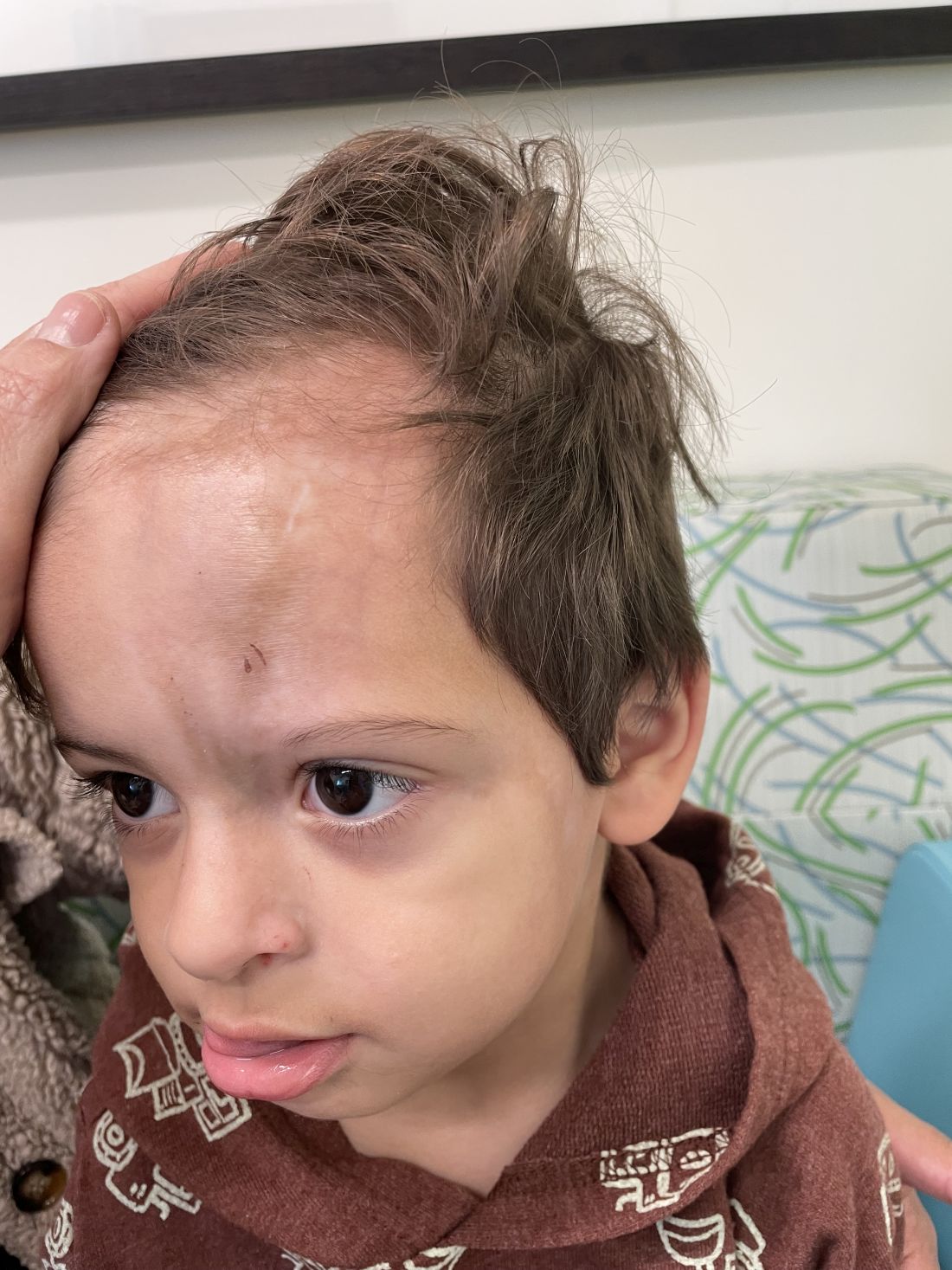
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
On examination, a faint pink patch was observed on the right forehead, frontal scalp, and nose. The lesion paled under pressure, with small areas of hair loss on the scalp. No atrophy was noted.
Low-dose naltrexone falls short for fibromyalgia
TOPLINE:
Women with fibromyalgia who received low-dose naltrexone showed no significant improvement in pain at 12 weeks, compared with those who received placebo in a randomized trial.
METHODOLOGY:
- The researchers randomly assigned 99 women with fibromyalgia at a single center in Denmark to a daily dose of 6 mg of naltrexone or a placebo for 12 weeks.
- The primary outcome was within-group change in pain intensity from baseline to 12 weeks, measured using an 11-point numeric rating scale (NRS) and the Fibromyalgia Impact Questionnaire-Revised (FIQR); outcomes were measured at 4, 8, and 12 weeks.
- Secondary outcomes included the global impact of FIQR total scores, as well as FIQR scores for tenderness, fatigue, , , anxiety, memory, stiffness, and physical function.
TAKEAWAY:
- The patients ranged in age from 18 to 64 years, with a mean age of 50.6 years, and all but one was White.
- At 12 weeks, the mean change in pain intensity was greater in the naltrexone group compared with the placebo group (−1.3 points vs −0.9 points, respectively), but the difference was not statistically significant.
- Of the secondary outcomes, only memory problems related to fibromyalgia showed significant improvement with naltrexone compared with placebo (−0.93 vs −0.30; P = .004), although the significance was lost after adjusting for multiplicity.
- Adverse events were infrequent and similar between the groups; four of 49 patients in the naltrexone group and three of 50 in the placebo group discontinued their assigned treatments because of side effects, and no safety concerns appeared related to the 6-mg dose.
IN PRACTICE:
“At this time we recommend that off-label treatment of patients who have responded to low-dose naltrexone should not be terminated, but we recommend against initiating low-dose naltrexone for low-dose naltrexone-naive patients with fibromyalgia pending the results of additional adequately powered studies with distinctive inflammatory and autoantibody patient profiles,” Winfried Häuser, MD, of the Center for Pain Medicine and Mental Health, Saarbrücken, Germany, and Mary-Ann Fitzcharles, MD, of McGill University Health Centre, Montreal, Canada, wrote in an accompanying editorial.
SOURCE:
The lead author of the study was Karin Due Bruun, MD, of Odense University Hospital, Denmark. The full study and accompanying editorial were published online in The Lancet Rheumatology.
LIMITATIONS:
The study was only powered to detect a difference of 1.0 NRS points for pain intensity; other limitations include the homogenous population that prevents generalizability to other groups and the relatively short follow-up period.
DISCLOSURES:
The study was supported by the Danish Rheumatism Association, Odense University Hospital, Danielsen’s Foundation, and the Oak Foundation. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Women with fibromyalgia who received low-dose naltrexone showed no significant improvement in pain at 12 weeks, compared with those who received placebo in a randomized trial.
METHODOLOGY:
- The researchers randomly assigned 99 women with fibromyalgia at a single center in Denmark to a daily dose of 6 mg of naltrexone or a placebo for 12 weeks.
- The primary outcome was within-group change in pain intensity from baseline to 12 weeks, measured using an 11-point numeric rating scale (NRS) and the Fibromyalgia Impact Questionnaire-Revised (FIQR); outcomes were measured at 4, 8, and 12 weeks.
- Secondary outcomes included the global impact of FIQR total scores, as well as FIQR scores for tenderness, fatigue, , , anxiety, memory, stiffness, and physical function.
TAKEAWAY:
- The patients ranged in age from 18 to 64 years, with a mean age of 50.6 years, and all but one was White.
- At 12 weeks, the mean change in pain intensity was greater in the naltrexone group compared with the placebo group (−1.3 points vs −0.9 points, respectively), but the difference was not statistically significant.
- Of the secondary outcomes, only memory problems related to fibromyalgia showed significant improvement with naltrexone compared with placebo (−0.93 vs −0.30; P = .004), although the significance was lost after adjusting for multiplicity.
- Adverse events were infrequent and similar between the groups; four of 49 patients in the naltrexone group and three of 50 in the placebo group discontinued their assigned treatments because of side effects, and no safety concerns appeared related to the 6-mg dose.
IN PRACTICE:
“At this time we recommend that off-label treatment of patients who have responded to low-dose naltrexone should not be terminated, but we recommend against initiating low-dose naltrexone for low-dose naltrexone-naive patients with fibromyalgia pending the results of additional adequately powered studies with distinctive inflammatory and autoantibody patient profiles,” Winfried Häuser, MD, of the Center for Pain Medicine and Mental Health, Saarbrücken, Germany, and Mary-Ann Fitzcharles, MD, of McGill University Health Centre, Montreal, Canada, wrote in an accompanying editorial.
SOURCE:
The lead author of the study was Karin Due Bruun, MD, of Odense University Hospital, Denmark. The full study and accompanying editorial were published online in The Lancet Rheumatology.
LIMITATIONS:
The study was only powered to detect a difference of 1.0 NRS points for pain intensity; other limitations include the homogenous population that prevents generalizability to other groups and the relatively short follow-up period.
DISCLOSURES:
The study was supported by the Danish Rheumatism Association, Odense University Hospital, Danielsen’s Foundation, and the Oak Foundation. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Women with fibromyalgia who received low-dose naltrexone showed no significant improvement in pain at 12 weeks, compared with those who received placebo in a randomized trial.
METHODOLOGY:
- The researchers randomly assigned 99 women with fibromyalgia at a single center in Denmark to a daily dose of 6 mg of naltrexone or a placebo for 12 weeks.
- The primary outcome was within-group change in pain intensity from baseline to 12 weeks, measured using an 11-point numeric rating scale (NRS) and the Fibromyalgia Impact Questionnaire-Revised (FIQR); outcomes were measured at 4, 8, and 12 weeks.
- Secondary outcomes included the global impact of FIQR total scores, as well as FIQR scores for tenderness, fatigue, , , anxiety, memory, stiffness, and physical function.
TAKEAWAY:
- The patients ranged in age from 18 to 64 years, with a mean age of 50.6 years, and all but one was White.
- At 12 weeks, the mean change in pain intensity was greater in the naltrexone group compared with the placebo group (−1.3 points vs −0.9 points, respectively), but the difference was not statistically significant.
- Of the secondary outcomes, only memory problems related to fibromyalgia showed significant improvement with naltrexone compared with placebo (−0.93 vs −0.30; P = .004), although the significance was lost after adjusting for multiplicity.
- Adverse events were infrequent and similar between the groups; four of 49 patients in the naltrexone group and three of 50 in the placebo group discontinued their assigned treatments because of side effects, and no safety concerns appeared related to the 6-mg dose.
IN PRACTICE:
“At this time we recommend that off-label treatment of patients who have responded to low-dose naltrexone should not be terminated, but we recommend against initiating low-dose naltrexone for low-dose naltrexone-naive patients with fibromyalgia pending the results of additional adequately powered studies with distinctive inflammatory and autoantibody patient profiles,” Winfried Häuser, MD, of the Center for Pain Medicine and Mental Health, Saarbrücken, Germany, and Mary-Ann Fitzcharles, MD, of McGill University Health Centre, Montreal, Canada, wrote in an accompanying editorial.
SOURCE:
The lead author of the study was Karin Due Bruun, MD, of Odense University Hospital, Denmark. The full study and accompanying editorial were published online in The Lancet Rheumatology.
LIMITATIONS:
The study was only powered to detect a difference of 1.0 NRS points for pain intensity; other limitations include the homogenous population that prevents generalizability to other groups and the relatively short follow-up period.
DISCLOSURES:
The study was supported by the Danish Rheumatism Association, Odense University Hospital, Danielsen’s Foundation, and the Oak Foundation. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Telemedicine in diabetes care associated with worse outcomes
TOPLINE:
Adult patients with type 2 diabetes and complex care needs receiving endocrinology treatment through telemedicine alone show worse glycemic outcomes compared with those receiving treatment either in-person or in mixed-care models.
The findings contrast with some previous studies showing similar glycemic outcomes with telemedicine care vs in-person care for type 2 diabetes management.
The study is believed to be the first to examine telemedicine care outcomes specifically in the endocrinology setting and based on clinical factors that affect treatment complexity.
METHODOLOGY:
- The retrospective cohort study included 3778 adults with type 2 diabetes in a single, large integrated US health system who had received either telemedicine-only, in-person, or a mix of telemedicine and in-person care between May and October 2020.
- Patients were followed up through May 2022 and evaluated for estimated A1c change after 12 months within each treatment cohort, as well as factors associated with any changes.
- Of the patients, 1182 received telemedicine-only, 1049 received in-person, and 1547 received mixed care. Mean ages in the groups ranged from 57 to 63 years, and women made up between 55% and 63%.
TAKEAWAY:
- Over the 12-month evaluation period, patients receiving telemedicine-only care had no significant changes or improvements in adjusted A1c (−0.06; P = .55), those receiving in-person care had an improvement of 0.37% (P < .001), and those receiving mixed care had an improvement of 0.22% (P = .004).
- The glycemic outcome patterns were similar among patients with a baseline A1c of 8% or higher.
- Of those prescribed multiple daily injections vs no insulin, estimated changes in A1c were 0.25% higher for those receiving telemedicine than for those receiving in-person care (P = .03).
- No associations were observed between changes in A1c and comorbidities.
- Regarding reasons for the differences, the authors noted that “the strategies to support glycemic improvement that are available during in-person appointments have not consistently been translated to telemedicine care.”
- Essential components of telemedicine such as self-management education support may not currently be routinely available through telemedicine or at the point-of-care during telemedicine visits, they added.
- “In our prior work in this care setting, practitioners described how inferior availability of glucose data limited their ability to intensify treatment through telemedicine.”
- “Implementation of approaches to overcome these differences, such as team-based virtual care and technological tools to automate blood glucose data sharing, are needed to ensure all patients receive high-quality diabetes care regardless of care modality.”
IN PRACTICE:
“These findings suggest that patients with type 2 diabetes who rely on telemedicine alone to access endocrinology care may require additional support to achieve glycemic goals,” the authors reported.
“Since some patients with barriers to in-person endocrinology care will continue to rely on telemedicine to access care, structured approaches to ensure routine delivery of high-quality team-based diabetes care are needed,” they asserted.
“Translation of successful strategies from clinical trials into routine telemedicine care, especially targeted toward adults with more complex diabetes, is critical to improve clinical outcomes for patients who rely on this care modality.”
SOURCE:
The study was conducted by first author Margaret F. Zupa, MD, of the division of endocrinology and metabolism, University of Pittsburgh School of Medicine, Pennsylvania, and colleagues.
It was published in JAMA Network Open.
LIMITATIONS:
While demographic differences between the groups were included as covariates, the treatment modality cohorts were not balanced based on baseline characteristics that could be confounders.
Various factors, such as treatment complexity, glycemic control, and transportation barriers, could have affected whether patients received care with telemedicine; therefore, causal associations could not be established.
DISCLOSURES:
The study received funding from the National Center for Advancing Translational Sciences, National Institutes of Health, Pittsburgh Foundation, and Fraternal Order of the Eagles Charity Foundation Diabetes Fund. The authors’ disclosures are detailed in the study.
A version of this article appeared on Medscape.com.
TOPLINE:
Adult patients with type 2 diabetes and complex care needs receiving endocrinology treatment through telemedicine alone show worse glycemic outcomes compared with those receiving treatment either in-person or in mixed-care models.
The findings contrast with some previous studies showing similar glycemic outcomes with telemedicine care vs in-person care for type 2 diabetes management.
The study is believed to be the first to examine telemedicine care outcomes specifically in the endocrinology setting and based on clinical factors that affect treatment complexity.
METHODOLOGY:
- The retrospective cohort study included 3778 adults with type 2 diabetes in a single, large integrated US health system who had received either telemedicine-only, in-person, or a mix of telemedicine and in-person care between May and October 2020.
- Patients were followed up through May 2022 and evaluated for estimated A1c change after 12 months within each treatment cohort, as well as factors associated with any changes.
- Of the patients, 1182 received telemedicine-only, 1049 received in-person, and 1547 received mixed care. Mean ages in the groups ranged from 57 to 63 years, and women made up between 55% and 63%.
TAKEAWAY:
- Over the 12-month evaluation period, patients receiving telemedicine-only care had no significant changes or improvements in adjusted A1c (−0.06; P = .55), those receiving in-person care had an improvement of 0.37% (P < .001), and those receiving mixed care had an improvement of 0.22% (P = .004).
- The glycemic outcome patterns were similar among patients with a baseline A1c of 8% or higher.
- Of those prescribed multiple daily injections vs no insulin, estimated changes in A1c were 0.25% higher for those receiving telemedicine than for those receiving in-person care (P = .03).
- No associations were observed between changes in A1c and comorbidities.
- Regarding reasons for the differences, the authors noted that “the strategies to support glycemic improvement that are available during in-person appointments have not consistently been translated to telemedicine care.”
- Essential components of telemedicine such as self-management education support may not currently be routinely available through telemedicine or at the point-of-care during telemedicine visits, they added.
- “In our prior work in this care setting, practitioners described how inferior availability of glucose data limited their ability to intensify treatment through telemedicine.”
- “Implementation of approaches to overcome these differences, such as team-based virtual care and technological tools to automate blood glucose data sharing, are needed to ensure all patients receive high-quality diabetes care regardless of care modality.”
IN PRACTICE:
“These findings suggest that patients with type 2 diabetes who rely on telemedicine alone to access endocrinology care may require additional support to achieve glycemic goals,” the authors reported.
“Since some patients with barriers to in-person endocrinology care will continue to rely on telemedicine to access care, structured approaches to ensure routine delivery of high-quality team-based diabetes care are needed,” they asserted.
“Translation of successful strategies from clinical trials into routine telemedicine care, especially targeted toward adults with more complex diabetes, is critical to improve clinical outcomes for patients who rely on this care modality.”
SOURCE:
The study was conducted by first author Margaret F. Zupa, MD, of the division of endocrinology and metabolism, University of Pittsburgh School of Medicine, Pennsylvania, and colleagues.
It was published in JAMA Network Open.
LIMITATIONS:
While demographic differences between the groups were included as covariates, the treatment modality cohorts were not balanced based on baseline characteristics that could be confounders.
Various factors, such as treatment complexity, glycemic control, and transportation barriers, could have affected whether patients received care with telemedicine; therefore, causal associations could not be established.
DISCLOSURES:
The study received funding from the National Center for Advancing Translational Sciences, National Institutes of Health, Pittsburgh Foundation, and Fraternal Order of the Eagles Charity Foundation Diabetes Fund. The authors’ disclosures are detailed in the study.
A version of this article appeared on Medscape.com.
TOPLINE:
Adult patients with type 2 diabetes and complex care needs receiving endocrinology treatment through telemedicine alone show worse glycemic outcomes compared with those receiving treatment either in-person or in mixed-care models.
The findings contrast with some previous studies showing similar glycemic outcomes with telemedicine care vs in-person care for type 2 diabetes management.
The study is believed to be the first to examine telemedicine care outcomes specifically in the endocrinology setting and based on clinical factors that affect treatment complexity.
METHODOLOGY:
- The retrospective cohort study included 3778 adults with type 2 diabetes in a single, large integrated US health system who had received either telemedicine-only, in-person, or a mix of telemedicine and in-person care between May and October 2020.
- Patients were followed up through May 2022 and evaluated for estimated A1c change after 12 months within each treatment cohort, as well as factors associated with any changes.
- Of the patients, 1182 received telemedicine-only, 1049 received in-person, and 1547 received mixed care. Mean ages in the groups ranged from 57 to 63 years, and women made up between 55% and 63%.
TAKEAWAY:
- Over the 12-month evaluation period, patients receiving telemedicine-only care had no significant changes or improvements in adjusted A1c (−0.06; P = .55), those receiving in-person care had an improvement of 0.37% (P < .001), and those receiving mixed care had an improvement of 0.22% (P = .004).
- The glycemic outcome patterns were similar among patients with a baseline A1c of 8% or higher.
- Of those prescribed multiple daily injections vs no insulin, estimated changes in A1c were 0.25% higher for those receiving telemedicine than for those receiving in-person care (P = .03).
- No associations were observed between changes in A1c and comorbidities.
- Regarding reasons for the differences, the authors noted that “the strategies to support glycemic improvement that are available during in-person appointments have not consistently been translated to telemedicine care.”
- Essential components of telemedicine such as self-management education support may not currently be routinely available through telemedicine or at the point-of-care during telemedicine visits, they added.
- “In our prior work in this care setting, practitioners described how inferior availability of glucose data limited their ability to intensify treatment through telemedicine.”
- “Implementation of approaches to overcome these differences, such as team-based virtual care and technological tools to automate blood glucose data sharing, are needed to ensure all patients receive high-quality diabetes care regardless of care modality.”
IN PRACTICE:
“These findings suggest that patients with type 2 diabetes who rely on telemedicine alone to access endocrinology care may require additional support to achieve glycemic goals,” the authors reported.
“Since some patients with barriers to in-person endocrinology care will continue to rely on telemedicine to access care, structured approaches to ensure routine delivery of high-quality team-based diabetes care are needed,” they asserted.
“Translation of successful strategies from clinical trials into routine telemedicine care, especially targeted toward adults with more complex diabetes, is critical to improve clinical outcomes for patients who rely on this care modality.”
SOURCE:
The study was conducted by first author Margaret F. Zupa, MD, of the division of endocrinology and metabolism, University of Pittsburgh School of Medicine, Pennsylvania, and colleagues.
It was published in JAMA Network Open.
LIMITATIONS:
While demographic differences between the groups were included as covariates, the treatment modality cohorts were not balanced based on baseline characteristics that could be confounders.
Various factors, such as treatment complexity, glycemic control, and transportation barriers, could have affected whether patients received care with telemedicine; therefore, causal associations could not be established.
DISCLOSURES:
The study received funding from the National Center for Advancing Translational Sciences, National Institutes of Health, Pittsburgh Foundation, and Fraternal Order of the Eagles Charity Foundation Diabetes Fund. The authors’ disclosures are detailed in the study.
A version of this article appeared on Medscape.com.
LGBTQI+: Special considerations for reproductive health care
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
CASE A new patient office visit
A new patient is waiting for you in the exam room. You review the chart and see the sex demographic field is blank, and the patient’s name is Alex. As an ObGyn, most of your patients are female, but you have treated your patients’ partners for sexually transmitted infections. As you enter the room, you see 2 androgynously dressed individuals; you introduce yourself and ask,
“What brings you in today, and who is your friend?”
“This is my partner Charlie, and we are worried I have an STD.”
Estimates suggest that between 7% to 12% of the US population identifies as lesbian, gay, bisexual, transgender/non-binary, queer/questioning, intersex, or asexual (LGBTQI+).1 If you practice in an urban area, the odds are quite high that you have encountered an LGBTQI+ person who openly identified as such; if you are in a rural area, you also likely have had an LGBTQI+ patient, but they may not have disclosed this about themselves.2 Maybe you have had training in cultural relevance or are a member of this community and you feel confident in providing quality care to LGBTQI+ patients. Or maybe you think that, as a responsibly practicing health care clinician, you treat all patients the same, so whether or not you know their sexual orientation or gender identity does not impact the care you provide. As the proportion of US adults who identify as LGBTQI+ increases,1 it becomes more important for health care clinicians to understand the challenges these patients face when trying to access health care. To start, let’s review the meaning of LGBTQI+, the history of the community, what it means to be culturally relevant or humble, and how to create a welcoming and safe practice environment.
LGBTQI+ terms and definitions
The first step in providing quality care to LGBTQI+ patients is to understand the terminology associated with sexual orientation, gender identity, and gender expression.3–5
Sexual orientation refers to whom a person is sexually attracted. The term straight/heterosexual suggests a person is sexually attracted to a person of the opposite gender. Lesbian or gay refers to those who are attracted to their same gender. Some people use bisexual (attracted to both the same and opposite gender) and pansexual (attracted to all humans regardless of gender). Still others refer to themselves as queer—people who identify as someone who is not heterosexual or cisgender. A variety of other terms exist to describe one’s sexual attraction. There are also some people who identify as asexual, which suggests they are not sexually attracted to anyone.
Gender identity relates to how one views their own gender. If you were assigned female at birth and identify as a woman, you are cisgender. If you were assigned male at birth and identify as a woman, you may identify as transgender whether or not you have had gender transitioning surgery or have taken hormones. Some people do not identify with the terms male or female and may view themselves as nonbinary. The terms gender queer, gender fluid, gender diverse, and gender non-conforming also may be used to describe various ways that an individual may not identify as male or female. We also can refer to people as “assigned female at birth” or “assigned male at birth”. People with intersex conditions may require taking a unique medical history that includes asking about genetic testing (eg, 46,XX congenital adrenal hyperplasia or 46,XY complete gonadal dysgenesis).
Gender expression refers to how one pre-sents themselves to others through appearance, dress, and behavior. A person may be assigned female at birth, dress in a conventional male fashion, and still identify as a woman. Still others may choose to express their gender in a variety of ways that may not have anything to do with their sexual orientation or gender identity, such as dressing in ways that represent their culture.
People may be fluid in their sexual orientation or gender identity; it may change from day to day, month to month, or even year to year.6,7
*The term LGBTQI+ is not used consistently in the literature. Throughout this article, the terminology used matches that used in the cited reference(s).
Continue to: Health care and the LGBTQI+ community...
Health care and the LGBTQI+ community
The LGBTQI+ community has a history of experiencing societal discrimination and stigma, which stems from medical mistrust often due to a lack of understanding of their medical and psychosocial needs.8,9 A 2019 survey of US LGBTQ adults, found that about 50% of people who identified as transgender reported having negative or discriminatory experiences with a health care clinician.10 About 18% of transgender people anticipated being refused medical care due to their gender identity.10 About 18% of LGBTQ individuals avoid any type of medical care, fearing discrimination.10 Lesbian women are 3 times more likely to have not seen an ObGyn than women who identify as straight.11 Sixty-two percent of lesbian women have biological children and received prenatal care; however, of those, 47% do not receive routine cancer screenings.10,11 Only 45% of age-eligible lesbian women have received at least 1 dose of the HPV vaccine, compared with 60% of straight women.10,11
Due to societal stigma, more than 40% of transgender people have attempted suicide.12 Felt or perceived stigma is also associated with risky health behaviors that contribute to health disparities. LGBTQI+ people are more likely to use substances,13 lesbian women are more likely to be obese,14 and 19% of transgender men are living with HIV/AIDS.15 Rates of unintended pregnancy among lesbian women and transgender men are 28%, compared with 6% in straight women, and 12% in heterosexual teens.15,16
In addition to real or perceived discrimination, there are medical misperceptions among the LGBTQI+ community. For instance, sexual minority women (SMW) are less likely to receive regular screening for cervical cancer. In one survey of more than 400 SMW, about 25% reported not receiving regular screening. SMW may mistakenly believe they do not need Pap testing and pelvic exams because they do not have penile-vaginal intercourse.17,18 Transgender men may not identify with having a cervix, or may perceive ObGyns to be “gendered” toward people who identify as women.18
Embracing cultural humility
Cultural humility expands upon the term cultural competence, with the idea that one can never be fully competent in the culture of another person.19,20 The National Institutes of Health defines cultural humility as “a lifelong process of self-reflection and self-critique whereby the individual not only learns about another’s culture, but one starts with an examination of his/her own beliefs and cultural identities.”21
Having cultural humility is the recognition that, in order to treat your ObGyn patient as a whole person and engage in shared medical decision making in the office setting, you need to know their sexual orientation and gender identity. Treating each patient the same is not providing equitable care (equality does not equal equity) because each patient has different medical and psychosocial needs. Embracing cultural humility is the first step in creating safe and welcoming spaces in the ObGyn office.20
CASE Ways to better introduce yourself
To revisit the case, what options does the clinician have to start off on a best foot to create a safe space for Alex?
- Open with your own preferred pronouns. For instance, for an introduction, consider: “I’m Dr. X, my pronouns are she/her.”
- Don’t assume. Do not make assumptions about the relationship between Alex and the person accompanying them.
4 ways for creating welcoming and affirming spaces in ObGyn
- Make sure your intake form is inclusive. Include a space for pronouns and the patient’s preferred name (which may differ from their legal name). Also allow patients to choose more than 1 sexual orientation and gender identity.20 (An example form is available from the LGBT National Health Education Center: https://www.lgbtqiahealtheducation.org/publication/focus-forms-policy-creating-inclusive-environment-lgbt-patients/.)
- Create a safe environment in the waiting area. Try to ensure that at least 1 bathroom is labeled “All Gender” or “Family.” Gendered bathrooms (eg, Ladies’ or Men’s rooms) are not welcoming. Make sure your non-discrimination policy is displayed and includes sexual orientation and gender identity. Review the patient education and reading materials in your waiting room to ensure they are inclusive. Do they show people with varied gender expression? Do they show same-sex couples or interracial couples?
- Use a trauma-informed approach when taking a sexual history and while conducting a physical exam. Determine if a pelvic exam is necessary at this visit or can it be postponed for another visit, when trust has been established with the patient. Explain each part of the pelvic/vaginal exam prior to conducting and again while performing the exam. Before taking a sexual history, explain why you are asking the questions and be sure to remain neutral with your questioning. For instance, you can say, “It’s important for me to understand your medical history in detail to provide you with the best health care possible.” Instead of asking, “Do you have sex with men, women, or both?” ask, “Do you have sex with people with a penis, vagina, or both? Do you have anal sex?” Recognize that some patients may be in a polyamorous relationship and may have more than 1 committed partner. For sexually active patients consider asking if they have ever exchanged sex for money or other goods, making sure to avoid judgmental body language or wording. Patients who do engage in “survival sex” may benefit from a discussion on pre-exposure prophylaxis to reduce HIV transmission.22
- Provide appropriate counsel based on their feedback.
- Explain their risk for HPV infection and vaccination options.
- Respectfully ask if there is a need for contraception and review options appropriate for their situation.
- Ask about the use of “toys” and provide guidance on sanitation and risk of infection with shared toys.
- Determine current or past hormone use for patients who identify as transgender and nonbinary (although many do not take hormones and have not had gender-affirming procedures, some may be considering these procedures). Be sure to ask these patients if they have had any surgeries or other procedures.
The receipt of gynecologic care can be traumatic for some LGBTQI+ people. Explain to the patient why you are doing everything during your examination and how it might feel. If a pelvic exam is not absolutely necessary that day, perhaps the patient can return another time. For transgender men who have been taking testosterone,vaginal atrophy may be a concern, and you could consider a pediatric speculum.
In summary, the number of people who identify as lesbian, gay, bisexual, transgender/nonbinary, queer/questioning, intersex, or asexual is not insignificant. Many of these patients or their partners may present for ObGyn care at your office. Clinicians need to understand that there is a new language relative to sexual orientation and gender identity. Incorporating cultural humility into one’s practice requires personal introspection and is a first step to creating safe and welcoming spaces in the ObGyn office. ●
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
- Jones JM. LGBT identification in US ticks up to 7.1%. Gallup News. February 17, 2022. Accessed July 11, 2023. https://news.gallup .com/poll/389792/lgbt-identification-ticks -up.aspx
- Patterson JG, Tree JMJ, and Kamen C. Cultural competency and microaggressions in the provision of care to LGBT patients in rural and Appalachian Tennessee. Patient Educ Couns. 2019;102:2081-2090. doi: 10.1016/j.pec .2019.06.003
- Grasso C, Funk D. Collecting sexual orientation and gender identity (SO/GI) data in electronic health records. The National LGBT Health Education Center. Accessed October 12, 2023. https://fenwayhealth.org/wp-content/uploads /4.-Collecting-SOGI-Data.pdf
- Glossary of terms: LGBTQ. GLAAD website. Accessed October 16, 2023. https://glaad.org /reference/terms.
- LGBTQI+. Social protection and human rights website. Accessed November 2, 2023. https ://socialprotection-humanrights.org/key -issues/disadvantaged-and-vulnerable-groups /lgbtqi/
- Goldberg AE, Manley MH, Ellawala T, et al. Sexuality and sexual identity across the first year of parenthood among male-partnered plurisexual women. Psychol Sex Orientat Gend Divers. 2019;6:75.
- Campbell A, Perales F, Hughes TL, et al. Sexual fluidity and psychological distress: what happens when young women’s sexual identities change? J Health Soc Behav. 2022;63:577-593.
- Gessner M, Bishop MD, Martos A, et al. Sexual minority people’s perspectives of sexual health care: understanding minority stress in sexual health settings. Sex Res Social Policy. 2020;17:607618. doi: 10.1007/s13178-019-00418-9
- Carpenter E. “The health system just wasn’t built for us”: queer cisgender women and gender expansive individuals’ strategies for navigating reproductive health care. Womens Health Issues. 2021;31:478-484. doi: 10.1016 /j.whi.2021.06.004
- Casey LS, Reisner SL, Findling MG, et al. Discrimination in the United States: experiences of lesbian, gay, bisexual, transgender, and queer Americans. Health Serv Res. 2019;54(suppl 2):1454-1466. doi: 10.1111/1475-6773.13229
- Grasso C, Goldhammer H, Brown RJ, et al. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020:142:104245. doi: 10.1016 /j.ijmedinf.2020.104245
- Austin A, Craig SL, D’Souza S, et al. Suicidality among transgender youth: elucidating the role of interpersonal risk factors. J Interpers Violence. 2022;37:NP2696-NP2718. doi: 10.1177 /0886260520915554. Published correction appears in J Interpers Violence. 2020:8862 60520946128.
- Hibbert MP, Hillis A, Brett CE, et al. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. Int J Drug Policy. 2021;93:103187. doi: 10.1016 /j.drugpo.2021.103187
- Azagba S, Shan L, Latham K. Overweight and obesity among sexual minority adults in the United States. Int J Environ Res Public Health. 2019;16:1828. doi: 10.3390/ijerph16101828
- Klein PW, Psihopaidas D, Xavier J, et al. HIVrelated outcome disparities between transgender women living with HIV and cisgender people living with HIV served by the Health Resources and Services Administration’s Ryan White HIV/ AIDS Program: a retrospective study. PLoS Med. 2020;17:e1003125. doi: 10.1371/journal.pmed .1003125
- Jung C, Hunter A, Saleh M, et al. Breaking the binary: how clinicians can ensure everyone receives high quality reproductive health services. Open Access J Contracept. 2023:14:23-39. doi: 10.2147/OAJC.S368621
- Bustamante G, Reiter PL, McRee AL. Cervical cancer screening among sexual minority women: findings from a national survey. Cancer Causes Control. 2021;32:911-917. doi: 10.1007 /s10552-021-01442-0
- Dhillon N, Oliffe JL, Kelly MT, et al. Bridging barriers to cervical cancer screening in transgender men: a scoping review. Am J Mens Health. 2020;14:1557988320925691. doi: 10.1177/1557988320925691
- Stubbe DE. Practicing cultural competence and cultural humility in the care of diverse patients. Focus (Am Psychiatr Publ). 2020;18:49-51. doi: 10.1176/appi.focus.20190041
- Alpert A, Kamen C, Schabath MB, et al. What exactly are we measuring? Evaluating sexual and gender minority cultural humility training for oncology care clinicians. J Clin Oncol. 2020;38:2605-2609. doi: 10.1200/JCO.19.03300
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi: 10.1016 /j.apnr.2013.06.008
- Nagle-Yang S, Sachdeva J, Zhao LX, et al. Traumainformed care for obstetric and gynecologic settings. Matern Child Health J. 2022;26:2362-2369.
Announcement from the publisher
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
Dear OBG Management Reader:
Frontline Medical Communications Inc has made the difficult decision to discontinue publication of
The online archive of clinical content for
For the latest news and information on obstetrics and gynecology, continue to turn to MDedge ObGyn.
Goodbye to OBG Management
Robert L. Barbieri, MD
OBG
Over 4 decades, the work of the
Our editorial board members are nationally recognized experts in our field and innovators in clinical care. Our editorial members include: Arnold P. Advincula, MD; Linda D. Bradley, MD; Amy L. Garcia, MD; Steven R. Goldstein, MD, MSCP, CCD; Andrew M. Kaunitz, MD, MSCP; Barbara Levy, MD; David G. Mutch, MD; Errol R. Norwitz, MD, PhD, MBA; Jaimey Pauli, MD; JoAnn V. Pinkerton, MD, MSCP; Joseph S. Sanfilippo, MD; and James A. Simon, MD, CCD, IF, MSCP. Prior to his retirement, Dr. John Repke was an important member of our editorial board. Over the past decade our editorial team—Lila O’Connor, Editorial Manager, and Kathy Christie, Senior Medical Content Editor—have ensured that the articles written by our authors are expertly prepared for publication and presentation to our readers.
In clinical practice, we sometimes do not achieve the optimal patient outcomes we desire. Over the past 4 decades, the
The clinical utility of newly approved angiogenic markers for identifying patients at risk for adverse outcomes due to preeclampsia
In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.

In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The

In the United States there is an epidemic of hypertensive disorders in pregnancy, with 16% of pregnant people being diagnosed with preeclampsia, gestational hypertension, chronic hypertension, preeclampsia superimposed on chronic hypertension, HELLP, or eclampsia.1 Preeclampsia with severe features increases the maternal risk for stroke, pulmonary edema, kidney injury, abruption, and fetal and maternal death. Preeclampsia also increases the fetal risk for growth restriction, oligohydramnios, and preterm birth.
Angiogenic factors and the pathophysiology of preeclampsia—From bench to bedside
The pathophysiology of preeclampsia is not fully characterized, but a leading theory is that placental ischemia causes increased placental production of anti-angiogenesis factors and a decrease in placental production of pro-angiogenesis factors.2-4 Clinical studies support the theory that preeclampsia is associated with an increase in placental production of anti-angiogenesis factors, including soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, and a decrease in the placental production of pro-angiogenesis factors, including placental growth factor (PlGF).5-15
The US Food and Drug Administration (FDA) has recently approved an assay for the measurement of sFlt-1 (Brahms sFlt-1 Kryptor) and PlGF (Brahms sFlt-1 Kryptor) (Thermo Fisher Scientific; Waltham, Massachusetts).16 This editorial focuses on the current and evolving indications for the measurement of sFlt-1 and PlGF in obstetric practice.
FDA approval of a preeclampsia blood test
The FDA approval of the tests to measure sFlt-1 and PlGF is narrowly tailored and focused on using the sFlt-1/PlGF ratio to assess the risk of progression to preeclampsia with severe features within 2 weeks among hospitalized patients with a hypertensive disorder in pregnancy with a singleton pregnancy between 23 weeks 0 days (23w0d) and 34w6d gestation.16 The test is meant to be used in conjunction with other laboratory tests and clinical assessment. The FDA advises that the test results should not be used to diagnose preeclampsia, nor should they be used to determine the timing of delivery or timing of patient discharge.16 The sFlt-1 and PIGF measurements are both reported as pg/mL, and the sFlt-1/PlGF ratio has no units.
The FDA approval is based on clinical studies that demonstrate the effectiveness of the test in predicting the progression of a hypertensive disorder in pregnancy to preeclampsia with severe features within 2 weeks of testing. In one study, the sFlt-1/PlGF ratio was measured in 556 pregnant patients with a singleton pregnancy who were between 23w0d and 34w6d gestation and hospitalized with a hypertensivedisorder in pregnancy without severe features at study enrollment.15 Those patients receiving intravenous heparin were excluded because of the effect of heparin on sFlt-1 levels. Participants’ mean age was 31.7 years, and their mean gestational age was 30w3d. The patients’ mean body mass index (BMI) was 34.2 kg/m2, with mean maximal blood pressure (BP) at enrollment of 159 mm Hg systolic and 95 mm Hg diastolic.
In this cohort, 31% of enrolled patients progressed to preeclampsia with severe features within 2 weeks. At enrollment, the median sFlt-1/PlGF ratio was greater among the patients who progressed to preeclampsia with severe features than among those who did not have progression to preeclampsia with severe features (291 vs 7). An elevated sFlt-1/PlGF ratio (determined to be a ratio ≥ 40) predicted that patients would progress to severe preeclampsiawith severe features—with positive and negative predictive values of 65% and 96%, respectively. Among the subgroup of patients with a history of chronic hypertension, an sFlt-1/PlGF ratio ≥ 40 had positive and negative predictive values of 59% and 94%, respectively. Focusing the analysis on patients who self-reported their race as Black, representing 30% of the cohort, the positive and negative predictive values for a sFlt-1/PlGF ratio ≥ 40 were 66% and 99%, respectively.15
Receiver-operating curve analyses were used to compare the predictive performance of sFlt-1/PlGF measurement versus standard clinical factors and standard laboratory results, including systolic and diastolic BP; levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine; and platelet count.15 The area under the curve for predicting progression to preeclampsia with severe features was much greater for the sFlt-1/PlGF test (0.92) than for systolic (0.67) and diastolic BP (0.70), AST level (0.66), ALT level (0.61), creatinine level (0.65), and platelet count (0.57).15 These results demonstrate that measuring sFlt-1/PlGF ratios is a much better way to predict the progression of preeclampsia to severe disease than measuring standard clinical and laboratory results.
Patients with a sFlt-1/PlGF ratio ≥ 40 had higher rates of adverse maternal outcomes including severe hypertension, abruption, stroke, eclampsia, pulmonary edema, thrombocytopenia, low platelets, and/or coagulation disorder, than those patients with a ratio < 40, (16.1% vs 2.8%, respectively; relative risk [RR], 5.8; 95% confidence interval [CI], 2.8 to 12.2).15 Adverse fetal and neonatal outcomes (including fetal death, small for gestational age and early delivery due to progression of disease) were more common among patients with a sFlt-1/PlGF ratio of ≥ 40 (80% vs 26%; RR, 3.1; 95% CI, 2.5–3.8).15 Many other studies support the hypothesis that the sFlt-1/PlGF ratio is predictive of adverse outcomes among patients with hypertensive disorders in pregnancy.6-15
Applying the bottom-line study findings. Patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF ratio < 40 have a low risk of progressing to preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 96%. The remarkably high negative predictive value of a sFlt-1/PlGF ratio < 40 will help obstetricians generate a care plan that optimizes the use of limited health care resources. Conversely, about two-thirds of patients with a hypertensive disorder in pregnancy and a sFlt-1/PlGF test ≥ 40 will progress to preeclampsia with severe features and may need to prepare for a preterm delivery.
Continue to: Clinical utility of the sFlt-1/PlGF ratio in obstetric triage...
Clinical utility of the sFlt-1/PlGF ratio in obstetric triage
Measurement of the sFlt-1/PlGF ratio may help guide clinical care among patients referred to obstetric triage or admitted to the hospital for the evaluation of suspected preeclampsia. In one study, 402 patients with a singleton pregnancy referred to the hospital for evaluation of suspected preeclampsia, had a standard evaluation plus measurement of an sFlt-1/PlGF ratio.13 The clinicians caring for the patients did not have access to the sFlt-1/PlGF test results. In this cohort, 16% of the patients developed preeclampsia with severe features in the 2 weeks following the initial assessment in triage. In this cohort, a normal sFlt-1/PlGF ratio reliably predicted which patients were not going to develop preeclampsia with severe features over the following 2 weeks, with a negative predictive value of 98%. Among the patients with an elevated sFlt-1/PlGF ratio, however, the positive predictive value of the test was 47% for developing preeclampsia with severe features within the 2 weeks following initial evaluation. Among patients < 34 weeks’ gestation, an elevated sFlt-1/PlGF ratio had a positive predictive value of 65%, and a negative predictive value of 98%. Other studies also have reported that the sFlt-1/PlGF ratio is of value for assessing the risk for progression to preeclampsia with severe features in patients being evaluated for suspected preeclampsia.6,17,18
In obstetric triage, it is difficult to predict the clinical course of patients referred for the evaluation of suspected preeclampsia based on BP measurements or standard laboratory tests. The sFlt-1/PlGF test will help clinicians identify patients at low and high risk of progressing to preeclampsia with severe features.19 Patients with a normal sFlt-1/PlGF test are at low risk of developing preeclampsia with severe features over the following 2 weeks. Patients with an elevated sFlt-1/PlGF test are at higher risk of progressing to preeclampsia with severe features and may warrant more intensive obstetric care. An enhanced care program might include:
- patient education
- remote monitoring of BP or hospitalization
- more frequent assessment of fetal well-being and growth
- administration of glucocorticoids to advance fetal maturity, if indicated by the gestational age.
Twin pregnancy complicated by preeclampsia
Twin pregnancy is associated with a high risk of developing preeclampsia and fetal growth restriction. For patients with a twin pregnancy and a hypertensive disorder in pregnancy, an elevated sFlt-1/PlGF ratio is associated with the need for delivery within 2 weeks and an increased rate of adverse maternal and neonatal outcomes. In a retrospective study involving 164 patients with twin pregnancy first evaluated for suspected preeclampsia at a median gestational age of 33w4d, the sFlt-1/PlGF ratio was positively correlated with progression of preeclampsia without severe features to severe features within 2 weeks.20 In this cohort, at the initial evaluation for suspected preeclampsia, the sFlt-1/PlGF ratio was lower among patients who did not need delivery within 2 weeks compared with those who were delivered within 2 weeks, 24 versus 84 (P<.001). The mean sFlt-1/PlGF ratio was 99 among patients who needed delivery within 1 week following the initial evaluation for suspected preeclampsia. Among patients who delivered within 1 week of presentation, the reasons for delivery were the development of severe hypertension, severe dyspnea, placental abruption, rising levels of serum liver function enzymes, and/or onset of the HELLP syndrome.
An important finding in this study is that a normal
The sFlt-1/PlGF test is a welcome addition to OB care
FDA approval of laboratory tests to measure circulating levels of sFlt-1 and PlGF will advance obstetric practice by identifying patients with a hypertensive disorder in pregnancy who are at low and high risk of developing preeclampsia with severe features within 2 weeks of the test. No laboratory test can replace the clinical judgment of obstetricians who are responsible for balancing the maternal and fetal risks that can occur in the management of a patient with a hypertensive disorder in pregnancy. The
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization-United States 2017-2019. Morb Mortal Week Report. 2022;71:585-591.
- Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838-4445.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges and perspectives. Circ Res. 2019;124:1094-1112.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022(2S):S1019-S1034.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
- Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/ anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187-1207.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911-919.
- Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651-2657.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/ PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1-e8.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346-352.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1322.
- Duckworth S, Griffin M, Seed PT, et al. Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol. 2016;128:245-252.
- Rana S, Salahuddin S, Mueller A, et al. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hyertens. 2018;13:100-106.
- Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1/PlGF ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164-172.
- Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med Evidence. 2022. doi 10.1056/EVIDoa2200161.
- US Food and Drug Administration. FDA approval letter for an assay to measure sFlt-1 and PlGF. May 18, 2023. https://www.accessdata.fda.gov/cdrh _docs/pdf22/DEN220027.pdf
- Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/ anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132-144.
- Palomaki GE, Haddow JE, Haddow HR, et al. Modeling risk for severe adverse outcomes using angiogenic factor measurements in women with suspected preterm preeclampsia. Prenat Diagn. 2015;35:386-393.
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hyper. 2022;27:42-50.
- Binder J, Palmrich P, Pateisky P, et al. The prognostic value of angiogenic markers in twin pregnancies to predict delivery due to maternal complications of preeclampsia. Hypertension. 2020;76:176-183.
- Sapantzoglou I, Rouvali A, Koutras A, et al. sFlt-1, PlGF, the sFlt-1/PlGF ratio and their association with pre-eclampsia in twin pregnancies- a review of the literature. Medicina. 2023;59:1232.
- Satorres E, Martinez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514.
- Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451-458.
Answering the unknowns of taxanes for breast cancer during pregnancy
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
FROM SABCS 2023
Specific personality traits may influence dementia risk
TOPLINE:
, whereas those who score highly for neuroticism and have a negative outlook may be at increased risk, new research suggests.
METHODOLOGY:
- Researchers examined the link between the “big five” personality traits (conscientiousness, extraversion, openness to experience, neuroticism, and agreeableness) and subjective well-being (positive and negative affect and life satisfaction) and clinical symptoms of dementia (cognitive test performance) and neuropathology at autopsy.
- Data for the meta-analysis came from eight longitudinal studies with 44,531 adults (aged 49-81 years at baseline; 26%-61% women) followed for up to 21 years, during which 1703 incident cases of dementia occurred.
- Bayesian multilevel models tested whether personality traits and subjective well-being differentially predicted neuropsychological and neuropathologic characteristics of dementia.
TAKEAWAY:
- High neuroticism, negative affect, and low conscientiousness were risk factors for dementia, whereas conscientiousness, extraversion, and positive affect were protective.
- Across all analyses, there was directional consistency in estimates across samples, which is noteworthy given between-study differences in sociodemographic and design characteristics.
- No consistent associations were found between psychological factors and neuropathology.
- However, individuals higher in conscientiousness who did not receive a clinical diagnosis tended to have a lower Braak stage at autopsy, suggesting the possibility that conscientiousness is related to cognitive resilience.
IN PRACTICE:
“These results replicate and extend evidence that personality traits may assist in early identification and dementia-care planning strategies, as well as risk stratification for dementia diagnosis. Moreover, our findings provide further support for recommendations to incorporate psychological trait measures into clinical screening or diagnosis criteria,” the authors write. SOURCE:
The study, with first author Emorie Beck, PhD, Department of Psychology, University of California, Davis, was published online on November 29, 2023, in Alzheimer’s & Dementia.
LIMITATIONS:
Access to autopsy data was limited. The findings may not generalize across racial groups. The analysis did not examine dynamic associations between changing personality and cognition and neuropathology over time.
DISCLOSURES:
The study was supported by grants from the National Institute on Aging. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, whereas those who score highly for neuroticism and have a negative outlook may be at increased risk, new research suggests.
METHODOLOGY:
- Researchers examined the link between the “big five” personality traits (conscientiousness, extraversion, openness to experience, neuroticism, and agreeableness) and subjective well-being (positive and negative affect and life satisfaction) and clinical symptoms of dementia (cognitive test performance) and neuropathology at autopsy.
- Data for the meta-analysis came from eight longitudinal studies with 44,531 adults (aged 49-81 years at baseline; 26%-61% women) followed for up to 21 years, during which 1703 incident cases of dementia occurred.
- Bayesian multilevel models tested whether personality traits and subjective well-being differentially predicted neuropsychological and neuropathologic characteristics of dementia.
TAKEAWAY:
- High neuroticism, negative affect, and low conscientiousness were risk factors for dementia, whereas conscientiousness, extraversion, and positive affect were protective.
- Across all analyses, there was directional consistency in estimates across samples, which is noteworthy given between-study differences in sociodemographic and design characteristics.
- No consistent associations were found between psychological factors and neuropathology.
- However, individuals higher in conscientiousness who did not receive a clinical diagnosis tended to have a lower Braak stage at autopsy, suggesting the possibility that conscientiousness is related to cognitive resilience.
IN PRACTICE:
“These results replicate and extend evidence that personality traits may assist in early identification and dementia-care planning strategies, as well as risk stratification for dementia diagnosis. Moreover, our findings provide further support for recommendations to incorporate psychological trait measures into clinical screening or diagnosis criteria,” the authors write. SOURCE:
The study, with first author Emorie Beck, PhD, Department of Psychology, University of California, Davis, was published online on November 29, 2023, in Alzheimer’s & Dementia.
LIMITATIONS:
Access to autopsy data was limited. The findings may not generalize across racial groups. The analysis did not examine dynamic associations between changing personality and cognition and neuropathology over time.
DISCLOSURES:
The study was supported by grants from the National Institute on Aging. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, whereas those who score highly for neuroticism and have a negative outlook may be at increased risk, new research suggests.
METHODOLOGY:
- Researchers examined the link between the “big five” personality traits (conscientiousness, extraversion, openness to experience, neuroticism, and agreeableness) and subjective well-being (positive and negative affect and life satisfaction) and clinical symptoms of dementia (cognitive test performance) and neuropathology at autopsy.
- Data for the meta-analysis came from eight longitudinal studies with 44,531 adults (aged 49-81 years at baseline; 26%-61% women) followed for up to 21 years, during which 1703 incident cases of dementia occurred.
- Bayesian multilevel models tested whether personality traits and subjective well-being differentially predicted neuropsychological and neuropathologic characteristics of dementia.
TAKEAWAY:
- High neuroticism, negative affect, and low conscientiousness were risk factors for dementia, whereas conscientiousness, extraversion, and positive affect were protective.
- Across all analyses, there was directional consistency in estimates across samples, which is noteworthy given between-study differences in sociodemographic and design characteristics.
- No consistent associations were found between psychological factors and neuropathology.
- However, individuals higher in conscientiousness who did not receive a clinical diagnosis tended to have a lower Braak stage at autopsy, suggesting the possibility that conscientiousness is related to cognitive resilience.
IN PRACTICE:
“These results replicate and extend evidence that personality traits may assist in early identification and dementia-care planning strategies, as well as risk stratification for dementia diagnosis. Moreover, our findings provide further support for recommendations to incorporate psychological trait measures into clinical screening or diagnosis criteria,” the authors write. SOURCE:
The study, with first author Emorie Beck, PhD, Department of Psychology, University of California, Davis, was published online on November 29, 2023, in Alzheimer’s & Dementia.
LIMITATIONS:
Access to autopsy data was limited. The findings may not generalize across racial groups. The analysis did not examine dynamic associations between changing personality and cognition and neuropathology over time.
DISCLOSURES:
The study was supported by grants from the National Institute on Aging. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.