User login
Younger heart disease onset tied to higher dementia risk
TOPLINE:
, with the risk highest — at 36% — if onset is before age 45, results of a large observational study show.
METHODOLOGY:
- The study included 432,667 of the more than 500,000 participants in the UK Biobank, with a mean age of 56.9 years, 50,685 (11.7%) of whom had CHD and 50,445 had data on age at CHD onset.
- Researchers divided participants into three groups according to age at CHD onset (below 45 years, 45-59 years, and 60 years and older), and carried out a propensity score matching analysis.
- Outcomes included all-cause dementia, AD, and VD.
- Covariates included age, sex, race, educational level, body mass index, low-density lipoprotein cholesterol, smoking status, alcohol intake, exercise, depressed mood, hypertension, diabetes, statin use, and apolipoprotein E4 status.
TAKEAWAY:
- During a median follow-up of 12.8 years, researchers identified 5876 cases of all-cause dementia, 2540 cases of AD, and 1220 cases of VD.
- Fully adjusted models showed participants with CHD had significantly higher risks than those without CHD of developing all-cause dementia (hazard ratio [HR], 1.36; 95% CI, 1.28-1.45; P < .001), AD (HR, 1.13; 95% CI, 1.02-1.24; P = .019), and VD (HR, 1.78; 95% CI, 1.56-2.02; P < .001). The higher risk for VD suggests CHD has a more profound influence on neuropathologic changes involved in this dementia type, said the authors.
- Those with CHD diagnosed at a younger age had higher risks of developing dementia (HR per 10-year decrease in age, 1.25; 95% CI, 1.20-1.30 for all-cause dementia, 1.29; 95% CI, 1.20-1.38 for AD, and 1.22; 95% CI, 1.13-1.31 for VD; P for all < .001).
- Propensity score matching analysis showed patients with CHD had significantly higher risks for dementia compared with matched controls, with the highest risk seen in patients diagnosed before age 45 (HR, 2.40; 95% CI, 1.79-3.20; P < .001), followed by those diagnosed between 45 and 59 years (HR, 1.46; 95% CI, 1.32-1.62; P < .001) and at or above 60 years (HR, 1.11; 95% CI, 1.03-1.19; P = .005), with similar results for AD and VD.
IN PRACTICE:
The findings suggest “additional attention should be paid to the cognitive status of patients with CHD, especially the ones diagnosed with CHD at a young age,” the authors conclude, noting that “timely intervention, such as cognitive training, could be implemented once signs of cognitive deteriorations are detected.”
SOURCE:
The study was conducted by Jie Liang, BS, School of Nursing, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, and colleagues. It was published online on November 29, 2023, in the Journal of the American Heart Association.
LIMITATIONS:
As this is an observational study, it can’t conclude a causal relationship. Although the authors adjusted for many potential confounders, unknown risk factors that also contribute to CHD can’t be ruled out. As the study excluded 69,744 participants, selection bias is possible. The study included a mostly White population.
DISCLOSURES:
The study was supported by the National Natural Science Foundation of China, the Non-Profit Central Research Institute Fund of the Chinese Academy of Medical Sciences, and the China Medical Board. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, with the risk highest — at 36% — if onset is before age 45, results of a large observational study show.
METHODOLOGY:
- The study included 432,667 of the more than 500,000 participants in the UK Biobank, with a mean age of 56.9 years, 50,685 (11.7%) of whom had CHD and 50,445 had data on age at CHD onset.
- Researchers divided participants into three groups according to age at CHD onset (below 45 years, 45-59 years, and 60 years and older), and carried out a propensity score matching analysis.
- Outcomes included all-cause dementia, AD, and VD.
- Covariates included age, sex, race, educational level, body mass index, low-density lipoprotein cholesterol, smoking status, alcohol intake, exercise, depressed mood, hypertension, diabetes, statin use, and apolipoprotein E4 status.
TAKEAWAY:
- During a median follow-up of 12.8 years, researchers identified 5876 cases of all-cause dementia, 2540 cases of AD, and 1220 cases of VD.
- Fully adjusted models showed participants with CHD had significantly higher risks than those without CHD of developing all-cause dementia (hazard ratio [HR], 1.36; 95% CI, 1.28-1.45; P < .001), AD (HR, 1.13; 95% CI, 1.02-1.24; P = .019), and VD (HR, 1.78; 95% CI, 1.56-2.02; P < .001). The higher risk for VD suggests CHD has a more profound influence on neuropathologic changes involved in this dementia type, said the authors.
- Those with CHD diagnosed at a younger age had higher risks of developing dementia (HR per 10-year decrease in age, 1.25; 95% CI, 1.20-1.30 for all-cause dementia, 1.29; 95% CI, 1.20-1.38 for AD, and 1.22; 95% CI, 1.13-1.31 for VD; P for all < .001).
- Propensity score matching analysis showed patients with CHD had significantly higher risks for dementia compared with matched controls, with the highest risk seen in patients diagnosed before age 45 (HR, 2.40; 95% CI, 1.79-3.20; P < .001), followed by those diagnosed between 45 and 59 years (HR, 1.46; 95% CI, 1.32-1.62; P < .001) and at or above 60 years (HR, 1.11; 95% CI, 1.03-1.19; P = .005), with similar results for AD and VD.
IN PRACTICE:
The findings suggest “additional attention should be paid to the cognitive status of patients with CHD, especially the ones diagnosed with CHD at a young age,” the authors conclude, noting that “timely intervention, such as cognitive training, could be implemented once signs of cognitive deteriorations are detected.”
SOURCE:
The study was conducted by Jie Liang, BS, School of Nursing, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, and colleagues. It was published online on November 29, 2023, in the Journal of the American Heart Association.
LIMITATIONS:
As this is an observational study, it can’t conclude a causal relationship. Although the authors adjusted for many potential confounders, unknown risk factors that also contribute to CHD can’t be ruled out. As the study excluded 69,744 participants, selection bias is possible. The study included a mostly White population.
DISCLOSURES:
The study was supported by the National Natural Science Foundation of China, the Non-Profit Central Research Institute Fund of the Chinese Academy of Medical Sciences, and the China Medical Board. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, with the risk highest — at 36% — if onset is before age 45, results of a large observational study show.
METHODOLOGY:
- The study included 432,667 of the more than 500,000 participants in the UK Biobank, with a mean age of 56.9 years, 50,685 (11.7%) of whom had CHD and 50,445 had data on age at CHD onset.
- Researchers divided participants into three groups according to age at CHD onset (below 45 years, 45-59 years, and 60 years and older), and carried out a propensity score matching analysis.
- Outcomes included all-cause dementia, AD, and VD.
- Covariates included age, sex, race, educational level, body mass index, low-density lipoprotein cholesterol, smoking status, alcohol intake, exercise, depressed mood, hypertension, diabetes, statin use, and apolipoprotein E4 status.
TAKEAWAY:
- During a median follow-up of 12.8 years, researchers identified 5876 cases of all-cause dementia, 2540 cases of AD, and 1220 cases of VD.
- Fully adjusted models showed participants with CHD had significantly higher risks than those without CHD of developing all-cause dementia (hazard ratio [HR], 1.36; 95% CI, 1.28-1.45; P < .001), AD (HR, 1.13; 95% CI, 1.02-1.24; P = .019), and VD (HR, 1.78; 95% CI, 1.56-2.02; P < .001). The higher risk for VD suggests CHD has a more profound influence on neuropathologic changes involved in this dementia type, said the authors.
- Those with CHD diagnosed at a younger age had higher risks of developing dementia (HR per 10-year decrease in age, 1.25; 95% CI, 1.20-1.30 for all-cause dementia, 1.29; 95% CI, 1.20-1.38 for AD, and 1.22; 95% CI, 1.13-1.31 for VD; P for all < .001).
- Propensity score matching analysis showed patients with CHD had significantly higher risks for dementia compared with matched controls, with the highest risk seen in patients diagnosed before age 45 (HR, 2.40; 95% CI, 1.79-3.20; P < .001), followed by those diagnosed between 45 and 59 years (HR, 1.46; 95% CI, 1.32-1.62; P < .001) and at or above 60 years (HR, 1.11; 95% CI, 1.03-1.19; P = .005), with similar results for AD and VD.
IN PRACTICE:
The findings suggest “additional attention should be paid to the cognitive status of patients with CHD, especially the ones diagnosed with CHD at a young age,” the authors conclude, noting that “timely intervention, such as cognitive training, could be implemented once signs of cognitive deteriorations are detected.”
SOURCE:
The study was conducted by Jie Liang, BS, School of Nursing, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, and colleagues. It was published online on November 29, 2023, in the Journal of the American Heart Association.
LIMITATIONS:
As this is an observational study, it can’t conclude a causal relationship. Although the authors adjusted for many potential confounders, unknown risk factors that also contribute to CHD can’t be ruled out. As the study excluded 69,744 participants, selection bias is possible. The study included a mostly White population.
DISCLOSURES:
The study was supported by the National Natural Science Foundation of China, the Non-Profit Central Research Institute Fund of the Chinese Academy of Medical Sciences, and the China Medical Board. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Case Q: How can I best remove my patient’s difficult-to-find implant?
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
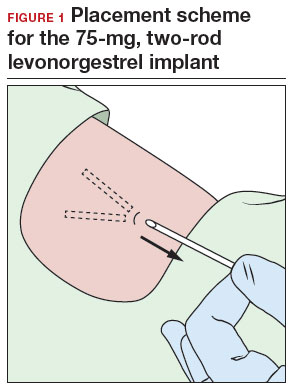
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
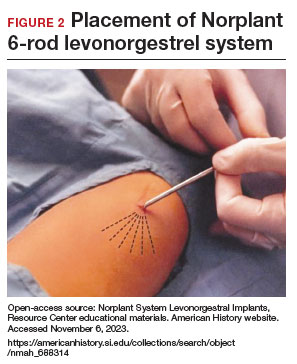
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).
Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
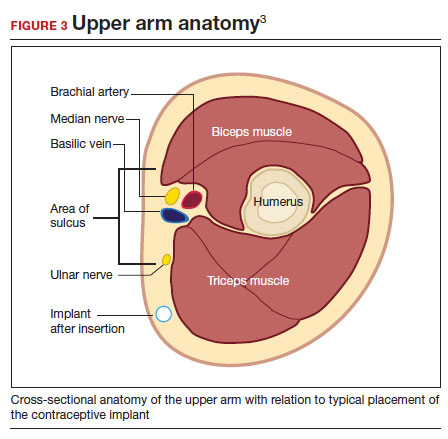
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).
Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
Quotes to live by: Paving the way to personal and professional success
In the first 2 years of medical school, the most common reasons for unsuccessful performance are a deficiency in cognitive knowledge, inefficient time management, and poor study skills. Thereafter, however, the principal reasons for poor performance in training or practice are personality issues and/or unprofessional behavior.
In this article, I review the attributes expected of a physician and the factors that undermine professionalism. I then offer suggestions for smoothing the pathway for personal and professional success. I crafted these suggestions with the “help” of some unlikely medical philosophers. (Note: Some variations of the cited quotations may exist.) I have tempered their guidance with my own personal experiences as a spouse, parent, and grandparent and my professional experiences over almost 50 years, during which I served as a career military officer, student clerkship director, residency program director, fellowship program director, and associate dean for student affairs. I readily acknowledge that, as major league baseball player Yogi Berra reputedly said, “I made too many wrong mistakes,” and that bad experiences are a tough way to ultimately learn good judgment. I hope these suggestions will help you avoid many of my “wrong mistakes.”
High expectations for the medical professional
“To whom much is given, much shall be required.”
—Luke 12:48
Medicine is a higher calling. It is not the usual type of business, and our patients certainly are not just customers or clients. In the unique moment of personal contact, we are asked to put the interest and well-being of our patient above all else. Our patients rightly have high expectations for what type of person their physician should be. The personal strengths expected of a physician include:
- humility
- honesty—personal and fiscal
- integrity
- strong moral compass
- fairness
- responsible
- diligent
- accountable
- insightful
- wise
- technically competent
- perseverant
- sympathetic
- empathetic
- inspiring.
To exhibit all these characteristics consistently is a herculean task and one that is impossible to fulfill. Many factors conspire to undermine our ability to steadfastly be all that we can be. Among these factors are:
- time constraints
- financial pressures
- physical illness
- emotional illness
- the explosion of information technology and scientific knowledge
- bureaucratic inefficiencies.
Therefore, we need to acknowledge with the philosopher Voltaire that “Perfect is the enemy of good.” We need to set our performance bar at excellence, not perfection. If we expect perfection of ourselves, we are destined to be consistently disappointed.
What follows is a series of well-intentioned and good-natured suggestions for keeping ourselves on an even keel, personally and professionally, and maintaining our compass setting on true north.
Continue to: Practical suggestions...
Practical suggestions
“It may not be that the race always goes to the swift nor the battle to the strong, but that is the way to bet.”
—Damon Runyon, journalist
The message is to study hard, work hard, practice our technical skills, and stay on top of our game. We must commit ourselves to a lifetime of learning.
“Chance favors the prepared mind.”
—Louis Pasteur, scientist
One of the best examples of this adage is Alexander Fleming’s “chance” discovery of the bactericidal effect of a mold growing on a culture plate in his laboratory. This observation led to the development of penicillin, an amazing antibiotic that, over the course of the past century, has saved the lives of literally hundreds of thousands of patients. We need to sustain our scientific curiosity throughout our careers and always remain open to new discoveries. Moreover, we need to maintain our capacity for awe and wonder as we consider the exquisite beauty of the scientific world.
“I have a dream.”
—Martin Luther King Jr, civil rights leader
Like Reverend King, we must aspire to a world where civility, peace, and social justice prevail, a world where we embrace diversity and inclusiveness and eschew prejudice, mean-spiritedness, and narrow-mindedness. We must acknowledge that some truths and moral principles are absolute, not relative.
“Once you learn to quit, it becomes a habit.”
—Vince Lombardi, professional football coach
Our lesson: Never quit. We must be fiercely determined to do the right thing, even in troubled and confusing times.
“A pessimist sees the difficulty in every opportunity; an optimist sees the opportunity in every difficulty.”
—Winston Churchill, British prime minister
Until proven wrong, always think the best of everyone. The bright side is far superior to the dark side. We must strive to consistently have a positive attitude and to be part of the solution to a problem, not the problem itself.
“It’s all such a delicate balance.”
—From “It’s a Delicate Balance” by Tom Dundee, folk singer and songwriter
Our top 3 priorities should always be our own emotional and physical well-being, the well-being and security of our loved ones, and the well-being of our patients. The order of these priorities may change, depending upon circumstances. When urgent patient care demands our presence and we miss a birthday celebration, anniversary dinner, soccer game, or dance recital, we need to make certain that, the next time a conflict arises, we arrange to have a colleague cover our clinical or administrative responsibilities.
We must learn to say no when our plate is too full. Failure to say no inevitably leads to life-work imbalance. It is always flattering to be asked to make a presentation, serve on a committee, or prepare a textbook chapter, and it is natural to be concerned that, if we decline, we will not be invited again. However, that concern is unwarranted. Rather, others will respect us for acknowledging when we are too busy and will be grateful that we did not accept an invitation and then miss important deadlines. Conversely, when we do say yes, we need to honor that commitment in a timely manner.
Continue to: The importance of time...
The importance of time
Perhaps the most common complaints that patients have with respect to their interactions with physicians are that they were forced to wait too long and then felt rushed through their appointment. Therefore:
- We must respect our patients’ time and recognize that their time is as valuable as ours.
- We must schedule our patient appointments appropriately and allow different amounts of time depending upon the complexity of a patient’s condition. We should not consistently overschedule. We need to offer a genuine apology when we keep a patient waiting for more than 15 minutes in the absence of an outright emergency that requires our attention elsewhere.
- When we interact with patients, we should sit down, establish eye-to-eye contact, and never appear hurried.
“You don’t make your character in a crisis; you exhibit it.”
—Oren Arnold, journalist and novelist
In the often-chaotic environment of the operating room or the labor and delivery suite, we must be the calm voice of reason at the center of the storm. We should not yell and make demands of others. We must strive to be unflappable. The other members of the team will be appreciative if they recognize that we have a steady hand on the tiller.
“To do good is noble. To teach others to do good is nobler—and less trouble.”
—Mark Twain, humorist
We need to teach our patients about their condition(s) so that they can assume more responsibility for their own care. We also need to teach our students and colleagues so that they can help us provide the best possible care for our patients. Being a good teacher is inherent in being a good physician. As the famous scientist Albert Einstein said, “If you cannot explain it simply, you do not understand it well enough.”
“It ain’t the things you don’t know that get you. It’s the things you think you know that ain’t so.”
—Artemus Ward, humorist
We must constantly strive to practice evidence-based medicine. We should not be the first to embrace the new or the last to give up the old. In medicine, as opposed to the highway, the best place to be is usually in the middle of the road. However, our commitment to evidence-based medicine cannot be absolute. In fact, no more than half of all our present treatment guidelines are based on level 1 evidence. At times, good old-fashioned common sense tempered by years of sobering experience should carry the day.
“We may be lost, but we’re making good time.”
—Yogi Berra, major league baseball player
In my experience, only the minority of mistakes in medicine result from lack of fundamental knowledge or a deficiency in technical skill. Rather, most result from imprudent haste and/or attempts to multitask. Therefore, our lesson is to slow down, concentrate on one task at a time, complete that task, and then refocus on the next challenge.
“The single greatest problem in communication is the illusion that it has taken place.”
—George Bernard Shaw, playwright
We must be sure that we always “close the loop” in our written and verbal communication so that we can avoid misunderstandings that threaten personal relationships and/or patient safety.
“You raise me up so I can stand on mountains.”
—From “You Raise Me Up” as sung by Josh Groban
All of us need a mentor to raise us up. We must choose our mentors carefully and recognize that we may need different mentors at different stages of our career. As we benefit from effective mentoring, we must pay it forward and be a good mentor to others.
“Worrying is a total waste of time. It accomplishes nothing, changes nothing, and robs you of joy. It is like paying a debt that you don’t owe.”
—Mark Twain, humorist
We have to assiduously cultivate the strength of resilience. We must accept that mistakes inevitably will occur and that perfection in practice is simply not possible, despite our best intentions. We then have to learn from these errors and ensure that they never occur again. We need to apologize for our mistakes and move on. If we carry our last strikeout into our next at bat, we are likely doomed to more misfortune.
“Feeling gratitude and not expressing it is like wrapping a present and not giving it.”
—William Arthur Ward, motivational writer
Our lesson is to be keenly aware of the importance of showing gratitude to those around us. The height of our success will depend directly on the depth of our gratitude. The higher we rise in the hierarchy of the medical profession, the more gracious and kind we need to be.
“Kindness is the language which the deaf can hear and the blind can see.”
—Mark Twain, humorist
“Kindness is the only service that will stand the storm of life and not wash out.”
—Abraham Lincoln, American president
There is never an excuse for rudeness or hubris. We should never teach or conduct business by intimidation. The words please, thank you, and I’m sorry should be front and center in our vocabulary. We must learn not to take ourselves too seriously, to remember that the best part of life is the laughter, and to always strive for grace and humility.
“The secret of the care of the patient is in caring for the patient.”
—Francis Peabody, physician
Patients may quickly forget what we say to them or even what we do for them, but they will never forget how we made them feel. Observe intently, listen carefully, talk less. Most people do not listen with the intent to understand. Rather, they listen with the intent to reply. We need to break this pattern by learning to listen with our heart. In fact, the quieter we become, the more we can hear. There is great symbolism in the fact that we have two ears and only one mouth.
“You got to know when to hold ‘em, know when to fold ‘em.”
—From “The Gambler” as sung by Kenny Rogers
Sometimes the best medicine is no medicine at all, but rather a soft shoulder, an open ear, a kind heart, and a compassionate soul.
“Do small things with great love.”
—Mother Teresa, Catholic missionary
The vast majority of us will not rise to lofty political or administrative positions or ever achieve celebrity status. We are unlikely to win the Nobel Prize and unlikely to find the cure for cancer or preeclampsia. However, we can work diligently to complete each small task with precision so that, like a great artist views his or her work, we, too, will want to sign our name to the patient care plan we have created and implemented.
“Earn this.”
—From Saving Private Ryan, a Steven Spielberg movie
At the end of this movie, the mortally wounded infantry captain (played by Tom Hanks) looks up at Private Ryan (played by Matt Damon) and says, “Earn this,” meaning make sure that you live your life in a way to justify the sacrifices so many made to save you. Like Private Ryan, we have to recognize that our MD degree does not constitute a lifetime entitlement to respect and honor. Rather, we have to practice each day so we continue to earn the respect of our patients, students, and colleagues and, so that, with confidence, we can then say to our patients, “How can I be of help to you?” ●
In the first 2 years of medical school, the most common reasons for unsuccessful performance are a deficiency in cognitive knowledge, inefficient time management, and poor study skills. Thereafter, however, the principal reasons for poor performance in training or practice are personality issues and/or unprofessional behavior.
In this article, I review the attributes expected of a physician and the factors that undermine professionalism. I then offer suggestions for smoothing the pathway for personal and professional success. I crafted these suggestions with the “help” of some unlikely medical philosophers. (Note: Some variations of the cited quotations may exist.) I have tempered their guidance with my own personal experiences as a spouse, parent, and grandparent and my professional experiences over almost 50 years, during which I served as a career military officer, student clerkship director, residency program director, fellowship program director, and associate dean for student affairs. I readily acknowledge that, as major league baseball player Yogi Berra reputedly said, “I made too many wrong mistakes,” and that bad experiences are a tough way to ultimately learn good judgment. I hope these suggestions will help you avoid many of my “wrong mistakes.”
High expectations for the medical professional
“To whom much is given, much shall be required.”
—Luke 12:48
Medicine is a higher calling. It is not the usual type of business, and our patients certainly are not just customers or clients. In the unique moment of personal contact, we are asked to put the interest and well-being of our patient above all else. Our patients rightly have high expectations for what type of person their physician should be. The personal strengths expected of a physician include:
- humility
- honesty—personal and fiscal
- integrity
- strong moral compass
- fairness
- responsible
- diligent
- accountable
- insightful
- wise
- technically competent
- perseverant
- sympathetic
- empathetic
- inspiring.
To exhibit all these characteristics consistently is a herculean task and one that is impossible to fulfill. Many factors conspire to undermine our ability to steadfastly be all that we can be. Among these factors are:
- time constraints
- financial pressures
- physical illness
- emotional illness
- the explosion of information technology and scientific knowledge
- bureaucratic inefficiencies.
Therefore, we need to acknowledge with the philosopher Voltaire that “Perfect is the enemy of good.” We need to set our performance bar at excellence, not perfection. If we expect perfection of ourselves, we are destined to be consistently disappointed.
What follows is a series of well-intentioned and good-natured suggestions for keeping ourselves on an even keel, personally and professionally, and maintaining our compass setting on true north.
Continue to: Practical suggestions...
Practical suggestions
“It may not be that the race always goes to the swift nor the battle to the strong, but that is the way to bet.”
—Damon Runyon, journalist
The message is to study hard, work hard, practice our technical skills, and stay on top of our game. We must commit ourselves to a lifetime of learning.
“Chance favors the prepared mind.”
—Louis Pasteur, scientist
One of the best examples of this adage is Alexander Fleming’s “chance” discovery of the bactericidal effect of a mold growing on a culture plate in his laboratory. This observation led to the development of penicillin, an amazing antibiotic that, over the course of the past century, has saved the lives of literally hundreds of thousands of patients. We need to sustain our scientific curiosity throughout our careers and always remain open to new discoveries. Moreover, we need to maintain our capacity for awe and wonder as we consider the exquisite beauty of the scientific world.
“I have a dream.”
—Martin Luther King Jr, civil rights leader
Like Reverend King, we must aspire to a world where civility, peace, and social justice prevail, a world where we embrace diversity and inclusiveness and eschew prejudice, mean-spiritedness, and narrow-mindedness. We must acknowledge that some truths and moral principles are absolute, not relative.
“Once you learn to quit, it becomes a habit.”
—Vince Lombardi, professional football coach
Our lesson: Never quit. We must be fiercely determined to do the right thing, even in troubled and confusing times.
“A pessimist sees the difficulty in every opportunity; an optimist sees the opportunity in every difficulty.”
—Winston Churchill, British prime minister
Until proven wrong, always think the best of everyone. The bright side is far superior to the dark side. We must strive to consistently have a positive attitude and to be part of the solution to a problem, not the problem itself.
“It’s all such a delicate balance.”
—From “It’s a Delicate Balance” by Tom Dundee, folk singer and songwriter
Our top 3 priorities should always be our own emotional and physical well-being, the well-being and security of our loved ones, and the well-being of our patients. The order of these priorities may change, depending upon circumstances. When urgent patient care demands our presence and we miss a birthday celebration, anniversary dinner, soccer game, or dance recital, we need to make certain that, the next time a conflict arises, we arrange to have a colleague cover our clinical or administrative responsibilities.
We must learn to say no when our plate is too full. Failure to say no inevitably leads to life-work imbalance. It is always flattering to be asked to make a presentation, serve on a committee, or prepare a textbook chapter, and it is natural to be concerned that, if we decline, we will not be invited again. However, that concern is unwarranted. Rather, others will respect us for acknowledging when we are too busy and will be grateful that we did not accept an invitation and then miss important deadlines. Conversely, when we do say yes, we need to honor that commitment in a timely manner.
Continue to: The importance of time...
The importance of time
Perhaps the most common complaints that patients have with respect to their interactions with physicians are that they were forced to wait too long and then felt rushed through their appointment. Therefore:
- We must respect our patients’ time and recognize that their time is as valuable as ours.
- We must schedule our patient appointments appropriately and allow different amounts of time depending upon the complexity of a patient’s condition. We should not consistently overschedule. We need to offer a genuine apology when we keep a patient waiting for more than 15 minutes in the absence of an outright emergency that requires our attention elsewhere.
- When we interact with patients, we should sit down, establish eye-to-eye contact, and never appear hurried.
“You don’t make your character in a crisis; you exhibit it.”
—Oren Arnold, journalist and novelist
In the often-chaotic environment of the operating room or the labor and delivery suite, we must be the calm voice of reason at the center of the storm. We should not yell and make demands of others. We must strive to be unflappable. The other members of the team will be appreciative if they recognize that we have a steady hand on the tiller.
“To do good is noble. To teach others to do good is nobler—and less trouble.”
—Mark Twain, humorist
We need to teach our patients about their condition(s) so that they can assume more responsibility for their own care. We also need to teach our students and colleagues so that they can help us provide the best possible care for our patients. Being a good teacher is inherent in being a good physician. As the famous scientist Albert Einstein said, “If you cannot explain it simply, you do not understand it well enough.”
“It ain’t the things you don’t know that get you. It’s the things you think you know that ain’t so.”
—Artemus Ward, humorist
We must constantly strive to practice evidence-based medicine. We should not be the first to embrace the new or the last to give up the old. In medicine, as opposed to the highway, the best place to be is usually in the middle of the road. However, our commitment to evidence-based medicine cannot be absolute. In fact, no more than half of all our present treatment guidelines are based on level 1 evidence. At times, good old-fashioned common sense tempered by years of sobering experience should carry the day.
“We may be lost, but we’re making good time.”
—Yogi Berra, major league baseball player
In my experience, only the minority of mistakes in medicine result from lack of fundamental knowledge or a deficiency in technical skill. Rather, most result from imprudent haste and/or attempts to multitask. Therefore, our lesson is to slow down, concentrate on one task at a time, complete that task, and then refocus on the next challenge.
“The single greatest problem in communication is the illusion that it has taken place.”
—George Bernard Shaw, playwright
We must be sure that we always “close the loop” in our written and verbal communication so that we can avoid misunderstandings that threaten personal relationships and/or patient safety.
“You raise me up so I can stand on mountains.”
—From “You Raise Me Up” as sung by Josh Groban
All of us need a mentor to raise us up. We must choose our mentors carefully and recognize that we may need different mentors at different stages of our career. As we benefit from effective mentoring, we must pay it forward and be a good mentor to others.
“Worrying is a total waste of time. It accomplishes nothing, changes nothing, and robs you of joy. It is like paying a debt that you don’t owe.”
—Mark Twain, humorist
We have to assiduously cultivate the strength of resilience. We must accept that mistakes inevitably will occur and that perfection in practice is simply not possible, despite our best intentions. We then have to learn from these errors and ensure that they never occur again. We need to apologize for our mistakes and move on. If we carry our last strikeout into our next at bat, we are likely doomed to more misfortune.
“Feeling gratitude and not expressing it is like wrapping a present and not giving it.”
—William Arthur Ward, motivational writer
Our lesson is to be keenly aware of the importance of showing gratitude to those around us. The height of our success will depend directly on the depth of our gratitude. The higher we rise in the hierarchy of the medical profession, the more gracious and kind we need to be.
“Kindness is the language which the deaf can hear and the blind can see.”
—Mark Twain, humorist
“Kindness is the only service that will stand the storm of life and not wash out.”
—Abraham Lincoln, American president
There is never an excuse for rudeness or hubris. We should never teach or conduct business by intimidation. The words please, thank you, and I’m sorry should be front and center in our vocabulary. We must learn not to take ourselves too seriously, to remember that the best part of life is the laughter, and to always strive for grace and humility.
“The secret of the care of the patient is in caring for the patient.”
—Francis Peabody, physician
Patients may quickly forget what we say to them or even what we do for them, but they will never forget how we made them feel. Observe intently, listen carefully, talk less. Most people do not listen with the intent to understand. Rather, they listen with the intent to reply. We need to break this pattern by learning to listen with our heart. In fact, the quieter we become, the more we can hear. There is great symbolism in the fact that we have two ears and only one mouth.
“You got to know when to hold ‘em, know when to fold ‘em.”
—From “The Gambler” as sung by Kenny Rogers
Sometimes the best medicine is no medicine at all, but rather a soft shoulder, an open ear, a kind heart, and a compassionate soul.
“Do small things with great love.”
—Mother Teresa, Catholic missionary
The vast majority of us will not rise to lofty political or administrative positions or ever achieve celebrity status. We are unlikely to win the Nobel Prize and unlikely to find the cure for cancer or preeclampsia. However, we can work diligently to complete each small task with precision so that, like a great artist views his or her work, we, too, will want to sign our name to the patient care plan we have created and implemented.
“Earn this.”
—From Saving Private Ryan, a Steven Spielberg movie
At the end of this movie, the mortally wounded infantry captain (played by Tom Hanks) looks up at Private Ryan (played by Matt Damon) and says, “Earn this,” meaning make sure that you live your life in a way to justify the sacrifices so many made to save you. Like Private Ryan, we have to recognize that our MD degree does not constitute a lifetime entitlement to respect and honor. Rather, we have to practice each day so we continue to earn the respect of our patients, students, and colleagues and, so that, with confidence, we can then say to our patients, “How can I be of help to you?” ●
In the first 2 years of medical school, the most common reasons for unsuccessful performance are a deficiency in cognitive knowledge, inefficient time management, and poor study skills. Thereafter, however, the principal reasons for poor performance in training or practice are personality issues and/or unprofessional behavior.
In this article, I review the attributes expected of a physician and the factors that undermine professionalism. I then offer suggestions for smoothing the pathway for personal and professional success. I crafted these suggestions with the “help” of some unlikely medical philosophers. (Note: Some variations of the cited quotations may exist.) I have tempered their guidance with my own personal experiences as a spouse, parent, and grandparent and my professional experiences over almost 50 years, during which I served as a career military officer, student clerkship director, residency program director, fellowship program director, and associate dean for student affairs. I readily acknowledge that, as major league baseball player Yogi Berra reputedly said, “I made too many wrong mistakes,” and that bad experiences are a tough way to ultimately learn good judgment. I hope these suggestions will help you avoid many of my “wrong mistakes.”
High expectations for the medical professional
“To whom much is given, much shall be required.”
—Luke 12:48
Medicine is a higher calling. It is not the usual type of business, and our patients certainly are not just customers or clients. In the unique moment of personal contact, we are asked to put the interest and well-being of our patient above all else. Our patients rightly have high expectations for what type of person their physician should be. The personal strengths expected of a physician include:
- humility
- honesty—personal and fiscal
- integrity
- strong moral compass
- fairness
- responsible
- diligent
- accountable
- insightful
- wise
- technically competent
- perseverant
- sympathetic
- empathetic
- inspiring.
To exhibit all these characteristics consistently is a herculean task and one that is impossible to fulfill. Many factors conspire to undermine our ability to steadfastly be all that we can be. Among these factors are:
- time constraints
- financial pressures
- physical illness
- emotional illness
- the explosion of information technology and scientific knowledge
- bureaucratic inefficiencies.
Therefore, we need to acknowledge with the philosopher Voltaire that “Perfect is the enemy of good.” We need to set our performance bar at excellence, not perfection. If we expect perfection of ourselves, we are destined to be consistently disappointed.
What follows is a series of well-intentioned and good-natured suggestions for keeping ourselves on an even keel, personally and professionally, and maintaining our compass setting on true north.
Continue to: Practical suggestions...
Practical suggestions
“It may not be that the race always goes to the swift nor the battle to the strong, but that is the way to bet.”
—Damon Runyon, journalist
The message is to study hard, work hard, practice our technical skills, and stay on top of our game. We must commit ourselves to a lifetime of learning.
“Chance favors the prepared mind.”
—Louis Pasteur, scientist
One of the best examples of this adage is Alexander Fleming’s “chance” discovery of the bactericidal effect of a mold growing on a culture plate in his laboratory. This observation led to the development of penicillin, an amazing antibiotic that, over the course of the past century, has saved the lives of literally hundreds of thousands of patients. We need to sustain our scientific curiosity throughout our careers and always remain open to new discoveries. Moreover, we need to maintain our capacity for awe and wonder as we consider the exquisite beauty of the scientific world.
“I have a dream.”
—Martin Luther King Jr, civil rights leader
Like Reverend King, we must aspire to a world where civility, peace, and social justice prevail, a world where we embrace diversity and inclusiveness and eschew prejudice, mean-spiritedness, and narrow-mindedness. We must acknowledge that some truths and moral principles are absolute, not relative.
“Once you learn to quit, it becomes a habit.”
—Vince Lombardi, professional football coach
Our lesson: Never quit. We must be fiercely determined to do the right thing, even in troubled and confusing times.
“A pessimist sees the difficulty in every opportunity; an optimist sees the opportunity in every difficulty.”
—Winston Churchill, British prime minister
Until proven wrong, always think the best of everyone. The bright side is far superior to the dark side. We must strive to consistently have a positive attitude and to be part of the solution to a problem, not the problem itself.
“It’s all such a delicate balance.”
—From “It’s a Delicate Balance” by Tom Dundee, folk singer and songwriter
Our top 3 priorities should always be our own emotional and physical well-being, the well-being and security of our loved ones, and the well-being of our patients. The order of these priorities may change, depending upon circumstances. When urgent patient care demands our presence and we miss a birthday celebration, anniversary dinner, soccer game, or dance recital, we need to make certain that, the next time a conflict arises, we arrange to have a colleague cover our clinical or administrative responsibilities.
We must learn to say no when our plate is too full. Failure to say no inevitably leads to life-work imbalance. It is always flattering to be asked to make a presentation, serve on a committee, or prepare a textbook chapter, and it is natural to be concerned that, if we decline, we will not be invited again. However, that concern is unwarranted. Rather, others will respect us for acknowledging when we are too busy and will be grateful that we did not accept an invitation and then miss important deadlines. Conversely, when we do say yes, we need to honor that commitment in a timely manner.
Continue to: The importance of time...
The importance of time
Perhaps the most common complaints that patients have with respect to their interactions with physicians are that they were forced to wait too long and then felt rushed through their appointment. Therefore:
- We must respect our patients’ time and recognize that their time is as valuable as ours.
- We must schedule our patient appointments appropriately and allow different amounts of time depending upon the complexity of a patient’s condition. We should not consistently overschedule. We need to offer a genuine apology when we keep a patient waiting for more than 15 minutes in the absence of an outright emergency that requires our attention elsewhere.
- When we interact with patients, we should sit down, establish eye-to-eye contact, and never appear hurried.
“You don’t make your character in a crisis; you exhibit it.”
—Oren Arnold, journalist and novelist
In the often-chaotic environment of the operating room or the labor and delivery suite, we must be the calm voice of reason at the center of the storm. We should not yell and make demands of others. We must strive to be unflappable. The other members of the team will be appreciative if they recognize that we have a steady hand on the tiller.
“To do good is noble. To teach others to do good is nobler—and less trouble.”
—Mark Twain, humorist
We need to teach our patients about their condition(s) so that they can assume more responsibility for their own care. We also need to teach our students and colleagues so that they can help us provide the best possible care for our patients. Being a good teacher is inherent in being a good physician. As the famous scientist Albert Einstein said, “If you cannot explain it simply, you do not understand it well enough.”
“It ain’t the things you don’t know that get you. It’s the things you think you know that ain’t so.”
—Artemus Ward, humorist
We must constantly strive to practice evidence-based medicine. We should not be the first to embrace the new or the last to give up the old. In medicine, as opposed to the highway, the best place to be is usually in the middle of the road. However, our commitment to evidence-based medicine cannot be absolute. In fact, no more than half of all our present treatment guidelines are based on level 1 evidence. At times, good old-fashioned common sense tempered by years of sobering experience should carry the day.
“We may be lost, but we’re making good time.”
—Yogi Berra, major league baseball player
In my experience, only the minority of mistakes in medicine result from lack of fundamental knowledge or a deficiency in technical skill. Rather, most result from imprudent haste and/or attempts to multitask. Therefore, our lesson is to slow down, concentrate on one task at a time, complete that task, and then refocus on the next challenge.
“The single greatest problem in communication is the illusion that it has taken place.”
—George Bernard Shaw, playwright
We must be sure that we always “close the loop” in our written and verbal communication so that we can avoid misunderstandings that threaten personal relationships and/or patient safety.
“You raise me up so I can stand on mountains.”
—From “You Raise Me Up” as sung by Josh Groban
All of us need a mentor to raise us up. We must choose our mentors carefully and recognize that we may need different mentors at different stages of our career. As we benefit from effective mentoring, we must pay it forward and be a good mentor to others.
“Worrying is a total waste of time. It accomplishes nothing, changes nothing, and robs you of joy. It is like paying a debt that you don’t owe.”
—Mark Twain, humorist
We have to assiduously cultivate the strength of resilience. We must accept that mistakes inevitably will occur and that perfection in practice is simply not possible, despite our best intentions. We then have to learn from these errors and ensure that they never occur again. We need to apologize for our mistakes and move on. If we carry our last strikeout into our next at bat, we are likely doomed to more misfortune.
“Feeling gratitude and not expressing it is like wrapping a present and not giving it.”
—William Arthur Ward, motivational writer
Our lesson is to be keenly aware of the importance of showing gratitude to those around us. The height of our success will depend directly on the depth of our gratitude. The higher we rise in the hierarchy of the medical profession, the more gracious and kind we need to be.
“Kindness is the language which the deaf can hear and the blind can see.”
—Mark Twain, humorist
“Kindness is the only service that will stand the storm of life and not wash out.”
—Abraham Lincoln, American president
There is never an excuse for rudeness or hubris. We should never teach or conduct business by intimidation. The words please, thank you, and I’m sorry should be front and center in our vocabulary. We must learn not to take ourselves too seriously, to remember that the best part of life is the laughter, and to always strive for grace and humility.
“The secret of the care of the patient is in caring for the patient.”
—Francis Peabody, physician
Patients may quickly forget what we say to them or even what we do for them, but they will never forget how we made them feel. Observe intently, listen carefully, talk less. Most people do not listen with the intent to understand. Rather, they listen with the intent to reply. We need to break this pattern by learning to listen with our heart. In fact, the quieter we become, the more we can hear. There is great symbolism in the fact that we have two ears and only one mouth.
“You got to know when to hold ‘em, know when to fold ‘em.”
—From “The Gambler” as sung by Kenny Rogers
Sometimes the best medicine is no medicine at all, but rather a soft shoulder, an open ear, a kind heart, and a compassionate soul.
“Do small things with great love.”
—Mother Teresa, Catholic missionary
The vast majority of us will not rise to lofty political or administrative positions or ever achieve celebrity status. We are unlikely to win the Nobel Prize and unlikely to find the cure for cancer or preeclampsia. However, we can work diligently to complete each small task with precision so that, like a great artist views his or her work, we, too, will want to sign our name to the patient care plan we have created and implemented.
“Earn this.”
—From Saving Private Ryan, a Steven Spielberg movie
At the end of this movie, the mortally wounded infantry captain (played by Tom Hanks) looks up at Private Ryan (played by Matt Damon) and says, “Earn this,” meaning make sure that you live your life in a way to justify the sacrifices so many made to save you. Like Private Ryan, we have to recognize that our MD degree does not constitute a lifetime entitlement to respect and honor. Rather, we have to practice each day so we continue to earn the respect of our patients, students, and colleagues and, so that, with confidence, we can then say to our patients, “How can I be of help to you?” ●
Women over 50 may safely de-escalate mammogram frequency following surgery
SAN ANTONIO – , according to results from a new randomized trial.
In the UK study, researchers found similar recurrence rates and overall survival between patients who continued to undergo annual screening versus women who underwent screening every 2 years after breast-conserving surgery or every 3 years after a mastectomy.
Current US guidelines recommend annual screening with no stopping point, while UK guidelines recommend annual screening for 5 years, followed by every 3 years after that.
The study was commissioned by the UK government after a previously commissioned systematic review showed lack of evidence for the existing frequency and duration of mammograms in this patient population, and a survey showed wide-ranging clinical practices. In response, the UK government funded the new study to evaluate whether it would be safe to reduce screening, according to Janet Dunn, PhD, who presented the study at the San Antonio Breast Cancer Symposium.
“Ladies are going back for years and years and years [for mammograms], and it’s inappropriate, it’s not necessary,” Dr. Dunn, professor of clinical trials and head of cancer trials at University of Warwick in the UK, said in an interview.
The lower frequency schedule requires that the patient be cancer free at 3 years following curative treatment. “We know for anybody going through breast cancer there are a couple of peaks of recurrence. One peak is 2 to 3 years [after curative treatment] for high-risk patients. The other peak is when they start hormone therapy, so at 5 to 6 years you get the peak. But this particular set of patients are at low to moderate risk, so they’re not the high-risk patients going into treatment trials,” said Dr. Dunn.
When asked if the findings should change practice, Dr. Dunn suggested that they could. “We would say that this is providing clinical evidence and probably changing guidance for the management of these patients: Instead of giving annual mammograms for years and years and years, after three years post curative surgery, and having gone through treatment with a baseline mammogram that’s clear, in the UK we can manage these patients back within the screening program, certainly for the mastectomy patients,” said Dr. Dunn.
She emphasized the potential mental health impact. “Women who are going through mammograms waiting for the results are much more anxious if they’ve had breast cancer before than those who were who were just going through normal screening. So [deescalation] is reducing anxiety. It’s also reducing the cost. It’s just reducing the burden on the whole health care system,” said Dr. Dunn.
During the Q&A session after the talk, Bruce Mann, MBBS, PhD, asked if there were differences in the pathology of the cancers that occurred in the less frequent group than those that arose in the annual group. If more frequent screening leads to earlier diagnosis of a tumor, “you would expect that those who have less [frequent screening] may be diagnosed with more advanced recurrences or new cancers, and that would eventually lead to a difference in outcome,” Dr. Mann said in an interview. He is director of breast cancer services at the Royal Melbourne Hospital in Australia.
He acknowledged the need to reconsider screening frequency, and complimented the researchers. “This is a nice pragmatic study. It’s interesting. It’s provocative. I think it’s a bit too early [to change practice]. I think we do need to see more information,” he said.
The researchers randomized 5235 women aged 50 and older to annual or less frequent mammography. The disease type was invasive in 87% of women, while 13% had ductal carcinoma in situ.
Over a median follow-up of 8.7 years, 7% experienced a recurrence. At 5 years, breast cancer–specific survival was 98.1% and 98.3% in the annual and less frequent groups, respectively (hazard ratio [HR], 0.92; 95% CI, 0.64-1.32). There was also no difference in recurrence-free survival or overall survival.
Analyses showed noninferiority of less frequent mammograms at a 3% margin (P < .0001) and a 1% margin (P = .003).
The researchers found that 83% of women were compliant with the mammogram schedule in the annual group, versus 69% in the less frequent arm. The COVID-19 pandemic was responsible for 35% of missed mammograms overall.
Quality of life measures showed that levels of distress over mammograms was similar across time and between the two groups, with 24% of women reporting medium or high levels of distress.
Dr. Dunn and Dr. Mann have no relevant financial disclosures.
SAN ANTONIO – , according to results from a new randomized trial.
In the UK study, researchers found similar recurrence rates and overall survival between patients who continued to undergo annual screening versus women who underwent screening every 2 years after breast-conserving surgery or every 3 years after a mastectomy.
Current US guidelines recommend annual screening with no stopping point, while UK guidelines recommend annual screening for 5 years, followed by every 3 years after that.
The study was commissioned by the UK government after a previously commissioned systematic review showed lack of evidence for the existing frequency and duration of mammograms in this patient population, and a survey showed wide-ranging clinical practices. In response, the UK government funded the new study to evaluate whether it would be safe to reduce screening, according to Janet Dunn, PhD, who presented the study at the San Antonio Breast Cancer Symposium.
“Ladies are going back for years and years and years [for mammograms], and it’s inappropriate, it’s not necessary,” Dr. Dunn, professor of clinical trials and head of cancer trials at University of Warwick in the UK, said in an interview.
The lower frequency schedule requires that the patient be cancer free at 3 years following curative treatment. “We know for anybody going through breast cancer there are a couple of peaks of recurrence. One peak is 2 to 3 years [after curative treatment] for high-risk patients. The other peak is when they start hormone therapy, so at 5 to 6 years you get the peak. But this particular set of patients are at low to moderate risk, so they’re not the high-risk patients going into treatment trials,” said Dr. Dunn.
When asked if the findings should change practice, Dr. Dunn suggested that they could. “We would say that this is providing clinical evidence and probably changing guidance for the management of these patients: Instead of giving annual mammograms for years and years and years, after three years post curative surgery, and having gone through treatment with a baseline mammogram that’s clear, in the UK we can manage these patients back within the screening program, certainly for the mastectomy patients,” said Dr. Dunn.
She emphasized the potential mental health impact. “Women who are going through mammograms waiting for the results are much more anxious if they’ve had breast cancer before than those who were who were just going through normal screening. So [deescalation] is reducing anxiety. It’s also reducing the cost. It’s just reducing the burden on the whole health care system,” said Dr. Dunn.
During the Q&A session after the talk, Bruce Mann, MBBS, PhD, asked if there were differences in the pathology of the cancers that occurred in the less frequent group than those that arose in the annual group. If more frequent screening leads to earlier diagnosis of a tumor, “you would expect that those who have less [frequent screening] may be diagnosed with more advanced recurrences or new cancers, and that would eventually lead to a difference in outcome,” Dr. Mann said in an interview. He is director of breast cancer services at the Royal Melbourne Hospital in Australia.
He acknowledged the need to reconsider screening frequency, and complimented the researchers. “This is a nice pragmatic study. It’s interesting. It’s provocative. I think it’s a bit too early [to change practice]. I think we do need to see more information,” he said.
The researchers randomized 5235 women aged 50 and older to annual or less frequent mammography. The disease type was invasive in 87% of women, while 13% had ductal carcinoma in situ.
Over a median follow-up of 8.7 years, 7% experienced a recurrence. At 5 years, breast cancer–specific survival was 98.1% and 98.3% in the annual and less frequent groups, respectively (hazard ratio [HR], 0.92; 95% CI, 0.64-1.32). There was also no difference in recurrence-free survival or overall survival.
Analyses showed noninferiority of less frequent mammograms at a 3% margin (P < .0001) and a 1% margin (P = .003).
The researchers found that 83% of women were compliant with the mammogram schedule in the annual group, versus 69% in the less frequent arm. The COVID-19 pandemic was responsible for 35% of missed mammograms overall.
Quality of life measures showed that levels of distress over mammograms was similar across time and between the two groups, with 24% of women reporting medium or high levels of distress.
Dr. Dunn and Dr. Mann have no relevant financial disclosures.
SAN ANTONIO – , according to results from a new randomized trial.
In the UK study, researchers found similar recurrence rates and overall survival between patients who continued to undergo annual screening versus women who underwent screening every 2 years after breast-conserving surgery or every 3 years after a mastectomy.
Current US guidelines recommend annual screening with no stopping point, while UK guidelines recommend annual screening for 5 years, followed by every 3 years after that.
The study was commissioned by the UK government after a previously commissioned systematic review showed lack of evidence for the existing frequency and duration of mammograms in this patient population, and a survey showed wide-ranging clinical practices. In response, the UK government funded the new study to evaluate whether it would be safe to reduce screening, according to Janet Dunn, PhD, who presented the study at the San Antonio Breast Cancer Symposium.
“Ladies are going back for years and years and years [for mammograms], and it’s inappropriate, it’s not necessary,” Dr. Dunn, professor of clinical trials and head of cancer trials at University of Warwick in the UK, said in an interview.
The lower frequency schedule requires that the patient be cancer free at 3 years following curative treatment. “We know for anybody going through breast cancer there are a couple of peaks of recurrence. One peak is 2 to 3 years [after curative treatment] for high-risk patients. The other peak is when they start hormone therapy, so at 5 to 6 years you get the peak. But this particular set of patients are at low to moderate risk, so they’re not the high-risk patients going into treatment trials,” said Dr. Dunn.
When asked if the findings should change practice, Dr. Dunn suggested that they could. “We would say that this is providing clinical evidence and probably changing guidance for the management of these patients: Instead of giving annual mammograms for years and years and years, after three years post curative surgery, and having gone through treatment with a baseline mammogram that’s clear, in the UK we can manage these patients back within the screening program, certainly for the mastectomy patients,” said Dr. Dunn.
She emphasized the potential mental health impact. “Women who are going through mammograms waiting for the results are much more anxious if they’ve had breast cancer before than those who were who were just going through normal screening. So [deescalation] is reducing anxiety. It’s also reducing the cost. It’s just reducing the burden on the whole health care system,” said Dr. Dunn.
During the Q&A session after the talk, Bruce Mann, MBBS, PhD, asked if there were differences in the pathology of the cancers that occurred in the less frequent group than those that arose in the annual group. If more frequent screening leads to earlier diagnosis of a tumor, “you would expect that those who have less [frequent screening] may be diagnosed with more advanced recurrences or new cancers, and that would eventually lead to a difference in outcome,” Dr. Mann said in an interview. He is director of breast cancer services at the Royal Melbourne Hospital in Australia.
He acknowledged the need to reconsider screening frequency, and complimented the researchers. “This is a nice pragmatic study. It’s interesting. It’s provocative. I think it’s a bit too early [to change practice]. I think we do need to see more information,” he said.
The researchers randomized 5235 women aged 50 and older to annual or less frequent mammography. The disease type was invasive in 87% of women, while 13% had ductal carcinoma in situ.
Over a median follow-up of 8.7 years, 7% experienced a recurrence. At 5 years, breast cancer–specific survival was 98.1% and 98.3% in the annual and less frequent groups, respectively (hazard ratio [HR], 0.92; 95% CI, 0.64-1.32). There was also no difference in recurrence-free survival or overall survival.
Analyses showed noninferiority of less frequent mammograms at a 3% margin (P < .0001) and a 1% margin (P = .003).
The researchers found that 83% of women were compliant with the mammogram schedule in the annual group, versus 69% in the less frequent arm. The COVID-19 pandemic was responsible for 35% of missed mammograms overall.
Quality of life measures showed that levels of distress over mammograms was similar across time and between the two groups, with 24% of women reporting medium or high levels of distress.
Dr. Dunn and Dr. Mann have no relevant financial disclosures.
FROM SABCS 2023
Sickle Cell Gene Therapy ‘Truly Transformative’
.
More specifically, a single infusion of lovo-cel led to complete resolution of vaso-occlusive events in 88% of patients, with 94% achieving complete resolution of severe events. All 10 adolescents in the study achieved complete resolution of vaso-occlusive events. Most patients remained free of vaso-occlusive events at their last follow-up.
“This is a one-time, truly transformative treatment with lovo-cel,” lead author Julie Kanter, MD, director of the adult sickle cell clinic at the University of Alabama in Birmingham, said in a media briefing at the annual meeting of the American Society of Hematology. The gene therapy can essentially eliminate vaso-occlusive events in patients with sickle cell disease and lead to normal hemoglobin levels, Dr. Kanter added.
For “anybody who has rounded on the inpatient floor and taken care of adolescents admitted with a pain crisis multiple times a year,” seeing these results “is so compelling,” commented Sarah O’Brien, MD, a pediatric hematologist at Nationwide Children’s Hospital in Columbus, Ohio, who moderated the briefing but was not involved in the study.
One and Done
Sickle cell disease, a debilitating and potentially life-threatening blood disorder, affects an estimated 100,000 people in the US.
People with the condition have a mutation in hemoglobin, which causes red blood cells to develop an abnormal sickle shape. These sickled cells block the flow of blood, ultimately depriving tissues of oxygen and leading to organ damage and severe pain, known as vaso-occlusive events.
On Dec. 8, the U.S. Food and Drug Administration (FDA) approved lovo-cel for patients aged 12 years or older with severe sickle cell disease alongside another gene-editing therapy called exagamglogene autotemcel or exa-cel (Casgevy, Vertex Pharmaceuticals and Crispr Therapeutics). The two therapies use different gene-editing approaches — exa-cel is the first to use the gene-editing tool CRISPR while lovo-cel uses a lentiviral vector.
Both are one-time, single-dose cell-based gene therapies.
With lovo-cel, patients first undergo a transfusion regimen and myeloablative conditioning with busulfan to collect cells that can then be genetically modified. A patient’s harvested cells are modified with an anti-sickling version of hemoglobin A, HbAT87Q. Patients then receive an infusion of these edited cells and remain in the hospital during engraftment and reconstitution.
Dr. Kanter presented long-term follow-up data on 47 patients enrolled in phase 1/2 and phase 3 studies of lovo-cel.
All patients had stable HbAT87Q levels from 6 months to their last follow-up at a median of 35.5 months.
Most patients achieved a durable globin response through their final follow-up visit.
Among the 34 evaluable patients, 88% had complete resolution of vaso-occlusive events 6 to 18 months after their infusion, including all 10 adolescent patients. Almost all patients (94%) achieved complete resolution of serious vaso-occlusive events.
In the few patients who experienced posttreatment vaso-occlusive events, these individuals still achieved major reductions in hospital admissions and hospital days.
Among 20 patients followed for at least 3 years, more than half had clinically meaningful improvements in pain intensity, pain interference, and fatigue.
Most treatment-related adverse events occurred within 1 year of lovo-cel infusions and were primarily related to busulfan conditioning. No cases of veno-occlusive liver disease, graft failure, or graft vs host disease occurred, and patients did not have complications related to the viral vector. No patients who had a history of stroke prior to lovo-cel therapy experienced a post-therapy stroke.
One patient died at baseline from significant cardiopulmonary disease related to sickle cell disease, but the death was considered unrelated to lovo-cel therapy.
To see a one-time treatment that essentially eradicates vaso-occlusive events is “really unparalleled,” said Steven Pipe, MD, from the University of Michigan School of Medicine in Ann Arbor, who presented data on a different study at the briefing.
However, Dr. Kanter noted, “it’s important to highlight that many of these individuals come into this therapy with significant disease and end-organ complications, and this will be something we will really need to follow long-term to understand how much this therapy can stabilize or reverse these complications.”
The studies were funded by bluebird bio. Dr. Kanter disclosed honoraria from the company and consulting/advising activities and receipt of research funding from multiple other entities. Dr. O’Brien disclosed consultancy for AstraZeneca, honoraria from Pharmacosmos, and research funding from Bristol Myers Squibb. Dr. Pipe disclosed consulting activities from multiple companies, not including bluebird bio.
A version of this article appeared on Medscape.com.
.
More specifically, a single infusion of lovo-cel led to complete resolution of vaso-occlusive events in 88% of patients, with 94% achieving complete resolution of severe events. All 10 adolescents in the study achieved complete resolution of vaso-occlusive events. Most patients remained free of vaso-occlusive events at their last follow-up.
“This is a one-time, truly transformative treatment with lovo-cel,” lead author Julie Kanter, MD, director of the adult sickle cell clinic at the University of Alabama in Birmingham, said in a media briefing at the annual meeting of the American Society of Hematology. The gene therapy can essentially eliminate vaso-occlusive events in patients with sickle cell disease and lead to normal hemoglobin levels, Dr. Kanter added.
For “anybody who has rounded on the inpatient floor and taken care of adolescents admitted with a pain crisis multiple times a year,” seeing these results “is so compelling,” commented Sarah O’Brien, MD, a pediatric hematologist at Nationwide Children’s Hospital in Columbus, Ohio, who moderated the briefing but was not involved in the study.
One and Done
Sickle cell disease, a debilitating and potentially life-threatening blood disorder, affects an estimated 100,000 people in the US.
People with the condition have a mutation in hemoglobin, which causes red blood cells to develop an abnormal sickle shape. These sickled cells block the flow of blood, ultimately depriving tissues of oxygen and leading to organ damage and severe pain, known as vaso-occlusive events.
On Dec. 8, the U.S. Food and Drug Administration (FDA) approved lovo-cel for patients aged 12 years or older with severe sickle cell disease alongside another gene-editing therapy called exagamglogene autotemcel or exa-cel (Casgevy, Vertex Pharmaceuticals and Crispr Therapeutics). The two therapies use different gene-editing approaches — exa-cel is the first to use the gene-editing tool CRISPR while lovo-cel uses a lentiviral vector.
Both are one-time, single-dose cell-based gene therapies.
With lovo-cel, patients first undergo a transfusion regimen and myeloablative conditioning with busulfan to collect cells that can then be genetically modified. A patient’s harvested cells are modified with an anti-sickling version of hemoglobin A, HbAT87Q. Patients then receive an infusion of these edited cells and remain in the hospital during engraftment and reconstitution.
Dr. Kanter presented long-term follow-up data on 47 patients enrolled in phase 1/2 and phase 3 studies of lovo-cel.
All patients had stable HbAT87Q levels from 6 months to their last follow-up at a median of 35.5 months.
Most patients achieved a durable globin response through their final follow-up visit.
Among the 34 evaluable patients, 88% had complete resolution of vaso-occlusive events 6 to 18 months after their infusion, including all 10 adolescent patients. Almost all patients (94%) achieved complete resolution of serious vaso-occlusive events.
In the few patients who experienced posttreatment vaso-occlusive events, these individuals still achieved major reductions in hospital admissions and hospital days.
Among 20 patients followed for at least 3 years, more than half had clinically meaningful improvements in pain intensity, pain interference, and fatigue.
Most treatment-related adverse events occurred within 1 year of lovo-cel infusions and were primarily related to busulfan conditioning. No cases of veno-occlusive liver disease, graft failure, or graft vs host disease occurred, and patients did not have complications related to the viral vector. No patients who had a history of stroke prior to lovo-cel therapy experienced a post-therapy stroke.
One patient died at baseline from significant cardiopulmonary disease related to sickle cell disease, but the death was considered unrelated to lovo-cel therapy.
To see a one-time treatment that essentially eradicates vaso-occlusive events is “really unparalleled,” said Steven Pipe, MD, from the University of Michigan School of Medicine in Ann Arbor, who presented data on a different study at the briefing.
However, Dr. Kanter noted, “it’s important to highlight that many of these individuals come into this therapy with significant disease and end-organ complications, and this will be something we will really need to follow long-term to understand how much this therapy can stabilize or reverse these complications.”
The studies were funded by bluebird bio. Dr. Kanter disclosed honoraria from the company and consulting/advising activities and receipt of research funding from multiple other entities. Dr. O’Brien disclosed consultancy for AstraZeneca, honoraria from Pharmacosmos, and research funding from Bristol Myers Squibb. Dr. Pipe disclosed consulting activities from multiple companies, not including bluebird bio.
A version of this article appeared on Medscape.com.
.
More specifically, a single infusion of lovo-cel led to complete resolution of vaso-occlusive events in 88% of patients, with 94% achieving complete resolution of severe events. All 10 adolescents in the study achieved complete resolution of vaso-occlusive events. Most patients remained free of vaso-occlusive events at their last follow-up.
“This is a one-time, truly transformative treatment with lovo-cel,” lead author Julie Kanter, MD, director of the adult sickle cell clinic at the University of Alabama in Birmingham, said in a media briefing at the annual meeting of the American Society of Hematology. The gene therapy can essentially eliminate vaso-occlusive events in patients with sickle cell disease and lead to normal hemoglobin levels, Dr. Kanter added.
For “anybody who has rounded on the inpatient floor and taken care of adolescents admitted with a pain crisis multiple times a year,” seeing these results “is so compelling,” commented Sarah O’Brien, MD, a pediatric hematologist at Nationwide Children’s Hospital in Columbus, Ohio, who moderated the briefing but was not involved in the study.
One and Done
Sickle cell disease, a debilitating and potentially life-threatening blood disorder, affects an estimated 100,000 people in the US.
People with the condition have a mutation in hemoglobin, which causes red blood cells to develop an abnormal sickle shape. These sickled cells block the flow of blood, ultimately depriving tissues of oxygen and leading to organ damage and severe pain, known as vaso-occlusive events.
On Dec. 8, the U.S. Food and Drug Administration (FDA) approved lovo-cel for patients aged 12 years or older with severe sickle cell disease alongside another gene-editing therapy called exagamglogene autotemcel or exa-cel (Casgevy, Vertex Pharmaceuticals and Crispr Therapeutics). The two therapies use different gene-editing approaches — exa-cel is the first to use the gene-editing tool CRISPR while lovo-cel uses a lentiviral vector.
Both are one-time, single-dose cell-based gene therapies.
With lovo-cel, patients first undergo a transfusion regimen and myeloablative conditioning with busulfan to collect cells that can then be genetically modified. A patient’s harvested cells are modified with an anti-sickling version of hemoglobin A, HbAT87Q. Patients then receive an infusion of these edited cells and remain in the hospital during engraftment and reconstitution.
Dr. Kanter presented long-term follow-up data on 47 patients enrolled in phase 1/2 and phase 3 studies of lovo-cel.
All patients had stable HbAT87Q levels from 6 months to their last follow-up at a median of 35.5 months.
Most patients achieved a durable globin response through their final follow-up visit.
Among the 34 evaluable patients, 88% had complete resolution of vaso-occlusive events 6 to 18 months after their infusion, including all 10 adolescent patients. Almost all patients (94%) achieved complete resolution of serious vaso-occlusive events.
In the few patients who experienced posttreatment vaso-occlusive events, these individuals still achieved major reductions in hospital admissions and hospital days.
Among 20 patients followed for at least 3 years, more than half had clinically meaningful improvements in pain intensity, pain interference, and fatigue.
Most treatment-related adverse events occurred within 1 year of lovo-cel infusions and were primarily related to busulfan conditioning. No cases of veno-occlusive liver disease, graft failure, or graft vs host disease occurred, and patients did not have complications related to the viral vector. No patients who had a history of stroke prior to lovo-cel therapy experienced a post-therapy stroke.
One patient died at baseline from significant cardiopulmonary disease related to sickle cell disease, but the death was considered unrelated to lovo-cel therapy.
To see a one-time treatment that essentially eradicates vaso-occlusive events is “really unparalleled,” said Steven Pipe, MD, from the University of Michigan School of Medicine in Ann Arbor, who presented data on a different study at the briefing.
However, Dr. Kanter noted, “it’s important to highlight that many of these individuals come into this therapy with significant disease and end-organ complications, and this will be something we will really need to follow long-term to understand how much this therapy can stabilize or reverse these complications.”
The studies were funded by bluebird bio. Dr. Kanter disclosed honoraria from the company and consulting/advising activities and receipt of research funding from multiple other entities. Dr. O’Brien disclosed consultancy for AstraZeneca, honoraria from Pharmacosmos, and research funding from Bristol Myers Squibb. Dr. Pipe disclosed consulting activities from multiple companies, not including bluebird bio.
A version of this article appeared on Medscape.com.
FROM ASH 2023
CAR T-Cell Therapy: Cure for Systemic Autoimmune Diseases?
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
FROM ASH 2023
In real world, patients with myeloma have worse outcomes
The analysis, which included nearly 4,000 patients with multiple myeloma, revealed that patients in a real-world setting demonstrated worse progression-free and overall survival on six of seven standard treatments compared with patients evaluated in randomized controlled trials.
Lead author Alissa Visram, MD, MPH, who spoke about the study at the annual meeting of the American Society of Hematology, said the findings will likely change the way she speaks to patients about their potential outcomes.
“I’ll probably present both numbers [from real-life and clinical-trial data] and give them a sense of the best-case scenario,” Dr. Visram said during an ASH media briefing. But she said she will also caution her patients that the real-world numbers reflect how people on these drugs actually fare.
The effectiveness of multiple myeloma drugs remains unclear outside the clinical trial setting, explained Dr. Visram, of the Division of Hematology at the Ottawa Hospital Research Institute, Ottawa, Ontario, Canada. Outcomes from randomized controlled trials form the basis of drug approvals but many patients in the real world do not meet the “stringent” trial inclusion criteria.
Dr. Visram and colleagues launched the current study to better understand the potential differences between real-world and clinical trial outcomes. In the analysis, the researchers compared real-world outcomes among patients receiving seven standard multiple myeloma regimens covered by Ontario’s public health plan with patient outcomes reported in phase 3 randomized controlled trials.
The retrospective study included 3951 patients with newly diagnosed and refractory multiple myeloma treated from 2007 to 2020 in Ontario. Regimens for newly diagnosed transplant ineligible patients included lenalidomide plus dexamethasone and triple therapy with bortezomib, lenalidomide, and dexamethasone. Regimens for patients with relapsed disease included pomalidomide plus dexamethasone or carfilzomib plus dexamethasone as well as triple combinations including carfilzomib, lenalidomide, and dexamethasone.
Overall, Dr. Visram and colleagues found that patients in the real-world setting demonstrated worse overall survival for six of the seven regimens evaluated (pooled hazard ratio [HR], 1.75; P = .010).
The real-world patients also had worse progression-free survival for six of the seven regimens (pooled HR, 1.44; P = .034).
For these regimens, progression-free survival was at least 3-18 months longer in the clinical trial cohort, while median overall survival was at least 19 months longer compared with real-world patients, Dr. Visram explained.
The only regimen with comparable outcomes in the clinical trial and real-world settings was pomalidomide and dexamethasone, she said. One reason could be that patients receiving pomalidomide plus dexamethasone in the clinical trial setting had similar or more advanced disease than those in the real-world setting.
The study also found that adverse effects were similar between the clinical and real-world groups.
The next step, Dr. Visram said, would be to explore what’s driving the differences in outcomes.
Are patients in the real-world setting older or frailer? Do they have more advanced disease? Are providers using these regimens differently?
Mikkael A. Sekeres, MD, MS, explained that the difference likely comes down to the health of the patient.
Patients in these types of clinical trials “are just these pristine specimens of human beings except for the cancer that’s being treated,” Dr. Sekeres, of the Sylvester Comprehensive Cancer Center at the University of Miami, Florida, said in an earlier ASH press briefing.
Cynthia E. Dunbar, MD, noted that patients in clinical trials have other advantages as well.
“Patients who are able to enroll in clinical trials are more likely to be able to show up at the treatment center at the right time and for every dose, have transportation, and afford drugs to prevent side effects,” said Dr. Dunbar, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute and secretary of ASH. These patients also “might stay on the drug for longer, or they have nurses who are always encouraging them on how to make it through a toxicity.”
Dr. Dunbar said hematologists and patients should consider randomized controlled trials to be “the best possible outcome, and perhaps adjust their thinking if an individual patient is older, sicker, or less able to follow a regimen exactly.”
No study funding was reported. Dr. Visram reported consulting and honoraria relationships with Apotex, Janssen, and Sanofi. Other study authors reported multiple relationships with industry. Disclosures for Dr. Dunbar and Dr. Sekeres were unavailable.
A version of this article appeared on Medscape.com.
The analysis, which included nearly 4,000 patients with multiple myeloma, revealed that patients in a real-world setting demonstrated worse progression-free and overall survival on six of seven standard treatments compared with patients evaluated in randomized controlled trials.
Lead author Alissa Visram, MD, MPH, who spoke about the study at the annual meeting of the American Society of Hematology, said the findings will likely change the way she speaks to patients about their potential outcomes.
“I’ll probably present both numbers [from real-life and clinical-trial data] and give them a sense of the best-case scenario,” Dr. Visram said during an ASH media briefing. But she said she will also caution her patients that the real-world numbers reflect how people on these drugs actually fare.
The effectiveness of multiple myeloma drugs remains unclear outside the clinical trial setting, explained Dr. Visram, of the Division of Hematology at the Ottawa Hospital Research Institute, Ottawa, Ontario, Canada. Outcomes from randomized controlled trials form the basis of drug approvals but many patients in the real world do not meet the “stringent” trial inclusion criteria.
Dr. Visram and colleagues launched the current study to better understand the potential differences between real-world and clinical trial outcomes. In the analysis, the researchers compared real-world outcomes among patients receiving seven standard multiple myeloma regimens covered by Ontario’s public health plan with patient outcomes reported in phase 3 randomized controlled trials.
The retrospective study included 3951 patients with newly diagnosed and refractory multiple myeloma treated from 2007 to 2020 in Ontario. Regimens for newly diagnosed transplant ineligible patients included lenalidomide plus dexamethasone and triple therapy with bortezomib, lenalidomide, and dexamethasone. Regimens for patients with relapsed disease included pomalidomide plus dexamethasone or carfilzomib plus dexamethasone as well as triple combinations including carfilzomib, lenalidomide, and dexamethasone.
Overall, Dr. Visram and colleagues found that patients in the real-world setting demonstrated worse overall survival for six of the seven regimens evaluated (pooled hazard ratio [HR], 1.75; P = .010).
The real-world patients also had worse progression-free survival for six of the seven regimens (pooled HR, 1.44; P = .034).
For these regimens, progression-free survival was at least 3-18 months longer in the clinical trial cohort, while median overall survival was at least 19 months longer compared with real-world patients, Dr. Visram explained.
The only regimen with comparable outcomes in the clinical trial and real-world settings was pomalidomide and dexamethasone, she said. One reason could be that patients receiving pomalidomide plus dexamethasone in the clinical trial setting had similar or more advanced disease than those in the real-world setting.
The study also found that adverse effects were similar between the clinical and real-world groups.
The next step, Dr. Visram said, would be to explore what’s driving the differences in outcomes.
Are patients in the real-world setting older or frailer? Do they have more advanced disease? Are providers using these regimens differently?
Mikkael A. Sekeres, MD, MS, explained that the difference likely comes down to the health of the patient.
Patients in these types of clinical trials “are just these pristine specimens of human beings except for the cancer that’s being treated,” Dr. Sekeres, of the Sylvester Comprehensive Cancer Center at the University of Miami, Florida, said in an earlier ASH press briefing.
Cynthia E. Dunbar, MD, noted that patients in clinical trials have other advantages as well.
“Patients who are able to enroll in clinical trials are more likely to be able to show up at the treatment center at the right time and for every dose, have transportation, and afford drugs to prevent side effects,” said Dr. Dunbar, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute and secretary of ASH. These patients also “might stay on the drug for longer, or they have nurses who are always encouraging them on how to make it through a toxicity.”
Dr. Dunbar said hematologists and patients should consider randomized controlled trials to be “the best possible outcome, and perhaps adjust their thinking if an individual patient is older, sicker, or less able to follow a regimen exactly.”
No study funding was reported. Dr. Visram reported consulting and honoraria relationships with Apotex, Janssen, and Sanofi. Other study authors reported multiple relationships with industry. Disclosures for Dr. Dunbar and Dr. Sekeres were unavailable.
A version of this article appeared on Medscape.com.
The analysis, which included nearly 4,000 patients with multiple myeloma, revealed that patients in a real-world setting demonstrated worse progression-free and overall survival on six of seven standard treatments compared with patients evaluated in randomized controlled trials.
Lead author Alissa Visram, MD, MPH, who spoke about the study at the annual meeting of the American Society of Hematology, said the findings will likely change the way she speaks to patients about their potential outcomes.
“I’ll probably present both numbers [from real-life and clinical-trial data] and give them a sense of the best-case scenario,” Dr. Visram said during an ASH media briefing. But she said she will also caution her patients that the real-world numbers reflect how people on these drugs actually fare.
The effectiveness of multiple myeloma drugs remains unclear outside the clinical trial setting, explained Dr. Visram, of the Division of Hematology at the Ottawa Hospital Research Institute, Ottawa, Ontario, Canada. Outcomes from randomized controlled trials form the basis of drug approvals but many patients in the real world do not meet the “stringent” trial inclusion criteria.
Dr. Visram and colleagues launched the current study to better understand the potential differences between real-world and clinical trial outcomes. In the analysis, the researchers compared real-world outcomes among patients receiving seven standard multiple myeloma regimens covered by Ontario’s public health plan with patient outcomes reported in phase 3 randomized controlled trials.
The retrospective study included 3951 patients with newly diagnosed and refractory multiple myeloma treated from 2007 to 2020 in Ontario. Regimens for newly diagnosed transplant ineligible patients included lenalidomide plus dexamethasone and triple therapy with bortezomib, lenalidomide, and dexamethasone. Regimens for patients with relapsed disease included pomalidomide plus dexamethasone or carfilzomib plus dexamethasone as well as triple combinations including carfilzomib, lenalidomide, and dexamethasone.
Overall, Dr. Visram and colleagues found that patients in the real-world setting demonstrated worse overall survival for six of the seven regimens evaluated (pooled hazard ratio [HR], 1.75; P = .010).
The real-world patients also had worse progression-free survival for six of the seven regimens (pooled HR, 1.44; P = .034).
For these regimens, progression-free survival was at least 3-18 months longer in the clinical trial cohort, while median overall survival was at least 19 months longer compared with real-world patients, Dr. Visram explained.
The only regimen with comparable outcomes in the clinical trial and real-world settings was pomalidomide and dexamethasone, she said. One reason could be that patients receiving pomalidomide plus dexamethasone in the clinical trial setting had similar or more advanced disease than those in the real-world setting.
The study also found that adverse effects were similar between the clinical and real-world groups.
The next step, Dr. Visram said, would be to explore what’s driving the differences in outcomes.
Are patients in the real-world setting older or frailer? Do they have more advanced disease? Are providers using these regimens differently?
Mikkael A. Sekeres, MD, MS, explained that the difference likely comes down to the health of the patient.
Patients in these types of clinical trials “are just these pristine specimens of human beings except for the cancer that’s being treated,” Dr. Sekeres, of the Sylvester Comprehensive Cancer Center at the University of Miami, Florida, said in an earlier ASH press briefing.
Cynthia E. Dunbar, MD, noted that patients in clinical trials have other advantages as well.
“Patients who are able to enroll in clinical trials are more likely to be able to show up at the treatment center at the right time and for every dose, have transportation, and afford drugs to prevent side effects,” said Dr. Dunbar, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute and secretary of ASH. These patients also “might stay on the drug for longer, or they have nurses who are always encouraging them on how to make it through a toxicity.”
Dr. Dunbar said hematologists and patients should consider randomized controlled trials to be “the best possible outcome, and perhaps adjust their thinking if an individual patient is older, sicker, or less able to follow a regimen exactly.”
No study funding was reported. Dr. Visram reported consulting and honoraria relationships with Apotex, Janssen, and Sanofi. Other study authors reported multiple relationships with industry. Disclosures for Dr. Dunbar and Dr. Sekeres were unavailable.
A version of this article appeared on Medscape.com.
FROM ASH 2023
This test may guide AML therapy for Black pediatric patients
.
The score, dubbed ACS10 and initially highlighted in a 2022 report, predicts how well patients will respond to cytarabine based on their genetic make-up, and has the potential to personalize treatment for Black pediatric patients, a group that often has worse outcomes than White patients.
In the current study, presented at the annual meeting of the American Society of Hematology (ASH) , Black patients with low ACS10 scores had significantly worse outcomes compared with those with high scores when initially treated with low-dose cytarabine, daunorubicin, and etoposide.
The difference in outcomes disappeared, however, for patients who received high-dose cytarabine, daunorubicin, and etoposide or clofarabine and cytarabine.
The genetic traits revealed by the test likely help explain why Black patients with AML typically fare worse on certain regimens, Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute, commented in an ASH press preview briefing.
This study also suggests that clinicians should perform testing for genetic variants and biomarkers that impact outcomes “instead of assuming that a certain dose should be given simply based on perceived or reported race or ethnicity,” said Dr. Dunbar, also secretary of ASH.
The ACS10 test, derived from a combination of 10 single nucleotide polymorphisms, is not yet available, but one could be developed to help guide treatment decisions for clinicians, especially those in developing countries where AML treatment can be very expensive, said study lead author Jatinder Lamba, PhD, MSc, of the University of Florida College of Pharmacy, Gainesville, at an ASH press briefing on Thursday.
Prior research shows that Black pediatric patients with AML often have worse outcomes than White patients. A recent study , for instance, found Black patients with AML, especially those aged 18 to 29 years, had a higher early death rate compared with White patients (16% vs 3%) and significantly lower 5-year overall survival rates (22% vs 51%). The authors of this study suggested that genetic differences between young Black and White patients could help explain the disparity.
In the new analysis, Dr. Lamba and colleagues explored how outcomes by race and cytarabine pharmacogenomics varied in pediatric patients with AML.
The study included 86 Black patients and 359 White patients with newly diagnosed AML treated on two multi-institutional clinical trials. The patients received one of three initial treatments that included cytarabine: high-dose or low-dose cytarabine, daunorubicin, and etoposide, or clofarabine and cytarabine.
Most Black patients in the analysis (73%) had low ACS10 scores compared with 30% of White patients.
Unlike other recent reports, this study found that Black and White patients had similar complete remission rates following two courses of induction therapy (92.6% vs 95%) as well as similar rates of minimal residual disease negativity after one course (55.8% vs 55.4%).
Event-free survival (EFS) and overall survival rates were also similar, with 5-year EFS estimates at 58.3% for Black patients and 58.2% for White patients and overall survival rates at 63.8% vs 69.4%, respectively (P = .24).
However, when separating outcomes by ACS10 scores, Black patients with low scores had significantly worse EFS following low-dose cytarabine, daunorubicin, and etoposide compared with those with high ACS10 scores. And when these patients received high-dose cytarabine, daunorubicin, and etoposide or clofarabine and cytarabine induction therapy instead, the differences went away.
Overall, Black patients demonstrated significantly better EFS following treatment with clofarabine and cytarabine compared with the low-dose cytarabine triple therapy (hazard ratio, 0.17; P = .01). After adjusting for cofounders, clofarabine and cytarabine induction was the best treatment for Black patients with low ACS10 scores (HR for EFS, 0.2).
“Our results suggest that pharmacogenomics differences between Black and White patients should be considered when tailoring induction regimens to improve outcomes of Black patients and bridge the racial disparity gap in AML treatment,” the researchers concluded.
In developing countries, especially in Africa, starting patients on high-dose cytarabine, daunorubicin, and etoposide can lead to better results “without increasing much of the economic burden” since this treatment is the cheapest, Dr. Lamba said. “At the same time, if the patients have high ACS10 score, you can reduce their economic burden by giving them standard dose” cytarabine, daunorubicin, and etoposide and achieve similar results.
No study funding was reported. Dr. Lamba reported no relevant financial relationships, and three other authors reported various disclosures. Disclosures for Dr. Dunbar were unavailable..
A version of this article appeared on Medscape.com.
.
The score, dubbed ACS10 and initially highlighted in a 2022 report, predicts how well patients will respond to cytarabine based on their genetic make-up, and has the potential to personalize treatment for Black pediatric patients, a group that often has worse outcomes than White patients.
In the current study, presented at the annual meeting of the American Society of Hematology (ASH) , Black patients with low ACS10 scores had significantly worse outcomes compared with those with high scores when initially treated with low-dose cytarabine, daunorubicin, and etoposide.
The difference in outcomes disappeared, however, for patients who received high-dose cytarabine, daunorubicin, and etoposide or clofarabine and cytarabine.
The genetic traits revealed by the test likely help explain why Black patients with AML typically fare worse on certain regimens, Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute, commented in an ASH press preview briefing.
This study also suggests that clinicians should perform testing for genetic variants and biomarkers that impact outcomes “instead of assuming that a certain dose should be given simply based on perceived or reported race or ethnicity,” said Dr. Dunbar, also secretary of ASH.
The ACS10 test, derived from a combination of 10 single nucleotide polymorphisms, is not yet available, but one could be developed to help guide treatment decisions for clinicians, especially those in developing countries where AML treatment can be very expensive, said study lead author Jatinder Lamba, PhD, MSc, of the University of Florida College of Pharmacy, Gainesville, at an ASH press briefing on Thursday.
Prior research shows that Black pediatric patients with AML often have worse outcomes than White patients. A recent study , for instance, found Black patients with AML, especially those aged 18 to 29 years, had a higher early death rate compared with White patients (16% vs 3%) and significantly lower 5-year overall survival rates (22% vs 51%). The authors of this study suggested that genetic differences between young Black and White patients could help explain the disparity.
In the new analysis, Dr. Lamba and colleagues explored how outcomes by race and cytarabine pharmacogenomics varied in pediatric patients with AML.
The study included 86 Black patients and 359 White patients with newly diagnosed AML treated on two multi-institutional clinical trials. The patients received one of three initial treatments that included cytarabine: high-dose or low-dose cytarabine, daunorubicin, and etoposide, or clofarabine and cytarabine.
Most Black patients in the analysis (73%) had low ACS10 scores compared with 30% of White patients.
Unlike other recent reports, this study found that Black and White patients had similar complete remission rates following two courses of induction therapy (92.6% vs 95%) as well as similar rates of minimal residual disease negativity after one course (55.8% vs 55.4%).
Event-free survival (EFS) and overall survival rates were also similar, with 5-year EFS estimates at 58.3% for Black patients and 58.2% for White patients and overall survival rates at 63.8% vs 69.4%, respectively (P = .24).
However, when separating outcomes by ACS10 scores, Black patients with low scores had significantly worse EFS following low-dose cytarabine, daunorubicin, and etoposide compared with those with high ACS10 scores. And when these patients received high-dose cytarabine, daunorubicin, and etoposide or clofarabine and cytarabine induction therapy instead, the differences went away.
Overall, Black patients demonstrated significantly better EFS following treatment with clofarabine and cytarabine compared with the low-dose cytarabine triple therapy (hazard ratio, 0.17; P = .01). After adjusting for cofounders, clofarabine and cytarabine induction was the best treatment for Black patients with low ACS10 scores (HR for EFS, 0.2).
“Our results suggest that pharmacogenomics differences between Black and White patients should be considered when tailoring induction regimens to improve outcomes of Black patients and bridge the racial disparity gap in AML treatment,” the researchers concluded.
In developing countries, especially in Africa, starting patients on high-dose cytarabine, daunorubicin, and etoposide can lead to better results “without increasing much of the economic burden” since this treatment is the cheapest, Dr. Lamba said. “At the same time, if the patients have high ACS10 score, you can reduce their economic burden by giving them standard dose” cytarabine, daunorubicin, and etoposide and achieve similar results.
No study funding was reported. Dr. Lamba reported no relevant financial relationships, and three other authors reported various disclosures. Disclosures for Dr. Dunbar were unavailable..
A version of this article appeared on Medscape.com.
.
The score, dubbed ACS10 and initially highlighted in a 2022 report, predicts how well patients will respond to cytarabine based on their genetic make-up, and has the potential to personalize treatment for Black pediatric patients, a group that often has worse outcomes than White patients.
In the current study, presented at the annual meeting of the American Society of Hematology (ASH) , Black patients with low ACS10 scores had significantly worse outcomes compared with those with high scores when initially treated with low-dose cytarabine, daunorubicin, and etoposide.
The difference in outcomes disappeared, however, for patients who received high-dose cytarabine, daunorubicin, and etoposide or clofarabine and cytarabine.
The genetic traits revealed by the test likely help explain why Black patients with AML typically fare worse on certain regimens, Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute, commented in an ASH press preview briefing.
This study also suggests that clinicians should perform testing for genetic variants and biomarkers that impact outcomes “instead of assuming that a certain dose should be given simply based on perceived or reported race or ethnicity,” said Dr. Dunbar, also secretary of ASH.
The ACS10 test, derived from a combination of 10 single nucleotide polymorphisms, is not yet available, but one could be developed to help guide treatment decisions for clinicians, especially those in developing countries where AML treatment can be very expensive, said study lead author Jatinder Lamba, PhD, MSc, of the University of Florida College of Pharmacy, Gainesville, at an ASH press briefing on Thursday.
Prior research shows that Black pediatric patients with AML often have worse outcomes than White patients. A recent study , for instance, found Black patients with AML, especially those aged 18 to 29 years, had a higher early death rate compared with White patients (16% vs 3%) and significantly lower 5-year overall survival rates (22% vs 51%). The authors of this study suggested that genetic differences between young Black and White patients could help explain the disparity.
In the new analysis, Dr. Lamba and colleagues explored how outcomes by race and cytarabine pharmacogenomics varied in pediatric patients with AML.
The study included 86 Black patients and 359 White patients with newly diagnosed AML treated on two multi-institutional clinical trials. The patients received one of three initial treatments that included cytarabine: high-dose or low-dose cytarabine, daunorubicin, and etoposide, or clofarabine and cytarabine.
Most Black patients in the analysis (73%) had low ACS10 scores compared with 30% of White patients.
Unlike other recent reports, this study found that Black and White patients had similar complete remission rates following two courses of induction therapy (92.6% vs 95%) as well as similar rates of minimal residual disease negativity after one course (55.8% vs 55.4%).
Event-free survival (EFS) and overall survival rates were also similar, with 5-year EFS estimates at 58.3% for Black patients and 58.2% for White patients and overall survival rates at 63.8% vs 69.4%, respectively (P = .24).
However, when separating outcomes by ACS10 scores, Black patients with low scores had significantly worse EFS following low-dose cytarabine, daunorubicin, and etoposide compared with those with high ACS10 scores. And when these patients received high-dose cytarabine, daunorubicin, and etoposide or clofarabine and cytarabine induction therapy instead, the differences went away.
Overall, Black patients demonstrated significantly better EFS following treatment with clofarabine and cytarabine compared with the low-dose cytarabine triple therapy (hazard ratio, 0.17; P = .01). After adjusting for cofounders, clofarabine and cytarabine induction was the best treatment for Black patients with low ACS10 scores (HR for EFS, 0.2).
“Our results suggest that pharmacogenomics differences between Black and White patients should be considered when tailoring induction regimens to improve outcomes of Black patients and bridge the racial disparity gap in AML treatment,” the researchers concluded.
In developing countries, especially in Africa, starting patients on high-dose cytarabine, daunorubicin, and etoposide can lead to better results “without increasing much of the economic burden” since this treatment is the cheapest, Dr. Lamba said. “At the same time, if the patients have high ACS10 score, you can reduce their economic burden by giving them standard dose” cytarabine, daunorubicin, and etoposide and achieve similar results.
No study funding was reported. Dr. Lamba reported no relevant financial relationships, and three other authors reported various disclosures. Disclosures for Dr. Dunbar were unavailable..
A version of this article appeared on Medscape.com.
FROM ASH 2023
MASLD often is worse in slim patients
PARIS — Although metabolic liver diseases are mainly seen in patients with obesity or type 2 diabetes, studies have shown that non-alcoholic fatty liver disease, recently renamed metabolic dysfunction-associated steatotic liver disease (MASLD), also affects slim patients. Moreover, the condition could be particularly severe in this population.
A recent study carried out using data from the French Constance cohort showed that of the 25,753 patients with MASLD, 16.3% were lean (BMI of less than 25 kg/m²). In addition, 50% of these patients had no metabolic risk factors.
These slim patients with MASLD were most often young patients, for the most part female, and less likely to present with symptoms of metabolic syndrome. Asian patients were overrepresented in this group.
“These patients probably have genetic and/or environmental risk factors,” commented senior author Lawrence Serfaty, MD, PhD, head of the metabolic liver unit at the new Strasbourg public hospital, during a press conference at the Paris NASH meeting.
The disease was more severe in slim subjects. Overall, 3.6% of the slim subjects had advanced fibrosis (Forns index > 6.9) vs 1.7% of patients with overweight or obesity (P < .001), regardless of demographic variables, metabolic risk factors, and lifestyle. They also had higher alanine aminotransferase levels.
In addition, over the course of a mean follow-up of 3.8 years, liver events (eg, cirrhosis, decompensated cirrhosis, and liver cancer), chronic kidney diseases, and all-cause mortality were much more common in these patients than in patients with overweight or obesity (adjusted hazard ratios of 5.84, 2.49, and 3.01, respectively). It should be noted that these clinical results were linked to fibrosis severity in both slim and overweight subjects with MASLD.
Nonetheless, cardiovascular events remained more common in patients with overweight or obesity, suggesting that obesity itself is a major risk factor for cardiovascular diseases, regardless of MASLD.
“Armed with these results, which confirm those obtained from other studies, we must seek to understand the pathogenesis of the disease in slim patients and study the role of the microbiota, genetics, and diet, as well as determining the effects of alcohol and tobacco, consumption of which was slightly more common in this subpopulation,” said Dr. Serfaty.
According to the study authors, sarcopenia and bile acids could also be involved in the pathogenesis of MASLD in slim patients. The researchers concluded that “due to the relatively low rate of MASLD in slim subjects, screening should target patients presenting with metabolic anomalies and/or unexplained cytolysis.”
This article was translated from the Medscape French edition.
PARIS — Although metabolic liver diseases are mainly seen in patients with obesity or type 2 diabetes, studies have shown that non-alcoholic fatty liver disease, recently renamed metabolic dysfunction-associated steatotic liver disease (MASLD), also affects slim patients. Moreover, the condition could be particularly severe in this population.
A recent study carried out using data from the French Constance cohort showed that of the 25,753 patients with MASLD, 16.3% were lean (BMI of less than 25 kg/m²). In addition, 50% of these patients had no metabolic risk factors.
These slim patients with MASLD were most often young patients, for the most part female, and less likely to present with symptoms of metabolic syndrome. Asian patients were overrepresented in this group.
“These patients probably have genetic and/or environmental risk factors,” commented senior author Lawrence Serfaty, MD, PhD, head of the metabolic liver unit at the new Strasbourg public hospital, during a press conference at the Paris NASH meeting.
The disease was more severe in slim subjects. Overall, 3.6% of the slim subjects had advanced fibrosis (Forns index > 6.9) vs 1.7% of patients with overweight or obesity (P < .001), regardless of demographic variables, metabolic risk factors, and lifestyle. They also had higher alanine aminotransferase levels.
In addition, over the course of a mean follow-up of 3.8 years, liver events (eg, cirrhosis, decompensated cirrhosis, and liver cancer), chronic kidney diseases, and all-cause mortality were much more common in these patients than in patients with overweight or obesity (adjusted hazard ratios of 5.84, 2.49, and 3.01, respectively). It should be noted that these clinical results were linked to fibrosis severity in both slim and overweight subjects with MASLD.
Nonetheless, cardiovascular events remained more common in patients with overweight or obesity, suggesting that obesity itself is a major risk factor for cardiovascular diseases, regardless of MASLD.
“Armed with these results, which confirm those obtained from other studies, we must seek to understand the pathogenesis of the disease in slim patients and study the role of the microbiota, genetics, and diet, as well as determining the effects of alcohol and tobacco, consumption of which was slightly more common in this subpopulation,” said Dr. Serfaty.
According to the study authors, sarcopenia and bile acids could also be involved in the pathogenesis of MASLD in slim patients. The researchers concluded that “due to the relatively low rate of MASLD in slim subjects, screening should target patients presenting with metabolic anomalies and/or unexplained cytolysis.”
This article was translated from the Medscape French edition.
PARIS — Although metabolic liver diseases are mainly seen in patients with obesity or type 2 diabetes, studies have shown that non-alcoholic fatty liver disease, recently renamed metabolic dysfunction-associated steatotic liver disease (MASLD), also affects slim patients. Moreover, the condition could be particularly severe in this population.
A recent study carried out using data from the French Constance cohort showed that of the 25,753 patients with MASLD, 16.3% were lean (BMI of less than 25 kg/m²). In addition, 50% of these patients had no metabolic risk factors.
These slim patients with MASLD were most often young patients, for the most part female, and less likely to present with symptoms of metabolic syndrome. Asian patients were overrepresented in this group.
“These patients probably have genetic and/or environmental risk factors,” commented senior author Lawrence Serfaty, MD, PhD, head of the metabolic liver unit at the new Strasbourg public hospital, during a press conference at the Paris NASH meeting.
The disease was more severe in slim subjects. Overall, 3.6% of the slim subjects had advanced fibrosis (Forns index > 6.9) vs 1.7% of patients with overweight or obesity (P < .001), regardless of demographic variables, metabolic risk factors, and lifestyle. They also had higher alanine aminotransferase levels.
In addition, over the course of a mean follow-up of 3.8 years, liver events (eg, cirrhosis, decompensated cirrhosis, and liver cancer), chronic kidney diseases, and all-cause mortality were much more common in these patients than in patients with overweight or obesity (adjusted hazard ratios of 5.84, 2.49, and 3.01, respectively). It should be noted that these clinical results were linked to fibrosis severity in both slim and overweight subjects with MASLD.
Nonetheless, cardiovascular events remained more common in patients with overweight or obesity, suggesting that obesity itself is a major risk factor for cardiovascular diseases, regardless of MASLD.
“Armed with these results, which confirm those obtained from other studies, we must seek to understand the pathogenesis of the disease in slim patients and study the role of the microbiota, genetics, and diet, as well as determining the effects of alcohol and tobacco, consumption of which was slightly more common in this subpopulation,” said Dr. Serfaty.
According to the study authors, sarcopenia and bile acids could also be involved in the pathogenesis of MASLD in slim patients. The researchers concluded that “due to the relatively low rate of MASLD in slim subjects, screening should target patients presenting with metabolic anomalies and/or unexplained cytolysis.”
This article was translated from the Medscape French edition.
Are liquid biopsy tests cost-effective for CRC screening?
Blood-based liquid biopsy tests for colorectal cancer (CRC) are in development and may soon hit the market, expanding potential options for patients who refuse traditional colonoscopy. But would they be a cost-effective screening tool?
Not according to an economic analysis by researchers at Columbia University Irving Medical Center in New York.
There is “intense research and patient and public interest” in blood-based cancer tests.
“However, liquid biopsy tests may not have sufficient performance and cost too much for them to be a viable strategy at this time,” corresponding author Chin Hur, MD, MPH, told this news organization.
The study was published online on November 16, 2023 in JAMA Network Open..
Better, Cheaper Liquid Biopsies Needed
The researchers developed a Markov model to compare the cost effectiveness of no screening and five CRC screening strategies: colonoscopy, liquid biopsy, liquid biopsy after nonadherence to colonoscopy, stool DNA, and fecal immunochemical test (FIT).
The model simulated a hypothetical cohort of unscreened adults at average risk for CRC with screening starting at age 45 years, in line with current US Preventive Services Task Force advice.
A strategy was considered cost-effective if it had an incremental cost-effectiveness ratio (ICER) below the US willingness-to-pay threshold of $100,000 per life-year gained.
According to their model, colonoscopy was the preferred (most cost-effective) strategy with an ICER of $28,071 per life-year gained.
Offering liquid biopsy screening to adults who refuse colonoscopy was the most effective strategy in terms of number of life-years gained, but it greatly exceeded the accepted threshold of $100,000 per life-year gained coming in at $377,538 per life-year gained. The cost of liquid biopsy would have to drop by 66% for this approach to become a cost-effective option, the researchers write.
Compared with no screening, the cost of liquid biopsy would have to fall by 94% for its ICER to drop below the willingness-to-pay threshold of $100,000 per life-year gained. When compared with stool-based tests, the cost of liquid biopsy would have to drop by 43%-80% to be cost-effective.
Liquid biopsy and the liquid biopsy after refusal of colonoscopy strategies had more life-years gained when polyp detection was introduced, but they did not achieve cost-effectiveness at liquid biopsy’s current price even with perfect performance.
“With current estimate of performance and cost,” liquid biopsy for CRC screening is not cost-effective, Dr. Hur told this news organization.
Liquid biopsy tests for CRC screening may become cost-effective in the future if they are significantly less expensive or if polyp detection is introduced along with a decrease in cost, Dr. Hur said.
Making blood-based CRC screening more effective and cost-effective “is more likely to depend on the ability to detect precancerous polyps than on the detection of CRC itself,” writes John Inadomi, MD, with University of Utah School of Medicine, Salt Lake City, in an invited commentary, also published online in JAMA Network Open.
The sensitivity of FIT for detecting advanced polyps is roughly 20%, whereas stool multitarget tests for blood and DNA or RNA detect 40%-45% of advanced polyps, Dr. Inadomi notes.
Blood-based tests, on the other hand, have been reported to detect 12%-16% of advanced polyps, “which is close to probability of a false-positive test,” he writes. “Because of their high cost, blood-based CRC screening will not be cost-effective unless they detect a greater proportion of advanced polyps than FIT.”
The need for follow-up colonoscopy in the case of a positive noncolonoscopy CRC screening test result is “an important concept that is not emphasized enough in clinical practice,” Dr. Inadomi adds. “Unfortunately, only 40% to 80% of people with positive noncolonoscopy screening test results follow-up with the requisite colonoscopy. Clinicians need to emphasize the necessity of the follow-up colonoscopy when discussing CRC screening options and adherence.”
Dr. Hur reported receiving consulting fees from Value Analytics outside the submitted work. Dr. Hur and co-authors Fay Kastrinos, MD, MPH, and Sheila Rustgi, MD, are supported by a grant from the National Cancer Institute. Co-author William Grady, MD, reported receiving personal fees from SEngine, Guardant Health, Freenome, Diacarta, Natera, Helio, Guidepoint, and GLG and nonfinancial support from LucidDx outside the submitted work. Grady also disclosed a patent pending for methylated gene biomarker for esophageal cancer. Co-author Yoanna Pumpalova, MD, reported receiving stock from Pfizer outside the submitted work. Inadomi reported receiving grants from Exact Sciences.
A version of this article appeared on Medscape.com.
Blood-based liquid biopsy tests for colorectal cancer (CRC) are in development and may soon hit the market, expanding potential options for patients who refuse traditional colonoscopy. But would they be a cost-effective screening tool?
Not according to an economic analysis by researchers at Columbia University Irving Medical Center in New York.
There is “intense research and patient and public interest” in blood-based cancer tests.
“However, liquid biopsy tests may not have sufficient performance and cost too much for them to be a viable strategy at this time,” corresponding author Chin Hur, MD, MPH, told this news organization.
The study was published online on November 16, 2023 in JAMA Network Open..
Better, Cheaper Liquid Biopsies Needed
The researchers developed a Markov model to compare the cost effectiveness of no screening and five CRC screening strategies: colonoscopy, liquid biopsy, liquid biopsy after nonadherence to colonoscopy, stool DNA, and fecal immunochemical test (FIT).
The model simulated a hypothetical cohort of unscreened adults at average risk for CRC with screening starting at age 45 years, in line with current US Preventive Services Task Force advice.
A strategy was considered cost-effective if it had an incremental cost-effectiveness ratio (ICER) below the US willingness-to-pay threshold of $100,000 per life-year gained.
According to their model, colonoscopy was the preferred (most cost-effective) strategy with an ICER of $28,071 per life-year gained.
Offering liquid biopsy screening to adults who refuse colonoscopy was the most effective strategy in terms of number of life-years gained, but it greatly exceeded the accepted threshold of $100,000 per life-year gained coming in at $377,538 per life-year gained. The cost of liquid biopsy would have to drop by 66% for this approach to become a cost-effective option, the researchers write.
Compared with no screening, the cost of liquid biopsy would have to fall by 94% for its ICER to drop below the willingness-to-pay threshold of $100,000 per life-year gained. When compared with stool-based tests, the cost of liquid biopsy would have to drop by 43%-80% to be cost-effective.
Liquid biopsy and the liquid biopsy after refusal of colonoscopy strategies had more life-years gained when polyp detection was introduced, but they did not achieve cost-effectiveness at liquid biopsy’s current price even with perfect performance.
“With current estimate of performance and cost,” liquid biopsy for CRC screening is not cost-effective, Dr. Hur told this news organization.
Liquid biopsy tests for CRC screening may become cost-effective in the future if they are significantly less expensive or if polyp detection is introduced along with a decrease in cost, Dr. Hur said.
Making blood-based CRC screening more effective and cost-effective “is more likely to depend on the ability to detect precancerous polyps than on the detection of CRC itself,” writes John Inadomi, MD, with University of Utah School of Medicine, Salt Lake City, in an invited commentary, also published online in JAMA Network Open.
The sensitivity of FIT for detecting advanced polyps is roughly 20%, whereas stool multitarget tests for blood and DNA or RNA detect 40%-45% of advanced polyps, Dr. Inadomi notes.
Blood-based tests, on the other hand, have been reported to detect 12%-16% of advanced polyps, “which is close to probability of a false-positive test,” he writes. “Because of their high cost, blood-based CRC screening will not be cost-effective unless they detect a greater proportion of advanced polyps than FIT.”
The need for follow-up colonoscopy in the case of a positive noncolonoscopy CRC screening test result is “an important concept that is not emphasized enough in clinical practice,” Dr. Inadomi adds. “Unfortunately, only 40% to 80% of people with positive noncolonoscopy screening test results follow-up with the requisite colonoscopy. Clinicians need to emphasize the necessity of the follow-up colonoscopy when discussing CRC screening options and adherence.”
Dr. Hur reported receiving consulting fees from Value Analytics outside the submitted work. Dr. Hur and co-authors Fay Kastrinos, MD, MPH, and Sheila Rustgi, MD, are supported by a grant from the National Cancer Institute. Co-author William Grady, MD, reported receiving personal fees from SEngine, Guardant Health, Freenome, Diacarta, Natera, Helio, Guidepoint, and GLG and nonfinancial support from LucidDx outside the submitted work. Grady also disclosed a patent pending for methylated gene biomarker for esophageal cancer. Co-author Yoanna Pumpalova, MD, reported receiving stock from Pfizer outside the submitted work. Inadomi reported receiving grants from Exact Sciences.
A version of this article appeared on Medscape.com.
Blood-based liquid biopsy tests for colorectal cancer (CRC) are in development and may soon hit the market, expanding potential options for patients who refuse traditional colonoscopy. But would they be a cost-effective screening tool?
Not according to an economic analysis by researchers at Columbia University Irving Medical Center in New York.
There is “intense research and patient and public interest” in blood-based cancer tests.
“However, liquid biopsy tests may not have sufficient performance and cost too much for them to be a viable strategy at this time,” corresponding author Chin Hur, MD, MPH, told this news organization.
The study was published online on November 16, 2023 in JAMA Network Open..
Better, Cheaper Liquid Biopsies Needed
The researchers developed a Markov model to compare the cost effectiveness of no screening and five CRC screening strategies: colonoscopy, liquid biopsy, liquid biopsy after nonadherence to colonoscopy, stool DNA, and fecal immunochemical test (FIT).
The model simulated a hypothetical cohort of unscreened adults at average risk for CRC with screening starting at age 45 years, in line with current US Preventive Services Task Force advice.
A strategy was considered cost-effective if it had an incremental cost-effectiveness ratio (ICER) below the US willingness-to-pay threshold of $100,000 per life-year gained.
According to their model, colonoscopy was the preferred (most cost-effective) strategy with an ICER of $28,071 per life-year gained.
Offering liquid biopsy screening to adults who refuse colonoscopy was the most effective strategy in terms of number of life-years gained, but it greatly exceeded the accepted threshold of $100,000 per life-year gained coming in at $377,538 per life-year gained. The cost of liquid biopsy would have to drop by 66% for this approach to become a cost-effective option, the researchers write.
Compared with no screening, the cost of liquid biopsy would have to fall by 94% for its ICER to drop below the willingness-to-pay threshold of $100,000 per life-year gained. When compared with stool-based tests, the cost of liquid biopsy would have to drop by 43%-80% to be cost-effective.
Liquid biopsy and the liquid biopsy after refusal of colonoscopy strategies had more life-years gained when polyp detection was introduced, but they did not achieve cost-effectiveness at liquid biopsy’s current price even with perfect performance.
“With current estimate of performance and cost,” liquid biopsy for CRC screening is not cost-effective, Dr. Hur told this news organization.
Liquid biopsy tests for CRC screening may become cost-effective in the future if they are significantly less expensive or if polyp detection is introduced along with a decrease in cost, Dr. Hur said.
Making blood-based CRC screening more effective and cost-effective “is more likely to depend on the ability to detect precancerous polyps than on the detection of CRC itself,” writes John Inadomi, MD, with University of Utah School of Medicine, Salt Lake City, in an invited commentary, also published online in JAMA Network Open.
The sensitivity of FIT for detecting advanced polyps is roughly 20%, whereas stool multitarget tests for blood and DNA or RNA detect 40%-45% of advanced polyps, Dr. Inadomi notes.
Blood-based tests, on the other hand, have been reported to detect 12%-16% of advanced polyps, “which is close to probability of a false-positive test,” he writes. “Because of their high cost, blood-based CRC screening will not be cost-effective unless they detect a greater proportion of advanced polyps than FIT.”
The need for follow-up colonoscopy in the case of a positive noncolonoscopy CRC screening test result is “an important concept that is not emphasized enough in clinical practice,” Dr. Inadomi adds. “Unfortunately, only 40% to 80% of people with positive noncolonoscopy screening test results follow-up with the requisite colonoscopy. Clinicians need to emphasize the necessity of the follow-up colonoscopy when discussing CRC screening options and adherence.”
Dr. Hur reported receiving consulting fees from Value Analytics outside the submitted work. Dr. Hur and co-authors Fay Kastrinos, MD, MPH, and Sheila Rustgi, MD, are supported by a grant from the National Cancer Institute. Co-author William Grady, MD, reported receiving personal fees from SEngine, Guardant Health, Freenome, Diacarta, Natera, Helio, Guidepoint, and GLG and nonfinancial support from LucidDx outside the submitted work. Grady also disclosed a patent pending for methylated gene biomarker for esophageal cancer. Co-author Yoanna Pumpalova, MD, reported receiving stock from Pfizer outside the submitted work. Inadomi reported receiving grants from Exact Sciences.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
