User login
Making social media work for your practice
Social media use is ubiquitous and, in the digital age, it is the ascendant form of communication. Individuals and organizations, digital immigrants (those born before the widespread adoption of digital technology), and digital natives alike are leveraging social media platforms, such as blogs, Facebook, Twitter, YouTube, and LinkedIn, to curate, consume, and share information across the spectrum of demographics and target audiences. In the United States, 7 in 10 Americans are using social media and, although young adults were early adopters, use among older adults is increasing rapidly.1
Furthermore, social media has cultivated remarkable opportunities in the dissemination of health information and disrupted traditional methods of patient–provider communication. The days when medically trained health professionals were the gatekeepers of health information are long gone. Approximately 50% of Americans seek health information online before seeing a physician.2 Patients and other consumers regularly access social media to search for information about diseases and treatments, engage with other patients, identify providers, and to express or rate their satisfaction with providers, clinics, and health systems.3-5 In addition, they trust online health information from doctors more than that from hospitals, health insurers, and drug companies.6 Not surprisingly, this has led to tremendous growth in use of social media by health care providers, hospitals, and health centers. More than 90% of US hospitals have a Facebook page and 50% have a Twitter account.7
There is ample opportunity to close the gap between patient and health care provider engagement in Social media, equip providers with the tools they need to be competent consumers and sharers of information in this digital exchange, and increase the pool of evidence-based information on GI and liver diseases on social media.12 However, there is limited published literature tailored to gastroenterologists and hepatologists. The goal of this article, therefore, is to provide a broad overview of best practices in the professional use of social media and highlight examples of novel applications in clinical practice.
Best practices: Getting started and maintaining a presence on social media
Social media can magnify your professional image, amplify your voice, and extend your reach and influence much faster than other methods. It also can be damaging if not used responsibly. Thus, we recommend the following approaches to responsible use of social media and cultivating your social media presence based on current evidence, professional organizations’ policy statements, and our combined experience. We initially presented these strategies during a Meet-the-Professor Luncheon at Digestive Disease Week® in Chicago (http://www.ddw.org/education/session-recordings).
Second, as with other aspects of medical training and practice, find a mentor to provide hands-on advice. This is particularly true if your general familiarity with the social media platforms is limited. If this is not available through your network of colleagues or workplace, we recommend exploring opportunities offered through your professional organization(s) such as the aforementioned Meet-the-Professor Luncheon at Digestive Diseases Week.
Third, know the privacy setting options on your social media platform(s) of choice and use them to your advantage. For example, on Facebook and Twitter, you can select an option that requests your permission before a friend or follower is added to your network. You also can tailor who (such as friends or followers only) can access your posted content directly. However, know that your content still may be made public if it is shared by one of your friends or followers.
Fourth, nurture your social media presence by sharing credible content deliberately, regularly, and, when appropriate, with attribution.
Fifth, diversify your content within the realm of your predefined objectives and/or goals and avoid a singular focus of self-promotion or the appearance of self-promotion. Top social media users suggest, and the authors agree, that your content should be only 25%-33% of your posts.
Sixth, thoroughly vet all content that you share. Avoid automatically sharing articles or posts because of a catchy headline. Read them before you post them. There may be details buried in them that are not credible or with which you do not agree.
Seventh, build community by connecting and engaging with other users on your social media platform(s) of choice.
Eighth, integrate multiple media (i.e., photos, videos, infographics) and/or social media platforms (i.e., embed link to YouTube or a website) to increase engagement.
Ninth, adhere to the code of ethics, governance, and privacy of the profession and of your employer.
Best practices: Privacy and governance in patient-oriented communication on social media
Two factors that have been of pivotal concern with the adoption of social media in the health care arena and led to many health care professionals being laggards as opposed to early adopters are privacy and governance. Will it violate the patient–provider relationship? What about the Health Insurance Portability and Accountability Act? How do I maintain boundaries between myself and the public at large? These are just a few of the questions that commonly are asked by those who are unfamiliar with social media etiquette for health care professionals. We highly recommend reviewing the position paper regarding online medical professionalism issued by the American College of Physicians and the Federation of State Medical Boards as a starting point.13 We believe the following to be contemporary guiding principles for GI health providers for maintaining a digital footprint on social media that reflects the ethical and professional standards of the field.
First, avoid sharing information that could be construed as a patient identifier without documented consent. This includes, but is not limited to, an identifiable specimen or photograph, and stories of care, rare conditions, and complications. Note that dates and location of care can lead to identification of a patient or care episode.
Second, recognize that personal and professional online profiles/pages are discoverable. Many advocate for separating the two as a means of shielding the public from elements of a private persona (i.e., family pictures and controversial opinions). However, the capacity to share and find comments and images on social media is much more powerful than the privacy settings on the various social media platforms. If you establish distinct personal and professional profiles, exercise caution before accepting friend or follow requests from patients on your personal profile. In addition, be cautious with your posts on private social media accounts because they rarely truly are private.
Third, avoid providing specific medical recommendations to individuals. This creates a patient–provider relationship and legal duty. Instead, recommend consultation with a health care provider and consider providing a link to general information on the topic (e.g., AGA information for patients at www.gastro.org/patientinfo).
Fourth, declare conflicts of interest, if applicable, when sharing information involving your clinical, research, and/or business practice.
Fifth, routinely monitor your online presence for accuracy and appropriateness of content posted by you and by others in reference to you. Know that our profession’s ethical standards for behavior extend to social media and we can be held accountable to colleagues and our employer if we violate them.
Many employers have become savvy to issues of governance in use of social media and institute policy recommendations to which employees are expected to adhere. If you are an employee, we recommend checking with your marketing and/or human resources department(s) in regards to this. If you are an employer and do not have such a policy on online professionalism, it is our hope that this article serves as a launching pad.
Novel applications for social media in clinical practice
Social media has been shown to be an effective medium for medical education through virtual journal clubs, moderated discussions or chats, and video sharing for teaching procedures, to name a few applications. Social media is used to collect data via polls or surveys, and to disseminate and track the views and downloads of published works. It is also a source for unsolicited, real-time feedback on patient experience and engagement through data-mining techniques, such as natural language processing and, more simply, for solicited feedback for patient satisfaction ratings. However, its role in academic promotion is less clear and is an area for which we see a great opportunity for growth.
Summary
We have outlined a high-level overview for why you should consider establishing and maintaining a professional presence on social media and how to accomplish this. These reasons include sharing information with colleagues, patients, and the public; amplifying the voice of physicians, a view that has diminished in the often-volatile health care environment; and promotion of the value of your work, be it patient care, advocacy, research, or education. You will have a smoother experience if you learn your local rules and policies and abide by our suggestions to avoid adverse outcomes. You will be most effective if you establish goals for your social media participation and revisit these goals over time for continued relevance and success and if you have consistent and valuable output that will support attainment of these goals. Welcome to the GI social media community! Be sure to follow Clinical Gastroenterology and Hepatology and the American Gastroenterological Association on Facebook (facebook.com/cghjournal and facebook.com/amergastroassn) and Twitter (@AGA_CGH and @AmerGastroAssn), and the coauthors (@DMGrayMD and @DrDeborahFisher) on Twitter.
References
1. Social Media Fact Sheet. Pew Research Center [updated January 12, 2017]. Available from http://www.pewinternet.org/fact-sheet/social-media/. Accessed: June 20, 2017.
2. Hesse B.W., Nelson D.E., Kreps G.L., et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618-24.
3. Moorhead S.A., Hazlett D.E., Harrison L., et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
4. Chou W.Y., Hunt Y.M., Beckjord E.B. et al. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11:e48.
5. Chretien K.C., Kind T. Social media and clinical care: ethical, professional, and social implications. Circulation. 2013;27:1413-21.
6. Social Media ‘likes’ Healthcare. PwC Health Research Institute; 2012. Available from https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf. Accessed: June 20, 2017.
7. Griffis H.M., Kilaru A.S., Werner R.M., et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16:e264.
8. Davis E.D., Tang S.J., Glover P.H., et al. Impact of social media on gastroenterologists in the United States. Dig Liver Dis. 2015;47:258-9.
9. Chiang A.L., Vartabedian B., Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroenterol. 2016;111:1082-4.
10. Reich J., Guo L., Hall J., et al. A survey of social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2678-87.
11. Timms C., Forton D.M., Poullis A. Social media use in patients with inflammatory bowel disease and chronic viral hepatitis. Clin Med. 2014;14:215.
12. Prasad B. Social media, health care, and social networking. Gastrointest Endosc. 2013;77:492-5.
13. Farnan J.M., Snyder Sulmasy L., Worster B.K., et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158:620-7.
14. Cabrera D., Vartabedian B.S., Spinner R.J., et al. More than likes and tweets: creating social media portfolios for academic promotion and tenure. J Grad Med Educ. 2017;9:421-5.
15. Cabrera D. Mayo Clinic includes social media scholarship activities in academic advancement. Available from https://socialmedia.mayoclinic.org/2016/05/25/mayo-clinic-includes-social-media-scholarship-activities-in-academic-advancement/
Date: May 26, 2016. (Accessed: July 1, 2017).
16. Freitag C.E., Arnold M.A., Gardner J.M., et al. If you are not on social media, here’s what you’re missing! #DoTheThing. Arch Pathol Lab Med. 2017; (Epub ahead of print).
17. Stukus D.R. How I used twitter to get promoted in academic medicine. Available from http://www.kevinmd.com/blog/2016/10/used-twitter-get-promoted-academic-medicine.html. Date: October 9, 2016. (Accessed: July 1, 2017).
Dr. Gray is in the division of gastroenterology, hepatology, and nutrition, department of medicine, The Ohio State University College of Medicine, Columbus; Dr. Fisher is in the division of gastroenterology, department of medicine, Duke University, Durham, N.C. The authors disclose no conflicts of interest.
Social media use is ubiquitous and, in the digital age, it is the ascendant form of communication. Individuals and organizations, digital immigrants (those born before the widespread adoption of digital technology), and digital natives alike are leveraging social media platforms, such as blogs, Facebook, Twitter, YouTube, and LinkedIn, to curate, consume, and share information across the spectrum of demographics and target audiences. In the United States, 7 in 10 Americans are using social media and, although young adults were early adopters, use among older adults is increasing rapidly.1
Furthermore, social media has cultivated remarkable opportunities in the dissemination of health information and disrupted traditional methods of patient–provider communication. The days when medically trained health professionals were the gatekeepers of health information are long gone. Approximately 50% of Americans seek health information online before seeing a physician.2 Patients and other consumers regularly access social media to search for information about diseases and treatments, engage with other patients, identify providers, and to express or rate their satisfaction with providers, clinics, and health systems.3-5 In addition, they trust online health information from doctors more than that from hospitals, health insurers, and drug companies.6 Not surprisingly, this has led to tremendous growth in use of social media by health care providers, hospitals, and health centers. More than 90% of US hospitals have a Facebook page and 50% have a Twitter account.7
There is ample opportunity to close the gap between patient and health care provider engagement in Social media, equip providers with the tools they need to be competent consumers and sharers of information in this digital exchange, and increase the pool of evidence-based information on GI and liver diseases on social media.12 However, there is limited published literature tailored to gastroenterologists and hepatologists. The goal of this article, therefore, is to provide a broad overview of best practices in the professional use of social media and highlight examples of novel applications in clinical practice.
Best practices: Getting started and maintaining a presence on social media
Social media can magnify your professional image, amplify your voice, and extend your reach and influence much faster than other methods. It also can be damaging if not used responsibly. Thus, we recommend the following approaches to responsible use of social media and cultivating your social media presence based on current evidence, professional organizations’ policy statements, and our combined experience. We initially presented these strategies during a Meet-the-Professor Luncheon at Digestive Disease Week® in Chicago (http://www.ddw.org/education/session-recordings).
Second, as with other aspects of medical training and practice, find a mentor to provide hands-on advice. This is particularly true if your general familiarity with the social media platforms is limited. If this is not available through your network of colleagues or workplace, we recommend exploring opportunities offered through your professional organization(s) such as the aforementioned Meet-the-Professor Luncheon at Digestive Diseases Week.
Third, know the privacy setting options on your social media platform(s) of choice and use them to your advantage. For example, on Facebook and Twitter, you can select an option that requests your permission before a friend or follower is added to your network. You also can tailor who (such as friends or followers only) can access your posted content directly. However, know that your content still may be made public if it is shared by one of your friends or followers.
Fourth, nurture your social media presence by sharing credible content deliberately, regularly, and, when appropriate, with attribution.
Fifth, diversify your content within the realm of your predefined objectives and/or goals and avoid a singular focus of self-promotion or the appearance of self-promotion. Top social media users suggest, and the authors agree, that your content should be only 25%-33% of your posts.
Sixth, thoroughly vet all content that you share. Avoid automatically sharing articles or posts because of a catchy headline. Read them before you post them. There may be details buried in them that are not credible or with which you do not agree.
Seventh, build community by connecting and engaging with other users on your social media platform(s) of choice.
Eighth, integrate multiple media (i.e., photos, videos, infographics) and/or social media platforms (i.e., embed link to YouTube or a website) to increase engagement.
Ninth, adhere to the code of ethics, governance, and privacy of the profession and of your employer.
Best practices: Privacy and governance in patient-oriented communication on social media
Two factors that have been of pivotal concern with the adoption of social media in the health care arena and led to many health care professionals being laggards as opposed to early adopters are privacy and governance. Will it violate the patient–provider relationship? What about the Health Insurance Portability and Accountability Act? How do I maintain boundaries between myself and the public at large? These are just a few of the questions that commonly are asked by those who are unfamiliar with social media etiquette for health care professionals. We highly recommend reviewing the position paper regarding online medical professionalism issued by the American College of Physicians and the Federation of State Medical Boards as a starting point.13 We believe the following to be contemporary guiding principles for GI health providers for maintaining a digital footprint on social media that reflects the ethical and professional standards of the field.
First, avoid sharing information that could be construed as a patient identifier without documented consent. This includes, but is not limited to, an identifiable specimen or photograph, and stories of care, rare conditions, and complications. Note that dates and location of care can lead to identification of a patient or care episode.
Second, recognize that personal and professional online profiles/pages are discoverable. Many advocate for separating the two as a means of shielding the public from elements of a private persona (i.e., family pictures and controversial opinions). However, the capacity to share and find comments and images on social media is much more powerful than the privacy settings on the various social media platforms. If you establish distinct personal and professional profiles, exercise caution before accepting friend or follow requests from patients on your personal profile. In addition, be cautious with your posts on private social media accounts because they rarely truly are private.
Third, avoid providing specific medical recommendations to individuals. This creates a patient–provider relationship and legal duty. Instead, recommend consultation with a health care provider and consider providing a link to general information on the topic (e.g., AGA information for patients at www.gastro.org/patientinfo).
Fourth, declare conflicts of interest, if applicable, when sharing information involving your clinical, research, and/or business practice.
Fifth, routinely monitor your online presence for accuracy and appropriateness of content posted by you and by others in reference to you. Know that our profession’s ethical standards for behavior extend to social media and we can be held accountable to colleagues and our employer if we violate them.
Many employers have become savvy to issues of governance in use of social media and institute policy recommendations to which employees are expected to adhere. If you are an employee, we recommend checking with your marketing and/or human resources department(s) in regards to this. If you are an employer and do not have such a policy on online professionalism, it is our hope that this article serves as a launching pad.
Novel applications for social media in clinical practice
Social media has been shown to be an effective medium for medical education through virtual journal clubs, moderated discussions or chats, and video sharing for teaching procedures, to name a few applications. Social media is used to collect data via polls or surveys, and to disseminate and track the views and downloads of published works. It is also a source for unsolicited, real-time feedback on patient experience and engagement through data-mining techniques, such as natural language processing and, more simply, for solicited feedback for patient satisfaction ratings. However, its role in academic promotion is less clear and is an area for which we see a great opportunity for growth.

Summary
We have outlined a high-level overview for why you should consider establishing and maintaining a professional presence on social media and how to accomplish this. These reasons include sharing information with colleagues, patients, and the public; amplifying the voice of physicians, a view that has diminished in the often-volatile health care environment; and promotion of the value of your work, be it patient care, advocacy, research, or education. You will have a smoother experience if you learn your local rules and policies and abide by our suggestions to avoid adverse outcomes. You will be most effective if you establish goals for your social media participation and revisit these goals over time for continued relevance and success and if you have consistent and valuable output that will support attainment of these goals. Welcome to the GI social media community! Be sure to follow Clinical Gastroenterology and Hepatology and the American Gastroenterological Association on Facebook (facebook.com/cghjournal and facebook.com/amergastroassn) and Twitter (@AGA_CGH and @AmerGastroAssn), and the coauthors (@DMGrayMD and @DrDeborahFisher) on Twitter.
References
1. Social Media Fact Sheet. Pew Research Center [updated January 12, 2017]. Available from http://www.pewinternet.org/fact-sheet/social-media/. Accessed: June 20, 2017.
2. Hesse B.W., Nelson D.E., Kreps G.L., et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618-24.
3. Moorhead S.A., Hazlett D.E., Harrison L., et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
4. Chou W.Y., Hunt Y.M., Beckjord E.B. et al. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11:e48.
5. Chretien K.C., Kind T. Social media and clinical care: ethical, professional, and social implications. Circulation. 2013;27:1413-21.
6. Social Media ‘likes’ Healthcare. PwC Health Research Institute; 2012. Available from https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf. Accessed: June 20, 2017.
7. Griffis H.M., Kilaru A.S., Werner R.M., et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16:e264.
8. Davis E.D., Tang S.J., Glover P.H., et al. Impact of social media on gastroenterologists in the United States. Dig Liver Dis. 2015;47:258-9.
9. Chiang A.L., Vartabedian B., Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroenterol. 2016;111:1082-4.
10. Reich J., Guo L., Hall J., et al. A survey of social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2678-87.
11. Timms C., Forton D.M., Poullis A. Social media use in patients with inflammatory bowel disease and chronic viral hepatitis. Clin Med. 2014;14:215.
12. Prasad B. Social media, health care, and social networking. Gastrointest Endosc. 2013;77:492-5.
13. Farnan J.M., Snyder Sulmasy L., Worster B.K., et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158:620-7.
14. Cabrera D., Vartabedian B.S., Spinner R.J., et al. More than likes and tweets: creating social media portfolios for academic promotion and tenure. J Grad Med Educ. 2017;9:421-5.
15. Cabrera D. Mayo Clinic includes social media scholarship activities in academic advancement. Available from https://socialmedia.mayoclinic.org/2016/05/25/mayo-clinic-includes-social-media-scholarship-activities-in-academic-advancement/
Date: May 26, 2016. (Accessed: July 1, 2017).
16. Freitag C.E., Arnold M.A., Gardner J.M., et al. If you are not on social media, here’s what you’re missing! #DoTheThing. Arch Pathol Lab Med. 2017; (Epub ahead of print).
17. Stukus D.R. How I used twitter to get promoted in academic medicine. Available from http://www.kevinmd.com/blog/2016/10/used-twitter-get-promoted-academic-medicine.html. Date: October 9, 2016. (Accessed: July 1, 2017).
Dr. Gray is in the division of gastroenterology, hepatology, and nutrition, department of medicine, The Ohio State University College of Medicine, Columbus; Dr. Fisher is in the division of gastroenterology, department of medicine, Duke University, Durham, N.C. The authors disclose no conflicts of interest.
Social media use is ubiquitous and, in the digital age, it is the ascendant form of communication. Individuals and organizations, digital immigrants (those born before the widespread adoption of digital technology), and digital natives alike are leveraging social media platforms, such as blogs, Facebook, Twitter, YouTube, and LinkedIn, to curate, consume, and share information across the spectrum of demographics and target audiences. In the United States, 7 in 10 Americans are using social media and, although young adults were early adopters, use among older adults is increasing rapidly.1
Furthermore, social media has cultivated remarkable opportunities in the dissemination of health information and disrupted traditional methods of patient–provider communication. The days when medically trained health professionals were the gatekeepers of health information are long gone. Approximately 50% of Americans seek health information online before seeing a physician.2 Patients and other consumers regularly access social media to search for information about diseases and treatments, engage with other patients, identify providers, and to express or rate their satisfaction with providers, clinics, and health systems.3-5 In addition, they trust online health information from doctors more than that from hospitals, health insurers, and drug companies.6 Not surprisingly, this has led to tremendous growth in use of social media by health care providers, hospitals, and health centers. More than 90% of US hospitals have a Facebook page and 50% have a Twitter account.7
There is ample opportunity to close the gap between patient and health care provider engagement in Social media, equip providers with the tools they need to be competent consumers and sharers of information in this digital exchange, and increase the pool of evidence-based information on GI and liver diseases on social media.12 However, there is limited published literature tailored to gastroenterologists and hepatologists. The goal of this article, therefore, is to provide a broad overview of best practices in the professional use of social media and highlight examples of novel applications in clinical practice.
Best practices: Getting started and maintaining a presence on social media
Social media can magnify your professional image, amplify your voice, and extend your reach and influence much faster than other methods. It also can be damaging if not used responsibly. Thus, we recommend the following approaches to responsible use of social media and cultivating your social media presence based on current evidence, professional organizations’ policy statements, and our combined experience. We initially presented these strategies during a Meet-the-Professor Luncheon at Digestive Disease Week® in Chicago (http://www.ddw.org/education/session-recordings).
Second, as with other aspects of medical training and practice, find a mentor to provide hands-on advice. This is particularly true if your general familiarity with the social media platforms is limited. If this is not available through your network of colleagues or workplace, we recommend exploring opportunities offered through your professional organization(s) such as the aforementioned Meet-the-Professor Luncheon at Digestive Diseases Week.
Third, know the privacy setting options on your social media platform(s) of choice and use them to your advantage. For example, on Facebook and Twitter, you can select an option that requests your permission before a friend or follower is added to your network. You also can tailor who (such as friends or followers only) can access your posted content directly. However, know that your content still may be made public if it is shared by one of your friends or followers.
Fourth, nurture your social media presence by sharing credible content deliberately, regularly, and, when appropriate, with attribution.
Fifth, diversify your content within the realm of your predefined objectives and/or goals and avoid a singular focus of self-promotion or the appearance of self-promotion. Top social media users suggest, and the authors agree, that your content should be only 25%-33% of your posts.
Sixth, thoroughly vet all content that you share. Avoid automatically sharing articles or posts because of a catchy headline. Read them before you post them. There may be details buried in them that are not credible or with which you do not agree.
Seventh, build community by connecting and engaging with other users on your social media platform(s) of choice.
Eighth, integrate multiple media (i.e., photos, videos, infographics) and/or social media platforms (i.e., embed link to YouTube or a website) to increase engagement.
Ninth, adhere to the code of ethics, governance, and privacy of the profession and of your employer.
Best practices: Privacy and governance in patient-oriented communication on social media
Two factors that have been of pivotal concern with the adoption of social media in the health care arena and led to many health care professionals being laggards as opposed to early adopters are privacy and governance. Will it violate the patient–provider relationship? What about the Health Insurance Portability and Accountability Act? How do I maintain boundaries between myself and the public at large? These are just a few of the questions that commonly are asked by those who are unfamiliar with social media etiquette for health care professionals. We highly recommend reviewing the position paper regarding online medical professionalism issued by the American College of Physicians and the Federation of State Medical Boards as a starting point.13 We believe the following to be contemporary guiding principles for GI health providers for maintaining a digital footprint on social media that reflects the ethical and professional standards of the field.
First, avoid sharing information that could be construed as a patient identifier without documented consent. This includes, but is not limited to, an identifiable specimen or photograph, and stories of care, rare conditions, and complications. Note that dates and location of care can lead to identification of a patient or care episode.
Second, recognize that personal and professional online profiles/pages are discoverable. Many advocate for separating the two as a means of shielding the public from elements of a private persona (i.e., family pictures and controversial opinions). However, the capacity to share and find comments and images on social media is much more powerful than the privacy settings on the various social media platforms. If you establish distinct personal and professional profiles, exercise caution before accepting friend or follow requests from patients on your personal profile. In addition, be cautious with your posts on private social media accounts because they rarely truly are private.
Third, avoid providing specific medical recommendations to individuals. This creates a patient–provider relationship and legal duty. Instead, recommend consultation with a health care provider and consider providing a link to general information on the topic (e.g., AGA information for patients at www.gastro.org/patientinfo).
Fourth, declare conflicts of interest, if applicable, when sharing information involving your clinical, research, and/or business practice.
Fifth, routinely monitor your online presence for accuracy and appropriateness of content posted by you and by others in reference to you. Know that our profession’s ethical standards for behavior extend to social media and we can be held accountable to colleagues and our employer if we violate them.
Many employers have become savvy to issues of governance in use of social media and institute policy recommendations to which employees are expected to adhere. If you are an employee, we recommend checking with your marketing and/or human resources department(s) in regards to this. If you are an employer and do not have such a policy on online professionalism, it is our hope that this article serves as a launching pad.
Novel applications for social media in clinical practice
Social media has been shown to be an effective medium for medical education through virtual journal clubs, moderated discussions or chats, and video sharing for teaching procedures, to name a few applications. Social media is used to collect data via polls or surveys, and to disseminate and track the views and downloads of published works. It is also a source for unsolicited, real-time feedback on patient experience and engagement through data-mining techniques, such as natural language processing and, more simply, for solicited feedback for patient satisfaction ratings. However, its role in academic promotion is less clear and is an area for which we see a great opportunity for growth.

Summary
We have outlined a high-level overview for why you should consider establishing and maintaining a professional presence on social media and how to accomplish this. These reasons include sharing information with colleagues, patients, and the public; amplifying the voice of physicians, a view that has diminished in the often-volatile health care environment; and promotion of the value of your work, be it patient care, advocacy, research, or education. You will have a smoother experience if you learn your local rules and policies and abide by our suggestions to avoid adverse outcomes. You will be most effective if you establish goals for your social media participation and revisit these goals over time for continued relevance and success and if you have consistent and valuable output that will support attainment of these goals. Welcome to the GI social media community! Be sure to follow Clinical Gastroenterology and Hepatology and the American Gastroenterological Association on Facebook (facebook.com/cghjournal and facebook.com/amergastroassn) and Twitter (@AGA_CGH and @AmerGastroAssn), and the coauthors (@DMGrayMD and @DrDeborahFisher) on Twitter.
References
1. Social Media Fact Sheet. Pew Research Center [updated January 12, 2017]. Available from http://www.pewinternet.org/fact-sheet/social-media/. Accessed: June 20, 2017.
2. Hesse B.W., Nelson D.E., Kreps G.L., et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618-24.
3. Moorhead S.A., Hazlett D.E., Harrison L., et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
4. Chou W.Y., Hunt Y.M., Beckjord E.B. et al. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11:e48.
5. Chretien K.C., Kind T. Social media and clinical care: ethical, professional, and social implications. Circulation. 2013;27:1413-21.
6. Social Media ‘likes’ Healthcare. PwC Health Research Institute; 2012. Available from https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf. Accessed: June 20, 2017.
7. Griffis H.M., Kilaru A.S., Werner R.M., et al. Use of social media across US hospitals: descriptive analysis of adoption and utilization. J Med Internet Res. 2014;16:e264.
8. Davis E.D., Tang S.J., Glover P.H., et al. Impact of social media on gastroenterologists in the United States. Dig Liver Dis. 2015;47:258-9.
9. Chiang A.L., Vartabedian B., Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroenterol. 2016;111:1082-4.
10. Reich J., Guo L., Hall J., et al. A survey of social media use and preferences in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2678-87.
11. Timms C., Forton D.M., Poullis A. Social media use in patients with inflammatory bowel disease and chronic viral hepatitis. Clin Med. 2014;14:215.
12. Prasad B. Social media, health care, and social networking. Gastrointest Endosc. 2013;77:492-5.
13. Farnan J.M., Snyder Sulmasy L., Worster B.K., et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158:620-7.
14. Cabrera D., Vartabedian B.S., Spinner R.J., et al. More than likes and tweets: creating social media portfolios for academic promotion and tenure. J Grad Med Educ. 2017;9:421-5.
15. Cabrera D. Mayo Clinic includes social media scholarship activities in academic advancement. Available from https://socialmedia.mayoclinic.org/2016/05/25/mayo-clinic-includes-social-media-scholarship-activities-in-academic-advancement/
Date: May 26, 2016. (Accessed: July 1, 2017).
16. Freitag C.E., Arnold M.A., Gardner J.M., et al. If you are not on social media, here’s what you’re missing! #DoTheThing. Arch Pathol Lab Med. 2017; (Epub ahead of print).
17. Stukus D.R. How I used twitter to get promoted in academic medicine. Available from http://www.kevinmd.com/blog/2016/10/used-twitter-get-promoted-academic-medicine.html. Date: October 9, 2016. (Accessed: July 1, 2017).
Dr. Gray is in the division of gastroenterology, hepatology, and nutrition, department of medicine, The Ohio State University College of Medicine, Columbus; Dr. Fisher is in the division of gastroenterology, department of medicine, Duke University, Durham, N.C. The authors disclose no conflicts of interest.
RNS May Reduce the Risk of SUDEP
Responsive neurostimulation (RNS) appears to reduce the risk of sudden unexpected death in epilepsy (SUDEP), according to research published online ahead of print January 16 in Epilepsia. The results provide evidence that treatments that reduce seizures decrease the risk of SUDEP, according to the authors. The data also indicate that not every SUDEP follows a seizure.
Orrin Devinsky, MD, Director of the Comprehensive Epilepsy Center at New York University Langone Medical Center, and colleagues examined data for all deaths in patients with epilepsy who received RNS in clinical trials or following FDA approval through May 5, 2016, and adjudicated them for SUDEP. In all, 256 patients received treatment during trials, and 451 received treatment after FDA approval.
The investigators observed 14 deaths, including two possible, one probable, and four definite cases of SUDEP. The rate of probable or definite SUDEP was 2.0/1,000 over 2,036 patient stimulation years and 2.3/1,000 over 2,208 patient implant years. The age-adjusted standardized mortality ratio for definite and probable SUDEP was 0.75.
“The SUDEP rate of 2.0/1,000 patient stimulation years among patients undergoing treatment with RNS is favorable relative to patients [with treatment-resistant epilepsy] randomized to the placebo arm of add-on drug studies (SUDEP rate of 6.1/1,000 patient years), patients who were referred for epilepsy surgery but did not receive epilepsy surgery (SUDEP rate of 6.3/1,000 patient years), and patients with recurrent seizures after epilepsy surgery (SUDEP rate 6.3/1,000 patient years),” said the authors. “Future explorations can examine whether certain seizure patterns are particularly associated with SUDEP.”
—Erik Greb
Suggested Reading
Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia. 2018 Jan 16 [Epub ahead of print].
Responsive neurostimulation (RNS) appears to reduce the risk of sudden unexpected death in epilepsy (SUDEP), according to research published online ahead of print January 16 in Epilepsia. The results provide evidence that treatments that reduce seizures decrease the risk of SUDEP, according to the authors. The data also indicate that not every SUDEP follows a seizure.
Orrin Devinsky, MD, Director of the Comprehensive Epilepsy Center at New York University Langone Medical Center, and colleagues examined data for all deaths in patients with epilepsy who received RNS in clinical trials or following FDA approval through May 5, 2016, and adjudicated them for SUDEP. In all, 256 patients received treatment during trials, and 451 received treatment after FDA approval.
The investigators observed 14 deaths, including two possible, one probable, and four definite cases of SUDEP. The rate of probable or definite SUDEP was 2.0/1,000 over 2,036 patient stimulation years and 2.3/1,000 over 2,208 patient implant years. The age-adjusted standardized mortality ratio for definite and probable SUDEP was 0.75.
“The SUDEP rate of 2.0/1,000 patient stimulation years among patients undergoing treatment with RNS is favorable relative to patients [with treatment-resistant epilepsy] randomized to the placebo arm of add-on drug studies (SUDEP rate of 6.1/1,000 patient years), patients who were referred for epilepsy surgery but did not receive epilepsy surgery (SUDEP rate of 6.3/1,000 patient years), and patients with recurrent seizures after epilepsy surgery (SUDEP rate 6.3/1,000 patient years),” said the authors. “Future explorations can examine whether certain seizure patterns are particularly associated with SUDEP.”
—Erik Greb
Suggested Reading
Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia. 2018 Jan 16 [Epub ahead of print].
Responsive neurostimulation (RNS) appears to reduce the risk of sudden unexpected death in epilepsy (SUDEP), according to research published online ahead of print January 16 in Epilepsia. The results provide evidence that treatments that reduce seizures decrease the risk of SUDEP, according to the authors. The data also indicate that not every SUDEP follows a seizure.
Orrin Devinsky, MD, Director of the Comprehensive Epilepsy Center at New York University Langone Medical Center, and colleagues examined data for all deaths in patients with epilepsy who received RNS in clinical trials or following FDA approval through May 5, 2016, and adjudicated them for SUDEP. In all, 256 patients received treatment during trials, and 451 received treatment after FDA approval.
The investigators observed 14 deaths, including two possible, one probable, and four definite cases of SUDEP. The rate of probable or definite SUDEP was 2.0/1,000 over 2,036 patient stimulation years and 2.3/1,000 over 2,208 patient implant years. The age-adjusted standardized mortality ratio for definite and probable SUDEP was 0.75.
“The SUDEP rate of 2.0/1,000 patient stimulation years among patients undergoing treatment with RNS is favorable relative to patients [with treatment-resistant epilepsy] randomized to the placebo arm of add-on drug studies (SUDEP rate of 6.1/1,000 patient years), patients who were referred for epilepsy surgery but did not receive epilepsy surgery (SUDEP rate of 6.3/1,000 patient years), and patients with recurrent seizures after epilepsy surgery (SUDEP rate 6.3/1,000 patient years),” said the authors. “Future explorations can examine whether certain seizure patterns are particularly associated with SUDEP.”
—Erik Greb
Suggested Reading
Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia. 2018 Jan 16 [Epub ahead of print].
Diagnostic delays, morbidity, and epidural abscesses
Clinical question: What is the frequency of diagnostic delays in epidural abscesses, and what factors may contribute to these delays?
Background: Diagnostic evaluation of back pain can be challenging. Missed diagnosis of serious conditions such as epidural abscesses can lead to significant morbidity.
Setting: Veterans Affairs Electronic Medical Record database from more than 1,700 VA outpatient and inpatient facilities in the United States.
Synopsis: Of the 119 patients with a new diagnosis of spinal epidural abscess, 55.5% were felt to have experienced a diagnostic error, defined by the study authors as a missed opportunity to evaluate a red flag (e.g. weight loss, neurologic deficit, fever) in a timely or appropriate manner. There was a significant difference in the time to diagnosis between patients with and without a diagnostic error (4 versus 12 days, P less than .01). Of those cases involving diagnostic error, 60.6% were felt to have resulted in serious patient harm and 12.1% in patient death. The most commonly missed red flags were fever, focal neurological deficits, and signs of active infection.
Based on these findings, the authors suggest that future intervention focus on improving information gathering during patient-physician encounter and physician education about existing guidelines.
The limitations of this study include its use of data from a single health system, and the employment of chart reviews instead of a root cause analysis based on provider and patient interviews.
Bottom line: A delay in diagnosis resulting in patient harm or death may occur frequently in cases of epidural abscesses. Further work on targeted interventions to reduce error and prevent harm are needed.
Citation: Bhise V, Meyer A, Singh H, et al. Diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-81.
Dr. Pizza is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What is the frequency of diagnostic delays in epidural abscesses, and what factors may contribute to these delays?
Background: Diagnostic evaluation of back pain can be challenging. Missed diagnosis of serious conditions such as epidural abscesses can lead to significant morbidity.
Setting: Veterans Affairs Electronic Medical Record database from more than 1,700 VA outpatient and inpatient facilities in the United States.
Synopsis: Of the 119 patients with a new diagnosis of spinal epidural abscess, 55.5% were felt to have experienced a diagnostic error, defined by the study authors as a missed opportunity to evaluate a red flag (e.g. weight loss, neurologic deficit, fever) in a timely or appropriate manner. There was a significant difference in the time to diagnosis between patients with and without a diagnostic error (4 versus 12 days, P less than .01). Of those cases involving diagnostic error, 60.6% were felt to have resulted in serious patient harm and 12.1% in patient death. The most commonly missed red flags were fever, focal neurological deficits, and signs of active infection.
Based on these findings, the authors suggest that future intervention focus on improving information gathering during patient-physician encounter and physician education about existing guidelines.
The limitations of this study include its use of data from a single health system, and the employment of chart reviews instead of a root cause analysis based on provider and patient interviews.
Bottom line: A delay in diagnosis resulting in patient harm or death may occur frequently in cases of epidural abscesses. Further work on targeted interventions to reduce error and prevent harm are needed.
Citation: Bhise V, Meyer A, Singh H, et al. Diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-81.
Dr. Pizza is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What is the frequency of diagnostic delays in epidural abscesses, and what factors may contribute to these delays?
Background: Diagnostic evaluation of back pain can be challenging. Missed diagnosis of serious conditions such as epidural abscesses can lead to significant morbidity.
Setting: Veterans Affairs Electronic Medical Record database from more than 1,700 VA outpatient and inpatient facilities in the United States.
Synopsis: Of the 119 patients with a new diagnosis of spinal epidural abscess, 55.5% were felt to have experienced a diagnostic error, defined by the study authors as a missed opportunity to evaluate a red flag (e.g. weight loss, neurologic deficit, fever) in a timely or appropriate manner. There was a significant difference in the time to diagnosis between patients with and without a diagnostic error (4 versus 12 days, P less than .01). Of those cases involving diagnostic error, 60.6% were felt to have resulted in serious patient harm and 12.1% in patient death. The most commonly missed red flags were fever, focal neurological deficits, and signs of active infection.
Based on these findings, the authors suggest that future intervention focus on improving information gathering during patient-physician encounter and physician education about existing guidelines.
The limitations of this study include its use of data from a single health system, and the employment of chart reviews instead of a root cause analysis based on provider and patient interviews.
Bottom line: A delay in diagnosis resulting in patient harm or death may occur frequently in cases of epidural abscesses. Further work on targeted interventions to reduce error and prevent harm are needed.
Citation: Bhise V, Meyer A, Singh H, et al. Diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-81.
Dr. Pizza is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Parental leave not available to all academic surgeons
Paid parental leave policies have been unevenly adopted among academic medical centers, according to a study published in the Journal of Surgical Research. These policies, or their lack, may have important ramifications for recruiting and specialty selection by surgeons, and for women of child-rearing age in particular.
Parental leave for surgeons has been championed by the American College of Surgeons, among other professional societies, in formal statements and supportive policies in recent years.
Dina S. Itum, MD, a fifth-year resident in the department of surgery, University of Texas Southwestern Medical Center, Houston, and a research team looked into how widespread parental leave is for surgeons in US medical centers. Their sample was the 91 top-ranked academic medical centers identified by U.S. News & World Report in 2016. The method used by U.S. News & World Report for ranking medical centers is based on student selectivity, dean and residency directors’ peer assessment of national institutions, faculty to student ratio, and the dollar amount in NIH research grants received.
“Parental leave” was defined by the research team as any leave dedicated to new mothers, fathers and/or primary caregivers after childbirth or adoption. “Paid leave” was defined as some protected leave with salary without mandated use of sick leave or vacation leave.
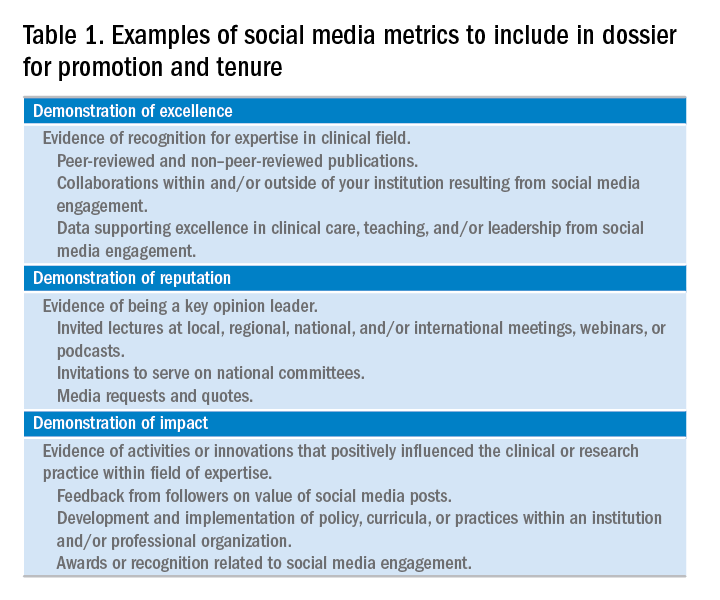
The study found that among the top-ranked 91 institutions, 48 (53%) offered some form of paid parental leave to faculty surgeons. The higher the rating, the more likely the institution offered paid parental leave: 77% of those in the top third of rankings vs. 53% in the middle third and 29% in the bottom third. Private institutions were more likely to offer paid leave of 6 weeks or longer; 67% vs. 33% of public institutions.
The investigators posed a question: Did these institutions implement the policy to attract the top talent, or did the policy improve faculty morale and productivity leading to a higher ranking? The study does not answer the question, but the investigators consider it an important issue for further study.
The investigators also suggested that surgeons of child-rearing age use parental leave information their in their own employment negotiations. “As physicians, we are aware of the health care benefits associated with parental leave, and as leaders within our communities, we should be at the forefront of supporting further advancement of this benefit,” the investigators wrote.
The investigators reported having no financial disclosures.
SOURCE: Itum DS et al. J Surg Res. 2018 Feb 3. doi. org/10.1016/j.jss.2018.01.001.
Paid parental leave policies have been unevenly adopted among academic medical centers, according to a study published in the Journal of Surgical Research. These policies, or their lack, may have important ramifications for recruiting and specialty selection by surgeons, and for women of child-rearing age in particular.
Parental leave for surgeons has been championed by the American College of Surgeons, among other professional societies, in formal statements and supportive policies in recent years.
Dina S. Itum, MD, a fifth-year resident in the department of surgery, University of Texas Southwestern Medical Center, Houston, and a research team looked into how widespread parental leave is for surgeons in US medical centers. Their sample was the 91 top-ranked academic medical centers identified by U.S. News & World Report in 2016. The method used by U.S. News & World Report for ranking medical centers is based on student selectivity, dean and residency directors’ peer assessment of national institutions, faculty to student ratio, and the dollar amount in NIH research grants received.
“Parental leave” was defined by the research team as any leave dedicated to new mothers, fathers and/or primary caregivers after childbirth or adoption. “Paid leave” was defined as some protected leave with salary without mandated use of sick leave or vacation leave.
The study found that among the top-ranked 91 institutions, 48 (53%) offered some form of paid parental leave to faculty surgeons. The higher the rating, the more likely the institution offered paid parental leave: 77% of those in the top third of rankings vs. 53% in the middle third and 29% in the bottom third. Private institutions were more likely to offer paid leave of 6 weeks or longer; 67% vs. 33% of public institutions.
The investigators posed a question: Did these institutions implement the policy to attract the top talent, or did the policy improve faculty morale and productivity leading to a higher ranking? The study does not answer the question, but the investigators consider it an important issue for further study.
The investigators also suggested that surgeons of child-rearing age use parental leave information their in their own employment negotiations. “As physicians, we are aware of the health care benefits associated with parental leave, and as leaders within our communities, we should be at the forefront of supporting further advancement of this benefit,” the investigators wrote.
The investigators reported having no financial disclosures.
SOURCE: Itum DS et al. J Surg Res. 2018 Feb 3. doi. org/10.1016/j.jss.2018.01.001.
Paid parental leave policies have been unevenly adopted among academic medical centers, according to a study published in the Journal of Surgical Research. These policies, or their lack, may have important ramifications for recruiting and specialty selection by surgeons, and for women of child-rearing age in particular.
Parental leave for surgeons has been championed by the American College of Surgeons, among other professional societies, in formal statements and supportive policies in recent years.
Dina S. Itum, MD, a fifth-year resident in the department of surgery, University of Texas Southwestern Medical Center, Houston, and a research team looked into how widespread parental leave is for surgeons in US medical centers. Their sample was the 91 top-ranked academic medical centers identified by U.S. News & World Report in 2016. The method used by U.S. News & World Report for ranking medical centers is based on student selectivity, dean and residency directors’ peer assessment of national institutions, faculty to student ratio, and the dollar amount in NIH research grants received.
“Parental leave” was defined by the research team as any leave dedicated to new mothers, fathers and/or primary caregivers after childbirth or adoption. “Paid leave” was defined as some protected leave with salary without mandated use of sick leave or vacation leave.
The study found that among the top-ranked 91 institutions, 48 (53%) offered some form of paid parental leave to faculty surgeons. The higher the rating, the more likely the institution offered paid parental leave: 77% of those in the top third of rankings vs. 53% in the middle third and 29% in the bottom third. Private institutions were more likely to offer paid leave of 6 weeks or longer; 67% vs. 33% of public institutions.
The investigators posed a question: Did these institutions implement the policy to attract the top talent, or did the policy improve faculty morale and productivity leading to a higher ranking? The study does not answer the question, but the investigators consider it an important issue for further study.
The investigators also suggested that surgeons of child-rearing age use parental leave information their in their own employment negotiations. “As physicians, we are aware of the health care benefits associated with parental leave, and as leaders within our communities, we should be at the forefront of supporting further advancement of this benefit,” the investigators wrote.
The investigators reported having no financial disclosures.
SOURCE: Itum DS et al. J Surg Res. 2018 Feb 3. doi. org/10.1016/j.jss.2018.01.001.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: Parental leave policies have been unevenly adopted by U.S. medical schools.
Major finding: Among the top 91 ranked medical schools, 53% offer paid parental leave.
Data source: Survey of 91 top academic medical centers identified in U.S. News & World Report 2016 listing.
Disclosures: The investigators reported having no financial disclosures.
SOURCE: Itum DS et al. J Surg Res. 2018 Feb 3. doi. org/10.1016/j.jss.2018.01.001.
FDA grants priority review for AML drug
The Food and Drug Administration has granted priority review status to ivosidenib for the treatment of patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase 1 mutation.
The drug, marketed by Agios Pharmaceuticals, was given a Prescription Drug User Free Act action date of Aug. 21, 2018.
Results from a phase 1 dose-escalation and expansion study (AG120-C-001) presented at the annual meeting of the American Society of Hematology showed a complete response and complete response with partial hematologic recovery rate of 30.4% in 125 patients with relapsed/refractory AML who received the drug, according to Agios.
The Food and Drug Administration has granted priority review status to ivosidenib for the treatment of patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase 1 mutation.
The drug, marketed by Agios Pharmaceuticals, was given a Prescription Drug User Free Act action date of Aug. 21, 2018.
Results from a phase 1 dose-escalation and expansion study (AG120-C-001) presented at the annual meeting of the American Society of Hematology showed a complete response and complete response with partial hematologic recovery rate of 30.4% in 125 patients with relapsed/refractory AML who received the drug, according to Agios.
The Food and Drug Administration has granted priority review status to ivosidenib for the treatment of patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase 1 mutation.
The drug, marketed by Agios Pharmaceuticals, was given a Prescription Drug User Free Act action date of Aug. 21, 2018.
Results from a phase 1 dose-escalation and expansion study (AG120-C-001) presented at the annual meeting of the American Society of Hematology showed a complete response and complete response with partial hematologic recovery rate of 30.4% in 125 patients with relapsed/refractory AML who received the drug, according to Agios.
Mortality estimates put pancreatic cancer on the map
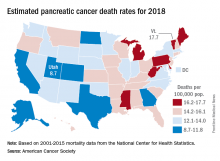
The alphabet may put Vermont right after Utah, but pancreatic cancer mortality has them on opposite sides of the country.
The expected number of deaths for 2018 and the current U.S. population estimate of nearly 326 million produce an expected death rate of 13.6 per 100,000 population. The Census Bureau estimates for the state populations, the deaths projected by the ACS, and a little math result in expected death rates of 8.7 per 100,000 for Utah and 17.7 for Vermont.
The incidence of pancreatic cancer increased by about 1% per year in whites and was stable in blacks from 2005 to 2014, although the rate of new cases in blacks is still about 25% higher than it is for whites. An estimated 55,440 new cases are expected in the United States in 2018, the ACS reported.
Although the survival rate for cancer of the pancreas has tripled since the mid-1970s, it is still much lower than for any other cancer. Five-year relative survival went from 3% in 1975-1977 to 9% in 2007-2013 for pancreatic cancer, while liver and intrahepatic bile duct cancer, which had the next-lowest survival rate, went from 3% to 19%, the ACS said.
The alphabet may put Vermont right after Utah, but pancreatic cancer mortality has them on opposite sides of the country.
The expected number of deaths for 2018 and the current U.S. population estimate of nearly 326 million produce an expected death rate of 13.6 per 100,000 population. The Census Bureau estimates for the state populations, the deaths projected by the ACS, and a little math result in expected death rates of 8.7 per 100,000 for Utah and 17.7 for Vermont.
The incidence of pancreatic cancer increased by about 1% per year in whites and was stable in blacks from 2005 to 2014, although the rate of new cases in blacks is still about 25% higher than it is for whites. An estimated 55,440 new cases are expected in the United States in 2018, the ACS reported.
Although the survival rate for cancer of the pancreas has tripled since the mid-1970s, it is still much lower than for any other cancer. Five-year relative survival went from 3% in 1975-1977 to 9% in 2007-2013 for pancreatic cancer, while liver and intrahepatic bile duct cancer, which had the next-lowest survival rate, went from 3% to 19%, the ACS said.
The alphabet may put Vermont right after Utah, but pancreatic cancer mortality has them on opposite sides of the country.
The expected number of deaths for 2018 and the current U.S. population estimate of nearly 326 million produce an expected death rate of 13.6 per 100,000 population. The Census Bureau estimates for the state populations, the deaths projected by the ACS, and a little math result in expected death rates of 8.7 per 100,000 for Utah and 17.7 for Vermont.
The incidence of pancreatic cancer increased by about 1% per year in whites and was stable in blacks from 2005 to 2014, although the rate of new cases in blacks is still about 25% higher than it is for whites. An estimated 55,440 new cases are expected in the United States in 2018, the ACS reported.
Although the survival rate for cancer of the pancreas has tripled since the mid-1970s, it is still much lower than for any other cancer. Five-year relative survival went from 3% in 1975-1977 to 9% in 2007-2013 for pancreatic cancer, while liver and intrahepatic bile duct cancer, which had the next-lowest survival rate, went from 3% to 19%, the ACS said.
Legacy Society members sustain research
AGA Legacy Society members share a desire to guarantee long-term support for digestive disease research. Through their foresight and generosity, they help ensure the continued momentum of discovery and patient education that has characterized GI medicine in recent decades. Legacy Society member donations directly support young GI investigators as they establish independent research careers.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
AGA members support young researchers at a critical decision point in their lives – when many consider giving up their research careers due to a lack of funding. “I am honored to be a recipient of the Research Scholar Award. I would like to thank the foundation for their generous contribution that will fund a crucial transition in my career,” said Jose Saenz, MD, PhD, Washington University School of Medicine and 2017 AGA – Gastric Cancer Foundation Research Scholar Award recipient.
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. Gifts to the foundation support researchers working towards developing new treatments and diagnostics for patients with GI conditions.
“I am extremely grateful to be selected for this award. I would like to thank the foundation donors for their generous support. This award will me build a research program to better understand mechanisms that promote growth of cholangiocarcinoma,” remarks Silvia Affe, PhD, Columbia University, 2017 AGA Research Scholar recipient.
Donors who make gifts at the Legacy Society level before DDW will receive an invitation to the annual Benefactors’ Dinner at the Folger Shakespeare Library in Washington, DC. Individuals interested in learning more about Legacy Society membership may contact Stacey Hinton Tuneski, Senior Director of Development at [email protected] or via phone (301) 222-4005. More information on the AGA Legacy Society including the current roster and acceptance form is available on the foundation’s website at www.gastro.org/legacysociety.
The makings of a grand celebration
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The Folger Shakespeare Library will be the location of the 2018 AGA Research Foundation Benefactors Dinner during DDW in Washington, DC. Just steps from the Capitol, the Great Hall and Pastor Reading room are a spectacular setting for an enjoyable evening with friends. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
AGA Legacy Society members share a desire to guarantee long-term support for digestive disease research. Through their foresight and generosity, they help ensure the continued momentum of discovery and patient education that has characterized GI medicine in recent decades. Legacy Society member donations directly support young GI investigators as they establish independent research careers.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
AGA members support young researchers at a critical decision point in their lives – when many consider giving up their research careers due to a lack of funding. “I am honored to be a recipient of the Research Scholar Award. I would like to thank the foundation for their generous contribution that will fund a crucial transition in my career,” said Jose Saenz, MD, PhD, Washington University School of Medicine and 2017 AGA – Gastric Cancer Foundation Research Scholar Award recipient.
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. Gifts to the foundation support researchers working towards developing new treatments and diagnostics for patients with GI conditions.
“I am extremely grateful to be selected for this award. I would like to thank the foundation donors for their generous support. This award will me build a research program to better understand mechanisms that promote growth of cholangiocarcinoma,” remarks Silvia Affe, PhD, Columbia University, 2017 AGA Research Scholar recipient.
Donors who make gifts at the Legacy Society level before DDW will receive an invitation to the annual Benefactors’ Dinner at the Folger Shakespeare Library in Washington, DC. Individuals interested in learning more about Legacy Society membership may contact Stacey Hinton Tuneski, Senior Director of Development at [email protected] or via phone (301) 222-4005. More information on the AGA Legacy Society including the current roster and acceptance form is available on the foundation’s website at www.gastro.org/legacysociety.
The makings of a grand celebration
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The Folger Shakespeare Library will be the location of the 2018 AGA Research Foundation Benefactors Dinner during DDW in Washington, DC. Just steps from the Capitol, the Great Hall and Pastor Reading room are a spectacular setting for an enjoyable evening with friends. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
AGA Legacy Society members share a desire to guarantee long-term support for digestive disease research. Through their foresight and generosity, they help ensure the continued momentum of discovery and patient education that has characterized GI medicine in recent decades. Legacy Society member donations directly support young GI investigators as they establish independent research careers.
Legacy Society members are the most generous individual donors to the AGA Research Foundation. Members of the AGA Legacy Society provide tax-deductible gifts to the AGA Research Foundation of $5,000 or more per year for 5 years ($25,000 total) or $50,000 or more in a planned gift, such as a bequest. All Legacy Society contributions go directly to support research awards.
AGA members support young researchers at a critical decision point in their lives – when many consider giving up their research careers due to a lack of funding. “I am honored to be a recipient of the Research Scholar Award. I would like to thank the foundation for their generous contribution that will fund a crucial transition in my career,” said Jose Saenz, MD, PhD, Washington University School of Medicine and 2017 AGA – Gastric Cancer Foundation Research Scholar Award recipient.
The AGA Research Foundation’s mission is to raise funds to support young researchers in gastroenterology and hepatology. Gifts to the foundation support researchers working towards developing new treatments and diagnostics for patients with GI conditions.
“I am extremely grateful to be selected for this award. I would like to thank the foundation donors for their generous support. This award will me build a research program to better understand mechanisms that promote growth of cholangiocarcinoma,” remarks Silvia Affe, PhD, Columbia University, 2017 AGA Research Scholar recipient.
Donors who make gifts at the Legacy Society level before DDW will receive an invitation to the annual Benefactors’ Dinner at the Folger Shakespeare Library in Washington, DC. Individuals interested in learning more about Legacy Society membership may contact Stacey Hinton Tuneski, Senior Director of Development at [email protected] or via phone (301) 222-4005. More information on the AGA Legacy Society including the current roster and acceptance form is available on the foundation’s website at www.gastro.org/legacysociety.
The makings of a grand celebration
Beginning with a memorable gathering at the United States Library of Congress in 2007, the AGA Benefactors’ Dinner has welcomed members of the AGA Legacy Society and other AGA dignitaries to special locations nationwide. The Folger Shakespeare Library will be the location of the 2018 AGA Research Foundation Benefactors Dinner during DDW in Washington, DC. Just steps from the Capitol, the Great Hall and Pastor Reading room are a spectacular setting for an enjoyable evening with friends. Members of the AGA Legacy Society will be among the distinguished honorees at the annual event.
MDedge Daily News: The Nonallergen of the Year
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Parabens are named “nonallergen” of the year, a gene test guides need for sentinel node biopsy in elderly melanoma patients, select atopic dermatitis patients need patch testing, and try these real solutions for delusional parasitosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Parabens are named “nonallergen” of the year, a gene test guides need for sentinel node biopsy in elderly melanoma patients, select atopic dermatitis patients need patch testing, and try these real solutions for delusional parasitosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Parabens are named “nonallergen” of the year, a gene test guides need for sentinel node biopsy in elderly melanoma patients, select atopic dermatitis patients need patch testing, and try these real solutions for delusional parasitosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
AGA Pres. Sheila Crowe Spends the Day on Capitol Hill
AGA President Sheila Crowe, MD, FRCPC, FACP, FACG, AGAF, recently spent the day on Capitol Hill meeting with lawmakers to advocate for AGA legislative priorities including increasing funding for NIH and biomedical research, support for the Removing Barriers to Colorectal Cancer Screening Act, and support for the Restoring the Patient’s Voice Act. Dr. Crowe met with eight congressional offices and received helpful feedback on the upcoming agenda in Congress and how it impacts AGA’s priorities.
NIH funding
Removing Barriers to Colorectal Cancer Screening Act
Fixing the current coinsurance problem for Medicare beneficiaries who undergo a screening colonoscopy that becomes therapeutic remains a top AGA priority. Most of the offices that Dr. Crowe met with were cosponsors of the legislation, the Removing Barriers to Colorectal Cancer Screening Act (HR 1017/S.479), that would waive coinsurance payment regardless of the screening outcome. Dr. Crowe shared her experience with patients and the financial burden this places on beneficiaries who need to be screened. Rep. Raul Ruiz, D-CA, and Rep. Scott Peters, D-CA, both members of the House Energy and Commerce Committee and supporters of the bill, will continue to advocate that the bill receive a hearing this year to help move it through Congress. The bill continues to have wide bipartisan support. Read more about the issue and how you can explain it to your patients.
Step therapy
More and more patients are being subject to step therapy protocols, also known as “fail first” under which they are required to try and fail sometimes two or three therapies before receiving coverage of the initial therapy recommended by their physician. With the emergence of new biologics to treat diseases like inflammatory bowel disease, more and more digestive disease patients are being subject to these protocols, which can have adverse effects on their health. Restoring the Patient’s Voice Act (HR 2077) would provide patients and providers with a fair and equitable appeals process when step therapy has been imposed and provides common sense exceptions for the provider to appeal. Dr. Crowe spoke of the impact this policy is having on digestive disease patients and the burden it puts on physician practices that have to take time away from patients to navigate the convoluted insurance appeals process. We are hopeful that many of the offices that we met with will support HR 2077. Read more about the issue.
Food is Medicine Working Group
Dr. Crowe also had a productive meeting with Rep. Jim McGovern’s, D-MA, office and learned more about the recently created Food is Medicine Working Group that he has initiated. McGovern is the Ranking Member of the Agriculture Committee’s Subcommittee on Nutrition which is responsible for our nation’s nutrition guidelines and the Supplemental Nutrition Assistance Program. The Working Group will focus on costs related to hunger and the importance of nutrition in treating chronic illness and disease. AGA looks forward to working with McGovern and members of the Working Group on this bipartisan initiative.
Capitol Hill needs to hear the voice of GI
In conjunction with Dr. Crowe’s visit, AGA launched a Virtual Advocacy Day to encourage members to contact their legislators in support of the issues that Dr. Crowe was advocating during her meetings. We thank those members who took time out of their schedules to take action.
AGA President Sheila Crowe, MD, FRCPC, FACP, FACG, AGAF, recently spent the day on Capitol Hill meeting with lawmakers to advocate for AGA legislative priorities including increasing funding for NIH and biomedical research, support for the Removing Barriers to Colorectal Cancer Screening Act, and support for the Restoring the Patient’s Voice Act. Dr. Crowe met with eight congressional offices and received helpful feedback on the upcoming agenda in Congress and how it impacts AGA’s priorities.
NIH funding
Removing Barriers to Colorectal Cancer Screening Act
Fixing the current coinsurance problem for Medicare beneficiaries who undergo a screening colonoscopy that becomes therapeutic remains a top AGA priority. Most of the offices that Dr. Crowe met with were cosponsors of the legislation, the Removing Barriers to Colorectal Cancer Screening Act (HR 1017/S.479), that would waive coinsurance payment regardless of the screening outcome. Dr. Crowe shared her experience with patients and the financial burden this places on beneficiaries who need to be screened. Rep. Raul Ruiz, D-CA, and Rep. Scott Peters, D-CA, both members of the House Energy and Commerce Committee and supporters of the bill, will continue to advocate that the bill receive a hearing this year to help move it through Congress. The bill continues to have wide bipartisan support. Read more about the issue and how you can explain it to your patients.
Step therapy
More and more patients are being subject to step therapy protocols, also known as “fail first” under which they are required to try and fail sometimes two or three therapies before receiving coverage of the initial therapy recommended by their physician. With the emergence of new biologics to treat diseases like inflammatory bowel disease, more and more digestive disease patients are being subject to these protocols, which can have adverse effects on their health. Restoring the Patient’s Voice Act (HR 2077) would provide patients and providers with a fair and equitable appeals process when step therapy has been imposed and provides common sense exceptions for the provider to appeal. Dr. Crowe spoke of the impact this policy is having on digestive disease patients and the burden it puts on physician practices that have to take time away from patients to navigate the convoluted insurance appeals process. We are hopeful that many of the offices that we met with will support HR 2077. Read more about the issue.
Food is Medicine Working Group
Dr. Crowe also had a productive meeting with Rep. Jim McGovern’s, D-MA, office and learned more about the recently created Food is Medicine Working Group that he has initiated. McGovern is the Ranking Member of the Agriculture Committee’s Subcommittee on Nutrition which is responsible for our nation’s nutrition guidelines and the Supplemental Nutrition Assistance Program. The Working Group will focus on costs related to hunger and the importance of nutrition in treating chronic illness and disease. AGA looks forward to working with McGovern and members of the Working Group on this bipartisan initiative.
Capitol Hill needs to hear the voice of GI
In conjunction with Dr. Crowe’s visit, AGA launched a Virtual Advocacy Day to encourage members to contact their legislators in support of the issues that Dr. Crowe was advocating during her meetings. We thank those members who took time out of their schedules to take action.
AGA President Sheila Crowe, MD, FRCPC, FACP, FACG, AGAF, recently spent the day on Capitol Hill meeting with lawmakers to advocate for AGA legislative priorities including increasing funding for NIH and biomedical research, support for the Removing Barriers to Colorectal Cancer Screening Act, and support for the Restoring the Patient’s Voice Act. Dr. Crowe met with eight congressional offices and received helpful feedback on the upcoming agenda in Congress and how it impacts AGA’s priorities.
NIH funding
Removing Barriers to Colorectal Cancer Screening Act
Fixing the current coinsurance problem for Medicare beneficiaries who undergo a screening colonoscopy that becomes therapeutic remains a top AGA priority. Most of the offices that Dr. Crowe met with were cosponsors of the legislation, the Removing Barriers to Colorectal Cancer Screening Act (HR 1017/S.479), that would waive coinsurance payment regardless of the screening outcome. Dr. Crowe shared her experience with patients and the financial burden this places on beneficiaries who need to be screened. Rep. Raul Ruiz, D-CA, and Rep. Scott Peters, D-CA, both members of the House Energy and Commerce Committee and supporters of the bill, will continue to advocate that the bill receive a hearing this year to help move it through Congress. The bill continues to have wide bipartisan support. Read more about the issue and how you can explain it to your patients.
Step therapy
More and more patients are being subject to step therapy protocols, also known as “fail first” under which they are required to try and fail sometimes two or three therapies before receiving coverage of the initial therapy recommended by their physician. With the emergence of new biologics to treat diseases like inflammatory bowel disease, more and more digestive disease patients are being subject to these protocols, which can have adverse effects on their health. Restoring the Patient’s Voice Act (HR 2077) would provide patients and providers with a fair and equitable appeals process when step therapy has been imposed and provides common sense exceptions for the provider to appeal. Dr. Crowe spoke of the impact this policy is having on digestive disease patients and the burden it puts on physician practices that have to take time away from patients to navigate the convoluted insurance appeals process. We are hopeful that many of the offices that we met with will support HR 2077. Read more about the issue.
Food is Medicine Working Group
Dr. Crowe also had a productive meeting with Rep. Jim McGovern’s, D-MA, office and learned more about the recently created Food is Medicine Working Group that he has initiated. McGovern is the Ranking Member of the Agriculture Committee’s Subcommittee on Nutrition which is responsible for our nation’s nutrition guidelines and the Supplemental Nutrition Assistance Program. The Working Group will focus on costs related to hunger and the importance of nutrition in treating chronic illness and disease. AGA looks forward to working with McGovern and members of the Working Group on this bipartisan initiative.
Capitol Hill needs to hear the voice of GI
In conjunction with Dr. Crowe’s visit, AGA launched a Virtual Advocacy Day to encourage members to contact their legislators in support of the issues that Dr. Crowe was advocating during her meetings. We thank those members who took time out of their schedules to take action.
Headlines from the 2018 Gastrointestinal Cancers Symposium
The 2018 Gastrointestinal Cancers Symposium took place Jan. 18-20, 2018, in San Francisco. During the meeting, investigators presented groundbreaking research designed to improve the diagnosis and treatment of GI cancers. Here are some of the most noteworthy headlines from the 2018 meeting.
Promising Results Using Liquid Biopsy to Improve CRC Early Detection
Researchers in Taiwan developed a screening test for early colorectal cancer (CRC) detection that requires a simple blood draw to assess for circulating tumor cells in the blood. The test demonstrates 88% accuracy to detect all stages of colorectal illness, including precancerous lesions. If validated and made commercially available, this test could be readily integrated into a patient’s routine physical exam, thereby increasing CRC screening compliance.
CELESTIAL Results May Lead to Cabozantinib Approval in Second-Line HCC
The phase III CELESTIAL trial met its primary endpoint by demonstrating a survival advantage with cabozantinib in patients with advanced hepatocellular carcinoma (HCC) that progressed following prior systemic therapy. Other outcomes included improvements in progression-free survival and objective response rate, as well as an acceptable safety profile, thus positioning cabozantinib for potential approval in the second-line setting in HCC.
RAINFALL Meets Primary Endpoint, But Ramucirumab Will Not Be Pursued for a First-Line Indication in G-GEJ Cancer
Results of the global, randomized, double-blind, placebo-controlled, phase III RAINFALL trial established the statistical benefit of ramucirumab, a monoclonal antibody targeting VEGFR-2, added to standard chemotherapy for patients with previously untreated metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. The findings revealed a significant 25% reduction in the risk of disease progression or death for the primary endpoint of progression-free survival (PFS). However, the reduction corresponded to only a 9-day improvement in median PFS, so the clinical benefit of frontline ramucirumab is debatable.
The Gastrointestinal Cancers Symposium is cosponsored by AGA, the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO).
More news from the 2018 Gastrointestinal Cancers Symposium is available at gicasym.org/daily-news.
The 2018 Gastrointestinal Cancers Symposium took place Jan. 18-20, 2018, in San Francisco. During the meeting, investigators presented groundbreaking research designed to improve the diagnosis and treatment of GI cancers. Here are some of the most noteworthy headlines from the 2018 meeting.
Promising Results Using Liquid Biopsy to Improve CRC Early Detection
Researchers in Taiwan developed a screening test for early colorectal cancer (CRC) detection that requires a simple blood draw to assess for circulating tumor cells in the blood. The test demonstrates 88% accuracy to detect all stages of colorectal illness, including precancerous lesions. If validated and made commercially available, this test could be readily integrated into a patient’s routine physical exam, thereby increasing CRC screening compliance.
CELESTIAL Results May Lead to Cabozantinib Approval in Second-Line HCC
The phase III CELESTIAL trial met its primary endpoint by demonstrating a survival advantage with cabozantinib in patients with advanced hepatocellular carcinoma (HCC) that progressed following prior systemic therapy. Other outcomes included improvements in progression-free survival and objective response rate, as well as an acceptable safety profile, thus positioning cabozantinib for potential approval in the second-line setting in HCC.
RAINFALL Meets Primary Endpoint, But Ramucirumab Will Not Be Pursued for a First-Line Indication in G-GEJ Cancer
Results of the global, randomized, double-blind, placebo-controlled, phase III RAINFALL trial established the statistical benefit of ramucirumab, a monoclonal antibody targeting VEGFR-2, added to standard chemotherapy for patients with previously untreated metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. The findings revealed a significant 25% reduction in the risk of disease progression or death for the primary endpoint of progression-free survival (PFS). However, the reduction corresponded to only a 9-day improvement in median PFS, so the clinical benefit of frontline ramucirumab is debatable.
The Gastrointestinal Cancers Symposium is cosponsored by AGA, the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO).
More news from the 2018 Gastrointestinal Cancers Symposium is available at gicasym.org/daily-news.
The 2018 Gastrointestinal Cancers Symposium took place Jan. 18-20, 2018, in San Francisco. During the meeting, investigators presented groundbreaking research designed to improve the diagnosis and treatment of GI cancers. Here are some of the most noteworthy headlines from the 2018 meeting.
Promising Results Using Liquid Biopsy to Improve CRC Early Detection
Researchers in Taiwan developed a screening test for early colorectal cancer (CRC) detection that requires a simple blood draw to assess for circulating tumor cells in the blood. The test demonstrates 88% accuracy to detect all stages of colorectal illness, including precancerous lesions. If validated and made commercially available, this test could be readily integrated into a patient’s routine physical exam, thereby increasing CRC screening compliance.
CELESTIAL Results May Lead to Cabozantinib Approval in Second-Line HCC
The phase III CELESTIAL trial met its primary endpoint by demonstrating a survival advantage with cabozantinib in patients with advanced hepatocellular carcinoma (HCC) that progressed following prior systemic therapy. Other outcomes included improvements in progression-free survival and objective response rate, as well as an acceptable safety profile, thus positioning cabozantinib for potential approval in the second-line setting in HCC.
RAINFALL Meets Primary Endpoint, But Ramucirumab Will Not Be Pursued for a First-Line Indication in G-GEJ Cancer
Results of the global, randomized, double-blind, placebo-controlled, phase III RAINFALL trial established the statistical benefit of ramucirumab, a monoclonal antibody targeting VEGFR-2, added to standard chemotherapy for patients with previously untreated metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. The findings revealed a significant 25% reduction in the risk of disease progression or death for the primary endpoint of progression-free survival (PFS). However, the reduction corresponded to only a 9-day improvement in median PFS, so the clinical benefit of frontline ramucirumab is debatable.
The Gastrointestinal Cancers Symposium is cosponsored by AGA, the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO).
More news from the 2018 Gastrointestinal Cancers Symposium is available at gicasym.org/daily-news.