User login
Case Study - New Onset Seizures in the Elderly
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Case
A 79-year-old woman had 2 generalized tonic-clonic seizures. She had no prior seizures. The patient’s family called 911.
Past medical history was remarkable for asthma, diet-controlled type 2 diabetes, gastroesophageal reflux disease, hypercholesterolemia, hypertension, and osteoporosis. She had a right-sided posterior communicating artery aneurysm coiled and stented 2 years previously.
Medications included aspirin, clopidogrel, an inhaler, ranitidine, and valsartan.
In the emergency room, the patient was initially confused but quickly returned to baseline. Vital signs and neurological examination were normal except for mild ptosis of the right eye, a chronic finding due to compression of the right 3rd nerve by the posterior communicating aneurysm.
Laboratories
Visible on head computed tomography (CT) scan was a thrombosed aneurysmal sac measuring 2.5 x 2 cm (Figure 1) which had increased in size from 2 years ago. Laboratory testing revealed an elevated lactic acid of 4.6 (normal: 0.871-2.1 mmol/L) and decreased CO2 of 20 (normal: 22-30 mEq/l) consistent with recent convulsions. Complete blood count, chemistry, urinalysis, and chest x-ray were normal.
Hospital Course
The patient was treated with levetiracetam and had no further seizures. After a diagnostic angiogram, a stent was placed to divert cerebral blood flow from the aneurysm. The patient was discharged with a prescription for levetiracetam 750 mg twice a day. A routine electroencephalogram (EEG) was unremarkable.
Discussion
Approximately 100,000 new cases of epilepsy are diagnosed in the United States each year.1 The incidence of new onset seizures is highest in the elderly.2 In this age group, the most common cause of provoked new onset epilepsy is acute stroke.2 Brain tumors, dementia, head trauma, and systemic disorders (eg, hepatic failure, hypoglycemia, hyperglycemia, hyponatremia, hypocalcemia, hypothyroidism, infections, and uremia) can all precipitate seizures.2
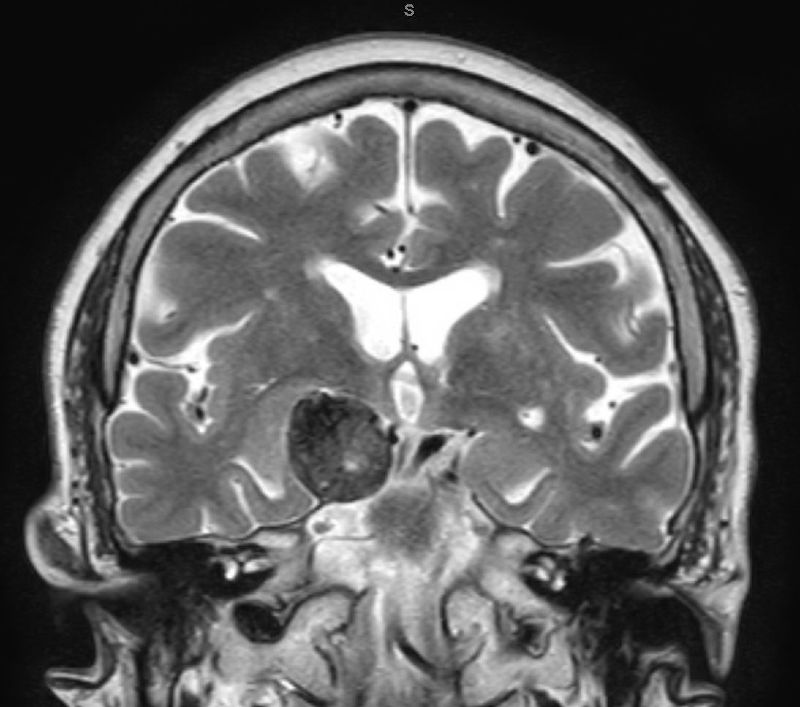
Seizures due to unruptured cerebral aneurysms are rare, but may be more common with giant aneurysms (2.5 cm or larger).3,4 Unruptured aneurysms may cause seizures due to subclinical hemorrhage, thrombus, or mass effect on the mesial temporal lobe.5 In this patient, mass effect on the right mesial temporal lobe is clearly evident on the magnetic resonance image (MRI) and is the likely explanation for her seizures (Figure 2).
Conclusion
This unusual case emphasizes the wide range of etiologies for new onset seizures in the elderly and need for a thorough diagnostic evaluation.3
References
1. Browne TR, Holmes GL. Epilepsy [published correction appears in N Engl J Med. 2001;344(25):1956]. N Engl J Med. 2001;344(15):1145-1151.
2. Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. 2005;331(7528):1317-1322.
3. Cagavi F, Kalayci M, Unal A, Atasoy HT, Cağavi Z, Açikgöz B. Giant unruptured anterior communicating artery aneurysm presenting with seizure. J Clin Neurosci. 2006;13(3):390-394.
4. Hänggi D, Winkler PA, Steiger JH. Primary epileptogenic unruptured intracranial aneurysms: incidence and effect of treatment on epilepsy. Neurosurgery. 2010;66(6):1161-1165.
5. Andereggen L, Andres RH. “Sentinel seizure” as a warning sign preceding fatal rupture of a giant middle cerebral artery aneurysm. World Neurosurg. 2017;100:709.e11-709.e13.
Figure 1. CT brain axial image without contrast demonstrating a giant aneurysm adjacent to right mesial temporal lobe
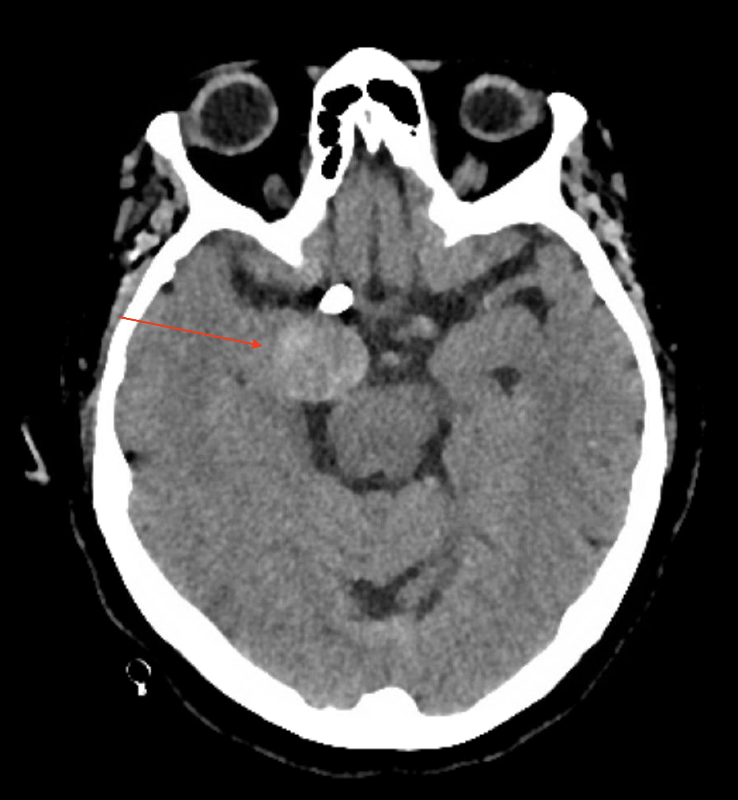
Figure 2. MRI brain coronal image T2W-TSE revealing a giant aneurysm compressing right mesial temporal lobe
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Case
A 79-year-old woman had 2 generalized tonic-clonic seizures. She had no prior seizures. The patient’s family called 911.
Past medical history was remarkable for asthma, diet-controlled type 2 diabetes, gastroesophageal reflux disease, hypercholesterolemia, hypertension, and osteoporosis. She had a right-sided posterior communicating artery aneurysm coiled and stented 2 years previously.
Medications included aspirin, clopidogrel, an inhaler, ranitidine, and valsartan.
In the emergency room, the patient was initially confused but quickly returned to baseline. Vital signs and neurological examination were normal except for mild ptosis of the right eye, a chronic finding due to compression of the right 3rd nerve by the posterior communicating aneurysm.
Laboratories
Visible on head computed tomography (CT) scan was a thrombosed aneurysmal sac measuring 2.5 x 2 cm (Figure 1) which had increased in size from 2 years ago. Laboratory testing revealed an elevated lactic acid of 4.6 (normal: 0.871-2.1 mmol/L) and decreased CO2 of 20 (normal: 22-30 mEq/l) consistent with recent convulsions. Complete blood count, chemistry, urinalysis, and chest x-ray were normal.
Hospital Course
The patient was treated with levetiracetam and had no further seizures. After a diagnostic angiogram, a stent was placed to divert cerebral blood flow from the aneurysm. The patient was discharged with a prescription for levetiracetam 750 mg twice a day. A routine electroencephalogram (EEG) was unremarkable.
Discussion
Approximately 100,000 new cases of epilepsy are diagnosed in the United States each year.1 The incidence of new onset seizures is highest in the elderly.2 In this age group, the most common cause of provoked new onset epilepsy is acute stroke.2 Brain tumors, dementia, head trauma, and systemic disorders (eg, hepatic failure, hypoglycemia, hyperglycemia, hyponatremia, hypocalcemia, hypothyroidism, infections, and uremia) can all precipitate seizures.2
Seizures due to unruptured cerebral aneurysms are rare, but may be more common with giant aneurysms (2.5 cm or larger).3,4 Unruptured aneurysms may cause seizures due to subclinical hemorrhage, thrombus, or mass effect on the mesial temporal lobe.5 In this patient, mass effect on the right mesial temporal lobe is clearly evident on the magnetic resonance image (MRI) and is the likely explanation for her seizures (Figure 2).
Conclusion
This unusual case emphasizes the wide range of etiologies for new onset seizures in the elderly and need for a thorough diagnostic evaluation.3
References
1. Browne TR, Holmes GL. Epilepsy [published correction appears in N Engl J Med. 2001;344(25):1956]. N Engl J Med. 2001;344(15):1145-1151.
2. Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. 2005;331(7528):1317-1322.
3. Cagavi F, Kalayci M, Unal A, Atasoy HT, Cağavi Z, Açikgöz B. Giant unruptured anterior communicating artery aneurysm presenting with seizure. J Clin Neurosci. 2006;13(3):390-394.
4. Hänggi D, Winkler PA, Steiger JH. Primary epileptogenic unruptured intracranial aneurysms: incidence and effect of treatment on epilepsy. Neurosurgery. 2010;66(6):1161-1165.
5. Andereggen L, Andres RH. “Sentinel seizure” as a warning sign preceding fatal rupture of a giant middle cerebral artery aneurysm. World Neurosurg. 2017;100:709.e11-709.e13.
Figure 1. CT brain axial image without contrast demonstrating a giant aneurysm adjacent to right mesial temporal lobe

Figure 2. MRI brain coronal image T2W-TSE revealing a giant aneurysm compressing right mesial temporal lobe

Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Case
A 79-year-old woman had 2 generalized tonic-clonic seizures. She had no prior seizures. The patient’s family called 911.
Past medical history was remarkable for asthma, diet-controlled type 2 diabetes, gastroesophageal reflux disease, hypercholesterolemia, hypertension, and osteoporosis. She had a right-sided posterior communicating artery aneurysm coiled and stented 2 years previously.
Medications included aspirin, clopidogrel, an inhaler, ranitidine, and valsartan.
In the emergency room, the patient was initially confused but quickly returned to baseline. Vital signs and neurological examination were normal except for mild ptosis of the right eye, a chronic finding due to compression of the right 3rd nerve by the posterior communicating aneurysm.
Laboratories
Visible on head computed tomography (CT) scan was a thrombosed aneurysmal sac measuring 2.5 x 2 cm (Figure 1) which had increased in size from 2 years ago. Laboratory testing revealed an elevated lactic acid of 4.6 (normal: 0.871-2.1 mmol/L) and decreased CO2 of 20 (normal: 22-30 mEq/l) consistent with recent convulsions. Complete blood count, chemistry, urinalysis, and chest x-ray were normal.
Hospital Course
The patient was treated with levetiracetam and had no further seizures. After a diagnostic angiogram, a stent was placed to divert cerebral blood flow from the aneurysm. The patient was discharged with a prescription for levetiracetam 750 mg twice a day. A routine electroencephalogram (EEG) was unremarkable.
Discussion
Approximately 100,000 new cases of epilepsy are diagnosed in the United States each year.1 The incidence of new onset seizures is highest in the elderly.2 In this age group, the most common cause of provoked new onset epilepsy is acute stroke.2 Brain tumors, dementia, head trauma, and systemic disorders (eg, hepatic failure, hypoglycemia, hyperglycemia, hyponatremia, hypocalcemia, hypothyroidism, infections, and uremia) can all precipitate seizures.2
Seizures due to unruptured cerebral aneurysms are rare, but may be more common with giant aneurysms (2.5 cm or larger).3,4 Unruptured aneurysms may cause seizures due to subclinical hemorrhage, thrombus, or mass effect on the mesial temporal lobe.5 In this patient, mass effect on the right mesial temporal lobe is clearly evident on the magnetic resonance image (MRI) and is the likely explanation for her seizures (Figure 2).
Conclusion
This unusual case emphasizes the wide range of etiologies for new onset seizures in the elderly and need for a thorough diagnostic evaluation.3
References
1. Browne TR, Holmes GL. Epilepsy [published correction appears in N Engl J Med. 2001;344(25):1956]. N Engl J Med. 2001;344(15):1145-1151.
2. Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. 2005;331(7528):1317-1322.
3. Cagavi F, Kalayci M, Unal A, Atasoy HT, Cağavi Z, Açikgöz B. Giant unruptured anterior communicating artery aneurysm presenting with seizure. J Clin Neurosci. 2006;13(3):390-394.
4. Hänggi D, Winkler PA, Steiger JH. Primary epileptogenic unruptured intracranial aneurysms: incidence and effect of treatment on epilepsy. Neurosurgery. 2010;66(6):1161-1165.
5. Andereggen L, Andres RH. “Sentinel seizure” as a warning sign preceding fatal rupture of a giant middle cerebral artery aneurysm. World Neurosurg. 2017;100:709.e11-709.e13.
Figure 1. CT brain axial image without contrast demonstrating a giant aneurysm adjacent to right mesial temporal lobe

Figure 2. MRI brain coronal image T2W-TSE revealing a giant aneurysm compressing right mesial temporal lobe

POEM found safe, effective for treating achalasia after failed Heller myotomy
Peroral endoscopic myotomy (POEM) safely and effectively treated achalasia in patients with persistent symptoms after Heller myotomy, according to the results of a retrospective study of 180 patients treated at 13 centers worldwide.
Rates of clinical success were 81% among patients who had previously undergone Heller myotomy and 94% among those who had not (P = .01), reported Saowanee Ngamruengphong, MD, of Johns Hopkins Medical Center, Baltimore, with her associates. The groups did not significantly differ in terms of rates of adverse events (8% and 13%, respectively), postprocedural symptomatic reflux (30% and 32%), or reflux esophagitis (44% and 52%). “Although the rate of clinical success in patients with prior Heller myotomy is lower than in those without [it], the safety profile of POEM is comparable,” they wrote in the October issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.01.031).
Heller myotomy achieves a long-term symptomatic response in about 90% of patients with achalasia and has a complication rate of only about 5%, according to Dr. Ngamruengphong and her associates. When this surgery does not successfully resolve symptoms, patients historically have chosen between repeating it or undergoing pneumatic dilation. However, POEM posted high success rates in several small, single-center case series. Thus, the researchers analyzed data on 180 adults with achalasia whose Eckardt scores were at least 3 and who underwent POEM at 13 tertiary care centers in Australia, France, Hong Kong, India, Italy, Japan, the United Kingdom, and the United States during 2009-2015.
POEM was a technical success for 98% for the group of patients who previously had undergone Heller myotomy and for 100% for those who had not, the researchers reported. In the univariate analysis, predictors of clinical failure included prior Heller myotomy (odds ratio, 3.6; 95% confidence interval, 1.3-10.4; P = .02) and prior pneumatic dilation (OR, 2.9; 95% CI, 1.2-7.4; P = .02). In the multivariable analysis, prior Heller myotomy significantly increased the chances of clinical failure (adjusted OR, 3.0; 95% confidence interval, 1.0-8.9; P = .04) after accounting for prior pneumatic dilation and baseline Eckardt score. Prior pneumatic dilation reached borderline significance (adjusted OR, 2.6; 95% CI, 0.99-7.0; P = .05). Clinical failure was not associated with age, sex, achalasia subtypes, previous therapy, baseline Eckardt score, length of myotomy, orientation of myotomy, or extent of lower esophageal sphincter myotomy.
“Previous studies have reported that the success rates of pneumatic dilation in patients who failed prior Heller myotomy ranged between 50% and 89%,” the researchers said. However, success is often short-lived, with up to 45% of patients needing another procedure within 2 years, putting them at risk of “potentially serious adverse events, such as esophageal perforation or aspiration,” they added.
Repeat surgical myotomy is reportedly successful in 73%-89% of cases; however, itis technically challenging because of adhesions and fibrosis from the previous surgery and is associated with a high risk of gastrointestinal perforation.
Clinicians should carefully investigate the reasons a Heller myotomy failed in order to elect a course of action, the researchers emphasized. “For instance, for patients with symptom relapse or failure to respond to surgical myotomy as a result of incomplete myotomy or myotomy fibrosis, POEM is likely to be effective,” they said. “On the other hand, when the cause of persistent symptoms after surgical myotomy is tight fundoplication, a redo fundoplication should be recommended.”
Dr. Ngamruengphong had no disclosures. Three coinvestigators disclosed consulting relationships with Boston Scientific, Medtronic, Sandhill Scientific, Erbe, and Cosmo Pharmaceuticals.
Peroral endoscopic myotomy (POEM) safely and effectively treated achalasia in patients with persistent symptoms after Heller myotomy, according to the results of a retrospective study of 180 patients treated at 13 centers worldwide.
Rates of clinical success were 81% among patients who had previously undergone Heller myotomy and 94% among those who had not (P = .01), reported Saowanee Ngamruengphong, MD, of Johns Hopkins Medical Center, Baltimore, with her associates. The groups did not significantly differ in terms of rates of adverse events (8% and 13%, respectively), postprocedural symptomatic reflux (30% and 32%), or reflux esophagitis (44% and 52%). “Although the rate of clinical success in patients with prior Heller myotomy is lower than in those without [it], the safety profile of POEM is comparable,” they wrote in the October issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.01.031).
Heller myotomy achieves a long-term symptomatic response in about 90% of patients with achalasia and has a complication rate of only about 5%, according to Dr. Ngamruengphong and her associates. When this surgery does not successfully resolve symptoms, patients historically have chosen between repeating it or undergoing pneumatic dilation. However, POEM posted high success rates in several small, single-center case series. Thus, the researchers analyzed data on 180 adults with achalasia whose Eckardt scores were at least 3 and who underwent POEM at 13 tertiary care centers in Australia, France, Hong Kong, India, Italy, Japan, the United Kingdom, and the United States during 2009-2015.
POEM was a technical success for 98% for the group of patients who previously had undergone Heller myotomy and for 100% for those who had not, the researchers reported. In the univariate analysis, predictors of clinical failure included prior Heller myotomy (odds ratio, 3.6; 95% confidence interval, 1.3-10.4; P = .02) and prior pneumatic dilation (OR, 2.9; 95% CI, 1.2-7.4; P = .02). In the multivariable analysis, prior Heller myotomy significantly increased the chances of clinical failure (adjusted OR, 3.0; 95% confidence interval, 1.0-8.9; P = .04) after accounting for prior pneumatic dilation and baseline Eckardt score. Prior pneumatic dilation reached borderline significance (adjusted OR, 2.6; 95% CI, 0.99-7.0; P = .05). Clinical failure was not associated with age, sex, achalasia subtypes, previous therapy, baseline Eckardt score, length of myotomy, orientation of myotomy, or extent of lower esophageal sphincter myotomy.
“Previous studies have reported that the success rates of pneumatic dilation in patients who failed prior Heller myotomy ranged between 50% and 89%,” the researchers said. However, success is often short-lived, with up to 45% of patients needing another procedure within 2 years, putting them at risk of “potentially serious adverse events, such as esophageal perforation or aspiration,” they added.
Repeat surgical myotomy is reportedly successful in 73%-89% of cases; however, itis technically challenging because of adhesions and fibrosis from the previous surgery and is associated with a high risk of gastrointestinal perforation.
Clinicians should carefully investigate the reasons a Heller myotomy failed in order to elect a course of action, the researchers emphasized. “For instance, for patients with symptom relapse or failure to respond to surgical myotomy as a result of incomplete myotomy or myotomy fibrosis, POEM is likely to be effective,” they said. “On the other hand, when the cause of persistent symptoms after surgical myotomy is tight fundoplication, a redo fundoplication should be recommended.”
Dr. Ngamruengphong had no disclosures. Three coinvestigators disclosed consulting relationships with Boston Scientific, Medtronic, Sandhill Scientific, Erbe, and Cosmo Pharmaceuticals.
Peroral endoscopic myotomy (POEM) safely and effectively treated achalasia in patients with persistent symptoms after Heller myotomy, according to the results of a retrospective study of 180 patients treated at 13 centers worldwide.
Rates of clinical success were 81% among patients who had previously undergone Heller myotomy and 94% among those who had not (P = .01), reported Saowanee Ngamruengphong, MD, of Johns Hopkins Medical Center, Baltimore, with her associates. The groups did not significantly differ in terms of rates of adverse events (8% and 13%, respectively), postprocedural symptomatic reflux (30% and 32%), or reflux esophagitis (44% and 52%). “Although the rate of clinical success in patients with prior Heller myotomy is lower than in those without [it], the safety profile of POEM is comparable,” they wrote in the October issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.01.031).
Heller myotomy achieves a long-term symptomatic response in about 90% of patients with achalasia and has a complication rate of only about 5%, according to Dr. Ngamruengphong and her associates. When this surgery does not successfully resolve symptoms, patients historically have chosen between repeating it or undergoing pneumatic dilation. However, POEM posted high success rates in several small, single-center case series. Thus, the researchers analyzed data on 180 adults with achalasia whose Eckardt scores were at least 3 and who underwent POEM at 13 tertiary care centers in Australia, France, Hong Kong, India, Italy, Japan, the United Kingdom, and the United States during 2009-2015.
POEM was a technical success for 98% for the group of patients who previously had undergone Heller myotomy and for 100% for those who had not, the researchers reported. In the univariate analysis, predictors of clinical failure included prior Heller myotomy (odds ratio, 3.6; 95% confidence interval, 1.3-10.4; P = .02) and prior pneumatic dilation (OR, 2.9; 95% CI, 1.2-7.4; P = .02). In the multivariable analysis, prior Heller myotomy significantly increased the chances of clinical failure (adjusted OR, 3.0; 95% confidence interval, 1.0-8.9; P = .04) after accounting for prior pneumatic dilation and baseline Eckardt score. Prior pneumatic dilation reached borderline significance (adjusted OR, 2.6; 95% CI, 0.99-7.0; P = .05). Clinical failure was not associated with age, sex, achalasia subtypes, previous therapy, baseline Eckardt score, length of myotomy, orientation of myotomy, or extent of lower esophageal sphincter myotomy.
“Previous studies have reported that the success rates of pneumatic dilation in patients who failed prior Heller myotomy ranged between 50% and 89%,” the researchers said. However, success is often short-lived, with up to 45% of patients needing another procedure within 2 years, putting them at risk of “potentially serious adverse events, such as esophageal perforation or aspiration,” they added.
Repeat surgical myotomy is reportedly successful in 73%-89% of cases; however, itis technically challenging because of adhesions and fibrosis from the previous surgery and is associated with a high risk of gastrointestinal perforation.
Clinicians should carefully investigate the reasons a Heller myotomy failed in order to elect a course of action, the researchers emphasized. “For instance, for patients with symptom relapse or failure to respond to surgical myotomy as a result of incomplete myotomy or myotomy fibrosis, POEM is likely to be effective,” they said. “On the other hand, when the cause of persistent symptoms after surgical myotomy is tight fundoplication, a redo fundoplication should be recommended.”
Dr. Ngamruengphong had no disclosures. Three coinvestigators disclosed consulting relationships with Boston Scientific, Medtronic, Sandhill Scientific, Erbe, and Cosmo Pharmaceuticals.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: Rates of clinical success were 81% when patients had previously undergone Heller myotomy and 94% when they had not (P = .01). Rates of adverse events (8% and 13%, respectively), as well as rates of postprocedural symptomatic reflux (30% and 32%) and reflux esophagitis (44% and 52%), were similar between groups.
Data source: A multicenter retrospective study of 180 patients with achalasia, half of whom had symptoms despite prior Heller myotomy.
Disclosures: Dr. Ngamruengphong had no disclosures. Three coinvestigators disclosed consulting relationships with Boston Scientific, Medtronic, Sandhill Scientific, Erbe, and Cosmo Pharmaceuticals.
FDA clears first 2D mammography device with patient-controlled compression
The Food and Drug Administration has granted premarket clearance to the first 2D digital mammography system that allows patients to control the amount of compression applied to their own breasts before the mammogram x-ray is taken.
Senographe Pristina with Self-Compression uses a handheld wireless remote control that allows women to adjust the compression force after breast positioning and during a mammography exam. The technologist positions the patient and initiates compression, then guides the patient to gradually increase compression using the remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted, according to the FDA’s Sept. 1 announcement.
“Regular mammograms are an important tool in detecting breast cancer,” Alberto Gutierrez, PhD, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health, said in a statement. “However, some patients may experience anxiety or stress about the discomfort from the compression during the mammogram. This device allows patients some control over the amount of compression for their exam.”
Read more details of the clearance on the FDA’s website.
The Food and Drug Administration has granted premarket clearance to the first 2D digital mammography system that allows patients to control the amount of compression applied to their own breasts before the mammogram x-ray is taken.
Senographe Pristina with Self-Compression uses a handheld wireless remote control that allows women to adjust the compression force after breast positioning and during a mammography exam. The technologist positions the patient and initiates compression, then guides the patient to gradually increase compression using the remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted, according to the FDA’s Sept. 1 announcement.
“Regular mammograms are an important tool in detecting breast cancer,” Alberto Gutierrez, PhD, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health, said in a statement. “However, some patients may experience anxiety or stress about the discomfort from the compression during the mammogram. This device allows patients some control over the amount of compression for their exam.”
Read more details of the clearance on the FDA’s website.
The Food and Drug Administration has granted premarket clearance to the first 2D digital mammography system that allows patients to control the amount of compression applied to their own breasts before the mammogram x-ray is taken.
Senographe Pristina with Self-Compression uses a handheld wireless remote control that allows women to adjust the compression force after breast positioning and during a mammography exam. The technologist positions the patient and initiates compression, then guides the patient to gradually increase compression using the remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted, according to the FDA’s Sept. 1 announcement.
“Regular mammograms are an important tool in detecting breast cancer,” Alberto Gutierrez, PhD, director of the FDA’s Office of In Vitro Diagnostics and Radiological Health, said in a statement. “However, some patients may experience anxiety or stress about the discomfort from the compression during the mammogram. This device allows patients some control over the amount of compression for their exam.”
Read more details of the clearance on the FDA’s website.
Hurricane Harvey tests Houston physicians’ mettle
As Houston-area citizens evacuated or hunkered down at home in anticipation of Hurricane Harvey, doctors like Mary L. Brandt, MD, FACS, packed a bag and headed to work.
“I came in on Saturday morning [Aug. 23] – I was on call – and so I packed a big suitcase and a big bag of food because I anticipated I would be here until Thursday,” Dr. Brandt said in an interview, “So I became part of the ‘ride-out crew.’ ”
Hospitals were hit hard by Hurricane Harvey, and many struggled against the effects of the Category 4 storm, which made landfall then stalled over Texas for almost a week, pummeling the area.

“We all know this [flooding] could happen, so all the facilities in the medical center have flood gates, and generators are out of the basement so that there is not any risk of losing all electricity, but then the issue becomes the staff,” Dr. Brandt said. “They can’t get to and from the facility, and that’s particularly true if they live in the periphery of Houston, which is common.”
The situation was the same for many area hospitals. Just 2 miles away from Texas Children’s Hospital, SreyRam Kuy, MD, FACS, associate chief of staff at the Michael E DeBakey VA Medical Center, and her colleagues prepared to run the hospital with a skeleton crew.
“We were preparing when it was still a tropical storm, and we talked to the staff ahead of time to let them know this would be a marathon, not a sprint,” Dr. Kuy said in an interview. “We had people staying in the hospital ahead of time because we were worried that when the hurricane hit, we would not be able to have people return.”
But when Harvey made landfall with Category 4 intensity, many medical facilities were caught by surprise.
“We didn’t know how bad it would be, I honestly don’t think anyone in the city or the state had any idea of how tremendous the impact would be, particularly with the flooding,” Dr. Kuy said. “We had staff going 5, 6 days here at the hospital, working continuously, sleeping on the floor, and because of that, we were able to perform multiple emergency surgeries during the disaster, including laparoscopic treatment of ruptured appendicitis and replacement of an infection aortic graft, which required massive transfusion.” The VA hospital broke from its core mission of caring for veterans, treating “homeless folks and nonveterans who were brought here by the Coast Guard, or the ambulances, or by air.”
At Texas Children’s Hospital, Dr. Brandt and her colleagues were dealing with similar situations, staying on their feet and moving quickly as rescued patients arrived by air.
“We were near the area that was flooding really terribly, and so the Coast Guard had been coming in and bringing kids,” Dr. Brandt said. “Sometimes, we knew what was coming and sometimes we didn’t. It was pretty much controlled chaos.”
Staff shared responsibilities, often taking on tasks far outside their usual roles.
“We didn’t have enough people working the cafeteria, and so, at one point, I put on my hair net, grabbed a ladle, and served in the lunch line,” Dr. Kuy said.
Throughout the storm and flooding, medical professionals fought through exhaustion and depleting supplies, all with little or no knowledge of how their own homes and families were faring.
“We had people here for so long, and they had no idea what was happening in their own homes,” Dr. Kuy said. “They were watching on the news, seeing the reports and watching their own neighborhoods flooded.”
Dr. Brandt and her colleagues would watch as reports came in of what was happening beyond the hospital walls.
“We have some meeting areas, we would watch the weather together and that goes from the janitors to the head of the hospital who was in the hospital with us,” she said.
Despite the chaos outside, morale did not waiver for either Dr. Kuy’s or Dr. Brandt’s crew.
“I remember walking throughout the hospital, doing my rounds, checking up on the units. I went to talk with some of the staff nurses, and what struck me was as I walk in I see these big smiles on their faces, I absolutely did not expect that,” Dr. Kuy said. “They had been in the hospital for 5 days, they were exhausted. It just makes me so proud to serve along these kinds of people.”
As travel became possible, Dr. Kuy and other area physicians – as well as volunteers from across the country – began to shift their focus to evacuation shelters, treating ambulatory patients there.
“The response has been phenomenal,” said Dr. Kuy. “I met an ER doctor from North Carolina who came to volunteer, we have FEMA [Federal Emergency Management Agency] doctors from all across the state, and then of course, all the people from the different VA [hospitals].”
Pediatricians have sent their support as well, offering time and supplies to help take care of the patients at Texas Children’s Hospital, Dr. Brandt said.
At presstime, volunteers were still needed. The Texas Department of State Health Services opened a web portal for volunteer opportunities, and lifted restriction on out-of-state doctors from practicing medicine without state registration.
While there is still much that needs to be done to recover, those on the ground said that they feel an overwhelming feeling of community as people face what will inevitably be a tough road ahead.
“Houston has a reputation and a culture of helping neighbors and it has been astounding to watch what’s happening,” said Dr. Brandt. “No matter how tired people are or how stressful any cases are, everyone’s morale stays high.”
[email protected]
On Twitter @eaztweets
As Houston-area citizens evacuated or hunkered down at home in anticipation of Hurricane Harvey, doctors like Mary L. Brandt, MD, FACS, packed a bag and headed to work.
“I came in on Saturday morning [Aug. 23] – I was on call – and so I packed a big suitcase and a big bag of food because I anticipated I would be here until Thursday,” Dr. Brandt said in an interview, “So I became part of the ‘ride-out crew.’ ”
Hospitals were hit hard by Hurricane Harvey, and many struggled against the effects of the Category 4 storm, which made landfall then stalled over Texas for almost a week, pummeling the area.

“We all know this [flooding] could happen, so all the facilities in the medical center have flood gates, and generators are out of the basement so that there is not any risk of losing all electricity, but then the issue becomes the staff,” Dr. Brandt said. “They can’t get to and from the facility, and that’s particularly true if they live in the periphery of Houston, which is common.”
The situation was the same for many area hospitals. Just 2 miles away from Texas Children’s Hospital, SreyRam Kuy, MD, FACS, associate chief of staff at the Michael E DeBakey VA Medical Center, and her colleagues prepared to run the hospital with a skeleton crew.
“We were preparing when it was still a tropical storm, and we talked to the staff ahead of time to let them know this would be a marathon, not a sprint,” Dr. Kuy said in an interview. “We had people staying in the hospital ahead of time because we were worried that when the hurricane hit, we would not be able to have people return.”
But when Harvey made landfall with Category 4 intensity, many medical facilities were caught by surprise.
“We didn’t know how bad it would be, I honestly don’t think anyone in the city or the state had any idea of how tremendous the impact would be, particularly with the flooding,” Dr. Kuy said. “We had staff going 5, 6 days here at the hospital, working continuously, sleeping on the floor, and because of that, we were able to perform multiple emergency surgeries during the disaster, including laparoscopic treatment of ruptured appendicitis and replacement of an infection aortic graft, which required massive transfusion.” The VA hospital broke from its core mission of caring for veterans, treating “homeless folks and nonveterans who were brought here by the Coast Guard, or the ambulances, or by air.”
At Texas Children’s Hospital, Dr. Brandt and her colleagues were dealing with similar situations, staying on their feet and moving quickly as rescued patients arrived by air.
“We were near the area that was flooding really terribly, and so the Coast Guard had been coming in and bringing kids,” Dr. Brandt said. “Sometimes, we knew what was coming and sometimes we didn’t. It was pretty much controlled chaos.”
Staff shared responsibilities, often taking on tasks far outside their usual roles.
“We didn’t have enough people working the cafeteria, and so, at one point, I put on my hair net, grabbed a ladle, and served in the lunch line,” Dr. Kuy said.
Throughout the storm and flooding, medical professionals fought through exhaustion and depleting supplies, all with little or no knowledge of how their own homes and families were faring.
“We had people here for so long, and they had no idea what was happening in their own homes,” Dr. Kuy said. “They were watching on the news, seeing the reports and watching their own neighborhoods flooded.”
Dr. Brandt and her colleagues would watch as reports came in of what was happening beyond the hospital walls.
“We have some meeting areas, we would watch the weather together and that goes from the janitors to the head of the hospital who was in the hospital with us,” she said.
Despite the chaos outside, morale did not waiver for either Dr. Kuy’s or Dr. Brandt’s crew.
“I remember walking throughout the hospital, doing my rounds, checking up on the units. I went to talk with some of the staff nurses, and what struck me was as I walk in I see these big smiles on their faces, I absolutely did not expect that,” Dr. Kuy said. “They had been in the hospital for 5 days, they were exhausted. It just makes me so proud to serve along these kinds of people.”
As travel became possible, Dr. Kuy and other area physicians – as well as volunteers from across the country – began to shift their focus to evacuation shelters, treating ambulatory patients there.
“The response has been phenomenal,” said Dr. Kuy. “I met an ER doctor from North Carolina who came to volunteer, we have FEMA [Federal Emergency Management Agency] doctors from all across the state, and then of course, all the people from the different VA [hospitals].”
Pediatricians have sent their support as well, offering time and supplies to help take care of the patients at Texas Children’s Hospital, Dr. Brandt said.
At presstime, volunteers were still needed. The Texas Department of State Health Services opened a web portal for volunteer opportunities, and lifted restriction on out-of-state doctors from practicing medicine without state registration.
While there is still much that needs to be done to recover, those on the ground said that they feel an overwhelming feeling of community as people face what will inevitably be a tough road ahead.
“Houston has a reputation and a culture of helping neighbors and it has been astounding to watch what’s happening,” said Dr. Brandt. “No matter how tired people are or how stressful any cases are, everyone’s morale stays high.”
[email protected]
On Twitter @eaztweets
As Houston-area citizens evacuated or hunkered down at home in anticipation of Hurricane Harvey, doctors like Mary L. Brandt, MD, FACS, packed a bag and headed to work.
“I came in on Saturday morning [Aug. 23] – I was on call – and so I packed a big suitcase and a big bag of food because I anticipated I would be here until Thursday,” Dr. Brandt said in an interview, “So I became part of the ‘ride-out crew.’ ”
Hospitals were hit hard by Hurricane Harvey, and many struggled against the effects of the Category 4 storm, which made landfall then stalled over Texas for almost a week, pummeling the area.

“We all know this [flooding] could happen, so all the facilities in the medical center have flood gates, and generators are out of the basement so that there is not any risk of losing all electricity, but then the issue becomes the staff,” Dr. Brandt said. “They can’t get to and from the facility, and that’s particularly true if they live in the periphery of Houston, which is common.”
The situation was the same for many area hospitals. Just 2 miles away from Texas Children’s Hospital, SreyRam Kuy, MD, FACS, associate chief of staff at the Michael E DeBakey VA Medical Center, and her colleagues prepared to run the hospital with a skeleton crew.
“We were preparing when it was still a tropical storm, and we talked to the staff ahead of time to let them know this would be a marathon, not a sprint,” Dr. Kuy said in an interview. “We had people staying in the hospital ahead of time because we were worried that when the hurricane hit, we would not be able to have people return.”
But when Harvey made landfall with Category 4 intensity, many medical facilities were caught by surprise.
“We didn’t know how bad it would be, I honestly don’t think anyone in the city or the state had any idea of how tremendous the impact would be, particularly with the flooding,” Dr. Kuy said. “We had staff going 5, 6 days here at the hospital, working continuously, sleeping on the floor, and because of that, we were able to perform multiple emergency surgeries during the disaster, including laparoscopic treatment of ruptured appendicitis and replacement of an infection aortic graft, which required massive transfusion.” The VA hospital broke from its core mission of caring for veterans, treating “homeless folks and nonveterans who were brought here by the Coast Guard, or the ambulances, or by air.”
At Texas Children’s Hospital, Dr. Brandt and her colleagues were dealing with similar situations, staying on their feet and moving quickly as rescued patients arrived by air.
“We were near the area that was flooding really terribly, and so the Coast Guard had been coming in and bringing kids,” Dr. Brandt said. “Sometimes, we knew what was coming and sometimes we didn’t. It was pretty much controlled chaos.”
Staff shared responsibilities, often taking on tasks far outside their usual roles.
“We didn’t have enough people working the cafeteria, and so, at one point, I put on my hair net, grabbed a ladle, and served in the lunch line,” Dr. Kuy said.
Throughout the storm and flooding, medical professionals fought through exhaustion and depleting supplies, all with little or no knowledge of how their own homes and families were faring.
“We had people here for so long, and they had no idea what was happening in their own homes,” Dr. Kuy said. “They were watching on the news, seeing the reports and watching their own neighborhoods flooded.”
Dr. Brandt and her colleagues would watch as reports came in of what was happening beyond the hospital walls.
“We have some meeting areas, we would watch the weather together and that goes from the janitors to the head of the hospital who was in the hospital with us,” she said.
Despite the chaos outside, morale did not waiver for either Dr. Kuy’s or Dr. Brandt’s crew.
“I remember walking throughout the hospital, doing my rounds, checking up on the units. I went to talk with some of the staff nurses, and what struck me was as I walk in I see these big smiles on their faces, I absolutely did not expect that,” Dr. Kuy said. “They had been in the hospital for 5 days, they were exhausted. It just makes me so proud to serve along these kinds of people.”
As travel became possible, Dr. Kuy and other area physicians – as well as volunteers from across the country – began to shift their focus to evacuation shelters, treating ambulatory patients there.
“The response has been phenomenal,” said Dr. Kuy. “I met an ER doctor from North Carolina who came to volunteer, we have FEMA [Federal Emergency Management Agency] doctors from all across the state, and then of course, all the people from the different VA [hospitals].”
Pediatricians have sent their support as well, offering time and supplies to help take care of the patients at Texas Children’s Hospital, Dr. Brandt said.
At presstime, volunteers were still needed. The Texas Department of State Health Services opened a web portal for volunteer opportunities, and lifted restriction on out-of-state doctors from practicing medicine without state registration.
While there is still much that needs to be done to recover, those on the ground said that they feel an overwhelming feeling of community as people face what will inevitably be a tough road ahead.
“Houston has a reputation and a culture of helping neighbors and it has been astounding to watch what’s happening,” said Dr. Brandt. “No matter how tired people are or how stressful any cases are, everyone’s morale stays high.”
[email protected]
On Twitter @eaztweets
More States Get Funding to Fight Opioid Epidemic
The CDC plans to award more than $12 million to 20 states and the District of Columbia to support responses to the opioid overdose epidemic. The new funding brings the number of recipients to 32.
States can use the funds to report nonfatal and fatal opioid overdose and risk factors linked to fatal overdoses more quickly, share data with key stakeholders, and share data with the CDC to improve multistate surveillance and response.
Fourteen states currently get funding under the Prescription Drug Overdose: Prevention for States (PfS) program, and another 8 will get $4.8 million. The money will allow states to enhance prescription drug-monitoring programs and implement and evaluate strategies to improve safe opioid prescribing practices.
The CDC plans to award more than $12 million to 20 states and the District of Columbia to support responses to the opioid overdose epidemic. The new funding brings the number of recipients to 32.
States can use the funds to report nonfatal and fatal opioid overdose and risk factors linked to fatal overdoses more quickly, share data with key stakeholders, and share data with the CDC to improve multistate surveillance and response.
Fourteen states currently get funding under the Prescription Drug Overdose: Prevention for States (PfS) program, and another 8 will get $4.8 million. The money will allow states to enhance prescription drug-monitoring programs and implement and evaluate strategies to improve safe opioid prescribing practices.
The CDC plans to award more than $12 million to 20 states and the District of Columbia to support responses to the opioid overdose epidemic. The new funding brings the number of recipients to 32.
States can use the funds to report nonfatal and fatal opioid overdose and risk factors linked to fatal overdoses more quickly, share data with key stakeholders, and share data with the CDC to improve multistate surveillance and response.
Fourteen states currently get funding under the Prescription Drug Overdose: Prevention for States (PfS) program, and another 8 will get $4.8 million. The money will allow states to enhance prescription drug-monitoring programs and implement and evaluate strategies to improve safe opioid prescribing practices.
Studies of donor CAR T cells placed on hold
The US Food and Drug Administration (FDA) has placed a clinical hold on both phase 1 studies of UCART123, a universal (allogeneic) chimeric antigen receptor (CAR) T-cell therapy targeting CD123.
One study was designed for patients with acute myeloid leukemia (AML), and the other was designed for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The clinical hold is due to the death of the first patient treated in the BPDCN trial.
The hold means no new subjects can be enrolled in either trial, and there can be no further dosing of subjects who are already enrolled.
BPDCN patient
The first patient treated in the BPDCN trial was a 78-year-old male who had received 1 prior therapy. He presented with relapsed/refractory BPDCN with 30% blasts in his bone marrow and cutaneous lesions (biopsy-proven BPDCN) at baseline.
The patient first received pre-conditioning with fludarabine (30 mg/m²/day for 4 days) and cyclophosphamide (1 g/m²/day for 3 days).
On August 16, 2017 (Day 0), the patient received UCART123 at 6.25 x 105 cells/kg, the first dose level explored in the protocol, without complication.
By Day 5, the patient had developed grade 2 cytokine release syndrome (CRS) and a grade 3 lung infection, which improved after a first dose of tocilizumab and the administration of broad-spectrum, intravenous antibiotics.
On Day 8, the patient was found to have more severe CRS (ultimately grade 5) and grade 4 capillary leak syndrome. Despite receiving treatment in keeping with CRS management, including the administration of corticosteroids and tociluzumab as well as intensive care unit support, the patient died on Day 9.
AML patient
The first patient treated in the AML study experienced similar adverse effects as the BPDCN patient but is still alive.
The AML patient was a 58-year-old woman with 84% blasts in her bone marrow at baseline.
On June 27, 2017 (Day 0), the patient received the same pre-conditioning regimen and the same dose of UCART123 as the BPDCN patient, without complication.
By Day 8, the AML patient had developed grade 2 CRS. This worsened to grade 3 on Day 9 and resolved on Day 11 with treatment in the intensive care unit.
The patient also experienced grade 4 capillary leak syndrome on Day 9 that resolved on Day 12.
Next steps
The data safety monitoring board for these trials met on August 28 and recommended lowering the dose of UCART123 to 6.25 x 104 cells/kg in both studies and capping cyclophosphamide to a total dose of 4 g over 3 days.
Cellectis, the company developing UCART123, said it is working with study investigators and the FDA to resume both trials with an amended protocol that includes these dose adjustments. ![]()
The US Food and Drug Administration (FDA) has placed a clinical hold on both phase 1 studies of UCART123, a universal (allogeneic) chimeric antigen receptor (CAR) T-cell therapy targeting CD123.
One study was designed for patients with acute myeloid leukemia (AML), and the other was designed for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The clinical hold is due to the death of the first patient treated in the BPDCN trial.
The hold means no new subjects can be enrolled in either trial, and there can be no further dosing of subjects who are already enrolled.
BPDCN patient
The first patient treated in the BPDCN trial was a 78-year-old male who had received 1 prior therapy. He presented with relapsed/refractory BPDCN with 30% blasts in his bone marrow and cutaneous lesions (biopsy-proven BPDCN) at baseline.
The patient first received pre-conditioning with fludarabine (30 mg/m²/day for 4 days) and cyclophosphamide (1 g/m²/day for 3 days).
On August 16, 2017 (Day 0), the patient received UCART123 at 6.25 x 105 cells/kg, the first dose level explored in the protocol, without complication.
By Day 5, the patient had developed grade 2 cytokine release syndrome (CRS) and a grade 3 lung infection, which improved after a first dose of tocilizumab and the administration of broad-spectrum, intravenous antibiotics.
On Day 8, the patient was found to have more severe CRS (ultimately grade 5) and grade 4 capillary leak syndrome. Despite receiving treatment in keeping with CRS management, including the administration of corticosteroids and tociluzumab as well as intensive care unit support, the patient died on Day 9.
AML patient
The first patient treated in the AML study experienced similar adverse effects as the BPDCN patient but is still alive.
The AML patient was a 58-year-old woman with 84% blasts in her bone marrow at baseline.
On June 27, 2017 (Day 0), the patient received the same pre-conditioning regimen and the same dose of UCART123 as the BPDCN patient, without complication.
By Day 8, the AML patient had developed grade 2 CRS. This worsened to grade 3 on Day 9 and resolved on Day 11 with treatment in the intensive care unit.
The patient also experienced grade 4 capillary leak syndrome on Day 9 that resolved on Day 12.
Next steps
The data safety monitoring board for these trials met on August 28 and recommended lowering the dose of UCART123 to 6.25 x 104 cells/kg in both studies and capping cyclophosphamide to a total dose of 4 g over 3 days.
Cellectis, the company developing UCART123, said it is working with study investigators and the FDA to resume both trials with an amended protocol that includes these dose adjustments. ![]()
The US Food and Drug Administration (FDA) has placed a clinical hold on both phase 1 studies of UCART123, a universal (allogeneic) chimeric antigen receptor (CAR) T-cell therapy targeting CD123.
One study was designed for patients with acute myeloid leukemia (AML), and the other was designed for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The clinical hold is due to the death of the first patient treated in the BPDCN trial.
The hold means no new subjects can be enrolled in either trial, and there can be no further dosing of subjects who are already enrolled.
BPDCN patient
The first patient treated in the BPDCN trial was a 78-year-old male who had received 1 prior therapy. He presented with relapsed/refractory BPDCN with 30% blasts in his bone marrow and cutaneous lesions (biopsy-proven BPDCN) at baseline.
The patient first received pre-conditioning with fludarabine (30 mg/m²/day for 4 days) and cyclophosphamide (1 g/m²/day for 3 days).
On August 16, 2017 (Day 0), the patient received UCART123 at 6.25 x 105 cells/kg, the first dose level explored in the protocol, without complication.
By Day 5, the patient had developed grade 2 cytokine release syndrome (CRS) and a grade 3 lung infection, which improved after a first dose of tocilizumab and the administration of broad-spectrum, intravenous antibiotics.
On Day 8, the patient was found to have more severe CRS (ultimately grade 5) and grade 4 capillary leak syndrome. Despite receiving treatment in keeping with CRS management, including the administration of corticosteroids and tociluzumab as well as intensive care unit support, the patient died on Day 9.
AML patient
The first patient treated in the AML study experienced similar adverse effects as the BPDCN patient but is still alive.
The AML patient was a 58-year-old woman with 84% blasts in her bone marrow at baseline.
On June 27, 2017 (Day 0), the patient received the same pre-conditioning regimen and the same dose of UCART123 as the BPDCN patient, without complication.
By Day 8, the AML patient had developed grade 2 CRS. This worsened to grade 3 on Day 9 and resolved on Day 11 with treatment in the intensive care unit.
The patient also experienced grade 4 capillary leak syndrome on Day 9 that resolved on Day 12.
Next steps
The data safety monitoring board for these trials met on August 28 and recommended lowering the dose of UCART123 to 6.25 x 104 cells/kg in both studies and capping cyclophosphamide to a total dose of 4 g over 3 days.
Cellectis, the company developing UCART123, said it is working with study investigators and the FDA to resume both trials with an amended protocol that includes these dose adjustments. ![]()
FDA grants orphan designation to product for CMV
The US Food and Drug Administration (FDA) has granted orphan drug designation to ATA230 for the treatment of cytomegalovirus (CMV) viremia and disease in immunocompromised patients.
ATA230 is an allogeneic, cytotoxic T-lymphocyte (CTL) product targeting antigens expressed by CMV.
The product is under investigation in phase 2 trials of patients with CMV viremia and disease who are refractory or resistant to antiviral treatment.
Atara Biotherapeutics, Inc., the company developing ATA230, said it will evaluate development plans for this therapy with the FDA and other global health authorities after beginning phase 3 studies of another product, ATA129.
The company said it decided to prioritize ATA129, which is being developed to treat patients with Epstein-Barr-virus-associated post-transplant lymphoproliferative disorder.
Phase 2 trial of ATA230
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to ATA230 for the treatment of cytomegalovirus (CMV) viremia and disease in immunocompromised patients.
ATA230 is an allogeneic, cytotoxic T-lymphocyte (CTL) product targeting antigens expressed by CMV.
The product is under investigation in phase 2 trials of patients with CMV viremia and disease who are refractory or resistant to antiviral treatment.
Atara Biotherapeutics, Inc., the company developing ATA230, said it will evaluate development plans for this therapy with the FDA and other global health authorities after beginning phase 3 studies of another product, ATA129.
The company said it decided to prioritize ATA129, which is being developed to treat patients with Epstein-Barr-virus-associated post-transplant lymphoproliferative disorder.
Phase 2 trial of ATA230
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to ATA230 for the treatment of cytomegalovirus (CMV) viremia and disease in immunocompromised patients.
ATA230 is an allogeneic, cytotoxic T-lymphocyte (CTL) product targeting antigens expressed by CMV.
The product is under investigation in phase 2 trials of patients with CMV viremia and disease who are refractory or resistant to antiviral treatment.
Atara Biotherapeutics, Inc., the company developing ATA230, said it will evaluate development plans for this therapy with the FDA and other global health authorities after beginning phase 3 studies of another product, ATA129.
The company said it decided to prioritize ATA129, which is being developed to treat patients with Epstein-Barr-virus-associated post-transplant lymphoproliferative disorder.
Phase 2 trial of ATA230
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
SCD drug receives rare pediatric disease designation
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to GBT440 for the treatment of sickle cell disease (SCD).
GBT440 is being developed by Global Blood Therapeutics, Inc. as a potentially disease-modifying therapy for SCD.
The drug works by increasing hemoglobin’s affinity for oxygen. Since oxygenated sickle hemoglobin does not polymerize, it is believed that GBT440 blocks polymerization and the resultant sickling of red blood cells.
If GBT440 can restore normal hemoglobin function and improve oxygen delivery, the therapy may be capable of modifying the progression of SCD.
The FDA previously granted GBT440 fast track and orphan drug designations.
About rare pediatric disease designation
Rare pediatric disease designation is granted to drugs that show promise to treat diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 or younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, if a drug with rare pediatric disease designation is approved, the drug’s developer may qualify for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
GBT440 trials
GBT440 is currently under investigation in a phase 1/2 trial (GBT440-001) of healthy subjects and adults with SCD. Data from this trial were presented at the 2016 ASH Annual Meeting.
At that time, there were 41 SCD patients who had been receiving GBT440 for up to 6 months.
All of these patients experienced a “profound and durable” reduction in hemolysis, as assessed by hemoglobin, reticulocytes, and/or bilirubin, according to Global Blood Therapeutics.
Patients treated with GBT440 for at least 90 days demonstrated a “clinically significant” increase in hemoglobin (greater than 1 g/dL increase) when compared with placebo-treated patients (46% vs 0%; P=0.006).
Patients treated with GBT440 also had a sustained reduction in irreversibly sickled cells when compared with placebo-treated patients (-76.6% vs +9.7%; P<0.001).
The most common treatment-related adverse events were grade 1/2 headache and gastrointestinal disorders. These events occurred in similar rates in the placebo and GBT440 arms. There were no drug-related serious or severe adverse events.
No sickle cell crises events occurred while participants were on GBT440. Exercise testing data showed normal tissue oxygen delivery (no change in oxygen consumption compared to placebo).
GBT440 is also under investigation in the phase 3 HOPE study, which includes SCD patients age 12 and older. And the drug is being tested in the phase 2 HOPE-KIDS 1 study, which includes pediatric patients (ages 6 to 17) with SCD. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to GBT440 for the treatment of sickle cell disease (SCD).
GBT440 is being developed by Global Blood Therapeutics, Inc. as a potentially disease-modifying therapy for SCD.
The drug works by increasing hemoglobin’s affinity for oxygen. Since oxygenated sickle hemoglobin does not polymerize, it is believed that GBT440 blocks polymerization and the resultant sickling of red blood cells.
If GBT440 can restore normal hemoglobin function and improve oxygen delivery, the therapy may be capable of modifying the progression of SCD.
The FDA previously granted GBT440 fast track and orphan drug designations.
About rare pediatric disease designation
Rare pediatric disease designation is granted to drugs that show promise to treat diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 or younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, if a drug with rare pediatric disease designation is approved, the drug’s developer may qualify for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
GBT440 trials
GBT440 is currently under investigation in a phase 1/2 trial (GBT440-001) of healthy subjects and adults with SCD. Data from this trial were presented at the 2016 ASH Annual Meeting.
At that time, there were 41 SCD patients who had been receiving GBT440 for up to 6 months.
All of these patients experienced a “profound and durable” reduction in hemolysis, as assessed by hemoglobin, reticulocytes, and/or bilirubin, according to Global Blood Therapeutics.
Patients treated with GBT440 for at least 90 days demonstrated a “clinically significant” increase in hemoglobin (greater than 1 g/dL increase) when compared with placebo-treated patients (46% vs 0%; P=0.006).
Patients treated with GBT440 also had a sustained reduction in irreversibly sickled cells when compared with placebo-treated patients (-76.6% vs +9.7%; P<0.001).
The most common treatment-related adverse events were grade 1/2 headache and gastrointestinal disorders. These events occurred in similar rates in the placebo and GBT440 arms. There were no drug-related serious or severe adverse events.
No sickle cell crises events occurred while participants were on GBT440. Exercise testing data showed normal tissue oxygen delivery (no change in oxygen consumption compared to placebo).
GBT440 is also under investigation in the phase 3 HOPE study, which includes SCD patients age 12 and older. And the drug is being tested in the phase 2 HOPE-KIDS 1 study, which includes pediatric patients (ages 6 to 17) with SCD. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to GBT440 for the treatment of sickle cell disease (SCD).
GBT440 is being developed by Global Blood Therapeutics, Inc. as a potentially disease-modifying therapy for SCD.
The drug works by increasing hemoglobin’s affinity for oxygen. Since oxygenated sickle hemoglobin does not polymerize, it is believed that GBT440 blocks polymerization and the resultant sickling of red blood cells.
If GBT440 can restore normal hemoglobin function and improve oxygen delivery, the therapy may be capable of modifying the progression of SCD.
The FDA previously granted GBT440 fast track and orphan drug designations.
About rare pediatric disease designation
Rare pediatric disease designation is granted to drugs that show promise to treat diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 or younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, if a drug with rare pediatric disease designation is approved, the drug’s developer may qualify for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
GBT440 trials
GBT440 is currently under investigation in a phase 1/2 trial (GBT440-001) of healthy subjects and adults with SCD. Data from this trial were presented at the 2016 ASH Annual Meeting.
At that time, there were 41 SCD patients who had been receiving GBT440 for up to 6 months.
All of these patients experienced a “profound and durable” reduction in hemolysis, as assessed by hemoglobin, reticulocytes, and/or bilirubin, according to Global Blood Therapeutics.
Patients treated with GBT440 for at least 90 days demonstrated a “clinically significant” increase in hemoglobin (greater than 1 g/dL increase) when compared with placebo-treated patients (46% vs 0%; P=0.006).
Patients treated with GBT440 also had a sustained reduction in irreversibly sickled cells when compared with placebo-treated patients (-76.6% vs +9.7%; P<0.001).
The most common treatment-related adverse events were grade 1/2 headache and gastrointestinal disorders. These events occurred in similar rates in the placebo and GBT440 arms. There were no drug-related serious or severe adverse events.
No sickle cell crises events occurred while participants were on GBT440. Exercise testing data showed normal tissue oxygen delivery (no change in oxygen consumption compared to placebo).
GBT440 is also under investigation in the phase 3 HOPE study, which includes SCD patients age 12 and older. And the drug is being tested in the phase 2 HOPE-KIDS 1 study, which includes pediatric patients (ages 6 to 17) with SCD. ![]()
FDA grants orphan designation to product for GVHD
The US Food and Drug Administration (FDA) has granted orphan drug designation to ApoGraft™ as prophylaxis for acute and chronic graft-versus-host disease (GVHD) in transplant recipients.
ApoGraft is a mobilized peripheral blood cell product collected via apheresis from a matched, related donor. The product is exposed to the apoptotic mediator Fas ligand prior to transplantation.
ApoGraft was designed to eliminate immune responses after transplantation of foreign cells and tissues.
ApoGraft is being developed by Cellect Biotechnology Ltd.
The company is testing ApoGraft as acute GVHD prophylaxis in a phase 1/2 trial.
The trial is currently enrolling patients with hemato-oncology disorders who are eligible for allogeneic, HLA-matched hematopoietic stem cell transplant (HSCT).
The study is expected to have 4 cohorts, each consisting of 3 patients.
The difference between the cohorts is the amount of apoptotic mediator Fas ligand (APO010) to which the graft is exposed during incubation prior to ApoGraft transplantation and HSCT:
- 10 ng/mL APO010 in Cohort 1
- 25 ng/mL APO010 in Cohort 2
- 50 ng/mL APO010 in Cohort 3
- 100 ng/mL APO010 in Cohort 4.
The study is expected to progress from one cohort to the next based on an independent data safety monitoring board review and analysis of safety data.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to ApoGraft™ as prophylaxis for acute and chronic graft-versus-host disease (GVHD) in transplant recipients.
ApoGraft is a mobilized peripheral blood cell product collected via apheresis from a matched, related donor. The product is exposed to the apoptotic mediator Fas ligand prior to transplantation.
ApoGraft was designed to eliminate immune responses after transplantation of foreign cells and tissues.
ApoGraft is being developed by Cellect Biotechnology Ltd.
The company is testing ApoGraft as acute GVHD prophylaxis in a phase 1/2 trial.
The trial is currently enrolling patients with hemato-oncology disorders who are eligible for allogeneic, HLA-matched hematopoietic stem cell transplant (HSCT).
The study is expected to have 4 cohorts, each consisting of 3 patients.
The difference between the cohorts is the amount of apoptotic mediator Fas ligand (APO010) to which the graft is exposed during incubation prior to ApoGraft transplantation and HSCT:
- 10 ng/mL APO010 in Cohort 1
- 25 ng/mL APO010 in Cohort 2
- 50 ng/mL APO010 in Cohort 3
- 100 ng/mL APO010 in Cohort 4.
The study is expected to progress from one cohort to the next based on an independent data safety monitoring board review and analysis of safety data.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to ApoGraft™ as prophylaxis for acute and chronic graft-versus-host disease (GVHD) in transplant recipients.
ApoGraft is a mobilized peripheral blood cell product collected via apheresis from a matched, related donor. The product is exposed to the apoptotic mediator Fas ligand prior to transplantation.
ApoGraft was designed to eliminate immune responses after transplantation of foreign cells and tissues.
ApoGraft is being developed by Cellect Biotechnology Ltd.
The company is testing ApoGraft as acute GVHD prophylaxis in a phase 1/2 trial.
The trial is currently enrolling patients with hemato-oncology disorders who are eligible for allogeneic, HLA-matched hematopoietic stem cell transplant (HSCT).
The study is expected to have 4 cohorts, each consisting of 3 patients.
The difference between the cohorts is the amount of apoptotic mediator Fas ligand (APO010) to which the graft is exposed during incubation prior to ApoGraft transplantation and HSCT:
- 10 ng/mL APO010 in Cohort 1
- 25 ng/mL APO010 in Cohort 2
- 50 ng/mL APO010 in Cohort 3
- 100 ng/mL APO010 in Cohort 4.
The study is expected to progress from one cohort to the next based on an independent data safety monitoring board review and analysis of safety data.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Respiratory infections in early years of life linked to celiac disease
in those with a family history of CD, according to Renata Auricchio, MD, University of Naples (Italy) Federico II, and her associates.
In a prospective cohort study, 373 newborns from families with at least one relative with CD were recruited. The cumulative incidence of new cases of CD was 6% at 3 years and 13.5% at 5 years of age, the researchers noted. In the first year when no child produced anti-tissue transglutaminase (anti-tTG) antibodies, respiratory infections (upper and lower tract) were more common among the case patients than among the controls (58% vs. 40%). During the second year, respiratory infections were again more frequent among the case patients than among controls (52% vs. 32%). And in the third year of life when most of the case patients were diagnosed with CD, no clinical event was more frequent in the case patients than in the control group.
“In this study, we report that early infections significantly contribute to the risk of developing CD,” Dr. Auricchio and her associates concluded. “It is possible that the exposure to early infection stimulates a genetically predisposed immune profile, which contributes to the switch from tolerance to intolerance to gluten, which is a common food antigen.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-4102).
in those with a family history of CD, according to Renata Auricchio, MD, University of Naples (Italy) Federico II, and her associates.
In a prospective cohort study, 373 newborns from families with at least one relative with CD were recruited. The cumulative incidence of new cases of CD was 6% at 3 years and 13.5% at 5 years of age, the researchers noted. In the first year when no child produced anti-tissue transglutaminase (anti-tTG) antibodies, respiratory infections (upper and lower tract) were more common among the case patients than among the controls (58% vs. 40%). During the second year, respiratory infections were again more frequent among the case patients than among controls (52% vs. 32%). And in the third year of life when most of the case patients were diagnosed with CD, no clinical event was more frequent in the case patients than in the control group.
“In this study, we report that early infections significantly contribute to the risk of developing CD,” Dr. Auricchio and her associates concluded. “It is possible that the exposure to early infection stimulates a genetically predisposed immune profile, which contributes to the switch from tolerance to intolerance to gluten, which is a common food antigen.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-4102).
in those with a family history of CD, according to Renata Auricchio, MD, University of Naples (Italy) Federico II, and her associates.
In a prospective cohort study, 373 newborns from families with at least one relative with CD were recruited. The cumulative incidence of new cases of CD was 6% at 3 years and 13.5% at 5 years of age, the researchers noted. In the first year when no child produced anti-tissue transglutaminase (anti-tTG) antibodies, respiratory infections (upper and lower tract) were more common among the case patients than among the controls (58% vs. 40%). During the second year, respiratory infections were again more frequent among the case patients than among controls (52% vs. 32%). And in the third year of life when most of the case patients were diagnosed with CD, no clinical event was more frequent in the case patients than in the control group.
“In this study, we report that early infections significantly contribute to the risk of developing CD,” Dr. Auricchio and her associates concluded. “It is possible that the exposure to early infection stimulates a genetically predisposed immune profile, which contributes to the switch from tolerance to intolerance to gluten, which is a common food antigen.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-4102).
FROM PEDIATRICS