User login
Defining Pharmacy Leadership in the VA
Ashley L. Adams, PharmD. What are the key leadership attributes of pharmacy leaders?
Julie A. Groppi, PharmD. As a pharmacy leader, you have to be confident in what you do as a pharmacist and not only look at what you are doing now but what you can do in the future. You always have to look for that next apple to pick, because you have to be willing to accept change and help influence change, even though many people do not like change. As a supervisor, I ran a large and growing clinical pharmacy program. I remember many colleagues saying, “You mean, I have to do this now?” I would always try to bring the conversation around with staff to ensure that the benefit of the change or ‘what is in it for you’ was included in the approach. If you are a leader, communicating with physicians, pharmacists, or VA leadership, you just need to sell it to show why it is important and how the change will improve the process. If you don’t, then you won’t be able facilitate or sustain the momentum needed for change.
One important aspect of being a change leader is to make sure you listen (and talk) to those working in the area on a daily basis when you are going through your processes and trying to create change on what is going happen. It is important to make sure your stakeholders are involved and heard while you think about all of your potential obstacles; this is something that I always have tried to do. Also, reflecting on where you have been and what you have done will help you to think differently and is something you should do both professionally and personally. I may not need to know every aspect of the process, but I need to know the obstacles to figure out ways to prevent or break down those walls and solve those underlying issues.
Dr. Adams. What are some of the challenges and opportunities you have found in pharmacy leadership
Dr. Groppi. I think the challenges [are related to] the sheer volume of work that is out there. Having the ability to be able to separate and think about where you want your team to go is the challenge of any leader. When you are right in the middle of it, you tend to focus on the task at hand to get the work done. One week, it is pain management, and then the next week it is hepatitis C, and then it’s assessing acute care services, then gaps or problems somewhere else. There are always different obstacles and different initiatives (pressures) coming at you. You have to not lose your sense of where you want to go. Often, many people cannot stop and look at the whole picture.
I joined the Clinical Pharmacy Practice office in 2011, and one of the first things we were challenged with when the office started was to write guidelines, create policies, and develop tools that would help guide the practice. However, when we started sending out resources to the field, many people were too busy with what was going on at their local facility to focus on what we had developed, so we had to step back. We brainstormed some ideas and looked at our peers in other offices who had demonstrated success. When we started discussing pharmacist scope of practice agreements, I looked at nursing service and their movement related to scope of practice and how it had impacted change in the profession over the past several years.
Nursing has great infrastructure and support for its program. They created many different types of clinical practice councils within nursing, and they were able to institute a lot of changes and spread their initiatives. We thought, “Why don’t we do this for clinical pharmacy?” So we started doing more outreach to the different sites and had discussions with our advisory board, which resulted in the development of the National Clinical Pharmacy Practice Council (NCPPC). We promoted facility and VISN councils to start talking about practice issues and regularly discussing our initiatives as a part of teleconferences, so we could gain support and keep the momentum. Now the NCPPC has grown and everyone is excited about what is happening. It is having a multipronged effect to impact clinical practice.
Dr. Adams. When you are starting on a new project, how do you and your fellow coworkers decide which one is the best to pursue?
Dr. Groppi. We just do them all—I’m joking... sometimes it feels that way. It’s really hard. There are a lot of different things happening at once and many competing priorities, so we try to do as many things as possible. We will assist with requests that come through the Central Office or questions coming from other program offices related to clinical pharmacy practice and we try to get involved and help support and share the success stories of our pharmacist roles as much as possible. For example, the National Nephrology Office contacted us, about the anticoagulation directive. They wanted to do something similar for nephrology since so many pharmacists were effectively and safely managing erythropoietin stimulating agents. This started a conversation.
Often, the priorities come from patient demand such as in primary care. When VA was implementing patient aligned care teams (PACTs), PBM had to ensure that we had conversations ready to describe clinical pharmacy practice in this area. The same thing occurred with hepatitis C. There were new drugs approved and roles for pharmacists, and often there were not enough providers to care for patients. It became an opportunity.
Frequently, choices are based on what we think will be the largest yield and the biggest gaps in care. Other times, it is based on national priorities. We look at the strategic plan for VA and develop our initiatives accordingly. What’s a new priority or component of the strategic plan for this year? What’s the plan for next year or moving forward? Telepharmacy a few years ago or telehealth is an example. We were making sure to describe our practice in the area and then set goals that are going to sustain the profession.
We focused on PACTs during the first few years as we had hundreds of pharmacists practicing. The next big area was specialty and acute care. We started leading workgroups and focused on policies and guidance to share strong practices. The past several years the focus has been on pain management because everyone is struggling with the number of veterans on opioids. When there is a big crisis, you have to hit it full force and look for opportunities that exist. Antimicrobial stewardship was another great example where we were able to provide help and describe the important role of pharmacists based on the strong practices we have across VA. Many times prioritization is on demand, but always keeping in mind what is happening around you and how it supports our VHA strategic plan.
Dr. Adams. What would be your main advice for future pharmacy leaders? Just taking those opportunities and going with them?
Dr. Groppi. Yes. Look for the spot where you might be able to make a positive im
Ashley L. Adams, PharmD. What are the key leadership attributes of pharmacy leaders?
Julie A. Groppi, PharmD. As a pharmacy leader, you have to be confident in what you do as a pharmacist and not only look at what you are doing now but what you can do in the future. You always have to look for that next apple to pick, because you have to be willing to accept change and help influence change, even though many people do not like change. As a supervisor, I ran a large and growing clinical pharmacy program. I remember many colleagues saying, “You mean, I have to do this now?” I would always try to bring the conversation around with staff to ensure that the benefit of the change or ‘what is in it for you’ was included in the approach. If you are a leader, communicating with physicians, pharmacists, or VA leadership, you just need to sell it to show why it is important and how the change will improve the process. If you don’t, then you won’t be able facilitate or sustain the momentum needed for change.
One important aspect of being a change leader is to make sure you listen (and talk) to those working in the area on a daily basis when you are going through your processes and trying to create change on what is going happen. It is important to make sure your stakeholders are involved and heard while you think about all of your potential obstacles; this is something that I always have tried to do. Also, reflecting on where you have been and what you have done will help you to think differently and is something you should do both professionally and personally. I may not need to know every aspect of the process, but I need to know the obstacles to figure out ways to prevent or break down those walls and solve those underlying issues.
Dr. Adams. What are some of the challenges and opportunities you have found in pharmacy leadership
Dr. Groppi. I think the challenges [are related to] the sheer volume of work that is out there. Having the ability to be able to separate and think about where you want your team to go is the challenge of any leader. When you are right in the middle of it, you tend to focus on the task at hand to get the work done. One week, it is pain management, and then the next week it is hepatitis C, and then it’s assessing acute care services, then gaps or problems somewhere else. There are always different obstacles and different initiatives (pressures) coming at you. You have to not lose your sense of where you want to go. Often, many people cannot stop and look at the whole picture.
I joined the Clinical Pharmacy Practice office in 2011, and one of the first things we were challenged with when the office started was to write guidelines, create policies, and develop tools that would help guide the practice. However, when we started sending out resources to the field, many people were too busy with what was going on at their local facility to focus on what we had developed, so we had to step back. We brainstormed some ideas and looked at our peers in other offices who had demonstrated success. When we started discussing pharmacist scope of practice agreements, I looked at nursing service and their movement related to scope of practice and how it had impacted change in the profession over the past several years.
Nursing has great infrastructure and support for its program. They created many different types of clinical practice councils within nursing, and they were able to institute a lot of changes and spread their initiatives. We thought, “Why don’t we do this for clinical pharmacy?” So we started doing more outreach to the different sites and had discussions with our advisory board, which resulted in the development of the National Clinical Pharmacy Practice Council (NCPPC). We promoted facility and VISN councils to start talking about practice issues and regularly discussing our initiatives as a part of teleconferences, so we could gain support and keep the momentum. Now the NCPPC has grown and everyone is excited about what is happening. It is having a multipronged effect to impact clinical practice.
Dr. Adams. When you are starting on a new project, how do you and your fellow coworkers decide which one is the best to pursue?
Dr. Groppi. We just do them all—I’m joking... sometimes it feels that way. It’s really hard. There are a lot of different things happening at once and many competing priorities, so we try to do as many things as possible. We will assist with requests that come through the Central Office or questions coming from other program offices related to clinical pharmacy practice and we try to get involved and help support and share the success stories of our pharmacist roles as much as possible. For example, the National Nephrology Office contacted us, about the anticoagulation directive. They wanted to do something similar for nephrology since so many pharmacists were effectively and safely managing erythropoietin stimulating agents. This started a conversation.
Often, the priorities come from patient demand such as in primary care. When VA was implementing patient aligned care teams (PACTs), PBM had to ensure that we had conversations ready to describe clinical pharmacy practice in this area. The same thing occurred with hepatitis C. There were new drugs approved and roles for pharmacists, and often there were not enough providers to care for patients. It became an opportunity.
Frequently, choices are based on what we think will be the largest yield and the biggest gaps in care. Other times, it is based on national priorities. We look at the strategic plan for VA and develop our initiatives accordingly. What’s a new priority or component of the strategic plan for this year? What’s the plan for next year or moving forward? Telepharmacy a few years ago or telehealth is an example. We were making sure to describe our practice in the area and then set goals that are going to sustain the profession.
We focused on PACTs during the first few years as we had hundreds of pharmacists practicing. The next big area was specialty and acute care. We started leading workgroups and focused on policies and guidance to share strong practices. The past several years the focus has been on pain management because everyone is struggling with the number of veterans on opioids. When there is a big crisis, you have to hit it full force and look for opportunities that exist. Antimicrobial stewardship was another great example where we were able to provide help and describe the important role of pharmacists based on the strong practices we have across VA. Many times prioritization is on demand, but always keeping in mind what is happening around you and how it supports our VHA strategic plan.
Dr. Adams. What would be your main advice for future pharmacy leaders? Just taking those opportunities and going with them?
Dr. Groppi. Yes. Look for the spot where you might be able to make a positive im
Ashley L. Adams, PharmD. What are the key leadership attributes of pharmacy leaders?
Julie A. Groppi, PharmD. As a pharmacy leader, you have to be confident in what you do as a pharmacist and not only look at what you are doing now but what you can do in the future. You always have to look for that next apple to pick, because you have to be willing to accept change and help influence change, even though many people do not like change. As a supervisor, I ran a large and growing clinical pharmacy program. I remember many colleagues saying, “You mean, I have to do this now?” I would always try to bring the conversation around with staff to ensure that the benefit of the change or ‘what is in it for you’ was included in the approach. If you are a leader, communicating with physicians, pharmacists, or VA leadership, you just need to sell it to show why it is important and how the change will improve the process. If you don’t, then you won’t be able facilitate or sustain the momentum needed for change.
One important aspect of being a change leader is to make sure you listen (and talk) to those working in the area on a daily basis when you are going through your processes and trying to create change on what is going happen. It is important to make sure your stakeholders are involved and heard while you think about all of your potential obstacles; this is something that I always have tried to do. Also, reflecting on where you have been and what you have done will help you to think differently and is something you should do both professionally and personally. I may not need to know every aspect of the process, but I need to know the obstacles to figure out ways to prevent or break down those walls and solve those underlying issues.
Dr. Adams. What are some of the challenges and opportunities you have found in pharmacy leadership
Dr. Groppi. I think the challenges [are related to] the sheer volume of work that is out there. Having the ability to be able to separate and think about where you want your team to go is the challenge of any leader. When you are right in the middle of it, you tend to focus on the task at hand to get the work done. One week, it is pain management, and then the next week it is hepatitis C, and then it’s assessing acute care services, then gaps or problems somewhere else. There are always different obstacles and different initiatives (pressures) coming at you. You have to not lose your sense of where you want to go. Often, many people cannot stop and look at the whole picture.
I joined the Clinical Pharmacy Practice office in 2011, and one of the first things we were challenged with when the office started was to write guidelines, create policies, and develop tools that would help guide the practice. However, when we started sending out resources to the field, many people were too busy with what was going on at their local facility to focus on what we had developed, so we had to step back. We brainstormed some ideas and looked at our peers in other offices who had demonstrated success. When we started discussing pharmacist scope of practice agreements, I looked at nursing service and their movement related to scope of practice and how it had impacted change in the profession over the past several years.
Nursing has great infrastructure and support for its program. They created many different types of clinical practice councils within nursing, and they were able to institute a lot of changes and spread their initiatives. We thought, “Why don’t we do this for clinical pharmacy?” So we started doing more outreach to the different sites and had discussions with our advisory board, which resulted in the development of the National Clinical Pharmacy Practice Council (NCPPC). We promoted facility and VISN councils to start talking about practice issues and regularly discussing our initiatives as a part of teleconferences, so we could gain support and keep the momentum. Now the NCPPC has grown and everyone is excited about what is happening. It is having a multipronged effect to impact clinical practice.
Dr. Adams. When you are starting on a new project, how do you and your fellow coworkers decide which one is the best to pursue?
Dr. Groppi. We just do them all—I’m joking... sometimes it feels that way. It’s really hard. There are a lot of different things happening at once and many competing priorities, so we try to do as many things as possible. We will assist with requests that come through the Central Office or questions coming from other program offices related to clinical pharmacy practice and we try to get involved and help support and share the success stories of our pharmacist roles as much as possible. For example, the National Nephrology Office contacted us, about the anticoagulation directive. They wanted to do something similar for nephrology since so many pharmacists were effectively and safely managing erythropoietin stimulating agents. This started a conversation.
Often, the priorities come from patient demand such as in primary care. When VA was implementing patient aligned care teams (PACTs), PBM had to ensure that we had conversations ready to describe clinical pharmacy practice in this area. The same thing occurred with hepatitis C. There were new drugs approved and roles for pharmacists, and often there were not enough providers to care for patients. It became an opportunity.
Frequently, choices are based on what we think will be the largest yield and the biggest gaps in care. Other times, it is based on national priorities. We look at the strategic plan for VA and develop our initiatives accordingly. What’s a new priority or component of the strategic plan for this year? What’s the plan for next year or moving forward? Telepharmacy a few years ago or telehealth is an example. We were making sure to describe our practice in the area and then set goals that are going to sustain the profession.
We focused on PACTs during the first few years as we had hundreds of pharmacists practicing. The next big area was specialty and acute care. We started leading workgroups and focused on policies and guidance to share strong practices. The past several years the focus has been on pain management because everyone is struggling with the number of veterans on opioids. When there is a big crisis, you have to hit it full force and look for opportunities that exist. Antimicrobial stewardship was another great example where we were able to provide help and describe the important role of pharmacists based on the strong practices we have across VA. Many times prioritization is on demand, but always keeping in mind what is happening around you and how it supports our VHA strategic plan.
Dr. Adams. What would be your main advice for future pharmacy leaders? Just taking those opportunities and going with them?
Dr. Groppi. Yes. Look for the spot where you might be able to make a positive im
BLA for CAR T-cell therapy granted priority review
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for axicabtagene ciloleucel (formerly KTE-C19), a chimeric antigen receptor (CAR) T-cell therapy.
Kite Pharma, Inc. is seeking approval for axicabtagene ciloleucel as a treatment for patients with relapsed or refractory, aggressive non-Hodgkin lymphoma (NHL) who are ineligible for autologous stem cell transplant.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA has set a review deadline of November 29, 2017, for the axicabtagene ciloleucel BLA.
Axicabtagene ciloleucel also has breakthrough therapy designation from the FDA as a treatment for diffuse large B-cell lymphoma, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma.
ZUMA-1 trial
The BLA for axicabtagene ciloleucel is supported by data from the phase 2 ZUMA-1 trial, which enrolled 111 patients with relapsed/refractory B-cell NHL.
After a single infusion of axicabtagene ciloleucel, the objective response rate was 82%. At a median follow-up of 8.7 months, 44% of patients were still in response, which included 39% of patients in complete response.
The most common grade 3 or higher adverse events were anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
There were 3 deaths during the trial that were not due to disease progression. Two of these deaths were deemed related to axicabtagene ciloleucel. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for axicabtagene ciloleucel (formerly KTE-C19), a chimeric antigen receptor (CAR) T-cell therapy.
Kite Pharma, Inc. is seeking approval for axicabtagene ciloleucel as a treatment for patients with relapsed or refractory, aggressive non-Hodgkin lymphoma (NHL) who are ineligible for autologous stem cell transplant.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA has set a review deadline of November 29, 2017, for the axicabtagene ciloleucel BLA.
Axicabtagene ciloleucel also has breakthrough therapy designation from the FDA as a treatment for diffuse large B-cell lymphoma, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma.
ZUMA-1 trial
The BLA for axicabtagene ciloleucel is supported by data from the phase 2 ZUMA-1 trial, which enrolled 111 patients with relapsed/refractory B-cell NHL.
After a single infusion of axicabtagene ciloleucel, the objective response rate was 82%. At a median follow-up of 8.7 months, 44% of patients were still in response, which included 39% of patients in complete response.
The most common grade 3 or higher adverse events were anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
There were 3 deaths during the trial that were not due to disease progression. Two of these deaths were deemed related to axicabtagene ciloleucel. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for axicabtagene ciloleucel (formerly KTE-C19), a chimeric antigen receptor (CAR) T-cell therapy.
Kite Pharma, Inc. is seeking approval for axicabtagene ciloleucel as a treatment for patients with relapsed or refractory, aggressive non-Hodgkin lymphoma (NHL) who are ineligible for autologous stem cell transplant.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA has set a review deadline of November 29, 2017, for the axicabtagene ciloleucel BLA.
Axicabtagene ciloleucel also has breakthrough therapy designation from the FDA as a treatment for diffuse large B-cell lymphoma, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma.
ZUMA-1 trial
The BLA for axicabtagene ciloleucel is supported by data from the phase 2 ZUMA-1 trial, which enrolled 111 patients with relapsed/refractory B-cell NHL.
After a single infusion of axicabtagene ciloleucel, the objective response rate was 82%. At a median follow-up of 8.7 months, 44% of patients were still in response, which included 39% of patients in complete response.
The most common grade 3 or higher adverse events were anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
There were 3 deaths during the trial that were not due to disease progression. Two of these deaths were deemed related to axicabtagene ciloleucel. ![]()
How Zika arrived and spread in Florida
A study published in Nature appears to explain how Zika virus entered the US via Florida in 2016 and how the virus might re-enter the country this year.
By sequencing the Zika virus genome at different points in the outbreak, researchers created a family tree showing where cases originated and how quickly they spread.
The team discovered that transmission of Zika virus began in Florida at least 4—and potentially up to 40—times last year.
The researchers also traced most of the Zika lineages back to strains of the virus in the Caribbean.
“Without these genomes, we wouldn’t be able to reconstruct the history of how the virus moved around,” said study author Kristian G. Andersen, PhD, of The Scripps Research Institute in La Jolla, California.
“Rapid viral genome sequencing during ongoing outbreaks is a new development that has only been made possible over the last couple of years.”
Why Miami?
By sequencing Zika virus genomes from humans and mosquitoes—and analyzing travel and mosquito abundance data—the researchers found that several factors created a “perfect storm” for the spread of Zika virus in Miami.
“This study shows why Miami is special,” said study author Nathan D. Grubaugh, PhD, of The Scripps Research Institute.
First, Dr Grubaugh explained, Miami is home to year-round populations of Aedes aegypti mosquitoes, the main species that transmits Zika virus.
The area is also a significant travel hub, bringing in more international air and sea traffic than any other city in the continental US in 2016. Finally, Miami is an especially popular destination for travelers who have visited Zika-afflicted areas.
The researchers found that travel from the Caribbean islands may have significantly contributed to cases of Zika reaching Miami.
Of the 5.7 million international travelers entering Miami by flights and cruise ships between January and June of 2016, more than half arrived from the Caribbean.
Killing mosquitos produces results
The researchers believe Zika virus may have started transmission in Miami up to 40 times, but most travel-related cases did not lead to any secondary infections locally. The virus was more likely to reach a dead end than keep spreading.
The researchers found that one reason for the dead-ends was a direct connection between mosquito control efforts and disease prevention.
“We show that if you decrease the mosquito population in an area, the number of Zika infections goes down proportionally,” Dr Andersen said. “This means we can significantly limit the risk of Zika virus by focusing on mosquito control. This is not too surprising, but it’s important to show that there is an almost perfect correlation between the number of mosquitos and the number of human infections.”
Based on data from the outbreak, the researchers see potential in stopping the virus through mosquito control efforts in the US and other infected countries, instead of, for example, through travel restrictions.
“Given how many times the introductions happened, trying to restrict traffic or movement of people obviously isn’t a solution,” Dr Andersen said. “Focusing on disease prevention and mosquito control in endemic areas is likely to be a much more successful strategy.”
When the virus did spread, the researchers found that splitting Miami into designated Zika zones—often done by neighborhood or city block—didn’t accurately represent how the virus was moving.
Within each Zika zone, the researchers discovered a mixing of multiple Zika lineages, suggesting the virus wasn’t well-confined, likely moving around with infected people.
Drs Andersen and Grubaugh hope these lessons from the 2016 epidemic will help researchers and health officials respond even faster to prevent Zika’s spread in 2017.
Behind the data
Understanding Zika’s timeline required an international team of researchers and partnerships with several health agencies.
The researchers also designed a new method of genomic sequencing just to study the Zika virus. Because Zika is hard to collect in the blood of those infected, it was a challenge for the researchers to isolate enough of its genetic material for sequencing.
To solve this problem, the researchers developed 2 different protocols to break apart the genetic material they could find and reassemble it in a useful way for analysis.
With these new protocols, the researchers sequenced the virus from 28 of the reported 256 Zika cases in Florida, as well as 7 mosquito pools, to model what happened in the larger patient group.
As they worked, the researchers released their data immediately publicly to help other researchers. They hope to release more data—and analysis—in real time as cases mount in 2017.
The research was supported by the National Institutes of Health, The Pew Charitable Trusts, The Ray Thomas Foundation, a Mahan Postdoctoral Fellowship from the Computational Biology Program at Fred Hutchinson Cancer Research Center, the US Centers for Disease Control and Prevention, the European Union Seventh Framework Programme, the United States Agency for International Development Emerging Pandemic Threats Program-2 PREDICT-2, and the Defense Advanced Research Projects Agency. ![]()
A study published in Nature appears to explain how Zika virus entered the US via Florida in 2016 and how the virus might re-enter the country this year.
By sequencing the Zika virus genome at different points in the outbreak, researchers created a family tree showing where cases originated and how quickly they spread.
The team discovered that transmission of Zika virus began in Florida at least 4—and potentially up to 40—times last year.
The researchers also traced most of the Zika lineages back to strains of the virus in the Caribbean.
“Without these genomes, we wouldn’t be able to reconstruct the history of how the virus moved around,” said study author Kristian G. Andersen, PhD, of The Scripps Research Institute in La Jolla, California.
“Rapid viral genome sequencing during ongoing outbreaks is a new development that has only been made possible over the last couple of years.”
Why Miami?
By sequencing Zika virus genomes from humans and mosquitoes—and analyzing travel and mosquito abundance data—the researchers found that several factors created a “perfect storm” for the spread of Zika virus in Miami.
“This study shows why Miami is special,” said study author Nathan D. Grubaugh, PhD, of The Scripps Research Institute.
First, Dr Grubaugh explained, Miami is home to year-round populations of Aedes aegypti mosquitoes, the main species that transmits Zika virus.
The area is also a significant travel hub, bringing in more international air and sea traffic than any other city in the continental US in 2016. Finally, Miami is an especially popular destination for travelers who have visited Zika-afflicted areas.
The researchers found that travel from the Caribbean islands may have significantly contributed to cases of Zika reaching Miami.
Of the 5.7 million international travelers entering Miami by flights and cruise ships between January and June of 2016, more than half arrived from the Caribbean.
Killing mosquitos produces results
The researchers believe Zika virus may have started transmission in Miami up to 40 times, but most travel-related cases did not lead to any secondary infections locally. The virus was more likely to reach a dead end than keep spreading.
The researchers found that one reason for the dead-ends was a direct connection between mosquito control efforts and disease prevention.
“We show that if you decrease the mosquito population in an area, the number of Zika infections goes down proportionally,” Dr Andersen said. “This means we can significantly limit the risk of Zika virus by focusing on mosquito control. This is not too surprising, but it’s important to show that there is an almost perfect correlation between the number of mosquitos and the number of human infections.”
Based on data from the outbreak, the researchers see potential in stopping the virus through mosquito control efforts in the US and other infected countries, instead of, for example, through travel restrictions.
“Given how many times the introductions happened, trying to restrict traffic or movement of people obviously isn’t a solution,” Dr Andersen said. “Focusing on disease prevention and mosquito control in endemic areas is likely to be a much more successful strategy.”
When the virus did spread, the researchers found that splitting Miami into designated Zika zones—often done by neighborhood or city block—didn’t accurately represent how the virus was moving.
Within each Zika zone, the researchers discovered a mixing of multiple Zika lineages, suggesting the virus wasn’t well-confined, likely moving around with infected people.
Drs Andersen and Grubaugh hope these lessons from the 2016 epidemic will help researchers and health officials respond even faster to prevent Zika’s spread in 2017.
Behind the data
Understanding Zika’s timeline required an international team of researchers and partnerships with several health agencies.
The researchers also designed a new method of genomic sequencing just to study the Zika virus. Because Zika is hard to collect in the blood of those infected, it was a challenge for the researchers to isolate enough of its genetic material for sequencing.
To solve this problem, the researchers developed 2 different protocols to break apart the genetic material they could find and reassemble it in a useful way for analysis.
With these new protocols, the researchers sequenced the virus from 28 of the reported 256 Zika cases in Florida, as well as 7 mosquito pools, to model what happened in the larger patient group.
As they worked, the researchers released their data immediately publicly to help other researchers. They hope to release more data—and analysis—in real time as cases mount in 2017.
The research was supported by the National Institutes of Health, The Pew Charitable Trusts, The Ray Thomas Foundation, a Mahan Postdoctoral Fellowship from the Computational Biology Program at Fred Hutchinson Cancer Research Center, the US Centers for Disease Control and Prevention, the European Union Seventh Framework Programme, the United States Agency for International Development Emerging Pandemic Threats Program-2 PREDICT-2, and the Defense Advanced Research Projects Agency. ![]()
A study published in Nature appears to explain how Zika virus entered the US via Florida in 2016 and how the virus might re-enter the country this year.
By sequencing the Zika virus genome at different points in the outbreak, researchers created a family tree showing where cases originated and how quickly they spread.
The team discovered that transmission of Zika virus began in Florida at least 4—and potentially up to 40—times last year.
The researchers also traced most of the Zika lineages back to strains of the virus in the Caribbean.
“Without these genomes, we wouldn’t be able to reconstruct the history of how the virus moved around,” said study author Kristian G. Andersen, PhD, of The Scripps Research Institute in La Jolla, California.
“Rapid viral genome sequencing during ongoing outbreaks is a new development that has only been made possible over the last couple of years.”
Why Miami?
By sequencing Zika virus genomes from humans and mosquitoes—and analyzing travel and mosquito abundance data—the researchers found that several factors created a “perfect storm” for the spread of Zika virus in Miami.
“This study shows why Miami is special,” said study author Nathan D. Grubaugh, PhD, of The Scripps Research Institute.
First, Dr Grubaugh explained, Miami is home to year-round populations of Aedes aegypti mosquitoes, the main species that transmits Zika virus.
The area is also a significant travel hub, bringing in more international air and sea traffic than any other city in the continental US in 2016. Finally, Miami is an especially popular destination for travelers who have visited Zika-afflicted areas.
The researchers found that travel from the Caribbean islands may have significantly contributed to cases of Zika reaching Miami.
Of the 5.7 million international travelers entering Miami by flights and cruise ships between January and June of 2016, more than half arrived from the Caribbean.
Killing mosquitos produces results
The researchers believe Zika virus may have started transmission in Miami up to 40 times, but most travel-related cases did not lead to any secondary infections locally. The virus was more likely to reach a dead end than keep spreading.
The researchers found that one reason for the dead-ends was a direct connection between mosquito control efforts and disease prevention.
“We show that if you decrease the mosquito population in an area, the number of Zika infections goes down proportionally,” Dr Andersen said. “This means we can significantly limit the risk of Zika virus by focusing on mosquito control. This is not too surprising, but it’s important to show that there is an almost perfect correlation between the number of mosquitos and the number of human infections.”
Based on data from the outbreak, the researchers see potential in stopping the virus through mosquito control efforts in the US and other infected countries, instead of, for example, through travel restrictions.
“Given how many times the introductions happened, trying to restrict traffic or movement of people obviously isn’t a solution,” Dr Andersen said. “Focusing on disease prevention and mosquito control in endemic areas is likely to be a much more successful strategy.”
When the virus did spread, the researchers found that splitting Miami into designated Zika zones—often done by neighborhood or city block—didn’t accurately represent how the virus was moving.
Within each Zika zone, the researchers discovered a mixing of multiple Zika lineages, suggesting the virus wasn’t well-confined, likely moving around with infected people.
Drs Andersen and Grubaugh hope these lessons from the 2016 epidemic will help researchers and health officials respond even faster to prevent Zika’s spread in 2017.
Behind the data
Understanding Zika’s timeline required an international team of researchers and partnerships with several health agencies.
The researchers also designed a new method of genomic sequencing just to study the Zika virus. Because Zika is hard to collect in the blood of those infected, it was a challenge for the researchers to isolate enough of its genetic material for sequencing.
To solve this problem, the researchers developed 2 different protocols to break apart the genetic material they could find and reassemble it in a useful way for analysis.
With these new protocols, the researchers sequenced the virus from 28 of the reported 256 Zika cases in Florida, as well as 7 mosquito pools, to model what happened in the larger patient group.
As they worked, the researchers released their data immediately publicly to help other researchers. They hope to release more data—and analysis—in real time as cases mount in 2017.
The research was supported by the National Institutes of Health, The Pew Charitable Trusts, The Ray Thomas Foundation, a Mahan Postdoctoral Fellowship from the Computational Biology Program at Fred Hutchinson Cancer Research Center, the US Centers for Disease Control and Prevention, the European Union Seventh Framework Programme, the United States Agency for International Development Emerging Pandemic Threats Program-2 PREDICT-2, and the Defense Advanced Research Projects Agency. ![]()
AstraZeneca recalls lot of Brilinta in US
AstraZeneca has announced a voluntary recall of 1 lot of professional (physician) sample bottles containing 8 tablets of Brilinta® (ticagrelor).
This recall follows a report that a professional sample bottle containing 8 tablets of Brilinta 90 mg also contained Zurampic® (lesinurad) 200 mg tablets, which is also manufactured by AstraZeneca.
The company said this precautionary measure is limited to 1 lot of Brilinta (Brilinta lot #JB5047) distributed to physicians in the US between March and April of 2017.
Other forms and dosage strengths of Brilinta, including medicine obtained via US retail or mail order pharmacies, are not affected by this recall. And this recall does not affect Zurampic.
Potential risks
Unintentional dosing with Zurampic has the potential to lead to adverse renal effects, including acute renal failure, which is more common when Zurampic is given alone, as it should be used in combination with a xanthine oxidase inhibitor.
Missed doses of Brilinta increase the risk of heart attack and stroke. People with a stent who miss doses of Brilinta have a higher risk of stent thrombosis, heart attack, and death. Patients should not stop taking Brilinta without talking to their prescribing doctor.
To date, AstraZeneca has not received any reports of adverse events related to this recall.
Next steps
AstraZeneca is notifying physicians by recall letter and is arranging for the return of all recalled products. Consumers who have medicine that is being recalled should contact their physician.
Consumers with questions regarding this recall can contact the AstraZeneca Information Center at 1-800-236-9933 between the hours of 8 am and 6 pm (Eastern time) Monday to Friday, excluding holidays.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using Brilinta.
Adverse reactions or quality problems related to Brilinta may also be reported to the FDA’s MedWatch Adverse Event Reporting Program.
About Brilinta and Zurampic
Brilinta is intended for use to reduce the rate of cardiovascular death, heart attack, and stroke in patients with acute coronary syndrome or a history of heart attack.
Brilinta is also intended to reduce the rate of stent thrombosis in patients who have been stented for treatment of acute coronary syndrome.
Zurampic is used together with a xanthine oxidase inhibitor, such as allopurinol or Uloric, in adults with gout who still have a high uric acid level.
Brilinta 90 mg tablets are supplied as a round, biconvex, yellow, film-coated tablet, and imprinted with a “90” above a “T” on one side of the pill.
Zurampic tablets 200 mg are blue in color and elliptical/oval in shape. They are imprinted with “LES200” on one side of the pill. ![]()
AstraZeneca has announced a voluntary recall of 1 lot of professional (physician) sample bottles containing 8 tablets of Brilinta® (ticagrelor).
This recall follows a report that a professional sample bottle containing 8 tablets of Brilinta 90 mg also contained Zurampic® (lesinurad) 200 mg tablets, which is also manufactured by AstraZeneca.
The company said this precautionary measure is limited to 1 lot of Brilinta (Brilinta lot #JB5047) distributed to physicians in the US between March and April of 2017.
Other forms and dosage strengths of Brilinta, including medicine obtained via US retail or mail order pharmacies, are not affected by this recall. And this recall does not affect Zurampic.
Potential risks
Unintentional dosing with Zurampic has the potential to lead to adverse renal effects, including acute renal failure, which is more common when Zurampic is given alone, as it should be used in combination with a xanthine oxidase inhibitor.
Missed doses of Brilinta increase the risk of heart attack and stroke. People with a stent who miss doses of Brilinta have a higher risk of stent thrombosis, heart attack, and death. Patients should not stop taking Brilinta without talking to their prescribing doctor.
To date, AstraZeneca has not received any reports of adverse events related to this recall.
Next steps
AstraZeneca is notifying physicians by recall letter and is arranging for the return of all recalled products. Consumers who have medicine that is being recalled should contact their physician.
Consumers with questions regarding this recall can contact the AstraZeneca Information Center at 1-800-236-9933 between the hours of 8 am and 6 pm (Eastern time) Monday to Friday, excluding holidays.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using Brilinta.
Adverse reactions or quality problems related to Brilinta may also be reported to the FDA’s MedWatch Adverse Event Reporting Program.
About Brilinta and Zurampic
Brilinta is intended for use to reduce the rate of cardiovascular death, heart attack, and stroke in patients with acute coronary syndrome or a history of heart attack.
Brilinta is also intended to reduce the rate of stent thrombosis in patients who have been stented for treatment of acute coronary syndrome.
Zurampic is used together with a xanthine oxidase inhibitor, such as allopurinol or Uloric, in adults with gout who still have a high uric acid level.
Brilinta 90 mg tablets are supplied as a round, biconvex, yellow, film-coated tablet, and imprinted with a “90” above a “T” on one side of the pill.
Zurampic tablets 200 mg are blue in color and elliptical/oval in shape. They are imprinted with “LES200” on one side of the pill. ![]()
AstraZeneca has announced a voluntary recall of 1 lot of professional (physician) sample bottles containing 8 tablets of Brilinta® (ticagrelor).
This recall follows a report that a professional sample bottle containing 8 tablets of Brilinta 90 mg also contained Zurampic® (lesinurad) 200 mg tablets, which is also manufactured by AstraZeneca.
The company said this precautionary measure is limited to 1 lot of Brilinta (Brilinta lot #JB5047) distributed to physicians in the US between March and April of 2017.
Other forms and dosage strengths of Brilinta, including medicine obtained via US retail or mail order pharmacies, are not affected by this recall. And this recall does not affect Zurampic.
Potential risks
Unintentional dosing with Zurampic has the potential to lead to adverse renal effects, including acute renal failure, which is more common when Zurampic is given alone, as it should be used in combination with a xanthine oxidase inhibitor.
Missed doses of Brilinta increase the risk of heart attack and stroke. People with a stent who miss doses of Brilinta have a higher risk of stent thrombosis, heart attack, and death. Patients should not stop taking Brilinta without talking to their prescribing doctor.
To date, AstraZeneca has not received any reports of adverse events related to this recall.
Next steps
AstraZeneca is notifying physicians by recall letter and is arranging for the return of all recalled products. Consumers who have medicine that is being recalled should contact their physician.
Consumers with questions regarding this recall can contact the AstraZeneca Information Center at 1-800-236-9933 between the hours of 8 am and 6 pm (Eastern time) Monday to Friday, excluding holidays.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using Brilinta.
Adverse reactions or quality problems related to Brilinta may also be reported to the FDA’s MedWatch Adverse Event Reporting Program.
About Brilinta and Zurampic
Brilinta is intended for use to reduce the rate of cardiovascular death, heart attack, and stroke in patients with acute coronary syndrome or a history of heart attack.
Brilinta is also intended to reduce the rate of stent thrombosis in patients who have been stented for treatment of acute coronary syndrome.
Zurampic is used together with a xanthine oxidase inhibitor, such as allopurinol or Uloric, in adults with gout who still have a high uric acid level.
Brilinta 90 mg tablets are supplied as a round, biconvex, yellow, film-coated tablet, and imprinted with a “90” above a “T” on one side of the pill.
Zurampic tablets 200 mg are blue in color and elliptical/oval in shape. They are imprinted with “LES200” on one side of the pill. ![]()
Genetic Insights Refine Prognostic Information in ALS
Researchers have shown that the negative prognostic characteristics of the C9orf72 repeat expansion in amyotrophic lateral sclerosis (ALS) apply primarily to males with spinal-onset disease, according to a report in the April issue of the Journal of Neurology, Neurosurgery and Psychiatry. This finding represents “a hitherto unrecognized gender-mediated effect of the genetic variant,” said lead author James Rooney, MD, a research fellow in the Academic Unit of Neurology at Trinity College Dublin.
The C9orf72 mutation represents the most common genetic cause of ALS. It affects up to 50% of familial cases and 20% of sporadic cases and is associated with a distinctive clinical phenotype that sometimes includes frontotemporal dementia. The C9orf72 repeat expansion previously has been reported as a negative prognostic factor in ALS, but studies have not been sufficiently powered to determine how the expanded variant affects subgroups of patients. To further explore the interaction of the genetic variant with demographic features, Dr. Rooney and colleagues examined the prognostic impact of the C9orf72 repeat expansion in European subgroups based on gender and site of onset.
C9orf72 status in combination with demographic and clinical data was drawn from 4,925 patients with ALS in three prospective national ALS registries (Ireland, Italy, and the Netherlands) and clinical data sets in the United Kingdom and Belgium. Flexible parametric survival models were built including known prognostic factors (eg, age, diagnostic delay, and site of onset), gender, and the presence of an expanded repeat in C9orf72. These models were used to explore the effects of C9orf72 on survival by gender and site of onset. Individual patient data meta-analysis was used to estimate hazard ratios for results of particular importance.
A total of 457 (8.95%) of the 4,925 patients carried the C9orf72 repeat expansion. A meta-analysis of C9orf72 estimated a survival hazard ratio of 1.36 (range, 1.18 to 1.57) for those carrying the expansion. Models evaluating interaction between gender and C9orf72 repeat expansions demonstrated that the reduced survival due to C9orf72 expansion primarily occurred in males with spinal-onset disease (hazard ratio, 1.56).
“Within this cohort, the median age of onset was 59.3, and the median survival was 2.29 years,” Dr. Rooney reported. These results compared with a median age of onset of 62.3 and median survival of 2.77 years in all other spinal-onset disease, with a median age of onset of 65 and median survival of 2.38 years in all bulbar-onset disease. “Moreover, and contrary to the usual pattern in young-onset disease, male spinal-onset C9orf72 expanded cases were also more likely to have experienced a shorter diagnostic delay, suggesting rapidly progressing disease,” he said.
“Dissecting prognostic factors associated with C9orf72 remains essential and highly relevant for the design of future therapeutic strategies,” said José Manuel Matamala, MD, and colleagues in an accompanying editorial. Dr. Matamala is affiliated with the Brain and Mind Centre at the University of Sydney.
—Glenn S. Williams
Suggested Reading
Rooney J, Fogh I, Westeneng HJ, et al. C9orf72 expansion differentially affects males with spinal onset amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(4):295-300.
Matamala JM, Dharmadasa T, Kiernan MC. Prognostic factors in C9orf72 amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(4):281
Researchers have shown that the negative prognostic characteristics of the C9orf72 repeat expansion in amyotrophic lateral sclerosis (ALS) apply primarily to males with spinal-onset disease, according to a report in the April issue of the Journal of Neurology, Neurosurgery and Psychiatry. This finding represents “a hitherto unrecognized gender-mediated effect of the genetic variant,” said lead author James Rooney, MD, a research fellow in the Academic Unit of Neurology at Trinity College Dublin.
The C9orf72 mutation represents the most common genetic cause of ALS. It affects up to 50% of familial cases and 20% of sporadic cases and is associated with a distinctive clinical phenotype that sometimes includes frontotemporal dementia. The C9orf72 repeat expansion previously has been reported as a negative prognostic factor in ALS, but studies have not been sufficiently powered to determine how the expanded variant affects subgroups of patients. To further explore the interaction of the genetic variant with demographic features, Dr. Rooney and colleagues examined the prognostic impact of the C9orf72 repeat expansion in European subgroups based on gender and site of onset.
C9orf72 status in combination with demographic and clinical data was drawn from 4,925 patients with ALS in three prospective national ALS registries (Ireland, Italy, and the Netherlands) and clinical data sets in the United Kingdom and Belgium. Flexible parametric survival models were built including known prognostic factors (eg, age, diagnostic delay, and site of onset), gender, and the presence of an expanded repeat in C9orf72. These models were used to explore the effects of C9orf72 on survival by gender and site of onset. Individual patient data meta-analysis was used to estimate hazard ratios for results of particular importance.
A total of 457 (8.95%) of the 4,925 patients carried the C9orf72 repeat expansion. A meta-analysis of C9orf72 estimated a survival hazard ratio of 1.36 (range, 1.18 to 1.57) for those carrying the expansion. Models evaluating interaction between gender and C9orf72 repeat expansions demonstrated that the reduced survival due to C9orf72 expansion primarily occurred in males with spinal-onset disease (hazard ratio, 1.56).
“Within this cohort, the median age of onset was 59.3, and the median survival was 2.29 years,” Dr. Rooney reported. These results compared with a median age of onset of 62.3 and median survival of 2.77 years in all other spinal-onset disease, with a median age of onset of 65 and median survival of 2.38 years in all bulbar-onset disease. “Moreover, and contrary to the usual pattern in young-onset disease, male spinal-onset C9orf72 expanded cases were also more likely to have experienced a shorter diagnostic delay, suggesting rapidly progressing disease,” he said.
“Dissecting prognostic factors associated with C9orf72 remains essential and highly relevant for the design of future therapeutic strategies,” said José Manuel Matamala, MD, and colleagues in an accompanying editorial. Dr. Matamala is affiliated with the Brain and Mind Centre at the University of Sydney.
—Glenn S. Williams
Suggested Reading
Rooney J, Fogh I, Westeneng HJ, et al. C9orf72 expansion differentially affects males with spinal onset amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(4):295-300.
Matamala JM, Dharmadasa T, Kiernan MC. Prognostic factors in C9orf72 amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(4):281
Researchers have shown that the negative prognostic characteristics of the C9orf72 repeat expansion in amyotrophic lateral sclerosis (ALS) apply primarily to males with spinal-onset disease, according to a report in the April issue of the Journal of Neurology, Neurosurgery and Psychiatry. This finding represents “a hitherto unrecognized gender-mediated effect of the genetic variant,” said lead author James Rooney, MD, a research fellow in the Academic Unit of Neurology at Trinity College Dublin.
The C9orf72 mutation represents the most common genetic cause of ALS. It affects up to 50% of familial cases and 20% of sporadic cases and is associated with a distinctive clinical phenotype that sometimes includes frontotemporal dementia. The C9orf72 repeat expansion previously has been reported as a negative prognostic factor in ALS, but studies have not been sufficiently powered to determine how the expanded variant affects subgroups of patients. To further explore the interaction of the genetic variant with demographic features, Dr. Rooney and colleagues examined the prognostic impact of the C9orf72 repeat expansion in European subgroups based on gender and site of onset.
C9orf72 status in combination with demographic and clinical data was drawn from 4,925 patients with ALS in three prospective national ALS registries (Ireland, Italy, and the Netherlands) and clinical data sets in the United Kingdom and Belgium. Flexible parametric survival models were built including known prognostic factors (eg, age, diagnostic delay, and site of onset), gender, and the presence of an expanded repeat in C9orf72. These models were used to explore the effects of C9orf72 on survival by gender and site of onset. Individual patient data meta-analysis was used to estimate hazard ratios for results of particular importance.
A total of 457 (8.95%) of the 4,925 patients carried the C9orf72 repeat expansion. A meta-analysis of C9orf72 estimated a survival hazard ratio of 1.36 (range, 1.18 to 1.57) for those carrying the expansion. Models evaluating interaction between gender and C9orf72 repeat expansions demonstrated that the reduced survival due to C9orf72 expansion primarily occurred in males with spinal-onset disease (hazard ratio, 1.56).
“Within this cohort, the median age of onset was 59.3, and the median survival was 2.29 years,” Dr. Rooney reported. These results compared with a median age of onset of 62.3 and median survival of 2.77 years in all other spinal-onset disease, with a median age of onset of 65 and median survival of 2.38 years in all bulbar-onset disease. “Moreover, and contrary to the usual pattern in young-onset disease, male spinal-onset C9orf72 expanded cases were also more likely to have experienced a shorter diagnostic delay, suggesting rapidly progressing disease,” he said.
“Dissecting prognostic factors associated with C9orf72 remains essential and highly relevant for the design of future therapeutic strategies,” said José Manuel Matamala, MD, and colleagues in an accompanying editorial. Dr. Matamala is affiliated with the Brain and Mind Centre at the University of Sydney.
—Glenn S. Williams
Suggested Reading
Rooney J, Fogh I, Westeneng HJ, et al. C9orf72 expansion differentially affects males with spinal onset amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(4):295-300.
Matamala JM, Dharmadasa T, Kiernan MC. Prognostic factors in C9orf72 amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(4):281
Study confirms new mutation, possible therapeutic target in epidermolysis bullosa
PORTLAND, ORE. – New research has uncovered “a new kid on the block” of genes underlying epidermolysis bullosa simplex (EBS), which may someday pave the way for new therapies, John McGrath, MD, said at the annual meeting of the Society for Investigative Dermatology.
The gene encodes kelch-like family member 24 (KLHL24), the substrate receptor of cullin 3-RBX1-KLHL24 complex, explained Dr. McGrath, professor of molecular dermatology, St. John’s Institute of Dermatology, King’s College London, where he studies heritable skin diseases. Keratin 14 is the substrate of this complex. In EBS, KLHL24 mutations affect methionine initiation codons, resulting in the loss of the first 28 amino acids of KLHL24 protein. As a result, the protein becomes overly stable, which leads to excessive ubiquitination and breakdown of keratin 14.
KLHL24 is the 19th gene to be implicated in EBS. News of the finding broke in late 2016, when two publications identified KLHL24 mutations in 19 patients with EBS from 10 different families. The first study, conducted in China, identified start-codon mutations in the KLHL24 gene in five patients, confirmed keratin 14 as the substrate of the protein, and established that KLHL24 mutations induce disproportionate ubiquitination and fragmentation of keratin 14. The researchers confirmed these results by using a knock-in mouse model (Nat Genet. 2016 Dec;48 [12]:1508-16).
Meanwhile, European researchers identified KLHL24 mutations in families from Israel, Germany, Switzerland, Finland, Qatar, and Italy, and confirmed that these mutations affect the equilibrium between intermediate keratin filaments and keratin breakdown that is necessary for skin integrity (Am J Hum Genet. 2016 Dec 1;99[6]:1395-1404).
EBS affects more than 400,000 individuals worldwide, and is usually linked to heterozygous missense mutations in genes encoding keratins 5 and 14, Dr. McGrath noted. “However, EBS is clinically and genetically heterogeneous,” he added. “About 20% of cases have no mutation in known genes.” Pursuing an accurate diagnosis of EBS subtype is important for genetic counseling and clinical care, he emphasized.
Therefore, to help confirm the findings of the studies in China and Europe, Dr. McGrath and his associates performed Sanger sequencing, skin pathology, clinical phenotyping, and electron microscopy of samples from undiagnosed cases of EB simplex that were banked at the U.K.’s National Diagnostic EB Laboratory. Among 183 cases (about 20% of all EBS referrals to the laboratory) that had not been linked to other mutations, 7 from six families had heterozygous KLHL24 mutations.
Together, the three studies name five KLHL24 mutations from 26 individuals, 16 families, and eight countries, Dr. McGrath said. “All these mutations target the initiation codon of methionine,” he added. As has previously been reported in EBS, immunostaining for keratin 14 is positive, outlining areas of cleavage within the basal cell layer of the skin. Semi-thin sections reveal pallor in the basal layer of keratinocytes, and electron microscopy of this layer shows blister formation.
“In nonlesional basal keratinocytes, there are few keratin filaments and mitochondria are prominent,” Dr. McGrath explained. “Higher magnification confirms the lack of identifiable keratin filaments, and reveals prominent microtubules.” Lesional skin shows not only the paucity of keratin filaments, but clear evidence of cytolysis, with numerous autophagosomes, autolysosomes, and disruption of organelles in the cellular cytoplasm. “The keratin has been chopped up, digested, and is being organically recycled. That’s essentially what’s going on here,” he said.
These studies show how clinician scientists can help advance the diagnosis and treatment of skin diseases by linking clinical and molecular pathology, Dr. McGrath said. But just as importantly, they might reveal a new approach to treating EBS. “Instead of gene editing or protein therapy, we have a new mechanism that may be ripe for proteasomal inhibitors or other targets of the ubiquitination pathway,” he added. “It’s an intriguing way forward when thinking about drugs and small molecules for EB therapy.”
The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
PORTLAND, ORE. – New research has uncovered “a new kid on the block” of genes underlying epidermolysis bullosa simplex (EBS), which may someday pave the way for new therapies, John McGrath, MD, said at the annual meeting of the Society for Investigative Dermatology.
The gene encodes kelch-like family member 24 (KLHL24), the substrate receptor of cullin 3-RBX1-KLHL24 complex, explained Dr. McGrath, professor of molecular dermatology, St. John’s Institute of Dermatology, King’s College London, where he studies heritable skin diseases. Keratin 14 is the substrate of this complex. In EBS, KLHL24 mutations affect methionine initiation codons, resulting in the loss of the first 28 amino acids of KLHL24 protein. As a result, the protein becomes overly stable, which leads to excessive ubiquitination and breakdown of keratin 14.
KLHL24 is the 19th gene to be implicated in EBS. News of the finding broke in late 2016, when two publications identified KLHL24 mutations in 19 patients with EBS from 10 different families. The first study, conducted in China, identified start-codon mutations in the KLHL24 gene in five patients, confirmed keratin 14 as the substrate of the protein, and established that KLHL24 mutations induce disproportionate ubiquitination and fragmentation of keratin 14. The researchers confirmed these results by using a knock-in mouse model (Nat Genet. 2016 Dec;48 [12]:1508-16).
Meanwhile, European researchers identified KLHL24 mutations in families from Israel, Germany, Switzerland, Finland, Qatar, and Italy, and confirmed that these mutations affect the equilibrium between intermediate keratin filaments and keratin breakdown that is necessary for skin integrity (Am J Hum Genet. 2016 Dec 1;99[6]:1395-1404).
EBS affects more than 400,000 individuals worldwide, and is usually linked to heterozygous missense mutations in genes encoding keratins 5 and 14, Dr. McGrath noted. “However, EBS is clinically and genetically heterogeneous,” he added. “About 20% of cases have no mutation in known genes.” Pursuing an accurate diagnosis of EBS subtype is important for genetic counseling and clinical care, he emphasized.
Therefore, to help confirm the findings of the studies in China and Europe, Dr. McGrath and his associates performed Sanger sequencing, skin pathology, clinical phenotyping, and electron microscopy of samples from undiagnosed cases of EB simplex that were banked at the U.K.’s National Diagnostic EB Laboratory. Among 183 cases (about 20% of all EBS referrals to the laboratory) that had not been linked to other mutations, 7 from six families had heterozygous KLHL24 mutations.
Together, the three studies name five KLHL24 mutations from 26 individuals, 16 families, and eight countries, Dr. McGrath said. “All these mutations target the initiation codon of methionine,” he added. As has previously been reported in EBS, immunostaining for keratin 14 is positive, outlining areas of cleavage within the basal cell layer of the skin. Semi-thin sections reveal pallor in the basal layer of keratinocytes, and electron microscopy of this layer shows blister formation.
“In nonlesional basal keratinocytes, there are few keratin filaments and mitochondria are prominent,” Dr. McGrath explained. “Higher magnification confirms the lack of identifiable keratin filaments, and reveals prominent microtubules.” Lesional skin shows not only the paucity of keratin filaments, but clear evidence of cytolysis, with numerous autophagosomes, autolysosomes, and disruption of organelles in the cellular cytoplasm. “The keratin has been chopped up, digested, and is being organically recycled. That’s essentially what’s going on here,” he said.
These studies show how clinician scientists can help advance the diagnosis and treatment of skin diseases by linking clinical and molecular pathology, Dr. McGrath said. But just as importantly, they might reveal a new approach to treating EBS. “Instead of gene editing or protein therapy, we have a new mechanism that may be ripe for proteasomal inhibitors or other targets of the ubiquitination pathway,” he added. “It’s an intriguing way forward when thinking about drugs and small molecules for EB therapy.”
The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
PORTLAND, ORE. – New research has uncovered “a new kid on the block” of genes underlying epidermolysis bullosa simplex (EBS), which may someday pave the way for new therapies, John McGrath, MD, said at the annual meeting of the Society for Investigative Dermatology.
The gene encodes kelch-like family member 24 (KLHL24), the substrate receptor of cullin 3-RBX1-KLHL24 complex, explained Dr. McGrath, professor of molecular dermatology, St. John’s Institute of Dermatology, King’s College London, where he studies heritable skin diseases. Keratin 14 is the substrate of this complex. In EBS, KLHL24 mutations affect methionine initiation codons, resulting in the loss of the first 28 amino acids of KLHL24 protein. As a result, the protein becomes overly stable, which leads to excessive ubiquitination and breakdown of keratin 14.
KLHL24 is the 19th gene to be implicated in EBS. News of the finding broke in late 2016, when two publications identified KLHL24 mutations in 19 patients with EBS from 10 different families. The first study, conducted in China, identified start-codon mutations in the KLHL24 gene in five patients, confirmed keratin 14 as the substrate of the protein, and established that KLHL24 mutations induce disproportionate ubiquitination and fragmentation of keratin 14. The researchers confirmed these results by using a knock-in mouse model (Nat Genet. 2016 Dec;48 [12]:1508-16).
Meanwhile, European researchers identified KLHL24 mutations in families from Israel, Germany, Switzerland, Finland, Qatar, and Italy, and confirmed that these mutations affect the equilibrium between intermediate keratin filaments and keratin breakdown that is necessary for skin integrity (Am J Hum Genet. 2016 Dec 1;99[6]:1395-1404).
EBS affects more than 400,000 individuals worldwide, and is usually linked to heterozygous missense mutations in genes encoding keratins 5 and 14, Dr. McGrath noted. “However, EBS is clinically and genetically heterogeneous,” he added. “About 20% of cases have no mutation in known genes.” Pursuing an accurate diagnosis of EBS subtype is important for genetic counseling and clinical care, he emphasized.
Therefore, to help confirm the findings of the studies in China and Europe, Dr. McGrath and his associates performed Sanger sequencing, skin pathology, clinical phenotyping, and electron microscopy of samples from undiagnosed cases of EB simplex that were banked at the U.K.’s National Diagnostic EB Laboratory. Among 183 cases (about 20% of all EBS referrals to the laboratory) that had not been linked to other mutations, 7 from six families had heterozygous KLHL24 mutations.
Together, the three studies name five KLHL24 mutations from 26 individuals, 16 families, and eight countries, Dr. McGrath said. “All these mutations target the initiation codon of methionine,” he added. As has previously been reported in EBS, immunostaining for keratin 14 is positive, outlining areas of cleavage within the basal cell layer of the skin. Semi-thin sections reveal pallor in the basal layer of keratinocytes, and electron microscopy of this layer shows blister formation.
“In nonlesional basal keratinocytes, there are few keratin filaments and mitochondria are prominent,” Dr. McGrath explained. “Higher magnification confirms the lack of identifiable keratin filaments, and reveals prominent microtubules.” Lesional skin shows not only the paucity of keratin filaments, but clear evidence of cytolysis, with numerous autophagosomes, autolysosomes, and disruption of organelles in the cellular cytoplasm. “The keratin has been chopped up, digested, and is being organically recycled. That’s essentially what’s going on here,” he said.
These studies show how clinician scientists can help advance the diagnosis and treatment of skin diseases by linking clinical and molecular pathology, Dr. McGrath said. But just as importantly, they might reveal a new approach to treating EBS. “Instead of gene editing or protein therapy, we have a new mechanism that may be ripe for proteasomal inhibitors or other targets of the ubiquitination pathway,” he added. “It’s an intriguing way forward when thinking about drugs and small molecules for EB therapy.”
The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
AT SID 2017
Key clinical point: Studies that have identified genes related to epidermolysis bullosa may lead to new treatment approaches for patients with epidermolysis bullosa simplex (EBS).
Major finding: Of 183 patients with EBS, not linked to other mutations, testing determined that 7 had heterozygous KLHL24 mutations, recently identified in patients with EB.
Data source: Samples from the 183 patients were banked at the United Kingdom’s National Diagnostic EB laboratory.
Disclosures: The National Institute for Health Research and the NHS Foundation Trust funded the study. Dr. McGrath had no relevant financial disclosures.
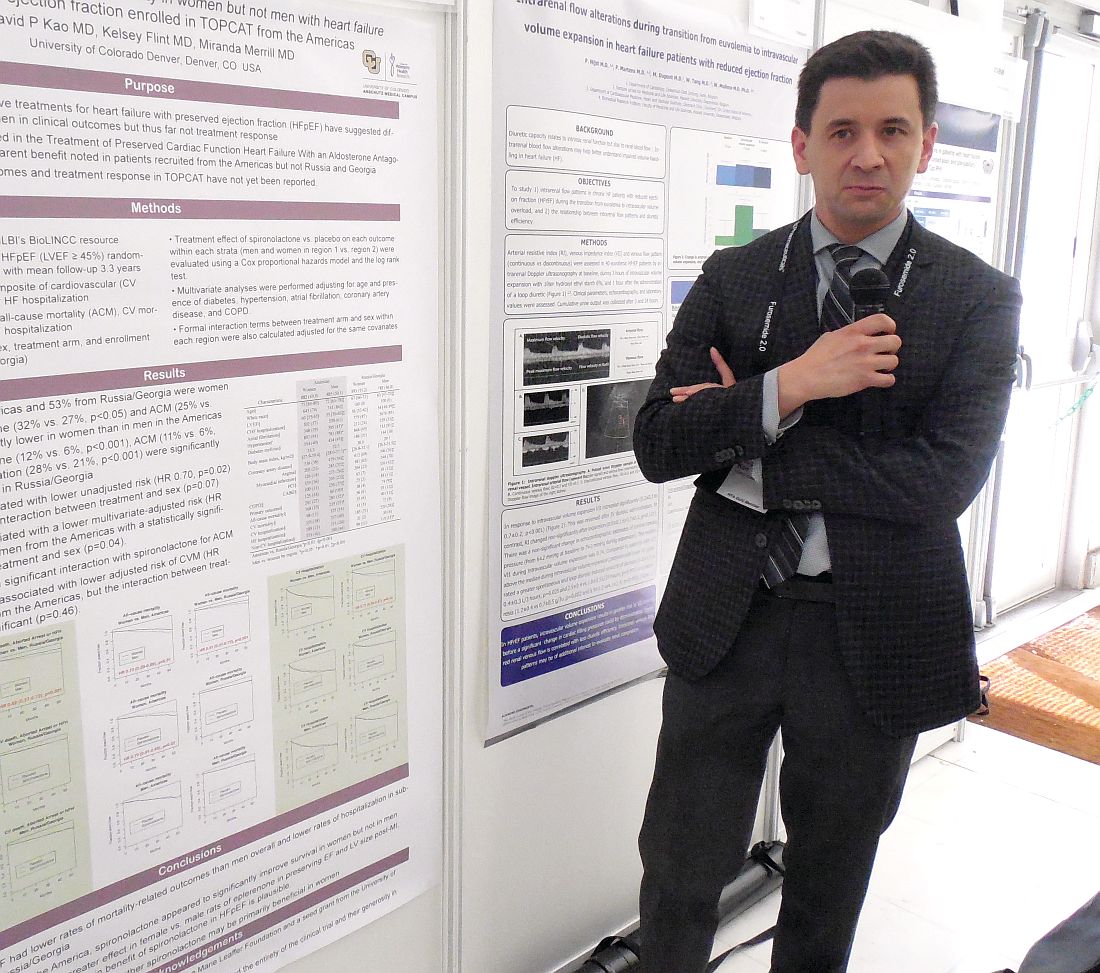
Spironolactone’s HFpEF benefit happens mostly in women
PARIS – Just when the aldosterone receptor antagonists spironolactone and eplerenone received official recognition in the 2017 U.S. heart failure guidelines as the only drug class that benefits patients with heart failure with preserved ejection fraction (HFpEF), a new post-hoc analysis of the pivotal evidence suggests the benefit is mostly in women, with little benefit to men.
The new analysis used data collected from TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), which randomized patients with HFpEF to treatment with spironolactone or placebo. Results from the overall study were neutral for the primary outcome of cardiovascular death, aborted cardiac arrest, and heart failure hospitalization (N Engl J Med. 2014 April 10;370[15]:1383-92). However, a series of post-hoc analyses showed that a high percentage of patients enrolled at centers in Russia or Georgia did not match the expected HFpEF profile, and these patients had poor responses to spironolactone. In contrast, patients enrolled at centers in the Americas more frequently matched the study’s target HFpEF profile, and they showed significant improvement for the primary endpoint (Circulation. 2015 Jan 6;131[1]:34-42).
“The observation that most of the benefit [from spironolactone treatment] may have been in women is interesting, but I don’t think that it would stop me from using [an aldosterone receptor antagonist] in men,” said Dr. Kao while presenting his report. The outcomes in both men and women “head in the same direction. It’s just that the mortality benefit is much clearer in women,” said Dr. Kao, a cardiologist at the University of Colorado at Denver, Aurora.
Among the patients enrolled at centers in North and South America, 882 were women, and 885 were men. Dr. Kao used the data collected in TOPCAT to calculate the impact of spironolactone treatment relative to placebo on outcomes just among women in the Americas and just among men.
The difference in all-cause mortality associated with spironolactone treatment had an even sharper sex disparity that in the primary outcome. Overall, all-cause mortality was 28% less common among women, compared with men, in the Americas. Among women, spironolactone treatment linked with a 30% reduced all-cause mortality rate, compared with placebo. Among men, the survival curves of those on spironolactone or placebo superimposed.
Dr. Kao said that published study results in rats had suggested that eplerenone (Inspra), an aldosterone receptor antagonist like spironolactone, had a more potent effect in females rats, compared with male rats, for preserving left ventricular function and size following myocardial damage. In addition, women with HFpEF often have more left ventricular hypertrophy, while men often have more diastolic dysfunction, and prior findings had suggested that aldosterone plays a role in left ventricular hypertrophy.
TOPCAT received no commercial funding. Dr. Kao had no disclosures.
[email protected]
On Twitter @mitchelzoler
PARIS – Just when the aldosterone receptor antagonists spironolactone and eplerenone received official recognition in the 2017 U.S. heart failure guidelines as the only drug class that benefits patients with heart failure with preserved ejection fraction (HFpEF), a new post-hoc analysis of the pivotal evidence suggests the benefit is mostly in women, with little benefit to men.
The new analysis used data collected from TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), which randomized patients with HFpEF to treatment with spironolactone or placebo. Results from the overall study were neutral for the primary outcome of cardiovascular death, aborted cardiac arrest, and heart failure hospitalization (N Engl J Med. 2014 April 10;370[15]:1383-92). However, a series of post-hoc analyses showed that a high percentage of patients enrolled at centers in Russia or Georgia did not match the expected HFpEF profile, and these patients had poor responses to spironolactone. In contrast, patients enrolled at centers in the Americas more frequently matched the study’s target HFpEF profile, and they showed significant improvement for the primary endpoint (Circulation. 2015 Jan 6;131[1]:34-42).
“The observation that most of the benefit [from spironolactone treatment] may have been in women is interesting, but I don’t think that it would stop me from using [an aldosterone receptor antagonist] in men,” said Dr. Kao while presenting his report. The outcomes in both men and women “head in the same direction. It’s just that the mortality benefit is much clearer in women,” said Dr. Kao, a cardiologist at the University of Colorado at Denver, Aurora.
Among the patients enrolled at centers in North and South America, 882 were women, and 885 were men. Dr. Kao used the data collected in TOPCAT to calculate the impact of spironolactone treatment relative to placebo on outcomes just among women in the Americas and just among men.
The difference in all-cause mortality associated with spironolactone treatment had an even sharper sex disparity that in the primary outcome. Overall, all-cause mortality was 28% less common among women, compared with men, in the Americas. Among women, spironolactone treatment linked with a 30% reduced all-cause mortality rate, compared with placebo. Among men, the survival curves of those on spironolactone or placebo superimposed.
Dr. Kao said that published study results in rats had suggested that eplerenone (Inspra), an aldosterone receptor antagonist like spironolactone, had a more potent effect in females rats, compared with male rats, for preserving left ventricular function and size following myocardial damage. In addition, women with HFpEF often have more left ventricular hypertrophy, while men often have more diastolic dysfunction, and prior findings had suggested that aldosterone plays a role in left ventricular hypertrophy.
TOPCAT received no commercial funding. Dr. Kao had no disclosures.
[email protected]
On Twitter @mitchelzoler
PARIS – Just when the aldosterone receptor antagonists spironolactone and eplerenone received official recognition in the 2017 U.S. heart failure guidelines as the only drug class that benefits patients with heart failure with preserved ejection fraction (HFpEF), a new post-hoc analysis of the pivotal evidence suggests the benefit is mostly in women, with little benefit to men.
The new analysis used data collected from TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), which randomized patients with HFpEF to treatment with spironolactone or placebo. Results from the overall study were neutral for the primary outcome of cardiovascular death, aborted cardiac arrest, and heart failure hospitalization (N Engl J Med. 2014 April 10;370[15]:1383-92). However, a series of post-hoc analyses showed that a high percentage of patients enrolled at centers in Russia or Georgia did not match the expected HFpEF profile, and these patients had poor responses to spironolactone. In contrast, patients enrolled at centers in the Americas more frequently matched the study’s target HFpEF profile, and they showed significant improvement for the primary endpoint (Circulation. 2015 Jan 6;131[1]:34-42).
“The observation that most of the benefit [from spironolactone treatment] may have been in women is interesting, but I don’t think that it would stop me from using [an aldosterone receptor antagonist] in men,” said Dr. Kao while presenting his report. The outcomes in both men and women “head in the same direction. It’s just that the mortality benefit is much clearer in women,” said Dr. Kao, a cardiologist at the University of Colorado at Denver, Aurora.
Among the patients enrolled at centers in North and South America, 882 were women, and 885 were men. Dr. Kao used the data collected in TOPCAT to calculate the impact of spironolactone treatment relative to placebo on outcomes just among women in the Americas and just among men.
The difference in all-cause mortality associated with spironolactone treatment had an even sharper sex disparity that in the primary outcome. Overall, all-cause mortality was 28% less common among women, compared with men, in the Americas. Among women, spironolactone treatment linked with a 30% reduced all-cause mortality rate, compared with placebo. Among men, the survival curves of those on spironolactone or placebo superimposed.
Dr. Kao said that published study results in rats had suggested that eplerenone (Inspra), an aldosterone receptor antagonist like spironolactone, had a more potent effect in females rats, compared with male rats, for preserving left ventricular function and size following myocardial damage. In addition, women with HFpEF often have more left ventricular hypertrophy, while men often have more diastolic dysfunction, and prior findings had suggested that aldosterone plays a role in left ventricular hypertrophy.
TOPCAT received no commercial funding. Dr. Kao had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: Women from the Americas in TOPCAT had an 18% lower rate of primary endpoint events, compared with men in the trial.
Data source: TOPCAT, a multicenter, randomized study with 3,445 patients.
Disclosures: TOPCAT received no commercial funding. Dr. Kao had no disclosures.
Complicated grief treatment gets better results than interpersonal psychotherapy
SAN FRANCISCO – The effectiveness of complicated grief treatment (CGT) rests, to a significant extent, on its capacity to reduce the grieving patient’s level of avoidance of reminders of the loss, Kim Glickman, PhD, said at the annual conference of the Anxiety and Depression Association of America.
Her psychotherapeutic mechanism-of-action study identified two other mediators of improvement in response to CGT: guilt related to the death and negative thoughts about the future. Patients who experienced significant reductions in levels of those variables during CGT were much more likely to ultimately be treatment responders.
The clinical implication of these findings is that psychotherapists should focus on reducing grief complications, such as avoidance behaviors and maladaptive thoughts, including blaming oneself or others for how the person died and seeing a hopeless future, according to Dr. Glickman, of City University of New York.
Complicated grief affects about 7% of bereaved individuals. It is characterized by prolonged emotional pain, intense sorrow, preoccupation with thoughts of the loved one, and persistent yearning. It is typically resistant to antidepressant therapy. In the DSM-5, it is called “persistent complex bereavement disorder” and is described in a chapter on provisional conditions for further study. Since it doesn’t have the status of a formal diagnostic entity, insurers typically will not pay for treatment of complicated grief reactions.
CGT has been shown to be effective in three randomized clinical trials. It is a manualized 16-session therapy that can be considered a form of cognitive-behavioral therapy with added elements of interpersonal psychotherapy and motivational interviewing. The focus is on encouraging adaptation to the loss by keeping grief center stage, honoring the person who died, and envisioning a future with possibilities for happiness, Dr. Glickman explained.
The mechanisms of action of CGT haven’t been well-characterized. This was the impetus for Dr. Glickman’s study, in which she analyzed data from the first randomized trial to demonstrate CGT’s effectiveness more than a decade ago (JAMA. 2005 Jun 1;293[21]:2601-8).
Among the 69 patients with complicated grief who completed 16 sessions of psychotherapy, the clinical response rate was 51% in the CGT group, compared with 28% in patients randomized to interpersonal psychotherapy. The number needed to treat with CGT was 4.3 in order to achieve a clinical response, defined as either a Clinical Global Impression–Improvement score of 1 or 2 or at least a 20-point improvement pre to post treatment on the self-rated Inventory of Complicated Grief.
In order to more closely examine potential mediators of clinical response, Dr. Glickman chose as her measure of change in feelings of guilt the study participants’ scores on the Structured Clinical Interview for Complicated Grief. To assess negative thoughts about the future, she relied on item two from the Beck Depression Inventory and, for avoidance behaviors, she used scores on the 15-item Grief-Related Avoidance Questionnaire.
CGT proved significantly more effective than interpersonal therapy at improving scores on all three of these instruments. The mediating effect was most robust for improvement in avoidance behaviors.
Dr. Glickman’s future research plans include looking at additional possible mediators of CGT’s efficacy, including change in emotion regulation, ideally assessed on a weekly basis during the course of treatment.
Complicated grief therapy was pioneered by therapists at Columbia University in New York. Dr. Glickman noted that more information about complicated grief and training in CGT is available at www.complicatedgrief.columbia.edu.
The randomized trial on which her analysis was based was funded by the National Institute of Mental Health. Dr. Glickman reported having no financial conflicts regarding her study.
SAN FRANCISCO – The effectiveness of complicated grief treatment (CGT) rests, to a significant extent, on its capacity to reduce the grieving patient’s level of avoidance of reminders of the loss, Kim Glickman, PhD, said at the annual conference of the Anxiety and Depression Association of America.
Her psychotherapeutic mechanism-of-action study identified two other mediators of improvement in response to CGT: guilt related to the death and negative thoughts about the future. Patients who experienced significant reductions in levels of those variables during CGT were much more likely to ultimately be treatment responders.
The clinical implication of these findings is that psychotherapists should focus on reducing grief complications, such as avoidance behaviors and maladaptive thoughts, including blaming oneself or others for how the person died and seeing a hopeless future, according to Dr. Glickman, of City University of New York.
Complicated grief affects about 7% of bereaved individuals. It is characterized by prolonged emotional pain, intense sorrow, preoccupation with thoughts of the loved one, and persistent yearning. It is typically resistant to antidepressant therapy. In the DSM-5, it is called “persistent complex bereavement disorder” and is described in a chapter on provisional conditions for further study. Since it doesn’t have the status of a formal diagnostic entity, insurers typically will not pay for treatment of complicated grief reactions.
CGT has been shown to be effective in three randomized clinical trials. It is a manualized 16-session therapy that can be considered a form of cognitive-behavioral therapy with added elements of interpersonal psychotherapy and motivational interviewing. The focus is on encouraging adaptation to the loss by keeping grief center stage, honoring the person who died, and envisioning a future with possibilities for happiness, Dr. Glickman explained.
The mechanisms of action of CGT haven’t been well-characterized. This was the impetus for Dr. Glickman’s study, in which she analyzed data from the first randomized trial to demonstrate CGT’s effectiveness more than a decade ago (JAMA. 2005 Jun 1;293[21]:2601-8).
Among the 69 patients with complicated grief who completed 16 sessions of psychotherapy, the clinical response rate was 51% in the CGT group, compared with 28% in patients randomized to interpersonal psychotherapy. The number needed to treat with CGT was 4.3 in order to achieve a clinical response, defined as either a Clinical Global Impression–Improvement score of 1 or 2 or at least a 20-point improvement pre to post treatment on the self-rated Inventory of Complicated Grief.
In order to more closely examine potential mediators of clinical response, Dr. Glickman chose as her measure of change in feelings of guilt the study participants’ scores on the Structured Clinical Interview for Complicated Grief. To assess negative thoughts about the future, she relied on item two from the Beck Depression Inventory and, for avoidance behaviors, she used scores on the 15-item Grief-Related Avoidance Questionnaire.
CGT proved significantly more effective than interpersonal therapy at improving scores on all three of these instruments. The mediating effect was most robust for improvement in avoidance behaviors.
Dr. Glickman’s future research plans include looking at additional possible mediators of CGT’s efficacy, including change in emotion regulation, ideally assessed on a weekly basis during the course of treatment.
Complicated grief therapy was pioneered by therapists at Columbia University in New York. Dr. Glickman noted that more information about complicated grief and training in CGT is available at www.complicatedgrief.columbia.edu.
The randomized trial on which her analysis was based was funded by the National Institute of Mental Health. Dr. Glickman reported having no financial conflicts regarding her study.
SAN FRANCISCO – The effectiveness of complicated grief treatment (CGT) rests, to a significant extent, on its capacity to reduce the grieving patient’s level of avoidance of reminders of the loss, Kim Glickman, PhD, said at the annual conference of the Anxiety and Depression Association of America.
Her psychotherapeutic mechanism-of-action study identified two other mediators of improvement in response to CGT: guilt related to the death and negative thoughts about the future. Patients who experienced significant reductions in levels of those variables during CGT were much more likely to ultimately be treatment responders.
The clinical implication of these findings is that psychotherapists should focus on reducing grief complications, such as avoidance behaviors and maladaptive thoughts, including blaming oneself or others for how the person died and seeing a hopeless future, according to Dr. Glickman, of City University of New York.
Complicated grief affects about 7% of bereaved individuals. It is characterized by prolonged emotional pain, intense sorrow, preoccupation with thoughts of the loved one, and persistent yearning. It is typically resistant to antidepressant therapy. In the DSM-5, it is called “persistent complex bereavement disorder” and is described in a chapter on provisional conditions for further study. Since it doesn’t have the status of a formal diagnostic entity, insurers typically will not pay for treatment of complicated grief reactions.
CGT has been shown to be effective in three randomized clinical trials. It is a manualized 16-session therapy that can be considered a form of cognitive-behavioral therapy with added elements of interpersonal psychotherapy and motivational interviewing. The focus is on encouraging adaptation to the loss by keeping grief center stage, honoring the person who died, and envisioning a future with possibilities for happiness, Dr. Glickman explained.
The mechanisms of action of CGT haven’t been well-characterized. This was the impetus for Dr. Glickman’s study, in which she analyzed data from the first randomized trial to demonstrate CGT’s effectiveness more than a decade ago (JAMA. 2005 Jun 1;293[21]:2601-8).
Among the 69 patients with complicated grief who completed 16 sessions of psychotherapy, the clinical response rate was 51% in the CGT group, compared with 28% in patients randomized to interpersonal psychotherapy. The number needed to treat with CGT was 4.3 in order to achieve a clinical response, defined as either a Clinical Global Impression–Improvement score of 1 or 2 or at least a 20-point improvement pre to post treatment on the self-rated Inventory of Complicated Grief.
In order to more closely examine potential mediators of clinical response, Dr. Glickman chose as her measure of change in feelings of guilt the study participants’ scores on the Structured Clinical Interview for Complicated Grief. To assess negative thoughts about the future, she relied on item two from the Beck Depression Inventory and, for avoidance behaviors, she used scores on the 15-item Grief-Related Avoidance Questionnaire.
CGT proved significantly more effective than interpersonal therapy at improving scores on all three of these instruments. The mediating effect was most robust for improvement in avoidance behaviors.
Dr. Glickman’s future research plans include looking at additional possible mediators of CGT’s efficacy, including change in emotion regulation, ideally assessed on a weekly basis during the course of treatment.
Complicated grief therapy was pioneered by therapists at Columbia University in New York. Dr. Glickman noted that more information about complicated grief and training in CGT is available at www.complicatedgrief.columbia.edu.
The randomized trial on which her analysis was based was funded by the National Institute of Mental Health. Dr. Glickman reported having no financial conflicts regarding her study.
AT THE ANXIETY AND DEPRESSION CONFERENCE 2017
Key clinical point:
Major finding: A key mediator of clinical improvement in response to cognitive grief treatment is a reduction in avoidance behaviors.
Data source: A secondary analysis of a randomized trial of complicated grief treatment versus interpersonal psychotherapy in 69 patients with complicated grief.
Disclosures: The study presenter reported having no financial conflicts.
First trimester use of inactivated flu vaccine didn’t cause birth defects
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
FROM THE JOURNAL OF PEDIATRICS
Cervical cancer screening adherence drops after HPV vaccination
SAN DIEGO – Young adult women who received the human papillomavirus (HPV) vaccination were less likely to adhere to the recommended screening schedule for cervical cancer, according to a recent study.
In looking at a cohort of women who received at least two Pap smears during a 4-year study period, Daniel Terk, MD, and his colleagues saw a significant drop-off in screening visits after women received their HPV vaccinations. The group of women who received their vaccine midstudy were significantly less likely to come in for annual screening after their vaccination than before (n = 140, adjusted odds ratio 0.19, 95% confidence interval, 0.08-0.49).
The retrospective chart review looked at rates of cervical cancer screening and HPV vaccination across 943 patient charts, beginning in 2006 when the HPV vaccine was released and ending in 2010. The inclusion criteria ensured that “participants were old enough to obtain cervical cancer screening and young enough to receive the HPV vaccine,” Dr. Terk and his collaborators wrote in the research abstract.
The drop-off in adherence for the subgroup who received their vaccines midstudy was an isolated finding, said Dr. Terk, noting that vaccination status did not affect adherence to the recommended screening schedule for the group as a whole.
Billing data and medical records were used to track HPV vaccination status and dates of administration, as well as the dates of testing and results for cervical cancer screening. Patients were included if they were born from 1980 to 1988, if they had two or more Pap smears during the study period, and if their HPV vaccination status could be verified. Patients with an initial abnormal Pap smear or a prior loop electrosurgical excision procedure were excluded, as were patients whose HPV vaccination status could not be confirmed.
In all, 943 patient charts were included in the review; 448 patients had no documented vaccination, with further review showing that 418 of these had, in fact, not been vaccinated, while the remaining 30 had actually had at least one HPV vaccine dose during the study period. Of the 495 patients with at least one documented vaccination, 175 had at least one Pap smear done before they were vaccinated, while 320 had their HPV vaccine before any documented cervical cancer screening.
Thus, there were two patient groups: 593 “unvaccinated” patients, who had at least one cervical cancer screening before receiving the vaccine, and 350 “vaccinated” patients, who had their HPV vaccine before any documented Pap smears.
Dr. Terk and his colleagues considered a screening interval of 18 months or less appropriate, while a longer interval than that between Pap smears was considered inappropriate timing. Individual patients were considered adherent if 76%-100% of their Pap smears were appropriately timed, partially adherent if 50%-75% of their Pap smears were appropriately timed, and not adherent if less than 50% of their Pap smears were appropriately timed.
Of those receiving their vaccine midstudy, 116 (82.9%) were adherent before vaccination, while 69 (49.3%) were adherent after vaccination.
The HPV vaccine is highly effective at preventing infection with the strains of HPV that are primarily responsible for cervical cancer. At the same time, about 70% of the cervical cancer cases that occur are associated with missed screening or inappropriate screening intervals, so screening is still an important part of the strategy to prevent cervical cancers, said Dr. Terk, a 4th-year ob.gyn. resident at the University of Rochester, N.Y.
The ACOG guidelines for cervical cancer screening have shifted through the years. During the period from 2003 to 2009, annual Pap smears were recommended for women under the age of 30 years, beginning at age 21 or 3 years after first sexual intercourse. Women 30 years and older who were at low risk could have a less frequent screening interval. ACOG currently recommends Pap testing alone every 3 years for women aged 21-29 years; co-testing with a Pap test and an HPV test every 5 years in women aged 30-65 years, or a Pap test alone every 3 years.
The study had a large sample size, and adherence rates track with national data, Dr. Terk said. However, screening guidelines have shifted in recent years, and vaccinations are now happening at a much younger age, so the findings should be interpreted with some caution. Still, he said, clinicians should incorporate a strong message about the importance of cervical cancer screening in their HPV vaccination and well-woman counseling.
Dr. Terk reported having no relevant financial disclosures and reported no outside sources of funding.
[email protected]
On Twitter @karioakes
SAN DIEGO – Young adult women who received the human papillomavirus (HPV) vaccination were less likely to adhere to the recommended screening schedule for cervical cancer, according to a recent study.
In looking at a cohort of women who received at least two Pap smears during a 4-year study period, Daniel Terk, MD, and his colleagues saw a significant drop-off in screening visits after women received their HPV vaccinations. The group of women who received their vaccine midstudy were significantly less likely to come in for annual screening after their vaccination than before (n = 140, adjusted odds ratio 0.19, 95% confidence interval, 0.08-0.49).
The retrospective chart review looked at rates of cervical cancer screening and HPV vaccination across 943 patient charts, beginning in 2006 when the HPV vaccine was released and ending in 2010. The inclusion criteria ensured that “participants were old enough to obtain cervical cancer screening and young enough to receive the HPV vaccine,” Dr. Terk and his collaborators wrote in the research abstract.
The drop-off in adherence for the subgroup who received their vaccines midstudy was an isolated finding, said Dr. Terk, noting that vaccination status did not affect adherence to the recommended screening schedule for the group as a whole.
Billing data and medical records were used to track HPV vaccination status and dates of administration, as well as the dates of testing and results for cervical cancer screening. Patients were included if they were born from 1980 to 1988, if they had two or more Pap smears during the study period, and if their HPV vaccination status could be verified. Patients with an initial abnormal Pap smear or a prior loop electrosurgical excision procedure were excluded, as were patients whose HPV vaccination status could not be confirmed.
In all, 943 patient charts were included in the review; 448 patients had no documented vaccination, with further review showing that 418 of these had, in fact, not been vaccinated, while the remaining 30 had actually had at least one HPV vaccine dose during the study period. Of the 495 patients with at least one documented vaccination, 175 had at least one Pap smear done before they were vaccinated, while 320 had their HPV vaccine before any documented cervical cancer screening.
Thus, there were two patient groups: 593 “unvaccinated” patients, who had at least one cervical cancer screening before receiving the vaccine, and 350 “vaccinated” patients, who had their HPV vaccine before any documented Pap smears.
Dr. Terk and his colleagues considered a screening interval of 18 months or less appropriate, while a longer interval than that between Pap smears was considered inappropriate timing. Individual patients were considered adherent if 76%-100% of their Pap smears were appropriately timed, partially adherent if 50%-75% of their Pap smears were appropriately timed, and not adherent if less than 50% of their Pap smears were appropriately timed.
Of those receiving their vaccine midstudy, 116 (82.9%) were adherent before vaccination, while 69 (49.3%) were adherent after vaccination.
The HPV vaccine is highly effective at preventing infection with the strains of HPV that are primarily responsible for cervical cancer. At the same time, about 70% of the cervical cancer cases that occur are associated with missed screening or inappropriate screening intervals, so screening is still an important part of the strategy to prevent cervical cancers, said Dr. Terk, a 4th-year ob.gyn. resident at the University of Rochester, N.Y.
The ACOG guidelines for cervical cancer screening have shifted through the years. During the period from 2003 to 2009, annual Pap smears were recommended for women under the age of 30 years, beginning at age 21 or 3 years after first sexual intercourse. Women 30 years and older who were at low risk could have a less frequent screening interval. ACOG currently recommends Pap testing alone every 3 years for women aged 21-29 years; co-testing with a Pap test and an HPV test every 5 years in women aged 30-65 years, or a Pap test alone every 3 years.
The study had a large sample size, and adherence rates track with national data, Dr. Terk said. However, screening guidelines have shifted in recent years, and vaccinations are now happening at a much younger age, so the findings should be interpreted with some caution. Still, he said, clinicians should incorporate a strong message about the importance of cervical cancer screening in their HPV vaccination and well-woman counseling.
Dr. Terk reported having no relevant financial disclosures and reported no outside sources of funding.
[email protected]
On Twitter @karioakes
SAN DIEGO – Young adult women who received the human papillomavirus (HPV) vaccination were less likely to adhere to the recommended screening schedule for cervical cancer, according to a recent study.
In looking at a cohort of women who received at least two Pap smears during a 4-year study period, Daniel Terk, MD, and his colleagues saw a significant drop-off in screening visits after women received their HPV vaccinations. The group of women who received their vaccine midstudy were significantly less likely to come in for annual screening after their vaccination than before (n = 140, adjusted odds ratio 0.19, 95% confidence interval, 0.08-0.49).
The retrospective chart review looked at rates of cervical cancer screening and HPV vaccination across 943 patient charts, beginning in 2006 when the HPV vaccine was released and ending in 2010. The inclusion criteria ensured that “participants were old enough to obtain cervical cancer screening and young enough to receive the HPV vaccine,” Dr. Terk and his collaborators wrote in the research abstract.
The drop-off in adherence for the subgroup who received their vaccines midstudy was an isolated finding, said Dr. Terk, noting that vaccination status did not affect adherence to the recommended screening schedule for the group as a whole.
Billing data and medical records were used to track HPV vaccination status and dates of administration, as well as the dates of testing and results for cervical cancer screening. Patients were included if they were born from 1980 to 1988, if they had two or more Pap smears during the study period, and if their HPV vaccination status could be verified. Patients with an initial abnormal Pap smear or a prior loop electrosurgical excision procedure were excluded, as were patients whose HPV vaccination status could not be confirmed.
In all, 943 patient charts were included in the review; 448 patients had no documented vaccination, with further review showing that 418 of these had, in fact, not been vaccinated, while the remaining 30 had actually had at least one HPV vaccine dose during the study period. Of the 495 patients with at least one documented vaccination, 175 had at least one Pap smear done before they were vaccinated, while 320 had their HPV vaccine before any documented cervical cancer screening.
Thus, there were two patient groups: 593 “unvaccinated” patients, who had at least one cervical cancer screening before receiving the vaccine, and 350 “vaccinated” patients, who had their HPV vaccine before any documented Pap smears.
Dr. Terk and his colleagues considered a screening interval of 18 months or less appropriate, while a longer interval than that between Pap smears was considered inappropriate timing. Individual patients were considered adherent if 76%-100% of their Pap smears were appropriately timed, partially adherent if 50%-75% of their Pap smears were appropriately timed, and not adherent if less than 50% of their Pap smears were appropriately timed.
Of those receiving their vaccine midstudy, 116 (82.9%) were adherent before vaccination, while 69 (49.3%) were adherent after vaccination.
The HPV vaccine is highly effective at preventing infection with the strains of HPV that are primarily responsible for cervical cancer. At the same time, about 70% of the cervical cancer cases that occur are associated with missed screening or inappropriate screening intervals, so screening is still an important part of the strategy to prevent cervical cancers, said Dr. Terk, a 4th-year ob.gyn. resident at the University of Rochester, N.Y.
The ACOG guidelines for cervical cancer screening have shifted through the years. During the period from 2003 to 2009, annual Pap smears were recommended for women under the age of 30 years, beginning at age 21 or 3 years after first sexual intercourse. Women 30 years and older who were at low risk could have a less frequent screening interval. ACOG currently recommends Pap testing alone every 3 years for women aged 21-29 years; co-testing with a Pap test and an HPV test every 5 years in women aged 30-65 years, or a Pap test alone every 3 years.
The study had a large sample size, and adherence rates track with national data, Dr. Terk said. However, screening guidelines have shifted in recent years, and vaccinations are now happening at a much younger age, so the findings should be interpreted with some caution. Still, he said, clinicians should incorporate a strong message about the importance of cervical cancer screening in their HPV vaccination and well-woman counseling.
Dr. Terk reported having no relevant financial disclosures and reported no outside sources of funding.
[email protected]
On Twitter @karioakes
AT ACOG 2017
Key clinical point:
Major finding: The odds ratio for adherence after immunization compared to before was 0.19 (95% confidence interval, 0.08-0.49).
Data source: Retrospective chart review of 495 women who received at least two Pap smears during the period from 2006 to 2010.
Disclosures: The study authors reported no outside sources of funding and no conflicts of interest.