User login
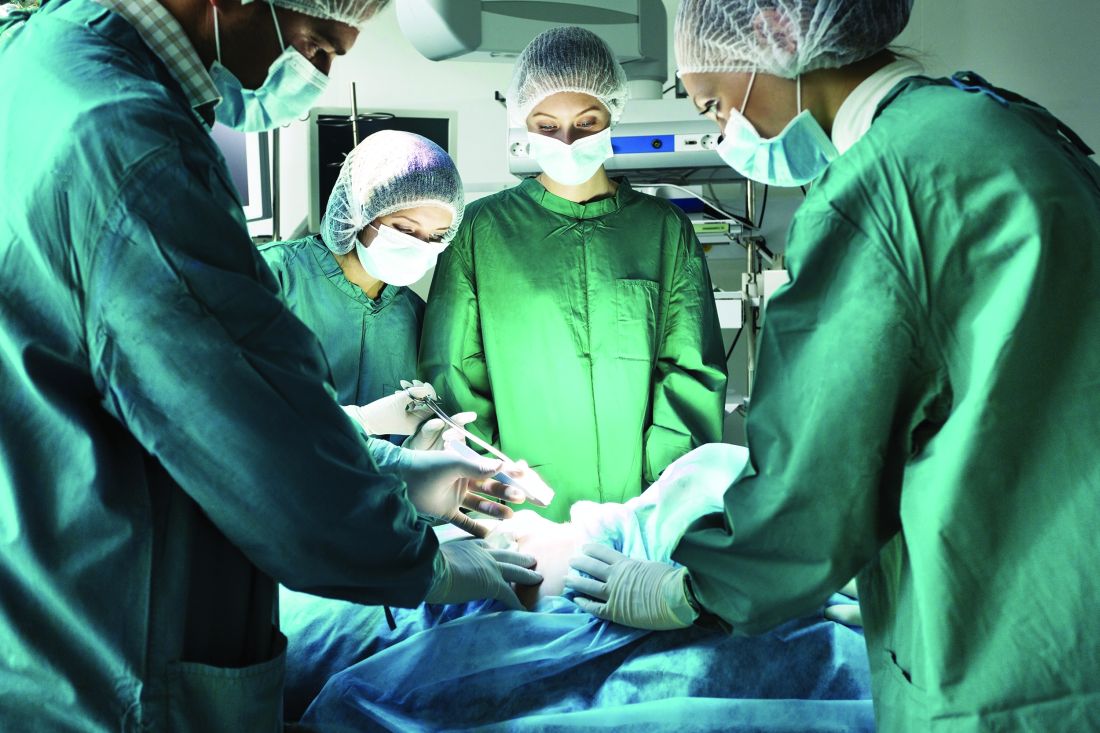
Clown therapy is community psychiatry in disguise
Ever had a patient who not only got better but used the insights she gained from talking with you to help others in distress? I have just such a patient in the Peruvian Amazon.
I’ve previously written about an annual clown trip to Peru that I make with my friend Patch Adams, MD, and 100 other humanitarian clowns from all over the world. We have been going there for a decade to spread cheer, and revitalize the impoverished community of Belén, which is situated in the Amazon floodplain in the city of Iquitos. We conduct workshops, perform street theater, create community art installations, visit hospitals, and work with local grass-roots organizations. For the last 5 years, we also have been conducting mental health clinics in the streets.
To provide a brief overview ... we go to a neighborhood, set up our space, and walk the streets with a bull horn. We announce our presence – “we are mental health professionals, and we’re meeting over at” ... and we talk with anybody, young and old, who wants to discuss health problems, family issues, or other concerns.
We sit in a public place and speak to individuals/couples/families for 20 minutes, while around us, support clowns entertain the kids. We neither make diagnoses nor give drugs; we come with a clown nose and an open heart, and we listen actively without judgment and focus on solutions. We help people identify their strengths and resilience, and give them practical advice. This is community psychiatry disguised as “clown therapy,” which is just another phrase for solution-oriented therapy/ positive psychology/reality therapy/resilience-based therapy, logotherapy, existential psychotherapy, or kitchen table wisdom. These street clinics have had a profound impact on patients and clinicians.
Three years ago, I met a middle-aged woman who was suicidally depressed, and together we negotiated a successful intervention. In summary, she emerged from a church that happened to be across the street from where we were setting up our clinic. A clown saw her weeping and approached her, and after talking with her assured her that there was somebody here right now – a mental health professional – who would talk with her.
Maria sat down and told an unbelievably painful story that was happening within her family. On that day, after 8 months of prayer and receiving no sign from God, she had decided to kill herself. After listening to her, I actually believed she could do it.
There are no treatment centers or emergency shelters for the poor in Belén, so at our closure, I made her promise that she would not try to kill herself until I could see her again at our next clinic 2 days away, and close by. I gave her an amulet that was blessed and told her it was a reminder of her promise, and that my smiling face would be with her until she saw me again. She returned with her daughters to the next clinic, and together, they found a way to take a step forward.
Last year, I made my first home visit, and met with Maria, her daughters, and new grandson in their “new” home where they were happily sustaining themselves . When I left, the love and appreciation was so overwhelming that I told them as long as I returned I would come visit every year.
I just got back from this year’s annual visit, and was again greeted with passionate tears of joy. We sat and talked, and Maria told me her story. It seems that people in the community were now coming to her as a resource when they were deeply depressed. People know that she had walked a similar path and moved beyond it.
She is a warm, good listener, and tells them a story about walking out of church and deciding she wanted to kill herself, and meeting with a tall gringo, a clown/doctor who miraculously saved her life. She gives them simple, practical advice, tells them how important it is to stay connected to their children, speak your truth with them openly; to pray for miracles and recognize them when they occur. She tells them to reach out for help, and people will reach out to them. She is a credible, inspiring friend who gives hope.
For those who remember when community psychiatry was actually a subspecialty, this is my vision of community mental health: People talking to credible witnesses/healers/resources in their community, whom they respect, who will listen without judgment, and maybe even say something that inspires a light in the darkness. It’s at least as effective as psychotropic drugs, and all its benefits come without side effects.
Once a year we come together, listen to each other’s stories, and continue our healing work together. Maria tells me her friends want to meet me. “They want to steal you away,” she says, “but I tell them I am not afraid.”
Maria, a lay therapist of sorts, is the community mental health consultant. Once a year, she consults with her gringo, the clown/doctor, to compare notes. We laugh and love, hug and cry, and give each other hope. No matter how divisive and polarizing the times, it is possible to come together in community and promote healing.
Dr. Hammerschlag is chief of community mental health of the Gesundheit! Institute and a faculty member at the University of Arizona, Phoenix. He is the author of several books on healing and spirituality, including “Kindling Spirit: Healing from Within” (New York: Turtle Island Press, 2010) and “The Dancing Healers: A Doctor’s Journey of Healing With Native Americans” (San Francisco: Harper, 1988). Dr. Hammerschlag’s website is healingdoc.com.
Ever had a patient who not only got better but used the insights she gained from talking with you to help others in distress? I have just such a patient in the Peruvian Amazon.
I’ve previously written about an annual clown trip to Peru that I make with my friend Patch Adams, MD, and 100 other humanitarian clowns from all over the world. We have been going there for a decade to spread cheer, and revitalize the impoverished community of Belén, which is situated in the Amazon floodplain in the city of Iquitos. We conduct workshops, perform street theater, create community art installations, visit hospitals, and work with local grass-roots organizations. For the last 5 years, we also have been conducting mental health clinics in the streets.
To provide a brief overview ... we go to a neighborhood, set up our space, and walk the streets with a bull horn. We announce our presence – “we are mental health professionals, and we’re meeting over at” ... and we talk with anybody, young and old, who wants to discuss health problems, family issues, or other concerns.
We sit in a public place and speak to individuals/couples/families for 20 minutes, while around us, support clowns entertain the kids. We neither make diagnoses nor give drugs; we come with a clown nose and an open heart, and we listen actively without judgment and focus on solutions. We help people identify their strengths and resilience, and give them practical advice. This is community psychiatry disguised as “clown therapy,” which is just another phrase for solution-oriented therapy/ positive psychology/reality therapy/resilience-based therapy, logotherapy, existential psychotherapy, or kitchen table wisdom. These street clinics have had a profound impact on patients and clinicians.
Three years ago, I met a middle-aged woman who was suicidally depressed, and together we negotiated a successful intervention. In summary, she emerged from a church that happened to be across the street from where we were setting up our clinic. A clown saw her weeping and approached her, and after talking with her assured her that there was somebody here right now – a mental health professional – who would talk with her.
Maria sat down and told an unbelievably painful story that was happening within her family. On that day, after 8 months of prayer and receiving no sign from God, she had decided to kill herself. After listening to her, I actually believed she could do it.
There are no treatment centers or emergency shelters for the poor in Belén, so at our closure, I made her promise that she would not try to kill herself until I could see her again at our next clinic 2 days away, and close by. I gave her an amulet that was blessed and told her it was a reminder of her promise, and that my smiling face would be with her until she saw me again. She returned with her daughters to the next clinic, and together, they found a way to take a step forward.
Last year, I made my first home visit, and met with Maria, her daughters, and new grandson in their “new” home where they were happily sustaining themselves . When I left, the love and appreciation was so overwhelming that I told them as long as I returned I would come visit every year.
I just got back from this year’s annual visit, and was again greeted with passionate tears of joy. We sat and talked, and Maria told me her story. It seems that people in the community were now coming to her as a resource when they were deeply depressed. People know that she had walked a similar path and moved beyond it.
She is a warm, good listener, and tells them a story about walking out of church and deciding she wanted to kill herself, and meeting with a tall gringo, a clown/doctor who miraculously saved her life. She gives them simple, practical advice, tells them how important it is to stay connected to their children, speak your truth with them openly; to pray for miracles and recognize them when they occur. She tells them to reach out for help, and people will reach out to them. She is a credible, inspiring friend who gives hope.
For those who remember when community psychiatry was actually a subspecialty, this is my vision of community mental health: People talking to credible witnesses/healers/resources in their community, whom they respect, who will listen without judgment, and maybe even say something that inspires a light in the darkness. It’s at least as effective as psychotropic drugs, and all its benefits come without side effects.
Once a year we come together, listen to each other’s stories, and continue our healing work together. Maria tells me her friends want to meet me. “They want to steal you away,” she says, “but I tell them I am not afraid.”
Maria, a lay therapist of sorts, is the community mental health consultant. Once a year, she consults with her gringo, the clown/doctor, to compare notes. We laugh and love, hug and cry, and give each other hope. No matter how divisive and polarizing the times, it is possible to come together in community and promote healing.
Dr. Hammerschlag is chief of community mental health of the Gesundheit! Institute and a faculty member at the University of Arizona, Phoenix. He is the author of several books on healing and spirituality, including “Kindling Spirit: Healing from Within” (New York: Turtle Island Press, 2010) and “The Dancing Healers: A Doctor’s Journey of Healing With Native Americans” (San Francisco: Harper, 1988). Dr. Hammerschlag’s website is healingdoc.com.
Ever had a patient who not only got better but used the insights she gained from talking with you to help others in distress? I have just such a patient in the Peruvian Amazon.
I’ve previously written about an annual clown trip to Peru that I make with my friend Patch Adams, MD, and 100 other humanitarian clowns from all over the world. We have been going there for a decade to spread cheer, and revitalize the impoverished community of Belén, which is situated in the Amazon floodplain in the city of Iquitos. We conduct workshops, perform street theater, create community art installations, visit hospitals, and work with local grass-roots organizations. For the last 5 years, we also have been conducting mental health clinics in the streets.
To provide a brief overview ... we go to a neighborhood, set up our space, and walk the streets with a bull horn. We announce our presence – “we are mental health professionals, and we’re meeting over at” ... and we talk with anybody, young and old, who wants to discuss health problems, family issues, or other concerns.
We sit in a public place and speak to individuals/couples/families for 20 minutes, while around us, support clowns entertain the kids. We neither make diagnoses nor give drugs; we come with a clown nose and an open heart, and we listen actively without judgment and focus on solutions. We help people identify their strengths and resilience, and give them practical advice. This is community psychiatry disguised as “clown therapy,” which is just another phrase for solution-oriented therapy/ positive psychology/reality therapy/resilience-based therapy, logotherapy, existential psychotherapy, or kitchen table wisdom. These street clinics have had a profound impact on patients and clinicians.
Three years ago, I met a middle-aged woman who was suicidally depressed, and together we negotiated a successful intervention. In summary, she emerged from a church that happened to be across the street from where we were setting up our clinic. A clown saw her weeping and approached her, and after talking with her assured her that there was somebody here right now – a mental health professional – who would talk with her.
Maria sat down and told an unbelievably painful story that was happening within her family. On that day, after 8 months of prayer and receiving no sign from God, she had decided to kill herself. After listening to her, I actually believed she could do it.
There are no treatment centers or emergency shelters for the poor in Belén, so at our closure, I made her promise that she would not try to kill herself until I could see her again at our next clinic 2 days away, and close by. I gave her an amulet that was blessed and told her it was a reminder of her promise, and that my smiling face would be with her until she saw me again. She returned with her daughters to the next clinic, and together, they found a way to take a step forward.
Last year, I made my first home visit, and met with Maria, her daughters, and new grandson in their “new” home where they were happily sustaining themselves . When I left, the love and appreciation was so overwhelming that I told them as long as I returned I would come visit every year.
I just got back from this year’s annual visit, and was again greeted with passionate tears of joy. We sat and talked, and Maria told me her story. It seems that people in the community were now coming to her as a resource when they were deeply depressed. People know that she had walked a similar path and moved beyond it.
She is a warm, good listener, and tells them a story about walking out of church and deciding she wanted to kill herself, and meeting with a tall gringo, a clown/doctor who miraculously saved her life. She gives them simple, practical advice, tells them how important it is to stay connected to their children, speak your truth with them openly; to pray for miracles and recognize them when they occur. She tells them to reach out for help, and people will reach out to them. She is a credible, inspiring friend who gives hope.
For those who remember when community psychiatry was actually a subspecialty, this is my vision of community mental health: People talking to credible witnesses/healers/resources in their community, whom they respect, who will listen without judgment, and maybe even say something that inspires a light in the darkness. It’s at least as effective as psychotropic drugs, and all its benefits come without side effects.
Once a year we come together, listen to each other’s stories, and continue our healing work together. Maria tells me her friends want to meet me. “They want to steal you away,” she says, “but I tell them I am not afraid.”
Maria, a lay therapist of sorts, is the community mental health consultant. Once a year, she consults with her gringo, the clown/doctor, to compare notes. We laugh and love, hug and cry, and give each other hope. No matter how divisive and polarizing the times, it is possible to come together in community and promote healing.
Dr. Hammerschlag is chief of community mental health of the Gesundheit! Institute and a faculty member at the University of Arizona, Phoenix. He is the author of several books on healing and spirituality, including “Kindling Spirit: Healing from Within” (New York: Turtle Island Press, 2010) and “The Dancing Healers: A Doctor’s Journey of Healing With Native Americans” (San Francisco: Harper, 1988). Dr. Hammerschlag’s website is healingdoc.com.
Survival, QoL better with nivolumab for recurrent head and neck cancer
COPENHAGEN – Among patients with recurrent or metastatic squamous cell carcinoma of the head and neck, those treated with the programmed death ligand–1 checkpoint inhibitor nivolumab reported consistently better quality of life than patients treated with a standard second-line agent of the investigator’s choice.
In a substudy of the randomized phase III Checkmate 141 trial, patients treated with nivolumab (Opdivo) had consistently stable outcomes across three separate, validated quality of life (QoL) instruments, reported Kevin Harrington, MBBS, of Royal Marsden Hospital in London.
“Nivolumab is the first PD-L1 [programmed death ligand–1] inhibitor to demonstrate a significant improvement in overall survival, with greater tolerability and a quality of life benefit, compared with standard of care therapy,” he said at the European Society of Medical Oncology Congress.
The overall trial results were reported at the 2016 annual meeting of the American Association for Cancer Research, and published online in the New England Journal of Medicine to coincide with Dr. Harrington’s presentation.
In this open-label trial, 361 patients with squamous cell carcinoma of the head and neck (HNSCC) that recurred or progressed within 6 months of completion of platinum-based chemotherapy were randomly assigned on a 2:1 basis to receive either nivolumab 3 mg/kg every 2 weeks, or standard, single-agent systemic chemotherapy with either methotrexate, docetaxel, or cetuximab.
After a median follow-up of 5.1 months (range, 0-16.8 months), the median overall survival, the primary endpoint, was 7.5 months for patients on nivolumab, compared with 5.1 months for those on standard therapy. The hazard ratio for death with nivolumab was 0.70 (P = .01). Estimates of 1-year survival were 36% vs. 16.6%, respectively. The median progression-free survival was similar between the groups, at 2 months and 2.3 months, respectively (a nonsignificant finding).
Treatment-related adverse events of grade 3 or 4 occurred in 13.1% of patients on nivolumab, compared with 35.1% of those on standard therapy.
Patient-reported outcomes
Dr. Harrington reviewed changes from baseline in symptoms and function for three different patient-reported instruments:
• The cancer-specific EORTC (European Organization for Research and Treatment of Cancer) QLQ-C30.
• The head and neck cancer–specific QLQ-H&N35.
• The generic health status EQ-5D-3L.
Patients were assessed on day 1 of the first treatment cycle, at week 9 and every 6 weeks thereafter of the study, at the first and second follow-up visits, and at survival follow-up visits (with the EQ-5D-3L only).
For the QLQ-C30 domains of physical, role, and social functioning, patients on nivolumab had either slight improvements from baseline or stayed the same, whereas patients on standard therapies had significant declines at both 9 and 15 weeks for all three domains.
There were no differences in functional domains on this instrument according to expression of PD-L1 (on either 1% or more of tumor cells or less than 1%).
For the EORTC QLQ-C30 symptom burden scale, patients assigned to the investigator’s choice had significantly and clinically meaningful worse symptoms, compared with patients on nivolumab at 9 and 15 weeks, and the median time to deterioration of function favored nivolumab on all subscales except for emotional functioning.
Similar differences in favor of nivolumab were seen in the EORTC QLQ-H&N35 instrument domains of pain, sensory problems, and social contact problems.
Lastly, on the EQ-5D-3L instrument, nivolumab-treated patients had stable health status, compared with worsening health status, with the difference statistically significant at week 15 (P = .037).
Anthony T.C. Chan, MD, professor of clinical oncology at the Chinese University of Hong Kong, the invited discussant, said that some of the differences in QoL noted in the study may have been due to differences between the therapies.
“The kinetics of adverse events with checkpoint blockade is now well described, and it’s quite different from systemic chemotherapy, where you get the side effects immediately. Quite often with checkpoint inhibitors, you can get adverse events up to 6-8 weeks or even longer after the start of therapy,” he said.
Nonetheless, “I think from [Checkmate 141] with the very positive results we’ve just seen with nivolumab, both in improving overall survival as well as improving patient-reported outcomes, after patients have failed platinum-based chemotherapy, nivolumab should be considered standard second-line therapy in this setting,” he said.
The trial was supported by Bristol-Myers Squibb. Dr. Harrington and Dr. Chan disclosed receiving research funding, serving as an adviser, and/or receiving travel, accommodations, and other expenses from the company.
[email protected]
COPENHAGEN – Among patients with recurrent or metastatic squamous cell carcinoma of the head and neck, those treated with the programmed death ligand–1 checkpoint inhibitor nivolumab reported consistently better quality of life than patients treated with a standard second-line agent of the investigator’s choice.
In a substudy of the randomized phase III Checkmate 141 trial, patients treated with nivolumab (Opdivo) had consistently stable outcomes across three separate, validated quality of life (QoL) instruments, reported Kevin Harrington, MBBS, of Royal Marsden Hospital in London.
“Nivolumab is the first PD-L1 [programmed death ligand–1] inhibitor to demonstrate a significant improvement in overall survival, with greater tolerability and a quality of life benefit, compared with standard of care therapy,” he said at the European Society of Medical Oncology Congress.
The overall trial results were reported at the 2016 annual meeting of the American Association for Cancer Research, and published online in the New England Journal of Medicine to coincide with Dr. Harrington’s presentation.
In this open-label trial, 361 patients with squamous cell carcinoma of the head and neck (HNSCC) that recurred or progressed within 6 months of completion of platinum-based chemotherapy were randomly assigned on a 2:1 basis to receive either nivolumab 3 mg/kg every 2 weeks, or standard, single-agent systemic chemotherapy with either methotrexate, docetaxel, or cetuximab.
After a median follow-up of 5.1 months (range, 0-16.8 months), the median overall survival, the primary endpoint, was 7.5 months for patients on nivolumab, compared with 5.1 months for those on standard therapy. The hazard ratio for death with nivolumab was 0.70 (P = .01). Estimates of 1-year survival were 36% vs. 16.6%, respectively. The median progression-free survival was similar between the groups, at 2 months and 2.3 months, respectively (a nonsignificant finding).
Treatment-related adverse events of grade 3 or 4 occurred in 13.1% of patients on nivolumab, compared with 35.1% of those on standard therapy.
Patient-reported outcomes
Dr. Harrington reviewed changes from baseline in symptoms and function for three different patient-reported instruments:
• The cancer-specific EORTC (European Organization for Research and Treatment of Cancer) QLQ-C30.
• The head and neck cancer–specific QLQ-H&N35.
• The generic health status EQ-5D-3L.
Patients were assessed on day 1 of the first treatment cycle, at week 9 and every 6 weeks thereafter of the study, at the first and second follow-up visits, and at survival follow-up visits (with the EQ-5D-3L only).
For the QLQ-C30 domains of physical, role, and social functioning, patients on nivolumab had either slight improvements from baseline or stayed the same, whereas patients on standard therapies had significant declines at both 9 and 15 weeks for all three domains.
There were no differences in functional domains on this instrument according to expression of PD-L1 (on either 1% or more of tumor cells or less than 1%).
For the EORTC QLQ-C30 symptom burden scale, patients assigned to the investigator’s choice had significantly and clinically meaningful worse symptoms, compared with patients on nivolumab at 9 and 15 weeks, and the median time to deterioration of function favored nivolumab on all subscales except for emotional functioning.
Similar differences in favor of nivolumab were seen in the EORTC QLQ-H&N35 instrument domains of pain, sensory problems, and social contact problems.
Lastly, on the EQ-5D-3L instrument, nivolumab-treated patients had stable health status, compared with worsening health status, with the difference statistically significant at week 15 (P = .037).
Anthony T.C. Chan, MD, professor of clinical oncology at the Chinese University of Hong Kong, the invited discussant, said that some of the differences in QoL noted in the study may have been due to differences between the therapies.
“The kinetics of adverse events with checkpoint blockade is now well described, and it’s quite different from systemic chemotherapy, where you get the side effects immediately. Quite often with checkpoint inhibitors, you can get adverse events up to 6-8 weeks or even longer after the start of therapy,” he said.
Nonetheless, “I think from [Checkmate 141] with the very positive results we’ve just seen with nivolumab, both in improving overall survival as well as improving patient-reported outcomes, after patients have failed platinum-based chemotherapy, nivolumab should be considered standard second-line therapy in this setting,” he said.
The trial was supported by Bristol-Myers Squibb. Dr. Harrington and Dr. Chan disclosed receiving research funding, serving as an adviser, and/or receiving travel, accommodations, and other expenses from the company.
[email protected]
COPENHAGEN – Among patients with recurrent or metastatic squamous cell carcinoma of the head and neck, those treated with the programmed death ligand–1 checkpoint inhibitor nivolumab reported consistently better quality of life than patients treated with a standard second-line agent of the investigator’s choice.
In a substudy of the randomized phase III Checkmate 141 trial, patients treated with nivolumab (Opdivo) had consistently stable outcomes across three separate, validated quality of life (QoL) instruments, reported Kevin Harrington, MBBS, of Royal Marsden Hospital in London.
“Nivolumab is the first PD-L1 [programmed death ligand–1] inhibitor to demonstrate a significant improvement in overall survival, with greater tolerability and a quality of life benefit, compared with standard of care therapy,” he said at the European Society of Medical Oncology Congress.
The overall trial results were reported at the 2016 annual meeting of the American Association for Cancer Research, and published online in the New England Journal of Medicine to coincide with Dr. Harrington’s presentation.
In this open-label trial, 361 patients with squamous cell carcinoma of the head and neck (HNSCC) that recurred or progressed within 6 months of completion of platinum-based chemotherapy were randomly assigned on a 2:1 basis to receive either nivolumab 3 mg/kg every 2 weeks, or standard, single-agent systemic chemotherapy with either methotrexate, docetaxel, or cetuximab.
After a median follow-up of 5.1 months (range, 0-16.8 months), the median overall survival, the primary endpoint, was 7.5 months for patients on nivolumab, compared with 5.1 months for those on standard therapy. The hazard ratio for death with nivolumab was 0.70 (P = .01). Estimates of 1-year survival were 36% vs. 16.6%, respectively. The median progression-free survival was similar between the groups, at 2 months and 2.3 months, respectively (a nonsignificant finding).
Treatment-related adverse events of grade 3 or 4 occurred in 13.1% of patients on nivolumab, compared with 35.1% of those on standard therapy.
Patient-reported outcomes
Dr. Harrington reviewed changes from baseline in symptoms and function for three different patient-reported instruments:
• The cancer-specific EORTC (European Organization for Research and Treatment of Cancer) QLQ-C30.
• The head and neck cancer–specific QLQ-H&N35.
• The generic health status EQ-5D-3L.
Patients were assessed on day 1 of the first treatment cycle, at week 9 and every 6 weeks thereafter of the study, at the first and second follow-up visits, and at survival follow-up visits (with the EQ-5D-3L only).
For the QLQ-C30 domains of physical, role, and social functioning, patients on nivolumab had either slight improvements from baseline or stayed the same, whereas patients on standard therapies had significant declines at both 9 and 15 weeks for all three domains.
There were no differences in functional domains on this instrument according to expression of PD-L1 (on either 1% or more of tumor cells or less than 1%).
For the EORTC QLQ-C30 symptom burden scale, patients assigned to the investigator’s choice had significantly and clinically meaningful worse symptoms, compared with patients on nivolumab at 9 and 15 weeks, and the median time to deterioration of function favored nivolumab on all subscales except for emotional functioning.
Similar differences in favor of nivolumab were seen in the EORTC QLQ-H&N35 instrument domains of pain, sensory problems, and social contact problems.
Lastly, on the EQ-5D-3L instrument, nivolumab-treated patients had stable health status, compared with worsening health status, with the difference statistically significant at week 15 (P = .037).
Anthony T.C. Chan, MD, professor of clinical oncology at the Chinese University of Hong Kong, the invited discussant, said that some of the differences in QoL noted in the study may have been due to differences between the therapies.
“The kinetics of adverse events with checkpoint blockade is now well described, and it’s quite different from systemic chemotherapy, where you get the side effects immediately. Quite often with checkpoint inhibitors, you can get adverse events up to 6-8 weeks or even longer after the start of therapy,” he said.
Nonetheless, “I think from [Checkmate 141] with the very positive results we’ve just seen with nivolumab, both in improving overall survival as well as improving patient-reported outcomes, after patients have failed platinum-based chemotherapy, nivolumab should be considered standard second-line therapy in this setting,” he said.
The trial was supported by Bristol-Myers Squibb. Dr. Harrington and Dr. Chan disclosed receiving research funding, serving as an adviser, and/or receiving travel, accommodations, and other expenses from the company.
[email protected]
Key clinical point:
Major finding: Patient-reported outcomes were superior and consistent for nivolumab across three validated instruments.
Data source: A substudy from a randomized, controlled clinical trial comparing nivolumab with methotrexate, docetaxel, or cetuximab in patients with recurrent or metastatic squamous cell cancers of the head and neck following platinum-based chemotherapy.
Disclosures: The trial was supported by Bristol-Myers Squibb. Dr. Harrington and Dr. Chan disclosed receiving research funding, serving as an adviser, and/or receiving travel, accommodations, and other expenses from the company.
Yes, neoadjuvant chemo improves survival in soft tissue sarcomas
COPENHAGEN – Hard data are hard to come by in sarcoma, but interim results from a randomized trial show that combination chemotherapy with an anthracycline and an alkylating agent offers both a relapse-free and overall survival benefit for patients with soft tissue sarcomas of the trunk wall or extremities, compared with histology-driven therapies.
In a study comparing three cycles of a full-dose epirubicin and ifosfamide combination with three cycles of one of five regimens tailored to specific sarcoma subtypes, there was an average absolute benefit in both relapse-free survival (RFS) and overall survival (OS) of approximately 20% with the combination regimen, reported Alessandro Gronchi, MD, chair of sarcoma surgery at the National Cancer Institute of Italy in Milan.
The findings come as a surprise, however, because the trial was originally designed to detect a benefit for the tailored, histology-driven therapies, and is therefore, technically, a failure, Dr. Gronchi said at the European Society for Medical Oncology Congress.
“In a population of soft-tissue sarcoma patients selected by a risk of relapse averaging 60%-70%, the neoadjuvant administration of a short full-dose anthracycline plus ifosfamide chemotherapy increases relapse-free survival of more than 20% and overall survival of at least 10%,” he said.
Chemotherapy in both the neoadjuvant and adjuvant setting for patients with high-risk soft-tissue sarcomas has been controversial, with a wide range of practices worldwide. Some centers administer neoadjuvant chemotherapy and/or radiation, followed by surgery and adjuvant radiation and/or chemotherapy, whereas other centers do not use chemotherapy, either out of concerns about toxicities, or because the evidence supporting the practice has been equivocal.
The best argument in favor of neoadjuvant chemotherapy in this setting until now has been that it can increase the chances of successful surgery (R0 resection), which is associated with longer progression-free survival and better control of both recurrence and distant metastasis, Dr. Gronchi said at a briefing prior to his presentation of the data in a symposium session.
The multicenter trial compared a regimen of epirubicin 120 mg/m2 plus ifosfamide 9 g/m2 (standard combination arm) with one of five different regimens based on the following sarcoma histologies, which comprise approximately 80% of all sarcomas of the trunk wall or extremities:
• Undifferentiated pleomorphic sarcoma (UPS): gemcitabine+docetaxel.
• High grade myxoid liposarcoma: trabectedin (Yondelis).
• Synovial sarcoma: high-dose prolonged-infusion ifosfamide.
• Malignant peripheral nerve sheath tumors (MPNST): etoposide plus ifosfamide.
• Leiomyosarcoma: gemcitabine+dacarbazine in leiomyosarcoma.
All patients had localized, high-risk soft tissue sarcomas defined as grade 3, size greater than 5 cm, and deep tumor site; the median tumor size was 10 cm in each arm.
Following chemotherapy, all resectable patients went on to surgery with or without radiation therapy.
A total of 287 patients, median age 50, were randomized either to the regimen tailored to their tumor type (142 patients), or to the combination (145). A total of 240 patients were evaluable for response.
At a median follow-up of 12.34 months, RFS rates were 62% for patients who received epirubicin-ifosfamide, compared with 38% for those who received tailored therapy, In univariate analysis, the hazard ratio for tailored therapy was 1.955 (P = .007).
Overall survival rates were 89% vs. 64%, respectively (P = .033).
An analysis of responses by RECIST (Response Evaluation Criteria in Solid Tumors) showed that more patients in the standard arm had partial responses (16% vs. 10%). Stable disease rates were comparable, at 81% and 82%, respectively. Fewer patients on the standard arm had disease progression, at 3% vs. 8%. There were no complete responses in either group.
A look at RFS by histology subtype showed that for four of the five tumor types, standard chemotherapy was better. For patients with myxoid round cell liposarcoma, however, trabecdetin was equivalent in efficacy to combination chemotherapy.
“Since the safety profile of trabecdetin is much better, it’s a much better-tolerated drug than combination epirubicin and ifosfamide, we are now aiming to reopen that strata and looking into possibly moving the drug into the first line,” Dr. Gronchi said in an interview.
He recommended that based on these results, ESMO guidelines for soft tissue and visceral sarcoma guidelines should be updated to read as follows (additions or substitution to the existing text shown in italics):
“If the decision is made to use CT as upfront treatment, it may well be used preoperatively. A benefit may be gained when 3 full-dose anthracycline ifosfamide chemotherapy cycles is used as upfront treatment, facilitating surgery. This regimen has ‘proven’ to give a positive impact on RFS/OS in selected high-risk soft-tissue sarcomas of extremity/trunk wall.”
He added that the guidelines should emphasize management of patients with discussion in multidisciplinary tumor boards in specialized or “reference” sarcoma centers or within reference networks sharing multidisciplinary expertise and treating a high number of patients.
Eurosarc funded the study. Dr. Gronchi and Dr. Le Cesne disclosed ties with several companies
COPENHAGEN – Hard data are hard to come by in sarcoma, but interim results from a randomized trial show that combination chemotherapy with an anthracycline and an alkylating agent offers both a relapse-free and overall survival benefit for patients with soft tissue sarcomas of the trunk wall or extremities, compared with histology-driven therapies.
In a study comparing three cycles of a full-dose epirubicin and ifosfamide combination with three cycles of one of five regimens tailored to specific sarcoma subtypes, there was an average absolute benefit in both relapse-free survival (RFS) and overall survival (OS) of approximately 20% with the combination regimen, reported Alessandro Gronchi, MD, chair of sarcoma surgery at the National Cancer Institute of Italy in Milan.
The findings come as a surprise, however, because the trial was originally designed to detect a benefit for the tailored, histology-driven therapies, and is therefore, technically, a failure, Dr. Gronchi said at the European Society for Medical Oncology Congress.
“In a population of soft-tissue sarcoma patients selected by a risk of relapse averaging 60%-70%, the neoadjuvant administration of a short full-dose anthracycline plus ifosfamide chemotherapy increases relapse-free survival of more than 20% and overall survival of at least 10%,” he said.
Chemotherapy in both the neoadjuvant and adjuvant setting for patients with high-risk soft-tissue sarcomas has been controversial, with a wide range of practices worldwide. Some centers administer neoadjuvant chemotherapy and/or radiation, followed by surgery and adjuvant radiation and/or chemotherapy, whereas other centers do not use chemotherapy, either out of concerns about toxicities, or because the evidence supporting the practice has been equivocal.
The best argument in favor of neoadjuvant chemotherapy in this setting until now has been that it can increase the chances of successful surgery (R0 resection), which is associated with longer progression-free survival and better control of both recurrence and distant metastasis, Dr. Gronchi said at a briefing prior to his presentation of the data in a symposium session.
The multicenter trial compared a regimen of epirubicin 120 mg/m2 plus ifosfamide 9 g/m2 (standard combination arm) with one of five different regimens based on the following sarcoma histologies, which comprise approximately 80% of all sarcomas of the trunk wall or extremities:
• Undifferentiated pleomorphic sarcoma (UPS): gemcitabine+docetaxel.
• High grade myxoid liposarcoma: trabectedin (Yondelis).
• Synovial sarcoma: high-dose prolonged-infusion ifosfamide.
• Malignant peripheral nerve sheath tumors (MPNST): etoposide plus ifosfamide.
• Leiomyosarcoma: gemcitabine+dacarbazine in leiomyosarcoma.
All patients had localized, high-risk soft tissue sarcomas defined as grade 3, size greater than 5 cm, and deep tumor site; the median tumor size was 10 cm in each arm.
Following chemotherapy, all resectable patients went on to surgery with or without radiation therapy.
A total of 287 patients, median age 50, were randomized either to the regimen tailored to their tumor type (142 patients), or to the combination (145). A total of 240 patients were evaluable for response.
At a median follow-up of 12.34 months, RFS rates were 62% for patients who received epirubicin-ifosfamide, compared with 38% for those who received tailored therapy, In univariate analysis, the hazard ratio for tailored therapy was 1.955 (P = .007).
Overall survival rates were 89% vs. 64%, respectively (P = .033).
An analysis of responses by RECIST (Response Evaluation Criteria in Solid Tumors) showed that more patients in the standard arm had partial responses (16% vs. 10%). Stable disease rates were comparable, at 81% and 82%, respectively. Fewer patients on the standard arm had disease progression, at 3% vs. 8%. There were no complete responses in either group.
A look at RFS by histology subtype showed that for four of the five tumor types, standard chemotherapy was better. For patients with myxoid round cell liposarcoma, however, trabecdetin was equivalent in efficacy to combination chemotherapy.
“Since the safety profile of trabecdetin is much better, it’s a much better-tolerated drug than combination epirubicin and ifosfamide, we are now aiming to reopen that strata and looking into possibly moving the drug into the first line,” Dr. Gronchi said in an interview.
He recommended that based on these results, ESMO guidelines for soft tissue and visceral sarcoma guidelines should be updated to read as follows (additions or substitution to the existing text shown in italics):
“If the decision is made to use CT as upfront treatment, it may well be used preoperatively. A benefit may be gained when 3 full-dose anthracycline ifosfamide chemotherapy cycles is used as upfront treatment, facilitating surgery. This regimen has ‘proven’ to give a positive impact on RFS/OS in selected high-risk soft-tissue sarcomas of extremity/trunk wall.”
He added that the guidelines should emphasize management of patients with discussion in multidisciplinary tumor boards in specialized or “reference” sarcoma centers or within reference networks sharing multidisciplinary expertise and treating a high number of patients.
Eurosarc funded the study. Dr. Gronchi and Dr. Le Cesne disclosed ties with several companies
COPENHAGEN – Hard data are hard to come by in sarcoma, but interim results from a randomized trial show that combination chemotherapy with an anthracycline and an alkylating agent offers both a relapse-free and overall survival benefit for patients with soft tissue sarcomas of the trunk wall or extremities, compared with histology-driven therapies.
In a study comparing three cycles of a full-dose epirubicin and ifosfamide combination with three cycles of one of five regimens tailored to specific sarcoma subtypes, there was an average absolute benefit in both relapse-free survival (RFS) and overall survival (OS) of approximately 20% with the combination regimen, reported Alessandro Gronchi, MD, chair of sarcoma surgery at the National Cancer Institute of Italy in Milan.
The findings come as a surprise, however, because the trial was originally designed to detect a benefit for the tailored, histology-driven therapies, and is therefore, technically, a failure, Dr. Gronchi said at the European Society for Medical Oncology Congress.
“In a population of soft-tissue sarcoma patients selected by a risk of relapse averaging 60%-70%, the neoadjuvant administration of a short full-dose anthracycline plus ifosfamide chemotherapy increases relapse-free survival of more than 20% and overall survival of at least 10%,” he said.
Chemotherapy in both the neoadjuvant and adjuvant setting for patients with high-risk soft-tissue sarcomas has been controversial, with a wide range of practices worldwide. Some centers administer neoadjuvant chemotherapy and/or radiation, followed by surgery and adjuvant radiation and/or chemotherapy, whereas other centers do not use chemotherapy, either out of concerns about toxicities, or because the evidence supporting the practice has been equivocal.
The best argument in favor of neoadjuvant chemotherapy in this setting until now has been that it can increase the chances of successful surgery (R0 resection), which is associated with longer progression-free survival and better control of both recurrence and distant metastasis, Dr. Gronchi said at a briefing prior to his presentation of the data in a symposium session.
The multicenter trial compared a regimen of epirubicin 120 mg/m2 plus ifosfamide 9 g/m2 (standard combination arm) with one of five different regimens based on the following sarcoma histologies, which comprise approximately 80% of all sarcomas of the trunk wall or extremities:
• Undifferentiated pleomorphic sarcoma (UPS): gemcitabine+docetaxel.
• High grade myxoid liposarcoma: trabectedin (Yondelis).
• Synovial sarcoma: high-dose prolonged-infusion ifosfamide.
• Malignant peripheral nerve sheath tumors (MPNST): etoposide plus ifosfamide.
• Leiomyosarcoma: gemcitabine+dacarbazine in leiomyosarcoma.
All patients had localized, high-risk soft tissue sarcomas defined as grade 3, size greater than 5 cm, and deep tumor site; the median tumor size was 10 cm in each arm.
Following chemotherapy, all resectable patients went on to surgery with or without radiation therapy.
A total of 287 patients, median age 50, were randomized either to the regimen tailored to their tumor type (142 patients), or to the combination (145). A total of 240 patients were evaluable for response.
At a median follow-up of 12.34 months, RFS rates were 62% for patients who received epirubicin-ifosfamide, compared with 38% for those who received tailored therapy, In univariate analysis, the hazard ratio for tailored therapy was 1.955 (P = .007).
Overall survival rates were 89% vs. 64%, respectively (P = .033).
An analysis of responses by RECIST (Response Evaluation Criteria in Solid Tumors) showed that more patients in the standard arm had partial responses (16% vs. 10%). Stable disease rates were comparable, at 81% and 82%, respectively. Fewer patients on the standard arm had disease progression, at 3% vs. 8%. There were no complete responses in either group.
A look at RFS by histology subtype showed that for four of the five tumor types, standard chemotherapy was better. For patients with myxoid round cell liposarcoma, however, trabecdetin was equivalent in efficacy to combination chemotherapy.
“Since the safety profile of trabecdetin is much better, it’s a much better-tolerated drug than combination epirubicin and ifosfamide, we are now aiming to reopen that strata and looking into possibly moving the drug into the first line,” Dr. Gronchi said in an interview.
He recommended that based on these results, ESMO guidelines for soft tissue and visceral sarcoma guidelines should be updated to read as follows (additions or substitution to the existing text shown in italics):
“If the decision is made to use CT as upfront treatment, it may well be used preoperatively. A benefit may be gained when 3 full-dose anthracycline ifosfamide chemotherapy cycles is used as upfront treatment, facilitating surgery. This regimen has ‘proven’ to give a positive impact on RFS/OS in selected high-risk soft-tissue sarcomas of extremity/trunk wall.”
He added that the guidelines should emphasize management of patients with discussion in multidisciplinary tumor boards in specialized or “reference” sarcoma centers or within reference networks sharing multidisciplinary expertise and treating a high number of patients.
Eurosarc funded the study. Dr. Gronchi and Dr. Le Cesne disclosed ties with several companies
Key clinical point: This study provides the first clear randomized evidence for a survival benefit with neoadjuvant chemotherapy in the five most common types of soft-tissue sarcomas of the extremities or trunk wall.
Major finding: Combined epirubicin/ifosfamide was associated with 30% better relapse-free survival and 10% better overall survival compared with histology-tailored therapies.
Data source: Randomized trial of 240 patients with sarcomas of the extremities or trunk wall.
Disclosures: Eurosarc funded the study. Dr. Gronchi and Dr. Le Cesne disclosed ties with several companies.
Depression drops COPD medication adherence
Chronic obstructive pulmonary disease (COPD) patients with depression are less likely to take their maintenance medications, according to a review of Medicare claims by the University of Maryland, Baltimore.
“Clinicians who treat older adults newly diagnosed with COPD should be aware of the development of depression, especially during the first 6 months. As such, clinicians should consider the need to monitor their patients with COPD for … depression [treatment], as well as use of and adherence to prescribed COPD medications. Close management of these and other aspects of newly diagnosed older adults with COPD will help to ensure optimal clinical outcomes,” said the investigators, led by Jennifer Albrecht, PhD, of the department of epidemiology and public health at the University of Maryland.
The researchers ran a random sampling of Medicare data and identified 31,033 beneficiaries diagnosed with COPD between 2006 and 2010; 6,227 patients (20% of the study sample) were diagnosed with depression within 2 years of being diagnosed with COPD.
The investigators found that depression reduced the likelihood of chronic obstructive pulmonary disease patients filling their prescriptions. Maintenance medication adherence was low overall, peaking at 57% in the month after the first fill and decreasing every month for the next 9 months for both the patients with depression and those patients who had not been diagnosed with the condition. Depression made things worse; 20% of depressed patients filled 80% or more of their medications at the pharmacy, vs. 22% of nondepressed patients. Patients with newly diagnosed depression were about 7% less likely to have good adherence (odds ratio, 0.93; 95% confidence interval, 0.89-0.98). Women – 65% of the study sample and 75% of those with depression – were less likely than men to fill their scripts.
Meanwhile, adherence to COPD maintenance medication was more likely among patients on short-term inhalers and supplemental oxygen, as well as among nursing home patients and those with low-income subsidies.
Patients were 83% white. Those diagnosed with depression were slightly younger on average than those who were not (67 vs. 69 years old) and were more likely to have more than three comorbid conditions (33% vs. 23%). With the exception of asthma, comorbid conditions made adherence worse. Depressed patients also had more severe COPD symptoms, based on their higher rates of oxygen use (10% vs. 8%).
Dr. Albrecht reported receiving grants from the National Institutes of Health during the conduct of the study.
Chronic obstructive pulmonary disease (COPD) patients with depression are less likely to take their maintenance medications, according to a review of Medicare claims by the University of Maryland, Baltimore.
“Clinicians who treat older adults newly diagnosed with COPD should be aware of the development of depression, especially during the first 6 months. As such, clinicians should consider the need to monitor their patients with COPD for … depression [treatment], as well as use of and adherence to prescribed COPD medications. Close management of these and other aspects of newly diagnosed older adults with COPD will help to ensure optimal clinical outcomes,” said the investigators, led by Jennifer Albrecht, PhD, of the department of epidemiology and public health at the University of Maryland.
The researchers ran a random sampling of Medicare data and identified 31,033 beneficiaries diagnosed with COPD between 2006 and 2010; 6,227 patients (20% of the study sample) were diagnosed with depression within 2 years of being diagnosed with COPD.
The investigators found that depression reduced the likelihood of chronic obstructive pulmonary disease patients filling their prescriptions. Maintenance medication adherence was low overall, peaking at 57% in the month after the first fill and decreasing every month for the next 9 months for both the patients with depression and those patients who had not been diagnosed with the condition. Depression made things worse; 20% of depressed patients filled 80% or more of their medications at the pharmacy, vs. 22% of nondepressed patients. Patients with newly diagnosed depression were about 7% less likely to have good adherence (odds ratio, 0.93; 95% confidence interval, 0.89-0.98). Women – 65% of the study sample and 75% of those with depression – were less likely than men to fill their scripts.
Meanwhile, adherence to COPD maintenance medication was more likely among patients on short-term inhalers and supplemental oxygen, as well as among nursing home patients and those with low-income subsidies.
Patients were 83% white. Those diagnosed with depression were slightly younger on average than those who were not (67 vs. 69 years old) and were more likely to have more than three comorbid conditions (33% vs. 23%). With the exception of asthma, comorbid conditions made adherence worse. Depressed patients also had more severe COPD symptoms, based on their higher rates of oxygen use (10% vs. 8%).
Dr. Albrecht reported receiving grants from the National Institutes of Health during the conduct of the study.
Chronic obstructive pulmonary disease (COPD) patients with depression are less likely to take their maintenance medications, according to a review of Medicare claims by the University of Maryland, Baltimore.
“Clinicians who treat older adults newly diagnosed with COPD should be aware of the development of depression, especially during the first 6 months. As such, clinicians should consider the need to monitor their patients with COPD for … depression [treatment], as well as use of and adherence to prescribed COPD medications. Close management of these and other aspects of newly diagnosed older adults with COPD will help to ensure optimal clinical outcomes,” said the investigators, led by Jennifer Albrecht, PhD, of the department of epidemiology and public health at the University of Maryland.
The researchers ran a random sampling of Medicare data and identified 31,033 beneficiaries diagnosed with COPD between 2006 and 2010; 6,227 patients (20% of the study sample) were diagnosed with depression within 2 years of being diagnosed with COPD.
The investigators found that depression reduced the likelihood of chronic obstructive pulmonary disease patients filling their prescriptions. Maintenance medication adherence was low overall, peaking at 57% in the month after the first fill and decreasing every month for the next 9 months for both the patients with depression and those patients who had not been diagnosed with the condition. Depression made things worse; 20% of depressed patients filled 80% or more of their medications at the pharmacy, vs. 22% of nondepressed patients. Patients with newly diagnosed depression were about 7% less likely to have good adherence (odds ratio, 0.93; 95% confidence interval, 0.89-0.98). Women – 65% of the study sample and 75% of those with depression – were less likely than men to fill their scripts.
Meanwhile, adherence to COPD maintenance medication was more likely among patients on short-term inhalers and supplemental oxygen, as well as among nursing home patients and those with low-income subsidies.
Patients were 83% white. Those diagnosed with depression were slightly younger on average than those who were not (67 vs. 69 years old) and were more likely to have more than three comorbid conditions (33% vs. 23%). With the exception of asthma, comorbid conditions made adherence worse. Depressed patients also had more severe COPD symptoms, based on their higher rates of oxygen use (10% vs. 8%).
Dr. Albrecht reported receiving grants from the National Institutes of Health during the conduct of the study.
FROM THE ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point:
Major finding: Patients with newly diagnosed depression were about 7% less likely to have good adherence to their medications (OR 0.93; 95% CI, 0.89–0.98).
Data source: A review of 31,033 Medicare COPD patients, who had filled their COPD maintenance medication at least twice.
Disclosures: Dr. Albrecht reported receiving grants from the National Institutes of Health during the conduct of the study.
E. coli resistant to colistin and carbapenems found in U.S. patient
The first strain of Escherichia coli harboring the antibiotic-resistant genes mcr-1 and blaNDM-5 was isolated in the urine of a U.S. patient, according to a report in mBio.
A 76-year-old man was admitted to a tertiary-care hospital in New Jersey with a fever and flank pain in August 2014. The patient emigrated from India and resided in the United States for 1 year prior to this presentation. He had a history of prostate cancer treated with radiation therapy and subsequently developed recurrent urinary tract infections. He had also experienced bladder perforation requiring bilateral placement of nephrostomy tubes, which were clamped 5 days prior to presentation.
Using molecular analysis, the E. coli isolate from the study case (named MCR1_NJ) was shown to carry both mcr-1 and blaNDM-5 genes. In addition to mcr-1 and blaNDM-5, strain MCR1_NJ was found to harbor resistance genes for aminoglycosides, beta-lactams, chloramphenicol, fluoroquinolones, rifampin, sulfonamides, and tetracycline.
“This strain was isolated in August 2014, highlighting an earlier presence of mcr-1 within the region than previously known and raising the likelihood of ongoing undetected transmission,” wrote José R. Mediavilla, MBS, MPH of the New Jersey Medical School, Rutgers University, Newark, N.J., and his coauthors. “Active surveillance efforts involving all polymyxin- and carbapenem-resistant organisms are imperative in order to determine mcr-1 prevalence and prevent further dissemination.”
Find the full study in mBio (doi: 10.1128/mBio.01191-16).
The first strain of Escherichia coli harboring the antibiotic-resistant genes mcr-1 and blaNDM-5 was isolated in the urine of a U.S. patient, according to a report in mBio.
A 76-year-old man was admitted to a tertiary-care hospital in New Jersey with a fever and flank pain in August 2014. The patient emigrated from India and resided in the United States for 1 year prior to this presentation. He had a history of prostate cancer treated with radiation therapy and subsequently developed recurrent urinary tract infections. He had also experienced bladder perforation requiring bilateral placement of nephrostomy tubes, which were clamped 5 days prior to presentation.
Using molecular analysis, the E. coli isolate from the study case (named MCR1_NJ) was shown to carry both mcr-1 and blaNDM-5 genes. In addition to mcr-1 and blaNDM-5, strain MCR1_NJ was found to harbor resistance genes for aminoglycosides, beta-lactams, chloramphenicol, fluoroquinolones, rifampin, sulfonamides, and tetracycline.
“This strain was isolated in August 2014, highlighting an earlier presence of mcr-1 within the region than previously known and raising the likelihood of ongoing undetected transmission,” wrote José R. Mediavilla, MBS, MPH of the New Jersey Medical School, Rutgers University, Newark, N.J., and his coauthors. “Active surveillance efforts involving all polymyxin- and carbapenem-resistant organisms are imperative in order to determine mcr-1 prevalence and prevent further dissemination.”
Find the full study in mBio (doi: 10.1128/mBio.01191-16).
The first strain of Escherichia coli harboring the antibiotic-resistant genes mcr-1 and blaNDM-5 was isolated in the urine of a U.S. patient, according to a report in mBio.
A 76-year-old man was admitted to a tertiary-care hospital in New Jersey with a fever and flank pain in August 2014. The patient emigrated from India and resided in the United States for 1 year prior to this presentation. He had a history of prostate cancer treated with radiation therapy and subsequently developed recurrent urinary tract infections. He had also experienced bladder perforation requiring bilateral placement of nephrostomy tubes, which were clamped 5 days prior to presentation.
Using molecular analysis, the E. coli isolate from the study case (named MCR1_NJ) was shown to carry both mcr-1 and blaNDM-5 genes. In addition to mcr-1 and blaNDM-5, strain MCR1_NJ was found to harbor resistance genes for aminoglycosides, beta-lactams, chloramphenicol, fluoroquinolones, rifampin, sulfonamides, and tetracycline.
“This strain was isolated in August 2014, highlighting an earlier presence of mcr-1 within the region than previously known and raising the likelihood of ongoing undetected transmission,” wrote José R. Mediavilla, MBS, MPH of the New Jersey Medical School, Rutgers University, Newark, N.J., and his coauthors. “Active surveillance efforts involving all polymyxin- and carbapenem-resistant organisms are imperative in order to determine mcr-1 prevalence and prevent further dissemination.”
Find the full study in mBio (doi: 10.1128/mBio.01191-16).
Unexplained subfertility in RA linked to periconceptional NSAID use
Women with rheumatoid arthritis are more often diagnosed with unexplained subfertility, compared with women in the general population, according to a new study published in Arthritis Care & Research.
This finding may imply that fertility in female RA patients is influenced by disease-related factors, specifically the use of periconceptional NSAIDs, according to the investigators.
In addition, when women with RA try to conceive, their antirheumatic treatment regimens need to be adjusted, which increases risk for permanent joint damage, the investigators wrote, adding that “understanding the underlying mechanisms of subfertility in RA, and treatment of these mechanisms whenever possible, would be an important step forward in the care for these patients.”
To study the outcome of fertility assessments in women with RA and subfertility, Dr. Brouwer and her associates performed a cross-sectional study of 260 female RA patients who were recruited from the Pregnancy-induced Amelioration of RA study, a Dutch nationwide prospective observational trial of women diagnosed with RA who were in their first trimester of pregnancy or who were trying to conceive.
Each eligible participant received a questionnaire that included questions regarding reproductive history, time to pregnancy, mode of conception for prior pregnancies, fertility assessments, and fertility treatments. For the 178 (68%) women who returned completed questionnaires, additional gynecologic histories and diagnoses were collected from medical histories and/or patient files.
Analysis of these data revealed that 82 women (46%; 95% confidence interval, 39%-53%) with RA were considered subfertile. Subfertility was most often unexplained (48% of known diagnoses) or caused by anovulation (28%) or semen abnormalities (16%).
“In comparison to the general population, female RA patients appear to be more often diagnosed with unexplained subfertility, whereas the percentage of subfertile women with anovulation was equal or slightly increased compared to percentages found in the general population,” the investigators wrote.
The majority of subfertile RA patients received fertility treatments, and “a considerable number of all pregnancies were conceived after women had been treated for subfertility,” according to the researchers.
The significant association between periconceptional NSAIDs use and unexplained subfertility “is in concordance with a previous study within the PARA cohort where we have shown that a longer [time to pregnancy] was associated with the periconceptional use of NSAIDs,” Dr. Brouwer and her associates wrote.
“In daily practice, when an RA patient wishes to conceive, NSAIDs should be avoided, and early consultation with an expert rheumatologist and a fertility specialist should be considered to optimize the patient’s chance of a complete family,” the investigators recommended.
This study was funded by the Dutch Arthritis Foundation. One investigator reported received financial compensation from UCB Pharma; the other investigators reported having no relevant disclosures.
[email protected]
On Twitter @jessnicolecraig
Women with rheumatoid arthritis are more often diagnosed with unexplained subfertility, compared with women in the general population, according to a new study published in Arthritis Care & Research.
This finding may imply that fertility in female RA patients is influenced by disease-related factors, specifically the use of periconceptional NSAIDs, according to the investigators.
In addition, when women with RA try to conceive, their antirheumatic treatment regimens need to be adjusted, which increases risk for permanent joint damage, the investigators wrote, adding that “understanding the underlying mechanisms of subfertility in RA, and treatment of these mechanisms whenever possible, would be an important step forward in the care for these patients.”
To study the outcome of fertility assessments in women with RA and subfertility, Dr. Brouwer and her associates performed a cross-sectional study of 260 female RA patients who were recruited from the Pregnancy-induced Amelioration of RA study, a Dutch nationwide prospective observational trial of women diagnosed with RA who were in their first trimester of pregnancy or who were trying to conceive.
Each eligible participant received a questionnaire that included questions regarding reproductive history, time to pregnancy, mode of conception for prior pregnancies, fertility assessments, and fertility treatments. For the 178 (68%) women who returned completed questionnaires, additional gynecologic histories and diagnoses were collected from medical histories and/or patient files.
Analysis of these data revealed that 82 women (46%; 95% confidence interval, 39%-53%) with RA were considered subfertile. Subfertility was most often unexplained (48% of known diagnoses) or caused by anovulation (28%) or semen abnormalities (16%).
“In comparison to the general population, female RA patients appear to be more often diagnosed with unexplained subfertility, whereas the percentage of subfertile women with anovulation was equal or slightly increased compared to percentages found in the general population,” the investigators wrote.
The majority of subfertile RA patients received fertility treatments, and “a considerable number of all pregnancies were conceived after women had been treated for subfertility,” according to the researchers.
The significant association between periconceptional NSAIDs use and unexplained subfertility “is in concordance with a previous study within the PARA cohort where we have shown that a longer [time to pregnancy] was associated with the periconceptional use of NSAIDs,” Dr. Brouwer and her associates wrote.
“In daily practice, when an RA patient wishes to conceive, NSAIDs should be avoided, and early consultation with an expert rheumatologist and a fertility specialist should be considered to optimize the patient’s chance of a complete family,” the investigators recommended.
This study was funded by the Dutch Arthritis Foundation. One investigator reported received financial compensation from UCB Pharma; the other investigators reported having no relevant disclosures.
[email protected]
On Twitter @jessnicolecraig
Women with rheumatoid arthritis are more often diagnosed with unexplained subfertility, compared with women in the general population, according to a new study published in Arthritis Care & Research.
This finding may imply that fertility in female RA patients is influenced by disease-related factors, specifically the use of periconceptional NSAIDs, according to the investigators.
In addition, when women with RA try to conceive, their antirheumatic treatment regimens need to be adjusted, which increases risk for permanent joint damage, the investigators wrote, adding that “understanding the underlying mechanisms of subfertility in RA, and treatment of these mechanisms whenever possible, would be an important step forward in the care for these patients.”
To study the outcome of fertility assessments in women with RA and subfertility, Dr. Brouwer and her associates performed a cross-sectional study of 260 female RA patients who were recruited from the Pregnancy-induced Amelioration of RA study, a Dutch nationwide prospective observational trial of women diagnosed with RA who were in their first trimester of pregnancy or who were trying to conceive.
Each eligible participant received a questionnaire that included questions regarding reproductive history, time to pregnancy, mode of conception for prior pregnancies, fertility assessments, and fertility treatments. For the 178 (68%) women who returned completed questionnaires, additional gynecologic histories and diagnoses were collected from medical histories and/or patient files.
Analysis of these data revealed that 82 women (46%; 95% confidence interval, 39%-53%) with RA were considered subfertile. Subfertility was most often unexplained (48% of known diagnoses) or caused by anovulation (28%) or semen abnormalities (16%).
“In comparison to the general population, female RA patients appear to be more often diagnosed with unexplained subfertility, whereas the percentage of subfertile women with anovulation was equal or slightly increased compared to percentages found in the general population,” the investigators wrote.
The majority of subfertile RA patients received fertility treatments, and “a considerable number of all pregnancies were conceived after women had been treated for subfertility,” according to the researchers.
The significant association between periconceptional NSAIDs use and unexplained subfertility “is in concordance with a previous study within the PARA cohort where we have shown that a longer [time to pregnancy] was associated with the periconceptional use of NSAIDs,” Dr. Brouwer and her associates wrote.
“In daily practice, when an RA patient wishes to conceive, NSAIDs should be avoided, and early consultation with an expert rheumatologist and a fertility specialist should be considered to optimize the patient’s chance of a complete family,” the investigators recommended.
This study was funded by the Dutch Arthritis Foundation. One investigator reported received financial compensation from UCB Pharma; the other investigators reported having no relevant disclosures.
[email protected]
On Twitter @jessnicolecraig
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Of 178 women with RA, 46% were considered subfertile. Subfertility was most often unexplained (48% of known diagnoses) or caused by anovulation (28%).
Data source: Cross-sectional study of 260 female RA patients.
Disclosures: This study was funded by the Dutch Arthritis Foundation. One investigator reported received financial compensation from UCB Pharma; the other investigators reported having no relevant disclosures.
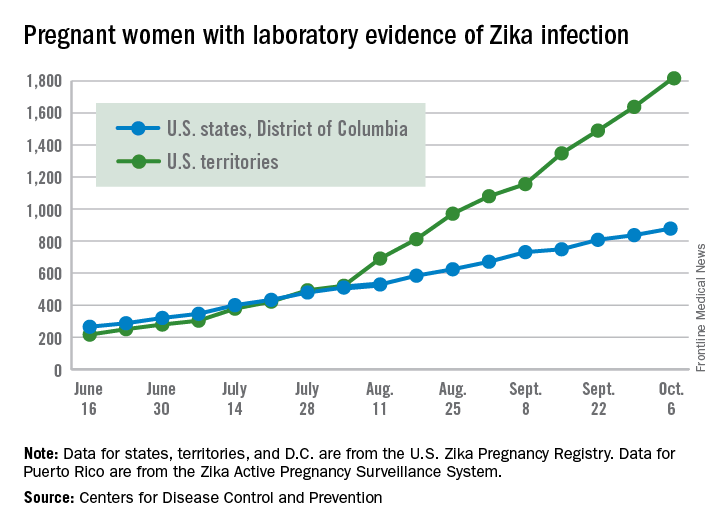
Number of Zika-infected pregnant women continues to climb
The number of pregnant women with laboratory evidence of Zika infection increased by 209 for the week ending Oct. 6, the second-largest weekly increase so far in the United States in 2016, according to the Centers for Disease Control and Prevention.
Another liveborn infant with Zika-related birth defects also was reported for the week, bringing the total for the year to 23 for the 50 states and the District of Columbia. There were no new cases of Zika-related pregnancy losses reported, so the 50-state/DC total remains at five.
Of the 209 new cases reported for the week ending Oct. 6, 41 were in the states/D.C. and 168 were in the territories. The total number of U.S. Zika cases in pregnant women for the year is 2,684: 878 in the states/D.C. and 1,806 in the territories, the CDC said.
Among all Americans, there were 29,891 cases reported as of Oct. 12: 3,936 in the states/D.C. and 25,955 in the territories. Of the territorial cases, 98% have been in Puerto Rico, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of pregnant women with laboratory evidence of Zika infection increased by 209 for the week ending Oct. 6, the second-largest weekly increase so far in the United States in 2016, according to the Centers for Disease Control and Prevention.
Another liveborn infant with Zika-related birth defects also was reported for the week, bringing the total for the year to 23 for the 50 states and the District of Columbia. There were no new cases of Zika-related pregnancy losses reported, so the 50-state/DC total remains at five.
Of the 209 new cases reported for the week ending Oct. 6, 41 were in the states/D.C. and 168 were in the territories. The total number of U.S. Zika cases in pregnant women for the year is 2,684: 878 in the states/D.C. and 1,806 in the territories, the CDC said.
Among all Americans, there were 29,891 cases reported as of Oct. 12: 3,936 in the states/D.C. and 25,955 in the territories. Of the territorial cases, 98% have been in Puerto Rico, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of pregnant women with laboratory evidence of Zika infection increased by 209 for the week ending Oct. 6, the second-largest weekly increase so far in the United States in 2016, according to the Centers for Disease Control and Prevention.
Another liveborn infant with Zika-related birth defects also was reported for the week, bringing the total for the year to 23 for the 50 states and the District of Columbia. There were no new cases of Zika-related pregnancy losses reported, so the 50-state/DC total remains at five.
Of the 209 new cases reported for the week ending Oct. 6, 41 were in the states/D.C. and 168 were in the territories. The total number of U.S. Zika cases in pregnant women for the year is 2,684: 878 in the states/D.C. and 1,806 in the territories, the CDC said.
Among all Americans, there were 29,891 cases reported as of Oct. 12: 3,936 in the states/D.C. and 25,955 in the territories. Of the territorial cases, 98% have been in Puerto Rico, the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Pancreaticobiliary potpourri
The session at the annual Digestive Disease Week entitled Pancreaticobiliary Potpourri encompassed three lectures. Suresh Chari, MD, from Mayo Clinic, Rochester, Minn., presented a lecture titled, “The cystic pancreas.” Gregory Gores, MD, AGAF, also of Mayo Clinic presented a lecture on “Managing the possibly malignant biliary stricture.” Finally, I, Todd H. Baron, MD, from the University of North Carolina at Chapel Hill delivered a lecture titled, “Preventing and managing complications of acute pancreatitis.”
Dr. Gores relayed that there are a variety of etiologies of biliary strictures. Discerning benign from malignant causes involves the use of cross-sectional imaging, PET-CT, serum tests, and endoscopy to include endoscopic retrograde cholangiopancreatography (ERCP) and EUS. IgG4, or autoimmune disease, is an important treatable cause of biliary obstruction. The diagnosis requires a high index of suspicion. Notably, an elevated serum IgG4 level can be seen in patients with cholangiocarcinoma. Fluorescence in situ hybridization (FISH) applied to biliary brush samples at the time of cytologic evaluation has been shown to markedly improve the sensitivity, compared with standard brush cytology. Cholangioscopy with targeted biopsies has been shown to have a sensitivity of 66% and specificity of 97%.
I emphasized the importance of preventing pancreatitis by careful selection of patients for ERCP, by limiting contrast injection during ERCP, and by the use of rectally administered nonsteroidal anti-inflammatory agents at the time of ERCP. Prevention of complications after onset of ERCP is the focus in patients with clinically severe acute pancreatitis, which is usually the result of pancreatic and/or peripancreatic necrosis. Early management consists of prompt and appropriate volume resuscitation, with recent evidence showing Lactated Ringer’s solution being superior to saline. Routine administration of antibiotics is not recommended, but early enteral feeding is recommended. Finally, interventions should be delayed as long as possible with minimally invasive techniques, including endoscopic drainage for walled-off pancreatic necrosis favored over traditional open procedures.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016. Dr. Baron is professor of medicine and director of advanced therapeutic endoscopy in the division of gastroenterology and hepatology in the school of medicine at the University of North Carolina at Chapel Hill. He has consulted and been a speaker for BSCI, Cook Endoscopy, and Olympus; and consulted for W.L. Gore.
The session at the annual Digestive Disease Week entitled Pancreaticobiliary Potpourri encompassed three lectures. Suresh Chari, MD, from Mayo Clinic, Rochester, Minn., presented a lecture titled, “The cystic pancreas.” Gregory Gores, MD, AGAF, also of Mayo Clinic presented a lecture on “Managing the possibly malignant biliary stricture.” Finally, I, Todd H. Baron, MD, from the University of North Carolina at Chapel Hill delivered a lecture titled, “Preventing and managing complications of acute pancreatitis.”
Dr. Gores relayed that there are a variety of etiologies of biliary strictures. Discerning benign from malignant causes involves the use of cross-sectional imaging, PET-CT, serum tests, and endoscopy to include endoscopic retrograde cholangiopancreatography (ERCP) and EUS. IgG4, or autoimmune disease, is an important treatable cause of biliary obstruction. The diagnosis requires a high index of suspicion. Notably, an elevated serum IgG4 level can be seen in patients with cholangiocarcinoma. Fluorescence in situ hybridization (FISH) applied to biliary brush samples at the time of cytologic evaluation has been shown to markedly improve the sensitivity, compared with standard brush cytology. Cholangioscopy with targeted biopsies has been shown to have a sensitivity of 66% and specificity of 97%.
I emphasized the importance of preventing pancreatitis by careful selection of patients for ERCP, by limiting contrast injection during ERCP, and by the use of rectally administered nonsteroidal anti-inflammatory agents at the time of ERCP. Prevention of complications after onset of ERCP is the focus in patients with clinically severe acute pancreatitis, which is usually the result of pancreatic and/or peripancreatic necrosis. Early management consists of prompt and appropriate volume resuscitation, with recent evidence showing Lactated Ringer’s solution being superior to saline. Routine administration of antibiotics is not recommended, but early enteral feeding is recommended. Finally, interventions should be delayed as long as possible with minimally invasive techniques, including endoscopic drainage for walled-off pancreatic necrosis favored over traditional open procedures.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016. Dr. Baron is professor of medicine and director of advanced therapeutic endoscopy in the division of gastroenterology and hepatology in the school of medicine at the University of North Carolina at Chapel Hill. He has consulted and been a speaker for BSCI, Cook Endoscopy, and Olympus; and consulted for W.L. Gore.
The session at the annual Digestive Disease Week entitled Pancreaticobiliary Potpourri encompassed three lectures. Suresh Chari, MD, from Mayo Clinic, Rochester, Minn., presented a lecture titled, “The cystic pancreas.” Gregory Gores, MD, AGAF, also of Mayo Clinic presented a lecture on “Managing the possibly malignant biliary stricture.” Finally, I, Todd H. Baron, MD, from the University of North Carolina at Chapel Hill delivered a lecture titled, “Preventing and managing complications of acute pancreatitis.”
Dr. Gores relayed that there are a variety of etiologies of biliary strictures. Discerning benign from malignant causes involves the use of cross-sectional imaging, PET-CT, serum tests, and endoscopy to include endoscopic retrograde cholangiopancreatography (ERCP) and EUS. IgG4, or autoimmune disease, is an important treatable cause of biliary obstruction. The diagnosis requires a high index of suspicion. Notably, an elevated serum IgG4 level can be seen in patients with cholangiocarcinoma. Fluorescence in situ hybridization (FISH) applied to biliary brush samples at the time of cytologic evaluation has been shown to markedly improve the sensitivity, compared with standard brush cytology. Cholangioscopy with targeted biopsies has been shown to have a sensitivity of 66% and specificity of 97%.
I emphasized the importance of preventing pancreatitis by careful selection of patients for ERCP, by limiting contrast injection during ERCP, and by the use of rectally administered nonsteroidal anti-inflammatory agents at the time of ERCP. Prevention of complications after onset of ERCP is the focus in patients with clinically severe acute pancreatitis, which is usually the result of pancreatic and/or peripancreatic necrosis. Early management consists of prompt and appropriate volume resuscitation, with recent evidence showing Lactated Ringer’s solution being superior to saline. Routine administration of antibiotics is not recommended, but early enteral feeding is recommended. Finally, interventions should be delayed as long as possible with minimally invasive techniques, including endoscopic drainage for walled-off pancreatic necrosis favored over traditional open procedures.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016. Dr. Baron is professor of medicine and director of advanced therapeutic endoscopy in the division of gastroenterology and hepatology in the school of medicine at the University of North Carolina at Chapel Hill. He has consulted and been a speaker for BSCI, Cook Endoscopy, and Olympus; and consulted for W.L. Gore.
Early menopause a risk factor for type 2 diabetes
“What we see in our data is that indeed, early onset of menopause is associated with increased risk of type 2 diabetes, and this association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also of estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, in an interview.
Dr. Muka, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands, presented the analysis from a large, prospective cohort of menopausal women at the annual meeting of the North American Menopause Society.
In an interview, Dr. Muka noted that the study investigated whether the association between early age at natural menopause (ANM) and type 2 diabetes is independent of intermediate risk factors for type 2 diabetes, such as obesity. Finally, the study also assessed whether endogenous sex hormone levels play a role in the link between early ANM and type 2 diabetes.
Enrollment in the Rotterdam study has been continuing in waves since the 1990s, with enrollment for participants in this particular study cohort occurring in 1997 and with additional cohorts enrolled in 2000-2001 and 2006-2008.
“I think this is the first prospective study with such long follow-up data and with a broad adjustment for confounding factors. Previous studies have been mainly cross-sectional, providing conflicting results,” said Dr. Muka.
Using self-reported age at menopause, the investigators excluded from the study women whose menopausal status was not known, who were actually not menopausal, or whose menopause had not occurred naturally. The study population also excluded women with prevalent type 2 diabetes or for whom no information about diabetes follow-up could be found.
The remaining 3,210 women who were included in the study had a median age of 67 years and had reached menopause at a median of 49.9 years. Most women (82.6%) had an ANM of 45-55 years; ANM for 8.8% was 40-44 years, while just 2.3% had an ANM of under 40 years. Mean body mass index was 27.1 kg/m2.
Participants were considered to have incident diabetes based on several sources: a general practitioner’s records, hospital discharge paperwork, or glucose measurements from visits during the Rotterdam study. Participants also were classified as having diabetes if they used a hypoglycemic medication or had a fasting blood glucose level of at least 7 mmol/L (126 mg/dL) or, in the absence of a fasting blood glucose measurement, a nonfasting blood glucose of at least 11.1 mmol/L (200 mg/dL).
Dr. Muka said he and his coinvestigators identified and adjusted for a large number of variables, using a series of three Cox proportion hazard models.
The first model adjusted for age, which wave of enrollment (cohort) participants were in, hormone therapy status, age at menarche, and the number of pregnancies that reached at least 6 months’ gestation.
The second model used all of the factors in model 1, and added BMI and glucose and insulin levels. The third model used all of the factors in model 2, and also added total cholesterol level, systolic blood pressure, the use of lipid-lowering or antihypertensive medications, alcohol and tobacco use, educational level, prevalent cardiovascular disease, and C-reactive protein levels.
The association of early ANM with the risk of type 2 diabetes was statistically significant in all three models (P less than .001), with very similar hazard ratios (HRs) in all models. For the third, the most comprehensive model, the HR was 1.42 (95% confidence interval, 0.83-2.45).
Extensive sensitivity analyses were carried out, and the association held independent of physical activity level, smoking status, use of hormone therapy, and age. The investigators also used multivariable analyses to ensure that the effect was not mediated by serum levels of thyroid-stimulating hormone, dihydroepiandosterone (DHEA) and DHEA sulfate, estradiol, testosterone, sex hormone–binding globulin (SHBG), or androstenedione.
This study was the first in this population to obtain and adjust for serum sex hormone and SHBG levels, said Dr. Muka.
The prospective design of the study and the long follow-up period were study strengths, said Dr. Muka. Additionally, blood glucose readings taken at study visits, together with electronically linked pharmacy dispensing records, were used for incident diabetes diagnoses.
Study limitations, Dr. Muka said, included the possibility of survival bias, since enrollees “may represent survivors of early menopause who did not develop [type 2 diabetes] or die prior to enrollment.” However, he said, this would mean that “we have underestimated the association, so the risk would be even higher.” Also, all study participants were white, so the results cannot be extrapolated to nonwhite populations.
Dr. Muka said that the largely American audience for his presentation was interested in the fact that the association existed independent of BMI, an obesity marker, based on questions and comments following the talk. “Indeed, we stratified the analysis, and we didn’t find any difference between participants who were obese and nonobese.” Also, he said that there were queries about whether the analyses had corrected for DEXA measurements, in order to assess lean versus fat body composition more accurately. This analysis has not been done, but Dr. Muka plans to complete it on his return to Rotterdam, he said.
Up to 10% of women will reach menopause before the age of 45, said Dr. Muka, so this analysis has the potential to impact primary care for millions of women. “Women who undergo early menopause may be a target for type 2 diabetes prevention measures and might need to be screened for other cardiovascular risk factors like high blood pressure and dyslipidemia, since they are also at risk for cardiovascular disease.”
Dr. Muka reported no outside funding sources and had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
“What we see in our data is that indeed, early onset of menopause is associated with increased risk of type 2 diabetes, and this association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also of estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, in an interview.
Dr. Muka, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands, presented the analysis from a large, prospective cohort of menopausal women at the annual meeting of the North American Menopause Society.
In an interview, Dr. Muka noted that the study investigated whether the association between early age at natural menopause (ANM) and type 2 diabetes is independent of intermediate risk factors for type 2 diabetes, such as obesity. Finally, the study also assessed whether endogenous sex hormone levels play a role in the link between early ANM and type 2 diabetes.
Enrollment in the Rotterdam study has been continuing in waves since the 1990s, with enrollment for participants in this particular study cohort occurring in 1997 and with additional cohorts enrolled in 2000-2001 and 2006-2008.
“I think this is the first prospective study with such long follow-up data and with a broad adjustment for confounding factors. Previous studies have been mainly cross-sectional, providing conflicting results,” said Dr. Muka.
Using self-reported age at menopause, the investigators excluded from the study women whose menopausal status was not known, who were actually not menopausal, or whose menopause had not occurred naturally. The study population also excluded women with prevalent type 2 diabetes or for whom no information about diabetes follow-up could be found.
The remaining 3,210 women who were included in the study had a median age of 67 years and had reached menopause at a median of 49.9 years. Most women (82.6%) had an ANM of 45-55 years; ANM for 8.8% was 40-44 years, while just 2.3% had an ANM of under 40 years. Mean body mass index was 27.1 kg/m2.
Participants were considered to have incident diabetes based on several sources: a general practitioner’s records, hospital discharge paperwork, or glucose measurements from visits during the Rotterdam study. Participants also were classified as having diabetes if they used a hypoglycemic medication or had a fasting blood glucose level of at least 7 mmol/L (126 mg/dL) or, in the absence of a fasting blood glucose measurement, a nonfasting blood glucose of at least 11.1 mmol/L (200 mg/dL).
Dr. Muka said he and his coinvestigators identified and adjusted for a large number of variables, using a series of three Cox proportion hazard models.
The first model adjusted for age, which wave of enrollment (cohort) participants were in, hormone therapy status, age at menarche, and the number of pregnancies that reached at least 6 months’ gestation.
The second model used all of the factors in model 1, and added BMI and glucose and insulin levels. The third model used all of the factors in model 2, and also added total cholesterol level, systolic blood pressure, the use of lipid-lowering or antihypertensive medications, alcohol and tobacco use, educational level, prevalent cardiovascular disease, and C-reactive protein levels.
The association of early ANM with the risk of type 2 diabetes was statistically significant in all three models (P less than .001), with very similar hazard ratios (HRs) in all models. For the third, the most comprehensive model, the HR was 1.42 (95% confidence interval, 0.83-2.45).
Extensive sensitivity analyses were carried out, and the association held independent of physical activity level, smoking status, use of hormone therapy, and age. The investigators also used multivariable analyses to ensure that the effect was not mediated by serum levels of thyroid-stimulating hormone, dihydroepiandosterone (DHEA) and DHEA sulfate, estradiol, testosterone, sex hormone–binding globulin (SHBG), or androstenedione.
This study was the first in this population to obtain and adjust for serum sex hormone and SHBG levels, said Dr. Muka.
The prospective design of the study and the long follow-up period were study strengths, said Dr. Muka. Additionally, blood glucose readings taken at study visits, together with electronically linked pharmacy dispensing records, were used for incident diabetes diagnoses.
Study limitations, Dr. Muka said, included the possibility of survival bias, since enrollees “may represent survivors of early menopause who did not develop [type 2 diabetes] or die prior to enrollment.” However, he said, this would mean that “we have underestimated the association, so the risk would be even higher.” Also, all study participants were white, so the results cannot be extrapolated to nonwhite populations.
Dr. Muka said that the largely American audience for his presentation was interested in the fact that the association existed independent of BMI, an obesity marker, based on questions and comments following the talk. “Indeed, we stratified the analysis, and we didn’t find any difference between participants who were obese and nonobese.” Also, he said that there were queries about whether the analyses had corrected for DEXA measurements, in order to assess lean versus fat body composition more accurately. This analysis has not been done, but Dr. Muka plans to complete it on his return to Rotterdam, he said.
Up to 10% of women will reach menopause before the age of 45, said Dr. Muka, so this analysis has the potential to impact primary care for millions of women. “Women who undergo early menopause may be a target for type 2 diabetes prevention measures and might need to be screened for other cardiovascular risk factors like high blood pressure and dyslipidemia, since they are also at risk for cardiovascular disease.”
Dr. Muka reported no outside funding sources and had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
“What we see in our data is that indeed, early onset of menopause is associated with increased risk of type 2 diabetes, and this association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also of estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, in an interview.
Dr. Muka, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands, presented the analysis from a large, prospective cohort of menopausal women at the annual meeting of the North American Menopause Society.
In an interview, Dr. Muka noted that the study investigated whether the association between early age at natural menopause (ANM) and type 2 diabetes is independent of intermediate risk factors for type 2 diabetes, such as obesity. Finally, the study also assessed whether endogenous sex hormone levels play a role in the link between early ANM and type 2 diabetes.
Enrollment in the Rotterdam study has been continuing in waves since the 1990s, with enrollment for participants in this particular study cohort occurring in 1997 and with additional cohorts enrolled in 2000-2001 and 2006-2008.
“I think this is the first prospective study with such long follow-up data and with a broad adjustment for confounding factors. Previous studies have been mainly cross-sectional, providing conflicting results,” said Dr. Muka.
Using self-reported age at menopause, the investigators excluded from the study women whose menopausal status was not known, who were actually not menopausal, or whose menopause had not occurred naturally. The study population also excluded women with prevalent type 2 diabetes or for whom no information about diabetes follow-up could be found.
The remaining 3,210 women who were included in the study had a median age of 67 years and had reached menopause at a median of 49.9 years. Most women (82.6%) had an ANM of 45-55 years; ANM for 8.8% was 40-44 years, while just 2.3% had an ANM of under 40 years. Mean body mass index was 27.1 kg/m2.
Participants were considered to have incident diabetes based on several sources: a general practitioner’s records, hospital discharge paperwork, or glucose measurements from visits during the Rotterdam study. Participants also were classified as having diabetes if they used a hypoglycemic medication or had a fasting blood glucose level of at least 7 mmol/L (126 mg/dL) or, in the absence of a fasting blood glucose measurement, a nonfasting blood glucose of at least 11.1 mmol/L (200 mg/dL).
Dr. Muka said he and his coinvestigators identified and adjusted for a large number of variables, using a series of three Cox proportion hazard models.
The first model adjusted for age, which wave of enrollment (cohort) participants were in, hormone therapy status, age at menarche, and the number of pregnancies that reached at least 6 months’ gestation.
The second model used all of the factors in model 1, and added BMI and glucose and insulin levels. The third model used all of the factors in model 2, and also added total cholesterol level, systolic blood pressure, the use of lipid-lowering or antihypertensive medications, alcohol and tobacco use, educational level, prevalent cardiovascular disease, and C-reactive protein levels.
The association of early ANM with the risk of type 2 diabetes was statistically significant in all three models (P less than .001), with very similar hazard ratios (HRs) in all models. For the third, the most comprehensive model, the HR was 1.42 (95% confidence interval, 0.83-2.45).
Extensive sensitivity analyses were carried out, and the association held independent of physical activity level, smoking status, use of hormone therapy, and age. The investigators also used multivariable analyses to ensure that the effect was not mediated by serum levels of thyroid-stimulating hormone, dihydroepiandosterone (DHEA) and DHEA sulfate, estradiol, testosterone, sex hormone–binding globulin (SHBG), or androstenedione.
This study was the first in this population to obtain and adjust for serum sex hormone and SHBG levels, said Dr. Muka.
The prospective design of the study and the long follow-up period were study strengths, said Dr. Muka. Additionally, blood glucose readings taken at study visits, together with electronically linked pharmacy dispensing records, were used for incident diabetes diagnoses.
Study limitations, Dr. Muka said, included the possibility of survival bias, since enrollees “may represent survivors of early menopause who did not develop [type 2 diabetes] or die prior to enrollment.” However, he said, this would mean that “we have underestimated the association, so the risk would be even higher.” Also, all study participants were white, so the results cannot be extrapolated to nonwhite populations.
Dr. Muka said that the largely American audience for his presentation was interested in the fact that the association existed independent of BMI, an obesity marker, based on questions and comments following the talk. “Indeed, we stratified the analysis, and we didn’t find any difference between participants who were obese and nonobese.” Also, he said that there were queries about whether the analyses had corrected for DEXA measurements, in order to assess lean versus fat body composition more accurately. This analysis has not been done, but Dr. Muka plans to complete it on his return to Rotterdam, he said.
Up to 10% of women will reach menopause before the age of 45, said Dr. Muka, so this analysis has the potential to impact primary care for millions of women. “Women who undergo early menopause may be a target for type 2 diabetes prevention measures and might need to be screened for other cardiovascular risk factors like high blood pressure and dyslipidemia, since they are also at risk for cardiovascular disease.”
Dr. Muka reported no outside funding sources and had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Key clinical point: Early menopause is associated with an increased risk for type 2 diabetes.
Major finding: Age at menopause between 40 and 45 was associated with a relative risk of 1.49 for type 2 diabetes.
Data source: A prospective cohort study of an initial cohort of 3,210 menopausal women.
Disclosures: No outside funding source was reported. Dr. Muka reported having no relevant financial conflicts.
Patients want surgery for ventral hernia despite risks and comorbidity obstacles
An in-depth qualitative survey of patients’ expectations and satisfaction regarding ventral hernia management showed that while most were satisfied with surgical outcomes, many were uninformed about postoperative adverse outcomes and many were dissatisfied with nonoperative management.
Zeinab M. Alawadi, MD, and a team of researchers at the University of Texas Health Sciences Center, Houston, initially interviewed 30 patients seeking care for ventral hernia at a safety-net hospital prior to their surgical consultation about factors guiding their decision-making framework. A second interview was conducted 6 months later, asking about their level of satisfaction with their care and outcomes. The study appeared online Oct. 11 in the Journal of the American College of Surgeons.
The initial interview revealed that most patients had limited knowledge about risks and potential adverse outcomes of surgery, but nearly three-quarters of them wanted to undergo surgery for their ventral hernias. Only 7 of the 30 patients were treated surgically and of those, 2 reported an unexpected level of postoperative pain and dissatisfaction with the surgery outcome. The remaining five patients who had surgery were extremely satisfied with their decision.
Most of those interviewed had nonoperative management of their hernias, due to factors such as obesity, diabetes, and smoking. These patients expressed dissatisfaction with the obstacles of meeting surgical criteria, in particular the difficulties of losing weight and coping with diabetes. “From patients’ perspectives, the additional challenges of managing their diabetes and difficulties with exercising due to painful hernias represent overwhelming barriers to treating their obesity. Patients’ accounts do not reflect a simple failure to adhere to medical recommendations but substantial obstacles to losing weight,” the researchers noted. But these patients also expressed willingness to try to meet surgical criteria and to take responsibility for recurrence prevention by self-management.
This study provides insight into patient perceptions and expectation of ventral hernia surgery. “Several findings in this study suggest a need for better education and counseling of patients regarding the natural history of hernias and the risks and benefits of different management strategies. Self-contradicting patient responses regarding knowledge of surgical risks and benefits may represent poor communication by the physicians or poor understanding by the patients. As a result, patients appeared to have unrealistic expectations of surgery. In addition, contrary to the literature, patients appeared to disregard the physicians’ risk assessment and persisted in their preferences for surgical management, even after counseling.”
This work was supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Sciences. The authors had no disclosures.
Read the complete study at goo.gl/pq4fjz.
An in-depth qualitative survey of patients’ expectations and satisfaction regarding ventral hernia management showed that while most were satisfied with surgical outcomes, many were uninformed about postoperative adverse outcomes and many were dissatisfied with nonoperative management.
Zeinab M. Alawadi, MD, and a team of researchers at the University of Texas Health Sciences Center, Houston, initially interviewed 30 patients seeking care for ventral hernia at a safety-net hospital prior to their surgical consultation about factors guiding their decision-making framework. A second interview was conducted 6 months later, asking about their level of satisfaction with their care and outcomes. The study appeared online Oct. 11 in the Journal of the American College of Surgeons.
The initial interview revealed that most patients had limited knowledge about risks and potential adverse outcomes of surgery, but nearly three-quarters of them wanted to undergo surgery for their ventral hernias. Only 7 of the 30 patients were treated surgically and of those, 2 reported an unexpected level of postoperative pain and dissatisfaction with the surgery outcome. The remaining five patients who had surgery were extremely satisfied with their decision.
Most of those interviewed had nonoperative management of their hernias, due to factors such as obesity, diabetes, and smoking. These patients expressed dissatisfaction with the obstacles of meeting surgical criteria, in particular the difficulties of losing weight and coping with diabetes. “From patients’ perspectives, the additional challenges of managing their diabetes and difficulties with exercising due to painful hernias represent overwhelming barriers to treating their obesity. Patients’ accounts do not reflect a simple failure to adhere to medical recommendations but substantial obstacles to losing weight,” the researchers noted. But these patients also expressed willingness to try to meet surgical criteria and to take responsibility for recurrence prevention by self-management.
This study provides insight into patient perceptions and expectation of ventral hernia surgery. “Several findings in this study suggest a need for better education and counseling of patients regarding the natural history of hernias and the risks and benefits of different management strategies. Self-contradicting patient responses regarding knowledge of surgical risks and benefits may represent poor communication by the physicians or poor understanding by the patients. As a result, patients appeared to have unrealistic expectations of surgery. In addition, contrary to the literature, patients appeared to disregard the physicians’ risk assessment and persisted in their preferences for surgical management, even after counseling.”
This work was supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Sciences. The authors had no disclosures.
Read the complete study at goo.gl/pq4fjz.
An in-depth qualitative survey of patients’ expectations and satisfaction regarding ventral hernia management showed that while most were satisfied with surgical outcomes, many were uninformed about postoperative adverse outcomes and many were dissatisfied with nonoperative management.
Zeinab M. Alawadi, MD, and a team of researchers at the University of Texas Health Sciences Center, Houston, initially interviewed 30 patients seeking care for ventral hernia at a safety-net hospital prior to their surgical consultation about factors guiding their decision-making framework. A second interview was conducted 6 months later, asking about their level of satisfaction with their care and outcomes. The study appeared online Oct. 11 in the Journal of the American College of Surgeons.
The initial interview revealed that most patients had limited knowledge about risks and potential adverse outcomes of surgery, but nearly three-quarters of them wanted to undergo surgery for their ventral hernias. Only 7 of the 30 patients were treated surgically and of those, 2 reported an unexpected level of postoperative pain and dissatisfaction with the surgery outcome. The remaining five patients who had surgery were extremely satisfied with their decision.
Most of those interviewed had nonoperative management of their hernias, due to factors such as obesity, diabetes, and smoking. These patients expressed dissatisfaction with the obstacles of meeting surgical criteria, in particular the difficulties of losing weight and coping with diabetes. “From patients’ perspectives, the additional challenges of managing their diabetes and difficulties with exercising due to painful hernias represent overwhelming barriers to treating their obesity. Patients’ accounts do not reflect a simple failure to adhere to medical recommendations but substantial obstacles to losing weight,” the researchers noted. But these patients also expressed willingness to try to meet surgical criteria and to take responsibility for recurrence prevention by self-management.
This study provides insight into patient perceptions and expectation of ventral hernia surgery. “Several findings in this study suggest a need for better education and counseling of patients regarding the natural history of hernias and the risks and benefits of different management strategies. Self-contradicting patient responses regarding knowledge of surgical risks and benefits may represent poor communication by the physicians or poor understanding by the patients. As a result, patients appeared to have unrealistic expectations of surgery. In addition, contrary to the literature, patients appeared to disregard the physicians’ risk assessment and persisted in their preferences for surgical management, even after counseling.”
This work was supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Sciences. The authors had no disclosures.
Read the complete study at goo.gl/pq4fjz.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS