User login
Update on informed consent
Question: Which of the following statements regarding the doctrine of informed consent is best?
A. All jurisdictions now require disclosure geared toward the reasonable patient rather than the reasonable doctor.
B. Disclosures are less important and sometimes unnecessary in global clinical research protocols.
C. The trend is toward the use of simplified and personalized consent forms.
D. A and C.
Answer: C. An important development in the doctrine of informed consent deals with the typical consent form, which is lengthy (going on 14 pages in one report), largely incomprehensible, and densely legalistic. Worse, the physician may misconstrue the form as a proxy for risk disclosure, whereas in fact the duly signed form merely purports to indicate that meaningful discussion of risks, benefits, and alternatives had taken place – if indeed that has been the case.
A review of more than 500 separate informed consent forms revealed these documents have limited educational value, go mostly unread, and are frequently misunderstood by patients (Arch. Surg. 2000;135:26-33).Thus, there is much to recommend the recent move toward shorter forms that use simpler language, bigger type fonts, and even graphics to improve patient and family understanding.
For example, a novel personalized approach currently being tested offers evidence-based benefits and risks to patients undergoing elective percutaneous coronary intervention. The consent document draws on the American College of Cardiology’s National Cardiovascular Data Registry to offer individualized risk estimates, and lists the experience of the health care team and the anticipated financial costs (JAMA 2010;303:1190-1).
Such approaches are consonant with the notion of patient-centered care, which the Institute of Medicine has identified as a core attribute of high-quality health care systems.
One of the continuing controversies in informed consent is the requisite standard of disclosure.
Historically, what needed to be disclosed was judged by what was ordinarily expected of the reasonable physician in the community. However, in 1972, Canterbury v. Spence (464 F.2d 772 [D.C. Cir. 1972]) persuasively argued for replacing such a professional standard (what doctors customarily would disclose) with a patient-oriented standard, i.e., what a reasonable patient would want to know.
California quickly followed, and an increasing number of jurisdictions such as Alaska, Hawaii, Massachusetts, Minnesota, Texas, West Virginia, and Wisconsin have since embraced this patient-centered standard. However, a number of other jurisdictions, including Arkansas, Indiana, Michigan, Montana, Nebraska, Nevada, and Wyoming, continue to adhere to the professional or physician-centered standard.
The momentum toward patient-centered disclosure appears to be growing, and has spread to other common-law jurisdictions abroad such as Canada and Australia. The United Kingdom was a steadfast opponent of patient-centric consent since Sidaway v. Board of Governors of the Bethlem Royal Hospital and the Maudsley Hospital ([1985] AC 871), its seminal House of Lords decision in the 1980s.
However, it finally abandoned its opposition on March 11, 2015, when its Supreme Court decided the case of a baby boy who sustained brain injury and arm paralysis at birth as a result of shoulder dystocia (Montgomery v. Lanarkshire Health Board [2015] UKSC 11).
The plaintiff alleged that being a diabetic mother, she should have been warned of the risks of shoulder dystocia and offered the alternative of a cesarean section. The obstetrician said she did not warn because the risks of a serious problem were very small. The court sided with the plaintiff, overturned Sidaway’s professional standard, and adopted the patient standard of disclosure as the new law.
What needs to be disclosed continues to plague the practitioner. The term “material risks” merely begs the question of the definition of “material.” It has been said that the crucial factor is the patient’s need.
The vexing issue surrounding the scope of risk disclosure is compounded by court decisions allowing inquiry outside the medical condition itself, such as a physician’s alcoholism or HIV status, and financial incentives amounting to a breach of fiduciary responsibility. However, courts have tended to reject requiring disclosure of practitioner experience or patient prognosis.
Novel issues continue to surface. For example, a recent Wisconsin Supreme Court case dealt with whether it was necessary for a physician to disclose the availability of a collateral test not directly linked to a patient’s diagnosis.
In Jandre v. Wis. Injured Patients & Families Comp. Fund (813 N.W.2d 627 [Wis. 2012]), a doctor diagnosed Bell’s palsy as the cause of a patient’s neurologic symptoms, because she heard no carotid bruits and the head CT scan was normal. Shortly thereafter, the patient developed a stroke. Although the doctor was found not negligent for the misdiagnosis, the plaintiff then proceeded on a lack of informed consent theory, asserting that the doctor should have advised him of the availability of a carotid ultrasound test to rule out a TIA.
In a momentous decision, the Wisconsin Supreme Court affirmed the lower court’s judgment in finding the physician liable for failing to disclose the availability of the ultrasound procedure.
Another area where informed consent is under renewed scrutiny is in research. Doctors in practice are increasingly participating as coinvestigators in industry-sponsored drug trials, and should be familiar with what is expected in the research context.
Augmented rules regarding informed consent govern all clinical research, with patient safety and free meaningful choice being paramount considerations. Federal and state regulations, implemented and monitored by institutional review boards, serve to enforce compliance and safeguard the safety of experimental subjects.
Still, as predicted nearly 50 years ago by Dr. Henry K. Beecher, “In any precise sense, statements regarding consent are meaningless unless one knows how fully the patient was informed of all risks, and if these are not known, that fact should also be made clear. A far more dependable safeguard than consent is the presence of a truly responsible investigator” (N. Engl. J. Med. 1966;274:1354-60).
Scandals in human research led by or in collaboration with U.S. investigators in foreign countries have been in the news. Global clinical trials are supposed to be carried out in compliance with the Nuremberg Code and the Declaration of Helsinki, but there may be occasional lapses or disregard for such international laws. A recent example is the trial using the antibiotic trovafloxacin, administered orally, versus FDA-approved parenteral ceftriaxone, during a meningitis outbreak in Nigeria (N. Engl. J. Med. 2009;360:2050-3).
Finally, the politics of abortion and first amendment rights have managed to insinuate themselves into the informed consent debate. Can states mandate disclosure in the name of securing meaningful informed consent, e.g., requiring physicians to provide information that might encourage a woman to reconsider her decision to have an abortion?
In 1992, the U.S. Supreme Court decided that this passes constitutional muster, because it did not place an undue burden on the woman. However, the Fourth Circuit Appellate Court recently ruled unconstitutional a North Carolina statute called Display of Real-Time View Requirement. The statute requires physicians to display ultrasound images of the unborn child to a mother contemplating an abortion, which the court ruled violated the First Amendment’s prohibition on state-compelled speech (N. Engl. J. Med. 2015;372:1285-7).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: Which of the following statements regarding the doctrine of informed consent is best?
A. All jurisdictions now require disclosure geared toward the reasonable patient rather than the reasonable doctor.
B. Disclosures are less important and sometimes unnecessary in global clinical research protocols.
C. The trend is toward the use of simplified and personalized consent forms.
D. A and C.
Answer: C. An important development in the doctrine of informed consent deals with the typical consent form, which is lengthy (going on 14 pages in one report), largely incomprehensible, and densely legalistic. Worse, the physician may misconstrue the form as a proxy for risk disclosure, whereas in fact the duly signed form merely purports to indicate that meaningful discussion of risks, benefits, and alternatives had taken place – if indeed that has been the case.
A review of more than 500 separate informed consent forms revealed these documents have limited educational value, go mostly unread, and are frequently misunderstood by patients (Arch. Surg. 2000;135:26-33).Thus, there is much to recommend the recent move toward shorter forms that use simpler language, bigger type fonts, and even graphics to improve patient and family understanding.
For example, a novel personalized approach currently being tested offers evidence-based benefits and risks to patients undergoing elective percutaneous coronary intervention. The consent document draws on the American College of Cardiology’s National Cardiovascular Data Registry to offer individualized risk estimates, and lists the experience of the health care team and the anticipated financial costs (JAMA 2010;303:1190-1).
Such approaches are consonant with the notion of patient-centered care, which the Institute of Medicine has identified as a core attribute of high-quality health care systems.
One of the continuing controversies in informed consent is the requisite standard of disclosure.
Historically, what needed to be disclosed was judged by what was ordinarily expected of the reasonable physician in the community. However, in 1972, Canterbury v. Spence (464 F.2d 772 [D.C. Cir. 1972]) persuasively argued for replacing such a professional standard (what doctors customarily would disclose) with a patient-oriented standard, i.e., what a reasonable patient would want to know.
California quickly followed, and an increasing number of jurisdictions such as Alaska, Hawaii, Massachusetts, Minnesota, Texas, West Virginia, and Wisconsin have since embraced this patient-centered standard. However, a number of other jurisdictions, including Arkansas, Indiana, Michigan, Montana, Nebraska, Nevada, and Wyoming, continue to adhere to the professional or physician-centered standard.
The momentum toward patient-centered disclosure appears to be growing, and has spread to other common-law jurisdictions abroad such as Canada and Australia. The United Kingdom was a steadfast opponent of patient-centric consent since Sidaway v. Board of Governors of the Bethlem Royal Hospital and the Maudsley Hospital ([1985] AC 871), its seminal House of Lords decision in the 1980s.
However, it finally abandoned its opposition on March 11, 2015, when its Supreme Court decided the case of a baby boy who sustained brain injury and arm paralysis at birth as a result of shoulder dystocia (Montgomery v. Lanarkshire Health Board [2015] UKSC 11).
The plaintiff alleged that being a diabetic mother, she should have been warned of the risks of shoulder dystocia and offered the alternative of a cesarean section. The obstetrician said she did not warn because the risks of a serious problem were very small. The court sided with the plaintiff, overturned Sidaway’s professional standard, and adopted the patient standard of disclosure as the new law.
What needs to be disclosed continues to plague the practitioner. The term “material risks” merely begs the question of the definition of “material.” It has been said that the crucial factor is the patient’s need.
The vexing issue surrounding the scope of risk disclosure is compounded by court decisions allowing inquiry outside the medical condition itself, such as a physician’s alcoholism or HIV status, and financial incentives amounting to a breach of fiduciary responsibility. However, courts have tended to reject requiring disclosure of practitioner experience or patient prognosis.
Novel issues continue to surface. For example, a recent Wisconsin Supreme Court case dealt with whether it was necessary for a physician to disclose the availability of a collateral test not directly linked to a patient’s diagnosis.
In Jandre v. Wis. Injured Patients & Families Comp. Fund (813 N.W.2d 627 [Wis. 2012]), a doctor diagnosed Bell’s palsy as the cause of a patient’s neurologic symptoms, because she heard no carotid bruits and the head CT scan was normal. Shortly thereafter, the patient developed a stroke. Although the doctor was found not negligent for the misdiagnosis, the plaintiff then proceeded on a lack of informed consent theory, asserting that the doctor should have advised him of the availability of a carotid ultrasound test to rule out a TIA.
In a momentous decision, the Wisconsin Supreme Court affirmed the lower court’s judgment in finding the physician liable for failing to disclose the availability of the ultrasound procedure.
Another area where informed consent is under renewed scrutiny is in research. Doctors in practice are increasingly participating as coinvestigators in industry-sponsored drug trials, and should be familiar with what is expected in the research context.
Augmented rules regarding informed consent govern all clinical research, with patient safety and free meaningful choice being paramount considerations. Federal and state regulations, implemented and monitored by institutional review boards, serve to enforce compliance and safeguard the safety of experimental subjects.
Still, as predicted nearly 50 years ago by Dr. Henry K. Beecher, “In any precise sense, statements regarding consent are meaningless unless one knows how fully the patient was informed of all risks, and if these are not known, that fact should also be made clear. A far more dependable safeguard than consent is the presence of a truly responsible investigator” (N. Engl. J. Med. 1966;274:1354-60).
Scandals in human research led by or in collaboration with U.S. investigators in foreign countries have been in the news. Global clinical trials are supposed to be carried out in compliance with the Nuremberg Code and the Declaration of Helsinki, but there may be occasional lapses or disregard for such international laws. A recent example is the trial using the antibiotic trovafloxacin, administered orally, versus FDA-approved parenteral ceftriaxone, during a meningitis outbreak in Nigeria (N. Engl. J. Med. 2009;360:2050-3).
Finally, the politics of abortion and first amendment rights have managed to insinuate themselves into the informed consent debate. Can states mandate disclosure in the name of securing meaningful informed consent, e.g., requiring physicians to provide information that might encourage a woman to reconsider her decision to have an abortion?
In 1992, the U.S. Supreme Court decided that this passes constitutional muster, because it did not place an undue burden on the woman. However, the Fourth Circuit Appellate Court recently ruled unconstitutional a North Carolina statute called Display of Real-Time View Requirement. The statute requires physicians to display ultrasound images of the unborn child to a mother contemplating an abortion, which the court ruled violated the First Amendment’s prohibition on state-compelled speech (N. Engl. J. Med. 2015;372:1285-7).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: Which of the following statements regarding the doctrine of informed consent is best?
A. All jurisdictions now require disclosure geared toward the reasonable patient rather than the reasonable doctor.
B. Disclosures are less important and sometimes unnecessary in global clinical research protocols.
C. The trend is toward the use of simplified and personalized consent forms.
D. A and C.
Answer: C. An important development in the doctrine of informed consent deals with the typical consent form, which is lengthy (going on 14 pages in one report), largely incomprehensible, and densely legalistic. Worse, the physician may misconstrue the form as a proxy for risk disclosure, whereas in fact the duly signed form merely purports to indicate that meaningful discussion of risks, benefits, and alternatives had taken place – if indeed that has been the case.
A review of more than 500 separate informed consent forms revealed these documents have limited educational value, go mostly unread, and are frequently misunderstood by patients (Arch. Surg. 2000;135:26-33).Thus, there is much to recommend the recent move toward shorter forms that use simpler language, bigger type fonts, and even graphics to improve patient and family understanding.
For example, a novel personalized approach currently being tested offers evidence-based benefits and risks to patients undergoing elective percutaneous coronary intervention. The consent document draws on the American College of Cardiology’s National Cardiovascular Data Registry to offer individualized risk estimates, and lists the experience of the health care team and the anticipated financial costs (JAMA 2010;303:1190-1).
Such approaches are consonant with the notion of patient-centered care, which the Institute of Medicine has identified as a core attribute of high-quality health care systems.
One of the continuing controversies in informed consent is the requisite standard of disclosure.
Historically, what needed to be disclosed was judged by what was ordinarily expected of the reasonable physician in the community. However, in 1972, Canterbury v. Spence (464 F.2d 772 [D.C. Cir. 1972]) persuasively argued for replacing such a professional standard (what doctors customarily would disclose) with a patient-oriented standard, i.e., what a reasonable patient would want to know.
California quickly followed, and an increasing number of jurisdictions such as Alaska, Hawaii, Massachusetts, Minnesota, Texas, West Virginia, and Wisconsin have since embraced this patient-centered standard. However, a number of other jurisdictions, including Arkansas, Indiana, Michigan, Montana, Nebraska, Nevada, and Wyoming, continue to adhere to the professional or physician-centered standard.
The momentum toward patient-centered disclosure appears to be growing, and has spread to other common-law jurisdictions abroad such as Canada and Australia. The United Kingdom was a steadfast opponent of patient-centric consent since Sidaway v. Board of Governors of the Bethlem Royal Hospital and the Maudsley Hospital ([1985] AC 871), its seminal House of Lords decision in the 1980s.
However, it finally abandoned its opposition on March 11, 2015, when its Supreme Court decided the case of a baby boy who sustained brain injury and arm paralysis at birth as a result of shoulder dystocia (Montgomery v. Lanarkshire Health Board [2015] UKSC 11).
The plaintiff alleged that being a diabetic mother, she should have been warned of the risks of shoulder dystocia and offered the alternative of a cesarean section. The obstetrician said she did not warn because the risks of a serious problem were very small. The court sided with the plaintiff, overturned Sidaway’s professional standard, and adopted the patient standard of disclosure as the new law.
What needs to be disclosed continues to plague the practitioner. The term “material risks” merely begs the question of the definition of “material.” It has been said that the crucial factor is the patient’s need.
The vexing issue surrounding the scope of risk disclosure is compounded by court decisions allowing inquiry outside the medical condition itself, such as a physician’s alcoholism or HIV status, and financial incentives amounting to a breach of fiduciary responsibility. However, courts have tended to reject requiring disclosure of practitioner experience or patient prognosis.
Novel issues continue to surface. For example, a recent Wisconsin Supreme Court case dealt with whether it was necessary for a physician to disclose the availability of a collateral test not directly linked to a patient’s diagnosis.
In Jandre v. Wis. Injured Patients & Families Comp. Fund (813 N.W.2d 627 [Wis. 2012]), a doctor diagnosed Bell’s palsy as the cause of a patient’s neurologic symptoms, because she heard no carotid bruits and the head CT scan was normal. Shortly thereafter, the patient developed a stroke. Although the doctor was found not negligent for the misdiagnosis, the plaintiff then proceeded on a lack of informed consent theory, asserting that the doctor should have advised him of the availability of a carotid ultrasound test to rule out a TIA.
In a momentous decision, the Wisconsin Supreme Court affirmed the lower court’s judgment in finding the physician liable for failing to disclose the availability of the ultrasound procedure.
Another area where informed consent is under renewed scrutiny is in research. Doctors in practice are increasingly participating as coinvestigators in industry-sponsored drug trials, and should be familiar with what is expected in the research context.
Augmented rules regarding informed consent govern all clinical research, with patient safety and free meaningful choice being paramount considerations. Federal and state regulations, implemented and monitored by institutional review boards, serve to enforce compliance and safeguard the safety of experimental subjects.
Still, as predicted nearly 50 years ago by Dr. Henry K. Beecher, “In any precise sense, statements regarding consent are meaningless unless one knows how fully the patient was informed of all risks, and if these are not known, that fact should also be made clear. A far more dependable safeguard than consent is the presence of a truly responsible investigator” (N. Engl. J. Med. 1966;274:1354-60).
Scandals in human research led by or in collaboration with U.S. investigators in foreign countries have been in the news. Global clinical trials are supposed to be carried out in compliance with the Nuremberg Code and the Declaration of Helsinki, but there may be occasional lapses or disregard for such international laws. A recent example is the trial using the antibiotic trovafloxacin, administered orally, versus FDA-approved parenteral ceftriaxone, during a meningitis outbreak in Nigeria (N. Engl. J. Med. 2009;360:2050-3).
Finally, the politics of abortion and first amendment rights have managed to insinuate themselves into the informed consent debate. Can states mandate disclosure in the name of securing meaningful informed consent, e.g., requiring physicians to provide information that might encourage a woman to reconsider her decision to have an abortion?
In 1992, the U.S. Supreme Court decided that this passes constitutional muster, because it did not place an undue burden on the woman. However, the Fourth Circuit Appellate Court recently ruled unconstitutional a North Carolina statute called Display of Real-Time View Requirement. The statute requires physicians to display ultrasound images of the unborn child to a mother contemplating an abortion, which the court ruled violated the First Amendment’s prohibition on state-compelled speech (N. Engl. J. Med. 2015;372:1285-7).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Witch hunts and hidden agendas
Witch hunt: An intensive effort to discover and expose disloyalty, subversion, dishonesty or the like, usually based on slight, doubtful or irrelevant evidence, and to punish accordingly. - Merriam-Webster Dictionary
Over the years, several excellent vascular surgeons have either lost their jobs or been pressured to resign for reasons that are unclear – although in some instances a few particular cases with bad outcomes have been cited as a reason.
Vascular Surgery is a difficult specialty. If one takes on challenging cases that need treatment – patients with large complex AAAs, extensive foot gangrene, or high-risk symptomatic carotid disease, an occasional poor outcome can be expected even if everything the vascular surgeon does is correct. One, two, or three bad outcomes per year in a busy vascular practice are inevitable, and should not be a reason for a vascular surgeon’s termination. Something more must be involved. Likely it is a hidden agenda.
That something can best be summarized by the term “medical politics.” This term may sound benign. In fact, medical politics are anything but benign. In the case of vascular surgeons, they are often subject to the governance and direction of administrators or senior surgical leaders who have little understanding of the risks of complex vascular surgery, and are interested only in the money generated by vascular admissions and procedures. Quantity and dollars trumps quality most times in the eyes of these administrative leaders.
Moreover, these leaders are not immune from the frailties of bias and jealousy which motivate much of human behavior. Throughout my career I have seen many instances when such administrative leaders resented a vascular surgeon because he or she used many hospital resources for a large practice, was successful academically, but behaved “too independently” from the larger department or its leader. It was one reason, vascular surgery needed to be its own independent specialty rather than a part of general surgery.
In the case of the vascular surgeons facing termination for reasons that they cannot understand, there is usually an administrative superior who is involved. Perhaps the vascular surgeon has questioned the authority or judgment of the superior on the grounds that they do not understand a particular vascular surgery issue. The resulting anger of the superior prompts him to want to eliminate the vascular surgeon who is under his administrative control. The witch hunt begins and may be inflamed by other unjustified motives such as jealousy or other hidden agendas which may never be known.
Once the witch hunt has begun, if the vascular surgeon has one or a few cases with bad outcomes, they make him or her vulnerable to the unjustified attack leading to termination by those who do not understand the nature of vascular surgery. Other factors, e.g., a disgruntled or jealous competitor in vascular surgery, can exacerbate the situation and accelerate the termination. The few bad-outcome cases, however, are the excuse or smoking gun which is used as the reason for termination. What goes unrecognized is the fact that every competent vascular surgeon who treats sick patients has such cases which can be used if uninformed enemies wish to focus on them.
How can a vascular surgeon protect him or herself from such witch hunts? First, they must be aware that the nature of vascular surgery and its requirement for treating challenging patients makes them vulnerable if one or another superior dislikes them or is “out to get them.” Second, if one is dealing with a case likely to have a bad outcome, it should be discussed beforehand with superiors, and it is wise to get help from a colleague who can share responsibility if things go poorly.
Third, try not to make enemies of important administrative leaders by challenging their authority or judgment.
Share successes with them, rather than taking all the glory. It will help to neutralize their jealousy. If possible, be humble. Make as many friends in other specialties as possible. They can sometimes diffuse the intensity of a witch hunt. And lastly be aware that any vascular surgeon, particularly a good one, can be the subject of a damaging witch hunt in the imperfect world in which we function.
Dr. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascsular Specialist do not necessarily reflect those of the Society or publisher.
Witch hunt: An intensive effort to discover and expose disloyalty, subversion, dishonesty or the like, usually based on slight, doubtful or irrelevant evidence, and to punish accordingly. - Merriam-Webster Dictionary
Over the years, several excellent vascular surgeons have either lost their jobs or been pressured to resign for reasons that are unclear – although in some instances a few particular cases with bad outcomes have been cited as a reason.
Vascular Surgery is a difficult specialty. If one takes on challenging cases that need treatment – patients with large complex AAAs, extensive foot gangrene, or high-risk symptomatic carotid disease, an occasional poor outcome can be expected even if everything the vascular surgeon does is correct. One, two, or three bad outcomes per year in a busy vascular practice are inevitable, and should not be a reason for a vascular surgeon’s termination. Something more must be involved. Likely it is a hidden agenda.
That something can best be summarized by the term “medical politics.” This term may sound benign. In fact, medical politics are anything but benign. In the case of vascular surgeons, they are often subject to the governance and direction of administrators or senior surgical leaders who have little understanding of the risks of complex vascular surgery, and are interested only in the money generated by vascular admissions and procedures. Quantity and dollars trumps quality most times in the eyes of these administrative leaders.
Moreover, these leaders are not immune from the frailties of bias and jealousy which motivate much of human behavior. Throughout my career I have seen many instances when such administrative leaders resented a vascular surgeon because he or she used many hospital resources for a large practice, was successful academically, but behaved “too independently” from the larger department or its leader. It was one reason, vascular surgery needed to be its own independent specialty rather than a part of general surgery.
In the case of the vascular surgeons facing termination for reasons that they cannot understand, there is usually an administrative superior who is involved. Perhaps the vascular surgeon has questioned the authority or judgment of the superior on the grounds that they do not understand a particular vascular surgery issue. The resulting anger of the superior prompts him to want to eliminate the vascular surgeon who is under his administrative control. The witch hunt begins and may be inflamed by other unjustified motives such as jealousy or other hidden agendas which may never be known.
Once the witch hunt has begun, if the vascular surgeon has one or a few cases with bad outcomes, they make him or her vulnerable to the unjustified attack leading to termination by those who do not understand the nature of vascular surgery. Other factors, e.g., a disgruntled or jealous competitor in vascular surgery, can exacerbate the situation and accelerate the termination. The few bad-outcome cases, however, are the excuse or smoking gun which is used as the reason for termination. What goes unrecognized is the fact that every competent vascular surgeon who treats sick patients has such cases which can be used if uninformed enemies wish to focus on them.
How can a vascular surgeon protect him or herself from such witch hunts? First, they must be aware that the nature of vascular surgery and its requirement for treating challenging patients makes them vulnerable if one or another superior dislikes them or is “out to get them.” Second, if one is dealing with a case likely to have a bad outcome, it should be discussed beforehand with superiors, and it is wise to get help from a colleague who can share responsibility if things go poorly.
Third, try not to make enemies of important administrative leaders by challenging their authority or judgment.
Share successes with them, rather than taking all the glory. It will help to neutralize their jealousy. If possible, be humble. Make as many friends in other specialties as possible. They can sometimes diffuse the intensity of a witch hunt. And lastly be aware that any vascular surgeon, particularly a good one, can be the subject of a damaging witch hunt in the imperfect world in which we function.
Dr. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascsular Specialist do not necessarily reflect those of the Society or publisher.
Witch hunt: An intensive effort to discover and expose disloyalty, subversion, dishonesty or the like, usually based on slight, doubtful or irrelevant evidence, and to punish accordingly. - Merriam-Webster Dictionary
Over the years, several excellent vascular surgeons have either lost their jobs or been pressured to resign for reasons that are unclear – although in some instances a few particular cases with bad outcomes have been cited as a reason.
Vascular Surgery is a difficult specialty. If one takes on challenging cases that need treatment – patients with large complex AAAs, extensive foot gangrene, or high-risk symptomatic carotid disease, an occasional poor outcome can be expected even if everything the vascular surgeon does is correct. One, two, or three bad outcomes per year in a busy vascular practice are inevitable, and should not be a reason for a vascular surgeon’s termination. Something more must be involved. Likely it is a hidden agenda.
That something can best be summarized by the term “medical politics.” This term may sound benign. In fact, medical politics are anything but benign. In the case of vascular surgeons, they are often subject to the governance and direction of administrators or senior surgical leaders who have little understanding of the risks of complex vascular surgery, and are interested only in the money generated by vascular admissions and procedures. Quantity and dollars trumps quality most times in the eyes of these administrative leaders.
Moreover, these leaders are not immune from the frailties of bias and jealousy which motivate much of human behavior. Throughout my career I have seen many instances when such administrative leaders resented a vascular surgeon because he or she used many hospital resources for a large practice, was successful academically, but behaved “too independently” from the larger department or its leader. It was one reason, vascular surgery needed to be its own independent specialty rather than a part of general surgery.
In the case of the vascular surgeons facing termination for reasons that they cannot understand, there is usually an administrative superior who is involved. Perhaps the vascular surgeon has questioned the authority or judgment of the superior on the grounds that they do not understand a particular vascular surgery issue. The resulting anger of the superior prompts him to want to eliminate the vascular surgeon who is under his administrative control. The witch hunt begins and may be inflamed by other unjustified motives such as jealousy or other hidden agendas which may never be known.
Once the witch hunt has begun, if the vascular surgeon has one or a few cases with bad outcomes, they make him or her vulnerable to the unjustified attack leading to termination by those who do not understand the nature of vascular surgery. Other factors, e.g., a disgruntled or jealous competitor in vascular surgery, can exacerbate the situation and accelerate the termination. The few bad-outcome cases, however, are the excuse or smoking gun which is used as the reason for termination. What goes unrecognized is the fact that every competent vascular surgeon who treats sick patients has such cases which can be used if uninformed enemies wish to focus on them.
How can a vascular surgeon protect him or herself from such witch hunts? First, they must be aware that the nature of vascular surgery and its requirement for treating challenging patients makes them vulnerable if one or another superior dislikes them or is “out to get them.” Second, if one is dealing with a case likely to have a bad outcome, it should be discussed beforehand with superiors, and it is wise to get help from a colleague who can share responsibility if things go poorly.
Third, try not to make enemies of important administrative leaders by challenging their authority or judgment.
Share successes with them, rather than taking all the glory. It will help to neutralize their jealousy. If possible, be humble. Make as many friends in other specialties as possible. They can sometimes diffuse the intensity of a witch hunt. And lastly be aware that any vascular surgeon, particularly a good one, can be the subject of a damaging witch hunt in the imperfect world in which we function.
Dr. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascsular Specialist do not necessarily reflect those of the Society or publisher.
Familial hypercholesterolemia: Clues to catching it early
› When one member of a family has early heart disease, screen the entire family for familial hypercholesterolemia (FH). A
› Consider all patients with FH as being at high risk for coronary heart disease, regardless of their Framingham Risk Score. C
› Treat FH patients with statins early to avoid cardiovascular events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Familial hypercholesterolemia (FH) poses a “silent” threat to patients with the condition, putting them at great risk of a coronary event. This genetic disorder, in which one or more mutations cause extremely high low-density lipoprotein (LDL) cholesterol levels, goes undiagnosed in approximately 80% of patients who have it.1 As a result, men with FH have a >50% risk of coronary heart disease (CHD) by age 50 and women with FH have a 30% risk of CHD by age 60.2 Patients with FH face a much higher risk of dying from a coronary event than those in the general population.3 For example, women between the ages of 20 and 39 who have this disorder are 125 times more likely to die of a coronary event than those who don’t.3
Unfortunately, FH can be difficult to diagnose. Some patients have physical findings, but these features can be subtle and easily missed. Typically, however, FH is diagnosed based on a patient’s cholesterol level and family history. By implementing screening and early treatment for FH, you may be able to initiate treatment that can temper the development of atherosclerosis and possibly extend a patient’s life.4
Two forms of the disorder, although one is more common
There are 2 types of FH:
Heterozygous FH (HeFH) occurs in about 1 in 300 to 500 people, which makes it more common than Down syndrome.5 More than a half a million people in the United States have HeFH.6
Homozygous FH (HoFH) is more serious than HeFH, and less common, affecting one in 1 million people. Homozygous carriers suffer from CHD much earlier than those with HeFH; some die within the first few years of life.7
Regardless of whether an affected individual inherited FH from one or both parents, more than one thousand mutations are known to cause inadequate clearance of LDL from the bloodstream.8 One of the most common mutations is a defective LDL receptor gene. Other abnormalities are known to occur with the proprotein convertase subtilisin/kexin type 9 (PCSK9) and apolipoprotein B genes.9
Start with screening
Suspect FH in patients who have a family history of premature heart disease. Also consider the patient’s ethnic background. The prevalence of FH is as high as one in 100 among certain groups, including French Canadians, Christian Lebanese, and 3 populations in South Africa (Ashkenazi Jews, Dutch Afrikaners, and Asian Indians).10
When there is high suspicion of FH based on a patient’s family history or ethnicity, additional screening is warranted for any patient older than age 2.11 If a patient’s family history is incomplete (eg, adoption, single-parent family), a lower threshold for screening is appropriate.
Lipid screening includes measuring serum total, LDL, and high-density lipoprotein cholesterol in either fasting or non-fasting samples. The United States Preventive Services Task Force (USPSTF) offers gender-specific recommendations for lipid disorder screening in the general population. For men, universal screening is recommended starting at age 35, and screening for those at increased risk of CHD should start at age 20.12
For women, the USPSTF recommends lipid screening only for those over age 20 who are at increased risk for CHD; such screening is strongly recommended for high-risk women ages 45 and older. In light of the serious consequences associated with FH, the National Lipid Association recommends lipid screening for all adults starting at age 20 (TABLE 1).13
What about kids? The recommendations for lipid screening in children and adolescents are mixed. Both the USPSTF and the American Academy of Family Physicians indicate that there is insufficient evidence to screen for lipid disorders in asymptomatic children and adolescents.14,15 However, in a set of recommendations based on expert opinion, the National Heart, Lung, and Blood Institute (NHLBI) suggests universal screening for younger patients with a non-fasting lipid profile once between ages 9 to 11 and again between ages 17 to 21.16 The American Academy of Pediatrics has adopted the NHLBI recommendations.17
Physical exam findings that suggest familial hypercholesterolemia
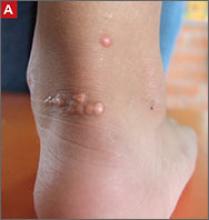
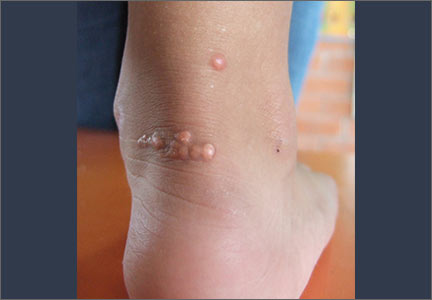
Tendon xanthomas (A), a thickening of the soft tissue as a result of infiltration by lipid-rich cells, most commonly occur at the Achilles and metacarpal tendons, but also can be seen at the patellar and triceps tendons.
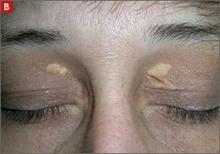
Tuberous xanthomas or xanthelasmas (B) are waxy-appearing growths that appear to be pasted on the skin in areas around the face, commonly the eyelids.
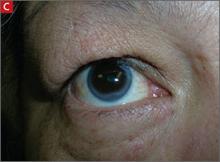
Arcus corneae (C) is an opaque ring around the outer edge of the cornea.
Use validated criteria to make the diagnosis
Include FH in your differential diagnosis when evaluating patients with very high LDL levels. However, rule out possible secondary causes of elevated LDL before rendering a conclusion. Hypothyroidism, nephrotic syndrome, diabetes, and liver disease are among the most common secondary causes of high LDL cholesterol.13
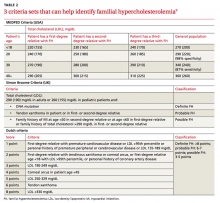
Several validated criteria sets can be used to establish an FH diagnosis. No single criteria set is more valid or more widely adopted around the world. All 3 of the most commonly used criteria sets take into account family history and a patient’s LDL level, and 2 of the 3 factor in physical findings (TABLE 2).9
Physical exam findings that suggest FH can be subtle (FIGURE). Tendon xanthomas are a thickening of the soft tissue as a result of infiltration by lipid-rich cells. They most commonly occur at the Achilles and metacarpal tendons, but can also be seen at the patellar and triceps tendons. Xanthomas may not be readily visible, so it’s important to run your fingers over these areas to detect nodularity or thickening. While the presence of a tendon xanthoma makes FH highly likely, they are present in less than half of patients with FH.17
Tuberous xanthomas or xanthelasmas are waxy-appearing growths that may look yellow or orange and appear to be pasted on the skin in areas around the face, commonly the eyelids. The presence of xanthelasmas in a patient younger than age 25 suggests FH.
Finally, arcus corneae is an opaque ring around the outer edge of the cornea. When this is seen in patients younger than age 45, it’s suggestive of FH.13 If you note tendon xanthomas, xanthelasmas, or arcus corneae while examining any of your patients, be sure to order an LDL level if it hasn’t already been done.
Is genetic testing necessary?
The only way to make a definitive diagnosis of FH is to find a mutation in a gene known to affect LDL metabolism. However, because genetic testing is expensive—and because more than one thousand different genetic defects can contribute to FH—it’s not practical to test every patient. Furthermore, since an estimated 20% of the mutations that contribute to FH have not yet been clearly delineated, a “normal” result on a genetic test might be misleading.5 Therefore, the diagnosis of FH usually is a clinical one. After clinically diagnosing a patient with FH, it’s imperative to screen first-degree family members by measuring their LDL cholesterol levels.
Lifestyle changes, statins can ward off CHD
Lifestyle modifications (ie, improved diet and exercise) and statins are the treatments of choice for patients with FH. Before starting pharmacotherapy, patients should undergo 3 months of lifestyle modification to assess how well this approach improves their lipid levels, assuming the patient doesn’t have additional risk factors such as hypertension or tobacco use, in which case he or she might require immediate pharmacotherapy. Statins can be initiated simultaneously with lifestyle choices in patients with an LDL >190 mg/dL.18
Lifestyle modification. Although FH is a genetic problem, patients should be encouraged to make healthy choices regarding diet and exercise. While the best choices may not get FH patients to their LDL goal, better choices may mean that patients can take fewer medications, or lower doses of them. Healthy lifestyle choices can also have other positive effects on cardiovascular risk (eg, lowering blood pressure).
Patients can’t be expected to navigate their food choices alone, and several visits with a dietician will likely be needed. It’s important to emphasize the family influence on diet and get the entire family involved with making healthy food choices.
In addition to addressing diet and exercise, be sure to encourage patients to abstain from tobacco and manage stress as part of their overall effort to reduce the likelihood of a cardiovascular event.
Statins. Early treatment of FH with statins can delay initial coronary events and prolong life.19 In a 12.5-year study of 2146 patients with FH, approximately 80% of patients treated with statins survived without experiencing CHD, compared to slightly less than 40% of those who were not treated with statins.19 Patients treated with statins had a 76% reduction in risk of CHD compared to those who didn’t receive statins.19 Even low doses of statins started early have been shown to help avoid myocardial infarction in adults with FH.20
The goal of treatment for FH is to reduced LDL levels by 50%.21 In pediatric patients, treating to an LDL level of 130 mg/dL is an alternative goal.21 Because it’s challenging to achieve this goal with improved diet and exercise alone, treatment with a statin is often necessary.22
Statins can be used in children as young as age 8, or even earlier in homozygous FH.6 While a physician might be hesitant to start a chronic medication in a young patient, research shows that earlier intervention results in additional years of life.23 To date, no significant adverse effects of statins in pediatric patients have been identified, and statins have not been shown to impair growth.24,25 Young female patients should be counseled about the adverse effects statins can have on a fetus if the patient becomes pregnant while taking the medication.
Navigating the waters of statin treatment
Musculoskeletal symptoms are the most common adverse effect reported by patients taking statins. A thorough assessment of a patient’s muscle complaints is necessary to avoid prematurely concluding that he or she cannot tolerate statins.
A study in which “statin-intolerant” patients were re-challenged found that more than 90% of patients could tolerate statins through the course of the one-year study and that it was likely that the patients’ initial muscle complaints were not due to statin use.23 (To read more about potential adverse events of statins, see “Statin adverse effects: Sorting out the evidence,” J Fam Pract. 2014;63:497-506.).
If LDL levels in a patient with HeFH remain at or above 160 mg/dL, intensifying treatment by adding another lipid-lowering medication might be warranted.22 For patients with HoFH, in whom the condition is more quickly life-threatening, there are additional choices, including LDL apheresis and medications such as mipomersen and lomitapide. Both of these medications can cause hepatotoxicity, and are available only through a Risk Evaluation and Mitigation Strategy program, which means they can only be prescribed by certified physicians. PCSK9 inhibitors are in the pipeline and may one day help patients with HoFH by addressing one of the genetic causes of this disorder.
CORRESPONDENCE
Richard Safeer, MD, 6704 Curtis Court, Glen Burnie, MD 21060; [email protected]
1. Datta BN, McDowell IF, Rees A. Integrating provision of specialist lipid services with cascade testing for familial hypercholesterolaemia. Curr Opin Lipidol. 2010;21:366-371.
2. DeMott K, Nherera L, Shaw EJ, et al. Clinical Guidelines and Evidence Review for Familial Hypercholesterolaemia: The Identification and Management of Adults and Children with Familial Hypercholesterolaemia. 2008. London, UK: National Collaborating Centre for Primary Care and Royal College of General Practitioners.
3. Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis. 1999;142:105-112.
4. Kavey RE, Allada V, Daniels SR, et al. American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention. Cardiovascular risk reduction in high-risk pediatric patients. Circulation. 2006;114:2710-2738.
5. Rees A. Familial hypercholesterolaemia: underdiagnosed and undertreated. Eur Heart J. 2008;29:2583-2584.
6. Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients. Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1-8.
7. Moriarty PM. LDL-apheresis therapy. Curr Treat Options Cardiovasc Med. 2006;8:282-288.
8. Goldstein JL, Brown MS. The LDL receptor locus and the genetics of familial hypercholesterolemia. Annu Rev Genet. 1979;13:259-289.
9. Fahed AC, Nemer GM. Familial hypercholesterolemia: the lipids or the genes? Nutr Metab (Lond). 2011;8:23.
10. Goldstein J, Hobbs H, Brown M. Familial hypercholesterolemia. In: Scriver C, Baudet A, Sly W, et al, eds. The Metabolic Basis of Inherited Disease. New York, NY: McGraw-Hill; 2001: 2863-2913.
11. Kwiterovich, PO. Clinical and laboratory assessment of cardiovascular risk in children: Guidelines for screening, evaluation and treatment. J Clin Lipidol. 2008;2:248-266.
12. US Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia): Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/lipid-disorders-in-adults-cholesterol-dyslipidemia-screening. Accessed July 6, 2015.
13. Hopkins PN, Toth PP, Ballantyne CM, et al; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S9-17.
14. US Preventive Services Task Force. Screening for lipid disorders in children: US Preventive Services Task Force recommendation statement. Pediatrics. 2007;120;e215-219.
15. American Academy of Family Physicians. Summary of recommendations for clinical preventive services. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/cps-recommendations.pdf. Accessed May 2, 2015.
16. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128:S213-56.
17. American Academy of Pediatrics. 2014 Recommendations for Pediatric Preventive Health Care. American Academy of Pediatrics Web site. Available at: http://pediatrics.aappublications.org/content/133/3/568.full.pdf+html. Accessed May 2, 2015.
18. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
19. Nordestgaard BG, Chapman MJ, Humphries SE, et al; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478-3490a.
20. Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423.
21. Daniels SR, Gidding SS, de Ferranti SD; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Pediatric aspects of familial hypercholesterolemias: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S30-37.
22. Robinson JG, Goldberg AC; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S18-29.
23. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
24. Eiland LS, Luttrell PK. Use of statins for dyslipidemia in the pediatric population. J Pediatr Pharmacol Ther. 2010;15:160-172.
25. O’Gorman CS, Higgins MF, O’Neill MB. Systematic review and metaanalysis of statins for heterozygous familial hypercholesterolemia in children: evaluation of cholesterol changes and side effects. Pediatr Cardiol. 2009;30:482-489.
› When one member of a family has early heart disease, screen the entire family for familial hypercholesterolemia (FH). A
› Consider all patients with FH as being at high risk for coronary heart disease, regardless of their Framingham Risk Score. C
› Treat FH patients with statins early to avoid cardiovascular events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Familial hypercholesterolemia (FH) poses a “silent” threat to patients with the condition, putting them at great risk of a coronary event. This genetic disorder, in which one or more mutations cause extremely high low-density lipoprotein (LDL) cholesterol levels, goes undiagnosed in approximately 80% of patients who have it.1 As a result, men with FH have a >50% risk of coronary heart disease (CHD) by age 50 and women with FH have a 30% risk of CHD by age 60.2 Patients with FH face a much higher risk of dying from a coronary event than those in the general population.3 For example, women between the ages of 20 and 39 who have this disorder are 125 times more likely to die of a coronary event than those who don’t.3
Unfortunately, FH can be difficult to diagnose. Some patients have physical findings, but these features can be subtle and easily missed. Typically, however, FH is diagnosed based on a patient’s cholesterol level and family history. By implementing screening and early treatment for FH, you may be able to initiate treatment that can temper the development of atherosclerosis and possibly extend a patient’s life.4
Two forms of the disorder, although one is more common
There are 2 types of FH:
Heterozygous FH (HeFH) occurs in about 1 in 300 to 500 people, which makes it more common than Down syndrome.5 More than a half a million people in the United States have HeFH.6
Homozygous FH (HoFH) is more serious than HeFH, and less common, affecting one in 1 million people. Homozygous carriers suffer from CHD much earlier than those with HeFH; some die within the first few years of life.7
Regardless of whether an affected individual inherited FH from one or both parents, more than one thousand mutations are known to cause inadequate clearance of LDL from the bloodstream.8 One of the most common mutations is a defective LDL receptor gene. Other abnormalities are known to occur with the proprotein convertase subtilisin/kexin type 9 (PCSK9) and apolipoprotein B genes.9
Start with screening
Suspect FH in patients who have a family history of premature heart disease. Also consider the patient’s ethnic background. The prevalence of FH is as high as one in 100 among certain groups, including French Canadians, Christian Lebanese, and 3 populations in South Africa (Ashkenazi Jews, Dutch Afrikaners, and Asian Indians).10
When there is high suspicion of FH based on a patient’s family history or ethnicity, additional screening is warranted for any patient older than age 2.11 If a patient’s family history is incomplete (eg, adoption, single-parent family), a lower threshold for screening is appropriate.
Lipid screening includes measuring serum total, LDL, and high-density lipoprotein cholesterol in either fasting or non-fasting samples. The United States Preventive Services Task Force (USPSTF) offers gender-specific recommendations for lipid disorder screening in the general population. For men, universal screening is recommended starting at age 35, and screening for those at increased risk of CHD should start at age 20.12
For women, the USPSTF recommends lipid screening only for those over age 20 who are at increased risk for CHD; such screening is strongly recommended for high-risk women ages 45 and older. In light of the serious consequences associated with FH, the National Lipid Association recommends lipid screening for all adults starting at age 20 (TABLE 1).13
What about kids? The recommendations for lipid screening in children and adolescents are mixed. Both the USPSTF and the American Academy of Family Physicians indicate that there is insufficient evidence to screen for lipid disorders in asymptomatic children and adolescents.14,15 However, in a set of recommendations based on expert opinion, the National Heart, Lung, and Blood Institute (NHLBI) suggests universal screening for younger patients with a non-fasting lipid profile once between ages 9 to 11 and again between ages 17 to 21.16 The American Academy of Pediatrics has adopted the NHLBI recommendations.17
Physical exam findings that suggest familial hypercholesterolemia
Tendon xanthomas (A), a thickening of the soft tissue as a result of infiltration by lipid-rich cells, most commonly occur at the Achilles and metacarpal tendons, but also can be seen at the patellar and triceps tendons.
Tuberous xanthomas or xanthelasmas (B) are waxy-appearing growths that appear to be pasted on the skin in areas around the face, commonly the eyelids.
Arcus corneae (C) is an opaque ring around the outer edge of the cornea.
Use validated criteria to make the diagnosis
Include FH in your differential diagnosis when evaluating patients with very high LDL levels. However, rule out possible secondary causes of elevated LDL before rendering a conclusion. Hypothyroidism, nephrotic syndrome, diabetes, and liver disease are among the most common secondary causes of high LDL cholesterol.13
Several validated criteria sets can be used to establish an FH diagnosis. No single criteria set is more valid or more widely adopted around the world. All 3 of the most commonly used criteria sets take into account family history and a patient’s LDL level, and 2 of the 3 factor in physical findings (TABLE 2).9
Physical exam findings that suggest FH can be subtle (FIGURE). Tendon xanthomas are a thickening of the soft tissue as a result of infiltration by lipid-rich cells. They most commonly occur at the Achilles and metacarpal tendons, but can also be seen at the patellar and triceps tendons. Xanthomas may not be readily visible, so it’s important to run your fingers over these areas to detect nodularity or thickening. While the presence of a tendon xanthoma makes FH highly likely, they are present in less than half of patients with FH.17
Tuberous xanthomas or xanthelasmas are waxy-appearing growths that may look yellow or orange and appear to be pasted on the skin in areas around the face, commonly the eyelids. The presence of xanthelasmas in a patient younger than age 25 suggests FH.
Finally, arcus corneae is an opaque ring around the outer edge of the cornea. When this is seen in patients younger than age 45, it’s suggestive of FH.13 If you note tendon xanthomas, xanthelasmas, or arcus corneae while examining any of your patients, be sure to order an LDL level if it hasn’t already been done.
Is genetic testing necessary?
The only way to make a definitive diagnosis of FH is to find a mutation in a gene known to affect LDL metabolism. However, because genetic testing is expensive—and because more than one thousand different genetic defects can contribute to FH—it’s not practical to test every patient. Furthermore, since an estimated 20% of the mutations that contribute to FH have not yet been clearly delineated, a “normal” result on a genetic test might be misleading.5 Therefore, the diagnosis of FH usually is a clinical one. After clinically diagnosing a patient with FH, it’s imperative to screen first-degree family members by measuring their LDL cholesterol levels.
Lifestyle changes, statins can ward off CHD
Lifestyle modifications (ie, improved diet and exercise) and statins are the treatments of choice for patients with FH. Before starting pharmacotherapy, patients should undergo 3 months of lifestyle modification to assess how well this approach improves their lipid levels, assuming the patient doesn’t have additional risk factors such as hypertension or tobacco use, in which case he or she might require immediate pharmacotherapy. Statins can be initiated simultaneously with lifestyle choices in patients with an LDL >190 mg/dL.18
Lifestyle modification. Although FH is a genetic problem, patients should be encouraged to make healthy choices regarding diet and exercise. While the best choices may not get FH patients to their LDL goal, better choices may mean that patients can take fewer medications, or lower doses of them. Healthy lifestyle choices can also have other positive effects on cardiovascular risk (eg, lowering blood pressure).
Patients can’t be expected to navigate their food choices alone, and several visits with a dietician will likely be needed. It’s important to emphasize the family influence on diet and get the entire family involved with making healthy food choices.
In addition to addressing diet and exercise, be sure to encourage patients to abstain from tobacco and manage stress as part of their overall effort to reduce the likelihood of a cardiovascular event.
Statins. Early treatment of FH with statins can delay initial coronary events and prolong life.19 In a 12.5-year study of 2146 patients with FH, approximately 80% of patients treated with statins survived without experiencing CHD, compared to slightly less than 40% of those who were not treated with statins.19 Patients treated with statins had a 76% reduction in risk of CHD compared to those who didn’t receive statins.19 Even low doses of statins started early have been shown to help avoid myocardial infarction in adults with FH.20
The goal of treatment for FH is to reduced LDL levels by 50%.21 In pediatric patients, treating to an LDL level of 130 mg/dL is an alternative goal.21 Because it’s challenging to achieve this goal with improved diet and exercise alone, treatment with a statin is often necessary.22
Statins can be used in children as young as age 8, or even earlier in homozygous FH.6 While a physician might be hesitant to start a chronic medication in a young patient, research shows that earlier intervention results in additional years of life.23 To date, no significant adverse effects of statins in pediatric patients have been identified, and statins have not been shown to impair growth.24,25 Young female patients should be counseled about the adverse effects statins can have on a fetus if the patient becomes pregnant while taking the medication.
Navigating the waters of statin treatment
Musculoskeletal symptoms are the most common adverse effect reported by patients taking statins. A thorough assessment of a patient’s muscle complaints is necessary to avoid prematurely concluding that he or she cannot tolerate statins.
A study in which “statin-intolerant” patients were re-challenged found that more than 90% of patients could tolerate statins through the course of the one-year study and that it was likely that the patients’ initial muscle complaints were not due to statin use.23 (To read more about potential adverse events of statins, see “Statin adverse effects: Sorting out the evidence,” J Fam Pract. 2014;63:497-506.).
If LDL levels in a patient with HeFH remain at or above 160 mg/dL, intensifying treatment by adding another lipid-lowering medication might be warranted.22 For patients with HoFH, in whom the condition is more quickly life-threatening, there are additional choices, including LDL apheresis and medications such as mipomersen and lomitapide. Both of these medications can cause hepatotoxicity, and are available only through a Risk Evaluation and Mitigation Strategy program, which means they can only be prescribed by certified physicians. PCSK9 inhibitors are in the pipeline and may one day help patients with HoFH by addressing one of the genetic causes of this disorder.
CORRESPONDENCE
Richard Safeer, MD, 6704 Curtis Court, Glen Burnie, MD 21060; [email protected]
› When one member of a family has early heart disease, screen the entire family for familial hypercholesterolemia (FH). A
› Consider all patients with FH as being at high risk for coronary heart disease, regardless of their Framingham Risk Score. C
› Treat FH patients with statins early to avoid cardiovascular events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Familial hypercholesterolemia (FH) poses a “silent” threat to patients with the condition, putting them at great risk of a coronary event. This genetic disorder, in which one or more mutations cause extremely high low-density lipoprotein (LDL) cholesterol levels, goes undiagnosed in approximately 80% of patients who have it.1 As a result, men with FH have a >50% risk of coronary heart disease (CHD) by age 50 and women with FH have a 30% risk of CHD by age 60.2 Patients with FH face a much higher risk of dying from a coronary event than those in the general population.3 For example, women between the ages of 20 and 39 who have this disorder are 125 times more likely to die of a coronary event than those who don’t.3
Unfortunately, FH can be difficult to diagnose. Some patients have physical findings, but these features can be subtle and easily missed. Typically, however, FH is diagnosed based on a patient’s cholesterol level and family history. By implementing screening and early treatment for FH, you may be able to initiate treatment that can temper the development of atherosclerosis and possibly extend a patient’s life.4
Two forms of the disorder, although one is more common
There are 2 types of FH:
Heterozygous FH (HeFH) occurs in about 1 in 300 to 500 people, which makes it more common than Down syndrome.5 More than a half a million people in the United States have HeFH.6
Homozygous FH (HoFH) is more serious than HeFH, and less common, affecting one in 1 million people. Homozygous carriers suffer from CHD much earlier than those with HeFH; some die within the first few years of life.7
Regardless of whether an affected individual inherited FH from one or both parents, more than one thousand mutations are known to cause inadequate clearance of LDL from the bloodstream.8 One of the most common mutations is a defective LDL receptor gene. Other abnormalities are known to occur with the proprotein convertase subtilisin/kexin type 9 (PCSK9) and apolipoprotein B genes.9
Start with screening
Suspect FH in patients who have a family history of premature heart disease. Also consider the patient’s ethnic background. The prevalence of FH is as high as one in 100 among certain groups, including French Canadians, Christian Lebanese, and 3 populations in South Africa (Ashkenazi Jews, Dutch Afrikaners, and Asian Indians).10
When there is high suspicion of FH based on a patient’s family history or ethnicity, additional screening is warranted for any patient older than age 2.11 If a patient’s family history is incomplete (eg, adoption, single-parent family), a lower threshold for screening is appropriate.
Lipid screening includes measuring serum total, LDL, and high-density lipoprotein cholesterol in either fasting or non-fasting samples. The United States Preventive Services Task Force (USPSTF) offers gender-specific recommendations for lipid disorder screening in the general population. For men, universal screening is recommended starting at age 35, and screening for those at increased risk of CHD should start at age 20.12
For women, the USPSTF recommends lipid screening only for those over age 20 who are at increased risk for CHD; such screening is strongly recommended for high-risk women ages 45 and older. In light of the serious consequences associated with FH, the National Lipid Association recommends lipid screening for all adults starting at age 20 (TABLE 1).13
What about kids? The recommendations for lipid screening in children and adolescents are mixed. Both the USPSTF and the American Academy of Family Physicians indicate that there is insufficient evidence to screen for lipid disorders in asymptomatic children and adolescents.14,15 However, in a set of recommendations based on expert opinion, the National Heart, Lung, and Blood Institute (NHLBI) suggests universal screening for younger patients with a non-fasting lipid profile once between ages 9 to 11 and again between ages 17 to 21.16 The American Academy of Pediatrics has adopted the NHLBI recommendations.17
Physical exam findings that suggest familial hypercholesterolemia
Tendon xanthomas (A), a thickening of the soft tissue as a result of infiltration by lipid-rich cells, most commonly occur at the Achilles and metacarpal tendons, but also can be seen at the patellar and triceps tendons.
Tuberous xanthomas or xanthelasmas (B) are waxy-appearing growths that appear to be pasted on the skin in areas around the face, commonly the eyelids.
Arcus corneae (C) is an opaque ring around the outer edge of the cornea.
Use validated criteria to make the diagnosis
Include FH in your differential diagnosis when evaluating patients with very high LDL levels. However, rule out possible secondary causes of elevated LDL before rendering a conclusion. Hypothyroidism, nephrotic syndrome, diabetes, and liver disease are among the most common secondary causes of high LDL cholesterol.13
Several validated criteria sets can be used to establish an FH diagnosis. No single criteria set is more valid or more widely adopted around the world. All 3 of the most commonly used criteria sets take into account family history and a patient’s LDL level, and 2 of the 3 factor in physical findings (TABLE 2).9
Physical exam findings that suggest FH can be subtle (FIGURE). Tendon xanthomas are a thickening of the soft tissue as a result of infiltration by lipid-rich cells. They most commonly occur at the Achilles and metacarpal tendons, but can also be seen at the patellar and triceps tendons. Xanthomas may not be readily visible, so it’s important to run your fingers over these areas to detect nodularity or thickening. While the presence of a tendon xanthoma makes FH highly likely, they are present in less than half of patients with FH.17
Tuberous xanthomas or xanthelasmas are waxy-appearing growths that may look yellow or orange and appear to be pasted on the skin in areas around the face, commonly the eyelids. The presence of xanthelasmas in a patient younger than age 25 suggests FH.
Finally, arcus corneae is an opaque ring around the outer edge of the cornea. When this is seen in patients younger than age 45, it’s suggestive of FH.13 If you note tendon xanthomas, xanthelasmas, or arcus corneae while examining any of your patients, be sure to order an LDL level if it hasn’t already been done.
Is genetic testing necessary?
The only way to make a definitive diagnosis of FH is to find a mutation in a gene known to affect LDL metabolism. However, because genetic testing is expensive—and because more than one thousand different genetic defects can contribute to FH—it’s not practical to test every patient. Furthermore, since an estimated 20% of the mutations that contribute to FH have not yet been clearly delineated, a “normal” result on a genetic test might be misleading.5 Therefore, the diagnosis of FH usually is a clinical one. After clinically diagnosing a patient with FH, it’s imperative to screen first-degree family members by measuring their LDL cholesterol levels.
Lifestyle changes, statins can ward off CHD
Lifestyle modifications (ie, improved diet and exercise) and statins are the treatments of choice for patients with FH. Before starting pharmacotherapy, patients should undergo 3 months of lifestyle modification to assess how well this approach improves their lipid levels, assuming the patient doesn’t have additional risk factors such as hypertension or tobacco use, in which case he or she might require immediate pharmacotherapy. Statins can be initiated simultaneously with lifestyle choices in patients with an LDL >190 mg/dL.18
Lifestyle modification. Although FH is a genetic problem, patients should be encouraged to make healthy choices regarding diet and exercise. While the best choices may not get FH patients to their LDL goal, better choices may mean that patients can take fewer medications, or lower doses of them. Healthy lifestyle choices can also have other positive effects on cardiovascular risk (eg, lowering blood pressure).
Patients can’t be expected to navigate their food choices alone, and several visits with a dietician will likely be needed. It’s important to emphasize the family influence on diet and get the entire family involved with making healthy food choices.
In addition to addressing diet and exercise, be sure to encourage patients to abstain from tobacco and manage stress as part of their overall effort to reduce the likelihood of a cardiovascular event.
Statins. Early treatment of FH with statins can delay initial coronary events and prolong life.19 In a 12.5-year study of 2146 patients with FH, approximately 80% of patients treated with statins survived without experiencing CHD, compared to slightly less than 40% of those who were not treated with statins.19 Patients treated with statins had a 76% reduction in risk of CHD compared to those who didn’t receive statins.19 Even low doses of statins started early have been shown to help avoid myocardial infarction in adults with FH.20
The goal of treatment for FH is to reduced LDL levels by 50%.21 In pediatric patients, treating to an LDL level of 130 mg/dL is an alternative goal.21 Because it’s challenging to achieve this goal with improved diet and exercise alone, treatment with a statin is often necessary.22
Statins can be used in children as young as age 8, or even earlier in homozygous FH.6 While a physician might be hesitant to start a chronic medication in a young patient, research shows that earlier intervention results in additional years of life.23 To date, no significant adverse effects of statins in pediatric patients have been identified, and statins have not been shown to impair growth.24,25 Young female patients should be counseled about the adverse effects statins can have on a fetus if the patient becomes pregnant while taking the medication.
Navigating the waters of statin treatment
Musculoskeletal symptoms are the most common adverse effect reported by patients taking statins. A thorough assessment of a patient’s muscle complaints is necessary to avoid prematurely concluding that he or she cannot tolerate statins.
A study in which “statin-intolerant” patients were re-challenged found that more than 90% of patients could tolerate statins through the course of the one-year study and that it was likely that the patients’ initial muscle complaints were not due to statin use.23 (To read more about potential adverse events of statins, see “Statin adverse effects: Sorting out the evidence,” J Fam Pract. 2014;63:497-506.).
If LDL levels in a patient with HeFH remain at or above 160 mg/dL, intensifying treatment by adding another lipid-lowering medication might be warranted.22 For patients with HoFH, in whom the condition is more quickly life-threatening, there are additional choices, including LDL apheresis and medications such as mipomersen and lomitapide. Both of these medications can cause hepatotoxicity, and are available only through a Risk Evaluation and Mitigation Strategy program, which means they can only be prescribed by certified physicians. PCSK9 inhibitors are in the pipeline and may one day help patients with HoFH by addressing one of the genetic causes of this disorder.
CORRESPONDENCE
Richard Safeer, MD, 6704 Curtis Court, Glen Burnie, MD 21060; [email protected]
1. Datta BN, McDowell IF, Rees A. Integrating provision of specialist lipid services with cascade testing for familial hypercholesterolaemia. Curr Opin Lipidol. 2010;21:366-371.
2. DeMott K, Nherera L, Shaw EJ, et al. Clinical Guidelines and Evidence Review for Familial Hypercholesterolaemia: The Identification and Management of Adults and Children with Familial Hypercholesterolaemia. 2008. London, UK: National Collaborating Centre for Primary Care and Royal College of General Practitioners.
3. Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis. 1999;142:105-112.
4. Kavey RE, Allada V, Daniels SR, et al. American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention. Cardiovascular risk reduction in high-risk pediatric patients. Circulation. 2006;114:2710-2738.
5. Rees A. Familial hypercholesterolaemia: underdiagnosed and undertreated. Eur Heart J. 2008;29:2583-2584.
6. Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients. Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1-8.
7. Moriarty PM. LDL-apheresis therapy. Curr Treat Options Cardiovasc Med. 2006;8:282-288.
8. Goldstein JL, Brown MS. The LDL receptor locus and the genetics of familial hypercholesterolemia. Annu Rev Genet. 1979;13:259-289.
9. Fahed AC, Nemer GM. Familial hypercholesterolemia: the lipids or the genes? Nutr Metab (Lond). 2011;8:23.
10. Goldstein J, Hobbs H, Brown M. Familial hypercholesterolemia. In: Scriver C, Baudet A, Sly W, et al, eds. The Metabolic Basis of Inherited Disease. New York, NY: McGraw-Hill; 2001: 2863-2913.
11. Kwiterovich, PO. Clinical and laboratory assessment of cardiovascular risk in children: Guidelines for screening, evaluation and treatment. J Clin Lipidol. 2008;2:248-266.
12. US Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia): Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/lipid-disorders-in-adults-cholesterol-dyslipidemia-screening. Accessed July 6, 2015.
13. Hopkins PN, Toth PP, Ballantyne CM, et al; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S9-17.
14. US Preventive Services Task Force. Screening for lipid disorders in children: US Preventive Services Task Force recommendation statement. Pediatrics. 2007;120;e215-219.
15. American Academy of Family Physicians. Summary of recommendations for clinical preventive services. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/cps-recommendations.pdf. Accessed May 2, 2015.
16. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128:S213-56.
17. American Academy of Pediatrics. 2014 Recommendations for Pediatric Preventive Health Care. American Academy of Pediatrics Web site. Available at: http://pediatrics.aappublications.org/content/133/3/568.full.pdf+html. Accessed May 2, 2015.
18. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
19. Nordestgaard BG, Chapman MJ, Humphries SE, et al; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478-3490a.
20. Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423.
21. Daniels SR, Gidding SS, de Ferranti SD; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Pediatric aspects of familial hypercholesterolemias: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S30-37.
22. Robinson JG, Goldberg AC; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S18-29.
23. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
24. Eiland LS, Luttrell PK. Use of statins for dyslipidemia in the pediatric population. J Pediatr Pharmacol Ther. 2010;15:160-172.
25. O’Gorman CS, Higgins MF, O’Neill MB. Systematic review and metaanalysis of statins for heterozygous familial hypercholesterolemia in children: evaluation of cholesterol changes and side effects. Pediatr Cardiol. 2009;30:482-489.
1. Datta BN, McDowell IF, Rees A. Integrating provision of specialist lipid services with cascade testing for familial hypercholesterolaemia. Curr Opin Lipidol. 2010;21:366-371.
2. DeMott K, Nherera L, Shaw EJ, et al. Clinical Guidelines and Evidence Review for Familial Hypercholesterolaemia: The Identification and Management of Adults and Children with Familial Hypercholesterolaemia. 2008. London, UK: National Collaborating Centre for Primary Care and Royal College of General Practitioners.
3. Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis. 1999;142:105-112.
4. Kavey RE, Allada V, Daniels SR, et al. American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention. Cardiovascular risk reduction in high-risk pediatric patients. Circulation. 2006;114:2710-2738.
5. Rees A. Familial hypercholesterolaemia: underdiagnosed and undertreated. Eur Heart J. 2008;29:2583-2584.
6. Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients. Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1-8.
7. Moriarty PM. LDL-apheresis therapy. Curr Treat Options Cardiovasc Med. 2006;8:282-288.
8. Goldstein JL, Brown MS. The LDL receptor locus and the genetics of familial hypercholesterolemia. Annu Rev Genet. 1979;13:259-289.
9. Fahed AC, Nemer GM. Familial hypercholesterolemia: the lipids or the genes? Nutr Metab (Lond). 2011;8:23.
10. Goldstein J, Hobbs H, Brown M. Familial hypercholesterolemia. In: Scriver C, Baudet A, Sly W, et al, eds. The Metabolic Basis of Inherited Disease. New York, NY: McGraw-Hill; 2001: 2863-2913.
11. Kwiterovich, PO. Clinical and laboratory assessment of cardiovascular risk in children: Guidelines for screening, evaluation and treatment. J Clin Lipidol. 2008;2:248-266.
12. US Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia): Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/lipid-disorders-in-adults-cholesterol-dyslipidemia-screening. Accessed July 6, 2015.
13. Hopkins PN, Toth PP, Ballantyne CM, et al; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S9-17.
14. US Preventive Services Task Force. Screening for lipid disorders in children: US Preventive Services Task Force recommendation statement. Pediatrics. 2007;120;e215-219.
15. American Academy of Family Physicians. Summary of recommendations for clinical preventive services. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/cps-recommendations.pdf. Accessed May 2, 2015.
16. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128:S213-56.
17. American Academy of Pediatrics. 2014 Recommendations for Pediatric Preventive Health Care. American Academy of Pediatrics Web site. Available at: http://pediatrics.aappublications.org/content/133/3/568.full.pdf+html. Accessed May 2, 2015.
18. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
19. Nordestgaard BG, Chapman MJ, Humphries SE, et al; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478-3490a.
20. Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423.
21. Daniels SR, Gidding SS, de Ferranti SD; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Pediatric aspects of familial hypercholesterolemias: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S30-37.
22. Robinson JG, Goldberg AC; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S18-29.
23. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
24. Eiland LS, Luttrell PK. Use of statins for dyslipidemia in the pediatric population. J Pediatr Pharmacol Ther. 2010;15:160-172.
25. O’Gorman CS, Higgins MF, O’Neill MB. Systematic review and metaanalysis of statins for heterozygous familial hypercholesterolemia in children: evaluation of cholesterol changes and side effects. Pediatr Cardiol. 2009;30:482-489.
Right foot pain while walking • no erythema or edema • no evidence of structural abnormalities • Dx?
THE CASE
A 24-year-old woman came to our clinic because she had pain in her right foot. Over the previous 4 weeks, she’d noticed increasing pain in the ball of her foot while walking and climbing stairs, particularly in the push-off portion of her gait. She described it as a nagging, localized pain that she rated as a 2 or 3 out of 10. It was an annoyance, but not unbearable. She felt no pain when standing in place or in a non-weight-bearing position.
She denied any trauma to the foot or change in activity, and had been exercising her usual amount (running 2-5 miles per week). Her medical and social histories were unremarkable, and her family history was negative for relevant conditions.
An examination of the right foot revealed no evidence of pes planus, pes cavus, hallux valgus, hammertoes, or other structural abnormalities of the foot or toes. She had no calluses, nor any erythema or edema of the foot or toes. Direct palpation of the medial sesamoid reproduced the patient’s symptoms. Passive dorsiflexion and plantar flexion of the first hallux elicited pain only at the extreme ends of range of motion. Active dorsiflexion and plantar flexion of the right first hallux showed 5 out of 5 strength. A mid-foot squeeze test was negative, and the remainder of the exam was normal.
THE DIAGNOSIS
Pain on palpation of the sesamoids prompted us to gather a more detailed history. The patient had never been a dancer or a long-distance or competitive runner. However, upon delving into possible causes of the pain, she admitted that she was a frequent “knuckle cracker,” and cracked many joints regularly, including the right first metatarsophalangeal joint (MTPJ). She explained that she cracked this joint by hyper-plantarflexing her big toe against the ground, and had been doing this multiple times a day for many years. In the past 4 weeks, she had noticed significant pain in the right first MTPJ while cracking the joint, but she was having difficulty breaking the longstanding habit.
The patient’s description of right foot pain associated with the push-off portion of her gait, and the fact that the pain was exacerbated by the extremes of dorsiflexion and plantar flexion of the great toe, was consistent with MTPJ pain. This, paired with our ability to reproduce the pain by direct palpation of the medial sesamoid, prompted us to make a clinical diagnosis of sesamoiditis. To our knowledge, this is the first case report of sesamoiditis caused by knuckle cracking.
DISCUSSION
Sesamoiditis—chronic pain and inflammation of the hallucal sesamoids—is an overuse or misuse injury that’s typically seen in runners and dancers.1 The hallucal sesamoids are 2 small bones located underneath the head of the first metatarsal and encased within the flexor hallucus brevis tendon that disperses weight from the head of the first metatarsal during the push-off portion of gait.2 Runners and dancers place significant, repetitive axial loading on the sesamoids, which often leads to injury.1 Although our patient initially seemed to have no typical risk factors for developing sesamoiditis, she later revealed that she regularly cracked the MTPJ, which we believe led to her injury.
Interestingly, despite the common assumption that long-term “cracking” of joints can lead to adverse effects such as osteoarthritis, research has not supported this assumption.3,4 A retrospective case-control study of patients with and without hand osteoarthritis found no association between knuckle cracking and osteoarthritis, and the prevalence of osteoarthritis was not higher in patients who cracked their knuckles more frequently and for more years.4
Nonetheless, there have been reports of acute injuries associated with knuckle cracking, consistent with forcing a joint past its normal range of motion, as is typically done in knuckle cracking.5 In forcefully plantarflexing her great toe against a surface until a “crack” was elicited, our patient may have injured the sesamoid by forcing it along the head of the first metatarsal. Conversely, her injury may have been caused by the repetitive displacement of the sesamoid past its usual location, resulting in chronic irritation.
Differential diagnosis includes fracture and stress injury
The differential diagnosis for subacute to chronic pain localized to the sesamoids includes repetitive stress injury (sesamoiditis or capsular strain), fracture or stress fracture, osteoarthritis, osteonecrosis, and gout.1,2 Given our patient’s age and lack of erythema and edema, osteoarthritis and gout were unlikely.
To treat the injury, eliminate the behavior that caused it
Imaging studies may not be necessary in cases of suspected sesamoiditis because such studies are often negative for sesamoiditis and stress fractures of the sesamoids, and because they typically would not affect how the injury is initially treated.1,2,6 In cases in which radiographic confirmation of sesamoiditis is necessary to rule out more serious pathology, 99mTc-methylene diphosphonate (99mTc-MDP) bone scan and magnetic resonance imaging (MRI) are far more sensitive than plain films.1 While a 99mTc-MDP bone scan will show increased uptake at the sesamoids, it has been replaced by MRI, which will show bone marrow edema of the sesamoids and can rule out fracture or osteoarthritis.1
Sesamoiditis is typically managed with a combination of ice, analgesics, activity modification, and/or orthoses.2 Of course, the key to successfully treating sesamoiditis (and all musculoskeletal injuries) is to not only make the diagnosis, but to find the underlying cause in order to prevent continued—or worsening—pain.
Our patient agreed to close follow-up rather than imaging. We established that the only inciting event was the cracking of her MTPJ, and that she should try to eliminate this action before trying other interventions. Our patient stopped cracking her MTPJ and her pain completely resolved in 2 weeks. She remains symptom-free.
THE TAKEAWAY
Ask about knuckle cracking when taking the history of a patient who presents with sesamoiditis, which is characterized by chronic pain and inflammation of the hallucal sesamoids.
1. Nwawka OK, Hayashi D, Diaz LE, et al. Sesamoids and accessory ossicles of the foot: anatomical variability and related pathology. Insights Imaging. 2013;4:581-593.
2. Boike A, Schnirring-Judge M, McMillin S. Sesamoid disorders of the first metatarsophalangeal joint. Clin Podiatr Med Surg. 2011;28:269-285.
3. Castellanos J, Axelrod D. Effect of habitual knuckle cracking on hand function. Ann Rheum Dis. 1990:49:308-309.
4. Deweber K, Olszewski M, Ortolano R. Knuckle cracking and hand osteoarthritis. J Am Board Fam Med. 2011;24:169-174.
5. Chan PS, Steinberg DR, Bozentka DJ. Consequences of knuckle cracking: a report of two acute injuries. Am J Orthop. 1999;28:113-114.
6. Yang RH, Chu YK. Hallucal sesamoiditis manifested on bone scan. Clin Nucl Med. 2013;38:1019-1021.
THE CASE
A 24-year-old woman came to our clinic because she had pain in her right foot. Over the previous 4 weeks, she’d noticed increasing pain in the ball of her foot while walking and climbing stairs, particularly in the push-off portion of her gait. She described it as a nagging, localized pain that she rated as a 2 or 3 out of 10. It was an annoyance, but not unbearable. She felt no pain when standing in place or in a non-weight-bearing position.
She denied any trauma to the foot or change in activity, and had been exercising her usual amount (running 2-5 miles per week). Her medical and social histories were unremarkable, and her family history was negative for relevant conditions.
An examination of the right foot revealed no evidence of pes planus, pes cavus, hallux valgus, hammertoes, or other structural abnormalities of the foot or toes. She had no calluses, nor any erythema or edema of the foot or toes. Direct palpation of the medial sesamoid reproduced the patient’s symptoms. Passive dorsiflexion and plantar flexion of the first hallux elicited pain only at the extreme ends of range of motion. Active dorsiflexion and plantar flexion of the right first hallux showed 5 out of 5 strength. A mid-foot squeeze test was negative, and the remainder of the exam was normal.
THE DIAGNOSIS
Pain on palpation of the sesamoids prompted us to gather a more detailed history. The patient had never been a dancer or a long-distance or competitive runner. However, upon delving into possible causes of the pain, she admitted that she was a frequent “knuckle cracker,” and cracked many joints regularly, including the right first metatarsophalangeal joint (MTPJ). She explained that she cracked this joint by hyper-plantarflexing her big toe against the ground, and had been doing this multiple times a day for many years. In the past 4 weeks, she had noticed significant pain in the right first MTPJ while cracking the joint, but she was having difficulty breaking the longstanding habit.
The patient’s description of right foot pain associated with the push-off portion of her gait, and the fact that the pain was exacerbated by the extremes of dorsiflexion and plantar flexion of the great toe, was consistent with MTPJ pain. This, paired with our ability to reproduce the pain by direct palpation of the medial sesamoid, prompted us to make a clinical diagnosis of sesamoiditis. To our knowledge, this is the first case report of sesamoiditis caused by knuckle cracking.
DISCUSSION
Sesamoiditis—chronic pain and inflammation of the hallucal sesamoids—is an overuse or misuse injury that’s typically seen in runners and dancers.1 The hallucal sesamoids are 2 small bones located underneath the head of the first metatarsal and encased within the flexor hallucus brevis tendon that disperses weight from the head of the first metatarsal during the push-off portion of gait.2 Runners and dancers place significant, repetitive axial loading on the sesamoids, which often leads to injury.1 Although our patient initially seemed to have no typical risk factors for developing sesamoiditis, she later revealed that she regularly cracked the MTPJ, which we believe led to her injury.
Interestingly, despite the common assumption that long-term “cracking” of joints can lead to adverse effects such as osteoarthritis, research has not supported this assumption.3,4 A retrospective case-control study of patients with and without hand osteoarthritis found no association between knuckle cracking and osteoarthritis, and the prevalence of osteoarthritis was not higher in patients who cracked their knuckles more frequently and for more years.4
Nonetheless, there have been reports of acute injuries associated with knuckle cracking, consistent with forcing a joint past its normal range of motion, as is typically done in knuckle cracking.5 In forcefully plantarflexing her great toe against a surface until a “crack” was elicited, our patient may have injured the sesamoid by forcing it along the head of the first metatarsal. Conversely, her injury may have been caused by the repetitive displacement of the sesamoid past its usual location, resulting in chronic irritation.
Differential diagnosis includes fracture and stress injury
The differential diagnosis for subacute to chronic pain localized to the sesamoids includes repetitive stress injury (sesamoiditis or capsular strain), fracture or stress fracture, osteoarthritis, osteonecrosis, and gout.1,2 Given our patient’s age and lack of erythema and edema, osteoarthritis and gout were unlikely.
To treat the injury, eliminate the behavior that caused it
Imaging studies may not be necessary in cases of suspected sesamoiditis because such studies are often negative for sesamoiditis and stress fractures of the sesamoids, and because they typically would not affect how the injury is initially treated.1,2,6 In cases in which radiographic confirmation of sesamoiditis is necessary to rule out more serious pathology, 99mTc-methylene diphosphonate (99mTc-MDP) bone scan and magnetic resonance imaging (MRI) are far more sensitive than plain films.1 While a 99mTc-MDP bone scan will show increased uptake at the sesamoids, it has been replaced by MRI, which will show bone marrow edema of the sesamoids and can rule out fracture or osteoarthritis.1
Sesamoiditis is typically managed with a combination of ice, analgesics, activity modification, and/or orthoses.2 Of course, the key to successfully treating sesamoiditis (and all musculoskeletal injuries) is to not only make the diagnosis, but to find the underlying cause in order to prevent continued—or worsening—pain.
Our patient agreed to close follow-up rather than imaging. We established that the only inciting event was the cracking of her MTPJ, and that she should try to eliminate this action before trying other interventions. Our patient stopped cracking her MTPJ and her pain completely resolved in 2 weeks. She remains symptom-free.
THE TAKEAWAY
Ask about knuckle cracking when taking the history of a patient who presents with sesamoiditis, which is characterized by chronic pain and inflammation of the hallucal sesamoids.
THE CASE
A 24-year-old woman came to our clinic because she had pain in her right foot. Over the previous 4 weeks, she’d noticed increasing pain in the ball of her foot while walking and climbing stairs, particularly in the push-off portion of her gait. She described it as a nagging, localized pain that she rated as a 2 or 3 out of 10. It was an annoyance, but not unbearable. She felt no pain when standing in place or in a non-weight-bearing position.
She denied any trauma to the foot or change in activity, and had been exercising her usual amount (running 2-5 miles per week). Her medical and social histories were unremarkable, and her family history was negative for relevant conditions.
An examination of the right foot revealed no evidence of pes planus, pes cavus, hallux valgus, hammertoes, or other structural abnormalities of the foot or toes. She had no calluses, nor any erythema or edema of the foot or toes. Direct palpation of the medial sesamoid reproduced the patient’s symptoms. Passive dorsiflexion and plantar flexion of the first hallux elicited pain only at the extreme ends of range of motion. Active dorsiflexion and plantar flexion of the right first hallux showed 5 out of 5 strength. A mid-foot squeeze test was negative, and the remainder of the exam was normal.
THE DIAGNOSIS
Pain on palpation of the sesamoids prompted us to gather a more detailed history. The patient had never been a dancer or a long-distance or competitive runner. However, upon delving into possible causes of the pain, she admitted that she was a frequent “knuckle cracker,” and cracked many joints regularly, including the right first metatarsophalangeal joint (MTPJ). She explained that she cracked this joint by hyper-plantarflexing her big toe against the ground, and had been doing this multiple times a day for many years. In the past 4 weeks, she had noticed significant pain in the right first MTPJ while cracking the joint, but she was having difficulty breaking the longstanding habit.
The patient’s description of right foot pain associated with the push-off portion of her gait, and the fact that the pain was exacerbated by the extremes of dorsiflexion and plantar flexion of the great toe, was consistent with MTPJ pain. This, paired with our ability to reproduce the pain by direct palpation of the medial sesamoid, prompted us to make a clinical diagnosis of sesamoiditis. To our knowledge, this is the first case report of sesamoiditis caused by knuckle cracking.
DISCUSSION
Sesamoiditis—chronic pain and inflammation of the hallucal sesamoids—is an overuse or misuse injury that’s typically seen in runners and dancers.1 The hallucal sesamoids are 2 small bones located underneath the head of the first metatarsal and encased within the flexor hallucus brevis tendon that disperses weight from the head of the first metatarsal during the push-off portion of gait.2 Runners and dancers place significant, repetitive axial loading on the sesamoids, which often leads to injury.1 Although our patient initially seemed to have no typical risk factors for developing sesamoiditis, she later revealed that she regularly cracked the MTPJ, which we believe led to her injury.
Interestingly, despite the common assumption that long-term “cracking” of joints can lead to adverse effects such as osteoarthritis, research has not supported this assumption.3,4 A retrospective case-control study of patients with and without hand osteoarthritis found no association between knuckle cracking and osteoarthritis, and the prevalence of osteoarthritis was not higher in patients who cracked their knuckles more frequently and for more years.4
Nonetheless, there have been reports of acute injuries associated with knuckle cracking, consistent with forcing a joint past its normal range of motion, as is typically done in knuckle cracking.5 In forcefully plantarflexing her great toe against a surface until a “crack” was elicited, our patient may have injured the sesamoid by forcing it along the head of the first metatarsal. Conversely, her injury may have been caused by the repetitive displacement of the sesamoid past its usual location, resulting in chronic irritation.
Differential diagnosis includes fracture and stress injury
The differential diagnosis for subacute to chronic pain localized to the sesamoids includes repetitive stress injury (sesamoiditis or capsular strain), fracture or stress fracture, osteoarthritis, osteonecrosis, and gout.1,2 Given our patient’s age and lack of erythema and edema, osteoarthritis and gout were unlikely.
To treat the injury, eliminate the behavior that caused it
Imaging studies may not be necessary in cases of suspected sesamoiditis because such studies are often negative for sesamoiditis and stress fractures of the sesamoids, and because they typically would not affect how the injury is initially treated.1,2,6 In cases in which radiographic confirmation of sesamoiditis is necessary to rule out more serious pathology, 99mTc-methylene diphosphonate (99mTc-MDP) bone scan and magnetic resonance imaging (MRI) are far more sensitive than plain films.1 While a 99mTc-MDP bone scan will show increased uptake at the sesamoids, it has been replaced by MRI, which will show bone marrow edema of the sesamoids and can rule out fracture or osteoarthritis.1
Sesamoiditis is typically managed with a combination of ice, analgesics, activity modification, and/or orthoses.2 Of course, the key to successfully treating sesamoiditis (and all musculoskeletal injuries) is to not only make the diagnosis, but to find the underlying cause in order to prevent continued—or worsening—pain.
Our patient agreed to close follow-up rather than imaging. We established that the only inciting event was the cracking of her MTPJ, and that she should try to eliminate this action before trying other interventions. Our patient stopped cracking her MTPJ and her pain completely resolved in 2 weeks. She remains symptom-free.
THE TAKEAWAY
Ask about knuckle cracking when taking the history of a patient who presents with sesamoiditis, which is characterized by chronic pain and inflammation of the hallucal sesamoids.
1. Nwawka OK, Hayashi D, Diaz LE, et al. Sesamoids and accessory ossicles of the foot: anatomical variability and related pathology. Insights Imaging. 2013;4:581-593.
2. Boike A, Schnirring-Judge M, McMillin S. Sesamoid disorders of the first metatarsophalangeal joint. Clin Podiatr Med Surg. 2011;28:269-285.
3. Castellanos J, Axelrod D. Effect of habitual knuckle cracking on hand function. Ann Rheum Dis. 1990:49:308-309.
4. Deweber K, Olszewski M, Ortolano R. Knuckle cracking and hand osteoarthritis. J Am Board Fam Med. 2011;24:169-174.
5. Chan PS, Steinberg DR, Bozentka DJ. Consequences of knuckle cracking: a report of two acute injuries. Am J Orthop. 1999;28:113-114.
6. Yang RH, Chu YK. Hallucal sesamoiditis manifested on bone scan. Clin Nucl Med. 2013;38:1019-1021.
1. Nwawka OK, Hayashi D, Diaz LE, et al. Sesamoids and accessory ossicles of the foot: anatomical variability and related pathology. Insights Imaging. 2013;4:581-593.
2. Boike A, Schnirring-Judge M, McMillin S. Sesamoid disorders of the first metatarsophalangeal joint. Clin Podiatr Med Surg. 2011;28:269-285.
3. Castellanos J, Axelrod D. Effect of habitual knuckle cracking on hand function. Ann Rheum Dis. 1990:49:308-309.
4. Deweber K, Olszewski M, Ortolano R. Knuckle cracking and hand osteoarthritis. J Am Board Fam Med. 2011;24:169-174.
5. Chan PS, Steinberg DR, Bozentka DJ. Consequences of knuckle cracking: a report of two acute injuries. Am J Orthop. 1999;28:113-114.
6. Yang RH, Chu YK. Hallucal sesamoiditis manifested on bone scan. Clin Nucl Med. 2013;38:1019-1021.
Targeting neuropathic pain: Consider these alternatives
Anticonvulsants, antidepressants, and opioids are the most frequently prescribed medications for neuropathic pain.1 But some patients are unable to tolerate the adverse effects of these drugs, and others achieve only partial pain relief. What can you offer them?
Combinations of prescription medications are generally considered more effective than monotherapy for painful peripheral neuropathy,1 but it is unclear which combinations are best. Alternative therapies—several of which have some evidence of safety and efficacy in treating peripheral neuropathy—are another option. Yet trials with alternative therapies, alone or in combination with prescription drugs, are rarely considered.
In fact, physicians are often unfamiliar with these therapies. Many are concerned about the absence of US Food and Drug Administration approval for alternative therapies and the variability in quality control associated with the lack of oversight, as well. Making recommendations about the duration of therapy also presents a challenge because most studies of supplements are relatively short. What’s more, alternative treatments are rarely covered by third-party payers.
Nonetheless, the therapies detailed in the text and TABLE2-12 that follow are generally well tolerated and appear to be safe. Adding them to your arsenal of therapeutic choices for patients with painful peripheral neuropathy may increase your ability to provide successful treatment.
Acetyl-L-carnitine (ALC)
ALC occurs naturally in the body as L-carnitine and acetyl-carnitine esters, which are converted to carnitines by intracellular enzymes and cell membrane transporters.2 ALC has been studied in patients with neuropathy associated with human immunodeficiency virus (HIV), cancer, and diabetes. Potential mechanisms of action include the correction of a deficiency that may be causing the neuropathy (which sometimes occurs in HIV-positive patients13 or those taking anticonvulsants14), a direct antioxidant effect, or an enhanced response to nerve growth factor.13
ALC can be given intramuscularly (IM) or orally in doses of 2000 to 3000 mg/d. In one randomized placebo-controlled trial (N=333), patients with diabetic neuropathy received 1000 mg IM followed by an oral dose of 2000 mg every day for a year.6 Mean pain scores decreased by 39%, with 67% of those receiving ALC vs 23% of those on placebo showing moderate to marked improvement.
In a pooled analysis (N=1257) of 2 randomized controlled trials (RCTs), patients with diabetes took 1000 mg ALC 3 times daily or placebo for a year.7 Cohort pain scores improved by 40% from baseline in the ALC group compared with a 24% improvement for those in the placebo group.
THE BOTTOM LINE ALC is well tolerated, with minor adverse effects such as headache and nausea reported.6,7 It should not be given to patients taking acenocoumarol or warfarin, however. A major interaction causing an elevated international normalized ratio has been found to occur when either agent is combined with L-carnitine2 and could theoretically occur with ALC, as well. No other drug-drug interactions have been documented.2
Alpha lipoic acid (ALA)
Both a fat- and a water-soluble vitamin that is usually obtained from the diet, ALA regenerates endogenous antioxidants like vitamins C and E and glutathione. It is this regenerative mechanism that it is believed to alleviate diabetic neuropathy.2 ALA 600 mg/d appears to be effective, although studies suggest that intravenous (IV) use is more effective than oral administration.
A meta-analysis of 4 RCTs (N=653), 2 with ALA taken orally and 2 involving IV administration, is a case in point.3 The pooled standardized mean difference estimated from all trials showed a reduction in total symptom scores of −2.26 (95% confidence interval [CI], −3.12 to −1.41; P=.00001), with 0 indicating no symptoms, 3 indicating severe symptoms, and a maximum score of 14.64 if all symptoms were severe and continuous. Subgroup analyses revealed a reduction of −1.78 (95% CI, −2.45 to −1.10; P=.00001) for oral ALA and −2.81 (95% CI, −4.16 to −1.46; P=.0001) for IV administration. Doses >600 mg/d did not improve efficacy, but did increase adverse effects such as nausea, vomiting, and dizziness.
In a multicenter RCT (N=460) of ALA 600 mg/d for 4 years, however, no improvement in the primary endpoint (a composite of neuropathy impairment scores and 7 neurophysiologic tests) was found.15 Although there was a statistically significant improvement in symptoms of neuropathy (−0.68 with ALA compared with +0.61 with placebo), the change was too small to be considered clinically significant.
ALA did slow the progression of neuropathy, however, with 29% of patients in the treatment group experiencing worsening symptoms compared with 38% of those on placebo. There was no difference in tolerability or discontinuation of treatment between the 2 groups.
A recent observational study (N=101) compared the efficacy of pregabalin, carbamazepine, and ALA over a 21-month period.4 Although those taking pregabalin had the best response rate, all 3 treatments led to significant improvement in the burning associated with neuropathic pain.
ALA 100 mg bid has been investigated as part of a 3-drug combination (with pregabalin 75 mg bid and methylcobalamin 750 mcg bid) compared with monotherapy (pregabalin 75 mg bid) in an open randomized study (N=30) for 12 weeks.16 While there was a trend toward improvement in pain relief, sleep interference, and nerve function in the combination therapy group, no statistically significant difference between the 2 groups was found. Nonetheless, more than a third (36%) had a global assessment rating of “excellent” vs one in 5 (20%) of those on pregabalin alone.
THE BOTTOM LINE Overall, ALA is well tolerated; the most common adverse effects are nausea and skin rash. IV administration is more effective than oral administration, but may cause nausea, headache, and an allergic reaction at the injection site.2 ALA does have the potential for an interaction with chemotherapy and thyroid hormone and may decrease the effectiveness of these therapies.2
B vitamins
Deficiencies of vitamin B1 (thiamine), B6 (pyridoxine), B12 (cyanocobalamin), and folate are known causes of neuropathy, and correcting them often improves or eliminates the symptoms.13 Vitamin B12 deficiency is commonly seen in patients taking metformin;14 these patients may benefit from supplementation with B12 1000 mcg/d.
Many of the B vitamins have been studied for treatment of neuropathy, but benfotiamine (a lipid-soluble form of thiamine) is thought to be the best option because it is better absorbed across cell membranes than other B vitamins.9 A Cochrane review found that benfotiamine alone may be effective for both diabetic and alcoholic neuropathy and that short-term use of higher doses of vitamin B complex (25 mg B1 or 320 mg benfotiamine + 50-720 mg B6 + 1000 mcg B12 daily) may reduce neuropathic pain.9
A randomized multicenter trial (N=214) found that adding a supplement containing L-methylfolate 3 mg, pyridoxal 5-phosphate 35 mg, and methylcobalamin 2 mg twice daily to other medications (eg, pregabalin, gabapentin, or duloxetine) improved symptoms of diabetic neuropathy.10 At 24 weeks, those receiving the combination therapy had a 26% decrease in pain symptoms compared with a 15% decrease for those on medication alone, with no significant adverse effects.
THE BOTTOM LINE Overall, vitamin B supplementation is well tolerated and appears to be more effective in relieving neuropathic pain than medication alone.9,14 But larger studies are needed before its efficacy in treating patients who do not have a deficiency can be established.
Capsaicin
Capsaicin, an ingredient found in peppers, works by binding to nociceptors to selectively stimulate afferent C fibers. This causes the release of substance P, a neurotransmitter that mediates pain, leading to its depletion and resulting in desensitization.2 Several meta-analyses and systematic reviews have found that topical capsaicin can be very effective, both as an adjunctive treatment and as monotherapy for neuropathic pain.11,17,18 The concentration used in the studies was 0.075% capsaicin cream, applied 3 to 4 times a day for 6 to 12 weeks, compared with placebo creams. In all categories studied, capsaicin was either statistically significant or trending in its favor, with the exception of adverse effects.
Capsaicin led to an improvement in daily activities and ability to sleep and a reduction in pain as measured with a visual analog scale and physician global evaluation.11,17,18
The most notable adverse effects were a burning sensation on the skin and coughing and sneezing caused by inhalation of dried cream. Although the adverse effects were expected to improve after 2 to 7 days of use, a significant number of participants withdrew from the study.
A 7-study meta-analysis showed the effectiveness of an 8% capsaicin patch for treatment of post-herpetic neuralgia and HIV-associated neuropathy.12 The patch, available only by prescription, was worn every day for 4 weeks (60 minutes daily for post-herpetic neuralgia and 30 minutes a day for HIV-associated neuropathy). The pooled results were statistically significant, but the patch was less effective for patients ages 18 to 40 years and for those of Asian descent. It can be used with other analgesics or as monotherapy, with few adverse reactions.12,19
THE BOTTOM LINE Since capsaicin is a topical medication, there are no relevant drug-drug interactions. Patients should be cautioned to wash their hands after application, however, and to avoid contact with eyes and open wounds.
Gamma linolenic acid (GLA)
Also known as evening primrose oil, GLA is an omega-6 fatty acid that’s an important constituent of neuronal cell membranes—and believed to decrease neuropathic pain by having some anti-inflammatory effects.2 This suggests that therapy with GLA has the potential to improve neuronal phospholipid structure and microcirculation.2
Two placebo-controlled trials (N=22,111) showed improvement in pain scores and multiple neurophysiologic assessments in patients with diabetes treated with GLA (360-480 mg/d).20,21 The treatment was well tolerated, but the beneficial effect was more pronounced in those with less severe diabetes.
THE BOTTOM LINE The dose of GLA studied (8 to 12 capsules daily) could lead to problems with patient adherence. In addition, GLA should be used with caution in patients who are taking antiplatelet medication or have seizure disorders.2
Magnesium (Mg)
Mg is highly involved in multiple enzyme systems throughout the body. Although it is very well absorbed from dietary sources,2 patients with diabetes, liver disease, and hormonal imbalances, as well as the elderly, are often deficient in Mg. It is unclear how this affects peripheral neuropathy.13
Mg may have an antinociceptive effect by decreasing intracellular calcium influx and antagonizing N-methyl-D-aspartate receptors and associated nerve signaling.22 A small RCT (N=80) showed Mg to decrease the severity of neuropathic back pain.22 Patients received Mg sulfate 1 g IV, given over 4 hours, every day for 2 weeks. The infusion was then replaced with Mg oxide 400 mg plus Mg gluconate 100 mg, taken orally twice daily for 4 weeks. An improvement in mean pain score was seen as early as 2 weeks, and scores had decreased by 2.8 points (on a 0-10-point scale) at 6 months.
Another small RCT (N=45) gave patients with neuropathy of postherpetic, traumatic, or surgical (but not diabetic) origin Mg chloride 838 mg orally 3 times a day for 4 weeks.23 The supplement was taken with meals. Mean pain scores in the treatment group decreased by 3 points, but this was not significantly different from the improvement seen in those on placebo.
In a similar study, patients (N=110) with type 1 diabetes and a normal serum Mg but an insufficiency as measured by erythrocyte Mg were given Mg gluconate 300 mg or placebo daily for 5 years.8 The supplement slowed the progression of peripheral neuropathy (only 12% of those receiving Mg gluconate experienced a significant worsening of symptoms over the course of the study, compared with 61% of those in the placebo group), but in most cases, it did not lead to an improvement.
No consistent approach to Mg supplementation has been studied, which makes recommending a particular route, dose, or formulation challenging. There is evidence that oral Mg, particularly in the form of Mg oxide, can cause diarrhea, especially in doses >350 mg/d. Mg gluconate and Mg chloride are better tolerated; Mg carbonate should be avoided due to poor oral absorption.2
BOTTOM LINE Mg supplementation appears to slow the progression of diabetic peripheral neuropathy, but is unsafe for patients with renal dysfunction, cardiac conduction abnormalities, or elevated Mg levels.2 Caution is required, too, when considering Mg supplementation for patients taking anticoagulants, bisphosphonates, digoxin, potassium-sparing diuretics, or tetracycline antibiotics.2
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
1. Chaparro LE, Wiffen PJ, Moore RA, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012:(7):CD008943.
2. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com. Accessed January 4, 2015.
3. Mijnhout GS, Kollen BJ, Alkhalaf A, et al. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279.
4. Patel N, Mishra V, Patel P, et al. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62.
5. Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12:29-34.
6. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3:223-231.
7. Sima AA, Calvani M, Mehra M, et al; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89-94.
8. De Leeuw, Engelen W, De Block C, et al. Long term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm). Magnes Res. 2004;17:109-114.
9. Ang CD, Alviar MJM, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573.
10. Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes
with peripheral neuropathy: a randomized trial. Am J Med. 2013;126:141-149.
11. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006; 11:294-329.
14. Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, et al. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol. 2011; 6:260-273.
15. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with a-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34:2054-2060.
16. Vasudevan D, Naik MM, Mukaddam QI. Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition. [MAINTAIN]: Results of a pilot study. Ann Indian Acad Neurol. 2014;17:19-24.
17. Halat KM, Dennehy CE. Botanicals and dietary supplements in diabetic peripheral neuropathy. J Am Board Fam Pract. 2003;16:47-57.
18. Donofrio P, Walker F, Hunt V, et al. Treatment of painful diabetic neuropathy with topical capsaicin: A multicenter, double-blind, vehicle-controlled study. Arch Int Med. 1991;151:2225-2229.
19. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
20. Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16:8-15.
21. Jamal GA, Carmichael H. The effect of gamma linolenic acid on human diabetic peripheral neuropathy: a double blind placebo controlled trial. Diabetic Med. 1990;7:319-323.
22. Yousef AA, Al-deeb AE. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68:260-266.
23. Pickering G, Morel V, Simen E. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011;24:28-35.
Anticonvulsants, antidepressants, and opioids are the most frequently prescribed medications for neuropathic pain.1 But some patients are unable to tolerate the adverse effects of these drugs, and others achieve only partial pain relief. What can you offer them?
Combinations of prescription medications are generally considered more effective than monotherapy for painful peripheral neuropathy,1 but it is unclear which combinations are best. Alternative therapies—several of which have some evidence of safety and efficacy in treating peripheral neuropathy—are another option. Yet trials with alternative therapies, alone or in combination with prescription drugs, are rarely considered.
In fact, physicians are often unfamiliar with these therapies. Many are concerned about the absence of US Food and Drug Administration approval for alternative therapies and the variability in quality control associated with the lack of oversight, as well. Making recommendations about the duration of therapy also presents a challenge because most studies of supplements are relatively short. What’s more, alternative treatments are rarely covered by third-party payers.
Nonetheless, the therapies detailed in the text and TABLE2-12 that follow are generally well tolerated and appear to be safe. Adding them to your arsenal of therapeutic choices for patients with painful peripheral neuropathy may increase your ability to provide successful treatment.
Acetyl-L-carnitine (ALC)
ALC occurs naturally in the body as L-carnitine and acetyl-carnitine esters, which are converted to carnitines by intracellular enzymes and cell membrane transporters.2 ALC has been studied in patients with neuropathy associated with human immunodeficiency virus (HIV), cancer, and diabetes. Potential mechanisms of action include the correction of a deficiency that may be causing the neuropathy (which sometimes occurs in HIV-positive patients13 or those taking anticonvulsants14), a direct antioxidant effect, or an enhanced response to nerve growth factor.13
ALC can be given intramuscularly (IM) or orally in doses of 2000 to 3000 mg/d. In one randomized placebo-controlled trial (N=333), patients with diabetic neuropathy received 1000 mg IM followed by an oral dose of 2000 mg every day for a year.6 Mean pain scores decreased by 39%, with 67% of those receiving ALC vs 23% of those on placebo showing moderate to marked improvement.
In a pooled analysis (N=1257) of 2 randomized controlled trials (RCTs), patients with diabetes took 1000 mg ALC 3 times daily or placebo for a year.7 Cohort pain scores improved by 40% from baseline in the ALC group compared with a 24% improvement for those in the placebo group.
THE BOTTOM LINE ALC is well tolerated, with minor adverse effects such as headache and nausea reported.6,7 It should not be given to patients taking acenocoumarol or warfarin, however. A major interaction causing an elevated international normalized ratio has been found to occur when either agent is combined with L-carnitine2 and could theoretically occur with ALC, as well. No other drug-drug interactions have been documented.2
Alpha lipoic acid (ALA)
Both a fat- and a water-soluble vitamin that is usually obtained from the diet, ALA regenerates endogenous antioxidants like vitamins C and E and glutathione. It is this regenerative mechanism that it is believed to alleviate diabetic neuropathy.2 ALA 600 mg/d appears to be effective, although studies suggest that intravenous (IV) use is more effective than oral administration.
A meta-analysis of 4 RCTs (N=653), 2 with ALA taken orally and 2 involving IV administration, is a case in point.3 The pooled standardized mean difference estimated from all trials showed a reduction in total symptom scores of −2.26 (95% confidence interval [CI], −3.12 to −1.41; P=.00001), with 0 indicating no symptoms, 3 indicating severe symptoms, and a maximum score of 14.64 if all symptoms were severe and continuous. Subgroup analyses revealed a reduction of −1.78 (95% CI, −2.45 to −1.10; P=.00001) for oral ALA and −2.81 (95% CI, −4.16 to −1.46; P=.0001) for IV administration. Doses >600 mg/d did not improve efficacy, but did increase adverse effects such as nausea, vomiting, and dizziness.
In a multicenter RCT (N=460) of ALA 600 mg/d for 4 years, however, no improvement in the primary endpoint (a composite of neuropathy impairment scores and 7 neurophysiologic tests) was found.15 Although there was a statistically significant improvement in symptoms of neuropathy (−0.68 with ALA compared with +0.61 with placebo), the change was too small to be considered clinically significant.
ALA did slow the progression of neuropathy, however, with 29% of patients in the treatment group experiencing worsening symptoms compared with 38% of those on placebo. There was no difference in tolerability or discontinuation of treatment between the 2 groups.
A recent observational study (N=101) compared the efficacy of pregabalin, carbamazepine, and ALA over a 21-month period.4 Although those taking pregabalin had the best response rate, all 3 treatments led to significant improvement in the burning associated with neuropathic pain.
ALA 100 mg bid has been investigated as part of a 3-drug combination (with pregabalin 75 mg bid and methylcobalamin 750 mcg bid) compared with monotherapy (pregabalin 75 mg bid) in an open randomized study (N=30) for 12 weeks.16 While there was a trend toward improvement in pain relief, sleep interference, and nerve function in the combination therapy group, no statistically significant difference between the 2 groups was found. Nonetheless, more than a third (36%) had a global assessment rating of “excellent” vs one in 5 (20%) of those on pregabalin alone.
THE BOTTOM LINE Overall, ALA is well tolerated; the most common adverse effects are nausea and skin rash. IV administration is more effective than oral administration, but may cause nausea, headache, and an allergic reaction at the injection site.2 ALA does have the potential for an interaction with chemotherapy and thyroid hormone and may decrease the effectiveness of these therapies.2
B vitamins
Deficiencies of vitamin B1 (thiamine), B6 (pyridoxine), B12 (cyanocobalamin), and folate are known causes of neuropathy, and correcting them often improves or eliminates the symptoms.13 Vitamin B12 deficiency is commonly seen in patients taking metformin;14 these patients may benefit from supplementation with B12 1000 mcg/d.
Many of the B vitamins have been studied for treatment of neuropathy, but benfotiamine (a lipid-soluble form of thiamine) is thought to be the best option because it is better absorbed across cell membranes than other B vitamins.9 A Cochrane review found that benfotiamine alone may be effective for both diabetic and alcoholic neuropathy and that short-term use of higher doses of vitamin B complex (25 mg B1 or 320 mg benfotiamine + 50-720 mg B6 + 1000 mcg B12 daily) may reduce neuropathic pain.9
A randomized multicenter trial (N=214) found that adding a supplement containing L-methylfolate 3 mg, pyridoxal 5-phosphate 35 mg, and methylcobalamin 2 mg twice daily to other medications (eg, pregabalin, gabapentin, or duloxetine) improved symptoms of diabetic neuropathy.10 At 24 weeks, those receiving the combination therapy had a 26% decrease in pain symptoms compared with a 15% decrease for those on medication alone, with no significant adverse effects.
THE BOTTOM LINE Overall, vitamin B supplementation is well tolerated and appears to be more effective in relieving neuropathic pain than medication alone.9,14 But larger studies are needed before its efficacy in treating patients who do not have a deficiency can be established.
Capsaicin
Capsaicin, an ingredient found in peppers, works by binding to nociceptors to selectively stimulate afferent C fibers. This causes the release of substance P, a neurotransmitter that mediates pain, leading to its depletion and resulting in desensitization.2 Several meta-analyses and systematic reviews have found that topical capsaicin can be very effective, both as an adjunctive treatment and as monotherapy for neuropathic pain.11,17,18 The concentration used in the studies was 0.075% capsaicin cream, applied 3 to 4 times a day for 6 to 12 weeks, compared with placebo creams. In all categories studied, capsaicin was either statistically significant or trending in its favor, with the exception of adverse effects.
Capsaicin led to an improvement in daily activities and ability to sleep and a reduction in pain as measured with a visual analog scale and physician global evaluation.11,17,18
The most notable adverse effects were a burning sensation on the skin and coughing and sneezing caused by inhalation of dried cream. Although the adverse effects were expected to improve after 2 to 7 days of use, a significant number of participants withdrew from the study.
A 7-study meta-analysis showed the effectiveness of an 8% capsaicin patch for treatment of post-herpetic neuralgia and HIV-associated neuropathy.12 The patch, available only by prescription, was worn every day for 4 weeks (60 minutes daily for post-herpetic neuralgia and 30 minutes a day for HIV-associated neuropathy). The pooled results were statistically significant, but the patch was less effective for patients ages 18 to 40 years and for those of Asian descent. It can be used with other analgesics or as monotherapy, with few adverse reactions.12,19
THE BOTTOM LINE Since capsaicin is a topical medication, there are no relevant drug-drug interactions. Patients should be cautioned to wash their hands after application, however, and to avoid contact with eyes and open wounds.
Gamma linolenic acid (GLA)
Also known as evening primrose oil, GLA is an omega-6 fatty acid that’s an important constituent of neuronal cell membranes—and believed to decrease neuropathic pain by having some anti-inflammatory effects.2 This suggests that therapy with GLA has the potential to improve neuronal phospholipid structure and microcirculation.2
Two placebo-controlled trials (N=22,111) showed improvement in pain scores and multiple neurophysiologic assessments in patients with diabetes treated with GLA (360-480 mg/d).20,21 The treatment was well tolerated, but the beneficial effect was more pronounced in those with less severe diabetes.
THE BOTTOM LINE The dose of GLA studied (8 to 12 capsules daily) could lead to problems with patient adherence. In addition, GLA should be used with caution in patients who are taking antiplatelet medication or have seizure disorders.2
Magnesium (Mg)
Mg is highly involved in multiple enzyme systems throughout the body. Although it is very well absorbed from dietary sources,2 patients with diabetes, liver disease, and hormonal imbalances, as well as the elderly, are often deficient in Mg. It is unclear how this affects peripheral neuropathy.13
Mg may have an antinociceptive effect by decreasing intracellular calcium influx and antagonizing N-methyl-D-aspartate receptors and associated nerve signaling.22 A small RCT (N=80) showed Mg to decrease the severity of neuropathic back pain.22 Patients received Mg sulfate 1 g IV, given over 4 hours, every day for 2 weeks. The infusion was then replaced with Mg oxide 400 mg plus Mg gluconate 100 mg, taken orally twice daily for 4 weeks. An improvement in mean pain score was seen as early as 2 weeks, and scores had decreased by 2.8 points (on a 0-10-point scale) at 6 months.
Another small RCT (N=45) gave patients with neuropathy of postherpetic, traumatic, or surgical (but not diabetic) origin Mg chloride 838 mg orally 3 times a day for 4 weeks.23 The supplement was taken with meals. Mean pain scores in the treatment group decreased by 3 points, but this was not significantly different from the improvement seen in those on placebo.
In a similar study, patients (N=110) with type 1 diabetes and a normal serum Mg but an insufficiency as measured by erythrocyte Mg were given Mg gluconate 300 mg or placebo daily for 5 years.8 The supplement slowed the progression of peripheral neuropathy (only 12% of those receiving Mg gluconate experienced a significant worsening of symptoms over the course of the study, compared with 61% of those in the placebo group), but in most cases, it did not lead to an improvement.
No consistent approach to Mg supplementation has been studied, which makes recommending a particular route, dose, or formulation challenging. There is evidence that oral Mg, particularly in the form of Mg oxide, can cause diarrhea, especially in doses >350 mg/d. Mg gluconate and Mg chloride are better tolerated; Mg carbonate should be avoided due to poor oral absorption.2
BOTTOM LINE Mg supplementation appears to slow the progression of diabetic peripheral neuropathy, but is unsafe for patients with renal dysfunction, cardiac conduction abnormalities, or elevated Mg levels.2 Caution is required, too, when considering Mg supplementation for patients taking anticoagulants, bisphosphonates, digoxin, potassium-sparing diuretics, or tetracycline antibiotics.2
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
Anticonvulsants, antidepressants, and opioids are the most frequently prescribed medications for neuropathic pain.1 But some patients are unable to tolerate the adverse effects of these drugs, and others achieve only partial pain relief. What can you offer them?
Combinations of prescription medications are generally considered more effective than monotherapy for painful peripheral neuropathy,1 but it is unclear which combinations are best. Alternative therapies—several of which have some evidence of safety and efficacy in treating peripheral neuropathy—are another option. Yet trials with alternative therapies, alone or in combination with prescription drugs, are rarely considered.
In fact, physicians are often unfamiliar with these therapies. Many are concerned about the absence of US Food and Drug Administration approval for alternative therapies and the variability in quality control associated with the lack of oversight, as well. Making recommendations about the duration of therapy also presents a challenge because most studies of supplements are relatively short. What’s more, alternative treatments are rarely covered by third-party payers.
Nonetheless, the therapies detailed in the text and TABLE2-12 that follow are generally well tolerated and appear to be safe. Adding them to your arsenal of therapeutic choices for patients with painful peripheral neuropathy may increase your ability to provide successful treatment.
Acetyl-L-carnitine (ALC)
ALC occurs naturally in the body as L-carnitine and acetyl-carnitine esters, which are converted to carnitines by intracellular enzymes and cell membrane transporters.2 ALC has been studied in patients with neuropathy associated with human immunodeficiency virus (HIV), cancer, and diabetes. Potential mechanisms of action include the correction of a deficiency that may be causing the neuropathy (which sometimes occurs in HIV-positive patients13 or those taking anticonvulsants14), a direct antioxidant effect, or an enhanced response to nerve growth factor.13
ALC can be given intramuscularly (IM) or orally in doses of 2000 to 3000 mg/d. In one randomized placebo-controlled trial (N=333), patients with diabetic neuropathy received 1000 mg IM followed by an oral dose of 2000 mg every day for a year.6 Mean pain scores decreased by 39%, with 67% of those receiving ALC vs 23% of those on placebo showing moderate to marked improvement.
In a pooled analysis (N=1257) of 2 randomized controlled trials (RCTs), patients with diabetes took 1000 mg ALC 3 times daily or placebo for a year.7 Cohort pain scores improved by 40% from baseline in the ALC group compared with a 24% improvement for those in the placebo group.
THE BOTTOM LINE ALC is well tolerated, with minor adverse effects such as headache and nausea reported.6,7 It should not be given to patients taking acenocoumarol or warfarin, however. A major interaction causing an elevated international normalized ratio has been found to occur when either agent is combined with L-carnitine2 and could theoretically occur with ALC, as well. No other drug-drug interactions have been documented.2
Alpha lipoic acid (ALA)
Both a fat- and a water-soluble vitamin that is usually obtained from the diet, ALA regenerates endogenous antioxidants like vitamins C and E and glutathione. It is this regenerative mechanism that it is believed to alleviate diabetic neuropathy.2 ALA 600 mg/d appears to be effective, although studies suggest that intravenous (IV) use is more effective than oral administration.
A meta-analysis of 4 RCTs (N=653), 2 with ALA taken orally and 2 involving IV administration, is a case in point.3 The pooled standardized mean difference estimated from all trials showed a reduction in total symptom scores of −2.26 (95% confidence interval [CI], −3.12 to −1.41; P=.00001), with 0 indicating no symptoms, 3 indicating severe symptoms, and a maximum score of 14.64 if all symptoms were severe and continuous. Subgroup analyses revealed a reduction of −1.78 (95% CI, −2.45 to −1.10; P=.00001) for oral ALA and −2.81 (95% CI, −4.16 to −1.46; P=.0001) for IV administration. Doses >600 mg/d did not improve efficacy, but did increase adverse effects such as nausea, vomiting, and dizziness.
In a multicenter RCT (N=460) of ALA 600 mg/d for 4 years, however, no improvement in the primary endpoint (a composite of neuropathy impairment scores and 7 neurophysiologic tests) was found.15 Although there was a statistically significant improvement in symptoms of neuropathy (−0.68 with ALA compared with +0.61 with placebo), the change was too small to be considered clinically significant.
ALA did slow the progression of neuropathy, however, with 29% of patients in the treatment group experiencing worsening symptoms compared with 38% of those on placebo. There was no difference in tolerability or discontinuation of treatment between the 2 groups.
A recent observational study (N=101) compared the efficacy of pregabalin, carbamazepine, and ALA over a 21-month period.4 Although those taking pregabalin had the best response rate, all 3 treatments led to significant improvement in the burning associated with neuropathic pain.
ALA 100 mg bid has been investigated as part of a 3-drug combination (with pregabalin 75 mg bid and methylcobalamin 750 mcg bid) compared with monotherapy (pregabalin 75 mg bid) in an open randomized study (N=30) for 12 weeks.16 While there was a trend toward improvement in pain relief, sleep interference, and nerve function in the combination therapy group, no statistically significant difference between the 2 groups was found. Nonetheless, more than a third (36%) had a global assessment rating of “excellent” vs one in 5 (20%) of those on pregabalin alone.
THE BOTTOM LINE Overall, ALA is well tolerated; the most common adverse effects are nausea and skin rash. IV administration is more effective than oral administration, but may cause nausea, headache, and an allergic reaction at the injection site.2 ALA does have the potential for an interaction with chemotherapy and thyroid hormone and may decrease the effectiveness of these therapies.2
B vitamins
Deficiencies of vitamin B1 (thiamine), B6 (pyridoxine), B12 (cyanocobalamin), and folate are known causes of neuropathy, and correcting them often improves or eliminates the symptoms.13 Vitamin B12 deficiency is commonly seen in patients taking metformin;14 these patients may benefit from supplementation with B12 1000 mcg/d.
Many of the B vitamins have been studied for treatment of neuropathy, but benfotiamine (a lipid-soluble form of thiamine) is thought to be the best option because it is better absorbed across cell membranes than other B vitamins.9 A Cochrane review found that benfotiamine alone may be effective for both diabetic and alcoholic neuropathy and that short-term use of higher doses of vitamin B complex (25 mg B1 or 320 mg benfotiamine + 50-720 mg B6 + 1000 mcg B12 daily) may reduce neuropathic pain.9
A randomized multicenter trial (N=214) found that adding a supplement containing L-methylfolate 3 mg, pyridoxal 5-phosphate 35 mg, and methylcobalamin 2 mg twice daily to other medications (eg, pregabalin, gabapentin, or duloxetine) improved symptoms of diabetic neuropathy.10 At 24 weeks, those receiving the combination therapy had a 26% decrease in pain symptoms compared with a 15% decrease for those on medication alone, with no significant adverse effects.
THE BOTTOM LINE Overall, vitamin B supplementation is well tolerated and appears to be more effective in relieving neuropathic pain than medication alone.9,14 But larger studies are needed before its efficacy in treating patients who do not have a deficiency can be established.
Capsaicin
Capsaicin, an ingredient found in peppers, works by binding to nociceptors to selectively stimulate afferent C fibers. This causes the release of substance P, a neurotransmitter that mediates pain, leading to its depletion and resulting in desensitization.2 Several meta-analyses and systematic reviews have found that topical capsaicin can be very effective, both as an adjunctive treatment and as monotherapy for neuropathic pain.11,17,18 The concentration used in the studies was 0.075% capsaicin cream, applied 3 to 4 times a day for 6 to 12 weeks, compared with placebo creams. In all categories studied, capsaicin was either statistically significant or trending in its favor, with the exception of adverse effects.
Capsaicin led to an improvement in daily activities and ability to sleep and a reduction in pain as measured with a visual analog scale and physician global evaluation.11,17,18
The most notable adverse effects were a burning sensation on the skin and coughing and sneezing caused by inhalation of dried cream. Although the adverse effects were expected to improve after 2 to 7 days of use, a significant number of participants withdrew from the study.
A 7-study meta-analysis showed the effectiveness of an 8% capsaicin patch for treatment of post-herpetic neuralgia and HIV-associated neuropathy.12 The patch, available only by prescription, was worn every day for 4 weeks (60 minutes daily for post-herpetic neuralgia and 30 minutes a day for HIV-associated neuropathy). The pooled results were statistically significant, but the patch was less effective for patients ages 18 to 40 years and for those of Asian descent. It can be used with other analgesics or as monotherapy, with few adverse reactions.12,19
THE BOTTOM LINE Since capsaicin is a topical medication, there are no relevant drug-drug interactions. Patients should be cautioned to wash their hands after application, however, and to avoid contact with eyes and open wounds.
Gamma linolenic acid (GLA)
Also known as evening primrose oil, GLA is an omega-6 fatty acid that’s an important constituent of neuronal cell membranes—and believed to decrease neuropathic pain by having some anti-inflammatory effects.2 This suggests that therapy with GLA has the potential to improve neuronal phospholipid structure and microcirculation.2
Two placebo-controlled trials (N=22,111) showed improvement in pain scores and multiple neurophysiologic assessments in patients with diabetes treated with GLA (360-480 mg/d).20,21 The treatment was well tolerated, but the beneficial effect was more pronounced in those with less severe diabetes.
THE BOTTOM LINE The dose of GLA studied (8 to 12 capsules daily) could lead to problems with patient adherence. In addition, GLA should be used with caution in patients who are taking antiplatelet medication or have seizure disorders.2
Magnesium (Mg)
Mg is highly involved in multiple enzyme systems throughout the body. Although it is very well absorbed from dietary sources,2 patients with diabetes, liver disease, and hormonal imbalances, as well as the elderly, are often deficient in Mg. It is unclear how this affects peripheral neuropathy.13
Mg may have an antinociceptive effect by decreasing intracellular calcium influx and antagonizing N-methyl-D-aspartate receptors and associated nerve signaling.22 A small RCT (N=80) showed Mg to decrease the severity of neuropathic back pain.22 Patients received Mg sulfate 1 g IV, given over 4 hours, every day for 2 weeks. The infusion was then replaced with Mg oxide 400 mg plus Mg gluconate 100 mg, taken orally twice daily for 4 weeks. An improvement in mean pain score was seen as early as 2 weeks, and scores had decreased by 2.8 points (on a 0-10-point scale) at 6 months.
Another small RCT (N=45) gave patients with neuropathy of postherpetic, traumatic, or surgical (but not diabetic) origin Mg chloride 838 mg orally 3 times a day for 4 weeks.23 The supplement was taken with meals. Mean pain scores in the treatment group decreased by 3 points, but this was not significantly different from the improvement seen in those on placebo.
In a similar study, patients (N=110) with type 1 diabetes and a normal serum Mg but an insufficiency as measured by erythrocyte Mg were given Mg gluconate 300 mg or placebo daily for 5 years.8 The supplement slowed the progression of peripheral neuropathy (only 12% of those receiving Mg gluconate experienced a significant worsening of symptoms over the course of the study, compared with 61% of those in the placebo group), but in most cases, it did not lead to an improvement.
No consistent approach to Mg supplementation has been studied, which makes recommending a particular route, dose, or formulation challenging. There is evidence that oral Mg, particularly in the form of Mg oxide, can cause diarrhea, especially in doses >350 mg/d. Mg gluconate and Mg chloride are better tolerated; Mg carbonate should be avoided due to poor oral absorption.2
BOTTOM LINE Mg supplementation appears to slow the progression of diabetic peripheral neuropathy, but is unsafe for patients with renal dysfunction, cardiac conduction abnormalities, or elevated Mg levels.2 Caution is required, too, when considering Mg supplementation for patients taking anticoagulants, bisphosphonates, digoxin, potassium-sparing diuretics, or tetracycline antibiotics.2
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
1. Chaparro LE, Wiffen PJ, Moore RA, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012:(7):CD008943.
2. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com. Accessed January 4, 2015.
3. Mijnhout GS, Kollen BJ, Alkhalaf A, et al. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279.
4. Patel N, Mishra V, Patel P, et al. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62.
5. Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12:29-34.
6. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3:223-231.
7. Sima AA, Calvani M, Mehra M, et al; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89-94.
8. De Leeuw, Engelen W, De Block C, et al. Long term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm). Magnes Res. 2004;17:109-114.
9. Ang CD, Alviar MJM, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573.
10. Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes
with peripheral neuropathy: a randomized trial. Am J Med. 2013;126:141-149.
11. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006; 11:294-329.
14. Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, et al. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol. 2011; 6:260-273.
15. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with a-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34:2054-2060.
16. Vasudevan D, Naik MM, Mukaddam QI. Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition. [MAINTAIN]: Results of a pilot study. Ann Indian Acad Neurol. 2014;17:19-24.
17. Halat KM, Dennehy CE. Botanicals and dietary supplements in diabetic peripheral neuropathy. J Am Board Fam Pract. 2003;16:47-57.
18. Donofrio P, Walker F, Hunt V, et al. Treatment of painful diabetic neuropathy with topical capsaicin: A multicenter, double-blind, vehicle-controlled study. Arch Int Med. 1991;151:2225-2229.
19. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
20. Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16:8-15.
21. Jamal GA, Carmichael H. The effect of gamma linolenic acid on human diabetic peripheral neuropathy: a double blind placebo controlled trial. Diabetic Med. 1990;7:319-323.
22. Yousef AA, Al-deeb AE. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68:260-266.
23. Pickering G, Morel V, Simen E. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011;24:28-35.
1. Chaparro LE, Wiffen PJ, Moore RA, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012:(7):CD008943.
2. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com. Accessed January 4, 2015.
3. Mijnhout GS, Kollen BJ, Alkhalaf A, et al. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279.
4. Patel N, Mishra V, Patel P, et al. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62.
5. Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12:29-34.
6. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3:223-231.
7. Sima AA, Calvani M, Mehra M, et al; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89-94.
8. De Leeuw, Engelen W, De Block C, et al. Long term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm). Magnes Res. 2004;17:109-114.
9. Ang CD, Alviar MJM, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573.
10. Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes
with peripheral neuropathy: a randomized trial. Am J Med. 2013;126:141-149.
11. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006; 11:294-329.
14. Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, et al. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol. 2011; 6:260-273.
15. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with a-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34:2054-2060.
16. Vasudevan D, Naik MM, Mukaddam QI. Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition. [MAINTAIN]: Results of a pilot study. Ann Indian Acad Neurol. 2014;17:19-24.
17. Halat KM, Dennehy CE. Botanicals and dietary supplements in diabetic peripheral neuropathy. J Am Board Fam Pract. 2003;16:47-57.
18. Donofrio P, Walker F, Hunt V, et al. Treatment of painful diabetic neuropathy with topical capsaicin: A multicenter, double-blind, vehicle-controlled study. Arch Int Med. 1991;151:2225-2229.
19. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
20. Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16:8-15.
21. Jamal GA, Carmichael H. The effect of gamma linolenic acid on human diabetic peripheral neuropathy: a double blind placebo controlled trial. Diabetic Med. 1990;7:319-323.
22. Yousef AA, Al-deeb AE. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68:260-266.
23. Pickering G, Morel V, Simen E. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011;24:28-35.
Player-to-Player Contact Is the Main Source of High School Soccer Concussions
Head contact with other players, not with the ball, is the main source of concussions among high school soccer players, according to research published online ahead of print July 13 in JAMA Pediatrics.
Several studies have shown that heading the ball is responsible for many soccer-related concussions. Some people have called for banning heading, especially among children and adolescents, to make the sport safer. No large study, however, had examined the exact mechanism of head injuries among school-aged soccer players, so such prevention efforts could not be considered evidence-based, said R. Dawn Comstock, PhD, an epidemiologist at the University of Colorado Denver in Aurora.
Dr. Comstock and colleagues performed a retrospective analysis of data from a large, Internet-based sports injury surveillance study, focusing on concussions sustained during soccer practices or games that required medical attention and restricted the athlete’s participation for one or more days. The investigators assessed nationally representative samples of 100 high schools every year for nine years. There were 627 concussions during 1,393,753 athlete exposures among girls (4.50 per 10,000 exposures) and 442 concussions during 1,592,238 athlete exposures among boys (2.78 per 10,000 exposures).
The most common mechanism of concussion was player-to-player contact among boys (68.8%) and girls (51.3%). Contact with the ball accounted for 17% of concussions among boys and 29% among girls.
The number and types of concussion symptoms were the same, regardless of whether the concussion was caused by player-to-player contact or player-to-ball contact. However, symptom resolution time was slightly but significantly longer for both boys and girls when the concussion was caused by collision with a ball or goal post.
“We postulate that banning heading from soccer will have limited effectiveness as a primary prevention mechanism unless such a ban is combined with concurrent efforts to reduce athlete–athlete contact throughout the game,” Dr. Comstock and her associates said.
“It may be culturally more tolerable to the soccer community to attempt to reduce athlete–athlete contact across all phases of play through better enforcement of existing rules, enhanced education of athletes on the rules of the game, and improved coaching of activities such as heading,” rather than simply banning heading, said the researchers.
—Mary Ann Moon
Head contact with other players, not with the ball, is the main source of concussions among high school soccer players, according to research published online ahead of print July 13 in JAMA Pediatrics.
Several studies have shown that heading the ball is responsible for many soccer-related concussions. Some people have called for banning heading, especially among children and adolescents, to make the sport safer. No large study, however, had examined the exact mechanism of head injuries among school-aged soccer players, so such prevention efforts could not be considered evidence-based, said R. Dawn Comstock, PhD, an epidemiologist at the University of Colorado Denver in Aurora.
Dr. Comstock and colleagues performed a retrospective analysis of data from a large, Internet-based sports injury surveillance study, focusing on concussions sustained during soccer practices or games that required medical attention and restricted the athlete’s participation for one or more days. The investigators assessed nationally representative samples of 100 high schools every year for nine years. There were 627 concussions during 1,393,753 athlete exposures among girls (4.50 per 10,000 exposures) and 442 concussions during 1,592,238 athlete exposures among boys (2.78 per 10,000 exposures).
The most common mechanism of concussion was player-to-player contact among boys (68.8%) and girls (51.3%). Contact with the ball accounted for 17% of concussions among boys and 29% among girls.
The number and types of concussion symptoms were the same, regardless of whether the concussion was caused by player-to-player contact or player-to-ball contact. However, symptom resolution time was slightly but significantly longer for both boys and girls when the concussion was caused by collision with a ball or goal post.
“We postulate that banning heading from soccer will have limited effectiveness as a primary prevention mechanism unless such a ban is combined with concurrent efforts to reduce athlete–athlete contact throughout the game,” Dr. Comstock and her associates said.
“It may be culturally more tolerable to the soccer community to attempt to reduce athlete–athlete contact across all phases of play through better enforcement of existing rules, enhanced education of athletes on the rules of the game, and improved coaching of activities such as heading,” rather than simply banning heading, said the researchers.
—Mary Ann Moon
Head contact with other players, not with the ball, is the main source of concussions among high school soccer players, according to research published online ahead of print July 13 in JAMA Pediatrics.
Several studies have shown that heading the ball is responsible for many soccer-related concussions. Some people have called for banning heading, especially among children and adolescents, to make the sport safer. No large study, however, had examined the exact mechanism of head injuries among school-aged soccer players, so such prevention efforts could not be considered evidence-based, said R. Dawn Comstock, PhD, an epidemiologist at the University of Colorado Denver in Aurora.
Dr. Comstock and colleagues performed a retrospective analysis of data from a large, Internet-based sports injury surveillance study, focusing on concussions sustained during soccer practices or games that required medical attention and restricted the athlete’s participation for one or more days. The investigators assessed nationally representative samples of 100 high schools every year for nine years. There were 627 concussions during 1,393,753 athlete exposures among girls (4.50 per 10,000 exposures) and 442 concussions during 1,592,238 athlete exposures among boys (2.78 per 10,000 exposures).
The most common mechanism of concussion was player-to-player contact among boys (68.8%) and girls (51.3%). Contact with the ball accounted for 17% of concussions among boys and 29% among girls.
The number and types of concussion symptoms were the same, regardless of whether the concussion was caused by player-to-player contact or player-to-ball contact. However, symptom resolution time was slightly but significantly longer for both boys and girls when the concussion was caused by collision with a ball or goal post.
“We postulate that banning heading from soccer will have limited effectiveness as a primary prevention mechanism unless such a ban is combined with concurrent efforts to reduce athlete–athlete contact throughout the game,” Dr. Comstock and her associates said.
“It may be culturally more tolerable to the soccer community to attempt to reduce athlete–athlete contact across all phases of play through better enforcement of existing rules, enhanced education of athletes on the rules of the game, and improved coaching of activities such as heading,” rather than simply banning heading, said the researchers.
—Mary Ann Moon
Getting in Shape May Help Reduce Irregular Heart Rhythm
(Reuters Health) - For overweight and obese people with atrial fibrillation, improving cardiorespiratory fitness with exercise may help to reduce or eliminate symptoms, a recent Australian study found.
Participants with the greatest improvements in their cardiorespiratory fitness were less burdened by symptoms of the arrhythmia and more likely to be symptom free during the study, compared to those who had smaller or no fitness improvements.
A possible side effect of the cardio exercise regimen, weight loss, may have also contributed to the improvements, researchers say.
Atrial fibrillation (AF) is a "growing epidemic" and obesity is a risk factor, the study's lead author Prash Sanders, director of the Center for Heart Rhythm Disorders at Royal Adelaide Hospital, told Reuters Health in an email.
The researchers examined the role of cardiorespiratory fitness and the incremental benefit of cardiorespiratory fitness improvement on rhythm control in 308 overweight and obese individuals with AF.
At the start of the study and four years later, participants completed questionnaires about their AF symptoms. The researchers also had patients wear a heart monitor for seven days at a time.
Based on baseline exercise stress testing, 95 people were classified as having low cardiorespiratory fitness, 134 had adequate fitness and 79 were classified as high fitness, Sanders and colleagues report in the Journal of the American College of Cardiology online June 22.
The research team prescribed the patients an exercise program tailored to their age and physical ability, which gradually increased in intensity.
By the end of four years, those who had increased their fitness levels by two metabolic equivalents (METs) or more, and those who lost weight were more likely to have reduced or no AF symptoms than those who improved by less than two METs or not at all.
Though increasing cardiorespiratory fitness seems to be beneficial, Dr. Michael Lloyd, a cardiologist at Emory University Hospital in Atlanta, Georgia, advises caution. "Overweight people should check with their doctor prior to embarking on any exercise program," said Lloyd, who was not involved in the study.
Sanders recommends a tailored exercise program, "in which consideration for age and physical ability should be made so that targets are achieved without risking injuries."
Dr. Waqas Qureshi, a cardiology researcher at Wake Forest University in Winston-Salem, North Carolina, who also was not involved in the study, noted that weight loss from exercise may add to the protection against heart irregularities.
Lloyd, however, emphasized that cardiorespiratory fitness is beneficial even apart from weight loss. "If you are overweight, it's not just the pounds that matter, it's how fit you are. This study shows that increasing your fitness through exercise and lifestyle choices does reduce your chance of having the most common arrhythmia."
(Reuters Health) - For overweight and obese people with atrial fibrillation, improving cardiorespiratory fitness with exercise may help to reduce or eliminate symptoms, a recent Australian study found.
Participants with the greatest improvements in their cardiorespiratory fitness were less burdened by symptoms of the arrhythmia and more likely to be symptom free during the study, compared to those who had smaller or no fitness improvements.
A possible side effect of the cardio exercise regimen, weight loss, may have also contributed to the improvements, researchers say.
Atrial fibrillation (AF) is a "growing epidemic" and obesity is a risk factor, the study's lead author Prash Sanders, director of the Center for Heart Rhythm Disorders at Royal Adelaide Hospital, told Reuters Health in an email.
The researchers examined the role of cardiorespiratory fitness and the incremental benefit of cardiorespiratory fitness improvement on rhythm control in 308 overweight and obese individuals with AF.
At the start of the study and four years later, participants completed questionnaires about their AF symptoms. The researchers also had patients wear a heart monitor for seven days at a time.
Based on baseline exercise stress testing, 95 people were classified as having low cardiorespiratory fitness, 134 had adequate fitness and 79 were classified as high fitness, Sanders and colleagues report in the Journal of the American College of Cardiology online June 22.
The research team prescribed the patients an exercise program tailored to their age and physical ability, which gradually increased in intensity.
By the end of four years, those who had increased their fitness levels by two metabolic equivalents (METs) or more, and those who lost weight were more likely to have reduced or no AF symptoms than those who improved by less than two METs or not at all.
Though increasing cardiorespiratory fitness seems to be beneficial, Dr. Michael Lloyd, a cardiologist at Emory University Hospital in Atlanta, Georgia, advises caution. "Overweight people should check with their doctor prior to embarking on any exercise program," said Lloyd, who was not involved in the study.
Sanders recommends a tailored exercise program, "in which consideration for age and physical ability should be made so that targets are achieved without risking injuries."
Dr. Waqas Qureshi, a cardiology researcher at Wake Forest University in Winston-Salem, North Carolina, who also was not involved in the study, noted that weight loss from exercise may add to the protection against heart irregularities.
Lloyd, however, emphasized that cardiorespiratory fitness is beneficial even apart from weight loss. "If you are overweight, it's not just the pounds that matter, it's how fit you are. This study shows that increasing your fitness through exercise and lifestyle choices does reduce your chance of having the most common arrhythmia."
(Reuters Health) - For overweight and obese people with atrial fibrillation, improving cardiorespiratory fitness with exercise may help to reduce or eliminate symptoms, a recent Australian study found.
Participants with the greatest improvements in their cardiorespiratory fitness were less burdened by symptoms of the arrhythmia and more likely to be symptom free during the study, compared to those who had smaller or no fitness improvements.
A possible side effect of the cardio exercise regimen, weight loss, may have also contributed to the improvements, researchers say.
Atrial fibrillation (AF) is a "growing epidemic" and obesity is a risk factor, the study's lead author Prash Sanders, director of the Center for Heart Rhythm Disorders at Royal Adelaide Hospital, told Reuters Health in an email.
The researchers examined the role of cardiorespiratory fitness and the incremental benefit of cardiorespiratory fitness improvement on rhythm control in 308 overweight and obese individuals with AF.
At the start of the study and four years later, participants completed questionnaires about their AF symptoms. The researchers also had patients wear a heart monitor for seven days at a time.
Based on baseline exercise stress testing, 95 people were classified as having low cardiorespiratory fitness, 134 had adequate fitness and 79 were classified as high fitness, Sanders and colleagues report in the Journal of the American College of Cardiology online June 22.
The research team prescribed the patients an exercise program tailored to their age and physical ability, which gradually increased in intensity.
By the end of four years, those who had increased their fitness levels by two metabolic equivalents (METs) or more, and those who lost weight were more likely to have reduced or no AF symptoms than those who improved by less than two METs or not at all.
Though increasing cardiorespiratory fitness seems to be beneficial, Dr. Michael Lloyd, a cardiologist at Emory University Hospital in Atlanta, Georgia, advises caution. "Overweight people should check with their doctor prior to embarking on any exercise program," said Lloyd, who was not involved in the study.
Sanders recommends a tailored exercise program, "in which consideration for age and physical ability should be made so that targets are achieved without risking injuries."
Dr. Waqas Qureshi, a cardiology researcher at Wake Forest University in Winston-Salem, North Carolina, who also was not involved in the study, noted that weight loss from exercise may add to the protection against heart irregularities.
Lloyd, however, emphasized that cardiorespiratory fitness is beneficial even apart from weight loss. "If you are overweight, it's not just the pounds that matter, it's how fit you are. This study shows that increasing your fitness through exercise and lifestyle choices does reduce your chance of having the most common arrhythmia."
PHM15: Physical Burnout for Hospitalists
Presenters: Allison Ballantine and Lisa Zaoutis
Physician burnout can be thought of as similar to water level in a reservoir during a drought – there is an imbalance between usage and replenishment. This leads to physician exhaustion, cynicism, and inefficiency. Burnout is a function of both the individual (younger, single, and less resilient are at higher risk) and the environment (high job demands with inadequate resources).
Burnout can affect:
- Job performance. With increased error rates, decreased cognitive function, decreased patient safety, and increased risk medmal litigation.
- Physician retention. A burned out physician is more likely to leave with subsequent practice disruption and cost.
- Physician health. Increased risk of depression, substance abuse, and suicidality.
Interventions can help prevent and mitigate burnout from both a personal and institutional perspective. Individuals can mitigate potential burnout through different strategies. These include having a strong support network and practicing mindfulness to decrease rumination about work related issues. Engaging in relaxing activities that provide reward with low effort as well as physical actions, such as ensuring private time with no calls, will lessen stresses.
Institutional efforts to decrease burnout need to focus on factors that address perceived fairness in the workplace, adequate financial compensation, social recognition, and an appropriate balance between responsibility and authority.
More resources can be found at the speakers’ website and through Christina Maslach, a leading researcher in the field.
Presenters: Allison Ballantine and Lisa Zaoutis
Physician burnout can be thought of as similar to water level in a reservoir during a drought – there is an imbalance between usage and replenishment. This leads to physician exhaustion, cynicism, and inefficiency. Burnout is a function of both the individual (younger, single, and less resilient are at higher risk) and the environment (high job demands with inadequate resources).
Burnout can affect:
- Job performance. With increased error rates, decreased cognitive function, decreased patient safety, and increased risk medmal litigation.
- Physician retention. A burned out physician is more likely to leave with subsequent practice disruption and cost.
- Physician health. Increased risk of depression, substance abuse, and suicidality.
Interventions can help prevent and mitigate burnout from both a personal and institutional perspective. Individuals can mitigate potential burnout through different strategies. These include having a strong support network and practicing mindfulness to decrease rumination about work related issues. Engaging in relaxing activities that provide reward with low effort as well as physical actions, such as ensuring private time with no calls, will lessen stresses.
Institutional efforts to decrease burnout need to focus on factors that address perceived fairness in the workplace, adequate financial compensation, social recognition, and an appropriate balance between responsibility and authority.
More resources can be found at the speakers’ website and through Christina Maslach, a leading researcher in the field.
Presenters: Allison Ballantine and Lisa Zaoutis
Physician burnout can be thought of as similar to water level in a reservoir during a drought – there is an imbalance between usage and replenishment. This leads to physician exhaustion, cynicism, and inefficiency. Burnout is a function of both the individual (younger, single, and less resilient are at higher risk) and the environment (high job demands with inadequate resources).
Burnout can affect:
- Job performance. With increased error rates, decreased cognitive function, decreased patient safety, and increased risk medmal litigation.
- Physician retention. A burned out physician is more likely to leave with subsequent practice disruption and cost.
- Physician health. Increased risk of depression, substance abuse, and suicidality.
Interventions can help prevent and mitigate burnout from both a personal and institutional perspective. Individuals can mitigate potential burnout through different strategies. These include having a strong support network and practicing mindfulness to decrease rumination about work related issues. Engaging in relaxing activities that provide reward with low effort as well as physical actions, such as ensuring private time with no calls, will lessen stresses.
Institutional efforts to decrease burnout need to focus on factors that address perceived fairness in the workplace, adequate financial compensation, social recognition, and an appropriate balance between responsibility and authority.
More resources can be found at the speakers’ website and through Christina Maslach, a leading researcher in the field.
Combo may overcome TKI resistance in CML

Schürch, MD, PhD, (left)
and Adrian Ochsenbein, MD
Photo by Susi Bürki
Combining a tyrosine kinase inhibitor (TKI) with a monoclonal antibody (mAb) may circumvent TKI resistance in chronic myeloid leukemia (CML), according to preclinical research published in Science Translational Medicine.
To understand how TKI resistance develops, researchers analyzed the effect of these drugs on BCR-ABL1+ leukemia cell lines, cells from patients with newly diagnosed CML, and mouse models of CML.
The team found that TKIs induce CD70 expression in leukemic stem cells (LSCs) by downregulating microRNA-29. This results in reduced CD70 promoter DNA methylation and upregulation of the transcription factor specificity protein 1 (SP1).
The increase in CD70 triggers CD27 signaling and compensatory Wnt pathway activation. The researchers said this suggests LSCs evade TKIs by activating Wnt signaling through this route.
So the team hypothesized that combination treatment with a TKI and a mAb blocking the CD70/CD27 interaction would eradicate LSCs.
First, they tested an αCD27 mAb alone or in combination with a TKI in leukemia cell lines. Compared to either agent alone, αCD27/imatinib cotreatment significantly (P<0.001) reduced cell growth by inhibiting proliferation and enhancing apoptosis in SD-1 cells.
The researchers observed similar results when they tested the αCD27 mAb and nilotinib in SD-1 cells, as well as when they tested the αCD27 mAb with imatinib or ponatinib in KBM5 and KBM5r cells.
The team noted that αCD27/imatinib cotreatment inhibited Wnt pathway activation significantly stronger than either compound alone (P<0.001) but had little to no effect on Notch, Hedgehog, and MAP kinase pathways.
The researchers also conducted in vitro tests with an αCD70 mAb (clone 41D12-D) that was specifically designed to block the CD70/CD27 interaction without inducing effector functions such as antibody-dependent cell- or complement-mediated cytotoxicity and antibody-dependent cell-mediated phagocytosis.
They found that αCD70/imatinib cotreatment “potently reduced” CD34+ CML stem/progenitor cells in liquid cultures by inhibiting proliferation and increasing apoptosis. The combination also significantly impaired colony formation in semisolid cultures when compared to either agent alone (P<0.05).
In addition, αCD70/imatinib cotreatment eliminated human CD34+ CML stem/progenitor cells in murine xenografts. The LSCs were completely eradicated in 9 of 12 mice treated.
In a murine CML model, combination treatment with imatinib and an αCD70 mAb (clone FR70) significantly improved survival (P<0.001) compared to either agent alone. And 60% of mice (9 of 15) that received the combination were alive 90 days after transplantation.
The researchers said this suggests the LSCs were completely eradicated or at least effectively controlled long-term. ![]()

Schürch, MD, PhD, (left)
and Adrian Ochsenbein, MD
Photo by Susi Bürki
Combining a tyrosine kinase inhibitor (TKI) with a monoclonal antibody (mAb) may circumvent TKI resistance in chronic myeloid leukemia (CML), according to preclinical research published in Science Translational Medicine.
To understand how TKI resistance develops, researchers analyzed the effect of these drugs on BCR-ABL1+ leukemia cell lines, cells from patients with newly diagnosed CML, and mouse models of CML.
The team found that TKIs induce CD70 expression in leukemic stem cells (LSCs) by downregulating microRNA-29. This results in reduced CD70 promoter DNA methylation and upregulation of the transcription factor specificity protein 1 (SP1).
The increase in CD70 triggers CD27 signaling and compensatory Wnt pathway activation. The researchers said this suggests LSCs evade TKIs by activating Wnt signaling through this route.
So the team hypothesized that combination treatment with a TKI and a mAb blocking the CD70/CD27 interaction would eradicate LSCs.
First, they tested an αCD27 mAb alone or in combination with a TKI in leukemia cell lines. Compared to either agent alone, αCD27/imatinib cotreatment significantly (P<0.001) reduced cell growth by inhibiting proliferation and enhancing apoptosis in SD-1 cells.
The researchers observed similar results when they tested the αCD27 mAb and nilotinib in SD-1 cells, as well as when they tested the αCD27 mAb with imatinib or ponatinib in KBM5 and KBM5r cells.
The team noted that αCD27/imatinib cotreatment inhibited Wnt pathway activation significantly stronger than either compound alone (P<0.001) but had little to no effect on Notch, Hedgehog, and MAP kinase pathways.
The researchers also conducted in vitro tests with an αCD70 mAb (clone 41D12-D) that was specifically designed to block the CD70/CD27 interaction without inducing effector functions such as antibody-dependent cell- or complement-mediated cytotoxicity and antibody-dependent cell-mediated phagocytosis.
They found that αCD70/imatinib cotreatment “potently reduced” CD34+ CML stem/progenitor cells in liquid cultures by inhibiting proliferation and increasing apoptosis. The combination also significantly impaired colony formation in semisolid cultures when compared to either agent alone (P<0.05).
In addition, αCD70/imatinib cotreatment eliminated human CD34+ CML stem/progenitor cells in murine xenografts. The LSCs were completely eradicated in 9 of 12 mice treated.
In a murine CML model, combination treatment with imatinib and an αCD70 mAb (clone FR70) significantly improved survival (P<0.001) compared to either agent alone. And 60% of mice (9 of 15) that received the combination were alive 90 days after transplantation.
The researchers said this suggests the LSCs were completely eradicated or at least effectively controlled long-term. ![]()

Schürch, MD, PhD, (left)
and Adrian Ochsenbein, MD
Photo by Susi Bürki
Combining a tyrosine kinase inhibitor (TKI) with a monoclonal antibody (mAb) may circumvent TKI resistance in chronic myeloid leukemia (CML), according to preclinical research published in Science Translational Medicine.
To understand how TKI resistance develops, researchers analyzed the effect of these drugs on BCR-ABL1+ leukemia cell lines, cells from patients with newly diagnosed CML, and mouse models of CML.
The team found that TKIs induce CD70 expression in leukemic stem cells (LSCs) by downregulating microRNA-29. This results in reduced CD70 promoter DNA methylation and upregulation of the transcription factor specificity protein 1 (SP1).
The increase in CD70 triggers CD27 signaling and compensatory Wnt pathway activation. The researchers said this suggests LSCs evade TKIs by activating Wnt signaling through this route.
So the team hypothesized that combination treatment with a TKI and a mAb blocking the CD70/CD27 interaction would eradicate LSCs.
First, they tested an αCD27 mAb alone or in combination with a TKI in leukemia cell lines. Compared to either agent alone, αCD27/imatinib cotreatment significantly (P<0.001) reduced cell growth by inhibiting proliferation and enhancing apoptosis in SD-1 cells.
The researchers observed similar results when they tested the αCD27 mAb and nilotinib in SD-1 cells, as well as when they tested the αCD27 mAb with imatinib or ponatinib in KBM5 and KBM5r cells.
The team noted that αCD27/imatinib cotreatment inhibited Wnt pathway activation significantly stronger than either compound alone (P<0.001) but had little to no effect on Notch, Hedgehog, and MAP kinase pathways.
The researchers also conducted in vitro tests with an αCD70 mAb (clone 41D12-D) that was specifically designed to block the CD70/CD27 interaction without inducing effector functions such as antibody-dependent cell- or complement-mediated cytotoxicity and antibody-dependent cell-mediated phagocytosis.
They found that αCD70/imatinib cotreatment “potently reduced” CD34+ CML stem/progenitor cells in liquid cultures by inhibiting proliferation and increasing apoptosis. The combination also significantly impaired colony formation in semisolid cultures when compared to either agent alone (P<0.05).
In addition, αCD70/imatinib cotreatment eliminated human CD34+ CML stem/progenitor cells in murine xenografts. The LSCs were completely eradicated in 9 of 12 mice treated.
In a murine CML model, combination treatment with imatinib and an αCD70 mAb (clone FR70) significantly improved survival (P<0.001) compared to either agent alone. And 60% of mice (9 of 15) that received the combination were alive 90 days after transplantation.
The researchers said this suggests the LSCs were completely eradicated or at least effectively controlled long-term. ![]()
What the Supreme Court ruling in King v. Burwell means for women’s health
In a widely anticipated judgment on the Affordable Care Act (ACA), the US Supreme Court ruled 6-3 in favor of the law on June 26, 2015. The case at hand, King v. Burwell, challenged whether individuals purchasing health insurance through federal exchanges were eligible for federal premium subsidies. This ruling cemented the ACA into law and avoided a potential calamity in the private health insurance market. Let’s take a closer look.
What the case was about
The ACA allows states to set up their own health insurance exchanges or participate in a federally run exchange. Although the drafters of the ACA had expected each state to set up its own exchange, two-thirds of the states declined to do so, many in opposition to the ACA. As a result, 7 million citizens in 34 states now purchase their health insurance through federally created exchanges.
The plaintiffs in King v. Burwell argued that, because the legislation refers to those enrolled “through an Exchange established by the State,” individuals in states with federally run exchanges are not eligible for subsidies.
The law states:
(A) the monthly premiums for such month for 1 or more qualified health plans offered in the individual market within a State which cover the taxpayer, the taxpayer’s spouse, or any dependent (as defined in section 152) of the taxpayer and which were enrolled in through an Exchange established by the State under 1311 of the Patient Protection and Affordable Care Act…[emphasis added].
The Supreme Court was asked to decide whether to adhere to those exact words or to honor Congress’ intent to allow individuals to purchase subsidized insurance on any type of exchange.
What might have happened
We’ve explored in previous articles the interconnectedness of many sections of the ACA. Nowhere is that interconnectedness more clearly demonstrated than here. In order to ensure that private health insurers provide better coverage, the law requires them to abide by important consumer protections, including the elimination of “preexisting condition” exclusions. In order to prevent adverse selection and keep insurers solvent under these new rules, all individuals are required to have health care coverage—the individual mandate. If everyone is required to purchase health insurance, it has to be affordable, so lower-income individuals were promised subsidies, paid for 100% by the federal government, to help them cover their premiums when insurance is purchased through an exchange. Take away the subsidies and the whole thing starts to unravel.
The Urban Institute estimated that a Supreme Court ruling in favor of King, which would have eliminated the subsidies in states using a federal exchange, would have reduced federal tax subsidies by $29 billion in 2016, making coverage unaffordable for many and increasing the ranks of the uninsured by 8.2 million people.1
Louise Sheiner and Brendan Mochoruk of the Brookings Institute speculated that healthy individuals would disproportionately leave the marketplace, triggering 35% increases in insurance premiums for those remaining, as well as significant increases in premiums for those who just lost their subsidies.2 Many observers, including these experts, forecast that insurance companies would exit the federal exchanges altogether, triggering a health insurance “death spiral”: As premiums rise, the healthiest customers leave the marketplace, causing premiums to rise more, causing more healthy people to leave, and so on.
Clearly, this Supreme Court decision has had dramatic, long-term, real-world effects on millions of Americans. On the national level, 6,387,789 individuals were at risk of losing their tax credits if the Supreme Court had ruled in favor of King. That number represents more than $1.7 billion in total monthly tax credits. For a look at how a judgment in favor of King would have affected subsidies on a state-by-state basis, see TABLE 1.
What other commentators are saying about the King v. Burwell decision
In his majority opinion, Chief Justice John Roberts noted that the “meaning of the phrase ‘established by the State’ is not so clear.” And as Amy Howe articulated on SCOTUSblog: “if the phrase…is in fact not clear…then the next step is to look at the Affordable Care Act more broadly to determine what Congress meant by the phrase. And when you do that, the Court reasoned, it becomes apparent that Congress actually intended for the subsidies to be available to everyone who buys health insurance on an exchange, no matter who created it. If the subsidies weren’t available in the states with federal exchanges, the Court explained, the insurance markets in those states simply wouldn’t work properly: without the subsidies, almost all of the people who purchased insurance on the exchanges would no longer be required to purchase insurance because it would be too expensive. This would create a ‘death spiral’….”
—Amy Howe, SCOTUSblog3
“Additional court challenges to other ACA provisions are still possible, but King’s six-member majority shows little appetite for challenges threatening the Act’s core structure. Even Scalia’s dissent recognizes that the ACA may one day ‘attain the enduring status of the Social Security Act.’ Thus, the decision may usher in a new era of policy maturity, in which efforts to undermine the ACA diminish, as focus shifts to efforts to implement and improve it.”
—Mark A. Hall, JD, New England Journal of Medicine4
“With the Court upholding the administration’s interpretation of the law, the Obama administration has little reason to accede to
Republican proposals. The Court’s decision effectively puts the future of the ACA on hold until the 2016 elections, when the people will decide whether to stay the course or to chart a very different path.”
—Timothy Jost, Health Affairs5
“A case that 6 months ago seemed to offer the Court’s conservatives a low-risk opportunity to accomplish what they almost did in 2012—kill the Affordable Care Act—became suffused with danger, for the millions of newly insured Americans, of course, but also for the Supreme Court itself. Ideology came face to face with reality, and reality prevailed.”
—Linda Greenhouse, New York Times6
How premium subsidies work
Premium subsidies are actually tax credits. Individuals and families can qualify for them to purchase any type of health insurance offered on an exchange, except catastrophic coverage. To receive the premium tax credit for coverage starting in 2015, a marketplace enrollee must:
- have a household income that is 1 to4 times the federal poverty level. In 2015, the range of incomes that qualify for subsidies is $11,670 for an individual and $23,850 for a family of 4 at 100% of the federal poverty level. At 400% of the federal poverty level, it is $46,680 for an individual and $95,400 for a family of 4.
- lack access to affordable coverage through an employer (including a family member’s employer)
- be ineligible for coverage through Medicare, Medicaid, the Children’s Health Insurance Program, or other forms of public assistance
- have US citizenship or proof of legal residency
- file taxes jointly if married.
The premium tax credit caps the amount that an individual or family must spend on their monthly payments for health insurance. The cap depends on the family’s income; lower-income families have a lower cap. The amount of the tax credit remains the same, so a person who purchases a more expensive plan pays the cost difference (TABLE 2).
The ruling’s effect on women’s health
On June 26, American College of Obstetricians and Gynecologists President Mark S. DeFrancesco, MD, MBA, hailed the Supreme Court decision, saying, “Importantly, recent data have shown that newly insured adults under the ACA were more likely to be women. Those who did gain coverage through the ACA reported better access to health care and better financial security from medical costs.”
“Without question, many women enrollees were able to purchase health insurance coverage due, in part, to the ACA subsidies that helped make this purchase affordable. In fact, government data have suggested that roughly 85% of health exchange enrollees received subsidies,” Dr. DeFrancesco said.
“If the Supreme Court had overturned this important assistance, approximately 4.8 million women would have been unable to afford the coverage that they need. The impact also would have been widespread; as these women were forced to leave the insurance marketplace, it is likely that premiums throughout the marketplace would have risen dramatically,” he continued.
“Instead, patients—especially the low- and moderate-income American women who have particularly benefited from ACA subsidies—will continue to have the peace of mind that comes with insurance coverage.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Blumberg LJ, Buettgens M, Holahan J. The implications of a Supreme Court finding for the plaintiff in King v. Burwell: 8.2 million more uninsured and 35% higher premiums. Urban Institute. http://www.urban.org/research/publication/implications-supreme-court-finding-plaintiff-king-vs-burwell-82-million-more-uninsured-and-35-higher-premiums. Published January 8, 2015. Accessed July 2, 2015.
2. Sheiner L, Mochoruk B. King v. Burwell explained. Brookings Institute. http://www.brookings.edu/blogs/health360/posts/2015/03/03-king-v-burwell-explainer-sheiner. Published March 3, 2015. Accessed July 2, 2015.
3. Howe A. Court backs Obama administration on health care subsidies: In plain English. SCOTUSblog. http://www.scotusblog.com/2015/06/court-backs-obama-administration-on-health-care-subsidies-in-plain-english/. Published June 25, 2015. Accessed July 1, 2015.
4. Hall MA. King v. Burwell—ACA Armageddon averted. N Engl J Med. http://www.nejm.org/doi/full/10.1056/NEJMp1504077. Published July 1, 2015. Accessed July 2, 2015.
5. Jost T. Implementing health reform: The Supreme Court upholds tax credits in the federal exchange. Health Affairs blog. http://healthaffairs.org/blog/2015/06/25/implementing-health-reform-the-supreme-court-upholds-tax-credits-in-the-federal-exchange/. Published June 25, 2015. Accessed July 1, 2015.
6. Greenhouse L. The Roberts Court’s reality check. New York Times. http://www.nytimes.com/2015/06/26/opinion/the-roberts-courts-reality-check.html. Published June 25, 2015. Accessed July 1, 2015.
7. Henry J. Kaiser Family Foundation. Explaining health care reform: questions about health insurance subsidies. Table 2. http://kff.org/health-reform/issue-brief/explaining-health-care-reform-questions-about-health/. Published October 27, 2014. Accessed July 2, 2015.
In a widely anticipated judgment on the Affordable Care Act (ACA), the US Supreme Court ruled 6-3 in favor of the law on June 26, 2015. The case at hand, King v. Burwell, challenged whether individuals purchasing health insurance through federal exchanges were eligible for federal premium subsidies. This ruling cemented the ACA into law and avoided a potential calamity in the private health insurance market. Let’s take a closer look.
What the case was about
The ACA allows states to set up their own health insurance exchanges or participate in a federally run exchange. Although the drafters of the ACA had expected each state to set up its own exchange, two-thirds of the states declined to do so, many in opposition to the ACA. As a result, 7 million citizens in 34 states now purchase their health insurance through federally created exchanges.
The plaintiffs in King v. Burwell argued that, because the legislation refers to those enrolled “through an Exchange established by the State,” individuals in states with federally run exchanges are not eligible for subsidies.
The law states:
(A) the monthly premiums for such month for 1 or more qualified health plans offered in the individual market within a State which cover the taxpayer, the taxpayer’s spouse, or any dependent (as defined in section 152) of the taxpayer and which were enrolled in through an Exchange established by the State under 1311 of the Patient Protection and Affordable Care Act…[emphasis added].
The Supreme Court was asked to decide whether to adhere to those exact words or to honor Congress’ intent to allow individuals to purchase subsidized insurance on any type of exchange.
What might have happened
We’ve explored in previous articles the interconnectedness of many sections of the ACA. Nowhere is that interconnectedness more clearly demonstrated than here. In order to ensure that private health insurers provide better coverage, the law requires them to abide by important consumer protections, including the elimination of “preexisting condition” exclusions. In order to prevent adverse selection and keep insurers solvent under these new rules, all individuals are required to have health care coverage—the individual mandate. If everyone is required to purchase health insurance, it has to be affordable, so lower-income individuals were promised subsidies, paid for 100% by the federal government, to help them cover their premiums when insurance is purchased through an exchange. Take away the subsidies and the whole thing starts to unravel.
The Urban Institute estimated that a Supreme Court ruling in favor of King, which would have eliminated the subsidies in states using a federal exchange, would have reduced federal tax subsidies by $29 billion in 2016, making coverage unaffordable for many and increasing the ranks of the uninsured by 8.2 million people.1
Louise Sheiner and Brendan Mochoruk of the Brookings Institute speculated that healthy individuals would disproportionately leave the marketplace, triggering 35% increases in insurance premiums for those remaining, as well as significant increases in premiums for those who just lost their subsidies.2 Many observers, including these experts, forecast that insurance companies would exit the federal exchanges altogether, triggering a health insurance “death spiral”: As premiums rise, the healthiest customers leave the marketplace, causing premiums to rise more, causing more healthy people to leave, and so on.
Clearly, this Supreme Court decision has had dramatic, long-term, real-world effects on millions of Americans. On the national level, 6,387,789 individuals were at risk of losing their tax credits if the Supreme Court had ruled in favor of King. That number represents more than $1.7 billion in total monthly tax credits. For a look at how a judgment in favor of King would have affected subsidies on a state-by-state basis, see TABLE 1.
What other commentators are saying about the King v. Burwell decision
In his majority opinion, Chief Justice John Roberts noted that the “meaning of the phrase ‘established by the State’ is not so clear.” And as Amy Howe articulated on SCOTUSblog: “if the phrase…is in fact not clear…then the next step is to look at the Affordable Care Act more broadly to determine what Congress meant by the phrase. And when you do that, the Court reasoned, it becomes apparent that Congress actually intended for the subsidies to be available to everyone who buys health insurance on an exchange, no matter who created it. If the subsidies weren’t available in the states with federal exchanges, the Court explained, the insurance markets in those states simply wouldn’t work properly: without the subsidies, almost all of the people who purchased insurance on the exchanges would no longer be required to purchase insurance because it would be too expensive. This would create a ‘death spiral’….”
—Amy Howe, SCOTUSblog3
“Additional court challenges to other ACA provisions are still possible, but King’s six-member majority shows little appetite for challenges threatening the Act’s core structure. Even Scalia’s dissent recognizes that the ACA may one day ‘attain the enduring status of the Social Security Act.’ Thus, the decision may usher in a new era of policy maturity, in which efforts to undermine the ACA diminish, as focus shifts to efforts to implement and improve it.”
—Mark A. Hall, JD, New England Journal of Medicine4
“With the Court upholding the administration’s interpretation of the law, the Obama administration has little reason to accede to
Republican proposals. The Court’s decision effectively puts the future of the ACA on hold until the 2016 elections, when the people will decide whether to stay the course or to chart a very different path.”
—Timothy Jost, Health Affairs5
“A case that 6 months ago seemed to offer the Court’s conservatives a low-risk opportunity to accomplish what they almost did in 2012—kill the Affordable Care Act—became suffused with danger, for the millions of newly insured Americans, of course, but also for the Supreme Court itself. Ideology came face to face with reality, and reality prevailed.”
—Linda Greenhouse, New York Times6
How premium subsidies work
Premium subsidies are actually tax credits. Individuals and families can qualify for them to purchase any type of health insurance offered on an exchange, except catastrophic coverage. To receive the premium tax credit for coverage starting in 2015, a marketplace enrollee must:
- have a household income that is 1 to4 times the federal poverty level. In 2015, the range of incomes that qualify for subsidies is $11,670 for an individual and $23,850 for a family of 4 at 100% of the federal poverty level. At 400% of the federal poverty level, it is $46,680 for an individual and $95,400 for a family of 4.
- lack access to affordable coverage through an employer (including a family member’s employer)
- be ineligible for coverage through Medicare, Medicaid, the Children’s Health Insurance Program, or other forms of public assistance
- have US citizenship or proof of legal residency
- file taxes jointly if married.
The premium tax credit caps the amount that an individual or family must spend on their monthly payments for health insurance. The cap depends on the family’s income; lower-income families have a lower cap. The amount of the tax credit remains the same, so a person who purchases a more expensive plan pays the cost difference (TABLE 2).
The ruling’s effect on women’s health
On June 26, American College of Obstetricians and Gynecologists President Mark S. DeFrancesco, MD, MBA, hailed the Supreme Court decision, saying, “Importantly, recent data have shown that newly insured adults under the ACA were more likely to be women. Those who did gain coverage through the ACA reported better access to health care and better financial security from medical costs.”
“Without question, many women enrollees were able to purchase health insurance coverage due, in part, to the ACA subsidies that helped make this purchase affordable. In fact, government data have suggested that roughly 85% of health exchange enrollees received subsidies,” Dr. DeFrancesco said.
“If the Supreme Court had overturned this important assistance, approximately 4.8 million women would have been unable to afford the coverage that they need. The impact also would have been widespread; as these women were forced to leave the insurance marketplace, it is likely that premiums throughout the marketplace would have risen dramatically,” he continued.
“Instead, patients—especially the low- and moderate-income American women who have particularly benefited from ACA subsidies—will continue to have the peace of mind that comes with insurance coverage.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In a widely anticipated judgment on the Affordable Care Act (ACA), the US Supreme Court ruled 6-3 in favor of the law on June 26, 2015. The case at hand, King v. Burwell, challenged whether individuals purchasing health insurance through federal exchanges were eligible for federal premium subsidies. This ruling cemented the ACA into law and avoided a potential calamity in the private health insurance market. Let’s take a closer look.
What the case was about
The ACA allows states to set up their own health insurance exchanges or participate in a federally run exchange. Although the drafters of the ACA had expected each state to set up its own exchange, two-thirds of the states declined to do so, many in opposition to the ACA. As a result, 7 million citizens in 34 states now purchase their health insurance through federally created exchanges.
The plaintiffs in King v. Burwell argued that, because the legislation refers to those enrolled “through an Exchange established by the State,” individuals in states with federally run exchanges are not eligible for subsidies.
The law states:
(A) the monthly premiums for such month for 1 or more qualified health plans offered in the individual market within a State which cover the taxpayer, the taxpayer’s spouse, or any dependent (as defined in section 152) of the taxpayer and which were enrolled in through an Exchange established by the State under 1311 of the Patient Protection and Affordable Care Act…[emphasis added].
The Supreme Court was asked to decide whether to adhere to those exact words or to honor Congress’ intent to allow individuals to purchase subsidized insurance on any type of exchange.
What might have happened
We’ve explored in previous articles the interconnectedness of many sections of the ACA. Nowhere is that interconnectedness more clearly demonstrated than here. In order to ensure that private health insurers provide better coverage, the law requires them to abide by important consumer protections, including the elimination of “preexisting condition” exclusions. In order to prevent adverse selection and keep insurers solvent under these new rules, all individuals are required to have health care coverage—the individual mandate. If everyone is required to purchase health insurance, it has to be affordable, so lower-income individuals were promised subsidies, paid for 100% by the federal government, to help them cover their premiums when insurance is purchased through an exchange. Take away the subsidies and the whole thing starts to unravel.
The Urban Institute estimated that a Supreme Court ruling in favor of King, which would have eliminated the subsidies in states using a federal exchange, would have reduced federal tax subsidies by $29 billion in 2016, making coverage unaffordable for many and increasing the ranks of the uninsured by 8.2 million people.1
Louise Sheiner and Brendan Mochoruk of the Brookings Institute speculated that healthy individuals would disproportionately leave the marketplace, triggering 35% increases in insurance premiums for those remaining, as well as significant increases in premiums for those who just lost their subsidies.2 Many observers, including these experts, forecast that insurance companies would exit the federal exchanges altogether, triggering a health insurance “death spiral”: As premiums rise, the healthiest customers leave the marketplace, causing premiums to rise more, causing more healthy people to leave, and so on.
Clearly, this Supreme Court decision has had dramatic, long-term, real-world effects on millions of Americans. On the national level, 6,387,789 individuals were at risk of losing their tax credits if the Supreme Court had ruled in favor of King. That number represents more than $1.7 billion in total monthly tax credits. For a look at how a judgment in favor of King would have affected subsidies on a state-by-state basis, see TABLE 1.
What other commentators are saying about the King v. Burwell decision
In his majority opinion, Chief Justice John Roberts noted that the “meaning of the phrase ‘established by the State’ is not so clear.” And as Amy Howe articulated on SCOTUSblog: “if the phrase…is in fact not clear…then the next step is to look at the Affordable Care Act more broadly to determine what Congress meant by the phrase. And when you do that, the Court reasoned, it becomes apparent that Congress actually intended for the subsidies to be available to everyone who buys health insurance on an exchange, no matter who created it. If the subsidies weren’t available in the states with federal exchanges, the Court explained, the insurance markets in those states simply wouldn’t work properly: without the subsidies, almost all of the people who purchased insurance on the exchanges would no longer be required to purchase insurance because it would be too expensive. This would create a ‘death spiral’….”
—Amy Howe, SCOTUSblog3
“Additional court challenges to other ACA provisions are still possible, but King’s six-member majority shows little appetite for challenges threatening the Act’s core structure. Even Scalia’s dissent recognizes that the ACA may one day ‘attain the enduring status of the Social Security Act.’ Thus, the decision may usher in a new era of policy maturity, in which efforts to undermine the ACA diminish, as focus shifts to efforts to implement and improve it.”
—Mark A. Hall, JD, New England Journal of Medicine4
“With the Court upholding the administration’s interpretation of the law, the Obama administration has little reason to accede to
Republican proposals. The Court’s decision effectively puts the future of the ACA on hold until the 2016 elections, when the people will decide whether to stay the course or to chart a very different path.”
—Timothy Jost, Health Affairs5
“A case that 6 months ago seemed to offer the Court’s conservatives a low-risk opportunity to accomplish what they almost did in 2012—kill the Affordable Care Act—became suffused with danger, for the millions of newly insured Americans, of course, but also for the Supreme Court itself. Ideology came face to face with reality, and reality prevailed.”
—Linda Greenhouse, New York Times6
How premium subsidies work
Premium subsidies are actually tax credits. Individuals and families can qualify for them to purchase any type of health insurance offered on an exchange, except catastrophic coverage. To receive the premium tax credit for coverage starting in 2015, a marketplace enrollee must:
- have a household income that is 1 to4 times the federal poverty level. In 2015, the range of incomes that qualify for subsidies is $11,670 for an individual and $23,850 for a family of 4 at 100% of the federal poverty level. At 400% of the federal poverty level, it is $46,680 for an individual and $95,400 for a family of 4.
- lack access to affordable coverage through an employer (including a family member’s employer)
- be ineligible for coverage through Medicare, Medicaid, the Children’s Health Insurance Program, or other forms of public assistance
- have US citizenship or proof of legal residency
- file taxes jointly if married.
The premium tax credit caps the amount that an individual or family must spend on their monthly payments for health insurance. The cap depends on the family’s income; lower-income families have a lower cap. The amount of the tax credit remains the same, so a person who purchases a more expensive plan pays the cost difference (TABLE 2).
The ruling’s effect on women’s health
On June 26, American College of Obstetricians and Gynecologists President Mark S. DeFrancesco, MD, MBA, hailed the Supreme Court decision, saying, “Importantly, recent data have shown that newly insured adults under the ACA were more likely to be women. Those who did gain coverage through the ACA reported better access to health care and better financial security from medical costs.”
“Without question, many women enrollees were able to purchase health insurance coverage due, in part, to the ACA subsidies that helped make this purchase affordable. In fact, government data have suggested that roughly 85% of health exchange enrollees received subsidies,” Dr. DeFrancesco said.
“If the Supreme Court had overturned this important assistance, approximately 4.8 million women would have been unable to afford the coverage that they need. The impact also would have been widespread; as these women were forced to leave the insurance marketplace, it is likely that premiums throughout the marketplace would have risen dramatically,” he continued.
“Instead, patients—especially the low- and moderate-income American women who have particularly benefited from ACA subsidies—will continue to have the peace of mind that comes with insurance coverage.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Blumberg LJ, Buettgens M, Holahan J. The implications of a Supreme Court finding for the plaintiff in King v. Burwell: 8.2 million more uninsured and 35% higher premiums. Urban Institute. http://www.urban.org/research/publication/implications-supreme-court-finding-plaintiff-king-vs-burwell-82-million-more-uninsured-and-35-higher-premiums. Published January 8, 2015. Accessed July 2, 2015.
2. Sheiner L, Mochoruk B. King v. Burwell explained. Brookings Institute. http://www.brookings.edu/blogs/health360/posts/2015/03/03-king-v-burwell-explainer-sheiner. Published March 3, 2015. Accessed July 2, 2015.
3. Howe A. Court backs Obama administration on health care subsidies: In plain English. SCOTUSblog. http://www.scotusblog.com/2015/06/court-backs-obama-administration-on-health-care-subsidies-in-plain-english/. Published June 25, 2015. Accessed July 1, 2015.
4. Hall MA. King v. Burwell—ACA Armageddon averted. N Engl J Med. http://www.nejm.org/doi/full/10.1056/NEJMp1504077. Published July 1, 2015. Accessed July 2, 2015.
5. Jost T. Implementing health reform: The Supreme Court upholds tax credits in the federal exchange. Health Affairs blog. http://healthaffairs.org/blog/2015/06/25/implementing-health-reform-the-supreme-court-upholds-tax-credits-in-the-federal-exchange/. Published June 25, 2015. Accessed July 1, 2015.
6. Greenhouse L. The Roberts Court’s reality check. New York Times. http://www.nytimes.com/2015/06/26/opinion/the-roberts-courts-reality-check.html. Published June 25, 2015. Accessed July 1, 2015.
7. Henry J. Kaiser Family Foundation. Explaining health care reform: questions about health insurance subsidies. Table 2. http://kff.org/health-reform/issue-brief/explaining-health-care-reform-questions-about-health/. Published October 27, 2014. Accessed July 2, 2015.
1. Blumberg LJ, Buettgens M, Holahan J. The implications of a Supreme Court finding for the plaintiff in King v. Burwell: 8.2 million more uninsured and 35% higher premiums. Urban Institute. http://www.urban.org/research/publication/implications-supreme-court-finding-plaintiff-king-vs-burwell-82-million-more-uninsured-and-35-higher-premiums. Published January 8, 2015. Accessed July 2, 2015.
2. Sheiner L, Mochoruk B. King v. Burwell explained. Brookings Institute. http://www.brookings.edu/blogs/health360/posts/2015/03/03-king-v-burwell-explainer-sheiner. Published March 3, 2015. Accessed July 2, 2015.
3. Howe A. Court backs Obama administration on health care subsidies: In plain English. SCOTUSblog. http://www.scotusblog.com/2015/06/court-backs-obama-administration-on-health-care-subsidies-in-plain-english/. Published June 25, 2015. Accessed July 1, 2015.
4. Hall MA. King v. Burwell—ACA Armageddon averted. N Engl J Med. http://www.nejm.org/doi/full/10.1056/NEJMp1504077. Published July 1, 2015. Accessed July 2, 2015.
5. Jost T. Implementing health reform: The Supreme Court upholds tax credits in the federal exchange. Health Affairs blog. http://healthaffairs.org/blog/2015/06/25/implementing-health-reform-the-supreme-court-upholds-tax-credits-in-the-federal-exchange/. Published June 25, 2015. Accessed July 1, 2015.
6. Greenhouse L. The Roberts Court’s reality check. New York Times. http://www.nytimes.com/2015/06/26/opinion/the-roberts-courts-reality-check.html. Published June 25, 2015. Accessed July 1, 2015.
7. Henry J. Kaiser Family Foundation. Explaining health care reform: questions about health insurance subsidies. Table 2. http://kff.org/health-reform/issue-brief/explaining-health-care-reform-questions-about-health/. Published October 27, 2014. Accessed July 2, 2015.