User login
Extra Pulmonary Small Cell Carcinoma: A Single Center Experience
Purpose: Extrapulmonary small cell carcinoma (ESCC) has been a difficult disease to manage since it was first described > 80 years ago. Definitive treatment recommendations are lacking, and the treatment strategies commonly utilized are extrapolated from our experience with pulmonary small cell carcinoma. A better understanding of this entity will improve our collective management. By pooling our collective knowledge, we will hopefully be able to draw some meaningful conclusions about this disease.
Methods: The University of Kansas tumor registry was reviewed from 1990-2013. Forty-two potential cases of ESCC were identified and the charts reviewed. Of these, 35 cases met the inclusion and exclusion criteria for review. Information gathered included age, gender, smoking status, weight loss, metastatic disease related data, stage, ECOG performance status (PS), treatment received, and survival data.
Results: Patients were evaluated with a variety of primary locations of disease including gastrointestinal (GI) tract, genitourinary (GU) tract, pelvic organs, head and neck, and unknown primary. Several sites of metastatic disease were noted, with 57% and 43% of patients meeting criteria for limited disease and extensive disease, respectively. Chemotherapy, surgery, and radiation were used in several different regimens, with small-cell lung cancer-type regimens incorporating a platinum and etoposide being the most common. Kaplan-Meier survival estimates were used to identify possible prognostic variables including stage, primary site, number of treatment modalities received, use of chemotherapy in limited stage disease, and ECOG PS.
Conclusions: In our review of 35 patients, GI (29%) and GU (31%) tract tumors were the most common primary sites of disease with a male to female predominance and 57% of patients presenting with limited disease. The majority of patients treated with chemotherapy received a platinum/etoposide doublet (74%), and almost half received radiation (43%). Statistically significant prognostic factors included stage, ECOG PS, site of primary disease, use of chemotherapy, and number of treatment modalities used. Additional clinical trials are needed to better understand this rare disorder to further our knowledge of the optimum management approach.
Purpose: Extrapulmonary small cell carcinoma (ESCC) has been a difficult disease to manage since it was first described > 80 years ago. Definitive treatment recommendations are lacking, and the treatment strategies commonly utilized are extrapolated from our experience with pulmonary small cell carcinoma. A better understanding of this entity will improve our collective management. By pooling our collective knowledge, we will hopefully be able to draw some meaningful conclusions about this disease.
Methods: The University of Kansas tumor registry was reviewed from 1990-2013. Forty-two potential cases of ESCC were identified and the charts reviewed. Of these, 35 cases met the inclusion and exclusion criteria for review. Information gathered included age, gender, smoking status, weight loss, metastatic disease related data, stage, ECOG performance status (PS), treatment received, and survival data.
Results: Patients were evaluated with a variety of primary locations of disease including gastrointestinal (GI) tract, genitourinary (GU) tract, pelvic organs, head and neck, and unknown primary. Several sites of metastatic disease were noted, with 57% and 43% of patients meeting criteria for limited disease and extensive disease, respectively. Chemotherapy, surgery, and radiation were used in several different regimens, with small-cell lung cancer-type regimens incorporating a platinum and etoposide being the most common. Kaplan-Meier survival estimates were used to identify possible prognostic variables including stage, primary site, number of treatment modalities received, use of chemotherapy in limited stage disease, and ECOG PS.
Conclusions: In our review of 35 patients, GI (29%) and GU (31%) tract tumors were the most common primary sites of disease with a male to female predominance and 57% of patients presenting with limited disease. The majority of patients treated with chemotherapy received a platinum/etoposide doublet (74%), and almost half received radiation (43%). Statistically significant prognostic factors included stage, ECOG PS, site of primary disease, use of chemotherapy, and number of treatment modalities used. Additional clinical trials are needed to better understand this rare disorder to further our knowledge of the optimum management approach.
Purpose: Extrapulmonary small cell carcinoma (ESCC) has been a difficult disease to manage since it was first described > 80 years ago. Definitive treatment recommendations are lacking, and the treatment strategies commonly utilized are extrapolated from our experience with pulmonary small cell carcinoma. A better understanding of this entity will improve our collective management. By pooling our collective knowledge, we will hopefully be able to draw some meaningful conclusions about this disease.
Methods: The University of Kansas tumor registry was reviewed from 1990-2013. Forty-two potential cases of ESCC were identified and the charts reviewed. Of these, 35 cases met the inclusion and exclusion criteria for review. Information gathered included age, gender, smoking status, weight loss, metastatic disease related data, stage, ECOG performance status (PS), treatment received, and survival data.
Results: Patients were evaluated with a variety of primary locations of disease including gastrointestinal (GI) tract, genitourinary (GU) tract, pelvic organs, head and neck, and unknown primary. Several sites of metastatic disease were noted, with 57% and 43% of patients meeting criteria for limited disease and extensive disease, respectively. Chemotherapy, surgery, and radiation were used in several different regimens, with small-cell lung cancer-type regimens incorporating a platinum and etoposide being the most common. Kaplan-Meier survival estimates were used to identify possible prognostic variables including stage, primary site, number of treatment modalities received, use of chemotherapy in limited stage disease, and ECOG PS.
Conclusions: In our review of 35 patients, GI (29%) and GU (31%) tract tumors were the most common primary sites of disease with a male to female predominance and 57% of patients presenting with limited disease. The majority of patients treated with chemotherapy received a platinum/etoposide doublet (74%), and almost half received radiation (43%). Statistically significant prognostic factors included stage, ECOG PS, site of primary disease, use of chemotherapy, and number of treatment modalities used. Additional clinical trials are needed to better understand this rare disorder to further our knowledge of the optimum management approach.
Geographic Distribution of Rural-Urban Status of Women With Breast Cancer in Veterans Health Administration, Using 2 Plans: Rural Urban Continuum and Rural Urban Commuting Areas
Purpose: Women with breast cancer (BC) are increasingly diagnosed and treated within the VHA. Breast cancer requires specialized care in tertiary settings such as VAMCs, typically located in urban settings, placing BC patients in rural areas at a disadvantage. Assigning rural-urban status is complicated by the presence of multiple classification plans. In this report, we compare rural-urban status of BC patients in the VHA and its association with distance to nearest VAMC, using 2 plans: USDA Economic Research Service (ERS) Rural Urban Continuum (RUC) and University of Washington’s Rural Urban Commuting Areas 2.0 (RUCA).
Methods: Between 2000 and 2012, 3,622 women were diagnosed with and/or treated for BC within the VHA and recorded in the VA Central Cancer Registry (VA CCR). The patient’s zip code of residence at the time of diagnosis and rural-urban status according to USDA ERS RUC were obtained from the VA CCR. Rural urban commuting status was aggregated into 3 categories: metropolitan, large nonmetropolitan, and rural. Using zip code of residence, rural-urban status of all but 63 women was determined using the University of Washington’s (RUCA) plan and aggregated into 3 categories: urban (metropolitan), large rural or micropolitan, and small rural/isolated small rural. The VHA is organized into 21 regional administrative service networks, or VISNs. The geographic distribution of BC in VHA was determined using the RUC and RUCA scheme, then reported by VISN Census Bureau geographic region: Northeast, Midwest, South, and West. The two plans were compared, using Cohen’s Kappa statistic. The distance between zip code of residence and the nearest within-VISN VAMC was obtained from the VA Planning Systems Support Group database. The association between rural-urban status according to RUC and RUCA and the distance to the nearest VAMC was determined using analysis of variance (ANOVA).
Results: Rural-urban status according to RUC and RUCA were strongly associated (Cohen’s Kappa 0.74, P < .001). About 80% of women with BC in VHA resided in metropolitan areas; the remaining women were split evenly between large nonmetropolitan/micropolitan and rural/small, isolated rural. The Midwest had the highest percentages of both large rural (14%) and small/isolated rural patients (17%), whereas patients in the Northeast had the smallest percentages of large rural (8%) and small/isolated rural patients (7%). Patients living in the Northeast had the shortest travel distances to the nearest within-VISN VAMC, whereas patients in the West had the longest distances. In the Northeast, the average distance to nearest VAMC increased from 11 miles for patients living in metropolitan areas, to 44 miles in small/isolated rural areas. In the West, patients living in metropolitan areas were on average, 37 miles from nearest VAMC. This increased to 124 miles for patients in small/isolated rural areas in the West. Both classifications were significantly associated with increased distance to nearest VAMC (P < .001). On multivariate analysis, rural residence remained significantly associated with increased distance to nearest VAMC (P = .01) even after adjusting for RUCA.
Conclusions: Women with BC living in rural areas must travel longer distances to their VHA facility to receive specialized cancer care. Various plans define rural-urban status, using different methodologies. The rural-urban status of women with BC in VHA was similar using either RUC or RUCA. Rural residence defined by RUC was significantly associated with longer distances to VAMC even after adjusting for RUCA. This suggests that the 2 methodologies are not identical but are highly related when being compared with distance from tertiary care. The choice of rural classification methodology should be considered carefully when researching rural status and cancer outcomes.
Purpose: Women with breast cancer (BC) are increasingly diagnosed and treated within the VHA. Breast cancer requires specialized care in tertiary settings such as VAMCs, typically located in urban settings, placing BC patients in rural areas at a disadvantage. Assigning rural-urban status is complicated by the presence of multiple classification plans. In this report, we compare rural-urban status of BC patients in the VHA and its association with distance to nearest VAMC, using 2 plans: USDA Economic Research Service (ERS) Rural Urban Continuum (RUC) and University of Washington’s Rural Urban Commuting Areas 2.0 (RUCA).
Methods: Between 2000 and 2012, 3,622 women were diagnosed with and/or treated for BC within the VHA and recorded in the VA Central Cancer Registry (VA CCR). The patient’s zip code of residence at the time of diagnosis and rural-urban status according to USDA ERS RUC were obtained from the VA CCR. Rural urban commuting status was aggregated into 3 categories: metropolitan, large nonmetropolitan, and rural. Using zip code of residence, rural-urban status of all but 63 women was determined using the University of Washington’s (RUCA) plan and aggregated into 3 categories: urban (metropolitan), large rural or micropolitan, and small rural/isolated small rural. The VHA is organized into 21 regional administrative service networks, or VISNs. The geographic distribution of BC in VHA was determined using the RUC and RUCA scheme, then reported by VISN Census Bureau geographic region: Northeast, Midwest, South, and West. The two plans were compared, using Cohen’s Kappa statistic. The distance between zip code of residence and the nearest within-VISN VAMC was obtained from the VA Planning Systems Support Group database. The association between rural-urban status according to RUC and RUCA and the distance to the nearest VAMC was determined using analysis of variance (ANOVA).
Results: Rural-urban status according to RUC and RUCA were strongly associated (Cohen’s Kappa 0.74, P < .001). About 80% of women with BC in VHA resided in metropolitan areas; the remaining women were split evenly between large nonmetropolitan/micropolitan and rural/small, isolated rural. The Midwest had the highest percentages of both large rural (14%) and small/isolated rural patients (17%), whereas patients in the Northeast had the smallest percentages of large rural (8%) and small/isolated rural patients (7%). Patients living in the Northeast had the shortest travel distances to the nearest within-VISN VAMC, whereas patients in the West had the longest distances. In the Northeast, the average distance to nearest VAMC increased from 11 miles for patients living in metropolitan areas, to 44 miles in small/isolated rural areas. In the West, patients living in metropolitan areas were on average, 37 miles from nearest VAMC. This increased to 124 miles for patients in small/isolated rural areas in the West. Both classifications were significantly associated with increased distance to nearest VAMC (P < .001). On multivariate analysis, rural residence remained significantly associated with increased distance to nearest VAMC (P = .01) even after adjusting for RUCA.
Conclusions: Women with BC living in rural areas must travel longer distances to their VHA facility to receive specialized cancer care. Various plans define rural-urban status, using different methodologies. The rural-urban status of women with BC in VHA was similar using either RUC or RUCA. Rural residence defined by RUC was significantly associated with longer distances to VAMC even after adjusting for RUCA. This suggests that the 2 methodologies are not identical but are highly related when being compared with distance from tertiary care. The choice of rural classification methodology should be considered carefully when researching rural status and cancer outcomes.
Purpose: Women with breast cancer (BC) are increasingly diagnosed and treated within the VHA. Breast cancer requires specialized care in tertiary settings such as VAMCs, typically located in urban settings, placing BC patients in rural areas at a disadvantage. Assigning rural-urban status is complicated by the presence of multiple classification plans. In this report, we compare rural-urban status of BC patients in the VHA and its association with distance to nearest VAMC, using 2 plans: USDA Economic Research Service (ERS) Rural Urban Continuum (RUC) and University of Washington’s Rural Urban Commuting Areas 2.0 (RUCA).
Methods: Between 2000 and 2012, 3,622 women were diagnosed with and/or treated for BC within the VHA and recorded in the VA Central Cancer Registry (VA CCR). The patient’s zip code of residence at the time of diagnosis and rural-urban status according to USDA ERS RUC were obtained from the VA CCR. Rural urban commuting status was aggregated into 3 categories: metropolitan, large nonmetropolitan, and rural. Using zip code of residence, rural-urban status of all but 63 women was determined using the University of Washington’s (RUCA) plan and aggregated into 3 categories: urban (metropolitan), large rural or micropolitan, and small rural/isolated small rural. The VHA is organized into 21 regional administrative service networks, or VISNs. The geographic distribution of BC in VHA was determined using the RUC and RUCA scheme, then reported by VISN Census Bureau geographic region: Northeast, Midwest, South, and West. The two plans were compared, using Cohen’s Kappa statistic. The distance between zip code of residence and the nearest within-VISN VAMC was obtained from the VA Planning Systems Support Group database. The association between rural-urban status according to RUC and RUCA and the distance to the nearest VAMC was determined using analysis of variance (ANOVA).
Results: Rural-urban status according to RUC and RUCA were strongly associated (Cohen’s Kappa 0.74, P < .001). About 80% of women with BC in VHA resided in metropolitan areas; the remaining women were split evenly between large nonmetropolitan/micropolitan and rural/small, isolated rural. The Midwest had the highest percentages of both large rural (14%) and small/isolated rural patients (17%), whereas patients in the Northeast had the smallest percentages of large rural (8%) and small/isolated rural patients (7%). Patients living in the Northeast had the shortest travel distances to the nearest within-VISN VAMC, whereas patients in the West had the longest distances. In the Northeast, the average distance to nearest VAMC increased from 11 miles for patients living in metropolitan areas, to 44 miles in small/isolated rural areas. In the West, patients living in metropolitan areas were on average, 37 miles from nearest VAMC. This increased to 124 miles for patients in small/isolated rural areas in the West. Both classifications were significantly associated with increased distance to nearest VAMC (P < .001). On multivariate analysis, rural residence remained significantly associated with increased distance to nearest VAMC (P = .01) even after adjusting for RUCA.
Conclusions: Women with BC living in rural areas must travel longer distances to their VHA facility to receive specialized cancer care. Various plans define rural-urban status, using different methodologies. The rural-urban status of women with BC in VHA was similar using either RUC or RUCA. Rural residence defined by RUC was significantly associated with longer distances to VAMC even after adjusting for RUCA. This suggests that the 2 methodologies are not identical but are highly related when being compared with distance from tertiary care. The choice of rural classification methodology should be considered carefully when researching rural status and cancer outcomes.
Incidence of Second Primary Malignancies in Patients Treated for Thyroid Cancer: A Review of 51 Cases
Purpose: The incidence of the second primary cancer after treatment with radioactive iodine (RAI) is a concern for patients and clinicians. Our objective was to examine the changes that have occurred over a 20-year period in a single institution, by comparing the lifetime observed incidence of the second primary cancer vs those patients with the incidence after the treatment with RAI.
Method: We reviewed all thyroid cancer cases (n = 51) between 1991 and 2010 in the VA New Jersey cancer registry. Patients were distributed into 4 groups consisting of 5-year intervals. These groups were based on accession year and then segregated again into groups that received radioactive iodine and groups that did not. Then, we reviewed the incidence of multiple primaries during the life of the patient and the incidence of a second primary after the diagnoses of thyroid cancer. The incidence of second primary malignancy and age distribution of these groups over the study period was also compared.
Results: An increase in the number of cases diagnosed over the last 20 years was noted. There were a total of 12 cases in the first 10 years compared with 39 cases in the last 10 years. During this period, the total number of all cancer cases accessioned by the registry per year remained stable and constant. However, the increases were significant in the last 5 years; comprising more than half of all cases diagnosed in this 20-year period. There was an observed difference in the average age at diagnosis with the mean age for the first 10 years being 56 years in comparison to the past 10 years, which was 61 years. Also, patients who received radioactive iodine were consistently younger than the group who did not receive RAI. The lifetime incidence of multiple primaries was about 50% in the entire group. The incidence remains similar in each period examined and in all the groups. However, there is an observed difference in the incidence of second primary in the RAI group after treatment compared with those who did not received RAI.
Conclusions: There was an increase in the incidence of thyroid cancer diagnosed over the 20-year period, and the overall incidence of multiple primaries during this time span remained consistent. However, the observed incidence of the second primary after the diagnoses of thyroid cancer was higher in the group who received RAI treatment.
Purpose: The incidence of the second primary cancer after treatment with radioactive iodine (RAI) is a concern for patients and clinicians. Our objective was to examine the changes that have occurred over a 20-year period in a single institution, by comparing the lifetime observed incidence of the second primary cancer vs those patients with the incidence after the treatment with RAI.
Method: We reviewed all thyroid cancer cases (n = 51) between 1991 and 2010 in the VA New Jersey cancer registry. Patients were distributed into 4 groups consisting of 5-year intervals. These groups were based on accession year and then segregated again into groups that received radioactive iodine and groups that did not. Then, we reviewed the incidence of multiple primaries during the life of the patient and the incidence of a second primary after the diagnoses of thyroid cancer. The incidence of second primary malignancy and age distribution of these groups over the study period was also compared.
Results: An increase in the number of cases diagnosed over the last 20 years was noted. There were a total of 12 cases in the first 10 years compared with 39 cases in the last 10 years. During this period, the total number of all cancer cases accessioned by the registry per year remained stable and constant. However, the increases were significant in the last 5 years; comprising more than half of all cases diagnosed in this 20-year period. There was an observed difference in the average age at diagnosis with the mean age for the first 10 years being 56 years in comparison to the past 10 years, which was 61 years. Also, patients who received radioactive iodine were consistently younger than the group who did not receive RAI. The lifetime incidence of multiple primaries was about 50% in the entire group. The incidence remains similar in each period examined and in all the groups. However, there is an observed difference in the incidence of second primary in the RAI group after treatment compared with those who did not received RAI.
Conclusions: There was an increase in the incidence of thyroid cancer diagnosed over the 20-year period, and the overall incidence of multiple primaries during this time span remained consistent. However, the observed incidence of the second primary after the diagnoses of thyroid cancer was higher in the group who received RAI treatment.
Purpose: The incidence of the second primary cancer after treatment with radioactive iodine (RAI) is a concern for patients and clinicians. Our objective was to examine the changes that have occurred over a 20-year period in a single institution, by comparing the lifetime observed incidence of the second primary cancer vs those patients with the incidence after the treatment with RAI.
Method: We reviewed all thyroid cancer cases (n = 51) between 1991 and 2010 in the VA New Jersey cancer registry. Patients were distributed into 4 groups consisting of 5-year intervals. These groups were based on accession year and then segregated again into groups that received radioactive iodine and groups that did not. Then, we reviewed the incidence of multiple primaries during the life of the patient and the incidence of a second primary after the diagnoses of thyroid cancer. The incidence of second primary malignancy and age distribution of these groups over the study period was also compared.
Results: An increase in the number of cases diagnosed over the last 20 years was noted. There were a total of 12 cases in the first 10 years compared with 39 cases in the last 10 years. During this period, the total number of all cancer cases accessioned by the registry per year remained stable and constant. However, the increases were significant in the last 5 years; comprising more than half of all cases diagnosed in this 20-year period. There was an observed difference in the average age at diagnosis with the mean age for the first 10 years being 56 years in comparison to the past 10 years, which was 61 years. Also, patients who received radioactive iodine were consistently younger than the group who did not receive RAI. The lifetime incidence of multiple primaries was about 50% in the entire group. The incidence remains similar in each period examined and in all the groups. However, there is an observed difference in the incidence of second primary in the RAI group after treatment compared with those who did not received RAI.
Conclusions: There was an increase in the incidence of thyroid cancer diagnosed over the 20-year period, and the overall incidence of multiple primaries during this time span remained consistent. However, the observed incidence of the second primary after the diagnoses of thyroid cancer was higher in the group who received RAI treatment.
New Developments in Chronic Lymphocytic Leukemia Treatment
Recently, Federal Practitioner talked with Sanjai Sharma, MD, about how signaling pathways in chronic lymphocytic leukemia (CLL) is critical to the development of therapeutic agents to treat this disease. Ibrutinib and idelalisib are therapeutic agents that block signaling pathways and, therefore, inhibit the growth of CLL cells.
For more information about CLL, read "Signaling Pathways and Novel Inhibitors in Chronic Lymphocytic Leukemia," in our August 2014 issue.
Dr. Sharma is a physician at the West Los Angeles VA Medical Center and associate professor in the Department of Medicine, Hematology/Oncology at UCLA, both in California.
Recently, Federal Practitioner talked with Sanjai Sharma, MD, about how signaling pathways in chronic lymphocytic leukemia (CLL) is critical to the development of therapeutic agents to treat this disease. Ibrutinib and idelalisib are therapeutic agents that block signaling pathways and, therefore, inhibit the growth of CLL cells.
For more information about CLL, read "Signaling Pathways and Novel Inhibitors in Chronic Lymphocytic Leukemia," in our August 2014 issue.
Dr. Sharma is a physician at the West Los Angeles VA Medical Center and associate professor in the Department of Medicine, Hematology/Oncology at UCLA, both in California.
Recently, Federal Practitioner talked with Sanjai Sharma, MD, about how signaling pathways in chronic lymphocytic leukemia (CLL) is critical to the development of therapeutic agents to treat this disease. Ibrutinib and idelalisib are therapeutic agents that block signaling pathways and, therefore, inhibit the growth of CLL cells.
For more information about CLL, read "Signaling Pathways and Novel Inhibitors in Chronic Lymphocytic Leukemia," in our August 2014 issue.
Dr. Sharma is a physician at the West Los Angeles VA Medical Center and associate professor in the Department of Medicine, Hematology/Oncology at UCLA, both in California.
Study explains BCR-ABL-independent imatinib resistance

A new study helps explain why some chronic myeloid leukemia (CML) patients develop resistance to imatinib despite the absence of BCR-ABL mutations.
Researchers discovered that a signaling pathway associated with cell division and growth acts as an alternative survival signal underlying imatinib resistance.
But blocking this pathway with an inhibitor known as trametinib can prevent resistance to imatinib and increase survival in mice.
The researchers recounted these discoveries in Science Translational Medicine.
Michael R. Green, MD, PhD, of the University of Massachusetts Medical School in Worcester, and his colleagues began this research with a large-scale RNA interference screen. This revealed a set of genes that promote imatinib sensitivity.
The team then set out to identify the regulatory pathways through which these genes promote imatinib sensitivity. They found that knocking down the genes in BCR-ABL+ cells results in sustained RAF/MEK/ERK signaling after treatment with imatinib.
Further investigation revealed it is PRKCH, which encodes the protein kinase C family member PKCη, that increases RAF/MEK/ERK signaling through phosphorylation and activation of CRAF.
Dr Green and his colleagues also found that PRKCH is upregulated in CML cell lines and patient samples that exhibit BCR-ABL-independent imatinib resistance. Experiments in mice revealed that PRKCH modulates the proliferation of BCR-ABL+ cells, CML progression, and imatinib sensitivity.
Furthermore, imatinib-resistant murine and human CML stem cells contained high levels of PRKCH. And experiments confirmed that high PRKCH expression contributed to the imatinib resistance observed in these cells.
Fortunately, the researchers discovered that combining imatinib with the MEK inhibitor trametinib can overcome BCR-ABL-independent imatinib resistance in CML cells. The combination also prolonged survival in mouse models of imatinib-resistant CML.
Dr Green and his colleagues said these results reveal a mechanism of BCR-ABL-independent imatinib resistance that can be targeted with therapy. And, as treatment with trametinib and imatinib kills CML stem cells but spares normal hematopoietic stem cells, it may be a feasible treatment option for CML patients. ![]()

A new study helps explain why some chronic myeloid leukemia (CML) patients develop resistance to imatinib despite the absence of BCR-ABL mutations.
Researchers discovered that a signaling pathway associated with cell division and growth acts as an alternative survival signal underlying imatinib resistance.
But blocking this pathway with an inhibitor known as trametinib can prevent resistance to imatinib and increase survival in mice.
The researchers recounted these discoveries in Science Translational Medicine.
Michael R. Green, MD, PhD, of the University of Massachusetts Medical School in Worcester, and his colleagues began this research with a large-scale RNA interference screen. This revealed a set of genes that promote imatinib sensitivity.
The team then set out to identify the regulatory pathways through which these genes promote imatinib sensitivity. They found that knocking down the genes in BCR-ABL+ cells results in sustained RAF/MEK/ERK signaling after treatment with imatinib.
Further investigation revealed it is PRKCH, which encodes the protein kinase C family member PKCη, that increases RAF/MEK/ERK signaling through phosphorylation and activation of CRAF.
Dr Green and his colleagues also found that PRKCH is upregulated in CML cell lines and patient samples that exhibit BCR-ABL-independent imatinib resistance. Experiments in mice revealed that PRKCH modulates the proliferation of BCR-ABL+ cells, CML progression, and imatinib sensitivity.
Furthermore, imatinib-resistant murine and human CML stem cells contained high levels of PRKCH. And experiments confirmed that high PRKCH expression contributed to the imatinib resistance observed in these cells.
Fortunately, the researchers discovered that combining imatinib with the MEK inhibitor trametinib can overcome BCR-ABL-independent imatinib resistance in CML cells. The combination also prolonged survival in mouse models of imatinib-resistant CML.
Dr Green and his colleagues said these results reveal a mechanism of BCR-ABL-independent imatinib resistance that can be targeted with therapy. And, as treatment with trametinib and imatinib kills CML stem cells but spares normal hematopoietic stem cells, it may be a feasible treatment option for CML patients. ![]()

A new study helps explain why some chronic myeloid leukemia (CML) patients develop resistance to imatinib despite the absence of BCR-ABL mutations.
Researchers discovered that a signaling pathway associated with cell division and growth acts as an alternative survival signal underlying imatinib resistance.
But blocking this pathway with an inhibitor known as trametinib can prevent resistance to imatinib and increase survival in mice.
The researchers recounted these discoveries in Science Translational Medicine.
Michael R. Green, MD, PhD, of the University of Massachusetts Medical School in Worcester, and his colleagues began this research with a large-scale RNA interference screen. This revealed a set of genes that promote imatinib sensitivity.
The team then set out to identify the regulatory pathways through which these genes promote imatinib sensitivity. They found that knocking down the genes in BCR-ABL+ cells results in sustained RAF/MEK/ERK signaling after treatment with imatinib.
Further investigation revealed it is PRKCH, which encodes the protein kinase C family member PKCη, that increases RAF/MEK/ERK signaling through phosphorylation and activation of CRAF.
Dr Green and his colleagues also found that PRKCH is upregulated in CML cell lines and patient samples that exhibit BCR-ABL-independent imatinib resistance. Experiments in mice revealed that PRKCH modulates the proliferation of BCR-ABL+ cells, CML progression, and imatinib sensitivity.
Furthermore, imatinib-resistant murine and human CML stem cells contained high levels of PRKCH. And experiments confirmed that high PRKCH expression contributed to the imatinib resistance observed in these cells.
Fortunately, the researchers discovered that combining imatinib with the MEK inhibitor trametinib can overcome BCR-ABL-independent imatinib resistance in CML cells. The combination also prolonged survival in mouse models of imatinib-resistant CML.
Dr Green and his colleagues said these results reveal a mechanism of BCR-ABL-independent imatinib resistance that can be targeted with therapy. And, as treatment with trametinib and imatinib kills CML stem cells but spares normal hematopoietic stem cells, it may be a feasible treatment option for CML patients. ![]()
Patch solves problem plaguing malaria vaccination

patches on her hand
Credit: Tómas Tyner
Delivering malaria vaccines via a skin patch can streamline the vaccination process, according to preclinical research published in Scientific Reports.
The patch has arrays of tiny silicon microneedles that painlessly create temporary pores in the outermost layer of the skin, permitting the vaccine to flow into the skin.
Researchers used the patch to vaccinate mice with a live adenovirus engineered to deliver a protein from the malaria parasite Plasmodium yoelii.
The team found the patch could overcome one of the main problems with this type of vaccine—namely, although it induces high levels of immunity to malaria, it can also induce a strong immune response to the adenovirus itself.
This anti-adenovirus immunity prevents its repeated use as a vaccine, as the immune system recognizes the adenovirus and prevents it from delivering the malaria protein. So another vaccine type or adenovirus strain needs to be used in the booster immunization.
“What’s exciting from this work is that administration of this vaccine with the microneedle patch did not induce this strong anti-adenovirus immunity, even though very potent immunity to the malaria antigen is generated,” said study author Anne Moore, PhD, of University College Cork in Ireland.
This suggests the patch can facilitate the repeated use of the same adenovirus vaccine, thereby potentially reducing manufacturing costs of multiple vaccines.
In their experiments with mice, Dr Moore and her colleagues demonstrated that using the microneedle patch in the primary immunization does indeed permit repeated use of the same adenovirus vaccine. And this immunization method induced potent and highly protective immune responses against malaria.
Specifically, the researchers delivered a vaccine known as HAdV5-PyMSP142 to mice. They found the patch induced equivalent or enhanced antibody responses but decreased anti-vector responses when compared to intradermal delivery of the vaccine.
The addition of a heterologous vaccine known as MVA-PyMSP142 also produced greater antibody responses in mice primed with HAdV5-PyMSP142 via the patch, compared to those vaccinated intradermally.
The researchers observed the highest protection against blood-stage malaria when mice were vaccinated first with the patch and then intradermally.
In an attempt to commercialize this research, Dr Moore is heading to Silicon Valley next week to meet with venture capitalists and technology companies. ![]()

patches on her hand
Credit: Tómas Tyner
Delivering malaria vaccines via a skin patch can streamline the vaccination process, according to preclinical research published in Scientific Reports.
The patch has arrays of tiny silicon microneedles that painlessly create temporary pores in the outermost layer of the skin, permitting the vaccine to flow into the skin.
Researchers used the patch to vaccinate mice with a live adenovirus engineered to deliver a protein from the malaria parasite Plasmodium yoelii.
The team found the patch could overcome one of the main problems with this type of vaccine—namely, although it induces high levels of immunity to malaria, it can also induce a strong immune response to the adenovirus itself.
This anti-adenovirus immunity prevents its repeated use as a vaccine, as the immune system recognizes the adenovirus and prevents it from delivering the malaria protein. So another vaccine type or adenovirus strain needs to be used in the booster immunization.
“What’s exciting from this work is that administration of this vaccine with the microneedle patch did not induce this strong anti-adenovirus immunity, even though very potent immunity to the malaria antigen is generated,” said study author Anne Moore, PhD, of University College Cork in Ireland.
This suggests the patch can facilitate the repeated use of the same adenovirus vaccine, thereby potentially reducing manufacturing costs of multiple vaccines.
In their experiments with mice, Dr Moore and her colleagues demonstrated that using the microneedle patch in the primary immunization does indeed permit repeated use of the same adenovirus vaccine. And this immunization method induced potent and highly protective immune responses against malaria.
Specifically, the researchers delivered a vaccine known as HAdV5-PyMSP142 to mice. They found the patch induced equivalent or enhanced antibody responses but decreased anti-vector responses when compared to intradermal delivery of the vaccine.
The addition of a heterologous vaccine known as MVA-PyMSP142 also produced greater antibody responses in mice primed with HAdV5-PyMSP142 via the patch, compared to those vaccinated intradermally.
The researchers observed the highest protection against blood-stage malaria when mice were vaccinated first with the patch and then intradermally.
In an attempt to commercialize this research, Dr Moore is heading to Silicon Valley next week to meet with venture capitalists and technology companies. ![]()

patches on her hand
Credit: Tómas Tyner
Delivering malaria vaccines via a skin patch can streamline the vaccination process, according to preclinical research published in Scientific Reports.
The patch has arrays of tiny silicon microneedles that painlessly create temporary pores in the outermost layer of the skin, permitting the vaccine to flow into the skin.
Researchers used the patch to vaccinate mice with a live adenovirus engineered to deliver a protein from the malaria parasite Plasmodium yoelii.
The team found the patch could overcome one of the main problems with this type of vaccine—namely, although it induces high levels of immunity to malaria, it can also induce a strong immune response to the adenovirus itself.
This anti-adenovirus immunity prevents its repeated use as a vaccine, as the immune system recognizes the adenovirus and prevents it from delivering the malaria protein. So another vaccine type or adenovirus strain needs to be used in the booster immunization.
“What’s exciting from this work is that administration of this vaccine with the microneedle patch did not induce this strong anti-adenovirus immunity, even though very potent immunity to the malaria antigen is generated,” said study author Anne Moore, PhD, of University College Cork in Ireland.
This suggests the patch can facilitate the repeated use of the same adenovirus vaccine, thereby potentially reducing manufacturing costs of multiple vaccines.
In their experiments with mice, Dr Moore and her colleagues demonstrated that using the microneedle patch in the primary immunization does indeed permit repeated use of the same adenovirus vaccine. And this immunization method induced potent and highly protective immune responses against malaria.
Specifically, the researchers delivered a vaccine known as HAdV5-PyMSP142 to mice. They found the patch induced equivalent or enhanced antibody responses but decreased anti-vector responses when compared to intradermal delivery of the vaccine.
The addition of a heterologous vaccine known as MVA-PyMSP142 also produced greater antibody responses in mice primed with HAdV5-PyMSP142 via the patch, compared to those vaccinated intradermally.
The researchers observed the highest protection against blood-stage malaria when mice were vaccinated first with the patch and then intradermally.
In an attempt to commercialize this research, Dr Moore is heading to Silicon Valley next week to meet with venture capitalists and technology companies. ![]()
Journals may fail to correct major errors in articles

Credit: CDC/James Gathany
A review of errata reports from medical publications revealed that nearly a quarter of the errors may have changed the way study data were interpreted.
And about half of the errors were not corrected in the original text, or the errata report did not specify whether a correction was made.
This suggests authors and journals must be more vigilant and consistent in identifying and reporting errors, said Paul Hauptman, MD, of Saint Louis University in Missouri.
He and his colleagues conducted this research and reported the results in The American Journal of Medicine.
The researchers reviewed 20 prominent English language journals in the fields of general medicine and cardiology. And they identified 577 error reports over an 18-month period.
More than 24% of these reports included an error the team rated as major. Major errors were associated with material changes in the interpretation of data in text, figures, or tables, or with significant alterations in the article’s conclusions.
One of the examples of a major error report in the study comes from a paper about depression. In the original article, the incidence of new depression cases was misquoted by a factor of 10. The article initially reported 15.8% of women with new depression, rather than the real figure of 1.58%, which was later corrected.
“As the volume of research publications continues to rise, the scientific community needs to examine how it manages its mistakes,” said Eric Armbrecht, PhD, of the Saint Louis University Center for Outcomes Research.
“Transparency, consistency, and clarity are essential. Our study found that these are not common among some of the top medical journals.”
In fact, the researchers found that 51% of the errors they identified were not corrected in the original text, or the errata report did not specify whether a correction was made.
The team noted that this study did not provide any definitive explanations for why such errors appear in publications, although one possibility is that most authors don’t read and edit the final version of their manuscripts prior to publication.
“It’s noteworthy that although final approval of an article may fall to the first or corresponding author, the criteria put forth by [the International Committee of Medical Journal Editors] specifies that each author must provide final approval of the version to be published,” Dr Hauptman said.
He added that, at this point, it’s not possible to measure how frequently a journal reader incorporates errata into clinical care or the extent to which patient outcomes may be affected. ![]()

Credit: CDC/James Gathany
A review of errata reports from medical publications revealed that nearly a quarter of the errors may have changed the way study data were interpreted.
And about half of the errors were not corrected in the original text, or the errata report did not specify whether a correction was made.
This suggests authors and journals must be more vigilant and consistent in identifying and reporting errors, said Paul Hauptman, MD, of Saint Louis University in Missouri.
He and his colleagues conducted this research and reported the results in The American Journal of Medicine.
The researchers reviewed 20 prominent English language journals in the fields of general medicine and cardiology. And they identified 577 error reports over an 18-month period.
More than 24% of these reports included an error the team rated as major. Major errors were associated with material changes in the interpretation of data in text, figures, or tables, or with significant alterations in the article’s conclusions.
One of the examples of a major error report in the study comes from a paper about depression. In the original article, the incidence of new depression cases was misquoted by a factor of 10. The article initially reported 15.8% of women with new depression, rather than the real figure of 1.58%, which was later corrected.
“As the volume of research publications continues to rise, the scientific community needs to examine how it manages its mistakes,” said Eric Armbrecht, PhD, of the Saint Louis University Center for Outcomes Research.
“Transparency, consistency, and clarity are essential. Our study found that these are not common among some of the top medical journals.”
In fact, the researchers found that 51% of the errors they identified were not corrected in the original text, or the errata report did not specify whether a correction was made.
The team noted that this study did not provide any definitive explanations for why such errors appear in publications, although one possibility is that most authors don’t read and edit the final version of their manuscripts prior to publication.
“It’s noteworthy that although final approval of an article may fall to the first or corresponding author, the criteria put forth by [the International Committee of Medical Journal Editors] specifies that each author must provide final approval of the version to be published,” Dr Hauptman said.
He added that, at this point, it’s not possible to measure how frequently a journal reader incorporates errata into clinical care or the extent to which patient outcomes may be affected. ![]()

Credit: CDC/James Gathany
A review of errata reports from medical publications revealed that nearly a quarter of the errors may have changed the way study data were interpreted.
And about half of the errors were not corrected in the original text, or the errata report did not specify whether a correction was made.
This suggests authors and journals must be more vigilant and consistent in identifying and reporting errors, said Paul Hauptman, MD, of Saint Louis University in Missouri.
He and his colleagues conducted this research and reported the results in The American Journal of Medicine.
The researchers reviewed 20 prominent English language journals in the fields of general medicine and cardiology. And they identified 577 error reports over an 18-month period.
More than 24% of these reports included an error the team rated as major. Major errors were associated with material changes in the interpretation of data in text, figures, or tables, or with significant alterations in the article’s conclusions.
One of the examples of a major error report in the study comes from a paper about depression. In the original article, the incidence of new depression cases was misquoted by a factor of 10. The article initially reported 15.8% of women with new depression, rather than the real figure of 1.58%, which was later corrected.
“As the volume of research publications continues to rise, the scientific community needs to examine how it manages its mistakes,” said Eric Armbrecht, PhD, of the Saint Louis University Center for Outcomes Research.
“Transparency, consistency, and clarity are essential. Our study found that these are not common among some of the top medical journals.”
In fact, the researchers found that 51% of the errors they identified were not corrected in the original text, or the errata report did not specify whether a correction was made.
The team noted that this study did not provide any definitive explanations for why such errors appear in publications, although one possibility is that most authors don’t read and edit the final version of their manuscripts prior to publication.
“It’s noteworthy that although final approval of an article may fall to the first or corresponding author, the criteria put forth by [the International Committee of Medical Journal Editors] specifies that each author must provide final approval of the version to be published,” Dr Hauptman said.
He added that, at this point, it’s not possible to measure how frequently a journal reader incorporates errata into clinical care or the extent to which patient outcomes may be affected. ![]()
Cellular RNA can template DNA repair in yeast
DNA has been repaired by
transcript RNA within the cells
Credit: Georgia Tech/Rob Felt
Scientists have shown that RNA produced within yeast cells can serve as a template for repairing the most devastating DNA damage—a break in both strands of a DNA helix.
The group believes their study is the first to show that a cell’s own RNA can be used for DNA recombination and repair.
If the phenomenon extends to human cells, it could potentially lead to new therapeutic or preventative strategies for genetic diseases.
The scientists described the phenomenon in Nature.
“We have found that genetic information can flow from RNA to DNA in a homology-driven manner, from cellular RNA to a homologous DNA sequence,” said study author Francesca Storici, PhD, of the Georgia Institute of Technology in Atlanta.
“This process is moving the genetic information in the opposite direction from which it normally flows. We have shown that when an endogenous RNA molecule can anneal to broken homologous DNA without being removed, the RNA can repair the damaged DNA. This finding reveals the existence of a novel mechanism of genetic recombination.”
Dr Storici’s team previously showed that synthetic RNA introduced into cells—including human cells—could repair DNA damage. But the process was inefficient, and there were questions about whether it could occur naturally.
To find out whether cells could use endogenous RNA transcripts to repair DNA damage, she and her colleagues devised experiments using the yeast Saccharomyces cerevisiae.
The team developed a strategy for distinguishing repair by endogenous RNA from repair by the normal DNA-based mechanisms in the budding yeast cells, including using mutants that lacked the ability to convert the RNA into a DNA copy.
They then induced a DNA double-strand break in the yeast genome and observed whether the organism could survive and grow by repairing the damage using only transcript RNA within the cells.
The DNA region that generates the transcript was constructed to contain a marker gene interrupted by an intron. Following intron removal during transcription, the transcript RNA sequence has no intron, while the DNA region that generates the transcript retains the intron.
Only the repair templated by the transcript devoid of the intron can restore the function of a homologous marker gene in which the DNA double-strand break is induced.
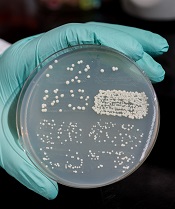
Dr Storici and her colleagues measured success by counting the number of yeast colonies growing on a Petri dish, indicating that the repair had been made by endogenous RNA.
They conducted testing on two types of breaks, one in the DNA from which the RNA transcript had been made, and the other in a homologous sequence from a different location in the DNA.
The team found that proximity of the RNA to the broken DNA increased the efficiency of the repair, and the repair occurred via a homologous recombination process. Dr Storici believes the repair mechanism may operate in cells beyond yeast, and many types of RNA can be used.
“We are showing that the flow of genetic information from RNA to DNA is not restricted to retro-elements and telomeres but occurs with a generic cellular transcript, making it more of a general phenomenon than had been anticipated,” she explained. “Potentially, any RNA in the cell could have this function.”
For the future, Dr Storici hopes to learn more about the mechanism, including what regulates it. She also wants to determine whether it takes place in human cells. If so, that could have implications for treating or preventing diseases caused by genetic damage.
“Cells synthesize lots of RNA transcripts during their life spans,” Dr Storici said. “Therefore, RNA may have an unanticipated impact on genomic stability and plasticity. We need to understand in which situations cells would activate RNA-DNA recombination. Better understanding this molecular process could also help us manipulate mechanisms for therapy, allowing us to treat a disease or prevent it altogether.” ![]()

DNA has been repaired by
transcript RNA within the cells
Credit: Georgia Tech/Rob Felt
Scientists have shown that RNA produced within yeast cells can serve as a template for repairing the most devastating DNA damage—a break in both strands of a DNA helix.
The group believes their study is the first to show that a cell’s own RNA can be used for DNA recombination and repair.
If the phenomenon extends to human cells, it could potentially lead to new therapeutic or preventative strategies for genetic diseases.
The scientists described the phenomenon in Nature.
“We have found that genetic information can flow from RNA to DNA in a homology-driven manner, from cellular RNA to a homologous DNA sequence,” said study author Francesca Storici, PhD, of the Georgia Institute of Technology in Atlanta.
“This process is moving the genetic information in the opposite direction from which it normally flows. We have shown that when an endogenous RNA molecule can anneal to broken homologous DNA without being removed, the RNA can repair the damaged DNA. This finding reveals the existence of a novel mechanism of genetic recombination.”
Dr Storici’s team previously showed that synthetic RNA introduced into cells—including human cells—could repair DNA damage. But the process was inefficient, and there were questions about whether it could occur naturally.
To find out whether cells could use endogenous RNA transcripts to repair DNA damage, she and her colleagues devised experiments using the yeast Saccharomyces cerevisiae.
The team developed a strategy for distinguishing repair by endogenous RNA from repair by the normal DNA-based mechanisms in the budding yeast cells, including using mutants that lacked the ability to convert the RNA into a DNA copy.
They then induced a DNA double-strand break in the yeast genome and observed whether the organism could survive and grow by repairing the damage using only transcript RNA within the cells.
The DNA region that generates the transcript was constructed to contain a marker gene interrupted by an intron. Following intron removal during transcription, the transcript RNA sequence has no intron, while the DNA region that generates the transcript retains the intron.
Only the repair templated by the transcript devoid of the intron can restore the function of a homologous marker gene in which the DNA double-strand break is induced.
Dr Storici and her colleagues measured success by counting the number of yeast colonies growing on a Petri dish, indicating that the repair had been made by endogenous RNA.
They conducted testing on two types of breaks, one in the DNA from which the RNA transcript had been made, and the other in a homologous sequence from a different location in the DNA.
The team found that proximity of the RNA to the broken DNA increased the efficiency of the repair, and the repair occurred via a homologous recombination process. Dr Storici believes the repair mechanism may operate in cells beyond yeast, and many types of RNA can be used.
“We are showing that the flow of genetic information from RNA to DNA is not restricted to retro-elements and telomeres but occurs with a generic cellular transcript, making it more of a general phenomenon than had been anticipated,” she explained. “Potentially, any RNA in the cell could have this function.”
For the future, Dr Storici hopes to learn more about the mechanism, including what regulates it. She also wants to determine whether it takes place in human cells. If so, that could have implications for treating or preventing diseases caused by genetic damage.
“Cells synthesize lots of RNA transcripts during their life spans,” Dr Storici said. “Therefore, RNA may have an unanticipated impact on genomic stability and plasticity. We need to understand in which situations cells would activate RNA-DNA recombination. Better understanding this molecular process could also help us manipulate mechanisms for therapy, allowing us to treat a disease or prevent it altogether.” ![]()

DNA has been repaired by
transcript RNA within the cells
Credit: Georgia Tech/Rob Felt
Scientists have shown that RNA produced within yeast cells can serve as a template for repairing the most devastating DNA damage—a break in both strands of a DNA helix.
The group believes their study is the first to show that a cell’s own RNA can be used for DNA recombination and repair.
If the phenomenon extends to human cells, it could potentially lead to new therapeutic or preventative strategies for genetic diseases.
The scientists described the phenomenon in Nature.
“We have found that genetic information can flow from RNA to DNA in a homology-driven manner, from cellular RNA to a homologous DNA sequence,” said study author Francesca Storici, PhD, of the Georgia Institute of Technology in Atlanta.
“This process is moving the genetic information in the opposite direction from which it normally flows. We have shown that when an endogenous RNA molecule can anneal to broken homologous DNA without being removed, the RNA can repair the damaged DNA. This finding reveals the existence of a novel mechanism of genetic recombination.”
Dr Storici’s team previously showed that synthetic RNA introduced into cells—including human cells—could repair DNA damage. But the process was inefficient, and there were questions about whether it could occur naturally.
To find out whether cells could use endogenous RNA transcripts to repair DNA damage, she and her colleagues devised experiments using the yeast Saccharomyces cerevisiae.
The team developed a strategy for distinguishing repair by endogenous RNA from repair by the normal DNA-based mechanisms in the budding yeast cells, including using mutants that lacked the ability to convert the RNA into a DNA copy.
They then induced a DNA double-strand break in the yeast genome and observed whether the organism could survive and grow by repairing the damage using only transcript RNA within the cells.
The DNA region that generates the transcript was constructed to contain a marker gene interrupted by an intron. Following intron removal during transcription, the transcript RNA sequence has no intron, while the DNA region that generates the transcript retains the intron.
Only the repair templated by the transcript devoid of the intron can restore the function of a homologous marker gene in which the DNA double-strand break is induced.
Dr Storici and her colleagues measured success by counting the number of yeast colonies growing on a Petri dish, indicating that the repair had been made by endogenous RNA.
They conducted testing on two types of breaks, one in the DNA from which the RNA transcript had been made, and the other in a homologous sequence from a different location in the DNA.
The team found that proximity of the RNA to the broken DNA increased the efficiency of the repair, and the repair occurred via a homologous recombination process. Dr Storici believes the repair mechanism may operate in cells beyond yeast, and many types of RNA can be used.
“We are showing that the flow of genetic information from RNA to DNA is not restricted to retro-elements and telomeres but occurs with a generic cellular transcript, making it more of a general phenomenon than had been anticipated,” she explained. “Potentially, any RNA in the cell could have this function.”
For the future, Dr Storici hopes to learn more about the mechanism, including what regulates it. She also wants to determine whether it takes place in human cells. If so, that could have implications for treating or preventing diseases caused by genetic damage.
“Cells synthesize lots of RNA transcripts during their life spans,” Dr Storici said. “Therefore, RNA may have an unanticipated impact on genomic stability and plasticity. We need to understand in which situations cells would activate RNA-DNA recombination. Better understanding this molecular process could also help us manipulate mechanisms for therapy, allowing us to treat a disease or prevent it altogether.” ![]()
VIDEO: Rivaroxaban provides advantages for cardioversion in AF
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2014
Successful Treatment of Schnitzler Syndrome With Canakinumab
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
To the Editor:
Schnitzler syndrome occurs with a triad of chronic urticaria, recurring fevers, and monoclonal gammopathy. It was recognized as a clinical entity in 1972; now nearly 200 patients are reported in the medical literature.1-3 Flulike symptoms, arthralgia, bone pain, lymphadenopathy, and hepatosplenomegaly also are clinical findings.4,5 The erythrocyte sedimentation rate (ESR) often is markedly elevated, as are other acute phase reactants. Leukocytosis with neutrophilia and IgM and IgG monoclonal gammopathies have been described.4
Schnitzler syndrome shares many clinical characteristics with a subset of autoinflammatory disorders referred to as cryopyrin-associated periodic syndromes (CAPS), which includes familial cold autoinflammatory syndrome and Muckle-Wellssyndrome. These syndromes are associated with mutations in the cold-induced autoinflammatory syndrome 1 gene, CIAS1, which encodes the NALP3 inflammasome, leading to overproduction of IL-1β.5 A gain-of-function mutation in CIAS1 has been described in a patient with Schnitzler syndrome.6
Treatment of urticaria and constitutional symptoms associated with Schnitzler syndrome is challenging. Antihistamines are ineffective, though high-dose systemic glucocorticosteroids control most of the clinical manifestations. Methotrexate sodium, cyclosporine, and tumor necrosis factor antagonists are utilized as glucocorticosteroid-sparing agents. Anakinra, an IL-1 receptor monoclonal antibody that is approved for use in CAPS, has been reported to induce complete resolution of Schnitzler syndrome when administered daily; however, it is not approved by the US Food and Drug Administration for this disorder.7 Canakinumab, an IL-1β monoclonal antibody that is dosed every 8 weeks, was approved by the US Food and Drug Administration in 2009 for the treatment of CAPS. Given the similar clinical characteristics and genetic mutations found in CAPS and Schnitzler syndrome, canakinumab may be an effective treatment of both disorders. We report successful treatment with this monoclonal antibody in 2 patients with Schnitzler syndrome.
A 63-year-old man reported having night sweats and fatigue but had no arthralgia or arthritis. He had a 1-year history of severe urticaria and recurrent fevers (temperature, up to 38.4°C) and he also had type 1 diabetes mellitus, hypothyroidism, and celiac disease. Physical examination revealed an elevated temperature (38.4°C) and generalized urticaria but no evidence of hepatosplenomegaly, adenopathy, or arthritis. Leukocytosis was revealed (white blood cell count, 12,400/μL [reference range, 4500–11,000/μL]) with neutrophilia (88.5% [reference range, 56%]), elevated ESR (81 mm/h [reference range, 0–20 mm/h]), and IgM κ monoclonal gammopathy (0.37 g/L [reference range, 0.4–2.3 g/L]). Clinical examination as well as laboratory and imaging studies did not show evidence of malignancy or autoimmune disease. A skin biopsy identified neutrophilic urticaria without vasculitis. Prednisone 20 mg daily controlled the urticaria and fever, but symptoms recurred within days of glucocorticosteroid withdrawal.
A 47-year-old woman presented with a 7-year history of severe urticaria, fever (temperature, 38.9°C), myalgia, and arthralgia. She had a medical history of allergic rhinitis, gastroesophageal reflux disease, chronic pain syndrome, and depression. Physical examination revealed generalized urticaria with cervical and axillary lymphadenopathy 1 to 2 cm in size but no hepatosplenomegaly or arthritis. Prior evaluations for fever of unknown origin as well as autoimmune and malignant disorders were negative. Skin biopsies reported neutrophilic urticaria without vasculitis, and a lymph node biopsy from the left axilla revealed neutrophilic inflammation. A white blood cell count of 17,800/μL with 61.6% neutrophils, elevated C-reactive protein (153.4 mg/L [reference range, 0.08–3.1 mg/L]) and ESR (90 mm/h), and an IgG λ monoclonal gammopathy were present. She was previously treated with etanercept, methotrexate sodium, golimumab, and adalimumab, with only a partial response. For more than 5 years, prednisone 20 to 50 mg daily was necessary to control her symptoms. Cyclosporine 200 mg twice daily was added as a corticosteroid-sparing drug with partial response.
Both patients were diagnosed with Schnitzler syndrome and were started on canakinumab 150 mg administered subcutaneously in the upper arm every 8 weeks. Resolution of the urticaria and fevers occurred within 2 weeks, and all other medications for the treatment of Schnitzler syndrome were withdrawn without recurrence of symptoms after 3 years. The neutrophil count and acute phase reactants returned within reference range in each patient, but the monoclonal gammopathies remained unchanged. Patient 2 noted worsening of arthralgia after initiation of canakinumab, but long-term corticosteroid withdrawal was considered the cause. Patient 1 has been able to increase the interval of dosing to every 3 to 4 months without recurrence of symptoms. Patient 2 has not tolerated similar changes in dosing interval.
Canakinumab given at 8-week intervals was a safe and effective treatment of Schnitzler syndrome in this open trial of 2 patients. Anakinra also induces remission, but daily dosing is required. Cost may be a notable factor in the choice of therapy, as canakinumab costs substantially more per year than anakinra. Further investigation is required to determine if treatment with canakinumab will result in long-term remission and if less-frequent dosing will provide continued efficacy.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.
1. Schnitzler L. Lésions urticariennes chroniques permanentes (érythème pétaloïde?). Cas cliniques. nº 46 B. Journee Dermatologique d’Angers. October 1972.
2. Schnitzler L, Schubert B, Boasson M, et al. Urticaire chronique, lésions osseuses, macroglobulinémie IgM: maladie de Waldenstrӧm. Bull Soc Fr Dermatol Syphiligr. 1974;81:363.
3. Simon A, Asli B, Braun-Falco M, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562-568.
4. de Koning HD, Bodar EJ, van der Meer JW, et al. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment [published online ahead of print June 21, 2007]. Semin Arthritis Rheum. 2007;37:137-148.
5. Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. four new cases and review of the literature. Medicine (Baltimore). 2001;80:37-44.
6. Loock J, Lamprecht P, Timmann C, et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J Allergy Clin Immunol. 2010;125:500-502.
7. Ryan JG, de Koning HD, Beck LA, et al. IL-1 blockade in Schnitzler syndrome: ex vivo findings correlate with clinical remission [published online ahead of print October 22, 2007]. J Allergy Clin Immunol. 2008;121:260-262.

